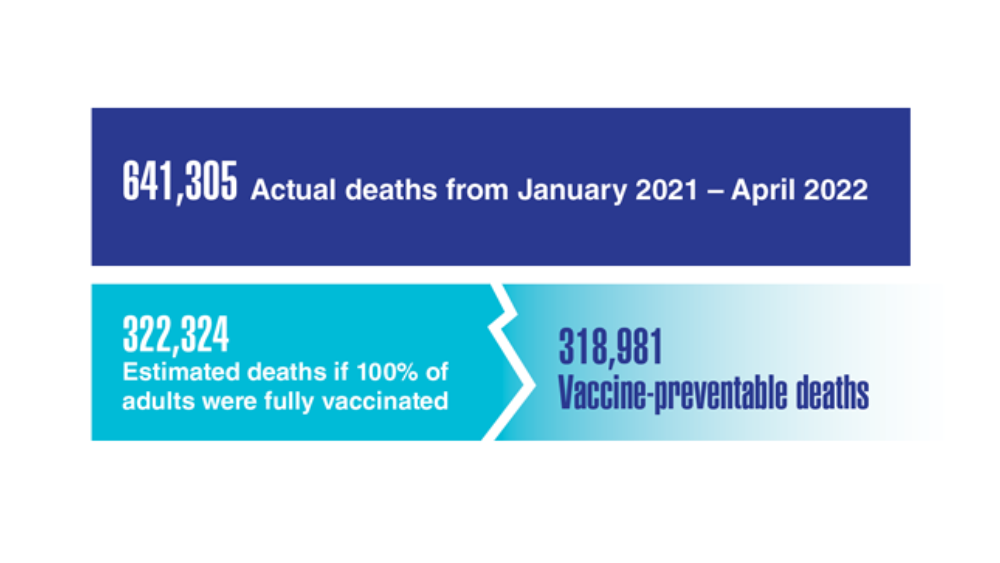
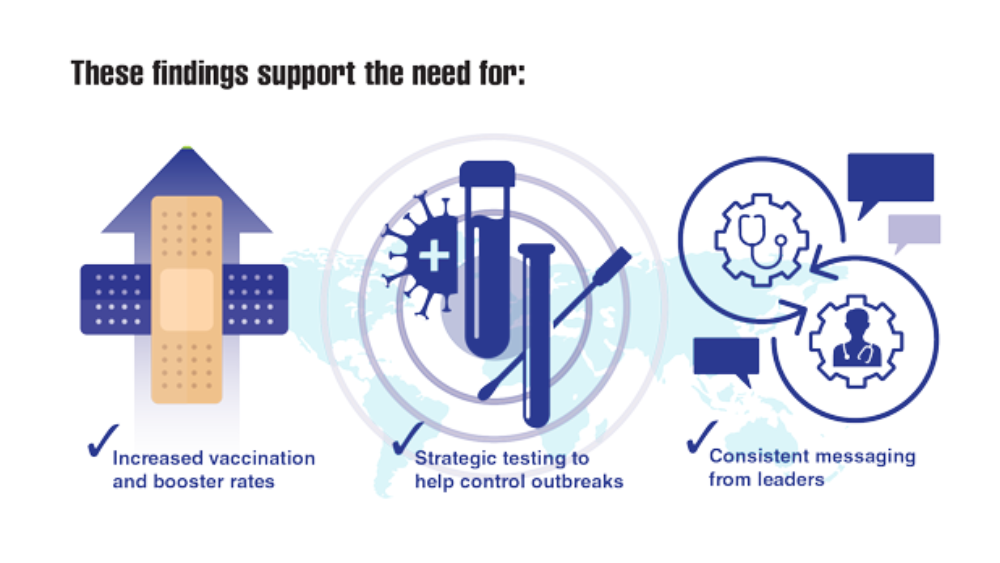
User login
Pro-life ob.gyns. say Dobbs not end of abortion struggle
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
After 49 years of labor, abortion foes received the ultimate victory in June when the United States Supreme Court struck down a federal right to terminate pregnancy. Among those most heartened by the ruling was a small organization of doctors who specialize in women’s reproductive health. The group’s leader, while grateful for the win, isn’t ready for a curtain call. Instead, she sees her task as moving from a national stage to 50 regional ones.
The decision in Dobbs v. Jackson, which overturned a woman’s constitutional right to obtain an abortion, was the biggest but not final quarry for the American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG). “It actually doesn’t change anything except to turn the whole discussion on abortion back to the states, which in our opinion is where it should have been 50 years ago,” Donna Harrison, MD, the group’s chief executive officer, said in a recent interview.
Dr. Harrison, an obstetrician-gynecologist and adjunct professor of bioethics at Trinity International University in Deerfield, Ind., said she was proud of “our small role in bringing science” to the top court’s attention, noting that the ruling incorporated some of AAPLOG’s medical arguments in reversing Roe v. Wade, the 1973 decision that created a right to abortion – and prompted her group’s founding. The ruling, for instance, agreed – in a departure from the generally accepted science – that a fetus is viable at 15 weeks, and the procedure is risky for mothers thereafter. “You could congratulate us for perseverance and for bringing that information, which has been in the peer-reviewed literature for a long time, to the justices’ attention,” she said.
Dr. Harrison said she was pleased that the Supreme Court agreed with the “science” that guided its decision to overturn Roe. That the court was willing to embrace that evidence troubles the American College of Obstetricians and Gynecologists (ACOG), the nation’s leading professional group for reproductive health experts.
Defending the ‘second patient’
AAPLOG operates under the belief that life begins at the moment of fertilization, at which point “we defend the life of our second patient, the human being in the womb,” Dr. Harrison said. “For a very long time, ob.gyns. who valued both patients were not given a voice, and I think now we’re finding our voice.” The group will continue supporting abortion restrictions at the state level.
AAPLOG, with 6,000 members, was considered a “special interest” group within ACOG until the college discontinued such subgroups in 2013. ACOG, numbering 60,000 members, calls the Dobbs ruling “a huge step back for women and everyone who is seeking access to ob.gyn. care,” said Molly Meegan, JD, ACOG’s chief legal officer. Ms. Meegan expressed concern over the newfound influence of AAPLOG, which she called “a single-issue, single-topic, single-advocacy organization.”
Pro-choice groups, including ACOG, worry that the reversal of Roe has provided AAPLOG with an undeserved veneer of medical expertise. The decision also allowed judges and legislators to “insert themselves into nuanced and complex situations” they know little about and will rely on groups like AAPLOG to exert influence, Ms. Meegan said.
In turn, Dr. Harrison described ACOG as engaging in “rabid, pro-abortion activism.”
The number of abortions in the United States had steadily declined from a peak of 1.4 million per year in 1990 until 2017, after which it has risen slightly. In 2019, according to the U.S. Centers for Disease Control and Prevention, 625,000 abortions occurred nationally. Of those, 42.3% were medication abortions performed in the first 9 weeks, using a combination of the drugs mifepristone and misoprostol. Medication abortions now account for more than half of all pregnancy terminations in the United States, according to the Guttmacher Institute.
Dr. Harrison said that medication abortions put women at an elevated risk of serious, sometimes deadly bleeding, while ACOG points to evidence that the risk of childbirth to women is significantly higher. She also is no fan of Plan B, the “morning after” pill, which is available to women without having to consult a doctor. She described abortifacients as “a huge danger to women being harmed” by medications available over the counter.
In Dr. Harrison’s view, the 10-year-old Ohio girl who traveled to Indiana to obtain an abortion after she became pregnant as the result of rape should have continued her pregnancy. So, too, should young girls who are the victims of incest. “Incest is a horrific crime,” she said, “but aborting a girl because of incest doesn’t make her un-raped. It just adds another trauma.”
When told of Dr. Harrison’s comment, Ms. Meegan paused for 5 seconds before saying, “I think that statement speaks for itself.”
Louise Perkins King, MD, JD, an ob.gyn. and director of reproductive bioethics at Harvard Medical School, Boston, said she had the “horrific” experience of delivering a baby to an 11-year-old girl.
“Children are not fully developed, and they should not be having children,” Dr. King said.
Anne-Marie E. Amies Oelschlager, MD, vice chair of ACOG’s Clinical Consensus Committee and an ob.gyn. at Seattle Children’s in Washington, said in a statement that adolescents who are sexually assaulted are at extremely high risk of depression and posttraumatic stress disorder. “Do we expect a fourth-grader to carry a pregnancy to term, deliver, and expect that child to carry on after this horror?,” she asked.
Dr. Harrison dismissed such concerns. “Somehow abortion is a mental health treatment? Abortion doesn’t treat mental health problems,” she said. “Is there any proof that aborting in those circumstances improves their mental health? I would tell you there is very little research about it. …There are human beings involved, and this child who was raped, who also had a child, who was a human being, who is no longer.”
Dr. Harrison said the Dobbs decision would have no effect on up to 93% of ob.gyns. who don’t perform abortions. Dr. King said the reason that most don’t perform the procedure is the “stigma” attached to abortion. “It’s still frowned upon,” she said. “We don’t talk about it as health care.”
Ms. Meegan added that ob.gyns. are fearful in the wake of the Dobbs decision because “they might find themselves subject to civil and criminal penalties.”
Dr. Harrison said that Roe was always a political decision and the science was always behind AAPLOG – something both Ms. Meegan and Dr. King dispute. Ms. Meegan and Dr. King said they are concerned about the chilling effects on both women and their clinicians, especially with laws that prevent referrals and travel to other states.
“You can’t compel me to give blood or bone marrow,” Dr. King said. “You can’t even compel me to give my hair for somebody, and you can’t compel me to give an organ. And all of a sudden when I’m pregnant, all my rights are out the window?”
A version of this article first appeared on Medscape.com.
