User login
MDedge Daily News: Could bimekizumab reshape psoriasis treatment?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Could bimekizumab reshape psoriasis treatment? Next-day discharge after TAVR shows promise. Are fewer ultrasounds safe in high-risk pregnancies? And Medicare’s readmissions penalties are working – or not.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Could bimekizumab reshape psoriasis treatment? Next-day discharge after TAVR shows promise. Are fewer ultrasounds safe in high-risk pregnancies? And Medicare’s readmissions penalties are working – or not.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Could bimekizumab reshape psoriasis treatment? Next-day discharge after TAVR shows promise. Are fewer ultrasounds safe in high-risk pregnancies? And Medicare’s readmissions penalties are working – or not.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Treating Cancer Fatigue With Placebo
Cancer-related fatigue can linger long after treatments are ended, making daily activities harder and diminishing quality of life (QOL). But researchers from University of Alabama in Birmingham and Harvard Medical School in Boston suggest a nonpharmaceutical way to help patients feel better: placebo.
They compared an open-label placebo with treatment as usual in patients with cancer-related fatigue in a 21-day controlled trial. The patients had completed cancer treatment 6 months to 10 years prior to enrollment. Of 74 patients, 28 reported a moderate level of fatigue and 46 reported a severe level. The mean fatigue scores at baseline were similar for both groups.
The participants randomly assigned to placebo took 2 placebo pills twice a day. At 21 days, the average difference in scores was statistically significant. The placebo group reported a 29% improvement in fatigue severity and a 39% improvement in fatigue-disrupted QOL. Put another way, 76% of the placebo group had a change score above the mean change score of the usual-treatment group. The results were clinically meaningful, the researchers say. Moreover, there were no reported adverse events or adverse effects.
After that main study, the researchers also conducted a 21-day exploratory crossover extension, which began 1 week later. Their findings supported the main study results, with the same magnitude of improvement. The usual-treatment patients who chose to try the placebo also reported a similar magnitude of reductions in fatigue severity (23%) and fatigue-disrupted QOL (35%).
Interestingly, the effects seemed to be sustained, the researchers say. At day 48, there was no significant change in fatigue scores compared with day 21, an “exciting” preliminary finding they say that needs further exploration.
Source:
Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. Scientific Reports. 2018;8:2784.
doi:10.1038/s41598-018-20993-y.
Cancer-related fatigue can linger long after treatments are ended, making daily activities harder and diminishing quality of life (QOL). But researchers from University of Alabama in Birmingham and Harvard Medical School in Boston suggest a nonpharmaceutical way to help patients feel better: placebo.
They compared an open-label placebo with treatment as usual in patients with cancer-related fatigue in a 21-day controlled trial. The patients had completed cancer treatment 6 months to 10 years prior to enrollment. Of 74 patients, 28 reported a moderate level of fatigue and 46 reported a severe level. The mean fatigue scores at baseline were similar for both groups.
The participants randomly assigned to placebo took 2 placebo pills twice a day. At 21 days, the average difference in scores was statistically significant. The placebo group reported a 29% improvement in fatigue severity and a 39% improvement in fatigue-disrupted QOL. Put another way, 76% of the placebo group had a change score above the mean change score of the usual-treatment group. The results were clinically meaningful, the researchers say. Moreover, there were no reported adverse events or adverse effects.
After that main study, the researchers also conducted a 21-day exploratory crossover extension, which began 1 week later. Their findings supported the main study results, with the same magnitude of improvement. The usual-treatment patients who chose to try the placebo also reported a similar magnitude of reductions in fatigue severity (23%) and fatigue-disrupted QOL (35%).
Interestingly, the effects seemed to be sustained, the researchers say. At day 48, there was no significant change in fatigue scores compared with day 21, an “exciting” preliminary finding they say that needs further exploration.
Source:
Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. Scientific Reports. 2018;8:2784.
doi:10.1038/s41598-018-20993-y.
Cancer-related fatigue can linger long after treatments are ended, making daily activities harder and diminishing quality of life (QOL). But researchers from University of Alabama in Birmingham and Harvard Medical School in Boston suggest a nonpharmaceutical way to help patients feel better: placebo.
They compared an open-label placebo with treatment as usual in patients with cancer-related fatigue in a 21-day controlled trial. The patients had completed cancer treatment 6 months to 10 years prior to enrollment. Of 74 patients, 28 reported a moderate level of fatigue and 46 reported a severe level. The mean fatigue scores at baseline were similar for both groups.
The participants randomly assigned to placebo took 2 placebo pills twice a day. At 21 days, the average difference in scores was statistically significant. The placebo group reported a 29% improvement in fatigue severity and a 39% improvement in fatigue-disrupted QOL. Put another way, 76% of the placebo group had a change score above the mean change score of the usual-treatment group. The results were clinically meaningful, the researchers say. Moreover, there were no reported adverse events or adverse effects.
After that main study, the researchers also conducted a 21-day exploratory crossover extension, which began 1 week later. Their findings supported the main study results, with the same magnitude of improvement. The usual-treatment patients who chose to try the placebo also reported a similar magnitude of reductions in fatigue severity (23%) and fatigue-disrupted QOL (35%).
Interestingly, the effects seemed to be sustained, the researchers say. At day 48, there was no significant change in fatigue scores compared with day 21, an “exciting” preliminary finding they say that needs further exploration.
Source:
Hoenemeyer TW, Kaptchuk TJ, Mehta TS, Fontaine KR. Scientific Reports. 2018;8:2784.
doi:10.1038/s41598-018-20993-y.
New C. difficile guidelines recommend fecal microbiota transplants
.
The updated Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children, published in the Feb. 15 edition of Clinical Infectious Diseases (doi: 10.1093/cid/cix1085), address changes in management and diagnosis of the infection, and include recommendations for pediatric infection. The guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America were lasted published in 2010.
One of the strongest recommendations was on the use of FMTs to treat recurrent C. difficile infection after the failure of antibiotic therapy.
“Anecdotal treatment success rates of fecal microbiota transplantation for recurrent CDI [C. difficile infection] have been high regardless of route of instillation of feces, and have ranged between 77% and 94% with administration via the proximal small bowel; the highest success rates (80%-100%) have been associated with instillation of feces via the colon,” they wrote.
The guidelines also addressed what the authors described as the “evolving controversy” over the best methods for diagnosis, pointing out that there is little consensus about the best laboratory testing method.
“Given these various conundrums and the paucity of large prospective studies, the recommendations, while strong in some instances, are based upon a very low to low quality of evidence,” the authors said.
That aside, they advised that patients with unexplained and new-onset diarrhea (three or more unformed stools in 24 hours) were the preferred target population for testing for C. difficile infection. The most sensitive method of diagnosis in patients with clinical symptoms likely to be C. difficile infection was a nucleic acid amplification test, or a multistep algorithm, rather than a toxin test alone.
The guidelines committee also strongly advised against repeat testing within 7 days during the same episode of diarrhea, and against testing stool from asymptomatic patients, except for the purpose of epidemiologic study. They also noted there was insufficient evidence for the use of biologic markers such as fecal lactoferrin as an adjunct to testing.
The guidelines’ authors found there was not enough evidence to recommend discontinuing proton pump inhibitors to reduce the incidence of C. difficile infection, despite epidemiologic evidence of an association between proton pump inhibitor use and C. difficile infection. Similarly, there was a lack of evidence for the use of probiotics for primary prevention, but the authors noted that meta-analyses suggest probiotics may help prevent C. difficile infection in patients on antibiotics without a history of C. difficile infection.
With respect to antibiotic treatment, they recommended that patients diagnosed with C. difficile infection should first discontinue the inciting antibiotic treatment and then begin therapy with either vancomycin or fidaxomicin. For recurrent infection, they advised a tapered and pulsed regimen of oral vancomycin or a 10-day course of fidaxomicin. If patients had received metronidazole for the primary episode, they should be given a standard 10-day course of vancomycin for recurrent infection, the authors said.
In terms of diagnosis and management of pediatric C. difficile, the guidelines advised against routinely testing infants under 2 years of age with diarrhea, as the rate of C. difficile colonization even among asymptomatic infants can be higher than 40%. Even in children older than age 2, there was only a “weak” recommendation for C. difficile testing in patients with prolonged or worsening diarrhea and other risk factors such as inflammatory bowel disease or recent antibiotic exposure.
Children with a first episode or first recurrence of nonsevere C. difficile should be treated with either metronidazole or vancomycin, the authors wrote, but in the case of more severe illness or second recurrence, oral vancomycin was preferred over metronidazole.
The authors also suggested clinicians consider FMTs for children with recurrent infection that had failed to respond to antibiotics, but noted the quality of evidence for this was very low.
The guidelines were funded by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Six authors declared grants, consultancies, board positions, and other payments from the pharmaceutical industry outside the submitted work. One author also held patents relating to the treatment and prevention of C. difficile infection.
SOURCE: McDonald CL et al. Clin Infect Dis. 2018 Feb 15. doi: 10.1093/cid/cix1085.
.
The updated Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children, published in the Feb. 15 edition of Clinical Infectious Diseases (doi: 10.1093/cid/cix1085), address changes in management and diagnosis of the infection, and include recommendations for pediatric infection. The guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America were lasted published in 2010.
One of the strongest recommendations was on the use of FMTs to treat recurrent C. difficile infection after the failure of antibiotic therapy.
“Anecdotal treatment success rates of fecal microbiota transplantation for recurrent CDI [C. difficile infection] have been high regardless of route of instillation of feces, and have ranged between 77% and 94% with administration via the proximal small bowel; the highest success rates (80%-100%) have been associated with instillation of feces via the colon,” they wrote.
The guidelines also addressed what the authors described as the “evolving controversy” over the best methods for diagnosis, pointing out that there is little consensus about the best laboratory testing method.
“Given these various conundrums and the paucity of large prospective studies, the recommendations, while strong in some instances, are based upon a very low to low quality of evidence,” the authors said.
That aside, they advised that patients with unexplained and new-onset diarrhea (three or more unformed stools in 24 hours) were the preferred target population for testing for C. difficile infection. The most sensitive method of diagnosis in patients with clinical symptoms likely to be C. difficile infection was a nucleic acid amplification test, or a multistep algorithm, rather than a toxin test alone.
