User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Panel Recommends Small Bump in 2025 Medicare Physician Pay
An influential panel is seeking an increase in Medicare’s 2025 payments for clinicians, adding to pressure on Congress to reconsider how the largest US purchaser of health services pays for office visits and related care of the nation’s older citizens and those with disabilities.
The Medicare Payment Advisory Commission (MedPAC) on Thursday voted unanimously in favor of a two-part recommendation on changes to the 2025 physician fee schedule:
- An increase in the base rate equal to half of the projected change in the Medicare Economic Index (MEI). Recent estimates have projected a 2.6% increase in MEI for 2025, which is intended to show how inflation affects the costs of running a medical practice.
- The creation of a safety-net add-on payment under the physician fee schedule to cover care of people with low incomes.
These recommendations echo the calls MedPAC made in a 2023 report to Congress.
Lawmakers and the Centers for Medicare and Medicaid Services (CMS) rely on MedPAC’s work in deciding how much to pay for services. About 1.3 million clinicians bill Medicare for their work, including about 670,000 physicians.
Thursday’s MedPAC vote comes amid continuing uncertainty about how much the federal government will actually pay clinicians this year through the physician fee schedule.
There are serious efforts underway to undo cuts already demanded by previously passed federal law. In an email, Rep. Larry Buchson, MD, (R-IN) said he remains committed to “eliminating the full 3.37% cut this year while also working toward a permanent solution to halt the downward spiral of physician reimbursement.”
“The Medicare payment cut to physicians will impede patients’ access to care and further accelerate the current path toward consolidation, physician burnout, and closure of medical practices,” Buchson told this news organization. “It’s past time that Congress provides much needed and deserved stability for America’s doctors.”
Congress this month is attempting to complete overdue budget legislation needed to fund federal operations for fiscal 2024, which began October 1, 2023. The pending expiration of a short-term stopgap continuing resolution could provide a vehicle that could also carry legislation that would address the physician fee schedule.
In a Thursday statement, Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, commended MedPAC for its recommendations and urged lawmakers to act.
“Long-term reforms from Congress are overdue to close the unsustainable gap between what Medicare pays physicians and the actual costs of delivering high-quality care,” Dr. Ehrenfeld said. “When adjusted for inflation in practice costs, Medicare physician pay declined 26% from 2001 to 2023.”
Continual Struggles
Congress has struggled for years in its attempts to set Medicare payments for office visits and other services covered by the physician fee schedule. A 1990s budget law set the stage for what proved to be untenable reductions in payment through the sustainable growth rate mechanism.
Between 2003 through April 2014, lawmakers passed “doc-fix” legislation 17 times to block the slated cuts, according to the Congressional Research Service. In 2015, Congress passed an intended overhaul of the physician fee schedule through the Medicare Access and CHIP Reauthorization Act (MACRA). As part of this law, Congress eliminated a base automatic inflation adjuster for the physician fee schedule.
In recent years, Congress has acted repeatedly to address MACRA’s mandates for flat base pay. MedPAC and members of both parties in Congress have called for a broad new look at how Medicare pays physicians.
At Thursday’s meeting, MedPAC member Lawrence Casalino, MD, PhD, MPH, noted that the struggles to keep up with inflation and the “unpredictability of what the payment rates are going to be from year to year really do affect physician morale.”
A version of this article appeared on Medscape.com.
An influential panel is seeking an increase in Medicare’s 2025 payments for clinicians, adding to pressure on Congress to reconsider how the largest US purchaser of health services pays for office visits and related care of the nation’s older citizens and those with disabilities.
The Medicare Payment Advisory Commission (MedPAC) on Thursday voted unanimously in favor of a two-part recommendation on changes to the 2025 physician fee schedule:
- An increase in the base rate equal to half of the projected change in the Medicare Economic Index (MEI). Recent estimates have projected a 2.6% increase in MEI for 2025, which is intended to show how inflation affects the costs of running a medical practice.
- The creation of a safety-net add-on payment under the physician fee schedule to cover care of people with low incomes.
These recommendations echo the calls MedPAC made in a 2023 report to Congress.
Lawmakers and the Centers for Medicare and Medicaid Services (CMS) rely on MedPAC’s work in deciding how much to pay for services. About 1.3 million clinicians bill Medicare for their work, including about 670,000 physicians.
Thursday’s MedPAC vote comes amid continuing uncertainty about how much the federal government will actually pay clinicians this year through the physician fee schedule.
There are serious efforts underway to undo cuts already demanded by previously passed federal law. In an email, Rep. Larry Buchson, MD, (R-IN) said he remains committed to “eliminating the full 3.37% cut this year while also working toward a permanent solution to halt the downward spiral of physician reimbursement.”
“The Medicare payment cut to physicians will impede patients’ access to care and further accelerate the current path toward consolidation, physician burnout, and closure of medical practices,” Buchson told this news organization. “It’s past time that Congress provides much needed and deserved stability for America’s doctors.”
Congress this month is attempting to complete overdue budget legislation needed to fund federal operations for fiscal 2024, which began October 1, 2023. The pending expiration of a short-term stopgap continuing resolution could provide a vehicle that could also carry legislation that would address the physician fee schedule.
In a Thursday statement, Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, commended MedPAC for its recommendations and urged lawmakers to act.
“Long-term reforms from Congress are overdue to close the unsustainable gap between what Medicare pays physicians and the actual costs of delivering high-quality care,” Dr. Ehrenfeld said. “When adjusted for inflation in practice costs, Medicare physician pay declined 26% from 2001 to 2023.”
Continual Struggles
Congress has struggled for years in its attempts to set Medicare payments for office visits and other services covered by the physician fee schedule. A 1990s budget law set the stage for what proved to be untenable reductions in payment through the sustainable growth rate mechanism.
Between 2003 through April 2014, lawmakers passed “doc-fix” legislation 17 times to block the slated cuts, according to the Congressional Research Service. In 2015, Congress passed an intended overhaul of the physician fee schedule through the Medicare Access and CHIP Reauthorization Act (MACRA). As part of this law, Congress eliminated a base automatic inflation adjuster for the physician fee schedule.
In recent years, Congress has acted repeatedly to address MACRA’s mandates for flat base pay. MedPAC and members of both parties in Congress have called for a broad new look at how Medicare pays physicians.
At Thursday’s meeting, MedPAC member Lawrence Casalino, MD, PhD, MPH, noted that the struggles to keep up with inflation and the “unpredictability of what the payment rates are going to be from year to year really do affect physician morale.”
A version of this article appeared on Medscape.com.
