User login
Rapid action or sustained effect? Methotrexate vs. ciclosporin for pediatric AD
MONTREAL – in the TREAT study, investigators reported at the annual meeting of the International Society of Atopic Dermatitis.
The findings are important, since many regulatory bodies require patients to have tried such first-line conventional systemic therapies before moving on to novel therapeutics, explained Carsten Flohr, MD, PhD, research and development lead at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust London.
“We don’t really have much pediatric trial data; very often the pediatric data that we have is buried in adult trials and when it comes to an adequately powered randomized controlled trial with conventional systemic medication in pediatric patients, we don’t have one – so we’re lacking that gold standard,” said Dr. Flohr, chair in dermatology and population health sciences at King’s College London.
In the TREAT trial, 103 patients with AD (mean age, 10 years) who had not responded to topical treatment, were randomly assigned to oral ciclosporin (4 mg/kg daily) or methotrexate (0.4 mg/kg weekly) for 36 weeks and then followed for another 24 weeks off therapy for the co-primary outcomes of change in objective Scoring Atopic Dermatitis (o-SCORAD) at 12 weeks, as well as time to first significant flare after treatment cessation, defined as returning to baseline o-SCORAD, or restarting a systemic treatment.
Secondary outcomes included disease severity and quality of life (QOL) measures, as well as safety. At baseline, the mean o-SCORAD was 46.81, with mean Eczema Area and Severity Index (EASI) and Patient Oriented Eczema Measure (POEM) scores of 28.05 and 20.62 respectively. The mean Children’s Dermatology Life Quality Index (CDLQI) score was 14.96.
Looking at change in eczema severity measured by o-SCORAD at 12 weeks, ciclosporin was superior to methotrexate, with a mean difference in o-SCORAD change of -5.69 (P =.01). For the co-primary endpoint of time to first significant flare during the 24 weeks after treatment cessation, “there was a trend toward more flare activity in the ciclosporin group, although with a hazard ratio of 1.55, this was statistically not significant,” Dr. Flohr said.
On a graph showing mean EASI scores from baseline through the 60-week study period, Dr. Flohr explained how the score first dropped more precipitously in patients treated with ciclosporin compared with those treated with methotrexate, reaching a statistically significant difference between the groups by 12 weeks (–3.13, P = .0145).
However, after that time, while the EASI score among those on methotrexate continued to drop, the ciclosporin score evened out, so that by 20 weeks, methotrexate EASI scores were better, and remained so until the end of treatment and further, out to 60 weeks (mean difference -6.36, P < .001). “The most interesting bit of this graph is [that] the curve is pointing downwards for methotrexate up to the 9-month point, suggesting these people had not reached their full therapeutic potential yet, whereas if you’re on ciclosporin you plateau and there’s not much additional improvement, if at all, and then people [on ciclosporin] start going up in their disease activity off therapy,” he said.
The same pattern was seen with all the other outcome measures, including o-SCORAD and POEM.
Quality of life significantly improved by about 8 points in both treatment groups, with no significant differences between groups, and this improvement was sustained through the 24 weeks following cessation of therapy. However, during this treatment-free phase, patients on methotrexate had fewer parent-reported flares compared with those on ciclosporin (mean 6.19 vs 5.40 flares, P =.0251), although there was no difference between groups in time to first flare.
Describing the treatment safety as “overall reassuring,” Dr. Flohr said there were slightly more nonserious adverse events in the methotrexate arm (407 vs. 369), with nausea occurring more often in this group (43.1% vs. 17.6%).
“I think we were seeing this clinically, but to see it in a clinical trial gives us more confidence in discussing with parents,” said session moderator Melinda Gooderham, MD, assistant professor at Queens University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology in Peterborough.
What she also took away from the study was safety of these treatments. “The discontinuation rate was not different with either drug, so it’s not like ciclosporin works fast but all these people have problems and discontinue,” Dr. Gooderham told this news organization. “That’s also reassuring.”
Asked which treatment she prefers, Dr. Gooderham, a consultant physician at Peterborough Regional Health Centre, picked methotrexate “because of the lasting effect. But there are times when you may need more rapid control ... where I might choose ciclosporin first, but for me it’s maybe 90% methotrexate first, 10% ciclosporin.”
Dr. Flohr and Dr. Gooderham report no relevant financial relationships. The study was funded by the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
MONTREAL – in the TREAT study, investigators reported at the annual meeting of the International Society of Atopic Dermatitis.
The findings are important, since many regulatory bodies require patients to have tried such first-line conventional systemic therapies before moving on to novel therapeutics, explained Carsten Flohr, MD, PhD, research and development lead at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust London.
“We don’t really have much pediatric trial data; very often the pediatric data that we have is buried in adult trials and when it comes to an adequately powered randomized controlled trial with conventional systemic medication in pediatric patients, we don’t have one – so we’re lacking that gold standard,” said Dr. Flohr, chair in dermatology and population health sciences at King’s College London.
In the TREAT trial, 103 patients with AD (mean age, 10 years) who had not responded to topical treatment, were randomly assigned to oral ciclosporin (4 mg/kg daily) or methotrexate (0.4 mg/kg weekly) for 36 weeks and then followed for another 24 weeks off therapy for the co-primary outcomes of change in objective Scoring Atopic Dermatitis (o-SCORAD) at 12 weeks, as well as time to first significant flare after treatment cessation, defined as returning to baseline o-SCORAD, or restarting a systemic treatment.
Secondary outcomes included disease severity and quality of life (QOL) measures, as well as safety. At baseline, the mean o-SCORAD was 46.81, with mean Eczema Area and Severity Index (EASI) and Patient Oriented Eczema Measure (POEM) scores of 28.05 and 20.62 respectively. The mean Children’s Dermatology Life Quality Index (CDLQI) score was 14.96.
Looking at change in eczema severity measured by o-SCORAD at 12 weeks, ciclosporin was superior to methotrexate, with a mean difference in o-SCORAD change of -5.69 (P =.01). For the co-primary endpoint of time to first significant flare during the 24 weeks after treatment cessation, “there was a trend toward more flare activity in the ciclosporin group, although with a hazard ratio of 1.55, this was statistically not significant,” Dr. Flohr said.
On a graph showing mean EASI scores from baseline through the 60-week study period, Dr. Flohr explained how the score first dropped more precipitously in patients treated with ciclosporin compared with those treated with methotrexate, reaching a statistically significant difference between the groups by 12 weeks (–3.13, P = .0145).
However, after that time, while the EASI score among those on methotrexate continued to drop, the ciclosporin score evened out, so that by 20 weeks, methotrexate EASI scores were better, and remained so until the end of treatment and further, out to 60 weeks (mean difference -6.36, P < .001). “The most interesting bit of this graph is [that] the curve is pointing downwards for methotrexate up to the 9-month point, suggesting these people had not reached their full therapeutic potential yet, whereas if you’re on ciclosporin you plateau and there’s not much additional improvement, if at all, and then people [on ciclosporin] start going up in their disease activity off therapy,” he said.
The same pattern was seen with all the other outcome measures, including o-SCORAD and POEM.
Quality of life significantly improved by about 8 points in both treatment groups, with no significant differences between groups, and this improvement was sustained through the 24 weeks following cessation of therapy. However, during this treatment-free phase, patients on methotrexate had fewer parent-reported flares compared with those on ciclosporin (mean 6.19 vs 5.40 flares, P =.0251), although there was no difference between groups in time to first flare.
Describing the treatment safety as “overall reassuring,” Dr. Flohr said there were slightly more nonserious adverse events in the methotrexate arm (407 vs. 369), with nausea occurring more often in this group (43.1% vs. 17.6%).
“I think we were seeing this clinically, but to see it in a clinical trial gives us more confidence in discussing with parents,” said session moderator Melinda Gooderham, MD, assistant professor at Queens University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology in Peterborough.
What she also took away from the study was safety of these treatments. “The discontinuation rate was not different with either drug, so it’s not like ciclosporin works fast but all these people have problems and discontinue,” Dr. Gooderham told this news organization. “That’s also reassuring.”
Asked which treatment she prefers, Dr. Gooderham, a consultant physician at Peterborough Regional Health Centre, picked methotrexate “because of the lasting effect. But there are times when you may need more rapid control ... where I might choose ciclosporin first, but for me it’s maybe 90% methotrexate first, 10% ciclosporin.”
Dr. Flohr and Dr. Gooderham report no relevant financial relationships. The study was funded by the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
MONTREAL – in the TREAT study, investigators reported at the annual meeting of the International Society of Atopic Dermatitis.
The findings are important, since many regulatory bodies require patients to have tried such first-line conventional systemic therapies before moving on to novel therapeutics, explained Carsten Flohr, MD, PhD, research and development lead at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust London.
“We don’t really have much pediatric trial data; very often the pediatric data that we have is buried in adult trials and when it comes to an adequately powered randomized controlled trial with conventional systemic medication in pediatric patients, we don’t have one – so we’re lacking that gold standard,” said Dr. Flohr, chair in dermatology and population health sciences at King’s College London.
In the TREAT trial, 103 patients with AD (mean age, 10 years) who had not responded to topical treatment, were randomly assigned to oral ciclosporin (4 mg/kg daily) or methotrexate (0.4 mg/kg weekly) for 36 weeks and then followed for another 24 weeks off therapy for the co-primary outcomes of change in objective Scoring Atopic Dermatitis (o-SCORAD) at 12 weeks, as well as time to first significant flare after treatment cessation, defined as returning to baseline o-SCORAD, or restarting a systemic treatment.
Secondary outcomes included disease severity and quality of life (QOL) measures, as well as safety. At baseline, the mean o-SCORAD was 46.81, with mean Eczema Area and Severity Index (EASI) and Patient Oriented Eczema Measure (POEM) scores of 28.05 and 20.62 respectively. The mean Children’s Dermatology Life Quality Index (CDLQI) score was 14.96.
Looking at change in eczema severity measured by o-SCORAD at 12 weeks, ciclosporin was superior to methotrexate, with a mean difference in o-SCORAD change of -5.69 (P =.01). For the co-primary endpoint of time to first significant flare during the 24 weeks after treatment cessation, “there was a trend toward more flare activity in the ciclosporin group, although with a hazard ratio of 1.55, this was statistically not significant,” Dr. Flohr said.
On a graph showing mean EASI scores from baseline through the 60-week study period, Dr. Flohr explained how the score first dropped more precipitously in patients treated with ciclosporin compared with those treated with methotrexate, reaching a statistically significant difference between the groups by 12 weeks (–3.13, P = .0145).
However, after that time, while the EASI score among those on methotrexate continued to drop, the ciclosporin score evened out, so that by 20 weeks, methotrexate EASI scores were better, and remained so until the end of treatment and further, out to 60 weeks (mean difference -6.36, P < .001). “The most interesting bit of this graph is [that] the curve is pointing downwards for methotrexate up to the 9-month point, suggesting these people had not reached their full therapeutic potential yet, whereas if you’re on ciclosporin you plateau and there’s not much additional improvement, if at all, and then people [on ciclosporin] start going up in their disease activity off therapy,” he said.
The same pattern was seen with all the other outcome measures, including o-SCORAD and POEM.
Quality of life significantly improved by about 8 points in both treatment groups, with no significant differences between groups, and this improvement was sustained through the 24 weeks following cessation of therapy. However, during this treatment-free phase, patients on methotrexate had fewer parent-reported flares compared with those on ciclosporin (mean 6.19 vs 5.40 flares, P =.0251), although there was no difference between groups in time to first flare.
Describing the treatment safety as “overall reassuring,” Dr. Flohr said there were slightly more nonserious adverse events in the methotrexate arm (407 vs. 369), with nausea occurring more often in this group (43.1% vs. 17.6%).
“I think we were seeing this clinically, but to see it in a clinical trial gives us more confidence in discussing with parents,” said session moderator Melinda Gooderham, MD, assistant professor at Queens University, Kingston, Ont., and medical director at the SKiN Centre for Dermatology in Peterborough.
