User login
Excoriated Papules and Plaques on the Arms and Legs
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
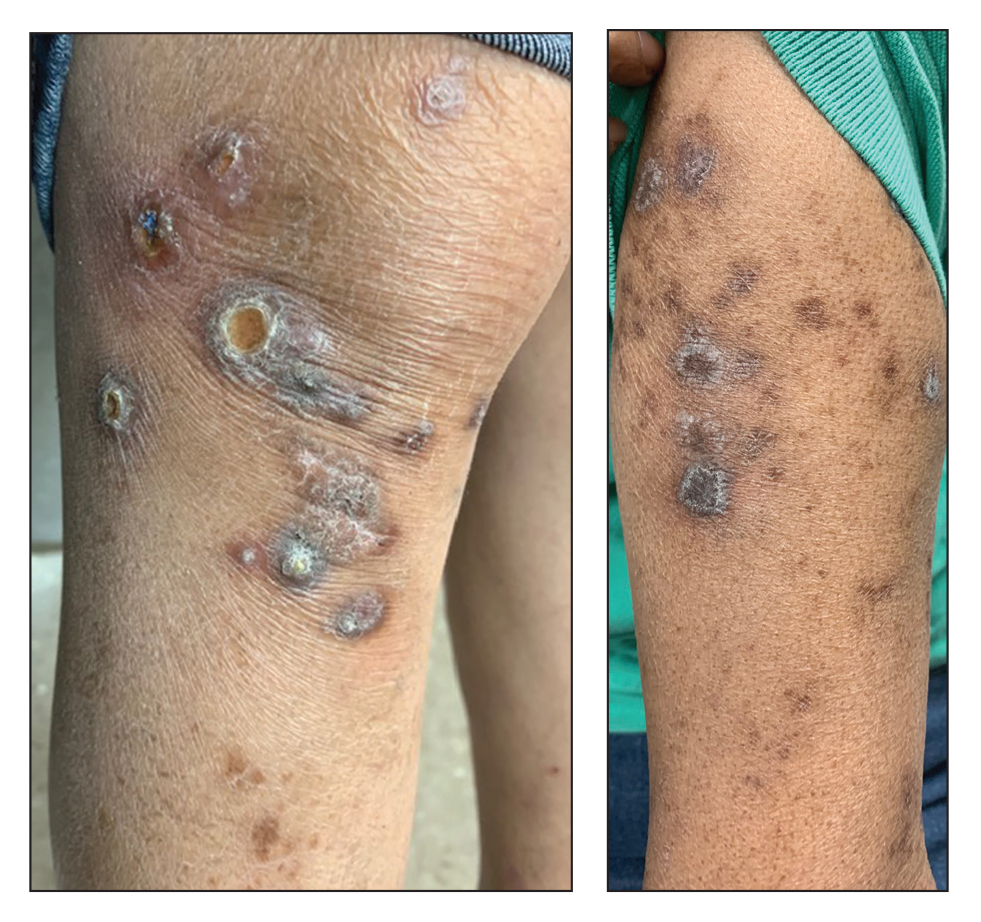
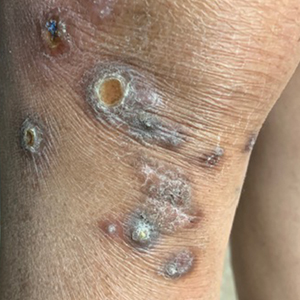
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) may be either acquired or inherited. It is 1 of 4 classical forms of transepithelial elimination, which also includes elastosis perforans serpiginosa (EPS) as well as perforating folliculitis and Kyrle disease. These 4 forms of transepithelial elimination share characteristics of the elimination of altered dermal components through the epidermis.1 The acquired subtype of RPC frequently occurs in patients with diabetes mellitus and end-stage renal disease,2 both present in our patient.
Clinical presentation typically shows pruritic hyperkeratotic papules with a central crater filled with crust that frequently are distributed on the extensor surfaces of the extremities, often in a linear pattern.3 The perforating papules and nodules occasionally may involve the trunk and face.4 Histopathologic examination is characterized by the elimination of altered collagen through the epidermis. Established lesions may show a cup-shaped depression of the epidermis filled with a keratin plug. The underlying dermis will show vertically oriented basophilic collagen fibers with focal extrusion through the epidermis, and elastic fibers will be absent.5 The exact pathophysiology of this disease is unknown, but it may represent a cutaneous response to superficial trauma caused by intense scratching.6
Standard treatment protocols are not well established for this condition, but some evidence shows that a combination of treatments can help ameliorate symptoms, even if they are not curative.7 Treatments without strong evidence have included a wide range of topical, systemic, and other therapies. Case series and anecdotal reports have used retinoids, corticosteroids, menthol, antibiotics, allopurinol antihistamines, cryotherapy, and lasers.8 One case was treated with a combination of narrowband UVB phototherapy and doxycycline with resolution in approximately 6 weeks.9 Other cases have been cured using triple therapy with antihistamines, topical or injected steroids, and emollients or oral antibiotics.7 Evidence shows that there may be benefit to combining multiple different treatment types that target pruritus, inflammation, and collagen damage.7,9 This disease usually cannot be cured, but it may be improved by the available treatments.
The differential diagnosis includes delusional parasitosis, EPS, perforating folliculitis, and prurigo nodularis. Delusional parasitosis also can be characterized by excoriated plaques and a sensation of parasites infesting the skin, as our patient described.10 However, it can be differentiated from RPC by the fact that it is a diagnosis of exclusion, which would not have the histopathologic findings of the elimination of collagen from the epidermis, as was demonstrated in our patient.11 Elastosis perforans serpiginosa is in the same family of perforating diseases as RPC; however, EPS typically appears in children or young adults and often is associated with other genetic disorders. Physical examination in a patient with EPS would reveal keratotic papules in a serpiginous pattern, whereas our patient had discrete lesions without any serpiginous pattern. The histopathologic appearance of EPS would reveal plugs of elastic fibers rather than collagen fibers, as was demonstrated in our patient.8 Perforating folliculitis, while also demonstrating transepithelial elimination similar to RPC, would appear as erythematous follicular papules with small central keratotic plugs and histopathologic findings of a widely dilated follicle with a mass of keratotic debris.12 Prurigo nodularis would appear as dome-shaped papulonodules with varying degrees of scale, crust, and erosion, with a histopathologic appearance of hyperplasia and thick hyperkeratosis.11
Overall, the histopathology is paramount in differentiating RPC from the alternative diagnoses, with the extrusion of collagen from the epidermis not being seen in these other conditions. The coupling of the medical history (type 2 diabetes mellitus and end-stage renal disease) with the clinical presentation and skin biopsy findings confirmed the diagnosis of RPC.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
- Fei C, Wang Y, Gong Y, et al. Acquired reactive perforating collagenosis: a report of a typical case. Medicine (Baltimore). 2016;95:E4305.
- Matsui A, Nakano H, Aizu T, et al. Treatment of acquired reactive perforating collagenosis with 308‐nm excimer laser. Clin Exp Dermatol. 2016;41:820-821.
- Dey AK. Reactive perforating collagenosis: an important differential diagnosis in hemodialysis patients. Saudi J Kidney Dis Transpl. 2018;29:422-425.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 9th ed. McGraw-Hill Education LLC; 2012.
- Plaza JA, Prieto VG. Inflammatory Skin Disorders. Demos Medical Publishing LLC; 2012.
- Kreuter A, Gambichler T. Acquired reactive perforating collagenosis. CMAJ. 2010;182:E184.
- Zhang X, Yang Y, Shao S. Acquired reactive perforating collagenosis: a case report and review of the literature. Medicine (Baltimore). 2020;99:E20391.
- Rapini RP. Perforating diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1690-1696.
- Gao L, Gu L, Chen Z, et al. Doxycycline combined with NB-UVB phototherapy for acquired reactive perforating collagenosis. Ther Clin Risk Manag. 2020;16:917-921.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Psychocutaneous disorders. Dermatology Essentials. Elsevier; 2014:50-55. 11. Bolognia JL, Schaffer JV, Duncan KO, et al. Pruritus and dysesthesia. Dermatology Essentials. Elsevier; 2014:39-49. 12. Rubio FA, Herranz P, Robayna G, et al. Perforating folliculitis: report of a case in an HIV-infected man. J Am Acad Dermatol. 1999;40:300-302.
A 73-year-old woman presented for evaluation of a rash on the arms and legs of 3 months’ duration. The rash had developed abruptly, and she believed it was caused by bugs in the skin; her husband noted that she constantly picked at her arms and legs. She had a medical history of hypertension, type 2 diabetes mellitus, and endstage renal disease on dialysis. Physical examination revealed multiple pigmented papules and plaques, some with keratotic scale, on the lower legs (left) and arms, with greater involvement on the left arm (right). The lesions were of various sizes and shapes, some with a central keratotic core, and several lesions demonstrated erosion, excoriation, or ulceration. Histopathologic examination revealed slight attenuation of the epidermis with loss of normal rete peg architecture, alternating areas of hypergranulosis and hypogranulosis, central ulceration with inflammatory cells, and a basophilic hue to the ulcer base with sweeping up of the collagen fibers.
Adding immunotherapy to chemo in lung cancer improves patient outcomes, new data show
according to an analysis presented at the annual European Lung Cancer Congress (ELCC) on March 30.
“Overall, it is very clear that chemotherapy plus immunotherapy prolongs the time to symptom deterioration and actually improves symptoms” in this patient population, said study discussant Luis Paz-Ares, MD, PhD, chair of medical oncology at the Hospital Universitario 12 de Octubre, Madrid, who was not involved in the research.
Last September, investigators reported efficacy outcomes from the phase 3 POSEIDON trial, which randomized 1,013 patients with EGFR/ALK wild-type mNSCLC to one of three first-line options: chemotherapy alone, chemotherapy plus the checkpoint inhibitor durvalumab, or chemotherapy plus two check-point inhibitors, durvalumab and tremelimumab. The analysis showed improved progression-free survival in both immunotherapy arms as well as a significant 2.3-month overall survival advantage with dual immunotherapy and a nonsignificant 1.6-month advantage with single agent durvalumab.
