User login
The Group Practice Manager in the VHA: A View From the Field
The Veterans Health Administration (VHA) provides care for 9 million veterans at 1,255 health care sites linked to one of 170 local medical systems.1 Recognizing that providing timely care requires effective access management, the US Congress mandated training of VHA staff to manage and improve access to care but did not provide additional local funds for new positions.2 In response, the VHA created the group practice manager (GPM), a new position responsible for improving clinical practice management and unifying access improvement across leadership levels, professions, and services within each local medical system.
In May 2015, the VHA began hiring and training GPMs to spearhead management of access to services. The US Department of Veterans Affairs (VA) Office of Veteran Access to Care spearheaded GPM training, including face-to-face sessions, national calls, webinars, and educational materials. Five local medical systems were selected by the VA Office of Veteran Access to Care to implement the GPM role to allow for an early evaluation of the program that would inform a subsequent nationwide rollout. Implementation of the GPM role remained in the hands of local medical systems.
Longer wait times are shown to impact patient health.3,4 Open access scheduling and other patient-centered access management interventions have been shown to improve availability of primary care appointments.5 However, little empirical evidence exists regarding the managers who focus on clinic access interventions. While the nonpeer-reviewed literature includes references to such roles, including GPMs, the empirical literature has focused on external practice faciliators,6-8 “mid-level managers,”9 and clinic staff.10 We found no peer-reviewed articles on the needs and experiences of practice managers who are focused on improving access. The purpose of this study was to examine GPM prototype sites to both enhance subsequent nationwide implementation and to advance empirical literature on managing patient access within health care.
Methods
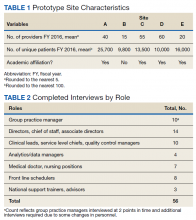
In 2015, the VA identified 5 prototype sites representing diverse geographic locations, size, and complexity for the implementation of the GPM role (Table 1). These sites self-identified as having clinical practice management experience. GPMs attended 4 training sessions between February and August 2015.
Data Collection
Participants from each prototype site included GPMs, national trainers, clinic leaders, and frontline staff. Table 2 includes the roles and sample size. Participants were recruited through purposive sampling followed by snowball sampling until thematic saturation was reached (the point at which subsequent data fail to produce new findings across sites and roles of interest).
Guided by the Consolidated Framework for Implementation Research (CFIR), the research team developed semistructured interview guides tailored to participants’ roles to elicit rich descriptions regarding overall impressions, practice management strategies, goals, activities, relationship to clinic roles, data analytics usage, challenges, barriers, and facilitators.11 These guides included open-ended questions and structured prompts utilizing participant language for follow-up probes to minimize interviewing bias (eAppendix:
Data Analysis
Data were analyzed using iterative deductive and inductive content analysis.12 Deductive content analysis consisted of identifying quotes that fit within preidentified categories (ie, perceptions of national effort, organizational structure for GPM, challenges, facilitators, metrics and tools, and mobilizing access culture) developed by the interdisciplinary research team. Further content analysis entailed open-coding and iteratively revisiting and reconciling codes associated within each preidentified category as new codes emerged. The team analyzed the resulting codes to inductively and iteratively identify and stabilize themes regarding the GPM role: roles and tasks, GPM characteristics, issues, and challenges. Through this process we moved coded data to reconciled descriptions suited to addressing the purposes of this study. Dedoose 7.0.23 software was used for qualitative data management and analysis.
Results
The study identified participants’ overall impressions of the GPM initiative and key themes within 4 major domains regarding implementing the GPM role: roles and tasks (implementing clinic practice management, leading patient access, supporting data analytics, and enabling self and staff); GPM characteristics (familiarity with clinical services, knowledge of VHA systems, ability to analyze patient data, communication skills, and the ability to work with others); and issues, and challenges (technical, social, and structural).
Overall Impressions
Interviewees perceived the GPM initiative as a consolidation of existing distributed responsibilities into one role that directly reported to local top-level management with indirect reporting to national leaders. Many of the sites reported that they had designated or planned to designate a role resembling the GPM prior to the initiative. “There are staff who’ve been doing some of this work all along,” a GPM noted. “We just didn’t have them grouped together. They weren’t necessarily all working in the same type of service under the same type of structure.”
Whether the GPM position was new or not, participants referenced the importance and challenges of engaging the local facility in recognizing the agency associated with the GPM position. According to national support, the staff are trying to get the facility to understand “why the group practice manager is so important… we’ve got to embed that standard position in the system.”
While the GPM was recognized as the hub of access management, respondents recognized that transformation regarding access involved many players. “We have to create [an] orchestrated team inside each facility,” an advisor argued.
Respondents discussed how the initiative allows local facilities to appoint a specific person with a specific title and role who helps facilitate, organize, and legitimize an access focus at their sites. One GPM interviewee noted how the initiative helped refocus some of their previously less centralized efforts. “We’ve always looked at productivity; we’ve always looked at access; we’ve always looked at efficiency. I think the bigger difference is now there are individuals identified in the clinics, as practice managers as well…I interact with them. They interact with individual clinic staff, and it’s more of a group process than a single individual.”
The value of having tools available and being able to track and manage patient care as a specific example of the positive impact of this new role was noted by participants. A GPM noted that many health care providers will be happy to have tools to better manage their services and a process “that flows from a service level all the way up to executive management, where there is a common interest in making those things happen—I think that’s going to be a tremendous help.”
Participants expressed concern that the national GPM rollout would be a one-size-fits-all approach. These respondents emphasized the need to have the flexibility to customize their activities to meet their unique site and patient needs.
GPM Roles and Tasks
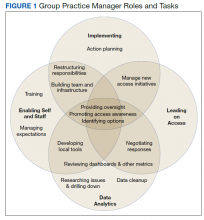
Participants described 4 primary roles that the GPM was expected to fill: implementing clinic practice management, leading patient access, supporting data analytics, and enabling self and staff. Some activities overlapped in that they served to support multiple role areas (Figure 1).
Implementing clinic practice management. In the early stages of the initiative, the GPM’s primary role was to prepare the facility to implement a standardized set of clinic practice management (CPM) team processes. Part of standardizing the CPM process was defining the scope and tasks of the GPM, which requires significant planning for the implementation. “My big job is to finalize what we think group practice management is going to look [like] here,” a GPM reported.
Each prototype site had latitude to interpret the GPM initiative in a way that would work in their context within given VHA boundaries and ongoing initiatives. To achieve the high-level vision and purpose, the GPM first had to develop action plans that accounted for the operating environment of the facility. According to one GPM, VA national officials are “constantly” asking for action plans, which required significant time by specific deadlines. “They want an action plan [and to] clean up all your consults, [and to] clean up all your recall reminders.”
Leading on improving access efforts. Participants saw the GPM as the central staff member responsible for providing oversight of any activities and people involved in improving access. “I ensure everybody is doing what they’re supposed to do,” one GPM reported. When the GPM sees areas that are not being addressed, the individual tries to develop a process or training to “close those gaps.”
GPMs promoted an awareness of their goals, changes in process, and new tools accompanying the initiative. However, other access initiatives were occurring simultaneously creating confusion for health care providers and patients; thus GPMs found they were managing a wide array of related initiatives.
GPMs have to negotiate with leaders across the VHA facility, many of whom operated at a higher leadership level and had different priorities, to address access problems.
“I’m a lieutenant as a GPM in a clinic, a GPM noted. “How is the lieutenant going to talk to a major or a colonel in the clinic and say your clinic has problems. How[‘s] that lieutenant...going to do that? With people skills!”
Managing expectations about the speed and to what extent a problem could be resolved was an important part of the GPM leadership role. “I see myself as managing expectations both up to the leadership and down to the frontline,” a GPM explained. “I find myself talking to leadership [about] our progress. But at the same time, we have to say, ‘not everything can be fixed overnight.’”
Providing leadership on access-related issues included developing a range of options for addressing patient access problems. One analytics manager recounted how the GPM role led to evaluating how physical space limited efficiency in clinic flow. The first step was identifying possible additional rooms to improve clinic flow. This required working with the space committee to “get someone to look at our overarching space and find someplace else for them to sit” to avoid adding to congestion in the clinic area.
Supporting data analytics.
GPMs routinely immersed themselves in the data to understand access issues. GPMs worked with clinic leaders to identify the underlying causes and various solutions. The GPMs maintained access through administrative scrubbing of the data and finding “smart ways to get patients scheduled,” a GPM explained. “I don’t think our facility would have taken care of as many veterans in the time frame as we did....We’ve cleared over 4,000 consults that were older than 90 days. We have cleared thousands and thousands of weekly reminders.”
GPMs expressed the need for aggregated (ie, dashboard) and standardized information to efficiently address access issues. “I would like to have some more standardization on what’s being reviewed; it seems to change frequently, and so [to] be able to track and trend and have something given to me to review,” one health care provider requested. On the other hand, participants also described a need for decision support tools that would lead to action aligned with best practices. “Instead of a dashboard or something that’s just measuring their performance, it’s more something that they can look at and take action” a national support staff advisor suggested.
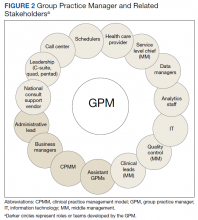
Enabling self and staff. GPMs felt they were most effective if they enabled themselves and stakeholders through training and by cultivating relationships and team building. Figure 2 illustrates the various stakeholders GPMs reported engaging with. The GPMS should be building relationships, bridging relationships, developing trust, and then providing a higher level of hands-on management. However, “that doesn’t really happen right now, day to day,” one member of leadership reported.
Key topics in GPM leadership training included both soft skills (change management, culture change, and negotiation skills) and crucial analytic/technical training (understanding each metric and dashboard available, data analytics, and supply/demand balancing techniques). The GPMs not only wanted to understand metrics as part of their training, but also want to know what to do about them.
An “operationalization” training approach (discerning the meaning of data, data-based decision making, and determining action from multiple options) inspired by real-life situations was preferred by participants. Other effective learning structures included job aids in the form of templated Gantt charts, process maps to guide GPMs through implementation of new processes, formalized peer learning (accumulated field insights shared during training courses), and informal peer sharing of direct experiences during calls.
GPMs also emphasized training for frontline clinical and support staff, including schedulers. VHA schedulers typically have less education and experience higher turnover rates than do other clinic staff, yet they carry out complex and critical tasks. Providing training and ensuring that any materials developed for training and education were appropriate to the level of education of schedulers was an important task for GPMs. “If they don’t understand all of the scheduling principles and potential,” one GPM explained, “we will not be maximizing the utilization of our parts.”
GPMs also provided informal education to clinicians. Participants noted GPMs have to avoid appearing to overstep their positions or presuming more knowledge and expertise than clinicians. They “have to be able to teach a physician without being overbearing, in a way a physician will accept it as advisement,” one program leader reported.
GPM knowledge, skills, and abilities. GPMs presented a complex range of knowledge, skills, and abilities, including clinical, administrative, analytics, and people skills. All interviewees reported that their prior education and experience did not sufficiently train them for the GPM role. GPMs identified a willingness to learn quickly as a critical characteristic. Many GPMs tended to have a formal education in health administration or business (eg, MBAs); others had administrative experience (eg, administrative assistance to executive leadership) or clinical training (eg, physician assistant). Detailed clinical knowledge was not expected, but clinical familiarity was helpful.
Some interviewees also mentioned previous experience and familiarity with the VHA system specifically as an advantage. This was especially true for VA outpatient flows, clinic flows, and understanding what an outpatient is in a VA context. Interviewees noted the importance of GPMs needing to be able to analyze patient demand metrics and underlying data in order to determine supply of providers and then to allocate adequate resources to complement providers. Forecasting skills were referenced as a key point. “They need to be able to be assured that they can recruit more providers if needed,” a national support staff advisor noted.
Given the importance of developing effective relationships, communication skills were mentioned by most participants and underscored as critical to establishing trust between GPMs and others as the initiative was being implemented. Interviewees indicated that relationship building was further enhanced when GPMs possessed the ability to “work with” rather than command clinicians and staff; navigate politics; and were respectful of other people’s knowledge, skills, abilities, and status. “They have to work with the nursing staff and teach them,” a leader described, “so that people understand that we are going to a different place to achieve our primary objectives and goals.”
Issues and Challenges
Participants identified several technical, social, and structural challenges and barriers to successfully implementing the GPM role.
Technical challenges. Recurring themes across all phases of data analytics were GPMs’ capability to challenge data use and use large volumes of information from multiple data sources (entering and accessing data; “drilling down” from summaries; generating reports; and analyzing and interpreting resulting metrics). Interviewees reported that information assessment and analytic support were not consistent. One GPM had a data analyst pulling reports needed to support clinical units while other GPMs trained staff to pull data. Even with support, some GPMs had issues due to limited information technology (IT) skills or access privileges leading to inefficiencies and delays. “Whenever I need anything from a programmer, I have to go through, you know, the IT gods in the sky,” one GPM remarked. “That usually takes a few months or more.”
Social challenges. Instituting the GPM role was a cultural change, and interviewees reported needing to address resistance to CPM model efforts. Resistance to change “is particularly hard in the VA just because it has a unique culture,” one leader noted. “There is a comfort in the legacy way of doing things.” The GPM initiative was introduced during a time when other national level initiatives were being implemented throughout the VHA. Fulfilling requests for information for these initiatives became the responsibility of the GPM and their team, which diverted attention from the mandate to improve access. Furthermore, GPMs were often considered the “change communicators” to clinics putting them in the role of “bad messenger,” which degraded trust and made it difficult to partner with clinicians.
Efforts to work through change management and build relationships included general program awareness presentation to internal stakeholders; including key stakeholders in GPM committees; pre-emptive conversations with unit chiefs; creating awareness of the GPM activities and progress through formal and informal update meetings; and identifying successes regarding access.
Structural challenges. The GPM role did not have direct supervision over clinical and administrative leaders, making it challenging to enact change. GPMs reported that “they do not always have authority over the area that they are being asked to manage,” which made their work difficult, requiring strong negotiation skills and political savvy to affect change. However, as the clinic staff and providers saw how the GPM could support and positively impact their practice, these challenges began to subside.
Discussion
This study provides empirical evidence regarding the implementation of a new access management strategy for health care systems focused on improving timeliness of care. First, the GPM position was seen as critical at each facility, as a single point person, to help local system leaders respond effectively to both national mandates and local context. Second, requiring the GPMs to report to the medical center director or chief of staff was important for integrating access perspectives across service lines and to facilitate a strong GPM role in strategic planning. Third, the intentional flexibility of the access management initiative, beyond the nationally specified aspects of the GPM role, was key for allowing individual sites to adapt to unique local challenges, resources, and population demands. Fourth, the initiative provided GPMs with opportunities to learn important skills and support the acquisition, utilization, and communication of a tremendous range of data toward responsive action.
According to our respondents, the GPM role demands functioning across a broad set of responsibilities; understanding the big picture as well as the complex underlying variables; engaging facility leaders, clinical and administrative staff; and prioritizing competing national and local demands. Consistent with previous findings, effective GPMs must possess a complex set of skills (interpersonal, analytic, and leadership) and the ability to create a supporting team.13
In practice, improving access at individual sites of care (VA medical centers and community-based outpatient clinics) poses significant challenges, especially in the early stages, even with the assistance of a GPM. For example, some respondents reported being overwhelmed by the volume of available data and dashboards, and responding to current requests for data analysis and dissemination sometimes impeded long range planning. Multiple national access-related initiatives and local pressures also generated excessive and potentially conflicting demands. Thus, while the creation of a GPM position seemed to be essential for the pilot sites to improve local access and timeliness to care, success also requires ongoing national and facility-wide communication, education, and support. Ongoing data analysis training and support will be critical to ensuring the sustainability of the position. Last, recruiting GPMs with the needed complex skill set may prove to be challenging, and it will be important to provide resources to identify, attract, and retain well-qualified GPMs.
Limitations and Future Work
This study was based on a small initial sample of pilot sites of varying sizes and, as such, may not reflect the experience of all VHA GPMs. In addition, the use of snowball sampling, while facilitating identification of key stakeholders, may introduce bias in participant sampling. Nonetheless, the results from this study provide findings that informed the national VHA GPM initiative and can inform further studies of practice management roles outside of the VA.
Further study of the VHA GPM implementation and similar roles in other health care systems is needed. As the GPM position is fully implemented across the VHA, large scale evaluation is needed to gain a more representative picture and allow for comparison of the GPM role at various types of facilities (eg, size, rurality, complexity, ranking based on access performance metrics).
Conclusion
Improving access to care is a central goal for health care systems. The incorporation of the GPM role is an innovative approach to improve access management strategies. Early study of prototype sites provided VHA leadership with valuable insights used to influence further rollout of this initiative. Based on our findings, national and local support are important to ongoing success. National access mandates, training, and resources should focus on ensuring sufficient GPM authority, enabling GPMs to use data, and ensuring GPMs engage with frontline clinical and administrative staff to improve veteran access to care.
1. US Department of Veterans Affairs. Veterans Health Administration. https://www.va.gov/health. Updated October 25, 2019. Accessed January 8, 2020.
2. Veterans Access, Choice, and Accountability Act of 2014. 38 CFR § 17.1500 (2014).
3. Fahmy N, Aprikian A, Al-Otaibi M, et al. Impact of treatment delay in patients with bladder cancer managed with partial cystectomy in Quebec: a population-based study. Can Urol Assoc J. 2009;3(2):131-135.
4. Hill CJ, Joonas K. The impact of unacceptable wait time on health care patients’ attitudes and actions. Health Mark Q. 2005;23(2):69-87.
5. Ansell D, Crispo JAG, Simard B, Bjerre LM. Interventions to reduce wait times for primary care appointments: a systematic review. BMC Health Serv Res. 2017;17(1):295.
6. Kotecha J, Han H, Green M, Russell G, Martin MI, Birtwhistle R. The role of the practice facilitators in Ontario primary healthcare quality improvement. BMC Fam Pract. 2015;16:93.
7. Taylor EF, Machta RM, Meyers DS, Genevro J, Peikes DN. Enhancing the primary care team to provide redesigned care: the roles of practice facilitators and care managers. Ann Fam Med. 2013;11(1):80-83.
8. Liddy C, Laferriere D, Baskerville B, Dahrouge S, Knox L, Hogg W. An overview of practice facilitation programs in Canada: current perspectives and future directions. Healthc Policy. 2013;8(3):58-67.
9. Birken SA, Lee SY, Weiner BJ, Chin MH, Schaefer CT. Improving the effectiveness of health care innovation implementation: middle managers as change agents. Med Care Res Rev. 2013;70(1):29-45.
10. Ahluwalia S, Offredy M. A qualitative study of the impact of the implementation of advanced access in primary healthcare on the working lives of general practice staff. BMC Fam Pract. 2005;6:39.
11. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
12. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
13. Stefl ME. Common competencies for all healthcare managers: the Healthcare Leadership Alliance model. J Healthc Manag. 2008;53(6):360-374.
The Veterans Health Administration (VHA) provides care for 9 million veterans at 1,255 health care sites linked to one of 170 local medical systems.1 Recognizing that providing timely care requires effective access management, the US Congress mandated training of VHA staff to manage and improve access to care but did not provide additional local funds for new positions.2 In response, the VHA created the group practice manager (GPM), a new position responsible for improving clinical practice management and unifying access improvement across leadership levels, professions, and services within each local medical system.
In May 2015, the VHA began hiring and training GPMs to spearhead management of access to services. The US Department of Veterans Affairs (VA) Office of Veteran Access to Care spearheaded GPM training, including face-to-face sessions, national calls, webinars, and educational materials. Five local medical systems were selected by the VA Office of Veteran Access to Care to implement the GPM role to allow for an early evaluation of the program that would inform a subsequent nationwide rollout. Implementation of the GPM role remained in the hands of local medical systems.
Longer wait times are shown to impact patient health.3,4 Open access scheduling and other patient-centered access management interventions have been shown to improve availability of primary care appointments.5 However, little empirical evidence exists regarding the managers who focus on clinic access interventions. While the nonpeer-reviewed literature includes references to such roles, including GPMs, the empirical literature has focused on external practice faciliators,6-8 “mid-level managers,”9 and clinic staff.10 We found no peer-reviewed articles on the needs and experiences of practice managers who are focused on improving access. The purpose of this study was to examine GPM prototype sites to both enhance subsequent nationwide implementation and to advance empirical literature on managing patient access within health care.
Methods
In 2015, the VA identified 5 prototype sites representing diverse geographic locations, size, and complexity for the implementation of the GPM role (Table 1). These sites self-identified as having clinical practice management experience. GPMs attended 4 training sessions between February and August 2015.
Data Collection
Participants from each prototype site included GPMs, national trainers, clinic leaders, and frontline staff. Table 2 includes the roles and sample size. Participants were recruited through purposive sampling followed by snowball sampling until thematic saturation was reached (the point at which subsequent data fail to produce new findings across sites and roles of interest).
Guided by the Consolidated Framework for Implementation Research (CFIR), the research team developed semistructured interview guides tailored to participants’ roles to elicit rich descriptions regarding overall impressions, practice management strategies, goals, activities, relationship to clinic roles, data analytics usage, challenges, barriers, and facilitators.11 These guides included open-ended questions and structured prompts utilizing participant language for follow-up probes to minimize interviewing bias (eAppendix:
Data Analysis
Data were analyzed using iterative deductive and inductive content analysis.12 Deductive content analysis consisted of identifying quotes that fit within preidentified categories (ie, perceptions of national effort, organizational structure for GPM, challenges, facilitators, metrics and tools, and mobilizing access culture) developed by the interdisciplinary research team. Further content analysis entailed open-coding and iteratively revisiting and reconciling codes associated within each preidentified category as new codes emerged. The team analyzed the resulting codes to inductively and iteratively identify and stabilize themes regarding the GPM role: roles and tasks, GPM characteristics, issues, and challenges. Through this process we moved coded data to reconciled descriptions suited to addressing the purposes of this study. Dedoose 7.0.23 software was used for qualitative data management and analysis.
Results
The study identified participants’ overall impressions of the GPM initiative and key themes within 4 major domains regarding implementing the GPM role: roles and tasks (implementing clinic practice management, leading patient access, supporting data analytics, and enabling self and staff); GPM characteristics (familiarity with clinical services, knowledge of VHA systems, ability to analyze patient data, communication skills, and the ability to work with others); and issues, and challenges (technical, social, and structural).
Overall Impressions
Interviewees perceived the GPM initiative as a consolidation of existing distributed responsibilities into one role that directly reported to local top-level management with indirect reporting to national leaders. Many of the sites reported that they had designated or planned to designate a role resembling the GPM prior to the initiative. “There are staff who’ve been doing some of this work all along,” a GPM noted. “We just didn’t have them grouped together. They weren’t necessarily all working in the same type of service under the same type of structure.”
Whether the GPM position was new or not, participants referenced the importance and challenges of engaging the local facility in recognizing the agency associated with the GPM position. According to national support, the staff are trying to get the facility to understand “why the group practice manager is so important… we’ve got to embed that standard position in the system.”
While the GPM was recognized as the hub of access management, respondents recognized that transformation regarding access involved many players. “We have to create [an] orchestrated team inside each facility,” an advisor argued.
Respondents discussed how the initiative allows local facilities to appoint a specific person with a specific title and role who helps facilitate, organize, and legitimize an access focus at their sites. One GPM interviewee noted how the initiative helped refocus some of their previously less centralized efforts. “We’ve always looked at productivity; we’ve always looked at access; we’ve always looked at efficiency. I think the bigger difference is now there are individuals identified in the clinics, as practice managers as well…I interact with them. They interact with individual clinic staff, and it’s more of a group process than a single individual.”
The value of having tools available and being able to track and manage patient care as a specific example of the positive impact of this new role was noted by participants. A GPM noted that many health care providers will be happy to have tools to better manage their services and a process “that flows from a service level all the way up to executive management, where there is a common interest in making those things happen—I think that’s going to be a tremendous help.”
Participants expressed concern that the national GPM rollout would be a one-size-fits-all approach. These respondents emphasized the need to have the flexibility to customize their activities to meet their unique site and patient needs.
GPM Roles and Tasks
Participants described 4 primary roles that the GPM was expected to fill: implementing clinic practice management, leading patient access, supporting data analytics, and enabling self and staff. Some activities overlapped in that they served to support multiple role areas (Figure 1).