What she also took away from the study was safety of these treatments. “The discontinuation rate was not different with either drug, so it’s not like ciclosporin works fast but all these people have problems and discontinue,” Dr. Gooderham told this news organization. “That’s also reassuring.”
Asked which treatment she prefers, Dr. Gooderham, a consultant physician at Peterborough Regional Health Centre, picked methotrexate “because of the lasting effect. But there are times when you may need more rapid control ... where I might choose ciclosporin first, but for me it’s maybe 90% methotrexate first, 10% ciclosporin.”
Dr. Flohr and Dr. Gooderham report no relevant financial relationships. The study was funded by the National Institute for Health and Care Research.
A version of this article first appeared on Medscape.com.
AT ISAD 2022
Access to abortion clinics declines sharply
Estimated travel time to abortion facilities in the United States has increased significantly since the Supreme Court overturned Roe v. Wade, according to results from an original investigation published online in JAMA.
In the wake of the ruling, many clinics have closed and now 33.3% of females of reproductive age live more than an hour from an abortion facility, more than double the 14.6% who lived that far before the Dobbs v. Jackson Women’s Health Organization court ruling, the paper states.
A 2022 study found that when people live 50 miles or more from an abortion facility they “were more likely to still be seeking an abortion on a 4-week follow-up than those who lived closer to an abortion facility,” wrote the authors, led by Benjamin Rader, MPH, from the Computational Epidemiology Lab at Boston Children’s Hospital.
Of 1,134 abortion facilities in the United States, 749 were considered active before the ruling and 671 were considered active in a simulated post-Dobbs period.
More than 15 states have total or partial bans
The researchers accounted for the closure of abortion facilities in states with total bans or 6-week abortion bans, compared with the period before the ruling, “during which all facilities providing abortions in 2021 were considered active.” The authors noted that more than 15 states have such bans.
Researchers found median and mean travel times to abortion facilities were estimated to be 10.9 minutes (interquartile ratio, 4.3-32.4) and 27.8 (standard deviation, 42.0) minutes before the ruling and used a paired sample t test (P < .001) to estimate the increase to a median of 17.0 (IQR, 4.9-124.5) minutes and a mean 100.4 (SD, 161.5) minutes after the ruling.
The numbers “highlight the catastrophe in terms of where we are,” Catherine Cansino, MD, MPH, professor, obstetrics and gynecology at the University of California, Davis, said in an interview.
Behind those numbers, she said, are brick walls for people who can’t take off work to drive that far or can’t leave their responsibilities of care for dependents or don’t have a car or even a driver’s license. It also calculates only land travel (car or public transportation) and doesn’t capture the financial and logistical burdens for some to fly to other states.
Dr. Cansino serves on the board of the Society of Family Planning, which publishes #WeCount, a national reporting effort that attempts to capture the effect of the Dobbs decision on abortion access. In a report published Oct. 28, #WeCount stated the numbers show that since the decision, there were 5,270 fewer abortions in July and 5,400 fewer in August, for a total of 10,670 fewer people in the United States who had abortions in the 2 months.
For Dr. Cansino, the numbers are only one measure of the wider problem.
“If it affects one person, it’s really the spirit of the consequence,” she said. “It’s difficult to wrap your mind around these numbers but the bottom line is that someone other than the person experiencing this health issue is making a decision for them.
“You will see physicians leaving states,” she said, “because their hands are tied in giving care.”
Glimpse of future from Texas example
The experience of abortion restrictions in Texas, described in another original investigation published in JAMA, provides a window into what could happen as access to abortions continues to decrease.
Texas has banned abortions after detectable embryonic cardiac activity since Sept. 1, 2021. Researchers obtained data on 80,107 abortions performed between September 2020 and February 2022.
In the first month following implementation of the Texas law, SB-8, the number of abortions in Texas dropped by 50%, compared with September 2020, and many pregnant Texas residents traveled out of state for abortion care.
But out-of-state abortions didn’t fully offset the overall drop in facility-based abortions.
“This decrease in facility-based abortion care suggests that many Texas residents continued their pregnancies, traveled beyond a neighboring state, or self-managed their abortion,” the authors wrote.
Increased time comes with costs
Sarah W. Prager, MD, professor in obstetrics and gynecology at University of Washington, Seattle, and director of the family planning division, explained that the travel time has to be seen in addition to the time it takes to complete the procedure.
Depending on state law, an abortion may take more than one visit to a clinic, which may mean adding lodging costs and overnight hours, or taking time off work, or finding childcare.
“A typical time to be at a clinic is upwards of 6 hours,” Dr. Prager explained, including paperwork, counseling, consent, the procedure, and recovery. That time is growing as active clinics overbook with others closing, she noted.
“We already know that 75% of people getting abortions are economically burdened at baseline. Gas is super expensive so the farther they have to drive – if they have their own car – that’s going to be expensive,” she noted.
In Washington, she said, abortion access is centralized in the western part of the state and located primarily between Seattle and Olympia. Though Oregon to the south has some of the nation’s most supportive laws for abortion, the other surrounding states have restrictive laws.
People in Alaska, Wyoming, Idaho, and Montana all have restrictive access, she noted, so people seeking abortions from those states have long distances to drive to western Washington and Oregon.
“Even for people living in eastern Washington, they are sometimes driving hours to get abortion care,” she said. “We’re really looking at health care that is dictated by geography, not by evidence, medicine, or science.”
The study by Dr. White and colleagues was supported by grants from the Susan Thompson Buffett Foundation and Collaborative for Gender + Reproductive Equity, as well as a center grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the Population Research Center at the University of Texas at Austin. One coauthor reported receiving compensation from the University of Texas at Austin for providing data during the conduct of the study, as well as grants from Merck and Gynuity Health Projects and personal fees from Merck and Organon outside the submitted work; another reported being named plaintiff in the case Planned Parenthood of Montana v State of Montana, a lawsuit challenging abortion restrictions in that state. No other disclosures were reported. Dr. Cansino and Dr. Prager reported no relevant financial relationships.
Estimated travel time to abortion facilities in the United States has increased significantly since the Supreme Court overturned Roe v. Wade, according to results from an original investigation published online in JAMA.
In the wake of the ruling, many clinics have closed and now 33.3% of females of reproductive age live more than an hour from an abortion facility, more than double the 14.6% who lived that far before the Dobbs v. Jackson Women’s Health Organization court ruling, the paper states.
A 2022 study found that when people live 50 miles or more from an abortion facility they “were more likely to still be seeking an abortion on a 4-week follow-up than those who lived closer to an abortion facility,” wrote the authors, led by Benjamin Rader, MPH, from the Computational Epidemiology Lab at Boston Children’s Hospital.
Of 1,134 abortion facilities in the United States, 749 were considered active before the ruling and 671 were considered active in a simulated post-Dobbs period.
More than 15 states have total or partial bans
The researchers accounted for the closure of abortion facilities in states with total bans or 6-week abortion bans, compared with the period before the ruling, “during which all facilities providing abortions in 2021 were considered active.” The authors noted that more than 15 states have such bans.
Researchers found median and mean travel times to abortion facilities were estimated to be 10.9 minutes (interquartile ratio, 4.3-32.4) and 27.8 (standard deviation, 42.0) minutes before the ruling and used a paired sample t test (P < .001) to estimate the increase to a median of 17.0 (IQR, 4.9-124.5) minutes and a mean 100.4 (SD, 161.5) minutes after the ruling.
The numbers “highlight the catastrophe in terms of where we are,” Catherine Cansino, MD, MPH, professor, obstetrics and gynecology at the University of California, Davis, said in an interview.
Behind those numbers, she said, are brick walls for people who can’t take off work to drive that far or can’t leave their responsibilities of care for dependents or don’t have a car or even a driver’s license. It also calculates only land travel (car or public transportation) and doesn’t capture the financial and logistical burdens for some to fly to other states.
Dr. Cansino serves on the board of the Society of Family Planning, which publishes #WeCount, a national reporting effort that attempts to capture the effect of the Dobbs decision on abortion access. In a report published Oct. 28, #WeCount stated the numbers show that since the decision, there were 5,270 fewer abortions in July and 5,400 fewer in August, for a total of 10,670 fewer people in the United States who had abortions in the 2 months.
For Dr. Cansino, the numbers are only one measure of the wider problem.
“If it affects one person, it’s really the spirit of the consequence,” she said. “It’s difficult to wrap your mind around these numbers but the bottom line is that someone other than the person experiencing this health issue is making a decision for them.
“You will see physicians leaving states,” she said, “because their hands are tied in giving care.”
Glimpse of future from Texas example
The experience of abortion restrictions in Texas, described in another original investigation published in JAMA, provides a window into what could happen as access to abortions continues to decrease.
Texas has banned abortions after detectable embryonic cardiac activity since Sept. 1, 2021. Researchers obtained data on 80,107 abortions performed between September 2020 and February 2022.
In the first month following implementation of the Texas law, SB-8, the number of abortions in Texas dropped by 50%, compared with September 2020, and many pregnant Texas residents traveled out of state for abortion care.
But out-of-state abortions didn’t fully offset the overall drop in facility-based abortions.
“This decrease in facility-based abortion care suggests that many Texas residents continued their pregnancies, traveled beyond a neighboring state, or self-managed their abortion,” the authors wrote.
Increased time comes with costs
Sarah W. Prager, MD, professor in obstetrics and gynecology at University of Washington, Seattle, and director of the family planning division, explained that the travel time has to be seen in addition to the time it takes to complete the procedure.
Depending on state law, an abortion may take more than one visit to a clinic, which may mean adding lodging costs and overnight hours, or taking time off work, or finding childcare.
“A typical time to be at a clinic is upwards of 6 hours,” Dr. Prager explained, including paperwork, counseling, consent, the procedure, and recovery. That time is growing as active clinics overbook with others closing, she noted.
“We already know that 75% of people getting abortions are economically burdened at baseline. Gas is super expensive so the farther they have to drive – if they have their own car – that’s going to be expensive,” she noted.
In Washington, she said, abortion access is centralized in the western part of the state and located primarily between Seattle and Olympia. Though Oregon to the south has some of the nation’s most supportive laws for abortion, the other surrounding states have restrictive laws.
People in Alaska, Wyoming, Idaho, and Montana all have restrictive access, she noted, so people seeking abortions from those states have long distances to drive to western Washington and Oregon.
“Even for people living in eastern Washington, they are sometimes driving hours to get abortion care,” she said. “We’re really looking at health care that is dictated by geography, not by evidence, medicine, or science.”
The study by Dr. White and colleagues was supported by grants from the Susan Thompson Buffett Foundation and Collaborative for Gender + Reproductive Equity, as well as a center grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the Population Research Center at the University of Texas at Austin. One coauthor reported receiving compensation from the University of Texas at Austin for providing data during the conduct of the study, as well as grants from Merck and Gynuity Health Projects and personal fees from Merck and Organon outside the submitted work; another reported being named plaintiff in the case Planned Parenthood of Montana v State of Montana, a lawsuit challenging abortion restrictions in that state. No other disclosures were reported. Dr. Cansino and Dr. Prager reported no relevant financial relationships.
Estimated travel time to abortion facilities in the United States has increased significantly since the Supreme Court overturned Roe v. Wade, according to results from an original investigation published online in JAMA.
In the wake of the ruling, many clinics have closed and now 33.3% of females of reproductive age live more than an hour from an abortion facility, more than double the 14.6% who lived that far before the Dobbs v. Jackson Women’s Health Organization court ruling, the paper states.
A 2022 study found that when people live 50 miles or more from an abortion facility they “were more likely to still be seeking an abortion on a 4-week follow-up than those who lived closer to an abortion facility,” wrote the authors, led by Benjamin Rader, MPH, from the Computational Epidemiology Lab at Boston Children’s Hospital.
Of 1,134 abortion facilities in the United States, 749 were considered active before the ruling and 671 were considered active in a simulated post-Dobbs period.
