User login
Myo-inositol is one of the components of an integrative approach to acne
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
, Jonette Elizabeth Keri, MD, PhD, professor of dermatology at the University of Miami, said during a presentation on therapies for acne at the annual Integrative Dermatology Symposium.
Probiotics and omega-3 fatty acids are among the other complementary therapies that have a role in acne treatment, she and others said during the meeting.
Myo-inositol has been well-studied in the gynecology-endocrinology community in patients with polycystic ovary syndrome (PCOS), demonstrating an ability to improve the metabolic profile and reduce acne and hirsutism, Dr. Keri said.
A study of 137 young, overweight women with PCOS and moderate acne, for example, found that compared with placebo, 6 months of myo-inositol or D-chiro-inositol, another isoform of inositol, significantly improved the acne score, endocrine and metabolic parameters, insulin resistance, and regularity of the menstrual cycle, Dr. Keri said. Both isoforms of inositol are second messengers in the signal transduction of insulin.
During a panel discussion, asked about a case of an adult female with acne, Dr. Keri said that many of her adult female patients “don’t want to do isotretinoin or antibiotics, and they don’t want to do any kind of hormonal treatment,” the options she would recommend. But for patients who do not want these treatments, she said, “I go down the route of supplements,” and myo-inositol is her “favorite” option. It’s safe to use during pregnancy, she emphasized, noting that myo-inositol is being studied for the prevention of preterm birth.
Dr. Keri, who described herself as “more of a traditionalist,” prescribes myo-inositol 2 gm twice a day in pill form. In Europe, she noted in her presentation, myo-inositol is also compounded for topical use.
Diet, probiotics, other nutraceuticals
A low-glycemic-load diet was among several complementary therapies reported in a 2015 Cochrane Database Systematic Review to have some evidence (though low-quality) of reducing total skin lesions in acne (along with tea tree oil and bee venom) and today, it is the most evidence-based dietary recommendation for acne, Dr. Keri said.
Omega-3 fatty acids and increased fruit and vegetable intake have also been reported to be acne-protective — and hyperglycemia, carbohydrates, milk and dairy products, and saturated fats and trans fats have been reported to be acne-promoting, she noted.
But, the low-glycemic-load data “is the strongest,” she said. The best advice for patients, she added, is to consume less sugar and fewer sugary drinks and “avoid white foods” such as white bread, rice, and pasta.
Probiotics can also be recommended, especially for patients on antibiotic therapy, Dr. Keri said. For “basic science evidence,” she pointed to a randomized, double-blinded, placebo-controlled study of 20 adults with acne, which evaluated the impact of a probiotic on improvement in acne and skin expression of genes involved with insulin signaling. Participants took either a liquid supplement containing Lactobacillus rhamnosus SP1 (LSP1) or placebo over a 12-week period. The investigators performed paired skin biopsies before and after 12 weeks of treatment and analyzed them for insulin-like growth factor 1 (IGF1) and forkhead box protein O1 (FOXO1) gene expression.
They found that compared with baseline, the probiotic group showed a 32% reduction in IGF1 and a 65% increase in FOXO1 gene expression (P < .0001 for both), with no such differences observed in the placebo group.
Clinically, patients in the probiotic group had an adjusted odds ratio of 28.4 (95% confidence interval, 2.2-411.1, P < .05) of acne being rated as improved or markedly improved compared with those on placebo.
Dr. Keri and others at the meeting also referenced a 2013 prospective, open-label trial that randomized 45 women with acne, ages 18-35 years, to one of three arms: Probiotic supplementation only, minocycline only, and both probiotic and minocycline. The probiotic used was a product containing Lactobacillus acidophilus, Lactobacillus bulgaricus, and Bifidobacterium bifidum. At 8 and 12 weeks, the combination group “did the best with the lowest total lesion count” compared with the probiotic group and the minocycline group, differences that were significant (P < .001 and P <.01, respectively), she said. “And they also had less candidiasis when using a probiotic than when using an antibiotic alone,” she said. Two patients in the minocycline-only group failed to complete the study because they developed vaginal candidiasis.
In addition to reducing potential adverse events secondary to chronic antibiotic use, probiotics can have synergistic anti-inflammatory effects, she said.
Dr. Keri said she recommends probiotics for patients taking antibiotics and encourages them “to get a branded probiotic,” such as Culturelle, “or if they prefer a food source, soy or almond milk–based yogurt.” As with other elements of a holistic approach to acne, she urged clinicians to consider the cost of treatment.
Probiotics (Lactobacillus plantarum) were one of four nutraceuticals determined in a 2023 systematic review to have “good-quality” evidence for potential efficacy, Dr. Keri noted, along with vitamin D, green tea extract, and cheongsangbangpoong-tang, the latter of which is an herbal therapeutic formula approved by the Korean Food and Drug Administration for use in acne.
“There were really no bad systemic effects for any of these,” she said. “The tricky part of this review is that each of the four have only one study” deemed to be a good-quality study. Omega-3 fatty acids were among several other nutraceuticals deemed to have “fair-quality” evidence for efficacy. Zinc was reported to be the most studied nutraceutical in acne, but didn’t rate as highly in the review. Dr. Keri said she likes to include zinc in her armamentarium because “it can be used in pregnant women,” noting that reviews and guidelines “are just that, a guide ... to combine with experience.”
Omega-3 fatty acids with isotretinoin
Several speakers at the meeting, including Steven Daveluy, MD, associate professor and residency program director in the department of dermatology, Wayne State University, Dearborn, Michigan, spoke about the value of omega-3 fatty acids in reducing side effects of isotretinoin. “In the FDA trials [of isotretinoin] they had patients take 50 grams of fat,” he said. “You can use the good fats to help you out.”
Research has shown that 1 gm per day of oral omega-3 reduces dryness of the lips, nose, eyes, and skin, “which are the big side effects we see with isotretinoin,” he said. An impact on triglyceride levels has also been demonstrated, Dr. Daveluy said, pointing to a longitudinal survey study of 39 patients treated with isotretinoin that showed a mean increase in triglyceride levels of 49% during treatment in patients who did not use omega-3 supplementation, compared with a mean increase of 13.9% (P =.04) in patients who used the supplements.“There is also evidence that omega-3 can decrease depression, which may or may not be a side effect of isotretinoin ... but it’s something we consider in our [acne] patients,” Dr. Daveluy said.
During a panel discussion at the meeting, Apple A. Bodemer, MD, associate professor of dermatology at the University of Wisconsin, Madison, said she usually prescribes 2 g of docosahexaenoic acid eicosapentaenoic acid combined in patients on isotretinoin because “at that dose, omega-3s have been found to be anti-inflammatory.”
Dr. Keri reported being an investigator and speaker for Galderma, and an advisory board member for Ortho Dermatologics and for Almirall. Dr. Daveluy reported no relevant disclosures.
FROM IDS 2023
Appetite loss and unusual agitation
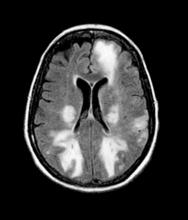
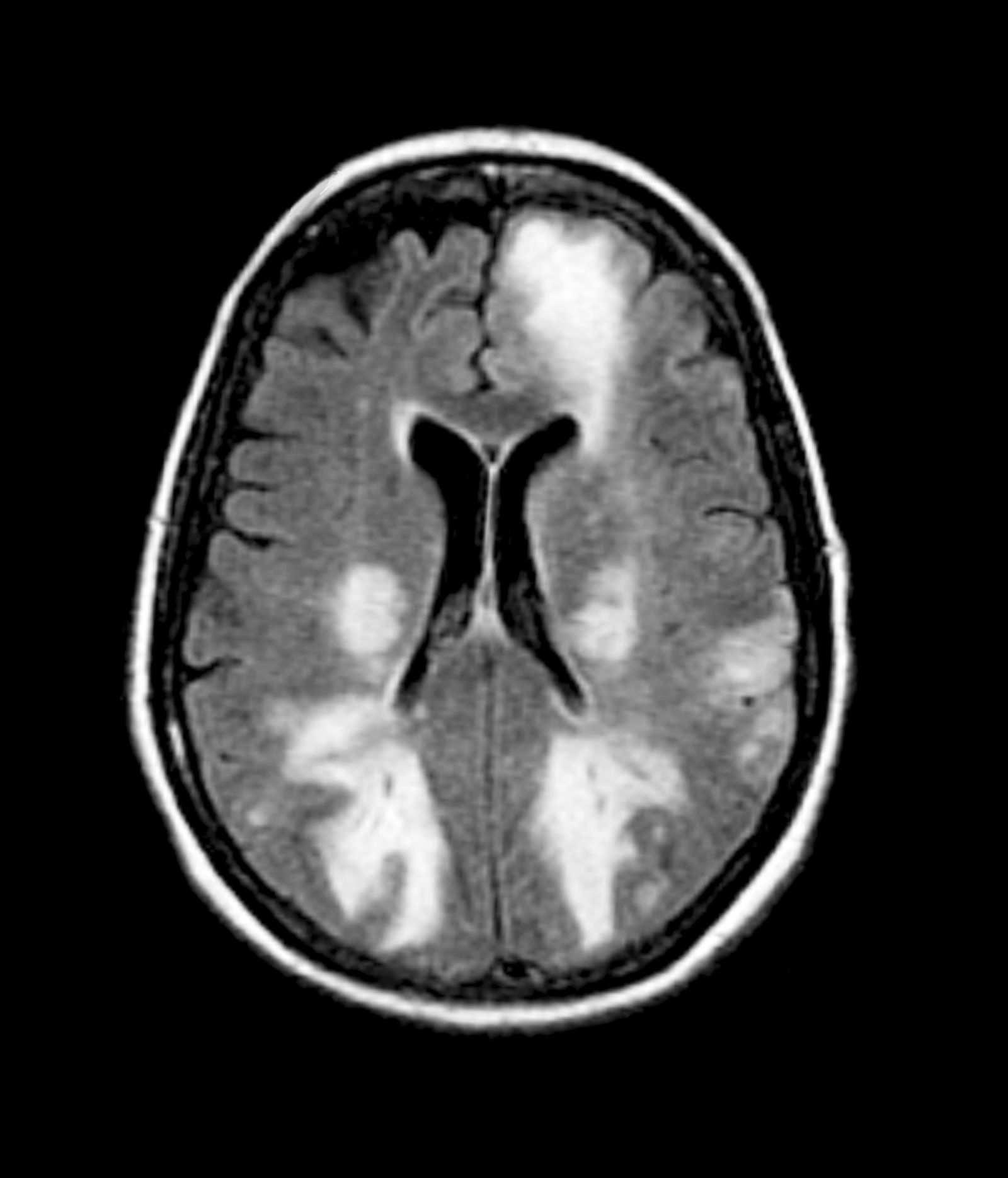
Given the patient's results on the genetic panel and MRI, as well as the noted cognitive decline and increased aggression, this patient is suspected of having limbic-predominant age-related TDP-43 encephalopathy (LATE) secondary to AD and is referred to the neurologist on her multidisciplinary care team for further consultation and testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. LATE is a new classification of dementia, identified in 2019, that mimics AD but is a unique disease entity driven by the misfolding of the protein TDP-43, which regulates gene expression in the brain. Misfolded TDP-43 protein is common among older adults aged ≥ 85 years, and about a quarter of this population has enough misfolded TDP-43 protein to affect their memory and cognition.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage. Initial mental status testing evaluates attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Because LATE is a newly discovered form of dementia, there are no set guidelines on diagnosing LATE and no robust biomarker for TDP-43. What is known about LATE has been gleaned mostly from retrospective clinicopathologic studies.
The LATE consensus working group reports that the clinical course of disease, as studied by autopsy-proven LATE neuropathologic change (LATE-NC), is described as an "amnestic cognitive syndrome that can evolve to incorporate multiple cognitive domains and ultimately to impair activities of daily living." Researchers are currently analyzing different clinical assessments and neuroimaging with MRI to characterize LATE. A group of international researchers recently published a set of clinical criteria for limbic-predominant amnestic neurodegenerative syndrome (LANS), which is associated with LATE-NC. Their criteria include "core, standard and advanced features that are measurable in vivo, including older age at evaluation, mild clinical syndrome, disproportionate hippocampal atrophy, impaired semantic memory, limbic hypometabolism, absence of neocortical degenerative patterns and low likelihood of neocortical tau, with degrees of certainty (highest, high, moderate, low)." Other neuroimaging studies of autopsy-confirmed LATE-NC have shown that atrophy is mostly focused in the medial temporal lobe with marked reduced hippocampal volume.
The group reports that LATE and AD probably share pathophysiologic mechanisms. One of the universally accepted hallmarks of AD is the formation of beta-amyloid plaques, which are dense, mostly insoluble deposits of beta-amyloid protein that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making. These plaques disrupt brain function and lead to brain atrophy. The LATE group also reports that this same pathology has been noted with LATE: "Many subjects with LATE-NC have comorbid brain pathologies, often including amyloid-beta plaques and tauopathy." That said, genetic studies have helped identify five genes with risk alleles for LATE (GRN, TMEM106B, ABCC9, KCNMB2, and APOE), suggesting disease-specific underlying mechanisms compared to AD.
Patient and caregiver education and guidance is vital with a dementia diagnosis. If LATE and/or AD are suspected, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Because LATE is a new classification of dementia, there are no known effective treatments. One ongoing study is testing the use of autologous bone marrow–derived stem cells to help improve cognitive impairment among patients with LATE, AD, and other dementias. Current AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the United States for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's results on the genetic panel and MRI, as well as the noted cognitive decline and increased aggression, this patient is suspected of having limbic-predominant age-related TDP-43 encephalopathy (LATE) secondary to AD and is referred to the neurologist on her multidisciplinary care team for further consultation and testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. LATE is a new classification of dementia, identified in 2019, that mimics AD but is a unique disease entity driven by the misfolding of the protein TDP-43, which regulates gene expression in the brain. Misfolded TDP-43 protein is common among older adults aged ≥ 85 years, and about a quarter of this population has enough misfolded TDP-43 protein to affect their memory and cognition.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage. Initial mental status testing evaluates attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Because LATE is a newly discovered form of dementia, there are no set guidelines on diagnosing LATE and no robust biomarker for TDP-43. What is known about LATE has been gleaned mostly from retrospective clinicopathologic studies.
The LATE consensus working group reports that the clinical course of disease, as studied by autopsy-proven LATE neuropathologic change (LATE-NC), is described as an "amnestic cognitive syndrome that can evolve to incorporate multiple cognitive domains and ultimately to impair activities of daily living." Researchers are currently analyzing different clinical assessments and neuroimaging with MRI to characterize LATE. A group of international researchers recently published a set of clinical criteria for limbic-predominant amnestic neurodegenerative syndrome (LANS), which is associated with LATE-NC. Their criteria include "core, standard and advanced features that are measurable in vivo, including older age at evaluation, mild clinical syndrome, disproportionate hippocampal atrophy, impaired semantic memory, limbic hypometabolism, absence of neocortical degenerative patterns and low likelihood of neocortical tau, with degrees of certainty (highest, high, moderate, low)." Other neuroimaging studies of autopsy-confirmed LATE-NC have shown that atrophy is mostly focused in the medial temporal lobe with marked reduced hippocampal volume.
The group reports that LATE and AD probably share pathophysiologic mechanisms. One of the universally accepted hallmarks of AD is the formation of beta-amyloid plaques, which are dense, mostly insoluble deposits of beta-amyloid protein that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making. These plaques disrupt brain function and lead to brain atrophy. The LATE group also reports that this same pathology has been noted with LATE: "Many subjects with LATE-NC have comorbid brain pathologies, often including amyloid-beta plaques and tauopathy." That said, genetic studies have helped identify five genes with risk alleles for LATE (GRN, TMEM106B, ABCC9, KCNMB2, and APOE), suggesting disease-specific underlying mechanisms compared to AD.
Patient and caregiver education and guidance is vital with a dementia diagnosis. If LATE and/or AD are suspected, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Because LATE is a new classification of dementia, there are no known effective treatments. One ongoing study is testing the use of autologous bone marrow–derived stem cells to help improve cognitive impairment among patients with LATE, AD, and other dementias. Current AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the United States for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Given the patient's results on the genetic panel and MRI, as well as the noted cognitive decline and increased aggression, this patient is suspected of having limbic-predominant age-related TDP-43 encephalopathy (LATE) secondary to AD and is referred to the neurologist on her multidisciplinary care team for further consultation and testing.
AD is one of the most common forms of dementia. More than 6 million people in the United States have clinical AD or mild cognitive impairment because of AD. LATE is a new classification of dementia, identified in 2019, that mimics AD but is a unique disease entity driven by the misfolding of the protein TDP-43, which regulates gene expression in the brain. Misfolded TDP-43 protein is common among older adults aged ≥ 85 years, and about a quarter of this population has enough misfolded TDP-43 protein to affect their memory and cognition.
Diagnosing AD currently relies on a clinical approach. A complete physical examination, with a detailed neurologic examination and a mental status examination, is used to evaluate disease stage. Initial mental status testing evaluates attention and concentration, recent and remote memory, language, praxis, executive function, and visuospatial function. Because LATE is a newly discovered form of dementia, there are no set guidelines on diagnosing LATE and no robust biomarker for TDP-43. What is known about LATE has been gleaned mostly from retrospective clinicopathologic studies.
The LATE consensus working group reports that the clinical course of disease, as studied by autopsy-proven LATE neuropathologic change (LATE-NC), is described as an "amnestic cognitive syndrome that can evolve to incorporate multiple cognitive domains and ultimately to impair activities of daily living." Researchers are currently analyzing different clinical assessments and neuroimaging with MRI to characterize LATE. A group of international researchers recently published a set of clinical criteria for limbic-predominant amnestic neurodegenerative syndrome (LANS), which is associated with LATE-NC. Their criteria include "core, standard and advanced features that are measurable in vivo, including older age at evaluation, mild clinical syndrome, disproportionate hippocampal atrophy, impaired semantic memory, limbic hypometabolism, absence of neocortical degenerative patterns and low likelihood of neocortical tau, with degrees of certainty (highest, high, moderate, low)." Other neuroimaging studies of autopsy-confirmed LATE-NC have shown that atrophy is mostly focused in the medial temporal lobe with marked reduced hippocampal volume.
The group reports that LATE and AD probably share pathophysiologic mechanisms. One of the universally accepted hallmarks of AD is the formation of beta-amyloid plaques, which are dense, mostly insoluble deposits of beta-amyloid protein that develop around neurons in the hippocampus and other regions in the cerebral cortex used for decision-making. These plaques disrupt brain function and lead to brain atrophy. The LATE group also reports that this same pathology has been noted with LATE: "Many subjects with LATE-NC have comorbid brain pathologies, often including amyloid-beta plaques and tauopathy." That said, genetic studies have helped identify five genes with risk alleles for LATE (GRN, TMEM106B, ABCC9, KCNMB2, and APOE), suggesting disease-specific underlying mechanisms compared to AD.
Patient and caregiver education and guidance is vital with a dementia diagnosis. If LATE and/or AD are suspected, physicians should encourage the involvement of family and friends who agree to become more involved in the patient's care as the disease progresses. These individuals need to understand the patient's wishes around care, especially for the future when the patient is no longer able to make decisions. The patient may also consider establishing medical advance directives and durable power of attorney for medical and financial decision-making. Caregivers supporting the patient are encouraged to help balance the physical needs of the patient while maintaining respect for them as a competent adult to the extent allowed by the progression of their disease.
Because LATE is a new classification of dementia, there are no known effective treatments. One ongoing study is testing the use of autologous bone marrow–derived stem cells to help improve cognitive impairment among patients with LATE, AD, and other dementias. Current AD treatments are focused on symptomatic therapies that modulate neurotransmitters — either acetylcholine or glutamate. The standard medical treatment includes cholinesterase inhibitors and a partial N-methyl-D-aspartate antagonist. Two amyloid-directed antibodies (aducanumab, lecanemab) are currently available in the United States for individuals with AD exhibiting mild cognitive impairment or mild dementia. A third agent currently in clinical trials (donanemab) has shown significantly slowed clinical progression after 1.5 years among clinical trial participants with early symptomatic AD and amyloid and tau pathology.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
An 85-year-old woman presents to her geriatrician with her daughter, who is her primary caregiver. Seven years ago, the patient was diagnosed with mild Alzheimer's disease (AD). Her symptoms at diagnosis were irritability, forgetfulness, and panic attacks. Cognitive, behavioral, and functional assessments showed levels of decline; neurologic examination revealed mild hyposmia. The patient has been living with her daughter ever since her AD diagnosis.
At today's visit, the daughter reports that her mother has been experiencing loss of appetite and wide mood fluctuations with moments of unusual agitation. In addition, she tells the geriatrician that her mother has had trouble maintaining her balance and seems to have lost her sense of time. The patient has difficulty remembering what month and day it is, and how long it's been since her brother came to visit — which has been every Sunday like clockwork since the patient moved in with her daughter. The daughter also notes that her mother loses track of the story line when she is watching movie and TV shows lately.
The physician orders a brain MRI and genetic panel. MRI reveals atrophy in the frontal cortex as well as the medial temporal lobe, with hippocampal sclerosis. The genetic panel shows APOE and TMEM106 mutations.
‘Fake Xanax’ Tied to Seizures, Coma Is Resistant to Naloxone
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
Bromazolam, a street drug that has been detected with increasing frequency in the United States, has reportedly caused protracted seizures, myocardial injury, comas, and multiday intensive care stays in three individuals, new data from the US Centers for Disease Control and Prevention (CDC) showed.
The substance is one of at least a dozen designer benzodiazepines created in the lab but not approved for any therapeutic use. The Center for Forensic Science Research and Education (CFSRE) reported that bromazolam was first detected in 2016 in recreational drugs in Europe and subsequently appeared in the United States.
It is sold under names such as “XLI-268,” “Xanax,” “Fake Xanax,” and “Dope.” Bromazolam may be sold in tablet or powder form, or sometimes as gummies, and is often taken with fentanyl by users.
The CDC report, published in the Morbidity and Mortality Weekly Report (MMWR), described three cases of “previously healthy young adults,” two 25-year-old men and a 20-year-old woman, who took tablets believing it was alprazolam, when it was actually bromazolam and were found unresponsive.
They could not be revived with naloxone and continued to be unresponsive upon arrival at the emergency department. One of the men was hypertensive (152/100 mmHg), tachycardic (heart rate of 124 beats per minute), and hyperthermic (101.7 °F [38.7 °C]) and experienced multiple generalized seizures. He was intubated and admitted to intensive care.
The other man also had an elevated temperature (100.4 °F) and was intubated and admitted to the ICU because of unresponsiveness and multiple generalized seizures.
The woman was also intubated and nonresponsive with focal seizures. All three had elevated troponin levels and had urine tests positive for benzodiazepines.
The first man was intubated for 5 days and discharged after 11 days, while the second man was discharged on the fourth day with mild hearing difficulty.
The woman progressed to status epilepticus despite administration of multiple antiepileptic medications and was in a persistent coma. She was transferred to a second hospital after 11 days and was subsequently lost to follow-up.
Toxicology testing by the Drug Enforcement Administration confirmed the presence of bromazolam (range = 31.1-207 ng/mL), without the presence of fentanyl or any other opioid.
The CDC said that “the constellation of findings reported should prompt close involvement with public health officials and regional poison centers, given the more severe findings in these reported cases compared with those expected from routine benzodiazepine overdoses.” In addition, it noted that clinicians and first responders should “consider bromazolam in cases of patients requiring treatment for seizures, myocardial injury, or hyperthermia after illicit drug use.”
Surging Supply, Increased Warnings
In 2022, the CDC warned that the drug was surging in the United States, noting that as of mid-2022, bromazolam was identified in more than 250 toxicology cases submitted to NMS Labs, and that it had been identified in more than 190 toxicology samples tested at CFSRE.
In early 2021, only 1% of samples were positive for bromazolam. By mid-2022, 13% of samples were positive for bromazolam, and 75% of the bromazolam samples were positive for fentanyl.
The combination is sold on the street as benzo-dope.
Health authorities across the globe have been warning about the dangers of designer benzodiazepines, and bromazolam in particular. They’ve noted that the overdose reversal agent naloxone does not combat the effects of a benzodiazepine overdose.
In December 2022, the Canadian province of New Brunswick said that bromazolam had been detected in nine sudden death investigations, and that fentanyl was detected in some of those cases. The provincial government of the Northwest Territories warned in May 2023 that bromazolam had been detected in the region’s drug supply and cautioned against combining it with opioids.
The Indiana Department of Health notified the public, first responders, law enforcement, and clinicians in August 2023 that the drug was increasingly being detected in the state. In the first half of the year, 35 people who had overdosed in Indiana tested positive for bromazolam. The state did not test for the presence of bromazolam before 2023.
According to the MMWR, the law enforcement seizures in the United States of bromazolam increased from no more than three per year during 2016-2018 to 2142 in 2022 and 2913 in 2023.
Illinois has been an area of increased use. Bromazolam-involved deaths increased from 10 in 2021 to 51 in 2022, the CDC researchers reported.
A version of this article appeared on Medscape.com.
FROM THE MORBIDITY AND MORTALITY WEEKLY REPORT
Side Effects of Local Treatment for Advanced Prostate Cancer May Linger for Years
TOPLINE:
METHODOLOGY:
Recent evidence suggested that in men with advanced prostate cancer, local therapy with radical prostatectomy or radiation may improve survival outcomes; however, data on the long-term side effects from these local options were limited.
The retrospective cohort included 5502 men (mean age, 68 years) diagnosed with advanced (T4, N1, and/or M1) prostate cancer.
A total of 1705 men (31%) received initial local treatment, consisting of radical prostatectomy, (55%), radiation (39%), or both (5.6%), while 3797 (69%) opted for initial nonlocal treatment (hormone therapy, chemotherapy, or both).
The main outcomes were treatment-related adverse effects, including GI, chronic pain, sexual dysfunction, and urinary symptoms, assessed at three timepoints after initial treatment — up to 1 year, between 1 and 2 years, and between 2 and 5 years.
TAKEAWAY:
Overall, 916 men (75%) who had initial local treatment and 897 men (67%) with initial nonlocal therapy reported at least one adverse condition up to 5 years after initial treatment.
In the first year after initial treatment, local therapy was associated with a higher prevalence of GI (9% vs 3%), pain (60% vs 38%), sexual (37% vs 8%), and urinary (46.5% vs 18%) conditions. Men receiving local therapy were more likely to experience GI (adjusted odds ratio [aOR], 4.08), pain (aOR, 1.57), sexual (aOR, 2.96), and urinary (aOR, 2.25) conditions.
Between 2 and 5 years after local therapy, certain conditions remained more prevalent — 7.8% vs 4.2% for GI, 40% vs 13% for sexual, and 40.5% vs 26% for urinary issues. Men receiving local vs nonlocal therapy were more likely to experience GI (aOR, 2.39), sexual (aOR, 3.36), and urinary (aOR, 1.39) issues over the long term.
The researchers found no difference in the prevalence of constitutional conditions such as hot flashes (36.5% vs 34.4%) in the first year following initial local or nonlocal therapy. However, local treatment followed by any secondary treatment was associated with a higher likelihood of developing constitutional conditions at 1-2 years (aOR, 1.50) and 2-5 years (aOR, 1.78) after initial treatment.
IN PRACTICE:
“These results suggest that patients and clinicians should consider the adverse effects of local treatment” alongside the potential for enhanced survival when making treatment decisions in the setting of advanced prostate cancer, the authors explained. Careful informed decision-making by both patients and practitioners is especially important because “there are currently no established guidelines regarding the use of local treatment among men with advanced prostate cancer.”
SOURCE:
The study, with first author Saira Khan, PhD, MPH, Washington University School of Medicine in St. Louis, Missouri, was published online in JAMA Network Open.
LIMITATIONS:
The authors noted that the study was limited by its retrospective design. Men who received local treatment were, on average, younger; older or lesser healthy patients who received local treatment may experience worse adverse effects than observed in the study. The study was limited to US veterans.
DISCLOSURES:
The study was supported by a grant from the US Department of Defense. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Recent evidence suggested that in men with advanced prostate cancer, local therapy with radical prostatectomy or radiation may improve survival outcomes; however, data on the long-term side effects from these local options were limited.
The retrospective cohort included 5502 men (mean age, 68 years) diagnosed with advanced (T4, N1, and/or M1) prostate cancer.
A total of 1705 men (31%) received initial local treatment, consisting of radical prostatectomy, (55%), radiation (39%), or both (5.6%), while 3797 (69%) opted for initial nonlocal treatment (hormone therapy, chemotherapy, or both).
The main outcomes were treatment-related adverse effects, including GI, chronic pain, sexual dysfunction, and urinary symptoms, assessed at three timepoints after initial treatment — up to 1 year, between 1 and 2 years, and between 2 and 5 years.
TAKEAWAY:
Overall, 916 men (75%) who had initial local treatment and 897 men (67%) with initial nonlocal therapy reported at least one adverse condition up to 5 years after initial treatment.
In the first year after initial treatment, local therapy was associated with a higher prevalence of GI (9% vs 3%), pain (60% vs 38%), sexual (37% vs 8%), and urinary (46.5% vs 18%) conditions. Men receiving local therapy were more likely to experience GI (adjusted odds ratio [aOR], 4.08), pain (aOR, 1.57), sexual (aOR, 2.96), and urinary (aOR, 2.25) conditions.
Between 2 and 5 years after local therapy, certain conditions remained more prevalent — 7.8% vs 4.2% for GI, 40% vs 13% for sexual, and 40.5% vs 26% for urinary issues. Men receiving local vs nonlocal therapy were more likely to experience GI (aOR, 2.39), sexual (aOR, 3.36), and urinary (aOR, 1.39) issues over the long term.
The researchers found no difference in the prevalence of constitutional conditions such as hot flashes (36.5% vs 34.4%) in the first year following initial local or nonlocal therapy. However, local treatment followed by any secondary treatment was associated with a higher likelihood of developing constitutional conditions at 1-2 years (aOR, 1.50) and 2-5 years (aOR, 1.78) after initial treatment.
IN PRACTICE:
“These results suggest that patients and clinicians should consider the adverse effects of local treatment” alongside the potential for enhanced survival when making treatment decisions in the setting of advanced prostate cancer, the authors explained. Careful informed decision-making by both patients and practitioners is especially important because “there are currently no established guidelines regarding the use of local treatment among men with advanced prostate cancer.”
SOURCE:
The study, with first author Saira Khan, PhD, MPH, Washington University School of Medicine in St. Louis, Missouri, was published online in JAMA Network Open.
LIMITATIONS:
The authors noted that the study was limited by its retrospective design. Men who received local treatment were, on average, younger; older or lesser healthy patients who received local treatment may experience worse adverse effects than observed in the study. The study was limited to US veterans.
DISCLOSURES:
The study was supported by a grant from the US Department of Defense. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Recent evidence suggested that in men with advanced prostate cancer, local therapy with radical prostatectomy or radiation may improve survival outcomes; however, data on the long-term side effects from these local options were limited.
The retrospective cohort included 5502 men (mean age, 68 years) diagnosed with advanced (T4, N1, and/or M1) prostate cancer.
A total of 1705 men (31%) received initial local treatment, consisting of radical prostatectomy, (55%), radiation (39%), or both (5.6%), while 3797 (69%) opted for initial nonlocal treatment (hormone therapy, chemotherapy, or both).
The main outcomes were treatment-related adverse effects, including GI, chronic pain, sexual dysfunction, and urinary symptoms, assessed at three timepoints after initial treatment — up to 1 year, between 1 and 2 years, and between 2 and 5 years.
TAKEAWAY:
Overall, 916 men (75%) who had initial local treatment and 897 men (67%) with initial nonlocal therapy reported at least one adverse condition up to 5 years after initial treatment.
In the first year after initial treatment, local therapy was associated with a higher prevalence of GI (9% vs 3%), pain (60% vs 38%), sexual (37% vs 8%), and urinary (46.5% vs 18%) conditions. Men receiving local therapy were more likely to experience GI (adjusted odds ratio [aOR], 4.08), pain (aOR, 1.57), sexual (aOR, 2.96), and urinary (aOR, 2.25) conditions.
Between 2 and 5 years after local therapy, certain conditions remained more prevalent — 7.8% vs 4.2% for GI, 40% vs 13% for sexual, and 40.5% vs 26% for urinary issues. Men receiving local vs nonlocal therapy were more likely to experience GI (aOR, 2.39), sexual (aOR, 3.36), and urinary (aOR, 1.39) issues over the long term.
The researchers found no difference in the prevalence of constitutional conditions such as hot flashes (36.5% vs 34.4%) in the first year following initial local or nonlocal therapy. However, local treatment followed by any secondary treatment was associated with a higher likelihood of developing constitutional conditions at 1-2 years (aOR, 1.50) and 2-5 years (aOR, 1.78) after initial treatment.
IN PRACTICE:
“These results suggest that patients and clinicians should consider the adverse effects of local treatment” alongside the potential for enhanced survival when making treatment decisions in the setting of advanced prostate cancer, the authors explained. Careful informed decision-making by both patients and practitioners is especially important because “there are currently no established guidelines regarding the use of local treatment among men with advanced prostate cancer.”
SOURCE:
The study, with first author Saira Khan, PhD, MPH, Washington University School of Medicine in St. Louis, Missouri, was published online in JAMA Network Open.
LIMITATIONS:
The authors noted that the study was limited by its retrospective design. Men who received local treatment were, on average, younger; older or lesser healthy patients who received local treatment may experience worse adverse effects than observed in the study. The study was limited to US veterans.
DISCLOSURES:
The study was supported by a grant from the US Department of Defense. The authors have no relevant disclosures.
A version of this article appeared on Medscape.com.
Rebranding NAFLD: Correcting ‘Flawed’ Conventions
Nonalcoholic fatty liver disease (NAFLD) should now be referred to as metabolic dysfunction–associated steatotic liver disease (MASLD), according to a recent commentary by leading hepatologists.
This update, which was determined by a panel of 236 panelists from 56 countries, is part of a broader effort to rebrand “fatty liver disease” as “steatotic liver disease” (SLD), reported lead author Alina M. Allen, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues.
Writing in Gastroenterology, they described a range of reasons for the nomenclature changes, from the need for better characterization of disease subtypes, to the concern that the term “fatty” may be perceived as stigmatizing by some patients.
“The scientific community and stakeholder organizations associated with liver diseases determined there was a need for new terminology to cover liver disease related to alcohol alone, metabolic risk factors (until recently termed NAFLD/nonalcoholic steatohepatitis [NASH]) alone, the combination of alcohol and metabolic risk factors, and hepatic steatosis due to other specific etiologies,” the authors wrote.
Naming conventions in this area have been flawed since inception, Dr. Allen and colleagues wrote, noting that “nonalcoholic” is exclusionary rather than descriptive, and is particularly misplaced in the pediatric setting. These shortcomings could explain why the term “NASH” took more than a decade to enter common usage, they suggested, and why the present effort is not the first of its kind.
“There have been several movements to change the nomenclature [of NAFLD], including most recently to ‘metabolic dysfunction–associated fatty liver disease’ (MAFLD), a term that received limited traction,” the authors wrote.
Still, a change is needed, they added, as metabolic dysfunction is becoming increasingly common on a global scale, driving up rates of liver disease. Furthermore, in some patients, alcohol consumption and metabolic factors concurrently drive steatosis, suggesting an intermediate condition between alcohol-related liver disease (ALD) and NAFLD that is indescribable via current naming conventions.
SLD (determined by imaging or biopsy) now comprises five disease subtypes that can be determined via an algorithm provided in the present publication.
If at least one metabolic criterion is present, but no other causes of steatosis, then that patient has MASLD. The three other metabolic subtypes include MetALD (2-3 drinks per day for women and 3-4 drinks per day for men), ALD (more than 3 drinks per day for women and more than 4 drinks per day for men), and monogenic miscellaneous drug-induced liver injury (DILI).
Patients without metabolic criteria can also be classified with monogenic miscellaneous DILI with no caveat, whereas patients with metabolic criteria need only consume 2 or 3 drinks per day for women or 3-4 drinks per day for men, respectively, to be diagnosed with ALD.
Finally, patients with no metabolic criteria or other cause of steatosis should be characterized by cryptogenic SLD.
“While renaming and redefining the disease was needed, the implementation is not without challenges,” Dr. Allen and colleagues wrote. “A more complex classification may add confusion in the mind of nonhepatology providers when awareness and understanding of the implications of SLD are already suboptimal.”
Still, they predicted that the new naming system could lead to several positive outcomes, including improved SLD screening among individuals with metabolic risk factors, more accurate phenotyping of patients with moderate alcohol consumption, increased disease awareness in nonhepatology practices, and improved multidisciplinary collaboration.
Only time will tell whether these benefits come to fruition, Dr. Allen and colleagues noted, before closing with a quote: “In the words of Jean Piaget, the developmental psychologist of the 20th century, who coincidentally died the year the term NASH was coined, ‘Scientific knowledge is in perpetual evolution; it finds itself changed from one day to the next.’”
The authors disclosed no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) should now be referred to as metabolic dysfunction–associated steatotic liver disease (MASLD), according to a recent commentary by leading hepatologists.
This update, which was determined by a panel of 236 panelists from 56 countries, is part of a broader effort to rebrand “fatty liver disease” as “steatotic liver disease” (SLD), reported lead author Alina M. Allen, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues.
Writing in Gastroenterology, they described a range of reasons for the nomenclature changes, from the need for better characterization of disease subtypes, to the concern that the term “fatty” may be perceived as stigmatizing by some patients.
“The scientific community and stakeholder organizations associated with liver diseases determined there was a need for new terminology to cover liver disease related to alcohol alone, metabolic risk factors (until recently termed NAFLD/nonalcoholic steatohepatitis [NASH]) alone, the combination of alcohol and metabolic risk factors, and hepatic steatosis due to other specific etiologies,” the authors wrote.
Naming conventions in this area have been flawed since inception, Dr. Allen and colleagues wrote, noting that “nonalcoholic” is exclusionary rather than descriptive, and is particularly misplaced in the pediatric setting. These shortcomings could explain why the term “NASH” took more than a decade to enter common usage, they suggested, and why the present effort is not the first of its kind.
“There have been several movements to change the nomenclature [of NAFLD], including most recently to ‘metabolic dysfunction–associated fatty liver disease’ (MAFLD), a term that received limited traction,” the authors wrote.
Still, a change is needed, they added, as metabolic dysfunction is becoming increasingly common on a global scale, driving up rates of liver disease. Furthermore, in some patients, alcohol consumption and metabolic factors concurrently drive steatosis, suggesting an intermediate condition between alcohol-related liver disease (ALD) and NAFLD that is indescribable via current naming conventions.
SLD (determined by imaging or biopsy) now comprises five disease subtypes that can be determined via an algorithm provided in the present publication.
If at least one metabolic criterion is present, but no other causes of steatosis, then that patient has MASLD. The three other metabolic subtypes include MetALD (2-3 drinks per day for women and 3-4 drinks per day for men), ALD (more than 3 drinks per day for women and more than 4 drinks per day for men), and monogenic miscellaneous drug-induced liver injury (DILI).
Patients without metabolic criteria can also be classified with monogenic miscellaneous DILI with no caveat, whereas patients with metabolic criteria need only consume 2 or 3 drinks per day for women or 3-4 drinks per day for men, respectively, to be diagnosed with ALD.
Finally, patients with no metabolic criteria or other cause of steatosis should be characterized by cryptogenic SLD.
“While renaming and redefining the disease was needed, the implementation is not without challenges,” Dr. Allen and colleagues wrote. “A more complex classification may add confusion in the mind of nonhepatology providers when awareness and understanding of the implications of SLD are already suboptimal.”
Still, they predicted that the new naming system could lead to several positive outcomes, including improved SLD screening among individuals with metabolic risk factors, more accurate phenotyping of patients with moderate alcohol consumption, increased disease awareness in nonhepatology practices, and improved multidisciplinary collaboration.
Only time will tell whether these benefits come to fruition, Dr. Allen and colleagues noted, before closing with a quote: “In the words of Jean Piaget, the developmental psychologist of the 20th century, who coincidentally died the year the term NASH was coined, ‘Scientific knowledge is in perpetual evolution; it finds itself changed from one day to the next.’”
The authors disclosed no conflicts of interest.
Nonalcoholic fatty liver disease (NAFLD) should now be referred to as metabolic dysfunction–associated steatotic liver disease (MASLD), according to a recent commentary by leading hepatologists.
