User login
Long-Term Oxygen Therapy and Risk of Fire-Related Events
Chronic obstructive pulmonary disease (COPD) has been the third leading cause of death in the US since 2008.1 Current management of COPD includes smoking cessation, adequate nutrition, medication therapy, pulmonary rehabilitation, and vaccines.2 Outside of pharmacologic management, oxygen therapy has become a staple treatment of chronic hypoxemic respiratory failure due to COPD. Landmark trials, including the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) study, demonstrated improved survival in patients with COPD and hypoxemia, particularly if these patients received oxygen for 18 hours per day.3,4 NOTT prospectively evaluated 203 patients at 6 centers who were randomly allocated to either continuous oxygen therapy or 12-hour nocturnal oxygen therapy. The overall mortality in the nocturnal oxygen therapy group was 1.94 times that in the continuous oxygen therapy group (P = .01).3 The MRC study included 87 patients who were randomized to oxygen therapy or no oxygen; risk of death was 12% per year in the treated group vs 29% per year in the control group (P = .04).4 The effectiveness of long-term oxygen therapy (LTOT) in active smokers continues to be a source of debate; although 50% of patients in the NOTT trial were smokers, there was no subgroup analysis of whether smoking status had an impact on survival in those on continuous oxygen therapy.
Although many therapies are available for the treatment of COPD, the most effective treatment to prevent the progression of COPD is smoking cessation. Resources like smoking cessation programs, nicotine patches, and medications, such as bupropion and varenicline, are available to aid smoking cessation.5 However, many patients are unable to quit tobacco use despite their best efforts using available resources, and they continue to smoke even with progressive COPD. Long-time smokers also are likely to continue smoking while on LTOT, which increases their risk for fire-related injury.6-8
Traditional indications are being scrutinized after the LTOT trial found no benefit with respect to time to death or first hospitalization among patients with stable COPD and resting or exercise-induced moderate desaturation.9
Although oxygen accelerates combustion and is a potential fire hazard, LTOT has been prescribed even to active smokers as the 2 landmark trials did not exclude patients who were active smokers from receiving oxygen therapy.3,4 Therefore, LTOT has traditionally been prescribed to veterans who are actively smoking, despite the fire hazard. Attempts at mitigating hazards related to oxygen therapy in active smokers include counseling extensively about safety measures (which includes avoiding open flames such as candles, large fires, or sparks when on LTOT and providing Home Safety Agreements—a written contract between prescriber and patient wherein the patient agrees to abide by the terms of the US Department of Veterans Affairs (VA) to mitigate hazards related to LTOT in order to receive LTOT (eAppendix
Methods
With this practice in mind, we conducted an institutional review board approved retrospective chart review of all veterans with diagnosis of COPD within the Central Texas Veterans Health Care System (CTVHCS) who were prescribed new LTOT between October 1, 2010 and September 30, 2015. Given the retrospective nature of the chart review, patient consent was not obtained. Inclusion criteria were veterans aged > 18 years who had a confirmed diagnosis of COPD by spirometry and who met criteria for either continuous or ambulation- only oxygen therapy.
Criteria for exclusion included patients with hypoxemia not solely attributable to COPD or due to diseases other than COPD. We reviewed encounters in these patients’ charts, including follow-up in the clinic of the providers prescribing oxygen, to assess for fire-related incidents, defined as events wherein fire was visualized by the patient or by individuals living with the patient and with report provided to medical equipment company providing oxygen; the patient did not have to seek medical care to qualify for fire-related incident. Of the 158 patients who met the criteria for inclusion in the study, 152 were male.
Statistics
Bayesian logistic regression was used to model the outcome variable fire-related incident with the predictors smoking status, age, race, depression, PTSD, and type of oxygen used. Mental health disorders have significant effect on substance use disorders, such as alcohol use. Depression and PTSD were more common mental health diagnoses found in our patient population. Additionally, due to the small sample size, these psychiatric diagnoses were chosen to evaluate the impact of mental health disorders on firerelated events.
Although the sample size of events was small, weakly informative normal priors (0, 2.5) were used to shrink parameter estimates toward 0 and minimize overfitting. Weakly informative normal priors have also been suggested to deal with the problem of quasi-complete separation, where in our case, both smoking and no-PTSD perfectly predicted the 9 fire-related incidents.10 All input variables were centered and scaled as recommended. 9 The model fit well as assessed by posterior predictive checks, and Rhat was 1.00 for all parameters, indicating that all chains converged. Analysis was completed in R version 3.5.1 using the ‘brms’ package for Bayesian modeling.11
Results
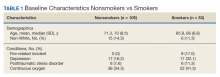
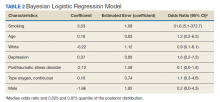
The mean age for the 158 included patients was 71.3 years in nonsmokers and 65.9 years in smokers. Fifty-three of the included patients were active smokers when LTOT was initiated. Nine veterans had fire-related incidents during the study period. All 9 patients were actively smoking (about 17%) at the time of the fire incidents. There were no deaths, and 5 patients required hospitalization due to facial burns resulting from the fire-related incidents. Our study focused on 5 baseline characteristics in our population (Table 1). After gathering data, our group inferred that these characteristics had a potential relationship to fire-related incidents compared with other variables that were studied. Future studies could look at other patient characteristics that may be linked to fire-related incidents in patients on LTOT. For example, not having PTSD also perfectly predicts fire-related incidents in our data (ie, none of the participants who had fire-related incidents had PTSD). Although this finding was not within the 95% confidence interval (CI) in the model, it does show that care must be taken when interpreting effects from small samples (Table 2). The modelestimated odds of a fire-related incident occurring in a smoker were 31.6 (5.1-372.7) times more likely than were the odds of a firerelated incident occurring in a nonsmoker, holding all other predictors at their reference level; 95% CI for the odds ratios for all other predictors in the model included a value of 1.
Discussion
This study showed evidence of increased odds of fire-related events in actively smoking patients receiving LTOT compared with patients who do not actively smoke while attempting to adjust for potential confounders. Of the 9 patients who had fire events, 5 required hospitalization for burns.
A similar retrospective cohort study by Sharma and colleagues in 2015 demonstrated an increased risk of burn-related injury when on LTOT but reiterated that the benefit of oxygen outweighs the risk of burn-related injury in patients requiring oxygen therapy.12 Interestingly, Sharma and colleagues were unable to identify smoking status for the patients studied but further identified factors associated with burn injury to include male sex, low socioeconomic status, oxygen therapy use, and ≥ 3 comorbidities. The study’s conclusion recommended continued education by health care professionals (HCPs) to their patients on LTOT regarding potential for burn injury. In the same vein, the VA National Center for Ethics in Health Care noted that “clinicians should familiarize themselves with the risks and benefits of LTOT; should inform their patients of the risks and benefits without exaggerating the risk associated with smoking; avoid undue coercion inherent in the clinician’s ability to withdraw LTOT; reduce the risk to the greatest degree possible; and consider termination of LTOT in very extreme cases and in consultation with a multidisciplinary committee.”13
This statement is in contrast to the guidelines and policies of other countries, such as Sweden, where smoking is a direct contraindication for prescription of oxygen therapy, or in Australia and New Zealand, where the Thoracic Society of Australia and New Zealand oxygen therapy guidelines recommend against prescription of LTOT, citing “increased fire risk and the probability that the poorer prognosis conferred by smoking will offset treatment benefit.”6,14
The prevalence of oxygen therapy introduces the potential for fire-related incidents with subsequent injury requiring medical care. There are few studies regarding home oxygen fire in the US due to the lack of a uniform reporting system. One study by Wendling and Pelletier analyzed deaths in Maine, Massachusetts, New Hampshire, and Oklahoma between 2000 and 2007 and found 38 deaths directly attributable to home oxygen fires as a result of smoking.15 Further, the Consumer Product Safety Commission’s National Electronic Injury Surveillance System between 2003 and 2006 attributed 1,190 thermal burns related to home oxygen fires; the majority of which were ignited by tobacco smoking.15 The Swedish National Register of Respiratory Failure (Swedevox) published prospective population-based, consecutive cohort study that collected data over 17 years and evaluated the risk of fire-related incident in those on LTOT. Of the 12,497 patients sampled, 17 had a burn injury and 2 patients died. The low incidence of burn injury on LTOT was attributed to the strict guidelines instituted in Sweden for doctors to avoid prescribing LTOT to actively smoking patients.6 A follow-up study by Tanash and colleagues compared the risk of burn injury in each country, respectively. The results found an increased number of burn injuries in those on oxygen therapy in Denmark, a country with fewer restrictions on smoking compared with those of Sweden.7 Similarly, our results showed that the rate of fire and burn injuries was exclusively among veterans who were active smokers. All patients who were prescribed oxygen therapy at CTVHCS received counseling and signed Home Safety Agreements. Despite following the recommendations set forth by the VA on counseling, extensive harm reduction techniques, and close follow-up, we found there was still a high incidence of fires in veterans with COPD on LTOT who continue to smoke.
The findings from our study concur with those previously published regarding the risk of home oxygen fire and concomitant smoking, supporting the idea for more regulated and concrete guidelines for prescribing LTOT to those requiring it.8
Limitations
The major limitation was the small sample size of our study. Another limitation was that our study population is predominantly male as is common in veteran cohorts. In fiscal year 2016, the veteran population of Texas was 1,434,361 males and 168,967 females.16 According to Franklin and colleagues, HCPs noticed an increase use of long-term oxygen among women compared with that of men.17
Conclusions
Our study showed an increased odds of firerelated incidents of patients while on LTOT, strengthening the argument that even with extensive education, those who smoke and are on LTOT continue to put themselves at risk of a fire-related incident. This finding stresses the importance of continuing patient education on the importance of smoking cessation prior to administration of LTOT or avoiding fire hazards while on LTOT. Further research into LTOT and fire hazards could help in implementing a more structured approval process for patients who want to obtain LTOT. We propose further studies evaluating risk factors for the incidence of fire events among patients prescribed LTOT. A growing and aging population with a need for LTOT necessitates examination of oxygen safe prescribing.
1. Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States 2000-2014. https:// www.cdc.gov/nchs/data/databriefs/db256.pdf. Published September 2016. Accessed September 10, 2020.
2. Itoh M, Tsuji T, Nemoto K, Nakamura H, Aoshiba K. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316-1335. doi:10.3390/nu5041316
3. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391. doi:10.7326/0003-4819-93-3-391
4. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681-686. doi:10.1016/S0140-6736(81)91970-X
5. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233-239. doi:10.7326/0003-4819-142-4 -200502150-00005
6. Tanash HA, Huss F, Ekström M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479-2484. doi:10.2147/COPD.S91508
7. Tanash HA, Ringbaek T, Huss F, Ekström M. Burn injury during long-term oxygen therapy in Denmark and Sweden: the potential role of smoking. Int J Chronic Obstruct Pulmon Dis. 2017;12:193-197. doi:10.2147/COPD.S119949
8. Kassis SA, Savetamal A, Assi R, et al. Characteristics of patients with injury secondary to smoking on home oxygen therapy transferred intubated to a burn center. J Am Coll Surg. 2014;218(6):1182-1186. doi:10.1016/j.jamcollsurg.2013.12.055
9. Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, et al. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617-1627. doi:10.1056/NEJMoa1604344
10. Ghosh J, Li Y, Mitra R. On the use of Cauchy prior distributions for Bayesian logistic regression. Bayesian Anal. 2018;13(2):359-383. doi:10.1214/17-ba1051
11. Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Software. 2017;80(1). doi:10.18637/jss.v080.i01
12. Sharma G, Meena R, Goodwin JS, Zhang W, Kuo Y-F, Duarte AG. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90(4):492-499. doi:10.1016/j.mayocp.2014.12.024
13. US Department of Veterans Affairs, National Ethics Committee. Ethical considerations that arise when a home care patient on long term oxygen therapy continues to smoke. http://vaww.ethics.va.gov/docs/necrpts/NEC_Report_20100301_Smoking_while_on_LTOT.pdf. Published March 2010. [Nonpublic, source not verified.]
14. McDonald C F, Whyte K, Jenkins S, Serginson J. Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76-78. doi:10.1111/resp.12678
15. Centers for Disease Control and Prevention (CDC). Fatal fires associated with smoking during long-term oxygen therapy--Maine, Massachusetts, New Hampshire, and Oklahoma, 2000-2007. MMWR Morb Mortal Wkly Rep. 2008;57(31):852-854.
16. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Population tables: the state, age/gender, 2016. https://www.va.gov/vetdata/Veteran_ Population.asp. Updated August 5, 2020. Accessed September 11, 2020.
17. Franklin KA, Gustafson T, Ranstam J, Ström K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease--gender differences. Respir Med. 2007;101(7):1506-1511. doi:10.1016/j.rmed.2007.01.009
Chronic obstructive pulmonary disease (COPD) has been the third leading cause of death in the US since 2008.1 Current management of COPD includes smoking cessation, adequate nutrition, medication therapy, pulmonary rehabilitation, and vaccines.2 Outside of pharmacologic management, oxygen therapy has become a staple treatment of chronic hypoxemic respiratory failure due to COPD. Landmark trials, including the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) study, demonstrated improved survival in patients with COPD and hypoxemia, particularly if these patients received oxygen for 18 hours per day.3,4 NOTT prospectively evaluated 203 patients at 6 centers who were randomly allocated to either continuous oxygen therapy or 12-hour nocturnal oxygen therapy. The overall mortality in the nocturnal oxygen therapy group was 1.94 times that in the continuous oxygen therapy group (P = .01).3 The MRC study included 87 patients who were randomized to oxygen therapy or no oxygen; risk of death was 12% per year in the treated group vs 29% per year in the control group (P = .04).4 The effectiveness of long-term oxygen therapy (LTOT) in active smokers continues to be a source of debate; although 50% of patients in the NOTT trial were smokers, there was no subgroup analysis of whether smoking status had an impact on survival in those on continuous oxygen therapy.
Although many therapies are available for the treatment of COPD, the most effective treatment to prevent the progression of COPD is smoking cessation. Resources like smoking cessation programs, nicotine patches, and medications, such as bupropion and varenicline, are available to aid smoking cessation.5 However, many patients are unable to quit tobacco use despite their best efforts using available resources, and they continue to smoke even with progressive COPD. Long-time smokers also are likely to continue smoking while on LTOT, which increases their risk for fire-related injury.6-8
Traditional indications are being scrutinized after the LTOT trial found no benefit with respect to time to death or first hospitalization among patients with stable COPD and resting or exercise-induced moderate desaturation.9
Although oxygen accelerates combustion and is a potential fire hazard, LTOT has been prescribed even to active smokers as the 2 landmark trials did not exclude patients who were active smokers from receiving oxygen therapy.3,4 Therefore, LTOT has traditionally been prescribed to veterans who are actively smoking, despite the fire hazard. Attempts at mitigating hazards related to oxygen therapy in active smokers include counseling extensively about safety measures (which includes avoiding open flames such as candles, large fires, or sparks when on LTOT and providing Home Safety Agreements—a written contract between prescriber and patient wherein the patient agrees to abide by the terms of the US Department of Veterans Affairs (VA) to mitigate hazards related to LTOT in order to receive LTOT (eAppendix
Methods
With this practice in mind, we conducted an institutional review board approved retrospective chart review of all veterans with diagnosis of COPD within the Central Texas Veterans Health Care System (CTVHCS) who were prescribed new LTOT between October 1, 2010 and September 30, 2015. Given the retrospective nature of the chart review, patient consent was not obtained. Inclusion criteria were veterans aged > 18 years who had a confirmed diagnosis of COPD by spirometry and who met criteria for either continuous or ambulation- only oxygen therapy.
Criteria for exclusion included patients with hypoxemia not solely attributable to COPD or due to diseases other than COPD. We reviewed encounters in these patients’ charts, including follow-up in the clinic of the providers prescribing oxygen, to assess for fire-related incidents, defined as events wherein fire was visualized by the patient or by individuals living with the patient and with report provided to medical equipment company providing oxygen; the patient did not have to seek medical care to qualify for fire-related incident. Of the 158 patients who met the criteria for inclusion in the study, 152 were male.
Statistics
Bayesian logistic regression was used to model the outcome variable fire-related incident with the predictors smoking status, age, race, depression, PTSD, and type of oxygen used. Mental health disorders have significant effect on substance use disorders, such as alcohol use. Depression and PTSD were more common mental health diagnoses found in our patient population. Additionally, due to the small sample size, these psychiatric diagnoses were chosen to evaluate the impact of mental health disorders on firerelated events.
Although the sample size of events was small, weakly informative normal priors (0, 2.5) were used to shrink parameter estimates toward 0 and minimize overfitting. Weakly informative normal priors have also been suggested to deal with the problem of quasi-complete separation, where in our case, both smoking and no-PTSD perfectly predicted the 9 fire-related incidents.10 All input variables were centered and scaled as recommended. 9 The model fit well as assessed by posterior predictive checks, and Rhat was 1.00 for all parameters, indicating that all chains converged. Analysis was completed in R version 3.5.1 using the ‘brms’ package for Bayesian modeling.11
Results
The mean age for the 158 included patients was 71.3 years in nonsmokers and 65.9 years in smokers. Fifty-three of the included patients were active smokers when LTOT was initiated. Nine veterans had fire-related incidents during the study period. All 9 patients were actively smoking (about 17%) at the time of the fire incidents. There were no deaths, and 5 patients required hospitalization due to facial burns resulting from the fire-related incidents. Our study focused on 5 baseline characteristics in our population (Table 1). After gathering data, our group inferred that these characteristics had a potential relationship to fire-related incidents compared with other variables that were studied. Future studies could look at other patient characteristics that may be linked to fire-related incidents in patients on LTOT. For example, not having PTSD also perfectly predicts fire-related incidents in our data (ie, none of the participants who had fire-related incidents had PTSD). Although this finding was not within the 95% confidence interval (CI) in the model, it does show that care must be taken when interpreting effects from small samples (Table 2). The modelestimated odds of a fire-related incident occurring in a smoker were 31.6 (5.1-372.7) times more likely than were the odds of a firerelated incident occurring in a nonsmoker, holding all other predictors at their reference level; 95% CI for the odds ratios for all other predictors in the model included a value of 1.
Discussion
This study showed evidence of increased odds of fire-related events in actively smoking patients receiving LTOT compared with patients who do not actively smoke while attempting to adjust for potential confounders. Of the 9 patients who had fire events, 5 required hospitalization for burns.
A similar retrospective cohort study by Sharma and colleagues in 2015 demonstrated an increased risk of burn-related injury when on LTOT but reiterated that the benefit of oxygen outweighs the risk of burn-related injury in patients requiring oxygen therapy.12 Interestingly, Sharma and colleagues were unable to identify smoking status for the patients studied but further identified factors associated with burn injury to include male sex, low socioeconomic status, oxygen therapy use, and ≥ 3 comorbidities. The study’s conclusion recommended continued education by health care professionals (HCPs) to their patients on LTOT regarding potential for burn injury. In the same vein, the VA National Center for Ethics in Health Care noted that “clinicians should familiarize themselves with the risks and benefits of LTOT; should inform their patients of the risks and benefits without exaggerating the risk associated with smoking; avoid undue coercion inherent in the clinician’s ability to withdraw LTOT; reduce the risk to the greatest degree possible; and consider termination of LTOT in very extreme cases and in consultation with a multidisciplinary committee.”13
This statement is in contrast to the guidelines and policies of other countries, such as Sweden, where smoking is a direct contraindication for prescription of oxygen therapy, or in Australia and New Zealand, where the Thoracic Society of Australia and New Zealand oxygen therapy guidelines recommend against prescription of LTOT, citing “increased fire risk and the probability that the poorer prognosis conferred by smoking will offset treatment benefit.”6,14
The prevalence of oxygen therapy introduces the potential for fire-related incidents with subsequent injury requiring medical care. There are few studies regarding home oxygen fire in the US due to the lack of a uniform reporting system. One study by Wendling and Pelletier analyzed deaths in Maine, Massachusetts, New Hampshire, and Oklahoma between 2000 and 2007 and found 38 deaths directly attributable to home oxygen fires as a result of smoking.15 Further, the Consumer Product Safety Commission’s National Electronic Injury Surveillance System between 2003 and 2006 attributed 1,190 thermal burns related to home oxygen fires; the majority of which were ignited by tobacco smoking.15 The Swedish National Register of Respiratory Failure (Swedevox) published prospective population-based, consecutive cohort study that collected data over 17 years and evaluated the risk of fire-related incident in those on LTOT. Of the 12,497 patients sampled, 17 had a burn injury and 2 patients died. The low incidence of burn injury on LTOT was attributed to the strict guidelines instituted in Sweden for doctors to avoid prescribing LTOT to actively smoking patients.6 A follow-up study by Tanash and colleagues compared the risk of burn injury in each country, respectively. The results found an increased number of burn injuries in those on oxygen therapy in Denmark, a country with fewer restrictions on smoking compared with those of Sweden.7 Similarly, our results showed that the rate of fire and burn injuries was exclusively among veterans who were active smokers. All patients who were prescribed oxygen therapy at CTVHCS received counseling and signed Home Safety Agreements. Despite following the recommendations set forth by the VA on counseling, extensive harm reduction techniques, and close follow-up, we found there was still a high incidence of fires in veterans with COPD on LTOT who continue to smoke.
The findings from our study concur with those previously published regarding the risk of home oxygen fire and concomitant smoking, supporting the idea for more regulated and concrete guidelines for prescribing LTOT to those requiring it.8
Limitations
The major limitation was the small sample size of our study. Another limitation was that our study population is predominantly male as is common in veteran cohorts. In fiscal year 2016, the veteran population of Texas was 1,434,361 males and 168,967 females.16 According to Franklin and colleagues, HCPs noticed an increase use of long-term oxygen among women compared with that of men.17
Conclusions
Our study showed an increased odds of firerelated incidents of patients while on LTOT, strengthening the argument that even with extensive education, those who smoke and are on LTOT continue to put themselves at risk of a fire-related incident. This finding stresses the importance of continuing patient education on the importance of smoking cessation prior to administration of LTOT or avoiding fire hazards while on LTOT. Further research into LTOT and fire hazards could help in implementing a more structured approval process for patients who want to obtain LTOT. We propose further studies evaluating risk factors for the incidence of fire events among patients prescribed LTOT. A growing and aging population with a need for LTOT necessitates examination of oxygen safe prescribing.
Chronic obstructive pulmonary disease (COPD) has been the third leading cause of death in the US since 2008.1 Current management of COPD includes smoking cessation, adequate nutrition, medication therapy, pulmonary rehabilitation, and vaccines.2 Outside of pharmacologic management, oxygen therapy has become a staple treatment of chronic hypoxemic respiratory failure due to COPD. Landmark trials, including the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) study, demonstrated improved survival in patients with COPD and hypoxemia, particularly if these patients received oxygen for 18 hours per day.3,4 NOTT prospectively evaluated 203 patients at 6 centers who were randomly allocated to either continuous oxygen therapy or 12-hour nocturnal oxygen therapy. The overall mortality in the nocturnal oxygen therapy group was 1.94 times that in the continuous oxygen therapy group (P = .01).3 The MRC study included 87 patients who were randomized to oxygen therapy or no oxygen; risk of death was 12% per year in the treated group vs 29% per year in the control group (P = .04).4 The effectiveness of long-term oxygen therapy (LTOT) in active smokers continues to be a source of debate; although 50% of patients in the NOTT trial were smokers, there was no subgroup analysis of whether smoking status had an impact on survival in those on continuous oxygen therapy.
Although many therapies are available for the treatment of COPD, the most effective treatment to prevent the progression of COPD is smoking cessation. Resources like smoking cessation programs, nicotine patches, and medications, such as bupropion and varenicline, are available to aid smoking cessation.5 However, many patients are unable to quit tobacco use despite their best efforts using available resources, and they continue to smoke even with progressive COPD. Long-time smokers also are likely to continue smoking while on LTOT, which increases their risk for fire-related injury.6-8
Traditional indications are being scrutinized after the LTOT trial found no benefit with respect to time to death or first hospitalization among patients with stable COPD and resting or exercise-induced moderate desaturation.9
Although oxygen accelerates combustion and is a potential fire hazard, LTOT has been prescribed even to active smokers as the 2 landmark trials did not exclude patients who were active smokers from receiving oxygen therapy.3,4 Therefore, LTOT has traditionally been prescribed to veterans who are actively smoking, despite the fire hazard. Attempts at mitigating hazards related to oxygen therapy in active smokers include counseling extensively about safety measures (which includes avoiding open flames such as candles, large fires, or sparks when on LTOT and providing Home Safety Agreements—a written contract between prescriber and patient wherein the patient agrees to abide by the terms of the US Department of Veterans Affairs (VA) to mitigate hazards related to LTOT in order to receive LTOT (eAppendix
Methods
With this practice in mind, we conducted an institutional review board approved retrospective chart review of all veterans with diagnosis of COPD within the Central Texas Veterans Health Care System (CTVHCS) who were prescribed new LTOT between October 1, 2010 and September 30, 2015. Given the retrospective nature of the chart review, patient consent was not obtained. Inclusion criteria were veterans aged > 18 years who had a confirmed diagnosis of COPD by spirometry and who met criteria for either continuous or ambulation- only oxygen therapy.
Criteria for exclusion included patients with hypoxemia not solely attributable to COPD or due to diseases other than COPD. We reviewed encounters in these patients’ charts, including follow-up in the clinic of the providers prescribing oxygen, to assess for fire-related incidents, defined as events wherein fire was visualized by the patient or by individuals living with the patient and with report provided to medical equipment company providing oxygen; the patient did not have to seek medical care to qualify for fire-related incident. Of the 158 patients who met the criteria for inclusion in the study, 152 were male.
Statistics
Bayesian logistic regression was used to model the outcome variable fire-related incident with the predictors smoking status, age, race, depression, PTSD, and type of oxygen used. Mental health disorders have significant effect on substance use disorders, such as alcohol use. Depression and PTSD were more common mental health diagnoses found in our patient population. Additionally, due to the small sample size, these psychiatric diagnoses were chosen to evaluate the impact of mental health disorders on firerelated events.
Although the sample size of events was small, weakly informative normal priors (0, 2.5) were used to shrink parameter estimates toward 0 and minimize overfitting. Weakly informative normal priors have also been suggested to deal with the problem of quasi-complete separation, where in our case, both smoking and no-PTSD perfectly predicted the 9 fire-related incidents.10 All input variables were centered and scaled as recommended. 9 The model fit well as assessed by posterior predictive checks, and Rhat was 1.00 for all parameters, indicating that all chains converged. Analysis was completed in R version 3.5.1 using the ‘brms’ package for Bayesian modeling.11
Results
The mean age for the 158 included patients was 71.3 years in nonsmokers and 65.9 years in smokers. Fifty-three of the included patients were active smokers when LTOT was initiated. Nine veterans had fire-related incidents during the study period. All 9 patients were actively smoking (about 17%) at the time of the fire incidents. There were no deaths, and 5 patients required hospitalization due to facial burns resulting from the fire-related incidents. Our study focused on 5 baseline characteristics in our population (Table 1). After gathering data, our group inferred that these characteristics had a potential relationship to fire-related incidents compared with other variables that were studied. Future studies could look at other patient characteristics that may be linked to fire-related incidents in patients on LTOT. For example, not having PTSD also perfectly predicts fire-related incidents in our data (ie, none of the participants who had fire-related incidents had PTSD). Although this finding was not within the 95% confidence interval (CI) in the model, it does show that care must be taken when interpreting effects from small samples (Table 2). The modelestimated odds of a fire-related incident occurring in a smoker were 31.6 (5.1-372.7) times more likely than were the odds of a firerelated incident occurring in a nonsmoker, holding all other predictors at their reference level; 95% CI for the odds ratios for all other predictors in the model included a value of 1.
Discussion
This study showed evidence of increased odds of fire-related events in actively smoking patients receiving LTOT compared with patients who do not actively smoke while attempting to adjust for potential confounders. Of the 9 patients who had fire events, 5 required hospitalization for burns.
A similar retrospective cohort study by Sharma and colleagues in 2015 demonstrated an increased risk of burn-related injury when on LTOT but reiterated that the benefit of oxygen outweighs the risk of burn-related injury in patients requiring oxygen therapy.12 Interestingly, Sharma and colleagues were unable to identify smoking status for the patients studied but further identified factors associated with burn injury to include male sex, low socioeconomic status, oxygen therapy use, and ≥ 3 comorbidities. The study’s conclusion recommended continued education by health care professionals (HCPs) to their patients on LTOT regarding potential for burn injury. In the same vein, the VA National Center for Ethics in Health Care noted that “clinicians should familiarize themselves with the risks and benefits of LTOT; should inform their patients of the risks and benefits without exaggerating the risk associated with smoking; avoid undue coercion inherent in the clinician’s ability to withdraw LTOT; reduce the risk to the greatest degree possible; and consider termination of LTOT in very extreme cases and in consultation with a multidisciplinary committee.”13
This statement is in contrast to the guidelines and policies of other countries, such as Sweden, where smoking is a direct contraindication for prescription of oxygen therapy, or in Australia and New Zealand, where the Thoracic Society of Australia and New Zealand oxygen therapy guidelines recommend against prescription of LTOT, citing “increased fire risk and the probability that the poorer prognosis conferred by smoking will offset treatment benefit.”6,14
The prevalence of oxygen therapy introduces the potential for fire-related incidents with subsequent injury requiring medical care. There are few studies regarding home oxygen fire in the US due to the lack of a uniform reporting system. One study by Wendling and Pelletier analyzed deaths in Maine, Massachusetts, New Hampshire, and Oklahoma between 2000 and 2007 and found 38 deaths directly attributable to home oxygen fires as a result of smoking.15 Further, the Consumer Product Safety Commission’s National Electronic Injury Surveillance System between 2003 and 2006 attributed 1,190 thermal burns related to home oxygen fires; the majority of which were ignited by tobacco smoking.15 The Swedish National Register of Respiratory Failure (Swedevox) published prospective population-based, consecutive cohort study that collected data over 17 years and evaluated the risk of fire-related incident in those on LTOT. Of the 12,497 patients sampled, 17 had a burn injury and 2 patients died. The low incidence of burn injury on LTOT was attributed to the strict guidelines instituted in Sweden for doctors to avoid prescribing LTOT to actively smoking patients.6 A follow-up study by Tanash and colleagues compared the risk of burn injury in each country, respectively. The results found an increased number of burn injuries in those on oxygen therapy in Denmark, a country with fewer restrictions on smoking compared with those of Sweden.7 Similarly, our results showed that the rate of fire and burn injuries was exclusively among veterans who were active smokers. All patients who were prescribed oxygen therapy at CTVHCS received counseling and signed Home Safety Agreements. Despite following the recommendations set forth by the VA on counseling, extensive harm reduction techniques, and close follow-up, we found there was still a high incidence of fires in veterans with COPD on LTOT who continue to smoke.
The findings from our study concur with those previously published regarding the risk of home oxygen fire and concomitant smoking, supporting the idea for more regulated and concrete guidelines for prescribing LTOT to those requiring it.8
Limitations
The major limitation was the small sample size of our study. Another limitation was that our study population is predominantly male as is common in veteran cohorts. In fiscal year 2016, the veteran population of Texas was 1,434,361 males and 168,967 females.16 According to Franklin and colleagues, HCPs noticed an increase use of long-term oxygen among women compared with that of men.17
Conclusions
Our study showed an increased odds of firerelated incidents of patients while on LTOT, strengthening the argument that even with extensive education, those who smoke and are on LTOT continue to put themselves at risk of a fire-related incident. This finding stresses the importance of continuing patient education on the importance of smoking cessation prior to administration of LTOT or avoiding fire hazards while on LTOT. Further research into LTOT and fire hazards could help in implementing a more structured approval process for patients who want to obtain LTOT. We propose further studies evaluating risk factors for the incidence of fire events among patients prescribed LTOT. A growing and aging population with a need for LTOT necessitates examination of oxygen safe prescribing.
1. Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States 2000-2014. https:// www.cdc.gov/nchs/data/databriefs/db256.pdf. Published September 2016. Accessed September 10, 2020.
2. Itoh M, Tsuji T, Nemoto K, Nakamura H, Aoshiba K. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316-1335. doi:10.3390/nu5041316
3. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391. doi:10.7326/0003-4819-93-3-391
4. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681-686. doi:10.1016/S0140-6736(81)91970-X
5. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233-239. doi:10.7326/0003-4819-142-4 -200502150-00005
6. Tanash HA, Huss F, Ekström M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479-2484. doi:10.2147/COPD.S91508
7. Tanash HA, Ringbaek T, Huss F, Ekström M. Burn injury during long-term oxygen therapy in Denmark and Sweden: the potential role of smoking. Int J Chronic Obstruct Pulmon Dis. 2017;12:193-197. doi:10.2147/COPD.S119949
8. Kassis SA, Savetamal A, Assi R, et al. Characteristics of patients with injury secondary to smoking on home oxygen therapy transferred intubated to a burn center. J Am Coll Surg. 2014;218(6):1182-1186. doi:10.1016/j.jamcollsurg.2013.12.055
9. Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, et al. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617-1627. doi:10.1056/NEJMoa1604344
10. Ghosh J, Li Y, Mitra R. On the use of Cauchy prior distributions for Bayesian logistic regression. Bayesian Anal. 2018;13(2):359-383. doi:10.1214/17-ba1051
11. Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Software. 2017;80(1). doi:10.18637/jss.v080.i01
12. Sharma G, Meena R, Goodwin JS, Zhang W, Kuo Y-F, Duarte AG. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90(4):492-499. doi:10.1016/j.mayocp.2014.12.024
13. US Department of Veterans Affairs, National Ethics Committee. Ethical considerations that arise when a home care patient on long term oxygen therapy continues to smoke. http://vaww.ethics.va.gov/docs/necrpts/NEC_Report_20100301_Smoking_while_on_LTOT.pdf. Published March 2010. [Nonpublic, source not verified.]
14. McDonald C F, Whyte K, Jenkins S, Serginson J. Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76-78. doi:10.1111/resp.12678
15. Centers for Disease Control and Prevention (CDC). Fatal fires associated with smoking during long-term oxygen therapy--Maine, Massachusetts, New Hampshire, and Oklahoma, 2000-2007. MMWR Morb Mortal Wkly Rep. 2008;57(31):852-854.
16. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Population tables: the state, age/gender, 2016. https://www.va.gov/vetdata/Veteran_ Population.asp. Updated August 5, 2020. Accessed September 11, 2020.
17. Franklin KA, Gustafson T, Ranstam J, Ström K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease--gender differences. Respir Med. 2007;101(7):1506-1511. doi:10.1016/j.rmed.2007.01.009
1. Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States 2000-2014. https:// www.cdc.gov/nchs/data/databriefs/db256.pdf. Published September 2016. Accessed September 10, 2020.
2. Itoh M, Tsuji T, Nemoto K, Nakamura H, Aoshiba K. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316-1335. doi:10.3390/nu5041316
3. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391. doi:10.7326/0003-4819-93-3-391
4. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681-686. doi:10.1016/S0140-6736(81)91970-X
5. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233-239. doi:10.7326/0003-4819-142-4 -200502150-00005
6. Tanash HA, Huss F, Ekström M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479-2484. doi:10.2147/COPD.S91508
7. Tanash HA, Ringbaek T, Huss F, Ekström M. Burn injury during long-term oxygen therapy in Denmark and Sweden: the potential role of smoking. Int J Chronic Obstruct Pulmon Dis. 2017;12:193-197. doi:10.2147/COPD.S119949
8. Kassis SA, Savetamal A, Assi R, et al. Characteristics of patients with injury secondary to smoking on home oxygen therapy transferred intubated to a burn center. J Am Coll Surg. 2014;218(6):1182-1186. doi:10.1016/j.jamcollsurg.2013.12.055
9. Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, et al. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617-1627. doi:10.1056/NEJMoa1604344
10. Ghosh J, Li Y, Mitra R. On the use of Cauchy prior distributions for Bayesian logistic regression. Bayesian Anal. 2018;13(2):359-383. doi:10.1214/17-ba1051
11. Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Software. 2017;80(1). doi:10.18637/jss.v080.i01
12. Sharma G, Meena R, Goodwin JS, Zhang W, Kuo Y-F, Duarte AG. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90(4):492-499. doi:10.1016/j.mayocp.2014.12.024
13. US Department of Veterans Affairs, National Ethics Committee. Ethical considerations that arise when a home care patient on long term oxygen therapy continues to smoke. http://vaww.ethics.va.gov/docs/necrpts/NEC_Report_20100301_Smoking_while_on_LTOT.pdf. Published March 2010. [Nonpublic, source not verified.]
14. McDonald C F, Whyte K, Jenkins S, Serginson J. Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76-78. doi:10.1111/resp.12678
15. Centers for Disease Control and Prevention (CDC). Fatal fires associated with smoking during long-term oxygen therapy--Maine, Massachusetts, New Hampshire, and Oklahoma, 2000-2007. MMWR Morb Mortal Wkly Rep. 2008;57(31):852-854.
16. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Population tables: the state, age/gender, 2016. https://www.va.gov/vetdata/Veteran_ Population.asp. Updated August 5, 2020. Accessed September 11, 2020.
17. Franklin KA, Gustafson T, Ranstam J, Ström K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease--gender differences. Respir Med. 2007;101(7):1506-1511. doi:10.1016/j.rmed.2007.01.009
Restarting breast cancer screening after disruption not so simple
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
according to a modeling study reported at the 12th European Breast Cancer Conference.
Fallout of the pandemic has included reductions in cancer screening and diagnosis, said study investigator Lindy M. Kregting, a PhD student in the department of public health at Erasmus Medical Center, University Medical Center Rotterdam (the Netherlands).
In the Netherlands, new breast cancer diagnoses fell dramatically from historical levels starting in February. The number in April was less than half of that expected.
Ms. Kregting and colleagues used modeling to assess the impact of four strategies for restarting breast cancer screening in the Netherlands. The strategies differed regarding the population affected, the duration of the effects, and changes in stopping age. The usual situation, without any disruption, served as the comparator.
Results showed wide variation across strategies with respect to the increase in screening capacity needed during the latter half of this year – from 0% to 100% – and the excess breast cancer mortality occurring during 2020-2030 – from as many as 181 excess breast cancer deaths to as few as 14.
“The effects of the disruption are dependent on the chosen restart strategy,” Ms. Kregting summarized. “It would be preferred to immediately catch up because this minimizes the impact, but it also requires a very high capacity, so it may not always be possible. A proper alternative would be to increase the stopping age, so no screens are omitted, because this requires a rather normal capacity, and it will result in only small effects on incidence and mortality.”
As screening programs restart in some countries, there are still a lot of unknowns that could affect outcomes, including how many women will attend given that some may stay away out of fear, Ms. Kregting cautioned.
“We plan to do further model calculations when we know exactly what has happened. ... For now, we just assumed some reasonable disruption periods, and we assumed that capacity would be back to the original, before COVID-19, but I think we can say this is probably not the case,” she added.
Study details
Ms. Kregting and colleagues used Dutch breast cancer screening program parameters (biennial digital mammography for women aged 50-75 years) and a microsimulation screening analysis model to simulate four strategies for restarting breast cancer screening after a 6-month disruption:
- “Everyone delay,” a strategy in which all screening continues in the order planned with no change in the stopping age of 75 years (so that one in four women ultimately miss a screening during their lifetime)
- “First rounds no delay,” in which there is a delay in screening except for women having their first screening
- “Continue after stopping age,” in which there is a delay in screening but temporary increasing of the stopping age (to 76.5 years) to ensure all women get their final screen
- “Catch-up after stop,” in which capacity is increased to ensure full catch-up, with all delayed screens caught up in a 6-month period (the second half of 2020).
Results showed that 5,872 women would be screened in the latter half of 2020 if screening proceeded as usual without disruption. The necessary capacity was essentially the same with all of the restarting strategies, except for the catch-up-after-stop strategy, which would require a doubling of that number.
The temporal pattern of breast cancer incidence varied according to restart strategy early on, but incidence essentially returned to that expected with undisrupted screening by 2025 for all four strategies, with some small fluctuations thereafter.
The impact on breast cancer mortality differed considerably long term. It increased slightly and transiently above the expected level with the catch-up-after-stop strategy, but there were sizable, long-lasting increases with the other strategies, with excess deaths still seen in 2060 for the everyone-delay strategy.
In absolute terms, the excess number of breast cancer deaths during 2020-2030, compared with undisrupted screening, was 181 with the everyone-delay strategy, 155 with the first-rounds-no-delay strategy, 145 with the continue-after-stopping-age strategy, and just 14 with the catch-up-after-stop strategy. Ms. Kregting declined to provide numbers for other countries, given that the model is based on the Dutch population and screening program.
Results in context
“The unprecedented burden of COVID-19 on health systems worldwide has important implications for cancer care,” said invited discussant Alessandra Gennari, MD, PhD, of the University of Eastern Piedmont and Maggiore della Carità Hospital, both in Novara, Italy.
“There is a delay in diagnosis due to the fact that screening programs and diagnostic programs have been decreased or suspended in many Western countries where this is standard of care. Patients also are more reluctant to present to health care services, delaying their diagnosis,” Dr. Gennari said.
Findings of this new study add to those of similar studies undertaken in Italy (published in In Vivo) and the United Kingdom (published in The Lancet Oncology) showing the likely marked toll of the pandemic on cancer diagnosis and mortality, Dr. Gennari noted. Taken together, the findings underscore the urgent need for policy interventions to mitigate this impact.
“These interventions should focus on increasing routine diagnostic capacity, through which up to 40% of patients with cancer are diagnosed,” Dr. Gennari recommended. “Public health messaging is needed that accurately conveys the risk of severe illness from COVID-19 versus the risks of not seeking health care advice if patients are symptomatic. Finally, there is a need for provision of evidence-based data on which clinicians can adequately base their decision on how to manage the risks of cancer patients and the risks and benefits of procedures during the pandemic.”
The current study did not have any specific funding, and Ms. Kregting disclosed no conflicts of interest. Dr. Gennari disclosed relationships with Roche, Eisai, Lilly, AstraZeneca, Daiichi Sankyo, Merck, Novartis, and Pfizer.
SOURCE: Kregting L et al. EBCC-12 Virtual Conference, Abstract 24.
FROM EBCC-12 VIRTUAL CONFERENCE
Chronic, preventive care fell as telemedicine soared during COVID-19
As the COVID-19 pandemic drove down the number of primary care visits and altered the method – moving many to telehealth appointments instead of in-person visits – the content of those appointments also changed, researchers reported in JAMA Network Open.
For the study, G. Caleb Alexander, MD, from the Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, and colleagues analyzed data from the IQVIA National Disease and Therapeutic Index, a nationally representative audit of outpatient care in the United States, from the first quarter of 2018 through the second quarter of 2020.
Most primary care visits in 2018 and 2019 were office based, the authors noted. In the second quarter (Q2, April-May) of 2020, as the COVID-19 pandemic spread across the country, the total number of primary care encounters decreased by 21.4%, and the number of office visits dropped by 50.2%, compared with the average of visits during Q2 in 2018 and 2019.
At the same time, telemedicine visits increased from just 1.1% of total visits in Q2 of 2018 and 2019 to 4.1% of visits in the first quarter (January through March) of 2020 and to 35.3% of visits in Q2 of 2020.
The authors also found that the use of telemedicine in the first half of 2020 varied by geographical region and was not associated with the regional COVID-19 burden. In the Pacific region (Washington, Oregon, and California), 26.8% of encounters were virtual. By contrast, the proportion of telemedicine encounters accounted for only 15.1% of visits in the East North Central states (Wisconsin, Michigan, Illinois, Indiana, and Ohio).
Adults between the ages of 19 and 55 years were more likely to attend telemedicine visits than were those younger or older. Additionally, adults who were commercially insured were more likely to adopt telemedicine versus those with public or no insurance. The study did not find substantial differences in telemedicine use by payer type, nor evidence of a racial disparity between Black and White people in their use of telemedicine.
Drop-off in preventive and chronic care
During the second quarter of this year, the authors reported, the number of visits that included blood pressure assessments dropped by 50.1% and the number of visits in which cholesterol levels were assessed fell by 36.9%, compared with the Q2 of 2018 and 2019.
Visits in which providers prescribed new antihypertensive or cholesterol-lowering medications decreased by 26% in Q2 of 2020 versus the same periods in the previous 2 years. The number of visits in which such prescriptions were renewed dropped by 8.9%.
New treatments also decreased significantly in Q2 of 2020 for patients with chronic conditions, including hypertension, diabetes, high cholesterol, asthma, depression, and insomnia.
When the authors compared the content of telemedicine versus in-person visits in Q2 of 2020, they found a substantial difference. Blood pressure was assessed in 69.7% of office visits, compared with 9.6% of telemedicine. Similarly, cholesterol levels were evaluated in 21.6% of office visits versus 13.5% of telemedicine encounters. New medications were ordered in similar proportions of office-based and telemedicine visits.
The authors concluded that “the COVID-19 pandemic has been associated with changes in the structure of primary care delivery, with the content of telemedicine visits differing from that of office-based encounters.”
While limited in scope, the authors noted, their study is one of the first to evaluate the changes in the content of primary care visits during the pandemic. They attributed the decline in evaluations of cardiovascular risk factors such as blood pressure and cholesterol to “fewer total visits and less frequent assessments during telemedicine encounters.”
While pointing to the inherent limitations of telemedicine, the study did not mention the availability of digital home blood pressure cuffs or home cholesterol test kits. Both kinds of devices are available at consumer-friendly price points and can help people track their indicators, but they’re not considered a substitute for sphygmomanometers used in offices or conventional lab tests. It’s not known how many consumers with cardiovascular risk factors have this kind of home monitoring equipment or how many doctors look at this kind of data.
Dr. Alexander reported serving as a paid adviser to IQVIA; that he is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and that he is a member of OptumRx’s National P&T Committee. One coauthor reported serving as an unpaid adviser to IQVIA and receiving personal fees from the states of California, Washington, and Alaska outside the submitted work. No other disclosures were reported.
A version of this article originally appeared on Medscape.com.
As the COVID-19 pandemic drove down the number of primary care visits and altered the method – moving many to telehealth appointments instead of in-person visits – the content of those appointments also changed, researchers reported in JAMA Network Open.
For the study, G. Caleb Alexander, MD, from the Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, and colleagues analyzed data from the IQVIA National Disease and Therapeutic Index, a nationally representative audit of outpatient care in the United States, from the first quarter of 2018 through the second quarter of 2020.
Most primary care visits in 2018 and 2019 were office based, the authors noted. In the second quarter (Q2, April-May) of 2020, as the COVID-19 pandemic spread across the country, the total number of primary care encounters decreased by 21.4%, and the number of office visits dropped by 50.2%, compared with the average of visits during Q2 in 2018 and 2019.
At the same time, telemedicine visits increased from just 1.1% of total visits in Q2 of 2018 and 2019 to 4.1% of visits in the first quarter (January through March) of 2020 and to 35.3% of visits in Q2 of 2020.
The authors also found that the use of telemedicine in the first half of 2020 varied by geographical region and was not associated with the regional COVID-19 burden. In the Pacific region (Washington, Oregon, and California), 26.8% of encounters were virtual. By contrast, the proportion of telemedicine encounters accounted for only 15.1% of visits in the East North Central states (Wisconsin, Michigan, Illinois, Indiana, and Ohio).
Adults between the ages of 19 and 55 years were more likely to attend telemedicine visits than were those younger or older. Additionally, adults who were commercially insured were more likely to adopt telemedicine versus those with public or no insurance. The study did not find substantial differences in telemedicine use by payer type, nor evidence of a racial disparity between Black and White people in their use of telemedicine.
Drop-off in preventive and chronic care
During the second quarter of this year, the authors reported, the number of visits that included blood pressure assessments dropped by 50.1% and the number of visits in which cholesterol levels were assessed fell by 36.9%, compared with the Q2 of 2018 and 2019.
Visits in which providers prescribed new antihypertensive or cholesterol-lowering medications decreased by 26% in Q2 of 2020 versus the same periods in the previous 2 years. The number of visits in which such prescriptions were renewed dropped by 8.9%.
New treatments also decreased significantly in Q2 of 2020 for patients with chronic conditions, including hypertension, diabetes, high cholesterol, asthma, depression, and insomnia.
When the authors compared the content of telemedicine versus in-person visits in Q2 of 2020, they found a substantial difference. Blood pressure was assessed in 69.7% of office visits, compared with 9.6% of telemedicine. Similarly, cholesterol levels were evaluated in 21.6% of office visits versus 13.5% of telemedicine encounters. New medications were ordered in similar proportions of office-based and telemedicine visits.
The authors concluded that “the COVID-19 pandemic has been associated with changes in the structure of primary care delivery, with the content of telemedicine visits differing from that of office-based encounters.”
While limited in scope, the authors noted, their study is one of the first to evaluate the changes in the content of primary care visits during the pandemic. They attributed the decline in evaluations of cardiovascular risk factors such as blood pressure and cholesterol to “fewer total visits and less frequent assessments during telemedicine encounters.”
While pointing to the inherent limitations of telemedicine, the study did not mention the availability of digital home blood pressure cuffs or home cholesterol test kits. Both kinds of devices are available at consumer-friendly price points and can help people track their indicators, but they’re not considered a substitute for sphygmomanometers used in offices or conventional lab tests. It’s not known how many consumers with cardiovascular risk factors have this kind of home monitoring equipment or how many doctors look at this kind of data.
Dr. Alexander reported serving as a paid adviser to IQVIA; that he is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and that he is a member of OptumRx’s National P&T Committee. One coauthor reported serving as an unpaid adviser to IQVIA and receiving personal fees from the states of California, Washington, and Alaska outside the submitted work. No other disclosures were reported.
A version of this article originally appeared on Medscape.com.
As the COVID-19 pandemic drove down the number of primary care visits and altered the method – moving many to telehealth appointments instead of in-person visits – the content of those appointments also changed, researchers reported in JAMA Network Open.
For the study, G. Caleb Alexander, MD, from the Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, and colleagues analyzed data from the IQVIA National Disease and Therapeutic Index, a nationally representative audit of outpatient care in the United States, from the first quarter of 2018 through the second quarter of 2020.
Most primary care visits in 2018 and 2019 were office based, the authors noted. In the second quarter (Q2, April-May) of 2020, as the COVID-19 pandemic spread across the country, the total number of primary care encounters decreased by 21.4%, and the number of office visits dropped by 50.2%, compared with the average of visits during Q2 in 2018 and 2019.
At the same time, telemedicine visits increased from just 1.1% of total visits in Q2 of 2018 and 2019 to 4.1% of visits in the first quarter (January through March) of 2020 and to 35.3% of visits in Q2 of 2020.
The authors also found that the use of telemedicine in the first half of 2020 varied by geographical region and was not associated with the regional COVID-19 burden. In the Pacific region (Washington, Oregon, and California), 26.8% of encounters were virtual. By contrast, the proportion of telemedicine encounters accounted for only 15.1% of visits in the East North Central states (Wisconsin, Michigan, Illinois, Indiana, and Ohio).
Adults between the ages of 19 and 55 years were more likely to attend telemedicine visits than were those younger or older. Additionally, adults who were commercially insured were more likely to adopt telemedicine versus those with public or no insurance. The study did not find substantial differences in telemedicine use by payer type, nor evidence of a racial disparity between Black and White people in their use of telemedicine.
Drop-off in preventive and chronic care
During the second quarter of this year, the authors reported, the number of visits that included blood pressure assessments dropped by 50.1% and the number of visits in which cholesterol levels were assessed fell by 36.9%, compared with the Q2 of 2018 and 2019.
Visits in which providers prescribed new antihypertensive or cholesterol-lowering medications decreased by 26% in Q2 of 2020 versus the same periods in the previous 2 years. The number of visits in which such prescriptions were renewed dropped by 8.9%.
New treatments also decreased significantly in Q2 of 2020 for patients with chronic conditions, including hypertension, diabetes, high cholesterol, asthma, depression, and insomnia.
When the authors compared the content of telemedicine versus in-person visits in Q2 of 2020, they found a substantial difference. Blood pressure was assessed in 69.7% of office visits, compared with 9.6% of telemedicine. Similarly, cholesterol levels were evaluated in 21.6% of office visits versus 13.5% of telemedicine encounters. New medications were ordered in similar proportions of office-based and telemedicine visits.
The authors concluded that “the COVID-19 pandemic has been associated with changes in the structure of primary care delivery, with the content of telemedicine visits differing from that of office-based encounters.”
While limited in scope, the authors noted, their study is one of the first to evaluate the changes in the content of primary care visits during the pandemic. They attributed the decline in evaluations of cardiovascular risk factors such as blood pressure and cholesterol to “fewer total visits and less frequent assessments during telemedicine encounters.”
While pointing to the inherent limitations of telemedicine, the study did not mention the availability of digital home blood pressure cuffs or home cholesterol test kits. Both kinds of devices are available at consumer-friendly price points and can help people track their indicators, but they’re not considered a substitute for sphygmomanometers used in offices or conventional lab tests. It’s not known how many consumers with cardiovascular risk factors have this kind of home monitoring equipment or how many doctors look at this kind of data.
Dr. Alexander reported serving as a paid adviser to IQVIA; that he is a cofounding principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and that he is a member of OptumRx’s National P&T Committee. One coauthor reported serving as an unpaid adviser to IQVIA and receiving personal fees from the states of California, Washington, and Alaska outside the submitted work. No other disclosures were reported.
A version of this article originally appeared on Medscape.com.
Meta-analysis: Acalabrutinib showed better PFS and OS than other frontline CLL therapies
Acalabrutinib, given alone or in combination with obinutuzumab, showed favorable progression-free survival (PFS) and overall survival (OS), compared with other frontline therapies for chronic lymphocytic leukemia (CLL) in fludarabine-ineligible patients, according to the results of a meta-analysis comparing clinical trial results.
Researchers conducted a systematic literature review for applicable CLL studies that examined frontline treatments in order to compare the results with data on acalabrutinib (monotherapy and in combination with obinutuzumab) from patients in the ELEVATE-TN study (NCT02475681), according to a report published in Clinical Therapeutics.
Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston, and colleagues performed a network meta-analysis (NMA) comparing acalabrutinib versus other standard frontline therapies for CLL in patients for whom fludarabine-based treatment is not appropriate.
“In the absence of head-to-head trial data, NMAs allow for simultaneous comparisons of a number of interventions with multiple comparators, by synthesizing direct and indirect evidence,” the authors stated.
Eight randomized controlled trials (RCTs) met the criteria for comparison.
The researchers constructed two evidence networks: Network A comprised solely RCTs that met the inclusion criteria, and Network B comprised seven RCTs and a published cross-trial comparison of ibrutinib from RESONATE-2 and chlorambucil plus obinutuzumab from iLLUMINATE. PFS and OS results were reported by using hazard ratios and 95% credible intervals.
Overall benefit
Both networks showed a significant improvement in PFS for acalabrutinib plus obinutuzumab over all comparators, according to the researchers. Both networks also showed a significant improvement in PFS for acalabrutinib monotherapy versus most comparators, with a significant difference to ibrutinib monotherapy found in Network A but not Network B.
Conversely, a significant difference in PFS was observed for acalabrutinib monotherapy versus venetoclax plus obinutuzumab in Network B but not Network A.
Overall survival hazard ratios ranged from 0.18 to 0.65 in favor of acalabrutinib-based treatment, but not all were significant. Acalabrutinib plus obinutuzumab ranked highest in terms of PFS and OS improvement, followed by acalabrutinib monotherapy.
“Although our NMAs provide useful insights into the relative efficacy of acalabrutinib, compared with other frontline treatments of CLL, the results cannot be considered confirmatory, and head-to-head randomized trials are needed, especially to compare the efficacy of acalabrutinib versus other targeted agents,” the researchers concluded.
AstraZeneca sponsored the study. The authors reported funding from AstraZeneca and numerous other pharmaceutical companies.
SOURCE: Davids MS et al. Clin Ther. 2020 Oct 5. doi: 10.1016/j.clinthera.2020.08.017.
Acalabrutinib, given alone or in combination with obinutuzumab, showed favorable progression-free survival (PFS) and overall survival (OS), compared with other frontline therapies for chronic lymphocytic leukemia (CLL) in fludarabine-ineligible patients, according to the results of a meta-analysis comparing clinical trial results.
Researchers conducted a systematic literature review for applicable CLL studies that examined frontline treatments in order to compare the results with data on acalabrutinib (monotherapy and in combination with obinutuzumab) from patients in the ELEVATE-TN study (NCT02475681), according to a report published in Clinical Therapeutics.
Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston, and colleagues performed a network meta-analysis (NMA) comparing acalabrutinib versus other standard frontline therapies for CLL in patients for whom fludarabine-based treatment is not appropriate.
“In the absence of head-to-head trial data, NMAs allow for simultaneous comparisons of a number of interventions with multiple comparators, by synthesizing direct and indirect evidence,” the authors stated.
Eight randomized controlled trials (RCTs) met the criteria for comparison.
The researchers constructed two evidence networks: Network A comprised solely RCTs that met the inclusion criteria, and Network B comprised seven RCTs and a published cross-trial comparison of ibrutinib from RESONATE-2 and chlorambucil plus obinutuzumab from iLLUMINATE. PFS and OS results were reported by using hazard ratios and 95% credible intervals.
Overall benefit
Both networks showed a significant improvement in PFS for acalabrutinib plus obinutuzumab over all comparators, according to the researchers. Both networks also showed a significant improvement in PFS for acalabrutinib monotherapy versus most comparators, with a significant difference to ibrutinib monotherapy found in Network A but not Network B.
Conversely, a significant difference in PFS was observed for acalabrutinib monotherapy versus venetoclax plus obinutuzumab in Network B but not Network A.
Overall survival hazard ratios ranged from 0.18 to 0.65 in favor of acalabrutinib-based treatment, but not all were significant. Acalabrutinib plus obinutuzumab ranked highest in terms of PFS and OS improvement, followed by acalabrutinib monotherapy.
“Although our NMAs provide useful insights into the relative efficacy of acalabrutinib, compared with other frontline treatments of CLL, the results cannot be considered confirmatory, and head-to-head randomized trials are needed, especially to compare the efficacy of acalabrutinib versus other targeted agents,” the researchers concluded.
AstraZeneca sponsored the study. The authors reported funding from AstraZeneca and numerous other pharmaceutical companies.
SOURCE: Davids MS et al. Clin Ther. 2020 Oct 5. doi: 10.1016/j.clinthera.2020.08.017.
Acalabrutinib, given alone or in combination with obinutuzumab, showed favorable progression-free survival (PFS) and overall survival (OS), compared with other frontline therapies for chronic lymphocytic leukemia (CLL) in fludarabine-ineligible patients, according to the results of a meta-analysis comparing clinical trial results.
Researchers conducted a systematic literature review for applicable CLL studies that examined frontline treatments in order to compare the results with data on acalabrutinib (monotherapy and in combination with obinutuzumab) from patients in the ELEVATE-TN study (NCT02475681), according to a report published in Clinical Therapeutics.
Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston, and colleagues performed a network meta-analysis (NMA) comparing acalabrutinib versus other standard frontline therapies for CLL in patients for whom fludarabine-based treatment is not appropriate.
“In the absence of head-to-head trial data, NMAs allow for simultaneous comparisons of a number of interventions with multiple comparators, by synthesizing direct and indirect evidence,” the authors stated.
Eight randomized controlled trials (RCTs) met the criteria for comparison.
The researchers constructed two evidence networks: Network A comprised solely RCTs that met the inclusion criteria, and Network B comprised seven RCTs and a published cross-trial comparison of ibrutinib from RESONATE-2 and chlorambucil plus obinutuzumab from iLLUMINATE. PFS and OS results were reported by using hazard ratios and 95% credible intervals.
Overall benefit
Both networks showed a significant improvement in PFS for acalabrutinib plus obinutuzumab over all comparators, according to the researchers. Both networks also showed a significant improvement in PFS for acalabrutinib monotherapy versus most comparators, with a significant difference to ibrutinib monotherapy found in Network A but not Network B.
Conversely, a significant difference in PFS was observed for acalabrutinib monotherapy versus venetoclax plus obinutuzumab in Network B but not Network A.
Overall survival hazard ratios ranged from 0.18 to 0.65 in favor of acalabrutinib-based treatment, but not all were significant. Acalabrutinib plus obinutuzumab ranked highest in terms of PFS and OS improvement, followed by acalabrutinib monotherapy.
“Although our NMAs provide useful insights into the relative efficacy of acalabrutinib, compared with other frontline treatments of CLL, the results cannot be considered confirmatory, and head-to-head randomized trials are needed, especially to compare the efficacy of acalabrutinib versus other targeted agents,” the researchers concluded.
AstraZeneca sponsored the study. The authors reported funding from AstraZeneca and numerous other pharmaceutical companies.
SOURCE: Davids MS et al. Clin Ther. 2020 Oct 5. doi: 10.1016/j.clinthera.2020.08.017.
FROM CLINICAL THERAPEUTICS
Atezolizumab strikes out in ovarian cancer
“Despite notable success with the incorporation of bevacizumab and atezolizumab in the treatment of other solid tumors ... the first such study in ovarian cancer did not meet its first primary endpoint” of extending PFS in the intention-to-treat population or in patients positive for programmed death-ligand 1 (PD-L1), said investigator Kathleen Moore, MD, of the University of Oklahoma in Oklahoma City.
Dr. Moore presented this study, IMagyn050/GOG 3015/ENGOT-OV39, at the European Society for Medical Oncology Virtual Congress 2020.
Explaining the negative results
The rationale for this study was good, said discussant Isabelle Ray-Coquard, MD, PhD, of the University Claude Bernard Lyon I in Villeurbanne, France, who was an investigator on the study but not an author on the meeting report.
Unfortunately, the study’s results were ultimately negative. A prior phase 3 trial of an immune checkpoint inhibitor in ovarian cancer also had negative results. In the JAVELIN OVARIAN 100 trial, adding avelumab to chemotherapy did not improve PFS, and the trial was stopped early for futility.
Dr. Ray-Coquard posed the question of whether checkpoint inhibitors are “dead” for epithelial ovarian cancer but said the answer isn’t clear.
There might be something unique about the tumor microenvironment that shields ovarian cancer from the immune system, or perhaps the PD-L1 pathway is the wrong target for immunotherapy.
It might also be that the first-line setting is the wrong place for checkpoint inhibitors, and they might work better in the second line, Dr. Ray-Coquard said.
Another possibility is that the delayed effect of immunotherapy means that overall survival – which isn’t yet mature for IMagyn050 – might be a better primary outcome than PFS.
Patient and treatment details
IMagyn050 enrolled 1,301 patients with newly diagnosed, stage III/IV epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Patients were randomized to atezolizumab (n = 651) or placebo (n = 650) in combination with bevacizumab, paclitaxel, and carboplatin every 3 weeks for six cycles. For cycles 7-22, patients received bevacizumab with placebo or atezolizumab.
Most patients had ovarian cancer (73% in the placebo arm and 75% in the atezolizumab arm), followed by fallopian tube cancer (17% and 15%, respectively) and primary peritoneal cancer (10% and 9%, respectively).
In both arms, 31% of patients had stage IV disease, and 60% were PD-L1 positive (at least 1% of tumor cells staining positive).
Treatment was given in the neoadjuvant setting for 25% of patients in both arms and after primary cytoreductive surgery for the remaining patients.
Efficacy and safety
In the intention-to-treat population, the median PFS was 19.5 months with atezolizumab and 18.4 months in the placebo arm. In PD-L1–positive patients, the median PFS was 20.8 months and 18.5 months, respectively.
The differences in PFS were not statistically significant or clinically meaningful, Dr. Ray-Coquard said.
She noted that results were not stratified by BRCA status, which has been linked to PFS in ovarian cancer, and a potential imbalance between the groups might have contributed to the negative results.
Overall survival data won’t be mature until 2023, but, in the first interim analysis, “there was no apparent difference in the curves,” Dr. Moore said.
In the intention-to-treat population, the median overall survival was not reached in either treatment arm. In the PD-L1–positive population, the median overall survival was 31.2 months in the placebo arm and was not reached in the atezolizumab arm.
Adverse events were consistent with the known safety profiles of atezolizumab and the other drugs, Dr. Moore said. There were more serious events and more events leading to discontinuation with atezolizumab.
Adverse events more common with atezolizumab included febrile neutropenia, pyrexia, thyroid abnormalities, and rash, including severe cutaneous reactions. Colitis, pancreatitis, and infusion-related reactions were relatively infrequent but more common with atezolizumab, Dr. Moore said.
What the future holds
Dr. Ray-Coquard said there are signals in IMagyn050 that warrant follow-up, including a trend toward improved PFS among women who had high-grade nonserous clear cell histology. In this group, the median PFS was 12.3 months in the placebo arm and 13.6 months in the atezolizumab arm (hazard ratio, 0.64).
Additionally, there was a significant PFS improvement in women with at least 5% of their tumor cells staining positive for PD-L1. The median PFS was 20.2 months in the placebo arm but was not reached in the atezolizumab arm (HR, 0.64; P = .0278).
Dr. Ray-Coquard said we need to know who these PD-L1–high patients are in terms of BRCA status, histology, and residual disease.
She went on to say that companies haven’t given up on checkpoint inhibitors for ovarian cancer. Phase 3 trials are testing the atezolizumab/bevacizumab/chemotherapy combination for late relapsed disease, atezolizumab with niraparib and chemotherapy for recurrent ovarian cancer, and nivolumab with rucaparib following response to chemotherapy.
The IMagyn050 study was funded by F. Hoffmann-La Roche, the company developing atezolizumab. Dr. Moore and Dr. Ray-Coquard disclosed relationships with Roche and many other companies.
SOURCE: Moore K et al. ESMO 2020, Abstract LBA31.
“Despite notable success with the incorporation of bevacizumab and atezolizumab in the treatment of other solid tumors ... the first such study in ovarian cancer did not meet its first primary endpoint” of extending PFS in the intention-to-treat population or in patients positive for programmed death-ligand 1 (PD-L1), said investigator Kathleen Moore, MD, of the University of Oklahoma in Oklahoma City.
Dr. Moore presented this study, IMagyn050/GOG 3015/ENGOT-OV39, at the European Society for Medical Oncology Virtual Congress 2020.
Explaining the negative results
The rationale for this study was good, said discussant Isabelle Ray-Coquard, MD, PhD, of the University Claude Bernard Lyon I in Villeurbanne, France, who was an investigator on the study but not an author on the meeting report.
Unfortunately, the study’s results were ultimately negative. A prior phase 3 trial of an immune checkpoint inhibitor in ovarian cancer also had negative results. In the JAVELIN OVARIAN 100 trial, adding avelumab to chemotherapy did not improve PFS, and the trial was stopped early for futility.
Dr. Ray-Coquard posed the question of whether checkpoint inhibitors are “dead” for epithelial ovarian cancer but said the answer isn’t clear.
There might be something unique about the tumor microenvironment that shields ovarian cancer from the immune system, or perhaps the PD-L1 pathway is the wrong target for immunotherapy.
It might also be that the first-line setting is the wrong place for checkpoint inhibitors, and they might work better in the second line, Dr. Ray-Coquard said.
Another possibility is that the delayed effect of immunotherapy means that overall survival – which isn’t yet mature for IMagyn050 – might be a better primary outcome than PFS.
Patient and treatment details
IMagyn050 enrolled 1,301 patients with newly diagnosed, stage III/IV epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Patients were randomized to atezolizumab (n = 651) or placebo (n = 650) in combination with bevacizumab, paclitaxel, and carboplatin every 3 weeks for six cycles. For cycles 7-22, patients received bevacizumab with placebo or atezolizumab.
Most patients had ovarian cancer (73% in the placebo arm and 75% in the atezolizumab arm), followed by fallopian tube cancer (17% and 15%, respectively) and primary peritoneal cancer (10% and 9%, respectively).
In both arms, 31% of patients had stage IV disease, and 60% were PD-L1 positive (at least 1% of tumor cells staining positive).
Treatment was given in the neoadjuvant setting for 25% of patients in both arms and after primary cytoreductive surgery for the remaining patients.
Efficacy and safety
In the intention-to-treat population, the median PFS was 19.5 months with atezolizumab and 18.4 months in the placebo arm. In PD-L1–positive patients, the median PFS was 20.8 months and 18.5 months, respectively.
The differences in PFS were not statistically significant or clinically meaningful, Dr. Ray-Coquard said.
She noted that results were not stratified by BRCA status, which has been linked to PFS in ovarian cancer, and a potential imbalance between the groups might have contributed to the negative results.
Overall survival data won’t be mature until 2023, but, in the first interim analysis, “there was no apparent difference in the curves,” Dr. Moore said.
In the intention-to-treat population, the median overall survival was not reached in either treatment arm. In the PD-L1–positive population, the median overall survival was 31.2 months in the placebo arm and was not reached in the atezolizumab arm.
Adverse events were consistent with the known safety profiles of atezolizumab and the other drugs, Dr. Moore said. There were more serious events and more events leading to discontinuation with atezolizumab.
Adverse events more common with atezolizumab included febrile neutropenia, pyrexia, thyroid abnormalities, and rash, including severe cutaneous reactions. Colitis, pancreatitis, and infusion-related reactions were relatively infrequent but more common with atezolizumab, Dr. Moore said.
What the future holds
Dr. Ray-Coquard said there are signals in IMagyn050 that warrant follow-up, including a trend toward improved PFS among women who had high-grade nonserous clear cell histology. In this group, the median PFS was 12.3 months in the placebo arm and 13.6 months in the atezolizumab arm (hazard ratio, 0.64).
Additionally, there was a significant PFS improvement in women with at least 5% of their tumor cells staining positive for PD-L1. The median PFS was 20.2 months in the placebo arm but was not reached in the atezolizumab arm (HR, 0.64; P = .0278).
Dr. Ray-Coquard said we need to know who these PD-L1–high patients are in terms of BRCA status, histology, and residual disease.
She went on to say that companies haven’t given up on checkpoint inhibitors for ovarian cancer. Phase 3 trials are testing the atezolizumab/bevacizumab/chemotherapy combination for late relapsed disease, atezolizumab with niraparib and chemotherapy for recurrent ovarian cancer, and nivolumab with rucaparib following response to chemotherapy.
The IMagyn050 study was funded by F. Hoffmann-La Roche, the company developing atezolizumab. Dr. Moore and Dr. Ray-Coquard disclosed relationships with Roche and many other companies.
SOURCE: Moore K et al. ESMO 2020, Abstract LBA31.
“Despite notable success with the incorporation of bevacizumab and atezolizumab in the treatment of other solid tumors ... the first such study in ovarian cancer did not meet its first primary endpoint” of extending PFS in the intention-to-treat population or in patients positive for programmed death-ligand 1 (PD-L1), said investigator Kathleen Moore, MD, of the University of Oklahoma in Oklahoma City.
Dr. Moore presented this study, IMagyn050/GOG 3015/ENGOT-OV39, at the European Society for Medical Oncology Virtual Congress 2020.
Explaining the negative results
The rationale for this study was good, said discussant Isabelle Ray-Coquard, MD, PhD, of the University Claude Bernard Lyon I in Villeurbanne, France, who was an investigator on the study but not an author on the meeting report.
Unfortunately, the study’s results were ultimately negative. A prior phase 3 trial of an immune checkpoint inhibitor in ovarian cancer also had negative results. In the JAVELIN OVARIAN 100 trial, adding avelumab to chemotherapy did not improve PFS, and the trial was stopped early for futility.
Dr. Ray-Coquard posed the question of whether checkpoint inhibitors are “dead” for epithelial ovarian cancer but said the answer isn’t clear.
There might be something unique about the tumor microenvironment that shields ovarian cancer from the immune system, or perhaps the PD-L1 pathway is the wrong target for immunotherapy.
It might also be that the first-line setting is the wrong place for checkpoint inhibitors, and they might work better in the second line, Dr. Ray-Coquard said.
Another possibility is that the delayed effect of immunotherapy means that overall survival – which isn’t yet mature for IMagyn050 – might be a better primary outcome than PFS.
Patient and treatment details
IMagyn050 enrolled 1,301 patients with newly diagnosed, stage III/IV epithelial ovarian, primary peritoneal, or fallopian tube cancer.
Patients were randomized to atezolizumab (n = 651) or placebo (n = 650) in combination with bevacizumab, paclitaxel, and carboplatin every 3 weeks for six cycles. For cycles 7-22, patients received bevacizumab with placebo or atezolizumab.
Most patients had ovarian cancer (73% in the placebo arm and 75% in the atezolizumab arm), followed by fallopian tube cancer (17% and 15%, respectively) and primary peritoneal cancer (10% and 9%, respectively).
In both arms, 31% of patients had stage IV disease, and 60% were PD-L1 positive (at least 1% of tumor cells staining positive).
Treatment was given in the neoadjuvant setting for 25% of patients in both arms and after primary cytoreductive surgery for the remaining patients.
Efficacy and safety
In the intention-to-treat population, the median PFS was 19.5 months with atezolizumab and 18.4 months in the placebo arm. In PD-L1–positive patients, the median PFS was 20.8 months and 18.5 months, respectively.
The differences in PFS were not statistically significant or clinically meaningful, Dr. Ray-Coquard said.
She noted that results were not stratified by BRCA status, which has been linked to PFS in ovarian cancer, and a potential imbalance between the groups might have contributed to the negative results.
Overall survival data won’t be mature until 2023, but, in the first interim analysis, “there was no apparent difference in the curves,” Dr. Moore said.
In the intention-to-treat population, the median overall survival was not reached in either treatment arm. In the PD-L1–positive population, the median overall survival was 31.2 months in the placebo arm and was not reached in the atezolizumab arm.
Adverse events were consistent with the known safety profiles of atezolizumab and the other drugs, Dr. Moore said. There were more serious events and more events leading to discontinuation with atezolizumab.
Adverse events more common with atezolizumab included febrile neutropenia, pyrexia, thyroid abnormalities, and rash, including severe cutaneous reactions. Colitis, pancreatitis, and infusion-related reactions were relatively infrequent but more common with atezolizumab, Dr. Moore said.
What the future holds
Dr. Ray-Coquard said there are signals in IMagyn050 that warrant follow-up, including a trend toward improved PFS among women who had high-grade nonserous clear cell histology. In this group, the median PFS was 12.3 months in the placebo arm and 13.6 months in the atezolizumab arm (hazard ratio, 0.64).
Additionally, there was a significant PFS improvement in women with at least 5% of their tumor cells staining positive for PD-L1. The median PFS was 20.2 months in the placebo arm but was not reached in the atezolizumab arm (HR, 0.64; P = .0278).
Dr. Ray-Coquard said we need to know who these PD-L1–high patients are in terms of BRCA status, histology, and residual disease.
She went on to say that companies haven’t given up on checkpoint inhibitors for ovarian cancer. Phase 3 trials are testing the atezolizumab/bevacizumab/chemotherapy combination for late relapsed disease, atezolizumab with niraparib and chemotherapy for recurrent ovarian cancer, and nivolumab with rucaparib following response to chemotherapy.
The IMagyn050 study was funded by F. Hoffmann-La Roche, the company developing atezolizumab. Dr. Moore and Dr. Ray-Coquard disclosed relationships with Roche and many other companies.
SOURCE: Moore K et al. ESMO 2020, Abstract LBA31.
FROM ESMO 2020
Deep brain stimulation ‘promising’ in severe schizophrenia
Deep brain stimulation (DBS) may be an effective option for patients with treatment-resistant schizophrenia (TRS), new research suggests. However, until further studies are conducted, the treatment should only be considered for the most severe cases.
The first clinical trial to assess DBS in this challenging patient population included eight patients initially randomly assigned to receive electrode placement in one of two locations in the brain. Once a clinical response was achieved and participants were stabilized, they were randomly assigned to a second crossover phase.
Preliminary findings from the first phase of the DBS-SCHIZO pilot study, which were reported in 2017, showed promising efficacy.
The newly released final results revealed an association between DBS and significant improvements in Positive and Negative Symptoms Scale (PANSS) scores, as well as reductions in doses of antipsychotic medication. Moreover, the effect reversed when the electrode was switched off.
“DBS may be a potential option for severe treatment-resistant schizophrenia patients,” lead investigator Iluminada Corripio, MD, PhD, department of psychiatry, Hospital de la Santa Creu i Sant Pau, Barcelona, said during her presentation at the virtual congress of the European College of Neuropsychopharmacology. The new data were updated results of a study published in EBioMedicine earlier this year.
Dr. Corripio underlined that it is important to balance the risks and benefits of the intervention. DBS is “not useful for all phenotypes,” and benefits have been seen in patients with hallucinations but not in those with a disorganized phenotype, she added.
High economic burden
Managing TRS is challenging and is associated with a high clinical and economic burden, Corripio noted. Relapse rates can reach 80%, increasing resource use by between 200% and 900%.
There is a strong rationale for studying the use of DBS in schizophrenia, because schizophrenia shares a neurologic basis with other neurologic and psychiatric disorders centered around the cortical-striatal-thalamic-cortical circuit, said Dr. Corripio.
The study included eight patients with a DSM-IV-TR diagnosis of schizophrenia whose conditions were resistant to at least two different atypical antipsychotics and who had not responded to clozapine monotherapy, combination therapy, or electroconvulsive therapy.
All were randomly assigned in a 1:1 ratio to DBS electrode implantation in one of two locations. Investigators chose the nucleus accumbens (NAcc), because recent studies have shown that DBS can increase dopamine levels there, and the subgenual anterior cingulate cortex (ACC). Deactivation failure in the ACC region has been observed in patients with schizophrenia and other mental illnesses.
Stimulation began 48-72 hours postoperatively with unilateral left stimulation at 2.5 volts. It was increased in 0.5 volt increments to a maximum of 7.5 volts. Patients who did not respond were switched to bilateral stimulation.
Follow-up was conducted every 2 weeks for up to 20 months. The study’s primary outcome was a symptomatic response, defined as an improvement of at least 25% on the PANSS.
Once that was achieved, patients could enter a second randomization phase in which they were assigned, in a 24-week, double-blind crossover design, to on- or off-treatment DBS arms such that patients received stimulation for 12 weeks before the device was turned off for 12 weeks, or vice versa.
Those who experienced relapse while off treatment were crossed over to the on-treatment arm; those who experienced relapsed while on treatment were withdrawn from the study. The patients’ average age was 42.5 years, and 50% were women. All were taking clozapine in combination with another antipsychotic.
Adverse events
Five patients experienced adverse events during the first phase, four of which were associated with rechargeable battery replacement. One experienced akathisia, another experienced behavioral changes, and a third experienced electrical disturbances.
A fourth patient experienced postsurgical hemorrhage of the right internal capsule on day 4, followed by encephalitis at week 8. He had a clinical improvement but experienced relapsed during follow-up.
The fifth patient accidentally switched off the device and withdrew from the study.
During the first randomization phase, DBS was associated with significant improvements on total, positive, and negative PANSS scores in comparison with the postoperative baseline measure in the seven remaining patients (P < .001).
When the team compared the baseline measure with the last observation, the improvement in PANSS scores remained significant for total scores (P = .007) and positive scores (P = .002), but not for negative scores (P = .18).
Three patients entered the second crossover phase of the study. Two began in the off-treatment arm and experienced relapsed within 1 and 2 weeks, respectively. Total PANSS scores increased from 79 to 98 for the first patient and from 47 to 93 for the second patient.
Neuroimaging showed that, among patients who responded to DBS, brain metabolism increased in some brain areas and decreased in others. Dr. Corripio said this suggests a “rebalancing” of neural circuits.
As of July 2020, one of three patients with an electrode placed in the NAcc had experienced remission of positive symptoms and now has predominant negative symptoms. Another experienced significant improvements in negative symptoms. Two patients currently require psychosocial rehabilitation.
Patients for whom an electrode was placed in the ACC required higher voltages and more time to achieve an effect in comparison with those for whom an electrode was placed in the NAcc. Two patients required bilateral stimulation.
However, for all three patients who remained in the study, their clozapine dose was reduced.
Dr. Corripio reported that the team has observed negative thoughts and obsessive symptoms in patients with electrodes in the ACC, and all have needed either psychosocial rehabilitation or cognitive-behavioral therapy.
The investigators are now planning another DBS study involving patients with TRS, although this one will include a clinical recovery program focusing on family interventions and cognitive-behavioral therapy.
“Last-resort” treatment
In the postpresentation debate, Damiaan Denys, PhD, professor and chair of the department of psychiatry at the Academic Medical Canter, University of Amsterdam, said that DBS remains a treatment of “last resort” in TRS.
This is because it is both costly and invasive, and although the associated risk of bleeding and infection is low, he noted that the consequences are significant.
Dr. Denys added that patients need to have the potential for improvement; electrodes can be easily implanted, and the approach may tempt clinicians who sometimes “struggle with a huge amount of treatment-refractory cases.”
He also pointed to results achieved in studies of obsessive-compulsive disorder and depression, in which around 50% of patients responded to DBS.
“I think that’s the reason why we should be reluctant and not treat anyone at any stage, but first look for the more severe cases,” Dr. Denys said.
Unmet need
Judith M. Gault, PhD, associate research professor of neurosurgery at the University of Colorado at Denver, Aurora, also took part in the debate.
She said in an interview that patients with TRS have a lot of unmet needs and that DBS is worth trying in this patient population, with the goal being to “conduct a really good clinical trial” similar to the current study.
Antipsychotic drugs work well in responsive patients, but “in some cases the person is treatment refractory ... and in other cases the patient relapses,” Dr. Gault said.
She believes that DBS has the “potential to be more potent than antipsychotics in modulating the circuit of interest” and so fulfills the unmet needs of these patients while alleviating their symptoms.
Dr. Gault added that some patients experience “breakthrough symptoms” even while they are medication adherent. “That is a call for an intervention that is more potent” and suggests another potential role for DBS.
Overall, there are “a lot of really compelling reasons to pursue” DBS. However, there are also questions about how motivated patients with TRS are to participate in a clinical trial, Dr. Gault noted.
Patients with schizophrenia “tend not to be very motivated, especially if they have negative symptoms.” However, “if you were able to consider more of the population and not just the most severely affected, eventually you would find more people who are interested,” she said.
Still, it will take a better understanding of the efficacy and safety of the intervention for more people to be interested in trying it, said Dr. Gault.
“I think it’s hard early on, when you don’t actually know what the outcomes would be, if it’s even effective at all. But as you get more and more data in the population and at the different targets, people would be more open to it,” she said.
Another issue in generating interest among patients with schizophrenia is that many have not considered DBS as an option.
“It takes a while to think about it,” she noted. “You don’t want to rush into something that you just heard about, and so part of it is just education.”
The study was funded by Instituto Carlos III. Dr. Corripio reported having received research grants and conducting consultancy for Otsuka, Ferrer, Janssen, and Lilly. No other relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Deep brain stimulation (DBS) may be an effective option for patients with treatment-resistant schizophrenia (TRS), new research suggests. However, until further studies are conducted, the treatment should only be considered for the most severe cases.
The first clinical trial to assess DBS in this challenging patient population included eight patients initially randomly assigned to receive electrode placement in one of two locations in the brain. Once a clinical response was achieved and participants were stabilized, they were randomly assigned to a second crossover phase.
Preliminary findings from the first phase of the DBS-SCHIZO pilot study, which were reported in 2017, showed promising efficacy.
The newly released final results revealed an association between DBS and significant improvements in Positive and Negative Symptoms Scale (PANSS) scores, as well as reductions in doses of antipsychotic medication. Moreover, the effect reversed when the electrode was switched off.
“DBS may be a potential option for severe treatment-resistant schizophrenia patients,” lead investigator Iluminada Corripio, MD, PhD, department of psychiatry, Hospital de la Santa Creu i Sant Pau, Barcelona, said during her presentation at the virtual congress of the European College of Neuropsychopharmacology. The new data were updated results of a study published in EBioMedicine earlier this year.
Dr. Corripio underlined that it is important to balance the risks and benefits of the intervention. DBS is “not useful for all phenotypes,” and benefits have been seen in patients with hallucinations but not in those with a disorganized phenotype, she added.
High economic burden
Managing TRS is challenging and is associated with a high clinical and economic burden, Corripio noted. Relapse rates can reach 80%, increasing resource use by between 200% and 900%.
There is a strong rationale for studying the use of DBS in schizophrenia, because schizophrenia shares a neurologic basis with other neurologic and psychiatric disorders centered around the cortical-striatal-thalamic-cortical circuit, said Dr. Corripio.
The study included eight patients with a DSM-IV-TR diagnosis of schizophrenia whose conditions were resistant to at least two different atypical antipsychotics and who had not responded to clozapine monotherapy, combination therapy, or electroconvulsive therapy.
All were randomly assigned in a 1:1 ratio to DBS electrode implantation in one of two locations. Investigators chose the nucleus accumbens (NAcc), because recent studies have shown that DBS can increase dopamine levels there, and the subgenual anterior cingulate cortex (ACC). Deactivation failure in the ACC region has been observed in patients with schizophrenia and other mental illnesses.
Stimulation began 48-72 hours postoperatively with unilateral left stimulation at 2.5 volts. It was increased in 0.5 volt increments to a maximum of 7.5 volts. Patients who did not respond were switched to bilateral stimulation.
Follow-up was conducted every 2 weeks for up to 20 months. The study’s primary outcome was a symptomatic response, defined as an improvement of at least 25% on the PANSS.
Once that was achieved, patients could enter a second randomization phase in which they were assigned, in a 24-week, double-blind crossover design, to on- or off-treatment DBS arms such that patients received stimulation for 12 weeks before the device was turned off for 12 weeks, or vice versa.
Those who experienced relapse while off treatment were crossed over to the on-treatment arm; those who experienced relapsed while on treatment were withdrawn from the study. The patients’ average age was 42.5 years, and 50% were women. All were taking clozapine in combination with another antipsychotic.
Adverse events
Five patients experienced adverse events during the first phase, four of which were associated with rechargeable battery replacement. One experienced akathisia, another experienced behavioral changes, and a third experienced electrical disturbances.
A fourth patient experienced postsurgical hemorrhage of the right internal capsule on day 4, followed by encephalitis at week 8. He had a clinical improvement but experienced relapsed during follow-up.
The fifth patient accidentally switched off the device and withdrew from the study.
During the first randomization phase, DBS was associated with significant improvements on total, positive, and negative PANSS scores in comparison with the postoperative baseline measure in the seven remaining patients (P < .001).
When the team compared the baseline measure with the last observation, the improvement in PANSS scores remained significant for total scores (P = .007) and positive scores (P = .002), but not for negative scores (P = .18).
Three patients entered the second crossover phase of the study. Two began in the off-treatment arm and experienced relapsed within 1 and 2 weeks, respectively. Total PANSS scores increased from 79 to 98 for the first patient and from 47 to 93 for the second patient.
Neuroimaging showed that, among patients who responded to DBS, brain metabolism increased in some brain areas and decreased in others. Dr. Corripio said this suggests a “rebalancing” of neural circuits.
As of July 2020, one of three patients with an electrode placed in the NAcc had experienced remission of positive symptoms and now has predominant negative symptoms. Another experienced significant improvements in negative symptoms. Two patients currently require psychosocial rehabilitation.
Patients for whom an electrode was placed in the ACC required higher voltages and more time to achieve an effect in comparison with those for whom an electrode was placed in the NAcc. Two patients required bilateral stimulation.
However, for all three patients who remained in the study, their clozapine dose was reduced.
Dr. Corripio reported that the team has observed negative thoughts and obsessive symptoms in patients with electrodes in the ACC, and all have needed either psychosocial rehabilitation or cognitive-behavioral therapy.
The investigators are now planning another DBS study involving patients with TRS, although this one will include a clinical recovery program focusing on family interventions and cognitive-behavioral therapy.
“Last-resort” treatment
In the postpresentation debate, Damiaan Denys, PhD, professor and chair of the department of psychiatry at the Academic Medical Canter, University of Amsterdam, said that DBS remains a treatment of “last resort” in TRS.
This is because it is both costly and invasive, and although the associated risk of bleeding and infection is low, he noted that the consequences are significant.
Dr. Denys added that patients need to have the potential for improvement; electrodes can be easily implanted, and the approach may tempt clinicians who sometimes “struggle with a huge amount of treatment-refractory cases.”
He also pointed to results achieved in studies of obsessive-compulsive disorder and depression, in which around 50% of patients responded to DBS.
“I think that’s the reason why we should be reluctant and not treat anyone at any stage, but first look for the more severe cases,” Dr. Denys said.
Unmet need
Judith M. Gault, PhD, associate research professor of neurosurgery at the University of Colorado at Denver, Aurora, also took part in the debate.
She said in an interview that patients with TRS have a lot of unmet needs and that DBS is worth trying in this patient population, with the goal being to “conduct a really good clinical trial” similar to the current study.
Antipsychotic drugs work well in responsive patients, but “in some cases the person is treatment refractory ... and in other cases the patient relapses,” Dr. Gault said.
She believes that DBS has the “potential to be more potent than antipsychotics in modulating the circuit of interest” and so fulfills the unmet needs of these patients while alleviating their symptoms.
Dr. Gault added that some patients experience “breakthrough symptoms” even while they are medication adherent. “That is a call for an intervention that is more potent” and suggests another potential role for DBS.
Overall, there are “a lot of really compelling reasons to pursue” DBS. However, there are also questions about how motivated patients with TRS are to participate in a clinical trial, Dr. Gault noted.
Patients with schizophrenia “tend not to be very motivated, especially if they have negative symptoms.” However, “if you were able to consider more of the population and not just the most severely affected, eventually you would find more people who are interested,” she said.
Still, it will take a better understanding of the efficacy and safety of the intervention for more people to be interested in trying it, said Dr. Gault.
“I think it’s hard early on, when you don’t actually know what the outcomes would be, if it’s even effective at all. But as you get more and more data in the population and at the different targets, people would be more open to it,” she said.
Another issue in generating interest among patients with schizophrenia is that many have not considered DBS as an option.
“It takes a while to think about it,” she noted. “You don’t want to rush into something that you just heard about, and so part of it is just education.”
The study was funded by Instituto Carlos III. Dr. Corripio reported having received research grants and conducting consultancy for Otsuka, Ferrer, Janssen, and Lilly. No other relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Deep brain stimulation (DBS) may be an effective option for patients with treatment-resistant schizophrenia (TRS), new research suggests. However, until further studies are conducted, the treatment should only be considered for the most severe cases.
The first clinical trial to assess DBS in this challenging patient population included eight patients initially randomly assigned to receive electrode placement in one of two locations in the brain. Once a clinical response was achieved and participants were stabilized, they were randomly assigned to a second crossover phase.
Preliminary findings from the first phase of the DBS-SCHIZO pilot study, which were reported in 2017, showed promising efficacy.
The newly released final results revealed an association between DBS and significant improvements in Positive and Negative Symptoms Scale (PANSS) scores, as well as reductions in doses of antipsychotic medication. Moreover, the effect reversed when the electrode was switched off.
“DBS may be a potential option for severe treatment-resistant schizophrenia patients,” lead investigator Iluminada Corripio, MD, PhD, department of psychiatry, Hospital de la Santa Creu i Sant Pau, Barcelona, said during her presentation at the virtual congress of the European College of Neuropsychopharmacology. The new data were updated results of a study published in EBioMedicine earlier this year.
Dr. Corripio underlined that it is important to balance the risks and benefits of the intervention. DBS is “not useful for all phenotypes,” and benefits have been seen in patients with hallucinations but not in those with a disorganized phenotype, she added.
High economic burden
Managing TRS is challenging and is associated with a high clinical and economic burden, Corripio noted. Relapse rates can reach 80%, increasing resource use by between 200% and 900%.
There is a strong rationale for studying the use of DBS in schizophrenia, because schizophrenia shares a neurologic basis with other neurologic and psychiatric disorders centered around the cortical-striatal-thalamic-cortical circuit, said Dr. Corripio.
The study included eight patients with a DSM-IV-TR diagnosis of schizophrenia whose conditions were resistant to at least two different atypical antipsychotics and who had not responded to clozapine monotherapy, combination therapy, or electroconvulsive therapy.
All were randomly assigned in a 1:1 ratio to DBS electrode implantation in one of two locations. Investigators chose the nucleus accumbens (NAcc), because recent studies have shown that DBS can increase dopamine levels there, and the subgenual anterior cingulate cortex (ACC). Deactivation failure in the ACC region has been observed in patients with schizophrenia and other mental illnesses.
Stimulation began 48-72 hours postoperatively with unilateral left stimulation at 2.5 volts. It was increased in 0.5 volt increments to a maximum of 7.5 volts. Patients who did not respond were switched to bilateral stimulation.
Follow-up was conducted every 2 weeks for up to 20 months. The study’s primary outcome was a symptomatic response, defined as an improvement of at least 25% on the PANSS.
Once that was achieved, patients could enter a second randomization phase in which they were assigned, in a 24-week, double-blind crossover design, to on- or off-treatment DBS arms such that patients received stimulation for 12 weeks before the device was turned off for 12 weeks, or vice versa.
Those who experienced relapse while off treatment were crossed over to the on-treatment arm; those who experienced relapsed while on treatment were withdrawn from the study. The patients’ average age was 42.5 years, and 50% were women. All were taking clozapine in combination with another antipsychotic.
Adverse events
Five patients experienced adverse events during the first phase, four of which were associated with rechargeable battery replacement. One experienced akathisia, another experienced behavioral changes, and a third experienced electrical disturbances.
A fourth patient experienced postsurgical hemorrhage of the right internal capsule on day 4, followed by encephalitis at week 8. He had a clinical improvement but experienced relapsed during follow-up.
The fifth patient accidentally switched off the device and withdrew from the study.
During the first randomization phase, DBS was associated with significant improvements on total, positive, and negative PANSS scores in comparison with the postoperative baseline measure in the seven remaining patients (P < .001).
When the team compared the baseline measure with the last observation, the improvement in PANSS scores remained significant for total scores (P = .007) and positive scores (P = .002), but not for negative scores (P = .18).
Three patients entered the second crossover phase of the study. Two began in the off-treatment arm and experienced relapsed within 1 and 2 weeks, respectively. Total PANSS scores increased from 79 to 98 for the first patient and from 47 to 93 for the second patient.
Neuroimaging showed that, among patients who responded to DBS, brain metabolism increased in some brain areas and decreased in others. Dr. Corripio said this suggests a “rebalancing” of neural circuits.
As of July 2020, one of three patients with an electrode placed in the NAcc had experienced remission of positive symptoms and now has predominant negative symptoms. Another experienced significant improvements in negative symptoms. Two patients currently require psychosocial rehabilitation.
Patients for whom an electrode was placed in the ACC required higher voltages and more time to achieve an effect in comparison with those for whom an electrode was placed in the NAcc. Two patients required bilateral stimulation.
However, for all three patients who remained in the study, their clozapine dose was reduced.
Dr. Corripio reported that the team has observed negative thoughts and obsessive symptoms in patients with electrodes in the ACC, and all have needed either psychosocial rehabilitation or cognitive-behavioral therapy.
The investigators are now planning another DBS study involving patients with TRS, although this one will include a clinical recovery program focusing on family interventions and cognitive-behavioral therapy.
“Last-resort” treatment
In the postpresentation debate, Damiaan Denys, PhD, professor and chair of the department of psychiatry at the Academic Medical Canter, University of Amsterdam, said that DBS remains a treatment of “last resort” in TRS.
This is because it is both costly and invasive, and although the associated risk of bleeding and infection is low, he noted that the consequences are significant.
Dr. Denys added that patients need to have the potential for improvement; electrodes can be easily implanted, and the approach may tempt clinicians who sometimes “struggle with a huge amount of treatment-refractory cases.”
He also pointed to results achieved in studies of obsessive-compulsive disorder and depression, in which around 50% of patients responded to DBS.
“I think that’s the reason why we should be reluctant and not treat anyone at any stage, but first look for the more severe cases,” Dr. Denys said.
Unmet need
Judith M. Gault, PhD, associate research professor of neurosurgery at the University of Colorado at Denver, Aurora, also took part in the debate.
She said in an interview that patients with TRS have a lot of unmet needs and that DBS is worth trying in this patient population, with the goal being to “conduct a really good clinical trial” similar to the current study.
Antipsychotic drugs work well in responsive patients, but “in some cases the person is treatment refractory ... and in other cases the patient relapses,” Dr. Gault said.
She believes that DBS has the “potential to be more potent than antipsychotics in modulating the circuit of interest” and so fulfills the unmet needs of these patients while alleviating their symptoms.
Dr. Gault added that some patients experience “breakthrough symptoms” even while they are medication adherent. “That is a call for an intervention that is more potent” and suggests another potential role for DBS.
Overall, there are “a lot of really compelling reasons to pursue” DBS. However, there are also questions about how motivated patients with TRS are to participate in a clinical trial, Dr. Gault noted.
Patients with schizophrenia “tend not to be very motivated, especially if they have negative symptoms.” However, “if you were able to consider more of the population and not just the most severely affected, eventually you would find more people who are interested,” she said.
Still, it will take a better understanding of the efficacy and safety of the intervention for more people to be interested in trying it, said Dr. Gault.
“I think it’s hard early on, when you don’t actually know what the outcomes would be, if it’s even effective at all. But as you get more and more data in the population and at the different targets, people would be more open to it,” she said.
Another issue in generating interest among patients with schizophrenia is that many have not considered DBS as an option.
“It takes a while to think about it,” she noted. “You don’t want to rush into something that you just heard about, and so part of it is just education.”
The study was funded by Instituto Carlos III. Dr. Corripio reported having received research grants and conducting consultancy for Otsuka, Ferrer, Janssen, and Lilly. No other relevant financial relationships were reported.
A version of this article originally appeared on Medscape.com.
Counterfeits: An ugly truth in aesthetic medicine
according to the results of two recent surveys of such providers.

“Counterfeit medical devices and injectables may be more prevalent in aesthetic medicine than most practitioners might estimate,” Jordan V. Wang, MD, of Sidney Kimmel Medical College, Philadelphia, and associates wrote in Dermatologic Surgery, even though “the vast majority [believe] that they are inferior and even potentially harmful.”
In one of the online surveys, conducted among members of the American Society of Dermatologic Surgery, 41.1% of the 616 respondents said they had encountered counterfeit injectables, more than half (56.5%) of whom were solicited to buy such products. Just over 10% had purchased counterfeit injectables, although nearly 80% did so unknowingly, the investigators said.
In the second survey, 37.4% of the 765 respondents (members of the ASDS as well as the American Society for Laser Medicine and Surgery) said that they had encountered counterfeit medical devices, and nearly half had been approached to purchase such devices. Of those who were approached, 4.6% had actually purchased a counterfeit, but only 6.1% did so unknowingly, Dr. Wang and associates reported.
In the medical device survey, almost a quarter (24.2%) acknowledged that they know of other providers using them, while 29.3% of those surveyed about injectables know of others who use counterfeits, they said.
Over 90% of practitioners in each survey agreed that counterfeits are worse in terms of safety, reliability, and effectiveness, but the proportions were smaller when they were asked if counterfeits were either very or extremely endangering to patient safety: 70.5% for injectables and 59.2% for devices, the investigators said.
Experience with adverse events from counterfeits in patients was reported by 39.7% of respondents to the injectables survey and by 20.1% of those in the device survey, they added.
Majorities in both surveys – 73.7% for injectables and 68.9% for devices – also said that they were either not familiar or only somewhat familiar with the Food and Drug Administration’s regulations on counterfeits. “This is especially problematic considering the potentially severe adverse events and steep punishments,” Dr. Wang and associates wrote.
The authors disclosed that they had no significant interest with commercial supporters. Dr. Wang is now a fellow at the Laser & Skin Surgery Center of New York.
SOURCE: Wang JV et al. Dermatol. Surg. 2020 Oct;46(10):1323-6.
according to the results of two recent surveys of such providers.

“Counterfeit medical devices and injectables may be more prevalent in aesthetic medicine than most practitioners might estimate,” Jordan V. Wang, MD, of Sidney Kimmel Medical College, Philadelphia, and associates wrote in Dermatologic Surgery, even though “the vast majority [believe] that they are inferior and even potentially harmful.”
In one of the online surveys, conducted among members of the American Society of Dermatologic Surgery, 41.1% of the 616 respondents said they had encountered counterfeit injectables, more than half (56.5%) of whom were solicited to buy such products. Just over 10% had purchased counterfeit injectables, although nearly 80% did so unknowingly, the investigators said.
In the second survey, 37.4% of the 765 respondents (members of the ASDS as well as the American Society for Laser Medicine and Surgery) said that they had encountered counterfeit medical devices, and nearly half had been approached to purchase such devices. Of those who were approached, 4.6% had actually purchased a counterfeit, but only 6.1% did so unknowingly, Dr. Wang and associates reported.
In the medical device survey, almost a quarter (24.2%) acknowledged that they know of other providers using them, while 29.3% of those surveyed about injectables know of others who use counterfeits, they said.
Over 90% of practitioners in each survey agreed that counterfeits are worse in terms of safety, reliability, and effectiveness, but the proportions were smaller when they were asked if counterfeits were either very or extremely endangering to patient safety: 70.5% for injectables and 59.2% for devices, the investigators said.
Experience with adverse events from counterfeits in patients was reported by 39.7% of respondents to the injectables survey and by 20.1% of those in the device survey, they added.
Majorities in both surveys – 73.7% for injectables and 68.9% for devices – also said that they were either not familiar or only somewhat familiar with the Food and Drug Administration’s regulations on counterfeits. “This is especially problematic considering the potentially severe adverse events and steep punishments,” Dr. Wang and associates wrote.
The authors disclosed that they had no significant interest with commercial supporters. Dr. Wang is now a fellow at the Laser & Skin Surgery Center of New York.
SOURCE: Wang JV et al. Dermatol. Surg. 2020 Oct;46(10):1323-6.
according to the results of two recent surveys of such providers.

“Counterfeit medical devices and injectables may be more prevalent in aesthetic medicine than most practitioners might estimate,” Jordan V. Wang, MD, of Sidney Kimmel Medical College, Philadelphia, and associates wrote in Dermatologic Surgery, even though “the vast majority [believe] that they are inferior and even potentially harmful.”
In one of the online surveys, conducted among members of the American Society of Dermatologic Surgery, 41.1% of the 616 respondents said they had encountered counterfeit injectables, more than half (56.5%) of whom were solicited to buy such products. Just over 10% had purchased counterfeit injectables, although nearly 80% did so unknowingly, the investigators said.
In the second survey, 37.4% of the 765 respondents (members of the ASDS as well as the American Society for Laser Medicine and Surgery) said that they had encountered counterfeit medical devices, and nearly half had been approached to purchase such devices. Of those who were approached, 4.6% had actually purchased a counterfeit, but only 6.1% did so unknowingly, Dr. Wang and associates reported.
In the medical device survey, almost a quarter (24.2%) acknowledged that they know of other providers using them, while 29.3% of those surveyed about injectables know of others who use counterfeits, they said.
Over 90% of practitioners in each survey agreed that counterfeits are worse in terms of safety, reliability, and effectiveness, but the proportions were smaller when they were asked if counterfeits were either very or extremely endangering to patient safety: 70.5% for injectables and 59.2% for devices, the investigators said.
Experience with adverse events from counterfeits in patients was reported by 39.7% of respondents to the injectables survey and by 20.1% of those in the device survey, they added.
Majorities in both surveys – 73.7% for injectables and 68.9% for devices – also said that they were either not familiar or only somewhat familiar with the Food and Drug Administration’s regulations on counterfeits. “This is especially problematic considering the potentially severe adverse events and steep punishments,” Dr. Wang and associates wrote.
The authors disclosed that they had no significant interest with commercial supporters. Dr. Wang is now a fellow at the Laser & Skin Surgery Center of New York.
SOURCE: Wang JV et al. Dermatol. Surg. 2020 Oct;46(10):1323-6.
FROM DERMATOLOGIC SURGERY
‘Dr. Pimple Popper’ shares her social media tips
The way Sandra Lee, MD, sees it, establishing a presence on Instagram, Twitter, and other social media channels may not float your boat, but its potential influence deserves your attention.
“We can no longer hide from social media; it is part of our lives now,” Dr. Lee, a dermatologist who practices in Upland, Calif., said at the virtual annual Masters of Aesthetics Symposium. “You’re missing some real opportunities without it.”
In October of 2014, Dr. Lee began using Instagram to provide followers a glimpse into her life as a dermatologist, everything from Mohs surgery and Botox to keloid removals and ear lobe repair surgeries. “Early on, I happened to post an extraction video,” she recalled. “It got a notable increase in attention. I thought it was weird. I did it again, and it happened again. I just started posting extraction videos every day: finding blackheads and whiteheads or milia or whatnot on my patients and just posting them. I watched in amazement as followers’ comments and attention grew.”
Soon after Dr. Lee started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious,” she said. “It’s a group of tens of thousands of people who share popping videos with each other,” she explained. “I thought that was really strange. I also thought that maybe I could be their queen, so I decided to share my videos there. This meant that I would have to start a YouTube channel where I could upload my videos.”
With this, Dr. Lee formed her alter ego, “Dr. Pimple Popper,” and became a YouTube sensation, building 6.6 million subscribers over the course of a few years. She also grew 4 million followers on Instagram, 2.9 million on Facebook, and more than 138,000 on Twitter. About 80% of her followers are women who range between 18 and 40 years of age. “They are very interested in skin care,” she said. “This is the target audience that advertisers want.”
Dr. Lee’s rapid rise to fame caused some soul-searching about her intentions. “What is really important to me is to not embarrass my patients and not embarrass myself or my specialty,” she said. “I wanted to show that we as dermatologists are so much more than pimple poppers, that we have an amazing specialty. Could I do this and still grow followers? Could I entertain them and keep their interest and educate them at the same time? Show them why we are experts?”
She added: “How could I reach people who have never seen a dermatologist and maybe teach them how to take care of their skin? And help them to know when the best time is to see a dermatologist. How can we distinguish ourselves from the rest of them: the estheticians, the nurse practitioners, the physician assistants, and the physicians who are board-certified in other specialties but who present themselves on social media as dermatologists? Our specialty is getting taken over by nondermatologists on social media from all angles, so it’s become important to me to remind people, in a positive way, that there’s a difference between a board-certified dermatologist and others.”
She offered the following six pearls of advice for building and maintaining your social media presence:
- Entertain, and secretly educate, without teaching them. “People want to learn about the world, and they want to know more about skin care,” said Dr. Lee, who also stars in her own TV reality show on TLC. “They want to know more about dermatology.”
- Know your audience. “Notice what posts get the most attention and try to figure out why that content resonates,” she advised. “Read your comments.”
- Show that you’re human. “They want to follow you because they like you as a person, not just because you’re a dermatologist,” she said. “Distinguish yourself amongst us dermatologists.”
- Don’t bad mouth other specialties or other so-called skin specialists. “Don’t invite the conflict,” she said. “In my opinion, the best way to fight this is to stay on the positive side and to showcase dermatology and how amazing it is to be a board-certified dermatologist.”
- Don’t hire someone to post for you, at least not initially. Handle your social media accounts yourself, “because otherwise you really can’t understand what is driving it,” said Dr. Lee, who launched her own skin care line, SLMD Skincare. “I don’t think it can grow to a large degree without you being directly involved.”
- Use the feedback and responses to make yourself a better dermatologist. “I think that social media has made my bedside manner better, my techniques better,” she said. “It has made me a better dermatologist and, I think, a better person, too.”
dbrunk@mdedge.com
The way Sandra Lee, MD, sees it, establishing a presence on Instagram, Twitter, and other social media channels may not float your boat, but its potential influence deserves your attention.
“We can no longer hide from social media; it is part of our lives now,” Dr. Lee, a dermatologist who practices in Upland, Calif., said at the virtual annual Masters of Aesthetics Symposium. “You’re missing some real opportunities without it.”
In October of 2014, Dr. Lee began using Instagram to provide followers a glimpse into her life as a dermatologist, everything from Mohs surgery and Botox to keloid removals and ear lobe repair surgeries. “Early on, I happened to post an extraction video,” she recalled. “It got a notable increase in attention. I thought it was weird. I did it again, and it happened again. I just started posting extraction videos every day: finding blackheads and whiteheads or milia or whatnot on my patients and just posting them. I watched in amazement as followers’ comments and attention grew.”
Soon after Dr. Lee started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious,” she said. “It’s a group of tens of thousands of people who share popping videos with each other,” she explained. “I thought that was really strange. I also thought that maybe I could be their queen, so I decided to share my videos there. This meant that I would have to start a YouTube channel where I could upload my videos.”
With this, Dr. Lee formed her alter ego, “Dr. Pimple Popper,” and became a YouTube sensation, building 6.6 million subscribers over the course of a few years. She also grew 4 million followers on Instagram, 2.9 million on Facebook, and more than 138,000 on Twitter. About 80% of her followers are women who range between 18 and 40 years of age. “They are very interested in skin care,” she said. “This is the target audience that advertisers want.”
Dr. Lee’s rapid rise to fame caused some soul-searching about her intentions. “What is really important to me is to not embarrass my patients and not embarrass myself or my specialty,” she said. “I wanted to show that we as dermatologists are so much more than pimple poppers, that we have an amazing specialty. Could I do this and still grow followers? Could I entertain them and keep their interest and educate them at the same time? Show them why we are experts?”
She added: “How could I reach people who have never seen a dermatologist and maybe teach them how to take care of their skin? And help them to know when the best time is to see a dermatologist. How can we distinguish ourselves from the rest of them: the estheticians, the nurse practitioners, the physician assistants, and the physicians who are board-certified in other specialties but who present themselves on social media as dermatologists? Our specialty is getting taken over by nondermatologists on social media from all angles, so it’s become important to me to remind people, in a positive way, that there’s a difference between a board-certified dermatologist and others.”
She offered the following six pearls of advice for building and maintaining your social media presence:
- Entertain, and secretly educate, without teaching them. “People want to learn about the world, and they want to know more about skin care,” said Dr. Lee, who also stars in her own TV reality show on TLC. “They want to know more about dermatology.”
- Know your audience. “Notice what posts get the most attention and try to figure out why that content resonates,” she advised. “Read your comments.”
- Show that you’re human. “They want to follow you because they like you as a person, not just because you’re a dermatologist,” she said. “Distinguish yourself amongst us dermatologists.”
- Don’t bad mouth other specialties or other so-called skin specialists. “Don’t invite the conflict,” she said. “In my opinion, the best way to fight this is to stay on the positive side and to showcase dermatology and how amazing it is to be a board-certified dermatologist.”
- Don’t hire someone to post for you, at least not initially. Handle your social media accounts yourself, “because otherwise you really can’t understand what is driving it,” said Dr. Lee, who launched her own skin care line, SLMD Skincare. “I don’t think it can grow to a large degree without you being directly involved.”
- Use the feedback and responses to make yourself a better dermatologist. “I think that social media has made my bedside manner better, my techniques better,” she said. “It has made me a better dermatologist and, I think, a better person, too.”
dbrunk@mdedge.com
The way Sandra Lee, MD, sees it, establishing a presence on Instagram, Twitter, and other social media channels may not float your boat, but its potential influence deserves your attention.
“We can no longer hide from social media; it is part of our lives now,” Dr. Lee, a dermatologist who practices in Upland, Calif., said at the virtual annual Masters of Aesthetics Symposium. “You’re missing some real opportunities without it.”
In October of 2014, Dr. Lee began using Instagram to provide followers a glimpse into her life as a dermatologist, everything from Mohs surgery and Botox to keloid removals and ear lobe repair surgeries. “Early on, I happened to post an extraction video,” she recalled. “It got a notable increase in attention. I thought it was weird. I did it again, and it happened again. I just started posting extraction videos every day: finding blackheads and whiteheads or milia or whatnot on my patients and just posting them. I watched in amazement as followers’ comments and attention grew.”
Soon after Dr. Lee started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious,” she said. “It’s a group of tens of thousands of people who share popping videos with each other,” she explained. “I thought that was really strange. I also thought that maybe I could be their queen, so I decided to share my videos there. This meant that I would have to start a YouTube channel where I could upload my videos.”
With this, Dr. Lee formed her alter ego, “Dr. Pimple Popper,” and became a YouTube sensation, building 6.6 million subscribers over the course of a few years. She also grew 4 million followers on Instagram, 2.9 million on Facebook, and more than 138,000 on Twitter. About 80% of her followers are women who range between 18 and 40 years of age. “They are very interested in skin care,” she said. “This is the target audience that advertisers want.”
Dr. Lee’s rapid rise to fame caused some soul-searching about her intentions. “What is really important to me is to not embarrass my patients and not embarrass myself or my specialty,” she said. “I wanted to show that we as dermatologists are so much more than pimple poppers, that we have an amazing specialty. Could I do this and still grow followers? Could I entertain them and keep their interest and educate them at the same time? Show them why we are experts?”
She added: “How could I reach people who have never seen a dermatologist and maybe teach them how to take care of their skin? And help them to know when the best time is to see a dermatologist. How can we distinguish ourselves from the rest of them: the estheticians, the nurse practitioners, the physician assistants, and the physicians who are board-certified in other specialties but who present themselves on social media as dermatologists? Our specialty is getting taken over by nondermatologists on social media from all angles, so it’s become important to me to remind people, in a positive way, that there’s a difference between a board-certified dermatologist and others.”
She offered the following six pearls of advice for building and maintaining your social media presence:
- Entertain, and secretly educate, without teaching them. “People want to learn about the world, and they want to know more about skin care,” said Dr. Lee, who also stars in her own TV reality show on TLC. “They want to know more about dermatology.”
- Know your audience. “Notice what posts get the most attention and try to figure out why that content resonates,” she advised. “Read your comments.”
- Show that you’re human. “They want to follow you because they like you as a person, not just because you’re a dermatologist,” she said. “Distinguish yourself amongst us dermatologists.”
- Don’t bad mouth other specialties or other so-called skin specialists. “Don’t invite the conflict,” she said. “In my opinion, the best way to fight this is to stay on the positive side and to showcase dermatology and how amazing it is to be a board-certified dermatologist.”
- Don’t hire someone to post for you, at least not initially. Handle your social media accounts yourself, “because otherwise you really can’t understand what is driving it,” said Dr. Lee, who launched her own skin care line, SLMD Skincare. “I don’t think it can grow to a large degree without you being directly involved.”
- Use the feedback and responses to make yourself a better dermatologist. “I think that social media has made my bedside manner better, my techniques better,” she said. “It has made me a better dermatologist and, I think, a better person, too.”
dbrunk@mdedge.com
FROM MOA 2020
Practicing cognitive techniques can help athletes reach optimal performance
Successful athletes exhibit positive mental health. This mental health is directly related to athletic success and high levels of performance.1 Mental skills are as important as natural physical ability and mechanical skills in the sport of tennis.
Research has shown that tennis is 85% mental and that players spend 80% of their time on the court handling emotions. Some players look good in practice when they are not under pressure but cannot win matches (they have the physical skill level to win) because they cannot handle their own emotions during the duress of a match. They are affected by anger, fear, stress, poor concentration, and other internal elements that interfere with their ability to perform at an optimal level. Competitors may also be affected by external factors such as the sun, wind, an opponent, and so on, and may use these situations as an excuse not to win.
Players normally practice physical skills but rarely practice cognitive techniques. Regardless of level of play – pro, collegiate, junior, or club – practicing mental skills will greatly improve the players’ arsenal of weapons, giving them an edge in matches and making them the best players they can be. Mental health professionals also can use these strategies to help motivate athletes who compete in other sports – and in other competitive endeavors.
Visualization is the formation of a mental image of something of your choice. Visualization imagery techniques can be used by players to calm themselves before playing a match so their emotions are not wasted on trying to quiet the minds and quell stress. Implementing the following visualization techniques will reduce a player’s anxiety during the match, allowing the player to direct energy toward optimal mental and physical performance on the court.
In advance of a match, encourage the player to learn and analyze the opponent’s strengths and weaknesses by watching the opponent play and/or from asking others. The night before the scheduled match, get the player to imagine how they will play points against their competitor. Play into the opponents’ vulnerabilities or first play to their strengths to expose shortcomings and – then attack their weakness. For example, if an opponent has a weak backhand, first play to the opponent’s forehand and, when the opponent is vulnerable, go into his backhand to get a short or weak ball – and attack. The following are specific strategies that mental health professionals who work with athletes can use to help them perform optimally.
Using visualization, shadowing
Visualize the correct way to hit a tennis stroke and repeat it over and over in your mind. On a tennis court or where ever you have adequate space, shadow a stroke by using a racket and repetitively performing the actual stroke without hitting a ball. At home, practice relaxation and deep breathing techniques at night before going to sleep. Put yourself in a relaxed state and visualize repetitively striking the ball correctly. The next time you actually hit the stroke, you will produce a better shot.
Focusing on, staying in the here and now
The “here” means to focus on what is happening on your own court, not what is happening on the court next to you. Players may be affected by external factors, such as the sun, wind, and their opponent and may use these conditions or situations as an excuse if they do not win. Ignore background chatter and distractions, and be a horse with blinders. Be responsible for yourself and your own actions; manage what you can and realize that you cannot control the weather or actions of your opponent.
The “now” refers to staying present and focusing only on the current point. Do not think of past mistakes. If you are winning a match, do not think about celebrating while the match is still in play. If you are losing, do not start to write a script of excuses why you lost the match. Instead, just concentrate on the present, point by point. Focusing will allow you to understand what is true and important in the here and now. Focusing will help alleviate stress and better equip you to make quick decisions and be clear about your intended actions.
Set realistic and achievable goals
It is always good to have goals and dreams; however, you as a player must understand the realities of your current level of play. Know your level; don’t be grandiose and think you are able to beat Rafael Nadal. Having an unrealistic attitude will result in frustration and poor performance during a match. Instead, set achievable, and realistic short- and long-term goals for yourself, which will aid in your overall tennis development. After the match is over, reflect upon and evaluate the points – and your overall performance.
Don’t devalue yourself if you lose a match. Do not feel too low from a loss or too high from a win. When you have a match loss, use it as an opportunity to learn from your mistakes and to improve by working on your weaknesses in future practice until you feel confident enough to use your new skills in a tournament.
Stay positive
Do not tie up your self-esteem as a person with your match outcome; in otherwords, separate feelings of self-worth from your match results. Cultivate an optimistic attitude and talk positively to yourself, strive to improve, and maintain positive self-esteem in practice and in matches. During practice, allocate 110% effort, and focus on the process, not the outcome. Arrange your practice matches so that one-third of them are against players of your same level, one-third against players worse than you, and one-third against players better than yourself.
Deal with adversity
It is important to be able to deal with external pressures going on in your life such as conflicts related to family, peers, school, work, and relationships. Deal with and manage this discord before your match so you can maintain control of your emotions and can give 100% effort on the court.
Learn mental techniques
Many athletes may have difficulty teaching themselves cognitive skills and would benefit from a few sessions with a sports psychologist/psychiatrist to understand and learn the techniques. Once the tactics are understood and learned, players can apply them to training and ultimately to their tournament arsenal, allowing them to play to their ultimate potential.
References
1. Morgan WP. Selected psychological factors limiting performance: A mental health model. In Clarke DH and Eckert HM (eds.), Limits of Human Performance. Champaign, Ill.: Human Kinetics Publishers, 1985.
Dr. Cohen had a private practice in psychiatry for more than 35 years. He is a former professor of psychiatry, family medicine, and otolaryngology at Thomas Jefferson University in Philadelphia. Dr. Cohen has been a nationally ranked tennis player from age 12 to the present and served as captain of the tennis team at the University of Pennsylvania, Philadelphia. Dr. Cohen, who was ranked No. 1 in tennis in the middle states section and in the country in various categories and times, was inducted into the Philadelphia Jewish Sports Hall of Fame in 2012. Dr. Cohen has no conflicts of interest.
Ms. Cohen, Dr. Cohen’s daughter, was No. 1 ranked in the United States in junior tennis and No. 4 in the world. In addition, Ms. Cohen was ranked among the top 100 players in the world by the professional World Tennis Association. She also was the No. 2 college player in United States, and an All-American at the University of Miami. She holds a master’s in sports psychology, and presently works as a sports psychologist and tennis professional in Philadelphia. Ms. Cohen has no conflicts of interest.
Successful athletes exhibit positive mental health. This mental health is directly related to athletic success and high levels of performance.1 Mental skills are as important as natural physical ability and mechanical skills in the sport of tennis.
Research has shown that tennis is 85% mental and that players spend 80% of their time on the court handling emotions. Some players look good in practice when they are not under pressure but cannot win matches (they have the physical skill level to win) because they cannot handle their own emotions during the duress of a match. They are affected by anger, fear, stress, poor concentration, and other internal elements that interfere with their ability to perform at an optimal level. Competitors may also be affected by external factors such as the sun, wind, an opponent, and so on, and may use these situations as an excuse not to win.
Players normally practice physical skills but rarely practice cognitive techniques. Regardless of level of play – pro, collegiate, junior, or club – practicing mental skills will greatly improve the players’ arsenal of weapons, giving them an edge in matches and making them the best players they can be. Mental health professionals also can use these strategies to help motivate athletes who compete in other sports – and in other competitive endeavors.
Visualization is the formation of a mental image of something of your choice. Visualization imagery techniques can be used by players to calm themselves before playing a match so their emotions are not wasted on trying to quiet the minds and quell stress. Implementing the following visualization techniques will reduce a player’s anxiety during the match, allowing the player to direct energy toward optimal mental and physical performance on the court.
In advance of a match, encourage the player to learn and analyze the opponent’s strengths and weaknesses by watching the opponent play and/or from asking others. The night before the scheduled match, get the player to imagine how they will play points against their competitor. Play into the opponents’ vulnerabilities or first play to their strengths to expose shortcomings and – then attack their weakness. For example, if an opponent has a weak backhand, first play to the opponent’s forehand and, when the opponent is vulnerable, go into his backhand to get a short or weak ball – and attack. The following are specific strategies that mental health professionals who work with athletes can use to help them perform optimally.
Using visualization, shadowing
Visualize the correct way to hit a tennis stroke and repeat it over and over in your mind. On a tennis court or where ever you have adequate space, shadow a stroke by using a racket and repetitively performing the actual stroke without hitting a ball. At home, practice relaxation and deep breathing techniques at night before going to sleep. Put yourself in a relaxed state and visualize repetitively striking the ball correctly. The next time you actually hit the stroke, you will produce a better shot.
Focusing on, staying in the here and now
The “here” means to focus on what is happening on your own court, not what is happening on the court next to you. Players may be affected by external factors, such as the sun, wind, and their opponent and may use these conditions or situations as an excuse if they do not win. Ignore background chatter and distractions, and be a horse with blinders. Be responsible for yourself and your own actions; manage what you can and realize that you cannot control the weather or actions of your opponent.
The “now” refers to staying present and focusing only on the current point. Do not think of past mistakes. If you are winning a match, do not think about celebrating while the match is still in play. If you are losing, do not start to write a script of excuses why you lost the match. Instead, just concentrate on the present, point by point. Focusing will allow you to understand what is true and important in the here and now. Focusing will help alleviate stress and better equip you to make quick decisions and be clear about your intended actions.
Set realistic and achievable goals
It is always good to have goals and dreams; however, you as a player must understand the realities of your current level of play. Know your level; don’t be grandiose and think you are able to beat Rafael Nadal. Having an unrealistic attitude will result in frustration and poor performance during a match. Instead, set achievable, and realistic short- and long-term goals for yourself, which will aid in your overall tennis development. After the match is over, reflect upon and evaluate the points – and your overall performance.
Don’t devalue yourself if you lose a match. Do not feel too low from a loss or too high from a win. When you have a match loss, use it as an opportunity to learn from your mistakes and to improve by working on your weaknesses in future practice until you feel confident enough to use your new skills in a tournament.
Stay positive
Do not tie up your self-esteem as a person with your match outcome; in otherwords, separate feelings of self-worth from your match results. Cultivate an optimistic attitude and talk positively to yourself, strive to improve, and maintain positive self-esteem in practice and in matches. During practice, allocate 110% effort, and focus on the process, not the outcome. Arrange your practice matches so that one-third of them are against players of your same level, one-third against players worse than you, and one-third against players better than yourself.
Deal with adversity
It is important to be able to deal with external pressures going on in your life such as conflicts related to family, peers, school, work, and relationships. Deal with and manage this discord before your match so you can maintain control of your emotions and can give 100% effort on the court.
Learn mental techniques
Many athletes may have difficulty teaching themselves cognitive skills and would benefit from a few sessions with a sports psychologist/psychiatrist to understand and learn the techniques. Once the tactics are understood and learned, players can apply them to training and ultimately to their tournament arsenal, allowing them to play to their ultimate potential.
References
1. Morgan WP. Selected psychological factors limiting performance: A mental health model. In Clarke DH and Eckert HM (eds.), Limits of Human Performance. Champaign, Ill.: Human Kinetics Publishers, 1985.
Dr. Cohen had a private practice in psychiatry for more than 35 years. He is a former professor of psychiatry, family medicine, and otolaryngology at Thomas Jefferson University in Philadelphia. Dr. Cohen has been a nationally ranked tennis player from age 12 to the present and served as captain of the tennis team at the University of Pennsylvania, Philadelphia. Dr. Cohen, who was ranked No. 1 in tennis in the middle states section and in the country in various categories and times, was inducted into the Philadelphia Jewish Sports Hall of Fame in 2012. Dr. Cohen has no conflicts of interest.
Ms. Cohen, Dr. Cohen’s daughter, was No. 1 ranked in the United States in junior tennis and No. 4 in the world. In addition, Ms. Cohen was ranked among the top 100 players in the world by the professional World Tennis Association. She also was the No. 2 college player in United States, and an All-American at the University of Miami. She holds a master’s in sports psychology, and presently works as a sports psychologist and tennis professional in Philadelphia. Ms. Cohen has no conflicts of interest.
Successful athletes exhibit positive mental health. This mental health is directly related to athletic success and high levels of performance.1 Mental skills are as important as natural physical ability and mechanical skills in the sport of tennis.
Research has shown that tennis is 85% mental and that players spend 80% of their time on the court handling emotions. Some players look good in practice when they are not under pressure but cannot win matches (they have the physical skill level to win) because they cannot handle their own emotions during the duress of a match. They are affected by anger, fear, stress, poor concentration, and other internal elements that interfere with their ability to perform at an optimal level. Competitors may also be affected by external factors such as the sun, wind, an opponent, and so on, and may use these situations as an excuse not to win.
Players normally practice physical skills but rarely practice cognitive techniques. Regardless of level of play – pro, collegiate, junior, or club – practicing mental skills will greatly improve the players’ arsenal of weapons, giving them an edge in matches and making them the best players they can be. Mental health professionals also can use these strategies to help motivate athletes who compete in other sports – and in other competitive endeavors.
Visualization is the formation of a mental image of something of your choice. Visualization imagery techniques can be used by players to calm themselves before playing a match so their emotions are not wasted on trying to quiet the minds and quell stress. Implementing the following visualization techniques will reduce a player’s anxiety during the match, allowing the player to direct energy toward optimal mental and physical performance on the court.
In advance of a match, encourage the player to learn and analyze the opponent’s strengths and weaknesses by watching the opponent play and/or from asking others. The night before the scheduled match, get the player to imagine how they will play points against their competitor. Play into the opponents’ vulnerabilities or first play to their strengths to expose shortcomings and – then attack their weakness. For example, if an opponent has a weak backhand, first play to the opponent’s forehand and, when the opponent is vulnerable, go into his backhand to get a short or weak ball – and attack. The following are specific strategies that mental health professionals who work with athletes can use to help them perform optimally.
Using visualization, shadowing
Visualize the correct way to hit a tennis stroke and repeat it over and over in your mind. On a tennis court or where ever you have adequate space, shadow a stroke by using a racket and repetitively performing the actual stroke without hitting a ball. At home, practice relaxation and deep breathing techniques at night before going to sleep. Put yourself in a relaxed state and visualize repetitively striking the ball correctly. The next time you actually hit the stroke, you will produce a better shot.
Focusing on, staying in the here and now
The “here” means to focus on what is happening on your own court, not what is happening on the court next to you. Players may be affected by external factors, such as the sun, wind, and their opponent and may use these conditions or situations as an excuse if they do not win. Ignore background chatter and distractions, and be a horse with blinders. Be responsible for yourself and your own actions; manage what you can and realize that you cannot control the weather or actions of your opponent.
The “now” refers to staying present and focusing only on the current point. Do not think of past mistakes. If you are winning a match, do not think about celebrating while the match is still in play. If you are losing, do not start to write a script of excuses why you lost the match. Instead, just concentrate on the present, point by point. Focusing will allow you to understand what is true and important in the here and now. Focusing will help alleviate stress and better equip you to make quick decisions and be clear about your intended actions.
Set realistic and achievable goals
It is always good to have goals and dreams; however, you as a player must understand the realities of your current level of play. Know your level; don’t be grandiose and think you are able to beat Rafael Nadal. Having an unrealistic attitude will result in frustration and poor performance during a match. Instead, set achievable, and realistic short- and long-term goals for yourself, which will aid in your overall tennis development. After the match is over, reflect upon and evaluate the points – and your overall performance.
Don’t devalue yourself if you lose a match. Do not feel too low from a loss or too high from a win. When you have a match loss, use it as an opportunity to learn from your mistakes and to improve by working on your weaknesses in future practice until you feel confident enough to use your new skills in a tournament.
Stay positive
Do not tie up your self-esteem as a person with your match outcome; in otherwords, separate feelings of self-worth from your match results. Cultivate an optimistic attitude and talk positively to yourself, strive to improve, and maintain positive self-esteem in practice and in matches. During practice, allocate 110% effort, and focus on the process, not the outcome. Arrange your practice matches so that one-third of them are against players of your same level, one-third against players worse than you, and one-third against players better than yourself.
Deal with adversity
It is important to be able to deal with external pressures going on in your life such as conflicts related to family, peers, school, work, and relationships. Deal with and manage this discord before your match so you can maintain control of your emotions and can give 100% effort on the court.
Learn mental techniques
Many athletes may have difficulty teaching themselves cognitive skills and would benefit from a few sessions with a sports psychologist/psychiatrist to understand and learn the techniques. Once the tactics are understood and learned, players can apply them to training and ultimately to their tournament arsenal, allowing them to play to their ultimate potential.
References
1. Morgan WP. Selected psychological factors limiting performance: A mental health model. In Clarke DH and Eckert HM (eds.), Limits of Human Performance. Champaign, Ill.: Human Kinetics Publishers, 1985.
Dr. Cohen had a private practice in psychiatry for more than 35 years. He is a former professor of psychiatry, family medicine, and otolaryngology at Thomas Jefferson University in Philadelphia. Dr. Cohen has been a nationally ranked tennis player from age 12 to the present and served as captain of the tennis team at the University of Pennsylvania, Philadelphia. Dr. Cohen, who was ranked No. 1 in tennis in the middle states section and in the country in various categories and times, was inducted into the Philadelphia Jewish Sports Hall of Fame in 2012. Dr. Cohen has no conflicts of interest.
Ms. Cohen, Dr. Cohen’s daughter, was No. 1 ranked in the United States in junior tennis and No. 4 in the world. In addition, Ms. Cohen was ranked among the top 100 players in the world by the professional World Tennis Association. She also was the No. 2 college player in United States, and an All-American at the University of Miami. She holds a master’s in sports psychology, and presently works as a sports psychologist and tennis professional in Philadelphia. Ms. Cohen has no conflicts of interest.
Psychosocial resilience associated with better cardiovascular health in Blacks
Resilience might deserve targeting
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
Resilience might deserve targeting
Resilience might deserve targeting
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
Increased psychosocial resilience, which captures a sense of purpose, optimism, and life-coping strategies, correlates with improved cardiovascular (CV) health in Black Americans, according to a study that might hold a key for identifying new strategies for CV disease prevention.
“Our findings highlight the importance of individual psychosocial factors that promote cardiovascular health among Black adults, traditionally considered to be a high-risk population,” according to a team of authors collaborating on a study produced by the Morehouse-Emory Cardiovascular Center for Health Equity in Atlanta.
Studies associating psychosocial resilience with improved health outcomes have been published before. In a 12-study review of this concept, it was emphasized that resilience is a dynamic process, not a personality trait, and has shown promise as a target of efforts to relieve the burden of disease (Johnston MC et al. Psychosomatics 2015;56:168-80).
In this study, which received partial support from the American Heart Association, psychosocial resilience was evaluated at both the individual level and at the community level among 389 Black adults living in Atlanta. The senior author was Tené T. Lewis, PhD, of the department of epidemiology at Emory’s Rollins School of Public Health (Circ Cardiovasc Qual Outcomes 2020 Oct 7;13:3006638).
Psychosocial resilience was calculated across the domains of environmental mastery, purpose of life, optimism, coping, and lack of depression with standardized tests, such as the Life Orientation Test-Revised questionnaire for optimism and the Ryff Scales of Psychological Well-Being for the domains of environmental mastery and purpose of life. A composite score for psychosocial resilience was reached by calculating the median score across the measured domains.
Patients with high psychosocial resilience, defined as a composite score above the median, or low resilience, defined as a lower score, were then compared for CV health based on the AHA’s Life’s Simple 7 (LS7) score.
LS7 scores incorporate measures for exercise, diet, smoking history, blood pressure, glucose, cholesterol, and body mass index. Composite LS7 scores range from 0 to 14. Prior work cited by the authors have associated each 1-unit increase in LS7 score with a 13% lower risk of CVD.
As a continuous variable for CV risk at the individual level, each higher standard-deviation increment in the composite psychosocial resilience score was associated with a highly significant 0.42-point increase in LS7 score (P < .001) for study participants. In other words, increasing resilience predicted lower CV risk scores.
Resilience was also calculated at the community level by looking at census tract-level rates of CV mortality and morbidity relative to socioeconomic status. Again, high CV resilience, defined as scores above the median, were compared with lower scores across neighborhoods with similar median household income. As a continuous variable in this analysis, each higher standard-deviation increment in the resilience score was associated with a 0.27-point increase in LS7 score (P = .01).
After adjustment for sociodemographic factors, the association between psychosocial resilience and CV health remained significant for both the individual and community calculations, according to the authors. When examined jointly, high individual psychosocial resilience remained independently associated with improved CV health, but living in a high-resilience neighborhood was not an independent predictor.
When evaluated individually, each of the domains in the psychosocial resistance score were positively correlated with higher LS7 scores, meaning lower CV risk. The strongest associations on a statistical level were low depressive symptoms (P = .001), environmental mastery (P = .006), and purpose in life (P = .009).
The impact of high psychosocial resistance scores was greatest in Black adults living in low-resilience neighborhoods. Among these subjects, high resilience was associated with a nearly 1-point increase in LS7 score relative to low resilience (8.38 vs. 7.42). This was unexpected, but it “is consistent with some broader conceptual literature that posits that individual psychosocial resilience matters more under conditions of adversity,” the authors reported.
Understanding disparities is key
Black race has repeatedly been associated with an increased risk of CV events, but this study is valuable for providing a fresh perspective on the potential reasons, according to the authors of an accompanying editorial, Amber E. Johnson, MD, and Jared Magnani, MD, who are both affiliated with the division of cardiology at the University of Pittsburgh (Circ Cardiovasc Qual Outcomes 2020 Oct 7. doi: 10.1161/CIRCOUTCOMES.120.007357.
“Clinicians increasingly recognize that race-based disparities do not stem inherently from race; instead, the disparities stem from the underlying social determinations of health,” they wrote, citing such variables as unequal access to pay and acceptable living conditions “and the structural racism that perpetuates them.”
They agreed with the authors that promotion of psychosocial resilience among Black people living in communities with poor CV health has the potential to improve CV outcomes, but they warned that this is complex. Although they contend that resilience techniques can be taught, they cautioned there might be limitations if the underlying factors associated with poor psychosocial resilience remain unchanged.
“Thus, the superficial application of positive psychology strategies is likely insufficient to bring parity to CV health outcomes,” they wrote, concluding that strategies to promote health equity would negate the need for interventions to bolster resilience.
Studies that focus on Black adults and cardiovascular health, including this investigation into the role of psychosocial factors “are much needed and very welcome,” said Harlan M. Krumholz, MD, a cardiologist and professor in the Institute for Social and Policy Studies at Yale University, New Haven, Conn.
He sees a broad array of potential directions of research.
“The study opens many questions about whether the resilience can be strengthened by interventions; whether addressing structural racism could reduce the need for such resilience, and whether this association is specific to Black adults in an urban center or is generally present in other settings and in other populations,” Dr. Krumholz said.
An effort is now needed to determine “whether this is a marker or a mediator of cardiovascular health,” he added.
In either case, resilience is a potentially important factor for understanding racial disparities in CV-disease prevalence and outcomes, according to the authors of the accompanying editorial and Dr. Krumholz.
SOURCE: Kim JH et al. Circ Cardiovasc Qual Outcomes. 2020 Oct 7;13:e006638.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES