User login
AGA says stay the course, despite the Delta variant
As COVID-19 cases rise in the United States due to the Delta variant, there is renewed concern about infection and transmission of SARS-CoV-2 during endoscopy. In May 2021, AGA released updated recommendations on preprocedure testing post vaccination in the setting of ongoing population-wide vaccination programs for the prevention of COVID-19–related morbidity. In vaccinated individuals, breakthrough infections occurred very infrequently. Weighing the evidence demonstrating extremely low rates of rates of infection and transmission with vaccination and PPE, and considering the downsides of routine testing (burden, cost, false test results, increased disparities), AGA made a conditional recommendation against routine preprocedure testing for elective cases. The highly contagious Delta variant has now emerged as the predominant SARS-CoV2 virus in the U.S. and some data suggests that it may cause more severe illness than previous strains. While more breakthrough infections may develop in fully vaccinated individuals, the greatest risk of infection, transmission and hospitalizations is among those who are unvaccinated.
- AGA suggests against reinstituting routine preprocedure testing prior to elective endoscopy. The downsides (delays in patient care, burden, inaccurate results) outweigh potential benefits. Infection and transmission of SARS-CoV-2 from asymptomatic individuals is rare especially among vaccinated health care workers using personal protective equipment (PPE), even with the emergence of the Delta variant.
- If PPE is available, AGA recommends using N95 for upper endoscopy and suggests using N95 or surgical masks for lower endoscopy (acknowledging that upper endoscopy is more aerosolizing than lower endoscopy) and continuation of elective and nonelective endoscopy.
- Based on local prevalence rates, PPE, and test availability, in intermediate- and high-prevalence settings, preprocedure testing may be used to inform PPE decisions (N95 versus surgical mask). Additional benefits to testing are small and include deferring elective endoscopy in individuals testing positive and reducing anxiety among staff and patients.
As COVID-19 cases rise in the United States due to the Delta variant, there is renewed concern about infection and transmission of SARS-CoV-2 during endoscopy. In May 2021, AGA released updated recommendations on preprocedure testing post vaccination in the setting of ongoing population-wide vaccination programs for the prevention of COVID-19–related morbidity. In vaccinated individuals, breakthrough infections occurred very infrequently. Weighing the evidence demonstrating extremely low rates of rates of infection and transmission with vaccination and PPE, and considering the downsides of routine testing (burden, cost, false test results, increased disparities), AGA made a conditional recommendation against routine preprocedure testing for elective cases. The highly contagious Delta variant has now emerged as the predominant SARS-CoV2 virus in the U.S. and some data suggests that it may cause more severe illness than previous strains. While more breakthrough infections may develop in fully vaccinated individuals, the greatest risk of infection, transmission and hospitalizations is among those who are unvaccinated.
- AGA suggests against reinstituting routine preprocedure testing prior to elective endoscopy. The downsides (delays in patient care, burden, inaccurate results) outweigh potential benefits. Infection and transmission of SARS-CoV-2 from asymptomatic individuals is rare especially among vaccinated health care workers using personal protective equipment (PPE), even with the emergence of the Delta variant.
- If PPE is available, AGA recommends using N95 for upper endoscopy and suggests using N95 or surgical masks for lower endoscopy (acknowledging that upper endoscopy is more aerosolizing than lower endoscopy) and continuation of elective and nonelective endoscopy.
- Based on local prevalence rates, PPE, and test availability, in intermediate- and high-prevalence settings, preprocedure testing may be used to inform PPE decisions (N95 versus surgical mask). Additional benefits to testing are small and include deferring elective endoscopy in individuals testing positive and reducing anxiety among staff and patients.
As COVID-19 cases rise in the United States due to the Delta variant, there is renewed concern about infection and transmission of SARS-CoV-2 during endoscopy. In May 2021, AGA released updated recommendations on preprocedure testing post vaccination in the setting of ongoing population-wide vaccination programs for the prevention of COVID-19–related morbidity. In vaccinated individuals, breakthrough infections occurred very infrequently. Weighing the evidence demonstrating extremely low rates of rates of infection and transmission with vaccination and PPE, and considering the downsides of routine testing (burden, cost, false test results, increased disparities), AGA made a conditional recommendation against routine preprocedure testing for elective cases. The highly contagious Delta variant has now emerged as the predominant SARS-CoV2 virus in the U.S. and some data suggests that it may cause more severe illness than previous strains. While more breakthrough infections may develop in fully vaccinated individuals, the greatest risk of infection, transmission and hospitalizations is among those who are unvaccinated.
- AGA suggests against reinstituting routine preprocedure testing prior to elective endoscopy. The downsides (delays in patient care, burden, inaccurate results) outweigh potential benefits. Infection and transmission of SARS-CoV-2 from asymptomatic individuals is rare especially among vaccinated health care workers using personal protective equipment (PPE), even with the emergence of the Delta variant.
- If PPE is available, AGA recommends using N95 for upper endoscopy and suggests using N95 or surgical masks for lower endoscopy (acknowledging that upper endoscopy is more aerosolizing than lower endoscopy) and continuation of elective and nonelective endoscopy.
- Based on local prevalence rates, PPE, and test availability, in intermediate- and high-prevalence settings, preprocedure testing may be used to inform PPE decisions (N95 versus surgical mask). Additional benefits to testing are small and include deferring elective endoscopy in individuals testing positive and reducing anxiety among staff and patients.
Autism prevalence in children as high as 10% in some New Jersey communities
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators found that up to 10% of children in some of the state’s school districts have an ASD diagnosis vs. the national average of just under 2%.
School districts with higher ASD prevalence in the study have expansive health and educational programs in place to diagnose and support children with ASD, which likely contributed to the higher caseloads, senior investigator Walter Zahorodny, PhD, associate professor of pediatrics at New Jersey Medical School in Newark, said in an interview.
“When you have those players on the ground, it’s likely we’re going to be finding more, if not almost all, of the children with autism in a given district,” said Dr. Zahorodny, director of the New Jersey Autism Study for the Centers for Disease Control and Prevention.
The study was published online Oct. 21 in the journal Autism Research.
