User login
Drug-resistant epilepsy needs earlier surgical referral
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
FROM EPILEPSIA
Funding of cosmetic clinical trials linked to racial/ethnic disparity
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.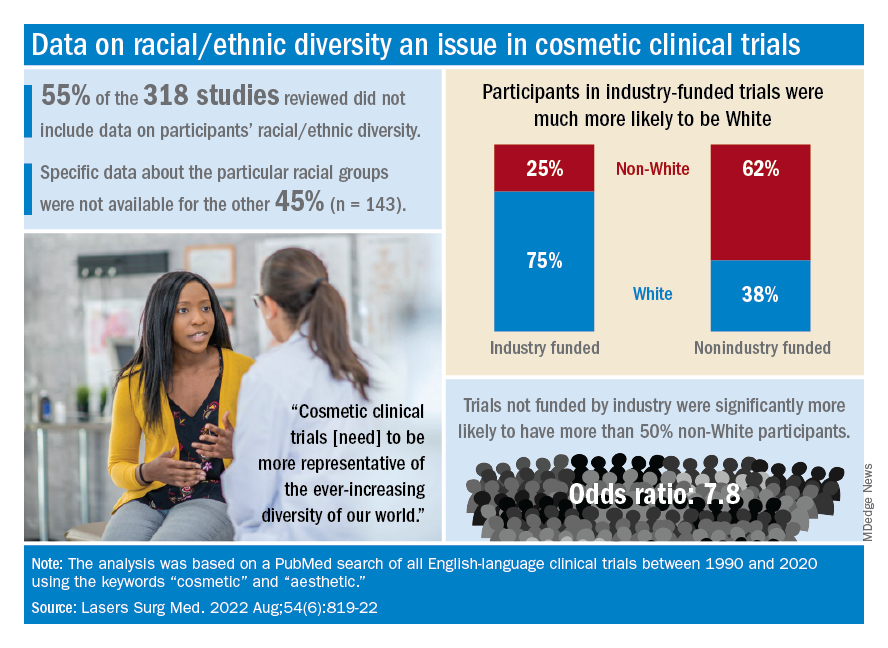
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
Individuals of nonwhite race/ethnicity are not underrepresented in cosmetic clinical trials, according to a recent literature review. The explanation for those contradictory conclusions comes down to money, or, more specifically, the source of the money.
Among the cosmetic studies funded by industry, non-Whites represented about 25% of the patient populations. That proportion, however, rose to 62% for studies that were funded by universities/governments or had no funding source reported, Lisa Akintilo, MD, and associates said in their review.
“Lack of inclusion of diverse patient populations is both a medical and moral issue as conclusions of such homogeneous studies may not be generalizable. In the realm of cosmetic dermatology, diverse research cohorts are needed to identify potential disparities in therapies for cosmetic concerns and fully investigate effective treatments for all,” wrote Dr. Akintilo of New York University and coauthors.
Data from the U.S. Census show that non-Hispanic Whites made up 60% of the population in 2019, with that proportion falling to about 50% by 2045, the investigators noted. A report from the American Society of Plastic Surgeons showed that about 34% of cosmetic patients identified as skin of color in 2020.
The availability of data was an issue in the review of the literature from 1990 to 2020, as 55% of the 318 randomized controlled trials that were reviewed did not include any information on racial/ethnic diversity and the other 143 studies offered only enough to determine White/non-White status, they explained.
That limitation meant that those 143 studies had to form the basis of the funding analysis, which also indicated that the studies with funding outside of industry were significantly more likely (odds ratio, 7.8) to have more than 50% non-White participants, compared with the industry-funded trials. The projects with industry backing, however, had a larger mean sample size than did those without: 139 vs. 81, Dr. Akintilo and associates said.
“The protocols of cosmetic trials should be questioned, as many target Caucasian‐centric treatment goals that may not be in alignment with the goals of skin of color patients,” they wrote. “It is important for cosmetic providers to recognize the well-established anatomical variations between different races and ethnicities and how they can inform desired cosmetic procedures.”
The investigators said that they had no conflicts of interest.
FROM LASERS IN SURGERY AND MEDICINE
Vaginal birth possible in 50% of women with low-lying placenta
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
About half of women with an asymptomatic low-lying placenta in the third trimester and an internal os distance of 11-20 mm can have a vaginal birth after 35 weeks without any higher risk of severe complications than if they had undergone elective cesarean delivery, a new study indicates.
The retrospective analysis of 128,233 births between 2007 and 2012 at six hospitals in France showed that of the 171 women (0.13%) with low-lying placenta, 70 underwent a trial of labor, and 101 had an elective cesarean delivery. The vaginal delivery rate was 50.0% in the group of 38 women with an internal os distance of 11-20 mm, and 18.5% among 27 women with an internal os distance of 1-10 mm.
Similar rates of severe postpartum hemorrhage (PPH) were observed whether the patient opted for a trial of labor or for elective cesarean delivery (22.9% vs. 23.0%), regardless of maternal age, prepregnancy body mass index, nulliparity, and previous cesarean delivery. Rates of severe maternal and neonatal morbidity were 2.9% vs. 2.0%, and 12.9% vs. 9.9%, respectively, both nonsignificantly different, the study showed.
These findings confirm results from an earlier study and could reduce the incidence of unnecessary cesarean deliveries in women with low-lying placenta, said researchers led by Loïc Sentilhes, MD, PhD, of the department of obstetrics and gynecology at Bordeaux (France) University Hospital Center.
“Our results support a policy of offering a trial of labor to women with low-lying placenta at or after 35 weeks of gestation and a distance of 11-20 mm between the placental edge and the internal os on ultrasonography,” they wrote in Obstetrics & Gynecology.
Although an internal os distance of 1-10 mm did not increase the incidence of severe PPH or other severe maternal morbidity, 80% of these patients went on to have an emergency cesarean section. For this reason, the high risk of emergency cesarean should be discussed during shared decision-making, the study authors said.
Avoiding unnecessary cesarean deliveries is crucial to limiting the occurrence of low-lying placenta, placenta previa, vasa previa, and placenta accreta spectrum in subsequent pregnancies, Dr. Sentilhes told this news organization. “We hope that our results will help caregivers to objectively advise their patients with low-lying placenta regarding the choice of their mode of delivery.”
“This is further evidence to reassure clinicians that managing such patients with labor is a reasonable approach,” said Aaron B. Caughey, MD, MPH, PhD, professor and chair of the department of obstetrics and gynecology at Oregon Health & Science University, Portland. He was not involved in the study.
Many obstetricians have practiced this for decades, noted Dr. Caughey, associate dean for women’s health research and policy at Oregon Health. “We manage these patients expectantly with a plan for a trial of labor.”
“I am absolutely in agreement,” said Sarah L. Pachtman, MD, an obstetrician-gynecologist at Long Island Jewish Medical Center in New York, who is an independent expert. Dr. Pachtman noted that since she works at a hospital equipped for emergency cesarean deliveries, “I can get a baby out in 5 minutes if necessary.”
Dr. Pachtman’s practice consists of “a very large population of women who strongly desire vaginal delivery.
“It’s a better recovery for them, avoids the risks of abdominal surgery, gives them quicker skin-to-skin contact with their newborn and they can start breastfeeding sooner,” she said in an interview. “And the risk of bleeding is actually lower compared to elective cesarean delivery.”
Deciding on the mode of delivery should be based on patient preference and physician comfort, shared decision-making, and where the patient delivers, Dr. Pachtman said. “If the placental edge is between 1 mm and 10 mm or abutting the internal os, I explain to the patient that there is a risk of bleeding even before labor starts, and they would most likely want to choose an elective cesarean delivery.”
Although low-lying placenta can be associated with significant maternal and neonatal morbidity and mortality, particularly when diagnosed at delivery, universal cervical length screening during routine anatomic ultrasound is identifying the presence of low-lying placenta much earlier in pregnancy.
“We’re identifying it more, following it more, and reporting it more,” Dr. Pachtman said. And in the vast majority of patients, she emphasized, the 28-week follow-up transvaginal ultrasound shows that the low-lying placenta has resolved.
Dr. Sentilhes reported a relationship with Ferring Laboratories. No other study authors disclosed having conflicts of interest. Dr. Caughey and Dr. Pachtman reported having no conflicts of interest.
FROM OBSTETRICS & GYNECOLOGY
Vonoprazan-based therapy for resistant H. pylori superior to standard care
A look at the data behind the FDA approval
Vonoprazan, a potassium-competitive acid blocker, appears to be superior to standard proton pump inhibitor–based therapy in clarithromycin-resistant Helicobacter pylori strains, as well as noninferior to standard care in nonresistant infections, according to a recent study that supported a Food and Drug Administration approval of vonoprazan dual and triple therapies in May 2022.
For decades, H. pylori has been mostly treated by proton pump inhibitor–based triple therapy, which includes a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. However, eradication rates have dropped below 80% in the United States and Europe, according to the authors, mainly because of rising rates of clarithromycin resistance.
Since H. pylori is a leading cause of peptic ulcer, gastric adenocarcinoma, and gastric mucosa–associated lymphoid tissue lymphoma, better eradication methods should be highlighted, researchers led by William Chey, MD, professor of medicine and director of the GI Physiology Laboratory at Michigan Medicine in Ann Arbor, wrote in Gastroenterology.
In a multicenter, randomized, controlled, phase 3 trial, the research team studied 1,046 treatment-naive adults with H. pylori infection at 103 sites in the U.S., the U.K., Bulgaria, the Czech Republic, Hungary, and Poland between December 2019 and January 2021.
The patients were randomized to receive open-label vonoprazan dual therapy or a double-blind triple therapy twice a day for 14 days. The vonoprazan dual therapy consisted of 20 mg of vonoprazan twice daily and 1 gram of amoxicillin three times per day. The triple therapy consisted of 20 mg of vonoprazan or 30 mg of lansoprazole (standard care), each given with 1 gram of amoxicillin and 500 mg of clarithromycin.
The primary outcome assessed noninferiority in eradication rates in patients without clarithromycin- and amoxicillin-resistant strains, with a noninferiority margin of 10%. Secondary outcomes assessed the superiority in eradication rates in clarithromycin-resistant infections, as well as in all patients.
Eradication rates for nonresistant strains were 84.7% for vonoprazan triple therapy and 78.5% for vonoprazan dual therapy, compared with 78.8% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered noninferior to standard therapy.
The eradication rates in clarithromycin-resistant infections were 65.8% for vonoprazan triple therapy and 69.6% in vonoprazan dual therapy, compared with 31.9% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior to standard therapy, with a difference of 33.9 percentage points for triple therapy and 37.7 percentage points for dual therapy.
In all patients, the eradication rates were 80.8% for vonoprazan triple therapy and 77.2% for vonoprazan dual therapy, compared with 68.5% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior, with a difference of 12.3 percentage points for triple therapy and 8.7 percentage points for dual therapy.
Treatment-emergent adverse events were reported in 34.1% of patients in the vonoprazan triple therapy group and 29.9% of patients in the vonoprazan dual therapy group, compared with 34.5% in the lansoprazole triple-therapy group. Most adverse events were mild to moderate.
Serious adverse events occurred in 1.3% of the overall study population, including 1.7% of the vonoprazan triple therapy group, 1.4% of the vonoprazan dual therapy group, and 0.9% of the lansoprazole triple therapy group. None were considered related to the study drugs.
Vonoprazan was approved for the treatment of H. pylori infections by the FDA in May 2022, and had already been approved for treatment of H. pylori infections and other acid-related diseases in several other countries. It decreases intragastric pH and maintains it to a greater degree than that of proton pump inhibitors, which has been associated with higher eradication rates, the authors wrote.
“Optimizing current regimens offers the potential to increase eradication rates and reduce additional antibiotic usage, thereby promoting and improving antimicrobial stewardship,” the study authors wrote.
The study was funded by Phathom Pharmaceuticals, which contributed to the design and conduct of the trial, collection and interpretation of the data, preparation and review of the manuscript, and the decision to submit the manuscript for publication. The study authors declared various conflicts of interest, including some who have received compensation as a consultant, advisory committee member, or employee for Phathom Pharmaceuticals.
Gastric acid inhibition plays a fundamental role for H. pylori eradication. Proton pump inhibitors (PPIs) are generally used, combined with antibiotics, in this scenario. More recently, vonoprazan, a potassium-competitive acid blocker, has been suggested to enhance H. pylori therapy by optimizing gastric acid suppression. However, clinical experience with vonoprazan has been limited to East Asian countries. The study by Chey et al. reports data from the first clinical trial from the United States and Europe, concluding that vonoprazan triple (together with amoxicillin and clarithromycin) and dual (together with amoxicillin) therapies were superior to PPI-based triple therapy, especially in clarithromycin-resistant strains.
However, some aspects deserve to be taken into consideration. The first one is that the cure rate with the standard triple therapy (with lansoprazole) was as low as 68%, underlining what has been known for a long time: This regimen should no longer be considered standard treatment in Europe or the United States and that it should not be recommended in areas with high (>15%) clarithromycin resistance, such as the United States and most European countries.
Secondly, the overall efficacy considering all patients (both with clarithromycin-susceptible and -resistant strains) with vonoprazan dual and triple regimens were of only 77% and 81%, not reaching the recommended target (≥ 90%) for first-line treatment. Therefore, the fair conclusion of the present article should have been not only that vonoprazan regimens are more effective than PPI ones, but also that all of them are insufficiently effective.
Finally, eradication rates in clarithromycin-resistant infections with the vonoprazan regimens (≤ 70%), although superior to those with lansoprazole (32%), were still clearly suboptimal, emphasizing that both PPI and vonoprazan based treatments would be inadequate if used in high-clarithromycin resistance regions.
Javier P. Gisbert, MD, PhD, is with the Hospital Universitario de La Princesa and the Universidad Autónoma de Madrid, both in Madrid. Dr. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen.
A look at the data behind the FDA approval
A look at the data behind the FDA approval
Gastric acid inhibition plays a fundamental role for H. pylori eradication. Proton pump inhibitors (PPIs) are generally used, combined with antibiotics, in this scenario. More recently, vonoprazan, a potassium-competitive acid blocker, has been suggested to enhance H. pylori therapy by optimizing gastric acid suppression. However, clinical experience with vonoprazan has been limited to East Asian countries. The study by Chey et al. reports data from the first clinical trial from the United States and Europe, concluding that vonoprazan triple (together with amoxicillin and clarithromycin) and dual (together with amoxicillin) therapies were superior to PPI-based triple therapy, especially in clarithromycin-resistant strains.
However, some aspects deserve to be taken into consideration. The first one is that the cure rate with the standard triple therapy (with lansoprazole) was as low as 68%, underlining what has been known for a long time: This regimen should no longer be considered standard treatment in Europe or the United States and that it should not be recommended in areas with high (>15%) clarithromycin resistance, such as the United States and most European countries.
Secondly, the overall efficacy considering all patients (both with clarithromycin-susceptible and -resistant strains) with vonoprazan dual and triple regimens were of only 77% and 81%, not reaching the recommended target (≥ 90%) for first-line treatment. Therefore, the fair conclusion of the present article should have been not only that vonoprazan regimens are more effective than PPI ones, but also that all of them are insufficiently effective.
Finally, eradication rates in clarithromycin-resistant infections with the vonoprazan regimens (≤ 70%), although superior to those with lansoprazole (32%), were still clearly suboptimal, emphasizing that both PPI and vonoprazan based treatments would be inadequate if used in high-clarithromycin resistance regions.
Javier P. Gisbert, MD, PhD, is with the Hospital Universitario de La Princesa and the Universidad Autónoma de Madrid, both in Madrid. Dr. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen.
Gastric acid inhibition plays a fundamental role for H. pylori eradication. Proton pump inhibitors (PPIs) are generally used, combined with antibiotics, in this scenario. More recently, vonoprazan, a potassium-competitive acid blocker, has been suggested to enhance H. pylori therapy by optimizing gastric acid suppression. However, clinical experience with vonoprazan has been limited to East Asian countries. The study by Chey et al. reports data from the first clinical trial from the United States and Europe, concluding that vonoprazan triple (together with amoxicillin and clarithromycin) and dual (together with amoxicillin) therapies were superior to PPI-based triple therapy, especially in clarithromycin-resistant strains.
However, some aspects deserve to be taken into consideration. The first one is that the cure rate with the standard triple therapy (with lansoprazole) was as low as 68%, underlining what has been known for a long time: This regimen should no longer be considered standard treatment in Europe or the United States and that it should not be recommended in areas with high (>15%) clarithromycin resistance, such as the United States and most European countries.
Secondly, the overall efficacy considering all patients (both with clarithromycin-susceptible and -resistant strains) with vonoprazan dual and triple regimens were of only 77% and 81%, not reaching the recommended target (≥ 90%) for first-line treatment. Therefore, the fair conclusion of the present article should have been not only that vonoprazan regimens are more effective than PPI ones, but also that all of them are insufficiently effective.
Finally, eradication rates in clarithromycin-resistant infections with the vonoprazan regimens (≤ 70%), although superior to those with lansoprazole (32%), were still clearly suboptimal, emphasizing that both PPI and vonoprazan based treatments would be inadequate if used in high-clarithromycin resistance regions.
Javier P. Gisbert, MD, PhD, is with the Hospital Universitario de La Princesa and the Universidad Autónoma de Madrid, both in Madrid. Dr. Gisbert has served as speaker, consultant, and advisory member for or has received research funding from Mayoly, Allergan, Diasorin, Gebro Pharma, and Richen.
Vonoprazan, a potassium-competitive acid blocker, appears to be superior to standard proton pump inhibitor–based therapy in clarithromycin-resistant Helicobacter pylori strains, as well as noninferior to standard care in nonresistant infections, according to a recent study that supported a Food and Drug Administration approval of vonoprazan dual and triple therapies in May 2022.
For decades, H. pylori has been mostly treated by proton pump inhibitor–based triple therapy, which includes a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. However, eradication rates have dropped below 80% in the United States and Europe, according to the authors, mainly because of rising rates of clarithromycin resistance.
Since H. pylori is a leading cause of peptic ulcer, gastric adenocarcinoma, and gastric mucosa–associated lymphoid tissue lymphoma, better eradication methods should be highlighted, researchers led by William Chey, MD, professor of medicine and director of the GI Physiology Laboratory at Michigan Medicine in Ann Arbor, wrote in Gastroenterology.
In a multicenter, randomized, controlled, phase 3 trial, the research team studied 1,046 treatment-naive adults with H. pylori infection at 103 sites in the U.S., the U.K., Bulgaria, the Czech Republic, Hungary, and Poland between December 2019 and January 2021.
The patients were randomized to receive open-label vonoprazan dual therapy or a double-blind triple therapy twice a day for 14 days. The vonoprazan dual therapy consisted of 20 mg of vonoprazan twice daily and 1 gram of amoxicillin three times per day. The triple therapy consisted of 20 mg of vonoprazan or 30 mg of lansoprazole (standard care), each given with 1 gram of amoxicillin and 500 mg of clarithromycin.
The primary outcome assessed noninferiority in eradication rates in patients without clarithromycin- and amoxicillin-resistant strains, with a noninferiority margin of 10%. Secondary outcomes assessed the superiority in eradication rates in clarithromycin-resistant infections, as well as in all patients.
Eradication rates for nonresistant strains were 84.7% for vonoprazan triple therapy and 78.5% for vonoprazan dual therapy, compared with 78.8% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered noninferior to standard therapy.
The eradication rates in clarithromycin-resistant infections were 65.8% for vonoprazan triple therapy and 69.6% in vonoprazan dual therapy, compared with 31.9% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior to standard therapy, with a difference of 33.9 percentage points for triple therapy and 37.7 percentage points for dual therapy.
In all patients, the eradication rates were 80.8% for vonoprazan triple therapy and 77.2% for vonoprazan dual therapy, compared with 68.5% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior, with a difference of 12.3 percentage points for triple therapy and 8.7 percentage points for dual therapy.
Treatment-emergent adverse events were reported in 34.1% of patients in the vonoprazan triple therapy group and 29.9% of patients in the vonoprazan dual therapy group, compared with 34.5% in the lansoprazole triple-therapy group. Most adverse events were mild to moderate.
Serious adverse events occurred in 1.3% of the overall study population, including 1.7% of the vonoprazan triple therapy group, 1.4% of the vonoprazan dual therapy group, and 0.9% of the lansoprazole triple therapy group. None were considered related to the study drugs.
Vonoprazan was approved for the treatment of H. pylori infections by the FDA in May 2022, and had already been approved for treatment of H. pylori infections and other acid-related diseases in several other countries. It decreases intragastric pH and maintains it to a greater degree than that of proton pump inhibitors, which has been associated with higher eradication rates, the authors wrote.
“Optimizing current regimens offers the potential to increase eradication rates and reduce additional antibiotic usage, thereby promoting and improving antimicrobial stewardship,” the study authors wrote.
The study was funded by Phathom Pharmaceuticals, which contributed to the design and conduct of the trial, collection and interpretation of the data, preparation and review of the manuscript, and the decision to submit the manuscript for publication. The study authors declared various conflicts of interest, including some who have received compensation as a consultant, advisory committee member, or employee for Phathom Pharmaceuticals.
Vonoprazan, a potassium-competitive acid blocker, appears to be superior to standard proton pump inhibitor–based therapy in clarithromycin-resistant Helicobacter pylori strains, as well as noninferior to standard care in nonresistant infections, according to a recent study that supported a Food and Drug Administration approval of vonoprazan dual and triple therapies in May 2022.
For decades, H. pylori has been mostly treated by proton pump inhibitor–based triple therapy, which includes a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole. However, eradication rates have dropped below 80% in the United States and Europe, according to the authors, mainly because of rising rates of clarithromycin resistance.
Since H. pylori is a leading cause of peptic ulcer, gastric adenocarcinoma, and gastric mucosa–associated lymphoid tissue lymphoma, better eradication methods should be highlighted, researchers led by William Chey, MD, professor of medicine and director of the GI Physiology Laboratory at Michigan Medicine in Ann Arbor, wrote in Gastroenterology.
In a multicenter, randomized, controlled, phase 3 trial, the research team studied 1,046 treatment-naive adults with H. pylori infection at 103 sites in the U.S., the U.K., Bulgaria, the Czech Republic, Hungary, and Poland between December 2019 and January 2021.
The patients were randomized to receive open-label vonoprazan dual therapy or a double-blind triple therapy twice a day for 14 days. The vonoprazan dual therapy consisted of 20 mg of vonoprazan twice daily and 1 gram of amoxicillin three times per day. The triple therapy consisted of 20 mg of vonoprazan or 30 mg of lansoprazole (standard care), each given with 1 gram of amoxicillin and 500 mg of clarithromycin.
The primary outcome assessed noninferiority in eradication rates in patients without clarithromycin- and amoxicillin-resistant strains, with a noninferiority margin of 10%. Secondary outcomes assessed the superiority in eradication rates in clarithromycin-resistant infections, as well as in all patients.
Eradication rates for nonresistant strains were 84.7% for vonoprazan triple therapy and 78.5% for vonoprazan dual therapy, compared with 78.8% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered noninferior to standard therapy.
The eradication rates in clarithromycin-resistant infections were 65.8% for vonoprazan triple therapy and 69.6% in vonoprazan dual therapy, compared with 31.9% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior to standard therapy, with a difference of 33.9 percentage points for triple therapy and 37.7 percentage points for dual therapy.
In all patients, the eradication rates were 80.8% for vonoprazan triple therapy and 77.2% for vonoprazan dual therapy, compared with 68.5% for lansoprazole triple therapy. The rates for both vonoprazan therapies were considered superior, with a difference of 12.3 percentage points for triple therapy and 8.7 percentage points for dual therapy.
Treatment-emergent adverse events were reported in 34.1% of patients in the vonoprazan triple therapy group and 29.9% of patients in the vonoprazan dual therapy group, compared with 34.5% in the lansoprazole triple-therapy group. Most adverse events were mild to moderate.
Serious adverse events occurred in 1.3% of the overall study population, including 1.7% of the vonoprazan triple therapy group, 1.4% of the vonoprazan dual therapy group, and 0.9% of the lansoprazole triple therapy group. None were considered related to the study drugs.
Vonoprazan was approved for the treatment of H. pylori infections by the FDA in May 2022, and had already been approved for treatment of H. pylori infections and other acid-related diseases in several other countries. It decreases intragastric pH and maintains it to a greater degree than that of proton pump inhibitors, which has been associated with higher eradication rates, the authors wrote.
“Optimizing current regimens offers the potential to increase eradication rates and reduce additional antibiotic usage, thereby promoting and improving antimicrobial stewardship,” the study authors wrote.
The study was funded by Phathom Pharmaceuticals, which contributed to the design and conduct of the trial, collection and interpretation of the data, preparation and review of the manuscript, and the decision to submit the manuscript for publication. The study authors declared various conflicts of interest, including some who have received compensation as a consultant, advisory committee member, or employee for Phathom Pharmaceuticals.
FROM GASTROENTEROLOGY
Jury out on synbiotics for kids with GI disorders
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION
Genetic counseling for cancer often costs patients nothing
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
But even among those who do, the cost is $16 or less, a cohort study shows.
“The findings highlight the relatively low financial costs of genetic counseling, a form of care with potentially substantial implications for cancer treatment,” lead author Mya Roberson, PhD, Vanderbilt University, Nashville, Tenn., and colleagues explained.
The study was published online in JAMA Health Forum.
Genetic counseling is an important feature of cancer care that can affect treatment decisions and surveillance. But coverage of genetic counseling services varies across insurance types.
To understand the costs to patients, the investigators used data from IBM Watson Health MarketScan to create a cohort of privately insured patients with breast, prostate, endometrial, ovarian, colorectal, and pancreatic cancer who had at least one genetic counseling session from 2013 to the end of 2019.
Dr. Roberson and colleagues then calculated out-of-pocket costs – including coinsurance, copayments, and deductibles – and total costs paid on claims for genetic counseling encounters. The cohort included 16,791 patients, the majority of whom had breast cancer.
Although the median insurance payment for genetic counseling encounters was $118 ($58-$211), most patients paid nothing out of pocket for these services. Among the 31% of patients with an out-of-pocket expense, the cost was $16 or less.
Compared with breast cancer patients, men with prostate cancer were 28% more likely to have out-of-pocket costs for genetic counseling, which may “reflect a lack of awareness about the medical necessity of genetic counseling,” the authors suggested.
Overall, the study highlights the value of genetic counseling in cancer care.
“Cancer genetic counseling not only promotes informed decision-making about genetic testing and cancer treatment in the era of precision medicine, but it also is a form of low-cost, high-value care,” the authors wrote.
The study was funded by a grant from the National Cancer Institute. Dr. Roberson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA HEALTH FORUM
Updates on treatment/prevention of VTE in cancer patients
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
Updated clinical practice guidelines for the treatment and prevention of venous thromboembolism for patients with cancer, including those with cancer and COVID-19, have been released by the International Initiative on Thrombosis and Cancer (ITAC), an academic working group of VTE experts.
“Because patients with cancer have a baseline increased risk of VTE, compared with patients without cancer, the combination of both COVID-19 and cancer – and its effect on VTE risk and treatment – is of concern,” said the authors, led by Dominique Farge, MD, PhD, Nord Universite de Paris.
they added.
The new guidelines were published online in The Lancet Oncology.
“Cancer-associated VTE remains an important clinical problem, associated with increased morbidity and mortality,” Dr. Farge and colleagues observed.
“The ITAC guidelines’ companion free web-based mobile application will assist the practicing clinician with decision making at various levels to provide optimal care of patients with cancer to treat and prevent VTE,” they emphasized. More information is available at itaccme.com.
Cancer patients with COVID
The new section of the guidelines notes that the treatment and prevention of VTE for cancer patients infected with SARS-CoV-2 remain the same as for patients without COVID.
Whether or not cancer patients with COVID-19 are hospitalized, have been discharged, or are ambulatory, they should be assessed for the risk of VTE, as should any other patient. For cancer patients with COVID-19 who are hospitalized, pharmacologic prophylaxis should be given at the same dose and anticoagulant type as for hospitalized cancer patients who do not have COVID-19.
Following discharge, VTE prophylaxis is not advised for cancer patients infected with SARS-CoV-2, and routine primary pharmacologic prophylaxis of VTE for ambulatory patients with COVID-19 is also not recommended, the authors noted.
Initial treatment of established VTE
Initial treatment of established VTE for up to 10 days of anticoagulation should include low-molecular-weight heparin (LMWH) when creatinine clearance is at least 30 mL/min.
“A regimen of LMWH, taken once per day, is recommended unless a twice-per-day regimen is required because of patients’ characteristics,” the authors noted. These characteristics include a high risk of bleeding, moderate renal failure, and the need for technical intervention, including surgery.
If a twice-a-day regimen is required, only enoxaparin at a dose of 1 mg/kg twice daily can be used, the authors cautioned.
For patients with a low risk of gastrointestinal or genitourinary bleeding, rivaroxaban (Xarelto) or apixaban (Eliquis) can be given in the first 10 days, as well as edoxaban (Lixiana). The latter should be started after at least 5 days of parenteral anticoagulation, provided creatinine clearance is at least 30 mL/min.
“Unfractionated heparin as well as fondaparinux (GlaxoSmithKline) can be also used for the initial treatment of established VTE when LMWH or direct oral anticoagulants are contraindicated,” Dr. Farge and colleagues wrote.
Thrombolysis can be considered on a case-by-case basis, although physicians must pay attention to specific contraindications, especially bleeding risk.
“In the initial treatment of VTE, inferior vena cava filters might be considered when anticoagulant treatment is contraindicated or, in the case of pulmonary embolism, when recurrence occurs under optimal anticoagulation,” the authors noted.
Maintenance VTE treatment
For maintenance therapy, which the authors define as early maintenance for up to 6 months and long-term maintenance beyond 6 months, they point out that LMWHs are preferred over vitamin K antagonists for the treatment of VTE when the creatinine clearance is again at least 30 mL/min.
Any of the direct oral anticoagulants (DOAs) – edoxaban, rivaroxaban, or apixaban – is also recommended for the same patients, provided there is no risk of inducing a strong drug-drug interaction or GI absorption is impaired.
However, the DOAs should be used with caution for patients with GI malignancies, especially upper GI cancers, because data show there is an increased risk of GI bleeding with both edoxaban and rivaroxaban.
“LMWH or direct oral anticoagulants should be used for a minimum of 6 months to treat established VTE in patients with cancer,” the authors wrote.
“After 6 months, termination or continuation of anticoagulation (LMWH, direct oral anticoagulants, or vitamin K antagonists) should be based on individual evaluation of the benefit-risk ratio,” they added.
Treatment of VTE recurrence
The guideline authors explain that three options can be considered in the event of VTE recurrence. These include an increase in the LMWH dose by 20%-25%, or a switch to a DOA, or, if patients are taking a DOA, a switch to an LMWH. If the patient is taking a vitamin K antagonist, it can be switched to either an LMWH or a DOA.
For treatment of catheter-related thrombosis, anticoagulant treatment is recommended for a minimum of 3 months and as long as the central venous catheter is in place. In this setting, the LMWHs are recommended.
The central venous catheter can be kept in place if it is functional, well positioned, and is not infected, provided there is good resolution of symptoms under close surveillance while anticoagulants are being administered.
In surgically treated patients, the LMWH, given once a day, to patients with a serum creatinine concentration of at least 30 mL/min can be used to prevent VTE. Alternatively, VTE can be prevented by the use low-dose unfractionated heparin, given three times a day.
“Pharmacological prophylaxis should be started 2-12 h preoperatively and continued for at least 7–10 days,” Dr. Farge and colleagues advised. In this setting, there is insufficient evidence to support the use of fondaparinux or a DOA as an alternative to an LMWH for the prophylaxis of postoperative VTE. “Use of the highest prophylactic dose of LMWH to prevent postoperative VTE in patients with cancer is recommended,” the authors advised.
Furthermore, extended prophylaxis of at least 4 weeks with LMWH is advised to prevent postoperative VTE after major abdominal or pelvic surgery. Mechanical methods are not recommended except when pharmacologic methods are contraindicated. Inferior vena cava filters are also not recommended for routine prophylaxis.
Patients with reduced mobility
For medically treated hospitalized patients with cancer whose mobility is reduced, the authors recommend prophylaxis with either an LMWH or fondaparinux, provided their creatinine clearance is at least 30 mL/min. These patients can also be treated with unfractionated heparin, they add.
In contrast, DOAs are not recommended – at least not routinely – in this setting, the authors cautioned. Primary pharmacologic prophylaxis of VTE with either LMWH or DOAs – either rivaroxaban or apixaban – is indicated in ambulatory patients with locally advanced or metastatic pancreatic cancer who are receiving systemic anticancer therapy, provided they are at low risk of bleeding.
However, primary pharmacologic prophylaxis with LMWH is not recommended outside of a clinical trial for patients with locally advanced or metastatic lung cancer who are undergoing systemic anticancer therapy, even for patients who are at low risk of bleeding.
For ambulatory patients who are receiving systemic anticancer therapy and who are at intermediate risk of VTE, primary prophylaxis with rivaroxaban or apixaban is recommended for those with myeloma who are receiving immunomodulatory therapy plus steroids or other systemic therapies.
In this setting, oral anticoagulants should consist of a vitamin K antagonist, given at low or therapeutic doses, or apixaban, given at prophylactic doses. Alternatively, LMWH, given at prophylactic doses, or low-dose aspirin, given at a dose of 100 mg/day, can be used.
Catheter-related thrombosis
Use of anticoagulation for routine prophylaxis of catheter-related thrombosis is not recommended. Catheters should be inserted on the right side in the jugular vein, and the distal extremity of the central catheter should be located at the junction of the superior vena cava and the right atrium. “In patients requiring central venous catheters, we suggest the use of implanted ports over peripheral inserted central catheter lines,” the authors noted.
The authors described a number of unique situations regarding the treatment of VTE. These situations include patients with a brain tumor, for whom treatment of established VTE should favor either LMWH or a DOA. The authors also recommended the use of LMWH or unfractionated heparin, started postoperatively, for the prevention of VTE for patients undergoing neurosurgery.
In contrast, pharmacologic prophylaxis of VTE in medically treated patients with a brain tumor who are not undergoing neurosurgery is not recommended. “In the presence of severe renal failure...we suggest using unfractionated heparin followed by early vitamin K antagonists (possibly from day 1) or LMWH adjusted to anti-Xa concentration of the treatment of established VTE,” Dr. Farge and colleagues wrote.
Anticoagulant treatment is also recommended for a minimum of 3 months for children with symptomatic catheter-related thrombosis and as long as the central venous catheter is in place. For children with acute lymphoblastic leukemia who are undergoing induction chemotherapy, LMWH is also recommended as thromboprophylaxis.
For children who require a central venous catheter, the authors suggested that physicians use implanted ports over peripherally inserted central lines.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Firm Exophytic Tumor on the Shin
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
A 62-year-old man presented with a firm, exophytic, 2.8×1.5-cm tumor on the left shin of 6 to 7 years’ duration. An excisional biopsy was obtained for histopathologic evaluation.
Blood test for cancer available, but is it ready for prime time?
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
The Galleri blood test is being now offered by a number of United States health networks.
The company marketing the test, GRAIL, has established partnerships with the U.S. Department of Veterans Affairs, Mercy Health, Ochsner Health, Intermountain Healthcare, Community Health Network, Knight Cancer Institute at Oregon Health & Science University, Premier, and Cleveland Clinic, among others.
Cleveland Clinic’s Eric Klein, MD, emeritus chair of the Glickman Urological Kidney Institute, is enthusiastic about the test, describing it as a “game-changer” and emphasizing that it can detect many different cancers and at a very early stage.
“It completely changes the way we think about screening for cancer,” commented Jeff Venstrom, MD, chief medical officer at GRAIL. He joined the company because “there are not many things in life where you can be part of a disruptive paradigm and disruptive technology, and this really is disruptive,” he said in an interview.
‘The devil is in the details’
But there is some concern among clinicians that widespread clinical use of the test may be premature.
Having a blood test for multiple cancers is a “very good idea, and the scientific basis for this platform is sound,” commented Timothy R. Rebbeck, PhD, professor of cancer prevention, Harvard T.H. Chan School of Public Health, and Division of Population Sciences, Dana-Farber Cancer Institute, both in Boston.
“But the devil is in the details to ensure the test can accurately detect very early cancers and there is a pathway for subsequent workup (diagnosis, monitoring, treatment, etc.),” Dr. Rebbeck told this news organization.
Galleri is offering the test to individuals who are older than 50 and have a family history of cancer or those who are high risk for cancer or immunocompromised. They suggest that interested individuals get in touch with their health care professional, who then needs to register with GRAIL and order the test.
As well as needing a prescription, interested individuals will have to pay for it out of pocket, around $950. The test is not covered by medical insurance and is not approved by the U.S. Food and Drug Administration.
Falls into primary care setting
Dr. Rebbeck commented that Galleri is a screening test for individuals who don’t have cancer, so the test is intended to fall into the primary care setting. But he warned that “clinical pathways are not yet in place (but are being developed) so that primary care providers can effectively use them.”
The test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA in a blood sample.
The methylation turns genes on or off, explains Cleveland Clinic’s Dr. Klein in his post. “It’s like fingerprints and how fingerprints tell the difference between two people,” he wrote. “The methylation patterns are fingerprints that are characteristic of each kind of cancer. They look one way for lung cancer and different for colon cancer.”
The test returns one of two possible results: either “positive, cancer signal detected” or “negative, no cancer signal detected.”
According to the company, when a cancer signal is detected, the Galleri test predicts the cancer signal origin “with high accuracy, to help guide the next steps to diagnosis.”
However, one problem for clinical practice is all the follow-up tests an individual may undergo if their test comes back positive, said Sameek Roychowdhury, MD, PhD, an oncologist with Ohio State University Comprehensive Cancer Center, Columbus.
“Not everybody will have an actual cancer, but they may undergo many tests, with a lot of stress and cost and still not find anything. I can tell you every time someone undergoes a test looking for cancer, that is not an easy day,” Dr. Roychowdhury said in an interview.
In a large-scale validation study, the Galleri test had a specificity of 99.5% (false-positive rate of 0.5%), meaning in roughly 200 people tested without cancer, only one person received a false-positive result (that is, “cancer signal detected” when cancer is not present).
The overall sensitivity of the test for any stage of cancer was 51.5%, although it was higher for later-stage cancers (77% for stage III and 90.1% for stage IV) and lower for early-stage cancers (16.8% for stage I and 40.4% for stage II).
Exacerbate health disparities?
In Dr. Rebbeck’s view, the characteristics of the test are still “relatively poor for detecting very early cancers, so it will need additional tweaking before it really achieves the goal of multi-cancer EARLY detection,” he said.
Dr. Venstrom acknowledges that the test is “not perfect yet” and says the company will continue to update and improve its performance. “We have some new data coming out in September,” he said.
Clinical data are being accumulated in the United Kingdom, where the Galleri test is being investigated in a large trial run by the National Health Service (NHS). The company recently announced that the enrollment of 140,000 healthy cancer-free volunteers aged 50-77 into this trial has now been completed and claimed this the largest-ever study of a multi-cancer early detection test.
Dr. Roychowdhury said he would encourage anyone interested in the test to join a clinical trial.
Another expert approached for comment last year, when GRAIL first started marketing the test, was in agreement. This test should be viewed as one that is still under clinical investigation, commented William Grady, MD, a member of the clinical research division and public health sciences division at the Fred Hutchinson Cancer Research Center, Seattle.
“The Galleri test is still unproven in the clinical care setting and ... I am concerned that many of the results will be false-positives and will cause many unnecessary follow-up tests and imaging studies as well as anxiety in the people getting the test done,” Dr. Grady said.
Dr. Rebbeck said another issue that needs to be addressed is whether all populations will have access to and benefit from these types of blood tests to screen for cancer, given that they are expensive.
“There is a great danger – as we have seen with many other technological innovations – that the wealthy and connected benefit, but the majority of the population, and particularly those who are underserved, do not,” Dr. Rebbeck said.
“As a result, health disparities are created or exacerbated. This is something that needs to be addressed so that the future use of these tests will provide equitable benefits,” he added.
Dr. Rebbeck and Dr. Roychowdhury have reported no relevant financial relationships. Dr. Venstrom is an employee of GRAIL.
A version of this article first appeared on Medscape.com.
FDA acts against sales of unapproved mole and skin tag products on Amazon, other sites
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.
according to a press release issued on Aug. 9.
In addition to Amazon.com, the other two companies are Ariella Naturals, and Justified Laboratories.
Currently, no over-the-counter products are FDA-approved for the at-home removal of moles and skin tags, and use of unapproved products could be dangerous to consumers, according to the statement. These products may be sold as ointments, gels, sticks, or liquids, and may contain high concentrations of salicylic acid or other harmful ingredients. Introducing unapproved products in to interstate commerce violates the Federal Food, Drug, and Cosmetic Act.
Two products sold on Amazon are the “Deisana Skin Tag Remover, Mole Remover and Repair Gel Set” and “Skincell Mole Skin Tag Corrector Serum,” according to the letter sent to Amazon.
The warning letters alert the three companies that they have 15 days from receipt to address any violations. However, warning letters are not a final FDA action, according to the statement.
“The agency’s rigorous surveillance works to identify threats to public health and stop these products from reaching our communities,” Donald D. Ashley, JD, director of the Office of Compliance in the FDA’s Center for Drug Evaluation and Research, said in the press release. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law,” he added.
The statement emphasized that moles should be evaluated by a health care professional, as attempts at self-diagnosis and at-home treatment could lead to a delayed cancer diagnosis, and potentially to cancer progression.
Products marketed to consumers for at-home removal of moles, skin tags, and other skin lesions could cause injuries, infections, and scarring, according to a related consumer update first posted by the FDA in June, which was updated after the warning letters were sent out.
Consumers and health care professionals are encouraged to report any adverse events related to mole removal or skin tag removal products to the agency’s MedWatch Adverse Event Reporting program.
The FDA also offers an online guide, BeSafeRx, with advice for consumers about potential risks of using online pharmacies and how to do so safely.