User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
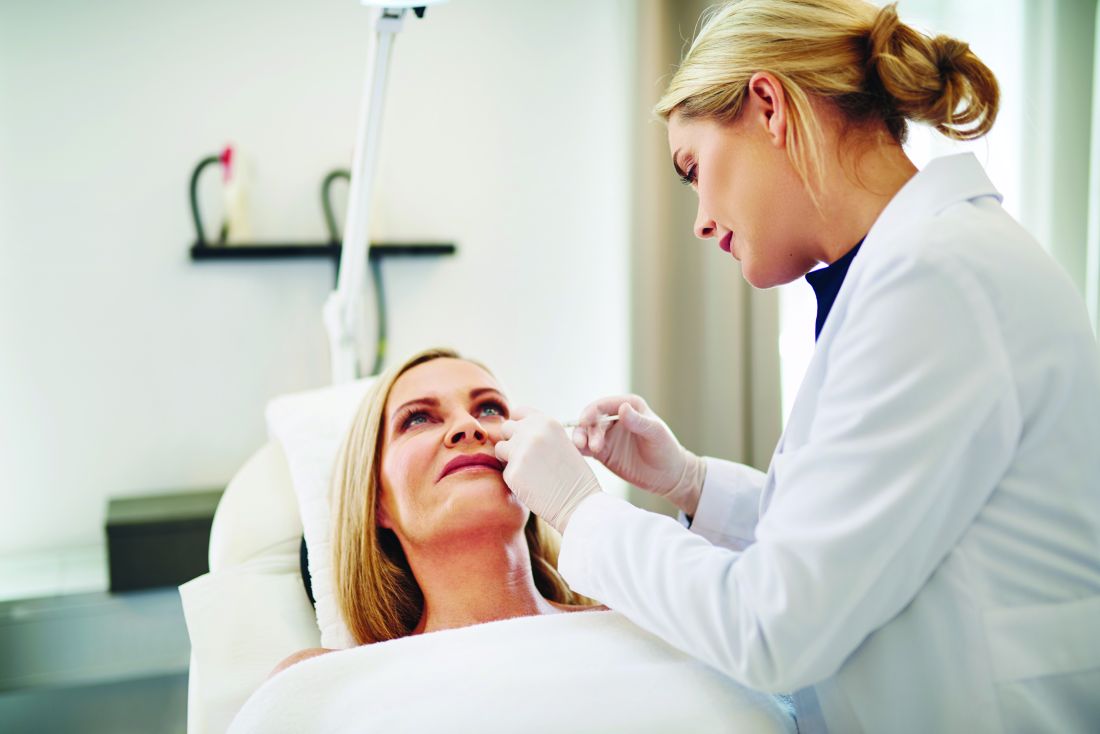
ASDS issues first filler safety recommendations
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
FDA grants emergency use authorization to Johnson & Johnson COVID-19 vaccine
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
And then there were three.
More vaccine availability at a time of high demand and limited supply could help officials vaccinate more Americans, more quickly. In addition, the J&J vaccine offers one-dose convenience and storage at conventional refrigeration temperatures.
Initial reactions to the EUA for the J&J vaccine have been positive.
“The advantages of having a third vaccine, especially one that is a single shot and can be stored without special refrigeration requirements, will be a major contribution in getting the general public vaccinated sooner, both in the U.S. and around the world,” Phyllis Tien, MD, professor of medicine in the division of infectious diseases at the University of California, San Francisco, told Medscape Medical News.
“It’s great news. We have yet a third vaccine that is highly effective at preventing COVID, and even more effective at preventing severe COVID,” said Paul Goepfert, MD. It’s a “tremendous boon for our country and other countries as well.”
“This vaccine has also been shown to be effective against the B.1.351 strain that was first described in South Africa,” added Dr. Goepfert, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama at Birmingham.
The EUA “is indeed exciting news,” Colleen Kraft, MD, associate chief medical officer at Emory University Hospital and associate professor at Emory University School of Medicine in Atlanta, said during a February 25 media briefing.
One recent concern centers on people aged 60 years and older. Documents the FDA released earlier this week suggest a lower efficacy, 42%, for the J&J immunization among people in this age group with certain relevant comorbidities. In contrast, without underlying conditions like heart disease or diabetes, efficacy in this cohort was 72%.
The more the merrier
The scope and urgency of the COVID-19 pandemic necessitates as many protective measures as possible, said Raj Shah, MD, geriatrician, and associate professor of family medicine and codirector of the Center for Community Health Equity at Rush University in Chicago.
“Trying to vaccinate as many individuals living in the United States to prevent the spread of COVID is such a big project that no one company or one vaccine was going to be able to ramp up fast enough on its own,” Dr. Shah told Medscape Medical News.“This has been the hope for us,” he added, “to get to multiple vaccines with slightly different properties that will provide more options.”
Experience with the J&J vaccine so far suggests reactions are less severe. “The nice thing about the Johnson and Johnson [vaccine] is that it definitely has less side effects,” Dr. Kraft said.
On the other hand, low-grade fever, chills, or fatigue after vaccination can be considered a positive because they can reflect how well the immune system is responding, she added.
One and done?
Single-dose administration could be more than a convenience — it could also help clinicians vaccinate members of underserved communities and rural locations, where returning for a second dose could be more difficult for some people.
“In a controlled setting, in a clinical trial, we do a lot to make sure people get all the treatment they need,” Dr. Shah said. “We’re not seeing it right now, but we’re always worried when we have more than one dose that has to be administered, that some people will drop off and not come back for the second vaccine.”
This group could include the needle-phobic, he added. “For them, having it done once alleviates a lot of the anxiety.”
Looking beyond the numbers
The phase 3 ENSEMBLE study of the J&J vaccine revealed a 72% efficacy for preventing moderate-to-severe COVID-19 among U.S. participants. In contrast, researchers reported 94% to 95% efficacy for the Pfizer/BioNTech and Moderna vaccines.
However, experts agreed that focusing solely on these numbers can miss more important points. For example, no participants who received the J&J vaccine in the phase 3 trial died from COVID-19-related illness. There were five such deaths in the placebo cohort.
“One of the things that these vaccines do very well is they minimize severe disease,” Dr. Kraft said. “As somebody that has spent an inordinate time in the hospital taking care of patients with severe disease from COVID, this is very much a welcome addition to our armamentarium to fight this virus.”
“If you can give something that prevents people from dying, that is a true path to normalcy,” Dr. Goepfert added.
More work to do
“The demand is strong from all groups right now. We just have to work on getting more vaccines out there,” Dr. Shah said.
“We are at a point in this country where we are getting better with the distribution of the vaccine,” he added, “but we are nowhere close to achieving that distribution of vaccines to get to everybody.”
Dr. Goepfert, Dr. Shah, and Dr. Kraft disclosed no relevant financial relationships. Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the San Francisco VA Health Care System.
A version of this article first appeared on Medscape.com.
