User login
The best crystalloid for the critically ill
Hemodynamic instability is rewarded with a sojourn in the intensive care unit (ICU). When the intensivists see it, they’re going to throw fluids at it. Most likely a crystalloid of some type. This has been true for decades, centuries even. When I was a medical student, which was decades but not centuries ago, I used crystalloids every day on the surgical wards, in the operating room, in the emergency department, or on the medicine wards. Medicine docs preferred normal saline (NS) and surgeons used lactated Ringer’s solution (LR). I never gave this a second thought.
During medical school, I was drawn to internal medicine by the heavy emphasis on evidence-based medicine in the field. Prior to 2015 though, there wasn’t much data to support using one crystalloid formulation over another. Pre-2010, we had an American Thoracic Society (ATS) consensus statement on using crystalloid vs. colloid, making recommendations largely drawn from the SAFE trial. The ATS statement also suggested starches may be harmful, a view that was confirmed in a series of articles published in 2012 and 2013. There was less discussion about what type of crystalloid was best.
In 2014, I finally read a paper that compared crystalloid formulations. It was a network meta-analysis, which is “statistician speak” for combining disparate trials to make indirect comparisons. In the absence of large, randomized trials, this approach was a welcome addition to the data we had at the time. The authors concluded that “balanced” (typically LR or Plasma-Lyte) are superior to “unbalanced” (another term for NS) crystalloids. Balanced fluids typically have acetate or lactate and have a higher pH and lower chloride than NS. I found the signal for balanced fluids interesting at the time but promptly forgot about it.
Since 2015, the critical care community has rallied to produce a bevy of large trials comparing balanced vs. unbalanced crystalloids. The first was the SPLIT trial, which showed equivalence. Then came the SMART trial in 2018, which showed balanced fluids were better. Of note, another trial with an identical design (SALT-ED) was published in the same issue of The New England Journal of Medicine as SMART. SALT-ED enrolled patients in the emergency department, not the ICU, but also found benefit to using balanced fluids, albeit not for their primary outcome. I admit, after SMART and SALT-ED were published, I made the switch to LR. A secondary analysis of patients with sepsis pushed me further toward LR, while others withheld judgment.
Then we saw publication of the BaSICs trial, another large, randomized study evaluating crystalloid composition. I was hoping this one might put the issue to rest. That nephrologist who perseverated on every patient’s chloride during morning report would be vindicated. NS would prove to be too unbalanced and would finally be retired. No such luck. This is critical care medicine, where the initial signal is rarely confirmed in the follow-up trials. BaSICs found no difference between crystalloids for most important outcomes. The study did find balanced fluids may worsen outcomes for patients with head injuries.
Finally, there’s the PLUS trial, a large, multicenter randomized controlled trial comparing Plasma-Lyte vs. NS in the ICU. I could make the argument that this trial was the best of the bunch, and it was negative. The researchers did an excellent job of showing that serum pH and chloride levels did vary by fluid composition, but despite this, mortality and renal outcomes did not differ. Case closed? Crystalloid composition doesn’t matter, right?
An editorial that accompanies the BaSICs trial does an outstanding job of reviewing SPLIT, SMART, and BaSICs. The authors discuss design and population differences that may have led to differing results, and there are many. They conclude for most patients in the ICU, there’s no compelling reason to choose one crystalloid over another. Perhaps they’re right.
An updated meta-analysis that included all the studies I’ve mentioned concluded there was an 89% probability that balanced fluid reduces mortality for ICU patients. How could the meta-analysis authors reach this conclusion given all the negative trials? It has to do with their statistical methods – they performed both standard, frequentist (if statistical significance isn’t reached the study, is considered negative) and Bayesian analyses (posterior probability of benefit is calculated, regardless of P value). The frequentist approach was negative, but the posterior probability for benefit remained high.
Personally, I see no reason not to favor LR when resuscitating ICU patients without head injuries. In particular, it seems that medical patients (who made up almost 80% of those in the SMART trial) and those with sepsis may benefit. The critical care community has again outdone itself by performing large, well-designed trials to address important questions. Despite not having a definitive answer on crystalloid resuscitation, we know a lot more than we did when I was a medical student.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He reported receiving research grant from: Fisher-Paykel and receiving income from the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
Hemodynamic instability is rewarded with a sojourn in the intensive care unit (ICU). When the intensivists see it, they’re going to throw fluids at it. Most likely a crystalloid of some type. This has been true for decades, centuries even. When I was a medical student, which was decades but not centuries ago, I used crystalloids every day on the surgical wards, in the operating room, in the emergency department, or on the medicine wards. Medicine docs preferred normal saline (NS) and surgeons used lactated Ringer’s solution (LR). I never gave this a second thought.
During medical school, I was drawn to internal medicine by the heavy emphasis on evidence-based medicine in the field. Prior to 2015 though, there wasn’t much data to support using one crystalloid formulation over another. Pre-2010, we had an American Thoracic Society (ATS) consensus statement on using crystalloid vs. colloid, making recommendations largely drawn from the SAFE trial. The ATS statement also suggested starches may be harmful, a view that was confirmed in a series of articles published in 2012 and 2013. There was less discussion about what type of crystalloid was best.
In 2014, I finally read a paper that compared crystalloid formulations. It was a network meta-analysis, which is “statistician speak” for combining disparate trials to make indirect comparisons. In the absence of large, randomized trials, this approach was a welcome addition to the data we had at the time. The authors concluded that “balanced” (typically LR or Plasma-Lyte) are superior to “unbalanced” (another term for NS) crystalloids. Balanced fluids typically have acetate or lactate and have a higher pH and lower chloride than NS. I found the signal for balanced fluids interesting at the time but promptly forgot about it.
Since 2015, the critical care community has rallied to produce a bevy of large trials comparing balanced vs. unbalanced crystalloids. The first was the SPLIT trial, which showed equivalence. Then came the SMART trial in 2018, which showed balanced fluids were better. Of note, another trial with an identical design (SALT-ED) was published in the same issue of The New England Journal of Medicine as SMART. SALT-ED enrolled patients in the emergency department, not the ICU, but also found benefit to using balanced fluids, albeit not for their primary outcome. I admit, after SMART and SALT-ED were published, I made the switch to LR. A secondary analysis of patients with sepsis pushed me further toward LR, while others withheld judgment.
Then we saw publication of the BaSICs trial, another large, randomized study evaluating crystalloid composition. I was hoping this one might put the issue to rest. That nephrologist who perseverated on every patient’s chloride during morning report would be vindicated. NS would prove to be too unbalanced and would finally be retired. No such luck. This is critical care medicine, where the initial signal is rarely confirmed in the follow-up trials. BaSICs found no difference between crystalloids for most important outcomes. The study did find balanced fluids may worsen outcomes for patients with head injuries.
Finally, there’s the PLUS trial, a large, multicenter randomized controlled trial comparing Plasma-Lyte vs. NS in the ICU. I could make the argument that this trial was the best of the bunch, and it was negative. The researchers did an excellent job of showing that serum pH and chloride levels did vary by fluid composition, but despite this, mortality and renal outcomes did not differ. Case closed? Crystalloid composition doesn’t matter, right?
An editorial that accompanies the BaSICs trial does an outstanding job of reviewing SPLIT, SMART, and BaSICs. The authors discuss design and population differences that may have led to differing results, and there are many. They conclude for most patients in the ICU, there’s no compelling reason to choose one crystalloid over another. Perhaps they’re right.
An updated meta-analysis that included all the studies I’ve mentioned concluded there was an 89% probability that balanced fluid reduces mortality for ICU patients. How could the meta-analysis authors reach this conclusion given all the negative trials? It has to do with their statistical methods – they performed both standard, frequentist (if statistical significance isn’t reached the study, is considered negative) and Bayesian analyses (posterior probability of benefit is calculated, regardless of P value). The frequentist approach was negative, but the posterior probability for benefit remained high.
Personally, I see no reason not to favor LR when resuscitating ICU patients without head injuries. In particular, it seems that medical patients (who made up almost 80% of those in the SMART trial) and those with sepsis may benefit. The critical care community has again outdone itself by performing large, well-designed trials to address important questions. Despite not having a definitive answer on crystalloid resuscitation, we know a lot more than we did when I was a medical student.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He reported receiving research grant from: Fisher-Paykel and receiving income from the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
Hemodynamic instability is rewarded with a sojourn in the intensive care unit (ICU). When the intensivists see it, they’re going to throw fluids at it. Most likely a crystalloid of some type. This has been true for decades, centuries even. When I was a medical student, which was decades but not centuries ago, I used crystalloids every day on the surgical wards, in the operating room, in the emergency department, or on the medicine wards. Medicine docs preferred normal saline (NS) and surgeons used lactated Ringer’s solution (LR). I never gave this a second thought.
During medical school, I was drawn to internal medicine by the heavy emphasis on evidence-based medicine in the field. Prior to 2015 though, there wasn’t much data to support using one crystalloid formulation over another. Pre-2010, we had an American Thoracic Society (ATS) consensus statement on using crystalloid vs. colloid, making recommendations largely drawn from the SAFE trial. The ATS statement also suggested starches may be harmful, a view that was confirmed in a series of articles published in 2012 and 2013. There was less discussion about what type of crystalloid was best.
In 2014, I finally read a paper that compared crystalloid formulations. It was a network meta-analysis, which is “statistician speak” for combining disparate trials to make indirect comparisons. In the absence of large, randomized trials, this approach was a welcome addition to the data we had at the time. The authors concluded that “balanced” (typically LR or Plasma-Lyte) are superior to “unbalanced” (another term for NS) crystalloids. Balanced fluids typically have acetate or lactate and have a higher pH and lower chloride than NS. I found the signal for balanced fluids interesting at the time but promptly forgot about it.
Since 2015, the critical care community has rallied to produce a bevy of large trials comparing balanced vs. unbalanced crystalloids. The first was the SPLIT trial, which showed equivalence. Then came the SMART trial in 2018, which showed balanced fluids were better. Of note, another trial with an identical design (SALT-ED) was published in the same issue of The New England Journal of Medicine as SMART. SALT-ED enrolled patients in the emergency department, not the ICU, but also found benefit to using balanced fluids, albeit not for their primary outcome. I admit, after SMART and SALT-ED were published, I made the switch to LR. A secondary analysis of patients with sepsis pushed me further toward LR, while others withheld judgment.
Then we saw publication of the BaSICs trial, another large, randomized study evaluating crystalloid composition. I was hoping this one might put the issue to rest. That nephrologist who perseverated on every patient’s chloride during morning report would be vindicated. NS would prove to be too unbalanced and would finally be retired. No such luck. This is critical care medicine, where the initial signal is rarely confirmed in the follow-up trials. BaSICs found no difference between crystalloids for most important outcomes. The study did find balanced fluids may worsen outcomes for patients with head injuries.
Finally, there’s the PLUS trial, a large, multicenter randomized controlled trial comparing Plasma-Lyte vs. NS in the ICU. I could make the argument that this trial was the best of the bunch, and it was negative. The researchers did an excellent job of showing that serum pH and chloride levels did vary by fluid composition, but despite this, mortality and renal outcomes did not differ. Case closed? Crystalloid composition doesn’t matter, right?
An editorial that accompanies the BaSICs trial does an outstanding job of reviewing SPLIT, SMART, and BaSICs. The authors discuss design and population differences that may have led to differing results, and there are many. They conclude for most patients in the ICU, there’s no compelling reason to choose one crystalloid over another. Perhaps they’re right.
An updated meta-analysis that included all the studies I’ve mentioned concluded there was an 89% probability that balanced fluid reduces mortality for ICU patients. How could the meta-analysis authors reach this conclusion given all the negative trials? It has to do with their statistical methods – they performed both standard, frequentist (if statistical significance isn’t reached the study, is considered negative) and Bayesian analyses (posterior probability of benefit is calculated, regardless of P value). The frequentist approach was negative, but the posterior probability for benefit remained high.
Personally, I see no reason not to favor LR when resuscitating ICU patients without head injuries. In particular, it seems that medical patients (who made up almost 80% of those in the SMART trial) and those with sepsis may benefit. The critical care community has again outdone itself by performing large, well-designed trials to address important questions. Despite not having a definitive answer on crystalloid resuscitation, we know a lot more than we did when I was a medical student.
Dr. Holley is associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center. He reported receiving research grant from: Fisher-Paykel and receiving income from the American College of Chest Physicians. A version of this article first appeared on Medscape.com.
Mortality 12 times higher in children with congenital Zika
About 80% of people infected with Zika virus show no symptoms, and that’s particularly problematic during pregnancy. The infection can cause birth defects and is the origin of numerous cases of microcephaly and other neurologic impairments.
The large amount of Aedes aegypti mosquitoes in Brazilian cities, in addition to social and political problems, facilitated the spread of Zika to the point that the country recorded its highest number of congenital Zika syndrome notifications from 2015 to 2018. Since then, researchers have investigated the extent of the problem.
One of the most compelling findings about the dramatic legacy of Zika in Brazil was published Feb. 24 in The New England Journal of Medicine: After tracking 11,481,215 children born alive in Brazil up to 36 months of age between the years 2015 and 2018, the researchers found that the mortality rate was about 12 times higher among children with congenital Zika syndrome in comparison to children without the syndrome. The study is the first to follow children with congenital Zika syndrome for 3 years and to report mortality in this group.
“This difference persisted throughout the first 3 years of life,” Enny S. Paixão, PhD, of the London School of Hygiene and Tropical Medicine, and Fiocruz-Bahia’s Instituto Gonçalo Moniz, in Brazil, said in an interview.
At the end of the study period, the mortality rate was 52.6 deaths (95% confidence interval, 47.6-58.0) per 1,000 person-years among children with congenital Zika syndrome and 5.6 deaths (95% CI, 5.6-5.7) per 1,000 person-years among those without the syndrome. The mortality rate ratio among children with congenital Zika syndrome, compared with those without it, was 11.3 (95% CI, 10.2-12.4). Data analysis also showed that the 3,308 children with the syndrome were born to mothers who were younger and had fewer years of study when compared to the mothers of their 11,477,907 counterparts without the syndrome.
“If the children survived the first month of life, they had a greater chance of surviving during childhood, because the mortality rates drop,” said Dr. Paixão. “In children with congenital Zika syndrome, this rate also drops, but slowly. The more we stratified by period – neonatal, post neonatal, and the period from the first to the third year of life – the more we saw the relative risk increase. After the first year of life, children with the syndrome were almost 22 times more likely to die compared to children without it. It was hard to believe the data.” Dr. Paixão added that the mortality observed in this study is comparable with the findings of previous studies.
In addition to the large sample size – more than 11 million children – another unique aspect of the work was the comparison with healthy live births. “Previous studies didn’t have this comparison group,” Dr. said Paixão.
Perhaps the major challenge of the study, Dr. Paixão explained, was the fragmentation of the data. “In Brazil we have high-quality data systems, but they are not interconnected. We have a database with all live births, another with mortality records, and another with all children with congenital Zika syndrome. The first big challenge was putting all this information together.”
The solution found by the researchers was to use data linkage – bringing information about the same person from different data banks to create a richer dataset. Basically, they linked the data from the live births registry with the deaths that occurred in the studied age group plus around 18,000 children with congenital Zika syndrome. This was done, said Dr. Paixão, by choosing some identifying variables (such as mother’s name, address, and age) and using an algorithm that evaluates the probability that the “N” in one database is the same person in another database.
“This is expensive, complex, [and] involves super-powerful computers and a lot of researchers,” she said.
The impressive mortality data for children with congenital Zika syndrome obtained by the group of researchers made it inevitable to think about how the country should address this terrible legacy.
“The first and most important recommendation is that the country needs to invest in primary care, so that women don’t get Zika during pregnancy and children aren’t at risk of getting the syndrome,” said Dr. Paixão.
As for the affected population, she highlighted the need to deepen the understanding of the syndrome’s natural history to improve survival and quality of life of affected children and their families. One possibility that was recently discussed by the group of researchers is to carry out a study on the causes of hospitalization of children with the syndrome to develop appropriate protocols and procedures that reduce admissions and death in this population.
A version of this article first appeared on Medscape.com.
About 80% of people infected with Zika virus show no symptoms, and that’s particularly problematic during pregnancy. The infection can cause birth defects and is the origin of numerous cases of microcephaly and other neurologic impairments.
The large amount of Aedes aegypti mosquitoes in Brazilian cities, in addition to social and political problems, facilitated the spread of Zika to the point that the country recorded its highest number of congenital Zika syndrome notifications from 2015 to 2018. Since then, researchers have investigated the extent of the problem.
One of the most compelling findings about the dramatic legacy of Zika in Brazil was published Feb. 24 in The New England Journal of Medicine: After tracking 11,481,215 children born alive in Brazil up to 36 months of age between the years 2015 and 2018, the researchers found that the mortality rate was about 12 times higher among children with congenital Zika syndrome in comparison to children without the syndrome. The study is the first to follow children with congenital Zika syndrome for 3 years and to report mortality in this group.
“This difference persisted throughout the first 3 years of life,” Enny S. Paixão, PhD, of the London School of Hygiene and Tropical Medicine, and Fiocruz-Bahia’s Instituto Gonçalo Moniz, in Brazil, said in an interview.
At the end of the study period, the mortality rate was 52.6 deaths (95% confidence interval, 47.6-58.0) per 1,000 person-years among children with congenital Zika syndrome and 5.6 deaths (95% CI, 5.6-5.7) per 1,000 person-years among those without the syndrome. The mortality rate ratio among children with congenital Zika syndrome, compared with those without it, was 11.3 (95% CI, 10.2-12.4). Data analysis also showed that the 3,308 children with the syndrome were born to mothers who were younger and had fewer years of study when compared to the mothers of their 11,477,907 counterparts without the syndrome.
“If the children survived the first month of life, they had a greater chance of surviving during childhood, because the mortality rates drop,” said Dr. Paixão. “In children with congenital Zika syndrome, this rate also drops, but slowly. The more we stratified by period – neonatal, post neonatal, and the period from the first to the third year of life – the more we saw the relative risk increase. After the first year of life, children with the syndrome were almost 22 times more likely to die compared to children without it. It was hard to believe the data.” Dr. Paixão added that the mortality observed in this study is comparable with the findings of previous studies.
In addition to the large sample size – more than 11 million children – another unique aspect of the work was the comparison with healthy live births. “Previous studies didn’t have this comparison group,” Dr. said Paixão.
Perhaps the major challenge of the study, Dr. Paixão explained, was the fragmentation of the data. “In Brazil we have high-quality data systems, but they are not interconnected. We have a database with all live births, another with mortality records, and another with all children with congenital Zika syndrome. The first big challenge was putting all this information together.”
The solution found by the researchers was to use data linkage – bringing information about the same person from different data banks to create a richer dataset. Basically, they linked the data from the live births registry with the deaths that occurred in the studied age group plus around 18,000 children with congenital Zika syndrome. This was done, said Dr. Paixão, by choosing some identifying variables (such as mother’s name, address, and age) and using an algorithm that evaluates the probability that the “N” in one database is the same person in another database.
“This is expensive, complex, [and] involves super-powerful computers and a lot of researchers,” she said.
The impressive mortality data for children with congenital Zika syndrome obtained by the group of researchers made it inevitable to think about how the country should address this terrible legacy.
“The first and most important recommendation is that the country needs to invest in primary care, so that women don’t get Zika during pregnancy and children aren’t at risk of getting the syndrome,” said Dr. Paixão.
As for the affected population, she highlighted the need to deepen the understanding of the syndrome’s natural history to improve survival and quality of life of affected children and their families. One possibility that was recently discussed by the group of researchers is to carry out a study on the causes of hospitalization of children with the syndrome to develop appropriate protocols and procedures that reduce admissions and death in this population.
A version of this article first appeared on Medscape.com.
About 80% of people infected with Zika virus show no symptoms, and that’s particularly problematic during pregnancy. The infection can cause birth defects and is the origin of numerous cases of microcephaly and other neurologic impairments.
The large amount of Aedes aegypti mosquitoes in Brazilian cities, in addition to social and political problems, facilitated the spread of Zika to the point that the country recorded its highest number of congenital Zika syndrome notifications from 2015 to 2018. Since then, researchers have investigated the extent of the problem.
One of the most compelling findings about the dramatic legacy of Zika in Brazil was published Feb. 24 in The New England Journal of Medicine: After tracking 11,481,215 children born alive in Brazil up to 36 months of age between the years 2015 and 2018, the researchers found that the mortality rate was about 12 times higher among children with congenital Zika syndrome in comparison to children without the syndrome. The study is the first to follow children with congenital Zika syndrome for 3 years and to report mortality in this group.
“This difference persisted throughout the first 3 years of life,” Enny S. Paixão, PhD, of the London School of Hygiene and Tropical Medicine, and Fiocruz-Bahia’s Instituto Gonçalo Moniz, in Brazil, said in an interview.
At the end of the study period, the mortality rate was 52.6 deaths (95% confidence interval, 47.6-58.0) per 1,000 person-years among children with congenital Zika syndrome and 5.6 deaths (95% CI, 5.6-5.7) per 1,000 person-years among those without the syndrome. The mortality rate ratio among children with congenital Zika syndrome, compared with those without it, was 11.3 (95% CI, 10.2-12.4). Data analysis also showed that the 3,308 children with the syndrome were born to mothers who were younger and had fewer years of study when compared to the mothers of their 11,477,907 counterparts without the syndrome.
“If the children survived the first month of life, they had a greater chance of surviving during childhood, because the mortality rates drop,” said Dr. Paixão. “In children with congenital Zika syndrome, this rate also drops, but slowly. The more we stratified by period – neonatal, post neonatal, and the period from the first to the third year of life – the more we saw the relative risk increase. After the first year of life, children with the syndrome were almost 22 times more likely to die compared to children without it. It was hard to believe the data.” Dr. Paixão added that the mortality observed in this study is comparable with the findings of previous studies.
In addition to the large sample size – more than 11 million children – another unique aspect of the work was the comparison with healthy live births. “Previous studies didn’t have this comparison group,” Dr. said Paixão.
Perhaps the major challenge of the study, Dr. Paixão explained, was the fragmentation of the data. “In Brazil we have high-quality data systems, but they are not interconnected. We have a database with all live births, another with mortality records, and another with all children with congenital Zika syndrome. The first big challenge was putting all this information together.”
The solution found by the researchers was to use data linkage – bringing information about the same person from different data banks to create a richer dataset. Basically, they linked the data from the live births registry with the deaths that occurred in the studied age group plus around 18,000 children with congenital Zika syndrome. This was done, said Dr. Paixão, by choosing some identifying variables (such as mother’s name, address, and age) and using an algorithm that evaluates the probability that the “N” in one database is the same person in another database.
“This is expensive, complex, [and] involves super-powerful computers and a lot of researchers,” she said.
The impressive mortality data for children with congenital Zika syndrome obtained by the group of researchers made it inevitable to think about how the country should address this terrible legacy.
“The first and most important recommendation is that the country needs to invest in primary care, so that women don’t get Zika during pregnancy and children aren’t at risk of getting the syndrome,” said Dr. Paixão.
As for the affected population, she highlighted the need to deepen the understanding of the syndrome’s natural history to improve survival and quality of life of affected children and their families. One possibility that was recently discussed by the group of researchers is to carry out a study on the causes of hospitalization of children with the syndrome to develop appropriate protocols and procedures that reduce admissions and death in this population.
A version of this article first appeared on Medscape.com.
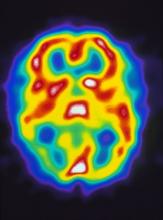
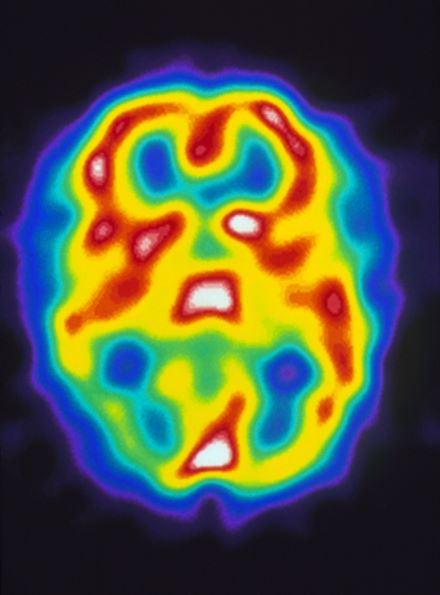
Pulsating unilateral headache and nausea
The patient is probably experiencing migraine without aura. Migraines are a complex disorder characterized by recurrent episodes of headache, most often unilateral. These attacks are associated with symptoms related to the central nervous system. Approximately 15% of patients with migraine experience aura (temporary visual, sensory, speech, or other motor disturbances). More research is needed to determine whether migraine with and without aura are potentially different diagnostic entities.
Classic migraine is a clinical diagnosis. When patients experience migraine symptoms routinely, however, it is important to consider whether these signs and symptoms can be accounted for by another diagnosis. Neuroimaging and, less commonly, lumbar puncture may be indicated in some presentations; red flags that call for additional workup are captured in the acronym SNOOP: systemic symptoms, neurologic symptoms, onset is acute, older patients, and previous history. In addition, classic migraine should be distinguished from common headaches as well as rare subtypes of migraine. For instance, hemiplegic migraine typically presents with temporary unilateral hemiparesis, sometimes accompanied by speech disturbance, and may be inherited (familial hemiplegic migraine). Basilar migraine is another rare subtype of migraine that manifests with signs of vertebrobasilar insufficiency. Attacks of chronic paroxysmal hemicrania are unilateral (just as migraines can be in about half of all cases); they are marked by their high intensity but short duration, and are accompanied by same-side facial autonomic symptoms (eg, tearing, congestion). Such patients' history and presentation do not fulfill criteria put forth by the American Headache Society (AHS) for chronic migraine, which specify having headaches 15 or more days per month for more than 3 months, and in which on at least 8 days per month those attacks are consistent with migraine or are relieved by a triptan or ergot derivative.
Migraine management must be personalized for each patient and is often associated with a marked trial-and-error period. Migraine without aura and migraine with aura are treated via similar approaches. AHS guidelines include several medications that may be effective in mitigating migraines, including both migraine-specific agents (ergotamine, ergotamine derivatives, and lasmiditan), and nonspecific agents (NSAIDs, combination analgesics, intravenous magnesium, isometheptene-containing compounds, and antiemetics). Triptans represent first-line acute treatment for migraine, but the FDA has approved five acute migraine treatments in total: celecoxib, lasmiditan, remote electrical neuromodulation (REN), rimegepant, and ubrogepant. For moderate or severe attacks, migraine-specific agents are recommended: beyond triptans, dihydroergotamine (DHE), small-molecule calcitonin gene-related peptide receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Patients should limit medication use to an average of two headache days per week, and those who do not find relief within these parameters are candidates for preventive migraine treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
The patient is probably experiencing migraine without aura. Migraines are a complex disorder characterized by recurrent episodes of headache, most often unilateral. These attacks are associated with symptoms related to the central nervous system. Approximately 15% of patients with migraine experience aura (temporary visual, sensory, speech, or other motor disturbances). More research is needed to determine whether migraine with and without aura are potentially different diagnostic entities.
Classic migraine is a clinical diagnosis. When patients experience migraine symptoms routinely, however, it is important to consider whether these signs and symptoms can be accounted for by another diagnosis. Neuroimaging and, less commonly, lumbar puncture may be indicated in some presentations; red flags that call for additional workup are captured in the acronym SNOOP: systemic symptoms, neurologic symptoms, onset is acute, older patients, and previous history. In addition, classic migraine should be distinguished from common headaches as well as rare subtypes of migraine. For instance, hemiplegic migraine typically presents with temporary unilateral hemiparesis, sometimes accompanied by speech disturbance, and may be inherited (familial hemiplegic migraine). Basilar migraine is another rare subtype of migraine that manifests with signs of vertebrobasilar insufficiency. Attacks of chronic paroxysmal hemicrania are unilateral (just as migraines can be in about half of all cases); they are marked by their high intensity but short duration, and are accompanied by same-side facial autonomic symptoms (eg, tearing, congestion). Such patients' history and presentation do not fulfill criteria put forth by the American Headache Society (AHS) for chronic migraine, which specify having headaches 15 or more days per month for more than 3 months, and in which on at least 8 days per month those attacks are consistent with migraine or are relieved by a triptan or ergot derivative.
Migraine management must be personalized for each patient and is often associated with a marked trial-and-error period. Migraine without aura and migraine with aura are treated via similar approaches. AHS guidelines include several medications that may be effective in mitigating migraines, including both migraine-specific agents (ergotamine, ergotamine derivatives, and lasmiditan), and nonspecific agents (NSAIDs, combination analgesics, intravenous magnesium, isometheptene-containing compounds, and antiemetics). Triptans represent first-line acute treatment for migraine, but the FDA has approved five acute migraine treatments in total: celecoxib, lasmiditan, remote electrical neuromodulation (REN), rimegepant, and ubrogepant. For moderate or severe attacks, migraine-specific agents are recommended: beyond triptans, dihydroergotamine (DHE), small-molecule calcitonin gene-related peptide receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Patients should limit medication use to an average of two headache days per week, and those who do not find relief within these parameters are candidates for preventive migraine treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
The patient is probably experiencing migraine without aura. Migraines are a complex disorder characterized by recurrent episodes of headache, most often unilateral. These attacks are associated with symptoms related to the central nervous system. Approximately 15% of patients with migraine experience aura (temporary visual, sensory, speech, or other motor disturbances). More research is needed to determine whether migraine with and without aura are potentially different diagnostic entities.
Classic migraine is a clinical diagnosis. When patients experience migraine symptoms routinely, however, it is important to consider whether these signs and symptoms can be accounted for by another diagnosis. Neuroimaging and, less commonly, lumbar puncture may be indicated in some presentations; red flags that call for additional workup are captured in the acronym SNOOP: systemic symptoms, neurologic symptoms, onset is acute, older patients, and previous history. In addition, classic migraine should be distinguished from common headaches as well as rare subtypes of migraine. For instance, hemiplegic migraine typically presents with temporary unilateral hemiparesis, sometimes accompanied by speech disturbance, and may be inherited (familial hemiplegic migraine). Basilar migraine is another rare subtype of migraine that manifests with signs of vertebrobasilar insufficiency. Attacks of chronic paroxysmal hemicrania are unilateral (just as migraines can be in about half of all cases); they are marked by their high intensity but short duration, and are accompanied by same-side facial autonomic symptoms (eg, tearing, congestion). Such patients' history and presentation do not fulfill criteria put forth by the American Headache Society (AHS) for chronic migraine, which specify having headaches 15 or more days per month for more than 3 months, and in which on at least 8 days per month those attacks are consistent with migraine or are relieved by a triptan or ergot derivative.
Migraine management must be personalized for each patient and is often associated with a marked trial-and-error period. Migraine without aura and migraine with aura are treated via similar approaches. AHS guidelines include several medications that may be effective in mitigating migraines, including both migraine-specific agents (ergotamine, ergotamine derivatives, and lasmiditan), and nonspecific agents (NSAIDs, combination analgesics, intravenous magnesium, isometheptene-containing compounds, and antiemetics). Triptans represent first-line acute treatment for migraine, but the FDA has approved five acute migraine treatments in total: celecoxib, lasmiditan, remote electrical neuromodulation (REN), rimegepant, and ubrogepant. For moderate or severe attacks, migraine-specific agents are recommended: beyond triptans, dihydroergotamine (DHE), small-molecule calcitonin gene-related peptide receptor antagonists (gepants), and selective serotonin (5-HT1F) receptor agonists (ditans). Patients should limit medication use to an average of two headache days per week, and those who do not find relief within these parameters are candidates for preventive migraine treatment.
Angeliki Vgontzas, MD, Instructor, Department of Neurology, Harvard Medical School; Associate Neurologist, Department of Neurology, Brigham and Women's Hospital/Brigham and Women's Faulkner Hospital, Boston, Massachusetts.
Angeliki Vgontzas, MD, has disclosed no relevant financial relationships.
A 22-year-old woman presents with a pulsating unilateral headache (right side) and is very nauseated. The patient reports that since childhood, she has been prone to headaches, with no other significant medical history. Over the past year or so, the headaches have been occurring about once or twice a month, have taken on a throbbing quality, and usually last for several days without relief from nonsteroidal anti-inflammatory drugs (NSAIDs). While taking part in a clinical trial, the patient undergoes a single photon emission CT scan which shows reduced blood flow (lower left).
MRI far safer than CT for guiding radiotherapy in prostate cancer
shows a study from the University of California, Los Angeles.
Among the first 100 men in the phase 3 MIRAGE trial (Magnetic Resonance Imaging–Guided Versus Computed Tomography–Guided Stereotactic Body Radiotherapy for Prostate Cancer), MRI guidance more than halved the incidence of grade 2 or higher physician-reported genitourinary toxicity within 90 days of the procedure, which fell from 47.1% with CT to 22.4% with MRI.
While 13.7% of men had gastrointestinal complications with CT guidance, there wasn’t a single case in the MRI arm. The findings were presented Feb. 17 at the American Society of Clinical Oncology Genitourinary Cancers Symposium.
The investigators thought they’d need 300 men to detect a safety difference, but the results are so strong that they’ve scaled back enrollment to 154. In the meantime, MRI-guided SBRT is now offered routinely to men with localized prostate cancer at UCLA.
“Our final results are expected later this year, but we are extremely optimistic by what we’re seeing, and hope this technology will soon begin to offer men undergoing radiotherapy for prostate cancer better outcomes,” said lead investigator Amar Upadhyaya Kishan, MD, a genitourinary oncology radiologist, in a UCLA press release.
The better outcomes are caused by the enhanced imaging capabilities of MRI, including real time tracking and automatic beam shutoff when the prostate moves too far outside of the treatment boundary, Dr. Kishan explained on Twitter.
Because of the extra precision, “we felt we could safely reduce the planning margins to only 2 mm” with MRI, down from 4 mm with CT. It translated to smaller treatment volumes and less collateral tissue damage, he said.
Across the first 100 subjects, 49 men were randomized to MRI-guided SBRT and 51 to SBRT with CT guidance. Their prostates and proximal seminal vesicles were dosed with 40 Gy of radiation in five fractions. Rectal spacing and nodal irradiation were at physician discretion.
Patients in the MRI arm also reported significantly fewer urinary symptoms, including urgency, incontinence, burning sensations, and bowel dysfunction, such as pain, diarrhea, and obstruction, among others, at 1 month with MRI guidance. The differences diminished at 3 months with adverse event management in the CT arm.
Lymph nodes were irradiated in 29% of men in the CT group versus 20% in the MRI arm, and 37% of the CT group versus 27% with MRI had rectal spacing.
Baseline gland size was a median of 39 mL in both groups. Baseline International Prostate Symptom Scores were a median of 8 points in the MRI group but 5 points in the CT arm.
The work was funded by UCLA, among others. Dr. Kishan has ownership interests in ViewRay, the company that makes the MRI-guiding technology used in the trial, and reported honoraria and research funding from the company.
shows a study from the University of California, Los Angeles.
Among the first 100 men in the phase 3 MIRAGE trial (Magnetic Resonance Imaging–Guided Versus Computed Tomography–Guided Stereotactic Body Radiotherapy for Prostate Cancer), MRI guidance more than halved the incidence of grade 2 or higher physician-reported genitourinary toxicity within 90 days of the procedure, which fell from 47.1% with CT to 22.4% with MRI.
While 13.7% of men had gastrointestinal complications with CT guidance, there wasn’t a single case in the MRI arm. The findings were presented Feb. 17 at the American Society of Clinical Oncology Genitourinary Cancers Symposium.
The investigators thought they’d need 300 men to detect a safety difference, but the results are so strong that they’ve scaled back enrollment to 154. In the meantime, MRI-guided SBRT is now offered routinely to men with localized prostate cancer at UCLA.
“Our final results are expected later this year, but we are extremely optimistic by what we’re seeing, and hope this technology will soon begin to offer men undergoing radiotherapy for prostate cancer better outcomes,” said lead investigator Amar Upadhyaya Kishan, MD, a genitourinary oncology radiologist, in a UCLA press release.
The better outcomes are caused by the enhanced imaging capabilities of MRI, including real time tracking and automatic beam shutoff when the prostate moves too far outside of the treatment boundary, Dr. Kishan explained on Twitter.
Because of the extra precision, “we felt we could safely reduce the planning margins to only 2 mm” with MRI, down from 4 mm with CT. It translated to smaller treatment volumes and less collateral tissue damage, he said.
Across the first 100 subjects, 49 men were randomized to MRI-guided SBRT and 51 to SBRT with CT guidance. Their prostates and proximal seminal vesicles were dosed with 40 Gy of radiation in five fractions. Rectal spacing and nodal irradiation were at physician discretion.
Patients in the MRI arm also reported significantly fewer urinary symptoms, including urgency, incontinence, burning sensations, and bowel dysfunction, such as pain, diarrhea, and obstruction, among others, at 1 month with MRI guidance. The differences diminished at 3 months with adverse event management in the CT arm.
Lymph nodes were irradiated in 29% of men in the CT group versus 20% in the MRI arm, and 37% of the CT group versus 27% with MRI had rectal spacing.
Baseline gland size was a median of 39 mL in both groups. Baseline International Prostate Symptom Scores were a median of 8 points in the MRI group but 5 points in the CT arm.
The work was funded by UCLA, among others. Dr. Kishan has ownership interests in ViewRay, the company that makes the MRI-guiding technology used in the trial, and reported honoraria and research funding from the company.
shows a study from the University of California, Los Angeles.
Among the first 100 men in the phase 3 MIRAGE trial (Magnetic Resonance Imaging–Guided Versus Computed Tomography–Guided Stereotactic Body Radiotherapy for Prostate Cancer), MRI guidance more than halved the incidence of grade 2 or higher physician-reported genitourinary toxicity within 90 days of the procedure, which fell from 47.1% with CT to 22.4% with MRI.
While 13.7% of men had gastrointestinal complications with CT guidance, there wasn’t a single case in the MRI arm. The findings were presented Feb. 17 at the American Society of Clinical Oncology Genitourinary Cancers Symposium.
The investigators thought they’d need 300 men to detect a safety difference, but the results are so strong that they’ve scaled back enrollment to 154. In the meantime, MRI-guided SBRT is now offered routinely to men with localized prostate cancer at UCLA.
“Our final results are expected later this year, but we are extremely optimistic by what we’re seeing, and hope this technology will soon begin to offer men undergoing radiotherapy for prostate cancer better outcomes,” said lead investigator Amar Upadhyaya Kishan, MD, a genitourinary oncology radiologist, in a UCLA press release.
The better outcomes are caused by the enhanced imaging capabilities of MRI, including real time tracking and automatic beam shutoff when the prostate moves too far outside of the treatment boundary, Dr. Kishan explained on Twitter.
Because of the extra precision, “we felt we could safely reduce the planning margins to only 2 mm” with MRI, down from 4 mm with CT. It translated to smaller treatment volumes and less collateral tissue damage, he said.
Across the first 100 subjects, 49 men were randomized to MRI-guided SBRT and 51 to SBRT with CT guidance. Their prostates and proximal seminal vesicles were dosed with 40 Gy of radiation in five fractions. Rectal spacing and nodal irradiation were at physician discretion.
Patients in the MRI arm also reported significantly fewer urinary symptoms, including urgency, incontinence, burning sensations, and bowel dysfunction, such as pain, diarrhea, and obstruction, among others, at 1 month with MRI guidance. The differences diminished at 3 months with adverse event management in the CT arm.
Lymph nodes were irradiated in 29% of men in the CT group versus 20% in the MRI arm, and 37% of the CT group versus 27% with MRI had rectal spacing.
Baseline gland size was a median of 39 mL in both groups. Baseline International Prostate Symptom Scores were a median of 8 points in the MRI group but 5 points in the CT arm.
The work was funded by UCLA, among others. Dr. Kishan has ownership interests in ViewRay, the company that makes the MRI-guiding technology used in the trial, and reported honoraria and research funding from the company.
FROM ASCO GU 2022
Your heart doesn’t like peas any more than you do
Big Vegetable has lied to us all
Hear this, children of the world: Your parents have betrayed you. They tell you day in and day out that vegetables are necessary, that they’re healthy, that you need them, but it is not the truth. Behind their foul taste is nothing but empty lies.
Okay, before we get a full-blown child rebellion on our hands, let’s reel things in. Eating vegetables has many benefits, and will help prevent many nasty medical conditions, such as diabetes or cancer. However, cardiovascular disease is not among them.
For their study published in Frontiers in Nutrition, researchers analyzed the diet, lifestyle, and medical history of nearly 400,000 U.K. adults over a 5-year period, finding that 4.5% developed heart disease and that the average adult consumed about 5 tablespoons of vegetables per day. Those who consumed the most vegetables had a reduction in heart disease incidence of about 15%, compared with those who ate the least.
Hang on, you’re thinking, we just said that vegetables didn’t prevent cardiovascular disease. But the data show otherwise! Ah, but the data are unadjusted. Once the researchers took socioeconomic status, information level, and general lifestyle into account, that benefit disappeared almost completely. The benefit seems to come not from the vegetables themselves, but from being able to afford better food and medical care in general.
The researchers were quick to note the other benefits of eating vegetables, and that people should probably keep eating those five servings a day. But we’re onto you, scientists. You can’t fool us with your vegetable-based lies. Unless we’re talking about pizza. Pizza is the best vegetable.
The good old days of surgery?
Modern surgical instruments, techniques, and technological innovations are amazing. It’s hard to imagine what surgery was like before laparoscopes came along, or x-ray machines, or even anesthesia. But those days weren’t really that long ago. Modern anesthesia, after all, dates back to just 1846. We’ve got socks almost that old.
But suppose we go back even further … say 5,300 years. Older than the oldest sock. Scientists studying a funerary chamber in Burgos, Spain, which was built in the 4th millennium B.C., have come across what looks like “the first known radical mastoidectomy in the history of humankind,” Sonia Díaz-Navarro of the University of Valladolid (Spain) and associates wrote in Scientific Reports.
One of the skulls they uncovered shows signs of trepanation. “Despite the [evidence] of cut marks, it is difficult to conclude the type of tool used to remove the bone tissue, most likely a sharp instrument with a circular movement,” they investigators said.
What is clear, though, is that the patient survived the surgery, because there is evidence of bone regeneration at the surgical sites. Sites? “Based on the differences in bone remodelling between the two temporals, it appears that the procedure was first conducted on the right ear, due to an ear pathology sufficiently alarming to require an intervention, which this prehistoric woman survived,” they explained.
The same procedure was then performed on the left ear, “but whether this was performed shortly after the right ear, or several months or even years later can’t be concluded from the existing evidence,” IFL Science reported.
Located nearby was a small section of tree bark with some scratches on it. That, ladies and gentlemen, was the first prior authorization form.
I hate that song, with reason
Do you have a favorite song? You may have a million reasons for loving that song. And past research can tell you why. But it’s only in a recent study that researchers were able to tell you why you dislike a song. And you know the song we’re talking about.
Dislike breaks down into three major categories of rationale: subject-related reasons (how the song makes you feel emotionally and/or physically), object-related reasons (the lyrics or composition), and social reasons (do you relate to this?). Researchers at the Max Planck Institute for Empirical Aesthetics in Frankfurt, Germany, interviewed 21 participants and asked them to come up with a prepared list of music that they disliked and why they didn’t like it. And there was a lot that they didn’t like: 277 dislikes worth, to be exact.
“The most often mentioned type of dislike was musical style, followed by artist and genre,” senior author Julia Merrill explained on Eurekalert. Just over 40% of those rationales for not liking the music just had to do with the music itself, but 85% involved the music combined with one of the other categories.
Social reasoning played a big part in dislike. If the listener didn’t feel like a part of the target in-group for the music or the music didn’t have the same social values as those of the listener, it had an impact on dislike, they said.
But our dislike of certain types of music doesn’t just separate us from people in a negative way. Looking at the dislike of certain types of music helps us define our terms of having good taste, the researchers explained. Saying that one type of music is better than another can bring us closer with like-minded people and becomes a piece of how we identify ourselves. Cue the music snobs.
So if you can blast Barry Manilow but can’t bring yourself to play the Rolling Stones, there’s a reason for that. And if you love Aretha Franklin but not Frank Sinatra, there’s a reason for that, too. It’s all very personal. Just as music is meant to be.
Big Vegetable has lied to us all
Hear this, children of the world: Your parents have betrayed you. They tell you day in and day out that vegetables are necessary, that they’re healthy, that you need them, but it is not the truth. Behind their foul taste is nothing but empty lies.
Okay, before we get a full-blown child rebellion on our hands, let’s reel things in. Eating vegetables has many benefits, and will help prevent many nasty medical conditions, such as diabetes or cancer. However, cardiovascular disease is not among them.
For their study published in Frontiers in Nutrition, researchers analyzed the diet, lifestyle, and medical history of nearly 400,000 U.K. adults over a 5-year period, finding that 4.5% developed heart disease and that the average adult consumed about 5 tablespoons of vegetables per day. Those who consumed the most vegetables had a reduction in heart disease incidence of about 15%, compared with those who ate the least.
Hang on, you’re thinking, we just said that vegetables didn’t prevent cardiovascular disease. But the data show otherwise! Ah, but the data are unadjusted. Once the researchers took socioeconomic status, information level, and general lifestyle into account, that benefit disappeared almost completely. The benefit seems to come not from the vegetables themselves, but from being able to afford better food and medical care in general.
The researchers were quick to note the other benefits of eating vegetables, and that people should probably keep eating those five servings a day. But we’re onto you, scientists. You can’t fool us with your vegetable-based lies. Unless we’re talking about pizza. Pizza is the best vegetable.
The good old days of surgery?
Modern surgical instruments, techniques, and technological innovations are amazing. It’s hard to imagine what surgery was like before laparoscopes came along, or x-ray machines, or even anesthesia. But those days weren’t really that long ago. Modern anesthesia, after all, dates back to just 1846. We’ve got socks almost that old.
But suppose we go back even further … say 5,300 years. Older than the oldest sock. Scientists studying a funerary chamber in Burgos, Spain, which was built in the 4th millennium B.C., have come across what looks like “the first known radical mastoidectomy in the history of humankind,” Sonia Díaz-Navarro of the University of Valladolid (Spain) and associates wrote in Scientific Reports.
One of the skulls they uncovered shows signs of trepanation. “Despite the [evidence] of cut marks, it is difficult to conclude the type of tool used to remove the bone tissue, most likely a sharp instrument with a circular movement,” they investigators said.
What is clear, though, is that the patient survived the surgery, because there is evidence of bone regeneration at the surgical sites. Sites? “Based on the differences in bone remodelling between the two temporals, it appears that the procedure was first conducted on the right ear, due to an ear pathology sufficiently alarming to require an intervention, which this prehistoric woman survived,” they explained.
The same procedure was then performed on the left ear, “but whether this was performed shortly after the right ear, or several months or even years later can’t be concluded from the existing evidence,” IFL Science reported.
Located nearby was a small section of tree bark with some scratches on it. That, ladies and gentlemen, was the first prior authorization form.
I hate that song, with reason
Do you have a favorite song? You may have a million reasons for loving that song. And past research can tell you why. But it’s only in a recent study that researchers were able to tell you why you dislike a song. And you know the song we’re talking about.
Dislike breaks down into three major categories of rationale: subject-related reasons (how the song makes you feel emotionally and/or physically), object-related reasons (the lyrics or composition), and social reasons (do you relate to this?). Researchers at the Max Planck Institute for Empirical Aesthetics in Frankfurt, Germany, interviewed 21 participants and asked them to come up with a prepared list of music that they disliked and why they didn’t like it. And there was a lot that they didn’t like: 277 dislikes worth, to be exact.
“The most often mentioned type of dislike was musical style, followed by artist and genre,” senior author Julia Merrill explained on Eurekalert. Just over 40% of those rationales for not liking the music just had to do with the music itself, but 85% involved the music combined with one of the other categories.
Social reasoning played a big part in dislike. If the listener didn’t feel like a part of the target in-group for the music or the music didn’t have the same social values as those of the listener, it had an impact on dislike, they said.
But our dislike of certain types of music doesn’t just separate us from people in a negative way. Looking at the dislike of certain types of music helps us define our terms of having good taste, the researchers explained. Saying that one type of music is better than another can bring us closer with like-minded people and becomes a piece of how we identify ourselves. Cue the music snobs.
So if you can blast Barry Manilow but can’t bring yourself to play the Rolling Stones, there’s a reason for that. And if you love Aretha Franklin but not Frank Sinatra, there’s a reason for that, too. It’s all very personal. Just as music is meant to be.
Big Vegetable has lied to us all
Hear this, children of the world: Your parents have betrayed you. They tell you day in and day out that vegetables are necessary, that they’re healthy, that you need them, but it is not the truth. Behind their foul taste is nothing but empty lies.
Okay, before we get a full-blown child rebellion on our hands, let’s reel things in. Eating vegetables has many benefits, and will help prevent many nasty medical conditions, such as diabetes or cancer. However, cardiovascular disease is not among them.
For their study published in Frontiers in Nutrition, researchers analyzed the diet, lifestyle, and medical history of nearly 400,000 U.K. adults over a 5-year period, finding that 4.5% developed heart disease and that the average adult consumed about 5 tablespoons of vegetables per day. Those who consumed the most vegetables had a reduction in heart disease incidence of about 15%, compared with those who ate the least.
Hang on, you’re thinking, we just said that vegetables didn’t prevent cardiovascular disease. But the data show otherwise! Ah, but the data are unadjusted. Once the researchers took socioeconomic status, information level, and general lifestyle into account, that benefit disappeared almost completely. The benefit seems to come not from the vegetables themselves, but from being able to afford better food and medical care in general.
The researchers were quick to note the other benefits of eating vegetables, and that people should probably keep eating those five servings a day. But we’re onto you, scientists. You can’t fool us with your vegetable-based lies. Unless we’re talking about pizza. Pizza is the best vegetable.
The good old days of surgery?
Modern surgical instruments, techniques, and technological innovations are amazing. It’s hard to imagine what surgery was like before laparoscopes came along, or x-ray machines, or even anesthesia. But those days weren’t really that long ago. Modern anesthesia, after all, dates back to just 1846. We’ve got socks almost that old.
But suppose we go back even further … say 5,300 years. Older than the oldest sock. Scientists studying a funerary chamber in Burgos, Spain, which was built in the 4th millennium B.C., have come across what looks like “the first known radical mastoidectomy in the history of humankind,” Sonia Díaz-Navarro of the University of Valladolid (Spain) and associates wrote in Scientific Reports.
One of the skulls they uncovered shows signs of trepanation. “Despite the [evidence] of cut marks, it is difficult to conclude the type of tool used to remove the bone tissue, most likely a sharp instrument with a circular movement,” they investigators said.
What is clear, though, is that the patient survived the surgery, because there is evidence of bone regeneration at the surgical sites. Sites? “Based on the differences in bone remodelling between the two temporals, it appears that the procedure was first conducted on the right ear, due to an ear pathology sufficiently alarming to require an intervention, which this prehistoric woman survived,” they explained.
The same procedure was then performed on the left ear, “but whether this was performed shortly after the right ear, or several months or even years later can’t be concluded from the existing evidence,” IFL Science reported.
Located nearby was a small section of tree bark with some scratches on it. That, ladies and gentlemen, was the first prior authorization form.
I hate that song, with reason
Do you have a favorite song? You may have a million reasons for loving that song. And past research can tell you why. But it’s only in a recent study that researchers were able to tell you why you dislike a song. And you know the song we’re talking about.
Dislike breaks down into three major categories of rationale: subject-related reasons (how the song makes you feel emotionally and/or physically), object-related reasons (the lyrics or composition), and social reasons (do you relate to this?). Researchers at the Max Planck Institute for Empirical Aesthetics in Frankfurt, Germany, interviewed 21 participants and asked them to come up with a prepared list of music that they disliked and why they didn’t like it. And there was a lot that they didn’t like: 277 dislikes worth, to be exact.
“The most often mentioned type of dislike was musical style, followed by artist and genre,” senior author Julia Merrill explained on Eurekalert. Just over 40% of those rationales for not liking the music just had to do with the music itself, but 85% involved the music combined with one of the other categories.
Social reasoning played a big part in dislike. If the listener didn’t feel like a part of the target in-group for the music or the music didn’t have the same social values as those of the listener, it had an impact on dislike, they said.
But our dislike of certain types of music doesn’t just separate us from people in a negative way. Looking at the dislike of certain types of music helps us define our terms of having good taste, the researchers explained. Saying that one type of music is better than another can bring us closer with like-minded people and becomes a piece of how we identify ourselves. Cue the music snobs.
So if you can blast Barry Manilow but can’t bring yourself to play the Rolling Stones, there’s a reason for that. And if you love Aretha Franklin but not Frank Sinatra, there’s a reason for that, too. It’s all very personal. Just as music is meant to be.
More exercise for people with hemophilia, experts advise
Clinicians should do more to encourage people with hemophilia to undertake regular physical activity and sporting activities, a panel of Italian experts has advised.
they wrote in a consensus paper published in Blood Transfusion.
Physical activity is not only recommended by the World Federation of Hemophilia for people with this bleeding disorder, but also recommended for everyone, depending on their age, by the World Health Organization.
People with hemophilia “are not exempt” from the WHO recommendations, noted Dr. Chiara Biasoli of the unit of transfusion medicine and Centre for Inherited Bleeding Disorders, Maurizio Bufalini Hospital in Cesena, Italy, and fellow expert panel members.
MEMO expert consensus project
To help clinicians decide when and how to recommend physical exercise to people with hemophilia, Dr. Biasoli and colleagues initiated the MEMO (Movement for Persons With Haemophilia) expert consensus project. The aim was to offer some clear practical guidance for routine practice.
The project began with a core group of 11 hemophilia experts meeting virtually in early 2020 because of the COVID-19 pandemic. The MEMO scientific committee, as they became known, formulated a set of consensus statements which they then put before members of the Italian Association of Hemophilia Centres, asking them to vote online on their level of agreement with each statement.
A modified Delphi approach was used to reach a consensus, with statements that scored 7 or higher on a 9-point rating scale moving forward into the next round of voting. A total of three voting rounds was made, which took into account the views of 40 experts, overall.
Overview of the MEMO consensus statements
The MEMO consensus statements cover three topic areas: the first four statements focus on the impact of hemophilia on movement, the next three give physical activity recommendations, and the final three look at choice and management of sporting activities.
Regarding the impact of hemophilia on movement, Dr. Biasoli and colleagues noted that “overweight and obesity are an increasing problem in PwH” and, due to their known association with poor physical health, urgently need to be addressed.
Perhaps “insufficient education by hematologists and other invoiced specialists” is at play, they suggested. Importantly, in children, “parents’ fears with consequent overprotection” may be contributing factors.
Not only is movement beneficial for improving joint function, they stated, but it’s also crucial to improving bone density and reducing the risk of joint bleeds.
Even people with inhibitors should be encouraged to be active more regularly, the expert panel said. This should of course be done with “particular caution and monitoring of the effectiveness of prophylaxis for the prevention of acute bleeding events, so that physical activity is conducted safely.”
The panel’s recommendations on sporting activities include the advice to work in a multidisciplinary team that involves hematologists, musculoskeletal specialists and specialists in sports medicine, with the latter helping decide on what sporting activity might be most appropriate. They also suggest that participation in sport should be encouraged from a young age, noting that the Canadian Hemophilia Society has issued some good tips in that regard.
Alongside the recommendations the MEMO expert panel has created four “pyramids of movement” to help clinicians visualize and discuss the recommendations with their patients.
“Physical activity can be considered as a low price intervention that can prevent/reduce the occurrence of chronic diseases and should be further encouraged,” Dr. Biasoli and fellow MEMO expert panel members concluded.
The members of the MEMO expert panel disclosed multiple financial ties with pharmaceutical companies, but none are relevant to the recommendations they made.
Clinicians should do more to encourage people with hemophilia to undertake regular physical activity and sporting activities, a panel of Italian experts has advised.
they wrote in a consensus paper published in Blood Transfusion.
Physical activity is not only recommended by the World Federation of Hemophilia for people with this bleeding disorder, but also recommended for everyone, depending on their age, by the World Health Organization.
People with hemophilia “are not exempt” from the WHO recommendations, noted Dr. Chiara Biasoli of the unit of transfusion medicine and Centre for Inherited Bleeding Disorders, Maurizio Bufalini Hospital in Cesena, Italy, and fellow expert panel members.
MEMO expert consensus project
To help clinicians decide when and how to recommend physical exercise to people with hemophilia, Dr. Biasoli and colleagues initiated the MEMO (Movement for Persons With Haemophilia) expert consensus project. The aim was to offer some clear practical guidance for routine practice.
The project began with a core group of 11 hemophilia experts meeting virtually in early 2020 because of the COVID-19 pandemic. The MEMO scientific committee, as they became known, formulated a set of consensus statements which they then put before members of the Italian Association of Hemophilia Centres, asking them to vote online on their level of agreement with each statement.
A modified Delphi approach was used to reach a consensus, with statements that scored 7 or higher on a 9-point rating scale moving forward into the next round of voting. A total of three voting rounds was made, which took into account the views of 40 experts, overall.
Overview of the MEMO consensus statements
The MEMO consensus statements cover three topic areas: the first four statements focus on the impact of hemophilia on movement, the next three give physical activity recommendations, and the final three look at choice and management of sporting activities.
Regarding the impact of hemophilia on movement, Dr. Biasoli and colleagues noted that “overweight and obesity are an increasing problem in PwH” and, due to their known association with poor physical health, urgently need to be addressed.
Perhaps “insufficient education by hematologists and other invoiced specialists” is at play, they suggested. Importantly, in children, “parents’ fears with consequent overprotection” may be contributing factors.
Not only is movement beneficial for improving joint function, they stated, but it’s also crucial to improving bone density and reducing the risk of joint bleeds.
Even people with inhibitors should be encouraged to be active more regularly, the expert panel said. This should of course be done with “particular caution and monitoring of the effectiveness of prophylaxis for the prevention of acute bleeding events, so that physical activity is conducted safely.”
The panel’s recommendations on sporting activities include the advice to work in a multidisciplinary team that involves hematologists, musculoskeletal specialists and specialists in sports medicine, with the latter helping decide on what sporting activity might be most appropriate. They also suggest that participation in sport should be encouraged from a young age, noting that the Canadian Hemophilia Society has issued some good tips in that regard.
Alongside the recommendations the MEMO expert panel has created four “pyramids of movement” to help clinicians visualize and discuss the recommendations with their patients.
“Physical activity can be considered as a low price intervention that can prevent/reduce the occurrence of chronic diseases and should be further encouraged,” Dr. Biasoli and fellow MEMO expert panel members concluded.
The members of the MEMO expert panel disclosed multiple financial ties with pharmaceutical companies, but none are relevant to the recommendations they made.
Clinicians should do more to encourage people with hemophilia to undertake regular physical activity and sporting activities, a panel of Italian experts has advised.
they wrote in a consensus paper published in Blood Transfusion.
Physical activity is not only recommended by the World Federation of Hemophilia for people with this bleeding disorder, but also recommended for everyone, depending on their age, by the World Health Organization.
People with hemophilia “are not exempt” from the WHO recommendations, noted Dr. Chiara Biasoli of the unit of transfusion medicine and Centre for Inherited Bleeding Disorders, Maurizio Bufalini Hospital in Cesena, Italy, and fellow expert panel members.
MEMO expert consensus project
To help clinicians decide when and how to recommend physical exercise to people with hemophilia, Dr. Biasoli and colleagues initiated the MEMO (Movement for Persons With Haemophilia) expert consensus project. The aim was to offer some clear practical guidance for routine practice.
The project began with a core group of 11 hemophilia experts meeting virtually in early 2020 because of the COVID-19 pandemic. The MEMO scientific committee, as they became known, formulated a set of consensus statements which they then put before members of the Italian Association of Hemophilia Centres, asking them to vote online on their level of agreement with each statement.
A modified Delphi approach was used to reach a consensus, with statements that scored 7 or higher on a 9-point rating scale moving forward into the next round of voting. A total of three voting rounds was made, which took into account the views of 40 experts, overall.
Overview of the MEMO consensus statements
The MEMO consensus statements cover three topic areas: the first four statements focus on the impact of hemophilia on movement, the next three give physical activity recommendations, and the final three look at choice and management of sporting activities.
Regarding the impact of hemophilia on movement, Dr. Biasoli and colleagues noted that “overweight and obesity are an increasing problem in PwH” and, due to their known association with poor physical health, urgently need to be addressed.
Perhaps “insufficient education by hematologists and other invoiced specialists” is at play, they suggested. Importantly, in children, “parents’ fears with consequent overprotection” may be contributing factors.
Not only is movement beneficial for improving joint function, they stated, but it’s also crucial to improving bone density and reducing the risk of joint bleeds.
Even people with inhibitors should be encouraged to be active more regularly, the expert panel said. This should of course be done with “particular caution and monitoring of the effectiveness of prophylaxis for the prevention of acute bleeding events, so that physical activity is conducted safely.”
The panel’s recommendations on sporting activities include the advice to work in a multidisciplinary team that involves hematologists, musculoskeletal specialists and specialists in sports medicine, with the latter helping decide on what sporting activity might be most appropriate. They also suggest that participation in sport should be encouraged from a young age, noting that the Canadian Hemophilia Society has issued some good tips in that regard.
Alongside the recommendations the MEMO expert panel has created four “pyramids of movement” to help clinicians visualize and discuss the recommendations with their patients.
“Physical activity can be considered as a low price intervention that can prevent/reduce the occurrence of chronic diseases and should be further encouraged,” Dr. Biasoli and fellow MEMO expert panel members concluded.
The members of the MEMO expert panel disclosed multiple financial ties with pharmaceutical companies, but none are relevant to the recommendations they made.
FROM BLOOD TRANSFUSION
NSCLC Management: Advanced by Science, Challenged by Human Barriers
New therapies developed to treat non-small cell lung cancer are not reaching all patients with this disease. Human-created barriers bar the way for those experiencing real or perceived stigma and those who reside in remote places or live on little income.
In this supplement, Abbie Begnaud, MD, FCCP, hones in on this human-created dichotomy and discusses the problems and possible solutions, along with diagnostic and treatment options.
New therapies developed to treat non-small cell lung cancer are not reaching all patients with this disease. Human-created barriers bar the way for those experiencing real or perceived stigma and those who reside in remote places or live on little income.
In this supplement, Abbie Begnaud, MD, FCCP, hones in on this human-created dichotomy and discusses the problems and possible solutions, along with diagnostic and treatment options.
New therapies developed to treat non-small cell lung cancer are not reaching all patients with this disease. Human-created barriers bar the way for those experiencing real or perceived stigma and those who reside in remote places or live on little income.
In this supplement, Abbie Begnaud, MD, FCCP, hones in on this human-created dichotomy and discusses the problems and possible solutions, along with diagnostic and treatment options.
2021 in Review: Key Trials in Type 2 Diabetes (T2D)
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
Ronald Goldenberg, MD, completed his residency in Internal Medicine in 1987 at the University of Toronto, and his fellowship in Endocrinology & Metabolism in 1989 at the University of Toronto. Dr. Goldenberg is a past chair of the Ontario Medical Association Section on Endocrinology & Metabolism and a previous President of the Toronto Diabetes Association. Dr. Goldenberg has been an investigator in a wide array of clinical trials in the areas of diabetes, hypertension, obesity, and dyslipidemia. His major areas of interest include clinical care of diabetes, obesity, dyslipidemia and thyroid disorders. He has been actively involved in Continuing Medical Education for the last 3 decades, with a strong focus on translating evidence-based medicine into practical patient care.
As a consultant endocrinologist with an area of interest that includes clinical care of diabetes, can you briefly tell us what the top 5 studies of 2021 were that are most likely to influence diabetes or obesity practice?
Dr. Goldenberg: 2021 was a banner year for clinicians managing diabetes and or obesity. There were many key trials that were published and or presented. In my mind, the most important ones that will really influence practice include the STEP program of semaglutide 2.4 mg once weekly in the management of overweight or obesity. There is the FIGARO-DKD and FIDELITY analysis of finerenone in patients with type 2 diabetes and chronic kidney disease. Other top studies include the SURPASS trials of a novel dual incretin agonist called tirzepatide, the EMPEROR-Preserved trial with empagliflozin and a pooled analysis of empagliflozin in both HFrEF and HFpEF trials, and the AMPLITUDE-O trial, which is a cardiovascular outcome trial with an exendin-based GLP-1 receptor agonist known as efpeglenatide.
2021 was definitely a landmark year in diabetes. Let's start with the STEP program with semaglutide 2.4. What were the important findings in these studies?
Dr. Goldenberg: STEP is the Phase III program for 2.4 milligrams once weekly in the management of overweight or obesity. The STEP program studies that have been published and/or presented in 2021 include four Phase IIIa trials STEP 1 through STEP 4, as well as three Phase IIIb trials, STEP 5, 6, and 8. They're all rather similar, as they each enrolled patients with overweight and/or obesity. Patients were up-titrated to semaglutide 2.4 milligrams once weekly, and the top-line summary across all of these trials is that patients randomized to semaglutide 2.4 mg once weekly lost 15% to 17% of their body weight amongst those that did not have diabetes, which is really a tremendous amount of weight loss for an anti-obesity drug. And even those with type 2 diabetes lost almost 10% of their body weight, which is pretty impressive given that patients with type 2 diabetes are often somewhat refractory to weight loss.
There was a high percentage of body weight loss across these trials, as roughly 86 to 90% of patients without diabetes achieved at least a 5% body weight loss and even in those with diabetes, almost 70% achieved a 5% loss in their body weight. As far as overall safety, the safety profile of semaglutide 2.4 mg once weekly was generally similar to the GLP-1 receptor agonist class. The most common side effects were gastrointestinal. Nausea occurred anywhere from 20% to 58% of patients, but it was generally transient. Very few people withdrew because of gastrointestinal side effects.
I think the key thing for clinicians to know about the STEP program is that it's the results of these trials that led to the FDA approving semaglutide 2.4 mg once weekly as a new agent for the management of overweight and/or obesity.
You mentioned FIGARO-DKD and FIDELITY with Finerenone. Can you talk more about the relevance of this data and summarize the key findings?
Dr. Goldenberg: Finerenone is a new selective non-steroidal mineralocorticoid receptor antagonist that interacts with the mineralocorticoid receptor in a different way compared to traditional steroidal mineralocorticoid receptor agonists. We know from pre-clinical data that this agent targets inflammation and fibrosis in both the kidney and the heart. The finerenone Phase III program focused on patients with type 2 diabetes and chronic kidney disease. In 2021, they published the FIGARO-DKD trial. This enrolled almost 7,500 patients with type 2 diabetes and an eGFR of 25 ml/min or more along with albuminuria.
The key result of this trial is the primary outcome of CV death non-fatal MI, non-fatal stroke, or hospitalization for heart failure was reduced by 13%. The number needed to treat after 3 ½ years was 47. The primary outcome was mainly driven by a reduction for hospitalization for heart failure. Key secondary outcomes included composite kidney outcomes, one of which was defined by a sustained eGFR reduction of 40% or more along with end-stage kidney disease or renal death. This did not quite reach statistical significance, but a more stringent outcome that included a reduction in eGFR of at least 57% or more was in fact reduced by 23%.
End-stage kidney disease was reduced by 36% in the FIGARO-DKD trial. Finerenone was well tolerated. Hyperkalemia occurred in 10.8% of patients on finerenone and 5.3% on placebo but it was quite unusual to have to stop finerenone because of hyperkalemia. Building on FIGARO-DKD in 2021 was a prespecified meta-analysis of two large Phase III trials, the FIGARO-DKD trial and also the previously published FIDELIO-DKD trial and in this pooled analysis the composite cardiovascular outcome was reduced by 14%. The benefit on cardiovascular events was independent of the baseline eGFR or urine albumin to creatinine ratio, as well as independent of the use of SGLT2 inhibitors or GLP-1 receptor agonists.
There was also a 23% reduction in a composite kidney outcome that used a sustained 57% reduction in eGFR as part of that outcome and essentially each component of the composite kidney outcome was reduced, including kidney failure, end-stage kidney disease, eGFR of less than 15 ml/min in addition to a ≥57% decrease in eGFR. And this kidney outcome showed a benefit irrespective of the use of SGLT2 inhibitor at baseline although the number of patients taking an SGLT2 inhibitor in this analysis was relatively small. So overall, the results of finerenone in 2021 support the use of this agent in patients with type 2 diabetes and chronic kidney disease to improve both cardiovascular and kidney outcomes.
Thank you for this insight. Regarding the SURPASS trials with tirzepatide, what is tirzepatide and what was its impact on glycemia and weight?
Dr. Goldenberg. My pleasure. Tirzepatide is a unique dual GIP/GLP-1 receptor agonist that has been formulated to be given as a once weekly injection. In 2021, we heard the first results from the Phase III program including SURPASS-1 through SURPASS-5. In these trials, patients were randomized to tirzepatide 5 mg, 10 mg, or 15 mg, and often compared to placebo or an active comparator. Across the SURPASS trials, the A1C reduction from baseline was between 1.9% to 2.6%. Up to 97% of patients on tirzepatide achieved a HbA1c of less than 7%, and up to 62% achieve a normal HbA1c of less than 5.7%.
In addition to these rather robust glycemic outcomes, there was excellent weight loss in the SURPASS program with the weight reduction ranging from 6 to 13 kg from baseline. Interestingly, in the SURPASS studies, tirzepatide showed superiority to semaglutide 1 mg and also superiority to titrated basal insulin. As far as safety, the side effect profile was similar to all GLP-1 receptor agonists with transient nausea being the most common side effect. Overall, tirzepatide will definitely add to our ability to treat our patients with type 2 diabetes with an incretin agent, and when this agent gets approved, hopefully, it will provide robust glycemic lowering and weight loss.
The fourth key study you mentioned is the EMPEROR-Preserved along with the EMPEROR-Pooled with the empagliflozin. What did they find in this analysis?
Dr. Goldenberg: The EMPEROR-Preserved was the first completed large randomized clinical trial of an SGLT2 inhibitor in patients with heart failure with preserved ejection fraction. They enrolled almost 6,000 patients with HFpEF with or without type 2 diabetes and they were randomized to empagliflozin 10 mg or placebo. The primary outcome of cardiovascular death or hospitalization for heart failure was reduced by 21% with empagliflozin and the number needed to treat was 31. This primary outcome was largely driven by a reduction in hospitalization for heart failure. The primary outcome showed consistent benefit across 15 prespecified subgroups, including those with or without type 2 diabetes, and including a spectrum of baseline left ventricular ejection fractions from 40% to 50% to greater than 60%.
There were also some key secondary endpoints: total hospitalization for heart failure was reduced by 27% and empagliflozin also slowed the decline of eGFR over time in the EMPEROR-Preserved trial. The agent was well tolerated. There was a slight signal for more hypotension and genital mycotic infections, but otherwise really no concerning adverse effects.
Building on the EMPEROR-Preserved trial was a prespecified pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved, the two large outcome trials with empagliflozin in heart failure patients. The prespecified primary outcome of this analysis was a major renal outcome which included a GFR reduction of ≥40%, renal replacement therapy or sustained eGFR <10-15 ml/min. While in EMPEROR-Reduced there was a significant 49% reduction in this composite renal outcome, in EMPEROR-Preserved there was no significant reduction. Because of the heterogeneity across these two trials, it was not statistically valid to pool these two results for the composite renal outcome. However, what they found in EMPEROR-Pooled is that if you use a more robust renal outcome including at least a 50% decline in eGFR, then there seems to be a trend that varies depending on baseline left ventricular ejection fraction, suggesting a benefit on the renal outcome if your baseline left ventricular function ranges from 40% to 60%, but lack of benefit with a baseline left ventricular ejection fraction of over 60%. The top line summary of this data is that for the first time we have robust evidence that an SGLT2 inhibitor, in this case empagliflozin 10 mg, can provide a cardiovascular benefit in patients with HFpEF, in addition to the known benefit in HFrEF patients.
Finally, there's the AMPLITUDE-O with efpeglenatide, an international randomized controlled trial conducted at approximately 344 sites in 28 countries. What are the key learnings and messages for this specific study?
Dr. Goldenberg: Efpeglenatide is an exendin-4-based GLP-1 receptor agonist that is given once weekly and the AMPLITUDE-O trial is the cardiovascular outcome trial with efpeglenatide done in patients with type 2 diabetes and either cardiovascular disease or chronic kidney disease plus at least one cardiovascular risk factor. It was an important trial because prior to this cardiovascular outcome trial studies of exendin-4-based GLP-1 receptor agonist have been neutral. However, the AMPLITUDE-O study showed for the first-time superiority with an exendin-4-based GLP-1 receptor agonist. In this case, efpeglenatide 4 or 6 milligrams versus placebo was associated with a 27% reduction in the primary outcome of CV death, non-fatal MI or non-fatal stroke.
Importantly, there was a consistent benefit with efpeglenatide across a spectrum of prespecified subgroups, the most important one being those that entered the trial on a background SGLT2 inhibitor, which represented about 15% of the patients. They derived the same overall benefit as those not taking an SGLT2 inhibitor. It is important to appreciate that this is probably the most robust data we have for showing a cardiovascular benefit of adding a GLP-1 receptor agonist to an SGLT2 inhibitor in high risk patients with type 2 diabetes. AMPLITUDE-O also adds to the already appreciated knowledge of the cardiovascular benefit of GLP-1 receptor agonists and builds on this story by showing that you can get a cardiovascular benefit with an exendin-4-based GLP-1 receptor agonist and you can get a benefit as an add on to SGLT2 inhibitors.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
STEP Program
- Wilding et al. N Engl J Med 2021; doi:10.1056/NEJMoa2032183; 2. Davies et al. Lancet, 2021; doi.org/10.1016/S0140-6736(21)00213-0: 3. Wadden et al. JAMA. doi:10.1001/jama.2021.1831; 4. Rubino et al. JAMA. 2021 Apr 13;325(14):1414-1425. doi: 10.1001/jama.2021.3224. 5. Garvey et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021; 6. Kadowaki et al. Presented at the International Congress on Metabolic Syndrome hybrid meeting .September 2-4, 2021; 7. Rubino et al. Presented at the 39th Annual Meeting of The Obesity Society (TOS) held at ObesityWeek®, virtual meeting, November 1–5, 2021.
FIGARO-DKD and FIDELITY
- Pitt et al. N Engl J Med 2021; 385:2252-2263.DOI: 10.1056/NEJMoa2110956; 2. Agarwal et al. European Heart Journal 2021).https://doi.org/10.1093/eurheartj/ehab777.
SURPASS trials
- Rosenstock J, et al. Lancet. 2021;398(10295):143-155; 2. Frias JP, et al. N Eng J Med. 2021;385(6):503-515; 3. Ludvik B, et al. Lancet. 2021;398(10300):583-598; 4. Del Prato S, et al. Lancet. 2021; 5. Dahl D, et al. Poster presented at: ADA 2021. Poster LB-20.
EMPEROR-Preserved and EMPEROR-Pooled
- Anker S et al. N Engl J Med 2021; 385:1451-1461. DOI: 10.1056/NEJMoa2107038; 2. Packer M et al. N Engl J Med 2021; 385:1531-1533DOI: 10.1056/NEJMc2112411.
AMPLITUDE-O
- Gerstein H et al. N Engl J Med 2021; 385:896-907. DOI: 10.1056/NEJMoa2108269.
PsA: TNF-blockade does not downregulate IL-17 cytokine and receptor protein level
Key clinical point: Despite reducing cellular inflammation and improving clinical outcome for joint involvement, adalimumab, a tumor necrosis factor (TNF) inhibitor, did not affect the levels of interleukin (IL)-17 cytokines and its receptors in the skin and synovium of patients with psoriatic arthritis (PsA).
Major finding: At baseline, the skin of patients with PsA vs. healthy donors (HD) showed significantly lower levels of IL-17A (P = .017) and its receptor IL-17RA (P = .007), but higher levels of IL-17F (P = .0002) and its receptor IL-17RC (P = .024). After 4 weeks of treatment, patients recieving adalimumab and placebo showed similar levels of IL-17A, IL-17F, and IL-17RC.
Study details: Findings are from a double-blind, single-center study including 24 patients with PsA and mild psoriatic skin lesions who were randomly assigned to adalimumab or placebo.
Disclosures: This study was funded by the Innovative Medicines Initiatives European Union. The authors declared no conflicts of interest.
Source: Bolt JW et al. Biomedicines. 2022;10(2):324 (Jan 29). Doi: 10.3390/biomedicines10020324.
Key clinical point: Despite reducing cellular inflammation and improving clinical outcome for joint involvement, adalimumab, a tumor necrosis factor (TNF) inhibitor, did not affect the levels of interleukin (IL)-17 cytokines and its receptors in the skin and synovium of patients with psoriatic arthritis (PsA).
Major finding: At baseline, the skin of patients with PsA vs. healthy donors (HD) showed significantly lower levels of IL-17A (P = .017) and its receptor IL-17RA (P = .007), but higher levels of IL-17F (P = .0002) and its receptor IL-17RC (P = .024). After 4 weeks of treatment, patients recieving adalimumab and placebo showed similar levels of IL-17A, IL-17F, and IL-17RC.
Study details: Findings are from a double-blind, single-center study including 24 patients with PsA and mild psoriatic skin lesions who were randomly assigned to adalimumab or placebo.
Disclosures: This study was funded by the Innovative Medicines Initiatives European Union. The authors declared no conflicts of interest.
Source: Bolt JW et al. Biomedicines. 2022;10(2):324 (Jan 29). Doi: 10.3390/biomedicines10020324.
Key clinical point: Despite reducing cellular inflammation and improving clinical outcome for joint involvement, adalimumab, a tumor necrosis factor (TNF) inhibitor, did not affect the levels of interleukin (IL)-17 cytokines and its receptors in the skin and synovium of patients with psoriatic arthritis (PsA).
Major finding: At baseline, the skin of patients with PsA vs. healthy donors (HD) showed significantly lower levels of IL-17A (P = .017) and its receptor IL-17RA (P = .007), but higher levels of IL-17F (P = .0002) and its receptor IL-17RC (P = .024). After 4 weeks of treatment, patients recieving adalimumab and placebo showed similar levels of IL-17A, IL-17F, and IL-17RC.
Study details: Findings are from a double-blind, single-center study including 24 patients with PsA and mild psoriatic skin lesions who were randomly assigned to adalimumab or placebo.
Disclosures: This study was funded by the Innovative Medicines Initiatives European Union. The authors declared no conflicts of interest.
Source: Bolt JW et al. Biomedicines. 2022;10(2):324 (Jan 29). Doi: 10.3390/biomedicines10020324.
Sex-specific adjustments in management strategy may be beneficial in early PsA
Key clinical point: The disease burden of psoriatic arthritis (PsA) was higher in women vs. men even after 1 year of standard-of-care treatment.
Major finding: Women vs. men reported a significantly longer duration of symptoms, higher tender joint count (both P < .05) and enthesitis at baseline (P < .05), and higher disease activity, higher levels of pain, and a lower functional capacity even after 1 year of follow-up (all P < .05). Minimal disease activity was predominantly present among men vs. women at baseline (18% vs. 10%; P < .05) and at 1 year of follow-up (59% vs. 37%, P < .00).
Study details: This prospective cohort study included 307 men and 313 women newly diagnosed with PsA from the Dutch south-west Early Psoriatic Arthritis Registry (DEPAR), who were followed up for 1 year.
Disclosures: No source of funding was reported for the study. The DEPAR cohort received funding from the Dutch Government, Pfizer, and other sources. The authors declared no conflicts of interest.
Source: Passia E et al. Arthritis Res Ther. 2022;24:22 (Jan 11). Doi: 10.1186/s13075-021-02680-y.
Key clinical point: The disease burden of psoriatic arthritis (PsA) was higher in women vs. men even after 1 year of standard-of-care treatment.
Major finding: Women vs. men reported a significantly longer duration of symptoms, higher tender joint count (both P < .05) and enthesitis at baseline (P < .05), and higher disease activity, higher levels of pain, and a lower functional capacity even after 1 year of follow-up (all P < .05). Minimal disease activity was predominantly present among men vs. women at baseline (18% vs. 10%; P < .05) and at 1 year of follow-up (59% vs. 37%, P < .00).
Study details: This prospective cohort study included 307 men and 313 women newly diagnosed with PsA from the Dutch south-west Early Psoriatic Arthritis Registry (DEPAR), who were followed up for 1 year.
Disclosures: No source of funding was reported for the study. The DEPAR cohort received funding from the Dutch Government, Pfizer, and other sources. The authors declared no conflicts of interest.
Source: Passia E et al. Arthritis Res Ther. 2022;24:22 (Jan 11). Doi: 10.1186/s13075-021-02680-y.
Key clinical point: The disease burden of psoriatic arthritis (PsA) was higher in women vs. men even after 1 year of standard-of-care treatment.
Major finding: Women vs. men reported a significantly longer duration of symptoms, higher tender joint count (both P < .05) and enthesitis at baseline (P < .05), and higher disease activity, higher levels of pain, and a lower functional capacity even after 1 year of follow-up (all P < .05). Minimal disease activity was predominantly present among men vs. women at baseline (18% vs. 10%; P < .05) and at 1 year of follow-up (59% vs. 37%, P < .00).
Study details: This prospective cohort study included 307 men and 313 women newly diagnosed with PsA from the Dutch south-west Early Psoriatic Arthritis Registry (DEPAR), who were followed up for 1 year.
Disclosures: No source of funding was reported for the study. The DEPAR cohort received funding from the Dutch Government, Pfizer, and other sources. The authors declared no conflicts of interest.
Source: Passia E et al. Arthritis Res Ther. 2022;24:22 (Jan 11). Doi: 10.1186/s13075-021-02680-y.