User login
ASCO addresses financial barriers to cancer clinical trials
The American Society of Clinical Oncology (ASCO) has released a policy statement addressing financial barriers that may prevent cancer patients from participating in clinical trials.
The four main recommendations in ASCO’s policy statement are:
- Payers should create clear, consistent, streamlined, and transparent policies regarding clinical trial coverage.
- Patients should receive easy-to-understand information about potential out-of-pocket costs.
- “Ethically appropriate” financial compensation for out-of-pocket costs should be allowed.
- Researchers should be incentivized to investigate and “better characterize” costs incurred by cancer patients in clinical trials as well as identify ways to “mitigate the risk of trial-associated financial hardship.”
ASCO’s full policy statement, “Addressing Financial Barriers to Patient Participation in Clinical Trials,” is available on the Journal of Clinical Oncology website.
SOURCE: Winkfield KM et al. J Clin Oncol. 2018 Sep 13:JCO1801132. doi: 10.1200/JCO.18.01132.
The American Society of Clinical Oncology (ASCO) has released a policy statement addressing financial barriers that may prevent cancer patients from participating in clinical trials.
The four main recommendations in ASCO’s policy statement are:
- Payers should create clear, consistent, streamlined, and transparent policies regarding clinical trial coverage.
- Patients should receive easy-to-understand information about potential out-of-pocket costs.
- “Ethically appropriate” financial compensation for out-of-pocket costs should be allowed.
- Researchers should be incentivized to investigate and “better characterize” costs incurred by cancer patients in clinical trials as well as identify ways to “mitigate the risk of trial-associated financial hardship.”
ASCO’s full policy statement, “Addressing Financial Barriers to Patient Participation in Clinical Trials,” is available on the Journal of Clinical Oncology website.
SOURCE: Winkfield KM et al. J Clin Oncol. 2018 Sep 13:JCO1801132. doi: 10.1200/JCO.18.01132.
The American Society of Clinical Oncology (ASCO) has released a policy statement addressing financial barriers that may prevent cancer patients from participating in clinical trials.
The four main recommendations in ASCO’s policy statement are:
- Payers should create clear, consistent, streamlined, and transparent policies regarding clinical trial coverage.
- Patients should receive easy-to-understand information about potential out-of-pocket costs.
- “Ethically appropriate” financial compensation for out-of-pocket costs should be allowed.
- Researchers should be incentivized to investigate and “better characterize” costs incurred by cancer patients in clinical trials as well as identify ways to “mitigate the risk of trial-associated financial hardship.”
ASCO’s full policy statement, “Addressing Financial Barriers to Patient Participation in Clinical Trials,” is available on the Journal of Clinical Oncology website.
SOURCE: Winkfield KM et al. J Clin Oncol. 2018 Sep 13:JCO1801132. doi: 10.1200/JCO.18.01132.
FROM JOURNAL OF CLINICAL ONCOLOGY
One-step gestational diabetes screening doesn’t improve outcomes
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
Diabetes is a significant global public health concern, but is especially problematic for women of reproductive age because diabetes in pregnancy can cause significant health complications for the mother and baby. Gestational diabetes mellitus (GDM) affects up to 10% of pregnancies in the United States annually, and is associated with perinatal loss, operative delivery, macrosomia, hypoglycemia, respiratory distress syndrome, and metabolic derangements for the offspring. For the mother, GDM is associated with hypertensive disorders, infections, hydramnios, and increased risk for developing type 2 diabetes later in life. As the incidence of GDM continues to rise, studies examining how to reduce, manage or prevent this condition become increasingly important.
The authors’ conclusions, that adopting the one-step approach increased the number of women with diagnosed GDM but did not significantly improve maternal or neonatal outcomes, are not surprising. Since the initial publication of the Hyperglycemia and Adverse Pregnancy Outcome Study, upon which the International Association of the Diabetes in Pregnancy Study Groups based its recommendations to go to a one-step approach, much debate has ensued about the best method to diagnose GDM. Indeed, the National Institutes of Health convened a consensus panel to review the literature and determine whether the one-step approach should be universally adopted (the panel concluded that more information was needed, and that the current two-step approach should continue to be used).
As the authors concede, studies have shown conflicting results, and no large-scale randomized controlled trial has been conducted to date. However, the literature does not bear out the idea that the one-step approach is truly better. The current study, although including a significant number of women and a reasonable control group, only serves as yet another study to reinforce what has previously been published.
I would agree with the researchers’ conclusions that the one-step approach is not necessarily beneficial. Although the one-step approach may identify a subset of patients who might not otherwise be diagnosed with GDM, it still remains unclear whether the outcomes for these patients will be improved. Furthermore, additional testing, need for insulin or other oral antidiabetic medications, etc., would result in additional stress to the patient and the health care system. Based on the authors’ findings, and results of other studies, it remains to be determined if the effort (diagnosing additional patients with GDM) is justified medically, economically, or otherwise.
As ob.gyns., we must continually ask ourselves: “By not doing something, are we causing harm to our patients?” If we change the diagnostic criteria for GDM, thereby increasing the number of women with the condition who would then require additional care, medications, and, potentially, more complex decisions around timing and mode of delivery, we need to be certain that we are not doing harm. This, and other studies examining the use of the one- versus two-step approach have yet to demonstrate, unequivocally, that changing the criteria reduces harm, and, perhaps, might – unintentionally – cause more.
As the study authors and the NIH consensus panel concluded, more rigorous evaluation is needed; that is, a large, multicenter randomized controlled trial that examines not only the benefits during pregnancy but also the long-term benefits to women and their children.
E. Albert Reece, MD, PhD, MBA, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He provided commentary on the study by Pocobelli et al. Dr. Reece said he had no relevant financial disclosures.
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
according to data from a before-and-after cohort study of women in the state of Washington.
The one-step test, a 75-g 2-hour oral glucose tolerance test (OGTT), was recommended for all pregnant women in 2010, although the traditional two-step test – a 50-g screening glucose challenge test followed by a 100-g 3-hour OGTT – remains widely used, wrote Gaia Pocobelli, PhD, of Kaiser Permanente Washington Health Research Institute, Seattle, and her colleagues. “No randomized trial has been published comparing outcomes of the two approaches.”
In a study published in Obstetrics & Gynecology, the researchers compared data from 23,257 women who received prenatal care in Washington State between January 2009 and December 2014, including 8,363 women who received care before the guideline change, 4,103 who received care during a transition period, and 10,791 after the guideline change. Approximately 60% of the women received care from clinicians internal to Kaiser Permanente; 40% received care from external providers. Most (87%) of the internal clinicians switched to the one-step approach, the researchers said. Only 5% of external providers did so.
Overall, adopting the one-step approach was associated with a 41% increase in the diagnosis of GDM without improved maternal or neonatal outcomes, the researchers noted.
The incidence of GDM increased from 7% before the guideline change to 11% afterward for women seen by internal providers. For women seen by external providers, gestational diabetes incidence increased from 10% to 11%.
For women seen by internal providers, the use of insulin increased from 1% before the guideline change to 4% afterward; for women seen by external providers, use of insulin increased from 1.3% to 1.4% (change between the groups P less than .001).
In addition, women seen by internal providers were more likely to undergo induction of labor after the guideline change (25% to 29%), while labor induction decreased for women seen by external providers (31% to 29%) for a relative risk of 1.2.
Neonatal hypoglycemia increased from 1% to 2% among women seen by internal providers, but decreased slightly from 2.4% to 2.1% for women seen by external providers, for a relative risk of 1.77.
There were no significant differences between the women seen by internal and external providers in risk of primary cesarean section, large for gestational age, small for gestational age, or neonatal ICU admission.
The main limitation of the study was the potential confounding variables including maternal diet and exercise, and possible underreporting of risk factors such as smoking, the researchers noted. However, the results were strengthened by the large study population, and the results “do not suggest a benefit of adopting the one-step over the two-step approach.
“Kaiser Permanente Washington has revised [its] guidelines to return to a two-step process. We recommend that any health care system considering switching to the one-step approach incorporate a rigorous evaluation of changes in maternal and neonatal outcomes,” Dr. Pocobelli and her associates added.
Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Increased diagnoses of gestational diabetes did not significantly improve maternal or fetal outcomes.
Major finding: Adoption of a one-step screening process for gestational diabetes increased diagnoses by 41%.
Study details: The data come from a before-and-after cohort study with a population of 23,257 women.
Disclosures: Dr. Pocobelli disclosed funding from Jazz Pharmaceuticals for work unrelated to this study. The study was supported in part by a grant from the Group Health Foundation Momentum Fund.
Source: Pocobelli G et al. Obstet Gynecol. 2018;132:859-67.
Tau PET tracer distinguishes Alzheimer’s from other disorders
PET imaging with an investigational tau tracer, 18F-flortaucipir, distinguished Alzheimer’s disease from other neurodegenerative disorders with a high degree of accuracy in a multicenter study.
PET with 18F-flortaucipir also outperformed structural MRI markers for Alzheimer’s disease (AD), and when compared against amyloid-beta (Abeta) biomarkers, showed higher specificity for an AD dementia diagnosis, researchers wrote in a report published online Sept. 18 in JAMA.
“The accuracy and potential utility of this test in patient care require further research in clinically more representative populations,” they wrote.
The study included 719 participants recruited from memory disorder clinics in Sweden, Korea, and California. That cohort included 179 patients with AD dementia, 254 who had non-AD neurodegenerative diseases, 126 with mild cognitive impairment (MCI), and 160 controls who were cognitively normal. All participants underwent 18F-flortaucipir PET between June 2014 and November 2017.
They found that 18F-flortaucipir showed “high accuracy” for distinguishing AD dementia from non-AD neurodegenerative disorders across all five regions of interest (ROI) evaluated in the study.
For example, 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal meta-ROI, they reported.
By contrast, the accuracy of 18F-flortaucipir was lower for distinguishing MCI caused by AD versus non-AD neurodegenerative conditions. In the temporal meta-ROI, that translated into an 83.4% accuracy, 61.5% sensitivity, and 90.6% specificity, according to the report.
The 18F-flortaucipir tracer outperformed state-of-the-art MRI measures for distinguishing AD dementia from non-AD neurodegenerative disorders, including hippocampal volumes, AD-signature thickness, and whole-brain cortical thickness, Dr. Ossenkoppele and his colleagues found in a secondary analysis.
Likewise, certain analyses showed that specificity for AD dementia versus non-AD neurodegenerative disorders was higher with 18F-flortaucipir than with Abeta status.
While both MRI measures and Abeta status are increasingly used to evaluate cognitive impairment adjunctively in a clinical setting, the utility of 18F-flortaucipir PET as a diagnostic biomarker still must be determined, the investigators wrote in a discussion of their results.
“An intended clinical use of 18F-flortaucipir PET might be to improve the diagnostic work-up as an add-on test to Abeta biomarkers in patients with early-onset dementia and possibly as a triage or even replacement test in patients with late-onset dementia in whom incidental Abeta pathology is common,” they wrote.
