User login
Self-harm is a leading cause of death for new moms
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
Death by self-harm through suicide or overdose is a leading cause of death for women in the first year post partum, data indicate. Many of these deaths may be preventable, said Adrienne Griffen, MPP, executive director of the Maternal Mental Health Leadership Alliance.
Ms. Griffen discussed these findings and ways clinicians may be able to help at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
Women “visit a health care provider an average of 25 times during a healthy pregnancy and first year of baby’s life,” she said. “Obstetric and primary care providers who serve pregnant and postpartum women are uniquely positioned to intervene effectively to screen and assess women for mental health disorders.”
To that end, clinicians should discuss mental health “early and often,” Ms. Griffen said.
“Asking about mental health issues and suicide will not cause women to think these thoughts,” she said. “We cannot wait for women to raise their hand and ask for help because by the time they do that, they needed help many weeks ago.”
For example, a doctor might tell a patient: “Your mental health is just as important as your physical health, and anxiety and depression are the most common complications of pregnancy and childbirth,” Ms. Griffen suggested. “Every time I see you, I’m going to ask you how you are doing, and we’ll do a formal screening assessment periodically over the course of the pregnancy. … Your job is to answer us honestly so that we can connect you with resources as soon as possible to minimize the impact on you and your baby.”
Although the obstetric provider should introduce this topic, a nurse, lactation consultant, or social worker may conduct screenings and help patients who are experiencing distress, she said.
During the past decade, several medical associations have issued new guidance around screening new mothers for anxiety and depression. One recent ACOG committee opinion recommends screening for depression at least once during pregnancy and once post partum, and encourages doctors to initiate medical therapy if possible and provide resources and referrals.
Another committee opinion suggests that doctors should have contact with a patient between 2 and 3 weeks post partum, primarily to assess for mental health.
Limited data
In discussing maternal suicide statistics, Ms. Griffen focused on data from Maternal Mortality Review Committees (MMRCs).
Two other sources of data about maternal mortality – the National Vital Statistics System and the Pregnancy Mortality Surveillance System – do not include information about suicide, which may be a reason this cause of death is not discussed more often, Ms. Griffen noted.
MMRCs, on the other hand, include information about suicide and self-harm. About half of the states in the United States have these multidisciplinary committees. Committee members review deaths of all women during pregnancy or within 1 year of pregnancy. Members consider a range of clinical and nonclinical data, including reports from social services and police, to try to understand the circumstances of each death.
A report that examined pregnancy-related deaths using data from 14 U.S. MMRCs between 2008 and 2017 showed that mental health conditions were the leading cause of death for non-Hispanic White women. In all, 34% of pregnancy-related suicide deaths had a documented prior suicide attempt, and the majority of suicides happened in the late postpartum time frame (43-365 days post partum).
Some physicians cite a lack of education, time, reimbursement, or referral resources as barriers to maternal mental health screening and treatment, but there may be useful options available, Ms. Griffen said. Postpartum Support International provides resources for physicians, as well as mothers. The National Curriculum in Reproductive Psychiatry and the Seleni Institute also have educational resources.
Some states have psychiatry access programs, where psychiatrists educate obstetricians, family physicians, and pediatricians about how to assess for and treat maternal mental health issues, Ms. Griffen noted.
Self care, social support, and talk therapy may help patients. “Sometimes medication is needed, but a combination of all of these things … can help women recover from maternal mental health conditions,” Ms. Griffen said.
Need to intervene
Although medical societies have emphasized the importance of maternal mental health screening and treatment in recent years, the risk of self-harm has been a concern for obstetricians and gynecologists long before then, said Marc Alan Landsberg, MD, a member of the meeting’s scientific committee who moderated the session.
“We have been talking about this at ACOG for a long time,” Dr. Landsberg said in an interview.
The presentation highlighted why obstetricians, gynecologists, and other doctors who deliver babies and care for women post partum “have got to screen these people,” he said. The finding that 34% of pregnancy-related suicide deaths had a prior suicide attempt indicates that clinicians may be able to identify these patients, Dr. Landsberg said. Suicide and overdose are leading causes of death in the first year post partum and “probably 100% of these are preventable,” he said.
As a first step, screening may be relatively simple. The Edinburgh Postnatal Depression Scale, highlighted during the talk, is an easy and quick tool to use, Dr. Landsberg said. It contains 10 items and assesses for anxiety and depression. It also specifically asks about suicide.
Ms. Griffen and Dr. Landsberg had no conflicts of interest.
FROM ACOG 2021
Blood biomarker a ‘promising’ predictor of psychosis relapse
Copeptin, a small peptide secreted with the hormone vasopressin, appears to be one of the first promising biomarkers for predicting psychosis relapse, results of an observational study suggest.
An analysis of plasma copeptin levels in patients with schizophrenia showed those with high plasma levels of the peptide were about three times more likely to experience psychotic relapse, compared with their counterparts with lower levels.
The results suggest, “copeptin could be a promising biomarker in predicting psychotic relapse in schizophrenia spectrum disorder,” said study investigator Jennifer Küster, MD, University Psychiatric Clinics Basel (Switzerland). Measuring copeptin levels upon hospital admission “could help to intensify” the care of at-risk patients, she added.
The findings were presented at the virtual Congress of the Schizophrenia International Research Society 2021.
Relapse prevention important
Two-thirds of patients with schizophrenia experience at least one relapse of a psychotic episode, which in turn increases the risk of the disorder having a chronic course, Dr. Küster noted.
In addition, a psychotic relapse is associated with deterioration of function and cognition and reduced treatment response, “so relapse prevention is important,” she said.
Previous research has explored various methods of predicting schizophrenia outcomes. These include measuring inflammatory markers, catecholamines, oxytocin, and cortisol in combination with imaging markers, “but so far no reliable biomarker has been found,” Dr. Küster said.
She noted that psychotic relapse is associated with increased psychological stress – and vasopressin, which is secreted by the pituitary gland, is a known marker of stress. It is involved in sodium homeostasis and higher brain function and is also elevated in acute psychosis.
However, vasopressin “is challenging to measure because assays are complicated and unreliable,” Dr. Küster said.
As a result, the researchers turned their attention to copeptin, a more stable, more reliable surrogate marker for vasopressin. Copeptin has been shown previously to be a predictor of outcomes in somatic diseases and is also increased during psychological distress.
To measure the utility of copeptin in predicting psychotic relapse,
Baseline characteristics were collected and fasting serum copeptin levels were measured. Disease severity was measured using a range of validated assessment scales.
Predictive factor
Among 69 patients available for analysis, 30 experienced psychotic relapse at 1-year follow-up. Relapse was defined as rehospitalization because of an acute psychotic episode.
There were no differences in baseline demographic characteristics between patients with, and without, psychotic relapse. There were also no differences in baseline psychopathology, including scores on the Positive and Negative Syndrome Scale, the Beck Depression Inventory, and the Global Assessment of Function.
Dr. Küster noted that there were no overall differences between patients with and without psychotic relapse in terms of their plasma copeptin or cortisol levels at baseline.
“The only difference we saw was in diagnosis,” she reported. Patients with psychotic relapse were significantly more likely to have comorbid drug abuse – 43% in patients who relapsed versus 15% of those who did not (P = .02).
However, when the investigators calculated the area under the receiver operating characteristics curve for copeptin levels, they found there was a significant difference in relapse rates in those with copeptin levels >6 pmol/L vs. those with lower levels (hazard ratio, 2.3; P = .039).
When the focus was on only patients with schizophrenia spectrum disorder, the results were even more pronounced. The HR for psychotic relapse in patients with higher vs. lower copeptin levels was 3.2 (P = .028).
“We also looked for other possible predicting factors,” Dr. Küster said. This included sex, age, duration of disease, reason for hospitalization, psychopathology, medication, comorbidities, and cortisol levels. “But none of these factors was associated with psychotic relapse,” she added.
The only factor positively associated with relapse was drug abuse, primarily via marijuana. However, the association with copeptin remained significant even after taking this factor into account.
In future studies, the researchers plan to examine whether copeptin levels could identify which patients at ultra-high risk will transition to first-episode psychosis, as well as to predict development of posttraumatic stress disorder, Dr. Küster said.
A proxy for ‘something simpler’?
Commenting on the findings for this news organization, Leah H. Rubin, PhD, associate professor of neurology, Johns Hopkins University, Baltimore, described the study as “interesting” – and noted that her own research has included measuring vasopressin in patients with untreated first-episode psychosis.
Dr. Rubin’s findings showed that levels of the hormone were associated with psychosis severity, and thus she is “not surprised that they found a marker” that may be promising in psychosis relapse prediction.
However, she took issue with the notion that vasopressin is an unreliable marker, pointing out that the work of her team demonstrates that it can be measured. Dr. Rubin added that she found it to be “pretty stable.”
In addition, because the current study had a small sample size, Dr. Rubin said she would be interested to see whether the findings can be replicated on a larger scale.
She also noted that more than two-thirds of the study population were men. “Vasopressin and oxytocin are sexually dimorphic neuropeptides,” she explained, “so I think it becomes important to ensure ... whether it’s the same for men and women.”
“Just from a psychosocial perspective, what’s going on in those folks’ lives?” Dr. Rubin asked. “Is it truly copeptin” or is it high stress levels that facilitate a relapse? Copeptin levels, she added, may be “a proxy for something simpler.”
The study authors and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Copeptin, a small peptide secreted with the hormone vasopressin, appears to be one of the first promising biomarkers for predicting psychosis relapse, results of an observational study suggest.
An analysis of plasma copeptin levels in patients with schizophrenia showed those with high plasma levels of the peptide were about three times more likely to experience psychotic relapse, compared with their counterparts with lower levels.
The results suggest, “copeptin could be a promising biomarker in predicting psychotic relapse in schizophrenia spectrum disorder,” said study investigator Jennifer Küster, MD, University Psychiatric Clinics Basel (Switzerland). Measuring copeptin levels upon hospital admission “could help to intensify” the care of at-risk patients, she added.
The findings were presented at the virtual Congress of the Schizophrenia International Research Society 2021.
Relapse prevention important
Two-thirds of patients with schizophrenia experience at least one relapse of a psychotic episode, which in turn increases the risk of the disorder having a chronic course, Dr. Küster noted.
In addition, a psychotic relapse is associated with deterioration of function and cognition and reduced treatment response, “so relapse prevention is important,” she said.
Previous research has explored various methods of predicting schizophrenia outcomes. These include measuring inflammatory markers, catecholamines, oxytocin, and cortisol in combination with imaging markers, “but so far no reliable biomarker has been found,” Dr. Küster said.
She noted that psychotic relapse is associated with increased psychological stress – and vasopressin, which is secreted by the pituitary gland, is a known marker of stress. It is involved in sodium homeostasis and higher brain function and is also elevated in acute psychosis.
However, vasopressin “is challenging to measure because assays are complicated and unreliable,” Dr. Küster said.
As a result, the researchers turned their attention to copeptin, a more stable, more reliable surrogate marker for vasopressin. Copeptin has been shown previously to be a predictor of outcomes in somatic diseases and is also increased during psychological distress.
To measure the utility of copeptin in predicting psychotic relapse,
Baseline characteristics were collected and fasting serum copeptin levels were measured. Disease severity was measured using a range of validated assessment scales.
Predictive factor
Among 69 patients available for analysis, 30 experienced psychotic relapse at 1-year follow-up. Relapse was defined as rehospitalization because of an acute psychotic episode.
There were no differences in baseline demographic characteristics between patients with, and without, psychotic relapse. There were also no differences in baseline psychopathology, including scores on the Positive and Negative Syndrome Scale, the Beck Depression Inventory, and the Global Assessment of Function.
Dr. Küster noted that there were no overall differences between patients with and without psychotic relapse in terms of their plasma copeptin or cortisol levels at baseline.
“The only difference we saw was in diagnosis,” she reported. Patients with psychotic relapse were significantly more likely to have comorbid drug abuse – 43% in patients who relapsed versus 15% of those who did not (P = .02).
However, when the investigators calculated the area under the receiver operating characteristics curve for copeptin levels, they found there was a significant difference in relapse rates in those with copeptin levels >6 pmol/L vs. those with lower levels (hazard ratio, 2.3; P = .039).
When the focus was on only patients with schizophrenia spectrum disorder, the results were even more pronounced. The HR for psychotic relapse in patients with higher vs. lower copeptin levels was 3.2 (P = .028).
“We also looked for other possible predicting factors,” Dr. Küster said. This included sex, age, duration of disease, reason for hospitalization, psychopathology, medication, comorbidities, and cortisol levels. “But none of these factors was associated with psychotic relapse,” she added.
The only factor positively associated with relapse was drug abuse, primarily via marijuana. However, the association with copeptin remained significant even after taking this factor into account.
In future studies, the researchers plan to examine whether copeptin levels could identify which patients at ultra-high risk will transition to first-episode psychosis, as well as to predict development of posttraumatic stress disorder, Dr. Küster said.
A proxy for ‘something simpler’?
Commenting on the findings for this news organization, Leah H. Rubin, PhD, associate professor of neurology, Johns Hopkins University, Baltimore, described the study as “interesting” – and noted that her own research has included measuring vasopressin in patients with untreated first-episode psychosis.
Dr. Rubin’s findings showed that levels of the hormone were associated with psychosis severity, and thus she is “not surprised that they found a marker” that may be promising in psychosis relapse prediction.
However, she took issue with the notion that vasopressin is an unreliable marker, pointing out that the work of her team demonstrates that it can be measured. Dr. Rubin added that she found it to be “pretty stable.”
In addition, because the current study had a small sample size, Dr. Rubin said she would be interested to see whether the findings can be replicated on a larger scale.
She also noted that more than two-thirds of the study population were men. “Vasopressin and oxytocin are sexually dimorphic neuropeptides,” she explained, “so I think it becomes important to ensure ... whether it’s the same for men and women.”
“Just from a psychosocial perspective, what’s going on in those folks’ lives?” Dr. Rubin asked. “Is it truly copeptin” or is it high stress levels that facilitate a relapse? Copeptin levels, she added, may be “a proxy for something simpler.”
The study authors and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Copeptin, a small peptide secreted with the hormone vasopressin, appears to be one of the first promising biomarkers for predicting psychosis relapse, results of an observational study suggest.
An analysis of plasma copeptin levels in patients with schizophrenia showed those with high plasma levels of the peptide were about three times more likely to experience psychotic relapse, compared with their counterparts with lower levels.
The results suggest, “copeptin could be a promising biomarker in predicting psychotic relapse in schizophrenia spectrum disorder,” said study investigator Jennifer Küster, MD, University Psychiatric Clinics Basel (Switzerland). Measuring copeptin levels upon hospital admission “could help to intensify” the care of at-risk patients, she added.
The findings were presented at the virtual Congress of the Schizophrenia International Research Society 2021.
Relapse prevention important
Two-thirds of patients with schizophrenia experience at least one relapse of a psychotic episode, which in turn increases the risk of the disorder having a chronic course, Dr. Küster noted.
In addition, a psychotic relapse is associated with deterioration of function and cognition and reduced treatment response, “so relapse prevention is important,” she said.
Previous research has explored various methods of predicting schizophrenia outcomes. These include measuring inflammatory markers, catecholamines, oxytocin, and cortisol in combination with imaging markers, “but so far no reliable biomarker has been found,” Dr. Küster said.
She noted that psychotic relapse is associated with increased psychological stress – and vasopressin, which is secreted by the pituitary gland, is a known marker of stress. It is involved in sodium homeostasis and higher brain function and is also elevated in acute psychosis.
However, vasopressin “is challenging to measure because assays are complicated and unreliable,” Dr. Küster said.
As a result, the researchers turned their attention to copeptin, a more stable, more reliable surrogate marker for vasopressin. Copeptin has been shown previously to be a predictor of outcomes in somatic diseases and is also increased during psychological distress.
To measure the utility of copeptin in predicting psychotic relapse,
Baseline characteristics were collected and fasting serum copeptin levels were measured. Disease severity was measured using a range of validated assessment scales.
Predictive factor
Among 69 patients available for analysis, 30 experienced psychotic relapse at 1-year follow-up. Relapse was defined as rehospitalization because of an acute psychotic episode.
There were no differences in baseline demographic characteristics between patients with, and without, psychotic relapse. There were also no differences in baseline psychopathology, including scores on the Positive and Negative Syndrome Scale, the Beck Depression Inventory, and the Global Assessment of Function.
Dr. Küster noted that there were no overall differences between patients with and without psychotic relapse in terms of their plasma copeptin or cortisol levels at baseline.
“The only difference we saw was in diagnosis,” she reported. Patients with psychotic relapse were significantly more likely to have comorbid drug abuse – 43% in patients who relapsed versus 15% of those who did not (P = .02).
However, when the investigators calculated the area under the receiver operating characteristics curve for copeptin levels, they found there was a significant difference in relapse rates in those with copeptin levels >6 pmol/L vs. those with lower levels (hazard ratio, 2.3; P = .039).
When the focus was on only patients with schizophrenia spectrum disorder, the results were even more pronounced. The HR for psychotic relapse in patients with higher vs. lower copeptin levels was 3.2 (P = .028).
“We also looked for other possible predicting factors,” Dr. Küster said. This included sex, age, duration of disease, reason for hospitalization, psychopathology, medication, comorbidities, and cortisol levels. “But none of these factors was associated with psychotic relapse,” she added.
The only factor positively associated with relapse was drug abuse, primarily via marijuana. However, the association with copeptin remained significant even after taking this factor into account.
In future studies, the researchers plan to examine whether copeptin levels could identify which patients at ultra-high risk will transition to first-episode psychosis, as well as to predict development of posttraumatic stress disorder, Dr. Küster said.
A proxy for ‘something simpler’?
Commenting on the findings for this news organization, Leah H. Rubin, PhD, associate professor of neurology, Johns Hopkins University, Baltimore, described the study as “interesting” – and noted that her own research has included measuring vasopressin in patients with untreated first-episode psychosis.
Dr. Rubin’s findings showed that levels of the hormone were associated with psychosis severity, and thus she is “not surprised that they found a marker” that may be promising in psychosis relapse prediction.
However, she took issue with the notion that vasopressin is an unreliable marker, pointing out that the work of her team demonstrates that it can be measured. Dr. Rubin added that she found it to be “pretty stable.”
In addition, because the current study had a small sample size, Dr. Rubin said she would be interested to see whether the findings can be replicated on a larger scale.
She also noted that more than two-thirds of the study population were men. “Vasopressin and oxytocin are sexually dimorphic neuropeptides,” she explained, “so I think it becomes important to ensure ... whether it’s the same for men and women.”
“Just from a psychosocial perspective, what’s going on in those folks’ lives?” Dr. Rubin asked. “Is it truly copeptin” or is it high stress levels that facilitate a relapse? Copeptin levels, she added, may be “a proxy for something simpler.”
The study authors and Dr. Rubin have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cell phone, smart watch magnets can affect medical devices, FDA says
The Food and Drug Administration is recommending patients and caregivers keep cell phones and smart watches at least 6 inches away from implanted medical devices, such as pacemakers and defibrillators.
The warning, published on May 13, comes on the heels of recent research reporting that high–field strength magnets in newer smartphones may cause some implanted medical devices to switch to “magnet mode” and suspend normal lifesaving operations until the magnet is moved away.
This, for example, may cause a cardiac defibrillator to be unable to detect tachycardia events, the agency noted. The magnets may also change the operational mode such as turning on asynchronous mode in a pacemaker.
“The FDA is aware of published articles which describe the effect that sufficiently strong magnetic fields can turn on the magnetic safe mode when in close contact,” it said. “The FDA also conducted its own testing on some products that use the high–field strength magnet feature and have confirmed the magnetic field is both consistent with the publications and strong enough to turn on the magnetic safety mode of the medical devices in question.”
The FDA said it believes the risk to patients is low and is not aware of any adverse events associated with this issue at this time.
The American Heart Association has also cautioned that magnetic fields can inhibit the pulse generators for implantable cardioverter defibrillators and pacemakers.
The FDA offered the following simple precautions for individuals with implanted medical devices:
- Keep the consumer electronics, such as certain cell phones and smart watches, 6 inches away from implanted medical devices.
- Do not carry consumer electronics in a pocket over the medical device.
- Check your device using your home monitoring system, if you have one.
- Talk to your health care provider if you are experiencing any symptoms or have questions regarding magnets in consumer electronics and implanted medical devices.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is recommending patients and caregivers keep cell phones and smart watches at least 6 inches away from implanted medical devices, such as pacemakers and defibrillators.
The warning, published on May 13, comes on the heels of recent research reporting that high–field strength magnets in newer smartphones may cause some implanted medical devices to switch to “magnet mode” and suspend normal lifesaving operations until the magnet is moved away.
This, for example, may cause a cardiac defibrillator to be unable to detect tachycardia events, the agency noted. The magnets may also change the operational mode such as turning on asynchronous mode in a pacemaker.
“The FDA is aware of published articles which describe the effect that sufficiently strong magnetic fields can turn on the magnetic safe mode when in close contact,” it said. “The FDA also conducted its own testing on some products that use the high–field strength magnet feature and have confirmed the magnetic field is both consistent with the publications and strong enough to turn on the magnetic safety mode of the medical devices in question.”
The FDA said it believes the risk to patients is low and is not aware of any adverse events associated with this issue at this time.
The American Heart Association has also cautioned that magnetic fields can inhibit the pulse generators for implantable cardioverter defibrillators and pacemakers.
The FDA offered the following simple precautions for individuals with implanted medical devices:
- Keep the consumer electronics, such as certain cell phones and smart watches, 6 inches away from implanted medical devices.
- Do not carry consumer electronics in a pocket over the medical device.
- Check your device using your home monitoring system, if you have one.
- Talk to your health care provider if you are experiencing any symptoms or have questions regarding magnets in consumer electronics and implanted medical devices.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is recommending patients and caregivers keep cell phones and smart watches at least 6 inches away from implanted medical devices, such as pacemakers and defibrillators.
The warning, published on May 13, comes on the heels of recent research reporting that high–field strength magnets in newer smartphones may cause some implanted medical devices to switch to “magnet mode” and suspend normal lifesaving operations until the magnet is moved away.
This, for example, may cause a cardiac defibrillator to be unable to detect tachycardia events, the agency noted. The magnets may also change the operational mode such as turning on asynchronous mode in a pacemaker.
“The FDA is aware of published articles which describe the effect that sufficiently strong magnetic fields can turn on the magnetic safe mode when in close contact,” it said. “The FDA also conducted its own testing on some products that use the high–field strength magnet feature and have confirmed the magnetic field is both consistent with the publications and strong enough to turn on the magnetic safety mode of the medical devices in question.”
The FDA said it believes the risk to patients is low and is not aware of any adverse events associated with this issue at this time.
The American Heart Association has also cautioned that magnetic fields can inhibit the pulse generators for implantable cardioverter defibrillators and pacemakers.
The FDA offered the following simple precautions for individuals with implanted medical devices:
- Keep the consumer electronics, such as certain cell phones and smart watches, 6 inches away from implanted medical devices.
- Do not carry consumer electronics in a pocket over the medical device.
- Check your device using your home monitoring system, if you have one.
- Talk to your health care provider if you are experiencing any symptoms or have questions regarding magnets in consumer electronics and implanted medical devices.
A version of this article first appeared on Medscape.com.
Study points to best treatments for depression in primary care
according to a network meta-analysis (NMA) comparing either and both approaches with control conditions in the primary care setting.
The findings are important, since the majority of depressed patients are treated by primary care physicians, yet relatively few randomized trials of treatment have focused on this setting, noted senior study author Pim Cuijpers, PhD, from Vrije Universiteit Amsterdam, and colleagues, in the paper, which was published in Annals of Family Medicine.
“The main message is that clinicians should certainly consider psychotherapy instead of pharmacotherapy, because this is preferred by most patients, and when possible, combined treatments should be the preferred choice because the outcomes are considerably better,” he said in an interview. Either way, he emphasized that “preference of patients is very important and all three treatments are better than usual care.”
