User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Biden vaccine mandate rule could be ready within weeks
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
Seizure a first sign of COVID in kids?
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Growing proportion of cardiac arrests in U.S. considered opioid related
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
Observational data indicate that the number of hospitalizations for cardiac arrests linked to opioid use roughly doubled from 2012 to 2018.
“This was an observational study, so we cannot conclude that all of the arrests were caused by opioids, but the findings do suggest the opioid epidemic is a contributor to increasing rates,” Senada S. Malik, of the University of New England, Portland, Maine, reported at the virtual annual congress of the European Society of Cardiology.
The data were drawn from the Nationwide Inpatient Sample (NIS) from 2012 to 2018, the most recent period available. Cardiac arrests were considered opioid related if there was a secondary diagnosis of opioid disease. The rates of opioid-associated hospitalizations for these types of cardiac arrests climbed from about 800 per year in 2012 to 1,500 per year in 2018, a trend that was statistically significant (P < .05).
The profile of patients with an opioid-associated cardiac arrest was different from those without secondary diagnosis of opioid disease. This included a younger age and lower rates of comorbidities: heart failure (21.2% vs. 40.6%; P < .05), renal failure (14.3% vs. 30.2%; P < .05), diabetes (19.5% vs. 35.4%; P < .05), and hypertension (43.4% vs. 64.9%; P < .05).
Mortality from opioid-associated cardiac arrest is lower
These features might explain the lower rate of in-hospital mortality for opioid-associated cardiac arrests (56.7% vs. 61.2%), according to Ms. Malik, who performed this research in collaboration with Wilbert S. Aronow, MD, director of cardiology research, Westchester Medical Center, Valhalla, N.Y.
When compared to those without a history of opioid use on admission, those with opioid-associated cardiac arrest were more likely to be depressed (18.8% vs. 9.0%), to smoke (37.0% vs. 21.8%) and to abuse alcohol (16.9% vs. 7.1%), according to the NIS data.
While these findings are based on cardiac arrests brought to a hospital, some opioid-induced cardiac arrests never result in hospital admission, according to data included in a recently issued scientific statement from the American Heart Association.
Rate of opioid-associated cardiac arrests underestimated
In that statement, which was focused on opioid-associated out-of-hospital cardiac arrests (OA-OHCA), numerous studies were cited to support the conclusion that these events are common and underestimated. One problem is that opioid-induced cardiac arrests are not always accurately differentiated from cardiac arrests induced by use of other substances, such as barbiturates, cocaine, or alcohol.
For this and other reasons, the data are inconsistent. One study based on emergency medical service (EMS) response data concluded that 9% of all out-of-hospital cardiac arrests are opioid associated.
In another study using potentially more accurate autopsy data, 60% of the non–cardiac-associated cardiac arrests were found to occur in individuals with potentially lethal serum concentrations of opioids. As 40% of out-of-hospital cardiac arrests were considered non–cardiac related, this suggested that 15% of all out-of-hospital cardiac arrests are opioid related.
In the NIS data, the incident curves of opioid-related cardiac arrests appeared to be flattening in 2018, the last year of data collection, but there was no indication they were declining.
Patterns of opioid-induced cardiac arrests evolving
The patterns of opioid-induced cardiac arrest have changed and are likely to continue to change in response to the evolving opioid epidemic, according to the AHA scientific statement. The authors described three waves of opioid abuse. The first, which was related to the promotion of prescription opioids to treat chronic pain that ultimately led to high rates of opioid addiction, peaked in 2012 when rates of these prescriptions began to fall. At that time a second wave, attributed to patients switching to less expensive nonprescription heroin, was already underway. A third wave, attributed to growth in the use of synthetic opioids, such as fentanyl, began in 2013 and is ongoing, according to data cited in the AHA statement.
Recognizing the role of opioids in rising rates of cardiac arrest is important for promoting strategies of effective treatment and prevention, according to Cameron Dezfulian, MD, medical director of the adult congenital heart disease program at Texas Children’s Hospital, Houston. Dr. Dezfulian was vice chair and leader of the writing committee for the AHA scientific statement on OA-OHCA. He said there are plenty of data to support the need for greater attention to the role of opioids in cardiac arrest.
“The recent data affirms the trends many of us have observed without our emergency rooms and ICUs: a steady increase in the proportion of OA-OHCA, primarily in young and otherwise healthy individuals,” he said.
He calls not only for more awareness at the front lines of health are but also for a more comprehensive approach.
“Public health policies and community- and hospital-based interventions are needed to reduce the mortality due to OA-OHCA, which is distinct from the traditional cardiac etiology,” Dr. Dezfulian said.
In opioid-induced cardiac arrest, as in other types of cardiac arrest, prompt initiation of cardiopulmonary resuscitation is essential, but early administration of the opioid antagonist naloxone can also be lifesaving, according to treatment strategies outlined in the AHA scientific statement. The fact that OA-OHCA typically occur in patients with structurally and electrophysiologically normal hearts is emphasized in the AHA statement. So is the enormous public health toll of OA-OHCA.
Death due to opioid overdose, which includes cardiac arrests, is now the leading cause of mortality in the U.S. among individuals between the ages of 25 and 64 years, according to the statement.
Ms. Malik reports no potential conflicts of interest. Dr. Dezfulian reports a financial relationship with Mallinckrodt.
FROM ESC 2021
Sweeping new vaccine mandates will impact most U.S. workers
, including sweeping vaccine mandates that will affect 100 million American workers, nearly two-thirds of the country’s workforce.
“As your president, I’m announcing tonight a new plan to get more Americans vaccinated to combat those blocking public health,” he said Sept. 9.
As part of a six-part plan unveiled in a speech from the State Dining Room of the White House, President Biden said he would require vaccinations for nearly 4 million federal workers and the employees of companies that contract with the federal government.
He has also directed the Occupational Safety and Health Administration to develop a rule that will require large employers -- those with at least 100 employees -- to ensure their workers are vaccinated or tested weekly.
Nearly 17 million health care workers will face new vaccine mandates as part of the conditions of participation in the Medicare and Medicaid programs.
President Biden said the federal government will require staff at federally funded Head Start programs and schools to be vaccinated. He’s also calling on all states to mandate vaccines for teachers.
“A distinct minority of Americans, supported by a distinct minority of elected officials, are keeping us from turning the corner,” PresidentBiden said. “These pandemic politics, as I refer to them, are making people sick, causing unvaccinated people to die.”
One public health official said he was glad to see the president’s bold action.
“What I saw today was the federal government trying to use its powers to create greater safety in the American population,” said Ashish K. Jha, MD, dean of the school of public health at Brown University, Providence, R.I., in a call with reporters after the speech.
National Nurses United, the largest union of registered nurses in the United States, issued a statement in support of President Biden’s new vaccination requirements, but pushed back on his language.
“…as advocates for public health, registered nurses want to be extremely clear: There is no such thing as a pandemic of only the unvaccinated. The science of epidemiology tells us there is just one deadly, global pandemic that has not yet ended, and we are all in it together. To get out of it, we must act together. All of us,” the statement says.
A host of other professional groups, including the American Medical Association and the Association of State and Territorial Health Officials, also issued statements of support for President Biden’s plan.
But the plan was not well received by all.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden Administration,” said Georgia Governor Brian Kemp, a Republican, in a Tweet.
The National Council for Occupational Safety and Health called the plan “a missed opportunity” because it failed to include workplace protections for essential workers such as grocery, postal, and transit workers.
“Social distancing, improved ventilation, shift rotation, and protective equipment to reduce exposure are important components of an overall plan to reduce risk and stop the virus. These tools are missing from the new steps President Biden announced today,” said Jessica Martinez, co-executive director of the group.
In addition to the new vaccination requirements, President Biden said extra doses would be on the way for people who have already been fully vaccinated in order to protect against waning immunity, starting on Sept. 20. But he noted that those plans would be contingent on the Food and Drug Administration’s approval for third doses and the Centers for Disease Control and Prevention’s recommendation of the shots.
President Biden pledged to use the Defense Production Act to ramp up production of at-home tests, which have been selling out across the nation as the Delta variant spreads.
He also announced plans to expand access to COVID-19 testing, including offering testing for free at thousands of pharmacies nationwide and getting major retailers to sell at-home COVID-19 tests at cost.
The BinaxNow test kit, which currently retails for $23.99, will now cost about $15 for two tests at Kroger, Amazon, and Walmart, according to the White House. Food banks and community health centers will get free tests, too.
He called on states to set up COVID-19 testing programs at all schools.
Jha said that in his view, the big, game-changing news out of the president’s speech was the expansion of testing.
“Our country has failed to deploy tests in a way that can really bring this pandemic under control,” Jha said. “There are plenty of reasons, data, experience to indicate that if these were widely available, it would make a dramatic difference in reducing infection numbers across our country.”.
Dr. Jha said the private market had not worked effectively to make testing more widely available, so it was “absolutely a requirement of the federal government to step in and make testing more widely available,” he said.
President Biden also announced new economic stimulus programs, saying he’s expanding loan programs to small businesses and streamlining the loan forgiveness process.
President Biden said he’s boosting help for overburdened hospitals, doubling the number of federal surge response teams sent to hard-hit areas to reduce the strain on local health care workers. He said he would increase the pace of antibody treatments to states by 50%.
“We made so much progress during the past 7 months of this pandemic. Even so, we remain at a critical moment, a critical time,” he said. “We have the tools. Now, we just have to finish the job with truth, with science, with confidence and together as one nation.”
A version of this article first appeared on WebMD.com.
, including sweeping vaccine mandates that will affect 100 million American workers, nearly two-thirds of the country’s workforce.
“As your president, I’m announcing tonight a new plan to get more Americans vaccinated to combat those blocking public health,” he said Sept. 9.
As part of a six-part plan unveiled in a speech from the State Dining Room of the White House, President Biden said he would require vaccinations for nearly 4 million federal workers and the employees of companies that contract with the federal government.
He has also directed the Occupational Safety and Health Administration to develop a rule that will require large employers -- those with at least 100 employees -- to ensure their workers are vaccinated or tested weekly.
Nearly 17 million health care workers will face new vaccine mandates as part of the conditions of participation in the Medicare and Medicaid programs.
President Biden said the federal government will require staff at federally funded Head Start programs and schools to be vaccinated. He’s also calling on all states to mandate vaccines for teachers.
“A distinct minority of Americans, supported by a distinct minority of elected officials, are keeping us from turning the corner,” PresidentBiden said. “These pandemic politics, as I refer to them, are making people sick, causing unvaccinated people to die.”
One public health official said he was glad to see the president’s bold action.
“What I saw today was the federal government trying to use its powers to create greater safety in the American population,” said Ashish K. Jha, MD, dean of the school of public health at Brown University, Providence, R.I., in a call with reporters after the speech.
National Nurses United, the largest union of registered nurses in the United States, issued a statement in support of President Biden’s new vaccination requirements, but pushed back on his language.
“…as advocates for public health, registered nurses want to be extremely clear: There is no such thing as a pandemic of only the unvaccinated. The science of epidemiology tells us there is just one deadly, global pandemic that has not yet ended, and we are all in it together. To get out of it, we must act together. All of us,” the statement says.
A host of other professional groups, including the American Medical Association and the Association of State and Territorial Health Officials, also issued statements of support for President Biden’s plan.
But the plan was not well received by all.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden Administration,” said Georgia Governor Brian Kemp, a Republican, in a Tweet.
The National Council for Occupational Safety and Health called the plan “a missed opportunity” because it failed to include workplace protections for essential workers such as grocery, postal, and transit workers.
“Social distancing, improved ventilation, shift rotation, and protective equipment to reduce exposure are important components of an overall plan to reduce risk and stop the virus. These tools are missing from the new steps President Biden announced today,” said Jessica Martinez, co-executive director of the group.
In addition to the new vaccination requirements, President Biden said extra doses would be on the way for people who have already been fully vaccinated in order to protect against waning immunity, starting on Sept. 20. But he noted that those plans would be contingent on the Food and Drug Administration’s approval for third doses and the Centers for Disease Control and Prevention’s recommendation of the shots.
President Biden pledged to use the Defense Production Act to ramp up production of at-home tests, which have been selling out across the nation as the Delta variant spreads.
He also announced plans to expand access to COVID-19 testing, including offering testing for free at thousands of pharmacies nationwide and getting major retailers to sell at-home COVID-19 tests at cost.
The BinaxNow test kit, which currently retails for $23.99, will now cost about $15 for two tests at Kroger, Amazon, and Walmart, according to the White House. Food banks and community health centers will get free tests, too.
He called on states to set up COVID-19 testing programs at all schools.
Jha said that in his view, the big, game-changing news out of the president’s speech was the expansion of testing.
“Our country has failed to deploy tests in a way that can really bring this pandemic under control,” Jha said. “There are plenty of reasons, data, experience to indicate that if these were widely available, it would make a dramatic difference in reducing infection numbers across our country.”.
Dr. Jha said the private market had not worked effectively to make testing more widely available, so it was “absolutely a requirement of the federal government to step in and make testing more widely available,” he said.
President Biden also announced new economic stimulus programs, saying he’s expanding loan programs to small businesses and streamlining the loan forgiveness process.
President Biden said he’s boosting help for overburdened hospitals, doubling the number of federal surge response teams sent to hard-hit areas to reduce the strain on local health care workers. He said he would increase the pace of antibody treatments to states by 50%.
“We made so much progress during the past 7 months of this pandemic. Even so, we remain at a critical moment, a critical time,” he said. “We have the tools. Now, we just have to finish the job with truth, with science, with confidence and together as one nation.”
A version of this article first appeared on WebMD.com.
, including sweeping vaccine mandates that will affect 100 million American workers, nearly two-thirds of the country’s workforce.
“As your president, I’m announcing tonight a new plan to get more Americans vaccinated to combat those blocking public health,” he said Sept. 9.
As part of a six-part plan unveiled in a speech from the State Dining Room of the White House, President Biden said he would require vaccinations for nearly 4 million federal workers and the employees of companies that contract with the federal government.
He has also directed the Occupational Safety and Health Administration to develop a rule that will require large employers -- those with at least 100 employees -- to ensure their workers are vaccinated or tested weekly.
Nearly 17 million health care workers will face new vaccine mandates as part of the conditions of participation in the Medicare and Medicaid programs.
President Biden said the federal government will require staff at federally funded Head Start programs and schools to be vaccinated. He’s also calling on all states to mandate vaccines for teachers.
“A distinct minority of Americans, supported by a distinct minority of elected officials, are keeping us from turning the corner,” PresidentBiden said. “These pandemic politics, as I refer to them, are making people sick, causing unvaccinated people to die.”
One public health official said he was glad to see the president’s bold action.
“What I saw today was the federal government trying to use its powers to create greater safety in the American population,” said Ashish K. Jha, MD, dean of the school of public health at Brown University, Providence, R.I., in a call with reporters after the speech.
National Nurses United, the largest union of registered nurses in the United States, issued a statement in support of President Biden’s new vaccination requirements, but pushed back on his language.
“…as advocates for public health, registered nurses want to be extremely clear: There is no such thing as a pandemic of only the unvaccinated. The science of epidemiology tells us there is just one deadly, global pandemic that has not yet ended, and we are all in it together. To get out of it, we must act together. All of us,” the statement says.
A host of other professional groups, including the American Medical Association and the Association of State and Territorial Health Officials, also issued statements of support for President Biden’s plan.
But the plan was not well received by all.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden Administration,” said Georgia Governor Brian Kemp, a Republican, in a Tweet.
The National Council for Occupational Safety and Health called the plan “a missed opportunity” because it failed to include workplace protections for essential workers such as grocery, postal, and transit workers.
“Social distancing, improved ventilation, shift rotation, and protective equipment to reduce exposure are important components of an overall plan to reduce risk and stop the virus. These tools are missing from the new steps President Biden announced today,” said Jessica Martinez, co-executive director of the group.
In addition to the new vaccination requirements, President Biden said extra doses would be on the way for people who have already been fully vaccinated in order to protect against waning immunity, starting on Sept. 20. But he noted that those plans would be contingent on the Food and Drug Administration’s approval for third doses and the Centers for Disease Control and Prevention’s recommendation of the shots.
President Biden pledged to use the Defense Production Act to ramp up production of at-home tests, which have been selling out across the nation as the Delta variant spreads.
He also announced plans to expand access to COVID-19 testing, including offering testing for free at thousands of pharmacies nationwide and getting major retailers to sell at-home COVID-19 tests at cost.
The BinaxNow test kit, which currently retails for $23.99, will now cost about $15 for two tests at Kroger, Amazon, and Walmart, according to the White House. Food banks and community health centers will get free tests, too.
He called on states to set up COVID-19 testing programs at all schools.
Jha said that in his view, the big, game-changing news out of the president’s speech was the expansion of testing.
“Our country has failed to deploy tests in a way that can really bring this pandemic under control,” Jha said. “There are plenty of reasons, data, experience to indicate that if these were widely available, it would make a dramatic difference in reducing infection numbers across our country.”.
Dr. Jha said the private market had not worked effectively to make testing more widely available, so it was “absolutely a requirement of the federal government to step in and make testing more widely available,” he said.
President Biden also announced new economic stimulus programs, saying he’s expanding loan programs to small businesses and streamlining the loan forgiveness process.
President Biden said he’s boosting help for overburdened hospitals, doubling the number of federal surge response teams sent to hard-hit areas to reduce the strain on local health care workers. He said he would increase the pace of antibody treatments to states by 50%.
“We made so much progress during the past 7 months of this pandemic. Even so, we remain at a critical moment, a critical time,” he said. “We have the tools. Now, we just have to finish the job with truth, with science, with confidence and together as one nation.”
A version of this article first appeared on WebMD.com.
COVID-19 linked to rise in suicide-related ED visits among youth
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
FROM JAMA PSYCHIATRY
STOP-DAPT 2 ACS: 1 month of DAPT proves inadequate for patients with recent ACS
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
One month of dual antiplatelet therapy followed by 11 months of clopidogrel monotherapy failed to prove noninferiority to 12 unbroken months of DAPT for net clinical benefit in a multicenter Japanese trial that randomized more than 4,000 patients who underwent percutaneous coronary intervention (PCI) after a recent acute coronary syndrome episode.
The outcomes showed that while truncating DAPT duration could, as expected, cut major bleeding episodes roughly in half, it also led to a significant near doubling of myocardial infarction and showed a strong trend toward also increasing a composite tally of several types of ischemic events. These data were reported this week by Hirotoshi Watanabe, MD, PhD, at the virtual annual congress of the European Society of Cardiology. All study patients had undergone PCI with cobalt-chromium everolimus-eluting (CCEE) coronary stents (Xience).
These findings from the STOPDAPT-2 ACS trial highlighted the limits of minimizing DAPT after PCI in patients at high ischemic risk, such as after an acute coronary syndrome (ACS) event.
It also was a counterpoint to a somewhat similar study also reported at the congress, MASTER DAPT, which showed that 1 month of DAPT was noninferior to 3 or more months of DAPT for net clinical benefit in a distinctly different population of patients undergoing PCI (and using a different type of coronary stent) – those at high bleeding risk and with only about half the patients having had a recent ACS.
The results of STOPDAPT-2 ACS “do not support use of 1 month of DAPT followed by P2Y12 inhibitor monotherapy with clopidogrel compared with standard DAPT,” commented Robert A. Byrne, MBBCh, PhD, designated discussant for the report and professor at the RCSI University of Medicine and Health Sciences in Dublin.
“Although major bleeding was significantly reduced with this approach, there appeared to be a significant increase in adverse ischemic events, and there was a clear signal in relation to overall mortality, the ultimate arbiter of net clinical benefit,” added Dr. Byrne, who is also director of cardiology at Mater Private Hospital in Dublin.
He suggested that a mechanistic explanation for the signal of harm seem in STOPDAPT-2 ACS was the relatively low potency of clopidogrel (Plavix) as an antiplatelet agent, compared with other P2Y12 inhibitors such as prasugrel (Effient) and ticagrelor (Brilinta), as well as the genetically driven variability in response to clopidogrel that’s also absent with alternative agents.
These between-agent differences are of “particular clinical relevance in the early aftermath of an ACS event,” Dr. Byrne said.
12-month DAPT remains standard for PCI patients with recent ACS
The totality of clinical evidence “continues to support a standard 12-month duration of DAPT – using aspirin and either prasugrel or ticagrelor – as the preferred default approach,” Dr. Byrne concluded.
He acknowledged that an abbreviated duration of DAPT followed by P2Y12 inhibitor monotherapy “might be considered as an alternative.” In patients following an ACS event who do not have high risk for bleeding, he said, the minimum duration of DAPT should be at least 3 months and with preferential use of a more potent P2Y12 inhibitor.
Twelve months of DAPT treatment with aspirin and a P2Y12 inhibitor for patients following PCI “remains the standard of care in guidelines,” noted Marco Roffi, MD, a second discussant at the congress. But several questions remain, he added, such as which P2Y12 inhibitors work best and whether DAPT can be less than 12 months.
“The investigators [for STOPDAPT-2 ACS] pushed these questions to the limit with 1 month of DAPT and clopidogrel monotherapy,” said Dr. Roffi, professor and director of interventional cardiology at University Hospital, Geneva.
“This was a risky bet, and the investigators lost by not proving noninferiority and with excess ischemic events,” he commented.
First came STOPDAPT-2
Dr. Watanabe and colleagues designed STOPDAPT-2 ACS as a follow-up to their prior STOPDAPT-2 trial, which randomly assigned slightly more than 3000 patients at 90 Japanese centers to the identical two treatment options: 1 month of DAPT followed by 11 months of clopidogrel monotherapy or 12 months of DAPT, except the trial enrolled all types of patients undergoing PCI. This meant that a minority, 38%, had a recent ACS event, while the remaining patients had chronic coronary artery disease. As in STOPDAPT-2 ACS, all patients in STOPDAPT-2 had received a CCEE stent.
STOPDAPT-2 also used the same primary endpoint to tally net clinical benefit as STOPDAPT-2 ACS: cardiovascular death, MI, stroke of any type, definite stent thrombosis, or TIMI major or minor bleeding classification.
In STOPDAPT-2, using the mixed population with both recent ACS and chronic coronary disease, the regimen of 1 month of DAPT followed by 11 months of clopidogrel monotherapy was both noninferior to and superior to 12 months of DAPT, reducing the net adverse-event tally by 36% relative to 12-month DAPT and by an absolute reduction of 1.34%, as reported in 2019.
Despite this superiority, the results from STOPDAPT-2 had little impact on global practice, commented Kurt Huber, MD, professor and director of the cardiology ICU at the Medical University of Vienna.
“STOP-DAPT-2 did not give us a clear message with respect to reducing antiplatelet treatment after 1 month. I thought that for ACS patients 1 month might be too short,” Dr. Huber said during a press briefing.
Focusing on post-ACS
To directly address this issue, the investigators launched STOPDAPT-2 ACS, which used the same design as the preceding study but only enrolled patients soon after an ACS event. The trial included for its main analysis 3,008 newly enrolled patients with recent ACS, and 1,161 patients who had a recent ACS event and had been randomly assigned in STOPDAPT-2, creating a total study cohort for the new analysis of 4136 patients treated and followed for the study’s full 12 months.
The patients averaged 67 years old, 79% were men, and 30% had diabetes. About 56% had a recent ST-elevation MI, about 20% a recent non–ST-elevation MI, and the remaining 24% had unstable angina. For their unspecified P2Y12 inhibition, roughly half the patients received clopidogrel and the rest received prasugrel. Adherence to the two assigned treatment regimens was very good, with a very small number of patients not adhering to their assigned protocol.
The composite adverse event incidence over 12 months was 3.2% among those who received 1-month DAPT and 2.83% in those on DAPT for 12 months, a difference that failed to achieve the prespecified definition of noninferiority for 1-month DAPT, reported Dr. Watanabe, an interventional cardiologist at Kyoto University.
The ischemic event composite was 50% lower among those on 12-month DAPT, compared with 1 month of DAPT, a difference that just missed significance. The rate of MI was 91% higher with 1-month DAPT, compared with 12 months, a significant difference.
One-month DAPT also significantly reduced the primary measure of bleeding events – the combination of TIMI major and minor bleeds – by a significant 54%, compared with 12-month DAPT. A second metric of clinically meaningful bleeds, those that meet either the type 3 or 5 definition of the Bleeding Academic Research Consortium, were reduced by a significant 59% by 1-month DAPT, compared with 12 months of DAPT.
The new findings from STOPDAPT-2 ACS contrasted with those from MASTER DAPT, but in an explicable way that related to different patient types, different P2Y12 inhibitors, different treatment durations, and different stents.
“We’ve seen in MASTER DAPT that if you use the right stent and use ticagrelor for monotherapy there may be some ability to shorten DAPT, but we still do not know what would happen in patients with very high ischemic risk,” concluded Dr. Huber.
“A reduction in DAPT duration might work in patients without high bleeding risk, but I would exclude patients with very high ischemic risk,” he added. “I also can’t tell you whether 1 month or 3 months is the right approach, and I think clopidogrel is not the right drug to use for monotherapy after ACS.”
STOPDAPT-2 and STOPDAPT-2 ACS were both sponsored by Abbott Vascular, which markets the CCEE (Xience) stents used in both studies. Dr. Watanabe has received lecture fees from Abbott and from Daiichi-Sankyo. Dr. Byrne has received research funding from Abbott Vascular as well as from Biosensors, Biotronik, and Boston Scientific. Roffi has received research funding from Biotronik, Boston Scientific, GE Healthcare, Medtronic, and Terumo. Dr. Huber has received lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, Pfizer, Sanofi-Aventis, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Elderly mice receive the gift of warmth
Steal from the warm, give to the cold
If there’s one constant in life other than taxes, it’s elderly people moving to Florida. The Sunshine State’s reputation as a giant retirement home needs no elaboration, but why do senior citizens gravitate there? Well, many reasons, but a big one is that, the older you get, the more susceptible and sensitive you are to the cold. And now, according to a new study, we may have identified a culprit.
Researchers from Yale University examined a group of mice and found that the older ones lacked ICL2 cells in their fatty tissue. These cells, at least in younger mice, help restore body heat when exposed to cold temperatures. Lacking these cells meant that older mice had a limited ability to burn their fat and raise their temperature in response to cold.
Well, job done, all we need to do now is stimulate production of ICL2 cells in elderly people, and they’ll be able to go outside in 80-degree weather without a sweater again. Except there’s a problem. In a cruel twist of fate, when the elderly mice were given a molecule to boost ICL2 cell production, they actually became less tolerant of the cold than at baseline. Oops.
The scientists didn’t give up though, and gave their elderly mice ICL2 cells from young mice. This finally did the trick, though we have to admit, if that treatment does eventually scale up to humans, the prospect of a bunch of senior citizens taking ICL2 cells from young people to stay warm does sound a bit like a bad vampire movie premise. “I vant to suck your immune cell group 2 innate lymphoid cells!” Not the most pithy catch phrase in the world.
Grocery store tapping your subconscious? It’s a good thing
We all know there’s marketing and functionality elements to grocery stores and how they’re set up for your shopping pleasure. But what if I told you that the good old supermarket subconscious trick works on how healthy food decisions are?
In a recent study, researchers at the University of Southampton in England found that if you placed a wider selection of fruits and vegetables near the entrances and more nonfood items near checkouts, sales decreased on the sweets and increased on the produce. “The findings of our study suggest that a healthier store layout could lead to nearly 10,000 extra portions of fruit and vegetables and approximately 1,500 fewer portions of confectionery being sold on a weekly basis in each store,” lead author Dr. Christina Vogel explained.
You’re probably thinking that food placement studies aren’t new. That’s true, but this one went above and beyond. Instead of just looking at the influence placement has on purchase, this one took it further by trying to reduce the consumers’ “calorie opportunities” and examining the effect on sales. Also, customer loyalty, patterns, and diets were taken into account across multiple household members.
The researchers think shifting the layouts in grocery stores could shift people’s food choices, producing a domino effect on the population’s overall diet. With obesity, diabetes, and cardiology concerns always looming, swaying consumers toward healthier food choices makes for better public health overall.
So if you feel like you’re being subconsciously assaulted by veggies every time you walk into Trader Joe’s, just know it’s for your own good.
TikTokers take on tics
We know TikTok is what makes a lot of teens and young adults tick, but what if TikTokers are actually catching tic disorders from other TikTokers?
TikTok blew up during the pandemic. Many people were stuck at home and had nothing better to do than make and watch TikTok videos. The pandemic brought isolation, uncertainty, and anxiety. The stress that followed may have caused many people, mostly women and young girls, to develop tic disorders.
There’s a TikTok for everything, whether it’s a new dance or a recipe. Many people even use TikTok to speak out about their illnesses. Several TikTokers have Tourette’s syndrome and show their tics on their videos. It appears that some audience members actually “catch” the tics from watching the videos and are then unable to stop certain jerking movements or saying specific words.
Neurologists at the University of Calgary (Alta.), who were hearing from colleagues and getting referrals of such patients, called it “an epidemic within the pandemic.” The behavior is not actually Tourette’s, they told Vice, but the patients “cannot stop, and we have absolutely witnessed that.”
There is, of course, controversy over the issue. One individual with the condition said, “I feel like there’s a lot of really weird, backwards stigma on TikTok about tic disorders. Like, you aren’t allowed to have one unless it’s this one.”
Who would have guessed that people would disagree over stuff on the Internet?
Look on the bright side: Obesity edition
The pandemic may have postponed “Top Gun: Maverick” and “The Marvelous Mrs. Maisel” until who-knows-when, but we here at LOTME are happy to announce the nearly-as-anticipated return of Bacteria vs. the World.
As you may recall from our last edition of BVTW, bacteria battled the ghost of Charles Darwin, who had taken the earthly form of antibiotics capable of stopping bacterial evolution. Tonight, our prokaryotic protagonists take on an equally relentless and ubiquitous challenger: obesity.
Specifically, we’re putting bacteria up against the obesity survival paradox, that phenomenon in which obesity and overweight seem to protect against – yes, you guessed it – bacterial infections.
A Swedish research team observed a group of 2,196 individual adults who received care for suspected severe bacterial infection at Skaraborg Hospital in Skövde. One year after hospitalization, 26% of normal-weight (body mass index, 18.5-24.99) patients were dead, compared with 17% of overweight (BMI, 25.0-29.99), 16% of obese (BMI, 30.0-34.99), and 9% of very obese (BMI >35) patients.
These results confirm the obesity survival paradox, but “what we don’t know is how being overweight can benefit the patient with a bacterial infection, or whether it’s connected with functions in the immune system and how they’re regulated,” lead author Dr. Åsa Alsiö said in a written statement.
A spokes-cell for the bacteria disputed the results and challenged the legitimacy of the investigators. When asked if there should be some sort of reexamination of the findings, he/she/it replied: “You bet your flagella.” We then pointed out that humans don’t have flagellum, and the representative raised his/her/its flagella in what could only be considered an obscene gesture.
Steal from the warm, give to the cold
If there’s one constant in life other than taxes, it’s elderly people moving to Florida. The Sunshine State’s reputation as a giant retirement home needs no elaboration, but why do senior citizens gravitate there? Well, many reasons, but a big one is that, the older you get, the more susceptible and sensitive you are to the cold. And now, according to a new study, we may have identified a culprit.
Researchers from Yale University examined a group of mice and found that the older ones lacked ICL2 cells in their fatty tissue. These cells, at least in younger mice, help restore body heat when exposed to cold temperatures. Lacking these cells meant that older mice had a limited ability to burn their fat and raise their temperature in response to cold.
Well, job done, all we need to do now is stimulate production of ICL2 cells in elderly people, and they’ll be able to go outside in 80-degree weather without a sweater again. Except there’s a problem. In a cruel twist of fate, when the elderly mice were given a molecule to boost ICL2 cell production, they actually became less tolerant of the cold than at baseline. Oops.
The scientists didn’t give up though, and gave their elderly mice ICL2 cells from young mice. This finally did the trick, though we have to admit, if that treatment does eventually scale up to humans, the prospect of a bunch of senior citizens taking ICL2 cells from young people to stay warm does sound a bit like a bad vampire movie premise. “I vant to suck your immune cell group 2 innate lymphoid cells!” Not the most pithy catch phrase in the world.
Grocery store tapping your subconscious? It’s a good thing
We all know there’s marketing and functionality elements to grocery stores and how they’re set up for your shopping pleasure. But what if I told you that the good old supermarket subconscious trick works on how healthy food decisions are?
In a recent study, researchers at the University of Southampton in England found that if you placed a wider selection of fruits and vegetables near the entrances and more nonfood items near checkouts, sales decreased on the sweets and increased on the produce. “The findings of our study suggest that a healthier store layout could lead to nearly 10,000 extra portions of fruit and vegetables and approximately 1,500 fewer portions of confectionery being sold on a weekly basis in each store,” lead author Dr. Christina Vogel explained.
You’re probably thinking that food placement studies aren’t new. That’s true, but this one went above and beyond. Instead of just looking at the influence placement has on purchase, this one took it further by trying to reduce the consumers’ “calorie opportunities” and examining the effect on sales. Also, customer loyalty, patterns, and diets were taken into account across multiple household members.
The researchers think shifting the layouts in grocery stores could shift people’s food choices, producing a domino effect on the population’s overall diet. With obesity, diabetes, and cardiology concerns always looming, swaying consumers toward healthier food choices makes for better public health overall.
So if you feel like you’re being subconsciously assaulted by veggies every time you walk into Trader Joe’s, just know it’s for your own good.
TikTokers take on tics
We know TikTok is what makes a lot of teens and young adults tick, but what if TikTokers are actually catching tic disorders from other TikTokers?
TikTok blew up during the pandemic. Many people were stuck at home and had nothing better to do than make and watch TikTok videos. The pandemic brought isolation, uncertainty, and anxiety. The stress that followed may have caused many people, mostly women and young girls, to develop tic disorders.
There’s a TikTok for everything, whether it’s a new dance or a recipe. Many people even use TikTok to speak out about their illnesses. Several TikTokers have Tourette’s syndrome and show their tics on their videos. It appears that some audience members actually “catch” the tics from watching the videos and are then unable to stop certain jerking movements or saying specific words.
Neurologists at the University of Calgary (Alta.), who were hearing from colleagues and getting referrals of such patients, called it “an epidemic within the pandemic.” The behavior is not actually Tourette’s, they told Vice, but the patients “cannot stop, and we have absolutely witnessed that.”
There is, of course, controversy over the issue. One individual with the condition said, “I feel like there’s a lot of really weird, backwards stigma on TikTok about tic disorders. Like, you aren’t allowed to have one unless it’s this one.”
Who would have guessed that people would disagree over stuff on the Internet?
Look on the bright side: Obesity edition
The pandemic may have postponed “Top Gun: Maverick” and “The Marvelous Mrs. Maisel” until who-knows-when, but we here at LOTME are happy to announce the nearly-as-anticipated return of Bacteria vs. the World.
As you may recall from our last edition of BVTW, bacteria battled the ghost of Charles Darwin, who had taken the earthly form of antibiotics capable of stopping bacterial evolution. Tonight, our prokaryotic protagonists take on an equally relentless and ubiquitous challenger: obesity.
Specifically, we’re putting bacteria up against the obesity survival paradox, that phenomenon in which obesity and overweight seem to protect against – yes, you guessed it – bacterial infections.
A Swedish research team observed a group of 2,196 individual adults who received care for suspected severe bacterial infection at Skaraborg Hospital in Skövde. One year after hospitalization, 26% of normal-weight (body mass index, 18.5-24.99) patients were dead, compared with 17% of overweight (BMI, 25.0-29.99), 16% of obese (BMI, 30.0-34.99), and 9% of very obese (BMI >35) patients.
These results confirm the obesity survival paradox, but “what we don’t know is how being overweight can benefit the patient with a bacterial infection, or whether it’s connected with functions in the immune system and how they’re regulated,” lead author Dr. Åsa Alsiö said in a written statement.
A spokes-cell for the bacteria disputed the results and challenged the legitimacy of the investigators. When asked if there should be some sort of reexamination of the findings, he/she/it replied: “You bet your flagella.” We then pointed out that humans don’t have flagellum, and the representative raised his/her/its flagella in what could only be considered an obscene gesture.
Steal from the warm, give to the cold
If there’s one constant in life other than taxes, it’s elderly people moving to Florida. The Sunshine State’s reputation as a giant retirement home needs no elaboration, but why do senior citizens gravitate there? Well, many reasons, but a big one is that, the older you get, the more susceptible and sensitive you are to the cold. And now, according to a new study, we may have identified a culprit.
Researchers from Yale University examined a group of mice and found that the older ones lacked ICL2 cells in their fatty tissue. These cells, at least in younger mice, help restore body heat when exposed to cold temperatures. Lacking these cells meant that older mice had a limited ability to burn their fat and raise their temperature in response to cold.
Well, job done, all we need to do now is stimulate production of ICL2 cells in elderly people, and they’ll be able to go outside in 80-degree weather without a sweater again. Except there’s a problem. In a cruel twist of fate, when the elderly mice were given a molecule to boost ICL2 cell production, they actually became less tolerant of the cold than at baseline. Oops.
The scientists didn’t give up though, and gave their elderly mice ICL2 cells from young mice. This finally did the trick, though we have to admit, if that treatment does eventually scale up to humans, the prospect of a bunch of senior citizens taking ICL2 cells from young people to stay warm does sound a bit like a bad vampire movie premise. “I vant to suck your immune cell group 2 innate lymphoid cells!” Not the most pithy catch phrase in the world.
Grocery store tapping your subconscious? It’s a good thing
We all know there’s marketing and functionality elements to grocery stores and how they’re set up for your shopping pleasure. But what if I told you that the good old supermarket subconscious trick works on how healthy food decisions are?
In a recent study, researchers at the University of Southampton in England found that if you placed a wider selection of fruits and vegetables near the entrances and more nonfood items near checkouts, sales decreased on the sweets and increased on the produce. “The findings of our study suggest that a healthier store layout could lead to nearly 10,000 extra portions of fruit and vegetables and approximately 1,500 fewer portions of confectionery being sold on a weekly basis in each store,” lead author Dr. Christina Vogel explained.
You’re probably thinking that food placement studies aren’t new. That’s true, but this one went above and beyond. Instead of just looking at the influence placement has on purchase, this one took it further by trying to reduce the consumers’ “calorie opportunities” and examining the effect on sales. Also, customer loyalty, patterns, and diets were taken into account across multiple household members.
The researchers think shifting the layouts in grocery stores could shift people’s food choices, producing a domino effect on the population’s overall diet. With obesity, diabetes, and cardiology concerns always looming, swaying consumers toward healthier food choices makes for better public health overall.
So if you feel like you’re being subconsciously assaulted by veggies every time you walk into Trader Joe’s, just know it’s for your own good.
TikTokers take on tics
We know TikTok is what makes a lot of teens and young adults tick, but what if TikTokers are actually catching tic disorders from other TikTokers?
TikTok blew up during the pandemic. Many people were stuck at home and had nothing better to do than make and watch TikTok videos. The pandemic brought isolation, uncertainty, and anxiety. The stress that followed may have caused many people, mostly women and young girls, to develop tic disorders.
There’s a TikTok for everything, whether it’s a new dance or a recipe. Many people even use TikTok to speak out about their illnesses. Several TikTokers have Tourette’s syndrome and show their tics on their videos. It appears that some audience members actually “catch” the tics from watching the videos and are then unable to stop certain jerking movements or saying specific words.
Neurologists at the University of Calgary (Alta.), who were hearing from colleagues and getting referrals of such patients, called it “an epidemic within the pandemic.” The behavior is not actually Tourette’s, they told Vice, but the patients “cannot stop, and we have absolutely witnessed that.”
There is, of course, controversy over the issue. One individual with the condition said, “I feel like there’s a lot of really weird, backwards stigma on TikTok about tic disorders. Like, you aren’t allowed to have one unless it’s this one.”
Who would have guessed that people would disagree over stuff on the Internet?
Look on the bright side: Obesity edition
The pandemic may have postponed “Top Gun: Maverick” and “The Marvelous Mrs. Maisel” until who-knows-when, but we here at LOTME are happy to announce the nearly-as-anticipated return of Bacteria vs. the World.
As you may recall from our last edition of BVTW, bacteria battled the ghost of Charles Darwin, who had taken the earthly form of antibiotics capable of stopping bacterial evolution. Tonight, our prokaryotic protagonists take on an equally relentless and ubiquitous challenger: obesity.
Specifically, we’re putting bacteria up against the obesity survival paradox, that phenomenon in which obesity and overweight seem to protect against – yes, you guessed it – bacterial infections.
A Swedish research team observed a group of 2,196 individual adults who received care for suspected severe bacterial infection at Skaraborg Hospital in Skövde. One year after hospitalization, 26% of normal-weight (body mass index, 18.5-24.99) patients were dead, compared with 17% of overweight (BMI, 25.0-29.99), 16% of obese (BMI, 30.0-34.99), and 9% of very obese (BMI >35) patients.
These results confirm the obesity survival paradox, but “what we don’t know is how being overweight can benefit the patient with a bacterial infection, or whether it’s connected with functions in the immune system and how they’re regulated,” lead author Dr. Åsa Alsiö said in a written statement.
A spokes-cell for the bacteria disputed the results and challenged the legitimacy of the investigators. When asked if there should be some sort of reexamination of the findings, he/she/it replied: “You bet your flagella.” We then pointed out that humans don’t have flagellum, and the representative raised his/her/its flagella in what could only be considered an obscene gesture.
Walking 7,000 steps per day may be enough to reduce mortality risk
based on prospective data from more than 2,000 people.
Findings were consistent regardless of race or sex, and step intensity had no impact on mortality risk, reported lead author Amanda E. Paluch, PhD, of the University of Massachusetts Amherst, and colleagues.
“In response to the need for empirical data on the associations of step volume and intensity with mortality in younger and diverse populations, we conducted a prospective study in middle-aged Black and White adults followed up for mortality for approximately 11 years,” the investigators wrote in JAMA Network Open. “The objectives of our study were to examine the associations of step volume and intensity with mortality overall and by race and sex.”
Steps per day is easy to communicate
Dr. Paluch noted that steps per day is a “very appealing metric to quantify activity,” for both researchers and laypeople.
“Steps per day is simple and easy to communicate in public health and clinical settings,” Dr. Paluch said in an interview. “Additionally, the dramatic growth of wearable devices measuring steps makes it appealing and broadens the reach of promoting physical activity to many individuals. Walking is an activity that most of the general population can pursue. It can also be accumulated throughout daily living and may seem more achievable to fit into busy lives than a structured exercise session.”
The present investigation was conducted as part of the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The dataset included 2,110 participants ranging from 38-50 years of age, with a mean age of 45.2 years. A slightly higher proportion of the subjects were women (57.1%) and White (57.9%).
All participants wore an ActiGraph 7164 accelerometer for 1 week and were then followed for death of any cause, with a mean follow-up of 10.8 years. Multivariable-adjusted Cox proportional hazards models included a range of covariates, such as smoking history, body weight, alcohol intake, blood pressure, total cholesterol, and others. Step counts were grouped into low (less than 7,000 steps per day), moderate (7,000-9,999), and high (at least 10,000 steps per day) categories.
Compared with individuals who took less than 7,000 steps per day, those who took 7,000-9,000 steps per day had a 72% reduced risk of mortality (hazard ratio, 0.28; 95% confidence interval, 0.15-0.54). Going beyond 10,000 steps appeared to add no benefit, based on a 55% lower risk of all-cause mortality in the highly active group, compared with those taking less than 7,000 steps per day (HR, 0.45; 95% CI, 0.25-0.81).
Walking faster didn’t appear to help either, as stepping intensity was not associated with mortality risk; however, Dr. Paluch urged a cautious interpretation of this finding, calling it “inconclusive,” and suggesting that more research is needed.
“It is also important to note that this study only looked at premature all-cause mortality, and therefore the results may be different for other health outcomes, such as the risk of cardiovascular disease, or diabetes, cancer, or mental health outcomes,” Dr. Paluch said.
“The results from our study demonstrated that those who are least active have the most to gain,” Dr. Paluch said. “Even small incremental increases in steps per day are associated with a lower mortality risk during middle age. A walking plan that gradually works up toward 7,000-10,000 steps per day in middle-aged adults may have health benefits and lower the risk of premature mortality.”
Causality cannot be confirmed
According to Raed A. Joundi, MD, DPhil, of the University of Calgary (Alta.), the study size, diverse population, and length of follow-up should increase confidence in the findings, although a causal relationship remains elusive.
“As this study is observational, causality between step count and mortality cannot be confirmed; however, the authors accounted for many factors, and the association was consistent in different analyses and with prior literature,” Dr. Joundi said in an interview. “The authors did not assess the risk of other important events like stroke and heart attack, and these could be addressed in a future study.”
Dr. Joundi, who recently published a study linking exercise with a 50% reduction in mortality after stroke, noted that “physical activity has innumerable benefits, and it’s important that people engage in activity that can be regular and consistent, regardless of the type or intensity.”
To this end, he highlighted the use of “devices capable of monitoring step count, which can be an important motivational tool,” and suggested that these findings may bring a sigh of relief to step counters who come up a little short on a common daily goal.
“A target of 10,000 steps is often used for public health promotion, and this study now provides convincing observational evidence that it may be an optimal step count target for mortality reduction,” Dr. Joundi said. “However, if 10,000 steps per day is not feasible, 7,000 steps seems to be a very reasonable target given its association with markedly lower mortality in this study.”
Not all step counters are equal
Unfortunately, such recommendations are complicated by uncertainty in measurement, as widely used step counting devices, like smart watches, may not yield the same results as research-grade accelerometers, according to Nicole L. Spartano, PhD, of Boston University.
“Many comparison studies have been conducted in laboratory settings among young healthy adults, but these do not necessarily reflect real-life wear experiences that will be generalizable to the population as a whole,” Dr. Spartano wrote in an accompanying editorial.
She called for large-scale comparison studies to compare research-grade and consumer devices.
“The reason for conducting comparison studies is not to develop distinct guidelines for different devices or subgroups of the population, but rather to understand the variability so that we can develop one clear message that is most appropriate to the public,” Dr. Spartano wrote. “Some devices may have bias in terms of step measurement at different activity intensity and may not record steps as accurately in older adults or individuals with obesity or mobility disorders. For example, when adults who were obese wore an ActiGraph monitor in a laboratory setting, the device only recorded 80% of steps walked at a moderate pace, while other devices recorded close to 100% of steps walked. If we in the public health community are to move toward using these devices more for physical activity prescription, these details will need to be explored in more depth.”
CARDIA was conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Some study authors received grants from the National Institutes of Health and the Kaiser Foundation Research Institute. Dr Spartano disclosed relationships with Novo Nordisk, the American Heart Association, the Alzheimer’s Association, and the National Institutes of Health. Dr. Joundi and Dr. Paluch disclosed no relevant financial relationships.
based on prospective data from more than 2,000 people.
Findings were consistent regardless of race or sex, and step intensity had no impact on mortality risk, reported lead author Amanda E. Paluch, PhD, of the University of Massachusetts Amherst, and colleagues.
“In response to the need for empirical data on the associations of step volume and intensity with mortality in younger and diverse populations, we conducted a prospective study in middle-aged Black and White adults followed up for mortality for approximately 11 years,” the investigators wrote in JAMA Network Open. “The objectives of our study were to examine the associations of step volume and intensity with mortality overall and by race and sex.”
Steps per day is easy to communicate
Dr. Paluch noted that steps per day is a “very appealing metric to quantify activity,” for both researchers and laypeople.
“Steps per day is simple and easy to communicate in public health and clinical settings,” Dr. Paluch said in an interview. “Additionally, the dramatic growth of wearable devices measuring steps makes it appealing and broadens the reach of promoting physical activity to many individuals. Walking is an activity that most of the general population can pursue. It can also be accumulated throughout daily living and may seem more achievable to fit into busy lives than a structured exercise session.”
The present investigation was conducted as part of the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The dataset included 2,110 participants ranging from 38-50 years of age, with a mean age of 45.2 years. A slightly higher proportion of the subjects were women (57.1%) and White (57.9%).
All participants wore an ActiGraph 7164 accelerometer for 1 week and were then followed for death of any cause, with a mean follow-up of 10.8 years. Multivariable-adjusted Cox proportional hazards models included a range of covariates, such as smoking history, body weight, alcohol intake, blood pressure, total cholesterol, and others. Step counts were grouped into low (less than 7,000 steps per day), moderate (7,000-9,999), and high (at least 10,000 steps per day) categories.
Compared with individuals who took less than 7,000 steps per day, those who took 7,000-9,000 steps per day had a 72% reduced risk of mortality (hazard ratio, 0.28; 95% confidence interval, 0.15-0.54). Going beyond 10,000 steps appeared to add no benefit, based on a 55% lower risk of all-cause mortality in the highly active group, compared with those taking less than 7,000 steps per day (HR, 0.45; 95% CI, 0.25-0.81).
Walking faster didn’t appear to help either, as stepping intensity was not associated with mortality risk; however, Dr. Paluch urged a cautious interpretation of this finding, calling it “inconclusive,” and suggesting that more research is needed.
“It is also important to note that this study only looked at premature all-cause mortality, and therefore the results may be different for other health outcomes, such as the risk of cardiovascular disease, or diabetes, cancer, or mental health outcomes,” Dr. Paluch said.
“The results from our study demonstrated that those who are least active have the most to gain,” Dr. Paluch said. “Even small incremental increases in steps per day are associated with a lower mortality risk during middle age. A walking plan that gradually works up toward 7,000-10,000 steps per day in middle-aged adults may have health benefits and lower the risk of premature mortality.”
Causality cannot be confirmed
According to Raed A. Joundi, MD, DPhil, of the University of Calgary (Alta.), the study size, diverse population, and length of follow-up should increase confidence in the findings, although a causal relationship remains elusive.
“As this study is observational, causality between step count and mortality cannot be confirmed; however, the authors accounted for many factors, and the association was consistent in different analyses and with prior literature,” Dr. Joundi said in an interview. “The authors did not assess the risk of other important events like stroke and heart attack, and these could be addressed in a future study.”
Dr. Joundi, who recently published a study linking exercise with a 50% reduction in mortality after stroke, noted that “physical activity has innumerable benefits, and it’s important that people engage in activity that can be regular and consistent, regardless of the type or intensity.”
To this end, he highlighted the use of “devices capable of monitoring step count, which can be an important motivational tool,” and suggested that these findings may bring a sigh of relief to step counters who come up a little short on a common daily goal.
“A target of 10,000 steps is often used for public health promotion, and this study now provides convincing observational evidence that it may be an optimal step count target for mortality reduction,” Dr. Joundi said. “However, if 10,000 steps per day is not feasible, 7,000 steps seems to be a very reasonable target given its association with markedly lower mortality in this study.”
Not all step counters are equal
Unfortunately, such recommendations are complicated by uncertainty in measurement, as widely used step counting devices, like smart watches, may not yield the same results as research-grade accelerometers, according to Nicole L. Spartano, PhD, of Boston University.
“Many comparison studies have been conducted in laboratory settings among young healthy adults, but these do not necessarily reflect real-life wear experiences that will be generalizable to the population as a whole,” Dr. Spartano wrote in an accompanying editorial.
She called for large-scale comparison studies to compare research-grade and consumer devices.
“The reason for conducting comparison studies is not to develop distinct guidelines for different devices or subgroups of the population, but rather to understand the variability so that we can develop one clear message that is most appropriate to the public,” Dr. Spartano wrote. “Some devices may have bias in terms of step measurement at different activity intensity and may not record steps as accurately in older adults or individuals with obesity or mobility disorders. For example, when adults who were obese wore an ActiGraph monitor in a laboratory setting, the device only recorded 80% of steps walked at a moderate pace, while other devices recorded close to 100% of steps walked. If we in the public health community are to move toward using these devices more for physical activity prescription, these details will need to be explored in more depth.”
CARDIA was conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Some study authors received grants from the National Institutes of Health and the Kaiser Foundation Research Institute. Dr Spartano disclosed relationships with Novo Nordisk, the American Heart Association, the Alzheimer’s Association, and the National Institutes of Health. Dr. Joundi and Dr. Paluch disclosed no relevant financial relationships.
based on prospective data from more than 2,000 people.
Findings were consistent regardless of race or sex, and step intensity had no impact on mortality risk, reported lead author Amanda E. Paluch, PhD, of the University of Massachusetts Amherst, and colleagues.
“In response to the need for empirical data on the associations of step volume and intensity with mortality in younger and diverse populations, we conducted a prospective study in middle-aged Black and White adults followed up for mortality for approximately 11 years,” the investigators wrote in JAMA Network Open. “The objectives of our study were to examine the associations of step volume and intensity with mortality overall and by race and sex.”
Steps per day is easy to communicate
Dr. Paluch noted that steps per day is a “very appealing metric to quantify activity,” for both researchers and laypeople.
“Steps per day is simple and easy to communicate in public health and clinical settings,” Dr. Paluch said in an interview. “Additionally, the dramatic growth of wearable devices measuring steps makes it appealing and broadens the reach of promoting physical activity to many individuals. Walking is an activity that most of the general population can pursue. It can also be accumulated throughout daily living and may seem more achievable to fit into busy lives than a structured exercise session.”
The present investigation was conducted as part of the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The dataset included 2,110 participants ranging from 38-50 years of age, with a mean age of 45.2 years. A slightly higher proportion of the subjects were women (57.1%) and White (57.9%).
All participants wore an ActiGraph 7164 accelerometer for 1 week and were then followed for death of any cause, with a mean follow-up of 10.8 years. Multivariable-adjusted Cox proportional hazards models included a range of covariates, such as smoking history, body weight, alcohol intake, blood pressure, total cholesterol, and others. Step counts were grouped into low (less than 7,000 steps per day), moderate (7,000-9,999), and high (at least 10,000 steps per day) categories.
Compared with individuals who took less than 7,000 steps per day, those who took 7,000-9,000 steps per day had a 72% reduced risk of mortality (hazard ratio, 0.28; 95% confidence interval, 0.15-0.54). Going beyond 10,000 steps appeared to add no benefit, based on a 55% lower risk of all-cause mortality in the highly active group, compared with those taking less than 7,000 steps per day (HR, 0.45; 95% CI, 0.25-0.81).
Walking faster didn’t appear to help either, as stepping intensity was not associated with mortality risk; however, Dr. Paluch urged a cautious interpretation of this finding, calling it “inconclusive,” and suggesting that more research is needed.
“It is also important to note that this study only looked at premature all-cause mortality, and therefore the results may be different for other health outcomes, such as the risk of cardiovascular disease, or diabetes, cancer, or mental health outcomes,” Dr. Paluch said.
“The results from our study demonstrated that those who are least active have the most to gain,” Dr. Paluch said. “Even small incremental increases in steps per day are associated with a lower mortality risk during middle age. A walking plan that gradually works up toward 7,000-10,000 steps per day in middle-aged adults may have health benefits and lower the risk of premature mortality.”
Causality cannot be confirmed
According to Raed A. Joundi, MD, DPhil, of the University of Calgary (Alta.), the study size, diverse population, and length of follow-up should increase confidence in the findings, although a causal relationship remains elusive.
“As this study is observational, causality between step count and mortality cannot be confirmed; however, the authors accounted for many factors, and the association was consistent in different analyses and with prior literature,” Dr. Joundi said in an interview. “The authors did not assess the risk of other important events like stroke and heart attack, and these could be addressed in a future study.”
Dr. Joundi, who recently published a study linking exercise with a 50% reduction in mortality after stroke, noted that “physical activity has innumerable benefits, and it’s important that people engage in activity that can be regular and consistent, regardless of the type or intensity.”
To this end, he highlighted the use of “devices capable of monitoring step count, which can be an important motivational tool,” and suggested that these findings may bring a sigh of relief to step counters who come up a little short on a common daily goal.
“A target of 10,000 steps is often used for public health promotion, and this study now provides convincing observational evidence that it may be an optimal step count target for mortality reduction,” Dr. Joundi said. “However, if 10,000 steps per day is not feasible, 7,000 steps seems to be a very reasonable target given its association with markedly lower mortality in this study.”
Not all step counters are equal
Unfortunately, such recommendations are complicated by uncertainty in measurement, as widely used step counting devices, like smart watches, may not yield the same results as research-grade accelerometers, according to Nicole L. Spartano, PhD, of Boston University.
“Many comparison studies have been conducted in laboratory settings among young healthy adults, but these do not necessarily reflect real-life wear experiences that will be generalizable to the population as a whole,” Dr. Spartano wrote in an accompanying editorial.
She called for large-scale comparison studies to compare research-grade and consumer devices.
“The reason for conducting comparison studies is not to develop distinct guidelines for different devices or subgroups of the population, but rather to understand the variability so that we can develop one clear message that is most appropriate to the public,” Dr. Spartano wrote. “Some devices may have bias in terms of step measurement at different activity intensity and may not record steps as accurately in older adults or individuals with obesity or mobility disorders. For example, when adults who were obese wore an ActiGraph monitor in a laboratory setting, the device only recorded 80% of steps walked at a moderate pace, while other devices recorded close to 100% of steps walked. If we in the public health community are to move toward using these devices more for physical activity prescription, these details will need to be explored in more depth.”
CARDIA was conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Some study authors received grants from the National Institutes of Health and the Kaiser Foundation Research Institute. Dr Spartano disclosed relationships with Novo Nordisk, the American Heart Association, the Alzheimer’s Association, and the National Institutes of Health. Dr. Joundi and Dr. Paluch disclosed no relevant financial relationships.
FROM JAMA NETWORK OPEN
United States reaches 5 million cases of child COVID
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
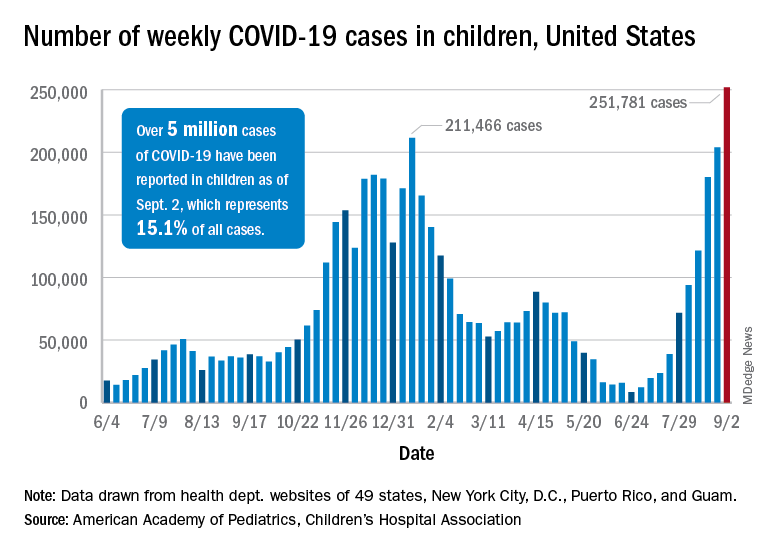
The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Anakinra improved survival in hospitalized COVID-19 patients
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
FROM NATURE MEDICINE