The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies that are marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
SOURCE: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
PET imaging with an investigational tau tracer, 18F-flortaucipir, distinguished Alzheimer’s disease from other neurodegenerative disorders with a high degree of accuracy in a multicenter study.
PET with 18F-flortaucipir also outperformed structural MRI markers for Alzheimer’s disease (AD), and when compared against amyloid-beta (Abeta) biomarkers, showed higher specificity for an AD dementia diagnosis, researchers wrote in a report published online Sept. 18 in JAMA.
“The accuracy and potential utility of this test in patient care require further research in clinically more representative populations,” they wrote.
The study included 719 participants recruited from memory disorder clinics in Sweden, Korea, and California. That cohort included 179 patients with AD dementia, 254 who had non-AD neurodegenerative diseases, 126 with mild cognitive impairment (MCI), and 160 controls who were cognitively normal. All participants underwent 18F-flortaucipir PET between June 2014 and November 2017.
They found that 18F-flortaucipir showed “high accuracy” for distinguishing AD dementia from non-AD neurodegenerative disorders across all five regions of interest (ROI) evaluated in the study.
For example, 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal meta-ROI, they reported.
By contrast, the accuracy of 18F-flortaucipir was lower for distinguishing MCI caused by AD versus non-AD neurodegenerative conditions. In the temporal meta-ROI, that translated into an 83.4% accuracy, 61.5% sensitivity, and 90.6% specificity, according to the report.
The 18F-flortaucipir tracer outperformed state-of-the-art MRI measures for distinguishing AD dementia from non-AD neurodegenerative disorders, including hippocampal volumes, AD-signature thickness, and whole-brain cortical thickness, Dr. Ossenkoppele and his colleagues found in a secondary analysis.
Likewise, certain analyses showed that specificity for AD dementia versus non-AD neurodegenerative disorders was higher with 18F-flortaucipir than with Abeta status.
While both MRI measures and Abeta status are increasingly used to evaluate cognitive impairment adjunctively in a clinical setting, the utility of 18F-flortaucipir PET as a diagnostic biomarker still must be determined, the investigators wrote in a discussion of their results.
“An intended clinical use of 18F-flortaucipir PET might be to improve the diagnostic work-up as an add-on test to Abeta biomarkers in patients with early-onset dementia and possibly as a triage or even replacement test in patients with late-onset dementia in whom incidental Abeta pathology is common,” they wrote.
The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies that are marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
SOURCE: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
PET imaging with an investigational tau tracer, 18F-flortaucipir, distinguished Alzheimer’s disease from other neurodegenerative disorders with a high degree of accuracy in a multicenter study.
PET with 18F-flortaucipir also outperformed structural MRI markers for Alzheimer’s disease (AD), and when compared against amyloid-beta (Abeta) biomarkers, showed higher specificity for an AD dementia diagnosis, researchers wrote in a report published online Sept. 18 in JAMA.
“The accuracy and potential utility of this test in patient care require further research in clinically more representative populations,” they wrote.
The study included 719 participants recruited from memory disorder clinics in Sweden, Korea, and California. That cohort included 179 patients with AD dementia, 254 who had non-AD neurodegenerative diseases, 126 with mild cognitive impairment (MCI), and 160 controls who were cognitively normal. All participants underwent 18F-flortaucipir PET between June 2014 and November 2017.
They found that 18F-flortaucipir showed “high accuracy” for distinguishing AD dementia from non-AD neurodegenerative disorders across all five regions of interest (ROI) evaluated in the study.
For example, 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal meta-ROI, they reported.
By contrast, the accuracy of 18F-flortaucipir was lower for distinguishing MCI caused by AD versus non-AD neurodegenerative conditions. In the temporal meta-ROI, that translated into an 83.4% accuracy, 61.5% sensitivity, and 90.6% specificity, according to the report.
The 18F-flortaucipir tracer outperformed state-of-the-art MRI measures for distinguishing AD dementia from non-AD neurodegenerative disorders, including hippocampal volumes, AD-signature thickness, and whole-brain cortical thickness, Dr. Ossenkoppele and his colleagues found in a secondary analysis.
Likewise, certain analyses showed that specificity for AD dementia versus non-AD neurodegenerative disorders was higher with 18F-flortaucipir than with Abeta status.
While both MRI measures and Abeta status are increasingly used to evaluate cognitive impairment adjunctively in a clinical setting, the utility of 18F-flortaucipir PET as a diagnostic biomarker still must be determined, the investigators wrote in a discussion of their results.
“An intended clinical use of 18F-flortaucipir PET might be to improve the diagnostic work-up as an add-on test to Abeta biomarkers in patients with early-onset dementia and possibly as a triage or even replacement test in patients with late-onset dementia in whom incidental Abeta pathology is common,” they wrote.
The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies that are marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
SOURCE: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
FROM JAMA
Key clinical point:
Major finding: PET with 18F-flortaucipir PET had 90.3% accuracy, 89.9% sensitivity, and 90.6% specificity in the temporal metaregion of interest.
Study details: A multicenter, cross-sectional study including 719 participants recruited from memory disorder clinics in Sweden, Korea, and California.
Disclosures: The study was sponsored by different sources at each of the clinic sites. Many authors disclosed relationships with companies marketing and/or developing PET imaging tracers, including Avid Radiopharmaceuticals/Eli Lilly, GE Healthcare, and Piramal, as well as other pharmaceutical companies.
Source: Ossenkoppele R et al. JAMA. 2018;320(11):1151-62.
Amantadine-Induced Livedo Reticularis in a Child Treated Off Label for Neurobehavioral Disorders
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
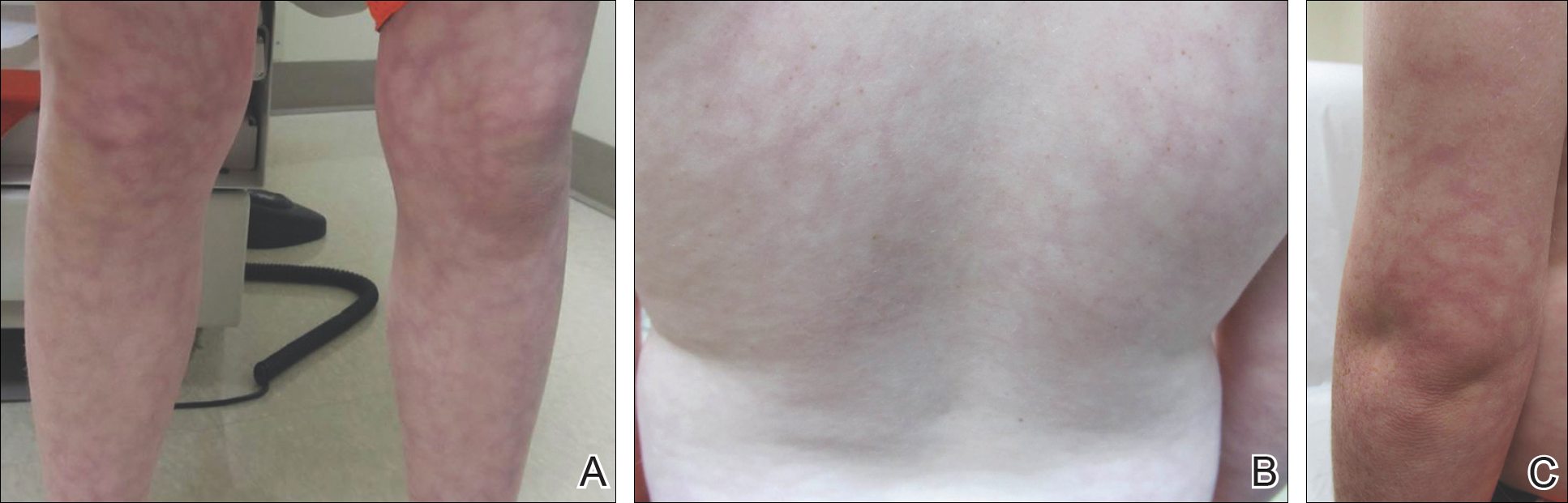
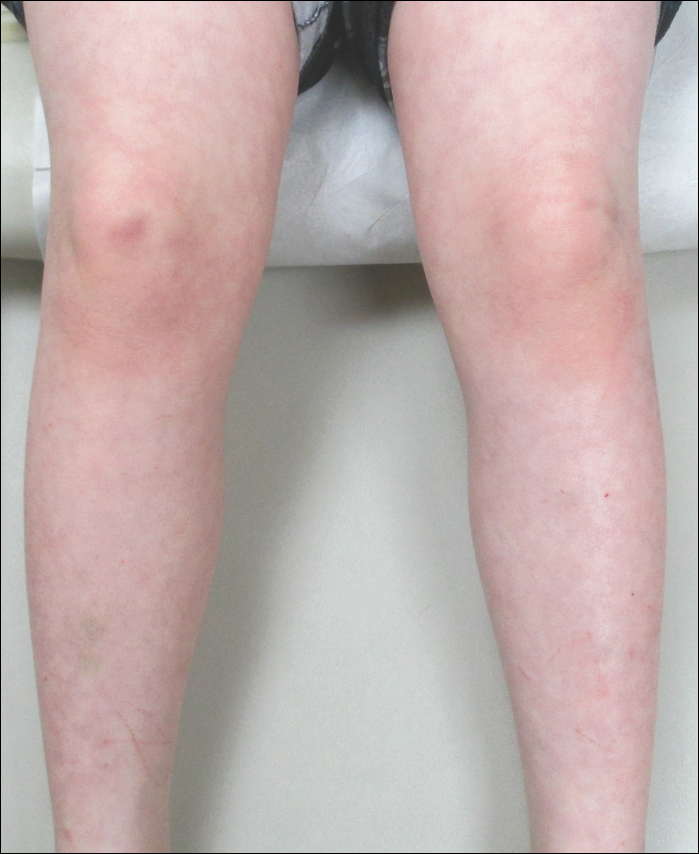
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).
Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Practice Points
- Amantadine is a generally well-tolerated medication that is more commonly used for off-label treatment of several pediatric neurobehavioral conditions such as attention deficit hyperactivity disorder, autism spectrum disorders, obsessive compulsive disorder, depression, and others.
- Livedo reticularis has known associations with several medications and diseases; however, the most common presentation is cutis marmorata, a benign condition that typically affects newborns.
Inframammary Macerated Erosion
The Diagnosis: Hailey-Hailey Disease (Benign Familial Chronic Pemphigus)
Our patient had a long-standing history of Hailey-Hailey disease, as confirmed by multiple prior skin biopsies at outside institutions as well as our affiliated site. He began treatment with oral doxycycline 50 mg twice daily for 2 weeks, triamcinolone cream 0.1% twice daily to the affected region, and aluminum acetate solution soaks and chlorhexidine wash daily along with petroleum jelly, which resulted in good control of the disease. The differential diagnosis of eroded plaques, particularly in the axillary, crural, and inframammary folds, is broad and includes candidiasis, inverse psoriasis, contact dermatitis, dermatophyte infection, pemphigus vegetans or foliaceus, and granular parakeratosis.
