User login
National Rapid Genome Testing Program Benefits NICU Care
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
A national study in Israel demonstrates the feasibility and diagnostic benefits of rapid trio genome sequencing in critically ill neonates.
METHODOLOGY:
- Researchers conducted a prospective, multicenter cohort study from October 2021 to December 2022, involving all Israeli medical genetics institutes and neonatal intensive care units.
- A total of 130 critically ill neonates suspected of having a genetic disorder were enrolled, with rapid genome sequencing results expected within 10 days.
TAKEAWAY:
- Rapid trio genome sequencing diagnosed 50% of the neonates with disease-causing variants, including 12 chromosomal and 52 monogenic conditions.
- Another 11% had variants of unknown significance that were suspected to be disease-causing, and 1% had a novel gene suspected of causing disease.
- The mean turnaround time for the rapid reports was 7 days, demonstrating the feasibility of implementing rapid genome sequencing in a national healthcare setting, the researchers said.
- Genomic testing led to a change in clinical management for 22% of the neonates, which shows the clinical utility of this approach to diagnosis, they said.
IN PRACTICE:
Genetic testing may identify patients who are candidates for precision medical treatment and inform family planning, which is “critical for families with a severely affected or deceased child,” the study authors wrote.
SOURCE:
The corresponding author for the study was Daphna Marom, MD, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. It was published online on February 22, 2024, in JAMA Network Open.
LIMITATIONS:
The study’s reliance on voluntary participation may have introduced referral bias, potentially affecting the diagnostic rates. The long-term impact of diagnosis on survival, growth, and development remains to be evaluated. Bioinformatics tools have limitations, as shown by the missed detection of maternal uniparental disomy in one case of a hypotonic infant with Prader-Willi syndrome, the researchers noted. Clinical judgment is still essential, they said.
DISCLOSURES:
The study was sponsored by a collaboration between the Israeli Ministry of Health, Illumina, and the Genomics Center at the Tel Aviv Sourasky Medical Center. Illumina provided reagents, bioinformatics tools, and editorial assistance. Study authors disclosed financial ties to Illumina.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Dupilumab Earns FDA Priority Review for Add-On COPD Care
The Food and Drug Administration (FDA) has accepted an application for Priority Review for dupilumab as an add-on therapy for adults with uncontrolled chronic obstructive pulmonary disease (COPD), according to a press release from manufacturer Regeneron.
If approved, dupilumab would be the only biologic option for COPD and the first new treatment option in approximately 10 years, according to the company.
Dupilumab works by blocking signaling by the interleukin (IL) 4 and IL-13 pathways, and Regeneron’s development program focuses on a population of COPD patients who also have type 2 inflammation.
The supplemental Biologics License Application was based on data from a pair of clinical trials in the company’s phase 3 COPD clinical research program.
In the studies, known as BOREAS and NOTUS, adults with uncontrolled COPD and type 2 inflammation who were current or former smokers were randomized to 300 mg of subcutaneous dupilumab or placebo once every 2 weeks. Type 2 inflammation was defined as blood eosinophil counts of at least 300 cells per microliter.
All patients received standard-of-care therapy. The primary endpoint of reduced annualized moderate or severe acute COPD exacerbations was 30% and 34% greater in the dupilumab groups in the two studies, respectively, compared with the placebo groups, and the significant differences in improvement persisted at 52 weeks.
Safety data were similar to previous studies of dupilumab for its approved indications. The most common adverse events seen in 5% or more of dupilumab patients compared with placebo patients across the two studies included back pain, COVID-19, diarrhea, headache, and nasopharyngitis.
Priority Review status is granted to applications for approval for therapies that may offer significant improvements, although the therapies are still in clinical development. The target action date for the FDA decision is June 27, 2024, and regulatory submissions for dupilumab for COPD are under consideration in China and Europe in addition to the United States, according to the company.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has accepted an application for Priority Review for dupilumab as an add-on therapy for adults with uncontrolled chronic obstructive pulmonary disease (COPD), according to a press release from manufacturer Regeneron.
If approved, dupilumab would be the only biologic option for COPD and the first new treatment option in approximately 10 years, according to the company.
Dupilumab works by blocking signaling by the interleukin (IL) 4 and IL-13 pathways, and Regeneron’s development program focuses on a population of COPD patients who also have type 2 inflammation.
The supplemental Biologics License Application was based on data from a pair of clinical trials in the company’s phase 3 COPD clinical research program.
In the studies, known as BOREAS and NOTUS, adults with uncontrolled COPD and type 2 inflammation who were current or former smokers were randomized to 300 mg of subcutaneous dupilumab or placebo once every 2 weeks. Type 2 inflammation was defined as blood eosinophil counts of at least 300 cells per microliter.
All patients received standard-of-care therapy. The primary endpoint of reduced annualized moderate or severe acute COPD exacerbations was 30% and 34% greater in the dupilumab groups in the two studies, respectively, compared with the placebo groups, and the significant differences in improvement persisted at 52 weeks.
Safety data were similar to previous studies of dupilumab for its approved indications. The most common adverse events seen in 5% or more of dupilumab patients compared with placebo patients across the two studies included back pain, COVID-19, diarrhea, headache, and nasopharyngitis.
Priority Review status is granted to applications for approval for therapies that may offer significant improvements, although the therapies are still in clinical development. The target action date for the FDA decision is June 27, 2024, and regulatory submissions for dupilumab for COPD are under consideration in China and Europe in addition to the United States, according to the company.
A version of this article appeared on Medscape.com.
The Food and Drug Administration (FDA) has accepted an application for Priority Review for dupilumab as an add-on therapy for adults with uncontrolled chronic obstructive pulmonary disease (COPD), according to a press release from manufacturer Regeneron.
If approved, dupilumab would be the only biologic option for COPD and the first new treatment option in approximately 10 years, according to the company.
Dupilumab works by blocking signaling by the interleukin (IL) 4 and IL-13 pathways, and Regeneron’s development program focuses on a population of COPD patients who also have type 2 inflammation.
The supplemental Biologics License Application was based on data from a pair of clinical trials in the company’s phase 3 COPD clinical research program.
In the studies, known as BOREAS and NOTUS, adults with uncontrolled COPD and type 2 inflammation who were current or former smokers were randomized to 300 mg of subcutaneous dupilumab or placebo once every 2 weeks. Type 2 inflammation was defined as blood eosinophil counts of at least 300 cells per microliter.
All patients received standard-of-care therapy. The primary endpoint of reduced annualized moderate or severe acute COPD exacerbations was 30% and 34% greater in the dupilumab groups in the two studies, respectively, compared with the placebo groups, and the significant differences in improvement persisted at 52 weeks.
Safety data were similar to previous studies of dupilumab for its approved indications. The most common adverse events seen in 5% or more of dupilumab patients compared with placebo patients across the two studies included back pain, COVID-19, diarrhea, headache, and nasopharyngitis.
Priority Review status is granted to applications for approval for therapies that may offer significant improvements, although the therapies are still in clinical development. The target action date for the FDA decision is June 27, 2024, and regulatory submissions for dupilumab for COPD are under consideration in China and Europe in addition to the United States, according to the company.
A version of this article appeared on Medscape.com.
FDA Clears Medical Grade Over-the-Counter Pulse Oximeter
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
The MightySat Medical, an over-the-counter medical fingertip pulse oximeter, has received clearance from the US Food and Drug Administration (FDA) for use without a prescription, according to a press release from manufacturer Masimo.
The device is the first medical fingertip pulse oximeter available directly to consumers without a prescription that includes the same technology used by many hospitals, according to the company.
According to the FDA, home pulse oximeters are currently generally of two classes: hospital-grade prescription devices which have been vetted for accuracy through clinical trials, and over-the-counter devices which are sold direct to consumers but often estimate oxygen saturation. FDA communication on pulse oximeter accuracy states "OTC oximeters that are sold as either general wellness or sporting/aviation products are not intended for medical purposes, so they do not undergo FDA review."
Pulse oximeter use is important for patients diagnosed with breathing problems or lung diseases such as asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, lung cancer, flu, pneumonia, or COVID-19 to collect accurate data on arterial blood oxygen saturation that they can share with their healthcare providers, according to the company. Patients with cardiac conditions, including pulmonary hypertension and heart failure may also benefit from pulse oximeter monitoring.
However, challenges of pulse oximeter use include measuring accuracy when patients are moving, measuring patients with poor circulation, and measuring patients with cool, thick, or darker skin. The MightySat Medical is designed to provide reliable measures of oxygen saturation and pulse rate across all patient groups, the manufacturers wrote in the press release.
