User login
Review of Strategies to Reduce Central Line-Associated Bloodstream Infection (CLABSI) and Catheter-Associated Urinary Tract Infection (CAUTI) in Adult ICUs
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
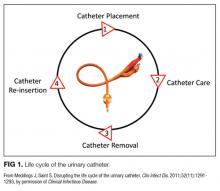
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
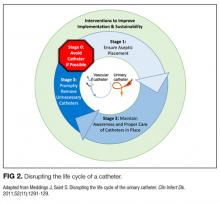
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
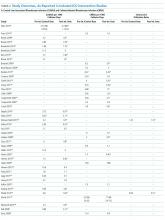
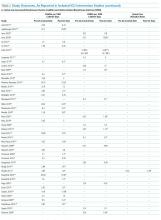
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
Central line–associated bloodstream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI) are morbid and expensive healthcare-associated infections (HAIs).1-8 While these HAIs are prevalent in intensive care units (ICUs) and general wards, most of the research, prevention efforts, and financial penalties have been focused in the ICU.9,10 For hospitalists, who are taking a larger role in caring for the critically ill,11,12 it is optimal to understand best preventive practices.
There has been a national puTash to standardize procedures and products to prevent CLABSI and CAUTI.2,13-16 CLABSI has transitioned from a common ICU complication to a “never event.” Success has been reflected in the prevention of 25,000 CLABSIs over the last decade, translating to a 58% reduction in infections, with 6000 deaths prevented and $414 million saved.2 CLABSI prevention principles have been applied to CAUTI prevention (ie, aseptic insertion, maintenance care, prompting removal) but with slower adoption17 and fewer dramatic CAUTI reductions,18 due in part to weaker recognition19 of CAUTI as a serious clinical event, despite its morbidity20 and cost.21
Despite recent improvements in preventing HAIs, there is a marked variability in how hospitals perform in preventing these infections.22 To inform infection prevention strategies for a large-scale implementation project funded by the Agency for Healthcare Research and Quality and focused on ICUs with persistently elevated CLABSI and/or CAUTI rates,23 we performed a systematic search of interventions to prevent CLABSI and CAUTI in the ICU setting. This evidence was synthesized to help units select and prioritize interventions to prevent these HAIs.
METHODS
Literature Search Strategy
We performed a systematic search to identify CLABSI and CAUTI prevention studies and synthesized findings using a narrative review process. Using criteria developed and refined from seminal articles on the topic,10,14,24-34 we searched the PubMed and Cochrane databases from their inception to October of 2015 using Medical Subject Headings (MeSHs) for “central venous catheters,” “CLABSI,” “central line associated bloodstream infection,” “catheter related bloodstream infection,” “intravascular devices,” “urinary catheterization,” “urinary catheters,” “urinary tract infections,” “CAUTI,” and “catheter associated urinary tract infections” and filtered for articles containing the MeSHs “intensive care unit” and “ICU.” Supplemental Figure 1 details the search, yielding 102 studies for CLABSI and 28 studies for CAUTI, including 7 studies with CLABSI and CAUTI interventions.
Eligibility Criteria Review
Study Design
We included randomized and nonrandomized studies that implemented at least 1 intervention to prevent CLABSI or CAUTI in an adult ICU setting and reported the preintervention or control group data to compare with the postintervention data. We excluded general ward, outpatient/ambulatory, and neonatal/pediatric settings. Interventions to prevent CLABSI or CAUTI were included. We excluded interventions focused on diagnosis or treatment or those that lacked adequate description of the intervention for replication. Studies with interventions that are no longer standard of care in the United States (US) were excluded, as were studies not available in English.
Outcomes
Primary Outcomes for Central Vascular Catheter Infection
- CLABSI: A lab-confirmed bloodstream infection in a patient who has had a central line for at least 48 hours on the date of the development of the bloodstream infection and without another known source of infection. We included studies that reported CLABSIs per 1000 central line days or those that provided data to permit calculation of this ratio. This measure is similar to current National Healthcare Safety Network (NHSN) surveillance definitions.22
- Catheter-related bloodstream infection (CRBSI): A lab-confirmed bloodstream infection attributed to an intravascular catheter by a quantitative culture of the catheter tip or by differences in growth between catheter and peripheral venipuncture blood culture specimens.35 This microbiologic definition of a central line bloodstream infection was often used prior to NHSN reporting, with rates provided as the number of CRBSIs per 1000 central line days.
Primary Outcome for Urinary Catheter Infection
- CAUTI: Urinary tract infection occurring in patients during or after the recent use of an indwelling urinary catheter. We included studies that reported CAUTIs per 1000 urinary catheter days or those that provided data to permit calculation of this ratio (similar to the current NHSN surveillance definitions).22 We excluded studies where CAUTI was defined as bacteriuria alone, without symptoms.
Secondary Outcomes
- Central line utilization ratio: The device utilization ratio (DUR) measure of central line use is calculated as central line days divided by patient days.
- Urinary catheter utilization ratio: The DUR measure of urinary catheter use is calculated as indwelling urinary catheter days divided by patient days, as used in NHSN surveillance, excluding other catheter types.22 We excluded other measures of urinary catheter use because of a large variation in definitions, which limits the ability to compare measures across studies.
Data Synthesis and Analysis
Information on the ICU and intervention type, intervention components, outcomes, and whether interventions were in use prior to the study was abstracted by CAUTI and CLABSI experts (JM and PKP) and confirmed by a second author.
We compared interventions found in the literature to components of the previously published urinary catheter “life cycle,” a conceptual model used to organize and prioritize interventions for a reduction in CAUTI (Figure 1).36
RESULTS
Conceptual Model for Disrupting the Life Cycle of a Catheter
Our data analysis demonstrated that components of the urinary catheter life cycle (Figure 1) were useful and could be applied to vascular catheters, but changes were needed to make the model more valuable to hospitalists implementing CLABSI and CAUTI prevention interventions. We found that the previously named stage 1 (catheter placement) is better described in 2 stages: stage 0, avoid catheter if possible, and stage 1, ensure aseptic placement. Additionally, we tailored the model to include actionable language, describing ways to disrupt the life cycle. Finally, we added a component to represent interventions to improve implementation and sustainability, such as auditing compliance and timely feedback to clinicians. Thus, we introduce a new conceptual model, “Disrupting the Life Cycle of a Catheter” (Figure 2)
Central Vascular Catheter Interventional Study Results
Characteristics of Included Central Vascular Catheter Infection Studies
Of the 102 central vascular catheter (CVC) studies that met the inclusion criteria (reporting outcomes for 105 intervention cohorts), 59 studies10,14,16,24-27,38-89 reporting outcomes for 61 intervention cohorts were performed in the US. Study designs included 14 randomized controlled trials (RCTs)48,64,68,74,79,90-98 and 88 before–after studies (Appendix Table 1). 10,14,16,24-27,33,38-47,49-63,69-73,75-78,80-89,99-131 Many RCTs evaluated antimicrobial products (CVCs, hubs, bathing) as interventions,48,68,74,90-95,97,98 but a few RCTs studied interventions64,79,93 impacting catheter care or use (Appendix Table 1). Fifty-one studies took place in tertiary care hospitals and 55 in academic hospitals. Thirty-one studies were multicenter; the largest included 792 hospitals and 1071 ICUs.24 ICU bed size ranged from 5 to 59.
CVC Study Outcomes
Sixty-three studies reported CLABSI outcomes, and 39 reported CRBSI outcomes (Table 2). Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles,22 which varied by ICU type. Preintervention or control infection rates per 1000 catheter days varied widely (means: CLABSI 7.5, CRBSI 6.3); US studies reported ranges of 1.1 to 12.1 CLABSI and 1.2 to 11.0 CRBSI per 1000 catheter days; non-US studies reported ranges of 1.4 to 45.9 CLABSI and 1.6 to 22.7 CRBSI per 1000 catheter days. Postintervention rates varied widely, with overall means of 2.8 CLABSI and 2.5 CRBSI per 1000 catheter days, including US study ranges of 0 to 8.9 CLABSI and 0 to 5.4 CRBSI, and non-US study ranges of 0 to 17.1 CLABSI and 0 to 15.9 CRBSI.