The NMA included studies comparing psychotherapy, antidepressant medication, or a combination of both, with control conditions (defined as usual care, wait list, or pill placebo) in adult primary care patients with depression.
Patients could have major depression, persistent mood disorders (dysthymia), both, or high scores on self-rating depression scales. The primary outcome of the NMA was response, defined as a 50% improvement in the Hamilton Depression Rating scores (HAM-D).
A total of 58 studies met inclusion criteria, involving 9,301 patients.
Treatment options compared
Compared with usual care, both psychotherapy alone and pharmacotherapy alone had significantly better response rates, with no significant difference between them (relative risk, 1.60 and RR, 1.65, respectively). The combination of psychotherapy and pharmacotherapy was even better (RR, 2.15), whereas the wait list was less effective (RR, 0.68).
When comparing combined therapy with psychotherapy or pharmacotherapy, the superiority of combination therapy over psychotherapy was only slightly statistically significant (RR, 1.35; 95% confidence interval, 1.00-1.81), while pharmacotherapy was only slightly inferior (RR, 1.30; 95% CI, 0.98-1.73).
“The significance level is not very high, which is related to statistical power,” said Dr. Cuijpers. “But the mean benefit is quite substantial in my opinion, with a 35% higher chance of response in the combined treatment, compared to psychotherapy alone.”
Looking at the outcome of remission, (normally defined as a score of 7 or less on the HAM-D), the outcomes were “comparable to those for response, with the exception that combined treatment was not significantly different from psychotherapy,” they wrote.
One important caveat is that several studies included in the NMA included patients with moderate to severe depression, a population that is different from the usual primary care population of depressed patients who have mild to moderate symptoms. Antidepressant medications are also assumed to work better against more severe symptoms, added the authors. “The inclusion of these studies might therefore have resulted in an overestimation of the effects of pharmacotherapy in the present NMA.”
Among other limitations, the authors noted that studies included mixed populations of patients with dysthymia and major depression; they also made no distinction between different types of antidepressants.
Psychotherapies unknown, but meta-analysis is still useful
Commenting on these findings, Neil Skolnik, MD, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, said this is “an important study, confirming and extending the conclusions” of a systematic review published in 2016 as a Clinical Practice Guideline from the American College of Physicians.
“Unfortunately, the authors did not specify what type of psychotherapy was studied in the meta-analysis, so we have to look elsewhere if we want to advise our patients on what type of psychotherapy to seek, since there are important differences between different types of therapy,” he said.
Still, he described the study as providing “helpful information for the practicing clinician, as it gives us solid information with which to engage and advise patients in a shared decision-making process for effective treatment of depression.”
“Some patients will choose psychotherapy, some will choose medications. They can make either choice with the confidence that both approaches are effective,” Dr. Skolnik elaborated. “In addition, if psychotherapy does not seem to be sufficiently helping we are on solid ground adding an antidepressant medication to psychotherapy, with this data showing that the combined treatment works better than psychotherapy alone.”
Dr. Cuijpers receives allowances for his memberships on the board of directors of Mind, Fonds Psychische Gezondheid, and Korrelatie, and for being chair of the PACO committee of the Raad voor Civiel-militaire Zorg en Onderzoek of the Dutch Ministry of Defense. He also serves as deputy editor of Depression and Anxiety and associate editor of Psychological Bulletin, and he receives royalties for books he has authored or coauthored. He received grants from the European Union, ZonMw, and PFGV. Another study author reported receiving personal fees from Mitsubishi-Tanabe, MSD, and Shionogi and a grant from Mitsubishi-Tanabe outside the submitted work. One author has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, and Angelini Pharmam, while another reported receiving personal fees from Boehringer Ingelheim, Kyowa Kirin, ASKA Pharmaceutical, and Toyota Motor Corporation outside the submitted work. The other authors and Dr. Skolnik reported no conflicts.
according to a network meta-analysis (NMA) comparing either and both approaches with control conditions in the primary care setting.
The findings are important, since the majority of depressed patients are treated by primary care physicians, yet relatively few randomized trials of treatment have focused on this setting, noted senior study author Pim Cuijpers, PhD, from Vrije Universiteit Amsterdam, and colleagues, in the paper, which was published in Annals of Family Medicine.
“The main message is that clinicians should certainly consider psychotherapy instead of pharmacotherapy, because this is preferred by most patients, and when possible, combined treatments should be the preferred choice because the outcomes are considerably better,” he said in an interview. Either way, he emphasized that “preference of patients is very important and all three treatments are better than usual care.”
The NMA included studies comparing psychotherapy, antidepressant medication, or a combination of both, with control conditions (defined as usual care, wait list, or pill placebo) in adult primary care patients with depression.
Patients could have major depression, persistent mood disorders (dysthymia), both, or high scores on self-rating depression scales. The primary outcome of the NMA was response, defined as a 50% improvement in the Hamilton Depression Rating scores (HAM-D).
A total of 58 studies met inclusion criteria, involving 9,301 patients.
Treatment options compared
Compared with usual care, both psychotherapy alone and pharmacotherapy alone had significantly better response rates, with no significant difference between them (relative risk, 1.60 and RR, 1.65, respectively). The combination of psychotherapy and pharmacotherapy was even better (RR, 2.15), whereas the wait list was less effective (RR, 0.68).
When comparing combined therapy with psychotherapy or pharmacotherapy, the superiority of combination therapy over psychotherapy was only slightly statistically significant (RR, 1.35; 95% confidence interval, 1.00-1.81), while pharmacotherapy was only slightly inferior (RR, 1.30; 95% CI, 0.98-1.73).
“The significance level is not very high, which is related to statistical power,” said Dr. Cuijpers. “But the mean benefit is quite substantial in my opinion, with a 35% higher chance of response in the combined treatment, compared to psychotherapy alone.”
Looking at the outcome of remission, (normally defined as a score of 7 or less on the HAM-D), the outcomes were “comparable to those for response, with the exception that combined treatment was not significantly different from psychotherapy,” they wrote.
One important caveat is that several studies included in the NMA included patients with moderate to severe depression, a population that is different from the usual primary care population of depressed patients who have mild to moderate symptoms. Antidepressant medications are also assumed to work better against more severe symptoms, added the authors. “The inclusion of these studies might therefore have resulted in an overestimation of the effects of pharmacotherapy in the present NMA.”
Among other limitations, the authors noted that studies included mixed populations of patients with dysthymia and major depression; they also made no distinction between different types of antidepressants.
Psychotherapies unknown, but meta-analysis is still useful
Commenting on these findings, Neil Skolnik, MD, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, said this is “an important study, confirming and extending the conclusions” of a systematic review published in 2016 as a Clinical Practice Guideline from the American College of Physicians.
“Unfortunately, the authors did not specify what type of psychotherapy was studied in the meta-analysis, so we have to look elsewhere if we want to advise our patients on what type of psychotherapy to seek, since there are important differences between different types of therapy,” he said.
Still, he described the study as providing “helpful information for the practicing clinician, as it gives us solid information with which to engage and advise patients in a shared decision-making process for effective treatment of depression.”
“Some patients will choose psychotherapy, some will choose medications. They can make either choice with the confidence that both approaches are effective,” Dr. Skolnik elaborated. “In addition, if psychotherapy does not seem to be sufficiently helping we are on solid ground adding an antidepressant medication to psychotherapy, with this data showing that the combined treatment works better than psychotherapy alone.”
Dr. Cuijpers receives allowances for his memberships on the board of directors of Mind, Fonds Psychische Gezondheid, and Korrelatie, and for being chair of the PACO committee of the Raad voor Civiel-militaire Zorg en Onderzoek of the Dutch Ministry of Defense. He also serves as deputy editor of Depression and Anxiety and associate editor of Psychological Bulletin, and he receives royalties for books he has authored or coauthored. He received grants from the European Union, ZonMw, and PFGV. Another study author reported receiving personal fees from Mitsubishi-Tanabe, MSD, and Shionogi and a grant from Mitsubishi-Tanabe outside the submitted work. One author has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, and Angelini Pharmam, while another reported receiving personal fees from Boehringer Ingelheim, Kyowa Kirin, ASKA Pharmaceutical, and Toyota Motor Corporation outside the submitted work. The other authors and Dr. Skolnik reported no conflicts.
according to a network meta-analysis (NMA) comparing either and both approaches with control conditions in the primary care setting.
The findings are important, since the majority of depressed patients are treated by primary care physicians, yet relatively few randomized trials of treatment have focused on this setting, noted senior study author Pim Cuijpers, PhD, from Vrije Universiteit Amsterdam, and colleagues, in the paper, which was published in Annals of Family Medicine.
“The main message is that clinicians should certainly consider psychotherapy instead of pharmacotherapy, because this is preferred by most patients, and when possible, combined treatments should be the preferred choice because the outcomes are considerably better,” he said in an interview. Either way, he emphasized that “preference of patients is very important and all three treatments are better than usual care.”
The NMA included studies comparing psychotherapy, antidepressant medication, or a combination of both, with control conditions (defined as usual care, wait list, or pill placebo) in adult primary care patients with depression.
Patients could have major depression, persistent mood disorders (dysthymia), both, or high scores on self-rating depression scales. The primary outcome of the NMA was response, defined as a 50% improvement in the Hamilton Depression Rating scores (HAM-D).
A total of 58 studies met inclusion criteria, involving 9,301 patients.
Treatment options compared
Compared with usual care, both psychotherapy alone and pharmacotherapy alone had significantly better response rates, with no significant difference between them (relative risk, 1.60 and RR, 1.65, respectively). The combination of psychotherapy and pharmacotherapy was even better (RR, 2.15), whereas the wait list was less effective (RR, 0.68).
When comparing combined therapy with psychotherapy or pharmacotherapy, the superiority of combination therapy over psychotherapy was only slightly statistically significant (RR, 1.35; 95% confidence interval, 1.00-1.81), while pharmacotherapy was only slightly inferior (RR, 1.30; 95% CI, 0.98-1.73).
“The significance level is not very high, which is related to statistical power,” said Dr. Cuijpers. “But the mean benefit is quite substantial in my opinion, with a 35% higher chance of response in the combined treatment, compared to psychotherapy alone.”
Looking at the outcome of remission, (normally defined as a score of 7 or less on the HAM-D), the outcomes were “comparable to those for response, with the exception that combined treatment was not significantly different from psychotherapy,” they wrote.
One important caveat is that several studies included in the NMA included patients with moderate to severe depression, a population that is different from the usual primary care population of depressed patients who have mild to moderate symptoms. Antidepressant medications are also assumed to work better against more severe symptoms, added the authors. “The inclusion of these studies might therefore have resulted in an overestimation of the effects of pharmacotherapy in the present NMA.”
Among other limitations, the authors noted that studies included mixed populations of patients with dysthymia and major depression; they also made no distinction between different types of antidepressants.
Psychotherapies unknown, but meta-analysis is still useful
Commenting on these findings, Neil Skolnik, MD, professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, said this is “an important study, confirming and extending the conclusions” of a systematic review published in 2016 as a Clinical Practice Guideline from the American College of Physicians.
“Unfortunately, the authors did not specify what type of psychotherapy was studied in the meta-analysis, so we have to look elsewhere if we want to advise our patients on what type of psychotherapy to seek, since there are important differences between different types of therapy,” he said.
Still, he described the study as providing “helpful information for the practicing clinician, as it gives us solid information with which to engage and advise patients in a shared decision-making process for effective treatment of depression.”
“Some patients will choose psychotherapy, some will choose medications. They can make either choice with the confidence that both approaches are effective,” Dr. Skolnik elaborated. “In addition, if psychotherapy does not seem to be sufficiently helping we are on solid ground adding an antidepressant medication to psychotherapy, with this data showing that the combined treatment works better than psychotherapy alone.”