Asked for additional comment, Diego J. Maselli, MD, FCCP, Professor and Chief in the division of Pulmonary Diseases and Critical Care at UT Health at San Antonio, noted, "Over the past decades, there has been an increased interest in home monitoring of medical conditions, particulrly with the development of more portable and accessible technology."
"This was heightended by the COVID-19 pandemic where telemedicine was frequently required as a means of delivering care," Dr. Maselli continued. "One of the important characteristics to monitor was the oxgen saturation in patients that had an active COVID-19 infection as it would dictate management and was part of the protocol for monitoring the clinical course of infection. Because of this need, many companies developed portable pulse oximeters for home use. This resulted in widespread use of pulse oximeters at home and other places outside clinic or hospital."
Other over-the-counter pulse oximeters that are not cleared by the FDA may create confusion among patients about the accuracy of their measurements, according to the company.
Dr. Maselli also commented that pulse oximeters' value can vary. "Unfortunately, these devices vary in quality and reliability and patients may not be fully aware of this. Most recently, the FDA approved a hospital-grade pulse oximeter that requires no prescription. This device may provide a more accurate reading in a wide range of clinical situations outside the healthcare setting. Patients should be aware that there are different grades of pulse oximeter before selecting one for home use. In addition, patients should work closely with their providers to better select the monitoring modaility that best fits their clinical situation," he said.
MightySat Medical is indicated for individuals aged 18 years and older who are well or poorly perfused under no motion conditions and is not intended as a diagnostic or screening tool for lung disease, according to the release. Treatment decisions based on data from the device should be made only in consultation with a healthcare provider, the company said. Dr. Maselli serves as a member of the CHEST Physician editorial board.
The FDA’s website offers further guidance related to at-home pulse oximeter use, with recommendations and limitations, as well as information on initiatives to ensure accurate and equitable pulse oximetry for all patients.
A version of this article appeared on Medscape.com.
Florida’s Stance on Measles Upends Expert Guidance
Amid an ongoing measles outbreak in Florida possibly sparked by vaccine hesitancy, the state’s surgeon general Joseph Ladapo, MD, is contradicting public health guidance of encouraging quarantine of unvaccinated children.
Rather than requesting that parents keep children unvaccinated against measles home from school or to get their children vaccinated, both critical tools in containing an outbreak, Dr. Ladapo has advised parents to do whatever they think is best. Pediatricians and infectious disease specialists fear a free-for-all will fuel the spread of the highly infectious virus, including in their own clinics.
The outbreak has been traced to an elementary school in Weston and has so far sickened at least eight children, one of whom is younger than 5 years. According to the Centers for Disease Control and Prevention, roughly 91% of the 230,000-odd kindergarteners in Florida had received the requisite doses of the MMR vaccine, which also protects against mumps and rubella, for the 2022-2023 school year, below the 95% vaccination level which public health authorities believes confers herd immunity against measles. An estimated 4.5% of kindergarteners in the state have received an exemption for the vaccine, which prevents measles in 97% of the people who get the shots, for a lifetime. The first dose is given around age 13 months and the second when people are age 4 or 5 years and soon to enter school.
“If you’re vaccinated you have a very slim chance of getting the virus,” said Rana Alissa, MD, a pediatrician at University of Florida Health in Jacksonville.
An unvaccinated child has no protection against measles, and could spread it to others merely by sneezing or touching a surface. In a school setting, infection could spread to a teacher who cannot receive the measles vaccine due to a weakened immune system, or the unvaccinated child could spread the virus at a pediatric clinic or hospital when seeking care for measles unless the clinic staff takes rigorous steps to separate the child from other children. Some children at the clinic won’t be able to get the measles vaccine either because of immunodeficiency or perhaps having had a bone marrow transplant.
Assuming the unvaccinated child is healthy, the measles infection will run its course, and the child will then be immune to the disease, Dr. Alissa said. But meanwhile, the child could pose a significant risk to others.
“We’re not worried about the unvaccinated kids who are very healthy. We’re worried about the adults who did not get vaccinated and who are very sick,” said Dr. Alissa, vice president of the Florida chapter of the American Academy of Pediatrics (AAP). “We’re worried about the little kids who are less than 13 months old. We’re worried about the kids with immunodeficiency disorders.” The Florida chapter of the AAP encourages parents to get their children vaccinated against measles amid the ongoing outbreak.
“I wish our surgeon general was on the same page as us,” Dr. Alissa added, noting that she thinks misplaced vaccine hesitancy has caused some parents to forego a safe and effective vaccine for their children.
Never Too Late to Vaxx
Measles symptoms appear 10-14 days after exposure and can include sore throat, cough, runny nose, inflamed eyes, fever, and blotchy skin rashes. According to the Centers for Disease Control and Prevention (CDC), 20% of people who are unvaccinated against measles will be hospitalized for the virus if they contract it.
Given the incubation period for the virus, clinicians and public health officials recommend unvaccinated children isolate for 21 days after being exposed to measles at school. The advice applies to any unvaccinated child, whether because their parent opted against the vaccine or because they cannot safely receive the immunization.
This is the guidance that Surgeon General Ladapo is flouting.
“We have a public health system. They’re awesome. They’re the experts. Let’s use them,” Dr. Alissa noted. “Their recommendation is to keep the unvaccinated kids at home for 21 days when you have an outbreak.”
“We’re not calling him doctor anymore,” said Andrew Pavia, MD, chief of the Division of Pediatric Infectious Diseases at the University of Utah in Salt Lake City.
“Getting your kids immunized before they enter school is so critical,” added Dr. Pavia, because the 21-day quarantine period is onerous for children and parents alike.
In a February 26 statement, Marcus Plescia, MD, MPH, chief medical officer of the Association of State and Territorial Health Officials, said “well-established public health practice recommends that unvaccinated persons exposed to measles stay home for at least 21 days to prevent further growth of the outbreak. While this is undoubtedly disruptive to the persons impacted, imagine how much more disruptive it would be if measles takes hold again in the United States, spreading widely, and impacting children and communities across the entire nation.”
During an outbreak, it’s still possible to give a measles vaccine to a child who has not yet received the shots, Dr. Pavia stressed. But time is of the essence: Vaccination should occur within 72 hours of the first known measles case in a school.
“It’s not perfect, they may still get measles, but it will greatly decrease the severity,” Dr. Pavia said.
If some children won’t get vaccinated during an outbreak, their parents may call a pediatrician or hospital staff for help as measles symptoms take hold. Clinicians should advise everyone in the home who is older than 2 years to begin wearing N95 masks and gloves, Dr. Alissa said. And when the child comes into the clinic he or she should be examined in a separate room, ideally one with negative air pressure and frequent filtration, Dr. Alissa added. If not, any private room will do if nobody else uses the room for at least 2 hours afterward.
“Measles is phenomenally transmissible,” Dr. Pavia said. A person with the virus can infect 12 to 18 others who are not protected against the pathogen.
Someone with a severe reaction to measles could get an injection of intramuscular immunoglobulin, Dr. Pavia said, although this tends to be uncomfortable and expensive.
“The vaccine works. We almost got rid of measles,” Dr. Alissa said, although parents who choose to send their unvaccinated children to school can do so if they choose to.
“The fear of every pediatrician is to have a child die from this,” she said. “People who are sick, please stay at home.”
Dr. Pavia reported an advisory relationship with Sanofi Pasteur regarding an RSV vaccine. Dr. Alissa reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
Amid an ongoing measles outbreak in Florida possibly sparked by vaccine hesitancy, the state’s surgeon general Joseph Ladapo, MD, is contradicting public health guidance of encouraging quarantine of unvaccinated children.
Rather than requesting that parents keep children unvaccinated against measles home from school or to get their children vaccinated, both critical tools in containing an outbreak, Dr. Ladapo has advised parents to do whatever they think is best. Pediatricians and infectious disease specialists fear a free-for-all will fuel the spread of the highly infectious virus, including in their own clinics.
The outbreak has been traced to an elementary school in Weston and has so far sickened at least eight children, one of whom is younger than 5 years. According to the Centers for Disease Control and Prevention, roughly 91% of the 230,000-odd kindergarteners in Florida had received the requisite doses of the MMR vaccine, which also protects against mumps and rubella, for the 2022-2023 school year, below the 95% vaccination level which public health authorities believes confers herd immunity against measles. An estimated 4.5% of kindergarteners in the state have received an exemption for the vaccine, which prevents measles in 97% of the people who get the shots, for a lifetime. The first dose is given around age 13 months and the second when people are age 4 or 5 years and soon to enter school.