Central line DURs were reported in only 5 studies; 3 reported decreased postintervention DURs (2 with statistical significance), with a mean 11.7% reduction (Table 2).
CVC Interventions
CVC study interventions are summarized in Table 1, categorized by catheter life cycle component (Figure 2). Thirty-two included studies used a single intervention to prevent CVC infection. Interventions to avoid placement when possible were infrequent. Insertion-stage interventions were common and included avoiding the femoral site during placement, ensuring maximal sterile barriers, and chlorhexidine skin preparation. Standardizing basic products for central line insertion was often done by providing ICUs with a CLABSI insertion kit or stocked cart. In some studies, this was implemented prior to the intervention, and in others, the kit or cart itself was the intervention. Maintenance-stage interventions included scrubbing the hub prior to use, replacing wet or soiled dressings, accessing the catheter with sterile devices, and performing aseptic dressing changes. A recent systematic review and meta-analysis of CVC infection prevention studies indicated that implementing care bundles and/or checklists appears to yield stronger risk reductions than interventions without these components.132 The most common catheter removal interventions were daily audits of line removal and CLABSI rounds focused on ongoing catheter necessity.
Common implementation and sustainability interventions included outcome surveillance, such as feedback on CLABSI, and socio-adaptive interventions to prompt improvements in patient safety culture. Process and outcome surveillance as interventions were implemented in about one-quarter of the studies reviewed (AppendixTable 1).
CAUTI Interventional Study Results
Characteristics of Included CAUTI Studies
Of the 28 CAUTI studies that met the inclusion criteria (reporting outcomes for 30 intervention cohorts), 14 studies (reporting outcomes for 16 intervention cohorts) were performed in the US.28,34,53,66,68,133-141 Study designs included 2 RCTs (focused on urinary catheter avoidance or removal142 and chlorhexidine bathing68) and 26 nonrandomized, before–after studies28,30,33,34,53,66,109,114-116,133-141,143-149 (Appendix Table 1). The number of hospitals per study varied from 1 to 53, with the majority being single-hospital interventions.
CAUTI Study Outcomes
All 28 studies reported CAUTIs per 1000 catheter days for both intervention and comparison groups (Table 2). Preintervention or control CAUTI rates varied widely, with an overall mean of 12.5 CAUTIs per 1000 catheter days; US studies reported a range from 1.4 to 15.8 CAUTIs per 1000 catheter days; non-US studies reported a range from 0.8 to 90.1 CAUTIs per 1000 catheter days. Many studies had preintervention or control rates above the 2013 NHSN 75th percentiles.22 Postintervention CAUTI rates varied widely, with an overall mean of 7.0 CAUTIs per 1000 catheter days, including a US study range from 0 to 11.2 and a non-US study range from 1.9 to 65.7.
Overall (Table 2), 27 of the 30 intervention cohorts described in the 28 studies reported fewer CAUTIs, including all ICU types. Lower postintervention CAUTI rates were reported in 25 studies, with a mean 49.4% reduction, including 11 statistically significant reductions; many studies did not report the level of statistical significance or described inadequate power to detect a significant change (Table 2).
Urinary catheter utilization rates were reported for 11 studies (Table 2). A decreased urinary catheter utilization rate was reported in 7 studies (4 with statistically signficiant reductions), with a mean 16% reduction (Table 2). Other outcomes included cost savings, the potential for unintended negative outcomes, and clinician compliance with intervention components. Positive cost savings were reported in 5 studies.30,34,133,141,149
CAUTI Interventions
Of the 28 included CAUTI prevention studies, only 5 studied single interventions. Interventions were categorized in Table 1 by “life cycle” stages or as interventions to improve implementation and sustainability (Figure 2). Interventions to restrict indwelling urinary catheter use were common, including creating lists of approved indications selected by unit or hospital policy and requiring catheter orders with approved indications. Eight studies published approved indication lists.28,34,133-135,138,142,146 Although several studies describe the encouragement and use of bladder scanners and urinary catheter alternatives, none described purchasing these catheter alternatives.
Interventions to avoid indwelling urinary catheters included education about external catheters,28,34,109,133,140,144-146 urinary retention protocols,34,144,135,141 and bladder scanner simulation training.133 Interventions to improve aseptic insertion28,34,66,109,116,139-141-143-146,150 and maintenance care28,34,66,109,116,133,135,136,139-141,143-146,150 of urinary catheters were common. Four studies used a standardized urinary catheter kit or cart,28,34,139,142 and 2 studies used a commercial urinary catheter securement device.34,140 A CAUTI bundle checklist in daily patient care rounds was tested in 3 studies (Table 1).66,136,150 Reminder and stop order strategies, with the potential to reduce CAUTI rates by >50%,151 were included in 15 studies, with inteventions such as nurse-empowered stop orders. Several implementation and sustainability interventions were described, including socio-adaptive strategies such as holding multidisciplinary meetings to obtain unit or clinician feedback to inform design and improve buy-in and providing frequent feedback to ICU clinicians, including audits of catheter use appropriateness and catheter-associated infections.
DISCUSSION
This extensive literature review yielded a large body of literature demonstrating success in preventing CLABSI and CAUTI in all types of adult ICUs, including in general medical and surgical ICUs and in specialized units with historically higher rates, such as trauma, burn, and neurosurgical. Reported reductions in catheter infections were impressive (>65% for CLABSI or CRBSI and nearly 50% for CAUTI), though several studies had limited power to detect statistical significance. DURs were reported more rarely (particularly for vascular catheters) and often without power to detect statistical significance. Nevertheless, 7 studies reported reduced urinary catheter use (16% mean reduction), which would be anticipated to be clinically significant.
The conceptual model introduced for “Disrupting the Life Cycle of a Catheter” (Figure 2) can be a helpful tool for hospitalists and intensivists to assess and prioritize potential strategies for reducing catheter-associated infections. This study’s results indicate that CLABSI prevention studies often used interventions that optimize best practices during aseptic insertion and maintenance, but few studies emphasized reducing inappropriate central line use. Conversely, CAUTI prevention often targeted avoiding placement and prompting the removal of urinary catheters, with fewer studies evaluating innovative products or technical skill advancement for aseptic insertion or maintenance, though educational interventions to standardize aseptic catheter use were common. Recently, recommendations for reducing the inappropriate use of urinary catheters and intravenous catheters, including scenarios common in ICUs, were developed by using the rigorous RAND/UCLA Appropriateness Method152,153; these resources may be helpful to hospitalists designing and implementing interventions to reduce catheter use.
In reviewing the US studies of 5 units demonstrating the greatest success in preventing CLABSI56,62,65,78,83 and CAUTI,28,34,66,134 several shared features emerged. Interventions that addressed multiple steps within the life cycle of a catheter (avoidance, insertion, maintenance, and removal) were common. Previous work has shown that assuring compliance in infection prevention efforts is a key to success,154 and in both CLABSI and CAUTI studies, auditing was included in these successful interventions. Specifically for CLABSI, the checklist, a central quality improvement tool, was frequently associated with success. Unique to CAUTI, engaging a multidisciplinary team including nurse leadership seemed critical to optimize implementation and sustainability efforts. In addition, a focus on stage 3 (removal), including protocols to remove by default, was associated with success in CAUTI studies.
Our review was limited by a frequent lack of reporting of statistical significance or by inadequate power to detect a significant change and great variety. The ability to compare the impact of specific interventions is limited because studies varied greatly with respect to the type of intervention, duration of data collection, and outcomes assessed. We also anticipate that successful interventions are more likely to be published than are trials without success. Strengths include the use of a rigorous search process and the inclusion and review of several types of interventions implemented in ICUs.
In conclusion, despite high catheter use in ICUs, the literature includes many successful interventions for the prevention of vascular and urinary catheter infections in multiple ICU types. This review indicates that targeting multiple steps within the life cycle of a catheter, particularly when combined with interventions to optimize implementation and sustainability, can improve success in reducing CLABSI and CAUTI in the ICU.
Acknowledgments
The authors thank all members of the National Project Team for the AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI.
Disclosure
Agency for Healthcare Research and Quality (AHRQ) contract #HHSP233201500016I/HHSP23337002T provided funding for this study. J.M.’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, VA Ann Arbor Patient Safety Center of Inquiry, the Health Research and Educational Trust, American Hospital Association and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the US Department of Veterans Affairs. All authors report no conflicts of interest relevant to this article.
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
1. National and state healthcare-associated infections progress report. Centers for Disease Control and Prevention website. http://www.cdc.gov/hai/progress-report/. 2016. Accessed January 10, 2016.
2. Srinivasan A, Wise M, Bell M, et al. Vital signs: central line-associated blood stream infections-United States, 2001, 2008, and 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):243-248. PubMed
3. Abramczyk ML, Carvalho WB, Carvalho ES, Medeiros EA. Nosocomial infection in a pediatric intensive care unit in a developing country. Braz J Infect Dis. 2003;7(6):375-380. PubMed
4. Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28(1):68-75. PubMed
5. Ziegler MJ, Pellegrini DC, Safdar N. Attributable mortality of central line associated bloodstream infection: systematic review and meta-analysis. Infection. 2015;43(1):29-36. PubMed
6. Siempos, II, Kopterides P, Tsangaris I, Dimopoulou I, Armaganidis AE. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: a meta-analysis. Crit Care Med. 2009;37(7):2283-2289. PubMed
7. Zingg W, Sax H, Inan C, et al. Hospital-wide surveillance of catheter-related bloodstream infection: from the expected to the unexpected. J Hosp Infect. 2009;73(1):41-46. PubMed
8. Chant C, Smith OM, Marshall JC, Friedrich JO. Relationship of catheter-associated urinary tract infection to mortality and length of stay in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Med. 2011;39(5):1167-1173. PubMed
9. Lee GM, Kleinman K, Soumerai SB, et al. Effect of nonpayment for preventable infections in US hospitals. N Engl J Med. 2012;367(15):1428-1437.
10. Muto C, Herbert C, Harrison E, Edwards JR, et al. Reduction in central line-associated bloodstream infections among patients in intensive care units - Pennsylvania, April 2001-March 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1013-1016. PubMed
11. Heisler M. Hospitalists and intensivists: partners in caring for the critically ill--the time has come. J Hosp Med. 2010;5(1):1-3. PubMed
12. Siegal EM, Dressler DD, Dichter JR, Gorman MJ, Lipsett PA. Training a hospitalist workforce to address the intensivist shortage in American hospitals: a position paper from the Society of Hospital Medicine and the Society of Critical Care Medicine. J Hosp Med. 2012;7(5):359-364. PubMed
13. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. 2009. Accessed May 26, 2017.
14. Hong AL, Sawyer MD, Shore A, et al. Decreasing central‐line–associated bloodstream infections in Connecticut intensive care units. J Healthc Qual. 2013;35(5):78-87. PubMed
15. Weaver SJ, Weeks K, Pham JC, Pronovost PJ. On the CUSP: Stop BSI: evaluating the relationship between central line-associated bloodstream infection rate and patient safety climate profile. Am J Infect Control. 2014;42(10 Suppl):S203-S208. PubMed
16. Lin DM, Weeks K, Holzmueller CG, Pronovost PJ, Pham JC. Maintaining and sustaining the On the CUSP: stop BSI model in Hawaii. Jt Comm J Qual Patient Saf. 2013;39(2):51-60. PubMed
17. Krein SL, Fowler KE, Ratz D, Meddings J, Saint S. Preventing device-associated infections in US hospitals: national surveys from 2005 to 2013. BMJ Qual Saf. 2015;24(6):385-392. PubMed
18. Department of Health and Human Services Action Plan to Prevent Healthcare-Associated Infections. Current progress on meeting these targets reviewed in 2013. https://health.gov/hcq/prevent-hai.asp. Accessed October 28, 2016.
19. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. PubMed
20. Nicolle LE. Catheter associated urinary tract infections. Antimicrob Resist Infect Control. 2014;3:23. PubMed
21. Kennedy EH, Greene MT, Saint S. Estimating hospital costs of catheter-associated urinary tract infection. J Hosp Med. 2013;8(9):519-522. PubMed
22. Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network Report, data summary for 2013, Device-associated Module. Am J Infect Control. 2015;43:206-221. PubMed
23. AHRQ Safety Program for Intensive Care Units: Preventing CLABSI and CAUTI. Agency for Healthcare Research and Quality website. http://www.ahrq.gov/professionals/quality-patient-safety/hais/tools/preventing/index.html. 2017. Accessed August 24, 2017.
24. Berenholtz SM, Lubomski LH, Weeks K, et al. Eliminating central line-associated bloodstream infections: a national patient safety imperative. Infect Control Hosp Epidemiol. 2014;35(1):56-62. PubMed
25. Lin DM, Weeks K, Bauer L, et al. Eradicating central line-associated bloodstream infections statewide: the Hawaii experience. Am J Med Qual. 2012;27(2):124-129. PubMed
26. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725-2732. PubMed
27. DePalo VA, McNicoll L, Cornell M, Rocha JM, Adams L, Pronovost PJ. The Rhode Island ICU collaborative: a model for reducing central line-associated bloodstream infection and ventilator-associated pneumonia statewide. Qual Saf Health Care. 2010;19(6):555-561. PubMed
28. Dumigan DG, Kohan CA, Reed CR, Jekel JF, Fikrig MK. Utilizing national nosocomial infection surveillance system data to improve urinary tract infection rates in three intensive-care units. Clin Perform Qual Health Care. 1998;6(4):172-178. PubMed
29. Eggimann P, Harbarth S, Constantin MN, Touveneau S, Chevrolet JC, Pittet D. Impact of a prevention strategy targeted at vascular-access care on incidence of infections acquired in intensive care. Lancet. 2000;355(9218):1864-1868. PubMed
30. Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978. PubMed
31. McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40(2):388-393. PubMed
32. Miller SE, Maragakis LL. Central line-associated bloodstream infection prevention. Curr Opin Infect Dis. 2012;25(4):412-422. PubMed
33. Seguin P, Laviolle B, Isslame S, Coué A, Mallédant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36(7):1202-1206. PubMed
34. Titsworth WL, Hester J, Correia T, et al. Reduction of catheter-associated urinary tract infections among patients in a neurological intensive care unit: a single institution’s success. J Neurosurg. 2012;116(4):911-920. PubMed
35. Bouza E, Muñoz P, López-Rodríguez J, et al. A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003;54(4):279-287. PubMed
36. Meddings J, Saint S. Disrupting the life cycle of the urinary catheter. Clin Infect Dis. 2011;52(11):1291-1293. PubMed
37. O’Grady NP, Alexander M, Burns L, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections 2011. Healthcare Infection Control Practices Advisory Committee (HICPAC). Centers for Disease Control and Prevention website. https://www.cdc.gov/infectioncontrol/guidelines/BSI/index.html. 2011. Accessed May 26, 2017.
