User login
Omics analysis links blood type to COVID-19
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Checkpoint inhibitors earn spot in new ESMO SCLC guidelines
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
FROM ANNALS OF ONCOLOGY
Opioid addiction meds may curb growing problem of kratom dependence
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
Medications typically used to treat opioid use disorder (OUD) may* also be effective for the growing public health problem of kratom addiction, new research shows.
Results of a comprehensive literature review and an expert survey suggest buprenorphine, naltrexone, and methadone may be effective for patients seeking help for kratom addiction, and if further research confirms these findings, the indication for OUD medications could potentially be expanded to include moderate-to-severe kratom addiction, study investigator Saeed Ahmed, MD, medical director of West Ridge Center at Rutland Regional Medical Center, Rutland, Vermont, said in an interview.
Dr. Ahmed, who practices general psychiatry and addiction psychiatry, presented the findings at the virtual American Psychiatric Association 2021 Annual Meeting.
Emerging public health problem
Kratom can be ingested in pill or capsule form or as an extract. Its leaves can be chewed or dried and powdered to make a tea. It can also be incorporated into topical creams, balms, or tinctures.
Products containing the substance are “readily available and legal for sale in many states and cities in the U.S.,” said Dr. Ahmed, adding that it can be purchased online or at local smoke shops and is increasingly used by individuals to self-treat a variety of conditions including pain, anxiety, and mood conditions and as an opioid substitute.
As reported by this news organization, a 2018 analysis conducted by the U.S. Food and Drug Administration showed kratom is, in fact, an opioid, a finding that garnered significant push-back from the American Kratom Association.
Kratom addiction is an “emerging public health problem,” said Dr. Ahmed, adding that in recent years the number of calls to poison control centers across the country has increased 52-fold – from one per month to two per day. He believes misinformation through social media has helped fuel its use.
Kratom use, the investigators note, can lead to muscle pain, weight loss, insomnia, hallucinations and, in some cases (particularly when combined with synthetic opioids or benzodiazepines), it can lead to respiratory depression, seizures, coma, and death.
In addition,
To investigate, the researchers conducted a systematic literature search for cases pertaining to maintenance treatment for kratom dependence. They also tapped into case reports and scientific posters from reliable online sources and conference proceedings. In addition, they conducted a survey of members from the American Society of Addiction Medicine (ASAM).
The researchers found 14 reports of long-term management of kratom addiction, half of which did not involve an OUD. It’s important to exclude OUDs to avoid possible confounding.
In most cases, buprenorphine was used, but in a few cases naltrexone or methadone were prescribed. All cases had a favorable outcome. Dr. Ahmed noted that buprenorphine maintenance doses appear to be lower than those required to effectively treat OUD.
With a response rate of 11.5% (82 respondents) the ASAM survey results showed 82.6% of respondents (n = 57) had experience managing KUD, including 27.5% (n = 19) who had kratom addiction only. Of these, 89.5% (n = 17-19), used buprenorphine to manage KUD and of these, 6 combined it with talk therapy.
Dr. Ahmed cautioned that the included cases varied significantly in terms of relevant data, including kratom dose and route of administration, toxicology screening used to monitor abstinence, and duration of maintenance follow-up.
Despite these limitations, the review and survey underscore the importance of including moderate to severe kratom dependence as an indication for current OUD medications, the researchers note.
Including kratom addiction as an indication for these medications is important, especially for patients who are heavily addicted, to meet DSM-5 diagnostic criteria for moderate or severe SUD, they add.
In addition, the researchers recommend that clinicians consider referring patients with moderate to severe kratom dependence for counseling or enrollment in 12-step addiction treatment programs.
A separate diagnosis?
Dr. Ahmed said he would like to see kratom dependence included in the DSM-5 as a separate entity because it is a botanical with properties similar to, but different from, traditional opioids.
“This will not only help to better inform clinicians about a diagnostic criteria encompassing problematic use and facilitate screening, but it will also pave the way for treatments to be explored for this diagnosable condition,” he said. Dr. Ahmed pointed to a review published in the Wisconsin Medical Journal earlier this year that explored potential treatments for kratom dependence.
Commenting on the study for an interview, Petros Levounis, MD, professor and chair, department of psychiatry, and associate dean for professional development, Rutgers New Jersey Medical School, Newark, said the authors “have done a great job reviewing the literature and asking experts” about kratom addiction treatment.
“The punchline of their study is that kratom behaves very much like an opioid and is treated like an opioid.”
Dr. Levounis noted that kratom dependence is so new that experts don’t know much about it. However, he added, emerging evidence suggests that kratom “should be considered an opioid more than anything else,” but specified that he does not believe it warrants its own diagnosis.
He noted that individual opioids don’t have their own diagnostic category and that opioid use disorder is an umbrella term that covers all of these drugs.
Dr. Ahmed and Dr. Levounis have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
*Updated 5/18/2021
FDA preparing an environmental impact statement for 2 sunscreen ingredients
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
The Food and Drug Administration is launching a process to prepare an environmental impact statement (EIS) regarding the use oxybenzone and octinoxate in over-the-counter sunscreen products.
According to the “Intent to Prepare an Environmental Impact Statement for Certain Sunscreen Drug Products for Over-The-Counter Use,” which was published in the Federal Register on May 13, 2021, the FDA will prepare an EIS “when data or information in an environmental assessment or otherwise available to the Agency leads to a finding that the proposed agency action may significantly affect the quality of the human environment.” The first step in this effort involves a “public scoping process” to evaluate any potential environmental impacts associated with the use of oxybenzone and octinoxate in sunscreens so that an EIS, if required, “can be completed prior to issuance of a final sunscreen order addressing sunscreens containing these ingredients.”
The American Academy of Dermatology Association weighed in on the FDA’s announcement, noting that it “appreciates the efforts of the agency to thoroughly examine all relevant science before issuing a final sunscreen order on these ingredients,” according to a statement released by the AADA on May 13, 2021.
The statement added: “Skin cancer is the most common cancer in the U.S., and unprotected exposure to the sun’s harmful ultraviolet rays is a major risk factor. The AADA continues to focus on encouraging members of the public to protect themselves by seeking shade, wearing protective clothing – including a lightweight and long-sleeved shirt, pants, a wide-brimmed hat and sunglasses – and applying a broad-spectrum sunscreen with an SPF of 30 or higher to all exposed skin.”
According to the FDA document, a series of developments regarding oxybenzone and octinoxate prompted the agency to take this step, including comments the agency received in response to the 2019 proposed rule titled “Sunscreen Drug Products for Over-The-Counter Human Use,” which raised concern about the potential effects of the two ingredients on coral and/or coral reefs, as well as research efforts by the National Oceanic and Atmospheric Administration Coral Reef Conservation Programs on the potential impacts of sunscreen products that include oxybenzone and octinoxate on coral reefs and other aquatic systems. Hawaii’s 2018 state law prohibiting the sale, offer of sale, and distribution of sunscreens that contain oxybenzone and/or octinoxate also influenced the agency’s decision to further evaluate the topic.
“The purpose of the public scoping process is to determine relevant issues that will influence the scope of the environmental analysis, including potential alternatives and the extent to which those issues and impacts will be analyzed,” the FDA document states. “At this initial stage of the scoping process, we have identified the following four alternatives: FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA [new drug application] is impermissible; FDA will conclude that the inclusion of oxybenzone and octinoxate in sunscreens marketed without an NDA is permissible; FDA will conclude that inclusion of oxybenzone in sunscreens marketed without an NDA is permissible but that the inclusion of octinoxate in sunscreens marketed without an NDA is impermissible; or FDA will conclude that inclusion of octinoxate in sunscreens marketed without an NDA is permissible but that the inclusion of oxybenzone in sunscreens marketed without an NDA is impermissible.”
Until June 14, the FDA is accepting comments from the public electronically via the Federal eRulemaking Portal at www.regulations.gov (search for Docket No. FDA-2021-N-0352) or by mail to: Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, Md., 20852. Refer to Docket No. FDA-2021-N-0352.
Telemedicine is popular among Mohs surgeons – for now
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
A majority of
A variety of factors combine to make it “very difficult for surgeons to make long-term plans for implementing telemedicine in their practices,” said Mario Maruthur, MD, who presented the findings at the annual meeting of the American College of Mohs Surgery. “Telemedicine likely has a role in Mohs practices, particularly with postop follow-up visits. However, postpandemic reimbursement and regulatory issues need to be formally laid out before Mohs surgeons are able to incorporate it into their permanent work flow.”
Dr. Maruthur, a Mohs surgery and dermatologic oncology fellow at Memorial Sloan Kettering Cancer Center, New York, and colleagues sent a survey to ACMS members in September and October 2020. “We saw first-hand in our surgical practice that telemedicine quickly became an important tool when the pandemic surged in the spring of 2020,” he said. Considering that surgical practices are highly dependent on in-person visits, the impetus for this study was to assess to what degree Mohs practices from across the spectrum, including academic and private practices, embraced telemedicine during the pandemic, and “what these surgical practices used telemedicine for, how it was received by their patients, which telemedicine platforms were most often utilized, and lastly, what are their plans if any for incorporating telemedicine into their surgical practices after the pandemic subsides.”
The researchers received responses from 115 surgeons representing all regions of the country (40% Northeast, 21% South, 21% Midwest, and 18% West). Half practiced in urban areas (37%) and large cities (13%), and 40% were in an academic setting versus 36% in a single-specialty private practice.
More than 70% of the respondents said their case load fell by at least 75% during the initial surge of the pandemic; 80% turned to telemedicine, compared with just 23% who relied on the technology prior to the pandemic. The most commonly used telemedicine technologies were FaceTime, Zoom, Doximity, and Epic.
Mohs surgeons reported most commonly using telemedicine for postsurgery management (77% of the total 115 responses). “Telemedicine is a great fit for this category of visits as they allow the surgeon to view the surgical site and answer any questions they patient may have,” Dr. Maruthur said. “If the surgeon does suspect a postop infection or other concern based on a patient’s signs or symptoms, they can easily schedule the patient for an in-person assessment. We suspect that postop follow-up visits may be the best candidate for long-term use of telemedicine in Mohs surgery practices.”
Surgeons also reported using telemedicine for “spot checks” (61%) and surgical consultations (59%).
However, Dr. Maruther noted that preoperative assessments and spot checks can be difficult to perform using telemedicine. “The quality of the video image is not always great, patients can have a difficult time pointing the camera at the right spot and at the right distance. Even appreciating the actual size of the lesion are all difficult over a video encounter. And there is a lot of information gleaned from in-person physical examination, such as whether the lesion is fixed to a deeper structure and whether there are any nearby scars or other suspicious lesions.”
Nearly three-quarters of the surgeons using the technology said most or all patients were receptive to telemedicine.
However, the surgeons reported multiple barriers to the use of telemedicine: Limitations when compared with physical exams (88%), fitting it into the work flow (58%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
In an interview, Sumaira Z. Aasi, MD, director of Mohs and dermatologic surgery, Stanford University, agreed that there are many obstacles to routine use of telemedicine by Mohs surgeons. “As surgeons, we rely on the physical and tactile exam to get a sense of the size and extent of the cancer and characteristics such as the laxity of the surrounding tissue whether the tumor is fixed,” she said. “It is very difficult to access this on a telemedicine visit.”
In addition, she said, “many of our patients are in the elderly population, and some may not be comfortable using this technology. Also, it’s not a work flow that we are comfortable or familiar with. And I think that the technology has to improve to allow for better resolution of images as we ‘examine’ patients through a telemedicine visit.”
She added that “another con is there is a reliance on having the patient point out lesions of concern. Many cancers are picked by a careful in-person examination by a qualified physician/dermatologist/Mohs surgeon when the lesion is quite small or subtle and not even noticed by the patient themselves. This approach invariably leads to earlier biopsies and earlier treatments that can prevent morbidity and save health care money.”
On the other hand, she said, telemedicine “may save patients some time and money in terms of the effort and cost of transportation to come in for simpler postoperative medical visits that are often short in their very nature, such as postop check-ups.”
Most of the surgeons surveyed (69%) said telemedicine probably or definitely deserves a place in the practice Mohs surgery, but only 50% said they’d like to or would definitely pursue giving telemedicine a role in their practices once the pandemic is over.
“At the start of the pandemic, many regulations in areas such as HIPAA were eased, and reimbursements were increased, which allowed telemedicine to be quickly adopted,” Dr. Maruther said. “The government and payers have yet to decide which regulations and reimbursements will be in place after the pandemic. That makes it very difficult for surgeons to make long-term plans for implementing telemedicine in their practices.”
Dr. Aasi predicted that telemedicine will become more appealing to patients and physicians as it its technology and usability improves. More familiarity with its use will also be helpful, she said, and surgeons will be more receptive as it’s incorporated into efficient daily work flow.
The study was funded in part by the National Institutes of Health.
FROM THE ACMS ANNUAL MEETING
Pediatricians see drop in income during the pandemic
The average income for pediatricians declined slightly from 2019 to 2020, according to the Medscape Pediatrician Compensation Report 2021.

The report, which was conducted between October 2020 and February 2021, found that the average pediatrician income was down $11,000 – from $232,000 in 2019 to $221,000 in 2020, with 48% of pediatricians reporting at least some decline in compensation.
The specialty also earned the least amount of money in 2020, compared with all of the other specialties, which isn’t surprising since pediatricians have been among the lowest-paid physician specialties since 2013. The highest-earning specialty was plastic surgery with an average income of $526,000 annually.
Most pediatricians who saw a drop in income cited pandemic-related issues such as job loss, fewer hours, and fewer patients.
Jesse Hackell, MD, vice president and chief operating officer of Ponoma Pediatrics in New York, said in an interview the reduced wages pediatricians saw in 2020 didn’t surprise him because many pediatric offices saw a huge drop in visits that were not urgent.
“[The report] shows that procedural specialties tended to do a lot better than the nonprocedural specialties,” Dr. Hackell said. “That’s because, during the shutdown, if you broke your leg, you still needed the orthopedist. And even though the hospitals weren’t doing elective surgeries, they were certainly doing the emergency stuff.”
Meanwhile, in pediatrician offices, where Dr. Hackell said office visits dropped 70%-80% at the beginning of the pandemic, “parents weren’t going to bring a healthy kid out for routine visits and they weren’t going to bring a kid out for minor illnesses and expose them to possibly communicable diseases in the office.”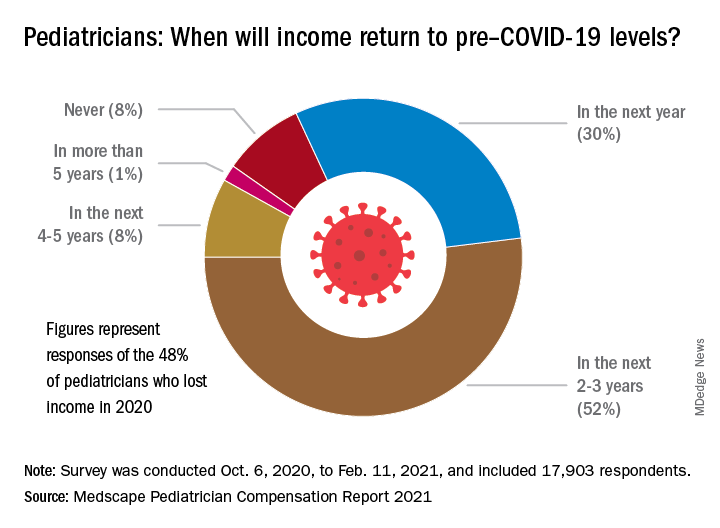
About 52% of pediatricians who lost income because of the pandemic believe their income levels will return to normal in 2-3 years. Meanwhile 30% of pediatricians expect their income to return to normal within a year, and 8% believe it will take 4 years for them to bounce back.
Physician work hours generally declined for some time during the pandemic, according to the report. However, most pediatricians are working about the same number of hours as they did before the pandemic, which is 47 hours per week.
Despite working the same number of hours per week that they did prepandemic, they are seeing fewer patients. They are currently seeing on average 64 patients per week, compared with the 78 patients they used to see weekly before the pandemic.
Dr. Hackell said that might be because pediatric offices are trying to make up the loss of revenue during the beginning of the pandemic, from the reduced number of well visits and immunizations, in the second half of the year with outreach.
“Since about June 2020, we’ve been making concerted efforts to remind parents that preventing other infectious diseases is critically important,” Dr. Hackell explained. “And so actually, for the second half of the year, many of us saw more well visits and immunization volume than in 2019 as we sought to make up the gap. It wasn’t that we were seeing more overall, but we’re trying to make up the gap that happened from March, April, May, [and] June.”
Most pediatricians find their work rewarding. One-third say the most rewarding part of their job is gratitude from and relationships with their patients. Meanwhile, 31% of pediatricians said knowing they are making the world a better place was a rewarding part of their job. Only 8% of them said making money was a rewarding part of their job.
Dr. Hackell said he did not go into pediatrics to make money, it was because he found it stimulating and has “no complaints.”
“I’ve been a pediatrician for 40 years and I wouldn’t do anything else,” Dr. Hackell said. “I don’t know that there’s anything that I would find as rewarding as the relationships that I’ve had over 40 years with my patients. You know, getting invited to weddings of kids who I saw when they were newborns is pretty impressive. It’s the gratification of having ongoing relationships with families.”
Furthermore, the report revealed that 77% of pediatricians said they would pick medicine again if they had a choice, and 82% said they would choose the same specialty.
The experts disclosed no relevant financial interests.
*This story was updated on 5/18/2021.
The average income for pediatricians declined slightly from 2019 to 2020, according to the Medscape Pediatrician Compensation Report 2021.

The report, which was conducted between October 2020 and February 2021, found that the average pediatrician income was down $11,000 – from $232,000 in 2019 to $221,000 in 2020, with 48% of pediatricians reporting at least some decline in compensation.
The specialty also earned the least amount of money in 2020, compared with all of the other specialties, which isn’t surprising since pediatricians have been among the lowest-paid physician specialties since 2013. The highest-earning specialty was plastic surgery with an average income of $526,000 annually.
Most pediatricians who saw a drop in income cited pandemic-related issues such as job loss, fewer hours, and fewer patients.
Jesse Hackell, MD, vice president and chief operating officer of Ponoma Pediatrics in New York, said in an interview the reduced wages pediatricians saw in 2020 didn’t surprise him because many pediatric offices saw a huge drop in visits that were not urgent.
“[The report] shows that procedural specialties tended to do a lot better than the nonprocedural specialties,” Dr. Hackell said. “That’s because, during the shutdown, if you broke your leg, you still needed the orthopedist. And even though the hospitals weren’t doing elective surgeries, they were certainly doing the emergency stuff.”
Meanwhile, in pediatrician offices, where Dr. Hackell said office visits dropped 70%-80% at the beginning of the pandemic, “parents weren’t going to bring a healthy kid out for routine visits and they weren’t going to bring a kid out for minor illnesses and expose them to possibly communicable diseases in the office.”
About 52% of pediatricians who lost income because of the pandemic believe their income levels will return to normal in 2-3 years. Meanwhile 30% of pediatricians expect their income to return to normal within a year, and 8% believe it will take 4 years for them to bounce back.
Physician work hours generally declined for some time during the pandemic, according to the report. However, most pediatricians are working about the same number of hours as they did before the pandemic, which is 47 hours per week.
Despite working the same number of hours per week that they did prepandemic, they are seeing fewer patients. They are currently seeing on average 64 patients per week, compared with the 78 patients they used to see weekly before the pandemic.
Dr. Hackell said that might be because pediatric offices are trying to make up the loss of revenue during the beginning of the pandemic, from the reduced number of well visits and immunizations, in the second half of the year with outreach.
“Since about June 2020, we’ve been making concerted efforts to remind parents that preventing other infectious diseases is critically important,” Dr. Hackell explained. “And so actually, for the second half of the year, many of us saw more well visits and immunization volume than in 2019 as we sought to make up the gap. It wasn’t that we were seeing more overall, but we’re trying to make up the gap that happened from March, April, May, [and] June.”
Most pediatricians find their work rewarding. One-third say the most rewarding part of their job is gratitude from and relationships with their patients. Meanwhile, 31% of pediatricians said knowing they are making the world a better place was a rewarding part of their job. Only 8% of them said making money was a rewarding part of their job.
Dr. Hackell said he did not go into pediatrics to make money, it was because he found it stimulating and has “no complaints.”
“I’ve been a pediatrician for 40 years and I wouldn’t do anything else,” Dr. Hackell said. “I don’t know that there’s anything that I would find as rewarding as the relationships that I’ve had over 40 years with my patients. You know, getting invited to weddings of kids who I saw when they were newborns is pretty impressive. It’s the gratification of having ongoing relationships with families.”
Furthermore, the report revealed that 77% of pediatricians said they would pick medicine again if they had a choice, and 82% said they would choose the same specialty.
The experts disclosed no relevant financial interests.
*This story was updated on 5/18/2021.
The average income for pediatricians declined slightly from 2019 to 2020, according to the Medscape Pediatrician Compensation Report 2021.

The report, which was conducted between October 2020 and February 2021, found that the average pediatrician income was down $11,000 – from $232,000 in 2019 to $221,000 in 2020, with 48% of pediatricians reporting at least some decline in compensation.
The specialty also earned the least amount of money in 2020, compared with all of the other specialties, which isn’t surprising since pediatricians have been among the lowest-paid physician specialties since 2013. The highest-earning specialty was plastic surgery with an average income of $526,000 annually.
Most pediatricians who saw a drop in income cited pandemic-related issues such as job loss, fewer hours, and fewer patients.
Jesse Hackell, MD, vice president and chief operating officer of Ponoma Pediatrics in New York, said in an interview the reduced wages pediatricians saw in 2020 didn’t surprise him because many pediatric offices saw a huge drop in visits that were not urgent.
“[The report] shows that procedural specialties tended to do a lot better than the nonprocedural specialties,” Dr. Hackell said. “That’s because, during the shutdown, if you broke your leg, you still needed the orthopedist. And even though the hospitals weren’t doing elective surgeries, they were certainly doing the emergency stuff.”
Meanwhile, in pediatrician offices, where Dr. Hackell said office visits dropped 70%-80% at the beginning of the pandemic, “parents weren’t going to bring a healthy kid out for routine visits and they weren’t going to bring a kid out for minor illnesses and expose them to possibly communicable diseases in the office.”
About 52% of pediatricians who lost income because of the pandemic believe their income levels will return to normal in 2-3 years. Meanwhile 30% of pediatricians expect their income to return to normal within a year, and 8% believe it will take 4 years for them to bounce back.
Physician work hours generally declined for some time during the pandemic, according to the report. However, most pediatricians are working about the same number of hours as they did before the pandemic, which is 47 hours per week.
Despite working the same number of hours per week that they did prepandemic, they are seeing fewer patients. They are currently seeing on average 64 patients per week, compared with the 78 patients they used to see weekly before the pandemic.
Dr. Hackell said that might be because pediatric offices are trying to make up the loss of revenue during the beginning of the pandemic, from the reduced number of well visits and immunizations, in the second half of the year with outreach.
“Since about June 2020, we’ve been making concerted efforts to remind parents that preventing other infectious diseases is critically important,” Dr. Hackell explained. “And so actually, for the second half of the year, many of us saw more well visits and immunization volume than in 2019 as we sought to make up the gap. It wasn’t that we were seeing more overall, but we’re trying to make up the gap that happened from March, April, May, [and] June.”
Most pediatricians find their work rewarding. One-third say the most rewarding part of their job is gratitude from and relationships with their patients. Meanwhile, 31% of pediatricians said knowing they are making the world a better place was a rewarding part of their job. Only 8% of them said making money was a rewarding part of their job.
Dr. Hackell said he did not go into pediatrics to make money, it was because he found it stimulating and has “no complaints.”
“I’ve been a pediatrician for 40 years and I wouldn’t do anything else,” Dr. Hackell said. “I don’t know that there’s anything that I would find as rewarding as the relationships that I’ve had over 40 years with my patients. You know, getting invited to weddings of kids who I saw when they were newborns is pretty impressive. It’s the gratification of having ongoing relationships with families.”
Furthermore, the report revealed that 77% of pediatricians said they would pick medicine again if they had a choice, and 82% said they would choose the same specialty.
The experts disclosed no relevant financial interests.
*This story was updated on 5/18/2021.
Are more naturopaths trying to compete with docs?
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
Jon Hislop, MD, PhD, hadn’t been in practice very long before patients began coming to him with requests to order tests that their naturopaths had recommended.
The family physician in North Vancouver, British Columbia, knew little about naturopathy but began researching it.
“I was finding that some of what the naturopaths were telling them was a little odd. Some of the tests they were asking for were unnecessary,” Dr. Hislop said.
The more he learned about naturopathy, the more appalled he became. He eventually took to Twitter, where he wages a campaign against naturopathy and alternative medicine.
“There is no alternative medicine,” he said. “There’s medicine and there’s other stuff. We need to stick to medicine and stay away from the other stuff.”
Dr. Hislop is not alone in his criticism of naturopathic medicine. Professional medical societies almost universally oppose naturopathy, but that has not stopped its spread or prevented it from becoming part of some health care systems.
Americans spent $30.2 billion on out-of-pocket complementary health care, according to a 2016 report from the National Institutes of Health. That includes everything from herbal supplements and massage therapy to chiropractic care.
What is naturopathic medicine?
Naturopathy came to the United States from Germany in the 1800s, but some of its practices are thousands of years old. Naturopathic treatments include homeopathy, IV vitamin infusions, acupuncture, Reiki, and herbal supplements.
Naturopathy is based on the belief that the body has an innate ability to heal itself. It discourages drugs and surgery in favor of supplements, herbs, and other so-called natural treatments. Much of it centers around addressing lifestyle issues and counseling patients to improve their diets, quit smoking, exercise more, lose weight, etc., in order to address the root causes of some health problems.
Practitioners are critical of Western medicine for what they regard as an over-reliance on drugs and technology and for treating symptoms rather than the causes of disease.
“We get a lot of people who are at the end of their ropes, people with hard-to-diagnose diseases who know they are sick but whose labs are normal,” said Jaquel Patterson, ND, former president of the American Association of Naturopathic Physicians (AANP) and medical director of a naturopathic practice in Connecticut.
Separate training and licensing
There are major differences among naturopaths.
At one extreme are unlicensed, self-taught “healers,” who can embrace everything from homeopathy to aromatherapy.
At the other end are naturopathic doctors (NDs), who are more likely to become part of health care systems. These caregivers are trained and licensed, though not by the same institutions as traditional physicians.
To be licensed, NDs must graduate from one of seven accredited naturopathic medical schools in the United States and Canada. In addition to a standard medical curriculum, schools require graduates to complete 4 years of training in clinical nutrition, acupuncture, homeopathic medicine, botanical medicine, physical medicine, and counseling. Medical students intern in clinical settings for 2 years.
NDs are eager to distinguish themselves from their uncredentialed counterparts.
“Some people go to a weekend class and call themselves naturopaths. That’s very concerning. I don’t want those people to be licensed either,” said Hallie Armstrong, ND, who practices in Michigan.
In the United States, there are 6,000 practicing NDs and an unknown number of unlicensed naturopathic healers.
Can naturopaths call themselves ‘physicians’?
Twenty-two states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands have licensing or registration laws for naturopathic doctors. Three states – South Carolina, Tennessee, and Florida – prohibit practicing naturopathic medicine without a license, according to the AANP.
States that license NDs differ in what they permit them to do.
Nine states allow licensed NDs to use the term “physician,” although this is prohibited in seven states. Most licensed states allow naturopathic practitioners some prescribing authority, including the prescribing of many controlled substances, although only a few states permit full prescribing rights. Most states that license NDs allow them to prescribe and administer nonprescription therapeutic substances, drugs, and therapies.
Twelve states and the District of Columbia allow licensed naturopathic doctors to perform some minor procedures, such as stitching up wounds. Additionally, 13 states allow NDs to order diagnostic tests.
Although the AANP lobbies to get licensure in more states and to expand the activities that NDs can perform, the medical establishment in those states nearly always opposes the legislation, as do national organizations, such as the American Academy of Family Physicians and the American College of Physicians.
“They absolutely will not stop until they get licenses. They’ve done a really good job of selling themselves as legitimate health care professionals to state legislatures,” said David Gorski, MD, PhD, FACS, a surgical oncologist and managing editor of Science-Based Medicine, a blog that attacks unproven medical claims and defends traditional medicine. Naturopathy is a favorite target.
Are naturopaths gaining ground anyway?
Despite the opposition of the medical establishment and many individual health care professionals, a growing number of health care systems are adopting alternative medicine.
In 2018, the AANP stated that 28 prominent health systems, hospitals, and cancer treatment centers had one or more licensed NDs on staff. Among them were Cancer Treatment Centers of America, Cedars-Sinai, Columbia University’s Herbert Irving Comprehensive Cancer Center, and the Fred Hutchinson Cancer Research Center.
Other health care systems may not have NDs on staff but provide naturopathic treatments, usually under the heading of “complementary medicine” or “integrative medicine.” For example, the Cleveland Clinic’s Center for Integrative and Lifestyle Medicine offers acupuncture, Chinese herbal medicine, Reiki, yoga, and culinary medicine.
Critics find this appalling.
“I think it’s a mistake to integrate that kind of practice into a science-based health care setting. If we learned anything over the past year, it’s that medicine based on magical thinking is dangerous,” said Timothy Caulfield, LLM, FCAHS, research director at the Health Law Institute of the University of Alberta, Edmonton.
Dr. Gorski added: “I’m not exactly sure why doctors who should know better have become more accepting of practices that aren’t science-based or are outright quackery.”
Becoming part of the system
Beaumont Health, Michigan’s largest health care system, added integrative medicine in 2006 and hired its first naturopathic practitioners a year later.
The integrative practitioners began in oncology, offering such things as massage therapy, acupuncture, guided imagery, and Reiki. “Very quickly, people outside oncology began saying, ‘I’ve got a cardiology patient who would really benefit from this ... I’ve got a GI patient who could benefit from this...,’” said Maureen Anderson, MD, medical director of Beaumont Integrative Medicine.
Beaumont now offers integrative medicine at three locations. They average 20,000 visits a year and work with 50 to 60 practitioners, many of whom work part-time.
Because Michigan does not license NDs, their scope of practice at Beaumont is limited. They take patient histories, provide advice on nutrition, diet, and exercise, and prescribe herbs and supplements. Beaumont operates its own herbal and supplement pharmacy.
NDs work under the medical supervision of Dr. Anderson, an emergency medicine physician who became interested in naturopathy because she thought traditional medicine doesn’t do a good job of providing care for chronic conditions. Any initial skepticism on the part of the medical staff has been overcome by seeing the benefits naturopathy provides, Dr. Anderson said. The claim is echoed by Mr. Armstrong, an ND who works in the system part-time: “As soon as [doctors] understand our schooling and where we’re coming from and understand that we want to do the same things, then they’re very accepting.”
The University of California, Irvine, health care system has one of the largest naturopathic medicine programs in the country, the result of a $200 million donation in 2017 from a couple who champion alternative medicine. The Susan Samueli Integrative Health Institute includes 28 health care professionals, including MDs, NDs, RNs, acupuncturists, dietitians, yoga instructors, and others. It includes a research arm, which is focused primarily on acupuncture.
The alternative medicine offerings benefit the system, said Kim Hecht, DO, medical director of inpatient and ambulatory services at the Samueli Institute.
“I’m not against traditional medicine, because I think everything has a time and a place,” Dr. Hecht said. However, she rejects the idea that MDs can offer the same holistic approach as NDs.
“Medical science likes to say we’re interested in treating the whole person, but if you look at medical school courses, that’s not what’s being taught,” she said.
The chance to work within a traditional health care system was attractive to Arvin Jenab, ND, medical director of naturopathic medicine at the institute.
“It offers the opportunity to refine our medicine and trim the things that aren’t necessary or are controversial and concentrate on the things at the core of what we do,” he said.
UCI Health practices a conservative model of naturopathy that supports traditional practitioners, Mr. Jenab said.
Is there any harm?
Some patients clearly want what naturopathy offers. So what’s the harm?
Health care systems that integrate alternative medicine legitimize it and lower the overall standard of care, Mr. Caulfield said. Most naturopathy claims are not backed by evidence, and making it available to patients amounts to deceiving them, he said.
“If there’s good science behind it, it’s not going to be alternative medicine; it’s going to be medicine,” Mr. Caulfield said.
Family physician Dr. Hislop said that refusing to order naturopath-recommended tests interferes with his relationships with patients and often requires lengthy conversations to explain the problems with naturopathy.
Naturopathic medicine can deter patients from seeking proven conventional treatments, which can put their health at risk, Dr. Gorski said.
Some naturopaths could potentially be harmful.
In 2017, a California woman died after receiving an IV preparation of curcumin, a chemical constituent in the Indian spice turmeric featured in alternative medicine. The U.S. Food and Drug Administration found that the treating ND mixed the curcumin emulsion product with ungraded castor oil that had a warning label stating: “CAUTION: For manufacturing or laboratory use only.”
Because naturopathic care is generally not covered by insurance, it can also be expensive for patients who pay out of pocket.
Ironically, the mainstream health care system helps create the environment in which naturopathic medicine thrives.
It offers patients a more relaxed and personal alternative to rushed visits with harried doctors scrambling to see the required number of patients in a day. By contrast, an initial visit with an ND might last a leisurely 60 minutes, with 30-minute follow-up appointments.
Mr. Caulfield acknowledged that the relaxed naturopathic approach can be more attractive to patients but said the answer is to reform the current system: “You don’t fix a broken arm by acupuncture.”
A version of this article first appeared on Medscape.com.
New STRENGTH analysis reignites debate on omega-3 CV benefits
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
Ultrasound renal denervation drops BP in patients on triple therapy
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
Renal denervation’s comeback as a potential treatment for patients with drug-resistant hypertension rolls on.
Renal denervation with ultrasound energy produced a significant, median 4.5–mm Hg incremental drop in daytime, ambulatory, systolic blood pressure, compared with sham-treatment after 2 months follow-up in a randomized study of 136 patients with drug-resistant hypertension maintained on a standardized, single-pill, triple-drug regimen during the study.
The results “confirm that ultrasound renal denervation can lower blood pressure across a spectrum of hypertension,” concluded Ajay J. Kirtane, MD, at the annual scientific sessions of the American College of Cardiology. Renal denervation procedures involve percutaneously placing an endovascular catheter bilaterally inside a patient’s renal arteries and using brief pulses of energy to ablate neurons involved in blood pressure regulation.
A former ‘hot concept’
“Renal denervation was a hot concept a number of years ago, but had been tested only in studies without a sham control,” and initial testing using sham controls failed to show a significant benefit from the intervention, noted Deepak L. Bhatt, MD, an interventional cardiologist and professor of medicine at Harvard Medical School in Boston who was not involved with the study. The significant reductions in systolic blood pressure reported with renal denervation, compared with control patients in this study, “are believable” because of inclusion of a true control cohort, he added. “This really exciting finding puts renal denervation squarely back on the map,” commented Dr. Bhatt during a press briefing.
Dr. Bhatt added that, while the median 4.5–mm Hg incremental reduction in daytime, ambulatory, systolic blood pressure, compared with control patients – the study’s primary endpoint – may seem modest, “in the world of hypertension it’s a meaningful reduction” that, if sustained over the long term, would be expected to produce meaningful cuts in adverse cardiovascular events such as heart failure, stroke, and MI.
“The question is whether the effects are durable,” highlighted Dr. Bhatt, who helped lead the first sham-controlled trial of renal denervation, SYMPLICITY HTN-3, which failed to show a significant blood pressure reduction, compared with controls using radiofrequency energy to ablate renal nerves. A more recent study that used a different radiofrequency catheter and sham controls showed a significant effect on reducing systolic blood pressure in the SPYRAL HTN-OFF MED Pivotal trial, which by design did not maintain patients on any antihypertensive medications following their renal denervation procedure.
Dr. Kirtane noted that, although the median systolic blood pressure reduction, compared with controls treated by a sham procedure, was 4.5 mm Hg, the total median systolic pressure reduction after 2 months in the actively treated patients was 8.0 mm Hg when compared with their baseline blood pressure.
Concurrently with his report the results also appeared in an article posted online (Lancet. 2021 May 16;doi: 10.1016/S0140-6736(21)00788-1).
Denervation coupled with a single, daily three-drug pill
The RADIANCE-HTN TRIO study ran at 53 centers in the United States and Europe, and randomized 136 adults with an office-measured blood pressure of at least 140/90 mm Hg despite being on a stable regimen of at least three antihypertensive drugs including a diuretic. The enrolled cohort averaged 52 years of age and had an average office-measured pressure of about 162/104 mm Hg despite being on an average of four agents, although only about a third of enrolled patients were on treatment with a mineralocorticoid-receptor antagonist (MRA) such as spironolactone.
At the time of enrollment and 4 weeks before their denervation procedure, all patients switched to a uniform drug regimen of a single, daily, oral pill containing the calcium channel blocker amlodipine, the angiotensin receptor blocker valsartan or olmesartan, and the diuretic hydrochlorothiazide with no other drug treatment allowed except for unusual, prespecified clinical circumstances. All patients remained on this drug regimen for the initial 2-month follow-up period unless their blood pressure exceeded 180/110 mm Hg during in-office measurement.
The denervation treatment was well tolerated, although patients reported brief, transient, and “minor” pain associated with the procedure that did not affect treatment blinding or have any lingering consequences, said Dr. Kirtane, professor of medicine at Columbia University Vagelos College of Physicians and Surgeons in New York.
A reason to use energy delivery by ultrasound rather than by radiofrequency to ablate nerves in the renal arteries is that the ultrasound approach exerts a more uniform effect, allowing effective treatment delivery without need for catheter repositioning into more distal branches of the renal arteries, said Dr. Kirtane, who is also an interventional cardiologist at NewYork-Presbyterian/Columbia University Irving Medical Center.
But each method has its advantages, he added.
He also conceded that additional questions need to be addressed regarding which patients are most appropriate for renal denervation. “We need to figure out in which patients we can apply a device-based treatment,” Dr. Kirtane said during the press briefing. Patients with what appears to be drug-resistant hypertension often do not receive treatment with a MRA because of adverse effects, and many of these patients are not usually assessed for primary aldosteronism.
In SYMPLICITY HTN-3, “about half the patients who were seemingly eligible became ineligible” when they started treatment with a MRA, noted Dr. Bhatt. “A little spironolactone can go a long way” toward resolving treatment-resistant hypertension in many patients, he said.
RADIANCE-HTN TRIO was sponsored by ReCor Medical, the company developing the tested ultrasound catheter. Dr. Kirtane has received travel expenses and meals from ReCor Medical and several other companies, and Columbia has received research funding from ReCor Medical and several other companies related to research he has conducted. Dr. Bhatt has no relationship with ReCor Medical. He has been a consultant to and received honoraria from K2P, Level Ex, and MJH Life Sciences; he has been an advisor to Cardax, Cereno Scientific, Myokardia, Novo Nordisk, Phase Bio, and PLx Pharma; and he has received research funding from numerous companies.
FROM ACC 2021
FLOWER-MI: FFR-guided complete revascularization shows no advantage in STEMI
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
For patients with ST-elevated myocardial infarction (STEMI) undergoing complete revascularization, percutaneous coronary interventions (PCI) guided by fractional flow reserve (FFR) relative to angiography-guided PCI do not result in significantly lower risk of death or events, according to data from the randomized FLOWER-MI trial.
Rather, the events at 1 year were numerically lower among those randomized to the angiography-guided approach, according to the principal investigator of the trial, Etienne Puymirat, MD, PhD.
Prior studies showing an advantage for FFR-guided PCI in patients with coronary syndromes provided the hypothesis that FFR-guided PCI would also be superior for guiding PCI in STEMI patients. In the multicenter FAME trial, for example, FFR-guided PCI for patients with multivessel disease was associated with fewer stent placements (P < .001) and a nearly 30% lower rate of events at 1 year (P = .02).
While the advantage of complete revascularization, meaning PCI treatment of nonculprit as well as culprit lesions, has already been shown to be a better strategy than treatment of culprit lesions alone, FLOWER-MI is the first large study to compare FFR to angiography for guiding this approach to STEMI patients with multivessel disease, said Dr. Puymirat of Hôpital Européen George Pompidou, Paris, at the annual scientific sessions of the American College of Cardiology.
In this trial, involving multiple centers in France, STEMI patients were eligible for randomization if they had successful PCI of a culprit lesion and 50% or greater stenosis in at least one additional nonculprit lesion. The complete revascularization, whether patients were randomized to PCI guided by angiography or FFR, was performed during the index hospital admission. Patient management and follow-up was otherwise the same.
After a small number of exclusions, the intention-to-treat populations were 577 patients in the angiography-guided group and 586 in the FFR-guided group. The characteristics of the groups were well matched with an average age of about 62 years and similar rates of risk factors, such as hypertension and diabetes.
Angiography guidance just as good
The primary outcome was a composite of all-cause mortality, nonfatal MI, and unplanned revascularization. By hazard ratio, the risk of having one of these events within 1 year of PCI was numerically greater, at 32 in the FFR-guided group and 24 in the angiography-guided group, but the difference was not statistically significant (1.32; P = .31).
However, the total rate of events was low (5.5% vs. 4.2% for the angiography-guided and FFR-guided groups, respectively) and the confidence intervals were wide (95% CI, 0.78-2.23). This was also true of the components of the primary outcome.
No signal for a difference between strategies could be derived from these components, which included a higher rate of MI in the FFR-guided group (3.1% vs. 1.7%) but a lower rate of death (1.5% vs. 1.7%).
Unplanned hospitalizations leading to revascularization rates were also low (1.9% and 2.6% for angiography-guided and FFR-guided PCI, respectively), although it was reported that the rate of revascularization for nonculprit lesions was about twice as high in the FFR group (53.3% vs. 27.3%).
At 1 year, there were also low rates and no significant differences in a list of secondary outcomes that included hospitalization for recurrent ischemia or heart failure, stent thrombosis, and revascularization. As within the primary composite outcome, no pattern could be seen in the secondary events, some of which were numerically more common in the FFR-guided group and some numerically lower.
In a cost-efficacy analysis, the median per-patient cost of the FFR-guided strategy was about 500 Euros ($607) greater (8,832 vs. 8,322; P < .01), leading Dr. Puymirat to conclude that “the use of FFR for nonculprit lesions appears to be less effective but more expensive,” at least by costs derived in France.
Lack of statistical power limits interpretation
The conclusion of FLOWER-MI is that FFR-guided PCI in complete revascularization of nonculprit lesions in STEMI patients is not superior to an angiography-guided approach, but Dr. Puymirat cautioned that the low number of events precludes a definitive message.
William Fearon, MD, professor of cardiovascular medicine at Stanford (Calif.) University Medical Center, agreed. Based on his calculations, the trial was substantially underpowered. Evaluating the details of treatment in the FFR group, Dr. Fearon pointed out that a nonculprit lesion with a FFR of 0.80 or less was identified in about 55% of patients. Ultimately, 66% in the FFR group received PCI, eliminating the key distinction between strategies for the majority of patients enrolled.
“Only about one-third of the FFR-guided patients, or about 200 patients, did not receive nonculprit PCI, and therefore only in this small group could we expect a difference in outcomes from the angio-guided group,” Dr. Fearon said.
Fewer stents were placed in the FFR-guided than angiography-guided group (1.01 vs. 1.5), but Dr. Fearon suggested that it would be very difficult to show a difference in risk of events in a study of this size when event rates at 1 year reached only about 5%.
In response, Dr. Puymirat acknowledged that the rate of events for this trial, which was designed in 2015, were lower than expected. In recalculating the power needed based on the rate of events observed in FLOWER-MI, he estimated that about 8,000 patients would have been needed to show a meaningful difference in these PCI strategies.
Dr. Puymirat reports financial relationships with more than a dozen pharmaceutical companies, including Abbott, which provided some of the funding for this trial. Dr. Fearon reports financial relationships with Abbott, CathWorks, HeartFlow, and Medtronic.
FROM ACC 2021