“If you’re vaccinated you have a very slim chance of getting the virus,” said Rana Alissa, MD, a pediatrician at University of Florida Health in Jacksonville.
An unvaccinated child has no protection against measles, and could spread it to others merely by sneezing or touching a surface. In a school setting, infection could spread to a teacher who cannot receive the measles vaccine due to a weakened immune system, or the unvaccinated child could spread the virus at a pediatric clinic or hospital when seeking care for measles unless the clinic staff takes rigorous steps to separate the child from other children. Some children at the clinic won’t be able to get the measles vaccine either because of immunodeficiency or perhaps having had a bone marrow transplant.
Assuming the unvaccinated child is healthy, the measles infection will run its course, and the child will then be immune to the disease, Dr. Alissa said. But meanwhile, the child could pose a significant risk to others.
“We’re not worried about the unvaccinated kids who are very healthy. We’re worried about the adults who did not get vaccinated and who are very sick,” said Dr. Alissa, vice president of the Florida chapter of the American Academy of Pediatrics (AAP). “We’re worried about the little kids who are less than 13 months old. We’re worried about the kids with immunodeficiency disorders.” The Florida chapter of the AAP encourages parents to get their children vaccinated against measles amid the ongoing outbreak.
“I wish our surgeon general was on the same page as us,” Dr. Alissa added, noting that she thinks misplaced vaccine hesitancy has caused some parents to forego a safe and effective vaccine for their children.
Never Too Late to Vaxx
Measles symptoms appear 10-14 days after exposure and can include sore throat, cough, runny nose, inflamed eyes, fever, and blotchy skin rashes. According to the Centers for Disease Control and Prevention (CDC), 20% of people who are unvaccinated against measles will be hospitalized for the virus if they contract it.
Given the incubation period for the virus, clinicians and public health officials recommend unvaccinated children isolate for 21 days after being exposed to measles at school. The advice applies to any unvaccinated child, whether because their parent opted against the vaccine or because they cannot safely receive the immunization.
This is the guidance that Surgeon General Ladapo is flouting.
“We have a public health system. They’re awesome. They’re the experts. Let’s use them,” Dr. Alissa noted. “Their recommendation is to keep the unvaccinated kids at home for 21 days when you have an outbreak.”
“We’re not calling him doctor anymore,” said Andrew Pavia, MD, chief of the Division of Pediatric Infectious Diseases at the University of Utah in Salt Lake City.
“Getting your kids immunized before they enter school is so critical,” added Dr. Pavia, because the 21-day quarantine period is onerous for children and parents alike.
In a February 26 statement, Marcus Plescia, MD, MPH, chief medical officer of the Association of State and Territorial Health Officials, said “well-established public health practice recommends that unvaccinated persons exposed to measles stay home for at least 21 days to prevent further growth of the outbreak. While this is undoubtedly disruptive to the persons impacted, imagine how much more disruptive it would be if measles takes hold again in the United States, spreading widely, and impacting children and communities across the entire nation.”
During an outbreak, it’s still possible to give a measles vaccine to a child who has not yet received the shots, Dr. Pavia stressed. But time is of the essence: Vaccination should occur within 72 hours of the first known measles case in a school.
“It’s not perfect, they may still get measles, but it will greatly decrease the severity,” Dr. Pavia said.
If some children won’t get vaccinated during an outbreak, their parents may call a pediatrician or hospital staff for help as measles symptoms take hold. Clinicians should advise everyone in the home who is older than 2 years to begin wearing N95 masks and gloves, Dr. Alissa said. And when the child comes into the clinic he or she should be examined in a separate room, ideally one with negative air pressure and frequent filtration, Dr. Alissa added. If not, any private room will do if nobody else uses the room for at least 2 hours afterward.
“Measles is phenomenally transmissible,” Dr. Pavia said. A person with the virus can infect 12 to 18 others who are not protected against the pathogen.
Someone with a severe reaction to measles could get an injection of intramuscular immunoglobulin, Dr. Pavia said, although this tends to be uncomfortable and expensive.
“The vaccine works. We almost got rid of measles,” Dr. Alissa said, although parents who choose to send their unvaccinated children to school can do so if they choose to.
“The fear of every pediatrician is to have a child die from this,” she said. “People who are sick, please stay at home.”
Dr. Pavia reported an advisory relationship with Sanofi Pasteur regarding an RSV vaccine. Dr. Alissa reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
Amid an ongoing measles outbreak in Florida possibly sparked by vaccine hesitancy, the state’s surgeon general Joseph Ladapo, MD, is contradicting public health guidance of encouraging quarantine of unvaccinated children.
Rather than requesting that parents keep children unvaccinated against measles home from school or to get their children vaccinated, both critical tools in containing an outbreak, Dr. Ladapo has advised parents to do whatever they think is best. Pediatricians and infectious disease specialists fear a free-for-all will fuel the spread of the highly infectious virus, including in their own clinics.
The outbreak has been traced to an elementary school in Weston and has so far sickened at least eight children, one of whom is younger than 5 years. According to the Centers for Disease Control and Prevention, roughly 91% of the 230,000-odd kindergarteners in Florida had received the requisite doses of the MMR vaccine, which also protects against mumps and rubella, for the 2022-2023 school year, below the 95% vaccination level which public health authorities believes confers herd immunity against measles. An estimated 4.5% of kindergarteners in the state have received an exemption for the vaccine, which prevents measles in 97% of the people who get the shots, for a lifetime. The first dose is given around age 13 months and the second when people are age 4 or 5 years and soon to enter school.
“If you’re vaccinated you have a very slim chance of getting the virus,” said Rana Alissa, MD, a pediatrician at University of Florida Health in Jacksonville.
An unvaccinated child has no protection against measles, and could spread it to others merely by sneezing or touching a surface. In a school setting, infection could spread to a teacher who cannot receive the measles vaccine due to a weakened immune system, or the unvaccinated child could spread the virus at a pediatric clinic or hospital when seeking care for measles unless the clinic staff takes rigorous steps to separate the child from other children. Some children at the clinic won’t be able to get the measles vaccine either because of immunodeficiency or perhaps having had a bone marrow transplant.
Assuming the unvaccinated child is healthy, the measles infection will run its course, and the child will then be immune to the disease, Dr. Alissa said. But meanwhile, the child could pose a significant risk to others.
“We’re not worried about the unvaccinated kids who are very healthy. We’re worried about the adults who did not get vaccinated and who are very sick,” said Dr. Alissa, vice president of the Florida chapter of the American Academy of Pediatrics (AAP). “We’re worried about the little kids who are less than 13 months old. We’re worried about the kids with immunodeficiency disorders.” The Florida chapter of the AAP encourages parents to get their children vaccinated against measles amid the ongoing outbreak.
“I wish our surgeon general was on the same page as us,” Dr. Alissa added, noting that she thinks misplaced vaccine hesitancy has caused some parents to forego a safe and effective vaccine for their children.
Never Too Late to Vaxx
Measles symptoms appear 10-14 days after exposure and can include sore throat, cough, runny nose, inflamed eyes, fever, and blotchy skin rashes. According to the Centers for Disease Control and Prevention (CDC), 20% of people who are unvaccinated against measles will be hospitalized for the virus if they contract it.
Given the incubation period for the virus, clinicians and public health officials recommend unvaccinated children isolate for 21 days after being exposed to measles at school. The advice applies to any unvaccinated child, whether because their parent opted against the vaccine or because they cannot safely receive the immunization.
This is the guidance that Surgeon General Ladapo is flouting.
“We have a public health system. They’re awesome. They’re the experts. Let’s use them,” Dr. Alissa noted. “Their recommendation is to keep the unvaccinated kids at home for 21 days when you have an outbreak.”
“We’re not calling him doctor anymore,” said Andrew Pavia, MD, chief of the Division of Pediatric Infectious Diseases at the University of Utah in Salt Lake City.
“Getting your kids immunized before they enter school is so critical,” added Dr. Pavia, because the 21-day quarantine period is onerous for children and parents alike.
In a February 26 statement, Marcus Plescia, MD, MPH, chief medical officer of the Association of State and Territorial Health Officials, said “well-established public health practice recommends that unvaccinated persons exposed to measles stay home for at least 21 days to prevent further growth of the outbreak. While this is undoubtedly disruptive to the persons impacted, imagine how much more disruptive it would be if measles takes hold again in the United States, spreading widely, and impacting children and communities across the entire nation.”
