User login
News and Views that Matter to Physicians
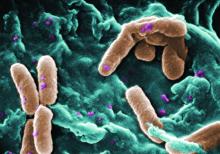
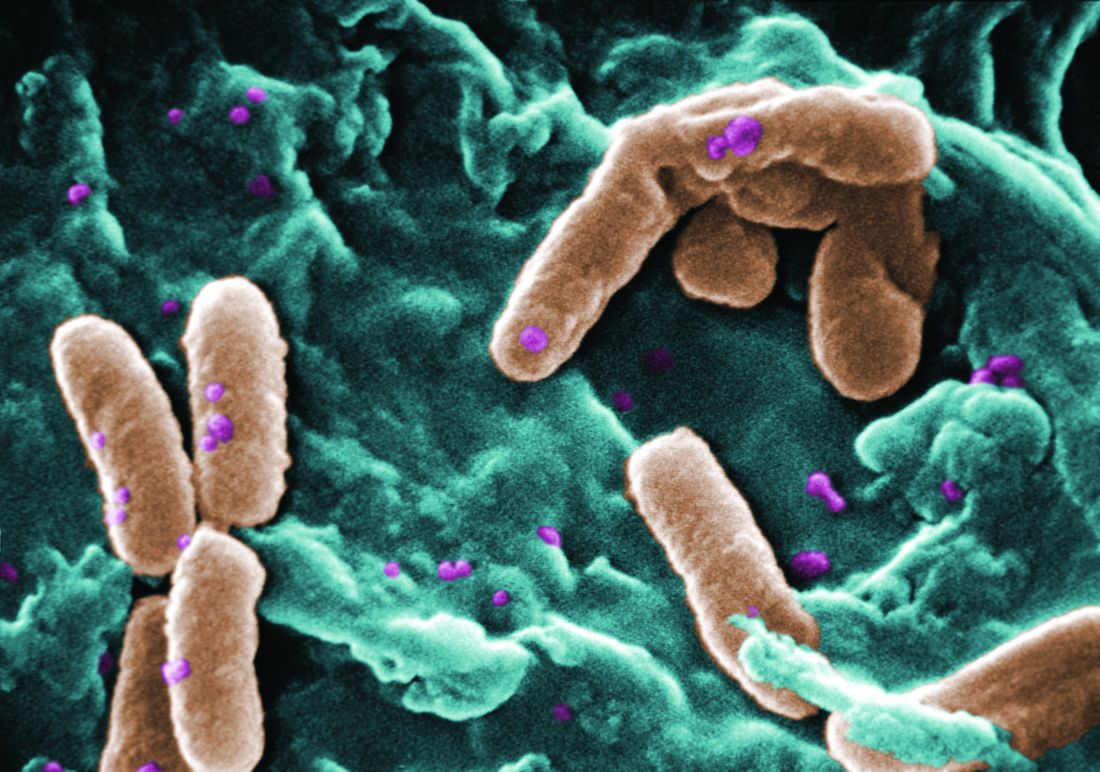
Rate of resistant P. aeruginosa in children rising steadily
The rate of infection with resistant Pseudomonas aeruginosa among children has risen steadily about 4% per year since 1999, according to a report.
Infections with P aeruginosa in children occur most often associated with pulmonary disease in patients with cystic fibrosis, but healthy children also can experience a variety of P aeruginosa infection types.
They focused on 77,349 P. aeruginosa isolates that were tested for resistance for all five antibiotic classes: cephalosporins, beta-lactam and beta-lactamase inhibitors, carbapenems, fluoroquinolones, and aminoglycosides. The investigators were specifically interested in multidrug- and carbapenem-resistant P. aeruginosa. They described the organism as “arguably the most resistance-prone health care–related pathogen.”
The study samples were obtained from the respiratory tract, urinary tract, ear, sinuses, wounds, skin, and connective tissues of children aged 1-17 years who were treated in 1999-2012; 2012 was the last year for which this information was collected in this database. Patients were treated in ambulatory, inpatient, ICU, and long-term-care settings. The study excluded children with cystic fibrosis and children under 1 year of age, so as to improve the applicability of the results to the general pediatric population.
The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%. This represents an average annual increase of approximately 4% for both types of resistance, Dr. Logan and her associates said (J Ped Infect Dis. 2016 Nov 16. doi: 10.1093/jpids/piw064).
These increases were consistent across all but one age group and all but one treatment setting, the exception being inpatients aged 13-17 years. The prevalence of resistant P. aeruginosa was highest among adolescents in ambulatory, ICU, or long-term-care settings; their rates were three times higher than those in children aged 1 year and two times higher than those in children aged 5 years. It is possible that this pattern reflects “an increasing number of older children with a medically complex condition who have frequent exposure to the health care environment,” the investigators noted.
“The results of our study highlight the need for bacterial surveillance, strategies for implementing effective infection prevention, and antimicrobial stewardship programs.” In addition, all health care facilities should consider using rapid molecular diagnostic platforms to guide antibiotic treatment decisions, “to reduce the burden of the persistent and continually evolving global threat of extensively drug-resistant organisms,” Dr. Logan and her associates added.
This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
The rate of infection with resistant Pseudomonas aeruginosa among children has risen steadily about 4% per year since 1999, according to a report.
Infections with P aeruginosa in children occur most often associated with pulmonary disease in patients with cystic fibrosis, but healthy children also can experience a variety of P aeruginosa infection types.
They focused on 77,349 P. aeruginosa isolates that were tested for resistance for all five antibiotic classes: cephalosporins, beta-lactam and beta-lactamase inhibitors, carbapenems, fluoroquinolones, and aminoglycosides. The investigators were specifically interested in multidrug- and carbapenem-resistant P. aeruginosa. They described the organism as “arguably the most resistance-prone health care–related pathogen.”
The study samples were obtained from the respiratory tract, urinary tract, ear, sinuses, wounds, skin, and connective tissues of children aged 1-17 years who were treated in 1999-2012; 2012 was the last year for which this information was collected in this database. Patients were treated in ambulatory, inpatient, ICU, and long-term-care settings. The study excluded children with cystic fibrosis and children under 1 year of age, so as to improve the applicability of the results to the general pediatric population.
The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%. This represents an average annual increase of approximately 4% for both types of resistance, Dr. Logan and her associates said (J Ped Infect Dis. 2016 Nov 16. doi: 10.1093/jpids/piw064).
These increases were consistent across all but one age group and all but one treatment setting, the exception being inpatients aged 13-17 years. The prevalence of resistant P. aeruginosa was highest among adolescents in ambulatory, ICU, or long-term-care settings; their rates were three times higher than those in children aged 1 year and two times higher than those in children aged 5 years. It is possible that this pattern reflects “an increasing number of older children with a medically complex condition who have frequent exposure to the health care environment,” the investigators noted.
“The results of our study highlight the need for bacterial surveillance, strategies for implementing effective infection prevention, and antimicrobial stewardship programs.” In addition, all health care facilities should consider using rapid molecular diagnostic platforms to guide antibiotic treatment decisions, “to reduce the burden of the persistent and continually evolving global threat of extensively drug-resistant organisms,” Dr. Logan and her associates added.
This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
The rate of infection with resistant Pseudomonas aeruginosa among children has risen steadily about 4% per year since 1999, according to a report.
Infections with P aeruginosa in children occur most often associated with pulmonary disease in patients with cystic fibrosis, but healthy children also can experience a variety of P aeruginosa infection types.
They focused on 77,349 P. aeruginosa isolates that were tested for resistance for all five antibiotic classes: cephalosporins, beta-lactam and beta-lactamase inhibitors, carbapenems, fluoroquinolones, and aminoglycosides. The investigators were specifically interested in multidrug- and carbapenem-resistant P. aeruginosa. They described the organism as “arguably the most resistance-prone health care–related pathogen.”
The study samples were obtained from the respiratory tract, urinary tract, ear, sinuses, wounds, skin, and connective tissues of children aged 1-17 years who were treated in 1999-2012; 2012 was the last year for which this information was collected in this database. Patients were treated in ambulatory, inpatient, ICU, and long-term-care settings. The study excluded children with cystic fibrosis and children under 1 year of age, so as to improve the applicability of the results to the general pediatric population.
The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%. This represents an average annual increase of approximately 4% for both types of resistance, Dr. Logan and her associates said (J Ped Infect Dis. 2016 Nov 16. doi: 10.1093/jpids/piw064).
These increases were consistent across all but one age group and all but one treatment setting, the exception being inpatients aged 13-17 years. The prevalence of resistant P. aeruginosa was highest among adolescents in ambulatory, ICU, or long-term-care settings; their rates were three times higher than those in children aged 1 year and two times higher than those in children aged 5 years. It is possible that this pattern reflects “an increasing number of older children with a medically complex condition who have frequent exposure to the health care environment,” the investigators noted.
“The results of our study highlight the need for bacterial surveillance, strategies for implementing effective infection prevention, and antimicrobial stewardship programs.” In addition, all health care facilities should consider using rapid molecular diagnostic platforms to guide antibiotic treatment decisions, “to reduce the burden of the persistent and continually evolving global threat of extensively drug-resistant organisms,” Dr. Logan and her associates added.
This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
FROM THE JOURNAL OF PEDIATRIC INFECTIOUS DISEASES
Key clinical point:
Major finding: The proportion of multidrug-resistant P. aeruginosa samples rose from 15% to 26% during the 13-year study period, and the proportion of carbapenem-resistant P. aeruginosa samples rose from 9% to 20%.
Data source: A longitudinal analysis of information in a nationally representative database of microbiology laboratories serving approximately 300 U.S. hospitals.
Disclosures: This study was supported by the National Institute of Allergy and Infectious Diseases; the Global Antibiotic Resistance Partnership, funded by the Bill and Melinda Gates Foundation; and the Health Grand Challenges Program at Princeton University. Dr. Logan and her associates reported having no relevant financial disclosures.
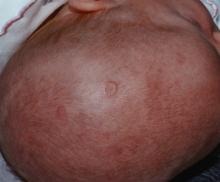
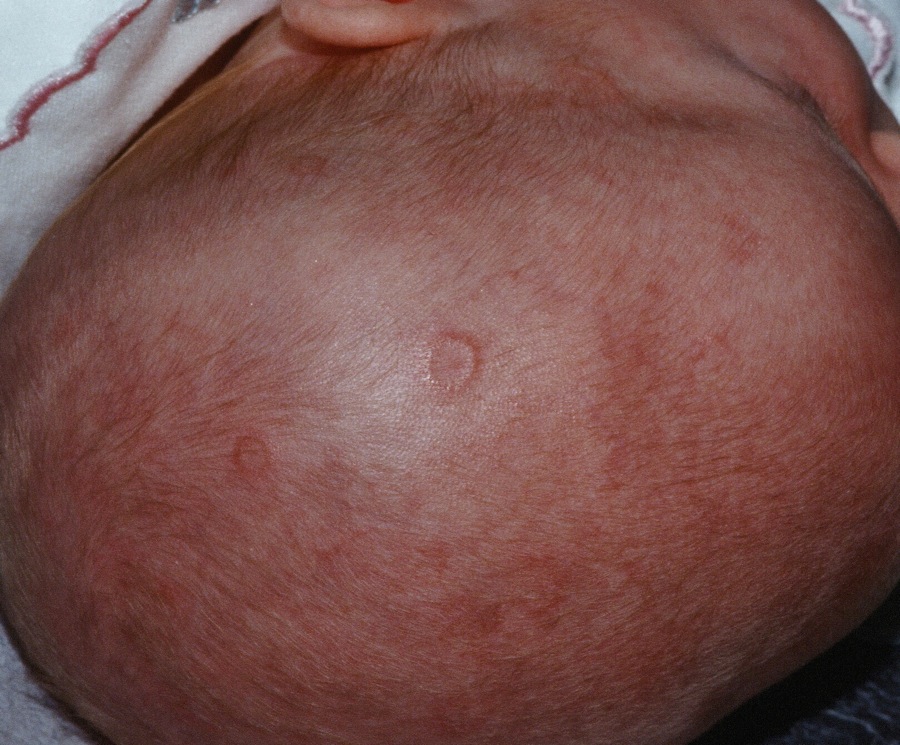
Prenatal exposure to hydroxychloroquine cuts risk of neonatal cutaneous lupus
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The drug was associated with a 60% decreased risk of developing the disorder.
Data source: The case-control study involved 545 infants.
Disclosures: Dr. Barsalou had no financial disclosures.
House passes 21st Century Cures bill
A pared-down version of the 21st Century Cures Act passed the House Nov. 30 by an overwhelming 392-26 vote, setting the stage for a quick Senate vote on the compromise legislation.
H.R. 34 gained more support on the House floor than did a version of the legislation that passed the House in 2015. In order to gain that additional support and ensure Senate approval, funding for key biomedical research efforts – the BRAIN Initiative, the Cancer Moonshot, and the Precision Medicine Initiative – was reduced from $9.3 billion to $4.8 billion over 10 years. Further, those funds are not guaranteed but will need to be appropriated through the federal budget process.
Other provisions include creation of an NIH program to support new researchers; funds to accelerate improved methods for prevention, diagnosis, and treatment of tick-borne diseases; the development of a national neurologic condition surveillance system; and the establishment of a task force on research specific to pregnant and breastfeeding women.
“More women with chronic diseases are becoming pregnant, yet safe and effective medications to manage these ongoing conditions throughout their pregnancy and beyond are needed,” Mary Norton, MD, president of the Society for Maternal-Fetal Medicine, said in a statement. “This legislation is a great first step toward greater collaboration and communication among federal agencies and public stakeholders.”
The 21st Century Cures bill also “takes concrete steps to help women and families suffering from postpartum depression,” Thomas Gellhaus, MD, president of the American College of Obstetricians and Gynecologists, said in a statement. “Postpartum depression is one of the most common medical complications following pregnancy. ... Cures expands state programs to better identify, treat, and support women and families at risk for or facing postpartum depression.”
The bill provides $500 million to the Food and Drug Administration to help the agency speed up the drug approval process, focusing on identifying biomarkers and developing targeted drugs for rare diseases. It also reauthorizes the pediatric rare disease priority review voucher program; requires drug companies to have a publicly accessible compassionate use policy for drugs treating serious or life-threatening conditions; and provides flexibility to get new antimicrobial drugs to market quickly.
Changes in the drug approval process were contentious during debate on the House floor.
“In its attempt to speed up the drug and device approval process, this legislation neglects the very people whom clinical trials are meant to help, that is, the patients,” Rep. Rosa DeLauro (D-Conn.) said. “Rather than protect those who rely on the health care system, it reduces the already weak regulation on medical devices, allows drugs with only limited evidence of the drug’s safety and efficacy, and rushes the use of new and unproven antibiotics.”
Other legislators expressed disappointment at the bill’s mental health care provisions. Rep. Joseph Kennedy III (D-Mass.) said that his “real concerns with the legislation lie with the mental health reform proposals, which don’t go nearly far enough. Mental health parity is already the law, thanks to the Mental Health Parity and Addiction Equity Act and the Affordable Care Act; but each study we read, Mr. Speaker, and each story we hear proves that insurance companies are skirting those rules.
“We need enforcement and transparency today,” Rep. Kennedy continued. “We need random audits before there have been violations, not after. We need insurers to publicly disclose the rates and reasons for denials in a way that patients and their families can understand, not in away that mental health advocates can’t even obtain. We need to increase Medicaid reimbursements in order to expand access to care, not to reduce them or roll back expansion.”
The pediatric provisions drew mixed reviews from the American Academy of Pediatrics.
“The 21st Century Cures Act includes three new programs that, if funded, would improve infant and child mental health: one that supports behavioral and mental health integration into the pediatric primary care setting, one that increases screening and treatment for maternal depression, and one that enhances infant and early childhood mental health,” AAP President Benard Dreyer, MD, said in a statement. “Of additional note is a provision that incentivizes the certification of health information technology for use by pediatricians, and a provision that ensures children in [psychiatric facilities] receive Medicaid’s early periodic screening, treatment, and diagnosis gold standard of care. Finally, the AAP supports the 21st Century Cures Act’s reauthorization of a bill to prevent underage drinking, which includes a new program to train pediatric health professionals in substance use screening, intervention, and referrals.”
However, Dr. Dreyer noted that more work needs to be done.
“The Family First Prevention Services Act of 2016, a comprehensive, bipartisan effort to improve how the child welfare system serves children and families in adversity, was connected to the 21st Century Cures Act until earlier this week,” he said. “Family First represents more than 2 years of work, and is a pivotal opportunity for a major federal policy shift away from placing children in out-of-home care and toward keeping families together. Removing it from this legislative package could mean losing the chance to pass it at all, an unacceptable and undeserved setback for the nation’s most vulnerable children.”
21st Century Cures also contains health IT-related provisions, mostly aimed at improving the interoperability of electronic health records. It also reduces the documentation burden on providers and establishes the authority for the HHS Office of Inspector General to penalize those engaged in information blocking between EHRs.
The bill also increases the transparency around Medicare local coverage decisions and exempts certain transfers of value from reporting requirements related to continuing education. It sets reimbursement for Medicare Part B drugs infused through durable medical equipment at 106% of average sales price.
Sen. Lamar Alexander, chairman of the Health, Education, Labor and Pensions Committee, said that a vote in that chamber could happen as early as Dec. 5. Upon House passage, President Obama signaled his intention to sign the bill, according to a statement from the White House Press Secretary.
A pared-down version of the 21st Century Cures Act passed the House Nov. 30 by an overwhelming 392-26 vote, setting the stage for a quick Senate vote on the compromise legislation.
H.R. 34 gained more support on the House floor than did a version of the legislation that passed the House in 2015. In order to gain that additional support and ensure Senate approval, funding for key biomedical research efforts – the BRAIN Initiative, the Cancer Moonshot, and the Precision Medicine Initiative – was reduced from $9.3 billion to $4.8 billion over 10 years. Further, those funds are not guaranteed but will need to be appropriated through the federal budget process.
Other provisions include creation of an NIH program to support new researchers; funds to accelerate improved methods for prevention, diagnosis, and treatment of tick-borne diseases; the development of a national neurologic condition surveillance system; and the establishment of a task force on research specific to pregnant and breastfeeding women.
“More women with chronic diseases are becoming pregnant, yet safe and effective medications to manage these ongoing conditions throughout their pregnancy and beyond are needed,” Mary Norton, MD, president of the Society for Maternal-Fetal Medicine, said in a statement. “This legislation is a great first step toward greater collaboration and communication among federal agencies and public stakeholders.”
The 21st Century Cures bill also “takes concrete steps to help women and families suffering from postpartum depression,” Thomas Gellhaus, MD, president of the American College of Obstetricians and Gynecologists, said in a statement. “Postpartum depression is one of the most common medical complications following pregnancy. ... Cures expands state programs to better identify, treat, and support women and families at risk for or facing postpartum depression.”
The bill provides $500 million to the Food and Drug Administration to help the agency speed up the drug approval process, focusing on identifying biomarkers and developing targeted drugs for rare diseases. It also reauthorizes the pediatric rare disease priority review voucher program; requires drug companies to have a publicly accessible compassionate use policy for drugs treating serious or life-threatening conditions; and provides flexibility to get new antimicrobial drugs to market quickly.
Changes in the drug approval process were contentious during debate on the House floor.
“In its attempt to speed up the drug and device approval process, this legislation neglects the very people whom clinical trials are meant to help, that is, the patients,” Rep. Rosa DeLauro (D-Conn.) said. “Rather than protect those who rely on the health care system, it reduces the already weak regulation on medical devices, allows drugs with only limited evidence of the drug’s safety and efficacy, and rushes the use of new and unproven antibiotics.”
Other legislators expressed disappointment at the bill’s mental health care provisions. Rep. Joseph Kennedy III (D-Mass.) said that his “real concerns with the legislation lie with the mental health reform proposals, which don’t go nearly far enough. Mental health parity is already the law, thanks to the Mental Health Parity and Addiction Equity Act and the Affordable Care Act; but each study we read, Mr. Speaker, and each story we hear proves that insurance companies are skirting those rules.
“We need enforcement and transparency today,” Rep. Kennedy continued. “We need random audits before there have been violations, not after. We need insurers to publicly disclose the rates and reasons for denials in a way that patients and their families can understand, not in away that mental health advocates can’t even obtain. We need to increase Medicaid reimbursements in order to expand access to care, not to reduce them or roll back expansion.”
The pediatric provisions drew mixed reviews from the American Academy of Pediatrics.
“The 21st Century Cures Act includes three new programs that, if funded, would improve infant and child mental health: one that supports behavioral and mental health integration into the pediatric primary care setting, one that increases screening and treatment for maternal depression, and one that enhances infant and early childhood mental health,” AAP President Benard Dreyer, MD, said in a statement. “Of additional note is a provision that incentivizes the certification of health information technology for use by pediatricians, and a provision that ensures children in [psychiatric facilities] receive Medicaid’s early periodic screening, treatment, and diagnosis gold standard of care. Finally, the AAP supports the 21st Century Cures Act’s reauthorization of a bill to prevent underage drinking, which includes a new program to train pediatric health professionals in substance use screening, intervention, and referrals.”
However, Dr. Dreyer noted that more work needs to be done.
“The Family First Prevention Services Act of 2016, a comprehensive, bipartisan effort to improve how the child welfare system serves children and families in adversity, was connected to the 21st Century Cures Act until earlier this week,” he said. “Family First represents more than 2 years of work, and is a pivotal opportunity for a major federal policy shift away from placing children in out-of-home care and toward keeping families together. Removing it from this legislative package could mean losing the chance to pass it at all, an unacceptable and undeserved setback for the nation’s most vulnerable children.”
21st Century Cures also contains health IT-related provisions, mostly aimed at improving the interoperability of electronic health records. It also reduces the documentation burden on providers and establishes the authority for the HHS Office of Inspector General to penalize those engaged in information blocking between EHRs.
The bill also increases the transparency around Medicare local coverage decisions and exempts certain transfers of value from reporting requirements related to continuing education. It sets reimbursement for Medicare Part B drugs infused through durable medical equipment at 106% of average sales price.
Sen. Lamar Alexander, chairman of the Health, Education, Labor and Pensions Committee, said that a vote in that chamber could happen as early as Dec. 5. Upon House passage, President Obama signaled his intention to sign the bill, according to a statement from the White House Press Secretary.
A pared-down version of the 21st Century Cures Act passed the House Nov. 30 by an overwhelming 392-26 vote, setting the stage for a quick Senate vote on the compromise legislation.
H.R. 34 gained more support on the House floor than did a version of the legislation that passed the House in 2015. In order to gain that additional support and ensure Senate approval, funding for key biomedical research efforts – the BRAIN Initiative, the Cancer Moonshot, and the Precision Medicine Initiative – was reduced from $9.3 billion to $4.8 billion over 10 years. Further, those funds are not guaranteed but will need to be appropriated through the federal budget process.
Other provisions include creation of an NIH program to support new researchers; funds to accelerate improved methods for prevention, diagnosis, and treatment of tick-borne diseases; the development of a national neurologic condition surveillance system; and the establishment of a task force on research specific to pregnant and breastfeeding women.
“More women with chronic diseases are becoming pregnant, yet safe and effective medications to manage these ongoing conditions throughout their pregnancy and beyond are needed,” Mary Norton, MD, president of the Society for Maternal-Fetal Medicine, said in a statement. “This legislation is a great first step toward greater collaboration and communication among federal agencies and public stakeholders.”
The 21st Century Cures bill also “takes concrete steps to help women and families suffering from postpartum depression,” Thomas Gellhaus, MD, president of the American College of Obstetricians and Gynecologists, said in a statement. “Postpartum depression is one of the most common medical complications following pregnancy. ... Cures expands state programs to better identify, treat, and support women and families at risk for or facing postpartum depression.”
The bill provides $500 million to the Food and Drug Administration to help the agency speed up the drug approval process, focusing on identifying biomarkers and developing targeted drugs for rare diseases. It also reauthorizes the pediatric rare disease priority review voucher program; requires drug companies to have a publicly accessible compassionate use policy for drugs treating serious or life-threatening conditions; and provides flexibility to get new antimicrobial drugs to market quickly.
Changes in the drug approval process were contentious during debate on the House floor.
“In its attempt to speed up the drug and device approval process, this legislation neglects the very people whom clinical trials are meant to help, that is, the patients,” Rep. Rosa DeLauro (D-Conn.) said. “Rather than protect those who rely on the health care system, it reduces the already weak regulation on medical devices, allows drugs with only limited evidence of the drug’s safety and efficacy, and rushes the use of new and unproven antibiotics.”
Other legislators expressed disappointment at the bill’s mental health care provisions. Rep. Joseph Kennedy III (D-Mass.) said that his “real concerns with the legislation lie with the mental health reform proposals, which don’t go nearly far enough. Mental health parity is already the law, thanks to the Mental Health Parity and Addiction Equity Act and the Affordable Care Act; but each study we read, Mr. Speaker, and each story we hear proves that insurance companies are skirting those rules.
“We need enforcement and transparency today,” Rep. Kennedy continued. “We need random audits before there have been violations, not after. We need insurers to publicly disclose the rates and reasons for denials in a way that patients and their families can understand, not in away that mental health advocates can’t even obtain. We need to increase Medicaid reimbursements in order to expand access to care, not to reduce them or roll back expansion.”
The pediatric provisions drew mixed reviews from the American Academy of Pediatrics.
“The 21st Century Cures Act includes three new programs that, if funded, would improve infant and child mental health: one that supports behavioral and mental health integration into the pediatric primary care setting, one that increases screening and treatment for maternal depression, and one that enhances infant and early childhood mental health,” AAP President Benard Dreyer, MD, said in a statement. “Of additional note is a provision that incentivizes the certification of health information technology for use by pediatricians, and a provision that ensures children in [psychiatric facilities] receive Medicaid’s early periodic screening, treatment, and diagnosis gold standard of care. Finally, the AAP supports the 21st Century Cures Act’s reauthorization of a bill to prevent underage drinking, which includes a new program to train pediatric health professionals in substance use screening, intervention, and referrals.”
However, Dr. Dreyer noted that more work needs to be done.
“The Family First Prevention Services Act of 2016, a comprehensive, bipartisan effort to improve how the child welfare system serves children and families in adversity, was connected to the 21st Century Cures Act until earlier this week,” he said. “Family First represents more than 2 years of work, and is a pivotal opportunity for a major federal policy shift away from placing children in out-of-home care and toward keeping families together. Removing it from this legislative package could mean losing the chance to pass it at all, an unacceptable and undeserved setback for the nation’s most vulnerable children.”
21st Century Cures also contains health IT-related provisions, mostly aimed at improving the interoperability of electronic health records. It also reduces the documentation burden on providers and establishes the authority for the HHS Office of Inspector General to penalize those engaged in information blocking between EHRs.
The bill also increases the transparency around Medicare local coverage decisions and exempts certain transfers of value from reporting requirements related to continuing education. It sets reimbursement for Medicare Part B drugs infused through durable medical equipment at 106% of average sales price.
Sen. Lamar Alexander, chairman of the Health, Education, Labor and Pensions Committee, said that a vote in that chamber could happen as early as Dec. 5. Upon House passage, President Obama signaled his intention to sign the bill, according to a statement from the White House Press Secretary.
Slavitt to Trump administration: Keep the CMS Innovation Center
WASHINGTON – Acting Administrator Andy Slavitt has some advice for his successors at the CMS: Keep the Center for Medicare & Medicaid Innovation, even if you trash the Affordable Care Act.
The innovation center is vital to the success of the Quality Payment Program, the value-based payment framework set up by the Medicare Access and CHIP Reauthorization Act (MACRA), Mr. Slavitt said Dec. 1 at the National MACRA MIPS/APM Summit.
“MACRA can’t work as well without a CMS Innovation Center that can move quickly to develop and expand new approaches to paying for care,” Mr. Slavitt said. “With changes to the Innovation Center, the advanced alternative payment approaches could slow significantly. We will have a much narrower path with fewer specialty options and approaches, which take in patient and physician feedback. Medicare and commercial payers would then fall further out of alignment, and more importantly, less patients would have access to innovative care methods.”
Mr. Slavitt offered a few other recommendations to the next regime. First, he called on the Trump administration to ensure that the 20 million people who have obtained health care coverage under the ACA do not lose it as a key to continued delivery system reform.
“Build from a foundation of progress, do not head backwards,” Mr. Slavitt advised. “There can be no delivery system reform without building on the foundation of reaching universal coverage.”
To that end, he advised keeping other key ACA provisions, including no-cost preventive care, the elimination of annual and lifetime coverage caps, and the end of pre-existing condition exclusions.
“If we want to fix how care is delivered, so that we’re providing value, then we must ensure that Americans can afford and access quality care at every point in their lives,” he said. “If we lose even some of the coverage gains made under the ACA, or leave people in limbo, people will lose access to regular care and we will drive up long-term costs.”
He also called for more improvements in the health IT space, including a demand for affordable systems and technologies that can exchange data and support quality health care.
“MACRA is an opportunity to move the focus away from paperwork and reporting and toward paying for what works,” Mr. Slavitt said. “For a variety of reasons, EHRs became an industry before they became a useful tool. The technology community must be held accountable ... to make room for new innovators and to give clinicians more freedom and more flexibility to focus on their patients, to practice medicine, and deliver better care.”
President-elect Trump has designated Seema Verma, a health care consultant who helped design the Indiana’s ACA Medicaid expansion, to be the next CMS administrator.
WASHINGTON – Acting Administrator Andy Slavitt has some advice for his successors at the CMS: Keep the Center for Medicare & Medicaid Innovation, even if you trash the Affordable Care Act.
The innovation center is vital to the success of the Quality Payment Program, the value-based payment framework set up by the Medicare Access and CHIP Reauthorization Act (MACRA), Mr. Slavitt said Dec. 1 at the National MACRA MIPS/APM Summit.
“MACRA can’t work as well without a CMS Innovation Center that can move quickly to develop and expand new approaches to paying for care,” Mr. Slavitt said. “With changes to the Innovation Center, the advanced alternative payment approaches could slow significantly. We will have a much narrower path with fewer specialty options and approaches, which take in patient and physician feedback. Medicare and commercial payers would then fall further out of alignment, and more importantly, less patients would have access to innovative care methods.”
Mr. Slavitt offered a few other recommendations to the next regime. First, he called on the Trump administration to ensure that the 20 million people who have obtained health care coverage under the ACA do not lose it as a key to continued delivery system reform.
“Build from a foundation of progress, do not head backwards,” Mr. Slavitt advised. “There can be no delivery system reform without building on the foundation of reaching universal coverage.”
To that end, he advised keeping other key ACA provisions, including no-cost preventive care, the elimination of annual and lifetime coverage caps, and the end of pre-existing condition exclusions.
“If we want to fix how care is delivered, so that we’re providing value, then we must ensure that Americans can afford and access quality care at every point in their lives,” he said. “If we lose even some of the coverage gains made under the ACA, or leave people in limbo, people will lose access to regular care and we will drive up long-term costs.”
He also called for more improvements in the health IT space, including a demand for affordable systems and technologies that can exchange data and support quality health care.
“MACRA is an opportunity to move the focus away from paperwork and reporting and toward paying for what works,” Mr. Slavitt said. “For a variety of reasons, EHRs became an industry before they became a useful tool. The technology community must be held accountable ... to make room for new innovators and to give clinicians more freedom and more flexibility to focus on their patients, to practice medicine, and deliver better care.”
President-elect Trump has designated Seema Verma, a health care consultant who helped design the Indiana’s ACA Medicaid expansion, to be the next CMS administrator.
WASHINGTON – Acting Administrator Andy Slavitt has some advice for his successors at the CMS: Keep the Center for Medicare & Medicaid Innovation, even if you trash the Affordable Care Act.
The innovation center is vital to the success of the Quality Payment Program, the value-based payment framework set up by the Medicare Access and CHIP Reauthorization Act (MACRA), Mr. Slavitt said Dec. 1 at the National MACRA MIPS/APM Summit.
“MACRA can’t work as well without a CMS Innovation Center that can move quickly to develop and expand new approaches to paying for care,” Mr. Slavitt said. “With changes to the Innovation Center, the advanced alternative payment approaches could slow significantly. We will have a much narrower path with fewer specialty options and approaches, which take in patient and physician feedback. Medicare and commercial payers would then fall further out of alignment, and more importantly, less patients would have access to innovative care methods.”
Mr. Slavitt offered a few other recommendations to the next regime. First, he called on the Trump administration to ensure that the 20 million people who have obtained health care coverage under the ACA do not lose it as a key to continued delivery system reform.
“Build from a foundation of progress, do not head backwards,” Mr. Slavitt advised. “There can be no delivery system reform without building on the foundation of reaching universal coverage.”
To that end, he advised keeping other key ACA provisions, including no-cost preventive care, the elimination of annual and lifetime coverage caps, and the end of pre-existing condition exclusions.
“If we want to fix how care is delivered, so that we’re providing value, then we must ensure that Americans can afford and access quality care at every point in their lives,” he said. “If we lose even some of the coverage gains made under the ACA, or leave people in limbo, people will lose access to regular care and we will drive up long-term costs.”
He also called for more improvements in the health IT space, including a demand for affordable systems and technologies that can exchange data and support quality health care.
“MACRA is an opportunity to move the focus away from paperwork and reporting and toward paying for what works,” Mr. Slavitt said. “For a variety of reasons, EHRs became an industry before they became a useful tool. The technology community must be held accountable ... to make room for new innovators and to give clinicians more freedom and more flexibility to focus on their patients, to practice medicine, and deliver better care.”
President-elect Trump has designated Seema Verma, a health care consultant who helped design the Indiana’s ACA Medicaid expansion, to be the next CMS administrator.
AT THE NATIONAL MACRA MIPS/APM SUMMIT
Legislators commit to bipartisan support of Alzheimer’s funding
WASHINGTON – The nation’s political sea-change won’t wash Alzheimer’s disease research funding offtrack, two legislators vowed at a Washington briefing.
Rather than descend into partisan budget-bickering under the new administration, lawmakers should reach across the aisle and pass funding bills to vigorously propel the nation toward its goal of having an effective disease-modifying Alzheimer’s disease therapy by 2025.
“If we make this a high-enough priority, we can meet that goal,” Rep. Paul Tonko (D-NY) said at a briefing sponsored by The Hill newspaper, with support by Eli Lilly. “We should prioritize the work we need to do to achieve it in both the House and Senate, and move forward both aggressively and progressively.”
“I am a strong proponent of investing in research. We simply must continue to put research dollars into Alzheimer’s. If we don’t invest, try to find a cure or treatment, Alzheimer’s will become the single largest driver of health care costs on both a federal and state level. We have to recognize this: We could, potentially, not even be able to provide care for all patients we will have, unless we find a treatment or a cure.”
After a decade of struggle, federal dollars for Alzheimer’s research have begun to creep up. Last year, Congress passed a historic $350 million increase in Alzheimer’s research funding at the National Institutes of Health, raising the total spending to $991 million for fiscal year 2016. Then, in June, the Senate Appropriations Committee approved a landmark $400 million funding increase. In July, the House Appropriations Committee approved its own $350 million bump.
In August, the NIH recommended a $414 million increase for fiscal year 2018. If both the fiscal year 2017 increase and fiscal year 2018 request are ultimately passed, funding levels would be very close to the $2 billion/year federal commitment that researchers and Alzheimer’s policy mavens say is necessary to achieve the 2025 goal, set forth in the National Plan to Address Alzheimer’s Disease.
“There’s no such thing as too much research funding,” Sen. Tillis said during the briefing. But, he added, that federal generosity must be wisely husbanded.
“There must also be a sense of discipline along with the funding. Money like that should be targeted in terms of which diseases we go after – they should be areas that have the broadest impact. In a world of scarce resources, we can’t afford to simply throw money around.”
Alzheimer’s research is a perfect example of this careful resource management, he said. The NIH has prepared its second “bypass budget,” a funding proposal that passes the normal legislative channels and goes directly to the President.
Based on the consensus of scientists involved in search for a disease-modifying therapy, this budget proposal estimates the additional money needed to meet the 2025 goal, above the NIH’s baseline Alzheimer’s funding.
Only two other areas of medicine have such a budget: cancer and HIV-AIDS, said Robert J. Egge, chief public policy officer of the Alzheimer’s Association.
“This budget goes right from the scientists to Congress with no filter by the Office of Management and Budget, and lands directly on the President’s desk,” he said in an interview. “It tells legislators what scientists need in order to accomplish that 2025 goal.”
The $2 billion/year figure, Mr. Egge noted, is a ground-floor suggestion. “That’s what we need right now to stay on track. We think the best way to figure out where we need to be in the long-term is to let the scientists at NIH tell us what they need, and we need Congress and the Administration to follow this year to year.”
Like Sen. Tillis and Rep. Tonko, Mr. Egge was upbeat in anticipating steady funding progress.
“The champions of Alzheimer’s funding who have made such a difference for us are all back and in their same positions,” with unstinting commitment to the cause.
Those legislators include:
• Rep. Tom Cole (R-OK), chairman of the House Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, which funds the NIH. In 2015, Rep. Cole shepherded through a $300 million appropriation for Alzheimer’s research, and the pending $350 million appropriation for fiscal year 2017.
• Sen. Roy Blunt (R-MO), who, as chairman of the Senate Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, secured June’s $400 million and a $350 million bump in 2015.
• Ranking member Sen. Patty Murray (D-WA), who cosponsored the Alzheimer’s Breakthrough Act of 2009, and who worked with Sen. Blunt to secure the recent Alzheimer’s funding increases.
Mr. Egge is not overly troubled about the antiscience rhetoric bandied about by some potential members of President-elect Trump’s administration. He predicted those rumblings will settle down and not negatively affect Alzheimer’s funding.
“I think all the talk of not believing in the science of biomedical research was premature,” he said. “I think it’s completely appropriate to say ‘We are going to watch closely and form opinions,’ but I have never been overly alarmed by this. As an Alzheimer’s advocate for strong science, I am waiting to see what the next steps are. President-elect Trump has said on the campaign trail that Alzheimer’s would be a top priority. Our champions from both parties are fully committed to this fight and recognize that science is the fundamental path to do so. I share this optimism. It’s not complacent optimism – it’s vigilant optimism that we will continue with the momentum we need to finally, squarely and effectively, address this disease.”
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The nation’s political sea-change won’t wash Alzheimer’s disease research funding offtrack, two legislators vowed at a Washington briefing.
Rather than descend into partisan budget-bickering under the new administration, lawmakers should reach across the aisle and pass funding bills to vigorously propel the nation toward its goal of having an effective disease-modifying Alzheimer’s disease therapy by 2025.
“If we make this a high-enough priority, we can meet that goal,” Rep. Paul Tonko (D-NY) said at a briefing sponsored by The Hill newspaper, with support by Eli Lilly. “We should prioritize the work we need to do to achieve it in both the House and Senate, and move forward both aggressively and progressively.”
“I am a strong proponent of investing in research. We simply must continue to put research dollars into Alzheimer’s. If we don’t invest, try to find a cure or treatment, Alzheimer’s will become the single largest driver of health care costs on both a federal and state level. We have to recognize this: We could, potentially, not even be able to provide care for all patients we will have, unless we find a treatment or a cure.”
After a decade of struggle, federal dollars for Alzheimer’s research have begun to creep up. Last year, Congress passed a historic $350 million increase in Alzheimer’s research funding at the National Institutes of Health, raising the total spending to $991 million for fiscal year 2016. Then, in June, the Senate Appropriations Committee approved a landmark $400 million funding increase. In July, the House Appropriations Committee approved its own $350 million bump.
In August, the NIH recommended a $414 million increase for fiscal year 2018. If both the fiscal year 2017 increase and fiscal year 2018 request are ultimately passed, funding levels would be very close to the $2 billion/year federal commitment that researchers and Alzheimer’s policy mavens say is necessary to achieve the 2025 goal, set forth in the National Plan to Address Alzheimer’s Disease.
“There’s no such thing as too much research funding,” Sen. Tillis said during the briefing. But, he added, that federal generosity must be wisely husbanded.
“There must also be a sense of discipline along with the funding. Money like that should be targeted in terms of which diseases we go after – they should be areas that have the broadest impact. In a world of scarce resources, we can’t afford to simply throw money around.”
Alzheimer’s research is a perfect example of this careful resource management, he said. The NIH has prepared its second “bypass budget,” a funding proposal that passes the normal legislative channels and goes directly to the President.
Based on the consensus of scientists involved in search for a disease-modifying therapy, this budget proposal estimates the additional money needed to meet the 2025 goal, above the NIH’s baseline Alzheimer’s funding.
Only two other areas of medicine have such a budget: cancer and HIV-AIDS, said Robert J. Egge, chief public policy officer of the Alzheimer’s Association.
“This budget goes right from the scientists to Congress with no filter by the Office of Management and Budget, and lands directly on the President’s desk,” he said in an interview. “It tells legislators what scientists need in order to accomplish that 2025 goal.”
The $2 billion/year figure, Mr. Egge noted, is a ground-floor suggestion. “That’s what we need right now to stay on track. We think the best way to figure out where we need to be in the long-term is to let the scientists at NIH tell us what they need, and we need Congress and the Administration to follow this year to year.”
Like Sen. Tillis and Rep. Tonko, Mr. Egge was upbeat in anticipating steady funding progress.
“The champions of Alzheimer’s funding who have made such a difference for us are all back and in their same positions,” with unstinting commitment to the cause.
Those legislators include:
• Rep. Tom Cole (R-OK), chairman of the House Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, which funds the NIH. In 2015, Rep. Cole shepherded through a $300 million appropriation for Alzheimer’s research, and the pending $350 million appropriation for fiscal year 2017.
• Sen. Roy Blunt (R-MO), who, as chairman of the Senate Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, secured June’s $400 million and a $350 million bump in 2015.
• Ranking member Sen. Patty Murray (D-WA), who cosponsored the Alzheimer’s Breakthrough Act of 2009, and who worked with Sen. Blunt to secure the recent Alzheimer’s funding increases.
Mr. Egge is not overly troubled about the antiscience rhetoric bandied about by some potential members of President-elect Trump’s administration. He predicted those rumblings will settle down and not negatively affect Alzheimer’s funding.
“I think all the talk of not believing in the science of biomedical research was premature,” he said. “I think it’s completely appropriate to say ‘We are going to watch closely and form opinions,’ but I have never been overly alarmed by this. As an Alzheimer’s advocate for strong science, I am waiting to see what the next steps are. President-elect Trump has said on the campaign trail that Alzheimer’s would be a top priority. Our champions from both parties are fully committed to this fight and recognize that science is the fundamental path to do so. I share this optimism. It’s not complacent optimism – it’s vigilant optimism that we will continue with the momentum we need to finally, squarely and effectively, address this disease.”
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The nation’s political sea-change won’t wash Alzheimer’s disease research funding offtrack, two legislators vowed at a Washington briefing.
Rather than descend into partisan budget-bickering under the new administration, lawmakers should reach across the aisle and pass funding bills to vigorously propel the nation toward its goal of having an effective disease-modifying Alzheimer’s disease therapy by 2025.
“If we make this a high-enough priority, we can meet that goal,” Rep. Paul Tonko (D-NY) said at a briefing sponsored by The Hill newspaper, with support by Eli Lilly. “We should prioritize the work we need to do to achieve it in both the House and Senate, and move forward both aggressively and progressively.”
“I am a strong proponent of investing in research. We simply must continue to put research dollars into Alzheimer’s. If we don’t invest, try to find a cure or treatment, Alzheimer’s will become the single largest driver of health care costs on both a federal and state level. We have to recognize this: We could, potentially, not even be able to provide care for all patients we will have, unless we find a treatment or a cure.”
After a decade of struggle, federal dollars for Alzheimer’s research have begun to creep up. Last year, Congress passed a historic $350 million increase in Alzheimer’s research funding at the National Institutes of Health, raising the total spending to $991 million for fiscal year 2016. Then, in June, the Senate Appropriations Committee approved a landmark $400 million funding increase. In July, the House Appropriations Committee approved its own $350 million bump.
In August, the NIH recommended a $414 million increase for fiscal year 2018. If both the fiscal year 2017 increase and fiscal year 2018 request are ultimately passed, funding levels would be very close to the $2 billion/year federal commitment that researchers and Alzheimer’s policy mavens say is necessary to achieve the 2025 goal, set forth in the National Plan to Address Alzheimer’s Disease.
“There’s no such thing as too much research funding,” Sen. Tillis said during the briefing. But, he added, that federal generosity must be wisely husbanded.
“There must also be a sense of discipline along with the funding. Money like that should be targeted in terms of which diseases we go after – they should be areas that have the broadest impact. In a world of scarce resources, we can’t afford to simply throw money around.”
Alzheimer’s research is a perfect example of this careful resource management, he said. The NIH has prepared its second “bypass budget,” a funding proposal that passes the normal legislative channels and goes directly to the President.
Based on the consensus of scientists involved in search for a disease-modifying therapy, this budget proposal estimates the additional money needed to meet the 2025 goal, above the NIH’s baseline Alzheimer’s funding.
Only two other areas of medicine have such a budget: cancer and HIV-AIDS, said Robert J. Egge, chief public policy officer of the Alzheimer’s Association.
“This budget goes right from the scientists to Congress with no filter by the Office of Management and Budget, and lands directly on the President’s desk,” he said in an interview. “It tells legislators what scientists need in order to accomplish that 2025 goal.”
The $2 billion/year figure, Mr. Egge noted, is a ground-floor suggestion. “That’s what we need right now to stay on track. We think the best way to figure out where we need to be in the long-term is to let the scientists at NIH tell us what they need, and we need Congress and the Administration to follow this year to year.”
Like Sen. Tillis and Rep. Tonko, Mr. Egge was upbeat in anticipating steady funding progress.
“The champions of Alzheimer’s funding who have made such a difference for us are all back and in their same positions,” with unstinting commitment to the cause.
Those legislators include:
• Rep. Tom Cole (R-OK), chairman of the House Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, which funds the NIH. In 2015, Rep. Cole shepherded through a $300 million appropriation for Alzheimer’s research, and the pending $350 million appropriation for fiscal year 2017.
• Sen. Roy Blunt (R-MO), who, as chairman of the Senate Appropriations Committee’s Subcommittee on Labor, Health and Human Services, Education and Related Agencies, secured June’s $400 million and a $350 million bump in 2015.
• Ranking member Sen. Patty Murray (D-WA), who cosponsored the Alzheimer’s Breakthrough Act of 2009, and who worked with Sen. Blunt to secure the recent Alzheimer’s funding increases.
Mr. Egge is not overly troubled about the antiscience rhetoric bandied about by some potential members of President-elect Trump’s administration. He predicted those rumblings will settle down and not negatively affect Alzheimer’s funding.
“I think all the talk of not believing in the science of biomedical research was premature,” he said. “I think it’s completely appropriate to say ‘We are going to watch closely and form opinions,’ but I have never been overly alarmed by this. As an Alzheimer’s advocate for strong science, I am waiting to see what the next steps are. President-elect Trump has said on the campaign trail that Alzheimer’s would be a top priority. Our champions from both parties are fully committed to this fight and recognize that science is the fundamental path to do so. I share this optimism. It’s not complacent optimism – it’s vigilant optimism that we will continue with the momentum we need to finally, squarely and effectively, address this disease.”
msullivan@frontlinemedcom.com
On Twitter @alz_gal
GOLD: Base COPD treatment on symptoms, exacerbation risk
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
As health care moves toward individualized care plans for patients, the updated GOLD recommendations enhance the possibility of personalized COPD treatment. This means more symptom-focused treatment for patients and, as Dr. Criner points out, more freedom for providers to manage patients based on what seems best.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has uncoupled spirometry results from the ABCD treatment algorithm; this move marks the organization’s first announcing of major COPD guidance since 2011.
Spirometry now stands apart from GOLD’s ABCD symptom/exacerbation risk score with its own grade, with possibilities ranging from 1 to 4. A forced expiratory volume in 1 second (FEV1) of 80% or more of the predicted value rates a 1; the score degrades to 4 with an FEV1 below 30%.
“In previous GOLD documents, recommendations for management of COPD were based solely on spirometric category. However, there is considerable evidence that the level of FEV1 is a poor descriptor of disease status, and, for this reason, the management of stable COPD based on … disease impact (determined mainly by symptom burden and activity limitation) and future risk of disease progression (especially of exacerbations) is recommended. ... ABCD groups are now proposed to be derived exclusively from patient symptoms and their history of exacerbations,” GOLD said.
The clear focus on symptoms and exacerbations is “the major accomplishment” of the new report, which has been downloaded more than 45,000 times since it’s release, a testament to GOLD’s importance to clinicians trying to help COPD patients.
“We are trying to do a better job of personalizing treatment,” said GOLD board member Gerard Criner, MD, chair and professor of thoracic medicine and surgery at Temple University in Philadelphia.
The change “allows you to plan treatment based on symptoms [even] if you don’t have immediate access to spirometry, and then refine treatment once you have spirometry results. It also allows you to escalate and deescalate treatment because you are not boxed into a letter grade group” forced by spirometry. “You can also take a better look at pharmacologic versus nonpharmacologic therapy” when deciding what to do, he said.
In short, “we think it gives more freedom” to manage patients based on what seems best, Dr. Criner said.
GOLD included an example of how the new assessment can help. “Consider two patients,” it said, both with an FEV1 less than 30% and a COPD Assessment Test result of 18, but one with no exacerbations in the past year and the other with three. Both would have scored a GOLD D in the old system, and been treated similarly.
“However, with the new proposed scheme, the subject with three exacerbations ... would be labeled GOLD [spirometry] grade 4, group D,” and their treatment would focus on exacerbations. The no-exacerbation patient would be classified as GOLD grade 4, group B. Treatment would focus on symptoms. Drugs are still an option, but also lung volume reduction and lung transplant, GOLD said. Spirometry, in other words, is less important than how the patient is doing.
The group incorporated “every major study up to the first week of November” in the new report, Dr. Criner said, so there’s more to consider.
For instance, it’s clear now that patients benefit from home oxygen if they are severely hypoxemic while sitting on the couch watching TV, but not if they desaturate only when they get up and walk around, or come into the clinic to exercise. “We did not” know that in 2011, he said.
GOLD also recommended pulmonary rehabilitation and palliative care when indicated, as well as ongoing evaluation to make sure patients are able to use their inhalers, a major problem in COPD.
GOLD said that group A patients - those with few symptoms and low exacerbation risk - should be offered a bronchodilator. Initial therapy for group B - more symptoms, but low exacerbation risk - and group C - higher exacerbation risk but fewer symptoms - “should consist of a single long-acting bronchodilator. There is no evidence to recommend one class of long-acting bronchodilator over another.”
For group D - highly symptomatic with frequent exacerbations - “we recommend starting therapy with a [long-acting beta-2 agonist]/[long-acting antimuscarinic antagonist] combination,” the group said.
There was no industry involvement in GOLD’s report, but numerous authors and board members had pharmaceutical company ties, and GOLD’s treatment advice relies on drug company studies. Dr. Criner reported personal payments from Holaria, and research funding and other nonpersonal payments from AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Johnson and Johnson, and others.
New guidelines provide standardized hypoglycemia values for clinical evaluation
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
, joining with the European Association for the Study of Diabetes to specify that a level of less than 3 mmol/L (54 mg/dL) should be considered “clinically important hypoglycemia.”
“A single glucose level should be agreed to that has serious clinical and health-economic consequences,” the ADA and EASD stated in the new guidelines. “This would enable the diabetes and regulatory communities to compare the effectiveness of interventions in reducing hypoglycemia, be they pharmacological, technological, or educational. It would also permit the use of meta-analysis as a statistical tool to increase power when comparing interventions.”
An international, multidisciplinary group – the International Hypoglycemia Study Group – was formed to create distinct definitions of the various levels of severity that hypoglycemia can have. The new guidelines contain three levels, which should be used by clinicians to determine what amounts of blood glucose are significant enough to be clinically reported.
“Currently, there is no uniform agreement to what constitutes reportable hypoglycemia in clinical trials,” she said. “In some studies, it is defined as a blood glucose level of less than 70 mg/dL, whereas in others it is defined as a blood glucose level less than 54 mg/dL.”
The guidelines define first-level hypoglycemia as any glucose level of 3.9 mmol/L (70 mg/dL) or less. This is not considered low enough to be reported on a consistent basis in clinical studies; however, that determination must ultimately be made by the investigators, as the parameters for what is significant often vary from study to study.
The second level is the 3 mmol/L (54 mg/dL), which now is deemed to be a clinically significant level of hypoglycemia. Because it is “sufficiently low to indicate serious, clinically important hypoglycemia,” it should be reported as part of any clinical studies. Finally, the third level, less than 2.8 mmol/L (50 mg/dL), indicates severe hypoglycemia and is classified as any individual with “severe cognitive impairment requiring external assistance for recovery,” according to the guidelines (Diabetes Care. 2016 Dec 1. doi: 10.2337/dc16-2215).
With a new standard of hypoglycemic values that are deemed clinically significant, the ADA and EASD hope that comparing different insulins, medications, technologies, and educational interventions will now become easier and more standardized, leading to better care worldwide.
Although there is general agreement as to where severe hypoglycemia really begins, the newly defined glucose levels are “a step in the right direction,” according to Dr. Rodbard.
The International Hypoglycaemia Study Group developed these guidelines through a grant from Novo Nordisk, awarded to the Six Degrees Academy of Toronto. Dr. Heller has received advisory or consultation fees from Lilly, Novo Nordisk, Takeda, Merck, Sharp & Dohme, and Becton Dickinson; has served as a speaker for AstraZeneca, Lilly, Novo Nordisk, Boehringer Ingelheim, and Takeda; and has received research support from Medtronic U.K. Dr. Rodbard did not report any financial disclosures.
New PAD guidelines expected to boost awareness and treatment
NEW ORLEANS – The latest revision of U.S. guidelines for diagnosing and managing lower-extremity peripheral artery disease is seen by several experts as primarily a renewed call to action by American physicians to more diligently identify at-risk people in their practices, diagnose the disease with ankle-brachial index measurement, and appropriately treat patients who have the disease.
The new lower-extremity peripheral artery disease (PAD) guidelines “give us a platform to get something done,” said Heather L. Gornik, MD, vice chair of the guidelines panel and medical director of the noninvasive vascular lab at the Cleveland Clinic. Improved PAD identification and care “starts with the fundamentals of recognizing who is at risk for PAD and then doing some clinical investigation to find it, because it’s there. You just need to ask patients if they have leg symptoms, have them take off their socks and examine their feet,” Dr. Gornik said in an interview following a program devoted to the revised guidelines at the American Heart Association scientific sessions.
The new guidelines are “a clarion call to action,” commented Alan T. Hirsch, MD, professor of medicine, epidemiology, and community health and director of the vascular medicine program at the University of Minnesota in Minneapolis. PAD “is the single most morbid and fatal of all cardiovascular diseases, so why in 2016 are we challenged to have every cardiologist trained in basic PAD competency?” asked Dr. Hirsch, who chaired the 2005 guidelines panel.
The AHA wants to “elevate awareness of PAD among the public and health professionals, and we want to better understand how we can be a catalyst for change,” said Terri Wiggins, the organization’s vice president for vascular health programs. AHA publications now stress that clinicians need to have patients “take off their socks and look at the patient’s feet,” she said.
The problem with the way many U.S. physicians handle PAD goes beyond a failure to properly screen and diagnose the disease. Analysis of data collected by the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey during 2006-2013 showed that, among an average of 3.9 million patients seen each year with PAD, just 38% received treatment with an antiplatelet drug, 35% received a statin, and among those patients who smoked, 36% received counseling for smoking cessation, Jeffrey S. Berger, MD, reported in a separate talk at the meeting. These rates were roughly constant throughout the 8-year period he examined.
“These data are eye-opening. They highlight a clear opportunity to improve the care of patients with PAD with guideline-directed therapy. PAD is problematic because only 10%-15% of patients have typical symptoms, so many physicians have a false perception that these patients are not at high risk,” Dr. Berger said in an interview. “What the AHA is doing is great” for raising awareness, he added.
The revised 2016 PAD guidelines made several changes, compared with what had been on the books from the 2005 guidelines and 2011 update, including classifying vorapaxar (Zontivity) treatment a class IIb recommendation with “uncertain” incremental benefit when used as an add-on agent on top of standard antiplatelet therapy, endorsement of annual influenza vaccination as something every PAD patient should receive and a class I recommendation, and acknowledgment that selected patients with critical limb ischemia are candidates for an “endovascular first” approach to revascularization,
Perhaps just as important was inclusion for the first time in the new guidelines of three advocacy priorities for initiatives by various professional societies with an interest in PAD: easy availability of ankle-brachial index measurement as the initial test to establish a diagnosis of PAD in patients with physical examination findings suggestive of the disease; access to supervised exercise programs for patients diagnosed with PAD; and incorporation of patient-centered outcomes into the regulatory approval process of new medical therapies and revascularization technologies for treating PAD.
The new guidelines “ form the basis for the AHA establishing a Get With The Guidelines program for PAD so that clinicians can be held accountable for delivering these treatments,” said Naomi M. Hamburg, MD, chief of vascular biology at Boston University and a member of the guidelines panel.
Dr. Gornik has an ownership interest in Summit Doppler Systems and Zin Medical and has received research support from AstraZeneca and Theravasc. Dr. Hamburg has been a consultant to Acceleron and has received research support from Everest Genomics, Hershey’s, Unex, and Welch’s. Dr. Hirsch, Ms. Wiggins, and Dr. Berger had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW ORLEANS – The latest revision of U.S. guidelines for diagnosing and managing lower-extremity peripheral artery disease is seen by several experts as primarily a renewed call to action by American physicians to more diligently identify at-risk people in their practices, diagnose the disease with ankle-brachial index measurement, and appropriately treat patients who have the disease.
The new lower-extremity peripheral artery disease (PAD) guidelines “give us a platform to get something done,” said Heather L. Gornik, MD, vice chair of the guidelines panel and medical director of the noninvasive vascular lab at the Cleveland Clinic. Improved PAD identification and care “starts with the fundamentals of recognizing who is at risk for PAD and then doing some clinical investigation to find it, because it’s there. You just need to ask patients if they have leg symptoms, have them take off their socks and examine their feet,” Dr. Gornik said in an interview following a program devoted to the revised guidelines at the American Heart Association scientific sessions.
The new guidelines are “a clarion call to action,” commented Alan T. Hirsch, MD, professor of medicine, epidemiology, and community health and director of the vascular medicine program at the University of Minnesota in Minneapolis. PAD “is the single most morbid and fatal of all cardiovascular diseases, so why in 2016 are we challenged to have every cardiologist trained in basic PAD competency?” asked Dr. Hirsch, who chaired the 2005 guidelines panel.
The AHA wants to “elevate awareness of PAD among the public and health professionals, and we want to better understand how we can be a catalyst for change,” said Terri Wiggins, the organization’s vice president for vascular health programs. AHA publications now stress that clinicians need to have patients “take off their socks and look at the patient’s feet,” she said.
The problem with the way many U.S. physicians handle PAD goes beyond a failure to properly screen and diagnose the disease. Analysis of data collected by the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey during 2006-2013 showed that, among an average of 3.9 million patients seen each year with PAD, just 38% received treatment with an antiplatelet drug, 35% received a statin, and among those patients who smoked, 36% received counseling for smoking cessation, Jeffrey S. Berger, MD, reported in a separate talk at the meeting. These rates were roughly constant throughout the 8-year period he examined.
“These data are eye-opening. They highlight a clear opportunity to improve the care of patients with PAD with guideline-directed therapy. PAD is problematic because only 10%-15% of patients have typical symptoms, so many physicians have a false perception that these patients are not at high risk,” Dr. Berger said in an interview. “What the AHA is doing is great” for raising awareness, he added.
The revised 2016 PAD guidelines made several changes, compared with what had been on the books from the 2005 guidelines and 2011 update, including classifying vorapaxar (Zontivity) treatment a class IIb recommendation with “uncertain” incremental benefit when used as an add-on agent on top of standard antiplatelet therapy, endorsement of annual influenza vaccination as something every PAD patient should receive and a class I recommendation, and acknowledgment that selected patients with critical limb ischemia are candidates for an “endovascular first” approach to revascularization,
Perhaps just as important was inclusion for the first time in the new guidelines of three advocacy priorities for initiatives by various professional societies with an interest in PAD: easy availability of ankle-brachial index measurement as the initial test to establish a diagnosis of PAD in patients with physical examination findings suggestive of the disease; access to supervised exercise programs for patients diagnosed with PAD; and incorporation of patient-centered outcomes into the regulatory approval process of new medical therapies and revascularization technologies for treating PAD.
The new guidelines “ form the basis for the AHA establishing a Get With The Guidelines program for PAD so that clinicians can be held accountable for delivering these treatments,” said Naomi M. Hamburg, MD, chief of vascular biology at Boston University and a member of the guidelines panel.
Dr. Gornik has an ownership interest in Summit Doppler Systems and Zin Medical and has received research support from AstraZeneca and Theravasc. Dr. Hamburg has been a consultant to Acceleron and has received research support from Everest Genomics, Hershey’s, Unex, and Welch’s. Dr. Hirsch, Ms. Wiggins, and Dr. Berger had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW ORLEANS – The latest revision of U.S. guidelines for diagnosing and managing lower-extremity peripheral artery disease is seen by several experts as primarily a renewed call to action by American physicians to more diligently identify at-risk people in their practices, diagnose the disease with ankle-brachial index measurement, and appropriately treat patients who have the disease.
The new lower-extremity peripheral artery disease (PAD) guidelines “give us a platform to get something done,” said Heather L. Gornik, MD, vice chair of the guidelines panel and medical director of the noninvasive vascular lab at the Cleveland Clinic. Improved PAD identification and care “starts with the fundamentals of recognizing who is at risk for PAD and then doing some clinical investigation to find it, because it’s there. You just need to ask patients if they have leg symptoms, have them take off their socks and examine their feet,” Dr. Gornik said in an interview following a program devoted to the revised guidelines at the American Heart Association scientific sessions.
The new guidelines are “a clarion call to action,” commented Alan T. Hirsch, MD, professor of medicine, epidemiology, and community health and director of the vascular medicine program at the University of Minnesota in Minneapolis. PAD “is the single most morbid and fatal of all cardiovascular diseases, so why in 2016 are we challenged to have every cardiologist trained in basic PAD competency?” asked Dr. Hirsch, who chaired the 2005 guidelines panel.
The AHA wants to “elevate awareness of PAD among the public and health professionals, and we want to better understand how we can be a catalyst for change,” said Terri Wiggins, the organization’s vice president for vascular health programs. AHA publications now stress that clinicians need to have patients “take off their socks and look at the patient’s feet,” she said.
The problem with the way many U.S. physicians handle PAD goes beyond a failure to properly screen and diagnose the disease. Analysis of data collected by the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey during 2006-2013 showed that, among an average of 3.9 million patients seen each year with PAD, just 38% received treatment with an antiplatelet drug, 35% received a statin, and among those patients who smoked, 36% received counseling for smoking cessation, Jeffrey S. Berger, MD, reported in a separate talk at the meeting. These rates were roughly constant throughout the 8-year period he examined.
“These data are eye-opening. They highlight a clear opportunity to improve the care of patients with PAD with guideline-directed therapy. PAD is problematic because only 10%-15% of patients have typical symptoms, so many physicians have a false perception that these patients are not at high risk,” Dr. Berger said in an interview. “What the AHA is doing is great” for raising awareness, he added.
The revised 2016 PAD guidelines made several changes, compared with what had been on the books from the 2005 guidelines and 2011 update, including classifying vorapaxar (Zontivity) treatment a class IIb recommendation with “uncertain” incremental benefit when used as an add-on agent on top of standard antiplatelet therapy, endorsement of annual influenza vaccination as something every PAD patient should receive and a class I recommendation, and acknowledgment that selected patients with critical limb ischemia are candidates for an “endovascular first” approach to revascularization,
Perhaps just as important was inclusion for the first time in the new guidelines of three advocacy priorities for initiatives by various professional societies with an interest in PAD: easy availability of ankle-brachial index measurement as the initial test to establish a diagnosis of PAD in patients with physical examination findings suggestive of the disease; access to supervised exercise programs for patients diagnosed with PAD; and incorporation of patient-centered outcomes into the regulatory approval process of new medical therapies and revascularization technologies for treating PAD.
The new guidelines “ form the basis for the AHA establishing a Get With The Guidelines program for PAD so that clinicians can be held accountable for delivering these treatments,” said Naomi M. Hamburg, MD, chief of vascular biology at Boston University and a member of the guidelines panel.
Dr. Gornik has an ownership interest in Summit Doppler Systems and Zin Medical and has received research support from AstraZeneca and Theravasc. Dr. Hamburg has been a consultant to Acceleron and has received research support from Everest Genomics, Hershey’s, Unex, and Welch’s. Dr. Hirsch, Ms. Wiggins, and Dr. Berger had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Age correlated with occult cancer in VTE patients
A newly devised risk score may help identify which patients with acute venous thromboembolism (VTE) are most likely to have occult cancer and where they are likely to have it, according to a report published online in CHEST.
Although VTE is known to occur before an occult cancer becomes symptomatic in a minority of patients, clinicians don’t agree on which patients with VTE should be screened for occult cancer or on how extensive that screening should be. Some favor a basic screening with only a clinical history, physical exam, simple lab tests, and a chest X-ray, while others advocate a more thorough work-up to improve the patient’s chance for a cure. “The potential benefits and harms of such screening are controversial, partly because there is little evidence” concerning which patients are at highest risk and which cancer types/sites should be assessed, said Luis Jara-Palomares, MD, PhD, of the medical surgical unit of respiratory diseases, Virgen del Rocio Hospital, Seville, Spain, and his associates.
Dr. Jara-Palomares and his associates devised a prognostic score by assigning points to the variables they found to be most significantly associated with occult cancer. For example, male sex was accorded 1 point, chronic lung disease or a high platelet count was accorded 1 point, age over 70 years or anemia was accorded 2 points, and postoperative or prior VTE was accorded negative 2 points. The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
The mean age of these study participants was 63 years, and approximately half were women. A total of 444 (7.6%) were diagnosed as having cancer at 1-24 months after presenting with VTE. Most of these cancers were discovered within 6 months of the VTE.
Patients who had occult cancer were significantly more likely than those who did not to be male and older than 70 years of age; to have chronic lung disease, an elevated platelet count, and/or anemia; and to have a history of recent surgery and/or prior VTE.
The percentage of VTE patients who had occult cancer increased with advancing age, from 2%-3% in the youngest age group (younger than 50 years) to 8%-12% in the oldest age group (older than 70 years). Among men with occult cancer, the most frequently affected sites were the lung (26%), prostate (17%), and colon/rectum (10%). Among women, the most frequent types of cancer were colorectal (19%), breast (12%), uterine (9.1%), hematologic (8.6%), pancreatic (7.6%), and stomach (6.6%).
Overall, more than half of the men who had occult cancer had cancer affecting the lung, prostate, or colon/rectum, while two-thirds of the women who had occult cancer had cancer affecting the colon/rectum, breast, or abdomen. “This is important because [cancer] screening is not necessary in all VTE patients, but any information suggesting [which] patients are at increased risk and [which] sites are more common may be of help to decide the most appropriate work-up for each patient,” the investigators noted.
To determine which patients are most likely to have occult cancer and which cancer types are most likely to develop, the investigators analyzed data from RIETE (the Computerized Registry of Patients With Venous Thromboembolism), an international registry of more than 50,000 consecutive patients with confirmed acute VTE. They focused on 5,863 patients who presented with pulmonary embolism (34%), deep vein thrombosis (48%), or both disorders (18%) and were followed for at least 2 years for the development of cancer.
The accuracy of this risk scoring system must be tested further in a large validation cohort before it can be widely adopted. If it proves to be accurate, then patients with low total risk scores could forgo the expense, discomfort, and psychological stress of extensive cancer screening, Dr. Jara-Palomares and his associates said.
Men whose total score is 3 points or more could benefit from a rectal exam and fecal occult blood test to rule out colorectal cancer, a rectal exam and prostate-specific antigen test to rule out prostate cancer, and a chest CT to rule out lung cancer. Women whose total score is 3 points or higher could benefit from a fecal occult blood test to rule out colorectal cancer, a mammogram to rule out breast cancer, and abdominopelvic CT to rule out uterine, pancreatic, and stomach cancer, they noted.
The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.
A newly devised risk score may help identify which patients with acute venous thromboembolism (VTE) are most likely to have occult cancer and where they are likely to have it, according to a report published online in CHEST.
Although VTE is known to occur before an occult cancer becomes symptomatic in a minority of patients, clinicians don’t agree on which patients with VTE should be screened for occult cancer or on how extensive that screening should be. Some favor a basic screening with only a clinical history, physical exam, simple lab tests, and a chest X-ray, while others advocate a more thorough work-up to improve the patient’s chance for a cure. “The potential benefits and harms of such screening are controversial, partly because there is little evidence” concerning which patients are at highest risk and which cancer types/sites should be assessed, said Luis Jara-Palomares, MD, PhD, of the medical surgical unit of respiratory diseases, Virgen del Rocio Hospital, Seville, Spain, and his associates.
Dr. Jara-Palomares and his associates devised a prognostic score by assigning points to the variables they found to be most significantly associated with occult cancer. For example, male sex was accorded 1 point, chronic lung disease or a high platelet count was accorded 1 point, age over 70 years or anemia was accorded 2 points, and postoperative or prior VTE was accorded negative 2 points. The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
The mean age of these study participants was 63 years, and approximately half were women. A total of 444 (7.6%) were diagnosed as having cancer at 1-24 months after presenting with VTE. Most of these cancers were discovered within 6 months of the VTE.
Patients who had occult cancer were significantly more likely than those who did not to be male and older than 70 years of age; to have chronic lung disease, an elevated platelet count, and/or anemia; and to have a history of recent surgery and/or prior VTE.
The percentage of VTE patients who had occult cancer increased with advancing age, from 2%-3% in the youngest age group (younger than 50 years) to 8%-12% in the oldest age group (older than 70 years). Among men with occult cancer, the most frequently affected sites were the lung (26%), prostate (17%), and colon/rectum (10%). Among women, the most frequent types of cancer were colorectal (19%), breast (12%), uterine (9.1%), hematologic (8.6%), pancreatic (7.6%), and stomach (6.6%).
Overall, more than half of the men who had occult cancer had cancer affecting the lung, prostate, or colon/rectum, while two-thirds of the women who had occult cancer had cancer affecting the colon/rectum, breast, or abdomen. “This is important because [cancer] screening is not necessary in all VTE patients, but any information suggesting [which] patients are at increased risk and [which] sites are more common may be of help to decide the most appropriate work-up for each patient,” the investigators noted.
To determine which patients are most likely to have occult cancer and which cancer types are most likely to develop, the investigators analyzed data from RIETE (the Computerized Registry of Patients With Venous Thromboembolism), an international registry of more than 50,000 consecutive patients with confirmed acute VTE. They focused on 5,863 patients who presented with pulmonary embolism (34%), deep vein thrombosis (48%), or both disorders (18%) and were followed for at least 2 years for the development of cancer.
The accuracy of this risk scoring system must be tested further in a large validation cohort before it can be widely adopted. If it proves to be accurate, then patients with low total risk scores could forgo the expense, discomfort, and psychological stress of extensive cancer screening, Dr. Jara-Palomares and his associates said.
Men whose total score is 3 points or more could benefit from a rectal exam and fecal occult blood test to rule out colorectal cancer, a rectal exam and prostate-specific antigen test to rule out prostate cancer, and a chest CT to rule out lung cancer. Women whose total score is 3 points or higher could benefit from a fecal occult blood test to rule out colorectal cancer, a mammogram to rule out breast cancer, and abdominopelvic CT to rule out uterine, pancreatic, and stomach cancer, they noted.
The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.
A newly devised risk score may help identify which patients with acute venous thromboembolism (VTE) are most likely to have occult cancer and where they are likely to have it, according to a report published online in CHEST.
Although VTE is known to occur before an occult cancer becomes symptomatic in a minority of patients, clinicians don’t agree on which patients with VTE should be screened for occult cancer or on how extensive that screening should be. Some favor a basic screening with only a clinical history, physical exam, simple lab tests, and a chest X-ray, while others advocate a more thorough work-up to improve the patient’s chance for a cure. “The potential benefits and harms of such screening are controversial, partly because there is little evidence” concerning which patients are at highest risk and which cancer types/sites should be assessed, said Luis Jara-Palomares, MD, PhD, of the medical surgical unit of respiratory diseases, Virgen del Rocio Hospital, Seville, Spain, and his associates.
Dr. Jara-Palomares and his associates devised a prognostic score by assigning points to the variables they found to be most significantly associated with occult cancer. For example, male sex was accorded 1 point, chronic lung disease or a high platelet count was accorded 1 point, age over 70 years or anemia was accorded 2 points, and postoperative or prior VTE was accorded negative 2 points. The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
The mean age of these study participants was 63 years, and approximately half were women. A total of 444 (7.6%) were diagnosed as having cancer at 1-24 months after presenting with VTE. Most of these cancers were discovered within 6 months of the VTE.
Patients who had occult cancer were significantly more likely than those who did not to be male and older than 70 years of age; to have chronic lung disease, an elevated platelet count, and/or anemia; and to have a history of recent surgery and/or prior VTE.
The percentage of VTE patients who had occult cancer increased with advancing age, from 2%-3% in the youngest age group (younger than 50 years) to 8%-12% in the oldest age group (older than 70 years). Among men with occult cancer, the most frequently affected sites were the lung (26%), prostate (17%), and colon/rectum (10%). Among women, the most frequent types of cancer were colorectal (19%), breast (12%), uterine (9.1%), hematologic (8.6%), pancreatic (7.6%), and stomach (6.6%).
Overall, more than half of the men who had occult cancer had cancer affecting the lung, prostate, or colon/rectum, while two-thirds of the women who had occult cancer had cancer affecting the colon/rectum, breast, or abdomen. “This is important because [cancer] screening is not necessary in all VTE patients, but any information suggesting [which] patients are at increased risk and [which] sites are more common may be of help to decide the most appropriate work-up for each patient,” the investigators noted.
To determine which patients are most likely to have occult cancer and which cancer types are most likely to develop, the investigators analyzed data from RIETE (the Computerized Registry of Patients With Venous Thromboembolism), an international registry of more than 50,000 consecutive patients with confirmed acute VTE. They focused on 5,863 patients who presented with pulmonary embolism (34%), deep vein thrombosis (48%), or both disorders (18%) and were followed for at least 2 years for the development of cancer.
The accuracy of this risk scoring system must be tested further in a large validation cohort before it can be widely adopted. If it proves to be accurate, then patients with low total risk scores could forgo the expense, discomfort, and psychological stress of extensive cancer screening, Dr. Jara-Palomares and his associates said.
Men whose total score is 3 points or more could benefit from a rectal exam and fecal occult blood test to rule out colorectal cancer, a rectal exam and prostate-specific antigen test to rule out prostate cancer, and a chest CT to rule out lung cancer. Women whose total score is 3 points or higher could benefit from a fecal occult blood test to rule out colorectal cancer, a mammogram to rule out breast cancer, and abdominopelvic CT to rule out uterine, pancreatic, and stomach cancer, they noted.
The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.
FROM CHEST
Key clinical point:
Major finding: The incidence of occult cancer was significantly lower among patients whose total score was 2 points or less (5.8%) than among those whose total score was 3 points or higher (12%).
Data source: A retrospective cohort study involving 5,863 consecutive VTE patients in an international observational registry.
Disclosures: The RIETE Registry is supported by an unrestricted educational grant from Sanofi Spain and by Bayer Pharma AG. Dr. Jara-Palomares reported having no relevant financial disclosures; his associates reported ties to Sanofi, Bayer, and LEO Pharma.
VIDEO: No difference between PPI and H2RA for low-dose aspirin gastroprotection
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Aspirin is widely used for primary and secondary prophylaxis of cardiovascular disease. Dr. Chan and colleagues present a randomized, controlled trial comparing rabeprazole 20 mg once a day to famotidine 40 mg once a day in preventing recurrent GI hemorrhage and endoscopic ulcers in low-dose (less than 325 mg) aspirin users. The authors conclude that no statistical difference was found between the two agents. The study contrasts with another study from Hong Kong, which found that proton pump inhibitors were more effective.
The authors are to be complimented on this important addition to the literature but the reader should not conclude that H2-receptor antagonists and proton pump inhibitors are equivalent in preventing recurrent bleeding from aspirin-induced ulcers.
Nimish Vakil, MD, AGAF, is clinical professor of medicine at the University of Wisconsin–Madison. He has consulted for Ironwood and AstraZeneca.
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
Among patients on low-dose aspirin at risk for recurrent GI bleeding, there were slightly fewer GI bleeds or ulcers when patients were on the proton pump inhibitor rabeprazole (Aciphex) instead of the histamine2-receptor antagonist famotidine (Pepcid), but the difference was not statistically significant according to a study reported in the January issue of Gastroenterology.
SOURCE: American Gastroenterological Association
In a 270-subject, double-blind, randomized trial in Hong Kong and Japan led by Francis Chan, MD, of the Chinese University of Hong Kong, investigators found, “Among high-risk aspirin users, the incidence of recurrent bleeding is comparable with either use of PPI [proton pump inhibitor] or H2RA [H2-receptor antagonist].” However, “since a small difference in efficacy cannot be excluded, PPI probably remains the preferred treatment for long-term protection against upper GI bleeding in high-risk aspirin users” (Gastroenterology. 2016 Sep 15. doi: 10.1053/j.gastro.2016.09.006).
Even so, “our findings suggest that famotidine may be a reasonable alternative option for aspirin users who disfavor long-term PPI therapy,” they said.
Because of concerns about the long-term safety of PPIs, including the association between PPIs and increased risk of serious cardiovascular events in patients on clopidogrel (Plavix), clinicians have been looking for alternatives. The findings reassure that H2RAs are a reasonable choice; many clinicians have already turned to them.
All 270 subjects had previously had endoscopically confirmed ulcer bleeding while on low-dose aspirin (325 mg or less per day). “Considering clinicians will be most concerned with the adequacy of gastroprotective treatment effect in aspirin users with the highest risk, we exclusively enrolled patients with endoscopy-proven upper GI bleeding,” Dr. Chan and his colleagues said.
After the ulcers healed, the subjects resumed aspirin (80 mg) daily and were randomized to either famotidine 40 mg once daily (n = 132) or rabeprazole 20 mg daily (n = 138) for up to 12 months. Helicobacter pylori was eradicated prior to randomization in patients who tested positive. Subjects were evaluated every 2 months, with repeat endoscopy for symptoms of upper GI bleeding or significant drops in hemoglobin, as well as at the end of the study.
During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
“Our findings indicate that both treatments are comparable in preventing recurrent upper GI bleeding in high-risk aspirin users, although a small difference in efficacy cannot be excluded,” the investigators said.
Over two-thirds of the subjects were men, and the mean age was 73 years. About a quarter in the PPI group and almost 40% in the H2RA group had H. pylori cleared before randomization.
The Research Grant Council of Hong Kong funded the work. Dr. Chan has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.
FROM GASTROENTEROLOGY
Key clinical point:
Major finding: During the 12-month study period, upper GI bleeding recurred in one patient receiving rabeprazole (0.7%) and four receiving famotidine (3.1%; P = .16). The composite endpoint of recurrent bleeding or endoscopic ulcers at month 12 was reached by nine patients in the rabeprazole group (7.9%) and 13 receiving famotidine (12.4%; P = .26).
Data source: A 270-subject, double-blind, randomized trial in Hong Kong and Japan.
Disclosures: The Research Grant Council of Hong Kong funded the work. The lead investigator has served as a consultant to Pfizer, Eisai, Takeda, and Otsuka, and has received research grants from Pfizer and lecture fees from Pfizer, AstraZeneca, and Takeda. Several other authors reported similar industry disclosures.