Dr. Cuijpers receives allowances for his memberships on the board of directors of Mind, Fonds Psychische Gezondheid, and Korrelatie, and for being chair of the PACO committee of the Raad voor Civiel-militaire Zorg en Onderzoek of the Dutch Ministry of Defense. He also serves as deputy editor of Depression and Anxiety and associate editor of Psychological Bulletin, and he receives royalties for books he has authored or coauthored. He received grants from the European Union, ZonMw, and PFGV. Another study author reported receiving personal fees from Mitsubishi-Tanabe, MSD, and Shionogi and a grant from Mitsubishi-Tanabe outside the submitted work. One author has received research and consultancy fees from INCiPiT (Italian Network for Paediatric Trials), CARIPLO Foundation, and Angelini Pharmam, while another reported receiving personal fees from Boehringer Ingelheim, Kyowa Kirin, ASKA Pharmaceutical, and Toyota Motor Corporation outside the submitted work. The other authors and Dr. Skolnik reported no conflicts.
FROM ANNALS OF FAMILY MEDICINE
IBD online: What do patients search for?
A new online survey of inflammatory bowel disease (IBD) patients found that individuals seeking information on social media are generally satisfied with the care that they get from their health care providers. However, the online activity suggested a desire for more information, especially with respect to supportive needs like diet and complementary/alternative medicine (CAM).
The study was led by Idan Goren, MD, and Henit Yanai, MD, of Rabin Medical Center, Petah Tikva, Israel.
The researchers suspected that social media users with IBD were looking for information they weren’t getting from their provider, so the researchers set out to identify those specific unmet needs. In a pilot exploratory phase of their investigation, they conducted an initial survey followed by an analysis of social media posts, then they conducted a second phase with a survey based on the findings in the pilot exploration.
The initial survey was conducted within a social media platform in Israel called Camoni, where patients can interact with each other and with health care providers who have experience treating IBD, including gastroenterologists, dietitians, and psychologists. The survey included 10 items about disease characteristics, information needs, information search habits, and other factors. The subsequent analysis step included individual posts on the network between January 2014 and January 2019; the investigators categorized posts by the topics of interest brought up in the initial survey and determined the frequency of posts related to each category.
Out of the 255 respondents to this initial survey, 72% reported satisfaction with the information they received in person. In addition, 67% said that search engines like Google were their most important source of disease-related information, 58% reported relying heavily on websites, and 53% reported relying on health care providers. The most common topics of interest were diet (65%), medications and their potential adverse effects (58%), disease management (48%), and CAM (43%).
After this pilot exploratory phase, the researchers developed a structured survey that they used in IBD-based forums on Facebook and other social networks. Data were collected from this survey during a 4-week period in November 2019.
About half of the 534 respondents to the more widely distributed follow-up survey were in Israel. Overall, 83% reported using IBD-related medications, 45% of which were biologics. Out of the 534 respondents, 70% primarily received treatment from IBD referral centers. Interestingly, 77% said that they would prefer to rely on social media that is guided by health care providers, but only 22% reported that they actually used such a network. Responding along a visual analog scale, they reported general satisfaction with their routine IBD care (mean score, 79 ± 27 out of 100), their providers’ effectiveness of communication (82 ± 24), and the providers’ ability to understand patient concerns (73 ± 28). Those who were active in social media rated accessibility of IBD service as 68 ± 30. Exploration of topical interest found the most common to be diet (46%), lifestyle (45%), CAM (43%), diagnostic test interpretation (34%), and specialist referrals and reviews (31%).
The general satisfaction with information from health care providers contrasted with some previous studies that had shown that patients seeking information online often felt the opposite: For example, a 2019 Canadian survey found that only 10%-36% of IBD patients believed they received adequate information on IBD issues during clinical visits. The authors of the current study speculated that the incongruence might be explained by the fact that the current survey included patients with greater disease burden, who might get more attention during clinic visits than might patients with milder illness.
“In conclusion, our results indicate that patients’ activity on [social media] appears to be independent of their satisfaction with formal IBD care and rather reflects the contemporary need for ongoing information, particularly focused on supportive needs, such as diet and CAM,” the investigators wrote.
“Try not to Google everything”
The findings weren’t surprising, but the researchers found that patients seeking information online often have a high level of disease burden, as evidenced by biologics use and a majority being seen by specialists. That’s worrisome, said Jason Reich, MD, a gastroenterologist in Fall River, Mass., who has also studied social media use among IBD patients but was not involved in this study. “The last person you want getting poor-quality information is someone with pretty active disease,” said Dr. Reich in an interview.
Dr. Reich agreed with the authors that IBD specialists should consider having a dietitian in their clinic, or at least refer patients to dietitians early on. He also advocated for gastroenterologists (and all physicians, really) to have an online presence, if possible. “At least make themselves and their office accessible. I always tell my patients, if you have questions, try not to Google everything online and just shoot me a message through the portal instead,” said Dr. Reich. He added that nurses can handle such duties, especially those trained in IBD. “Personally, I don’t mind sending my short messages back and forth. Especially if it’s just a question. That’s easy enough to do when it takes maybe a minute or 2.”
The authors disclosed no funding sources. Dr. Reich has no relevant financial disclosures.
A new online survey of inflammatory bowel disease (IBD) patients found that individuals seeking information on social media are generally satisfied with the care that they get from their health care providers. However, the online activity suggested a desire for more information, especially with respect to supportive needs like diet and complementary/alternative medicine (CAM).
The study was led by Idan Goren, MD, and Henit Yanai, MD, of Rabin Medical Center, Petah Tikva, Israel.
The researchers suspected that social media users with IBD were looking for information they weren’t getting from their provider, so the researchers set out to identify those specific unmet needs. In a pilot exploratory phase of their investigation, they conducted an initial survey followed by an analysis of social media posts, then they conducted a second phase with a survey based on the findings in the pilot exploration.
The initial survey was conducted within a social media platform in Israel called Camoni, where patients can interact with each other and with health care providers who have experience treating IBD, including gastroenterologists, dietitians, and psychologists. The survey included 10 items about disease characteristics, information needs, information search habits, and other factors. The subsequent analysis step included individual posts on the network between January 2014 and January 2019; the investigators categorized posts by the topics of interest brought up in the initial survey and determined the frequency of posts related to each category.
Out of the 255 respondents to this initial survey, 72% reported satisfaction with the information they received in person. In addition, 67% said that search engines like Google were their most important source of disease-related information, 58% reported relying heavily on websites, and 53% reported relying on health care providers. The most common topics of interest were diet (65%), medications and their potential adverse effects (58%), disease management (48%), and CAM (43%).
After this pilot exploratory phase, the researchers developed a structured survey that they used in IBD-based forums on Facebook and other social networks. Data were collected from this survey during a 4-week period in November 2019.
About half of the 534 respondents to the more widely distributed follow-up survey were in Israel. Overall, 83% reported using IBD-related medications, 45% of which were biologics. Out of the 534 respondents, 70% primarily received treatment from IBD referral centers. Interestingly, 77% said that they would prefer to rely on social media that is guided by health care providers, but only 22% reported that they actually used such a network. Responding along a visual analog scale, they reported general satisfaction with their routine IBD care (mean score, 79 ± 27 out of 100), their providers’ effectiveness of communication (82 ± 24), and the providers’ ability to understand patient concerns (73 ± 28). Those who were active in social media rated accessibility of IBD service as 68 ± 30. Exploration of topical interest found the most common to be diet (46%), lifestyle (45%), CAM (43%), diagnostic test interpretation (34%), and specialist referrals and reviews (31%).
The general satisfaction with information from health care providers contrasted with some previous studies that had shown that patients seeking information online often felt the opposite: For example, a 2019 Canadian survey found that only 10%-36% of IBD patients believed they received adequate information on IBD issues during clinical visits. The authors of the current study speculated that the incongruence might be explained by the fact that the current survey included patients with greater disease burden, who might get more attention during clinic visits than might patients with milder illness.
“In conclusion, our results indicate that patients’ activity on [social media] appears to be independent of their satisfaction with formal IBD care and rather reflects the contemporary need for ongoing information, particularly focused on supportive needs, such as diet and CAM,” the investigators wrote.
“Try not to Google everything”
The findings weren’t surprising, but the researchers found that patients seeking information online often have a high level of disease burden, as evidenced by biologics use and a majority being seen by specialists. That’s worrisome, said Jason Reich, MD, a gastroenterologist in Fall River, Mass., who has also studied social media use among IBD patients but was not involved in this study. “The last person you want getting poor-quality information is someone with pretty active disease,” said Dr. Reich in an interview.
Dr. Reich agreed with the authors that IBD specialists should consider having a dietitian in their clinic, or at least refer patients to dietitians early on. He also advocated for gastroenterologists (and all physicians, really) to have an online presence, if possible. “At least make themselves and their office accessible. I always tell my patients, if you have questions, try not to Google everything online and just shoot me a message through the portal instead,” said Dr. Reich. He added that nurses can handle such duties, especially those trained in IBD. “Personally, I don’t mind sending my short messages back and forth. Especially if it’s just a question. That’s easy enough to do when it takes maybe a minute or 2.”
The authors disclosed no funding sources. Dr. Reich has no relevant financial disclosures.
A new online survey of inflammatory bowel disease (IBD) patients found that individuals seeking information on social media are generally satisfied with the care that they get from their health care providers. However, the online activity suggested a desire for more information, especially with respect to supportive needs like diet and complementary/alternative medicine (CAM).
The study was led by Idan Goren, MD, and Henit Yanai, MD, of Rabin Medical Center, Petah Tikva, Israel.
The researchers suspected that social media users with IBD were looking for information they weren’t getting from their provider, so the researchers set out to identify those specific unmet needs. In a pilot exploratory phase of their investigation, they conducted an initial survey followed by an analysis of social media posts, then they conducted a second phase with a survey based on the findings in the pilot exploration.
The initial survey was conducted within a social media platform in Israel called Camoni, where patients can interact with each other and with health care providers who have experience treating IBD, including gastroenterologists, dietitians, and psychologists. The survey included 10 items about disease characteristics, information needs, information search habits, and other factors. The subsequent analysis step included individual posts on the network between January 2014 and January 2019; the investigators categorized posts by the topics of interest brought up in the initial survey and determined the frequency of posts related to each category.
Out of the 255 respondents to this initial survey, 72% reported satisfaction with the information they received in person. In addition, 67% said that search engines like Google were their most important source of disease-related information, 58% reported relying heavily on websites, and 53% reported relying on health care providers. The most common topics of interest were diet (65%), medications and their potential adverse effects (58%), disease management (48%), and CAM (43%).
After this pilot exploratory phase, the researchers developed a structured survey that they used in IBD-based forums on Facebook and other social networks. Data were collected from this survey during a 4-week period in November 2019.
About half of the 534 respondents to the more widely distributed follow-up survey were in Israel. Overall, 83% reported using IBD-related medications, 45% of which were biologics. Out of the 534 respondents, 70% primarily received treatment from IBD referral centers. Interestingly, 77% said that they would prefer to rely on social media that is guided by health care providers, but only 22% reported that they actually used such a network. Responding along a visual analog scale, they reported general satisfaction with their routine IBD care (mean score, 79 ± 27 out of 100), their providers’ effectiveness of communication (82 ± 24), and the providers’ ability to understand patient concerns (73 ± 28). Those who were active in social media rated accessibility of IBD service as 68 ± 30. Exploration of topical interest found the most common to be diet (46%), lifestyle (45%), CAM (43%), diagnostic test interpretation (34%), and specialist referrals and reviews (31%).
The general satisfaction with information from health care providers contrasted with some previous studies that had shown that patients seeking information online often felt the opposite: For example, a 2019 Canadian survey found that only 10%-36% of IBD patients believed they received adequate information on IBD issues during clinical visits. The authors of the current study speculated that the incongruence might be explained by the fact that the current survey included patients with greater disease burden, who might get more attention during clinic visits than might patients with milder illness.
“In conclusion, our results indicate that patients’ activity on [social media] appears to be independent of their satisfaction with formal IBD care and rather reflects the contemporary need for ongoing information, particularly focused on supportive needs, such as diet and CAM,” the investigators wrote.
“Try not to Google everything”
The findings weren’t surprising, but the researchers found that patients seeking information online often have a high level of disease burden, as evidenced by biologics use and a majority being seen by specialists. That’s worrisome, said Jason Reich, MD, a gastroenterologist in Fall River, Mass., who has also studied social media use among IBD patients but was not involved in this study. “The last person you want getting poor-quality information is someone with pretty active disease,” said Dr. Reich in an interview.
Dr. Reich agreed with the authors that IBD specialists should consider having a dietitian in their clinic, or at least refer patients to dietitians early on. He also advocated for gastroenterologists (and all physicians, really) to have an online presence, if possible. “At least make themselves and their office accessible. I always tell my patients, if you have questions, try not to Google everything online and just shoot me a message through the portal instead,” said Dr. Reich. He added that nurses can handle such duties, especially those trained in IBD. “Personally, I don’t mind sending my short messages back and forth. Especially if it’s just a question. That’s easy enough to do when it takes maybe a minute or 2.”
The authors disclosed no funding sources. Dr. Reich has no relevant financial disclosures.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Focus on prepregnancy care
Improving maternal morbidity and mortality begins prior to conception. Numerous modifiable and nonmodifiable factors—lifestyle behaviors, chronic medical conditions, medications, immunizations, prior pregnancy events—have been shown to improve pregnancy outcomes if they are reviewed, identified, and optimized before conception.
Laying a solid foundation for a healthy pregnancy requires a comprehensive approach to patient counseling. However, the national Pregnancy Risk Assessment Monitoring System (PRAMS; a surveillance program of the Centers for Disease Control and Prevention) data from 2014 show that only about 20% of women receive counseling on at least 5 out of 11 healthy lifestyle behaviors and prevention strategies before pregnancy. The ability to leverage technology-enabled smart device applications can provide clinicians with immediate access to information necessary to address with patients at a preconception visit. Apps built specifically for physicians offer a convenient, thorough, and peer-vetted reference that can increase the efficiency and quality of consultation in a busy practice.
Prepregnancy care app considerations
When applying the ACOG-recommended rubric to evaluate the quality of an app targeted to address preconception counseling, the accuracy and objectivity of the content, as well as the app’s ease of use, are vital characteristics to consider, and these criteria should score 4 out of 4 on the rubric.
Several apps offer suggestions on how to address important components of health, including counseling and intervention strategies and evidence-based recommendations. The most efficacious apps offer embedded references to more detailed resources for use when complexities inevitably arise during consultation. Truly comprehensive prepregnancy care requires clinicians to take a step beyond the review of patients’ medications and comorbidities. It is therefore helpful to implement point-of-care apps that prompt evaluation of the often-overlooked aspects of prepregnancy counseling, including risk of interpersonal violence and infectious diseases, occupational exposures, and immunization status.
Physician-focused prepregnancy apps that provide reminders, prompts, and strategies for addressing a comprehensive set of health components prior to conception can be valuable tools to incorporate into both educational environments and busy practices to address maternal morbidity and mortality. ●
Improving maternal morbidity and mortality begins prior to conception. Numerous modifiable and nonmodifiable factors—lifestyle behaviors, chronic medical conditions, medications, immunizations, prior pregnancy events—have been shown to improve pregnancy outcomes if they are reviewed, identified, and optimized before conception.
Laying a solid foundation for a healthy pregnancy requires a comprehensive approach to patient counseling. However, the national Pregnancy Risk Assessment Monitoring System (PRAMS; a surveillance program of the Centers for Disease Control and Prevention) data from 2014 show that only about 20% of women receive counseling on at least 5 out of 11 healthy lifestyle behaviors and prevention strategies before pregnancy. The ability to leverage technology-enabled smart device applications can provide clinicians with immediate access to information necessary to address with patients at a preconception visit. Apps built specifically for physicians offer a convenient, thorough, and peer-vetted reference that can increase the efficiency and quality of consultation in a busy practice.
Prepregnancy care app considerations
When applying the ACOG-recommended rubric to evaluate the quality of an app targeted to address preconception counseling, the accuracy and objectivity of the content, as well as the app’s ease of use, are vital characteristics to consider, and these criteria should score 4 out of 4 on the rubric.
Several apps offer suggestions on how to address important components of health, including counseling and intervention strategies and evidence-based recommendations. The most efficacious apps offer embedded references to more detailed resources for use when complexities inevitably arise during consultation. Truly comprehensive prepregnancy care requires clinicians to take a step beyond the review of patients’ medications and comorbidities. It is therefore helpful to implement point-of-care apps that prompt evaluation of the often-overlooked aspects of prepregnancy counseling, including risk of interpersonal violence and infectious diseases, occupational exposures, and immunization status.
Physician-focused prepregnancy apps that provide reminders, prompts, and strategies for addressing a comprehensive set of health components prior to conception can be valuable tools to incorporate into both educational environments and busy practices to address maternal morbidity and mortality. ●
Improving maternal morbidity and mortality begins prior to conception. Numerous modifiable and nonmodifiable factors—lifestyle behaviors, chronic medical conditions, medications, immunizations, prior pregnancy events—have been shown to improve pregnancy outcomes if they are reviewed, identified, and optimized before conception.
Laying a solid foundation for a healthy pregnancy requires a comprehensive approach to patient counseling. However, the national Pregnancy Risk Assessment Monitoring System (PRAMS; a surveillance program of the Centers for Disease Control and Prevention) data from 2014 show that only about 20% of women receive counseling on at least 5 out of 11 healthy lifestyle behaviors and prevention strategies before pregnancy. The ability to leverage technology-enabled smart device applications can provide clinicians with immediate access to information necessary to address with patients at a preconception visit. Apps built specifically for physicians offer a convenient, thorough, and peer-vetted reference that can increase the efficiency and quality of consultation in a busy practice.
Prepregnancy care app considerations
When applying the ACOG-recommended rubric to evaluate the quality of an app targeted to address preconception counseling, the accuracy and objectivity of the content, as well as the app’s ease of use, are vital characteristics to consider, and these criteria should score 4 out of 4 on the rubric.
Several apps offer suggestions on how to address important components of health, including counseling and intervention strategies and evidence-based recommendations. The most efficacious apps offer embedded references to more detailed resources for use when complexities inevitably arise during consultation. Truly comprehensive prepregnancy care requires clinicians to take a step beyond the review of patients’ medications and comorbidities. It is therefore helpful to implement point-of-care apps that prompt evaluation of the often-overlooked aspects of prepregnancy counseling, including risk of interpersonal violence and infectious diseases, occupational exposures, and immunization status.
Physician-focused prepregnancy apps that provide reminders, prompts, and strategies for addressing a comprehensive set of health components prior to conception can be valuable tools to incorporate into both educational environments and busy practices to address maternal morbidity and mortality. ●
Championing preventive care in ObGyn: A tool to evaluate for useful medical apps
Personalizing care is at the heart of the American College of Obstetricians and Gynecologists (ACOG) 2020–2021 President Dr. Eva Chalas’ initiative to “Revisit the Visit.” As obstetrician-gynecologists, we care for patients across the entirety of their life. This role gives us the opportunity to form long-term partnerships with women to address important preventive health care measures.
Dr. Chalas established a Presidential Task Force that identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. The annual visit can serve as a particularly impactful point of care to achieve specific preventive care objectives and offer mitigation strategies based on patient-specific risk factors. We are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas.
Harnessing modern technology
To adopt these health topics into practice, we need improved, more effective tools both to increase productivity during the office visit and to provide more personalized care. Notably, the widespread adoption of and proliferation of mobile devices—and the medical apps accessible on them—is creating new and innovative ways to improve health and health care delivery. More than 90% of physicians use a smartphone at work, and 62% of smartphone users have used their device to gather health data.1
In addition, according to a US Food and Drug Administration (FDA) report, in 2017, 325,000 health care applications were available on smartphones; this equates to an expected 3.7 billion mobile health application downloads that year by 1.7 billion smartphone users worldwide.2 As of October 2020, 48,000-plus health apps were available on the iOS mobile operating system alone.3
For patients and clinicians, picking the most suitable apps can be challenging in the face of evolving clinical evidence, emerging privacy risks, functionality concerns, and the fact that apps constantly update and change. Many have relied on star rating systems and user reviews in app stores to guide their selection process despite mounting evidence that suggests that such evaluation methods are misleading, not always addressing such important parameters as usability, validity, security, and privacy.4,5
Approaches for evaluating medical apps
Many app evaluation frameworks have emerged, but none is universally accepted within the health care field.
The American Psychiatric Association’s (APA) App Evaluation Model represents a comprehensive resource to consider when evaluating medical apps. It stratifies numerous variables into 5 levels that form a pyramid. In this model, background information forms the base of this pyramid and includes factors such as business model, credibility, cost, and advertising of the app. The top of the pyramid is comprised of data integration that considers data ownership and therapeutic alliance.6 Although this model is beneficial in that it provides a framework, it is not practical for point-of-care purposes as it offers no objective way to rate or score an app for quick and easy comparison.
The privately owned and operated Health On the Net (HON) Foundation is well known for its HONcode, an ethical standard for quality medical information on the internet. It uses 8 principles to certify a health website. However, the HON website itself states that it cannot guarantee the accuracy or completeness of medical information presented by a site.7 Although HON certification by a website is a sign of good intention, it is not beneficial to the practicing clinician who is looking to use an app to directly assist in clinical care.
The Agency for Healthcare Research and Quality (AHRQ) is another well-respected body that has delineated essential details to consider when using a health website. The AHRQ identifies features (similar to those of the APA pyramid and HONcode) for users to consider, such as credibility, content, design, and disclosures.8 However, this model too lacks a concise user-friendly evaluating system.
Although the FDA plans to apply some regulatory authority to the evaluation of a certain subset of high-risk mobile medical apps, it is not planning to evaluate or regulate many of the medical apps that clinicians use in daily practice. This leaves us, and our patients, to be guided by the principle of caveat emptor, or “let the buyer beware.”
Thus, Dr. Chalas’ Presidential Task Force carefully considered various resources to provide a useful tool that would help obstetrician-gynecologists objectively vet a medical app in practice.
Continue to: The Task Force’s recommended rubric...
The Task Force’s recommended rubric
The rubric shown for evaluating mobile drug information apps was developed by the American Society of Health-System Pharmacists (ASHP). The ASHP rubric takes into account the criteria recognized by the APA pyramid, the HON Foundation, and the AHRQ and incorporates them into a user-friendly tool and scoring system that can be applied as an evaluation checklist.9 This tool is meant to aid clinicians in evaluating medical apps, but it ultimately is the user’s decision to determine if an app’s deficiencies should deter its use.
While all of the criteria are relevant and important, it is incumbent on us as medical experts to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice. A low score on these criteria would belie any perceived usefulness or value the app may have.
When applying the rubric to evaluate the quality of an app, we should be mindful of the primary user and which characteristics are more important than others to effect positive changes in health. For example, in addressing obesity, it is the patient who will be interacting with the app. Therefore, it’s important that the app should score, on a 1- to 4-point scale (1 point being major deficiencies, 4 points being no deficiencies), a 4 out of 4 on features like usefulness, functionality, and design. Coveted design features that enhance the user’s experience will appeal to patients and keep them engaged and motivated. However, when addressing a woman’s health with respect to cancer risk, the principal features on which the app should score 4 out of 4 would be authority, objectivity, timeliness, and accuracy.
In the upcoming articles in this series, a member of the Presidential Task Force will reference the ASHP rubric to guide clinicians in choosing apps to address one of the critical health areas with their patients. The author of the piece will highlight key features of an app to consider what would add the most value in incorporating its use in clinical practice.
It would be impossible to evaluate all health care apps even if we focused only on the medical apps relevant to obstetrics and gynecology. There is much value in having a framework for efficiently measuring an app’s benefit in clinical practice. The objective of this article series is to help clinicians Revisit the Visit by providing an effective tool to evaluate a medical app. ●
- Mobius MD website. 11 Surprising mobile health statistics. http://www.mobius.md/blog/2019/03/11-mobile-health -statistics/. Accessed January 19, 2021.
- US Food and Drug Administration website. Device software functions including medical applications. November 5, 2019. https://www.fda.gov/medical-devices/digital-health-center -excellence/device-software-functions-including-mobile -medical-applications. Accessed March 10, 2021.
- Statista website. Number of mHealth apps available in the Apple App Store from 1st quarter 2015 to 4th quarter 2020. https://www.statista.com/statistics/779910/health-apps -available-ios-worldwide/. Accessed January 19, 2021.
