User login
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
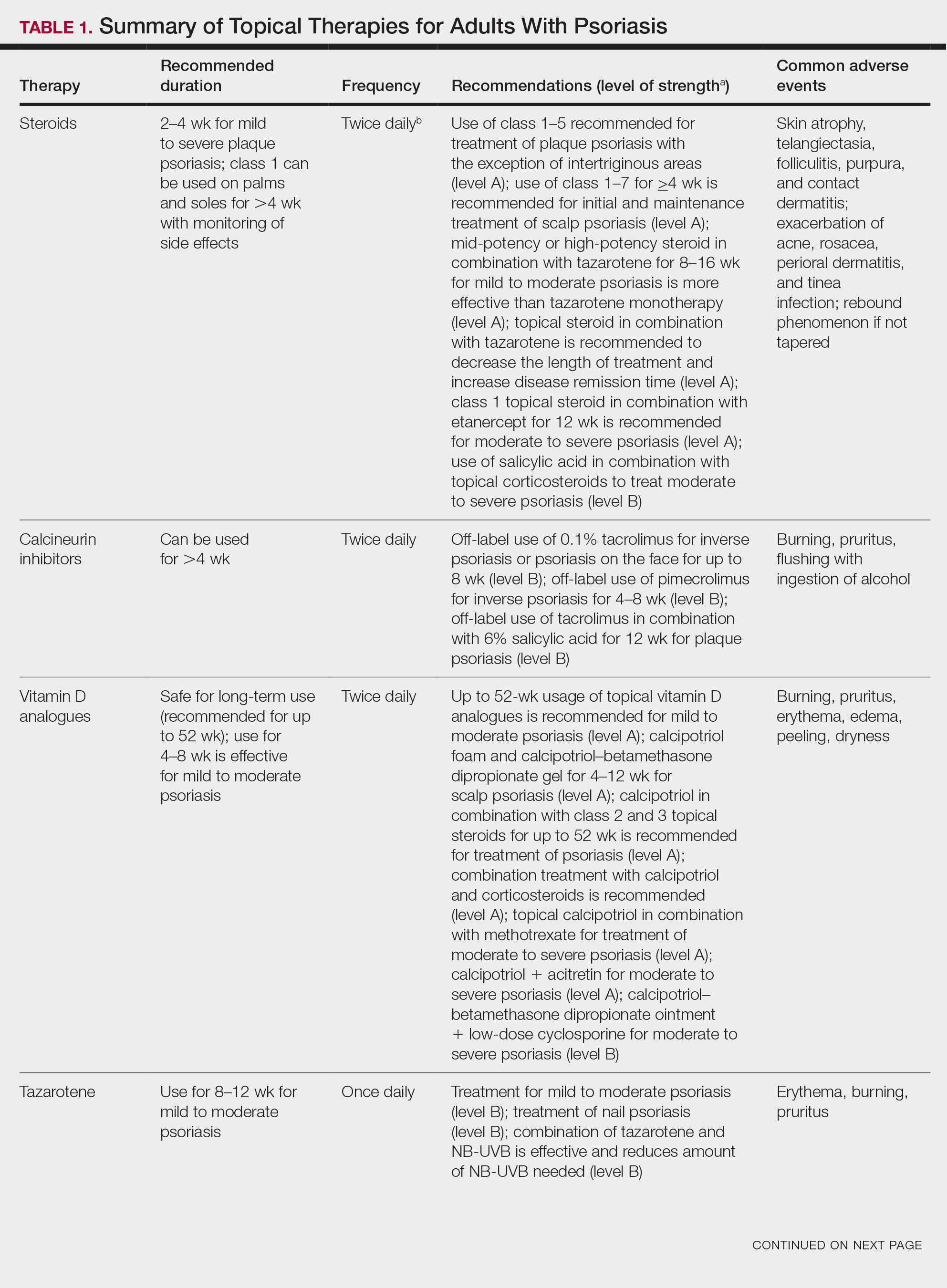
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.
Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
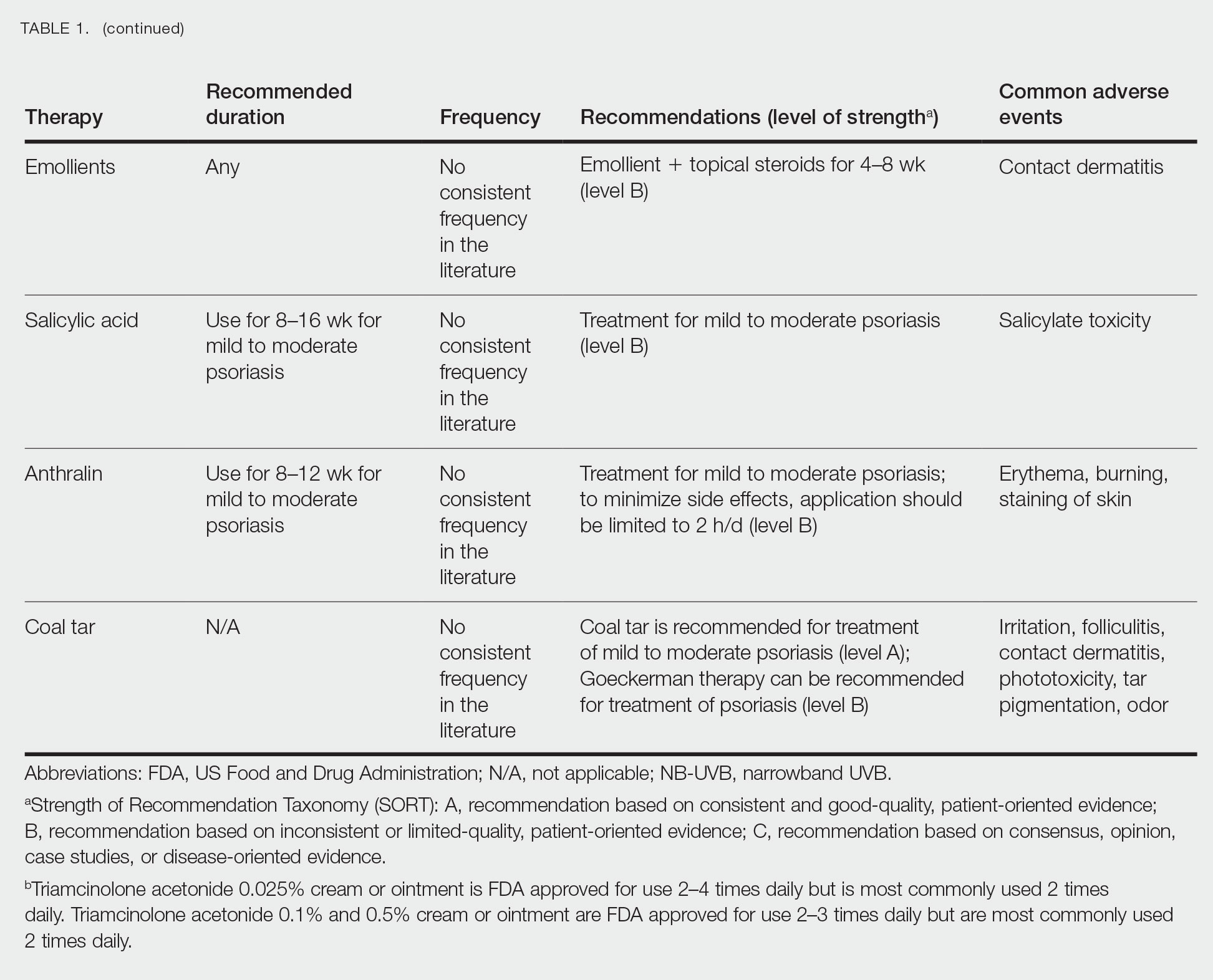
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.