During an outbreak, it’s still possible to give a measles vaccine to a child who has not yet received the shots, Dr. Pavia stressed. But time is of the essence: Vaccination should occur within 72 hours of the first known measles case in a school.
“It’s not perfect, they may still get measles, but it will greatly decrease the severity,” Dr. Pavia said.
If some children won’t get vaccinated during an outbreak, their parents may call a pediatrician or hospital staff for help as measles symptoms take hold. Clinicians should advise everyone in the home who is older than 2 years to begin wearing N95 masks and gloves, Dr. Alissa said. And when the child comes into the clinic he or she should be examined in a separate room, ideally one with negative air pressure and frequent filtration, Dr. Alissa added. If not, any private room will do if nobody else uses the room for at least 2 hours afterward.
“Measles is phenomenally transmissible,” Dr. Pavia said. A person with the virus can infect 12 to 18 others who are not protected against the pathogen.
Someone with a severe reaction to measles could get an injection of intramuscular immunoglobulin, Dr. Pavia said, although this tends to be uncomfortable and expensive.
“The vaccine works. We almost got rid of measles,” Dr. Alissa said, although parents who choose to send their unvaccinated children to school can do so if they choose to.
“The fear of every pediatrician is to have a child die from this,” she said. “People who are sick, please stay at home.”
Dr. Pavia reported an advisory relationship with Sanofi Pasteur regarding an RSV vaccine. Dr. Alissa reported no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
It Sure Looks Like Cannabis Is Bad for the Heart, Doesn’t It?
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.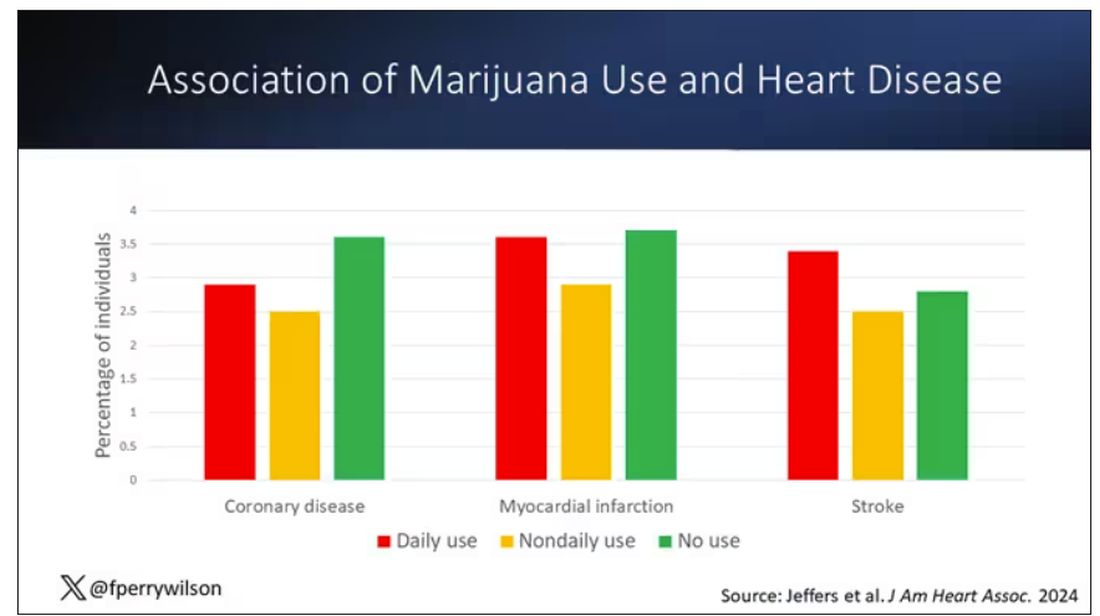
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.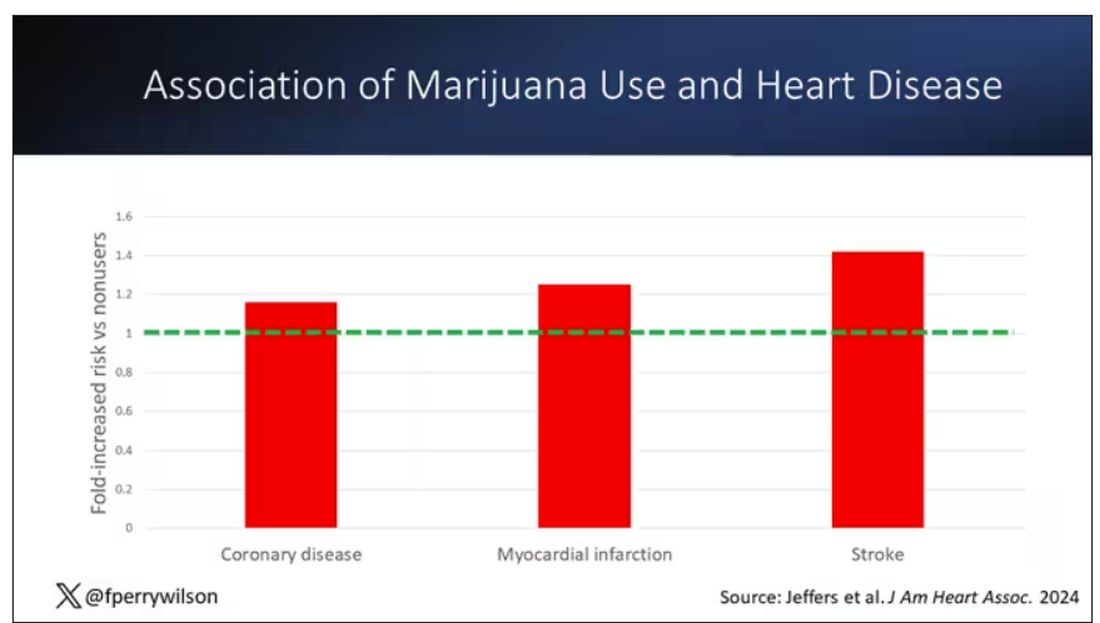
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.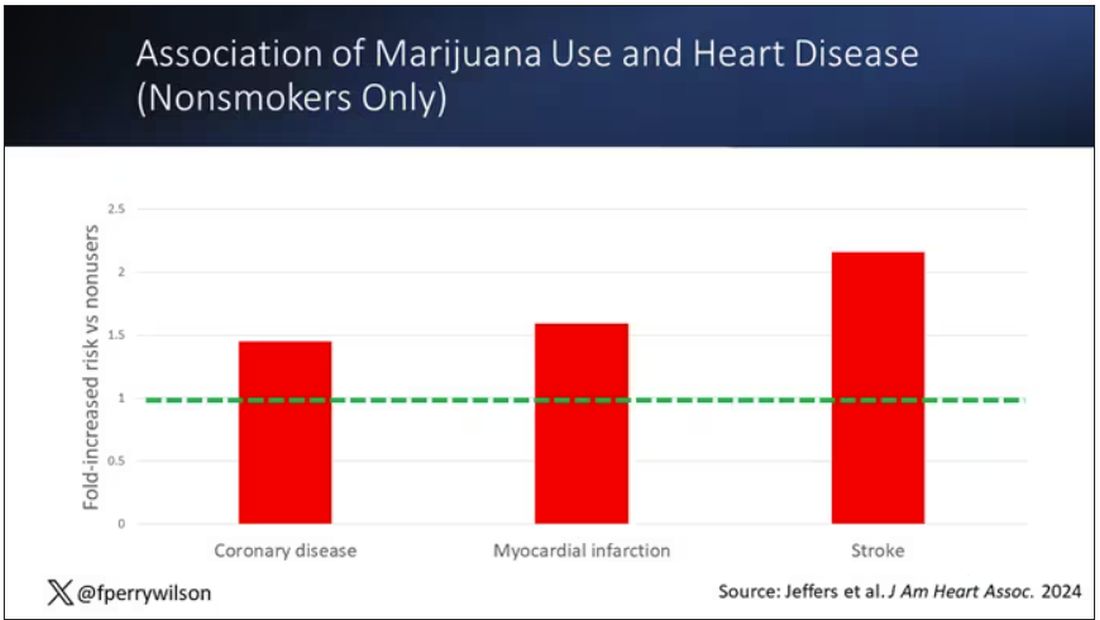
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.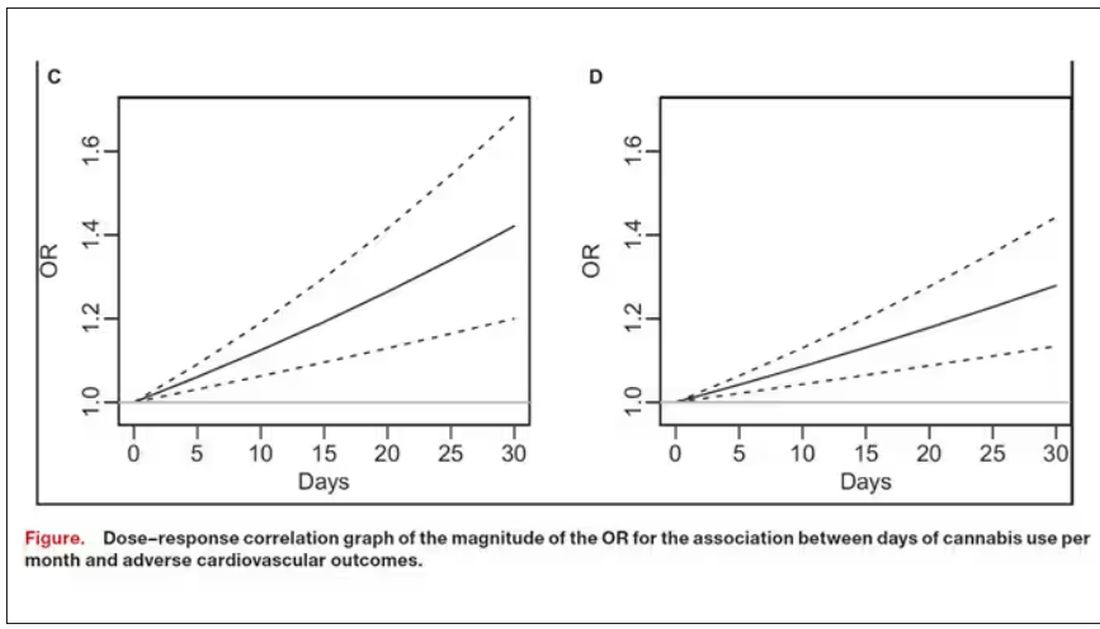
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
If you’re an epidemiologist trying to explore whether some exposure is a risk factor for a disease, you can run into a tough problem when your exposure of interest is highly correlated with another risk factor for the disease. For decades, this stymied investigations into the link, if any, between marijuana use and cardiovascular disease because, for decades, most people who used marijuana in some way also smoked cigarettes — which is a very clear risk factor for heart disease.
But the times they are a-changing.
Thanks to the legalization of marijuana for recreational use in many states, and even broader social trends, there is now a large population of people who use marijuana but do not use cigarettes. That means we can start to determine whether marijuana use is an independent risk factor for heart disease.
And this week, we have the largest study yet to attempt to answer that question, though, as I’ll explain momentarily, the smoke hasn’t entirely cleared yet.
The centerpiece of the study we are discussing this week, “Association of Cannabis Use With Cardiovascular Outcomes Among US Adults,” which appeared in the Journal of the American Heart Association, is the Behavioral Risk Factor Surveillance System, an annual telephone survey conducted by the Centers for Disease Control and Prevention since 1984 that gathers data on all sorts of stuff that we do to ourselves: our drinking habits, our smoking habits, and, more recently, our marijuana habits.
The paper combines annual data from 2016 to 2020 representing 27 states and two US territories for a total sample size of more than 430,000 individuals. The key exposure? Marijuana use, which was coded as the number of days of marijuana use in the past 30 days. The key outcome? Coronary heart disease, collected through questions such as “Has a doctor, nurse, or other health professional ever told you that you had a heart attack?”
Right away you might detect a couple of problems here. But let me show you the results before we worry about what they mean.
You can see the rates of the major cardiovascular outcomes here, stratified by daily use of marijuana, nondaily use, and no use. Broadly speaking, the risk was highest for daily users, lowest for occasional users, and in the middle for non-users.
Of course, non-users and users are different in lots of other ways; non-users were quite a bit older, for example. Adjusting for all those factors showed that, independent of age, smoking status, the presence of diabetes, and so on, there was an independently increased risk for cardiovascular outcomes in people who used marijuana.
Importantly, 60% of people in this study were never smokers, and the results in that group looked pretty similar to the results overall.
But I said there were a couple of problems, so let’s dig into those a bit.
First, like most survey studies, this one requires honest and accurate reporting from its subjects. There was no verification of heart disease using electronic health records or of marijuana usage based on biosamples. Broadly, miscategorization of exposure and outcomes in surveys tends to bias the results toward the null hypothesis, toward concluding that there is no link between exposure and outcome, so perhaps this is okay.
The bigger problem is the fact that this is a cross-sectional design. If you really wanted to know whether marijuana led to heart disease, you’d do a longitudinal study following users and non-users for some number of decades and see who developed heart disease and who didn’t. (For the pedants out there, I suppose you’d actually want to randomize people to use marijuana or not and then see who had a heart attack, but the IRB keeps rejecting my protocol when I submit it.)
Here, though, we literally can’t tell whether people who use marijuana have more heart attacks or whether people who have heart attacks use more marijuana. The authors argue that there are no data that show that people are more likely to use marijuana after a heart attack or stroke, but at the time the survey was conducted, they had already had their heart attack or stroke.
The authors also imply that they found a dose-response relationship between marijuana use and these cardiovascular outcomes. This is an important statement because dose response is one factor that we use to determine whether a risk factor may actually be causative as opposed to just correlative.
But I take issue with the dose-response language here. The model used to make these graphs classifies marijuana use as a single continuous variable ranging from 0 (no days of use in the past 30 days) to 1 (30 days of use in the past 30 days). The model is thus constrained to monotonically increase or decrease with respect to the outcome. To prove a dose response, you have to give the model the option to find something that isn’t a dose response — for example, by classifying marijuana use into discrete, independent categories rather than a single continuous number.
Am I arguing here that marijuana use is good for you? Of course not. Nor am I even arguing that it has no effect on the cardiovascular system. There are endocannabinoid receptors all over your vasculature. But a cross-sectional survey study, while a good start, is not quite the right way to answer the question. So, while the jury is still out, it’s high time for more research.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Connecticut. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Galantamine Supplements Found Mislabeled, Contaminated
TOPLINE:
Galantamine purchased as a dietary supplement may be more likely to contain bacterial contaminants and an incorrect amount of the product vs when it is prescribed as a generic drug, new research showed.
METHODOLOGY:
- Galantamine, a plant alkaloid, is approved for treating mild to moderate Alzheimer’s dementia but is also marketed as a dietary supplement for cognitive enhancement.
- In June 2023, researchers purchased all 10 galantamine dietary supplements available on Amazon.com that had a Supplement Facts panel.
- In September 2023, they acquired all 11 generic immediate-release formulations of prescription galantamine available in the United States.
- They analyzed the content of galantamine in each product using ultrahigh-performance liquid chromatography-mass spectrometry and quantified any microorganisms present.
TAKEAWAY:
- Generic galantamine drugs were found to contain 97.5%-104.2% of the labeled content, with no microbial contamination.
- , according to the authors of the study.
IN PRACTICE:
“Clinicians should query patients with memory concerns about the use of dietary supplements and advise patients not to use galantamine supplements,” the researchers wrote.
SOURCE:
The corresponding author of the study was Pieter A. Cohen, MD, with Broadway Clinic, Cambridge Health Alliance, in Somerville, Massachusetts. The paper was published online as a research letter in JAMA.
LIMITATIONS:
The products were purchased at a single point in time and may not reflect current options, the researchers noted. The generalizability of the findings to other supplement ingredients or generic drugs is unknown.
DISCLOSURES:
Dr. Cohen has received grants from the Consumers Union and PEW Charitable Trust and personal fees from UpToDate and the Centers for Disease Control and Prevention. He has been sued by a supplement company in a case where the jury found in his favor.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Galantamine purchased as a dietary supplement may be more likely to contain bacterial contaminants and an incorrect amount of the product vs when it is prescribed as a generic drug, new research showed.
METHODOLOGY:
- Galantamine, a plant alkaloid, is approved for treating mild to moderate Alzheimer’s dementia but is also marketed as a dietary supplement for cognitive enhancement.
- In June 2023, researchers purchased all 10 galantamine dietary supplements available on Amazon.com that had a Supplement Facts panel.
