User login
How Motivational Interviewing Helps Patients with Diabetes
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
In 2019, 30.3 million US adults were reported to have diabetes—an epidemic according to some public health experts.1,2 Even more sobering, an estimated 84.1 million (or more than 1 in 3) American adults have prediabetes.1 Diabetes is associated with multiple complications, including an increased risk for heart disease or stroke.3 In 2015, it was the seventh leading cause of death and a major cause of kidney failure, lower limb amputations, stroke, and blindness.2,4
As clinicians we often ask ourselves, “How can I help my patients become more effective managers of their diabetes, so that they can maximize their quality of life over both the short and long term?” Unfortunately, management of diabetes is fraught with difficulty, both for the provider and the patient. Medications for glycemic control can be expensive and inconvenient and can have adverse effects—all of which may lead to inconsistent adherence. Lifestyle changes—including diet, regular physical activity, exercise, and weight management—are important low-risk interventions that help patients maintain glycemic values and reduce the risk for diabetic complications. However, some patients may find it difficult to make or are ambivalent to behavioral change.
These patients may benefit from having structured verbal encouragement—such as motivational interviewing (MI)—incorporated into their visits. The following discussion will explain how MI can be an effective communication tool for encouraging patients with diabetes or prediabetes to make important behavioral changes and improve health outcomes.
Q What is MI?
First created by William R. Miller and Stephen Rollnick in the 1980s as a counseling method to help patients with substance use disorders, MI was eventually expanded to address other clinical challenges, including tobacco cessation, weight management, and diabetes care. MI helps patients identify their motivations and goals to improve long-term outcomes and work through any ambivalence to change. It utilizes an empathic approach with open-ended questions.5 This helps reduce the resistance frequently encountered during an average “lecture-style” interaction and facilitates a collaborative relationship that empowers the patient to make positive lifestyle changes.
MI affirms the patient’s experience while exploring any discrepancies between goals and actions. Two important components for conducting MI are (1) verbally reflecting the patient’s motivations and thoughts about change and (2) allowing the patient to “voice the arguments for change.”6 These components help the patient take ownership of the overarching goal for behavioral change and in the development of an action plan.
MI involves 4 primary processes: engaging, focusing, evoking, and planning (defined in the Table).7 MI begins with building rapport and a trusting relationship by engaging with empathic responses that reflect the patient’s concerns and focusing on what is important to him or her. The clinician should evoke the patient’s reasons and motivations for change. During the planning process, the clinician highlights the salient points of the conversation and works with the patient to identify an action he or she could take as a first step toward change.7
Table
Motivational Interviewing Processes
Engaging: Demonstrating empathy |
Focusing: Identifying what is important to the patient |
Evoking: Eliciting patient’s internal motivations for change |
Planning: Reinforcing the patient’s commitment to change |
Source: Arkowitz H, et al. Motivational Interviewing in the Treatment of Psychological Problems. 2015. 7
Continue to: Q How can I use MI with my patients with diabetes?
Q How can I use MI with my patients with diabetes?
MI can be used in a variety of clinical settings, including primary care and behavioral health, and can be effective when employed even in short periods of time.8,9 This communication style can be incorporated into regular follow-up appointments to help the clinician and the patient work toward better glycemic control and improved long-term outcomes.
For clinicians who are new users of MI, consider the mnemonic OARS (Open-ended questions, Affirmations, accurate empathic Reflections, Summarizing) to utilize the core components of MI.10 The OARS techniques are vital MI tools that can help the clinician explore the patient’s motivation for pursuing change, and they help the clinician recognize and appreciate the patient’s perspective on the challenges of initiating change.10 The following sample conversation illustrates how OARS can be used.
Open-ended question:
Clinician: What do you think are the greatest challenges when it comes to controlling your diabetes?
Patient: It’s just so frustrating, I keep avoiding bad food and trying to eat healthy, but my sugar still goes up.
Affirmations:
Clinician: Thank you for sharing that with me. It sounds like you are persistent and have been working hard to make healthier choices.
Patient: Yes, but I’m so tired of trying. It just doesn’t seem to work.
Accurate empathic reflections:
Clinician: It is important for you to control your diabetes, but you feel discouraged by the results that you’ve seen.
Patient: Yeah, I just don’t know what else to do to make my sugar better.
Continue to: Summarizing
Summarizing:
Clinician: You’ve said that controlling your blood sugar is important to you and that you’ve tried eating healthily, but it just isn’t working well enough. It sounds like you are ready to explore alternatives that might help you gain better control of the situation. Is that right?
Patient: Well, yes, it is.
Here the patient recognizes the need for help in controlling his or her diabetes, and the clinician can then move the conversation to additional treatment options, such as medication changes or support group intervention. Using OARS, the provider can focus on what is important to the patient and evaluate any discrepancies between the patient’s goals and actions.
Q Does the research support MI for patients with diabetes?
Many studies have evaluated the efficacy of MI on behavioral change and health care–related outcomes.8,11-15 Since its inception, MI has shown great promise in addictive behavior modification.16 Multiple studies also show support for its beneficial effect on weight management as well as on physical activity level, which are 2 factors strongly associated with improved outcomes in patients with prediabetes and diabetes.8,11-15,17 In a 2017 meta-analysis of MI for patients with obesity, prediabetes, and type 2 diabetes, Phillips and Guarnaccia found significant support for behavioral change leading to improvements in quantifiable medical measurements.18
Systematic reviews of MI in health care settings have produced some conflicting findings. While there is evidence for the usefulness of MI in bringing about positive lifestyle changes, data supporting the effective use of MI in specific diabetes-related outcomes (eg, A1C levels) have been less robust.8,11-15,19 However, this is a particularly challenging area of study due in part to limitations of research designs and the inherent difficulties in assuring high-quality, consistent MI approaches. Despite these limitations, MI has significant positive results in improving patient adherence to treatment regimens.9,16,20,21
Conclusion
MI is a promising method that empowers patients to make modifications to their lifestyle choices, work through ambivalence, and better align goals with actions. Although the data on patient outcomes is inconclusive, evidence suggests that MI conducted across appointments holds benefit and that it is even more effective when combined with additional nonpharmacologic techniques, such as cognitive behavioral therapy.17,22 Additionally, research suggests that MI strengthens the clinician-patient relationship, with patients reporting greater empathy from their clinicians and overall satisfaction with interactions.23 Improved communication and mutual respect in clinician-patient interactions help maintain the therapeutic alliance for the future. For additional guidance and resources on MI, visit the Motivational Interviewing Network of Trainers website at motivationalinterviewing.org.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
1. CDC. About diabetes. www.cdc.gov/diabetes/basics/diabetes.html. Reviewed August 6, 2019. Accessed December 2, 2019.
2. World Health Organization. Diabetes. www.who.int/news-room/fact-sheets/detail/diabetes. Published October 3, 2018. Accessed December 2, 2019.
3. CDC. Put the brakes on diabetes complications. www.cdc.gov/features/preventing-diabetes-complications/index.html. Reviewed October 21, 2019. Accessed December 2, 2019.
4. CDC. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2017. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed December 2, 2019.
5. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
6. Miller WR, Rose GS. Toward a theory of motivational interviewing. Am Psychol. 2009;64(6):527-537.
7. Arkowitz H, Miller WR, Rollnick S, eds. Motivational Interviewing in the Treatment of Psychological Problems. 2nd ed. New York, NY: The Guilford Press; 2015.
8. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37(4):768-780.
9. Palacio A, Garay D, Langer B, et al. Motivational interviewing improves medication adherence: a systematic review and meta-analysis. J Gen Intern Med. 2016;31(8):929-940.
10. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
11. Armstrong MJ, Mottershead TA, Ronksley PE, et al. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12(9):709-723.
12. Frost H, Campbell P, Maxwell M, et al. Effectiveness of motivational interviewing on adult behaviour change in health and social care settings: a systematic review of reviews. PLoS One. 2018;13(10):e0204890.
13. Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003;71(5):843-861.
14. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305-312.
15. Hardcastle S, Taylor A, Bailey M, Castle R. A randomised controlled trial on the effectiveness of a primary health care based counselling intervention on physical activity, diet and CHD risk factors. Patient Educ Couns. 2008:70(1):31-39.
16. Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. 2005;1:91-111.
17. Morton K, Beauchamp M, Prothero A, et al. The effectiveness of motivational interviewing for health behaviour change in primary care settings: a systematic review. Health Psychol Rev. 2015;9(2):205-223.
18. Phillips AS, Guarnaccia CA. Self-determination theory and motivational interviewing interventions for type 2 diabetes prevention and treatment: a systematic review. J Health Psychol. 2017:135910531773760.
19. Mathiesen AS, Egerod I, Jensen T, et al. Psychosocial interventions for reducing diabetes distress in vulnerable people with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Metab Syndr Obes. 2018;12:19-33.
20. Skolasky RL, Maggard AM, Wegener ST, Riley LH 3rd. Telephone-based intervention to improve rehabilitation engagement after spinal stenosis surgery: a prospective lagged controlled trial. J Bone Joint Surg Am. 2018;100(1):21-30.
21. Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: a systematic review. Patient Educ Couns. 2017;100(12):2190-2199.
22. Barrett, S, Begg, S, O’Halloran, P, et al. Integrated motivational interviewing and cognitive behaviour therapy for lifestyle mediators of overweight and obesity in community-dwelling adults: a systematic review and meta-analyses. BMC Public Health. 2018;18:1160.
23. Wagoner ST, Kavookjian J. The influence of motivational interviewing on patients with inflammatory bowel disease: a systematic review of the literature. J Clin Med Res. 2017;9(8):659-666.
Tender Papules on the Bilateral Dorsal Hands
The Diagnosis: Interstitial Granulomatous Dermatitis
Interstitial granulomatous dermatitis (IGD) is rare, and the exact incidence is unknown, with only a few cases reported in the literature annually.1 Although IGD may arise in both children and adults, it occurs more commonly in adults, with an age of onset of 52 to 58.5 years. Interstitial granulomatous dermatitis also shows a female predominance.1
Interstitial granulomatous dermatitis may present as annular flesh-colored or erythematous to violaceous papules and plaques, or less commonly erythematous linear cordlike subcutaneous nodules (called the rope sign).1 Lesions often are asymptomatic but may be pruritic or tender. Interstitial granulomatous dermatitis has been associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, and primary biliary cholangitis, and rarely malignancy.2 Interstitial granulomatous drug reactions can occur months to years after initiation of therapy with offending agents, and common causes include calcium channel blockers, statins, and tumor necrosis factor α inhibitors.3
Interstitial granulomatous dermatitis and palisaded neutrophilic and granulomatous dermatitis (PNGD) demonstrate overlapping clinical features and are thought to be part of the same spectrum of granulomatous dermatitis.4 Both IGD and PNGD may present with symmetric flesh-colored to erythematous papules or erythematous annular or linear plaques.5 Interstitial granulomatous dermatitis and PNGD may be differentiated through histopathologic examination.
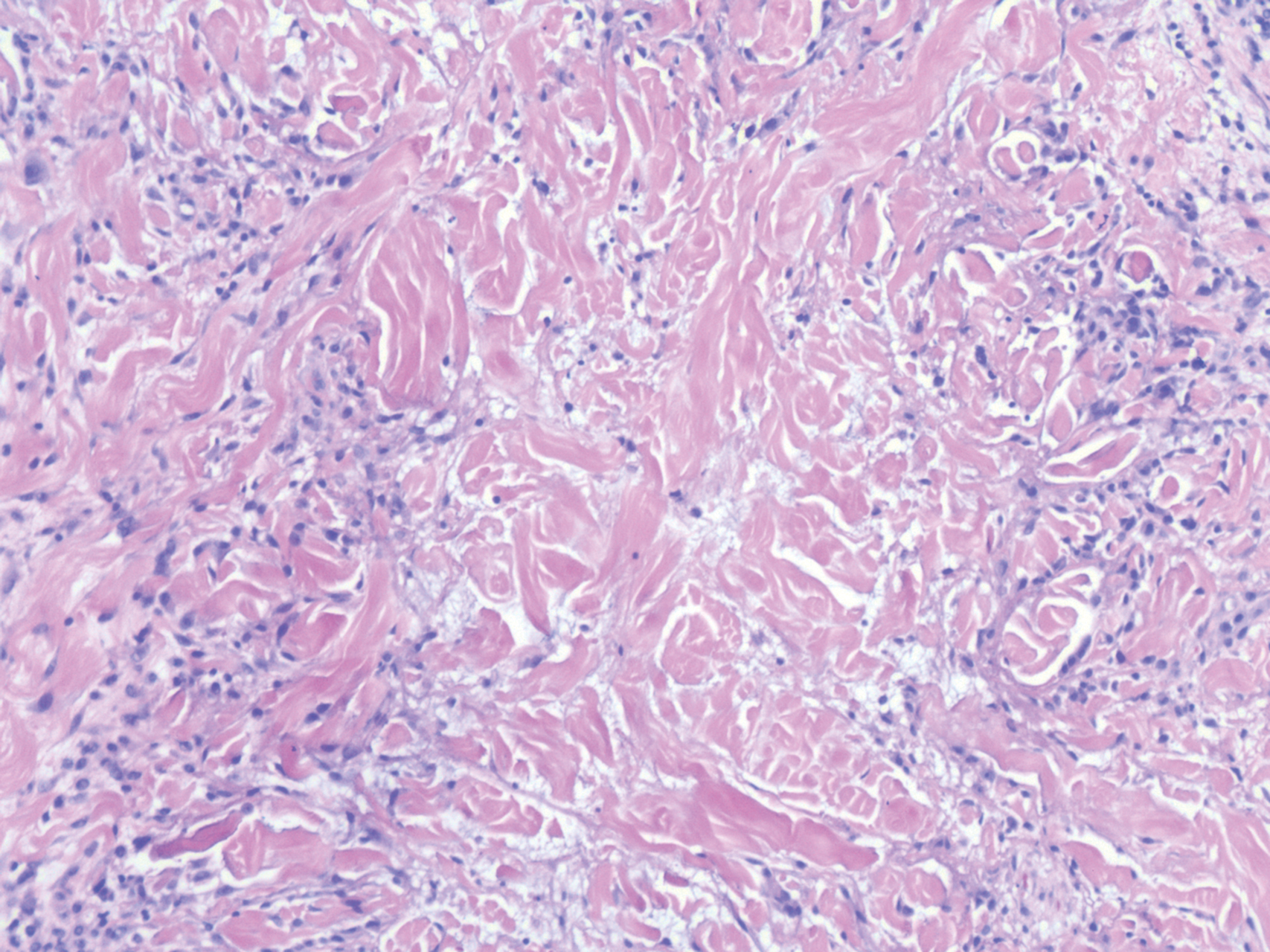
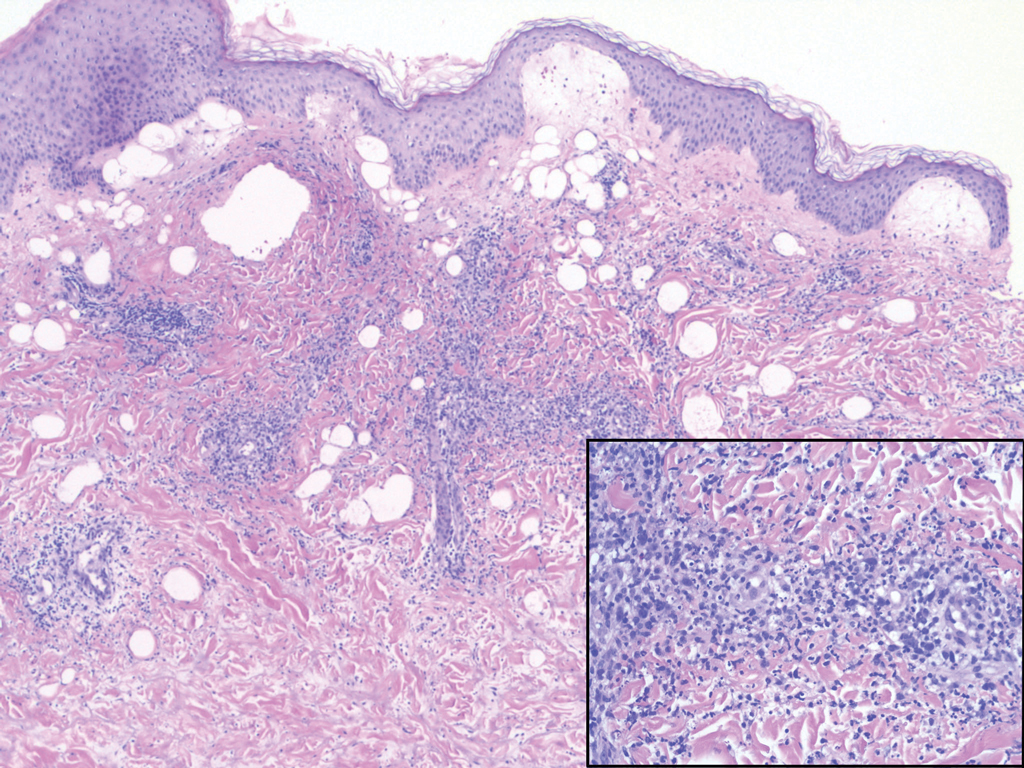
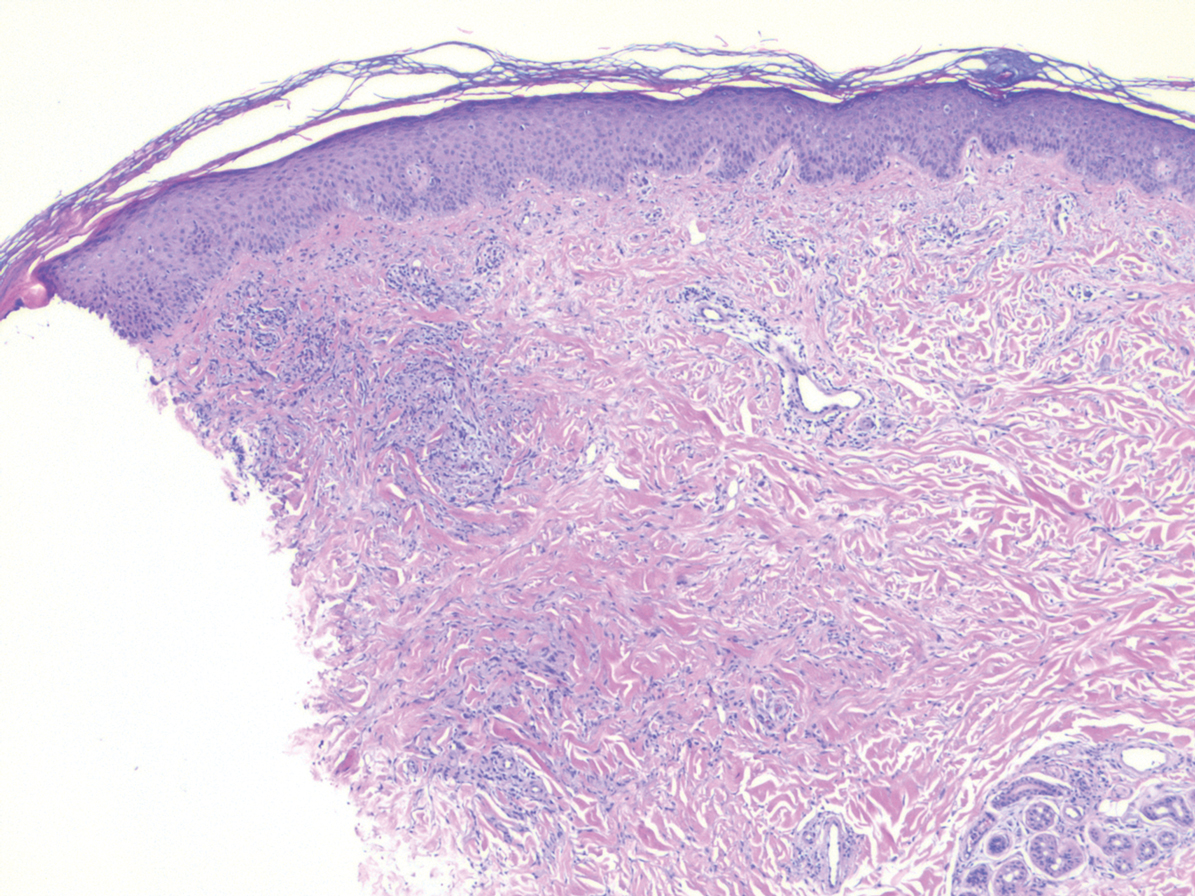
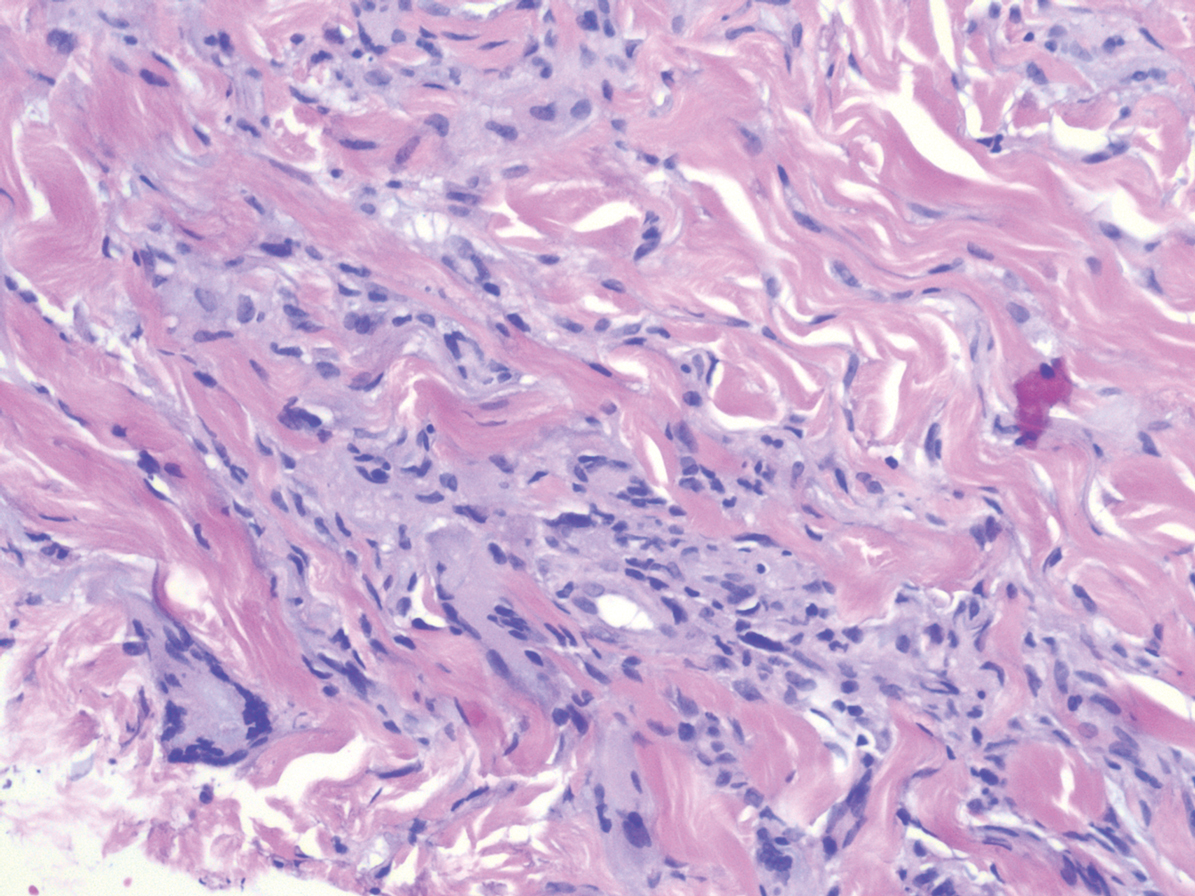
Histopathology of IGD shows an interstitial infiltrate of epithelioid histiocytes in the dermis, often surrounding foci of degenerated collagen resembling palisading granulomas (quiz images).1 Perivascular and interstitial lymphocytic infiltrates also are present in most cases. Epidermal changes are minimal in IGD but can be associated with interstitial granulomatous drug reactions.1 There usually is no vasculitis, and mucin typically is absent, unlike granuloma annulare (GA).3,6 In comparison, histopathologic examination of PNGD shows basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris with focal areas of leukocytoclastic vasculitis and rare mucin.5
No specific treatment is recommended, and lesions may resolve without any therapy. Reported treatments include topical, intralesional, or systemic steroids; nonsteroidal anti-inflammatory drugs; methotrexate; hydroxychloroquine; and cyclosporine.6 Due to the strong association with systemic diseases, it is important to evaluate patients with IGD for autoimmune diseases and conduct age-appropriate cancer screening. Furthermore, a review of medications is warranted to assess the possibility of interstitial granulomatous drug reactions.6 In our patient, rheumatologic workup and age-appropriate cancer screenings were negative, and the rash spontaneously resolved without treatment.
Granuloma annulare presents with asymptomatic flesh-colored to erythematous papules and plaques in an annular configuration. In the localized variant of GA, plaques frequently localize to the distal extremities, especially the dorsal hands, as in our patient. Other variants include generalized GA, subcutaneous GA, and perforating GA. Mucin and a palisading or interstitial pattern of granulomatous inflammation are key features on histopathology in all subtypes of GA (Figure 1).7 Patch GA is a rare variant that presents with asymptomatic erythematous to brown patches, is associated with interstitial-type inflammation on histopathology, and can be difficult to distinguish from IGD.8 Granuloma annulare with interstitial inflammation on histology can be differentiated from IGD by the comparative lack of mucin in IGD.7
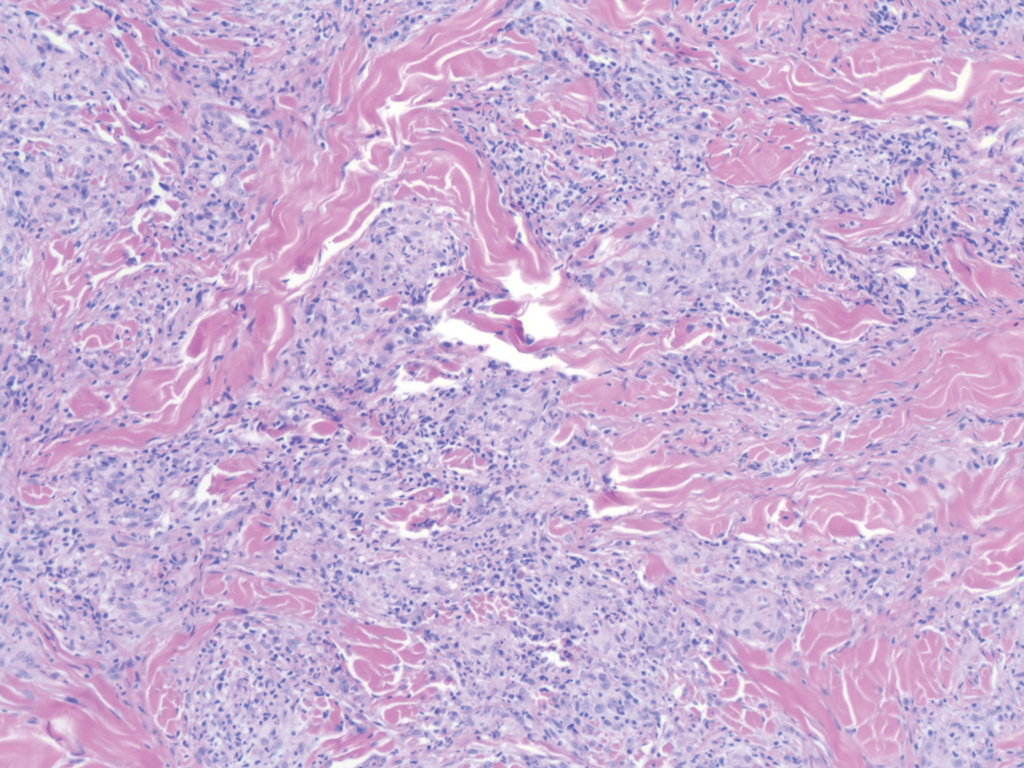
Sweet syndrome (SS) is characterized by sudden-onset, painful, erythematous plaques and/or nodules, commonly associated with fever and leukocytosis. Clinical variants of SS include pustular and bullous SS; giant cellulitis-like SS; necrotizing SS; and neutrophilic dermatosis of the dorsal hands presenting with hemorrhagic bullae, plaques, and pustules.7-9 Histopathologic examination shows dense nodular or perivascular neutrophilic infiltrate in the dermis without evidence of vasculitis (Figure 2).10 Histopathologic variants include histiocytoid, lymphocytic, subcutaneous, and cryptococcoid.9 The classic variant of SS has a bandlike, predominantly neutrophilic infiltrate with marked leukocytoclasia, which can be differentiated from the histiocytoid infiltrate of IGD.11 It has been shown that the infiltrate of the histiocytoid variant of SS is composed of myeloperoxidase-positive, immature myeloid cells rather than true histiocytes, and therefore can be differentiated from IGD.12 Lastly, all variants of SS have dermal edema, which typically is absent in IGD, and SS has no evidence of necrobiosis.
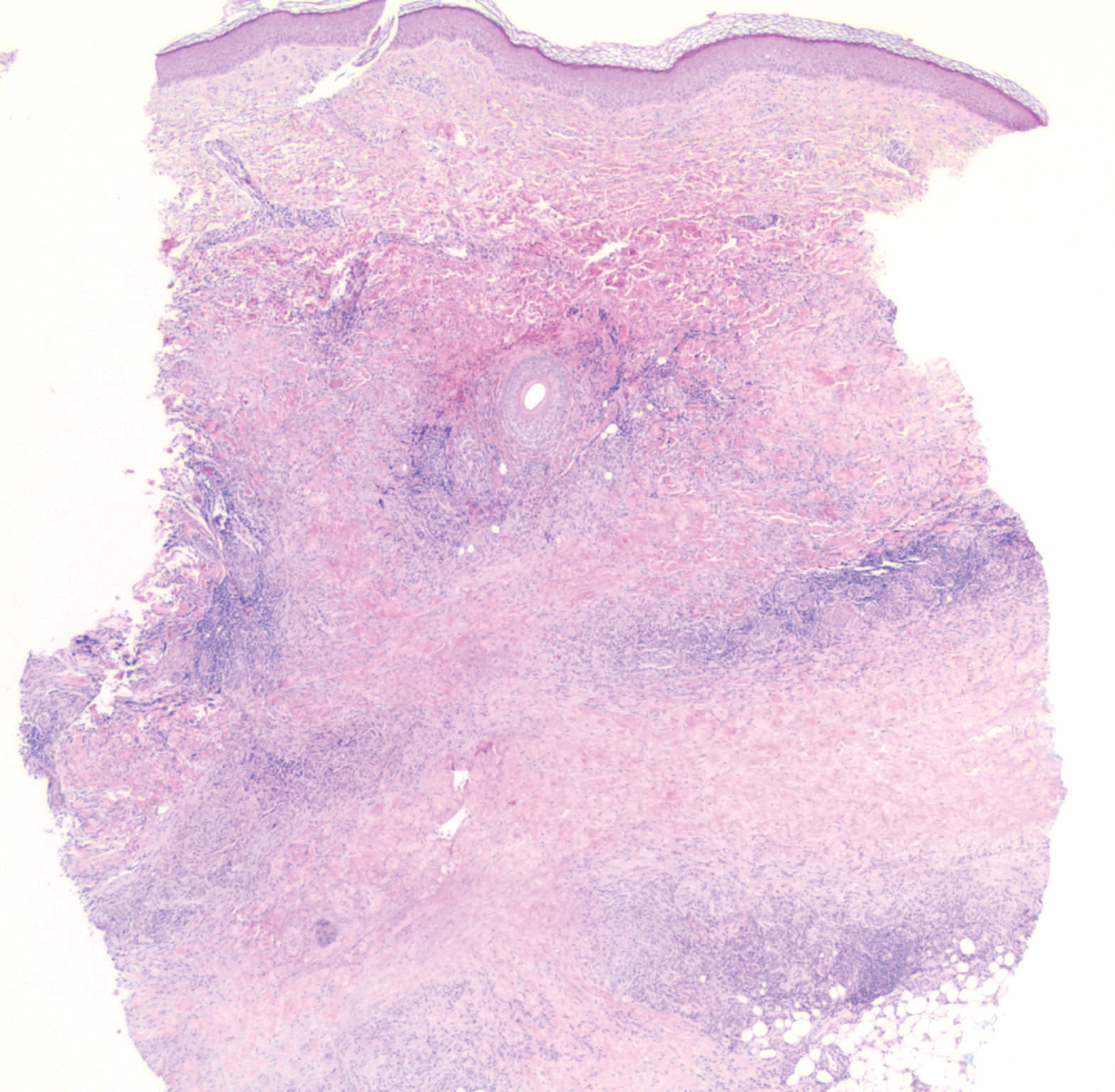
Erythema elevatum diutinum (EED) is a rare disease that presents with bilateral violaceous or erythematous to brown papules, plaques, or nodules. Lesions frequently localize to extensor surfaces, including the hands and fingers, and may be asymptomatic or associated with pruritus, burning, or tingling.13 Early EED lesions are characterized by leukocytoclastic vasculitis of the papillary and mid-dermal vessels with a perivascular neutrophilic infiltrate and perivascular fibrinoid necrosis. With older EED lesions, dermal and perivascular onion skin-like fibrosis become more prominent (Figure 3).14 The neutrophilic infiltrate, dermal fibrosis, and chronic vasculitic changes distinguish EED from IGD.
Necrobiosis lipoidica (NL) is a rare disease that presents with well-demarcated, yellow to red-brown papules and nodules most commonly localized to the bilateral lower extremities on the pretibial area. Papules and nodules evolve into plaques over time, and ulceration is common.15 On histopathology, NL primarily exhibits granulomatous inflammation with parallel palisading (Figure 4). The hallmark feature is necrobiosis--or degeneration--of collagen; the alternation of necrobiotic collagen and inflammatory infiltrate creates a layered cake-like appearance on low power.16 The clinical presentation as well as the dermal necrobiotic granuloma consisting of a large confluent area of necrobiosis centered in the superficial dermis and subcutaneous tissue of NL distinguishes it from the histiocytic infiltrate of IGD.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1644-1663.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Huizenga T, Kado JA, Pellicane B, et al. Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis. Cutis. 2018;101:E19-E21.
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Mutasim DF, Bridges AG. Patch granuloma annulare: clinicopathologic study of 6 patients. J Am Acad Dermatol. 2000;42:417-421.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Dabade TS, Davis MD. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet's syndrome). Dermatol Ther. 2011;24:273-284.
- Davis M, Moschella L. Neutrophilic dermatoses. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:2102-2112.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Sardiña LA, Jour G, Piliang MP, et al. Erythema elevatum diutinum a rare and poorly understood cutaneous vasculitis: a single institution experience. J Cutan Pathol. 2019;46:97-101.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360.
The Diagnosis: Interstitial Granulomatous Dermatitis
Interstitial granulomatous dermatitis (IGD) is rare, and the exact incidence is unknown, with only a few cases reported in the literature annually.1 Although IGD may arise in both children and adults, it occurs more commonly in adults, with an age of onset of 52 to 58.5 years. Interstitial granulomatous dermatitis also shows a female predominance.1
Interstitial granulomatous dermatitis may present as annular flesh-colored or erythematous to violaceous papules and plaques, or less commonly erythematous linear cordlike subcutaneous nodules (called the rope sign).1 Lesions often are asymptomatic but may be pruritic or tender. Interstitial granulomatous dermatitis has been associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, and primary biliary cholangitis, and rarely malignancy.2 Interstitial granulomatous drug reactions can occur months to years after initiation of therapy with offending agents, and common causes include calcium channel blockers, statins, and tumor necrosis factor α inhibitors.3
Interstitial granulomatous dermatitis and palisaded neutrophilic and granulomatous dermatitis (PNGD) demonstrate overlapping clinical features and are thought to be part of the same spectrum of granulomatous dermatitis.4 Both IGD and PNGD may present with symmetric flesh-colored to erythematous papules or erythematous annular or linear plaques.5 Interstitial granulomatous dermatitis and PNGD may be differentiated through histopathologic examination.
Histopathology of IGD shows an interstitial infiltrate of epithelioid histiocytes in the dermis, often surrounding foci of degenerated collagen resembling palisading granulomas (quiz images).1 Perivascular and interstitial lymphocytic infiltrates also are present in most cases. Epidermal changes are minimal in IGD but can be associated with interstitial granulomatous drug reactions.1 There usually is no vasculitis, and mucin typically is absent, unlike granuloma annulare (GA).3,6 In comparison, histopathologic examination of PNGD shows basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris with focal areas of leukocytoclastic vasculitis and rare mucin.5
No specific treatment is recommended, and lesions may resolve without any therapy. Reported treatments include topical, intralesional, or systemic steroids; nonsteroidal anti-inflammatory drugs; methotrexate; hydroxychloroquine; and cyclosporine.6 Due to the strong association with systemic diseases, it is important to evaluate patients with IGD for autoimmune diseases and conduct age-appropriate cancer screening. Furthermore, a review of medications is warranted to assess the possibility of interstitial granulomatous drug reactions.6 In our patient, rheumatologic workup and age-appropriate cancer screenings were negative, and the rash spontaneously resolved without treatment.
Granuloma annulare presents with asymptomatic flesh-colored to erythematous papules and plaques in an annular configuration. In the localized variant of GA, plaques frequently localize to the distal extremities, especially the dorsal hands, as in our patient. Other variants include generalized GA, subcutaneous GA, and perforating GA. Mucin and a palisading or interstitial pattern of granulomatous inflammation are key features on histopathology in all subtypes of GA (Figure 1).7 Patch GA is a rare variant that presents with asymptomatic erythematous to brown patches, is associated with interstitial-type inflammation on histopathology, and can be difficult to distinguish from IGD.8 Granuloma annulare with interstitial inflammation on histology can be differentiated from IGD by the comparative lack of mucin in IGD.7
Sweet syndrome (SS) is characterized by sudden-onset, painful, erythematous plaques and/or nodules, commonly associated with fever and leukocytosis. Clinical variants of SS include pustular and bullous SS; giant cellulitis-like SS; necrotizing SS; and neutrophilic dermatosis of the dorsal hands presenting with hemorrhagic bullae, plaques, and pustules.7-9 Histopathologic examination shows dense nodular or perivascular neutrophilic infiltrate in the dermis without evidence of vasculitis (Figure 2).10 Histopathologic variants include histiocytoid, lymphocytic, subcutaneous, and cryptococcoid.9 The classic variant of SS has a bandlike, predominantly neutrophilic infiltrate with marked leukocytoclasia, which can be differentiated from the histiocytoid infiltrate of IGD.11 It has been shown that the infiltrate of the histiocytoid variant of SS is composed of myeloperoxidase-positive, immature myeloid cells rather than true histiocytes, and therefore can be differentiated from IGD.12 Lastly, all variants of SS have dermal edema, which typically is absent in IGD, and SS has no evidence of necrobiosis.
Erythema elevatum diutinum (EED) is a rare disease that presents with bilateral violaceous or erythematous to brown papules, plaques, or nodules. Lesions frequently localize to extensor surfaces, including the hands and fingers, and may be asymptomatic or associated with pruritus, burning, or tingling.13 Early EED lesions are characterized by leukocytoclastic vasculitis of the papillary and mid-dermal vessels with a perivascular neutrophilic infiltrate and perivascular fibrinoid necrosis. With older EED lesions, dermal and perivascular onion skin-like fibrosis become more prominent (Figure 3).14 The neutrophilic infiltrate, dermal fibrosis, and chronic vasculitic changes distinguish EED from IGD.
Necrobiosis lipoidica (NL) is a rare disease that presents with well-demarcated, yellow to red-brown papules and nodules most commonly localized to the bilateral lower extremities on the pretibial area. Papules and nodules evolve into plaques over time, and ulceration is common.15 On histopathology, NL primarily exhibits granulomatous inflammation with parallel palisading (Figure 4). The hallmark feature is necrobiosis--or degeneration--of collagen; the alternation of necrobiotic collagen and inflammatory infiltrate creates a layered cake-like appearance on low power.16 The clinical presentation as well as the dermal necrobiotic granuloma consisting of a large confluent area of necrobiosis centered in the superficial dermis and subcutaneous tissue of NL distinguishes it from the histiocytic infiltrate of IGD.
The Diagnosis: Interstitial Granulomatous Dermatitis
Interstitial granulomatous dermatitis (IGD) is rare, and the exact incidence is unknown, with only a few cases reported in the literature annually.1 Although IGD may arise in both children and adults, it occurs more commonly in adults, with an age of onset of 52 to 58.5 years. Interstitial granulomatous dermatitis also shows a female predominance.1
Interstitial granulomatous dermatitis may present as annular flesh-colored or erythematous to violaceous papules and plaques, or less commonly erythematous linear cordlike subcutaneous nodules (called the rope sign).1 Lesions often are asymptomatic but may be pruritic or tender. Interstitial granulomatous dermatitis has been associated with autoimmune conditions such as rheumatoid arthritis, systemic lupus erythematosus, and primary biliary cholangitis, and rarely malignancy.2 Interstitial granulomatous drug reactions can occur months to years after initiation of therapy with offending agents, and common causes include calcium channel blockers, statins, and tumor necrosis factor α inhibitors.3
Interstitial granulomatous dermatitis and palisaded neutrophilic and granulomatous dermatitis (PNGD) demonstrate overlapping clinical features and are thought to be part of the same spectrum of granulomatous dermatitis.4 Both IGD and PNGD may present with symmetric flesh-colored to erythematous papules or erythematous annular or linear plaques.5 Interstitial granulomatous dermatitis and PNGD may be differentiated through histopathologic examination.
Histopathology of IGD shows an interstitial infiltrate of epithelioid histiocytes in the dermis, often surrounding foci of degenerated collagen resembling palisading granulomas (quiz images).1 Perivascular and interstitial lymphocytic infiltrates also are present in most cases. Epidermal changes are minimal in IGD but can be associated with interstitial granulomatous drug reactions.1 There usually is no vasculitis, and mucin typically is absent, unlike granuloma annulare (GA).3,6 In comparison, histopathologic examination of PNGD shows basophilic degenerated collagen surrounded by palisades of histiocytes, neutrophils, and nuclear debris with focal areas of leukocytoclastic vasculitis and rare mucin.5
No specific treatment is recommended, and lesions may resolve without any therapy. Reported treatments include topical, intralesional, or systemic steroids; nonsteroidal anti-inflammatory drugs; methotrexate; hydroxychloroquine; and cyclosporine.6 Due to the strong association with systemic diseases, it is important to evaluate patients with IGD for autoimmune diseases and conduct age-appropriate cancer screening. Furthermore, a review of medications is warranted to assess the possibility of interstitial granulomatous drug reactions.6 In our patient, rheumatologic workup and age-appropriate cancer screenings were negative, and the rash spontaneously resolved without treatment.
Granuloma annulare presents with asymptomatic flesh-colored to erythematous papules and plaques in an annular configuration. In the localized variant of GA, plaques frequently localize to the distal extremities, especially the dorsal hands, as in our patient. Other variants include generalized GA, subcutaneous GA, and perforating GA. Mucin and a palisading or interstitial pattern of granulomatous inflammation are key features on histopathology in all subtypes of GA (Figure 1).7 Patch GA is a rare variant that presents with asymptomatic erythematous to brown patches, is associated with interstitial-type inflammation on histopathology, and can be difficult to distinguish from IGD.8 Granuloma annulare with interstitial inflammation on histology can be differentiated from IGD by the comparative lack of mucin in IGD.7
Sweet syndrome (SS) is characterized by sudden-onset, painful, erythematous plaques and/or nodules, commonly associated with fever and leukocytosis. Clinical variants of SS include pustular and bullous SS; giant cellulitis-like SS; necrotizing SS; and neutrophilic dermatosis of the dorsal hands presenting with hemorrhagic bullae, plaques, and pustules.7-9 Histopathologic examination shows dense nodular or perivascular neutrophilic infiltrate in the dermis without evidence of vasculitis (Figure 2).10 Histopathologic variants include histiocytoid, lymphocytic, subcutaneous, and cryptococcoid.9 The classic variant of SS has a bandlike, predominantly neutrophilic infiltrate with marked leukocytoclasia, which can be differentiated from the histiocytoid infiltrate of IGD.11 It has been shown that the infiltrate of the histiocytoid variant of SS is composed of myeloperoxidase-positive, immature myeloid cells rather than true histiocytes, and therefore can be differentiated from IGD.12 Lastly, all variants of SS have dermal edema, which typically is absent in IGD, and SS has no evidence of necrobiosis.
Erythema elevatum diutinum (EED) is a rare disease that presents with bilateral violaceous or erythematous to brown papules, plaques, or nodules. Lesions frequently localize to extensor surfaces, including the hands and fingers, and may be asymptomatic or associated with pruritus, burning, or tingling.13 Early EED lesions are characterized by leukocytoclastic vasculitis of the papillary and mid-dermal vessels with a perivascular neutrophilic infiltrate and perivascular fibrinoid necrosis. With older EED lesions, dermal and perivascular onion skin-like fibrosis become more prominent (Figure 3).14 The neutrophilic infiltrate, dermal fibrosis, and chronic vasculitic changes distinguish EED from IGD.
Necrobiosis lipoidica (NL) is a rare disease that presents with well-demarcated, yellow to red-brown papules and nodules most commonly localized to the bilateral lower extremities on the pretibial area. Papules and nodules evolve into plaques over time, and ulceration is common.15 On histopathology, NL primarily exhibits granulomatous inflammation with parallel palisading (Figure 4). The hallmark feature is necrobiosis--or degeneration--of collagen; the alternation of necrobiotic collagen and inflammatory infiltrate creates a layered cake-like appearance on low power.16 The clinical presentation as well as the dermal necrobiotic granuloma consisting of a large confluent area of necrobiosis centered in the superficial dermis and subcutaneous tissue of NL distinguishes it from the histiocytic infiltrate of IGD.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1644-1663.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Huizenga T, Kado JA, Pellicane B, et al. Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis. Cutis. 2018;101:E19-E21.
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Mutasim DF, Bridges AG. Patch granuloma annulare: clinicopathologic study of 6 patients. J Am Acad Dermatol. 2000;42:417-421.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Dabade TS, Davis MD. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet's syndrome). Dermatol Ther. 2011;24:273-284.
- Davis M, Moschella L. Neutrophilic dermatoses. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:2102-2112.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Sardiña LA, Jour G, Piliang MP, et al. Erythema elevatum diutinum a rare and poorly understood cutaneous vasculitis: a single institution experience. J Cutan Pathol. 2019;46:97-101.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Terziroli Beretta-Piccoli B, Mainetti C, Peeters MA, et al. Cutaneous granulomatosis: a comprehensive review. Clin Rev Allergy Immunol. 2018;54:131-146.
- Rosenbach MA, Wanat KA, Reisenauer A, et al. Non-infectious granulomas. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:1644-1663.
- Chu P, Connolly MK, LeBoit PE. The histopathologic spectrum of palisaded neutrophilic and granulomatous dermatitis in patients with collagen vascular disease. Arch Dermatol. 1994;130:1278-1283.
- Huizenga T, Kado JA, Pellicane B, et al. Interstitial granulomatous dermatitis and palisaded neutrophilic granulomatous dermatitis. Cutis. 2018;101:E19-E21.
- Rosenbach M, English JC 3rd. Reactive granulomatous dermatitis: a review of palisaded neutrophilic and granulomatous dermatitis, interstitial granulomatous dermatitis, interstitial granulomatous drug reaction, and a proposed reclassification. Dermatol Clin. 2015;33:373-387.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- Mutasim DF, Bridges AG. Patch granuloma annulare: clinicopathologic study of 6 patients. J Am Acad Dermatol. 2000;42:417-421.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Dabade TS, Davis MD. Diagnosis and treatment of the neutrophilic dermatoses (pyoderma gangrenosum, Sweet's syndrome). Dermatol Ther. 2011;24:273-284.
- Davis M, Moschella L. Neutrophilic dermatoses. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier Saunders; 2018:2102-2112.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Gibson LE, el-Azhary RA. Erythema elevatum diutinum. Clin Dermatol. 2000;18:295-299.
- Sardiña LA, Jour G, Piliang MP, et al. Erythema elevatum diutinum a rare and poorly understood cutaneous vasculitis: a single institution experience. J Cutan Pathol. 2019;46:97-101.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Sibbald C, Reid S, Alavi A. Necrobiosis lipoidica. Dermatol Clin. 2015;33:343-360.
A 58-year-old woman with a medical history of asthma, hypertension, hypothyroidism, and hyperlipidemia presented with a painful rash of 10 days' duration. The rash was associated with fever at home (temperature, 38.5.2 °C), and a review of systems was positive for joint pain. Physical examination revealed numerous 8- to 10-mm, erythematous, discus-shaped papules on the bilateral dorsal hands, bilateral palms, right knee, and right dorsal foot with slight tenderness to palpation. A papule on the right dorsal hand was biopsied.
ADA2 is a potent new biomarker for macrophage activation syndrome
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
ATLANTA – Adenosine deaminase 2 above the upper limit of normal is 86% sensitive and 94% specific for distinguishing macrophage activation syndrome from active systemic juvenile idiopathic arthritis, making it perhaps the most potent blood marker yet identified to differentiate the two, according to a report presented at the annual meeting of the American College of Rheumatology.
The upper limit of normal was 27.8 U/L, two standard deviations above the median of 13 U/L (interquartile range, 10.6-16.1) in 174 healthy children. The work was published simultaneously in Annals of the Rheumatic Diseases.
In children with active systemic juvenile idiopathic arthritis (JIA), adenosine deaminase 2 (ADA2) “beyond the upper limit of normal is strong evidence for concomitant” macrophage activation syndrome (MAS). “Our work represents a new method to diagnose this condition,” said lead investigator Pui Y. Lee, MD, PhD, a pediatric rheumatologist at Boston Children’s Hospital.
The hope, he said, is that the finding will lead to quicker recognition and treatment of MAS, a devastating complication of systemic JIA in which rampant inflammation begets further inflammation in a downward spiral that ultimately proves fatal in about 20% of cases. The problem is that the clinical features of MAS overlap with those of active systemic JIA, which makes early diagnosis difficult.
Ferritin and other common markers are not very specific unless “the cutoff is raised significantly to distinguish MAS from general inflammation. Most labs will not tell you ‘this is an active systemic JIA range; this is an MAS-like range.’ It’s hard for them to define that for you. ADA2 is more black and white; if you go above the upper limit, you most likely have MAS,” Dr. Lee explained at the meeting.
Potentially, “we can combine this test with other tests to define a single MAS panel,” he said.
ADA2 is measured by a simple, inexpensive enzyme assay that’s been around for 20 years, but it hasn’t caught on because the protein’s function is unknown and the clinical relevance of ADA2 levels has been uncertain. With the new findings, “it is our hope that ADA2 testing will become more available,” Dr. Lee said.
The protein appears to be a product of monocytes and macrophages, and a genetic deficiency has recently been linked to congenital vasculitis, which made Dr. Lee and colleagues curious about ADA2 in other rheumatic diseases. The first step was to define normal limits in healthy controls; the 13 U/L median in children proved to be a bit higher than in 150 healthy adults.
The team then found that levels were completely normal in 25 children with active Kawasaki disease, and only mildly elevated in 13 children with systemic lupus and 13 with juvenile dermatomyositis. The Kawasaki children, in particular “were highly inflamed, so this protein is not just simply a marker of inflammation,” Dr. Lee said.
They next turned to 120 children with JIA, with a mix of systemic and nonsystemic cases. “The ones with very high levels, far beyond the upper limit of normal, were” almost exclusively the 23 children with systemic JIA and clinically diagnosed MAS. “As long as [JIA children] didn’t have MAS, their levels were pretty much close to normal,” he said.
In eight MAS children with repeat testing, levels fell below the upper limit of normal with treatment and remission, but children prone to repeat MAS seemed to hover closer to the limit even when they were well.
Blood sample testing showed that interleukin-18 and interferon-gamma were the main drivers of ADA2 expression in the periphery, “which makes sense because these two cytokines are very involved in the process of MAS,” Dr. Lee said.
The work was funded by the National Institutes of Health, among others. Dr. Lee didn’t have any disclosures.
SOURCE: Lee PY et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract 920.
REPORTING FROM ACR 2019
Reduced kidney function linked to fractures in older women
Moderate reductions in kidney function in older women are associated with an increased short-term risk of fractures, according to a study published in Osteoporosis International.
However, the longitudinal, population-based cohort study did not find an association with fracture risk either in women older than 80 years or in those with worse kidney function.
“Since the kidneys regulate homeostasis of PTH [parathyroid hormone], phosphate, calcium, and vitamin D, any disruption in function can be expected to disturb bone remodeling and have implications for skeletal health,” wrote Linnea Malmgren, MD, and colleagues from Skåne University Hospital in Malmö, Sweden.
Previous studies have found that a large proportion of older women have reduced kidney function equivalent to a diagnosis of chronic kidney disease and that it is associated with bone loss. However, there have been few studies exploring that association in a population without a diagnosis of chronic kidney disease.
In their study, Dr. Malmgren and colleagues followed 981 women aged 75 years and 685 women aged 80 years, who underwent assessment of kidney function and bone mineral density and were followed up for fracture.
They found that women who experienced an osteoporotic fracture between the ages of 75 and 80 years had significantly lower baseline kidney function, compared with those who did not experience a fracture.
Compared with women with normal kidney function at age 75, women with intermediate kidney function (estimated glomerular filtration rate 45-59 mL/min per 1.73m2) had a significant 2.21-fold higher risk of osteoporotic fracture within 2 years, a 1.51-fold higher risk up to 5 years, and an elevated risk of hip fracture.
A similar trend was seen in women with poor kidney function (eGFR less than 45 mL/min per 1.73m2), but it was not statistically significant.
The analysis also found that kidney function at age 80 years was not significantly associated with long-term fracture risk, nor was there a significant association for 10-year fracture risk in those aged 75 years.
Reduced kidney function was also associated with a higher fracture risk even in women without osteoporosis.
“As expected, fracture risk was high among those with osteoporosis, but risk seemed to further increase in women with both osteoporosis and reduced kidney function, compared with those with osteoporosis and normal function,” the authors reported.
“These findings indicate that implications for bone health and fracture risk might occur in the very common modest reduction of kidney function in the elderly, and also possibly before a diagnosis of CKD-MBD [chronic kidney disease–mineral and bone disorder].”
The study was supported by Lund University; the Swedish Research Council; Forte, Greta and Johan Kock Foundation; A. Påhlsson Foundation; A. Osterlund Foundation; H Järnhardt Foundation; King Gustav V 80-year Fund; Thelma Zoegas Foundation; Swedish Rheumatism Foundation; Skåne University Hospital Research Fund; and the Research and Development Council of Region Skåne, Sweden. No conflicts of interest were declared.
SOURCE: Malmgren L et al. Osteoporos Int. 2019 Nov 21. doi: 10.1007/s00198-019-05152-x.
Moderate reductions in kidney function in older women are associated with an increased short-term risk of fractures, according to a study published in Osteoporosis International.
However, the longitudinal, population-based cohort study did not find an association with fracture risk either in women older than 80 years or in those with worse kidney function.
“Since the kidneys regulate homeostasis of PTH [parathyroid hormone], phosphate, calcium, and vitamin D, any disruption in function can be expected to disturb bone remodeling and have implications for skeletal health,” wrote Linnea Malmgren, MD, and colleagues from Skåne University Hospital in Malmö, Sweden.
Previous studies have found that a large proportion of older women have reduced kidney function equivalent to a diagnosis of chronic kidney disease and that it is associated with bone loss. However, there have been few studies exploring that association in a population without a diagnosis of chronic kidney disease.
In their study, Dr. Malmgren and colleagues followed 981 women aged 75 years and 685 women aged 80 years, who underwent assessment of kidney function and bone mineral density and were followed up for fracture.
They found that women who experienced an osteoporotic fracture between the ages of 75 and 80 years had significantly lower baseline kidney function, compared with those who did not experience a fracture.
Compared with women with normal kidney function at age 75, women with intermediate kidney function (estimated glomerular filtration rate 45-59 mL/min per 1.73m2) had a significant 2.21-fold higher risk of osteoporotic fracture within 2 years, a 1.51-fold higher risk up to 5 years, and an elevated risk of hip fracture.
A similar trend was seen in women with poor kidney function (eGFR less than 45 mL/min per 1.73m2), but it was not statistically significant.
The analysis also found that kidney function at age 80 years was not significantly associated with long-term fracture risk, nor was there a significant association for 10-year fracture risk in those aged 75 years.
Reduced kidney function was also associated with a higher fracture risk even in women without osteoporosis.
“As expected, fracture risk was high among those with osteoporosis, but risk seemed to further increase in women with both osteoporosis and reduced kidney function, compared with those with osteoporosis and normal function,” the authors reported.
“These findings indicate that implications for bone health and fracture risk might occur in the very common modest reduction of kidney function in the elderly, and also possibly before a diagnosis of CKD-MBD [chronic kidney disease–mineral and bone disorder].”
The study was supported by Lund University; the Swedish Research Council; Forte, Greta and Johan Kock Foundation; A. Påhlsson Foundation; A. Osterlund Foundation; H Järnhardt Foundation; King Gustav V 80-year Fund; Thelma Zoegas Foundation; Swedish Rheumatism Foundation; Skåne University Hospital Research Fund; and the Research and Development Council of Region Skåne, Sweden. No conflicts of interest were declared.
SOURCE: Malmgren L et al. Osteoporos Int. 2019 Nov 21. doi: 10.1007/s00198-019-05152-x.
Moderate reductions in kidney function in older women are associated with an increased short-term risk of fractures, according to a study published in Osteoporosis International.
However, the longitudinal, population-based cohort study did not find an association with fracture risk either in women older than 80 years or in those with worse kidney function.
“Since the kidneys regulate homeostasis of PTH [parathyroid hormone], phosphate, calcium, and vitamin D, any disruption in function can be expected to disturb bone remodeling and have implications for skeletal health,” wrote Linnea Malmgren, MD, and colleagues from Skåne University Hospital in Malmö, Sweden.
Previous studies have found that a large proportion of older women have reduced kidney function equivalent to a diagnosis of chronic kidney disease and that it is associated with bone loss. However, there have been few studies exploring that association in a population without a diagnosis of chronic kidney disease.
In their study, Dr. Malmgren and colleagues followed 981 women aged 75 years and 685 women aged 80 years, who underwent assessment of kidney function and bone mineral density and were followed up for fracture.
They found that women who experienced an osteoporotic fracture between the ages of 75 and 80 years had significantly lower baseline kidney function, compared with those who did not experience a fracture.
Compared with women with normal kidney function at age 75, women with intermediate kidney function (estimated glomerular filtration rate 45-59 mL/min per 1.73m2) had a significant 2.21-fold higher risk of osteoporotic fracture within 2 years, a 1.51-fold higher risk up to 5 years, and an elevated risk of hip fracture.
A similar trend was seen in women with poor kidney function (eGFR less than 45 mL/min per 1.73m2), but it was not statistically significant.
The analysis also found that kidney function at age 80 years was not significantly associated with long-term fracture risk, nor was there a significant association for 10-year fracture risk in those aged 75 years.
Reduced kidney function was also associated with a higher fracture risk even in women without osteoporosis.
“As expected, fracture risk was high among those with osteoporosis, but risk seemed to further increase in women with both osteoporosis and reduced kidney function, compared with those with osteoporosis and normal function,” the authors reported.
“These findings indicate that implications for bone health and fracture risk might occur in the very common modest reduction of kidney function in the elderly, and also possibly before a diagnosis of CKD-MBD [chronic kidney disease–mineral and bone disorder].”
The study was supported by Lund University; the Swedish Research Council; Forte, Greta and Johan Kock Foundation; A. Påhlsson Foundation; A. Osterlund Foundation; H Järnhardt Foundation; King Gustav V 80-year Fund; Thelma Zoegas Foundation; Swedish Rheumatism Foundation; Skåne University Hospital Research Fund; and the Research and Development Council of Region Skåne, Sweden. No conflicts of interest were declared.
SOURCE: Malmgren L et al. Osteoporos Int. 2019 Nov 21. doi: 10.1007/s00198-019-05152-x.
FROM OSTEOPOROSIS INTERNATIONAL
FDA panel rejects vernakalant bid for AFib cardioversion indication
for cardioversion of recent-onset atrial fibrillation (AFib).
It was the second time before an FDA advisory panel for vernakalant (Brinavess, Correvio International Sàrl), which the agency had declined to approve in 2008 due to safety concerns. That time, however, its advisors had given the agency a decidedly positive recommendation.
Since then, registry data collected for the drug’s resubmission seemed only to raise further safety issues, especially evidence that a single infusion may cause severe hypotension and suppress left ventricular function.
Some members of the Cardiovascular and Renal Drugs Advisory Committee (CRDAC), including a number who voted against approval, expressed hopes for further research aimed at identifying specific AFib patient groups who might safely benefit from vernakalant.
Of note, the drug has long been available for AFib cardioversion in Europe, where there are a number of other pharmacologic options, and was recently approved in Canada.
“We do recognize there’s a significant clinical need here,” observed Paul M. Ridker, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital Boston, a CRDAC panelist.
The results of the safety study that Correvio presented to the panel were “pretty marginal,” Dr. Ridker said. Given the negative safety signals and the available cardioversion alternatives, he questioned whether vernakalant represented a “substantial advance versus just another option. Right now, I’m not convinced it’s a substantial advance.”
FDA representatives were skeptical about vernakalant when they walked into the meeting room, as noted in briefing documents they had circulated beforehand. The drug’s safety experience under consideration included one case of ventricular arrhythmia and cardiogenic shock in a treated patient without apparent structural heart disease, who subsequently died. That case was much discussed throughout the meeting.
In its resubmission of vernakalant to regulators, Correvio also pointed to a significant unmet need for AFib cardioversion options in the United States, given the few alternatives.
For example, ibutilide is FDA-approved for recent-onset AFib or atrial flutter; but as the company and panelists noted, the drug isn’t often used for that indication. Patients with recent-onset AFib are often put on rate-control meds without cardioversion. Or clinicians may resort to electrical cardioversion, which can be logistically cumbersome and require anesthesia and generally a hospital stay.
Oral or intravenous amiodarone and oral dofetilide, flecainide, and propafenone are guideline-recommended but not actually FDA-approved for recent-onset AFib, the company noted.
Correvio made its “pre-infusion checklist” a core feature of its case. It was designed to guide selection of patients for vernakalant cardioversion based on contraindications such as a systolic blood pressure under 100 mm Hg, severe heart failure, aortic stenosis, severe bradycardia or heart block, or a prolonged QT interval.
In his presentation to the panel, FDA medical officer Preston Dunnmon, MD, said the safety results from the SPECTRUM registry, another main pillar of support for the vernakalant resubmission, “are not reassuring.”
As reasons, Dr. Dunnmon cited likely patient-selection bias and its high proportion of patients who were not prospectively enrolled; 21% were retrospectively entered from records.
Moreover, “the proposed preinfusion checklist will not reliably predict which subjects will experience serious cardiovascular adverse events with vernakalant,” he said.
“Vernakalant has induced harm that cannot be reliably predicted, prevented, or in some cases, treated. In contrast to vernakalant, electrical cardioversion and ibutilide pharmacologic cardioversion can cause adverse events, but these are transient and treatable,” he said.
Many on the panel agreed. “I thought the totality of evidence supported the hypothesis that this drug has a potential for a fatal side effect in a disease that you can live with, potentially, and that there are other treatments for,” said Julia B. Lewis, MD, Vanderbilt Medical Center, Nashville, Tenn., who chaired the CRDAC panel.
“The drug clearly converts atrial fibrillation, although it’s only transient,” observed John H. Alexander, MD, MHSc, Duke University, Durham, N.C., one of the two panelists who voted to recommend approval of vernakalant.
“And, there clearly is a serious safety signal in some populations of patients,” he agreed. “However, I was more reassured by the SPECTRUM data.” There is likely to be a low-risk group of patients for whom vernakalant could represent an important option that “outweighs the relatively low risk of serious complications,” Dr. Alexander said.
“So more work needs to be done to clarify who are the low risk patients where it would be favorable.”
Panelist Matthew Needleman, MD, Walter Reed National Military Medical Center, Bethesda, Md., also voted in favor of approval.
“We’ve all known patients with normal ejection fractions who keep coming in with symptomatic atrial fib, want to get out of it quickly, and get back to their lives. So having an option like this I think would be good for a very select group of patients,” Dr. Needleman said.
But the preinfusion checklist and other potential ways to select low-risk patients for vernakalant could potentially backfire, warned John M. Mandrola, MD, Baptist Medical Associates, Louisville, Ky., from the panel.
The FDA representatives had presented evidence that the drug can seriously depress ventricular function, and that the lower cardiac output is what leads to hypotension, he elaborated in an interview after the meeting.
If the checklist is used to exclude hemodynamically unstable patients from receiving vernakalant, he said, “Then you’re really giving this drug to relatively healthy patients for convenience, to decrease hospitalization or the hospital stay.”
The signal for substantial harm, Dr. Mandrola said, has to be balanced against that modest benefit.
Moreover, those in whom the drug doesn’t work may be left in a worse situation, he proposed. Only about half of patients are successfully converted on vernakalant, the company and FDA data suggested. The other half of patients who don’t achieve sinus rhythm on the drug still must face the significant hazards of depressed ejection fraction and hypotension, a high price to pay for an unsuccessful treatment.
Dr. Mandrola is Chief Cardiology Correspondent for theheart.org | Medscape Cardiology; his disclosure statement states no relevant financial relationships.
This article first appeared on Medscape.com.
for cardioversion of recent-onset atrial fibrillation (AFib).
It was the second time before an FDA advisory panel for vernakalant (Brinavess, Correvio International Sàrl), which the agency had declined to approve in 2008 due to safety concerns. That time, however, its advisors had given the agency a decidedly positive recommendation.
Since then, registry data collected for the drug’s resubmission seemed only to raise further safety issues, especially evidence that a single infusion may cause severe hypotension and suppress left ventricular function.
Some members of the Cardiovascular and Renal Drugs Advisory Committee (CRDAC), including a number who voted against approval, expressed hopes for further research aimed at identifying specific AFib patient groups who might safely benefit from vernakalant.
Of note, the drug has long been available for AFib cardioversion in Europe, where there are a number of other pharmacologic options, and was recently approved in Canada.
“We do recognize there’s a significant clinical need here,” observed Paul M. Ridker, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital Boston, a CRDAC panelist.
The results of the safety study that Correvio presented to the panel were “pretty marginal,” Dr. Ridker said. Given the negative safety signals and the available cardioversion alternatives, he questioned whether vernakalant represented a “substantial advance versus just another option. Right now, I’m not convinced it’s a substantial advance.”
FDA representatives were skeptical about vernakalant when they walked into the meeting room, as noted in briefing documents they had circulated beforehand. The drug’s safety experience under consideration included one case of ventricular arrhythmia and cardiogenic shock in a treated patient without apparent structural heart disease, who subsequently died. That case was much discussed throughout the meeting.
In its resubmission of vernakalant to regulators, Correvio also pointed to a significant unmet need for AFib cardioversion options in the United States, given the few alternatives.
For example, ibutilide is FDA-approved for recent-onset AFib or atrial flutter; but as the company and panelists noted, the drug isn’t often used for that indication. Patients with recent-onset AFib are often put on rate-control meds without cardioversion. Or clinicians may resort to electrical cardioversion, which can be logistically cumbersome and require anesthesia and generally a hospital stay.
Oral or intravenous amiodarone and oral dofetilide, flecainide, and propafenone are guideline-recommended but not actually FDA-approved for recent-onset AFib, the company noted.
Correvio made its “pre-infusion checklist” a core feature of its case. It was designed to guide selection of patients for vernakalant cardioversion based on contraindications such as a systolic blood pressure under 100 mm Hg, severe heart failure, aortic stenosis, severe bradycardia or heart block, or a prolonged QT interval.
In his presentation to the panel, FDA medical officer Preston Dunnmon, MD, said the safety results from the SPECTRUM registry, another main pillar of support for the vernakalant resubmission, “are not reassuring.”
As reasons, Dr. Dunnmon cited likely patient-selection bias and its high proportion of patients who were not prospectively enrolled; 21% were retrospectively entered from records.
Moreover, “the proposed preinfusion checklist will not reliably predict which subjects will experience serious cardiovascular adverse events with vernakalant,” he said.
“Vernakalant has induced harm that cannot be reliably predicted, prevented, or in some cases, treated. In contrast to vernakalant, electrical cardioversion and ibutilide pharmacologic cardioversion can cause adverse events, but these are transient and treatable,” he said.
Many on the panel agreed. “I thought the totality of evidence supported the hypothesis that this drug has a potential for a fatal side effect in a disease that you can live with, potentially, and that there are other treatments for,” said Julia B. Lewis, MD, Vanderbilt Medical Center, Nashville, Tenn., who chaired the CRDAC panel.
“The drug clearly converts atrial fibrillation, although it’s only transient,” observed John H. Alexander, MD, MHSc, Duke University, Durham, N.C., one of the two panelists who voted to recommend approval of vernakalant.
“And, there clearly is a serious safety signal in some populations of patients,” he agreed. “However, I was more reassured by the SPECTRUM data.” There is likely to be a low-risk group of patients for whom vernakalant could represent an important option that “outweighs the relatively low risk of serious complications,” Dr. Alexander said.
“So more work needs to be done to clarify who are the low risk patients where it would be favorable.”
Panelist Matthew Needleman, MD, Walter Reed National Military Medical Center, Bethesda, Md., also voted in favor of approval.
“We’ve all known patients with normal ejection fractions who keep coming in with symptomatic atrial fib, want to get out of it quickly, and get back to their lives. So having an option like this I think would be good for a very select group of patients,” Dr. Needleman said.
But the preinfusion checklist and other potential ways to select low-risk patients for vernakalant could potentially backfire, warned John M. Mandrola, MD, Baptist Medical Associates, Louisville, Ky., from the panel.
The FDA representatives had presented evidence that the drug can seriously depress ventricular function, and that the lower cardiac output is what leads to hypotension, he elaborated in an interview after the meeting.
If the checklist is used to exclude hemodynamically unstable patients from receiving vernakalant, he said, “Then you’re really giving this drug to relatively healthy patients for convenience, to decrease hospitalization or the hospital stay.”
The signal for substantial harm, Dr. Mandrola said, has to be balanced against that modest benefit.
Moreover, those in whom the drug doesn’t work may be left in a worse situation, he proposed. Only about half of patients are successfully converted on vernakalant, the company and FDA data suggested. The other half of patients who don’t achieve sinus rhythm on the drug still must face the significant hazards of depressed ejection fraction and hypotension, a high price to pay for an unsuccessful treatment.
Dr. Mandrola is Chief Cardiology Correspondent for theheart.org | Medscape Cardiology; his disclosure statement states no relevant financial relationships.
This article first appeared on Medscape.com.
for cardioversion of recent-onset atrial fibrillation (AFib).
It was the second time before an FDA advisory panel for vernakalant (Brinavess, Correvio International Sàrl), which the agency had declined to approve in 2008 due to safety concerns. That time, however, its advisors had given the agency a decidedly positive recommendation.
Since then, registry data collected for the drug’s resubmission seemed only to raise further safety issues, especially evidence that a single infusion may cause severe hypotension and suppress left ventricular function.
Some members of the Cardiovascular and Renal Drugs Advisory Committee (CRDAC), including a number who voted against approval, expressed hopes for further research aimed at identifying specific AFib patient groups who might safely benefit from vernakalant.
Of note, the drug has long been available for AFib cardioversion in Europe, where there are a number of other pharmacologic options, and was recently approved in Canada.
“We do recognize there’s a significant clinical need here,” observed Paul M. Ridker, MD, MPH, of Harvard Medical School and Brigham and Women’s Hospital Boston, a CRDAC panelist.
The results of the safety study that Correvio presented to the panel were “pretty marginal,” Dr. Ridker said. Given the negative safety signals and the available cardioversion alternatives, he questioned whether vernakalant represented a “substantial advance versus just another option. Right now, I’m not convinced it’s a substantial advance.”
FDA representatives were skeptical about vernakalant when they walked into the meeting room, as noted in briefing documents they had circulated beforehand. The drug’s safety experience under consideration included one case of ventricular arrhythmia and cardiogenic shock in a treated patient without apparent structural heart disease, who subsequently died. That case was much discussed throughout the meeting.
In its resubmission of vernakalant to regulators, Correvio also pointed to a significant unmet need for AFib cardioversion options in the United States, given the few alternatives.
For example, ibutilide is FDA-approved for recent-onset AFib or atrial flutter; but as the company and panelists noted, the drug isn’t often used for that indication. Patients with recent-onset AFib are often put on rate-control meds without cardioversion. Or clinicians may resort to electrical cardioversion, which can be logistically cumbersome and require anesthesia and generally a hospital stay.
Oral or intravenous amiodarone and oral dofetilide, flecainide, and propafenone are guideline-recommended but not actually FDA-approved for recent-onset AFib, the company noted.
Correvio made its “pre-infusion checklist” a core feature of its case. It was designed to guide selection of patients for vernakalant cardioversion based on contraindications such as a systolic blood pressure under 100 mm Hg, severe heart failure, aortic stenosis, severe bradycardia or heart block, or a prolonged QT interval.
In his presentation to the panel, FDA medical officer Preston Dunnmon, MD, said the safety results from the SPECTRUM registry, another main pillar of support for the vernakalant resubmission, “are not reassuring.”
As reasons, Dr. Dunnmon cited likely patient-selection bias and its high proportion of patients who were not prospectively enrolled; 21% were retrospectively entered from records.
Moreover, “the proposed preinfusion checklist will not reliably predict which subjects will experience serious cardiovascular adverse events with vernakalant,” he said.
“Vernakalant has induced harm that cannot be reliably predicted, prevented, or in some cases, treated. In contrast to vernakalant, electrical cardioversion and ibutilide pharmacologic cardioversion can cause adverse events, but these are transient and treatable,” he said.
Many on the panel agreed. “I thought the totality of evidence supported the hypothesis that this drug has a potential for a fatal side effect in a disease that you can live with, potentially, and that there are other treatments for,” said Julia B. Lewis, MD, Vanderbilt Medical Center, Nashville, Tenn., who chaired the CRDAC panel.
“The drug clearly converts atrial fibrillation, although it’s only transient,” observed John H. Alexander, MD, MHSc, Duke University, Durham, N.C., one of the two panelists who voted to recommend approval of vernakalant.
“And, there clearly is a serious safety signal in some populations of patients,” he agreed. “However, I was more reassured by the SPECTRUM data.” There is likely to be a low-risk group of patients for whom vernakalant could represent an important option that “outweighs the relatively low risk of serious complications,” Dr. Alexander said.
“So more work needs to be done to clarify who are the low risk patients where it would be favorable.”
Panelist Matthew Needleman, MD, Walter Reed National Military Medical Center, Bethesda, Md., also voted in favor of approval.
“We’ve all known patients with normal ejection fractions who keep coming in with symptomatic atrial fib, want to get out of it quickly, and get back to their lives. So having an option like this I think would be good for a very select group of patients,” Dr. Needleman said.
But the preinfusion checklist and other potential ways to select low-risk patients for vernakalant could potentially backfire, warned John M. Mandrola, MD, Baptist Medical Associates, Louisville, Ky., from the panel.
The FDA representatives had presented evidence that the drug can seriously depress ventricular function, and that the lower cardiac output is what leads to hypotension, he elaborated in an interview after the meeting.
If the checklist is used to exclude hemodynamically unstable patients from receiving vernakalant, he said, “Then you’re really giving this drug to relatively healthy patients for convenience, to decrease hospitalization or the hospital stay.”
The signal for substantial harm, Dr. Mandrola said, has to be balanced against that modest benefit.
Moreover, those in whom the drug doesn’t work may be left in a worse situation, he proposed. Only about half of patients are successfully converted on vernakalant, the company and FDA data suggested. The other half of patients who don’t achieve sinus rhythm on the drug still must face the significant hazards of depressed ejection fraction and hypotension, a high price to pay for an unsuccessful treatment.
Dr. Mandrola is Chief Cardiology Correspondent for theheart.org | Medscape Cardiology; his disclosure statement states no relevant financial relationships.
This article first appeared on Medscape.com.
Cardiac arrhythmia heightens mortality risk during epilepsy hospitalizations
BALTIMORE – Patients hospitalized for epilepsy may have higher odds of death if they have a secondary diagnosis of arrhythmia, whereas the presence of apnea alone may not significantly increase mortality, according to an analysis of data from the Nationwide Inpatient Sample presented at the annual meeting of the American Epilepsy Society.
“If you have someone with arrhythmia and epilepsy, you have to be more concerned about possible SUDEP [sudden unexpected death in epilepsy],” relative to someone with apnea and epilepsy, said senior study author Sanjay P. Singh, MD, professor of neurology at Creighton University, Omaha, Neb.
Research indicates that apnea and cardiac arrhythmias may contribute to SUDEP, and the incidence of SUDEP is higher in patients with intractable epilepsy.
To identify the prevalence of apnea, arrhythmia, and both conditions in epilepsy hospitalizations, as well as the prevalence of intractable epilepsy and mortality, Dr. Singh and colleagues performed a retrospective, cross-sectional analysis of pediatric and adult epilepsy hospitalizations between 2003 and 2014 in the Nationwide Inpatient Sample. They determined apnea and arrhythmia diagnoses using ICD-9-CM codes.
Among more than 2.6 million epilepsy hospitalizations, the prevalence of apnea was 2.75%, the prevalence of arrhythmia was 8.91%, and the prevalence of both was 0.49%. The proportion of patients with intractable epilepsy was 7.7%. Among the more than 207,000 hospitalizations with intractable epilepsy, the prevalence of apnea was 3.62%, the prevalence of arrhythmia was 3.34%, and the prevalence of both was 0.36%. The prevalence trend of apnea, arrhythmia, and both together increased between 2003 and 2014.
“In univariate analysis, prevalence of mortality was highest among patients with arrhythmia,” the researchers reported, at – 3.1% in patients with arrhythmia versus 0.48% in patients with apnea, 2.91% in patients with both, and 0.46% in patients without apnea or arrhythmia.
In a multivariable regression analysis, significant and independent predictors of death included intractable epilepsy (odds ratio, 1.17), apnea (OR, 0.84), arrhythmia (OR, 3.29), and the presence of both apnea and arrhythmia (OR, 3.24). When hospitalization was complicated by intractable epilepsy, the odds of death rose with the presence of apnea (OR, 2.07), arrhythmia (OR, 8.39), and with both apnea and arrhythmia (OR, 11.64).
The results highlight the importance of effective epilepsy management, said first author Urvish K. Patel, MBBS, also with Creighton University. “If we can stop [conversion to intractable epilepsy], then this odds ratio can go down.”
Attention to arrhythmias, as well as the combination of arrhythmias and apnea, may “be important in identifying patients at risk for SUDEP,” the authors concluded.
The researchers had no disclosures and reported receiving no outside funding for their work.
SOURCE: Patel UK et al. AES 2019, Abstract 2.140.
BALTIMORE – Patients hospitalized for epilepsy may have higher odds of death if they have a secondary diagnosis of arrhythmia, whereas the presence of apnea alone may not significantly increase mortality, according to an analysis of data from the Nationwide Inpatient Sample presented at the annual meeting of the American Epilepsy Society.
“If you have someone with arrhythmia and epilepsy, you have to be more concerned about possible SUDEP [sudden unexpected death in epilepsy],” relative to someone with apnea and epilepsy, said senior study author Sanjay P. Singh, MD, professor of neurology at Creighton University, Omaha, Neb.
Research indicates that apnea and cardiac arrhythmias may contribute to SUDEP, and the incidence of SUDEP is higher in patients with intractable epilepsy.
To identify the prevalence of apnea, arrhythmia, and both conditions in epilepsy hospitalizations, as well as the prevalence of intractable epilepsy and mortality, Dr. Singh and colleagues performed a retrospective, cross-sectional analysis of pediatric and adult epilepsy hospitalizations between 2003 and 2014 in the Nationwide Inpatient Sample. They determined apnea and arrhythmia diagnoses using ICD-9-CM codes.
Among more than 2.6 million epilepsy hospitalizations, the prevalence of apnea was 2.75%, the prevalence of arrhythmia was 8.91%, and the prevalence of both was 0.49%. The proportion of patients with intractable epilepsy was 7.7%. Among the more than 207,000 hospitalizations with intractable epilepsy, the prevalence of apnea was 3.62%, the prevalence of arrhythmia was 3.34%, and the prevalence of both was 0.36%. The prevalence trend of apnea, arrhythmia, and both together increased between 2003 and 2014.
“In univariate analysis, prevalence of mortality was highest among patients with arrhythmia,” the researchers reported, at – 3.1% in patients with arrhythmia versus 0.48% in patients with apnea, 2.91% in patients with both, and 0.46% in patients without apnea or arrhythmia.
In a multivariable regression analysis, significant and independent predictors of death included intractable epilepsy (odds ratio, 1.17), apnea (OR, 0.84), arrhythmia (OR, 3.29), and the presence of both apnea and arrhythmia (OR, 3.24). When hospitalization was complicated by intractable epilepsy, the odds of death rose with the presence of apnea (OR, 2.07), arrhythmia (OR, 8.39), and with both apnea and arrhythmia (OR, 11.64).
The results highlight the importance of effective epilepsy management, said first author Urvish K. Patel, MBBS, also with Creighton University. “If we can stop [conversion to intractable epilepsy], then this odds ratio can go down.”
Attention to arrhythmias, as well as the combination of arrhythmias and apnea, may “be important in identifying patients at risk for SUDEP,” the authors concluded.
The researchers had no disclosures and reported receiving no outside funding for their work.
SOURCE: Patel UK et al. AES 2019, Abstract 2.140.
BALTIMORE – Patients hospitalized for epilepsy may have higher odds of death if they have a secondary diagnosis of arrhythmia, whereas the presence of apnea alone may not significantly increase mortality, according to an analysis of data from the Nationwide Inpatient Sample presented at the annual meeting of the American Epilepsy Society.
“If you have someone with arrhythmia and epilepsy, you have to be more concerned about possible SUDEP [sudden unexpected death in epilepsy],” relative to someone with apnea and epilepsy, said senior study author Sanjay P. Singh, MD, professor of neurology at Creighton University, Omaha, Neb.
Research indicates that apnea and cardiac arrhythmias may contribute to SUDEP, and the incidence of SUDEP is higher in patients with intractable epilepsy.
To identify the prevalence of apnea, arrhythmia, and both conditions in epilepsy hospitalizations, as well as the prevalence of intractable epilepsy and mortality, Dr. Singh and colleagues performed a retrospective, cross-sectional analysis of pediatric and adult epilepsy hospitalizations between 2003 and 2014 in the Nationwide Inpatient Sample. They determined apnea and arrhythmia diagnoses using ICD-9-CM codes.
Among more than 2.6 million epilepsy hospitalizations, the prevalence of apnea was 2.75%, the prevalence of arrhythmia was 8.91%, and the prevalence of both was 0.49%. The proportion of patients with intractable epilepsy was 7.7%. Among the more than 207,000 hospitalizations with intractable epilepsy, the prevalence of apnea was 3.62%, the prevalence of arrhythmia was 3.34%, and the prevalence of both was 0.36%. The prevalence trend of apnea, arrhythmia, and both together increased between 2003 and 2014.
“In univariate analysis, prevalence of mortality was highest among patients with arrhythmia,” the researchers reported, at – 3.1% in patients with arrhythmia versus 0.48% in patients with apnea, 2.91% in patients with both, and 0.46% in patients without apnea or arrhythmia.
In a multivariable regression analysis, significant and independent predictors of death included intractable epilepsy (odds ratio, 1.17), apnea (OR, 0.84), arrhythmia (OR, 3.29), and the presence of both apnea and arrhythmia (OR, 3.24). When hospitalization was complicated by intractable epilepsy, the odds of death rose with the presence of apnea (OR, 2.07), arrhythmia (OR, 8.39), and with both apnea and arrhythmia (OR, 11.64).
The results highlight the importance of effective epilepsy management, said first author Urvish K. Patel, MBBS, also with Creighton University. “If we can stop [conversion to intractable epilepsy], then this odds ratio can go down.”
Attention to arrhythmias, as well as the combination of arrhythmias and apnea, may “be important in identifying patients at risk for SUDEP,” the authors concluded.
The researchers had no disclosures and reported receiving no outside funding for their work.
SOURCE: Patel UK et al. AES 2019, Abstract 2.140.
REPORTING FROM AES 2019
Poll: Do you agree that hormonal contraception (OCPs, progesterone-only pills, the patch, vaginal rings, and DMPA) should be offered OTC?
[polldaddy:10476065]
[polldaddy:10476065]
[polldaddy:10476065]
Tofacitinib improves disease activity in patients with polyarticular-course JIA
ATLANTA – Treatment of polyarticular-course juvenile idiopathic arthritis with tofacitinib led to significantly fewer disease flares and greater improvement in disease activity than with placebo in a phase 3, multinational, randomized, double-blind, controlled withdrawal study presented at the annual meeting of the American College of Rheumatology.
Hermine I. Brunner, MD, director of the division of rheumatology at Cincinnati Children’s Hospital Medical Center, and colleagues conducted the study in 225 patients between 2 and less than 18 years old with polyarticular-course juvenile idiopathic arthritis (pJIA; n = 184), psoriatic arthritis (PsA; n = 20), or enthesitis-related arthritis (ERA; n = 21). Patients were included if they had an inadequate response or intolerance to a disease-modifying antirheumatic drug and active disease with five or more active joints in the case of pJIA and three or more active joints in PsA or ERA.
Dr. Brunner presented results only for pJIA patients; the results for PsA and ERA patients will be assessed and presented separately.
The researchers divided their study into two sections. In the open-label portion of the study, patients received twice-daily tofacitinib (Xeljanz) at a dose of 5 mg or a weight-based lower dose in patients under 40 kg for 18 weeks. A total of 173 patients met JIA ACR30 response criteria, defined as 30% or greater improvement in three of six JIA core set variables and worsening in no more than one of the core set variables, and then were randomized in part 2 of the study to continue the same dose of tofacitinib or receive placebo until 44 weeks. Dr. Brunner noted that most patients who discontinued treatment in parts 1 and 2 did so because of insufficient clinical response rather than from adverse events.
Disease flare occurrence between 18 and 44 weeks was measured as a primary endpoint, and key secondary endpoints included JIA ACR30/50/70 response and change in Childhood Health Assessment Questionnaire Disability Index (CHAQ-DI) scores from part 2 baseline. The researchers used a “gatekeeping approach” that sequenced outcome measures in their statistical analysis to control for false positives in primary and secondary outcomes, where statistical significance could be achieved only if the previous outcome in the sequence was statistically significant.
Patients had a median age of 13 years, and most were female, white (about 87%), and between one-third and one-half of patients were based in North America. JIA disease duration was a median of about 2.5 years, C-reactive protein was about 0.3 mg/dL, and median CHAQ-DI scores were about 0.9 across tofacitinib and placebo groups. Other baseline characteristics were balanced between the two groups, Dr. Brunner said.
“Patients with polyarticular-course JIA in the open-label study experienced a rapid improvement of their disease activity from very high to moderate within 18 weeks,” Dr. Brunner said in her presentation. “[T]ofacitinib demonstrated significantly greater efficacy versus placebo in patients with polyarticular-course JIA based on occurrence of fewer flares in part 2.”
Specifically, disease flare occurred in 29.2% of patients by 44 weeks in the tofacitinib group, compared with 52.9% of patients in the placebo group (P = .0031), for an overall 54% lower risk of flare among patients receiving tofacitinib (hazard ratio, 0.459; 95% confidence interval, 0.268-0.785; P = .0037). The response rate was higher for patients receiving tofacitinib at 44 weeks when measured by JIA ACR30 (70.8% vs. 47.1% with placebo; P = .0031) or by JIA ACR50 (66.7% vs. 47.1%; P = .0166) and JIA ACR70 criteria (54.2% vs. 37.1%; P = .0387). The change in CHAQ-DI score also improved at 44 weeks to a significantly greater extent in the tofacitinib group than with placebo (–0.09 vs. 0.03; P = .0292).
“The safety profile of tofacitinib in children with JIA was comparable to what you have seen or known in the [rheumatoid arthritis] population, and no new safety risks were identified in this pediatric population,” Dr. Brunner said.
The researchers reported ties with Pfizer, which funded the study, and more than two dozen other pharmaceutical companies.
SOURCE: Brunner HI et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L22.
ATLANTA – Treatment of polyarticular-course juvenile idiopathic arthritis with tofacitinib led to significantly fewer disease flares and greater improvement in disease activity than with placebo in a phase 3, multinational, randomized, double-blind, controlled withdrawal study presented at the annual meeting of the American College of Rheumatology.
Hermine I. Brunner, MD, director of the division of rheumatology at Cincinnati Children’s Hospital Medical Center, and colleagues conducted the study in 225 patients between 2 and less than 18 years old with polyarticular-course juvenile idiopathic arthritis (pJIA; n = 184), psoriatic arthritis (PsA; n = 20), or enthesitis-related arthritis (ERA; n = 21). Patients were included if they had an inadequate response or intolerance to a disease-modifying antirheumatic drug and active disease with five or more active joints in the case of pJIA and three or more active joints in PsA or ERA.
Dr. Brunner presented results only for pJIA patients; the results for PsA and ERA patients will be assessed and presented separately.
The researchers divided their study into two sections. In the open-label portion of the study, patients received twice-daily tofacitinib (Xeljanz) at a dose of 5 mg or a weight-based lower dose in patients under 40 kg for 18 weeks. A total of 173 patients met JIA ACR30 response criteria, defined as 30% or greater improvement in three of six JIA core set variables and worsening in no more than one of the core set variables, and then were randomized in part 2 of the study to continue the same dose of tofacitinib or receive placebo until 44 weeks. Dr. Brunner noted that most patients who discontinued treatment in parts 1 and 2 did so because of insufficient clinical response rather than from adverse events.
Disease flare occurrence between 18 and 44 weeks was measured as a primary endpoint, and key secondary endpoints included JIA ACR30/50/70 response and change in Childhood Health Assessment Questionnaire Disability Index (CHAQ-DI) scores from part 2 baseline. The researchers used a “gatekeeping approach” that sequenced outcome measures in their statistical analysis to control for false positives in primary and secondary outcomes, where statistical significance could be achieved only if the previous outcome in the sequence was statistically significant.
Patients had a median age of 13 years, and most were female, white (about 87%), and between one-third and one-half of patients were based in North America. JIA disease duration was a median of about 2.5 years, C-reactive protein was about 0.3 mg/dL, and median CHAQ-DI scores were about 0.9 across tofacitinib and placebo groups. Other baseline characteristics were balanced between the two groups, Dr. Brunner said.
“Patients with polyarticular-course JIA in the open-label study experienced a rapid improvement of their disease activity from very high to moderate within 18 weeks,” Dr. Brunner said in her presentation. “[T]ofacitinib demonstrated significantly greater efficacy versus placebo in patients with polyarticular-course JIA based on occurrence of fewer flares in part 2.”
Specifically, disease flare occurred in 29.2% of patients by 44 weeks in the tofacitinib group, compared with 52.9% of patients in the placebo group (P = .0031), for an overall 54% lower risk of flare among patients receiving tofacitinib (hazard ratio, 0.459; 95% confidence interval, 0.268-0.785; P = .0037). The response rate was higher for patients receiving tofacitinib at 44 weeks when measured by JIA ACR30 (70.8% vs. 47.1% with placebo; P = .0031) or by JIA ACR50 (66.7% vs. 47.1%; P = .0166) and JIA ACR70 criteria (54.2% vs. 37.1%; P = .0387). The change in CHAQ-DI score also improved at 44 weeks to a significantly greater extent in the tofacitinib group than with placebo (–0.09 vs. 0.03; P = .0292).
“The safety profile of tofacitinib in children with JIA was comparable to what you have seen or known in the [rheumatoid arthritis] population, and no new safety risks were identified in this pediatric population,” Dr. Brunner said.
The researchers reported ties with Pfizer, which funded the study, and more than two dozen other pharmaceutical companies.
SOURCE: Brunner HI et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L22.
ATLANTA – Treatment of polyarticular-course juvenile idiopathic arthritis with tofacitinib led to significantly fewer disease flares and greater improvement in disease activity than with placebo in a phase 3, multinational, randomized, double-blind, controlled withdrawal study presented at the annual meeting of the American College of Rheumatology.
Hermine I. Brunner, MD, director of the division of rheumatology at Cincinnati Children’s Hospital Medical Center, and colleagues conducted the study in 225 patients between 2 and less than 18 years old with polyarticular-course juvenile idiopathic arthritis (pJIA; n = 184), psoriatic arthritis (PsA; n = 20), or enthesitis-related arthritis (ERA; n = 21). Patients were included if they had an inadequate response or intolerance to a disease-modifying antirheumatic drug and active disease with five or more active joints in the case of pJIA and three or more active joints in PsA or ERA.
Dr. Brunner presented results only for pJIA patients; the results for PsA and ERA patients will be assessed and presented separately.
The researchers divided their study into two sections. In the open-label portion of the study, patients received twice-daily tofacitinib (Xeljanz) at a dose of 5 mg or a weight-based lower dose in patients under 40 kg for 18 weeks. A total of 173 patients met JIA ACR30 response criteria, defined as 30% or greater improvement in three of six JIA core set variables and worsening in no more than one of the core set variables, and then were randomized in part 2 of the study to continue the same dose of tofacitinib or receive placebo until 44 weeks. Dr. Brunner noted that most patients who discontinued treatment in parts 1 and 2 did so because of insufficient clinical response rather than from adverse events.
Disease flare occurrence between 18 and 44 weeks was measured as a primary endpoint, and key secondary endpoints included JIA ACR30/50/70 response and change in Childhood Health Assessment Questionnaire Disability Index (CHAQ-DI) scores from part 2 baseline. The researchers used a “gatekeeping approach” that sequenced outcome measures in their statistical analysis to control for false positives in primary and secondary outcomes, where statistical significance could be achieved only if the previous outcome in the sequence was statistically significant.
Patients had a median age of 13 years, and most were female, white (about 87%), and between one-third and one-half of patients were based in North America. JIA disease duration was a median of about 2.5 years, C-reactive protein was about 0.3 mg/dL, and median CHAQ-DI scores were about 0.9 across tofacitinib and placebo groups. Other baseline characteristics were balanced between the two groups, Dr. Brunner said.
“Patients with polyarticular-course JIA in the open-label study experienced a rapid improvement of their disease activity from very high to moderate within 18 weeks,” Dr. Brunner said in her presentation. “[T]ofacitinib demonstrated significantly greater efficacy versus placebo in patients with polyarticular-course JIA based on occurrence of fewer flares in part 2.”
Specifically, disease flare occurred in 29.2% of patients by 44 weeks in the tofacitinib group, compared with 52.9% of patients in the placebo group (P = .0031), for an overall 54% lower risk of flare among patients receiving tofacitinib (hazard ratio, 0.459; 95% confidence interval, 0.268-0.785; P = .0037). The response rate was higher for patients receiving tofacitinib at 44 weeks when measured by JIA ACR30 (70.8% vs. 47.1% with placebo; P = .0031) or by JIA ACR50 (66.7% vs. 47.1%; P = .0166) and JIA ACR70 criteria (54.2% vs. 37.1%; P = .0387). The change in CHAQ-DI score also improved at 44 weeks to a significantly greater extent in the tofacitinib group than with placebo (–0.09 vs. 0.03; P = .0292).
“The safety profile of tofacitinib in children with JIA was comparable to what you have seen or known in the [rheumatoid arthritis] population, and no new safety risks were identified in this pediatric population,” Dr. Brunner said.
The researchers reported ties with Pfizer, which funded the study, and more than two dozen other pharmaceutical companies.
SOURCE: Brunner HI et al. Arthritis Rheumatol. 2019;71(suppl 10), Abstract L22.
REPORTING FROM ACR 2019
Brains of MDD patients show alterations in areas tied to positive emotions
The brains of medication-naive patients with major depressive disorder (MDD) exhibit interhemispheric structural imbalances in areas that regulate positive emotional processes, results of a small cross-sectional study suggest.
“To the best of our knowledge, asymmetric alterations in cortical thickness and subcortical volume in patients with MDD have not yet been reported,” wrote Zhiwei Zuo, of the department of radiology, Southwest Hospital, Army Medical University, Chongqing, China, and associates. “A comprehensive understanding of the cerebral pathophysiology changes in depression is essential and may lead to more targeted approaches for the prevention and treatment of MDD.”
The investigators enrolled 35 medication-naive, untreated patients with MDD from the hospital’s department of psychology, and 35 age-, gender-, and education-matched controls. Using whole-brain analysis, the investigators identified asymmetry in cortical thickness and subcortical volume that was mostly present in the cortical-striatal-pallidal-thalamic circuit. This part of the brain helps translate underlying positive affect processes into conscious feelings of pleasure, the authors reported. in recent years (Clin Psychol Psychother. 2012 Jul-Aug;19[4]:326-40). The current study results were published in NeuroImage: Clinical.
Some limitations of the study include its small sample size and its cross-sectional nature. Nevertheless, they said, the findings could provide possible targets for therapeutic monitoring of the illness.
“These alterations were independent of depressive symptom severity, suggesting that cerebral asymmetry could be an appropriate indicator of morphological variations in mental disease,” the investigators noted.
The study was funded by the National Nature Science Foundation of China, the National Key Research and Development Plan of China, and the Innovative Talents Project of Southwest Hospital. The authors had no conflicts of interest to disclose.
SOURCE: Zuo Z et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2018.101614.
The brains of medication-naive patients with major depressive disorder (MDD) exhibit interhemispheric structural imbalances in areas that regulate positive emotional processes, results of a small cross-sectional study suggest.
“To the best of our knowledge, asymmetric alterations in cortical thickness and subcortical volume in patients with MDD have not yet been reported,” wrote Zhiwei Zuo, of the department of radiology, Southwest Hospital, Army Medical University, Chongqing, China, and associates. “A comprehensive understanding of the cerebral pathophysiology changes in depression is essential and may lead to more targeted approaches for the prevention and treatment of MDD.”
The investigators enrolled 35 medication-naive, untreated patients with MDD from the hospital’s department of psychology, and 35 age-, gender-, and education-matched controls. Using whole-brain analysis, the investigators identified asymmetry in cortical thickness and subcortical volume that was mostly present in the cortical-striatal-pallidal-thalamic circuit. This part of the brain helps translate underlying positive affect processes into conscious feelings of pleasure, the authors reported. in recent years (Clin Psychol Psychother. 2012 Jul-Aug;19[4]:326-40). The current study results were published in NeuroImage: Clinical.
Some limitations of the study include its small sample size and its cross-sectional nature. Nevertheless, they said, the findings could provide possible targets for therapeutic monitoring of the illness.
“These alterations were independent of depressive symptom severity, suggesting that cerebral asymmetry could be an appropriate indicator of morphological variations in mental disease,” the investigators noted.
The study was funded by the National Nature Science Foundation of China, the National Key Research and Development Plan of China, and the Innovative Talents Project of Southwest Hospital. The authors had no conflicts of interest to disclose.
SOURCE: Zuo Z et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2018.101614.
The brains of medication-naive patients with major depressive disorder (MDD) exhibit interhemispheric structural imbalances in areas that regulate positive emotional processes, results of a small cross-sectional study suggest.
“To the best of our knowledge, asymmetric alterations in cortical thickness and subcortical volume in patients with MDD have not yet been reported,” wrote Zhiwei Zuo, of the department of radiology, Southwest Hospital, Army Medical University, Chongqing, China, and associates. “A comprehensive understanding of the cerebral pathophysiology changes in depression is essential and may lead to more targeted approaches for the prevention and treatment of MDD.”
The investigators enrolled 35 medication-naive, untreated patients with MDD from the hospital’s department of psychology, and 35 age-, gender-, and education-matched controls. Using whole-brain analysis, the investigators identified asymmetry in cortical thickness and subcortical volume that was mostly present in the cortical-striatal-pallidal-thalamic circuit. This part of the brain helps translate underlying positive affect processes into conscious feelings of pleasure, the authors reported. in recent years (Clin Psychol Psychother. 2012 Jul-Aug;19[4]:326-40). The current study results were published in NeuroImage: Clinical.
Some limitations of the study include its small sample size and its cross-sectional nature. Nevertheless, they said, the findings could provide possible targets for therapeutic monitoring of the illness.
“These alterations were independent of depressive symptom severity, suggesting that cerebral asymmetry could be an appropriate indicator of morphological variations in mental disease,” the investigators noted.
The study was funded by the National Nature Science Foundation of China, the National Key Research and Development Plan of China, and the Innovative Talents Project of Southwest Hospital. The authors had no conflicts of interest to disclose.
SOURCE: Zuo Z et al. Neuroimage Clin. 2019. doi: 10.1016/j.nicl.2018.101614.
FROM NEUROIMAGE: CLINICAL
FDA investigates NDMA contamination in metformin
This follows reports of low-level NDMA contamination of metformin in other countries and of a few regulatory agencies issuing recalls for the drug, according to a statement from Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
“There are no metformin recalls affecting the U.S. market at this time,” the agency emphasized in the statement. It said NDMA levels in affected medication have been low, at or even below the acceptable intake limit, and there is currently no evidence indicating that metformin drugs within the United States or European Union have been contaminated.
The FDA advised that patients should continue taking metformin alone or in combination with other drugs to control their diabetes and that it would be dangerous for them to stop taking the medication without first discussing it with their providers. It also recommended that providers continue to use metformin when “clinically appropriate” while the investigation is underway as there are no alternative therapies to treat the disease in the same way.
NDMA is a common contaminant that is found in water and some foods and has probable carcinogenic effects when exposure is too high. The acceptable daily intake for NDMA in the United States is 96 ng/day, according to the statement, though people who take in that amount or less every day for 70 years are not expected to have an increased risk of cancer.
Both the FDA and its counterpart, the European Medicines Agency, have recently investigated the presence of NDMA impurities in ranitidine, a drug used to reduce production of stomach acid, which led to several manufacturers issuing recalls for it.
The agencies have also investigated angiotensin II receptor blockers, which are used to treat hypertension, heart failure, and high blood pressure.
The presence of NDMA “can be related to the drug’s manufacturing process or its chemical structure or even the conditions in which they are stored or packaged. As food and drugs are processed in the body, nitrosamines, including NDMA, can be formed,” Dr. Woodcock noted in the statement.
“We are monitoring this issue closely to assess any potential impact on patients with diabetes,” said Robert W. Lash, MD, chief professional and clinical affairs officer of the Endocrine Society. “We have members around the world and are concerned about the possibility of carcinogenic impurities in medications, both in the United States and elsewhere.”
This follows reports of low-level NDMA contamination of metformin in other countries and of a few regulatory agencies issuing recalls for the drug, according to a statement from Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
“There are no metformin recalls affecting the U.S. market at this time,” the agency emphasized in the statement. It said NDMA levels in affected medication have been low, at or even below the acceptable intake limit, and there is currently no evidence indicating that metformin drugs within the United States or European Union have been contaminated.
The FDA advised that patients should continue taking metformin alone or in combination with other drugs to control their diabetes and that it would be dangerous for them to stop taking the medication without first discussing it with their providers. It also recommended that providers continue to use metformin when “clinically appropriate” while the investigation is underway as there are no alternative therapies to treat the disease in the same way.
NDMA is a common contaminant that is found in water and some foods and has probable carcinogenic effects when exposure is too high. The acceptable daily intake for NDMA in the United States is 96 ng/day, according to the statement, though people who take in that amount or less every day for 70 years are not expected to have an increased risk of cancer.
Both the FDA and its counterpart, the European Medicines Agency, have recently investigated the presence of NDMA impurities in ranitidine, a drug used to reduce production of stomach acid, which led to several manufacturers issuing recalls for it.
The agencies have also investigated angiotensin II receptor blockers, which are used to treat hypertension, heart failure, and high blood pressure.
The presence of NDMA “can be related to the drug’s manufacturing process or its chemical structure or even the conditions in which they are stored or packaged. As food and drugs are processed in the body, nitrosamines, including NDMA, can be formed,” Dr. Woodcock noted in the statement.
“We are monitoring this issue closely to assess any potential impact on patients with diabetes,” said Robert W. Lash, MD, chief professional and clinical affairs officer of the Endocrine Society. “We have members around the world and are concerned about the possibility of carcinogenic impurities in medications, both in the United States and elsewhere.”
This follows reports of low-level NDMA contamination of metformin in other countries and of a few regulatory agencies issuing recalls for the drug, according to a statement from Janet Woodcock, MD, director of the FDA’s Center for Drug Evaluation and Research.
“There are no metformin recalls affecting the U.S. market at this time,” the agency emphasized in the statement. It said NDMA levels in affected medication have been low, at or even below the acceptable intake limit, and there is currently no evidence indicating that metformin drugs within the United States or European Union have been contaminated.
The FDA advised that patients should continue taking metformin alone or in combination with other drugs to control their diabetes and that it would be dangerous for them to stop taking the medication without first discussing it with their providers. It also recommended that providers continue to use metformin when “clinically appropriate” while the investigation is underway as there are no alternative therapies to treat the disease in the same way.
NDMA is a common contaminant that is found in water and some foods and has probable carcinogenic effects when exposure is too high. The acceptable daily intake for NDMA in the United States is 96 ng/day, according to the statement, though people who take in that amount or less every day for 70 years are not expected to have an increased risk of cancer.
Both the FDA and its counterpart, the European Medicines Agency, have recently investigated the presence of NDMA impurities in ranitidine, a drug used to reduce production of stomach acid, which led to several manufacturers issuing recalls for it.
The agencies have also investigated angiotensin II receptor blockers, which are used to treat hypertension, heart failure, and high blood pressure.
The presence of NDMA “can be related to the drug’s manufacturing process or its chemical structure or even the conditions in which they are stored or packaged. As food and drugs are processed in the body, nitrosamines, including NDMA, can be formed,” Dr. Woodcock noted in the statement.
“We are monitoring this issue closely to assess any potential impact on patients with diabetes,” said Robert W. Lash, MD, chief professional and clinical affairs officer of the Endocrine Society. “We have members around the world and are concerned about the possibility of carcinogenic impurities in medications, both in the United States and elsewhere.”