More than 15 states have total or partial bans
The researchers accounted for the closure of abortion facilities in states with total bans or 6-week abortion bans, compared with the period before the ruling, “during which all facilities providing abortions in 2021 were considered active.” The authors noted that more than 15 states have such bans.
Researchers found median and mean travel times to abortion facilities were estimated to be 10.9 minutes (interquartile ratio, 4.3-32.4) and 27.8 (standard deviation, 42.0) minutes before the ruling and used a paired sample t test (P < .001) to estimate the increase to a median of 17.0 (IQR, 4.9-124.5) minutes and a mean 100.4 (SD, 161.5) minutes after the ruling.
The numbers “highlight the catastrophe in terms of where we are,” Catherine Cansino, MD, MPH, professor, obstetrics and gynecology at the University of California, Davis, said in an interview.
Behind those numbers, she said, are brick walls for people who can’t take off work to drive that far or can’t leave their responsibilities of care for dependents or don’t have a car or even a driver’s license. It also calculates only land travel (car or public transportation) and doesn’t capture the financial and logistical burdens for some to fly to other states.
Dr. Cansino serves on the board of the Society of Family Planning, which publishes #WeCount, a national reporting effort that attempts to capture the effect of the Dobbs decision on abortion access. In a report published Oct. 28, #WeCount stated the numbers show that since the decision, there were 5,270 fewer abortions in July and 5,400 fewer in August, for a total of 10,670 fewer people in the United States who had abortions in the 2 months.
For Dr. Cansino, the numbers are only one measure of the wider problem.
“If it affects one person, it’s really the spirit of the consequence,” she said. “It’s difficult to wrap your mind around these numbers but the bottom line is that someone other than the person experiencing this health issue is making a decision for them.
“You will see physicians leaving states,” she said, “because their hands are tied in giving care.”
Glimpse of future from Texas example
The experience of abortion restrictions in Texas, described in another original investigation published in JAMA, provides a window into what could happen as access to abortions continues to decrease.
Texas has banned abortions after detectable embryonic cardiac activity since Sept. 1, 2021. Researchers obtained data on 80,107 abortions performed between September 2020 and February 2022.
In the first month following implementation of the Texas law, SB-8, the number of abortions in Texas dropped by 50%, compared with September 2020, and many pregnant Texas residents traveled out of state for abortion care.
But out-of-state abortions didn’t fully offset the overall drop in facility-based abortions.
“This decrease in facility-based abortion care suggests that many Texas residents continued their pregnancies, traveled beyond a neighboring state, or self-managed their abortion,” the authors wrote.
Increased time comes with costs
Sarah W. Prager, MD, professor in obstetrics and gynecology at University of Washington, Seattle, and director of the family planning division, explained that the travel time has to be seen in addition to the time it takes to complete the procedure.
Depending on state law, an abortion may take more than one visit to a clinic, which may mean adding lodging costs and overnight hours, or taking time off work, or finding childcare.
“A typical time to be at a clinic is upwards of 6 hours,” Dr. Prager explained, including paperwork, counseling, consent, the procedure, and recovery. That time is growing as active clinics overbook with others closing, she noted.
“We already know that 75% of people getting abortions are economically burdened at baseline. Gas is super expensive so the farther they have to drive – if they have their own car – that’s going to be expensive,” she noted.
In Washington, she said, abortion access is centralized in the western part of the state and located primarily between Seattle and Olympia. Though Oregon to the south has some of the nation’s most supportive laws for abortion, the other surrounding states have restrictive laws.
People in Alaska, Wyoming, Idaho, and Montana all have restrictive access, she noted, so people seeking abortions from those states have long distances to drive to western Washington and Oregon.
“Even for people living in eastern Washington, they are sometimes driving hours to get abortion care,” she said. “We’re really looking at health care that is dictated by geography, not by evidence, medicine, or science.”
The study by Dr. White and colleagues was supported by grants from the Susan Thompson Buffett Foundation and Collaborative for Gender + Reproductive Equity, as well as a center grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development awarded to the Population Research Center at the University of Texas at Austin. One coauthor reported receiving compensation from the University of Texas at Austin for providing data during the conduct of the study, as well as grants from Merck and Gynuity Health Projects and personal fees from Merck and Organon outside the submitted work; another reported being named plaintiff in the case Planned Parenthood of Montana v State of Montana, a lawsuit challenging abortion restrictions in that state. No other disclosures were reported. Dr. Cansino and Dr. Prager reported no relevant financial relationships.
FROM JAMA
Online support tool improves AD self-management
MONTREAL – for up to 1 year, according to two randomized controlled trials presented at the annual meeting of the International Society of Atopic Dermatitis.
The intervention, directed either at parents of children with AD or young adults with AD, “is very low cost, evidence based, easily accessible, and free from possible commercial bias,” said investigator Kim Thomas, MD, professor of applied dermatology research and codirector of the Centre of Evidence Based Dermatology, faculty of medicine & health sciences, University of Nottingham (England).
The main focus of the intervention, along with general education, is “getting control” of the condition with flare-control creams and “keeping control” with regular emollient use.
Efficacy of the intervention, available free online, was compared with “usual eczema care” in 340 parents of children with AD up to age 12 and 337 young patients with AD aged 13-25. Participants were randomized to the intervention plus usual care or usual care alone. The primary outcome was the Patient-Oriented Eczema Measure(POEM) at 24 weeks, with a further measurement at 52 weeks.
In the parent group, about half were women and 83% were White, and the median age of their children was 4 years. About 50% of parents had a university degree, making them “possibly better educated than we might want our target audience for this type of intervention,” Dr. Thomas commented. Most of the children had moderate AD.
In the young patient group, the mean age was 19 years, more than three-quarters were female, 83% were White, and most had moderate AD.
At 24 weeks, both intervention groups had improved POEM scores, compared with controls, with a mean difference of 1.5 points in the parent group (P = .002) and 1.7 points in the young patient group (P = .04). “A small difference, but statistically significant and sustained,” Dr. Thomas said, adding that this difference was sustained up to 52 weeks.
In terms of mechanism of action, a secondary outcome looked at the concept of enablement, “which again, seemed to be improved in the intervention group, which suggests it’s something to do with being able to understand and cope with their disease better,” she said. The tool is targeted to “people who wouldn’t normally get to a dermatologist and certainly wouldn’t get access to group interventions.”
An additional aim of the intervention was “to provide a single, consistent message received from every point of contact that people might engage with ... [from] community doctors, pharmacists, dermatologists, and importantly, eczema charities all signposting [the intervention] and sharing a consistent message.”
While the intervention is free and available to patients anywhere, Dr. Thomas emphasized that it is tailored to the U.K. health care system. “If people would like to get in touch and help work with us to maybe adapt it slightly to make it more suitable for your own health care systems, that’s something we’d be very happy to look at with you.”
Asked for comment, Natalie Cunningham, MD, panel moderator, was lukewarm about the tool. “It can be a supplement, but you can never replace the one-on-one patient–health care provider interaction,” she told this news organization. “That could be provided by a nondermatologist and supplemented by an online component,” said Dr. Cunningham, from the Izaak Walton Killam Hospital for Children in Halifax, N.S.
“First-line treatment for eczema, no matter what kind of eczema, is topical steroids, and that is something that requires a lot of education – and something you want to do one on one in person because everyone comes to it with a different experience, baggage, or understanding,” she said. “We need to figure out what the barrier is so that you can do the right education.”
In addition, with systemic AD therapies currently approved for children, parents and young patients need to be able to advocate for specialist care to access these medications, she noted.
Dr. Thomas and Dr. Cunningham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – for up to 1 year, according to two randomized controlled trials presented at the annual meeting of the International Society of Atopic Dermatitis.
The intervention, directed either at parents of children with AD or young adults with AD, “is very low cost, evidence based, easily accessible, and free from possible commercial bias,” said investigator Kim Thomas, MD, professor of applied dermatology research and codirector of the Centre of Evidence Based Dermatology, faculty of medicine & health sciences, University of Nottingham (England).
The main focus of the intervention, along with general education, is “getting control” of the condition with flare-control creams and “keeping control” with regular emollient use.
Efficacy of the intervention, available free online, was compared with “usual eczema care” in 340 parents of children with AD up to age 12 and 337 young patients with AD aged 13-25. Participants were randomized to the intervention plus usual care or usual care alone. The primary outcome was the Patient-Oriented Eczema Measure(POEM) at 24 weeks, with a further measurement at 52 weeks.
In the parent group, about half were women and 83% were White, and the median age of their children was 4 years. About 50% of parents had a university degree, making them “possibly better educated than we might want our target audience for this type of intervention,” Dr. Thomas commented. Most of the children had moderate AD.
In the young patient group, the mean age was 19 years, more than three-quarters were female, 83% were White, and most had moderate AD.
At 24 weeks, both intervention groups had improved POEM scores, compared with controls, with a mean difference of 1.5 points in the parent group (P = .002) and 1.7 points in the young patient group (P = .04). “A small difference, but statistically significant and sustained,” Dr. Thomas said, adding that this difference was sustained up to 52 weeks.
In terms of mechanism of action, a secondary outcome looked at the concept of enablement, “which again, seemed to be improved in the intervention group, which suggests it’s something to do with being able to understand and cope with their disease better,” she said. The tool is targeted to “people who wouldn’t normally get to a dermatologist and certainly wouldn’t get access to group interventions.”
An additional aim of the intervention was “to provide a single, consistent message received from every point of contact that people might engage with ... [from] community doctors, pharmacists, dermatologists, and importantly, eczema charities all signposting [the intervention] and sharing a consistent message.”
While the intervention is free and available to patients anywhere, Dr. Thomas emphasized that it is tailored to the U.K. health care system. “If people would like to get in touch and help work with us to maybe adapt it slightly to make it more suitable for your own health care systems, that’s something we’d be very happy to look at with you.”
Asked for comment, Natalie Cunningham, MD, panel moderator, was lukewarm about the tool. “It can be a supplement, but you can never replace the one-on-one patient–health care provider interaction,” she told this news organization. “That could be provided by a nondermatologist and supplemented by an online component,” said Dr. Cunningham, from the Izaak Walton Killam Hospital for Children in Halifax, N.S.
“First-line treatment for eczema, no matter what kind of eczema, is topical steroids, and that is something that requires a lot of education – and something you want to do one on one in person because everyone comes to it with a different experience, baggage, or understanding,” she said. “We need to figure out what the barrier is so that you can do the right education.”
In addition, with systemic AD therapies currently approved for children, parents and young patients need to be able to advocate for specialist care to access these medications, she noted.
Dr. Thomas and Dr. Cunningham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MONTREAL – for up to 1 year, according to two randomized controlled trials presented at the annual meeting of the International Society of Atopic Dermatitis.
The intervention, directed either at parents of children with AD or young adults with AD, “is very low cost, evidence based, easily accessible, and free from possible commercial bias,” said investigator Kim Thomas, MD, professor of applied dermatology research and codirector of the Centre of Evidence Based Dermatology, faculty of medicine & health sciences, University of Nottingham (England).
The main focus of the intervention, along with general education, is “getting control” of the condition with flare-control creams and “keeping control” with regular emollient use.
Efficacy of the intervention, available free online, was compared with “usual eczema care” in 340 parents of children with AD up to age 12 and 337 young patients with AD aged 13-25. Participants were randomized to the intervention plus usual care or usual care alone. The primary outcome was the Patient-Oriented Eczema Measure(POEM) at 24 weeks, with a further measurement at 52 weeks.
In the parent group, about half were women and 83% were White, and the median age of their children was 4 years. About 50% of parents had a university degree, making them “possibly better educated than we might want our target audience for this type of intervention,” Dr. Thomas commented. Most of the children had moderate AD.
In the young patient group, the mean age was 19 years, more than three-quarters were female, 83% were White, and most had moderate AD.
At 24 weeks, both intervention groups had improved POEM scores, compared with controls, with a mean difference of 1.5 points in the parent group (P = .002) and 1.7 points in the young patient group (P = .04). “A small difference, but statistically significant and sustained,” Dr. Thomas said, adding that this difference was sustained up to 52 weeks.