For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.
Guidance From the National Psoriasis Foundation COVID-19 Task Force
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
FDA authorizes intradermal use of Jynneos vaccine for monkeypox
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on Aug. 9 authorized intradermal administration of the Jynneos vaccine for the treatment of monkeypox. The process, approved specifically for high-risk patients, was passed under the administration’s Emergency Use Authorization. It follows the decision on Aug. 4 by the U.S. Department of Health and Human Services to declare monkeypox a public health emergency. Intradermal administration will allow providers to get five doses out of a one-dose vial.
This news organization will update this article as more information becomes available.
A version of this article first appeared on Medscape.com.
Tobramycin tames infection in bronchiectasis
Nebulized tobramycin significantly reduced the density of Pseudomonas aeruginosa in sputum and improved quality of life for adults with bronchiectasis in a study with more than 300 individuals.
Chronic P. aeruginosa infection remains a challenge for bronchiectasis patients, and treatment options are limited, wrote Wei-jie Guan, MD, of the First Affiliated Hospital of Guangzhou Medical University, Guangdong, China, and colleagues. Tobramycin has demonstrated antipseudomonal effects, but previous studies have been small, results have been inconclusive, and there are safety concerns with the currently approved method of intravenous injection.
In a study published in the journal Chest, the researchers randomly assigned 167 patients to receive nebulized tobramycin inhalation solution (TIS) and 172 patients to receive placebo. Patients in the active-treatment group received 300 mg/5 mL of TIS twice daily in two cycles of 28 days on- and off-treatment alternating periods. The primary endpoints were changes in P. aeruginosa density from baseline and scores on the Quality of Life–Bronchiectasis questionnaire at day 29. Follow-up data were collected every 4 weeks for 16 weeks. Secondary endpoints included rate of negative P. aeruginosa culture at day 29; change in P. aeruginosa density from baseline; quality of life at day 85; and 24-hour sputum volume and purulence at day 29, 57, and 85.
The study population included adults aged 18-75 years with symptomatic bronchiectasis. The participants’ conditions had been clinically stable for 4 weeks. Sputum cultures tested positive for P. aeruginosa at two consecutive screening visits prior to randomization. The study was conducted at 33 sites within mainland China.
Overall, with an adjusted mean difference of 1.74 Log10 colony-forming units/g (P < .001). TIS patients also showed significantly greater improvement in Quality of Life–Bronchiectasis respiratory symptom scores, with an adjusted mean difference of 7.91 (P < .001) at day 29.
In addition, more TIS patients became culture negative for P. aeruginosa by day 29, compared with placebo patients (29.3% vs. 10.6%), and 24-hour sputum volume and sputum purulence scores were significantly lower for TIS patients at day 29, day 57, and day 85, compared with placebo patients.
Adverse events were similar and occurred in 81.5% of TIS patients and 81.6% of placebo patients. The most common were hemoptysis, chest discomfort, and acute upper respiratory tract infections. A total of 10 patients in the TIS group experienced transient wheezing that resolved within 30 minutes. A total of 11 TIS patients and 5 placebo patients experienced an adverse event that caused them to discontinue participation in the study. These events included blurred vision and dizziness, which occurred in two TIS patients and was deemed related to the study drug. One TIS patient died as a result of acute myocardial infarction, but this was deemed to be unrelated to the study drug.
The findings were limited by several factors, including the short duration of treatment and relatively young population, which might affect generalizability, the researchers noted. Other limitations include a lack of data on the effects of TIS on microorganisms other than P. aeruginosa, as well as limited outpatient visits, owing to COVID-19 restrictions.
However, the results confirm the ability of TIS nebulization to reduce P. aeruginosa and improve quality of life for adult patients with bronchiectasis, the authors concluded.
The study was funded by grants to multiple researchers from the National Science and Technology Major Project of the Ministry of Science and Technology of China and other government sources. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nebulized tobramycin significantly reduced the density of Pseudomonas aeruginosa in sputum and improved quality of life for adults with bronchiectasis in a study with more than 300 individuals.
Chronic P. aeruginosa infection remains a challenge for bronchiectasis patients, and treatment options are limited, wrote Wei-jie Guan, MD, of the First Affiliated Hospital of Guangzhou Medical University, Guangdong, China, and colleagues. Tobramycin has demonstrated antipseudomonal effects, but previous studies have been small, results have been inconclusive, and there are safety concerns with the currently approved method of intravenous injection.
In a study published in the journal Chest, the researchers randomly assigned 167 patients to receive nebulized tobramycin inhalation solution (TIS) and 172 patients to receive placebo. Patients in the active-treatment group received 300 mg/5 mL of TIS twice daily in two cycles of 28 days on- and off-treatment alternating periods. The primary endpoints were changes in P. aeruginosa density from baseline and scores on the Quality of Life–Bronchiectasis questionnaire at day 29. Follow-up data were collected every 4 weeks for 16 weeks. Secondary endpoints included rate of negative P. aeruginosa culture at day 29; change in P. aeruginosa density from baseline; quality of life at day 85; and 24-hour sputum volume and purulence at day 29, 57, and 85.
The study population included adults aged 18-75 years with symptomatic bronchiectasis. The participants’ conditions had been clinically stable for 4 weeks. Sputum cultures tested positive for P. aeruginosa at two consecutive screening visits prior to randomization. The study was conducted at 33 sites within mainland China.
Overall, with an adjusted mean difference of 1.74 Log10 colony-forming units/g (P < .001). TIS patients also showed significantly greater improvement in Quality of Life–Bronchiectasis respiratory symptom scores, with an adjusted mean difference of 7.91 (P < .001) at day 29.
In addition, more TIS patients became culture negative for P. aeruginosa by day 29, compared with placebo patients (29.3% vs. 10.6%), and 24-hour sputum volume and sputum purulence scores were significantly lower for TIS patients at day 29, day 57, and day 85, compared with placebo patients.
Adverse events were similar and occurred in 81.5% of TIS patients and 81.6% of placebo patients. The most common were hemoptysis, chest discomfort, and acute upper respiratory tract infections. A total of 10 patients in the TIS group experienced transient wheezing that resolved within 30 minutes. A total of 11 TIS patients and 5 placebo patients experienced an adverse event that caused them to discontinue participation in the study. These events included blurred vision and dizziness, which occurred in two TIS patients and was deemed related to the study drug. One TIS patient died as a result of acute myocardial infarction, but this was deemed to be unrelated to the study drug.
The findings were limited by several factors, including the short duration of treatment and relatively young population, which might affect generalizability, the researchers noted. Other limitations include a lack of data on the effects of TIS on microorganisms other than P. aeruginosa, as well as limited outpatient visits, owing to COVID-19 restrictions.
However, the results confirm the ability of TIS nebulization to reduce P. aeruginosa and improve quality of life for adult patients with bronchiectasis, the authors concluded.
The study was funded by grants to multiple researchers from the National Science and Technology Major Project of the Ministry of Science and Technology of China and other government sources. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nebulized tobramycin significantly reduced the density of Pseudomonas aeruginosa in sputum and improved quality of life for adults with bronchiectasis in a study with more than 300 individuals.
Chronic P. aeruginosa infection remains a challenge for bronchiectasis patients, and treatment options are limited, wrote Wei-jie Guan, MD, of the First Affiliated Hospital of Guangzhou Medical University, Guangdong, China, and colleagues. Tobramycin has demonstrated antipseudomonal effects, but previous studies have been small, results have been inconclusive, and there are safety concerns with the currently approved method of intravenous injection.
In a study published in the journal Chest, the researchers randomly assigned 167 patients to receive nebulized tobramycin inhalation solution (TIS) and 172 patients to receive placebo. Patients in the active-treatment group received 300 mg/5 mL of TIS twice daily in two cycles of 28 days on- and off-treatment alternating periods. The primary endpoints were changes in P. aeruginosa density from baseline and scores on the Quality of Life–Bronchiectasis questionnaire at day 29. Follow-up data were collected every 4 weeks for 16 weeks. Secondary endpoints included rate of negative P. aeruginosa culture at day 29; change in P. aeruginosa density from baseline; quality of life at day 85; and 24-hour sputum volume and purulence at day 29, 57, and 85.
The study population included adults aged 18-75 years with symptomatic bronchiectasis. The participants’ conditions had been clinically stable for 4 weeks. Sputum cultures tested positive for P. aeruginosa at two consecutive screening visits prior to randomization. The study was conducted at 33 sites within mainland China.
Overall, with an adjusted mean difference of 1.74 Log10 colony-forming units/g (P < .001). TIS patients also showed significantly greater improvement in Quality of Life–Bronchiectasis respiratory symptom scores, with an adjusted mean difference of 7.91 (P < .001) at day 29.
In addition, more TIS patients became culture negative for P. aeruginosa by day 29, compared with placebo patients (29.3% vs. 10.6%), and 24-hour sputum volume and sputum purulence scores were significantly lower for TIS patients at day 29, day 57, and day 85, compared with placebo patients.
Adverse events were similar and occurred in 81.5% of TIS patients and 81.6% of placebo patients. The most common were hemoptysis, chest discomfort, and acute upper respiratory tract infections. A total of 10 patients in the TIS group experienced transient wheezing that resolved within 30 minutes. A total of 11 TIS patients and 5 placebo patients experienced an adverse event that caused them to discontinue participation in the study. These events included blurred vision and dizziness, which occurred in two TIS patients and was deemed related to the study drug. One TIS patient died as a result of acute myocardial infarction, but this was deemed to be unrelated to the study drug.
The findings were limited by several factors, including the short duration of treatment and relatively young population, which might affect generalizability, the researchers noted. Other limitations include a lack of data on the effects of TIS on microorganisms other than P. aeruginosa, as well as limited outpatient visits, owing to COVID-19 restrictions.
However, the results confirm the ability of TIS nebulization to reduce P. aeruginosa and improve quality of life for adult patients with bronchiectasis, the authors concluded.
The study was funded by grants to multiple researchers from the National Science and Technology Major Project of the Ministry of Science and Technology of China and other government sources. The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CHEST
In one state, pandemic tamped down lice and scabies cases
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
FROM PEDIATRIC DERMATOLOGY
CT scan changes indicate increased mortality risk in ever-smokers
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
Longitudinal progression of parenchymal changes on CT images — also referred to as quantitative interstitial abnormalities (QIA) – is independently associated with decreased lung function and an increased all-cause mortality risk, an analysis of two cohorts of ever-smokers indicates. And among the main risk factors for QIA progression is smoking.
“These abnormalities have gone by a few different names but fundamentally, they are high density findings of chest CT that in some cases represent early or subtle evidence of pulmonary fibrosis,” Samuel Ash, MD, MPH, assistant professor of medicine, Brigham and Women’s Hospital, Boston, told this news organization.
So when I see someone with visual evidence of this type of change on their chest CT, I make sure to emphasize that while they don’t have interstitial lung disease [ILD] yet, these findings suggest they may be susceptible to lung injury from tobacco smoke and that if they don’t stop smoking now, they are at risk for a disease like interstitial pulmonary fibrosis [IPF] which is a highly morbid disease with a high mortality risk,” he added.
The study was published online in the journal CHEST.
Ever-smoking cohorts
Analysis of QIA progression on CT chest scans was carried out on participants from the Genetic Epidemiology of COPD (COPDGene) study as well as those from the Pittsburgh Lung Screening Study (PLuSS). COPDGene was a prospective cohort of over 10,300 ever-smokers with at least a 10–pack-year smoking history between the ages of 45 and 80. Participants underwent a series of tests including chest CT scans at baseline between 2006 and 2011 and again approximately 5 years later.