This update, which was determined by a panel of 236 panelists from 56 countries, is part of a broader effort to rebrand “fatty liver disease” as “steatotic liver disease” (SLD), reported lead author Alina M. Allen, MD, of Mayo Clinic, Rochester, Minnesota, and colleagues.
Writing in Gastroenterology, they described a range of reasons for the nomenclature changes, from the need for better characterization of disease subtypes, to the concern that the term “fatty” may be perceived as stigmatizing by some patients.
“The scientific community and stakeholder organizations associated with liver diseases determined there was a need for new terminology to cover liver disease related to alcohol alone, metabolic risk factors (until recently termed NAFLD/nonalcoholic steatohepatitis [NASH]) alone, the combination of alcohol and metabolic risk factors, and hepatic steatosis due to other specific etiologies,” the authors wrote.
Naming conventions in this area have been flawed since inception, Dr. Allen and colleagues wrote, noting that “nonalcoholic” is exclusionary rather than descriptive, and is particularly misplaced in the pediatric setting. These shortcomings could explain why the term “NASH” took more than a decade to enter common usage, they suggested, and why the present effort is not the first of its kind.
“There have been several movements to change the nomenclature [of NAFLD], including most recently to ‘metabolic dysfunction–associated fatty liver disease’ (MAFLD), a term that received limited traction,” the authors wrote.
Still, a change is needed, they added, as metabolic dysfunction is becoming increasingly common on a global scale, driving up rates of liver disease. Furthermore, in some patients, alcohol consumption and metabolic factors concurrently drive steatosis, suggesting an intermediate condition between alcohol-related liver disease (ALD) and NAFLD that is indescribable via current naming conventions.
SLD (determined by imaging or biopsy) now comprises five disease subtypes that can be determined via an algorithm provided in the present publication.
If at least one metabolic criterion is present, but no other causes of steatosis, then that patient has MASLD. The three other metabolic subtypes include MetALD (2-3 drinks per day for women and 3-4 drinks per day for men), ALD (more than 3 drinks per day for women and more than 4 drinks per day for men), and monogenic miscellaneous drug-induced liver injury (DILI).
Patients without metabolic criteria can also be classified with monogenic miscellaneous DILI with no caveat, whereas patients with metabolic criteria need only consume 2 or 3 drinks per day for women or 3-4 drinks per day for men, respectively, to be diagnosed with ALD.
Finally, patients with no metabolic criteria or other cause of steatosis should be characterized by cryptogenic SLD.
“While renaming and redefining the disease was needed, the implementation is not without challenges,” Dr. Allen and colleagues wrote. “A more complex classification may add confusion in the mind of nonhepatology providers when awareness and understanding of the implications of SLD are already suboptimal.”
Still, they predicted that the new naming system could lead to several positive outcomes, including improved SLD screening among individuals with metabolic risk factors, more accurate phenotyping of patients with moderate alcohol consumption, increased disease awareness in nonhepatology practices, and improved multidisciplinary collaboration.
Only time will tell whether these benefits come to fruition, Dr. Allen and colleagues noted, before closing with a quote: “In the words of Jean Piaget, the developmental psychologist of the 20th century, who coincidentally died the year the term NASH was coined, ‘Scientific knowledge is in perpetual evolution; it finds itself changed from one day to the next.’”
The authors disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Zoom: Convenient and Imperfect
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Inflammatory Responses to a Common Food Additive May Depend On the Microbiome
Inflammatory responses to the food additive carboxymethylcellulose (CMC) may depend on the unique characteristics of an individual’s microbiome, according to recent research.
These findings suggest that CMC, which is commonly used as a thickener and emulsifier to improve texture and shelf life of food, could potentially trigger chronic inflammation in genetically prone individuals, although more work is needed to pinpoint the exact microbiota involved, reported lead author Noëmie Daniel, PhD, of the French National Institute of Health and Medical Research (INSERM), Paris, and colleagues.“Preclinical work has shown that [CMC] consumption detrimentally impacts the intestinal microbiota in a way that promotes chronic inflammation,” the investigators wrote in a research letter in Cellular and Molecular Gastroenterology and Hepatology.
They published the results of a randomized, double-blind controlled trial that showed that the seven individuals exposed to a CMC-supplemented diet had “significant alterations in microbiota composition and metabolome” compared with the nine control subjects.
Yet responses to CMC varied widely. In the treatment group, some participants were relatively insensitive to CMC, while two participants had “stark alterations” in their microbiome.
“Such CMC sensitivity was not associated with overt signs of intestinal inflammation but nonetheless might mark proneness to chronic inflammation, compelling us to better understand mechanisms that mediate CMC sensitivity,” the investigators wrote.
To learn more, Dr. Daniel and colleagues conducted the present study, which involved a series of analyses and experiments.
They first compared inflammatory bowel disease-associated mutations and basal gene expression between CMC-sensitive and CMC-insensitive individuals from their previous trial. Neither were associated with CMC sensitivity, they found.
Evaluating microbiota was a more fruitful approach. Microbiome multivariable association with linear models analysis revealed 11 amplicon sequence variants (ASVs) that differed between groups.
“This algorithm did not detect differences between randomly selected subjects, arguing that ASVs that are associated with CMC sensitivity were not false discoveries but rather had marked, and perhaps contributed to, CMC sensitivity status,” the investigators wrote.
Next, they transplanted pre-CMC fecal samples from 2 CMC-sensitive and 2 CMC-insensitive individuals into germ-free, colitis-prone, interleukin 10-/- mice. Exposing these mice to CMC led to distinct changes in microbiota that was not clearly associated with the sensitivity status of the donor. However, mice that received transplants from CMC-sensitive individuals demonstrated increased microbiota-derived proinflammatory markers, increased microbiota encroachment, and “stark” intestinal inflammation after CMC consumption, suggesting that these responses were somehow mediated by the microbiome.
“These [results] indicate a role for basal microbiotas in influencing CMC impact on this cardinal feature of intestinal inflammation and suspected driver of chronic diseases,” the investigators wrote.
“Our findings suggest that the microbiota participate in the extent to which an individual harbors proneness to CMC-induced inflammatory diseases,” they added. “Accordingly, CMC consumption may be one trigger of chronic inflammation in genetically prone individuals colonized with a given microbial ecosystem.”
Research on a larger number of participants appears needed to substantiate their observations and determine the exact microbiota contributor(s) driving CMC sensitivity, the researchers concluded.
The investigators disclosed no conflicts of interest. Funding support came from a starting grant from the European Research Council under the European Union’s Horizon 2020 research and innovation program, a Chaire d’Excellence from IdEx Université de Paris, an award from the Fondation de l’Avenir, ANR grants EMULBIONT and DREAM, and the national program Microbiote from INSERM (B.C.). Supported also came from National Institutes of Health grants, the Penn Center for Nutritional Science and Medicine, and the Max Planck Society.
The consumption of highly processed foods, enriched with food additives, is associated with an increased risk of developing inflammatory bowel disease (IBD). Alteration of the intestinal barrier and microbiota encroachment on epithelial cells is thought to be one of the mechanisms leading to inappropriate mucosal immune activation in response to food additive intake. However, we still do not know why some exposed individuals develop IBD while others do not. The findings of Daniel and colleagues suggest that proinflammatory sensitivity to the food additive carboxymethylcellulose (CMC) is primarily dependent on the composition of the gut microbiota, and that this sensitivity can be, at least partially, transferable, using fecal microbiota transfers in a mouse model of IBD. In particular, they identified 11 taxa of the host basal microbiota associated with the development of intestinal inflammation in response to CMC.
Finally, this study also illustrates the relevance of systematically assessing the impact of food additives and emulsifiers on the gut microbiota and intestinal physiology in order to evaluate their safety using translational approaches similar to those applied by Daniel and colleagues.
Nicolas Benech, MD, PhD is an assistant professor at the Lyon 1 University and Gastroenterology department, Hôpital de la Croix-Rousse, Hospices Civils de Lyon, Lyon, France, the director of the Lyon Fecal Microbiota transplantation Center, and cofounder of the Lyon GEM Microbiota Study Group. He has no conflicts.
The consumption of highly processed foods, enriched with food additives, is associated with an increased risk of developing inflammatory bowel disease (IBD). Alteration of the intestinal barrier and microbiota encroachment on epithelial cells is thought to be one of the mechanisms leading to inappropriate mucosal immune activation in response to food additive intake. However, we still do not know why some exposed individuals develop IBD while others do not. The findings of Daniel and colleagues suggest that proinflammatory sensitivity to the food additive carboxymethylcellulose (CMC) is primarily dependent on the composition of the gut microbiota, and that this sensitivity can be, at least partially, transferable, using fecal microbiota transfers in a mouse model of IBD. In particular, they identified 11 taxa of the host basal microbiota associated with the development of intestinal inflammation in response to CMC.
Finally, this study also illustrates the relevance of systematically assessing the impact of food additives and emulsifiers on the gut microbiota and intestinal physiology in order to evaluate their safety using translational approaches similar to those applied by Daniel and colleagues.
Nicolas Benech, MD, PhD is an assistant professor at the Lyon 1 University and Gastroenterology department, Hôpital de la Croix-Rousse, Hospices Civils de Lyon, Lyon, France, the director of the Lyon Fecal Microbiota transplantation Center, and cofounder of the Lyon GEM Microbiota Study Group. He has no conflicts.
The consumption of highly processed foods, enriched with food additives, is associated with an increased risk of developing inflammatory bowel disease (IBD). Alteration of the intestinal barrier and microbiota encroachment on epithelial cells is thought to be one of the mechanisms leading to inappropriate mucosal immune activation in response to food additive intake. However, we still do not know why some exposed individuals develop IBD while others do not. The findings of Daniel and colleagues suggest that proinflammatory sensitivity to the food additive carboxymethylcellulose (CMC) is primarily dependent on the composition of the gut microbiota, and that this sensitivity can be, at least partially, transferable, using fecal microbiota transfers in a mouse model of IBD. In particular, they identified 11 taxa of the host basal microbiota associated with the development of intestinal inflammation in response to CMC.
Finally, this study also illustrates the relevance of systematically assessing the impact of food additives and emulsifiers on the gut microbiota and intestinal physiology in order to evaluate their safety using translational approaches similar to those applied by Daniel and colleagues.