The guidelines committee also strongly advised against repeat testing within 7 days during the same episode of diarrhea, and against testing stool from asymptomatic patients, except for the purpose of epidemiologic study. They also noted there was insufficient evidence for the use of biologic markers such as fecal lactoferrin as an adjunct to testing.
The guidelines’ authors found there was not enough evidence to recommend discontinuing proton pump inhibitors to reduce the incidence of C. difficile infection, despite epidemiologic evidence of an association between proton pump inhibitor use and C. difficile infection. Similarly, there was a lack of evidence for the use of probiotics for primary prevention, but the authors noted that meta-analyses suggest probiotics may help prevent C. difficile infection in patients on antibiotics without a history of C. difficile infection.
With respect to antibiotic treatment, they recommended that patients diagnosed with C. difficile infection should first discontinue the inciting antibiotic treatment and then begin therapy with either vancomycin or fidaxomicin. For recurrent infection, they advised a tapered and pulsed regimen of oral vancomycin or a 10-day course of fidaxomicin. If patients had received metronidazole for the primary episode, they should be given a standard 10-day course of vancomycin for recurrent infection, the authors said.
In terms of diagnosis and management of pediatric C. difficile, the guidelines advised against routinely testing infants under 2 years of age with diarrhea, as the rate of C. difficile colonization even among asymptomatic infants can be higher than 40%. Even in children older than age 2, there was only a “weak” recommendation for C. difficile testing in patients with prolonged or worsening diarrhea and other risk factors such as inflammatory bowel disease or recent antibiotic exposure.
Children with a first episode or first recurrence of nonsevere C. difficile should be treated with either metronidazole or vancomycin, the authors wrote, but in the case of more severe illness or second recurrence, oral vancomycin was preferred over metronidazole.
The authors also suggested clinicians consider FMTs for children with recurrent infection that had failed to respond to antibiotics, but noted the quality of evidence for this was very low.
The guidelines were funded by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Six authors declared grants, consultancies, board positions, and other payments from the pharmaceutical industry outside the submitted work. One author also held patents relating to the treatment and prevention of C. difficile infection.
SOURCE: McDonald CL et al. Clin Infect Dis. 2018 Feb 15. doi: 10.1093/cid/cix1085.
.
The updated Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children, published in the Feb. 15 edition of Clinical Infectious Diseases (doi: 10.1093/cid/cix1085), address changes in management and diagnosis of the infection, and include recommendations for pediatric infection. The guidelines from the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America were lasted published in 2010.
One of the strongest recommendations was on the use of FMTs to treat recurrent C. difficile infection after the failure of antibiotic therapy.
“Anecdotal treatment success rates of fecal microbiota transplantation for recurrent CDI [C. difficile infection] have been high regardless of route of instillation of feces, and have ranged between 77% and 94% with administration via the proximal small bowel; the highest success rates (80%-100%) have been associated with instillation of feces via the colon,” they wrote.
The guidelines also addressed what the authors described as the “evolving controversy” over the best methods for diagnosis, pointing out that there is little consensus about the best laboratory testing method.
“Given these various conundrums and the paucity of large prospective studies, the recommendations, while strong in some instances, are based upon a very low to low quality of evidence,” the authors said.
That aside, they advised that patients with unexplained and new-onset diarrhea (three or more unformed stools in 24 hours) were the preferred target population for testing for C. difficile infection. The most sensitive method of diagnosis in patients with clinical symptoms likely to be C. difficile infection was a nucleic acid amplification test, or a multistep algorithm, rather than a toxin test alone.
The guidelines committee also strongly advised against repeat testing within 7 days during the same episode of diarrhea, and against testing stool from asymptomatic patients, except for the purpose of epidemiologic study. They also noted there was insufficient evidence for the use of biologic markers such as fecal lactoferrin as an adjunct to testing.
The guidelines’ authors found there was not enough evidence to recommend discontinuing proton pump inhibitors to reduce the incidence of C. difficile infection, despite epidemiologic evidence of an association between proton pump inhibitor use and C. difficile infection. Similarly, there was a lack of evidence for the use of probiotics for primary prevention, but the authors noted that meta-analyses suggest probiotics may help prevent C. difficile infection in patients on antibiotics without a history of C. difficile infection.
With respect to antibiotic treatment, they recommended that patients diagnosed with C. difficile infection should first discontinue the inciting antibiotic treatment and then begin therapy with either vancomycin or fidaxomicin. For recurrent infection, they advised a tapered and pulsed regimen of oral vancomycin or a 10-day course of fidaxomicin. If patients had received metronidazole for the primary episode, they should be given a standard 10-day course of vancomycin for recurrent infection, the authors said.
In terms of diagnosis and management of pediatric C. difficile, the guidelines advised against routinely testing infants under 2 years of age with diarrhea, as the rate of C. difficile colonization even among asymptomatic infants can be higher than 40%. Even in children older than age 2, there was only a “weak” recommendation for C. difficile testing in patients with prolonged or worsening diarrhea and other risk factors such as inflammatory bowel disease or recent antibiotic exposure.
Children with a first episode or first recurrence of nonsevere C. difficile should be treated with either metronidazole or vancomycin, the authors wrote, but in the case of more severe illness or second recurrence, oral vancomycin was preferred over metronidazole.
The authors also suggested clinicians consider FMTs for children with recurrent infection that had failed to respond to antibiotics, but noted the quality of evidence for this was very low.
The guidelines were funded by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Six authors declared grants, consultancies, board positions, and other payments from the pharmaceutical industry outside the submitted work. One author also held patents relating to the treatment and prevention of C. difficile infection.
SOURCE: McDonald CL et al. Clin Infect Dis. 2018 Feb 15. doi: 10.1093/cid/cix1085.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point: Fecal microbiota transplants should be considered for use in patients with recurrent Clostridium difficile infection that has not responded to antibiotic therapy.
Major finding: One of the strongest recommendations in the new guidelines on C. difficile infection is to consider use of fecal microbiota transplants in patients with recurrent infection.
Data source: Clinical practice guidelines.
Disclosures: The guidelines were funded by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Six authors declared grants, consultancies, board positions, and other payments from the pharmaceutical industry outside the submitted work. One author also held patents relating to the treatment and prevention of C. difficile infection.
Source: McDonald CL et al. Clin Infect Dis. 2018 Feb 15. doi: 10.1093/cid/cix1085.
Antibody has ‘very promising activity’ in rel/ref CTCL
LA JOLLA, CA—An antibody targeting KIR3DL2 has “very promising activity” in relapsed/refractory cutaneous T-cell lymphoma (CTCL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
The antibody, IPH4102, produced an overall response rate (ORR) of 44% in a phase 1 trial of CTCL patients, with an ORR of 50% in patients with Sézary syndrome (SS).
There were 2 serious adverse events (AEs) that were considered possibly related to IPH4102, and 1 of these was fatal.
Youn H. Kim, MD, of Stanford Cancer Institute in Palo Alto, California, presented these results at this year’s T-cell Lymphoma Forum. The research was sponsored by Innate Pharma, the company developing IPH4102.
“KIR3DL2 is a target that’s a member of the killer immunoglobulin-like receptor [KIR] family, and it’s also very specifically expressed in CTCL,” Dr Kim explained. “The antibody [IPH4102] is Fc-modified to enhance the antibody-dependent cell cytotoxicity and also works by antibody-dependent cell phagocytosis. It binds and, with the immunologic effect, kills the cancer cells.”
“[IPH4102] is specific for cancer cells because [KIR3DL2] is only minimally expressed in normal T cells as well as natural killer [NK] cells. About a third of NK cells that do express [KIR3DL2] don’t get affected [by IPH4102]. So it looks like the killer cells don’t kill each other, and they specifically target the neoplastic cells.”
Patients
Dr Kim and her colleagues have tested IPH4102 in 25 patients with relapsed/refractory CTCL—20 with SS, 4 with mycosis fungoides (MF), and 1 with CD4+ CTCL not otherwise specified. At baseline, patients had a median age of 71 (range, 42-90).
The patients had received a median of 4 prior systemic regimens (range, 2-10). Among the MF and SS patients, 1 patient had stage IB disease, 3 had stage IIB, and 20 had stage IVA disease.
Treatment
This study has a dose-escalation portion (accelerated 3+3 design) and an expansion portion. Dr Kim presented results for the 25 patients in the dose-escalation portion, which was completed in May 2017.
Patients received IPH4102 at 10 dose levels, ranging from 0.0001 mg/kg to 10 mg/kg. They received the drug once weekly for 4 doses, every 2 weeks for 10 doses, and every 4 weeks thereafter. They were treated until progression or unacceptable toxicity.
The expansion cohorts (which include SS and transformed MF patients) started enrolling in July 2017, with patients receiving IPH4102 at the recommended phase 2 dose—750 mg.
Safety
There were no dose-limiting toxicities with IPH4102. So the equivalent of 10 mg/kg—a 750 mg flat dose—was deemed the recommended phase 2 (and expansion) dose.
The incidence of AEs was 92% (n=23), and the incidence of treatment-related AEs was 52% (n=13).
Treatment-related AEs included lymphopenia (16%, n=4), asthenia (12%, n=3), nausea (8%, n=2), chills (8%, n=2), pyrexia (8%, n=2), arthralgia (8%, n=2), and muscle spasms (8%, n=2).
Eight patients had serious AEs, and 2 had serious AEs that were considered possibly related to treatment.
One patient had grade 2 atrial flutter diagnosed 1 hour after the first dose of IPH4102. The patient had a history of cardiac arrhythmia. She was hospitalized after the atrial flutter, received amiodarone, and the arrhythmia resolved. She went on to receive 15 more doses of IPH4102 without recurrence.
The other patient with a serious AE considered possibly related to treatment had hepatitis that occurred 6 weeks after the last dose of IPH4102. The patient had discontinued treatment due to progression after receiving IPH4102 for a year and achieving a partial response (PR).
This patient ultimately died of the hepatitis. Another fatal AE, considered unrelated to treatment, was Staphylococcus aureus sepsis.
“The safety looks very solid,” Dr Kim said, “[with] very low numbers of any significant severe adverse effects.”
Efficacy
The best global ORR was 44%, with 10 PRs and 1 complete response (CR). Twelve patients had stable disease, and 2 progressed.
In patients with SS, the best global ORR was 50%. One patient had a CR, 9 had PRs, and 8 had stable disease. The ORR was 60% in the skin and a 65% in the blood compartment for patients with SS.