At the ELCC meeting, study presenter and lead investigator Edward Garon, MD, reported the latest data on the trial’s secondary endpoints: patient-reported outcomes. Global health status, functioning, and symptom scores were assessed using two questionnaires, the EORTC QLQ-C30 and EORTC QLQ-LC13.
Overall, Dr. Garon and colleagues reported a longer time to deterioration in all three areas – global health status, functioning, and symptoms – for patients who received immunotherapy versus chemotherapy alone, with similar results in both immunotherapy arms.
Time to deterioration in global health status, for instance, was a median of about 8 months on both immunotherapy regimens versus 5.6 months with chemotherapy alone. The positive findings held for many patient-reported treatment side effects, including dyspnea, hemoptysis, nausea/vomiting, and insomnia, but the benefits of adding immunotherapy weren’t always statistically significant.
Adding one or both checkpoint inhibitors to chemotherapy “improved efficacy while delaying deterioration in symptoms, functioning, and [health-related quality of life] versus chemotherapy alone in patients with mNSCLC,” concluded Dr. Garon, a thoracic medical oncologist at the University of California, Los Angeles. Plus, he added, “the pattern was observed across nearly all prespecified symptoms and domains of interest.”
According to study discussant Dr. Paz-Ares, “the data seem to be very consistent with all the trials asking similar questions.” The important thing here is figuring out the ideal candidates for dual inhibitor therapy, he said.
With positive efficacy and patient-reported outcomes for single and dual immunotherapy in this trial, it’s a “relatively straightforward” decision to add immunotherapy to chemotherapy for patients with mNSCLC, Massimo Di Maio, a medical oncologist at the University of Turin, Italy, said in an editorial on the ELCC’s news site.
However, that’s not always the case for every cancer type, which makes patient-reported outcomes “crucial” for determining the right treatment for each patient. Some might opt for a modest survival benefit regardless of the side effects, while others might favor a less toxic approach, even it means not living quite as long, he said.
The problem, he stressed, is that trials often release efficacy data well before patient-reported outcomes, which makes weighing the benefits and risks of a treat-ment option more difficult. The delay between efficacy and patient-reported outcome data was about 6 months in the POSEIDON trial.
“Timing is key when it comes to using [patient reported outcomes] for decision-making in oncology,” Dr. Di Maio said. “In fact, to enable a full assessment of a treatment, results should be published concurrently with the efficacy and safety data. Unfortunately, this is generally not the case.”
POSEIDON was funded by AstraZeneca, which markets durvalumab and is developing tremelimumab. Dr. Garon reported grants from the company. Dr. Paz-Ares reported honoraria and institutional research grants from AstraZeneca. Dr. Di Maio is a consultant for AstraZeneca and reported receiving honoraria and personal fees from the company.
according to an analysis presented at the annual European Lung Cancer Congress (ELCC) on March 30.
“Overall, it is very clear that chemotherapy plus immunotherapy prolongs the time to symptom deterioration and actually improves symptoms” in this patient population, said study discussant Luis Paz-Ares, MD, PhD, chair of medical oncology at the Hospital Universitario 12 de Octubre, Madrid, who was not involved in the research.
Last September, investigators reported efficacy outcomes from the phase 3 POSEIDON trial, which randomized 1,013 patients with EGFR/ALK wild-type mNSCLC to one of three first-line options: chemotherapy alone, chemotherapy plus the checkpoint inhibitor durvalumab, or chemotherapy plus two check-point inhibitors, durvalumab and tremelimumab. The analysis showed improved progression-free survival in both immunotherapy arms as well as a significant 2.3-month overall survival advantage with dual immunotherapy and a nonsignificant 1.6-month advantage with single agent durvalumab.
At the ELCC meeting, study presenter and lead investigator Edward Garon, MD, reported the latest data on the trial’s secondary endpoints: patient-reported outcomes. Global health status, functioning, and symptom scores were assessed using two questionnaires, the EORTC QLQ-C30 and EORTC QLQ-LC13.
Overall, Dr. Garon and colleagues reported a longer time to deterioration in all three areas – global health status, functioning, and symptoms – for patients who received immunotherapy versus chemotherapy alone, with similar results in both immunotherapy arms.
Time to deterioration in global health status, for instance, was a median of about 8 months on both immunotherapy regimens versus 5.6 months with chemotherapy alone. The positive findings held for many patient-reported treatment side effects, including dyspnea, hemoptysis, nausea/vomiting, and insomnia, but the benefits of adding immunotherapy weren’t always statistically significant.
Adding one or both checkpoint inhibitors to chemotherapy “improved efficacy while delaying deterioration in symptoms, functioning, and [health-related quality of life] versus chemotherapy alone in patients with mNSCLC,” concluded Dr. Garon, a thoracic medical oncologist at the University of California, Los Angeles. Plus, he added, “the pattern was observed across nearly all prespecified symptoms and domains of interest.”
According to study discussant Dr. Paz-Ares, “the data seem to be very consistent with all the trials asking similar questions.” The important thing here is figuring out the ideal candidates for dual inhibitor therapy, he said.
With positive efficacy and patient-reported outcomes for single and dual immunotherapy in this trial, it’s a “relatively straightforward” decision to add immunotherapy to chemotherapy for patients with mNSCLC, Massimo Di Maio, a medical oncologist at the University of Turin, Italy, said in an editorial on the ELCC’s news site.
However, that’s not always the case for every cancer type, which makes patient-reported outcomes “crucial” for determining the right treatment for each patient. Some might opt for a modest survival benefit regardless of the side effects, while others might favor a less toxic approach, even it means not living quite as long, he said.
The problem, he stressed, is that trials often release efficacy data well before patient-reported outcomes, which makes weighing the benefits and risks of a treat-ment option more difficult. The delay between efficacy and patient-reported outcome data was about 6 months in the POSEIDON trial.
“Timing is key when it comes to using [patient reported outcomes] for decision-making in oncology,” Dr. Di Maio said. “In fact, to enable a full assessment of a treatment, results should be published concurrently with the efficacy and safety data. Unfortunately, this is generally not the case.”
POSEIDON was funded by AstraZeneca, which markets durvalumab and is developing tremelimumab. Dr. Garon reported grants from the company. Dr. Paz-Ares reported honoraria and institutional research grants from AstraZeneca. Dr. Di Maio is a consultant for AstraZeneca and reported receiving honoraria and personal fees from the company.
according to an analysis presented at the annual European Lung Cancer Congress (ELCC) on March 30.
“Overall, it is very clear that chemotherapy plus immunotherapy prolongs the time to symptom deterioration and actually improves symptoms” in this patient population, said study discussant Luis Paz-Ares, MD, PhD, chair of medical oncology at the Hospital Universitario 12 de Octubre, Madrid, who was not involved in the research.
Last September, investigators reported efficacy outcomes from the phase 3 POSEIDON trial, which randomized 1,013 patients with EGFR/ALK wild-type mNSCLC to one of three first-line options: chemotherapy alone, chemotherapy plus the checkpoint inhibitor durvalumab, or chemotherapy plus two check-point inhibitors, durvalumab and tremelimumab. The analysis showed improved progression-free survival in both immunotherapy arms as well as a significant 2.3-month overall survival advantage with dual immunotherapy and a nonsignificant 1.6-month advantage with single agent durvalumab.
At the ELCC meeting, study presenter and lead investigator Edward Garon, MD, reported the latest data on the trial’s secondary endpoints: patient-reported outcomes. Global health status, functioning, and symptom scores were assessed using two questionnaires, the EORTC QLQ-C30 and EORTC QLQ-LC13.
Overall, Dr. Garon and colleagues reported a longer time to deterioration in all three areas – global health status, functioning, and symptoms – for patients who received immunotherapy versus chemotherapy alone, with similar results in both immunotherapy arms.
Time to deterioration in global health status, for instance, was a median of about 8 months on both immunotherapy regimens versus 5.6 months with chemotherapy alone. The positive findings held for many patient-reported treatment side effects, including dyspnea, hemoptysis, nausea/vomiting, and insomnia, but the benefits of adding immunotherapy weren’t always statistically significant.
Adding one or both checkpoint inhibitors to chemotherapy “improved efficacy while delaying deterioration in symptoms, functioning, and [health-related quality of life] versus chemotherapy alone in patients with mNSCLC,” concluded Dr. Garon, a thoracic medical oncologist at the University of California, Los Angeles. Plus, he added, “the pattern was observed across nearly all prespecified symptoms and domains of interest.”
According to study discussant Dr. Paz-Ares, “the data seem to be very consistent with all the trials asking similar questions.” The important thing here is figuring out the ideal candidates for dual inhibitor therapy, he said.
With positive efficacy and patient-reported outcomes for single and dual immunotherapy in this trial, it’s a “relatively straightforward” decision to add immunotherapy to chemotherapy for patients with mNSCLC, Massimo Di Maio, a medical oncologist at the University of Turin, Italy, said in an editorial on the ELCC’s news site.
However, that’s not always the case for every cancer type, which makes patient-reported outcomes “crucial” for determining the right treatment for each patient. Some might opt for a modest survival benefit regardless of the side effects, while others might favor a less toxic approach, even it means not living quite as long, he said.
The problem, he stressed, is that trials often release efficacy data well before patient-reported outcomes, which makes weighing the benefits and risks of a treat-ment option more difficult. The delay between efficacy and patient-reported outcome data was about 6 months in the POSEIDON trial.
“Timing is key when it comes to using [patient reported outcomes] for decision-making in oncology,” Dr. Di Maio said. “In fact, to enable a full assessment of a treatment, results should be published concurrently with the efficacy and safety data. Unfortunately, this is generally not the case.”
POSEIDON was funded by AstraZeneca, which markets durvalumab and is developing tremelimumab. Dr. Garon reported grants from the company. Dr. Paz-Ares reported honoraria and institutional research grants from AstraZeneca. Dr. Di Maio is a consultant for AstraZeneca and reported receiving honoraria and personal fees from the company.
FROM ELCC 2022
Experimental drug may boost executive function in patients with Alzheimer’s disease
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients performed better in cognitive testing after just 2 weeks, especially in areas of executive functioning. Clinicians involved in the study also reported improvements in patients’ ability to complete daily activities, especially in complex tasks such as using a computer, carrying out household chores, and managing their medications.
“It’s pretty incredible to see improvement over the course of a week to a week and a half,” said study investigator Aaron Koenig, MD, vice president of Early Clinical Development at Sage Therapeutics in Cambridge, Mass. “Not only are we seeing objective improvement, we’re also seeing a subjective benefit.”
The drug, SAGE-718, is also under study for MCI in patients with Huntington’s disease, the drug’s primary indication, and Parkinson’s disease.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Improved executive function
SAGE-718 is in a new class of drugs called positive allosteric modulator of N-methyl-D-aspartate (NMDA) receptors, which are thought to improve neuroplasticity.
For the phase 2a open-label LUMINARY trial, researchers enrolled 26 patients ages 50-80 years with Alzheimer’s disease who had MCI. Patients completed a battery of cognitive tests at the study outset, again at the end of treatment, and again after 28 days.
Participants received SAGE-718 daily for 2 weeks and were followed for another 2 weeks.
The study’s primary outcome was safety. Seven patients (26.9%) reported mild or moderate treatment-emergent adverse events (AEs), and there were no serious AEs or deaths.
However, after 14 days, researchers also noted improvements from baseline on multiple tests of executive functioning, learning, and memory. And at 28 days, participants demonstrated significantly better Montreal Cognitive Assessment scores, compared with baseline (+2.3 points; P < .05), suggesting improvement in global cognition.
“We know that in Alzheimer’s and other neurodegenerative conditions there is a change in cognition, the ability to think, the ability to do things,” Dr. Koenig said. “What we’ve seen with SAGE-718 to date, all the way back to our phase 1 studies, is a cognitively beneficial effect, but more specifically an improvement in executive functioning.”
Intentional small study design
Commenting on the findings, Percy Griffin, PhD, MSc, director of scientific engagement for the Alzheimer’s Association, said that because of the small study size, short follow-up, and limited data available in the conference abstract, “we cannot speculate about the efficacy of this investigational therapy.”
“Bigger picture, the real-world clinical meaningfulness of research results that are generated in highly controlled circumstances is an important question that is being discussed right now throughout the Alzheimer’s field,” he added.
However, Dr. Koenig countered that the small study design was intentional. “Over the course of a year, we can get to the answer in different patient populations rather than running these rather large and arduous trials that may pan out to not be positive,” he said.
“The purpose here is to say, directionally, are we seeing improvement that warrants further investigation? If you don’t see an effect in a small number of patients, if you don’t see that effect rather quickly, and if you don’t see an effect that should translate into something meaningful, we at SAGE believe that you may not have a drug there,” he added.
Sage Therapeutics plans to launch a phase 2b placebo-controlled trial later this year to study SAGE-718 in more Alzheimer’s patients over a longer period of time.
The study was funded by SAGE Therapeutics. Dr. Koenig is an employee of SAGE and reports no other conflicts. Dr. Griffin has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Novel drug significantly reduces tics in Tourette syndrome – without side effects
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new study shows.
Importantly, unlike current medications for the disorder, ecocipam does not lead to weight gain, anxiety, depression, or tardive dyskinesia, compared with placebo – a factor that may lead to better adherence.
For clinicians treating children with Tourette syndrome, the results suggest “help is on the way,” said study investigator Donald Gilbert, MD, professor of pediatrics and neurology, University of Cincinnati Children’s Hospital Medical Center.
“There may be a drug available with a new mechanism of action that is effective to suppress tics without causing weight gain or unwanted neuropsychiatric side effects,” Dr. Gilbert said.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
First-in-class agent
Tourette syndrome is a neurologic condition that causes sudden repetitive involuntary muscle movements and sounds known as tics. These movements typically develop in childhood and worsen during adolescence.
“There’s a risk of injury, such as to the neck, with tics in childhood, so it’s good to have something that makes tics less severe and less socially impairing in junior high,” said Dr. Gilbert.
While tics generally diminish by adulthood, “about 10% of the patients we see as kids persist into adulthood enough to need medical interventions,” said Dr. Gilbert.
Ecopipam is a first-in-class selective D1 receptor antagonist in clinical development for pediatric patients with Tourette syndrome. The compound was previously tested without success in schizophrenia and in obesity, the idea being that because dopamine is linked to pleasure or reward, blocking it might result in weight loss, said Dr. Gilbert.
However, earlier studies in Tourette syndrome suggested that ecopipam reduces tics in children and adults and had low metabolic and movement-related adverse effects.
Drugs currently used to treat tics include haloperidol, pimocide, and aripiprazole. All of these agents block the dopamine-2 (D2) receptor and can cause weight gain and tardive dyskinesia, said Dr. Gilbert.
Placebo-controlled trial
The new study included 149 patients with Tourette syndrome who had a score of at least 20 on the Yale Global Tic Severity Total Tic Score (YGTSS-TTS). The scale measures five aspects of motor and vocal tics: the number, frequency, intensity, complexity, and interference.
With that scale, intensity assesses whether tics cause injury, complexity evaluates the number of muscle group, and interference assesses whether tics interrupt functions, such as speaking or walking.
For each of the five areas, scores range from 0-5, with higher scores indicating greater severity. The motor and vocal parts have a maximum of 25 points each, for a maximum total of 50.
Participants were randomly assigned to receive once-daily oral ecopipam or placebo. A 4-week titration period was followed by an 8-week maintenance period and then a 1-week tapering period.
The primary endpoint was mean change from baseline to week 12 in scores on the YGTSS-TTS.
Results on the YGTSS-TTS showed a significant improvement in the ecopipam group, compared with placebo groups (least square [LS] mean difference: -3.44; 95% confidence interval: -6.09 to -0.79; P = .011).
The analysis indicated a 30% reduction, with an effect size of 0.48, which is “pretty good,” said Dr. Gilbert. “The amount of change is comparable to other drugs that are marketed” to treat tics.
The drug was effective for younger as well as older children. Among those aged 6-11 years, the LS mean difference was -4.95 (95% CI: -9.99 to 0.10; P = .054), and for those aged 12 to 17 years, the LS mean difference was -3.37 (95% CI: -6.51 to -0.24; P = .035).
A key secondary endpoint was the score on the Clinical Global Impression of Tourette Syndrome Severity, which Dr. Gilbert said is a more subjective measure of whether a patient’s life has improved. Here, the mean change at week 12 was significant (P = .001) for the treated group (improvement of 0.91 points), compared with the placebo group (improvement of 0.5 points).
Researchers also assessed safety and tolerability. Treatment-related adverse events (AEs) occurred in 34% of patients taking ecopipam and in 21% of those taking placebo. The most common AEs were headache (9.2%), fatigue (6.6%), somnolence (6.6%), and restlessness (5.3%).
There were no metabolic or movement-related AEs or treatment-related serious AEs.
“This drug doesn’t cause weight gain at all,” said Dr. Gilbert. He noted that there was also no difference in the groups in terms of rates of depression, anxiety, or tardive dyskinesia.
Significant tic reduction
Commenting on the findings, Jessica Frey, MD, a movement disorders fellow at the University of Florida, said the new double-blind, placebo-controlled study “is promising” in that it demonstrates significant tic reduction, compared with placebo without significant side effects.
“Ecopipam could potentially expand pharmacologic treatment options for children and adolescents with Tourette syndrome in the near future,” she said.
Dr. Frey will also be presenting results at the meeting of a study showing a significant correlation between tic severity and social media use among adolescents with Tourette syndrome during the COVID pandemic.
She noted that dopamine is an important neurotransmitter in the underlying pathophysiology of Tourette syndrome. In addition, although D2 receptor blockade can provide significant tic reduction, the “intolerable” side effects often linked to medications with this mechanism “can lead to discontinuation,” said Dr. Frey.
She also noted that ecopipam has previously been evaluated in an open-label study and a follow-up placebo-controlled study that demonstrated safety as well as significant tic reduction.
The study was supported by Emalex Biosciences. Dr. Gilbert and Dr. Frey report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Anticoagulation not routinely needed after TAVR: ADAPT-TAVR
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
In patients undergoing transcatheter aortic valve replacement (TAVR), the incidence of leaflet thrombosis was numerically lower in those treated with the anticoagulant edoxaban for 6 months after the procedure than in those who received dual antiplatelet therapy, although the difference was not statistically significant, in the ADAPT-TAVR study.
There was no difference in new cerebral thromboembolism or neurologic/neurocognitive function between the two groups in the study.
Also, there was no significant relation between subclinical leaflet thrombosis and increased risk for cerebral thromboembolism and neurologic dysfunction.
The ADAPT-TAVR trial was presented April 4 at the American College of Cardiology (ACC) 2022 Scientific Session by Duk-Woo Park, MD, Asan Medical Center, Seoul, South Korea. It was simultaneously published online in Circulation.
“The key messages from this study are that subclinical leaflet thrombosis has not been proven to affect clinical outcomes for patients undergoing valve replacement and that in patients in whom leaflet thrombosis causes no symptoms or complications, its presence should not dictate the type of antithrombotic therapy that patients receive following the implantation of an artificial heart valve,” Dr. Park said.
“These findings do not support the routine use of computed tomography scans to detect subclinical leaflet thrombosis,” he added.
Commenting on the study at an ACC press conference, Megan Coylewright, MD, director of the Structural Heart Program at Erlanger Health System, Chattanooga, Tennessee, said: “Oftentimes when studies are negative, we’re disappointed. In this case, I think we are pleased that the study is negative because it suggests we do not have to expose our TAVR patients to anticoagulation for benefit.”
Dr. Coylewright explained that the ADAPT-TAVR study was asking whether clots form on the valve, as defined by CT.
“We are worried about that for two reasons: could that clot cause a stroke, and could that clot cause the valve to break down over time. This study looked at the first issue. And it found that there was some clot build up on the valve, but that it wasn’t significantly different between the anticoagulant and dual antiplatelet groups. And there was no correlation with embolic events, she noted.
“It shows how fast our field moves. In the U.S. now, we are using aspirin alone at 81 mg for patients who do not have an indication for oral anticoagulation after TAVR. We are moving away from dual antiplatelet therapy because the bleeding risk is so bad,” Dr. Coylewright said.
In his presentation, Dr. Park explained that it is believed that oral anticoagulants are more effective than antiplatelet therapy at reducing subclinical leaflet thrombosis, but it is not known whether there is a causal association between subclinical leaflet thrombosis and cerebral embolism, or whether oral anticoagulation can reduce cerebral embolism related to subclinical leaflet thrombosis.
The ADAPT-TAVR was conducted to look at these issues. The open-label randomized trial was conducted in five centers in Hong Kong, South Korea, and Taiwan.
For the study, 229 patients who had undergone successful TAVR and did not have an indication for anticoagulation were randomized to edoxaban 60 mg once daily, edoxaban 30 mg once daily for patients needing a reduced dose, or dual antiplatelet therapy for 6 months.
The primary endpoint was an incidence of leaflet thrombosis on four-dimensional CT at 6 months.
Results showed a strong trend toward a lower incidence of leaflet thrombosis in the edoxaban groups than in the dual antiplatelet group (9.8% vs. 18.4%; P = .076).
There was a nonsignificant difference in the percentage of patients with new cerebral lesions identified on brain MRI between the edoxaban and dual antiplatelet groups (25.0% vs. 20.2%).
The percentage of patients with worsening of neurologic and neurocognitive function was not different among the groups.
The incidence of any or major bleeding events was not different between two therapies.
There was also no significant association of the presence or extent of leaflet thrombosis with new cerebral lesions or change of neurologic or neurocognitive function.
Dr. Park noted that the trial had several limitations, including an open-label design, use of surrogate imaging outcomes for the primary outcome, and the relatively short follow-up period, so the study was underpowered to detect any meaningful differences in clinical efficacy and safety outcomes. The results should thus be considered hypothesis-generating, highlighting the need for further research, he added.
The long-term effect of leaflet thrombosis or different antithrombotic strategies on bioprosthetic valve durability is still unknown, Dr. Park said.
He also pointed out that the findings cannot be directly extrapolated to patients with an established indication for oral anticoagulant therapy.
The ADAPT-TAVR trial was an investigator-initiated trial and was funded by the CardioVascular Research Foundation (Seoul, Korea) and Daiichi Sankyo Korea.
A version of this article first appeared on Medscape.com.
Early PCSK9 inhibition in AMI yields plaque regression
When the PCSK9 inhibitor alirocumab is added to high-intensity statins soon after an acute myocardial infarction (AMI), the reduction in atheroma volume is doubled at 12 months, compared with placebo, while other key signs of plaque stabilization, such as fibrous cap thickness, are also significantly and substantially improved, according to the results of the PACMAN-AMI trial.
The study is consistent with other PCSK9 inhibitor trials, supporting the concept that “we should be seeking very low levels of LDL-C in high-risk patients,” reported Lorenz Räber, MD, PhD, of Bern (Switz.) University Hospital, at the annual scientific sessions of the American College of Cardiology.
The low LCL-C target, the data from PACMAN-AMI suggest, is below 50 mg/dL, but even lower is better. When displayed graphically, the improvements in remodeling characteristics “get very steep” as levels descend below a 50 mg/dL threshold, Dr. Räber reported. This was true regardless of study arm.
In PACMAN-AMI, 300 AMI patients (with either ST-elevation or non-ST-elevaion) were randomized to 150 mg alirocumab or placebo administered by subcutaneous injection within 24 hours after an urgent percutaneous intervention (PCI) and stent placement. All patients received their assigned therapy on top of a high-intensity statin in the form of 20 mg of rosuvastatin daily.
Primary outcome was atheroma volume
The primary endpoint was atheroma volume as determined by intravenous ultrasound (IVUS), but the secondary endpoints of maximum lipid core burden, as determined by near infrared spectroscopy (NIRS), and fibrous cap thickness, as determined by optical coherence tomography (OCT), were also adequately powered, according to Dr. Räber.
The imaging measures taken at baseline were repeated in exactly the same spot after 52 weeks on treatment.
For the primary outcome of atheroma volume, the mean 2.1% reduction among those randomized to alirocumab was more than double the 0.9% reduction in the placebo group (P = .001).
The mean reduction in lipid core volume based on a maximum lipid core burden index was also more than doubled (-79.42 vs. -37.60 maxLCBI4mm; P = .006). The increase in fibrous cap thickness was not quite twofold greater but very close (62.67 vs. 33.19 mcm; P = .001).
From baseline, the relative reductions in LDL-C, which were reached about 4 weeks after starting treatment and maintained over the course of the study, were greater in the group randomized to alirocumab (-84.8% vs. -50.7%). This was expected, but the more important finding was a near linear relationship between reductions of LDL-C and each of these endpoints regardless of treatment, fully explaining the advantage of alirocumab, according to Dr. Räber.
For the addition of alirocumab, “these findings indicate incremental coronary plaque regression, lipid core reduction, and plaque stabilization, and provide a mechanistic rationale in favor of early initiation of very intensive LDL-C lower in the setting of an acute MI,” he said.
The results of the PACMAN-AMI trial were published simultaneously at the time of the ACC presentation.
Results consistent with earlier trials
Alirocumab was well tolerated. Injection site reactions (6.1% vs. 3.3%) and general allergic reactions (3.4% vs. 0%) were more common on alirocumab, but there were no significant differences between the arms of this study for serious adverse events. There were slightly more neurocognitive events (2.0 vs. 0%) and abnormal alanine transferase levels (0.7% vs. 0%) in the alirocumab group.
The data are generally consistent with two previously published trials with another PCSK9 inhibitor, according to Dr. Räber. In the randomized GLAGOV trial published more than 5 years ago, evolocumab also produced about a 1% absolute reduction (P < .001) in plaque volume at the end of 78 weeks of follow-up relative to placebo.
However, that trial was limited to patients with coronary artery disease without a recent cardiovascular event. The more recent HUYGENS trial, which was presented virtually at the 2021 annual meeting of the European Society of Cardiology meeting and has not yet been published, looked at one of the endpoints also evaluated in PACMAN-AMI. In that study of 161 randomized NSTEMI patients, there was also about a doubling of fibrous cap thickness (42.7 vs. 21.5 mcm) for the PCSK9 inhibitor relative to placebo.
Clinical endpoints were not compared in either the PACMAN-AMI or HUYGENS trial.
PACMAN-AMI confirms plaque stabilization
Nevertheless, the message of plaque stabilization is important, according to Anthony N. DeMaria, MD, Founding Director of the Sulpizio Cardiovascular Center at the University of San Diego. Although he acknowledged that a 1% absolute reduction in mean plaque volume might “make you want to yawn,” he argued that this is a misreading of important changes observed in plaque physiology.
“What we have now is evidence that very low lipid levels result in plaque remodeling. The plaques might not get a whole lot smaller, but the changes are important,” he said, noting, for example, that a thicker fibrous cap and increased plaque stability “clearly plays a role in reducing risk of subsequent events.”
“You cannot help but be impressed by the relationship of lipid lowering and the favorable effect on remodeling,” he added.
The data associating PCSK9 inhibitors with protection from cardiovascular events is already extensive, according to Michael J. Blaha, MD, Director of Clinical Research for Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, but he called PACMAN-ACS “an extremely relevant study.”
“This provides more evidence of the mechanism of benefit, which I think is extremely important when talking to patients about the goals of therapy,” he said.
PACMAN-AMI provided a very simple take home message for Pamela B. Morris, MD, Director of Preventive Cardiology, Medical University of South Carolina, Charleston.
“This study shows that if you get LCL-C under 50 mg/dL regardless of treatment, there is a favorable remodeling effect,” Dr. Morris said. In AMI patients, the data confirm “go early and go low,” she added. “You should do whatever is necessary [go get to these lower targets].”
Dr. Räber has financial relationships with Abbott, Amgen, AstraZeneca, Boston Scientific, Biotronik, Canon, Heartflow, Medtronic, Occlutech, Regeneron, Sanofi, and Vifor. Dr. Blaha reports financial relationships with Akcea, Amgen, Bayer, Inozyme, Kaleido, Kowa, Medimmune, Novartis, Novo Nordisk, Regeneron, Roche, Sanofi, Siemens, and 89Bio. Dr. DeMaria reports no potential conflicts of interest. Dr. Morris reports a financial relationship with Amgen. The investigator-initiated trial received research grants from Infraredx, Regeneron, and Sanofi.
When the PCSK9 inhibitor alirocumab is added to high-intensity statins soon after an acute myocardial infarction (AMI), the reduction in atheroma volume is doubled at 12 months, compared with placebo, while other key signs of plaque stabilization, such as fibrous cap thickness, are also significantly and substantially improved, according to the results of the PACMAN-AMI trial.
The study is consistent with other PCSK9 inhibitor trials, supporting the concept that “we should be seeking very low levels of LDL-C in high-risk patients,” reported Lorenz Räber, MD, PhD, of Bern (Switz.) University Hospital, at the annual scientific sessions of the American College of Cardiology.
The low LCL-C target, the data from PACMAN-AMI suggest, is below 50 mg/dL, but even lower is better. When displayed graphically, the improvements in remodeling characteristics “get very steep” as levels descend below a 50 mg/dL threshold, Dr. Räber reported. This was true regardless of study arm.
In PACMAN-AMI, 300 AMI patients (with either ST-elevation or non-ST-elevaion) were randomized to 150 mg alirocumab or placebo administered by subcutaneous injection within 24 hours after an urgent percutaneous intervention (PCI) and stent placement. All patients received their assigned therapy on top of a high-intensity statin in the form of 20 mg of rosuvastatin daily.
Primary outcome was atheroma volume
The primary endpoint was atheroma volume as determined by intravenous ultrasound (IVUS), but the secondary endpoints of maximum lipid core burden, as determined by near infrared spectroscopy (NIRS), and fibrous cap thickness, as determined by optical coherence tomography (OCT), were also adequately powered, according to Dr. Räber.
The imaging measures taken at baseline were repeated in exactly the same spot after 52 weeks on treatment.
For the primary outcome of atheroma volume, the mean 2.1% reduction among those randomized to alirocumab was more than double the 0.9% reduction in the placebo group (P = .001).
The mean reduction in lipid core volume based on a maximum lipid core burden index was also more than doubled (-79.42 vs. -37.60 maxLCBI4mm; P = .006). The increase in fibrous cap thickness was not quite twofold greater but very close (62.67 vs. 33.19 mcm; P = .001).
From baseline, the relative reductions in LDL-C, which were reached about 4 weeks after starting treatment and maintained over the course of the study, were greater in the group randomized to alirocumab (-84.8% vs. -50.7%). This was expected, but the more important finding was a near linear relationship between reductions of LDL-C and each of these endpoints regardless of treatment, fully explaining the advantage of alirocumab, according to Dr. Räber.
For the addition of alirocumab, “these findings indicate incremental coronary plaque regression, lipid core reduction, and plaque stabilization, and provide a mechanistic rationale in favor of early initiation of very intensive LDL-C lower in the setting of an acute MI,” he said.
The results of the PACMAN-AMI trial were published simultaneously at the time of the ACC presentation.
Results consistent with earlier trials
Alirocumab was well tolerated. Injection site reactions (6.1% vs. 3.3%) and general allergic reactions (3.4% vs. 0%) were more common on alirocumab, but there were no significant differences between the arms of this study for serious adverse events. There were slightly more neurocognitive events (2.0 vs. 0%) and abnormal alanine transferase levels (0.7% vs. 0%) in the alirocumab group.
The data are generally consistent with two previously published trials with another PCSK9 inhibitor, according to Dr. Räber. In the randomized GLAGOV trial published more than 5 years ago, evolocumab also produced about a 1% absolute reduction (P < .001) in plaque volume at the end of 78 weeks of follow-up relative to placebo.
However, that trial was limited to patients with coronary artery disease without a recent cardiovascular event. The more recent HUYGENS trial, which was presented virtually at the 2021 annual meeting of the European Society of Cardiology meeting and has not yet been published, looked at one of the endpoints also evaluated in PACMAN-AMI. In that study of 161 randomized NSTEMI patients, there was also about a doubling of fibrous cap thickness (42.7 vs. 21.5 mcm) for the PCSK9 inhibitor relative to placebo.
Clinical endpoints were not compared in either the PACMAN-AMI or HUYGENS trial.
PACMAN-AMI confirms plaque stabilization
Nevertheless, the message of plaque stabilization is important, according to Anthony N. DeMaria, MD, Founding Director of the Sulpizio Cardiovascular Center at the University of San Diego. Although he acknowledged that a 1% absolute reduction in mean plaque volume might “make you want to yawn,” he argued that this is a misreading of important changes observed in plaque physiology.
“What we have now is evidence that very low lipid levels result in plaque remodeling. The plaques might not get a whole lot smaller, but the changes are important,” he said, noting, for example, that a thicker fibrous cap and increased plaque stability “clearly plays a role in reducing risk of subsequent events.”
“You cannot help but be impressed by the relationship of lipid lowering and the favorable effect on remodeling,” he added.
The data associating PCSK9 inhibitors with protection from cardiovascular events is already extensive, according to Michael J. Blaha, MD, Director of Clinical Research for Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, but he called PACMAN-ACS “an extremely relevant study.”
“This provides more evidence of the mechanism of benefit, which I think is extremely important when talking to patients about the goals of therapy,” he said.
PACMAN-AMI provided a very simple take home message for Pamela B. Morris, MD, Director of Preventive Cardiology, Medical University of South Carolina, Charleston.
“This study shows that if you get LCL-C under 50 mg/dL regardless of treatment, there is a favorable remodeling effect,” Dr. Morris said. In AMI patients, the data confirm “go early and go low,” she added. “You should do whatever is necessary [go get to these lower targets].”
Dr. Räber has financial relationships with Abbott, Amgen, AstraZeneca, Boston Scientific, Biotronik, Canon, Heartflow, Medtronic, Occlutech, Regeneron, Sanofi, and Vifor. Dr. Blaha reports financial relationships with Akcea, Amgen, Bayer, Inozyme, Kaleido, Kowa, Medimmune, Novartis, Novo Nordisk, Regeneron, Roche, Sanofi, Siemens, and 89Bio. Dr. DeMaria reports no potential conflicts of interest. Dr. Morris reports a financial relationship with Amgen. The investigator-initiated trial received research grants from Infraredx, Regeneron, and Sanofi.
When the PCSK9 inhibitor alirocumab is added to high-intensity statins soon after an acute myocardial infarction (AMI), the reduction in atheroma volume is doubled at 12 months, compared with placebo, while other key signs of plaque stabilization, such as fibrous cap thickness, are also significantly and substantially improved, according to the results of the PACMAN-AMI trial.
The study is consistent with other PCSK9 inhibitor trials, supporting the concept that “we should be seeking very low levels of LDL-C in high-risk patients,” reported Lorenz Räber, MD, PhD, of Bern (Switz.) University Hospital, at the annual scientific sessions of the American College of Cardiology.
The low LCL-C target, the data from PACMAN-AMI suggest, is below 50 mg/dL, but even lower is better. When displayed graphically, the improvements in remodeling characteristics “get very steep” as levels descend below a 50 mg/dL threshold, Dr. Räber reported. This was true regardless of study arm.
In PACMAN-AMI, 300 AMI patients (with either ST-elevation or non-ST-elevaion) were randomized to 150 mg alirocumab or placebo administered by subcutaneous injection within 24 hours after an urgent percutaneous intervention (PCI) and stent placement. All patients received their assigned therapy on top of a high-intensity statin in the form of 20 mg of rosuvastatin daily.
Primary outcome was atheroma volume
The primary endpoint was atheroma volume as determined by intravenous ultrasound (IVUS), but the secondary endpoints of maximum lipid core burden, as determined by near infrared spectroscopy (NIRS), and fibrous cap thickness, as determined by optical coherence tomography (OCT), were also adequately powered, according to Dr. Räber.
The imaging measures taken at baseline were repeated in exactly the same spot after 52 weeks on treatment.
For the primary outcome of atheroma volume, the mean 2.1% reduction among those randomized to alirocumab was more than double the 0.9% reduction in the placebo group (P = .001).
The mean reduction in lipid core volume based on a maximum lipid core burden index was also more than doubled (-79.42 vs. -37.60 maxLCBI4mm; P = .006). The increase in fibrous cap thickness was not quite twofold greater but very close (62.67 vs. 33.19 mcm; P = .001).
From baseline, the relative reductions in LDL-C, which were reached about 4 weeks after starting treatment and maintained over the course of the study, were greater in the group randomized to alirocumab (-84.8% vs. -50.7%). This was expected, but the more important finding was a near linear relationship between reductions of LDL-C and each of these endpoints regardless of treatment, fully explaining the advantage of alirocumab, according to Dr. Räber.
For the addition of alirocumab, “these findings indicate incremental coronary plaque regression, lipid core reduction, and plaque stabilization, and provide a mechanistic rationale in favor of early initiation of very intensive LDL-C lower in the setting of an acute MI,” he said.
The results of the PACMAN-AMI trial were published simultaneously at the time of the ACC presentation.
Results consistent with earlier trials
Alirocumab was well tolerated. Injection site reactions (6.1% vs. 3.3%) and general allergic reactions (3.4% vs. 0%) were more common on alirocumab, but there were no significant differences between the arms of this study for serious adverse events. There were slightly more neurocognitive events (2.0 vs. 0%) and abnormal alanine transferase levels (0.7% vs. 0%) in the alirocumab group.
The data are generally consistent with two previously published trials with another PCSK9 inhibitor, according to Dr. Räber. In the randomized GLAGOV trial published more than 5 years ago, evolocumab also produced about a 1% absolute reduction (P < .001) in plaque volume at the end of 78 weeks of follow-up relative to placebo.
However, that trial was limited to patients with coronary artery disease without a recent cardiovascular event. The more recent HUYGENS trial, which was presented virtually at the 2021 annual meeting of the European Society of Cardiology meeting and has not yet been published, looked at one of the endpoints also evaluated in PACMAN-AMI. In that study of 161 randomized NSTEMI patients, there was also about a doubling of fibrous cap thickness (42.7 vs. 21.5 mcm) for the PCSK9 inhibitor relative to placebo.
Clinical endpoints were not compared in either the PACMAN-AMI or HUYGENS trial.
PACMAN-AMI confirms plaque stabilization
Nevertheless, the message of plaque stabilization is important, according to Anthony N. DeMaria, MD, Founding Director of the Sulpizio Cardiovascular Center at the University of San Diego. Although he acknowledged that a 1% absolute reduction in mean plaque volume might “make you want to yawn,” he argued that this is a misreading of important changes observed in plaque physiology.
“What we have now is evidence that very low lipid levels result in plaque remodeling. The plaques might not get a whole lot smaller, but the changes are important,” he said, noting, for example, that a thicker fibrous cap and increased plaque stability “clearly plays a role in reducing risk of subsequent events.”
“You cannot help but be impressed by the relationship of lipid lowering and the favorable effect on remodeling,” he added.
The data associating PCSK9 inhibitors with protection from cardiovascular events is already extensive, according to Michael J. Blaha, MD, Director of Clinical Research for Ciccarone Center for Prevention of Cardiovascular Disease, Johns Hopkins University, Baltimore, but he called PACMAN-ACS “an extremely relevant study.”
“This provides more evidence of the mechanism of benefit, which I think is extremely important when talking to patients about the goals of therapy,” he said.
PACMAN-AMI provided a very simple take home message for Pamela B. Morris, MD, Director of Preventive Cardiology, Medical University of South Carolina, Charleston.
“This study shows that if you get LCL-C under 50 mg/dL regardless of treatment, there is a favorable remodeling effect,” Dr. Morris said. In AMI patients, the data confirm “go early and go low,” she added. “You should do whatever is necessary [go get to these lower targets].”
Dr. Räber has financial relationships with Abbott, Amgen, AstraZeneca, Boston Scientific, Biotronik, Canon, Heartflow, Medtronic, Occlutech, Regeneron, Sanofi, and Vifor. Dr. Blaha reports financial relationships with Akcea, Amgen, Bayer, Inozyme, Kaleido, Kowa, Medimmune, Novartis, Novo Nordisk, Regeneron, Roche, Sanofi, Siemens, and 89Bio. Dr. DeMaria reports no potential conflicts of interest. Dr. Morris reports a financial relationship with Amgen. The investigator-initiated trial received research grants from Infraredx, Regeneron, and Sanofi.
FROM ACC 2022
FDA approves leadless, single-chamber pacemaker system
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
The Food and Drug Administration has granted approval to Abbott’s Aveir leadless, single-chamber pacemaker system for patients with bradycardia.
In a press release, Abbott said the device has a unique mapping capability that allows interventionists implanting the device to measure electrical signals within the heart to determine the correct placement before final implantation. Aveir is implanted directly into the right ventricle via a catheter.
The company also said Aveir has a battery life that’s up to twice as long as other commercially available leadless pacemakers when following International Association for Standardization (ISO) standard settings. And the device can be retrieved if necessary, the press release said.
“Leadless pacemakers address known complications associated with traditional pacemakers,” Rahul Doshi, MD, director of electrophysiology at Honor Health in Scottsdale, Ariz., said in the press release. “In addition, the Aveir leadless pacemaker brings unique innovations we’ve been seeking, such as the ability to ensure electrical performance before we commit to placement.”
Investigators of the LEADLESS II phase 2 study reported last year on what they called “key design improvements” of the Aveir device compared to the first leadless pacemaker, the discontinued Nanostim. They included a 12% longer battery life, a shorter and wider form factor, a modified docking button that allows for retrievability, a modified delivery system, and an application-specific integrated circuit chip that can support a dual-chamber pacing system in the future.
The study reported that 96% of the 200 enrolled patients met the primary safety endpoint of no serious device-related adverse events at 6 weeks after implantation. A similar percentage achieved therapeutic pacing and sensing amplitude.
The study also reported that interventionists accurately positioned Aveir the first time or with a single repositioning in 96% of cases.
More evidence that COVID ‘brain fog’ is biologically based
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found elevated levels of CSF immune activation and immunovascular markers in individuals with cognitive postacute sequelae of SARS-CoV-2 infection (PASC). Patients whose cognitive symptoms developed during the acute phase of COVID-19 had the highest levels of brain inflammation.
The findings add to a growing body of evidence that suggests the condition often referred to as “brain fog” has a neurologic basis, said lead author Joanna Hellmuth, MD, MHS, assistant professor of neurology at the University of California, San Francisco Weill Institute of Neurosciences and the UCSF Memory and Aging Center.
The findings will be presented at the 2022 annual meeting of the American Academy of Neurology.
Inflammatory response
There are no effective diagnostic tests or treatments for cognitive PASC, which prompted the investigators to study inflammation in patients with the condition. Initial findings were reported earlier in 2022, which showed abnormalities in the CSF in 77% of patients with cognitive impairment. Patients without cognitive impairments had normal CSF.
Extending that work in this new study, researchers studied patients from the Long-term Impact of Infection With Novel Coronavirus (LIINC) study with confirmed SARS-CoV-2 infection who were not hospitalized. They conducted 2-hour neurocognitive interviews and identified 23 people with new, persistent cognitive symptoms (cognitive PASC) and 10 with no cognitive symptoms who served as controls.
All participants underwent additional neurologic examination and neuropsychological testing, and half agreed to a lumbar puncture to allow researchers to collect CSF samples. The CSF was collected a median of 10.2 months after initial COVID symptoms began.
Participants with cognitive PASC had higher median levels of CSF acute phase reactants C-reactive protein (0.007 mg/L vs. 0.000 mg/L; P =.004) and serum amyloid A (0.001 mg/L vs. 0.000 mg/L; P = .001), compared with COVID controls.
The PASC group also had elevated levels of CSF immune activation markers interferon gamma–inducible protein (IP-10), interleukin-8, and immunovascular markers vascular endothelial growth factor-C and VEGFR-1, although the differences with the control group were not statistically significant.
The timing of the onset of cognitive problems was also associated with higher levels of immune activation and immunovascular markers. Patients with brain fog that developed during the acute phase of COVID-19 had higher levels of CSF VEGF-C, compared with patients whose cognitive symptoms developed more than a month after initial COVID symptoms (173 pg/mL vs. 99 pg/mL; P = .048) and COVID controls (79 pg/mL; P = .048).
Acute onset cognitive PASC participants had higher CSF levels of IP-10 (P = .030), IL-8 (P = .048), placental growth factor (P = .030) and intercellular adhesion molecule-1 (P = .045), compared with COVID controls.
Researchers believe these new findings could mean that intrathecal immune activation and endothelial activation/dysfunction may contribute to cognitive PASC and that the mechanisms involved may be different in patients with acute cognitive PASC versus those with delayed onset.
“Our data suggests that perhaps in these people with more acute cognitive changes they don’t have the return to homeostasis,” Dr. Hellmuth said, while patients with delayed onset cognitive PASC had levels more in line with COVID patients who had no cognitive issues.
Moving the needle forward
Commenting on the findings, William Schaffner, MD, professor of infectious diseases, Vanderbilt University Medical Center, Nashville, Tenn., said that, while the study doesn’t rule out a possible psychological basis for cognitive PASC, it adds more weight to the biological argument.
“When you have nonspecific symptoms for which specific tests are unavailable,” Dr. Schaffner explained, “there is a natural question that always comes up: Is this principally a biologically induced phenomenon or psychological? This moves the needle substantially in the direction of a biological phenomenon.”
Another important element to the study, Dr. Schaffner said, is that the patients involved had mild COVID.
“Not every patient with long COVID symptoms had been hospitalized with severe disease,” he said. “There are inflammatory phenomenon in various organ systems such that even if the inflammatory response in the lung was not severe enough to get you into the hospital, there were inflammatory responses in other organ systems that could persist once the acute infection resolved.”
Although the small size of the study is a limitation, Dr. Schaffner said that shouldn’t minimize the importance of these findings.
“That it’s small doesn’t diminish its value,” he said. “The next step forward might be to try to associate the markers more specifically with COVID. The more precise we can be, the more convincing the story will become.”
The study was funded by the National Institutes of Health. Dr. Hellmuth received grant support from the National Institutes of Health/National Institute of Mental Health supporting this work and personal fees for medical-legal consultation outside of the submitted work. Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAN 2022
Postmenopausal women may benefit from vaginal estradiol for treatment of symptoms
Vaginal estradiol tablets promoted significant changes in the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but reduction in bothersome symptoms were similar, based on data from 144 individuals.
“In the Menopause Strategies–Finding Lasting Answers and Health (MsFLASH) trial network’s Vaginal Health Trial of treatment for moderate to severe vaginal symptoms of menopause, there were no significant differences in reduction of vaginal symptoms among women using the estradiol vaginal tablet or vaginal moisturizer compared to women using the placebo regimen; all three groups had a reduction in vaginal symptoms,” lead author Sujatha Srinivasan, PhD, of the Fred Hutchinson Cancer Center, Seattle, said in an interview.
“However, the impact of these treatments on the vaginal microenvironment are poorly understood,” she said.
In a study published in JAMA Network Open, Dr. Srinivasan and colleagues conducted a secondary analysis to examine the effects of estradiol or a low-pH vaginal moisturizer on the vaginal microbiota, metabolome, and pH after 12 weeks of treatment vs. a low-pH placebo.
“Changes, or lack thereof, in the vaginal microenvironment might have implications beyond symptoms, and might be linked to risk for cervical cancer, genital infections, or other outcomes, though our study did not evaluate those associations,” Dr. Srinivasan said in an interview. Dr. Srinivasan’s comments were corroborated by coauthor Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston.
The study population included postmenopausal women with moderate to severe genitourinary symptoms who were enrolled in a randomized, controlled trial between April 2016 and February 2017. The average age of the women was 61 years, and 90% were White. The women were randomized to 10 mcg vaginal estradiol plus placebo gel, placebo tablet plus vaginal moisturizer, or a dual placebo.
The primary outcome in the original study was a change in the reported most bothersome symptoms (MBS) selected by the participants at the time of study enrollment; these included pain with penetration, vaginal dryness, and vulvovaginal irritation, itching, and pain. The main outcomes in the secondary analysis were changes in the diversity and composition of the vaginal microbiota, changes in the metabolome, and pH. Microbiota diversity was calculated via the Shannon Diversity Index (SDI).
After 12 weeks, the bacterial microbiota were dominated by Lactobacillus and Bifidobacterium in 80% of the estradiol group, 36% of the moisturizer group, and 26% of the placebo group (P < .001).
In addition, diversity analysis showed significant changes in bacterial composition in women in the estradiol group compared with the placebo group, but no significant differences between the moisturizer and placebo groups.
The composition of vaginal fluid small molecule metabolites changed significantly in 90 of 171 metabolites measured in the estradiol group from baseline to 12 weeks. Changes in the moisturizer and placebo groups were not significant.
Vaginal pH among women in the estradiol group was significantly lower than placebo at 12 weeks, with a median of 5 vs. 6 (P = .005). No significant difference in pH occurred for women in the moisturizer group. “However, pH significantly decreased over 12 weeks within each treatment group, reflecting the low-pH formulations of both the moisturizer and the placebo,” the researchers wrote.
Overall, women with high-diversity bacterial communities at baseline showed a greater median change in pH compared with women with low-diversity communities (median change of −1 vs. −0.3, P = .007).
Improvement in MBS symptoms by at least 2 points occurred in 53% of the estradiol group, 44% of the moisturizer group, and 49% of the placebo group. The similarity in severe symptom improvement among the groups confirms the lack of a causal association between microbiota and postmenopausal vaginal symptom severity, the researchers wrote.
“This study demonstrated that a decrease in vaginal pH alone was insufficient to change the vaginal microbiota,” Dr. Srinivasan said. “While the changes with estrogen were somewhat expected, the observation that low-pH vaginal products don’t change the vaginal microbiota is contrary to some expectations, and suggests that “low-pH” products may not be as helpful as their marketing claims,” she added. “A vaginal microbiota with an abundance of lactobacilli, a vaginal microenvironment with high concentrations of lactate, and a low vaginal pH is associated with health in premenopausal women. We also know that such a microenvironment is typically associated with low inflammation,” said Dr. Srinivasan. “At this time, we don’t have specific information as to how this is beneficial to postmenopausal women,” she noted. However, “If we extrapolate from the data on premenopausal women, the data from this secondary analysis suggests that vaginal estradiol may have positive impacts on the vaginal microenvironment regardless of impact on symptoms,” she said.
“Future areas of investigation should focus on understanding potential benefits of a Lactobacillus-dominant microbiota in postmenopausal women,” Dr. Srinivasan said.
The study findings were limited by several factors including the relatively small number of participants, and collection of data samples at only three time periods, as well as the lack of data on whether the observed changes are durable over longer treatment times, the researchers noted.
“The need to increase participant diversity in studies of postmenopausal women is highlighted by our finding that the 6 Black women in our analysis were all categorized in the low-diversity subgroup; data from premenopausal women suggest that Black women have diverse bacterial communities,” they added.
However, the results suggest that “a significant decrease in pH over the course of a trial may not reflect the same underlying biological processes among different interventions, and thus, lowering pH should not be a primary goal,” they concluded.
Estradiol may have limited clinical impact
“For postmenopausal women with dyspareunia, vaginal dryness, and/or burning/itching/irritation, the question of appropriate treatment is common,” Constance Bohon, MD, a gynecologist in private practice in Washington, said in an interview. “It is helpful to have a study that focuses on the benefit of a moisturizer as compared with vaginal estrogen for these women,” she said.
Dr. Bohon said she was not surprised with the benefits of the moisturizer for dyspareunia and vaginal dryness. “What did surprise me was that the complaint of vaginal itch, burn, or irritation was not significantly improved in the vaginal estrogen group compared with the moisturizer group. I assumed that estrogen would have been more beneficial in this group because these symptoms are more likely to be caused by a vaginal infection that would not be improved with moisturizer alone,” she said. “I expected that the change in the vaginal flora to increase Lactobacillus would have had a greater impact on an infection than the moisturizer, which did not significantly change the flora.”
For clinicians, the take-home message is that, for these patients, use of a moisturizer may be sufficient, Dr. Bohon said.
“Additional research should be done to assess each issue,” she noted. “For example, in the women who have pain with sex, what is the frequency of intercourse?” she asked. Other research should address the questions of whether women who have intercourse at least once a week have less dyspareunia than those who have less frequent sex, and whether a lubricant decreases dyspareunia as well as a moisturizer or vaginal estrogen, she added.
The study was supported by the National Institutes of Health. Dr. Srinivasan disclosed personal fees from Lupin unrelated to the current study. Dr. Bohon had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Vaginal estradiol tablets promoted significant changes in the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but reduction in bothersome symptoms were similar, based on data from 144 individuals.
“In the Menopause Strategies–Finding Lasting Answers and Health (MsFLASH) trial network’s Vaginal Health Trial of treatment for moderate to severe vaginal symptoms of menopause, there were no significant differences in reduction of vaginal symptoms among women using the estradiol vaginal tablet or vaginal moisturizer compared to women using the placebo regimen; all three groups had a reduction in vaginal symptoms,” lead author Sujatha Srinivasan, PhD, of the Fred Hutchinson Cancer Center, Seattle, said in an interview.
“However, the impact of these treatments on the vaginal microenvironment are poorly understood,” she said.
In a study published in JAMA Network Open, Dr. Srinivasan and colleagues conducted a secondary analysis to examine the effects of estradiol or a low-pH vaginal moisturizer on the vaginal microbiota, metabolome, and pH after 12 weeks of treatment vs. a low-pH placebo.
“Changes, or lack thereof, in the vaginal microenvironment might have implications beyond symptoms, and might be linked to risk for cervical cancer, genital infections, or other outcomes, though our study did not evaluate those associations,” Dr. Srinivasan said in an interview. Dr. Srinivasan’s comments were corroborated by coauthor Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston.
The study population included postmenopausal women with moderate to severe genitourinary symptoms who were enrolled in a randomized, controlled trial between April 2016 and February 2017. The average age of the women was 61 years, and 90% were White. The women were randomized to 10 mcg vaginal estradiol plus placebo gel, placebo tablet plus vaginal moisturizer, or a dual placebo.
The primary outcome in the original study was a change in the reported most bothersome symptoms (MBS) selected by the participants at the time of study enrollment; these included pain with penetration, vaginal dryness, and vulvovaginal irritation, itching, and pain. The main outcomes in the secondary analysis were changes in the diversity and composition of the vaginal microbiota, changes in the metabolome, and pH. Microbiota diversity was calculated via the Shannon Diversity Index (SDI).
After 12 weeks, the bacterial microbiota were dominated by Lactobacillus and Bifidobacterium in 80% of the estradiol group, 36% of the moisturizer group, and 26% of the placebo group (P < .001).
In addition, diversity analysis showed significant changes in bacterial composition in women in the estradiol group compared with the placebo group, but no significant differences between the moisturizer and placebo groups.
The composition of vaginal fluid small molecule metabolites changed significantly in 90 of 171 metabolites measured in the estradiol group from baseline to 12 weeks. Changes in the moisturizer and placebo groups were not significant.
Vaginal pH among women in the estradiol group was significantly lower than placebo at 12 weeks, with a median of 5 vs. 6 (P = .005). No significant difference in pH occurred for women in the moisturizer group. “However, pH significantly decreased over 12 weeks within each treatment group, reflecting the low-pH formulations of both the moisturizer and the placebo,” the researchers wrote.
Overall, women with high-diversity bacterial communities at baseline showed a greater median change in pH compared with women with low-diversity communities (median change of −1 vs. −0.3, P = .007).
Improvement in MBS symptoms by at least 2 points occurred in 53% of the estradiol group, 44% of the moisturizer group, and 49% of the placebo group. The similarity in severe symptom improvement among the groups confirms the lack of a causal association between microbiota and postmenopausal vaginal symptom severity, the researchers wrote.
“This study demonstrated that a decrease in vaginal pH alone was insufficient to change the vaginal microbiota,” Dr. Srinivasan said. “While the changes with estrogen were somewhat expected, the observation that low-pH vaginal products don’t change the vaginal microbiota is contrary to some expectations, and suggests that “low-pH” products may not be as helpful as their marketing claims,” she added. “A vaginal microbiota with an abundance of lactobacilli, a vaginal microenvironment with high concentrations of lactate, and a low vaginal pH is associated with health in premenopausal women. We also know that such a microenvironment is typically associated with low inflammation,” said Dr. Srinivasan. “At this time, we don’t have specific information as to how this is beneficial to postmenopausal women,” she noted. However, “If we extrapolate from the data on premenopausal women, the data from this secondary analysis suggests that vaginal estradiol may have positive impacts on the vaginal microenvironment regardless of impact on symptoms,” she said.
“Future areas of investigation should focus on understanding potential benefits of a Lactobacillus-dominant microbiota in postmenopausal women,” Dr. Srinivasan said.
The study findings were limited by several factors including the relatively small number of participants, and collection of data samples at only three time periods, as well as the lack of data on whether the observed changes are durable over longer treatment times, the researchers noted.
“The need to increase participant diversity in studies of postmenopausal women is highlighted by our finding that the 6 Black women in our analysis were all categorized in the low-diversity subgroup; data from premenopausal women suggest that Black women have diverse bacterial communities,” they added.
However, the results suggest that “a significant decrease in pH over the course of a trial may not reflect the same underlying biological processes among different interventions, and thus, lowering pH should not be a primary goal,” they concluded.
Estradiol may have limited clinical impact
“For postmenopausal women with dyspareunia, vaginal dryness, and/or burning/itching/irritation, the question of appropriate treatment is common,” Constance Bohon, MD, a gynecologist in private practice in Washington, said in an interview. “It is helpful to have a study that focuses on the benefit of a moisturizer as compared with vaginal estrogen for these women,” she said.
Dr. Bohon said she was not surprised with the benefits of the moisturizer for dyspareunia and vaginal dryness. “What did surprise me was that the complaint of vaginal itch, burn, or irritation was not significantly improved in the vaginal estrogen group compared with the moisturizer group. I assumed that estrogen would have been more beneficial in this group because these symptoms are more likely to be caused by a vaginal infection that would not be improved with moisturizer alone,” she said. “I expected that the change in the vaginal flora to increase Lactobacillus would have had a greater impact on an infection than the moisturizer, which did not significantly change the flora.”
For clinicians, the take-home message is that, for these patients, use of a moisturizer may be sufficient, Dr. Bohon said.
“Additional research should be done to assess each issue,” she noted. “For example, in the women who have pain with sex, what is the frequency of intercourse?” she asked. Other research should address the questions of whether women who have intercourse at least once a week have less dyspareunia than those who have less frequent sex, and whether a lubricant decreases dyspareunia as well as a moisturizer or vaginal estrogen, she added.
The study was supported by the National Institutes of Health. Dr. Srinivasan disclosed personal fees from Lupin unrelated to the current study. Dr. Bohon had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Vaginal estradiol tablets promoted significant changes in the vaginal microbiota in postmenopausal women compared with vaginal moisturizer or placebo, but reduction in bothersome symptoms were similar, based on data from 144 individuals.
“In the Menopause Strategies–Finding Lasting Answers and Health (MsFLASH) trial network’s Vaginal Health Trial of treatment for moderate to severe vaginal symptoms of menopause, there were no significant differences in reduction of vaginal symptoms among women using the estradiol vaginal tablet or vaginal moisturizer compared to women using the placebo regimen; all three groups had a reduction in vaginal symptoms,” lead author Sujatha Srinivasan, PhD, of the Fred Hutchinson Cancer Center, Seattle, said in an interview.
“However, the impact of these treatments on the vaginal microenvironment are poorly understood,” she said.
In a study published in JAMA Network Open, Dr. Srinivasan and colleagues conducted a secondary analysis to examine the effects of estradiol or a low-pH vaginal moisturizer on the vaginal microbiota, metabolome, and pH after 12 weeks of treatment vs. a low-pH placebo.
“Changes, or lack thereof, in the vaginal microenvironment might have implications beyond symptoms, and might be linked to risk for cervical cancer, genital infections, or other outcomes, though our study did not evaluate those associations,” Dr. Srinivasan said in an interview. Dr. Srinivasan’s comments were corroborated by coauthor Caroline M. Mitchell, MD, of Massachusetts General Hospital, Boston.
The study population included postmenopausal women with moderate to severe genitourinary symptoms who were enrolled in a randomized, controlled trial between April 2016 and February 2017. The average age of the women was 61 years, and 90% were White. The women were randomized to 10 mcg vaginal estradiol plus placebo gel, placebo tablet plus vaginal moisturizer, or a dual placebo.
The primary outcome in the original study was a change in the reported most bothersome symptoms (MBS) selected by the participants at the time of study enrollment; these included pain with penetration, vaginal dryness, and vulvovaginal irritation, itching, and pain. The main outcomes in the secondary analysis were changes in the diversity and composition of the vaginal microbiota, changes in the metabolome, and pH. Microbiota diversity was calculated via the Shannon Diversity Index (SDI).
After 12 weeks, the bacterial microbiota were dominated by Lactobacillus and Bifidobacterium in 80% of the estradiol group, 36% of the moisturizer group, and 26% of the placebo group (P < .001).
In addition, diversity analysis showed significant changes in bacterial composition in women in the estradiol group compared with the placebo group, but no significant differences between the moisturizer and placebo groups.
The composition of vaginal fluid small molecule metabolites changed significantly in 90 of 171 metabolites measured in the estradiol group from baseline to 12 weeks. Changes in the moisturizer and placebo groups were not significant.
Vaginal pH among women in the estradiol group was significantly lower than placebo at 12 weeks, with a median of 5 vs. 6 (P = .005). No significant difference in pH occurred for women in the moisturizer group. “However, pH significantly decreased over 12 weeks within each treatment group, reflecting the low-pH formulations of both the moisturizer and the placebo,” the researchers wrote.
Overall, women with high-diversity bacterial communities at baseline showed a greater median change in pH compared with women with low-diversity communities (median change of −1 vs. −0.3, P = .007).
Improvement in MBS symptoms by at least 2 points occurred in 53% of the estradiol group, 44% of the moisturizer group, and 49% of the placebo group. The similarity in severe symptom improvement among the groups confirms the lack of a causal association between microbiota and postmenopausal vaginal symptom severity, the researchers wrote.
“This study demonstrated that a decrease in vaginal pH alone was insufficient to change the vaginal microbiota,” Dr. Srinivasan said. “While the changes with estrogen were somewhat expected, the observation that low-pH vaginal products don’t change the vaginal microbiota is contrary to some expectations, and suggests that “low-pH” products may not be as helpful as their marketing claims,” she added. “A vaginal microbiota with an abundance of lactobacilli, a vaginal microenvironment with high concentrations of lactate, and a low vaginal pH is associated with health in premenopausal women. We also know that such a microenvironment is typically associated with low inflammation,” said Dr. Srinivasan. “At this time, we don’t have specific information as to how this is beneficial to postmenopausal women,” she noted. However, “If we extrapolate from the data on premenopausal women, the data from this secondary analysis suggests that vaginal estradiol may have positive impacts on the vaginal microenvironment regardless of impact on symptoms,” she said.
“Future areas of investigation should focus on understanding potential benefits of a Lactobacillus-dominant microbiota in postmenopausal women,” Dr. Srinivasan said.
The study findings were limited by several factors including the relatively small number of participants, and collection of data samples at only three time periods, as well as the lack of data on whether the observed changes are durable over longer treatment times, the researchers noted.
“The need to increase participant diversity in studies of postmenopausal women is highlighted by our finding that the 6 Black women in our analysis were all categorized in the low-diversity subgroup; data from premenopausal women suggest that Black women have diverse bacterial communities,” they added.
However, the results suggest that “a significant decrease in pH over the course of a trial may not reflect the same underlying biological processes among different interventions, and thus, lowering pH should not be a primary goal,” they concluded.
Estradiol may have limited clinical impact
“For postmenopausal women with dyspareunia, vaginal dryness, and/or burning/itching/irritation, the question of appropriate treatment is common,” Constance Bohon, MD, a gynecologist in private practice in Washington, said in an interview. “It is helpful to have a study that focuses on the benefit of a moisturizer as compared with vaginal estrogen for these women,” she said.
Dr. Bohon said she was not surprised with the benefits of the moisturizer for dyspareunia and vaginal dryness. “What did surprise me was that the complaint of vaginal itch, burn, or irritation was not significantly improved in the vaginal estrogen group compared with the moisturizer group. I assumed that estrogen would have been more beneficial in this group because these symptoms are more likely to be caused by a vaginal infection that would not be improved with moisturizer alone,” she said. “I expected that the change in the vaginal flora to increase Lactobacillus would have had a greater impact on an infection than the moisturizer, which did not significantly change the flora.”
For clinicians, the take-home message is that, for these patients, use of a moisturizer may be sufficient, Dr. Bohon said.
“Additional research should be done to assess each issue,” she noted. “For example, in the women who have pain with sex, what is the frequency of intercourse?” she asked. Other research should address the questions of whether women who have intercourse at least once a week have less dyspareunia than those who have less frequent sex, and whether a lubricant decreases dyspareunia as well as a moisturizer or vaginal estrogen, she added.
The study was supported by the National Institutes of Health. Dr. Srinivasan disclosed personal fees from Lupin unrelated to the current study. Dr. Bohon had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
FROM JAMA NETWORK OPEN
It’s a gimmick
March 30 was National Doctor’s Day, which resulted in my getting all kinds of generic emails from pharmaceutical reps, market research places, insurance companies, and the two hospitals I’m on staff at.
They all had similar meaningless platitudes thanking me for what I do, reassuring me that I’m appreciated, that I make the world a better place, yadda yadda yadda. The hospital even said I could swing by the medical staff office and pick up an “appreciation bag,” which I’m told contained a T-shirt, bottle of hand sanitizer, and a few other trinkets.
Spare me.
I’m not looking for any of that. In fact, I really don’t care.
Wishing me a “Happy Doctors Day” after spending the other 364 days denying my claims, refusing to cover tests or medications for my patients who need them (I don’t order these things for the hell of it, you know), telling me that I’m bringing down your Press Ganey scores, complaining about the copay that I have no control over, yelling at my staff for doing their jobs ... is pretty damn hollow.
It’s kind of like Mother’s Day: If you’re a jackass to your mom most of the year, sending her flowers on a Sunday in May doesn’t make it all right.
People also seem to forget that, in a small practice, my awesome staff is an extension of myself. Mistreating them, then wishing me a “Happy Doctor’s Day,” is also worthless.
I still like what I do. All the hassles from insurance companies, various administrators, the occasional angry patient … after all these years, they put a dent in it, but I still have no regrets about the course I’ve chosen. They can’t take away the happiness I get from helping those who need me.
It’s a job I love that’s allowed me to support my family and work with two wonderful staff members I’d never have met otherwise.
And that’s all I need.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
March 30 was National Doctor’s Day, which resulted in my getting all kinds of generic emails from pharmaceutical reps, market research places, insurance companies, and the two hospitals I’m on staff at.
They all had similar meaningless platitudes thanking me for what I do, reassuring me that I’m appreciated, that I make the world a better place, yadda yadda yadda. The hospital even said I could swing by the medical staff office and pick up an “appreciation bag,” which I’m told contained a T-shirt, bottle of hand sanitizer, and a few other trinkets.
Spare me.
I’m not looking for any of that. In fact, I really don’t care.
Wishing me a “Happy Doctors Day” after spending the other 364 days denying my claims, refusing to cover tests or medications for my patients who need them (I don’t order these things for the hell of it, you know), telling me that I’m bringing down your Press Ganey scores, complaining about the copay that I have no control over, yelling at my staff for doing their jobs ... is pretty damn hollow.
It’s kind of like Mother’s Day: If you’re a jackass to your mom most of the year, sending her flowers on a Sunday in May doesn’t make it all right.
People also seem to forget that, in a small practice, my awesome staff is an extension of myself. Mistreating them, then wishing me a “Happy Doctor’s Day,” is also worthless.
I still like what I do. All the hassles from insurance companies, various administrators, the occasional angry patient … after all these years, they put a dent in it, but I still have no regrets about the course I’ve chosen. They can’t take away the happiness I get from helping those who need me.
It’s a job I love that’s allowed me to support my family and work with two wonderful staff members I’d never have met otherwise.
And that’s all I need.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
March 30 was National Doctor’s Day, which resulted in my getting all kinds of generic emails from pharmaceutical reps, market research places, insurance companies, and the two hospitals I’m on staff at.
They all had similar meaningless platitudes thanking me for what I do, reassuring me that I’m appreciated, that I make the world a better place, yadda yadda yadda. The hospital even said I could swing by the medical staff office and pick up an “appreciation bag,” which I’m told contained a T-shirt, bottle of hand sanitizer, and a few other trinkets.
Spare me.
I’m not looking for any of that. In fact, I really don’t care.
Wishing me a “Happy Doctors Day” after spending the other 364 days denying my claims, refusing to cover tests or medications for my patients who need them (I don’t order these things for the hell of it, you know), telling me that I’m bringing down your Press Ganey scores, complaining about the copay that I have no control over, yelling at my staff for doing their jobs ... is pretty damn hollow.
It’s kind of like Mother’s Day: If you’re a jackass to your mom most of the year, sending her flowers on a Sunday in May doesn’t make it all right.
People also seem to forget that, in a small practice, my awesome staff is an extension of myself. Mistreating them, then wishing me a “Happy Doctor’s Day,” is also worthless.
I still like what I do. All the hassles from insurance companies, various administrators, the occasional angry patient … after all these years, they put a dent in it, but I still have no regrets about the course I’ve chosen. They can’t take away the happiness I get from helping those who need me.
It’s a job I love that’s allowed me to support my family and work with two wonderful staff members I’d never have met otherwise.
And that’s all I need.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.