Implementing clinic practice management. In the early stages of the initiative, the GPM’s primary role was to prepare the facility to implement a standardized set of clinic practice management (CPM) team processes. Part of standardizing the CPM process was defining the scope and tasks of the GPM, which requires significant planning for the implementation. “My big job is to finalize what we think group practice management is going to look [like] here,” a GPM reported.
Each prototype site had latitude to interpret the GPM initiative in a way that would work in their context within given VHA boundaries and ongoing initiatives. To achieve the high-level vision and purpose, the GPM first had to develop action plans that accounted for the operating environment of the facility. According to one GPM, VA national officials are “constantly” asking for action plans, which required significant time by specific deadlines. “They want an action plan [and to] clean up all your consults, [and to] clean up all your recall reminders.”
Leading on improving access efforts. Participants saw the GPM as the central staff member responsible for providing oversight of any activities and people involved in improving access. “I ensure everybody is doing what they’re supposed to do,” one GPM reported. When the GPM sees areas that are not being addressed, the individual tries to develop a process or training to “close those gaps.”
GPMs promoted an awareness of their goals, changes in process, and new tools accompanying the initiative. However, other access initiatives were occurring simultaneously creating confusion for health care providers and patients; thus GPMs found they were managing a wide array of related initiatives.
GPMs have to negotiate with leaders across the VHA facility, many of whom operated at a higher leadership level and had different priorities, to address access problems.
“I’m a lieutenant as a GPM in a clinic, a GPM noted. “How is the lieutenant going to talk to a major or a colonel in the clinic and say your clinic has problems. How[‘s] that lieutenant...going to do that? With people skills!”
Managing expectations about the speed and to what extent a problem could be resolved was an important part of the GPM leadership role. “I see myself as managing expectations both up to the leadership and down to the frontline,” a GPM explained. “I find myself talking to leadership [about] our progress. But at the same time, we have to say, ‘not everything can be fixed overnight.’”
Providing leadership on access-related issues included developing a range of options for addressing patient access problems. One analytics manager recounted how the GPM role led to evaluating how physical space limited efficiency in clinic flow. The first step was identifying possible additional rooms to improve clinic flow. This required working with the space committee to “get someone to look at our overarching space and find someplace else for them to sit” to avoid adding to congestion in the clinic area.
Supporting data analytics.
GPMs routinely immersed themselves in the data to understand access issues. GPMs worked with clinic leaders to identify the underlying causes and various solutions. The GPMs maintained access through administrative scrubbing of the data and finding “smart ways to get patients scheduled,” a GPM explained. “I don’t think our facility would have taken care of as many veterans in the time frame as we did....We’ve cleared over 4,000 consults that were older than 90 days. We have cleared thousands and thousands of weekly reminders.”
GPMs expressed the need for aggregated (ie, dashboard) and standardized information to efficiently address access issues. “I would like to have some more standardization on what’s being reviewed; it seems to change frequently, and so [to] be able to track and trend and have something given to me to review,” one health care provider requested. On the other hand, participants also described a need for decision support tools that would lead to action aligned with best practices. “Instead of a dashboard or something that’s just measuring their performance, it’s more something that they can look at and take action” a national support staff advisor suggested.
Enabling self and staff. GPMs felt they were most effective if they enabled themselves and stakeholders through training and by cultivating relationships and team building. Figure 2 illustrates the various stakeholders GPMs reported engaging with. The GPMS should be building relationships, bridging relationships, developing trust, and then providing a higher level of hands-on management. However, “that doesn’t really happen right now, day to day,” one member of leadership reported.
Key topics in GPM leadership training included both soft skills (change management, culture change, and negotiation skills) and crucial analytic/technical training (understanding each metric and dashboard available, data analytics, and supply/demand balancing techniques). The GPMs not only wanted to understand metrics as part of their training, but also want to know what to do about them.
An “operationalization” training approach (discerning the meaning of data, data-based decision making, and determining action from multiple options) inspired by real-life situations was preferred by participants. Other effective learning structures included job aids in the form of templated Gantt charts, process maps to guide GPMs through implementation of new processes, formalized peer learning (accumulated field insights shared during training courses), and informal peer sharing of direct experiences during calls.
GPMs also emphasized training for frontline clinical and support staff, including schedulers. VHA schedulers typically have less education and experience higher turnover rates than do other clinic staff, yet they carry out complex and critical tasks. Providing training and ensuring that any materials developed for training and education were appropriate to the level of education of schedulers was an important task for GPMs. “If they don’t understand all of the scheduling principles and potential,” one GPM explained, “we will not be maximizing the utilization of our parts.”
GPMs also provided informal education to clinicians. Participants noted GPMs have to avoid appearing to overstep their positions or presuming more knowledge and expertise than clinicians. They “have to be able to teach a physician without being overbearing, in a way a physician will accept it as advisement,” one program leader reported.
GPM knowledge, skills, and abilities. GPMs presented a complex range of knowledge, skills, and abilities, including clinical, administrative, analytics, and people skills. All interviewees reported that their prior education and experience did not sufficiently train them for the GPM role. GPMs identified a willingness to learn quickly as a critical characteristic. Many GPMs tended to have a formal education in health administration or business (eg, MBAs); others had administrative experience (eg, administrative assistance to executive leadership) or clinical training (eg, physician assistant). Detailed clinical knowledge was not expected, but clinical familiarity was helpful.
Some interviewees also mentioned previous experience and familiarity with the VHA system specifically as an advantage. This was especially true for VA outpatient flows, clinic flows, and understanding what an outpatient is in a VA context. Interviewees noted the importance of GPMs needing to be able to analyze patient demand metrics and underlying data in order to determine supply of providers and then to allocate adequate resources to complement providers. Forecasting skills were referenced as a key point. “They need to be able to be assured that they can recruit more providers if needed,” a national support staff advisor noted.
Given the importance of developing effective relationships, communication skills were mentioned by most participants and underscored as critical to establishing trust between GPMs and others as the initiative was being implemented. Interviewees indicated that relationship building was further enhanced when GPMs possessed the ability to “work with” rather than command clinicians and staff; navigate politics; and were respectful of other people’s knowledge, skills, abilities, and status. “They have to work with the nursing staff and teach them,” a leader described, “so that people understand that we are going to a different place to achieve our primary objectives and goals.”
Issues and Challenges
Participants identified several technical, social, and structural challenges and barriers to successfully implementing the GPM role.
Technical challenges. Recurring themes across all phases of data analytics were GPMs’ capability to challenge data use and use large volumes of information from multiple data sources (entering and accessing data; “drilling down” from summaries; generating reports; and analyzing and interpreting resulting metrics). Interviewees reported that information assessment and analytic support were not consistent. One GPM had a data analyst pulling reports needed to support clinical units while other GPMs trained staff to pull data. Even with support, some GPMs had issues due to limited information technology (IT) skills or access privileges leading to inefficiencies and delays. “Whenever I need anything from a programmer, I have to go through, you know, the IT gods in the sky,” one GPM remarked. “That usually takes a few months or more.”
Social challenges. Instituting the GPM role was a cultural change, and interviewees reported needing to address resistance to CPM model efforts. Resistance to change “is particularly hard in the VA just because it has a unique culture,” one leader noted. “There is a comfort in the legacy way of doing things.” The GPM initiative was introduced during a time when other national level initiatives were being implemented throughout the VHA. Fulfilling requests for information for these initiatives became the responsibility of the GPM and their team, which diverted attention from the mandate to improve access. Furthermore, GPMs were often considered the “change communicators” to clinics putting them in the role of “bad messenger,” which degraded trust and made it difficult to partner with clinicians.
Efforts to work through change management and build relationships included general program awareness presentation to internal stakeholders; including key stakeholders in GPM committees; pre-emptive conversations with unit chiefs; creating awareness of the GPM activities and progress through formal and informal update meetings; and identifying successes regarding access.
Structural challenges. The GPM role did not have direct supervision over clinical and administrative leaders, making it challenging to enact change. GPMs reported that “they do not always have authority over the area that they are being asked to manage,” which made their work difficult, requiring strong negotiation skills and political savvy to affect change. However, as the clinic staff and providers saw how the GPM could support and positively impact their practice, these challenges began to subside.
Discussion
This study provides empirical evidence regarding the implementation of a new access management strategy for health care systems focused on improving timeliness of care. First, the GPM position was seen as critical at each facility, as a single point person, to help local system leaders respond effectively to both national mandates and local context. Second, requiring the GPMs to report to the medical center director or chief of staff was important for integrating access perspectives across service lines and to facilitate a strong GPM role in strategic planning. Third, the intentional flexibility of the access management initiative, beyond the nationally specified aspects of the GPM role, was key for allowing individual sites to adapt to unique local challenges, resources, and population demands. Fourth, the initiative provided GPMs with opportunities to learn important skills and support the acquisition, utilization, and communication of a tremendous range of data toward responsive action.
According to our respondents, the GPM role demands functioning across a broad set of responsibilities; understanding the big picture as well as the complex underlying variables; engaging facility leaders, clinical and administrative staff; and prioritizing competing national and local demands. Consistent with previous findings, effective GPMs must possess a complex set of skills (interpersonal, analytic, and leadership) and the ability to create a supporting team.13
In practice, improving access at individual sites of care (VA medical centers and community-based outpatient clinics) poses significant challenges, especially in the early stages, even with the assistance of a GPM. For example, some respondents reported being overwhelmed by the volume of available data and dashboards, and responding to current requests for data analysis and dissemination sometimes impeded long range planning. Multiple national access-related initiatives and local pressures also generated excessive and potentially conflicting demands. Thus, while the creation of a GPM position seemed to be essential for the pilot sites to improve local access and timeliness to care, success also requires ongoing national and facility-wide communication, education, and support. Ongoing data analysis training and support will be critical to ensuring the sustainability of the position. Last, recruiting GPMs with the needed complex skill set may prove to be challenging, and it will be important to provide resources to identify, attract, and retain well-qualified GPMs.
Limitations and Future Work
This study was based on a small initial sample of pilot sites of varying sizes and, as such, may not reflect the experience of all VHA GPMs. In addition, the use of snowball sampling, while facilitating identification of key stakeholders, may introduce bias in participant sampling. Nonetheless, the results from this study provide findings that informed the national VHA GPM initiative and can inform further studies of practice management roles outside of the VA.
Further study of the VHA GPM implementation and similar roles in other health care systems is needed. As the GPM position is fully implemented across the VHA, large scale evaluation is needed to gain a more representative picture and allow for comparison of the GPM role at various types of facilities (eg, size, rurality, complexity, ranking based on access performance metrics).
Conclusion
Improving access to care is a central goal for health care systems. The incorporation of the GPM role is an innovative approach to improve access management strategies. Early study of prototype sites provided VHA leadership with valuable insights used to influence further rollout of this initiative. Based on our findings, national and local support are important to ongoing success. National access mandates, training, and resources should focus on ensuring sufficient GPM authority, enabling GPMs to use data, and ensuring GPMs engage with frontline clinical and administrative staff to improve veteran access to care.
The Veterans Health Administration (VHA) provides care for 9 million veterans at 1,255 health care sites linked to one of 170 local medical systems.1 Recognizing that providing timely care requires effective access management, the US Congress mandated training of VHA staff to manage and improve access to care but did not provide additional local funds for new positions.2 In response, the VHA created the group practice manager (GPM), a new position responsible for improving clinical practice management and unifying access improvement across leadership levels, professions, and services within each local medical system.
In May 2015, the VHA began hiring and training GPMs to spearhead management of access to services. The US Department of Veterans Affairs (VA) Office of Veteran Access to Care spearheaded GPM training, including face-to-face sessions, national calls, webinars, and educational materials. Five local medical systems were selected by the VA Office of Veteran Access to Care to implement the GPM role to allow for an early evaluation of the program that would inform a subsequent nationwide rollout. Implementation of the GPM role remained in the hands of local medical systems.
Longer wait times are shown to impact patient health.3,4 Open access scheduling and other patient-centered access management interventions have been shown to improve availability of primary care appointments.5 However, little empirical evidence exists regarding the managers who focus on clinic access interventions. While the nonpeer-reviewed literature includes references to such roles, including GPMs, the empirical literature has focused on external practice faciliators,6-8 “mid-level managers,”9 and clinic staff.10 We found no peer-reviewed articles on the needs and experiences of practice managers who are focused on improving access. The purpose of this study was to examine GPM prototype sites to both enhance subsequent nationwide implementation and to advance empirical literature on managing patient access within health care.
Methods
In 2015, the VA identified 5 prototype sites representing diverse geographic locations, size, and complexity for the implementation of the GPM role (Table 1). These sites self-identified as having clinical practice management experience. GPMs attended 4 training sessions between February and August 2015.
Data Collection
Participants from each prototype site included GPMs, national trainers, clinic leaders, and frontline staff. Table 2 includes the roles and sample size. Participants were recruited through purposive sampling followed by snowball sampling until thematic saturation was reached (the point at which subsequent data fail to produce new findings across sites and roles of interest).
Guided by the Consolidated Framework for Implementation Research (CFIR), the research team developed semistructured interview guides tailored to participants’ roles to elicit rich descriptions regarding overall impressions, practice management strategies, goals, activities, relationship to clinic roles, data analytics usage, challenges, barriers, and facilitators.11 These guides included open-ended questions and structured prompts utilizing participant language for follow-up probes to minimize interviewing bias (eAppendix:
Data Analysis
Data were analyzed using iterative deductive and inductive content analysis.12 Deductive content analysis consisted of identifying quotes that fit within preidentified categories (ie, perceptions of national effort, organizational structure for GPM, challenges, facilitators, metrics and tools, and mobilizing access culture) developed by the interdisciplinary research team. Further content analysis entailed open-coding and iteratively revisiting and reconciling codes associated within each preidentified category as new codes emerged. The team analyzed the resulting codes to inductively and iteratively identify and stabilize themes regarding the GPM role: roles and tasks, GPM characteristics, issues, and challenges. Through this process we moved coded data to reconciled descriptions suited to addressing the purposes of this study. Dedoose 7.0.23 software was used for qualitative data management and analysis.
Results
The study identified participants’ overall impressions of the GPM initiative and key themes within 4 major domains regarding implementing the GPM role: roles and tasks (implementing clinic practice management, leading patient access, supporting data analytics, and enabling self and staff); GPM characteristics (familiarity with clinical services, knowledge of VHA systems, ability to analyze patient data, communication skills, and the ability to work with others); and issues, and challenges (technical, social, and structural).
Overall Impressions
Interviewees perceived the GPM initiative as a consolidation of existing distributed responsibilities into one role that directly reported to local top-level management with indirect reporting to national leaders. Many of the sites reported that they had designated or planned to designate a role resembling the GPM prior to the initiative. “There are staff who’ve been doing some of this work all along,” a GPM noted. “We just didn’t have them grouped together. They weren’t necessarily all working in the same type of service under the same type of structure.”
Whether the GPM position was new or not, participants referenced the importance and challenges of engaging the local facility in recognizing the agency associated with the GPM position. According to national support, the staff are trying to get the facility to understand “why the group practice manager is so important… we’ve got to embed that standard position in the system.”
While the GPM was recognized as the hub of access management, respondents recognized that transformation regarding access involved many players. “We have to create [an] orchestrated team inside each facility,” an advisor argued.
Respondents discussed how the initiative allows local facilities to appoint a specific person with a specific title and role who helps facilitate, organize, and legitimize an access focus at their sites. One GPM interviewee noted how the initiative helped refocus some of their previously less centralized efforts. “We’ve always looked at productivity; we’ve always looked at access; we’ve always looked at efficiency. I think the bigger difference is now there are individuals identified in the clinics, as practice managers as well…I interact with them. They interact with individual clinic staff, and it’s more of a group process than a single individual.”
The value of having tools available and being able to track and manage patient care as a specific example of the positive impact of this new role was noted by participants. A GPM noted that many health care providers will be happy to have tools to better manage their services and a process “that flows from a service level all the way up to executive management, where there is a common interest in making those things happen—I think that’s going to be a tremendous help.”
Participants expressed concern that the national GPM rollout would be a one-size-fits-all approach. These respondents emphasized the need to have the flexibility to customize their activities to meet their unique site and patient needs.
GPM Roles and Tasks
Participants described 4 primary roles that the GPM was expected to fill: implementing clinic practice management, leading patient access, supporting data analytics, and enabling self and staff. Some activities overlapped in that they served to support multiple role areas (Figure 1).
Implementing clinic practice management. In the early stages of the initiative, the GPM’s primary role was to prepare the facility to implement a standardized set of clinic practice management (CPM) team processes. Part of standardizing the CPM process was defining the scope and tasks of the GPM, which requires significant planning for the implementation. “My big job is to finalize what we think group practice management is going to look [like] here,” a GPM reported.
Each prototype site had latitude to interpret the GPM initiative in a way that would work in their context within given VHA boundaries and ongoing initiatives. To achieve the high-level vision and purpose, the GPM first had to develop action plans that accounted for the operating environment of the facility. According to one GPM, VA national officials are “constantly” asking for action plans, which required significant time by specific deadlines. “They want an action plan [and to] clean up all your consults, [and to] clean up all your recall reminders.”
Leading on improving access efforts. Participants saw the GPM as the central staff member responsible for providing oversight of any activities and people involved in improving access. “I ensure everybody is doing what they’re supposed to do,” one GPM reported. When the GPM sees areas that are not being addressed, the individual tries to develop a process or training to “close those gaps.”
GPMs promoted an awareness of their goals, changes in process, and new tools accompanying the initiative. However, other access initiatives were occurring simultaneously creating confusion for health care providers and patients; thus GPMs found they were managing a wide array of related initiatives.
GPMs have to negotiate with leaders across the VHA facility, many of whom operated at a higher leadership level and had different priorities, to address access problems.
“I’m a lieutenant as a GPM in a clinic, a GPM noted. “How is the lieutenant going to talk to a major or a colonel in the clinic and say your clinic has problems. How[‘s] that lieutenant...going to do that? With people skills!”
Managing expectations about the speed and to what extent a problem could be resolved was an important part of the GPM leadership role. “I see myself as managing expectations both up to the leadership and down to the frontline,” a GPM explained. “I find myself talking to leadership [about] our progress. But at the same time, we have to say, ‘not everything can be fixed overnight.’”
Providing leadership on access-related issues included developing a range of options for addressing patient access problems. One analytics manager recounted how the GPM role led to evaluating how physical space limited efficiency in clinic flow. The first step was identifying possible additional rooms to improve clinic flow. This required working with the space committee to “get someone to look at our overarching space and find someplace else for them to sit” to avoid adding to congestion in the clinic area.
Supporting data analytics.
GPMs routinely immersed themselves in the data to understand access issues. GPMs worked with clinic leaders to identify the underlying causes and various solutions. The GPMs maintained access through administrative scrubbing of the data and finding “smart ways to get patients scheduled,” a GPM explained. “I don’t think our facility would have taken care of as many veterans in the time frame as we did....We’ve cleared over 4,000 consults that were older than 90 days. We have cleared thousands and thousands of weekly reminders.”
GPMs expressed the need for aggregated (ie, dashboard) and standardized information to efficiently address access issues. “I would like to have some more standardization on what’s being reviewed; it seems to change frequently, and so [to] be able to track and trend and have something given to me to review,” one health care provider requested. On the other hand, participants also described a need for decision support tools that would lead to action aligned with best practices. “Instead of a dashboard or something that’s just measuring their performance, it’s more something that they can look at and take action” a national support staff advisor suggested.
Enabling self and staff. GPMs felt they were most effective if they enabled themselves and stakeholders through training and by cultivating relationships and team building. Figure 2 illustrates the various stakeholders GPMs reported engaging with. The GPMS should be building relationships, bridging relationships, developing trust, and then providing a higher level of hands-on management. However, “that doesn’t really happen right now, day to day,” one member of leadership reported.
Key topics in GPM leadership training included both soft skills (change management, culture change, and negotiation skills) and crucial analytic/technical training (understanding each metric and dashboard available, data analytics, and supply/demand balancing techniques). The GPMs not only wanted to understand metrics as part of their training, but also want to know what to do about them.
An “operationalization” training approach (discerning the meaning of data, data-based decision making, and determining action from multiple options) inspired by real-life situations was preferred by participants. Other effective learning structures included job aids in the form of templated Gantt charts, process maps to guide GPMs through implementation of new processes, formalized peer learning (accumulated field insights shared during training courses), and informal peer sharing of direct experiences during calls.
GPMs also emphasized training for frontline clinical and support staff, including schedulers. VHA schedulers typically have less education and experience higher turnover rates than do other clinic staff, yet they carry out complex and critical tasks. Providing training and ensuring that any materials developed for training and education were appropriate to the level of education of schedulers was an important task for GPMs. “If they don’t understand all of the scheduling principles and potential,” one GPM explained, “we will not be maximizing the utilization of our parts.”
GPMs also provided informal education to clinicians. Participants noted GPMs have to avoid appearing to overstep their positions or presuming more knowledge and expertise than clinicians. They “have to be able to teach a physician without being overbearing, in a way a physician will accept it as advisement,” one program leader reported.
GPM knowledge, skills, and abilities. GPMs presented a complex range of knowledge, skills, and abilities, including clinical, administrative, analytics, and people skills. All interviewees reported that their prior education and experience did not sufficiently train them for the GPM role. GPMs identified a willingness to learn quickly as a critical characteristic. Many GPMs tended to have a formal education in health administration or business (eg, MBAs); others had administrative experience (eg, administrative assistance to executive leadership) or clinical training (eg, physician assistant). Detailed clinical knowledge was not expected, but clinical familiarity was helpful.
Some interviewees also mentioned previous experience and familiarity with the VHA system specifically as an advantage. This was especially true for VA outpatient flows, clinic flows, and understanding what an outpatient is in a VA context. Interviewees noted the importance of GPMs needing to be able to analyze patient demand metrics and underlying data in order to determine supply of providers and then to allocate adequate resources to complement providers. Forecasting skills were referenced as a key point. “They need to be able to be assured that they can recruit more providers if needed,” a national support staff advisor noted.
Given the importance of developing effective relationships, communication skills were mentioned by most participants and underscored as critical to establishing trust between GPMs and others as the initiative was being implemented. Interviewees indicated that relationship building was further enhanced when GPMs possessed the ability to “work with” rather than command clinicians and staff; navigate politics; and were respectful of other people’s knowledge, skills, abilities, and status. “They have to work with the nursing staff and teach them,” a leader described, “so that people understand that we are going to a different place to achieve our primary objectives and goals.”
Issues and Challenges
Participants identified several technical, social, and structural challenges and barriers to successfully implementing the GPM role.
Technical challenges. Recurring themes across all phases of data analytics were GPMs’ capability to challenge data use and use large volumes of information from multiple data sources (entering and accessing data; “drilling down” from summaries; generating reports; and analyzing and interpreting resulting metrics). Interviewees reported that information assessment and analytic support were not consistent. One GPM had a data analyst pulling reports needed to support clinical units while other GPMs trained staff to pull data. Even with support, some GPMs had issues due to limited information technology (IT) skills or access privileges leading to inefficiencies and delays. “Whenever I need anything from a programmer, I have to go through, you know, the IT gods in the sky,” one GPM remarked. “That usually takes a few months or more.”
Social challenges. Instituting the GPM role was a cultural change, and interviewees reported needing to address resistance to CPM model efforts. Resistance to change “is particularly hard in the VA just because it has a unique culture,” one leader noted. “There is a comfort in the legacy way of doing things.” The GPM initiative was introduced during a time when other national level initiatives were being implemented throughout the VHA. Fulfilling requests for information for these initiatives became the responsibility of the GPM and their team, which diverted attention from the mandate to improve access. Furthermore, GPMs were often considered the “change communicators” to clinics putting them in the role of “bad messenger,” which degraded trust and made it difficult to partner with clinicians.
Efforts to work through change management and build relationships included general program awareness presentation to internal stakeholders; including key stakeholders in GPM committees; pre-emptive conversations with unit chiefs; creating awareness of the GPM activities and progress through formal and informal update meetings; and identifying successes regarding access.
Structural challenges. The GPM role did not have direct supervision over clinical and administrative leaders, making it challenging to enact change. GPMs reported that “they do not always have authority over the area that they are being asked to manage,” which made their work difficult, requiring strong negotiation skills and political savvy to affect change. However, as the clinic staff and providers saw how the GPM could support and positively impact their practice, these challenges began to subside.
Discussion
This study provides empirical evidence regarding the implementation of a new access management strategy for health care systems focused on improving timeliness of care. First, the GPM position was seen as critical at each facility, as a single point person, to help local system leaders respond effectively to both national mandates and local context. Second, requiring the GPMs to report to the medical center director or chief of staff was important for integrating access perspectives across service lines and to facilitate a strong GPM role in strategic planning. Third, the intentional flexibility of the access management initiative, beyond the nationally specified aspects of the GPM role, was key for allowing individual sites to adapt to unique local challenges, resources, and population demands. Fourth, the initiative provided GPMs with opportunities to learn important skills and support the acquisition, utilization, and communication of a tremendous range of data toward responsive action.
According to our respondents, the GPM role demands functioning across a broad set of responsibilities; understanding the big picture as well as the complex underlying variables; engaging facility leaders, clinical and administrative staff; and prioritizing competing national and local demands. Consistent with previous findings, effective GPMs must possess a complex set of skills (interpersonal, analytic, and leadership) and the ability to create a supporting team.13
In practice, improving access at individual sites of care (VA medical centers and community-based outpatient clinics) poses significant challenges, especially in the early stages, even with the assistance of a GPM. For example, some respondents reported being overwhelmed by the volume of available data and dashboards, and responding to current requests for data analysis and dissemination sometimes impeded long range planning. Multiple national access-related initiatives and local pressures also generated excessive and potentially conflicting demands. Thus, while the creation of a GPM position seemed to be essential for the pilot sites to improve local access and timeliness to care, success also requires ongoing national and facility-wide communication, education, and support. Ongoing data analysis training and support will be critical to ensuring the sustainability of the position. Last, recruiting GPMs with the needed complex skill set may prove to be challenging, and it will be important to provide resources to identify, attract, and retain well-qualified GPMs.
Limitations and Future Work
This study was based on a small initial sample of pilot sites of varying sizes and, as such, may not reflect the experience of all VHA GPMs. In addition, the use of snowball sampling, while facilitating identification of key stakeholders, may introduce bias in participant sampling. Nonetheless, the results from this study provide findings that informed the national VHA GPM initiative and can inform further studies of practice management roles outside of the VA.
Further study of the VHA GPM implementation and similar roles in other health care systems is needed. As the GPM position is fully implemented across the VHA, large scale evaluation is needed to gain a more representative picture and allow for comparison of the GPM role at various types of facilities (eg, size, rurality, complexity, ranking based on access performance metrics).
Conclusion
Improving access to care is a central goal for health care systems. The incorporation of the GPM role is an innovative approach to improve access management strategies. Early study of prototype sites provided VHA leadership with valuable insights used to influence further rollout of this initiative. Based on our findings, national and local support are important to ongoing success. National access mandates, training, and resources should focus on ensuring sufficient GPM authority, enabling GPMs to use data, and ensuring GPMs engage with frontline clinical and administrative staff to improve veteran access to care.
1. US Department of Veterans Affairs. Veterans Health Administration. https://www.va.gov/health. Updated October 25, 2019. Accessed January 8, 2020.
2. Veterans Access, Choice, and Accountability Act of 2014. 38 CFR § 17.1500 (2014).
3. Fahmy N, Aprikian A, Al-Otaibi M, et al. Impact of treatment delay in patients with bladder cancer managed with partial cystectomy in Quebec: a population-based study. Can Urol Assoc J. 2009;3(2):131-135.
4. Hill CJ, Joonas K. The impact of unacceptable wait time on health care patients’ attitudes and actions. Health Mark Q. 2005;23(2):69-87.
5. Ansell D, Crispo JAG, Simard B, Bjerre LM. Interventions to reduce wait times for primary care appointments: a systematic review. BMC Health Serv Res. 2017;17(1):295.
6. Kotecha J, Han H, Green M, Russell G, Martin MI, Birtwhistle R. The role of the practice facilitators in Ontario primary healthcare quality improvement. BMC Fam Pract. 2015;16:93.
7. Taylor EF, Machta RM, Meyers DS, Genevro J, Peikes DN. Enhancing the primary care team to provide redesigned care: the roles of practice facilitators and care managers. Ann Fam Med. 2013;11(1):80-83.
8. Liddy C, Laferriere D, Baskerville B, Dahrouge S, Knox L, Hogg W. An overview of practice facilitation programs in Canada: current perspectives and future directions. Healthc Policy. 2013;8(3):58-67.
9. Birken SA, Lee SY, Weiner BJ, Chin MH, Schaefer CT. Improving the effectiveness of health care innovation implementation: middle managers as change agents. Med Care Res Rev. 2013;70(1):29-45.
10. Ahluwalia S, Offredy M. A qualitative study of the impact of the implementation of advanced access in primary healthcare on the working lives of general practice staff. BMC Fam Pract. 2005;6:39.
11. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
12. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
13. Stefl ME. Common competencies for all healthcare managers: the Healthcare Leadership Alliance model. J Healthc Manag. 2008;53(6):360-374.
1. US Department of Veterans Affairs. Veterans Health Administration. https://www.va.gov/health. Updated October 25, 2019. Accessed January 8, 2020.
2. Veterans Access, Choice, and Accountability Act of 2014. 38 CFR § 17.1500 (2014).
3. Fahmy N, Aprikian A, Al-Otaibi M, et al. Impact of treatment delay in patients with bladder cancer managed with partial cystectomy in Quebec: a population-based study. Can Urol Assoc J. 2009;3(2):131-135.
4. Hill CJ, Joonas K. The impact of unacceptable wait time on health care patients’ attitudes and actions. Health Mark Q. 2005;23(2):69-87.
5. Ansell D, Crispo JAG, Simard B, Bjerre LM. Interventions to reduce wait times for primary care appointments: a systematic review. BMC Health Serv Res. 2017;17(1):295.
6. Kotecha J, Han H, Green M, Russell G, Martin MI, Birtwhistle R. The role of the practice facilitators in Ontario primary healthcare quality improvement. BMC Fam Pract. 2015;16:93.
7. Taylor EF, Machta RM, Meyers DS, Genevro J, Peikes DN. Enhancing the primary care team to provide redesigned care: the roles of practice facilitators and care managers. Ann Fam Med. 2013;11(1):80-83.
8. Liddy C, Laferriere D, Baskerville B, Dahrouge S, Knox L, Hogg W. An overview of practice facilitation programs in Canada: current perspectives and future directions. Healthc Policy. 2013;8(3):58-67.
9. Birken SA, Lee SY, Weiner BJ, Chin MH, Schaefer CT. Improving the effectiveness of health care innovation implementation: middle managers as change agents. Med Care Res Rev. 2013;70(1):29-45.
10. Ahluwalia S, Offredy M. A qualitative study of the impact of the implementation of advanced access in primary healthcare on the working lives of general practice staff. BMC Fam Pract. 2005;6:39.
11. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
12. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107-115.
13. Stefl ME. Common competencies for all healthcare managers: the Healthcare Leadership Alliance model. J Healthc Manag. 2008;53(6):360-374.
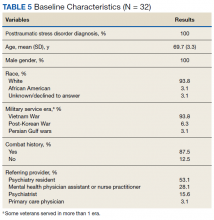
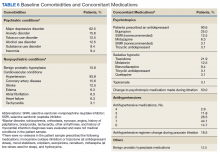
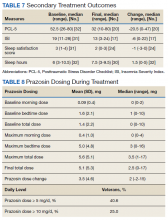
Prazosin Outcomes in Older Veterans With Posttraumatic Stress Disorder
Posttraumatic stress disorder (PTSD) is a common psychiatric condition in the veteran population and is associated with significant sleep disturbances and trauma-related nightmares.1 PTSD can present with intrusive symptoms, such as recurrent memories or dreams, which are associated with traumatic events.2 Clinical studies have described an increase in central nervous system (CNS) noradrenergic activity in PTSD; specifically, noradrenergic outflow and/or postsynaptic adrenoreceptor responsiveness is increased.3,4 Targeting a reduction in noradrenergic activity via antagonism of noradrenergic receptors has been a therapeutic treatment strategy in PTSD.
Prazosin crosses the blood-brain barrier and works to antagonize α-1 adrenoreceptors to decrease noradrenergic outflow.5 It has been shown in multiple trials to effectively reduce nightmares and improve sleep quality in the veteran population.6-12 However, a recent negative trial contributed to a downgraded recommendation for prazosin in the treatment of PTSD-related nightmares in the joint PTSD guideline from the US Department of Veterans Affairs (VA) and US Department of Defense (DoD).13,14
The diagnosis of PTSD in veterans aged ≥ 65 years has been increasing due to improved recognition.15 As a result, prazosin may be considered more frequently as a treatment option for those patients who report PTSD-related nightmares. It is important to recognize that the normal physiologic process of aging is associated with increased noradrenergic outflow, which may change the pharmacodynamics of prazosin in geriatric patients.12,16 This may necessitate increased doses to adequately antagonize the α-1 adenoreceptor.17 High doses of prazosin may increase the risk of hypotension in older patients.12 This increased risk is especially concerning for patients who already receive multiple medications or have comorbid conditions that impact blood pressure (BP).
The existing literature has few studies that have reported on outcomes with prazosin use in older veterans.11,12 The few existing reports provide clinically valuable descriptions of tolerability and efficacy with prazosin. For example, Peskind and colleagues showed prazosin to be an effective agent in the treatment of PTSD-related nightmares.12 However, in older veterans prazosin dosing > 4 mg has not been described or reported in the literature.
There appears to be a lack of clinical guidance with regards to dosing of prazosin in older patients. The goal of the current study was to assess the outcomes of older veterans with PTSD under pharmacist management of prazosin at our outpatient Prazosin Titration Clinic (PTC) in order to contribute to the minimal, yet valuable, existing clinical literature.
Methods
This study was approved by the University of Iowa Institutional Review Board and Iowa City Veterans Affairs Health Care System (ICVAHCS) research and development committee. The study was a retrospective chart review of older patients with consultations referred to the ICVAHCS PTC. To be eligible for inclusion, veterans with a PTSD diagnosis must have been evaluated at an initial consult appointment with a mental health clinical pharmacy specialist (MH CPS) from February 1, 2016 to August 31, 2018, and had at least 1 follow-up appointment. Follow-up visits were conducted either by telephone or in a face-to-face clinic visit.
Prazosin Titration Clinics
VA health care systems use pharmacists to manage veterans prescribed prazosin through PTC consultations. PTCs provide a process for close follow-up and assessment of PTSD-related outcomes. Due to the frequency of follow-up, this service may be beneficial for older veterans with more complex comorbidities and medication regimens. Any veteran with PTSD-related nightmares may be referred to the PTC for a consultation by any health care provider. Once referred to the clinic, MH CPSs assume responsibility for the prazosin prescription, including dose adjustments. For example, if a veteran reported no issues with tolerability but continued to have frequent and distressing nightmares, the dose may be increased, typically by 1-mg to 2-mg increments. Once the veteran reaches a stable and tolerable dose of prazosin, they are discharged from the PTC, and the referring health care provider resumes responsibility for the prazosin prescription.
Clinically Measured Outcomes
Nightmare frequency and intensity were measured using the Recurrent Distressing Dreams item B2 of the Clinician Administered PTSD Scale (CAPS) (Table 1). The PTSD Checklist (PCL-5), Insomnia Severity Index (ISI), and total sleep hours were used to determine the effect of prazosin on symptom severity (Table 2). The PCL-5 is a 20-item self-report used to monitor and quantify symptom level and change over time. It evaluates the frequency over the past month that a patient was bothered by any of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) PTSD criterion.2 Scores range from 0 (not at all) to 4 (extreme), with a maximum score of 80. The ISI is a 7-item self-report of sleep symptoms, with a total score of 28, where increasing scores indicate increasing severity of insomnia (Table 3).
Clinically measured outcome scales were performed and assessed by MH CPSs. CAPS frequency and intensity were measured at each clinic visit. PCL-5 and ISI scores were assessed at baseline and at the endpoint of study or discharge from clinic (Table 4). Patients who continued in the PTC after the end of the study date or who were lost to follow-up did not complete these measures at time of discharge.
Data Analysis
The primary outcome was change in CAPS nightmare frequency and intensity from time of initial clinic visit to time of discharge or end of study. The secondary outcomes included change in PCL-5, ISI, and sleep hours. Other secondary outcomes included measures of tolerability: BP changes, adverse effects (AEs) reported, and outcome of prazosin therapy when AEs were reported. Change in PTSD symptoms, PCL-5, and ISI were assessed using the Wilcoxon signed rank tests. Findings were considered to be statistically significant at P ≤ .05. Other variables were reported descriptively.
Results
Thirty-two veterans, aged ≥ 65 years, with clinical diagnosis of PTSD at the time of referral to the PTC were reviewed (Table 5). All patients were male and 93.8% were white. Thirty were Vietnam era veterans, 1 served in the Persian Gulf era, and 2 served in the post-Korean War era. Twenty-eight veterans had a combat history. Severe PTSD symptoms were reported as indicated by baseline PCL-5 scores, and moderate severity insomnia symptoms as indicated by baseline ISI scores.
All veterans had at least 1 comorbid medical condition, and the majority had multiple medical comorbidities. All were taking multiple medical and psychiatric medications. More than 80% of veterans were taking antihypertensive agents at baseline (Table 6). Twenty-two of the 32 veterans were prescribed a VA/DoD PTSD guideline-recommended antidepressant.
Primary Outcomes
The baseline, final, and changes in the primary outcomes are included in the Figure. Treatment with prazosin was associated with significant improvement in median scores from baseline to endpoint for CAPS nightmare frequency (-2, P = .0001), CAPS nightmare intensity (-2, P = .001), and total CAPS item score (-4, P < .001).
Secondary Outcomes
Of the 32 patients included in the study, PCL-5 was obtained from 20 veterans and ISI from 17 veterans at discharge from clinic. Thirty veterans reported final sleep hours, 2 veterans were unable to quantify average sleep hours per night at their final visit. PTSD symptom severity showed significant median change from baseline to endpoint of management in PTC for PCL-5 (-20.5, P = .0002) and ISI (-6.5, P = .002). Total sleep hours also showed significant improvement from baseline to endpoint (1.5, P = .003) (Table 7).
Prazosin Dosing
Maximum prazosin total daily doses were evaluated from the study baseline to the endpoint (Table 8). The mean (SD) maximum total daily dose of prazosin reached was 5.6 (5.1) mg (median, 3.5 mg; range, 1-17 mg). The mean (SD) total daily dose of prazosin at endpoint of study was 5.1 (5.3) mg (median, 2.5 mg; range, 0-17 mg). The average (SD) change of prazosin dose from baseline to endpoint was 3.5 (4.6) mg (median, 2 mg; range, -2 to 15 mg).
Tolerability
The average (SD) baseline systolic BP (SBP) was 135.8 (20.5) mm Hg and diastolic BP (DBP) was 77.2 (11.0) mm Hg. The average SBP and DBP at study endpoint were 131.8 (16.6) mm Hg and 75.9 (13.7) mm Hg, respectively. Endpoint BP values were missing for 6 patients.
Nine of 32 veterans reported AEs during PTC management of prazosin. Dizziness was the most common AE reported. Other AEs noted included orthostatic hypotension, headache, and falls. Of 12 reported AEs, 8 were related to dizziness, 5 of which were transient or tolerable. One veteran had a dose reduction of prazosin due to dizziness, and 3 veterans discontinued prazosin due to orthostasis. Several veterans had changes made to their antihypertensive medication regimen during prazosin titration, including dose reductions and/or decreased number of medications. If indicated, the MH CPS collaborated with the antihypertensive prescriber to make dosing adjustments. Two veterans reported a fall during prazosin titration; 1 veteran had other mobility-related factors thought to precipitate to their fall, and neither veterans were injured because of the falls.
Twenty-eight veterans (87.5%) treated in the PTC continued prazosin therapy after discharge. Six months postdischarge, 70% of veterans had maintained prazosin therapy. Two veterans required a dose increase postdischarge from PTC, and 1 veteran required a dose reduction. About one-third of veterans included in this study continued in the PTC beyond the end of the study period. Common reasons for clinic discharge were symptom resolution (37.5%), adverse reactions (12.5%), lost to follow-up (6.3%), or nonadherence (3.1%).
Discussion
The existing literature reports few outcomes for older veterans prescribed prazosin for PTSD. One report included a 75-year-old otherwise-healthy veteran, who received 2-mg prazosin at bedtime. At this dose, he reported good tolerability and response, as indicated by a reduction in his CAPS nightmare severity score.11 An open-label trial assessed prazosin in 9 geriatric men with chronic PTSD and found low-dose prazosin (average [SD] maximum prazosin dose reported was 2.3 [0.7] mg, range 2-4 mg per day) greatly reduced nightmares and overall PTSD severity in 8 of 9 subjects.12 Despite the veterans in that study having multiple medical comorbid conditions and taking concomitant medications, prazosin was reported to be well tolerated, and changes in BP were determined to be clinically insignificant.12 A recent study of middle-aged veterans (average [SD] age 52 [14] years) reported prazosin did not significantly alleviate PTSD-related nightmares.13 However, we observed prazosin therapy significantly reduced nightmares and sleep disturbances, and significantly improved PTSD severity in our older veteran population.
To our knowledge, the current study is the largest retrospective study that evaluates prazosin therapy for the treatment of PTSD-related nightmares in older veterans. The findings of this study are similar to a previous study in older veterans as well as studies of prazosin in younger and middle-aged adult veterans, with the average age ranging from 30 to 56 years.6-12 Like the previously reported studies, prazosin also was well tolerated in our sample of veterans with multiple comorbidities and concomitant medications. Changes in BP were not clinically significant.
Studies have demonstrated increased noradrenergic activity as a component of the normal aging process.16,17 This may require utilizing caution during prazosin dose titration and frequent patient assessment, due to the concern for risk of hypotension in older patients and in particular those who may require increased doses to achieve efficacy. In our study, favorable outcomes were achieved at an average (SD) total daily dose of 5.1 (5.3) mg (median, 2.5 mg; range 0-17 mg). A previous report showed efficacy of prazosin around an average (SD) maximum dose of 2.3 (0.7) mg, which is lower than the doses reported in the current study.12 In addition, 13 veterans (40.6%) from our sample reached doses of ≥ 5 mg per day, and 8 veterans (25.0%) reached doses of ≥ 10 mg per day.
The doses reached in this study were reflective of a management approach using assessment of patient-reported symptoms at weekly to biweekly follow-up visits. The individualized management approach applied in the PTC by MH CPSs aids in uncovering the most efficacious and tolerated dose of prazosin for each veteran. Evaluation of symptom change during treatment in PTC was facilitated use of objective rating scales, which helped measure nightmare frequency and intensity, sleep satisfaction, and global PTSD severity. Given the variability in dosing of prazosin reported in the literature, further studies may be warranted to provide more definitive clinical guidance as far as dosing prazosin in older patients.
The study by Peskind and colleaguesrationalized that lower doses of prazosin may be used in older patients given pharmacokinetic effects of aging, age-associated changes in PTSD pathophysiology, and effects and interactions of concomitant medications.12 However, our study found that prazosin could be well tolerated at higher doses. The rate of discontinuation due to intolerable AEs was low. AEs reported were consistent with the established AE profile of prazosin, with dizziness, orthostasis, and headache most commonly reported. Similar to the Peskind and colleagues study, BP had a tendency to decrease in this current study; however, the change was not clinically significant.12 That study also reported transient dizziness with prazosin titration, which was shown to be tolerable in the majority of our veterans reporting dizziness.12 Other common AEs with prazosin, such as rash, priapism, sedation, syncope, other cardiac AEs, and sleep disturbance were not reported in our study population.
MH CPS-managed PTCs are one venue that may allow veterans to achieve favorable outcomes through frequent follow-up. As prazosin dosing is specific to each individual patient, frequent follow-up visits are helpful in determining optimal doses that maximize efficacy while minimizing intolerable AEs. The majority of veterans treated in our PTC continued use of prazosin 6 months postdischarge, while 3 veterans required a postdischarge dose change.
The 2017 VA/DoD PTSD guidelines recommend individual, trauma-focused psychotherapy over pharmacologic therapy for the primary treatment of PTSD.14 About half of the veterans in the current study participated in either group or individual psychotherapy during enrollment in the PTC. A systematic review of psychotherapy in older veterans reported mixed results, with 4 studies indicating positive effects of therapy, while the other 3 studies reported no benefit or mixed effects for PTSD symptoms. The review concluded that fewer older adults experience complete remission of symptoms with psychotherapy alone.18 A previous study of older veterans described improvement in PTSD-related symptoms with prazosin without concurrent psychotherapy.12
Limitations and Strengths
While this study is the largest study to evaluate outcomes of prazosin in older patients with PTSD, there are several important limitations. The study population was small and all were male. The results of this study may not be applicable to women. Another limitation was several missing values in our data set, as some secondary outcomes were not collected via telephone follow-up visits. This could potentially contribute a measurement bias in the reported secondary outcomes results, specifically for the PCL-5 and ISI. Additionally, some veterans in this study may have reported symptomatic improvement based on the additional supportive intervention that clinical pharmacists were able to offer, as well as concomitant participation in psychotherapy. This may be reflected in the study results. This study did not have a true placebo group, as we may find a reduction in symptoms with placebo.
Strengths of this study include multiple data points for assessment of prazosin tolerability and a pre- and poststudy design, which allowed for the veterans to serve as their own control. Another strength of this study is that data were complete for primary outcome measures, including the CAPS Recurrent and Distressing Dreams Item, where prazosin showed significant benefit in reduction of PTSD-related nightmares. While the results of this study are reassuring, further randomized, double-blind, placebo-controlled trials are likely needed in order to establish efficacy and tolerability of prazosin in older veterans for PTSD related nightmares.
Conclusion
These results demonstrate prazosin therapy in older veterans can significantly improve PTSD-related nightmares and PTSD severity. Prazosin was well tolerated in this population at doses higher than previously reported in other studies. This study shows that prazosin therapy can be effectively managed and tolerated in older veterans with complex medical and psychiatric comorbidities to provide favorable patient outcomes.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Iowa City VA Health Care System and by the Health Services Research and Development Service, US Department of Veterans Affairs.
1. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington VA: American Psychiatric Association; 2013.
3. Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):266-274.
4. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
5. Friedman MJ. Posttraumatic and Acute Stress Disorders. 6th ed. New York: Springer Publishing; 2015.
6. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
10. Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716-722.
11. Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
12. Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
13. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
14. The Management of Posttraumatic Stress Disorder Work Group. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0–2017. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal.pdf. Published June 2017. Accessed January 7, 2020.
15. Nichols BL, Czirr R. 24/Post-traumatic stress disorder: hidden syndrome in elders. Clin Gerontol. 1986;5(3-4):417-433.
16. Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am J Physiol. 1990;259(3, pt 1):E422-E431.
17. Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46(6):756-765.
18. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
Posttraumatic stress disorder (PTSD) is a common psychiatric condition in the veteran population and is associated with significant sleep disturbances and trauma-related nightmares.1 PTSD can present with intrusive symptoms, such as recurrent memories or dreams, which are associated with traumatic events.2 Clinical studies have described an increase in central nervous system (CNS) noradrenergic activity in PTSD; specifically, noradrenergic outflow and/or postsynaptic adrenoreceptor responsiveness is increased.3,4 Targeting a reduction in noradrenergic activity via antagonism of noradrenergic receptors has been a therapeutic treatment strategy in PTSD.
Prazosin crosses the blood-brain barrier and works to antagonize α-1 adrenoreceptors to decrease noradrenergic outflow.5 It has been shown in multiple trials to effectively reduce nightmares and improve sleep quality in the veteran population.6-12 However, a recent negative trial contributed to a downgraded recommendation for prazosin in the treatment of PTSD-related nightmares in the joint PTSD guideline from the US Department of Veterans Affairs (VA) and US Department of Defense (DoD).13,14
The diagnosis of PTSD in veterans aged ≥ 65 years has been increasing due to improved recognition.15 As a result, prazosin may be considered more frequently as a treatment option for those patients who report PTSD-related nightmares. It is important to recognize that the normal physiologic process of aging is associated with increased noradrenergic outflow, which may change the pharmacodynamics of prazosin in geriatric patients.12,16 This may necessitate increased doses to adequately antagonize the α-1 adenoreceptor.17 High doses of prazosin may increase the risk of hypotension in older patients.12 This increased risk is especially concerning for patients who already receive multiple medications or have comorbid conditions that impact blood pressure (BP).
The existing literature has few studies that have reported on outcomes with prazosin use in older veterans.11,12 The few existing reports provide clinically valuable descriptions of tolerability and efficacy with prazosin. For example, Peskind and colleagues showed prazosin to be an effective agent in the treatment of PTSD-related nightmares.12 However, in older veterans prazosin dosing > 4 mg has not been described or reported in the literature.
There appears to be a lack of clinical guidance with regards to dosing of prazosin in older patients. The goal of the current study was to assess the outcomes of older veterans with PTSD under pharmacist management of prazosin at our outpatient Prazosin Titration Clinic (PTC) in order to contribute to the minimal, yet valuable, existing clinical literature.
Methods
This study was approved by the University of Iowa Institutional Review Board and Iowa City Veterans Affairs Health Care System (ICVAHCS) research and development committee. The study was a retrospective chart review of older patients with consultations referred to the ICVAHCS PTC. To be eligible for inclusion, veterans with a PTSD diagnosis must have been evaluated at an initial consult appointment with a mental health clinical pharmacy specialist (MH CPS) from February 1, 2016 to August 31, 2018, and had at least 1 follow-up appointment. Follow-up visits were conducted either by telephone or in a face-to-face clinic visit.
Prazosin Titration Clinics
VA health care systems use pharmacists to manage veterans prescribed prazosin through PTC consultations. PTCs provide a process for close follow-up and assessment of PTSD-related outcomes. Due to the frequency of follow-up, this service may be beneficial for older veterans with more complex comorbidities and medication regimens. Any veteran with PTSD-related nightmares may be referred to the PTC for a consultation by any health care provider. Once referred to the clinic, MH CPSs assume responsibility for the prazosin prescription, including dose adjustments. For example, if a veteran reported no issues with tolerability but continued to have frequent and distressing nightmares, the dose may be increased, typically by 1-mg to 2-mg increments. Once the veteran reaches a stable and tolerable dose of prazosin, they are discharged from the PTC, and the referring health care provider resumes responsibility for the prazosin prescription.
Clinically Measured Outcomes
Nightmare frequency and intensity were measured using the Recurrent Distressing Dreams item B2 of the Clinician Administered PTSD Scale (CAPS) (Table 1). The PTSD Checklist (PCL-5), Insomnia Severity Index (ISI), and total sleep hours were used to determine the effect of prazosin on symptom severity (Table 2). The PCL-5 is a 20-item self-report used to monitor and quantify symptom level and change over time. It evaluates the frequency over the past month that a patient was bothered by any of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) PTSD criterion.2 Scores range from 0 (not at all) to 4 (extreme), with a maximum score of 80. The ISI is a 7-item self-report of sleep symptoms, with a total score of 28, where increasing scores indicate increasing severity of insomnia (Table 3).
Clinically measured outcome scales were performed and assessed by MH CPSs. CAPS frequency and intensity were measured at each clinic visit. PCL-5 and ISI scores were assessed at baseline and at the endpoint of study or discharge from clinic (Table 4). Patients who continued in the PTC after the end of the study date or who were lost to follow-up did not complete these measures at time of discharge.
Data Analysis
The primary outcome was change in CAPS nightmare frequency and intensity from time of initial clinic visit to time of discharge or end of study. The secondary outcomes included change in PCL-5, ISI, and sleep hours. Other secondary outcomes included measures of tolerability: BP changes, adverse effects (AEs) reported, and outcome of prazosin therapy when AEs were reported. Change in PTSD symptoms, PCL-5, and ISI were assessed using the Wilcoxon signed rank tests. Findings were considered to be statistically significant at P ≤ .05. Other variables were reported descriptively.
Results
Thirty-two veterans, aged ≥ 65 years, with clinical diagnosis of PTSD at the time of referral to the PTC were reviewed (Table 5). All patients were male and 93.8% were white. Thirty were Vietnam era veterans, 1 served in the Persian Gulf era, and 2 served in the post-Korean War era. Twenty-eight veterans had a combat history. Severe PTSD symptoms were reported as indicated by baseline PCL-5 scores, and moderate severity insomnia symptoms as indicated by baseline ISI scores.
All veterans had at least 1 comorbid medical condition, and the majority had multiple medical comorbidities. All were taking multiple medical and psychiatric medications. More than 80% of veterans were taking antihypertensive agents at baseline (Table 6). Twenty-two of the 32 veterans were prescribed a VA/DoD PTSD guideline-recommended antidepressant.
Primary Outcomes
The baseline, final, and changes in the primary outcomes are included in the Figure. Treatment with prazosin was associated with significant improvement in median scores from baseline to endpoint for CAPS nightmare frequency (-2, P = .0001), CAPS nightmare intensity (-2, P = .001), and total CAPS item score (-4, P < .001).
Secondary Outcomes
Of the 32 patients included in the study, PCL-5 was obtained from 20 veterans and ISI from 17 veterans at discharge from clinic. Thirty veterans reported final sleep hours, 2 veterans were unable to quantify average sleep hours per night at their final visit. PTSD symptom severity showed significant median change from baseline to endpoint of management in PTC for PCL-5 (-20.5, P = .0002) and ISI (-6.5, P = .002). Total sleep hours also showed significant improvement from baseline to endpoint (1.5, P = .003) (Table 7).
Prazosin Dosing
Maximum prazosin total daily doses were evaluated from the study baseline to the endpoint (Table 8). The mean (SD) maximum total daily dose of prazosin reached was 5.6 (5.1) mg (median, 3.5 mg; range, 1-17 mg). The mean (SD) total daily dose of prazosin at endpoint of study was 5.1 (5.3) mg (median, 2.5 mg; range, 0-17 mg). The average (SD) change of prazosin dose from baseline to endpoint was 3.5 (4.6) mg (median, 2 mg; range, -2 to 15 mg).
Tolerability
The average (SD) baseline systolic BP (SBP) was 135.8 (20.5) mm Hg and diastolic BP (DBP) was 77.2 (11.0) mm Hg. The average SBP and DBP at study endpoint were 131.8 (16.6) mm Hg and 75.9 (13.7) mm Hg, respectively. Endpoint BP values were missing for 6 patients.
Nine of 32 veterans reported AEs during PTC management of prazosin. Dizziness was the most common AE reported. Other AEs noted included orthostatic hypotension, headache, and falls. Of 12 reported AEs, 8 were related to dizziness, 5 of which were transient or tolerable. One veteran had a dose reduction of prazosin due to dizziness, and 3 veterans discontinued prazosin due to orthostasis. Several veterans had changes made to their antihypertensive medication regimen during prazosin titration, including dose reductions and/or decreased number of medications. If indicated, the MH CPS collaborated with the antihypertensive prescriber to make dosing adjustments. Two veterans reported a fall during prazosin titration; 1 veteran had other mobility-related factors thought to precipitate to their fall, and neither veterans were injured because of the falls.
Twenty-eight veterans (87.5%) treated in the PTC continued prazosin therapy after discharge. Six months postdischarge, 70% of veterans had maintained prazosin therapy. Two veterans required a dose increase postdischarge from PTC, and 1 veteran required a dose reduction. About one-third of veterans included in this study continued in the PTC beyond the end of the study period. Common reasons for clinic discharge were symptom resolution (37.5%), adverse reactions (12.5%), lost to follow-up (6.3%), or nonadherence (3.1%).
Discussion
The existing literature reports few outcomes for older veterans prescribed prazosin for PTSD. One report included a 75-year-old otherwise-healthy veteran, who received 2-mg prazosin at bedtime. At this dose, he reported good tolerability and response, as indicated by a reduction in his CAPS nightmare severity score.11 An open-label trial assessed prazosin in 9 geriatric men with chronic PTSD and found low-dose prazosin (average [SD] maximum prazosin dose reported was 2.3 [0.7] mg, range 2-4 mg per day) greatly reduced nightmares and overall PTSD severity in 8 of 9 subjects.12 Despite the veterans in that study having multiple medical comorbid conditions and taking concomitant medications, prazosin was reported to be well tolerated, and changes in BP were determined to be clinically insignificant.12 A recent study of middle-aged veterans (average [SD] age 52 [14] years) reported prazosin did not significantly alleviate PTSD-related nightmares.13 However, we observed prazosin therapy significantly reduced nightmares and sleep disturbances, and significantly improved PTSD severity in our older veteran population.
To our knowledge, the current study is the largest retrospective study that evaluates prazosin therapy for the treatment of PTSD-related nightmares in older veterans. The findings of this study are similar to a previous study in older veterans as well as studies of prazosin in younger and middle-aged adult veterans, with the average age ranging from 30 to 56 years.6-12 Like the previously reported studies, prazosin also was well tolerated in our sample of veterans with multiple comorbidities and concomitant medications. Changes in BP were not clinically significant.
Studies have demonstrated increased noradrenergic activity as a component of the normal aging process.16,17 This may require utilizing caution during prazosin dose titration and frequent patient assessment, due to the concern for risk of hypotension in older patients and in particular those who may require increased doses to achieve efficacy. In our study, favorable outcomes were achieved at an average (SD) total daily dose of 5.1 (5.3) mg (median, 2.5 mg; range 0-17 mg). A previous report showed efficacy of prazosin around an average (SD) maximum dose of 2.3 (0.7) mg, which is lower than the doses reported in the current study.12 In addition, 13 veterans (40.6%) from our sample reached doses of ≥ 5 mg per day, and 8 veterans (25.0%) reached doses of ≥ 10 mg per day.
The doses reached in this study were reflective of a management approach using assessment of patient-reported symptoms at weekly to biweekly follow-up visits. The individualized management approach applied in the PTC by MH CPSs aids in uncovering the most efficacious and tolerated dose of prazosin for each veteran. Evaluation of symptom change during treatment in PTC was facilitated use of objective rating scales, which helped measure nightmare frequency and intensity, sleep satisfaction, and global PTSD severity. Given the variability in dosing of prazosin reported in the literature, further studies may be warranted to provide more definitive clinical guidance as far as dosing prazosin in older patients.
The study by Peskind and colleaguesrationalized that lower doses of prazosin may be used in older patients given pharmacokinetic effects of aging, age-associated changes in PTSD pathophysiology, and effects and interactions of concomitant medications.12 However, our study found that prazosin could be well tolerated at higher doses. The rate of discontinuation due to intolerable AEs was low. AEs reported were consistent with the established AE profile of prazosin, with dizziness, orthostasis, and headache most commonly reported. Similar to the Peskind and colleagues study, BP had a tendency to decrease in this current study; however, the change was not clinically significant.12 That study also reported transient dizziness with prazosin titration, which was shown to be tolerable in the majority of our veterans reporting dizziness.12 Other common AEs with prazosin, such as rash, priapism, sedation, syncope, other cardiac AEs, and sleep disturbance were not reported in our study population.
MH CPS-managed PTCs are one venue that may allow veterans to achieve favorable outcomes through frequent follow-up. As prazosin dosing is specific to each individual patient, frequent follow-up visits are helpful in determining optimal doses that maximize efficacy while minimizing intolerable AEs. The majority of veterans treated in our PTC continued use of prazosin 6 months postdischarge, while 3 veterans required a postdischarge dose change.
The 2017 VA/DoD PTSD guidelines recommend individual, trauma-focused psychotherapy over pharmacologic therapy for the primary treatment of PTSD.14 About half of the veterans in the current study participated in either group or individual psychotherapy during enrollment in the PTC. A systematic review of psychotherapy in older veterans reported mixed results, with 4 studies indicating positive effects of therapy, while the other 3 studies reported no benefit or mixed effects for PTSD symptoms. The review concluded that fewer older adults experience complete remission of symptoms with psychotherapy alone.18 A previous study of older veterans described improvement in PTSD-related symptoms with prazosin without concurrent psychotherapy.12
Limitations and Strengths
While this study is the largest study to evaluate outcomes of prazosin in older patients with PTSD, there are several important limitations. The study population was small and all were male. The results of this study may not be applicable to women. Another limitation was several missing values in our data set, as some secondary outcomes were not collected via telephone follow-up visits. This could potentially contribute a measurement bias in the reported secondary outcomes results, specifically for the PCL-5 and ISI. Additionally, some veterans in this study may have reported symptomatic improvement based on the additional supportive intervention that clinical pharmacists were able to offer, as well as concomitant participation in psychotherapy. This may be reflected in the study results. This study did not have a true placebo group, as we may find a reduction in symptoms with placebo.
Strengths of this study include multiple data points for assessment of prazosin tolerability and a pre- and poststudy design, which allowed for the veterans to serve as their own control. Another strength of this study is that data were complete for primary outcome measures, including the CAPS Recurrent and Distressing Dreams Item, where prazosin showed significant benefit in reduction of PTSD-related nightmares. While the results of this study are reassuring, further randomized, double-blind, placebo-controlled trials are likely needed in order to establish efficacy and tolerability of prazosin in older veterans for PTSD related nightmares.
Conclusion
These results demonstrate prazosin therapy in older veterans can significantly improve PTSD-related nightmares and PTSD severity. Prazosin was well tolerated in this population at doses higher than previously reported in other studies. This study shows that prazosin therapy can be effectively managed and tolerated in older veterans with complex medical and psychiatric comorbidities to provide favorable patient outcomes.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Iowa City VA Health Care System and by the Health Services Research and Development Service, US Department of Veterans Affairs.
Posttraumatic stress disorder (PTSD) is a common psychiatric condition in the veteran population and is associated with significant sleep disturbances and trauma-related nightmares.1 PTSD can present with intrusive symptoms, such as recurrent memories or dreams, which are associated with traumatic events.2 Clinical studies have described an increase in central nervous system (CNS) noradrenergic activity in PTSD; specifically, noradrenergic outflow and/or postsynaptic adrenoreceptor responsiveness is increased.3,4 Targeting a reduction in noradrenergic activity via antagonism of noradrenergic receptors has been a therapeutic treatment strategy in PTSD.
Prazosin crosses the blood-brain barrier and works to antagonize α-1 adrenoreceptors to decrease noradrenergic outflow.5 It has been shown in multiple trials to effectively reduce nightmares and improve sleep quality in the veteran population.6-12 However, a recent negative trial contributed to a downgraded recommendation for prazosin in the treatment of PTSD-related nightmares in the joint PTSD guideline from the US Department of Veterans Affairs (VA) and US Department of Defense (DoD).13,14
The diagnosis of PTSD in veterans aged ≥ 65 years has been increasing due to improved recognition.15 As a result, prazosin may be considered more frequently as a treatment option for those patients who report PTSD-related nightmares. It is important to recognize that the normal physiologic process of aging is associated with increased noradrenergic outflow, which may change the pharmacodynamics of prazosin in geriatric patients.12,16 This may necessitate increased doses to adequately antagonize the α-1 adenoreceptor.17 High doses of prazosin may increase the risk of hypotension in older patients.12 This increased risk is especially concerning for patients who already receive multiple medications or have comorbid conditions that impact blood pressure (BP).
The existing literature has few studies that have reported on outcomes with prazosin use in older veterans.11,12 The few existing reports provide clinically valuable descriptions of tolerability and efficacy with prazosin. For example, Peskind and colleagues showed prazosin to be an effective agent in the treatment of PTSD-related nightmares.12 However, in older veterans prazosin dosing > 4 mg has not been described or reported in the literature.
There appears to be a lack of clinical guidance with regards to dosing of prazosin in older patients. The goal of the current study was to assess the outcomes of older veterans with PTSD under pharmacist management of prazosin at our outpatient Prazosin Titration Clinic (PTC) in order to contribute to the minimal, yet valuable, existing clinical literature.
Methods
This study was approved by the University of Iowa Institutional Review Board and Iowa City Veterans Affairs Health Care System (ICVAHCS) research and development committee. The study was a retrospective chart review of older patients with consultations referred to the ICVAHCS PTC. To be eligible for inclusion, veterans with a PTSD diagnosis must have been evaluated at an initial consult appointment with a mental health clinical pharmacy specialist (MH CPS) from February 1, 2016 to August 31, 2018, and had at least 1 follow-up appointment. Follow-up visits were conducted either by telephone or in a face-to-face clinic visit.
Prazosin Titration Clinics
VA health care systems use pharmacists to manage veterans prescribed prazosin through PTC consultations. PTCs provide a process for close follow-up and assessment of PTSD-related outcomes. Due to the frequency of follow-up, this service may be beneficial for older veterans with more complex comorbidities and medication regimens. Any veteran with PTSD-related nightmares may be referred to the PTC for a consultation by any health care provider. Once referred to the clinic, MH CPSs assume responsibility for the prazosin prescription, including dose adjustments. For example, if a veteran reported no issues with tolerability but continued to have frequent and distressing nightmares, the dose may be increased, typically by 1-mg to 2-mg increments. Once the veteran reaches a stable and tolerable dose of prazosin, they are discharged from the PTC, and the referring health care provider resumes responsibility for the prazosin prescription.
Clinically Measured Outcomes
Nightmare frequency and intensity were measured using the Recurrent Distressing Dreams item B2 of the Clinician Administered PTSD Scale (CAPS) (Table 1). The PTSD Checklist (PCL-5), Insomnia Severity Index (ISI), and total sleep hours were used to determine the effect of prazosin on symptom severity (Table 2). The PCL-5 is a 20-item self-report used to monitor and quantify symptom level and change over time. It evaluates the frequency over the past month that a patient was bothered by any of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) PTSD criterion.2 Scores range from 0 (not at all) to 4 (extreme), with a maximum score of 80. The ISI is a 7-item self-report of sleep symptoms, with a total score of 28, where increasing scores indicate increasing severity of insomnia (Table 3).
Clinically measured outcome scales were performed and assessed by MH CPSs. CAPS frequency and intensity were measured at each clinic visit. PCL-5 and ISI scores were assessed at baseline and at the endpoint of study or discharge from clinic (Table 4). Patients who continued in the PTC after the end of the study date or who were lost to follow-up did not complete these measures at time of discharge.
Data Analysis
The primary outcome was change in CAPS nightmare frequency and intensity from time of initial clinic visit to time of discharge or end of study. The secondary outcomes included change in PCL-5, ISI, and sleep hours. Other secondary outcomes included measures of tolerability: BP changes, adverse effects (AEs) reported, and outcome of prazosin therapy when AEs were reported. Change in PTSD symptoms, PCL-5, and ISI were assessed using the Wilcoxon signed rank tests. Findings were considered to be statistically significant at P ≤ .05. Other variables were reported descriptively.
Results
Thirty-two veterans, aged ≥ 65 years, with clinical diagnosis of PTSD at the time of referral to the PTC were reviewed (Table 5). All patients were male and 93.8% were white. Thirty were Vietnam era veterans, 1 served in the Persian Gulf era, and 2 served in the post-Korean War era. Twenty-eight veterans had a combat history. Severe PTSD symptoms were reported as indicated by baseline PCL-5 scores, and moderate severity insomnia symptoms as indicated by baseline ISI scores.
All veterans had at least 1 comorbid medical condition, and the majority had multiple medical comorbidities. All were taking multiple medical and psychiatric medications. More than 80% of veterans were taking antihypertensive agents at baseline (Table 6). Twenty-two of the 32 veterans were prescribed a VA/DoD PTSD guideline-recommended antidepressant.
Primary Outcomes
The baseline, final, and changes in the primary outcomes are included in the Figure. Treatment with prazosin was associated with significant improvement in median scores from baseline to endpoint for CAPS nightmare frequency (-2, P = .0001), CAPS nightmare intensity (-2, P = .001), and total CAPS item score (-4, P < .001).
Secondary Outcomes
Of the 32 patients included in the study, PCL-5 was obtained from 20 veterans and ISI from 17 veterans at discharge from clinic. Thirty veterans reported final sleep hours, 2 veterans were unable to quantify average sleep hours per night at their final visit. PTSD symptom severity showed significant median change from baseline to endpoint of management in PTC for PCL-5 (-20.5, P = .0002) and ISI (-6.5, P = .002). Total sleep hours also showed significant improvement from baseline to endpoint (1.5, P = .003) (Table 7).
Prazosin Dosing
Maximum prazosin total daily doses were evaluated from the study baseline to the endpoint (Table 8). The mean (SD) maximum total daily dose of prazosin reached was 5.6 (5.1) mg (median, 3.5 mg; range, 1-17 mg). The mean (SD) total daily dose of prazosin at endpoint of study was 5.1 (5.3) mg (median, 2.5 mg; range, 0-17 mg). The average (SD) change of prazosin dose from baseline to endpoint was 3.5 (4.6) mg (median, 2 mg; range, -2 to 15 mg).
Tolerability
The average (SD) baseline systolic BP (SBP) was 135.8 (20.5) mm Hg and diastolic BP (DBP) was 77.2 (11.0) mm Hg. The average SBP and DBP at study endpoint were 131.8 (16.6) mm Hg and 75.9 (13.7) mm Hg, respectively. Endpoint BP values were missing for 6 patients.
Nine of 32 veterans reported AEs during PTC management of prazosin. Dizziness was the most common AE reported. Other AEs noted included orthostatic hypotension, headache, and falls. Of 12 reported AEs, 8 were related to dizziness, 5 of which were transient or tolerable. One veteran had a dose reduction of prazosin due to dizziness, and 3 veterans discontinued prazosin due to orthostasis. Several veterans had changes made to their antihypertensive medication regimen during prazosin titration, including dose reductions and/or decreased number of medications. If indicated, the MH CPS collaborated with the antihypertensive prescriber to make dosing adjustments. Two veterans reported a fall during prazosin titration; 1 veteran had other mobility-related factors thought to precipitate to their fall, and neither veterans were injured because of the falls.
Twenty-eight veterans (87.5%) treated in the PTC continued prazosin therapy after discharge. Six months postdischarge, 70% of veterans had maintained prazosin therapy. Two veterans required a dose increase postdischarge from PTC, and 1 veteran required a dose reduction. About one-third of veterans included in this study continued in the PTC beyond the end of the study period. Common reasons for clinic discharge were symptom resolution (37.5%), adverse reactions (12.5%), lost to follow-up (6.3%), or nonadherence (3.1%).
Discussion
The existing literature reports few outcomes for older veterans prescribed prazosin for PTSD. One report included a 75-year-old otherwise-healthy veteran, who received 2-mg prazosin at bedtime. At this dose, he reported good tolerability and response, as indicated by a reduction in his CAPS nightmare severity score.11 An open-label trial assessed prazosin in 9 geriatric men with chronic PTSD and found low-dose prazosin (average [SD] maximum prazosin dose reported was 2.3 [0.7] mg, range 2-4 mg per day) greatly reduced nightmares and overall PTSD severity in 8 of 9 subjects.12 Despite the veterans in that study having multiple medical comorbid conditions and taking concomitant medications, prazosin was reported to be well tolerated, and changes in BP were determined to be clinically insignificant.12 A recent study of middle-aged veterans (average [SD] age 52 [14] years) reported prazosin did not significantly alleviate PTSD-related nightmares.13 However, we observed prazosin therapy significantly reduced nightmares and sleep disturbances, and significantly improved PTSD severity in our older veteran population.
To our knowledge, the current study is the largest retrospective study that evaluates prazosin therapy for the treatment of PTSD-related nightmares in older veterans. The findings of this study are similar to a previous study in older veterans as well as studies of prazosin in younger and middle-aged adult veterans, with the average age ranging from 30 to 56 years.6-12 Like the previously reported studies, prazosin also was well tolerated in our sample of veterans with multiple comorbidities and concomitant medications. Changes in BP were not clinically significant.
Studies have demonstrated increased noradrenergic activity as a component of the normal aging process.16,17 This may require utilizing caution during prazosin dose titration and frequent patient assessment, due to the concern for risk of hypotension in older patients and in particular those who may require increased doses to achieve efficacy. In our study, favorable outcomes were achieved at an average (SD) total daily dose of 5.1 (5.3) mg (median, 2.5 mg; range 0-17 mg). A previous report showed efficacy of prazosin around an average (SD) maximum dose of 2.3 (0.7) mg, which is lower than the doses reported in the current study.12 In addition, 13 veterans (40.6%) from our sample reached doses of ≥ 5 mg per day, and 8 veterans (25.0%) reached doses of ≥ 10 mg per day.
The doses reached in this study were reflective of a management approach using assessment of patient-reported symptoms at weekly to biweekly follow-up visits. The individualized management approach applied in the PTC by MH CPSs aids in uncovering the most efficacious and tolerated dose of prazosin for each veteran. Evaluation of symptom change during treatment in PTC was facilitated use of objective rating scales, which helped measure nightmare frequency and intensity, sleep satisfaction, and global PTSD severity. Given the variability in dosing of prazosin reported in the literature, further studies may be warranted to provide more definitive clinical guidance as far as dosing prazosin in older patients.
The study by Peskind and colleaguesrationalized that lower doses of prazosin may be used in older patients given pharmacokinetic effects of aging, age-associated changes in PTSD pathophysiology, and effects and interactions of concomitant medications.12 However, our study found that prazosin could be well tolerated at higher doses. The rate of discontinuation due to intolerable AEs was low. AEs reported were consistent with the established AE profile of prazosin, with dizziness, orthostasis, and headache most commonly reported. Similar to the Peskind and colleagues study, BP had a tendency to decrease in this current study; however, the change was not clinically significant.12 That study also reported transient dizziness with prazosin titration, which was shown to be tolerable in the majority of our veterans reporting dizziness.12 Other common AEs with prazosin, such as rash, priapism, sedation, syncope, other cardiac AEs, and sleep disturbance were not reported in our study population.
MH CPS-managed PTCs are one venue that may allow veterans to achieve favorable outcomes through frequent follow-up. As prazosin dosing is specific to each individual patient, frequent follow-up visits are helpful in determining optimal doses that maximize efficacy while minimizing intolerable AEs. The majority of veterans treated in our PTC continued use of prazosin 6 months postdischarge, while 3 veterans required a postdischarge dose change.
The 2017 VA/DoD PTSD guidelines recommend individual, trauma-focused psychotherapy over pharmacologic therapy for the primary treatment of PTSD.14 About half of the veterans in the current study participated in either group or individual psychotherapy during enrollment in the PTC. A systematic review of psychotherapy in older veterans reported mixed results, with 4 studies indicating positive effects of therapy, while the other 3 studies reported no benefit or mixed effects for PTSD symptoms. The review concluded that fewer older adults experience complete remission of symptoms with psychotherapy alone.18 A previous study of older veterans described improvement in PTSD-related symptoms with prazosin without concurrent psychotherapy.12
Limitations and Strengths
While this study is the largest study to evaluate outcomes of prazosin in older patients with PTSD, there are several important limitations. The study population was small and all were male. The results of this study may not be applicable to women. Another limitation was several missing values in our data set, as some secondary outcomes were not collected via telephone follow-up visits. This could potentially contribute a measurement bias in the reported secondary outcomes results, specifically for the PCL-5 and ISI. Additionally, some veterans in this study may have reported symptomatic improvement based on the additional supportive intervention that clinical pharmacists were able to offer, as well as concomitant participation in psychotherapy. This may be reflected in the study results. This study did not have a true placebo group, as we may find a reduction in symptoms with placebo.
Strengths of this study include multiple data points for assessment of prazosin tolerability and a pre- and poststudy design, which allowed for the veterans to serve as their own control. Another strength of this study is that data were complete for primary outcome measures, including the CAPS Recurrent and Distressing Dreams Item, where prazosin showed significant benefit in reduction of PTSD-related nightmares. While the results of this study are reassuring, further randomized, double-blind, placebo-controlled trials are likely needed in order to establish efficacy and tolerability of prazosin in older veterans for PTSD related nightmares.
Conclusion
These results demonstrate prazosin therapy in older veterans can significantly improve PTSD-related nightmares and PTSD severity. Prazosin was well tolerated in this population at doses higher than previously reported in other studies. This study shows that prazosin therapy can be effectively managed and tolerated in older veterans with complex medical and psychiatric comorbidities to provide favorable patient outcomes.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Iowa City VA Health Care System and by the Health Services Research and Development Service, US Department of Veterans Affairs.
1. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington VA: American Psychiatric Association; 2013.
3. Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):266-274.
4. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
5. Friedman MJ. Posttraumatic and Acute Stress Disorders. 6th ed. New York: Springer Publishing; 2015.
6. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
10. Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716-722.
11. Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
12. Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
13. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
14. The Management of Posttraumatic Stress Disorder Work Group. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0–2017. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal.pdf. Published June 2017. Accessed January 7, 2020.
15. Nichols BL, Czirr R. 24/Post-traumatic stress disorder: hidden syndrome in elders. Clin Gerontol. 1986;5(3-4):417-433.
16. Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am J Physiol. 1990;259(3, pt 1):E422-E431.
17. Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46(6):756-765.
18. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
1. Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697-707.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington VA: American Psychiatric Association; 2013.
3. Southwick SM, Krystal JH, Morgan CA, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50(4):266-274.
4. Geracioti TD Jr, Baker DG, Ekhator NN, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227-1230.
5. Friedman MJ. Posttraumatic and Acute Stress Disorders. 6th ed. New York: Springer Publishing; 2015.
6. Raskind MA, Peterson K, Williams T, et al. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003-1010.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934.
8. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373.
9. Germain A, Richardson R, Moul DE, et al. Placebo-controlled comparison of prazosin and cognitive-behavioral treatments for sleep disturbances in US military veterans. J Psychosom Res. 2012;72(2):89-96.
10. Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716-722.
11. Raskind MA, Dobie DJ, Kanter ED, Petrie EC, Thompson CE, Peskind ER. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-133.
12. Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171.
13. Raskind MA, Peskind ER, Chow B, et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018;378(6):507-517.
14. The Management of Posttraumatic Stress Disorder Work Group. VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. Version 3.0–2017. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal.pdf. Published June 2017. Accessed January 7, 2020.
15. Nichols BL, Czirr R. 24/Post-traumatic stress disorder: hidden syndrome in elders. Clin Gerontol. 1986;5(3-4):417-433.
16. Supiano MA, Linares OA, Smith MJ, Halter JB. Age-related differences in norepinephrine kinetics: effect of posture and sodium-restricted diet. Am J Physiol. 1990;259(3, pt 1):E422-E431.
17. Raskind MA, Peskind ER, Holmes C, Goldstein DS. Patterns of cerebrospinal fluid catechols support increased central noradrenergic responsiveness in aging and Alzheimer’s disease. Biol Psychiatry. 1999;46(6):756-765.
18. Dinnen S, Simiola V, Cook JM. Post-traumatic stress disorder in older adults: a systematic review of the psychotherapy treatment literature. Aging Ment Health. 2015;19(2):144-150.
A Case-Based Review of Iron Overload With an Emphasis on Porphyria Cutanea Tarda, Hepatitis C, C282Y Heterozygosity, and Coronary Artery Disease
Sporadic porphyria cutanea tarda (PCT) is the most common cause of porphyria worldwide.1,2 Unlike other forms of porphyria, PCT usually is an acquired disease precipitated by extrinsic risk factors that commonly include excessive alcohol consumption, smoking, and chronic hepatitis C virus (HCV) infection. Additional risk factors include myeloproliferative disorders, exposure to polyhalogenated compounds, estrogen therapy, diseases of iron overload like hereditary hemochromatosis (HH), and potentially, HIV infection.1-3
In this case report, we present a patient with an iron overload (due in part to an HFE gene mutation) and concomitant PCT,
Case Presentation
Mr. M is a 59-year-old white male of Irish background with a medical history that includes coronary artery disease. He is status post ST-elevation myocardial infarction and percutaneous coronary intervention with placement of 2 drug-eluting stents. Additional medical issues include PCT and HCV infection with cirrhosis. He is an active smoker.
The patient has a long history of developing blisters with minor trauma, such as rubbing against his mattress/bed sheets or bumping into doors. These blisters primarily occur on his upper extremities, but also can occur on his face after shaving. Mr. M was diagnosed with HCV infection in 1979 while on active military duty. At that time, he had an acute HCV infection and jaundice that required a prolonged hospitalization. He reported no IV drug use and that many others on his military base had similar manifestations. He drinks 1 to 2 beers daily, but reports no binge drinking.
His laboratory studies were notable for ferritin, 2,069 ng/mL; serum iron, 317 mcg/dL; total iron binding capacity, 320 mcg/dL; transferrin, 239 mg/dl; liver function test alanine aminotransferase, 151 U/L; aspartate aminotransferase, 159 U/L; total bilirubin, 1.73 mg/dL; albumin, 3.6 g/dL; alkaline phosphatase, 119 U/L; INR, 1.1; and transferrin saturation, 99%. Mr. M’s HCV viral load was 28,700 IU/L with genotype 1b. Hemochromatosis genetic studies were notable for a heterozygous C282Y gene mutation and negative for H63D and S65C mutations. He repeatedly declined completing a 24-hour urine study of porphyrins. Ultrasonography was consistent with cirrhosis and splenomegaly. The patient was treatment naïve for HCV. He declined multiple offers for treatment of his HCV, citing financial considerations.
Porphyria Cutanea Tarda
The pathogenesis of PCT is related to the intrahepatic deficiency of uroporphyrinogen decarboxylase (UROD), an enzyme in the heme biosynthetic pathway (Figure 1). Decreased activity of UROD leads to accumulation of uroporphyrinogen and its derivatives, which most likely are oxidized in presence of cytochrome P450 1A2. Up to 80% of PCT cases are sporadic, in which the deficiency of UROD is acquired by exogenous risk factors as mentioned above. However, the remaining 20% of PCT cases are due to an autosomal dominant mutation of UROD that causes the partial deficiency (up to 50%) of UROD. In these cases, additional risk factors are needed to decrease UROD activity to < 75% for symptoms to occur.
Clinical Manifestation
Patients with PCT typically develop blisters, skin fragility, and peeling with sun exposure or minor trauma. They also may experience delayed wound healing in sun-exposed skin.3 The photosensitivity of PCT is believed to be related to the saturation of highly carboxylated uroporphyrins in the liver, which are then released into the circulation. Sun exposure then activates these products facilitating an immune reaction and subsequent skin damage.2 In chronic cases, fibrotic reactions and scaring occur which can be mistaken for scleroderma. Other skin manifestations include hyperpigmentation, hypertrichosis, alopecia due to scaring and purplish heliotrope suffusion of periorbital areas.
Patients can develop cirrhosis due to accumulation of porphyria in the hepatocytes and subsequent parenchymal damage. Hepatocellular carcinoma surveillance is recommended for patients with PCT, although its incidence is rare in those patients.
Diagnosis and Treatment
PCT is mainly a clinical diagnosis. Physicians should consider PCT in patients with photosensitivity and blisters after minor trauma (Figure 2). The urine of a patient with PCT is often pink or red when exposed to air or light due to its high concentration of porphyrin products. Mild elevation of liver enzymes and fatty liver on ultrasound are also noted. Evidence of iron overload is seen in most cases. Screening for risk factors like HCV, HIV, hepatitis B virus, and HH is recommended. Confirmation of PCT typically requires measurement of the porphyria level in a 24-hour urine collection.
Avoiding sun exposure is fundamental in decreasing the development of skin lesions and scaring. Additionally, patients should be advised about the adverse effects of alcohol, smoking, and estrogen therapy on PCT. Treatment of PCT is frequently focused on iron overload and subsequent increased porphyrin oxidation.1,2 Iron can increase reactive oxygen species (ROS), which, in turn, increases the rate of oxidation of uroporphyrinogens. Excess iron also decreases the activity of UROD and increases δ-aminolevulinic acid (ALA) production (the precursor of uroporphyrinogen). Phlebotomy to treat iron overload should be done to a target ferritin level of 20 ng/mL. Clinical manifestations, including skin lesions, typically will normalize before the laboratory findings. Therapeutic remission is expected after 6 to 7 phlebotomy attempts, while clinical improvement can occur after 2 to 3 phlebotomies.
In addition to phlebotomy, 4-aminoquinoline medications (chloroquine and hydroxychloroquine) can be used effectively to treat PCT. Hydroxychloroquine is generally preferred due to its better safety profile. Although the exact mechanism of action of 4-aminoquinolines is not clear, it has been suggested that they bind to porphyrins and form water-soluble products, which are then excreted in the urine. Again, clinical remission occurs much sooner than chemical remission, (3 months vs 12 months). A 4-aminoquinoline should not be used in patients with severe liver disease, renal insufficiency, pregnancy, or G6PD deficiency. When used, they should be used in lower than typical doses due to the rapid removal of accumulated porphyrin from the hepatocytes potentially causing necrosis and acute hepatitis.
Iron chelation also is effective, but it is slower in achieving remission and more expensive than phlebotomy. Treatment of PCT should be individualized. For example, 4-aminoquinolines are contraindicated for patients with end-stage renal disease (ESRD), while phlebotomy could present a problem for patients with preexisting anemia. In this instance, removing 50 cc of blood every 2 weeks may be safe and effective. Furthermore, 4-aminoquinolines in patients with severe iron overload and phlebotomy have been used together. Plasmapheresis is still another option in patients with ESRD.
The use of direct antiviral agents (DAA) in the treatment of HCV has shown promising results in maintaining undetectable viral loads and concurrent remission of PCT. Several studies have shown that treatment of HCV with a DAA obviates the need for treatment PCT.3-5 Treatment of HCV with interferon (IFN) and ribavirin have shown mixed results in controlling PCT, possibly due to their ineffectiveness in maintaining a suppressed viral load. Some studies even showed worsening of PCT with IFN/ribavirin.6
Hemochromatosis
Human cells need iron for aerobic respiration. The intestinal mucosa controls iron uptake and its transfer to the blood stream. Aside from variations in intestinal absorption with fecal excretion, humans do not have another pathway to excrete excess iron. HH is the most common genetic disorder in whites.7 It is an autosomal recessive disorder that increases the intestinal absorption of iron. The most common mutation in the hemochromatosis (HFE) gene results in a substitution of tyrosine for cysteine at amino acid number 282 and is referred to as the C282Y mutation. A second mutation changes histidine at position 63 to aspartic acid and is referred to as a H63D mutation. H63D is present in a minority of the patients with phenotypically expressed HH and its clinical impact is unknown.
Homozygosity of the C282Y mutation is the most common genotype associated with clinical hemochromatosis. While carriers of the C282Y gene heterozygote mutation typically do not develop enough iron overload to cause clinical hemochromatosis, they can if other risk factors, such as PCT, excess alcohol use, liver disease, or HCV, are present.8 Additionally, an associated genetic defect, like a compound heterozygotes C282Y/H63D mutation, a private HFE mutation in trans, or other iron-related genes, can cause manifestations of iron overload. Lastly, about 20% of patients that are heterozygous for both mutations can express the HH phenotype.8
Clinical Manifestation
Patients with HH absorb only a few extra milligrams of iron daily. The clinical manifestation begins to occur when the total body iron store reaches 15-40 g (normal, 4 g). While the genetic mutation is present from birth, iron stores start to rise slowly to around 10 g > age 15 years, at which point serum iron levels are elevated. After age 20 years, the speed with which the iron is stored increases, and by 30 years, liver damage and tissue injury will occur. Cirrhosis is possible by 40 years.7 Age, sex, dietary iron intake, blood loss (menstruation), pregnancy, and other unknown factors greatly influence the disease progression. Homozygote C282Y mutation is as common in women as it is in men, but women are less likely to express the HH phenotype, presumably due, in part, to menstruation. When diagnosed early, most of the clinical manifestations of HH are preventable. Additional manifestations of HH include hyperpigmentation, cardiomyopathy, diabetes mellitus, hypogonadism, hypothyroidism, and arthropathy due to pseudogout.
Iron overload due to HH should be distinguished from other causes of iron overload including exogenous iron overload, anemia (thalassemia, sideroblastic), and chronic liver diseases like PCT, viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease.
Diagnosis
HH should be suspected in patients with a high serum transferrin saturation and elevated serum ferritin concentrations. Typically, transferrin saturation is > 50% and ferritin levels are > 300 ng/mL in men and > 200 ng/mL in women. In early stages of the disease, transferrin saturation can be normal. Additionally, in patients with chronic inflammation, ferritin may be high due to acute-phase reactants and the iron panel should be interpreted with caution. When the secondary causes of abnormalities in a patient’s iron studies are excluded, genetic testing for HFE gene is recommended.
The majority of patients (60-93%) with clinically evident hemochromatosis are homozygous for C282Y mutation. In a heterozygous C282Y mutation with a high transferrin saturation and HH phenotype, additional genetic testing for a heterozygous compound mutation C282Y/H63D is recommended.8 Additional studies could include evaluation for a private HFE mutation in trans or other iron-related genes. Liver biopsy is the gold standard for assessing the degree of hepatic fibrosis. Determining the degree of fibrosis by some means is needed due to the increased risk of hepatocellular carcinoma (HCC) in HH patients with advanced fibrosis and cirrhosis.9
Treatment
Iron depletion with phlebotomy is the cornerstone of treatment in HH. Phlebotomy initially is done weekly with goal of achieving a transferrin saturation < 50%, a serum ferritin level < 50 ng/mL, and a hemoglobin of 12 to 13 ng/mL. When these goals are achieved, patients typically need 4 to 8 phlebotomies per year to maintain a transferrin saturation < 50% (Figure 3).
Hemochromatosis and PCT
Many studies have investigated the relevance of C282Y and/or H63D mutations in patients with PCT.9,10 It appears that ≥ 1 mutation of the HFE gene in PCT may be an important susceptibility factor in the development of clinical PCT. Various studies have shown an incidence of C282Y mutations of 44 to 47% in patients with PCT, compared with 9 to 12% in control populations.9,10 The incidence of the H63D mutation in PCT has been more variable, with some studies showing no difference between patients with PCT and a control group, while other studies showed 31% incidence of H63D mutation in patients with PCT.9,10 A higher incidence of C282Y and H63D mutations in PCT may be a sign that the HFE mutation could be an important factor in developing PCT.
Hemochromatosis and Hepatitis C
Transferrin saturation is frequently elevated in patients with HCV. It is yet unclear whether the pathology of liver disease in patients with HCV is influenced by iron overload or limited to the direct cell damage from replication of the virus and subsequent inflammation. It is believed that the pathology of iron overload in the patients with HCV is different from HH. Like other secondary causes of iron overload, the excess iron is stored in the Kupffer cells of patients with HCV. In HH, excess iron is stored in hepatocytes.
The prevalence of the HFE mutation is the same in the patients with chronic HCV and healthy individuals.10,11 However, HFE mutations are more prevalent in 30 to 60% of the patients with chronic HCV who have elevated transferrin saturations. Alone, C282Y heterozygosity, H63D heterozygosity, or C282Y/H63D compound heterozygosity could not lead to clinically significant iron overload in otherwise healthy individuals; however, these could be a significant cause of iron overload in patients with chronic HCV. Theoretically, the combination of iron overload and HFE gene mutations could increase the rate of advanced fibrosis/cirrhosis in chronic HCV. An increase serum ferritin level of 200 ng/dL in women and 250 ng/dL in men has been observed in 32% of patients with chronic HCV. In this subset of patients, phlebotomy reduced the progression of their liver disease and reduction in their liver enzymes.
Iron Overload and Cardiovascular Risk
In 1987, a Framingham cohort of > 2,800 patients showed a higher incident of CAD in postmenopausal women when compared with premenopausal women.12 In the 1980s, Sullivan hypothesized that the reason for higher incidence of CAD in men when compared with premenopausal women was due to their higher body iron storage.13-16 A study of 1,930 Finnish men reported that the men with ferritin level ≥ 200 ng/dL had a risk 2.2 times higher of acute myocardial infarction when compared to men with lower serum ferritin level.17
A prospective study published in 1997 by Klechl showed the role of iron stores in early atherogenesis via promotion of lipid oxidation.18 Other epidemiological studies have shown a decreased risk of myocardial infarction in blood donors, and while arguments have been made that the blood donors tend to be healthier individuals, 2 studies were published in 1997 matching healthy blood donors to healthy nonblood donors, and both showed a lower risk of CVD in the donors when compared with nondonors.19,20 Furthermore, in an animal model of atherosclerosis, an iron depleted diet showed a reduction of atherosclerosis progression.21 Multiple studies have shown that the heterozygosity for HFE is significantly linked to the risk of cardiovascular events, including the fact that heterozygosity for C282Y has been shown to be a risk factor for myocardial infarction in men and cerebrovascular death in women.22-25
Conclusion
Multiple studies have shown an association between the elevated iron levels associated with the HFE genotype and the disease states of our patient. These include an increased risk of CAD, the increased risk of cirrhosis in HCV and the development of PCT. Indeed, in this case, our patient likely acquired PCT from the combined risks of HCV and his heterozygous HFE genetic mutation.
With regard to Mr. M’s treatment, the use of an antiviral agent in the treatment of his HCV is fundamental, along with avoidance of alcohol and smoking. If he were to accept HCV treatment, we would anticipate resolution of the PCT, but the ongoing progression of his liver and cardiovascular conditions, due perhaps in part, to relative iron overload from his heterozygous HFE mutation. In this situation, we expect that an ongoing course of therapeutic phlebotomy could help to delay the progression of his chronic liver and cardiovascular diseases.
1. Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab. 2019;128(3):271-281.
2. Ryan Caballes F, Sendi H, Bonkovsky HL. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
3. Wiznia LE, Laird ME, Franks AG Jr. Hepatitis C virus and its cutaneous manifestations: treatment in the direct-acting antiviral era. J Eur Acad Dermatol Venereol. 2017;31(8):1260-1270.
4. Nihei T, Kiniwa Y, Mikoshiba Y, Joshita S, Okuyama R. Improvement of porphyria cutanea tarda following treatment of hepatitis C virus by direct-acting antivirals: a case report. J Dermatol. 2019;46(5):e149-e151.
5. Combalia A, To-Figueras J, Laguno M, Martínez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184. 6. Singal AK, Venkata KVR, Jampana S, Islam FU, Anderson KE. Hepatitis C treatment in patients with porphyria cutanea tarda. Am J Med Sci. 2017;353 (6):523-528.
7. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65(5):853-860.
8. Aguilar-Martinez P, Grandchamp B, Cunat S, et al. Iron overload in HFE C282Y heterozygotes at first genetic testing: a strategy for identifying rare HFE variants. Haematologica. 2011;96(4):507-514.
9. Erhardt A, Maschner-Olberg A, Mellenthin C, et al. HFE mutations and chronic hepatitis C: H63D and C282Y heterozygosity are independent risk factors for liver fibrosis and cirrhosis. J Hepatol. 2003;38(3):335-342.
10. Mehrany K, Drage LA, Brandhagen DJ, Pittelkow MR. Association of porphyria cutanea tarda with hereditary hemochromatosis. J Am Acad Dermatol. 2004;51(2):205-211.
11. Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124(5):1509-1523.
12. Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157-161.
13. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):1293-1294.
14. Sullivan JL. The sex difference in ischemic heart disease. Perspect Biol Med. 1983;26(4):657-671.
15. Sullivan JL. The iron paradigm of ischemic heart disease. Am Heart J. 1989;117(5):1177-1188.
16. Sullivan JL. Stored iron and ischemic heart disease: empirical support for a new paradigm. Circulation. 1992;86(3):1036-1037.
17. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803-811.
18. Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300-3307.
19. Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793-794.
20. Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78(2):188-193.
21. Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99(9):1222-1229.
22. Surber R, Sigusch HH, Kuehnert H, Figulla HR. Haemochromatosis (HFE) gene C282Y mutation and the risk of coronary artery disease and myocardial infarction: a study in 1279 patients undergoing coronary angiography. J Med Genet. 2003;40(5):e58.
23. Tuomainen TP, Kontula K, Nyyssönen K, Lakka TA, Heliö T, Salonen JT. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation. 1999;100(12):1274-1279.
24. Roest M, van der Schouw YT, de Valk B, et al. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation. 1999;100(12):1268-1273.
25. Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, Gharaati M. The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler. 2014;10(1):32-36.
Sporadic porphyria cutanea tarda (PCT) is the most common cause of porphyria worldwide.1,2 Unlike other forms of porphyria, PCT usually is an acquired disease precipitated by extrinsic risk factors that commonly include excessive alcohol consumption, smoking, and chronic hepatitis C virus (HCV) infection. Additional risk factors include myeloproliferative disorders, exposure to polyhalogenated compounds, estrogen therapy, diseases of iron overload like hereditary hemochromatosis (HH), and potentially, HIV infection.1-3
In this case report, we present a patient with an iron overload (due in part to an HFE gene mutation) and concomitant PCT,
Case Presentation
Mr. M is a 59-year-old white male of Irish background with a medical history that includes coronary artery disease. He is status post ST-elevation myocardial infarction and percutaneous coronary intervention with placement of 2 drug-eluting stents. Additional medical issues include PCT and HCV infection with cirrhosis. He is an active smoker.
The patient has a long history of developing blisters with minor trauma, such as rubbing against his mattress/bed sheets or bumping into doors. These blisters primarily occur on his upper extremities, but also can occur on his face after shaving. Mr. M was diagnosed with HCV infection in 1979 while on active military duty. At that time, he had an acute HCV infection and jaundice that required a prolonged hospitalization. He reported no IV drug use and that many others on his military base had similar manifestations. He drinks 1 to 2 beers daily, but reports no binge drinking.
His laboratory studies were notable for ferritin, 2,069 ng/mL; serum iron, 317 mcg/dL; total iron binding capacity, 320 mcg/dL; transferrin, 239 mg/dl; liver function test alanine aminotransferase, 151 U/L; aspartate aminotransferase, 159 U/L; total bilirubin, 1.73 mg/dL; albumin, 3.6 g/dL; alkaline phosphatase, 119 U/L; INR, 1.1; and transferrin saturation, 99%. Mr. M’s HCV viral load was 28,700 IU/L with genotype 1b. Hemochromatosis genetic studies were notable for a heterozygous C282Y gene mutation and negative for H63D and S65C mutations. He repeatedly declined completing a 24-hour urine study of porphyrins. Ultrasonography was consistent with cirrhosis and splenomegaly. The patient was treatment naïve for HCV. He declined multiple offers for treatment of his HCV, citing financial considerations.
Porphyria Cutanea Tarda
The pathogenesis of PCT is related to the intrahepatic deficiency of uroporphyrinogen decarboxylase (UROD), an enzyme in the heme biosynthetic pathway (Figure 1). Decreased activity of UROD leads to accumulation of uroporphyrinogen and its derivatives, which most likely are oxidized in presence of cytochrome P450 1A2. Up to 80% of PCT cases are sporadic, in which the deficiency of UROD is acquired by exogenous risk factors as mentioned above. However, the remaining 20% of PCT cases are due to an autosomal dominant mutation of UROD that causes the partial deficiency (up to 50%) of UROD. In these cases, additional risk factors are needed to decrease UROD activity to < 75% for symptoms to occur.
Clinical Manifestation
Patients with PCT typically develop blisters, skin fragility, and peeling with sun exposure or minor trauma. They also may experience delayed wound healing in sun-exposed skin.3 The photosensitivity of PCT is believed to be related to the saturation of highly carboxylated uroporphyrins in the liver, which are then released into the circulation. Sun exposure then activates these products facilitating an immune reaction and subsequent skin damage.2 In chronic cases, fibrotic reactions and scaring occur which can be mistaken for scleroderma. Other skin manifestations include hyperpigmentation, hypertrichosis, alopecia due to scaring and purplish heliotrope suffusion of periorbital areas.
Patients can develop cirrhosis due to accumulation of porphyria in the hepatocytes and subsequent parenchymal damage. Hepatocellular carcinoma surveillance is recommended for patients with PCT, although its incidence is rare in those patients.
Diagnosis and Treatment
PCT is mainly a clinical diagnosis. Physicians should consider PCT in patients with photosensitivity and blisters after minor trauma (Figure 2). The urine of a patient with PCT is often pink or red when exposed to air or light due to its high concentration of porphyrin products. Mild elevation of liver enzymes and fatty liver on ultrasound are also noted. Evidence of iron overload is seen in most cases. Screening for risk factors like HCV, HIV, hepatitis B virus, and HH is recommended. Confirmation of PCT typically requires measurement of the porphyria level in a 24-hour urine collection.
Avoiding sun exposure is fundamental in decreasing the development of skin lesions and scaring. Additionally, patients should be advised about the adverse effects of alcohol, smoking, and estrogen therapy on PCT. Treatment of PCT is frequently focused on iron overload and subsequent increased porphyrin oxidation.1,2 Iron can increase reactive oxygen species (ROS), which, in turn, increases the rate of oxidation of uroporphyrinogens. Excess iron also decreases the activity of UROD and increases δ-aminolevulinic acid (ALA) production (the precursor of uroporphyrinogen). Phlebotomy to treat iron overload should be done to a target ferritin level of 20 ng/mL. Clinical manifestations, including skin lesions, typically will normalize before the laboratory findings. Therapeutic remission is expected after 6 to 7 phlebotomy attempts, while clinical improvement can occur after 2 to 3 phlebotomies.
In addition to phlebotomy, 4-aminoquinoline medications (chloroquine and hydroxychloroquine) can be used effectively to treat PCT. Hydroxychloroquine is generally preferred due to its better safety profile. Although the exact mechanism of action of 4-aminoquinolines is not clear, it has been suggested that they bind to porphyrins and form water-soluble products, which are then excreted in the urine. Again, clinical remission occurs much sooner than chemical remission, (3 months vs 12 months). A 4-aminoquinoline should not be used in patients with severe liver disease, renal insufficiency, pregnancy, or G6PD deficiency. When used, they should be used in lower than typical doses due to the rapid removal of accumulated porphyrin from the hepatocytes potentially causing necrosis and acute hepatitis.
Iron chelation also is effective, but it is slower in achieving remission and more expensive than phlebotomy. Treatment of PCT should be individualized. For example, 4-aminoquinolines are contraindicated for patients with end-stage renal disease (ESRD), while phlebotomy could present a problem for patients with preexisting anemia. In this instance, removing 50 cc of blood every 2 weeks may be safe and effective. Furthermore, 4-aminoquinolines in patients with severe iron overload and phlebotomy have been used together. Plasmapheresis is still another option in patients with ESRD.
The use of direct antiviral agents (DAA) in the treatment of HCV has shown promising results in maintaining undetectable viral loads and concurrent remission of PCT. Several studies have shown that treatment of HCV with a DAA obviates the need for treatment PCT.3-5 Treatment of HCV with interferon (IFN) and ribavirin have shown mixed results in controlling PCT, possibly due to their ineffectiveness in maintaining a suppressed viral load. Some studies even showed worsening of PCT with IFN/ribavirin.6
Hemochromatosis
Human cells need iron for aerobic respiration. The intestinal mucosa controls iron uptake and its transfer to the blood stream. Aside from variations in intestinal absorption with fecal excretion, humans do not have another pathway to excrete excess iron. HH is the most common genetic disorder in whites.7 It is an autosomal recessive disorder that increases the intestinal absorption of iron. The most common mutation in the hemochromatosis (HFE) gene results in a substitution of tyrosine for cysteine at amino acid number 282 and is referred to as the C282Y mutation. A second mutation changes histidine at position 63 to aspartic acid and is referred to as a H63D mutation. H63D is present in a minority of the patients with phenotypically expressed HH and its clinical impact is unknown.
Homozygosity of the C282Y mutation is the most common genotype associated with clinical hemochromatosis. While carriers of the C282Y gene heterozygote mutation typically do not develop enough iron overload to cause clinical hemochromatosis, they can if other risk factors, such as PCT, excess alcohol use, liver disease, or HCV, are present.8 Additionally, an associated genetic defect, like a compound heterozygotes C282Y/H63D mutation, a private HFE mutation in trans, or other iron-related genes, can cause manifestations of iron overload. Lastly, about 20% of patients that are heterozygous for both mutations can express the HH phenotype.8
Clinical Manifestation
Patients with HH absorb only a few extra milligrams of iron daily. The clinical manifestation begins to occur when the total body iron store reaches 15-40 g (normal, 4 g). While the genetic mutation is present from birth, iron stores start to rise slowly to around 10 g > age 15 years, at which point serum iron levels are elevated. After age 20 years, the speed with which the iron is stored increases, and by 30 years, liver damage and tissue injury will occur. Cirrhosis is possible by 40 years.7 Age, sex, dietary iron intake, blood loss (menstruation), pregnancy, and other unknown factors greatly influence the disease progression. Homozygote C282Y mutation is as common in women as it is in men, but women are less likely to express the HH phenotype, presumably due, in part, to menstruation. When diagnosed early, most of the clinical manifestations of HH are preventable. Additional manifestations of HH include hyperpigmentation, cardiomyopathy, diabetes mellitus, hypogonadism, hypothyroidism, and arthropathy due to pseudogout.
Iron overload due to HH should be distinguished from other causes of iron overload including exogenous iron overload, anemia (thalassemia, sideroblastic), and chronic liver diseases like PCT, viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease.
Diagnosis
HH should be suspected in patients with a high serum transferrin saturation and elevated serum ferritin concentrations. Typically, transferrin saturation is > 50% and ferritin levels are > 300 ng/mL in men and > 200 ng/mL in women. In early stages of the disease, transferrin saturation can be normal. Additionally, in patients with chronic inflammation, ferritin may be high due to acute-phase reactants and the iron panel should be interpreted with caution. When the secondary causes of abnormalities in a patient’s iron studies are excluded, genetic testing for HFE gene is recommended.
The majority of patients (60-93%) with clinically evident hemochromatosis are homozygous for C282Y mutation. In a heterozygous C282Y mutation with a high transferrin saturation and HH phenotype, additional genetic testing for a heterozygous compound mutation C282Y/H63D is recommended.8 Additional studies could include evaluation for a private HFE mutation in trans or other iron-related genes. Liver biopsy is the gold standard for assessing the degree of hepatic fibrosis. Determining the degree of fibrosis by some means is needed due to the increased risk of hepatocellular carcinoma (HCC) in HH patients with advanced fibrosis and cirrhosis.9
Treatment
Iron depletion with phlebotomy is the cornerstone of treatment in HH. Phlebotomy initially is done weekly with goal of achieving a transferrin saturation < 50%, a serum ferritin level < 50 ng/mL, and a hemoglobin of 12 to 13 ng/mL. When these goals are achieved, patients typically need 4 to 8 phlebotomies per year to maintain a transferrin saturation < 50% (Figure 3).
Hemochromatosis and PCT
Many studies have investigated the relevance of C282Y and/or H63D mutations in patients with PCT.9,10 It appears that ≥ 1 mutation of the HFE gene in PCT may be an important susceptibility factor in the development of clinical PCT. Various studies have shown an incidence of C282Y mutations of 44 to 47% in patients with PCT, compared with 9 to 12% in control populations.9,10 The incidence of the H63D mutation in PCT has been more variable, with some studies showing no difference between patients with PCT and a control group, while other studies showed 31% incidence of H63D mutation in patients with PCT.9,10 A higher incidence of C282Y and H63D mutations in PCT may be a sign that the HFE mutation could be an important factor in developing PCT.
Hemochromatosis and Hepatitis C
Transferrin saturation is frequently elevated in patients with HCV. It is yet unclear whether the pathology of liver disease in patients with HCV is influenced by iron overload or limited to the direct cell damage from replication of the virus and subsequent inflammation. It is believed that the pathology of iron overload in the patients with HCV is different from HH. Like other secondary causes of iron overload, the excess iron is stored in the Kupffer cells of patients with HCV. In HH, excess iron is stored in hepatocytes.
The prevalence of the HFE mutation is the same in the patients with chronic HCV and healthy individuals.10,11 However, HFE mutations are more prevalent in 30 to 60% of the patients with chronic HCV who have elevated transferrin saturations. Alone, C282Y heterozygosity, H63D heterozygosity, or C282Y/H63D compound heterozygosity could not lead to clinically significant iron overload in otherwise healthy individuals; however, these could be a significant cause of iron overload in patients with chronic HCV. Theoretically, the combination of iron overload and HFE gene mutations could increase the rate of advanced fibrosis/cirrhosis in chronic HCV. An increase serum ferritin level of 200 ng/dL in women and 250 ng/dL in men has been observed in 32% of patients with chronic HCV. In this subset of patients, phlebotomy reduced the progression of their liver disease and reduction in their liver enzymes.
Iron Overload and Cardiovascular Risk
In 1987, a Framingham cohort of > 2,800 patients showed a higher incident of CAD in postmenopausal women when compared with premenopausal women.12 In the 1980s, Sullivan hypothesized that the reason for higher incidence of CAD in men when compared with premenopausal women was due to their higher body iron storage.13-16 A study of 1,930 Finnish men reported that the men with ferritin level ≥ 200 ng/dL had a risk 2.2 times higher of acute myocardial infarction when compared to men with lower serum ferritin level.17
A prospective study published in 1997 by Klechl showed the role of iron stores in early atherogenesis via promotion of lipid oxidation.18 Other epidemiological studies have shown a decreased risk of myocardial infarction in blood donors, and while arguments have been made that the blood donors tend to be healthier individuals, 2 studies were published in 1997 matching healthy blood donors to healthy nonblood donors, and both showed a lower risk of CVD in the donors when compared with nondonors.19,20 Furthermore, in an animal model of atherosclerosis, an iron depleted diet showed a reduction of atherosclerosis progression.21 Multiple studies have shown that the heterozygosity for HFE is significantly linked to the risk of cardiovascular events, including the fact that heterozygosity for C282Y has been shown to be a risk factor for myocardial infarction in men and cerebrovascular death in women.22-25
Conclusion
Multiple studies have shown an association between the elevated iron levels associated with the HFE genotype and the disease states of our patient. These include an increased risk of CAD, the increased risk of cirrhosis in HCV and the development of PCT. Indeed, in this case, our patient likely acquired PCT from the combined risks of HCV and his heterozygous HFE genetic mutation.
With regard to Mr. M’s treatment, the use of an antiviral agent in the treatment of his HCV is fundamental, along with avoidance of alcohol and smoking. If he were to accept HCV treatment, we would anticipate resolution of the PCT, but the ongoing progression of his liver and cardiovascular conditions, due perhaps in part, to relative iron overload from his heterozygous HFE mutation. In this situation, we expect that an ongoing course of therapeutic phlebotomy could help to delay the progression of his chronic liver and cardiovascular diseases.
Sporadic porphyria cutanea tarda (PCT) is the most common cause of porphyria worldwide.1,2 Unlike other forms of porphyria, PCT usually is an acquired disease precipitated by extrinsic risk factors that commonly include excessive alcohol consumption, smoking, and chronic hepatitis C virus (HCV) infection. Additional risk factors include myeloproliferative disorders, exposure to polyhalogenated compounds, estrogen therapy, diseases of iron overload like hereditary hemochromatosis (HH), and potentially, HIV infection.1-3
In this case report, we present a patient with an iron overload (due in part to an HFE gene mutation) and concomitant PCT,
Case Presentation
Mr. M is a 59-year-old white male of Irish background with a medical history that includes coronary artery disease. He is status post ST-elevation myocardial infarction and percutaneous coronary intervention with placement of 2 drug-eluting stents. Additional medical issues include PCT and HCV infection with cirrhosis. He is an active smoker.
The patient has a long history of developing blisters with minor trauma, such as rubbing against his mattress/bed sheets or bumping into doors. These blisters primarily occur on his upper extremities, but also can occur on his face after shaving. Mr. M was diagnosed with HCV infection in 1979 while on active military duty. At that time, he had an acute HCV infection and jaundice that required a prolonged hospitalization. He reported no IV drug use and that many others on his military base had similar manifestations. He drinks 1 to 2 beers daily, but reports no binge drinking.
His laboratory studies were notable for ferritin, 2,069 ng/mL; serum iron, 317 mcg/dL; total iron binding capacity, 320 mcg/dL; transferrin, 239 mg/dl; liver function test alanine aminotransferase, 151 U/L; aspartate aminotransferase, 159 U/L; total bilirubin, 1.73 mg/dL; albumin, 3.6 g/dL; alkaline phosphatase, 119 U/L; INR, 1.1; and transferrin saturation, 99%. Mr. M’s HCV viral load was 28,700 IU/L with genotype 1b. Hemochromatosis genetic studies were notable for a heterozygous C282Y gene mutation and negative for H63D and S65C mutations. He repeatedly declined completing a 24-hour urine study of porphyrins. Ultrasonography was consistent with cirrhosis and splenomegaly. The patient was treatment naïve for HCV. He declined multiple offers for treatment of his HCV, citing financial considerations.
Porphyria Cutanea Tarda
The pathogenesis of PCT is related to the intrahepatic deficiency of uroporphyrinogen decarboxylase (UROD), an enzyme in the heme biosynthetic pathway (Figure 1). Decreased activity of UROD leads to accumulation of uroporphyrinogen and its derivatives, which most likely are oxidized in presence of cytochrome P450 1A2. Up to 80% of PCT cases are sporadic, in which the deficiency of UROD is acquired by exogenous risk factors as mentioned above. However, the remaining 20% of PCT cases are due to an autosomal dominant mutation of UROD that causes the partial deficiency (up to 50%) of UROD. In these cases, additional risk factors are needed to decrease UROD activity to < 75% for symptoms to occur.
Clinical Manifestation
Patients with PCT typically develop blisters, skin fragility, and peeling with sun exposure or minor trauma. They also may experience delayed wound healing in sun-exposed skin.3 The photosensitivity of PCT is believed to be related to the saturation of highly carboxylated uroporphyrins in the liver, which are then released into the circulation. Sun exposure then activates these products facilitating an immune reaction and subsequent skin damage.2 In chronic cases, fibrotic reactions and scaring occur which can be mistaken for scleroderma. Other skin manifestations include hyperpigmentation, hypertrichosis, alopecia due to scaring and purplish heliotrope suffusion of periorbital areas.
Patients can develop cirrhosis due to accumulation of porphyria in the hepatocytes and subsequent parenchymal damage. Hepatocellular carcinoma surveillance is recommended for patients with PCT, although its incidence is rare in those patients.
Diagnosis and Treatment
PCT is mainly a clinical diagnosis. Physicians should consider PCT in patients with photosensitivity and blisters after minor trauma (Figure 2). The urine of a patient with PCT is often pink or red when exposed to air or light due to its high concentration of porphyrin products. Mild elevation of liver enzymes and fatty liver on ultrasound are also noted. Evidence of iron overload is seen in most cases. Screening for risk factors like HCV, HIV, hepatitis B virus, and HH is recommended. Confirmation of PCT typically requires measurement of the porphyria level in a 24-hour urine collection.
Avoiding sun exposure is fundamental in decreasing the development of skin lesions and scaring. Additionally, patients should be advised about the adverse effects of alcohol, smoking, and estrogen therapy on PCT. Treatment of PCT is frequently focused on iron overload and subsequent increased porphyrin oxidation.1,2 Iron can increase reactive oxygen species (ROS), which, in turn, increases the rate of oxidation of uroporphyrinogens. Excess iron also decreases the activity of UROD and increases δ-aminolevulinic acid (ALA) production (the precursor of uroporphyrinogen). Phlebotomy to treat iron overload should be done to a target ferritin level of 20 ng/mL. Clinical manifestations, including skin lesions, typically will normalize before the laboratory findings. Therapeutic remission is expected after 6 to 7 phlebotomy attempts, while clinical improvement can occur after 2 to 3 phlebotomies.
In addition to phlebotomy, 4-aminoquinoline medications (chloroquine and hydroxychloroquine) can be used effectively to treat PCT. Hydroxychloroquine is generally preferred due to its better safety profile. Although the exact mechanism of action of 4-aminoquinolines is not clear, it has been suggested that they bind to porphyrins and form water-soluble products, which are then excreted in the urine. Again, clinical remission occurs much sooner than chemical remission, (3 months vs 12 months). A 4-aminoquinoline should not be used in patients with severe liver disease, renal insufficiency, pregnancy, or G6PD deficiency. When used, they should be used in lower than typical doses due to the rapid removal of accumulated porphyrin from the hepatocytes potentially causing necrosis and acute hepatitis.
Iron chelation also is effective, but it is slower in achieving remission and more expensive than phlebotomy. Treatment of PCT should be individualized. For example, 4-aminoquinolines are contraindicated for patients with end-stage renal disease (ESRD), while phlebotomy could present a problem for patients with preexisting anemia. In this instance, removing 50 cc of blood every 2 weeks may be safe and effective. Furthermore, 4-aminoquinolines in patients with severe iron overload and phlebotomy have been used together. Plasmapheresis is still another option in patients with ESRD.
The use of direct antiviral agents (DAA) in the treatment of HCV has shown promising results in maintaining undetectable viral loads and concurrent remission of PCT. Several studies have shown that treatment of HCV with a DAA obviates the need for treatment PCT.3-5 Treatment of HCV with interferon (IFN) and ribavirin have shown mixed results in controlling PCT, possibly due to their ineffectiveness in maintaining a suppressed viral load. Some studies even showed worsening of PCT with IFN/ribavirin.6
Hemochromatosis
Human cells need iron for aerobic respiration. The intestinal mucosa controls iron uptake and its transfer to the blood stream. Aside from variations in intestinal absorption with fecal excretion, humans do not have another pathway to excrete excess iron. HH is the most common genetic disorder in whites.7 It is an autosomal recessive disorder that increases the intestinal absorption of iron. The most common mutation in the hemochromatosis (HFE) gene results in a substitution of tyrosine for cysteine at amino acid number 282 and is referred to as the C282Y mutation. A second mutation changes histidine at position 63 to aspartic acid and is referred to as a H63D mutation. H63D is present in a minority of the patients with phenotypically expressed HH and its clinical impact is unknown.
Homozygosity of the C282Y mutation is the most common genotype associated with clinical hemochromatosis. While carriers of the C282Y gene heterozygote mutation typically do not develop enough iron overload to cause clinical hemochromatosis, they can if other risk factors, such as PCT, excess alcohol use, liver disease, or HCV, are present.8 Additionally, an associated genetic defect, like a compound heterozygotes C282Y/H63D mutation, a private HFE mutation in trans, or other iron-related genes, can cause manifestations of iron overload. Lastly, about 20% of patients that are heterozygous for both mutations can express the HH phenotype.8
Clinical Manifestation
Patients with HH absorb only a few extra milligrams of iron daily. The clinical manifestation begins to occur when the total body iron store reaches 15-40 g (normal, 4 g). While the genetic mutation is present from birth, iron stores start to rise slowly to around 10 g > age 15 years, at which point serum iron levels are elevated. After age 20 years, the speed with which the iron is stored increases, and by 30 years, liver damage and tissue injury will occur. Cirrhosis is possible by 40 years.7 Age, sex, dietary iron intake, blood loss (menstruation), pregnancy, and other unknown factors greatly influence the disease progression. Homozygote C282Y mutation is as common in women as it is in men, but women are less likely to express the HH phenotype, presumably due, in part, to menstruation. When diagnosed early, most of the clinical manifestations of HH are preventable. Additional manifestations of HH include hyperpigmentation, cardiomyopathy, diabetes mellitus, hypogonadism, hypothyroidism, and arthropathy due to pseudogout.
Iron overload due to HH should be distinguished from other causes of iron overload including exogenous iron overload, anemia (thalassemia, sideroblastic), and chronic liver diseases like PCT, viral hepatitis, nonalcoholic steatohepatitis, and alcoholic liver disease.
Diagnosis
HH should be suspected in patients with a high serum transferrin saturation and elevated serum ferritin concentrations. Typically, transferrin saturation is > 50% and ferritin levels are > 300 ng/mL in men and > 200 ng/mL in women. In early stages of the disease, transferrin saturation can be normal. Additionally, in patients with chronic inflammation, ferritin may be high due to acute-phase reactants and the iron panel should be interpreted with caution. When the secondary causes of abnormalities in a patient’s iron studies are excluded, genetic testing for HFE gene is recommended.
The majority of patients (60-93%) with clinically evident hemochromatosis are homozygous for C282Y mutation. In a heterozygous C282Y mutation with a high transferrin saturation and HH phenotype, additional genetic testing for a heterozygous compound mutation C282Y/H63D is recommended.8 Additional studies could include evaluation for a private HFE mutation in trans or other iron-related genes. Liver biopsy is the gold standard for assessing the degree of hepatic fibrosis. Determining the degree of fibrosis by some means is needed due to the increased risk of hepatocellular carcinoma (HCC) in HH patients with advanced fibrosis and cirrhosis.9
Treatment
Iron depletion with phlebotomy is the cornerstone of treatment in HH. Phlebotomy initially is done weekly with goal of achieving a transferrin saturation < 50%, a serum ferritin level < 50 ng/mL, and a hemoglobin of 12 to 13 ng/mL. When these goals are achieved, patients typically need 4 to 8 phlebotomies per year to maintain a transferrin saturation < 50% (Figure 3).
Hemochromatosis and PCT
Many studies have investigated the relevance of C282Y and/or H63D mutations in patients with PCT.9,10 It appears that ≥ 1 mutation of the HFE gene in PCT may be an important susceptibility factor in the development of clinical PCT. Various studies have shown an incidence of C282Y mutations of 44 to 47% in patients with PCT, compared with 9 to 12% in control populations.9,10 The incidence of the H63D mutation in PCT has been more variable, with some studies showing no difference between patients with PCT and a control group, while other studies showed 31% incidence of H63D mutation in patients with PCT.9,10 A higher incidence of C282Y and H63D mutations in PCT may be a sign that the HFE mutation could be an important factor in developing PCT.
Hemochromatosis and Hepatitis C
Transferrin saturation is frequently elevated in patients with HCV. It is yet unclear whether the pathology of liver disease in patients with HCV is influenced by iron overload or limited to the direct cell damage from replication of the virus and subsequent inflammation. It is believed that the pathology of iron overload in the patients with HCV is different from HH. Like other secondary causes of iron overload, the excess iron is stored in the Kupffer cells of patients with HCV. In HH, excess iron is stored in hepatocytes.
The prevalence of the HFE mutation is the same in the patients with chronic HCV and healthy individuals.10,11 However, HFE mutations are more prevalent in 30 to 60% of the patients with chronic HCV who have elevated transferrin saturations. Alone, C282Y heterozygosity, H63D heterozygosity, or C282Y/H63D compound heterozygosity could not lead to clinically significant iron overload in otherwise healthy individuals; however, these could be a significant cause of iron overload in patients with chronic HCV. Theoretically, the combination of iron overload and HFE gene mutations could increase the rate of advanced fibrosis/cirrhosis in chronic HCV. An increase serum ferritin level of 200 ng/dL in women and 250 ng/dL in men has been observed in 32% of patients with chronic HCV. In this subset of patients, phlebotomy reduced the progression of their liver disease and reduction in their liver enzymes.
Iron Overload and Cardiovascular Risk
In 1987, a Framingham cohort of > 2,800 patients showed a higher incident of CAD in postmenopausal women when compared with premenopausal women.12 In the 1980s, Sullivan hypothesized that the reason for higher incidence of CAD in men when compared with premenopausal women was due to their higher body iron storage.13-16 A study of 1,930 Finnish men reported that the men with ferritin level ≥ 200 ng/dL had a risk 2.2 times higher of acute myocardial infarction when compared to men with lower serum ferritin level.17
A prospective study published in 1997 by Klechl showed the role of iron stores in early atherogenesis via promotion of lipid oxidation.18 Other epidemiological studies have shown a decreased risk of myocardial infarction in blood donors, and while arguments have been made that the blood donors tend to be healthier individuals, 2 studies were published in 1997 matching healthy blood donors to healthy nonblood donors, and both showed a lower risk of CVD in the donors when compared with nondonors.19,20 Furthermore, in an animal model of atherosclerosis, an iron depleted diet showed a reduction of atherosclerosis progression.21 Multiple studies have shown that the heterozygosity for HFE is significantly linked to the risk of cardiovascular events, including the fact that heterozygosity for C282Y has been shown to be a risk factor for myocardial infarction in men and cerebrovascular death in women.22-25
Conclusion
Multiple studies have shown an association between the elevated iron levels associated with the HFE genotype and the disease states of our patient. These include an increased risk of CAD, the increased risk of cirrhosis in HCV and the development of PCT. Indeed, in this case, our patient likely acquired PCT from the combined risks of HCV and his heterozygous HFE genetic mutation.
With regard to Mr. M’s treatment, the use of an antiviral agent in the treatment of his HCV is fundamental, along with avoidance of alcohol and smoking. If he were to accept HCV treatment, we would anticipate resolution of the PCT, but the ongoing progression of his liver and cardiovascular conditions, due perhaps in part, to relative iron overload from his heterozygous HFE mutation. In this situation, we expect that an ongoing course of therapeutic phlebotomy could help to delay the progression of his chronic liver and cardiovascular diseases.
1. Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab. 2019;128(3):271-281.
2. Ryan Caballes F, Sendi H, Bonkovsky HL. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
3. Wiznia LE, Laird ME, Franks AG Jr. Hepatitis C virus and its cutaneous manifestations: treatment in the direct-acting antiviral era. J Eur Acad Dermatol Venereol. 2017;31(8):1260-1270.
4. Nihei T, Kiniwa Y, Mikoshiba Y, Joshita S, Okuyama R. Improvement of porphyria cutanea tarda following treatment of hepatitis C virus by direct-acting antivirals: a case report. J Dermatol. 2019;46(5):e149-e151.
5. Combalia A, To-Figueras J, Laguno M, Martínez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184. 6. Singal AK, Venkata KVR, Jampana S, Islam FU, Anderson KE. Hepatitis C treatment in patients with porphyria cutanea tarda. Am J Med Sci. 2017;353 (6):523-528.
7. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65(5):853-860.
8. Aguilar-Martinez P, Grandchamp B, Cunat S, et al. Iron overload in HFE C282Y heterozygotes at first genetic testing: a strategy for identifying rare HFE variants. Haematologica. 2011;96(4):507-514.
9. Erhardt A, Maschner-Olberg A, Mellenthin C, et al. HFE mutations and chronic hepatitis C: H63D and C282Y heterozygosity are independent risk factors for liver fibrosis and cirrhosis. J Hepatol. 2003;38(3):335-342.
10. Mehrany K, Drage LA, Brandhagen DJ, Pittelkow MR. Association of porphyria cutanea tarda with hereditary hemochromatosis. J Am Acad Dermatol. 2004;51(2):205-211.
11. Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124(5):1509-1523.
12. Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157-161.
13. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):1293-1294.
14. Sullivan JL. The sex difference in ischemic heart disease. Perspect Biol Med. 1983;26(4):657-671.
15. Sullivan JL. The iron paradigm of ischemic heart disease. Am Heart J. 1989;117(5):1177-1188.
16. Sullivan JL. Stored iron and ischemic heart disease: empirical support for a new paradigm. Circulation. 1992;86(3):1036-1037.
17. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803-811.
18. Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300-3307.
19. Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793-794.
20. Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78(2):188-193.
21. Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99(9):1222-1229.
22. Surber R, Sigusch HH, Kuehnert H, Figulla HR. Haemochromatosis (HFE) gene C282Y mutation and the risk of coronary artery disease and myocardial infarction: a study in 1279 patients undergoing coronary angiography. J Med Genet. 2003;40(5):e58.
23. Tuomainen TP, Kontula K, Nyyssönen K, Lakka TA, Heliö T, Salonen JT. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation. 1999;100(12):1274-1279.
24. Roest M, van der Schouw YT, de Valk B, et al. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation. 1999;100(12):1268-1273.
25. Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, Gharaati M. The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler. 2014;10(1):32-36.
1. Singal AK. Porphyria cutanea tarda: Recent update. Mol Genet Metab. 2019;128(3):271-281.
2. Ryan Caballes F, Sendi H, Bonkovsky HL. Hepatitis C, porphyria cutanea tarda and liver iron: an update. Liver Int. 2012;32(6):880-893.
3. Wiznia LE, Laird ME, Franks AG Jr. Hepatitis C virus and its cutaneous manifestations: treatment in the direct-acting antiviral era. J Eur Acad Dermatol Venereol. 2017;31(8):1260-1270.
4. Nihei T, Kiniwa Y, Mikoshiba Y, Joshita S, Okuyama R. Improvement of porphyria cutanea tarda following treatment of hepatitis C virus by direct-acting antivirals: a case report. J Dermatol. 2019;46(5):e149-e151.
5. Combalia A, To-Figueras J, Laguno M, Martínez-Rebollar M, Aguilera P. Direct-acting antivirals for hepatitis C virus induce a rapid clinical and biochemical remission of porphyria cutanea tarda. Br J Dermatol. 2017;177(5):e183-e184. 6. Singal AK, Venkata KVR, Jampana S, Islam FU, Anderson KE. Hepatitis C treatment in patients with porphyria cutanea tarda. Am J Med Sci. 2017;353 (6):523-528.
7. Brandhagen DJ, Fairbanks VF, Baldus W. Recognition and management of hereditary hemochromatosis. Am Fam Physician. 2002;65(5):853-860.
8. Aguilar-Martinez P, Grandchamp B, Cunat S, et al. Iron overload in HFE C282Y heterozygotes at first genetic testing: a strategy for identifying rare HFE variants. Haematologica. 2011;96(4):507-514.
9. Erhardt A, Maschner-Olberg A, Mellenthin C, et al. HFE mutations and chronic hepatitis C: H63D and C282Y heterozygosity are independent risk factors for liver fibrosis and cirrhosis. J Hepatol. 2003;38(3):335-342.
10. Mehrany K, Drage LA, Brandhagen DJ, Pittelkow MR. Association of porphyria cutanea tarda with hereditary hemochromatosis. J Am Acad Dermatol. 2004;51(2):205-211.
11. Pietrangelo A. Hemochromatosis gene modifies course of hepatitis C viral infection. Gastroenterology. 2003;124(5):1509-1523.
12. Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157-161.
13. Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1(8233):1293-1294.
14. Sullivan JL. The sex difference in ischemic heart disease. Perspect Biol Med. 1983;26(4):657-671.
15. Sullivan JL. The iron paradigm of ischemic heart disease. Am Heart J. 1989;117(5):1177-1188.
16. Sullivan JL. Stored iron and ischemic heart disease: empirical support for a new paradigm. Circulation. 1992;86(3):1036-1037.
17. Salonen JT, Nyyssönen K, Korpela H, Tuomilehto J, Seppänen R, Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992;86(3):803-811.
18. Kiechl S, Willeit J, Egger G, Poewe W, Oberhollenzer F. Body iron stores and the risk of carotid atherosclerosis: prospective results from the Bruneck study. Circulation. 1997;96(10):3300-3307.
19. Tuomainen TP, Salonen R, Nyyssönen K, Salonen JT. Cohort study of relation between donating blood and risk of myocardial infarction in 2682 men in eastern Finland. BMJ. 1997;314(7083):793-794.
20. Meyers DG, Strickland D, Maloley PA, Seburg JK, Wilson JE, McManus BF. Possible association of a reduction in cardiovascular events with blood donation. Heart. 1997;78(2):188-193.
21. Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99(9):1222-1229.
22. Surber R, Sigusch HH, Kuehnert H, Figulla HR. Haemochromatosis (HFE) gene C282Y mutation and the risk of coronary artery disease and myocardial infarction: a study in 1279 patients undergoing coronary angiography. J Med Genet. 2003;40(5):e58.
23. Tuomainen TP, Kontula K, Nyyssönen K, Lakka TA, Heliö T, Salonen JT. Increased risk of acute myocardial infarction in carriers of the hemochromatosis gene Cys282Tyr mutation: a prospective cohort study in men in eastern Finland. Circulation. 1999;100(12):1274-1279.
24. Roest M, van der Schouw YT, de Valk B, et al. Heterozygosity for a hereditary hemochromatosis gene is associated with cardiovascular death in women. Circulation. 1999;100(12):1268-1273.
25. Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, Gharaati M. The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler. 2014;10(1):32-36.
Lenvatinib/pembrolizumab has good activity in advanced RCC, other solid tumors
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
A combination of the tyrosine kinase inhibitor lenvatinib (Lenvima) and the immune checkpoint inhibitor pembrolizumab (Keytruda) was safe and showed promising activity against advanced renal cell carcinoma and other solid tumors in a phase 1b/2 study.
Overall response rates (ORR) at 24 weeks ranged from 63% for patients with advanced renal cell carcinomas (RCC) to 25% for patients with urothelial cancers, reported Matthew H. Taylor, MD, of Knight Cancer Institute at Oregon Health & Science University in Portland, and colleagues.
The findings from this study sparked additional clinical trials for patients with gastric cancer, gastroesophageal cancer, and differentiated thyroid cancer, and set the stage for larger phase 3 trials in patients with advanced RCC, endometrial cancer, malignant melanoma, and non–small cell lung cancer (NSCLC).
“In the future, we also plan to study lenvatinib plus pembrolizumab in patients with RCC who have had disease progression after treatment with immune checkpoint inhibitors,” they wrote. The report was published in Journal of Clinical Oncology.
Lenvatinib is a multitargeted tyrosine kinase inhibitor (TKI) with action against vascular endothelial growth factor (VEGF) receptors 1-3, fibroblast growth factor (FGF) receptors 1-4, platelet-derived growth factor receptors alpha and the RET and KIT kinases.
“Preclinical and clinical studies suggest that modulation of VEGF-mediated immune suppression via angiogenesis inhibition could potentially augment the immunotherapeutic activity of immune checkpoint inhibitors,” the investigators wrote.
They reported results from the dose finding (1b) phase including 13 patients and initial phase 2 expansion cohorts with a total of 124 patients.
The maximum tolerated dose of lenvatinib in combination with pembrolizumab was established as 20 mg/day.
At 24 weeks of follow-up, the ORR for 30 patients with RCC was 63%; two additional patients had responses after week 24, for a total ORR at study cutoff in this cohort of 70%. The median duration of response for these patients was 20 months, and the median progression-free survival (PFS) was 19.8 months. At the time of data cutoff for this analysis, 9 of the 30 patients with RCC were still on treatment.
For 23 patients with endometrial cancer, the 24-week and overall ORR were 52%, with a median duration of response not reached, and a median PFS of 9.7 months. Seven patients were still on treatment at data cutoff.
For 21 patients with melanoma, the 24-week and overall ORR were 48%, median duration of response was 12.5 months, and median PFS was 5.5 months. Two of the patients were still on treatment at data cutoff.
For the 22 patients with squamous cell cancer of the head and neck, the 24-week ORR was 36%, with two patients having a response after week 24 for a total ORR at data cutoff of 46%. The median duration of response was 8.2 months and the median PFS was 4.7 months. Three patients remained on treatment at data cutoff.
For 21 patients with NSCLC, the 24-week and overall ORR were 33%, the median duration of response was 10.9 months, and median PFS was 5.9 months. Six of the patients were still receiving treatment at data cutoff.
For 20 patients with urothelial cancer, the 24-week and overall ORR were 25%, with a median duration of response not reached, and a median PFS of 5.4 months. Three patients were still receiving the combination at the time of data cutoff.
Treatment related adverse events (TRAEs) occurred in 133 of all 137 patients enrolled in the two study phases. The adverse events were similar across all cohorts, with any grade of events including fatigue in 58%, diarrhea in 52%, hypertension in 47%, hypothyroidism in 42%, and decreased appetite in 39%.
The most frequent grade 3 or 4 TRAEs were hypertension in 20%, fatigue in 12%, diarrhea in 9%, proteinuria in 8%, and increased lipase levels in 7%.
In all, 85% of patients had a TRAE leading to lenvatinib dose reduction and/or interruption, and 13% required lenvatinib discontinuation.
Events leading to pembrolizumab dose interruption occurred in 45% of patients, and pembrolizumab discontinuation in 15%.
The study was sponsored by Eisai with collaboration from Merck Sharp & Dohme. Dr. Taylor disclosed a consulting or advisory role for Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, and Sanofi/Genzyme, and speakers bureau activities for BMS and Eisai.
SOURCE: Taylor MH et al. J Clin Oncol. 2020 Jan. 21 doi: 10.1200/JCO.19.01598.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Get familiar with evidence on these supplements
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 19
New frontier: Transgender men yield eggs, babies, even after testosterone
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
Transgender men who were assigned female sex at birth show a similar response to ovarian stimulation as cisgender women, even after using testosterone, shows the first formal study of its kind in this patient group.
The transgender patients each had an average of 20 eggs retrieved, and all who transferred embryos eventually achieved a successful pregnancy and delivery, “representing the largest cohort of transgender male patients to be described in the literature thus far,” wrote Nina Resetkova, MD, and colleagues in their article, published in Fertility and Sterility.
The research has been hailed as groundbreaking.
Dr. Resetkova, a reproductive endocrinologist at Boston IVF, Beth Israel Deaconess Medical Center, said in an interview that “these new data show it is reasonable for transmen [female-to-male transition], even those who have used testosterone for some time, to undergo assisted reproductive technology [ART].
“We’ve found that there isn’t a decrease in oocyte retrieval and may actually be a slight increase. We found this to be remarkable,” she said, emphasizing that these findings should be very reassuring for transgender male patients concerned about fertility.
“Transmales worry that they’ve thrown in the towel, and by committing to testosterone have started on a pathway with no return, but these data suggest they still have options,” Dr. Resetkova explained.
“Our study shows that these patients can have ovarian stimulation outcomes that are similar to those of cisgender counterparts, and this seems to be true even in cases of patients who have already initiated hormonal transition with the use of testosterone,” she said.
The researcher hopes the results will encourage more referrals for transgender men wishing to explore their fertility options. “Previously, many doctors were reluctant to refer to a fertility practice if their transmale patient had already started testosterone therapy or they had been on it for several years,” she said.
In a comment, Joshua Safer, MD, a spokesperson on transgender issues for the Endocrine Society, said that “fertility compromise may represent the single largest risk of medical treatment for some transgender persons. At meetings and in personal communications, several clinical groups have reported successful egg harvest from transgender men.
“However, this is the first careful study of a defined cohort published formally. As such, it serves as an important reference in advancing transgender medical care,” noted Dr. Safer, executive director of the Mount Sinai Center for Transgender Medicine and Surgery, New York.
Need for guidance
Previously, there has been an assumption that transgender individuals were not interested in maintaining their reproductive potential, but this has proven untrue. “Several recent studies have demonstrated that transgender people do desire parenthood, or at the least wish to preserve that possibility,” noted Dr. Resetkova and colleagues.
Both the American Society for Reproductive Medicine and the European Society of Human Reproduction and Embryology have issued opinions that transgender patients should have the same access to fertility options as cisgender patients and that fertility preservation options should be discussed before gender transition, they noted.
The first and key intervention needed is the ability to preserve fertility through the cryopreservation of gametes before medical or surgical transition. In transgender men, this can be done via oocyte, embryo, or ovarian tissue cryopreservation.
Dr. Resetkova and the team at Boston IVF realized there was no published evidence, bar a couple of case reports, to guide clinicians caring for transgender men who wanted to preserve their fertility.
To help fill the research gap, they drew data from a retrospective cohort using electronic medical records from a single large in vitro fertilization (IVF) clinic. The search was conducted from January 2010 to July 2018, because the first transgender man was treated at the clinic in 2010.
To be included in the study, the patient had to identify as a transgender man and have completed an ovarian stimulation cycle for oocyte cryopreservation, embryo cryopreservation, or intended uterine transfer.
“This is the first study to describe transgender cycle parameters and outcomes in such detail and scope,” the authors noted in their article.
The study aimed to investigate ART outcomes in a female-to-male transgender cohort (n = 26) who wished to preserve fertility through egg freezing and/or undergo IVF with the intention of pregnancy.
Each transgender man was matched with five cisgender women for age, body mass index, and anti-Müllerian hormone level, and egg yield was compared. The 130 cisgender women were in straight relationships where there was difficulty conceiving, mostly because of male-factor, or tubal-factor, infertility; cisgender women with ovulatory dysfunction were excluded.
Egg harvest and T
The transgender patients were aged 14-39 years, with an average age at cycle start of 28 years. Some patients had not yet undergone any form of medical transition but planned to do so after ART.
The majority (61%) had received testosterone hormonal therapy, and a small number had undergone surgery, for example mastectomy and reconstruction, but none had undergone a hysterectomy or ovary removal (so-called “bottom” or gender-reassignment surgery).
Prior to ART, all patients taking testosterone came off the hormone on average 4 months prior to starting treatment. The mean time on testosterone before seeking ART treatment was 3.7 years and ranged from 3 months to 17 years.
“All patients had intact uterus and ovaries, and all patients had gone through puberty and had not received puberty blocking. This was required for ovarian stimulation and egg freezing,” Dr. Resetkova explained in an interview.
Researchers tracked patient records for outcomes, including oocyte yield, number of mature oocytes, total gonadotropin dose, and peak estradiol levels.
A mean of 19.9 +/– 8.7 oocytes were retrieved per cycle in the transgender cohort, compared with 15.9 +/– 9.6 in the cisgender female group; peak estradiol levels were similar between the two groups. However, the total dose of gonadotropins used was higher in the transgender group compared with the cisgender group (3,892 IU vs. 2,599 IU).
Of the 26 transgender men, 16 had egg preservation (oocyte banking) only. Seven couples had fresh or frozen embryo transfers, with all achieving live births.
Among the patients who planned for IVF with embryo transfer, two intended to carry the pregnancy themselves and the remaining five transferred embryos to their cisgender female partner.
The authors noted that many of the transgender patients who ultimately did not choose to proceed with treatment did so because of the need to stop testosterone therapy before initiating a cycle or the burden of cost.
“For many transgender patients, stopping androgen therapy can be both physically and psychologically distressing, especially because many experience the resumption of menses,” they observed.
“A logical follow-up question is whether ovarian stimulation can be done with any measure of success without the cessation of testosterone,” they noted.
“Although our findings are certainly reassuring for patients who have already initiated androgens, they were still all required to stop therapy to proceed with stimulation. This is a barrier to access that should be investigated, and if overcome may increase utilization of ART by transgender male patients,” they wrote.
Dr. Safer said that, to his knowledge, “a couple of fertility groups ... have been clear that the egg harvest could take place while the transgender men were using testosterone.”
Higher yield
The results with regard to the use of testosterone prior to ART were particularly enlightening, said Dr. Resetkova, who noted that testosterone therapy did not seem to affect ovarian stimulation.
“Before this study, we did not know if long-term testosterone use had a negative impact on egg reserve but, remarkably, testosterone does not appear to have an effect on the ovarian reserve as measured by egg count,” she noted, although she acknowledged that the “study is small.”
“In fact, in some ways, it looks like testosterone might even have a beneficial effect on egg count with a trend towards a higher number of eggs in the transmales who used it,” she added.
But this is “speculative,” she acknowledged, given the low numbers.
Reflecting on why long-term testosterone use may have shown a trend towards greater egg retrieval, Dr. Resetkova explained that the environment might be more similar to an individual with underlying elevated testosterone as seen in polycystic ovarian syndrome, and she noted these patients typically have a higher egg yield during IVF therapy.
Commenting on the higher doses of gonadotropins used in transgender patients, Dr. Resetkova suggested there could be various reasons for this, given that dosing was at the physician’s discretion, including the possibility that they knew the patient only had one chance and therefore higher doses of gonadotropins may have been administered.
Furthermore, each round of treatment is expensive. The researcher stressed, however, there was no conclusion in this respect based on their data.
Puberty blocking and fertility
When asked whether a transgender man who had undergone puberty blocking before transitioning (i.e., someone who had not gone through natural puberty) would be able to follow a similar course to pregnancy as the study participants, Dr. Resetkova acknowledged that is a more challenging area.
“We have little data so it’s hard to be conclusive, but it’s unlikely these patients would have mature hormonal responses and the ovaries might be in a naive state,” she hypothesized. “I don’t know that they would retain so many options as someone who had gone through natural puberty.”
“However, there are research protocols in place at some academic institutions for transgender patients planning to undergo puberty blocking,” she observed.
Finally, referring to individuals who transition from male to female using estrogen therapy, Dr. Resetkova said that the quality of sperm production might be impaired with long-term estrogen exposure. She added that other centers are looking at this.
“As transgender individuals increasingly seek access to reproductive services, we seek to shed light on the optimal way to provide effective care to these patients,” Dr. Dr. Resetkova and colleagues conclude.
Dr. Resetkova has reported no relevant financial relationships.
This article first appeared on Medscape.com.
SOURCE: Resetkova N et al. Fertil Steril. 2019;112:858-65.
Periorbital Swelling and Rash Following Trauma
The Diagnosis: Herpes Zoster Opthalmicus
Due to the potential concern of vision loss, the patient was directed to a local emergency department for immediate ophthalmologic evaluation. He was diagnosed with herpes zoster ophthalmicus (HZO) and treated with oral acyclovir and prednisone. The rash and periorbital swelling resolved within 2 weeks of treatment, and he remained asymptomatic at follow-up 3 months later.
Herpes zoster ophthalmicus presents with an erythematous and vesicular rash in the distribution of cranial nerve V1. The herpetiform grouping of lesions on the forehead is diagnostic of HZO. Varicella-zoster virus (VZV) infection presents in 2 distinct forms. Primary infection (commonly known as chickenpox) presents clinically as a vesicular rash usually located on the face, arms, and trunk. Although the initial presentation usually occurs in childhood and is self-limited, the virus becomes latent in the dorsal root ganglia of sensory neurons. Varicella-zoster virus may become reactivated later in life and is termed herpes zoster (commonly known as shingles). It most often presents as a painful vesicular rash that may later form pustules.
Zoster outbreaks typically do not cross the midline but may in disseminated disease. Patients may experience a prodrome in the form of pain or less commonly pruritus or paresthesia along the dermatome between 1 and 10 days before the rash appears. Triggers for herpes zoster include illness, medications, malnutrition, surgery, or the natural decline in immune function due to aging. Trauma is another important precipitating event for VZV reactivation; one case-control study showed that zoster patients were 3.4 times more likely than controls to have had trauma the week prior.1 Patients with cranial zoster are more than 25 times more likely to have experienced trauma in the preceding week. Local trauma may predispose these patients to VZV reactivation by stimulating local sensory nerves or by disrupting local cutaneous immunity.2
Herpes zoster ophthalmicus occurs when zoster presents in the ophthalmic division of the fifth cranial nerve. It is a serious, vision-threatening condition with a presentation that can include conjunctivitis, scleritis, keratitis, optic neuritis, exophthalmos, lid retraction, ptosis, and extraocular muscle palsies. Treatment includes antiviral medication (eg, acyclovir, valacyclovir, famciclovir) and prompt ophthalmologic consultation due to potential vision-threatening complications, such as acute retinal necrosis. Acute pain control may be necessary with nonsteroidal anti-inflammatory drugs, opioids, steroids, tricyclic antidepressants, or anticonvulsants.3 Wet-to-dry dressings with sterile saline or Burow solution and/or calamine lotion can provide symptomatic relief of itching.
Periorbital and preseptal cellulitis typically present with more erythema of the skin surrounding the eye and without the accompanying rash. Periorbital cellulitis is the more serious infection and may be clinically distinguished by the presence of pain with extraocular muscle movement. Contact dermatitis and pemphigus vulgaris are possibilities, but both were less likely than HZO in this case presentation given the distribution of the rash and the patient history. Contact dermatitis typically presents with no prodrome with a main concern of pruritus. Pemphigus vulgaris nearly always includes involvement of the oral mucous membranes.
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667-672.
- Zhang JX, Joesoef RM, Bialek S, et al. Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis. 2013;207:1007-1011.
- Rousseau A, Bourcier T, Colin J, et al. Herpes zoster ophthalmicus--diagnosis and management. US Ophthalm Rev. 2013;6:119-124.
The Diagnosis: Herpes Zoster Opthalmicus
Due to the potential concern of vision loss, the patient was directed to a local emergency department for immediate ophthalmologic evaluation. He was diagnosed with herpes zoster ophthalmicus (HZO) and treated with oral acyclovir and prednisone. The rash and periorbital swelling resolved within 2 weeks of treatment, and he remained asymptomatic at follow-up 3 months later.
Herpes zoster ophthalmicus presents with an erythematous and vesicular rash in the distribution of cranial nerve V1. The herpetiform grouping of lesions on the forehead is diagnostic of HZO. Varicella-zoster virus (VZV) infection presents in 2 distinct forms. Primary infection (commonly known as chickenpox) presents clinically as a vesicular rash usually located on the face, arms, and trunk. Although the initial presentation usually occurs in childhood and is self-limited, the virus becomes latent in the dorsal root ganglia of sensory neurons. Varicella-zoster virus may become reactivated later in life and is termed herpes zoster (commonly known as shingles). It most often presents as a painful vesicular rash that may later form pustules.
Zoster outbreaks typically do not cross the midline but may in disseminated disease. Patients may experience a prodrome in the form of pain or less commonly pruritus or paresthesia along the dermatome between 1 and 10 days before the rash appears. Triggers for herpes zoster include illness, medications, malnutrition, surgery, or the natural decline in immune function due to aging. Trauma is another important precipitating event for VZV reactivation; one case-control study showed that zoster patients were 3.4 times more likely than controls to have had trauma the week prior.1 Patients with cranial zoster are more than 25 times more likely to have experienced trauma in the preceding week. Local trauma may predispose these patients to VZV reactivation by stimulating local sensory nerves or by disrupting local cutaneous immunity.2
Herpes zoster ophthalmicus occurs when zoster presents in the ophthalmic division of the fifth cranial nerve. It is a serious, vision-threatening condition with a presentation that can include conjunctivitis, scleritis, keratitis, optic neuritis, exophthalmos, lid retraction, ptosis, and extraocular muscle palsies. Treatment includes antiviral medication (eg, acyclovir, valacyclovir, famciclovir) and prompt ophthalmologic consultation due to potential vision-threatening complications, such as acute retinal necrosis. Acute pain control may be necessary with nonsteroidal anti-inflammatory drugs, opioids, steroids, tricyclic antidepressants, or anticonvulsants.3 Wet-to-dry dressings with sterile saline or Burow solution and/or calamine lotion can provide symptomatic relief of itching.
Periorbital and preseptal cellulitis typically present with more erythema of the skin surrounding the eye and without the accompanying rash. Periorbital cellulitis is the more serious infection and may be clinically distinguished by the presence of pain with extraocular muscle movement. Contact dermatitis and pemphigus vulgaris are possibilities, but both were less likely than HZO in this case presentation given the distribution of the rash and the patient history. Contact dermatitis typically presents with no prodrome with a main concern of pruritus. Pemphigus vulgaris nearly always includes involvement of the oral mucous membranes.
The Diagnosis: Herpes Zoster Opthalmicus
Due to the potential concern of vision loss, the patient was directed to a local emergency department for immediate ophthalmologic evaluation. He was diagnosed with herpes zoster ophthalmicus (HZO) and treated with oral acyclovir and prednisone. The rash and periorbital swelling resolved within 2 weeks of treatment, and he remained asymptomatic at follow-up 3 months later.
Herpes zoster ophthalmicus presents with an erythematous and vesicular rash in the distribution of cranial nerve V1. The herpetiform grouping of lesions on the forehead is diagnostic of HZO. Varicella-zoster virus (VZV) infection presents in 2 distinct forms. Primary infection (commonly known as chickenpox) presents clinically as a vesicular rash usually located on the face, arms, and trunk. Although the initial presentation usually occurs in childhood and is self-limited, the virus becomes latent in the dorsal root ganglia of sensory neurons. Varicella-zoster virus may become reactivated later in life and is termed herpes zoster (commonly known as shingles). It most often presents as a painful vesicular rash that may later form pustules.
Zoster outbreaks typically do not cross the midline but may in disseminated disease. Patients may experience a prodrome in the form of pain or less commonly pruritus or paresthesia along the dermatome between 1 and 10 days before the rash appears. Triggers for herpes zoster include illness, medications, malnutrition, surgery, or the natural decline in immune function due to aging. Trauma is another important precipitating event for VZV reactivation; one case-control study showed that zoster patients were 3.4 times more likely than controls to have had trauma the week prior.1 Patients with cranial zoster are more than 25 times more likely to have experienced trauma in the preceding week. Local trauma may predispose these patients to VZV reactivation by stimulating local sensory nerves or by disrupting local cutaneous immunity.2
Herpes zoster ophthalmicus occurs when zoster presents in the ophthalmic division of the fifth cranial nerve. It is a serious, vision-threatening condition with a presentation that can include conjunctivitis, scleritis, keratitis, optic neuritis, exophthalmos, lid retraction, ptosis, and extraocular muscle palsies. Treatment includes antiviral medication (eg, acyclovir, valacyclovir, famciclovir) and prompt ophthalmologic consultation due to potential vision-threatening complications, such as acute retinal necrosis. Acute pain control may be necessary with nonsteroidal anti-inflammatory drugs, opioids, steroids, tricyclic antidepressants, or anticonvulsants.3 Wet-to-dry dressings with sterile saline or Burow solution and/or calamine lotion can provide symptomatic relief of itching.
Periorbital and preseptal cellulitis typically present with more erythema of the skin surrounding the eye and without the accompanying rash. Periorbital cellulitis is the more serious infection and may be clinically distinguished by the presence of pain with extraocular muscle movement. Contact dermatitis and pemphigus vulgaris are possibilities, but both were less likely than HZO in this case presentation given the distribution of the rash and the patient history. Contact dermatitis typically presents with no prodrome with a main concern of pruritus. Pemphigus vulgaris nearly always includes involvement of the oral mucous membranes.
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667-672.
- Zhang JX, Joesoef RM, Bialek S, et al. Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis. 2013;207:1007-1011.
- Rousseau A, Bourcier T, Colin J, et al. Herpes zoster ophthalmicus--diagnosis and management. US Ophthalm Rev. 2013;6:119-124.
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol. 1997;36:667-672.
- Zhang JX, Joesoef RM, Bialek S, et al. Association of physical trauma with risk of herpes zoster among Medicare beneficiaries in the United States. J Infect Dis. 2013;207:1007-1011.
- Rousseau A, Bourcier T, Colin J, et al. Herpes zoster ophthalmicus--diagnosis and management. US Ophthalm Rev. 2013;6:119-124.
A 56-year-old man presented to an urgent care clinic with right periorbital swelling. He reported hitting his head on the door to a storage unit 2 days prior but did not lose consciousness. The swelling presented 2 days later. He reported mild headache and swelling around the right eye that coincided with an uncomfortable rash on the face and scalp. He also reported visual disruption due to the swelling but denied any eye pain, discharge from the eye, or painful eye movements. He had no lesions on the lips or inside the mouth. He denied any history of skin conditions. He further denied fever, joint pain, or any other systemic symptoms. His chronic medical conditions included diabetes mellitus, hypertension, and hyperlipidemia that were stable on metformin, carvedilol, amlodipine, enalapril, and simvastatin, which he had taken for several years. He had not started any new medications, and there were no recent changes in the dosing of his medications.
For pediatric use of supplements, rely on resources, evidence
NEW ORLEANS – More than 1 in 10 children (12%) have received complementary or alternative medicine (CAM), according to the 2012 National Health Interview Survey. It’s therefore vital that you are familiar with the options and evidence on these treatments, according to Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
“Use of CAM by a parent was strongly associated with the child’s use of CAM,” Dr. Breuner told attendees at the annual meeting of the American Academy of Pediatrics. Parents of children using CAM were more likely to have a college education and to use prescription medication, the National Health Interview Survey found, and teens were more frequent users of CAM than infants.
The most common conditions treated in children with CAM were back and neck pain, colds, anxiety, stress, ADHD, insomnia, and general musculoskeletal conditions or complaints. Fish oil, melatonin, probiotics, and chiropractic and osteopathic manipulation were used more frequently than any other CAM treatments, but Dr. Breuner’s presentation focused specifically on supplements, including vitamins and herbs.
of how lax the law is when it comes to the safety and effectiveness of vitamins, minerals, herbs, and other dietary supplements.
“Products can go on the market with no testing of efficacy, and companies do not have to prove that their products are safe – only offer reasonable assurance of safety,” Dr. Breuner explained. “Supplements do not have to be manufactured to any standards, and FDA [Food and Drug Administration] approval is not needed for package or marketing claims,” although the reputable manufacturers favor standards.
She cited a 2011 study of popular supplement products on the market that found 75% of them did not include key safety messages (BMC Med. 2011 Aug 9;9:94). The study focused on St. John’s wort, ginkgo, ginseng, garlic, and echinacea products, and it’s likely other products lack such safety information as well. Yet researchers have identified a wide range of potential adverse effects from herbal medicines (Clin Med [Lond]. 2013 Feb;13[1]:7-12).
Physicians and consumers can rely on a handful of voluntary standards and online databases to guide therapeutic decisions and learn more about the evidence on specific products. The U.S. Pharmacopeia Dietary Supplement Verification Program is a seal consumers can look for on supplement products that indicates the product meets stricter standards than what the FDA allows.
Other resources include ConsumerLab.com, the Natural Medicines Research Collaboration, and the Pubmed Dietary Supplement Subset database from the National Institute of Medicine. The latter contains more than 676,000 unique scientific citations on published studies about vitamins, minerals, and botanicals, Dr. Breuner said.
Dr. Breuner presented an overview of more than a dozen popular supplements that included their uses and the evidence related to their use. Although not exhaustive, her list included the most common supplements for which some research has been done: butterbur, caffeine, cannabidiol, coenzyme Q10, echinacea, magnesium, melatonin, N-acetylcysteine, omega 3 fatty acids, St. John’s wort, turmeric (curcumin), and zinc.
The findings from these studies, however, vary greatly, and the studies themselves are often small and limited to adults. Shared decision making is key in working with families interested in using CAM, and families should be aware that supplements can have side effects just as FDA-approved drugs do.
Dr. Breuner reported that she had no relevant financial disclosures.
NEW ORLEANS – More than 1 in 10 children (12%) have received complementary or alternative medicine (CAM), according to the 2012 National Health Interview Survey. It’s therefore vital that you are familiar with the options and evidence on these treatments, according to Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
“Use of CAM by a parent was strongly associated with the child’s use of CAM,” Dr. Breuner told attendees at the annual meeting of the American Academy of Pediatrics. Parents of children using CAM were more likely to have a college education and to use prescription medication, the National Health Interview Survey found, and teens were more frequent users of CAM than infants.
The most common conditions treated in children with CAM were back and neck pain, colds, anxiety, stress, ADHD, insomnia, and general musculoskeletal conditions or complaints. Fish oil, melatonin, probiotics, and chiropractic and osteopathic manipulation were used more frequently than any other CAM treatments, but Dr. Breuner’s presentation focused specifically on supplements, including vitamins and herbs.
of how lax the law is when it comes to the safety and effectiveness of vitamins, minerals, herbs, and other dietary supplements.
“Products can go on the market with no testing of efficacy, and companies do not have to prove that their products are safe – only offer reasonable assurance of safety,” Dr. Breuner explained. “Supplements do not have to be manufactured to any standards, and FDA [Food and Drug Administration] approval is not needed for package or marketing claims,” although the reputable manufacturers favor standards.
She cited a 2011 study of popular supplement products on the market that found 75% of them did not include key safety messages (BMC Med. 2011 Aug 9;9:94). The study focused on St. John’s wort, ginkgo, ginseng, garlic, and echinacea products, and it’s likely other products lack such safety information as well. Yet researchers have identified a wide range of potential adverse effects from herbal medicines (Clin Med [Lond]. 2013 Feb;13[1]:7-12).
Physicians and consumers can rely on a handful of voluntary standards and online databases to guide therapeutic decisions and learn more about the evidence on specific products. The U.S. Pharmacopeia Dietary Supplement Verification Program is a seal consumers can look for on supplement products that indicates the product meets stricter standards than what the FDA allows.
Other resources include ConsumerLab.com, the Natural Medicines Research Collaboration, and the Pubmed Dietary Supplement Subset database from the National Institute of Medicine. The latter contains more than 676,000 unique scientific citations on published studies about vitamins, minerals, and botanicals, Dr. Breuner said.
Dr. Breuner presented an overview of more than a dozen popular supplements that included their uses and the evidence related to their use. Although not exhaustive, her list included the most common supplements for which some research has been done: butterbur, caffeine, cannabidiol, coenzyme Q10, echinacea, magnesium, melatonin, N-acetylcysteine, omega 3 fatty acids, St. John’s wort, turmeric (curcumin), and zinc.
The findings from these studies, however, vary greatly, and the studies themselves are often small and limited to adults. Shared decision making is key in working with families interested in using CAM, and families should be aware that supplements can have side effects just as FDA-approved drugs do.
Dr. Breuner reported that she had no relevant financial disclosures.
NEW ORLEANS – More than 1 in 10 children (12%) have received complementary or alternative medicine (CAM), according to the 2012 National Health Interview Survey. It’s therefore vital that you are familiar with the options and evidence on these treatments, according to Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
“Use of CAM by a parent was strongly associated with the child’s use of CAM,” Dr. Breuner told attendees at the annual meeting of the American Academy of Pediatrics. Parents of children using CAM were more likely to have a college education and to use prescription medication, the National Health Interview Survey found, and teens were more frequent users of CAM than infants.
The most common conditions treated in children with CAM were back and neck pain, colds, anxiety, stress, ADHD, insomnia, and general musculoskeletal conditions or complaints. Fish oil, melatonin, probiotics, and chiropractic and osteopathic manipulation were used more frequently than any other CAM treatments, but Dr. Breuner’s presentation focused specifically on supplements, including vitamins and herbs.
of how lax the law is when it comes to the safety and effectiveness of vitamins, minerals, herbs, and other dietary supplements.
“Products can go on the market with no testing of efficacy, and companies do not have to prove that their products are safe – only offer reasonable assurance of safety,” Dr. Breuner explained. “Supplements do not have to be manufactured to any standards, and FDA [Food and Drug Administration] approval is not needed for package or marketing claims,” although the reputable manufacturers favor standards.
She cited a 2011 study of popular supplement products on the market that found 75% of them did not include key safety messages (BMC Med. 2011 Aug 9;9:94). The study focused on St. John’s wort, ginkgo, ginseng, garlic, and echinacea products, and it’s likely other products lack such safety information as well. Yet researchers have identified a wide range of potential adverse effects from herbal medicines (Clin Med [Lond]. 2013 Feb;13[1]:7-12).
Physicians and consumers can rely on a handful of voluntary standards and online databases to guide therapeutic decisions and learn more about the evidence on specific products. The U.S. Pharmacopeia Dietary Supplement Verification Program is a seal consumers can look for on supplement products that indicates the product meets stricter standards than what the FDA allows.
Other resources include ConsumerLab.com, the Natural Medicines Research Collaboration, and the Pubmed Dietary Supplement Subset database from the National Institute of Medicine. The latter contains more than 676,000 unique scientific citations on published studies about vitamins, minerals, and botanicals, Dr. Breuner said.
Dr. Breuner presented an overview of more than a dozen popular supplements that included their uses and the evidence related to their use. Although not exhaustive, her list included the most common supplements for which some research has been done: butterbur, caffeine, cannabidiol, coenzyme Q10, echinacea, magnesium, melatonin, N-acetylcysteine, omega 3 fatty acids, St. John’s wort, turmeric (curcumin), and zinc.
The findings from these studies, however, vary greatly, and the studies themselves are often small and limited to adults. Shared decision making is key in working with families interested in using CAM, and families should be aware that supplements can have side effects just as FDA-approved drugs do.
Dr. Breuner reported that she had no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Zoledronate promotes postdenosumab bone retention
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
Women with osteoporosis who received a single infusion of zoledronate after discontinuing denosumab (Prolia) maintained bone mineral density at both the lumbar spine and the total hip, based on data from 120 individuals.
Although denosumab is often prescribed for postmenopausal osteoporosis, its effects disappear when treatment ends, wrote Judith Everts-Graber, MD, of OsteoRheuma Bern (Switzerland), and colleagues. In addition, recent reports of increased fractures in osteoporotic women after denosumab discontinuation highlight the need for subsequent therapy, but no protocol has been established.
In a study published in the Journal of Bone and Mineral Research, the investigators reviewed data from women aged older than 48 years with postmenopausal osteoporosis who were treated with denosumab between Aug. 1, 2010, and March 31, 2019. The women received four or more injections of 60 mg denosumab administered at 6-month intervals, followed by a single infusion of 5 mg zoledronate 6 months after the final denosumab injection. Patients were evaluated using dual-energy x-ray absorptiometry and vertebral fracture assessment every 2 years after starting denosumab; the average duration of treatment was 3 years.
At an average of 2.5 years after discontinuing denosumab, women who received zoledronate retained 66% of bone mineral density (BMD) gains at the lumbar spine, 49% at the total hip, and 57% at the femoral neck. In addition, three patients developed symptomatic single vertebral fractures and four patients developed peripheral fractures between 1 and 3 years after their last denosumab injections, but none of these patients sustained multiple fractures.
All bone loss occurred within 18 months of denosumab discontinuation, and no significant differences appeared between patients with gains in BMD greater than or less than 9%.
The study findings were limited by several factors, including the retrospective design and the lack of a control group, the researchers noted. However, they collected data from 11 of 28 patients who did not follow the treatment recommendations and did not receive zoledronate after discontinuing denosumab. “As expected, BMD of the lumbar spine and total hip decreased to baseline,” they wrote. In addition, 2 of the 11 patients experienced multiple vertebral fractures.
A single 5-mg infusion of zoledronate “may be a promising step in identifying sequential long-term treatment strategies for osteoporosis,” the researchers concluded. “Nevertheless, each patient requires an individualized surveillance and treatment plan after denosumab discontinuation, including BMD assessment, evaluation of bone turnover markers and consideration of individual clinical risk factors, in particular prevalent fragility fractures.”
The study was funded by OsteoRheuma Bern. The researchers reported having no financial conflicts.
SOURCE: Everts-Graber J et al. J Bone Miner Res. 2020 Jan 28. doi: 10.1002/jbmr.3962.
FROM THE JOURNAL OF BONE AND MINERAL RESEARCH
WHO declares public health emergency for novel coronavirus
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.
Amid the rising spread of the 2019 Novel Coronavirus (2019-nCoV),
The declaration was made during a press briefing on Jan. 30 after a week of growing concern and pressure on WHO to designate the virus at a higher emergency level. WHO’s Emergency Committee made the nearly unanimous decision after considering the increasing number of coronavirus cases in China, the rising infections outside of China, and the questionable measures some countries are taking regarding travel, said committee chair Didier Houssin, MD, said during the press conference.
As of Jan. 30, there were 8,236 confirmed cases of the coronavirus in China and 171 deaths, with another 112 cases identified outside of China in 21 other countries.
“Declaring a Public Health Emergency of International Concern is likely to facilitate [WHO’s] leadership role for public health measures, holding countries to account concerning additional measures they may take regarding travel, trade, quarantine or screening, research efforts, global coordination and anticipation of economic impact [and] support to vulnerable states,” Dr. Houssin said during the press conference. “Declaring a PHEIC should certainly not be seen as manifestation of distrust in the Chinese authorities and people which are doing tremendous efforts on the frontlines of this outbreak, with transparency, and let us hope, with success.”
What happens next?
Once a PHEIC is declared, WHO launches a series of steps, including the release of temporary recommendations for the affected country on health measures to implement and guidance for other countries on preventing and reducing the international spread of the disease, WHO spokesman Tarik Jasarevic said in an interview.
“The purpose of declaring a PHEIC is to advise the world on what measures need to be taken to enhance global health security by preventing international transmission of an infectious hazard,” he said.
Following the Jan. 30 press conference, WHO released temporary guidance for China and for other countries regarding identifying, managing, containing, and preventing the virus. China is advised to continue updating the population about the outbreak, continue enhancing its public health measures for containment and surveillance of cases, and to continue collaboration with WHO and other partners to investigate the epidemiology and evolution of the outbreak and share data on all human cases.
Other countries should be prepared for containment, including the active surveillance, early detection, isolation, case management, and prevention of virus transmission and to share full data with WHO, according to the recommendations.
Under the International Health Regulations (IHR), countries are required to share information and data with WHO. Additionally, WHO leaders advised the global community to support low- and middle-income countries with their response to the coronavirus and to facilitate diagnostics, potential vaccines, and therapeutics in these areas.
The IHR requires that countries implementing health measures that go beyond what WHO recommends must send to WHO the public health rationale and justification within 48 hours of their implementation for WHO review, Mr. Jasarevic noted.
“WHO is obliged to share the information about measures and the justification received with other countries involved,” he said.
PHEIC travel and resource impact
Declaration of a PHEIC means WHO will now oversee any travel restrictions made by other countries in response to 2019-nCoV. The agency recommends that countries conduct a risk and cost-benefit analysis before enacting travel restrictions and other countries are required to inform WHO about any travel measures taken.
“Countries will be asked to provide public health justification for any travel or trade measures that are not scientifically based, such as refusal of entry of suspect cases or unaffected persons to affected areas,” Mr. Jasarevic said in an interview.
As far as resources, the PHEIC mechanism is not a fundraising mechanism, but some donors might consider a PHEIC declaration as a trigger for releasing additional funding to respond to the health threat, he said.
Allison T. Chamberlain, PhD, acting director for the Emory Center for Public Health Preparedness and Research at the Emory Rollins School of Public Health in Atlanta, said national governments and nongovernmental aid organizations are among the most affected by a PHEIC because they are looked at to provide assistance to the most heavily affected areas and to bolster public health preparedness within their own borders.
“In terms of resources that are deployed, a Public Health Emergency of International Concern raises levels of international support and commitment to stopping the emergency,” Dr. Chamberlain said in an interview. “By doing so, it gives countries the needed flexibility to release financial resources of their own accord to support things like response teams that might go into heavily affected areas to assist, for instance.”
WHO Director-General Dr. Tedros Adhanom Ghebreyesus stressed that cooperation among countries is key during the PHEIC.
“We can only stop it together,” he said during the press conference. “This is the time for facts, not fear. This is the time for science, not rumors. This is the time for solidarity, not stigma.”
This is the sixth PHEIC declared by WHO in the last 10 years. Such declarations were made for the 2009 H1NI influenza pandemic, the 2014 polio resurgence, the 2014 Ebola outbreak in West Africa, the 2016 Zika virus, and the 2019 Kivu Ebola outbreak in the Democratic Republic of Congo.