Patients with a postbronchodilator forced expiratory volume in 1 second (FEV1) of 80% or more of predicted and a FEV1-to-FVC (forced vital capacity) ratio of at least 0.7 were defined to have GOLD stage 0 disease while those with a postbronchodilator FEV1 of 80% or less than predicted and a FEV1-to-FVC ratio of at least 0.7 were defined to have preserved ratio impaired spirometry (PRISm) disease.
PLuSS involved 3,642 ever-smokers between the ages of 50 years and 79 years with at least a 12.5–pack-year history with no prior history of lung cancer. Participants again underwent a series of tests including a CT scan on visit 1 between 2002 and 2005 and then a second CT scan at a second visit almost 9 years later. “In the COPDGene cohort, 4,635 participants had complete clinical data, CT scans and spirometry from visits 1 and 2 for analysis,” the authors reported.
At visit 1 almost 48% of participants were current smokers and the mean pack-year history of the cohort was 41.9 years. The mean time between visits 1 and 2 was 5.6 years. Both the mean prebronchodilator FEV1 as well as the mean FVC decreased between visits 1 and 2. For example, the mean prebronchodilator FEV1 dropped from 2.2 liters to 2.0 liters between visits 1 and 2 while the mean prebronchodilator FVC decreased from 3.2 liters to 3.0 liters between the first and second visits.
In the PLuSS cohort, 1,307 participants had complete imaging and spirometry data available for visits 1 and 2 for analysis. The mean time between visits 1 and 2 was 8.6 years. Over 59% of the cohort were current smokers with a mean pack-year history of 65. Again, the mean prebronchodilator FEV1 and FVC both dropped between visit 1 and 2, as the authors note.
The mean prebronchodilator FEV1, for example, decreased from 2.5 liters to 2.1 liters between visits 1 and 2 while the mean prebronchodilator FVC dropped from 3.6 liters to 3.2 liters during the same interval. Looking at risk factors associated with QIA progression, investigators note that each additional year of baseline age was associated with a higher annual increase in QIA by 0.01% per year (95% confidence interval, 0.01%-0.02%; P < .001) in the COPDGene cohort and a 0.02% increase (95% CI, 0.01%-0.02%; P < .001) in the PLuSS cohort.
Female sex in turn was associated with a 0.07% per year (95% CI, 0.02%-0.12%; P = .003) higher increase in the QIA, compared with men in the COPDGene cohort and a 0.14% (95% CI, 0.02%-0.26%; P = .025) per year higher increase in the QIA in the PLuSS cohort. Current smoking status was only associated with a higher rate of QIA progression in the COPDGene cohort at a rate of 0.10% per year (95% CI, 0.06%-0.15%; P < .001).
Lastly, every copy of the minor allele of the MUIC5B promoter polymorphism was associated with a 0.12% per year (95% CI, 0.07%-0.16%; P < .0001) increase in QIA in the COPDGene cohort as well.
Smoking cessation
Smoking cessation is the obvious first step for patients with evidence of QIA progression but physicians can probably do more for these patients sooner, Dr. Ash said. “If we use heart disease as an analogy, we don’t want to start treating someone until they have a heart attack or are in heart failure, we start by checking their cholesterol and blood pressure and treating them with medications to prevent progression.”
Similarly, physicians need to start thinking about IPF and other lung diseases in the same way. For IPF, medications such as pirfenidone (Esbriet) and nintedanib (Ofev) do not reverse prior lung damage but they do slow disease progression and physicians need to initiate treatment before patients are short of breath, not after. Meantime, Dr. Ash advised physicians that, if they have a patient who is getting a CT scan for whatever reason, they should keep a close eye on whether or not patients have any of these interstitial changes and, if they do, then if the changes are getting worse.
“These patients are likely to be the ones who are going to develop IPF and who may benefit from ongoing imaging surveillance,” he said. And while clinicians may not yet be ready to use a quantitative tool at the bedside, “this tool – or one like it – is coming and we have to start thinking about how to incorporate these types of devices into our clinical practice.”
Temporal changes
Asked to comment on the findings, Surya Bhatt, MD, associate professor of medicine at the University of Alabama at Birmingham, said that the study advances the community’s understanding of the relationship between temporal changes in objectively measured interstitial lung abnormalities and several important clinical outcomes, including lung function decline and mortality. “Several risk factors for progression were also identified,” he noted.
“And these results make a case for initiating clinical trials to determine whether early treatment with existing antifibrotic medications in these high risk individuals can decrease the perpetuation of these permanent lung changes,” Dr. Bhatt said.
The COPDGene study was supported in part by contributions made by an industry advisory board. Dr. Ash was supported in part by Quantitative Imaging Solutions. Dr. Bhatt declared that he has receiving consulting fees or has service on advisory boards for Boehringer Ingelheim and Sanofi/Regeneron. He ha also received fee for CME from IntegrityCE.
FROM CHEST
Climate change can worsen more than half of infectious diseases
An extensive new study shows that climate change can aggravate over half of known human pathogenic diseases. This comprehensive systematic review of the literature narrowed down 3,213 cases, linking 286 infectious diseases to specific climate change hazards. Of these, 58% were worsened, and only 9 conditions showed any benefit associated with environmental change.
The study was published online in Nature Climate Change. The complete list of cases, transmission pathways, and associated papers can be explored in detail – a remarkable, interactive data visualization.
To compile the data, investigators searched 10 keywords on the Global Infectious Disease and Epidemiology Network (GIDEON) and Center for Disease Control and Prevention databases. They then filled gaps by examining alternative names of the diseases, pathogens, and hazards.
Coauthor Tristan McKenzie, PhD, a postdoctoral researcher at the University of Gothenburg, Sweden, told this news organization: “If someone is interested in a certain pathway, it’s a beautiful starting point.” Or if someone wants to “do a modeling study and they want to focus on a specific area, the specific examples in the literature are already there” in the extensive database.
An early key finding is that warming and increased precipitation broadened the range of many pathogens through expansion of their habitat. This shift brings many pathogens closer to people. Examples are viruses (dengue, Chikungunya), bacteria (Lyme), protozoans (trypanosomes), and more. Warming has affected aquatic systems (for example, Vibrio) and higher altitudes and latitudes (malaria, dengue).
Pathogenic hazards are not just moving closer to people. People are also moving closer to the pathogenic hazards, with heat waves causing people to seek refuge with water activities, for example. This increases their exposure to pathogens, such as Vibrio, hepatitis, and water-borne gastroenteritis.
Some hazards, such as warming, can even make pathogens more virulent. Heat can upregulate Vibrio’s gene expression of proteins affecting transmission, adhesion, penetration, and host injury.
Heat and rainfall can increase stagnant water, enhancing mosquitoes’ breeding and growing grounds and enabling them to transmit many more infections.
People’s capacity to respond to climate hazards can also be impaired. For example, there is a reduced concentration of nutrients in crops under high CO2 levels, which can result in malnutrition. Lower crop yields can further fuel outbreaks of measles, cholera, or Cryptosporidium. Drought also likely forces people to drink contaminated water.
Among all this bad news, the authors found a small number of cases where climate hazards reduced the risk of infection. For example, droughts reduced the breeding grounds of mosquitoes, reducing the prevalence of malaria and chikungunya. But in other cases, the density of mosquitoes increased in some pools, causing an increased local risk of infection.
Naomi Hauser, MD, MPH, assistant clinical professor at UC Davis, Sacramento, told this news organization she was particularly impressed with the data visualization. “It really emphasizes the magnitude of what we’re dealing with. It makes you feel the weight of what they’re trying to represent,” she said.
On the other hand, Dr. Hauser said she would have liked “more emphasis on how the climate hazards interact with each other. It sort of made it sound like each of these climate hazards is in a vacuum – like when there’s floods, and that’s the problem. But there are a lot of other things ... like when we have warming and surface water temperature changes, it can also change the pH of the water and the salinity of the water, and those can also impact what we see with pathogens in the water.”
Dr. McKenzie explained one limitation: The study looked only at 10 keywords. So an example of a dust storm in Africa causing an increase in Vibrio in the United States could not be identified by this approach. “This also goes back to the scale of the problem, because we have something going on in the Sahara that’s impacting the East Coast of the United States,” he said. “And finding that link is not necessarily obvious – or at least not as obvious as [if] there [were] a hurricane and a bunch of people got sick from waterborne disease. So I think that really highlights the scale of this problem.”
Instead of looking at only one individual or group of pathogens, the study provided a much broader review of infections caused by an array of climate hazards. As Dr. McKenzie said, “no one’s actually done the work previously to really just try and get a comprehensive picture of what we might be dealing with. And so that was the goal for us.” The 58% estimate of diseases worsened by climate change is conservative, and, he says, “arguably, this is an even bigger problem than what we present.”
Dr. McKenzie concluded: “If we’re looking at the spread of some more serious or rare diseases in areas, to me then the answer is ... we need to be aggressively mitigating greenhouse gas emissions. Let’s start with the source.”
Dr. McKenzie and Dr. Hauser report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An extensive new study shows that climate change can aggravate over half of known human pathogenic diseases. This comprehensive systematic review of the literature narrowed down 3,213 cases, linking 286 infectious diseases to specific climate change hazards. Of these, 58% were worsened, and only 9 conditions showed any benefit associated with environmental change.
The study was published online in Nature Climate Change. The complete list of cases, transmission pathways, and associated papers can be explored in detail – a remarkable, interactive data visualization.
To compile the data, investigators searched 10 keywords on the Global Infectious Disease and Epidemiology Network (GIDEON) and Center for Disease Control and Prevention databases. They then filled gaps by examining alternative names of the diseases, pathogens, and hazards.
Coauthor Tristan McKenzie, PhD, a postdoctoral researcher at the University of Gothenburg, Sweden, told this news organization: “If someone is interested in a certain pathway, it’s a beautiful starting point.” Or if someone wants to “do a modeling study and they want to focus on a specific area, the specific examples in the literature are already there” in the extensive database.
An early key finding is that warming and increased precipitation broadened the range of many pathogens through expansion of their habitat. This shift brings many pathogens closer to people. Examples are viruses (dengue, Chikungunya), bacteria (Lyme), protozoans (trypanosomes), and more. Warming has affected aquatic systems (for example, Vibrio) and higher altitudes and latitudes (malaria, dengue).
Pathogenic hazards are not just moving closer to people. People are also moving closer to the pathogenic hazards, with heat waves causing people to seek refuge with water activities, for example. This increases their exposure to pathogens, such as Vibrio, hepatitis, and water-borne gastroenteritis.
Some hazards, such as warming, can even make pathogens more virulent. Heat can upregulate Vibrio’s gene expression of proteins affecting transmission, adhesion, penetration, and host injury.
Heat and rainfall can increase stagnant water, enhancing mosquitoes’ breeding and growing grounds and enabling them to transmit many more infections.
People’s capacity to respond to climate hazards can also be impaired. For example, there is a reduced concentration of nutrients in crops under high CO2 levels, which can result in malnutrition. Lower crop yields can further fuel outbreaks of measles, cholera, or Cryptosporidium. Drought also likely forces people to drink contaminated water.
Among all this bad news, the authors found a small number of cases where climate hazards reduced the risk of infection. For example, droughts reduced the breeding grounds of mosquitoes, reducing the prevalence of malaria and chikungunya. But in other cases, the density of mosquitoes increased in some pools, causing an increased local risk of infection.
Naomi Hauser, MD, MPH, assistant clinical professor at UC Davis, Sacramento, told this news organization she was particularly impressed with the data visualization. “It really emphasizes the magnitude of what we’re dealing with. It makes you feel the weight of what they’re trying to represent,” she said.
On the other hand, Dr. Hauser said she would have liked “more emphasis on how the climate hazards interact with each other. It sort of made it sound like each of these climate hazards is in a vacuum – like when there’s floods, and that’s the problem. But there are a lot of other things ... like when we have warming and surface water temperature changes, it can also change the pH of the water and the salinity of the water, and those can also impact what we see with pathogens in the water.”
Dr. McKenzie explained one limitation: The study looked only at 10 keywords. So an example of a dust storm in Africa causing an increase in Vibrio in the United States could not be identified by this approach. “This also goes back to the scale of the problem, because we have something going on in the Sahara that’s impacting the East Coast of the United States,” he said. “And finding that link is not necessarily obvious – or at least not as obvious as [if] there [were] a hurricane and a bunch of people got sick from waterborne disease. So I think that really highlights the scale of this problem.”
Instead of looking at only one individual or group of pathogens, the study provided a much broader review of infections caused by an array of climate hazards. As Dr. McKenzie said, “no one’s actually done the work previously to really just try and get a comprehensive picture of what we might be dealing with. And so that was the goal for us.” The 58% estimate of diseases worsened by climate change is conservative, and, he says, “arguably, this is an even bigger problem than what we present.”
Dr. McKenzie concluded: “If we’re looking at the spread of some more serious or rare diseases in areas, to me then the answer is ... we need to be aggressively mitigating greenhouse gas emissions. Let’s start with the source.”
Dr. McKenzie and Dr. Hauser report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An extensive new study shows that climate change can aggravate over half of known human pathogenic diseases. This comprehensive systematic review of the literature narrowed down 3,213 cases, linking 286 infectious diseases to specific climate change hazards. Of these, 58% were worsened, and only 9 conditions showed any benefit associated with environmental change.
The study was published online in Nature Climate Change. The complete list of cases, transmission pathways, and associated papers can be explored in detail – a remarkable, interactive data visualization.
To compile the data, investigators searched 10 keywords on the Global Infectious Disease and Epidemiology Network (GIDEON) and Center for Disease Control and Prevention databases. They then filled gaps by examining alternative names of the diseases, pathogens, and hazards.
Coauthor Tristan McKenzie, PhD, a postdoctoral researcher at the University of Gothenburg, Sweden, told this news organization: “If someone is interested in a certain pathway, it’s a beautiful starting point.” Or if someone wants to “do a modeling study and they want to focus on a specific area, the specific examples in the literature are already there” in the extensive database.
An early key finding is that warming and increased precipitation broadened the range of many pathogens through expansion of their habitat. This shift brings many pathogens closer to people. Examples are viruses (dengue, Chikungunya), bacteria (Lyme), protozoans (trypanosomes), and more. Warming has affected aquatic systems (for example, Vibrio) and higher altitudes and latitudes (malaria, dengue).
Pathogenic hazards are not just moving closer to people. People are also moving closer to the pathogenic hazards, with heat waves causing people to seek refuge with water activities, for example. This increases their exposure to pathogens, such as Vibrio, hepatitis, and water-borne gastroenteritis.
Some hazards, such as warming, can even make pathogens more virulent. Heat can upregulate Vibrio’s gene expression of proteins affecting transmission, adhesion, penetration, and host injury.
Heat and rainfall can increase stagnant water, enhancing mosquitoes’ breeding and growing grounds and enabling them to transmit many more infections.
People’s capacity to respond to climate hazards can also be impaired. For example, there is a reduced concentration of nutrients in crops under high CO2 levels, which can result in malnutrition. Lower crop yields can further fuel outbreaks of measles, cholera, or Cryptosporidium. Drought also likely forces people to drink contaminated water.
Among all this bad news, the authors found a small number of cases where climate hazards reduced the risk of infection. For example, droughts reduced the breeding grounds of mosquitoes, reducing the prevalence of malaria and chikungunya. But in other cases, the density of mosquitoes increased in some pools, causing an increased local risk of infection.
Naomi Hauser, MD, MPH, assistant clinical professor at UC Davis, Sacramento, told this news organization she was particularly impressed with the data visualization. “It really emphasizes the magnitude of what we’re dealing with. It makes you feel the weight of what they’re trying to represent,” she said.
On the other hand, Dr. Hauser said she would have liked “more emphasis on how the climate hazards interact with each other. It sort of made it sound like each of these climate hazards is in a vacuum – like when there’s floods, and that’s the problem. But there are a lot of other things ... like when we have warming and surface water temperature changes, it can also change the pH of the water and the salinity of the water, and those can also impact what we see with pathogens in the water.”
Dr. McKenzie explained one limitation: The study looked only at 10 keywords. So an example of a dust storm in Africa causing an increase in Vibrio in the United States could not be identified by this approach. “This also goes back to the scale of the problem, because we have something going on in the Sahara that’s impacting the East Coast of the United States,” he said. “And finding that link is not necessarily obvious – or at least not as obvious as [if] there [were] a hurricane and a bunch of people got sick from waterborne disease. So I think that really highlights the scale of this problem.”
Instead of looking at only one individual or group of pathogens, the study provided a much broader review of infections caused by an array of climate hazards. As Dr. McKenzie said, “no one’s actually done the work previously to really just try and get a comprehensive picture of what we might be dealing with. And so that was the goal for us.” The 58% estimate of diseases worsened by climate change is conservative, and, he says, “arguably, this is an even bigger problem than what we present.”
Dr. McKenzie concluded: “If we’re looking at the spread of some more serious or rare diseases in areas, to me then the answer is ... we need to be aggressively mitigating greenhouse gas emissions. Let’s start with the source.”
Dr. McKenzie and Dr. Hauser report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Severe illness rising as vaccination effort stalls
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
ORATOR2 mute on best de-escalation therapy for low-risk HPV+ oropharynx cancers
The question of whether primary transoral surgery or radiation is a better treatment deescalation option for patients with low-risk human papillomavirus (HPV)-related oropharyngeal squamous cell carcinomas (OPSCCs) is still unanswered, despite the best efforts of investigators in the randomized phase 2 ORATOR2 trial.
, the study found.
The trial, begun in early 2018, was halted in late 2020 because of safety concerns after 2 of 31 patients randomized to surgery died from treatment-related causes.
But the story doesn’t end there, investigators and observers say.
In both trial arms, patients had good swallowing outcomes and other favorable quality-of-life measures at 1 year, and it’s too early to tell whether the transoral surgery (TOS) was associated with an unacceptable risk of grade 5 toxic effects, but patients in both trial arms achieved good swallowing outcomes at 1 year, wrote investigators Daniel A. Palma, MD, from Western University in London, Ontario, and colleagues in JAMA Oncology.
Nonetheless, “the results of this randomized clinical trial suggest that a primary [surgery] approach was associated with an up-front risk of treatment-related mortality, and caution is warranted with this approach,” the investigators wrote.
Hard to interpret
“It’s challenging to do that study in Canada, frankly, and it’s hard to make much of it, with the trial being terminated early,” said Neil D. Gross, MD, a head and neck cancer surgeon-scientist with MD Anderson Cancer Center, Houston, in an interview.
Dr. Gross and Sewit Teckie, MD, system chief of radiation oncology at NYC Health and Hospitals, cowrote an editorial accompanying the ORATOR2 results that was published in JAMA Oncology.
“There’s a huge difference in the volume of transoral robotic surgery performed in the United States, compared with Canada – it’s night and day, and that’s due to the different kind of healthcare systems that we have,” Dr. Gross said.
As he and Dr. Teckie noted, the combined mortality rate for surgical patients in the multicenter ORATOR2 and the earlier ORATOR trial, which was not limited to patients with HPV-related cancers, was 3.6%.
In contrast, in the ECOG-ACRIN 3311 trial comparing standard radiation with reduced dose radiation following TOS in patients with intermediate-risk HPV-positive oropharynx cancer, there was only one death among 495 patients, for a mortality rate of 0.2%.
“In the United States, mortality after transoral robotic surgery compares favorably with nonsurgical treatment and is lowest at high-volume centers. ORATOR2 also mandated prophylactic tracheostomy, a practice rarely used in contemporary transoral surgery for low-risk HPV-related OPSCC,” the authors wrote.
In defense of surgery
In an interview posted on the JAMA Network website, ORATOR2 co-investigator Anthony C. Nichols, MD, from the department of otolaryngology, head and neck surgery at Western University, London, Ontario, said that despite the findings of ORATOR2, transoral surgery is a good option for patients with low-risk disease and favorable anatomy.
“When you even look at the subset of these early T-stage patients that have anatomy that’s favorable towards transoral surgery, they do better. Their burden of disease is smaller, there’s less extensive neck disease ... so what happens very frequently, including even in the discussion of ECOG-ACRIN 3311, is comparisons to these large cooperative group studies that include T3, T4 tumors that no one on the planet would think about removing transorally,” he said.
“Everyone focuses on the surgical stopping, but what we should also focus on is how outstanding the patients did in the RT arm in both these studies,” he added.
Dr. Nichols also noted that quality-of-life metrics for patients randomized to surgery are comparable with those of patients randomized to radiation and that swallowing outcomes with surgery may be superior.
“In our minds, I think the issue is resolved, and we’re just moving on to the next concept, and the debate will rage on,” he said.
ORATOR2 study methodology
The primary endpoint of the trial was OS, compared with historical controls, with secondary endpoints of PFS, quality of life, and toxicity.
A total of 30 patients were randomized to receive RT, and 31 to receive TOS and neck dissection, with adjuvant reduced-dose RT depending on pathologic findings.
At a median follow-up of 17 months, there were 3 deaths in the surgery arm, including the 2 previously mentioned patients who died from treatment-related causes at 0.7 and 4.3 months after randomization, and 1 patient who died from myocardial infarction at 8.5 months. As noted before, OS and PFS data were not mature at the time of study termination.
Quality of life and functional outcomes were generally similar between the trial arms, except for worse scores among patients randomized to TOS and neck dissection in subdomains of coughing and weight loss on the European Organisation for Research and Treatment of Cancer H&N35 scale.
The trial was supported by an Ontario Institute for Cancer Research clinician-scientist operating grant and Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers Fund. Dr. Nichols reported grants from Novartis Canada outside the submitted work. Dr. Gross reported grants and personal fees from Regeneron, personal fees from Sanofi-Genzyme, Intuitive Surgical, and DragonFly Therapeutics, as well as advisory board service for PDS Biotechnology, Shattuck Labs, and Sanofi-Genzyme outside the submitted work.
The question of whether primary transoral surgery or radiation is a better treatment deescalation option for patients with low-risk human papillomavirus (HPV)-related oropharyngeal squamous cell carcinomas (OPSCCs) is still unanswered, despite the best efforts of investigators in the randomized phase 2 ORATOR2 trial.
, the study found.
The trial, begun in early 2018, was halted in late 2020 because of safety concerns after 2 of 31 patients randomized to surgery died from treatment-related causes.
But the story doesn’t end there, investigators and observers say.
In both trial arms, patients had good swallowing outcomes and other favorable quality-of-life measures at 1 year, and it’s too early to tell whether the transoral surgery (TOS) was associated with an unacceptable risk of grade 5 toxic effects, but patients in both trial arms achieved good swallowing outcomes at 1 year, wrote investigators Daniel A. Palma, MD, from Western University in London, Ontario, and colleagues in JAMA Oncology.
Nonetheless, “the results of this randomized clinical trial suggest that a primary [surgery] approach was associated with an up-front risk of treatment-related mortality, and caution is warranted with this approach,” the investigators wrote.
Hard to interpret
“It’s challenging to do that study in Canada, frankly, and it’s hard to make much of it, with the trial being terminated early,” said Neil D. Gross, MD, a head and neck cancer surgeon-scientist with MD Anderson Cancer Center, Houston, in an interview.
Dr. Gross and Sewit Teckie, MD, system chief of radiation oncology at NYC Health and Hospitals, cowrote an editorial accompanying the ORATOR2 results that was published in JAMA Oncology.
“There’s a huge difference in the volume of transoral robotic surgery performed in the United States, compared with Canada – it’s night and day, and that’s due to the different kind of healthcare systems that we have,” Dr. Gross said.
As he and Dr. Teckie noted, the combined mortality rate for surgical patients in the multicenter ORATOR2 and the earlier ORATOR trial, which was not limited to patients with HPV-related cancers, was 3.6%.
In contrast, in the ECOG-ACRIN 3311 trial comparing standard radiation with reduced dose radiation following TOS in patients with intermediate-risk HPV-positive oropharynx cancer, there was only one death among 495 patients, for a mortality rate of 0.2%.
“In the United States, mortality after transoral robotic surgery compares favorably with nonsurgical treatment and is lowest at high-volume centers. ORATOR2 also mandated prophylactic tracheostomy, a practice rarely used in contemporary transoral surgery for low-risk HPV-related OPSCC,” the authors wrote.
In defense of surgery
In an interview posted on the JAMA Network website, ORATOR2 co-investigator Anthony C. Nichols, MD, from the department of otolaryngology, head and neck surgery at Western University, London, Ontario, said that despite the findings of ORATOR2, transoral surgery is a good option for patients with low-risk disease and favorable anatomy.
“When you even look at the subset of these early T-stage patients that have anatomy that’s favorable towards transoral surgery, they do better. Their burden of disease is smaller, there’s less extensive neck disease ... so what happens very frequently, including even in the discussion of ECOG-ACRIN 3311, is comparisons to these large cooperative group studies that include T3, T4 tumors that no one on the planet would think about removing transorally,” he said.
“Everyone focuses on the surgical stopping, but what we should also focus on is how outstanding the patients did in the RT arm in both these studies,” he added.
Dr. Nichols also noted that quality-of-life metrics for patients randomized to surgery are comparable with those of patients randomized to radiation and that swallowing outcomes with surgery may be superior.
“In our minds, I think the issue is resolved, and we’re just moving on to the next concept, and the debate will rage on,” he said.
ORATOR2 study methodology
The primary endpoint of the trial was OS, compared with historical controls, with secondary endpoints of PFS, quality of life, and toxicity.
A total of 30 patients were randomized to receive RT, and 31 to receive TOS and neck dissection, with adjuvant reduced-dose RT depending on pathologic findings.
At a median follow-up of 17 months, there were 3 deaths in the surgery arm, including the 2 previously mentioned patients who died from treatment-related causes at 0.7 and 4.3 months after randomization, and 1 patient who died from myocardial infarction at 8.5 months. As noted before, OS and PFS data were not mature at the time of study termination.
Quality of life and functional outcomes were generally similar between the trial arms, except for worse scores among patients randomized to TOS and neck dissection in subdomains of coughing and weight loss on the European Organisation for Research and Treatment of Cancer H&N35 scale.
The trial was supported by an Ontario Institute for Cancer Research clinician-scientist operating grant and Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers Fund. Dr. Nichols reported grants from Novartis Canada outside the submitted work. Dr. Gross reported grants and personal fees from Regeneron, personal fees from Sanofi-Genzyme, Intuitive Surgical, and DragonFly Therapeutics, as well as advisory board service for PDS Biotechnology, Shattuck Labs, and Sanofi-Genzyme outside the submitted work.
The question of whether primary transoral surgery or radiation is a better treatment deescalation option for patients with low-risk human papillomavirus (HPV)-related oropharyngeal squamous cell carcinomas (OPSCCs) is still unanswered, despite the best efforts of investigators in the randomized phase 2 ORATOR2 trial.
, the study found.
The trial, begun in early 2018, was halted in late 2020 because of safety concerns after 2 of 31 patients randomized to surgery died from treatment-related causes.
But the story doesn’t end there, investigators and observers say.
In both trial arms, patients had good swallowing outcomes and other favorable quality-of-life measures at 1 year, and it’s too early to tell whether the transoral surgery (TOS) was associated with an unacceptable risk of grade 5 toxic effects, but patients in both trial arms achieved good swallowing outcomes at 1 year, wrote investigators Daniel A. Palma, MD, from Western University in London, Ontario, and colleagues in JAMA Oncology.
Nonetheless, “the results of this randomized clinical trial suggest that a primary [surgery] approach was associated with an up-front risk of treatment-related mortality, and caution is warranted with this approach,” the investigators wrote.
Hard to interpret
“It’s challenging to do that study in Canada, frankly, and it’s hard to make much of it, with the trial being terminated early,” said Neil D. Gross, MD, a head and neck cancer surgeon-scientist with MD Anderson Cancer Center, Houston, in an interview.
Dr. Gross and Sewit Teckie, MD, system chief of radiation oncology at NYC Health and Hospitals, cowrote an editorial accompanying the ORATOR2 results that was published in JAMA Oncology.
“There’s a huge difference in the volume of transoral robotic surgery performed in the United States, compared with Canada – it’s night and day, and that’s due to the different kind of healthcare systems that we have,” Dr. Gross said.
As he and Dr. Teckie noted, the combined mortality rate for surgical patients in the multicenter ORATOR2 and the earlier ORATOR trial, which was not limited to patients with HPV-related cancers, was 3.6%.
In contrast, in the ECOG-ACRIN 3311 trial comparing standard radiation with reduced dose radiation following TOS in patients with intermediate-risk HPV-positive oropharynx cancer, there was only one death among 495 patients, for a mortality rate of 0.2%.
“In the United States, mortality after transoral robotic surgery compares favorably with nonsurgical treatment and is lowest at high-volume centers. ORATOR2 also mandated prophylactic tracheostomy, a practice rarely used in contemporary transoral surgery for low-risk HPV-related OPSCC,” the authors wrote.
In defense of surgery
In an interview posted on the JAMA Network website, ORATOR2 co-investigator Anthony C. Nichols, MD, from the department of otolaryngology, head and neck surgery at Western University, London, Ontario, said that despite the findings of ORATOR2, transoral surgery is a good option for patients with low-risk disease and favorable anatomy.
“When you even look at the subset of these early T-stage patients that have anatomy that’s favorable towards transoral surgery, they do better. Their burden of disease is smaller, there’s less extensive neck disease ... so what happens very frequently, including even in the discussion of ECOG-ACRIN 3311, is comparisons to these large cooperative group studies that include T3, T4 tumors that no one on the planet would think about removing transorally,” he said.
“Everyone focuses on the surgical stopping, but what we should also focus on is how outstanding the patients did in the RT arm in both these studies,” he added.
Dr. Nichols also noted that quality-of-life metrics for patients randomized to surgery are comparable with those of patients randomized to radiation and that swallowing outcomes with surgery may be superior.
“In our minds, I think the issue is resolved, and we’re just moving on to the next concept, and the debate will rage on,” he said.
ORATOR2 study methodology
The primary endpoint of the trial was OS, compared with historical controls, with secondary endpoints of PFS, quality of life, and toxicity.
A total of 30 patients were randomized to receive RT, and 31 to receive TOS and neck dissection, with adjuvant reduced-dose RT depending on pathologic findings.
At a median follow-up of 17 months, there were 3 deaths in the surgery arm, including the 2 previously mentioned patients who died from treatment-related causes at 0.7 and 4.3 months after randomization, and 1 patient who died from myocardial infarction at 8.5 months. As noted before, OS and PFS data were not mature at the time of study termination.
Quality of life and functional outcomes were generally similar between the trial arms, except for worse scores among patients randomized to TOS and neck dissection in subdomains of coughing and weight loss on the European Organisation for Research and Treatment of Cancer H&N35 scale.
The trial was supported by an Ontario Institute for Cancer Research clinician-scientist operating grant and Wolfe Surgical Research Professorship in the Biology of Head and Neck Cancers Fund. Dr. Nichols reported grants from Novartis Canada outside the submitted work. Dr. Gross reported grants and personal fees from Regeneron, personal fees from Sanofi-Genzyme, Intuitive Surgical, and DragonFly Therapeutics, as well as advisory board service for PDS Biotechnology, Shattuck Labs, and Sanofi-Genzyme outside the submitted work.
FROM JAMA ONCOLOGY
Drug-resistant epilepsy needs earlier surgical referral
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
FROM EPILEPSIA