38. Allen GB, Miller V, Nicholas C, et al. A multitiered strategy of simulation training, kit consolidation, and electronic documentation is associated with a reduction in central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):643-648. PubMed
39. Arora N, Patel K, Engell CA, LaRosa JA. The effect of interdisciplinary team rounds on urinary catheter and central venous catheter days and rates of infection. Am J Med Qual. 2014;29(4):329-334. PubMed
40. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169(15):1420-1423. PubMed
41. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
42. Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32(10):2014-2020. PubMed
43. Bonne S, Mazuski JE, Sona C, et al. Effectiveness of minocycline and rifampin vs chlorhexidine and silver sulfadiazine-impregnated central venous catheters in preventing central line-associated bloodstream infection in a high-volume academic intensive care unit: a before and after trial. J Am Coll Surg. 2015;221(3):739-747. PubMed
44. Borschel DM, Chenoweth CE, Kaufman SR, et al. Are antiseptic-coated central venous catheters effective in a real-world setting? Am J Infect Control. 2006;34(6):388-393. PubMed
45. Burden AR, Torjman MC, Dy GE, et al. Prevention of central venous catheter-related bloodstream infections: is it time to add simulation training to the prevention bundle? J Clin Anesth. 2012;24(7):555-560. PubMed
46. Cherry RA, West CE, Hamilton MC, Rafferty CM, Hollenbeak CS, Caputo GM. Reduction of central venous catheter associated blood stream infections following implementation of a resident oversight and credentialing policy. Patient Saf Surg. 2011;5(1):15. PubMed
47. Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17(3):163-166. PubMed
48. Collin GR. Decreasing catheter colonization through the use of an antiseptic-impregnated catheter: a continuous quality improvement project. Chest. 1999;115(6):1632-1640. PubMed
49. Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30(1):59-64. PubMed
50. Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139(2):131-136. PubMed
51. Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38(10):817-821. PubMed
52. Exline MC, Ali NA, Zikri N, et al. Beyond the bundle--journey of a tertiary care medical intensive care unit to zero central line-associated bloodstream infections. Crit Care. 2013;17(2):R41. PubMed
53. Fox C, Wavra T, Drake DA, et al. Use of a patient hand hygiene protocol to reduce hospital-acquired infections and improve nurses’ hand washing. Am J Crit Care. 2015;24(3):216-224. PubMed
54. Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201(3):349-358. PubMed
55. Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144(4):492-495. PubMed
56. Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32(6):619-622. PubMed
57. Hanna HA, Raad II, Hackett B, et al. Antibiotic-impregnated catheters associated with significant decrease in nosocomial and multidrug-resistant bacteremias in critically ill patients. Chest. 2003;124(3):1030-1038. PubMed
58. Hatler CW, Mast D, Corderella J, et al. Using evidence and process improvement strategies to enhance healthcare outcomes for the critically ill: a pilot project. Am J Crit Care. 2006;15(6):549-555. PubMed
59. Kamboj M, Blair R, Bell N, et al. Use of disinfection cap to reduce central-line-associated bloodstream infection and blood culture contamination among hematology-oncology patients. Infect Control Hosp Epidemiol. 2015;36:1401-1408. PubMed
60. Khouli H, Jahnes K, Shapiro J, et al. Performance of medical residents in sterile techniques during central vein catheterization: randomized trial of efficacy of simulation-based training. Chest. 2011;139(1):80-87. PubMed
61. Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34(12):713-723. PubMed
62. Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30(5):293-298. PubMed
63. Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127(4):257-266. PubMed
64. Marsteller JA, Sexton JB, Hsu YJ, et al. A multicenter, phased, cluster-randomized controlled trial to reduce central line-associated bloodstream infections in intensive care units. Crit Care Med. 2012;40(11):2933-2939. PubMed
65. McMullan C, Propper G, Schuhmacher C, et al. A multidisciplinary approach to reduce central line-associated bloodstream infections. Jt Comm J Qual Patient Saf. 2013;39(2):61-69. PubMed
66. Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68(1):23-31. PubMed
67. Montecalvo MA, McKenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505-511. PubMed
68. Noto MJ, Domenico HJ, Byrne DW, et al. Chlorhexidine bathing and health care-associated infections: a randomized clinical trial. JAMA. 2015;313(4):369-378. PubMed
69. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959-963. PubMed
70. Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854-858. PubMed
71. Pronovost PJ, Watson SR, Goeschel CA, Hyzy RC, Berenholtz SM. Sustaining reductions in central line-associated bloodstream infections in Michigan intensive care units: A 10-year analysis. Am J Med Qual. 2016;31(3):197-202. PubMed
72. Rangachari P, Madaio M, Rethemeyer RK, et al. Cumulative impact of periodic top-down communications on infection prevention practices and outcomes in two units. Health Care Manage Rev. 2015;40(4):324-336. PubMed
73. Render ML, Hasselbeck R, Freyberg RW, et al. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20(8):725-732. PubMed
74. Rupp ME, Lisco SJ, Lipsett PA, et al. Effect of a second-generation venous catheter impregnated with chlorhexidine and silver sulfadiazine on central catheter-related infections: a randomized, controlled trial. Ann Intern Med. 2005;143(8):570-580. PubMed
75. Sacks GD, Diggs BS, Hadjizacharia P, Green D, Salim A, Malinoski DJ. Reducing the rate of catheter-associated bloodstream infections in a surgical intensive care unit using the Institute for Healthcare Improvement Central Line Bundle. Am J Surg. 2014;207(6):817-823. PubMed
76. Salemi C, Canola MT, Eck EK. Hand washing and physicians: how to get them together. Infect Control Hosp Epidemiol. 2002;23(1):32-35. PubMed
77. Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32(9):479-487. PubMed
78. Sopirala MM, Smyer J, Fawley L, et al. Sustained reduction of central line-associated bloodstream infections in an intensive care unit using a top-down and bottom-up approach. Am J Infect Control. 2013;41(2):183-184. PubMed
79. Speroff T, Ely EW, Greevy R, et al. Quality improvement projects targeting health care-associated infections: comparing Virtual Collaborative and Toolkit approaches. J Hosp Med. 2011;6(5):271-278. PubMed
80. Thom KA, Li S, Custer M, et al. Successful implementation of a unit-based quality nurse to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(2):139-143. PubMed
81. Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of “bundles” in a closed-model medical intensive care unit. J Crit Care. 2010;25(1):174.e11-174.e18. PubMed
82. Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14(4):295-302. PubMed
83. Walz JM, Ellison RT 3rd, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg. 2015;120(4):868-876. PubMed
84. Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27(7):662-669. PubMed
85. Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126(5):1612-1618. PubMed
86. Watson SR, George C, Martin M, Bogan B, Goeschel C, Pronovost PJ. Preventing central line-associated bloodstream infections and improving safety culture: a statewide experience. Jt Comm J Qual Patient Saf. 2009;35(12):593-597. PubMed
87. Mueller JT, Wright AJ, Fedraw LA, et al. Standardizing central line safety: lessons learned for physician leaders. Am J Med Qual. 2014;29(3):191-199. PubMed
88. Vigorito MC, McNicoll L, Adams L, Sexton B. Improving safety culture results in Rhode Island ICUs: lessons learned from the development of action-oriented plans. Jt Comm J Qual Patient Saf. 2011;37(11):509-514. PubMed
89. Zack J. Zeroing in on zero tolerance for central line-associated bacteremia. Am J Infect Control. 2008;36(10):S176.e1-S176.e2. PubMed
90. Brun-Buisson C, Doyon F, Sollet JP, Cochard JF, Cohen Y, Nitenberg G. Prevention of intravascular catheter-related infection with newer chlorhexidine-silver sulfadiazine-coated catheters: a randomized controlled trial. Intensive Care Med. 2004;30(5):837-843. PubMed
91. Carrasco MN, Bueno A, de las Cuevas C, et al. Evaluation of a triple-lumen central venous heparin-coated catheter versus a catheter coated with chlorhexidine and silver sulfadiazine in critically ill patients. Intensive Care Med. 2004;30(4):633-638 PubMed
92. Corral L, Nolla-Salas M, Ibañez-Nolla J, et al. A prospective, randomized study in critically ill patients using the Oligon Vantex catheter. J Hosp Infect. 2003;55(3):212-219. PubMed
93. Hagau N, Studnicska D, Gavrus RL, Csipak G, Hagau R, Slavcovici AV. Central venous catheter colonization and catheter-related bloodstream infections in critically ill patients: a comparison between standard and silver-integrated catheters. Eur J Anaesthesiol. 2009;26(9):752-758. PubMed
94. Kalfon P, de Vaumas C, Samba D, et al. Comparison of silver-impregnated with standard multi-lumen central venous catheters in critically ill patients. Crit Care Med. 2007;35(4):1032-1039. PubMed
95. Kurtz P, Rosa P, Penna G, et al. Antibiotic coated catheter to decrease infection: pilot study. Rev Bras Ter Intensiva. 2008;20(2):160-164. PubMed
96. Osma S, Kahveci SF, Kaya FN, et al. Efficacy of antiseptic-impregnated catheters on catheter colonization and catheter-related bloodstream infections in patients in an intensive care unit. J Hosp Infect. 2006;62(2):156-162. PubMed
97. León C, Alvarez-Lerma F, Ruiz-Santana S, et al. Antiseptic chamber-containing hub reduces central venous catheter-related infection: a prospective, randomized study. Crit Care Med. 2003;31(5):1318-1324. PubMed
98. León C, Ruiz-Santana S, Rello J, et al. Benefits of minocycline and rifampin-impregnated central venous catheters. A prospective, randomized, double-blind, controlled, multicenter trial. Intensive Care Med. 2004;30(10):1891-1899. PubMed
99. Bion J, Richardson A, Hibbert P, et al. ‘Matching Michigan’: a 2-year stepped interventional programme to minimise central venous catheter-blood stream infections in intensive care units in England. BMJ Qual Saf. 2013;22(2):110-123. PubMed
100. Cherifi S, Gerard M, Arias S, Byl B. A multicenter quasi-experimental study: impact of a central line infection control program using auditing and performance feedback in five Belgian intensive care units. Antimicrob Resist Infect Control. 2013;2(1):33. PubMed
101. Entesari-Tatafi D, Orford N, Bailey MJ, Chonghaile MN, Lamb-Jenkins J, Athan E. Effectiveness of a care bundle to reduce central line-associated bloodstream infections. Med J Aust. 2015;202(5):247-250. PubMed
102. Hakko E, Guvenc S, Karaman I, Cakmak A, Erdem T, Cakmakci M. Long-term sustainability of zero central-line associated bloodstream infections is possible with high compliance with care bundle elements. East Mediterr Health J. 2015;21(4):293-298. PubMed
103. Hansen S, Schwab F, Schneider S, Sohr D, Gastmeier P, Geffers C. Time-series analysis to observe the impact of a centrally organized educational intervention on the prevention of central-line-associated bloodstream infections in 32 German intensive care units. J Hosp Infect. 2014;87(4):220-226. PubMed
104. Hermon A, Pain T, Beckett P, et al. Improving compliance with central venous catheter care bundles using electronic records. Nurs Crit Care. 2015;20(4):196-203. PubMed
105. Jaggi N, Rodrigues C, Rosenthal VD, et al. Impact of an international nosocomial infection control consortium multidimensional approach on central line-associated bloodstream infection rates in adult intensive care units in eight cities in India. Int J Infect Dis. 2013;17(12):e1218-e1224. PubMed
106. Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining “zero” central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41(12):1209-1213. PubMed
107. Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control. 2013;41(8):710-716. PubMed
108. Klintworth G, Stafford J, O’Connor M, et al. Beyond the intensive care unit bundle: Implementation of a successful hospital-wide initiative to reduce central line-associated bloodstream infections. Am J Infect Control. 2014;42(6):685-687. PubMed
109. Leblebicioglu H, Ersoz G, Rosenthal VD, et al. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in adult intensive care units in 10 cities of Turkey: International Nosocomial Infection Control Consortium findings (INICC). Am J Infect Control. 2013;41(10):885-891. PubMed
110. Latif A, Kelly B, Edrees H, et al. Implementing a multifaceted intervention to decrease central line-associated bloodstream infections in SEHA (Abu Dhabi Health Services Company) intensive care units: the Abu Dhabi experience. Infect Control Hosp Epidemiol. 2015;36(7):816-822. PubMed
111. Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20(2):174-180. PubMed
112. Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38(6):440-448. PubMed
113. Lorente L, Lecuona M, Jiménez A, et al. Chlorhexidine-silver sulfadiazine-impregnated venous catheters save costs. Am J Infect Control. 2014;42(3):321-324. PubMed
114. Marra AR, Cal RG, Durão MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38(6):434-439. PubMed
115. Martínez-Reséndez MF, Garza-González E, Mendoza-Olazaran S, et al. Impact of daily chlorhexidine baths and hand hygiene compliance on nosocomial infection rates in critically ill patients. Am J Infect Control. 2014;42(7):713-717. PubMed
116. Mathur P, Tak V, Gunjiyal J, et al. Device-associated infections at a level-1 trauma centre of a developing nation: impact of automated surveillance, training and feedbacks. Indian J Med Microbiol. 2015;33(1):51-62. PubMed
117. Mazi W, Begum Z, Abdulla D, et al. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42(8):865-867. PubMed
118. Menegueti MG, Ardison KM, Bellissimo-Rodrigues F, et al. The impact of implementation of bundle to reduce catheter-related bloodstream infection rates. J Clin Med Res. 2015;7(11):857-861. PubMed
119. Paula AP, Oliveira PR, Miranda EP, et al. The long-term impact of a program to prevent central line-associated bloodstream infections in a surgical intensive care unit. Clinics (Sao Paulo). 2012;67(8):969-970. PubMed
120. Reddy KK, Samuel A, Smiley KA, Weber S, Hon H. Reducing central line-associated bloodstream infections in three ICUs at a tertiary care hospital in the United Arab Emirates. Jt Comm J Qual Patient Saf. 2014;40(12):559-561. PubMed
121. Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med. 2013;41(10):2364-2372. PubMed
122. Peredo R, Sabatier C, Villagrá A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29(9):1173-1177. PubMed
123. Pérez Parra A, Cruz Menárguez M, Pérez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31(9):964-967. PubMed
124. Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31(7):405-409. PubMed
125. Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line-associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31(12):1264-1272. PubMed
126. Salama MF, Jamal W, Mousa HA, Rotimi V. Implementation of central venous catheter bundle in an intensive care unit in Kuwait: Effect on central line-associated bloodstream infections. J Infect Public Health. 2016;9(1):34-41. PubMed
127. Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29(12):1171-1173. PubMed
128. Scheithauer S, Lewalter K, Schröder J, et al. Reduction of central venous line-associated bloodstream infection rates by using a chlorhexidine-containing dressing. Infection. 2014;42(1):155-159. PubMed
129. Singh S, Kumar RK, Sundaram KR, et al. Improving outcomes and reducing costs by modular training in infection control in a resource-limited setting. Int J Qual Health Care. 2012;24(6):641-648. PubMed
130. Zingg W, Cartier V, Inan C, et al. Hospital-wide multidisciplinary, multimodal intervention programme to reduce central venous catheter-associated bloodstream infection. PLoS One. 2014;9(4):e93898. PubMed
131. Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37(7):2167-2173. PubMed
132. Blot K, Bergs J, Vogelaers D, Blot S, Vandijck D. Prevention of central line-associated bloodstream infections through quality improvement interventions: a systematic review and meta-analysis. Clin Infect Dis. 2014;59(1):96-105. PubMed
133. Alexaitis I, Broome B. Implementation of a nurse-driven protocol to prevent catheter-associated urinary tract infections. J Nurs Care Qual. 2014;29(3):245-252. PubMed
134. Elpern EH, Killeen K, Ketchem A, Wiley A, Patel G, Lateef O. Reducing use of indwelling urinary catheters and associated urinary tract infections. Am J Crit Care. 2009;18(6):535-541. PubMed
135. Fuchs MA, Sexton DJ, Thornlow DK, Champagne MT. Evaluation of an evidence-based, nurse-driven checklist to prevent hospital-acquired catheter-associated urinary tract infections in intensive care units. J Nurs Care Qual. 2011;26(2):101-109. PubMed
136. Jain M, Miller L, Belt D, King D, Berwick DM. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care. 2006;15(4):235-239. PubMed
137. Popp JA, Layon AJ, Nappo R, Richards WT, Mozingo DW. Hospital-acquired infections and thermally injured patients: chlorhexidine gluconate baths work. Am J Infect Control. 2014;42(2):129-132. PubMed
138. Reilly L, Sullivan P, Ninni S, Fochesto D, Williams K, Fetherman B. Reducing foley catheter device days in an intensive care unit: using the evidence to change practice. AACN Adv Crit Care. 2006;17(3):272-283. PubMed
139. Saint S, Fowler KE, Sermak K, et al. Introducing the No Preventable Harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. PubMed
140. Schelling K, Palamone J, Thomas K, et al. Reducing catheter-associated urinary tract infections in a neuro-spine intensive care unit. Am J Infect Control. 2015;43(8):892-894. PubMed
141. Sutherland T, Beloff J, McGrath C, et al. A single-center multidisciplinary initiative to reduce catheter-associated urinary tract infection rates: Quality and financial implications. Health Care Manag (Frederick). 2015;34(3):218-224. PubMed
142. Chen YY, Chi MM, Chen YC, Chan YJ, Chou SS, Wang FD. Using a criteria-based reminder to reduce use of indwelling urinary catheters and decrease urinary tract infections. Am J Crit Care. 2013;22(2):105-114. PubMed
143. Amine AE, Helal MO, Bakr WM. Evaluation of an intervention program to prevent hospital-acquired catheter-associated urinary tract infections in an ICU in a rural Egypt hospital. GMS Hyg Infect Control. 2014;9(2):Doc15. PubMed
144. Kanj SS, Zahreddine N, Rosenthal VD, Alamuddin L, Kanafani Z, Molaeb B. Impact of a multidimensional infection control approach on catheter-associated urinary tract infection rates in an adult intensive care unit in Lebanon: International Nosocomial Infection Control Consortium (INICC) findings. Int J Infect Dis. 2013;17(9):e686-e690. PubMed
145. Navoa-Ng JA, Berba R, Rosenthal VD, et al. Impact of an International Nosocomial Infection Control Consortium multidimensional approach on catheter-associated urinary tract infections in adult intensive care units in the Philippines: International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2013;6(5):389-399. PubMed
146. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2012;40(5):517-526. PubMed
147. Salama MF, Jamal WY, Mousa HA, Al-Abdulghani KA, Rotimi VO. The effect of hand hygiene compliance on hospital-acquired infections in an ICU setting in a Kuwaiti teaching hospital. J Infect Public Health. 2013;6(1):27-34. PubMed
148. Seyman D, Oztoprak N, Berk H, Kizilates F, Emek M. Weekly chlorhexidine douche: does it reduce healthcare-associated bloodstream infections? Scand J Infect Dis. 2014;46(10):697-703. PubMed
149. Apisarnthanarak A, Thongphubeth K, Sirinvaravong S, et al. Effectiveness of multifaceted hospitalwide quality improvement programs featuring an intervention to remove unnecessary urinary catheters at a tertiary care center in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):791-798. PubMed
150. Marra AR, Sampaio Camargo TZ, Gonçalves P, et al. Preventing catheter-associated urinary tract infection in the zero-tolerance era. Am J Infect Control. 2011;39(10):817-822. PubMed
151. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2014;23(4):277-289. PubMed
152. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. PubMed
153. Meddings J, Saint S, Fowler KE, et al. The Ann Arbor Criteria for appropriate urinary catheter use in hospitalized medical patients: results obtained by using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;162(9 Suppl):S1-S34. PubMed
154. Furuya EY, Dick AW, Herzig CT, Pogorzelska-Maziarz M, Larson EL, Stone PW. Central Line-Associated Bloodstream Infection Reduction and Bundle Compliance in Intensive Care Units: A National Study. Infect Control Hosp Epidemiol. 2016;37(7):805-810. PubMed
© 2018 Society of Hospital Medicine
Fuller Road, Ann Arbor, MI 48105; Telephone: 734-845-3460; Fax: 734-845-3290, E-mail: payalkp@umich.edu
Developing machines that detect disease
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Smells – of skin, breath, or bodily fluids – can, in some cases, reveal the presence of disease. This fact has led researchers to try to build an odor sensor that could make a fast, reliable diagnosis, and now the field may be on the verge of a breakthrough, according to a recent article in the New York Times.
In addition to various efforts in Austria, Switzerland, and Japan, an English manufacturer – Owlstone Medical – has been making headway with an odor analysis technology. It will be part of a National Health Service trial that will test the sensor for diagnosing lung cancer. The company also is conducting a trial using urine samples to detect colon cancer; its program allows changing the software to change what disease you detect.
Meanwhile, an Israeli chemical engineer, Hossam Haick, is using similar technology, with molecular receptors that have an affinity for certain biomarkers of disease found in the breath. Artificial intelligence allows the sensors to improve with each use, and a paper published last year showed that this system could distinguish among 17 different diseases with up to 86% accuracy.
And in the United States, researchers from the Monell Chemical Senses Center and the University of Pennsylvania are working on an odor sensor that detects ovarian cancer in samples of blood plasma. They chose plasma because it is less likely than breath or urine to be affected by other factors such as diet or environmental chemicals.
These technologies could be available to doctors in 3-5 years, experts say.
Reference
Murphy K. One Day, a Machine Will Smell Whether You’re Sick . New York Times. May 1, 2017. Accessed May 29, 2017.
Ustekinumab may reduce risk of nonmelanoma skin cancer
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
GENEVA – Ustekinumab therapy appears to protect psoriasis patients against nonmelanoma skin cancer (NMSC), according to a new analysis from the PSOLAR registry.
Compared with psoriasis patients on methotrexate, the risk of developing on-treatment NMSC was lower among patients on the interleukin-12-/23 inhibitor ustekinumab (Stelara) and those on the three tumor necrosis factor (TNF) inhibitors included in the PSOLAR registry – infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira). The lower risk was statistically significant only for ustekinumab, although there was a favorable trend with the TNF inhibitors showing a 19% relative risk reduction, Bhaskar Srivastava, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
PSOLAR (Psoriasis Longitudinal Assessment and Registry) is an ongoing international prospective observational study evaluating long-term safety and clinical outcomes in psoriasis patients eligible for systemic therapies. The study is now fully enrolled, with 12,090 psoriasis patients and 48,870 patient-years of follow-up and climbing, noted Dr. Srivastava, an employee of Janssen Scientific Affairs, Spring House, Pa.
This analysis focused on 6,782 PSOLAR participants with a mean 18-year history of psoriasis and no history of NMSC at enrollment: 2,623 patients on ustekinumab with 7,900 patient-years of prospective follow-up, 3,727 on a TNF inhibitor with 10,580 patient-years of follow-up, and 432 controls on methotrexate with 781 patient-years of follow-up.
Patients on a biologic were significantly younger, with a mean age of 46.7 years, versus 53.6 years for those on methotrexate. Rates of past or current smoking were similar, in the 55%-60% range, regardless of which systemic agent patients were using.
The crude unadjusted incidence rate for NMSC among all patients on a biologic was 0.33 cancers/100 patient-years, compared with 1.41/100 patient-years for psoriasis patients on methotrexate.
Patients on ustekinumab had an NMSC incidence rate of 0.19/100 patient-years, with a basal cell carcinoma rate of 0.13/100 patient-years and a squamous cell carcinoma rate of 0.06/100 patient-years. Psoriasis patients on a TNF inhibitor had an NMSC incidence rate of 0.43/100 patient-years, with a basal cell carcinoma rate of 0.26/100 patient-years and a squamous cell carcinoma rate of 0.17/100 patient-years.
In a multivariate analysis adjusted for age, sex, race, location, duration of psoriasis, smoking, prior malignancy, skin type, and history of treatment with cyclosporine, methotrexate, other systemic agents, or phototherapy, patients taking ustekinumab had a statistically significant 65% reduction in the risk of NMSC compared with patients on methotrexate and a 74% relative risk reduction for basal cell carcinoma; however, the squamous cell carcinoma risk in the two patient groups was similar.
Dr. Srivastava said the PSOLAR data shouldn’t be taken as the final word regarding NMSC risk and the use of biologics. He noted that psoriasis itself is associated with an increased risk of NMSC. And methotrexate, which was used as the reference standard in this analysis, may alter the risk of NMSC.
“Overall, these results require further validation in psoriasis populations with larger numbers of exposed patients,” he said.
The PSOLAR registry is funded by Janssen, where Dr. Srivastava is employed.
AT THE EADV CONGRESS
Key clinical point:
Major finding: Psoriasis patients on ustekinumab had an adjusted 65% reduction in the risk of developing nonmelanoma skin cancer compared with patients on methotrexate.
Data source: An analysis of 6,782 psoriasis patients participating in an international prospective observational registry evaluating the long-term safety and clinical outcomes of systemic therapies.
Disclosures: The PSOLAR registry is funded by ustekinumab manufacturer Janssen; the study presenter is a company employee.
Understanding the Causes of Venous Pressure
Developing an effective treatment plan for patients with venous disease hinges on an thorough clinical evaluation and a keen understanding of venous anatomy and physiology, both of which will be the focus of the Thursday morning session, “Venous Clinical Examination and Hemodynamics.”
“Less invasive treatments are now available because of the advent of new technologies,” said co-moderator Dr. Jose I. Almeida, director of the Miami Vein Center and voluntary professor of surgery at the University of Miami School of Medicine. “However, the proper treatment of patients with advanced venous disease has little to do with the new toys available on the shelf. Rather, it depends on a proper understanding of the fundamental problem: What causes increased venous pressure?”
Presentations will focus on answering that question. “Without an accurate physician assessment, one cannot apply an effective treatment plan for a patient with venous disease,” said Dr. Almeida. Session co-moderators include Dr. Lowell S. Kabnick, director of the New York University Vein Center, and Dr. Thomas W. Wakefield, vascular surgery section head at the University of Michigan Cardiovascular Center.
“The session begins with identifying symptoms and signs of venous disease, scoring the disease severity with a validated tool, and classifying the severity on a scale of one to six,” said Dr. Almeida, who starts off the session with his own review of the CEAP classification for venous disease – Clinical, Etiologic, Anatomic and Pathophysiologic – and the Venous Clinical Severity Score system. “Patient reported outcomes are now increasingly important to all stakeholders in venous disease space, and these principles will be summarized,” he said.
The session then moves into the anatomy and physiology of venous disorders. “Proper treatment hinges on the understanding of superficial reflux pathways and how to identify them with the ultrasound imaging,” said Dr. Almeida “Venous hypertension can also be caused by obstruction. The hemodynamics from the perspective of our European colleagues will be presented – and the controversial treatments used based on these hemodynamic principles will be reviewed.”
Dr. Bo G. Eklof of the University of Lund will provide the European perspective with an update on the Venous Symposium Consensus (SYMVein), a collaboration of the European Venous Forum and International Working Group to develop a consensus on venous symptoms.
Dr. Kabnick will then explore outcome assessment of central venous disease, and Dr. Wakefield will review the evidence on the pathophysiology of varicose veins. Dr. Seshadri Raju, vascular surgeon at St. Dominic Hospital will explore emerging concepts in venous flow and pressure.
“This will be the talk that ties in the concepts of flow and pressure and how each contributes to the final common pathway of venous disease—namely, venous hypertension,” Dr. Almeida said of Dr. Raju’s presentation.
In keeping with a deeper dive into venous anatomy, Dr. Neil M. Khilnani, associate professor of radiology at Weill Medical College of Cornell University, will discuss the role of duplex ultrasound in identifying reflux pathways, and Dr. Brajesh K. Lal, professor at University of Maryland School of Medicine, will present the latest evidence on venous return pathology. Then Dr. Byung-Boong Lee, professor of surgery at George Washington School of Medicine, will provide an update on the International Union of Phlebology consensus on chronic venous disease
Two presenters from the Riviera Vein Institute in the Principality of Monaco will close out the session. Research chair Dr. Sylvain Chastanet will present 10-year results of the ASVAL – the French acronym for selective ablation of varicose veins under local anesthesia – method for treating varicose veins. And Dr. Paul Pittaluga will wrap things up by analyzing the role of the saphenofemoral junction in planning treatment for varicose veins.
Developing an effective treatment plan for patients with venous disease hinges on an thorough clinical evaluation and a keen understanding of venous anatomy and physiology, both of which will be the focus of the Thursday morning session, “Venous Clinical Examination and Hemodynamics.”
“Less invasive treatments are now available because of the advent of new technologies,” said co-moderator Dr. Jose I. Almeida, director of the Miami Vein Center and voluntary professor of surgery at the University of Miami School of Medicine. “However, the proper treatment of patients with advanced venous disease has little to do with the new toys available on the shelf. Rather, it depends on a proper understanding of the fundamental problem: What causes increased venous pressure?”
Presentations will focus on answering that question. “Without an accurate physician assessment, one cannot apply an effective treatment plan for a patient with venous disease,” said Dr. Almeida. Session co-moderators include Dr. Lowell S. Kabnick, director of the New York University Vein Center, and Dr. Thomas W. Wakefield, vascular surgery section head at the University of Michigan Cardiovascular Center.
“The session begins with identifying symptoms and signs of venous disease, scoring the disease severity with a validated tool, and classifying the severity on a scale of one to six,” said Dr. Almeida, who starts off the session with his own review of the CEAP classification for venous disease – Clinical, Etiologic, Anatomic and Pathophysiologic – and the Venous Clinical Severity Score system. “Patient reported outcomes are now increasingly important to all stakeholders in venous disease space, and these principles will be summarized,” he said.
The session then moves into the anatomy and physiology of venous disorders. “Proper treatment hinges on the understanding of superficial reflux pathways and how to identify them with the ultrasound imaging,” said Dr. Almeida “Venous hypertension can also be caused by obstruction. The hemodynamics from the perspective of our European colleagues will be presented – and the controversial treatments used based on these hemodynamic principles will be reviewed.”
Dr. Bo G. Eklof of the University of Lund will provide the European perspective with an update on the Venous Symposium Consensus (SYMVein), a collaboration of the European Venous Forum and International Working Group to develop a consensus on venous symptoms.
Dr. Kabnick will then explore outcome assessment of central venous disease, and Dr. Wakefield will review the evidence on the pathophysiology of varicose veins. Dr. Seshadri Raju, vascular surgeon at St. Dominic Hospital will explore emerging concepts in venous flow and pressure.
“This will be the talk that ties in the concepts of flow and pressure and how each contributes to the final common pathway of venous disease—namely, venous hypertension,” Dr. Almeida said of Dr. Raju’s presentation.
In keeping with a deeper dive into venous anatomy, Dr. Neil M. Khilnani, associate professor of radiology at Weill Medical College of Cornell University, will discuss the role of duplex ultrasound in identifying reflux pathways, and Dr. Brajesh K. Lal, professor at University of Maryland School of Medicine, will present the latest evidence on venous return pathology. Then Dr. Byung-Boong Lee, professor of surgery at George Washington School of Medicine, will provide an update on the International Union of Phlebology consensus on chronic venous disease
Two presenters from the Riviera Vein Institute in the Principality of Monaco will close out the session. Research chair Dr. Sylvain Chastanet will present 10-year results of the ASVAL – the French acronym for selective ablation of varicose veins under local anesthesia – method for treating varicose veins. And Dr. Paul Pittaluga will wrap things up by analyzing the role of the saphenofemoral junction in planning treatment for varicose veins.
Developing an effective treatment plan for patients with venous disease hinges on an thorough clinical evaluation and a keen understanding of venous anatomy and physiology, both of which will be the focus of the Thursday morning session, “Venous Clinical Examination and Hemodynamics.”
“Less invasive treatments are now available because of the advent of new technologies,” said co-moderator Dr. Jose I. Almeida, director of the Miami Vein Center and voluntary professor of surgery at the University of Miami School of Medicine. “However, the proper treatment of patients with advanced venous disease has little to do with the new toys available on the shelf. Rather, it depends on a proper understanding of the fundamental problem: What causes increased venous pressure?”
Presentations will focus on answering that question. “Without an accurate physician assessment, one cannot apply an effective treatment plan for a patient with venous disease,” said Dr. Almeida. Session co-moderators include Dr. Lowell S. Kabnick, director of the New York University Vein Center, and Dr. Thomas W. Wakefield, vascular surgery section head at the University of Michigan Cardiovascular Center.
