User login
Mavacamten controlled hypertrophic cardiomyopathy for over 1 year
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
WASHINGTON – Treatment of patients with symptomatic obstructive hypertrophic cardiomyopathy who remained on treatment with the investigational agent mavacamten for a median of 62 weeks continued to show the same level of safe response to the drug as seen after the first 30 weeks on treatment in the pivotal trial for this agent.
The new findings from longer-term treatment bode well for mavacamten. That’s because if the drug is used in routine practice to avoid the need for surgery or an invasive intervention to reduce blockage of a patient’s left ventricular outflow tract, the duration of mavacamten treatment will likely need to continue for many years and even for decades, said Florian Rader, MD, who presented the results at the annual scientific sessions of the American College of Cardiology.
“In practice, mavacamten will probably be used for many, many years, especially as it replaces septal-reduction therapy, so we need long-term data,” noted Dr. Rader during a press briefing on his report. “I’m very happy with the long-term data” in the follow-up study.
The Food and Drug Administration is currently considering whether to approve mavacamten for routine marketing to treat patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM), with a decision expected by the end of April 2022.
The study Dr. Rader reported followed 231 patients with symptomatic oHCM who had completed the 30-week pivotal trial of mavacamten, EXPLORER-HCM, and opted to continue on open-label extended treatment with mavacamten, either continuing the treatment they started during the trial or crossing over to receive mavacamten after receiving placebo during the trial.
The major findings from EXPLORER-LTE (long-term extension) were that continued treatment for a median of about 62 weeks maintained the safety and efficacy findings seen at the end of the blinded, randomized, initial 30-week phase, said Dr. Rader, codirector of the Clinic for Hypertrophic Cardiomyopathy and Aortopathies at Cedars-Sinai Medical Center in Los Angeles.
‘Almost revolutionary’
Mavacamten represents “an almost revolutionary change” for treating oHCM, commented Maya E. Guglin, MD, professor of clinical medicine and an advanced heart failure physician at Indiana University, Indianapolis. “Until now, there was no good medical treatment for symptomatic oHCM. This will change the landscape, and without question it will change guidelines for treating oHCM,” said Dr. Guglin during the press briefing.
“All of us who care for patients with oHCM have looked forward to having a disease-specific therapy. It is encouraging to see that the safety and efficacy remained high with long-term follow-up,” commented Kyle W. Klarich, MD, a professor and cardiologist who specializes in treating patients with HCM at the Mayo Clinic in Rochester, Minn.
Mavacamten is a direct myosin inhibitor that reduces the excess number of myosin-actin cross bridges that form in patients with oHCM, and thereby directly targets the pathophysiology that underlies the disorder, explained Dr. Rader.
The patients on mavacamten included in the long-term extension reported by Dr. Rader averaged 60 years of age, and 61% were men. They averaged a 35.6–mm Hg drop in their resting left ventricular outflow tract (LVOT) gradient after 48 weeks on treatment, and a 32.8–mm Hg reduction after 84 weeks. When the investigators measured their LVOT gradient during a valsalva maneuver, their reductions from baseline averaged 45.3 mm Hg after 48 weeks and 46.4 mm Hg after 84 weeks.
Resting left ventricular ejection fraction also fell, by an average of 7.0 percentage points from baseline after 48 weeks, and by an average of 9.0 percentage points after 84 weeks. After 48 weeks on treatment, 68% of patients had at least a one-class improvement from baseline in their New York Heart Association functional class.
The safety results showed that most treatment-related adverse events were mild or moderate, and about 2% of patients had a serious drug-related adverse event. Ten of the 231 patients discontinued mavacamten because of a treated-related adverse event.
EXPLORER-HCM and EXPLORER-LTE were sponsored by MyoKardia, the company that is developing mavacamten and which is now owned by Bristol-Myers Squibb. Dr. Rader has been a consultant to MyoKardia as well as to Medtronic and ReCor. Dr. Guglin and Dr. Klarich had no disclosures.
AT ACC 2022
Novel cholesterol drug disappoints: TRANSLATE-TIMI 70
An investigational drug targeting a novel cholesterol pathway has shown disappointing results in the TRANSLATE-TIMI 70 phase 2b study.
Vupanorsen is an antisense oligonucleotide targeting hepatic angiopoietin-like protein 3 (ANGPTL3), a protein that inhibits enzymes involved in the metabolism of triglyceride and cholesterol. Inhibition of ANGPTL3 is one of several novel targets for lowering triglycerides and non-HDL cholesterol.
Results of the TRANSLATE-TIMI 70 study were presented at the annual scientific sessions of the American College of Cardiology by Brian Bergmark, MD, a cardiologist at Brigham and Women’s Hospital, Boston. They were also simultaneously published online in Circulation.
“While vupanorsen significantly reduced triglycerides and non-HDL cholesterol, the reduction in non-HDL cholesterol of 22%-27% was not to a degree that was clinically meaningful for cardiovascular risk reduction, and there were also some potentially important safety issues,” Dr. Bergmark said in an interview.
Pfizer has announced that, after reviewing the results of this study, it is discontinuing development of vupanorsen and will return rights to Ionis, from which it licensed the investigational therapy in 2019.
In response to a question at an ACC press conference on whether there could be any future for the drug, Dr. Bergmark said that “the degree of lipid lowering was not as much as what had been suggested was potentially possible by acting on this pathway, and then there are the additional safety concerns. So, for the specific question of what we were looking at – cardiovascular risk reduction by impacting non-HDL cholesterol and apo [apolipoprotein] B – the modest efficacy paired with the safety concerns does not look favorable for future development of this drug.”
But he added: “Whether some other person or company wants to think about triglyceride lowering and try to find a dose that is a bit safer, that is not for me to say.”
In his ACC presentation, Dr. Bergmark explained that ANGPTL3 is a protein secreted by the liver that inhibits lipases, including lipoprotein lipase. Loss-of-function variants in ANGPTL3 are associated with lower levels of plasma lipids and a monoclonal antibody targeting ANGPTL3, evinacumab (Evkeeza, Regeneron), is approved as an intravenous infusion for the treatment of familial hypercholesterolemia. Vupanorsen is a second-generation antisense oligonucleotide targeting hepatic ANGPTL3 messenger RNA with a potential role for cardiovascular risk reduction.
A previous phase 2a study of vupanorsen in patients with hypertriglyceridemia, hepatic steatosis, and type 2 diabetes mellitus showed significant reductions in triglycerides at all doses studied, as well as reductions in non-HDL cholesterol at the highest doses (80 mg per month given by subcutaneous injection).
Dr. Bergmark noted that, because a potential cardiovascular benefit of vupanorsen would best be reflected by its effects on non-HDL cholesterol, the current TRANSLATE-TIMI 70 trial was designed to assess the effect of escalating doses of vupanorsen on non-HDL cholesterol levels in statin-treated adults with hyperlipidemia.
For the study, 286 adults with non-HDL cholesterol levels of 100 mg/dL or greater (median, 132 mg/dL) and triglyceride levels of 150-500 mg/dL (median, 216 mg/dL) who were receiving statin therapy were randomly assigned to placebo or one of seven vupanorsen dose regimens (80, 120, or 160 mg every 4 weeks or 60, 80, 120, or 160 mg every 2 weeks). All doses were given by subcutaneous injection.
The study population was said to reflect “a typical cohort intended for cardiovascular risk reduction, with type 2 diabetes in approximately one-half of patients and prevalent atherosclerotic cardiovascular disease in a substantial portion,” the researchers wrote in the published report.
The primary endpoint was placebo-adjusted percentage change from baseline in non-HDL cholesterol at 24 weeks. Secondary endpoints included placebo-adjusted percentage changes from baseline in triglycerides, LDL cholesterol, apo B, and ANGPTL3.
Vupanorsen resulted in significant decreases from baseline over placebo in non-HDL cholesterol ranging from 22.0% in the group receiving 60 mg every 2 weeks to 27.7% in the group receiving 80 mg every 2 weeks, but there did not appear to be a dose response.
Regarding additional lipid endpoints, vupanorsen reduced triglyceride levels in a dose-dependent manner, ranging from 41.3% in the group receiving 120 mg every 4 weeks to 56.8% in the group receiving 160 mg every 2 weeks.
The effects of vupanorsen on LDL cholesterol and apo B were more modest and without a clear dose response. Vupanorsen also lowered HDL cholesterol levels at all doses studied, and there was no significant change in high-sensitivity C-reactive protein at any dose.
Liver enzymes and hepatic fat increases of concern
In terms of safety, vupanorsen treatment was linked to liver enzyme elevations; more than three-times elevations of alanine aminotransferase or aspartate aminotransferase were more common at higher total monthly doses (up to 33.3% and 44.4%, respectively). Injection site reactions were also an issue, including recall reactions at sites of previous injections when subsequent injections were given. In addition, there was a dose-related increase (up to 76%) in hepatic fat fraction.
In the Circulation paper, the researchers say it is unclear whether the increases in hepatic fat fraction and liver enzymes reflect a metabolic effect of vupanorsen specifically or an off-target effect resulting from hepatic targeting of ANGPTL3. “Regardless, these are medically meaningful findings with important safety ramifications,” they wrote.
They pointed out that, whereas the reduction in ANGPTL3 levels increased with total monthly dose of vupanorsen, there was no clear dose-response reduction in LDL cholesterol, apo B, or non-HDL cholesterol.
In comparison, evinacumab, a monoclonal antibody against ANGPTL3 that is thought to cause near-total suppression of ANGPTL3 activity, reduces apo B levels by more than 40% in adults with refractory hypercholesterolemia or homozygous familial hypercholesterolemia.
Asked why vupanorsen showed less of an effect on non-HDL cholesterol than evinacumab, Dr. Bergmark suggested that the monoclonal antibody may achieve greater inhibition of ANGPTL3. “It may be that near complete suppression is needed to obtain clinically meaningful reductions in apo B and non-HDL cholesterol. That is a speculative and simplistic explanation,” he commented.
Conversely, reductions in triglycerides with vupanorsen showed a dose-response relationship, mirroring the reduction in ANGPTL3 and consistent with the expected increases in lipoprotein lipase activity, the researchers reported.
They note that the “relatively muted effect on apo B levels” suggests that vupanorsen is primarily decreasing the triglyceride and, to a lesser extent, cholesterol content of very low-density lipoprotein cholesterol particles rather than reducing the number of such particles.
“These observations have important implications for the potential ability of this mechanism to reduce lipid-mediated cardiovascular risk, which largely appears to be a function of the number of ApoB-containing lipoproteins,” they said.
Designated discussant of the study at the ACC late-breaking session, Pradeep Natarajan, MD, director of preventive cardiology at Massachusetts General Hospital in Boston, asked Dr. Bergmark what minimum degree of non-HDL cholesterol reduction would be compelling for a new drug to be considered for wide-scale use.
Dr. Bergmark replied there was no clear to answer to that question, as it would depend on many factors, including the risk of the population and the time horizon involved. But he added: “I think a minimum of at least a 30%-40% reduction in non-HDL cholesterol would be needed for a meaningful reduction in cardiovascular risk across a variety of settings.”
The TRANSLATE-TIMI 70 study was funded by Pfizer. Dr. Bergmark is a member of the TIMI Study Group, which has received institutional grant support through Brigham and Women’s Hospital from numerous pharmaceutical companies. Dr. Bergmark also reported receiving grant support through Brigham and Women’s Hospital from Pfizer, Ionis, AstraZeneca, and Abbott Vascular and consulting/personal fees from Abiomed, CSI, Philips, Abbott Vascular, Servier, DaiichiSankyo, Janssen, and Quark.
A version of this article first appeared on Medscape.com.
An investigational drug targeting a novel cholesterol pathway has shown disappointing results in the TRANSLATE-TIMI 70 phase 2b study.
Vupanorsen is an antisense oligonucleotide targeting hepatic angiopoietin-like protein 3 (ANGPTL3), a protein that inhibits enzymes involved in the metabolism of triglyceride and cholesterol. Inhibition of ANGPTL3 is one of several novel targets for lowering triglycerides and non-HDL cholesterol.
Results of the TRANSLATE-TIMI 70 study were presented at the annual scientific sessions of the American College of Cardiology by Brian Bergmark, MD, a cardiologist at Brigham and Women’s Hospital, Boston. They were also simultaneously published online in Circulation.
“While vupanorsen significantly reduced triglycerides and non-HDL cholesterol, the reduction in non-HDL cholesterol of 22%-27% was not to a degree that was clinically meaningful for cardiovascular risk reduction, and there were also some potentially important safety issues,” Dr. Bergmark said in an interview.
Pfizer has announced that, after reviewing the results of this study, it is discontinuing development of vupanorsen and will return rights to Ionis, from which it licensed the investigational therapy in 2019.
In response to a question at an ACC press conference on whether there could be any future for the drug, Dr. Bergmark said that “the degree of lipid lowering was not as much as what had been suggested was potentially possible by acting on this pathway, and then there are the additional safety concerns. So, for the specific question of what we were looking at – cardiovascular risk reduction by impacting non-HDL cholesterol and apo [apolipoprotein] B – the modest efficacy paired with the safety concerns does not look favorable for future development of this drug.”
But he added: “Whether some other person or company wants to think about triglyceride lowering and try to find a dose that is a bit safer, that is not for me to say.”
In his ACC presentation, Dr. Bergmark explained that ANGPTL3 is a protein secreted by the liver that inhibits lipases, including lipoprotein lipase. Loss-of-function variants in ANGPTL3 are associated with lower levels of plasma lipids and a monoclonal antibody targeting ANGPTL3, evinacumab (Evkeeza, Regeneron), is approved as an intravenous infusion for the treatment of familial hypercholesterolemia. Vupanorsen is a second-generation antisense oligonucleotide targeting hepatic ANGPTL3 messenger RNA with a potential role for cardiovascular risk reduction.
A previous phase 2a study of vupanorsen in patients with hypertriglyceridemia, hepatic steatosis, and type 2 diabetes mellitus showed significant reductions in triglycerides at all doses studied, as well as reductions in non-HDL cholesterol at the highest doses (80 mg per month given by subcutaneous injection).
Dr. Bergmark noted that, because a potential cardiovascular benefit of vupanorsen would best be reflected by its effects on non-HDL cholesterol, the current TRANSLATE-TIMI 70 trial was designed to assess the effect of escalating doses of vupanorsen on non-HDL cholesterol levels in statin-treated adults with hyperlipidemia.
For the study, 286 adults with non-HDL cholesterol levels of 100 mg/dL or greater (median, 132 mg/dL) and triglyceride levels of 150-500 mg/dL (median, 216 mg/dL) who were receiving statin therapy were randomly assigned to placebo or one of seven vupanorsen dose regimens (80, 120, or 160 mg every 4 weeks or 60, 80, 120, or 160 mg every 2 weeks). All doses were given by subcutaneous injection.
The study population was said to reflect “a typical cohort intended for cardiovascular risk reduction, with type 2 diabetes in approximately one-half of patients and prevalent atherosclerotic cardiovascular disease in a substantial portion,” the researchers wrote in the published report.
The primary endpoint was placebo-adjusted percentage change from baseline in non-HDL cholesterol at 24 weeks. Secondary endpoints included placebo-adjusted percentage changes from baseline in triglycerides, LDL cholesterol, apo B, and ANGPTL3.
Vupanorsen resulted in significant decreases from baseline over placebo in non-HDL cholesterol ranging from 22.0% in the group receiving 60 mg every 2 weeks to 27.7% in the group receiving 80 mg every 2 weeks, but there did not appear to be a dose response.
Regarding additional lipid endpoints, vupanorsen reduced triglyceride levels in a dose-dependent manner, ranging from 41.3% in the group receiving 120 mg every 4 weeks to 56.8% in the group receiving 160 mg every 2 weeks.
The effects of vupanorsen on LDL cholesterol and apo B were more modest and without a clear dose response. Vupanorsen also lowered HDL cholesterol levels at all doses studied, and there was no significant change in high-sensitivity C-reactive protein at any dose.
Liver enzymes and hepatic fat increases of concern
In terms of safety, vupanorsen treatment was linked to liver enzyme elevations; more than three-times elevations of alanine aminotransferase or aspartate aminotransferase were more common at higher total monthly doses (up to 33.3% and 44.4%, respectively). Injection site reactions were also an issue, including recall reactions at sites of previous injections when subsequent injections were given. In addition, there was a dose-related increase (up to 76%) in hepatic fat fraction.
In the Circulation paper, the researchers say it is unclear whether the increases in hepatic fat fraction and liver enzymes reflect a metabolic effect of vupanorsen specifically or an off-target effect resulting from hepatic targeting of ANGPTL3. “Regardless, these are medically meaningful findings with important safety ramifications,” they wrote.
They pointed out that, whereas the reduction in ANGPTL3 levels increased with total monthly dose of vupanorsen, there was no clear dose-response reduction in LDL cholesterol, apo B, or non-HDL cholesterol.
In comparison, evinacumab, a monoclonal antibody against ANGPTL3 that is thought to cause near-total suppression of ANGPTL3 activity, reduces apo B levels by more than 40% in adults with refractory hypercholesterolemia or homozygous familial hypercholesterolemia.
Asked why vupanorsen showed less of an effect on non-HDL cholesterol than evinacumab, Dr. Bergmark suggested that the monoclonal antibody may achieve greater inhibition of ANGPTL3. “It may be that near complete suppression is needed to obtain clinically meaningful reductions in apo B and non-HDL cholesterol. That is a speculative and simplistic explanation,” he commented.
Conversely, reductions in triglycerides with vupanorsen showed a dose-response relationship, mirroring the reduction in ANGPTL3 and consistent with the expected increases in lipoprotein lipase activity, the researchers reported.
They note that the “relatively muted effect on apo B levels” suggests that vupanorsen is primarily decreasing the triglyceride and, to a lesser extent, cholesterol content of very low-density lipoprotein cholesterol particles rather than reducing the number of such particles.
“These observations have important implications for the potential ability of this mechanism to reduce lipid-mediated cardiovascular risk, which largely appears to be a function of the number of ApoB-containing lipoproteins,” they said.
Designated discussant of the study at the ACC late-breaking session, Pradeep Natarajan, MD, director of preventive cardiology at Massachusetts General Hospital in Boston, asked Dr. Bergmark what minimum degree of non-HDL cholesterol reduction would be compelling for a new drug to be considered for wide-scale use.
Dr. Bergmark replied there was no clear to answer to that question, as it would depend on many factors, including the risk of the population and the time horizon involved. But he added: “I think a minimum of at least a 30%-40% reduction in non-HDL cholesterol would be needed for a meaningful reduction in cardiovascular risk across a variety of settings.”
The TRANSLATE-TIMI 70 study was funded by Pfizer. Dr. Bergmark is a member of the TIMI Study Group, which has received institutional grant support through Brigham and Women’s Hospital from numerous pharmaceutical companies. Dr. Bergmark also reported receiving grant support through Brigham and Women’s Hospital from Pfizer, Ionis, AstraZeneca, and Abbott Vascular and consulting/personal fees from Abiomed, CSI, Philips, Abbott Vascular, Servier, DaiichiSankyo, Janssen, and Quark.
A version of this article first appeared on Medscape.com.
An investigational drug targeting a novel cholesterol pathway has shown disappointing results in the TRANSLATE-TIMI 70 phase 2b study.
Vupanorsen is an antisense oligonucleotide targeting hepatic angiopoietin-like protein 3 (ANGPTL3), a protein that inhibits enzymes involved in the metabolism of triglyceride and cholesterol. Inhibition of ANGPTL3 is one of several novel targets for lowering triglycerides and non-HDL cholesterol.
Results of the TRANSLATE-TIMI 70 study were presented at the annual scientific sessions of the American College of Cardiology by Brian Bergmark, MD, a cardiologist at Brigham and Women’s Hospital, Boston. They were also simultaneously published online in Circulation.
“While vupanorsen significantly reduced triglycerides and non-HDL cholesterol, the reduction in non-HDL cholesterol of 22%-27% was not to a degree that was clinically meaningful for cardiovascular risk reduction, and there were also some potentially important safety issues,” Dr. Bergmark said in an interview.
Pfizer has announced that, after reviewing the results of this study, it is discontinuing development of vupanorsen and will return rights to Ionis, from which it licensed the investigational therapy in 2019.
In response to a question at an ACC press conference on whether there could be any future for the drug, Dr. Bergmark said that “the degree of lipid lowering was not as much as what had been suggested was potentially possible by acting on this pathway, and then there are the additional safety concerns. So, for the specific question of what we were looking at – cardiovascular risk reduction by impacting non-HDL cholesterol and apo [apolipoprotein] B – the modest efficacy paired with the safety concerns does not look favorable for future development of this drug.”
But he added: “Whether some other person or company wants to think about triglyceride lowering and try to find a dose that is a bit safer, that is not for me to say.”
In his ACC presentation, Dr. Bergmark explained that ANGPTL3 is a protein secreted by the liver that inhibits lipases, including lipoprotein lipase. Loss-of-function variants in ANGPTL3 are associated with lower levels of plasma lipids and a monoclonal antibody targeting ANGPTL3, evinacumab (Evkeeza, Regeneron), is approved as an intravenous infusion for the treatment of familial hypercholesterolemia. Vupanorsen is a second-generation antisense oligonucleotide targeting hepatic ANGPTL3 messenger RNA with a potential role for cardiovascular risk reduction.
A previous phase 2a study of vupanorsen in patients with hypertriglyceridemia, hepatic steatosis, and type 2 diabetes mellitus showed significant reductions in triglycerides at all doses studied, as well as reductions in non-HDL cholesterol at the highest doses (80 mg per month given by subcutaneous injection).
Dr. Bergmark noted that, because a potential cardiovascular benefit of vupanorsen would best be reflected by its effects on non-HDL cholesterol, the current TRANSLATE-TIMI 70 trial was designed to assess the effect of escalating doses of vupanorsen on non-HDL cholesterol levels in statin-treated adults with hyperlipidemia.
For the study, 286 adults with non-HDL cholesterol levels of 100 mg/dL or greater (median, 132 mg/dL) and triglyceride levels of 150-500 mg/dL (median, 216 mg/dL) who were receiving statin therapy were randomly assigned to placebo or one of seven vupanorsen dose regimens (80, 120, or 160 mg every 4 weeks or 60, 80, 120, or 160 mg every 2 weeks). All doses were given by subcutaneous injection.
The study population was said to reflect “a typical cohort intended for cardiovascular risk reduction, with type 2 diabetes in approximately one-half of patients and prevalent atherosclerotic cardiovascular disease in a substantial portion,” the researchers wrote in the published report.
The primary endpoint was placebo-adjusted percentage change from baseline in non-HDL cholesterol at 24 weeks. Secondary endpoints included placebo-adjusted percentage changes from baseline in triglycerides, LDL cholesterol, apo B, and ANGPTL3.
Vupanorsen resulted in significant decreases from baseline over placebo in non-HDL cholesterol ranging from 22.0% in the group receiving 60 mg every 2 weeks to 27.7% in the group receiving 80 mg every 2 weeks, but there did not appear to be a dose response.
Regarding additional lipid endpoints, vupanorsen reduced triglyceride levels in a dose-dependent manner, ranging from 41.3% in the group receiving 120 mg every 4 weeks to 56.8% in the group receiving 160 mg every 2 weeks.
The effects of vupanorsen on LDL cholesterol and apo B were more modest and without a clear dose response. Vupanorsen also lowered HDL cholesterol levels at all doses studied, and there was no significant change in high-sensitivity C-reactive protein at any dose.
Liver enzymes and hepatic fat increases of concern
In terms of safety, vupanorsen treatment was linked to liver enzyme elevations; more than three-times elevations of alanine aminotransferase or aspartate aminotransferase were more common at higher total monthly doses (up to 33.3% and 44.4%, respectively). Injection site reactions were also an issue, including recall reactions at sites of previous injections when subsequent injections were given. In addition, there was a dose-related increase (up to 76%) in hepatic fat fraction.
In the Circulation paper, the researchers say it is unclear whether the increases in hepatic fat fraction and liver enzymes reflect a metabolic effect of vupanorsen specifically or an off-target effect resulting from hepatic targeting of ANGPTL3. “Regardless, these are medically meaningful findings with important safety ramifications,” they wrote.
They pointed out that, whereas the reduction in ANGPTL3 levels increased with total monthly dose of vupanorsen, there was no clear dose-response reduction in LDL cholesterol, apo B, or non-HDL cholesterol.
In comparison, evinacumab, a monoclonal antibody against ANGPTL3 that is thought to cause near-total suppression of ANGPTL3 activity, reduces apo B levels by more than 40% in adults with refractory hypercholesterolemia or homozygous familial hypercholesterolemia.
Asked why vupanorsen showed less of an effect on non-HDL cholesterol than evinacumab, Dr. Bergmark suggested that the monoclonal antibody may achieve greater inhibition of ANGPTL3. “It may be that near complete suppression is needed to obtain clinically meaningful reductions in apo B and non-HDL cholesterol. That is a speculative and simplistic explanation,” he commented.
Conversely, reductions in triglycerides with vupanorsen showed a dose-response relationship, mirroring the reduction in ANGPTL3 and consistent with the expected increases in lipoprotein lipase activity, the researchers reported.
They note that the “relatively muted effect on apo B levels” suggests that vupanorsen is primarily decreasing the triglyceride and, to a lesser extent, cholesterol content of very low-density lipoprotein cholesterol particles rather than reducing the number of such particles.
“These observations have important implications for the potential ability of this mechanism to reduce lipid-mediated cardiovascular risk, which largely appears to be a function of the number of ApoB-containing lipoproteins,” they said.
Designated discussant of the study at the ACC late-breaking session, Pradeep Natarajan, MD, director of preventive cardiology at Massachusetts General Hospital in Boston, asked Dr. Bergmark what minimum degree of non-HDL cholesterol reduction would be compelling for a new drug to be considered for wide-scale use.
Dr. Bergmark replied there was no clear to answer to that question, as it would depend on many factors, including the risk of the population and the time horizon involved. But he added: “I think a minimum of at least a 30%-40% reduction in non-HDL cholesterol would be needed for a meaningful reduction in cardiovascular risk across a variety of settings.”
The TRANSLATE-TIMI 70 study was funded by Pfizer. Dr. Bergmark is a member of the TIMI Study Group, which has received institutional grant support through Brigham and Women’s Hospital from numerous pharmaceutical companies. Dr. Bergmark also reported receiving grant support through Brigham and Women’s Hospital from Pfizer, Ionis, AstraZeneca, and Abbott Vascular and consulting/personal fees from Abiomed, CSI, Philips, Abbott Vascular, Servier, DaiichiSankyo, Janssen, and Quark.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Diltiazem fails to improve vasomotor dysfunction, angina in ANOCA: EDIT-CMD
In a randomized trial of patients with angina and no obstructive coronary artery disease (ANOCA), 6 weeks of treatment with diltiazem did not improve coronary vasomotor dysfunction – apart from epicardial spasm – or angina symptoms and quality of life.
The trial investigated whether this therapy would improve these outcomes in patients with two mutually exclusive subgroups, or endotypes, of coronary vasomotor dysfunction: coronary artery spasm (epicardial spasm, microvascular spasm) or coronary microvascular dysfunction indicated by coronary flow reserve (CFR) and index of microvascular resistance (IMR) values.
Treatment success, the primary study endpoint – defined as normalization of one of the abnormal endotypes and no normal endotype becoming abnormal – was similar after treatment with diltiazem, compared with placebo. Nor were there significant differences for secondary endpoints apart from improvements in epicardial spasm in the two groups.
Tijn Jansen, MD, presented these findings from the EDIT-CMD trial in a featured clinical research session at the annual scientific sessions of the American College of Cardiology. The study was simultaneously published online April 2, 2022, in JACC: Cardiovascular Imaging.
“This first study using repeated coronary function testing provides a platform for future research,” concluded Dr. Jansen, a PhD candidate in the department of cardiology, Radboud University, Nijmegen, the Netherlands.
“We were surprised indeed” that diltiazem did not meet its primary endpoint for successful treatment and did not reduce symptoms or improve quality of life, compared with placebo, unlike results of the CorMicA trial, he said in an interview.
“We did find a treatment success, however, of 21%, which was slightly lower than expected, but it was not better than just giving placebo. This was similar regarding symptoms and quality of life, where we did find an overall improvement with diltiazem, but again not higher than using placebo,” he added. “It seems that giving the diagnosis to these patients itself creates a reduction in symptoms,” that might be caused by a reduction in stress, Dr. Jansen suggested.
The clinical implication, he said, is that more randomized controlled trials in this patient population are needed to permit evidence-based patient-tailored treatment, based on the different endotypes. “It might even be imaginable to test effectiveness in each individual patient using coronary function testing,” he said.
These tests are more and more commonly used in clinical practice, Dr. Jansen noted. “In the Netherlands, we recently launched the NL-CFT registry, which enables the participating centers to perform the CFT with a standardized protocol, with the goal to collect data and increase knowledge in this patient population.”
Heterogeneous population?
“I think probably the reason this trial was negative is [that coronary vasomotor dysfunction is] just too heterogeneous,” assigned discussant, C. Noel Bairey Merz, MD, commented.
“The problem with effectiveness trials is that you get a very heterogeneous population, and not everything works for everyone,” she said.
“This was a strategy trial – too heterogenous and too small to assess each endotype response,” Dr. Bairey Merz elaborated in an interview.
“Calcium channel blockers [CCBs] will not [effectively] treat all endotypes of coronary microvascular dysfunction,” she added, noting that the 6-month CorMIcA trial demonstrated in a larger, more rigorous trial design that CCBs are effective for epicardial and microvascular spasm.
“If you were going to do this study again, would you allow physicians to do up-titration and/or go a little bit longer?” Dr. Bairey Merz asked Dr. Jansen during the discussion.
“I do think this is a very heterogeneous group,” he agreed. However, the protocol allowed researchers to titrate diltiazem from 120 mg/day to 360 mg/day.
“If I were to do it again,” Dr. Jansen said, “I would focus on one specific endotype, probably epicardial spasm.”
First RCT of diltiazem in patients with ANOCA
Up to 40% of patients undergoing coronary angiography for stable angina do not have obstructive coronary artery disease (CAD), and 60%-90% of these patients have coronary vasomotor dysfunction, Dr. Jansen noted.
The landmark CorMicA trial showed that diagnosing the specific endotype of coronary vasomotor dysfunction using coronary function testing allows for tailored medication that decreased angina and improved quality of life, the researchers noted.
A recent European Society of Cardiology position paper on ANOCA “recommends the use of various pharmacological treatments including calcium-channel blockers, beta-blockers, ACE inhibitors, statins, and nitric oxide modulators, of which CCBs have the most prominent role in both endotypes of coronary vasospasms” and coronary microvascular dysfunction, they wrote.
“However, evidence substantiating these recommendations is lacking,” the researchers added, “since it is based on studies in a different population, with small sample sizes, or not placebo controlled.”
To investigate this, between 2019 and 2021, EDIT-CMD enrolled 126 adults aged 18 years and older who had two or more chronic angina episodes per week and no signs of obstructive CAD, who were seen at three hospitals specializing in ANOCA in the Netherlands.
The participants underwent coronary function testing that consisted of an acetylcholine spasm provocation test to evaluate for epicardial spasm and microvascular spasm, and a bolus thermodilution test with adenosine, to assess CFR and IMR. Coronary microvascular dysfunction was defined as CFR less than 2.0 and IMR of 25 or greater.
Of 99 patients with vasospasm or microvascular dysfunction, 85 patients were randomly assigned to receive diltiazem (n = 41) or placebo (n = 44) for 6 weeks.
The patients in both groups had a mean age of 58 years, and 29% were male; 22% had previously undergone percutaneous coronary intervention, and 48% had severe angina (Canadian Cardiovascular Society grade III/IV).
At baseline, about 50% had epicardial spasm, 25% had microvascular spasm and 25% had no spasm, and 54% in the diltiazem group and 73% in the placebo group had microvascular dysfunction.
After 6 weeks, 73 patients (35 in the placebo group and 38 in the diltiazem group) were available for repeat coronary function testing.
For the primary outcome, after 6 weeks of treatment, the proportion of patients with normalization of one abnormal parameter of coronary vasomotor dysfunction, without any normal parameter becoming abnormal, occurred in 8 patients (21%) in the diltiazem group versus 10 patients (29%) in the placebo group (P = .46)
In secondary outcomes, after 6 weeks of treatment, there were no significant differences in the prevalence of microvascular dysfunction, in Seattle Angina Questionnaire scores for angina symptoms, or RAND-36 scores for quality of life between patients who received diltiazem vs those who received placebo.
However, more patients in the diltiazem group than in the placebo group progressed from epicardial spasm to microvascular or no spasm (47% vs. 6%; P = .006).
The EDIT-CMD trial was sponsored by Abbott. Dr. Jansen has no relevant financial disclosures. Dr. Bairey Merz discloses having a fiduciary role and shares in iRhythm and being on the advisory board for Sanofi.
A version of this article first appeared on Medscape.com.
In a randomized trial of patients with angina and no obstructive coronary artery disease (ANOCA), 6 weeks of treatment with diltiazem did not improve coronary vasomotor dysfunction – apart from epicardial spasm – or angina symptoms and quality of life.
The trial investigated whether this therapy would improve these outcomes in patients with two mutually exclusive subgroups, or endotypes, of coronary vasomotor dysfunction: coronary artery spasm (epicardial spasm, microvascular spasm) or coronary microvascular dysfunction indicated by coronary flow reserve (CFR) and index of microvascular resistance (IMR) values.
Treatment success, the primary study endpoint – defined as normalization of one of the abnormal endotypes and no normal endotype becoming abnormal – was similar after treatment with diltiazem, compared with placebo. Nor were there significant differences for secondary endpoints apart from improvements in epicardial spasm in the two groups.
Tijn Jansen, MD, presented these findings from the EDIT-CMD trial in a featured clinical research session at the annual scientific sessions of the American College of Cardiology. The study was simultaneously published online April 2, 2022, in JACC: Cardiovascular Imaging.
“This first study using repeated coronary function testing provides a platform for future research,” concluded Dr. Jansen, a PhD candidate in the department of cardiology, Radboud University, Nijmegen, the Netherlands.
“We were surprised indeed” that diltiazem did not meet its primary endpoint for successful treatment and did not reduce symptoms or improve quality of life, compared with placebo, unlike results of the CorMicA trial, he said in an interview.
“We did find a treatment success, however, of 21%, which was slightly lower than expected, but it was not better than just giving placebo. This was similar regarding symptoms and quality of life, where we did find an overall improvement with diltiazem, but again not higher than using placebo,” he added. “It seems that giving the diagnosis to these patients itself creates a reduction in symptoms,” that might be caused by a reduction in stress, Dr. Jansen suggested.
The clinical implication, he said, is that more randomized controlled trials in this patient population are needed to permit evidence-based patient-tailored treatment, based on the different endotypes. “It might even be imaginable to test effectiveness in each individual patient using coronary function testing,” he said.
These tests are more and more commonly used in clinical practice, Dr. Jansen noted. “In the Netherlands, we recently launched the NL-CFT registry, which enables the participating centers to perform the CFT with a standardized protocol, with the goal to collect data and increase knowledge in this patient population.”
Heterogeneous population?
“I think probably the reason this trial was negative is [that coronary vasomotor dysfunction is] just too heterogeneous,” assigned discussant, C. Noel Bairey Merz, MD, commented.
“The problem with effectiveness trials is that you get a very heterogeneous population, and not everything works for everyone,” she said.
“This was a strategy trial – too heterogenous and too small to assess each endotype response,” Dr. Bairey Merz elaborated in an interview.
“Calcium channel blockers [CCBs] will not [effectively] treat all endotypes of coronary microvascular dysfunction,” she added, noting that the 6-month CorMIcA trial demonstrated in a larger, more rigorous trial design that CCBs are effective for epicardial and microvascular spasm.
“If you were going to do this study again, would you allow physicians to do up-titration and/or go a little bit longer?” Dr. Bairey Merz asked Dr. Jansen during the discussion.
“I do think this is a very heterogeneous group,” he agreed. However, the protocol allowed researchers to titrate diltiazem from 120 mg/day to 360 mg/day.
“If I were to do it again,” Dr. Jansen said, “I would focus on one specific endotype, probably epicardial spasm.”
First RCT of diltiazem in patients with ANOCA
Up to 40% of patients undergoing coronary angiography for stable angina do not have obstructive coronary artery disease (CAD), and 60%-90% of these patients have coronary vasomotor dysfunction, Dr. Jansen noted.
The landmark CorMicA trial showed that diagnosing the specific endotype of coronary vasomotor dysfunction using coronary function testing allows for tailored medication that decreased angina and improved quality of life, the researchers noted.
A recent European Society of Cardiology position paper on ANOCA “recommends the use of various pharmacological treatments including calcium-channel blockers, beta-blockers, ACE inhibitors, statins, and nitric oxide modulators, of which CCBs have the most prominent role in both endotypes of coronary vasospasms” and coronary microvascular dysfunction, they wrote.
“However, evidence substantiating these recommendations is lacking,” the researchers added, “since it is based on studies in a different population, with small sample sizes, or not placebo controlled.”
To investigate this, between 2019 and 2021, EDIT-CMD enrolled 126 adults aged 18 years and older who had two or more chronic angina episodes per week and no signs of obstructive CAD, who were seen at three hospitals specializing in ANOCA in the Netherlands.
The participants underwent coronary function testing that consisted of an acetylcholine spasm provocation test to evaluate for epicardial spasm and microvascular spasm, and a bolus thermodilution test with adenosine, to assess CFR and IMR. Coronary microvascular dysfunction was defined as CFR less than 2.0 and IMR of 25 or greater.
Of 99 patients with vasospasm or microvascular dysfunction, 85 patients were randomly assigned to receive diltiazem (n = 41) or placebo (n = 44) for 6 weeks.
The patients in both groups had a mean age of 58 years, and 29% were male; 22% had previously undergone percutaneous coronary intervention, and 48% had severe angina (Canadian Cardiovascular Society grade III/IV).
At baseline, about 50% had epicardial spasm, 25% had microvascular spasm and 25% had no spasm, and 54% in the diltiazem group and 73% in the placebo group had microvascular dysfunction.
After 6 weeks, 73 patients (35 in the placebo group and 38 in the diltiazem group) were available for repeat coronary function testing.
For the primary outcome, after 6 weeks of treatment, the proportion of patients with normalization of one abnormal parameter of coronary vasomotor dysfunction, without any normal parameter becoming abnormal, occurred in 8 patients (21%) in the diltiazem group versus 10 patients (29%) in the placebo group (P = .46)
In secondary outcomes, after 6 weeks of treatment, there were no significant differences in the prevalence of microvascular dysfunction, in Seattle Angina Questionnaire scores for angina symptoms, or RAND-36 scores for quality of life between patients who received diltiazem vs those who received placebo.
However, more patients in the diltiazem group than in the placebo group progressed from epicardial spasm to microvascular or no spasm (47% vs. 6%; P = .006).
The EDIT-CMD trial was sponsored by Abbott. Dr. Jansen has no relevant financial disclosures. Dr. Bairey Merz discloses having a fiduciary role and shares in iRhythm and being on the advisory board for Sanofi.
A version of this article first appeared on Medscape.com.
In a randomized trial of patients with angina and no obstructive coronary artery disease (ANOCA), 6 weeks of treatment with diltiazem did not improve coronary vasomotor dysfunction – apart from epicardial spasm – or angina symptoms and quality of life.
The trial investigated whether this therapy would improve these outcomes in patients with two mutually exclusive subgroups, or endotypes, of coronary vasomotor dysfunction: coronary artery spasm (epicardial spasm, microvascular spasm) or coronary microvascular dysfunction indicated by coronary flow reserve (CFR) and index of microvascular resistance (IMR) values.
Treatment success, the primary study endpoint – defined as normalization of one of the abnormal endotypes and no normal endotype becoming abnormal – was similar after treatment with diltiazem, compared with placebo. Nor were there significant differences for secondary endpoints apart from improvements in epicardial spasm in the two groups.
Tijn Jansen, MD, presented these findings from the EDIT-CMD trial in a featured clinical research session at the annual scientific sessions of the American College of Cardiology. The study was simultaneously published online April 2, 2022, in JACC: Cardiovascular Imaging.
“This first study using repeated coronary function testing provides a platform for future research,” concluded Dr. Jansen, a PhD candidate in the department of cardiology, Radboud University, Nijmegen, the Netherlands.
“We were surprised indeed” that diltiazem did not meet its primary endpoint for successful treatment and did not reduce symptoms or improve quality of life, compared with placebo, unlike results of the CorMicA trial, he said in an interview.
“We did find a treatment success, however, of 21%, which was slightly lower than expected, but it was not better than just giving placebo. This was similar regarding symptoms and quality of life, where we did find an overall improvement with diltiazem, but again not higher than using placebo,” he added. “It seems that giving the diagnosis to these patients itself creates a reduction in symptoms,” that might be caused by a reduction in stress, Dr. Jansen suggested.
The clinical implication, he said, is that more randomized controlled trials in this patient population are needed to permit evidence-based patient-tailored treatment, based on the different endotypes. “It might even be imaginable to test effectiveness in each individual patient using coronary function testing,” he said.
These tests are more and more commonly used in clinical practice, Dr. Jansen noted. “In the Netherlands, we recently launched the NL-CFT registry, which enables the participating centers to perform the CFT with a standardized protocol, with the goal to collect data and increase knowledge in this patient population.”
Heterogeneous population?
“I think probably the reason this trial was negative is [that coronary vasomotor dysfunction is] just too heterogeneous,” assigned discussant, C. Noel Bairey Merz, MD, commented.
“The problem with effectiveness trials is that you get a very heterogeneous population, and not everything works for everyone,” she said.
“This was a strategy trial – too heterogenous and too small to assess each endotype response,” Dr. Bairey Merz elaborated in an interview.
“Calcium channel blockers [CCBs] will not [effectively] treat all endotypes of coronary microvascular dysfunction,” she added, noting that the 6-month CorMIcA trial demonstrated in a larger, more rigorous trial design that CCBs are effective for epicardial and microvascular spasm.
“If you were going to do this study again, would you allow physicians to do up-titration and/or go a little bit longer?” Dr. Bairey Merz asked Dr. Jansen during the discussion.
“I do think this is a very heterogeneous group,” he agreed. However, the protocol allowed researchers to titrate diltiazem from 120 mg/day to 360 mg/day.
“If I were to do it again,” Dr. Jansen said, “I would focus on one specific endotype, probably epicardial spasm.”
First RCT of diltiazem in patients with ANOCA
Up to 40% of patients undergoing coronary angiography for stable angina do not have obstructive coronary artery disease (CAD), and 60%-90% of these patients have coronary vasomotor dysfunction, Dr. Jansen noted.
The landmark CorMicA trial showed that diagnosing the specific endotype of coronary vasomotor dysfunction using coronary function testing allows for tailored medication that decreased angina and improved quality of life, the researchers noted.
A recent European Society of Cardiology position paper on ANOCA “recommends the use of various pharmacological treatments including calcium-channel blockers, beta-blockers, ACE inhibitors, statins, and nitric oxide modulators, of which CCBs have the most prominent role in both endotypes of coronary vasospasms” and coronary microvascular dysfunction, they wrote.
“However, evidence substantiating these recommendations is lacking,” the researchers added, “since it is based on studies in a different population, with small sample sizes, or not placebo controlled.”
To investigate this, between 2019 and 2021, EDIT-CMD enrolled 126 adults aged 18 years and older who had two or more chronic angina episodes per week and no signs of obstructive CAD, who were seen at three hospitals specializing in ANOCA in the Netherlands.
The participants underwent coronary function testing that consisted of an acetylcholine spasm provocation test to evaluate for epicardial spasm and microvascular spasm, and a bolus thermodilution test with adenosine, to assess CFR and IMR. Coronary microvascular dysfunction was defined as CFR less than 2.0 and IMR of 25 or greater.
Of 99 patients with vasospasm or microvascular dysfunction, 85 patients were randomly assigned to receive diltiazem (n = 41) or placebo (n = 44) for 6 weeks.
The patients in both groups had a mean age of 58 years, and 29% were male; 22% had previously undergone percutaneous coronary intervention, and 48% had severe angina (Canadian Cardiovascular Society grade III/IV).
At baseline, about 50% had epicardial spasm, 25% had microvascular spasm and 25% had no spasm, and 54% in the diltiazem group and 73% in the placebo group had microvascular dysfunction.
After 6 weeks, 73 patients (35 in the placebo group and 38 in the diltiazem group) were available for repeat coronary function testing.
For the primary outcome, after 6 weeks of treatment, the proportion of patients with normalization of one abnormal parameter of coronary vasomotor dysfunction, without any normal parameter becoming abnormal, occurred in 8 patients (21%) in the diltiazem group versus 10 patients (29%) in the placebo group (P = .46)
In secondary outcomes, after 6 weeks of treatment, there were no significant differences in the prevalence of microvascular dysfunction, in Seattle Angina Questionnaire scores for angina symptoms, or RAND-36 scores for quality of life between patients who received diltiazem vs those who received placebo.
However, more patients in the diltiazem group than in the placebo group progressed from epicardial spasm to microvascular or no spasm (47% vs. 6%; P = .006).
The EDIT-CMD trial was sponsored by Abbott. Dr. Jansen has no relevant financial disclosures. Dr. Bairey Merz discloses having a fiduciary role and shares in iRhythm and being on the advisory board for Sanofi.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
VALOR-HCM: Novel drug may delay, avert invasive therapy in OHCM
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
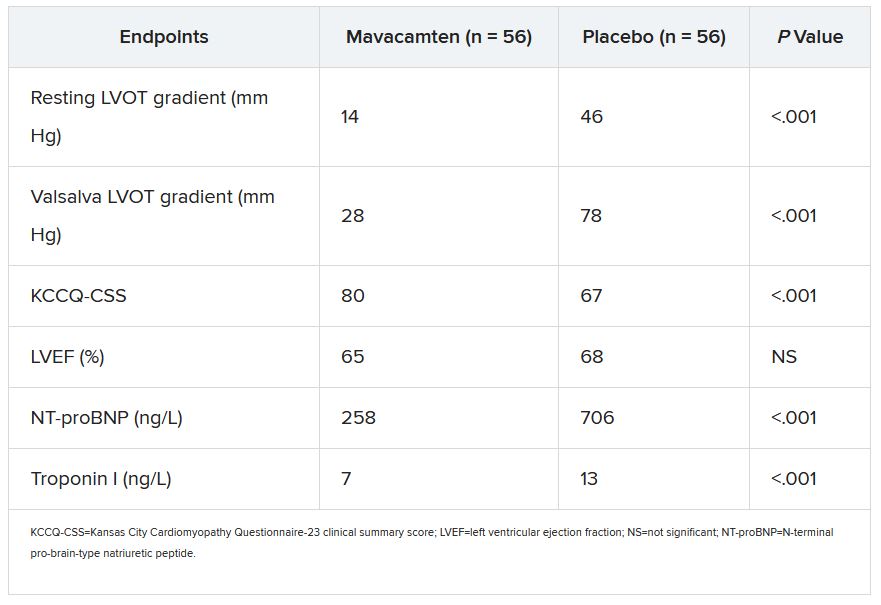
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.
The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.
The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
Treatment with a novel myosin-inhibiting agent may improve symptoms and hemodynamics enough in patients with obstructive hypertrophic cardiomyopathy (OHCM) so that they can avoid or at least delay septal reduction therapy (SRT), suggests a randomized trial of modest size and duration.
Of 112 patients with OHCM who were sick enough while receiving standard medications to qualify for SRT, those assigned to take mavacamten (MyoKardia) instead of placebo were far less likely to still be eligible for SRT 16 weeks later.
In other words, their OHCM had improved enough during therapy with mavacamten such that SRT, either surgical septal myectomy or transcatheter alcohol septal ablation, could no longer be recommended per guidelines.
Mavacamten, which lessens myocardial contractility by selective inhibition of cardiac myosin, is the first agent tested in prospective trials to appear as a viable medical option in patients with severe, symptomatic OHCM, observed principal investigator Milind Y. Desai, MD, MBA, of the Cleveland Clinic.
“There’s clearly an unmet need for noninvasive therapies, medical therapies, that work in OHCM,” he said in an interview. Mavacamten “adds to the armamentarium” of OHCM management options and may give patients with symptoms despite conventional medications an alternative to SRT, which is considered definitive but has drawbacks.
The goal of SRT is to alleviate obstruction of the left ventricular outflow tract (LVOT), but surgical SRT requires a sternotomy, with all the risks and recovery time that entails. Catheter-based alcohol septal ablation is a less common alternative for some patients with suitable anatomy, Dr. Desai noted.
But those procedures “are not uniformly available, and even when available, the outcomes are fairly heterogeneous,” he said. “The guidelines recommend that you should go to a center with a mortality rate of less than 1% with these procedures. Centers like that are very few across the world,” and procedural mortality can be much higher at centers with less SRT experience.
Dr. Desai presented the results of VALOR-HCM at the annual scientific sessions of the American College of Cardiology. Of the 56 patients assigned to mavacamten, 10 (17.9%) decided to undergo SRT by the end of the trial, or otherwise still met guideline-recommended criteria for receiving SRT, the primary endpoint. In comparison, 43 of the 56 patients (76.8%) in the control group (P < .0001) met that endpoint.
More patients receiving mavacamten improved by at least one New York Heart Association (NYHA) functional class during the trial’s 16 weeks: 63% versus 21% for those assigned to placebo. And 27% and 2%, respectively, improved by at least two NYHA classes, Dr. Desai said.
Guidelines recommend that SRT be reserved for patients in NYHA class III or IV heart failure with a resting or provoked LVOT gradient of at least 50 mm Hg.
Of note, Desai said, only two patients in each group elected to undergo SRT during the study. “The primary endpoint was driven by reduction in guideline eligibility for SRT, but 95% of patients in the study chose to continue with medical therapy.”
Speaking as a panelist after Dr. Desai’s presentation, Lynne W. Stevenson, MD, lauded the phase 3 trial’s “brave design,” which featured a highly unusual subjective primary endpoint and framed it as an advantage.
That the trial showed a significant mavacamten effect for that endpoint “answered, in one step, the question of what does this actually mean to the patient – which often takes much longer,” observed Dr. Stevenson, from Vanderbilt University, Nashville, Tenn.
Even so, she added, whether patients still qualified for SRT in the trial at least had to be supported by objective measures of LVOT gradient and NT-proBNP levels.
“My perspective is that of a cardiac surgeon who performs septal myectomies,” said John Cleveland, MD, University of Colorado at Denver, Aurora, who said he was impressed at how few patients receiving mavacamten went on to undergo SRT, while the rest were able to at least defer that decision.
Current recommendations are that patients who go to SRT “should be maximally medically treated and still symptomatic,” Dr. Cleveland observed at a press conference on VALOR-HCM. Should mavacamten be added to the list of agents to use before resorting to invasive therapy? “My answer would be yes,” he said, and patients who remain symptomatic even while receiving the myosin inhibitor and other medications should proceed to SRT.
The trial’s patients had documented OHCM, severe symptoms, and a resting or provoked LVOT gradient of at least 50 mm Hg despite maximally tolerated medications – which could include disopyramide, beta-blockers, and calcium channel blockers. About half the study population was female, and 89% were White. All had been referred for SRT.
Active therapy consisted of mavacamten initiated at 5 mg/day, with up-titrations at 8 and 12 weeks as tolerated, guided by echocardiographic left ventricular ejection fraction and LVOT gradient.
Most secondary endpoints improved significantly in patients receiving the drug, compared with placebo. They included measures of quality of life, symptom status, ventricular function, natriuretic peptides, and troponin I.
The secondary outcomes are consistent with what was observed in the EXPLORER-HCM trial, which in 2020 suggested that mavacamten could improve measures of quality of life, NYHA functional class, LVOT gradient, peak VO2, and other metrics in patients with OHCM.
Dr. Desai said mavacamten was well tolerated. “There were two patients who had a transient drop in ejection fraction to less than 50%, so the drug was temporarily discontinued, but resumed at a lower dose and they were able to complete the study.”
Dr. Stevenson commented on the “pretty quick” up-titration of mavacamten dosages in a study lasting only 4 months, which could have been a concern given the drug’s limited track record and its mechanism of action targeting contractility. “Fortunately, no serious safety signals” were observed.
Dr. Desai emphasized that mavacamten up-titrations were strictly guided by regular echocardiographic monitoring and assessment of LVOT gradients, in addition to clinical responses. And that, he said, is likely how up-titrations should be carried out if mavacamten is approved for OHCM.
VALOR-HCM was supported by MyoKardia. Dr. Desai disclosed receiving honoraria or consulting fees from Caristo Diagnostics, Medtronic, and MyoKardia. Dr. Stevenson disclosed receiving honoraria or consulting fees from Novartis; serving on a data safety monitoring board for Livanova; and other relationships with Abbott Medical, Biotronik, Boston Scientific, Bristol-Myers Squibb, Endotronic, Gore Medical, and Johnson & Johnson. Dr. Cleveland had no disclosures.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
FAME 3 subanalysis adds twist to negative primary results
A new subanalysis of the FAME 3 trial, which failed to show that percutaneous intervention (PCI) guided by fractional flow reserve (FFR) is noninferior to coronary artery bypass grafting (CABG) for treating three-vessel coronary artery disease, has associated PCI with early quality of life (QOL) advantages, according to findings presented at the annual scientific sessions of the American College of Cardiology.
Despite a modestly greater risk of major adverse cardiac events (MACE) at the end of 12 months’ follow-up among those treated with FFR-guided PCI, the greater QOL early after the procedure might be relevant to patients weighing these options, according to Frederik M. Zimmerman, MD, of Catharina Hospital in Eindhoven, the Netherlands.
“FFR-guided PCI results in a faster improvement in quality of life than CABG during the first year after revascularization, and it improved working status in patients younger than 65 years of age,” Dr. Zimmermann said.
The primary results of FAME 3 were presented at the 2021 Transcatheter Cardiovascular Therapeutics annual meeting by lead author William F. Fearon, MD, of Stanford (Calif.) University and published simultaneously in the New England Journal of Medicine.
Rather than confirming the hypothesis that FFR-guided PCI is comparable with CABG for the primary composite MACE outcome death from any cause, myocardial infarction, stroke, or revascularization, the incidence of MACE at 12 months was 10.6% in those randomized to PCI and 6.9% in the group assigned to CABG.
This translated into a hazard ratio for MACE of 1.5, signifying a 50% increase in risk for FFR-guided PCI relative to CABG for the primary outcome, a difference that negated the study definition of noninferiority (P = .35).
In this new health-related subanalysis, which was published simultaneously with his ACC presentation, the groups were compared over 12 months for QOL as measured with European Quality of Life–5 dimensions (EQ-5D) scale, angina as measured with the Canadian Cardiovascular Classification (CCC) system, and employment.
Outcomes data available in >85% of patients
Of the 1,500 patients enrolled and randomized in FAME 3 (757 to FFR-guided PCI and 743 to CABG), this health outcomes subanalysis was performed with complete data at 12 months from 89% of those in the PCI group and 88% of those in the CABG group.
Ultimately, the study did not show differences in any of these measures at the end of 12 months, but there were significant differences in QOL and employment at earlier time points. In particular, the significantly different (P < .001) trajectory for QOL improvement at 1 and 6 months favored FFR-guided PCI whether evaluated with the EQ-5D instrument or an EQ visual analog scale.
Rates of angina defined by as CCC class of at least 2 were low after revascularization in both arms of the study, negating any opportunity for differences, but patients aged younger than 65 years were almost twice as likely to have returned to full- or part-time work 1 month after revascularization (60.2% vs. 33.1%), and they remained at higher odds for working at 12 months (68.1% vs. 57.4%).
In patients aged older than 65 years, return-to-work rates did not differ significantly at any time point.
These results suggest potentially clinically meaningful early advantages for FFR-guided PCI, but some experts questioned the rationale for reporting positive secondary findings from a negative trial.
“This subanalysis is curious,” said Allen Jeremias, MD, director of interventional cardiology research, Saint Francis Hospital, Roslyn, N.Y. He pointed out that reporting these data is an anomaly.
Subanalyses uncommon in negative trials
“CABG was found to be better, so why look at QOL,” said Dr. Jeremias, who was an ACC-invited expert to discuss the results. However, he went on to say, “this could be an exception to the rule.”
The reason, according to Dr. Jeremias, is that the absolute difference at 12 months between FFR-guided PCI and CABG for the MACE events of greatest concern – death, MI, or stroke – was only about 2% greater in the FFR-guided PCI group (7.3% vs. 5.2%). The biggest contributor to the difference in MACE in FAME 3 at 12 months was the higher rate of repeat revascularization (5.9% vs. 3.9%).
Moreover, patients randomized to FFR-guided PCI had lower rates of many adverse events. This included risk of bleeding (1.6% vs. 3.8%; P = .009 as defined by type ≥3 Bleeding Academic Research Consortium , acute kidney injury (0.1% vs. 0.9%; P = .04), atrial fibrillation (2.4% vs. 14.1%; P < .001) and rehospitalization within 30 days (5.5% vs. 10.2%; P < .001).
In the context of a modest increase in risk of MACE and the lower rate of several important treatment-related adverse events, the QOL advantages identified in this subanalysis “might be a reasonable topic for patient-shared decision-making,” Dr. Jeremias suggested.
New data might inform patient decision-making
He granted the possibility that well-informed patients might accept the modestly increased risk of MACE for one or more of the outcomes, such as a higher likelihood of an early return to work, that favored FFR-guided PCI.
This is the point of this subanalysis, agreed Dr. Zimmermann.
“It is all about shared decision-making,” he said. Also emphasizing that the negative trial endpoint of FAME 3 “was driven largely by an increased risk of revascularization,” he believes that these new data might be a basis for discussions with patients weighing relative risks and benefits.
There are more data to come, according to Dr. Zimmermann, who said that follow-up of up to 5 years is planned. The 3-year data will be made available in 2023.
Dr. Zimmermann reported no potential conflicts of interest. Dr. Jeremias reported financial relationships with Abbott, ACIST, Boston Scientific, and Volcano. The investigator-initiated trial received research grants from Abbott Vascular and Medtronic.
A new subanalysis of the FAME 3 trial, which failed to show that percutaneous intervention (PCI) guided by fractional flow reserve (FFR) is noninferior to coronary artery bypass grafting (CABG) for treating three-vessel coronary artery disease, has associated PCI with early quality of life (QOL) advantages, according to findings presented at the annual scientific sessions of the American College of Cardiology.
Despite a modestly greater risk of major adverse cardiac events (MACE) at the end of 12 months’ follow-up among those treated with FFR-guided PCI, the greater QOL early after the procedure might be relevant to patients weighing these options, according to Frederik M. Zimmerman, MD, of Catharina Hospital in Eindhoven, the Netherlands.
“FFR-guided PCI results in a faster improvement in quality of life than CABG during the first year after revascularization, and it improved working status in patients younger than 65 years of age,” Dr. Zimmermann said.
The primary results of FAME 3 were presented at the 2021 Transcatheter Cardiovascular Therapeutics annual meeting by lead author William F. Fearon, MD, of Stanford (Calif.) University and published simultaneously in the New England Journal of Medicine.
Rather than confirming the hypothesis that FFR-guided PCI is comparable with CABG for the primary composite MACE outcome death from any cause, myocardial infarction, stroke, or revascularization, the incidence of MACE at 12 months was 10.6% in those randomized to PCI and 6.9% in the group assigned to CABG.
This translated into a hazard ratio for MACE of 1.5, signifying a 50% increase in risk for FFR-guided PCI relative to CABG for the primary outcome, a difference that negated the study definition of noninferiority (P = .35).
In this new health-related subanalysis, which was published simultaneously with his ACC presentation, the groups were compared over 12 months for QOL as measured with European Quality of Life–5 dimensions (EQ-5D) scale, angina as measured with the Canadian Cardiovascular Classification (CCC) system, and employment.
Outcomes data available in >85% of patients
Of the 1,500 patients enrolled and randomized in FAME 3 (757 to FFR-guided PCI and 743 to CABG), this health outcomes subanalysis was performed with complete data at 12 months from 89% of those in the PCI group and 88% of those in the CABG group.
Ultimately, the study did not show differences in any of these measures at the end of 12 months, but there were significant differences in QOL and employment at earlier time points. In particular, the significantly different (P < .001) trajectory for QOL improvement at 1 and 6 months favored FFR-guided PCI whether evaluated with the EQ-5D instrument or an EQ visual analog scale.
Rates of angina defined by as CCC class of at least 2 were low after revascularization in both arms of the study, negating any opportunity for differences, but patients aged younger than 65 years were almost twice as likely to have returned to full- or part-time work 1 month after revascularization (60.2% vs. 33.1%), and they remained at higher odds for working at 12 months (68.1% vs. 57.4%).
In patients aged older than 65 years, return-to-work rates did not differ significantly at any time point.
These results suggest potentially clinically meaningful early advantages for FFR-guided PCI, but some experts questioned the rationale for reporting positive secondary findings from a negative trial.
“This subanalysis is curious,” said Allen Jeremias, MD, director of interventional cardiology research, Saint Francis Hospital, Roslyn, N.Y. He pointed out that reporting these data is an anomaly.
Subanalyses uncommon in negative trials
“CABG was found to be better, so why look at QOL,” said Dr. Jeremias, who was an ACC-invited expert to discuss the results. However, he went on to say, “this could be an exception to the rule.”
The reason, according to Dr. Jeremias, is that the absolute difference at 12 months between FFR-guided PCI and CABG for the MACE events of greatest concern – death, MI, or stroke – was only about 2% greater in the FFR-guided PCI group (7.3% vs. 5.2%). The biggest contributor to the difference in MACE in FAME 3 at 12 months was the higher rate of repeat revascularization (5.9% vs. 3.9%).
Moreover, patients randomized to FFR-guided PCI had lower rates of many adverse events. This included risk of bleeding (1.6% vs. 3.8%; P = .009 as defined by type ≥3 Bleeding Academic Research Consortium , acute kidney injury (0.1% vs. 0.9%; P = .04), atrial fibrillation (2.4% vs. 14.1%; P < .001) and rehospitalization within 30 days (5.5% vs. 10.2%; P < .001).
In the context of a modest increase in risk of MACE and the lower rate of several important treatment-related adverse events, the QOL advantages identified in this subanalysis “might be a reasonable topic for patient-shared decision-making,” Dr. Jeremias suggested.
New data might inform patient decision-making
He granted the possibility that well-informed patients might accept the modestly increased risk of MACE for one or more of the outcomes, such as a higher likelihood of an early return to work, that favored FFR-guided PCI.
This is the point of this subanalysis, agreed Dr. Zimmermann.
“It is all about shared decision-making,” he said. Also emphasizing that the negative trial endpoint of FAME 3 “was driven largely by an increased risk of revascularization,” he believes that these new data might be a basis for discussions with patients weighing relative risks and benefits.
There are more data to come, according to Dr. Zimmermann, who said that follow-up of up to 5 years is planned. The 3-year data will be made available in 2023.
Dr. Zimmermann reported no potential conflicts of interest. Dr. Jeremias reported financial relationships with Abbott, ACIST, Boston Scientific, and Volcano. The investigator-initiated trial received research grants from Abbott Vascular and Medtronic.
A new subanalysis of the FAME 3 trial, which failed to show that percutaneous intervention (PCI) guided by fractional flow reserve (FFR) is noninferior to coronary artery bypass grafting (CABG) for treating three-vessel coronary artery disease, has associated PCI with early quality of life (QOL) advantages, according to findings presented at the annual scientific sessions of the American College of Cardiology.
Despite a modestly greater risk of major adverse cardiac events (MACE) at the end of 12 months’ follow-up among those treated with FFR-guided PCI, the greater QOL early after the procedure might be relevant to patients weighing these options, according to Frederik M. Zimmerman, MD, of Catharina Hospital in Eindhoven, the Netherlands.
“FFR-guided PCI results in a faster improvement in quality of life than CABG during the first year after revascularization, and it improved working status in patients younger than 65 years of age,” Dr. Zimmermann said.
The primary results of FAME 3 were presented at the 2021 Transcatheter Cardiovascular Therapeutics annual meeting by lead author William F. Fearon, MD, of Stanford (Calif.) University and published simultaneously in the New England Journal of Medicine.
Rather than confirming the hypothesis that FFR-guided PCI is comparable with CABG for the primary composite MACE outcome death from any cause, myocardial infarction, stroke, or revascularization, the incidence of MACE at 12 months was 10.6% in those randomized to PCI and 6.9% in the group assigned to CABG.
This translated into a hazard ratio for MACE of 1.5, signifying a 50% increase in risk for FFR-guided PCI relative to CABG for the primary outcome, a difference that negated the study definition of noninferiority (P = .35).
In this new health-related subanalysis, which was published simultaneously with his ACC presentation, the groups were compared over 12 months for QOL as measured with European Quality of Life–5 dimensions (EQ-5D) scale, angina as measured with the Canadian Cardiovascular Classification (CCC) system, and employment.
Outcomes data available in >85% of patients
Of the 1,500 patients enrolled and randomized in FAME 3 (757 to FFR-guided PCI and 743 to CABG), this health outcomes subanalysis was performed with complete data at 12 months from 89% of those in the PCI group and 88% of those in the CABG group.
Ultimately, the study did not show differences in any of these measures at the end of 12 months, but there were significant differences in QOL and employment at earlier time points. In particular, the significantly different (P < .001) trajectory for QOL improvement at 1 and 6 months favored FFR-guided PCI whether evaluated with the EQ-5D instrument or an EQ visual analog scale.
Rates of angina defined by as CCC class of at least 2 were low after revascularization in both arms of the study, negating any opportunity for differences, but patients aged younger than 65 years were almost twice as likely to have returned to full- or part-time work 1 month after revascularization (60.2% vs. 33.1%), and they remained at higher odds for working at 12 months (68.1% vs. 57.4%).
In patients aged older than 65 years, return-to-work rates did not differ significantly at any time point.
These results suggest potentially clinically meaningful early advantages for FFR-guided PCI, but some experts questioned the rationale for reporting positive secondary findings from a negative trial.
“This subanalysis is curious,” said Allen Jeremias, MD, director of interventional cardiology research, Saint Francis Hospital, Roslyn, N.Y. He pointed out that reporting these data is an anomaly.
Subanalyses uncommon in negative trials
“CABG was found to be better, so why look at QOL,” said Dr. Jeremias, who was an ACC-invited expert to discuss the results. However, he went on to say, “this could be an exception to the rule.”
The reason, according to Dr. Jeremias, is that the absolute difference at 12 months between FFR-guided PCI and CABG for the MACE events of greatest concern – death, MI, or stroke – was only about 2% greater in the FFR-guided PCI group (7.3% vs. 5.2%). The biggest contributor to the difference in MACE in FAME 3 at 12 months was the higher rate of repeat revascularization (5.9% vs. 3.9%).
Moreover, patients randomized to FFR-guided PCI had lower rates of many adverse events. This included risk of bleeding (1.6% vs. 3.8%; P = .009 as defined by type ≥3 Bleeding Academic Research Consortium , acute kidney injury (0.1% vs. 0.9%; P = .04), atrial fibrillation (2.4% vs. 14.1%; P < .001) and rehospitalization within 30 days (5.5% vs. 10.2%; P < .001).
In the context of a modest increase in risk of MACE and the lower rate of several important treatment-related adverse events, the QOL advantages identified in this subanalysis “might be a reasonable topic for patient-shared decision-making,” Dr. Jeremias suggested.
New data might inform patient decision-making
He granted the possibility that well-informed patients might accept the modestly increased risk of MACE for one or more of the outcomes, such as a higher likelihood of an early return to work, that favored FFR-guided PCI.
This is the point of this subanalysis, agreed Dr. Zimmermann.
“It is all about shared decision-making,” he said. Also emphasizing that the negative trial endpoint of FAME 3 “was driven largely by an increased risk of revascularization,” he believes that these new data might be a basis for discussions with patients weighing relative risks and benefits.
There are more data to come, according to Dr. Zimmermann, who said that follow-up of up to 5 years is planned. The 3-year data will be made available in 2023.
Dr. Zimmermann reported no potential conflicts of interest. Dr. Jeremias reported financial relationships with Abbott, ACIST, Boston Scientific, and Volcano. The investigator-initiated trial received research grants from Abbott Vascular and Medtronic.
FROM ACC 2021
POISE-3 backs wider use of tranexamic acid in noncardiac surgery
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
The antifibrinolytic tranexamic acid (TXA) reduced serious bleeding without a significant effect on major vascular outcomes in patients undergoing noncardiac surgery at risk for these complications in the POISE-3 trial.
TXA cut the primary efficacy outcome of life-threatening, major, and critical organ bleeding at 30 days by 24% compared with placebo (9.1% vs. 11.7%; hazard ratio [HR], 0.76; P < .0001).
The primary safety outcome of myocardial injury after noncardiac surgery (MINS), nonhemorrhagic stroke, peripheral arterial thrombosis, and symptomatic proximal venous thromboembolism (VTE) at 30 days occurred in 14.2% vs.. 13.9% of patients, respectively (HR, 1.023). This failed, however, to meet the study›s threshold to prove TXA noninferior to placebo (one-sided P = .044).
There was no increased risk for death or stroke with TXA, according to results published April 2 in the New England Journal of Medicine.
Principal investigator P.J. Devereaux, MD, PhD, Population Health Research Institute and McMaster University, Hamilton, Ontario, Canada, pointed out that there is only a 4.4% probability that the composite vascular outcome hazard ratio was above the noninferiority margin and that just 10 events separated the two groups (649 vs.. 639).
“Healthcare providers and patients will have to weigh a clear beneficial reduction in the composite bleeding outcome, which is an absolute difference of 2.7%, a result that was highly statistically significant, versus a low probability of a small increase in risk of the composite vascular endpoint, with an absolute difference of 0.3%,” a nonsignificant result, Dr. Devereaux said during the formal presentation of the results at the hybrid annual scientific sessions of the American College of Cardiology.
The findings, he said, should also be put in the context that 300 million adults have a major surgery each year worldwide and most don’t receive TXA. At the same time, there’s an annual global shortage of 30 million blood product units, and surgical bleeding accounts for up to 40% of all transfusions.
“POISE-3 identifies that use of TXA could avoid upwards of 8 million bleeding events resulting in transfusion on an annual basis, indicating potential for large public health and clinical benefit if TXA become standard practice in noncardiac surgery,” Dr. Devereaux said during the late-breaking trial session.
TXA is indicated for heavy menstrual bleeding and hemophilia and has been used in cardiac surgery, but it is increasingly being used in noncardiac surgeries. As previously reported, POISE showed that the beta-blocker metoprolol lowered the risk for myocardial infarction (MI) but increased the risk for severe stroke and overall death, whereas in POISE-2, perioperative low-dose aspirin lowered the risk for MI but was linked to more major bleeding.
The cumulative data have not shown an increased risk for thrombotic events in other settings, Dr. Devereaux told this news organization.
“I’m a cardiologist, and I think that we’ve been guilty at times of always only focusing on the thrombotic side of the equation and ignoring that bleeding is a very important aspect of the circulatory system,” he said. “And I think this shows for the first time clear unequivocal evidence that there’s a cheap, very encouraging, safe way to prevent this.”
“An important point is that if you can give tranexamic acid and prevent bleeding in your cardiac patients having noncardiac surgery, then you can prevent the delay of reinitiating their anticoagulants and their antiplatelets after surgery and getting them back on the medications that are important for them to prevent their cardiovascular event,” Dr. Devereaux added.
Discussant Michael J. Mack, MD, commented that TXA, widely used in cardiac surgery, is an old, inexpensive drug that “should be more widely used in noncardiac surgery.” Dr. Mack, from Baylor Scott & White Health, Dallas, added that he would limit it to major noncardiac surgery.
International trial
PeriOperative ISchemic Evaluation-3 (POISE-3) investigators at 114 hospitals in 22 countries (including countries in North and South America, Europe, and Africa; Russia; India; and Australia) randomly assigned 9,535 patients, aged 45 years or older, with or at risk for cardiovascular and bleeding complications to receive a TXA 1-g intravenous bolus or placebo at the start and end of inpatient noncardiac surgery.
Patients taking at least one long-term antihypertensive medication were also randomly assigned to a perioperative hypotension- or hypertension-avoidance strategy, which differ in the use of antihypertensives on the morning of surgery and the first 2 days after surgery, and in the target mean arterial pressure during surgery. Results from these cohorts will be presented in a separate session on April 4.
The study had planned to enroll 10,000 patients but was stopped early by the steering committee because of financial constraints resulting from slow enrollment during the pandemic. The decision was made without knowledge of the trial results but with knowledge that aggregate composite bleeding and vascular outcomes were higher than originally estimated, Dr. Devereaux noted.
Among all participants, the mean age was 70 years, 56% were male, almost a third had coronary artery disease, 15% had peripheral artery disease, and 8% had a prior stroke. About 80% were undergoing major surgery. Adherence to the study medications was 96.3% in both groups.
Secondary bleeding outcomes were lower in the TXA and placebo groups, including bleeding independently associated with mortality after surgery (8.7% vs. 11.3%), life-threatening bleeding (1.6% vs. 1.7%), major bleeding (7.6% vs. 10.4%), and critical organ bleeding (0.3% vs. 0.4%).
Importantly, the TXA group had significantly lower rates of International Society on Thrombosis and Haemostasis major bleeding (6.6% vs. 8.7%; P = .0001) and the need for transfusion of 1 or more units of packed red blood cells (9.4% vs. 12.0%; P <.0001), Dr. Devereaux noted.
In terms of secondary vascular outcomes, there were no significant differences between the TXA and placebo groups in rates of MINS (12.8% vs. 12.6%), MINS not fulfilling definition of MI (both 11.5%), MI (1.4% vs. 1.1%), and the net risk-benefit outcome (a composite of vascular death and nonfatal life-threatening, major, or critical organ bleeding, MINS, stroke, peripheral arterial thrombosis, and symptomatic proximal VTE; 20.7% vs. 21.9%).
The two groups had similar rates of all-cause (1.1% vs. 1.2%) and vascular (0.5% vs. 0.6%) mortality.
There also were no significant differences in other tertiary outcomes, such as acute kidney injury (14.1% vs. 13.7%), rehospitalization for vascular reasons (1.8% vs. 1.6%), or seizures (0.2% vs. <0.1%). The latter has been a concern, with the risk reported to increase with higher doses.
Subgroup analyses
Preplanned subgroup analyses showed a benefit for TXA over placebo for the primary efficacy outcome in orthopedic and nonorthopedic surgery and in patients with hemoglobin level below 120 g/L or 120 g/L or higher, with an estimated glomerular filtration rate less than 45 mL/min/1.73 m 2 or 45 mL/min/1.73 m 2 or higher, or with an N-terminal pro– B-type natriuretic peptide level below 200 ng/L or 200 ng/L or higher.
For the primary safety outcome, the benefit favored placebo but the interaction was not statistically significant for any of the four subgroups.
A post hoc subgroup analysis also showed similar results across the major categories of surgery, including general, vascular, urologic, and gynecologic, Dr. Devereaux told this news organization.
Although TXA is commonly used in orthopedic procedures, Dr. Devereaux noted, in other types of surgeries, “it’s not used at all.” But because TXA “is so cheap, and we can apply it to a broad population, even at an economic level it looks like it’s a winner to give to almost all patients having noncardiac surgery.”
The team also recently published a risk prediction tool that can help estimate a patient’s baseline risk for bleeding.
“So just using a model, which will bring together the patient’s type of surgery and their risk factors, you can look to see, okay, this is enough risk of bleeding, I’m just going to give tranexamic acid,” he said. “We will also be doing economic analyses because blood is also not cheap.”
The study was funded by the Canadian Institutes of Health Research, National Health and Medical Research Council (Australia), and the Research Grant Council (Hong Kong). Dr. Devereaux reports research/research grants from Abbott Diagnostics, Philips Healthcare, Roche Diagnostics, and Siemens. Dr. Mack reports receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
SCORED: Sotagliflozin shows robust MACE benefit
WASHINGTON – Results from new analyses further fleshed out the potent effect by the investigational SGLT1&2 inhibitor sotagliflozin on major cardiovascular adverse events in patients with type 2 diabetes, chronic kidney disease, and at high risk for cardiovascular disease in the SCORED trial that randomized more than 10,000 patients.
In prespecified, secondary analyses of the SCORED results, treatment with sotagliflozin during a median of 16 months was linked to a significant 21% risk reduction relative to placebo for the combined incidence of total major adverse cardiovascular events (MACE), which included cardiovascular death, first and recurrent episodes of nonfatal MI, and nonfatal stroke among the 5,144 randomized patients who entered the trial with a history of cardiovascular disease (CVD), Deepak L. Bhatt, MD, said at the annual scientific sessions of the American College of Cardiology.
Among the 5,440 patients in the study who did not have a history of CVD (although they did have at least one major risk factor or at least two minor risk factors), treatment with sotagliflozin was linked to a significant 26% relative risk reduction in total MACE events.
Part of these overall MACE benefits resulted from similar improvements from sotagliflozin treatment on the individual outcomes of total nonfatal MI and total nonfatal strokes. Treatment with sotagliflozin cut these MIs by a significant 31% in patients with a history of CVD relative to patients who received placebo, and by a relative 34% in those without a CVD event in their history, a difference compared with placebo that fell short of significance, said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Health, both in Boston.
Treatment with sotagliflozin also cut total nonfatal strokes by 31% relative to placebo in patients with a history of CVD, and by a relative 38% in those without a CVD history. Both differences fell short of significance.
An early MACE benefit and a stroke benefit
“This stroke benefit has not been clearly seen” with any agent from the closely related sodium-glucose cotransport-2 (SGLT2) inhibitor class, and “the MACE benefit appeared very early,” within 3 months from the start of sotagliflozin treatment, “which may be because of the SGLT1 inhibition,” Dr. Bhatt said during his report.
The SGLT1 receptor is the primary mechanism cells in the gut use to absorb glucose and galactose in the human gastrointestinal tract, Dr. Bhatt explained, while the SGLT2 receptor appears on kidney cells and is the major player in the reabsorption of filtered glucose. The SGLT2 inhibitor class includes the agents canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), while sotagliflozin inhibits both SGLT1 and SGLT2.
Main results from SCORED appeared in a report first released in late 2020, and showed that for the study’s primary endpoint treatment with sotagliflozin linked with a significant 26% relative risk reduction for the composite of cardiovascular deaths, hospitalizations for heart failure, and urgent visits for heart failure (N Engl J Med. 2021 Jan 14;384[2]:129-39). Patient follow-up in SCORED was not as long as originally planned when the study stopped early due to a loss of funding from a sponsor that was triggered by the COVID-19 pandemic.
MACE results ‘heterogeneous’ from SGLT2 inhibitors
Sotagliflozin and agents from the SGLT2 inhibitor class “have been consistent” in their benefits for reducing cardiovascular death and hospitalization for heart failure, but for MACE, the results from the SGLT2 inhibitors “have been more heterogeneous,” and the effect of sotagliflozin on MACE “were different in SCORED,” commented Michelle L. O’Donoghue, MD, MPH, a cardiologist at Brigham and Women’s Hospital in Boston who was not involved with this work.
“The results suggest a benefit [from sotagliflozin] on atherosclerotic events, which could be a potential advantage” compared with the SGLT2 inhibitors, “but the heterogeneity of this effect” among these agents means that more confirmatory data are needed for sotagliflozin, Dr. O’Donoghue said in an interview.
“There is a lot of enthusiasm for the concept” of combined inhibition of the SGLT1 and 2 receptors, and if more evidence for unique benefits of this effect accumulate “it may lead to increased enthusiasm for sotagliflozin,” she said. “A lot will also depend on pricing decisions” for sotagliflozin, if it receives U.S. marketing approval from the Food and Drug Administration. Decisions about which agent from the SGLT2 inhibitor class to prescribe “are often being made based on price right now,” Dr. O’Donoghue said.
Lexicon Pharmaceuticals, the company developing sotagliflozin, has announced plans to resubmit its new drug application for sotagliflozin to the FDA later in 2022, with the agency’s approval decision likely occurring late in 2022 or sometime during 2023. In February, the company withdrew its December 2021 application to correct a “technical issue” it had found.
An additional analysis reported by Dr. Bhatt used combined data from SCORED as well as several additional randomized trials of sotagliflozin involving a total of more than 20,000 patients that showed a significant 21% reduction in the incidence of MACE compared with placebo.
During his talk, Dr. Bhatt said that sotagliflozin was potentially superior to the agents that inhibit only SGLT2. In an interview, he based this tentative assessment on at least four attributes of sotagliflozin that have emerged from trial results:
- The drug’s ability to significantly reduce MACE and to have this effect apparent within a few months of treatment onset;
- The significantly reduced rate of stroke with sotagliflozin (when patients are not subdivided into those with or without a history of CVD) that has not yet been seen with any SGLT2 inhibitor;
- The ability of sotagliflozin to reduce hemoglobin A1c levels in patients with type 2 diabetes even when their estimated glomerular filtration rate is less than 30 mL/min per 1.73 m2, an effect not seen with SGLT2 inhibitors and possibly explained by sotagliflozin having an effect on gut absorption of glucose in addition to its SGLT2 inhibitory effect in the kidney;
- And the proven ability of sotagliflozin to be safe and effective when initiated in patients hospitalized for heart failure, a property that so far has only also been shown for the SGLT2 inhibitor empagliflozin in the EMPULSE trial (Nature Med. 2022 Mar;28: 568-74).
SCORED was sponsored by Sanofi and Lexicon Pharmaceuticals, the companies originally developing sotagliflozin, although with the withdrawal of Sanofi’s support, further development is now sponsored entirely by Lexicon. Dr. Bhatt received research funding from Sanofi and Lexicon that was paid to Brigham and Women’s Health, and he has been an advisor to numerous companies. Dr. O’Donoghue has been a consultant to Amgen, Janssen, and Novartis, and has received research funding from Amgen, AZ MedImmune, Intarcia, Janssen, Merck, and Novartis.
WASHINGTON – Results from new analyses further fleshed out the potent effect by the investigational SGLT1&2 inhibitor sotagliflozin on major cardiovascular adverse events in patients with type 2 diabetes, chronic kidney disease, and at high risk for cardiovascular disease in the SCORED trial that randomized more than 10,000 patients.
In prespecified, secondary analyses of the SCORED results, treatment with sotagliflozin during a median of 16 months was linked to a significant 21% risk reduction relative to placebo for the combined incidence of total major adverse cardiovascular events (MACE), which included cardiovascular death, first and recurrent episodes of nonfatal MI, and nonfatal stroke among the 5,144 randomized patients who entered the trial with a history of cardiovascular disease (CVD), Deepak L. Bhatt, MD, said at the annual scientific sessions of the American College of Cardiology.
Among the 5,440 patients in the study who did not have a history of CVD (although they did have at least one major risk factor or at least two minor risk factors), treatment with sotagliflozin was linked to a significant 26% relative risk reduction in total MACE events.
Part of these overall MACE benefits resulted from similar improvements from sotagliflozin treatment on the individual outcomes of total nonfatal MI and total nonfatal strokes. Treatment with sotagliflozin cut these MIs by a significant 31% in patients with a history of CVD relative to patients who received placebo, and by a relative 34% in those without a CVD event in their history, a difference compared with placebo that fell short of significance, said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Health, both in Boston.
Treatment with sotagliflozin also cut total nonfatal strokes by 31% relative to placebo in patients with a history of CVD, and by a relative 38% in those without a CVD history. Both differences fell short of significance.
An early MACE benefit and a stroke benefit
“This stroke benefit has not been clearly seen” with any agent from the closely related sodium-glucose cotransport-2 (SGLT2) inhibitor class, and “the MACE benefit appeared very early,” within 3 months from the start of sotagliflozin treatment, “which may be because of the SGLT1 inhibition,” Dr. Bhatt said during his report.
The SGLT1 receptor is the primary mechanism cells in the gut use to absorb glucose and galactose in the human gastrointestinal tract, Dr. Bhatt explained, while the SGLT2 receptor appears on kidney cells and is the major player in the reabsorption of filtered glucose. The SGLT2 inhibitor class includes the agents canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), while sotagliflozin inhibits both SGLT1 and SGLT2.
Main results from SCORED appeared in a report first released in late 2020, and showed that for the study’s primary endpoint treatment with sotagliflozin linked with a significant 26% relative risk reduction for the composite of cardiovascular deaths, hospitalizations for heart failure, and urgent visits for heart failure (N Engl J Med. 2021 Jan 14;384[2]:129-39). Patient follow-up in SCORED was not as long as originally planned when the study stopped early due to a loss of funding from a sponsor that was triggered by the COVID-19 pandemic.
MACE results ‘heterogeneous’ from SGLT2 inhibitors
Sotagliflozin and agents from the SGLT2 inhibitor class “have been consistent” in their benefits for reducing cardiovascular death and hospitalization for heart failure, but for MACE, the results from the SGLT2 inhibitors “have been more heterogeneous,” and the effect of sotagliflozin on MACE “were different in SCORED,” commented Michelle L. O’Donoghue, MD, MPH, a cardiologist at Brigham and Women’s Hospital in Boston who was not involved with this work.
“The results suggest a benefit [from sotagliflozin] on atherosclerotic events, which could be a potential advantage” compared with the SGLT2 inhibitors, “but the heterogeneity of this effect” among these agents means that more confirmatory data are needed for sotagliflozin, Dr. O’Donoghue said in an interview.
“There is a lot of enthusiasm for the concept” of combined inhibition of the SGLT1 and 2 receptors, and if more evidence for unique benefits of this effect accumulate “it may lead to increased enthusiasm for sotagliflozin,” she said. “A lot will also depend on pricing decisions” for sotagliflozin, if it receives U.S. marketing approval from the Food and Drug Administration. Decisions about which agent from the SGLT2 inhibitor class to prescribe “are often being made based on price right now,” Dr. O’Donoghue said.
Lexicon Pharmaceuticals, the company developing sotagliflozin, has announced plans to resubmit its new drug application for sotagliflozin to the FDA later in 2022, with the agency’s approval decision likely occurring late in 2022 or sometime during 2023. In February, the company withdrew its December 2021 application to correct a “technical issue” it had found.
An additional analysis reported by Dr. Bhatt used combined data from SCORED as well as several additional randomized trials of sotagliflozin involving a total of more than 20,000 patients that showed a significant 21% reduction in the incidence of MACE compared with placebo.
During his talk, Dr. Bhatt said that sotagliflozin was potentially superior to the agents that inhibit only SGLT2. In an interview, he based this tentative assessment on at least four attributes of sotagliflozin that have emerged from trial results:
- The drug’s ability to significantly reduce MACE and to have this effect apparent within a few months of treatment onset;
- The significantly reduced rate of stroke with sotagliflozin (when patients are not subdivided into those with or without a history of CVD) that has not yet been seen with any SGLT2 inhibitor;
- The ability of sotagliflozin to reduce hemoglobin A1c levels in patients with type 2 diabetes even when their estimated glomerular filtration rate is less than 30 mL/min per 1.73 m2, an effect not seen with SGLT2 inhibitors and possibly explained by sotagliflozin having an effect on gut absorption of glucose in addition to its SGLT2 inhibitory effect in the kidney;
- And the proven ability of sotagliflozin to be safe and effective when initiated in patients hospitalized for heart failure, a property that so far has only also been shown for the SGLT2 inhibitor empagliflozin in the EMPULSE trial (Nature Med. 2022 Mar;28: 568-74).
SCORED was sponsored by Sanofi and Lexicon Pharmaceuticals, the companies originally developing sotagliflozin, although with the withdrawal of Sanofi’s support, further development is now sponsored entirely by Lexicon. Dr. Bhatt received research funding from Sanofi and Lexicon that was paid to Brigham and Women’s Health, and he has been an advisor to numerous companies. Dr. O’Donoghue has been a consultant to Amgen, Janssen, and Novartis, and has received research funding from Amgen, AZ MedImmune, Intarcia, Janssen, Merck, and Novartis.
WASHINGTON – Results from new analyses further fleshed out the potent effect by the investigational SGLT1&2 inhibitor sotagliflozin on major cardiovascular adverse events in patients with type 2 diabetes, chronic kidney disease, and at high risk for cardiovascular disease in the SCORED trial that randomized more than 10,000 patients.
In prespecified, secondary analyses of the SCORED results, treatment with sotagliflozin during a median of 16 months was linked to a significant 21% risk reduction relative to placebo for the combined incidence of total major adverse cardiovascular events (MACE), which included cardiovascular death, first and recurrent episodes of nonfatal MI, and nonfatal stroke among the 5,144 randomized patients who entered the trial with a history of cardiovascular disease (CVD), Deepak L. Bhatt, MD, said at the annual scientific sessions of the American College of Cardiology.
Among the 5,440 patients in the study who did not have a history of CVD (although they did have at least one major risk factor or at least two minor risk factors), treatment with sotagliflozin was linked to a significant 26% relative risk reduction in total MACE events.
Part of these overall MACE benefits resulted from similar improvements from sotagliflozin treatment on the individual outcomes of total nonfatal MI and total nonfatal strokes. Treatment with sotagliflozin cut these MIs by a significant 31% in patients with a history of CVD relative to patients who received placebo, and by a relative 34% in those without a CVD event in their history, a difference compared with placebo that fell short of significance, said Dr. Bhatt, professor of medicine at Harvard Medical School and executive director of interventional cardiovascular programs at Brigham and Women’s Health, both in Boston.
Treatment with sotagliflozin also cut total nonfatal strokes by 31% relative to placebo in patients with a history of CVD, and by a relative 38% in those without a CVD history. Both differences fell short of significance.
An early MACE benefit and a stroke benefit
“This stroke benefit has not been clearly seen” with any agent from the closely related sodium-glucose cotransport-2 (SGLT2) inhibitor class, and “the MACE benefit appeared very early,” within 3 months from the start of sotagliflozin treatment, “which may be because of the SGLT1 inhibition,” Dr. Bhatt said during his report.
The SGLT1 receptor is the primary mechanism cells in the gut use to absorb glucose and galactose in the human gastrointestinal tract, Dr. Bhatt explained, while the SGLT2 receptor appears on kidney cells and is the major player in the reabsorption of filtered glucose. The SGLT2 inhibitor class includes the agents canagliflozin (Invokana), dapagliflozin (Farxiga), and empagliflozin (Jardiance), while sotagliflozin inhibits both SGLT1 and SGLT2.
Main results from SCORED appeared in a report first released in late 2020, and showed that for the study’s primary endpoint treatment with sotagliflozin linked with a significant 26% relative risk reduction for the composite of cardiovascular deaths, hospitalizations for heart failure, and urgent visits for heart failure (N Engl J Med. 2021 Jan 14;384[2]:129-39). Patient follow-up in SCORED was not as long as originally planned when the study stopped early due to a loss of funding from a sponsor that was triggered by the COVID-19 pandemic.
MACE results ‘heterogeneous’ from SGLT2 inhibitors
Sotagliflozin and agents from the SGLT2 inhibitor class “have been consistent” in their benefits for reducing cardiovascular death and hospitalization for heart failure, but for MACE, the results from the SGLT2 inhibitors “have been more heterogeneous,” and the effect of sotagliflozin on MACE “were different in SCORED,” commented Michelle L. O’Donoghue, MD, MPH, a cardiologist at Brigham and Women’s Hospital in Boston who was not involved with this work.
“The results suggest a benefit [from sotagliflozin] on atherosclerotic events, which could be a potential advantage” compared with the SGLT2 inhibitors, “but the heterogeneity of this effect” among these agents means that more confirmatory data are needed for sotagliflozin, Dr. O’Donoghue said in an interview.
“There is a lot of enthusiasm for the concept” of combined inhibition of the SGLT1 and 2 receptors, and if more evidence for unique benefits of this effect accumulate “it may lead to increased enthusiasm for sotagliflozin,” she said. “A lot will also depend on pricing decisions” for sotagliflozin, if it receives U.S. marketing approval from the Food and Drug Administration. Decisions about which agent from the SGLT2 inhibitor class to prescribe “are often being made based on price right now,” Dr. O’Donoghue said.
Lexicon Pharmaceuticals, the company developing sotagliflozin, has announced plans to resubmit its new drug application for sotagliflozin to the FDA later in 2022, with the agency’s approval decision likely occurring late in 2022 or sometime during 2023. In February, the company withdrew its December 2021 application to correct a “technical issue” it had found.
An additional analysis reported by Dr. Bhatt used combined data from SCORED as well as several additional randomized trials of sotagliflozin involving a total of more than 20,000 patients that showed a significant 21% reduction in the incidence of MACE compared with placebo.
During his talk, Dr. Bhatt said that sotagliflozin was potentially superior to the agents that inhibit only SGLT2. In an interview, he based this tentative assessment on at least four attributes of sotagliflozin that have emerged from trial results:
- The drug’s ability to significantly reduce MACE and to have this effect apparent within a few months of treatment onset;
- The significantly reduced rate of stroke with sotagliflozin (when patients are not subdivided into those with or without a history of CVD) that has not yet been seen with any SGLT2 inhibitor;
- The ability of sotagliflozin to reduce hemoglobin A1c levels in patients with type 2 diabetes even when their estimated glomerular filtration rate is less than 30 mL/min per 1.73 m2, an effect not seen with SGLT2 inhibitors and possibly explained by sotagliflozin having an effect on gut absorption of glucose in addition to its SGLT2 inhibitory effect in the kidney;
- And the proven ability of sotagliflozin to be safe and effective when initiated in patients hospitalized for heart failure, a property that so far has only also been shown for the SGLT2 inhibitor empagliflozin in the EMPULSE trial (Nature Med. 2022 Mar;28: 568-74).
SCORED was sponsored by Sanofi and Lexicon Pharmaceuticals, the companies originally developing sotagliflozin, although with the withdrawal of Sanofi’s support, further development is now sponsored entirely by Lexicon. Dr. Bhatt received research funding from Sanofi and Lexicon that was paid to Brigham and Women’s Health, and he has been an advisor to numerous companies. Dr. O’Donoghue has been a consultant to Amgen, Janssen, and Novartis, and has received research funding from Amgen, AZ MedImmune, Intarcia, Janssen, Merck, and Novartis.
AT ACC 2022
Hypertension control during pregnancy validated in major trial
Pregnant women with even mild hypertension should receive blood pressure–lowering medications to reduce the likelihood of adverse outcomes for the mother and the child, according to a large, open-label, randomized trial.
“Treating to the blood pressure goal in this study reduced the risk of adverse events associated with pregnancy but did not impair fetal growth,” Alan T. Tita, MD, PhD, associate dean for Global and Women’s Health, University of Alabama, Birmingham, reported at the annual scientific sessions of the American College of Cardiology.
The question of whether to treat chronic hypertension during pregnancy has been “an international controversy for decades,” said Dr. Tita, who led the investigator-initiated Chronic Hypertension and Pregnancy (CHAP) trial.
For the composite primary outcome of severe preeclampsia, medically indicated preterm birth at less than 35 weeks of gestation, placental abruption, or fetal/neonatal death, the treatment of hypertension versus no treatment showed a relative risk reduction of 18% (30.2% vs. 37%, (hazard ratio, 0.82; P < .001).
Small for gestational age is primary safety endpoint
An increase in preeclampsia risk in women whose fetus was small for gestational age (SGA), a theoretical consequence of reductions in arterial pressure, was not seen. The rate of SGA, defined as below the 10th percentile, was slightly higher in the treatment group (11.2% vs. 10.4%), but the difference did not approach significance (P = 0.76).
By answering this long-pending question, the CHAP data are “practice changing,” declared an ACC-invited commentator, Athena Poppas, MD, chief of cardiology and director of the Lifespan Cardiovascular Institute, Providence, R.I. She agreed that the need for treatment of mild chronic hypertension has been a dilemma for clinicians that is now acceptably resolved.
In this trial, 2,408 pregnant women with chronic mild hypertension defined as a blood pressure of 160/90 mm Hg were randomized to treatment with a goal blood pressure of less than 140/90 mm Hg or no treatment unless the blood pressure rose to at least 160/105. All women had singleton pregnancies. Enrollment before 23 weeks of gestation was required. Severe hypertension (at least 160/105 mm Hg) was an exclusion criterion, as were several comorbidities, such as kidney disease.
Combination therapy accepted for <140/90 mm Hg goal
The beta-blocker labetalol or the calcium channel blocker nifedipine as single agents were the preferred antihypertensive medications in the protocol, but other medications were permitted. To reach the blood pressure goal, the single-agent therapy was titrated to the maximum dose before starting a second agent.
After randomization the systolic and diastolic blood pressures fell in both groups, but they fell more and remained consistently lower in the active treatment group, particularly during the first 20 weeks after randomization, according to graphs displayed by Dr. Tita. Over the course of the study, the mean diastolic blood pressures were 129.5 and 132.6 mm Hg in the active treatment and control groups, respectively, while the systolic pressures were 79.1 vs. 81.5 mm Hg.
When the components of the primary outcome were evaluated separately, the greatest advantage of treatment was the reduction in the rate of severe eclampsia (23.3% vs. 29.1%; HR, 0.80: 95% confidence interval, 0.70-0.92) and preterm birth (12.2% vs. 16.7%; HR, 0.73: 95% CI, 0.60-0.89).
Across a large array of subgroups, including those with or without diabetes and those treated before or after 14 weeks of gestation, there was a consistent advantage for treatment, even if not statistically different. It is notable that 48% of patients were Black and 35% had a body mass index of at least 40. The active treatment was favored across all groups stratified by these characteristics.
Although the incidences of placental abruption (1.7% on treatment vs. 1.9% without) and fetal or neonatal death (3.5% vs. 4.3%) were lower in the active treatment group, they were uncommon events in both arms of the study. The differences did not reach statistical significance.
Maternal morbidity rates lower on treatment
Severe SGA, which was defined as below the 5th percentile, was also numerically but not significantly higher in the control arm than in the group receiving treatment (5.1% vs. 5.5%), but the incidence of composite adverse maternal events was numerically lower (2.1% vs. 2.8%). The incidences of all components of maternal morbidity, such as maternal death (0.1% vs. 0.2%) pulmonary edema (0.4% vs. 0.9%), heart failure (0.1% vs. 0.1%), and acute kidney injury (0.8% vs. 1.2%), were either lower or the same on active treatment versus no treatment.
According to Dr. Tita, who called CHAP one of the largest and most diverse studies to address the value of treating mild hypertension in pregnancy, the American College of Obstetricians and Gynecologists (ACOG) is evaluating these data for changing their current guidelines for managing hypertension during pregnancy.
“The rate of chronic hypertension during pregnancy has been rising in the United States due to the increase in the average age of pregnant women and the rising rates of obesity,” Dr. Tita commented.
“We definitely needed these data,” said Mary Norine Walsh, MD, medical director, Ascension Saint Vincent Cardiovascular Research Institute, Indianapolis. Not only has the value of treating mild hypertension been unresolved, but Dr. Walsh pointed out that the rates of maternal mortality in the United States are rising and now generally exceed those of many other developed countries.
There are several features in the design of this trial that make the results even more salient to clinical practice, according to Dr. Walsh. This includes the fact that about half of patients enrolled were on Medicaid. As a result, the study confirmed benefit in what Dr. Walsh characterized as a “vulnerable” population.
“We will be busy now to make sure that our [pregnant] patients are achieving these target blood pressures,” Dr. Walsh said. She indicated that CHAP validates the treatment target of 140/90 mm Hg as a standard of care.
The results were published in the New England Journal of Medicine simultaneously with its ACC presentation.
The trial was funded by the National Heart, Lung, and Blood Institute. Dr. Tita reports research grants from Pfizer. Dr. Walsh reports a financial relationship with EBR Systems. Dr. Poppas reports no potential conflicts of interest.
Pregnant women with even mild hypertension should receive blood pressure–lowering medications to reduce the likelihood of adverse outcomes for the mother and the child, according to a large, open-label, randomized trial.
“Treating to the blood pressure goal in this study reduced the risk of adverse events associated with pregnancy but did not impair fetal growth,” Alan T. Tita, MD, PhD, associate dean for Global and Women’s Health, University of Alabama, Birmingham, reported at the annual scientific sessions of the American College of Cardiology.
The question of whether to treat chronic hypertension during pregnancy has been “an international controversy for decades,” said Dr. Tita, who led the investigator-initiated Chronic Hypertension and Pregnancy (CHAP) trial.
For the composite primary outcome of severe preeclampsia, medically indicated preterm birth at less than 35 weeks of gestation, placental abruption, or fetal/neonatal death, the treatment of hypertension versus no treatment showed a relative risk reduction of 18% (30.2% vs. 37%, (hazard ratio, 0.82; P < .001).
Small for gestational age is primary safety endpoint
An increase in preeclampsia risk in women whose fetus was small for gestational age (SGA), a theoretical consequence of reductions in arterial pressure, was not seen. The rate of SGA, defined as below the 10th percentile, was slightly higher in the treatment group (11.2% vs. 10.4%), but the difference did not approach significance (P = 0.76).
By answering this long-pending question, the CHAP data are “practice changing,” declared an ACC-invited commentator, Athena Poppas, MD, chief of cardiology and director of the Lifespan Cardiovascular Institute, Providence, R.I. She agreed that the need for treatment of mild chronic hypertension has been a dilemma for clinicians that is now acceptably resolved.
In this trial, 2,408 pregnant women with chronic mild hypertension defined as a blood pressure of 160/90 mm Hg were randomized to treatment with a goal blood pressure of less than 140/90 mm Hg or no treatment unless the blood pressure rose to at least 160/105. All women had singleton pregnancies. Enrollment before 23 weeks of gestation was required. Severe hypertension (at least 160/105 mm Hg) was an exclusion criterion, as were several comorbidities, such as kidney disease.
Combination therapy accepted for <140/90 mm Hg goal
The beta-blocker labetalol or the calcium channel blocker nifedipine as single agents were the preferred antihypertensive medications in the protocol, but other medications were permitted. To reach the blood pressure goal, the single-agent therapy was titrated to the maximum dose before starting a second agent.
After randomization the systolic and diastolic blood pressures fell in both groups, but they fell more and remained consistently lower in the active treatment group, particularly during the first 20 weeks after randomization, according to graphs displayed by Dr. Tita. Over the course of the study, the mean diastolic blood pressures were 129.5 and 132.6 mm Hg in the active treatment and control groups, respectively, while the systolic pressures were 79.1 vs. 81.5 mm Hg.
When the components of the primary outcome were evaluated separately, the greatest advantage of treatment was the reduction in the rate of severe eclampsia (23.3% vs. 29.1%; HR, 0.80: 95% confidence interval, 0.70-0.92) and preterm birth (12.2% vs. 16.7%; HR, 0.73: 95% CI, 0.60-0.89).
Across a large array of subgroups, including those with or without diabetes and those treated before or after 14 weeks of gestation, there was a consistent advantage for treatment, even if not statistically different. It is notable that 48% of patients were Black and 35% had a body mass index of at least 40. The active treatment was favored across all groups stratified by these characteristics.
Although the incidences of placental abruption (1.7% on treatment vs. 1.9% without) and fetal or neonatal death (3.5% vs. 4.3%) were lower in the active treatment group, they were uncommon events in both arms of the study. The differences did not reach statistical significance.
Maternal morbidity rates lower on treatment
Severe SGA, which was defined as below the 5th percentile, was also numerically but not significantly higher in the control arm than in the group receiving treatment (5.1% vs. 5.5%), but the incidence of composite adverse maternal events was numerically lower (2.1% vs. 2.8%). The incidences of all components of maternal morbidity, such as maternal death (0.1% vs. 0.2%) pulmonary edema (0.4% vs. 0.9%), heart failure (0.1% vs. 0.1%), and acute kidney injury (0.8% vs. 1.2%), were either lower or the same on active treatment versus no treatment.
According to Dr. Tita, who called CHAP one of the largest and most diverse studies to address the value of treating mild hypertension in pregnancy, the American College of Obstetricians and Gynecologists (ACOG) is evaluating these data for changing their current guidelines for managing hypertension during pregnancy.
“The rate of chronic hypertension during pregnancy has been rising in the United States due to the increase in the average age of pregnant women and the rising rates of obesity,” Dr. Tita commented.
“We definitely needed these data,” said Mary Norine Walsh, MD, medical director, Ascension Saint Vincent Cardiovascular Research Institute, Indianapolis. Not only has the value of treating mild hypertension been unresolved, but Dr. Walsh pointed out that the rates of maternal mortality in the United States are rising and now generally exceed those of many other developed countries.
There are several features in the design of this trial that make the results even more salient to clinical practice, according to Dr. Walsh. This includes the fact that about half of patients enrolled were on Medicaid. As a result, the study confirmed benefit in what Dr. Walsh characterized as a “vulnerable” population.
“We will be busy now to make sure that our [pregnant] patients are achieving these target blood pressures,” Dr. Walsh said. She indicated that CHAP validates the treatment target of 140/90 mm Hg as a standard of care.
The results were published in the New England Journal of Medicine simultaneously with its ACC presentation.
The trial was funded by the National Heart, Lung, and Blood Institute. Dr. Tita reports research grants from Pfizer. Dr. Walsh reports a financial relationship with EBR Systems. Dr. Poppas reports no potential conflicts of interest.
Pregnant women with even mild hypertension should receive blood pressure–lowering medications to reduce the likelihood of adverse outcomes for the mother and the child, according to a large, open-label, randomized trial.
“Treating to the blood pressure goal in this study reduced the risk of adverse events associated with pregnancy but did not impair fetal growth,” Alan T. Tita, MD, PhD, associate dean for Global and Women’s Health, University of Alabama, Birmingham, reported at the annual scientific sessions of the American College of Cardiology.
The question of whether to treat chronic hypertension during pregnancy has been “an international controversy for decades,” said Dr. Tita, who led the investigator-initiated Chronic Hypertension and Pregnancy (CHAP) trial.
For the composite primary outcome of severe preeclampsia, medically indicated preterm birth at less than 35 weeks of gestation, placental abruption, or fetal/neonatal death, the treatment of hypertension versus no treatment showed a relative risk reduction of 18% (30.2% vs. 37%, (hazard ratio, 0.82; P < .001).
Small for gestational age is primary safety endpoint
An increase in preeclampsia risk in women whose fetus was small for gestational age (SGA), a theoretical consequence of reductions in arterial pressure, was not seen. The rate of SGA, defined as below the 10th percentile, was slightly higher in the treatment group (11.2% vs. 10.4%), but the difference did not approach significance (P = 0.76).
By answering this long-pending question, the CHAP data are “practice changing,” declared an ACC-invited commentator, Athena Poppas, MD, chief of cardiology and director of the Lifespan Cardiovascular Institute, Providence, R.I. She agreed that the need for treatment of mild chronic hypertension has been a dilemma for clinicians that is now acceptably resolved.
In this trial, 2,408 pregnant women with chronic mild hypertension defined as a blood pressure of 160/90 mm Hg were randomized to treatment with a goal blood pressure of less than 140/90 mm Hg or no treatment unless the blood pressure rose to at least 160/105. All women had singleton pregnancies. Enrollment before 23 weeks of gestation was required. Severe hypertension (at least 160/105 mm Hg) was an exclusion criterion, as were several comorbidities, such as kidney disease.
Combination therapy accepted for <140/90 mm Hg goal
The beta-blocker labetalol or the calcium channel blocker nifedipine as single agents were the preferred antihypertensive medications in the protocol, but other medications were permitted. To reach the blood pressure goal, the single-agent therapy was titrated to the maximum dose before starting a second agent.
After randomization the systolic and diastolic blood pressures fell in both groups, but they fell more and remained consistently lower in the active treatment group, particularly during the first 20 weeks after randomization, according to graphs displayed by Dr. Tita. Over the course of the study, the mean diastolic blood pressures were 129.5 and 132.6 mm Hg in the active treatment and control groups, respectively, while the systolic pressures were 79.1 vs. 81.5 mm Hg.
When the components of the primary outcome were evaluated separately, the greatest advantage of treatment was the reduction in the rate of severe eclampsia (23.3% vs. 29.1%; HR, 0.80: 95% confidence interval, 0.70-0.92) and preterm birth (12.2% vs. 16.7%; HR, 0.73: 95% CI, 0.60-0.89).
Across a large array of subgroups, including those with or without diabetes and those treated before or after 14 weeks of gestation, there was a consistent advantage for treatment, even if not statistically different. It is notable that 48% of patients were Black and 35% had a body mass index of at least 40. The active treatment was favored across all groups stratified by these characteristics.
Although the incidences of placental abruption (1.7% on treatment vs. 1.9% without) and fetal or neonatal death (3.5% vs. 4.3%) were lower in the active treatment group, they were uncommon events in both arms of the study. The differences did not reach statistical significance.
Maternal morbidity rates lower on treatment
Severe SGA, which was defined as below the 5th percentile, was also numerically but not significantly higher in the control arm than in the group receiving treatment (5.1% vs. 5.5%), but the incidence of composite adverse maternal events was numerically lower (2.1% vs. 2.8%). The incidences of all components of maternal morbidity, such as maternal death (0.1% vs. 0.2%) pulmonary edema (0.4% vs. 0.9%), heart failure (0.1% vs. 0.1%), and acute kidney injury (0.8% vs. 1.2%), were either lower or the same on active treatment versus no treatment.
According to Dr. Tita, who called CHAP one of the largest and most diverse studies to address the value of treating mild hypertension in pregnancy, the American College of Obstetricians and Gynecologists (ACOG) is evaluating these data for changing their current guidelines for managing hypertension during pregnancy.
“The rate of chronic hypertension during pregnancy has been rising in the United States due to the increase in the average age of pregnant women and the rising rates of obesity,” Dr. Tita commented.
“We definitely needed these data,” said Mary Norine Walsh, MD, medical director, Ascension Saint Vincent Cardiovascular Research Institute, Indianapolis. Not only has the value of treating mild hypertension been unresolved, but Dr. Walsh pointed out that the rates of maternal mortality in the United States are rising and now generally exceed those of many other developed countries.
There are several features in the design of this trial that make the results even more salient to clinical practice, according to Dr. Walsh. This includes the fact that about half of patients enrolled were on Medicaid. As a result, the study confirmed benefit in what Dr. Walsh characterized as a “vulnerable” population.
“We will be busy now to make sure that our [pregnant] patients are achieving these target blood pressures,” Dr. Walsh said. She indicated that CHAP validates the treatment target of 140/90 mm Hg as a standard of care.
The results were published in the New England Journal of Medicine simultaneously with its ACC presentation.
The trial was funded by the National Heart, Lung, and Blood Institute. Dr. Tita reports research grants from Pfizer. Dr. Walsh reports a financial relationship with EBR Systems. Dr. Poppas reports no potential conflicts of interest.
FROM ACC 2022
Low-sodium diet did not cut clinical events in heart failure trial
A low-sodium diet was not associated with a reduction in future clinical events in a new study in ambulatory patients with heart failure. But there was a moderate benefit on quality of life and New York Heart Association (NYHA) functional class.
The results of the SODIUM-HF trial were presented April 2 at the annual scientific sessions of the American College of Cardiology, conducted virtually and in person in Washington. They were also simultaneously published online in the Lancet.
The study found that a strategy to reduce dietary sodium intake to less than 1,500 mg daily was not more effective than usual care in reducing the primary endpoint of risk for hospitalization or emergency department visits due to cardiovascular causes or all-cause death at 12 months.
“This is the largest and longest trial to look at the question of reducing dietary sodium in heart failure patients,” lead author Justin Ezekowitz, MBBCh, from the Canadian VIGOUR Center at the University of Alberta, Edmonton, Canada, told this news organization.
But he pointed out that there were fewer events than expected in the study, which was stopped early because of a combination of futility and practical difficulties caused by the COVID pandemic, so it could have been underpowered. Dr. Ezekowitz also suggested that a greater reduction in sodium than achieved in this study or a longer follow-up may be required to show an effect on clinical events.
“We hope others will do additional studies of sodium as well as other dietary recommendations as part of a comprehensive diet for heart failure patients,” he commented.
Dr. Ezekowitz said that the study results did not allow blanket recommendations to be made on reducing sodium intake in heart failure.
But he added: “I don’t think we should write off sodium reduction in this population. I think we can tell patients that reducing dietary sodium may potentially improve symptoms and quality of life, and I will continue to recommend reducing sodium as part of an overall healthy diet. We don’t want to throw the baby out with the bathwater.”
Dr. Ezekowitz noted that heart failure is associated with neurohormonal activation and abnormalities in autonomic control that lead to sodium and water retention; thus, dietary restriction of sodium has been historically endorsed as a mechanism to prevent fluid overload and subsequent clinical outcomes; however, clinical trials so far have shown mixed results.
“The guidelines used to strongly recommend a reduction in sodium intake in heart failure patients, but this advice has backed off in recent years because of the lack of data. Most heart failure guidelines now do not make any recommendations on dietary sodium,” he said.
SODIUM-HF was a pragmatic, multinational, open-label, randomized trial conducted in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand), which included 809 patients (median age, 67 years) with chronic heart failure (NYHA functional class II–III) who were receiving optimally tolerated guideline-directed medical treatment. They were randomly assigned to usual care according to local guidelines or a low-sodium diet of less than 100 mmol (<1,500 mg/day). Patients with a baseline sodium intake of less than 1,500 mg/day were excluded.
In the intervention group, patients were asked to follow low-sodium menus developed by dietitians localized to each region. They also received behavioral counseling by trained dietitians or physicians or nurses.
Dietary sodium intake was assessed by using a 3-day food record (including 1 weekend day) at baseline, 6 months, and 12 months in both groups and, for the intervention group, also at 3 and 9 months to monitor and support dietary adherence.
Dr. Ezekowitz explained that although the best method for measuring sodium levels would normally be a 24-hour urine sodium, this would be impractical in a large clinical trial. In addition, he pointed out that urinary sodium is not an accurate measure of actual sodium levels in patients taking diuretics, so it is not a good measure to use in a heart failure population.
“The food record method of assessing sodium levels has been well validated; I think we measured it as accurately as we could have done,” he added.
Results showed that between baseline and 12 months, the median sodium intake decreased from 2,286 mg/day to 1,658 mg/day in the low-sodium group and from 2,119 mg/day to 2,073 mg/day in the usual care group. The median difference between groups was 415 mg/day at 12 months.
By 12 months, events comprising the primary outcome (hospitalization or emergency department visits due to cardiovascular causes or all-cause death) had occurred in 15% of patients in the low-sodium diet group and 17% of those in the usual care group (hazard ratio [HR], 0.89 [95% CI, 0.63 - 1.26]; P = .53).
All-cause death occurred in 6% of patients in the low-sodium diet group and 4% of those in the usual care group (HR, 1.38; P = .32). Cardiovascular-related hospitalization occurred in 10% of the low-sodium group and 12% of the usual care group (HR, 0.82; P = .36), and cardiovascular-related emergency department visits occurred in 4% of both groups (HR, 1.21; P = .60).
The absence of treatment effect for the primary outcome was consistent across most prespecified subgroups, including those with higher vs lower baseline sodium intake. But there was a suggestion of a greater reduction in the primary outcome in individuals younger than age 65 years than in those age 65 years and older.
Quality-of-life measures on the Kansas City Cardiomyopathy Questionnaire (KCCQ) suggested a benefit in the low-sodium group, with mean between-group differences in the change from baseline to 12 months of 3.38 points in the overall summary score, 3.29 points in the clinical summary score, and 3.77 points in the physical limitation score (all differences were statistically significant).
There was no significant difference in 6-minute-walk distance at 12 months between the low-sodium diet group and the usual care group.
NYHA functional class at 12 months differed significantly between groups; the low-sodium diet group had a greater likelihood of improving by one NYHA class than the usual care group (odds ratio, 0.59; P = .0061).
No safety events related to the study treatment were reported in either group.
Dr. Ezekowitz said that to investigate whether longer follow-up may show a difference in events, further analyses are planned at 2 years and 5 years.
Questions on food recall and blinding
Commenting on the findings at the late-breaking clinical trials session at the ACC meeting, Biykem Bozkurt, MD, professor of medicine at Baylor College of Medicine, Houston, congratulated Dr. Ezekowitz on conducting this trial.
“We have been chasing the holy grail of sodium reduction in heart failure for a very long time, so I have to commend you and your team for taking on this challenge, especially during the pandemic,” she said.
But Dr. Bozkurt questioned whether the intervention group actually had a meaningful sodium reduction given that this was measured by food recall and this may have been accounted for by under-reporting of certain food intakes.
Dr. Ezekowitz responded that patients acted as their own controls in that calorie intake, fluid intake, and weight were also assessed and did not change. “So I think we did have a meaningful reduction in sodium,” he said.
Dr. Bozkurt also queried whether the improvements in quality of life and functional status were reliable given that this was an unblinded study.
To this point, Dr. Ezekowitz pointed out that the KCCQ quality-of-life measure was a highly validated instrument and that improvements were seen in these measures at 3, 6, and 12 months. “It is not like these were spurious findings, so I think we have to look at this as a real result,” he argued.
Commenting on the study at an ACC press conference, Mary Norine Walsh, MD, director of the heart failure and cardiac transplantation programs at St. Vincent Heart Center in Indianapolis, said the trial had answered two important questions: that sodium reduction in heart failure may not reduce heart failure hospitalization/death but that patients feel better.
“I think we can safely tell patients that if they slip up a bit they may not end up in hospital,” she added.
This study was funded by the Canadian Institutes of Health Research and the University Hospital Foundation (Edmonton, Alberta, Canada) and the Health Research Council of New Zealand. Dr. Ezekowitz reports research grants from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, eko.ai, US2.ai, Merck, Novartis, Otsuka, Sanofi, and Servier and consulting fees from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Merck, Novartis, Otsuka, Sanofi, and Servier.
A version of this article first appeared on Medscape.com.
A low-sodium diet was not associated with a reduction in future clinical events in a new study in ambulatory patients with heart failure. But there was a moderate benefit on quality of life and New York Heart Association (NYHA) functional class.
The results of the SODIUM-HF trial were presented April 2 at the annual scientific sessions of the American College of Cardiology, conducted virtually and in person in Washington. They were also simultaneously published online in the Lancet.
The study found that a strategy to reduce dietary sodium intake to less than 1,500 mg daily was not more effective than usual care in reducing the primary endpoint of risk for hospitalization or emergency department visits due to cardiovascular causes or all-cause death at 12 months.
“This is the largest and longest trial to look at the question of reducing dietary sodium in heart failure patients,” lead author Justin Ezekowitz, MBBCh, from the Canadian VIGOUR Center at the University of Alberta, Edmonton, Canada, told this news organization.
But he pointed out that there were fewer events than expected in the study, which was stopped early because of a combination of futility and practical difficulties caused by the COVID pandemic, so it could have been underpowered. Dr. Ezekowitz also suggested that a greater reduction in sodium than achieved in this study or a longer follow-up may be required to show an effect on clinical events.
“We hope others will do additional studies of sodium as well as other dietary recommendations as part of a comprehensive diet for heart failure patients,” he commented.
Dr. Ezekowitz said that the study results did not allow blanket recommendations to be made on reducing sodium intake in heart failure.
But he added: “I don’t think we should write off sodium reduction in this population. I think we can tell patients that reducing dietary sodium may potentially improve symptoms and quality of life, and I will continue to recommend reducing sodium as part of an overall healthy diet. We don’t want to throw the baby out with the bathwater.”
Dr. Ezekowitz noted that heart failure is associated with neurohormonal activation and abnormalities in autonomic control that lead to sodium and water retention; thus, dietary restriction of sodium has been historically endorsed as a mechanism to prevent fluid overload and subsequent clinical outcomes; however, clinical trials so far have shown mixed results.
“The guidelines used to strongly recommend a reduction in sodium intake in heart failure patients, but this advice has backed off in recent years because of the lack of data. Most heart failure guidelines now do not make any recommendations on dietary sodium,” he said.
SODIUM-HF was a pragmatic, multinational, open-label, randomized trial conducted in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand), which included 809 patients (median age, 67 years) with chronic heart failure (NYHA functional class II–III) who were receiving optimally tolerated guideline-directed medical treatment. They were randomly assigned to usual care according to local guidelines or a low-sodium diet of less than 100 mmol (<1,500 mg/day). Patients with a baseline sodium intake of less than 1,500 mg/day were excluded.
In the intervention group, patients were asked to follow low-sodium menus developed by dietitians localized to each region. They also received behavioral counseling by trained dietitians or physicians or nurses.
Dietary sodium intake was assessed by using a 3-day food record (including 1 weekend day) at baseline, 6 months, and 12 months in both groups and, for the intervention group, also at 3 and 9 months to monitor and support dietary adherence.
Dr. Ezekowitz explained that although the best method for measuring sodium levels would normally be a 24-hour urine sodium, this would be impractical in a large clinical trial. In addition, he pointed out that urinary sodium is not an accurate measure of actual sodium levels in patients taking diuretics, so it is not a good measure to use in a heart failure population.
“The food record method of assessing sodium levels has been well validated; I think we measured it as accurately as we could have done,” he added.
Results showed that between baseline and 12 months, the median sodium intake decreased from 2,286 mg/day to 1,658 mg/day in the low-sodium group and from 2,119 mg/day to 2,073 mg/day in the usual care group. The median difference between groups was 415 mg/day at 12 months.
By 12 months, events comprising the primary outcome (hospitalization or emergency department visits due to cardiovascular causes or all-cause death) had occurred in 15% of patients in the low-sodium diet group and 17% of those in the usual care group (hazard ratio [HR], 0.89 [95% CI, 0.63 - 1.26]; P = .53).
All-cause death occurred in 6% of patients in the low-sodium diet group and 4% of those in the usual care group (HR, 1.38; P = .32). Cardiovascular-related hospitalization occurred in 10% of the low-sodium group and 12% of the usual care group (HR, 0.82; P = .36), and cardiovascular-related emergency department visits occurred in 4% of both groups (HR, 1.21; P = .60).
The absence of treatment effect for the primary outcome was consistent across most prespecified subgroups, including those with higher vs lower baseline sodium intake. But there was a suggestion of a greater reduction in the primary outcome in individuals younger than age 65 years than in those age 65 years and older.
Quality-of-life measures on the Kansas City Cardiomyopathy Questionnaire (KCCQ) suggested a benefit in the low-sodium group, with mean between-group differences in the change from baseline to 12 months of 3.38 points in the overall summary score, 3.29 points in the clinical summary score, and 3.77 points in the physical limitation score (all differences were statistically significant).
There was no significant difference in 6-minute-walk distance at 12 months between the low-sodium diet group and the usual care group.
NYHA functional class at 12 months differed significantly between groups; the low-sodium diet group had a greater likelihood of improving by one NYHA class than the usual care group (odds ratio, 0.59; P = .0061).
No safety events related to the study treatment were reported in either group.
Dr. Ezekowitz said that to investigate whether longer follow-up may show a difference in events, further analyses are planned at 2 years and 5 years.
Questions on food recall and blinding
Commenting on the findings at the late-breaking clinical trials session at the ACC meeting, Biykem Bozkurt, MD, professor of medicine at Baylor College of Medicine, Houston, congratulated Dr. Ezekowitz on conducting this trial.
“We have been chasing the holy grail of sodium reduction in heart failure for a very long time, so I have to commend you and your team for taking on this challenge, especially during the pandemic,” she said.
But Dr. Bozkurt questioned whether the intervention group actually had a meaningful sodium reduction given that this was measured by food recall and this may have been accounted for by under-reporting of certain food intakes.
Dr. Ezekowitz responded that patients acted as their own controls in that calorie intake, fluid intake, and weight were also assessed and did not change. “So I think we did have a meaningful reduction in sodium,” he said.
Dr. Bozkurt also queried whether the improvements in quality of life and functional status were reliable given that this was an unblinded study.
To this point, Dr. Ezekowitz pointed out that the KCCQ quality-of-life measure was a highly validated instrument and that improvements were seen in these measures at 3, 6, and 12 months. “It is not like these were spurious findings, so I think we have to look at this as a real result,” he argued.
Commenting on the study at an ACC press conference, Mary Norine Walsh, MD, director of the heart failure and cardiac transplantation programs at St. Vincent Heart Center in Indianapolis, said the trial had answered two important questions: that sodium reduction in heart failure may not reduce heart failure hospitalization/death but that patients feel better.
“I think we can safely tell patients that if they slip up a bit they may not end up in hospital,” she added.
This study was funded by the Canadian Institutes of Health Research and the University Hospital Foundation (Edmonton, Alberta, Canada) and the Health Research Council of New Zealand. Dr. Ezekowitz reports research grants from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, eko.ai, US2.ai, Merck, Novartis, Otsuka, Sanofi, and Servier and consulting fees from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Merck, Novartis, Otsuka, Sanofi, and Servier.
A version of this article first appeared on Medscape.com.
A low-sodium diet was not associated with a reduction in future clinical events in a new study in ambulatory patients with heart failure. But there was a moderate benefit on quality of life and New York Heart Association (NYHA) functional class.
The results of the SODIUM-HF trial were presented April 2 at the annual scientific sessions of the American College of Cardiology, conducted virtually and in person in Washington. They were also simultaneously published online in the Lancet.
The study found that a strategy to reduce dietary sodium intake to less than 1,500 mg daily was not more effective than usual care in reducing the primary endpoint of risk for hospitalization or emergency department visits due to cardiovascular causes or all-cause death at 12 months.
“This is the largest and longest trial to look at the question of reducing dietary sodium in heart failure patients,” lead author Justin Ezekowitz, MBBCh, from the Canadian VIGOUR Center at the University of Alberta, Edmonton, Canada, told this news organization.
But he pointed out that there were fewer events than expected in the study, which was stopped early because of a combination of futility and practical difficulties caused by the COVID pandemic, so it could have been underpowered. Dr. Ezekowitz also suggested that a greater reduction in sodium than achieved in this study or a longer follow-up may be required to show an effect on clinical events.
“We hope others will do additional studies of sodium as well as other dietary recommendations as part of a comprehensive diet for heart failure patients,” he commented.
Dr. Ezekowitz said that the study results did not allow blanket recommendations to be made on reducing sodium intake in heart failure.
But he added: “I don’t think we should write off sodium reduction in this population. I think we can tell patients that reducing dietary sodium may potentially improve symptoms and quality of life, and I will continue to recommend reducing sodium as part of an overall healthy diet. We don’t want to throw the baby out with the bathwater.”
Dr. Ezekowitz noted that heart failure is associated with neurohormonal activation and abnormalities in autonomic control that lead to sodium and water retention; thus, dietary restriction of sodium has been historically endorsed as a mechanism to prevent fluid overload and subsequent clinical outcomes; however, clinical trials so far have shown mixed results.
“The guidelines used to strongly recommend a reduction in sodium intake in heart failure patients, but this advice has backed off in recent years because of the lack of data. Most heart failure guidelines now do not make any recommendations on dietary sodium,” he said.
SODIUM-HF was a pragmatic, multinational, open-label, randomized trial conducted in six countries (Australia, Canada, Chile, Colombia, Mexico, and New Zealand), which included 809 patients (median age, 67 years) with chronic heart failure (NYHA functional class II–III) who were receiving optimally tolerated guideline-directed medical treatment. They were randomly assigned to usual care according to local guidelines or a low-sodium diet of less than 100 mmol (<1,500 mg/day). Patients with a baseline sodium intake of less than 1,500 mg/day were excluded.
In the intervention group, patients were asked to follow low-sodium menus developed by dietitians localized to each region. They also received behavioral counseling by trained dietitians or physicians or nurses.
Dietary sodium intake was assessed by using a 3-day food record (including 1 weekend day) at baseline, 6 months, and 12 months in both groups and, for the intervention group, also at 3 and 9 months to monitor and support dietary adherence.
Dr. Ezekowitz explained that although the best method for measuring sodium levels would normally be a 24-hour urine sodium, this would be impractical in a large clinical trial. In addition, he pointed out that urinary sodium is not an accurate measure of actual sodium levels in patients taking diuretics, so it is not a good measure to use in a heart failure population.
“The food record method of assessing sodium levels has been well validated; I think we measured it as accurately as we could have done,” he added.
Results showed that between baseline and 12 months, the median sodium intake decreased from 2,286 mg/day to 1,658 mg/day in the low-sodium group and from 2,119 mg/day to 2,073 mg/day in the usual care group. The median difference between groups was 415 mg/day at 12 months.
By 12 months, events comprising the primary outcome (hospitalization or emergency department visits due to cardiovascular causes or all-cause death) had occurred in 15% of patients in the low-sodium diet group and 17% of those in the usual care group (hazard ratio [HR], 0.89 [95% CI, 0.63 - 1.26]; P = .53).
All-cause death occurred in 6% of patients in the low-sodium diet group and 4% of those in the usual care group (HR, 1.38; P = .32). Cardiovascular-related hospitalization occurred in 10% of the low-sodium group and 12% of the usual care group (HR, 0.82; P = .36), and cardiovascular-related emergency department visits occurred in 4% of both groups (HR, 1.21; P = .60).
The absence of treatment effect for the primary outcome was consistent across most prespecified subgroups, including those with higher vs lower baseline sodium intake. But there was a suggestion of a greater reduction in the primary outcome in individuals younger than age 65 years than in those age 65 years and older.
Quality-of-life measures on the Kansas City Cardiomyopathy Questionnaire (KCCQ) suggested a benefit in the low-sodium group, with mean between-group differences in the change from baseline to 12 months of 3.38 points in the overall summary score, 3.29 points in the clinical summary score, and 3.77 points in the physical limitation score (all differences were statistically significant).
There was no significant difference in 6-minute-walk distance at 12 months between the low-sodium diet group and the usual care group.
NYHA functional class at 12 months differed significantly between groups; the low-sodium diet group had a greater likelihood of improving by one NYHA class than the usual care group (odds ratio, 0.59; P = .0061).
No safety events related to the study treatment were reported in either group.
Dr. Ezekowitz said that to investigate whether longer follow-up may show a difference in events, further analyses are planned at 2 years and 5 years.
Questions on food recall and blinding
Commenting on the findings at the late-breaking clinical trials session at the ACC meeting, Biykem Bozkurt, MD, professor of medicine at Baylor College of Medicine, Houston, congratulated Dr. Ezekowitz on conducting this trial.
“We have been chasing the holy grail of sodium reduction in heart failure for a very long time, so I have to commend you and your team for taking on this challenge, especially during the pandemic,” she said.
But Dr. Bozkurt questioned whether the intervention group actually had a meaningful sodium reduction given that this was measured by food recall and this may have been accounted for by under-reporting of certain food intakes.
Dr. Ezekowitz responded that patients acted as their own controls in that calorie intake, fluid intake, and weight were also assessed and did not change. “So I think we did have a meaningful reduction in sodium,” he said.
Dr. Bozkurt also queried whether the improvements in quality of life and functional status were reliable given that this was an unblinded study.
To this point, Dr. Ezekowitz pointed out that the KCCQ quality-of-life measure was a highly validated instrument and that improvements were seen in these measures at 3, 6, and 12 months. “It is not like these were spurious findings, so I think we have to look at this as a real result,” he argued.
Commenting on the study at an ACC press conference, Mary Norine Walsh, MD, director of the heart failure and cardiac transplantation programs at St. Vincent Heart Center in Indianapolis, said the trial had answered two important questions: that sodium reduction in heart failure may not reduce heart failure hospitalization/death but that patients feel better.
“I think we can safely tell patients that if they slip up a bit they may not end up in hospital,” she added.
This study was funded by the Canadian Institutes of Health Research and the University Hospital Foundation (Edmonton, Alberta, Canada) and the Health Research Council of New Zealand. Dr. Ezekowitz reports research grants from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, eko.ai, US2.ai, Merck, Novartis, Otsuka, Sanofi, and Servier and consulting fees from American Regent, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Merck, Novartis, Otsuka, Sanofi, and Servier.
A version of this article first appeared on Medscape.com.
FROM ACC 2022
Breast cancer therapy toxicities: Education and communication
This transcript of a video roundtable, which is available on Medscape.com, has been edited for clarity.
Hope S. Rugo, MD: Hello. I’m Hope Rugo, a breast medical oncologist from the University of California, San Francisco. I’m joined here by three of my friends and colleagues to discuss the toxicity of new agents in the treatment of breast cancer. Fatima, do you want to start by introducing yourself?
Fatima F. Cardoso, MD: Sure. Hello, everyone. I’m Fatima Cardoso, a breast medical oncologist in Lisbon, Portugal.
Dr. Rugo: Sheila.
Sheila Pettiford: Hi, I’m Sheila Pettiford. I am a metastatic [breast cancer] patient and have been for almost 8 years in April. I used to live in Philadelphia, Pennsylvania, but moved to Delaware in the last couple of years during the pandemic. I’m happy to be here.
Dr. Rugo: Julia.
Julia Maués: Hi, everyone. I also am a person living with metastatic breast cancer. I was diagnosed in 2013, so it’s going to be 9 years, also in April.
Effective monitoring and management of side effects: A team effort
Dr. Rugo: We have an amazing group and an international representation, which is also really nice because we get different perspectives. What we’re going to talk about is important to providers and patients across the board. With the host of new agents for the treatment of breast cancer – most of which have really moved us forward in terms of having effective treatment options – we’ve also been faced with a lot of new toxicities or side effects that we haven’t seen before or that we might not have expected from the specific agent.
Those toxicities across the board include side effects that are quite familiar to us, like low blood counts, but we may not advise people well enough about other side effects such as mouth sores, inflammation of the lungs, immune toxicities, and skin toxicities.
Fatima, do you want to start and talk about how we can think about these toxicities and address them?
Dr. Cardoso: Sure. Thank you. From the health care provider point of view, what I would highlight is to educate. Educate before we start the treatment. It’s very important to inform the patient but in a balanced way, so we don’t overexaggerate certain types of side effects or underestimate certain types of side effects.
It’s very important because an informed patient will be attentive to the types of side effects that can happen. Also, teach the patient when it is a [cause for] alarm or something for which they might need to contact their health care team and when that’s not the case. I think this is one crucial topic.
The other one is to monitor. Find ways how to best communicate between the patient and the health care team but in a way that you can monitor, so you can act very early on. Most of these new side effects, if you act early on, will not become severe. It is very important to know about them and to act early on.
I believe there is something important that we don’t think about all the time, and that is prophylaxis. Do not be shy about using prophylactic measures, be it for the mouth sores, nausea and vomiting, diarrhea, and other things that really impact the quality of life of patients. Those, to start, are my three major points of attention for health care professionals.
Dr. Rugo: I think that’s so incredibly important – the comments that you’ve made – and also that prevention and prophylaxis are so important. You don’t want to have a patient have diarrhea in the middle of the night and not have any antipropulsive agents at home. Just as a very straightforward example, it’s really important.
Also, the ability to know what you should be looking for and how you can manage it [is important]. There are many examples of times when, even with some education, providers may not have communicated well to the patient. Then the patient is surprised and unhappy with the situation and unable to manage it.
The importance of education
Sheila, your comments on this from the patient perspective are so important. How important is the education piece, and how do you manage the fear of side effects vs actually managing the side effects that might be caused by the treatment you’re taking?
Ms. Pettiford: Thank you for that question. I really think it’s a dance. It’s a dance between the patient and the health care team. Yes, education is absolutely important. However, the health care professionals have to establish a relationship of trust with the patient. My own circumstances were that – and I was very fortunate in that my oncologist, who I chose just by looking and not by a recommendation – I did find an oncologist who listened to me.
When it came time for me to deal with a new medication, the education she provided me was sufficient because of the fact that there was a lot of listening that had gone on prior to the new medicine being given to me. I trusted what I was hearing, and it felt like there was a balanced situation that came about from what I was being told. I could look it up, too.
There still is that part of the patient who will be participating in the process, as well. They can still look up things, and that’s one of the downfalls of the information age we are in. It is a dance. I just want to go back to that. There’s a dance between the patient and the health care providers.
Dr. Rugo: Julia, from the patient’s side, how do you balance the benefits you might get from a treatment versus the side effects and how best to manage them?
Ms. Maués: I think it’s interesting that when we talk with our doctors, and especially when we read about a certain treatment, the attention is focused on the very severe and unlikely side effects that a drug has. We don’t talk as much about the side effects that are most likely to happen and will affect us but may not be life threatening.
Especially for those of us with metastatic cancer who are going to ideally be on a drug for a very long time, we’re then faced with low-grade nausea for the rest of our lives. That’s not okay either, right? I think it’s important to talk about all of the levels of toxicities and everything that can be done to avoid this.
Communication is key
Ms. Pettiford: I just want to add something that Dr. Cardoso said about monitoring that is absolutely important. We’re in a day and time when it’s very difficult to get someone on the telephone, but we do have digital charts and other ways that monitoring can take place. I was at a large teaching university, and I had to go monthly for my treatments. Every month, there were questions that were asked about my life and my condition. I could always get in contact with somebody through the digital chart.
Dr. Rugo: That’s an incredibly important comment, Sheila, about communication and how patients can feel like they have someone to go to in real time who can help manage things. Fatima, I’m interested in your comment on that.
Also, just to go to the next step, which is that when we see data reported on clinical trials and how the agent we’ve added or substituted is better than the standard, the toxicity tables are side effects that occur in at least 10% or more patients and sometimes even 20%. Then they’re graded, where often the division is grade 3 or greater. That may not actually reflect much about what the individual patient experience is. How do we interpret these data? Communication and interpretation?
Dr. Cardoso: Absolutely. I always call attention that perhaps, since we focus so much on grade 3 and 4 [side effects], that is the reason why we don’t see in the usual reporting differences quality of life between treatments. Quality of life is affected also significantly by grade 2 side effects. Or, like Julia was mentioning, even grade 1, if they are persistent, will eventually affect your quality of life.
Sometimes, like I was saying, don’t underestimate – it’s a little bit like that. We focus on explaining, “Look, this new immunotherapy can give you all these different side effects.” But then we forget to say: “Oh, by the way, it may also give you some nausea.” Actually, the nausea will affect the patient’s quality of life. I think that’s why it is so important to balance the way we provide the information.
I would like also to take on what Sheila said that sometimes too much information is not very helpful. That’s why sometimes we have to go stepwise. The first time you’re about to start the treatment, advise [the patient] on the most frequent side effects. Later on, you have time to say: “Okay, by the way, this can also give a rare side effect. This is what you should look for. If you have it, please contact your health care team.”
I think the most difficult part, at least from my experience, is for patients to understand what is really a sign of a severe side effect and what is normal for that type of treatment. Some of the new ways of communicating, like using some patient-reported outcome (PRO) apps, actually help the patient by saying, “This that you are feeling is normal. It can wait for your next appointment. This that you are feeling, it’s better if you try to reach your health care team right away. Or, this is an urgent thing and go to the emergency room near you.”
For this kind of triage, there are now new apps that can help. I think this is the most difficult part because when you are a patient, you don’t know if what you are feeling is actually a sign of something very severe or if it’s normal for the type of treatment you are receiving.
Dr. Rugo: I think that’s so important, and these new PRO apps may help with this. Of course, nothing substitutes for talking in the end if you’re confused or it doesn’t fit into whatever’s in that paradigm. I think it’s important.
Best practices in focusing on the individual patient
Julia, what do you think the best way of educating the patient is when you’re going to start a new treatment? You might be newly diagnosed with cancer or you might have had cancer for a number of years. You’re going to start a new treatment. What’s the best way to know what to look for and how to manage it?
Ms. Maués: I think the key here is that everyone’s different, so have that conversation, the doctor and the patient, about what the best way [of education] is for that specific person. Do they want a flyer listing all of the side effects? Do they want a link to a video they can watch and understand? Do they want someone to come in and give an extra explanation about things? Everyone learns so differently, and I think it’s really hard to assume there’s one way that all patients will understand.
I think the PRO apps are great, and also another benefit is that you keep track of your side effects. Sometimes we don’t even remember well. When did you have nausea? Was it in the morning? Was it in the evening? Is it every day? If you track it with these apps, then you will have the data stored there in the form to answer those questions.
Dr. Cardoso: There was recently a publication – I found it quite interesting – from Lesley Fallowfield’s group saying that the majority of patients would better absorb the information if it is not just text, but if it somehow has a video component, an image, or an infographic that would help them memorize a little bit more information.
Dr. Rugo: There’s been a move toward trying to make videos because the amount of education that’s needed on the providers’ side from our nurses and advanced practice providers may be overwhelming, so things might get missed. The idea of having videos to get everybody on the same page is very popular right now for this reason, and Lesley’s work is really groundbreaking.
Sheila, what do you think is the best way to communicate information?
Ms. Pettiford: Well, I definitely think it’s important for the doctors to recognize, as Julia said, that everyone is different, and all their patients are different. They could come with the same exact subtype of whatever cancer they have – in this situation, breast cancer – and still have so many different reactions. It’s so important for everybody on the health care team to listen to what the patient says because the patient is the one who is living with the illness and knows their body, hopefully.
It’s just one of those things. It’s not a one-size-fits-all situation. You give the standards, but I think it’s important to offer various ways of communicating to a patient because some people are visual. Some people want an overwhelming amount of information so they can sort through it. Then, you have some people who just want the bullet points. Again, it is important not to try to do it as a one-size-fits-all type thing.
Dr. Rugo: Yes, that’s such a good point. I’m always struck by the fact that some patients are totally on top of it and listen to it all, and then other people, we just can’t get them to even call in regarding their side effects. In some ways, it’s frightening for people to call in with issues. Maybe they’re afraid they won’t get the treatment, or that it is related to their cancer progressing, too. Trying to meet people on their own level is a real challenge and an important one.
We talked about education for providers. Fatima, how should we be best educating for these new drugs and new side effects? So many different manifestations can occur, and as we talked about, they might be quite uncommon. We just want people to keep their ears up for any kind of unusual toxicity we see. We all know that the presentation of efficacy data is not adequate for education.
Dr. Cardoso: When we present a new treatment, we focus usually on efficacy, right? Then we say a few things about safety, particularly if there is a new or a severe side effect, but we don’t go through details on how to best manage this in clinical practice.
Anecdotally, I remember that I contacted you because I was going to start using a new treatment and you had some experience. I asked, “What about nausea and vomiting? What do you do for prophylaxis?” I couldn’t find it anywhere in the manuscripts or the presentations. I think we need to focus a little bit more on practical tips. If you are about to start this new treatment, what you should think about and not just the very severe and rare side effects?
Of course, as health care professionals, we need to keep this in our minds. For example, with immunotherapy, side effects can often occur even after stopping the treatment. For other types of new treatments, we need to gain knowledge about endocrinology, for example, which is something that oncologists wouldn’t have to deal with that often in the past. Now, new skills are needed.
It’s also what makes our profession so exciting. There’s always something new to learn, and I like to look at it from that perspective. It’s not boring at all. We are always learning new things.
Dr. Rugo: Indeed. Certainly, you and I have worked together on trying to encourage our pharmaceutical colleagues to publish these papers alongside their urgency of distributing the efficacy data and publishing the papers on efficacy, and also to do a nitty-gritty review of safety and talk about management strategies. I’m really pleased that there seems to be a little more focus on that earlier now in the drug process – although still not early enough – but it’s getting there. That’s a good thing.
Ms. Pettiford: Julia, you mentioned earlier how important it is for the individual patient’s quality of life to understand how these side effects can affect them. It really is one of those things in which we have to make personal decisions. What might be good for one person in terms of what happens with side effects, and their ability to function might not work with someone else.
If you are a person who’s dealing with metastatic disease who has children, a household, a dog, and a cat to take care of, what I can handle being that I’m a single person is not what they can handle. That’s all a part of the education piece. That’s all part of the teamwork. That’s all part of the communication process. It all comes into play.
Dr. Rugo: That’s such an incredibly important point. As we’re wrapping up, it would be great if everybody had some points to make that pulled together some of our conversation. Julia, do you want to start?
Ms. Maués: Yes, I was going to add specifically about the topic you were just discussing, with all that an oncologist’s team has to know and all the different areas of our health being affected by these new treatments. One tip for patients and their teams is that the other providers around the patients may not be as informed about the disease and the treatments they are on. Sometimes we patients end up getting information that isn’t up to date with the latest drugs and things like that.
When we do talk with someone about our issues, make sure they are informed about the new drugs. For example, we often have skin issues. There are dermatologists that work with cancer patients often, and they’re very informed about the side effects that come with these drugs. There are others who never see these sorts of issues and may assume it’s something completely different.
I usually just go to doctors that my oncologist’s team collaborates with and gets referrals from because they send their patients to these doctors often. These are doctors that see cancer patients. We’re a very unique group.
Dr. Rugo: That’s a really good point. I have the same thing. We all have a little stable of people we refer to for various issues that we can reach on speed dial.
The importance of diversity in clinical trials to obtain the most useful outcomes
Fatima, there’s recently been, appropriately so, more of a push to try and evaluate side effects by racial and ethnic subgroups. I think we’re still pretty crummy at it, but we are making some progress. How important is that to you when you think about patients and managing them?
Dr. Cardoso: I think this is quite important. One area of research that is underused, really, is all the new genomics and sequencing technologies to understand why people react differently to the same treatment. Why is it that for some people, either for ethnic or other reasons, you have a different metabolism or something else that justifies a very high rate of side effects from a certain treatment, whereas in other regions of the world this doesn’t happen?
Not to go into these new drugs, but when using a very old drug like a taxane, I found a difference in reaction between the Portuguese patients and the Belgian patients, the two countries where I’ve worked. I even found that the cause might be genetic because the Portuguese living in Belgium reacted differently than the Belgians themselves.
Maybe there is something in the genetics that justifies the type of side effects that you have. I make a plea also for us to dedicate research to understanding why certain side effects are related to race and others are related to maybe some other types of genetic alterations that will lead to an increased side effect.
Dr. Rugo: Sheila, comments?
Ms. Pettiford: That is just excellent. It’s excellent to even consider it because it is so obvious. To me, it’s an obvious situation because there are things that are underneath the skin that we don’t understand. We have to take that into consideration when we are dealing with all these wonderful – I call them miracle – drugs that have come about in the last 20 years.
There still is much more to be done, and I try to participate in any type of organization that’s encouraging diversity in clinical trials because you need to have people of all different ethnicities in order for us to get to these answers. It’s fascinating that you found this out, doctor.
The patient-centered dosing initiative
Ms. Maués: I have the pleasure of being a member of a patient-led initiative called the Patient-Centered Dosing Initiative (PCDI). We are highlighting the discussion around dosages of drugs, especially in the metastatic setting. Metastatic breast cancer is what we’re focusing on, although it could apply to any type of cancer. We are advised by a number of wonderful, world-renowned physicians, Dr. Rugo being one of them. Anne Loeser, the leader of our group, has spoken at ASCO about this topic of dosage. What we’re seeing is that the dosage determination for oncology drugs is still done in the same way it used to be done decades ago and mostly with the curative intent of early-stage disease — metastatic cancer patients back then really didn’t live long at all. What we’re seeing right now is people with metastatic breast cancer that are able to, in some cases, live a long life managing their disease.
Patients are put on doses that are too high for them to be able to manage the side effects, and then they end up having to go off the drug, which means they have lost one of the tools in their toolbox. So, what we like to say about dosing is that, for metastatic cancer patients, it’s a marathon and not a sprint. If we throw all the poison at the patient from the very beginning, they won’t be able to take this for a very long time. And in the metastatic setting, the goal is to stay on each therapy for as long as possible. If we burn one of the cards early on, you have to move on to the next one. This is finite because at some point, there are not enough drugs that can help a particular patient. The PCDI is really getting a lot of visibility with the FDA and experts. People are talking more about dosages, and the FDA is now providing guidance for pharmaceutical companies to study different dosages in the clinical trials from the very beginning. This initiative is almost 3 years old, and we have made a tremendous impact since then.
Dr. Rugo: I think this is an incredibly important area moving forward, and thankfully, there’s so much interest now in not only promoting diversity, enrollment in trials, and education to promote diversity but also in looking at differences in efficacy and side effects.
I’ll just thank everybody for your contributions and amazing perspectives in this incredibly important area. As we move forward with better agents, we need to also make sure we’re understanding what the side effects are, managing them, and hearing the voices of our patients. Thanks very much.
Ms. Pettiford: Thank you so much.
Dr. Cardoso: Thank you.
Ms. Maués: Thank you.
Editor’s note: Our panelists would like to highlight these points:
- The patient and the health care team must build trust with each other.
- African Americans have historical reasons for not trusting the health care industry. Much outreach is still needed.
- Inform and educate before the start of treatment and during the treatment.
- Be balanced and do not underestimate common side effects or overestimate rare ones. Adapt the amount and the detail of the information to the wishes of the individual patient. Offer various methods of delivery (e.g., videos, pamphlets, fact sheets).
- Patients will research their condition and treatments online. Instead of trying to stop this, help them find the best sources.
- Patients will connect with others in the patient community and learn from each other’s experiences. Keep in mind that everyone is different, and decisions should always be made together with the medical team.
- Monitor patients regularly, especially during the first few treatment cycles.
- Use different forms of communication between the patient and health care providers (e.g., apps, digital charts, oncology nurses/nurse navigators, responsive oncologists, different forms of telemedicine), but don’t forget to speak directly with the patient.
- The use of new PRO apps can be very useful to help patients differentiate between urgent and nonurgent signs and symptoms.
- As much as possible, use preventive/prophylactic measures, namely for nausea, vomiting, diarrhea/constipation, and mucositis.
- Be aware of late side effects, especially with immunotherapy.
- Don’t forget that grade 1-2 side effects can substantially impact quality of life, particularly if they are persistent.
- Consider quality-of-life issues for each patient. What is acceptable for one patient may not be for another.
- Learn how to manage new and specific side effects (e.g., endocrine, skin related, pneumonitis).
- Keep an open dialogue about treatment and side effects. Things can change, and there are different ways to address issues such as medications for side effects and dosing changes.
- Listen to your patient and respond in a timely fashion.
- Ethnicity and genetics should be studied as a factor for individual side effects. Standard industry dosages of a new anticancer medication might not be as effective in one ethnic group as another due to the lack of diversity in clinical trials.
- Medications with hard-to-manage or dangerous side effects may be counterproductive regardless of effectiveness.
- Cancer treatment varies vastly depending on region and type of treatment facility. There are many unmet needs in rural areas because of lack of oncology personnel, finances, transportation, etc.
Dr. Rugo is a professor in the department of medicine, University of California San Francisco Comprehensive Cancer Center; director, Breast Oncology and Clinical Trials Education, Cancer Infusion Services, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco. Dr. Cardoso is director, breast unit, Champalimaud Clinical Centre, Lisbon. Financial disclosures for both Dr. Rugo and Dr. Cardoso are available on Medscape.com, where this article first appeared. Julia Maués is a patient in Washington. She has disclosed no relevant financial relationships. Sheila Pettiford is a patient in Middletown, Del. She has disclosed no relevant financial relationships.
This transcript of a video roundtable, which is available on Medscape.com, has been edited for clarity.
Hope S. Rugo, MD: Hello. I’m Hope Rugo, a breast medical oncologist from the University of California, San Francisco. I’m joined here by three of my friends and colleagues to discuss the toxicity of new agents in the treatment of breast cancer. Fatima, do you want to start by introducing yourself?
Fatima F. Cardoso, MD: Sure. Hello, everyone. I’m Fatima Cardoso, a breast medical oncologist in Lisbon, Portugal.
Dr. Rugo: Sheila.
Sheila Pettiford: Hi, I’m Sheila Pettiford. I am a metastatic [breast cancer] patient and have been for almost 8 years in April. I used to live in Philadelphia, Pennsylvania, but moved to Delaware in the last couple of years during the pandemic. I’m happy to be here.
Dr. Rugo: Julia.
Julia Maués: Hi, everyone. I also am a person living with metastatic breast cancer. I was diagnosed in 2013, so it’s going to be 9 years, also in April.
Effective monitoring and management of side effects: A team effort
Dr. Rugo: We have an amazing group and an international representation, which is also really nice because we get different perspectives. What we’re going to talk about is important to providers and patients across the board. With the host of new agents for the treatment of breast cancer – most of which have really moved us forward in terms of having effective treatment options – we’ve also been faced with a lot of new toxicities or side effects that we haven’t seen before or that we might not have expected from the specific agent.
Those toxicities across the board include side effects that are quite familiar to us, like low blood counts, but we may not advise people well enough about other side effects such as mouth sores, inflammation of the lungs, immune toxicities, and skin toxicities.
Fatima, do you want to start and talk about how we can think about these toxicities and address them?
Dr. Cardoso: Sure. Thank you. From the health care provider point of view, what I would highlight is to educate. Educate before we start the treatment. It’s very important to inform the patient but in a balanced way, so we don’t overexaggerate certain types of side effects or underestimate certain types of side effects.
It’s very important because an informed patient will be attentive to the types of side effects that can happen. Also, teach the patient when it is a [cause for] alarm or something for which they might need to contact their health care team and when that’s not the case. I think this is one crucial topic.
The other one is to monitor. Find ways how to best communicate between the patient and the health care team but in a way that you can monitor, so you can act very early on. Most of these new side effects, if you act early on, will not become severe. It is very important to know about them and to act early on.
I believe there is something important that we don’t think about all the time, and that is prophylaxis. Do not be shy about using prophylactic measures, be it for the mouth sores, nausea and vomiting, diarrhea, and other things that really impact the quality of life of patients. Those, to start, are my three major points of attention for health care professionals.
Dr. Rugo: I think that’s so incredibly important – the comments that you’ve made – and also that prevention and prophylaxis are so important. You don’t want to have a patient have diarrhea in the middle of the night and not have any antipropulsive agents at home. Just as a very straightforward example, it’s really important.
Also, the ability to know what you should be looking for and how you can manage it [is important]. There are many examples of times when, even with some education, providers may not have communicated well to the patient. Then the patient is surprised and unhappy with the situation and unable to manage it.
The importance of education
Sheila, your comments on this from the patient perspective are so important. How important is the education piece, and how do you manage the fear of side effects vs actually managing the side effects that might be caused by the treatment you’re taking?
Ms. Pettiford: Thank you for that question. I really think it’s a dance. It’s a dance between the patient and the health care team. Yes, education is absolutely important. However, the health care professionals have to establish a relationship of trust with the patient. My own circumstances were that – and I was very fortunate in that my oncologist, who I chose just by looking and not by a recommendation – I did find an oncologist who listened to me.
When it came time for me to deal with a new medication, the education she provided me was sufficient because of the fact that there was a lot of listening that had gone on prior to the new medicine being given to me. I trusted what I was hearing, and it felt like there was a balanced situation that came about from what I was being told. I could look it up, too.
There still is that part of the patient who will be participating in the process, as well. They can still look up things, and that’s one of the downfalls of the information age we are in. It is a dance. I just want to go back to that. There’s a dance between the patient and the health care providers.
Dr. Rugo: Julia, from the patient’s side, how do you balance the benefits you might get from a treatment versus the side effects and how best to manage them?
Ms. Maués: I think it’s interesting that when we talk with our doctors, and especially when we read about a certain treatment, the attention is focused on the very severe and unlikely side effects that a drug has. We don’t talk as much about the side effects that are most likely to happen and will affect us but may not be life threatening.
Especially for those of us with metastatic cancer who are going to ideally be on a drug for a very long time, we’re then faced with low-grade nausea for the rest of our lives. That’s not okay either, right? I think it’s important to talk about all of the levels of toxicities and everything that can be done to avoid this.
Communication is key
Ms. Pettiford: I just want to add something that Dr. Cardoso said about monitoring that is absolutely important. We’re in a day and time when it’s very difficult to get someone on the telephone, but we do have digital charts and other ways that monitoring can take place. I was at a large teaching university, and I had to go monthly for my treatments. Every month, there were questions that were asked about my life and my condition. I could always get in contact with somebody through the digital chart.
Dr. Rugo: That’s an incredibly important comment, Sheila, about communication and how patients can feel like they have someone to go to in real time who can help manage things. Fatima, I’m interested in your comment on that.
Also, just to go to the next step, which is that when we see data reported on clinical trials and how the agent we’ve added or substituted is better than the standard, the toxicity tables are side effects that occur in at least 10% or more patients and sometimes even 20%. Then they’re graded, where often the division is grade 3 or greater. That may not actually reflect much about what the individual patient experience is. How do we interpret these data? Communication and interpretation?
Dr. Cardoso: Absolutely. I always call attention that perhaps, since we focus so much on grade 3 and 4 [side effects], that is the reason why we don’t see in the usual reporting differences quality of life between treatments. Quality of life is affected also significantly by grade 2 side effects. Or, like Julia was mentioning, even grade 1, if they are persistent, will eventually affect your quality of life.
Sometimes, like I was saying, don’t underestimate – it’s a little bit like that. We focus on explaining, “Look, this new immunotherapy can give you all these different side effects.” But then we forget to say: “Oh, by the way, it may also give you some nausea.” Actually, the nausea will affect the patient’s quality of life. I think that’s why it is so important to balance the way we provide the information.
I would like also to take on what Sheila said that sometimes too much information is not very helpful. That’s why sometimes we have to go stepwise. The first time you’re about to start the treatment, advise [the patient] on the most frequent side effects. Later on, you have time to say: “Okay, by the way, this can also give a rare side effect. This is what you should look for. If you have it, please contact your health care team.”
I think the most difficult part, at least from my experience, is for patients to understand what is really a sign of a severe side effect and what is normal for that type of treatment. Some of the new ways of communicating, like using some patient-reported outcome (PRO) apps, actually help the patient by saying, “This that you are feeling is normal. It can wait for your next appointment. This that you are feeling, it’s better if you try to reach your health care team right away. Or, this is an urgent thing and go to the emergency room near you.”
For this kind of triage, there are now new apps that can help. I think this is the most difficult part because when you are a patient, you don’t know if what you are feeling is actually a sign of something very severe or if it’s normal for the type of treatment you are receiving.
Dr. Rugo: I think that’s so important, and these new PRO apps may help with this. Of course, nothing substitutes for talking in the end if you’re confused or it doesn’t fit into whatever’s in that paradigm. I think it’s important.
Best practices in focusing on the individual patient
Julia, what do you think the best way of educating the patient is when you’re going to start a new treatment? You might be newly diagnosed with cancer or you might have had cancer for a number of years. You’re going to start a new treatment. What’s the best way to know what to look for and how to manage it?
Ms. Maués: I think the key here is that everyone’s different, so have that conversation, the doctor and the patient, about what the best way [of education] is for that specific person. Do they want a flyer listing all of the side effects? Do they want a link to a video they can watch and understand? Do they want someone to come in and give an extra explanation about things? Everyone learns so differently, and I think it’s really hard to assume there’s one way that all patients will understand.
I think the PRO apps are great, and also another benefit is that you keep track of your side effects. Sometimes we don’t even remember well. When did you have nausea? Was it in the morning? Was it in the evening? Is it every day? If you track it with these apps, then you will have the data stored there in the form to answer those questions.
Dr. Cardoso: There was recently a publication – I found it quite interesting – from Lesley Fallowfield’s group saying that the majority of patients would better absorb the information if it is not just text, but if it somehow has a video component, an image, or an infographic that would help them memorize a little bit more information.
Dr. Rugo: There’s been a move toward trying to make videos because the amount of education that’s needed on the providers’ side from our nurses and advanced practice providers may be overwhelming, so things might get missed. The idea of having videos to get everybody on the same page is very popular right now for this reason, and Lesley’s work is really groundbreaking.
Sheila, what do you think is the best way to communicate information?
Ms. Pettiford: Well, I definitely think it’s important for the doctors to recognize, as Julia said, that everyone is different, and all their patients are different. They could come with the same exact subtype of whatever cancer they have – in this situation, breast cancer – and still have so many different reactions. It’s so important for everybody on the health care team to listen to what the patient says because the patient is the one who is living with the illness and knows their body, hopefully.
It’s just one of those things. It’s not a one-size-fits-all situation. You give the standards, but I think it’s important to offer various ways of communicating to a patient because some people are visual. Some people want an overwhelming amount of information so they can sort through it. Then, you have some people who just want the bullet points. Again, it is important not to try to do it as a one-size-fits-all type thing.
Dr. Rugo: Yes, that’s such a good point. I’m always struck by the fact that some patients are totally on top of it and listen to it all, and then other people, we just can’t get them to even call in regarding their side effects. In some ways, it’s frightening for people to call in with issues. Maybe they’re afraid they won’t get the treatment, or that it is related to their cancer progressing, too. Trying to meet people on their own level is a real challenge and an important one.
We talked about education for providers. Fatima, how should we be best educating for these new drugs and new side effects? So many different manifestations can occur, and as we talked about, they might be quite uncommon. We just want people to keep their ears up for any kind of unusual toxicity we see. We all know that the presentation of efficacy data is not adequate for education.
Dr. Cardoso: When we present a new treatment, we focus usually on efficacy, right? Then we say a few things about safety, particularly if there is a new or a severe side effect, but we don’t go through details on how to best manage this in clinical practice.
Anecdotally, I remember that I contacted you because I was going to start using a new treatment and you had some experience. I asked, “What about nausea and vomiting? What do you do for prophylaxis?” I couldn’t find it anywhere in the manuscripts or the presentations. I think we need to focus a little bit more on practical tips. If you are about to start this new treatment, what you should think about and not just the very severe and rare side effects?
Of course, as health care professionals, we need to keep this in our minds. For example, with immunotherapy, side effects can often occur even after stopping the treatment. For other types of new treatments, we need to gain knowledge about endocrinology, for example, which is something that oncologists wouldn’t have to deal with that often in the past. Now, new skills are needed.
It’s also what makes our profession so exciting. There’s always something new to learn, and I like to look at it from that perspective. It’s not boring at all. We are always learning new things.
Dr. Rugo: Indeed. Certainly, you and I have worked together on trying to encourage our pharmaceutical colleagues to publish these papers alongside their urgency of distributing the efficacy data and publishing the papers on efficacy, and also to do a nitty-gritty review of safety and talk about management strategies. I’m really pleased that there seems to be a little more focus on that earlier now in the drug process – although still not early enough – but it’s getting there. That’s a good thing.
Ms. Pettiford: Julia, you mentioned earlier how important it is for the individual patient’s quality of life to understand how these side effects can affect them. It really is one of those things in which we have to make personal decisions. What might be good for one person in terms of what happens with side effects, and their ability to function might not work with someone else.
If you are a person who’s dealing with metastatic disease who has children, a household, a dog, and a cat to take care of, what I can handle being that I’m a single person is not what they can handle. That’s all a part of the education piece. That’s all part of the teamwork. That’s all part of the communication process. It all comes into play.
Dr. Rugo: That’s such an incredibly important point. As we’re wrapping up, it would be great if everybody had some points to make that pulled together some of our conversation. Julia, do you want to start?
Ms. Maués: Yes, I was going to add specifically about the topic you were just discussing, with all that an oncologist’s team has to know and all the different areas of our health being affected by these new treatments. One tip for patients and their teams is that the other providers around the patients may not be as informed about the disease and the treatments they are on. Sometimes we patients end up getting information that isn’t up to date with the latest drugs and things like that.
When we do talk with someone about our issues, make sure they are informed about the new drugs. For example, we often have skin issues. There are dermatologists that work with cancer patients often, and they’re very informed about the side effects that come with these drugs. There are others who never see these sorts of issues and may assume it’s something completely different.
I usually just go to doctors that my oncologist’s team collaborates with and gets referrals from because they send their patients to these doctors often. These are doctors that see cancer patients. We’re a very unique group.
Dr. Rugo: That’s a really good point. I have the same thing. We all have a little stable of people we refer to for various issues that we can reach on speed dial.
The importance of diversity in clinical trials to obtain the most useful outcomes
Fatima, there’s recently been, appropriately so, more of a push to try and evaluate side effects by racial and ethnic subgroups. I think we’re still pretty crummy at it, but we are making some progress. How important is that to you when you think about patients and managing them?
Dr. Cardoso: I think this is quite important. One area of research that is underused, really, is all the new genomics and sequencing technologies to understand why people react differently to the same treatment. Why is it that for some people, either for ethnic or other reasons, you have a different metabolism or something else that justifies a very high rate of side effects from a certain treatment, whereas in other regions of the world this doesn’t happen?
Not to go into these new drugs, but when using a very old drug like a taxane, I found a difference in reaction between the Portuguese patients and the Belgian patients, the two countries where I’ve worked. I even found that the cause might be genetic because the Portuguese living in Belgium reacted differently than the Belgians themselves.
Maybe there is something in the genetics that justifies the type of side effects that you have. I make a plea also for us to dedicate research to understanding why certain side effects are related to race and others are related to maybe some other types of genetic alterations that will lead to an increased side effect.
Dr. Rugo: Sheila, comments?
Ms. Pettiford: That is just excellent. It’s excellent to even consider it because it is so obvious. To me, it’s an obvious situation because there are things that are underneath the skin that we don’t understand. We have to take that into consideration when we are dealing with all these wonderful – I call them miracle – drugs that have come about in the last 20 years.
There still is much more to be done, and I try to participate in any type of organization that’s encouraging diversity in clinical trials because you need to have people of all different ethnicities in order for us to get to these answers. It’s fascinating that you found this out, doctor.
The patient-centered dosing initiative
Ms. Maués: I have the pleasure of being a member of a patient-led initiative called the Patient-Centered Dosing Initiative (PCDI). We are highlighting the discussion around dosages of drugs, especially in the metastatic setting. Metastatic breast cancer is what we’re focusing on, although it could apply to any type of cancer. We are advised by a number of wonderful, world-renowned physicians, Dr. Rugo being one of them. Anne Loeser, the leader of our group, has spoken at ASCO about this topic of dosage. What we’re seeing is that the dosage determination for oncology drugs is still done in the same way it used to be done decades ago and mostly with the curative intent of early-stage disease — metastatic cancer patients back then really didn’t live long at all. What we’re seeing right now is people with metastatic breast cancer that are able to, in some cases, live a long life managing their disease.
Patients are put on doses that are too high for them to be able to manage the side effects, and then they end up having to go off the drug, which means they have lost one of the tools in their toolbox. So, what we like to say about dosing is that, for metastatic cancer patients, it’s a marathon and not a sprint. If we throw all the poison at the patient from the very beginning, they won’t be able to take this for a very long time. And in the metastatic setting, the goal is to stay on each therapy for as long as possible. If we burn one of the cards early on, you have to move on to the next one. This is finite because at some point, there are not enough drugs that can help a particular patient. The PCDI is really getting a lot of visibility with the FDA and experts. People are talking more about dosages, and the FDA is now providing guidance for pharmaceutical companies to study different dosages in the clinical trials from the very beginning. This initiative is almost 3 years old, and we have made a tremendous impact since then.
Dr. Rugo: I think this is an incredibly important area moving forward, and thankfully, there’s so much interest now in not only promoting diversity, enrollment in trials, and education to promote diversity but also in looking at differences in efficacy and side effects.
I’ll just thank everybody for your contributions and amazing perspectives in this incredibly important area. As we move forward with better agents, we need to also make sure we’re understanding what the side effects are, managing them, and hearing the voices of our patients. Thanks very much.
Ms. Pettiford: Thank you so much.
Dr. Cardoso: Thank you.
Ms. Maués: Thank you.
Editor’s note: Our panelists would like to highlight these points:
- The patient and the health care team must build trust with each other.
- African Americans have historical reasons for not trusting the health care industry. Much outreach is still needed.
- Inform and educate before the start of treatment and during the treatment.
- Be balanced and do not underestimate common side effects or overestimate rare ones. Adapt the amount and the detail of the information to the wishes of the individual patient. Offer various methods of delivery (e.g., videos, pamphlets, fact sheets).
- Patients will research their condition and treatments online. Instead of trying to stop this, help them find the best sources.
- Patients will connect with others in the patient community and learn from each other’s experiences. Keep in mind that everyone is different, and decisions should always be made together with the medical team.
- Monitor patients regularly, especially during the first few treatment cycles.
- Use different forms of communication between the patient and health care providers (e.g., apps, digital charts, oncology nurses/nurse navigators, responsive oncologists, different forms of telemedicine), but don’t forget to speak directly with the patient.
- The use of new PRO apps can be very useful to help patients differentiate between urgent and nonurgent signs and symptoms.
- As much as possible, use preventive/prophylactic measures, namely for nausea, vomiting, diarrhea/constipation, and mucositis.
- Be aware of late side effects, especially with immunotherapy.
- Don’t forget that grade 1-2 side effects can substantially impact quality of life, particularly if they are persistent.
- Consider quality-of-life issues for each patient. What is acceptable for one patient may not be for another.
- Learn how to manage new and specific side effects (e.g., endocrine, skin related, pneumonitis).
- Keep an open dialogue about treatment and side effects. Things can change, and there are different ways to address issues such as medications for side effects and dosing changes.
- Listen to your patient and respond in a timely fashion.
- Ethnicity and genetics should be studied as a factor for individual side effects. Standard industry dosages of a new anticancer medication might not be as effective in one ethnic group as another due to the lack of diversity in clinical trials.
- Medications with hard-to-manage or dangerous side effects may be counterproductive regardless of effectiveness.
- Cancer treatment varies vastly depending on region and type of treatment facility. There are many unmet needs in rural areas because of lack of oncology personnel, finances, transportation, etc.
Dr. Rugo is a professor in the department of medicine, University of California San Francisco Comprehensive Cancer Center; director, Breast Oncology and Clinical Trials Education, Cancer Infusion Services, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco. Dr. Cardoso is director, breast unit, Champalimaud Clinical Centre, Lisbon. Financial disclosures for both Dr. Rugo and Dr. Cardoso are available on Medscape.com, where this article first appeared. Julia Maués is a patient in Washington. She has disclosed no relevant financial relationships. Sheila Pettiford is a patient in Middletown, Del. She has disclosed no relevant financial relationships.
This transcript of a video roundtable, which is available on Medscape.com, has been edited for clarity.
Hope S. Rugo, MD: Hello. I’m Hope Rugo, a breast medical oncologist from the University of California, San Francisco. I’m joined here by three of my friends and colleagues to discuss the toxicity of new agents in the treatment of breast cancer. Fatima, do you want to start by introducing yourself?
Fatima F. Cardoso, MD: Sure. Hello, everyone. I’m Fatima Cardoso, a breast medical oncologist in Lisbon, Portugal.
Dr. Rugo: Sheila.
Sheila Pettiford: Hi, I’m Sheila Pettiford. I am a metastatic [breast cancer] patient and have been for almost 8 years in April. I used to live in Philadelphia, Pennsylvania, but moved to Delaware in the last couple of years during the pandemic. I’m happy to be here.
Dr. Rugo: Julia.
Julia Maués: Hi, everyone. I also am a person living with metastatic breast cancer. I was diagnosed in 2013, so it’s going to be 9 years, also in April.
Effective monitoring and management of side effects: A team effort
Dr. Rugo: We have an amazing group and an international representation, which is also really nice because we get different perspectives. What we’re going to talk about is important to providers and patients across the board. With the host of new agents for the treatment of breast cancer – most of which have really moved us forward in terms of having effective treatment options – we’ve also been faced with a lot of new toxicities or side effects that we haven’t seen before or that we might not have expected from the specific agent.
Those toxicities across the board include side effects that are quite familiar to us, like low blood counts, but we may not advise people well enough about other side effects such as mouth sores, inflammation of the lungs, immune toxicities, and skin toxicities.
Fatima, do you want to start and talk about how we can think about these toxicities and address them?
Dr. Cardoso: Sure. Thank you. From the health care provider point of view, what I would highlight is to educate. Educate before we start the treatment. It’s very important to inform the patient but in a balanced way, so we don’t overexaggerate certain types of side effects or underestimate certain types of side effects.
It’s very important because an informed patient will be attentive to the types of side effects that can happen. Also, teach the patient when it is a [cause for] alarm or something for which they might need to contact their health care team and when that’s not the case. I think this is one crucial topic.
The other one is to monitor. Find ways how to best communicate between the patient and the health care team but in a way that you can monitor, so you can act very early on. Most of these new side effects, if you act early on, will not become severe. It is very important to know about them and to act early on.
I believe there is something important that we don’t think about all the time, and that is prophylaxis. Do not be shy about using prophylactic measures, be it for the mouth sores, nausea and vomiting, diarrhea, and other things that really impact the quality of life of patients. Those, to start, are my three major points of attention for health care professionals.
Dr. Rugo: I think that’s so incredibly important – the comments that you’ve made – and also that prevention and prophylaxis are so important. You don’t want to have a patient have diarrhea in the middle of the night and not have any antipropulsive agents at home. Just as a very straightforward example, it’s really important.
Also, the ability to know what you should be looking for and how you can manage it [is important]. There are many examples of times when, even with some education, providers may not have communicated well to the patient. Then the patient is surprised and unhappy with the situation and unable to manage it.
The importance of education
Sheila, your comments on this from the patient perspective are so important. How important is the education piece, and how do you manage the fear of side effects vs actually managing the side effects that might be caused by the treatment you’re taking?
Ms. Pettiford: Thank you for that question. I really think it’s a dance. It’s a dance between the patient and the health care team. Yes, education is absolutely important. However, the health care professionals have to establish a relationship of trust with the patient. My own circumstances were that – and I was very fortunate in that my oncologist, who I chose just by looking and not by a recommendation – I did find an oncologist who listened to me.
When it came time for me to deal with a new medication, the education she provided me was sufficient because of the fact that there was a lot of listening that had gone on prior to the new medicine being given to me. I trusted what I was hearing, and it felt like there was a balanced situation that came about from what I was being told. I could look it up, too.
There still is that part of the patient who will be participating in the process, as well. They can still look up things, and that’s one of the downfalls of the information age we are in. It is a dance. I just want to go back to that. There’s a dance between the patient and the health care providers.
Dr. Rugo: Julia, from the patient’s side, how do you balance the benefits you might get from a treatment versus the side effects and how best to manage them?
Ms. Maués: I think it’s interesting that when we talk with our doctors, and especially when we read about a certain treatment, the attention is focused on the very severe and unlikely side effects that a drug has. We don’t talk as much about the side effects that are most likely to happen and will affect us but may not be life threatening.
Especially for those of us with metastatic cancer who are going to ideally be on a drug for a very long time, we’re then faced with low-grade nausea for the rest of our lives. That’s not okay either, right? I think it’s important to talk about all of the levels of toxicities and everything that can be done to avoid this.
Communication is key
Ms. Pettiford: I just want to add something that Dr. Cardoso said about monitoring that is absolutely important. We’re in a day and time when it’s very difficult to get someone on the telephone, but we do have digital charts and other ways that monitoring can take place. I was at a large teaching university, and I had to go monthly for my treatments. Every month, there were questions that were asked about my life and my condition. I could always get in contact with somebody through the digital chart.
Dr. Rugo: That’s an incredibly important comment, Sheila, about communication and how patients can feel like they have someone to go to in real time who can help manage things. Fatima, I’m interested in your comment on that.
Also, just to go to the next step, which is that when we see data reported on clinical trials and how the agent we’ve added or substituted is better than the standard, the toxicity tables are side effects that occur in at least 10% or more patients and sometimes even 20%. Then they’re graded, where often the division is grade 3 or greater. That may not actually reflect much about what the individual patient experience is. How do we interpret these data? Communication and interpretation?
Dr. Cardoso: Absolutely. I always call attention that perhaps, since we focus so much on grade 3 and 4 [side effects], that is the reason why we don’t see in the usual reporting differences quality of life between treatments. Quality of life is affected also significantly by grade 2 side effects. Or, like Julia was mentioning, even grade 1, if they are persistent, will eventually affect your quality of life.
Sometimes, like I was saying, don’t underestimate – it’s a little bit like that. We focus on explaining, “Look, this new immunotherapy can give you all these different side effects.” But then we forget to say: “Oh, by the way, it may also give you some nausea.” Actually, the nausea will affect the patient’s quality of life. I think that’s why it is so important to balance the way we provide the information.
I would like also to take on what Sheila said that sometimes too much information is not very helpful. That’s why sometimes we have to go stepwise. The first time you’re about to start the treatment, advise [the patient] on the most frequent side effects. Later on, you have time to say: “Okay, by the way, this can also give a rare side effect. This is what you should look for. If you have it, please contact your health care team.”
I think the most difficult part, at least from my experience, is for patients to understand what is really a sign of a severe side effect and what is normal for that type of treatment. Some of the new ways of communicating, like using some patient-reported outcome (PRO) apps, actually help the patient by saying, “This that you are feeling is normal. It can wait for your next appointment. This that you are feeling, it’s better if you try to reach your health care team right away. Or, this is an urgent thing and go to the emergency room near you.”
For this kind of triage, there are now new apps that can help. I think this is the most difficult part because when you are a patient, you don’t know if what you are feeling is actually a sign of something very severe or if it’s normal for the type of treatment you are receiving.
Dr. Rugo: I think that’s so important, and these new PRO apps may help with this. Of course, nothing substitutes for talking in the end if you’re confused or it doesn’t fit into whatever’s in that paradigm. I think it’s important.
Best practices in focusing on the individual patient
Julia, what do you think the best way of educating the patient is when you’re going to start a new treatment? You might be newly diagnosed with cancer or you might have had cancer for a number of years. You’re going to start a new treatment. What’s the best way to know what to look for and how to manage it?
Ms. Maués: I think the key here is that everyone’s different, so have that conversation, the doctor and the patient, about what the best way [of education] is for that specific person. Do they want a flyer listing all of the side effects? Do they want a link to a video they can watch and understand? Do they want someone to come in and give an extra explanation about things? Everyone learns so differently, and I think it’s really hard to assume there’s one way that all patients will understand.
I think the PRO apps are great, and also another benefit is that you keep track of your side effects. Sometimes we don’t even remember well. When did you have nausea? Was it in the morning? Was it in the evening? Is it every day? If you track it with these apps, then you will have the data stored there in the form to answer those questions.
Dr. Cardoso: There was recently a publication – I found it quite interesting – from Lesley Fallowfield’s group saying that the majority of patients would better absorb the information if it is not just text, but if it somehow has a video component, an image, or an infographic that would help them memorize a little bit more information.
Dr. Rugo: There’s been a move toward trying to make videos because the amount of education that’s needed on the providers’ side from our nurses and advanced practice providers may be overwhelming, so things might get missed. The idea of having videos to get everybody on the same page is very popular right now for this reason, and Lesley’s work is really groundbreaking.
Sheila, what do you think is the best way to communicate information?
Ms. Pettiford: Well, I definitely think it’s important for the doctors to recognize, as Julia said, that everyone is different, and all their patients are different. They could come with the same exact subtype of whatever cancer they have – in this situation, breast cancer – and still have so many different reactions. It’s so important for everybody on the health care team to listen to what the patient says because the patient is the one who is living with the illness and knows their body, hopefully.
It’s just one of those things. It’s not a one-size-fits-all situation. You give the standards, but I think it’s important to offer various ways of communicating to a patient because some people are visual. Some people want an overwhelming amount of information so they can sort through it. Then, you have some people who just want the bullet points. Again, it is important not to try to do it as a one-size-fits-all type thing.
Dr. Rugo: Yes, that’s such a good point. I’m always struck by the fact that some patients are totally on top of it and listen to it all, and then other people, we just can’t get them to even call in regarding their side effects. In some ways, it’s frightening for people to call in with issues. Maybe they’re afraid they won’t get the treatment, or that it is related to their cancer progressing, too. Trying to meet people on their own level is a real challenge and an important one.
We talked about education for providers. Fatima, how should we be best educating for these new drugs and new side effects? So many different manifestations can occur, and as we talked about, they might be quite uncommon. We just want people to keep their ears up for any kind of unusual toxicity we see. We all know that the presentation of efficacy data is not adequate for education.
Dr. Cardoso: When we present a new treatment, we focus usually on efficacy, right? Then we say a few things about safety, particularly if there is a new or a severe side effect, but we don’t go through details on how to best manage this in clinical practice.
Anecdotally, I remember that I contacted you because I was going to start using a new treatment and you had some experience. I asked, “What about nausea and vomiting? What do you do for prophylaxis?” I couldn’t find it anywhere in the manuscripts or the presentations. I think we need to focus a little bit more on practical tips. If you are about to start this new treatment, what you should think about and not just the very severe and rare side effects?
Of course, as health care professionals, we need to keep this in our minds. For example, with immunotherapy, side effects can often occur even after stopping the treatment. For other types of new treatments, we need to gain knowledge about endocrinology, for example, which is something that oncologists wouldn’t have to deal with that often in the past. Now, new skills are needed.
It’s also what makes our profession so exciting. There’s always something new to learn, and I like to look at it from that perspective. It’s not boring at all. We are always learning new things.
Dr. Rugo: Indeed. Certainly, you and I have worked together on trying to encourage our pharmaceutical colleagues to publish these papers alongside their urgency of distributing the efficacy data and publishing the papers on efficacy, and also to do a nitty-gritty review of safety and talk about management strategies. I’m really pleased that there seems to be a little more focus on that earlier now in the drug process – although still not early enough – but it’s getting there. That’s a good thing.
Ms. Pettiford: Julia, you mentioned earlier how important it is for the individual patient’s quality of life to understand how these side effects can affect them. It really is one of those things in which we have to make personal decisions. What might be good for one person in terms of what happens with side effects, and their ability to function might not work with someone else.
If you are a person who’s dealing with metastatic disease who has children, a household, a dog, and a cat to take care of, what I can handle being that I’m a single person is not what they can handle. That’s all a part of the education piece. That’s all part of the teamwork. That’s all part of the communication process. It all comes into play.
Dr. Rugo: That’s such an incredibly important point. As we’re wrapping up, it would be great if everybody had some points to make that pulled together some of our conversation. Julia, do you want to start?
Ms. Maués: Yes, I was going to add specifically about the topic you were just discussing, with all that an oncologist’s team has to know and all the different areas of our health being affected by these new treatments. One tip for patients and their teams is that the other providers around the patients may not be as informed about the disease and the treatments they are on. Sometimes we patients end up getting information that isn’t up to date with the latest drugs and things like that.
When we do talk with someone about our issues, make sure they are informed about the new drugs. For example, we often have skin issues. There are dermatologists that work with cancer patients often, and they’re very informed about the side effects that come with these drugs. There are others who never see these sorts of issues and may assume it’s something completely different.
I usually just go to doctors that my oncologist’s team collaborates with and gets referrals from because they send their patients to these doctors often. These are doctors that see cancer patients. We’re a very unique group.
Dr. Rugo: That’s a really good point. I have the same thing. We all have a little stable of people we refer to for various issues that we can reach on speed dial.
The importance of diversity in clinical trials to obtain the most useful outcomes
Fatima, there’s recently been, appropriately so, more of a push to try and evaluate side effects by racial and ethnic subgroups. I think we’re still pretty crummy at it, but we are making some progress. How important is that to you when you think about patients and managing them?
Dr. Cardoso: I think this is quite important. One area of research that is underused, really, is all the new genomics and sequencing technologies to understand why people react differently to the same treatment. Why is it that for some people, either for ethnic or other reasons, you have a different metabolism or something else that justifies a very high rate of side effects from a certain treatment, whereas in other regions of the world this doesn’t happen?
Not to go into these new drugs, but when using a very old drug like a taxane, I found a difference in reaction between the Portuguese patients and the Belgian patients, the two countries where I’ve worked. I even found that the cause might be genetic because the Portuguese living in Belgium reacted differently than the Belgians themselves.
Maybe there is something in the genetics that justifies the type of side effects that you have. I make a plea also for us to dedicate research to understanding why certain side effects are related to race and others are related to maybe some other types of genetic alterations that will lead to an increased side effect.
Dr. Rugo: Sheila, comments?
Ms. Pettiford: That is just excellent. It’s excellent to even consider it because it is so obvious. To me, it’s an obvious situation because there are things that are underneath the skin that we don’t understand. We have to take that into consideration when we are dealing with all these wonderful – I call them miracle – drugs that have come about in the last 20 years.
There still is much more to be done, and I try to participate in any type of organization that’s encouraging diversity in clinical trials because you need to have people of all different ethnicities in order for us to get to these answers. It’s fascinating that you found this out, doctor.
The patient-centered dosing initiative
Ms. Maués: I have the pleasure of being a member of a patient-led initiative called the Patient-Centered Dosing Initiative (PCDI). We are highlighting the discussion around dosages of drugs, especially in the metastatic setting. Metastatic breast cancer is what we’re focusing on, although it could apply to any type of cancer. We are advised by a number of wonderful, world-renowned physicians, Dr. Rugo being one of them. Anne Loeser, the leader of our group, has spoken at ASCO about this topic of dosage. What we’re seeing is that the dosage determination for oncology drugs is still done in the same way it used to be done decades ago and mostly with the curative intent of early-stage disease — metastatic cancer patients back then really didn’t live long at all. What we’re seeing right now is people with metastatic breast cancer that are able to, in some cases, live a long life managing their disease.
Patients are put on doses that are too high for them to be able to manage the side effects, and then they end up having to go off the drug, which means they have lost one of the tools in their toolbox. So, what we like to say about dosing is that, for metastatic cancer patients, it’s a marathon and not a sprint. If we throw all the poison at the patient from the very beginning, they won’t be able to take this for a very long time. And in the metastatic setting, the goal is to stay on each therapy for as long as possible. If we burn one of the cards early on, you have to move on to the next one. This is finite because at some point, there are not enough drugs that can help a particular patient. The PCDI is really getting a lot of visibility with the FDA and experts. People are talking more about dosages, and the FDA is now providing guidance for pharmaceutical companies to study different dosages in the clinical trials from the very beginning. This initiative is almost 3 years old, and we have made a tremendous impact since then.
Dr. Rugo: I think this is an incredibly important area moving forward, and thankfully, there’s so much interest now in not only promoting diversity, enrollment in trials, and education to promote diversity but also in looking at differences in efficacy and side effects.
I’ll just thank everybody for your contributions and amazing perspectives in this incredibly important area. As we move forward with better agents, we need to also make sure we’re understanding what the side effects are, managing them, and hearing the voices of our patients. Thanks very much.
Ms. Pettiford: Thank you so much.
Dr. Cardoso: Thank you.
Ms. Maués: Thank you.
Editor’s note: Our panelists would like to highlight these points:
- The patient and the health care team must build trust with each other.
- African Americans have historical reasons for not trusting the health care industry. Much outreach is still needed.
- Inform and educate before the start of treatment and during the treatment.
- Be balanced and do not underestimate common side effects or overestimate rare ones. Adapt the amount and the detail of the information to the wishes of the individual patient. Offer various methods of delivery (e.g., videos, pamphlets, fact sheets).
- Patients will research their condition and treatments online. Instead of trying to stop this, help them find the best sources.
- Patients will connect with others in the patient community and learn from each other’s experiences. Keep in mind that everyone is different, and decisions should always be made together with the medical team.
- Monitor patients regularly, especially during the first few treatment cycles.
- Use different forms of communication between the patient and health care providers (e.g., apps, digital charts, oncology nurses/nurse navigators, responsive oncologists, different forms of telemedicine), but don’t forget to speak directly with the patient.
- The use of new PRO apps can be very useful to help patients differentiate between urgent and nonurgent signs and symptoms.
- As much as possible, use preventive/prophylactic measures, namely for nausea, vomiting, diarrhea/constipation, and mucositis.
- Be aware of late side effects, especially with immunotherapy.
- Don’t forget that grade 1-2 side effects can substantially impact quality of life, particularly if they are persistent.
- Consider quality-of-life issues for each patient. What is acceptable for one patient may not be for another.
- Learn how to manage new and specific side effects (e.g., endocrine, skin related, pneumonitis).
- Keep an open dialogue about treatment and side effects. Things can change, and there are different ways to address issues such as medications for side effects and dosing changes.
- Listen to your patient and respond in a timely fashion.
- Ethnicity and genetics should be studied as a factor for individual side effects. Standard industry dosages of a new anticancer medication might not be as effective in one ethnic group as another due to the lack of diversity in clinical trials.
- Medications with hard-to-manage or dangerous side effects may be counterproductive regardless of effectiveness.
- Cancer treatment varies vastly depending on region and type of treatment facility. There are many unmet needs in rural areas because of lack of oncology personnel, finances, transportation, etc.
Dr. Rugo is a professor in the department of medicine, University of California San Francisco Comprehensive Cancer Center; director, Breast Oncology and Clinical Trials Education, Cancer Infusion Services, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco. Dr. Cardoso is director, breast unit, Champalimaud Clinical Centre, Lisbon. Financial disclosures for both Dr. Rugo and Dr. Cardoso are available on Medscape.com, where this article first appeared. Julia Maués is a patient in Washington. She has disclosed no relevant financial relationships. Sheila Pettiford is a patient in Middletown, Del. She has disclosed no relevant financial relationships.