J&J COVID-19 vaccine wins unanimous backing of FDA panel
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration (FDA) is expected to quickly provide an emergency use authorization (EUA) for the vaccine following the recommendation by the panel. The FDA’s Vaccines and Related Biological Products Advisory Committee voted 22-0 on this question: Based on the totality of scientific evidence available, do the benefits of the Johnson & Johnson COVID-19 Vaccine outweigh its risks for use in individuals 18 years of age and older?
The Johnson & Johnson vaccine is expected to offer more convenient dosing and be easier to distribute than the two rival products already available in the United States. Janssen’s vaccine is intended to be given in a single dose. In December, the FDA granted EUAs for the Pfizer/BioNTech and Moderna COVID-19 vaccines, which are each two-dose regimens.
Johnson & Johnson’s vaccine can be stored for at least 3 months at normal refrigerator temperatures of 2°C to 8°C (36°F to 46°F). Its shipping and storage fits into the existing medical supply infrastructure, the company said in its briefing materials for the FDA advisory committee meeting. In contrast, Pfizer’s vaccine is stored in ultracold freezers at temperatures between -80°C and -60°C (-112°F and -76°F), according to the Centers for Disease Control and Prevention. Moderna’s vaccine may be stored in a freezer between -25°C and -15°C (-13°F and 5°F).
But FDA advisers focused more in their deliberations on concerns about Janssen’s vaccine, including emerging reports of allergic reactions.
The advisers also discussed how patients might respond to the widely reported gap between Johnson & Johnson’s topline efficacy rates compared with rivals. The company’s initial unveiling last month of key results for its vaccine caused an initial wave of disappointment, with its overall efficacy against moderate-to-severe COVID-19 28 days postvaccination first reported at about 66% globally. By contrast, results for the Pfizer and Moderna vaccines suggest they have efficacy rates of 95% and 94%.
But in concluding, the advisers spoke of the Janssen vaccine as a much-needed tool to address the COVID-19 pandemic. The death toll in the United States attributed to the virus has reached 501,414, according to the World Health Organization.
“Despite the concerns that were raised during the discussion. I think what we have to keep in mind is that we’re still in the midst of this deadly pandemic,” said FDA adviser Archana Chatterjee, MD, PhD, from Rosalind Franklin University. “There is a shortage of vaccines that are currently authorized, and I think authorization of this vaccine will help meet the needs at the moment.”
The FDA is not bound to accept the recommendations of its advisers, but it often does so.
Anaphylaxis case
FDA advisers raised only a few questions for Johnson & Johnson and FDA staff ahead of their vote. The committee’s deliberations were less contentious and heated than had been during its December reviews of the Pfizer and Moderna vaccines. In those meetings, the panel voted 17-4, with one abstention, in favor of Pfizer’s vaccine and 20-0, with one abstention, on the Moderna vaccine.
“We are very comfortable now with the procedure, as well as the vaccines,” said Arnold Monto, MD, after the Feb. 26 vote on the Janssen vaccine. Dr. Monto, from the University of Michigan School of Public Health in Ann Arbor, has served as the chairman of the FDA panel through its review of all three COVID-19 vaccines.
Among the issues noted in the deliberations was the emergence of a concern about anaphylaxis with the vaccine.
This serious allergic reaction has been seen in people who have taken the Pfizer and Moderna vaccines. Before the week of the panel meeting, though, there had not been reports of anaphylaxis with the Johnson & Johnson vaccine, said Macaya Douoguih, MD, MPH, head of clinical development and medical affairs for Janssen/ Johnson & Johnson’s vaccines division.
However, on February 24, Johnson & Johnson received preliminary reports about two cases of severe allergic reaction from an open-label study in South Africa, with one of these being anaphylaxis, Dr. Douoguih said. The company will continue to closely monitor for these events as outlined in their pharmacovigilance plan, Dr. Douoguih said.
Federal health officials have sought to make clinicians aware of the rare risk for anaphylaxis with COVID vaccines, while reminding the public that this reaction can be managed.
The FDA had Tom Shimabukuro, MD, MPH, MBA, from the CDC, give an update on postmarketing surveillance for the Pfizer and Moderna vaccines as part of the review of the Johnson & Johnson application. Dr. Shimabukuro and CDC colleagues published a report in JAMA on February 14 that looked at an anaphylaxis case reported connected with COVID vaccines between December 14, 2020, and January 18, 2021.
The CDC identified 66 case reports received that met Brighton Collaboration case definition criteria for anaphylaxis (levels 1, 2, or 3): 47 following Pfizer/BioNTech vaccine, for a reporting rate of 4.7 cases/million doses administered, and 19 following Moderna vaccine, for a reporting rate of 2.5 cases/million doses administered, Dr. Shimabukuro and CDC colleagues wrote.
The CDC has published materials to help clinicians prepare for the possibility of this rare event, Dr. Shimabukuro told the FDA advisers.
“The take-home message here is that these are rare events and anaphylaxis, although clinically serious, is treatable,” Dr. Shimabukuro said.
At the conclusion of the meeting, FDA panelist Patrick Moore, MD, MPH, from the University of Pittsburgh in Pennsylvania, stressed the need to convey to the public that the COVID vaccines appear so far to be safe. Many people earlier had doubts about how the FDA could both safely and quickly review the applications for EUAs for these products.
“As of February 26, things are looking good. That could change tomorrow,” Dr. Moore said. But “this whole EUA process does seem to have worked, despite my own personal concerns about it.”
No second-class vaccines
The Johnson & Johnson vaccine, known as Ad26.COV2.S, is composed of a recombinant, replication-incompetent human adenovirus type 26 (Ad26) vector. It’s intended to encode a stabilized form of SARS-CoV-2 spike (S) protein. The Pfizer and Moderna vaccines use a different mechanism. They rely on mRNA.
The FDA advisers also discussed how patients might respond to the widely reported gap between Janssen’s topline efficacy rates compared with rivals. They urged against people parsing study details too finely and seeking to pick and choose their shots.
“It’s important that people do not think that one vaccine is better than another,” said FDA adviser H. Cody Meissner, MD, from Tufts University School of Medicine in Boston.
Dr. Monto agreed, noting that many people in the United States are still waiting for their turn to get COVID vaccines because of the limited early supply.
Trying to game the system to get one vaccine instead of another would not be wise. “In this environment, whatever you can get, get,” Dr. Monto said.
During an open public hearing, Sarah Christopherson, policy advocacy director of the National Women’s Health Network, said that press reports are fueling a damaging impression in the public that there are “first and second-class” vaccines.
“That has the potential to exacerbate existing mistrust” in vaccines, she said. “Public health authorities must address these perceptions head on.”
She urged against attempts to compare the Janssen vaccine to others, noting the potential effects of emerging variants of the virus.
“It’s difficult to make an apples-to-apples comparison between vaccines,” she said.
Johnson & Johnson’s efficacy results, which are lower than those of the mRNA vaccines, may be a reflection of the ways in which SARS-Co-V-2 is mutating and thus becoming more of a threat, according to the company. A key study of the new vaccine, involving about 44,000 people, coincided with the emergence of new SARS-CoV-2 variants, which were emerging in some of the countries where the pivotal COV3001 study was being conducted, the company said.
