User login
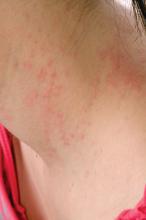
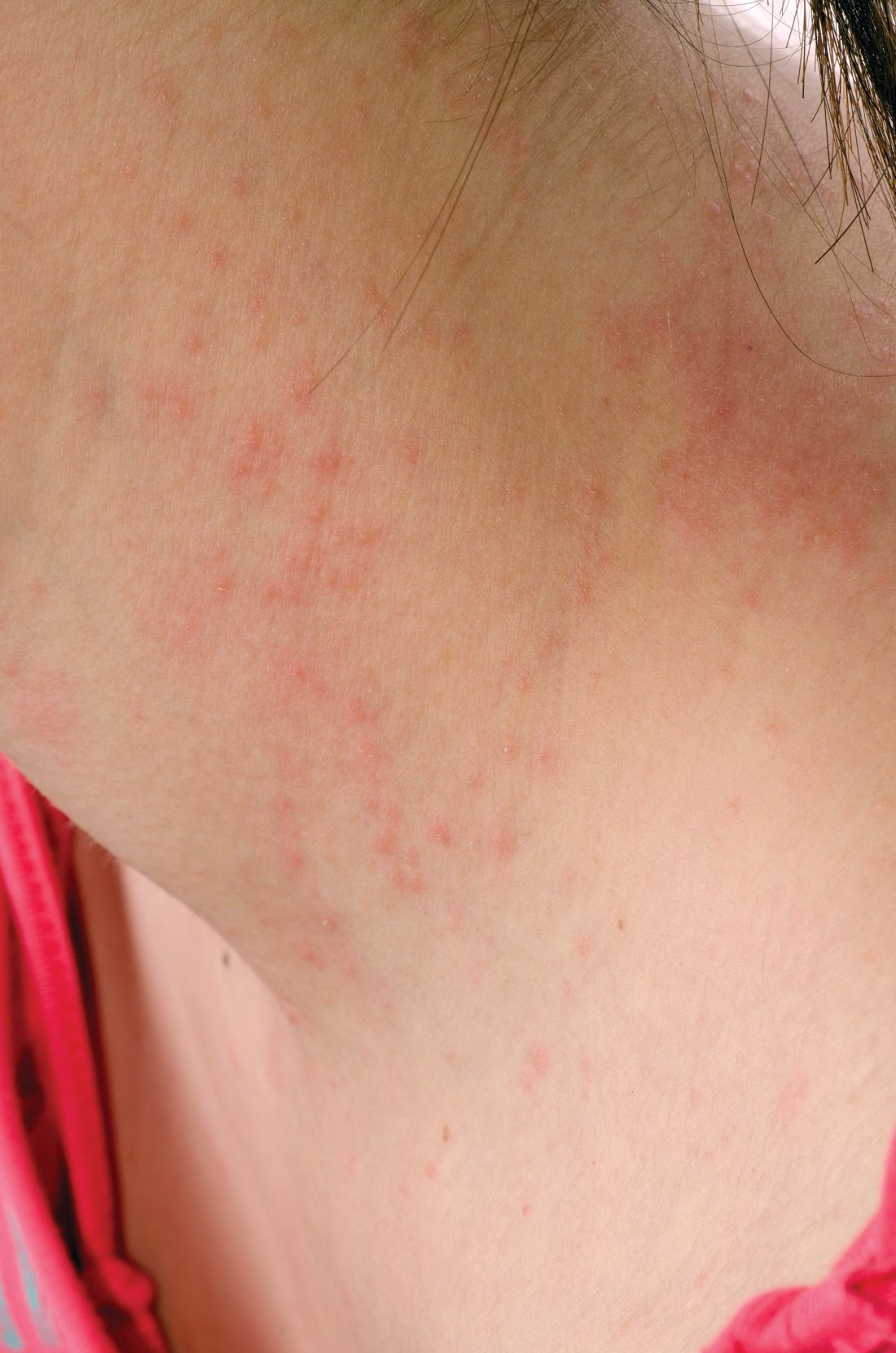
Teen tanning bed bans keep patients from phototherapy
said Daniel J. Lewis and Madeleine Duvic, MD, of the University of Texas MD Anderson Cancer Center, Houston.
To prevent skin cancer, many states and the District of Columbia have set in place age restrictions on the use of commercial tanning beds and banned minors from indoor tanning; another 12 states put in place bans at younger ages. The Food and Drug Administration proposed a policy in 2015 that would keep all minors from indoor tanning, according to Mr. Lewis and Dr. Duvic.
Phototherapy is known to be an effective treatment for psoriasis, mycosis fungoides, and vitiligo. Phototherapy usually is given in physician offices and administered in ultraviolet B for treatment of psoriasis, but studies have found that tanning beds, which emit primarily ultraviolet A, can produce a clinical response in 80% of psoriasis patients, and 53% of patients report using tanning beds for psoriasis treatment, Mr. Lewis and Dr. Duvic said in a letter to the editor in Clinics in Dermatology.
The demanding schedule of visiting a physician’s office three times a week for phototherapy can be hard for some patients, especially children attending school during regular office hours and patients in rural areas. High copayments also can limit access, the authors said.
Despite the risks, making tanning beds “uniformly illegal for minors would be a disservice to patients with limited access to phototherapy. A more effective approach might be to collaborate with the tanning industry to achieve reasonable limits on age and exposure as opposed to an outright ban. At the very least, patients who receive a prescription from a dermatologist should be exempted from a universal ban,” they concluded.
SOURCE: Lewis, DJ and Duvic, M. Clin Dermatol. 2018 Jan-Feb;36(1):104-5
said Daniel J. Lewis and Madeleine Duvic, MD, of the University of Texas MD Anderson Cancer Center, Houston.
To prevent skin cancer, many states and the District of Columbia have set in place age restrictions on the use of commercial tanning beds and banned minors from indoor tanning; another 12 states put in place bans at younger ages. The Food and Drug Administration proposed a policy in 2015 that would keep all minors from indoor tanning, according to Mr. Lewis and Dr. Duvic.
Phototherapy is known to be an effective treatment for psoriasis, mycosis fungoides, and vitiligo. Phototherapy usually is given in physician offices and administered in ultraviolet B for treatment of psoriasis, but studies have found that tanning beds, which emit primarily ultraviolet A, can produce a clinical response in 80% of psoriasis patients, and 53% of patients report using tanning beds for psoriasis treatment, Mr. Lewis and Dr. Duvic said in a letter to the editor in Clinics in Dermatology.
The demanding schedule of visiting a physician’s office three times a week for phototherapy can be hard for some patients, especially children attending school during regular office hours and patients in rural areas. High copayments also can limit access, the authors said.
Despite the risks, making tanning beds “uniformly illegal for minors would be a disservice to patients with limited access to phototherapy. A more effective approach might be to collaborate with the tanning industry to achieve reasonable limits on age and exposure as opposed to an outright ban. At the very least, patients who receive a prescription from a dermatologist should be exempted from a universal ban,” they concluded.
SOURCE: Lewis, DJ and Duvic, M. Clin Dermatol. 2018 Jan-Feb;36(1):104-5
said Daniel J. Lewis and Madeleine Duvic, MD, of the University of Texas MD Anderson Cancer Center, Houston.
To prevent skin cancer, many states and the District of Columbia have set in place age restrictions on the use of commercial tanning beds and banned minors from indoor tanning; another 12 states put in place bans at younger ages. The Food and Drug Administration proposed a policy in 2015 that would keep all minors from indoor tanning, according to Mr. Lewis and Dr. Duvic.
Phototherapy is known to be an effective treatment for psoriasis, mycosis fungoides, and vitiligo. Phototherapy usually is given in physician offices and administered in ultraviolet B for treatment of psoriasis, but studies have found that tanning beds, which emit primarily ultraviolet A, can produce a clinical response in 80% of psoriasis patients, and 53% of patients report using tanning beds for psoriasis treatment, Mr. Lewis and Dr. Duvic said in a letter to the editor in Clinics in Dermatology.
The demanding schedule of visiting a physician’s office three times a week for phototherapy can be hard for some patients, especially children attending school during regular office hours and patients in rural areas. High copayments also can limit access, the authors said.
Despite the risks, making tanning beds “uniformly illegal for minors would be a disservice to patients with limited access to phototherapy. A more effective approach might be to collaborate with the tanning industry to achieve reasonable limits on age and exposure as opposed to an outright ban. At the very least, patients who receive a prescription from a dermatologist should be exempted from a universal ban,” they concluded.
SOURCE: Lewis, DJ and Duvic, M. Clin Dermatol. 2018 Jan-Feb;36(1):104-5
FROM CLINICS IN DERMATOLOGY
Topical steroid reduces atopic dermatitis relapse in children
according to Elena Rubio-Gomis of the Consorcio Hospital General Universitario de Valencia, Spain, and her associates.
Most atopic disease management is based on managing active disease, but atopic dermatitis is a chronic relapsing disease for which long-term maintenance therapies are being sought.
“Fluticasone 0.05% cream is a potent topical corticosteroid with a low systemic absorption and rapid metabolism and clearance,” which argues for low likelihood of causing systemic side effects and possible use in preventing atopic dermatitis relapse, the investigators wrote. And it is not very expensive.
In a randomized, double-blind, placebo-controlled study of 54 children with mild to moderate atopic dermatitis treated with twice-daily fluticasone propionate 0.05% cream up to 2 weeks, 49 children aged 2-10 years entered the second phase of the study. These children were randomly assigned to receive fluticasone propionate or vehicle twice weekly on consecutive days for 16 weeks; treatment was stopped then or at relapse.
Children treated with fluticasone propionate had a 2.7-fold lower risk of relapse than that of children treated with vehicle; 7 of 26 (27%) in the fluticasone propionate group and 13 of 23 patients (57%) in the vehicle group experienced relapse, the investigators wrote. The report was published in Allergologia et Immunopathologia. The median time to relapse with fluticasone propionate exceeded 16 weeks (the duration of the study), while in the vehicle group it was 65 days (a little over 9 weeks).
The treatment with fluticasone propionate and vehicle both were well tolerated. During the second phase of the study, 9 patients (35%) reported at least one adverse event in the fluticasone propionate group and 11 (48%) in the vehicle group. None of these were believed to be related to the drug studied, the investigators said.
SOURCE: Rubio-Gomis E et al. Allergol Immunopathol (Madr). 2018. doi: 10.1016/j.aller.2017.12.001.
according to Elena Rubio-Gomis of the Consorcio Hospital General Universitario de Valencia, Spain, and her associates.