Local analysis
Researchers used the Autism and Developmental Disabilities Monitoring (ADDM) Network, a surveillance method developed by the CDC that includes data collected from health and special education records of children living in 11 communities across the United States. New Jersey is one of the ADDM participating sites.
National data are important, but Dr. Zahorodny and colleagues wanted to examine ASD prevalence at a more granular level, comparing prevalence district by district.
They examined data from 5,453 children who were 8 years old in 2016 and attended public school in Essex, Hudson, Ocean, and Union counties.
The prevalence of ASD was 36 children per 1,000 overall. Hudson County reported the lowest rate, at 31 cases per 1,000 children, and Ocean County reported the highest, at 54 cases per 1,000 children.
Across the region, ASD prevalence was four times higher in boys vs. girls, mirroring national statistics.
High ASD prevalence was more likely in mid-socioeconomic status districts (prevalence ratio [PR], 1.2; P = .01) and in larger school districts (PR, 1.3; P = .004).
Hispanic children had significantly lower ASD prevalence overall compared with White children (PR, 0.6; P < .001). In fact, prevalence was 30%-60% lower among Hispanic children in three of four counties compared with White children.
Another study is underway to better understand why autism rates were lower in specific districts and Hispanic children overall, but Dr. Zahorodny said one possibility is a lack of resources in those districts.
Will new methodology miss cases?
The study’s methodology was used by the CDC from 2000 to 2016 and includes assessment of children who have an ASD diagnosis, and children who haven’t received a diagnosis but have documented behaviors consistent with ASD.
In 2018, it was replaced with a less comprehensive approach that relies only on children with an ASD diagnosis. Data using this new methodology have not yet been reported.
In the new study from New Jersey, 767 children with autism were diagnosed by a pediatrician, neurologist, or other community provider. The remaining 175 children with autism, 18.6% of the total cohort, did not have an ASD diagnosis but met the ADDM case definition.
Under the new methodology, those children would not be counted.
“Something could be lost in the new methodology in terms of usefulness of the information because when the estimates are incomplete or low, that might lead people to make the wrong judgments when they make decisions about resources,” Dr. Zahorodny said.
The study was funded by the Centers for Disease Control and Prevention and the National Institutes of Health. The study authors report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ohio records more deaths than births for first time
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
In 2020, around 143,661 Ohioans died and 129,313 Ohioans were born, according to The Columbus Dispatch. The trend appears to have continued so far this year, with 107,462 deaths and 100,781 births reported to date.
Deaths haven’t surpassed births in the 112 years since the state began compiling data in 1909, the newspaper reported. The state’s birth rate has been declining for years while the number of deaths has risen, though data shows that the COVID-19 pandemic accelerated the shift.
“It doesn’t surprise me at all,” Joseph Gastaldo, MD, the medical director of infectious diseases for OhioHealth, told the newspaper.
Ohio’s birth rate fell by 4% in 2020, which defied public expectations about a pandemic birth boom. In addition, the state reported 13,927 COVID-19 deaths throughout the year.
“It’s COVID, clearly,” he noted.
Alabama also recorded more deaths than births for the first time last year, according to The New York Times. The state reported 64,714 deaths and 57,641 births in 2020.
“Our state literally shrunk in 2020,” Scott Harris, MD, the state health officer for Alabama, said at a news conference in September.
The state had never recorded a gap that large, even during World War I, World War II, and the 1918 flu pandemic, he said. Alabama has kept records on the numbers since 1900.
“We’ve never had a time when deaths exceeded births,” Dr. Harris said.
In fact, about half of U.S. states reported death rates higher than birth rates in 2020, according to a recent study from researchers at the University of New Hampshire. In 2019, only five states --Maine, New Hampshire, Rhode Island, Vermont, and West Virginia -- reported more deaths than births.
In 2020, the United States reported a record of nearly 3.4 million deaths, which was 18% more than in 2019, the researchers found. COVID-19 was the primary reason for the increase in deaths, accounting for about 11% of total deaths. Meanwhile, births dropped by 4% to about 3.6 million.
The surplus of births over deaths added 229,000 people to the U.S. population in 2020, as compared to 892,000 in 2019, which means the country’s population growth slowed last year. The decline, paired with lower immigration rates during the pandemic, led to the smallest annual percentage population gain in at least 100 years.
“Deaths will likely exceed births again in many states in 2021,” Kenneth Johnson, PhD, a senior demographer and study author, wrote in a statement.
“How large or protracted these fertility declines and mortality increases will be remains to be seen, but they have already dramatically reduced population growth in the United States,” he said.
A version of this article first appeared on WebMD.com.
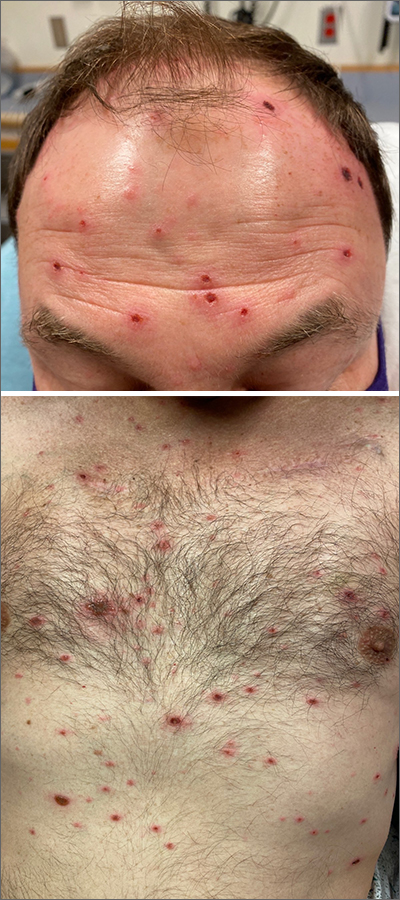
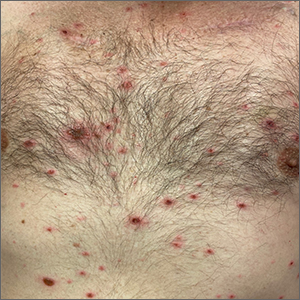
Rash in an immunocompromised patient
The patient’s history and presentation, as well as a positive varicella zoster virus (VZV) culture of vesicular fluid, led to a diagnosis of disseminated herpes zoster (DHZ). The patient’s laboratory studies were remarkable for mild lymphopenia, thrombocytopenia, and elevated liver function tests. Shave and punch biopsies of the lesion showed ballooning epithelial necrosis with multinucleated giant cells.
