User login
Pain in MS: Focus on flexibility, multiple strategies, and nondrug treatments
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
FROM CMSC 2021
AGA leaders met with federal regulators
AGA President John Inadomi, MD, and former AGA President David Lieberman, MD, along with American Cancer Society Cancer Action Network and Fight CRC, met with Assistant Secretary of Labor, Ali Khawar, and representatives from the U.S. Department of Health & Human Services and U.S. Department of Treasury to request they direct private health plans to cover colonoscopy after a positive noninvasive colorectal cancer (CRC) screening test.
The meeting was in response to an appeal sent to the three agencies, which provided guidance to health plans to ensure that workers have the benefits that have been agreed upon by their employers. As part of the Affordable Care Act, plans are mandated to cover colorectal cancer screening without cost sharing.
In May 2021, when the United States Preventive Services Task Force (USPFTF) lowered the recommended CRC screening age to 45, it also stated that “positive results on stool-based screening tests require follow-up with colonoscopy for the screening benefits to be achieved.”
To ensure that privately insured Americans receive proper CRC screening, AGA, ACS, and Fight CRC are pushing the government to provide written guidance to private plans clarifying that follow-up colonoscopies conducted after a positive noninvasive screening test are part of the colorectal cancer screening process and, therefore, patients should not face out-of-pocket costs when completing colorectal cancer screening.
Colorectal cancer remains the second leading killer in cancer in the United States despite the availability of preventive screening options. In 2018, just 68.8% of those eligible were screened for colorectal cancer. The challenge of getting people screened was exacerbated in 2020 when it is estimated that colorectal cancer screening declined by 86% during the first few months of the COVID-19 pandemic.
AGA President John Inadomi, MD, and former AGA President David Lieberman, MD, along with American Cancer Society Cancer Action Network and Fight CRC, met with Assistant Secretary of Labor, Ali Khawar, and representatives from the U.S. Department of Health & Human Services and U.S. Department of Treasury to request they direct private health plans to cover colonoscopy after a positive noninvasive colorectal cancer (CRC) screening test.
The meeting was in response to an appeal sent to the three agencies, which provided guidance to health plans to ensure that workers have the benefits that have been agreed upon by their employers. As part of the Affordable Care Act, plans are mandated to cover colorectal cancer screening without cost sharing.
In May 2021, when the United States Preventive Services Task Force (USPFTF) lowered the recommended CRC screening age to 45, it also stated that “positive results on stool-based screening tests require follow-up with colonoscopy for the screening benefits to be achieved.”
To ensure that privately insured Americans receive proper CRC screening, AGA, ACS, and Fight CRC are pushing the government to provide written guidance to private plans clarifying that follow-up colonoscopies conducted after a positive noninvasive screening test are part of the colorectal cancer screening process and, therefore, patients should not face out-of-pocket costs when completing colorectal cancer screening.
Colorectal cancer remains the second leading killer in cancer in the United States despite the availability of preventive screening options. In 2018, just 68.8% of those eligible were screened for colorectal cancer. The challenge of getting people screened was exacerbated in 2020 when it is estimated that colorectal cancer screening declined by 86% during the first few months of the COVID-19 pandemic.
AGA President John Inadomi, MD, and former AGA President David Lieberman, MD, along with American Cancer Society Cancer Action Network and Fight CRC, met with Assistant Secretary of Labor, Ali Khawar, and representatives from the U.S. Department of Health & Human Services and U.S. Department of Treasury to request they direct private health plans to cover colonoscopy after a positive noninvasive colorectal cancer (CRC) screening test.
The meeting was in response to an appeal sent to the three agencies, which provided guidance to health plans to ensure that workers have the benefits that have been agreed upon by their employers. As part of the Affordable Care Act, plans are mandated to cover colorectal cancer screening without cost sharing.
In May 2021, when the United States Preventive Services Task Force (USPFTF) lowered the recommended CRC screening age to 45, it also stated that “positive results on stool-based screening tests require follow-up with colonoscopy for the screening benefits to be achieved.”
To ensure that privately insured Americans receive proper CRC screening, AGA, ACS, and Fight CRC are pushing the government to provide written guidance to private plans clarifying that follow-up colonoscopies conducted after a positive noninvasive screening test are part of the colorectal cancer screening process and, therefore, patients should not face out-of-pocket costs when completing colorectal cancer screening.
Colorectal cancer remains the second leading killer in cancer in the United States despite the availability of preventive screening options. In 2018, just 68.8% of those eligible were screened for colorectal cancer. The challenge of getting people screened was exacerbated in 2020 when it is estimated that colorectal cancer screening declined by 86% during the first few months of the COVID-19 pandemic.
Unvaccinated pregnant women have more severe COVID
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
An increasing number of people who are unvaccinated and pregnant are being hospitalized for COVID-19, report investigators who saw hospital admissions double in a single year.
“With the surge, we had expected to begin treating patients who developed severe or critical illness again in pregnancy,” says Emily Adhikari, MD, from the University of Texas Southwestern Medical Center in Dallas. “But we did not expect the level of respiratory illness that we began to see in our patients. That was a surprise and an alarming finding that we felt was really important to get out there.”
The researchers followed more than 1,500 pregnant women diagnosed with COVID-19 who received care from Parkland Health and Hospital System in Dallas County, one of the nation’s busiest for deliveries. After the emergence of the Delta variant, the number of pregnant women hospitalized with COVID-19 more than doubled over the previous year.
And 82 pregnant women went on to develop severe or critical COVID, they report in their study, published online in the American Journal of Obstetrics and Gynecology. All but 1 of these patients were unvaccinated, 10 needed a ventilator, and two died.
The proportion of cases that were critical was about 5% in 2020. However, in April 2021, even though the number of total cases remained low, the number of severe illnesses started to rise. After the Delta variant became dominant, both the number and severity of cases increased, and after August 2021, more than 25% of pregnant people diagnosed with COVID-19 required hospitalization.
Hospitalizations Double
“We need to focus and really act urgently to recommend vaccination in pregnancy because that is the primary prevention tool that we have,” says Dr. Adhikari. “We do not have a proven cure for this illness, and that is important to know.”
These findings, which focus on a vulnerable population, are especially important given the elevated prevalence of COVID-19 in pregnant people of lower economic status, said Lissette Tanner, MD, MPH, from Emory University in Atlanta, who was not involved with the study.
“There are higher rates of hospitalization and death among Black, Hispanic, and Native American communities,” she reported. “It is essential to know how the virus is affecting those most affected and often most disadvantaged to deal with the pandemic.”
Vaccination rates are low in this population; just 19.2% of pregnant women receive at least one dose during pregnancy, according to the CDC. But pregnancy confers a higher risk for severe COVID-19 illness and for adverse outcomes, such as preterm birth and stillbirth.
Of the 665 people in the study cohort who were pregnant or had given birth when the vaccines were available, only 21.4% received at least one dose of a COVID-19 vaccine.
Given the increased risk for COVID-19 during pregnancy, the American College of Obstetricians and Gynecologists, the Society for Maternal-Fetal Medicine, and the CDC recommend vaccination for people who are pregnant, breastfeeding, or trying to get pregnant.
According to ACOG, pregnant women who are fully vaccinated can follow the same guidelines as everyone else who is fully vaccinated; however, to prevent breakthrough infections, they might want to continue wearing a mask. ACOG also recommends that those not fully vaccinated follow physical-distancing guidelines and limit contact with people as much as possible to avoid infection.
A version of this article first appeared on WebMD.com.
Disinclined to offer laser hair removal? An expert makes the case to think otherwise
Omar A. Ibrahimi, MD, PhD, hears some dermatology colleagues say they don’t bother to offer laser hair removal in their practices because they figure that the procedure is under the purview of medical spas, but he sees it differently.
“I offer laser hair removal in my practice as a way to protect my patients from being picked off by medical spas,” Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, said during a virtual course on laser and aesthetic skin therapy. “These patients are going to want to get laser hair removal. If they’re not going to have the opportunity to get it at your practice, they’re going to seek it elsewhere. When they go elsewhere, they’re going to be picked off for other procedures as well.”
First developed in 1995 by R. Rox Anderson, MD, and colleagues at The Wellman Center for Photomedicine, laser hair removal has become the gold standard for permanent hair destruction, and ranks as the most common energy-based procedure performed in the world, Dr. Ibrahimi said. “Results are very long lasting and durable beyond 2 years after treatment,” he said. “These patients tend to be highly satisfied and have permanence with these treatments.”
Treatment goal, patient selection
While the target chromophore for the procedure is melanin, the goal is to destroy the stem cells located in the hair bulge and the hair bulb. “This is technically called the extended theory of selective photothermolysis, but it’s the same concept except that our target chromophore and our desired target for destruction are slightly spatially separated,” he said.
Proper patient selection is key, so a focused medical history and physical exam are essential prior to the procedure. If unwanted hair is located on the face, jawline, or chest of a female, consider and ask about potential endocrine-related dysfunctions such as polycystic ovary syndrome (PCOS). “Getting those addressed can often help the hypertrichosis as well,” he said. “Another condition is explosive hypertrichosis where hair growth starts very suddenly. It’s uncommon but it’s something to think about.”
Pregnancy is not an absolute contraindication for laser hair removal, Dr. Ibrahimi continued, but he elects not to perform the procedure on pregnant patients. He also asks patients about any history of photosensitivity, active infection at the intended treatment site, keloids, or hypertrophic scarring. Past methods of hair removal also matter. “What we’re targeting is the pigment in the hair shafts,” he said. “So, if your patient is waxing or plucking or epilating or removing the hair in some manner, they’re actually removing the target chromophore.”
Patients with darker Fitzpatrick skin types can be treated safely but tanned individuals face a risk of complications because of active melanocytes. “As we approach summer in New England, we slow down the amount of hair removal we do because it’s a riskier procedure,” he said. “I recommend that my patients not get any significant amount of sun exposure a month before or after treatment.”
The color and quality of hair also drive treatment success. Black and brown terminal hairs absorb the millisecond laser energy, but white, gray, red, and light blond hairs lack adequate melanin to make them suitable target chromophores.
Excessive and unwanted body hair ranges in severity and can usually be classified as either hypertrichosis or hirsutism.
The desired clinical endpoint is perifollicular edema and erythema. Treatment parameters that can be varied with Food and Drug Administration–cleared devices include wavelength, fluence, pulse duration, spot size, and skin cooling. The most popular devices are the Alexandrite 755 nm laser; the diode 800 nm laser; and the 1064 nm Nd:YAG laser, which is safe for all skin types. “Often you have to use higher relative fluences to treat patients with the 1064 nm Nd:YAG because on the absorption spectrum, the 1064-nm wavelength has a relatively lower absorption for melanin compared to the alexandrite. However, you can still get effective, long-term hair reduction with the Nd:YAG laser,” he said (Arch Dermatol. 2008 Oct;144[10]:1323-7).
More recently, Dr. Ibrahimi and colleagues found that a 1060-nm diode laser system with multiple handpieces for permanent hair reduction was safe for all skin types, in an open label prospective study.
Higher fluences have been correlated with greater rates of permanent hair removal, but they also are more likely to cause undesired side effects. Dr. Ibrahimi advises clinicians new to laser hair removal to conduct a few different test spots and look for the desired clinical endpoint of perifollicular erythema and edema. “The highest fluence that gives you that endpoint without any adverse reactions is going to the best fluence for treatment,” he said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Do a few test spots, bring them back a week later and see which ones were tolerated well without any side effects and which weren’t. That gives you a good starting point for your treatment.”
Cooling down the epidermal melanin not only keeps the procedure safe, it’s a salve for pain. “There are a variety of methods of passive and active cooling,” said Dr. Ibrahimi, a member of the American Society for Dermatologic Surgery board of directors. “You can use something as simple as cold gel, but the active methods are better because once the method of passive application of cold gel warms up, you lose that cooling effect. You can use forced chilled air. Many commercial devices come with a cold tip which cools down the epidermal melanin. Others use dynamic cooling, which emit cryogen spray from a separate part of the handpiece. It hits right where the laser pulse is going to go, is absorbed by the skin, and it cools down the epidermal melanin.”
Treatment complications
Complications that can occur from treatment include pigmentary changes such as hyperpigmentation and hypopigmentation. “In lighter skinned individuals, sometimes excess fluence can lead to an erythematous appearance,” he said. “In darker-skinned individuals, this often manifests as hyperpigmentation and can be very long-lasting.” Dr. Ibrahimi ranks improper technique as a complication, “because ideally you want to lay your pulses down with 10%-15% overlap during treatment,” he explained. “If you don’t overlap, you’re going to have zones that don’t get any of the laser photons. If you do, then your patient is not going to be happy with you.”
Paradoxical hypertrichosis occurs in 1%-5% of patients, typically in women from Mediterranean, Middle Eastern, or South Asian ethnic backgrounds. This tends to develop on the lateral or jawline part of the face. “Often it occurs in the setting where they come in and want these vellus hairs treated,” he said. “Somehow the laser, instead of destroying the hair shaft, triggers it and stimulates it and can’t differentiate a vellus hair from a terminal hair. This is important to discuss during your informed consent, especially when you’re treating on the lateral jawline or the sideburn area. If this happens, you can treat through it.”
Transgender patients and future directions
Dr. Ibrahimi pointed out that increasing numbers of transgender patients are visiting dermatologists seeking laser hair removal. About 16 million Americans are estimated to have a gender identity that differs from the one assigned to them at birth, yet they face several barriers to care, “ranging from ignorance on our end to maybe our own biases being transposed onto these patients,” he said. “We really need to do a better job for them. We really have an obligation to provide good care for all of our patients.”
Transgender women typically seek hair removal on the face and neck as well as in the genital area to remove hairs in preparation for vaginoplasty. Transgender men typically seek hair reduction on the forearm or on the thigh, because those are graft sites in preparation for phalloplasty. As a resource for transgender care, he recommends the UCSF Transgender Care website.
As for future directions in the field, Dr. Ibrahimi predicted that hair removal devices for home use will continue to improve and become more widespread. “This raises a host of considerations, from the risk of eye damage to the risk for paradoxical hypertrichosis, and what happens when pigmented lesions get treated with these low-powered machines compared to the ones we have in our office,” he said. “I also think we’re going to see office-based devices with larger spot sizes, smarter devices that are capable of taking over more of the functions we do. I’m most excited about the potential for treating nonpigmented white hair or poorly pigmented blond or reddish hair in the future.”
Dr. Ibrahimi disclosed that he has received research funding and speaker honoraria from Lutronic, Lumenis, Cutera, and Syneron-Candela. He also holds stock in AVAVA Inc.
Omar A. Ibrahimi, MD, PhD, hears some dermatology colleagues say they don’t bother to offer laser hair removal in their practices because they figure that the procedure is under the purview of medical spas, but he sees it differently.
“I offer laser hair removal in my practice as a way to protect my patients from being picked off by medical spas,” Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, said during a virtual course on laser and aesthetic skin therapy. “These patients are going to want to get laser hair removal. If they’re not going to have the opportunity to get it at your practice, they’re going to seek it elsewhere. When they go elsewhere, they’re going to be picked off for other procedures as well.”
First developed in 1995 by R. Rox Anderson, MD, and colleagues at The Wellman Center for Photomedicine, laser hair removal has become the gold standard for permanent hair destruction, and ranks as the most common energy-based procedure performed in the world, Dr. Ibrahimi said. “Results are very long lasting and durable beyond 2 years after treatment,” he said. “These patients tend to be highly satisfied and have permanence with these treatments.”
Treatment goal, patient selection
While the target chromophore for the procedure is melanin, the goal is to destroy the stem cells located in the hair bulge and the hair bulb. “This is technically called the extended theory of selective photothermolysis, but it’s the same concept except that our target chromophore and our desired target for destruction are slightly spatially separated,” he said.
Proper patient selection is key, so a focused medical history and physical exam are essential prior to the procedure. If unwanted hair is located on the face, jawline, or chest of a female, consider and ask about potential endocrine-related dysfunctions such as polycystic ovary syndrome (PCOS). “Getting those addressed can often help the hypertrichosis as well,” he said. “Another condition is explosive hypertrichosis where hair growth starts very suddenly. It’s uncommon but it’s something to think about.”
Pregnancy is not an absolute contraindication for laser hair removal, Dr. Ibrahimi continued, but he elects not to perform the procedure on pregnant patients. He also asks patients about any history of photosensitivity, active infection at the intended treatment site, keloids, or hypertrophic scarring. Past methods of hair removal also matter. “What we’re targeting is the pigment in the hair shafts,” he said. “So, if your patient is waxing or plucking or epilating or removing the hair in some manner, they’re actually removing the target chromophore.”
Patients with darker Fitzpatrick skin types can be treated safely but tanned individuals face a risk of complications because of active melanocytes. “As we approach summer in New England, we slow down the amount of hair removal we do because it’s a riskier procedure,” he said. “I recommend that my patients not get any significant amount of sun exposure a month before or after treatment.”
The color and quality of hair also drive treatment success. Black and brown terminal hairs absorb the millisecond laser energy, but white, gray, red, and light blond hairs lack adequate melanin to make them suitable target chromophores.
Excessive and unwanted body hair ranges in severity and can usually be classified as either hypertrichosis or hirsutism.
The desired clinical endpoint is perifollicular edema and erythema. Treatment parameters that can be varied with Food and Drug Administration–cleared devices include wavelength, fluence, pulse duration, spot size, and skin cooling. The most popular devices are the Alexandrite 755 nm laser; the diode 800 nm laser; and the 1064 nm Nd:YAG laser, which is safe for all skin types. “Often you have to use higher relative fluences to treat patients with the 1064 nm Nd:YAG because on the absorption spectrum, the 1064-nm wavelength has a relatively lower absorption for melanin compared to the alexandrite. However, you can still get effective, long-term hair reduction with the Nd:YAG laser,” he said (Arch Dermatol. 2008 Oct;144[10]:1323-7).
More recently, Dr. Ibrahimi and colleagues found that a 1060-nm diode laser system with multiple handpieces for permanent hair reduction was safe for all skin types, in an open label prospective study.
Higher fluences have been correlated with greater rates of permanent hair removal, but they also are more likely to cause undesired side effects. Dr. Ibrahimi advises clinicians new to laser hair removal to conduct a few different test spots and look for the desired clinical endpoint of perifollicular erythema and edema. “The highest fluence that gives you that endpoint without any adverse reactions is going to the best fluence for treatment,” he said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Do a few test spots, bring them back a week later and see which ones were tolerated well without any side effects and which weren’t. That gives you a good starting point for your treatment.”
Cooling down the epidermal melanin not only keeps the procedure safe, it’s a salve for pain. “There are a variety of methods of passive and active cooling,” said Dr. Ibrahimi, a member of the American Society for Dermatologic Surgery board of directors. “You can use something as simple as cold gel, but the active methods are better because once the method of passive application of cold gel warms up, you lose that cooling effect. You can use forced chilled air. Many commercial devices come with a cold tip which cools down the epidermal melanin. Others use dynamic cooling, which emit cryogen spray from a separate part of the handpiece. It hits right where the laser pulse is going to go, is absorbed by the skin, and it cools down the epidermal melanin.”
Treatment complications
Complications that can occur from treatment include pigmentary changes such as hyperpigmentation and hypopigmentation. “In lighter skinned individuals, sometimes excess fluence can lead to an erythematous appearance,” he said. “In darker-skinned individuals, this often manifests as hyperpigmentation and can be very long-lasting.” Dr. Ibrahimi ranks improper technique as a complication, “because ideally you want to lay your pulses down with 10%-15% overlap during treatment,” he explained. “If you don’t overlap, you’re going to have zones that don’t get any of the laser photons. If you do, then your patient is not going to be happy with you.”
Paradoxical hypertrichosis occurs in 1%-5% of patients, typically in women from Mediterranean, Middle Eastern, or South Asian ethnic backgrounds. This tends to develop on the lateral or jawline part of the face. “Often it occurs in the setting where they come in and want these vellus hairs treated,” he said. “Somehow the laser, instead of destroying the hair shaft, triggers it and stimulates it and can’t differentiate a vellus hair from a terminal hair. This is important to discuss during your informed consent, especially when you’re treating on the lateral jawline or the sideburn area. If this happens, you can treat through it.”
Transgender patients and future directions
Dr. Ibrahimi pointed out that increasing numbers of transgender patients are visiting dermatologists seeking laser hair removal. About 16 million Americans are estimated to have a gender identity that differs from the one assigned to them at birth, yet they face several barriers to care, “ranging from ignorance on our end to maybe our own biases being transposed onto these patients,” he said. “We really need to do a better job for them. We really have an obligation to provide good care for all of our patients.”
Transgender women typically seek hair removal on the face and neck as well as in the genital area to remove hairs in preparation for vaginoplasty. Transgender men typically seek hair reduction on the forearm or on the thigh, because those are graft sites in preparation for phalloplasty. As a resource for transgender care, he recommends the UCSF Transgender Care website.
As for future directions in the field, Dr. Ibrahimi predicted that hair removal devices for home use will continue to improve and become more widespread. “This raises a host of considerations, from the risk of eye damage to the risk for paradoxical hypertrichosis, and what happens when pigmented lesions get treated with these low-powered machines compared to the ones we have in our office,” he said. “I also think we’re going to see office-based devices with larger spot sizes, smarter devices that are capable of taking over more of the functions we do. I’m most excited about the potential for treating nonpigmented white hair or poorly pigmented blond or reddish hair in the future.”
Dr. Ibrahimi disclosed that he has received research funding and speaker honoraria from Lutronic, Lumenis, Cutera, and Syneron-Candela. He also holds stock in AVAVA Inc.
Omar A. Ibrahimi, MD, PhD, hears some dermatology colleagues say they don’t bother to offer laser hair removal in their practices because they figure that the procedure is under the purview of medical spas, but he sees it differently.
“I offer laser hair removal in my practice as a way to protect my patients from being picked off by medical spas,” Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, said during a virtual course on laser and aesthetic skin therapy. “These patients are going to want to get laser hair removal. If they’re not going to have the opportunity to get it at your practice, they’re going to seek it elsewhere. When they go elsewhere, they’re going to be picked off for other procedures as well.”
First developed in 1995 by R. Rox Anderson, MD, and colleagues at The Wellman Center for Photomedicine, laser hair removal has become the gold standard for permanent hair destruction, and ranks as the most common energy-based procedure performed in the world, Dr. Ibrahimi said. “Results are very long lasting and durable beyond 2 years after treatment,” he said. “These patients tend to be highly satisfied and have permanence with these treatments.”
Treatment goal, patient selection
While the target chromophore for the procedure is melanin, the goal is to destroy the stem cells located in the hair bulge and the hair bulb. “This is technically called the extended theory of selective photothermolysis, but it’s the same concept except that our target chromophore and our desired target for destruction are slightly spatially separated,” he said.
Proper patient selection is key, so a focused medical history and physical exam are essential prior to the procedure. If unwanted hair is located on the face, jawline, or chest of a female, consider and ask about potential endocrine-related dysfunctions such as polycystic ovary syndrome (PCOS). “Getting those addressed can often help the hypertrichosis as well,” he said. “Another condition is explosive hypertrichosis where hair growth starts very suddenly. It’s uncommon but it’s something to think about.”
Pregnancy is not an absolute contraindication for laser hair removal, Dr. Ibrahimi continued, but he elects not to perform the procedure on pregnant patients. He also asks patients about any history of photosensitivity, active infection at the intended treatment site, keloids, or hypertrophic scarring. Past methods of hair removal also matter. “What we’re targeting is the pigment in the hair shafts,” he said. “So, if your patient is waxing or plucking or epilating or removing the hair in some manner, they’re actually removing the target chromophore.”
Patients with darker Fitzpatrick skin types can be treated safely but tanned individuals face a risk of complications because of active melanocytes. “As we approach summer in New England, we slow down the amount of hair removal we do because it’s a riskier procedure,” he said. “I recommend that my patients not get any significant amount of sun exposure a month before or after treatment.”
The color and quality of hair also drive treatment success. Black and brown terminal hairs absorb the millisecond laser energy, but white, gray, red, and light blond hairs lack adequate melanin to make them suitable target chromophores.
Excessive and unwanted body hair ranges in severity and can usually be classified as either hypertrichosis or hirsutism.
The desired clinical endpoint is perifollicular edema and erythema. Treatment parameters that can be varied with Food and Drug Administration–cleared devices include wavelength, fluence, pulse duration, spot size, and skin cooling. The most popular devices are the Alexandrite 755 nm laser; the diode 800 nm laser; and the 1064 nm Nd:YAG laser, which is safe for all skin types. “Often you have to use higher relative fluences to treat patients with the 1064 nm Nd:YAG because on the absorption spectrum, the 1064-nm wavelength has a relatively lower absorption for melanin compared to the alexandrite. However, you can still get effective, long-term hair reduction with the Nd:YAG laser,” he said (Arch Dermatol. 2008 Oct;144[10]:1323-7).
More recently, Dr. Ibrahimi and colleagues found that a 1060-nm diode laser system with multiple handpieces for permanent hair reduction was safe for all skin types, in an open label prospective study.
Higher fluences have been correlated with greater rates of permanent hair removal, but they also are more likely to cause undesired side effects. Dr. Ibrahimi advises clinicians new to laser hair removal to conduct a few different test spots and look for the desired clinical endpoint of perifollicular erythema and edema. “The highest fluence that gives you that endpoint without any adverse reactions is going to the best fluence for treatment,” he said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Do a few test spots, bring them back a week later and see which ones were tolerated well without any side effects and which weren’t. That gives you a good starting point for your treatment.”
Cooling down the epidermal melanin not only keeps the procedure safe, it’s a salve for pain. “There are a variety of methods of passive and active cooling,” said Dr. Ibrahimi, a member of the American Society for Dermatologic Surgery board of directors. “You can use something as simple as cold gel, but the active methods are better because once the method of passive application of cold gel warms up, you lose that cooling effect. You can use forced chilled air. Many commercial devices come with a cold tip which cools down the epidermal melanin. Others use dynamic cooling, which emit cryogen spray from a separate part of the handpiece. It hits right where the laser pulse is going to go, is absorbed by the skin, and it cools down the epidermal melanin.”
Treatment complications
Complications that can occur from treatment include pigmentary changes such as hyperpigmentation and hypopigmentation. “In lighter skinned individuals, sometimes excess fluence can lead to an erythematous appearance,” he said. “In darker-skinned individuals, this often manifests as hyperpigmentation and can be very long-lasting.” Dr. Ibrahimi ranks improper technique as a complication, “because ideally you want to lay your pulses down with 10%-15% overlap during treatment,” he explained. “If you don’t overlap, you’re going to have zones that don’t get any of the laser photons. If you do, then your patient is not going to be happy with you.”
Paradoxical hypertrichosis occurs in 1%-5% of patients, typically in women from Mediterranean, Middle Eastern, or South Asian ethnic backgrounds. This tends to develop on the lateral or jawline part of the face. “Often it occurs in the setting where they come in and want these vellus hairs treated,” he said. “Somehow the laser, instead of destroying the hair shaft, triggers it and stimulates it and can’t differentiate a vellus hair from a terminal hair. This is important to discuss during your informed consent, especially when you’re treating on the lateral jawline or the sideburn area. If this happens, you can treat through it.”
Transgender patients and future directions
Dr. Ibrahimi pointed out that increasing numbers of transgender patients are visiting dermatologists seeking laser hair removal. About 16 million Americans are estimated to have a gender identity that differs from the one assigned to them at birth, yet they face several barriers to care, “ranging from ignorance on our end to maybe our own biases being transposed onto these patients,” he said. “We really need to do a better job for them. We really have an obligation to provide good care for all of our patients.”
Transgender women typically seek hair removal on the face and neck as well as in the genital area to remove hairs in preparation for vaginoplasty. Transgender men typically seek hair reduction on the forearm or on the thigh, because those are graft sites in preparation for phalloplasty. As a resource for transgender care, he recommends the UCSF Transgender Care website.
As for future directions in the field, Dr. Ibrahimi predicted that hair removal devices for home use will continue to improve and become more widespread. “This raises a host of considerations, from the risk of eye damage to the risk for paradoxical hypertrichosis, and what happens when pigmented lesions get treated with these low-powered machines compared to the ones we have in our office,” he said. “I also think we’re going to see office-based devices with larger spot sizes, smarter devices that are capable of taking over more of the functions we do. I’m most excited about the potential for treating nonpigmented white hair or poorly pigmented blond or reddish hair in the future.”
Dr. Ibrahimi disclosed that he has received research funding and speaker honoraria from Lutronic, Lumenis, Cutera, and Syneron-Candela. He also holds stock in AVAVA Inc.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Risk-based antenatal type-and-screen blood testing safe and economical
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
Implementing a selective type-and-screen blood testing policy in the labor and delivery unit was associated with projected annual savings of close to $200,000, a large single-center study found. Furthermore, there was no evidence of increased maternal morbidity in the university-based facility performing more than 4,400 deliveries per year, according to Ashley E. Benson, MD, MA, of the department of obstetrics and gynecology at the University of Utah, Salt Lake City, and colleagues.
The study, published in Obstetrics & Gynecology, evaluated patient safety, resource utilization, and transfusion-related costs after a policy change from universal type and screen to selective, risk-based type and screen on admission to labor and delivery.
“There had been some national interest in moving toward decreased resource utilization, and findings that universal screening was not cost effective,” Dr. Benson, who has since relocated to Oregon Health & Science University, Portland, said in an interview. An earlier cost-effective modeling study at her center had suggested that universal test and screen was not cost effective and likely not safer either. “So based on that data we felt an implementation study was warranted.”
The switch to a selective policy was made in 2018, after which her group compared outcomes from October 2017 to September 2019, looking those both 1 year preimplementation and 1 year post implementation.
One year post implementation, the following outcomes emerged, compared with preimplementation:
- Overall projected saving of $181,000 a year in the maternity unit
- Lower mean monthly type- and screen-related costs, such as those for ABO typing, antibody screen, and antibody workup. cross-matches, hold clots, and transfused products: $9,753 vs. $20,676 in the preimplementation year (P < .001)
- A lower mean monthly cost of total transfusion preparedness: $25,090 vs. $39,211 (P < .001)
- No differences in emergency-release transfusion events (four vs. three, P = .99),the study’s primary safety outcome
- Fewer emergency-release red blood cell units transfused (9 vs. 24, P = .002) and O-negative RBC units transfused (8 vs. 18, P = .016)
- No differences in hysterectomies (0.05% vs. 0.1%, P = .44) and ICU admissions (0.45% vs. 0.51%, P = .43)
“In a year of selective type and screen, we saw a 51% reduction in costs related to type and screen, and a 38% reduction in overall transfusion-related costs,” the authors wrote. “This study supports other literature suggesting that more judicious use of type and screen may be safe and cost effective.”
Dr. Benson said the results were positively received when presented a meeting 2 years ago but the published version has yet to prompt feedback.
The study
Antepartum patients underwent transfusion preparedness tests according to the center’s standard antenatal admission order sets and were risk stratified in alignment with California Maternal Quality Care Collaborative recommendations. The mean maternal age of patients in both time periods was similar at just over 29 years and the mean gestational age at delivery was just under 38 weeks.
Under the new policy, a “hold clot” is obtained for women stratified as low or medium risk on admission. In this instance, a tube of patient blood is held in the blood bank but processed only if needed, as in the event of active hemorrhage or an order for transfusion. A blood cross-match is obtained on all women stratified as high risk or having a prior positive antibody screen.
Relevant costs were the direct costs of transfusion-related testing in the labor and delivery unit from a health system perspective.
Obstetric hemorrhage is the leading cause of maternal death worldwide, the authors pointed out. While transfusion in obstetric patients occurs in only 1% or 2% of all deliveries it is nevertheless difficult to predict which patients will need transfusion, with only 2%-8% of those stratified as high risk ultimately requiring transfusion. Although obstetric hemorrhage safety bundles recommend risk stratification on admission to labor and delivery with selective type and screen for higher-risk individuals, for safety and simplicity’s sake, many labor and delivery units perform universal type and screen.
The authors cautioned that these results occurred in an academic tertiary care center with systems fine-tuned to deal with active hemorrhage and deliver timely transfusable blood. “At the moment we don’t have enough data to say whether the selective approach would be safe in hospitals with more limited blood bank capacity and access and fewer transfusion specialists in a setting optimized to respond to emergent needs, Dr. Benson said.
Katayoun F. M. Fomani, MD, a transfusion medicine specialist and medical director of blood bank and transfusion services at Long Island Jewish Medical Center, New York, agreed. “This approach only works in a controlled environment such as in this study where eligible women were assessed antenatally at the same center, but it would not work at every institution,” she said in an interview. “In addition, all patients were assessed according to the California Collaborative guideline, which itself increases the safety level but is not followed everywhere.”
The obstetric division at her hospital in New York adheres to the universal type and screen. “We have patients coming in from outside whose antenatal testing was not done at our hospital,” she said. “For this selective approach to work you need a controlled population and the electronic resources and personnel to follow each patient carefully.”
The authors indicated no specific funding for this study and disclosed no potential conflicts of interest. Dr. Fomani had no potential competing interests to declare.
FROM OBSTETRICS & GYNECOLOGY
The missing puzzle piece
Mrs. Stevens died last week. She was 87.
That’s nothing new. The nature of medicine is such that you’ll see patients pass on.
But Mrs. Stevens bothers me, because even to the end I’m not sure I ever had an answer.
Her case began with somewhat nebulous, but clearly neurological, symptoms. An initial workup was normal, as was the secondary one.
The third stage of increasingly esoteric tests turned up some clues as to what was going wrong, even as she continued to dwindle. I could at least start working on a differential, even if none of it was good.
I met with her and her husband, and they wanted an answer, good or bad.
I pulled some strings at a local tertiary subspecialty center and got her in. They agreed with my suspicions, though also couldn’t find something definitive. They even repeated the tests, and came to the same conclusions – narrowed down to a few things, but no smoking gun.
Throughout all of this Mrs. Stevens kept spiraling down. After a few hospital admissions and even a biopsy of an abdominal mass we thought would give us the answer, we still didn’t solve the puzzle.
At some point she and her husband grew tired of looking and accepted that it wouldn’t change anything. Her internist called hospice in. They kept her comfortable for her last few weeks.
They didn’t want an autopsy, so the secret stayed with her.
Looking back, I agree with their decision to stop the workup. When looking further won’t change anything, why bother?
But, as a doctor, it’s frustrating. There’s a degree of intellectual curiosity that drives us. We want answers. We want to solve puzzles.
And sometimes we never get that final piece. Even if it’s the right decision for the patient, at the end of the day it’s still an unsolved crime to us. A reminder that,
We probably never will.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Stevens died last week. She was 87.
That’s nothing new. The nature of medicine is such that you’ll see patients pass on.
But Mrs. Stevens bothers me, because even to the end I’m not sure I ever had an answer.
Her case began with somewhat nebulous, but clearly neurological, symptoms. An initial workup was normal, as was the secondary one.
The third stage of increasingly esoteric tests turned up some clues as to what was going wrong, even as she continued to dwindle. I could at least start working on a differential, even if none of it was good.
I met with her and her husband, and they wanted an answer, good or bad.
I pulled some strings at a local tertiary subspecialty center and got her in. They agreed with my suspicions, though also couldn’t find something definitive. They even repeated the tests, and came to the same conclusions – narrowed down to a few things, but no smoking gun.
Throughout all of this Mrs. Stevens kept spiraling down. After a few hospital admissions and even a biopsy of an abdominal mass we thought would give us the answer, we still didn’t solve the puzzle.
At some point she and her husband grew tired of looking and accepted that it wouldn’t change anything. Her internist called hospice in. They kept her comfortable for her last few weeks.
They didn’t want an autopsy, so the secret stayed with her.
Looking back, I agree with their decision to stop the workup. When looking further won’t change anything, why bother?
But, as a doctor, it’s frustrating. There’s a degree of intellectual curiosity that drives us. We want answers. We want to solve puzzles.
And sometimes we never get that final piece. Even if it’s the right decision for the patient, at the end of the day it’s still an unsolved crime to us. A reminder that,
We probably never will.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Stevens died last week. She was 87.
That’s nothing new. The nature of medicine is such that you’ll see patients pass on.
But Mrs. Stevens bothers me, because even to the end I’m not sure I ever had an answer.
Her case began with somewhat nebulous, but clearly neurological, symptoms. An initial workup was normal, as was the secondary one.
The third stage of increasingly esoteric tests turned up some clues as to what was going wrong, even as she continued to dwindle. I could at least start working on a differential, even if none of it was good.
I met with her and her husband, and they wanted an answer, good or bad.
I pulled some strings at a local tertiary subspecialty center and got her in. They agreed with my suspicions, though also couldn’t find something definitive. They even repeated the tests, and came to the same conclusions – narrowed down to a few things, but no smoking gun.
Throughout all of this Mrs. Stevens kept spiraling down. After a few hospital admissions and even a biopsy of an abdominal mass we thought would give us the answer, we still didn’t solve the puzzle.
At some point she and her husband grew tired of looking and accepted that it wouldn’t change anything. Her internist called hospice in. They kept her comfortable for her last few weeks.
They didn’t want an autopsy, so the secret stayed with her.
Looking back, I agree with their decision to stop the workup. When looking further won’t change anything, why bother?
But, as a doctor, it’s frustrating. There’s a degree of intellectual curiosity that drives us. We want answers. We want to solve puzzles.
And sometimes we never get that final piece. Even if it’s the right decision for the patient, at the end of the day it’s still an unsolved crime to us. A reminder that,
We probably never will.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Vitamin D status may play a pivotal role in colon cancer prevention
This is according to an observational study published in the journal Gastroenterology. The study included 94,205 women (aged 25-42 years) who were followed between 1991 and 2015 during which 111 incident cases of early-onset colorectal cancer were diagnosed. Among 29,186 women who had at least one lower endoscopy from 1991 to 2011, 1,439 newly diagnosed conventional adenomas and 1,878 serrated polyps were found.
Women who consumed the highest average levels of total vitamin D of 450 IU per day, compared with those consuming less than 300 IU per day, showed a significantly reduced risk of early-onset colorectal cancer. Consuming 400 IU each day was associated with a 54% reduced risk of early-onset colorectal cancer.
“If confirmed, our findings could potentially lead to recommendations for higher vitamin D intake as an inexpensive low-risk complement to colorectal cancer screening as a prevention strategy for adults younger than age 50,” wrote the study authors, led by Edward L. Giovannucci, MD, ScD, of the Harvard School of Public Health, Boston.
Associations between vitamin D levels and colorectal cancer have been documented in review articles over the years. The link is the subject of 10 recently completed or ongoing clinical trials. Few studies have focused on early colorectal cancer and vitamin D intake. Unlike advanced colorectal cancer, the early-onset form of the disease is not as strongly associated with the traditional risk factors of a family history of colorectal cancer and it is therefore believed to be more strongly linked to other factors, such as lifestyle and diet – including vitamin D supplementation.
The evidence is in, but it’s incomplete
In addition to the new study in Gastroenterology, other observational studies, as well as laboratory and animal studies, suggest that vitamin D plays a role in inhibiting carcinogenesis. Vitamin D, researchers theorize, contains anti-inflammatory, immunomodulatory, and tumor angiogenesis properties that can slow the growth of tumors, but the evidence is mixed.
A meta-analysis of 137,567 patients published in 2013 in Preventive Medicine found an inverse association between 25-hydroxyvitamin D (25[OH]D) and total cancer mortality in women, but not among men. Three meta-analyses published in 2014 and 2019 found that vitamin D supplementation does not affect cancer incidence but does significantly reduce total cancer mortality rates by 12%-13%.
In 2019, researchers led by Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research for the American Cancer Society, described a causal relationship between circulating vitamin D and colorectal cancer risk among 17 cohorts from a pooled analysis. “Our study suggests that optimal circulating 25(OH)D concentrations for colorectal cancer risk reduction are 75-100 nmol/L, [which is] higher than current Institute of Medicine recommendations for bone health,” she and colleagues wrote. Their findings were published in the Journal of the National Cancer Institute.
The Vitamin D and Omega-3 Trial (VITAL) published in 2019 in the New England Journal of Medicine, showed no significant effect of vitamin D3 supplementation of 2,000 IU/day in lowering the risk of invasive cancer or cardiovascular events.
Despite the mixed results, studies offer valuable insights into cancer risks, said Scott Kopetz, MD, PhD, codirector of the colorectal cancer moon shot research program at the University of Texas MD Anderson Cancer Center, Houston.
The Gastroenterology study is noteworthy because it focuses on early-onset colorectal cancer, he said.
“[The authors] demonstrate for the first time that there is an association of vitamin D intake with early-onset colorectal incidence, especially in the left side of the colon and rectum where the increase in early onset colorectal cancer manifests,” Dr. Kopetz said. “The analysis suggests that it may require long-term vitamin D intake to derive the benefit, which may explain why some shorter-term randomized studies failed to demonstrate.”
In animal models, vitamin D3 is “estimated to lower the incidence of colorectal cancer by 50%,” according to Lidija Klampfer, PhD, formerly a molecular biologist and senior research scientist with the Southern Research Institute, Birmingham, Ala.
Dr. Klampfer, a founding partner of ProteXase Therapeutics, is the author of an article on vitamin D and colon cancer published in 2014 in the World Journal of Gastrointestinal Oncology.
“The levels of vitamin D3 appear to be an essential determinant for the development and progression of colon cancer and supplementation with vitamin D3 is effective in suppressing intestinal tumorigenesis in animal models,” she wrote. “Studies have shown that 1,25 dihydroxyvitamin D3 can inhibit tumor-promoting inflammation leading to the development and progression of colon cancer.”
The hazards of a vitamin D deficiency
A severe vitamin D deficiency is associated with compromised bone and muscle health, calcium absorption, immunity, heart function and it can affect mood. Other studies have linked vitamin D deficiency to colorectal cancer, blood cancers, and bowel cancer.
Serum 25(OH)D is the primary circulating form of vitamin D and is considered the best marker for assessing vitamin D status, says Karin Amrein, MD, MSc, an endocrinologist with the Medical University of Graz (Austria). She was the lead author of a review on vitamin D deficiency published in January 2020 in the European Journal of Clinical Nutrition.
The Global Consensus Recommendations define vitamin D insufficiency as 12-20 ng/mL (30-50 nmol/L) and a deficiency as a serum 25OHD concentration less than 12 ng/mL (30 nmol/L). A deficiency in adults is usually treated with 50,000 IU of vitamin D2 or D3 once weekly for 8 weeks followed by maintenance dosages of cholecalciferol (vitamin D3) at 800-1,000 IU daily from dietary and supplemental sources.
Screening is recommended for individuals who exhibit symptoms and conditions associated with a vitamin D deficiency, but there is little agreement on recommended serum levels because every individual is different, according to the U.S. Preventive Services Task Force which updated its vitamin D recommendations in April for the first time in 7 years.
This is according to an observational study published in the journal Gastroenterology. The study included 94,205 women (aged 25-42 years) who were followed between 1991 and 2015 during which 111 incident cases of early-onset colorectal cancer were diagnosed. Among 29,186 women who had at least one lower endoscopy from 1991 to 2011, 1,439 newly diagnosed conventional adenomas and 1,878 serrated polyps were found.
Women who consumed the highest average levels of total vitamin D of 450 IU per day, compared with those consuming less than 300 IU per day, showed a significantly reduced risk of early-onset colorectal cancer. Consuming 400 IU each day was associated with a 54% reduced risk of early-onset colorectal cancer.
“If confirmed, our findings could potentially lead to recommendations for higher vitamin D intake as an inexpensive low-risk complement to colorectal cancer screening as a prevention strategy for adults younger than age 50,” wrote the study authors, led by Edward L. Giovannucci, MD, ScD, of the Harvard School of Public Health, Boston.
Associations between vitamin D levels and colorectal cancer have been documented in review articles over the years. The link is the subject of 10 recently completed or ongoing clinical trials. Few studies have focused on early colorectal cancer and vitamin D intake. Unlike advanced colorectal cancer, the early-onset form of the disease is not as strongly associated with the traditional risk factors of a family history of colorectal cancer and it is therefore believed to be more strongly linked to other factors, such as lifestyle and diet – including vitamin D supplementation.
The evidence is in, but it’s incomplete
In addition to the new study in Gastroenterology, other observational studies, as well as laboratory and animal studies, suggest that vitamin D plays a role in inhibiting carcinogenesis. Vitamin D, researchers theorize, contains anti-inflammatory, immunomodulatory, and tumor angiogenesis properties that can slow the growth of tumors, but the evidence is mixed.
A meta-analysis of 137,567 patients published in 2013 in Preventive Medicine found an inverse association between 25-hydroxyvitamin D (25[OH]D) and total cancer mortality in women, but not among men. Three meta-analyses published in 2014 and 2019 found that vitamin D supplementation does not affect cancer incidence but does significantly reduce total cancer mortality rates by 12%-13%.
In 2019, researchers led by Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research for the American Cancer Society, described a causal relationship between circulating vitamin D and colorectal cancer risk among 17 cohorts from a pooled analysis. “Our study suggests that optimal circulating 25(OH)D concentrations for colorectal cancer risk reduction are 75-100 nmol/L, [which is] higher than current Institute of Medicine recommendations for bone health,” she and colleagues wrote. Their findings were published in the Journal of the National Cancer Institute.
The Vitamin D and Omega-3 Trial (VITAL) published in 2019 in the New England Journal of Medicine, showed no significant effect of vitamin D3 supplementation of 2,000 IU/day in lowering the risk of invasive cancer or cardiovascular events.
Despite the mixed results, studies offer valuable insights into cancer risks, said Scott Kopetz, MD, PhD, codirector of the colorectal cancer moon shot research program at the University of Texas MD Anderson Cancer Center, Houston.
The Gastroenterology study is noteworthy because it focuses on early-onset colorectal cancer, he said.
“[The authors] demonstrate for the first time that there is an association of vitamin D intake with early-onset colorectal incidence, especially in the left side of the colon and rectum where the increase in early onset colorectal cancer manifests,” Dr. Kopetz said. “The analysis suggests that it may require long-term vitamin D intake to derive the benefit, which may explain why some shorter-term randomized studies failed to demonstrate.”
In animal models, vitamin D3 is “estimated to lower the incidence of colorectal cancer by 50%,” according to Lidija Klampfer, PhD, formerly a molecular biologist and senior research scientist with the Southern Research Institute, Birmingham, Ala.
Dr. Klampfer, a founding partner of ProteXase Therapeutics, is the author of an article on vitamin D and colon cancer published in 2014 in the World Journal of Gastrointestinal Oncology.
“The levels of vitamin D3 appear to be an essential determinant for the development and progression of colon cancer and supplementation with vitamin D3 is effective in suppressing intestinal tumorigenesis in animal models,” she wrote. “Studies have shown that 1,25 dihydroxyvitamin D3 can inhibit tumor-promoting inflammation leading to the development and progression of colon cancer.”
The hazards of a vitamin D deficiency
A severe vitamin D deficiency is associated with compromised bone and muscle health, calcium absorption, immunity, heart function and it can affect mood. Other studies have linked vitamin D deficiency to colorectal cancer, blood cancers, and bowel cancer.
Serum 25(OH)D is the primary circulating form of vitamin D and is considered the best marker for assessing vitamin D status, says Karin Amrein, MD, MSc, an endocrinologist with the Medical University of Graz (Austria). She was the lead author of a review on vitamin D deficiency published in January 2020 in the European Journal of Clinical Nutrition.
The Global Consensus Recommendations define vitamin D insufficiency as 12-20 ng/mL (30-50 nmol/L) and a deficiency as a serum 25OHD concentration less than 12 ng/mL (30 nmol/L). A deficiency in adults is usually treated with 50,000 IU of vitamin D2 or D3 once weekly for 8 weeks followed by maintenance dosages of cholecalciferol (vitamin D3) at 800-1,000 IU daily from dietary and supplemental sources.
Screening is recommended for individuals who exhibit symptoms and conditions associated with a vitamin D deficiency, but there is little agreement on recommended serum levels because every individual is different, according to the U.S. Preventive Services Task Force which updated its vitamin D recommendations in April for the first time in 7 years.
This is according to an observational study published in the journal Gastroenterology. The study included 94,205 women (aged 25-42 years) who were followed between 1991 and 2015 during which 111 incident cases of early-onset colorectal cancer were diagnosed. Among 29,186 women who had at least one lower endoscopy from 1991 to 2011, 1,439 newly diagnosed conventional adenomas and 1,878 serrated polyps were found.
Women who consumed the highest average levels of total vitamin D of 450 IU per day, compared with those consuming less than 300 IU per day, showed a significantly reduced risk of early-onset colorectal cancer. Consuming 400 IU each day was associated with a 54% reduced risk of early-onset colorectal cancer.
“If confirmed, our findings could potentially lead to recommendations for higher vitamin D intake as an inexpensive low-risk complement to colorectal cancer screening as a prevention strategy for adults younger than age 50,” wrote the study authors, led by Edward L. Giovannucci, MD, ScD, of the Harvard School of Public Health, Boston.
Associations between vitamin D levels and colorectal cancer have been documented in review articles over the years. The link is the subject of 10 recently completed or ongoing clinical trials. Few studies have focused on early colorectal cancer and vitamin D intake. Unlike advanced colorectal cancer, the early-onset form of the disease is not as strongly associated with the traditional risk factors of a family history of colorectal cancer and it is therefore believed to be more strongly linked to other factors, such as lifestyle and diet – including vitamin D supplementation.
The evidence is in, but it’s incomplete
In addition to the new study in Gastroenterology, other observational studies, as well as laboratory and animal studies, suggest that vitamin D plays a role in inhibiting carcinogenesis. Vitamin D, researchers theorize, contains anti-inflammatory, immunomodulatory, and tumor angiogenesis properties that can slow the growth of tumors, but the evidence is mixed.
A meta-analysis of 137,567 patients published in 2013 in Preventive Medicine found an inverse association between 25-hydroxyvitamin D (25[OH]D) and total cancer mortality in women, but not among men. Three meta-analyses published in 2014 and 2019 found that vitamin D supplementation does not affect cancer incidence but does significantly reduce total cancer mortality rates by 12%-13%.
In 2019, researchers led by Marjorie McCullough, ScD, RD, senior scientific director of epidemiology research for the American Cancer Society, described a causal relationship between circulating vitamin D and colorectal cancer risk among 17 cohorts from a pooled analysis. “Our study suggests that optimal circulating 25(OH)D concentrations for colorectal cancer risk reduction are 75-100 nmol/L, [which is] higher than current Institute of Medicine recommendations for bone health,” she and colleagues wrote. Their findings were published in the Journal of the National Cancer Institute.
The Vitamin D and Omega-3 Trial (VITAL) published in 2019 in the New England Journal of Medicine, showed no significant effect of vitamin D3 supplementation of 2,000 IU/day in lowering the risk of invasive cancer or cardiovascular events.
Despite the mixed results, studies offer valuable insights into cancer risks, said Scott Kopetz, MD, PhD, codirector of the colorectal cancer moon shot research program at the University of Texas MD Anderson Cancer Center, Houston.
The Gastroenterology study is noteworthy because it focuses on early-onset colorectal cancer, he said.
“[The authors] demonstrate for the first time that there is an association of vitamin D intake with early-onset colorectal incidence, especially in the left side of the colon and rectum where the increase in early onset colorectal cancer manifests,” Dr. Kopetz said. “The analysis suggests that it may require long-term vitamin D intake to derive the benefit, which may explain why some shorter-term randomized studies failed to demonstrate.”
In animal models, vitamin D3 is “estimated to lower the incidence of colorectal cancer by 50%,” according to Lidija Klampfer, PhD, formerly a molecular biologist and senior research scientist with the Southern Research Institute, Birmingham, Ala.
Dr. Klampfer, a founding partner of ProteXase Therapeutics, is the author of an article on vitamin D and colon cancer published in 2014 in the World Journal of Gastrointestinal Oncology.
“The levels of vitamin D3 appear to be an essential determinant for the development and progression of colon cancer and supplementation with vitamin D3 is effective in suppressing intestinal tumorigenesis in animal models,” she wrote. “Studies have shown that 1,25 dihydroxyvitamin D3 can inhibit tumor-promoting inflammation leading to the development and progression of colon cancer.”
The hazards of a vitamin D deficiency
A severe vitamin D deficiency is associated with compromised bone and muscle health, calcium absorption, immunity, heart function and it can affect mood. Other studies have linked vitamin D deficiency to colorectal cancer, blood cancers, and bowel cancer.
Serum 25(OH)D is the primary circulating form of vitamin D and is considered the best marker for assessing vitamin D status, says Karin Amrein, MD, MSc, an endocrinologist with the Medical University of Graz (Austria). She was the lead author of a review on vitamin D deficiency published in January 2020 in the European Journal of Clinical Nutrition.
The Global Consensus Recommendations define vitamin D insufficiency as 12-20 ng/mL (30-50 nmol/L) and a deficiency as a serum 25OHD concentration less than 12 ng/mL (30 nmol/L). A deficiency in adults is usually treated with 50,000 IU of vitamin D2 or D3 once weekly for 8 weeks followed by maintenance dosages of cholecalciferol (vitamin D3) at 800-1,000 IU daily from dietary and supplemental sources.
Screening is recommended for individuals who exhibit symptoms and conditions associated with a vitamin D deficiency, but there is little agreement on recommended serum levels because every individual is different, according to the U.S. Preventive Services Task Force which updated its vitamin D recommendations in April for the first time in 7 years.
FROM GASTROENTEROLOGY
Association of Healthcare Access With Intensive Care Unit Utilization and Mortality in Patients of Hispanic Ethnicity Hospitalized With COVID-19
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).
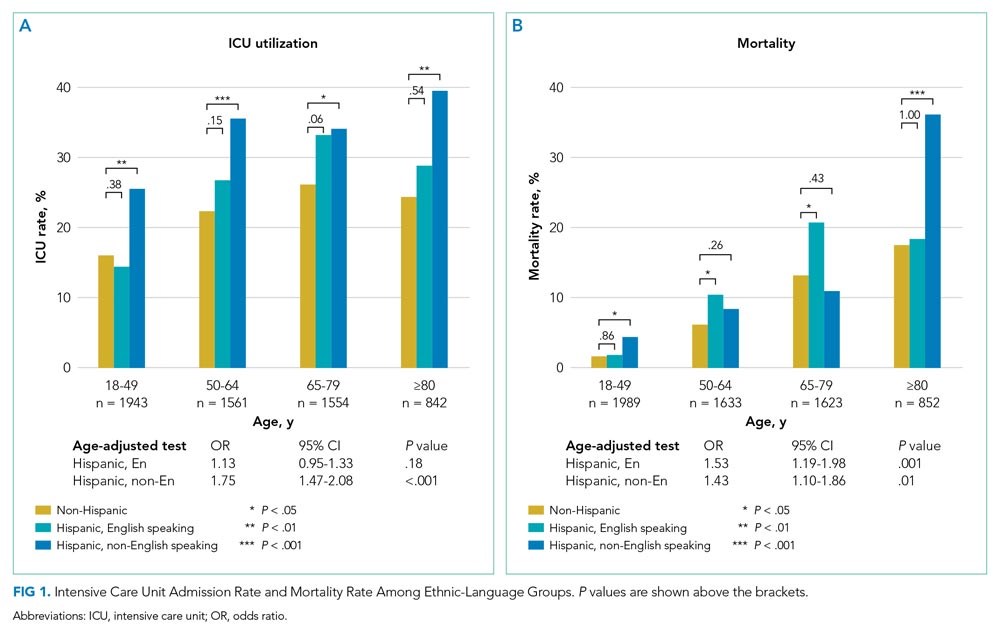
To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.
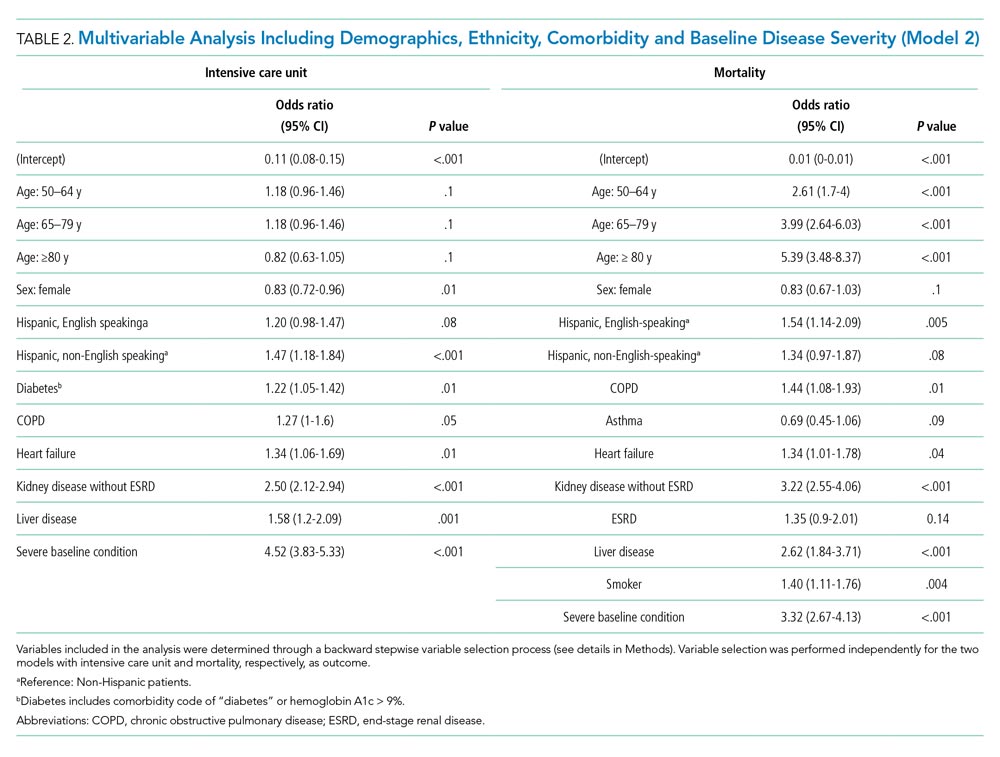
Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).

To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.

Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
In the United States, health disparities in COVID-19 outcomes (including morbidity and mortality) based on race and ethnicity have been described in the scientific literature and mainstream media.1-7 According to the US Centers for Disease Control and Prevention (CDC), Hispanic people are 3.2 times more likely to be hospitalized with COVID-19 than non-Hispanic White people.8 Further, Hispanic people diagnosed with COVID-19 are 2.3 times more likely to die, adjusted for age, than non-Hispanic White people.9 As the epicenter of the COVID-19 pandemic shifted from the Northeast to the South, the CDC reported that, among people who died from COVID-19 in the United States from May to August 2020, the percentage of Hispanic people increased from 16.3% to 26.4%.10
Published studies on the effect of ethnicity on critical illness or mortality for hospitalized COVID-19 patients are limited and inconsistent. While some studies reported a higher mortality rate for Hispanic patients,11-15 others showed no difference.4,16,17 A recent meta-analysis found that intensive care unit (ICU) utilization and mortality were slightly higher among Hispanic COVID-19 inpatients, but this finding did not reach statistical significance.18 Past studies from different healthcare systems were limited by the small sample size of hospitalized Hispanic patients and the heterogeneity of patients. A comprehensive analysis from a large healthcare system with sufficient sample size is needed to understand the impact of ethnicity on clinical outcomes of hospitalized COVID-19 patients.
Texas Health Resources (THR) is a large integrated healthcare system serving the Dallas-Fort Worth-Arlington (DFW) metropolitan area. According to the 2019 US Census Bureau American Community Survey, Hispanic people comprise 18.4% of the population of this geographic area.19 Congruent with the CDC’s findings, Hispanic patients account for a disproportionate share (32.2%) of hospitalized COVID-19 patients at THR relative to the area’s demographic composition. Aware of the increased risk, we undertook an analysis of the clinical outcomes and the clinical, social, and demographic characteristics of Hispanic patients hospitalized at THR with COVID-19. Our primary goal was to investigate whether clinical outcomes differ by ethnicity among patients hospitalized with COVID-19 and, if so, whether inpatient care or preadmission factors contribute to this difference.
Methods
Study Setting and Overview
We collected data from the single electronic health record (EHR) used by 20 THR hospitals located across the DFW metropolitan area. THR is the largest faith-based, nonprofit health system in North Texas, operating 20 acute care hospitals. Including all access points, such as outpatient facilities and physician group practices, THR serves 7 million residents in 16 counties in North Texas, of whom 16.8% are Hispanic, 73.3% are non-Hispanic, and 9.9% are unclassified, congruent with demographics in the DFW area.
The institutional review boards at THR and UT Southwestern Medical Center approved the study under a waiver of informed consent (as a minimal-risk medical record review). After collection, all data were de-identified prior to statistical analysis.
Cohort, Outcomes, and Covariables
The study cohort included 6097 adult patients with laboratory-confirmed COVID-19 (age ≥18 years) who were admitted as inpatients from March 3 to November 5, 2020. The primary outcomes included ICU utilization and death during hospitalization. We described demographic characteristics using the following variables: age (18–49, 50–64, 65–79, ≥80 years), sex, self-reported ethnicity, and primary spoken language.
We defined a severe baseline condition as an elevated respiratory subscore parsed from the overall MSOFA (Modified Sequential Organ Failure Assessment),20 an elevated Epic Deterioration Index (EDI),21 or an elevated C-reactive protein level (CRP) at baseline (any elevated CRP). Baseline referred to the variable mean during the first available 12-hour window of measurement during the COVID-19 hospital admission, including variables obtained in the emergency department (ED). An elevated MSOFA referred to a score of 4, corresponding to an SpO2/FiO2 < 150. Elevated EDI referred to a baseline EDI > 45. An elevated CRP referred to a baseline CRP > 20 mg/dL.22
Variables reflecting access to healthcare included: THR EHR creation year (representing the first time patients accessed the THR health system), insurance payor type, and presence of a primary care provider (PCP). The federal government established the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured program. The insurance payor for patients covered by this program is designated as COVID-19 HRSA. Presence of a PCP reflects any documented PCP, regardless of affiliation with THR. We selected these access metrics opportunistically, as they were consistently documented in the EHR and readily available for analysis.
We used 12 variables to describe comorbidities or underlying conditions that, according to the CDC, increased patients’ risk of severe illness from COVID-1923: diagnoses of diabetes, hypertension, obesity, chronic obstructive pulmonary disease (COPD), asthma, smoking, other lung disease, heart failure, kidney disease without end-stage renal disease (ESRD), ESRD, liver disease, and cancer. We identified comorbidities by mining the structured diagnosis codes documented in the EHR prior to and during the COVID-19 admission. Sources for diagnoses included final billed diagnosis codes, working diagnosis codes, problem list, and reason for visit. The definition of diabetes included previously recorded diabetes or baseline hemoglobin A1c > 9%. We also recorded the presence of four major COVID-19 treatments: steroids, remdesivir, tocilizumab, and fresh frozen plasma (FFP) from convalescent patients.24-26 Each treatment variable was defined by receipt of one or more doses.
Statistical Analysis
To analyze patient outcomes based on ethnicity, we divided the study cohort into a Hispanic group and a non-Hispanic group based on self-reported ethnicity in the EHR. To study the potential impact of primary language among Hispanic patients, we divided them into English-speaking and non-English-speaking patients based on their self-reported primary language. As a result, we analyzed three groups of patients: (1) non-Hispanic, (2) Hispanic and English speaking, and (3) Hispanic and non-English speaking. We tested differences of a given categorical variable across the three groups using the chi-square test for each age subgroup (18–49, 50–64, 65–79, ≥80 years). The Cochran-Mantel-Haenszel test was used for the overall difference adjusted for age. To assess whether an observed disparity in treatment existed across the three groups, we tested the difference in the administration of four major therapeutics for COVID-19, including steroids, remdesivir, tocilizumab, and convalescent plasma. To determine whether any groups had elevated disease severity at hospital admission (baseline), we tested the difference in four disease-severity metrics across the ethnic-language groups: (1) elevated respiratory MSOFA score, (2) elevated EDI, (3) elevated CRP level, and (4) any of the three conditions.
To study the associations with ICU utilization and death, respectively, we performed a multivariable analysis using a generalized linear mixed model with binomial distribution and a logit link function. In each analysis model, the hospital of admission was included as a random-effect variable to account for the potential treatment variations among different hospitals, while other variables were regarded as fixed effects. In the first multivariable analysis (Model 1), all demographic variables, including age, sex, and ethnicity, and different types of comorbidities and underlying conditions, were included as fixed-effect variables in the initial model, and then backward stepwise variable selection was performed to establish the final model (Model 1). We performed the backward stepwise variable selection separately for the outcome of ICU use or mortality. Based on Akaike information criterion (AIC), during each iteration the fixed-effect variable that led to the largest decrease in the AIC value was removed, and the variable selection process was completed when the AIC value stopped decreasing. In Model 2, we added the disease-severity variable at baseline to the selected variable set derived from Model 1 to explore its effect on the associations between ethnicity and clinical outcomes. In Model 3, we added healthcare access–related variables, including first-time healthsystem access, payor type, and PCP availability to Model 2. We performed all statistical analyses using R, version 4.0.2 (R Foundation for Statistical Computing) in RStudio (version 1.3.1093).
Results
Distinct Demographic and Comorbidity Patterns for Three Ethnic-Language Groups
We identified 6097 adult patients (age ≥18 years) who had confirmed COVID-19 disease and were hospitalized between March 3 and November 5, 2020. Demographic characteristics and comorbidity for these patients are summarized in Table 1. Among these patients, 4139 (67.9%) were non-Hispanic and 1958 (32.1%) were Hispanic. Among the Hispanic patients, 1203 (61.4%) identified English as their primary language and 755 (38.6%) identified a non-English primary language. Age distribution was vastly different among the three ethnic-language groups (Table 1). Unlike the relatively balanced distribution across different age groups in the non-Hispanic group, more than half (55.8%) of the English-speaking Hispanic patients were in the youngest age group (18-49 years). A much lower fraction of Hispanic patients was among the oldest (≥80 years) age group (P < .001). Because COVID-19 clinical outcome is strongly associated with age,27 we used age-stratified analysis when comparing group-level differences in patient outcomes.

Sex distribution also was different among the three groups, with the non-English-speaking Hispanic group having more male patients (53.0%). Diabetes and obesity, which are associated with clinical outcomes of COVID-19 patients, were more prevalent in Hispanic patients (Table 1). Non-English-speaking Hispanic patients had the highest diabetes rate (48.7% with documented diabetes; 15.8% with baseline HbA1c > 9%; P < .001). English-speaking Hispanic patients presented with the highest obesity rate (62.8%; P < .001). Appendix Table 1 provides detailed age-group-specific comorbidity distributions among ethnic-language groups.
Patients of Hispanic Ethnicity Experienced a Higher Rate of ICU Utilization and Mortality
Of the 6097 patients overall, 1365 (22.4%) were admitted to the ICU and 543 (8.9%) died in hospital. For non-Hispanic patients (n = 4139), 883 (21.3%) were admitted to the ICU and 373 (9.0%) died in hospital. For English-speaking Hispanic patients (n = 1203), 241 (20.0%) were admitted to the ICU and 91 (7.6%) died in hospital. For non-English-speaking Hispanic patients (n = 755), 241 (31.9%) were admitted to the ICU and 79 (10.5%) died in hospital. Figure 1 summarizes the age-stratified comparison of ICU utilization and mortality across the three ethnic-language patient groups. In all age groups, non-English-speaking Hispanic patients experienced a significantly higher ICU utilization rate compared to non-Hispanic patients (age-adjusted OR, 1.75; 95% CI, 1.47-2.08; P < .001). English-speaking and non-English-speaking Hispanic patients had a significantly higher mortality rate compared to non-Hispanic patients (age-adjusted OR, 1.53; 95% CI, 1.19-1.98; P = .001 for English-speaking Hispanic patients; age-adjusted OR, 1.43; 95% CI,: 1.10-1.86; P = .01 for non-English-speaking Hispanic patients).

To delineate the risk factors associated with ICU utilization and death, we performed multivariable logistic regression with stepwise variable selection. After adjusting for age, sex, and comorbidity (Model 1), the factors ethnicity and primary language were still strongly associated with ICU utilization and mortality (Appendix Table 2). Non-English-speaking Hispanic patients had an OR of 1.74 (95% CI, 1.41-2.15; P < .001) for ICU utilization and an OR of 1.54 (95% CI, 1.12-2.12; P = .008) for mortality compared to non-Hispanic patients. Similarly, English-speaking Hispanic patients had higher ICU utilization (OR, 1.28; 95% CI, 1.05-1.55; P = .01) and a higher mortality rate (OR, 1.60; 95% CI, 1.19-2.14; P = .002).
No Disparity in COVID-19 Therapeutics Observed Across Three Ethnic-Language Groups
Appendix Figure 1 summarizes the comparison of the administration of four major treatments across the three ethnic-language groups. We did not observe any underuse of COVID-19 therapeutics for Hispanic patients. Usage rates for these therapies were significantly higher, after adjusting for age, in Hispanic groups when compared to non-Hispanic patients (OR ranged from 1.21 to 1.96). Steroids were the most common treatment in all patient groups. Tocilizumab was used almost twice as frequently (OR, 1.96; 95% CI, 1.64-2.33; P < .001) in non-English-speaking Hispanic patients compared to non-Hispanic patients.
Patients of Hispanic Ethnicity Had More Severe Disease at Hospital Admission
Figure 2 shows that non-English-speaking Hispanic patients had a higher rate of severe illness at admission based on each of these metrics: high respiratory MSOFA score (OR, 2.43; 95% CI, 1.77-3.33; P < .001), high EDI (OR, 1.85; 95% CI, 1.41-2.41; P < .001), and high CRP level (OR, 2.06; 95% CI, 1.64-2.58; P < .001). English-speaking Hispanic patients also had a greater rate of high CRP level (OR, 1.48; 95% CI, 1.17-1.86; P = .001) compared to non-Hispanic patients. When considering the presentation of any one of these clinical indicators, the English-speaking and non-English-speaking Hispanic patients had a higher rate of severe baseline condition (OR, 1.33; 95% CI, 1.10-1.61; P = .004 for English-speaking patients; OR, 2.27; 95% CI, 1.89-2.72; P < .001 for non-English-speaking patients).

We then studied how the baseline disease condition affects the association between ethnicity and clinical outcomes. We performed a multivariable analysis including baseline disease severity as a covariable (Model 2, Table 2), which showed that baseline disease severity was strongly associated with ICU admission (OR, 4.52; 95% CI, 3.83-5.33; P < .001) and mortality (OR, 3.32; 95% CI, 2.67-4.13; P < .001). The associations between ethnicity and clinical outcomes were reduced after considering the baseline disease condition. The OR dropped to 1.47 (95% CI, 1.18-1.84; P < .001) and 1.34 (95% CI, 0.97-1.87; P = .08) for ICU utilization and mortality, respectively, when comparing non-English-speaking Hispanic patients to non-Hispanic patients. A similar reduction was observed for English-speaking Hispanic patients. Model comparison showed a significant improvement of Model 2 over Model 1 based on ANOVA test (P < .001) as well as AIC.

Hispanic Patients Had Worse Healthcare Access
To explore the etiology for the more severe disease conditions at hospital admission among Hispanic patients, we analyzed variables related to healthcare access. We found that Hispanic patients were likely to have reduced access to healthcare (Table 1; Appendix Figure 2). For a large proportion (16.9%) of the COVID-19 patients in this study, their medical records were first created at THR in 2020, corresponding to the initial time these patients accessed THR for their healthcare. This surge in 2020, compared to previous years with data (2005–2019), corresponds to the number of new patients seen because of COVID-19 (Appendix Figure 2A). Among this new patient population, the proportion of non-English-speaking Hispanic patients in 2020 was 28.3%, compared to 9.1% from 2005 to 2019 (P < .001). The proportion of new English-speaking Hispanic patients in 2020 was 22.1%, compared to an average of 19.2% from 2005 to 2019 (P < .001). In addition, a much smaller proportion of Hispanic patients had a PCP (P < .001) (Table 1; Appendix Figure 2B), with non-English-speaking Hispanic patients having the smallest proportion (58.5%).
Appendix Figure 2C illustrates the comparison of payor types across the three patient groups. A much higher proportion of Hispanic patients used COVID-19 HRSA (P < .001) compared to non-Hispanic patients. Breaking this down further by primary language, 29.1% of non-English-speaking Hispanic patients relied on COVID-19 HRSA due to otherwise uninsured status, compared to 12.7% of English-speaking Hispanic patients and only 5.1% of non-Hispanic patients. Similarly, non-English-speaking Hispanic patients have the highest self-pay rates (2.3%) compared to English-speaking Hispanic patients (1.4%) and non-Hispanic patients (0.7%). In summary, more Hispanic patients, and especially non-English-speaking Hispanic patients, lacked conventional health insurance and experienced limited access to healthcare.
Further evidence showed a trend of correlation between presentation of severe COVID-19 conditions when arriving at the hospital and each of the healthcare access factors analyzed (Appendix Figure 3).
Discussion
With a large sample size of hospitalized COVID-19 patients at an integrated health system in the DFW metropolitan area, we observed an increased rate of ICU utilization and mortality among Hispanic inpatients. After adjusting for age, we found that non-English-speaking Hispanic patients were 75% more likely to require critical care compared with non-Hispanic patients. English-speaking and non-English-speaking Hispanic patients had an increased mortality rate (age-adjusted) compared to non-Hispanic patients. The association between ethnicity and clinical outcomes remained significant after adjusting for age, sex, and comorbidities. We did not observe any underuse of major COVID-19 therapeutics in Hispanic patients, and excluded in-hospital treatments from the contributors to the outcome differences.
Hispanic patients, especially non-English-speaking Hispanic patients, had a higher rate of severe COVID-19 disease at the time of hospital admission (Figure 2). After including baseline disease severity into the multivariable analysis (Model 2), the overall model improved (P < .001) while the associations between ethnicity and outcomes decreased (Table 2). This suggests disease severity at admission was a main contributor to the observed associations between ethnicity and clinical outcomes. The higher rate of baseline COVID-19 severity in Hispanic patients might also explain their higher rate of receiving major COVID-19 therapeutics (Appendix Figure 1).
This study found that Hispanic patients were less likely to have a PCP and insurance coverage compared with non-Hispanic patients (P < .001). This disparity was more pronounced among non-English-speaking Hispanic patients (Appendix Figure 2). We also observed that a disproportionately larger proportion (50.4%) of patients who visited the healthcare system for the first time in 2020 (the year of the COVID-19 pandemic) was composed of Hispanic patients, compared to merely 28.4% prior to 2020. While there is a possibility that patients had primary care outside THR, the staggering number of Hispanic patients who were new to the health system in 2020, in conjunction with the fact that immigrants tend to be “healthier” compared to their native-born peers (the so-called immigrant paradox),28 led us to conclude that there were few other primary care options for these patients, making THR’s ED the primary care option of choice. The systemic, structural barriers to routine care might be a possible cause for delayed admission and, in turn, elevated baseline COVID-19 severity for Hispanic patients (Appendix Figure 3).
Recent studies have investigated the impact of socioeconomic factors on racial/ethnic disparities in the COVID-19 pandemic.7,16,17 To our knowledge, no study has directly analyzed the link between healthcare access metrics, COVID-19 severity at admission, and the Hispanic population stratified by primary language. Studies exist on this subject for other diseases, however. For example, healthcare access factors have been associated with sepsis-related mortality.29,30 In fact, a recent study that explored the potential effect of language barriers on healthcare access demonstrated an association between limited English proficiency and sepsis-related mortality.31 Our study found that Hispanic patients whose primary language is not English had the worst clinical outcomes, including more severe baseline COVID-19 conditions, and the least access to healthcare, highlighting the importance of addressing language barriers in COVID-19 care. Further research is needed to confirm the relationship between limited English proficiency and clinical outcomes, as well as potential factors that contribute to such a relationship in different types of diseases.
Our study has a number of limitations. First, it was limited to only one large healthcare system, which means the results may not be generalizable. Because THR is an open system, comorbidity data may be incomplete, and we cannot exclude the possibility that patients accessed care outside THR prior to or during the pandemic. We may overcome this limitation in the future with cross-system health information exchange data. Second, we did not have data for the time of symptom onset, so we were unable to analyze the direct evidence of the possible delayed care. As a result, we were unable to analyze whether treatments were administered in a timely manner or appropriately. Third, our analysis was not adjusted for other socioeconomic factors (eg, income, education) due to lack of data. We used self-identification for ethnicity, but unlike new approaches by the U.S. Census Bureau,32 our survey allowed only one choice to be selected.
Conclusion
Sociodemographic factors among Hispanic inpatients hospitalized for COVID-19 at a large integrated health system—including a primary non-English language, lack of a PCP, and insurance status—were associated with measures of reduced access to care and more severe illness at admission. Structural barriers to care, which may be associated with reduced health literacy and less access to health insurance, can result in delayed treatment and more severe illness at admission and underdiagnosis of medical conditions, contributing to worse outcomes in this population. Our findings suggest that interventions to promote early recognition of signs and symptoms of COVID-19 and to encourage prompt clinical care at the community level may reduce the burden of COVID-19 deaths in racial or ethnic minority communities with language and socioeconomic barriers.
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
1. Lopez L III, Hart LH III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325(8):719-720. https://doi.org/10.1001/jama.2020.26443
2. Cooper LA, Williams DR. Excess deaths from COVID-19, community bereavement, and restorative justice for communities of color. JAMA. 2020;324(15):1491-1492. https://doi.org/10.1001/jama.2020.19567
3. Clay LA, Rogus S. Primary and secondary health impacts of COVID-19 among minority individuals in New York State. Int J Environ Res Public Health. 2021;18(2):683. https://doi.org/10.3390/ijerph18020683
4. Rodriguez F, Solomon N, de Lemos JA, et al. Racial and ethnic differences in presentation and outcomes for patients hospitalized with COVID-19: findings from the American Heart Association’s COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143(24):2332-2342. https://doi.org/10.1161/CIRCULATIONAHA.120.052278
5. Moreira A, Chorath K, Rajasekaran K, Burmeister F, Ahmed M, Moreira A. Demographic predictors of hospitalization and mortality in US children with COVID-19. Eur J Pediatr. 2021;180(5):1659-1663. https://doi.org/10.1007/s00431-021-03955-x
6. Kolata G. Social inequities explain racial gaps in pandemic, studies find. The New York Times. December 9, 2020. https://www.nytimes.com/2020/12/09/health/coronavirus-black-hispanic.html
7. Liao TF, De Maio F. Association of social and economic inequality with coronavirus disease 2019 incidence and mortality across US counties. JAMA Netw Open. 2021;4(1):e2034578. https://doi.org/10.1001/jamanetworkopen.2020.34578
8. Centers for Disease Control and Prevention. A Weekly Surveillance Summary of U.S. COVID-19 Activity: Key Updates for Week 2. January 21, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/pdf/covidview-01-22-2021.pdf
9. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by race/ethnicity. Updated September 9, 2021. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html
10. Gold JAW, Rossen LM, Ahmad FB, et al. Race, ethnicity, and age trends in persons who died from COVID-19 – United States, May-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(42):1517-1521. https://doi.org/10.15585/mmwr.mm6942e1
11. Pennington AF, Kompaniyets L, Summers AD, et al. Risk of clinical severity by age and race/ethnicity among adults hospitalized for COVID-19 – United States, March-September 2020. Open Forum Infect Dis. 2021;8(2):ofaa638. https://doi.org/10.1093/ofid/ofaa638.
12. Renelus BD, Khoury NC, Chandrasekaran K, et al. Racial disparities in COVID-19 hospitalization and in-hospital mortality at the height of the New York City pandemic. J Racial Ethn Health Disparities. 2021;8(5):1161-1167. https://doi.org/10.1007/s40615-020-00872-x
13. Wiley Z, Ross-Driscoll K, Wang Z, Smothers L, Mehta AK, Patzer RE. Racial and ethnic differences and clinical outcomes of COVID-19 patients presenting to the emergency department. Clin Infect Dis. 2021 Apr 2. [Epub ahead of print] https://doi.org/10.1093/cid/ciab290
14. Dai CL, Kornilov SA, Roper RT, et al. Characteristics and factors associated with COVID-19 infection, hospitalization, and mortality across race and ethnicity. Clin Infect Dis. 2021 Feb 20. [Epub ahead of print] https://doi.org/10.1093/cid/ciab154
15. Pan AP, Khan O, Meeks JR, et al. Disparities in COVID-19 hospitalizations and mortality among black and Hispanic patients: cross-sectional analysis from the greater Houston metropolitan area. BMC Public Health. 2021;21(1):1330. https://doi.org/10.1186/s12889-021-11431-2
16. Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3(12):e2026881. https://doi.org/10.1001/jamanetworkopen.2020.26881
17. Gershengorn HB, Patel S, Shukla B, et al. Association of race and ethnicity with COVID-19 test positivity and hospitalization is mediated by socioeconomic factors. Ann Am Thorac Soc. 2021;18(8):1326-1334. https://doi.org/10.1513/AnnalsATS.202011-1448OC
18. Sze S, Pan D, Nevill CR, et al. Ethnicity and clinical outcomes in COVID-19: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100630. https://doi.org/10.1016/j.eclinm.2020.100630
19. U.S. Census Bureau. 2019 U.S Census Bureau American Community Survey. https://www.census.gov/programs-surveys/acs
20. North Texas Mass Critical Care Task Force. North Texas Mass Critical Care Guidelines Document. Hospital and ICU Triage Guidelines for ADULTS. January 2014. https://www.dallas-cms.org/tmaimis/dcms/assets/files/communityhealth/MCC/GuidelinesAdult_JAN2014.pdf
21. Singh K, Valley TS, Tang S, et al. Evaluating a widely implemented proprietary deterioration index model among hospitalized COVID-19 patients. Ann Am Thorac Soc. 2021;18(7):1129-1137. https://doi.org/10.1513/AnnalsATS.202006-698OC
22. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489-493. https://doi.org/10.12788/jhm.3497
23. Centers for Disease Control and Prevention. Science Brief: Evidence used to update the list of underlying medical conditions that increase a person’s risk of severe illness from COVID-19. Updated May 12, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/underlying-evidence-table.html
24. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181(1):41-51. https://doi.org/10.1001/jamainternmed.2020.6252
25. Baroutjian A, Sanchez C, Boneva D, McKenney M, Elkbuli A. SARS-CoV-2 pharmacologic therapies and their safety/effectiveness according to level of evidence. Am J Emerg Med. 2020;38(11):2405-2415. https://doi.org/10.1016/j.ajem.2020.08.091
26. Janiaud P, Axfors C, Schmitt AM, et al. Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA. 2021;325(12):1185-1195. https://doi.org/10.1001/jama.2021.2747
27. Panagiotou OA, Kosar CM, White EM, et al. Risk factors associated with all-cause 30-day mortality in nursing home residents with COVID-19. JAMA Intern Med. 2021;181(4):439-448. https://doi.org/10.1001/jamainternmed.2020.7968
28. Bacong AM, Menjívar C. Recasting the immigrant health paradox through intersections of legal status and race. J Immigr Minor Health. 2021;23(5):1092-1104. https://doi.org/10.1007/s10903-021-01162-2
29. Plopper GE, Sciarretta KL, Buchman TG. Disparities in sepsis outcomes may be attributable to access to care. Crit Care Med. 2021;49(8):1358-1360. https://doi.org/10.1097/CCM.0000000000005126
30. Jones JM, Fingar KR, Miller MA, et al. Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004-2013. Crit Care Med. 2017;45(12):e1209-e1217. https://doi.org/10.1097/CCM.0000000000002699
31. Jacobs ZG, Prasad PA, Fang MC, Abe-Jones Y, Kangelaris KN. The association between limited English proficiency and sepsis mortality. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3334
32. Cohn D. Census considers new approach to asking about race – by not using the term at all. June 18, 2015. https://www.pewresearch.org/fact-tank/2015/06/18/census-considers-new-approach-to-asking-about-race-by-not-using-the-term-at-all/
© 2021 Society of Hospital Medicine
Top case
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Vikrant Parihar, MD, wrote the following in “COVID-19 and UC”:
A 43-year-old man with an index presentation of distal colitis (Montreal E2) (Mayo endoscopic score 2-3) was discharged home on tapering doses of oral steroids. He was being worked up to commence anti-TNF likely initially as combo therapy. Fully vaccinated against COVID – had both doses of vaccine way back in May. Attended a match and looks to have got mild symptoms and on testing turned out to be COVID+. Rx himself by self-quarantine.
What would be the optimal strategy?
1. Stop steroids completely and immediately given the adverse signal in registry data?
2. When can anti-TNF’s be safely started?
3. How to manage him in the interim?
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25172.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Vikrant Parihar, MD, wrote the following in “COVID-19 and UC”:
A 43-year-old man with an index presentation of distal colitis (Montreal E2) (Mayo endoscopic score 2-3) was discharged home on tapering doses of oral steroids. He was being worked up to commence anti-TNF likely initially as combo therapy. Fully vaccinated against COVID – had both doses of vaccine way back in May. Attended a match and looks to have got mild symptoms and on testing turned out to be COVID+. Rx himself by self-quarantine.
What would be the optimal strategy?
1. Stop steroids completely and immediately given the adverse signal in registry data?
2. When can anti-TNF’s be safely started?
3. How to manage him in the interim?
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25172.
Physicians with difficult patient scenarios regularly bring their questions to the AGA Community to seek advice from colleagues about therapy and disease management options, best practices, and diagnoses. Here’s a preview of a recent popular clinical discussion:
Vikrant Parihar, MD, wrote the following in “COVID-19 and UC”:
A 43-year-old man with an index presentation of distal colitis (Montreal E2) (Mayo endoscopic score 2-3) was discharged home on tapering doses of oral steroids. He was being worked up to commence anti-TNF likely initially as combo therapy. Fully vaccinated against COVID – had both doses of vaccine way back in May. Attended a match and looks to have got mild symptoms and on testing turned out to be COVID+. Rx himself by self-quarantine.
What would be the optimal strategy?
1. Stop steroids completely and immediately given the adverse signal in registry data?
2. When can anti-TNF’s be safely started?
3. How to manage him in the interim?
See how AGA members responded and join the discussion: https://community.gastro.org/posts/25172.