At least 14 days after vaccination, the Johnson & Johnson COVID vaccine efficacy (95% confidence interval) was 72.0% (58.2, 81.7) in the United States, 68.1% (48.8, 80.7) in Brazil, and 64.0% (41.2, 78.7) in South Africa.
Weakened standards?
Several researchers called on the FDA to maintain a critical attitude when assessing Johnson & Johnson’s application for the EUA, warning of a potential for a permanent erosion of agency rules due to hasty action on COVID vaccines.
They raised concerns about the FDA demanding too little in terms of follow-up studies on COVID vaccines and with persisting murkiness resulting in attempts to determine how well these treatments work beyond the initial study period.
“I worry about FDA lowering its approval standards,” said Peter Doshi, PhD, from The BMJ and a faculty member at the University of Maryland School of Medicine in Baltimore, during an open public hearing at the meeting.
“There’s a real urgency to stand back right now and look at the forest here, as well as the trees, and I urge the committee to consider the effects FDA decisions may have on the entire regulatory approval process,” Dr. Doshi said.
Dr. Doshi asked why Johnson & Johnson did not seek a standard full approval — a biologics license application (BLA) — instead of aiming for the lower bar of an EUA. The FDA already has allowed wide distribution of the Pfizer/BioNTech and Moderna vaccines through EUAs. That removes the sense of urgency that FDA faced last year in his view.
The FDA’s June 2020 guidance on the development of COVID vaccines had asked drugmakers to plan on following participants in COVID vaccine trials for “ideally at least one to two years.” Yet people who got placebo in Moderna and Pfizer trials already are being vaccinated, Dr. Doshi said. And Johnson & Johnson said in its presentation to the FDA that if the Ad26.COV2.S vaccine were granted an EUA, the COV3001 study design would be amended to “facilitate cross-over of placebo participants in all participating countries to receive one dose of active study vaccine as fast as operationally feasible.”
“I’m nervous about the prospect of there never being a COVID vaccine that meets the FDA’s approval standard” for a BLA instead of the more limited EUA, Dr. Doshi said.
Diana Zuckerman, PhD, president of the nonprofit National Center for Health Research, noted that the FDA’s subsequent guidance tailored for EUAs for COVID vaccines “drastically shortened” the follow-up time to a median of 2 months. Dr. Zuckerman said that a crossover design would be “a reasonable compromise, but only if the placebo group has at least 6 months of data.” Dr. Zuckerman opened her remarks in the open public hearing by saying she had inherited Johnson & Johnson stock, so was speaking at the meeting against her own financial interest.
“As soon as a vaccine is authorized, we start losing the placebo group. If FDA lets that happen, that’s a huge loss for public health and a huge loss of information about how we can all stay safe,” Dr. Zuckerman said.
A version of this article first appeared on Medscape.com.
Data on atopic dermatitis risk factors are accumulating
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
, according to Zelma Chiesa Fuxench, MD.
This gene codes for profilaggrin, a protein, which is then cleaved to form filaggrin, which helps to organize the cytoskeleton of the skin and is an important structural component of the skin. The understanding is that patients who have filaggrin mutations tend to have earlier onset and more persistent disease, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said during the Revolutionizing Atopic Dermatitis virtual symposium.
“Prior studies have shown that mutations in the FLG gene can confer a risk of developed AD that is two- to sevenfold with variants R501X and the 22804del4 frequently described. It is important to note that most of these findings have been described primarily in populations of European descent, with other variants being found in populations of African nation descent, and seem to be more prevalent in populations with early onset disease.”
Environmental factors
Other AD-related risk factors that have been previously described in the literature include environmental factors such as climate, diet, breastfeeding, obesity, pollution, tobacco smoke, pet ownership, and microbiome or gut microflora. “The list of culprits is ever increasing,” she said. “However, it’s important to recognize that data to support some of these associations are lacking, and oftentimes, a lot of the results are contradictory.”
As part of the International Study of Asthma and Allergies in Childhood, researchers evaluated the association between climate factors with the 12-month period prevalence rates of symptoms of atopic eczema in children. They found that patients who lived at higher latitudes and those who lived in areas where there were lower mean outdoor temperatures tended to have a higher prevalence of eczema symptoms. Worldwide, they found that symptoms of eczema were also prevalent in areas where there was lower indoor humidity.
“The authors concluded that they can’t really demonstrate a cause and effect, and that while latitude and temperature changes appear to affect the prevalence of eczema, they may do so indirectly, perhaps to changes in behavior and differences in sun exposure,” said Dr. Chiesa Fuxench, who was not involved with the study. “For example, we know that vitamin D is a protective risk factor for AD. Low vitamin D has been associated with more severe disease in some studies. We also know that UV exposure leads to the conversion of filaggrin degradation products such as trans-urocanic acid into cis-urocanic acid, which has been demonstrated to have immunosuppressive effects.”
A systematic review and meta-analysis of nine articles found small associations, which were significant, between being born in the winter (odds ratio, 1.15) and fall (OR, 1.16) and the risk of developing AD, compared with being born in the spring and summer. However, an analysis of satellite-derived data on air temperature across the United States from 1993 to 2011 found that as ambient air temperature increases, so did the risk for an ambulatory visit for AD to physicians from the National Ambulatory Medical Care Survey.
In all areas but the south, the largest number of AD visits occur in the spring. In the south, more AD visits occur in the summer. “This raises the point that we don’t really know everything when it comes to the influence of temperature and climate change on AD,” Dr. Chiesa Fuxench said.
Several maternal and neonatal risk factors for AD have been described in the literature, including the effect of prenatal exposure to antibiotics. In one large analysis, investigators assessed the association among 18-month-old children in the Danish National Birth Cohort, which included 62,560 mother-child pairs. They found that prenatal antibiotic use was associated with an increased odds of AD among children born to atopic mothers but only when used during all three trimesters (adjusted OR, 1.45). When they further stratified these analyses by type of birth (vaginal versus C-section), the association persisted in both groups, but was stronger among those delivered by C-section.
Probiotics
The role of probiotics to reduce the risk for AD has also been investigated. “We do know that probiotics could potentially be helpful, and it is often a readily available intervention,” Dr. Chiesa Fuxench said. “But the question still is how and when to supplement.”
In a systematic review and meta-analysis, researchers examined supplementation with probiotics given to breastfeeding mothers, pregnant mothers, or directly given to infants, and the risk of developing AD up to 18 months of age. They found that overall, probiotic exposure resulted in decreased risk of developing AD. In stratified analyses, the strongest association was observed for those who received probiotics during their pregnancy, during breastfeeding, and as an infant, which conferred about a 25% reduced risk.
Antibiotic exposure
What about early-life exposure to antibiotics on one’s risk for developing AD? A meta-analysis of 22 studies found that children who had been exposed to antibiotics during the first 2 years of life had an increased risk of eczema (OR, 1.26), compared with children who had not been exposed during the same period of time. “Interesting hypotheses can be generated from this study,” she said. “Perhaps future steps should focus on the impact of antibiotic exposure, the gut microbiome, and maternal risk factors for AD.”
In a separate study that supported these findings, researchers evaluated the association between the use of acid-suppressive medications and antibiotics during infancy and the development of allergic disease in early childhood. They found that exposure to either of these medications during the first six months of infancy resulted in a mild increased risk of developing AD, and concluded that they should be used during infancy only in situations of clear clinical benefit. “We should be good stewards of antibiotic use, in particular due to concern for antibiotic resistance in the population overall,” Dr. Chiesa Fuxench said.
Prevention strategies
Several AD prevention strategies have also been described in the medical literature, including the use of daily emollients during infancy. In a multicenter trial carried out in the United Kingdom, researchers tested whether daily use of emollient in the first year of life could prevent eczema in high-risk children, which was defined as having at least one first-degree relative with parent-reported eczema, allergic rhinitis, or asthma. The primary outcome was eczema at age 2 years. The researchers found no evidence to suggest that daily emollient use during the first year of life prevents eczema.
Another study, the PreventADALL trial of 2,397 infants, consisted of four treatment arms: a control group advised to follow national guidelines on infant nutrition; a skin intervention group that was asked to use skin emollients, a food intervention group with early introduction of peanut, cow’s milk, wheat, and egg, and a combined skin and food intervention. The investigators found no difference in the risk reduction of developing AD among patients who were treated with skin emollients or early complementary feeding, and concluded that these types of interventions should not be considered as interventions to prevent AD in this cohort of patients.
However, Dr. Chiesa Fuxench emphasized that emollients and moisturizers are an important part of the treatment regimen for AD patients. A Cochrane systematic review of nearly 80 randomized, controlled trials evaluating the use of emollients in eczema found that most moisturizers showed some beneficial effects in addition to active treatment, including prolonging the time to flare, reducing the number of flares, and reducing the amount of topical corticosteroids used.
For treatment, Dr. Chiesa Fuxench recommends a proactive approach focused on short-term induction therapy with intensive topical anti-inflammatories until the affected area is almost healed, followed by maintenance therapy that involves use of a long-term, low- to mid-potency steroid or a topical calcineurin inhibitor to previously affected areas. “These interventions have been shown to decrease the risk of recurrence and can shorten the treatment duration in the event of a flare,” she said.
She also favors a time-contingent approach to treating patients with AD. “As physicians, we tend to do our visits more as symptom contingent, which means when a patient is flaring. This reinforces the view that this is a difficult disease to treat, and that there is no hope,” she pointed out. But for chronic diseases, she added, “a time-contingent approach with appointments at set intervals leads to a different perception. It can result in better compliance, because skin care might be performed more regularly. It’s analogous to when you know you’re going to see the dentist so you floss more regularly the week before your appointment. There also seems to be less pressure on physicians and patients because you are seeing each other more frequently; you can talk more openly about what’s working and what’s not.”
Dr. Chiesa Fuxench reported having no disclosures relevant to her presentation.
FROM REVOLUTIONIZING AD 2020
AI detects ugly-duckling skin lesions for melanoma follow-up
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
.
The system could use photographs of large areas of patients’ bodies taken with ordinary cameras in primary care or by the patients themselves to screen for early-stage melanoma, said Luis R. Soenksen, PhD, a postdoctoral associate and venture builder at Massachusetts Institute of Technology in Cambridge, Mass.
“We believe we’re providing technology for that to happen at a massive scale, which is what is needed to reduce mortality rates,” he said in an interview.
He and his colleagues published their findings in Science Translational Medicine.
Diagnosing skin lesions has already proved one of the most promising medical applications of AI. In a 2017 paper, researchers reported that a deep neural network had classified skin lesions more accurately than did dermatologists. But so far, most such programs depend on experts to preselect the lesions worthy of analysis. And they use images from dermoscopy or single-lesion near-field photography.
Dr. Soenksen and colleagues wanted a system that could use a variety of cameras such as those in smartphones under a variety of conditions to assess lesions over wide areas of anatomy.
So they programmed their convolutional neural network to simultaneously use two approaches for screening lesions. Like the earlier systems, theirs looks for characteristics of individual lesions, such as asymmetry, border unevenness, color distribution, diameter, and evolution (ABCDE.) But it also looks for lesion saliency, a comparison of the lesions on the skin of one individual to identify the “ugly ducklings” that stand out from the rest.
They trained the system using 20,388 wide-field images from 133 patients at the Hospital Gregorio Marañón in Madrid, as well as publicly available images. The images were taken with a variety of consumer-grade cameras, about half of them nondermoscopy, and included backgrounds, skin edges, bare skin sections, nonsuspicious pigmented lesions, and suspicious pigmented lesions. The lesions in the images were visually classified by a consensus of three board-certified dermatologists.
Once they trained the system, the researchers tested it on another 6,796 images from the same patients, using the dermatologists’ classification as the gold standard. The system distinguished the suspicious lesions with 90.3% sensitivity (true positive), 89.9% specificity (true negative), and 86.56% accuracy.
Dr. Soenksen said he could envision photos acquired for screening in three scenarios. First, people could photograph themselves, or someone else at their homes could photograph them. These photos could even include whole nude bodies.
Second, clinicians could photograph patients’ body parts during medical visits for other purposes. “It makes sense to do these evaluations in the point of care where a referral can actually happen, like the primary care office,” said Dr. Soenksen.
Third, photos could be taken at places where people show up in bathing suits.
In each scenario, the system would then tell patients whether any lesions needed evaluation by a dermatologist.
To ensure privacy, Dr. Soenksen envisions using devices that do not transmit all the data to the cloud but instead do at least some of the calculations on their own. High-end smartphones have sufficient computing capacity for that, he said.
In their next phase of this work, the researchers would like to test the system on more skin of color cases and in more varied conditions, said Dr. Soenksen. And they would like to put it through randomized clinical trials, potentially using biopsies to validate the results.
That’s a key step, said Veronica Rotemberg, MD, PhD, director of the dermatology imaging informatics program at Memorial Sloan Kettering Cancer Center, New York.
“Usually when we think about melanoma, we think of histology as the gold standard, or specific subtypes of melanoma as a gold standard,” she said in an interview.
The technology also raises the question of excessive screening, she said. “Identifying the ugly duckling could be extremely important in finding more melanoma,” she said. “But in a patient who doesn’t have melanoma, it could lead to a lot of unnecessary biopsies.”
The sheer number of referrals generated by such a system could overwhelm the dermatologists assigned to follow up on them, she added.
Still, Dr. Rotemberg said, the study is “a good proof of concept.” Ugly duckling analysis is a very active area of AI research with thousands of teams of researchers worldwide working on systems similar to this one, she added. “I’m so excited for the authors.”
Neither Dr. Soenksen nor Dr. Rotemberg disclosed any relevant financial interests.
Methotrexate-associated hepatotoxicity risk differs between psoriasis, PsA, and RA patients
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
, in a large population-based study published in the Journal of the American Academy of Dermatology.
“These findings suggest that conservative liver monitoring is warranted in patients receiving methotrexate for psoriatic disease,” particularly psoriasis, the investigators concluded.
Joel M. Gelfand, MD, professor of dermatology at the University of Pennsylvania, Philadelphia, and colleagues performed a population-based cohort study of patients in Denmark in a hospital clinic with psoriasis, PsA, or RA who received methotrexate between 1997 and 2015; they compared rates of mild liver disease, moderate to severe liver disease, cirrhosis, and cirrhosis-related hospitalization between the groups.
In total, 5,687 patients with psoriasis, 6,520 patients with PsA, and 28,030 patients with RA met inclusion criteria: receiving one or more methotrexate prescriptions or having been dispensed methotrexate at the hospital clinic during the study period. Patients with RA tended to be older (mean, 59.7 years) and the group consisted of more women (71.6%) than the psoriasis patients (47.7 years; 45.3% women) or PsA patients (50.7 years; 57.3% women). In the groups, 17.9% to 23.5% had a history of smoking, and 2.8% to 7.4% had a history of alcohol abuse; the rates of diabetes were between 7.0% and 8.3%, and hyperlipidemia or statin use between 13.6% and 16.4%.
The average weekly methotrexate dose was similar in the three patient groups (a mean of 19.2-19.9 mg). However, the duration of methotrexate use among patients with RA was longer (a mean of 72.1 weeks) compared with the PsA (56.3 weeks) and psoriasis (43.0 weeks) groups. In addition, 50% of the patients in the RA group discontinued treatment after 80 months, 50% in the PsA group discontinued after 54 months, and 50% of patients with psoriasis discontinued after 26 months.
Patients with RA also had a higher cumulative methotrexate dose (a mean of 4.0 g) compared with PsA (3.0 g) and psoriasis (2.1) groups.
When the researchers looked at the incidence rate (IR) for the different categories of liver disease, they found the following differences:
- Mild liver disease: The IR per 1,000 person-years for patients with psoriasis was 4.22 per 1,000 person-years (95% confidence interval, 3.61-4.91), compared with 2.39 per 1,000 person-years (95% CI, 1.95-2.91) for patients with PsA, and 1.39 per 1,000 person-years (95% CI, 1.25-1.55) for patients with RA.
- Moderate to severe liver disease: The IR for patients with psoriasis was 0.98 per 1,000 person years (95% CI, 0.70-1.33), compared with 0.51 (95% CI, 0.32-0.77) for patients with PsA, and 0.46 (95% CI, 0.37-0.55) for patients with RA.
- Cirrhosis: The IR for patients with psoriasis was 1.89 per 1,000 person years (95% CI, 1.49-2.37), compared with 0.84 (95% CI, 0.59-1.16) for patients with PsA, and 0.42 (95% CI, 0.34-0.51) for patients with RA.
- Cirrhosis-related hospitalization: This was the least common outcome, with an IR per 1,000 person years of 0.73 (95% CI, 0.49-1.05) for patients with psoriasis, 0.32 (95% CI, 0.18-0.54) for patients with PsA, and 0.22 (95% CI, 0.17-0.29) for patients with RA.
When results were adjusted with Cox regression analyses, the psoriasis group had a significantly increased risk compared with the RA group with regard to mild liver disease (hazard ratio, 2.22; 95% CI, 1.81-2.72), moderate to-severe liver disease (HR, 1.56; 95% CI, 1.05-2.31), cirrhosis (HR, 3.38; 95% CI, 2.44-4.68), and cirrhosis-related hospitalization (HR, 2.25; 95% CI, 1.37-3.69). Compared with patients with RA, patients with PsA had a significantly increased risk of mild liver disease (HR, 1.27; 95% CI, 1.01-1.60) and cirrhosis (HR, 1.63; 95% CI, 1.10-2.42), but not moderate to severe liver disease or hospitalizations related to cirrhosis.
The researchers noted it is unclear why there was a difference in risk between the three groups of patients.
“While such differences in hepatotoxicity risk were previously attributed to differences in rates of alcoholism, obesity, diabetes, and other comorbidities between the disease populations, our study finds that the underlying disease influences liver disease risk independent of age, sex, smoking, alcohol use, diabetes, hyperlipidemia, overall comorbidity, and weekly methotrexate dose,” wrote Dr. Gelfand and colleagues.
As far as they know, their study “ is one of the first and largest population-based studies to directly compare” liver disease in these three groups of patients on methotrexate, they wrote, noting that earlier studies were smaller and frequently used indirect hepatic injury measures.
Limitations of the study included the inability to account for disease severity as well as the potential for disease misclassification, surveillance bias, and confounding by unmeasured variables such as body mass index. Further, the results do not show whether “liver disease is attributed to methotrexate use, the underlying disease, or a combination of both,” the researchers noted.
Four authors report relationships in the form of consultancies, continuing medical information payments, deputy editor positions, fellowship support, individual or spousal honoraria, patents, research grants, and/or speaker positions with various pharmaceutical companies, medical journals, societies, and other organizations; two authors had no disclosures. There was no funding source.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Janssen/J&J COVID-19 vaccine cuts transmission, new data show
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The single-dose vaccine reduces the risk of asymptomatic transmission by 74% at 71 days, compared with placebo, according to documents released today by the U.S. Food and Drug Administration.
“The decrease in asymptomatic transmission is very welcome news too in curbing the spread of the virus,” Phyllis Tien, MD, told this news organization.
“While the earlier press release reported that the vaccine was effective against preventing severe COVID-19 disease, as well as hospitalizations and death, this new data shows that the vaccine can also decrease transmission, which is very important on a public health level,” said Dr. Tien, professor of medicine in the division of infectious diseases at the University of California, San Francisco.
“It is extremely important in terms of getting to herd immunity,” Paul Goepfert, MD, director of the Alabama Vaccine Research Clinic and infectious disease specialist at the University of Alabama, Birmingham, said in an interview. “It means that this vaccine is likely preventing subsequent transmission after a single dose, which could have huge implications once we get the majority of folks vaccinated.”
The FDA cautioned that the numbers of participants included in the study are relatively small and need to be verified. However, the Johnson & Johnson vaccine might not be the only product offering this advantage. Early data suggest that the Pfizer/BioNTech vaccine also decreases transmission, providing further evidence that the protection offered by immunization goes beyond the individual.
The new analyses were provided by the FDA in advance of its review of the Janssen/Johnson & Johnson vaccine. The agency plans to fully address the Ad26.COV2.S vaccine at its Vaccines and Related Biological Products Advisory Committee Meeting on Friday, including evaluating its safety and efficacy.
The agency’s decision on whether or not to grant emergency use authorization (EUA) to the Johnson & Johnson vaccine could come as early as Friday evening or Saturday.
In addition to the newly released data, officials are likely to discuss phase 3 data, released Jan. 29, that reveal an 85% efficacy for the vaccine against severe COVID-19 illness globally, including data from South America, South Africa, and the United States. When the analysis was restricted to data from U.S. participants, the trial showed a 73% efficacy against moderate to severe COVID-19.
If and when the FDA grants an EUA, it remains unclear how much of the new vaccine will be immediately available. Initially, Johnson & Johnson predicted 18 million doses would be ready by the end of February, but others stated the figure will be closer to 2-4 million. The manufacturer’s contract with the U.S. government stipulates production of 100-million doses by the end of June.
Dr. Tien received support from Johnson & Johnson to conduct the J&J COVID-19 vaccine trial in the SF VA HealthCare System. Dr. Goepfert has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Emerging treatments for molluscum contagiosum and acne show promise
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
dbrunk@mdedge.com
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
dbrunk@mdedge.com
, but that could soon change, according to Leon H. Kircik, MD.
“The treatment of molluscum is still an unmet need,” Dr. Kircik, clinical professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, said at the Orlando Dermatology Aesthetic and Clinical Conference. However, a proprietary drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, which has been tested in phase 3 studies, is currently under FDA review. The manufacturer, Verrica Pharmaceuticals resubmitted a new drug application for the product, VP-102, in December 2020.
“VP-102 features a visualization agent so the injector can see which lesions have been treated, as well as a bittering agent to mitigate oral ingestion by children. Complete clearance at 12 weeks ranged from 46% to 54% of patients, while lesion count reduction compared with baseline ranged from 69% to 82%.”
Acne
In August, 2020, clascoterone 1% cream was approved for the treatment of acne in patients 12 years and older, a development that Dr. Kircik said “can be a game changer in acne treatment.” Clascoterone cream 1% exhibits strong, selective anti-androgen activity by targeting androgen receptors in the skin, not systemically. “It limits or blocks transcription of androgen responsive genes, but it also has an anti-inflammatory effect and an anti-sebum effect,” he explained.
According to results from two phase 3 trials of the product, a response of clear or almost clear on the IGA scale at week 12 was achieved in 18.4% of those on treatment vs. 9% of those on vehicle in one study (P less than .001) and 20.3% vs. 6.5%, respectively, in the second study (P less than .001). Clascoterone is also being evaluated for treating androgenetic alopecia.
In Dr. Kircik’s clinical experience, retinoids can be helpful for patients with moderate to severe acne. “We always use them for anticomedogenic effects, but we also know that they have anti-inflammatory effects,” he said. “They actually inhibit toll-like receptor activity. They also inhibit the AP-1 pathway by causing a reduction in inflammatory signaling associated with collagen degradation and scarring.”
The most recent retinoid to be approved for the topical treatment of acne was 0.005% trifarotene cream, in 2019, for patients aged 9 years and older. “But when we got the results, it was not that exciting,” a difference of about 3.6 (mean) inflammatory lesion reduction between the active and the vehicle arm, said Dr. Kircik, medical director of Physicians Skin Care in Louisville, Ky. “According to the package insert, treatment side effects included mild to moderate erythema in 59% of patients, scaling in 65%, dryness in 69%, and stinging/burning in 56%, which makes it difficult to use in our clinical practice.”
The drug was also tested for treating truncal acne. However, one comparative study showed that tazarotene 0.045% lotion spread an average of 36.7 square centimeters farther than the trifarotene cream, which makes the tazarotene lotion easier to use on the chest and back, he said.
Dr. Kircik also discussed 4% minocycline, a hydrophobic, topical foam formulation of minocycline that was approved by the FDA in 2019 for the treatment of moderate to severe acne, for patients aged 9 and older. In a 12-week study that involved 1,488 patients (mean age was about 20 years), investigators observed a 56% reduction in inflammatory lesion count among those treated with minocycline 4%, compared with 43% in the vehicle group.
Dr. Kircik, one of the authors of the study, noted that the hydrophobic composition of minocycline 4% allows for stable and efficient delivery of an inherently unstable active pharmaceutical ingredient such as minocycline. “It’s free of primary irritants such as surfactants and short chain alcohols, which makes it much more tolerable,” he said. “The unique physical foam characteristics facilitate ease of application and absorption at target sites.”
Dr. Kircik reported that he serves as a consultant and/or adviser to numerous pharmaceutical companies, including Galderma, the manufacturer of trifarotene cream.
dbrunk@mdedge.com
FROM ODAC 2021
What’s best for filler injection, needle or cannula?
“You may be a needle person or a cannula person, but it doesn’t have to be that way,” he said during the Orlando Dermatology Aesthetic and Clinical Conference. “There are pros and cons of both techniques, but I think there’s a place for both.”
With a sharp needle, placement of the tip is considered precise, especially when delivering a supraperiosteal injection. “From a learning curve, especially for us as dermatologists, it’s easier because we’re used to injecting lidocaine, and we’re using needles on a day-to-day basis for injectables and other applications,” said Dr. Keaney, a dermatologist who is founder and director of SkinDC in Arlington, Va. “However, use of a needle is traumatic; it creates an increased risk of bruising and we’re cutting through tissue. We can potentially puncture a blood vessel and create a vascular event.”
Clinicians may consider filler delivery by sharp needle as being more precise, but in an observational cadaver study of cannula vs. sharp needle for placement of tissue fillers, investigators found an increased risk for spread of filler to more superficial layers along the needle trajectory, as well as a higher risk of intra-arterial injection. “This may explain why, when you’re injecting on a tear trough, you may still get some swelling in the area,” Dr. Keaney said. “You may see some swelling and some product, because it’s tracking along the injection point. One can argue that you can reduce this risk by using a longer needle or cannula.”
With a longer cannula, the blunt tips may act to displace blood vessels rather than to lacerate them. “They allow for greater coverage through fewer injection points and they approach the injection site at a more oblique angle, so it’s harder for the product to track,” he explained. “Cannula patients tend to faint on me a lot more than my needle patients do, so while the cannula may be more comfortable, it can be more nerve-wracking for patients.”
Recent studies have shown that with cannula proficiency, clinicians can achieve results on par with using sharp needles for dermal filler treatments (Dermatol Surg. 2020 Apr;46[4]:465-72; Dermatol Surg. 2012 Feb;38[2]:207-14). A recent head-to-head comparison found no significant differences in the use of needles vs. cannulas for the treatment of the dorsal hand with diluted calcium hydroxylapatite, though patients reported 12% greater satisfaction with the cannula technique (Dermatol Surg. 2020 Oct;46 Suppl 1:S54-61).
“Based on these articles, we can feel comfortable that with proficient use, you can deliver similar results with the cannula as you would with the needle,” said Dr. Keaney, a clinical associate faculty member in the department of dermatology at George Washington University, Washington. “As a result, we are seeing Food and Drug Administration–approved indications for the use of cannulas for dermal fillers: Restylane Silk for lips, Restylane Lyft for cheeks, Juvederm Voluma for cheeks, and Juvederm Voluma for the chin.”
Such emerging data present a conundrum, though. If someone is comfortable injecting dermal filler with needles, why switch to using cannulas? After all, a case study reported arterial penetration with blunt-tipped cannulas using injectables . “Cannulas are not 100% safe,” Dr. Keaney said. “One of my mentors once said, “If a vascular event has not happened to you yet, you have not injected enough. These things can happen even in the most experienced hands, whether you use a needle or a cannula.”
However, safety data from a recently published retrospective study demonstrated that cannulas are less likely to be associated with occlusions compared with needles (a risk of 1 occlusion per 40,882 injections vs. 1 occlusion per 6,410 injections (P less than .001).
“Cannulas are generally safer because the blunt tip kind of dissects tissue and pushes vessels away,” he said. “That doesn’t mean it can’t get into a vessel, it just requires greater force to penetrate facial arteries with a cannula. Finer tips may be easier to use.”
Larger cannulas can tear into an arterial wall when the artery wall is relatively fixed, so it cannot slide aside enough to avoid injury, Dr. Keaney continued. “Arterial location perpendicular to cannula trajectory carries the most risk,” he said. Meanwhile, filler-induced blindness, which he characterized as “the worst possible outcome,” is often due to the retrograde embolization of the product. This can occur with injection pressures greater than the sum of the systolic arterial pressure and the frictional forces due to viscous flow.
Dr. Keaney said he uses both needles and cannulas in his clinical practice. “I use a needle to inject on bone if I want to mimic bony projections along the zygomatic arch or jawline or chin,” he said. “I think a needle can get those boluses to develop that projection that you want. If I’m injecting within soft tissue plane, I use a 22 G cannula and keep the cannula moving within the tissue. I inject slowly and less than 0.2 cc per bolus. I compress when injecting on the nose and I’m cautious to inject previous patients who have undergone plastic surgery or in areas of previous scarring.”
Dr. Keaney reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies.
“You may be a needle person or a cannula person, but it doesn’t have to be that way,” he said during the Orlando Dermatology Aesthetic and Clinical Conference. “There are pros and cons of both techniques, but I think there’s a place for both.”
With a sharp needle, placement of the tip is considered precise, especially when delivering a supraperiosteal injection. “From a learning curve, especially for us as dermatologists, it’s easier because we’re used to injecting lidocaine, and we’re using needles on a day-to-day basis for injectables and other applications,” said Dr. Keaney, a dermatologist who is founder and director of SkinDC in Arlington, Va. “However, use of a needle is traumatic; it creates an increased risk of bruising and we’re cutting through tissue. We can potentially puncture a blood vessel and create a vascular event.”
Clinicians may consider filler delivery by sharp needle as being more precise, but in an observational cadaver study of cannula vs. sharp needle for placement of tissue fillers, investigators found an increased risk for spread of filler to more superficial layers along the needle trajectory, as well as a higher risk of intra-arterial injection. “This may explain why, when you’re injecting on a tear trough, you may still get some swelling in the area,” Dr. Keaney said. “You may see some swelling and some product, because it’s tracking along the injection point. One can argue that you can reduce this risk by using a longer needle or cannula.”
With a longer cannula, the blunt tips may act to displace blood vessels rather than to lacerate them. “They allow for greater coverage through fewer injection points and they approach the injection site at a more oblique angle, so it’s harder for the product to track,” he explained. “Cannula patients tend to faint on me a lot more than my needle patients do, so while the cannula may be more comfortable, it can be more nerve-wracking for patients.”
Recent studies have shown that with cannula proficiency, clinicians can achieve results on par with using sharp needles for dermal filler treatments (Dermatol Surg. 2020 Apr;46[4]:465-72; Dermatol Surg. 2012 Feb;38[2]:207-14). A recent head-to-head comparison found no significant differences in the use of needles vs. cannulas for the treatment of the dorsal hand with diluted calcium hydroxylapatite, though patients reported 12% greater satisfaction with the cannula technique (Dermatol Surg. 2020 Oct;46 Suppl 1:S54-61).
“Based on these articles, we can feel comfortable that with proficient use, you can deliver similar results with the cannula as you would with the needle,” said Dr. Keaney, a clinical associate faculty member in the department of dermatology at George Washington University, Washington. “As a result, we are seeing Food and Drug Administration–approved indications for the use of cannulas for dermal fillers: Restylane Silk for lips, Restylane Lyft for cheeks, Juvederm Voluma for cheeks, and Juvederm Voluma for the chin.”
Such emerging data present a conundrum, though. If someone is comfortable injecting dermal filler with needles, why switch to using cannulas? After all, a case study reported arterial penetration with blunt-tipped cannulas using injectables . “Cannulas are not 100% safe,” Dr. Keaney said. “One of my mentors once said, “If a vascular event has not happened to you yet, you have not injected enough. These things can happen even in the most experienced hands, whether you use a needle or a cannula.”
However, safety data from a recently published retrospective study demonstrated that cannulas are less likely to be associated with occlusions compared with needles (a risk of 1 occlusion per 40,882 injections vs. 1 occlusion per 6,410 injections (P less than .001).
“Cannulas are generally safer because the blunt tip kind of dissects tissue and pushes vessels away,” he said. “That doesn’t mean it can’t get into a vessel, it just requires greater force to penetrate facial arteries with a cannula. Finer tips may be easier to use.”
Larger cannulas can tear into an arterial wall when the artery wall is relatively fixed, so it cannot slide aside enough to avoid injury, Dr. Keaney continued. “Arterial location perpendicular to cannula trajectory carries the most risk,” he said. Meanwhile, filler-induced blindness, which he characterized as “the worst possible outcome,” is often due to the retrograde embolization of the product. This can occur with injection pressures greater than the sum of the systolic arterial pressure and the frictional forces due to viscous flow.
Dr. Keaney said he uses both needles and cannulas in his clinical practice. “I use a needle to inject on bone if I want to mimic bony projections along the zygomatic arch or jawline or chin,” he said. “I think a needle can get those boluses to develop that projection that you want. If I’m injecting within soft tissue plane, I use a 22 G cannula and keep the cannula moving within the tissue. I inject slowly and less than 0.2 cc per bolus. I compress when injecting on the nose and I’m cautious to inject previous patients who have undergone plastic surgery or in areas of previous scarring.”
Dr. Keaney reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies.
“You may be a needle person or a cannula person, but it doesn’t have to be that way,” he said during the Orlando Dermatology Aesthetic and Clinical Conference. “There are pros and cons of both techniques, but I think there’s a place for both.”
With a sharp needle, placement of the tip is considered precise, especially when delivering a supraperiosteal injection. “From a learning curve, especially for us as dermatologists, it’s easier because we’re used to injecting lidocaine, and we’re using needles on a day-to-day basis for injectables and other applications,” said Dr. Keaney, a dermatologist who is founder and director of SkinDC in Arlington, Va. “However, use of a needle is traumatic; it creates an increased risk of bruising and we’re cutting through tissue. We can potentially puncture a blood vessel and create a vascular event.”
Clinicians may consider filler delivery by sharp needle as being more precise, but in an observational cadaver study of cannula vs. sharp needle for placement of tissue fillers, investigators found an increased risk for spread of filler to more superficial layers along the needle trajectory, as well as a higher risk of intra-arterial injection. “This may explain why, when you’re injecting on a tear trough, you may still get some swelling in the area,” Dr. Keaney said. “You may see some swelling and some product, because it’s tracking along the injection point. One can argue that you can reduce this risk by using a longer needle or cannula.”
With a longer cannula, the blunt tips may act to displace blood vessels rather than to lacerate them. “They allow for greater coverage through fewer injection points and they approach the injection site at a more oblique angle, so it’s harder for the product to track,” he explained. “Cannula patients tend to faint on me a lot more than my needle patients do, so while the cannula may be more comfortable, it can be more nerve-wracking for patients.”
Recent studies have shown that with cannula proficiency, clinicians can achieve results on par with using sharp needles for dermal filler treatments (Dermatol Surg. 2020 Apr;46[4]:465-72; Dermatol Surg. 2012 Feb;38[2]:207-14). A recent head-to-head comparison found no significant differences in the use of needles vs. cannulas for the treatment of the dorsal hand with diluted calcium hydroxylapatite, though patients reported 12% greater satisfaction with the cannula technique (Dermatol Surg. 2020 Oct;46 Suppl 1:S54-61).
“Based on these articles, we can feel comfortable that with proficient use, you can deliver similar results with the cannula as you would with the needle,” said Dr. Keaney, a clinical associate faculty member in the department of dermatology at George Washington University, Washington. “As a result, we are seeing Food and Drug Administration–approved indications for the use of cannulas for dermal fillers: Restylane Silk for lips, Restylane Lyft for cheeks, Juvederm Voluma for cheeks, and Juvederm Voluma for the chin.”
Such emerging data present a conundrum, though. If someone is comfortable injecting dermal filler with needles, why switch to using cannulas? After all, a case study reported arterial penetration with blunt-tipped cannulas using injectables . “Cannulas are not 100% safe,” Dr. Keaney said. “One of my mentors once said, “If a vascular event has not happened to you yet, you have not injected enough. These things can happen even in the most experienced hands, whether you use a needle or a cannula.”
However, safety data from a recently published retrospective study demonstrated that cannulas are less likely to be associated with occlusions compared with needles (a risk of 1 occlusion per 40,882 injections vs. 1 occlusion per 6,410 injections (P less than .001).
“Cannulas are generally safer because the blunt tip kind of dissects tissue and pushes vessels away,” he said. “That doesn’t mean it can’t get into a vessel, it just requires greater force to penetrate facial arteries with a cannula. Finer tips may be easier to use.”
Larger cannulas can tear into an arterial wall when the artery wall is relatively fixed, so it cannot slide aside enough to avoid injury, Dr. Keaney continued. “Arterial location perpendicular to cannula trajectory carries the most risk,” he said. Meanwhile, filler-induced blindness, which he characterized as “the worst possible outcome,” is often due to the retrograde embolization of the product. This can occur with injection pressures greater than the sum of the systolic arterial pressure and the frictional forces due to viscous flow.
Dr. Keaney said he uses both needles and cannulas in his clinical practice. “I use a needle to inject on bone if I want to mimic bony projections along the zygomatic arch or jawline or chin,” he said. “I think a needle can get those boluses to develop that projection that you want. If I’m injecting within soft tissue plane, I use a 22 G cannula and keep the cannula moving within the tissue. I inject slowly and less than 0.2 cc per bolus. I compress when injecting on the nose and I’m cautious to inject previous patients who have undergone plastic surgery or in areas of previous scarring.”
Dr. Keaney reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies.
FROM ODAC 2021
Cellulitis treatment recommendations
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.
He noticed discomfort today and saw that his left lower leg had redness and was warm. He does not recall scratches or injury to his leg. He has not had fever or chills. He has no other symptoms. His diabetes has been well controlled with diet and metformin.
On exam, his blood pressure is 120/70, pulse is 80, temperature is 37 degrees Celsius.
In the left lower extremity, the patient had 1+ edema at the ankle, with a 14-cm x 20-cm warm, erythematous area just above the ankle and extending proximally.
His labs found an HCT of 44 and a WBC of 12,000. What do you recommend?
A) Vascular duplex exam
B) 1st generation cephalosporin
C) 1st generation cephalosporin + TMP/Sulfa
D) Oral clindamycin
E) IV vancomycin
This patient has cellulitis and should receive a beta lactam antibiotic, which will have the best coverage and lowest minimal inhibitory concentration for the likely organism, beta hemolytic streptococci. Clindamycin would likely work, but it has greater side effects. This patient does not need coverage for methicillin-resistant staphylococcus aureus (MRSA). I know many of you, if not most, know this, but I want to go through relevant data and formal recommendations, because of a recent call I received from a patient.
My patient had a full body rash after receiving cephalexin + TMP/sulfa [trimethoprim-sulfamethoxazole] treatment for cellulitis. In recent years the addition of TMP/sulfa to strep treatment to also cover MRSA has become popular, especially in emergency department and urgent care settings.
Moran and colleagues studied cephalexin + TMP/sulfa vs. cephalexin and placebo in patients with uncomplicated cellulitis.1 The outcome measured was clinical cure, and there was no difference between groups; clinical cure occurred in 182 (83.5%) of 218 participants in the cephalexin plus TMP/sulfa group vs. 165 (85.5%) of 193 in the cephalexin group (difference, −2.0%; 95% confidence interval, −9.7% to 5.7%; P = .50).
Jeng and colleagues studied patients admitted for a cellulitis, and evaluated the patients’ response to beta-lactam antibiotics.2 Patients had acute and convalescent serologies for beta hemolytic strep. Almost all evaluable patients with positive strep studies (97%) responded to beta-lactams, and 21 of 23 (91%) with negative studies responded to beta-lactams (overall response rate 95%). This study was done during a time of high MRSA prevalence.
The most recent Infectious Diseases Society of America guidelines for skin and soft tissue infections, recommend oral penicillin, cephalexin, dicloxacillin, or clindamycin for mild cellulitis, and IV equivalent if patients have moderate cellulitis.3 If abscesses are present, then drainage is recommended and MRSA coverage. Kamath and colleagues reported on how closely guidelines for skin and soft tissue infections were followed.4 In patients with mild cellulitis, only 36% received guideline-suggested antibiotics. The most common antibiotic prescribed that was outside the guidelines was trimethoprim-sulfamethoxazole.
Myth: Cellulitis treatment should include MRSA coverage.
My advice: Stick with beta-lactam antibiotics, unless an abscess is present. There is no need to add MRSA coverage for initial treatment of mild to moderate cellulitis.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Moran GJ et al. Effect of cephalexin plus trimethoprim-sulfamethoxazole vs. cephalexin alone on clinical cure of uncomplicated cellulitis: A randomized clinical trial. JAMA 2017 May 23;317(20):2088-96.
2. Jeng Arthur et al. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis. Medicine. 2010;July;89(4):217-26.
3. Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-e52.
4. Kamath RS et al. Guidelines vs. actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. 2018 Jan 12;5(1):ofx188.