User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Hospitalists and medical malpractice
A look at some sobering trends
Among the pressures felt by hospitalists are concerns about being subject to a malpractice claim. Anxiety about malpractice influences the way hospitalists practice, giving rise to defensive medicine.
One survey, which asked hospitalists to retrospectively rate which of their orders represented defensive medicine, found that 28% of orders were deemed defensive.1 Defensive medicine can lead to low-value medical care, drive up health care costs, and potentially subject patients to unnecessary testing.2,3
Encouragingly, medical malpractice claims rates have, overall, been downtrending. An analysis of data from the National Practitioner Data Bank, which is a repository of all paid malpractice claims against individual physicians, found that malpractice claims rates decreased by 55.7% from 1992 to 2014 among all specialties, and by 46.1% for internal medicine physicians.4 The data used in this analysis did not separate hospitalists from other internal medicine physicians. An older study of malpractice claims against hospitalists found that hospitalists had significantly lower claims rates than non-hospitalist internal medicine physicians.5
Current malpractice environment for hospitalists
Seeking to shed light on the current malpractice environment faced by hospitalists, a recent study examined claims against hospitalists using the Comparative Benchmarking System (CBS), a national database of malpractice claims containing approximately 30% of all U.S. malpractice claims, which is maintained by CRICO, the malpractice insurer for the Harvard-affiliated medical institutions.6
Claims in the CBS database are examined by trained nurse coders who review the claims, along with the associated medical and legal records, to understand the contributing factors behind the adverse event leading to the claim.
Contrary to the trends for nearly all other physician specialties, the malpractice claims rates of hospitalists were not downtrending, going from 1.77 claims per 100 physician-years from 2009-2013 to 2.08 claims per 100 physician-years from 2014-2018. The overall claims rate for hospitalists was significantly higher than that for internal medicine subspecialists (though roughly the same as the claims rate for non-hospitalist general internal medicine physicians). These sobering findings raise the important question of why hospitalists claims rates are heading in the wrong direction.
One possible answer relates the ever-broadening scope of hospitalist practice. Hospitalists are being asked to care for surgical patients and other patient populations that they may have less familiarity with, increasing the risk of medical errors. Among the other specialties most commonly also named in hospitalist claims, general surgery and orthopedic surgery are in the top five. The extraordinary growth in the field of hospital medicine has meant a need to hire an increasing number of hospitalists, leading to less-experienced physicians entering the field.
Making hospital medicine safer
A more urgent question than what is driving the trends in hospitalist claims rates is what can be done to avoid adverse events and make hospital medicine safer. One potential answer is thoughtful collaboration arrangements with the surgical and other specialties with whom hospitalists may be co-managing patients.
Questions about who responds to what types of clinical issues that might arise and specific domains of responsibility should be defined in advance, so that a lack of role clarity does not negatively impact patient care. Given that hospitalists will be less comfortable addressing more technical surgical issues, expectations about surgeons’ availability should be established. Nocturnists may be tasked with overnight cross-coverage of patients on services, such as oncology and cardiology, that subspecialty physicians have responsibility for during the day. Agreeing upon triggers for when the nocturnist should contact the daytime subspecialty attending (for example, if a rapid response is called on their patient) should be considered, so that nocturnists are not left deciding, in the moment, whether to call the daytime attending. Measures such as this ensure that everyone’s expectations are aligned. In addition, new hospitalists need to be offered support, in the form of training and mentorship.
CBS malpractice data, which includes the contributing factors underlying what went wrong, illuminates potential targets for programs designed to enhance patient safety. In the recent hospitalist malpractice study, the two contributing factors that were the best predictors of a hospitalist malpractice claim closing with payment to the claimant were clinical judgment errors and communication breakdowns. Identifying measures that are effective in promoting patient safety by refining the clinical judgement of clinicians is a challenge, and there are limited data demonstrating what programs are effective in this area.
Clinical decision support (CDS) systems have shown promise in promoting guideline-concordant care.7 However, the role of CDS in aiding the higher-stakes clinical decisions that may be called into question after an adverse outcome is not well defined. Alerts that a patient may be developing sepsis is one type of CDS that has been extensively studied and has been shown to be of some benefit.8 The importance of clinical judgment to whether payment is made on a malpractice claim can inform risk management strategies. Hospitalists should document the thought process behind their decision making in the chart, especially for important clinical decisions. A note showing that the clinician was thoughtfully weighing the risks and benefits using the data available at the time will help make a case defensible if an adverse outcome occurs.
The effect of communication breakdowns on hospitalist case outcomes highlights the importance of measures to improve and systematize communication among clinicians, particularly at vulnerable junctures – such as handoffs from the day team to the night team, and transitions from one care setting to another. An example of an intervention to improve handoffs with cogent evidence to support it is I-PASS, which is an approach to handoffs between teams in which information about the patient’s illness severity, clinical background, and contingencies is conveyed and synthesized in a structured manner. A study of the effect of implementation of I-PASS among nine pediatric residency programs demonstrated a 30% reduction in preventable adverse events.9
Applying insights from malpractice claims analysis to clinical practice
The systematic review of malpractice cases to determine the contributing factors and other case attributes is an important source of patient safety insights. The process breakdowns described by the contributing factors can inform the design of patient safety initiatives. In addition, malpractice data provides information on which specialties and what types of clinicians are being named together in malpractice claims.
In the hospitalist malpractice study, in addition to general surgery and orthopedic surgery, the other clinical services most commonly subject to claims along with hospitalists were nursing, emergency medicine, and cardiology. Another observation was that physician assistants and nurse practitioners are increasingly being named in hospitalist claims. This information is crucial to guiding who needs to be in the room with hospitalists when efforts are undertaken to enhance patient safety within hospital medicine.
An understandable response to the finding that hospitalist claims rates are not decreasing is for hospitalists to seek ways to lower their risk of being named in a malpractice claim. Of course, avoiding adverse events by providing the safest possible care is paramount. Even when patients do suffer adverse events due to a physician negligence, only rarely, less than 5% of the time, does this result in a malpractice claim.10 Important lessons in risk management can be learned from examining why patients decide to sue when mistakes lead to bad outcomes.
An analysis of plaintiffs’ depositions found that the key reasons that patients decided to file a malpractice claim include a poor relationship with the physician – specifically, a lack of empathy from the physician, feeling deserted by the physician, and feeling devalued by the physician.11 These findings support the use of programs that assist physicians in compassionately disclosing adverse events to patients. Among inpatient physicians, patient satisfaction survey questions about the time the physician spent with the patient and the physician’s concern for the patient are better predictors of the physicians’ risk management performance than is the question about the skill of the physician.12 In the aftermath of an adverse event, focusing on maintaining a strong patient-physician relationship is not only the right the thing to do, the data tell us that it is also a sensible approach to reducing medicolegal risk.
Dr. Schaffer practices as a member of the Hospital Medicine Unit at Brigham and Women’s Hospital, Boston, where he serves as an attending physician on the inpatient general medicine services. An instructor at Harvard Medical School, his academic interests include research using large medical malpractice databases to examine temporal trends in medical malpractice.
References
1. Rothberg MB, et al. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. doi:10.1001/jamainternmed.2014.4649.
2. Kachalia A, et al. Overuse of testing in preoperative evaluation and syncope: A survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. doi: 10.7326/M14-0694.
3. Mello MM, et al. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. doi: 10.1377/hlthaff.2009.0807.
4. Schaffer AC, et al. Rates and characteristics of paid malpractice claims among U.S. physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. doi:10.1001/jamainternmed.2017.0311.
5. Schaffer AC, et al. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. doi: 10.1002/jhm.2244.
6. Schaffer AC, et al. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021 Jul;16(7):390-396. doi: 10.12788/jhm.3557.
7. Poon EG. Clinical decision support: a tool of the hospital trade. J Hosp Med. 2015;10(1):60-61. doi: 10.1002/jhm.2295.
8. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med. 2015;10(6):396-402. doi: 10.1002/jhm.2347.
9. Starmer AJ, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMsa1405556.
10. Localio AR, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi: 10.1056/NEJM199107253250405.
11. Beckman HB, et al. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. doi:10.1001/archinte.1994.00420120093010.
12. Stelfox HT, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. doi: 10.1016/j.amjmed.2005.01.060.
A look at some sobering trends
A look at some sobering trends
Among the pressures felt by hospitalists are concerns about being subject to a malpractice claim. Anxiety about malpractice influences the way hospitalists practice, giving rise to defensive medicine.
One survey, which asked hospitalists to retrospectively rate which of their orders represented defensive medicine, found that 28% of orders were deemed defensive.1 Defensive medicine can lead to low-value medical care, drive up health care costs, and potentially subject patients to unnecessary testing.2,3
Encouragingly, medical malpractice claims rates have, overall, been downtrending. An analysis of data from the National Practitioner Data Bank, which is a repository of all paid malpractice claims against individual physicians, found that malpractice claims rates decreased by 55.7% from 1992 to 2014 among all specialties, and by 46.1% for internal medicine physicians.4 The data used in this analysis did not separate hospitalists from other internal medicine physicians. An older study of malpractice claims against hospitalists found that hospitalists had significantly lower claims rates than non-hospitalist internal medicine physicians.5
Current malpractice environment for hospitalists
Seeking to shed light on the current malpractice environment faced by hospitalists, a recent study examined claims against hospitalists using the Comparative Benchmarking System (CBS), a national database of malpractice claims containing approximately 30% of all U.S. malpractice claims, which is maintained by CRICO, the malpractice insurer for the Harvard-affiliated medical institutions.6
Claims in the CBS database are examined by trained nurse coders who review the claims, along with the associated medical and legal records, to understand the contributing factors behind the adverse event leading to the claim.
Contrary to the trends for nearly all other physician specialties, the malpractice claims rates of hospitalists were not downtrending, going from 1.77 claims per 100 physician-years from 2009-2013 to 2.08 claims per 100 physician-years from 2014-2018. The overall claims rate for hospitalists was significantly higher than that for internal medicine subspecialists (though roughly the same as the claims rate for non-hospitalist general internal medicine physicians). These sobering findings raise the important question of why hospitalists claims rates are heading in the wrong direction.
One possible answer relates the ever-broadening scope of hospitalist practice. Hospitalists are being asked to care for surgical patients and other patient populations that they may have less familiarity with, increasing the risk of medical errors. Among the other specialties most commonly also named in hospitalist claims, general surgery and orthopedic surgery are in the top five. The extraordinary growth in the field of hospital medicine has meant a need to hire an increasing number of hospitalists, leading to less-experienced physicians entering the field.
Making hospital medicine safer
A more urgent question than what is driving the trends in hospitalist claims rates is what can be done to avoid adverse events and make hospital medicine safer. One potential answer is thoughtful collaboration arrangements with the surgical and other specialties with whom hospitalists may be co-managing patients.
Questions about who responds to what types of clinical issues that might arise and specific domains of responsibility should be defined in advance, so that a lack of role clarity does not negatively impact patient care. Given that hospitalists will be less comfortable addressing more technical surgical issues, expectations about surgeons’ availability should be established. Nocturnists may be tasked with overnight cross-coverage of patients on services, such as oncology and cardiology, that subspecialty physicians have responsibility for during the day. Agreeing upon triggers for when the nocturnist should contact the daytime subspecialty attending (for example, if a rapid response is called on their patient) should be considered, so that nocturnists are not left deciding, in the moment, whether to call the daytime attending. Measures such as this ensure that everyone’s expectations are aligned. In addition, new hospitalists need to be offered support, in the form of training and mentorship.
CBS malpractice data, which includes the contributing factors underlying what went wrong, illuminates potential targets for programs designed to enhance patient safety. In the recent hospitalist malpractice study, the two contributing factors that were the best predictors of a hospitalist malpractice claim closing with payment to the claimant were clinical judgment errors and communication breakdowns. Identifying measures that are effective in promoting patient safety by refining the clinical judgement of clinicians is a challenge, and there are limited data demonstrating what programs are effective in this area.
Clinical decision support (CDS) systems have shown promise in promoting guideline-concordant care.7 However, the role of CDS in aiding the higher-stakes clinical decisions that may be called into question after an adverse outcome is not well defined. Alerts that a patient may be developing sepsis is one type of CDS that has been extensively studied and has been shown to be of some benefit.8 The importance of clinical judgment to whether payment is made on a malpractice claim can inform risk management strategies. Hospitalists should document the thought process behind their decision making in the chart, especially for important clinical decisions. A note showing that the clinician was thoughtfully weighing the risks and benefits using the data available at the time will help make a case defensible if an adverse outcome occurs.
The effect of communication breakdowns on hospitalist case outcomes highlights the importance of measures to improve and systematize communication among clinicians, particularly at vulnerable junctures – such as handoffs from the day team to the night team, and transitions from one care setting to another. An example of an intervention to improve handoffs with cogent evidence to support it is I-PASS, which is an approach to handoffs between teams in which information about the patient’s illness severity, clinical background, and contingencies is conveyed and synthesized in a structured manner. A study of the effect of implementation of I-PASS among nine pediatric residency programs demonstrated a 30% reduction in preventable adverse events.9
Applying insights from malpractice claims analysis to clinical practice
The systematic review of malpractice cases to determine the contributing factors and other case attributes is an important source of patient safety insights. The process breakdowns described by the contributing factors can inform the design of patient safety initiatives. In addition, malpractice data provides information on which specialties and what types of clinicians are being named together in malpractice claims.
In the hospitalist malpractice study, in addition to general surgery and orthopedic surgery, the other clinical services most commonly subject to claims along with hospitalists were nursing, emergency medicine, and cardiology. Another observation was that physician assistants and nurse practitioners are increasingly being named in hospitalist claims. This information is crucial to guiding who needs to be in the room with hospitalists when efforts are undertaken to enhance patient safety within hospital medicine.
An understandable response to the finding that hospitalist claims rates are not decreasing is for hospitalists to seek ways to lower their risk of being named in a malpractice claim. Of course, avoiding adverse events by providing the safest possible care is paramount. Even when patients do suffer adverse events due to a physician negligence, only rarely, less than 5% of the time, does this result in a malpractice claim.10 Important lessons in risk management can be learned from examining why patients decide to sue when mistakes lead to bad outcomes.
An analysis of plaintiffs’ depositions found that the key reasons that patients decided to file a malpractice claim include a poor relationship with the physician – specifically, a lack of empathy from the physician, feeling deserted by the physician, and feeling devalued by the physician.11 These findings support the use of programs that assist physicians in compassionately disclosing adverse events to patients. Among inpatient physicians, patient satisfaction survey questions about the time the physician spent with the patient and the physician’s concern for the patient are better predictors of the physicians’ risk management performance than is the question about the skill of the physician.12 In the aftermath of an adverse event, focusing on maintaining a strong patient-physician relationship is not only the right the thing to do, the data tell us that it is also a sensible approach to reducing medicolegal risk.
Dr. Schaffer practices as a member of the Hospital Medicine Unit at Brigham and Women’s Hospital, Boston, where he serves as an attending physician on the inpatient general medicine services. An instructor at Harvard Medical School, his academic interests include research using large medical malpractice databases to examine temporal trends in medical malpractice.
References
1. Rothberg MB, et al. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. doi:10.1001/jamainternmed.2014.4649.
2. Kachalia A, et al. Overuse of testing in preoperative evaluation and syncope: A survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. doi: 10.7326/M14-0694.
3. Mello MM, et al. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. doi: 10.1377/hlthaff.2009.0807.
4. Schaffer AC, et al. Rates and characteristics of paid malpractice claims among U.S. physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. doi:10.1001/jamainternmed.2017.0311.
5. Schaffer AC, et al. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. doi: 10.1002/jhm.2244.
6. Schaffer AC, et al. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021 Jul;16(7):390-396. doi: 10.12788/jhm.3557.
7. Poon EG. Clinical decision support: a tool of the hospital trade. J Hosp Med. 2015;10(1):60-61. doi: 10.1002/jhm.2295.
8. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med. 2015;10(6):396-402. doi: 10.1002/jhm.2347.
9. Starmer AJ, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMsa1405556.
10. Localio AR, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi: 10.1056/NEJM199107253250405.
11. Beckman HB, et al. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. doi:10.1001/archinte.1994.00420120093010.
12. Stelfox HT, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. doi: 10.1016/j.amjmed.2005.01.060.
Among the pressures felt by hospitalists are concerns about being subject to a malpractice claim. Anxiety about malpractice influences the way hospitalists practice, giving rise to defensive medicine.
One survey, which asked hospitalists to retrospectively rate which of their orders represented defensive medicine, found that 28% of orders were deemed defensive.1 Defensive medicine can lead to low-value medical care, drive up health care costs, and potentially subject patients to unnecessary testing.2,3
Encouragingly, medical malpractice claims rates have, overall, been downtrending. An analysis of data from the National Practitioner Data Bank, which is a repository of all paid malpractice claims against individual physicians, found that malpractice claims rates decreased by 55.7% from 1992 to 2014 among all specialties, and by 46.1% for internal medicine physicians.4 The data used in this analysis did not separate hospitalists from other internal medicine physicians. An older study of malpractice claims against hospitalists found that hospitalists had significantly lower claims rates than non-hospitalist internal medicine physicians.5
Current malpractice environment for hospitalists
Seeking to shed light on the current malpractice environment faced by hospitalists, a recent study examined claims against hospitalists using the Comparative Benchmarking System (CBS), a national database of malpractice claims containing approximately 30% of all U.S. malpractice claims, which is maintained by CRICO, the malpractice insurer for the Harvard-affiliated medical institutions.6
Claims in the CBS database are examined by trained nurse coders who review the claims, along with the associated medical and legal records, to understand the contributing factors behind the adverse event leading to the claim.
Contrary to the trends for nearly all other physician specialties, the malpractice claims rates of hospitalists were not downtrending, going from 1.77 claims per 100 physician-years from 2009-2013 to 2.08 claims per 100 physician-years from 2014-2018. The overall claims rate for hospitalists was significantly higher than that for internal medicine subspecialists (though roughly the same as the claims rate for non-hospitalist general internal medicine physicians). These sobering findings raise the important question of why hospitalists claims rates are heading in the wrong direction.
One possible answer relates the ever-broadening scope of hospitalist practice. Hospitalists are being asked to care for surgical patients and other patient populations that they may have less familiarity with, increasing the risk of medical errors. Among the other specialties most commonly also named in hospitalist claims, general surgery and orthopedic surgery are in the top five. The extraordinary growth in the field of hospital medicine has meant a need to hire an increasing number of hospitalists, leading to less-experienced physicians entering the field.
Making hospital medicine safer
A more urgent question than what is driving the trends in hospitalist claims rates is what can be done to avoid adverse events and make hospital medicine safer. One potential answer is thoughtful collaboration arrangements with the surgical and other specialties with whom hospitalists may be co-managing patients.
Questions about who responds to what types of clinical issues that might arise and specific domains of responsibility should be defined in advance, so that a lack of role clarity does not negatively impact patient care. Given that hospitalists will be less comfortable addressing more technical surgical issues, expectations about surgeons’ availability should be established. Nocturnists may be tasked with overnight cross-coverage of patients on services, such as oncology and cardiology, that subspecialty physicians have responsibility for during the day. Agreeing upon triggers for when the nocturnist should contact the daytime subspecialty attending (for example, if a rapid response is called on their patient) should be considered, so that nocturnists are not left deciding, in the moment, whether to call the daytime attending. Measures such as this ensure that everyone’s expectations are aligned. In addition, new hospitalists need to be offered support, in the form of training and mentorship.
CBS malpractice data, which includes the contributing factors underlying what went wrong, illuminates potential targets for programs designed to enhance patient safety. In the recent hospitalist malpractice study, the two contributing factors that were the best predictors of a hospitalist malpractice claim closing with payment to the claimant were clinical judgment errors and communication breakdowns. Identifying measures that are effective in promoting patient safety by refining the clinical judgement of clinicians is a challenge, and there are limited data demonstrating what programs are effective in this area.
Clinical decision support (CDS) systems have shown promise in promoting guideline-concordant care.7 However, the role of CDS in aiding the higher-stakes clinical decisions that may be called into question after an adverse outcome is not well defined. Alerts that a patient may be developing sepsis is one type of CDS that has been extensively studied and has been shown to be of some benefit.8 The importance of clinical judgment to whether payment is made on a malpractice claim can inform risk management strategies. Hospitalists should document the thought process behind their decision making in the chart, especially for important clinical decisions. A note showing that the clinician was thoughtfully weighing the risks and benefits using the data available at the time will help make a case defensible if an adverse outcome occurs.
The effect of communication breakdowns on hospitalist case outcomes highlights the importance of measures to improve and systematize communication among clinicians, particularly at vulnerable junctures – such as handoffs from the day team to the night team, and transitions from one care setting to another. An example of an intervention to improve handoffs with cogent evidence to support it is I-PASS, which is an approach to handoffs between teams in which information about the patient’s illness severity, clinical background, and contingencies is conveyed and synthesized in a structured manner. A study of the effect of implementation of I-PASS among nine pediatric residency programs demonstrated a 30% reduction in preventable adverse events.9
Applying insights from malpractice claims analysis to clinical practice
The systematic review of malpractice cases to determine the contributing factors and other case attributes is an important source of patient safety insights. The process breakdowns described by the contributing factors can inform the design of patient safety initiatives. In addition, malpractice data provides information on which specialties and what types of clinicians are being named together in malpractice claims.
In the hospitalist malpractice study, in addition to general surgery and orthopedic surgery, the other clinical services most commonly subject to claims along with hospitalists were nursing, emergency medicine, and cardiology. Another observation was that physician assistants and nurse practitioners are increasingly being named in hospitalist claims. This information is crucial to guiding who needs to be in the room with hospitalists when efforts are undertaken to enhance patient safety within hospital medicine.
An understandable response to the finding that hospitalist claims rates are not decreasing is for hospitalists to seek ways to lower their risk of being named in a malpractice claim. Of course, avoiding adverse events by providing the safest possible care is paramount. Even when patients do suffer adverse events due to a physician negligence, only rarely, less than 5% of the time, does this result in a malpractice claim.10 Important lessons in risk management can be learned from examining why patients decide to sue when mistakes lead to bad outcomes.
An analysis of plaintiffs’ depositions found that the key reasons that patients decided to file a malpractice claim include a poor relationship with the physician – specifically, a lack of empathy from the physician, feeling deserted by the physician, and feeling devalued by the physician.11 These findings support the use of programs that assist physicians in compassionately disclosing adverse events to patients. Among inpatient physicians, patient satisfaction survey questions about the time the physician spent with the patient and the physician’s concern for the patient are better predictors of the physicians’ risk management performance than is the question about the skill of the physician.12 In the aftermath of an adverse event, focusing on maintaining a strong patient-physician relationship is not only the right the thing to do, the data tell us that it is also a sensible approach to reducing medicolegal risk.
Dr. Schaffer practices as a member of the Hospital Medicine Unit at Brigham and Women’s Hospital, Boston, where he serves as an attending physician on the inpatient general medicine services. An instructor at Harvard Medical School, his academic interests include research using large medical malpractice databases to examine temporal trends in medical malpractice.
References
1. Rothberg MB, et al. The cost of defensive medicine on 3 hospital medicine services. JAMA Intern Med. 2014;174(11):1867-1868. doi:10.1001/jamainternmed.2014.4649.
2. Kachalia A, et al. Overuse of testing in preoperative evaluation and syncope: A survey of hospitalists. Ann Intern Med. 2015;162(2):100-108. doi: 10.7326/M14-0694.
3. Mello MM, et al. National costs of the medical liability system. Health Aff (Millwood). 2010;29(9):1569-1577. doi: 10.1377/hlthaff.2009.0807.
4. Schaffer AC, et al. Rates and characteristics of paid malpractice claims among U.S. physicians by specialty, 1992-2014. JAMA Intern Med. 2017;177(5):710-718. doi:10.1001/jamainternmed.2017.0311.
5. Schaffer AC, et al. Liability impact of the hospitalist model of care. J Hosp Med. 2014;9(12):750-755. doi: 10.1002/jhm.2244.
6. Schaffer AC, et al. Rates and characteristics of medical malpractice claims against hospitalists. J Hosp Med. 2021 Jul;16(7):390-396. doi: 10.12788/jhm.3557.
7. Poon EG. Clinical decision support: a tool of the hospital trade. J Hosp Med. 2015;10(1):60-61. doi: 10.1002/jhm.2295.
8. Makam AN, Nguyen OK, Auerbach AD. Diagnostic accuracy and effectiveness of automated electronic sepsis alert systems: A systematic review. J Hosp Med. 2015;10(6):396-402. doi: 10.1002/jhm.2347.
9. Starmer AJ, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803-1812. doi: 10.1056/NEJMsa1405556.
10. Localio AR, et al. Relation between malpractice claims and adverse events due to negligence. Results of the Harvard Medical Practice Study III. N Engl J Med. 1991;325(4):245-251. doi: 10.1056/NEJM199107253250405.
11. Beckman HB, et al. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365-1370. doi:10.1001/archinte.1994.00420120093010.
12. Stelfox HT, et al. The relation of patient satisfaction with complaints against physicians and malpractice lawsuits. Am J Med. 2005;118(10):1126-1133. doi: 10.1016/j.amjmed.2005.01.060.
Untreatable, drug-resistant fungus found in Texas and Washington, D.C.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Increases in new COVID cases among children far outpace vaccinations
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.
Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
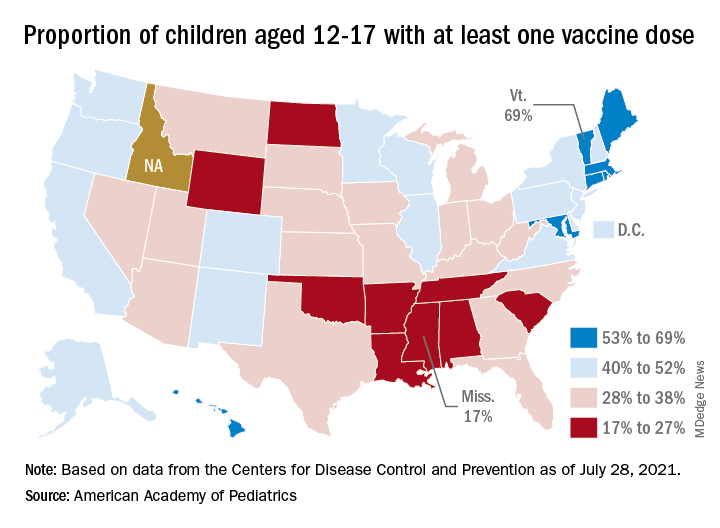
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
New COVID-19 cases in children soared by almost 86% over the course of just 1 week, while the number of 12- to 17-year-old children who have received at least one dose of vaccine rose by 5.4%, according to two separate sources.

Meanwhile, the increase over the past 2 weeks – from 23,551 new cases for July 16-22 to almost 72,000 – works out to almost 205%, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Children represented 19.0% of the cases reported during the week of July 23-29, and they have made up 14.3% of all cases since the pandemic began, with the total number of cases in children now approaching 4.2 million, the AAP and CHA said in their weekly COVID report. About 22% of the U.S. population is under the age of 18 years.
As of Aug. 2, just over 9.8 million children aged 12-17 years had received at least one dose of the COVID vaccine, which was up by about 500,000, or 5.4%, from a week earlier, based on data from the Centers for Disease Control and Prevention.
Children aged 16-17 have reached a notable milestone on the journey that started with vaccine approval in December: 50.2% have gotten at least one dose and 40.3% are fully vaccinated. Among children aged 12-15 years, the proportion with at least one dose of vaccine is up to 39.5%, compared with 37.1% the previous week, while 29.0% are fully vaccinated (27.8% the week before), the CDC said on its COVID Data Tracker.
The national rates for child vaccination, however, tend to hide the disparities between states. There is a gap between Mississippi (lowest), where just 17% of children aged 12-17 years have gotten at least one dose, and Vermont (highest), which is up to 69%. Vermont also has the highest rate of vaccine completion (60%), while Alabama and Mississippi have the lowest (10%), according to a solo report from the AAP.
Never prouder to be a hospitalist
I have been a proud hospitalist for more than 20 years, and yet I have never been prouder to be a hospitalist than now. The pandemic has been brutal, killing more than 600,000 Americans as of this writing. It has stretched the health care system, its doctors, nurses, and other providers to the limit. Yet we will get through it, we are getting through it, and hospitalists deserve a huge portion of the credit.
According to the CDC, there have been over 2.3 million COVID-19 hospitalizations. In my home state of Maryland, between two-thirds and three-quarters of hospitalized COVID patients are cared for on general medical floors, the domain of hospitalists. When hospitals needed COVID units, hospitalists stepped up to design and staff them. When our ICU colleagues needed support, especially in those early dark days, hospitalists stepped in. When our outpatient colleagues were called into the hospital, hospitalists were there to help them on board. When the House of Medicine was in chaos due to COVID-19, hospitalists ran towards that fire. Our previous 20+ years of collective experience made us the ideal specialty to manage the inpatient challenges over the last 18 months.
Need a new clinical schedule by Sunday? Check.
Need help with new clinical protocols? Check.
Need to help other colleagues? Check.
Need to reprogram the EMR? Check.
Need a new way to teach residents and students on the wards? Check.
Need a whole new unit – no, wait – a new hospital wing? No, scratch that – a whole new COVID hospital in a few weeks? Check. (I personally did that last one at the Baltimore Convention Center!)
For me and many hospitalists like me, it is as if the last 20 years were prep work for the pandemic.
Here at SHM, we know the pandemic is hard work – exhausting, even. SHM has been actively focused on supporting hospitalists during this crisis so that hospitalists can focus on patients. Early in the pandemic, SHM quickly pivoted to supply hospitalists with COVID-19 resources in their fight against the coronavirus. Numerous COVID-19 webinars, a COVID addendum to the State of Hospital Medicine Report, and a dedicated COVID issue of the Journal of Hospital Medicine were early and successful information dissemination strategies.
As the world – and hospitalists – dug in for a multi-year pandemic, SHM continued to advance the care of patients by opening our library of educational content for free to anyone. Our Public Policy Committee was active around both COVID-19- and hospitalist-related topics: immigration, telehealth, wellbeing, and financial impacts, to name a few.
As the pandemic slogged on, our Wellbeing Task Force came up with innovative support measures, including a check-in guide for hospitalists and fellow health care workers and dedicated wellness sessions complete with a licensed therapist for members. All the while, despite the restrictions and hurdles the pandemic has thrown our way, SHM members keep meeting and collaborating through virtual chapter events, committee work, special interest groups, and our annual conference, SHM Converge. Thank you to the countless members who donated their time to SHM, so that SHM could support hospitalists and their patients.
Now, we are transitioning into a new phase of the pandemic. The medical miracles that are the COVID-19 vaccines have made that possible. Fully vaccinated, I no longer worry that every time someone sneezes, or when I care for patients with a fever, that I am playing a high stakes poker game with my life. Don’t get me wrong; as I write, the Delta variant has a hold on the nation, and I know it’s not over yet. But it does appear as if the medical war on COVID is shifting from national to regional (or even local) responses.
During this new phase, we must rebuild our personal and professional lives. If you haven’t read Retired Lieutenant General Mark Hertling’s perspective piece in the August issue of the Journal of Hospital Medicine, I strongly encourage you to do so. He shares profound lessons on transitioning from active combat that are directly applicable to hospitalists who have been “deployed” battling COVID-19.
SHM will continue to pivot to meet our members’ needs too. We are already gearing up for more in-person education and networking. Chapters are starting to meet in person, and SHM is happy to provide visiting faculty. I will visit members from Florida to Maine and places in between starting this fall! Our Board of Directors and other SHM leaders are also starting to meet with members in person. Our own Leadership Academy will take place at Amelia Island in Florida in October, where we can learn, network, and even decompress. We also can’t wait for SHM Converge 2022 in Nashville, where we hope to reunite with many of you after 2 years of virtual conferences.
Our response to the pandemic, a once in a century crisis where our own safety was at risk, where doing the right thing might mean death or harming loved ones, our response of running into the fire to save lives is truly inspiring. The power of care – for our patients, for our family and friends, and for our hospital medicine community and the community at large – is evident more now than ever.
There have always been good reasons to be proud of being a hospitalist: taking care of the acutely ill, helping hospitals improve, teaching young doctors, and watching my specialty grow by leaps and bounds, to name just a few. But I’ve never been prouder than I am now.
Dr. Howell is the CEO of the Society of Hospital Medicine.
I have been a proud hospitalist for more than 20 years, and yet I have never been prouder to be a hospitalist than now. The pandemic has been brutal, killing more than 600,000 Americans as of this writing. It has stretched the health care system, its doctors, nurses, and other providers to the limit. Yet we will get through it, we are getting through it, and hospitalists deserve a huge portion of the credit.
According to the CDC, there have been over 2.3 million COVID-19 hospitalizations. In my home state of Maryland, between two-thirds and three-quarters of hospitalized COVID patients are cared for on general medical floors, the domain of hospitalists. When hospitals needed COVID units, hospitalists stepped up to design and staff them. When our ICU colleagues needed support, especially in those early dark days, hospitalists stepped in. When our outpatient colleagues were called into the hospital, hospitalists were there to help them on board. When the House of Medicine was in chaos due to COVID-19, hospitalists ran towards that fire. Our previous 20+ years of collective experience made us the ideal specialty to manage the inpatient challenges over the last 18 months.
Need a new clinical schedule by Sunday? Check.
Need help with new clinical protocols? Check.
Need to help other colleagues? Check.
Need to reprogram the EMR? Check.
Need a new way to teach residents and students on the wards? Check.
Need a whole new unit – no, wait – a new hospital wing? No, scratch that – a whole new COVID hospital in a few weeks? Check. (I personally did that last one at the Baltimore Convention Center!)
For me and many hospitalists like me, it is as if the last 20 years were prep work for the pandemic.
Here at SHM, we know the pandemic is hard work – exhausting, even. SHM has been actively focused on supporting hospitalists during this crisis so that hospitalists can focus on patients. Early in the pandemic, SHM quickly pivoted to supply hospitalists with COVID-19 resources in their fight against the coronavirus. Numerous COVID-19 webinars, a COVID addendum to the State of Hospital Medicine Report, and a dedicated COVID issue of the Journal of Hospital Medicine were early and successful information dissemination strategies.
As the world – and hospitalists – dug in for a multi-year pandemic, SHM continued to advance the care of patients by opening our library of educational content for free to anyone. Our Public Policy Committee was active around both COVID-19- and hospitalist-related topics: immigration, telehealth, wellbeing, and financial impacts, to name a few.
As the pandemic slogged on, our Wellbeing Task Force came up with innovative support measures, including a check-in guide for hospitalists and fellow health care workers and dedicated wellness sessions complete with a licensed therapist for members. All the while, despite the restrictions and hurdles the pandemic has thrown our way, SHM members keep meeting and collaborating through virtual chapter events, committee work, special interest groups, and our annual conference, SHM Converge. Thank you to the countless members who donated their time to SHM, so that SHM could support hospitalists and their patients.
Now, we are transitioning into a new phase of the pandemic. The medical miracles that are the COVID-19 vaccines have made that possible. Fully vaccinated, I no longer worry that every time someone sneezes, or when I care for patients with a fever, that I am playing a high stakes poker game with my life. Don’t get me wrong; as I write, the Delta variant has a hold on the nation, and I know it’s not over yet. But it does appear as if the medical war on COVID is shifting from national to regional (or even local) responses.
During this new phase, we must rebuild our personal and professional lives. If you haven’t read Retired Lieutenant General Mark Hertling’s perspective piece in the August issue of the Journal of Hospital Medicine, I strongly encourage you to do so. He shares profound lessons on transitioning from active combat that are directly applicable to hospitalists who have been “deployed” battling COVID-19.
SHM will continue to pivot to meet our members’ needs too. We are already gearing up for more in-person education and networking. Chapters are starting to meet in person, and SHM is happy to provide visiting faculty. I will visit members from Florida to Maine and places in between starting this fall! Our Board of Directors and other SHM leaders are also starting to meet with members in person. Our own Leadership Academy will take place at Amelia Island in Florida in October, where we can learn, network, and even decompress. We also can’t wait for SHM Converge 2022 in Nashville, where we hope to reunite with many of you after 2 years of virtual conferences.
Our response to the pandemic, a once in a century crisis where our own safety was at risk, where doing the right thing might mean death or harming loved ones, our response of running into the fire to save lives is truly inspiring. The power of care – for our patients, for our family and friends, and for our hospital medicine community and the community at large – is evident more now than ever.
There have always been good reasons to be proud of being a hospitalist: taking care of the acutely ill, helping hospitals improve, teaching young doctors, and watching my specialty grow by leaps and bounds, to name just a few. But I’ve never been prouder than I am now.
Dr. Howell is the CEO of the Society of Hospital Medicine.
I have been a proud hospitalist for more than 20 years, and yet I have never been prouder to be a hospitalist than now. The pandemic has been brutal, killing more than 600,000 Americans as of this writing. It has stretched the health care system, its doctors, nurses, and other providers to the limit. Yet we will get through it, we are getting through it, and hospitalists deserve a huge portion of the credit.
According to the CDC, there have been over 2.3 million COVID-19 hospitalizations. In my home state of Maryland, between two-thirds and three-quarters of hospitalized COVID patients are cared for on general medical floors, the domain of hospitalists. When hospitals needed COVID units, hospitalists stepped up to design and staff them. When our ICU colleagues needed support, especially in those early dark days, hospitalists stepped in. When our outpatient colleagues were called into the hospital, hospitalists were there to help them on board. When the House of Medicine was in chaos due to COVID-19, hospitalists ran towards that fire. Our previous 20+ years of collective experience made us the ideal specialty to manage the inpatient challenges over the last 18 months.
Need a new clinical schedule by Sunday? Check.
Need help with new clinical protocols? Check.
Need to help other colleagues? Check.
Need to reprogram the EMR? Check.
Need a new way to teach residents and students on the wards? Check.
Need a whole new unit – no, wait – a new hospital wing? No, scratch that – a whole new COVID hospital in a few weeks? Check. (I personally did that last one at the Baltimore Convention Center!)
For me and many hospitalists like me, it is as if the last 20 years were prep work for the pandemic.
Here at SHM, we know the pandemic is hard work – exhausting, even. SHM has been actively focused on supporting hospitalists during this crisis so that hospitalists can focus on patients. Early in the pandemic, SHM quickly pivoted to supply hospitalists with COVID-19 resources in their fight against the coronavirus. Numerous COVID-19 webinars, a COVID addendum to the State of Hospital Medicine Report, and a dedicated COVID issue of the Journal of Hospital Medicine were early and successful information dissemination strategies.
As the world – and hospitalists – dug in for a multi-year pandemic, SHM continued to advance the care of patients by opening our library of educational content for free to anyone. Our Public Policy Committee was active around both COVID-19- and hospitalist-related topics: immigration, telehealth, wellbeing, and financial impacts, to name a few.
As the pandemic slogged on, our Wellbeing Task Force came up with innovative support measures, including a check-in guide for hospitalists and fellow health care workers and dedicated wellness sessions complete with a licensed therapist for members. All the while, despite the restrictions and hurdles the pandemic has thrown our way, SHM members keep meeting and collaborating through virtual chapter events, committee work, special interest groups, and our annual conference, SHM Converge. Thank you to the countless members who donated their time to SHM, so that SHM could support hospitalists and their patients.
Now, we are transitioning into a new phase of the pandemic. The medical miracles that are the COVID-19 vaccines have made that possible. Fully vaccinated, I no longer worry that every time someone sneezes, or when I care for patients with a fever, that I am playing a high stakes poker game with my life. Don’t get me wrong; as I write, the Delta variant has a hold on the nation, and I know it’s not over yet. But it does appear as if the medical war on COVID is shifting from national to regional (or even local) responses.
During this new phase, we must rebuild our personal and professional lives. If you haven’t read Retired Lieutenant General Mark Hertling’s perspective piece in the August issue of the Journal of Hospital Medicine, I strongly encourage you to do so. He shares profound lessons on transitioning from active combat that are directly applicable to hospitalists who have been “deployed” battling COVID-19.
SHM will continue to pivot to meet our members’ needs too. We are already gearing up for more in-person education and networking. Chapters are starting to meet in person, and SHM is happy to provide visiting faculty. I will visit members from Florida to Maine and places in between starting this fall! Our Board of Directors and other SHM leaders are also starting to meet with members in person. Our own Leadership Academy will take place at Amelia Island in Florida in October, where we can learn, network, and even decompress. We also can’t wait for SHM Converge 2022 in Nashville, where we hope to reunite with many of you after 2 years of virtual conferences.
Our response to the pandemic, a once in a century crisis where our own safety was at risk, where doing the right thing might mean death or harming loved ones, our response of running into the fire to save lives is truly inspiring. The power of care – for our patients, for our family and friends, and for our hospital medicine community and the community at large – is evident more now than ever.
There have always been good reasons to be proud of being a hospitalist: taking care of the acutely ill, helping hospitals improve, teaching young doctors, and watching my specialty grow by leaps and bounds, to name just a few. But I’ve never been prouder than I am now.
Dr. Howell is the CEO of the Society of Hospital Medicine.
COVID-19: Delta variant is raising the stakes
Empathetic conversations with unvaccinated people desperately needed
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Empathetic conversations with unvaccinated people desperately needed
Empathetic conversations with unvaccinated people desperately needed
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Mental illness admissions: 18-44 is the age of prevalence
More mental and/or substance use disorders are ranked among the top-five diagnoses for hospitalized men and women aged 18-44 years than for any other age group, according to a recent report from the Agency for Healthcare Research and Quality.
aged 18-44, while depressive disorders were the third-most common (195.0 stays per 100,000) and alcohol-related disorders were fifth at 153.2 per 100,000, Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in an AHRQ statistical brief.
Prevalence was somewhat lower in women aged 18-44 years, with two mental illnesses appearing among the top five nonmaternal diagnoses: Depressive disorders were second at 222.5 stays per 100,000 and bipolar and related disorders were fourth at 142.0 per 100,000. The leading primary diagnosis in women in 2018 was septicemia, which was the most common cause overall in the age group at a rate of 279.3 per 100,000, the investigators reported.
There were no mental and/or substance use disorders in the top five primary diagnoses for any of the other adult age groups – 45-64, 65-74, and ≥75 – included in the report. Septicemia was the leading diagnosis for men in all three groups and for women in two of three (45-64 and ≥75), with osteoarthritis first among women aged 65-74 years, they said.
There was one mental illness among the top-five diagnoses for children under age 18 years, as depressive disorders were the most common reason for stays in girls (176.6 per 100,000 population) and the fifth most common for boys (74.0 per 100,000), said Dr. McDermott of IBM Watson Health and Mr. Roemer of AHRQ.
Septicemia was the leading nonmaternal, nonneonatal diagnosis for all inpatient stays and all ages in 2018 with a rate of 679.5 per 100,000, followed by heart failure (347.9), osteoarthritis (345.5), pneumonia not related to tuberculosis (226.8), and diabetes mellitus (207.8), based on data from the National Inpatient Sample.
Depressive disorders were most common mental health diagnosis in those admitted to hospitals and the 12th most common diagnosis overall; schizophrenia, in 16th place overall, was the only other mental illness among the top 20, the investigators said.
“This information can help establish national health priorities, initiatives, and action plans,” Dr. McDermott and Mr. Roemer wrote, and “at the hospital level, administrators can use diagnosis-related information to inform planning and resource allocation, such as optimizing subspecialty services or units for the care of high-priority conditions.”
More mental and/or substance use disorders are ranked among the top-five diagnoses for hospitalized men and women aged 18-44 years than for any other age group, according to a recent report from the Agency for Healthcare Research and Quality.

aged 18-44, while depressive disorders were the third-most common (195.0 stays per 100,000) and alcohol-related disorders were fifth at 153.2 per 100,000, Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in an AHRQ statistical brief.
Prevalence was somewhat lower in women aged 18-44 years, with two mental illnesses appearing among the top five nonmaternal diagnoses: Depressive disorders were second at 222.5 stays per 100,000 and bipolar and related disorders were fourth at 142.0 per 100,000. The leading primary diagnosis in women in 2018 was septicemia, which was the most common cause overall in the age group at a rate of 279.3 per 100,000, the investigators reported.
There were no mental and/or substance use disorders in the top five primary diagnoses for any of the other adult age groups – 45-64, 65-74, and ≥75 – included in the report. Septicemia was the leading diagnosis for men in all three groups and for women in two of three (45-64 and ≥75), with osteoarthritis first among women aged 65-74 years, they said.
There was one mental illness among the top-five diagnoses for children under age 18 years, as depressive disorders were the most common reason for stays in girls (176.6 per 100,000 population) and the fifth most common for boys (74.0 per 100,000), said Dr. McDermott of IBM Watson Health and Mr. Roemer of AHRQ.
Septicemia was the leading nonmaternal, nonneonatal diagnosis for all inpatient stays and all ages in 2018 with a rate of 679.5 per 100,000, followed by heart failure (347.9), osteoarthritis (345.5), pneumonia not related to tuberculosis (226.8), and diabetes mellitus (207.8), based on data from the National Inpatient Sample.
Depressive disorders were most common mental health diagnosis in those admitted to hospitals and the 12th most common diagnosis overall; schizophrenia, in 16th place overall, was the only other mental illness among the top 20, the investigators said.
“This information can help establish national health priorities, initiatives, and action plans,” Dr. McDermott and Mr. Roemer wrote, and “at the hospital level, administrators can use diagnosis-related information to inform planning and resource allocation, such as optimizing subspecialty services or units for the care of high-priority conditions.”
More mental and/or substance use disorders are ranked among the top-five diagnoses for hospitalized men and women aged 18-44 years than for any other age group, according to a recent report from the Agency for Healthcare Research and Quality.

aged 18-44, while depressive disorders were the third-most common (195.0 stays per 100,000) and alcohol-related disorders were fifth at 153.2 per 100,000, Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in an AHRQ statistical brief.
Prevalence was somewhat lower in women aged 18-44 years, with two mental illnesses appearing among the top five nonmaternal diagnoses: Depressive disorders were second at 222.5 stays per 100,000 and bipolar and related disorders were fourth at 142.0 per 100,000. The leading primary diagnosis in women in 2018 was septicemia, which was the most common cause overall in the age group at a rate of 279.3 per 100,000, the investigators reported.
There were no mental and/or substance use disorders in the top five primary diagnoses for any of the other adult age groups – 45-64, 65-74, and ≥75 – included in the report. Septicemia was the leading diagnosis for men in all three groups and for women in two of three (45-64 and ≥75), with osteoarthritis first among women aged 65-74 years, they said.
There was one mental illness among the top-five diagnoses for children under age 18 years, as depressive disorders were the most common reason for stays in girls (176.6 per 100,000 population) and the fifth most common for boys (74.0 per 100,000), said Dr. McDermott of IBM Watson Health and Mr. Roemer of AHRQ.
Septicemia was the leading nonmaternal, nonneonatal diagnosis for all inpatient stays and all ages in 2018 with a rate of 679.5 per 100,000, followed by heart failure (347.9), osteoarthritis (345.5), pneumonia not related to tuberculosis (226.8), and diabetes mellitus (207.8), based on data from the National Inpatient Sample.
Depressive disorders were most common mental health diagnosis in those admitted to hospitals and the 12th most common diagnosis overall; schizophrenia, in 16th place overall, was the only other mental illness among the top 20, the investigators said.
“This information can help establish national health priorities, initiatives, and action plans,” Dr. McDermott and Mr. Roemer wrote, and “at the hospital level, administrators can use diagnosis-related information to inform planning and resource allocation, such as optimizing subspecialty services or units for the care of high-priority conditions.”
Hospital disaster preparation confronts COVID
Hospitalist groups should have disaster response plans
Jason Persoff, MD, SFHM, now a hospitalist at University of Colorado Hospital in Aurora and an amateur storm chaser, got a close look at how natural disasters can impact hospital care when a tornado destroyed St. John’s Regional Medical Center in Joplin, Mo., on May 22, 2011.
He and a colleague who had been following the storm responded to injuries on the highway before reporting for a long day’s service at the other hospital in Joplin, Freeman Hospital West, caring for patients transferred from St. John’s on an impromptu unit without access to their medical records.
“During my medical training, I had done emergency medicine as an EMT, so I was interested in how the system responds to emergencies,” he explained. “At Joplin I learned how it feels when the boots on the ground in a crisis are not connected to an incident command structure.” Another thing he learned was the essential role for hospitalists in a hospital’s response to a crisis – and thus the need to involve them well in advance in the hospital’s planning for future emergencies.
“Disaster preparation – when done right – helps you ‘herd cats’ in a crisis situation,” he said. “The tornado and its wake served as defining moments for me. I used them as the impetus to improve health care’s response to disasters.” Part of that commitment was to help hospitalists understand their part in emergency preparation.1
Dr. Persoff is now the assistant medical director of emergency preparedness at University of Colorado Hospital. He also helped to create a position called physician support supervisor, which is filled by physicians who have held leadership positions in a hospital to help coordinate the disparate needs of all clinicians in a crisis and facilitate rapid response.2
But then along came the COVID pandemic – which in many locales around the world was unprecedented in scope. Dr. Persoff said his hospital was fairly well prepared, after a decade of engagement with emergency planning. It drew on experience with H1N1, also known as swine flu, and the Ebola virus, which killed 11,323 people, primarily in West Africa, from 2013 to 2016, as models. In a matter of days, the CU division of hospital medicine was able to modify and deploy its existing disaster plans to quickly respond to an influx of COVID patients.3
“Basically, what we set out to do was to treat COVID patients as if they were Ebola patients, cordoning them off in a small area of the hospital. That was naive of us,” he said. “We weren’t able to grasp the scale at the outset. It does defy the imagination – how the hospital could fill up with just one type of patient.”
What is disaster planning?
Emergency preparation for hospitals emerged as a recognized medical specialization in the 1970s. Initially it was largely considered the realm of emergency physicians, trauma services, or critical care doctors. Resources such as the World Health Organization, the Federal Emergency Management Agency, and similar groups recommend an all-hazards approach, a broad and flexible strategy for managing emergencies that could include natural disasters – earthquakes, storms, tornadoes, or wildfires – or human-caused events, such as mass shootings or terrorist attacks. The Joint Commission requires accredited hospitals to conduct several disaster drills annually.
The U.S. Hospital Preparedness Program was created in 2002 to enhance the ability of hospitals and health systems to prepare for and respond to bioterrorism attacks on civilians and other public health emergencies, including natural disasters and pandemics. It offers a foundation for national preparedness and a primary source of federal funding for health care system preparedness. The hospital, at the heart of the health care system, is expected to receive the injured and infected, because patients know they can obtain care there.
One of the fundamental tools for crisis response is the incident command system (ICS), which spells out how to quickly establish a command structure and assign responsibility for key tasks as well as overall leadership. The National Incident Management System organizes emergency management across all government levels and the private sector to ensure that the most pressing needs are met and precious resources are used without duplication. ICS is a standardized approach to command, control, and coordination of emergency response using a common hierarchy recognized across organizations, with advance training in how it should be deployed.
A crisis like never before
Nearly every hospital or health system goes through drills for an emergency, said Hassan Khouli, MD, chair of the department of critical care medicine at the Cleveland Clinic, and coauthor of an article in the journal Chest last year outlining 10 principles of emergency preparedness derived from its experience with the COVID pandemic.4 Some of these include: don’t wait; engage a variety of stakeholders; identify sources of truth; and prioritize hospital employees’ safety and well-being.
Part of the preparation is doing table-top exercises, with case scenarios or actual situations presented, working with clinicians on brainstorming and identifying opportunities for improvement, Dr. Khouli said. “These drills are so important, regardless of what the disaster turns out to be. We’ve done that over the years. We are a large health system, very process and detail oriented. Our emergency incident command structure was activated before we saw our first COVID patient,” he said.
“This was a crisis like never before, with huge amounts of uncertainty,” he noted. “But I believe the Cleveland Clinic system did very well, measured by outcomes such as surveys of health care teams across the system, which gave us reassuring results, and clinical outcomes with lower ICU and hospital mortality rates.”
Christopher Whinney, MD, SFHM, department chair of hospital medicine at Cleveland Clinic, said hospitalists worked hand in hand with the health system’s incident command structure and took responsibility for managing non-ICU COVID patients at six hospitals in the system.
“Hospitalists had a place at the table, and we collaborated well with incident command, enterprise redeployment committees, and emergency and critical care colleagues,” he noted. Hospitalists were on the leadership team for a number of planning meetings, and key stakeholders for bringing information back to their groups.
“First thing we did was to look at our workforce. The challenge was how to respond to up to a hundred COVID admissions per day – how to mobilize providers and build surge teams that incorporated primary care providers and medical trainees. We onboarded 200 providers to do hospital care within 60 days,” he said.
“We realized that communication with patients and families was a big part of the challenge, so we assigned people with good communication skills to fill this role. While we were fortunate not to get the terrible surges they had in other places, we felt we were prepared for the worst.”
Challenges of surge capacity
Every disaster is different, said Srikant Polepalli, MD, associate hospitalist medical director for Staten Island University Hospital in New York, part of the Northwell Health system. He brought the experience of being part of the response to Superstorm Sandy in October 2012 to the COVID pandemic.
“Specifically for hospitalists, the biggest challenge is working on surge capacity for a sudden influx of patients,” he said. “But with Northwell as our umbrella, we can triage and load-balance to move patients from hospital to hospital as needed. With the pandemic, we started with one COVID unit and then expanded to fill the entire hospital.”
Dr. Polepalli was appointed medical director for a temporary field hospital installed at South Beach Psychiatric Center, also in Staten Island. “We were able to acquire help and bring in people ranging from hospitalists to ER physicians, travel nurses, operation managers and the National Guard. Our command center did a phenomenal job of allocating and obtaining resources. It helped to have a structure that was already established and to rely on the resources of the health system,” Dr. Polepalli said. Not every hospital has a structure like Northwell’s.
“We’re not out of the pandemic yet, but we’ll continue with disaster drills and planning,” he said. “We must continue to adapt and have converted our temporary facilities to COVID testing centers, antibody infusion centers, and vaccination centers.”
For Alfred Burger, MD, SFHM, a hospitalist at Mount Sinai’s Beth Israel campus in New York, hospital medicine, now in its maturing phase, is still feeling its way through hospital and health care system transformation.
“My group is an academic, multicampus hospitalist group employed by the hospital system. When I meet other hospitalists at SHM conferences, whether they come from privately owned, corporately owned, or contracted models, they vary widely in terms of how involved the hospitalists are in crisis planning and their ability to respond to crises. At large academic medical centers like ours, one or more doctors is tasked with being involved in preparing for the next disaster,” he said.
“I think we responded the best we could, although it was difficult as we lost many patients to COVID. We were trying to save lives using the tools we knew from treating pneumonias and other forms of acute inflammatory lung injuries. We used every bit of our training in situations where no one had the right answers. But disasters teach us how to be flexible and pivot on the fly, and what to do when things don’t go our way.”
What is disaster response?
Medical response to a disaster essentially boils down to three main things: stuff, staff, and space, Dr. Persoff said. Those are the cornerstones of an emergency plan.
“There is not a hazard that exists that you can’t take an all-hazards approach to dealing with fundamental realities on the ground. No plan can be comprehensive enough to deal with all the intricacies of an emergency. But many plans can have the bones of a response that will allow you to face adverse circumstances,” he said.
“We actually became quite efficient early on in the pandemic, able to adapt in the moment. We were able to build an effective bridge between workers on the ground and our incident command structure, which seemed to reduce a lot of stress and create situational awareness. We implemented ICS as soon as we heard that China was building a COVID hospital, back in February of 2020.”
When one thinks about mass trauma, such as a 747 crash, Dr. Persoff said, the need is to treat burn victims and trauma victims in large numbers. At that point, the ED downstairs is filled with medical patients. Hospital medicine can rapidly admit those patients to clear out room in the ED. Surgeons are also dedicated to rapidly treating those patients, but what about patients who are on the floor following their surgeries? Hospitalists can offer consultations or primary management so the surgeons can stay in the OR, and the same in the ICU, while safely discharging hospitalized patients in a timely manner to make room for incoming patients.
“The lessons of COVID have been hard-taught and hard-earned. No good plan survives contact with the enemy,” he said. “But I think we’ll be better prepared for the next pandemic.”
Maria Frank, MD, FACP, SFHM, a hospitalist at Denver Health who chairs SHM’s Disaster Management Special Interest Group, says she got the bug for disaster preparation during postresidency training as an internist in emergency medicine. “I’m also the medical director for our biocontainment unit, created for infections like Ebola.” SHM’s SIG, which has 150 members, is now writing a review article on disaster planning for the field.
“I got a call on Dec. 27, 2019, about this new pneumonia, and they said, ‘We don’t know what it is, but it’s a coronavirus,’” she recalled. “When I got off the phone, I said, ‘Let’s make sure our response plan works and we have enough of everything on hand.’” Dr. Frank said she was expecting something more like SARS (severe acute respiratory syndrome). “When they called the public health emergency of international concern for COVID, I was at a Centers for Disease Control and Prevention meeting in Atlanta. It really wasn’t a surprise for us.”
All hospitals plan for disasters, although they use different names and have different levels of commitment, Dr. Frank said. What’s not consistent is the participation of hospitalists. “Even when a disaster is 100% trauma related, consider a hospital like mine that has at least four times as many hospitalists as surgeons at any given time. The hospitalists need to take overall management for the patients who aren’t actually in the operating room.”
Time to debrief
Dr. Frank recommends debriefing on the hospital’s and the hospitalist group’s experience with COVID. “Look at the biggest challenges your group faced. Was it staffing, or time off, or the need for day care? Was it burnout, lack of knowledge, lack of [personal protective equipment]?” Each hospital could use its own COVID experience to work on identifying the challenges and the problems, she said. “I’d encourage each department and division to do this exercise individually. Then come together to find common ground with other departments in the hospital.”
This debriefing exercise isn’t just for doctors – it’s also for nurses, environmental services, security, and many other departments, she said. “COVID showed us how crisis response is a group effort. What will bring us together is to learn the challenges each of us faced. It was amazing to see hospitalists doing what they do best.” Post pandemic, hospitalists should also consider getting involved in research and publications, in order to share their lessons.
“One of the things we learned is that hospitalists are very versatile,” Dr. Frank added. But it’s also good for the group to have members specialize, for example, in biocontainment. “We are experts in discharging patients, in patient flow and operations, in coordinating complex medical care. So we would naturally take the lead in, for example, opening a geographic unit or collaborating with other specialists to create innovative models. That’s our job. It’s essential that we’re involved well in advance.”
COVID may be a once-in-a-lifetime experience, but there will be other disasters to come, she said. “If your hospital doesn’t have a disaster plan for hospitalists, get involved in establishing one. Each hospitalist group should have its own response plan. Talk to your peers at other hospitals, and get involved at the institutional level. I’m happy to share our plan; just contact me.” Readers can contact Dr. Frank at maria.frank@dhha.org.
References
1. Persoff J et al. The role of hospital medicine in emergency preparedness: A framework for hospitalist leadership in disaster preparedness, response and recovery. J Hosp Med. 2018 Oct;13(10):713-7. doi: 10.12788/jhm.3073.
2. Persoff J et al. Expanding the hospital incident command system with a physician-centric role during a pandemic: The role of the physician clinical support supervisor. J Hosp Adm. 2020;9(3):7-10. doi: 10.5430/jha.v9n3p7.
3. Bowden K et al. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J Gen Intern Med. 2020 Sep;35(9):273-7. doi: 10.1007/s11606-020-05952-6.
4. Orsini E et al. Lessons on outbreak preparedness from the Cleveland Clinic. Chest. 2020;158(5):2090-6. doi: 10.1016/j.chest.2020.06.009.
Hospitalist groups should have disaster response plans
Hospitalist groups should have disaster response plans
Jason Persoff, MD, SFHM, now a hospitalist at University of Colorado Hospital in Aurora and an amateur storm chaser, got a close look at how natural disasters can impact hospital care when a tornado destroyed St. John’s Regional Medical Center in Joplin, Mo., on May 22, 2011.
He and a colleague who had been following the storm responded to injuries on the highway before reporting for a long day’s service at the other hospital in Joplin, Freeman Hospital West, caring for patients transferred from St. John’s on an impromptu unit without access to their medical records.
“During my medical training, I had done emergency medicine as an EMT, so I was interested in how the system responds to emergencies,” he explained. “At Joplin I learned how it feels when the boots on the ground in a crisis are not connected to an incident command structure.” Another thing he learned was the essential role for hospitalists in a hospital’s response to a crisis – and thus the need to involve them well in advance in the hospital’s planning for future emergencies.
“Disaster preparation – when done right – helps you ‘herd cats’ in a crisis situation,” he said. “The tornado and its wake served as defining moments for me. I used them as the impetus to improve health care’s response to disasters.” Part of that commitment was to help hospitalists understand their part in emergency preparation.1
Dr. Persoff is now the assistant medical director of emergency preparedness at University of Colorado Hospital. He also helped to create a position called physician support supervisor, which is filled by physicians who have held leadership positions in a hospital to help coordinate the disparate needs of all clinicians in a crisis and facilitate rapid response.2
But then along came the COVID pandemic – which in many locales around the world was unprecedented in scope. Dr. Persoff said his hospital was fairly well prepared, after a decade of engagement with emergency planning. It drew on experience with H1N1, also known as swine flu, and the Ebola virus, which killed 11,323 people, primarily in West Africa, from 2013 to 2016, as models. In a matter of days, the CU division of hospital medicine was able to modify and deploy its existing disaster plans to quickly respond to an influx of COVID patients.3
“Basically, what we set out to do was to treat COVID patients as if they were Ebola patients, cordoning them off in a small area of the hospital. That was naive of us,” he said. “We weren’t able to grasp the scale at the outset. It does defy the imagination – how the hospital could fill up with just one type of patient.”
What is disaster planning?
Emergency preparation for hospitals emerged as a recognized medical specialization in the 1970s. Initially it was largely considered the realm of emergency physicians, trauma services, or critical care doctors. Resources such as the World Health Organization, the Federal Emergency Management Agency, and similar groups recommend an all-hazards approach, a broad and flexible strategy for managing emergencies that could include natural disasters – earthquakes, storms, tornadoes, or wildfires – or human-caused events, such as mass shootings or terrorist attacks. The Joint Commission requires accredited hospitals to conduct several disaster drills annually.
The U.S. Hospital Preparedness Program was created in 2002 to enhance the ability of hospitals and health systems to prepare for and respond to bioterrorism attacks on civilians and other public health emergencies, including natural disasters and pandemics. It offers a foundation for national preparedness and a primary source of federal funding for health care system preparedness. The hospital, at the heart of the health care system, is expected to receive the injured and infected, because patients know they can obtain care there.
One of the fundamental tools for crisis response is the incident command system (ICS), which spells out how to quickly establish a command structure and assign responsibility for key tasks as well as overall leadership. The National Incident Management System organizes emergency management across all government levels and the private sector to ensure that the most pressing needs are met and precious resources are used without duplication. ICS is a standardized approach to command, control, and coordination of emergency response using a common hierarchy recognized across organizations, with advance training in how it should be deployed.
A crisis like never before
Nearly every hospital or health system goes through drills for an emergency, said Hassan Khouli, MD, chair of the department of critical care medicine at the Cleveland Clinic, and coauthor of an article in the journal Chest last year outlining 10 principles of emergency preparedness derived from its experience with the COVID pandemic.4 Some of these include: don’t wait; engage a variety of stakeholders; identify sources of truth; and prioritize hospital employees’ safety and well-being.
Part of the preparation is doing table-top exercises, with case scenarios or actual situations presented, working with clinicians on brainstorming and identifying opportunities for improvement, Dr. Khouli said. “These drills are so important, regardless of what the disaster turns out to be. We’ve done that over the years. We are a large health system, very process and detail oriented. Our emergency incident command structure was activated before we saw our first COVID patient,” he said.
“This was a crisis like never before, with huge amounts of uncertainty,” he noted. “But I believe the Cleveland Clinic system did very well, measured by outcomes such as surveys of health care teams across the system, which gave us reassuring results, and clinical outcomes with lower ICU and hospital mortality rates.”
Christopher Whinney, MD, SFHM, department chair of hospital medicine at Cleveland Clinic, said hospitalists worked hand in hand with the health system’s incident command structure and took responsibility for managing non-ICU COVID patients at six hospitals in the system.
“Hospitalists had a place at the table, and we collaborated well with incident command, enterprise redeployment committees, and emergency and critical care colleagues,” he noted. Hospitalists were on the leadership team for a number of planning meetings, and key stakeholders for bringing information back to their groups.
“First thing we did was to look at our workforce. The challenge was how to respond to up to a hundred COVID admissions per day – how to mobilize providers and build surge teams that incorporated primary care providers and medical trainees. We onboarded 200 providers to do hospital care within 60 days,” he said.
“We realized that communication with patients and families was a big part of the challenge, so we assigned people with good communication skills to fill this role. While we were fortunate not to get the terrible surges they had in other places, we felt we were prepared for the worst.”
Challenges of surge capacity
Every disaster is different, said Srikant Polepalli, MD, associate hospitalist medical director for Staten Island University Hospital in New York, part of the Northwell Health system. He brought the experience of being part of the response to Superstorm Sandy in October 2012 to the COVID pandemic.
“Specifically for hospitalists, the biggest challenge is working on surge capacity for a sudden influx of patients,” he said. “But with Northwell as our umbrella, we can triage and load-balance to move patients from hospital to hospital as needed. With the pandemic, we started with one COVID unit and then expanded to fill the entire hospital.”
Dr. Polepalli was appointed medical director for a temporary field hospital installed at South Beach Psychiatric Center, also in Staten Island. “We were able to acquire help and bring in people ranging from hospitalists to ER physicians, travel nurses, operation managers and the National Guard. Our command center did a phenomenal job of allocating and obtaining resources. It helped to have a structure that was already established and to rely on the resources of the health system,” Dr. Polepalli said. Not every hospital has a structure like Northwell’s.
“We’re not out of the pandemic yet, but we’ll continue with disaster drills and planning,” he said. “We must continue to adapt and have converted our temporary facilities to COVID testing centers, antibody infusion centers, and vaccination centers.”
For Alfred Burger, MD, SFHM, a hospitalist at Mount Sinai’s Beth Israel campus in New York, hospital medicine, now in its maturing phase, is still feeling its way through hospital and health care system transformation.
“My group is an academic, multicampus hospitalist group employed by the hospital system. When I meet other hospitalists at SHM conferences, whether they come from privately owned, corporately owned, or contracted models, they vary widely in terms of how involved the hospitalists are in crisis planning and their ability to respond to crises. At large academic medical centers like ours, one or more doctors is tasked with being involved in preparing for the next disaster,” he said.
“I think we responded the best we could, although it was difficult as we lost many patients to COVID. We were trying to save lives using the tools we knew from treating pneumonias and other forms of acute inflammatory lung injuries. We used every bit of our training in situations where no one had the right answers. But disasters teach us how to be flexible and pivot on the fly, and what to do when things don’t go our way.”
What is disaster response?
Medical response to a disaster essentially boils down to three main things: stuff, staff, and space, Dr. Persoff said. Those are the cornerstones of an emergency plan.
“There is not a hazard that exists that you can’t take an all-hazards approach to dealing with fundamental realities on the ground. No plan can be comprehensive enough to deal with all the intricacies of an emergency. But many plans can have the bones of a response that will allow you to face adverse circumstances,” he said.
“We actually became quite efficient early on in the pandemic, able to adapt in the moment. We were able to build an effective bridge between workers on the ground and our incident command structure, which seemed to reduce a lot of stress and create situational awareness. We implemented ICS as soon as we heard that China was building a COVID hospital, back in February of 2020.”
When one thinks about mass trauma, such as a 747 crash, Dr. Persoff said, the need is to treat burn victims and trauma victims in large numbers. At that point, the ED downstairs is filled with medical patients. Hospital medicine can rapidly admit those patients to clear out room in the ED. Surgeons are also dedicated to rapidly treating those patients, but what about patients who are on the floor following their surgeries? Hospitalists can offer consultations or primary management so the surgeons can stay in the OR, and the same in the ICU, while safely discharging hospitalized patients in a timely manner to make room for incoming patients.
“The lessons of COVID have been hard-taught and hard-earned. No good plan survives contact with the enemy,” he said. “But I think we’ll be better prepared for the next pandemic.”
Maria Frank, MD, FACP, SFHM, a hospitalist at Denver Health who chairs SHM’s Disaster Management Special Interest Group, says she got the bug for disaster preparation during postresidency training as an internist in emergency medicine. “I’m also the medical director for our biocontainment unit, created for infections like Ebola.” SHM’s SIG, which has 150 members, is now writing a review article on disaster planning for the field.
“I got a call on Dec. 27, 2019, about this new pneumonia, and they said, ‘We don’t know what it is, but it’s a coronavirus,’” she recalled. “When I got off the phone, I said, ‘Let’s make sure our response plan works and we have enough of everything on hand.’” Dr. Frank said she was expecting something more like SARS (severe acute respiratory syndrome). “When they called the public health emergency of international concern for COVID, I was at a Centers for Disease Control and Prevention meeting in Atlanta. It really wasn’t a surprise for us.”
All hospitals plan for disasters, although they use different names and have different levels of commitment, Dr. Frank said. What’s not consistent is the participation of hospitalists. “Even when a disaster is 100% trauma related, consider a hospital like mine that has at least four times as many hospitalists as surgeons at any given time. The hospitalists need to take overall management for the patients who aren’t actually in the operating room.”
Time to debrief
Dr. Frank recommends debriefing on the hospital’s and the hospitalist group’s experience with COVID. “Look at the biggest challenges your group faced. Was it staffing, or time off, or the need for day care? Was it burnout, lack of knowledge, lack of [personal protective equipment]?” Each hospital could use its own COVID experience to work on identifying the challenges and the problems, she said. “I’d encourage each department and division to do this exercise individually. Then come together to find common ground with other departments in the hospital.”
This debriefing exercise isn’t just for doctors – it’s also for nurses, environmental services, security, and many other departments, she said. “COVID showed us how crisis response is a group effort. What will bring us together is to learn the challenges each of us faced. It was amazing to see hospitalists doing what they do best.” Post pandemic, hospitalists should also consider getting involved in research and publications, in order to share their lessons.
“One of the things we learned is that hospitalists are very versatile,” Dr. Frank added. But it’s also good for the group to have members specialize, for example, in biocontainment. “We are experts in discharging patients, in patient flow and operations, in coordinating complex medical care. So we would naturally take the lead in, for example, opening a geographic unit or collaborating with other specialists to create innovative models. That’s our job. It’s essential that we’re involved well in advance.”
COVID may be a once-in-a-lifetime experience, but there will be other disasters to come, she said. “If your hospital doesn’t have a disaster plan for hospitalists, get involved in establishing one. Each hospitalist group should have its own response plan. Talk to your peers at other hospitals, and get involved at the institutional level. I’m happy to share our plan; just contact me.” Readers can contact Dr. Frank at maria.frank@dhha.org.
References
1. Persoff J et al. The role of hospital medicine in emergency preparedness: A framework for hospitalist leadership in disaster preparedness, response and recovery. J Hosp Med. 2018 Oct;13(10):713-7. doi: 10.12788/jhm.3073.
2. Persoff J et al. Expanding the hospital incident command system with a physician-centric role during a pandemic: The role of the physician clinical support supervisor. J Hosp Adm. 2020;9(3):7-10. doi: 10.5430/jha.v9n3p7.
3. Bowden K et al. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J Gen Intern Med. 2020 Sep;35(9):273-7. doi: 10.1007/s11606-020-05952-6.
4. Orsini E et al. Lessons on outbreak preparedness from the Cleveland Clinic. Chest. 2020;158(5):2090-6. doi: 10.1016/j.chest.2020.06.009.
Jason Persoff, MD, SFHM, now a hospitalist at University of Colorado Hospital in Aurora and an amateur storm chaser, got a close look at how natural disasters can impact hospital care when a tornado destroyed St. John’s Regional Medical Center in Joplin, Mo., on May 22, 2011.
He and a colleague who had been following the storm responded to injuries on the highway before reporting for a long day’s service at the other hospital in Joplin, Freeman Hospital West, caring for patients transferred from St. John’s on an impromptu unit without access to their medical records.
“During my medical training, I had done emergency medicine as an EMT, so I was interested in how the system responds to emergencies,” he explained. “At Joplin I learned how it feels when the boots on the ground in a crisis are not connected to an incident command structure.” Another thing he learned was the essential role for hospitalists in a hospital’s response to a crisis – and thus the need to involve them well in advance in the hospital’s planning for future emergencies.
“Disaster preparation – when done right – helps you ‘herd cats’ in a crisis situation,” he said. “The tornado and its wake served as defining moments for me. I used them as the impetus to improve health care’s response to disasters.” Part of that commitment was to help hospitalists understand their part in emergency preparation.1
Dr. Persoff is now the assistant medical director of emergency preparedness at University of Colorado Hospital. He also helped to create a position called physician support supervisor, which is filled by physicians who have held leadership positions in a hospital to help coordinate the disparate needs of all clinicians in a crisis and facilitate rapid response.2
But then along came the COVID pandemic – which in many locales around the world was unprecedented in scope. Dr. Persoff said his hospital was fairly well prepared, after a decade of engagement with emergency planning. It drew on experience with H1N1, also known as swine flu, and the Ebola virus, which killed 11,323 people, primarily in West Africa, from 2013 to 2016, as models. In a matter of days, the CU division of hospital medicine was able to modify and deploy its existing disaster plans to quickly respond to an influx of COVID patients.3
“Basically, what we set out to do was to treat COVID patients as if they were Ebola patients, cordoning them off in a small area of the hospital. That was naive of us,” he said. “We weren’t able to grasp the scale at the outset. It does defy the imagination – how the hospital could fill up with just one type of patient.”
What is disaster planning?
Emergency preparation for hospitals emerged as a recognized medical specialization in the 1970s. Initially it was largely considered the realm of emergency physicians, trauma services, or critical care doctors. Resources such as the World Health Organization, the Federal Emergency Management Agency, and similar groups recommend an all-hazards approach, a broad and flexible strategy for managing emergencies that could include natural disasters – earthquakes, storms, tornadoes, or wildfires – or human-caused events, such as mass shootings or terrorist attacks. The Joint Commission requires accredited hospitals to conduct several disaster drills annually.
The U.S. Hospital Preparedness Program was created in 2002 to enhance the ability of hospitals and health systems to prepare for and respond to bioterrorism attacks on civilians and other public health emergencies, including natural disasters and pandemics. It offers a foundation for national preparedness and a primary source of federal funding for health care system preparedness. The hospital, at the heart of the health care system, is expected to receive the injured and infected, because patients know they can obtain care there.
One of the fundamental tools for crisis response is the incident command system (ICS), which spells out how to quickly establish a command structure and assign responsibility for key tasks as well as overall leadership. The National Incident Management System organizes emergency management across all government levels and the private sector to ensure that the most pressing needs are met and precious resources are used without duplication. ICS is a standardized approach to command, control, and coordination of emergency response using a common hierarchy recognized across organizations, with advance training in how it should be deployed.
A crisis like never before
Nearly every hospital or health system goes through drills for an emergency, said Hassan Khouli, MD, chair of the department of critical care medicine at the Cleveland Clinic, and coauthor of an article in the journal Chest last year outlining 10 principles of emergency preparedness derived from its experience with the COVID pandemic.4 Some of these include: don’t wait; engage a variety of stakeholders; identify sources of truth; and prioritize hospital employees’ safety and well-being.
Part of the preparation is doing table-top exercises, with case scenarios or actual situations presented, working with clinicians on brainstorming and identifying opportunities for improvement, Dr. Khouli said. “These drills are so important, regardless of what the disaster turns out to be. We’ve done that over the years. We are a large health system, very process and detail oriented. Our emergency incident command structure was activated before we saw our first COVID patient,” he said.
“This was a crisis like never before, with huge amounts of uncertainty,” he noted. “But I believe the Cleveland Clinic system did very well, measured by outcomes such as surveys of health care teams across the system, which gave us reassuring results, and clinical outcomes with lower ICU and hospital mortality rates.”
Christopher Whinney, MD, SFHM, department chair of hospital medicine at Cleveland Clinic, said hospitalists worked hand in hand with the health system’s incident command structure and took responsibility for managing non-ICU COVID patients at six hospitals in the system.
“Hospitalists had a place at the table, and we collaborated well with incident command, enterprise redeployment committees, and emergency and critical care colleagues,” he noted. Hospitalists were on the leadership team for a number of planning meetings, and key stakeholders for bringing information back to their groups.
“First thing we did was to look at our workforce. The challenge was how to respond to up to a hundred COVID admissions per day – how to mobilize providers and build surge teams that incorporated primary care providers and medical trainees. We onboarded 200 providers to do hospital care within 60 days,” he said.
“We realized that communication with patients and families was a big part of the challenge, so we assigned people with good communication skills to fill this role. While we were fortunate not to get the terrible surges they had in other places, we felt we were prepared for the worst.”
Challenges of surge capacity
Every disaster is different, said Srikant Polepalli, MD, associate hospitalist medical director for Staten Island University Hospital in New York, part of the Northwell Health system. He brought the experience of being part of the response to Superstorm Sandy in October 2012 to the COVID pandemic.
“Specifically for hospitalists, the biggest challenge is working on surge capacity for a sudden influx of patients,” he said. “But with Northwell as our umbrella, we can triage and load-balance to move patients from hospital to hospital as needed. With the pandemic, we started with one COVID unit and then expanded to fill the entire hospital.”
Dr. Polepalli was appointed medical director for a temporary field hospital installed at South Beach Psychiatric Center, also in Staten Island. “We were able to acquire help and bring in people ranging from hospitalists to ER physicians, travel nurses, operation managers and the National Guard. Our command center did a phenomenal job of allocating and obtaining resources. It helped to have a structure that was already established and to rely on the resources of the health system,” Dr. Polepalli said. Not every hospital has a structure like Northwell’s.
“We’re not out of the pandemic yet, but we’ll continue with disaster drills and planning,” he said. “We must continue to adapt and have converted our temporary facilities to COVID testing centers, antibody infusion centers, and vaccination centers.”
For Alfred Burger, MD, SFHM, a hospitalist at Mount Sinai’s Beth Israel campus in New York, hospital medicine, now in its maturing phase, is still feeling its way through hospital and health care system transformation.
“My group is an academic, multicampus hospitalist group employed by the hospital system. When I meet other hospitalists at SHM conferences, whether they come from privately owned, corporately owned, or contracted models, they vary widely in terms of how involved the hospitalists are in crisis planning and their ability to respond to crises. At large academic medical centers like ours, one or more doctors is tasked with being involved in preparing for the next disaster,” he said.
“I think we responded the best we could, although it was difficult as we lost many patients to COVID. We were trying to save lives using the tools we knew from treating pneumonias and other forms of acute inflammatory lung injuries. We used every bit of our training in situations where no one had the right answers. But disasters teach us how to be flexible and pivot on the fly, and what to do when things don’t go our way.”
What is disaster response?
Medical response to a disaster essentially boils down to three main things: stuff, staff, and space, Dr. Persoff said. Those are the cornerstones of an emergency plan.
“There is not a hazard that exists that you can’t take an all-hazards approach to dealing with fundamental realities on the ground. No plan can be comprehensive enough to deal with all the intricacies of an emergency. But many plans can have the bones of a response that will allow you to face adverse circumstances,” he said.
“We actually became quite efficient early on in the pandemic, able to adapt in the moment. We were able to build an effective bridge between workers on the ground and our incident command structure, which seemed to reduce a lot of stress and create situational awareness. We implemented ICS as soon as we heard that China was building a COVID hospital, back in February of 2020.”
When one thinks about mass trauma, such as a 747 crash, Dr. Persoff said, the need is to treat burn victims and trauma victims in large numbers. At that point, the ED downstairs is filled with medical patients. Hospital medicine can rapidly admit those patients to clear out room in the ED. Surgeons are also dedicated to rapidly treating those patients, but what about patients who are on the floor following their surgeries? Hospitalists can offer consultations or primary management so the surgeons can stay in the OR, and the same in the ICU, while safely discharging hospitalized patients in a timely manner to make room for incoming patients.
“The lessons of COVID have been hard-taught and hard-earned. No good plan survives contact with the enemy,” he said. “But I think we’ll be better prepared for the next pandemic.”
Maria Frank, MD, FACP, SFHM, a hospitalist at Denver Health who chairs SHM’s Disaster Management Special Interest Group, says she got the bug for disaster preparation during postresidency training as an internist in emergency medicine. “I’m also the medical director for our biocontainment unit, created for infections like Ebola.” SHM’s SIG, which has 150 members, is now writing a review article on disaster planning for the field.
“I got a call on Dec. 27, 2019, about this new pneumonia, and they said, ‘We don’t know what it is, but it’s a coronavirus,’” she recalled. “When I got off the phone, I said, ‘Let’s make sure our response plan works and we have enough of everything on hand.’” Dr. Frank said she was expecting something more like SARS (severe acute respiratory syndrome). “When they called the public health emergency of international concern for COVID, I was at a Centers for Disease Control and Prevention meeting in Atlanta. It really wasn’t a surprise for us.”
All hospitals plan for disasters, although they use different names and have different levels of commitment, Dr. Frank said. What’s not consistent is the participation of hospitalists. “Even when a disaster is 100% trauma related, consider a hospital like mine that has at least four times as many hospitalists as surgeons at any given time. The hospitalists need to take overall management for the patients who aren’t actually in the operating room.”
Time to debrief
Dr. Frank recommends debriefing on the hospital’s and the hospitalist group’s experience with COVID. “Look at the biggest challenges your group faced. Was it staffing, or time off, or the need for day care? Was it burnout, lack of knowledge, lack of [personal protective equipment]?” Each hospital could use its own COVID experience to work on identifying the challenges and the problems, she said. “I’d encourage each department and division to do this exercise individually. Then come together to find common ground with other departments in the hospital.”
This debriefing exercise isn’t just for doctors – it’s also for nurses, environmental services, security, and many other departments, she said. “COVID showed us how crisis response is a group effort. What will bring us together is to learn the challenges each of us faced. It was amazing to see hospitalists doing what they do best.” Post pandemic, hospitalists should also consider getting involved in research and publications, in order to share their lessons.
“One of the things we learned is that hospitalists are very versatile,” Dr. Frank added. But it’s also good for the group to have members specialize, for example, in biocontainment. “We are experts in discharging patients, in patient flow and operations, in coordinating complex medical care. So we would naturally take the lead in, for example, opening a geographic unit or collaborating with other specialists to create innovative models. That’s our job. It’s essential that we’re involved well in advance.”
COVID may be a once-in-a-lifetime experience, but there will be other disasters to come, she said. “If your hospital doesn’t have a disaster plan for hospitalists, get involved in establishing one. Each hospitalist group should have its own response plan. Talk to your peers at other hospitals, and get involved at the institutional level. I’m happy to share our plan; just contact me.” Readers can contact Dr. Frank at maria.frank@dhha.org.
References
1. Persoff J et al. The role of hospital medicine in emergency preparedness: A framework for hospitalist leadership in disaster preparedness, response and recovery. J Hosp Med. 2018 Oct;13(10):713-7. doi: 10.12788/jhm.3073.
2. Persoff J et al. Expanding the hospital incident command system with a physician-centric role during a pandemic: The role of the physician clinical support supervisor. J Hosp Adm. 2020;9(3):7-10. doi: 10.5430/jha.v9n3p7.
3. Bowden K et al. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J Gen Intern Med. 2020 Sep;35(9):273-7. doi: 10.1007/s11606-020-05952-6.
4. Orsini E et al. Lessons on outbreak preparedness from the Cleveland Clinic. Chest. 2020;158(5):2090-6. doi: 10.1016/j.chest.2020.06.009.
DOACs best aspirin after ventricular ablation: STROKE-VT
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Bronchitis the leader at putting children in the hospital
About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.
Early transition to oral beta-lactams for low-risk S. aureus bacteremia may be acceptable
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.