User login
COVID-19 Wellbeing
Resources for hospitalists
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
Resources for hospitalists
Resources for hospitalists
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
Monoclonal antibody drops fat, ups muscle in obesity, diabetes
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
In a phase 2 randomized clinical trial of adults with type 2 diabetes and obesity, investigational drug bimagrumab (BYM338, Novartis) – a monoclonal antibody that blocks activin type II receptors and stimulates skeletal muscle growth – led to big reductions in total body fat mass and A1c and significant increases in lean mass compared with placebo.
The efficacy and safety findings “suggest that blockade of the activin receptor with bimagrumab could provide a novel pharmacologic approach for managing patients with type 2 diabetes with excess adiposity,” Steven B. Heymsfield, MD, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, and colleagues reported in their study, published online Jan. 13 in JAMA Network Open.
Preliminary findings from the study of 75 patients treated for 48 weeks – in which neither group ate less despite intensive nutrition advice – were presented at Obesity Week in 2019.
As reported then, Lee M. Kaplan, MD, PhD, noted that the 6.5% weight loss in the bimagrumab group was similar to that seen with antiobesity medications that suppress appetite.
“What it suggests,” he said in an interview, “is that there may be a completely new mechanism at play here,” because patients receiving bimagrumab weren’t eating less but were losing the same amount of weight as reported for weight-loss drugs that work by decreasing appetite.
“Is this going to be the kind of complementary drug with a different mechanism that’s going to augment the effects of other drugs?” wondered Dr. Kaplan, director of the Obesity, Metabolism & Nutrition Institute at Massachusetts General Hospital, Boston, who has previously served as a scientific consultant to Novartis.
Asked about future plans for bimagrumab, a Novartis spokesperson said in an interview, “We are currently reviewing the program strategy and considering next steps.”
Four FDA-approved weight-loss drugs now approved
The Food and Drug Administration approval for lorcaserin (Belviq, Belviq XR, Eisai) for weight loss was rescinded on Feb. 13, 2020, when a postmarketing trial revealed an increased occurrence of cancer, leaving four drugs approved for weight loss in the United States, plus several drugs in development, Dr. Heymsfield and colleagues wrote.
The current phase 2 trial was designed to determine the safety and efficacy of bimagrumab – which had originally been studied to see if it would increase lean muscle mass in people with sarcopenia – on total body fat mass and glycemic control in patients with type 2 diabetes and overweight or obesity.
Researchers enrolled 75 adults at eight sites in the United States and one in Wales, United Kingdom, from 2017 to 2019.
On average, patients were 60 years old with an A1c of 7.8% and a body mass index of 32.9 kg/m2; they weighed 93.6 kg and had a fat mass of 35 kg.
Patients received an intravenous infusion of bimagrumab (10 mg/kg up to 1,200 mg in 5% dextrose solution) or placebo (5% dextrose solution) every 4 weeks for 48 weeks. They met with a registered dietitian at each monthly study visit and had a virtual check-in between visits.
Participants were advised to follow a diet that would cut 500 calories a day and encouraged to follow the American Diabetes Association walking program.
Body fat mass was measured by dual-energy x-ray absorptiometry (DEXA).
There were more women in the bimagrumab group than in the placebo group (62% vs. 32%), but baseline BMI, total body fat mass, and A1c were similar in both groups.
Same caloric intake, less fat tissue, more muscle, smaller waist
At 48 weeks in the bimagrumab vs. placebo group, there was on average (all P < .001):
- A loss of 20.5% vs. 0.5% (−7.5 vs. −0.2 kg) of total body fat mass.
- A loss of 6.5% vs. 0.8% (−5.9 vs. −0.8 kg) of body weight.
- A gain of 3.6% vs. a loss of 0.8% (1.7 vs. −0.4 kg) of lean mass.
Similarly, the relatively large between-group differences in total body fat mass and body weight at 48 weeks with bimagrumab were accompanied by favorable differences in BMI (−2.19 vs. −0.28 kg/m2; P < .001) and waist circumference (−9.0 vs. 0.5 cm; P < .001), the investigators pointed out.
Moreover, the reduction of abdominal visceral adipose tissue and waist circumference with bimagrumab “was nearly twice that observed in a recently published study of patients with type 2 diabetes treated with an intensive lifestyle program and the glucagon-like peptide 1 (GLP-1) agonist liraglutide,” they noted.
This highlights “the importance of moving away from body weight as a primary efficacy marker of drugs to more metabolically relevant endpoints.”
Also, A1c decreased by 0.76% in the bimagrumab group and increased by 0.04% in the placebo group (P = .005).
Serious adverse events occurred in three patients (8%) in the bimagrumab group (elevated lipase, epigastric pain, pancreatitis, pneumonia) and three patients (8%) in the placebo group (cellulitis, acute coronary syndrome, acute myocardial infarction, worsening gastroparesis, thermal burn).
Adverse events were reported by 31 of 37 patients in the bimagrumab group, most often mild diarrhea (41%) and muscle spasms (41%), and 31 of 38 patients in the placebo group, most often headache (13%) and upper respiratory tract infection (13%).
The study was funded by Novartis. Dr. Heymsfield has reported receiving personal fees from Tanita and Medifast outside the submitted work. Disclosures for the other authors are listed in the article. Dr. Kaplan has reported previously serving as a scientific consultant to Novartis.
A version of this article first appeared on Medscape.com.
Call for Myeloma, Leukemia, Lymphoma and Other Hematology Papers
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to a future special issue on hematology topics, including myelomas, lymphomas, leukemias, and other bloodborne dieases.
Interested authors should submit a brief 2 to 3 sentence abstract to fedprac@frontlinemedcom.com. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
Federal Practitioner uses Editorial Manager , a web-based manuscript submission and review system. All manuscripts must be submitted through this system.