Hailey-Hailey disease is a genetic disorder with a prevalence of 1 in 50,000 individuals. Most patients develop symptoms during the second or third decades of life.1 Hailey-Hailey disease exhibits an autosomal-dominant pattern of inheritance secondary to mutation in the human ATP2C1 gene, which codes for the ATPase secretory pathway of the Ca2+ transporting pump type 1 (SPCA1) localized in the Golgi apparatus.2 Altered SPCA1 protein reduces concentration of Ca2+ within the Golgi lumen, which in turn impairs the processing of junctional proteins needed for normal cell-to-cell adhesion.1
Clinically, Hailey-Hailey disease is characterized by vesicular or erosive plaques that have a predilection for intertriginous areas of the body.1 The primary lesions often are flaccid vesicles that easily rupture, leaving behind crusted erosions that spread peripherally. The lesions also can appear as macerated plaques resembling torn tissue paper, as in our case. Friction, heat, and sweat exacerbate the disease. Complications occur from secondary bacterial, fungal, and viral colonization. Malodor and vegetations can indicate bacterial or fungal infections and can lead to persistence of skin lesions. Herpes simplex virus infections can exacerbate preexisting lesions.3 Hailey-Hailey disease of the anogenital region also can be complicated by infection with oncogenic strains of human papillomavirus and lead to cutaneous squamous cell carcinoma.4
Hailey-Hailey disease histologically appears as suprabasal and intraepidermal keratinocyte acantholysis,5 which typically is widespread in the epidermis, with large areas of dyscohesion with a dilapidated brick wall-like appearance.1 In more chronic lesions, epidermal hyperplasia, parakeratosis, and focal crusts may be observed. A moderate perivascular lymphocytic infiltrate can be observed in the superficial dermis. Direct immunofluorescence typically is negative.
Topical corticosteroids and antimicrobials are first-line therapies that often only provide temporary suppression. When the disease is refractory to topical therapies, intralesional corticosteroids may be attempted. There is no strong evidence to support the use of systemic therapy, aside from antimicrobial agents (eg, doxycycline) for the use of superinfections. In severe cases, immunomodulating therapies such as prednisone, cyclosporine, methotrexate, dapsone, alefacept, and oral retinoids may be effective.6-8 Surgical therapy also can be considered for recalcitrant disease, including wide excision and grafting, though these techniques can be associated with morbidity.9
Superficial ablative techniques including dermabrasion, laser therapy with CO2 and erbium-doped YAG, photodynamic therapy, and electron beam radiation have been shown to be effective modalities in severe cases.5,9-11 It has been hypothesized that keratinocytes expressing the molecular defect are ablated, while the surrounding normal adnexal epithelium can regenerate normal epithelium. It also is thought that dermal fibrosis leads to better support of the diseased epidermis and decreases the risk for ulceration and fissuring.9
- Hohl D. Darier disease and Hailey-Hailey disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:887-896.
- Micaroni M, Giacchetti G, Plebani R, et al. ATP2C1 gene mutations in Hailey-Hailey disease and possible roles of SPCA1 isoforms in membrane trafficking. Cell Death Dis. 2016;7:E2259.
- Peppiatt T, Keefe M,White JE. Hailey-Hailey disease--exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 1992;17:201-202.
- Chen MY, Chiu HC, Su LH, et al. Presence of human papillomavirus type 6 DNA in the perineal verrucoid lesions of Hailey-Hailey disease. J Eur Acad Dermatol Venereol. 2006;20:1356-1357.
- Graham PM, Melkonian A, Fivenson D. Familial benign chronic pemphigus (Hailey-Hailey disease) treated with electron beam radiation. JAAD Case Rep. 2016;2:159-161.
- Berth-Jones J, Smith SG, Graham-Brown RA, et al. Benign familial chronic pemphigus (Hailey-Hailey disease) responds to cyclosporin. Clin Exp Dermatol. 1995;20:70-72.
- Sire DJ, Johnson BL. Benign familial chronic pemphigus treated with dapsone. Arch Dermatol. 1971;103:262-265.
- Hunt MJ, Salisbury EL, Painter DM. Vesiculobullous Hailey-Hailey disease: successful treatment with oral retinoids. Australas J Dermatol. 1996;37:196-198.
- Ortiz AE, Zachary CB. Laser therapy for Hailey-Hailey disease: review of the literature and a case report. Dermatol Reports. 2011;3:E28.
- Don PC, Carney PS, Lynch WS, et al. Carbon dioxide laserabrasion: a new approach to management of familial benign chronic pemphigus (Hailey-Hailey disease). J Dermatol Surg Oncol. 1987;13:1187-1194.
- Beier C, Kaufmann R. Efficacy of erbium:YAG laser ablation in Darier disease and Hailey-Hailey disease. Arch Dermatol. 1999;135:423-427.
The Diagnosis: Hailey-Hailey Disease (Benign Familial Chronic Pemphigus)
Our patient had a long-standing history of Hailey-Hailey disease, as confirmed by multiple prior skin biopsies at outside institutions as well as our affiliated site. He began treatment with oral doxycycline 50 mg twice daily for 2 weeks, triamcinolone cream 0.1% twice daily to the affected region, and aluminum acetate solution soaks and chlorhexidine wash daily along with petroleum jelly, which resulted in good control of the disease. The differential diagnosis of eroded plaques, particularly in the axillary, crural, and inframammary folds, is broad and includes candidiasis, inverse psoriasis, contact dermatitis, dermatophyte infection, pemphigus vegetans or foliaceus, and granular parakeratosis.
Hailey-Hailey disease is a genetic disorder with a prevalence of 1 in 50,000 individuals. Most patients develop symptoms during the second or third decades of life.1 Hailey-Hailey disease exhibits an autosomal-dominant pattern of inheritance secondary to mutation in the human ATP2C1 gene, which codes for the ATPase secretory pathway of the Ca2+ transporting pump type 1 (SPCA1) localized in the Golgi apparatus.2 Altered SPCA1 protein reduces concentration of Ca2+ within the Golgi lumen, which in turn impairs the processing of junctional proteins needed for normal cell-to-cell adhesion.1
Clinically, Hailey-Hailey disease is characterized by vesicular or erosive plaques that have a predilection for intertriginous areas of the body.1 The primary lesions often are flaccid vesicles that easily rupture, leaving behind crusted erosions that spread peripherally. The lesions also can appear as macerated plaques resembling torn tissue paper, as in our case. Friction, heat, and sweat exacerbate the disease. Complications occur from secondary bacterial, fungal, and viral colonization. Malodor and vegetations can indicate bacterial or fungal infections and can lead to persistence of skin lesions. Herpes simplex virus infections can exacerbate preexisting lesions.3 Hailey-Hailey disease of the anogenital region also can be complicated by infection with oncogenic strains of human papillomavirus and lead to cutaneous squamous cell carcinoma.4
Hailey-Hailey disease histologically appears as suprabasal and intraepidermal keratinocyte acantholysis,5 which typically is widespread in the epidermis, with large areas of dyscohesion with a dilapidated brick wall-like appearance.1 In more chronic lesions, epidermal hyperplasia, parakeratosis, and focal crusts may be observed. A moderate perivascular lymphocytic infiltrate can be observed in the superficial dermis. Direct immunofluorescence typically is negative.
Topical corticosteroids and antimicrobials are first-line therapies that often only provide temporary suppression. When the disease is refractory to topical therapies, intralesional corticosteroids may be attempted. There is no strong evidence to support the use of systemic therapy, aside from antimicrobial agents (eg, doxycycline) for the use of superinfections. In severe cases, immunomodulating therapies such as prednisone, cyclosporine, methotrexate, dapsone, alefacept, and oral retinoids may be effective.6-8 Surgical therapy also can be considered for recalcitrant disease, including wide excision and grafting, though these techniques can be associated with morbidity.9
Superficial ablative techniques including dermabrasion, laser therapy with CO2 and erbium-doped YAG, photodynamic therapy, and electron beam radiation have been shown to be effective modalities in severe cases.5,9-11 It has been hypothesized that keratinocytes expressing the molecular defect are ablated, while the surrounding normal adnexal epithelium can regenerate normal epithelium. It also is thought that dermal fibrosis leads to better support of the diseased epidermis and decreases the risk for ulceration and fissuring.9
The Diagnosis: Hailey-Hailey Disease (Benign Familial Chronic Pemphigus)
Our patient had a long-standing history of Hailey-Hailey disease, as confirmed by multiple prior skin biopsies at outside institutions as well as our affiliated site. He began treatment with oral doxycycline 50 mg twice daily for 2 weeks, triamcinolone cream 0.1% twice daily to the affected region, and aluminum acetate solution soaks and chlorhexidine wash daily along with petroleum jelly, which resulted in good control of the disease. The differential diagnosis of eroded plaques, particularly in the axillary, crural, and inframammary folds, is broad and includes candidiasis, inverse psoriasis, contact dermatitis, dermatophyte infection, pemphigus vegetans or foliaceus, and granular parakeratosis.
Hailey-Hailey disease is a genetic disorder with a prevalence of 1 in 50,000 individuals. Most patients develop symptoms during the second or third decades of life.1 Hailey-Hailey disease exhibits an autosomal-dominant pattern of inheritance secondary to mutation in the human ATP2C1 gene, which codes for the ATPase secretory pathway of the Ca2+ transporting pump type 1 (SPCA1) localized in the Golgi apparatus.2 Altered SPCA1 protein reduces concentration of Ca2+ within the Golgi lumen, which in turn impairs the processing of junctional proteins needed for normal cell-to-cell adhesion.1
Clinically, Hailey-Hailey disease is characterized by vesicular or erosive plaques that have a predilection for intertriginous areas of the body.1 The primary lesions often are flaccid vesicles that easily rupture, leaving behind crusted erosions that spread peripherally. The lesions also can appear as macerated plaques resembling torn tissue paper, as in our case. Friction, heat, and sweat exacerbate the disease. Complications occur from secondary bacterial, fungal, and viral colonization. Malodor and vegetations can indicate bacterial or fungal infections and can lead to persistence of skin lesions. Herpes simplex virus infections can exacerbate preexisting lesions.3 Hailey-Hailey disease of the anogenital region also can be complicated by infection with oncogenic strains of human papillomavirus and lead to cutaneous squamous cell carcinoma.4
Hailey-Hailey disease histologically appears as suprabasal and intraepidermal keratinocyte acantholysis,5 which typically is widespread in the epidermis, with large areas of dyscohesion with a dilapidated brick wall-like appearance.1 In more chronic lesions, epidermal hyperplasia, parakeratosis, and focal crusts may be observed. A moderate perivascular lymphocytic infiltrate can be observed in the superficial dermis. Direct immunofluorescence typically is negative.