Nicolas Benech, MD, PhD is an assistant professor at the Lyon 1 University and Gastroenterology department, Hôpital de la Croix-Rousse, Hospices Civils de Lyon, Lyon, France, the director of the Lyon Fecal Microbiota transplantation Center, and cofounder of the Lyon GEM Microbiota Study Group. He has no conflicts.
Inflammatory responses to the food additive carboxymethylcellulose (CMC) may depend on the unique characteristics of an individual’s microbiome, according to recent research.
These findings suggest that CMC, which is commonly used as a thickener and emulsifier to improve texture and shelf life of food, could potentially trigger chronic inflammation in genetically prone individuals, although more work is needed to pinpoint the exact microbiota involved, reported lead author Noëmie Daniel, PhD, of the French National Institute of Health and Medical Research (INSERM), Paris, and colleagues.“Preclinical work has shown that [CMC] consumption detrimentally impacts the intestinal microbiota in a way that promotes chronic inflammation,” the investigators wrote in a research letter in Cellular and Molecular Gastroenterology and Hepatology.
They published the results of a randomized, double-blind controlled trial that showed that the seven individuals exposed to a CMC-supplemented diet had “significant alterations in microbiota composition and metabolome” compared with the nine control subjects.
Yet responses to CMC varied widely. In the treatment group, some participants were relatively insensitive to CMC, while two participants had “stark alterations” in their microbiome.
“Such CMC sensitivity was not associated with overt signs of intestinal inflammation but nonetheless might mark proneness to chronic inflammation, compelling us to better understand mechanisms that mediate CMC sensitivity,” the investigators wrote.
To learn more, Dr. Daniel and colleagues conducted the present study, which involved a series of analyses and experiments.
They first compared inflammatory bowel disease-associated mutations and basal gene expression between CMC-sensitive and CMC-insensitive individuals from their previous trial. Neither were associated with CMC sensitivity, they found.
Evaluating microbiota was a more fruitful approach. Microbiome multivariable association with linear models analysis revealed 11 amplicon sequence variants (ASVs) that differed between groups.
“This algorithm did not detect differences between randomly selected subjects, arguing that ASVs that are associated with CMC sensitivity were not false discoveries but rather had marked, and perhaps contributed to, CMC sensitivity status,” the investigators wrote.
Next, they transplanted pre-CMC fecal samples from 2 CMC-sensitive and 2 CMC-insensitive individuals into germ-free, colitis-prone, interleukin 10-/- mice. Exposing these mice to CMC led to distinct changes in microbiota that was not clearly associated with the sensitivity status of the donor. However, mice that received transplants from CMC-sensitive individuals demonstrated increased microbiota-derived proinflammatory markers, increased microbiota encroachment, and “stark” intestinal inflammation after CMC consumption, suggesting that these responses were somehow mediated by the microbiome.
“These [results] indicate a role for basal microbiotas in influencing CMC impact on this cardinal feature of intestinal inflammation and suspected driver of chronic diseases,” the investigators wrote.
“Our findings suggest that the microbiota participate in the extent to which an individual harbors proneness to CMC-induced inflammatory diseases,” they added. “Accordingly, CMC consumption may be one trigger of chronic inflammation in genetically prone individuals colonized with a given microbial ecosystem.”
Research on a larger number of participants appears needed to substantiate their observations and determine the exact microbiota contributor(s) driving CMC sensitivity, the researchers concluded.
The investigators disclosed no conflicts of interest. Funding support came from a starting grant from the European Research Council under the European Union’s Horizon 2020 research and innovation program, a Chaire d’Excellence from IdEx Université de Paris, an award from the Fondation de l’Avenir, ANR grants EMULBIONT and DREAM, and the national program Microbiote from INSERM (B.C.). Supported also came from National Institutes of Health grants, the Penn Center for Nutritional Science and Medicine, and the Max Planck Society.
Inflammatory responses to the food additive carboxymethylcellulose (CMC) may depend on the unique characteristics of an individual’s microbiome, according to recent research.
These findings suggest that CMC, which is commonly used as a thickener and emulsifier to improve texture and shelf life of food, could potentially trigger chronic inflammation in genetically prone individuals, although more work is needed to pinpoint the exact microbiota involved, reported lead author Noëmie Daniel, PhD, of the French National Institute of Health and Medical Research (INSERM), Paris, and colleagues.“Preclinical work has shown that [CMC] consumption detrimentally impacts the intestinal microbiota in a way that promotes chronic inflammation,” the investigators wrote in a research letter in Cellular and Molecular Gastroenterology and Hepatology.
They published the results of a randomized, double-blind controlled trial that showed that the seven individuals exposed to a CMC-supplemented diet had “significant alterations in microbiota composition and metabolome” compared with the nine control subjects.
Yet responses to CMC varied widely. In the treatment group, some participants were relatively insensitive to CMC, while two participants had “stark alterations” in their microbiome.
“Such CMC sensitivity was not associated with overt signs of intestinal inflammation but nonetheless might mark proneness to chronic inflammation, compelling us to better understand mechanisms that mediate CMC sensitivity,” the investigators wrote.
To learn more, Dr. Daniel and colleagues conducted the present study, which involved a series of analyses and experiments.
They first compared inflammatory bowel disease-associated mutations and basal gene expression between CMC-sensitive and CMC-insensitive individuals from their previous trial. Neither were associated with CMC sensitivity, they found.
Evaluating microbiota was a more fruitful approach. Microbiome multivariable association with linear models analysis revealed 11 amplicon sequence variants (ASVs) that differed between groups.
“This algorithm did not detect differences between randomly selected subjects, arguing that ASVs that are associated with CMC sensitivity were not false discoveries but rather had marked, and perhaps contributed to, CMC sensitivity status,” the investigators wrote.
Next, they transplanted pre-CMC fecal samples from 2 CMC-sensitive and 2 CMC-insensitive individuals into germ-free, colitis-prone, interleukin 10-/- mice. Exposing these mice to CMC led to distinct changes in microbiota that was not clearly associated with the sensitivity status of the donor. However, mice that received transplants from CMC-sensitive individuals demonstrated increased microbiota-derived proinflammatory markers, increased microbiota encroachment, and “stark” intestinal inflammation after CMC consumption, suggesting that these responses were somehow mediated by the microbiome.
“These [results] indicate a role for basal microbiotas in influencing CMC impact on this cardinal feature of intestinal inflammation and suspected driver of chronic diseases,” the investigators wrote.
“Our findings suggest that the microbiota participate in the extent to which an individual harbors proneness to CMC-induced inflammatory diseases,” they added. “Accordingly, CMC consumption may be one trigger of chronic inflammation in genetically prone individuals colonized with a given microbial ecosystem.”
Research on a larger number of participants appears needed to substantiate their observations and determine the exact microbiota contributor(s) driving CMC sensitivity, the researchers concluded.
The investigators disclosed no conflicts of interest. Funding support came from a starting grant from the European Research Council under the European Union’s Horizon 2020 research and innovation program, a Chaire d’Excellence from IdEx Université de Paris, an award from the Fondation de l’Avenir, ANR grants EMULBIONT and DREAM, and the national program Microbiote from INSERM (B.C.). Supported also came from National Institutes of Health grants, the Penn Center for Nutritional Science and Medicine, and the Max Planck Society.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
More comprehensive testing needed to characterize esophageal dysphagia
The current approach to esophageal function testing is insufficient to characterize esophageal motility disorders, as many patients with esophageal dysphagia have abnormalities that are undetectable with routine tests, according to investigators.
More nuanced assessments of esophageal motility disorders could potentially lead to more accurate diagnoses, and more effective treatments, reported Ravinder K. Mittal, MD, and Ali Zifan, PhD, of the University of California San Diego.
Esophageal motility disorders are currently divided into major and minor variants based on the contraction phase of peristalsis, Dr. Mittal and Dr. Zifan wrote in their report in Gastro Hep Advances. Yet the reason for dysphagia in many of these patients remains a puzzle, particularly in patients with supernormal contraction during peristalsis, like those with nutcracker esophagus. What’s more, up to half of patients with dysphagia have normal findings on high-resolution manometry impedance (HRMZ), the typical diagnostic modality, leaving many with the broad label of functional dysphagia.
This lack of clarity “suggests that the etiology in many patients remains unknown,” according to the investigators, which prompted them to publish the present review article.
After describing the shortcomings of current test methods, the investigators provided an overview of the physiology of esophageal peristalsis, then dove deeper into available data concerning luminal cross section measurements, esophageal distension during peristalsis, bolus flow, and distension contraction patterns in normal patients versus those with various kinds of dysphagia.
They highlighted two key findings.
First, in patients with functional dysphagia, esophagogastric junction outflow obstruction (EGJOO), and high amplitude esophageal peristaltic contractions (HAEC), the bolus must travel through a narrow esophageal lumen. Second, in patients with nonobstructive dysphagia and type 3 achalasia, the bolus moves against distal luminal occlusion.
“These findings indicate a relative dynamic obstruction to bolus flow and reduced distensibility of the esophageal wall in patients with several primary esophageal motility disorders,” the investigators wrote. “We speculate that the dysphagia sensation experienced by many patients may result from a normal or supernormal contraction wave pushing the bolus against resistance.”
Yet routine esophageal function testing fails to capture these abnormalities, Dr. Mittal and Dr. Zifan noted.
“[C]urrent techniques used to measure esophageal distension during peristalsis are not adequate,” they wrote. “The high-resolution manometry and current scheme of classifying esophageal motor disorders in the current format emphasize only half of the story of peristalsis, probably the less important of the two halves, i.e., the contraction phase of peristalsis.”
More focus is needed on esophageal distension, they suggested, noting that relaxation is first needed to accommodate a bolus before contraction, no matter how powerful, can push it down the esophagus.
“A simple analogy is that of a car — it cannot get through a roadway that is smaller than its own width, irrespective of the horsepower of its engine,” they wrote.
The solution may lie in a more comprehensive approach to esophageal function testing.
“Integrating representations of distension and contraction, along with objective assessments of flow timing and distensibility, complements the current classification of esophageal motility disorders that are based on the contraction characteristics,” the investigators wrote, predicting that these efforts could improve diagnostic accuracy.
What to do about those diagnoses is another mystery.
“The question though remains regarding the optimal treatment for the impaired distension function of the esophagus, and whether improvement in the distension function will lead to improvement in dysphagia symptoms,” the investigators concluded.
The review was supported by the National Institutes of Health. The investigators reported copyright/patent protection for the computer software (Dplots) used to evaluate the distension contraction plots.
Medicine is strewn with diseases first labeled as functional or psychologically induced that have been recategorized into clear non-sensory disorders of which functional dysphagia is one.
In this review article, Dr. Mittal and Dr. Zifan discuss a summary and new paradigm for esophageal motility disorders and the origin of functional dysphagia (FD). As with other functional disorders, the predominance of research has suggested that functional dysphagia is in large part a sensory disorder in which patient are sensing normally sub-threshold events of normal bolus transit interpreted as dysphagia.
Will this lead to recategorization of all functional dysphagia as a perturbation in motor function and the discovery of new therapies? Certainly, to some degree, though sensory dysfunction will likely remain a prominent mechanism in some patients. Nevertheless, it is always exciting when a new approach to an old disorder emerges. With the work from Dr. Mittal’s laboratory and many others, functional dysphagia may soon drop the functional!
David A. Katzka, MD, is a gastroenterologist at New York–Presbyterian/Columbia University Irving Medical Center, New York, where he leads the Esophagology and Swallowing Center. He has performed research for Medtronic, but has no other relevant disclosures.
Medicine is strewn with diseases first labeled as functional or psychologically induced that have been recategorized into clear non-sensory disorders of which functional dysphagia is one.
In this review article, Dr. Mittal and Dr. Zifan discuss a summary and new paradigm for esophageal motility disorders and the origin of functional dysphagia (FD). As with other functional disorders, the predominance of research has suggested that functional dysphagia is in large part a sensory disorder in which patient are sensing normally sub-threshold events of normal bolus transit interpreted as dysphagia.
Will this lead to recategorization of all functional dysphagia as a perturbation in motor function and the discovery of new therapies? Certainly, to some degree, though sensory dysfunction will likely remain a prominent mechanism in some patients. Nevertheless, it is always exciting when a new approach to an old disorder emerges. With the work from Dr. Mittal’s laboratory and many others, functional dysphagia may soon drop the functional!
David A. Katzka, MD, is a gastroenterologist at New York–Presbyterian/Columbia University Irving Medical Center, New York, where he leads the Esophagology and Swallowing Center. He has performed research for Medtronic, but has no other relevant disclosures.
Medicine is strewn with diseases first labeled as functional or psychologically induced that have been recategorized into clear non-sensory disorders of which functional dysphagia is one.
In this review article, Dr. Mittal and Dr. Zifan discuss a summary and new paradigm for esophageal motility disorders and the origin of functional dysphagia (FD). As with other functional disorders, the predominance of research has suggested that functional dysphagia is in large part a sensory disorder in which patient are sensing normally sub-threshold events of normal bolus transit interpreted as dysphagia.
Will this lead to recategorization of all functional dysphagia as a perturbation in motor function and the discovery of new therapies? Certainly, to some degree, though sensory dysfunction will likely remain a prominent mechanism in some patients. Nevertheless, it is always exciting when a new approach to an old disorder emerges. With the work from Dr. Mittal’s laboratory and many others, functional dysphagia may soon drop the functional!
David A. Katzka, MD, is a gastroenterologist at New York–Presbyterian/Columbia University Irving Medical Center, New York, where he leads the Esophagology and Swallowing Center. He has performed research for Medtronic, but has no other relevant disclosures.
The current approach to esophageal function testing is insufficient to characterize esophageal motility disorders, as many patients with esophageal dysphagia have abnormalities that are undetectable with routine tests, according to investigators.
More nuanced assessments of esophageal motility disorders could potentially lead to more accurate diagnoses, and more effective treatments, reported Ravinder K. Mittal, MD, and Ali Zifan, PhD, of the University of California San Diego.
Esophageal motility disorders are currently divided into major and minor variants based on the contraction phase of peristalsis, Dr. Mittal and Dr. Zifan wrote in their report in Gastro Hep Advances. Yet the reason for dysphagia in many of these patients remains a puzzle, particularly in patients with supernormal contraction during peristalsis, like those with nutcracker esophagus. What’s more, up to half of patients with dysphagia have normal findings on high-resolution manometry impedance (HRMZ), the typical diagnostic modality, leaving many with the broad label of functional dysphagia.
This lack of clarity “suggests that the etiology in many patients remains unknown,” according to the investigators, which prompted them to publish the present review article.
After describing the shortcomings of current test methods, the investigators provided an overview of the physiology of esophageal peristalsis, then dove deeper into available data concerning luminal cross section measurements, esophageal distension during peristalsis, bolus flow, and distension contraction patterns in normal patients versus those with various kinds of dysphagia.
They highlighted two key findings.
First, in patients with functional dysphagia, esophagogastric junction outflow obstruction (EGJOO), and high amplitude esophageal peristaltic contractions (HAEC), the bolus must travel through a narrow esophageal lumen. Second, in patients with nonobstructive dysphagia and type 3 achalasia, the bolus moves against distal luminal occlusion.
“These findings indicate a relative dynamic obstruction to bolus flow and reduced distensibility of the esophageal wall in patients with several primary esophageal motility disorders,” the investigators wrote. “We speculate that the dysphagia sensation experienced by many patients may result from a normal or supernormal contraction wave pushing the bolus against resistance.”
Yet routine esophageal function testing fails to capture these abnormalities, Dr. Mittal and Dr. Zifan noted.
“[C]urrent techniques used to measure esophageal distension during peristalsis are not adequate,” they wrote. “The high-resolution manometry and current scheme of classifying esophageal motor disorders in the current format emphasize only half of the story of peristalsis, probably the less important of the two halves, i.e., the contraction phase of peristalsis.”
More focus is needed on esophageal distension, they suggested, noting that relaxation is first needed to accommodate a bolus before contraction, no matter how powerful, can push it down the esophagus.
“A simple analogy is that of a car — it cannot get through a roadway that is smaller than its own width, irrespective of the horsepower of its engine,” they wrote.
The solution may lie in a more comprehensive approach to esophageal function testing.
“Integrating representations of distension and contraction, along with objective assessments of flow timing and distensibility, complements the current classification of esophageal motility disorders that are based on the contraction characteristics,” the investigators wrote, predicting that these efforts could improve diagnostic accuracy.
What to do about those diagnoses is another mystery.
“The question though remains regarding the optimal treatment for the impaired distension function of the esophagus, and whether improvement in the distension function will lead to improvement in dysphagia symptoms,” the investigators concluded.
The review was supported by the National Institutes of Health. The investigators reported copyright/patent protection for the computer software (Dplots) used to evaluate the distension contraction plots.
The current approach to esophageal function testing is insufficient to characterize esophageal motility disorders, as many patients with esophageal dysphagia have abnormalities that are undetectable with routine tests, according to investigators.
More nuanced assessments of esophageal motility disorders could potentially lead to more accurate diagnoses, and more effective treatments, reported Ravinder K. Mittal, MD, and Ali Zifan, PhD, of the University of California San Diego.
Esophageal motility disorders are currently divided into major and minor variants based on the contraction phase of peristalsis, Dr. Mittal and Dr. Zifan wrote in their report in Gastro Hep Advances. Yet the reason for dysphagia in many of these patients remains a puzzle, particularly in patients with supernormal contraction during peristalsis, like those with nutcracker esophagus. What’s more, up to half of patients with dysphagia have normal findings on high-resolution manometry impedance (HRMZ), the typical diagnostic modality, leaving many with the broad label of functional dysphagia.
This lack of clarity “suggests that the etiology in many patients remains unknown,” according to the investigators, which prompted them to publish the present review article.
After describing the shortcomings of current test methods, the investigators provided an overview of the physiology of esophageal peristalsis, then dove deeper into available data concerning luminal cross section measurements, esophageal distension during peristalsis, bolus flow, and distension contraction patterns in normal patients versus those with various kinds of dysphagia.
They highlighted two key findings.
First, in patients with functional dysphagia, esophagogastric junction outflow obstruction (EGJOO), and high amplitude esophageal peristaltic contractions (HAEC), the bolus must travel through a narrow esophageal lumen. Second, in patients with nonobstructive dysphagia and type 3 achalasia, the bolus moves against distal luminal occlusion.
“These findings indicate a relative dynamic obstruction to bolus flow and reduced distensibility of the esophageal wall in patients with several primary esophageal motility disorders,” the investigators wrote. “We speculate that the dysphagia sensation experienced by many patients may result from a normal or supernormal contraction wave pushing the bolus against resistance.”
Yet routine esophageal function testing fails to capture these abnormalities, Dr. Mittal and Dr. Zifan noted.
“[C]urrent techniques used to measure esophageal distension during peristalsis are not adequate,” they wrote. “The high-resolution manometry and current scheme of classifying esophageal motor disorders in the current format emphasize only half of the story of peristalsis, probably the less important of the two halves, i.e., the contraction phase of peristalsis.”
More focus is needed on esophageal distension, they suggested, noting that relaxation is first needed to accommodate a bolus before contraction, no matter how powerful, can push it down the esophagus.
“A simple analogy is that of a car — it cannot get through a roadway that is smaller than its own width, irrespective of the horsepower of its engine,” they wrote.
The solution may lie in a more comprehensive approach to esophageal function testing.
“Integrating representations of distension and contraction, along with objective assessments of flow timing and distensibility, complements the current classification of esophageal motility disorders that are based on the contraction characteristics,” the investigators wrote, predicting that these efforts could improve diagnostic accuracy.
What to do about those diagnoses is another mystery.
“The question though remains regarding the optimal treatment for the impaired distension function of the esophagus, and whether improvement in the distension function will lead to improvement in dysphagia symptoms,” the investigators concluded.
The review was supported by the National Institutes of Health. The investigators reported copyright/patent protection for the computer software (Dplots) used to evaluate the distension contraction plots.
FROM GASTRO HEP ADVANCES
Modifiable Risk Factors for Young-Onset Dementia Flagged
TOPLINE:
In addition to better known risk factors such as diabetes, stroke, heart disease, and depression, findings of a large study suggested vitamin D deficiency, elevated C-reactive protein (CRP) levels, and social isolation increase the risk for young-onset dementia (YOD).
METHODOLOGY:
- The study included 356,052 participants younger than 65 years (mean baseline age, 54.6 years) without dementia from the UK Biobank, an ongoing prospective cohort study.
- Participants underwent a comprehensive baseline assessment, provided biological samples, completed touch screen questionnaires, and underwent a physical examination.
- Researchers identified incident all-cause YOD cases from hospital inpatient registers or death register linkage.
- The researchers detected 39 potential risk factors and grouped them into domains of sociodemographic, genetic, lifestyle, environmental, vitamin D and CRP levels, cardiometabolic, psychiatric, and other factors.
- Researchers analyzed incidence rates of YOD for 5-year age bands starting at age 40 years and separately for men and women.
TAKEAWAY:
- During a mean follow-up of 8.12 years, there were 485 incident YOD cases (incidence rate of 16.8 per 100,000 person-years; 95% CI 15.4-18.3).
- The final analysis identified 15 risk factors associated with significantly higher incidence of YOD, including traditional factors like stroke (hazard ratio [HR], 2.07), heart disease (HR, 1.61), diabetes (HR, 1.65), and depression (HR, 3.25) but also less-recognized risk factors like vitamin D deficiency (< 10 ng/mL; HR, 1.59), high CRP levels (> 1 mg/dL; HR, 1.54), and social isolation (infrequent visits to friends or family; HR, 1.53), with lower socioeconomic status (HR, 1.82), having two apolipoprotein E epsilon-4 alleles (HR, 1.87), orthostatic hypotension, which the authors said may be an early sign of Parkinson dementia or Lewy body dementia (HR, 4.20), and hearing impairment (HR, 1.56) also increasing risk.
- Interestingly, some alcohol use seemed to be protective (moderate or heavy alcohol use had a lower association with YOD than alcohol abstinence, possibly due to the “healthy drinker effect” where people who drink are healthier than abstainers who may have illnesses preventing them from drinking, said the authors), as was higher education level and higher than normative handgrip strength (less strength is a proxy for physical frailty).
- Men with diabetes had higher YOD risk than those without diabetes, while there was no association with diabetes in women; on the other hand, women with high CRP levels had greater YOD risk than those with low levels, while there was no association with CRP in men.
IN PRACTICE:
“While further exploration of these risk factors is necessary to identify potential underlying mechanisms, addressing these modifiable factors may prove effective in mitigating the risk of developing YOD and can be readily integrated in current dementia prevention initiatives,” the investigators wrote.
SOURCE:
The study was led by Stevie Hendriks, PhD, Department of Psychiatry and Neuropsychology, Maastricht University, Maastricht, the Netherlands. It was published online in JAMA Neurology.
LIMITATIONS:
The study was observational and so can’t infer causality. Several factors were based on self-reported data, which might be a source of response bias. Factors not considered in the study, for example, family history of dementia and drug (other than alcohol) use disorder, may have confounded associations. Some factors including orthostatic hypotension had few exposed cases, leading to decreased power to detect associations. Hospital and death records may not have captured all YOD cases. The UK Biobank is overrepresented by healthy and White participants, so results may not be generalizable to other racial and ethnic groups. The analyses only focused on all-cause dementia.
DISCLOSURES:
The study was supported by Alzheimer Netherlands. Hendriks has no relevant conflicts of interest; see paper for disclosures of other authors.
A version of this article appeared on Medscape.com.
TOPLINE:
In addition to better known risk factors such as diabetes, stroke, heart disease, and depression, findings of a large study suggested vitamin D deficiency, elevated C-reactive protein (CRP) levels, and social isolation increase the risk for young-onset dementia (YOD).
METHODOLOGY:
- The study included 356,052 participants younger than 65 years (mean baseline age, 54.6 years) without dementia from the UK Biobank, an ongoing prospective cohort study.
- Participants underwent a comprehensive baseline assessment, provided biological samples, completed touch screen questionnaires, and underwent a physical examination.
- Researchers identified incident all-cause YOD cases from hospital inpatient registers or death register linkage.
- The researchers detected 39 potential risk factors and grouped them into domains of sociodemographic, genetic, lifestyle, environmental, vitamin D and CRP levels, cardiometabolic, psychiatric, and other factors.
- Researchers analyzed incidence rates of YOD for 5-year age bands starting at age 40 years and separately for men and women.
TAKEAWAY:
- During a mean follow-up of 8.12 years, there were 485 incident YOD cases (incidence rate of 16.8 per 100,000 person-years; 95% CI 15.4-18.3).
- The final analysis identified 15 risk factors associated with significantly higher incidence of YOD, including traditional factors like stroke (hazard ratio [HR], 2.07), heart disease (HR, 1.61), diabetes (HR, 1.65), and depression (HR, 3.25) but also less-recognized risk factors like vitamin D deficiency (< 10 ng/mL; HR, 1.59), high CRP levels (> 1 mg/dL; HR, 1.54), and social isolation (infrequent visits to friends or family; HR, 1.53), with lower socioeconomic status (HR, 1.82), having two apolipoprotein E epsilon-4 alleles (HR, 1.87), orthostatic hypotension, which the authors said may be an early sign of Parkinson dementia or Lewy body dementia (HR, 4.20), and hearing impairment (HR, 1.56) also increasing risk.
- Interestingly, some alcohol use seemed to be protective (moderate or heavy alcohol use had a lower association with YOD than alcohol abstinence, possibly due to the “healthy drinker effect” where people who drink are healthier than abstainers who may have illnesses preventing them from drinking, said the authors), as was higher education level and higher than normative handgrip strength (less strength is a proxy for physical frailty).
- Men with diabetes had higher YOD risk than those without diabetes, while there was no association with diabetes in women; on the other hand, women with high CRP levels had greater YOD risk than those with low levels, while there was no association with CRP in men.
IN PRACTICE:
“While further exploration of these risk factors is necessary to identify potential underlying mechanisms, addressing these modifiable factors may prove effective in mitigating the risk of developing YOD and can be readily integrated in current dementia prevention initiatives,” the investigators wrote.
SOURCE:
The study was led by Stevie Hendriks, PhD, Department of Psychiatry and Neuropsychology, Maastricht University, Maastricht, the Netherlands. It was published online in JAMA Neurology.
LIMITATIONS:
The study was observational and so can’t infer causality. Several factors were based on self-reported data, which might be a source of response bias. Factors not considered in the study, for example, family history of dementia and drug (other than alcohol) use disorder, may have confounded associations. Some factors including orthostatic hypotension had few exposed cases, leading to decreased power to detect associations. Hospital and death records may not have captured all YOD cases. The UK Biobank is overrepresented by healthy and White participants, so results may not be generalizable to other racial and ethnic groups. The analyses only focused on all-cause dementia.
DISCLOSURES:
The study was supported by Alzheimer Netherlands. Hendriks has no relevant conflicts of interest; see paper for disclosures of other authors.
A version of this article appeared on Medscape.com.
TOPLINE:
In addition to better known risk factors such as diabetes, stroke, heart disease, and depression, findings of a large study suggested vitamin D deficiency, elevated C-reactive protein (CRP) levels, and social isolation increase the risk for young-onset dementia (YOD).
METHODOLOGY:
- The study included 356,052 participants younger than 65 years (mean baseline age, 54.6 years) without dementia from the UK Biobank, an ongoing prospective cohort study.
- Participants underwent a comprehensive baseline assessment, provided biological samples, completed touch screen questionnaires, and underwent a physical examination.
- Researchers identified incident all-cause YOD cases from hospital inpatient registers or death register linkage.
- The researchers detected 39 potential risk factors and grouped them into domains of sociodemographic, genetic, lifestyle, environmental, vitamin D and CRP levels, cardiometabolic, psychiatric, and other factors.
- Researchers analyzed incidence rates of YOD for 5-year age bands starting at age 40 years and separately for men and women.
TAKEAWAY:
- During a mean follow-up of 8.12 years, there were 485 incident YOD cases (incidence rate of 16.8 per 100,000 person-years; 95% CI 15.4-18.3).
- The final analysis identified 15 risk factors associated with significantly higher incidence of YOD, including traditional factors like stroke (hazard ratio [HR], 2.07), heart disease (HR, 1.61), diabetes (HR, 1.65), and depression (HR, 3.25) but also less-recognized risk factors like vitamin D deficiency (< 10 ng/mL; HR, 1.59), high CRP levels (> 1 mg/dL; HR, 1.54), and social isolation (infrequent visits to friends or family; HR, 1.53), with lower socioeconomic status (HR, 1.82), having two apolipoprotein E epsilon-4 alleles (HR, 1.87), orthostatic hypotension, which the authors said may be an early sign of Parkinson dementia or Lewy body dementia (HR, 4.20), and hearing impairment (HR, 1.56) also increasing risk.
- Interestingly, some alcohol use seemed to be protective (moderate or heavy alcohol use had a lower association with YOD than alcohol abstinence, possibly due to the “healthy drinker effect” where people who drink are healthier than abstainers who may have illnesses preventing them from drinking, said the authors), as was higher education level and higher than normative handgrip strength (less strength is a proxy for physical frailty).
- Men with diabetes had higher YOD risk than those without diabetes, while there was no association with diabetes in women; on the other hand, women with high CRP levels had greater YOD risk than those with low levels, while there was no association with CRP in men.
IN PRACTICE:
“While further exploration of these risk factors is necessary to identify potential underlying mechanisms, addressing these modifiable factors may prove effective in mitigating the risk of developing YOD and can be readily integrated in current dementia prevention initiatives,” the investigators wrote.
SOURCE:
The study was led by Stevie Hendriks, PhD, Department of Psychiatry and Neuropsychology, Maastricht University, Maastricht, the Netherlands. It was published online in JAMA Neurology.
LIMITATIONS:
The study was observational and so can’t infer causality. Several factors were based on self-reported data, which might be a source of response bias. Factors not considered in the study, for example, family history of dementia and drug (other than alcohol) use disorder, may have confounded associations. Some factors including orthostatic hypotension had few exposed cases, leading to decreased power to detect associations. Hospital and death records may not have captured all YOD cases. The UK Biobank is overrepresented by healthy and White participants, so results may not be generalizable to other racial and ethnic groups. The analyses only focused on all-cause dementia.
DISCLOSURES:
The study was supported by Alzheimer Netherlands. Hendriks has no relevant conflicts of interest; see paper for disclosures of other authors.
A version of this article appeared on Medscape.com.