Most atopic disease management is based on managing active disease, but atopic dermatitis is a chronic relapsing disease for which long-term maintenance therapies are being sought.
“Fluticasone 0.05% cream is a potent topical corticosteroid with a low systemic absorption and rapid metabolism and clearance,” which argues for low likelihood of causing systemic side effects and possible use in preventing atopic dermatitis relapse, the investigators wrote. And it is not very expensive.
In a randomized, double-blind, placebo-controlled study of 54 children with mild to moderate atopic dermatitis treated with twice-daily fluticasone propionate 0.05% cream up to 2 weeks, 49 children aged 2-10 years entered the second phase of the study. These children were randomly assigned to receive fluticasone propionate or vehicle twice weekly on consecutive days for 16 weeks; treatment was stopped then or at relapse.
Children treated with fluticasone propionate had a 2.7-fold lower risk of relapse than that of children treated with vehicle; 7 of 26 (27%) in the fluticasone propionate group and 13 of 23 patients (57%) in the vehicle group experienced relapse, the investigators wrote. The report was published in Allergologia et Immunopathologia. The median time to relapse with fluticasone propionate exceeded 16 weeks (the duration of the study), while in the vehicle group it was 65 days (a little over 9 weeks).
The treatment with fluticasone propionate and vehicle both were well tolerated. During the second phase of the study, 9 patients (35%) reported at least one adverse event in the fluticasone propionate group and 11 (48%) in the vehicle group. None of these were believed to be related to the drug studied, the investigators said.
SOURCE: Rubio-Gomis E et al. Allergol Immunopathol (Madr). 2018. doi: 10.1016/j.aller.2017.12.001.
according to Elena Rubio-Gomis of the Consorcio Hospital General Universitario de Valencia, Spain, and her associates.
Most atopic disease management is based on managing active disease, but atopic dermatitis is a chronic relapsing disease for which long-term maintenance therapies are being sought.
“Fluticasone 0.05% cream is a potent topical corticosteroid with a low systemic absorption and rapid metabolism and clearance,” which argues for low likelihood of causing systemic side effects and possible use in preventing atopic dermatitis relapse, the investigators wrote. And it is not very expensive.
In a randomized, double-blind, placebo-controlled study of 54 children with mild to moderate atopic dermatitis treated with twice-daily fluticasone propionate 0.05% cream up to 2 weeks, 49 children aged 2-10 years entered the second phase of the study. These children were randomly assigned to receive fluticasone propionate or vehicle twice weekly on consecutive days for 16 weeks; treatment was stopped then or at relapse.
Children treated with fluticasone propionate had a 2.7-fold lower risk of relapse than that of children treated with vehicle; 7 of 26 (27%) in the fluticasone propionate group and 13 of 23 patients (57%) in the vehicle group experienced relapse, the investigators wrote. The report was published in Allergologia et Immunopathologia. The median time to relapse with fluticasone propionate exceeded 16 weeks (the duration of the study), while in the vehicle group it was 65 days (a little over 9 weeks).
The treatment with fluticasone propionate and vehicle both were well tolerated. During the second phase of the study, 9 patients (35%) reported at least one adverse event in the fluticasone propionate group and 11 (48%) in the vehicle group. None of these were believed to be related to the drug studied, the investigators said.
SOURCE: Rubio-Gomis E et al. Allergol Immunopathol (Madr). 2018. doi: 10.1016/j.aller.2017.12.001.
FROM ALLERGOLOGIA ET IMMUNOPATHOLOGIA
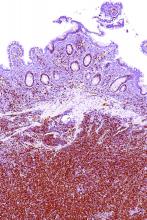
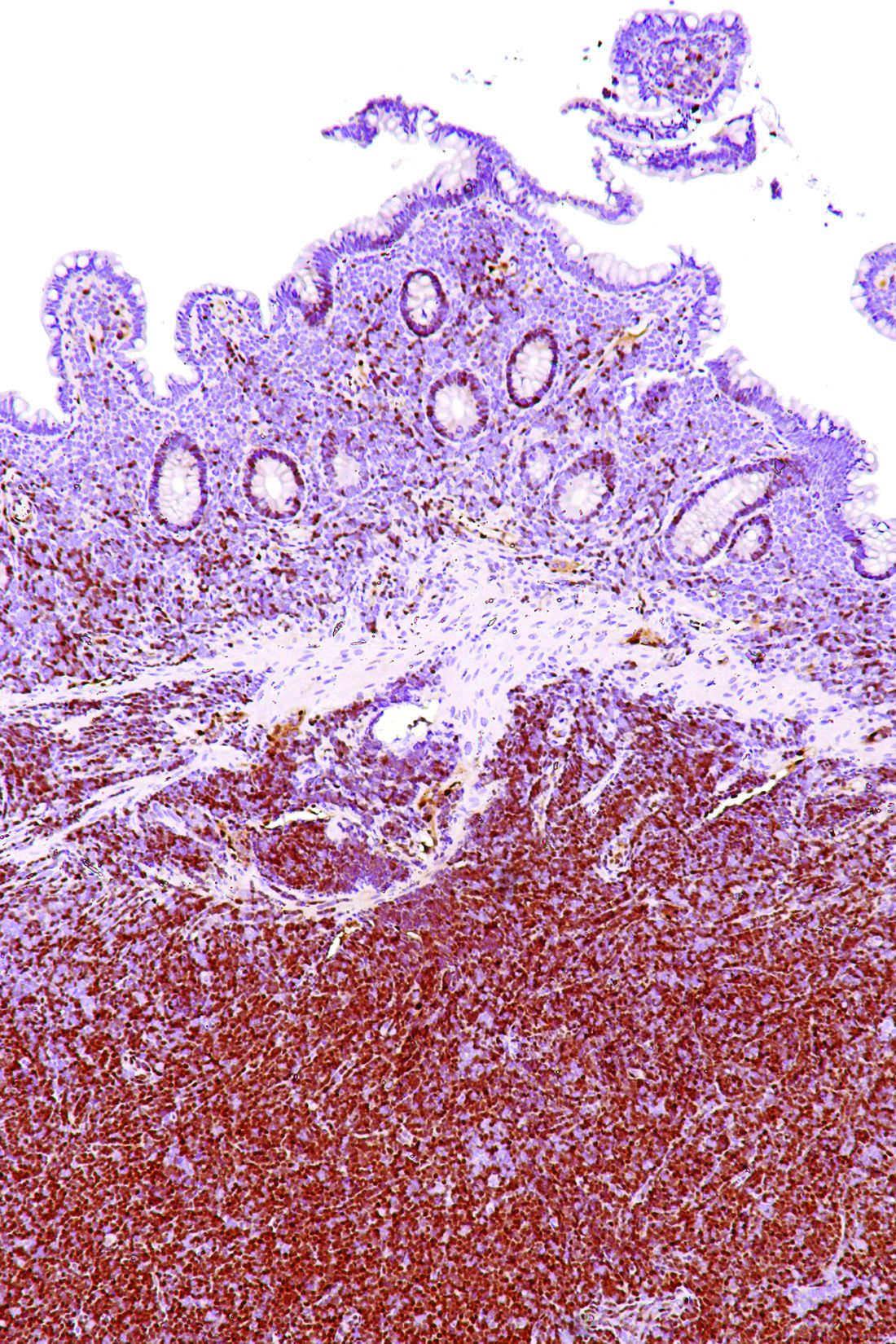
AML immune profiles correlate with relapse-free survival
SAN FRANCISCO – Immune-enriched acute myeloid leukemias might be amenable to immunotherapy that is tailored to the bone marrow tumor microenvironment, according to findings from a pan-cancer analysis of bone marrow samples.
The analysis, performed with 3D biology technology and an RNA pan-cancer immune profiling panel to characterize bone marrow specimens from 46 children and 28 adults with acute myeloid leukemia (AML), identified heterogeneous immune profiles that correlated with relapse-free survival (RFS) and overall survival (OS), Jayakumar Vadakekolathu, PhD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The specimens, including 63 from nonpromyelocytic de novo AML, 7 from AML in children with complete remission, 3 from adults with secondary AML, and 1 from an adult with treatment-related AML, were analyzed with the nCounter system from NanoString Technologies, and were visualized via digital spatial profiling, said Dr. Vadakekolathu of Nottingham Trent University in England.
The investigators identified two distinct immune gene expression profiles (GEPs) that were largely age-differentiated: Cluster A (myeloid-enriched specimens) included 26 children and 8 adults, and cluster B (myeloid-depleted specimens) included 9 children and 18 adults. These GEPs predicted clinical outcome; relapse free survival was 2.2 months in cluster A versus 18.3 months in cluster B (hazard ratio, 2.58) and overall survival was 6.3 months in cluster A, compared with 22.4 months in cluster B (HR, 2.39), Dr. Vadakekolathu reported.
The findings could have implications for the development of new treatment strategies, he said, noting that AML is a highly heterogeneous disease in terms of genetics, clinical manifestations, and outcome.
“Prognosis is determined by cytogenetic and molecular abnormalities, as well as by response to chemotherapy. De novo AML is cured in roughly 70% of children, 35%-40% of adults, and 5%-15% of elderly patients,” he said. Some patients with AML fail to respond to induction chemotherapy, and others eventually relapse despite the lack of adverse risk factors, he added.
The general therapeutic strategy in patients with AML has not changed substantially in more than 30 years, he said.
“High degrees of molecular complexity in AML present a considerable challenge in clinical implementation, and there is an urgent need to discover better biomarkers to identify high-risk patients before starting chemotherapy, which would enable testing of investigational therapeutic strategies in clinical trials,” he said.
In an effort to identify immune gene signatures across the spectrum of AML genotypes and to correlate transcriptomic and proteomic profiles with patient outcomes, he and his colleagues used a pediatric cohort from Children’s Hospital of Philadelphia (median age at diagnosis of 10 years), and an adult cohort from the Technical University of Dresden, Germany (median age at diagnosis, 55.5 years). Bone marrow samples were collected and analyzed at diagnosis.
Hierarchical clustering identified the two distinct clusters. The immune-enriched cluster A had heightened expression of T cells, natural killer cells, and cytotoxic cells, and also expressed CD8A, IFNG, FOXP3, the cell chemoattractants CXCL9 and CXCL10, and inhibitory molecules including IDO1 and the immune checkpoints LAG3, CTLA4, and PD-L1. The immune-depleted cluster B overexpressed genes associated with mast cell functions and CD8 T-cell exhaustion, and showed low expression of T-cell and B-cell genes.
Further analysis of 10 of the inflamed samples from patients with newly diagnosed AML was performed with digital spatial profiling (DSP) to visualize in situ leukemia–immune system interactions, Dr. Vadakekolathu said.
Surface antigens CD123 and CD3 were used as a visualization marker for leukemia cells and to identify bone marrow-infiltrating T cells, respectively. Protein quantification showed a higher concentration of CD3 counts in T-cell-rich versus T-cell-poor areas, and protein expression profiles showed strong correlations with various immunologically relevant molecules. The co-localization of CD8 T cells with FoxP3 Treg cells and PD-L1- and VISTA-expressing cell types evident on DSP represents an immune landscape consistent with the establishment of adaptive immune resistance mechanisms of immune escape, he noted.
The findings suggest immune enriched AMLs might be amenable to combination immunotherapies tailored to the bone marrow tumor microenvironment, such as IDO1 inhibitors and checkpoint blockade, Dr. Vadakekolathu said. “Immune gene expression profiles of AML might support rapid prediction of patient outcomes, discovery of novel immune biomarkers and therapeutic targets, and development of integrated patient stratifications,” he said.
This study was supported by grants from the Roger Counter Foundation and the Qatar National Research Fund. Dr. Vadakekolathu reported having no disclosures. Some authors reported employment or other financial relationships with NanoString Technologies.
sworcester@frontlinemedcom.com
SOURCE: Rutella S et al. ASCO-SITC Abstract 50
SAN FRANCISCO – Immune-enriched acute myeloid leukemias might be amenable to immunotherapy that is tailored to the bone marrow tumor microenvironment, according to findings from a pan-cancer analysis of bone marrow samples.
The analysis, performed with 3D biology technology and an RNA pan-cancer immune profiling panel to characterize bone marrow specimens from 46 children and 28 adults with acute myeloid leukemia (AML), identified heterogeneous immune profiles that correlated with relapse-free survival (RFS) and overall survival (OS), Jayakumar Vadakekolathu, PhD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The specimens, including 63 from nonpromyelocytic de novo AML, 7 from AML in children with complete remission, 3 from adults with secondary AML, and 1 from an adult with treatment-related AML, were analyzed with the nCounter system from NanoString Technologies, and were visualized via digital spatial profiling, said Dr. Vadakekolathu of Nottingham Trent University in England.
The investigators identified two distinct immune gene expression profiles (GEPs) that were largely age-differentiated: Cluster A (myeloid-enriched specimens) included 26 children and 8 adults, and cluster B (myeloid-depleted specimens) included 9 children and 18 adults. These GEPs predicted clinical outcome; relapse free survival was 2.2 months in cluster A versus 18.3 months in cluster B (hazard ratio, 2.58) and overall survival was 6.3 months in cluster A, compared with 22.4 months in cluster B (HR, 2.39), Dr. Vadakekolathu reported.
The findings could have implications for the development of new treatment strategies, he said, noting that AML is a highly heterogeneous disease in terms of genetics, clinical manifestations, and outcome.
“Prognosis is determined by cytogenetic and molecular abnormalities, as well as by response to chemotherapy. De novo AML is cured in roughly 70% of children, 35%-40% of adults, and 5%-15% of elderly patients,” he said. Some patients with AML fail to respond to induction chemotherapy, and others eventually relapse despite the lack of adverse risk factors, he added.
The general therapeutic strategy in patients with AML has not changed substantially in more than 30 years, he said.
“High degrees of molecular complexity in AML present a considerable challenge in clinical implementation, and there is an urgent need to discover better biomarkers to identify high-risk patients before starting chemotherapy, which would enable testing of investigational therapeutic strategies in clinical trials,” he said.
In an effort to identify immune gene signatures across the spectrum of AML genotypes and to correlate transcriptomic and proteomic profiles with patient outcomes, he and his colleagues used a pediatric cohort from Children’s Hospital of Philadelphia (median age at diagnosis of 10 years), and an adult cohort from the Technical University of Dresden, Germany (median age at diagnosis, 55.5 years). Bone marrow samples were collected and analyzed at diagnosis.
Hierarchical clustering identified the two distinct clusters. The immune-enriched cluster A had heightened expression of T cells, natural killer cells, and cytotoxic cells, and also expressed CD8A, IFNG, FOXP3, the cell chemoattractants CXCL9 and CXCL10, and inhibitory molecules including IDO1 and the immune checkpoints LAG3, CTLA4, and PD-L1. The immune-depleted cluster B overexpressed genes associated with mast cell functions and CD8 T-cell exhaustion, and showed low expression of T-cell and B-cell genes.
Further analysis of 10 of the inflamed samples from patients with newly diagnosed AML was performed with digital spatial profiling (DSP) to visualize in situ leukemia–immune system interactions, Dr. Vadakekolathu said.
Surface antigens CD123 and CD3 were used as a visualization marker for leukemia cells and to identify bone marrow-infiltrating T cells, respectively. Protein quantification showed a higher concentration of CD3 counts in T-cell-rich versus T-cell-poor areas, and protein expression profiles showed strong correlations with various immunologically relevant molecules. The co-localization of CD8 T cells with FoxP3 Treg cells and PD-L1- and VISTA-expressing cell types evident on DSP represents an immune landscape consistent with the establishment of adaptive immune resistance mechanisms of immune escape, he noted.
The findings suggest immune enriched AMLs might be amenable to combination immunotherapies tailored to the bone marrow tumor microenvironment, such as IDO1 inhibitors and checkpoint blockade, Dr. Vadakekolathu said. “Immune gene expression profiles of AML might support rapid prediction of patient outcomes, discovery of novel immune biomarkers and therapeutic targets, and development of integrated patient stratifications,” he said.
This study was supported by grants from the Roger Counter Foundation and the Qatar National Research Fund. Dr. Vadakekolathu reported having no disclosures. Some authors reported employment or other financial relationships with NanoString Technologies.
sworcester@frontlinemedcom.com
SOURCE: Rutella S et al. ASCO-SITC Abstract 50
SAN FRANCISCO – Immune-enriched acute myeloid leukemias might be amenable to immunotherapy that is tailored to the bone marrow tumor microenvironment, according to findings from a pan-cancer analysis of bone marrow samples.
The analysis, performed with 3D biology technology and an RNA pan-cancer immune profiling panel to characterize bone marrow specimens from 46 children and 28 adults with acute myeloid leukemia (AML), identified heterogeneous immune profiles that correlated with relapse-free survival (RFS) and overall survival (OS), Jayakumar Vadakekolathu, PhD, reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
The specimens, including 63 from nonpromyelocytic de novo AML, 7 from AML in children with complete remission, 3 from adults with secondary AML, and 1 from an adult with treatment-related AML, were analyzed with the nCounter system from NanoString Technologies, and were visualized via digital spatial profiling, said Dr. Vadakekolathu of Nottingham Trent University in England.
The investigators identified two distinct immune gene expression profiles (GEPs) that were largely age-differentiated: Cluster A (myeloid-enriched specimens) included 26 children and 8 adults, and cluster B (myeloid-depleted specimens) included 9 children and 18 adults. These GEPs predicted clinical outcome; relapse free survival was 2.2 months in cluster A versus 18.3 months in cluster B (hazard ratio, 2.58) and overall survival was 6.3 months in cluster A, compared with 22.4 months in cluster B (HR, 2.39), Dr. Vadakekolathu reported.
The findings could have implications for the development of new treatment strategies, he said, noting that AML is a highly heterogeneous disease in terms of genetics, clinical manifestations, and outcome.
“Prognosis is determined by cytogenetic and molecular abnormalities, as well as by response to chemotherapy. De novo AML is cured in roughly 70% of children, 35%-40% of adults, and 5%-15% of elderly patients,” he said. Some patients with AML fail to respond to induction chemotherapy, and others eventually relapse despite the lack of adverse risk factors, he added.
The general therapeutic strategy in patients with AML has not changed substantially in more than 30 years, he said.
“High degrees of molecular complexity in AML present a considerable challenge in clinical implementation, and there is an urgent need to discover better biomarkers to identify high-risk patients before starting chemotherapy, which would enable testing of investigational therapeutic strategies in clinical trials,” he said.
In an effort to identify immune gene signatures across the spectrum of AML genotypes and to correlate transcriptomic and proteomic profiles with patient outcomes, he and his colleagues used a pediatric cohort from Children’s Hospital of Philadelphia (median age at diagnosis of 10 years), and an adult cohort from the Technical University of Dresden, Germany (median age at diagnosis, 55.5 years). Bone marrow samples were collected and analyzed at diagnosis.
Hierarchical clustering identified the two distinct clusters. The immune-enriched cluster A had heightened expression of T cells, natural killer cells, and cytotoxic cells, and also expressed CD8A, IFNG, FOXP3, the cell chemoattractants CXCL9 and CXCL10, and inhibitory molecules including IDO1 and the immune checkpoints LAG3, CTLA4, and PD-L1. The immune-depleted cluster B overexpressed genes associated with mast cell functions and CD8 T-cell exhaustion, and showed low expression of T-cell and B-cell genes.
Further analysis of 10 of the inflamed samples from patients with newly diagnosed AML was performed with digital spatial profiling (DSP) to visualize in situ leukemia–immune system interactions, Dr. Vadakekolathu said.
Surface antigens CD123 and CD3 were used as a visualization marker for leukemia cells and to identify bone marrow-infiltrating T cells, respectively. Protein quantification showed a higher concentration of CD3 counts in T-cell-rich versus T-cell-poor areas, and protein expression profiles showed strong correlations with various immunologically relevant molecules. The co-localization of CD8 T cells with FoxP3 Treg cells and PD-L1- and VISTA-expressing cell types evident on DSP represents an immune landscape consistent with the establishment of adaptive immune resistance mechanisms of immune escape, he noted.
The findings suggest immune enriched AMLs might be amenable to combination immunotherapies tailored to the bone marrow tumor microenvironment, such as IDO1 inhibitors and checkpoint blockade, Dr. Vadakekolathu said. “Immune gene expression profiles of AML might support rapid prediction of patient outcomes, discovery of novel immune biomarkers and therapeutic targets, and development of integrated patient stratifications,” he said.
This study was supported by grants from the Roger Counter Foundation and the Qatar National Research Fund. Dr. Vadakekolathu reported having no disclosures. Some authors reported employment or other financial relationships with NanoString Technologies.
sworcester@frontlinemedcom.com
SOURCE: Rutella S et al. ASCO-SITC Abstract 50
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYMPOSIUM
Key clinical point:
Major finding: Relapse-free survival was 2.2 months in a cluster of myeloid-enriched specimens, compared with 18.3 months in a cluster of myeloid-depleted specimens (hazard ratio, 2.58).
Study details: A pan-cancer analysis of bone marrow specimens from 74 patients.
Disclosures: This study was supported by grants from the Roger Counter Foundation and the Qatar National Research Fund. Dr. Vadakekolathu reported having no disclosures. Some authors reported employment and other financial relationships with NanoString Technologies.
Source: Rutella S et al. ASCO-SITC Abstract 50.
Maternal short cervix and twins? Consider a pessary
DALLAS – Though cervical pessaries didn’t significantly reduce the rate of births before 34 weeks’ gestation when compared to vaginal progesterone for women with short cervixes and twin pregnancies, neonatal outcomes were improved with pessary use, according to the first randomized controlled trial that directly compared the two therapies.
Of 148 women who received pessaries, 24 (16.2%) delivered before 34 weeks’ gestation, compared with 33 of 149 women (22.1%) who received vaginal progesterone (relative risk 0.73, 95% confidence interval, 0.46-1.18; P = .24). Women who received pessaries were slightly more likely to carry their twin gestations to at least 37 weeks (79/148 versus 91/149; P = .05).
Certain perinatal outcomes were also better among the pessary group. The study measured a composite poor perinatal outcome of neonatal intensive care unit (NICU) admission, intraventricular hemorrhage, respiratory distress syndrome, necrotizing enterocolitis, and neonatal sepsis. This composite poor outcome occurred in 18.6% of the pessary group, compared with 26.5% of the progesterone group (P = .02). Neonatal admissions were more common in the progesterone than in the pessary group (22.1% vs. 13.2%, P = .01)
The odds of having a baby with birth weight less than 2,500 g was also higher for those receiving progesterone, with 22.1% of the progesterone group and 13.2% of the pessary group delivering infants in this range (P less than .001).
Previous work has shown that vaginal progesterone was efficacious when compared with placebo in preventing preterm births for women who have a twin gestation and a short cervix, according to Vinh Dang, MD, the study’s first author. Similarly, he said, studies have shown that placement of a cervical pessary also reduces preterm births in the scenario of twin gestation and a short cervix.
“No randomized controlled trial has directly compared cervical pessary versus vaginal progesterone for the prevention of preterm birth” in this population, Dr. Dang said during a presentation at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Accordingly, he and his colleagues at My Duc Hospital in Ho Chi Minh City, Vietnam, conducted a comparative effectiveness trial to provide a head-to-head comparison of the use of cervical pessaries versus vaginal progesterone for women with twin gestations and short cervixes.
The investigators conducted a single-center, randomized controlled trial, enrolling women with either monochorionic or dichorionic twin pregnancies at between 16 and 22 weeks of gestation, with a cervix length of less than 38 mm, as assessed by two trained ultrasonographers.
At My Duc Hospital, 28.4% of women with twin pregnancies and cervical length less than 38 mm delivered earlier than 34 weeks. This meant that in order to detect a 14% reduction in preterm birth, the study would need to include 290 women for sufficient statistical power, Dr. Dang said.
Patients (n = 300) were randomized 1:1 to receive vaginal progesterone, begun on the day of enrollment, or cervical pessary placed within 1 week of study enrollment. Two patients in the pessary arm and one in the progesterone arm were lost to follow-up.
Patients were permitted to receive other therapies; 14 women in the pessary arm also received antibiotics and 1 received progesterone. In the progesterone group, 12 received antibiotics, and 4 patients were cotreated with cervical cerclage.
The study excluded women under 18 years of age, and those with a prior history of cervical surgery or cervical cerclage. Major known complications in the current pregnancy, such as twin-to-twin transfusion syndrome, major congenital anomaly, fetal demise, and any symptoms of labor or late miscarriage were also grounds for exclusion.
Patient characteristics were similar between groups. The average age was 32 years. Most of the patients (83%-90%) were nulliparous, and more than 90% of the pregnancies were the result of assisted reproduction.
In addition to the primary outcome measure of preterm birth earlier than 34 weeks, the investigators tracked a number of obstetric and neonatal outcomes as secondary endpoints.
For the obstetric measures, Dr. Dang and his colleagues recorded deliveries that occurred before 28, 32, and 27 weeks’ gestation, whether labor was induced and if tocolytics or corticosteroids were used, mode of delivery, and proportion of live births. The number of days of admission for preterm labor, presence of chorioamnionitis, and occurrence of other maternal morbidity were also measured.
Dr. Dang and his colleagues also tracked birth weight, congenital anomalies, and 1- and 5-minute Apgar scores.
A prespecified subgroup analysis of women with even shorter cervixes – less than 28 mm – showed a significant reduction in preterm birth before 34 weeks in addition to the significant improvement in neonatal outcomes.
One author reported being a consultant for ObsEva, Merck, and Guerbet. The other authors had no disclosures.
SOURCE: Dang V et al. The Pregnancy Meeting Abstract LB03
DALLAS – Though cervical pessaries didn’t significantly reduce the rate of births before 34 weeks’ gestation when compared to vaginal progesterone for women with short cervixes and twin pregnancies, neonatal outcomes were improved with pessary use, according to the first randomized controlled trial that directly compared the two therapies.
Of 148 women who received pessaries, 24 (16.2%) delivered before 34 weeks’ gestation, compared with 33 of 149 women (22.1%) who received vaginal progesterone (relative risk 0.73, 95% confidence interval, 0.46-1.18; P = .24). Women who received pessaries were slightly more likely to carry their twin gestations to at least 37 weeks (79/148 versus 91/149; P = .05).
Certain perinatal outcomes were also better among the pessary group. The study measured a composite poor perinatal outcome of neonatal intensive care unit (NICU) admission, intraventricular hemorrhage, respiratory distress syndrome, necrotizing enterocolitis, and neonatal sepsis. This composite poor outcome occurred in 18.6% of the pessary group, compared with 26.5% of the progesterone group (P = .02). Neonatal admissions were more common in the progesterone than in the pessary group (22.1% vs. 13.2%, P = .01)
The odds of having a baby with birth weight less than 2,500 g was also higher for those receiving progesterone, with 22.1% of the progesterone group and 13.2% of the pessary group delivering infants in this range (P less than .001).
Previous work has shown that vaginal progesterone was efficacious when compared with placebo in preventing preterm births for women who have a twin gestation and a short cervix, according to Vinh Dang, MD, the study’s first author. Similarly, he said, studies have shown that placement of a cervical pessary also reduces preterm births in the scenario of twin gestation and a short cervix.
“No randomized controlled trial has directly compared cervical pessary versus vaginal progesterone for the prevention of preterm birth” in this population, Dr. Dang said during a presentation at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Accordingly, he and his colleagues at My Duc Hospital in Ho Chi Minh City, Vietnam, conducted a comparative effectiveness trial to provide a head-to-head comparison of the use of cervical pessaries versus vaginal progesterone for women with twin gestations and short cervixes.
The investigators conducted a single-center, randomized controlled trial, enrolling women with either monochorionic or dichorionic twin pregnancies at between 16 and 22 weeks of gestation, with a cervix length of less than 38 mm, as assessed by two trained ultrasonographers.
At My Duc Hospital, 28.4% of women with twin pregnancies and cervical length less than 38 mm delivered earlier than 34 weeks. This meant that in order to detect a 14% reduction in preterm birth, the study would need to include 290 women for sufficient statistical power, Dr. Dang said.
Patients (n = 300) were randomized 1:1 to receive vaginal progesterone, begun on the day of enrollment, or cervical pessary placed within 1 week of study enrollment. Two patients in the pessary arm and one in the progesterone arm were lost to follow-up.
Patients were permitted to receive other therapies; 14 women in the pessary arm also received antibiotics and 1 received progesterone. In the progesterone group, 12 received antibiotics, and 4 patients were cotreated with cervical cerclage.
The study excluded women under 18 years of age, and those with a prior history of cervical surgery or cervical cerclage. Major known complications in the current pregnancy, such as twin-to-twin transfusion syndrome, major congenital anomaly, fetal demise, and any symptoms of labor or late miscarriage were also grounds for exclusion.
Patient characteristics were similar between groups. The average age was 32 years. Most of the patients (83%-90%) were nulliparous, and more than 90% of the pregnancies were the result of assisted reproduction.
In addition to the primary outcome measure of preterm birth earlier than 34 weeks, the investigators tracked a number of obstetric and neonatal outcomes as secondary endpoints.
For the obstetric measures, Dr. Dang and his colleagues recorded deliveries that occurred before 28, 32, and 27 weeks’ gestation, whether labor was induced and if tocolytics or corticosteroids were used, mode of delivery, and proportion of live births. The number of days of admission for preterm labor, presence of chorioamnionitis, and occurrence of other maternal morbidity were also measured.
Dr. Dang and his colleagues also tracked birth weight, congenital anomalies, and 1- and 5-minute Apgar scores.
A prespecified subgroup analysis of women with even shorter cervixes – less than 28 mm – showed a significant reduction in preterm birth before 34 weeks in addition to the significant improvement in neonatal outcomes.
One author reported being a consultant for ObsEva, Merck, and Guerbet. The other authors had no disclosures.
SOURCE: Dang V et al. The Pregnancy Meeting Abstract LB03
DALLAS – Though cervical pessaries didn’t significantly reduce the rate of births before 34 weeks’ gestation when compared to vaginal progesterone for women with short cervixes and twin pregnancies, neonatal outcomes were improved with pessary use, according to the first randomized controlled trial that directly compared the two therapies.
Of 148 women who received pessaries, 24 (16.2%) delivered before 34 weeks’ gestation, compared with 33 of 149 women (22.1%) who received vaginal progesterone (relative risk 0.73, 95% confidence interval, 0.46-1.18; P = .24). Women who received pessaries were slightly more likely to carry their twin gestations to at least 37 weeks (79/148 versus 91/149; P = .05).
Certain perinatal outcomes were also better among the pessary group. The study measured a composite poor perinatal outcome of neonatal intensive care unit (NICU) admission, intraventricular hemorrhage, respiratory distress syndrome, necrotizing enterocolitis, and neonatal sepsis. This composite poor outcome occurred in 18.6% of the pessary group, compared with 26.5% of the progesterone group (P = .02). Neonatal admissions were more common in the progesterone than in the pessary group (22.1% vs. 13.2%, P = .01)
The odds of having a baby with birth weight less than 2,500 g was also higher for those receiving progesterone, with 22.1% of the progesterone group and 13.2% of the pessary group delivering infants in this range (P less than .001).