- Campbell L. Using star ratings to choose a medical app? There’s a better way. Healthline website. Updated August 3, 2018. http://healthline.com/health-news/using-ratings-to -choose-medical-app-theres-a-better-way. Accessed April 22, 2021.
- Levine DM, Co Z, Newmark LP, et al. Design and testing of a mobile health application rating tool. NPJ Digit Med. 2020;3:74.
- Torous JB, Chan SR, Gipson SY, et al. A hierarchical framework for evaluation and informed decision making regarding smartphone apps for clinical care. Psychiatr Serv. 2018;69:498-500.
- Health On the Net website. The commitment to reliable health and medical information on the internet. https:// www.hon.ch/HONcode/Patients/Visitor/visitor.html. Accessed January 19, 2021.
- Agency for Healthcare Research and Quality. Assessing the quality of internet health information. June 1999. http:// www.ahrq.gov/research/data/infoqual.html. Accessed April 22, 2021.
- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp.org/-/media/store-files /mobile-medical-apps.ashx. Accessed January 22, 2021.
Personalizing care is at the heart of the American College of Obstetricians and Gynecologists (ACOG) 2020–2021 President Dr. Eva Chalas’ initiative to “Revisit the Visit.” As obstetrician-gynecologists, we care for patients across the entirety of their life. This role gives us the opportunity to form long-term partnerships with women to address important preventive health care measures.
Dr. Chalas established a Presidential Task Force that identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. The annual visit can serve as a particularly impactful point of care to achieve specific preventive care objectives and offer mitigation strategies based on patient-specific risk factors. We are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas.
Harnessing modern technology
To adopt these health topics into practice, we need improved, more effective tools both to increase productivity during the office visit and to provide more personalized care. Notably, the widespread adoption of and proliferation of mobile devices—and the medical apps accessible on them—is creating new and innovative ways to improve health and health care delivery. More than 90% of physicians use a smartphone at work, and 62% of smartphone users have used their device to gather health data.1
In addition, according to a US Food and Drug Administration (FDA) report, in 2017, 325,000 health care applications were available on smartphones; this equates to an expected 3.7 billion mobile health application downloads that year by 1.7 billion smartphone users worldwide.2 As of October 2020, 48,000-plus health apps were available on the iOS mobile operating system alone.3
For patients and clinicians, picking the most suitable apps can be challenging in the face of evolving clinical evidence, emerging privacy risks, functionality concerns, and the fact that apps constantly update and change. Many have relied on star rating systems and user reviews in app stores to guide their selection process despite mounting evidence that suggests that such evaluation methods are misleading, not always addressing such important parameters as usability, validity, security, and privacy.4,5
Approaches for evaluating medical apps
Many app evaluation frameworks have emerged, but none is universally accepted within the health care field.
The American Psychiatric Association’s (APA) App Evaluation Model represents a comprehensive resource to consider when evaluating medical apps. It stratifies numerous variables into 5 levels that form a pyramid. In this model, background information forms the base of this pyramid and includes factors such as business model, credibility, cost, and advertising of the app. The top of the pyramid is comprised of data integration that considers data ownership and therapeutic alliance.6 Although this model is beneficial in that it provides a framework, it is not practical for point-of-care purposes as it offers no objective way to rate or score an app for quick and easy comparison.
The privately owned and operated Health On the Net (HON) Foundation is well known for its HONcode, an ethical standard for quality medical information on the internet. It uses 8 principles to certify a health website. However, the HON website itself states that it cannot guarantee the accuracy or completeness of medical information presented by a site.7 Although HON certification by a website is a sign of good intention, it is not beneficial to the practicing clinician who is looking to use an app to directly assist in clinical care.
The Agency for Healthcare Research and Quality (AHRQ) is another well-respected body that has delineated essential details to consider when using a health website. The AHRQ identifies features (similar to those of the APA pyramid and HONcode) for users to consider, such as credibility, content, design, and disclosures.8 However, this model too lacks a concise user-friendly evaluating system.
Although the FDA plans to apply some regulatory authority to the evaluation of a certain subset of high-risk mobile medical apps, it is not planning to evaluate or regulate many of the medical apps that clinicians use in daily practice. This leaves us, and our patients, to be guided by the principle of caveat emptor, or “let the buyer beware.”
Thus, Dr. Chalas’ Presidential Task Force carefully considered various resources to provide a useful tool that would help obstetrician-gynecologists objectively vet a medical app in practice.
Continue to: The Task Force’s recommended rubric...
The Task Force’s recommended rubric
The rubric shown for evaluating mobile drug information apps was developed by the American Society of Health-System Pharmacists (ASHP). The ASHP rubric takes into account the criteria recognized by the APA pyramid, the HON Foundation, and the AHRQ and incorporates them into a user-friendly tool and scoring system that can be applied as an evaluation checklist.9 This tool is meant to aid clinicians in evaluating medical apps, but it ultimately is the user’s decision to determine if an app’s deficiencies should deter its use.
While all of the criteria are relevant and important, it is incumbent on us as medical experts to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice. A low score on these criteria would belie any perceived usefulness or value the app may have.
When applying the rubric to evaluate the quality of an app, we should be mindful of the primary user and which characteristics are more important than others to effect positive changes in health. For example, in addressing obesity, it is the patient who will be interacting with the app. Therefore, it’s important that the app should score, on a 1- to 4-point scale (1 point being major deficiencies, 4 points being no deficiencies), a 4 out of 4 on features like usefulness, functionality, and design. Coveted design features that enhance the user’s experience will appeal to patients and keep them engaged and motivated. However, when addressing a woman’s health with respect to cancer risk, the principal features on which the app should score 4 out of 4 would be authority, objectivity, timeliness, and accuracy.
In the upcoming articles in this series, a member of the Presidential Task Force will reference the ASHP rubric to guide clinicians in choosing apps to address one of the critical health areas with their patients. The author of the piece will highlight key features of an app to consider what would add the most value in incorporating its use in clinical practice.
It would be impossible to evaluate all health care apps even if we focused only on the medical apps relevant to obstetrics and gynecology. There is much value in having a framework for efficiently measuring an app’s benefit in clinical practice. The objective of this article series is to help clinicians Revisit the Visit by providing an effective tool to evaluate a medical app. ●
Personalizing care is at the heart of the American College of Obstetricians and Gynecologists (ACOG) 2020–2021 President Dr. Eva Chalas’ initiative to “Revisit the Visit.” As obstetrician-gynecologists, we care for patients across the entirety of their life. This role gives us the opportunity to form long-term partnerships with women to address important preventive health care measures.
Dr. Chalas established a Presidential Task Force that identified 5 areas of preventive health that significantly influence the long-term morbidity of women: obesity, cardiovascular disease, preconception counseling, diabetes, and cancer risk. The annual visit can serve as a particularly impactful point of care to achieve specific preventive care objectives and offer mitigation strategies based on patient-specific risk factors. We are uniquely positioned to identify and initiate the conversation and subsequently manage, treat, and address these critical health areas.
Harnessing modern technology
To adopt these health topics into practice, we need improved, more effective tools both to increase productivity during the office visit and to provide more personalized care. Notably, the widespread adoption of and proliferation of mobile devices—and the medical apps accessible on them—is creating new and innovative ways to improve health and health care delivery. More than 90% of physicians use a smartphone at work, and 62% of smartphone users have used their device to gather health data.1
In addition, according to a US Food and Drug Administration (FDA) report, in 2017, 325,000 health care applications were available on smartphones; this equates to an expected 3.7 billion mobile health application downloads that year by 1.7 billion smartphone users worldwide.2 As of October 2020, 48,000-plus health apps were available on the iOS mobile operating system alone.3
For patients and clinicians, picking the most suitable apps can be challenging in the face of evolving clinical evidence, emerging privacy risks, functionality concerns, and the fact that apps constantly update and change. Many have relied on star rating systems and user reviews in app stores to guide their selection process despite mounting evidence that suggests that such evaluation methods are misleading, not always addressing such important parameters as usability, validity, security, and privacy.4,5
Approaches for evaluating medical apps
Many app evaluation frameworks have emerged, but none is universally accepted within the health care field.
The American Psychiatric Association’s (APA) App Evaluation Model represents a comprehensive resource to consider when evaluating medical apps. It stratifies numerous variables into 5 levels that form a pyramid. In this model, background information forms the base of this pyramid and includes factors such as business model, credibility, cost, and advertising of the app. The top of the pyramid is comprised of data integration that considers data ownership and therapeutic alliance.6 Although this model is beneficial in that it provides a framework, it is not practical for point-of-care purposes as it offers no objective way to rate or score an app for quick and easy comparison.
The privately owned and operated Health On the Net (HON) Foundation is well known for its HONcode, an ethical standard for quality medical information on the internet. It uses 8 principles to certify a health website. However, the HON website itself states that it cannot guarantee the accuracy or completeness of medical information presented by a site.7 Although HON certification by a website is a sign of good intention, it is not beneficial to the practicing clinician who is looking to use an app to directly assist in clinical care.
The Agency for Healthcare Research and Quality (AHRQ) is another well-respected body that has delineated essential details to consider when using a health website. The AHRQ identifies features (similar to those of the APA pyramid and HONcode) for users to consider, such as credibility, content, design, and disclosures.8 However, this model too lacks a concise user-friendly evaluating system.
Although the FDA plans to apply some regulatory authority to the evaluation of a certain subset of high-risk mobile medical apps, it is not planning to evaluate or regulate many of the medical apps that clinicians use in daily practice. This leaves us, and our patients, to be guided by the principle of caveat emptor, or “let the buyer beware.”
Thus, Dr. Chalas’ Presidential Task Force carefully considered various resources to provide a useful tool that would help obstetrician-gynecologists objectively vet a medical app in practice.
Continue to: The Task Force’s recommended rubric...
The Task Force’s recommended rubric
The rubric shown for evaluating mobile drug information apps was developed by the American Society of Health-System Pharmacists (ASHP). The ASHP rubric takes into account the criteria recognized by the APA pyramid, the HON Foundation, and the AHRQ and incorporates them into a user-friendly tool and scoring system that can be applied as an evaluation checklist.9 This tool is meant to aid clinicians in evaluating medical apps, but it ultimately is the user’s decision to determine if an app’s deficiencies should deter its use.
While all of the criteria are relevant and important, it is incumbent on us as medical experts to pay careful attention to the accuracy, authority, objectivity, timeliness, and security of any app we consider incorporating into clinical practice. A low score on these criteria would belie any perceived usefulness or value the app may have.
When applying the rubric to evaluate the quality of an app, we should be mindful of the primary user and which characteristics are more important than others to effect positive changes in health. For example, in addressing obesity, it is the patient who will be interacting with the app. Therefore, it’s important that the app should score, on a 1- to 4-point scale (1 point being major deficiencies, 4 points being no deficiencies), a 4 out of 4 on features like usefulness, functionality, and design. Coveted design features that enhance the user’s experience will appeal to patients and keep them engaged and motivated. However, when addressing a woman’s health with respect to cancer risk, the principal features on which the app should score 4 out of 4 would be authority, objectivity, timeliness, and accuracy.
In the upcoming articles in this series, a member of the Presidential Task Force will reference the ASHP rubric to guide clinicians in choosing apps to address one of the critical health areas with their patients. The author of the piece will highlight key features of an app to consider what would add the most value in incorporating its use in clinical practice.
It would be impossible to evaluate all health care apps even if we focused only on the medical apps relevant to obstetrics and gynecology. There is much value in having a framework for efficiently measuring an app’s benefit in clinical practice. The objective of this article series is to help clinicians Revisit the Visit by providing an effective tool to evaluate a medical app. ●
- Mobius MD website. 11 Surprising mobile health statistics. http://www.mobius.md/blog/2019/03/11-mobile-health -statistics/. Accessed January 19, 2021.
- US Food and Drug Administration website. Device software functions including medical applications. November 5, 2019. https://www.fda.gov/medical-devices/digital-health-center -excellence/device-software-functions-including-mobile -medical-applications. Accessed March 10, 2021.
- Statista website. Number of mHealth apps available in the Apple App Store from 1st quarter 2015 to 4th quarter 2020. https://www.statista.com/statistics/779910/health-apps -available-ios-worldwide/. Accessed January 19, 2021.
- Campbell L. Using star ratings to choose a medical app? There’s a better way. Healthline website. Updated August 3, 2018. http://healthline.com/health-news/using-ratings-to -choose-medical-app-theres-a-better-way. Accessed April 22, 2021.
- Levine DM, Co Z, Newmark LP, et al. Design and testing of a mobile health application rating tool. NPJ Digit Med. 2020;3:74.
- Torous JB, Chan SR, Gipson SY, et al. A hierarchical framework for evaluation and informed decision making regarding smartphone apps for clinical care. Psychiatr Serv. 2018;69:498-500.
- Health On the Net website. The commitment to reliable health and medical information on the internet. https:// www.hon.ch/HONcode/Patients/Visitor/visitor.html. Accessed January 19, 2021.
- Agency for Healthcare Research and Quality. Assessing the quality of internet health information. June 1999. http:// www.ahrq.gov/research/data/infoqual.html. Accessed April 22, 2021.
- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp.org/-/media/store-files /mobile-medical-apps.ashx. Accessed January 22, 2021.
- Mobius MD website. 11 Surprising mobile health statistics. http://www.mobius.md/blog/2019/03/11-mobile-health -statistics/. Accessed January 19, 2021.
- US Food and Drug Administration website. Device software functions including medical applications. November 5, 2019. https://www.fda.gov/medical-devices/digital-health-center -excellence/device-software-functions-including-mobile -medical-applications. Accessed March 10, 2021.
- Statista website. Number of mHealth apps available in the Apple App Store from 1st quarter 2015 to 4th quarter 2020. https://www.statista.com/statistics/779910/health-apps -available-ios-worldwide/. Accessed January 19, 2021.
- Campbell L. Using star ratings to choose a medical app? There’s a better way. Healthline website. Updated August 3, 2018. http://healthline.com/health-news/using-ratings-to -choose-medical-app-theres-a-better-way. Accessed April 22, 2021.
- Levine DM, Co Z, Newmark LP, et al. Design and testing of a mobile health application rating tool. NPJ Digit Med. 2020;3:74.
- Torous JB, Chan SR, Gipson SY, et al. A hierarchical framework for evaluation and informed decision making regarding smartphone apps for clinical care. Psychiatr Serv. 2018;69:498-500.
- Health On the Net website. The commitment to reliable health and medical information on the internet. https:// www.hon.ch/HONcode/Patients/Visitor/visitor.html. Accessed January 19, 2021.
- Agency for Healthcare Research and Quality. Assessing the quality of internet health information. June 1999. http:// www.ahrq.gov/research/data/infoqual.html. Accessed April 22, 2021.
- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp.org/-/media/store-files /mobile-medical-apps.ashx. Accessed January 22, 2021.
A reliable rubric for evaluating medical apps
To help ObGyns evaluate mobile apps for use in clinical practice, the American College of Obstetricians and Gynecologists Presidential Task Force of Dr. Eva Chalas recommends a quantitative rubric that was developed by the American Society of Health-System Pharmacists (ASHP) for evaluating drug information apps (TABLE).1 Criteria are graded on a point scale of 1 to 4, with 1 point indicating major deficiencies and 4 points indicating no deficiencies.
The ASHP used the following criteria in evaluating mobile apps:
- Usefulness: the app’s overall usefulness in a particular practice setting
- Accuracy: overall accuracy of the app should be thoroughly examined
- Authority: it is critical to assess authority or authorship to determine that the developers are reputable, qualified, and authoritative enough to create the medical content in question
- Objectivity: to determine if content is fair, balanced, and unbiased
- Timeliness: given that medical information is continually changing, an app must be evaluated based on the timeliness of its content
- Functionality: how the app downloads, deploys, and operates across devices and software platforms (that is, iOS, Android)
- Design: well-designed apps are generally more user friendly and, therefore, useful. They should require minimal or no training and have easily discernible buttons, a clean and uncluttered format, consistent graphics layout, terminology appropriate for the intended audience, streamlined navigation without extraneous steps/gestures, appropriate-sized text, and sufficient white space to improve readability.
- Security: Many apps collect a wide array of personal and device data. Collected data has the potential for being sold to third parties for marketing and advertising purposes. Apps should disclose their privacy policy and provide an explanation as to why personal data are being collected. If personal identifiable information (PII) is collected, then the app should be encrypted. If protected health information (PHI) is collected, the app must follow compliance with HIPAA/HITECH (Health Insurance Portability and Accountability Act/Health Information Technology for Economic and Clinical Health Act). Additionally, apps should not compromise the security or functionality of the mobile device being used.
- Value: appropriateness of an app's cost. ●
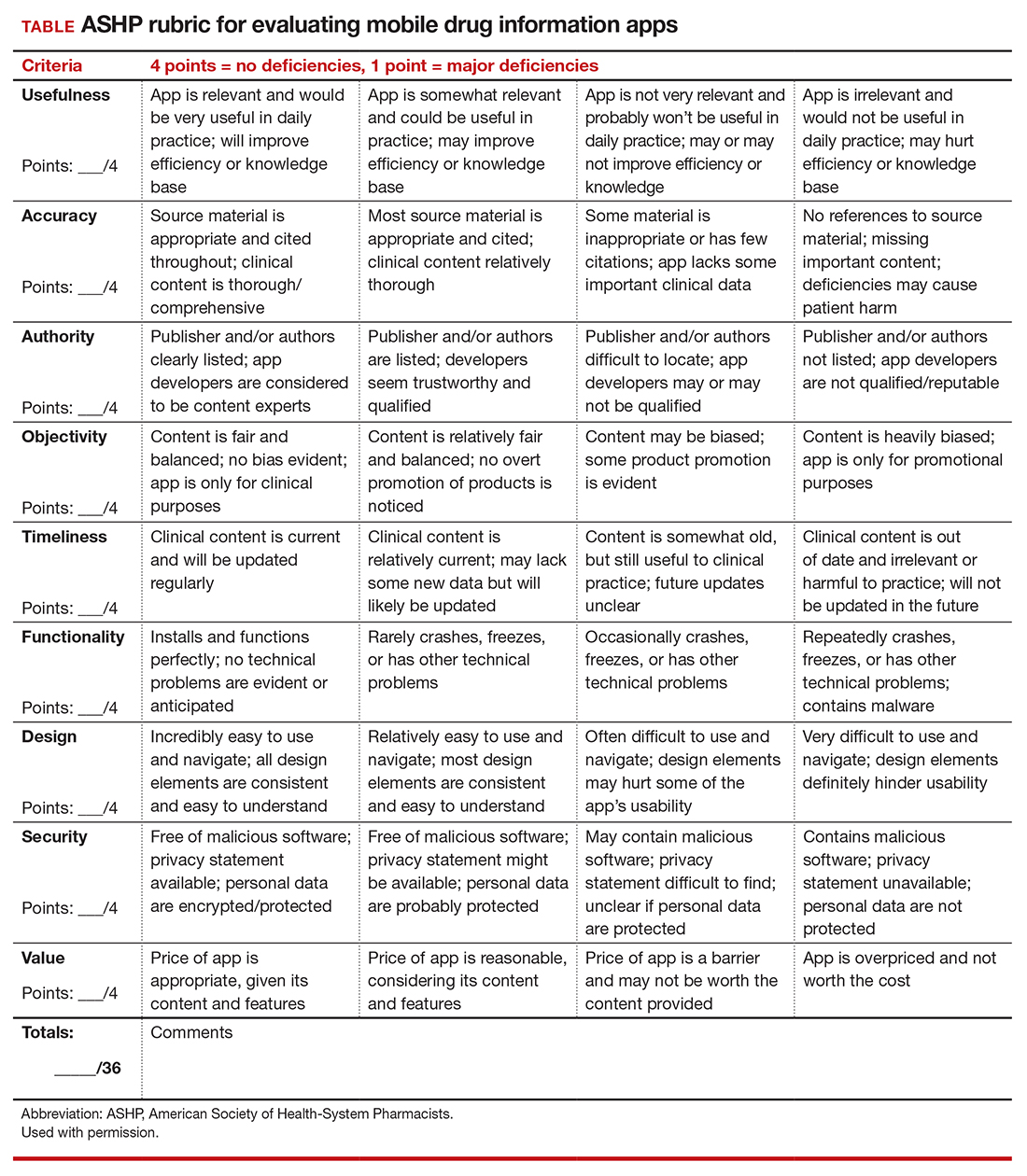
- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp .org/-/media/store-files/mobile-medical-apps. ashx. Accessed January 22, 2021.
To help ObGyns evaluate mobile apps for use in clinical practice, the American College of Obstetricians and Gynecologists Presidential Task Force of Dr. Eva Chalas recommends a quantitative rubric that was developed by the American Society of Health-System Pharmacists (ASHP) for evaluating drug information apps (TABLE).1 Criteria are graded on a point scale of 1 to 4, with 1 point indicating major deficiencies and 4 points indicating no deficiencies.
The ASHP used the following criteria in evaluating mobile apps:
- Usefulness: the app’s overall usefulness in a particular practice setting
- Accuracy: overall accuracy of the app should be thoroughly examined
- Authority: it is critical to assess authority or authorship to determine that the developers are reputable, qualified, and authoritative enough to create the medical content in question
- Objectivity: to determine if content is fair, balanced, and unbiased
- Timeliness: given that medical information is continually changing, an app must be evaluated based on the timeliness of its content
- Functionality: how the app downloads, deploys, and operates across devices and software platforms (that is, iOS, Android)
- Design: well-designed apps are generally more user friendly and, therefore, useful. They should require minimal or no training and have easily discernible buttons, a clean and uncluttered format, consistent graphics layout, terminology appropriate for the intended audience, streamlined navigation without extraneous steps/gestures, appropriate-sized text, and sufficient white space to improve readability.
- Security: Many apps collect a wide array of personal and device data. Collected data has the potential for being sold to third parties for marketing and advertising purposes. Apps should disclose their privacy policy and provide an explanation as to why personal data are being collected. If personal identifiable information (PII) is collected, then the app should be encrypted. If protected health information (PHI) is collected, the app must follow compliance with HIPAA/HITECH (Health Insurance Portability and Accountability Act/Health Information Technology for Economic and Clinical Health Act). Additionally, apps should not compromise the security or functionality of the mobile device being used.
- Value: appropriateness of an app's cost. ●

To help ObGyns evaluate mobile apps for use in clinical practice, the American College of Obstetricians and Gynecologists Presidential Task Force of Dr. Eva Chalas recommends a quantitative rubric that was developed by the American Society of Health-System Pharmacists (ASHP) for evaluating drug information apps (TABLE).1 Criteria are graded on a point scale of 1 to 4, with 1 point indicating major deficiencies and 4 points indicating no deficiencies.
The ASHP used the following criteria in evaluating mobile apps:
- Usefulness: the app’s overall usefulness in a particular practice setting
- Accuracy: overall accuracy of the app should be thoroughly examined
- Authority: it is critical to assess authority or authorship to determine that the developers are reputable, qualified, and authoritative enough to create the medical content in question
- Objectivity: to determine if content is fair, balanced, and unbiased
- Timeliness: given that medical information is continually changing, an app must be evaluated based on the timeliness of its content
- Functionality: how the app downloads, deploys, and operates across devices and software platforms (that is, iOS, Android)
- Design: well-designed apps are generally more user friendly and, therefore, useful. They should require minimal or no training and have easily discernible buttons, a clean and uncluttered format, consistent graphics layout, terminology appropriate for the intended audience, streamlined navigation without extraneous steps/gestures, appropriate-sized text, and sufficient white space to improve readability.
- Security: Many apps collect a wide array of personal and device data. Collected data has the potential for being sold to third parties for marketing and advertising purposes. Apps should disclose their privacy policy and provide an explanation as to why personal data are being collected. If personal identifiable information (PII) is collected, then the app should be encrypted. If protected health information (PHI) is collected, the app must follow compliance with HIPAA/HITECH (Health Insurance Portability and Accountability Act/Health Information Technology for Economic and Clinical Health Act). Additionally, apps should not compromise the security or functionality of the mobile device being used.
- Value: appropriateness of an app's cost. ●

- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp .org/-/media/store-files/mobile-medical-apps. ashx. Accessed January 22, 2021.
- Hanrahan C, Aungst TD, Cole S. Evaluating mobile medical applications. American Society of Health-System Pharmacists eReports. https://www.ashp .org/-/media/store-files/mobile-medical-apps. ashx. Accessed January 22, 2021.
PHM groups issue Choosing Wisely® recommendations
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
SHM members involved from the start
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
Doctors prescribe fewer statins in the afternoon
Primary care physicians are more likely to write a prescription for statins for their patients at risk for cardiovascular adverse events in the morning than in the afternoon, new research suggests.
In an observational cohort study, researchers from the nudge unit, University of Pennsylvania, Philadelphia, found that patients who had the first appointments of the day were most likely to have statins prescribed for them, and that this likelihood decreased as the day went on.
The study was published online May 11, 2021, in JAMA Network Open.
“Physicians are faced with decision fatigue, where they are seeing 20 patients in a day and may not have the mental bandwidth or cognitive bandwidth to fully think through every decision for every patient and to make all the appropriate decisions all of the time,” lead author Allison J. Hare, medical student and clinical informatics fellow in the nudge unit, said in an interview.
The Penn Medicine nudge unit attempts to better align clinician decision-making with current standards in best practices for the provision of various therapies, Ms. Hare explained.
“As we see more and more best-practice guidelines come out, we also see that there is a gap in the intention to treat and actual provision of these therapies,” she said. “There are also increasing expectations for clinicians to provide all of these different evidence-backed therapies. It can be hard to keep up with all these guidelines, especially when you are expected to take care of more and more patients, more and more efficiently.”
Guideline-directed statin therapy has been demonstrated to reduce the risk for major adverse cardiovascular events, yet 50% of statin-eligible patients have not been prescribed one.
“In our prior work at the nudge unit, we observed that rates of preventive care, including flu vaccination and cancer screening, declined as the clinic day progressed. We wanted to see if this occurred with statin scripts,” Ms. Hare said.
The researchers obtained data from 28 Penn Medicine primary care practices that included 10,757 patients at risk for heart disease for the period from March 2019 to February 2020.
Their mean age was 66.0 years (standard deviation, 10.5 years), 5,072 (47.2%) were female, and 7,071 (65.7%) were White. Patient characteristics were similar between morning and afternoon appointments.
All patients had clinical atherosclerotic cardiovascular disease, familial hypercholesterolemia, or LDL cholesterol of at least 190 mg/dL, conditions which qualified them for statins based on the U.S. Preventive Services Task Force guidelines.
The appointment times for each patient were broken down into hour blocks, ranging from the 8:00 a.m. hour to the 4:00 p.m. hour, which bookend open times in most practices.
Overall, statins were prescribed in 36% (n = 3,864) of visits.
The data showed a clear decline in statin prescribing as the day went on. For example, compared with patients who came in at 8:00 a.m. (the reference group), patients who came in at 9:00 a.m. were 12% less likely to get a prescription.
Patients coming in for noon appointments were 37% less likely to get a statin prescription, which made them the least likely to get a script. After the noon visits, there was a slight increase, but the likelihood of a statin prescription remained 27% less likely or worse for the rest of the day.
“In the context of the myriad tasks that clinicians are faced with doing for a single patient, and then also within the context of seeing 20 patients in 15-minute increments, it is easy to see how certain things fall through the cracks,” Ms. Hare said. “It’s impossible for any clinician to remember every single little thing for their patient every single time, so if we can augment the clinician’s ability to make those appropriate decisions with electronic tools, we can narrow that gap a little bit.”
Why the variability?
“The nudge unit uses prompts to ask the physician about prescribing statins. The question is, what is causing the variability in statin prescriptions?” Nieca Goldberg, MD, medical director of the New York University women’s heart program, said in an interview.
“Is it fatigue, lack of familiarity of guidelines, or is this due to the volume of patients and lack of time to discuss the therapy and make a shared decision with their patient? The answer to these questions was not part of the study,” said Dr. Goldberg, who is also an American Heart Association volunteer expert. “It would be interesting to know the thoughts of the physicians who were studied after they were informed of the results. Also, having a nudge to write the prescription will increase the prescriptions of statins, but will patients take the medication?”
The study was funded in part by a grant from the National Institute on Aging. Ms. Hare and Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians are more likely to write a prescription for statins for their patients at risk for cardiovascular adverse events in the morning than in the afternoon, new research suggests.
In an observational cohort study, researchers from the nudge unit, University of Pennsylvania, Philadelphia, found that patients who had the first appointments of the day were most likely to have statins prescribed for them, and that this likelihood decreased as the day went on.
The study was published online May 11, 2021, in JAMA Network Open.
“Physicians are faced with decision fatigue, where they are seeing 20 patients in a day and may not have the mental bandwidth or cognitive bandwidth to fully think through every decision for every patient and to make all the appropriate decisions all of the time,” lead author Allison J. Hare, medical student and clinical informatics fellow in the nudge unit, said in an interview.
The Penn Medicine nudge unit attempts to better align clinician decision-making with current standards in best practices for the provision of various therapies, Ms. Hare explained.
“As we see more and more best-practice guidelines come out, we also see that there is a gap in the intention to treat and actual provision of these therapies,” she said. “There are also increasing expectations for clinicians to provide all of these different evidence-backed therapies. It can be hard to keep up with all these guidelines, especially when you are expected to take care of more and more patients, more and more efficiently.”
Guideline-directed statin therapy has been demonstrated to reduce the risk for major adverse cardiovascular events, yet 50% of statin-eligible patients have not been prescribed one.
“In our prior work at the nudge unit, we observed that rates of preventive care, including flu vaccination and cancer screening, declined as the clinic day progressed. We wanted to see if this occurred with statin scripts,” Ms. Hare said.
The researchers obtained data from 28 Penn Medicine primary care practices that included 10,757 patients at risk for heart disease for the period from March 2019 to February 2020.
Their mean age was 66.0 years (standard deviation, 10.5 years), 5,072 (47.2%) were female, and 7,071 (65.7%) were White. Patient characteristics were similar between morning and afternoon appointments.
All patients had clinical atherosclerotic cardiovascular disease, familial hypercholesterolemia, or LDL cholesterol of at least 190 mg/dL, conditions which qualified them for statins based on the U.S. Preventive Services Task Force guidelines.
The appointment times for each patient were broken down into hour blocks, ranging from the 8:00 a.m. hour to the 4:00 p.m. hour, which bookend open times in most practices.
Overall, statins were prescribed in 36% (n = 3,864) of visits.
The data showed a clear decline in statin prescribing as the day went on. For example, compared with patients who came in at 8:00 a.m. (the reference group), patients who came in at 9:00 a.m. were 12% less likely to get a prescription.
Patients coming in for noon appointments were 37% less likely to get a statin prescription, which made them the least likely to get a script. After the noon visits, there was a slight increase, but the likelihood of a statin prescription remained 27% less likely or worse for the rest of the day.
“In the context of the myriad tasks that clinicians are faced with doing for a single patient, and then also within the context of seeing 20 patients in 15-minute increments, it is easy to see how certain things fall through the cracks,” Ms. Hare said. “It’s impossible for any clinician to remember every single little thing for their patient every single time, so if we can augment the clinician’s ability to make those appropriate decisions with electronic tools, we can narrow that gap a little bit.”
Why the variability?
“The nudge unit uses prompts to ask the physician about prescribing statins. The question is, what is causing the variability in statin prescriptions?” Nieca Goldberg, MD, medical director of the New York University women’s heart program, said in an interview.
“Is it fatigue, lack of familiarity of guidelines, or is this due to the volume of patients and lack of time to discuss the therapy and make a shared decision with their patient? The answer to these questions was not part of the study,” said Dr. Goldberg, who is also an American Heart Association volunteer expert. “It would be interesting to know the thoughts of the physicians who were studied after they were informed of the results. Also, having a nudge to write the prescription will increase the prescriptions of statins, but will patients take the medication?”
The study was funded in part by a grant from the National Institute on Aging. Ms. Hare and Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Primary care physicians are more likely to write a prescription for statins for their patients at risk for cardiovascular adverse events in the morning than in the afternoon, new research suggests.
In an observational cohort study, researchers from the nudge unit, University of Pennsylvania, Philadelphia, found that patients who had the first appointments of the day were most likely to have statins prescribed for them, and that this likelihood decreased as the day went on.
The study was published online May 11, 2021, in JAMA Network Open.
“Physicians are faced with decision fatigue, where they are seeing 20 patients in a day and may not have the mental bandwidth or cognitive bandwidth to fully think through every decision for every patient and to make all the appropriate decisions all of the time,” lead author Allison J. Hare, medical student and clinical informatics fellow in the nudge unit, said in an interview.
The Penn Medicine nudge unit attempts to better align clinician decision-making with current standards in best practices for the provision of various therapies, Ms. Hare explained.
“As we see more and more best-practice guidelines come out, we also see that there is a gap in the intention to treat and actual provision of these therapies,” she said. “There are also increasing expectations for clinicians to provide all of these different evidence-backed therapies. It can be hard to keep up with all these guidelines, especially when you are expected to take care of more and more patients, more and more efficiently.”
Guideline-directed statin therapy has been demonstrated to reduce the risk for major adverse cardiovascular events, yet 50% of statin-eligible patients have not been prescribed one.
“In our prior work at the nudge unit, we observed that rates of preventive care, including flu vaccination and cancer screening, declined as the clinic day progressed. We wanted to see if this occurred with statin scripts,” Ms. Hare said.
The researchers obtained data from 28 Penn Medicine primary care practices that included 10,757 patients at risk for heart disease for the period from March 2019 to February 2020.
Their mean age was 66.0 years (standard deviation, 10.5 years), 5,072 (47.2%) were female, and 7,071 (65.7%) were White. Patient characteristics were similar between morning and afternoon appointments.
All patients had clinical atherosclerotic cardiovascular disease, familial hypercholesterolemia, or LDL cholesterol of at least 190 mg/dL, conditions which qualified them for statins based on the U.S. Preventive Services Task Force guidelines.
The appointment times for each patient were broken down into hour blocks, ranging from the 8:00 a.m. hour to the 4:00 p.m. hour, which bookend open times in most practices.
Overall, statins were prescribed in 36% (n = 3,864) of visits.
The data showed a clear decline in statin prescribing as the day went on. For example, compared with patients who came in at 8:00 a.m. (the reference group), patients who came in at 9:00 a.m. were 12% less likely to get a prescription.
Patients coming in for noon appointments were 37% less likely to get a statin prescription, which made them the least likely to get a script. After the noon visits, there was a slight increase, but the likelihood of a statin prescription remained 27% less likely or worse for the rest of the day.
“In the context of the myriad tasks that clinicians are faced with doing for a single patient, and then also within the context of seeing 20 patients in 15-minute increments, it is easy to see how certain things fall through the cracks,” Ms. Hare said. “It’s impossible for any clinician to remember every single little thing for their patient every single time, so if we can augment the clinician’s ability to make those appropriate decisions with electronic tools, we can narrow that gap a little bit.”
Why the variability?
“The nudge unit uses prompts to ask the physician about prescribing statins. The question is, what is causing the variability in statin prescriptions?” Nieca Goldberg, MD, medical director of the New York University women’s heart program, said in an interview.
“Is it fatigue, lack of familiarity of guidelines, or is this due to the volume of patients and lack of time to discuss the therapy and make a shared decision with their patient? The answer to these questions was not part of the study,” said Dr. Goldberg, who is also an American Heart Association volunteer expert. “It would be interesting to know the thoughts of the physicians who were studied after they were informed of the results. Also, having a nudge to write the prescription will increase the prescriptions of statins, but will patients take the medication?”
The study was funded in part by a grant from the National Institute on Aging. Ms. Hare and Dr. Goldberg reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.

















