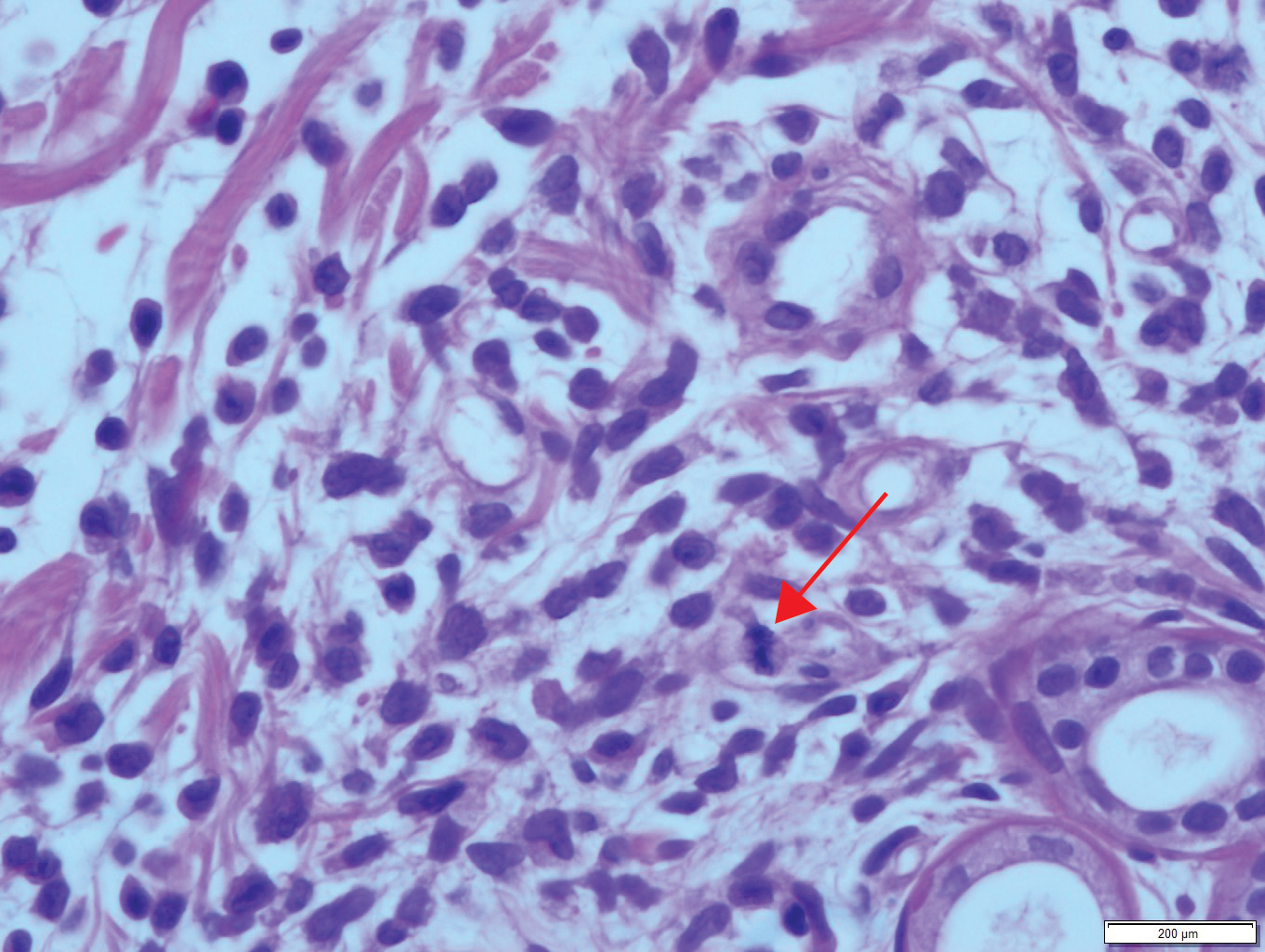
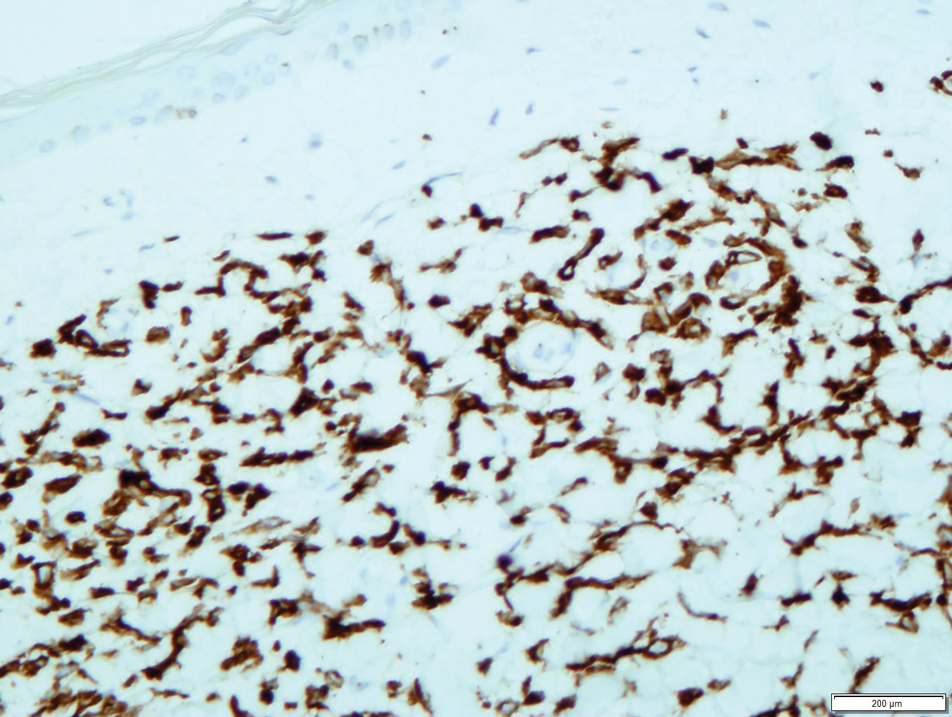
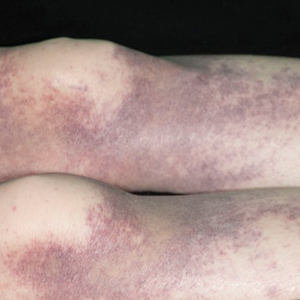
User login
Variants of nine breast cancer genes associated with severe disease
Breast cancer risk genes are generally associated with triple-negative and high-grade disease, but differ substantially in their associated pathology, a large case-control analysis shows.
The findings have potential implications for genetic testing, risk prediction, variant classification, and screening, the authors noted.
To assess links between pathogenic germline variants in nine major breast cancer (BC) susceptibility genes and pathological features of nonmetastasized breast tumors, investigators from the Breast Cancer Association Consortium compared 42,680 women with breast cancer and 42,387 population-matched controls from the BRIDGES large-scale sequencing study.
They looked specifically at features relevant to prognosis and distinct therapeutic options, including tumor subtype, morphology, size, stage, and lymph node involvement, and found substantial differences in tumor subtype distribution by gene.
“Pathogenic [protein-truncating variants and pathogenic missense variants] in these ... they wrote, noting that carriers of rare genetic variants in the nine genes comprised more than 27% of women diagnosed at age 40 or younger with triple-negative disease (driven mainly by BRCA1) and about 16% of those diagnosed with hormone receptor (HR)–positive, HER2-negative high-grade disease.
All together, the nine genes were associated with 14.4% of tumors in women aged 40 years and younger, but less than 4% in women over age 60 years, and among younger women, the prevalence of variants combined was higher in those with triple-negative and HR-positive, HER2-negative disease versus other subtypes, they said.
The findings were published online on Jan. 27, 2022, in JAMA Oncology.
Study subjects were women aged 18-79 years who were sampled, independently of family history, from 38 international population- or hospital-based studies conducted between 1991 and 2016.
The genes assessed included ATM, BARD1, BRCA1, BRCA2, CHEK2, PALB2, RAD51C, RAD51D, and TP53.
Substantial heterogeneity was observed in the distribution of intrinsic subtypes by gene. For example, RAD51C, RAD51D, and BARD1 variants were associated mainly with triple-negative disease (odds ratios, 6.19, 6.19, and 10.05 respectively), and CHEK2 variants were associated with all subtypes (with ORs ranging from 2.21-3.17) except for triple-negative disease, the authors found.
“For ATM variants, the association was strongest for [HR-positive, HER2-negative]high-grade subtype (OR, 4.99). BRCA1 was associated with increased risk of all subtypes, but the ORs varied widely, being highest for triple-negative disease (OR, 55.32),” they wrote.
BRCA2 and PALB2 variants were also associated with triple-negative disease, TP53 variants were most strongly associated with HR-positive, HER2-negative and HR-negative, HER2-positive subtypes, and tumors occurring in pathogenic variant carriers were of higher grade, they added, noting that for most genes and subtypes, a decline in ORs was observed with increasing age.
All genes except CHEK2 were more strongly associated with high-grade disease.
These and other findings from the study can “inform guidelines for eligibility for gene panel sequencing and breast cancer surveillance in the general population,” the authors explained, adding that tumor characteristics can also be used to determine whether variants of uncertain significance are likely to be pathogenic.
The data should therefore “improve the precision of variant classification algorithms and extend them to a larger set of genes,” they noted.
“This case-control study suggests that rare variants in BC susceptibility genes display marked heterogeneity with respect to tumor phenotype, but also similarities between genes that are consistent with known biological functions. This present study provides detailed quantification of subtype-specific breast cancer risks; these can potentially improve risk prediction models and breast cancer prevention strategies,” they concluded.
More specifically, women found to be carrying variants in these genes may be offered enhanced screening, including by MRI, risk-reducing surgery, chemoprevention, and genetic counseling, corresponding author Nasim Mavaddat, MBBS, PhD, explained in an interview.
“Understanding tumor pathology associated with the genes can inform breast cancer risk prediction models, and management and treatment of women harboring pathogenic variants,” she noted.
The findings “enhance our understanding of breast cancer biology and aid efforts to classify whether genetic variants of uncertain significance are also likely to be pathogenic,” added Dr. Mavaddat, a senior research associate at the University of Cambridge (England).
She also said the investigators are taking steps to include the results in “CanRisk,” a cancer risk prediction model used by genetic counselors and other clinicians, and are “exploring whether variants in these genes are associated with contralateral breast cancer risk and survival after a breast cancer.”
As most participants were European, analyses should be extended to women of other racial and ethnic groups, she added.
The BRIDGES panel sequencing was supported by the European Union Horizon 2020 research and innovation program BRIDGES and the Wellcome Trust. The Breast Cancer Association Consortium is also funded by the European Union Horizon 2020 research and innovation program, the PERSPECTIVE I&I project, which is funded by the Government of Canada, and the Confluence project, which is funded with intramural funds from the National Cancer Institute. Dr. Mavaddat reported grants from the University of Cambridge, European Union Horizon 2020, Wellcome Trust, Genome Canada, Canadian Institutes of Health Research, and National Cancer Institute during the conduct of the study.
Breast cancer risk genes are generally associated with triple-negative and high-grade disease, but differ substantially in their associated pathology, a large case-control analysis shows.
The findings have potential implications for genetic testing, risk prediction, variant classification, and screening, the authors noted.
To assess links between pathogenic germline variants in nine major breast cancer (BC) susceptibility genes and pathological features of nonmetastasized breast tumors, investigators from the Breast Cancer Association Consortium compared 42,680 women with breast cancer and 42,387 population-matched controls from the BRIDGES large-scale sequencing study.
They looked specifically at features relevant to prognosis and distinct therapeutic options, including tumor subtype, morphology, size, stage, and lymph node involvement, and found substantial differences in tumor subtype distribution by gene.
“Pathogenic [protein-truncating variants and pathogenic missense variants] in these ... they wrote, noting that carriers of rare genetic variants in the nine genes comprised more than 27% of women diagnosed at age 40 or younger with triple-negative disease (driven mainly by BRCA1) and about 16% of those diagnosed with hormone receptor (HR)–positive, HER2-negative high-grade disease.
All together, the nine genes were associated with 14.4% of tumors in women aged 40 years and younger, but less than 4% in women over age 60 years, and among younger women, the prevalence of variants combined was higher in those with triple-negative and HR-positive, HER2-negative disease versus other subtypes, they said.
The findings were published online on Jan. 27, 2022, in JAMA Oncology.
Study subjects were women aged 18-79 years who were sampled, independently of family history, from 38 international population- or hospital-based studies conducted between 1991 and 2016.
The genes assessed included ATM, BARD1, BRCA1, BRCA2, CHEK2, PALB2, RAD51C, RAD51D, and TP53.
Substantial heterogeneity was observed in the distribution of intrinsic subtypes by gene. For example, RAD51C, RAD51D, and BARD1 variants were associated mainly with triple-negative disease (odds ratios, 6.19, 6.19, and 10.05 respectively), and CHEK2 variants were associated with all subtypes (with ORs ranging from 2.21-3.17) except for triple-negative disease, the authors found.
“For ATM variants, the association was strongest for [HR-positive, HER2-negative]high-grade subtype (OR, 4.99). BRCA1 was associated with increased risk of all subtypes, but the ORs varied widely, being highest for triple-negative disease (OR, 55.32),” they wrote.