Previous work has shown that vaginal progesterone was efficacious when compared with placebo in preventing preterm births for women who have a twin gestation and a short cervix, according to Vinh Dang, MD, the study’s first author. Similarly, he said, studies have shown that placement of a cervical pessary also reduces preterm births in the scenario of twin gestation and a short cervix.
“No randomized controlled trial has directly compared cervical pessary versus vaginal progesterone for the prevention of preterm birth” in this population, Dr. Dang said during a presentation at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine.
Accordingly, he and his colleagues at My Duc Hospital in Ho Chi Minh City, Vietnam, conducted a comparative effectiveness trial to provide a head-to-head comparison of the use of cervical pessaries versus vaginal progesterone for women with twin gestations and short cervixes.
The investigators conducted a single-center, randomized controlled trial, enrolling women with either monochorionic or dichorionic twin pregnancies at between 16 and 22 weeks of gestation, with a cervix length of less than 38 mm, as assessed by two trained ultrasonographers.
At My Duc Hospital, 28.4% of women with twin pregnancies and cervical length less than 38 mm delivered earlier than 34 weeks. This meant that in order to detect a 14% reduction in preterm birth, the study would need to include 290 women for sufficient statistical power, Dr. Dang said.
Patients (n = 300) were randomized 1:1 to receive vaginal progesterone, begun on the day of enrollment, or cervical pessary placed within 1 week of study enrollment. Two patients in the pessary arm and one in the progesterone arm were lost to follow-up.
Patients were permitted to receive other therapies; 14 women in the pessary arm also received antibiotics and 1 received progesterone. In the progesterone group, 12 received antibiotics, and 4 patients were cotreated with cervical cerclage.
The study excluded women under 18 years of age, and those with a prior history of cervical surgery or cervical cerclage. Major known complications in the current pregnancy, such as twin-to-twin transfusion syndrome, major congenital anomaly, fetal demise, and any symptoms of labor or late miscarriage were also grounds for exclusion.
Patient characteristics were similar between groups. The average age was 32 years. Most of the patients (83%-90%) were nulliparous, and more than 90% of the pregnancies were the result of assisted reproduction.
In addition to the primary outcome measure of preterm birth earlier than 34 weeks, the investigators tracked a number of obstetric and neonatal outcomes as secondary endpoints.
For the obstetric measures, Dr. Dang and his colleagues recorded deliveries that occurred before 28, 32, and 27 weeks’ gestation, whether labor was induced and if tocolytics or corticosteroids were used, mode of delivery, and proportion of live births. The number of days of admission for preterm labor, presence of chorioamnionitis, and occurrence of other maternal morbidity were also measured.
Dr. Dang and his colleagues also tracked birth weight, congenital anomalies, and 1- and 5-minute Apgar scores.
A prespecified subgroup analysis of women with even shorter cervixes – less than 28 mm – showed a significant reduction in preterm birth before 34 weeks in addition to the significant improvement in neonatal outcomes.
One author reported being a consultant for ObsEva, Merck, and Guerbet. The other authors had no disclosures.
SOURCE: Dang V et al. The Pregnancy Meeting Abstract LB03
REPORTING FROM THE PREGNANCY MEETING
Key clinical point:
Major finding: Poor neonatal outcome occurred in 18.6% of those receiving pessary, compared with 26.5% of those receiving vaginal progesterone (P = .02).
Study details: Randomized controlled trial of cervical pessary versus vaginal progesterone for women with twin gestation and cervical length less than 38 mm.
Disclosures: One author reported funding from Obseva, Merck, and Guerbet. The other authors reported no conflicts of interest.
Source: Dang V et al. The Pregnancy Meeting Abstract LB03.
Triple therapy ups response in refractory mantle cell lymphoma
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The overall response from for the combination of the three drugs was 76% at 17.8 months median follow-up.
Study details: An open-label, single-arm, phase 2 trial of 50 adults with relapsed/refractory MCL.
Disclosures: Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
Source: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
MedPAC mulls boost to payments for E&M visits
WASHINGTON – Members of the Medicare Payment Advisory Commission, which advises Congress, are weighing whether to recommend a 10% increase in payments for evaluation and management services.
The boost would be offset by a 4.5% decrease in all other payments on the Medicare physician fee schedule.
“The fee schedule underprices ambulatory [evaluation and management] services relative to other services,” MedPAC staff member Ariel Winter said during a Jan. 12 presentation of a draft proposal.
Payments in the physician fee schedule are based on the relative amount of time and intensity required for each service, she noted. “E&M services are labor intensive,” she said. “A clinician takes the patient’s history, examines the patient, engages in medical decision-making and so forth. These activities do not lend themselves to reductions in the time it takes to provide the visit.”
In contrast, “the time needed for other services, such as procedures, often declines over time due to productivity gains in clinical practice and technology,” Ms. Winter continued. “Ideally, the prices for these services would also be reduced to reflect these efficiency gains.”
Ms. Winter pointed out that the payment reduction that captures the efficiency gains rarely happens, and because of budget neutral requirements, it means that “payment rates for ambulatory E&M services are too low relative to other services.”
MedPAC looked at the fee schedule time estimates across four specialties (cardiology, family medicine, orthopedics, and urology) and found that across the board, the fee schedule assumed it took the specialist more time than what was actually needed.
“However, the discrepancy was much greater for the practices that focus on procedures, which suggests that the services they provide may be based on inflated time estimates,” Ms. Winter said. “For example, the hours assumed in the fee schedule were 24% higher than actual hours worked for family medicine, but 64% higher in cardiology and 92% higher in orthopedics.”
To account for this, and psychiatric services, regardless of specialty, resulting in an increase of $2.7 billion in spending on these codes. That would force a reduction of 4.5% in payments for everything else in the fee schedule under budget neutrality requirements.
Based on this change, licensed clinical social workers would see the full 10% increase to their physician fee schedule payments and clinical psychologists would see an 8% increase, based on 2016 Medicare claims. Endocrinology would see a 6.5% increase, family practice a 5.7% increase, and rheumatology a 5.4% increase.
Of the specialties displayed on a presentation slide that showed an increase, internal medicine fared the worst, with a 2% increase. “That is because this specialty performs a lot of services other than ambulatory E&M” and the 4.5% reduction in those other services would have an offsetting effect, Ms. Winter said.
Ms. Winter noted that a number of specialties would see a reduction in their physician fee schedule payments greater than 4% “because they provide very few E&M or psychiatric services, and these include radiology, pathology, physical therapy, and occupational therapy.”
Another option presented to the MedPAC commissioners was offering a special incentive payment equal to a 10% increase to clinicians who bill 60% of their claims for E&M services. That idea, which is similar to the now-expired primary care incentive payment program, would require a 1.7% reduction in other services to maintain budget neutrality.
But MedPAC commissioners questioned the overall aim of this type of payment rebalancing.
“Are we hoping that enhancing payments for primary care will actually improve coordination of care and management of patients?” asked Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson. “I don’t think either of these options really does that. So that leads me to ask the question, ‘should we be thinking more about that issue?’”
Commissioner David Nerenz, PhD, director of the Center for Health Policy and Health Services Research at the Henry Ford Health System in Detroit, pointed out that a 10% uptick is not going to solve the problem of payment disparities. “And that doesn’t mean it should not be done. It just means that other things have to be considered and addressed at the same time if ultimately we are talking about people’s choice, for example, to go under primary care.”
Dr. Nerenz also noted that providers such as physical therapists and occupational therapists have to spend their time in face-to-face settings in the same way E&M visits are structured, but because it is billed as a procedure, they would get penalized under the draft proposal.
“I have the same concern about a procedure like colonoscopy,” he added. “You know, for the appropriate people, it’s a lifesaving procedure. Should that payment go down 4.5%? I don’t know.”
Commissioner Rita Redberg, MD, of the University of California San Francisco, suggested that some of these issues would go away on their own as physicians move into advanced alternative payment models in the Quality Payment Program, something MedPAC hopes will happen if their repeal and replacement of the Merit-based Incentive Payment System portion of the Quality Payment Program is adopted.
Commissioner Dana Gelb Safran, ScD, of Blue Cross Blue Shield of Massachusetts, looked at payment adequacy from three perspectives: better equity, better access, and better alignment with value. “I guess on all three counts, I am finding both of these approaches coming up short,” Dr. Safran said. “It maybe helps the most with equity, but then there’s the challenge about how payment actually gets shaped by the organizations providers are a part of,” she said, referring to whether employed physicians would see any benefit trickle down to them in the form of increased salary and benefits. “So I struggle with whether it even accomplished that, but maybe it’s not a bad idea for that.”
MedPAC is in the early stages of developing a recommendation on this subject and more discussions are expected at upcoming meetings. The next scheduled meeting is in March 2018.
WASHINGTON – Members of the Medicare Payment Advisory Commission, which advises Congress, are weighing whether to recommend a 10% increase in payments for evaluation and management services.
The boost would be offset by a 4.5% decrease in all other payments on the Medicare physician fee schedule.
“The fee schedule underprices ambulatory [evaluation and management] services relative to other services,” MedPAC staff member Ariel Winter said during a Jan. 12 presentation of a draft proposal.
Payments in the physician fee schedule are based on the relative amount of time and intensity required for each service, she noted. “E&M services are labor intensive,” she said. “A clinician takes the patient’s history, examines the patient, engages in medical decision-making and so forth. These activities do not lend themselves to reductions in the time it takes to provide the visit.”
In contrast, “the time needed for other services, such as procedures, often declines over time due to productivity gains in clinical practice and technology,” Ms. Winter continued. “Ideally, the prices for these services would also be reduced to reflect these efficiency gains.”
Ms. Winter pointed out that the payment reduction that captures the efficiency gains rarely happens, and because of budget neutral requirements, it means that “payment rates for ambulatory E&M services are too low relative to other services.”
MedPAC looked at the fee schedule time estimates across four specialties (cardiology, family medicine, orthopedics, and urology) and found that across the board, the fee schedule assumed it took the specialist more time than what was actually needed.
“However, the discrepancy was much greater for the practices that focus on procedures, which suggests that the services they provide may be based on inflated time estimates,” Ms. Winter said. “For example, the hours assumed in the fee schedule were 24% higher than actual hours worked for family medicine, but 64% higher in cardiology and 92% higher in orthopedics.”
To account for this, and psychiatric services, regardless of specialty, resulting in an increase of $2.7 billion in spending on these codes. That would force a reduction of 4.5% in payments for everything else in the fee schedule under budget neutrality requirements.
Based on this change, licensed clinical social workers would see the full 10% increase to their physician fee schedule payments and clinical psychologists would see an 8% increase, based on 2016 Medicare claims. Endocrinology would see a 6.5% increase, family practice a 5.7% increase, and rheumatology a 5.4% increase.
Of the specialties displayed on a presentation slide that showed an increase, internal medicine fared the worst, with a 2% increase. “That is because this specialty performs a lot of services other than ambulatory E&M” and the 4.5% reduction in those other services would have an offsetting effect, Ms. Winter said.
Ms. Winter noted that a number of specialties would see a reduction in their physician fee schedule payments greater than 4% “because they provide very few E&M or psychiatric services, and these include radiology, pathology, physical therapy, and occupational therapy.”
Another option presented to the MedPAC commissioners was offering a special incentive payment equal to a 10% increase to clinicians who bill 60% of their claims for E&M services. That idea, which is similar to the now-expired primary care incentive payment program, would require a 1.7% reduction in other services to maintain budget neutrality.
But MedPAC commissioners questioned the overall aim of this type of payment rebalancing.
“Are we hoping that enhancing payments for primary care will actually improve coordination of care and management of patients?” asked Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson. “I don’t think either of these options really does that. So that leads me to ask the question, ‘should we be thinking more about that issue?’”
Commissioner David Nerenz, PhD, director of the Center for Health Policy and Health Services Research at the Henry Ford Health System in Detroit, pointed out that a 10% uptick is not going to solve the problem of payment disparities. “And that doesn’t mean it should not be done. It just means that other things have to be considered and addressed at the same time if ultimately we are talking about people’s choice, for example, to go under primary care.”
Dr. Nerenz also noted that providers such as physical therapists and occupational therapists have to spend their time in face-to-face settings in the same way E&M visits are structured, but because it is billed as a procedure, they would get penalized under the draft proposal.
“I have the same concern about a procedure like colonoscopy,” he added. “You know, for the appropriate people, it’s a lifesaving procedure. Should that payment go down 4.5%? I don’t know.”
Commissioner Rita Redberg, MD, of the University of California San Francisco, suggested that some of these issues would go away on their own as physicians move into advanced alternative payment models in the Quality Payment Program, something MedPAC hopes will happen if their repeal and replacement of the Merit-based Incentive Payment System portion of the Quality Payment Program is adopted.
Commissioner Dana Gelb Safran, ScD, of Blue Cross Blue Shield of Massachusetts, looked at payment adequacy from three perspectives: better equity, better access, and better alignment with value. “I guess on all three counts, I am finding both of these approaches coming up short,” Dr. Safran said. “It maybe helps the most with equity, but then there’s the challenge about how payment actually gets shaped by the organizations providers are a part of,” she said, referring to whether employed physicians would see any benefit trickle down to them in the form of increased salary and benefits. “So I struggle with whether it even accomplished that, but maybe it’s not a bad idea for that.”
MedPAC is in the early stages of developing a recommendation on this subject and more discussions are expected at upcoming meetings. The next scheduled meeting is in March 2018.
WASHINGTON – Members of the Medicare Payment Advisory Commission, which advises Congress, are weighing whether to recommend a 10% increase in payments for evaluation and management services.
The boost would be offset by a 4.5% decrease in all other payments on the Medicare physician fee schedule.
“The fee schedule underprices ambulatory [evaluation and management] services relative to other services,” MedPAC staff member Ariel Winter said during a Jan. 12 presentation of a draft proposal.
Payments in the physician fee schedule are based on the relative amount of time and intensity required for each service, she noted. “E&M services are labor intensive,” she said. “A clinician takes the patient’s history, examines the patient, engages in medical decision-making and so forth. These activities do not lend themselves to reductions in the time it takes to provide the visit.”
In contrast, “the time needed for other services, such as procedures, often declines over time due to productivity gains in clinical practice and technology,” Ms. Winter continued. “Ideally, the prices for these services would also be reduced to reflect these efficiency gains.”
Ms. Winter pointed out that the payment reduction that captures the efficiency gains rarely happens, and because of budget neutral requirements, it means that “payment rates for ambulatory E&M services are too low relative to other services.”
MedPAC looked at the fee schedule time estimates across four specialties (cardiology, family medicine, orthopedics, and urology) and found that across the board, the fee schedule assumed it took the specialist more time than what was actually needed.
“However, the discrepancy was much greater for the practices that focus on procedures, which suggests that the services they provide may be based on inflated time estimates,” Ms. Winter said. “For example, the hours assumed in the fee schedule were 24% higher than actual hours worked for family medicine, but 64% higher in cardiology and 92% higher in orthopedics.”
To account for this, and psychiatric services, regardless of specialty, resulting in an increase of $2.7 billion in spending on these codes. That would force a reduction of 4.5% in payments for everything else in the fee schedule under budget neutrality requirements.
Based on this change, licensed clinical social workers would see the full 10% increase to their physician fee schedule payments and clinical psychologists would see an 8% increase, based on 2016 Medicare claims. Endocrinology would see a 6.5% increase, family practice a 5.7% increase, and rheumatology a 5.4% increase.
Of the specialties displayed on a presentation slide that showed an increase, internal medicine fared the worst, with a 2% increase. “That is because this specialty performs a lot of services other than ambulatory E&M” and the 4.5% reduction in those other services would have an offsetting effect, Ms. Winter said.
Ms. Winter noted that a number of specialties would see a reduction in their physician fee schedule payments greater than 4% “because they provide very few E&M or psychiatric services, and these include radiology, pathology, physical therapy, and occupational therapy.”
Another option presented to the MedPAC commissioners was offering a special incentive payment equal to a 10% increase to clinicians who bill 60% of their claims for E&M services. That idea, which is similar to the now-expired primary care incentive payment program, would require a 1.7% reduction in other services to maintain budget neutrality.
But MedPAC commissioners questioned the overall aim of this type of payment rebalancing.
“Are we hoping that enhancing payments for primary care will actually improve coordination of care and management of patients?” asked Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson. “I don’t think either of these options really does that. So that leads me to ask the question, ‘should we be thinking more about that issue?’”
Commissioner David Nerenz, PhD, director of the Center for Health Policy and Health Services Research at the Henry Ford Health System in Detroit, pointed out that a 10% uptick is not going to solve the problem of payment disparities. “And that doesn’t mean it should not be done. It just means that other things have to be considered and addressed at the same time if ultimately we are talking about people’s choice, for example, to go under primary care.”
Dr. Nerenz also noted that providers such as physical therapists and occupational therapists have to spend their time in face-to-face settings in the same way E&M visits are structured, but because it is billed as a procedure, they would get penalized under the draft proposal.
“I have the same concern about a procedure like colonoscopy,” he added. “You know, for the appropriate people, it’s a lifesaving procedure. Should that payment go down 4.5%? I don’t know.”
Commissioner Rita Redberg, MD, of the University of California San Francisco, suggested that some of these issues would go away on their own as physicians move into advanced alternative payment models in the Quality Payment Program, something MedPAC hopes will happen if their repeal and replacement of the Merit-based Incentive Payment System portion of the Quality Payment Program is adopted.
Commissioner Dana Gelb Safran, ScD, of Blue Cross Blue Shield of Massachusetts, looked at payment adequacy from three perspectives: better equity, better access, and better alignment with value. “I guess on all three counts, I am finding both of these approaches coming up short,” Dr. Safran said. “It maybe helps the most with equity, but then there’s the challenge about how payment actually gets shaped by the organizations providers are a part of,” she said, referring to whether employed physicians would see any benefit trickle down to them in the form of increased salary and benefits. “So I struggle with whether it even accomplished that, but maybe it’s not a bad idea for that.”
MedPAC is in the early stages of developing a recommendation on this subject and more discussions are expected at upcoming meetings. The next scheduled meeting is in March 2018.
REPORTING FROM A MEDPAC MEETING
Latest PANS trials suggest cause may be treatable
NEW YORK – New controlled trials in the treatment of pediatric acute neuropsychiatric syndrome (PANS) support the hypothesis that the cause may, in fact, be treatable, an expert said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
PANS and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) are controversial diagnoses. But ongoing research has expanded the body of literature describing symptoms and evaluating treatments, according to an overview from Barbara J. Coffey, MD, division chief in child and adolescent psychiatry at the University of Miami.
PANS and PANDAS, which are clinical diagnoses of exclusion, are characterized by an abrupt onset of one or more neuropsychiatric symptoms in children that can include signs of obsessive-compulsive disorder (OCD), tics, anxiety, irritability, and behavioral changes. Despite more than 15 years of clinical and experimental studies, almost all aspects of PANS and PANDAS remain controversial, Dr. Coffey said.
“Many pediatricians I work with do not want to see these kids, because they really do not know what to do,” she reported.
This prevailing conception has led to treatment trials with antibiotics for the underlying infection and, most recently, with intravenous immunoglobulins (IVIG) to prevent cross-reactive antibodies induced by infection. Dr. Coffey, reviewing several recently published studies, noted that there have been signals of activity suggesting “there may be something there.” However, she cautioned that the data are inconclusive.
Of recent studies, a trial with azithromycin in children with PANS generated somewhat positive findings (J Child Adolesc Psychopharmacol. 2017;27[7]:640-51). In this study, 31 children aged 4-14 years were randomized to receive azithromycin or placebo for 4 weeks. They were evaluated via the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS) and the OCD Clinical Global Impression Severity (CGI-S OCD) scale.
The study associated azithromycin with a significant reduction in symptoms as measured with CGI-S OCD (41.2% vs. 7.1%; P = .045), but no difference was found in response as measured with the CY-BOCS, which Dr. Coffey described as the more conservative measure. Even when measured with the CGI-S OCD scale, symptom improvements were modest. But the authors concluded that azithromycin “may be helpful” for the control of neuropsychiatric symptoms.
In a trial with IVIG, 35 children with PANDAS were randomized to IVIG or placebo (J Am Acad Child Adolesc Psychiatry. 2016;55[10]:860-7). The primary outcome was at least a 30% reduction in CY-BOCS at 12 weeks. Nonresponders at 12 weeks were permitted 12 more weeks of treatment with open-label IVIG.
Twice as many patients receiving IVIG, compared with those on placebo, reached the primary outcome (24% vs. 12%), but the study was small and the difference did not reach significance. However, the authors reported that the mean CY-BOCS improvement from baseline on IVIG, which was well tolerated, was 55% at week 12 and 62% at week 24. The authors suggested that larger trials are warranted.
“One critique of this study is that all of the patients were placed on prophylactic antibiotics, which may have attenuated the response,” Dr. Coffey said. In outlining these results, she emphasized: “This is not something I am recommending. I just want to acquaint you with what is going on out there.”
None of the most recent trials provide conclusive support for therapy targeted at infection or an autoimmune process in PANS or PANDAS, but Dr. Coffey indicated that . One of the most recent sets of data come from a recently published Scandinavian cohort study with 17 years of follow-up (JAMA Psychiatry. 2017;74[7]:740-6). That study associated pediatric infections of any kind, not just streptococcal infections, with an elevated risk of mental disorders – particularly OCD and tics.
Overall, the evidence base is growing to suggest “antibiotics and immune therapy may be beneficial, but the jury is not in yet for which patients, how much, and how long,” Dr. Coffey said. She is aware of clinicians who are now using antibiotics empirically to control neuropsychiatric symptoms in PANDAS but cautioned that more investigation is needed.
Dr. Coffey reports financial relationships with Genco Sciences, Neurocrine Biosciences, Shire, and Teva/Nuvelution.
NEW YORK – New controlled trials in the treatment of pediatric acute neuropsychiatric syndrome (PANS) support the hypothesis that the cause may, in fact, be treatable, an expert said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
PANS and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) are controversial diagnoses. But ongoing research has expanded the body of literature describing symptoms and evaluating treatments, according to an overview from Barbara J. Coffey, MD, division chief in child and adolescent psychiatry at the University of Miami.
PANS and PANDAS, which are clinical diagnoses of exclusion, are characterized by an abrupt onset of one or more neuropsychiatric symptoms in children that can include signs of obsessive-compulsive disorder (OCD), tics, anxiety, irritability, and behavioral changes. Despite more than 15 years of clinical and experimental studies, almost all aspects of PANS and PANDAS remain controversial, Dr. Coffey said.
“Many pediatricians I work with do not want to see these kids, because they really do not know what to do,” she reported.
This prevailing conception has led to treatment trials with antibiotics for the underlying infection and, most recently, with intravenous immunoglobulins (IVIG) to prevent cross-reactive antibodies induced by infection. Dr. Coffey, reviewing several recently published studies, noted that there have been signals of activity suggesting “there may be something there.” However, she cautioned that the data are inconclusive.
Of recent studies, a trial with azithromycin in children with PANS generated somewhat positive findings (J Child Adolesc Psychopharmacol. 2017;27[7]:640-51). In this study, 31 children aged 4-14 years were randomized to receive azithromycin or placebo for 4 weeks. They were evaluated via the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS) and the OCD Clinical Global Impression Severity (CGI-S OCD) scale.
The study associated azithromycin with a significant reduction in symptoms as measured with CGI-S OCD (41.2% vs. 7.1%; P = .045), but no difference was found in response as measured with the CY-BOCS, which Dr. Coffey described as the more conservative measure. Even when measured with the CGI-S OCD scale, symptom improvements were modest. But the authors concluded that azithromycin “may be helpful” for the control of neuropsychiatric symptoms.
In a trial with IVIG, 35 children with PANDAS were randomized to IVIG or placebo (J Am Acad Child Adolesc Psychiatry. 2016;55[10]:860-7). The primary outcome was at least a 30% reduction in CY-BOCS at 12 weeks. Nonresponders at 12 weeks were permitted 12 more weeks of treatment with open-label IVIG.
Twice as many patients receiving IVIG, compared with those on placebo, reached the primary outcome (24% vs. 12%), but the study was small and the difference did not reach significance. However, the authors reported that the mean CY-BOCS improvement from baseline on IVIG, which was well tolerated, was 55% at week 12 and 62% at week 24. The authors suggested that larger trials are warranted.
“One critique of this study is that all of the patients were placed on prophylactic antibiotics, which may have attenuated the response,” Dr. Coffey said. In outlining these results, she emphasized: “This is not something I am recommending. I just want to acquaint you with what is going on out there.”
None of the most recent trials provide conclusive support for therapy targeted at infection or an autoimmune process in PANS or PANDAS, but Dr. Coffey indicated that . One of the most recent sets of data come from a recently published Scandinavian cohort study with 17 years of follow-up (JAMA Psychiatry. 2017;74[7]:740-6). That study associated pediatric infections of any kind, not just streptococcal infections, with an elevated risk of mental disorders – particularly OCD and tics.
Overall, the evidence base is growing to suggest “antibiotics and immune therapy may be beneficial, but the jury is not in yet for which patients, how much, and how long,” Dr. Coffey said. She is aware of clinicians who are now using antibiotics empirically to control neuropsychiatric symptoms in PANDAS but cautioned that more investigation is needed.
Dr. Coffey reports financial relationships with Genco Sciences, Neurocrine Biosciences, Shire, and Teva/Nuvelution.
NEW YORK – New controlled trials in the treatment of pediatric acute neuropsychiatric syndrome (PANS) support the hypothesis that the cause may, in fact, be treatable, an expert said at a pediatric psychopharmacology update held by the American Academy of Child and Adolescent Psychiatry.
PANS and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS) are controversial diagnoses. But ongoing research has expanded the body of literature describing symptoms and evaluating treatments, according to an overview from Barbara J. Coffey, MD, division chief in child and adolescent psychiatry at the University of Miami.
PANS and PANDAS, which are clinical diagnoses of exclusion, are characterized by an abrupt onset of one or more neuropsychiatric symptoms in children that can include signs of obsessive-compulsive disorder (OCD), tics, anxiety, irritability, and behavioral changes. Despite more than 15 years of clinical and experimental studies, almost all aspects of PANS and PANDAS remain controversial, Dr. Coffey said.
“Many pediatricians I work with do not want to see these kids, because they really do not know what to do,” she reported.
This prevailing conception has led to treatment trials with antibiotics for the underlying infection and, most recently, with intravenous immunoglobulins (IVIG) to prevent cross-reactive antibodies induced by infection. Dr. Coffey, reviewing several recently published studies, noted that there have been signals of activity suggesting “there may be something there.” However, she cautioned that the data are inconclusive.
Of recent studies, a trial with azithromycin in children with PANS generated somewhat positive findings (J Child Adolesc Psychopharmacol. 2017;27[7]:640-51). In this study, 31 children aged 4-14 years were randomized to receive azithromycin or placebo for 4 weeks. They were evaluated via the Children’s Yale–Brown Obsessive Compulsive Scale (CY-BOCS) and the OCD Clinical Global Impression Severity (CGI-S OCD) scale.
The study associated azithromycin with a significant reduction in symptoms as measured with CGI-S OCD (41.2% vs. 7.1%; P = .045), but no difference was found in response as measured with the CY-BOCS, which Dr. Coffey described as the more conservative measure. Even when measured with the CGI-S OCD scale, symptom improvements were modest. But the authors concluded that azithromycin “may be helpful” for the control of neuropsychiatric symptoms.
In a trial with IVIG, 35 children with PANDAS were randomized to IVIG or placebo (J Am Acad Child Adolesc Psychiatry. 2016;55[10]:860-7). The primary outcome was at least a 30% reduction in CY-BOCS at 12 weeks. Nonresponders at 12 weeks were permitted 12 more weeks of treatment with open-label IVIG.
Twice as many patients receiving IVIG, compared with those on placebo, reached the primary outcome (24% vs. 12%), but the study was small and the difference did not reach significance. However, the authors reported that the mean CY-BOCS improvement from baseline on IVIG, which was well tolerated, was 55% at week 12 and 62% at week 24. The authors suggested that larger trials are warranted.
“One critique of this study is that all of the patients were placed on prophylactic antibiotics, which may have attenuated the response,” Dr. Coffey said. In outlining these results, she emphasized: “This is not something I am recommending. I just want to acquaint you with what is going on out there.”
None of the most recent trials provide conclusive support for therapy targeted at infection or an autoimmune process in PANS or PANDAS, but Dr. Coffey indicated that . One of the most recent sets of data come from a recently published Scandinavian cohort study with 17 years of follow-up (JAMA Psychiatry. 2017;74[7]:740-6). That study associated pediatric infections of any kind, not just streptococcal infections, with an elevated risk of mental disorders – particularly OCD and tics.
Overall, the evidence base is growing to suggest “antibiotics and immune therapy may be beneficial, but the jury is not in yet for which patients, how much, and how long,” Dr. Coffey said. She is aware of clinicians who are now using antibiotics empirically to control neuropsychiatric symptoms in PANDAS but cautioned that more investigation is needed.
Dr. Coffey reports financial relationships with Genco Sciences, Neurocrine Biosciences, Shire, and Teva/Nuvelution.
REPORTING FROM THE PSYCHOPHARMACOLOGY UPDATE INSTITUTE
Insights revealed into rheumatologist-patient discussions about biologics
A small study of the discussions of biologic treatment options between rheumatoid arthritis patients and their rheumatologists suggests they are limited in scope and may be influenced by clinicians’ preconceived notions about patient preferences.
In an analysis of video footage from office visits of 48 rheumatoid arthritis (RA) patients initiating biologics with 16 rheumatologists, only 5.6 minutes out of visits with an average duration of 15 minutes were spent discussing biologic therapy options. Postvisit interviews with patients highlighted problems in rheumatologists’ awareness of patients’ familiarity with biologic treatment options, particularly a lack of knowledge about intravenous (IV) options, according to findings from the ethnographic, observational study conducted by Nicholas Kottak, PhD, of Ethnographic Solutions LLC, and his colleagues and published in Arthritis Care & Research.
Of the 48 visits, the researchers found that subcutaneous (SC) therapy options were discussed in 45 visits and IV options were discussed in 35. In 27 of the 35 visits in which IV options were mentioned, the rheumatologists did not define or describe IV administration.
In postvisit interviews, all patients said they were previously aware that biologic therapy was available subcutaneously, but 22 entered their visit with no previous knowledge of IV options, 19 of whom remained unfamiliar after their visit.
According to the researchers, some patients said that they would prefer to receive IV biologic treatment and some would prefer SC delivery. However, all 16 participating rheumatologists thought that IV therapy would be less convenient for the patient than SC therapy, but only 22 of 48 patients said inconvenience was the main barrier to IV therapy. A total of 23 patients thought SC therapy would be easier, 17 thought IV therapy would be easier, 4 thought they would be the same, and 4 were unsure.
The way in which mode of biologic administration is discussed may also have financial implications, particularly for Medicare patients. “During 26 visits with Medicare patients, IV and SC therapies were mentioned during 22 and 23 visits, respectively. This is noteworthy as Medicare with supplemental insurance may preferably cover IV over SC therapy, since IV therapy qualifies as a medical benefit (Part B) and SC therapy falls under pharmacy benefits (Part D),” Dr. Kottak and his associates wrote.
• Preparing patients for biologic therapy at an earlier disease stage.
• Providing educational tools such as pamphlets and flyers.
• Increasing time spent with the patient and/or including nurse practitioners and physician assistants in the follow-up portion of the patient visit.
• Creating a treatment alliance with a patient to make the process more collaborative.
• Allowing patients to choose which biologic medication they start with while emphasizing the potential for positive outcomes and relative safety.
• Researching shared decision making between patients and physicians in a randomized, outcomes-based setting.
Janssen provided funding for the study as well as the writing of the report. Ethnographic Solutions received financial compensation from Janssen. Three of the six authors are employees of Janssen. The other researchers reported various financial disclosures, including, but not limited to, stock ownership, speaking fees, and advisory board positions.
SOURCE: Kottak N et al. Arthritis Care Res. 2018 Feb 5. doi: 10/1002/acr.23527
A small study of the discussions of biologic treatment options between rheumatoid arthritis patients and their rheumatologists suggests they are limited in scope and may be influenced by clinicians’ preconceived notions about patient preferences.
In an analysis of video footage from office visits of 48 rheumatoid arthritis (RA) patients initiating biologics with 16 rheumatologists, only 5.6 minutes out of visits with an average duration of 15 minutes were spent discussing biologic therapy options. Postvisit interviews with patients highlighted problems in rheumatologists’ awareness of patients’ familiarity with biologic treatment options, particularly a lack of knowledge about intravenous (IV) options, according to findings from the ethnographic, observational study conducted by Nicholas Kottak, PhD, of Ethnographic Solutions LLC, and his colleagues and published in Arthritis Care & Research.
Of the 48 visits, the researchers found that subcutaneous (SC) therapy options were discussed in 45 visits and IV options were discussed in 35. In 27 of the 35 visits in which IV options were mentioned, the rheumatologists did not define or describe IV administration.
In postvisit interviews, all patients said they were previously aware that biologic therapy was available subcutaneously, but 22 entered their visit with no previous knowledge of IV options, 19 of whom remained unfamiliar after their visit.
According to the researchers, some patients said that they would prefer to receive IV biologic treatment and some would prefer SC delivery. However, all 16 participating rheumatologists thought that IV therapy would be less convenient for the patient than SC therapy, but only 22 of 48 patients said inconvenience was the main barrier to IV therapy. A total of 23 patients thought SC therapy would be easier, 17 thought IV therapy would be easier, 4 thought they would be the same, and 4 were unsure.
The way in which mode of biologic administration is discussed may also have financial implications, particularly for Medicare patients. “During 26 visits with Medicare patients, IV and SC therapies were mentioned during 22 and 23 visits, respectively. This is noteworthy as Medicare with supplemental insurance may preferably cover IV over SC therapy, since IV therapy qualifies as a medical benefit (Part B) and SC therapy falls under pharmacy benefits (Part D),” Dr. Kottak and his associates wrote.
• Preparing patients for biologic therapy at an earlier disease stage.
• Providing educational tools such as pamphlets and flyers.
• Increasing time spent with the patient and/or including nurse practitioners and physician assistants in the follow-up portion of the patient visit.
• Creating a treatment alliance with a patient to make the process more collaborative.
• Allowing patients to choose which biologic medication they start with while emphasizing the potential for positive outcomes and relative safety.
• Researching shared decision making between patients and physicians in a randomized, outcomes-based setting.
Janssen provided funding for the study as well as the writing of the report. Ethnographic Solutions received financial compensation from Janssen. Three of the six authors are employees of Janssen. The other researchers reported various financial disclosures, including, but not limited to, stock ownership, speaking fees, and advisory board positions.
SOURCE: Kottak N et al. Arthritis Care Res. 2018 Feb 5. doi: 10/1002/acr.23527
A small study of the discussions of biologic treatment options between rheumatoid arthritis patients and their rheumatologists suggests they are limited in scope and may be influenced by clinicians’ preconceived notions about patient preferences.
In an analysis of video footage from office visits of 48 rheumatoid arthritis (RA) patients initiating biologics with 16 rheumatologists, only 5.6 minutes out of visits with an average duration of 15 minutes were spent discussing biologic therapy options. Postvisit interviews with patients highlighted problems in rheumatologists’ awareness of patients’ familiarity with biologic treatment options, particularly a lack of knowledge about intravenous (IV) options, according to findings from the ethnographic, observational study conducted by Nicholas Kottak, PhD, of Ethnographic Solutions LLC, and his colleagues and published in Arthritis Care & Research.
Of the 48 visits, the researchers found that subcutaneous (SC) therapy options were discussed in 45 visits and IV options were discussed in 35. In 27 of the 35 visits in which IV options were mentioned, the rheumatologists did not define or describe IV administration.
In postvisit interviews, all patients said they were previously aware that biologic therapy was available subcutaneously, but 22 entered their visit with no previous knowledge of IV options, 19 of whom remained unfamiliar after their visit.
According to the researchers, some patients said that they would prefer to receive IV biologic treatment and some would prefer SC delivery. However, all 16 participating rheumatologists thought that IV therapy would be less convenient for the patient than SC therapy, but only 22 of 48 patients said inconvenience was the main barrier to IV therapy. A total of 23 patients thought SC therapy would be easier, 17 thought IV therapy would be easier, 4 thought they would be the same, and 4 were unsure.
The way in which mode of biologic administration is discussed may also have financial implications, particularly for Medicare patients. “During 26 visits with Medicare patients, IV and SC therapies were mentioned during 22 and 23 visits, respectively. This is noteworthy as Medicare with supplemental insurance may preferably cover IV over SC therapy, since IV therapy qualifies as a medical benefit (Part B) and SC therapy falls under pharmacy benefits (Part D),” Dr. Kottak and his associates wrote.
• Preparing patients for biologic therapy at an earlier disease stage.
• Providing educational tools such as pamphlets and flyers.
• Increasing time spent with the patient and/or including nurse practitioners and physician assistants in the follow-up portion of the patient visit.
• Creating a treatment alliance with a patient to make the process more collaborative.
• Allowing patients to choose which biologic medication they start with while emphasizing the potential for positive outcomes and relative safety.
• Researching shared decision making between patients and physicians in a randomized, outcomes-based setting.
Janssen provided funding for the study as well as the writing of the report. Ethnographic Solutions received financial compensation from Janssen. Three of the six authors are employees of Janssen. The other researchers reported various financial disclosures, including, but not limited to, stock ownership, speaking fees, and advisory board positions.
SOURCE: Kottak N et al. Arthritis Care Res. 2018 Feb 5. doi: 10/1002/acr.23527
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Provide educational resources about biologics and don’t assume that subcutaneous therapy is more convenient or that patients understand IV therapy terminology and procedures.
Major finding: All 16 participating rheumatologists thought that intravenous therapy would be less convenient for the patient than subcutaneous therapy, but only 22 of 48 patients said inconvenience was the main barrier to IV therapy.
Study details: An ethnographic, observational study of 48 patients and 16 rheumatologists.
Disclosures: Janssen Pharmaceuticals funded the research. Dr. Kottak is president of Ethnographic Solutions LLC, which received compensation from Janssen Pharmaceuticals for this project.
Source: Kottak N et al. Arthritis Care Res. 2018 Feb 5. doi: 10/1002/acr.23527
Drug combo indicated for bacterial pneumonia
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
(Avycaz) to include hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP) in adults.
Specifically, the approved indication is for infections caused by certain Gram-negative bacteria – some of which are increasingly resistant to available antibiotics – including, Klebsiella pneumoniae, Enterobacter cloacae, Escherichia coli, Serratia marcescens, Proteus mirabilis, Pseudomonas aeruginosa, and Haemophilus influenzae.
There have not been new treatment options for HABP/VABP caused by Gram-negative bacteria in more than 15 years, according to Allergan, the drug’s manufacturer.
This is the third approved indication for ceftazidime/avibactam; the other two indications are for complicated intra-abdominal infections (in combination with metronidazole) and for complicated urinary tract infections.
Socioeconomic deprivation tied to survival in ALL
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()
Socioeconomic deprivation may decrease survival in adults with acute lymphoblastic leukemia (ALL), according to research published in BMC Cancer.
Researchers found that ALL patients living in more deprived areas of England had a 16% to 21% greater risk of dying than patients living in the least deprived areas.
The researchers also observed a 33% higher risk of mortality in patients who were treated at hospitals that manage few ALL patients.
“The findings are likely to have significant implications for the organization of NHS [National Health Service] services for the treatment of adults with this rare but serious condition,” said study author Ravi Maheswaran, MD, of the University of Sheffield in Sheffield, UK.
To conduct this study, Dr Maheswaran and Nick Morley, MBBS, of Royal Hallamshire Hospital in Sheffield, analyzed anonymized NHS data on hospital admissions.
The researchers identified 2921 adults (age 18 and older) who were diagnosed with ALL from 2001 to 2012 and assessed follow-up data on survival rates up to 2013.
There were 1870 deaths during follow-up, and the 5-year survival rate was 32%.
As expected, survival decreased with age but increased over time. The mortality hazard ratio (HR) was 1.38 for patients ages 30 to 39, 3.72 for patients ages 60 to 69, and 9.02 for patients age 80 and older.
The HR was 0.98 for patients diagnosed from 2005 to 2008 and 0.70 for patients diagnosed from 2009 to 2012, compared to 1.00 for patients diagnosed from 2001 to 2004.
Patients living in areas of socioeconomic deprivation had a greater risk of death, but it did not seem to matter whether the patients lived in rural or urban areas.
The mortality HR was 1.16 for patients living in the most deprived areas and 1.21 for patients in intermediate areas (with the least deprived areas as the reference, 1.00). The HR was 1.00 for both rural and urban areas.
The risk of death was higher for patients treated at hospitals with low volumes of adults with ALL. The mortality HR was 1.33 for low-volume hospitals, which were defined as hospitals with 15 or fewer ALL patients admitted over a 3-year time period for which data were available.
“These results, although concerning, are from a single study, and further work is needed to confirm our findings,” Dr Maheswaran said.
“If the association between high deprivation and poorer survival is confirmed, more investigation will be needed to understand why adults with this type of leukemia living in deprived areas have poorer survival and what can be done to address this inequality.”
“Confirmation that hospitals treating few patients with this rare condition have worse outcomes would mean that the NHS should seriously consider if treatment services for adults with acute lymphoblastic leukemia should mainly be provided by specialist centers in order to improve survival.” ![]()