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editor-in-Chief (or, in the event of a potential conflict of interest, a designated surrogate from the journal’s Editorial Advisory Association).
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to a future special issue on hematology topics, including myelomas, lymphomas, leukemias, and other bloodborne dieases.
Interested authors should submit a brief 2 to 3 sentence abstract to fedprac@frontlinemedcom.com. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
Federal Practitioner uses Editorial Manager , a web-based manuscript submission and review system. All manuscripts must be submitted through this system.
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editor-in-Chief (or, in the event of a potential conflict of interest, a designated surrogate from the journal’s Editorial Advisory Association).
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to a future special issue on hematology topics, including myelomas, lymphomas, leukemias, and other bloodborne dieases.
Interested authors should submit a brief 2 to 3 sentence abstract to fedprac@frontlinemedcom.com. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
Federal Practitioner uses Editorial Manager , a web-based manuscript submission and review system. All manuscripts must be submitted through this system.
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editor-in-Chief (or, in the event of a potential conflict of interest, a designated surrogate from the journal’s Editorial Advisory Association).
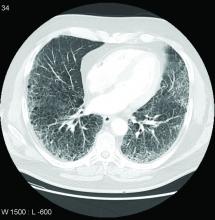
Lung disease raises mortality risk in older RA patients
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
Patients with rheumatoid arthritis–associated interstitial lung disease showed increases in overall mortality, respiratory mortality, and cancer mortality, compared with RA patients without interstitial lung disease, based on data from more than 500,000 patients in a nationwide cohort study.
RA-associated interstitial lung disease (RA-ILD) has been associated with worse survival rates as well as reduced quality of life, functional impairment, and increased health care use and costs, wrote Jeffrey A. Sparks, MD, of Brigham and Women’s Hospital, Boston, and colleagues. However, data on the incidence and prevalence of RA-ILD have been inconsistent and large studies are lacking.
In a study published online in Rheumatology, the researchers identified 509,787 RA patients aged 65 years and older from Medicare claims data. The average age of the patients was 72.6 years, and 76.2% were women.
At baseline, 10,306 (2%) of the study population had RA-ILD, and 13,372 (2.7%) developed RA-ILD over an average of 3.8 years’ follow-up per person (total of 1,873,127 person-years of follow-up). The overall incidence of RA-ILD was 7.14 per 1,000 person-years.
Overall mortality was significantly higher among RA-ILD patients than in those with RA alone in a multivariate analysis (38.7% vs. 20.7%; hazard ratio, 1.66).
In addition, RA-ILD was associated with an increased risk of respiratory mortality (HR, 4.39) and cancer mortality (HR, 1.56), compared with RA without ILD. For these hazard regression analyses, the researchers used Fine and Gray subdistribution HRs “to handle competing risks of alternative causes of mortality. For example, the risk of respiratory mortality for patients with RA-ILD, compared with RA without ILD also accounted for the competing risk of cardiovascular, cancer, infection and other types of mortality.”
In another multivariate analysis, male gender, smoking, asthma, chronic obstructive pulmonary disorder, and medication use (specifically biologic disease-modifying antirheumatic drugs, targeted synthetic DMARDs, and glucocorticoids) were independently associated with increased incident RA-ILD at baseline. However, “the associations of RA-related medications with incident RA-ILD risk should be interpreted with caution since they may be explained by unmeasured factors, including RA disease activity, severity, comorbidities, and prior or concomitant medication use,” the researchers noted.
The study findings were limited by several factors, including the lack of data on disease activity, disease duration, disease severity, and RA-related autoantibodies, the researchers noted. However, the results support data from previous studies and were strengthened by the large sample size and data on demographics and health care use.
“Ours is the first to study the epidemiology and mortality outcomes of RA-ILD using a validated claims algorithm to identify RA and RA-ILD,” and “to quantify the mortality burden of RA-ILD and to identify a potentially novel association of RA-ILD with cancer mortality,” they noted.
The study was supported by an investigator-initiated grant from Bristol-Myers Squibb. Lead author Dr. Sparks disclosed support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, the Brigham Research Institute, and the R. Bruce and Joan M. Mickey Research Scholar Fund. Dr. Sparks also disclosed serving as a consultant to Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer for work unrelated to the current study. Other authors reported research funding from Bristol-Myers Squibb, involvement in a clinical trial funded by Genentech and Bristol-Myers Squibb, and receiving research support to Brigham and Women’s Hospital for other studies from AbbVie, Bayer, Bristol-Myers Squibb, Novartis, Pfizer, Roche, and Vertex.
FROM RHEUMATOLOGY
U.K. variant spreading in the U.S. as COVID mutations raise stakes
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
The U.K.’s B117 variant is circulating in at least 24 states, according to new data from the Centers for Disease Control and Prevention COVID-19 variant surveillance. The CDC projects that the U.K. variant will become the dominant strain in the United States by March.
From any vantage point, the United Kingdom appears to be in the crosshairs of COVID-19: Weeks after a new, highly contagious variant emerged that fueled a surge in cases and fresh lockdowns, the United Kingdom was revealed to have the world’s highest coronavirus death rate.
But the United Kingdom also has a not-so-secret weapon of its own: A genomic sequencing program widely believed to be the most coordinated and advanced any nation has forged. In the vise grip of the virus, the Brits have gleaned key insights into the behavior and consequences of SARS-CoV-2.
But B117 is also notable for what it is missing: In this case, producing a negative result on certain polymerase chain reaction (PCR) tests in the spike protein, or S-gene.
One of the S-gene mutations specific to the variant deletes two amino acids, causing that portion of the PCR test to show up negative. The coincidental finding known as an S-gene target failure has become an integral proxy to help track where and when the variant is spreading in the United Kingdom, where about 5% of samples from COVID-19–infected patients are sequenced, said Sharon Peacock, PhD, executive director and chair of the COVID-19 Genomics U.K. Consortium.
That same tactic could prove valuable to clinicians similarly overwhelmed with cases and deaths but lacking high-level sequencing information on the virus, Dr. Peacock said in an interview. A British report released Friday stated that there is a “realistic possibility” that the variant has a higher death rate than other cases of SARS-CoV-2.
“In this particular variant, a deletion in the genome leads to one part of the diagnostic test failing,” Dr. Peacock explained. “Several targets are positive, but this is negative. In the U.K., this has been used as a surrogate marker.”
Targeting an invisible adversary
B117 is not the only variant that produces this result, Dr. Peacock cautioned, “but in screening for it, you can have this in mind.”
“Since the U.K. is sequencing about 5% of the cases they detect, this gives them really important clues about what’s happening there,” said Anderson Brito, PhD, a virologist and postdoctoral researcher at Yale University, New Haven, Conn., where investigators are creating custom PCR tests to detect the B117 variant.
Dr. Brito, who lived in the United Kingdom for 4 years while studying for his doctorate at Imperial College London, said a “major advantage” is the more unified process to collect and sequence samples. Crucial information – including the date and place of collection – comes with each sample, which fuels not only sequencing, but an epidemiologic perspective.
“They’re not in the dark at all,” Dr. Brito said in an interview. “I think no other country in the world knows better which virus lineages are circulating.”
The CDC launched the SPHERES consortium in May 2020 to coordinate the sequencing of SARS-CoV-2 genomes across the United States.
But American genomic efforts are “not as centralized,” said Dr. Brito, whose lab detected the first two cases of the U.K. variant in Connecticut on Jan. 6. “We struggle to get samples, because they’re decentralized to a level where there’s little coordination between hospitals and research centers. They’re not as connected as in the U.K. If we just get a sample and it has no date of collection and no origin information, for example, it’s basically useless.”
Global genomic collaborations include GISAID, an international database where researchers share new genomes from various coronaviruses. As of mid-January, the United States had submitted about 68,000 sequences to GISAID, adding about 3,000 new samples every week and expecting even more from commercial labs in coming days, according to the CDC.
“The U.K. is definitely much more on top of looking for variants as they pop up,” said Gigi Gronvall, PhD, an immunologist and senior scholar at Johns Hopkins Center for Health Security in Baltimore. “The U.S. has now turned that up.”
Warning from British scientists to the world
Despite these genomic accomplishments, some British scientists said they have regrets too, wishing they’d known just how rapidly SARS-CoV-2 was actually spreading a year ago, when it hit western Europe.
That information was crucial not only for preventive efforts, but because viruses inevitably mutate faster the more people who are infected, said Igor Rudan, MD, PhD, director of the Center for Global Health Research at University of Edinburgh.
“Italy showed us just how fast it was spreading and how deadly it is for the very old and people with multiple comorbidities,” said Dr. Rudan, who also editor in chief of the Journal of Global Health. “We wish we knew it was spreading so fast, and we wish we knew the threshold of cases we could allow to be infected before the virus would mutate.”
More mutations mean more new strains of SARS-CoV-2, Dr. Rudan said in an interview. “We’ve reached that threshold now and will see more of these mutations.”
Despite its current struggles, the United Kingdom is reaching beyond tracking its new variant’s spread and trying to identify new mutations that might change the way the virus behaves.
Three features of any emerging variant are particularly important, Dr. Peacock explained: Is it more transmissible? Is it more lethal? And does it cut the ability of natural- or vaccine-induced immunity to protect people from infection?
“We need to sequence people coming to the hospital who are sicker,” said Dr. Peacock, also a professor of public health and microbiology at the University of Cambridge (England). “Also, if anyone has the infection after they’ve already been sick or had the vaccine, we really want to know what that looks like” genomically.
SARS-CoV-2 has already logged more than 4,000 mutations, Dr. Peacock said. But “knowing that viruses mutate all the time is not sufficient reason not to look. We really want to know if mutations lead to changes in amino acids, and if that can lead to changes in functionality.”
For the moment, however, experts say they’re relieved that the U.K. strain doesn’t seem able to evade COVID-19 vaccines or render them less effective.
“Even though mutations are common, those able to change the viral coding are rare,” Dr. Brito explained. If necessary, vaccines could be tweaked to replace the spike gene sequence “within a matter of weeks. We already do this for flu vaccines. Every year, we have to monitor variants of the virus circulating to develop a vaccine that covers most of them. If we end up having to do it for SARS-CoV-2, I would not be surprised.”
But variant-fueled increases in infections will require more people to be vaccinated before herd immunity can be achieved, Dr. Rudan warned. “If it spreads faster, we’ll need to vaccinate probably 85% of people versus 70% to reach herd immunity.”
One lesson the COVID-19 pandemic has driven home “is to always be on your guard about what happens next,” Dr. Peacock said. Although confident about the genomic efforts in the United Kingdom to date, she and her colleagues feel they’re still reaching for a complete understanding of the evolutionary changes of the virus.
“We’re ahead of the curve right now, but we want to get in front of the curve,” Dr. Peacock said. “It’s essential to get ahead of what might be around the corner because we don’t know how the virus is going to evolve.”
A version of this article first appeared on Medscape.com.
Male Genital Examinations: Special Considerations and Pearls for Dermatologists
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
Practice Points
- Genital examinations are an important aspect of comprehensive dermatologic care for male patients.
- Unintentional patient erections are unique to male patients and should be addressed professionally, depending on the patient’s reaction.
- In addition to being mindful of body language and tone, dermatologists may consider involving a chaperone when performing genital examinations to optimize patient experience.
Brazilian researchers tracking reinfection by new virus variant
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
Just as Brazil surpassed 200,000 deaths from COVID-19 on Jan. 7, news from Bahia added another layer of concern: A platform case report in a preprint detailed the first case of reinfection in that state, apparently caused by a new strain, one having the E484K mutation.
That variant, now called Brazil P.1, has migrated to the United States. The Minnesota Department of Health announced on Jan. 25 the nation’s first known COVID-19 case associated with it.
The mutation is located in the protein gene of the virus’ spike, which forms the crown structure of coronaviruses and is responsible for the virus’ binding to human cells. The E484K mutation is now the focus because it’s associated with mutations that escape the immune system’s neutralizing antibodies.
“This mutation is at the center of worldwide concern, and it is the first time that it has appeared in a reinfection,” the study’s first author, Bruno Solano de Freitas Souza, MD, a researcher at the Salvador regional unit of Instituto D’Or of Teaching and Research, based at Hospital São Rafael, Salvador, Brazil, explained in an interview.
“We will wait for the sample from Bahia to confirm the case from the perspective of the Ministry of Health’s surveillance network,” said Fernando Motta, PhD, deputy head of the Laboratory for Respiratory Virus and Measles at the Oswaldo Cruz Institute in Rio de Janeiro, which acts as a national reference center for respiratory viruses with the Brazilian Ministry of Health (MS) and as a reference for the World Health Organization.
A case of reinfection
The case patient that led to the alarm was a 45-year-old woman who is a health care executive. She had no comorbidities. The team had been following health care professionals and patients who had tested positive on reverse transcription–polymerase chain reaction (RT-PCR) testing more than once to understand whether they represented cases of prolonged viral persistence or new infections.
The woman had symptoms of viral infection on two occasions (May 26 and Oct. 26). On both occasions, results of RT-PCR testing for SARS-CoV-2 on nasopharyngeal samples were positive. In the first episode, the patient had diarrhea, myalgia, asthenia, and odynophagia for about 7 days. She returned to activities 21 days later. In the second episode, she had more severe symptoms that lasted longer, but she still did not require hospitalization.
“It was the first confirmed case of reinfection in Bahia, and in the second episode, we observed a mutation that could have an impact on the ability of antibodies to neutralize the virus,” Dr. Souza said. “The research continues with the investigation of cases in which the patient has a positive SARS-CoV-2 RT-PCR more than once in an interval greater than 45 days, to have a higher level of evidence.”
He stressed that “it is very important to reinforce measures to control the pandemic, social distance, use of masks, and speed up vaccination to be able to control the circulation of the virus, while monitoring the evolution of it.”
On alert for more cases
A person who twice tests positive for SARS-CoV-2 on real-time RT-PCR is suspected of having been reinfected, provided 90 or more days have elapsed between the two episodes, regardless of the condition observed. To confirm the suspected case, the samples must be sent to reference laboratories according to a plan established by the Ministry of Health in Brazil.
A health professional living in the Brazilian city of Natal represented the first confirmed case of reinfection by the new coronavirus in Brazil. That case was announced on Dec. 10, 2020.
“We communicated this case of reinfection to the MS in early December 2020. And the second sample already had the E484K mutation on the spike, as in the case of Bahia,” said Dr. Motta.
The first step in differentiating reinfection from persistence is to observe differences in the genotyping of the virus. For the technique to be successful, Dr. Souza said, researchers need a large amount of viral genetic material, which usually cannot be obtained.
“That is why there are many more suspected than confirmed cases,” Dr. Souza explained. He admitted that, although there are few cases, “it is increasingly clear that reinfection is a reality.”
Markers of mutations
What worried the researchers most was not only the possibility of reinfection but also the fact that preliminary analyses showed a specific mutation.
“The E484K mutation is present in a group of variants identified in South Africa that have been associated with increased infectivity and has been observed in a strain recently described in Brazil,” Dr. Souza said.
Mutations are expected, appear spontaneously, and in most cases have no effects on transmission or clinical outcome – they are simply used as markers and are useful for contact tracing or studying transmission routes. But some mutations can last because they provide an advantage for the pathogen, even if only momentary. In the case of SARS-CoV-2, mutations in the protein spike gene (S) are relevant because they may give clues to that advantage – as well as to changes in infectivity, transmission potential, antibodies, and response to vaccines.
A variant of the virus that has eight changes that affect the protein S gene – and several others in different genes – is behind the increase in the number of cases in London and southeastern England. Researchers from the University of São Paulo identified one of the factors that made this new variant – classified as B.1.1.7 – more infectious.
With bioinformatics tools, they found that the protein S gene in the new viral strain has a stronger molecular interaction with the ACE2 receptor, which is on the surface of human cells and to which the virus binds, making infection possible. The variant has already spread to the rest of the world, and the first two cases have been confirmed in Brazil by the Adolf Lutz Institute.
The alert for a new variant in Africa – similar to B.1.1.7 in the United Kingdom in that it carries nine changes in protein S at position 501 – was made by the Brazilian virologist Tulio de Oliveira, PhD.
“We found that this strain seems to be spreading much faster,” Dr. Oliveira, who is with the University of KwaZulu Natal, told the journal Science. His work first alerted British scientists to the importance of the position N501Y.
“The new variants just described in the United Kingdom and South Africa are slightly more transmissible and have already been identified in cases imported into Brazil,” Dr. Motta said. “Unfortunately, we believe it is only a matter of time before it becomes indigenous.”
The viral family grows
Viruses such as SARS-CoV-2 are classified into strains on the basis of small differences in their genetic material. Since Dec. 26, 2020, in addition to the British and South African variants, it appears the Carioca lineage also is a player.
In a preprint article, researchers analyzed the evolution of the epidemic in Rio de Janeiro from April 2020 until just before the new increase in incidence in December. They compared the complete sequences of the viral genome of 180 patients from different municipalities. The study, which is being jointly conducted by members of the Federal University of Rio de Janeiro and the National Laboratory for Scientific Computing, identified a new variant of SARS-CoV-2 that has five unique mutations (from one of the predominant strains). Concern arose because, in addition to those five genetic changes, many of the samples had a sixth – the well-known E484K mutation.
“The three lines – the U.K., South Africa, and Brazil – were almost synchronous publications, but there is no clear evidence that they have any kind of common ancestry,” Carolina M. Voloch, PhD, the article’s first author and a biologist and researcher at the Molecular Virology Laboratory and associate professor in the department of genetics at the Federal University of Rio de Janeiro, said in an interview.
Dr. Voloch’s research focuses on the use of bioinformatics tools to study the molecular, phylogenetic, and genomic evolution of viruses.
“The emergence of new strains is common for viruses,” she said. “It can be happening anywhere in the world at any time.”
She stressed that identifying when mutations emerge will help to define the new Brazilian lineage. Researchers are working to determine whether the neutralizing antibodies of patients who have been infected with other strains respond to this Rio de Janeiro strain.
“We hope to soon be sharing these results,” Dr. Voloch said.
The article’s authors estimated that the new strain likely appeared in early July. They say more analysis is needed to predict whether the changes have a major effect on viral infectivity, the host’s immune response, or the severity of the disease. Asked about the lineage that caused the reinfection in Bahia, Dr. Voloch said she hadn’t yet contacted the authors to conduct a joint analysis but added that the data disclosed in the preprint would not represent the same variant.
“There are only two of the five mutations that characterize the Rio de Janeiro lineage. However, it has the E484K mutation that is present in more than 94% of the samples of the new variant of Rio,” she said.
She added that there’s a possibility of reinfection by the lineage that’s circulating in Rio de Janeiro and in other states, as well as countries such as the United States, the United Kingdom, and Japan.
“The Carioca virus is being exported to the rest of the world,” Dr. Voloch said.
Virus’ diversity still unknown
Researchers now know that SARS-CoV-2 probably circulated silently in Brazil as early as February 2020 and reached all the nation’s regions before air travel was restricted. Since the first half of 2020, there have been two predominant strains.
“More than a dozen strains have been identified in Brazil, but more important than counting strains to identify the speed with which they arise – which is directly associated with the rate of infection, which is very high in the country,” said Dr. Motta.
The so-called variant of Rio de Janeiro, he said, has also been detected in other states in four regions of Brazil. The key to documenting variants is to get a more representative sample with genomes from other parts of the country.
As of Jan. 10, a total of 347,000 complete genome sequences had been shared globally through open databases since SARS-CoV-2 was first identified, but the contribution of countries is uneven. Although the cost and complexity of genetic sequencing has dropped significantly over time, effective sequencing programs still require substantial investments in personnel, equipment, reagents, and bioinformatics infrastructure.
According to Dr. Voloch, it will only be possible to combat the new coronavirus by knowing its diversity and understanding how it evolves. The Fiocruz Genomic Network has made an infographic available so researchers can track the strains circulating in Brazil. It›s the result of collaboration between researchers from Fiocruz and the GISAID Initiative, an international partnership that promotes rapid data sharing.
As of Jan. 5, researchers in Brazil had studied 1,897 genomes – not nearly enough.
“In Brazil, there is little testing and even less sequencing,” lamented Dr. Souza.
“In the U.K., 1 in 600 cases is sequenced. In Brazil it is less than 1 in 10 million cases,” Dr. Voloch added.
So far, no decisive factors for public health, such as greater virulence or greater transmissibility, have been identified in any of the strains established in Brazil. The million-dollar question is whether the emergence of new strains could have an impact on the effectiveness of vaccines being administered today.
“In one way or another, the vaccine is our best bet ever, even if in the future we identify escapist mutants and have to modify it,” Dr. Motta said. “It is what we do annually with influenza.”
Dr. Voloch, Dr. Motta, and Dr. Souza disclosed no relevant financial relationships.
A version of this article first appeared on the Portuguese edition of Medscape.com.
Hypertrophic Lichen Planus–like Eruption Following Pembrolizumab
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
Practice Points
- With an increased use of immunotherapy medications such as pembrolizumab for various cancers, it is important that dermatologists are aware of the wide range of adverse cutaneous reactions that can occur, including lichenoid reactions.
- Hypertrophic lichen planus should be considered in the differential diagnosis of patients with cutaneous lesions in addition to nail findings developing after starting programmed cell death protein 1 inhibitor therapy.
President Biden to up states’ vaccine supplies, targets more doses
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Seven days into his presidency, Joe Biden announced that he is taking new steps to speed vaccines to Americans.
The president said he would increase the supply of vaccines to states from 8.6 million doses to 10 million doses per week, a 16% increase, for at least the next 3 weeks.
He said he was working to give states more advanced notice of their allotments so they could better plan their campaigns. He also said doses would be doled out based on population.
“We will both increase the supply and give our state and local partners more certainty about when doses will arrive,” he said Tuesday.
Finally, Mr. Biden announced that the United States would “soon be able to confirm” the purchase of 200 million more doses of the Pfizer and Moderna vaccines – 100 million of each – to effectively double the nation’s supply by “early summer.” That would increase the nation’s supply enough to fully vaccinate 300 million Americans by fall.
Mr. Biden said he was also working to shift the focus to getting more doses to economically disadvantaged communities and rural areas, which have fallen further behind as the vaccine rollout has faltered.
Even with these steps, Mr. Biden stressed that it would take months for vaccines to curb infections and deaths. He said, for the time being, masks, not vaccines, are the best way to save lives.
“The brutal truth is its going to take months before we get the majority of Americans vaccinated. Months,” he said, adding that wearing masks until at least April could save to save 50,000 lives.
“Let me be clear,” Mr. Biden said, “Things are going to get worse before they get better.
“We didn’t get into this mess overnight. It’s going to take months for us to turn things around. But let me be equally clear we’re going to get through this. We will defeat this pandemic,” he said.
A version of this article first appeared on WebMD.com.
Racial, social inequities persist in IBD
Although inflammatory bowel disease (IBD) affects primarily White patients, about one-quarter of cases are found in non-White racial and ethnic groups. Various factors have combined to lead to disparities in treatment and outcomes for non-Whites with IBD.
Ethnic and racial disparities, along with socioeconomic factors, were the subject of a presentation by Ruby Greywoode, MD, at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Historical and present-day realities of racial inequity and factors that contribute to socioeconomic status [include] educational and housing policies, employment practices, and generational wealth. Addressing health disparities requires acknowledging these systemic factors,” said Dr. Greywoode, who is with Montefiore Medical Center in New York.
An important concept in discussing health disparity is social determinants of health, which refers to nonbiological factors that affect health and health outcomes. These are “the conditions in which people live, work, learn, and play that affect their health and their quality of life,” said Dr. Greywoode.
Dr. Greywoode shared examples of social determinants that affect economic stability and financial worry. One study found that one in six IBD patients reported not taking their medications because of cost considerations. A survey of about 900 adults showed that 1 in 4 delayed medical care – half of those because of cost; patients who delayed care were 2.5 times more likely to report an IBD flare in the previous year.
Another important issue is food insecurity. Other presenters at the session emphasized the importance of high-quality nutrition in IBD, and Dr. Greywoode presented one survey showing that only 9% of patients who had both food security and social support reported cost-related medication nonadherence. Among those that had either food insecurity or poor social support, 12% reported cost-related medication nonadherence, but the proportion jumped to 57% among patients who had both food insecurity and lack of social support.
Session comoderator Kelly Issokson noted that socioeconomic factors often interfere with adoption of healthy diets. Whole foods and plant-based foods are expensive, and the financial pressures of the COVID-19 epidemic have made that worse. “Millions of people are slipping into poverty and food insecurity. This is one of the things she highlighted as factors in medication nonadherence,” said Ms. Issokson, who is the clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Greywoode also described studies that looked at race, socioeconomic status, and IBD outcomes. A review from 2013 showed disparities among Whites, African Americans, and Hispanics with respect to undergoing ulcerative colitis–related colectomy and Crohn’s disease–related bowel resection. Ulcerative colitis patients on Medicaid had 230% greater in-patient mortality, compared with patients with private insurance, even after adjustment for multiple confounders.
But inequities are not static. “Since this publication, we have numerous other studies drawing conclusions that sometimes agree with and sometimes conflict with it. My belief is that health disparities in IBD will continue to be an active area of research. We know that it takes vigilance to identify, track, and address any disparities when they do arise,” said Dr. Greywoode.
Dr. Greywoode also noted that phenotypic differences based on race and ethnicity influence disparities. She showed results from a meta-analysis that found a difference in the frequency of perianal Crohn’s disease by race and ethnicity; the highest frequency occurred in Black patients (31%), followed by Asians (22%), Whites (14%), and Hispanics (13%). Another study showed that African American patients with Crohn’s disease were more likely to develop a new abscess (adjusted odds ratio, 2.27; 95% confidence interval, 1.31-3.93) or anal fissure (aOR, 1.76; 95% CI, 1.01-3.07), and were also more likely to be initiated on an anti–tumor necrosis factor drug (aOR, 1.85; 95% CI, 1.09-3.14).
Those differences underscore the need to recognize that IBD is not just a disease for White patients. “As we move forward in IBD research, we recognize that individuals of European ancestry are not the only ones who have IBD. There is a growing diverse racial and ethnic population with IBD,” said Dr. Greywoode.
She noted that, in the United States, it is estimated that about one in four adult patients are non-Hispanic African American, Hispanic, Asians, or other ethnicities. Nevertheless, Whites are overrepresented among participants in IBD clinical trials. Some trials are composed of as much as 95% White patients, and sometimes race isn’t even listed. “It’s unclear if [race/ethnicity data are] not collected or not deemed important, but we know that what is not collected is not measured, and what is not measured can’t be evaluated, either to praise or constructively criticize,” said Dr. Greywoode.
Fortunately, there are efforts in place to improve representation in clinical trials. There has been a mandate for almost 3 decades that federally funded research must include racial and ethnic minorities who have been traditionally underrepresented. The Food and Drug Administration has also provided guidance to industry to improve diversity in clinical trial participation, and industry groups have developed strategies, including improved representation among investigators and related early-career development programs. At the community and independent health care practice levels, clinical trial networks encourage patient participation with regulatory and data management support to bolster practices with insufficient resources.
Underrepresentation in clinical trials resonated with comoderator Tina Aswani Omprakash, who is a patient advocate and a master’s in public health student at the Icahn School of Medicine at Mount Sinai, New York. She called for greater awareness among physicians that IBD can occur among people of all backgrounds. “[Providers] would look at me and [say]: ‘There’s no way that, as a South Asian woman, you have that kind of disease.’ There’s that lack of believability,” said Ms. Aswani Omprakash.
Greater recognition of the diversity of patients, as well as the phenotypic differences found among ethnicities, could also inform clinical trial participation and, ultimately, more personalized medicine. “We have to look at these things, observe how they’re affecting populations differently, so that we can have proper medication solutions,” said Ms. Aswani Omprakash.
Dr. Greywoode and Ms. Issokson have no relevant financial disclosures. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena.
The AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. The AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
Updated Feb. 17, 2021.
Although inflammatory bowel disease (IBD) affects primarily White patients, about one-quarter of cases are found in non-White racial and ethnic groups. Various factors have combined to lead to disparities in treatment and outcomes for non-Whites with IBD.
Ethnic and racial disparities, along with socioeconomic factors, were the subject of a presentation by Ruby Greywoode, MD, at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Historical and present-day realities of racial inequity and factors that contribute to socioeconomic status [include] educational and housing policies, employment practices, and generational wealth. Addressing health disparities requires acknowledging these systemic factors,” said Dr. Greywoode, who is with Montefiore Medical Center in New York.
An important concept in discussing health disparity is social determinants of health, which refers to nonbiological factors that affect health and health outcomes. These are “the conditions in which people live, work, learn, and play that affect their health and their quality of life,” said Dr. Greywoode.
Dr. Greywoode shared examples of social determinants that affect economic stability and financial worry. One study found that one in six IBD patients reported not taking their medications because of cost considerations. A survey of about 900 adults showed that 1 in 4 delayed medical care – half of those because of cost; patients who delayed care were 2.5 times more likely to report an IBD flare in the previous year.
Another important issue is food insecurity. Other presenters at the session emphasized the importance of high-quality nutrition in IBD, and Dr. Greywoode presented one survey showing that only 9% of patients who had both food security and social support reported cost-related medication nonadherence. Among those that had either food insecurity or poor social support, 12% reported cost-related medication nonadherence, but the proportion jumped to 57% among patients who had both food insecurity and lack of social support.
Session comoderator Kelly Issokson noted that socioeconomic factors often interfere with adoption of healthy diets. Whole foods and plant-based foods are expensive, and the financial pressures of the COVID-19 epidemic have made that worse. “Millions of people are slipping into poverty and food insecurity. This is one of the things she highlighted as factors in medication nonadherence,” said Ms. Issokson, who is the clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Greywoode also described studies that looked at race, socioeconomic status, and IBD outcomes. A review from 2013 showed disparities among Whites, African Americans, and Hispanics with respect to undergoing ulcerative colitis–related colectomy and Crohn’s disease–related bowel resection. Ulcerative colitis patients on Medicaid had 230% greater in-patient mortality, compared with patients with private insurance, even after adjustment for multiple confounders.
But inequities are not static. “Since this publication, we have numerous other studies drawing conclusions that sometimes agree with and sometimes conflict with it. My belief is that health disparities in IBD will continue to be an active area of research. We know that it takes vigilance to identify, track, and address any disparities when they do arise,” said Dr. Greywoode.
Dr. Greywoode also noted that phenotypic differences based on race and ethnicity influence disparities. She showed results from a meta-analysis that found a difference in the frequency of perianal Crohn’s disease by race and ethnicity; the highest frequency occurred in Black patients (31%), followed by Asians (22%), Whites (14%), and Hispanics (13%). Another study showed that African American patients with Crohn’s disease were more likely to develop a new abscess (adjusted odds ratio, 2.27; 95% confidence interval, 1.31-3.93) or anal fissure (aOR, 1.76; 95% CI, 1.01-3.07), and were also more likely to be initiated on an anti–tumor necrosis factor drug (aOR, 1.85; 95% CI, 1.09-3.14).
Those differences underscore the need to recognize that IBD is not just a disease for White patients. “As we move forward in IBD research, we recognize that individuals of European ancestry are not the only ones who have IBD. There is a growing diverse racial and ethnic population with IBD,” said Dr. Greywoode.
She noted that, in the United States, it is estimated that about one in four adult patients are non-Hispanic African American, Hispanic, Asians, or other ethnicities. Nevertheless, Whites are overrepresented among participants in IBD clinical trials. Some trials are composed of as much as 95% White patients, and sometimes race isn’t even listed. “It’s unclear if [race/ethnicity data are] not collected or not deemed important, but we know that what is not collected is not measured, and what is not measured can’t be evaluated, either to praise or constructively criticize,” said Dr. Greywoode.
Fortunately, there are efforts in place to improve representation in clinical trials. There has been a mandate for almost 3 decades that federally funded research must include racial and ethnic minorities who have been traditionally underrepresented. The Food and Drug Administration has also provided guidance to industry to improve diversity in clinical trial participation, and industry groups have developed strategies, including improved representation among investigators and related early-career development programs. At the community and independent health care practice levels, clinical trial networks encourage patient participation with regulatory and data management support to bolster practices with insufficient resources.
Underrepresentation in clinical trials resonated with comoderator Tina Aswani Omprakash, who is a patient advocate and a master’s in public health student at the Icahn School of Medicine at Mount Sinai, New York. She called for greater awareness among physicians that IBD can occur among people of all backgrounds. “[Providers] would look at me and [say]: ‘There’s no way that, as a South Asian woman, you have that kind of disease.’ There’s that lack of believability,” said Ms. Aswani Omprakash.
Greater recognition of the diversity of patients, as well as the phenotypic differences found among ethnicities, could also inform clinical trial participation and, ultimately, more personalized medicine. “We have to look at these things, observe how they’re affecting populations differently, so that we can have proper medication solutions,” said Ms. Aswani Omprakash.
Dr. Greywoode and Ms. Issokson have no relevant financial disclosures. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena.
The AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. The AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
Updated Feb. 17, 2021.
Although inflammatory bowel disease (IBD) affects primarily White patients, about one-quarter of cases are found in non-White racial and ethnic groups. Various factors have combined to lead to disparities in treatment and outcomes for non-Whites with IBD.
Ethnic and racial disparities, along with socioeconomic factors, were the subject of a presentation by Ruby Greywoode, MD, at the annual congress of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
“Historical and present-day realities of racial inequity and factors that contribute to socioeconomic status [include] educational and housing policies, employment practices, and generational wealth. Addressing health disparities requires acknowledging these systemic factors,” said Dr. Greywoode, who is with Montefiore Medical Center in New York.
An important concept in discussing health disparity is social determinants of health, which refers to nonbiological factors that affect health and health outcomes. These are “the conditions in which people live, work, learn, and play that affect their health and their quality of life,” said Dr. Greywoode.
Dr. Greywoode shared examples of social determinants that affect economic stability and financial worry. One study found that one in six IBD patients reported not taking their medications because of cost considerations. A survey of about 900 adults showed that 1 in 4 delayed medical care – half of those because of cost; patients who delayed care were 2.5 times more likely to report an IBD flare in the previous year.
Another important issue is food insecurity. Other presenters at the session emphasized the importance of high-quality nutrition in IBD, and Dr. Greywoode presented one survey showing that only 9% of patients who had both food security and social support reported cost-related medication nonadherence. Among those that had either food insecurity or poor social support, 12% reported cost-related medication nonadherence, but the proportion jumped to 57% among patients who had both food insecurity and lack of social support.
Session comoderator Kelly Issokson noted that socioeconomic factors often interfere with adoption of healthy diets. Whole foods and plant-based foods are expensive, and the financial pressures of the COVID-19 epidemic have made that worse. “Millions of people are slipping into poverty and food insecurity. This is one of the things she highlighted as factors in medication nonadherence,” said Ms. Issokson, who is the clinical nutritional coordinator at the digestive disease clinic at Cedars-Sinai Medical Center in Los Angeles.
Dr. Greywoode also described studies that looked at race, socioeconomic status, and IBD outcomes. A review from 2013 showed disparities among Whites, African Americans, and Hispanics with respect to undergoing ulcerative colitis–related colectomy and Crohn’s disease–related bowel resection. Ulcerative colitis patients on Medicaid had 230% greater in-patient mortality, compared with patients with private insurance, even after adjustment for multiple confounders.
But inequities are not static. “Since this publication, we have numerous other studies drawing conclusions that sometimes agree with and sometimes conflict with it. My belief is that health disparities in IBD will continue to be an active area of research. We know that it takes vigilance to identify, track, and address any disparities when they do arise,” said Dr. Greywoode.
Dr. Greywoode also noted that phenotypic differences based on race and ethnicity influence disparities. She showed results from a meta-analysis that found a difference in the frequency of perianal Crohn’s disease by race and ethnicity; the highest frequency occurred in Black patients (31%), followed by Asians (22%), Whites (14%), and Hispanics (13%). Another study showed that African American patients with Crohn’s disease were more likely to develop a new abscess (adjusted odds ratio, 2.27; 95% confidence interval, 1.31-3.93) or anal fissure (aOR, 1.76; 95% CI, 1.01-3.07), and were also more likely to be initiated on an anti–tumor necrosis factor drug (aOR, 1.85; 95% CI, 1.09-3.14).
Those differences underscore the need to recognize that IBD is not just a disease for White patients. “As we move forward in IBD research, we recognize that individuals of European ancestry are not the only ones who have IBD. There is a growing diverse racial and ethnic population with IBD,” said Dr. Greywoode.
She noted that, in the United States, it is estimated that about one in four adult patients are non-Hispanic African American, Hispanic, Asians, or other ethnicities. Nevertheless, Whites are overrepresented among participants in IBD clinical trials. Some trials are composed of as much as 95% White patients, and sometimes race isn’t even listed. “It’s unclear if [race/ethnicity data are] not collected or not deemed important, but we know that what is not collected is not measured, and what is not measured can’t be evaluated, either to praise or constructively criticize,” said Dr. Greywoode.
Fortunately, there are efforts in place to improve representation in clinical trials. There has been a mandate for almost 3 decades that federally funded research must include racial and ethnic minorities who have been traditionally underrepresented. The Food and Drug Administration has also provided guidance to industry to improve diversity in clinical trial participation, and industry groups have developed strategies, including improved representation among investigators and related early-career development programs. At the community and independent health care practice levels, clinical trial networks encourage patient participation with regulatory and data management support to bolster practices with insufficient resources.
Underrepresentation in clinical trials resonated with comoderator Tina Aswani Omprakash, who is a patient advocate and a master’s in public health student at the Icahn School of Medicine at Mount Sinai, New York. She called for greater awareness among physicians that IBD can occur among people of all backgrounds. “[Providers] would look at me and [say]: ‘There’s no way that, as a South Asian woman, you have that kind of disease.’ There’s that lack of believability,” said Ms. Aswani Omprakash.
Greater recognition of the diversity of patients, as well as the phenotypic differences found among ethnicities, could also inform clinical trial participation and, ultimately, more personalized medicine. “We have to look at these things, observe how they’re affecting populations differently, so that we can have proper medication solutions,” said Ms. Aswani Omprakash.
Dr. Greywoode and Ms. Issokson have no relevant financial disclosures. Ms. Aswani Omprakash has consulted for Genentech, AbbVie, Janssen, and Arena.
The AGA applauds researchers who are working to raise our awareness of health disparities in digestive diseases. The AGA is committed to addressing this important societal issue head on. Learn more about AGA’s commitment through the AGA Equity Project.
Updated Feb. 17, 2021.
FROM THE CROHN’S & COLITIS CONGRESS