Topical corticosteroids and antimicrobials are first-line therapies that often only provide temporary suppression. When the disease is refractory to topical therapies, intralesional corticosteroids may be attempted. There is no strong evidence to support the use of systemic therapy, aside from antimicrobial agents (eg, doxycycline) for the use of superinfections. In severe cases, immunomodulating therapies such as prednisone, cyclosporine, methotrexate, dapsone, alefacept, and oral retinoids may be effective.6-8 Surgical therapy also can be considered for recalcitrant disease, including wide excision and grafting, though these techniques can be associated with morbidity.9
Superficial ablative techniques including dermabrasion, laser therapy with CO2 and erbium-doped YAG, photodynamic therapy, and electron beam radiation have been shown to be effective modalities in severe cases.5,9-11 It has been hypothesized that keratinocytes expressing the molecular defect are ablated, while the surrounding normal adnexal epithelium can regenerate normal epithelium. It also is thought that dermal fibrosis leads to better support of the diseased epidermis and decreases the risk for ulceration and fissuring.9
- Hohl D. Darier disease and Hailey-Hailey disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:887-896.
- Micaroni M, Giacchetti G, Plebani R, et al. ATP2C1 gene mutations in Hailey-Hailey disease and possible roles of SPCA1 isoforms in membrane trafficking. Cell Death Dis. 2016;7:E2259.
- Peppiatt T, Keefe M,White JE. Hailey-Hailey disease--exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 1992;17:201-202.
- Chen MY, Chiu HC, Su LH, et al. Presence of human papillomavirus type 6 DNA in the perineal verrucoid lesions of Hailey-Hailey disease. J Eur Acad Dermatol Venereol. 2006;20:1356-1357.
- Graham PM, Melkonian A, Fivenson D. Familial benign chronic pemphigus (Hailey-Hailey disease) treated with electron beam radiation. JAAD Case Rep. 2016;2:159-161.
- Berth-Jones J, Smith SG, Graham-Brown RA, et al. Benign familial chronic pemphigus (Hailey-Hailey disease) responds to cyclosporin. Clin Exp Dermatol. 1995;20:70-72.
- Sire DJ, Johnson BL. Benign familial chronic pemphigus treated with dapsone. Arch Dermatol. 1971;103:262-265.
- Hunt MJ, Salisbury EL, Painter DM. Vesiculobullous Hailey-Hailey disease: successful treatment with oral retinoids. Australas J Dermatol. 1996;37:196-198.
- Ortiz AE, Zachary CB. Laser therapy for Hailey-Hailey disease: review of the literature and a case report. Dermatol Reports. 2011;3:E28.
- Don PC, Carney PS, Lynch WS, et al. Carbon dioxide laserabrasion: a new approach to management of familial benign chronic pemphigus (Hailey-Hailey disease). J Dermatol Surg Oncol. 1987;13:1187-1194.
- Beier C, Kaufmann R. Efficacy of erbium:YAG laser ablation in Darier disease and Hailey-Hailey disease. Arch Dermatol. 1999;135:423-427.
- Hohl D. Darier disease and Hailey-Hailey disease. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. China: Elsevier Saunders; 2012:887-896.
- Micaroni M, Giacchetti G, Plebani R, et al. ATP2C1 gene mutations in Hailey-Hailey disease and possible roles of SPCA1 isoforms in membrane trafficking. Cell Death Dis. 2016;7:E2259.
- Peppiatt T, Keefe M,White JE. Hailey-Hailey disease--exacerbation by herpes simplex virus and patch tests. Clin Exp Dermatol. 1992;17:201-202.
- Chen MY, Chiu HC, Su LH, et al. Presence of human papillomavirus type 6 DNA in the perineal verrucoid lesions of Hailey-Hailey disease. J Eur Acad Dermatol Venereol. 2006;20:1356-1357.
- Graham PM, Melkonian A, Fivenson D. Familial benign chronic pemphigus (Hailey-Hailey disease) treated with electron beam radiation. JAAD Case Rep. 2016;2:159-161.
- Berth-Jones J, Smith SG, Graham-Brown RA, et al. Benign familial chronic pemphigus (Hailey-Hailey disease) responds to cyclosporin. Clin Exp Dermatol. 1995;20:70-72.
- Sire DJ, Johnson BL. Benign familial chronic pemphigus treated with dapsone. Arch Dermatol. 1971;103:262-265.
- Hunt MJ, Salisbury EL, Painter DM. Vesiculobullous Hailey-Hailey disease: successful treatment with oral retinoids. Australas J Dermatol. 1996;37:196-198.
- Ortiz AE, Zachary CB. Laser therapy for Hailey-Hailey disease: review of the literature and a case report. Dermatol Reports. 2011;3:E28.
- Don PC, Carney PS, Lynch WS, et al. Carbon dioxide laserabrasion: a new approach to management of familial benign chronic pemphigus (Hailey-Hailey disease). J Dermatol Surg Oncol. 1987;13:1187-1194.
- Beier C, Kaufmann R. Efficacy of erbium:YAG laser ablation in Darier disease and Hailey-Hailey disease. Arch Dermatol. 1999;135:423-427.
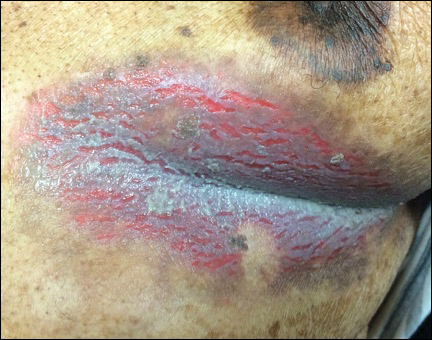
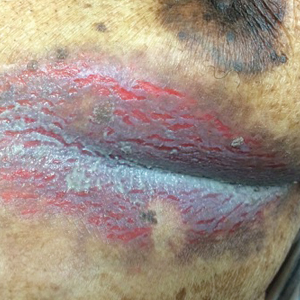
An 81-year-old man presented with a painful erosion in the left inframammary region of 2 weeks' duration. He described the lesion as pruritic and burning. He reported having prior similar episodes in the bilateral groin, axilla, and lower abdomen that often were malodorous. Use of triamcinolone cream 0.1% up to 4 times daily resulted in little relief of the erosion. Of note, the patient reported therapies for prior sites had included oral doxycycline 50 mg twice daily, clobetasol cream, and clindamycin solution, which provided limited relief but eventual resolution. Application of cold aluminum acetate solution compresses for 5 minutes daily irritated the skin even further and led to bleeding at the affected sites. The patient's father had a history of similar skin lesions.
Best practices defined for proton therapy in mediastinal lymphomas
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but only when this modality offers a clear advantage over intensity-modulated radiotherapy, according to new guidelines from the International Lymphoma Radiation Oncology Group.
Proton therapy reduces radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart. The advantages are not always clear in other situations, such as when the target spans the right side of the heart, or when the target is above the heart with no axillary involvement, according to guideline authors Bouthaina Dabaja, MD, professor and section chief of hematology in the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” Dr. Dabaja and her coauthors said in the guidelines, which were published in the journal Blood (doi: 10.1182/blood-2018-03-837633).
Along with intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy, proton therapy presents “another opportunity” for more conformal dose distribution and better sparing of organs at risk, according to the consensus recommendations. Proton therapy can greatly benefit certain patients with mediastinal disease. These include young female patients to reduce breast dose and risk of a secondary breast cancer, patients at high risk of radiation-related toxicity due to previous treatment, and patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines. However, the consideration of proton therapy needs to factor the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy (PSPT) is the least complex delivery technique, it is challenging because beams can conform only to one side of the target; by contrast, the experts said, active mode pencil beam scanning proton therapy (PBSPT) potentially provides better conformity and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said in their report.
However, motion management is “of prime importance” with PBSPT, which is more sensitive to density changes in the beam path as compared to PSPT, they added. Toward that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
The guidelines list a total of 11 authors representing The University of Texas MD Anderson Cancer Center; University of Florida, Jacksonville; University of Pennsylvania, Philadelphia; University of Louisiana, Baton Rouge; Proton Therapy Center Czech, Prague; Motol University Hospital, Czech Republic; St. Thomas and Guy Hospital, London; Institut Curie, Paris; and Rigshospitalet, Copenhagen.
Dr. Dabaja and her guideline coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but only when this modality offers a clear advantage over intensity-modulated radiotherapy, according to new guidelines from the International Lymphoma Radiation Oncology Group.
Proton therapy reduces radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart. The advantages are not always clear in other situations, such as when the target spans the right side of the heart, or when the target is above the heart with no axillary involvement, according to guideline authors Bouthaina Dabaja, MD, professor and section chief of hematology in the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” Dr. Dabaja and her coauthors said in the guidelines, which were published in the journal Blood (doi: 10.1182/blood-2018-03-837633).
Along with intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy, proton therapy presents “another opportunity” for more conformal dose distribution and better sparing of organs at risk, according to the consensus recommendations. Proton therapy can greatly benefit certain patients with mediastinal disease. These include young female patients to reduce breast dose and risk of a secondary breast cancer, patients at high risk of radiation-related toxicity due to previous treatment, and patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines. However, the consideration of proton therapy needs to factor the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy (PSPT) is the least complex delivery technique, it is challenging because beams can conform only to one side of the target; by contrast, the experts said, active mode pencil beam scanning proton therapy (PBSPT) potentially provides better conformity and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said in their report.
However, motion management is “of prime importance” with PBSPT, which is more sensitive to density changes in the beam path as compared to PSPT, they added. Toward that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
The guidelines list a total of 11 authors representing The University of Texas MD Anderson Cancer Center; University of Florida, Jacksonville; University of Pennsylvania, Philadelphia; University of Louisiana, Baton Rouge; Proton Therapy Center Czech, Prague; Motol University Hospital, Czech Republic; St. Thomas and Guy Hospital, London; Institut Curie, Paris; and Rigshospitalet, Copenhagen.
Dr. Dabaja and her guideline coauthors reported no funding or conflicts of interest.
Proton therapy can help mitigate toxicity in adults with mediastinal lymphomas, but only when this modality offers a clear advantage over intensity-modulated radiotherapy, according to new guidelines from the International Lymphoma Radiation Oncology Group.
Proton therapy reduces radiation dose to organs at risk in certain clinical presentations, such as when the mediastinal target is on both sides of the heart. The advantages are not always clear in other situations, such as when the target spans the right side of the heart, or when the target is above the heart with no axillary involvement, according to guideline authors Bouthaina Dabaja, MD, professor and section chief of hematology in the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and her colleagues.
“The limited availability of proton therapy calls for case selection based on a clear understanding of which cases will derive most benefit from proton therapy as compared to advanced photon techniques,” Dr. Dabaja and her coauthors said in the guidelines, which were published in the journal Blood (doi: 10.1182/blood-2018-03-837633).
Along with intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy, proton therapy presents “another opportunity” for more conformal dose distribution and better sparing of organs at risk, according to the consensus recommendations. Proton therapy can greatly benefit certain patients with mediastinal disease. These include young female patients to reduce breast dose and risk of a secondary breast cancer, patients at high risk of radiation-related toxicity due to previous treatment, and patients with disease spanning below the origin of the left main stem coronary artery that is anterior to, posterior to, or on the left side of the heart.
“The relation of disease to organs at risk determines the situations in which proton therapy is most beneficial,” the experts said in the guidelines. However, the consideration of proton therapy needs to factor the complexities of proton therapy planning, the need to manage uncertainties, and the “evolving nature of the technology,” which includes the development of pencil beam scanning.
While passive scattering proton therapy (PSPT) is the least complex delivery technique, it is challenging because beams can conform only to one side of the target; by contrast, the experts said, active mode pencil beam scanning proton therapy (PBSPT) potentially provides better conformity and sparing of organs at risk.
“Because treatment involves delivery of individual controlled spots, inhomogenous doses can be created deliberately,” the guideline authors said in their report.
However, motion management is “of prime importance” with PBSPT, which is more sensitive to density changes in the beam path as compared to PSPT, they added. Toward that end, physicians should pay close attention to evaluating intrafractional movement, which is frequently tied to the breathing cycle.
The guidelines list a total of 11 authors representing The University of Texas MD Anderson Cancer Center; University of Florida, Jacksonville; University of Pennsylvania, Philadelphia; University of Louisiana, Baton Rouge; Proton Therapy Center Czech, Prague; Motol University Hospital, Czech Republic; St. Thomas and Guy Hospital, London; Institut Curie, Paris; and Rigshospitalet, Copenhagen.
Dr. Dabaja and her guideline coauthors reported no funding or conflicts of interest.
FROM BLOOD
U.S. perspective: Euro hypertension guidelines look a lot like ours
MUNICH – The “overwhelming impression” that Paul K. Whelton, MD, has of the newly revised hypertension diagnosis and management guidelines of the European Society of Cardiology is their similarity to hypertension guidelines released by the American College of Cardiology and American Heart Association in November 2017.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“We both recommend the same treatment target, of less than 130/80 mm Hg,” noted Dr. Whelton, professor at Tulane University in New Orleans, although the European guidelines (Euro J Cardiology. 2018 Sep 1; 39[33]:3021-104) put more qualifications on this target and specify treating to no lower than 130 mm Hg systolic pressure in patients who are at least 65 years old as well as in patients with chronic kidney disease at any age. In a video interview, Dr. Whelton also cited areas of disagreement, such as how patients with an untreated blood pressure of 130-139 mm Hg are classified (high normal in the European guidelines, stage 1 hypertension in the U.S. guidelines), and whether initial drug monotherapy is a reasonable treatment strategy (U.S. says yes, Europe says no).
Dr. Whelton noted that recent modeling studies have documented the potential public health benefits from following the diagnosis and management approaches set forth in the 2017 U.S. guidelines (J Am Coll Cardiol. 2018 May;71[19]:e127-e248). For example, an analysis based on data collected by the U.S. National Health and Nutrition Examination Survey during 2013-2016 showed that following the 2017 guidelines for diagnosing and treating hypertension would have resulted in prevention of more than twice the number of cardiovascular disease events nationally as compared with application of the prior, 2014 U.S. hypertension guideline (JAMA. 2014 Feb 5;311[5]:507-20): 610,000 events prevented, compared with 270,000 events prevented. The same study showed that the 2017 guidelines would have nearly doubled the number of all-cause deaths prevented, with 334,000 deaths prevented, compared with 177,000 prevented by applying the 2014 guidelines (JAMA Cardiology. 2018 July;3[7]:572-81).
Dr. Whelton had no commercial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MUNICH – The “overwhelming impression” that Paul K. Whelton, MD, has of the newly revised hypertension diagnosis and management guidelines of the European Society of Cardiology is their similarity to hypertension guidelines released by the American College of Cardiology and American Heart Association in November 2017.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“We both recommend the same treatment target, of less than 130/80 mm Hg,” noted Dr. Whelton, professor at Tulane University in New Orleans, although the European guidelines (Euro J Cardiology. 2018 Sep 1; 39[33]:3021-104) put more qualifications on this target and specify treating to no lower than 130 mm Hg systolic pressure in patients who are at least 65 years old as well as in patients with chronic kidney disease at any age. In a video interview, Dr. Whelton also cited areas of disagreement, such as how patients with an untreated blood pressure of 130-139 mm Hg are classified (high normal in the European guidelines, stage 1 hypertension in the U.S. guidelines), and whether initial drug monotherapy is a reasonable treatment strategy (U.S. says yes, Europe says no).
Dr. Whelton noted that recent modeling studies have documented the potential public health benefits from following the diagnosis and management approaches set forth in the 2017 U.S. guidelines (J Am Coll Cardiol. 2018 May;71[19]:e127-e248). For example, an analysis based on data collected by the U.S. National Health and Nutrition Examination Survey during 2013-2016 showed that following the 2017 guidelines for diagnosing and treating hypertension would have resulted in prevention of more than twice the number of cardiovascular disease events nationally as compared with application of the prior, 2014 U.S. hypertension guideline (JAMA. 2014 Feb 5;311[5]:507-20): 610,000 events prevented, compared with 270,000 events prevented. The same study showed that the 2017 guidelines would have nearly doubled the number of all-cause deaths prevented, with 334,000 deaths prevented, compared with 177,000 prevented by applying the 2014 guidelines (JAMA Cardiology. 2018 July;3[7]:572-81).
Dr. Whelton had no commercial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MUNICH – The “overwhelming impression” that Paul K. Whelton, MD, has of the newly revised hypertension diagnosis and management guidelines of the European Society of Cardiology is their similarity to hypertension guidelines released by the American College of Cardiology and American Heart Association in November 2017.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“We both recommend the same treatment target, of less than 130/80 mm Hg,” noted Dr. Whelton, professor at Tulane University in New Orleans, although the European guidelines (Euro J Cardiology. 2018 Sep 1; 39[33]:3021-104) put more qualifications on this target and specify treating to no lower than 130 mm Hg systolic pressure in patients who are at least 65 years old as well as in patients with chronic kidney disease at any age. In a video interview, Dr. Whelton also cited areas of disagreement, such as how patients with an untreated blood pressure of 130-139 mm Hg are classified (high normal in the European guidelines, stage 1 hypertension in the U.S. guidelines), and whether initial drug monotherapy is a reasonable treatment strategy (U.S. says yes, Europe says no).
Dr. Whelton noted that recent modeling studies have documented the potential public health benefits from following the diagnosis and management approaches set forth in the 2017 U.S. guidelines (J Am Coll Cardiol. 2018 May;71[19]:e127-e248). For example, an analysis based on data collected by the U.S. National Health and Nutrition Examination Survey during 2013-2016 showed that following the 2017 guidelines for diagnosing and treating hypertension would have resulted in prevention of more than twice the number of cardiovascular disease events nationally as compared with application of the prior, 2014 U.S. hypertension guideline (JAMA. 2014 Feb 5;311[5]:507-20): 610,000 events prevented, compared with 270,000 events prevented. The same study showed that the 2017 guidelines would have nearly doubled the number of all-cause deaths prevented, with 334,000 deaths prevented, compared with 177,000 prevented by applying the 2014 guidelines (JAMA Cardiology. 2018 July;3[7]:572-81).
Dr. Whelton had no commercial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM THE ESC CONGRESS 2018
New Euro hypertension guidelines target most adults to less than 130/80 mm Hg
MUNICH – The European Society of Cardiology joined other international cardiology groups in endorsing lower targets for blood pressure treatment and lower pressure thresholds for starting drug treatment in its revised hypertension diagnosis and management guidelines released in August during the Society’s annual congress here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Provided that treatment is well tolerated, treated blood pressure should be targeted to 130/80 mm Hg or lower in most patients,” Giuseppe Mancia, MD, said as he and his colleagues presented the new guidelines at the annual congress of the European Society of Cardiology.
Although the new European guidelines further buttress this more aggressive approach to blood pressure management that first appeared almost a year ago in U.S. guidelines from the American College of Cardiology and the American Heart Association, an approach that remains controversial among U.S. primary care physicians, the European strategy (Eur Heart J. 2018 Sep 1;39[33]:3021-104) was generally more cautious than the broader endorsement of lower blood pressure goals advanced by the U.S. recommendations (J Am Coll Cardiol. 2018 May;71[19]:e127-e248).
In the European approach, “the first objective is to treat to less than 140/90 mm Hg. If this is well tolerated, then treat to less than 130/80 mm Hg in most patients,” said Dr. Mancia, cochair of the European writing panel. Further pressure reductions to less than 130/80 mm Hg are usually harder, the incremental benefit from further reduction is less than when pressures first fall below 140/90 mm Hg, and the evidence for incremental benefit of any size from further pressure reduction is less strong for certain key patient subgroups: people at least 80 years old, and patients with diabetes, chronic kidney disease, or coronary artery disease, said Dr. Mancia, an emeritus professor at the University of Milan.
“The consistency between two major guidelines is important, but there are differences that may look subtle but are not subtle,” he said in a video interview. “If a patient gets to less than 140 mm Hg, the doctor should not think that’s a failure; it’s a very important result.”
One striking example of how the two guidelines differ on treatment targets is for people at least 80 years old. The overall blood pressure threshold for starting drug treatment in patients this age in the European guidelines is 160/90 mm Hg, although it remains at 140/90 for people aged 65-79 years old “provided that treatment is well tolerated” and the patients are “fit.” The 2017 U.S. guidelines, by contrast, say that considering drug treatment for everyone with a pressure at or above 130/80 mm Hg is a class I recommendation regardless of age as long as the person is “noninstitutionalized, ambulatory, [and] community-dwelling.” Once an older patient of any age, 65 years or older, starts drug treatment to reduce blood pressure, the European guidelines allow for treating to a target systolic blood pressure of 130-139 mm Hg as long as the regimen is well tolerated, and the guidelines say that a diastolic pressure target of less than 80 mm Hg should be considered for all adults regardless of age.
The new European guidelines also define adults with an untreated pressure of 130-139/85-89 mm Hg as “high normal,” rather than the “stage 1 hypertension” designation given to people with pressures of 130-139/80-89 mm Hg in the U.S. guidelines. Robert M. Carey, MD, vice chair of the U.S. guidelines panel, minimized this as a “semantic” difference, and he highlighted that management of people with pressures in this range is roughly similar in the two sets of recommendations. “The name is different, but treatment is the same,” Dr. Carey said.
The European guidelines call for initial lifestyle interventions, followed by drug treatment “that may be considered” for patients who have “very-high” cardiovascular risk because of established cardiovascular disease, especially coronary artery disease, and detail the specific clinical conditions that fall into the very-high-risk category. The U.S. guidelines say that stage 1 hypertension patients should get lifestyle interventions, followed by drug treatment for the roughly 30% of patients in this category who score at least a 10% 10-year risk on the American College of Cardiology’s Atherosclerotic Cardiovascular Disease Risk Estimator Plus.The new European guidelines are a “validation” of the ACC/AHA 2017 guidelines, commented Dr. Carey, a professor of medicine at the University of Virginia in Charlottesville. “Overall, we’re delighted to have these two major groups” agree, he said in an interview. “There is a tremendous amount of concurrence.”
Other areas of agreement between the two guidelines include their emphasis on careful and repeated blood pressure measurement, including out-of-office measurement, before settling on a diagnosis of hypertension; systematic assessment of possible masked or white-coat hypertension in selected people; and frequent use of combined drug treatment including initiation of a dual-drug, single-pill regimen when starting drug treatment and aggressive follow-up by adding a third drug when needed. However, in another divergence the U.S. guidelines give a much stronger endorsement to starting treatment with monotherapy, a strategy the European guidelines scrapped.
Dr. Carey also noted that the European endorsement of three antihypertensives formulated into a single pill for patients who need more than two drugs would be difficult for American clinicians to follow as virtually no such formulations are approved for U.S. use.
Dr. Mancini has received honoraria from Boehringer Ingelheim, CVRx, Daiichi Sankyo, Ferrer, Medtronic, Menarini, Merck, Novartis, Recordati, and Servier. Dr. Williams has been a consultant to Novartis, Relypsa, and Vascular Dynamics, and he has spoken on behalf of Boehringer Ingelheim, Daiichi Sankyo, Novartis, and Servier. Dr. Carey and Dr. Whelton had no commercial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MUNICH – The European Society of Cardiology joined other international cardiology groups in endorsing lower targets for blood pressure treatment and lower pressure thresholds for starting drug treatment in its revised hypertension diagnosis and management guidelines released in August during the Society’s annual congress here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Provided that treatment is well tolerated, treated blood pressure should be targeted to 130/80 mm Hg or lower in most patients,” Giuseppe Mancia, MD, said as he and his colleagues presented the new guidelines at the annual congress of the European Society of Cardiology.
Although the new European guidelines further buttress this more aggressive approach to blood pressure management that first appeared almost a year ago in U.S. guidelines from the American College of Cardiology and the American Heart Association, an approach that remains controversial among U.S. primary care physicians, the European strategy (Eur Heart J. 2018 Sep 1;39[33]:3021-104) was generally more cautious than the broader endorsement of lower blood pressure goals advanced by the U.S. recommendations (J Am Coll Cardiol. 2018 May;71[19]:e127-e248).
In the European approach, “the first objective is to treat to less than 140/90 mm Hg. If this is well tolerated, then treat to less than 130/80 mm Hg in most patients,” said Dr. Mancia, cochair of the European writing panel. Further pressure reductions to less than 130/80 mm Hg are usually harder, the incremental benefit from further reduction is less than when pressures first fall below 140/90 mm Hg, and the evidence for incremental benefit of any size from further pressure reduction is less strong for certain key patient subgroups: people at least 80 years old, and patients with diabetes, chronic kidney disease, or coronary artery disease, said Dr. Mancia, an emeritus professor at the University of Milan.
“The consistency between two major guidelines is important, but there are differences that may look subtle but are not subtle,” he said in a video interview. “If a patient gets to less than 140 mm Hg, the doctor should not think that’s a failure; it’s a very important result.”
One striking example of how the two guidelines differ on treatment targets is for people at least 80 years old. The overall blood pressure threshold for starting drug treatment in patients this age in the European guidelines is 160/90 mm Hg, although it remains at 140/90 for people aged 65-79 years old “provided that treatment is well tolerated” and the patients are “fit.” The 2017 U.S. guidelines, by contrast, say that considering drug treatment for everyone with a pressure at or above 130/80 mm Hg is a class I recommendation regardless of age as long as the person is “noninstitutionalized, ambulatory, [and] community-dwelling.” Once an older patient of any age, 65 years or older, starts drug treatment to reduce blood pressure, the European guidelines allow for treating to a target systolic blood pressure of 130-139 mm Hg as long as the regimen is well tolerated, and the guidelines say that a diastolic pressure target of less than 80 mm Hg should be considered for all adults regardless of age.
The new European guidelines also define adults with an untreated pressure of 130-139/85-89 mm Hg as “high normal,” rather than the “stage 1 hypertension” designation given to people with pressures of 130-139/80-89 mm Hg in the U.S. guidelines. Robert M. Carey, MD, vice chair of the U.S. guidelines panel, minimized this as a “semantic” difference, and he highlighted that management of people with pressures in this range is roughly similar in the two sets of recommendations. “The name is different, but treatment is the same,” Dr. Carey said.
The European guidelines call for initial lifestyle interventions, followed by drug treatment “that may be considered” for patients who have “very-high” cardiovascular risk because of established cardiovascular disease, especially coronary artery disease, and detail the specific clinical conditions that fall into the very-high-risk category. The U.S. guidelines say that stage 1 hypertension patients should get lifestyle interventions, followed by drug treatment for the roughly 30% of patients in this category who score at least a 10% 10-year risk on the American College of Cardiology’s Atherosclerotic Cardiovascular Disease Risk Estimator Plus.The new European guidelines are a “validation” of the ACC/AHA 2017 guidelines, commented Dr. Carey, a professor of medicine at the University of Virginia in Charlottesville. “Overall, we’re delighted to have these two major groups” agree, he said in an interview. “There is a tremendous amount of concurrence.”
Other areas of agreement between the two guidelines include their emphasis on careful and repeated blood pressure measurement, including out-of-office measurement, before settling on a diagnosis of hypertension; systematic assessment of possible masked or white-coat hypertension in selected people; and frequent use of combined drug treatment including initiation of a dual-drug, single-pill regimen when starting drug treatment and aggressive follow-up by adding a third drug when needed. However, in another divergence the U.S. guidelines give a much stronger endorsement to starting treatment with monotherapy, a strategy the European guidelines scrapped.
Dr. Carey also noted that the European endorsement of three antihypertensives formulated into a single pill for patients who need more than two drugs would be difficult for American clinicians to follow as virtually no such formulations are approved for U.S. use.
Dr. Mancini has received honoraria from Boehringer Ingelheim, CVRx, Daiichi Sankyo, Ferrer, Medtronic, Menarini, Merck, Novartis, Recordati, and Servier. Dr. Williams has been a consultant to Novartis, Relypsa, and Vascular Dynamics, and he has spoken on behalf of Boehringer Ingelheim, Daiichi Sankyo, Novartis, and Servier. Dr. Carey and Dr. Whelton had no commercial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
MUNICH – The European Society of Cardiology joined other international cardiology groups in endorsing lower targets for blood pressure treatment and lower pressure thresholds for starting drug treatment in its revised hypertension diagnosis and management guidelines released in August during the Society’s annual congress here.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Provided that treatment is well tolerated, treated blood pressure should be targeted to 130/80 mm Hg or lower in most patients,” Giuseppe Mancia, MD, said as he and his colleagues presented the new guidelines at the annual congress of the European Society of Cardiology.
Although the new European guidelines further buttress this more aggressive approach to blood pressure management that first appeared almost a year ago in U.S. guidelines from the American College of Cardiology and the American Heart Association, an approach that remains controversial among U.S. primary care physicians, the European strategy (Eur Heart J. 2018 Sep 1;39[33]:3021-104) was generally more cautious than the broader endorsement of lower blood pressure goals advanced by the U.S. recommendations (J Am Coll Cardiol. 2018 May;71[19]:e127-e248).
In the European approach, “the first objective is to treat to less than 140/90 mm Hg. If this is well tolerated, then treat to less than 130/80 mm Hg in most patients,” said Dr. Mancia, cochair of the European writing panel. Further pressure reductions to less than 130/80 mm Hg are usually harder, the incremental benefit from further reduction is less than when pressures first fall below 140/90 mm Hg, and the evidence for incremental benefit of any size from further pressure reduction is less strong for certain key patient subgroups: people at least 80 years old, and patients with diabetes, chronic kidney disease, or coronary artery disease, said Dr. Mancia, an emeritus professor at the University of Milan.
“The consistency between two major guidelines is important, but there are differences that may look subtle but are not subtle,” he said in a video interview. “If a patient gets to less than 140 mm Hg, the doctor should not think that’s a failure; it’s a very important result.”
One striking example of how the two guidelines differ on treatment targets is for people at least 80 years old. The overall blood pressure threshold for starting drug treatment in patients this age in the European guidelines is 160/90 mm Hg, although it remains at 140/90 for people aged 65-79 years old “provided that treatment is well tolerated” and the patients are “fit.” The 2017 U.S. guidelines, by contrast, say that considering drug treatment for everyone with a pressure at or above 130/80 mm Hg is a class I recommendation regardless of age as long as the person is “noninstitutionalized, ambulatory, [and] community-dwelling.” Once an older patient of any age, 65 years or older, starts drug treatment to reduce blood pressure, the European guidelines allow for treating to a target systolic blood pressure of 130-139 mm Hg as long as the regimen is well tolerated, and the guidelines say that a diastolic pressure target of less than 80 mm Hg should be considered for all adults regardless of age.
The new European guidelines also define adults with an untreated pressure of 130-139/85-89 mm Hg as “high normal,” rather than the “stage 1 hypertension” designation given to people with pressures of 130-139/80-89 mm Hg in the U.S. guidelines. Robert M. Carey, MD, vice chair of the U.S. guidelines panel, minimized this as a “semantic” difference, and he highlighted that management of people with pressures in this range is roughly similar in the two sets of recommendations. “The name is different, but treatment is the same,” Dr. Carey said.
The European guidelines call for initial lifestyle interventions, followed by drug treatment “that may be considered” for patients who have “very-high” cardiovascular risk because of established cardiovascular disease, especially coronary artery disease, and detail the specific clinical conditions that fall into the very-high-risk category. The U.S. guidelines say that stage 1 hypertension patients should get lifestyle interventions, followed by drug treatment for the roughly 30% of patients in this category who score at least a 10% 10-year risk on the American College of Cardiology’s Atherosclerotic Cardiovascular Disease Risk Estimator Plus.The new European guidelines are a “validation” of the ACC/AHA 2017 guidelines, commented Dr. Carey, a professor of medicine at the University of Virginia in Charlottesville. “Overall, we’re delighted to have these two major groups” agree, he said in an interview. “There is a tremendous amount of concurrence.”
Other areas of agreement between the two guidelines include their emphasis on careful and repeated blood pressure measurement, including out-of-office measurement, before settling on a diagnosis of hypertension; systematic assessment of possible masked or white-coat hypertension in selected people; and frequent use of combined drug treatment including initiation of a dual-drug, single-pill regimen when starting drug treatment and aggressive follow-up by adding a third drug when needed. However, in another divergence the U.S. guidelines give a much stronger endorsement to starting treatment with monotherapy, a strategy the European guidelines scrapped.
Dr. Carey also noted that the European endorsement of three antihypertensives formulated into a single pill for patients who need more than two drugs would be difficult for American clinicians to follow as virtually no such formulations are approved for U.S. use.
Dr. Mancini has received honoraria from Boehringer Ingelheim, CVRx, Daiichi Sankyo, Ferrer, Medtronic, Menarini, Merck, Novartis, Recordati, and Servier. Dr. Williams has been a consultant to Novartis, Relypsa, and Vascular Dynamics, and he has spoken on behalf of Boehringer Ingelheim, Daiichi Sankyo, Novartis, and Servier. Dr. Carey and Dr. Whelton had no commercial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM THE ESC CONGRESS 2018
FDA clears gold microparticles for acne vulgaris
The Food and Drug Administration has granted clearance to “Sebacia Microparticles” for the treatment of acne vulgaris, for use with 1,064 nm lasers , according to a press release from Sebacia, the company developing the product.
The product, gold-coated silica microparticles, in a topical suspension, is cleared for use “as an accessory to 1064 nm lasers to facilitate photothermal heating of sebaceous glands for the treatment of mild to moderate inflammatory acne vulgaris,” the release said.
FDA clearance is based on a randomized, blinded, controlled trial of 168 patients with mild to moderate acne vulgaris, according to the company. Patients were treated with either laser and microparticles or with laser alone. The primary endpoint – noninferiority – was met with a median 53% reduction in inflammatory lesion count in the group receiving microparticles plus laser versus 45% in the laser-only group, at 12 weeks. All adverse events were mild to moderate, with none that were serious, the company statement said.
The Food and Drug Administration has granted clearance to “Sebacia Microparticles” for the treatment of acne vulgaris, for use with 1,064 nm lasers , according to a press release from Sebacia, the company developing the product.
The product, gold-coated silica microparticles, in a topical suspension, is cleared for use “as an accessory to 1064 nm lasers to facilitate photothermal heating of sebaceous glands for the treatment of mild to moderate inflammatory acne vulgaris,” the release said.
FDA clearance is based on a randomized, blinded, controlled trial of 168 patients with mild to moderate acne vulgaris, according to the company. Patients were treated with either laser and microparticles or with laser alone. The primary endpoint – noninferiority – was met with a median 53% reduction in inflammatory lesion count in the group receiving microparticles plus laser versus 45% in the laser-only group, at 12 weeks. All adverse events were mild to moderate, with none that were serious, the company statement said.
The Food and Drug Administration has granted clearance to “Sebacia Microparticles” for the treatment of acne vulgaris, for use with 1,064 nm lasers , according to a press release from Sebacia, the company developing the product.
The product, gold-coated silica microparticles, in a topical suspension, is cleared for use “as an accessory to 1064 nm lasers to facilitate photothermal heating of sebaceous glands for the treatment of mild to moderate inflammatory acne vulgaris,” the release said.
FDA clearance is based on a randomized, blinded, controlled trial of 168 patients with mild to moderate acne vulgaris, according to the company. Patients were treated with either laser and microparticles or with laser alone. The primary endpoint – noninferiority – was met with a median 53% reduction in inflammatory lesion count in the group receiving microparticles plus laser versus 45% in the laser-only group, at 12 weeks. All adverse events were mild to moderate, with none that were serious, the company statement said.
Updated AHA recommendations favor nonstatin therapy for cholesterol control
Importance
While statins remain the foundation for treating high cholesterol in order to reduce cardiovascular risk, new evidence has lead to important revisions in the American Heart Association’s recommendations for treatment of hypercholesterolemia in patients at very high cardiovascular risk (secondary prevention) with the addition of specific nonstatin agents. We will briefly review the AHA 2013 guideline recommendations, the relevant new information, and the updated AHA recommendations.
American Heart Association 2013 guidelines
The 2013 American College of Cardiology/AHA cholesterol guidelines recommend either high- or moderate-intensity statin therapy for patients in the four statin benefit groups:
1. Adult patients older than 21 years of age with clinical atherosclerotic cardiovascular disease (ASCVD).
2. Adults older than 21 years of age with low-density lipoprotein cholesterol (LDL-C) above 190 mg/dL.
3. Adults aged 40-75 years without ASCVD but with diabetes and with LDL-C 70-189 mg/dL.
4. Adults aged 40-75 years without either ASCVD or diabetes, with LDL-C 70-189 mg/dL and an estimated 10-year risk for ASCVD of over 7.5% as determined by the Pooled Cohort Equations.
At the time of the 2013 guidelines, there was little evidence to recommend the use of medications other than statins.
Recent evidence
The IMPROVE-IT trial1 was a double-blind, randomized trial involving 18,144 men and women who were older than 50 years and hospitalized for an acute coronary syndrome within the preceding 10 days. They were randomized to either simvastatin plus ezetimibe or simvastatin plus placebo. The primary endpoints were a composite of death from cardiovascular disease, a major coronary event (nonfatal MI, unstable angina requiring admission, or coronary revascularization), or nonfatal stroke. At 1 year, the mean LDL was 69.9 mg/dL in the simvastatin-monotherapy group and 53.2 mg/dL in the simvastatin-ezetimibe group (P under .001), representing a 24% decrease in LDL between the two groups. The rate of the primary endpoints was significantly lower in the simvastatin plus ezetimibe group with a hazard ratio of 0.936 (P = .016). The risk of MI was significantly decreased with an HR of 0.87 (P = .002), and the risk of ischemic stroke significantly decreased with an HR of 0.79 (P = .008). Prespecified safety endpoints showed no significant difference between the two groups.
The FOURIER trial2 examined the PCSK-9 inhibitor, evolocumab. FOURIER was a randomized, double-blind, placebo-controlled study involving 27,564 patients with atherosclerotic cardiovascular disease and LDL levels of 70 mg/dL or higher who were receiving statin therapy (at least atorvastatin 20 mg or equivalent with/without ezetimibe). Patients were between 45 and 80 years old with a history of history of MI, nonhemorrhagic stroke, or symptomatic peripheral artery disease. Patients were randomized to receive subcutaneous injections of evolocumab or matching placebo. The primary endpoints were similar to that of IMPROVE-IT: a composite of cardiovascular death, myocardial infarction, stroke, unstable angina hospitalization, and coronary revascularization. The median LDL on entry was 92 mg/dL for both groups. At 48 weeks, the evolocumab group showed a 59% decrease in LDL, compared with placebo, with a decrease in median LDL from 92 mg/dL to 30 mg/dL. The primary endpoint occurred in 9.8% of the evolocumab group and 11.3% in the placebo group for a hazard ratio of 0.85 (P less than .001) representing a total risk reduction of 13.2%. The risk of MI or stroke and need for revascularization were significantly lower values in the evolocumab group, compared with placebo. Cardiovascular death did not show significant changes. There was no significant difference in rate of serious events.
The ODYSSEY trial3 reported on another PCSK-9 inhibitor, alirocumab, in a randomized, double-blind, placebo-controlled trial involving 18,924 patients who had an ACS in the prior 12 months. At the median follow-up (2.8 years), the LDL of the alirocumab group was 53.3 mg/dL, compared with 101.4 mg/dL in the placebo group. The primary endpoints for cardiovascular risks were similar to those in the FOURIER trial: a risk of 9.5% in the alirocumab group and 11.1% in the placebo group for a total risk reduction of 14.4%. This suggests the class of PCSK-9 inhibitors have a strong correlation with reducing LDL levels 54%-59% and reducing major cardiac adverse events by 13%-15%.
Recommendations
The American College of Cardiology released a focused update that integrated the new evidence regarding the use of nonstatin therapy. The current focused update recommends an overall 50% or greater reduction in LDL for patients with clinical ASCVD. If this reduction is not achieved, ACC suggests that one consider the addition of nonstatin therapy with either ezetimibe or a PCSK-9 inhibitor.4 If a patient requires less than 25% additional LDL reduction, consider ezetimibe; if a patient requires more than 25% additional LDL reduction, consider a PCSK-9 inhibitor. Specifically, the guideline states: “If the patient still has less than 50% reduction in LDL-C (and may consider LDL-C above 70 mg/dL or non–HDL-C above 100 mg/dL), the patient and clinician should enter into a discussion focused on shared decision making regarding the addition of a nonstatin medication to the current regimen.”
The other group that is mentioned in the recommendations, with an acknowledgment that the evidence for benefit in primary prevention is not available, is individuals who have an LDL above 190 mg/dL even while compliant with a maximally effective statin regimen. The guidelines make further but less strong recommendations about a number of risk groups, but the largest and strongest change, based on strong evidence, is the recommendation to consider nonstatin therapy in individuals with established ASCVD, as described above.
Bottom line
Recent trials show significant reductions in LDL, leading to significant reductions in cardiovascular endpoints with ezetimibe and PCSK-9 inhibitors. This has led to an additional ACC recommendation to consider the use of nonstatin therapy in addition to maximal statin therapy in selected patients with established cardiovascular disease.
References
1. Cannon C et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
2. Sabatine M et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-22.
3. ODYSSEY Outcomes: Results suggest use of PCSK9 inhibitor reduces CV events, LDL-C in ACS patients. Article from American College of Cardiology. ACC News Story. 2018 Mar 10.
4. Lloyd-Jones DM et al. 2017 Focused update of the 2016 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017 Oct 3;70(14):1785-1822. Epub 2017 Sep 5.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Plako is a second-year resident in the family medicine residency program at Abington Jefferson Hospital.
Importance
While statins remain the foundation for treating high cholesterol in order to reduce cardiovascular risk, new evidence has lead to important revisions in the American Heart Association’s recommendations for treatment of hypercholesterolemia in patients at very high cardiovascular risk (secondary prevention) with the addition of specific nonstatin agents. We will briefly review the AHA 2013 guideline recommendations, the relevant new information, and the updated AHA recommendations.
American Heart Association 2013 guidelines
The 2013 American College of Cardiology/AHA cholesterol guidelines recommend either high- or moderate-intensity statin therapy for patients in the four statin benefit groups:
1. Adult patients older than 21 years of age with clinical atherosclerotic cardiovascular disease (ASCVD).
2. Adults older than 21 years of age with low-density lipoprotein cholesterol (LDL-C) above 190 mg/dL.
3. Adults aged 40-75 years without ASCVD but with diabetes and with LDL-C 70-189 mg/dL.
4. Adults aged 40-75 years without either ASCVD or diabetes, with LDL-C 70-189 mg/dL and an estimated 10-year risk for ASCVD of over 7.5% as determined by the Pooled Cohort Equations.
At the time of the 2013 guidelines, there was little evidence to recommend the use of medications other than statins.
Recent evidence
The IMPROVE-IT trial1 was a double-blind, randomized trial involving 18,144 men and women who were older than 50 years and hospitalized for an acute coronary syndrome within the preceding 10 days. They were randomized to either simvastatin plus ezetimibe or simvastatin plus placebo. The primary endpoints were a composite of death from cardiovascular disease, a major coronary event (nonfatal MI, unstable angina requiring admission, or coronary revascularization), or nonfatal stroke. At 1 year, the mean LDL was 69.9 mg/dL in the simvastatin-monotherapy group and 53.2 mg/dL in the simvastatin-ezetimibe group (P under .001), representing a 24% decrease in LDL between the two groups. The rate of the primary endpoints was significantly lower in the simvastatin plus ezetimibe group with a hazard ratio of 0.936 (P = .016). The risk of MI was significantly decreased with an HR of 0.87 (P = .002), and the risk of ischemic stroke significantly decreased with an HR of 0.79 (P = .008). Prespecified safety endpoints showed no significant difference between the two groups.
The FOURIER trial2 examined the PCSK-9 inhibitor, evolocumab. FOURIER was a randomized, double-blind, placebo-controlled study involving 27,564 patients with atherosclerotic cardiovascular disease and LDL levels of 70 mg/dL or higher who were receiving statin therapy (at least atorvastatin 20 mg or equivalent with/without ezetimibe). Patients were between 45 and 80 years old with a history of history of MI, nonhemorrhagic stroke, or symptomatic peripheral artery disease. Patients were randomized to receive subcutaneous injections of evolocumab or matching placebo. The primary endpoints were similar to that of IMPROVE-IT: a composite of cardiovascular death, myocardial infarction, stroke, unstable angina hospitalization, and coronary revascularization. The median LDL on entry was 92 mg/dL for both groups. At 48 weeks, the evolocumab group showed a 59% decrease in LDL, compared with placebo, with a decrease in median LDL from 92 mg/dL to 30 mg/dL. The primary endpoint occurred in 9.8% of the evolocumab group and 11.3% in the placebo group for a hazard ratio of 0.85 (P less than .001) representing a total risk reduction of 13.2%. The risk of MI or stroke and need for revascularization were significantly lower values in the evolocumab group, compared with placebo. Cardiovascular death did not show significant changes. There was no significant difference in rate of serious events.
The ODYSSEY trial3 reported on another PCSK-9 inhibitor, alirocumab, in a randomized, double-blind, placebo-controlled trial involving 18,924 patients who had an ACS in the prior 12 months. At the median follow-up (2.8 years), the LDL of the alirocumab group was 53.3 mg/dL, compared with 101.4 mg/dL in the placebo group. The primary endpoints for cardiovascular risks were similar to those in the FOURIER trial: a risk of 9.5% in the alirocumab group and 11.1% in the placebo group for a total risk reduction of 14.4%. This suggests the class of PCSK-9 inhibitors have a strong correlation with reducing LDL levels 54%-59% and reducing major cardiac adverse events by 13%-15%.
Recommendations
The American College of Cardiology released a focused update that integrated the new evidence regarding the use of nonstatin therapy. The current focused update recommends an overall 50% or greater reduction in LDL for patients with clinical ASCVD. If this reduction is not achieved, ACC suggests that one consider the addition of nonstatin therapy with either ezetimibe or a PCSK-9 inhibitor.4 If a patient requires less than 25% additional LDL reduction, consider ezetimibe; if a patient requires more than 25% additional LDL reduction, consider a PCSK-9 inhibitor. Specifically, the guideline states: “If the patient still has less than 50% reduction in LDL-C (and may consider LDL-C above 70 mg/dL or non–HDL-C above 100 mg/dL), the patient and clinician should enter into a discussion focused on shared decision making regarding the addition of a nonstatin medication to the current regimen.”
The other group that is mentioned in the recommendations, with an acknowledgment that the evidence for benefit in primary prevention is not available, is individuals who have an LDL above 190 mg/dL even while compliant with a maximally effective statin regimen. The guidelines make further but less strong recommendations about a number of risk groups, but the largest and strongest change, based on strong evidence, is the recommendation to consider nonstatin therapy in individuals with established ASCVD, as described above.
Bottom line
Recent trials show significant reductions in LDL, leading to significant reductions in cardiovascular endpoints with ezetimibe and PCSK-9 inhibitors. This has led to an additional ACC recommendation to consider the use of nonstatin therapy in addition to maximal statin therapy in selected patients with established cardiovascular disease.
References
1. Cannon C et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
2. Sabatine M et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-22.
3. ODYSSEY Outcomes: Results suggest use of PCSK9 inhibitor reduces CV events, LDL-C in ACS patients. Article from American College of Cardiology. ACC News Story. 2018 Mar 10.
4. Lloyd-Jones DM et al. 2017 Focused update of the 2016 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017 Oct 3;70(14):1785-1822. Epub 2017 Sep 5.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Plako is a second-year resident in the family medicine residency program at Abington Jefferson Hospital.
Importance
While statins remain the foundation for treating high cholesterol in order to reduce cardiovascular risk, new evidence has lead to important revisions in the American Heart Association’s recommendations for treatment of hypercholesterolemia in patients at very high cardiovascular risk (secondary prevention) with the addition of specific nonstatin agents. We will briefly review the AHA 2013 guideline recommendations, the relevant new information, and the updated AHA recommendations.
American Heart Association 2013 guidelines
The 2013 American College of Cardiology/AHA cholesterol guidelines recommend either high- or moderate-intensity statin therapy for patients in the four statin benefit groups:
1. Adult patients older than 21 years of age with clinical atherosclerotic cardiovascular disease (ASCVD).
2. Adults older than 21 years of age with low-density lipoprotein cholesterol (LDL-C) above 190 mg/dL.
3. Adults aged 40-75 years without ASCVD but with diabetes and with LDL-C 70-189 mg/dL.
4. Adults aged 40-75 years without either ASCVD or diabetes, with LDL-C 70-189 mg/dL and an estimated 10-year risk for ASCVD of over 7.5% as determined by the Pooled Cohort Equations.
At the time of the 2013 guidelines, there was little evidence to recommend the use of medications other than statins.
Recent evidence
The IMPROVE-IT trial1 was a double-blind, randomized trial involving 18,144 men and women who were older than 50 years and hospitalized for an acute coronary syndrome within the preceding 10 days. They were randomized to either simvastatin plus ezetimibe or simvastatin plus placebo. The primary endpoints were a composite of death from cardiovascular disease, a major coronary event (nonfatal MI, unstable angina requiring admission, or coronary revascularization), or nonfatal stroke. At 1 year, the mean LDL was 69.9 mg/dL in the simvastatin-monotherapy group and 53.2 mg/dL in the simvastatin-ezetimibe group (P under .001), representing a 24% decrease in LDL between the two groups. The rate of the primary endpoints was significantly lower in the simvastatin plus ezetimibe group with a hazard ratio of 0.936 (P = .016). The risk of MI was significantly decreased with an HR of 0.87 (P = .002), and the risk of ischemic stroke significantly decreased with an HR of 0.79 (P = .008). Prespecified safety endpoints showed no significant difference between the two groups.
The FOURIER trial2 examined the PCSK-9 inhibitor, evolocumab. FOURIER was a randomized, double-blind, placebo-controlled study involving 27,564 patients with atherosclerotic cardiovascular disease and LDL levels of 70 mg/dL or higher who were receiving statin therapy (at least atorvastatin 20 mg or equivalent with/without ezetimibe). Patients were between 45 and 80 years old with a history of history of MI, nonhemorrhagic stroke, or symptomatic peripheral artery disease. Patients were randomized to receive subcutaneous injections of evolocumab or matching placebo. The primary endpoints were similar to that of IMPROVE-IT: a composite of cardiovascular death, myocardial infarction, stroke, unstable angina hospitalization, and coronary revascularization. The median LDL on entry was 92 mg/dL for both groups. At 48 weeks, the evolocumab group showed a 59% decrease in LDL, compared with placebo, with a decrease in median LDL from 92 mg/dL to 30 mg/dL. The primary endpoint occurred in 9.8% of the evolocumab group and 11.3% in the placebo group for a hazard ratio of 0.85 (P less than .001) representing a total risk reduction of 13.2%. The risk of MI or stroke and need for revascularization were significantly lower values in the evolocumab group, compared with placebo. Cardiovascular death did not show significant changes. There was no significant difference in rate of serious events.
The ODYSSEY trial3 reported on another PCSK-9 inhibitor, alirocumab, in a randomized, double-blind, placebo-controlled trial involving 18,924 patients who had an ACS in the prior 12 months. At the median follow-up (2.8 years), the LDL of the alirocumab group was 53.3 mg/dL, compared with 101.4 mg/dL in the placebo group. The primary endpoints for cardiovascular risks were similar to those in the FOURIER trial: a risk of 9.5% in the alirocumab group and 11.1% in the placebo group for a total risk reduction of 14.4%. This suggests the class of PCSK-9 inhibitors have a strong correlation with reducing LDL levels 54%-59% and reducing major cardiac adverse events by 13%-15%.
Recommendations
The American College of Cardiology released a focused update that integrated the new evidence regarding the use of nonstatin therapy. The current focused update recommends an overall 50% or greater reduction in LDL for patients with clinical ASCVD. If this reduction is not achieved, ACC suggests that one consider the addition of nonstatin therapy with either ezetimibe or a PCSK-9 inhibitor.4 If a patient requires less than 25% additional LDL reduction, consider ezetimibe; if a patient requires more than 25% additional LDL reduction, consider a PCSK-9 inhibitor. Specifically, the guideline states: “If the patient still has less than 50% reduction in LDL-C (and may consider LDL-C above 70 mg/dL or non–HDL-C above 100 mg/dL), the patient and clinician should enter into a discussion focused on shared decision making regarding the addition of a nonstatin medication to the current regimen.”
The other group that is mentioned in the recommendations, with an acknowledgment that the evidence for benefit in primary prevention is not available, is individuals who have an LDL above 190 mg/dL even while compliant with a maximally effective statin regimen. The guidelines make further but less strong recommendations about a number of risk groups, but the largest and strongest change, based on strong evidence, is the recommendation to consider nonstatin therapy in individuals with established ASCVD, as described above.
Bottom line
Recent trials show significant reductions in LDL, leading to significant reductions in cardiovascular endpoints with ezetimibe and PCSK-9 inhibitors. This has led to an additional ACC recommendation to consider the use of nonstatin therapy in addition to maximal statin therapy in selected patients with established cardiovascular disease.
References
1. Cannon C et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387-97.
2. Sabatine M et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-22.
3. ODYSSEY Outcomes: Results suggest use of PCSK9 inhibitor reduces CV events, LDL-C in ACS patients. Article from American College of Cardiology. ACC News Story. 2018 Mar 10.
4. Lloyd-Jones DM et al. 2017 Focused update of the 2016 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on expert consensus decision pathways. J Am Coll Cardiol. 2017 Oct 3;70(14):1785-1822. Epub 2017 Sep 5.
Dr. Skolnik is a professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Plako is a second-year resident in the family medicine residency program at Abington Jefferson Hospital.