Federal Health Care Data Trends 2022: Sleep Disorders
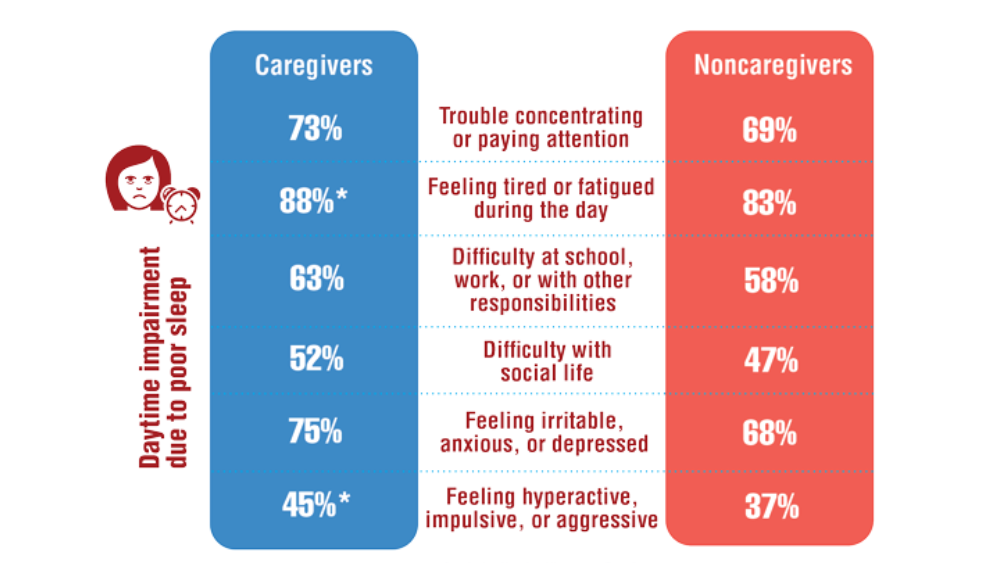
- Song Y, Carlson GC, McGowan SK, et al. Sleep disruption due to stress in women veterans: a comparison between caregivers and noncaregivers. Behav Sleep Med. 2021;19(2):243-254. http://doi.org/10.1080/15402002.2020.1732981
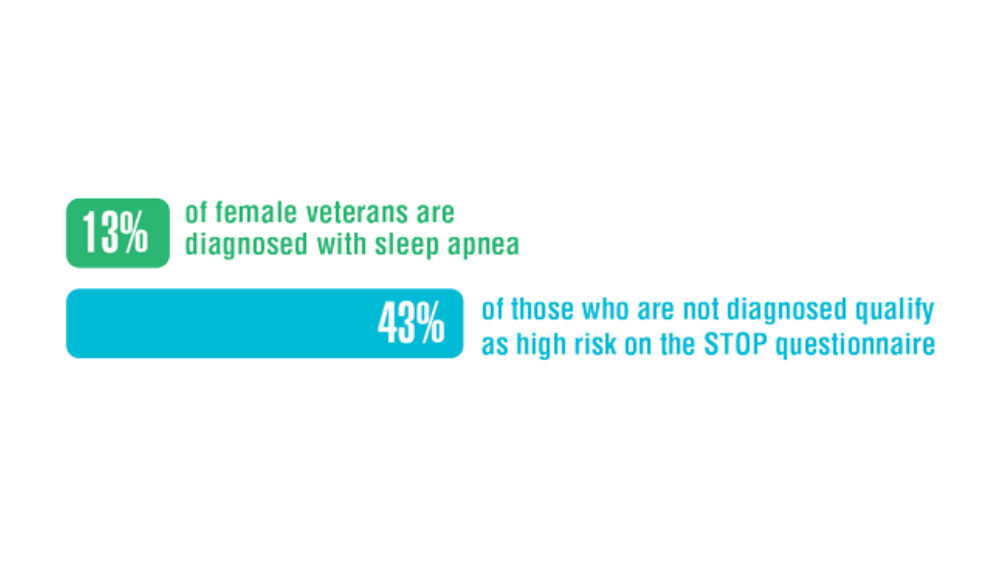
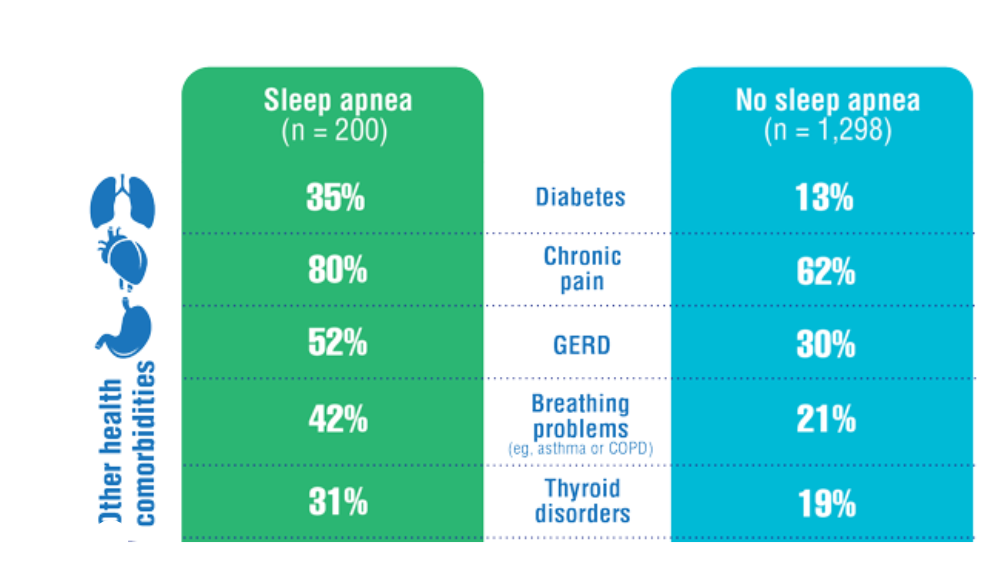
- Martin JL, Carlson G, Kelly M, et al. Sleep apnea in women veterans: results of a national survey of VA health care users. J Clin Sleep Med. 2021;17(3):555-565. http://doi.org/10.5664/jcsm.8956
- Song Y, Carlson GC, McGowan SK, et al. Sleep disruption due to stress in women veterans: a comparison between caregivers and noncaregivers. Behav Sleep Med. 2021;19(2):243-254. http://doi.org/10.1080/15402002.2020.1732981
- Martin JL, Carlson G, Kelly M, et al. Sleep apnea in women veterans: results of a national survey of VA health care users. J Clin Sleep Med. 2021;17(3):555-565. http://doi.org/10.5664/jcsm.8956
- Song Y, Carlson GC, McGowan SK, et al. Sleep disruption due to stress in women veterans: a comparison between caregivers and noncaregivers. Behav Sleep Med. 2021;19(2):243-254. http://doi.org/10.1080/15402002.2020.1732981
- Martin JL, Carlson G, Kelly M, et al. Sleep apnea in women veterans: results of a national survey of VA health care users. J Clin Sleep Med. 2021;17(3):555-565. http://doi.org/10.5664/jcsm.8956
Impact of Race on Outcomes of High-Risk Patients With Prostate Cancer Treated With Moderately Hypofractionated Radiotherapy in an Equal Access Setting
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
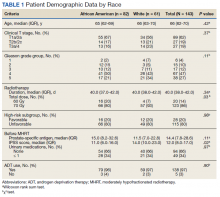
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
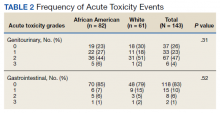
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
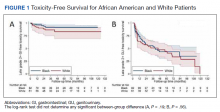
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
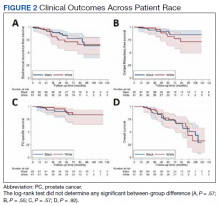
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
Although moderately hypofractionated radiotherapy (MHRT) is an accepted treatment for localized prostate cancer, its adaptation remains limited in the United States.1,2 MHRT theoretically exploits α/β ratio differences between the prostate (1.5 Gy), bladder (5-10 Gy), and rectum (3 Gy), thereby reducing late treatment-related adverse effects compared with those of conventional fractionation at biologically equivalent doses.3-8 Multiple randomized noninferiority trials have demonstrated equivalent outcomes between MHRT and conventional fraction with no appreciable increase in patient-reported toxicity.9-14 Although these studies have led to the acceptance of MHRT as a standard treatment, the majority of these trials involve individuals with low- and intermediate-risk disease.
There are less phase 3 data addressing MHRT for high-risk prostate cancer (HRPC).10,12,14-17 Only 2 studies examined predominately high-risk populations, accounting for 83 and 292 patients, respectively.15,16 Additional phase 3 trials with small proportions of high-risk patients (n = 126, 12%; n = 53, 35%) offer limited additional information regarding clinical outcomes and toxicity rates specific to high-risk disease.10-12 Numerous phase 1 and 2 studies report various field designs and fractionation plans for MHRT in the context of high-risk disease, although the applicability of these data to off-trial populations remains limited.18-20
Furthermore, African American individuals are underrepresented in the trials establishing the role of MHRT despite higher rates of prostate cancer incidence, more advanced disease stage at diagnosis, and higher rates of prostate cancer–specific survival (PCSS) when compared with White patients.21 Racial disparities across patients with prostate cancer and their management are multifactorial across health care literacy, education level, access to care (including transportation issues), and issues of adherence and distrust.22-25 Correlation of patient race to prostate cancer outcomes varies greatly across health care systems, with the US Department of Veterans Affairs (VA) equal access system providing robust mental health services and transportation services for some patients, while demonstrating similar rates of stage-adjusted PCSS between African American and White patients across a broad range of treatment modalities.26-28 Given the paucity of data exploring outcomes following MHRT for African American patients with HRPC, the present analysis provides long-term clinical outcomes and toxicity profiles for an off-trial majority African American population with HRPC treated with MHRT within the VA.
Methods
Records were retrospectively reviewed under an institutional review board–approved protocol for all patients with HRPC treated with definitive MHRT at the Durham Veterans Affairs Healthcare System in North Carolina between November 2008 and August 2018. Exclusion criteria included < 12 months of follow-up or elective nodal irradiation. Demographic variables obtained included age at diagnosis, race, clinical T stage, pre-MHRT prostate-specific antigen (PSA), Gleason grade group at diagnosis, favorable vs unfavorable high-risk disease, pre-MHRT international prostate symptom score (IPSS), and pre-MHRT urinary medication usage (yes/no).29
Concurrent androgen deprivation therapy (ADT) was initiated 6 to 8 weeks before MHRT unless medically contraindicated per the discretion of the treating radiation oncologist. Patients generally received 18 to 24 months of ADT, with those with favorable HRPC (ie, T1c disease with either Gleason 4+4 and PSA < 10 mg/mL or Gleason 3+3 and PSA > 20 ng/mL) receiving 6 months after 2015.29 Patients were simulated supine in either standard or custom immobilization with a full bladder and empty rectum. MHRT fractionation plans included 70 Gy at 2.5 Gy per fraction and 60 Gy at 3 Gy per fraction. Radiotherapy targets included the prostate and seminal vesicles without elective nodal coverage per institutional practice. Treatments were delivered following image guidance, either prostate matching with cone beam computed tomography or fiducial matching with kilo voltage imaging. All patients received intensity-modulated radiotherapy. For plans delivering 70 Gy at 2.5 Gy per fraction, constraints included bladder V (volume receiving) 70 < 10 cc, V65 ≤ 15%, V40 ≤ 35%, rectum V70 < 10 cc, V65 ≤ 10%, V40 ≤ 35%, femoral heads maximum point dose ≤ 40 Gy, penile bulb mean dose ≤ 50 Gy, and small bowel V40 ≤ 1%. For plans delivering 60 Gy at 3 Gy per fraction, constraints included rectum V57 ≤ 15%, V46 ≤ 30%, V37 ≤ 50%, bladder V60 ≤ 5%, V46 ≤ 30%, V37 ≤ 50%, and femoral heads V43 ≤ 5%.
Gastrointestinal (GI) and genitourinary (GU) toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, with acute toxicity defined as on-treatment < 3 months following completion of MHRT. Late toxicity was defined as ≥ 3 months following completion of MHRT. Individuals were seen in follow-up at 6 weeks and 3 months with PSA and testosterone after MHRT completion, then every 6 to 12 months for 5 years and annually thereafter. Each follow-up visit included history, physical examination, IPSS, and CTCAE grading for GI and GU toxicity.
The Wilcoxon rank sum test and χ2 test were used to compare differences in demographic data, dosimetric parameters, and frequency of toxicity events with respect to patient race. Clinical endpoints including biochemical recurrence-free survival (BRFS; defined by Phoenix criteria as 2.0 above PSA nadir), distant metastases-free survival (DMFS), PCSS, and overall survival (OS) were estimated from time of radiotherapy completion by the Kaplan-Meier method and compared between African American and White race by log-rank testing.30 Late GI and GU toxicity-free survival were estimated by Kaplan-Meier plots and compared between African American and White patients by the log-rank test. Statistical analysis was performed using SAS 9.4.