An influential panel is seeking an increase in Medicare’s 2025 payments for clinicians, adding to pressure on Congress to reconsider how the largest US purchaser of health services pays for office visits and related care of the nation’s older citizens and those with disabilities.
The Medicare Payment Advisory Commission (MedPAC) on Thursday voted unanimously in favor of a two-part recommendation on changes to the 2025 physician fee schedule:
- An increase in the base rate equal to half of the projected change in the Medicare Economic Index (MEI). Recent estimates have projected a 2.6% increase in MEI for 2025, which is intended to show how inflation affects the costs of running a medical practice.
- The creation of a safety-net add-on payment under the physician fee schedule to cover care of people with low incomes.
These recommendations echo the calls MedPAC made in a 2023 report to Congress.
Lawmakers and the Centers for Medicare and Medicaid Services (CMS) rely on MedPAC’s work in deciding how much to pay for services. About 1.3 million clinicians bill Medicare for their work, including about 670,000 physicians.
Thursday’s MedPAC vote comes amid continuing uncertainty about how much the federal government will actually pay clinicians this year through the physician fee schedule.
There are serious efforts underway to undo cuts already demanded by previously passed federal law. In an email, Rep. Larry Buchson, MD, (R-IN) said he remains committed to “eliminating the full 3.37% cut this year while also working toward a permanent solution to halt the downward spiral of physician reimbursement.”
“The Medicare payment cut to physicians will impede patients’ access to care and further accelerate the current path toward consolidation, physician burnout, and closure of medical practices,” Buchson told this news organization. “It’s past time that Congress provides much needed and deserved stability for America’s doctors.”
Congress this month is attempting to complete overdue budget legislation needed to fund federal operations for fiscal 2024, which began October 1, 2023. The pending expiration of a short-term stopgap continuing resolution could provide a vehicle that could also carry legislation that would address the physician fee schedule.
In a Thursday statement, Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, commended MedPAC for its recommendations and urged lawmakers to act.
“Long-term reforms from Congress are overdue to close the unsustainable gap between what Medicare pays physicians and the actual costs of delivering high-quality care,” Dr. Ehrenfeld said. “When adjusted for inflation in practice costs, Medicare physician pay declined 26% from 2001 to 2023.”
Continual Struggles
Congress has struggled for years in its attempts to set Medicare payments for office visits and other services covered by the physician fee schedule. A 1990s budget law set the stage for what proved to be untenable reductions in payment through the sustainable growth rate mechanism.
Between 2003 through April 2014, lawmakers passed “doc-fix” legislation 17 times to block the slated cuts, according to the Congressional Research Service. In 2015, Congress passed an intended overhaul of the physician fee schedule through the Medicare Access and CHIP Reauthorization Act (MACRA). As part of this law, Congress eliminated a base automatic inflation adjuster for the physician fee schedule.
In recent years, Congress has acted repeatedly to address MACRA’s mandates for flat base pay. MedPAC and members of both parties in Congress have called for a broad new look at how Medicare pays physicians.
At Thursday’s meeting, MedPAC member Lawrence Casalino, MD, PhD, MPH, noted that the struggles to keep up with inflation and the “unpredictability of what the payment rates are going to be from year to year really do affect physician morale.”
A version of this article appeared on Medscape.com.
Analysis Finds Risk of Alopecia Areata After COVID-19 Infection
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
“There is a growing number of reports on new onset, exacerbation, and recurrence of AA after COVID-19,” corresponding author Jin Park, MD, PhD, of the department of dermatology at Jeonbuk National University Medical School, South Korea, and colleagues wrote in a research letter published online January 10, 2024, in JAMA Dermatology. “However, evidence supporting an association between COVID-19 and AA is limited.”
To investigate the association between COVID-19 and AA, the researchers used data from the Korea Disease Control and Prevention Agency–COVID-19–National Health Insurance Service cohort to conduct a propensity score–matched, nationwide, population-based cohort study from October 8, 2020, to September 30, 2021. They used Cox proportional hazards regression to calculate the incidence, prevalence, and adjusted hazard ratios (AHRs) for AA.
The cohort consisted of 259,369 patients with COVID-19 and 259,369 uninfected controls. The researchers observed an increased risk of telogen effluvium in patients with COVID-19 compared with the uninfected controls (AHR, 6.40; 95% CI, 4.92-8.33), while the incidence of epidermal cysts, benign skin tumors, and other negative control outcomes did not differ between groups.
Meanwhile, the incidence of AA in patients with COVID-19 was significantly higher compared with the uninfected controls (43.19 per 10,000 person-years [PY]), regardless of clinical subtype. This translated into an AHR of 1.82 (95% CI, 1.60-2.07). In other findings, the incidence of patchy AA and alopecia totalis and alopecia universalis (AT/AU) was 35.94 and 7.24 per 10,000 PY in patients with COVID-19 compared with 19.43 and 4.18 per 10,000 PY in uninfected controls, respectively.
“These findings support the possible role of COVID-19 in AA occurrence and exacerbation, although other environmental factors, such as psychological stress, may have also contributed to AA development during the pandemic,” the authors concluded. “Plausible mechanisms of AA following COVID-19 include antigenic molecular mimicry between SARS-CoV-2 and hair follicle autoantigens, cytokine shifting, and bystander activation.”
They acknowledged certain limitations of the analysis, including the potential for detection or misclassification bias and the fact that it did not evaluate causality between the two conditions.
Shari Lipner, MD, PhD, associate professor of dermatology at Weill Cornell Medicine, New York, who was asked to comment on the study, said that strengths of the study include the large sample size, and the use of positive and negative outcome controls, and that the incidence and prevalence of AA in Korea was stable during the prepandemic period. “A weakness of the study is that all alopecia areata cases may not have necessarily been confirmed,” Dr. Lipner told this news organization.
“Based on this study, dermatologists may consider AA in the differential diagnosis for a patient presenting with hair loss with recent COVID-19 diagnosis,” she added, noting that the potential for prevention of AA flares is also a reason to recommend COVID-19 vaccination for patients with a history of AA.
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was also asked to comment on the study, said that while the analysis suggests a definite epidemiologic association between COVID-19 and AA, “any causal relationship needs further study.” She added that she has no specific advice for patients who develop AA following a COVID-19 infection. “Any conversation about AA can be difficult because there is no way to prognosticate if someone will just have one small, localized area of hair loss,” or several small areas, versus loss of all hair on the head or even the body as well, Dr. Ko explained.
The study was supported with grants from the National Research Foundation of the Korean Government and the Ministry of Health and Welfare, Republic of Korea. The authors, as well as Dr. Lipner and Dr. Ko, reported having no relevant disclosures.
FROM JAMA DERMATOLOGY
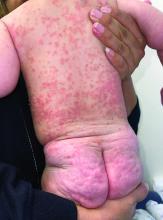
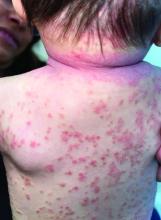
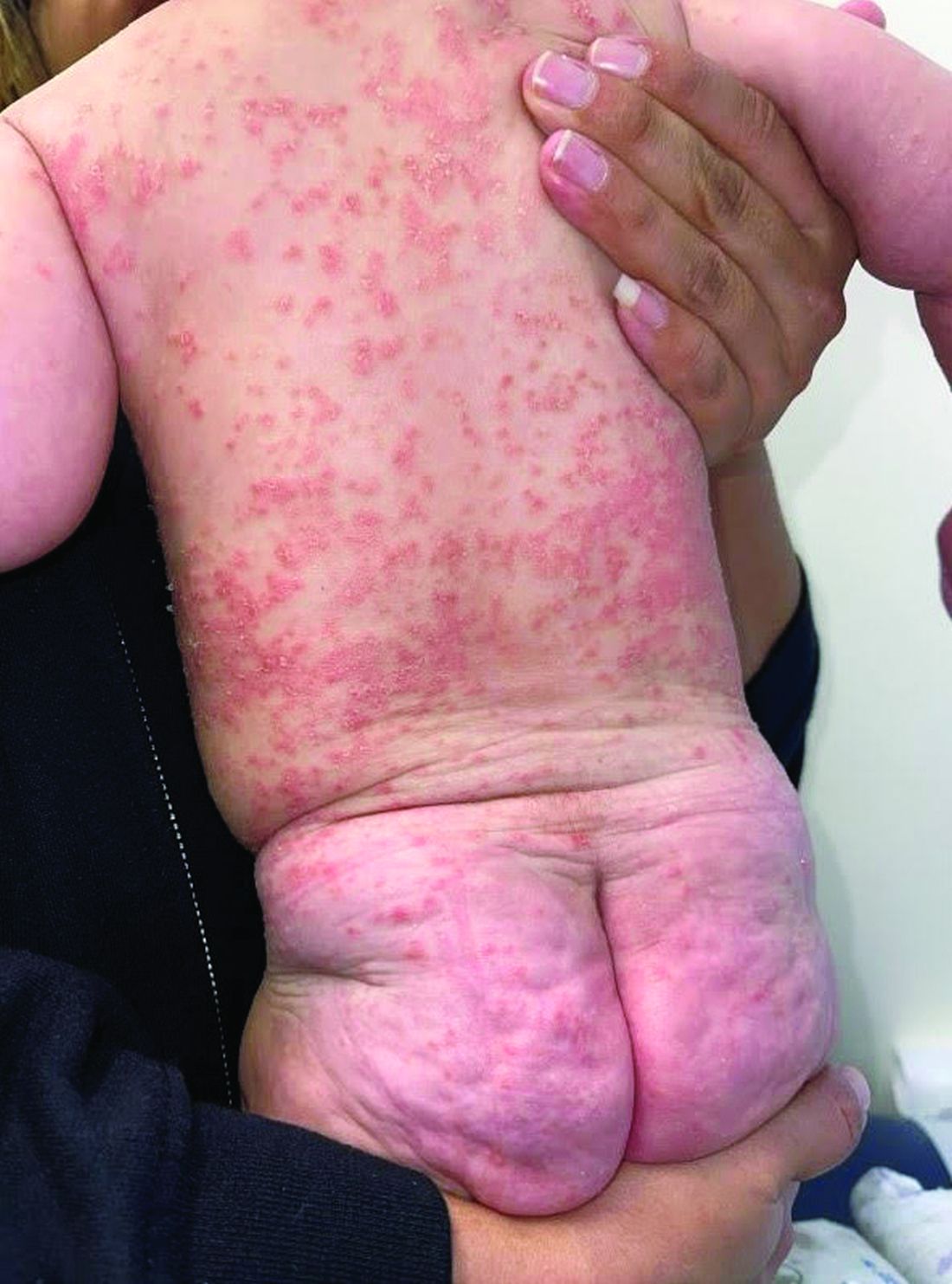
A 4-month-old male was referred for a 3-week history of an itchy generalized rash that started on the neck
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
Diagnosis: Infection-induced psoriasis (guttate-type, induced by streptococcal intertrigo)
Psoriasis is a chronic inflammatory disorder characterized by well-defined, scaly, erythematous plaques. Guttate psoriasis is a distinct variant of psoriasis that is more common in children and adolescents. Guttate psoriasis usually presents with multiple, scattered, small, drop-like (“guttate”), scaly, erythematous papules and plaques.
The pathophysiology of psoriasis involves an interplay between genetic and environmental factors. Guttate psoriasis is a chronic T-cell–mediated inflammatory disease in which there is an altered balance between T-helper-1 (TH1) and TH2 cells, transcription factor genes, and their products. HLA B-13, B-17, and Cw6 are human leukocyte antigen alleles implicated in genetic susceptibility. It is hypothesized that streptococcal infection precipitates guttate psoriasis by streptococcal superantigen–driven activation of cutaneous lymphocyte-associated antigen (CLA)–positive lymphocytes. It has been shown that streptococcal exotoxins and streptococcal M proteins act as superantigens.
Diagnosis is often made clinically based on characteristic physical findings and a possible preceding history of streptococcal infection. In patients with streptococcal infection, culture from an appropriate site and measurement of serum antistreptococcal antibody titers (for example, anti-DNase, antihyaluronidase and antistreptolysin-O) can help. A skin biopsy is usually not necessary but may be considered.
This patient presented with intertrigo of the neck and axillae at the time of presentation with the papulosquamous rash. Culture of the intertrigo yielded 4+ Group A beta streptococcus.
Treatment
Although there is currently no cure for guttate psoriasis, various treatment options can relieve symptoms and clear skin lesions, and infection-triggered lesions may remit, usually within several months. However, guttate psoriasis may persist and progress to chronic plaque psoriasis. Many treatment options are based mainly on clinical trials targeted for plaque psoriasis treatment.
For mild psoriasis, topical corticosteroids are first-line treatment. Other topical steroids include vitamin D analogs (calcipotriene), topical retinoids (tazarotene), topical calcineurin inhibitors (tacrolimus and pimecrolimus), and newer non-steroidal anti-inflammatory agents (roflumilast or tapinarof), neither approved yet in this young age group. In more severe cases, phototherapy with UVB light, traditional systemic immunosuppressive agents (methotrexate, cyclosporine) or targeted biologic therapies may be considered.
Differential Diagnosis
The differential diagnosis may include generalized intertrigo, pityriasis rubra pilaris, tinea corporis, atopic dermatitis, and staphylococcal scalded skin syndrome. Guttate psoriasis can be distinguished by history and physical exam. Further studies such as potassium hydroxide (KOH) scrapings may be helpful in ruling out the other disorders.
Intertrigo is an inflammatory condition of the flexural surfaces irritated by warm temperatures, friction, moisture, and poor ventilation that is commonly associated with Candida infection and/or streptococcal infection. Candidal intertrigo can present with erythematous patches or plaques in an intertriginous area that may develop erosions, macerations, fissures, crust, and weeping. Satellite papules and pustules are pathognomonic for Candida species. Streptococcal intertrigo usually presents with bright red color and may be painful or pruritic. Perianal streptococcal infection is reported as a trigger of guttate psoriasis in pediatric patients.
Pityriasis rubra pilaris is a rare inflammatory papulosquamous disorder with an unknown etiology. Red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis are primary features. Diagnosis is based on clinical and histopathology. Pityriasis rubra pilaris is self-limited and asymptomatic in many cases. Treatment may not be required, but combination therapy with topical agents includes emollients, keratolytic agents (for example, urea, salicylic acid, alpha-hydroxy acids), topical corticosteroids, tazarotene, and topical calcineurin inhibitors. Systemic agents include oral retinoids and methotrexate.
Atopic dermatitis is a chronic inflammatory skin disease that involves genetic and environmental factors, leading to abnormalities in the epidermis and the immune system presenting with its typical morphology and distribution. The morphology of eczematous lesions is distinct from papulosquamous lesions of psoriasis.
Staphylococcal scalded skin syndrome is a toxin-mediated skin disorder which presents with denuded, peeling skin due to epidermolytic exotoxin producing Staphylococcus species. Fever, erythematous rash, malaise, skin pain, and irritability presents initially. Progressive desquamation with accentuation in folds is typical, with progression usually within 1-2 days. Systemic antibiotics covering Staphylococcus should be administered early. Emollients and nonadherent dressings should be applied to affected areas to promote healing. Supportive care includes dehydration management, temperature regulation, and nutrition. Skin desquamation usually occurs within 5 days with resolution within 2 weeks.
This infant displayed streptococcal intertrigo which triggered an early presentation of guttate psoriasis. The patient was managed with completion of a course of oral cephalexin, midstrength topical corticosteroids to the truncal lesions, and mild topical corticosteroids to the face and diaper area with good clinical response.
Danny Lee and Samuel Le serve as research fellows in the Pediatric Dermatology Division of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. Dr. Eichenfield is Distinguished Professor of Dermatology and Pediatrics and Vice-Chair of the Department of Dermatology at the University of California San Diego and Rady Children’s Hospital, San Diego. The authors have no relevant financial disclosures.
Suggested Reading
Leung AK et al. Childhood guttate psoriasis: An updated review. Drugs Context. 2023 Oct 23:12:2023-8-2. doi: 10.7573/dic.2023-8-2.
Galili E et al. New-onset guttate psoriasis: A long-term follow-up study. Dermatology. 2023;239(2):188-194. doi: 10.1159/000527737.
Duffin KC et al. Advances and controversies in our understanding of guttate and plaque psoriasis. J Rheumatol. 2023 Nov;50(Suppl 2):4-7. doi: 10.3899/jrheum.2023-0500.
Saleh D, Tanner LS. Guttate Psoriasis. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK482498/
Dupire G et al. Antistreptococcal interventions for guttate and chronic plaque psoriasis. Cochrane Database Syst Rev. 2019 Mar 5;3(3):CD011571. doi: 10.1002/14651858.CD011571.pub2.
On physical exam, there was an erythematous patch with overlying areas of macerations on the neck and axilla. The trunk, extremities, and diaper area had multiple psoriasiform erythematous thin plaques with overlying scales.
Shingles Vaccine Offers 4 Years of Protection
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
Two doses of the recombinant zoster vaccine (RZV) are effective against herpes zoster (HZ) for 4 years after vaccination, according to a new study published in Annals of Internal Medicine.
Findings from the prospective cohort study showed that people who received two doses of the vaccine, regardless of when they received their second dose, experienced 79% vaccine effectiveness (VE) during the first year, with effectiveness decreasing to 73% by year 4. By contrast, the rate of effectiveness during the first year was 70% for people who received a single dose, falling to 52% effectiveness by year 4.
The findings also showed that the rate of effectiveness was 65% for those taking corticosteroids.
The study was conducted between 2018 and 2022 using data from the Vaccine Safety Datalink, a collaboration between the US Centers for Disease Control and Prevention (CDC) and nine healthcare systems across the country.
Researchers evaluated the incidence of HZ, as determined by a diagnosis and prescription for antiviral medication within 7 days of diagnosis, and monitored RZV status over time.
The findings may quell fears that waiting too long for the second dose reduces the effectiveness of the herpes vaccine, according to Nicola Klein, MD, PhD, director of the Vaccine Study Center at Kaiser Permanente in Oakland, California, who led the study.
The long-term efficacy of the vaccine is especially important because older adults are now living much longer than in previous years, according to Alexandra Tien, MD, a family physician at Medical Associates of Rhode Island in Providence.
“People live these days into their 80s and even 90s,” Dr. Tien said. “That’s a large number of years to need protection for, so it’s really important to have a long-lasting vaccine.”
The CDC currently recommends two doses of RZV separated by 2-6 months for patients aged 50 years and older. Adults older than 19 years who are immunocompromised should receive two doses of RZV separated by 1-2 months, the agency said.
According to Dr. Klein, research does not show whether VE for RZV wanes after 4 years. But interim findings from another study following people in clinical trials found VE levels remained high after 7 years.
The risk for HZ increases with age, reaching a lifetime risk of 50% among adults aged 85 years. Complications like postherpetic neuralgia (PHN) — characterized by long-term tingling, numbness, and disabling pain at the site of the rash — can interfere with the quality of life and ability to function in older adults. The CDC estimates that up to 18% of people with shingles experience PHN, and the risk increases with age.
Just like with any other vaccine, patients sometimes have concerns about the potential side effects of RZV, said Dr. Tien. But those effects, such as muscle pain, nausea, and fever, are mild compared to shingles.
“I always tell patients, with any vaccine, immunization is one of the biggest bangs for your buck in healthcare because you’re preventing a problem,” Dr. Tien said.
This study was funded by the CDC through contracts with participating sites. Study authors reported no disclosures. Dr. Tien reported no disclosures.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Impact of Pregnancy on Rosacea Unpredictable, Study Suggests
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women diagnosed with rosacea, the impact of pregnancy on the disease is unpredictable.
METHODOLOGY:
- Researchers conducted a telephone survey of 39 women with a diagnosis of rosacea in the electronic medical records prior to the onset of pregnancy who had been admitted to Oregon Health & Science University for labor and delivery from June 27, 2015, to June 27, 2020.
- Patient global assessment of clear (0), mild (1), moderate (2), or severe (3) rosacea was rated across five timepoints: 1-3 months preconception; first, second, and third trimesters; and 6 weeks postpartum.
TAKEAWAY:
- The mean age of the survey participants was 35.5 years, the mean gestational age at delivery was 39.4 weeks, and most had singleton pregnancies.
- All but one study participant (97.4%) reported symptoms of erythematotelangiectatic rosacea, while 26 (67%) reported symptoms of papulopustular rosacea.
- Nearly half of the participants (19, 48.7%) said their rosacea worsened during pregnancy, 13 (33.3%) reported no change in rosacea severity during pregnancy, and 7 (17.9%) reported that their rosacea improved during pregnancy.
- Before conceiving, the mean rosacea severity score among participants was mild (1.10; 95% CI, 0.92-1.29) and did not change significantly over time, a reflection of individual variations. In addition, 83.3% of participants did not use prescription rosacea treatments prior to pregnancy, and 89.6% did not use them during pregnancy.
IN PRACTICE:
“Rosacea, like acne, lacks a predictable group effect, and instead, each individual may have a different response to the physiologic changes of pregnancy,” the authors concluded.
SOURCE:
Genevieve Benedetti, MD, MPP, of the Department of Dermatology at Oregon Health & Science University, Portland, Oregon, led the research, published as a research letter in the International Journal of Women’s Dermatology.
LIMITATIONS:
The small sample size, single-center design, and overall prevalence of mild disease limit the ability to detect change.
DISCLOSURES:
The researchers reported having no disclosures.
A version of this article appeared on Medscape.com.
Study Identifies Cardiovascular Comorbidities Associated With Dermatomyositis
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- DM is associated with cardiovascular disease (CVD), but US-based data studies on CVD comorbidities in patients with DM are lacking.
- In a cross-sectional analysis of participants in the All of Us research program aged 18 years and older with at least 1 year of electronic health record (EHR) data, researchers identified DM cases and controls with nearest neighbor propensity score matching by age, sex, race/ethnicity, EHR duration, and healthcare visit quantity.
- They used the Pearson’s chi-squared test, Fisher’s exact test, unpaired t-test, or Mann-Whitney U test to compare clinical characteristics and traditional CV comorbidities.
- Multivariable conditional logistic regression was used with backward elimination of comorbidities with P > .1 or evidence of collinearity.
TAKEAWAY:
- Among 235,161 All of Us participants, researchers identified 206 DM cases and 824 matched controls with largely similar demographic characteristics, including smoking status, obesity, and indicators of socioeconomic status.
- Participants with DM were more likely to have a history of atrial fibrillation (10.1% vs 16.0%, respectively), chronic kidney disease (15.2% vs 29.1%), congestive heart failure (9.6% vs 18.0%), coronary artery disease (CAD) (18.2% vs 34.0%), hypertension (52.5% vs 60.7%), myocardial infarction (7.4% vs 15.0), type 2 diabetes (27.3% vs 47.6%), and valvular heart disease (8.7% vs 16.5%) than matched controls.
- In a multivariable analysis that adjusted for potential confounders, three comorbidities remained associated with DM: CAD (odds ratio [OR], 2.0; P < .001), type 2 diabetes (OR, 2.2; P < .001), and chronic kidney disease (OR, 1.7; P = .015).
IN PRACTICE:
“Our findings are important both for prognosis and clinical care, suggesting DM patients should be screened for CVD risk factors to potentially reduce the increased risk for cardiovascular events and CVD-related mortality in DM,” the authors concluded.
SOURCE:
Corresponding author Alisa N. Femia, MD, of the department of dermatology at NYU Grossman School of Medicine, led the research. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
How DM treatments might influence CVD development was not addressed. EHRs may have diagnostic inaccuracies and omissions and lack data on clinical features and severity.
DISCLOSURES:
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Femia reported consulting fees from Octagon Therapeutics, Timber Pharmaceuticals, and Guidepoint. Study author Michael S. Garshick, MD, reported consulting fees from AbbVie and Horizon Therapeutics. The remaining authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
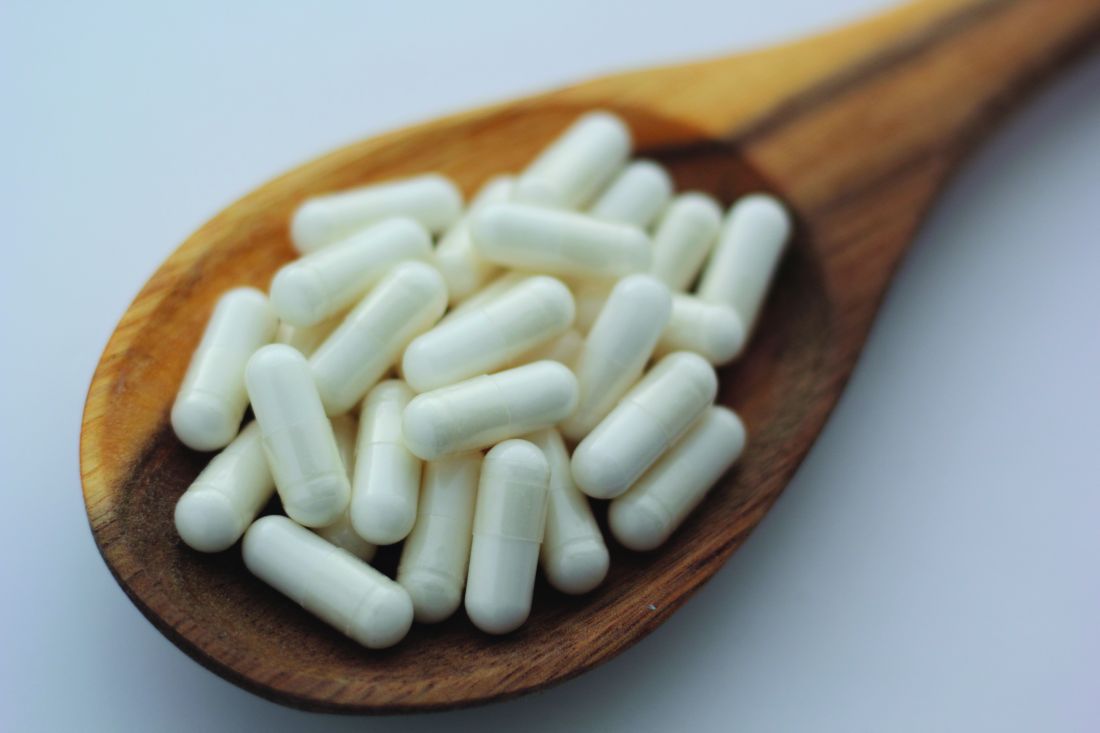
Myo-inositol is one of the components of an integrative approach to acne
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
FROM IDS 2023
Yes, Patients Are Getting More Complicated
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
The first time I saw a patient in the hospital was in 2004, twenty years ago, when I was a third-year med student. I mean, look at that guy. The things I could tell him.
Since that time, I have spent countless hours in the hospital as a resident, a renal fellow, and finally as an attending. And I’m sure many of you in the medical community feel the same thing I do, which is that patients are much more complicated now than they used to be. I’ll listen to an intern present a new case on rounds and she’ll have an assessment and plan that encompasses a dozen individual medical problems. Sometimes I have to literally be like, “Wait, why is this patient here again?”
But until now, I had no data to convince myself that this feeling was real — that hospitalized patients are getting more and more complicated, or that they only seem more complicated because I’m getting older. Maybe I was better able to keep track of things when I was an intern rather than now as an attending, spending just a couple months of the year in the hospital. I mean, after all, if patients were getting more complicated, surely hospitals would know this and allocate more resources to patient care, right?
Right?
It’s not an illusion. At least not according to this paper, Population-Based Trends in Complexity of Hospital Inpatients, appearing in JAMA Internal Medicine, which examines about 15 years of inpatient hospital admissions in British Columbia.
I like Canada for this study for two reasons: First, their electronic health record system is province-wide, so they don’t have issues of getting data from hospital A vs hospital B. All the data are there — in this case, more than 3 million nonelective hospital admissions from British Columbia. Second, there is universal healthcare. We don’t have to worry about insurance companies changing, or the start of a new program like the Affordable Care Act. It’s just a cleaner set-up.
Of course, complexity is hard to define, and the authors here decide to look at a variety of metrics I think we can agree are tied into complexity. These include things like patient age, comorbidities, medications, frequency of hospitalization, and so on. They also looked at outcomes associated with hospitalization: Did the patient require the ICU? Did they survive? Were they readmitted?
And the tale of the tape is as clear as that British Columbian air: Over the past 15 years, your average hospitalized patient is about 3 years older, is twice as likely to have kidney disease, 70% more likely to have diabetes, is on more medications (particularly anticoagulants), and is much more likely to be admitted through the emergency room. They’ve also spent more time in the hospital in the past year.
Given the increased complexity, you might expect that the outcomes for these patients are worse than years ago, but the data do not bear that out. In fact, inpatient mortality is lower now than it was 15 years ago, although 30-day postdischarge mortality is higher. Put those together and it turns out that death rates are pretty stable: 9% of people admitted for nonelective reasons to the hospital will die within 30 days. It’s just that nowadays, we tend to discharge them before that happens.
Why are our patients getting more complex? Some of it is demographics; the population is aging, after all. Some of it relates to the increasing burden of comorbidities like diabetes and kidney disease, which are associated with the obesity epidemic. But in some ways, we’re a victim of our own success.
Given all that, does it make any sense that many of our hospitals are at skeleton-crew staffing levels? That hospitalists report taking care of more patients than they ever have before?
There’s been so much talk about burnout in the health professions lately. Maybe something people need to start acknowledging — particularly those who haven’t practiced on the front lines for a decade or two — is that the job is, quite simply, harder now. As patients become more complex, we need more resources, human and otherwise, to care for them.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and here on Medscape. He tweets @fperrywilson and his book, How Medicine Works and When It Doesn’t, is available now. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA Gives Nod to Berdazimer Gel for Molluscum Contagiosum
On January 5, the Food and Drug Administration (FDA) approved berdazimer gel 10.3% for the treatment of molluscum contagiosum (MC) in adults and children aged 1 year or older.
Approval of berdazimer, a topical nitric oxide–releasing agent, was based largely on a 12-week pivotal phase 3 trial known as B-SIMPLE4, in which 891 patients with a mean age of 6.6 years (range, 0.9-47.5 years) were randomly assigned to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily. At 12 weeks, 32.4% of patients in the berdazimer group achieved complete clearance of MC lesions compared with 19.7% of those in the vehicle group (P < .001).
Only 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events in both groups were application-site pain and erythema, and most of these were mild or moderate.
According to a press release announcing the approval from Ligand Pharmaceuticals, which acquired berdazimer topical gel from Novan in September 2023, the development makes berdazimer topical gel 10.3% the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home; outside of a physician›s office; or outside of other medical settings to treat MC. Nitric oxide has been shown to have antiviral effects, although the mechanism of action of berdazimer for treating molluscum “is unknown,” the company said in the release.
The drug will be marketed under the name Zelsuvmi and is expected to be available in the second half of 2024.
On July 21, 2023, topical cantharidin became the first approved treatment of MC for adults and pediatric patients aged 2 years or older, with the FDA approval of a drug-device combination (Ycanth) that contains a formulation of cantharidin solution 0.7% and is administered by healthcare professionals.
A version of this article appeared on Medscape.com.
On January 5, the Food and Drug Administration (FDA) approved berdazimer gel 10.3% for the treatment of molluscum contagiosum (MC) in adults and children aged 1 year or older.
Approval of berdazimer, a topical nitric oxide–releasing agent, was based largely on a 12-week pivotal phase 3 trial known as B-SIMPLE4, in which 891 patients with a mean age of 6.6 years (range, 0.9-47.5 years) were randomly assigned to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily. At 12 weeks, 32.4% of patients in the berdazimer group achieved complete clearance of MC lesions compared with 19.7% of those in the vehicle group (P < .001).
Only 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events in both groups were application-site pain and erythema, and most of these were mild or moderate.
According to a press release announcing the approval from Ligand Pharmaceuticals, which acquired berdazimer topical gel from Novan in September 2023, the development makes berdazimer topical gel 10.3% the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home; outside of a physician›s office; or outside of other medical settings to treat MC. Nitric oxide has been shown to have antiviral effects, although the mechanism of action of berdazimer for treating molluscum “is unknown,” the company said in the release.
The drug will be marketed under the name Zelsuvmi and is expected to be available in the second half of 2024.
On July 21, 2023, topical cantharidin became the first approved treatment of MC for adults and pediatric patients aged 2 years or older, with the FDA approval of a drug-device combination (Ycanth) that contains a formulation of cantharidin solution 0.7% and is administered by healthcare professionals.
A version of this article appeared on Medscape.com.
On January 5, the Food and Drug Administration (FDA) approved berdazimer gel 10.3% for the treatment of molluscum contagiosum (MC) in adults and children aged 1 year or older.
Approval of berdazimer, a topical nitric oxide–releasing agent, was based largely on a 12-week pivotal phase 3 trial known as B-SIMPLE4, in which 891 patients with a mean age of 6.6 years (range, 0.9-47.5 years) were randomly assigned to treatment with berdazimer gel 10.3% or a vehicle gel applied in a thin layer to all lesions once daily. At 12 weeks, 32.4% of patients in the berdazimer group achieved complete clearance of MC lesions compared with 19.7% of those in the vehicle group (P < .001).
Only 4.1% of patients on berdazimer and 0.7% of those on the vehicle experienced adverse events that led to discontinuation of treatment. The most common adverse events in both groups were application-site pain and erythema, and most of these were mild or moderate.
According to a press release announcing the approval from Ligand Pharmaceuticals, which acquired berdazimer topical gel from Novan in September 2023, the development makes berdazimer topical gel 10.3% the first and only topical prescription medication that can be applied by patients, parents, or caregivers at home; outside of a physician›s office; or outside of other medical settings to treat MC. Nitric oxide has been shown to have antiviral effects, although the mechanism of action of berdazimer for treating molluscum “is unknown,” the company said in the release.
The drug will be marketed under the name Zelsuvmi and is expected to be available in the second half of 2024.
On July 21, 2023, topical cantharidin became the first approved treatment of MC for adults and pediatric patients aged 2 years or older, with the FDA approval of a drug-device combination (Ycanth) that contains a formulation of cantharidin solution 0.7% and is administered by healthcare professionals.
A version of this article appeared on Medscape.com.
Study: Early Tecovirimat Stops Mpox Progression in HIV Patients
A new analysis supports using the smallpox antiviral tecovirimat (TPOXX/ST-246) in HIV patients showing the first symptoms of the human smallpox disease mpox (monkeypox), caused by the variola virus.
In a small prospective matched cohort analysis, people with HIV (PWH) and mpox disease who received tecovirimat within 7 days of symptom onset were 13 times less likely to experience progression, compared with PWH not prescribed tecovirimat within that window. In a matched cohort of 112 PWH, mpox disease progression occurred in 5.4% in an early tecovirimat group and in 26.8% in a late- or no-tecovirimat group, for a paired odds ratio of 13.00 (95% CI, 1.71-99.40; P = .002).
“Results of the present study suggest that tecovirimat treatment should be started early at the time of suspected mpox diagnosis in all PWH, especially in those with nonsuppressed HIV viremia or mucosal site involvement,” wrote a team led by Bruce Aldred, MD, of the Division of Infectious Diseases in the Department of Medicine at Emory University School of Medicine in Atlanta, in JAMA Internal Medicine. Early symptoms of mpox include skin rash and mucosal lesions, along with viral symptoms such as fever, headache, muscle aches, back pain, low energy, and swollen lymph nodes.
As of March 1 of last year, the United States reported more than 30,000 cases, while cases numbered more than 86,000 worldwide.
Despite a lack of effectiveness data in humans, tecovirimat was widely prescribed to PWH with mpox during the 2022 epidemic, which disproportionately affected PWH, particularly those with low CD4+ T-cell counts or severe mpox clinical manifestations who needed urgent therapy. Developed to treat smallpox, tecovirimat has antiviral activity against other orthopoxviruses, and has reduced mpox-related morbidity and mortality in animals.
Based on the animal data, approval was granted by the US Food and Drug Administration (FDA) for human mpox treatment. Dr. Aldred and colleagues undertook this cohort analysis in the absence of human data and with the postoutbreak decline in cases impeding recruitment to a full-scale clinical trial.
Study design
The preponderantly Black cohort included 112 PWH diagnosed with mpox at four Atlanta hospitals from June 1 to October 7, 2022. Patients were grouped in an early cohort receiving tecovirimat within 7 days of symptom onset or a no or late cohort (no tecovirimat or treatment more than 7 days after symptom onset. Multivariate logistic regression models identified factors associated with progression, defined as development of at least one severe CDC mpox criterion after symptom day 7.
The cohorts were then matched 1:1 using propensity scores based on the identified factors, and mpox disease progression was compared.
Of 112 PWH, 56 receive early tecovirimat and 56 received no or late treatment. In the early group, the median (interquartile range [IQR]) age was 35 (30-42) years; 54 individuals (96.4%) were cisgender men, 46 (82.1%) were Black, and 10 (17.9%) were, variously, White, American Indian, Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or of unknown race.
In the late- or no-tecovirimat group, the median (IQR) age was 36 (32-43) years; 54 (96.4%) were cisgender men, 49 (87.5%) were Black, and 7 (12.5%) were individuals of other or unknown race. Mpox disease progression occurred in 3 PWH in the early-tecovirimat group and 15 PWH (26.8%) in the late- or no-tecovirimat group.
Dr. Aldred and colleagues acknowledged that more research is needed to confirm the findings and cited several study limitations. These included the small sample size, the preponderance of Black participants, and the possibility that unmatched confounding variables could have led to the observation of fewer cases of severe disease in the early-tecovirimat cohort.
This study was supported by a grant from the Emory Center for AIDS Research. Coauthors reported grants from various institutes at the National Institutes of Health as well as from multiple pharmaceutical companies.
A new analysis supports using the smallpox antiviral tecovirimat (TPOXX/ST-246) in HIV patients showing the first symptoms of the human smallpox disease mpox (monkeypox), caused by the variola virus.
In a small prospective matched cohort analysis, people with HIV (PWH) and mpox disease who received tecovirimat within 7 days of symptom onset were 13 times less likely to experience progression, compared with PWH not prescribed tecovirimat within that window. In a matched cohort of 112 PWH, mpox disease progression occurred in 5.4% in an early tecovirimat group and in 26.8% in a late- or no-tecovirimat group, for a paired odds ratio of 13.00 (95% CI, 1.71-99.40; P = .002).
“Results of the present study suggest that tecovirimat treatment should be started early at the time of suspected mpox diagnosis in all PWH, especially in those with nonsuppressed HIV viremia or mucosal site involvement,” wrote a team led by Bruce Aldred, MD, of the Division of Infectious Diseases in the Department of Medicine at Emory University School of Medicine in Atlanta, in JAMA Internal Medicine. Early symptoms of mpox include skin rash and mucosal lesions, along with viral symptoms such as fever, headache, muscle aches, back pain, low energy, and swollen lymph nodes.
As of March 1 of last year, the United States reported more than 30,000 cases, while cases numbered more than 86,000 worldwide.
Despite a lack of effectiveness data in humans, tecovirimat was widely prescribed to PWH with mpox during the 2022 epidemic, which disproportionately affected PWH, particularly those with low CD4+ T-cell counts or severe mpox clinical manifestations who needed urgent therapy. Developed to treat smallpox, tecovirimat has antiviral activity against other orthopoxviruses, and has reduced mpox-related morbidity and mortality in animals.
Based on the animal data, approval was granted by the US Food and Drug Administration (FDA) for human mpox treatment. Dr. Aldred and colleagues undertook this cohort analysis in the absence of human data and with the postoutbreak decline in cases impeding recruitment to a full-scale clinical trial.
Study design
The preponderantly Black cohort included 112 PWH diagnosed with mpox at four Atlanta hospitals from June 1 to October 7, 2022. Patients were grouped in an early cohort receiving tecovirimat within 7 days of symptom onset or a no or late cohort (no tecovirimat or treatment more than 7 days after symptom onset. Multivariate logistic regression models identified factors associated with progression, defined as development of at least one severe CDC mpox criterion after symptom day 7.
The cohorts were then matched 1:1 using propensity scores based on the identified factors, and mpox disease progression was compared.
Of 112 PWH, 56 receive early tecovirimat and 56 received no or late treatment. In the early group, the median (interquartile range [IQR]) age was 35 (30-42) years; 54 individuals (96.4%) were cisgender men, 46 (82.1%) were Black, and 10 (17.9%) were, variously, White, American Indian, Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or of unknown race.
In the late- or no-tecovirimat group, the median (IQR) age was 36 (32-43) years; 54 (96.4%) were cisgender men, 49 (87.5%) were Black, and 7 (12.5%) were individuals of other or unknown race. Mpox disease progression occurred in 3 PWH in the early-tecovirimat group and 15 PWH (26.8%) in the late- or no-tecovirimat group.
Dr. Aldred and colleagues acknowledged that more research is needed to confirm the findings and cited several study limitations. These included the small sample size, the preponderance of Black participants, and the possibility that unmatched confounding variables could have led to the observation of fewer cases of severe disease in the early-tecovirimat cohort.
This study was supported by a grant from the Emory Center for AIDS Research. Coauthors reported grants from various institutes at the National Institutes of Health as well as from multiple pharmaceutical companies.
A new analysis supports using the smallpox antiviral tecovirimat (TPOXX/ST-246) in HIV patients showing the first symptoms of the human smallpox disease mpox (monkeypox), caused by the variola virus.
In a small prospective matched cohort analysis, people with HIV (PWH) and mpox disease who received tecovirimat within 7 days of symptom onset were 13 times less likely to experience progression, compared with PWH not prescribed tecovirimat within that window. In a matched cohort of 112 PWH, mpox disease progression occurred in 5.4% in an early tecovirimat group and in 26.8% in a late- or no-tecovirimat group, for a paired odds ratio of 13.00 (95% CI, 1.71-99.40; P = .002).
“Results of the present study suggest that tecovirimat treatment should be started early at the time of suspected mpox diagnosis in all PWH, especially in those with nonsuppressed HIV viremia or mucosal site involvement,” wrote a team led by Bruce Aldred, MD, of the Division of Infectious Diseases in the Department of Medicine at Emory University School of Medicine in Atlanta, in JAMA Internal Medicine. Early symptoms of mpox include skin rash and mucosal lesions, along with viral symptoms such as fever, headache, muscle aches, back pain, low energy, and swollen lymph nodes.
As of March 1 of last year, the United States reported more than 30,000 cases, while cases numbered more than 86,000 worldwide.
Despite a lack of effectiveness data in humans, tecovirimat was widely prescribed to PWH with mpox during the 2022 epidemic, which disproportionately affected PWH, particularly those with low CD4+ T-cell counts or severe mpox clinical manifestations who needed urgent therapy. Developed to treat smallpox, tecovirimat has antiviral activity against other orthopoxviruses, and has reduced mpox-related morbidity and mortality in animals.
Based on the animal data, approval was granted by the US Food and Drug Administration (FDA) for human mpox treatment. Dr. Aldred and colleagues undertook this cohort analysis in the absence of human data and with the postoutbreak decline in cases impeding recruitment to a full-scale clinical trial.
Study design
The preponderantly Black cohort included 112 PWH diagnosed with mpox at four Atlanta hospitals from June 1 to October 7, 2022. Patients were grouped in an early cohort receiving tecovirimat within 7 days of symptom onset or a no or late cohort (no tecovirimat or treatment more than 7 days after symptom onset. Multivariate logistic regression models identified factors associated with progression, defined as development of at least one severe CDC mpox criterion after symptom day 7.
The cohorts were then matched 1:1 using propensity scores based on the identified factors, and mpox disease progression was compared.
Of 112 PWH, 56 receive early tecovirimat and 56 received no or late treatment. In the early group, the median (interquartile range [IQR]) age was 35 (30-42) years; 54 individuals (96.4%) were cisgender men, 46 (82.1%) were Black, and 10 (17.9%) were, variously, White, American Indian, Alaska Native, Asian, Native Hawaiian or Other Pacific Islander, or of unknown race.
In the late- or no-tecovirimat group, the median (IQR) age was 36 (32-43) years; 54 (96.4%) were cisgender men, 49 (87.5%) were Black, and 7 (12.5%) were individuals of other or unknown race. Mpox disease progression occurred in 3 PWH in the early-tecovirimat group and 15 PWH (26.8%) in the late- or no-tecovirimat group.
Dr. Aldred and colleagues acknowledged that more research is needed to confirm the findings and cited several study limitations. These included the small sample size, the preponderance of Black participants, and the possibility that unmatched confounding variables could have led to the observation of fewer cases of severe disease in the early-tecovirimat cohort.
This study was supported by a grant from the Emory Center for AIDS Research. Coauthors reported grants from various institutes at the National Institutes of Health as well as from multiple pharmaceutical companies.
FROM JAMA INTERNAL MEDICINE