- In September 2023, they acquired all 11 generic immediate-release formulations of prescription galantamine available in the United States.
- They analyzed the content of galantamine in each product using ultrahigh-performance liquid chromatography-mass spectrometry and quantified any microorganisms present.
TAKEAWAY:
- Generic galantamine drugs were found to contain 97.5%-104.2% of the labeled content, with no microbial contamination.
- , according to the authors of the study.
IN PRACTICE:
“Clinicians should query patients with memory concerns about the use of dietary supplements and advise patients not to use galantamine supplements,” the researchers wrote.
SOURCE:
The corresponding author of the study was Pieter A. Cohen, MD, with Broadway Clinic, Cambridge Health Alliance, in Somerville, Massachusetts. The paper was published online as a research letter in JAMA.
LIMITATIONS:
The products were purchased at a single point in time and may not reflect current options, the researchers noted. The generalizability of the findings to other supplement ingredients or generic drugs is unknown.
DISCLOSURES:
Dr. Cohen has received grants from the Consumers Union and PEW Charitable Trust and personal fees from UpToDate and the Centers for Disease Control and Prevention. He has been sued by a supplement company in a case where the jury found in his favor.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Galantamine purchased as a dietary supplement may be more likely to contain bacterial contaminants and an incorrect amount of the product vs when it is prescribed as a generic drug, new research showed.
METHODOLOGY:
- Galantamine, a plant alkaloid, is approved for treating mild to moderate Alzheimer’s dementia but is also marketed as a dietary supplement for cognitive enhancement.
- In June 2023, researchers purchased all 10 galantamine dietary supplements available on Amazon.com that had a Supplement Facts panel.
- In September 2023, they acquired all 11 generic immediate-release formulations of prescription galantamine available in the United States.
- They analyzed the content of galantamine in each product using ultrahigh-performance liquid chromatography-mass spectrometry and quantified any microorganisms present.
TAKEAWAY:
- Generic galantamine drugs were found to contain 97.5%-104.2% of the labeled content, with no microbial contamination.
- , according to the authors of the study.
IN PRACTICE:
“Clinicians should query patients with memory concerns about the use of dietary supplements and advise patients not to use galantamine supplements,” the researchers wrote.
SOURCE:
The corresponding author of the study was Pieter A. Cohen, MD, with Broadway Clinic, Cambridge Health Alliance, in Somerville, Massachusetts. The paper was published online as a research letter in JAMA.
LIMITATIONS:
The products were purchased at a single point in time and may not reflect current options, the researchers noted. The generalizability of the findings to other supplement ingredients or generic drugs is unknown.
DISCLOSURES:
Dr. Cohen has received grants from the Consumers Union and PEW Charitable Trust and personal fees from UpToDate and the Centers for Disease Control and Prevention. He has been sued by a supplement company in a case where the jury found in his favor.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
New Trials in Leukemia and Lymphoma: Could Your Patient Benefit?
Several clinical trials in leukemia and lymphoma have started enrolling recently. Maybe one of your patients could benefit from taking part?
run by the Center for International Blood and Bone Marrow Transplant Research.
The purpose of the study is to test whether cyclophosphamide, which is given to prevent a dreaded complication of stem cell transplantation called graft-versus-host disease, can be safely reduced without increasing infection or reducing protection. All participants will receive cyclophosphamide on days 3 and 4 post transplant. One group will receive a reduced dose of cyclophosphamide (25 mg/kg per dose), and the other will be given a usual dose (37.5 mg/kg).
Sites in Michigan, Missouri, Oregon, Virginia, and Washington started recruiting for 190 participants in December 2023. Study centers in Florida, Massachusetts, New York, and Wisconsin are also planned. Infection-free survival is the primary endpoint, and overall survival is a secondary measure. Quality of life (QoL) is not recorded. More details at clinicaltrials.gov.
Untreated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Adults who are newly diagnosed with this type of cancer and have active disease may wish to consider a randomized, open-label, phase 3 trial testing an experimental Bruton tyrosine kinase (BTK) inhibitor, nemtabrutinib (from Merck Sharp & Dohme), against standard-of-care BTK inhibitors ibrutinib (Imbruvica) and acalabrutinib (Calquence).
BTK inhibitors target B-cell proliferation in B-cell cancers such as CLL/SLL and allow for chemotherapy-free treatment of some hematological malignancies. In this study, until disease progression, unacceptable toxicity, or another reason for discontinuation occurs, participants will take daily oral nemtabrutinib, ibrutinib, or acalabrutinib.
The study opened in December 2023 in Pennsylvania, Washington, Taiwan, Israel, and the United Kingdom seeking 1200 participants. The primary outcomes are objective response rate and progression-free survival. Overall survival is a secondary outcome, and QoL is not measured. More details at clinicaltrials.gov.
Relapsed or refractory leukemia with a KMT2A-gene rearrangement (KMT2A-r). Children aged 1 month to younger than 6 years with this diagnosis may be able to join an open-label, nonrandomized, Children’s Oncology Group phase 2 study to determine the most tolerable and/or effective dose of an experimental oral drug called revumenib when added to chemotherapy.
KMT2A-gene alterations are associated with a poor prognosis in leukemia. These alterations cause blood cells to dedifferentiate and start proliferating uncontrollably as leukemia cells. The expression of the damaged KMT2A gene relies on a protein called menin. Revumenib, from Syndax Pharmaceuticals, blocks menin and prevents expression of KMT2A.
Children in the study will receive two different regimens of revumenib in combination with chemotherapy for up to a year, or until disease progression or unacceptable toxicity, and will then be followed for up to 5 years. Trial centers in 12 US states opened their doors in January 2024 looking for 78 participants. Toxicities and minimal residual disease are the primary outcomes; overall survival is a secondary outcome, and QoL is not assessed. More details at clinicaltrials.gov.
Previously untreated follicular lymphoma or diffuse large B-cell lymphoma. Adults with one of these types of lymphoma may be eligible for one of three open-label, randomized, phase 3 trials testing odronextamab (from Regeneron). This bispecific antibody is designed to ‘lock together’ CD20 on cancer cells with CD3-expressing cancer-killing T cells. It has shown anti-lymphoma activity in heavily pretreated patients.
Late in 2023, three phase 3 trials turned the spotlight on treatment-naive patients and started recruiting 2115 participants to assess odronextamab in this setting. The trial OLYMPIA-1 will compare odronextamab with standard-of-care rituximab (Rituxan) plus chemotherapy in follicular lymphoma. OLYMPIA-2 will test the drug in combination with chemotherapy, also in follicular lymphoma. OLYMPIA-3 will evaluate odronextamab plus chemotherapy against rituximab and chemotherapy in people with large B-cell lymphoma.
All study drugs, including odronextamab, will be administered by intravenous infusion, and participants will be followed for up to 5 years. Research centers across eight US states and Australia, Czechia, France, Italy, Poland, Spain, Turkey, and Thailand are currently accepting participants for the three trials. The primary outcomes are various measures of toxicity and complete response at 30 months in the follicular lymphoma studies and toxicity and progression-free survival in large B-cell lymphoma. All three trials are measuring overall survival and QoL as secondary endpoints.
Previously untreated stage II, III, or IV follicular lymphoma. Adults with this type of cancer may be eligible to participate in a randomized, open-label, phase 3 study testing whether an experimental therapy called epcoritamab (from AbbVie) improves disease response and is tolerable when added to standard therapy. For up to 120 weeks, one group of participants will receive a combination of intravenous rituximab and oral lenalidomide (Revlimid), while a second group will also receive subcutaneous injections of epcoritamab. Some participants may be offered investigators’ choice of chemotherapy as well.
Sites across Iowa, Maryland, Missouri, Ohio, Washington, and Montana started welcoming their 900 participants in February 2024. The primary outcome is complete response at 30 months. Overall survival and QoL are secondary outcomes. More details at clinicaltrials.gov.
Relapsed or refractory mantle cell lymphoma. Adults facing one of these clinical scenarios can join an Academic and Community Cancer Research United open label, phase 2 trial examining the effectiveness of combining tafasitamab (Monjuvi), lenalidomide, and venetoclax (Venclexta) for such patients.
Frontline therapy does not cure mantle cell lymphoma, and continued relapses are common. In this situation, treatments can include acalabrutinib, ibrutinib, stem cell transplantation, venetoclax, lenalidomide, and rituximab.