Four responses were ongoing at last follow-up. The median follow-up was 15 months.
The median duration of response for the entire cohort was 8.2 months (range, 64 days to 519+ days). For SS patients, the median duration of response was 9.9 months (range, 64 days to 519+ days).
The median progression-free survival was 9.8 months overall (range, 28 days to 610+ days). For SS patients, the median progression-free survival was 10.8 months (range, 28 days to 610+ days).
“So with the efficacy and the safety profile, we are really hoping to get this drug to the phase 2 level and are excited to move forward,” Dr Kim concluded.
LA JOLLA, CA—An antibody targeting KIR3DL2 has “very promising activity” in relapsed/refractory cutaneous T-cell lymphoma (CTCL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
The antibody, IPH4102, produced an overall response rate (ORR) of 44% in a phase 1 trial of CTCL patients, with an ORR of 50% in patients with Sézary syndrome (SS).
There were 2 serious adverse events (AEs) that were considered possibly related to IPH4102, and 1 of these was fatal.
Youn H. Kim, MD, of Stanford Cancer Institute in Palo Alto, California, presented these results at this year’s T-cell Lymphoma Forum. The research was sponsored by Innate Pharma, the company developing IPH4102.
“KIR3DL2 is a target that’s a member of the killer immunoglobulin-like receptor [KIR] family, and it’s also very specifically expressed in CTCL,” Dr Kim explained. “The antibody [IPH4102] is Fc-modified to enhance the antibody-dependent cell cytotoxicity and also works by antibody-dependent cell phagocytosis. It binds and, with the immunologic effect, kills the cancer cells.”
“[IPH4102] is specific for cancer cells because [KIR3DL2] is only minimally expressed in normal T cells as well as natural killer [NK] cells. About a third of NK cells that do express [KIR3DL2] don’t get affected [by IPH4102]. So it looks like the killer cells don’t kill each other, and they specifically target the neoplastic cells.”
Patients
Dr Kim and her colleagues have tested IPH4102 in 25 patients with relapsed/refractory CTCL—20 with SS, 4 with mycosis fungoides (MF), and 1 with CD4+ CTCL not otherwise specified. At baseline, patients had a median age of 71 (range, 42-90).
The patients had received a median of 4 prior systemic regimens (range, 2-10). Among the MF and SS patients, 1 patient had stage IB disease, 3 had stage IIB, and 20 had stage IVA disease.
Treatment
This study has a dose-escalation portion (accelerated 3+3 design) and an expansion portion. Dr Kim presented results for the 25 patients in the dose-escalation portion, which was completed in May 2017.
Patients received IPH4102 at 10 dose levels, ranging from 0.0001 mg/kg to 10 mg/kg. They received the drug once weekly for 4 doses, every 2 weeks for 10 doses, and every 4 weeks thereafter. They were treated until progression or unacceptable toxicity.
The expansion cohorts (which include SS and transformed MF patients) started enrolling in July 2017, with patients receiving IPH4102 at the recommended phase 2 dose—750 mg.
Safety
There were no dose-limiting toxicities with IPH4102. So the equivalent of 10 mg/kg—a 750 mg flat dose—was deemed the recommended phase 2 (and expansion) dose.
The incidence of AEs was 92% (n=23), and the incidence of treatment-related AEs was 52% (n=13).
Treatment-related AEs included lymphopenia (16%, n=4), asthenia (12%, n=3), nausea (8%, n=2), chills (8%, n=2), pyrexia (8%, n=2), arthralgia (8%, n=2), and muscle spasms (8%, n=2).
Eight patients had serious AEs, and 2 had serious AEs that were considered possibly related to treatment.
One patient had grade 2 atrial flutter diagnosed 1 hour after the first dose of IPH4102. The patient had a history of cardiac arrhythmia. She was hospitalized after the atrial flutter, received amiodarone, and the arrhythmia resolved. She went on to receive 15 more doses of IPH4102 without recurrence.
The other patient with a serious AE considered possibly related to treatment had hepatitis that occurred 6 weeks after the last dose of IPH4102. The patient had discontinued treatment due to progression after receiving IPH4102 for a year and achieving a partial response (PR).
This patient ultimately died of the hepatitis. Another fatal AE, considered unrelated to treatment, was Staphylococcus aureus sepsis.
“The safety looks very solid,” Dr Kim said, “[with] very low numbers of any significant severe adverse effects.”
Efficacy
The best global ORR was 44%, with 10 PRs and 1 complete response (CR). Twelve patients had stable disease, and 2 progressed.
In patients with SS, the best global ORR was 50%. One patient had a CR, 9 had PRs, and 8 had stable disease. The ORR was 60% in the skin and a 65% in the blood compartment for patients with SS.
Four responses were ongoing at last follow-up. The median follow-up was 15 months.
The median duration of response for the entire cohort was 8.2 months (range, 64 days to 519+ days). For SS patients, the median duration of response was 9.9 months (range, 64 days to 519+ days).
The median progression-free survival was 9.8 months overall (range, 28 days to 610+ days). For SS patients, the median progression-free survival was 10.8 months (range, 28 days to 610+ days).
“So with the efficacy and the safety profile, we are really hoping to get this drug to the phase 2 level and are excited to move forward,” Dr Kim concluded.
LA JOLLA, CA—An antibody targeting KIR3DL2 has “very promising activity” in relapsed/refractory cutaneous T-cell lymphoma (CTCL), according to a speaker at the 10th Annual T-cell Lymphoma Forum.
The antibody, IPH4102, produced an overall response rate (ORR) of 44% in a phase 1 trial of CTCL patients, with an ORR of 50% in patients with Sézary syndrome (SS).
There were 2 serious adverse events (AEs) that were considered possibly related to IPH4102, and 1 of these was fatal.
Youn H. Kim, MD, of Stanford Cancer Institute in Palo Alto, California, presented these results at this year’s T-cell Lymphoma Forum. The research was sponsored by Innate Pharma, the company developing IPH4102.
“KIR3DL2 is a target that’s a member of the killer immunoglobulin-like receptor [KIR] family, and it’s also very specifically expressed in CTCL,” Dr Kim explained. “The antibody [IPH4102] is Fc-modified to enhance the antibody-dependent cell cytotoxicity and also works by antibody-dependent cell phagocytosis. It binds and, with the immunologic effect, kills the cancer cells.”
“[IPH4102] is specific for cancer cells because [KIR3DL2] is only minimally expressed in normal T cells as well as natural killer [NK] cells. About a third of NK cells that do express [KIR3DL2] don’t get affected [by IPH4102]. So it looks like the killer cells don’t kill each other, and they specifically target the neoplastic cells.”
Patients
Dr Kim and her colleagues have tested IPH4102 in 25 patients with relapsed/refractory CTCL—20 with SS, 4 with mycosis fungoides (MF), and 1 with CD4+ CTCL not otherwise specified. At baseline, patients had a median age of 71 (range, 42-90).
The patients had received a median of 4 prior systemic regimens (range, 2-10). Among the MF and SS patients, 1 patient had stage IB disease, 3 had stage IIB, and 20 had stage IVA disease.
Treatment
This study has a dose-escalation portion (accelerated 3+3 design) and an expansion portion. Dr Kim presented results for the 25 patients in the dose-escalation portion, which was completed in May 2017.
Patients received IPH4102 at 10 dose levels, ranging from 0.0001 mg/kg to 10 mg/kg. They received the drug once weekly for 4 doses, every 2 weeks for 10 doses, and every 4 weeks thereafter. They were treated until progression or unacceptable toxicity.
The expansion cohorts (which include SS and transformed MF patients) started enrolling in July 2017, with patients receiving IPH4102 at the recommended phase 2 dose—750 mg.
Safety
There were no dose-limiting toxicities with IPH4102. So the equivalent of 10 mg/kg—a 750 mg flat dose—was deemed the recommended phase 2 (and expansion) dose.
The incidence of AEs was 92% (n=23), and the incidence of treatment-related AEs was 52% (n=13).
Treatment-related AEs included lymphopenia (16%, n=4), asthenia (12%, n=3), nausea (8%, n=2), chills (8%, n=2), pyrexia (8%, n=2), arthralgia (8%, n=2), and muscle spasms (8%, n=2).
Eight patients had serious AEs, and 2 had serious AEs that were considered possibly related to treatment.
One patient had grade 2 atrial flutter diagnosed 1 hour after the first dose of IPH4102. The patient had a history of cardiac arrhythmia. She was hospitalized after the atrial flutter, received amiodarone, and the arrhythmia resolved. She went on to receive 15 more doses of IPH4102 without recurrence.
The other patient with a serious AE considered possibly related to treatment had hepatitis that occurred 6 weeks after the last dose of IPH4102. The patient had discontinued treatment due to progression after receiving IPH4102 for a year and achieving a partial response (PR).
This patient ultimately died of the hepatitis. Another fatal AE, considered unrelated to treatment, was Staphylococcus aureus sepsis.
“The safety looks very solid,” Dr Kim said, “[with] very low numbers of any significant severe adverse effects.”
Efficacy
The best global ORR was 44%, with 10 PRs and 1 complete response (CR). Twelve patients had stable disease, and 2 progressed.
In patients with SS, the best global ORR was 50%. One patient had a CR, 9 had PRs, and 8 had stable disease. The ORR was 60% in the skin and a 65% in the blood compartment for patients with SS.
Four responses were ongoing at last follow-up. The median follow-up was 15 months.
The median duration of response for the entire cohort was 8.2 months (range, 64 days to 519+ days). For SS patients, the median duration of response was 9.9 months (range, 64 days to 519+ days).
The median progression-free survival was 9.8 months overall (range, 28 days to 610+ days). For SS patients, the median progression-free survival was 10.8 months (range, 28 days to 610+ days).
“So with the efficacy and the safety profile, we are really hoping to get this drug to the phase 2 level and are excited to move forward,” Dr Kim concluded.
ASCO, NCCN release guidelines on checkpoint inhibitors
Two cancer organizations have released guidelines for managing the side effects of immune checkpoint inhibitors.
The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network® (NCCN) developed these guidelines because patients who receive immune checkpoint inhibitors experience unique side effects that can be severe, irreversible, and life-threatening.
Given that checkpoint inhibitors have entered the clinic fairly recently, clinicians may need guidance in recognizing and treating these side effects.
