User login
Inflation will be the end of medicine
Inflation is the senility of democracies.
–Sylvia Townsend Warner
Electronic medical records? No, all of these are minor annoyances in the face of the practice killer, inflation.
Physicians live in a closed box where medical reimbursements are fixed, directly or by contract proxy to the government (Medicare) pay rate. Inflation is projected to be between 5% and 10% this year. We cannot increase our rates to increase the salaries of our employees, cover our increased medical disposable costs, and pay more for our state licensures and DEA registrations. No, we must try to find savings in our budget, which we have been squeezing for years.
Currently, medicine is facing a 9.75% cut in Medicare reimbursements, which will reset most private insurance rates, based on a percentage of Medicare. The temporary 3.75% conversion factor (CF) increase for all services is expiring. Also expiring is the 2% sequester from the Budget Control Act of 2011 (BCA), signed into law in August 2011. This was originally scheduled to sunset in 2021, but is going to continue to 2030.
A 4% statutory pay-as-you-go (PAYGO) sequester resulting from passage of the American Rescue Plan Act is being imposed. Statutory PAYGO is a policy written into law (it can be changed only through new legislation) that requires deficit neutrality overall in the laws (other than annual appropriations) enacted by Congress and imposes automatic spending reductions at the end of the year if such laws increase the deficit when they are added together.
There is a statutory freeze on Medicare Physician Fee Schedule (PFS) updates until 2026, at which time an annual increase of 0.25%, which is lower than inflation, will be enacted. This adds up to a 9.75% cut in Medicare pay until at least 2026. Recall that almost all of your private insurance contracts are tied to Medicare (some more, some less) and that this cut to the physician is doubled if your overhead is fixed at the typical 50% for most practices. This means an almost 20% cut in take-home pay for most physicians.
Now, when considering the most recent inflation number, which projects 5%-10% inflation for this year and at least 2% annually in the future, which compounds yearly (the Fed target), you are looking at catastrophic numbers.
The conversion factor – the pool of money doled out to physicians – has failed to keep pace with inflation – even at 2%-3% a year – and reimbursement is only 50% of what it was when created in 1998, despite small increases by Congress along the way. A recent Wall Street Journal guest editorial claimed that Medicare payments benefited from cost of living adjustments, same as Social Security. I do not agree, hence the 50% pay gap since 1998.
In addition, the costs of running a practice have increased by 37% between 2001 and 2020, 1.7% per year, according to the Medicare Economic Index.
Some of this may include general inflation, but certainly new OSHA rules, electronic medical records, Medicare quality improvement measures, and assorted other costs do not. So based on my own conservative estimate, on top of the 50% decline in the payment pool, physicians’ noninflationary operating costs increased by at least another 10% over the last 20 odd years. This is a 60% decline in reimbursements!
Medicare payments have been under pressure from the Centers for Medicare & Medicaid Services (CMS) anti-inflationary payment policies for more than 20 years. While physician services represent a very modest portion of the overall growth in health care costs, they are an easy target for cuts when policymakers seek to limit spending. Although we avoided direct cuts to reimbursements caused by the Medicare sustainable growth rate formula (SGR) – which was enacted in 1997 and repealed in 2015 – Medicare provider payments have remained constrained by a budget-neutral financing system.
There used to be ways out of the box. Physicians could go to work for hospitals or have their practices acquired by them, resulting in much better hospital-based reimbursement. This has been eliminated by site-neutral payments, which while instituted by President Trump, are unopposed by President Biden. You could also join larger groups with some loss of autonomy, which could presumably negotiate better rates with private insurers as another way out, but these rates are almost always based on a percentage of Medicare as noted above.
There may be a bit of good news, with price transparency being instituted, which again is unopposed by the Biden administration. At least private practice physicians may be able to show their services are a bargain compared to hospitals.
One could also take the low road, and sell out to private equity, but I suspect these deals will become much less attractive since some of these entities are going broke and all will feel the bite of lower reimbursements.
Physicians and patients should rise up and demand better reimbursements for physicians, or there will be no physicians to see. This is not greed, a bigger house, or a newer car, this is becoming a matter of practice survival. And seniors are not greedy, they have paid hundreds of thousands of dollars into Medicare in taxes for health insurance in retirement.
Physicians and retirees should contact their federal legislators and let them know a 9.75% cut is untenable and ask for Medicare rates to be fixed to the cost of living, just as Social Security is. Before we fund trillions of dollars in new government programs, perhaps we should look to the solvency of the existing ones we have.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Inflation is the senility of democracies.
–Sylvia Townsend Warner
Electronic medical records? No, all of these are minor annoyances in the face of the practice killer, inflation.
Physicians live in a closed box where medical reimbursements are fixed, directly or by contract proxy to the government (Medicare) pay rate. Inflation is projected to be between 5% and 10% this year. We cannot increase our rates to increase the salaries of our employees, cover our increased medical disposable costs, and pay more for our state licensures and DEA registrations. No, we must try to find savings in our budget, which we have been squeezing for years.
Currently, medicine is facing a 9.75% cut in Medicare reimbursements, which will reset most private insurance rates, based on a percentage of Medicare. The temporary 3.75% conversion factor (CF) increase for all services is expiring. Also expiring is the 2% sequester from the Budget Control Act of 2011 (BCA), signed into law in August 2011. This was originally scheduled to sunset in 2021, but is going to continue to 2030.
A 4% statutory pay-as-you-go (PAYGO) sequester resulting from passage of the American Rescue Plan Act is being imposed. Statutory PAYGO is a policy written into law (it can be changed only through new legislation) that requires deficit neutrality overall in the laws (other than annual appropriations) enacted by Congress and imposes automatic spending reductions at the end of the year if such laws increase the deficit when they are added together.
There is a statutory freeze on Medicare Physician Fee Schedule (PFS) updates until 2026, at which time an annual increase of 0.25%, which is lower than inflation, will be enacted. This adds up to a 9.75% cut in Medicare pay until at least 2026. Recall that almost all of your private insurance contracts are tied to Medicare (some more, some less) and that this cut to the physician is doubled if your overhead is fixed at the typical 50% for most practices. This means an almost 20% cut in take-home pay for most physicians.
Now, when considering the most recent inflation number, which projects 5%-10% inflation for this year and at least 2% annually in the future, which compounds yearly (the Fed target), you are looking at catastrophic numbers.
The conversion factor – the pool of money doled out to physicians – has failed to keep pace with inflation – even at 2%-3% a year – and reimbursement is only 50% of what it was when created in 1998, despite small increases by Congress along the way. A recent Wall Street Journal guest editorial claimed that Medicare payments benefited from cost of living adjustments, same as Social Security. I do not agree, hence the 50% pay gap since 1998.
In addition, the costs of running a practice have increased by 37% between 2001 and 2020, 1.7% per year, according to the Medicare Economic Index.
Some of this may include general inflation, but certainly new OSHA rules, electronic medical records, Medicare quality improvement measures, and assorted other costs do not. So based on my own conservative estimate, on top of the 50% decline in the payment pool, physicians’ noninflationary operating costs increased by at least another 10% over the last 20 odd years. This is a 60% decline in reimbursements!
Medicare payments have been under pressure from the Centers for Medicare & Medicaid Services (CMS) anti-inflationary payment policies for more than 20 years. While physician services represent a very modest portion of the overall growth in health care costs, they are an easy target for cuts when policymakers seek to limit spending. Although we avoided direct cuts to reimbursements caused by the Medicare sustainable growth rate formula (SGR) – which was enacted in 1997 and repealed in 2015 – Medicare provider payments have remained constrained by a budget-neutral financing system.
There used to be ways out of the box. Physicians could go to work for hospitals or have their practices acquired by them, resulting in much better hospital-based reimbursement. This has been eliminated by site-neutral payments, which while instituted by President Trump, are unopposed by President Biden. You could also join larger groups with some loss of autonomy, which could presumably negotiate better rates with private insurers as another way out, but these rates are almost always based on a percentage of Medicare as noted above.
There may be a bit of good news, with price transparency being instituted, which again is unopposed by the Biden administration. At least private practice physicians may be able to show their services are a bargain compared to hospitals.
One could also take the low road, and sell out to private equity, but I suspect these deals will become much less attractive since some of these entities are going broke and all will feel the bite of lower reimbursements.
Physicians and patients should rise up and demand better reimbursements for physicians, or there will be no physicians to see. This is not greed, a bigger house, or a newer car, this is becoming a matter of practice survival. And seniors are not greedy, they have paid hundreds of thousands of dollars into Medicare in taxes for health insurance in retirement.
Physicians and retirees should contact their federal legislators and let them know a 9.75% cut is untenable and ask for Medicare rates to be fixed to the cost of living, just as Social Security is. Before we fund trillions of dollars in new government programs, perhaps we should look to the solvency of the existing ones we have.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Inflation is the senility of democracies.
–Sylvia Townsend Warner
Electronic medical records? No, all of these are minor annoyances in the face of the practice killer, inflation.
Physicians live in a closed box where medical reimbursements are fixed, directly or by contract proxy to the government (Medicare) pay rate. Inflation is projected to be between 5% and 10% this year. We cannot increase our rates to increase the salaries of our employees, cover our increased medical disposable costs, and pay more for our state licensures and DEA registrations. No, we must try to find savings in our budget, which we have been squeezing for years.
Currently, medicine is facing a 9.75% cut in Medicare reimbursements, which will reset most private insurance rates, based on a percentage of Medicare. The temporary 3.75% conversion factor (CF) increase for all services is expiring. Also expiring is the 2% sequester from the Budget Control Act of 2011 (BCA), signed into law in August 2011. This was originally scheduled to sunset in 2021, but is going to continue to 2030.
A 4% statutory pay-as-you-go (PAYGO) sequester resulting from passage of the American Rescue Plan Act is being imposed. Statutory PAYGO is a policy written into law (it can be changed only through new legislation) that requires deficit neutrality overall in the laws (other than annual appropriations) enacted by Congress and imposes automatic spending reductions at the end of the year if such laws increase the deficit when they are added together.
There is a statutory freeze on Medicare Physician Fee Schedule (PFS) updates until 2026, at which time an annual increase of 0.25%, which is lower than inflation, will be enacted. This adds up to a 9.75% cut in Medicare pay until at least 2026. Recall that almost all of your private insurance contracts are tied to Medicare (some more, some less) and that this cut to the physician is doubled if your overhead is fixed at the typical 50% for most practices. This means an almost 20% cut in take-home pay for most physicians.
Now, when considering the most recent inflation number, which projects 5%-10% inflation for this year and at least 2% annually in the future, which compounds yearly (the Fed target), you are looking at catastrophic numbers.
The conversion factor – the pool of money doled out to physicians – has failed to keep pace with inflation – even at 2%-3% a year – and reimbursement is only 50% of what it was when created in 1998, despite small increases by Congress along the way. A recent Wall Street Journal guest editorial claimed that Medicare payments benefited from cost of living adjustments, same as Social Security. I do not agree, hence the 50% pay gap since 1998.
In addition, the costs of running a practice have increased by 37% between 2001 and 2020, 1.7% per year, according to the Medicare Economic Index.
Some of this may include general inflation, but certainly new OSHA rules, electronic medical records, Medicare quality improvement measures, and assorted other costs do not. So based on my own conservative estimate, on top of the 50% decline in the payment pool, physicians’ noninflationary operating costs increased by at least another 10% over the last 20 odd years. This is a 60% decline in reimbursements!
Medicare payments have been under pressure from the Centers for Medicare & Medicaid Services (CMS) anti-inflationary payment policies for more than 20 years. While physician services represent a very modest portion of the overall growth in health care costs, they are an easy target for cuts when policymakers seek to limit spending. Although we avoided direct cuts to reimbursements caused by the Medicare sustainable growth rate formula (SGR) – which was enacted in 1997 and repealed in 2015 – Medicare provider payments have remained constrained by a budget-neutral financing system.
There used to be ways out of the box. Physicians could go to work for hospitals or have their practices acquired by them, resulting in much better hospital-based reimbursement. This has been eliminated by site-neutral payments, which while instituted by President Trump, are unopposed by President Biden. You could also join larger groups with some loss of autonomy, which could presumably negotiate better rates with private insurers as another way out, but these rates are almost always based on a percentage of Medicare as noted above.
There may be a bit of good news, with price transparency being instituted, which again is unopposed by the Biden administration. At least private practice physicians may be able to show their services are a bargain compared to hospitals.
One could also take the low road, and sell out to private equity, but I suspect these deals will become much less attractive since some of these entities are going broke and all will feel the bite of lower reimbursements.
Physicians and patients should rise up and demand better reimbursements for physicians, or there will be no physicians to see. This is not greed, a bigger house, or a newer car, this is becoming a matter of practice survival. And seniors are not greedy, they have paid hundreds of thousands of dollars into Medicare in taxes for health insurance in retirement.
Physicians and retirees should contact their federal legislators and let them know a 9.75% cut is untenable and ask for Medicare rates to be fixed to the cost of living, just as Social Security is. Before we fund trillions of dollars in new government programs, perhaps we should look to the solvency of the existing ones we have.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at dermnews@mdedge.com.
Connecticut chapter of ACC at center of Twitter dustup
Tweets from a black female medical student about the perils of being on call after lengthy hospital shifts was met with a stinging rebuke from the Twitter account of the Connecticut chapter of the American College of Cardiology – prompting an apology and some high-octane exchanges on medical Twitter.
In a series of Tweets, “queen of anonymous medicine” @QueenMD202X describes one friend “working 87 hours this week and 13 days straight” and a second, a third-year medical student working a 15-hour surgical shift. “That is cruel,” she writes, “15-hour shift? For what?????”
In response to a Tweet suggesting that being on call can be a valuable experience for students to know what they’re facing once they get to residency, @QueenMD202X pointed out the 15-hour shifts aren’t just a one-off.
In a now-deleted Tweet that nevertheless appears in several additional tweets as a screenshot, @ConnecticutACC replied: “You might be in the wrong field. You sound very angry probably unsuitable for patient care when your mental state is as you describe it. Emotions are contagious.”
The response from the medical and broader Twitter community was swift, with several tweets calling the chapter’s reply insensitive and racist.
In another Tweet, @BrittGratreak responded by stating: “I think institutions need to be more transparent how they basically weigh the costs & benefits of writing a memorial statement for students who die by suicide rather than investing in changing the toxic culture of medical education to prevent deaths & producing harmed physicians.”
Within hours, Connecticut-ACC issued an apology from their now-deleted account and questioned the origins of the Tweet. “We sincerely apologize for the earlier post as the views do not represent the values or beliefs of the Chapter or broader ACC. We are working to ID its origins. Burnout & well-being are critical issues [that] ACC/CCACC is working to address on behalf of members at all career stages.”
Speaking to this news organization, Connecticut-ACC president and governor Craig McPherson, MD, Yale University, New Haven, Conn., said the chapter believes its account was hacked.
“We provide limited password access to our Twitter account, and we assume, since we’ve contacted most of the individuals who had access to the current password and all of the them deny any knowledge, the account got hacked … it’s just one of those unfortunate aspects of social media,” he said.
The password was quickly changed after the chapter learned of the Tweet on Wednesday and the account has since been closed, at Dr. McPherson’s request.
“We don’t condone that kind of language, that kind of remark. It’s highly inappropriate, and I certainly agree with anyone that voiced that opinion in the Twitterstorm that followed,” he said. “But as I said at the outset, I have no control over what people say on social media once it’s out there. All we can do is apologize for the fact our Twitter feed was used as a vehicle for those comments, which we consider inappropriate.”
Asked whether he considered the remarks racist, Dr. McPherson replied: “That’s not for me to judge.”
ACC president Dipti Itchhaporia, MD, however, weighed in this afternoon with a Tweet citing the need to address clinician well-being and an inclusive workplace.
Some on Twitter recalled their own long hours as a medical student or defended the need to inculcate students in the long hours they’ll face as physicians. Others observed that neither ACC nor its Connecticut chapter addressed the issue of medical student hours in their response. Although fellow and resident hours are regulated, Dr. McPherson pointed out that it’s up to each individual medical school to set the hours for their students.
A version of this article first appeared on Medscape.com.
Tweets from a black female medical student about the perils of being on call after lengthy hospital shifts was met with a stinging rebuke from the Twitter account of the Connecticut chapter of the American College of Cardiology – prompting an apology and some high-octane exchanges on medical Twitter.
In a series of Tweets, “queen of anonymous medicine” @QueenMD202X describes one friend “working 87 hours this week and 13 days straight” and a second, a third-year medical student working a 15-hour surgical shift. “That is cruel,” she writes, “15-hour shift? For what?????”
In response to a Tweet suggesting that being on call can be a valuable experience for students to know what they’re facing once they get to residency, @QueenMD202X pointed out the 15-hour shifts aren’t just a one-off.
In a now-deleted Tweet that nevertheless appears in several additional tweets as a screenshot, @ConnecticutACC replied: “You might be in the wrong field. You sound very angry probably unsuitable for patient care when your mental state is as you describe it. Emotions are contagious.”
The response from the medical and broader Twitter community was swift, with several tweets calling the chapter’s reply insensitive and racist.
In another Tweet, @BrittGratreak responded by stating: “I think institutions need to be more transparent how they basically weigh the costs & benefits of writing a memorial statement for students who die by suicide rather than investing in changing the toxic culture of medical education to prevent deaths & producing harmed physicians.”
Within hours, Connecticut-ACC issued an apology from their now-deleted account and questioned the origins of the Tweet. “We sincerely apologize for the earlier post as the views do not represent the values or beliefs of the Chapter or broader ACC. We are working to ID its origins. Burnout & well-being are critical issues [that] ACC/CCACC is working to address on behalf of members at all career stages.”
Speaking to this news organization, Connecticut-ACC president and governor Craig McPherson, MD, Yale University, New Haven, Conn., said the chapter believes its account was hacked.
“We provide limited password access to our Twitter account, and we assume, since we’ve contacted most of the individuals who had access to the current password and all of the them deny any knowledge, the account got hacked … it’s just one of those unfortunate aspects of social media,” he said.
The password was quickly changed after the chapter learned of the Tweet on Wednesday and the account has since been closed, at Dr. McPherson’s request.
“We don’t condone that kind of language, that kind of remark. It’s highly inappropriate, and I certainly agree with anyone that voiced that opinion in the Twitterstorm that followed,” he said. “But as I said at the outset, I have no control over what people say on social media once it’s out there. All we can do is apologize for the fact our Twitter feed was used as a vehicle for those comments, which we consider inappropriate.”
Asked whether he considered the remarks racist, Dr. McPherson replied: “That’s not for me to judge.”
ACC president Dipti Itchhaporia, MD, however, weighed in this afternoon with a Tweet citing the need to address clinician well-being and an inclusive workplace.
Some on Twitter recalled their own long hours as a medical student or defended the need to inculcate students in the long hours they’ll face as physicians. Others observed that neither ACC nor its Connecticut chapter addressed the issue of medical student hours in their response. Although fellow and resident hours are regulated, Dr. McPherson pointed out that it’s up to each individual medical school to set the hours for their students.
A version of this article first appeared on Medscape.com.
Tweets from a black female medical student about the perils of being on call after lengthy hospital shifts was met with a stinging rebuke from the Twitter account of the Connecticut chapter of the American College of Cardiology – prompting an apology and some high-octane exchanges on medical Twitter.
In a series of Tweets, “queen of anonymous medicine” @QueenMD202X describes one friend “working 87 hours this week and 13 days straight” and a second, a third-year medical student working a 15-hour surgical shift. “That is cruel,” she writes, “15-hour shift? For what?????”
In response to a Tweet suggesting that being on call can be a valuable experience for students to know what they’re facing once they get to residency, @QueenMD202X pointed out the 15-hour shifts aren’t just a one-off.
In a now-deleted Tweet that nevertheless appears in several additional tweets as a screenshot, @ConnecticutACC replied: “You might be in the wrong field. You sound very angry probably unsuitable for patient care when your mental state is as you describe it. Emotions are contagious.”
The response from the medical and broader Twitter community was swift, with several tweets calling the chapter’s reply insensitive and racist.
In another Tweet, @BrittGratreak responded by stating: “I think institutions need to be more transparent how they basically weigh the costs & benefits of writing a memorial statement for students who die by suicide rather than investing in changing the toxic culture of medical education to prevent deaths & producing harmed physicians.”
Within hours, Connecticut-ACC issued an apology from their now-deleted account and questioned the origins of the Tweet. “We sincerely apologize for the earlier post as the views do not represent the values or beliefs of the Chapter or broader ACC. We are working to ID its origins. Burnout & well-being are critical issues [that] ACC/CCACC is working to address on behalf of members at all career stages.”
Speaking to this news organization, Connecticut-ACC president and governor Craig McPherson, MD, Yale University, New Haven, Conn., said the chapter believes its account was hacked.
“We provide limited password access to our Twitter account, and we assume, since we’ve contacted most of the individuals who had access to the current password and all of the them deny any knowledge, the account got hacked … it’s just one of those unfortunate aspects of social media,” he said.
The password was quickly changed after the chapter learned of the Tweet on Wednesday and the account has since been closed, at Dr. McPherson’s request.
“We don’t condone that kind of language, that kind of remark. It’s highly inappropriate, and I certainly agree with anyone that voiced that opinion in the Twitterstorm that followed,” he said. “But as I said at the outset, I have no control over what people say on social media once it’s out there. All we can do is apologize for the fact our Twitter feed was used as a vehicle for those comments, which we consider inappropriate.”
Asked whether he considered the remarks racist, Dr. McPherson replied: “That’s not for me to judge.”
ACC president Dipti Itchhaporia, MD, however, weighed in this afternoon with a Tweet citing the need to address clinician well-being and an inclusive workplace.
Some on Twitter recalled their own long hours as a medical student or defended the need to inculcate students in the long hours they’ll face as physicians. Others observed that neither ACC nor its Connecticut chapter addressed the issue of medical student hours in their response. Although fellow and resident hours are regulated, Dr. McPherson pointed out that it’s up to each individual medical school to set the hours for their students.
A version of this article first appeared on Medscape.com.
Doctor wins restraining order against CVS after prescription ban
In an Aug. 11 decision, District Court Judge William Bertelsman ordered CVS to stop refusing prescriptions written by Kendall E. Hansen, MD. Judge Bertelsman ruled that Dr. Hansen is likely to succeed in his claim that CVS barred his prescriptions without evidence that he violated any law or professional protocol. The restraining order will remain in place while Dr. Hansen’s lawsuit against CVS Pharmacy proceeds.
Ronald W. Chapman II, an attorney representing Dr. Hansen, said the order is groundbreaking and that, to his knowledge, it’s the first time a federal court has overturned a pharmacy’s decision to block a prescriber.
“We believe that CVS’ decision was based solely on algorithms they use to analyze prescriber practices and not an any individual review of patient records,” Mr. Chapman said. “In fact, we invited CVS to come out to Dr. Hansen’s practice and look at how he was treating patients and ensure things were compliant, but they refused. Instead, they had a phone call with him then cut his patients off.”
Michael DeAngelis, a spokesman for CVS, said the court’s order illustrates the proverbial rock and hard place that pharmacies are placed between in the country’s fight against the misuse of prescription opioids.
“It is alleged in many lawsuits that pharmacies fill too many opioid prescriptions and should operate programs that use data to block prescriptions written by some doctors,” Mr. DeAngelis told this news organization. “And yet other lawsuits, including this one, argue that we should not operate programs that may block prescriptions. Such contradictions are grossly unfair to the pharmacy profession.”
Mr. DeAngelis declined to comment about Dr. Hansen’s claims or specify what led CVS to refuse his prescriptions.
Dr. Hansen declined to comment for this story through his attorney.
Dr. Hansen is no stranger to the spotlight. The Northern Kentucky pain doctor made headlines in 2012 when two of his horses, Fast and Accurate, and Hansen, ran in the Kentucky Derby. In February 2019, he drew media attention when his practice, Interventional Pain Specialists in Crestview Hills, Ky., was raided by federal agents. Dr. Hansen owns and operates the facility, which serves patients in Kentucky, Ohio, and Indiana.
The search yielded no findings, and no charges were filed, according to Mr. Chapman. Scott Hardcorn, director of the Northern Kentucky Drug Strike Force, confirmed that his agency assisted in the operation but said he was unaware of the outcome and that his officers generated no reports from the investigation. A spokesperson for the Drug Enforcement Administration would not comment about the investigation and directed a reporter for this news organization to the DEA website where enforcement actions are listed. No records or actions against Dr. Hansen can be found.
The CVS complaint stems from actions taken by the pharmacy against Dr. Hansen earlier this year. In June, a pharmacy representative allegedly contacted Dr. Hansen by phone and asked him questions about his practice and his prescribing practices, according to his lawsuit filed in U.S. District Court for the Eastern District of Kentucky. During the call, the representative did not inform Dr. Hansen that any of his prescriptions were in question or were suspected of being medically unnecessary, the complaint alleges.
On July 28, 2021, CVS sent Dr. Hansen a letter announcing that its pharmacies would no longer be honoring his prescriptions. The letter, entered as an exhibit in the lawsuit, states that CVS contacted Dr. Hansen twice in 2021 about his prescribing practices, once in May and again in June.
“Despite our attempts to resolve the concerns with your controlled substance prescribing patterns, these concerns persist,” Kahra Lutkiewicz, director of CVS’ retail pharmacy professional practice, wrote in the letter. “Thus, we are writing to inform you that effective Aug. 5, 2021, CVS/pharmacy stores will no longer be able to fill prescriptions that you write for controlled substances. We take our compliance obligations very seriously, and after careful consideration, find it necessary to take this action.”
The letter does not explain the details behind CVS’ concerns.
Dr. Hansen sued CVS on Aug. 4 for tortious interference with a business relationship and defamation, among other claims. His complaint alleges that Dr. Hansen and his patients will suffer irreparable injury if the prescription decision stands. More than 250 of Dr. Hansen’s patients use CVS pharmacies for their prescriptions, and some are locked into using the pharmacy because of insurance contracts, Mr. Chapman said.
“There really is nowhere else for these patients to go,” Mr. Chapman said. “They would have to go to a new doctor and establish a new relationship, and obviously that has devastating consequences when we’re talking about people who need their medication.”
CVS has not yet issued a written response to the lawsuit. In his order, Judge Bertelsman stated that a preliminary conference was held in which all parties were represented and stated their positions to the judge.
“Plaintiffs are likely to succeed on the merits of their claims that defendant has interfered with plaintiffs’ relationships with their patients by refusing to fill prescriptions written by plaintiffs, and defendant has done so without evidence that plaintiffs have violated any law or professional protocol related to such prescriptions,” Judge Bertelsman wrote. “The balance of the hardships between the parties weighs in favor of issuing a temporary restraining order inasmuch as defendant’s actions pose a threat to plaintiffs’ professional reputation and livelihood and ... because plaintiffs’ patients’ medical care is implicated by defendant’s actions, the public interest weighs in favor of issuance of the temporary restraining order.”
Dr. Hansen is currently embroiled in several other legal battles as both a plaintiff and a defendant.
In 2019, a patient sued him for negligence and fraud for allegedly performing medically unnecessary and excessive injection therapy. The suit claims the patient was required to undergo injection therapy on a continuing basis in order to receive her narcotic pain medication, according to the lawsuit filed in Kenton Circuit Court. The complaint alleges that Dr. Hansen made false representations to the patient and to her insurers that the injections were necessary for the treatment of the patient’s chronic pain.
The federal government is not involved in the case.
The negligence lawsuit is in the discovery stage, and attorneys plan to collect Dr. Hansen’s deposition soon, said Eric Deters, a spokesman for Deters Law, a law firm based in Independence, Ky., that is representing the patient.
“The crux is that he performs unnecessary pain procedures and forces you to get an unnecessary procedure before giving you your medication,” Mr. Deters said.
However, Dr. Hansen’s and Mr. Deters’ history together includes a recent riff, according to an August 2021 lawsuit filed by Dr. Hansen against the law firm. Dr. Hansen was a former medical expert in cases for Deters and Associates, but the relationship turned sour when attorneys believed Dr. Hansen was retained as an expert in a case against their clients, according to Dr. Hansen’s suit. Dr. Hansen claims that as retribution, Deters and Associates issued a medical malpractice lawsuit against him in 2020, even though attorneys allegedly knew the statute of limitations had run out. A trial court dismissed the 2020 lawsuit against Dr. Hansen as being untimely filed. Dr. Hansen’s lawsuit alleges wrongful use of civil proceedings and requests compensatory, punitive damages and court costs from the law firm.
The law firm has faced trouble in the past. In August 2021, the Ohio Supreme Court ordered that Mr. Deters pay a $6,500 fine for engaging in the unauthorized practice of law. Mr. Deters’ Kentucky law license has been suspended since 2013 for ethics infractions, according to court records. He retired from law in 2014 and now acts as a spokesperson and office manager for the law firm. The fine resulted from legal advice given by Mr. Deters to two clients at the law firm, according to the Ohio Supreme Court decision.
As for the CVS lawsuit, an upcoming hearing will determine whether the federal court issues a permanent injunction against CVS’s actions. CVS officials have not said whether they will fight the temporary restraining order or the withdrawal of their prescription ban against Dr. Hansen.
A version of this article first appeared on Medscape.com.
In an Aug. 11 decision, District Court Judge William Bertelsman ordered CVS to stop refusing prescriptions written by Kendall E. Hansen, MD. Judge Bertelsman ruled that Dr. Hansen is likely to succeed in his claim that CVS barred his prescriptions without evidence that he violated any law or professional protocol. The restraining order will remain in place while Dr. Hansen’s lawsuit against CVS Pharmacy proceeds.
Ronald W. Chapman II, an attorney representing Dr. Hansen, said the order is groundbreaking and that, to his knowledge, it’s the first time a federal court has overturned a pharmacy’s decision to block a prescriber.
“We believe that CVS’ decision was based solely on algorithms they use to analyze prescriber practices and not an any individual review of patient records,” Mr. Chapman said. “In fact, we invited CVS to come out to Dr. Hansen’s practice and look at how he was treating patients and ensure things were compliant, but they refused. Instead, they had a phone call with him then cut his patients off.”
Michael DeAngelis, a spokesman for CVS, said the court’s order illustrates the proverbial rock and hard place that pharmacies are placed between in the country’s fight against the misuse of prescription opioids.
“It is alleged in many lawsuits that pharmacies fill too many opioid prescriptions and should operate programs that use data to block prescriptions written by some doctors,” Mr. DeAngelis told this news organization. “And yet other lawsuits, including this one, argue that we should not operate programs that may block prescriptions. Such contradictions are grossly unfair to the pharmacy profession.”
Mr. DeAngelis declined to comment about Dr. Hansen’s claims or specify what led CVS to refuse his prescriptions.
Dr. Hansen declined to comment for this story through his attorney.
Dr. Hansen is no stranger to the spotlight. The Northern Kentucky pain doctor made headlines in 2012 when two of his horses, Fast and Accurate, and Hansen, ran in the Kentucky Derby. In February 2019, he drew media attention when his practice, Interventional Pain Specialists in Crestview Hills, Ky., was raided by federal agents. Dr. Hansen owns and operates the facility, which serves patients in Kentucky, Ohio, and Indiana.
The search yielded no findings, and no charges were filed, according to Mr. Chapman. Scott Hardcorn, director of the Northern Kentucky Drug Strike Force, confirmed that his agency assisted in the operation but said he was unaware of the outcome and that his officers generated no reports from the investigation. A spokesperson for the Drug Enforcement Administration would not comment about the investigation and directed a reporter for this news organization to the DEA website where enforcement actions are listed. No records or actions against Dr. Hansen can be found.
The CVS complaint stems from actions taken by the pharmacy against Dr. Hansen earlier this year. In June, a pharmacy representative allegedly contacted Dr. Hansen by phone and asked him questions about his practice and his prescribing practices, according to his lawsuit filed in U.S. District Court for the Eastern District of Kentucky. During the call, the representative did not inform Dr. Hansen that any of his prescriptions were in question or were suspected of being medically unnecessary, the complaint alleges.
On July 28, 2021, CVS sent Dr. Hansen a letter announcing that its pharmacies would no longer be honoring his prescriptions. The letter, entered as an exhibit in the lawsuit, states that CVS contacted Dr. Hansen twice in 2021 about his prescribing practices, once in May and again in June.
“Despite our attempts to resolve the concerns with your controlled substance prescribing patterns, these concerns persist,” Kahra Lutkiewicz, director of CVS’ retail pharmacy professional practice, wrote in the letter. “Thus, we are writing to inform you that effective Aug. 5, 2021, CVS/pharmacy stores will no longer be able to fill prescriptions that you write for controlled substances. We take our compliance obligations very seriously, and after careful consideration, find it necessary to take this action.”
The letter does not explain the details behind CVS’ concerns.
Dr. Hansen sued CVS on Aug. 4 for tortious interference with a business relationship and defamation, among other claims. His complaint alleges that Dr. Hansen and his patients will suffer irreparable injury if the prescription decision stands. More than 250 of Dr. Hansen’s patients use CVS pharmacies for their prescriptions, and some are locked into using the pharmacy because of insurance contracts, Mr. Chapman said.
“There really is nowhere else for these patients to go,” Mr. Chapman said. “They would have to go to a new doctor and establish a new relationship, and obviously that has devastating consequences when we’re talking about people who need their medication.”
CVS has not yet issued a written response to the lawsuit. In his order, Judge Bertelsman stated that a preliminary conference was held in which all parties were represented and stated their positions to the judge.
“Plaintiffs are likely to succeed on the merits of their claims that defendant has interfered with plaintiffs’ relationships with their patients by refusing to fill prescriptions written by plaintiffs, and defendant has done so without evidence that plaintiffs have violated any law or professional protocol related to such prescriptions,” Judge Bertelsman wrote. “The balance of the hardships between the parties weighs in favor of issuing a temporary restraining order inasmuch as defendant’s actions pose a threat to plaintiffs’ professional reputation and livelihood and ... because plaintiffs’ patients’ medical care is implicated by defendant’s actions, the public interest weighs in favor of issuance of the temporary restraining order.”
Dr. Hansen is currently embroiled in several other legal battles as both a plaintiff and a defendant.
In 2019, a patient sued him for negligence and fraud for allegedly performing medically unnecessary and excessive injection therapy. The suit claims the patient was required to undergo injection therapy on a continuing basis in order to receive her narcotic pain medication, according to the lawsuit filed in Kenton Circuit Court. The complaint alleges that Dr. Hansen made false representations to the patient and to her insurers that the injections were necessary for the treatment of the patient’s chronic pain.
The federal government is not involved in the case.
The negligence lawsuit is in the discovery stage, and attorneys plan to collect Dr. Hansen’s deposition soon, said Eric Deters, a spokesman for Deters Law, a law firm based in Independence, Ky., that is representing the patient.
“The crux is that he performs unnecessary pain procedures and forces you to get an unnecessary procedure before giving you your medication,” Mr. Deters said.
However, Dr. Hansen’s and Mr. Deters’ history together includes a recent riff, according to an August 2021 lawsuit filed by Dr. Hansen against the law firm. Dr. Hansen was a former medical expert in cases for Deters and Associates, but the relationship turned sour when attorneys believed Dr. Hansen was retained as an expert in a case against their clients, according to Dr. Hansen’s suit. Dr. Hansen claims that as retribution, Deters and Associates issued a medical malpractice lawsuit against him in 2020, even though attorneys allegedly knew the statute of limitations had run out. A trial court dismissed the 2020 lawsuit against Dr. Hansen as being untimely filed. Dr. Hansen’s lawsuit alleges wrongful use of civil proceedings and requests compensatory, punitive damages and court costs from the law firm.
The law firm has faced trouble in the past. In August 2021, the Ohio Supreme Court ordered that Mr. Deters pay a $6,500 fine for engaging in the unauthorized practice of law. Mr. Deters’ Kentucky law license has been suspended since 2013 for ethics infractions, according to court records. He retired from law in 2014 and now acts as a spokesperson and office manager for the law firm. The fine resulted from legal advice given by Mr. Deters to two clients at the law firm, according to the Ohio Supreme Court decision.
As for the CVS lawsuit, an upcoming hearing will determine whether the federal court issues a permanent injunction against CVS’s actions. CVS officials have not said whether they will fight the temporary restraining order or the withdrawal of their prescription ban against Dr. Hansen.
A version of this article first appeared on Medscape.com.
In an Aug. 11 decision, District Court Judge William Bertelsman ordered CVS to stop refusing prescriptions written by Kendall E. Hansen, MD. Judge Bertelsman ruled that Dr. Hansen is likely to succeed in his claim that CVS barred his prescriptions without evidence that he violated any law or professional protocol. The restraining order will remain in place while Dr. Hansen’s lawsuit against CVS Pharmacy proceeds.
Ronald W. Chapman II, an attorney representing Dr. Hansen, said the order is groundbreaking and that, to his knowledge, it’s the first time a federal court has overturned a pharmacy’s decision to block a prescriber.
“We believe that CVS’ decision was based solely on algorithms they use to analyze prescriber practices and not an any individual review of patient records,” Mr. Chapman said. “In fact, we invited CVS to come out to Dr. Hansen’s practice and look at how he was treating patients and ensure things were compliant, but they refused. Instead, they had a phone call with him then cut his patients off.”
Michael DeAngelis, a spokesman for CVS, said the court’s order illustrates the proverbial rock and hard place that pharmacies are placed between in the country’s fight against the misuse of prescription opioids.
“It is alleged in many lawsuits that pharmacies fill too many opioid prescriptions and should operate programs that use data to block prescriptions written by some doctors,” Mr. DeAngelis told this news organization. “And yet other lawsuits, including this one, argue that we should not operate programs that may block prescriptions. Such contradictions are grossly unfair to the pharmacy profession.”
Mr. DeAngelis declined to comment about Dr. Hansen’s claims or specify what led CVS to refuse his prescriptions.
Dr. Hansen declined to comment for this story through his attorney.
Dr. Hansen is no stranger to the spotlight. The Northern Kentucky pain doctor made headlines in 2012 when two of his horses, Fast and Accurate, and Hansen, ran in the Kentucky Derby. In February 2019, he drew media attention when his practice, Interventional Pain Specialists in Crestview Hills, Ky., was raided by federal agents. Dr. Hansen owns and operates the facility, which serves patients in Kentucky, Ohio, and Indiana.
The search yielded no findings, and no charges were filed, according to Mr. Chapman. Scott Hardcorn, director of the Northern Kentucky Drug Strike Force, confirmed that his agency assisted in the operation but said he was unaware of the outcome and that his officers generated no reports from the investigation. A spokesperson for the Drug Enforcement Administration would not comment about the investigation and directed a reporter for this news organization to the DEA website where enforcement actions are listed. No records or actions against Dr. Hansen can be found.
The CVS complaint stems from actions taken by the pharmacy against Dr. Hansen earlier this year. In June, a pharmacy representative allegedly contacted Dr. Hansen by phone and asked him questions about his practice and his prescribing practices, according to his lawsuit filed in U.S. District Court for the Eastern District of Kentucky. During the call, the representative did not inform Dr. Hansen that any of his prescriptions were in question or were suspected of being medically unnecessary, the complaint alleges.
On July 28, 2021, CVS sent Dr. Hansen a letter announcing that its pharmacies would no longer be honoring his prescriptions. The letter, entered as an exhibit in the lawsuit, states that CVS contacted Dr. Hansen twice in 2021 about his prescribing practices, once in May and again in June.
“Despite our attempts to resolve the concerns with your controlled substance prescribing patterns, these concerns persist,” Kahra Lutkiewicz, director of CVS’ retail pharmacy professional practice, wrote in the letter. “Thus, we are writing to inform you that effective Aug. 5, 2021, CVS/pharmacy stores will no longer be able to fill prescriptions that you write for controlled substances. We take our compliance obligations very seriously, and after careful consideration, find it necessary to take this action.”
The letter does not explain the details behind CVS’ concerns.
Dr. Hansen sued CVS on Aug. 4 for tortious interference with a business relationship and defamation, among other claims. His complaint alleges that Dr. Hansen and his patients will suffer irreparable injury if the prescription decision stands. More than 250 of Dr. Hansen’s patients use CVS pharmacies for their prescriptions, and some are locked into using the pharmacy because of insurance contracts, Mr. Chapman said.
“There really is nowhere else for these patients to go,” Mr. Chapman said. “They would have to go to a new doctor and establish a new relationship, and obviously that has devastating consequences when we’re talking about people who need their medication.”
CVS has not yet issued a written response to the lawsuit. In his order, Judge Bertelsman stated that a preliminary conference was held in which all parties were represented and stated their positions to the judge.
“Plaintiffs are likely to succeed on the merits of their claims that defendant has interfered with plaintiffs’ relationships with their patients by refusing to fill prescriptions written by plaintiffs, and defendant has done so without evidence that plaintiffs have violated any law or professional protocol related to such prescriptions,” Judge Bertelsman wrote. “The balance of the hardships between the parties weighs in favor of issuing a temporary restraining order inasmuch as defendant’s actions pose a threat to plaintiffs’ professional reputation and livelihood and ... because plaintiffs’ patients’ medical care is implicated by defendant’s actions, the public interest weighs in favor of issuance of the temporary restraining order.”
Dr. Hansen is currently embroiled in several other legal battles as both a plaintiff and a defendant.
In 2019, a patient sued him for negligence and fraud for allegedly performing medically unnecessary and excessive injection therapy. The suit claims the patient was required to undergo injection therapy on a continuing basis in order to receive her narcotic pain medication, according to the lawsuit filed in Kenton Circuit Court. The complaint alleges that Dr. Hansen made false representations to the patient and to her insurers that the injections were necessary for the treatment of the patient’s chronic pain.
The federal government is not involved in the case.
The negligence lawsuit is in the discovery stage, and attorneys plan to collect Dr. Hansen’s deposition soon, said Eric Deters, a spokesman for Deters Law, a law firm based in Independence, Ky., that is representing the patient.
“The crux is that he performs unnecessary pain procedures and forces you to get an unnecessary procedure before giving you your medication,” Mr. Deters said.
However, Dr. Hansen’s and Mr. Deters’ history together includes a recent riff, according to an August 2021 lawsuit filed by Dr. Hansen against the law firm. Dr. Hansen was a former medical expert in cases for Deters and Associates, but the relationship turned sour when attorneys believed Dr. Hansen was retained as an expert in a case against their clients, according to Dr. Hansen’s suit. Dr. Hansen claims that as retribution, Deters and Associates issued a medical malpractice lawsuit against him in 2020, even though attorneys allegedly knew the statute of limitations had run out. A trial court dismissed the 2020 lawsuit against Dr. Hansen as being untimely filed. Dr. Hansen’s lawsuit alleges wrongful use of civil proceedings and requests compensatory, punitive damages and court costs from the law firm.
The law firm has faced trouble in the past. In August 2021, the Ohio Supreme Court ordered that Mr. Deters pay a $6,500 fine for engaging in the unauthorized practice of law. Mr. Deters’ Kentucky law license has been suspended since 2013 for ethics infractions, according to court records. He retired from law in 2014 and now acts as a spokesperson and office manager for the law firm. The fine resulted from legal advice given by Mr. Deters to two clients at the law firm, according to the Ohio Supreme Court decision.
As for the CVS lawsuit, an upcoming hearing will determine whether the federal court issues a permanent injunction against CVS’s actions. CVS officials have not said whether they will fight the temporary restraining order or the withdrawal of their prescription ban against Dr. Hansen.
A version of this article first appeared on Medscape.com.
Mediterranean diet slows progression of atherosclerosis in CHD
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with coronary heart disease (CHD), following a Mediterranean diet is more effective in reducing progression of atherosclerosis than following a low-fat diet, according to new data from the CORDIOPREV randomized, controlled trial.
“The current study is, to our knowledge, the first to establish an effective dietary strategy for secondary cardiovascular prevention, reinforcing the fact that the Mediterranean diet rich in extra virgin olive oil (EVOO) could prevent the progression of atherosclerosis,” the study team said.
The data also show that patients with a higher atherosclerotic burden might benefit the most from the Mediterranean diet.
The study was published online Aug. 10, 2021, in Stroke.
Mediterranean or low fat?
“It is well established that lifestyle and dietary habits powerfully affect cardiovascular risk,” study investigator Elena M. Yubero-Serrano, PhD, with Reina Sofia University Hospital/University of Cordoba (Spain), told this news organization.
“The effectiveness of the Mediterranean diet in reducing cardiovascular risk has been seen in primary prevention. However, currently there is no consensus about a recommended dietary model for the secondary prevention of cardiovascular disease,” she said.
The Coronary Diet Intervention With Olive Oil and Cardiovascular Prevention (CORDIOPREV) study is an ongoing prospective study comparing the effects of two healthy diets for secondary prevention of cardiovascular disease (CVD) in 1002 patients.
The comparative effect of the diets in reducing CVD risk, assessed by quantification of intima-media thickness of the common carotid arteries (IMT-CC), is a key secondary endpoint of the study.
During the study, half of the patients follow a Mediterranean diet rich in EVOO, fruit and vegetables, whole grains, fish, and nuts. The other half follow a diet low in fat and rich in complex carbohydrates.
A total of 939 participants (459 in the low-fat diet group and 480 in the Mediterranean diet group) completed IMT-CC evaluation at baseline, and 809 (377 and 432, respectively) completed the IMT-CC evaluation at 5 years; 731 (335 and 396, respectively) did so at 7 years.
The Mediterranean diet significantly decreased IMT-CC both after 5 years (–0.027; P < .001) and after 7 years (–0.031 mm; P < .001), relative to baseline. In contrast, the low-fat diet did not exert any change on IMT-CC after 5 or 7 years, the researchers report.
The higher the IMT-CC at baseline, the greater the reduction in this parameter.
The Mediterranean diet also produced a greater decrease in IMT-CC and carotid plaque maximum height, compared with the low-fat diet throughout follow-up.
There were no between-group differences in carotid plaque numbers during follow-up.
“Our findings, in addition to reinforcing the clinical benefits of the Mediterranean diet, provide a beneficial dietary strategy as a clinical and therapeutic tool that could reduce the high cardiovascular recurrence in the context of secondary prevention,” Dr. Yubero-Serrano said in an interview.
Earlier data from CORDIOPREV showed that, after 1 year of eating a Mediterranean diet, compared with the low-fat diet, endothelial function was improved among patients with CHD, even those with type 2 diabetes, which was associated with a better balance of vascular homeostasis.
The Mediterranean diet may also modulate the lipid profile, particularly by increasing HDL cholesterol levels. The anti-inflammatory capacity of the Mediterranean diet could be another factor that contributes to reducing the progression of atherosclerosis, the researchers say.
Important study
Reached for comment, Alan Rozanski, MD, professor of medicine, Icahn School of Medicine at Mount Sinai and cardiologist at Mount Sinai Morningside, New York, said: “We know very well that lifestyle factors, diet, and exercise in particular are extremely important in promoting health, vitality, and decreasing risk for chronic diseases, including heart attack and stroke.
“But a lot of the studies depend on epidemiological work. Until now, we haven’t had important prospective studies evaluating different kinds of dietary approaches and how they affect carotid intimal thickening assessments that we can do by ultrasound. So having this kind of imaging study which shows that diet can halt progression of atherosclerosis is important,” said Dr. Rozanski.
“Changing one’s diet is extremely important and potentially beneficial in many ways, and being able to say to a patient with atherosclerosis that we have data that shows you can halt the progression of the disease can be extraordinarily encouraging to many patients,” he noted.
“When people have disease, they very often gravitate toward drugs, but continuing to emphasize lifestyle changes in these people is extremely important,” he added.
The CORDIOPREV study was supported by the Fundación Patrimonio Comunal Olivarero. Dr. Yubero-Serrano and Dr. Rozanski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cutaneous Chaetomium globosum Infection in a Vedolizumab-Treated Patient
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
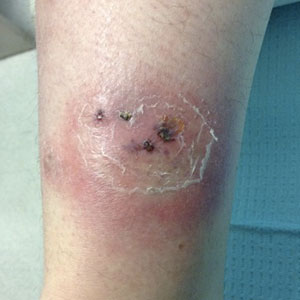
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.
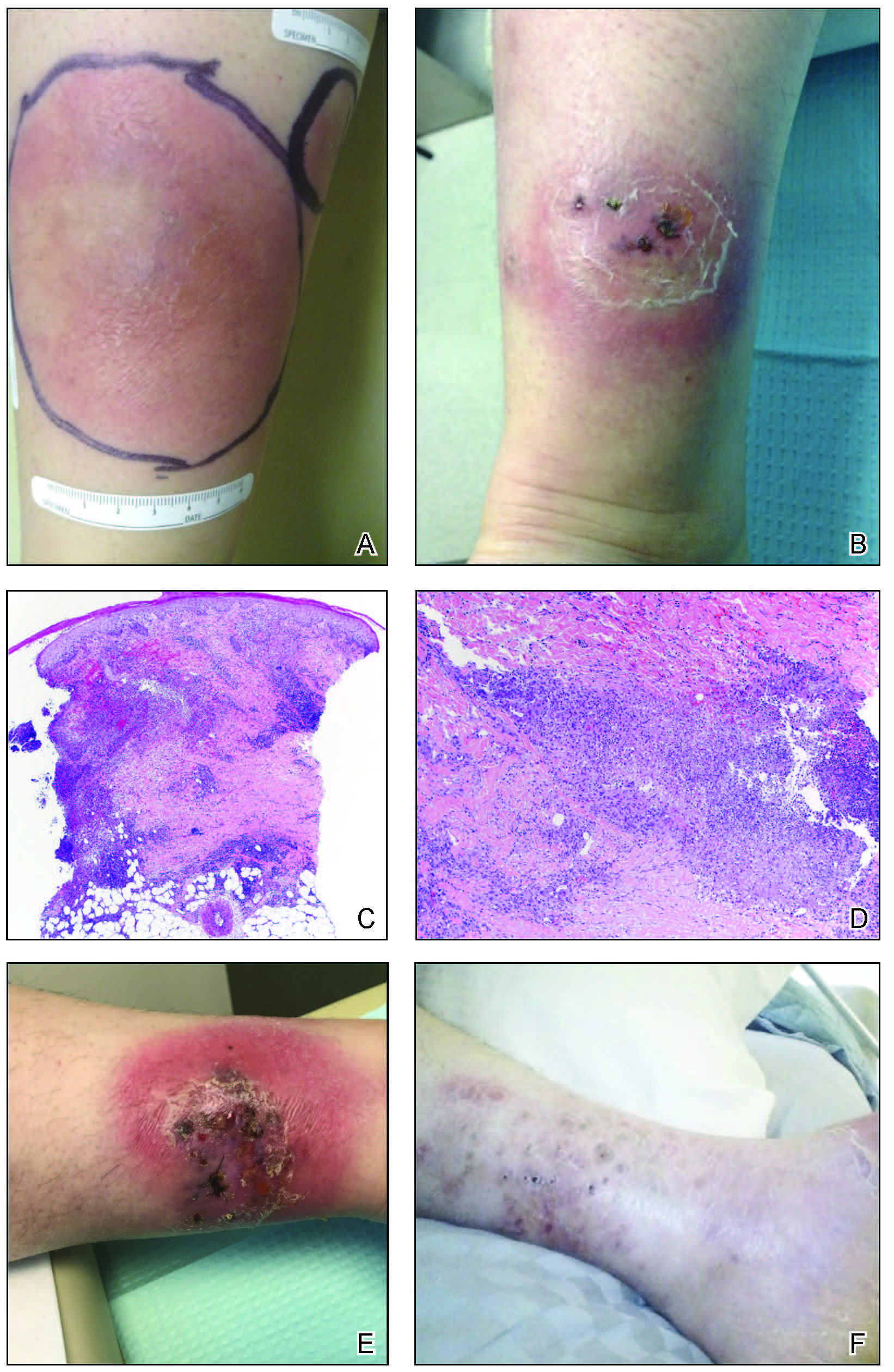
Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
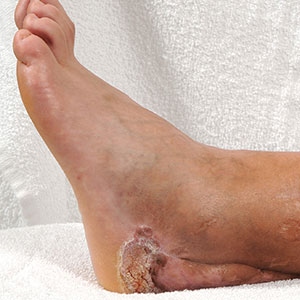
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.

Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.

Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
Practice Points
- Tissue culture remains the gold standard for deep fungal infections.
- Physicians must maintain a high index of suspicion for alternate diagnoses when a disease progresses along an unexpected course.
- Biologic medications may have low-incidence side effects that emerge in postmarket use.
Verrucous Carcinoma in a Wounded Military Amputee
To the Editor:
Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma first described by Ackerman in 1948.1 There are 4 main clinicopathologic types: oral florid papillomatosis or Ackerman tumor, giant condyloma acuminatum or Buschke-Lowenstein tumor, plantar verrucous carcinoma, and cutaneous verrucous carcinoma.2,3 Historically, most patients are older white men. The lesion commonly occurs in sites of inflammation4 or chronic irritation/trauma. Clinically, patients present with a slowly enlarging, exophytic, verrucous plaque violating the skin, fascia, and occasionally bone. Although these lesions have little tendency to metastasize, substantial morbidity can be seen due to local invasion. Despite surgical excision, recurrence is not uncommon and is associated with a poor prognosis and higher infiltrative potential.5
A 45-year-old male veteran initially presented to our dermatology clinic with a 4-cm, macerated, verrucous plaque on the left lateral ankle in the area of a skin graft placed during a prior limb salvage surgery (Figure 1). The patient experienced a traumatic blast injury while deployed 7 years prior with a subsequent right-sided below-the-knee amputation and left lower limb salvage. The lesion was clinically diagnosed as verruca vulgaris and treated with daily salicylic acid. Six weeks after the initial presentation, the lesion remained largely unchanged. A biopsy subsequently was obtained to confirm the diagnosis. At that time, the histopathology was consistent with verruca vulgaris without evidence of carcinoma. Due to the persistence of the lesion, lack of improvement with topical treatment, and overall size, the patient opted for surgical excision.
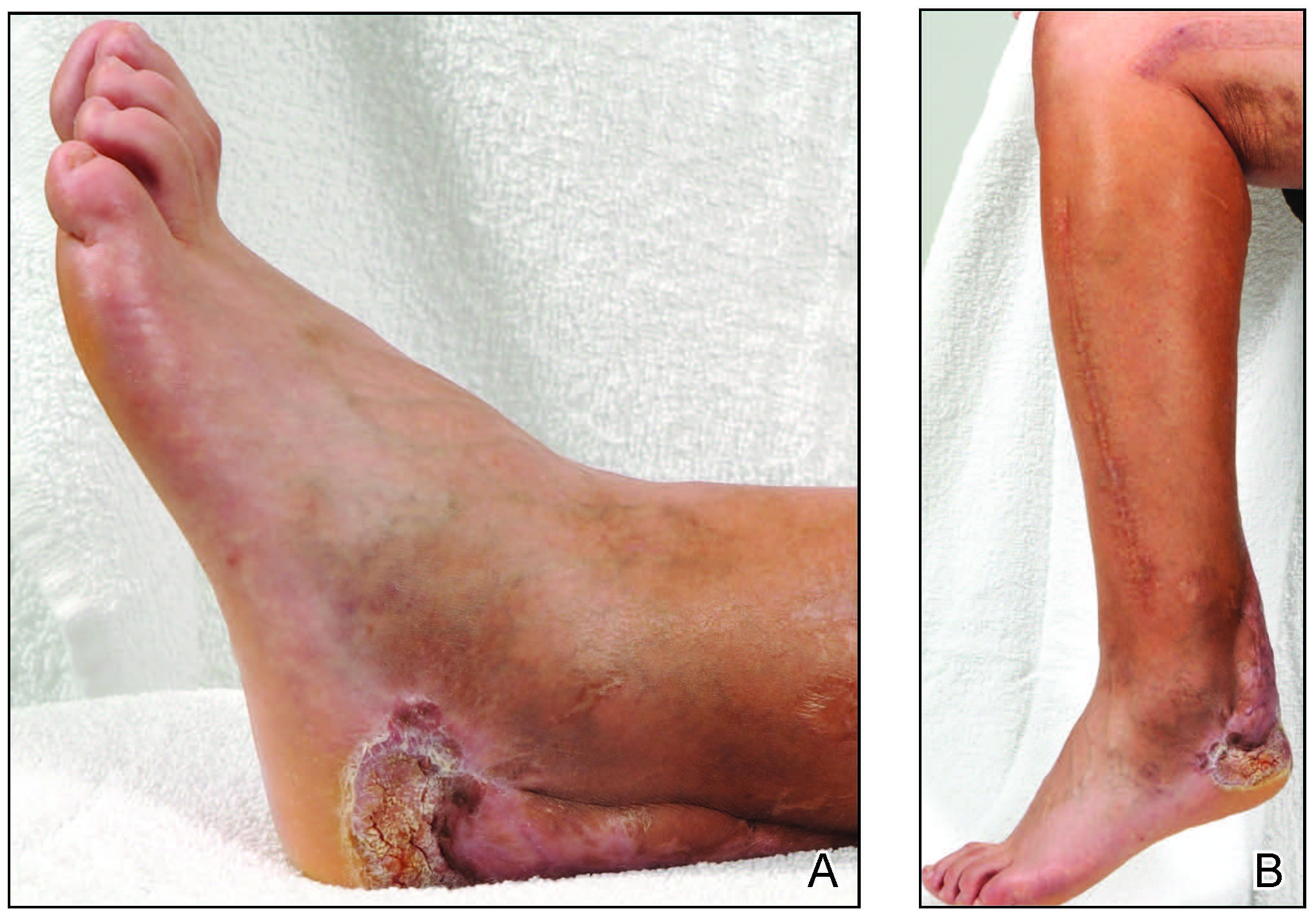
A year later, the lesion was excised again by orthopedic surgery, and the tissue was submitted for histopathologic evaluation, which was suggestive of a verrucous neoplasm with some disagreement on whether it was consistent with verrucous hyperplasia or verrucous carcinoma. Following excision, the patient sustained a nonhealing chronic ulcer that required wound care for a total of 6 months. The lesion recurred a year later and was surgically excised a third time. A split-thickness skin graft was utilized to repair the defect. Histopathology again was consistent with verrucous carcinoma. With a fourth and final recurrence of the verrucous plaque 6 months later, the patient elected to undergo a left-sided below-the-knee amputation.
Verrucous carcinoma can represent a diagnostic dilemma, as histologic sections may mimic benign entities. The features of a well-differentiated squamous epithelium with hyperkeratosis, papillomatosis, and acanthosis can be mistaken for verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia,6 which are characteristic of verrucous hyperplasia. Accurate diagnosis can be difficult with a superficial biopsy because of the mature appearance of the epithelium,7 prompting the need for multiple and deeper biopsies8 to include sampling of the base of the hyperplastic epithelium in which the characteristic bulbous pushing growth pattern of the rete ridges is visualized. Precise histologic diagnosis can be further confounded by external mechanical factors, such as pressure, which can distort the classic histopathology.7 The histopathologic features leading to the diagnosis of verrucous carcinoma in our specimen were minimal squamous atypia present in a predominantly exophytic squamous proliferation with human papillomavirus cytopathic effect and focal endophytic pushing borders by rounded bulbous rete ridges into the mid and deep dermis (Figure 2).

Diagnostic uncertainty can delay surgical excision and lead to progression of verrucous carcinoma. Unfortunately, even with appropriate surgical intervention, recurrence has been documented; therefore, close clinical follow-up is recommended. The tumor spreads by local invasion and may follow the path of least resistance.4 In our patient, the frequent tissue manipulation may have facilitated aggressive infiltration of the tumor, ultimately resulting in the loss of his remaining leg. Therefore, it is important for clinicians to recognize that verrucous carcinoma, especially one that develops on a refractory ulcer or scar tissue, may be a complex malignant neoplasm that requires extensive treatment at onset to prevent the amputation of a limb.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Yoshitasu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Schwartz R. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-14.
- Bernstein SC, Lim KK, Brodland DG, et al. The many faces of squamous cell carcinoma. Dermatol Surg. 1996;22:243-254.
- Costache M, Tatiana D, Mitrache L, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Shenoy A, Waghmare R, Kavishwar V, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Klima M, Kurtis B, Jordan P. Verrucous carcinoma of skin. J Cutan Pathol.1980;7:88-98.
- Pleat J, Sacks L, Rigby H. Cutaneous verrucous carcinoma. Br J Plast Surg. 2001;54:554-555.
To the Editor:
Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma first described by Ackerman in 1948.1 There are 4 main clinicopathologic types: oral florid papillomatosis or Ackerman tumor, giant condyloma acuminatum or Buschke-Lowenstein tumor, plantar verrucous carcinoma, and cutaneous verrucous carcinoma.2,3 Historically, most patients are older white men. The lesion commonly occurs in sites of inflammation4 or chronic irritation/trauma. Clinically, patients present with a slowly enlarging, exophytic, verrucous plaque violating the skin, fascia, and occasionally bone. Although these lesions have little tendency to metastasize, substantial morbidity can be seen due to local invasion. Despite surgical excision, recurrence is not uncommon and is associated with a poor prognosis and higher infiltrative potential.5
A 45-year-old male veteran initially presented to our dermatology clinic with a 4-cm, macerated, verrucous plaque on the left lateral ankle in the area of a skin graft placed during a prior limb salvage surgery (Figure 1). The patient experienced a traumatic blast injury while deployed 7 years prior with a subsequent right-sided below-the-knee amputation and left lower limb salvage. The lesion was clinically diagnosed as verruca vulgaris and treated with daily salicylic acid. Six weeks after the initial presentation, the lesion remained largely unchanged. A biopsy subsequently was obtained to confirm the diagnosis. At that time, the histopathology was consistent with verruca vulgaris without evidence of carcinoma. Due to the persistence of the lesion, lack of improvement with topical treatment, and overall size, the patient opted for surgical excision.

A year later, the lesion was excised again by orthopedic surgery, and the tissue was submitted for histopathologic evaluation, which was suggestive of a verrucous neoplasm with some disagreement on whether it was consistent with verrucous hyperplasia or verrucous carcinoma. Following excision, the patient sustained a nonhealing chronic ulcer that required wound care for a total of 6 months. The lesion recurred a year later and was surgically excised a third time. A split-thickness skin graft was utilized to repair the defect. Histopathology again was consistent with verrucous carcinoma. With a fourth and final recurrence of the verrucous plaque 6 months later, the patient elected to undergo a left-sided below-the-knee amputation.
Verrucous carcinoma can represent a diagnostic dilemma, as histologic sections may mimic benign entities. The features of a well-differentiated squamous epithelium with hyperkeratosis, papillomatosis, and acanthosis can be mistaken for verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia,6 which are characteristic of verrucous hyperplasia. Accurate diagnosis can be difficult with a superficial biopsy because of the mature appearance of the epithelium,7 prompting the need for multiple and deeper biopsies8 to include sampling of the base of the hyperplastic epithelium in which the characteristic bulbous pushing growth pattern of the rete ridges is visualized. Precise histologic diagnosis can be further confounded by external mechanical factors, such as pressure, which can distort the classic histopathology.7 The histopathologic features leading to the diagnosis of verrucous carcinoma in our specimen were minimal squamous atypia present in a predominantly exophytic squamous proliferation with human papillomavirus cytopathic effect and focal endophytic pushing borders by rounded bulbous rete ridges into the mid and deep dermis (Figure 2).

Diagnostic uncertainty can delay surgical excision and lead to progression of verrucous carcinoma. Unfortunately, even with appropriate surgical intervention, recurrence has been documented; therefore, close clinical follow-up is recommended. The tumor spreads by local invasion and may follow the path of least resistance.4 In our patient, the frequent tissue manipulation may have facilitated aggressive infiltration of the tumor, ultimately resulting in the loss of his remaining leg. Therefore, it is important for clinicians to recognize that verrucous carcinoma, especially one that develops on a refractory ulcer or scar tissue, may be a complex malignant neoplasm that requires extensive treatment at onset to prevent the amputation of a limb.
To the Editor:
Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma first described by Ackerman in 1948.1 There are 4 main clinicopathologic types: oral florid papillomatosis or Ackerman tumor, giant condyloma acuminatum or Buschke-Lowenstein tumor, plantar verrucous carcinoma, and cutaneous verrucous carcinoma.2,3 Historically, most patients are older white men. The lesion commonly occurs in sites of inflammation4 or chronic irritation/trauma. Clinically, patients present with a slowly enlarging, exophytic, verrucous plaque violating the skin, fascia, and occasionally bone. Although these lesions have little tendency to metastasize, substantial morbidity can be seen due to local invasion. Despite surgical excision, recurrence is not uncommon and is associated with a poor prognosis and higher infiltrative potential.5
A 45-year-old male veteran initially presented to our dermatology clinic with a 4-cm, macerated, verrucous plaque on the left lateral ankle in the area of a skin graft placed during a prior limb salvage surgery (Figure 1). The patient experienced a traumatic blast injury while deployed 7 years prior with a subsequent right-sided below-the-knee amputation and left lower limb salvage. The lesion was clinically diagnosed as verruca vulgaris and treated with daily salicylic acid. Six weeks after the initial presentation, the lesion remained largely unchanged. A biopsy subsequently was obtained to confirm the diagnosis. At that time, the histopathology was consistent with verruca vulgaris without evidence of carcinoma. Due to the persistence of the lesion, lack of improvement with topical treatment, and overall size, the patient opted for surgical excision.

A year later, the lesion was excised again by orthopedic surgery, and the tissue was submitted for histopathologic evaluation, which was suggestive of a verrucous neoplasm with some disagreement on whether it was consistent with verrucous hyperplasia or verrucous carcinoma. Following excision, the patient sustained a nonhealing chronic ulcer that required wound care for a total of 6 months. The lesion recurred a year later and was surgically excised a third time. A split-thickness skin graft was utilized to repair the defect. Histopathology again was consistent with verrucous carcinoma. With a fourth and final recurrence of the verrucous plaque 6 months later, the patient elected to undergo a left-sided below-the-knee amputation.
Verrucous carcinoma can represent a diagnostic dilemma, as histologic sections may mimic benign entities. The features of a well-differentiated squamous epithelium with hyperkeratosis, papillomatosis, and acanthosis can be mistaken for verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia,6 which are characteristic of verrucous hyperplasia. Accurate diagnosis can be difficult with a superficial biopsy because of the mature appearance of the epithelium,7 prompting the need for multiple and deeper biopsies8 to include sampling of the base of the hyperplastic epithelium in which the characteristic bulbous pushing growth pattern of the rete ridges is visualized. Precise histologic diagnosis can be further confounded by external mechanical factors, such as pressure, which can distort the classic histopathology.7 The histopathologic features leading to the diagnosis of verrucous carcinoma in our specimen were minimal squamous atypia present in a predominantly exophytic squamous proliferation with human papillomavirus cytopathic effect and focal endophytic pushing borders by rounded bulbous rete ridges into the mid and deep dermis (Figure 2).

Diagnostic uncertainty can delay surgical excision and lead to progression of verrucous carcinoma. Unfortunately, even with appropriate surgical intervention, recurrence has been documented; therefore, close clinical follow-up is recommended. The tumor spreads by local invasion and may follow the path of least resistance.4 In our patient, the frequent tissue manipulation may have facilitated aggressive infiltration of the tumor, ultimately resulting in the loss of his remaining leg. Therefore, it is important for clinicians to recognize that verrucous carcinoma, especially one that develops on a refractory ulcer or scar tissue, may be a complex malignant neoplasm that requires extensive treatment at onset to prevent the amputation of a limb.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Yoshitasu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Schwartz R. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-14.
- Bernstein SC, Lim KK, Brodland DG, et al. The many faces of squamous cell carcinoma. Dermatol Surg. 1996;22:243-254.
- Costache M, Tatiana D, Mitrache L, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Shenoy A, Waghmare R, Kavishwar V, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Klima M, Kurtis B, Jordan P. Verrucous carcinoma of skin. J Cutan Pathol.1980;7:88-98.
- Pleat J, Sacks L, Rigby H. Cutaneous verrucous carcinoma. Br J Plast Surg. 2001;54:554-555.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Yoshitasu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Schwartz R. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-14.
- Bernstein SC, Lim KK, Brodland DG, et al. The many faces of squamous cell carcinoma. Dermatol Surg. 1996;22:243-254.
- Costache M, Tatiana D, Mitrache L, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Shenoy A, Waghmare R, Kavishwar V, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Klima M, Kurtis B, Jordan P. Verrucous carcinoma of skin. J Cutan Pathol.1980;7:88-98.
- Pleat J, Sacks L, Rigby H. Cutaneous verrucous carcinoma. Br J Plast Surg. 2001;54:554-555.
Practice Points
- Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma that commonly occurs in sites of inflammation or chronic irritation.
- Histologically, verrucous carcinoma can be mistaken for other entities including verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia, often delaying the appropriate diagnosis and treatment.
Business Education in Dermatology Residency: A Survey of Program Directors
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
Practice Points
- In our survey of dermatology program directors, most felt inclusion of business education in residency training was important.
- Approximately half of the dermatology programs that responded to our survey offer business training to their residents.
- Economics and finance, management, and health care policy were the most important topics identified to include in a business curriculum for dermatology residents
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Psoriatic Arthritis Diagnosis and Management in the Era of Telehealth
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
Update on Biologics for Psoriasis in Clinical Practice
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Practice Points
- Choosing a biologic best fit for each patient’s individual health profile can reduce psoriasis disease burden.
- Clinicians should educate psoriasis patients that biologics are safe for most comorbidities, and conditions such as obesity have been associated with poorer therapeutic response.
- It is important to discuss possible side effects of biologics with patients and reassure them that mild side effects are the most common during therapy.