Results
We identified 143 patients with HRPC treated with definitive MHRT between November 2008 and August 2018 (Table 1). Mean age was 65 years (range, 36-80 years); 57% were African American men. Eighty percent of individuals had unfavorable high-risk disease. Median (IQR) PSA was 14.4 (7.8-28.6). Twenty-six percent had grade group 1-3 disease, 47% had grade group 4 disease, and 27% had grade group 5 disease. African American patients had significantly lower pre-MHRT IPSS scores than White patients (mean IPSS, 11 vs 14, respectively; P = .02) despite similar rates of preradiotherapy urinary medication usage (66% and 66%, respectively).
Eighty-six percent received 70 Gy over 28 fractions, with institutional protocol shifting to 60 Gy over 20 fractions (14%) in June 2017. The median (IQR) duration of radiotherapy was 39 (38-42) days, with 97% of individuals undergoing ADT for a median (IQR) duration of 24 (24-36) months. The median follow-up time was 38 months, with 57 (40%) patients followed for at least 60 months.
Grade 3 GI and GU acute toxicity events were observed in 1% and 4% of all individuals, respectively (Table 2). No acute GI or GU grade 4+ events were observed. No significant differences in acute GU or GI toxicity were observed between African American and White patients.
No significant differences between African American and White patients were observed for late grade 2+ GI (P = .19) or GU (P = .55) toxicity. Late grade 2+ GI toxicity was observed in 17 (12%) patients overall (Figure 1A). One grade 3 and 1 grade 4 late GI event were observed following MHRT completion: The latter involved hospitalization for bleeding secondary to radiation proctitis in the context of cirrhosis predating MHRT. Late grade 2+ GU toxicity was observed in 80 (56%) patients, with late grade 2 events steadily increasing over time (Figure 1B). Nine late grade 3 GU toxicity events were observed at a median of 13 months following completion of MHRT, 2 of which occurred more than 24 months after MHRT completion. No late grade 4 or 5 GU events were observed. IPSS values both before MHRT and at time of last follow-up were available for 65 (40%) patients, with a median (IQR) IPSS of 10 (6-16) before MHRT and 12 (8-16) at last follow-up at a median (IQR) interval of 36 months (26-76) from radiation completion.
No significant differences were observed between African American and White patients with respect to BRFS, DMFS, PCSS, or OS (Figure 2). Overall, 21 of 143 (15%) patients experienced biochemical recurrence: 5-year BRFS was 77% (95% CI, 67%-85%) for all patients, 83% (95% CI, 70%-91%) for African American patients, and 71% (95% CI, 53%-82%) for White patients. Five-year DMFS was 87% (95% CI, 77%-92%) for all individuals, 91% (95% CI, 80%-96%) for African American patients, and 81% (95% CI, 62%-91%) for White patients. Five-year PCSS was 89% (95% CI, 80%-94%) for all patients, with 5-year PCSS rates of 90% (95% CI, 79%-95%) for African American patients and 87% (95% CI, 70%-95%) for White patients. Five-year OS was 75% overall (95% CI, 64%-82%), with 5-year OS rates of 73% (95% CI, 58%-83%) for African American patients and 77% (95% CI, 60%-87%) for White patients.
Discussion
In this study, we reported acute and late GI and GU toxicity rates as well as clinical outcomes for a majority African American population with predominately unfavorable HRPC treated with MHRT in an equal access health care environment. We found that MHRT was well tolerated with high rates of biochemical control, PCSS, and OS. Additionally, outcomes were not significantly different across patient race. To our knowledge, this is the first report of MHRT for HRPC in a majority African American population.
We found that MHRT was an effective treatment for patients with HRPC, in particular those with unfavorable high-risk disease. While prior prospective and randomized studies have investigated the use of MHRT, our series was larger than most and had a predominately unfavorable high-risk population.12,15-17 Our biochemical and PCSS rates compare favorably with those of HRPC trial populations, particularly given the high proportion of unfavorable high-risk disease.12,15,16 Despite similar rates of biochemical control, OS was lower in the present cohort than in HRPC trial populations, even with a younger median age at diagnosis. The similarly high rates of non–HRPC-related death across race may reflect differences in baseline comorbidities compared with trial populations as well as reported differences between individuals in the VA and the private sector.31 This suggests that MHRT can be an effective treatment for patients with unfavorable HRPC.
We did not find any differences in outcomes between African American and White individuals with HRPC treated with MHRT. Furthermore, our study demonstrates long-term rates of BRFS and PCSS in a majority African American population with predominately unfavorable HRPC that are comparable with those of prior randomized MHRT studies in high-risk, predominately White populations.12,15,16 Prior reports have found that African American men with HRPC may be at increased risk for inferior clinical outcomes due to a number of socioeconomic, biologic, and cultural mediators.26,27,32 Such individuals may disproportionally benefit from shorter treatment courses that improve access to radiotherapy, a well-documented disparity for African American men with localized prostate cancer.33-36 The VA is an ideal system for studying racial disparities within prostate cancer, as accessibility of mental health and transportation services, income, and insurance status are not barriers to preventative or acute care.37 Our results are concordant with those previously seen for African American patients with prostate cancer seen in the VA, which similarly demonstrate equal outcomes with those of other races.28,36 Incorporation of the earlier mentioned VA services into oncologic care across other health care systems could better characterize determinants of racial disparities in prostate cancer, including the prognostic significance of shortening treatment duration and number of patient visits via MHRT.
Despite widespread acceptance in prostate cancer radiotherapy guidelines, routine use of MHRT seems limited across all stages of localized prostate cancer.1,2 Late toxicity is a frequently noted concern regarding MHRT use. Higher rates of late grade 2+ GI toxicity were observed in the hypofractionation arm of the HYPRO trial.17 While RTOG 0415 did not include patients with HRPC, significantly higher rates of physician-reported (but not patient-reported) late grade 2+ GI and GU toxicity were observed using the same MHRT fractionation regimen used for the majority of individuals in our cohort.9 In our study, the steady increase in late grade 2 GU toxicity is consistent with what is seen following conventionally fractionated radiotherapy and is likely multifactorial.38 The mean IPSS difference of 2/35 from pre-MHRT baseline to the time of last follow-up suggests minimal quality of life decline. The relatively stable IPSSs over time alongside the > 50% prevalence of late grade 2 GU toxicity per CTCAE grading seems consistent with the discrepancy noted in RTOG 0415 between increased physician-reported late toxicity and favorable patient-reported quality of life scores.9 Moreover, significant variance exists in toxicity grading across scoring systems, revised editions of CTCAE, and physician-specific toxicity classification, particularly with regard to the use of adrenergic receptor blocker medications. In light of these factors, the high rate of late grade 2 GU toxicity in our study should be interpreted in the context of largely stable post-MHRT IPSSs and favorable rates of late GI grade 2+ and late GU grade 3+ toxicity.
Limitations
This study has several inherent limitations. While the size of the current HRPC cohort is notably larger than similar populations within the majority of phase 3 MHRT trials, these data derive from a single VA hospital. It is unclear whether these outcomes would be representative in a similar high-risk population receiving care outside of the VA equal access system. Follow-up data beyond 5 years was available for less than half of patients, partially due to nonprostate cancer–related mortality at a higher rate than observed in HRPC trial populations.12,15,16 Furthermore, all GI toxicity events were exclusively physician reported, and GU toxicity reporting was limited in the off-trial setting with not all patients routinely completing IPSS questionnaires following MHRT completion. However, all patients were treated similarly, and radiation quality was verified over the treatment period with mandated accreditation, frequent standardized output checks, and systematic treatment review.39
Conclusions
Patients with HRPC treated with MHRT in an equal access, off-trial setting demonstrated favorable rates of biochemical control with acceptable rates of acute and late GI and GU toxicities. Clinical outcomes, including biochemical control, were not significantly different between African American and White patients, which may reflect equal access to care within the VA irrespective of income and insurance status. Incorporating VA services, such as access to primary care, mental health services, and transportation across other health care systems may aid in characterizing and mitigating racial and gender disparities in oncologic care.
Acknowledgments
Portions of this work were presented at the November 2020 ASTRO conference. 40
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
1. Stokes WA, Kavanagh BD, Raben D, Pugh TJ. Implementation of hypofractionated prostate radiation therapy in the United States: a National Cancer Database analysis. Pract Radiat Oncol. 2017;7:270-278. doi:10.1016/j.prro.2017.03.011
2. Jaworski L, Dominello MM, Heimburger DK, et al. Contemporary practice patterns for intact and post-operative prostate cancer: results from a statewide collaborative. Int J Radiat Oncol Biol Phys. 2019;105(1):E282. doi:10.1016/j.ijrobp.2019.06.1915
3. Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):e17-e24. doi:10.1016/j.ijrobp.2010.10.075
4. Tree AC, Khoo VS, van As NJ, Partridge M. Is biochemical relapse-free survival after profoundly hypofractionated radiotherapy consistent with current radiobiological models? Clin Oncol (R Coll Radiol). 2014;26(4):216-229. doi:10.1016/j.clon.2014.01.008
5. Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-1015. doi:10.1016/j.ijrobp.2004.04.014
6. Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81(2):600-605. doi:10.1016/j.ijrobp.2010.11.080
7. Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963-974. doi:10.3109/0284186X.2012.719635 start
8. Proust-Lima C, Taylor JMG, Sécher S, et al. Confirmation of a Low α/β ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys. 2011;79(1):195-201. doi:10.1016/j.ijrobp.2009.10.008
9. Lee WR, Dignam JJ, Amin MB, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20): 2325-2332. doi:10.1200/JCO.2016.67.0448
10. Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi:10.1016/S1470-2045(16)30102-4
11. Catton CN, Lukka H, Gu C-S, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol. 2017;35(17):1884-1890. doi:10.1200/JCO.2016.71.7397
12. Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-3868. doi:10.1200/JCO.2013.51.1972
13. Hoffman KE, Voong KR, Levy LB, et al. Randomized trial of hypofractionated, dose-escalated, intensity-modulated radiation therapy (IMRT) versus conventionally fractionated IMRT for localized prostate cancer. J Clin Oncol. 2018;36(29):2943-2949. doi:10.1200/JCO.2018.77.9868
14. Wilkins A, Mossop H, Syndikus I, et al. Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2015;16(16):1605-1616. doi:10.1016/S1470-2045(15)00280-6
15. Incrocci L, Wortel RC, Alemayehu WG, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016;17(8):1061-1069. doi.10.1016/S1470-2045(16)30070-5
16. Arcangeli G, Saracino B, Arcangeli S, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol. 2017;35(17):1891-1897. doi:10.1200/JCO.2016.70.4189
17. Aluwini S, Pos F, Schimmel E, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17(4):464-474. doi:10.1016/S1470-2045(15)00567-7
18. Pervez N, Small C, MacKenzie M, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(1):57-64. doi:10.1016/j.ijrobp.2009.01.048
19. Magli A, Moretti E, Tullio A, Giannarini G. Hypofractionated simultaneous integrated boost (IMRT- cancer: results of a prospective phase II trial SIB) with pelvic nodal irradiation and concurrent androgen deprivation therapy for high-risk prostate cancer: results of a prospective phase II trial. Prostate Cancer Prostatic Dis. 2018;21(2):269-276. doi:10.1038/s41391-018-0034-0
20. Di Muzio NG, Fodor A, Noris Chiorda B, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I–II study. Clin Oncol (R Coll Radiol). 2016;28(8):490-500. doi:10.1016/j.clon.2016.02.005
21. DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):21-233. doi:10.3322/caac.21555
22. Wolf MS, Knight SJ, Lyons EA, et al. Literacy, race, and PSA level among low-income men newly diagnosed with prostate cancer. Urology. 2006(1);68:89-93. doi:10.1016/j.urology.2006.01.064
23. Rebbeck TR. Prostate cancer disparities by race and ethnicity: from nucleotide to neighborhood. Cold Spring Harb Perspect Med. 2018;8(9):a030387. doi:10.1101/cshperspect.a030387
24. Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366.
25. Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34(5):449-460. doi:10.1007/s10900-009-9167-3
26. Connell PP, Ignacio L, Haraf D, et al. Equivalent racial outcome after conformal radiotherapy for prostate cancer: a single departmental experience. J Clin Oncol. 2001;19(1):54-61. doi:10.1200/JCO.2001.19.1.54
27. Dess RT, Hartman HE, Mahal BA, et al. Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol. 2019;5(1):975-983. doi:10.1200/JCO.2001.19.1.54
28. McKay RR, Sarkar RR, Kumar A, et al. Outcomes of Black men with prostate cancer treated with radiation therapy in the Veterans Health Administration. Cancer. 2021;127(3):403-411. doi:10.1002/cncr.33224

29. Muralidhar V, Chen M-H, Reznor G, et al. Definition and validation of “favorable high-risk prostate cancer”: implications for personalizing treatment of radiation-managed patients. Int J Radiat Oncol Biol Phys. 2015;93(4):828-835. doi:10.1016/j.ijrobp.2015.07.2281
30. Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965-974. doi:10.1016/j.ijrobp.2006.04.029
31. Freeman VL, Durazo-Arvizu R, Arozullah AM, Keys LC. Determinants of mortality following a diagnosis of prostate cancer in Veterans Affairs and private sector health care systems. Am J Public Health. 2003;93(100):1706-1712. doi:10.2105/ajph.93.10.1706
32. Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi:10.3322/canjclin.54.2.78
33. Zemplenyi AT, Kaló Z, Kovacs G, et al. Cost-effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care. 2018;27(1):e12430. doi:10.1111/ecc.12430
34. Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36(9):1337-1348. doi:10.1097/00005650-199809000-00006
35. Harlan L, Brawley O, Pommerenke F, Wali P, Kramer B. Geographic, age, and racial variation in the treatment of local/regional carcinoma of the prostate. J Clin Oncol. 1995;13(1):93-100. doi:10.1200/JCO.1995.13.1.93
36. Riviere P, Luterstein E, Kumar A, et al. Racial equity among African-American and non-Hispanic white men diagnosed with prostate cancer in the veterans affairs healthcare system. Int J Radiat Oncol Biol Phys. 2019;105:E305.
37. Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1-e11. doi:10.2105/AJPH.2017.304246
38. Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233-1239. doi:10.1001/jama.294.10.1233
39. Hagan M, Kapoor R, Michalski J, et al. VA-Radiation Oncology Quality Surveillance program. Int J Radiat Oncol Biol Phys. 2020;106(3):639-647. doi.10.1016/j.ijrobp.2019.08.064
40. Carpenter DJ, Natesan D, Floyd W, et al. Long-term experience in an equal access health care system using moderately hypofractionated radiotherapy for high risk prostate cancer in a predominately African American population with unfavorable disease. Int J Radiat Oncol Biol Phys. 2020;108(3):E417. https://www.redjournal.org/article/S0360-3016(20)33923-7/fulltext
MERIT: Endoscopic sleeve gastroplasty shows ‘very impressive’ outcomes in randomized clinical trial
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
In a randomized, controlled trial, endoscopic sleeve gastroplasty (ESG) combined with lifestyle modifications was safe and effective for weight loss among individuals with class I and class II obesity, compared with lifestyle modifications alone.
“Lifestyle modifications and pharmacological therapy have several limitations, and the use of bariatric surgery is hampered by its invasive nature and patient perceptions,” the study authors wrote. ESG is a minimally invasive, reversible, organ-sparing bariatric procedure that might be able to fill those care gaps, they explained.
Previous retrospective studies have suggested that ESG is effective, and a meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%). However, according to the authors of the current study, known as MERIT and published in the Lancet, there have been no randomized clinical trials investigating ESG's efficacy to date.
“[This is] the kind of study that we have been looking forward to. The outcomes were very impressive,” said Danny Issa, MD, who was asked to comment on the study. He is a clinical assistant professor of medicine at the University of California, Los Angeles. meta-analysis of 1,772 patients found an average total body weight loss of 15.1% at 6 months (95% confidence interval, 14.3%-16.0%) and 16.5% at 12 months (95% CI, 15.2%-17.8%).
Understanding the study and its results
Between December 2017 and June 2019, the researchers randomized 209 participants to ESG plus lifestyle modification or lifestyle modification only, which served as the control. The mean age was 47.3 in the ESG group (88% female) and 45.7 in the control group (84% female). The mean body mass index (BMI) was 35.5 kg/m2 in the ESG group and 35.7 among controls.
After 1 year, the intervention group had a mean percentage of excess weight loss (EWL) of 49.2% , compared with 3.2% for the control group (P < .0001). The mean percentage of total body weight lost was 13.6% in the ESG group and 0.8% in the control group (P < .0001). After adjustment for age, sex, type 2 diabetes, hypertension, and baseline BMI, the ESG group had a mean difference of excess weight loss of 44.7% (95% CI, 37.5%-51.9%) and a mean difference of total weight loss of 12.6% (95% CI, 10.7%-14.5%), compared with the control group at 52 weeks. At 52 weeks, 77% of the ESG group had at least a 25% excess weight loss, which was the secondary endpoint, compared with 12% of the control group (P < .0001).
Overall, 80% of the ESG group had an improvement in at least one metabolic comorbidity, while 12% experienced a worsening. Among the control group, 45% had an improvement and 50% worsened. Among 27 patients in the treatment group with diabetes, 93% experienced an improvement in hemoglobin A1c levels, compared with 15% of patients with diabetes in the control group. Similarly among patients with hypertension, 60% in the intervention group had an improvement, compared with 40% of controls. Of those with metabolic syndrome, 83% improved after undergoing surgery, compared with 35% of controls.
At 2 years, 68% of the ESG group who achieved a 25% EWL continued to have at least 25% EWL; 2% in the treatment group had a serious ESG-related adverse event, but there was no mortality or need for intensive care or follow-up surgery.
Aiming for level I evidence
“The results are very encouraging, so I think it’s good news for the field of bariatric endoscopy. I think it’s going to provide more confidence to patients and physicians, and for new trainees who are interested in this field, I think it’s going to inspire them,” said Shailendra Singh, MD, who was asked to comment on the study. Dr. Singh is an associate professor of medicine and director of bariatric medicine at West Virginia University, Morgantown.
The study could also improve insurance coverage of the procedure, said Dr. Singh. “I think this study will help us reach out to the payers and give them the data behind this because they always look for level I evidence. ESG is a relatively new endoscopic procedure; I think this is a step forward in that direction,” he said.
The study underlines the applicability of the procedure to patients who don’t want more invasive surgery, or who can’t tolerate some of the higher efficacy medications that are increasingly available.
It is also just one of various options for obesity treatment, which are increasingly being used in combination, according to Avlin Imaeda, MD. “Just like we see in hypertension, where you progressively add more and more medications, I think we’re going to see obesity treatment go that way too. I see this as adding choice for patients and adding to this potentially multimodal approach,” said Dr. Imaeda, an associate professor of medicine at Yale University, New Haven, Conn., who was not involved in the study.
The study authors report various financial relationships, including some with Apollo Endosurgery, which funded this study. Dr. Issa and Dr. Imaeda have no relevant financial disclosures. Dr. Singh is a consultant for Apollo Endosurgery.
This article was updated Aug. 18, 2022.
FROM THE LANCET
Federal Health Care Data Trends 2022: Vaccinations
- National Center for Health Statistics. National Health Interview Survey, 2015-2018. Veterans health statistics Table 11a. Updated June 19, 2020. Accessed March 29, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
Britten SA. Contributions of the Armed Forces Epidemiological Board to military progress. Mil Med. 1965;130:149-157.
Armed Forces Health Surveillance Division. Surveillance snapshot: influenza immunization among U.S. Armed Forces health care workers, August 2016–April 2021. October 1, 2021. Accessed April 7, 2022. https://health.mil/News/Articles/2021/10/01/Snap-Influenza-MSMR
Centers for Disease Control and Prevention. Influenza (flu): coverage by season. Updated March 16, 2021. Accessed April 7, 2022. https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. http://doi.org/10.1126/science.abm0620
Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. http://doi.org/10.15585/mmwr.mm7049a2
Vaccine preventable death analysis. Global Epidemics. Published May 13, 2022. Accessed May 19, 2022. https://globalepidemics.org/vaccinations/
- National Center for Health Statistics. National Health Interview Survey, 2015-2018. Veterans health statistics Table 11a. Updated June 19, 2020. Accessed March 29, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
Britten SA. Contributions of the Armed Forces Epidemiological Board to military progress. Mil Med. 1965;130:149-157.
Armed Forces Health Surveillance Division. Surveillance snapshot: influenza immunization among U.S. Armed Forces health care workers, August 2016–April 2021. October 1, 2021. Accessed April 7, 2022. https://health.mil/News/Articles/2021/10/01/Snap-Influenza-MSMR
Centers for Disease Control and Prevention. Influenza (flu): coverage by season. Updated March 16, 2021. Accessed April 7, 2022. https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. http://doi.org/10.1126/science.abm0620
Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. http://doi.org/10.15585/mmwr.mm7049a2
Vaccine preventable death analysis. Global Epidemics. Published May 13, 2022. Accessed May 19, 2022. https://globalepidemics.org/vaccinations/
- National Center for Health Statistics. National Health Interview Survey, 2015-2018. Veterans health statistics Table 11a. Updated June 19, 2020. Accessed March 29, 2022. https://www.cdc.gov/nchs/nhis/veterans_health_statistics/tables.htm
Britten SA. Contributions of the Armed Forces Epidemiological Board to military progress. Mil Med. 1965;130:149-157.
Armed Forces Health Surveillance Division. Surveillance snapshot: influenza immunization among U.S. Armed Forces health care workers, August 2016–April 2021. October 1, 2021. Accessed April 7, 2022. https://health.mil/News/Articles/2021/10/01/Snap-Influenza-MSMR
Centers for Disease Control and Prevention. Influenza (flu): coverage by season. Updated March 16, 2021. Accessed April 7, 2022. https://www.cdc.gov/flu/fluvaxview/coverage-by-season.htm
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375(6578):331-336. http://doi.org/10.1126/science.abm0620
Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans — five Veterans Affairs Medical Centers, United States, February 1–September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. http://doi.org/10.15585/mmwr.mm7049a2
Vaccine preventable death analysis. Global Epidemics. Published May 13, 2022. Accessed May 19, 2022. https://globalepidemics.org/vaccinations/
Does hidradenitis suppurativa worsen during pregnancy?
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
PORTLAND, ORE. – The recurrent boils, abscesses, and nodules of the chronic inflammatory skin condition hidradenitis suppurativa (HS) may improve during pregnancy for a subset of women, but for many, pregnancy does not change the disease course and may worsen symptoms.