“With rapidly increasing use of immune checkpoint inhibitors, it is imperative that clinicians are knowledgeable about their unique toxicity profiles,” said ASCO Chief Executive Officer Clifford A. Hudis, MD.
“These new guidelines from ASCO and NCCN will help our community continue to provide the highest quality of care to all patients as they incorporate these agents into routine care.”
To the develop their guidelines, ASCO and NCCN convened multidisciplinary panels with representation from hematology, oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, emergency medicine, and nursing, as well as patient advocacy experts.
The clinical recommendations are based on a systematic review of the literature and an informal consensus process. The recommendations pertain only to checkpoint inhibitors currently approved in the US—pembrolizumab, nivolumab, atezolizumab, avelumab, ipilimumab, and durvalumab.
Key recommendations from the guidelines include:
- In general, checkpoint inhibitors can be continued with close monitoring if patients experience grade 1 toxicities, with the exception of some neurologic, cardiac, and hematologic toxicities.
- For grade 2 toxicities, checkpoint inhibitors should be held until symptoms and/or lab values revert to grade 1 levels or lower. Corticosteroids may be offered.
- For grade 3 toxicities, patients should receive high-dose corticosteroids for at least 6 weeks. Extreme caution is recommended when restarting immunotherapy after grade 3 toxicity, if it is restarted at all.
- In general, grade 4 toxicities necessitate stopping checkpoint inhibitor therapy permanently.
Consult the guidelines for specific recommendations.
ASCO’s guidelines, “Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline,” have been published in the Journal of Clinical Oncology.
NCCN’s guidelines, “Management of Immunotherapy-Related Toxicities (Immune Checkpoint Inhibitor-Related Toxicities),” are available on the NCCN website.
Two cancer organizations have released guidelines for managing the side effects of immune checkpoint inhibitors.
The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network® (NCCN) developed these guidelines because patients who receive immune checkpoint inhibitors experience unique side effects that can be severe, irreversible, and life-threatening.
Given that checkpoint inhibitors have entered the clinic fairly recently, clinicians may need guidance in recognizing and treating these side effects.
“With rapidly increasing use of immune checkpoint inhibitors, it is imperative that clinicians are knowledgeable about their unique toxicity profiles,” said ASCO Chief Executive Officer Clifford A. Hudis, MD.
“These new guidelines from ASCO and NCCN will help our community continue to provide the highest quality of care to all patients as they incorporate these agents into routine care.”
To the develop their guidelines, ASCO and NCCN convened multidisciplinary panels with representation from hematology, oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, emergency medicine, and nursing, as well as patient advocacy experts.
The clinical recommendations are based on a systematic review of the literature and an informal consensus process. The recommendations pertain only to checkpoint inhibitors currently approved in the US—pembrolizumab, nivolumab, atezolizumab, avelumab, ipilimumab, and durvalumab.
Key recommendations from the guidelines include:
- In general, checkpoint inhibitors can be continued with close monitoring if patients experience grade 1 toxicities, with the exception of some neurologic, cardiac, and hematologic toxicities.
- For grade 2 toxicities, checkpoint inhibitors should be held until symptoms and/or lab values revert to grade 1 levels or lower. Corticosteroids may be offered.
- For grade 3 toxicities, patients should receive high-dose corticosteroids for at least 6 weeks. Extreme caution is recommended when restarting immunotherapy after grade 3 toxicity, if it is restarted at all.
- In general, grade 4 toxicities necessitate stopping checkpoint inhibitor therapy permanently.
Consult the guidelines for specific recommendations.
ASCO’s guidelines, “Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline,” have been published in the Journal of Clinical Oncology.
NCCN’s guidelines, “Management of Immunotherapy-Related Toxicities (Immune Checkpoint Inhibitor-Related Toxicities),” are available on the NCCN website.
Two cancer organizations have released guidelines for managing the side effects of immune checkpoint inhibitors.
The American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network® (NCCN) developed these guidelines because patients who receive immune checkpoint inhibitors experience unique side effects that can be severe, irreversible, and life-threatening.
Given that checkpoint inhibitors have entered the clinic fairly recently, clinicians may need guidance in recognizing and treating these side effects.
“With rapidly increasing use of immune checkpoint inhibitors, it is imperative that clinicians are knowledgeable about their unique toxicity profiles,” said ASCO Chief Executive Officer Clifford A. Hudis, MD.
“These new guidelines from ASCO and NCCN will help our community continue to provide the highest quality of care to all patients as they incorporate these agents into routine care.”
To the develop their guidelines, ASCO and NCCN convened multidisciplinary panels with representation from hematology, oncology, dermatology, gastroenterology, rheumatology, pulmonology, endocrinology, urology, neurology, emergency medicine, and nursing, as well as patient advocacy experts.
The clinical recommendations are based on a systematic review of the literature and an informal consensus process. The recommendations pertain only to checkpoint inhibitors currently approved in the US—pembrolizumab, nivolumab, atezolizumab, avelumab, ipilimumab, and durvalumab.
Key recommendations from the guidelines include:
- In general, checkpoint inhibitors can be continued with close monitoring if patients experience grade 1 toxicities, with the exception of some neurologic, cardiac, and hematologic toxicities.
- For grade 2 toxicities, checkpoint inhibitors should be held until symptoms and/or lab values revert to grade 1 levels or lower. Corticosteroids may be offered.
- For grade 3 toxicities, patients should receive high-dose corticosteroids for at least 6 weeks. Extreme caution is recommended when restarting immunotherapy after grade 3 toxicity, if it is restarted at all.
- In general, grade 4 toxicities necessitate stopping checkpoint inhibitor therapy permanently.
Consult the guidelines for specific recommendations.
ASCO’s guidelines, “Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline,” have been published in the Journal of Clinical Oncology.
NCCN’s guidelines, “Management of Immunotherapy-Related Toxicities (Immune Checkpoint Inhibitor-Related Toxicities),” are available on the NCCN website.
Product seems effective for ITI in severe hemophilia A
Results of a retrospective study suggest a recombinant factor VIII Fc fusion protein (rFVIIIFc) can be effective for immune tolerance induction (ITI) in patients with severe hemophilia A and inhibitors.
Four of 7 first-time ITI patients achieved tolerization with rFVIIIFc at a median of 7.8 months.
Of 12 patients who had previously received treatment for ITI, 7 achieved a negative inhibitor level with rFVIIIFc. However, only 3 of these patients were still negative at the time of analysis.
In all, 16 of the 19 patients studied remained on rFVIIIFc for prophylaxis or ITI, and researchers said longer follow-up was needed.
No adverse events were reported in this study.
Manuel Carcao, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada, and his colleagues conducted this study and published the results in Haemophilia.
The research was sponsored by Bioverativ Therapeutics, Inc. Bioverativ markets rFVIIIFc as Eloctate in some countries. Sobi markets rFVIIIFc (or efmoroctocog alfa) as Elocta in other countries.
The study included 19 patients—7 first-time ITI patients and 12 rescue patients—with severe hemophilia A and FVIII inhibitors.
First-timer results
Four of the 7 first-timers were tolerized and had transitioned to rFVIIIFc prophylaxis at the time of analysis.
Three patients met the definition of tolerization at 5 months, 7 months, and 9 months, respectively. All were all on a daily rFVIIIFc (85-200 IU/kg) regimen.
The fourth patient, who had been on a 3-times-per-week (50 IU/kg) ITI regimen, was considered tolerized at 14.8 months.
Of the 3 remaining patients, 2 had a decrease in Bethesda titer. The first patient had a decrease from 32 Bethesda units (BU) to 18 BU after 18 weeks of ITI. The second patient had a decrease from 378 BU to 23 BU after 58 weeks of ITI.
The third patient had an initial increase in titer from 3 BU to 16 BU after 15 weeks of rFVIIIFc. He went on to receive ITI with a different factor but did not respond. He resumed rFVIIIFc ITI after 27 weeks and had been receiving it for 7 weeks at the time of analysis. His most recent Bethesda titer had fallen. The researchers said this patient had been poorly compliant with ITI.
All 7 first-time ITI patients were still receiving rFVIIIFc—4 as prophylaxis and 3 for ITI—at the time of analysis.
Rescue results
Twelve patients had previously failed ITI. Seven of them achieved negative inhibitor levels with rFVIIIFc ITI. The median time to negativity was 14.1 weeks (range, 3 weeks to 67.6 weeks).
Three of the patients were still negative at the time of analysis. Two of them were still on rFVIIIFc ITI, and 1 was on rFVIIIFc prophylaxis.
Four patients who initially achieved negativity later developed a titer greater than 0.6 BU. Two of these patients were still on rFVIIIFc ITI at analysis, and 2 switched to other factors.
There were 5 patients who did not achieve negative inhibitor levels. One of these patients had a decrease in titer from 36 BU to 22 BU after 10 weeks. For the other 4 patients, titer was either unchanged or increased while on ITI.
Four of the 5 patients who did not achieve negative inhibitor levels remained on rFVIIIFc ITI at analysis, and 1 was placed on bypass therapy.
In total, 9 of the 12 rescue patients were still on rFVIIIFc at analysis—9 for ITI and 1 as prophylaxis.
“The development of inhibitors is a tremendous challenge and significant burden for people with severe hemophilia A, and the goal of treatment should be eradication of inhibitors,” said Maha Radhakrishnan, MD, of Bioverativ.
“The results of this analysis are encouraging and support the need for additional and ongoing scientific research on [rFVIIIFc ] in ITI to determine whether an Fc-based recombinant factor VIII therapy can rapidly tolerize patients with inhibitors.”
Bioverativ and Sobi have initiated 2 prospective studies designed to further evaluate the use of rFVIIIFc for ITI in patients with severe hemophilia A and inhibitors (NCT03093480 and NCT03103542).
Results of a retrospective study suggest a recombinant factor VIII Fc fusion protein (rFVIIIFc) can be effective for immune tolerance induction (ITI) in patients with severe hemophilia A and inhibitors.
Four of 7 first-time ITI patients achieved tolerization with rFVIIIFc at a median of 7.8 months.
Of 12 patients who had previously received treatment for ITI, 7 achieved a negative inhibitor level with rFVIIIFc. However, only 3 of these patients were still negative at the time of analysis.
In all, 16 of the 19 patients studied remained on rFVIIIFc for prophylaxis or ITI, and researchers said longer follow-up was needed.
No adverse events were reported in this study.
Manuel Carcao, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada, and his colleagues conducted this study and published the results in Haemophilia.
The research was sponsored by Bioverativ Therapeutics, Inc. Bioverativ markets rFVIIIFc as Eloctate in some countries. Sobi markets rFVIIIFc (or efmoroctocog alfa) as Elocta in other countries.
The study included 19 patients—7 first-time ITI patients and 12 rescue patients—with severe hemophilia A and FVIII inhibitors.
First-timer results
Four of the 7 first-timers were tolerized and had transitioned to rFVIIIFc prophylaxis at the time of analysis.
Three patients met the definition of tolerization at 5 months, 7 months, and 9 months, respectively. All were all on a daily rFVIIIFc (85-200 IU/kg) regimen.
The fourth patient, who had been on a 3-times-per-week (50 IU/kg) ITI regimen, was considered tolerized at 14.8 months.
Of the 3 remaining patients, 2 had a decrease in Bethesda titer. The first patient had a decrease from 32 Bethesda units (BU) to 18 BU after 18 weeks of ITI. The second patient had a decrease from 378 BU to 23 BU after 58 weeks of ITI.
The third patient had an initial increase in titer from 3 BU to 16 BU after 15 weeks of rFVIIIFc. He went on to receive ITI with a different factor but did not respond. He resumed rFVIIIFc ITI after 27 weeks and had been receiving it for 7 weeks at the time of analysis. His most recent Bethesda titer had fallen. The researchers said this patient had been poorly compliant with ITI.
All 7 first-time ITI patients were still receiving rFVIIIFc—4 as prophylaxis and 3 for ITI—at the time of analysis.
Rescue results
Twelve patients had previously failed ITI. Seven of them achieved negative inhibitor levels with rFVIIIFc ITI. The median time to negativity was 14.1 weeks (range, 3 weeks to 67.6 weeks).
Three of the patients were still negative at the time of analysis. Two of them were still on rFVIIIFc ITI, and 1 was on rFVIIIFc prophylaxis.
Four patients who initially achieved negativity later developed a titer greater than 0.6 BU. Two of these patients were still on rFVIIIFc ITI at analysis, and 2 switched to other factors.
There were 5 patients who did not achieve negative inhibitor levels. One of these patients had a decrease in titer from 36 BU to 22 BU after 10 weeks. For the other 4 patients, titer was either unchanged or increased while on ITI.
Four of the 5 patients who did not achieve negative inhibitor levels remained on rFVIIIFc ITI at analysis, and 1 was placed on bypass therapy.
In total, 9 of the 12 rescue patients were still on rFVIIIFc at analysis—9 for ITI and 1 as prophylaxis.
“The development of inhibitors is a tremendous challenge and significant burden for people with severe hemophilia A, and the goal of treatment should be eradication of inhibitors,” said Maha Radhakrishnan, MD, of Bioverativ.
“The results of this analysis are encouraging and support the need for additional and ongoing scientific research on [rFVIIIFc ] in ITI to determine whether an Fc-based recombinant factor VIII therapy can rapidly tolerize patients with inhibitors.”
Bioverativ and Sobi have initiated 2 prospective studies designed to further evaluate the use of rFVIIIFc for ITI in patients with severe hemophilia A and inhibitors (NCT03093480 and NCT03103542).
Results of a retrospective study suggest a recombinant factor VIII Fc fusion protein (rFVIIIFc) can be effective for immune tolerance induction (ITI) in patients with severe hemophilia A and inhibitors.
Four of 7 first-time ITI patients achieved tolerization with rFVIIIFc at a median of 7.8 months.
Of 12 patients who had previously received treatment for ITI, 7 achieved a negative inhibitor level with rFVIIIFc. However, only 3 of these patients were still negative at the time of analysis.
In all, 16 of the 19 patients studied remained on rFVIIIFc for prophylaxis or ITI, and researchers said longer follow-up was needed.
No adverse events were reported in this study.
Manuel Carcao, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada, and his colleagues conducted this study and published the results in Haemophilia.
The research was sponsored by Bioverativ Therapeutics, Inc. Bioverativ markets rFVIIIFc as Eloctate in some countries. Sobi markets rFVIIIFc (or efmoroctocog alfa) as Elocta in other countries.
The study included 19 patients—7 first-time ITI patients and 12 rescue patients—with severe hemophilia A and FVIII inhibitors.
First-timer results
Four of the 7 first-timers were tolerized and had transitioned to rFVIIIFc prophylaxis at the time of analysis.
Three patients met the definition of tolerization at 5 months, 7 months, and 9 months, respectively. All were all on a daily rFVIIIFc (85-200 IU/kg) regimen.
The fourth patient, who had been on a 3-times-per-week (50 IU/kg) ITI regimen, was considered tolerized at 14.8 months.
Of the 3 remaining patients, 2 had a decrease in Bethesda titer. The first patient had a decrease from 32 Bethesda units (BU) to 18 BU after 18 weeks of ITI. The second patient had a decrease from 378 BU to 23 BU after 58 weeks of ITI.
The third patient had an initial increase in titer from 3 BU to 16 BU after 15 weeks of rFVIIIFc. He went on to receive ITI with a different factor but did not respond. He resumed rFVIIIFc ITI after 27 weeks and had been receiving it for 7 weeks at the time of analysis. His most recent Bethesda titer had fallen. The researchers said this patient had been poorly compliant with ITI.
All 7 first-time ITI patients were still receiving rFVIIIFc—4 as prophylaxis and 3 for ITI—at the time of analysis.
Rescue results
Twelve patients had previously failed ITI. Seven of them achieved negative inhibitor levels with rFVIIIFc ITI. The median time to negativity was 14.1 weeks (range, 3 weeks to 67.6 weeks).
Three of the patients were still negative at the time of analysis. Two of them were still on rFVIIIFc ITI, and 1 was on rFVIIIFc prophylaxis.
Four patients who initially achieved negativity later developed a titer greater than 0.6 BU. Two of these patients were still on rFVIIIFc ITI at analysis, and 2 switched to other factors.
There were 5 patients who did not achieve negative inhibitor levels. One of these patients had a decrease in titer from 36 BU to 22 BU after 10 weeks. For the other 4 patients, titer was either unchanged or increased while on ITI.
Four of the 5 patients who did not achieve negative inhibitor levels remained on rFVIIIFc ITI at analysis, and 1 was placed on bypass therapy.
In total, 9 of the 12 rescue patients were still on rFVIIIFc at analysis—9 for ITI and 1 as prophylaxis.
“The development of inhibitors is a tremendous challenge and significant burden for people with severe hemophilia A, and the goal of treatment should be eradication of inhibitors,” said Maha Radhakrishnan, MD, of Bioverativ.
“The results of this analysis are encouraging and support the need for additional and ongoing scientific research on [rFVIIIFc ] in ITI to determine whether an Fc-based recombinant factor VIII therapy can rapidly tolerize patients with inhibitors.”
Bioverativ and Sobi have initiated 2 prospective studies designed to further evaluate the use of rFVIIIFc for ITI in patients with severe hemophilia A and inhibitors (NCT03093480 and NCT03103542).
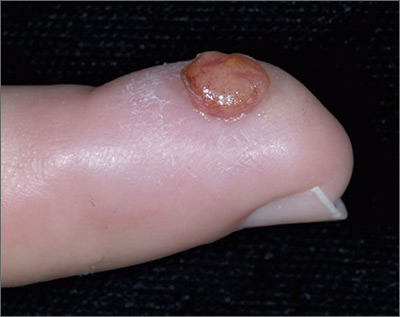
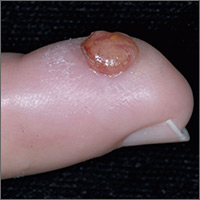
Growth on finger
The FP diagnosed pyogenic granuloma (PG), a common, benign, acquired vascular lesion of the skin and mucous membranes. PGs are erythematous, dome-shaped papules or nodules that bleed easily. They are prone to ulceration, erosion, and crusting, and rapid growth may occur over a period of weeks. The etiology for PG is unknown, but may be the result of trauma, infection, or preceding dermatoses. A more up-to-date and appropriate term for PG is lobular capillary hemangioma, because these lesions are neither pyogenic nor granulomas.
PGs are most often found on the fingers, lips, and hands. They may resemble a number of malignancies including basal cell carcinoma, Kaposi’s sarcoma, metastatic cutaneous lesions, squamous cell carcinoma, and amelanotic melanoma. For that reason, it’s especially important to send the excised lesion for pathology to ensure that malignancy isn’t missed.
The patient was eager to have the PG removed at this visit, as a number of previous visits to urgent care centers resulted in courses of antibiotics that didn’t help. After performing a digital block, the FP removed the PG with a shave excision, followed by electrodesiccation and curettage. The electrodesiccation and curettage are important to prevent recurrence. In this case, the pathology confirmed the diagnosis, and the PG did not recur.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Pyogenic Granuloma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 940-944.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed pyogenic granuloma (PG), a common, benign, acquired vascular lesion of the skin and mucous membranes. PGs are erythematous, dome-shaped papules or nodules that bleed easily. They are prone to ulceration, erosion, and crusting, and rapid growth may occur over a period of weeks. The etiology for PG is unknown, but may be the result of trauma, infection, or preceding dermatoses. A more up-to-date and appropriate term for PG is lobular capillary hemangioma, because these lesions are neither pyogenic nor granulomas.
PGs are most often found on the fingers, lips, and hands. They may resemble a number of malignancies including basal cell carcinoma, Kaposi’s sarcoma, metastatic cutaneous lesions, squamous cell carcinoma, and amelanotic melanoma. For that reason, it’s especially important to send the excised lesion for pathology to ensure that malignancy isn’t missed.
The patient was eager to have the PG removed at this visit, as a number of previous visits to urgent care centers resulted in courses of antibiotics that didn’t help. After performing a digital block, the FP removed the PG with a shave excision, followed by electrodesiccation and curettage. The electrodesiccation and curettage are important to prevent recurrence. In this case, the pathology confirmed the diagnosis, and the PG did not recur.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Pyogenic Granuloma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 940-944.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed pyogenic granuloma (PG), a common, benign, acquired vascular lesion of the skin and mucous membranes. PGs are erythematous, dome-shaped papules or nodules that bleed easily. They are prone to ulceration, erosion, and crusting, and rapid growth may occur over a period of weeks. The etiology for PG is unknown, but may be the result of trauma, infection, or preceding dermatoses. A more up-to-date and appropriate term for PG is lobular capillary hemangioma, because these lesions are neither pyogenic nor granulomas.
PGs are most often found on the fingers, lips, and hands. They may resemble a number of malignancies including basal cell carcinoma, Kaposi’s sarcoma, metastatic cutaneous lesions, squamous cell carcinoma, and amelanotic melanoma. For that reason, it’s especially important to send the excised lesion for pathology to ensure that malignancy isn’t missed.
The patient was eager to have the PG removed at this visit, as a number of previous visits to urgent care centers resulted in courses of antibiotics that didn’t help. After performing a digital block, the FP removed the PG with a shave excision, followed by electrodesiccation and curettage. The electrodesiccation and curettage are important to prevent recurrence. In this case, the pathology confirmed the diagnosis, and the PG did not recur.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M. Usatine R. Pyogenic Granuloma. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 940-944.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
CPR decision support videos can serve as a supplement to CPR preference discussions for inpatients
Clinical question: Does the use of a CPR decision support video impact patients’ code status preferences?
Background: Discussions about cardiopulmonary resuscitation are an important aspect of inpatient care but may be difficult to complete for several reasons, including poor patient understanding of the CPR process and its expected outcomes. This study sought to evaluate the impact of a CPR decision support video on patient CPR preferences.
Setting: General medicine wards at the Minneapolis Veterans Affairs from Sept. 28, 2015, to Oct. 23, 2015.
Synopsis: One hundred and nineteen patients older than 65 were randomized to receive standard care plus viewing a CPR decision support video or standard care alone. The primary outcome was patient code status preference. Patients completed a survey assessing trust in their care team.
Among the patients who viewed the video, 37% chose full code, compared with 71% of patients in the usual care arm. Patients who viewed the video were more likely to choose DNR/DNI (56%, compared with 17% in the control group). There was no significant difference in patient trust of the care team.
Study conclusions are limited by a study population consisting predominantly of white males. Though the study was randomized, it was not blinded.
Bottom line: A CPR decision support video led to a decrease in full code preference and an increase in DNR/DNI preference among hospitalized patients.
Citation: Merino AM et al. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017;12(9):700-4.
Dr. Rodriguez is a hospitalist and a clinical informatics fellow, Beth Israel Deaconess Medical Center, Boston.
Clinical question: Does the use of a CPR decision support video impact patients’ code status preferences?
Background: Discussions about cardiopulmonary resuscitation are an important aspect of inpatient care but may be difficult to complete for several reasons, including poor patient understanding of the CPR process and its expected outcomes. This study sought to evaluate the impact of a CPR decision support video on patient CPR preferences.
Setting: General medicine wards at the Minneapolis Veterans Affairs from Sept. 28, 2015, to Oct. 23, 2015.
Synopsis: One hundred and nineteen patients older than 65 were randomized to receive standard care plus viewing a CPR decision support video or standard care alone. The primary outcome was patient code status preference. Patients completed a survey assessing trust in their care team.
Among the patients who viewed the video, 37% chose full code, compared with 71% of patients in the usual care arm. Patients who viewed the video were more likely to choose DNR/DNI (56%, compared with 17% in the control group). There was no significant difference in patient trust of the care team.
Study conclusions are limited by a study population consisting predominantly of white males. Though the study was randomized, it was not blinded.
Bottom line: A CPR decision support video led to a decrease in full code preference and an increase in DNR/DNI preference among hospitalized patients.
Citation: Merino AM et al. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017;12(9):700-4.
Dr. Rodriguez is a hospitalist and a clinical informatics fellow, Beth Israel Deaconess Medical Center, Boston.
Clinical question: Does the use of a CPR decision support video impact patients’ code status preferences?
Background: Discussions about cardiopulmonary resuscitation are an important aspect of inpatient care but may be difficult to complete for several reasons, including poor patient understanding of the CPR process and its expected outcomes. This study sought to evaluate the impact of a CPR decision support video on patient CPR preferences.
Setting: General medicine wards at the Minneapolis Veterans Affairs from Sept. 28, 2015, to Oct. 23, 2015.
Synopsis: One hundred and nineteen patients older than 65 were randomized to receive standard care plus viewing a CPR decision support video or standard care alone. The primary outcome was patient code status preference. Patients completed a survey assessing trust in their care team.
Among the patients who viewed the video, 37% chose full code, compared with 71% of patients in the usual care arm. Patients who viewed the video were more likely to choose DNR/DNI (56%, compared with 17% in the control group). There was no significant difference in patient trust of the care team.
Study conclusions are limited by a study population consisting predominantly of white males. Though the study was randomized, it was not blinded.
Bottom line: A CPR decision support video led to a decrease in full code preference and an increase in DNR/DNI preference among hospitalized patients.
Citation: Merino AM et al. A randomized controlled trial of a CPR decision support video for patients admitted to the general medicine service. J Hosp Med. 2017;12(9):700-4.
Dr. Rodriguez is a hospitalist and a clinical informatics fellow, Beth Israel Deaconess Medical Center, Boston.
From the ACS President: The joy and privilege of a surgical career
As a Fellow of the American College of Surgeons (ACS), only you can recall the personal sacrifices you have made to attain the skills and knowledge necessary to enjoy the privilege of being a surgeon – years of missed time with family and friends, sleepless nights, and endless formative hours of deep experiential learning in the hospital. Someone else could have been there instead; you could have made a different career choice. But, no – surgery chose you, and you dove in. Thank you, thank you.
I hope you never lose sight of the lives you touched during those “lost” times – injured people, previously unknown neighbors with deadly diseases, or simply patients needing a little “repair.” People with a surgical disease are experiencing a rare life event: an operation. Never forget that each of those individuals, each patient, came to you – you personally – to help them.
Challenges
Regrettably, however, at times the cherished bond between a surgeon and a patient can get lost in our busy, burdened lives. It can get lost in physical fatigue, regulatory hoops, frustrations of the electronic health record, contract negotiations, challenging reimbursement policies, and on and on.
Add to that list other challenges that will surely arise in the course of your career: You will face various forms of threatened obsolescence in knowledge, skills, and technology. You will age. You will suffer personal tragedy and loss. You will become ill. That you may stumble when facing such challenges is not a sign of weakness. It is life.
I believe there is a bit of light ahead as our health care industry begins to recognize that this thing we call burnout is not a personal failing, but rather a function of our flawed work environments – and a significant threat not only to the surgeon, but also to patient safety, quality of care, and institutional financial stability. An active voice and actions in these essential domains of our work environment are mission critical for our College, as are efforts that are pursued on many fronts by Fellows and professionals in the Division of Advocacy and Health Policy in Washington, DC, and the Divisions of Research and Optimal Patient Care, Education, and Member Services in Chicago, IL. Fostering an environment to optimally support the care of the surgical patient – and surgeons – is core business in all we do in the ACS.
Let’s tackle a few other challenges. First, consider your personal and society’s investment in surgical training. Getting you to this skilled and knowledgeable point reflects an investment of more than $1 million dollars in costs of medical school and graduate medical education, and inestimable time and effort.
Second, the dire anticipated shortage of surgeons of many disciplines – general surgeons, orthopedists, urologists – appears to be real. If we are to keep our surgical pipeline full, we need to offer careers that are attractive to men and women equally. The U.S. general surgery pathway has entering classes of 40% women; however, other high-demand disciplines, such as neurosurgery, orthopedics, and cardiothoracic surgery, have not yet attracted women to their ranks in sufficient numbers, despite the fact that 50% of our medical school graduates are women. We need to examine the pathways to those surgical disciplines to ensure that gender- and ethnicity-based barriers are receding. Efforts are underway to address these challenges by the leadership in these disciplines that our College can help with.
Although much has changed for women in surgery in recent years, there are still differences in the lives of many female surgeons compared to their male colleagues. They remain at risk for pay inequity, being in aggregate compensated 10-17% less than their male colleagues for equal work. Despite a mature gender pipeline in some surgical specialties, women are still less likely to rise to leadership roles in their group practices, hospital structures, professional organizations, and academic institutions. The ACS can serve as a professional home to develop strategies to highlight and remedy these imbalances.
Parenting, to engage as fully and successfully as one may wish, is a challenge for many who choose our careers, regardless of gender. However, for most female surgeons beginning a family, the first step often comes with pregnancy and infant care, conditions that we have yet to embrace and support as a societal good rather than an individual’s gauntlet to run. Given our long and arduous educational pathways, these women often find themselves starting a family, be it by pregnancy or adoption, at the same moment they are beginning their busy early years of practice. Policies and practices to support surgeons who choose parenthood in the workforce are sorely needed and will, in fact, benefit all in the long run.
Our College, with guidance from the Women in Surgery Committee and the Association of Women Surgeons, has advanced that goal, issuing a statement that acknowledges the need for appropriate pregnancy and parental leave and that clearly articulates that the choice to become a parent in no way diminishes a surgeon’s commitment to career. The next steps will be building the institutional, financial, and community infrastructure to foster success in both career and parenting for all.
Retooling reimagined
Let’s ponder another challenge: the need to add to your repertoire a new, potentially transformative skill. How do we safely retool?
Twenty years ago, in the flawed early adoption of laparoscopic surgery, the ACS Committee on Emerging Surgical Technology Education articulated the principles of new skill acquisition: didactic learning, coupled with simulation-based training, and then proctored early experience, leading to independent practice and assessment of outcomes.
Subsequently, the College took the visionary step of establishing the ACS Accredited Education Institutes (AEI) program to develop a network of centers that would leverage emerging simulation technologies to enhance surgical training. The 96 national and international AEIs now serve as both educational and research centers to teach technical and nontechnical skills to surgeons and other health care professionals. At Houston Methodist Hospital, for example, we have built MITIE (Methodist Institute for Technology Innovation and Education), a comprehensive center with a focus on retooling surgeons in practice. We have hosted more than 13,000 surgeons in practice for retooling hands-on courses, along with more than 30,000 other health care professionals.
To ensure our surgical workforce stays at the top of their performance over a 40-year career, our College has convened a group of stakeholders, including payers, consumers, liability carriers, surgical technology industries, hospital executives, and, of course, surgeons, to define the infrastructure – facilities, faculty, curricula, assessment tools, and finances – needed to incorporate retooling and retraining into our health care system. Work to do.
Shape your future
The retooling reimagined initiative is but one example of how we can shape our professional futures. Remember, the ACS was founded nearly 105 years ago by surgeons who sought to improve the care of the surgical patient. Since then, individual surgeons, banding together within our College, have created some of the most effective systems in the world to improve surgical care – including the formation of the Committee on Trauma and the Commission on Cancer, which have led initiatives that have vastly improved care for their respective patient populations.
The ACS National Surgical Quality Improvement Program, born of the vision of Shukri Khuri, MD, FACS, who, when tasked with resolving a perceived problem in surgical care in the Veterans Affairs Health Care System, launched a research study to measure quality. Soon thereafter, he led an army of surgeons to improve surgical care in their own hospitals, founding a nationwide movement that now flourishes in thousands of hospitals as the world’s most effective surgical quality measurement and improvement system.
We can go on and on. A surgeon identifies a gap and with a good idea, and coupled to abundant College focus and the engagement of our Fellows, a valuable new program is launched. These initiatives were not delivered from on high. They were created by regular surgeons, like you and me, who saw gaps in their professional worlds and took steps to effect meaningful change.
Caring for each other
I have one more request: I want you to be aware of your colleagues. I want you to watch them for signs of stress and disturbances in their forces. And if you see something, ask a supportive question or offer needed assistance. Be aware of help that is available in your institution; know how to move a concern up the chain with sensitivity, but also with efficacy, coupled with compassionate concern for your colleague.
These are not easy discussions and may prove fruitless, but they are worth the effort to try, for we surgeons are a high-risk group for depression, substance abuse, and suicide – and for failing to seek assistance. This situation must change, but doing so will require that we destigmatize these conditions in ourselves and our colleagues, and destigmatize seeking assistance.
But, for now, on a joyful or a challenging day in your surgical life, I hope you are proud of your Fellowship in the ACS and your FACS initials that signify your commitment to the values of our profession. I hope you will draw endless support and friendship from those around you and that you will contribute more than you receive. And I hope that you will forever treasure your opportunity to practice as a surgeon, an exceptional joy and privilege.
Dr. Bass is the John F. and Carolyn Bookout Presidential Endowed Chair, professor of surgery and chair, department of surgery, Houston Methodist Hospital, TX, and the President of the American College of Surgeons (ACS).
As a Fellow of the American College of Surgeons (ACS), only you can recall the personal sacrifices you have made to attain the skills and knowledge necessary to enjoy the privilege of being a surgeon – years of missed time with family and friends, sleepless nights, and endless formative hours of deep experiential learning in the hospital. Someone else could have been there instead; you could have made a different career choice. But, no – surgery chose you, and you dove in. Thank you, thank you.
I hope you never lose sight of the lives you touched during those “lost” times – injured people, previously unknown neighbors with deadly diseases, or simply patients needing a little “repair.” People with a surgical disease are experiencing a rare life event: an operation. Never forget that each of those individuals, each patient, came to you – you personally – to help them.
Challenges
Regrettably, however, at times the cherished bond between a surgeon and a patient can get lost in our busy, burdened lives. It can get lost in physical fatigue, regulatory hoops, frustrations of the electronic health record, contract negotiations, challenging reimbursement policies, and on and on.
Add to that list other challenges that will surely arise in the course of your career: You will face various forms of threatened obsolescence in knowledge, skills, and technology. You will age. You will suffer personal tragedy and loss. You will become ill. That you may stumble when facing such challenges is not a sign of weakness. It is life.
I believe there is a bit of light ahead as our health care industry begins to recognize that this thing we call burnout is not a personal failing, but rather a function of our flawed work environments – and a significant threat not only to the surgeon, but also to patient safety, quality of care, and institutional financial stability. An active voice and actions in these essential domains of our work environment are mission critical for our College, as are efforts that are pursued on many fronts by Fellows and professionals in the Division of Advocacy and Health Policy in Washington, DC, and the Divisions of Research and Optimal Patient Care, Education, and Member Services in Chicago, IL. Fostering an environment to optimally support the care of the surgical patient – and surgeons – is core business in all we do in the ACS.
Let’s tackle a few other challenges. First, consider your personal and society’s investment in surgical training. Getting you to this skilled and knowledgeable point reflects an investment of more than $1 million dollars in costs of medical school and graduate medical education, and inestimable time and effort.
Second, the dire anticipated shortage of surgeons of many disciplines – general surgeons, orthopedists, urologists – appears to be real. If we are to keep our surgical pipeline full, we need to offer careers that are attractive to men and women equally. The U.S. general surgery pathway has entering classes of 40% women; however, other high-demand disciplines, such as neurosurgery, orthopedics, and cardiothoracic surgery, have not yet attracted women to their ranks in sufficient numbers, despite the fact that 50% of our medical school graduates are women. We need to examine the pathways to those surgical disciplines to ensure that gender- and ethnicity-based barriers are receding. Efforts are underway to address these challenges by the leadership in these disciplines that our College can help with.
Although much has changed for women in surgery in recent years, there are still differences in the lives of many female surgeons compared to their male colleagues. They remain at risk for pay inequity, being in aggregate compensated 10-17% less than their male colleagues for equal work. Despite a mature gender pipeline in some surgical specialties, women are still less likely to rise to leadership roles in their group practices, hospital structures, professional organizations, and academic institutions. The ACS can serve as a professional home to develop strategies to highlight and remedy these imbalances.
Parenting, to engage as fully and successfully as one may wish, is a challenge for many who choose our careers, regardless of gender. However, for most female surgeons beginning a family, the first step often comes with pregnancy and infant care, conditions that we have yet to embrace and support as a societal good rather than an individual’s gauntlet to run. Given our long and arduous educational pathways, these women often find themselves starting a family, be it by pregnancy or adoption, at the same moment they are beginning their busy early years of practice. Policies and practices to support surgeons who choose parenthood in the workforce are sorely needed and will, in fact, benefit all in the long run.
Our College, with guidance from the Women in Surgery Committee and the Association of Women Surgeons, has advanced that goal, issuing a statement that acknowledges the need for appropriate pregnancy and parental leave and that clearly articulates that the choice to become a parent in no way diminishes a surgeon’s commitment to career. The next steps will be building the institutional, financial, and community infrastructure to foster success in both career and parenting for all.
Retooling reimagined
Let’s ponder another challenge: the need to add to your repertoire a new, potentially transformative skill. How do we safely retool?
Twenty years ago, in the flawed early adoption of laparoscopic surgery, the ACS Committee on Emerging Surgical Technology Education articulated the principles of new skill acquisition: didactic learning, coupled with simulation-based training, and then proctored early experience, leading to independent practice and assessment of outcomes.
Subsequently, the College took the visionary step of establishing the ACS Accredited Education Institutes (AEI) program to develop a network of centers that would leverage emerging simulation technologies to enhance surgical training. The 96 national and international AEIs now serve as both educational and research centers to teach technical and nontechnical skills to surgeons and other health care professionals. At Houston Methodist Hospital, for example, we have built MITIE (Methodist Institute for Technology Innovation and Education), a comprehensive center with a focus on retooling surgeons in practice. We have hosted more than 13,000 surgeons in practice for retooling hands-on courses, along with more than 30,000 other health care professionals.
To ensure our surgical workforce stays at the top of their performance over a 40-year career, our College has convened a group of stakeholders, including payers, consumers, liability carriers, surgical technology industries, hospital executives, and, of course, surgeons, to define the infrastructure – facilities, faculty, curricula, assessment tools, and finances – needed to incorporate retooling and retraining into our health care system. Work to do.
Shape your future
The retooling reimagined initiative is but one example of how we can shape our professional futures. Remember, the ACS was founded nearly 105 years ago by surgeons who sought to improve the care of the surgical patient. Since then, individual surgeons, banding together within our College, have created some of the most effective systems in the world to improve surgical care – including the formation of the Committee on Trauma and the Commission on Cancer, which have led initiatives that have vastly improved care for their respective patient populations.
The ACS National Surgical Quality Improvement Program, born of the vision of Shukri Khuri, MD, FACS, who, when tasked with resolving a perceived problem in surgical care in the Veterans Affairs Health Care System, launched a research study to measure quality. Soon thereafter, he led an army of surgeons to improve surgical care in their own hospitals, founding a nationwide movement that now flourishes in thousands of hospitals as the world’s most effective surgical quality measurement and improvement system.
We can go on and on. A surgeon identifies a gap and with a good idea, and coupled to abundant College focus and the engagement of our Fellows, a valuable new program is launched. These initiatives were not delivered from on high. They were created by regular surgeons, like you and me, who saw gaps in their professional worlds and took steps to effect meaningful change.
Caring for each other
I have one more request: I want you to be aware of your colleagues. I want you to watch them for signs of stress and disturbances in their forces. And if you see something, ask a supportive question or offer needed assistance. Be aware of help that is available in your institution; know how to move a concern up the chain with sensitivity, but also with efficacy, coupled with compassionate concern for your colleague.
These are not easy discussions and may prove fruitless, but they are worth the effort to try, for we surgeons are a high-risk group for depression, substance abuse, and suicide – and for failing to seek assistance. This situation must change, but doing so will require that we destigmatize these conditions in ourselves and our colleagues, and destigmatize seeking assistance.
But, for now, on a joyful or a challenging day in your surgical life, I hope you are proud of your Fellowship in the ACS and your FACS initials that signify your commitment to the values of our profession. I hope you will draw endless support and friendship from those around you and that you will contribute more than you receive. And I hope that you will forever treasure your opportunity to practice as a surgeon, an exceptional joy and privilege.
Dr. Bass is the John F. and Carolyn Bookout Presidential Endowed Chair, professor of surgery and chair, department of surgery, Houston Methodist Hospital, TX, and the President of the American College of Surgeons (ACS).
As a Fellow of the American College of Surgeons (ACS), only you can recall the personal sacrifices you have made to attain the skills and knowledge necessary to enjoy the privilege of being a surgeon – years of missed time with family and friends, sleepless nights, and endless formative hours of deep experiential learning in the hospital. Someone else could have been there instead; you could have made a different career choice. But, no – surgery chose you, and you dove in. Thank you, thank you.
I hope you never lose sight of the lives you touched during those “lost” times – injured people, previously unknown neighbors with deadly diseases, or simply patients needing a little “repair.” People with a surgical disease are experiencing a rare life event: an operation. Never forget that each of those individuals, each patient, came to you – you personally – to help them.
Challenges
Regrettably, however, at times the cherished bond between a surgeon and a patient can get lost in our busy, burdened lives. It can get lost in physical fatigue, regulatory hoops, frustrations of the electronic health record, contract negotiations, challenging reimbursement policies, and on and on.
Add to that list other challenges that will surely arise in the course of your career: You will face various forms of threatened obsolescence in knowledge, skills, and technology. You will age. You will suffer personal tragedy and loss. You will become ill. That you may stumble when facing such challenges is not a sign of weakness. It is life.
I believe there is a bit of light ahead as our health care industry begins to recognize that this thing we call burnout is not a personal failing, but rather a function of our flawed work environments – and a significant threat not only to the surgeon, but also to patient safety, quality of care, and institutional financial stability. An active voice and actions in these essential domains of our work environment are mission critical for our College, as are efforts that are pursued on many fronts by Fellows and professionals in the Division of Advocacy and Health Policy in Washington, DC, and the Divisions of Research and Optimal Patient Care, Education, and Member Services in Chicago, IL. Fostering an environment to optimally support the care of the surgical patient – and surgeons – is core business in all we do in the ACS.
Let’s tackle a few other challenges. First, consider your personal and society’s investment in surgical training. Getting you to this skilled and knowledgeable point reflects an investment of more than $1 million dollars in costs of medical school and graduate medical education, and inestimable time and effort.
Second, the dire anticipated shortage of surgeons of many disciplines – general surgeons, orthopedists, urologists – appears to be real. If we are to keep our surgical pipeline full, we need to offer careers that are attractive to men and women equally. The U.S. general surgery pathway has entering classes of 40% women; however, other high-demand disciplines, such as neurosurgery, orthopedics, and cardiothoracic surgery, have not yet attracted women to their ranks in sufficient numbers, despite the fact that 50% of our medical school graduates are women. We need to examine the pathways to those surgical disciplines to ensure that gender- and ethnicity-based barriers are receding. Efforts are underway to address these challenges by the leadership in these disciplines that our College can help with.
Although much has changed for women in surgery in recent years, there are still differences in the lives of many female surgeons compared to their male colleagues. They remain at risk for pay inequity, being in aggregate compensated 10-17% less than their male colleagues for equal work. Despite a mature gender pipeline in some surgical specialties, women are still less likely to rise to leadership roles in their group practices, hospital structures, professional organizations, and academic institutions. The ACS can serve as a professional home to develop strategies to highlight and remedy these imbalances.
Parenting, to engage as fully and successfully as one may wish, is a challenge for many who choose our careers, regardless of gender. However, for most female surgeons beginning a family, the first step often comes with pregnancy and infant care, conditions that we have yet to embrace and support as a societal good rather than an individual’s gauntlet to run. Given our long and arduous educational pathways, these women often find themselves starting a family, be it by pregnancy or adoption, at the same moment they are beginning their busy early years of practice. Policies and practices to support surgeons who choose parenthood in the workforce are sorely needed and will, in fact, benefit all in the long run.
Our College, with guidance from the Women in Surgery Committee and the Association of Women Surgeons, has advanced that goal, issuing a statement that acknowledges the need for appropriate pregnancy and parental leave and that clearly articulates that the choice to become a parent in no way diminishes a surgeon’s commitment to career. The next steps will be building the institutional, financial, and community infrastructure to foster success in both career and parenting for all.
Retooling reimagined
Let’s ponder another challenge: the need to add to your repertoire a new, potentially transformative skill. How do we safely retool?
Twenty years ago, in the flawed early adoption of laparoscopic surgery, the ACS Committee on Emerging Surgical Technology Education articulated the principles of new skill acquisition: didactic learning, coupled with simulation-based training, and then proctored early experience, leading to independent practice and assessment of outcomes.
Subsequently, the College took the visionary step of establishing the ACS Accredited Education Institutes (AEI) program to develop a network of centers that would leverage emerging simulation technologies to enhance surgical training. The 96 national and international AEIs now serve as both educational and research centers to teach technical and nontechnical skills to surgeons and other health care professionals. At Houston Methodist Hospital, for example, we have built MITIE (Methodist Institute for Technology Innovation and Education), a comprehensive center with a focus on retooling surgeons in practice. We have hosted more than 13,000 surgeons in practice for retooling hands-on courses, along with more than 30,000 other health care professionals.
To ensure our surgical workforce stays at the top of their performance over a 40-year career, our College has convened a group of stakeholders, including payers, consumers, liability carriers, surgical technology industries, hospital executives, and, of course, surgeons, to define the infrastructure – facilities, faculty, curricula, assessment tools, and finances – needed to incorporate retooling and retraining into our health care system. Work to do.
Shape your future
The retooling reimagined initiative is but one example of how we can shape our professional futures. Remember, the ACS was founded nearly 105 years ago by surgeons who sought to improve the care of the surgical patient. Since then, individual surgeons, banding together within our College, have created some of the most effective systems in the world to improve surgical care – including the formation of the Committee on Trauma and the Commission on Cancer, which have led initiatives that have vastly improved care for their respective patient populations.
The ACS National Surgical Quality Improvement Program, born of the vision of Shukri Khuri, MD, FACS, who, when tasked with resolving a perceived problem in surgical care in the Veterans Affairs Health Care System, launched a research study to measure quality. Soon thereafter, he led an army of surgeons to improve surgical care in their own hospitals, founding a nationwide movement that now flourishes in thousands of hospitals as the world’s most effective surgical quality measurement and improvement system.
We can go on and on. A surgeon identifies a gap and with a good idea, and coupled to abundant College focus and the engagement of our Fellows, a valuable new program is launched. These initiatives were not delivered from on high. They were created by regular surgeons, like you and me, who saw gaps in their professional worlds and took steps to effect meaningful change.
Caring for each other
I have one more request: I want you to be aware of your colleagues. I want you to watch them for signs of stress and disturbances in their forces. And if you see something, ask a supportive question or offer needed assistance. Be aware of help that is available in your institution; know how to move a concern up the chain with sensitivity, but also with efficacy, coupled with compassionate concern for your colleague.
These are not easy discussions and may prove fruitless, but they are worth the effort to try, for we surgeons are a high-risk group for depression, substance abuse, and suicide – and for failing to seek assistance. This situation must change, but doing so will require that we destigmatize these conditions in ourselves and our colleagues, and destigmatize seeking assistance.
But, for now, on a joyful or a challenging day in your surgical life, I hope you are proud of your Fellowship in the ACS and your FACS initials that signify your commitment to the values of our profession. I hope you will draw endless support and friendship from those around you and that you will contribute more than you receive. And I hope that you will forever treasure your opportunity to practice as a surgeon, an exceptional joy and privilege.
Dr. Bass is the John F. and Carolyn Bookout Presidential Endowed Chair, professor of surgery and chair, department of surgery, Houston Methodist Hospital, TX, and the President of the American College of Surgeons (ACS).
From the Editors: An unexpected call to action
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.
In the waning weeks of 2017, still another disaster was added to the long list of natural and man-made tragedies of the year: the derailment of an Amtrak train near Tacoma, Washington. Although the cause of this event has not yet been determined and will not be for at least several months, we do know that safety equipment that has been recommended for years had not been installed in the train. I can only hope that this accident reminds our governmental leaders and institutional officials of the costs in lives and injury of ignoring deteriorating infrastructure and neglecting known safety measures.
An eyewitness and participant in the response to the accident was the Oregon Health & Science University Chair of Neurological Surgery, Nathan Selden, MD, PhD, FACS, who was driving north on Interstate 5 that morning with his 18-year-old son. I spoke recently with Dr. Selden to obtain his first-hand impressions of the experience.
They came upon the scene of the derailment shortly after it had occurred. He recognized immediately the horrifying potential for serious injuries and fatalities. First responders were already arriving on the scene and Dr. Selden offered his services to assist the injured. The first responders eagerly accepted his offer, and he spent the next two hours working with another MD and one RN from nearby Joint Base Lewis-McChord and a large number of EMTs and firefighters mobilized from nearby communities. The team of emergency workers removed almost 80 victims from precariously dangling train cars, provided first aid and basic trauma care, and triaged the victims to the most appropriate next site for treatment. Dr. Selden was most impressed by the courage of the firefighters who climbed into two train cars hanging off the highway overpass. He commented, “They were awesome, working in incredibly risky conditions.”
A pediatric neurosurgeon in his daily work, Dr. Selden is not in the habit of performing the duties that he did that day, but he used his expertise in trauma to assess the victims’ injuries, listing their problems on tags hung around their necks and advising the scene commander about what kind of specialist each patient would likely need. The commander could then direct ambulances to the most appropriate nearby facility for definitive care.
Although most of the hastily assembled emergency response team were strangers to one another, Dr. Selden remarked that “they all worked together efficiently” at a scene that he described as “orderly, purposeful chaos” to stop bleeding, bandage cuts, splint fractures, apply cervical collars, place the injured on backboards, and reassure and calm the victims, who were understandably scared and in shock. Dr. Selden modestly downplayed his role at the scene, and praised the EMTs, firefighters, and police for their leadership and professionalism in organizing and coordinating everyone efficiently and expertly. His comments about the experience focused on the effective way that the caregivers at the scene did their jobs and emphasized how long the road to healing will be for many of the injured. These victims will be in need of support and healing long after the public’s attention has moved on from the drama of that remarkably devastating event. For many of them and their families, he soberly noted, “their lives will be changed forever.” His comments reflect his deep understanding of the implications of the victims’ injuries, many of which involved his area of expertise, neurosurgical trauma.
Few of us will be called on during our careers to step out of our comfort zone to provide emergency care in a situation as far from our normal daily environment as this one was. But if we are, we would do well to follow the lead exemplified by Dr. Selden: call on the basic skills that we as surgeons all possess, work collaboratively with those trained to be first responders and rescuers, and acknowledge the profound and long-lasting effect such a calamity has on all of those who experience it.
Dr. Deveney is professor of surgery and vice chair of education in the department of surgery, Oregon Health & Science University, Portland. She is the coeditor of ACS Surgery News.