BRCA2 and PALB2 variants were also associated with triple-negative disease, TP53 variants were most strongly associated with HR-positive, HER2-negative and HR-negative, HER2-positive subtypes, and tumors occurring in pathogenic variant carriers were of higher grade, they added, noting that for most genes and subtypes, a decline in ORs was observed with increasing age.
All genes except CHEK2 were more strongly associated with high-grade disease.
These and other findings from the study can “inform guidelines for eligibility for gene panel sequencing and breast cancer surveillance in the general population,” the authors explained, adding that tumor characteristics can also be used to determine whether variants of uncertain significance are likely to be pathogenic.
The data should therefore “improve the precision of variant classification algorithms and extend them to a larger set of genes,” they noted.
“This case-control study suggests that rare variants in BC susceptibility genes display marked heterogeneity with respect to tumor phenotype, but also similarities between genes that are consistent with known biological functions. This present study provides detailed quantification of subtype-specific breast cancer risks; these can potentially improve risk prediction models and breast cancer prevention strategies,” they concluded.
More specifically, women found to be carrying variants in these genes may be offered enhanced screening, including by MRI, risk-reducing surgery, chemoprevention, and genetic counseling, corresponding author Nasim Mavaddat, MBBS, PhD, explained in an interview.
“Understanding tumor pathology associated with the genes can inform breast cancer risk prediction models, and management and treatment of women harboring pathogenic variants,” she noted.
The findings “enhance our understanding of breast cancer biology and aid efforts to classify whether genetic variants of uncertain significance are also likely to be pathogenic,” added Dr. Mavaddat, a senior research associate at the University of Cambridge (England).
She also said the investigators are taking steps to include the results in “CanRisk,” a cancer risk prediction model used by genetic counselors and other clinicians, and are “exploring whether variants in these genes are associated with contralateral breast cancer risk and survival after a breast cancer.”
As most participants were European, analyses should be extended to women of other racial and ethnic groups, she added.
The BRIDGES panel sequencing was supported by the European Union Horizon 2020 research and innovation program BRIDGES and the Wellcome Trust. The Breast Cancer Association Consortium is also funded by the European Union Horizon 2020 research and innovation program, the PERSPECTIVE I&I project, which is funded by the Government of Canada, and the Confluence project, which is funded with intramural funds from the National Cancer Institute. Dr. Mavaddat reported grants from the University of Cambridge, European Union Horizon 2020, Wellcome Trust, Genome Canada, Canadian Institutes of Health Research, and National Cancer Institute during the conduct of the study.
Breast cancer risk genes are generally associated with triple-negative and high-grade disease, but differ substantially in their associated pathology, a large case-control analysis shows.
The findings have potential implications for genetic testing, risk prediction, variant classification, and screening, the authors noted.
To assess links between pathogenic germline variants in nine major breast cancer (BC) susceptibility genes and pathological features of nonmetastasized breast tumors, investigators from the Breast Cancer Association Consortium compared 42,680 women with breast cancer and 42,387 population-matched controls from the BRIDGES large-scale sequencing study.
They looked specifically at features relevant to prognosis and distinct therapeutic options, including tumor subtype, morphology, size, stage, and lymph node involvement, and found substantial differences in tumor subtype distribution by gene.
“Pathogenic [protein-truncating variants and pathogenic missense variants] in these ... they wrote, noting that carriers of rare genetic variants in the nine genes comprised more than 27% of women diagnosed at age 40 or younger with triple-negative disease (driven mainly by BRCA1) and about 16% of those diagnosed with hormone receptor (HR)–positive, HER2-negative high-grade disease.
All together, the nine genes were associated with 14.4% of tumors in women aged 40 years and younger, but less than 4% in women over age 60 years, and among younger women, the prevalence of variants combined was higher in those with triple-negative and HR-positive, HER2-negative disease versus other subtypes, they said.
The findings were published online on Jan. 27, 2022, in JAMA Oncology.
Study subjects were women aged 18-79 years who were sampled, independently of family history, from 38 international population- or hospital-based studies conducted between 1991 and 2016.
The genes assessed included ATM, BARD1, BRCA1, BRCA2, CHEK2, PALB2, RAD51C, RAD51D, and TP53.
Substantial heterogeneity was observed in the distribution of intrinsic subtypes by gene. For example, RAD51C, RAD51D, and BARD1 variants were associated mainly with triple-negative disease (odds ratios, 6.19, 6.19, and 10.05 respectively), and CHEK2 variants were associated with all subtypes (with ORs ranging from 2.21-3.17) except for triple-negative disease, the authors found.
“For ATM variants, the association was strongest for [HR-positive, HER2-negative]high-grade subtype (OR, 4.99). BRCA1 was associated with increased risk of all subtypes, but the ORs varied widely, being highest for triple-negative disease (OR, 55.32),” they wrote.
BRCA2 and PALB2 variants were also associated with triple-negative disease, TP53 variants were most strongly associated with HR-positive, HER2-negative and HR-negative, HER2-positive subtypes, and tumors occurring in pathogenic variant carriers were of higher grade, they added, noting that for most genes and subtypes, a decline in ORs was observed with increasing age.
All genes except CHEK2 were more strongly associated with high-grade disease.
These and other findings from the study can “inform guidelines for eligibility for gene panel sequencing and breast cancer surveillance in the general population,” the authors explained, adding that tumor characteristics can also be used to determine whether variants of uncertain significance are likely to be pathogenic.
The data should therefore “improve the precision of variant classification algorithms and extend them to a larger set of genes,” they noted.
“This case-control study suggests that rare variants in BC susceptibility genes display marked heterogeneity with respect to tumor phenotype, but also similarities between genes that are consistent with known biological functions. This present study provides detailed quantification of subtype-specific breast cancer risks; these can potentially improve risk prediction models and breast cancer prevention strategies,” they concluded.
More specifically, women found to be carrying variants in these genes may be offered enhanced screening, including by MRI, risk-reducing surgery, chemoprevention, and genetic counseling, corresponding author Nasim Mavaddat, MBBS, PhD, explained in an interview.
“Understanding tumor pathology associated with the genes can inform breast cancer risk prediction models, and management and treatment of women harboring pathogenic variants,” she noted.
The findings “enhance our understanding of breast cancer biology and aid efforts to classify whether genetic variants of uncertain significance are also likely to be pathogenic,” added Dr. Mavaddat, a senior research associate at the University of Cambridge (England).
She also said the investigators are taking steps to include the results in “CanRisk,” a cancer risk prediction model used by genetic counselors and other clinicians, and are “exploring whether variants in these genes are associated with contralateral breast cancer risk and survival after a breast cancer.”
As most participants were European, analyses should be extended to women of other racial and ethnic groups, she added.
The BRIDGES panel sequencing was supported by the European Union Horizon 2020 research and innovation program BRIDGES and the Wellcome Trust. The Breast Cancer Association Consortium is also funded by the European Union Horizon 2020 research and innovation program, the PERSPECTIVE I&I project, which is funded by the Government of Canada, and the Confluence project, which is funded with intramural funds from the National Cancer Institute. Dr. Mavaddat reported grants from the University of Cambridge, European Union Horizon 2020, Wellcome Trust, Genome Canada, Canadian Institutes of Health Research, and National Cancer Institute during the conduct of the study.
FROM JAMA ONCOLOGY
Why dermatologists should support artificial intelligence efforts
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
“AI is meant to be an enhancement strategy, a support tool to improve our diagnostic abilities,” Dr. Patel, a Mohs surgeon who is director of cutaneous oncology at the George Washington University Cancer Center, Washington, said during the ODAC Dermatology, Aesthetic & Surgical Conference. “Dermatologists should embrace AI and drive how it is utilized – be the captain of the plane (technology) and the passenger (patient). If we’re not in the forefront of the plane, we’re not to be able to dictate which way we are going with this.”
In 2019, a group of German researchers found that AI can improve accuracy and efficiency of specialists in classifying skin cancer based on dermoscopic images. “I really do believe this is going to be the future,” said Dr. Patel, who was not involved with the study. “Current research involves using supervised learning on known outcomes to determine inputs to predict them. In dermatology, think of identifying melanoma from clinical or dermoscopic images or predicting metastasis risk from digitized pathology slides.”
However, there are currently no universal guidelines on how large an AI dataset needs to be to yield accurate results. In the dermatology literature, most AI datasets range between 600 and 14,000 examples, Dr. Patel said, with a large study-specific variation in performance. “Misleading results can result from unanticipated training errors,” he said.
“The AI network may learn its intended task or an unrelated situational cue. For example, you can use great images to predict melanoma, but you may have an unintended poor outcome related to images that have, say, a ruler inside of them clustered within the melanoma diagnoses.” And unbeknown to the system’s developer, “the algorithm picks up that the ruler is predictive of an image being a melanoma and not the pigmented lesion itself.” In other words, the algorithm is only as good as the dataset being used, he said. “This is the key element, to ask what the dataset is that’s training the tool that you may one day use.”
Convolutional neural network
In 2017, a seminal study published in Nature showed that for classification of melanoma and epidermal lesions, a type of AI used in image processing known as a convolutional neural network (CNN) was on par with dermatologists and outperformed the average. For epidermal lesions, the network was one standard deviation higher above the average for dermatologists, while for melanocytic lesions, the network was just below one standard deviation above the average of the dermatologists. A CNN “clearly can perform well because it works on a different level than how our brains work,” Dr. Patel said.
In a separate study, a CNN trained to recognize melanoma in dermoscopic images was compared to 58 international dermatologists with varying levels of dermoscopy experience; 29% were “beginners,” with less than 2 years of experience; 19% were “skilled,” with 2-5 years of experience; and 52% were “experts,” with at least 5 years of experience. The analysis consisted of two experiments: In level I, dermatologists classified lesions based on dermoscopy only. In level II, dermatologists were provided dermoscopy, clinical images, and additional clinical information, while the CNN was trained on images only. The researchers found that most dermatologists were outperformed by the CNN. “Physicians of all different levels of training and experience may benefit from assistance by a CNN’s image classification,” they concluded.
Gene expression profiling
Another aspect of AI is gene expression profiling (GEP), which Dr. Patel defined as the evaluation of frequency and intensity of genetic activity at once to create a global picture of cellular function. “It’s AI that uses machine learning to evaluate genetic expression to assess lesion behavior,” he explained.
One GEP test on the market is the Pigmented Lesion Assay (PLA) from DermTech, a noninvasive test that looks at the expression of two genes to predict if a lesion is malignant or not. “Based on their validation set, they have shown some impressive numbers,” with sensitivities above 90%, and published registry data that have shown higher sensitivities “and even specificities above 90%,” he said.
“On the surface, it looks like this would be a useful test,” Dr. Patel said. A study published in 2021 looked at the evidence of applying real-world evidence with this test to see if results held up. Based on the authors’ analysis, he noted, “you would need a sensitivity and specificity of 95% to yield a positivity rate of 9.5% for the PLA test, which is what has been reported in real-world use. So, there’s a disconnect somewhere and we are not quite there yet.” That may be a result of the dataset itself not being as uniform between the validation and the training datasets, he continued. Also, the expression of certain genes is different “if you don’t have a clean input variable” of what the test is being used for, he added.
“If you’re not mirroring the dataset, you’re not going to get clean data,” he said. “So, if you’re using this on younger patients or for sun-damaged lesional skin or nonmelanocytic lesions around sun-damaged areas, there are variable expressions that may not be accurately captured by that algorithm. This might help explain the real-world variation that we’re seeing.”
Another GEP test in use is the 31-Gene Expression Profile Test for Melanoma, which evaluates gene expressions in melanoma tumors and what the behavior of that tumor may be. The test has been available for more than a decade “and there is a lot of speculation about its use,” Dr. Patel said. “A recent paper attempted to come up with an algorithm of how to use this, but there’s a lot of concern about the endpoints of what changes in management might result from this test. That is what we need to be thinking about. There’s a lot of back and forth about this.”
In 2020, authors of a consensus statement on prognostic GEP in cutaneous melanoma concluded that before GEP testing is routinely used, the clinical benefit in the management of patients with melanoma should be established through further clinical investigation. Dr. Patel recommended the accompanying editorial on GEP in melanoma, written by Hensin Tsao, MD, PhD, and Warren H. Chan, MS, in JAMA Dermatology.
In Dr. Patel’s opinion, T1a melanomas (0.8 mm, nonulcerated) do not need routine GEP, but the GEP test may be useful in cases that are in the “gray zone,” such as those with T1b or some borderline T2a melanomas (> 0.8 mm, < 1.2mm, nonulcerated, but with high mitosis, etc.); patients with unique coexisting conditions such as pregnancy, and patients who may not tolerate sentinel lymph node biopsy (SLNB) or adjuvant therapy.
Echoing sentiments expressed in the JAMA Dermatology editorial, he advised dermatologists to “remember your training and know the data. GEP predicting survival is not the same as SLNB positive rate. GEP should not replace standard guidelines in T2a and higher melanomas. Nodal sampling remains part of all major guidelines and determines adjuvant therapy.”
He cited the characterization of GEP in the editorial as “a powerful technology” that heralds the age of personalized medicine, but it is not ready for ubiquitous use. Prospective studies and time will lead to highly accurate tools.”
Dr. Patel disclosed that he is chief medical officer for Lazarus AI.
FROM ODAC 2022
Prep shortages, coverage restrictions create new colonoscopy barriers
In April 2021, Express Scripts stopped covering low-volume bowel preparations in its National Preferred Formulary, a move that had the potential to affect many of the 75 million Americans covered by that pharmacy benefit manager’s programs, according to the American Society for Gastrointestinal Endoscopy (ASGE).
For gastroenterologists and their patients, it was an action that added insult to injury. The COVID-19 pandemic had already led to shortages of various preps, causing many thousands of Americans to forego colonoscopies. One study estimated that there was a 95% decline in weekly colorectal cancer screenings in the first half of 2020.
Just as screenings were returning to prepandemic levels, the Express Scripts coverage change threatened to create a new barrier for those already hesitant, especially since the prep is what patients most loathe about colonoscopy, said the gastroenterologists interviewed for this story.
Almost a year later, not much has changed. or instituting higher copays.
Gastroenterologists are having to delay procedures and patients are canceling appointments; some never return.
“I and many of my colleagues are very concerned that we are going to see an increase in advanced colon polyps and colon cancer,” said Jennifer A. Christie, MD, professor of medicine in the digestive diseases division at Emory University School of Medicine, Atlanta, and vice president of the ASGE.
Obstacles to getting the right prep “not only [delay] the care, but the negative outcomes could be horrible,” agreed Tauseef Ali, MD, clinical assistant professor at the University of Oklahoma and a member of the American College of Gastroenterology’s board of governors.
For the majority of patients, a wait might not be an issue, said Christian Stevoff, MD, assistant professor of medicine at Northwestern University Feinberg School of Medicine, Chicago. But a delayed diagnosis would be significant for those with larger polyps or cancer in the colon, he said.
“It’s a major problem for those people that it does affect,” Dr. Stevoff told this news organization.
He noted that his practice had to delay around 3,000 procedures in 2020, and while they have since caught up, approximately 25% of cases are being delayed right now for a variety of reasons. Most of those are in patients deemed to be low risk, though, he said.
PBMs: ‘a parasitic infection to our health care system’
Shortages of preps have been a persistent headache, but restrictions such as those instituted by Express Scripts have become a bigger problem, said some gastroenterologists.
Express Scripts did have several exceptions to its prohibition on coverage of low-volume preps. First, it could be approved if the patient had failed with a polyethylene glycol (PEG)–based prep like GoLYTELY. It could also be approved if the patient had tried MoviPrep and failed, if MoviPrep was unavailable, or if the patient has phenylketonuria or glucose-6-phosphate dehydrogenase deficiency.
Cigna-owned Express Scripts is one of three PBMs – along with CVS Caremark and OptumRX (owned by UnitedHealth Group) – that control 85% of prescription drug benefits in the United States, according to a 2019 investigation of the industry by the New York State Senate.
Express Scripts did not return requests for comment on its bowel prep coverage, and CVS Caremark declined to participate. A spokesperson for OptumRX told this news organization that the PBM provides bowel preps at “$0 cost-share” but only for health plan sponsors that are subject to Affordable Care Act regulations that require providing colonoscopies under such a payment structure. The company did not provide further information.
For some gastroenterologists, the anger toward PBMs is palpable. Dr. Ali calls PBMs “a parasitic infection to our health care system.”
“Keeping track of these bowel prep coverages has become a nightmare,” he said, noting that every payer seems to have its own preferred prep. “We have a dedicated nurse whose only job is to keep tabs on this, and she’s unable to because it’s just getting out of control.”
Some preps are contraindicated for patients, Dr. Ali said. Yet even in those cases, it’s difficult to get the alternatives covered. It often comes down to a joint effort by a pharmacist, the patient, and Dr. Ali’s office staff to get coverage for a medically necessary prep.
If it’s an emergency, Dr. Ali said, “either our patients bite the bullet and pay the price, or we have to come up with alternative solutions that may not lead to an optimal bowel preparation. It defeats the whole purpose of having a good bowel preparation and giving them a good outcome.”
He added that “there are a lot of patients who cancel their colonoscopies out of frustration,” because the bowel prep is not available or too expensive and that some patients choose to simply not reschedule.
Dr. Christie’s experience is similar. Sometimes patients must be rescheduled because “we could not get the prep in a timely fashion for them to be ready for their procedure.” Bringing back patients can be hard: They are busy, or they can’t get a ride, or time off work, or coverage for caregiving, she said. Ultimately, “some patients do decide to either defer or decline screening.”
The hassles also have the potential to exacerbate existing health disparities, she added.
Dr. Stevoff, the gastroenterologist at Northwestern, said cancellations are a concern, but to his knowledge, none of his patients have quit in frustration.
He said that because “most of the [preps] are equivalent to each other,” he often gives preference to what’s available.
He does tell patients that they may have a higher copay. For some that may be fine for what is only a once every 5- or 10-year payment. For others who cannot afford the cost, it may mean spending time trying to convince the insurer to pay for a prep that is not normally covered, he said.
A step backwards
Dr. Stevoff understands why payers might have preferred preps.
“As long as the outcomes are equivalent, I don’t think they’re going to be willing to pay for a prep that’s three or four times more expensive for a night of inconvenience for the patient,” he said.
David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, said he’s mystified by moves to limit bowel preps.
“Having to do an end-run to figure out what preps we can get for a patient is a step backwards,” he said.
Douglas Rex, MD, distinguished professor emeritus of medicine at Indiana University School of Medicine, Indianapolis, said it was dangerous to limit options.
“Trying to save relatively small amounts of money by restricting access to specific preps is seriously wrong-headed and a mistake,” he said. “If you keep the big picture in mind, we’re trying to keep people from getting colon cancer.”
Dr. Johnson also noted that a poor-quality prep might lead to a poor-quality exam, which is associated with not only reduced adenoma detection rates but also a shortened interval for a repeat exam, which just adds costs to the system.
“The whole impact of colon cancer screening is to discover polyps and remove them to prevent cancer,” Dr. Johnson said.
No rhyme or reason to ongoing shortages
Pandemic-related supply chain headaches have trickled down to bowel preps, which then lead to occasional delays in procedures.
“We have definitely seen shortages throughout most of the pandemic,” Dr. Rex said. At various times, he added, low-volume or high-volume prescription preps have not been available in all pharmacies or not available in certain pharmacies.
The spotty supplies have created a hassle and added costs because his office staff spends time making calls to find an available prep, he said.
Dr. Christie described similar issues at her practice, where the shortages have been “a significant challenge and issue.”
As of late January, some polyethylene glycol 3,350-based preps were still in short supply or had been discontinued, according to the American Society of Health-System Pharmacists (ASHP). Two companies – Teva and Lupin – did not provide a reason for the lack of product. However, ASHP said the companies anticipated being able to provide supplies in February.
Many PEG-based prescription products have been on back order for some time, said Dr. Johnson, who tries to avoid the higher-volume PEG-based preps in favor of low-volume preps that are more tolerable to patients.
The lack of information about a reason for the shortages has led to speculation.
At the beginning of the COVID-19 pandemic, GoLYTELY, one of the more commonly used PEG-based preps, was not available at all, Dr. Ali said. It was his understanding that PEG was being used as an ingredient in COVID vaccines, which helped explain the shortage, at least initially.
Dr. Stevoff also heard this explanation but said he had come to believe it was an “urban myth,” and added that he “never got confirmation from any of the companies” that they couldn’t get PEG because it was being used for vaccines. He noted that shortages of some PEG-based preps have continued even though vaccine production has stabilized.
Over-the-counter alternatives can be ‘hit or miss’
With the price for all bowel preps – and co-pays – increasing in the last 2 years, some practices are directing patients on how to mix up their own using over-the-counter (OTC) ingredients, which is likely to be less expensive.
The out-of-pocket cost for a four-liter, high-volume PEG-based prep might be $35-$50, according to GoodRx. Alternatives such as Suprep (sodium/potassium/magnesium) run $110-$120, according to the website, and a sodium phosphate-based prep, such as OsmoPrep, runs close to $300.
The OTC prep uses MiraLAX (PEG-based but without additional electrolytes mixed in) and bisacodyl (Dulcolax). Johns Hopkins University, Cleveland Clinic, and Memorial Sloan Kettering, among many other institutions, have advised patients to use the OTC do-it-yourself preps. It is a split prep, using 238 grams of MiraLAX mixed with 64 ounces of water or a sports drink (for example, Gatorade). The day before the procedure, patients take 2 bisacodyl (5 mg) tablets, followed by four 8-ounce glasses of a MiraLAX/water mixture. The same regimen is followed the day of the procedure.
There are mixed results on how adequately the regimen cleans the colon. A 2014 meta-analysis found that the MiraLAX-based prep was inferior in terms of bowel cleansing to PEG-based formulations premixed with electrolyte solutions (PEG-ELS). There was no statistically significant difference in polyp detection between the two. In a 2011 analysis, researchers concluded that GoLYTELY was superior to MiraLAX in colon prep and adenoma detection.
The 2014 U.S. Multi-Society Task Force on Colorectal Cancer Guidelines reported that the MiraLAX-based prep had less effective bowel preparation than 4-liter PEG-ELS solutions in at least one study but that it appeared to be more tolerable for patients and associated with few adverse events. More study of its safety is “warranted and desirable,” write the authors.
Even before the pandemic, Dr. Rex said his practice used the MiraLAX-based prep because it was less expensive, and “anecdotally it tends to be very well tolerated.”
However, he noted that the regimen is not approved by the Food and Drug Administration.
“A lot of people don’t like to use non–FDA approved preps because they’re afraid of some liability if there is a complication,” Dr. Rex said.
Dr. Ali added that he has advised patients to use the OTC preps, but the results can be “hit or miss.”
Some patients can easily comply with the instructions and will have good results, but others may not have the education or understanding or may have underlying medical conditions that lessen the OTC formulation’s effectiveness in getting a good cleanout, said Dr. Ali.
Dr. Stevoff has occasionally used the OTC prep but agrees that not all patients will be able to follow the directions.
“The more complicated a process is, the more likely it is that somebody will make a mistake,” he said.
A version of this article first appeared on Medscape.com.
In April 2021, Express Scripts stopped covering low-volume bowel preparations in its National Preferred Formulary, a move that had the potential to affect many of the 75 million Americans covered by that pharmacy benefit manager’s programs, according to the American Society for Gastrointestinal Endoscopy (ASGE).
For gastroenterologists and their patients, it was an action that added insult to injury. The COVID-19 pandemic had already led to shortages of various preps, causing many thousands of Americans to forego colonoscopies. One study estimated that there was a 95% decline in weekly colorectal cancer screenings in the first half of 2020.
Just as screenings were returning to prepandemic levels, the Express Scripts coverage change threatened to create a new barrier for those already hesitant, especially since the prep is what patients most loathe about colonoscopy, said the gastroenterologists interviewed for this story.
Almost a year later, not much has changed. or instituting higher copays.
Gastroenterologists are having to delay procedures and patients are canceling appointments; some never return.
“I and many of my colleagues are very concerned that we are going to see an increase in advanced colon polyps and colon cancer,” said Jennifer A. Christie, MD, professor of medicine in the digestive diseases division at Emory University School of Medicine, Atlanta, and vice president of the ASGE.
Obstacles to getting the right prep “not only [delay] the care, but the negative outcomes could be horrible,” agreed Tauseef Ali, MD, clinical assistant professor at the University of Oklahoma and a member of the American College of Gastroenterology’s board of governors.
For the majority of patients, a wait might not be an issue, said Christian Stevoff, MD, assistant professor of medicine at Northwestern University Feinberg School of Medicine, Chicago. But a delayed diagnosis would be significant for those with larger polyps or cancer in the colon, he said.
“It’s a major problem for those people that it does affect,” Dr. Stevoff told this news organization.
He noted that his practice had to delay around 3,000 procedures in 2020, and while they have since caught up, approximately 25% of cases are being delayed right now for a variety of reasons. Most of those are in patients deemed to be low risk, though, he said.
PBMs: ‘a parasitic infection to our health care system’
Shortages of preps have been a persistent headache, but restrictions such as those instituted by Express Scripts have become a bigger problem, said some gastroenterologists.
Express Scripts did have several exceptions to its prohibition on coverage of low-volume preps. First, it could be approved if the patient had failed with a polyethylene glycol (PEG)–based prep like GoLYTELY. It could also be approved if the patient had tried MoviPrep and failed, if MoviPrep was unavailable, or if the patient has phenylketonuria or glucose-6-phosphate dehydrogenase deficiency.
Cigna-owned Express Scripts is one of three PBMs – along with CVS Caremark and OptumRX (owned by UnitedHealth Group) – that control 85% of prescription drug benefits in the United States, according to a 2019 investigation of the industry by the New York State Senate.
Express Scripts did not return requests for comment on its bowel prep coverage, and CVS Caremark declined to participate. A spokesperson for OptumRX told this news organization that the PBM provides bowel preps at “$0 cost-share” but only for health plan sponsors that are subject to Affordable Care Act regulations that require providing colonoscopies under such a payment structure. The company did not provide further information.
For some gastroenterologists, the anger toward PBMs is palpable. Dr. Ali calls PBMs “a parasitic infection to our health care system.”
“Keeping track of these bowel prep coverages has become a nightmare,” he said, noting that every payer seems to have its own preferred prep. “We have a dedicated nurse whose only job is to keep tabs on this, and she’s unable to because it’s just getting out of control.”
Some preps are contraindicated for patients, Dr. Ali said. Yet even in those cases, it’s difficult to get the alternatives covered. It often comes down to a joint effort by a pharmacist, the patient, and Dr. Ali’s office staff to get coverage for a medically necessary prep.
If it’s an emergency, Dr. Ali said, “either our patients bite the bullet and pay the price, or we have to come up with alternative solutions that may not lead to an optimal bowel preparation. It defeats the whole purpose of having a good bowel preparation and giving them a good outcome.”
He added that “there are a lot of patients who cancel their colonoscopies out of frustration,” because the bowel prep is not available or too expensive and that some patients choose to simply not reschedule.
Dr. Christie’s experience is similar. Sometimes patients must be rescheduled because “we could not get the prep in a timely fashion for them to be ready for their procedure.” Bringing back patients can be hard: They are busy, or they can’t get a ride, or time off work, or coverage for caregiving, she said. Ultimately, “some patients do decide to either defer or decline screening.”
The hassles also have the potential to exacerbate existing health disparities, she added.
Dr. Stevoff, the gastroenterologist at Northwestern, said cancellations are a concern, but to his knowledge, none of his patients have quit in frustration.
He said that because “most of the [preps] are equivalent to each other,” he often gives preference to what’s available.
He does tell patients that they may have a higher copay. For some that may be fine for what is only a once every 5- or 10-year payment. For others who cannot afford the cost, it may mean spending time trying to convince the insurer to pay for a prep that is not normally covered, he said.
A step backwards
Dr. Stevoff understands why payers might have preferred preps.
“As long as the outcomes are equivalent, I don’t think they’re going to be willing to pay for a prep that’s three or four times more expensive for a night of inconvenience for the patient,” he said.
David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, said he’s mystified by moves to limit bowel preps.
“Having to do an end-run to figure out what preps we can get for a patient is a step backwards,” he said.
Douglas Rex, MD, distinguished professor emeritus of medicine at Indiana University School of Medicine, Indianapolis, said it was dangerous to limit options.
“Trying to save relatively small amounts of money by restricting access to specific preps is seriously wrong-headed and a mistake,” he said. “If you keep the big picture in mind, we’re trying to keep people from getting colon cancer.”
Dr. Johnson also noted that a poor-quality prep might lead to a poor-quality exam, which is associated with not only reduced adenoma detection rates but also a shortened interval for a repeat exam, which just adds costs to the system.
“The whole impact of colon cancer screening is to discover polyps and remove them to prevent cancer,” Dr. Johnson said.
No rhyme or reason to ongoing shortages
Pandemic-related supply chain headaches have trickled down to bowel preps, which then lead to occasional delays in procedures.
“We have definitely seen shortages throughout most of the pandemic,” Dr. Rex said. At various times, he added, low-volume or high-volume prescription preps have not been available in all pharmacies or not available in certain pharmacies.
The spotty supplies have created a hassle and added costs because his office staff spends time making calls to find an available prep, he said.
Dr. Christie described similar issues at her practice, where the shortages have been “a significant challenge and issue.”
As of late January, some polyethylene glycol 3,350-based preps were still in short supply or had been discontinued, according to the American Society of Health-System Pharmacists (ASHP). Two companies – Teva and Lupin – did not provide a reason for the lack of product. However, ASHP said the companies anticipated being able to provide supplies in February.
Many PEG-based prescription products have been on back order for some time, said Dr. Johnson, who tries to avoid the higher-volume PEG-based preps in favor of low-volume preps that are more tolerable to patients.
The lack of information about a reason for the shortages has led to speculation.
At the beginning of the COVID-19 pandemic, GoLYTELY, one of the more commonly used PEG-based preps, was not available at all, Dr. Ali said. It was his understanding that PEG was being used as an ingredient in COVID vaccines, which helped explain the shortage, at least initially.
Dr. Stevoff also heard this explanation but said he had come to believe it was an “urban myth,” and added that he “never got confirmation from any of the companies” that they couldn’t get PEG because it was being used for vaccines. He noted that shortages of some PEG-based preps have continued even though vaccine production has stabilized.
Over-the-counter alternatives can be ‘hit or miss’
With the price for all bowel preps – and co-pays – increasing in the last 2 years, some practices are directing patients on how to mix up their own using over-the-counter (OTC) ingredients, which is likely to be less expensive.
The out-of-pocket cost for a four-liter, high-volume PEG-based prep might be $35-$50, according to GoodRx. Alternatives such as Suprep (sodium/potassium/magnesium) run $110-$120, according to the website, and a sodium phosphate-based prep, such as OsmoPrep, runs close to $300.
The OTC prep uses MiraLAX (PEG-based but without additional electrolytes mixed in) and bisacodyl (Dulcolax). Johns Hopkins University, Cleveland Clinic, and Memorial Sloan Kettering, among many other institutions, have advised patients to use the OTC do-it-yourself preps. It is a split prep, using 238 grams of MiraLAX mixed with 64 ounces of water or a sports drink (for example, Gatorade). The day before the procedure, patients take 2 bisacodyl (5 mg) tablets, followed by four 8-ounce glasses of a MiraLAX/water mixture. The same regimen is followed the day of the procedure.
There are mixed results on how adequately the regimen cleans the colon. A 2014 meta-analysis found that the MiraLAX-based prep was inferior in terms of bowel cleansing to PEG-based formulations premixed with electrolyte solutions (PEG-ELS). There was no statistically significant difference in polyp detection between the two. In a 2011 analysis, researchers concluded that GoLYTELY was superior to MiraLAX in colon prep and adenoma detection.
The 2014 U.S. Multi-Society Task Force on Colorectal Cancer Guidelines reported that the MiraLAX-based prep had less effective bowel preparation than 4-liter PEG-ELS solutions in at least one study but that it appeared to be more tolerable for patients and associated with few adverse events. More study of its safety is “warranted and desirable,” write the authors.
Even before the pandemic, Dr. Rex said his practice used the MiraLAX-based prep because it was less expensive, and “anecdotally it tends to be very well tolerated.”
However, he noted that the regimen is not approved by the Food and Drug Administration.
“A lot of people don’t like to use non–FDA approved preps because they’re afraid of some liability if there is a complication,” Dr. Rex said.
Dr. Ali added that he has advised patients to use the OTC preps, but the results can be “hit or miss.”
Some patients can easily comply with the instructions and will have good results, but others may not have the education or understanding or may have underlying medical conditions that lessen the OTC formulation’s effectiveness in getting a good cleanout, said Dr. Ali.
Dr. Stevoff has occasionally used the OTC prep but agrees that not all patients will be able to follow the directions.
“The more complicated a process is, the more likely it is that somebody will make a mistake,” he said.
A version of this article first appeared on Medscape.com.
In April 2021, Express Scripts stopped covering low-volume bowel preparations in its National Preferred Formulary, a move that had the potential to affect many of the 75 million Americans covered by that pharmacy benefit manager’s programs, according to the American Society for Gastrointestinal Endoscopy (ASGE).
For gastroenterologists and their patients, it was an action that added insult to injury. The COVID-19 pandemic had already led to shortages of various preps, causing many thousands of Americans to forego colonoscopies. One study estimated that there was a 95% decline in weekly colorectal cancer screenings in the first half of 2020.
Just as screenings were returning to prepandemic levels, the Express Scripts coverage change threatened to create a new barrier for those already hesitant, especially since the prep is what patients most loathe about colonoscopy, said the gastroenterologists interviewed for this story.
Almost a year later, not much has changed. or instituting higher copays.
Gastroenterologists are having to delay procedures and patients are canceling appointments; some never return.
“I and many of my colleagues are very concerned that we are going to see an increase in advanced colon polyps and colon cancer,” said Jennifer A. Christie, MD, professor of medicine in the digestive diseases division at Emory University School of Medicine, Atlanta, and vice president of the ASGE.
Obstacles to getting the right prep “not only [delay] the care, but the negative outcomes could be horrible,” agreed Tauseef Ali, MD, clinical assistant professor at the University of Oklahoma and a member of the American College of Gastroenterology’s board of governors.
For the majority of patients, a wait might not be an issue, said Christian Stevoff, MD, assistant professor of medicine at Northwestern University Feinberg School of Medicine, Chicago. But a delayed diagnosis would be significant for those with larger polyps or cancer in the colon, he said.
“It’s a major problem for those people that it does affect,” Dr. Stevoff told this news organization.
He noted that his practice had to delay around 3,000 procedures in 2020, and while they have since caught up, approximately 25% of cases are being delayed right now for a variety of reasons. Most of those are in patients deemed to be low risk, though, he said.
PBMs: ‘a parasitic infection to our health care system’
Shortages of preps have been a persistent headache, but restrictions such as those instituted by Express Scripts have become a bigger problem, said some gastroenterologists.
Express Scripts did have several exceptions to its prohibition on coverage of low-volume preps. First, it could be approved if the patient had failed with a polyethylene glycol (PEG)–based prep like GoLYTELY. It could also be approved if the patient had tried MoviPrep and failed, if MoviPrep was unavailable, or if the patient has phenylketonuria or glucose-6-phosphate dehydrogenase deficiency.
Cigna-owned Express Scripts is one of three PBMs – along with CVS Caremark and OptumRX (owned by UnitedHealth Group) – that control 85% of prescription drug benefits in the United States, according to a 2019 investigation of the industry by the New York State Senate.
Express Scripts did not return requests for comment on its bowel prep coverage, and CVS Caremark declined to participate. A spokesperson for OptumRX told this news organization that the PBM provides bowel preps at “$0 cost-share” but only for health plan sponsors that are subject to Affordable Care Act regulations that require providing colonoscopies under such a payment structure. The company did not provide further information.
For some gastroenterologists, the anger toward PBMs is palpable. Dr. Ali calls PBMs “a parasitic infection to our health care system.”
“Keeping track of these bowel prep coverages has become a nightmare,” he said, noting that every payer seems to have its own preferred prep. “We have a dedicated nurse whose only job is to keep tabs on this, and she’s unable to because it’s just getting out of control.”
Some preps are contraindicated for patients, Dr. Ali said. Yet even in those cases, it’s difficult to get the alternatives covered. It often comes down to a joint effort by a pharmacist, the patient, and Dr. Ali’s office staff to get coverage for a medically necessary prep.
If it’s an emergency, Dr. Ali said, “either our patients bite the bullet and pay the price, or we have to come up with alternative solutions that may not lead to an optimal bowel preparation. It defeats the whole purpose of having a good bowel preparation and giving them a good outcome.”
He added that “there are a lot of patients who cancel their colonoscopies out of frustration,” because the bowel prep is not available or too expensive and that some patients choose to simply not reschedule.
Dr. Christie’s experience is similar. Sometimes patients must be rescheduled because “we could not get the prep in a timely fashion for them to be ready for their procedure.” Bringing back patients can be hard: They are busy, or they can’t get a ride, or time off work, or coverage for caregiving, she said. Ultimately, “some patients do decide to either defer or decline screening.”
The hassles also have the potential to exacerbate existing health disparities, she added.
Dr. Stevoff, the gastroenterologist at Northwestern, said cancellations are a concern, but to his knowledge, none of his patients have quit in frustration.
He said that because “most of the [preps] are equivalent to each other,” he often gives preference to what’s available.
He does tell patients that they may have a higher copay. For some that may be fine for what is only a once every 5- or 10-year payment. For others who cannot afford the cost, it may mean spending time trying to convince the insurer to pay for a prep that is not normally covered, he said.
A step backwards
Dr. Stevoff understands why payers might have preferred preps.
“As long as the outcomes are equivalent, I don’t think they’re going to be willing to pay for a prep that’s three or four times more expensive for a night of inconvenience for the patient,” he said.
David Johnson, MD, professor of medicine and chief of gastroenterology at Eastern Virginia Medical School, Norfolk, said he’s mystified by moves to limit bowel preps.
“Having to do an end-run to figure out what preps we can get for a patient is a step backwards,” he said.
Douglas Rex, MD, distinguished professor emeritus of medicine at Indiana University School of Medicine, Indianapolis, said it was dangerous to limit options.
“Trying to save relatively small amounts of money by restricting access to specific preps is seriously wrong-headed and a mistake,” he said. “If you keep the big picture in mind, we’re trying to keep people from getting colon cancer.”
Dr. Johnson also noted that a poor-quality prep might lead to a poor-quality exam, which is associated with not only reduced adenoma detection rates but also a shortened interval for a repeat exam, which just adds costs to the system.
“The whole impact of colon cancer screening is to discover polyps and remove them to prevent cancer,” Dr. Johnson said.
No rhyme or reason to ongoing shortages
Pandemic-related supply chain headaches have trickled down to bowel preps, which then lead to occasional delays in procedures.
“We have definitely seen shortages throughout most of the pandemic,” Dr. Rex said. At various times, he added, low-volume or high-volume prescription preps have not been available in all pharmacies or not available in certain pharmacies.
The spotty supplies have created a hassle and added costs because his office staff spends time making calls to find an available prep, he said.
Dr. Christie described similar issues at her practice, where the shortages have been “a significant challenge and issue.”
As of late January, some polyethylene glycol 3,350-based preps were still in short supply or had been discontinued, according to the American Society of Health-System Pharmacists (ASHP). Two companies – Teva and Lupin – did not provide a reason for the lack of product. However, ASHP said the companies anticipated being able to provide supplies in February.
Many PEG-based prescription products have been on back order for some time, said Dr. Johnson, who tries to avoid the higher-volume PEG-based preps in favor of low-volume preps that are more tolerable to patients.
The lack of information about a reason for the shortages has led to speculation.
At the beginning of the COVID-19 pandemic, GoLYTELY, one of the more commonly used PEG-based preps, was not available at all, Dr. Ali said. It was his understanding that PEG was being used as an ingredient in COVID vaccines, which helped explain the shortage, at least initially.
Dr. Stevoff also heard this explanation but said he had come to believe it was an “urban myth,” and added that he “never got confirmation from any of the companies” that they couldn’t get PEG because it was being used for vaccines. He noted that shortages of some PEG-based preps have continued even though vaccine production has stabilized.
Over-the-counter alternatives can be ‘hit or miss’
With the price for all bowel preps – and co-pays – increasing in the last 2 years, some practices are directing patients on how to mix up their own using over-the-counter (OTC) ingredients, which is likely to be less expensive.
The out-of-pocket cost for a four-liter, high-volume PEG-based prep might be $35-$50, according to GoodRx. Alternatives such as Suprep (sodium/potassium/magnesium) run $110-$120, according to the website, and a sodium phosphate-based prep, such as OsmoPrep, runs close to $300.
The OTC prep uses MiraLAX (PEG-based but without additional electrolytes mixed in) and bisacodyl (Dulcolax). Johns Hopkins University, Cleveland Clinic, and Memorial Sloan Kettering, among many other institutions, have advised patients to use the OTC do-it-yourself preps. It is a split prep, using 238 grams of MiraLAX mixed with 64 ounces of water or a sports drink (for example, Gatorade). The day before the procedure, patients take 2 bisacodyl (5 mg) tablets, followed by four 8-ounce glasses of a MiraLAX/water mixture. The same regimen is followed the day of the procedure.
There are mixed results on how adequately the regimen cleans the colon. A 2014 meta-analysis found that the MiraLAX-based prep was inferior in terms of bowel cleansing to PEG-based formulations premixed with electrolyte solutions (PEG-ELS). There was no statistically significant difference in polyp detection between the two. In a 2011 analysis, researchers concluded that GoLYTELY was superior to MiraLAX in colon prep and adenoma detection.
The 2014 U.S. Multi-Society Task Force on Colorectal Cancer Guidelines reported that the MiraLAX-based prep had less effective bowel preparation than 4-liter PEG-ELS solutions in at least one study but that it appeared to be more tolerable for patients and associated with few adverse events. More study of its safety is “warranted and desirable,” write the authors.
Even before the pandemic, Dr. Rex said his practice used the MiraLAX-based prep because it was less expensive, and “anecdotally it tends to be very well tolerated.”
However, he noted that the regimen is not approved by the Food and Drug Administration.
“A lot of people don’t like to use non–FDA approved preps because they’re afraid of some liability if there is a complication,” Dr. Rex said.
Dr. Ali added that he has advised patients to use the OTC preps, but the results can be “hit or miss.”
Some patients can easily comply with the instructions and will have good results, but others may not have the education or understanding or may have underlying medical conditions that lessen the OTC formulation’s effectiveness in getting a good cleanout, said Dr. Ali.
Dr. Stevoff has occasionally used the OTC prep but agrees that not all patients will be able to follow the directions.
“The more complicated a process is, the more likely it is that somebody will make a mistake,” he said.
A version of this article first appeared on Medscape.com.
Could the protective effect on heart disease of eating more veg be exaggerated?
Eating sufficient amounts of vegetables might be good for overall health, but surprising results from a study suggested that their inclusion in the diet might have little or no effect on the risk of developing cardiovascular disease (CVD).
An investigation, led by the Nuffield department of population health at the University of Oxford, found that They said their findings, published in the journal Frontiers in Nutrition might mean that advice on vegetable intake and heart disease in high-income countries should be reappraised.
However, leading experts commented that the findings confirmed that higher overall vegetable consumption did lower the risk of cardiovascular disease.
UK Biobank data
Boosting health through a diet rich in vegetables has been backed by a large body of evidence, with guidelines consistently recommending them as a valuable source of macronutrients and micronutrients, such as dietary fiber, vitamins, and phytochemicals. However, the research team, which included the Chinese University of Hong Kong and the University of Bristol, set out to probe the independent effects of cooked and raw vegetables on health outcomes. Previous epidemiological studies had demonstrated inconsistent findings, they said.
They based their research on 399,586 people with no history of angina, stroke, and myocardial infarction, who enrolled in the UK Biobank. Of those, 55.4% were women, and 90.9% were of White ethnicity. The average body mass index was 27.3.
Raw and cooked vegetables
From their enrollment questionnaire, the mean intake of vegetables was found to be 2.3 heaped tablespoons per day of raw vegetables, and 2.8 of cooked vegetables. During an average follow-up of 12.1 years, 4.5% of the participants went on to develop CVD.
There was an inverse association between incident CVD and total and raw vegetable intake, but not cooked vegetable intake. Those who ate the most vegetables – both cooked and raw – had a 10% lower incidence of CVD, compared with those who ate the least. However, whereas raw vegetable intake was associated with an 11% reduction in CVD for those who ate the most, compared with the least, no reduction was seen for cooked vegetables.
Consuming two or more heaped tablespoons each day of cooked and raw vegetables was associated with a lower risk of dying from CVD, but little evidence was seen that a higher intake increased protection further. Similarly, there was evidence of an inverse association of CVD mortality with raw vegetable intake.
Researcher Qi Feng, from the Nuffield department of population health, said: “Our large study did not find evidence for a protective effect of vegetable intake on the occurrence of CVD. Instead, our analyses show that the seemingly protective effect of vegetable intake against CVD risk is very likely to be accounted for by bias from residual confounding factors, related to differences in socioeconomic situation and lifestyle.”
Expert opinions
Some clinical specialists took issue with the interpretation of the findings.
Dr. Dipender Gill, BMBCh, PhD, National Institute for Health Research clinical lecturer at St George’s, University of London, told the Science Media Centre that: “Many of the considered confounders that were adjusted for may actually represent mediating mechanisms. For example, vegetable consumption may reduce cardiovascular risk by lowering blood pressure and bodyweight, and improving glycaemic control.
“By adjusting for such traits, the authors may inadvertently be negating some of the mechanisms by which vegetable consumption is exerting beneficial effects.”
Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics at King’s College London, said: “The conclusion that cooked vegetables may not be effective in reducing risk of cardiovascular disease may not be justified, especially as the group consuming the highest levels of vegetables were more likely to be receiving medication for high blood cholesterol and high blood pressure (i.e. this group was at higher risk of CVD), compared with those consuming the lowest intake.” He added: “These findings should not be taken to indicate that eating more vegetables has no benefit to health, especially cardiovascular health.”
Naveed Sattar, FMedSci, FRCPath, FRCPGlas, professor of metabolic medicine at the University of Glasgow, agreed. “In short, this paper should in no way change advice to eat at least five portions of fruit and vegetables a day,” he said. “Many living in the U.K. fall well short of this, sadly, and more needs to be done to encourage better intake of vegetables.
“In fact, I suspect we may have underestimated the importance of a healthy diet on health and disease in general.”
A version of this article first appeared on Medscape.com.
Eating sufficient amounts of vegetables might be good for overall health, but surprising results from a study suggested that their inclusion in the diet might have little or no effect on the risk of developing cardiovascular disease (CVD).
An investigation, led by the Nuffield department of population health at the University of Oxford, found that They said their findings, published in the journal Frontiers in Nutrition might mean that advice on vegetable intake and heart disease in high-income countries should be reappraised.
However, leading experts commented that the findings confirmed that higher overall vegetable consumption did lower the risk of cardiovascular disease.
UK Biobank data
Boosting health through a diet rich in vegetables has been backed by a large body of evidence, with guidelines consistently recommending them as a valuable source of macronutrients and micronutrients, such as dietary fiber, vitamins, and phytochemicals. However, the research team, which included the Chinese University of Hong Kong and the University of Bristol, set out to probe the independent effects of cooked and raw vegetables on health outcomes. Previous epidemiological studies had demonstrated inconsistent findings, they said.
They based their research on 399,586 people with no history of angina, stroke, and myocardial infarction, who enrolled in the UK Biobank. Of those, 55.4% were women, and 90.9% were of White ethnicity. The average body mass index was 27.3.
Raw and cooked vegetables
From their enrollment questionnaire, the mean intake of vegetables was found to be 2.3 heaped tablespoons per day of raw vegetables, and 2.8 of cooked vegetables. During an average follow-up of 12.1 years, 4.5% of the participants went on to develop CVD.
There was an inverse association between incident CVD and total and raw vegetable intake, but not cooked vegetable intake. Those who ate the most vegetables – both cooked and raw – had a 10% lower incidence of CVD, compared with those who ate the least. However, whereas raw vegetable intake was associated with an 11% reduction in CVD for those who ate the most, compared with the least, no reduction was seen for cooked vegetables.
Consuming two or more heaped tablespoons each day of cooked and raw vegetables was associated with a lower risk of dying from CVD, but little evidence was seen that a higher intake increased protection further. Similarly, there was evidence of an inverse association of CVD mortality with raw vegetable intake.
Researcher Qi Feng, from the Nuffield department of population health, said: “Our large study did not find evidence for a protective effect of vegetable intake on the occurrence of CVD. Instead, our analyses show that the seemingly protective effect of vegetable intake against CVD risk is very likely to be accounted for by bias from residual confounding factors, related to differences in socioeconomic situation and lifestyle.”
Expert opinions
Some clinical specialists took issue with the interpretation of the findings.
Dr. Dipender Gill, BMBCh, PhD, National Institute for Health Research clinical lecturer at St George’s, University of London, told the Science Media Centre that: “Many of the considered confounders that were adjusted for may actually represent mediating mechanisms. For example, vegetable consumption may reduce cardiovascular risk by lowering blood pressure and bodyweight, and improving glycaemic control.
“By adjusting for such traits, the authors may inadvertently be negating some of the mechanisms by which vegetable consumption is exerting beneficial effects.”
Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics at King’s College London, said: “The conclusion that cooked vegetables may not be effective in reducing risk of cardiovascular disease may not be justified, especially as the group consuming the highest levels of vegetables were more likely to be receiving medication for high blood cholesterol and high blood pressure (i.e. this group was at higher risk of CVD), compared with those consuming the lowest intake.” He added: “These findings should not be taken to indicate that eating more vegetables has no benefit to health, especially cardiovascular health.”
Naveed Sattar, FMedSci, FRCPath, FRCPGlas, professor of metabolic medicine at the University of Glasgow, agreed. “In short, this paper should in no way change advice to eat at least five portions of fruit and vegetables a day,” he said. “Many living in the U.K. fall well short of this, sadly, and more needs to be done to encourage better intake of vegetables.
“In fact, I suspect we may have underestimated the importance of a healthy diet on health and disease in general.”
A version of this article first appeared on Medscape.com.
Eating sufficient amounts of vegetables might be good for overall health, but surprising results from a study suggested that their inclusion in the diet might have little or no effect on the risk of developing cardiovascular disease (CVD).
An investigation, led by the Nuffield department of population health at the University of Oxford, found that They said their findings, published in the journal Frontiers in Nutrition might mean that advice on vegetable intake and heart disease in high-income countries should be reappraised.
However, leading experts commented that the findings confirmed that higher overall vegetable consumption did lower the risk of cardiovascular disease.
UK Biobank data
Boosting health through a diet rich in vegetables has been backed by a large body of evidence, with guidelines consistently recommending them as a valuable source of macronutrients and micronutrients, such as dietary fiber, vitamins, and phytochemicals. However, the research team, which included the Chinese University of Hong Kong and the University of Bristol, set out to probe the independent effects of cooked and raw vegetables on health outcomes. Previous epidemiological studies had demonstrated inconsistent findings, they said.
They based their research on 399,586 people with no history of angina, stroke, and myocardial infarction, who enrolled in the UK Biobank. Of those, 55.4% were women, and 90.9% were of White ethnicity. The average body mass index was 27.3.
Raw and cooked vegetables
From their enrollment questionnaire, the mean intake of vegetables was found to be 2.3 heaped tablespoons per day of raw vegetables, and 2.8 of cooked vegetables. During an average follow-up of 12.1 years, 4.5% of the participants went on to develop CVD.
There was an inverse association between incident CVD and total and raw vegetable intake, but not cooked vegetable intake. Those who ate the most vegetables – both cooked and raw – had a 10% lower incidence of CVD, compared with those who ate the least. However, whereas raw vegetable intake was associated with an 11% reduction in CVD for those who ate the most, compared with the least, no reduction was seen for cooked vegetables.
Consuming two or more heaped tablespoons each day of cooked and raw vegetables was associated with a lower risk of dying from CVD, but little evidence was seen that a higher intake increased protection further. Similarly, there was evidence of an inverse association of CVD mortality with raw vegetable intake.
Researcher Qi Feng, from the Nuffield department of population health, said: “Our large study did not find evidence for a protective effect of vegetable intake on the occurrence of CVD. Instead, our analyses show that the seemingly protective effect of vegetable intake against CVD risk is very likely to be accounted for by bias from residual confounding factors, related to differences in socioeconomic situation and lifestyle.”
Expert opinions
Some clinical specialists took issue with the interpretation of the findings.
Dr. Dipender Gill, BMBCh, PhD, National Institute for Health Research clinical lecturer at St George’s, University of London, told the Science Media Centre that: “Many of the considered confounders that were adjusted for may actually represent mediating mechanisms. For example, vegetable consumption may reduce cardiovascular risk by lowering blood pressure and bodyweight, and improving glycaemic control.
“By adjusting for such traits, the authors may inadvertently be negating some of the mechanisms by which vegetable consumption is exerting beneficial effects.”
Tom Sanders, DSc, PhD, professor emeritus of nutrition and dietetics at King’s College London, said: “The conclusion that cooked vegetables may not be effective in reducing risk of cardiovascular disease may not be justified, especially as the group consuming the highest levels of vegetables were more likely to be receiving medication for high blood cholesterol and high blood pressure (i.e. this group was at higher risk of CVD), compared with those consuming the lowest intake.” He added: “These findings should not be taken to indicate that eating more vegetables has no benefit to health, especially cardiovascular health.”
Naveed Sattar, FMedSci, FRCPath, FRCPGlas, professor of metabolic medicine at the University of Glasgow, agreed. “In short, this paper should in no way change advice to eat at least five portions of fruit and vegetables a day,” he said. “Many living in the U.K. fall well short of this, sadly, and more needs to be done to encourage better intake of vegetables.
“In fact, I suspect we may have underestimated the importance of a healthy diet on health and disease in general.”
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN NUTRITION
Doc says sobbing attorney and crying medical expert led to unfair million-dollar verdict
Jurors found obstetrician-gynecologist Charles H. Marks, DO, negligent for failing to follow up on a patient’s complex cyst and hyperechoic nodule, which resulted in a delayed ovarian cancer diagnosis. But during the doctor’s 4-day trial, several parties had emotional outbursts in front of the jury, including the plaintiff’s’ attorney, a physician expert witness, the patient, and a family member.
According to trial transcripts, a gynecologic oncologist expert began crying on the stand after providing a clinical description of the symptoms that plaintiff, Chasidy Plunkard, would probably experience leading up to her death. When asked how long the patient had to live, the oncologist said “months” and later added, “I think she is living for this trial.”
After these comments, Ms. Plunkard’s attorney, Kila Baldwin, also began crying and requested a break to regain her composure, according to district court documents. During the 3 minutes the attorney was gone, the courtroom was silent other than the sound of Ms. Plunkard and her cousin sobbing.
The following day, U.S. District Judge Jennifer P. Wilson warned Ms. Baldwin that she would consider declaring a mistrial if the outburst happened again, according to trial transcripts.
“I expect counsel to maintain a professional demeanor even when eliciting emotionally laden testimony,” Judge Wilson said. “…I will not allow another recess. Further, if we have another incident like we had yesterday, I would have to entertain, if a motion for mistrial is made, I would have to seriously consider that, because I am concerned that this jury already has had a demonstration of a level of emotion that may make it difficult for them to set that aside and render a verdict that’s based only on a dispassionate consideration of what I perceive to be a legitimate dispute regarding liability.”
Judge Wilson later instructed jurors to disregard certain testimony at the request of Dr. Marks’ attorneys and reminded them not to be influenced by sympathy. After jurors rendered their $1.3 million verdict against Dr. Marks, he requested a new trial, claiming the witness’s testimony and the emotional displays of the witness and the attorney unfairly influenced the jury.
The expert witness “and plaintiff’s counsel undoubtedly affected the jury’s ability to decide this case in a dispassionate and impartial way and denied Dr. Marks his right to a fair trial,” attorney Matthew Rappleye wrote in Dr. Marks’ motion for a retrial. “Just as the court feared, as a result of these events, the jury was ‘tainted’ and could no longer decide this case divorced of sympathy for Ms. Plunkard.”
In response, an attorney for Ms. Plunkard emphasized that Dr. Marks did not request a retrial during the trial and that he was granted objections to the relevant testimony that he sought.
“In any event, the jury had the benefit of substantial evidence concerning Dr. Marks’ negligence, and the jury was entitled to construe that evidence in Plaintiff’s favor” attorney Charles Becker wrote. “…Indeed, the jury’s economic damages award of $585,000 not only fell well below the projection of plaintiff’s expert, but also ran at the low-end of the projection provided by Defendant’s economist. As to the non-economic damages, the jury’s award of $750,000 could have been far higher…. Nothing about either verdict or the damages award suggests a jury that was influenced by impermissible displays of emotion by the trial participants.”
In a December 13, 2021, decision, the U.S. District Court for the Middle District Court of Pennsylvania denied Dr. Marks’ request for a retrial. In her decision, Judge Wilson wrote that nothing in the jury’s verdict appeared to indicate that jurors were swayed by sympathy and that the verdict appeared to be conservative in light of the testimony presented.
Attorneys for Dr. Marks did not respond to a request for comment. In a statement, Ms. Baldwin said that the judge’s decision was correct.
“The district court got it exactly right, and we look forward to Ms. Plunkard receiving the compensation awarded by the jury in this tragic case,” she said.
Why did the patient sue?
Ms. Plunkard’s lawsuit against Dr. Marks stemmed from a January 2016 visit for complaints of bloating, pelvic pain, irregular periods, and an abnormal pelvic ultrasound. The ultrasound, ordered by her primary care physician, showed a thickened endometrial lining; a normal left ovary; and an enlarged, abnormal right ovary containing a complicated cyst, according to Ms. Plunkard’s complaint. Dr. Marks performed an endometrial biopsy and advised Ms. Plunkard that she would not need to take further measures if the result was benign, according to her lawsuit.
The result was benign, so Ms. Plunkard said she sought no further treatment for the cyst and none of her treating physicians followed up on the cyst. In February 2017, Ms. Plunkard presented to an emergency department with severe right upper quadrant abdominal pain and an ultrasound showed possible gallstones and pleural effusion, according to court documents.
After laparoscopic cholecystectomy, it was discovered that Ms. Plunkard had an inflamed pelvis and an omental lymph node was removed and biopsied. Surgeons reported that the lymph node showed metastatic cancer of probable gynecologic origin.
The patient underwent exploratory laparotomy, resulting in a radical abdominal hysterectomy, appendectomy, resection of the rectosigmoid with end-to-end anastomosis, and removal of cancerous implants. She was ultimately diagnosed with stage IVB low-grade metastatic ovarian cancer and went through six chemotherapy courses.
The patent’s cancer briefly went into remission but returned. In her complaint, Ms. Plunkard said Dr. Marks’ negligence allowed the cancer to spread from stage I to stage IVB, increasing her risk for harm and untimely death.
Dr. Marks argued that a follow-up ultrasound was not required, that the cyst on the patient’s right ovary was benign and resolved itself, that Ms. Plunkard’s cancer originated on the left, and that a follow-up ultrasound would not have detected primary cancer on the left ovary, according to legal documents.
On January 7, 2022, Dr. Marks appealed the jury’s verdict and the order denying his request for a retrial to the U.S. Court of Appeals for the Third Circuit.
A version of this article first appeared on Medscape.com.
Jurors found obstetrician-gynecologist Charles H. Marks, DO, negligent for failing to follow up on a patient’s complex cyst and hyperechoic nodule, which resulted in a delayed ovarian cancer diagnosis. But during the doctor’s 4-day trial, several parties had emotional outbursts in front of the jury, including the plaintiff’s’ attorney, a physician expert witness, the patient, and a family member.
According to trial transcripts, a gynecologic oncologist expert began crying on the stand after providing a clinical description of the symptoms that plaintiff, Chasidy Plunkard, would probably experience leading up to her death. When asked how long the patient had to live, the oncologist said “months” and later added, “I think she is living for this trial.”
After these comments, Ms. Plunkard’s attorney, Kila Baldwin, also began crying and requested a break to regain her composure, according to district court documents. During the 3 minutes the attorney was gone, the courtroom was silent other than the sound of Ms. Plunkard and her cousin sobbing.
The following day, U.S. District Judge Jennifer P. Wilson warned Ms. Baldwin that she would consider declaring a mistrial if the outburst happened again, according to trial transcripts.
“I expect counsel to maintain a professional demeanor even when eliciting emotionally laden testimony,” Judge Wilson said. “…I will not allow another recess. Further, if we have another incident like we had yesterday, I would have to entertain, if a motion for mistrial is made, I would have to seriously consider that, because I am concerned that this jury already has had a demonstration of a level of emotion that may make it difficult for them to set that aside and render a verdict that’s based only on a dispassionate consideration of what I perceive to be a legitimate dispute regarding liability.”
Judge Wilson later instructed jurors to disregard certain testimony at the request of Dr. Marks’ attorneys and reminded them not to be influenced by sympathy. After jurors rendered their $1.3 million verdict against Dr. Marks, he requested a new trial, claiming the witness’s testimony and the emotional displays of the witness and the attorney unfairly influenced the jury.
The expert witness “and plaintiff’s counsel undoubtedly affected the jury’s ability to decide this case in a dispassionate and impartial way and denied Dr. Marks his right to a fair trial,” attorney Matthew Rappleye wrote in Dr. Marks’ motion for a retrial. “Just as the court feared, as a result of these events, the jury was ‘tainted’ and could no longer decide this case divorced of sympathy for Ms. Plunkard.”
In response, an attorney for Ms. Plunkard emphasized that Dr. Marks did not request a retrial during the trial and that he was granted objections to the relevant testimony that he sought.
“In any event, the jury had the benefit of substantial evidence concerning Dr. Marks’ negligence, and the jury was entitled to construe that evidence in Plaintiff’s favor” attorney Charles Becker wrote. “…Indeed, the jury’s economic damages award of $585,000 not only fell well below the projection of plaintiff’s expert, but also ran at the low-end of the projection provided by Defendant’s economist. As to the non-economic damages, the jury’s award of $750,000 could have been far higher…. Nothing about either verdict or the damages award suggests a jury that was influenced by impermissible displays of emotion by the trial participants.”
In a December 13, 2021, decision, the U.S. District Court for the Middle District Court of Pennsylvania denied Dr. Marks’ request for a retrial. In her decision, Judge Wilson wrote that nothing in the jury’s verdict appeared to indicate that jurors were swayed by sympathy and that the verdict appeared to be conservative in light of the testimony presented.
Attorneys for Dr. Marks did not respond to a request for comment. In a statement, Ms. Baldwin said that the judge’s decision was correct.
“The district court got it exactly right, and we look forward to Ms. Plunkard receiving the compensation awarded by the jury in this tragic case,” she said.
Why did the patient sue?
Ms. Plunkard’s lawsuit against Dr. Marks stemmed from a January 2016 visit for complaints of bloating, pelvic pain, irregular periods, and an abnormal pelvic ultrasound. The ultrasound, ordered by her primary care physician, showed a thickened endometrial lining; a normal left ovary; and an enlarged, abnormal right ovary containing a complicated cyst, according to Ms. Plunkard’s complaint. Dr. Marks performed an endometrial biopsy and advised Ms. Plunkard that she would not need to take further measures if the result was benign, according to her lawsuit.
The result was benign, so Ms. Plunkard said she sought no further treatment for the cyst and none of her treating physicians followed up on the cyst. In February 2017, Ms. Plunkard presented to an emergency department with severe right upper quadrant abdominal pain and an ultrasound showed possible gallstones and pleural effusion, according to court documents.
After laparoscopic cholecystectomy, it was discovered that Ms. Plunkard had an inflamed pelvis and an omental lymph node was removed and biopsied. Surgeons reported that the lymph node showed metastatic cancer of probable gynecologic origin.
The patient underwent exploratory laparotomy, resulting in a radical abdominal hysterectomy, appendectomy, resection of the rectosigmoid with end-to-end anastomosis, and removal of cancerous implants. She was ultimately diagnosed with stage IVB low-grade metastatic ovarian cancer and went through six chemotherapy courses.
The patent’s cancer briefly went into remission but returned. In her complaint, Ms. Plunkard said Dr. Marks’ negligence allowed the cancer to spread from stage I to stage IVB, increasing her risk for harm and untimely death.
Dr. Marks argued that a follow-up ultrasound was not required, that the cyst on the patient’s right ovary was benign and resolved itself, that Ms. Plunkard’s cancer originated on the left, and that a follow-up ultrasound would not have detected primary cancer on the left ovary, according to legal documents.
On January 7, 2022, Dr. Marks appealed the jury’s verdict and the order denying his request for a retrial to the U.S. Court of Appeals for the Third Circuit.
A version of this article first appeared on Medscape.com.
Jurors found obstetrician-gynecologist Charles H. Marks, DO, negligent for failing to follow up on a patient’s complex cyst and hyperechoic nodule, which resulted in a delayed ovarian cancer diagnosis. But during the doctor’s 4-day trial, several parties had emotional outbursts in front of the jury, including the plaintiff’s’ attorney, a physician expert witness, the patient, and a family member.
According to trial transcripts, a gynecologic oncologist expert began crying on the stand after providing a clinical description of the symptoms that plaintiff, Chasidy Plunkard, would probably experience leading up to her death. When asked how long the patient had to live, the oncologist said “months” and later added, “I think she is living for this trial.”
After these comments, Ms. Plunkard’s attorney, Kila Baldwin, also began crying and requested a break to regain her composure, according to district court documents. During the 3 minutes the attorney was gone, the courtroom was silent other than the sound of Ms. Plunkard and her cousin sobbing.
The following day, U.S. District Judge Jennifer P. Wilson warned Ms. Baldwin that she would consider declaring a mistrial if the outburst happened again, according to trial transcripts.
“I expect counsel to maintain a professional demeanor even when eliciting emotionally laden testimony,” Judge Wilson said. “…I will not allow another recess. Further, if we have another incident like we had yesterday, I would have to entertain, if a motion for mistrial is made, I would have to seriously consider that, because I am concerned that this jury already has had a demonstration of a level of emotion that may make it difficult for them to set that aside and render a verdict that’s based only on a dispassionate consideration of what I perceive to be a legitimate dispute regarding liability.”
Judge Wilson later instructed jurors to disregard certain testimony at the request of Dr. Marks’ attorneys and reminded them not to be influenced by sympathy. After jurors rendered their $1.3 million verdict against Dr. Marks, he requested a new trial, claiming the witness’s testimony and the emotional displays of the witness and the attorney unfairly influenced the jury.
The expert witness “and plaintiff’s counsel undoubtedly affected the jury’s ability to decide this case in a dispassionate and impartial way and denied Dr. Marks his right to a fair trial,” attorney Matthew Rappleye wrote in Dr. Marks’ motion for a retrial. “Just as the court feared, as a result of these events, the jury was ‘tainted’ and could no longer decide this case divorced of sympathy for Ms. Plunkard.”
In response, an attorney for Ms. Plunkard emphasized that Dr. Marks did not request a retrial during the trial and that he was granted objections to the relevant testimony that he sought.
“In any event, the jury had the benefit of substantial evidence concerning Dr. Marks’ negligence, and the jury was entitled to construe that evidence in Plaintiff’s favor” attorney Charles Becker wrote. “…Indeed, the jury’s economic damages award of $585,000 not only fell well below the projection of plaintiff’s expert, but also ran at the low-end of the projection provided by Defendant’s economist. As to the non-economic damages, the jury’s award of $750,000 could have been far higher…. Nothing about either verdict or the damages award suggests a jury that was influenced by impermissible displays of emotion by the trial participants.”
In a December 13, 2021, decision, the U.S. District Court for the Middle District Court of Pennsylvania denied Dr. Marks’ request for a retrial. In her decision, Judge Wilson wrote that nothing in the jury’s verdict appeared to indicate that jurors were swayed by sympathy and that the verdict appeared to be conservative in light of the testimony presented.
Attorneys for Dr. Marks did not respond to a request for comment. In a statement, Ms. Baldwin said that the judge’s decision was correct.
“The district court got it exactly right, and we look forward to Ms. Plunkard receiving the compensation awarded by the jury in this tragic case,” she said.
Why did the patient sue?
Ms. Plunkard’s lawsuit against Dr. Marks stemmed from a January 2016 visit for complaints of bloating, pelvic pain, irregular periods, and an abnormal pelvic ultrasound. The ultrasound, ordered by her primary care physician, showed a thickened endometrial lining; a normal left ovary; and an enlarged, abnormal right ovary containing a complicated cyst, according to Ms. Plunkard’s complaint. Dr. Marks performed an endometrial biopsy and advised Ms. Plunkard that she would not need to take further measures if the result was benign, according to her lawsuit.
The result was benign, so Ms. Plunkard said she sought no further treatment for the cyst and none of her treating physicians followed up on the cyst. In February 2017, Ms. Plunkard presented to an emergency department with severe right upper quadrant abdominal pain and an ultrasound showed possible gallstones and pleural effusion, according to court documents.
After laparoscopic cholecystectomy, it was discovered that Ms. Plunkard had an inflamed pelvis and an omental lymph node was removed and biopsied. Surgeons reported that the lymph node showed metastatic cancer of probable gynecologic origin.
The patient underwent exploratory laparotomy, resulting in a radical abdominal hysterectomy, appendectomy, resection of the rectosigmoid with end-to-end anastomosis, and removal of cancerous implants. She was ultimately diagnosed with stage IVB low-grade metastatic ovarian cancer and went through six chemotherapy courses.
The patent’s cancer briefly went into remission but returned. In her complaint, Ms. Plunkard said Dr. Marks’ negligence allowed the cancer to spread from stage I to stage IVB, increasing her risk for harm and untimely death.
Dr. Marks argued that a follow-up ultrasound was not required, that the cyst on the patient’s right ovary was benign and resolved itself, that Ms. Plunkard’s cancer originated on the left, and that a follow-up ultrasound would not have detected primary cancer on the left ovary, according to legal documents.
On January 7, 2022, Dr. Marks appealed the jury’s verdict and the order denying his request for a retrial to the U.S. Court of Appeals for the Third Circuit.
A version of this article first appeared on Medscape.com.
Mask mandates ending in all but one state
As COVID-19 cases and hospitalizations continue to decline across the United States,
Retailers and cruises are following along, with Apple and Target stores lifting their own mask mandates this week. Cruise lines such as Norwegian and Royal Caribbean International have said mask requirements will be relaxed for vaccinated passengers, according to the Washington Post.
But guidance from the Centers for Disease Control and Prevention hasn’t changed even as the Omicron variant recedes across the country. Vaccinated people should wear masks when indoors in areas of “substantial or high transmission,” which still covers more than 95% of the country, according to a CDC map.
As daily cases continue to fall, the CDC is reviewing its recommendations, Rochelle Walensky, MD, the CDC director, said during a briefing last week.
“We want to give people a break from things like mask-wearing, when these metrics are better, and then have the ability to reach for them again should things worsen,” she said.
As states relax mask rules, county and city officials are now deciding what to do in their jurisdictions. Vaccinated residents in Los Angeles County may soon be able to go maskless in indoor settings that check for proof of vaccination, according to the Los Angeles Times.
Chicago will also end its mask and COVID-19 vaccine mandates for public places such as restaurants Feb. 28, according to the Chicago Tribune. Illinois will end a statewide indoor mask mandate on the same day. Masks will still be required in health care settings and public transmit.
State and local school boards are debating their mask policies as well. The Maryland State Board of Education voted Feb. 22 to allow local school districts to decide whether students must wear face coverings in school, according to the Associated Press. The update will take effect on March 1 if approved by a Maryland General Assembly committee that oversees the rule.
In New York, state officials have begun lifting mask rules. At the same time, 58% of New York voters want to see early March data before school mask mandates are ended, according to a new poll, released Feb. 22 by the Siena College Research Institute. About 45% of those polled said the state’s indoor public mask mandate should also still be in place.
The debate about wearing masks in schools will likely continue, especially as districts get caught between health authorities and parents, according to the Wall Street Journal. District officials in several states are receiving hundreds of emails daily from both sides, with parents calling for mask rules to end or saying that requirements should remain in place for now to keep kids safe.
A version of this article first appeared on WebMD.com.
As COVID-19 cases and hospitalizations continue to decline across the United States,
Retailers and cruises are following along, with Apple and Target stores lifting their own mask mandates this week. Cruise lines such as Norwegian and Royal Caribbean International have said mask requirements will be relaxed for vaccinated passengers, according to the Washington Post.
But guidance from the Centers for Disease Control and Prevention hasn’t changed even as the Omicron variant recedes across the country. Vaccinated people should wear masks when indoors in areas of “substantial or high transmission,” which still covers more than 95% of the country, according to a CDC map.
As daily cases continue to fall, the CDC is reviewing its recommendations, Rochelle Walensky, MD, the CDC director, said during a briefing last week.
“We want to give people a break from things like mask-wearing, when these metrics are better, and then have the ability to reach for them again should things worsen,” she said.
As states relax mask rules, county and city officials are now deciding what to do in their jurisdictions. Vaccinated residents in Los Angeles County may soon be able to go maskless in indoor settings that check for proof of vaccination, according to the Los Angeles Times.
Chicago will also end its mask and COVID-19 vaccine mandates for public places such as restaurants Feb. 28, according to the Chicago Tribune. Illinois will end a statewide indoor mask mandate on the same day. Masks will still be required in health care settings and public transmit.
State and local school boards are debating their mask policies as well. The Maryland State Board of Education voted Feb. 22 to allow local school districts to decide whether students must wear face coverings in school, according to the Associated Press. The update will take effect on March 1 if approved by a Maryland General Assembly committee that oversees the rule.
In New York, state officials have begun lifting mask rules. At the same time, 58% of New York voters want to see early March data before school mask mandates are ended, according to a new poll, released Feb. 22 by the Siena College Research Institute. About 45% of those polled said the state’s indoor public mask mandate should also still be in place.
The debate about wearing masks in schools will likely continue, especially as districts get caught between health authorities and parents, according to the Wall Street Journal. District officials in several states are receiving hundreds of emails daily from both sides, with parents calling for mask rules to end or saying that requirements should remain in place for now to keep kids safe.
A version of this article first appeared on WebMD.com.
As COVID-19 cases and hospitalizations continue to decline across the United States,
Retailers and cruises are following along, with Apple and Target stores lifting their own mask mandates this week. Cruise lines such as Norwegian and Royal Caribbean International have said mask requirements will be relaxed for vaccinated passengers, according to the Washington Post.
But guidance from the Centers for Disease Control and Prevention hasn’t changed even as the Omicron variant recedes across the country. Vaccinated people should wear masks when indoors in areas of “substantial or high transmission,” which still covers more than 95% of the country, according to a CDC map.
As daily cases continue to fall, the CDC is reviewing its recommendations, Rochelle Walensky, MD, the CDC director, said during a briefing last week.
“We want to give people a break from things like mask-wearing, when these metrics are better, and then have the ability to reach for them again should things worsen,” she said.
As states relax mask rules, county and city officials are now deciding what to do in their jurisdictions. Vaccinated residents in Los Angeles County may soon be able to go maskless in indoor settings that check for proof of vaccination, according to the Los Angeles Times.
Chicago will also end its mask and COVID-19 vaccine mandates for public places such as restaurants Feb. 28, according to the Chicago Tribune. Illinois will end a statewide indoor mask mandate on the same day. Masks will still be required in health care settings and public transmit.
State and local school boards are debating their mask policies as well. The Maryland State Board of Education voted Feb. 22 to allow local school districts to decide whether students must wear face coverings in school, according to the Associated Press. The update will take effect on March 1 if approved by a Maryland General Assembly committee that oversees the rule.
In New York, state officials have begun lifting mask rules. At the same time, 58% of New York voters want to see early March data before school mask mandates are ended, according to a new poll, released Feb. 22 by the Siena College Research Institute. About 45% of those polled said the state’s indoor public mask mandate should also still be in place.
The debate about wearing masks in schools will likely continue, especially as districts get caught between health authorities and parents, according to the Wall Street Journal. District officials in several states are receiving hundreds of emails daily from both sides, with parents calling for mask rules to end or saying that requirements should remain in place for now to keep kids safe.
A version of this article first appeared on WebMD.com.
Toenail ridges
Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026
Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Transverse ridges that grow out with the nails are called Beau lines, also known as Beau’s ridges. This contrasts with Mees lines which are transverse white bands that grow out with the toenails, are nonpalpable, and are attributed to arsenic poisoning.
Beau lines are caused by a disruption in nail growth that can result from trauma, hypotension, or systemic or severe illness; they have also been reported in cases of COVID-19.1 Beau lines can occur on a single nail if the trauma or injury is isolated to 1 digit. If there was a systemic illness or stress, the lines can affect all 20 nails. The time of the inciting event can be approximated by how far the lines are from the cuticle. While there is some variability, it usually takes 12 to 18 months to grow an entirely new toenail. If the Beau lines have grown halfway out, then the stressor likely occurred 6 to 9 months earlier.
In this image, some asymmetry is visible between the right and left great toenails and there are some subtle distal changes, raising the possibility that there was more than 1 injury to this patient’s system (or prolonged difficulty). The patient said that to his knowledge, he had not been infected with COVID-19. However, hair and nail changes may be the only finding in some individuals who have been infected with COVID-19.1
This patient was counseled regarding the nature of this disorder and that without knowing what illness or injury caused the change, it was a benign finding. He was advised that it did not appear to be onychomycosis and did not require any medications or antifungal therapy. The patient was told to follow up if any changes developed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026
- Deng J, Ngo T, Zhu TH, Halverstam C. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140. doi: 10.1016/j.jdcr.2021.05.026
Leukemia Cutis Manifesting as Nonpalpable Purpura
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.
The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).
Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.
Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.
Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.
The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).
Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.
Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.
Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
To the Editor:
A 72-year-old man presented with symptomatic anemia and nonpalpable purpura of the legs, abdomen, and arms of 2 weeks’ duration (Figure 1). There were no associated perifollicular papules. Physical examination of the hair and gingiva were normal.
The patient’s medical history was notable for a poorly differentiated pancreatic adenocarcinoma (pT3N1M0) resected 7 months prior using a Whipple operation (pancreaticoduodenectomy). Adjuvant therapy consisted of 5 cycles of intravenous gemcitabine and paclitaxel. Treatment was discontinued 1 month prior due to progressive weight loss and the presence of new liver metastases on computed tomography. There was no recent history of corticosteroid, antiplatelet, or anticoagulant use. The patient had no known history of trauma at the affected sites.
The patient’s laboratory workup revealed the following results: hemoglobin, 5.5 g/dL (reference range, 13–18 g/dL); platelets, 128×109/L (reference range, 150–400×109/L); total white blood cell count (24.0×109/L [reference range, 4.0–11.0×109/L]), consisting of neutrophils (2.4×109/L [reference range, 2.0–7.5×109/L]), lymphocytes (3.1×109/L [reference range, 1.5–4.0×109/L]), and monocytes (18.5×109/L [reference range, 0.2–0.8×109/L]). Fibrinogen, activated partial thromboplastin time, and prothrombin time were within reference range. Results of a bone marrow biopsy showed 64% blasts. The lactate dehydrogenase level was 286 U/L (reference range, 135–220 U/L) and CA-19-9 antigen was 238 U/mL (reference range, 0–39 U/mL).
Results from a skin punch biopsy from the right leg showed a normal epidermis and papillary dermis. The reticular dermis was expanded by a diffuse cellular infiltrate with dermal edema and separation of collagen bundles (Figure 2), which consisted of small cells with irregular, cleaved, and notched nuclei, containing a variable amount of eosinophilic cytoplasm. Mitotic figures were present (Figure 3). There was no evidence of vasculitis, and Congo red stain for amyloid was negative. These atypical cells were positive for the leukocyte common antigen, favoring a hematopoietic infiltrate (Figure 4). Other positive markers included CD4 (associated with helper T cells, and mature and immature monocytes), CD68 (a monocyte/macrophage marker), and CD56 (associated with natural killer cells, myeloma, acute myeloid leukemia [AML], and neuroendocrine tumors). The cells were negative for CD3 (T-cell lineage–specific antigen), CD5 (marker of T cells and a subset of IgM-secreting B cells), CD34 (early hematopoietic marker), and CD20 (B-cell marker). Other negative myeloid markers included myeloperoxidase, CD117, and CD138. These findings suggested leukemic cell recruitment at the site of a reactive infiltrate. The patient completed 2 cycles of intravenous azacitidine with little response and subsequently opted for palliative measures.
Nonpalpable purpura has a broad differential diagnosis including primary and secondary thrombocytopenia; coagulopathies, including vitamin K deficiency, specific clotting factor deficiencies, and amyloid-related purpura; genetic or acquired collagen disorders, including vitamin C deficiency; and eruptions induced by drugs and herbal remedies.
Leukemia cutis is a relatively rare cause of purpura and is defined as cutaneous infiltration by neoplastic leucocytes.1 It most commonly is associated with AML and complicates approximately 5% to 15%of all adult cases.2 Cutaneous involvement occurs predominantly in monocytic variants; acute myelomonocytic leukemia and acute monocytic leukemia may arise in up to 50% of these cases.3 The clinical presentation may vary from papules, nodules, and plaques to rarer manifestations including purpura. A leukemic infiltrate often is associated with sites of inflammation, such as infection or ulceration,4 though there was no reported history of any known triggering events in our patient. Lesions usually involve the legs, followed by the arms, back, chest, scalp, and face.4 One-third of cases coincide with systemic symptoms, and approximately 10% precede bone marrow or peripheral blood involvement, referred to as aleukemic leukemia. The remainder of cases arise following an established diagnosis of systemic leukemia.5 Leukemia cutis is considered a marker of poor prognosis in AML,4,6 requiring treatment for the underlying systemic disease. Our case also was complicated by a concurrent pancreatic malignancy and relatively advanced age, which limited the feasibility of further treatment.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
- Strutton G. Cutaneous infiltrates: lymphomatous and leukemic. In: Weedon D, ed. Skin Pathology. 2nd ed. Churchill Livingstone; 2002:1118-1120.
- Cho-Vega JH, Medeiros LJ, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142.
- Kaddu S, Zenahlik P, Beham-Schmid C, et al. Specific cutaneous infiltrates in patients with myelogenous leukemia: a clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol. 1999;40:966-978.
- Paydas S, Zorludemir S. Leukaemia cutis and leukaemic vasculitis. Br J Dermatol. 2000;143:773-779.
- Shaikh BS, Frantz E, Lookingbill DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39:57-60.
- Su WP. Clinical, histopathologic, and immunohistochemical correlations in leukemia cutis. Semin Dermatol. 1994;13:223-230.
Practice Points
- Leukemia cutis complicates 5% to 15% of all cases of acute myeloid leukemia (AML) in adults.
- The appearance of leukemia cutis may be highly variable. Therefore, it should be included in the differential diagnosis for any cutaneous presentation in patients with an existing diagnosis or high likelihood of AML.
- Leukemic infiltrates are associated with sites of inflammation.
Twenty-three percent of health care workers likely to leave industry soon: Poll
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
according to a new poll.
About half of the respondents to the poll from USA Today/Ipsos reported feeling “burned out,” 43% said they were “anxious,” and 21% said they were “angry” about politics and abuse from patients and families.
“We’re trying to help people here, and we are getting verbally and physically abused for it,” Sarah Fried, a nurse in California who responded to the survey, told USA Today in a follow-up interview.
“Early in this pandemic, people were clapping for us and calling us heroes,” she said. “And what happened to that? What happened to them appreciating what nurses are doing?”
The poll was done Feb. 9-16 among 1,170 adults in the U.S. health care industry, including doctors, nurses, paramedics, therapists, home health aides, dentists, and other medical professionals.
A large majority of workers still reported being satisfied with their jobs, although that optimism has declined somewhat since early 2021 when the COVID-19 vaccine rollout was underway. About 80% of those in the recent poll said they were somewhat or very satisfied with their current job, which is down from 89% in an April 2021 poll from Kaiser Family Foundation/the Washington Post.
Most health care workers reported feeling “hopeful” (59%), “motivated” (59%), or “optimistic” (56%) about going to work. But “hopeful” is down from 76% and “optimistic” is down from 67%, compared with last year.
If they could pick a career over again, about 16% disagreed with the statement, “I would still decide to go into health care,” and 18% said they didn’t know how they felt about it.
“The pandemic has actually made me realize how important this career is and how I really do make a difference. I still love it,” Christina Rosa, a mental health counselor in Massachusetts, told USA Today.
During the pandemic, about 66% of those polled said they had treated a COVID-19 patient, which increased to 84% among nurses and 86% among hospital workers. Among those, 47% reported having a patient who died from COVID-19, including 53% of nurses and 55% of hospital workers.
What’s more, 81% of those who treated COVID-19 patients have cared for unvaccinated patients. Among those, 67% said their patients continued to express skepticism toward COVID-19 vaccines, and 38% said some patients expressed regret for not getting a vaccine. Beyond that, 26% said unvaccinated patients asked for unproven treatments, and 30% said the patient or family criticized the care they received.
Regarding coronavirus-related policy, most Americans working in health care expressed skepticism or criticism of the nation’s handling of the pandemic. About 39% agreed that the American health care system is “on the verge of collapse.”
Only 21% said the pandemic is mostly or completely under control. About 61% don’t think Americans are taking enough precautions to prevent the spread of the coronavirus.
Health care workers were slightly positive when it comes to the Centers for Disease Control and Prevention (54% approve, 34% disapprove), divided on the Biden administration (41% approve, 40% disapprove), and critical of the news media (20% approve, 61% disapprove) and the American public (18% approve, 68% disapprove).
Broadly, though, health care workers support public health efforts. About 85% back measures that provide N95 masks, and 83% back measures that provide COVID-19 tests.
A version of this article first appeared on WebMD.com.
Former APA president suspended by Columbia for ‘racist’ tweet
The university had not confirmed the suspension to this news organization by press time, but a letter from the school’s leadership notifying staff of the suspension was posted on Twitter the morning of Feb. 23 by addiction psychiatrist Jeremy Kidd, MD, who is a colleague of Dr. Lieberman’s at Columbia.
The suspension comes in the wake of Dr. Lieberman’s Feb. 21 tweet that drew immediate backlash by Twitter users who characterized it as racist and misogynist.
Dr. Lieberman, a former president of the American Psychiatric Association, reportedly deleted the tweet and his entire Twitter account soon after, according to NewsOne.
However, the tweet was captured by others, including Jack Turban, MD, a child psychiatry fellow at Stanford University. In Turban’s retweet, Dr. Lieberman commented on a tweet about a black model, noting, “whether a work of art or a freak of nature she’s a beautiful sight to behold.”
The response on Twitter was swift. “My ancestors would roll over in their graves if I refrained from commentary on how anti-Blackness shows up in ‘compliments,’” tweeted Jessica Isom, MD, MPH, a psychiatrist at Yale University.
Dr. Turban speculated that there will be no consequences for Dr. Lieberman, adding in his tweet, “He will continue to make the hiring decisions (including for faculty candidates who are women of color).”
Apology letter?
David Pagliaccio, a research scientist at the New York State Psychiatric Institute, posted what appeared to be an apology letter from Dr. Lieberman, although it could not be verified by this news organization.
In it, Dr. Lieberman was quoted as saying, “Yesterday, I tweeted from my personal account a message that was racist and sexist,” adding that prejudices he didn’t know he had held had been exposed, “and I’m deeply ashamed and very sorry.”
“I’ve hurt many, and I am beginning to understand the work ahead to make needed personal changes and over time to regain your trust,” Dr. Lieberman added.
Dr. Kidd called the suspension “absolutely the right move.” He added in his tweet that it “is only the beginning of what Columbia must do to heal & earn the trust our patients & trainees place in us every day.”
This news organization’s queries to Columbia University and to Dr. Lieberman were not returned by press time.
Dr. Lieberman is also director of the New York State Psychiatric Institute, was an advisory board member for Medscape Psychiatry and a frequent columnist for Medscape Medical News (sister organizations of MDedge.com), and was a consultant for Clinical Psychiatry.
A version of this article first appeared on Medscape.com.
The university had not confirmed the suspension to this news organization by press time, but a letter from the school’s leadership notifying staff of the suspension was posted on Twitter the morning of Feb. 23 by addiction psychiatrist Jeremy Kidd, MD, who is a colleague of Dr. Lieberman’s at Columbia.
The suspension comes in the wake of Dr. Lieberman’s Feb. 21 tweet that drew immediate backlash by Twitter users who characterized it as racist and misogynist.
Dr. Lieberman, a former president of the American Psychiatric Association, reportedly deleted the tweet and his entire Twitter account soon after, according to NewsOne.
However, the tweet was captured by others, including Jack Turban, MD, a child psychiatry fellow at Stanford University. In Turban’s retweet, Dr. Lieberman commented on a tweet about a black model, noting, “whether a work of art or a freak of nature she’s a beautiful sight to behold.”
The response on Twitter was swift. “My ancestors would roll over in their graves if I refrained from commentary on how anti-Blackness shows up in ‘compliments,’” tweeted Jessica Isom, MD, MPH, a psychiatrist at Yale University.
Dr. Turban speculated that there will be no consequences for Dr. Lieberman, adding in his tweet, “He will continue to make the hiring decisions (including for faculty candidates who are women of color).”
Apology letter?
David Pagliaccio, a research scientist at the New York State Psychiatric Institute, posted what appeared to be an apology letter from Dr. Lieberman, although it could not be verified by this news organization.
In it, Dr. Lieberman was quoted as saying, “Yesterday, I tweeted from my personal account a message that was racist and sexist,” adding that prejudices he didn’t know he had held had been exposed, “and I’m deeply ashamed and very sorry.”
“I’ve hurt many, and I am beginning to understand the work ahead to make needed personal changes and over time to regain your trust,” Dr. Lieberman added.
Dr. Kidd called the suspension “absolutely the right move.” He added in his tweet that it “is only the beginning of what Columbia must do to heal & earn the trust our patients & trainees place in us every day.”
This news organization’s queries to Columbia University and to Dr. Lieberman were not returned by press time.
Dr. Lieberman is also director of the New York State Psychiatric Institute, was an advisory board member for Medscape Psychiatry and a frequent columnist for Medscape Medical News (sister organizations of MDedge.com), and was a consultant for Clinical Psychiatry.
A version of this article first appeared on Medscape.com.
The university had not confirmed the suspension to this news organization by press time, but a letter from the school’s leadership notifying staff of the suspension was posted on Twitter the morning of Feb. 23 by addiction psychiatrist Jeremy Kidd, MD, who is a colleague of Dr. Lieberman’s at Columbia.
The suspension comes in the wake of Dr. Lieberman’s Feb. 21 tweet that drew immediate backlash by Twitter users who characterized it as racist and misogynist.
Dr. Lieberman, a former president of the American Psychiatric Association, reportedly deleted the tweet and his entire Twitter account soon after, according to NewsOne.
However, the tweet was captured by others, including Jack Turban, MD, a child psychiatry fellow at Stanford University. In Turban’s retweet, Dr. Lieberman commented on a tweet about a black model, noting, “whether a work of art or a freak of nature she’s a beautiful sight to behold.”
The response on Twitter was swift. “My ancestors would roll over in their graves if I refrained from commentary on how anti-Blackness shows up in ‘compliments,’” tweeted Jessica Isom, MD, MPH, a psychiatrist at Yale University.
Dr. Turban speculated that there will be no consequences for Dr. Lieberman, adding in his tweet, “He will continue to make the hiring decisions (including for faculty candidates who are women of color).”
Apology letter?
David Pagliaccio, a research scientist at the New York State Psychiatric Institute, posted what appeared to be an apology letter from Dr. Lieberman, although it could not be verified by this news organization.
In it, Dr. Lieberman was quoted as saying, “Yesterday, I tweeted from my personal account a message that was racist and sexist,” adding that prejudices he didn’t know he had held had been exposed, “and I’m deeply ashamed and very sorry.”
“I’ve hurt many, and I am beginning to understand the work ahead to make needed personal changes and over time to regain your trust,” Dr. Lieberman added.
Dr. Kidd called the suspension “absolutely the right move.” He added in his tweet that it “is only the beginning of what Columbia must do to heal & earn the trust our patients & trainees place in us every day.”
This news organization’s queries to Columbia University and to Dr. Lieberman were not returned by press time.
Dr. Lieberman is also director of the New York State Psychiatric Institute, was an advisory board member for Medscape Psychiatry and a frequent columnist for Medscape Medical News (sister organizations of MDedge.com), and was a consultant for Clinical Psychiatry.
A version of this article first appeared on Medscape.com.