In addition, HS appears to be a risk factor for adverse pregnancy and maternal outcomes.
“This is relevant, because in the United States, HS disproportionately impacts women compared with men by a ratio of about 3:1,” Jennifer Hsiao, MD, said at the annual meeting of the Pacific Dermatologic Association.
“Also, the highest prevalence of HS is among people in their 20s and 30s, so in their practice, clinicians will encounter female patients with HS who are either pregnant or actively thinking about getting pregnant,” she said.
During a wide-ranging presentation, Dr. Hsiao of the department of dermatology at the University of Southern California, Los Angeles, described the impact of pregnancy on HS, identified appropriate treatment options for this population of patients, and discussed HS comorbidities that may be exacerbated during pregnancy.
She began by noting that levels of progesterone and estrogen both rise during pregnancy. Progesterone is known to suppress development and function of Th1 and Th17 T cells, but the effect of estrogen on inflammation is less well known. At the same time, serum levels of interleukin (IL)-1 receptor antagonist and soluble TNF-alpha receptor both increase during pregnancy.
“This would lead to serum IL-1 and TNF-alpha falling, sort of like the way that we give anti–IL-1 and TNF blockers as HS treatments,” she explained. “So, presumably that might be helpful during HS in pregnancy. On the flip side, pregnancy weight gain can exacerbate HS, with increased friction between skin folds. In addition, just having more adipocytes can promote secretion of proinflammatory cytokines like TNF-alpha.”
To better understand the effect of pregnancy on patients with HS, Dr. Hsiao and colleagues conducted a systematic review and meta-analysis on the topic published in Dermatology. They included eight studies in which a total of 672 patients self-reported their HS disease course during pregnancy and 164 self-reported whether they had a postpartum HS flare or not. On pooled analyses, HS improved in 24% of patients but worsened in 20%. In addition, 60% of patients experienced a postpartum flare.
“So, at this point in time, based on the literature, it would be fair to tell your patient that during pregnancy, HS has a mixed response,” Dr. Hsiao said. “About 25% may have improvement, but for the rest, HS symptoms may be unchanged or even worsen. That’s why it’s so important to be in contact with your pregnant patients, because not only may they have to stay on treatment, but they might also have to escalate [their treatment] during pregnancy.”
Lifestyle modifications to discuss with pregnant HS patients include appropriate weight gain during pregnancy, smoking cessation, and avoidance of tight-fitting clothing, “since friction can make things worse,” she said. Topical antibiotics safe to use during pregnancy for patients with mild HS include clindamycin 1%, erythromycin 2%, and metronidazole 0.75% applied twice per day to active lesions, she continued.
As for systemic therapies, some data exist to support the use of metformin 500 mg once daily, titrating up to twice or – if needed and tolerated – three times daily for patients with mild to moderate HS, she said, referencing a paper published in the Journal of the European Academy of Dermatology and Venereology.
Zinc gluconate is another potential option. Of 22 nonpregnant HS patients with Hurley stage I-II disease who were treated with zinc gluconate 90 mg daily, 8 had a complete remission of HS and 14 had partial remission, according to a report in Dermatology.
“Zinc supplementation of up to 50 mg daily has shown no effect on neonatal or maternal outcomes at birth based on existing medical literature,” Dr. Hsiao added.
Among antibiotics, injections of intralesional Kenalog 5-10 mg/mL have been shown to decrease pain and inflammation in acute HS lesions and are unlikely to pose significant risks during pregnancy, but a course of systemic antibiotics may be warranted in moderate to severe disease, she said. These include, but are not limited to, clindamycin, erythromycin base, cephalexin, or metronidazole.
“In addition, some of my HS colleagues and I will also use other antibiotics such as Augmentin [amoxicillin/clavulanate] or cefdinir for HS and these are also generally considered safe to use in pregnancy,” she said. “Caution is advised with using rifampin, dapsone, and moxifloxacin during pregnancy.”
As for biologic agents, the first-line option is adalimumab, which is currently the only Food and Drug Administration–approved treatment for HS.
“There is also good efficacy data for infliximab,” she said. “Etanercept has less placental transfer than adalimumab or infliximab so it’s safer to use in pregnancy, but it has inconsistent data for efficacy in HS, so I would generally avoid using it to treat HS and reach for adalimumab or infliximab instead.”
Data on TNF-alpha inhibitors from the GI and rheumatology literature have demonstrated that there is minimal placental transport of maternal antibodies during the first two trimesters of pregnancy.
“It’s at the beginning of the third trimester that the placental transfer of antibodies picks up,” she said. “At that point in time, you can have a discussion with the patient: do you want to stay on treatment and treat through, or do you want to consider being taken off the medication? I think this is a discussion that needs to be had, because let’s say you peel off adalimumab or infliximab and they have severe HS flares. I’m not sure that leads to a better outcome. I usually treat through for my pregnant patients.”
To better understand clinician practice patterns on the management of HS in pregnancy, Dr. Hsiao and Erin Collier, MD, MPH, of University of California, Los Angeles, and colleagues distributed an online survey to HS specialists in North America. They reported the findings in the International Journal of Women’s Dermatology.
Of the 49 respondents, 36 (73%) directed an HS specialty clinic and 29 (59%) reported having prescribed or continued a biologic agent in a pregnant HS patient. The top three biologics prescribed were adalimumab (90%), infliximab (41%), and certolizumab pegol (34%). Dr. Hsiao noted that certolizumab pegol is a pegylated anti-TNF, so it lacks an Fc region on the medication.
“This means that it cannot be actively transported by the neonatal Fc receptor on the placenta, thus resulting in minimal placental transmission,” she said. “The main issue is that there is little data on its efficacy in HS, but it’s a reasonable option to consider in a pregnant patient, especially in a patient with severe HS who asks, ‘what’s the safest biologic that I can go on?’ But you’d have to discuss with the patient that in terms of efficacy data, there is much less in the literature compared to adalimumab or infliximab.”
Breastfeeding while on anti–TNF-alpha biologics is considered safe. “There are minimal amounts of medication in breast milk,” she said. “If any gets through, infant gastric digestion is thought to take care of the rest. Of note, babies born to mothers who are continually treated with biologic agents should not be given live vaccinations for 6 months after birth.”
In a single-center study, Dr. Hsiao and colleagues retrospectively examined pregnancy complications, pregnancy outcomes, and neonatal outcomes in patients with HS. The study population included 202 pregnancies in 127 HS patients. Of 134 babies born to mothers with HS, 74% were breastfed and 24% were bottle-fed, and presence of HS lesions on the breast was significantly associated with not breastfeeding.
“So, when we see these patients, if moms decide to breastfeed and they have lesions on the breast, it would be helpful to discuss expectations and perhaps treat HS breast lesions early, so the breastfeeding process may go more smoothly for them after they deliver,” said Dr. Hsiao, who is one of the editors of the textbook “A Comprehensive Guide to Hidradenitis Suppurativa” (Elsevier, 2021). Safety-related resources that she recommends for clinicians include Mother to Baby and the Drugs and Lactation Database (LactMed).
Dr. Hsiao concluded her presentation by spotlighting the influence of pregnancy on HS comorbidities. Patients with HS already have a higher prevalence of depression and anxiety compared to controls. “Pregnancy can exacerbate underlying mood disorders in patients,” she said. “That’s why monitoring the patient’s mood and coordinating mental health care with the patient’s primary care physician and ob.gyn. is important.”
In addition, pregnancy-related changes in body mass index, blood pressure, lipid metabolism, and glucose tolerance trend toward changes seen in metabolic syndrome, she said, and HS patients are already at higher risk of metabolic syndrome compared with the general population.
HS may also compromise a patient’s ability to have a healthy pregnancy. Dr. Hsiao worked with Amit Garg, MD, and colleagues on a study that drew from the IBM MarketScan Commercial Claims Database to evaluate adverse pregnancy and maternal outcomes in women with HS between Jan. 1, 2011, and Sept. 30, 2015.
After the researchers adjusted for age, race, smoking status, and other comorbidities, they found that HS pregnancies were independently associated with spontaneous abortion (odds ratio, 1.20), gestational diabetes (OR, 1.26), and cesarean section (OR, 1.09). The findings were published in the Journal of the American Academy of Dermatology.
A separate study that used the same database found comparable results, also published in the Journal of the American Academy of Dermatology. “What I say to patients right now is, ‘there are many women with HS who have healthy pregnancies and deliver healthy babies, but HS could be a risk factor for a higher-risk pregnancy.’ It’s important that these patients are established with an ob.gyn. and are closely monitored to make sure that we optimize their care and give them the best outcome possible for mom and baby.”
Dr. Hsiao disclosed that she is on the board of directors for the Hidradenitis Suppurativa Foundation. She has also served as an advisor for Novartis, UCB, and Boehringer Ingelheim and as a speaker and advisor for AbbVie.
AT PDA 2022
Stressed about weight gain? Well, stress causes weight gain
Stress, meet weight gain. Weight gain, meet stress
You’re not eating differently and you’re keeping active, but your waistline is expanding. How is that happening? Since eating healthy and exercising shouldn’t make you gain weight, there may be a hidden factor getting in your way. Stress. The one thing that can have a grip on your circadian rhythm stronger than any bodybuilder.
Investigators at Weill Cornell Medicine published two mouse studies that suggest stress and other factors that throw the body’s circadian clocks out of rhythm may contribute to weight gain.
In the first study, the researchers imitated disruptive condition effects like high cortisol exposure and chronic stress by implanting pellets under the skin that released glucocorticoid at a constant rate for 21 days. Mice that received the pellets had twice as much white and brown fat, as well as much higher insulin levels, regardless of their unchanged and still-healthy diet.
In the second study, they used tagged proteins as markers to monitor the daily fluctuations of a protein that regulates fat cell production and circadian gene expression in mouse fat cell precursors. The results showed “that fat cell precursors commit to becoming fat cells only during the circadian cycle phase corresponding to evening in humans,” they said in a written statement.
“Every cell in our body has an intrinsic cell clock, just like the fat cells, and we have a master clock in our brain, which controls hormone secretion,” said senior author Mary Teruel of Cornell University. “A lot of forces are working against a healthy metabolism when we are out of circadian rhythm. The more we understand, the more likely we will be able to do something about it.”
So if you’re stressing out that the scale is or isn’t moving in the direction you want, you could be standing in your own way. Take a chill pill.
Who can smell cancer? The locust nose
If you need to smell some gas, there’s nothing better than a nose. Just ask a scientist: “Noses are still state of the art,” said Debajit Saha, PhD, of Michigan State University. “There’s really nothing like them when it comes to gas sensing.”
And when it comes to noses, dogs are best, right? After all, there’s a reason we don’t have bomb-sniffing wombats and drug-sniffing ostriches. Dogs are better. Better, but not perfect. And if they’re not perfect, then human technology can do better.
Enter the electronic nose. Which is better than dogs … except that it isn’t. “People have been working on ‘electronic noses’ for more than 15 years, but they’re still not close to achieving what biology can do seamlessly,” Dr. Saha explained in a statement from the university.
Which brings us back to dogs. If you want to detect early-stage cancer using smell, you go to the dogs, right? Nope.
Here’s Christopher Contag, PhD, also of Michigan State, who recruited Dr. Saha to the university: “I told him, ‘When you come here, we’ll detect cancer. I’m sure your locusts can do it.’ ”
Yes, locusts. Dr. Contag and his research team were looking at mouth cancers and noticed that different cell lines had different appearances. Then they discovered that those different-looking cell lines produced different metabolites, some of which were volatile.
Enter Dr. Saha’s locusts. They were able to tell the difference between normal cells and cancer cells and could even distinguish between the different cell lines. And how they were able to share this information? Not voluntarily, that’s for sure. The researchers attached electrodes to the insects’ brains and recorded their responses to gas samples from both healthy and cancer cells. Those brain signals were then used to create chemical profiles of the different cells. Piece of cake.
The whole getting-electrodes-attached-to-their-brains thing seemed at least a bit ethically ambiguous, so we contacted the locusts’ PR office, which offered some positive spin: “Humans get their early cancer detection and we get that whole swarms-that-devour-entire-countrysides thing off our backs. Win win.”
Bad news for vampires everywhere
Pop culture has been extraordinarily kind to the vampire. A few hundred years ago, vampires were demon-possessed, often-inhuman monsters. Now? They’re suave, sophisticated, beautiful, and oh-so dramatic and angst-filled about their “curse.” Drink a little human blood, live and look young forever. Such monsters they are.
It does make sense in a morbid sort of way. An old person receiving the blood of the young does seem like a good idea for rejuvenation, right? A team of Ukrainian researchers sought to find out, conducting a study in which older mice were linked with young mice via heterochronic parabiosis. For 3 months, old-young mice pairs were surgically connected and shared blood. After 3 months, the mice were disconnected from each other and the effects of the blood link were studied.
For all the vampire enthusiasts out there, we have bad news and worse news. The bad news first: The older mice received absolutely no benefit from heterochronic parabiosis. No youthfulness, no increased lifespan, nothing. The worse news is that the younger mice were adversely affected by the older blood. They aged more and experienced a shortened lifespan, even after the connection was severed. The old blood, according to the investigators, contains factors capable of inducing aging in younger mice, but the opposite is not true. Further research into aging, they added, should focus on suppressing the aging factors in older blood.
Of note, the paper was written by doctors who are currently refugees, fleeing the war in Ukraine. We don’t want to speculate on the true cause of the war, but we’re onto you, Putin. We know you wanted the vampire research for yourself, but it won’t work. Your dream of becoming Vlad “Dracula” Putin will never come to pass.
Hearing is not always believing
Have you ever heard yourself on a voice mail, or from a recording you did at work? No matter how good you sound, you still might feel like the recording sounds nothing like you. It may even cause low self-esteem for those who don’t like how their voice sounds or don’t recognize it when it’s played back to them.
Since one possible symptom of schizophrenia is not recognizing one’s own speech and having a false sense of control over actions, and those with schizophrenia may hallucinate or hear voices, not being able to recognize their own voices may be alarming.
A recent study on the sense of agency, or sense of control, involved having volunteers speak with different pitches in their voices and then having it played back to them to gauge their reactions.
“Our results demonstrate that hearing one’s own voice is a critical factor to increased self-agency over speech. In other words, we do not strongly feel that ‘I’ am generating the speech if we hear someone else’s voice as an outcome of the speech. Our study provides empirical evidence of the tight link between the sense of agency and self-voice identity,” lead author Ryu Ohata, PhD, of the University of Tokyo, said in a written statement.
As social interaction becomes more digital through platforms such as FaceTime, Zoom, and voicemail, especially since the pandemic has promoted social distancing, it makes sense that people may be more aware and more surprised by how they sound on recordings.
So, if you ever promised someone something that you don’t want to do, and they play it back to you from the recording you made, maybe you can just say you don’t recognize the voice. And if it’s not you, then you don’t have to do it.
Stress, meet weight gain. Weight gain, meet stress
You’re not eating differently and you’re keeping active, but your waistline is expanding. How is that happening? Since eating healthy and exercising shouldn’t make you gain weight, there may be a hidden factor getting in your way. Stress. The one thing that can have a grip on your circadian rhythm stronger than any bodybuilder.
Investigators at Weill Cornell Medicine published two mouse studies that suggest stress and other factors that throw the body’s circadian clocks out of rhythm may contribute to weight gain.
In the first study, the researchers imitated disruptive condition effects like high cortisol exposure and chronic stress by implanting pellets under the skin that released glucocorticoid at a constant rate for 21 days. Mice that received the pellets had twice as much white and brown fat, as well as much higher insulin levels, regardless of their unchanged and still-healthy diet.
In the second study, they used tagged proteins as markers to monitor the daily fluctuations of a protein that regulates fat cell production and circadian gene expression in mouse fat cell precursors. The results showed “that fat cell precursors commit to becoming fat cells only during the circadian cycle phase corresponding to evening in humans,” they said in a written statement.
“Every cell in our body has an intrinsic cell clock, just like the fat cells, and we have a master clock in our brain, which controls hormone secretion,” said senior author Mary Teruel of Cornell University. “A lot of forces are working against a healthy metabolism when we are out of circadian rhythm. The more we understand, the more likely we will be able to do something about it.”
So if you’re stressing out that the scale is or isn’t moving in the direction you want, you could be standing in your own way. Take a chill pill.
Who can smell cancer? The locust nose
If you need to smell some gas, there’s nothing better than a nose. Just ask a scientist: “Noses are still state of the art,” said Debajit Saha, PhD, of Michigan State University. “There’s really nothing like them when it comes to gas sensing.”
And when it comes to noses, dogs are best, right? After all, there’s a reason we don’t have bomb-sniffing wombats and drug-sniffing ostriches. Dogs are better. Better, but not perfect. And if they’re not perfect, then human technology can do better.
Enter the electronic nose. Which is better than dogs … except that it isn’t. “People have been working on ‘electronic noses’ for more than 15 years, but they’re still not close to achieving what biology can do seamlessly,” Dr. Saha explained in a statement from the university.
Which brings us back to dogs. If you want to detect early-stage cancer using smell, you go to the dogs, right? Nope.
Here’s Christopher Contag, PhD, also of Michigan State, who recruited Dr. Saha to the university: “I told him, ‘When you come here, we’ll detect cancer. I’m sure your locusts can do it.’ ”
Yes, locusts. Dr. Contag and his research team were looking at mouth cancers and noticed that different cell lines had different appearances. Then they discovered that those different-looking cell lines produced different metabolites, some of which were volatile.
Enter Dr. Saha’s locusts. They were able to tell the difference between normal cells and cancer cells and could even distinguish between the different cell lines. And how they were able to share this information? Not voluntarily, that’s for sure. The researchers attached electrodes to the insects’ brains and recorded their responses to gas samples from both healthy and cancer cells. Those brain signals were then used to create chemical profiles of the different cells. Piece of cake.
The whole getting-electrodes-attached-to-their-brains thing seemed at least a bit ethically ambiguous, so we contacted the locusts’ PR office, which offered some positive spin: “Humans get their early cancer detection and we get that whole swarms-that-devour-entire-countrysides thing off our backs. Win win.”
Bad news for vampires everywhere
Pop culture has been extraordinarily kind to the vampire. A few hundred years ago, vampires were demon-possessed, often-inhuman monsters. Now? They’re suave, sophisticated, beautiful, and oh-so dramatic and angst-filled about their “curse.” Drink a little human blood, live and look young forever. Such monsters they are.
It does make sense in a morbid sort of way. An old person receiving the blood of the young does seem like a good idea for rejuvenation, right? A team of Ukrainian researchers sought to find out, conducting a study in which older mice were linked with young mice via heterochronic parabiosis. For 3 months, old-young mice pairs were surgically connected and shared blood. After 3 months, the mice were disconnected from each other and the effects of the blood link were studied.
For all the vampire enthusiasts out there, we have bad news and worse news. The bad news first: The older mice received absolutely no benefit from heterochronic parabiosis. No youthfulness, no increased lifespan, nothing. The worse news is that the younger mice were adversely affected by the older blood. They aged more and experienced a shortened lifespan, even after the connection was severed. The old blood, according to the investigators, contains factors capable of inducing aging in younger mice, but the opposite is not true. Further research into aging, they added, should focus on suppressing the aging factors in older blood.
Of note, the paper was written by doctors who are currently refugees, fleeing the war in Ukraine. We don’t want to speculate on the true cause of the war, but we’re onto you, Putin. We know you wanted the vampire research for yourself, but it won’t work. Your dream of becoming Vlad “Dracula” Putin will never come to pass.
Hearing is not always believing
Have you ever heard yourself on a voice mail, or from a recording you did at work? No matter how good you sound, you still might feel like the recording sounds nothing like you. It may even cause low self-esteem for those who don’t like how their voice sounds or don’t recognize it when it’s played back to them.
Since one possible symptom of schizophrenia is not recognizing one’s own speech and having a false sense of control over actions, and those with schizophrenia may hallucinate or hear voices, not being able to recognize their own voices may be alarming.
A recent study on the sense of agency, or sense of control, involved having volunteers speak with different pitches in their voices and then having it played back to them to gauge their reactions.
“Our results demonstrate that hearing one’s own voice is a critical factor to increased self-agency over speech. In other words, we do not strongly feel that ‘I’ am generating the speech if we hear someone else’s voice as an outcome of the speech. Our study provides empirical evidence of the tight link between the sense of agency and self-voice identity,” lead author Ryu Ohata, PhD, of the University of Tokyo, said in a written statement.
As social interaction becomes more digital through platforms such as FaceTime, Zoom, and voicemail, especially since the pandemic has promoted social distancing, it makes sense that people may be more aware and more surprised by how they sound on recordings.
So, if you ever promised someone something that you don’t want to do, and they play it back to you from the recording you made, maybe you can just say you don’t recognize the voice. And if it’s not you, then you don’t have to do it.
Stress, meet weight gain. Weight gain, meet stress
You’re not eating differently and you’re keeping active, but your waistline is expanding. How is that happening? Since eating healthy and exercising shouldn’t make you gain weight, there may be a hidden factor getting in your way. Stress. The one thing that can have a grip on your circadian rhythm stronger than any bodybuilder.
Investigators at Weill Cornell Medicine published two mouse studies that suggest stress and other factors that throw the body’s circadian clocks out of rhythm may contribute to weight gain.
In the first study, the researchers imitated disruptive condition effects like high cortisol exposure and chronic stress by implanting pellets under the skin that released glucocorticoid at a constant rate for 21 days. Mice that received the pellets had twice as much white and brown fat, as well as much higher insulin levels, regardless of their unchanged and still-healthy diet.
In the second study, they used tagged proteins as markers to monitor the daily fluctuations of a protein that regulates fat cell production and circadian gene expression in mouse fat cell precursors. The results showed “that fat cell precursors commit to becoming fat cells only during the circadian cycle phase corresponding to evening in humans,” they said in a written statement.
“Every cell in our body has an intrinsic cell clock, just like the fat cells, and we have a master clock in our brain, which controls hormone secretion,” said senior author Mary Teruel of Cornell University. “A lot of forces are working against a healthy metabolism when we are out of circadian rhythm. The more we understand, the more likely we will be able to do something about it.”
So if you’re stressing out that the scale is or isn’t moving in the direction you want, you could be standing in your own way. Take a chill pill.
Who can smell cancer? The locust nose
If you need to smell some gas, there’s nothing better than a nose. Just ask a scientist: “Noses are still state of the art,” said Debajit Saha, PhD, of Michigan State University. “There’s really nothing like them when it comes to gas sensing.”
And when it comes to noses, dogs are best, right? After all, there’s a reason we don’t have bomb-sniffing wombats and drug-sniffing ostriches. Dogs are better. Better, but not perfect. And if they’re not perfect, then human technology can do better.
