User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Children and COVID: New cases, vaccinations both decline
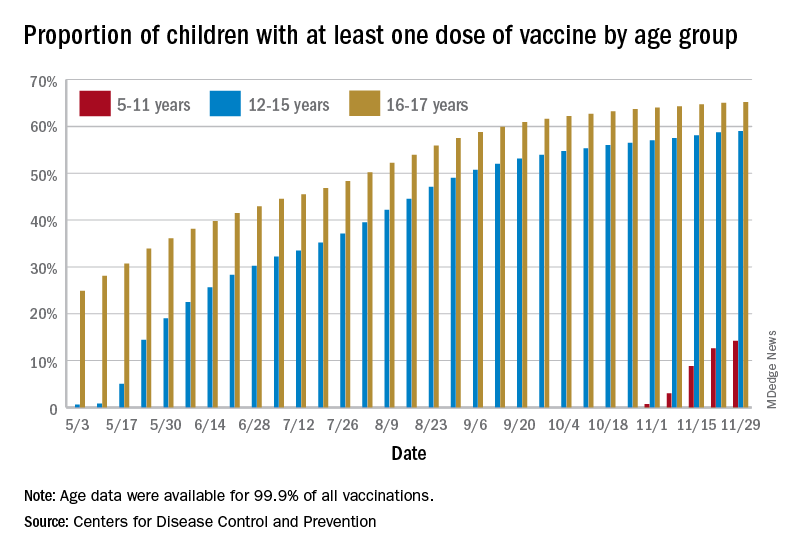
States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.

States reported 131,828 new pediatric cases for the week of Nov. 19-25, a decline of 7.1% over the previous week but still enough to surpass 100,000 for the 16th consecutive week. The weekly count had risen for 3 straight weeks since the last decrease in late October, the American Academy of Pediatrics and the Children’s Hospital Association said Nov. 30 in their weekly COVID report.
The AAP/CHA analysis, based on data from state and territorial health departments, puts the total number of cases in children at 6.9 million since the pandemic began, representing 17.0% of cases in Americans of all ages. The Centers for Disease Control and Prevention, which uses an age limit of 18 years to define a child, unlike some states, reports numbers of 6.1 million and 15.5%.
New vaccinations among the youngest eligible children, those aged 5-11 years, were down for the second week in a row after reaching almost 1.7 million during the first full week after approval on Nov. 2. Since then, the vaccination counts have been 1.2 million (Nov. 16-22) and 333,000 (Nov. 23-29), the CDC said on its COVID Data Tracker. A similar drop in the last week – from 127,000 to just 50,000 – also was seen for those aged 12-17 years.
Altogether, 14.2% of children aged 5-11, almost 4.1 million individuals, have received at least one dose of the vaccine, compared with 59.0% (10 million) of the 12- to 15-year-olds and 65.2% (5.5 million) of those aged 16-17. Just under 1% of the youngest group has been fully vaccinated, versus 49.0% and 55.8% for the older children, the CDC said.
It has been reported that Pfizer and BioNTech, which produce the only COVID vaccine approved for children, are planning to apply to the Food and Drug Administration during the first week of December for authorization for a booster dose for 16- and 17-year-olds.
Fauci: Omicron ‘very different from other variants’
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
The newly detected Omicron COVID-19 variant may be highly infectious and less responsive to available vaccines than other variants, but it is too early to know how it compares to the Delta variant, top infectious disease official Anthony S. Fauci, MD, said Nov. 30.
Dr. Fauci, speaking at a White House COVID-19 briefing, said there’s a “very unusual constellation of changes” across the COVID-19 genome that indicates it is unlike any variant we have seen so far.
“This mutational profile is very different from other variants of interest and concern, and although some mutations are also found in Delta, this is not Delta,” Dr. Fauci said. “These mutations have been associated with increased transmissibility and immune evasion.”
Omicron is the fifth designated COVID-19 variant of concern.
Detected first in South Africa, Omicron has been found in 20 countries so far. There are no known cases yet in the United States, but it has been detected in Canada.
Omicron has more than 30 mutations to the spike protein, the part of the virus that binds to human cells, Dr. Fauci said.
Cross-protection from boosters
Though the mutations suggest there is increased transmission of this variant, he said it is too soon to know how this compares to the Delta variant. And although the vaccines may not be as effective against Omicron, Dr. Fauci said there will likely be some protection.
“Remember, as with other variants, although partial immune escape may occur, vaccines, particularly boosters, give a level of antibodies that even with variants like Delta give you a degree of cross-protection, particularly against severe disease,” he said.
“When we say that although these mutations suggest a diminution of protection and a degree of immune evasion, we still, from experience with Delta, can make a reasonable conclusion that you would not eliminate all protection against this particular variant,” Dr. Fauci said.
So far, there is no reason to believe Omicron will cause more severe illness than other variants of concern.
“Although some preliminary information from South Africa suggests no unusual symptoms associated with variant, we do not know, and it is too early to tell,” Dr. Fauci said.
He recommended that people continue to wear masks, wash hands, and avoid crowded indoor venues. Most importantly, he recommended that everyone get their vaccines and boosters.
“One thing has become clear over the last 20 months: We can’t predict the future, but we can be prepared for it,” CDC Director Rochelle P. Walensky, MD, said at the briefing. “We have far more tools to fight the variant today than we did at this time last year.”
A version of this story first appeared on Medscape.com.
FDA panel backs first pill for COVID-19 by a small margin
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
, according to a panel of experts that advises the Food and Drug Administration on its regulatory decisions for these types of drugs.
The FDA’s Antimicrobial Drugs Advisory Committee narrowly voted to authorize the drug molnupiravir, voting 13 to 10 to support emergency use, which requires a medication to meet a lower standard of evidence than does full approval.
The FDA is not bound by the committee’s vote but typically follows its advice.
If authorized by the agency, molnupiravir would be the first antiviral agent available as a pill to treat COVID-19. Other therapies to treat the infection are available — monoclonal antibodies and the drug remdesivir — but they are given by infusion.
The United Kingdom has already authorized the use of Merck’s drug.
“This was clearly a difficult decision,” said committee member Michael Green, MD, a pediatric infectious disease expert at the University of Pittsburg School of Medicine.
Green said he voted yes, and that the drug’s ability to prevent deaths in the study weighed heavily on his decision. He said given uncertainties around the drug both the company and FDA should keep a close eye on patients taking the drug going forward.
“Should an alternative oral agent become available that had a better safety profile and equal or better efficacy profile, the agency might reconsider its authorization,” he said.
Others didn’t agree that the drug should be allowed onto the market.
“I voted no,” said Jennifer Le, PharmD, a professor of clinical pharmacy at the University of California. Dr. Le said the modest benefit of the medication didn’t outweigh all the potential safety issues. “I think I just need more efficacy and safety data,” she said.
Initial results from the first half of people enrolled in the clinical trial found the pill cut the risk of hospitalization or death by 50% in patients at higher risk of severe outcomes from COVID-19.
But later results, released just days before the meeting, showed that the drug’s effectiveness had dropped to about 30%.
In the updated analysis, 48 patients out of the 709 who were taking the drug were hospitalized or died within 29 days compared to 68 out of 699 who randomly got the placebo. There was one death in the group that got molnupiravir compared to nine in the placebo group. Nearly all those deaths occurred during the first phase of the study.
On Nov. 30 Merck explained that the drug’s efficacy appeared to fall, in part, because the placebo group had experienced fewer hospitalizations and deaths than expected during the second half of the study, making the drug look less beneficial by comparison.
The company said it wasn’t sure why patients in the placebo group had fared so much better in later trial enrollments.
“The efficacy of this product is not overwhelmingly good,” said committee member David Hardy, MD, an infectious disease expert at Charles Drew University School of Medicine in Los Angeles. “And I think that makes all of us a little uncomfortable about whether this is an advanced therapeutic because it’s an oral medication rather than an intravenous medication,” he said during the panel’s deliberations.
“I think we have to be very careful about how we’re going to allow people to use this,” Dr. Hardy said.
Many who voted for authorization thought use of the drug should be restricted to unvaccinated people who were at high risk of severe COVID-19 outcomes, the same population enrolled in the clinical trial. People in the trial were considered at higher risk if they were over age 60, had cancer, chronic kidney disease, chronic obstructive pulmonary disease, were obese, or had heart disease or diabetes.
There are some significant limitations of the study that may affect how the drug is used. Vaccinated people couldn’t enroll in the study, so it’s not known if the medication would have any benefit for them. Nearly two-thirds of the U.S. population is fully vaccinated. The study found no additional benefit of the medication compared to the placebo in people who had detectable antibodies, presumably from a prior infection.
Animal studies found that the drug — which kills the virus by forcing it to make errors as it copies its genetic material inside cells — could disrupt bone formation. For that reason, the manufacturer and the FDA agreed that it should not be used in anyone younger than age 18.
Animal studies also indicated that the drug could cause birth defects. For that reason, the company said the drug shouldn’t be given to women who are pregnant or breastfeeding and said doctors should make sure women of childbearing age aren’t pregnant before taking the medication.
Some members of the panel felt that pregnant women and their doctors should be given the choice of whether or not to use the drug, given that pregnant women are at high risk for severe COVID-19 outcomes and infused therapies may not be available in all settings.
Other members of the committee said they were uncomfortable authorizing the drug given its potential to mutate the virus.
The drug, which forces the virus to mutate as it copies its RNA, eventually causes the virus to make so many errors in its genetic material that it can no longer make more of itself and the immune system clears it out of the body.
But it takes a few days to work — the drug is designed to be taken for 5 consecutive days -- and studies of the viral loads of patients taking the drug show that through the first 2 days, viral loads remain detectable as these mutations occur.
Studies by the FDA show some of those mutations in the spike protein are the same ones that have helped the virus become more transmissible and escape the protection of vaccines.
So the question is whether someone taking the medication could develop a dangerous mutation and then infect someone else, sparking the spread of a new variant.
Nicholas Kartsonis, MD, a vice president at Merck, said that the company was still analyzing data.
“Even if the probability is very low — 1 in 10,000 or 1 in 100,000 -- that this drug would induce an escape mutant for which the vaccines we have would not cover, that would be catastrophic for the whole world, actually,” said committee member James Hildreth, MD, an immunologist and president of Meharry Medical College, Nashville. “Do you have sufficient data on the likelihood of that happening?” he asked Dr. Kartsonis of Merck.
“So we don’t,” Dr. Kartsonis said.
He said, in theory, the risk of mutation with molnupiravir is the same as seen with the use of vaccines or monoclonal antibody therapies. Dr. Hildreth wasn’t satisfied with that answer.
“With all respect, the mechanism of your drug is to drive [genetic mutations], so it’s not the same as the vaccine. It’s not the same as monoclonal antibodies,” he said.
Dr. Hildreth later said he didn’t feel comfortable voting for authorization given the uncertainties around escape mutants. He voted no.
“It was an easy vote for me,” he said.
A version of this article first appeared on Medscape.com.
Let’s talk about ‘chemsex’: Sexualized drug use among men who have sex with men
Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
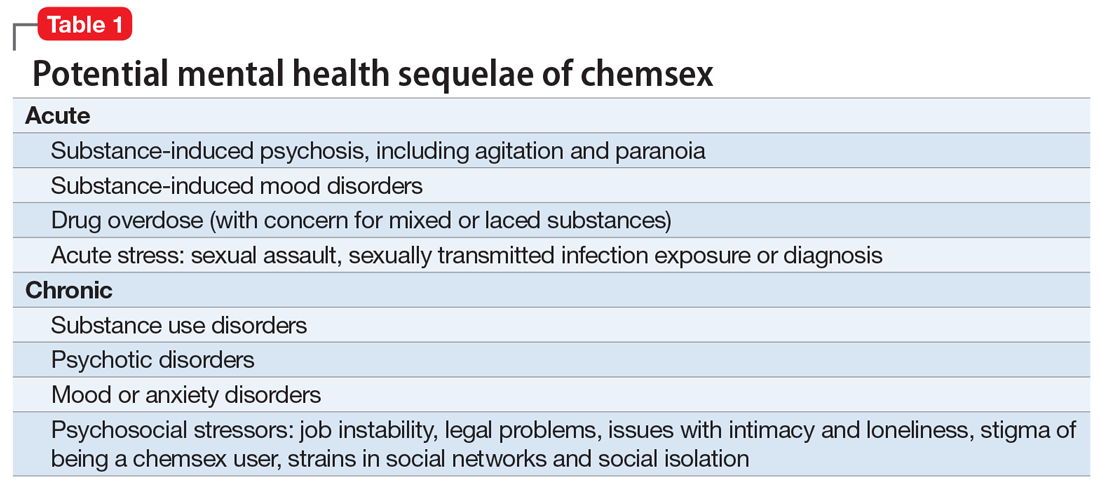
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).
What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).

What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

Consider the following patients who have presented to our hospital system:
- A 27-year-old gay man is brought to the emergency department by police after bizarre behavior in a hotel. He is paranoid, disorganized, and responding to internal stimuli. He admits to using methamphetamine before a potential “hookup” at the hotel
- A 35-year-old bisexual man presents to the psychiatric emergency department, worried he will lose his job and relationship after downloading a dating app on his work phone to buy methamphetamine
- A 30-year-old gay man divulges to his psychiatrist that he is insecure about his sexual performance and intimacy with his partner because most of their sexual contact involves using gamma-hydroxybutyric acid (GHB).
These are just some of the many psychiatric presentations we have encountered involving “chemsex” among men who have sex with men (MSM).
What is ‘chemsex?’
“Chemsex” refers to the use of specific drugs—mainly methamphetamine, mephedrone, or GHB—before or during sex to reduce sexual disinhibitions and to facilitate, initiate, prolong, sustain, and intensify the encounter.1 Chemsex participants report desired enhancements in:
- confidence and ability to engage with partners
- emotional awareness and shared experience with partners
- sexual performance and intensity of sensations.1
How prevalent is it?
Emerging in urban centers as a part of gay nightlife, chemsex has become increasingly prevalent among young MSM, fueled by a worldwide rise in methamphetamine use.1,2 In a large 2019 systematic review, Maxwell et al1 reported a wide range of chemsex prevalence estimates among MSM (3% to 29%). Higher estimates emerged from studies recruiting participants from sexual health clinics and through phone-based dating apps, while lower estimates tended to come from more representative samples of MSM. In studies from the United States, the prevalence of chemsex ranged from 9% to 10% in samples recruited from gay pride events, gay nightlife venues, and internet surveys. Across studies, MSM participating in chemsex were more likely to identify as gay, with mean ages ranging from 32 to 42 years, and were more likely to be HIV-positive.1
Methamphetamine was the most popular drug used, with GHB having higher prevalence in Western Europe, and mephedrone more common in the United Kingdom.1 Injection drug use was only examined in studies from the United Kingdom, the Netherlands, and Australia and showed a lower overall prevalence rate—1% to 9%. Methamphetamine was the most commonly injected drug. Other drugs used for chemsex included ketamine, 3,4-methylenedioxymethamphetamine (MDMA, aka “ecstasy”), cocaine, amyl nitrite (“poppers”), and erectile dysfunction medications.1It is important to remember that chemsex is a socially constructed concept and, as such, is subject to participant preferences and the popularity and availability of specific drugs. These features are likely to vary across geography, subcultures, and time. The above statistics ultimately represent a minority of MSM but highlight the importance of considering this phenomenon when caring for this population.1
Continue to: What makes chemsex unique?...
What makes chemsex unique?
Apps and access. Individuals who engage in chemsex report easy access to drugs via nightlife settings or through smartphone dating apps. Drugs are often shared during sexual encounters, which removes cost barriers for participants.1
Environment. Chemsex sometimes takes place in group settings at “sex-on-premises venues,” including clubs, bathhouses, and saunas. The rise of smartphone apps and closure of these venues has shifted much of chemsex to private settings.1Sexual behavior. Seventeen of the studies included in the Maxwell et al1 review showed an increased risk of condomless anal intercourse during chemsex. Several studies also reported increased rates of sex with multiple partners and new partners.1
What are the potential risks?
Physical health. High-risk sexual behaviors associated with chemsex increase the risk of sexually transmitted infections, including HIV and hepatitis C.1 Use of substances associated with chemsex can lead to overdose, cardiovascular events, and neurotoxicity.1,2
Mental health. In our clinical experience, the psychiatric implications of chemsex are numerous and exist on a spectrum from acute to chronic (Table 1).

What can clinicians do?
We encourage you to talk about chemsex with your patients. Table 2 provides a “tip sheet” to help you start the conversation, address risks, and provide support. We hope you continue to learn from your patients and keep up-to-date on this evolving topic.

1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
1. Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: a systematic review of the literature. Int J Drug Policy. 2019;63:74-89.
2. Paulus MP, Stewart JL. Neurobiology, clinical presentation, and treatment of methamphetamine use disorder: a review. JAMA Psychiatry. 2020;77(9):959-966.
Two questions can help establish a diagnosis of hidradenitis suppurativa
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
According to Iltefat H. Hamzavi, MD,
If the answer to the first question is “yes” and the patient has had at least two boils in intertriginous areas, that person likely has HS, a disease of apocrine gland–bearing skin that occurs in 1%-4% of people, has a higher prevalence in Blacks, compared with Whites, and affects more women than men by a 3:1 ratio.
“Current treatments offer limited efficacy, and the disease is chronic and recurrent,” Dr. Hamzavi, of the department of dermatology at Henry Ford Health System, Detroit, said during MedscapeLive’s annual Las Vegas Dermatology Seminar. “You often see nodules, abscesses, fistulae, and scarring,” with all different skin types represented in the majority of patients.
Typical HS lesions appear as inflamed nodules, abscesses, draining fistulas, and scars as well as double-headed “tombstone” comedones, he said. These are typically located in the axilla, intermammary folds, in the groin, around the genitals, and on the buttocks. Atypical lesions can also occur – often folliculitis and open comedones in locations such as the waistline, the neck, and behind the ears.
The differential diagnosis is wide-ranging and includes bacterial abscess, inflamed cyst, folliculitis, pilonidal sinus, cellulitis, and cutaneous Crohn’s disease. Pain may appear out of proportion to the physical examination.
“There is a window of opportunity to treat HS, early in the disease process,” Dr. Hamzavi said. “There are no definitive cures for HS but lots of treatment options.”
According to clinical management guidelines published by the United States and Canadian Hidradenitis Suppurativa Foundations, options for moderate stage disease include antibiotics, antiandrogens, retinoids, immunosuppression/biologics, deroofing, and limited excision with primary closure. Options for severe disease include radical excision.
“HS requires a mix of medical and procedural treatments based on the number of nodules,” Dr. Hamzavi said. “Because the disease has so many different phases, there is no perfect outcome measure yet, but progress is being made.”
In 2018, an effort to develop a consensus core outcome set of domains regarding what to measure in clinical trials of HS was launched; it is known as the Hidradenitis Suppurativa Core Outcomes Set International Collaboration (HISTORIC). It was formed as a collaboration between the International Dermatology Outcome Measures (IDEOM) initiative, the Cochrane Skin Group – Core Outcome Set Initiative (CSG-COUSIN), and Zealand University Hospital, Roskilde.
HISTORIC is now part of the partnership with CSG-COUSIN and this work continues onward. Core domains as defined by the group include pain, physical signs, HS-specific quality of life, global assessment, and disease progression. “For now, we are mostly using some objective measures and some patient-reported outcomes with the addition of ultrasound in some centers,” Dr. Hamzavi said.
He underscored the importance of lifestyle modifications in patients with HS, including smoking cessation and weight loss, as well as decreasing pressure/friction on lesions, using warm compresses, and modifying diet. “This generally involves a low-inflammatory diet: Low carbohydrate, low dairy, and higher protein content, but there is much work needed to understand the role of diet in HS,” he said.
“This is a tough disease, but the compassion you offer these patients will be paid back to you a thousandfold. They tend to be some of the happiest and most appreciative patients you will ever have in your practice.”
Dr. Hamzavi disclosed that he has been a clinical investigator for Clinuvel, Incyte, Pfizer, Avita, and Ferndale Labs. He has also been a consultant for Pfizer, AbbVie, Novartis, and Aclaris, and has received a grant from Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
NAFLD, ALD prevalent among teens, young adults
Two-fifths of adolescents and young adults in the United States may have nonalcoholic fatty liver disease (NAFLD), many with significant or advanced fibrosis, results of a nationwide surveillance study suggest.
In addition, among those who drink alcohol in excess, slightly more than half may have alcohol-associated fatty liver disease (ALD) that may lead to moderate to severe fibrosis in a substantial proportion, said Naim Alkhouri, MD, from Arizona Liver Health, Peoria, during a presentation of the findings at The Liver Meeting 2021: American Association for the Study of Liver Diseases (AASLD), held online.
“Efforts should focus on increasing awareness of the burden of ALD and NAFLD in this population and [mitigating] modifiable risk factors to prevent disease development and disease progression to potentially advanced fibrosis and cirrhosis,” he said.
Liver stiffness measured
Unlike previous studies that relied on liver enzyme levels or ultrasonography to estimate the prevalence of fatty liver disease among adolescents and young adults in the United States, Dr. Alkhouri and colleagues used valid liver ultrasonographic elastography (FibroScan) measurements, recorded during 2017-2018, from the National Health and Nutrition Examination Survey (NHANES) database.
The sample included participants aged 15 to 39 years. Those with viral hepatitis, alanine aminotransferase (ALT) levels greater than 500 U/L, or pregnancy were excluded.
The investigators divided the participants into those with excessive alcohol consumption, defined using the NHANES Alcohol Use Questionnaire as having more than two drinks per day for males or more than one drink per day for females, and those with no excessive alcohol consumption.
The authors used controlled attenuation parameters to identify participants with suspected ALD or NAFLD.
They then used liver stiffness measurement cutoffs of greater than or equal to 7.5 kPa to identify moderate fibrosis and greater than or equal to 9.5 kPa to identify severe fibrosis in those with evidence of ALD and cutoffs of greater than or equal to 6.1 kPa and greater than or equal to 7.1 kPa, respectively, in those with suspected NAFLD.
The cutoffs were chosen to maximize sensitivity, as determined from published literature, Dr. Alkhouri said.
Uncovering a high prevalence of ALD and NAFLD
The final sample comprised 1,319 participants, including 100 with excessive alcohol use and 1,219 without.
The heavy drinkers were significantly more likely to be older, male, White, current smokers, have lower platelet counts, higher aspartate aminotransferase (AST) and ALT levels, and higher mean corpuscular volumes.
Among the excessive drinkers, 52% had ALD. Of this group, 87.7% had either no or mild fibrosis, and 12.3% had moderate to severe fibrosis.
Among patients with excessive alcohol consumption, significant predictors of ALD included male sex, higher body mass index, ALT greater than the upper limit of normal, and higher A1c percentage.
Among those who were moderate drinkers or abstemious, 40% had NAFLD. Of this subgroup, 68.9% had no or mild fibrosis, and 31.1% had moderate to severe fibrosis.
Predictors of NAFLD in this group included older age, male sex, higher body mass index, and elevated ALT, AST, albumin, platelet counts, and A1c.
Is drinking underreported?
In a question-and-answer session following the presentation, co-moderator Miriam B. Vos, MD, a pediatric hepatologist at Children’s Healthcare of Atlanta, asked Dr. Alkhouri about his confidence in the accuracy of the measurements of alcohol consumption and whether there could be significant overlap between the ALD and NAFLD populations.
Dr. Alkhouri noted that he and his colleagues relied on items 121 and 130 of the NHANES Alcohol Use Questionnaire, which are self-reported by participants.
“Obviously, we’re not going to get honest answers all the time,” he said. “We’ve seen even in NASH [nonalcoholic steatohepatitis] clinical trials that when patients say they do not drink any alcohol, if you actually look for alcohol metabolites, up to 20% may have some evidence of alcohol consumption.
“I’m sure there’s a lot of overlap, but there’s no formal assessment,” he added.
Dr. Alkhouri noted that among the cohort with ALD, obesity and increased A1c were prevalent, “so it goes both ways. I think NAFLD can also contribute to progression of ALD, and that’s why we need to study another entity called ‘both alcoholic and nonalcoholic fatty liver disease.’”
Dr. Vos suggested that biomarkers may be useful for detecting alcohol use among patients with NAFLD and for further study of the progression of NAFLD to ALD.
No source of funding for the study has been disclosed. Dr. Alkhouri and Dr. Vos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two-fifths of adolescents and young adults in the United States may have nonalcoholic fatty liver disease (NAFLD), many with significant or advanced fibrosis, results of a nationwide surveillance study suggest.
In addition, among those who drink alcohol in excess, slightly more than half may have alcohol-associated fatty liver disease (ALD) that may lead to moderate to severe fibrosis in a substantial proportion, said Naim Alkhouri, MD, from Arizona Liver Health, Peoria, during a presentation of the findings at The Liver Meeting 2021: American Association for the Study of Liver Diseases (AASLD), held online.
“Efforts should focus on increasing awareness of the burden of ALD and NAFLD in this population and [mitigating] modifiable risk factors to prevent disease development and disease progression to potentially advanced fibrosis and cirrhosis,” he said.
Liver stiffness measured
Unlike previous studies that relied on liver enzyme levels or ultrasonography to estimate the prevalence of fatty liver disease among adolescents and young adults in the United States, Dr. Alkhouri and colleagues used valid liver ultrasonographic elastography (FibroScan) measurements, recorded during 2017-2018, from the National Health and Nutrition Examination Survey (NHANES) database.
The sample included participants aged 15 to 39 years. Those with viral hepatitis, alanine aminotransferase (ALT) levels greater than 500 U/L, or pregnancy were excluded.
The investigators divided the participants into those with excessive alcohol consumption, defined using the NHANES Alcohol Use Questionnaire as having more than two drinks per day for males or more than one drink per day for females, and those with no excessive alcohol consumption.
The authors used controlled attenuation parameters to identify participants with suspected ALD or NAFLD.
They then used liver stiffness measurement cutoffs of greater than or equal to 7.5 kPa to identify moderate fibrosis and greater than or equal to 9.5 kPa to identify severe fibrosis in those with evidence of ALD and cutoffs of greater than or equal to 6.1 kPa and greater than or equal to 7.1 kPa, respectively, in those with suspected NAFLD.
The cutoffs were chosen to maximize sensitivity, as determined from published literature, Dr. Alkhouri said.
Uncovering a high prevalence of ALD and NAFLD
The final sample comprised 1,319 participants, including 100 with excessive alcohol use and 1,219 without.
The heavy drinkers were significantly more likely to be older, male, White, current smokers, have lower platelet counts, higher aspartate aminotransferase (AST) and ALT levels, and higher mean corpuscular volumes.
Among the excessive drinkers, 52% had ALD. Of this group, 87.7% had either no or mild fibrosis, and 12.3% had moderate to severe fibrosis.
Among patients with excessive alcohol consumption, significant predictors of ALD included male sex, higher body mass index, ALT greater than the upper limit of normal, and higher A1c percentage.
Among those who were moderate drinkers or abstemious, 40% had NAFLD. Of this subgroup, 68.9% had no or mild fibrosis, and 31.1% had moderate to severe fibrosis.
Predictors of NAFLD in this group included older age, male sex, higher body mass index, and elevated ALT, AST, albumin, platelet counts, and A1c.
Is drinking underreported?
In a question-and-answer session following the presentation, co-moderator Miriam B. Vos, MD, a pediatric hepatologist at Children’s Healthcare of Atlanta, asked Dr. Alkhouri about his confidence in the accuracy of the measurements of alcohol consumption and whether there could be significant overlap between the ALD and NAFLD populations.
Dr. Alkhouri noted that he and his colleagues relied on items 121 and 130 of the NHANES Alcohol Use Questionnaire, which are self-reported by participants.
“Obviously, we’re not going to get honest answers all the time,” he said. “We’ve seen even in NASH [nonalcoholic steatohepatitis] clinical trials that when patients say they do not drink any alcohol, if you actually look for alcohol metabolites, up to 20% may have some evidence of alcohol consumption.
“I’m sure there’s a lot of overlap, but there’s no formal assessment,” he added.
Dr. Alkhouri noted that among the cohort with ALD, obesity and increased A1c were prevalent, “so it goes both ways. I think NAFLD can also contribute to progression of ALD, and that’s why we need to study another entity called ‘both alcoholic and nonalcoholic fatty liver disease.’”
Dr. Vos suggested that biomarkers may be useful for detecting alcohol use among patients with NAFLD and for further study of the progression of NAFLD to ALD.
No source of funding for the study has been disclosed. Dr. Alkhouri and Dr. Vos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two-fifths of adolescents and young adults in the United States may have nonalcoholic fatty liver disease (NAFLD), many with significant or advanced fibrosis, results of a nationwide surveillance study suggest.
In addition, among those who drink alcohol in excess, slightly more than half may have alcohol-associated fatty liver disease (ALD) that may lead to moderate to severe fibrosis in a substantial proportion, said Naim Alkhouri, MD, from Arizona Liver Health, Peoria, during a presentation of the findings at The Liver Meeting 2021: American Association for the Study of Liver Diseases (AASLD), held online.
“Efforts should focus on increasing awareness of the burden of ALD and NAFLD in this population and [mitigating] modifiable risk factors to prevent disease development and disease progression to potentially advanced fibrosis and cirrhosis,” he said.
Liver stiffness measured
Unlike previous studies that relied on liver enzyme levels or ultrasonography to estimate the prevalence of fatty liver disease among adolescents and young adults in the United States, Dr. Alkhouri and colleagues used valid liver ultrasonographic elastography (FibroScan) measurements, recorded during 2017-2018, from the National Health and Nutrition Examination Survey (NHANES) database.
The sample included participants aged 15 to 39 years. Those with viral hepatitis, alanine aminotransferase (ALT) levels greater than 500 U/L, or pregnancy were excluded.
The investigators divided the participants into those with excessive alcohol consumption, defined using the NHANES Alcohol Use Questionnaire as having more than two drinks per day for males or more than one drink per day for females, and those with no excessive alcohol consumption.
The authors used controlled attenuation parameters to identify participants with suspected ALD or NAFLD.
They then used liver stiffness measurement cutoffs of greater than or equal to 7.5 kPa to identify moderate fibrosis and greater than or equal to 9.5 kPa to identify severe fibrosis in those with evidence of ALD and cutoffs of greater than or equal to 6.1 kPa and greater than or equal to 7.1 kPa, respectively, in those with suspected NAFLD.
The cutoffs were chosen to maximize sensitivity, as determined from published literature, Dr. Alkhouri said.
Uncovering a high prevalence of ALD and NAFLD
The final sample comprised 1,319 participants, including 100 with excessive alcohol use and 1,219 without.
The heavy drinkers were significantly more likely to be older, male, White, current smokers, have lower platelet counts, higher aspartate aminotransferase (AST) and ALT levels, and higher mean corpuscular volumes.
Among the excessive drinkers, 52% had ALD. Of this group, 87.7% had either no or mild fibrosis, and 12.3% had moderate to severe fibrosis.
Among patients with excessive alcohol consumption, significant predictors of ALD included male sex, higher body mass index, ALT greater than the upper limit of normal, and higher A1c percentage.
Among those who were moderate drinkers or abstemious, 40% had NAFLD. Of this subgroup, 68.9% had no or mild fibrosis, and 31.1% had moderate to severe fibrosis.
Predictors of NAFLD in this group included older age, male sex, higher body mass index, and elevated ALT, AST, albumin, platelet counts, and A1c.
Is drinking underreported?
In a question-and-answer session following the presentation, co-moderator Miriam B. Vos, MD, a pediatric hepatologist at Children’s Healthcare of Atlanta, asked Dr. Alkhouri about his confidence in the accuracy of the measurements of alcohol consumption and whether there could be significant overlap between the ALD and NAFLD populations.
Dr. Alkhouri noted that he and his colleagues relied on items 121 and 130 of the NHANES Alcohol Use Questionnaire, which are self-reported by participants.
“Obviously, we’re not going to get honest answers all the time,” he said. “We’ve seen even in NASH [nonalcoholic steatohepatitis] clinical trials that when patients say they do not drink any alcohol, if you actually look for alcohol metabolites, up to 20% may have some evidence of alcohol consumption.
“I’m sure there’s a lot of overlap, but there’s no formal assessment,” he added.
Dr. Alkhouri noted that among the cohort with ALD, obesity and increased A1c were prevalent, “so it goes both ways. I think NAFLD can also contribute to progression of ALD, and that’s why we need to study another entity called ‘both alcoholic and nonalcoholic fatty liver disease.’”
Dr. Vos suggested that biomarkers may be useful for detecting alcohol use among patients with NAFLD and for further study of the progression of NAFLD to ALD.
No source of funding for the study has been disclosed. Dr. Alkhouri and Dr. Vos reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Merck’s COVID-19 pill may be less effective than first hoped
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
According to an analysis by scientists at the Food and Drug Administration, the experimental pill cut the risk of hospitalization or death from COVID-19 by about 30%, compared to a placebo, and the pill showed no benefit for people with antibodies against COVID-19 from prior infection.
The updated analysis showed 48 hospitalizations or deaths among study participants who were randomly assigned to take the antiviral drug, compared to 68 among those who took a placebo.
Those results come from the full set of 1,433 patients who were randomized in the clinical trial, which just became available last week.
Initial results from the first 775 patients enrolled in the clinical trial, which were issued in a company news release in October, had said the drug cut the risk of hospitalization or death for patients at high risk of severe disease by about 50%.
Merck has been producing millions of doses of molnupiravir, which is the first antiviral pill to treat COVID-19 infections. The United Kingdom’s drug regulator authorized use of the medication in early November. The company said it expected to distribute the medication globally by the end of 2021.
In October, two Indian drug companies halted late-stage clinical trials of a generic version of molnupiravir after the studies failed to find any benefit to patients with moderate COVID-19. Trials in patients with milder symptoms are still ongoing.
On Nov. 27, the New England Journal of Medicine postponed its planned early release of the molnupiravir study results, citing “new information.”
The medication is designed to be given as four pills taken every 12 hours for 5 days. It’s most effective when taken within the first few days of new symptoms, something that requires convenient and affordable testing.
The new results seem to put molnupiravir far below the effectiveness of existing treatments.
The infused monoclonal antibody cocktail REGEN-COV, which the FDA has already authorized for emergency use, is about 85% effective at preventing hospitalization or death in patients who are at risk for severe COVID-19 outcomes, and it appears to be just as effective in people who already have antibodies against COVID-19, which is why it is being given to both vaccinated and unvaccinated patients, the FDA said.
In early November, Pfizer said its experimental antiviral pill Paxlovid cut the risk of hospitalization or death by 89%.
In briefing documents posted ahead of an advisory committee meeting Nov. 30, the FDA highlights other potential safety issues with the Merck drug, which works by causing the virus to make mistakes as it copies itself, eventually causing the virus to mutate itself to death.
The agency has asked the advisory committee to weigh in on the right patient population for the drug: Should pregnant women get it? Could the drug harm a developing fetus?
Should vaccinated people with breakthrough infections get it? Would it work for them? People with reduced immune function are more likely to get a breakthrough infection. They’re also more likely to shed virus for a longer period of time, making them perfect incubators for variants. What could happen if we give this type of patient a drug that increases mutations?
And what about mutations caused by the medication? Could they increase the potential for more variants? The agency concluded the risk of this happening was low.
In animal studies, the drug impacted bone formation. For this reason, the agency has agreed with the drug company that molnupiravir should not be given to anyone under the age of 18.
Aside from these concerns, the FDA says there were no major safety issues among people who took part in the clinical trial, though they acknowledge that number is small.
A version of this article first appeared on WebMD.com.
Whole-food exclusion diet effective for adult Crohn’s disease
The Crohn’s disease exclusion diet (CDED), with or without partial enteral nutrition (PEN), effectively induced and maintained remission in adults with mild to moderate, biologic-naive CD, a randomized Israeli pilot study found.
The authors, led by Henit Yanai, MD, MBA, chief of the IBD Center at Rabin Medical Center in Petah Tikva, Israel, also suggested that dietary monotherapy might lead to durable endoscopic remission.
The study, published in The Lancet Gastroenterology & Hepatology, found about 60% of patients on CDED achieved clinical remission by week 6 without adding medications.
Furthermore, 80% of patients in remission at week 6 maintained clinical remission at week 24 on dietary monotherapy alone, allowing more than 50% of the intention-to-treat (ITT) population to achieve sustained remission at 6 months.
Dietary therapy resulted in a significant and progressive reduction in inflammatory markers such as C-reactive protein and fecal calprotectin. The benefit extended to mucosal healing, with 35% of the ITT population achieving endoscopic remission at 24 weeks.
Dr. Yanai explained the clinical context in which her group designed the study. “There were preliminary data regarding the efficacy of the dietary strategy for the induction of remission of mild CD in the pediatric population,” she said in an interview. “Additionally, there was an anecdotal experience in adults who reported benefits. Facing this and the lack of attractive alternatives for mild CD, we decided to examine the effectiveness of this therapeutic strategy in adults.”
Given the costs and side effects of medical treatment, interest in dietary monotherapy for IBD has been growing. As Dr. Yanai said, the CDED, a whole-foods regimen, plus PEN, has been found to help children with CD.
The CDED excludes proinflammatory food components associated with intestinal microbial dysbiosis, altered innate immunity, and impaired gut barrier function.
It involves increased consumption of fruits and vegetables, high-quality lean protein, complex carbohydrates, healthy oils, and fiber, while decreasing intake of inflammation-driving components such as animal and saturated vegetable fats, wheat, and dairy, as well as food additives such as emulsifiers, maltodextrin, and sulfites.
“The realization that exclusive enteral nutrition, which is based on liquid formulas, is effective for inducting remission in children PEN, which is a combination of liquid formulas and food, was less effective, led to the hypothesis that the mechanism might be the exclusion of dietary components that may lead to inflammation,” Dr. Yanai said. This theory, she added, derived from animal models showing that specific dietary components potentially drive inflammation.
The only current guideline-recommended nutritional therapy for remission induction is exclusive enteral nutrition (EEN) in pediatric CD.
The study
The open-label pilot trial led by Dr. Yanai, conducted at three medical centers, enrolled biologic-naive adults ages 18-55 with uncomplicated mild to moderate CD, with a disease duration of no more than 5 years. They had active disease on imaging and elevated C-reactive protein or fecal calprotectin.
During the period January 2017 to May 2020, eligible patients were randomly assigned 1 to 1 to CDED plus PEN enteral (n = 20) or CDED alone (n = 24) for 24 weeks. The primary endpoint was clinical remission, defined as a Harvey–Bradshaw Index score of less than 5 at week 6, an outcome was assessed in the ITT population, which consisted of those who used dietary therapy for at least 48 hours.
At week 6, 13 (68%) of 19 remaining patients in the CDED-plus-PEN group and 12 (57%) of 21 patients in the CDED-alone group had achieved clinical remission (P = .4618).
Among the 25 patients in remission 6 weeks out, 20 (80%) remained in sustained remission at week 24: 12 in the CDED-plus-PEN group and 8 in the CDED-alone group.
Moreover, 14 (35%) of 40 patients were in endoscopic remission at week 24: 8 on CDED plus PEN and 6 on CDED alone.
“CDED with or without partial enteral nutrition was effective for induction and maintenance of remission in adults with mild to moderate biologic-naive Crohn’s disease and might lead to endoscopic remission,” the authors wrote, adding that CDED for mild to moderate active disease should be assessed in a powered randomized controlled trial.
Compliance and adherence are obstacles to dietary therapies. Data for adults using exclusive enteral nutrition are conflicting, with poor compliance postulated to drive an inadequate response in some studies.
“Like every dietary treatment, adherence is challenging,” Dr. Yanai said. “However, when patients feel that it helps them, they have more incentive to follow the diet in the long run, and also once they quit and fare worse, they can go back and follow the first stage of CDED.”
She and her colleagues stressed the need for adequately powered randomized trials and recommended that the personalization of therapeutic diets in the future should take into account the need to deliver energy tailored to the nutritional and therapeutic goals of the patient.
Diets alone are not therapy
In an accompanying editorial, Alexa N. Sasson, MD, an IBD fellow at Massachusetts General Hospital and T.H. Chan Harvard School of Public Health, both in Boston, called diet “a promising and potentially modifiable risk factor with mounting evidence supporting its therapeutic benefit.”
She concurred that the Israeli findings indicate that CDED with or without PEN appears effective for inducing and maintaining remission in this cohort of patients. “Assessment of composite diets such as the CDED is important since they can be incorporated into daily life. The relative efficacy of each of the included and excluded foods, however, is not clear,” she wrote.
She cautioned, however, that dietary therapy does not constitute maintenance therapy and its effects are not sustained after the reintroduction of whole foods. “Identification of sustainable dietary interventions for the prevention and treatment of IBD is increasingly a focus of research,” she wrote.
Dr. Sasson agreed that dietary therapy for CD should be centered on patient interests, goals, and disease states. “Adjunctive dietary measures might be considered in all interested patients as a method of improving gastrointestinal-related symptoms and quality of life, with the potential to achieve a higher and more sustained level of remission,” she wrote.
Both the authors and the commentator agreed on the need for larger randomized trials with long-term follow-up to guide treatment decisions and identify patients who might benefit from dietary intervention.
This study was funded by the Azrieli Foundation and Nestlé Health Science. Dr. Yanai reported financial relationships with Pfizer, AbbVie, Ferring, Janssen, Neopharm, Pfizer, and Takeda. Several coauthors disclosed financial ties to multiple private-sector companies. Dr. Sasson had no competing interests to declare.
This article was updated Dec. 1, 2021.
The Crohn’s disease exclusion diet (CDED), with or without partial enteral nutrition (PEN), effectively induced and maintained remission in adults with mild to moderate, biologic-naive CD, a randomized Israeli pilot study found.
The authors, led by Henit Yanai, MD, MBA, chief of the IBD Center at Rabin Medical Center in Petah Tikva, Israel, also suggested that dietary monotherapy might lead to durable endoscopic remission.
The study, published in The Lancet Gastroenterology & Hepatology, found about 60% of patients on CDED achieved clinical remission by week 6 without adding medications.
Furthermore, 80% of patients in remission at week 6 maintained clinical remission at week 24 on dietary monotherapy alone, allowing more than 50% of the intention-to-treat (ITT) population to achieve sustained remission at 6 months.
Dietary therapy resulted in a significant and progressive reduction in inflammatory markers such as C-reactive protein and fecal calprotectin. The benefit extended to mucosal healing, with 35% of the ITT population achieving endoscopic remission at 24 weeks.
Dr. Yanai explained the clinical context in which her group designed the study. “There were preliminary data regarding the efficacy of the dietary strategy for the induction of remission of mild CD in the pediatric population,” she said in an interview. “Additionally, there was an anecdotal experience in adults who reported benefits. Facing this and the lack of attractive alternatives for mild CD, we decided to examine the effectiveness of this therapeutic strategy in adults.”
Given the costs and side effects of medical treatment, interest in dietary monotherapy for IBD has been growing. As Dr. Yanai said, the CDED, a whole-foods regimen, plus PEN, has been found to help children with CD.
The CDED excludes proinflammatory food components associated with intestinal microbial dysbiosis, altered innate immunity, and impaired gut barrier function.
It involves increased consumption of fruits and vegetables, high-quality lean protein, complex carbohydrates, healthy oils, and fiber, while decreasing intake of inflammation-driving components such as animal and saturated vegetable fats, wheat, and dairy, as well as food additives such as emulsifiers, maltodextrin, and sulfites.
“The realization that exclusive enteral nutrition, which is based on liquid formulas, is effective for inducting remission in children PEN, which is a combination of liquid formulas and food, was less effective, led to the hypothesis that the mechanism might be the exclusion of dietary components that may lead to inflammation,” Dr. Yanai said. This theory, she added, derived from animal models showing that specific dietary components potentially drive inflammation.
The only current guideline-recommended nutritional therapy for remission induction is exclusive enteral nutrition (EEN) in pediatric CD.
The study
The open-label pilot trial led by Dr. Yanai, conducted at three medical centers, enrolled biologic-naive adults ages 18-55 with uncomplicated mild to moderate CD, with a disease duration of no more than 5 years. They had active disease on imaging and elevated C-reactive protein or fecal calprotectin.
During the period January 2017 to May 2020, eligible patients were randomly assigned 1 to 1 to CDED plus PEN enteral (n = 20) or CDED alone (n = 24) for 24 weeks. The primary endpoint was clinical remission, defined as a Harvey–Bradshaw Index score of less than 5 at week 6, an outcome was assessed in the ITT population, which consisted of those who used dietary therapy for at least 48 hours.
At week 6, 13 (68%) of 19 remaining patients in the CDED-plus-PEN group and 12 (57%) of 21 patients in the CDED-alone group had achieved clinical remission (P = .4618).
Among the 25 patients in remission 6 weeks out, 20 (80%) remained in sustained remission at week 24: 12 in the CDED-plus-PEN group and 8 in the CDED-alone group.
Moreover, 14 (35%) of 40 patients were in endoscopic remission at week 24: 8 on CDED plus PEN and 6 on CDED alone.
“CDED with or without partial enteral nutrition was effective for induction and maintenance of remission in adults with mild to moderate biologic-naive Crohn’s disease and might lead to endoscopic remission,” the authors wrote, adding that CDED for mild to moderate active disease should be assessed in a powered randomized controlled trial.
Compliance and adherence are obstacles to dietary therapies. Data for adults using exclusive enteral nutrition are conflicting, with poor compliance postulated to drive an inadequate response in some studies.
“Like every dietary treatment, adherence is challenging,” Dr. Yanai said. “However, when patients feel that it helps them, they have more incentive to follow the diet in the long run, and also once they quit and fare worse, they can go back and follow the first stage of CDED.”
She and her colleagues stressed the need for adequately powered randomized trials and recommended that the personalization of therapeutic diets in the future should take into account the need to deliver energy tailored to the nutritional and therapeutic goals of the patient.
Diets alone are not therapy
In an accompanying editorial, Alexa N. Sasson, MD, an IBD fellow at Massachusetts General Hospital and T.H. Chan Harvard School of Public Health, both in Boston, called diet “a promising and potentially modifiable risk factor with mounting evidence supporting its therapeutic benefit.”
She concurred that the Israeli findings indicate that CDED with or without PEN appears effective for inducing and maintaining remission in this cohort of patients. “Assessment of composite diets such as the CDED is important since they can be incorporated into daily life. The relative efficacy of each of the included and excluded foods, however, is not clear,” she wrote.
She cautioned, however, that dietary therapy does not constitute maintenance therapy and its effects are not sustained after the reintroduction of whole foods. “Identification of sustainable dietary interventions for the prevention and treatment of IBD is increasingly a focus of research,” she wrote.
Dr. Sasson agreed that dietary therapy for CD should be centered on patient interests, goals, and disease states. “Adjunctive dietary measures might be considered in all interested patients as a method of improving gastrointestinal-related symptoms and quality of life, with the potential to achieve a higher and more sustained level of remission,” she wrote.
Both the authors and the commentator agreed on the need for larger randomized trials with long-term follow-up to guide treatment decisions and identify patients who might benefit from dietary intervention.
This study was funded by the Azrieli Foundation and Nestlé Health Science. Dr. Yanai reported financial relationships with Pfizer, AbbVie, Ferring, Janssen, Neopharm, Pfizer, and Takeda. Several coauthors disclosed financial ties to multiple private-sector companies. Dr. Sasson had no competing interests to declare.
This article was updated Dec. 1, 2021.
The Crohn’s disease exclusion diet (CDED), with or without partial enteral nutrition (PEN), effectively induced and maintained remission in adults with mild to moderate, biologic-naive CD, a randomized Israeli pilot study found.
The authors, led by Henit Yanai, MD, MBA, chief of the IBD Center at Rabin Medical Center in Petah Tikva, Israel, also suggested that dietary monotherapy might lead to durable endoscopic remission.
The study, published in The Lancet Gastroenterology & Hepatology, found about 60% of patients on CDED achieved clinical remission by week 6 without adding medications.
Furthermore, 80% of patients in remission at week 6 maintained clinical remission at week 24 on dietary monotherapy alone, allowing more than 50% of the intention-to-treat (ITT) population to achieve sustained remission at 6 months.
Dietary therapy resulted in a significant and progressive reduction in inflammatory markers such as C-reactive protein and fecal calprotectin. The benefit extended to mucosal healing, with 35% of the ITT population achieving endoscopic remission at 24 weeks.
Dr. Yanai explained the clinical context in which her group designed the study. “There were preliminary data regarding the efficacy of the dietary strategy for the induction of remission of mild CD in the pediatric population,” she said in an interview. “Additionally, there was an anecdotal experience in adults who reported benefits. Facing this and the lack of attractive alternatives for mild CD, we decided to examine the effectiveness of this therapeutic strategy in adults.”
Given the costs and side effects of medical treatment, interest in dietary monotherapy for IBD has been growing. As Dr. Yanai said, the CDED, a whole-foods regimen, plus PEN, has been found to help children with CD.
The CDED excludes proinflammatory food components associated with intestinal microbial dysbiosis, altered innate immunity, and impaired gut barrier function.
It involves increased consumption of fruits and vegetables, high-quality lean protein, complex carbohydrates, healthy oils, and fiber, while decreasing intake of inflammation-driving components such as animal and saturated vegetable fats, wheat, and dairy, as well as food additives such as emulsifiers, maltodextrin, and sulfites.
“The realization that exclusive enteral nutrition, which is based on liquid formulas, is effective for inducting remission in children PEN, which is a combination of liquid formulas and food, was less effective, led to the hypothesis that the mechanism might be the exclusion of dietary components that may lead to inflammation,” Dr. Yanai said. This theory, she added, derived from animal models showing that specific dietary components potentially drive inflammation.
The only current guideline-recommended nutritional therapy for remission induction is exclusive enteral nutrition (EEN) in pediatric CD.
The study
The open-label pilot trial led by Dr. Yanai, conducted at three medical centers, enrolled biologic-naive adults ages 18-55 with uncomplicated mild to moderate CD, with a disease duration of no more than 5 years. They had active disease on imaging and elevated C-reactive protein or fecal calprotectin.
During the period January 2017 to May 2020, eligible patients were randomly assigned 1 to 1 to CDED plus PEN enteral (n = 20) or CDED alone (n = 24) for 24 weeks. The primary endpoint was clinical remission, defined as a Harvey–Bradshaw Index score of less than 5 at week 6, an outcome was assessed in the ITT population, which consisted of those who used dietary therapy for at least 48 hours.
At week 6, 13 (68%) of 19 remaining patients in the CDED-plus-PEN group and 12 (57%) of 21 patients in the CDED-alone group had achieved clinical remission (P = .4618).
Among the 25 patients in remission 6 weeks out, 20 (80%) remained in sustained remission at week 24: 12 in the CDED-plus-PEN group and 8 in the CDED-alone group.
Moreover, 14 (35%) of 40 patients were in endoscopic remission at week 24: 8 on CDED plus PEN and 6 on CDED alone.
“CDED with or without partial enteral nutrition was effective for induction and maintenance of remission in adults with mild to moderate biologic-naive Crohn’s disease and might lead to endoscopic remission,” the authors wrote, adding that CDED for mild to moderate active disease should be assessed in a powered randomized controlled trial.
Compliance and adherence are obstacles to dietary therapies. Data for adults using exclusive enteral nutrition are conflicting, with poor compliance postulated to drive an inadequate response in some studies.
“Like every dietary treatment, adherence is challenging,” Dr. Yanai said. “However, when patients feel that it helps them, they have more incentive to follow the diet in the long run, and also once they quit and fare worse, they can go back and follow the first stage of CDED.”
She and her colleagues stressed the need for adequately powered randomized trials and recommended that the personalization of therapeutic diets in the future should take into account the need to deliver energy tailored to the nutritional and therapeutic goals of the patient.
Diets alone are not therapy
In an accompanying editorial, Alexa N. Sasson, MD, an IBD fellow at Massachusetts General Hospital and T.H. Chan Harvard School of Public Health, both in Boston, called diet “a promising and potentially modifiable risk factor with mounting evidence supporting its therapeutic benefit.”
She concurred that the Israeli findings indicate that CDED with or without PEN appears effective for inducing and maintaining remission in this cohort of patients. “Assessment of composite diets such as the CDED is important since they can be incorporated into daily life. The relative efficacy of each of the included and excluded foods, however, is not clear,” she wrote.
She cautioned, however, that dietary therapy does not constitute maintenance therapy and its effects are not sustained after the reintroduction of whole foods. “Identification of sustainable dietary interventions for the prevention and treatment of IBD is increasingly a focus of research,” she wrote.
Dr. Sasson agreed that dietary therapy for CD should be centered on patient interests, goals, and disease states. “Adjunctive dietary measures might be considered in all interested patients as a method of improving gastrointestinal-related symptoms and quality of life, with the potential to achieve a higher and more sustained level of remission,” she wrote.
Both the authors and the commentator agreed on the need for larger randomized trials with long-term follow-up to guide treatment decisions and identify patients who might benefit from dietary intervention.
This study was funded by the Azrieli Foundation and Nestlé Health Science. Dr. Yanai reported financial relationships with Pfizer, AbbVie, Ferring, Janssen, Neopharm, Pfizer, and Takeda. Several coauthors disclosed financial ties to multiple private-sector companies. Dr. Sasson had no competing interests to declare.
This article was updated Dec. 1, 2021.
FROM LANCET GASTROENTEROLOGY & HEPATOLOGY
Does vitamin D benefit only those who are deficient?
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
, suggests a new large-scale analysis.
Data on more than 380,000 participants gathered from 35 studies showed that, overall, there is no significant relationship between 25(OH)D concentrations, a clinical indicator of vitamin D status, and the incidence of coronary heart disease (CHD), stroke, or all-cause death, in a Mendelian randomization analysis.
However, Stephen Burgess, PhD, and colleagues showed that, in vitamin D–deficient individuals, each 10 nmol/L increase in 25(OH)D concentrations reduced the risk of all-cause mortality by 31%.
The research, published in The Lancet Diabetes & Endocrinology, also suggests there was a nonsignificant link between 25(OH)D concentrations and stroke and CHD, but again, only in vitamin D deficient individuals.
In an accompanying editorial, Guillaume Butler-Laporte, MD, and J. Brent Richards, MD, praise the researchers on their study methodology.
They add that the results “could have important public health and clinical consequences” and will “allow clinicians to better weigh the potential benefits of supplementation against its risk,” such as financial cost, “for better patient care – particularly among those with frank vitamin D deficiency.”
They continue: “Given that vitamin D deficiency is relatively common and vitamin D supplementation is safe, the rationale exists to test the effect of vitamin D supplementation in those with deficiency in large-scale randomized controlled trials.”
However, Dr. Butler-Laporte and Dr. Richards, of the Lady Davis Institute, Jewish General Hospital, Montreal, also note the study has several limitations, including the fact that the lifetime exposure to lower vitamin D levels captured by Mendelian randomization may result in larger effect sizes than in conventional trials.
Prior RCTS underpowered to detect effects of vitamin D supplements
“There are several potential mechanisms by which vitamin D could be protective for cardiovascular mortality, including mechanisms linking low vitamin D status with hyperparathyroidism and low serum calcium and phosphate,” write Dr. Burgess of the MRC Biostatistics Unit, University of Cambridge (England), and coauthors.
They also highlight that vitamin D is “further implicated in endothelial cell function” and affects the transcription of genes linked to cell division and apoptosis, providing “potential mechanisms implicating vitamin D for cancer.”
The researchers note that, while epidemiologic studies have “consistently” found a link between 25(OH)D levels and increased risk of cardiovascular disease, all-cause mortality, and other chronic diseases, several large trials of vitamin D supplementation have reported “null results.”
They argue, however, that many of these trials have recruited individuals “irrespective of baseline 25(OH)D concentration” and have been underpowered to detect the effects of supplementation.
To overcome these limitations, the team gathered data from the UK Biobank, the European Prospective Investigation Into Cancer and Nutrition Cardiovascular Disease (EPIC-CVD) study, 31 studies from the Vitamin D Studies Collaboration (VitDSC), and two Copenhagen population-based studies.
They first performed an observational study that included 384,721 individuals from the UK Biobank and 26,336 from EPIC-CVD who had a valid 25(OH)D measurement and no previously known cardiovascular disease at baseline.
Researchers also included 67,992 participants from the VitDSC studies who did not have previously known cardiovascular disease. They analyzed 25(OH)D concentrations, conventional cardiovascular risk factors, and major incident cardiovascular morbidity and mortality using individual participant data.
The results showed that, at low 25(OH)D concentrations, there was an inverse association between 25(OH)D and incident CHD, stroke, and all-cause mortality.
Next, the team conducted a Mendelian randomization analysis on 333,002 individuals from the UK Biobank and 26,336 from EPIC-CVD who were of European ancestry and had both a valid 25(OH)D measurement and genetic data that passed quality-control steps.
Information on 31,362 participants in the Copenhagen population-based studies was also included, giving a total of 386,406 individuals, of whom 33,546 had CHD, 18,166 had a stroke, and 27,885 died.
The mean age of participants ranged from 54.8 to 57.5 years, and between 53.4% and 55.4% were female.
Up to 7% of study participants were vitamin D deficient
The 25(OH)D analysis indicated that 3.9% of UK Biobank and 3.7% of Copenhagen study participants were deficient, compared with 6.9% in EPIC-CVD.
Across the full range of 25(OH)D concentrations, there was no significant association between genetically predicted 25(OH)D levels and CHD, stroke, or all-cause mortality.
However, restricting the analysis to individuals deemed vitamin D deficient (25[OH]D concentration < 25 nmol/L) revealed there was “strong evidence” for an inverse association with all-cause mortality, at an odds ratio per 10 nmol/L increase in genetically predicted 25(OH)D concentration of 0.69 (P < .0001), the team notes.
There were also nonsignificant associations between being in the deficient stratum and CHD, at an odds ratio of 0.89 (P = .14), and stroke, at an odds ratio of 0.85 (P = .09).
Further analysis suggests the association between 25(OH)D concentrations and all-cause mortality has a “clear threshold shape,” the researchers say, with evidence of an inverse association at concentrations below 40 nmol/L and null associations above that threshold.
They acknowledge, however, that their study has several potential limitations, including the assumption in their Mendelian randomization that the “only causal pathway from the genetic variants to the outcome is via 25(OH)D concentrations.”
Moreover, the genetic variants may affect 25(OH)D concentrations in a different way from “dietary supplementation or other clinical interventions.”
They also concede that their study was limited to middle-aged participants of European ancestries, which means the findings “might not be applicable to other populations.”
The study was funded by the British Heart Foundation, Medical Research Council, National Institute for Health Research, Health Data Research UK, Cancer Research UK, and International Agency for Research on Cancer. Dr. Burgess has reported no relevant financial relationships. Disclosures for the other authors are listed with the article.
A version of this article first appeared on Medscape.com.
U.S. obesity rates soar in early adulthood
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Obesity rates among “emerging adults” aged 18-25 have soared in the United States in recent decades with the mean body mass index (BMI) for these young adults now in the overweight category, according to research highlighting troubling trends in an often-overlooked age group.
While similar patterns have been observed in other age groups, including adolescents (ages 12-19) and young adults (ages 20-39) across recent decades, emerging adulthood tends to get less attention in the evaluation of obesity trends.
“Emerging adulthood may be a key period for preventing and treating obesity given that habits formed during this period often persist through the remainder of the life course,” write the authors of the study, which was published online Nov. 23 in JAMA.
“There is an urgent need for research on risk factors contributing to obesity during this developmental stage to inform the design of interventions as well as policies aimed at prevention,” they add.
They found that by 2018 a third of all young adults had obesity, compared with just 6% at the beginning of the study periods in 1976.
Studying the ages of transition
The findings are from an analysis of 8,015 emerging adults aged 18-25 in the cross-sectional National Health and Nutrition Examination Survey (NHANES), including NHANES II (1976-1980), NHANES III (1988-1994), and the continuous NHANES cycles from 1999 through 2018.
About half (3,965) of participants were female, 3,037 were non-Hispanic Black, and 2,386 met the criteria for household poverty.
The results showed substantial increases in mean BMI among emerging adults from a level in the normal range, at 23.1 kg/m2, in 1976-1980, increasing to 27.7 kg/m2 (overweight) in 2017-2018 (P = .006).
The prevalence of obesity (BMI 30.0 kg/m2 or higher) in the emerging adult age group soared from 6.2% between 1976-1980 to 32.7% in 2017-2018 (P = .007).
Meanwhile, the rate of those with normal/healthy weight (BMI 18.5-24.9 kg/m2) dropped from 68.7% to 37.5% (P = .005) over the same period.
Sensitivity analyses that were limited to continuous NHANES cycles showed similar results.
First author Alejandra Ellison-Barnes, MD, MPH, said the trends are consistent with rising obesity rates in the population as a whole – other studies have shown increases in obesity among children, adolescents, and adults over the same period – but are nevertheless striking, she stressed.
Young adults now fall into overweight category
“While we were not surprised by the general trend, given what is known about the increasing prevalence of obesity in both children and adults, we were surprised by the magnitude of the increase in prevalence and that the mean BMI in this age group now falls in the overweight range,” Dr. Ellison-Barnes, of the Division of General Internal Medicine, Johns Hopkins University School of Medicine, Baltimore, told this news organization.
She said she is not aware of other studies that have looked at obesity trends specifically among emerging adults.
However, considering the substantial life changes and growing independence, the life stage is important to understand in terms of dietary/lifestyle patterns.
“We theorize that emerging adulthood is a critical period for obesity development given that it is a time when individuals are often undergoing major life transitions such as leaving home, attending higher education, entering the workforce, and developing new relationships,” she emphasized.
As far as causes are concerned, “societal and cultural trends in these areas over the past several decades may have played a role in the observed changes,” she speculated.
The study population was limited to non-Hispanic Black and non-Hispanic White individuals due to changes in how NHANES assessed race and ethnicity over time. Therefore, a study limitation is that the patterns observed may not be generalizable to other races and ethnicities, the authors note.
However, considering the influence lifestyle changes can have, early adulthood “may be an ideal time to intervene in the clinical setting to prevent, manage, or reverse obesity to prevent adverse health outcomes in the future,” Dr. Ellison-Barnes said.
Dr. Ellison-Barnes has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.