DHZ is characterized by more than 20 vesicles outside of a primary or adjacent dermatome and is caused by extensive reactivation of VZV. DHZ is most often encountered in immunocompromised patients.1 Untreated DHZ can lead to encephalitis, myelitis, nerve palsies, pneumonitis, hepatitis, and ocular complications.
Diagnosis is usually made clinically and confirmed by a polymerase chain reaction test, direct fluorescent antibody testing, or viral culture.2 In immunocompetent patients, uncomplicated DHZ is treated with oral acyclovir 800 mg 5 times daily, valacyclovir 1 g 3 times daily, or famciclovir 500 mg 3 times daily for 7 days. Hospital admission for intravenous acyclovir is recommended for immunocompromised patients, especially those with internal organ involvement, such as hepatitis or encephalitis.3
Early treatment is imperative to avoid life-threatening complications. Active herpes zoster lesions are infectious by contact with vesicular fluid until they dry and crust over. Patients should be instructed to avoid contact with susceptible people, including those who are immunocompromised, babies who have not received their varicella vaccine, and pregnant women. It is important to keep DHZ on the differential—especially in an immunosuppressed patient with a diffuse vesicular rash—as it can lead to significant morbidity and mortality.
Our patient was treated with oral valacyclovir 1 g tid for 10 days and he improved without developing systemic symptoms.
Image courtesy of Christen B. Samaan, MD. Text courtesy of Christen B. Samaan, MD, and Matthew F. Helm, MD, Department of Dermatology, and Nanjiba Nawaz, BA, Penn State College of Medicine, Hershey.
1. Bollea-Garlatti ML, Bollea-Garlatti LA, Vacas AS, et al. Clinical characteristics and outcomes in a population with disseminated herpes zoster: a retrospective cohort study. Actas Dermosifiliogr. 2017;108:145-152. doi: 10.1016/j.ad.2016.10.009
2. Chiriac A, Chiriac AE, Podoleanu C, et al. Disseminated cutaneous herpes zoster—a frequently misdiagnosed entity. Int Wound J. 2020;17:1089-1091. doi: 10.1111/iwj.13370
3. Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321;324;330.
The patient’s history and presentation, as well as a positive varicella zoster virus (VZV) culture of vesicular fluid, led to a diagnosis of disseminated herpes zoster (DHZ). The patient’s laboratory studies were remarkable for mild lymphopenia, thrombocytopenia, and elevated liver function tests. Shave and punch biopsies of the lesion showed ballooning epithelial necrosis with multinucleated giant cells.
DHZ is characterized by more than 20 vesicles outside of a primary or adjacent dermatome and is caused by extensive reactivation of VZV. DHZ is most often encountered in immunocompromised patients.1 Untreated DHZ can lead to encephalitis, myelitis, nerve palsies, pneumonitis, hepatitis, and ocular complications.
Diagnosis is usually made clinically and confirmed by a polymerase chain reaction test, direct fluorescent antibody testing, or viral culture.2 In immunocompetent patients, uncomplicated DHZ is treated with oral acyclovir 800 mg 5 times daily, valacyclovir 1 g 3 times daily, or famciclovir 500 mg 3 times daily for 7 days. Hospital admission for intravenous acyclovir is recommended for immunocompromised patients, especially those with internal organ involvement, such as hepatitis or encephalitis.3
Early treatment is imperative to avoid life-threatening complications. Active herpes zoster lesions are infectious by contact with vesicular fluid until they dry and crust over. Patients should be instructed to avoid contact with susceptible people, including those who are immunocompromised, babies who have not received their varicella vaccine, and pregnant women. It is important to keep DHZ on the differential—especially in an immunosuppressed patient with a diffuse vesicular rash—as it can lead to significant morbidity and mortality.
Our patient was treated with oral valacyclovir 1 g tid for 10 days and he improved without developing systemic symptoms.
Image courtesy of Christen B. Samaan, MD. Text courtesy of Christen B. Samaan, MD, and Matthew F. Helm, MD, Department of Dermatology, and Nanjiba Nawaz, BA, Penn State College of Medicine, Hershey.
The patient’s history and presentation, as well as a positive varicella zoster virus (VZV) culture of vesicular fluid, led to a diagnosis of disseminated herpes zoster (DHZ). The patient’s laboratory studies were remarkable for mild lymphopenia, thrombocytopenia, and elevated liver function tests. Shave and punch biopsies of the lesion showed ballooning epithelial necrosis with multinucleated giant cells.
DHZ is characterized by more than 20 vesicles outside of a primary or adjacent dermatome and is caused by extensive reactivation of VZV. DHZ is most often encountered in immunocompromised patients.1 Untreated DHZ can lead to encephalitis, myelitis, nerve palsies, pneumonitis, hepatitis, and ocular complications.
Diagnosis is usually made clinically and confirmed by a polymerase chain reaction test, direct fluorescent antibody testing, or viral culture.2 In immunocompetent patients, uncomplicated DHZ is treated with oral acyclovir 800 mg 5 times daily, valacyclovir 1 g 3 times daily, or famciclovir 500 mg 3 times daily for 7 days. Hospital admission for intravenous acyclovir is recommended for immunocompromised patients, especially those with internal organ involvement, such as hepatitis or encephalitis.3
Early treatment is imperative to avoid life-threatening complications. Active herpes zoster lesions are infectious by contact with vesicular fluid until they dry and crust over. Patients should be instructed to avoid contact with susceptible people, including those who are immunocompromised, babies who have not received their varicella vaccine, and pregnant women. It is important to keep DHZ on the differential—especially in an immunosuppressed patient with a diffuse vesicular rash—as it can lead to significant morbidity and mortality.
Our patient was treated with oral valacyclovir 1 g tid for 10 days and he improved without developing systemic symptoms.
Image courtesy of Christen B. Samaan, MD. Text courtesy of Christen B. Samaan, MD, and Matthew F. Helm, MD, Department of Dermatology, and Nanjiba Nawaz, BA, Penn State College of Medicine, Hershey.
1. Bollea-Garlatti ML, Bollea-Garlatti LA, Vacas AS, et al. Clinical characteristics and outcomes in a population with disseminated herpes zoster: a retrospective cohort study. Actas Dermosifiliogr. 2017;108:145-152. doi: 10.1016/j.ad.2016.10.009
2. Chiriac A, Chiriac AE, Podoleanu C, et al. Disseminated cutaneous herpes zoster—a frequently misdiagnosed entity. Int Wound J. 2020;17:1089-1091. doi: 10.1111/iwj.13370
3. Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321;324;330.
1. Bollea-Garlatti ML, Bollea-Garlatti LA, Vacas AS, et al. Clinical characteristics and outcomes in a population with disseminated herpes zoster: a retrospective cohort study. Actas Dermosifiliogr. 2017;108:145-152. doi: 10.1016/j.ad.2016.10.009
2. Chiriac A, Chiriac AE, Podoleanu C, et al. Disseminated cutaneous herpes zoster—a frequently misdiagnosed entity. Int Wound J. 2020;17:1089-1091. doi: 10.1111/iwj.13370
3. Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321;324;330.
Hot temperatures in outdoor lockboxes increase sample errors
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: CRC November 2021
The tide may finally be shifting as drugs targeting specific KRAS mutations have made their way into the clinic, and there is particular excitement around the oral WEE1 inhibitor, adavosertib (AZD1775). WEE1 is a cell cycle regulatory protein and WEE1 inhibition may have increased activity in tumors with DNA repair deficiency. In a phase 2 maintenance study, Seligmann and colleagues randomized 69 patients with RAS- and TP53-mutated mCRC with stable disease or better after 16 weeks of induction chemotherapy 2:1 to receive adavosertib vs active monitoring. Median progression-free survival (mPFS) was significant improved with adavosertib (3.61 vs 1.68 months), and patients with left-sided primary tumors appears to derive more benefit. While this finding needs to be further explored in larger clinical trials, it is exciting that there may finally be a new treatment option for patients with this specific molecular subtype of mCRC.
Another maintenance mCRC trial of interest is the PANAMA trial, a phase 2 study in which 248 patients with RAS wild-type mCRC were randomized 1:1 to 5-fluorouracil/leucovorin with or without panitumumab, an anti-epidermal growth factor receptor antibody, following induction chemotherapy with 6 cycles FOLFOX/panitumumab. Modest and co-workers report that mPFS was significantly improved with continuing panitumumab in the maintenance setting (8.8 vs. 5.7 months), and there was a trend towards an overall survival benefit as well. This study further supports continuing anti-EGFR therapy with maintenance chemotherapy for patients with RAS wild-type mCRC.
Finally, in stage III CRC, there is a big movement towards using circulating tumor DNA (ctDNA) as a method to monitor disease recurrence by detecting minimal residual disease based on tumor DNA being shed into the bloodstream. Henriksen et al. evaluated 168 patients with stage III CRC who underwent surgical resection and plasma ctDNA testing using 16 patient-specific DNA variants (tumor tissue-informed testing). The rates of recurrence were much higher in patients with detectable ctDNA post-operatively and/or after the completion of adjuvant chemotherapy, whereas those patients with persistently undetectable ctDNA did not recur. ctDNA is a powerful new technology that we are still learning how to best harness in the clinic, and this study demonstrates its prognostic value and potential ability to detect recurrence prior to standard imaging surveillance. Moreover, the rate of ctDNA rise was also prognostic of survival. ctDNA testing is likely to become standard of care in the management of stage II/III colorectal cancer in the very near future, and we hope that eventually it may be able to predict who needs to receive adjuvant chemotherapy and who does not.
The tide may finally be shifting as drugs targeting specific KRAS mutations have made their way into the clinic, and there is particular excitement around the oral WEE1 inhibitor, adavosertib (AZD1775). WEE1 is a cell cycle regulatory protein and WEE1 inhibition may have increased activity in tumors with DNA repair deficiency. In a phase 2 maintenance study, Seligmann and colleagues randomized 69 patients with RAS- and TP53-mutated mCRC with stable disease or better after 16 weeks of induction chemotherapy 2:1 to receive adavosertib vs active monitoring. Median progression-free survival (mPFS) was significant improved with adavosertib (3.61 vs 1.68 months), and patients with left-sided primary tumors appears to derive more benefit. While this finding needs to be further explored in larger clinical trials, it is exciting that there may finally be a new treatment option for patients with this specific molecular subtype of mCRC.
Another maintenance mCRC trial of interest is the PANAMA trial, a phase 2 study in which 248 patients with RAS wild-type mCRC were randomized 1:1 to 5-fluorouracil/leucovorin with or without panitumumab, an anti-epidermal growth factor receptor antibody, following induction chemotherapy with 6 cycles FOLFOX/panitumumab. Modest and co-workers report that mPFS was significantly improved with continuing panitumumab in the maintenance setting (8.8 vs. 5.7 months), and there was a trend towards an overall survival benefit as well. This study further supports continuing anti-EGFR therapy with maintenance chemotherapy for patients with RAS wild-type mCRC.
Finally, in stage III CRC, there is a big movement towards using circulating tumor DNA (ctDNA) as a method to monitor disease recurrence by detecting minimal residual disease based on tumor DNA being shed into the bloodstream. Henriksen et al. evaluated 168 patients with stage III CRC who underwent surgical resection and plasma ctDNA testing using 16 patient-specific DNA variants (tumor tissue-informed testing). The rates of recurrence were much higher in patients with detectable ctDNA post-operatively and/or after the completion of adjuvant chemotherapy, whereas those patients with persistently undetectable ctDNA did not recur. ctDNA is a powerful new technology that we are still learning how to best harness in the clinic, and this study demonstrates its prognostic value and potential ability to detect recurrence prior to standard imaging surveillance. Moreover, the rate of ctDNA rise was also prognostic of survival. ctDNA testing is likely to become standard of care in the management of stage II/III colorectal cancer in the very near future, and we hope that eventually it may be able to predict who needs to receive adjuvant chemotherapy and who does not.
The tide may finally be shifting as drugs targeting specific KRAS mutations have made their way into the clinic, and there is particular excitement around the oral WEE1 inhibitor, adavosertib (AZD1775). WEE1 is a cell cycle regulatory protein and WEE1 inhibition may have increased activity in tumors with DNA repair deficiency. In a phase 2 maintenance study, Seligmann and colleagues randomized 69 patients with RAS- and TP53-mutated mCRC with stable disease or better after 16 weeks of induction chemotherapy 2:1 to receive adavosertib vs active monitoring. Median progression-free survival (mPFS) was significant improved with adavosertib (3.61 vs 1.68 months), and patients with left-sided primary tumors appears to derive more benefit. While this finding needs to be further explored in larger clinical trials, it is exciting that there may finally be a new treatment option for patients with this specific molecular subtype of mCRC.
Another maintenance mCRC trial of interest is the PANAMA trial, a phase 2 study in which 248 patients with RAS wild-type mCRC were randomized 1:1 to 5-fluorouracil/leucovorin with or without panitumumab, an anti-epidermal growth factor receptor antibody, following induction chemotherapy with 6 cycles FOLFOX/panitumumab. Modest and co-workers report that mPFS was significantly improved with continuing panitumumab in the maintenance setting (8.8 vs. 5.7 months), and there was a trend towards an overall survival benefit as well. This study further supports continuing anti-EGFR therapy with maintenance chemotherapy for patients with RAS wild-type mCRC.
Finally, in stage III CRC, there is a big movement towards using circulating tumor DNA (ctDNA) as a method to monitor disease recurrence by detecting minimal residual disease based on tumor DNA being shed into the bloodstream. Henriksen et al. evaluated 168 patients with stage III CRC who underwent surgical resection and plasma ctDNA testing using 16 patient-specific DNA variants (tumor tissue-informed testing). The rates of recurrence were much higher in patients with detectable ctDNA post-operatively and/or after the completion of adjuvant chemotherapy, whereas those patients with persistently undetectable ctDNA did not recur. ctDNA is a powerful new technology that we are still learning how to best harness in the clinic, and this study demonstrates its prognostic value and potential ability to detect recurrence prior to standard imaging surveillance. Moreover, the rate of ctDNA rise was also prognostic of survival. ctDNA testing is likely to become standard of care in the management of stage II/III colorectal cancer in the very near future, and we hope that eventually it may be able to predict who needs to receive adjuvant chemotherapy and who does not.
Treatment with novel laser in acne studies targets sebaceous glands
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Cannabis use: Messages remain mixed across diagnoses
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
Marijuana use is now a legal activity in many parts of the United States, but those managing patients with psychiatric disorders are in the difficult position of determining whether this use is helpful, harmful, or irrelevant to the underlying illness on the basis of limited and largely incomplete data, according to an overview of this issue presented at the virtual Psychopharmacology Update presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
While there is clear evidence that cannabis use relative to the general population “is more prevalent among patients with psychiatric disorders,” it is less certain how often this use is risky, said Diana M. Martinez, MD, professor of psychiatry at Columbia University in New York.
Independent of euphoric effects, cannabis can be perceived by individuals with psychiatric diagnosis as self-medication for feelings of stress, social anxiety, and insomnia, among other symptoms. These are the same reasons why many individuals without psychiatric conditions use cannabis-containing products.
The perception that cannabis use is generally benign presumably explains the successful efforts at legalization, but there are risks for those with or without psychiatric illnesses, Dr. Martinez pointed out at the meeting, sponsored by Medscape Live. Not least, about 20% of regular users of cannabis develop cannabis use disorder (CUD), a condition defined in the DSM-5 as the continued use of cannabis despite adverse consequences, such as dependence.
Impact of severe CUD ‘incapacitating’
“Of those who meet criteria for CUD, 23% have severe CUD, which is an incapacitating form,” reported Dr. Martinez, citing work led by Deborah Hasin, PhD, professor of clinical epidemiology at Columbia University.
However, relative to otherwise healthy individuals, those with a psychiatric diagnosis might face greater benefits or greater risks from cannabis use, according to Dr. Martinez, who cited a 2017 report from the National Academies of Science, Engineering, and Medicine (NASEM).
This report evaluated the potential risks and benefits on the basis of published studies.
There is limited evidence that regular cannabis increases rather than modifies symptoms of mania and hypomania in patients with bipolar disorder, according to the report. The report also cited limited evidence that cannabis use increases severity of posttraumatic stress disorder (PTSD). There was limited evidence of adverse effects on symptoms of anxiety, although this appeared to depend on daily or nearly daily use.
The report found no data of acceptable quality to draw conclusions about the effect of cannabis use on symptoms of depression.
In patients with attention-deficit/hyperactivity disorder (ADHD), “a recent study showed that daily but not occasional use of cannabis increased impulsivity but not inattention, working memory, or verbal intelligence,” said Dr. Martinez, citing a study published this year.
Some evidence also suggests that patients with a psychiatric disorder might benefit from cannabis use, but, again, this evidence is limited. For one example, it includes a potential reduction in symptoms of obsessive-compulsive disorder, Dr. Martinez said.
More support for cannabis in medical disease
Relative to the quality of evidence supporting benefit from cannabis in psychiatric disease, the data appear to be stronger for patients with medical illnesses, such as cancer. For example, Dr. Martinez cited evidence that tetrahydrocannabinol (THC), a major active ingredient in cannabis, improves sleep in the context of a medical illnesses. There is also evidence for anxiolytic effects in patients with a medical illness, although that is weaker.
In patients with or without a psychiatric disorder, marijuana does pose a risk of substance abuse disorder, and it shares the risks of intoxicants, such as inattention leading to increased risk of accidents, including motor vehicle accidents. This pertains to those with or without a psychiatric or medical condition, Dr. Martinez said.
While intermittent light use of cannabis appears to pose no risk or a very low risk of long-term adverse effects on cognition, at least in patients without psychiatric disorders, Dr. Martinez indicated that the risk-benefit ratio for any individual is use dependent. The risk of CUD, for example, increases with the frequency of exposure and the potency of the cannabis.
Empirical evidence for therapeutic role
In published studies, other researchers have expressed interest in a potential therapeutic role of cannabis for psychiatric disorders, but there appears to be a general consensus that the supportive data remain weak. One expert who has written on this topic, Jerome Sarris, PhD, professor of integrative mental health, NICM Health Research Institute, Western Sydney University, Westmead, Australia, said that empirical evidence does support a benefit in selected patients.
“Of course, high THC forms are strongly discouraged in people with schizophrenia or high risk of developing psychotic disorder, or in youths,” Dr. Sarris explained. “However, there is a potential role for use in people with sleep and pain issues, and many find it beneficial to also assist with affective disorder symptoms.”
In a systematic review he led that was published last year, the evidence to support cannabis for psychiatric disorders was characterized as “embryonic.” However, small studies and case reports appear to support benefit for such indications as ADHD if precautions are taken.
“I certainly would not discourage use of prescribed standardized medicinal cannabis therapeutics for all people with psychiatric disorders,” Dr. Sarris said. He suggested that attention should be made to the THC potency and terpene composition of the products that patients with psychiatric disorders are taking.
FROM PSYCHOPHARMACOLOGY UPDATE
Lesion morphology drives optical evaluation’s accuracy for predicting SMIC
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” first authors Sergei Vosko, MD, and Neal Shahidi, MD, of the department of gastroenterology and hepatology, Westmead Hospital, Sydney, and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Vosko and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of SMIC was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
Because endoscopists are becoming more proficient with endoscopic mucosal resection (EMR) and are pushing the bounds with endoscopic submucosal dissection (ESD), there is a need for high-quality endoscopic markers of submucosal invasion (SMI) to help guide decision-making for management.
This study by Dr. Vosko and colleagues demonstrated a high degree of accuracy in predicting SMI via optical evaluation for a select group of large nonpedunculated colorectal polyps (LNPCP). The features associated with SMI were Kudo Pit Pattern V, ulceration, depression (Paris 0-IIc morphology), and rigidity or fixation.
The authors demonstrated that optical evaluation was highly accurate in detecting submucosal invasion for flat LNPCP with a high sensitivity and specificity. The sensitivity was considerably lower when evaluating nodular LNPCPs with a higher miss rate in polyps >4.0 cm and those located in the rectosigmoid colon. Of the endoscopic features assessed, Kudo pit pattern had the highest reliability in predicting SMI.
These data further tip the scale in favor of EMR as the appropriate therapeutic option for flat LNPCP in absence of features of SMI outlined by the authors. It also highlights the need for all endoscopists to be well versed in Kudo Pit classification and proficient in assessing for rigidity, fixation, and depression as the therapeutic decision (namely EMR vs. ESD vs. surgery) is often made by the endoscopist discovering the polyp. More studies are needed to identify endoscopic characteristics that provide a high sensitivity and specificity for SMI in nodular LNPCPs.
Rehman Sheikh, MD, is a gastroenterologist at the Baylor College of Medicine in Houston. He has no conflicts to declare.
Because endoscopists are becoming more proficient with endoscopic mucosal resection (EMR) and are pushing the bounds with endoscopic submucosal dissection (ESD), there is a need for high-quality endoscopic markers of submucosal invasion (SMI) to help guide decision-making for management.
This study by Dr. Vosko and colleagues demonstrated a high degree of accuracy in predicting SMI via optical evaluation for a select group of large nonpedunculated colorectal polyps (LNPCP). The features associated with SMI were Kudo Pit Pattern V, ulceration, depression (Paris 0-IIc morphology), and rigidity or fixation.
The authors demonstrated that optical evaluation was highly accurate in detecting submucosal invasion for flat LNPCP with a high sensitivity and specificity. The sensitivity was considerably lower when evaluating nodular LNPCPs with a higher miss rate in polyps >4.0 cm and those located in the rectosigmoid colon. Of the endoscopic features assessed, Kudo pit pattern had the highest reliability in predicting SMI.
These data further tip the scale in favor of EMR as the appropriate therapeutic option for flat LNPCP in absence of features of SMI outlined by the authors. It also highlights the need for all endoscopists to be well versed in Kudo Pit classification and proficient in assessing for rigidity, fixation, and depression as the therapeutic decision (namely EMR vs. ESD vs. surgery) is often made by the endoscopist discovering the polyp. More studies are needed to identify endoscopic characteristics that provide a high sensitivity and specificity for SMI in nodular LNPCPs.
Rehman Sheikh, MD, is a gastroenterologist at the Baylor College of Medicine in Houston. He has no conflicts to declare.
Because endoscopists are becoming more proficient with endoscopic mucosal resection (EMR) and are pushing the bounds with endoscopic submucosal dissection (ESD), there is a need for high-quality endoscopic markers of submucosal invasion (SMI) to help guide decision-making for management.
This study by Dr. Vosko and colleagues demonstrated a high degree of accuracy in predicting SMI via optical evaluation for a select group of large nonpedunculated colorectal polyps (LNPCP). The features associated with SMI were Kudo Pit Pattern V, ulceration, depression (Paris 0-IIc morphology), and rigidity or fixation.
The authors demonstrated that optical evaluation was highly accurate in detecting submucosal invasion for flat LNPCP with a high sensitivity and specificity. The sensitivity was considerably lower when evaluating nodular LNPCPs with a higher miss rate in polyps >4.0 cm and those located in the rectosigmoid colon. Of the endoscopic features assessed, Kudo pit pattern had the highest reliability in predicting SMI.
These data further tip the scale in favor of EMR as the appropriate therapeutic option for flat LNPCP in absence of features of SMI outlined by the authors. It also highlights the need for all endoscopists to be well versed in Kudo Pit classification and proficient in assessing for rigidity, fixation, and depression as the therapeutic decision (namely EMR vs. ESD vs. surgery) is often made by the endoscopist discovering the polyp. More studies are needed to identify endoscopic characteristics that provide a high sensitivity and specificity for SMI in nodular LNPCPs.
Rehman Sheikh, MD, is a gastroenterologist at the Baylor College of Medicine in Houston. He has no conflicts to declare.
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” first authors Sergei Vosko, MD, and Neal Shahidi, MD, of the department of gastroenterology and hepatology, Westmead Hospital, Sydney, and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Vosko and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of SMIC was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
The diagnostic performance of optical evaluation for submucosal invasive cancer (SMIC) in patients with large (≥20 mm) nonpedunculated colorectal polyps (LNPCPs) may be dependent on lesion morphology. While optical evaluation featured excellent performance in the assessment of flat lesions, the assessment only featured decent performance in nodular lesions, underscoring the need for additional evaluation algorithms for these lesions.
Endoscopists rely on the accuracy of real-time optical evaluation to facilitate appropriate selection of treatment; however, in studies focusing on LNPCPs, the performance of optical evaluation is modest.
The stratification of optical evaluation by lesion morphology may enable more accurate “implementation of a selective resection algorithm by identifying lesion subgroups with accurate optical evaluation performance characteristics,” first authors Sergei Vosko, MD, and Neal Shahidi, MD, of the department of gastroenterology and hepatology, Westmead Hospital, Sydney, and colleagues wrote in Clinical Gastroenterology and Hepatology.
Given the potential importance of stratification in optical evaluation, Dr. Vosko and colleagues assessed the performance of the optical assessment modality based on lesion morphology in a prospective cohort of 1,583 LNPCPs measuring at least 20 mm in patients (median age, 69 years) referred for endoscopic resection.
In the observational cohort, centers performed optical evaluation before endoscopic resection. The optical prediction of SMIC was based on several different established features, including Kudo V pit pattern, depressed morphology, rigidity/fixation, and ulceration. The researchers calculated optical evaluation performance outcomes, which were reported by the dominant morphology, namely nodular (Paris 0–Is/0– IIaDIs) versus flat (Paris 0–IIa/0–IIb).
Across the overall cohort, the median lesion size was 35 mm. The investigators identified a total of 855 flat LNPCPs and 728 nodular LNPCPs, with 63.9% of LNPCPs considered granular. Additionally, the researchers reported submucosal invasive cancer in 146 LNPCPs (9.2%).
According to the investigators, the overall sensitivity of optical evaluation to diagnose submucosal invasive cancer was 67.1% (95% confidence interval, 59.2%-74.2%), while the overall specificity was 95.1% (95% CI, 93.9%-96.1%). The investigators reported significant differences between flat vs. nodular LNPCPs in terms of sensitivity (90.9% vs. 52.7%, respectively; P <.001) and specificity (96.3% vs. 93.7%; P =.027).
Overall, the SMIC miss rate was 3.0% (95% CI, 2.3%-4.0%). There was a significant difference in the SMIC miss rate between flat and nodular LNPCPs (0.6% vs. 5.9%, respectively; P < .001).
Independent predictors of missed SMIC on optical evaluation, as identified in the multiple logistic regression analysis, included nodular morphology (odds ratio, 7.2; 95% CI, 2.8-18.9; P < .001), rectosigmoid location (OR, 2.0; 95% CI, 1.1-3.7; P =.026), and size of at least 40 mm (OR, 2.0; 95% CI, 1.0-3.8; P =.039).
Based on the findings, the researchers suggested that all flat lesions, in the absence of optical features consistent with submucosal invasive cancer, should subsequently be removed by high-quality endoscopic mucosal resection, in conjunction with the application of “site-specific modifications and ancillary techniques where needed.”
One limitation of this study is how lesion morphology was classified, which can in some cases be subjective.
The researchers added that additional refinement is required “to robustly apply a selective resection algorithm irrespective of lesion morphology” given the modest performance value of optical evaluation in nodular lesions. “Nevertheless, it is imperative that all endoscopists embrace optical evaluation in everyday clinical practice, thus harnessing its proven ability to influence resection technique selection and the associated clinical and economic ramifications,” they concluded.
The study received financial support the Cancer Institute of New South Wales, in addition to funding from the Gallipoli Medical Research Foundation. One author reported receiving research support from Olympus Medical, Cook Medical, and Boston Scientific. The remaining authors disclosed no conflicts.
Selective cooling technology being used to remove age spots
, according to Arisa E. Ortiz, MD.
“What’s unique about this device is that I can see results without any downtime,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said during a virtual course on laser and aesthetic skin therapy. “Most other devices are not like that. It was well tolerated; there was minimal pain. There was no postinflammatory hyperpigmentation; it really is customizable to the patients’ needs.”
First cleared by the Food and Drug Administration in 2016 to remove benign lesions of the skin, Glacial Rx received an expanded indication in 2020 to temporarily reduce pain, swelling, and inflammation. The device, which was developed by R2 Technologies, relies on cryomodulation, a concept developed at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, to improve skin appearance and freeze melanin at the source. “Cryomodulation pauses melanin production, but the melanocyte function is preserved, the epidermal barrier is not disrupted, and there is no persistent inflammatory response, which is key, because it decreases the risk of postinflammatory hyperpigmentation, especially in darker skin types,” Dr. Ortiz said.
Here’s how it works: The handpiece of the device is placed on top of the skin and cooling is delivered to targeted solar lentigos and other benign lesions. Ice nucleation takes place within the dendrites. As cell turnover takes place, melanin-free cells migrate upward and appear as new skin. “Clinically, this appears as clearance of the lesion,” Dr. Ortiz said.
She discussed her clinical experience treating 15 patients with a beta version of the device. Since that time, Glacial Rx was redesigned to include a smaller-tipped handpiece, easier and faster prep time, and a proprietary water-based gel to facilitate ice crystal propagation, which is applied to the targeted lesions just prior to treatment.
For the trial at UCSD, the researchers performed 29 treatment sessions on 15 patients with Fitzpatrick skin types I-IV, to gain clinical experience and evaluate the effectiveness of the device. They found that the treatment was well tolerated, with minimal discomfort. The amount of heat extracted ranged from 107 to 166 kJ/cm2. No long-term dyschromia was observed, and some patients had lesion clearance after just one treatment.
“The settings are able to be titrated to where you have zero downtime, but you still get a lightening effect,” Dr. Ortiz said during the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “With other devices such as intense pulsed light, if you don’t see darkening than it probably didn’t work. With this device, you can titrate the length of the cooling and the temperature of the cooling.”
Posttreatment side effects commonly observed in the study were mild erythema, swelling, itching, and darkening. “There was minimal erythema in the higher settings, and some reports of itching and transient darkening in some of the higher settings,” she said.
Future indications for Glacial Rx may include psoriasis, acne, and rosacea. “We did try to use this for melasma,” she said. “It was effective, but I wouldn’t say it’s a cure for melasma. Melasma is very stubborn and requires a combination treatment, but it’s something we can use in our armamentarium.”
Dr. Ortiz reported having received consulting fees from R2 Technologies. She has been a paid consultant for and has received equipment from many device companies.
, according to Arisa E. Ortiz, MD.
“What’s unique about this device is that I can see results without any downtime,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said during a virtual course on laser and aesthetic skin therapy. “Most other devices are not like that. It was well tolerated; there was minimal pain. There was no postinflammatory hyperpigmentation; it really is customizable to the patients’ needs.”
First cleared by the Food and Drug Administration in 2016 to remove benign lesions of the skin, Glacial Rx received an expanded indication in 2020 to temporarily reduce pain, swelling, and inflammation. The device, which was developed by R2 Technologies, relies on cryomodulation, a concept developed at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, to improve skin appearance and freeze melanin at the source. “Cryomodulation pauses melanin production, but the melanocyte function is preserved, the epidermal barrier is not disrupted, and there is no persistent inflammatory response, which is key, because it decreases the risk of postinflammatory hyperpigmentation, especially in darker skin types,” Dr. Ortiz said.
Here’s how it works: The handpiece of the device is placed on top of the skin and cooling is delivered to targeted solar lentigos and other benign lesions. Ice nucleation takes place within the dendrites. As cell turnover takes place, melanin-free cells migrate upward and appear as new skin. “Clinically, this appears as clearance of the lesion,” Dr. Ortiz said.
She discussed her clinical experience treating 15 patients with a beta version of the device. Since that time, Glacial Rx was redesigned to include a smaller-tipped handpiece, easier and faster prep time, and a proprietary water-based gel to facilitate ice crystal propagation, which is applied to the targeted lesions just prior to treatment.
For the trial at UCSD, the researchers performed 29 treatment sessions on 15 patients with Fitzpatrick skin types I-IV, to gain clinical experience and evaluate the effectiveness of the device. They found that the treatment was well tolerated, with minimal discomfort. The amount of heat extracted ranged from 107 to 166 kJ/cm2. No long-term dyschromia was observed, and some patients had lesion clearance after just one treatment.
“The settings are able to be titrated to where you have zero downtime, but you still get a lightening effect,” Dr. Ortiz said during the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “With other devices such as intense pulsed light, if you don’t see darkening than it probably didn’t work. With this device, you can titrate the length of the cooling and the temperature of the cooling.”
Posttreatment side effects commonly observed in the study were mild erythema, swelling, itching, and darkening. “There was minimal erythema in the higher settings, and some reports of itching and transient darkening in some of the higher settings,” she said.
Future indications for Glacial Rx may include psoriasis, acne, and rosacea. “We did try to use this for melasma,” she said. “It was effective, but I wouldn’t say it’s a cure for melasma. Melasma is very stubborn and requires a combination treatment, but it’s something we can use in our armamentarium.”
Dr. Ortiz reported having received consulting fees from R2 Technologies. She has been a paid consultant for and has received equipment from many device companies.
, according to Arisa E. Ortiz, MD.
“What’s unique about this device is that I can see results without any downtime,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said during a virtual course on laser and aesthetic skin therapy. “Most other devices are not like that. It was well tolerated; there was minimal pain. There was no postinflammatory hyperpigmentation; it really is customizable to the patients’ needs.”
First cleared by the Food and Drug Administration in 2016 to remove benign lesions of the skin, Glacial Rx received an expanded indication in 2020 to temporarily reduce pain, swelling, and inflammation. The device, which was developed by R2 Technologies, relies on cryomodulation, a concept developed at Massachusetts General Hospital and the Wellman Center for Photomedicine, Boston, to improve skin appearance and freeze melanin at the source. “Cryomodulation pauses melanin production, but the melanocyte function is preserved, the epidermal barrier is not disrupted, and there is no persistent inflammatory response, which is key, because it decreases the risk of postinflammatory hyperpigmentation, especially in darker skin types,” Dr. Ortiz said.
Here’s how it works: The handpiece of the device is placed on top of the skin and cooling is delivered to targeted solar lentigos and other benign lesions. Ice nucleation takes place within the dendrites. As cell turnover takes place, melanin-free cells migrate upward and appear as new skin. “Clinically, this appears as clearance of the lesion,” Dr. Ortiz said.
She discussed her clinical experience treating 15 patients with a beta version of the device. Since that time, Glacial Rx was redesigned to include a smaller-tipped handpiece, easier and faster prep time, and a proprietary water-based gel to facilitate ice crystal propagation, which is applied to the targeted lesions just prior to treatment.
For the trial at UCSD, the researchers performed 29 treatment sessions on 15 patients with Fitzpatrick skin types I-IV, to gain clinical experience and evaluate the effectiveness of the device. They found that the treatment was well tolerated, with minimal discomfort. The amount of heat extracted ranged from 107 to 166 kJ/cm2. No long-term dyschromia was observed, and some patients had lesion clearance after just one treatment.
“The settings are able to be titrated to where you have zero downtime, but you still get a lightening effect,” Dr. Ortiz said during the meeting, named “Laser & Aesthetic Skin Therapy: What’s the Truth?” sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “With other devices such as intense pulsed light, if you don’t see darkening than it probably didn’t work. With this device, you can titrate the length of the cooling and the temperature of the cooling.”
Posttreatment side effects commonly observed in the study were mild erythema, swelling, itching, and darkening. “There was minimal erythema in the higher settings, and some reports of itching and transient darkening in some of the higher settings,” she said.
Future indications for Glacial Rx may include psoriasis, acne, and rosacea. “We did try to use this for melasma,” she said. “It was effective, but I wouldn’t say it’s a cure for melasma. Melasma is very stubborn and requires a combination treatment, but it’s something we can use in our armamentarium.”
Dr. Ortiz reported having received consulting fees from R2 Technologies. She has been a paid consultant for and has received equipment from many device companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE