User login
Tepotinib elicits responses in METex14 NSCLC
The objective response rate was 46.5% among the 99 patients followed for 9 or more months, as assessed by independent reviewers. There were no complete responders, according to the reviewers.
However, the response rate according to investigator assessment was 55.6%, including two responses that were judged to be complete.
The median duration of response was 11.1 months according to reviewers and 14 months according to investigators.
“The success of this trial, alongside other studies on the same class of drugs, establishes MET exon 14 as an actionable target for non–small cell lung cancer,” said senior author Xiuning Le, MD, PhD, assistant professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Le presented results from this trial as part of the American Society of Clinical Oncology virtual scientific program. Results were published simultaneously in the New England Journal of Medicine.
The trial, dubbed VISION, already won tepotinib approval in Japan to treat NSCLC patients with MET exon 14–skipping mutations. The Food and Drug Administration has granted tepotinib breakthrough status, and Merck, the drug’s manufacturer, plans to submit tepotinib for review this year.
The VISION trial enrolled 152 patients with NSCLC – 99 with at least 9 months of follow-up and 53 with shorter follow-up. The patients’ MET exon 14 mutations were confirmed by liquid or tissue biopsy.
The patients’ median age at baseline was 74 years, 54% were men, and almost half had no smoking history. Patients received tepotinib at 500 mg daily until disease progression or intolerable toxicity.
Overall, the VISION results “compare favorably” with those from studies of other MET inhibitors, the investigators wrote.
The 46.5% objective response rate (per independent reviewers) included the 99 patients with follow-up of at least 9 months who were liquid- or tissue-biopsy positive (combined group). The response rate was 48.5% among the 66 patients with positive liquid biopsies and 50% among the 60 patients with positive tissue biopsies.
The median progression-free survival was 8.5 months in the combined group, 8.5 months in the liquid-biopsy group, and 11 months in the tissue-biopsy group. The median overall survival was 17.1 months, 15.8 months, and 22.3 months, respectively.
The 11 patients with brain metastases at baseline had results that were in line with the other patients’ results. Patients with brain metastases had an objective response rate of 54.5%, a median response duration of 9.5 months, and a median progression-free survival of 10.9 months.
Overall, 88.8% of patients reported adverse events related to treatment, including peripheral edema in 63.2%, nausea in 25.7%, and diarrhea in 21.7%.
Grade 3-4 adverse events occurred in 27% of patients. Peripheral edema was the most common of these events, reported in 7.2% of patients.
“Proactive monitoring for peripheral edema is recommended and can be managed with temporary discontinuation of tepotinib or dose reduction,” the investigators wrote.
The death of a 79-year-old patient with respiratory failure and dyspnea, secondary to interstitial lung disease, was the only death considered to be treatment related.
The study was funded by Merck. Dr. Le and other investigators disclosed relationships, including employment, with the company.
SOURCE: Le X et al. ASCO 2020, Abstract 9556.
The objective response rate was 46.5% among the 99 patients followed for 9 or more months, as assessed by independent reviewers. There were no complete responders, according to the reviewers.
However, the response rate according to investigator assessment was 55.6%, including two responses that were judged to be complete.
The median duration of response was 11.1 months according to reviewers and 14 months according to investigators.
“The success of this trial, alongside other studies on the same class of drugs, establishes MET exon 14 as an actionable target for non–small cell lung cancer,” said senior author Xiuning Le, MD, PhD, assistant professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Le presented results from this trial as part of the American Society of Clinical Oncology virtual scientific program. Results were published simultaneously in the New England Journal of Medicine.
The trial, dubbed VISION, already won tepotinib approval in Japan to treat NSCLC patients with MET exon 14–skipping mutations. The Food and Drug Administration has granted tepotinib breakthrough status, and Merck, the drug’s manufacturer, plans to submit tepotinib for review this year.
The VISION trial enrolled 152 patients with NSCLC – 99 with at least 9 months of follow-up and 53 with shorter follow-up. The patients’ MET exon 14 mutations were confirmed by liquid or tissue biopsy.
The patients’ median age at baseline was 74 years, 54% were men, and almost half had no smoking history. Patients received tepotinib at 500 mg daily until disease progression or intolerable toxicity.
Overall, the VISION results “compare favorably” with those from studies of other MET inhibitors, the investigators wrote.
The 46.5% objective response rate (per independent reviewers) included the 99 patients with follow-up of at least 9 months who were liquid- or tissue-biopsy positive (combined group). The response rate was 48.5% among the 66 patients with positive liquid biopsies and 50% among the 60 patients with positive tissue biopsies.
The median progression-free survival was 8.5 months in the combined group, 8.5 months in the liquid-biopsy group, and 11 months in the tissue-biopsy group. The median overall survival was 17.1 months, 15.8 months, and 22.3 months, respectively.
The 11 patients with brain metastases at baseline had results that were in line with the other patients’ results. Patients with brain metastases had an objective response rate of 54.5%, a median response duration of 9.5 months, and a median progression-free survival of 10.9 months.
Overall, 88.8% of patients reported adverse events related to treatment, including peripheral edema in 63.2%, nausea in 25.7%, and diarrhea in 21.7%.
Grade 3-4 adverse events occurred in 27% of patients. Peripheral edema was the most common of these events, reported in 7.2% of patients.
“Proactive monitoring for peripheral edema is recommended and can be managed with temporary discontinuation of tepotinib or dose reduction,” the investigators wrote.
The death of a 79-year-old patient with respiratory failure and dyspnea, secondary to interstitial lung disease, was the only death considered to be treatment related.
The study was funded by Merck. Dr. Le and other investigators disclosed relationships, including employment, with the company.
SOURCE: Le X et al. ASCO 2020, Abstract 9556.
The objective response rate was 46.5% among the 99 patients followed for 9 or more months, as assessed by independent reviewers. There were no complete responders, according to the reviewers.
However, the response rate according to investigator assessment was 55.6%, including two responses that were judged to be complete.
The median duration of response was 11.1 months according to reviewers and 14 months according to investigators.
“The success of this trial, alongside other studies on the same class of drugs, establishes MET exon 14 as an actionable target for non–small cell lung cancer,” said senior author Xiuning Le, MD, PhD, assistant professor in the department of thoracic/head and neck medical oncology at the University of Texas MD Anderson Cancer Center, Houston.
Dr. Le presented results from this trial as part of the American Society of Clinical Oncology virtual scientific program. Results were published simultaneously in the New England Journal of Medicine.
The trial, dubbed VISION, already won tepotinib approval in Japan to treat NSCLC patients with MET exon 14–skipping mutations. The Food and Drug Administration has granted tepotinib breakthrough status, and Merck, the drug’s manufacturer, plans to submit tepotinib for review this year.
The VISION trial enrolled 152 patients with NSCLC – 99 with at least 9 months of follow-up and 53 with shorter follow-up. The patients’ MET exon 14 mutations were confirmed by liquid or tissue biopsy.
The patients’ median age at baseline was 74 years, 54% were men, and almost half had no smoking history. Patients received tepotinib at 500 mg daily until disease progression or intolerable toxicity.
Overall, the VISION results “compare favorably” with those from studies of other MET inhibitors, the investigators wrote.
The 46.5% objective response rate (per independent reviewers) included the 99 patients with follow-up of at least 9 months who were liquid- or tissue-biopsy positive (combined group). The response rate was 48.5% among the 66 patients with positive liquid biopsies and 50% among the 60 patients with positive tissue biopsies.
The median progression-free survival was 8.5 months in the combined group, 8.5 months in the liquid-biopsy group, and 11 months in the tissue-biopsy group. The median overall survival was 17.1 months, 15.8 months, and 22.3 months, respectively.
The 11 patients with brain metastases at baseline had results that were in line with the other patients’ results. Patients with brain metastases had an objective response rate of 54.5%, a median response duration of 9.5 months, and a median progression-free survival of 10.9 months.
Overall, 88.8% of patients reported adverse events related to treatment, including peripheral edema in 63.2%, nausea in 25.7%, and diarrhea in 21.7%.
Grade 3-4 adverse events occurred in 27% of patients. Peripheral edema was the most common of these events, reported in 7.2% of patients.
“Proactive monitoring for peripheral edema is recommended and can be managed with temporary discontinuation of tepotinib or dose reduction,” the investigators wrote.
The death of a 79-year-old patient with respiratory failure and dyspnea, secondary to interstitial lung disease, was the only death considered to be treatment related.
The study was funded by Merck. Dr. Le and other investigators disclosed relationships, including employment, with the company.
SOURCE: Le X et al. ASCO 2020, Abstract 9556.
FROM ASCO 2020
In-hospital formula feeding more than doubles odds of early weaning
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
Breastfed infants who receive formula in the hospital are more than twofold more likely to wean during the first year, compared with infants who are exclusively breastfed, according to research published online in Pediatrics.
The finding is based on an analysis of data from over 8,000 infants in the Minnesota Special Supplemental Nutrition Program for Women, Infants, and Children (WIC). The researchers used propensity scoring methods to match breastfed infants who received in-hospital formula to those who were exclusively breastfed. The researchers adjusted for potential confounders such as maternal age, cultural identity, marital status, education level, smoking, body mass index, diabetes mellitus, previous breastfeeding experience, and infant gestational age and birth weight.
“Our study strengthens the evidence that formula supplementation of breastfed infants negatively affects breastfeeding duration,” said Marcia Burton McCoy, MPH, of the Minnesota Department of Health’s WIC, and Pamela Heggie, MD, of the University of Minnesota in Minneapolis. “This finding has important clinical implications because breastfeeding duration has been shown to have a significant impact on numerous health outcomes, with a dose-response protective effect for sudden infant death syndrome, infection in infancy, and childhood obesity.”
Breastfeeding has various medical and neurodevelopmental benefits, and “even brief exposure to formula alters the infant microbiome long-term and increases the risk of allergy at 2 years of age,” the authors said.
In their study, one analysis that included 5,310 infants assumed that all bias was controlled through matching. A second, more conservative analysis that corrected for medically necessary supplementation included 4,836 infants. The researchers used data about in-hospital feeding which the Minnesota WIC staff collected in 2016 during WIC appointments.
In the first analysis, the hazard ratio of weaning across the first year was 6.1 among breastfed infants exposed to in-hospital formula feeding. In the second analysis, the hazard ratio was 2.5.
In-hospital formula feeding often leads to continued supplementation after discharge and may directly affect milk supply, Ms. McCoy and Dr. Heggie said. In-hospital formula feeding “is seldom medically necessary and, with rare exceptions, not medically indicated when the mother’s own milk or pasteurized donor milk is available.”
The study population was of lower income and more culturally diverse, compared with the general population, which may limit generalizability of the results, the authors noted.
With propensity scoring, the investigators found an association between in-hospital formula feeding and early weaning that “is analogous to previous estimates” that relied on more traditional observational methods, Lori B. Feldman-Winter, MD, MPH, professor of pediatrics at Cooper Medical School of Rowan University in Camden, N.J., and Ann L. Kellams, MD, professor of pediatrics at the University of Virginia in Charlottesville, said in an accompanying editorial.
“Maternal conditions such as obesity ... previous breast surgery, infertility, polycystic ovarian syndrome, and breast anomalies may lead to difficulties in establishing and maintaining sufficient milk supply as well as affect duration of continued breastfeeding,” the editorialists said. “Cultural, racial, and ethnic factors are also potential nonmedical reasons for breastfeeding supplementation.” In addition, implicit biases of health care practitioners may influence breastfeeding outcomes.
“The article by McCoy and Heggie gives us a compelling reason to avoid unnecessary supplementation, but there are also significant consequences of missing suboptimal intake in the newborn,” Dr. Feldman-Winter and Dr. Kellams emphasized. “Future research should be focused on methods of identifying both women and infants at risk for suboptimal intake, biological consequences of early formula supplementation, and best methods to preserve exclusive breastfeeding or human milk feeding.”
The study authors and the editorialists had no relevant financial disclosures.
SOURCES: McCoy MB et al. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2019-2946; Feldman-Winter LB and Kellams AL. Pediatrics. 2020 Jun 9. doi: 10.1542/peds.2020-1221.
FROM PEDIATRICS
Difluoroethane Inhalant Abuse, Skeletal Fluorosis, and Withdrawal
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
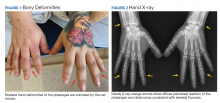
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
Difluoroethane (DFE) is an easily acquired and inexpensive volatile substance that can be inhaled recreationally. 1 It is found in common household items, including compressed air dusters, refrigerants, and propellants. DFE is a central nervous system (CNS) depressant associated with a brief sensation of euphoria when inhaled.2 Prolonged or excessive use is associated with toxicity, and abrupt cessation can induce withdrawal.3-5 We present a case of DFE abuse associated with skeletal fluorosis and withdrawal psychosis.
Case Presentation
A 39-year-old man with a 6-month history of inhaling 20 to 25 cans of DFE per day presented to the emergency department after abruptly stopping use 6 days prior. He described irritability, agitation, auditory hallucinations, and delusions of “demons trying to harm him.”
On presentation, the patient was afebrile with a mild sinus tachycardia. He was calm and cooperative but reported delusions and auditory hallucinations. He denied suicidal or homicidal ideation. His physical examination was remarkable for bony deformities of his hands (Figure 1).
The initial workup included a complete blood count; basic metabolic panel; liver function tests; urine toxicology; and testing for hepatitis B/C and HIV; all unremarkable. Psychiatry and poison control were consulted, and he was admitted.
After 72 hours, the patient's irritability, agitation, and sinus tachycardia resolved; however, his psychosis and hallucinations persisted. He was started on olanzapine and transferred to inpatient psychiatry. Additional laboratory tests revealed a serum fluoride of 0.35 mg/L (normal, 1-47 ug/L), C-telopeptide of 2,663 pg/mL (normal, 70-780 pg/mL), and hand X-rays showing diffuse bilateral periosteal reaction in the phalanges and distal ulnas (Figure 2).6
Discussion
DFE acts as a CNS depressant via glutamate and γ-aminobutyric acid receptors, causing a brief euphoria when inhaled.2 Acute toxicity can cause nausea, vomiting, abdominal pain, and altered mental status. Severe complications include loss of consciousness, mucosal frostbite, angioedema, cardiac arrhythmias, and skeletal fluorosis.2,7
Skeletal fluorosis is a rare ramification of excessive or prolonged DFE inhalation. DFE is metabolized into a fluorinated compound that accumulates and leaches calcium from bone, altering its structure. This can manifest as bony deformities with diffuse periosteal reaction and elevated serum fluoride levels. Furthermore, the elevated C-telopeptide level seen in this case may suggest increased bone turnover.
Approximately 50% of patients report withdrawal symptoms, but the timing, duration, and associated symptoms are not well understood.3 Withdrawal can include tremors, diaphoresis, nausea, vomiting, depression, anxiety, irritability, psychosis, and hallucinations. Symptoms typically start within 24 to 48 hours of cessation and last for 3 to 7 days.5 Psychotic symptoms often abate quickly; however, anxiety and insomnia can persist for weeks.5 There are no formal treatment guidelines, but poison control suggests observation and as-needed benzodiazepines. Although this patient’s irritability and agitation resolved, his psychosis and hallucinations persisted, raising concern for an underlying psychiatric diagnosis and prompting transfer to inpatient psychiatry.
Conslusion
Health care providers should recognize the symptoms of DFE toxicity, its complications, and withdrawal. Collaborating with psychiatry and poison control is beneficial in providing guidelines for supportive care.
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
1. Arroyo JP, Johnson DC, Lewis JB, et al. Treatment of acute intoxication from inhaled 1,2-difluoroethane. Ann Intern Med. 2018;169(11):820‐822. doi:10.7326/L18-0186
2. National Library of Medicine, PubChem. Hazardous Substance Data Bank (HSDB) 1,1-Difluoroethane. https:// pubchem.ncbi.nlm.nih.gov/source/hsdb/5205. Updated October 25, 2016. Accessed May 20, 2020.
3. Perron BE, Glass JE, Ahmedani BK, Vaughn MG, Roberts DE, Wu LT. The prevalence and clinical significance of inhalant withdrawal symptoms among a national sample. Subst Abuse Rehabil. 2011;2011(2):69‐76. doi:10.2147/SAR.S14937
4. Perron BE, Howard MO, Vaughn MG, Jarman CN. Inhalant withdrawal as a clinically significant feature of inhalant dependence disorder. Med Hypotheses. 2009;73(6):935‐937. doi:10.1016/j.mehy.2009.06.036
5. Addiction Center. Inhalant withdrawal and detox. https://www.addictioncenter.com/drugs/inhalants /withdrawal-detox. Accessed May 18, 2020.
6. Torra M, Rodamilans M, Corbella J. Serum and urine ionic fluoride: normal range in a nonexposed population. Biol Trace Elem Res. 1998;63(1):67‐71. doi:10.1007/BF02785278 7. Cohen E, Hsu RY, Evangelista P, Aaron R, Rubin LE. Rapid-onset diffuse skeletal fluorosis from inhalant abuse: a case report. JBJS Case Connect. 2014;4(4):e108. doi:10.2106/JBJS.CC.N.00085
Age leads COVID-19 hospitalization risk factors in RMDs
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
Being aged older than 65 years was associated with the highest risk of people with rheumatic and musculoskeletal diseases (RMDs) needing hospital treatment for COVID-19, according to the first results to be reported from ReCoVery, the German national COVID-19 registry.
Older patients with RMDs were five times more likely than younger patients to be hospitalized if they tested positive for SARS‑CoV‑2 and developed COVID-19 (odds ratio, 5.1; 95% confidence interval, 2.3-11.4).
The likelihood of hospitalization was also significantly increased by the current or prior use of glucocorticoids (OR, 2.59; 95% CI, 1.2-5.4) and by the presence of cardiovascular disease (OR, 2.27; 95% CI, 1.2-5.4).
“The register is a joint initiative of the German Society for Rheumatology and the Justus Liebig University in Giessen,” explained Anne Regierer, MD, during a live session of the annual European Congress of Rheumatology, held online this year due to COVID-19.
“The current pandemic has changed all of our lives. For patients it brought a lot of uncertainty and fears,” said Dr. Regierer, of the German Rheumatism Research Center Berlin.
“The risk of SARS-CoV-2 infection in patients with inflammatory rheumatic diseases [IRD] is still largely unknown. We still don’t know whether they have a high risk of getting the infection or whether they have a higher risk of a severer case ... therefore there’s an urgent need to have data to generate evidence for the management of our patients.”
Launched at the end of March 2020, the German registry now includes data on 251 patients – 194 of whom have recovered – provided by more than 200 registered rheumatologists. The registry data have now been integrated into the EULAR COVID-19 Database, which is itself part of a global effort to better understand and optimally manage RMD patients during the pandemic.
“The data presented by Dr. Regierer looked at similar outcomes and found quite similar results, which is reassuring,” Kimme Hyrich, MD, PhD, professor of epidemiology at the University of Manchester (England) and a consultant rheumatologist in the Kellgren Centre for Rheumatology at Manchester University Hospitals NHS Foundation Trust, said in an interview.
“We are very grateful for this collaboration [with the German society and others]. Our first publication has looked at hospitalization, but with more data we may have the opportunity to look at less-common outcomes [e.g. death, other COVID complications] or within individual diseases or treatments. So far I don’t think we will come to a different conclusion,” observed Dr. Hyrich, who is on the steering committee for the EULAR COVID-19 Database.
“These initial data are reassuring in that the majority of cases of COVID reported to our database have recovered, including those who were hospitalized,” she said.
Current EULAR advice is to continue treatment with glucocorticoids in patients who are being chronically treated, but to use them at the lowest possible dose.
The objectives of this first analysis of the German registry was to provide a description of the patients who did and did not require hospitalization and those who needed ventilation, as well as look at possible risk factors for hospitalization.
Dr. Regierer reported that, of 192 patients they included – all with a positive lab test for SARS-CoV-2 – 128 (67%) did not require hospital admission. Of those that did (n = 64), 43 (22%) did not need ventilation and 21 (11%) did. Fifteen patients died, all of whom had been hospitalized, and all but one of them had needed ventilation.
Concerning the characteristics of the patients, those who needed hospital treatment with and without ventilation were older than those who were not admitted (70 vs. 65 vs. 54 years, respectively).
“Looking at the sexes, the gender distribution is also interesting. We see 69% females in the nonhospitalized patients, 65% of the inpatients without ventilation, but only 43% females in the ventilated patients. So in this group, the male patients are the majority,” Dr. Regierer observed.
Just over half of all patients in the nonhospitalized and the hospitalized without ventilation groups had IRD in remission, but those in the hospitalized with ventilation group less than one-fifth had their IRD under control.
“Of course we have to keep in mind the small sample sizes,” Dr. Regierer said, but the distribution of patients by disease type was “what you’d expect in clinical care.” The majority of patients in each of the three groups had RA (47%, 56%, and 57%), followed by psoriatic arthritis (19%, 7%, and 14%), axial spondyloarthritis (11%, 5%, and 0%), systemic lupus erythematosus (6%, 2%, and 0%), and vasculitis (1%, 5%, and 5%).
Patients who were hospitalized with and without ventilation were more likely to have more than one comorbidity than those who were not hospitalized with COVID-19.
“The most frequent comorbidity was cardiovascular disease with 58% and 76% in the inpatient groups,” Dr. Regierer reported. One-third of the nonhospitalized patients had a cardiovascular comorbidity.
“If we look at pulmonary disease, we see that 38% of the ventilator patients had an underlying pulmonary disease,” she added. This was in comparison with 19% of the hospitalized without ventilation and 13% of the nonhospitalized patients. Diabetes was another common comorbidity in hospitalized patients with (16%) and without (19%) ventilation versus just 2% of nonhospitalized patients. While these and other comorbidities such as chronic renal insufficiency were associated with higher odds ratios in the multivariate risk factor analysis, they did not reach statistical significance.
With regard to RMD treatments, more than 60% of patients in the hospitalized group had received treatment with glucocorticoids versus 37% of those who did not get admitted. No differences were seen for the other treatments.
Interestingly, “female sex, remission, and use of NSAIDs have an odds ratio smaller than 1. So there might be a lower risk of hospitalization associated with these factors,” Dr. Regierer said.
Dr. Regierer has received grant support and is part of speaker’s bureaus for a variety of pharmaceutical companies. Dr. Hyrich disclosed grant income from Bristol-Myers Squibb, UCB, and Pfizer, and receiving speaker fees from AbbVie.
FROM THE EULAR 2020 E-CONGRESS
COVID-19 drives nursing homes to overhaul infection control efforts
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support.
“Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton, which has teaching affiliations with three senior communities.
Nursing home leaders are debating how to best use testing to guide transmission-based precautions and isolation strategies and how to keep residents safe while allowing some socialization after months of conflicting guidance from public health officials (on testing and on sites of care for patients discharged from the hospital, for instance), with a lack of adequate personal protective equipment (PPE) and testing supplies, and with nursing home resident deaths estimated to account for at least one-quarter of the total COVID-19–related mortality in the United States.
“COVID is not going away [over the next couple of years],” said Michael Wasserman, MD, medical director of the Eisenberg Village at the Los Angeles Jewish Home and president of the California Association of Long-Term Care Medicine.
Dr. Wasserman and other experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following:
- Full-time, well-trained “infection preventionists” – infection prevention managers, in essence – who can lead improvements in emergency preparedness and infection prevention and control (IPC)
- Medical directors who are well qualified and engaged
- A survey/inspection process that is educational and not solely punitive
- More resources and attention to structural reform
“If this pandemic doesn’t create significant change in the nursing home industry, nothing ever will,” Dr. Wasserman said.
Prepandemic experience
When Ghinwa Dumyati, MD, began working with nursing homes in early March to prevent and contain COVID-19 outbreaks, her focus was on PPE.
Nursing home staff were intimately familiar with standard precautions, and many had used contact precautions to prevent transmission of infections like Clostridioides difficile and Candida auris, as well as droplet precautions for influenza. With the threat of COVID-19, nursing homes “had a brand-new requirement to do both contact and droplet precautions – with a new need for eye protection – and in some situations, respiratory precautions with N95 masks,” said Dr. Dumyati, professor of medicine and director of communicable disease surveillance and prevention at the University of Rochester (N.Y.) Medical Center. “And on top of that, [staff] had to learn to conserve and reuse PPE.”
Staff had not been fit-tested for use of N95 respirators, she noted. “The only time an N95 was used in the nursing home prior to COVID-19,” she said, “was for a suspected tuberculosis patient [before hospital admission].”
Similarly, nursing homes had experience in quarantining units to prevent transmission of illnesses like influenza or norovirus – keeping residents in their rooms with no visitations or social activity, for instance – but never did they have to arrange “massive movements of residents to completely new units or parts of a unit,” said Dr. Dumyati, who also has led hospital and nursing home collaborative programs in Rochester to beat back C. difficile, and is now helping to formulate COVID-19 recommendations and guidance for members of AMDA – The Society for Post-Acute and Long-Term Care.
As the SARS-CoV-2 virus began its spread through the United States, efforts to strengthen IPC programs in nursing homes in Rochester and elsewhere had been focused largely on multidrug resistant organisms (MDROs) and antibiotic stewardship – not on pandemic preparedness.
Reducing antibiotic use had become a national priority, and a 2016 rule by the Centers for Medicare & Medicaid Services required nursing homes to develop, over a 3-year period, an IPC program that included an antibiotic stewardship component and employment of a trained infection preventionist on at least a half-time basis. Emergency preparedness (e.g., having alternate energy sources for a facility) was also included in the rule, but it was only in 2019 when CMS updated its “Requirements for Participation” rule to stipulate that emergency preparedness include planning for “emerging infectious diseases.”
“The 2016 regulations came about because infections were so problematic in nursing homes,” especially urinary tract infections, C. difficile, and drug-resistant infections, said Patricia Stone, PhD, RN, of the Center for Health Policy at the Columbia University School of Nursing, New York, who has published widely on infection prevention and control in nursing homes.
An analysis of IPC practices in 2014 and in 2018 suggests that the IPC-focused rules were helping, mainly with antibiotic stewardship programs but also with respect to some of the practices aimed at outbreak control, such as having policies in place for grouping infected residents together, instructing infected staff to stay home, and quarantining units on which outbreaks occur, Dr. Stone said. Policies for confining residents to rooms were reported by approximately 74% of nursing homes in 2014, and by approximately 87% in 2018, for instance. Overall, nursing homes were “getting better policies in place,” she said. The analysis compared data from two cross-sectional surveys of nursing homes conducted in 2014 and 2018 (945 and 888 facilities, respectively).
Nursing homes “have a long way to go,” however, with respect to the training of infection preventionists, Dr. Stone said. In 2014, her analysis shows, almost 65% of infection preventionists had no specific infection-control training and less than 3% were Certified in Infection Control (CIC) – a credential awarded by the Certification Board of Infection Control & Epidemiology. Of the 35% who had some form of official training, most completed state or local training courses.
The numbers improved slightly in 2018, with 7% of nursing homes reporting their infection preventionists had the highest-level certification, and 44% reporting that their infection preventionists had no specific infection-control training. Research has shown that infection-control training of any kind has a “strong effect” on IPC-related outcomes. While not demonstrated in research thus far, it seems plausible that “facilities with certified [infection preventionists] will have better processes in place,” said Dr. Stone, whose research has documented the need for more monitoring of staff compliance with hand-washing and other IPC procedures.
Infection preventionists in nursing homes typically have been directors of nursing or assistant directors of nursing who fold IPC responsibilities into a multitude of other responsibilities. Before the 2016 rules, some smaller facilities hired off-site consultants to do the job.
CMS upped the ante after several months of COVID-19, recommending in mid-May that nursing homes assign at least one individual with training in infection control “to provide on-site management of the IPC program.” The infection preventionists should be a “full-time role” in facilities that have more than 100 residents, the CMS guidance said. (Prior to the pandemic, CMS issued proposed regulations in 2019 that would modify the time an infection preventionist must devote to a facility from “part time” to “sufficient time.”)
However, neither the 2016 rule nor the most recent guidance on infection preventionists define the length or content of training.
Swati Gaur, MD, chair of the Infection Advisory Committee of AMDA and a certified medical director of two skilled nursing facilities in Gainesville, Ga., said that the pandemic “has really started to crystallize some of the limitations of having a very vague role, not just in terms of what an [infection preventionists] does [in the nursing home] but also the training,”
Fortunately, Dr. Gaur said, when SARS-CoV-2 struck, she had just transitioned her facilities’ designated infection preventionist to work full-time on the role. She had worked closely with her infection preventionist on IPC issues but wishes she had arranged for more rigorous independent training. “The role of the [infection preventionist] is huge and complicated,” now involving employee health, contract tracing, cohorting, isolation, and compliance with precautions and use of PPE, in addition to surveillance, data reporting, and communication with public health officials, she said.
“Facilities are finding out now that [the infection preventionist] cannot be an afterthought. And it won’t end with COVID. We have other respiratory illnesses like flu and other viruses that we struggle with all the time,” said Dr. Gaur, who is working alongside Dr. Dumyati and two other long-term care experts on AMDA’s COVID-19 guidance. The nursing homes that Dr. Gaur directs are part of the Northeast Georgia Health Care System and together include 271 beds.
Moving forward
IPC practices often collide with facilities’ role as a home, especially to those receiving long-term care. “We always have to measure what we do [to prevent and control infections] against patient autonomy and residents’ rights,” said Dr. Gaur. “We have struggled with these issues, prior to the pandemic. If patients are positive for multidrug resistant organisms [for instance], how long can they be isolated in their own rooms? You can’t for days and months put someone in a single room and create isolation. That’s where the science of infection prevention can collide with residents’ rights.”
Over the years, the Centers for Disease Control and Prevention has acknowledged this discordance, leaving it to facilities to decide, for instance, whether to actively screen for colonization with MDROs. In 2019, to help nursing homes prevent the transmission of MDROs from residents who are colonized but not actively infected, the CDC introduced new “enhanced barrier precautions” that require the use of gowns and gloves for specific resident activities identified as having a high risk of MDRO transmission. The new category of precautions is less restrictive than traditional contact precautions, which keep residents in their rooms.
Infection control in nursing homes “isn’t where it needs to be ... but we’re always going to have in nursing homes a situation where there’s a high potential for rapid transmission of infectious disease,” said Christopher Crnich, MD, PhD, an infectious disease specialist at the University of Wisconsin–Madison who chairs the long-term care special interest group of the Society of Healthcare Epidemiology of America and has offered COVID-19 advice to his state’s department of public health.
“Anytime you have a congregative community, particularly one that involves susceptible hosts, there will be an intrinsically susceptible environment ... I’m a bit disturbed by the emphasis on saying, ‘This nursing home had a COVID-19 outbreak, therefore this nursing home did something wrong,’ ” Dr. Crnich said.
“How we mitigate the size of the outbreaks is where we need to focus our attention,” he said. The goal with SARS-CoV-2, he said, is to recognize its introduction “as rapidly as possible” and stop its spread through empiric symptom- and exposure-based isolation, multiple waves of targeted testing, widespread use of contact and droplet precautions, and isolating staff as necessary.
As awareness grew this year among long-term care leaders that relying too heavily on symptom-based strategies may not be effective to prevent introduction and transmission of SARS-CoV-2, a study published in April in the New England Journal of Medicine cemented the need for a testing strategy not limited to symptomatic individuals.
The study documented that more than half of residents in a nursing home who had positive polymerase chain reaction (PCR) test results were asymptomatic at the time of testing, and that most went on to develop symptoms. The study was conducted after one case of COVID-19 had been identified.
Some states issued calls this spring for “universal testing” of all nursing home patients and staff, and the CMS recommendations issued to state and local officials in mid-May for phased nursing home “reopening” call for baseline testing of all residents and staff, followed by retesting all residents weekly until all residents test negative and by retesting all staff continuing every week.
However, the experts contacted for this story said that, without a highly accurate and accessible point-of-care test (and even with one, considering the virus’ incubation period), a universal approach that includes all nursing home residents may have more limited value than is being touted. In many scenarios, they said, it is most meaningful to focus still-limited testing supplies on the staff, many of whom work at more than one facility and are believed to be primary vectors of SARS-CoV-2.
Dr. Ouslander, Dr. Wasserman and other long-term care leaders have been discussing testing at length, trying to reach consensus on best policies. “I don’t think there’s any uniform approach or uniform agreement,” said Dr. Ouslander. “For me, under ideal circumstances what needs to be done to protect older people in nursing homes is to get access to as many accurate viral tests as possible and test staff at least once a week or every 10 days.”
In some facilities, there may be an unspoken barrier to the frequent testing of staff: Fear that staff who test positive will need to be quarantined, with no one to take their place on the front line. Dr. Ouslander said he knows of one county health department that has discouraged nursing homes from testing asymptomatic staff. “It’s insane and truly shocking,” he said.
At the University of Rochester Medical Center, Dr. Dumyati said, staffing agencies are running short of nurse aide substitutes, and staffing issues have become the “biggest challenge” facing a regional multidisciplinary group of medical directors, hospital leaders, and health department officials who are working to troubleshoot COVID-19 issues. “Some of our nursing homes have ended up sending some of their residents to other nursing homes or to the hospital [because of the loss of staff],” she said.
Currently in the state of New York, she noted, COVID-19 patients may not be discharged to nursing homes until they test negative for the virus through PCR testing. “And some people don’t clear by PCR for 4-6 weeks.”
The barriers
Staffing shortages – real in some locales, and anticipated in others as economic reopening grows – are reflective of underlying structural and financial factors that work against optimal IPC, experts said. It’s not uncommon for certified nurse assistants (CNAs) to be assigned to 10-15 residents. And according to AMDA, 30%-46% of CNAs are reported to receive some form of public assistance. Low wages force many CNAs to work other jobs, including shifts at other nursing homes.
Turnover of nursing home leadership also creates problems. Dr. Crnich calls it “one of the biggest barriers” to effective IPC in nursing homes. “Facilities can tolerate some turnover in their front line staff,” he said, “as long as their leadership structure remains relatively stable.” Dr. Stone and her coinvestigators have documented at least yearly turnover in top positions: They found that, in 2018, approximately one-quarter of facilities reported employing three or more infection preventionists, three or more administrators, and three or more directors of nursing during the prior 3 years.
Medical directors, moreover, are not uniformly qualified, engaged with their facilities, or supported by nursing home administrators. “It’s an open secret, I think, that a lot of facilities want a medical director who is a good referral source,” said Dr. Gaur. “A medical director needs to be completely engaged in [quality improvement and] infection control practices.”
Some nursing home chains, she noted, “have realized the value of the medical director, and have changed the way they’re paying them. They’re actually holding them accountable [for quality and outcomes].”
Medical directors such as Dr. Wasserman, who previously oversaw a 74-facility nursing home chain in California as chief medical officer and then chief executive officer and has worked on nursing home quality improvement processes for his state, said there is much that can be done clinically to prevent the spread of infections, such as more frequent use of telemedicine, more attention to “deprescribing” unnecessary medications (which reduces the number of medication passes and, thus, the number of “transmission opportunities”), and the use of continuous remote monitoring. He has been trying to secure Bluetooth-enabled pulse oximetry and temperature monitoring for the Los Angeles Jewish Home and other facilities.
Dr. Wasserman and other long-term care leaders believe that a more educational inspection process would also lead to improvements in IPC. “The punitive nature of the survey process is morally deflating to frontline staff [and] penalties take money away from operations,” Dr. Wasserman said. “It’s not a productive approach to quality improvement.”
Dr. Stone agreed. Infection control is now the primary focus of CMS’s inspection process, and she said that increased regulatory scrutiny of IPC beyond COVID-19 is a “good thing.” Her research has shown that most deficiencies identified by inspectors are infection control deficiencies, and that in 2014 and 2018, approximately one-third of nursing homes had infection control citations. (CMS recently increased penalties and fines for identified deficiencies.)
“But my hope would be that the survey process would be more educational [as it is for hospitals],” she said. “We need to be supporting nursing homes to do a better job.”
A silver lining of the COVID-19 pandemic, as Dr. Stone sees it, is that nursing homes may be more engaged with data reporting and infection surveillance going forward. Nursing homes are now required to report their COVID-19 cases to the CDC through its hospital-dominant National Healthcare Safety Network, and the CDC has made technical changes that now make it “easier [than it was in the past] for nursing homes to join and participate,” she said. “Now that all nursing homes are engaged, will they be engaged post-COVID, too? I hope so. Surveillance [of infections] is a first step toward better outcomes.”
For now, said Dr. Crnich, the intensive prevention and mitigation efforts that are being required of nursing homes to minimize COVID-19’s impact is “a big deal and will tax the resources of most nursing homes and exceed the resources of many” without outside support, Dr. Crnich said. “This has been the most illuminating part of all this, and will probably require us to reconsider how we’re resourcing our nursing homes moving forward into the future.”
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support.
“Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton, which has teaching affiliations with three senior communities.
Nursing home leaders are debating how to best use testing to guide transmission-based precautions and isolation strategies and how to keep residents safe while allowing some socialization after months of conflicting guidance from public health officials (on testing and on sites of care for patients discharged from the hospital, for instance), with a lack of adequate personal protective equipment (PPE) and testing supplies, and with nursing home resident deaths estimated to account for at least one-quarter of the total COVID-19–related mortality in the United States.
“COVID is not going away [over the next couple of years],” said Michael Wasserman, MD, medical director of the Eisenberg Village at the Los Angeles Jewish Home and president of the California Association of Long-Term Care Medicine.
Dr. Wasserman and other experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following:
- Full-time, well-trained “infection preventionists” – infection prevention managers, in essence – who can lead improvements in emergency preparedness and infection prevention and control (IPC)
- Medical directors who are well qualified and engaged
- A survey/inspection process that is educational and not solely punitive
- More resources and attention to structural reform
“If this pandemic doesn’t create significant change in the nursing home industry, nothing ever will,” Dr. Wasserman said.
Prepandemic experience
When Ghinwa Dumyati, MD, began working with nursing homes in early March to prevent and contain COVID-19 outbreaks, her focus was on PPE.
Nursing home staff were intimately familiar with standard precautions, and many had used contact precautions to prevent transmission of infections like Clostridioides difficile and Candida auris, as well as droplet precautions for influenza. With the threat of COVID-19, nursing homes “had a brand-new requirement to do both contact and droplet precautions – with a new need for eye protection – and in some situations, respiratory precautions with N95 masks,” said Dr. Dumyati, professor of medicine and director of communicable disease surveillance and prevention at the University of Rochester (N.Y.) Medical Center. “And on top of that, [staff] had to learn to conserve and reuse PPE.”
Staff had not been fit-tested for use of N95 respirators, she noted. “The only time an N95 was used in the nursing home prior to COVID-19,” she said, “was for a suspected tuberculosis patient [before hospital admission].”
Similarly, nursing homes had experience in quarantining units to prevent transmission of illnesses like influenza or norovirus – keeping residents in their rooms with no visitations or social activity, for instance – but never did they have to arrange “massive movements of residents to completely new units or parts of a unit,” said Dr. Dumyati, who also has led hospital and nursing home collaborative programs in Rochester to beat back C. difficile, and is now helping to formulate COVID-19 recommendations and guidance for members of AMDA – The Society for Post-Acute and Long-Term Care.
As the SARS-CoV-2 virus began its spread through the United States, efforts to strengthen IPC programs in nursing homes in Rochester and elsewhere had been focused largely on multidrug resistant organisms (MDROs) and antibiotic stewardship – not on pandemic preparedness.
Reducing antibiotic use had become a national priority, and a 2016 rule by the Centers for Medicare & Medicaid Services required nursing homes to develop, over a 3-year period, an IPC program that included an antibiotic stewardship component and employment of a trained infection preventionist on at least a half-time basis. Emergency preparedness (e.g., having alternate energy sources for a facility) was also included in the rule, but it was only in 2019 when CMS updated its “Requirements for Participation” rule to stipulate that emergency preparedness include planning for “emerging infectious diseases.”
“The 2016 regulations came about because infections were so problematic in nursing homes,” especially urinary tract infections, C. difficile, and drug-resistant infections, said Patricia Stone, PhD, RN, of the Center for Health Policy at the Columbia University School of Nursing, New York, who has published widely on infection prevention and control in nursing homes.
An analysis of IPC practices in 2014 and in 2018 suggests that the IPC-focused rules were helping, mainly with antibiotic stewardship programs but also with respect to some of the practices aimed at outbreak control, such as having policies in place for grouping infected residents together, instructing infected staff to stay home, and quarantining units on which outbreaks occur, Dr. Stone said. Policies for confining residents to rooms were reported by approximately 74% of nursing homes in 2014, and by approximately 87% in 2018, for instance. Overall, nursing homes were “getting better policies in place,” she said. The analysis compared data from two cross-sectional surveys of nursing homes conducted in 2014 and 2018 (945 and 888 facilities, respectively).
Nursing homes “have a long way to go,” however, with respect to the training of infection preventionists, Dr. Stone said. In 2014, her analysis shows, almost 65% of infection preventionists had no specific infection-control training and less than 3% were Certified in Infection Control (CIC) – a credential awarded by the Certification Board of Infection Control & Epidemiology. Of the 35% who had some form of official training, most completed state or local training courses.
The numbers improved slightly in 2018, with 7% of nursing homes reporting their infection preventionists had the highest-level certification, and 44% reporting that their infection preventionists had no specific infection-control training. Research has shown that infection-control training of any kind has a “strong effect” on IPC-related outcomes. While not demonstrated in research thus far, it seems plausible that “facilities with certified [infection preventionists] will have better processes in place,” said Dr. Stone, whose research has documented the need for more monitoring of staff compliance with hand-washing and other IPC procedures.
Infection preventionists in nursing homes typically have been directors of nursing or assistant directors of nursing who fold IPC responsibilities into a multitude of other responsibilities. Before the 2016 rules, some smaller facilities hired off-site consultants to do the job.
CMS upped the ante after several months of COVID-19, recommending in mid-May that nursing homes assign at least one individual with training in infection control “to provide on-site management of the IPC program.” The infection preventionists should be a “full-time role” in facilities that have more than 100 residents, the CMS guidance said. (Prior to the pandemic, CMS issued proposed regulations in 2019 that would modify the time an infection preventionist must devote to a facility from “part time” to “sufficient time.”)
However, neither the 2016 rule nor the most recent guidance on infection preventionists define the length or content of training.
Swati Gaur, MD, chair of the Infection Advisory Committee of AMDA and a certified medical director of two skilled nursing facilities in Gainesville, Ga., said that the pandemic “has really started to crystallize some of the limitations of having a very vague role, not just in terms of what an [infection preventionists] does [in the nursing home] but also the training,”
Fortunately, Dr. Gaur said, when SARS-CoV-2 struck, she had just transitioned her facilities’ designated infection preventionist to work full-time on the role. She had worked closely with her infection preventionist on IPC issues but wishes she had arranged for more rigorous independent training. “The role of the [infection preventionist] is huge and complicated,” now involving employee health, contract tracing, cohorting, isolation, and compliance with precautions and use of PPE, in addition to surveillance, data reporting, and communication with public health officials, she said.
“Facilities are finding out now that [the infection preventionist] cannot be an afterthought. And it won’t end with COVID. We have other respiratory illnesses like flu and other viruses that we struggle with all the time,” said Dr. Gaur, who is working alongside Dr. Dumyati and two other long-term care experts on AMDA’s COVID-19 guidance. The nursing homes that Dr. Gaur directs are part of the Northeast Georgia Health Care System and together include 271 beds.
Moving forward
IPC practices often collide with facilities’ role as a home, especially to those receiving long-term care. “We always have to measure what we do [to prevent and control infections] against patient autonomy and residents’ rights,” said Dr. Gaur. “We have struggled with these issues, prior to the pandemic. If patients are positive for multidrug resistant organisms [for instance], how long can they be isolated in their own rooms? You can’t for days and months put someone in a single room and create isolation. That’s where the science of infection prevention can collide with residents’ rights.”
Over the years, the Centers for Disease Control and Prevention has acknowledged this discordance, leaving it to facilities to decide, for instance, whether to actively screen for colonization with MDROs. In 2019, to help nursing homes prevent the transmission of MDROs from residents who are colonized but not actively infected, the CDC introduced new “enhanced barrier precautions” that require the use of gowns and gloves for specific resident activities identified as having a high risk of MDRO transmission. The new category of precautions is less restrictive than traditional contact precautions, which keep residents in their rooms.
Infection control in nursing homes “isn’t where it needs to be ... but we’re always going to have in nursing homes a situation where there’s a high potential for rapid transmission of infectious disease,” said Christopher Crnich, MD, PhD, an infectious disease specialist at the University of Wisconsin–Madison who chairs the long-term care special interest group of the Society of Healthcare Epidemiology of America and has offered COVID-19 advice to his state’s department of public health.
“Anytime you have a congregative community, particularly one that involves susceptible hosts, there will be an intrinsically susceptible environment ... I’m a bit disturbed by the emphasis on saying, ‘This nursing home had a COVID-19 outbreak, therefore this nursing home did something wrong,’ ” Dr. Crnich said.
“How we mitigate the size of the outbreaks is where we need to focus our attention,” he said. The goal with SARS-CoV-2, he said, is to recognize its introduction “as rapidly as possible” and stop its spread through empiric symptom- and exposure-based isolation, multiple waves of targeted testing, widespread use of contact and droplet precautions, and isolating staff as necessary.
As awareness grew this year among long-term care leaders that relying too heavily on symptom-based strategies may not be effective to prevent introduction and transmission of SARS-CoV-2, a study published in April in the New England Journal of Medicine cemented the need for a testing strategy not limited to symptomatic individuals.
The study documented that more than half of residents in a nursing home who had positive polymerase chain reaction (PCR) test results were asymptomatic at the time of testing, and that most went on to develop symptoms. The study was conducted after one case of COVID-19 had been identified.
Some states issued calls this spring for “universal testing” of all nursing home patients and staff, and the CMS recommendations issued to state and local officials in mid-May for phased nursing home “reopening” call for baseline testing of all residents and staff, followed by retesting all residents weekly until all residents test negative and by retesting all staff continuing every week.
However, the experts contacted for this story said that, without a highly accurate and accessible point-of-care test (and even with one, considering the virus’ incubation period), a universal approach that includes all nursing home residents may have more limited value than is being touted. In many scenarios, they said, it is most meaningful to focus still-limited testing supplies on the staff, many of whom work at more than one facility and are believed to be primary vectors of SARS-CoV-2.
Dr. Ouslander, Dr. Wasserman and other long-term care leaders have been discussing testing at length, trying to reach consensus on best policies. “I don’t think there’s any uniform approach or uniform agreement,” said Dr. Ouslander. “For me, under ideal circumstances what needs to be done to protect older people in nursing homes is to get access to as many accurate viral tests as possible and test staff at least once a week or every 10 days.”
In some facilities, there may be an unspoken barrier to the frequent testing of staff: Fear that staff who test positive will need to be quarantined, with no one to take their place on the front line. Dr. Ouslander said he knows of one county health department that has discouraged nursing homes from testing asymptomatic staff. “It’s insane and truly shocking,” he said.
At the University of Rochester Medical Center, Dr. Dumyati said, staffing agencies are running short of nurse aide substitutes, and staffing issues have become the “biggest challenge” facing a regional multidisciplinary group of medical directors, hospital leaders, and health department officials who are working to troubleshoot COVID-19 issues. “Some of our nursing homes have ended up sending some of their residents to other nursing homes or to the hospital [because of the loss of staff],” she said.
Currently in the state of New York, she noted, COVID-19 patients may not be discharged to nursing homes until they test negative for the virus through PCR testing. “And some people don’t clear by PCR for 4-6 weeks.”
The barriers
Staffing shortages – real in some locales, and anticipated in others as economic reopening grows – are reflective of underlying structural and financial factors that work against optimal IPC, experts said. It’s not uncommon for certified nurse assistants (CNAs) to be assigned to 10-15 residents. And according to AMDA, 30%-46% of CNAs are reported to receive some form of public assistance. Low wages force many CNAs to work other jobs, including shifts at other nursing homes.
Turnover of nursing home leadership also creates problems. Dr. Crnich calls it “one of the biggest barriers” to effective IPC in nursing homes. “Facilities can tolerate some turnover in their front line staff,” he said, “as long as their leadership structure remains relatively stable.” Dr. Stone and her coinvestigators have documented at least yearly turnover in top positions: They found that, in 2018, approximately one-quarter of facilities reported employing three or more infection preventionists, three or more administrators, and three or more directors of nursing during the prior 3 years.
Medical directors, moreover, are not uniformly qualified, engaged with their facilities, or supported by nursing home administrators. “It’s an open secret, I think, that a lot of facilities want a medical director who is a good referral source,” said Dr. Gaur. “A medical director needs to be completely engaged in [quality improvement and] infection control practices.”
Some nursing home chains, she noted, “have realized the value of the medical director, and have changed the way they’re paying them. They’re actually holding them accountable [for quality and outcomes].”
Medical directors such as Dr. Wasserman, who previously oversaw a 74-facility nursing home chain in California as chief medical officer and then chief executive officer and has worked on nursing home quality improvement processes for his state, said there is much that can be done clinically to prevent the spread of infections, such as more frequent use of telemedicine, more attention to “deprescribing” unnecessary medications (which reduces the number of medication passes and, thus, the number of “transmission opportunities”), and the use of continuous remote monitoring. He has been trying to secure Bluetooth-enabled pulse oximetry and temperature monitoring for the Los Angeles Jewish Home and other facilities.
Dr. Wasserman and other long-term care leaders believe that a more educational inspection process would also lead to improvements in IPC. “The punitive nature of the survey process is morally deflating to frontline staff [and] penalties take money away from operations,” Dr. Wasserman said. “It’s not a productive approach to quality improvement.”
Dr. Stone agreed. Infection control is now the primary focus of CMS’s inspection process, and she said that increased regulatory scrutiny of IPC beyond COVID-19 is a “good thing.” Her research has shown that most deficiencies identified by inspectors are infection control deficiencies, and that in 2014 and 2018, approximately one-third of nursing homes had infection control citations. (CMS recently increased penalties and fines for identified deficiencies.)
“But my hope would be that the survey process would be more educational [as it is for hospitals],” she said. “We need to be supporting nursing homes to do a better job.”
A silver lining of the COVID-19 pandemic, as Dr. Stone sees it, is that nursing homes may be more engaged with data reporting and infection surveillance going forward. Nursing homes are now required to report their COVID-19 cases to the CDC through its hospital-dominant National Healthcare Safety Network, and the CDC has made technical changes that now make it “easier [than it was in the past] for nursing homes to join and participate,” she said. “Now that all nursing homes are engaged, will they be engaged post-COVID, too? I hope so. Surveillance [of infections] is a first step toward better outcomes.”
For now, said Dr. Crnich, the intensive prevention and mitigation efforts that are being required of nursing homes to minimize COVID-19’s impact is “a big deal and will tax the resources of most nursing homes and exceed the resources of many” without outside support, Dr. Crnich said. “This has been the most illuminating part of all this, and will probably require us to reconsider how we’re resourcing our nursing homes moving forward into the future.”
The toll that COVID-19 has taken on nursing homes and their postacute and long-term care residents has a multilayered backstory involving underresourced organizational structures, inherent susceptibilities, minimally trained infection prevention staff, variable abilities to isolate and quarantine large numbers of patients and residents, and a lack of governmental support.
“Nursing homes have been trying their best to combat this pandemic using the best infection control procedures they have, but blindfolded and with their hands tied behind their backs,” said Joseph G. Ouslander, MD, professor of geriatric medicine at Florida Atlantic University, Boca Raton, which has teaching affiliations with three senior communities.
Nursing home leaders are debating how to best use testing to guide transmission-based precautions and isolation strategies and how to keep residents safe while allowing some socialization after months of conflicting guidance from public health officials (on testing and on sites of care for patients discharged from the hospital, for instance), with a lack of adequate personal protective equipment (PPE) and testing supplies, and with nursing home resident deaths estimated to account for at least one-quarter of the total COVID-19–related mortality in the United States.
“COVID is not going away [over the next couple of years],” said Michael Wasserman, MD, medical director of the Eisenberg Village at the Los Angeles Jewish Home and president of the California Association of Long-Term Care Medicine.
Dr. Wasserman and other experts in both long-term care and infectious disease said in interviews that, through the rest of the pandemic and beyond, nursing homes need the following:
- Full-time, well-trained “infection preventionists” – infection prevention managers, in essence – who can lead improvements in emergency preparedness and infection prevention and control (IPC)
- Medical directors who are well qualified and engaged
- A survey/inspection process that is educational and not solely punitive
- More resources and attention to structural reform
“If this pandemic doesn’t create significant change in the nursing home industry, nothing ever will,” Dr. Wasserman said.
Prepandemic experience
When Ghinwa Dumyati, MD, began working with nursing homes in early March to prevent and contain COVID-19 outbreaks, her focus was on PPE.
Nursing home staff were intimately familiar with standard precautions, and many had used contact precautions to prevent transmission of infections like Clostridioides difficile and Candida auris, as well as droplet precautions for influenza. With the threat of COVID-19, nursing homes “had a brand-new requirement to do both contact and droplet precautions – with a new need for eye protection – and in some situations, respiratory precautions with N95 masks,” said Dr. Dumyati, professor of medicine and director of communicable disease surveillance and prevention at the University of Rochester (N.Y.) Medical Center. “And on top of that, [staff] had to learn to conserve and reuse PPE.”
Staff had not been fit-tested for use of N95 respirators, she noted. “The only time an N95 was used in the nursing home prior to COVID-19,” she said, “was for a suspected tuberculosis patient [before hospital admission].”
Similarly, nursing homes had experience in quarantining units to prevent transmission of illnesses like influenza or norovirus – keeping residents in their rooms with no visitations or social activity, for instance – but never did they have to arrange “massive movements of residents to completely new units or parts of a unit,” said Dr. Dumyati, who also has led hospital and nursing home collaborative programs in Rochester to beat back C. difficile, and is now helping to formulate COVID-19 recommendations and guidance for members of AMDA – The Society for Post-Acute and Long-Term Care.
As the SARS-CoV-2 virus began its spread through the United States, efforts to strengthen IPC programs in nursing homes in Rochester and elsewhere had been focused largely on multidrug resistant organisms (MDROs) and antibiotic stewardship – not on pandemic preparedness.
Reducing antibiotic use had become a national priority, and a 2016 rule by the Centers for Medicare & Medicaid Services required nursing homes to develop, over a 3-year period, an IPC program that included an antibiotic stewardship component and employment of a trained infection preventionist on at least a half-time basis. Emergency preparedness (e.g., having alternate energy sources for a facility) was also included in the rule, but it was only in 2019 when CMS updated its “Requirements for Participation” rule to stipulate that emergency preparedness include planning for “emerging infectious diseases.”
“The 2016 regulations came about because infections were so problematic in nursing homes,” especially urinary tract infections, C. difficile, and drug-resistant infections, said Patricia Stone, PhD, RN, of the Center for Health Policy at the Columbia University School of Nursing, New York, who has published widely on infection prevention and control in nursing homes.
An analysis of IPC practices in 2014 and in 2018 suggests that the IPC-focused rules were helping, mainly with antibiotic stewardship programs but also with respect to some of the practices aimed at outbreak control, such as having policies in place for grouping infected residents together, instructing infected staff to stay home, and quarantining units on which outbreaks occur, Dr. Stone said. Policies for confining residents to rooms were reported by approximately 74% of nursing homes in 2014, and by approximately 87% in 2018, for instance. Overall, nursing homes were “getting better policies in place,” she said. The analysis compared data from two cross-sectional surveys of nursing homes conducted in 2014 and 2018 (945 and 888 facilities, respectively).
Nursing homes “have a long way to go,” however, with respect to the training of infection preventionists, Dr. Stone said. In 2014, her analysis shows, almost 65% of infection preventionists had no specific infection-control training and less than 3% were Certified in Infection Control (CIC) – a credential awarded by the Certification Board of Infection Control & Epidemiology. Of the 35% who had some form of official training, most completed state or local training courses.
The numbers improved slightly in 2018, with 7% of nursing homes reporting their infection preventionists had the highest-level certification, and 44% reporting that their infection preventionists had no specific infection-control training. Research has shown that infection-control training of any kind has a “strong effect” on IPC-related outcomes. While not demonstrated in research thus far, it seems plausible that “facilities with certified [infection preventionists] will have better processes in place,” said Dr. Stone, whose research has documented the need for more monitoring of staff compliance with hand-washing and other IPC procedures.
Infection preventionists in nursing homes typically have been directors of nursing or assistant directors of nursing who fold IPC responsibilities into a multitude of other responsibilities. Before the 2016 rules, some smaller facilities hired off-site consultants to do the job.
CMS upped the ante after several months of COVID-19, recommending in mid-May that nursing homes assign at least one individual with training in infection control “to provide on-site management of the IPC program.” The infection preventionists should be a “full-time role” in facilities that have more than 100 residents, the CMS guidance said. (Prior to the pandemic, CMS issued proposed regulations in 2019 that would modify the time an infection preventionist must devote to a facility from “part time” to “sufficient time.”)
However, neither the 2016 rule nor the most recent guidance on infection preventionists define the length or content of training.
Swati Gaur, MD, chair of the Infection Advisory Committee of AMDA and a certified medical director of two skilled nursing facilities in Gainesville, Ga., said that the pandemic “has really started to crystallize some of the limitations of having a very vague role, not just in terms of what an [infection preventionists] does [in the nursing home] but also the training,”
Fortunately, Dr. Gaur said, when SARS-CoV-2 struck, she had just transitioned her facilities’ designated infection preventionist to work full-time on the role. She had worked closely with her infection preventionist on IPC issues but wishes she had arranged for more rigorous independent training. “The role of the [infection preventionist] is huge and complicated,” now involving employee health, contract tracing, cohorting, isolation, and compliance with precautions and use of PPE, in addition to surveillance, data reporting, and communication with public health officials, she said.
“Facilities are finding out now that [the infection preventionist] cannot be an afterthought. And it won’t end with COVID. We have other respiratory illnesses like flu and other viruses that we struggle with all the time,” said Dr. Gaur, who is working alongside Dr. Dumyati and two other long-term care experts on AMDA’s COVID-19 guidance. The nursing homes that Dr. Gaur directs are part of the Northeast Georgia Health Care System and together include 271 beds.
Moving forward
IPC practices often collide with facilities’ role as a home, especially to those receiving long-term care. “We always have to measure what we do [to prevent and control infections] against patient autonomy and residents’ rights,” said Dr. Gaur. “We have struggled with these issues, prior to the pandemic. If patients are positive for multidrug resistant organisms [for instance], how long can they be isolated in their own rooms? You can’t for days and months put someone in a single room and create isolation. That’s where the science of infection prevention can collide with residents’ rights.”
Over the years, the Centers for Disease Control and Prevention has acknowledged this discordance, leaving it to facilities to decide, for instance, whether to actively screen for colonization with MDROs. In 2019, to help nursing homes prevent the transmission of MDROs from residents who are colonized but not actively infected, the CDC introduced new “enhanced barrier precautions” that require the use of gowns and gloves for specific resident activities identified as having a high risk of MDRO transmission. The new category of precautions is less restrictive than traditional contact precautions, which keep residents in their rooms.
Infection control in nursing homes “isn’t where it needs to be ... but we’re always going to have in nursing homes a situation where there’s a high potential for rapid transmission of infectious disease,” said Christopher Crnich, MD, PhD, an infectious disease specialist at the University of Wisconsin–Madison who chairs the long-term care special interest group of the Society of Healthcare Epidemiology of America and has offered COVID-19 advice to his state’s department of public health.
“Anytime you have a congregative community, particularly one that involves susceptible hosts, there will be an intrinsically susceptible environment ... I’m a bit disturbed by the emphasis on saying, ‘This nursing home had a COVID-19 outbreak, therefore this nursing home did something wrong,’ ” Dr. Crnich said.
“How we mitigate the size of the outbreaks is where we need to focus our attention,” he said. The goal with SARS-CoV-2, he said, is to recognize its introduction “as rapidly as possible” and stop its spread through empiric symptom- and exposure-based isolation, multiple waves of targeted testing, widespread use of contact and droplet precautions, and isolating staff as necessary.
As awareness grew this year among long-term care leaders that relying too heavily on symptom-based strategies may not be effective to prevent introduction and transmission of SARS-CoV-2, a study published in April in the New England Journal of Medicine cemented the need for a testing strategy not limited to symptomatic individuals.
The study documented that more than half of residents in a nursing home who had positive polymerase chain reaction (PCR) test results were asymptomatic at the time of testing, and that most went on to develop symptoms. The study was conducted after one case of COVID-19 had been identified.
Some states issued calls this spring for “universal testing” of all nursing home patients and staff, and the CMS recommendations issued to state and local officials in mid-May for phased nursing home “reopening” call for baseline testing of all residents and staff, followed by retesting all residents weekly until all residents test negative and by retesting all staff continuing every week.
However, the experts contacted for this story said that, without a highly accurate and accessible point-of-care test (and even with one, considering the virus’ incubation period), a universal approach that includes all nursing home residents may have more limited value than is being touted. In many scenarios, they said, it is most meaningful to focus still-limited testing supplies on the staff, many of whom work at more than one facility and are believed to be primary vectors of SARS-CoV-2.
Dr. Ouslander, Dr. Wasserman and other long-term care leaders have been discussing testing at length, trying to reach consensus on best policies. “I don’t think there’s any uniform approach or uniform agreement,” said Dr. Ouslander. “For me, under ideal circumstances what needs to be done to protect older people in nursing homes is to get access to as many accurate viral tests as possible and test staff at least once a week or every 10 days.”
In some facilities, there may be an unspoken barrier to the frequent testing of staff: Fear that staff who test positive will need to be quarantined, with no one to take their place on the front line. Dr. Ouslander said he knows of one county health department that has discouraged nursing homes from testing asymptomatic staff. “It’s insane and truly shocking,” he said.
At the University of Rochester Medical Center, Dr. Dumyati said, staffing agencies are running short of nurse aide substitutes, and staffing issues have become the “biggest challenge” facing a regional multidisciplinary group of medical directors, hospital leaders, and health department officials who are working to troubleshoot COVID-19 issues. “Some of our nursing homes have ended up sending some of their residents to other nursing homes or to the hospital [because of the loss of staff],” she said.
Currently in the state of New York, she noted, COVID-19 patients may not be discharged to nursing homes until they test negative for the virus through PCR testing. “And some people don’t clear by PCR for 4-6 weeks.”
The barriers
Staffing shortages – real in some locales, and anticipated in others as economic reopening grows – are reflective of underlying structural and financial factors that work against optimal IPC, experts said. It’s not uncommon for certified nurse assistants (CNAs) to be assigned to 10-15 residents. And according to AMDA, 30%-46% of CNAs are reported to receive some form of public assistance. Low wages force many CNAs to work other jobs, including shifts at other nursing homes.
Turnover of nursing home leadership also creates problems. Dr. Crnich calls it “one of the biggest barriers” to effective IPC in nursing homes. “Facilities can tolerate some turnover in their front line staff,” he said, “as long as their leadership structure remains relatively stable.” Dr. Stone and her coinvestigators have documented at least yearly turnover in top positions: They found that, in 2018, approximately one-quarter of facilities reported employing three or more infection preventionists, three or more administrators, and three or more directors of nursing during the prior 3 years.
Medical directors, moreover, are not uniformly qualified, engaged with their facilities, or supported by nursing home administrators. “It’s an open secret, I think, that a lot of facilities want a medical director who is a good referral source,” said Dr. Gaur. “A medical director needs to be completely engaged in [quality improvement and] infection control practices.”
Some nursing home chains, she noted, “have realized the value of the medical director, and have changed the way they’re paying them. They’re actually holding them accountable [for quality and outcomes].”
Medical directors such as Dr. Wasserman, who previously oversaw a 74-facility nursing home chain in California as chief medical officer and then chief executive officer and has worked on nursing home quality improvement processes for his state, said there is much that can be done clinically to prevent the spread of infections, such as more frequent use of telemedicine, more attention to “deprescribing” unnecessary medications (which reduces the number of medication passes and, thus, the number of “transmission opportunities”), and the use of continuous remote monitoring. He has been trying to secure Bluetooth-enabled pulse oximetry and temperature monitoring for the Los Angeles Jewish Home and other facilities.
Dr. Wasserman and other long-term care leaders believe that a more educational inspection process would also lead to improvements in IPC. “The punitive nature of the survey process is morally deflating to frontline staff [and] penalties take money away from operations,” Dr. Wasserman said. “It’s not a productive approach to quality improvement.”
Dr. Stone agreed. Infection control is now the primary focus of CMS’s inspection process, and she said that increased regulatory scrutiny of IPC beyond COVID-19 is a “good thing.” Her research has shown that most deficiencies identified by inspectors are infection control deficiencies, and that in 2014 and 2018, approximately one-third of nursing homes had infection control citations. (CMS recently increased penalties and fines for identified deficiencies.)
“But my hope would be that the survey process would be more educational [as it is for hospitals],” she said. “We need to be supporting nursing homes to do a better job.”
A silver lining of the COVID-19 pandemic, as Dr. Stone sees it, is that nursing homes may be more engaged with data reporting and infection surveillance going forward. Nursing homes are now required to report their COVID-19 cases to the CDC through its hospital-dominant National Healthcare Safety Network, and the CDC has made technical changes that now make it “easier [than it was in the past] for nursing homes to join and participate,” she said. “Now that all nursing homes are engaged, will they be engaged post-COVID, too? I hope so. Surveillance [of infections] is a first step toward better outcomes.”
For now, said Dr. Crnich, the intensive prevention and mitigation efforts that are being required of nursing homes to minimize COVID-19’s impact is “a big deal and will tax the resources of most nursing homes and exceed the resources of many” without outside support, Dr. Crnich said. “This has been the most illuminating part of all this, and will probably require us to reconsider how we’re resourcing our nursing homes moving forward into the future.”
Diffuse pustules
The presence of these sterile pustules with an erythematous base led to a diagnosis of acute generalized exanthematous pustulosis (AGEP), also known as a pustular drug eruption. Although pustules are present, AGEP is an allergic response to medications and not an infection.
AGEP can be associated with fever and leukocytosis. Interestingly, antibiotics are a frequent cause—not a treatment—since the pustules are sterile. It also is worth noting, in light of the COVID-19 pandemic, that hydroxychloroquine use has been linked to AGEP, although the number of cases cited in the literature is small.
Treatment is avoidance of the offending medication and symptomatic care. AGEP typically will resolve approximately 2 weeks after discontinuing the medication causing the reaction. Systemic steroids also may be used for treatment in severe cases.
This patient had a history of repeated episodes with his chemotherapy regimen, so he was treated symptomatically with diphenhydramine for the itching. Since chemotherapy was a priority to treat his colon cancer, avoidance of the offending agent was not an option. The Family Medicine Service recommended pretreatment with diphenhydramine 25 to 50 mg orally or intravenously for future rounds of chemotherapy to blunt future responses.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Mercogliano C, Khan M, Lin C, et al. AGEP overlap induced by hydroxychloroquine: a case report and literature review. J Community Hosp Intern Med Perspect. 2018;8:360-362.
The presence of these sterile pustules with an erythematous base led to a diagnosis of acute generalized exanthematous pustulosis (AGEP), also known as a pustular drug eruption. Although pustules are present, AGEP is an allergic response to medications and not an infection.
AGEP can be associated with fever and leukocytosis. Interestingly, antibiotics are a frequent cause—not a treatment—since the pustules are sterile. It also is worth noting, in light of the COVID-19 pandemic, that hydroxychloroquine use has been linked to AGEP, although the number of cases cited in the literature is small.
Treatment is avoidance of the offending medication and symptomatic care. AGEP typically will resolve approximately 2 weeks after discontinuing the medication causing the reaction. Systemic steroids also may be used for treatment in severe cases.
This patient had a history of repeated episodes with his chemotherapy regimen, so he was treated symptomatically with diphenhydramine for the itching. Since chemotherapy was a priority to treat his colon cancer, avoidance of the offending agent was not an option. The Family Medicine Service recommended pretreatment with diphenhydramine 25 to 50 mg orally or intravenously for future rounds of chemotherapy to blunt future responses.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
The presence of these sterile pustules with an erythematous base led to a diagnosis of acute generalized exanthematous pustulosis (AGEP), also known as a pustular drug eruption. Although pustules are present, AGEP is an allergic response to medications and not an infection.
AGEP can be associated with fever and leukocytosis. Interestingly, antibiotics are a frequent cause—not a treatment—since the pustules are sterile. It also is worth noting, in light of the COVID-19 pandemic, that hydroxychloroquine use has been linked to AGEP, although the number of cases cited in the literature is small.
Treatment is avoidance of the offending medication and symptomatic care. AGEP typically will resolve approximately 2 weeks after discontinuing the medication causing the reaction. Systemic steroids also may be used for treatment in severe cases.
This patient had a history of repeated episodes with his chemotherapy regimen, so he was treated symptomatically with diphenhydramine for the itching. Since chemotherapy was a priority to treat his colon cancer, avoidance of the offending agent was not an option. The Family Medicine Service recommended pretreatment with diphenhydramine 25 to 50 mg orally or intravenously for future rounds of chemotherapy to blunt future responses.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Mercogliano C, Khan M, Lin C, et al. AGEP overlap induced by hydroxychloroquine: a case report and literature review. J Community Hosp Intern Med Perspect. 2018;8:360-362.
Mercogliano C, Khan M, Lin C, et al. AGEP overlap induced by hydroxychloroquine: a case report and literature review. J Community Hosp Intern Med Perspect. 2018;8:360-362.
CMS issues interim final rule
On Thursday, April 30, 2020, CMS released a new interim final rule. During the COVID-19 Public Health Emergency, the Interim Final Rule makes several new, important temporary changes to Medicare regulations and payments. One important change retroactively (to March 1, 2020) increased payments for telephone-only visits to established patients:
- CPT 99441: a 5- to 10-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99212, about $46 (99441 is usually reimbursed at about $14).
- CPT 99442: an 11- to 20-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99213, about $76 (99442 is usually reimbursed at about $28).
- CPT 99443: a 21- to 30-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99212, about $110 (99443 is usually reimbursed at about $41).
These telephone codes may be used when addressing a new or old problem for established patients. Choose the code to reflect only the billing provider time communicating with the patient. There should not be another patient encounter for 7 calendar days before or after the telephone visit.
In addition, the new Interim Final Rule now allows attending physicians at teaching institutions providing supervision under the Primary Care Exception to report for telephone (using 99441-99443) or video (using 99212-99215) telemedicine encounters by residents, when the supervision is provided immediately after the resident encounter, rather than during the telephone or video visit. However, most chest physicians at teaching institutions do not supervise residents or fellows under the Primary Care Exception.
A CMS press release about the rule is available at cms.gov.
On Thursday, April 30, 2020, CMS released a new interim final rule. During the COVID-19 Public Health Emergency, the Interim Final Rule makes several new, important temporary changes to Medicare regulations and payments. One important change retroactively (to March 1, 2020) increased payments for telephone-only visits to established patients:
- CPT 99441: a 5- to 10-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99212, about $46 (99441 is usually reimbursed at about $14).
- CPT 99442: an 11- to 20-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99213, about $76 (99442 is usually reimbursed at about $28).
- CPT 99443: a 21- to 30-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99212, about $110 (99443 is usually reimbursed at about $41).
These telephone codes may be used when addressing a new or old problem for established patients. Choose the code to reflect only the billing provider time communicating with the patient. There should not be another patient encounter for 7 calendar days before or after the telephone visit.
In addition, the new Interim Final Rule now allows attending physicians at teaching institutions providing supervision under the Primary Care Exception to report for telephone (using 99441-99443) or video (using 99212-99215) telemedicine encounters by residents, when the supervision is provided immediately after the resident encounter, rather than during the telephone or video visit. However, most chest physicians at teaching institutions do not supervise residents or fellows under the Primary Care Exception.
A CMS press release about the rule is available at cms.gov.
On Thursday, April 30, 2020, CMS released a new interim final rule. During the COVID-19 Public Health Emergency, the Interim Final Rule makes several new, important temporary changes to Medicare regulations and payments. One important change retroactively (to March 1, 2020) increased payments for telephone-only visits to established patients:
- CPT 99441: a 5- to 10-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99212, about $46 (99441 is usually reimbursed at about $14).
- CPT 99442: an 11- to 20-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99213, about $76 (99442 is usually reimbursed at about $28).
- CPT 99443: a 21- to 30-minute telephone visit, in lieu of a face-to-face office visit, will be reimbursed at a similar rate to a 99212, about $110 (99443 is usually reimbursed at about $41).
These telephone codes may be used when addressing a new or old problem for established patients. Choose the code to reflect only the billing provider time communicating with the patient. There should not be another patient encounter for 7 calendar days before or after the telephone visit.
In addition, the new Interim Final Rule now allows attending physicians at teaching institutions providing supervision under the Primary Care Exception to report for telephone (using 99441-99443) or video (using 99212-99215) telemedicine encounters by residents, when the supervision is provided immediately after the resident encounter, rather than during the telephone or video visit. However, most chest physicians at teaching institutions do not supervise residents or fellows under the Primary Care Exception.
A CMS press release about the rule is available at cms.gov.
Your CHEST Foundation: Supporting communities during COVID-2019
The entire world has been affected by the COVID-19 crisis, yet many of our most vulnerable continue to suffer in silence. The CHEST Foundation is diligently working to help give voice to these all-too-often isolated and forgotten patients. Make a donation today, and help those who need it most: our family, friends, neighbors, and those most vulnerable to this devastating disease.
In addition to providing reliable and educational resources that address COVID-19 for both clinicians and patients, the CHEST Foundation is:
- Launching a series of public service announcement videos to empower patients and caregivers living with COPD and interstitial lung disease by providing information on necessary skills, such as cleaning medical equipment, and helping them stay safe and healthy while coping with isolation;
- Partnering with AMITA Health in Chicago to bring telehealth opportunities to patients and support groups; and
- Providing grant funding, in partnership with the Feldman Family Foundation, that supports projects such as providing supplies and groceries to patients and caregivers, expediting training and the means to get caregivers to NYC, and providing needed technology to continue hosting support group meetings in local communities.
The CHEST Foundation has rebranded and relaunched its website in an effort to make it more user-friendly, patient-focused, and clinician-centered. We’ve upgraded our current content, written new pieces, and carefully curated a complete collection of tools that will help patients, caregivers, and clinicians better navigate the complexities of lung disease. Information on all of the content previously listed will be available on the CHEST Foundation’s website at chestfoundation.org.
Thank you for helping as we fulfill the urgent needs of our community during this crisis. Help support your community by making a donation today.
The entire world has been affected by the COVID-19 crisis, yet many of our most vulnerable continue to suffer in silence. The CHEST Foundation is diligently working to help give voice to these all-too-often isolated and forgotten patients. Make a donation today, and help those who need it most: our family, friends, neighbors, and those most vulnerable to this devastating disease.
In addition to providing reliable and educational resources that address COVID-19 for both clinicians and patients, the CHEST Foundation is:
- Launching a series of public service announcement videos to empower patients and caregivers living with COPD and interstitial lung disease by providing information on necessary skills, such as cleaning medical equipment, and helping them stay safe and healthy while coping with isolation;
- Partnering with AMITA Health in Chicago to bring telehealth opportunities to patients and support groups; and
- Providing grant funding, in partnership with the Feldman Family Foundation, that supports projects such as providing supplies and groceries to patients and caregivers, expediting training and the means to get caregivers to NYC, and providing needed technology to continue hosting support group meetings in local communities.
The CHEST Foundation has rebranded and relaunched its website in an effort to make it more user-friendly, patient-focused, and clinician-centered. We’ve upgraded our current content, written new pieces, and carefully curated a complete collection of tools that will help patients, caregivers, and clinicians better navigate the complexities of lung disease. Information on all of the content previously listed will be available on the CHEST Foundation’s website at chestfoundation.org.
Thank you for helping as we fulfill the urgent needs of our community during this crisis. Help support your community by making a donation today.
The entire world has been affected by the COVID-19 crisis, yet many of our most vulnerable continue to suffer in silence. The CHEST Foundation is diligently working to help give voice to these all-too-often isolated and forgotten patients. Make a donation today, and help those who need it most: our family, friends, neighbors, and those most vulnerable to this devastating disease.
In addition to providing reliable and educational resources that address COVID-19 for both clinicians and patients, the CHEST Foundation is:
- Launching a series of public service announcement videos to empower patients and caregivers living with COPD and interstitial lung disease by providing information on necessary skills, such as cleaning medical equipment, and helping them stay safe and healthy while coping with isolation;
- Partnering with AMITA Health in Chicago to bring telehealth opportunities to patients and support groups; and
- Providing grant funding, in partnership with the Feldman Family Foundation, that supports projects such as providing supplies and groceries to patients and caregivers, expediting training and the means to get caregivers to NYC, and providing needed technology to continue hosting support group meetings in local communities.
The CHEST Foundation has rebranded and relaunched its website in an effort to make it more user-friendly, patient-focused, and clinician-centered. We’ve upgraded our current content, written new pieces, and carefully curated a complete collection of tools that will help patients, caregivers, and clinicians better navigate the complexities of lung disease. Information on all of the content previously listed will be available on the CHEST Foundation’s website at chestfoundation.org.
Thank you for helping as we fulfill the urgent needs of our community during this crisis. Help support your community by making a donation today.
Today’s best bet – Get involved with CHEST!
I am often overheard encouraging colleagues to become involved with CHEST. I am a strong believer that you get far more out of participation than you will ever put into it. I have now been fortunate to have many leadership roles within CHEST and currently serve on the Board of Regents and as Chair of the Council of NetWorks. I have been able to work with a growing number of people, including faculty and CHEST staff. The more invested I have become, the more CHEST truly feels like family.
I understand that while it may be easy for me to tell members to get involved, it often feels much more difficult to actually get appointed to a leadership position. Early in my career, I was given the advice, “When you are given a task, make sure you blow it out of the water. That will only open more doors for you.” Making the most of a position on a NetWork or committee can create future opportunities. We recently had self-nominations for leadership positions within the NetWork steering committees and committees at large. Some positions have one to two openings for 20 applications. It can be frustrating not to get a position the first time around. However, it is common for members to have to apply numerous times prior to being appointed. When applying to these positions, be sure to highlight any prior CHEST involvement, as this may weigh in on an appointment to specific positions. Some of the decisions to appoint a nominee are based on prior engagement with CHEST.
So how can one get involved without holding a leadership position? My first piece of advice is to ensure you are getting CHEST emails. Check them regularly to so that you do not miss any opportunities. Next, be a member of at least one NetWork that is of interest to you. The NetWorks provide a smaller community within CHEST for special interests within our field. You will get emailed updates throughout the year that include any projects in which input is needed. At the CHEST annual meeting, each NetWork holds an Open Forum that functions as their annual face-to-face business meeting. These meetings are open to everyone. This is an excellent way to meet the current steering committee members and become involved in plans for the upcoming year. This year, we have made the dates and times of the NetWork steering committee calls public on the CHEST website. Any NetWork member can join these calls, even if they are not officially on the steering committee. All ongoing projects are discussed on these calls, so participation on the call offers an excellent opportunity to volunteer. You can also get involved with the NetWorks on social media by using the appropriate NetWork hashtags, along with tagging @accpchest to communicate with your NetWork colleagues.

Finally, the easiest way to embrace CHEST, and possibly the most obvious, is to get involved with the CHEST annual meeting. The meeting is at its best when planned and orchestrated by a diverse group of people. Annual meeting planning usually starts in November or December of the prior year. Submitting a proposal for a session at the annual meeting is strongly encouraged. Tips for how to submit a strong, well-rounded session are offered on the submission website. Reviewing these tips first can help strengthen your proposal. An easy way to become involved, even as a student or as a trainee, is to submit an abstract to the annual meeting
Summing up, I would encourage everyone to simply be an active participant: raise your hand to ask questions, introduce yourself to those around you, and attend the social events at CHEST annual meeting. Before you know it, new friends will become old friends, and attending the CHEST annual meeting will start to feel like going to a family reunion.
I am often overheard encouraging colleagues to become involved with CHEST. I am a strong believer that you get far more out of participation than you will ever put into it. I have now been fortunate to have many leadership roles within CHEST and currently serve on the Board of Regents and as Chair of the Council of NetWorks. I have been able to work with a growing number of people, including faculty and CHEST staff. The more invested I have become, the more CHEST truly feels like family.
I understand that while it may be easy for me to tell members to get involved, it often feels much more difficult to actually get appointed to a leadership position. Early in my career, I was given the advice, “When you are given a task, make sure you blow it out of the water. That will only open more doors for you.” Making the most of a position on a NetWork or committee can create future opportunities. We recently had self-nominations for leadership positions within the NetWork steering committees and committees at large. Some positions have one to two openings for 20 applications. It can be frustrating not to get a position the first time around. However, it is common for members to have to apply numerous times prior to being appointed. When applying to these positions, be sure to highlight any prior CHEST involvement, as this may weigh in on an appointment to specific positions. Some of the decisions to appoint a nominee are based on prior engagement with CHEST.
So how can one get involved without holding a leadership position? My first piece of advice is to ensure you are getting CHEST emails. Check them regularly to so that you do not miss any opportunities. Next, be a member of at least one NetWork that is of interest to you. The NetWorks provide a smaller community within CHEST for special interests within our field. You will get emailed updates throughout the year that include any projects in which input is needed. At the CHEST annual meeting, each NetWork holds an Open Forum that functions as their annual face-to-face business meeting. These meetings are open to everyone. This is an excellent way to meet the current steering committee members and become involved in plans for the upcoming year. This year, we have made the dates and times of the NetWork steering committee calls public on the CHEST website. Any NetWork member can join these calls, even if they are not officially on the steering committee. All ongoing projects are discussed on these calls, so participation on the call offers an excellent opportunity to volunteer. You can also get involved with the NetWorks on social media by using the appropriate NetWork hashtags, along with tagging @accpchest to communicate with your NetWork colleagues.

Finally, the easiest way to embrace CHEST, and possibly the most obvious, is to get involved with the CHEST annual meeting. The meeting is at its best when planned and orchestrated by a diverse group of people. Annual meeting planning usually starts in November or December of the prior year. Submitting a proposal for a session at the annual meeting is strongly encouraged. Tips for how to submit a strong, well-rounded session are offered on the submission website. Reviewing these tips first can help strengthen your proposal. An easy way to become involved, even as a student or as a trainee, is to submit an abstract to the annual meeting
Summing up, I would encourage everyone to simply be an active participant: raise your hand to ask questions, introduce yourself to those around you, and attend the social events at CHEST annual meeting. Before you know it, new friends will become old friends, and attending the CHEST annual meeting will start to feel like going to a family reunion.
I am often overheard encouraging colleagues to become involved with CHEST. I am a strong believer that you get far more out of participation than you will ever put into it. I have now been fortunate to have many leadership roles within CHEST and currently serve on the Board of Regents and as Chair of the Council of NetWorks. I have been able to work with a growing number of people, including faculty and CHEST staff. The more invested I have become, the more CHEST truly feels like family.
I understand that while it may be easy for me to tell members to get involved, it often feels much more difficult to actually get appointed to a leadership position. Early in my career, I was given the advice, “When you are given a task, make sure you blow it out of the water. That will only open more doors for you.” Making the most of a position on a NetWork or committee can create future opportunities. We recently had self-nominations for leadership positions within the NetWork steering committees and committees at large. Some positions have one to two openings for 20 applications. It can be frustrating not to get a position the first time around. However, it is common for members to have to apply numerous times prior to being appointed. When applying to these positions, be sure to highlight any prior CHEST involvement, as this may weigh in on an appointment to specific positions. Some of the decisions to appoint a nominee are based on prior engagement with CHEST.
So how can one get involved without holding a leadership position? My first piece of advice is to ensure you are getting CHEST emails. Check them regularly to so that you do not miss any opportunities. Next, be a member of at least one NetWork that is of interest to you. The NetWorks provide a smaller community within CHEST for special interests within our field. You will get emailed updates throughout the year that include any projects in which input is needed. At the CHEST annual meeting, each NetWork holds an Open Forum that functions as their annual face-to-face business meeting. These meetings are open to everyone. This is an excellent way to meet the current steering committee members and become involved in plans for the upcoming year. This year, we have made the dates and times of the NetWork steering committee calls public on the CHEST website. Any NetWork member can join these calls, even if they are not officially on the steering committee. All ongoing projects are discussed on these calls, so participation on the call offers an excellent opportunity to volunteer. You can also get involved with the NetWorks on social media by using the appropriate NetWork hashtags, along with tagging @accpchest to communicate with your NetWork colleagues.

Finally, the easiest way to embrace CHEST, and possibly the most obvious, is to get involved with the CHEST annual meeting. The meeting is at its best when planned and orchestrated by a diverse group of people. Annual meeting planning usually starts in November or December of the prior year. Submitting a proposal for a session at the annual meeting is strongly encouraged. Tips for how to submit a strong, well-rounded session are offered on the submission website. Reviewing these tips first can help strengthen your proposal. An easy way to become involved, even as a student or as a trainee, is to submit an abstract to the annual meeting
Summing up, I would encourage everyone to simply be an active participant: raise your hand to ask questions, introduce yourself to those around you, and attend the social events at CHEST annual meeting. Before you know it, new friends will become old friends, and attending the CHEST annual meeting will start to feel like going to a family reunion.
Meet the FISH Bowl finalists
CHEST 2019 marked the inaugural FISH Bowl competition for attendees. Inspired by Shark Tank, our kinder, gentler, yet still competitive and cutting-edge FISH Bowl (Furthering Innovation and Science for Health) featured CHEST members disrupting our beliefs about how clinical care and education are performed. As health-care providers, they presented innovative ideas pertaining to education and clinical disease for pulmonary, critical care, and sleep medicine. Six finalists were chosen from dozens of submissions, and three emerged winners! In this new Meet the FISH Bowl Finalists series, CHEST introduces you to many of them – including winner Dr. Rachel Quaney.
Name: Rachel Quaney, MD
Institutional Affiliation: The Ohio State University
Position: Pulmonary and Critical Care Medicine Fellow
Title: Teaching Assessment Committee (TAC)
Brief Summary of Submission: Teaching Assessment Committee (TAC) is a novel approach to faculty feedback. We are modeling it after the success of the Clinical Competency Committees, but, in reverse, as fellows will give group-consensus-based feedback to faculty members.
Fellows will meet twice yearly with trained facilitators who help elicit constructive, nuanced feedback. The group setting ensures personal anonymity, which will serve to encourage more honest feedback. Then delivering this consensus-based information to program leadership and faculty members will hopefully provide helpful feedback regarding what is going well and what could be improved.
This pilot feasibility project is being employed at three fellowship programs this academic year. The goal will be to improve the feedback that faculty receive, while simultaneously increasing both faculty and fellow satisfaction with the process and the learning environment.
1. What inspired your innovation? More like who – and that would be the esteemed Dr. Gabe Bosslet of Indiana University. He brought the faculty perspective that attendings want better feedback. And, I supplied the fellow perspective—that even those of us who prioritize all things medical education often do a subpar job at providing effective feedback.
2. Who do you think can benefit most from it, and why? With some variation, almost all graduate medical education programs could benefit from the TAC method of faculty feedback. However, the most benefit would likely be seen in small programs or those that struggle with anonymity using current feedback methods.
3. What do you see as challenges to your innovation gaining widespread acceptance? How can they be overcome? I foresee two main challenges to implementation: time and buy-in. Fellows and residents are busy individuals with plenty on their plates, and this would require asking them for more time. This barrier could be solved by program and leadership buy-in or be exacerbated if it is lacking. If the process is endorsed by departmental and program leadership, this will provide credibility and ensure the necessary time is allotted.
4. What impact has winning FISH Bowl 2019 had on your vision for the innovation? The big picture vision I have for my innovation has not changed, but I am more acutely aware of the challenges and opportunities I will have to navigate, thanks to Drs. Morris, Niven, and Schulman. I am simultaneously more excited about this project but also feel the pressure to not disappoint!
5. How do you think your success at FISH Bowl 2019 will continue to impact your career overall in the months and years to come? It’s hard to imagine in what exact ways my career will be impacted, but I feel strongly that it will be positively influenced by this experience. I had the privilege of meeting a lot of individuals who feel passionate about medical education, both those established in our field and those at the beginning of their careers. These connections will likely lead to future collaborations and innovations.
CHEST 2019 marked the inaugural FISH Bowl competition for attendees. Inspired by Shark Tank, our kinder, gentler, yet still competitive and cutting-edge FISH Bowl (Furthering Innovation and Science for Health) featured CHEST members disrupting our beliefs about how clinical care and education are performed. As health-care providers, they presented innovative ideas pertaining to education and clinical disease for pulmonary, critical care, and sleep medicine. Six finalists were chosen from dozens of submissions, and three emerged winners! In this new Meet the FISH Bowl Finalists series, CHEST introduces you to many of them – including winner Dr. Rachel Quaney.
Name: Rachel Quaney, MD
Institutional Affiliation: The Ohio State University
Position: Pulmonary and Critical Care Medicine Fellow
Title: Teaching Assessment Committee (TAC)
Brief Summary of Submission: Teaching Assessment Committee (TAC) is a novel approach to faculty feedback. We are modeling it after the success of the Clinical Competency Committees, but, in reverse, as fellows will give group-consensus-based feedback to faculty members.
Fellows will meet twice yearly with trained facilitators who help elicit constructive, nuanced feedback. The group setting ensures personal anonymity, which will serve to encourage more honest feedback. Then delivering this consensus-based information to program leadership and faculty members will hopefully provide helpful feedback regarding what is going well and what could be improved.
This pilot feasibility project is being employed at three fellowship programs this academic year. The goal will be to improve the feedback that faculty receive, while simultaneously increasing both faculty and fellow satisfaction with the process and the learning environment.
1. What inspired your innovation? More like who – and that would be the esteemed Dr. Gabe Bosslet of Indiana University. He brought the faculty perspective that attendings want better feedback. And, I supplied the fellow perspective—that even those of us who prioritize all things medical education often do a subpar job at providing effective feedback.
2. Who do you think can benefit most from it, and why? With some variation, almost all graduate medical education programs could benefit from the TAC method of faculty feedback. However, the most benefit would likely be seen in small programs or those that struggle with anonymity using current feedback methods.
3. What do you see as challenges to your innovation gaining widespread acceptance? How can they be overcome? I foresee two main challenges to implementation: time and buy-in. Fellows and residents are busy individuals with plenty on their plates, and this would require asking them for more time. This barrier could be solved by program and leadership buy-in or be exacerbated if it is lacking. If the process is endorsed by departmental and program leadership, this will provide credibility and ensure the necessary time is allotted.
4. What impact has winning FISH Bowl 2019 had on your vision for the innovation? The big picture vision I have for my innovation has not changed, but I am more acutely aware of the challenges and opportunities I will have to navigate, thanks to Drs. Morris, Niven, and Schulman. I am simultaneously more excited about this project but also feel the pressure to not disappoint!
5. How do you think your success at FISH Bowl 2019 will continue to impact your career overall in the months and years to come? It’s hard to imagine in what exact ways my career will be impacted, but I feel strongly that it will be positively influenced by this experience. I had the privilege of meeting a lot of individuals who feel passionate about medical education, both those established in our field and those at the beginning of their careers. These connections will likely lead to future collaborations and innovations.
CHEST 2019 marked the inaugural FISH Bowl competition for attendees. Inspired by Shark Tank, our kinder, gentler, yet still competitive and cutting-edge FISH Bowl (Furthering Innovation and Science for Health) featured CHEST members disrupting our beliefs about how clinical care and education are performed. As health-care providers, they presented innovative ideas pertaining to education and clinical disease for pulmonary, critical care, and sleep medicine. Six finalists were chosen from dozens of submissions, and three emerged winners! In this new Meet the FISH Bowl Finalists series, CHEST introduces you to many of them – including winner Dr. Rachel Quaney.
Name: Rachel Quaney, MD
Institutional Affiliation: The Ohio State University
Position: Pulmonary and Critical Care Medicine Fellow
Title: Teaching Assessment Committee (TAC)
Brief Summary of Submission: Teaching Assessment Committee (TAC) is a novel approach to faculty feedback. We are modeling it after the success of the Clinical Competency Committees, but, in reverse, as fellows will give group-consensus-based feedback to faculty members.
Fellows will meet twice yearly with trained facilitators who help elicit constructive, nuanced feedback. The group setting ensures personal anonymity, which will serve to encourage more honest feedback. Then delivering this consensus-based information to program leadership and faculty members will hopefully provide helpful feedback regarding what is going well and what could be improved.
This pilot feasibility project is being employed at three fellowship programs this academic year. The goal will be to improve the feedback that faculty receive, while simultaneously increasing both faculty and fellow satisfaction with the process and the learning environment.
1. What inspired your innovation? More like who – and that would be the esteemed Dr. Gabe Bosslet of Indiana University. He brought the faculty perspective that attendings want better feedback. And, I supplied the fellow perspective—that even those of us who prioritize all things medical education often do a subpar job at providing effective feedback.
2. Who do you think can benefit most from it, and why? With some variation, almost all graduate medical education programs could benefit from the TAC method of faculty feedback. However, the most benefit would likely be seen in small programs or those that struggle with anonymity using current feedback methods.
3. What do you see as challenges to your innovation gaining widespread acceptance? How can they be overcome? I foresee two main challenges to implementation: time and buy-in. Fellows and residents are busy individuals with plenty on their plates, and this would require asking them for more time. This barrier could be solved by program and leadership buy-in or be exacerbated if it is lacking. If the process is endorsed by departmental and program leadership, this will provide credibility and ensure the necessary time is allotted.
4. What impact has winning FISH Bowl 2019 had on your vision for the innovation? The big picture vision I have for my innovation has not changed, but I am more acutely aware of the challenges and opportunities I will have to navigate, thanks to Drs. Morris, Niven, and Schulman. I am simultaneously more excited about this project but also feel the pressure to not disappoint!
5. How do you think your success at FISH Bowl 2019 will continue to impact your career overall in the months and years to come? It’s hard to imagine in what exact ways my career will be impacted, but I feel strongly that it will be positively influenced by this experience. I had the privilege of meeting a lot of individuals who feel passionate about medical education, both those established in our field and those at the beginning of their careers. These connections will likely lead to future collaborations and innovations.