Enter the electronic nose. Which is better than dogs … except that it isn’t. “People have been working on ‘electronic noses’ for more than 15 years, but they’re still not close to achieving what biology can do seamlessly,” Dr. Saha explained in a statement from the university.
Which brings us back to dogs. If you want to detect early-stage cancer using smell, you go to the dogs, right? Nope.
Here’s Christopher Contag, PhD, also of Michigan State, who recruited Dr. Saha to the university: “I told him, ‘When you come here, we’ll detect cancer. I’m sure your locusts can do it.’ ”
Yes, locusts. Dr. Contag and his research team were looking at mouth cancers and noticed that different cell lines had different appearances. Then they discovered that those different-looking cell lines produced different metabolites, some of which were volatile.
Enter Dr. Saha’s locusts. They were able to tell the difference between normal cells and cancer cells and could even distinguish between the different cell lines. And how they were able to share this information? Not voluntarily, that’s for sure. The researchers attached electrodes to the insects’ brains and recorded their responses to gas samples from both healthy and cancer cells. Those brain signals were then used to create chemical profiles of the different cells. Piece of cake.
The whole getting-electrodes-attached-to-their-brains thing seemed at least a bit ethically ambiguous, so we contacted the locusts’ PR office, which offered some positive spin: “Humans get their early cancer detection and we get that whole swarms-that-devour-entire-countrysides thing off our backs. Win win.”
Bad news for vampires everywhere
Pop culture has been extraordinarily kind to the vampire. A few hundred years ago, vampires were demon-possessed, often-inhuman monsters. Now? They’re suave, sophisticated, beautiful, and oh-so dramatic and angst-filled about their “curse.” Drink a little human blood, live and look young forever. Such monsters they are.
It does make sense in a morbid sort of way. An old person receiving the blood of the young does seem like a good idea for rejuvenation, right? A team of Ukrainian researchers sought to find out, conducting a study in which older mice were linked with young mice via heterochronic parabiosis. For 3 months, old-young mice pairs were surgically connected and shared blood. After 3 months, the mice were disconnected from each other and the effects of the blood link were studied.
For all the vampire enthusiasts out there, we have bad news and worse news. The bad news first: The older mice received absolutely no benefit from heterochronic parabiosis. No youthfulness, no increased lifespan, nothing. The worse news is that the younger mice were adversely affected by the older blood. They aged more and experienced a shortened lifespan, even after the connection was severed. The old blood, according to the investigators, contains factors capable of inducing aging in younger mice, but the opposite is not true. Further research into aging, they added, should focus on suppressing the aging factors in older blood.
Of note, the paper was written by doctors who are currently refugees, fleeing the war in Ukraine. We don’t want to speculate on the true cause of the war, but we’re onto you, Putin. We know you wanted the vampire research for yourself, but it won’t work. Your dream of becoming Vlad “Dracula” Putin will never come to pass.
Hearing is not always believing
Have you ever heard yourself on a voice mail, or from a recording you did at work? No matter how good you sound, you still might feel like the recording sounds nothing like you. It may even cause low self-esteem for those who don’t like how their voice sounds or don’t recognize it when it’s played back to them.
Since one possible symptom of schizophrenia is not recognizing one’s own speech and having a false sense of control over actions, and those with schizophrenia may hallucinate or hear voices, not being able to recognize their own voices may be alarming.
A recent study on the sense of agency, or sense of control, involved having volunteers speak with different pitches in their voices and then having it played back to them to gauge their reactions.
“Our results demonstrate that hearing one’s own voice is a critical factor to increased self-agency over speech. In other words, we do not strongly feel that ‘I’ am generating the speech if we hear someone else’s voice as an outcome of the speech. Our study provides empirical evidence of the tight link between the sense of agency and self-voice identity,” lead author Ryu Ohata, PhD, of the University of Tokyo, said in a written statement.
As social interaction becomes more digital through platforms such as FaceTime, Zoom, and voicemail, especially since the pandemic has promoted social distancing, it makes sense that people may be more aware and more surprised by how they sound on recordings.
So, if you ever promised someone something that you don’t want to do, and they play it back to you from the recording you made, maybe you can just say you don’t recognize the voice. And if it’s not you, then you don’t have to do it.
Popliteal plaques
Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733

Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Both a Wood lamp examination and a potassium hydroxide (KOH) prep returned negative results. Those findings, combined with the patient’s month-long antifungal medication adherence, helped to rule out other diagnoses. Based on history and examination, the patient was diagnosed with erythrasma.
Erythrasma is a skin infection caused by the gram-positive bacteria Corynebacterium minutissimum1 that usually manifests in moist intertriginous areas. Sometimes it is secondary to fungal or yeast infections, local skin irritation due to friction, or due to maceration of the skin from persistent moisture. The Wood lamp examination can show coral-red fluorescence in erythrasma, but recent bathing (as in this case) may limit this finding.1
The differential diagnosis of erythematous plaques in an intertriginous area includes inverse psoriasis. However, this patient had no nail changes, joint difficulties, or other rashes consistent with psoriasis. Macerated, erythematous inflammatory changes in intertriginous areas are always concerning for fungal infections (eg, yeast infection, tinea corporis), especially with the presence of any scale. In this case, the patient’s medication regimen helped to rule out these types of conditions.
First-line therapy for erythrasma includes topical antibiotics: clindamycin, erythromycin, mupirocin, and fusidic acid. Systemic antibiotics in the tetracycline family and macrolides may also be used but have a higher risk of adverse effects. Keeping the affected area dry is a useful adjunct to pharmacologic therapy.
The patient was treated with topical clindamycin bid for 7 days. By her 2-month follow-up appointment, there were no residual skin changes. Had the plaques persisted, the possibility of inverse psoriasis would have been revisited, with either presumptive treatment prescribed or biopsy performed to establish the diagnosis.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
1. Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment. Cureus. 2020;12:e10733. doi: 10.7759/cureus.10733
Plasma biomarkers predict COVID’s neurological sequelae
SAN DIEGO – Even after recovery of an acute COVID-19 infection, some patients experience extended or even long-term symptoms that can range from mild to debilitating. Some of these symptoms are neurological: headaches, brain fog, cognitive impairment, loss of taste or smell, and even cerebrovascular complications such stroke. There are even hints that COVID-19 infection could lead to future neurodegeneration.
Those issues have prompted efforts to identify biomarkers that can help track and monitor neurological complications of COVID-19. “Throughout the course of the pandemic, it has become apparent that COVID-19 can cause various neurological symptoms. Because of this, ,” Jennifer Cooper said during a lecture at the Alzheimer’s Association International Conference. She presented new research suggesting that neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) may prove useful.
Ms. Cooper is a master’s degree student at the University of British Columbia and Canada.
Looking for sensitivity and specificity in plasma biomarkers
The researchers turned to plasma-based markers because they can reflect underlying pathology in the central nervous system. They focused on NfL, which reflects axonal damage, and GFAP, which is a marker of astrocyte activation.
The researchers analyzed data from 209 patients with COVID-19 who were admitted to the Vancouver (B.C.) General Hospital intensive care unit. Sixty-four percent were male, and the median age was 61 years. Sixty percent were ventilated, and 17% died.
The researchers determined if an individual patient’s biomarker level at hospital admission fell within a normal biomarker reference interval. A total of 53% had NfL levels outside the normal range, and 42% had GFAP levels outside the normal range. In addition, 31% of patients had both GFAP and NfL levels outside of the normal range.
Among all patients, 12% experienced ischemia, 4% hemorrhage, 2% seizures, and 10% degeneration.
At admission, NfL predicted a neurological complication with an area under the curve (AUC) of 0.702. GFAP had an AUC of 0.722. In combination, they had an AUC of 0.743. At 1 week, NfL had an AUC of 0.802, GFAP an AUC of 0.733, and the combination an AUC of 0.812.
Using age-specific cutoff values, the researchers found increased risks for neurological complications at admission (NfL odds ratio [OR], 2.9; GFAP OR, 1.6; combined OR, 2.1) and at 1 week (NfL OR, not significant; GFAP OR, 4.8; combined OR, 6.6). “We can see that both NFL and GFAP have utility in detecting neurological complications. And combining both of our markers improves detection at both time points. NfL is a marker that provides more sensitivity, where in this cohort GFAP is a marker that provides a little bit more specificity,” said Ms. Cooper.
Will additional biomarkers help?
The researchers are continuing to follow up patients at 6 months and 18 months post diagnosis, using neuropsychiatric tests and additional biomarker analysis, as well as PET and MRI scans. The patient sample is being expanded to those in the general hospital ward and some who were not hospitalized.
During the Q&A session, Ms. Cooper was asked if the group had collected reference data from patients who were admitted to the ICU with non-COVID disease. She responded that the group has some of that data, but as the pandemic went on they had difficulty finding patients who had never been infected with COVID to serve as reliable controls. To date, they have identified 33 controls who had a respiratory condition when admitted to the ICU. “What we see is the neurological biomarker levels in COVID are slightly lower than those with another respiratory condition in the ICU. But the data has a massive spread and the significance is very small between the two groups,” said Ms. Cooper.
Unanswered questions
The study is interesting, but leaves a lot of unanswered questions, according to Wiesje van der Flier, PhD, who moderated the session where the study was presented. “There are a lot of unknowns still: Will [the biomarkers] become normal again, once the COVID is over? Also, there was an increased risk, but it was not a one-to-one correspondence, so you can also have the increased markers but not have the neurological signs or symptoms. So I thought there were lots of questions as well,” said Dr. van der Flier, professor of neurology at Amsterdam University Medical Center.
She noted that researchers at her institution in Amsterdam have observed similar relationships, and that the associations between neurological complications and plasma biomarkers over time will be an important topic of study.
The work could provide more information on neurological manifestations of long COVID, such as long-haul fatigue. “You might also think that’s some response in their brain. It would be great if we could actually capture that [using biomarkers],” said Dr. van der Flier.
Ms. Cooper and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – Even after recovery of an acute COVID-19 infection, some patients experience extended or even long-term symptoms that can range from mild to debilitating. Some of these symptoms are neurological: headaches, brain fog, cognitive impairment, loss of taste or smell, and even cerebrovascular complications such stroke. There are even hints that COVID-19 infection could lead to future neurodegeneration.
Those issues have prompted efforts to identify biomarkers that can help track and monitor neurological complications of COVID-19. “Throughout the course of the pandemic, it has become apparent that COVID-19 can cause various neurological symptoms. Because of this, ,” Jennifer Cooper said during a lecture at the Alzheimer’s Association International Conference. She presented new research suggesting that neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) may prove useful.
Ms. Cooper is a master’s degree student at the University of British Columbia and Canada.
Looking for sensitivity and specificity in plasma biomarkers
The researchers turned to plasma-based markers because they can reflect underlying pathology in the central nervous system. They focused on NfL, which reflects axonal damage, and GFAP, which is a marker of astrocyte activation.
The researchers analyzed data from 209 patients with COVID-19 who were admitted to the Vancouver (B.C.) General Hospital intensive care unit. Sixty-four percent were male, and the median age was 61 years. Sixty percent were ventilated, and 17% died.
The researchers determined if an individual patient’s biomarker level at hospital admission fell within a normal biomarker reference interval. A total of 53% had NfL levels outside the normal range, and 42% had GFAP levels outside the normal range. In addition, 31% of patients had both GFAP and NfL levels outside of the normal range.
Among all patients, 12% experienced ischemia, 4% hemorrhage, 2% seizures, and 10% degeneration.
At admission, NfL predicted a neurological complication with an area under the curve (AUC) of 0.702. GFAP had an AUC of 0.722. In combination, they had an AUC of 0.743. At 1 week, NfL had an AUC of 0.802, GFAP an AUC of 0.733, and the combination an AUC of 0.812.
Using age-specific cutoff values, the researchers found increased risks for neurological complications at admission (NfL odds ratio [OR], 2.9; GFAP OR, 1.6; combined OR, 2.1) and at 1 week (NfL OR, not significant; GFAP OR, 4.8; combined OR, 6.6). “We can see that both NFL and GFAP have utility in detecting neurological complications. And combining both of our markers improves detection at both time points. NfL is a marker that provides more sensitivity, where in this cohort GFAP is a marker that provides a little bit more specificity,” said Ms. Cooper.
Will additional biomarkers help?
The researchers are continuing to follow up patients at 6 months and 18 months post diagnosis, using neuropsychiatric tests and additional biomarker analysis, as well as PET and MRI scans. The patient sample is being expanded to those in the general hospital ward and some who were not hospitalized.
During the Q&A session, Ms. Cooper was asked if the group had collected reference data from patients who were admitted to the ICU with non-COVID disease. She responded that the group has some of that data, but as the pandemic went on they had difficulty finding patients who had never been infected with COVID to serve as reliable controls. To date, they have identified 33 controls who had a respiratory condition when admitted to the ICU. “What we see is the neurological biomarker levels in COVID are slightly lower than those with another respiratory condition in the ICU. But the data has a massive spread and the significance is very small between the two groups,” said Ms. Cooper.
Unanswered questions
The study is interesting, but leaves a lot of unanswered questions, according to Wiesje van der Flier, PhD, who moderated the session where the study was presented. “There are a lot of unknowns still: Will [the biomarkers] become normal again, once the COVID is over? Also, there was an increased risk, but it was not a one-to-one correspondence, so you can also have the increased markers but not have the neurological signs or symptoms. So I thought there were lots of questions as well,” said Dr. van der Flier, professor of neurology at Amsterdam University Medical Center.
She noted that researchers at her institution in Amsterdam have observed similar relationships, and that the associations between neurological complications and plasma biomarkers over time will be an important topic of study.
The work could provide more information on neurological manifestations of long COVID, such as long-haul fatigue. “You might also think that’s some response in their brain. It would be great if we could actually capture that [using biomarkers],” said Dr. van der Flier.
Ms. Cooper and Dr. van der Flier have no relevant financial disclosures.
SAN DIEGO – Even after recovery of an acute COVID-19 infection, some patients experience extended or even long-term symptoms that can range from mild to debilitating. Some of these symptoms are neurological: headaches, brain fog, cognitive impairment, loss of taste or smell, and even cerebrovascular complications such stroke. There are even hints that COVID-19 infection could lead to future neurodegeneration.
Those issues have prompted efforts to identify biomarkers that can help track and monitor neurological complications of COVID-19. “Throughout the course of the pandemic, it has become apparent that COVID-19 can cause various neurological symptoms. Because of this, ,” Jennifer Cooper said during a lecture at the Alzheimer’s Association International Conference. She presented new research suggesting that neurofilament light (NfL) and glial fibrillary acidic protein (GFAP) may prove useful.
Ms. Cooper is a master’s degree student at the University of British Columbia and Canada.
Looking for sensitivity and specificity in plasma biomarkers
The researchers turned to plasma-based markers because they can reflect underlying pathology in the central nervous system. They focused on NfL, which reflects axonal damage, and GFAP, which is a marker of astrocyte activation.
The researchers analyzed data from 209 patients with COVID-19 who were admitted to the Vancouver (B.C.) General Hospital intensive care unit. Sixty-four percent were male, and the median age was 61 years. Sixty percent were ventilated, and 17% died.
The researchers determined if an individual patient’s biomarker level at hospital admission fell within a normal biomarker reference interval. A total of 53% had NfL levels outside the normal range, and 42% had GFAP levels outside the normal range. In addition, 31% of patients had both GFAP and NfL levels outside of the normal range.
Among all patients, 12% experienced ischemia, 4% hemorrhage, 2% seizures, and 10% degeneration.
At admission, NfL predicted a neurological complication with an area under the curve (AUC) of 0.702. GFAP had an AUC of 0.722. In combination, they had an AUC of 0.743. At 1 week, NfL had an AUC of 0.802, GFAP an AUC of 0.733, and the combination an AUC of 0.812.
Using age-specific cutoff values, the researchers found increased risks for neurological complications at admission (NfL odds ratio [OR], 2.9; GFAP OR, 1.6; combined OR, 2.1) and at 1 week (NfL OR, not significant; GFAP OR, 4.8; combined OR, 6.6). “We can see that both NFL and GFAP have utility in detecting neurological complications. And combining both of our markers improves detection at both time points. NfL is a marker that provides more sensitivity, where in this cohort GFAP is a marker that provides a little bit more specificity,” said Ms. Cooper.
Will additional biomarkers help?
The researchers are continuing to follow up patients at 6 months and 18 months post diagnosis, using neuropsychiatric tests and additional biomarker analysis, as well as PET and MRI scans. The patient sample is being expanded to those in the general hospital ward and some who were not hospitalized.
During the Q&A session, Ms. Cooper was asked if the group had collected reference data from patients who were admitted to the ICU with non-COVID disease. She responded that the group has some of that data, but as the pandemic went on they had difficulty finding patients who had never been infected with COVID to serve as reliable controls. To date, they have identified 33 controls who had a respiratory condition when admitted to the ICU. “What we see is the neurological biomarker levels in COVID are slightly lower than those with another respiratory condition in the ICU. But the data has a massive spread and the significance is very small between the two groups,” said Ms. Cooper.
Unanswered questions
The study is interesting, but leaves a lot of unanswered questions, according to Wiesje van der Flier, PhD, who moderated the session where the study was presented. “There are a lot of unknowns still: Will [the biomarkers] become normal again, once the COVID is over? Also, there was an increased risk, but it was not a one-to-one correspondence, so you can also have the increased markers but not have the neurological signs or symptoms. So I thought there were lots of questions as well,” said Dr. van der Flier, professor of neurology at Amsterdam University Medical Center.
She noted that researchers at her institution in Amsterdam have observed similar relationships, and that the associations between neurological complications and plasma biomarkers over time will be an important topic of study.
The work could provide more information on neurological manifestations of long COVID, such as long-haul fatigue. “You might also think that’s some response in their brain. It would be great if we could actually capture that [using biomarkers],” said Dr. van der Flier.
Ms. Cooper and Dr. van der Flier have no relevant financial disclosures.
AT AAIC 2022
Cardiorespiratory fitness key to longevity for all?
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
Cardiorespiratory fitness emerged as a stronger predictor of all-cause mortality than did any traditional risk factor across the spectrum of age, sex, and race in a modeling study that included more than 750,000 U.S. veterans.
In addition, mortality risk was cut in half if individuals achieved a moderate cardiorespiratory fitness (CRF) level – that is, by meeting the current U.S. physical activity recommendations of 150 minutes per week, the authors note.
Furthermore, contrary to some previous research, “extremely high” fitness was not associated with an increased risk for mortality in the study, published online in the Journal of the American College of Cardiology.
“This study has been 15 years in the making,” lead author Peter Kokkinos, PhD, Rutgers University, New Brunswick, N.J., and the VA Medical Center, Washington, told this news organization. “We waited until we had the computer power and the right people to really assess this. We wanted to be very liberal in excluding patients we thought might contaminate the results, such as those with cardiovascular disease in the 6 months prior to a stress test.”
Figuring the time was right, the team analyzed data from the VA’s Exercise Testing and Health Outcomes Study (ETHOS) on individuals aged 30-95 years who underwent exercise treadmill tests between 1999 and 2020.
After exclusions, 750,302 individuals (from among 822,995) were included: 6.5% were women; 73.7% were White individuals; 19% were African American individuals; 4.7% were Hispanic individuals; and 2.1% were Native American, Asian, or Hawaiian individuals. Septuagenarians made up 14.7% of the cohort, and octogenarians made up 3.6%.
CRF categories for age and sex were determined by the peak metabolic equivalent of task (MET) achieved during the treadmill test. One MET is the energy spent at rest – that is the basal metabolic rate.
Although some physicians may resist putting patients through a stress test, “the amount of information we get from it is incredible,” Dr. Kokkinos noted. “We get blood pressure, we get heart rate, we get a response if you’re not doing exercise. This tells us a lot more than having you sit around so we can measure resting heart rate and blood pressure.”
Lowest mortality at 14.0 METs
During a median follow-up of 10.2 years (7,803,861 person-years), 23% of participants died, for an average of 22.4 events per 1,000 person-years.
Higher exercise capacity was inversely related to mortality risk across the cohort and within each age category. Specifically, every 1 MET increase in exercise capacity yielded an adjusted hazard ratio for mortality of 0.86 (95% confidence interval, 0.85-0.87; P < .001) for the entire cohort and similar HRs by sex and race.
The mortality risk for the least-fit individuals (20th percentile) was fourfold higher than for extremely fit individuals (HR, 4.09; 95% CI, 3.90-4.20), with the lowest mortality risk at about 14.0 METs for both men (HR, 0.24; 95% CI, 0.23-0.25) and women (HR, 0.23; 95% CI, 0.17-0.29). Extremely high CRF did not increase the risk.
In addition, at 20 years of follow-up, about 80% of men and 95% of women in the highest CRF category (98th percentile) were alive vs. less than 40% of men and approximately 75% of women in the least fit CRF category.
“We know CRF declines by 1% per year after age 30,” Dr. Kokkinos said. “But the age-related decline is cut in half if you are fit, meaning that an expected 10% decline over a decade will be only a 5% decline if you stay active. We cannot stop or reverse the decline, but we can kind of put the brakes on, and that’s a reason for clinicians to continue to encourage fitness.”
Indeed, “improving CRF should be considered a target in CVD prevention, similar to improving lipids, blood sugar, blood pressure, and weight,” Carl J. Lavie, MD, Ochsner Health, New Orleans, and colleagues affirm in a related editorial.
‘A difficult battle’
But that may not happen any time soon. “Unfortunately, despite having been recognized in an American Heart Association scientific statement as a clinical vital sign, aerobic fitness is undervalued and underutilized,” Claudio Gil Araújo, MD, PhD, research director of the Exercise Medicine Clinic-CLINIMEX, Rio de Janeiro, told this news organization.
Dr. Araújo led a recent study showing that the ability to stand on one leg for at least 10 seconds is strongly linked to the risk for death over the next 7 years.
Although physicians should be encouraging fitness, he said that “a substantial part of health professionals are physically unfit and feel uncomfortable talking about and prescribing exercise for their patients. Also, physicians tend to be better trained in treating diseases (using medications and/or prescribing procedures) than in preventing diseases by stimulating adoption of healthy habits. So, this a long road and a difficult battle.”
Nonetheless, he added, “Darwin said a long time ago that only the fittest will survive. If Darwin could read this study, he would surely smile.”
No commercial funding or conflicts of interest related to the study were reported. Dr. Lavie previously served as a speaker and consultant for PAI Health on their PAI (Personalized Activity Intelligence) applications.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY