User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
NCCN panel: Defer nonurgent skin cancer care during pandemic
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Amid the except when metastatic nodes are threatening vital structures or neoadjuvant therapy is not possible or has already failed, the National Comprehensive Cancer Network said in a new document about managing melanoma during the pandemic.
“The NCCN Melanoma Panel does not consider neoadjuvant therapy as a superior option to surgery followed by systemic adjuvant therapy for stage III melanoma, but available data suggest this is a reasonable resource-conserving option during the COVID-19 outbreak,” according to the panel. Surgery should be performed 8-9 weeks after initiation, said the group, an alliance of physicians from 30 U.S. cancer centers.
Echoing pandemic advice from other medical fields, the group’s melanoma recommendations focused on deferring nonurgent care until after the pandemic passes, and in the meantime limiting patient contact with the medical system and preserving hospital resources by, for instance, using telemedicine and opting for treatment regimens that require fewer trips to the clinic.
In a separate document on nonmelanoma skin cancer (NMSC), the group said that, with the exception of Merkel cell carcinoma, excisions for NMSC – including basal and squamous cell carcinoma, dermatofibrosarcoma protuberans, and rare tumors – should also generally be postponed during the pandemic.
The exception is if there is a risk of metastases within 3 months, but “such estimations of risks ... should be weighed against risks of the patient contracting COVID-19 infection or asymptomatically transmitting COVID-19 to health care workers,” the panel said.
Along the same lines, adjuvant therapy after surgical clearance of localized NMSC “should generally not be undertaken given the multiple visits required,” except for more extensive disease.
For primary cutaneous melanoma , “most time-to-treat studies show no adverse patient outcomes following a 90-day treatment delay, even for thicker [cutaneous melanoma],” the group said, so it recommended delaying wide excisions for melanoma in situ, lesions no thicker than 1 mm (T1) so long as the biopsy removed most of the lesion, and invasive melanomas of any depth if the biopsy had clear margins or only peripheral transection of the in situ component. They said sentinel lymph node biopsy can also be delayed for up to 3 months.
Resections for metastatic stage III-IV disease should also be put on hold unless the patient is symptomatic; systemic treatments should instead be continued. However, “given hospital-intensive resources, the use of talimogene laherparepvec for cutaneous/nodal/in-transit metastasis should be cautiously considered and, if possible, deferred until the COVID-19 crisis abates. A single dose of palliative radiation therapy may be useful for larger/symptomatic metastasis, as appropriate,” the group said.
If resection is still a go, the group noted that adjuvant therapy “has not been shown to improve melanoma-specific survival and should be deferred during the COVID-19 pandemic for patients with [a less than] 50% chance of disease relapse.” Dabrafenib/trametinib is the evidence-based choice if adjuvant treatment is opted for, but “alternative BRAF/MEK inhibitor regimens (encorafenib/binimetinib or vemurafenib/cobimetinib) may be substituted if drug supply is limited” by the pandemic, the group said.
For stage IV melanoma, “single-agent anti-PD-1 [programmed cell death 1] is recommended over combination ipilimumab/nivolumab at present” because there’s less inflammation and possible exacerbation of COVID-19, less need for steroids to counter adverse events, and less need for follow up to check for toxicities.
The group said evidence supports that 400 mg pembrolizumab administered intravenously every 6 weeks would likely be as effective as 200 mg intravenously every 3 weeks and would help keep people out of the hospital.
However, for stage IV melanoma with brain metastasis, there’s a strong rate of response to ipilimumab/nivolumab, so it may still be an option. In that case, “a regimen of ipilimumab 1 mg/kg and nivolumab 3 mg/kg every 3 weeks for four infusions, with subsequent consideration for nivolumab monotherapy, is associated with lower rates of immune-mediated toxicity,” compared with standard dosing.
Regarding potential drug shortages, the group noted that encorafenib/binimetinib or vemurafenib/cobimetinib combinations can be substituted for dabrafenib/trametinib for adjuvant therapy, and single-agent BRAF inhibitors can be used in the event of MEK inhibitor shortages.
In hospice, the group said oral temozolomide is the preferred option for palliative chemotherapy since it would limit resource utilization and contact with the medical system.
Nearly 24 tests for the novel coronavirus are available
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
according to the Infectious Diseases Society of America (IDSA).
“Based on what we know about influenza, it’s unlikely that all of these tests are going to perform exactly the same way,” said Angela M. Caliendo, MD, executive vice chair of the department of medicine at Brown University in Providence, R.I., at a press briefing. Although these tests are good, no test is perfect, she added.
The development and availability of testing has improved over time, but clinical laboratories still face challenges, said Kimberly E. Hanson, MD, associate professor of internal medicine at University of Utah, Salt Lake City. These challenges include shortages of devices for specimen collection, media, test tubes, and reagents. Although the goal is to test all symptomatic patients, these shortages require laboratories to prioritize health care workers and the sickest patients.
Tests are being approved through an abbreviated process
Two types of test, rapid tests and serology tests, are in use. Rapid tests use polymerase chain reactions to detect the virus in a clinical specimen. This type of testing is used to diagnose infection. Serology tests measure antibodies to the virus and are more appropriate for indicating whether a patient has been exposed to the virus.
The declaration of a national emergency enabled the FDA to activate its EUA policy, which allows for quicker approval of tests. Normally, a test must be assessed in the laboratory (such as with a mock specimen or an inactivated virus) and in a clinical study of patients. Under the EUA, clinical assessment is not required for the approval of a test. Consequently, the clinical performance of a test approved under EUA is unknown.
Collecting a specimen of good quality is critical to the quality of the test result, said Dr. Caliendo, the secretary of IDSA’s board of directors. Clinicians and investigators have used nasopharyngeal swabs, sputum, and specimens collected from deep within the lung. “We’re still collecting data to determine which is the best specimen type.” As coronavirus testing expands, particularly to drive-through testing sites, “we may be using people who are not as experienced, and so you might not get as high a quality specimen in that situation,” Dr. Caliendo added.
The timing of the test influences the quality of the result, as well, because the amount of virus is lower at the onset of symptoms than it is later. Another factor that affects the quality of the results is the test’s sensitivity.
The time to obtain results varies
The value of having several tests available is that it enables many patients to be tested simultaneously, said Dr. Hanson, a member of IDSA’s board of directors. It also helps to reduce potential problems with the supply of test kits. A test manufacturer, however, may supply parts of the test kit but not the whole kit. This requires the hospital or laboratory to obtain the remaining parts from other suppliers. Furthermore, test manufacturers may need to prioritize areas with high rates of infection or transmission when they ship their tests, which limits testing in other areas.
One reason for the lack of a national plan for testing is that the virus has affected different regions at different times, said Dr. Caliendo. Some tests are more difficult to perform than others, and not all laboratories are equally sophisticated, which can limit testing. It is necessary to test not only symptomatic patients who have been hospitalized, but also symptomatic patients in the community, said Dr. Caliendo. “Ideally, we’re going to need to couple acute diagnostics [testing while people are sick] with serologic testing. Serologic testing is going to be important for us to see who has been infected. That will give us an idea of who is left in our community who is at risk for developing infection.”
How quickly test results are available depends on the type of test and where it is administered. Recently established drive-through clinics can provide results in about 30 minutes. Tests performed in hospitals may take between 1 and 6 hours to yield results. “The issue is, do we have reagents that day?” said Dr. Caliendo. “We have to be careful whom we choose to test, and we screen that in the hospital so that we have enough tests to run as we need them.” But many locations have backlogs. “When you have a backlog of testing, you’re going to wait days, unfortunately, to get a result,” said Dr. Caliendo.
Dr. Caliendo and Dr. Hanson did not report disclosures for this briefing.
U.S. hospitals facing severe challenges from COVID-19, HHS report says
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
Hospitals across the country encountered severe challenges as the first wave of the COVID-19 pandemic swept over them, and they anticipated much worse to come, according to a new report from the Office of Inspector General of the Department of Health and Human Services (HHS).
From March 23 to 27, the OIG interviewed 323 hospitals of several types in 46 states, the District of Columbia, and Puerto Rico. The report it pulled together from these interviews is intended to help HHS manage the crisis, rather than to review its response to the pandemic, the OIG said.
The most significant hospital challenges, the report states, were testing and caring for patients with known or suspected COVID-19 and protecting staff members. In addition, the hospitals faced challenges in maintaining or expanding their capacities to treat COVID-19 patients and ensuring the adequacy of basic supplies.
The critical shortages of ventilators, personal protective equipment (PPE), and test kits in hospitals have been widely reported by the media. But the OIG report also focused on some areas that have received less press attention.
To begin with, the shortage of tests has not only slowed the national response to the pandemic, but has had a major impact on inpatient care, according to the report’s authors. The limited number of test kits means that only symptomatic staff members and patients can be tested; in some hospitals, there aren’t even enough tests for that, and some facilities subdivided the test kits they had, the report states.
Moreover, the test results often took 7 days or more to come back from commercial or government labs, the report states. In the meantime, symptomatic patients were presumed to have the coronavirus. While awaiting the results, they had to stay in the hospital, using beds and requiring staff who could otherwise have been assigned to other patients.
The doctors and nurse who cared for these presumptive COVID-19 patients also had to take time suiting up in PPE before seeing them; much of that scarce PPE was wasted on those who were later found not to have the illness.
As one administrator explained to OIG, “Sitting with 60 patients with presumed positives in our hospital isn’t healthy for anybody.”
Delayed test results also reduced hospitals’ ability to provide care by sidelining clinicians who reported COVID-19 symptoms. In one hospital, 20% to 25% of staff were determined to be presumptively positive for COVID-19. As a result of their tests not being analyzed promptly, these doctors and nurses were prevented from providing clinical services for longer than necessary.
Supply Shortages
The report also described some factors contributing to mask shortages. Because of the fear factor, for example, all staff members in one hospital were wearing masks, instead of just those in designated areas. An administrator said the hospital was using 2,000 masks a day, 10 times the number before the COVID-19 crisis.
Another hospital received 2,300 N95 masks from a state reserve, but they were unusable because the elastic bands had dry-rotted.
Meanwhile, some vendors were profiteering. Masks that used to cost 50 cents now sold for $6 each, one administrator said.
To combat the supply chain disruptions, some facilities were buying PPE from nontraditional sources such as online retailers, home supply stores, paint stores, autobody supply shops, and beauty salons. Other hospitals were using non–medical-grade PPE such as construction masks and handmade masks and gowns.
Other hospitals reported they were conserving and reusing PPE to stretch their supplies. In some cases, they had even changed policies to reduce the extent and frequency of patient interactions with clinicians so the latter would have to change their gear less often.
Shortages of other critical supplies and materials were also reported. Hospitals were running out of supplies that supported patient rooms, such as IV poles, medical gas, linens, toilet paper, and food.
Hospitals across the country were also expecting or experiencing a shortage of ventilators, although none said any patients had been denied access to them. Some institutions were adapting anesthesia machines and single-use emergency transport ventilators.
Also concerning to hospitals was the shortage of intensive-care specialists and nurses to operate the ventilators and care for critically ill patients. Some facilities were training anesthesiologists, hospitalists, and other nonintensivists on how to use the lifesaving equipment.
Meanwhile, patients with COVID-19 symptoms were continuing to show up in droves at emergency departments. Hospitals were concerned about potential shortages of ICU beds, negative-pressure rooms, and isolation units. Given limited bed availability, some administrators said, it was getting hard to separate COVID-19 from non–COVID-19 patients.
What Hospitals Want
As the COVID-19 crisis continues to mount, many hospitals are facing financial emergencies as well, the report noted.
“Hospitals described increasing costs and decreasing revenues as a threat to their financial viability. Hospitals reported that ceasing elective procedures and other services decreased revenues at the same time that their costs have increased as they prepare for a potential surge of patients. Many hospitals reported that their cash reserves were quickly depleting, which could disrupt ongoing hospital operations,” the authors write.
This report was conducted a few days before the passage of the CURES Act, which earmarked $100 billion for hospitals on the frontline of the crisis. As a recent analysis of financial hospital data revealed, however, even with the 20% bump in Medicare payments for COVID-19 care that this cash infusion represents, many hospitals will face a cash-flow crunch within 60 to 90 days, as reported by Medscape Medical News.
Besides higher Medicare payments, the OIG report said, hospitals wanted the government to drop the 14-day waiting period for reimbursement and to offer them loans and grants.
Hospitals also want federal and state governments to relax regulations on professional licensing of, and business relationships with, doctors and other clinicians. They’d like the government to:
- Let them reassign licensed professionals within their hospitals and across healthcare networks
- Provide flexibility with respect to licensed professionals practicing across state lines
- Provide relief from regulations that may restrict using contracted staff or physicians based on business relationships
This article first appeared on Medscape.com.
Many children with COVID-19 don’t have cough or fever
according to the Centers for Disease and Prevention Control.
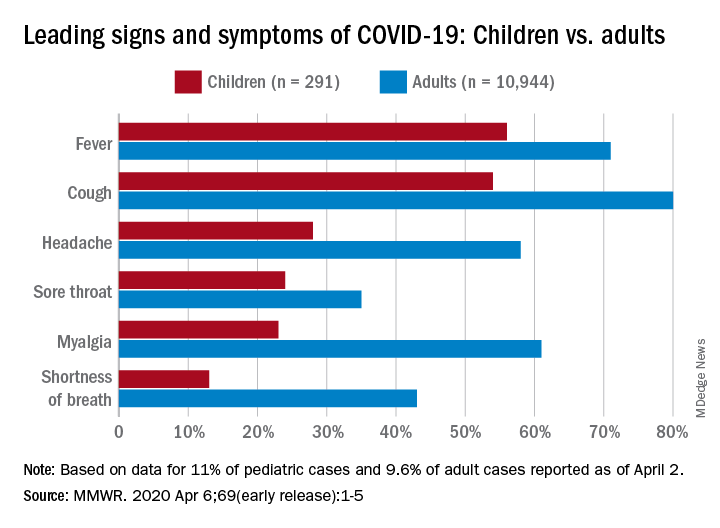
Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
according to the Centers for Disease and Prevention Control.

Among pediatric patients younger than 18 years in the United States, 73% had at least one of the trio of symptoms, compared with 93% of adults aged 18-64, noted Lucy A. McNamara, PhD, and the CDC’s COVID-19 response team, based on a preliminary analysis of the 149,082 cases reported as of April 2.
By a small margin, fever – present in 58% of pediatric patients – was the most common sign or symptom of COVID-19, compared with cough at 54% and shortness of breath in 13%. In adults, cough (81%) was seen most often, followed by fever (71%) and shortness of breath (43%), the investigators reported in the MMWR.
In both children and adults, headache and myalgia were more common than shortness of breath, as was sore throat in children, the team added.
“These findings are largely consistent with a report on pediatric COVID-19 patients aged <16 years in China, which found that only 41.5% of pediatric patients had fever [and] 48.5% had cough,” they wrote.
The CDC analysis of pediatric patients was limited by its small sample size, with data on signs and symptoms available for only 11% (291) of the 2,572 children known to have COVID-19 as of April 2. The adult population included 10,944 individuals, who represented 9.6% of the 113,985 U.S. patients aged 18-65, the response team said.
“As the number of COVID-19 cases continues to increase in many parts of the United States, it will be important to adapt COVID-19 surveillance strategies to maintain collection of critical case information without overburdening jurisdiction health departments,” they said.
SOURCE: McNamara LA et al. MMWR 2020 Apr 6;69(early release):1-5.
FROM MMWR
AAP issues guidance on managing infants born to mothers with COVID-19
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
Practicing solo and feeling grateful – despite COVID-19
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
I know that the world has gone upside down. It’s a nightmare, and people are filled with fear, and death is everywhere. In my little bubble of a world, however, I’ve been doing well.
I can’t lose my job, because I am my job. I’m a solo practitioner and have been for more than a decade. The restrictions to stay at home have not affected me, because I have a home office. Besides, I’m an introvert and see myself as a bit of a recluse, so the social distancing hasn’t been stressful. Conducting appointments by phone rather than face to face hasn’t undermined my work, since I can do everything that I do in my office over the phone. But I do it now in sweats and at my desk in my bedroom more often than not. I am prepared for a decrease in income as people lose their jobs, but that hasn’t happened yet. There are still people out there who are very motivated to come off their medications holistically. No rest for the wicked, as the saying goes.
On an emotional level, I feel calm because I’m not attached to material things, though I like them when they’re here. My children and friends have remained healthy, so I am grateful for that. I feel grounded in my belief that life goes on one way or another, and I trust in God to direct me wherever I need to go. Socially, I’ve been forced to be less lazy and cook more at home. As a result: less salt, MSG, and greasy food. I’ve spent a lot less on restaurants this past month and am eating less since I have to eat whatever I cook.
Can a person be more pandemic proof? I was joking with a friend about how pandemic-friendly my lifestyle is: spiritually, mentally, emotionally, physically, and socially. Oh, did I forget to mention the year supply of supplements in my office closet? They were for my patients, but those whole food green and red powders may come in handy, just in case.
So, that is how things are going for me. Please don’t hate me for not freaking out. When I read the news, I feel very sad for people who are suffering. I get angry at the politicians who can’t get their egos out of the way. But, I look at the sunshine outside my window, and I feel grateful that, at least in my case, I am not adding to the burden of suffering in the world. Not yet, anyway. I will keep trying to do the little bit that I do to help others for as long as I can.
Dr. Lee specializes in integrative and holistic psychiatry and has a private practice in Gaithersburg, Md. She has no disclosures.
Which of the changes that coronavirus has forced upon us will remain?
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Eventually this strange Twilight Zone world of coronavirus will end and life will return to normal.
But obviously it won’t be the same, and like everyone else I wonder what will be different.
Telemedicine is one obvious change in my world, though I don’t know how much yet (granted, no one else does, either). I’m seeing a handful of people that way, limited to established patients, where we’re discussing chronic issues or reviewing recent test results.
If I have to see a new patient or an established one with an urgent issue, I’m still willing to meet them at my office (wearing masks and washing hands frequently). In neurology, a lot still depends on a decent exam. It’s pretty hard to check reflexes, sensory modalities, and muscle tone over the phone. If you think a malpractice attorney is going to give you a pass because you missed something by not examining a patient because of coronavirus ... think again.
I’m not sure how the whole telemedicine thing will play out after the dust settles, at least not at my little practice. I’m currently seeing patients by FaceTime and Skype, neither of which is considered HIPAA compliant. The requirement has been waived during the crisis to make sure people can still see doctors, but I don’t see it lasting beyond that. Privacy will always be a central concern in medicine.
When they declare the pandemic over and say I can’t use FaceTime or Skype anymore, that will likely end my use of such. While there are HIPAA-compliant telemedicine services out there, in a small practice I don’t have the time or money to invest in them.
I also wonder how outcomes will change. I suspect the research-minded will be analyzing 2019 vs. 2020 data for years to come, trying to see if a sudden increase in telemedicine led to better or worse clinical outcomes. I’ll be curious to see what they find and how it breaks down by disease and specialty.
How will work change? Right now my staff of three (including me) are all working separately from home, handling phone calls as if it were another office day. In today’s era that’s easy to set up, and we’re used to the drill from when I’m out of town.
Maybe in the future, on lighter days, I’ll do this more often, and have my staff work from home (on typically busy days I’ll still need them to check patients in and out, fax things, file charts, and do all the other things they do to keep the practice running). The marked decrease in air pollution is certainly noticeable and good for all. When the year is over I’d like to see how non-coronavirus respiratory issues changed between 2019 and 2020.
Other businesses will be looking at that, too, with an increase in telecommuting. Why pay for a large office space when a lot can be done over the Internet? It saves rent, gas, and driving time. How it will affect us, as a socially-dependent species, I have no idea.
It’s the same with grocery delivery. While most of us will likely continue to shop at stores, many will stay with the ease of delivery services after this. It may cost more, but it certainly saves time.
There will be social changes, although how long they’ll last is anyone’s guess. Grocery baggers, stockers, and delivery staff, often seen as lower-level occupations, are now considered part of critical infrastructure in keeping people supplied with food and other necessities, as well as preventing fights from breaking out in the toilet paper and hand-sanitizer aisles.
I’d like to think that, in a country divided, the need to work together will help bring people of different opinions together again, but from the way things look I don’t see that happening, which is sad because viruses don’t discriminate, so we shouldn’t either in fighting them.
Like with other challenges that we face, big and little, I can only hope that we’ll learn something from this and have a better world after it’s over. Only time will tell.
Dr. Block has a solo neurology practice in Scottsdale, Ariz. He has no relevant disclosures.
Is protocol-driven COVID-19 respiratory therapy doing more harm than good?
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
Physicians in the COVID-19 trenches are beginning to question whether standard respiratory therapy protocols for acute respiratory distress syndrome (ARDS) are the best approach for treating patients with COVID-19 pneumonia.
At issue is the standard use of ventilators for a virus whose presentation has not followed the standard for ARDS, but is looking more like high-altitude pulmonary edema (HAPE) in some patients.
In a letter to the editor published in the American Journal of Respiratory and Critical Care Medicine on March 30, and in an editorial accepted for publication in Intensive Care Medicine, Luciano Gattinoni, MD, of the Medical University of Göttingen in Germany and colleagues make the case that protocol-driven ventilator use for patients with COVID-19 could be doing more harm than good.
Dr. Gattinoni noted that COVID-19 patients in ICUs in northern Italy had an atypical ARDS presentation with severe hypoxemia and well-preserved lung gas volume. He and colleagues suggested that instead of high positive end-expiratory pressure (PEEP), physicians should consider the lowest possible PEEP and gentle ventilation–practicing patience to “buy time with minimum additional damage.”
Similar observations were made by Cameron Kyle-Sidell, MD, a critical care physician working in New York City, who has been speaking out about this issue on Twitter and who shared his own experiences in this video interview with WebMD chief medical officer John Whyte, MD.
The bottom line, as Dr. Kyle-Sidell and Dr. Gattinoni agree, is that protocol-driven ventilator use may be causing lung injury in COVID-19 patients.
Consider disease phenotype
In the editorial, Dr. Gattinoni and colleagues explained further that ventilator settings should be based on physiological findings – with different respiratory treatment based on disease phenotype rather than using standard protocols.
‘“This, of course, is a conceptual model, but based on the observations we have this far, I don’t know of any model which is better,” he said in an interview.
Anecdotal evidence has increasingly demonstrated that this proposed physiological approach is associated with much lower mortality rates among COVID-19 patients, he said.
While not willing to name the hospitals at this time, he said that one center in Europe has had a 0% mortality rate among COVID-19 patients in the ICU when using this approach, compared with a 60% mortality rate at a nearby hospital using a protocol-driven approach.
In his editorial, Dr. Gattinoni disputed the recently published recommendation from the Surviving Sepsis Campaign that “mechanically ventilated patients with COVID-19 should be managed similarly to other patients with acute respiratory failure in the ICU.”
“Yet, COVID-19 pneumonia, despite falling in most of the circumstances under the Berlin definition of ARDS, is a specific disease, whose distinctive features are severe hypoxemia often associated with near normal respiratory system compliance,” Dr. Gattinoni and colleagues wrote, noting that this was true for more than half of the 150 patients he and his colleagues had assessed, and that several other colleagues in northern Italy reported similar findings. “This remarkable combination is almost never seen in severe ARDS.”
Dr. Gattinoni and colleagues hypothesized that COVID-19 patterns at patient presentation depend on interaction between three sets of factors: 1) disease severity, host response, physiological reserve and comorbidities; 2) ventilatory responsiveness of the patient to hypoxemia; and 3) time elapsed between disease onset and hospitalization.
They identified two primary phenotypes based on the interaction of these factors: Type L, characterized by low elastance, low ventilator perfusion ratio, low lung weight, and low recruitability; and Type H, characterized by high elastance, high right-to-left shunt, high lung weight, and high recruitability.
“Given this conceptual model, it follows that the respiratory treatment offered to Type L and Type H patients must be different,” Dr. Gattinoni said.
Patients may transition between phenotypes as their disease evolves. “If you start with the wrong protocol, at the end they become similar,” he said.
Rather, it is important to identify the phenotype at presentation to understand the pathophysiology and treat accordingly, he advised.
The phenotypes are best identified by CT scan, but signs implicit in each of the phenotypes, including respiratory system elastance and recruitability, can be used as surrogates if CT is unavailable, he noted.
“This is a kind of disease in which you don’t have to follow the protocol – you have to follow the physiology,” he said. “Unfortunately, many, many doctors around the world cannot think outside the protocol.”
In his interview with Dr. Whyte, Dr. Kyle-Sidell stressed that doctors must begin to consider other approaches. “We are desperate now, in the sense that everything we are doing does not seem to be working,” Dr. Kyle-Sidell said, noting that the first step toward improving outcomes is admitting that “this is something new.”
“I think it all starts from there, and I think we have the kind of scientific technology and the human capital in this country to solve this or at least have a very good shot at it,” he said.
Proposed treatment model
Dr. Gattinoni and his colleagues offered a proposed treatment model based on their conceptualization:
- Reverse hypoxemia through an increase in FiO2 to a level at which the Type L patient responds well, particularly for Type L patients who are not experiencing dyspnea.
- In Type L patients with dyspnea, try noninvasive options such as high-flow nasal cannula, continuous positive airway pressure, or noninvasive ventilation, and be sure to measure inspiratory esophageal pressure using esophageal manometry or surrogate measures. In intubated patients, determine P0.1 and P occlusion. High PEEP may decrease pleural pressure swings “and stop the vicious cycle that exacerbates lung injury,” but may be associated with high failure rates and delayed intubation.
- Intubate as soon as possible for esophageal pressure swings that increase from 5-10 cm H2O to above 15 cm H2O, which marks a transition from Type L to Type H phenotype and represents the level at which lung injury risk increases.
- For intubated and deeply sedated Type L patients who are hypercapnic, ventilate with volumes greater than 6 mL/kg up to 8-9 mL/kg as this high compliance results in tolerable strain without risk of ventilator-associated lung injury. Prone positioning should be used only as a rescue maneuver. Reduce PEEP to 8-10 cm H2O, given that the recruitability is low and the risk of hemodynamic failure increases at higher levels. Early intubation may avert the transition to Type H phenotype.
- Treat Type H phenotype like severe ARDS, including with higher PEEP if compatible with hemodynamics, and with prone positioning and extracorporeal support.
Dr. Gattinoni reported having no financial disclosures.
sworcester@mdedge.com
A decade of telemedicine policy has advanced in just 2 weeks
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
The rapid spread of , which he’d never used.
But as soon as he learned that telehealth regulations had been relaxed by the Centers for Medicare & Medicaid Services and that reimbursement had been broadened, Dr. Desai, a dermatologist in private practice and his staff began to mobilize.
“Kaboom! We made the decision to start doing it,” he said in an interview. “We drafted a consent form, uploaded it to our website, called patients, changed our voice greeting, and got clarity on insurance coverage. We’ve been flying by the seat of our pants.”
“I’m doing it because I don’t have a choice at this point,” said Dr. Desai, who is a member of the American Academy of Dermatology board of directors and its coronavirus task force. “I’m very worried about continuing to be able to meet our payroll expenses for staff and overhead to keep the office open.”
“Flying by the seat of our pants” to see patients virtually
Dermatologists have long been considered pioneers in telemedicine. They have, since the 1990s, capitalized on the visual nature of the specialty to diagnose and treat skin diseases by incorporating photos, videos, and virtual-patient visits. But the pandemic has forced the hands of even holdouts like Dr. Desai, who clung to in-person consults because of confusion related to HIPAA compliance issues and the sense that teledermatology “really dehumanizes patient interaction” for him.
In fact, as of 2017, only 15% of the nation’s 11,000 or so dermatologists had implemented telehealth into their practices, according to an AAD practice survey. In the wake of COVID-19, however, that percentage has likely more than tripled, experts estimate.
Now, dermatologists are assuming the mantle of educators for other specialists who never considered telehealth before in-person visits became fraught with concerns about the spread of the virus. And some are publishing guidelines for colleagues on how to prioritize teledermatology to stem transmission and conserve personal protective equipment (PPE) and hospital beds.
User-friendly technology and the relaxed telehealth restrictions have made it fairly simple for patients and physicians to connect. Facetime and other once-prohibited platforms are all currently permissible, although physicians are encouraged to notify patients about potential privacy risks, according to an AAD teledermatology tool kit.
Teledermatology innovators
“We’ve moved 10 years in telemedicine policy in 2 weeks,” said Karen Edison, MD, of the University of Missouri, Columbia. “The federal government has really loosened the reins.”
At least half of all dermatologists in the United States have adopted telehealth since the pandemic emerged, she estimated. And most, like Dr. Desai, have done so in just the last several weeks.
“You can do about 90% of what you need to do as a dermatologist using the technology,” said Dr. Edison, who launched the first dermatology Extension for Community Healthcare Outcomes, or ECHO, program in the Midwest. That telehealth model was originally developed to connect rural general practitioners with specialists at academic medical centers or large health systems.
“People are used to taking pictures with their phones. In some ways, this crisis may change the face of our specialty,” she said in an interview.
“As we’re all practicing social distancing, I think physicians and patients are rethinking how we can access healthcare without pursuing traditional face-to-face interactions,” said Ivy Lee, MD, from the University of California, San Francisco, who is past chair of the AAD telemedicine task force and current chair of the teledermatology committee at the American Telemedicine Association. “Virtual health and telemedicine fit perfectly with that.”
Even before the pandemic, the innovative ways dermatologists were using telehealth were garnering increasing acclaim. All four clinical groups short-listed for dermatology team of the year at the BMJ Awards 2020 employed telehealth to improve patient services in the United Kingdom.
In the United States, dermatologists are joining forces to boost understanding of how telehealth can protect patients and clinicians from some of the ravages of the virus.
The Society of Dermatology Hospitalists has developed an algorithm – built on experiences its members have had caring for hospitalized patients with acute dermatologic conditions – to provide a “logical way” to triage telemedicine consults in multiple hospital settings during the coronavirus crisis, said President-Elect Daniela Kroshinsky, MD, from Massachusetts General Hospital in Boston.
Telemedicine consultation is prioritized and patients at high risk for COVID-19 exposure are identified so that exposure time and resource use are limited and patient and staff safety are maximized.
“We want to empower our colleagues in community hospitals to play a role in safely providing care for patients in need but to be mindful about preserving resources,” said Dr. Kroshinsky, who reported that the algorithm will be published imminently.
“If you don’t have to see a patient in person and can offer recommendations through telederm, you don’t need to put on a gown, gloves, mask, or goggles,” she said in an interview. “If you’re unable to assess photos, then of course you’ll use the appropriate protective wear, but it will be better if you can obtain the same result” without having to do so.
Sharing expertise
After the first week of tracking data to gauge the effectiveness of the algorithm at Massachusetts General, Dr. Kroshinsky said she is buoyed.
Of the 35 patients assessed electronically – all of whom would previously have been seen in person – only 4 ended up needing a subsequent in-person consult, she reported.
“It’s worked out great,” said Dr. Kroshinsky, who noted that the pandemic is a “nice opportunity” to test different telehealth platforms and improve quality down the line. “We never had to use any excessive PPE, beyond what was routine, and the majority of patients were able to be staffed remotely. All patients had successful outcomes.”
With telehealth more firmly established in dermatology than in most other specialties, dermatologists are now helping clinicians in other fields who are rapidly ramping up their own telemedicine offerings.
These might include obstetrics and gynecology or “any medical specialty where they need to do checkups with their patients and don’t want them coming in for nonemergent visits,” said Carrie L. Kovarik, MD, of the University of Pennsylvania, Philadelphia.
In addition to fielding many recent calls and emails from physicians seeking guidance on telehealth, Dr. Kovarik, Dr. Lee, and colleagues have published the steps required to integrate the technology into outpatient practices.
“Now that there’s a time for broad implementation, our colleagues are looking to us for help and troubleshooting advice,” said Dr. Kovarik, who is also a member of the AAD COVID-19 response task force.
Various specialties “lend themselves to telehealth, depending on how image- or data-dependent they are,” Dr. Lee said in an interview. “But all specialists thinking of limiting or shutting down their practices are thinking about how they can provide continuity of care without exposing patients or staff to the risk of contracting the coronavirus.”
After-COVID goals
In his first week of virtual patient consults, Dr. Desai said he saw about 50 patients, which is still far fewer than the 160-180 he sees in person during a normal week.
“The problem is that patients don’t really want to do telehealth. You’d think it would be a good option,” he said, “but patients hesitate because they don’t really know how to use their device.” Some have instead rescheduled in-person appointments for months down the line.
Although telehealth has enabled Dr. Desai to readily assess patients with acne, hair loss, psoriasis, rashes, warts, and eczema, he’s concerned that necessary procedures, such as biopsies and dermoscopies, could be dangerously delayed. It’s also hard to assess the texture and thickness of certain skin lesions in photos or videos, he said.
“I’m trying to stay optimistic that this will get better and we’re able to move back to taking care of patients the way we need to,” he said.
Like Dr. Desai, other dermatologists who’ve implemented telemedicine during the pandemic have largely been swayed by the relaxed CMS regulations. “It’s made all the difference,” Dr. Kovarik said. “It has brought down the anxiety level and decreased questions about platforms and concentrated them on how to code the visits.”
And although it’s difficult to envision post-COVID medical practice in the thick of the pandemic, dermatologists expect the current strides in telemedicine will stick.
“I’m hoping that telehealth use isn’t dialed back all the way to baseline” after the pandemic eases, Dr. Kovarik said. “The cat’s out of the bag, and now that it is, hopefully it won’t be put back in.”
“If there’s a silver lining to this,” Dr. Kroshinsky said, “I hope it’s that we’ll be able to innovate around health care in a fashion we wouldn’t have seen otherwise.”
A version of this article originally appeared on Medscape.com.
Radial arteries show CABG survival advantage over saphenous veins
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
Coronary bypass surgery patients who received a radial artery as their second bypass conduit had significantly better 10-year survival than did patients who received a saphenous vein graft in a combined analysis of more than 1,000 randomized patients who had originally been enrolled in any of five independent studies.
“This is the first report of a survival benefit for CABG [coronary artery bypass grafting] using multiple arterial conduits based on randomized data,” Mario F.L. Gaudino, MD, said at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
Dr. Gaudino acknowledged several limitations of the finding. First, the analysis included studies that enrolled patients during 1997-2009 in five diverse sites worldwide and, therefore, involved varying surgical methods. Second, the sample size was “relatively low and underpowered, even after 10-year follow-up.” And third, the survival analysis was post hoc, although the statistically significant 27% relative reduction in mortality during follow-up with radial artery bypass, compared with saphenous vein bypass, was fully consistent with both the primary endpoint of the five studies and also with the combined hard endpoint of death and myocardial infarction.
The primary outcome of all-cause death, MI, and repeat revascularization also fell by a relative 27% with radial artery bypass, compared with saphenous vein bypass, and the combined rate of death and MI fell by 23% with radial artery bypass, both statistically significant differences, reported Dr. Gaudino, professor of cardiothoracic surgery at Weill Cornell Medicine, New York.
“The overall message is that radial bypass is associated with better long-term outcomes and potentially better survival,” he concluded.
The left interior thoracic artery (also known as the left internal mammary artery) is well established as the primary CABG conduit, usually used for bypassing the left anterior descending coronary artery, but when a second conduit is needed, several options exist: a saphenous vein, the left radial artery, or possibly the right internal thoracic artery (RITA) as a different arterial option.
Dr. Gaudino claimed that his new results placed the left radial artery clearly superior to the RITA as the second arterial conduit of choice for CABG. Not only does the RITA lack a similar efficacy evidence base from randomized trials, but the method poses a higher risk for surgical wound infections, he noted. The most recent society recommendations on conduit choice for CABG, issued less than 2 years ago by the European Society of Cardiology and European Association for Cardio-Thoracic Surgery, tapped the left radial artery as a level I recommendation over a saphenous vein graft when patients have high-grade coronary stenosis, while the RITA trailed as a level IIa recommendation specified only for patients with a low risk for sternal-wound infection.
“This was a great study that brings home the point that, in general, the radial artery is better than a saphenous vein graft,” commented Marc R. Moon, MD, a designated discussant and a professor of surgery and chief of cardiac surgery at Washington University, St. Louis.
The long-term follow-up that Dr. Gaudino reported came from extended, patient-level data collection for all 1,036 patients included in the original RADIAL (Radial Artery Database International Alliance) analysis of 5-year outcomes and reported in 2018. Dr. Gaudino and his associates tracked down survival information for all those patients, with outcome records out to at least 10 years for 91% of the original participants and with a mean follow-up that was also 10 years. The prior 2018 report based on 5-year results had also shown a statistically significant reduction in the primary endpoint with radial artery grafting, but at that point, follow-up had not yet collected enough mortality endpoints to produce a statistically significant between-group difference in death from any cause, a shortcoming that may have limited the ability of the prior report to influence U.S. practice.
“In the United States, there has been very little uptake of multiarterial grafting,” commented Frederick G.P. Welt, MD, a designated discussant, interventional cardiologist, professor of medicine, and associate chief of cardiovascular medicine at the University of Utah, Salt Lake City. The results from several earlier studies did not show much benefit for graft patency using radial arteries, but more recently clinicians have made advances in radial artery harvesting methods, implantation techniques, and adjunctive medications including antispasmodic treatments, Dr. Welt noted. Improved methods for using radial artery grafts and the risk of wound infection with RITA grafts were summarized in an editorial that accompanied the report of the 5-year RADIAL findings.
Dr. Welt said that, among the new findings reported by Dr. Gaudino, “the mortality effect is the most impressive.” Despite the limitations of the post hoc survival analysis, the reduced mortality finding is “nevertheless really important. The patient-level data lend it more credence.”
The study received no commercial funding. Dr. Gaudino had no disclosures. Dr. Moon has been a consultant to Medtronic. Dr. Welt has been an adviser to Medtronic.
SOURCE: Gaudino MFL et al. ACC 20, Abstract 410-11.
FROM ACC 2020