In this study, participants will take venetoclax and lenalidomide daily and receive intravenous tafasitamab every 2 weeks after an initial ramp-up period as per clinic standards. Participants will be followed for 5 years after entering the trial. The Mayo Clinic in Rochester, Minnesota, began recruiting the planned 100 trial participants in January 2024. The primary outcome is objective response rate; overall survival is a secondary outcome, and QoL will not be tracked. More details at clinicaltrials.gov.
All trial information is from the National Institutes of Health US National Library of Medicine (online at clinicaltrials.gov).
A version of this article appeared on Medscape.com .
Several clinical trials in leukemia and lymphoma have started enrolling recently. Maybe one of your patients could benefit from taking part?
run by the Center for International Blood and Bone Marrow Transplant Research.
The purpose of the study is to test whether cyclophosphamide, which is given to prevent a dreaded complication of stem cell transplantation called graft-versus-host disease, can be safely reduced without increasing infection or reducing protection. All participants will receive cyclophosphamide on days 3 and 4 post transplant. One group will receive a reduced dose of cyclophosphamide (25 mg/kg per dose), and the other will be given a usual dose (37.5 mg/kg).
Sites in Michigan, Missouri, Oregon, Virginia, and Washington started recruiting for 190 participants in December 2023. Study centers in Florida, Massachusetts, New York, and Wisconsin are also planned. Infection-free survival is the primary endpoint, and overall survival is a secondary measure. Quality of life (QoL) is not recorded. More details at clinicaltrials.gov.
Untreated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Adults who are newly diagnosed with this type of cancer and have active disease may wish to consider a randomized, open-label, phase 3 trial testing an experimental Bruton tyrosine kinase (BTK) inhibitor, nemtabrutinib (from Merck Sharp & Dohme), against standard-of-care BTK inhibitors ibrutinib (Imbruvica) and acalabrutinib (Calquence).
BTK inhibitors target B-cell proliferation in B-cell cancers such as CLL/SLL and allow for chemotherapy-free treatment of some hematological malignancies. In this study, until disease progression, unacceptable toxicity, or another reason for discontinuation occurs, participants will take daily oral nemtabrutinib, ibrutinib, or acalabrutinib.
The study opened in December 2023 in Pennsylvania, Washington, Taiwan, Israel, and the United Kingdom seeking 1200 participants. The primary outcomes are objective response rate and progression-free survival. Overall survival is a secondary outcome, and QoL is not measured. More details at clinicaltrials.gov.
Relapsed or refractory leukemia with a KMT2A-gene rearrangement (KMT2A-r). Children aged 1 month to younger than 6 years with this diagnosis may be able to join an open-label, nonrandomized, Children’s Oncology Group phase 2 study to determine the most tolerable and/or effective dose of an experimental oral drug called revumenib when added to chemotherapy.
KMT2A-gene alterations are associated with a poor prognosis in leukemia. These alterations cause blood cells to dedifferentiate and start proliferating uncontrollably as leukemia cells. The expression of the damaged KMT2A gene relies on a protein called menin. Revumenib, from Syndax Pharmaceuticals, blocks menin and prevents expression of KMT2A.
Children in the study will receive two different regimens of revumenib in combination with chemotherapy for up to a year, or until disease progression or unacceptable toxicity, and will then be followed for up to 5 years. Trial centers in 12 US states opened their doors in January 2024 looking for 78 participants. Toxicities and minimal residual disease are the primary outcomes; overall survival is a secondary outcome, and QoL is not assessed. More details at clinicaltrials.gov.
Previously untreated follicular lymphoma or diffuse large B-cell lymphoma. Adults with one of these types of lymphoma may be eligible for one of three open-label, randomized, phase 3 trials testing odronextamab (from Regeneron). This bispecific antibody is designed to ‘lock together’ CD20 on cancer cells with CD3-expressing cancer-killing T cells. It has shown anti-lymphoma activity in heavily pretreated patients.
Late in 2023, three phase 3 trials turned the spotlight on treatment-naive patients and started recruiting 2115 participants to assess odronextamab in this setting. The trial OLYMPIA-1 will compare odronextamab with standard-of-care rituximab (Rituxan) plus chemotherapy in follicular lymphoma. OLYMPIA-2 will test the drug in combination with chemotherapy, also in follicular lymphoma. OLYMPIA-3 will evaluate odronextamab plus chemotherapy against rituximab and chemotherapy in people with large B-cell lymphoma.
All study drugs, including odronextamab, will be administered by intravenous infusion, and participants will be followed for up to 5 years. Research centers across eight US states and Australia, Czechia, France, Italy, Poland, Spain, Turkey, and Thailand are currently accepting participants for the three trials. The primary outcomes are various measures of toxicity and complete response at 30 months in the follicular lymphoma studies and toxicity and progression-free survival in large B-cell lymphoma. All three trials are measuring overall survival and QoL as secondary endpoints.
Previously untreated stage II, III, or IV follicular lymphoma. Adults with this type of cancer may be eligible to participate in a randomized, open-label, phase 3 study testing whether an experimental therapy called epcoritamab (from AbbVie) improves disease response and is tolerable when added to standard therapy. For up to 120 weeks, one group of participants will receive a combination of intravenous rituximab and oral lenalidomide (Revlimid), while a second group will also receive subcutaneous injections of epcoritamab. Some participants may be offered investigators’ choice of chemotherapy as well.
Sites across Iowa, Maryland, Missouri, Ohio, Washington, and Montana started welcoming their 900 participants in February 2024. The primary outcome is complete response at 30 months. Overall survival and QoL are secondary outcomes. More details at clinicaltrials.gov.
Relapsed or refractory mantle cell lymphoma. Adults facing one of these clinical scenarios can join an Academic and Community Cancer Research United open label, phase 2 trial examining the effectiveness of combining tafasitamab (Monjuvi), lenalidomide, and venetoclax (Venclexta) for such patients.
Frontline therapy does not cure mantle cell lymphoma, and continued relapses are common. In this situation, treatments can include acalabrutinib, ibrutinib, stem cell transplantation, venetoclax, lenalidomide, and rituximab.
In this study, participants will take venetoclax and lenalidomide daily and receive intravenous tafasitamab every 2 weeks after an initial ramp-up period as per clinic standards. Participants will be followed for 5 years after entering the trial. The Mayo Clinic in Rochester, Minnesota, began recruiting the planned 100 trial participants in January 2024. The primary outcome is objective response rate; overall survival is a secondary outcome, and QoL will not be tracked. More details at clinicaltrials.gov.
All trial information is from the National Institutes of Health US National Library of Medicine (online at clinicaltrials.gov).
A version of this article appeared on Medscape.com .
Several clinical trials in leukemia and lymphoma have started enrolling recently. Maybe one of your patients could benefit from taking part?
run by the Center for International Blood and Bone Marrow Transplant Research.
The purpose of the study is to test whether cyclophosphamide, which is given to prevent a dreaded complication of stem cell transplantation called graft-versus-host disease, can be safely reduced without increasing infection or reducing protection. All participants will receive cyclophosphamide on days 3 and 4 post transplant. One group will receive a reduced dose of cyclophosphamide (25 mg/kg per dose), and the other will be given a usual dose (37.5 mg/kg).
Sites in Michigan, Missouri, Oregon, Virginia, and Washington started recruiting for 190 participants in December 2023. Study centers in Florida, Massachusetts, New York, and Wisconsin are also planned. Infection-free survival is the primary endpoint, and overall survival is a secondary measure. Quality of life (QoL) is not recorded. More details at clinicaltrials.gov.
Untreated chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Adults who are newly diagnosed with this type of cancer and have active disease may wish to consider a randomized, open-label, phase 3 trial testing an experimental Bruton tyrosine kinase (BTK) inhibitor, nemtabrutinib (from Merck Sharp & Dohme), against standard-of-care BTK inhibitors ibrutinib (Imbruvica) and acalabrutinib (Calquence).
BTK inhibitors target B-cell proliferation in B-cell cancers such as CLL/SLL and allow for chemotherapy-free treatment of some hematological malignancies. In this study, until disease progression, unacceptable toxicity, or another reason for discontinuation occurs, participants will take daily oral nemtabrutinib, ibrutinib, or acalabrutinib.
The study opened in December 2023 in Pennsylvania, Washington, Taiwan, Israel, and the United Kingdom seeking 1200 participants. The primary outcomes are objective response rate and progression-free survival. Overall survival is a secondary outcome, and QoL is not measured. More details at clinicaltrials.gov.
Relapsed or refractory leukemia with a KMT2A-gene rearrangement (KMT2A-r). Children aged 1 month to younger than 6 years with this diagnosis may be able to join an open-label, nonrandomized, Children’s Oncology Group phase 2 study to determine the most tolerable and/or effective dose of an experimental oral drug called revumenib when added to chemotherapy.
KMT2A-gene alterations are associated with a poor prognosis in leukemia. These alterations cause blood cells to dedifferentiate and start proliferating uncontrollably as leukemia cells. The expression of the damaged KMT2A gene relies on a protein called menin. Revumenib, from Syndax Pharmaceuticals, blocks menin and prevents expression of KMT2A.
Children in the study will receive two different regimens of revumenib in combination with chemotherapy for up to a year, or until disease progression or unacceptable toxicity, and will then be followed for up to 5 years. Trial centers in 12 US states opened their doors in January 2024 looking for 78 participants. Toxicities and minimal residual disease are the primary outcomes; overall survival is a secondary outcome, and QoL is not assessed. More details at clinicaltrials.gov.
Previously untreated follicular lymphoma or diffuse large B-cell lymphoma. Adults with one of these types of lymphoma may be eligible for one of three open-label, randomized, phase 3 trials testing odronextamab (from Regeneron). This bispecific antibody is designed to ‘lock together’ CD20 on cancer cells with CD3-expressing cancer-killing T cells. It has shown anti-lymphoma activity in heavily pretreated patients.
Late in 2023, three phase 3 trials turned the spotlight on treatment-naive patients and started recruiting 2115 participants to assess odronextamab in this setting. The trial OLYMPIA-1 will compare odronextamab with standard-of-care rituximab (Rituxan) plus chemotherapy in follicular lymphoma. OLYMPIA-2 will test the drug in combination with chemotherapy, also in follicular lymphoma. OLYMPIA-3 will evaluate odronextamab plus chemotherapy against rituximab and chemotherapy in people with large B-cell lymphoma.
All study drugs, including odronextamab, will be administered by intravenous infusion, and participants will be followed for up to 5 years. Research centers across eight US states and Australia, Czechia, France, Italy, Poland, Spain, Turkey, and Thailand are currently accepting participants for the three trials. The primary outcomes are various measures of toxicity and complete response at 30 months in the follicular lymphoma studies and toxicity and progression-free survival in large B-cell lymphoma. All three trials are measuring overall survival and QoL as secondary endpoints.
Previously untreated stage II, III, or IV follicular lymphoma. Adults with this type of cancer may be eligible to participate in a randomized, open-label, phase 3 study testing whether an experimental therapy called epcoritamab (from AbbVie) improves disease response and is tolerable when added to standard therapy. For up to 120 weeks, one group of participants will receive a combination of intravenous rituximab and oral lenalidomide (Revlimid), while a second group will also receive subcutaneous injections of epcoritamab. Some participants may be offered investigators’ choice of chemotherapy as well.
Sites across Iowa, Maryland, Missouri, Ohio, Washington, and Montana started welcoming their 900 participants in February 2024. The primary outcome is complete response at 30 months. Overall survival and QoL are secondary outcomes. More details at clinicaltrials.gov.
Relapsed or refractory mantle cell lymphoma. Adults facing one of these clinical scenarios can join an Academic and Community Cancer Research United open label, phase 2 trial examining the effectiveness of combining tafasitamab (Monjuvi), lenalidomide, and venetoclax (Venclexta) for such patients.
Frontline therapy does not cure mantle cell lymphoma, and continued relapses are common. In this situation, treatments can include acalabrutinib, ibrutinib, stem cell transplantation, venetoclax, lenalidomide, and rituximab.
In this study, participants will take venetoclax and lenalidomide daily and receive intravenous tafasitamab every 2 weeks after an initial ramp-up period as per clinic standards. Participants will be followed for 5 years after entering the trial. The Mayo Clinic in Rochester, Minnesota, began recruiting the planned 100 trial participants in January 2024. The primary outcome is objective response rate; overall survival is a secondary outcome, and QoL will not be tracked. More details at clinicaltrials.gov.
All trial information is from the National Institutes of Health US National Library of Medicine (online at clinicaltrials.gov).
A version of this article appeared on Medscape.com .
Myasthenia Gravis: Treating the Whole Patient
In the dynamic field of myasthenia gravis (MG) treatment, characterized by recent therapeutic advancements and a promising pipeline, Nicholas J. Silvestri, MD, advises early-career professionals to approach the whole patient, considering not only the disease manifestations but also its broader impact on their lives, including work and family.
Emphasizing the importance of tailoring therapies based on individual needs, Dr Silvestri encourages early and aggressive intervention, citing evidence supporting better long-term outcomes, and underscores the significance of treating the whole patient rather than just the disease.
In the dynamic field of myasthenia gravis (MG) treatment, characterized by recent therapeutic advancements and a promising pipeline, Nicholas J. Silvestri, MD, advises early-career professionals to approach the whole patient, considering not only the disease manifestations but also its broader impact on their lives, including work and family.
Emphasizing the importance of tailoring therapies based on individual needs, Dr Silvestri encourages early and aggressive intervention, citing evidence supporting better long-term outcomes, and underscores the significance of treating the whole patient rather than just the disease.
In the dynamic field of myasthenia gravis (MG) treatment, characterized by recent therapeutic advancements and a promising pipeline, Nicholas J. Silvestri, MD, advises early-career professionals to approach the whole patient, considering not only the disease manifestations but also its broader impact on their lives, including work and family.
Emphasizing the importance of tailoring therapies based on individual needs, Dr Silvestri encourages early and aggressive intervention, citing evidence supporting better long-term outcomes, and underscores the significance of treating the whole patient rather than just the disease.

Myasthenia Gravis: 3 Tips to Improve Patient-Centered Care
Kelly G. Gwathmey, MD, offers three key tips for clinicians early in their careers regarding myasthenia gravis (MG): First, prioritize listening to patients, as their experiences may not always align with clinical observations. Second, advocate for shared decision-making when starting or changing treatments, considering individual patient preferences and medical conditions. Third, understand the significance of ongoing monitoring using patient-reported outcome measures and MG scales to assess treatment response and optimize care for patients with MG.
Kelly G. Gwathmey, MD, offers three key tips for clinicians early in their careers regarding myasthenia gravis (MG): First, prioritize listening to patients, as their experiences may not always align with clinical observations. Second, advocate for shared decision-making when starting or changing treatments, considering individual patient preferences and medical conditions. Third, understand the significance of ongoing monitoring using patient-reported outcome measures and MG scales to assess treatment response and optimize care for patients with MG.
Kelly G. Gwathmey, MD, offers three key tips for clinicians early in their careers regarding myasthenia gravis (MG): First, prioritize listening to patients, as their experiences may not always align with clinical observations. Second, advocate for shared decision-making when starting or changing treatments, considering individual patient preferences and medical conditions. Third, understand the significance of ongoing monitoring using patient-reported outcome measures and MG scales to assess treatment response and optimize care for patients with MG.

Myasthenia Gravis: Reflections on Past Challenges and Evolving Strategies
Nicholas J. Silvestri, MD, recounts a memorable experience with a patient with myasthenia gravis (MG) during early neurology residency, detailing the diagnostic process and initial treatment with standard therapies. Despite the patient's positive response in terms of efficacy, tolerability issues and side effects posed challenges.
Dr Silvestri highlights the evolution in MG treatment, emphasizing the availability of newer, well-tolerated options with proven efficacy, suggesting a more balanced approach between effectiveness and patient safety in contemporary MG management.
Nicholas J. Silvestri, MD, recounts a memorable experience with a patient with myasthenia gravis (MG) during early neurology residency, detailing the diagnostic process and initial treatment with standard therapies. Despite the patient's positive response in terms of efficacy, tolerability issues and side effects posed challenges.
Dr Silvestri highlights the evolution in MG treatment, emphasizing the availability of newer, well-tolerated options with proven efficacy, suggesting a more balanced approach between effectiveness and patient safety in contemporary MG management.
Nicholas J. Silvestri, MD, recounts a memorable experience with a patient with myasthenia gravis (MG) during early neurology residency, detailing the diagnostic process and initial treatment with standard therapies. Despite the patient's positive response in terms of efficacy, tolerability issues and side effects posed challenges.
Dr Silvestri highlights the evolution in MG treatment, emphasizing the availability of newer, well-tolerated options with proven efficacy, suggesting a more balanced approach between effectiveness and patient safety in contemporary MG management.