In terms of mechanism of action, a secondary outcome looked at the concept of enablement, “which again, seemed to be improved in the intervention group, which suggests it’s something to do with being able to understand and cope with their disease better,” she said. The tool is targeted to “people who wouldn’t normally get to a dermatologist and certainly wouldn’t get access to group interventions.”
An additional aim of the intervention was “to provide a single, consistent message received from every point of contact that people might engage with ... [from] community doctors, pharmacists, dermatologists, and importantly, eczema charities all signposting [the intervention] and sharing a consistent message.”
While the intervention is free and available to patients anywhere, Dr. Thomas emphasized that it is tailored to the U.K. health care system. “If people would like to get in touch and help work with us to maybe adapt it slightly to make it more suitable for your own health care systems, that’s something we’d be very happy to look at with you.”
Asked for comment, Natalie Cunningham, MD, panel moderator, was lukewarm about the tool. “It can be a supplement, but you can never replace the one-on-one patient–health care provider interaction,” she told this news organization. “That could be provided by a nondermatologist and supplemented by an online component,” said Dr. Cunningham, from the Izaak Walton Killam Hospital for Children in Halifax, N.S.
“First-line treatment for eczema, no matter what kind of eczema, is topical steroids, and that is something that requires a lot of education – and something you want to do one on one in person because everyone comes to it with a different experience, baggage, or understanding,” she said. “We need to figure out what the barrier is so that you can do the right education.”
In addition, with systemic AD therapies currently approved for children, parents and young patients need to be able to advocate for specialist care to access these medications, she noted.
Dr. Thomas and Dr. Cunningham reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ISAD 2022
AHA 2022 to recapture in-person vibe but preserve global reach
That a bustling medical conference can have global reach as it unfolds is one of the COVID pandemic’s many lessons for science. Hybrid meetings such as the American Heart Association scientific sessions, getting underway Nov. 5 in Chicago and cyberspace, are one of its legacies.
The conference is set to recapture the magic of the in-person Scientific Sessions last experienced in Philadelphia in 2019. But planners are mindful of a special responsibility to younger clinicians and scientists who entered the field knowing only the virtual format and who may not know “what it’s like in a room when major science is presented or to present posters and have people come by for conversations,” Manesh R. Patel, MD, chair of the AHA 2022 Scientific Sessions program committee, told this news organization.
Still, the pandemic has underlined the value of live streaming for the great many who can’t attend in person, Dr. Patel said. At AHA 2022, virtual access doesn’t mean only late breaking and featured presentations; more than 70 full sessions will be streamed from Friday through Monday.
Overall, the conference has more than 800 sessions on the schedule, about a third are panels or invited lectures and two-thirds are original reports on the latest research. At the core of the research offerings, 78 studies and analyses are slated across 18 Late-Breaking Science (LBS) and Featured Science (FS) sessions from Saturday through Monday. At least 30 presentations and abstracts will enter the peer-reviewed literature right away with their simultaneous online publication, Dr. Patel said.
More a meet-and-greet than a presentation, the Puppy Snuggles Booth will make a return appearance in Chicago after earning rave reviews at the 2019 Sessions in Philadelphia. All are invited to take a breather from their schedules to pet, cuddle, and play with a passel of pups, all in need of homes and available for adoption. The experience’s favorable effect on blood pressure is almost guaranteed.
LBS and FS highlights
“It’s an amazing year for Late Breaking Science and Featured Science at the Scientific Sessions,” Dr. Patel said of the presentations selected for special attention after a rigorous review process. “We have science that is as broad and as deep as we’ve seen in years.”
Saturday’s two LBS sessions kick off the series with studies looking at agents long available in heart failure and hypertension but lacking solid supporting evidence, “pretty large randomized trials that are, we think, going to affect clinical practice as soon as they are presented,” Dr. Patel said.
They include TRANSFORM-HF, a comparison of the loop diuretics furosemide and torsemide in patients hospitalized with heart failure. And the Diuretic Comparison Project (DCP), with more than 13,000 patients with hypertension assigned to the diuretics chlorthalidone or hydrochlorothiazide, “is going to immediately impact how people think about blood pressure management,” Dr. Patel said.
Other highlights in the hypertension arena include the CRHCP trial, the MB-BP study, the Rich Life Project, and the polypill efficacy and safety trial QUARTET-USA, all in Sunday’s LBS-4; and the FRESH, PRECISION, and BrigHTN trials, all in LBS-9 on Monday.
Other heart failure trials joining TRANSFORM-HF in the line-up include IRONMAN, which revisited IV iron therapy in iron-deficient patients, in LBS-2 on Saturday and, in FS-4 on Monday, BETA3LVH and STRONG-HF, the latter a timely randomized test of pre- and post-discharge biomarker-driven uptitration of guideline-directed heart failure meds.
STRONG-HF was halted early, the trial’s nonprofit sponsor announced only weeks ago, after patients following the intensive uptitration strategy versus usual care showed a reduced risk of death or heart failure readmission; few other details were given.
Several sessions will be devoted to a rare breed of randomized trial, one that tests the efficacy of traditional herbal meds or nonprescription supplements against proven medications. “These are going to get a lot of people’s interest, one can imagine, because they are on common questions that patients bring to the clinic every day,” Dr. Patel said.
Such studies include CTS-AMI, which explored the traditional Chinese herbal medicine tongxinluo in ST-segment elevation myocardial infarction, in LBS-3 on Sunday, and SPORT in Sunday’s LBS-5, a small randomized comparison of low-dose rosuvastatin, cinnamon, garlic, turmeric, an omega-3 fish-oil supplement, a plant sterol, red yeast rice, and placebo for any effects on LDL-C levels.
Other novel approaches to dyslipidemia management are to be covered in RESPECT-EPA and OCEAN(a)-DOSE, both in LBS-5 on Sunday, and all five presentations in Monday’s FS-9, including ARCHES-2, SHASTA-2, FOURIER-OLE, and ORION-3.
The interplay of antiplatelets and coronary interventions will be explored in presentations called OPTION, in LBS-6 on Sunday, and HOST-EXAM and TWILIGHT, in FS-6 on Monday.
Coronary and peripheral-vascular interventions are center stage in reports on RAPCO in LBS-3 and BRIGHT-4 in LBS-6, both on Sunday, and BEST-CLI in LBS-7 and the After-80 Study in FS-6, both on Monday.
Several Monday reports will cover comorbidities and complications associated with COVID-19, including PREVENT-HD in LBS-7, and PANAMO, FERMIN, COVID-NET, and a secondary analysis of the DELIVER trial in FS-5.
Rebroadcasts for the Pacific Rim
The sessions will also feature several evening rebroadcasts of earlier LBS sessions that meeting planners scored highly for scientific merit and potential clinical impact but also for their “regional pull,” primarily for our colleagues in Asia, Dr. Patel said.
The first two LBS sessions presented live during the day in Chicago will be rebroadcast that evening as, for example, Sunday morning and afternoon fare in Tokyo and Singapore. And LBS-5 live Sunday afternoon will rebroadcast that night as a Monday mid-morning session in, say, Hong Kong or Seoul.
This year’s AHA meeting spans the range of cardiovascular care, from precision therapies, such as gene editing or specific drugs, to broad strategies that consider, for example, social determinants of health, Dr. Patel said. “I think people, when they leave the Scientific Sessions, will feel very engaged in the larger conversation about how you impact very common conditions globally.”
A version of this article first appeared on Medscape.com.
That a bustling medical conference can have global reach as it unfolds is one of the COVID pandemic’s many lessons for science. Hybrid meetings such as the American Heart Association scientific sessions, getting underway Nov. 5 in Chicago and cyberspace, are one of its legacies.
The conference is set to recapture the magic of the in-person Scientific Sessions last experienced in Philadelphia in 2019. But planners are mindful of a special responsibility to younger clinicians and scientists who entered the field knowing only the virtual format and who may not know “what it’s like in a room when major science is presented or to present posters and have people come by for conversations,” Manesh R. Patel, MD, chair of the AHA 2022 Scientific Sessions program committee, told this news organization.
Still, the pandemic has underlined the value of live streaming for the great many who can’t attend in person, Dr. Patel said. At AHA 2022, virtual access doesn’t mean only late breaking and featured presentations; more than 70 full sessions will be streamed from Friday through Monday.
Overall, the conference has more than 800 sessions on the schedule, about a third are panels or invited lectures and two-thirds are original reports on the latest research. At the core of the research offerings, 78 studies and analyses are slated across 18 Late-Breaking Science (LBS) and Featured Science (FS) sessions from Saturday through Monday. At least 30 presentations and abstracts will enter the peer-reviewed literature right away with their simultaneous online publication, Dr. Patel said.
More a meet-and-greet than a presentation, the Puppy Snuggles Booth will make a return appearance in Chicago after earning rave reviews at the 2019 Sessions in Philadelphia. All are invited to take a breather from their schedules to pet, cuddle, and play with a passel of pups, all in need of homes and available for adoption. The experience’s favorable effect on blood pressure is almost guaranteed.
LBS and FS highlights
“It’s an amazing year for Late Breaking Science and Featured Science at the Scientific Sessions,” Dr. Patel said of the presentations selected for special attention after a rigorous review process. “We have science that is as broad and as deep as we’ve seen in years.”
Saturday’s two LBS sessions kick off the series with studies looking at agents long available in heart failure and hypertension but lacking solid supporting evidence, “pretty large randomized trials that are, we think, going to affect clinical practice as soon as they are presented,” Dr. Patel said.
They include TRANSFORM-HF, a comparison of the loop diuretics furosemide and torsemide in patients hospitalized with heart failure. And the Diuretic Comparison Project (DCP), with more than 13,000 patients with hypertension assigned to the diuretics chlorthalidone or hydrochlorothiazide, “is going to immediately impact how people think about blood pressure management,” Dr. Patel said.
Other highlights in the hypertension arena include the CRHCP trial, the MB-BP study, the Rich Life Project, and the polypill efficacy and safety trial QUARTET-USA, all in Sunday’s LBS-4; and the FRESH, PRECISION, and BrigHTN trials, all in LBS-9 on Monday.
Other heart failure trials joining TRANSFORM-HF in the line-up include IRONMAN, which revisited IV iron therapy in iron-deficient patients, in LBS-2 on Saturday and, in FS-4 on Monday, BETA3LVH and STRONG-HF, the latter a timely randomized test of pre- and post-discharge biomarker-driven uptitration of guideline-directed heart failure meds.
STRONG-HF was halted early, the trial’s nonprofit sponsor announced only weeks ago, after patients following the intensive uptitration strategy versus usual care showed a reduced risk of death or heart failure readmission; few other details were given.
Several sessions will be devoted to a rare breed of randomized trial, one that tests the efficacy of traditional herbal meds or nonprescription supplements against proven medications. “These are going to get a lot of people’s interest, one can imagine, because they are on common questions that patients bring to the clinic every day,” Dr. Patel said.
Such studies include CTS-AMI, which explored the traditional Chinese herbal medicine tongxinluo in ST-segment elevation myocardial infarction, in LBS-3 on Sunday, and SPORT in Sunday’s LBS-5, a small randomized comparison of low-dose rosuvastatin, cinnamon, garlic, turmeric, an omega-3 fish-oil supplement, a plant sterol, red yeast rice, and placebo for any effects on LDL-C levels.
Other novel approaches to dyslipidemia management are to be covered in RESPECT-EPA and OCEAN(a)-DOSE, both in LBS-5 on Sunday, and all five presentations in Monday’s FS-9, including ARCHES-2, SHASTA-2, FOURIER-OLE, and ORION-3.
The interplay of antiplatelets and coronary interventions will be explored in presentations called OPTION, in LBS-6 on Sunday, and HOST-EXAM and TWILIGHT, in FS-6 on Monday.
Coronary and peripheral-vascular interventions are center stage in reports on RAPCO in LBS-3 and BRIGHT-4 in LBS-6, both on Sunday, and BEST-CLI in LBS-7 and the After-80 Study in FS-6, both on Monday.
Several Monday reports will cover comorbidities and complications associated with COVID-19, including PREVENT-HD in LBS-7, and PANAMO, FERMIN, COVID-NET, and a secondary analysis of the DELIVER trial in FS-5.
Rebroadcasts for the Pacific Rim
The sessions will also feature several evening rebroadcasts of earlier LBS sessions that meeting planners scored highly for scientific merit and potential clinical impact but also for their “regional pull,” primarily for our colleagues in Asia, Dr. Patel said.
The first two LBS sessions presented live during the day in Chicago will be rebroadcast that evening as, for example, Sunday morning and afternoon fare in Tokyo and Singapore. And LBS-5 live Sunday afternoon will rebroadcast that night as a Monday mid-morning session in, say, Hong Kong or Seoul.
This year’s AHA meeting spans the range of cardiovascular care, from precision therapies, such as gene editing or specific drugs, to broad strategies that consider, for example, social determinants of health, Dr. Patel said. “I think people, when they leave the Scientific Sessions, will feel very engaged in the larger conversation about how you impact very common conditions globally.”
A version of this article first appeared on Medscape.com.
That a bustling medical conference can have global reach as it unfolds is one of the COVID pandemic’s many lessons for science. Hybrid meetings such as the American Heart Association scientific sessions, getting underway Nov. 5 in Chicago and cyberspace, are one of its legacies.
The conference is set to recapture the magic of the in-person Scientific Sessions last experienced in Philadelphia in 2019. But planners are mindful of a special responsibility to younger clinicians and scientists who entered the field knowing only the virtual format and who may not know “what it’s like in a room when major science is presented or to present posters and have people come by for conversations,” Manesh R. Patel, MD, chair of the AHA 2022 Scientific Sessions program committee, told this news organization.
Still, the pandemic has underlined the value of live streaming for the great many who can’t attend in person, Dr. Patel said. At AHA 2022, virtual access doesn’t mean only late breaking and featured presentations; more than 70 full sessions will be streamed from Friday through Monday.
Overall, the conference has more than 800 sessions on the schedule, about a third are panels or invited lectures and two-thirds are original reports on the latest research. At the core of the research offerings, 78 studies and analyses are slated across 18 Late-Breaking Science (LBS) and Featured Science (FS) sessions from Saturday through Monday. At least 30 presentations and abstracts will enter the peer-reviewed literature right away with their simultaneous online publication, Dr. Patel said.
More a meet-and-greet than a presentation, the Puppy Snuggles Booth will make a return appearance in Chicago after earning rave reviews at the 2019 Sessions in Philadelphia. All are invited to take a breather from their schedules to pet, cuddle, and play with a passel of pups, all in need of homes and available for adoption. The experience’s favorable effect on blood pressure is almost guaranteed.
LBS and FS highlights
“It’s an amazing year for Late Breaking Science and Featured Science at the Scientific Sessions,” Dr. Patel said of the presentations selected for special attention after a rigorous review process. “We have science that is as broad and as deep as we’ve seen in years.”
Saturday’s two LBS sessions kick off the series with studies looking at agents long available in heart failure and hypertension but lacking solid supporting evidence, “pretty large randomized trials that are, we think, going to affect clinical practice as soon as they are presented,” Dr. Patel said.
They include TRANSFORM-HF, a comparison of the loop diuretics furosemide and torsemide in patients hospitalized with heart failure. And the Diuretic Comparison Project (DCP), with more than 13,000 patients with hypertension assigned to the diuretics chlorthalidone or hydrochlorothiazide, “is going to immediately impact how people think about blood pressure management,” Dr. Patel said.
Other highlights in the hypertension arena include the CRHCP trial, the MB-BP study, the Rich Life Project, and the polypill efficacy and safety trial QUARTET-USA, all in Sunday’s LBS-4; and the FRESH, PRECISION, and BrigHTN trials, all in LBS-9 on Monday.
Other heart failure trials joining TRANSFORM-HF in the line-up include IRONMAN, which revisited IV iron therapy in iron-deficient patients, in LBS-2 on Saturday and, in FS-4 on Monday, BETA3LVH and STRONG-HF, the latter a timely randomized test of pre- and post-discharge biomarker-driven uptitration of guideline-directed heart failure meds.
STRONG-HF was halted early, the trial’s nonprofit sponsor announced only weeks ago, after patients following the intensive uptitration strategy versus usual care showed a reduced risk of death or heart failure readmission; few other details were given.
Several sessions will be devoted to a rare breed of randomized trial, one that tests the efficacy of traditional herbal meds or nonprescription supplements against proven medications. “These are going to get a lot of people’s interest, one can imagine, because they are on common questions that patients bring to the clinic every day,” Dr. Patel said.
Such studies include CTS-AMI, which explored the traditional Chinese herbal medicine tongxinluo in ST-segment elevation myocardial infarction, in LBS-3 on Sunday, and SPORT in Sunday’s LBS-5, a small randomized comparison of low-dose rosuvastatin, cinnamon, garlic, turmeric, an omega-3 fish-oil supplement, a plant sterol, red yeast rice, and placebo for any effects on LDL-C levels.
Other novel approaches to dyslipidemia management are to be covered in RESPECT-EPA and OCEAN(a)-DOSE, both in LBS-5 on Sunday, and all five presentations in Monday’s FS-9, including ARCHES-2, SHASTA-2, FOURIER-OLE, and ORION-3.
The interplay of antiplatelets and coronary interventions will be explored in presentations called OPTION, in LBS-6 on Sunday, and HOST-EXAM and TWILIGHT, in FS-6 on Monday.
Coronary and peripheral-vascular interventions are center stage in reports on RAPCO in LBS-3 and BRIGHT-4 in LBS-6, both on Sunday, and BEST-CLI in LBS-7 and the After-80 Study in FS-6, both on Monday.
Several Monday reports will cover comorbidities and complications associated with COVID-19, including PREVENT-HD in LBS-7, and PANAMO, FERMIN, COVID-NET, and a secondary analysis of the DELIVER trial in FS-5.
Rebroadcasts for the Pacific Rim
The sessions will also feature several evening rebroadcasts of earlier LBS sessions that meeting planners scored highly for scientific merit and potential clinical impact but also for their “regional pull,” primarily for our colleagues in Asia, Dr. Patel said.
The first two LBS sessions presented live during the day in Chicago will be rebroadcast that evening as, for example, Sunday morning and afternoon fare in Tokyo and Singapore. And LBS-5 live Sunday afternoon will rebroadcast that night as a Monday mid-morning session in, say, Hong Kong or Seoul.
This year’s AHA meeting spans the range of cardiovascular care, from precision therapies, such as gene editing or specific drugs, to broad strategies that consider, for example, social determinants of health, Dr. Patel said. “I think people, when they leave the Scientific Sessions, will feel very engaged in the larger conversation about how you impact very common conditions globally.”
A version of this article first appeared on Medscape.com.
European research team to study drug resistance in psychiatry
Having secured 11 million euros in funding from the European Union’s Horizon Health program, an international team of pharmacology, pharmacogenetics, and psychiatry experts has set to work in hopes of helping patients with severe mental illnesses.
On this team is a group of researchers from the University of Cagliari in Sardinia, Italy. They are part of a network of international experts from 26 universities, research centers, and European associations, all of whom have vast experience in the fields of psychiatry, pharmacology, genetics, and statistics. Coordinating the project is Bernhard T. Baune, MD, PhD, professor of psychiatry at the University of Münster, Germany.
The problem of drug resistance is of great relevance to psychiatrists. About one-third of patients do not respond to pharmacologic therapies; as a result, their illness becomes more and more severe. This development has a major impact on these patients’ quality of life. In addition, health care and social services face a rise in the costs associated with managing the illnesses.
The research team from the University of Cagliari has two members from the department of biomedical sciences – Alessio Squassina, PhD, head of the pharmacogenetics laboratory, and Claudia Pisanu, MD, PhD – and two translational clinical researchers from the department of medical sciences and public health – Bernardo Carpiniello, MD, head of the psychiatry division, and Mirko Manchia, MD, PhD. They will be in charge of recruiting and collecting biological material from one set of patients with mental illnesses, collecting DNA and performing genetic screenings for all of the patients recruited by the network’s members in the various European countries, and conducting and coordinating clinical trials in which the pharmacologic therapies will be guided based on the molecular results.
“The process of figuring out whether someone has drug resistance is complex,” explained Dr. Squassina. “It may require very long periods of treatment and observation which, in the end, severely impact the patient’s chances of seeing a significant improvement in their symptoms and of being able to reintegrate themselves into society in the shortest possible time frame.” The goal of the Psych-STRATA project is to come up with a predictive algorithm – consisting of molecular markers and clinical data – that, before a specific antidepressant is even given, will be able to identify the patients who have a greater probability of responding and those who have a greater probability of not responding. Psych-STRATA’s findings could have a meaningful positive effect on the lives of patients with mental illnesses, as they would provide psychiatrists with guidance for managing pharmacologic therapies more precisely and in a way that is based on patients’ biological characteristics. This, in turn, would increase the efficacy of drugs, lower the risks of adverse effects, and significantly contribute to achieving quick remission of symptoms.
A version of this article appeared on Medscape.com. This article was translated from Univadis Italy.
Having secured 11 million euros in funding from the European Union’s Horizon Health program, an international team of pharmacology, pharmacogenetics, and psychiatry experts has set to work in hopes of helping patients with severe mental illnesses.
On this team is a group of researchers from the University of Cagliari in Sardinia, Italy. They are part of a network of international experts from 26 universities, research centers, and European associations, all of whom have vast experience in the fields of psychiatry, pharmacology, genetics, and statistics. Coordinating the project is Bernhard T. Baune, MD, PhD, professor of psychiatry at the University of Münster, Germany.
The problem of drug resistance is of great relevance to psychiatrists. About one-third of patients do not respond to pharmacologic therapies; as a result, their illness becomes more and more severe. This development has a major impact on these patients’ quality of life. In addition, health care and social services face a rise in the costs associated with managing the illnesses.
The research team from the University of Cagliari has two members from the department of biomedical sciences – Alessio Squassina, PhD, head of the pharmacogenetics laboratory, and Claudia Pisanu, MD, PhD – and two translational clinical researchers from the department of medical sciences and public health – Bernardo Carpiniello, MD, head of the psychiatry division, and Mirko Manchia, MD, PhD. They will be in charge of recruiting and collecting biological material from one set of patients with mental illnesses, collecting DNA and performing genetic screenings for all of the patients recruited by the network’s members in the various European countries, and conducting and coordinating clinical trials in which the pharmacologic therapies will be guided based on the molecular results.
“The process of figuring out whether someone has drug resistance is complex,” explained Dr. Squassina. “It may require very long periods of treatment and observation which, in the end, severely impact the patient’s chances of seeing a significant improvement in their symptoms and of being able to reintegrate themselves into society in the shortest possible time frame.” The goal of the Psych-STRATA project is to come up with a predictive algorithm – consisting of molecular markers and clinical data – that, before a specific antidepressant is even given, will be able to identify the patients who have a greater probability of responding and those who have a greater probability of not responding. Psych-STRATA’s findings could have a meaningful positive effect on the lives of patients with mental illnesses, as they would provide psychiatrists with guidance for managing pharmacologic therapies more precisely and in a way that is based on patients’ biological characteristics. This, in turn, would increase the efficacy of drugs, lower the risks of adverse effects, and significantly contribute to achieving quick remission of symptoms.
A version of this article appeared on Medscape.com. This article was translated from Univadis Italy.
Having secured 11 million euros in funding from the European Union’s Horizon Health program, an international team of pharmacology, pharmacogenetics, and psychiatry experts has set to work in hopes of helping patients with severe mental illnesses.
On this team is a group of researchers from the University of Cagliari in Sardinia, Italy. They are part of a network of international experts from 26 universities, research centers, and European associations, all of whom have vast experience in the fields of psychiatry, pharmacology, genetics, and statistics. Coordinating the project is Bernhard T. Baune, MD, PhD, professor of psychiatry at the University of Münster, Germany.
The problem of drug resistance is of great relevance to psychiatrists. About one-third of patients do not respond to pharmacologic therapies; as a result, their illness becomes more and more severe. This development has a major impact on these patients’ quality of life. In addition, health care and social services face a rise in the costs associated with managing the illnesses.
The research team from the University of Cagliari has two members from the department of biomedical sciences – Alessio Squassina, PhD, head of the pharmacogenetics laboratory, and Claudia Pisanu, MD, PhD – and two translational clinical researchers from the department of medical sciences and public health – Bernardo Carpiniello, MD, head of the psychiatry division, and Mirko Manchia, MD, PhD. They will be in charge of recruiting and collecting biological material from one set of patients with mental illnesses, collecting DNA and performing genetic screenings for all of the patients recruited by the network’s members in the various European countries, and conducting and coordinating clinical trials in which the pharmacologic therapies will be guided based on the molecular results.
“The process of figuring out whether someone has drug resistance is complex,” explained Dr. Squassina. “It may require very long periods of treatment and observation which, in the end, severely impact the patient’s chances of seeing a significant improvement in their symptoms and of being able to reintegrate themselves into society in the shortest possible time frame.” The goal of the Psych-STRATA project is to come up with a predictive algorithm – consisting of molecular markers and clinical data – that, before a specific antidepressant is even given, will be able to identify the patients who have a greater probability of responding and those who have a greater probability of not responding. Psych-STRATA’s findings could have a meaningful positive effect on the lives of patients with mental illnesses, as they would provide psychiatrists with guidance for managing pharmacologic therapies more precisely and in a way that is based on patients’ biological characteristics. This, in turn, would increase the efficacy of drugs, lower the risks of adverse effects, and significantly contribute to achieving quick remission of symptoms.
A version of this article appeared on Medscape.com. This article was translated from Univadis Italy.
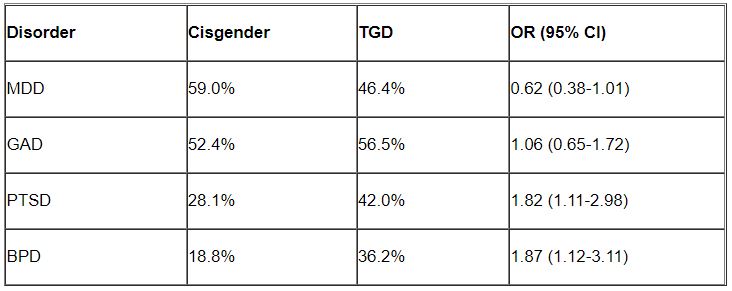
Higher rates of PTSD, BPD in transgender vs. cisgender psych patients
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).
“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).
“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although mood disorders, depression, and anxiety were the most common diagnoses in both TGD and cisgender patients, “when we compared the diagnostic profiles [of TGD patients] to those of cisgender patients, we found an increased prevalence of PTSD and BPD,” study investigator Mark Zimmerman, MD, professor of psychiatry and human behavior, Brown University, Providence, R.I., told this news organization.
“What we concluded is that psychiatric programs that wish to treat TGD patients should either have or should develop expertise in treating PTSD and BPD, not just mood and anxiety disorders,” Dr. Zimmerman said.
The study was published online September 26 in the Journal of Clinical Psychiatry.
‘Piecemeal literature’
TGD individuals “experience high rates of various forms of psychopathology in general and when compared with cisgender persons,” the investigators note.
They point out that most empirical evidence has relied upon the use of brief, unstructured psychodiagnostic assessment measures and assessment of a “limited constellation of psychiatric symptoms domains,” resulting in a “piecemeal literature wherein each piece of research documents elevations in one – or a few – diagnostic domains.”
Studies pointing to broader psychosocial health variables have often relied upon self-reported measures. In addition, in studies that utilized a structured interview approach, none “used a formal interview procedure to assess psychiatric diagnoses” and most focused only on a “limited number of psychiatric conditions based on self-reports of past diagnosis.”
The goal of the current study was to use semistructured interviews administered by professionals to compare the diagnostic profiles of a samples of TGD and cisgender patients who presented for treatment at a single naturalistic, clinically acute setting – a partial hospital program.
Dr. Zimmerman said that there was an additional motive for conducting the study. “There has been discussion in the field as to whether or not transgender or gender-diverse individuals all have borderline personality disorder, but that hasn’t been our clinical impression.”
Rather, Dr. Zimmerman and colleagues believe TGD people “may have had more difficult childhoods and more difficult adjustments in society because of societal attitudes and have to deal with that stress, whether it be microaggressions or overt bullying and aggression.” The study was designed to investigate this issue.
In addition, studies conducted in primary care programs in individuals seeking gender-affirming surgery have “reported a limited number of psychiatric diagnoses, but we were wondering whether, amongst psychiatric patients specifically, there were differences in diagnostic profiles between transgender and gender-diverse patients and cisgender patients. If so, what might the implications be for providing care for this population?”
TGD not synonymous with borderline
To investigate, the researchers administered semistructured diagnostic interviews for DSM-IV disorders to 2,212 psychiatric patients (66% cisgender women, 30.8% cisgender men, 3.1% TGD; mean [standard deviation] age 36.7 [14.4] years) presenting to the Rhode Island Hospital Department of Psychiatry Partial Hospital Program between April 2014 and January 2021.
Patients also completed a demographic questionnaire including their assigned sex at birth and their current gender identity.
Most patients (44.9%) were single, followed by 23.5% who were married, 14.1% living in a relationship as if married, 12.0% divorced, 3.6% separated, and 1.9% widowed.
Almost three-quarters of participants (73.2%) identified as White, followed by Hispanic (10.7%), Black (6.7%), “other” or a combination of racial/ethnic backgrounds (6.6%), and Asian (2.7%).
There were no differences between cisgender and TGD groups in terms of race or education, but the TGD patients were significantly younger compared with their cisgender counterparts and were significantly more likely to have never been married.
The average number of psychiatric diagnoses in the sample was 3.05 (± 1.73), with TGD patients having a larger number of psychiatric diagnoses than did their cisgender peers (an average of 3.54 ± 1.88 vs. 3.04 ± 1.72, respectively; t = 2.37; P = .02).
Major depressive disorder (MDD) and generalized anxiety disorder (GAD) were the most common disorders among both cisgender and TGD patients. However, after controlling for age, the researchers found that TGD patients were significantly more likely than were the cisgender patients to be diagnosed with PTSD and BPD (P < .05 for both).
“Of note, only about one-third of the TGD individuals were diagnosed with BPD, so it is important to realize that transgender or gender-diverse identity is not synonymous with BPD, as some have suggested,” noted Dr. Zimmerman, who is also the director of the outpatient division at the Partial Hospital Program, Rhode Island Hospital.
A representative sample?
Commenting on the study, Jack Drescher, MD, distinguished life fellow of the American Psychiatric Association and clinical professor of psychiatry, Columbia University, New York, called the findings “interesting” but noted that a limitation of the study is that it included “a patient population with likely more severe psychiatric illness, since they were all day hospital patients.”
The question is whether similar findings would be obtained in a less severely ill population, said Dr. Drescher, who is also a senior consulting analyst for sexuality and gender at Columbia University and was not involved with the study. “The patients in the study may not be representative of the general population, either cisgender or transgender.”
Dr. Drescher was “not surprised” by the finding regarding PTSD because the finding “is consistent with our understanding of the kinds of traumas that transgender people go through in day-to-day life.”
He noted that some people misunderstand the diagnostic criterion in BPD of identity confusion and think that because people with gender dysphoria may be confused about their identity, it means that all people who are transgender have borderline personality disorder, “but that’s not true.”
Dr. Zimmerman agreed. “The vast majority of individuals with BPD do not have a transgender or gender-diverse identity, and TGD should not be equated with BPD,” he said.
No source of study funding was disclosed. Dr. Zimmerman and coauthors and Dr. Drescher report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF CLINICAL PSYCHIATRY
Resection of infected sacrohysteropexy mesh
Pain in Cancer Survivors: Assess, Monitor, and Ask for Help
SAN DIEGO—As patients with cancer live longer, pain is going to become an even bigger challenge for clinicians, a palliative care specialist told cancer specialists in a presentation at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) in September, and decisions about treatment are becoming more complicated amid the opioid epidemic.
Fortunately, guidelines and clinical experience offer helpful insight into the best practices, said hematologist/oncologist Andrea Ruskin, MD, medical director of palliative care at Veterans Administration (VA) Connecticut Healthcare System (VACHS).
As Ruskin pointed out, two-thirds of newly diagnosed cancer patients are living for at least 5 years, “but with this progress comes challenges.” More than one-third (37%) of cancer survivors report cancer pain, 21% have noncancer pain, and 45% have both. About 5% to 8% of VA cancer survivors use opioids for the long term, she said, although there have been few studies in this population.
Among patients with head and neck cancer, specifically, chronic pain affects 45%, and severe pain affects 11%. Subclinical PTSD, depression, anxiety, and low quality of life are common in this population. “We may cure them, but they have a lot of issues going forward.”
One key strategy is to perform a comprehensive pain assessment at the first visit, and then address pain at every subsequent visit. She recommended a physician resource from the American Society of Clinical Oncology, and a template may be useful to provide helpful questions, Ruskin said.
At VACHS certain questions are routine. “Is pain interfering with your function? Sometimes people say it’s always a 10, but it’s not affecting function at all. Ask if the medicine is working. And how are they taking it? Sometimes they say, ‘I’m taking that for sleep,’ and we say ‘No, Mr. Smith, that is not a sleep medication.’”
Be aware that some patients may use nonmedical opioids, she said. And set expectations early on. “Safe opioid use starts with the very first prescription,” she said. “If I have somebody with myeloma or head and neck cancer, I make it very clear that my goal is that we want you off the opioids after the radiation or once the disease is in remission. I really make an effort at the very beginning to make sure that we're all on the same page.”
As you continue to see a patient, consider ordering urine tests, she said, not as a punitive measure but to make sure you’re offering the safest and most effective treatment. “We don’t do it to say ‘no, no, no.’ We do it for safety and to make sure they’re not getting meds elsewhere.”
What are the best practices when pain doesn’t go away? Should they stay on opioids? According to Ruskin, few evidence-based guidelines address the “more nuanced care” that patients need when their pain lasts for months or years.
But there are useful resources. Ruskin highlighted the National Comprehensive Cancer Network’s survivorship guidelines, and she summarized a few of the available painkiller options. “Opioids are great, and adjuvants are so-so. They work in some people, but we definitely have room for improvement.”
What if patients have persistent opioid use after cancer recovery? “I try to taper if I can, and I try to explain why I’m tapering. It could take months or years to taper patients,” she said. And consider transitioning the patient to buprenorphine, a drug that treats both pain and opioid use disorder, if appropriate. “You don’t need a waiver if you use it for pain. It’s definitely something we’re using more of.”
One important step is to bring in colleagues to help. Psychologists, chiropractors, physical therapists, physiatrists, and pain pharmacists can all be helpful, she said. “Learn about your VA resources and who can partner with you to help these complicated patients. They’re all at your fingertips.”
SAN DIEGO—As patients with cancer live longer, pain is going to become an even bigger challenge for clinicians, a palliative care specialist told cancer specialists in a presentation at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) in September, and decisions about treatment are becoming more complicated amid the opioid epidemic.
Fortunately, guidelines and clinical experience offer helpful insight into the best practices, said hematologist/oncologist Andrea Ruskin, MD, medical director of palliative care at Veterans Administration (VA) Connecticut Healthcare System (VACHS).
As Ruskin pointed out, two-thirds of newly diagnosed cancer patients are living for at least 5 years, “but with this progress comes challenges.” More than one-third (37%) of cancer survivors report cancer pain, 21% have noncancer pain, and 45% have both. About 5% to 8% of VA cancer survivors use opioids for the long term, she said, although there have been few studies in this population.
Among patients with head and neck cancer, specifically, chronic pain affects 45%, and severe pain affects 11%. Subclinical PTSD, depression, anxiety, and low quality of life are common in this population. “We may cure them, but they have a lot of issues going forward.”
One key strategy is to perform a comprehensive pain assessment at the first visit, and then address pain at every subsequent visit. She recommended a physician resource from the American Society of Clinical Oncology, and a template may be useful to provide helpful questions, Ruskin said.
At VACHS certain questions are routine. “Is pain interfering with your function? Sometimes people say it’s always a 10, but it’s not affecting function at all. Ask if the medicine is working. And how are they taking it? Sometimes they say, ‘I’m taking that for sleep,’ and we say ‘No, Mr. Smith, that is not a sleep medication.’”
Be aware that some patients may use nonmedical opioids, she said. And set expectations early on. “Safe opioid use starts with the very first prescription,” she said. “If I have somebody with myeloma or head and neck cancer, I make it very clear that my goal is that we want you off the opioids after the radiation or once the disease is in remission. I really make an effort at the very beginning to make sure that we're all on the same page.”
As you continue to see a patient, consider ordering urine tests, she said, not as a punitive measure but to make sure you’re offering the safest and most effective treatment. “We don’t do it to say ‘no, no, no.’ We do it for safety and to make sure they’re not getting meds elsewhere.”
What are the best practices when pain doesn’t go away? Should they stay on opioids? According to Ruskin, few evidence-based guidelines address the “more nuanced care” that patients need when their pain lasts for months or years.
But there are useful resources. Ruskin highlighted the National Comprehensive Cancer Network’s survivorship guidelines, and she summarized a few of the available painkiller options. “Opioids are great, and adjuvants are so-so. They work in some people, but we definitely have room for improvement.”
What if patients have persistent opioid use after cancer recovery? “I try to taper if I can, and I try to explain why I’m tapering. It could take months or years to taper patients,” she said. And consider transitioning the patient to buprenorphine, a drug that treats both pain and opioid use disorder, if appropriate. “You don’t need a waiver if you use it for pain. It’s definitely something we’re using more of.”
One important step is to bring in colleagues to help. Psychologists, chiropractors, physical therapists, physiatrists, and pain pharmacists can all be helpful, she said. “Learn about your VA resources and who can partner with you to help these complicated patients. They’re all at your fingertips.”
SAN DIEGO—As patients with cancer live longer, pain is going to become an even bigger challenge for clinicians, a palliative care specialist told cancer specialists in a presentation at the annual meeting of the Association of VA Hematology/Oncology (AVAHO) in September, and decisions about treatment are becoming more complicated amid the opioid epidemic.
Fortunately, guidelines and clinical experience offer helpful insight into the best practices, said hematologist/oncologist Andrea Ruskin, MD, medical director of palliative care at Veterans Administration (VA) Connecticut Healthcare System (VACHS).
As Ruskin pointed out, two-thirds of newly diagnosed cancer patients are living for at least 5 years, “but with this progress comes challenges.” More than one-third (37%) of cancer survivors report cancer pain, 21% have noncancer pain, and 45% have both. About 5% to 8% of VA cancer survivors use opioids for the long term, she said, although there have been few studies in this population.
Among patients with head and neck cancer, specifically, chronic pain affects 45%, and severe pain affects 11%. Subclinical PTSD, depression, anxiety, and low quality of life are common in this population. “We may cure them, but they have a lot of issues going forward.”
One key strategy is to perform a comprehensive pain assessment at the first visit, and then address pain at every subsequent visit. She recommended a physician resource from the American Society of Clinical Oncology, and a template may be useful to provide helpful questions, Ruskin said.
At VACHS certain questions are routine. “Is pain interfering with your function? Sometimes people say it’s always a 10, but it’s not affecting function at all. Ask if the medicine is working. And how are they taking it? Sometimes they say, ‘I’m taking that for sleep,’ and we say ‘No, Mr. Smith, that is not a sleep medication.’”
Be aware that some patients may use nonmedical opioids, she said. And set expectations early on. “Safe opioid use starts with the very first prescription,” she said. “If I have somebody with myeloma or head and neck cancer, I make it very clear that my goal is that we want you off the opioids after the radiation or once the disease is in remission. I really make an effort at the very beginning to make sure that we're all on the same page.”
As you continue to see a patient, consider ordering urine tests, she said, not as a punitive measure but to make sure you’re offering the safest and most effective treatment. “We don’t do it to say ‘no, no, no.’ We do it for safety and to make sure they’re not getting meds elsewhere.”
What are the best practices when pain doesn’t go away? Should they stay on opioids? According to Ruskin, few evidence-based guidelines address the “more nuanced care” that patients need when their pain lasts for months or years.
But there are useful resources. Ruskin highlighted the National Comprehensive Cancer Network’s survivorship guidelines, and she summarized a few of the available painkiller options. “Opioids are great, and adjuvants are so-so. They work in some people, but we definitely have room for improvement.”
What if patients have persistent opioid use after cancer recovery? “I try to taper if I can, and I try to explain why I’m tapering. It could take months or years to taper patients,” she said. And consider transitioning the patient to buprenorphine, a drug that treats both pain and opioid use disorder, if appropriate. “You don’t need a waiver if you use it for pain. It’s definitely something we’re using more of.”
One important step is to bring in colleagues to help. Psychologists, chiropractors, physical therapists, physiatrists, and pain pharmacists can all be helpful, she said. “Learn about your VA resources and who can partner with you to help these complicated patients. They’re all at your fingertips.”
HCC surveillance screening increased slightly with invitations, reminders
Mailing invitations for hepatocellular carcinoma (HCC) surveillance screening to patients with cirrhosis increased ultrasound uptake by 13 percentage points, but the majority of patients still did not receive the recommended semiannual screenings, according to findings published in Clinical Gastroenterology and Hepatology.
“These data highlight the need for more intensive interventions to further increase surveillance,” wrote Amit Singal, MD, of University of Texas Southwestern Medical Center and Parkland Health Hospital System in Dallas, and colleagues. “The underuse of HCC surveillance has been attributed to a combination of patient- and provider-level barriers, which can serve as future additional intervention targets.” These include transportation and financial barriers and possibly new blood-based screening modalities when they become available, thereby removing the need for a separate ultrasound appointment.
According to one study, more than 90% of hepatocellular carcinoma cases occur in people with chronic liver disease, and the cancer is a leading cause of death in those with compensated cirrhosis. Multiple medical associations therefore recommend an abdominal ultrasound every 6 months with or without alpha-fetoprotein (AFP) for surveillance in at-risk patients, including anyone with cirrhosis of any kind, but too few patients receive these surveillance ultrasounds, the authors write.
The researchers therefore conducted a pragmatic randomized clinical trial from March 2018 to September 2019 to compare surveillance ultrasound uptake for two groups of people with cirrhosis: 1,436 people who were mailed invitations to get a surveillance ultrasound and 1,436 people who received usual care, with surveillance recommended only at usual visits. The patients all received care at one of three health systems: a tertiary care referral center, a safety net health system, and a Veterans Affairs medical center. The primary outcome was semiannual surveillance in the patients over 1 year.
The researchers identified patients using ICD-9 and ICD-10 codes for cirrhosis and cirrhosis complications, as well as those with suspected but undocumented cirrhosis based on electronic medical record notes such as an elevated Fibrosis-4 index. They confirmed the diagnoses with chart review, confirmed that the patients had at least one outpatient visit in the previous year, and excluded those in whom surveillance is not recommended, who lacked contact information, or who spoke a language besides English or Spanish.
The mailing was a one-page letter in English and Spanish, written at a low literacy level, that explained hepatocellular carcinoma risk and recommended surveillance. Those who didn’t respond to the mailed invitation within 2 weeks received a reminder call to undergo surveillance, and those who scheduled an ultrasound received a reminder call about a week before the visit. Primary and subspecialty providers were blinded to the patients’ study arm assignments.
“We conducted the study as a pragmatic trial whereby patients in either arm could also be offered HCC surveillance by primary or specialty care providers during clinic visits,” the researchers wrote. “The frequency of the clinic visits and provider discussions regarding HCC surveillance were conducted per usual care and not dictated by the study protocol.”
Two-thirds of the patients (67.7%) were men, with a median age of 61.2 years. Just over a third (37.0%) were white, 31.9% were Hispanic, and 27.6% were Black. More than half the patients had hepatitis C (56.4%), 18.1% had alcohol-related liver disease, 14.5% had nonalcoholic fatty liver disease, and 2.4% had hepatitis B. Most of the patients had compensated cirrhosis, including 36.7% with ascites and 17.1% with hepatic encephalopathy.
Nearly a quarter of the patients in the outreach arm (23%) could not be contacted or lacked working phone numbers, but they remained in the intent-to-screen analysis. Just over a third of the patients who received mailed outreach (35.1%; 95% confidence interval, 32.6%-37.6%) received semiannual surveillance, compared to 21.9% (95% CI, 19.8%-24.2%) of the usual-care patients. The increased surveillance in the outreach group applied to most subgroups, including race/ethnicity and cirrhosis severity based on the Child-Turcotte-Pugh class.
“However, we observed site-level differences in the intervention effect, with significant increases in semiannual surveillance at the VA and safety net health systems (both P < .001) but not at the tertiary care referral center (P = .52),” the authors wrote. “In a post hoc subgroup analysis among patients with at least 1 primary care or gastroenterology outpatient visit during the study period, mailed outreach continued to increase semiannual surveillance, compared with usual care (46.8% vs. 32.7%; P < .001).”
Despite the improved rates from the intervention, the majority of patients still did not receive semiannual surveillance across all three sites, and almost 30% underwent no surveillance the entire year.
The research was funded by the National Cancer Institute, the Cancer Prevention Research Institute of Texas, and the Center for Innovations in Quality, Effectiveness and Safety. Dr. Singal has consulted for or served on the advisory boards of Bayer, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, and GRAIL. The other authors had no industry disclosures.
Hepatocellular carcinoma is a deadly cancer that is usually incurable unless detected at an early stage through regular surveillance. Current American guidelines support 6-monthly abdominal ultrasonography, with or without serum alpha-fetoprotein, for HCC surveillance in at-risk patients, such as those with cirrhosis. However, even in such a high-risk group, the uptake of and adherence to surveillance are far from satisfactory. This study by Dr. Singal and colleagues is therefore important and practical. Randomized controlled trials in HCC surveillance are rare. The authors clearly demonstrate that an outreach program comprising mail invitations followed by phone contacts if there was no response could increase the surveillance uptake by more than 10%.
None of these can work if chronic liver disease and cirrhosis are not diagnosed in the first place. Disease awareness, access to care (and racial discrepancies), and clinical care pathways are hurdles we need to overcome in order to make an impact on HCC mortality at the population level.
Vincent Wong, MD, is an academic hepatologist at the Chinese University of Hong Kong. He does not have relevant conflicts of interest in this article.
Hepatocellular carcinoma is a deadly cancer that is usually incurable unless detected at an early stage through regular surveillance. Current American guidelines support 6-monthly abdominal ultrasonography, with or without serum alpha-fetoprotein, for HCC surveillance in at-risk patients, such as those with cirrhosis. However, even in such a high-risk group, the uptake of and adherence to surveillance are far from satisfactory. This study by Dr. Singal and colleagues is therefore important and practical. Randomized controlled trials in HCC surveillance are rare. The authors clearly demonstrate that an outreach program comprising mail invitations followed by phone contacts if there was no response could increase the surveillance uptake by more than 10%.
None of these can work if chronic liver disease and cirrhosis are not diagnosed in the first place. Disease awareness, access to care (and racial discrepancies), and clinical care pathways are hurdles we need to overcome in order to make an impact on HCC mortality at the population level.
Vincent Wong, MD, is an academic hepatologist at the Chinese University of Hong Kong. He does not have relevant conflicts of interest in this article.
Hepatocellular carcinoma is a deadly cancer that is usually incurable unless detected at an early stage through regular surveillance. Current American guidelines support 6-monthly abdominal ultrasonography, with or without serum alpha-fetoprotein, for HCC surveillance in at-risk patients, such as those with cirrhosis. However, even in such a high-risk group, the uptake of and adherence to surveillance are far from satisfactory. This study by Dr. Singal and colleagues is therefore important and practical. Randomized controlled trials in HCC surveillance are rare. The authors clearly demonstrate that an outreach program comprising mail invitations followed by phone contacts if there was no response could increase the surveillance uptake by more than 10%.
None of these can work if chronic liver disease and cirrhosis are not diagnosed in the first place. Disease awareness, access to care (and racial discrepancies), and clinical care pathways are hurdles we need to overcome in order to make an impact on HCC mortality at the population level.
Vincent Wong, MD, is an academic hepatologist at the Chinese University of Hong Kong. He does not have relevant conflicts of interest in this article.
Mailing invitations for hepatocellular carcinoma (HCC) surveillance screening to patients with cirrhosis increased ultrasound uptake by 13 percentage points, but the majority of patients still did not receive the recommended semiannual screenings, according to findings published in Clinical Gastroenterology and Hepatology.
“These data highlight the need for more intensive interventions to further increase surveillance,” wrote Amit Singal, MD, of University of Texas Southwestern Medical Center and Parkland Health Hospital System in Dallas, and colleagues. “The underuse of HCC surveillance has been attributed to a combination of patient- and provider-level barriers, which can serve as future additional intervention targets.” These include transportation and financial barriers and possibly new blood-based screening modalities when they become available, thereby removing the need for a separate ultrasound appointment.
According to one study, more than 90% of hepatocellular carcinoma cases occur in people with chronic liver disease, and the cancer is a leading cause of death in those with compensated cirrhosis. Multiple medical associations therefore recommend an abdominal ultrasound every 6 months with or without alpha-fetoprotein (AFP) for surveillance in at-risk patients, including anyone with cirrhosis of any kind, but too few patients receive these surveillance ultrasounds, the authors write.
The researchers therefore conducted a pragmatic randomized clinical trial from March 2018 to September 2019 to compare surveillance ultrasound uptake for two groups of people with cirrhosis: 1,436 people who were mailed invitations to get a surveillance ultrasound and 1,436 people who received usual care, with surveillance recommended only at usual visits. The patients all received care at one of three health systems: a tertiary care referral center, a safety net health system, and a Veterans Affairs medical center. The primary outcome was semiannual surveillance in the patients over 1 year.
The researchers identified patients using ICD-9 and ICD-10 codes for cirrhosis and cirrhosis complications, as well as those with suspected but undocumented cirrhosis based on electronic medical record notes such as an elevated Fibrosis-4 index. They confirmed the diagnoses with chart review, confirmed that the patients had at least one outpatient visit in the previous year, and excluded those in whom surveillance is not recommended, who lacked contact information, or who spoke a language besides English or Spanish.
The mailing was a one-page letter in English and Spanish, written at a low literacy level, that explained hepatocellular carcinoma risk and recommended surveillance. Those who didn’t respond to the mailed invitation within 2 weeks received a reminder call to undergo surveillance, and those who scheduled an ultrasound received a reminder call about a week before the visit. Primary and subspecialty providers were blinded to the patients’ study arm assignments.
“We conducted the study as a pragmatic trial whereby patients in either arm could also be offered HCC surveillance by primary or specialty care providers during clinic visits,” the researchers wrote. “The frequency of the clinic visits and provider discussions regarding HCC surveillance were conducted per usual care and not dictated by the study protocol.”
Two-thirds of the patients (67.7%) were men, with a median age of 61.2 years. Just over a third (37.0%) were white, 31.9% were Hispanic, and 27.6% were Black. More than half the patients had hepatitis C (56.4%), 18.1% had alcohol-related liver disease, 14.5% had nonalcoholic fatty liver disease, and 2.4% had hepatitis B. Most of the patients had compensated cirrhosis, including 36.7% with ascites and 17.1% with hepatic encephalopathy.
Nearly a quarter of the patients in the outreach arm (23%) could not be contacted or lacked working phone numbers, but they remained in the intent-to-screen analysis. Just over a third of the patients who received mailed outreach (35.1%; 95% confidence interval, 32.6%-37.6%) received semiannual surveillance, compared to 21.9% (95% CI, 19.8%-24.2%) of the usual-care patients. The increased surveillance in the outreach group applied to most subgroups, including race/ethnicity and cirrhosis severity based on the Child-Turcotte-Pugh class.
“However, we observed site-level differences in the intervention effect, with significant increases in semiannual surveillance at the VA and safety net health systems (both P < .001) but not at the tertiary care referral center (P = .52),” the authors wrote. “In a post hoc subgroup analysis among patients with at least 1 primary care or gastroenterology outpatient visit during the study period, mailed outreach continued to increase semiannual surveillance, compared with usual care (46.8% vs. 32.7%; P < .001).”
Despite the improved rates from the intervention, the majority of patients still did not receive semiannual surveillance across all three sites, and almost 30% underwent no surveillance the entire year.
The research was funded by the National Cancer Institute, the Cancer Prevention Research Institute of Texas, and the Center for Innovations in Quality, Effectiveness and Safety. Dr. Singal has consulted for or served on the advisory boards of Bayer, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, and GRAIL. The other authors had no industry disclosures.
Mailing invitations for hepatocellular carcinoma (HCC) surveillance screening to patients with cirrhosis increased ultrasound uptake by 13 percentage points, but the majority of patients still did not receive the recommended semiannual screenings, according to findings published in Clinical Gastroenterology and Hepatology.
“These data highlight the need for more intensive interventions to further increase surveillance,” wrote Amit Singal, MD, of University of Texas Southwestern Medical Center and Parkland Health Hospital System in Dallas, and colleagues. “The underuse of HCC surveillance has been attributed to a combination of patient- and provider-level barriers, which can serve as future additional intervention targets.” These include transportation and financial barriers and possibly new blood-based screening modalities when they become available, thereby removing the need for a separate ultrasound appointment.
According to one study, more than 90% of hepatocellular carcinoma cases occur in people with chronic liver disease, and the cancer is a leading cause of death in those with compensated cirrhosis. Multiple medical associations therefore recommend an abdominal ultrasound every 6 months with or without alpha-fetoprotein (AFP) for surveillance in at-risk patients, including anyone with cirrhosis of any kind, but too few patients receive these surveillance ultrasounds, the authors write.
The researchers therefore conducted a pragmatic randomized clinical trial from March 2018 to September 2019 to compare surveillance ultrasound uptake for two groups of people with cirrhosis: 1,436 people who were mailed invitations to get a surveillance ultrasound and 1,436 people who received usual care, with surveillance recommended only at usual visits. The patients all received care at one of three health systems: a tertiary care referral center, a safety net health system, and a Veterans Affairs medical center. The primary outcome was semiannual surveillance in the patients over 1 year.
The researchers identified patients using ICD-9 and ICD-10 codes for cirrhosis and cirrhosis complications, as well as those with suspected but undocumented cirrhosis based on electronic medical record notes such as an elevated Fibrosis-4 index. They confirmed the diagnoses with chart review, confirmed that the patients had at least one outpatient visit in the previous year, and excluded those in whom surveillance is not recommended, who lacked contact information, or who spoke a language besides English or Spanish.
The mailing was a one-page letter in English and Spanish, written at a low literacy level, that explained hepatocellular carcinoma risk and recommended surveillance. Those who didn’t respond to the mailed invitation within 2 weeks received a reminder call to undergo surveillance, and those who scheduled an ultrasound received a reminder call about a week before the visit. Primary and subspecialty providers were blinded to the patients’ study arm assignments.
“We conducted the study as a pragmatic trial whereby patients in either arm could also be offered HCC surveillance by primary or specialty care providers during clinic visits,” the researchers wrote. “The frequency of the clinic visits and provider discussions regarding HCC surveillance were conducted per usual care and not dictated by the study protocol.”
Two-thirds of the patients (67.7%) were men, with a median age of 61.2 years. Just over a third (37.0%) were white, 31.9% were Hispanic, and 27.6% were Black. More than half the patients had hepatitis C (56.4%), 18.1% had alcohol-related liver disease, 14.5% had nonalcoholic fatty liver disease, and 2.4% had hepatitis B. Most of the patients had compensated cirrhosis, including 36.7% with ascites and 17.1% with hepatic encephalopathy.
Nearly a quarter of the patients in the outreach arm (23%) could not be contacted or lacked working phone numbers, but they remained in the intent-to-screen analysis. Just over a third of the patients who received mailed outreach (35.1%; 95% confidence interval, 32.6%-37.6%) received semiannual surveillance, compared to 21.9% (95% CI, 19.8%-24.2%) of the usual-care patients. The increased surveillance in the outreach group applied to most subgroups, including race/ethnicity and cirrhosis severity based on the Child-Turcotte-Pugh class.
“However, we observed site-level differences in the intervention effect, with significant increases in semiannual surveillance at the VA and safety net health systems (both P < .001) but not at the tertiary care referral center (P = .52),” the authors wrote. “In a post hoc subgroup analysis among patients with at least 1 primary care or gastroenterology outpatient visit during the study period, mailed outreach continued to increase semiannual surveillance, compared with usual care (46.8% vs. 32.7%; P < .001).”
Despite the improved rates from the intervention, the majority of patients still did not receive semiannual surveillance across all three sites, and almost 30% underwent no surveillance the entire year.
The research was funded by the National Cancer Institute, the Cancer Prevention Research Institute of Texas, and the Center for Innovations in Quality, Effectiveness and Safety. Dr. Singal has consulted for or served on the advisory boards of Bayer, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, and GRAIL. The other authors had no industry disclosures.
REPORTING FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Should health care be a right?
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is health care a human right?
This year voters in Oregon are being asked to decide that.
It brings up some interesting questions. Should it be a right? Food, water, shelter, and oxygen aren’t, as far as I know, considered such. So why health care?
Probably the main argument against the idea is that, if it’s a right, shouldn’t the government (and therefore taxpayers) be tasked with paying for it all?
Good question, and not one that I can answer. If my neighbor refuses to buy insurance, then has a health crisis he can’t afford, why should I have to pay for his obstinacy and lack of foresight? Isn’t it his problem?
Of course, the truth is that not everyone can afford health care, or insurance. They ain’t cheap. Even if you get coverage through your job, part of your earnings, and part of the company’s profits, are being taken out to pay for it.
This raises the question of whether health care is something that should be rationed only to the working, successfully retired, or wealthy. Heaven knows I have plenty of patients tell me that. Their point is that if you’re not contributing to society, why should society contribute to you?
One even said that our distant ancestors didn’t see an issue with this: If you were unable to hunt, or outrun a cave lion, you probably weren’t helping the rest of the tribe anyway and deserved what happened to you.
Perhaps true, but we aren’t our distant ancestors. Over the millennia we’ve developed into a remarkably social, and increasingly interconnected, species. Somewhat paradoxically we often care more about famines on the other side of the world than we do in our own cities. If you’re going to use the argument of “we didn’t used to do this,” we also didn’t used to have cars, planes, or computers, but I don’t see anyone giving them up.
Another thing to keep in mind is that we are all paying for the uninsured under pretty much any system of health care there is. Whether it’s through taxes, insurance premiums, or both, our own costs go up to pay the bills of those who don’t have coverage. So in that respect the financial aspect of declaring it a right probably doesn’t change the de facto truth of the situation. It just makes it more official-ish.
Maybe the statement has more philosophical or political meaning than it does practical. If it passes it may change a lot of things, or nothing at all, depending how it’s legally interpreted.
Like so many things, we won’t know where it goes unless it happens. And even then it’s uncertain where it will lead.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.