“The session begins with identifying symptoms and signs of venous disease, scoring the disease severity with a validated tool, and classifying the severity on a scale of one to six,” said Dr. Almeida, who starts off the session with his own review of the CEAP classification for venous disease – Clinical, Etiologic, Anatomic and Pathophysiologic – and the Venous Clinical Severity Score system. “Patient reported outcomes are now increasingly important to all stakeholders in venous disease space, and these principles will be summarized,” he said.
The session then moves into the anatomy and physiology of venous disorders. “Proper treatment hinges on the understanding of superficial reflux pathways and how to identify them with the ultrasound imaging,” said Dr. Almeida “Venous hypertension can also be caused by obstruction. The hemodynamics from the perspective of our European colleagues will be presented – and the controversial treatments used based on these hemodynamic principles will be reviewed.”
Dr. Bo G. Eklof of the University of Lund will provide the European perspective with an update on the Venous Symposium Consensus (SYMVein), a collaboration of the European Venous Forum and International Working Group to develop a consensus on venous symptoms.
Dr. Kabnick will then explore outcome assessment of central venous disease, and Dr. Wakefield will review the evidence on the pathophysiology of varicose veins. Dr. Seshadri Raju, vascular surgeon at St. Dominic Hospital will explore emerging concepts in venous flow and pressure.
“This will be the talk that ties in the concepts of flow and pressure and how each contributes to the final common pathway of venous disease—namely, venous hypertension,” Dr. Almeida said of Dr. Raju’s presentation.
In keeping with a deeper dive into venous anatomy, Dr. Neil M. Khilnani, associate professor of radiology at Weill Medical College of Cornell University, will discuss the role of duplex ultrasound in identifying reflux pathways, and Dr. Brajesh K. Lal, professor at University of Maryland School of Medicine, will present the latest evidence on venous return pathology. Then Dr. Byung-Boong Lee, professor of surgery at George Washington School of Medicine, will provide an update on the International Union of Phlebology consensus on chronic venous disease
Two presenters from the Riviera Vein Institute in the Principality of Monaco will close out the session. Research chair Dr. Sylvain Chastanet will present 10-year results of the ASVAL – the French acronym for selective ablation of varicose veins under local anesthesia – method for treating varicose veins. And Dr. Paul Pittaluga will wrap things up by analyzing the role of the saphenofemoral junction in planning treatment for varicose veins.
Nivolumab may extend survival in HCC patients
WASHINGTON – A multinational clinical trial has found that the metastatic cancer agent nivolumab can improve long-term survival and durable tumor responses in patients with advanced hepatocellular carcinoma (HCC) whether or not they’ve had previous treatment with a chemotherapy agent already approved for advanced primary liver cancer, a principal investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
“Nivolumab has demonstrated clinically meaningful efficacy across etiologies in sorafenib-naive and -experienced patients with extended follow-up,” Bruno Sangro, MD, of the University of Navarra in Pamplona, Spain, said in reporting results of the CheckMate-040 trial. “The median overall survival is 15 and 15.6 months in patients who were sorafenib-experienced in both the dose-escalation and expansion cohorts.”
The dose-escalation cohort received 0.1 to 10 mg/kg of nivolumab (Opdivo) while the dose-expansion group received a steady dose of 3 mg/kg. In all, 262 patients participated in the trial, 80 of whom had never been on sorafenib (Nexavar) therapy. The survival outcome in these subgroups, Dr. Sangro said, “really speaks for the consistency and the robustness of the results.”
Trial participants had inoperable, usually metastatic HCC, with Child-Pugh scores up to and including 7 in the escalation group or up to and including 6 in the expansion group. Most of them were progressing to treatment with one or more prior systemic therapies, including sorafenib. Their aspartate aminotransferase and alanine aminotransferase scores were in the upper limits of normal, and bilirubin was less than or equal to 3 mg/dL. If they had hepatitis B (HBV), their viral load had to be less than 100 IU/mL and they had to be on effective antiviral therapy. Any history of hepatic encephalopathy or clinically significant ascites and an active HBV and hepatitis C (HCV) coinfection were grounds for exclusion.
“Most patients had to discontinue nivolumab because of disease progression,” Dr. Sangro noted, so that only 36 patients, or 14%, were continuing treatment at the time of this analysis. Thirteen patients in the total population that discontinued nivolumab did so because of toxicity, he said.
“Around 20% of patients achieved an objective remission that included complete responses in all subgroups of patients; 15% of progressors and 23% of sorafenib-intolerant patients had an objective response,” Dr. Sangro said. In terms of overall response, about half of all patients in the sorafenib-experienced subgroups had a complete or partial response or stable disease: 51% in the dose-escalation subgroup and 54% in the dose-expansion subgroup.
Although tumor responses were associated with declines in alpha-fetoprotein levels, “it’s unlikely that these biomarkers will be useful either for monitoring or selecting patients for treatment,” he added. “Indeed, baseline alpha-fetoprotein levels were comparable between responders and nonresponders to nivolumab” Dr. Sangro said.
“We also showed there was some impact on HCV viral kinetics in infected individuals,” Dr. Sangro noted. “The overall safety profile for the HCC population is consistent with other tumor types in which nivolumab is approved; these include patients who are infected with hepatitis B or C viruses.”
The study showed that 36% (19/53) of HCV infected patients had a greater than 1 log decrease in viral load. No signs of additional antiviral activity were detected among HBV-infected patients already on effective antiviral treatment: only 5% (3/59) posted a up to 1 log decrease in HB surface antigen levels, and 11% (7/64) of patients had increases in viral load. “These increases occurred in the setting of low-level viremia.” Dr. Sangro said. “They were asymptomatic and [nivolumab] did not result in changes in hepatic parameters or other serious adverse events.”
With regard to adverse events (AEs), 77% of all patients had some treatment-related AEs, ranging from fatigue to rash to dry mouth to increased lab levels, but only 20% were grade 3 or 4, and 88% of those resolved in an average of 8 weeks, Dr. Sangro said.
More research into nivolumab for HCC is needed, Dr. Sangro said. “Ongoing and future studies in patients with advanced tumors will evaluate nivolumab in the first-line setting or in combination with other agents,” he said.
Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
WASHINGTON – A multinational clinical trial has found that the metastatic cancer agent nivolumab can improve long-term survival and durable tumor responses in patients with advanced hepatocellular carcinoma (HCC) whether or not they’ve had previous treatment with a chemotherapy agent already approved for advanced primary liver cancer, a principal investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
“Nivolumab has demonstrated clinically meaningful efficacy across etiologies in sorafenib-naive and -experienced patients with extended follow-up,” Bruno Sangro, MD, of the University of Navarra in Pamplona, Spain, said in reporting results of the CheckMate-040 trial. “The median overall survival is 15 and 15.6 months in patients who were sorafenib-experienced in both the dose-escalation and expansion cohorts.”
The dose-escalation cohort received 0.1 to 10 mg/kg of nivolumab (Opdivo) while the dose-expansion group received a steady dose of 3 mg/kg. In all, 262 patients participated in the trial, 80 of whom had never been on sorafenib (Nexavar) therapy. The survival outcome in these subgroups, Dr. Sangro said, “really speaks for the consistency and the robustness of the results.”
Trial participants had inoperable, usually metastatic HCC, with Child-Pugh scores up to and including 7 in the escalation group or up to and including 6 in the expansion group. Most of them were progressing to treatment with one or more prior systemic therapies, including sorafenib. Their aspartate aminotransferase and alanine aminotransferase scores were in the upper limits of normal, and bilirubin was less than or equal to 3 mg/dL. If they had hepatitis B (HBV), their viral load had to be less than 100 IU/mL and they had to be on effective antiviral therapy. Any history of hepatic encephalopathy or clinically significant ascites and an active HBV and hepatitis C (HCV) coinfection were grounds for exclusion.
“Most patients had to discontinue nivolumab because of disease progression,” Dr. Sangro noted, so that only 36 patients, or 14%, were continuing treatment at the time of this analysis. Thirteen patients in the total population that discontinued nivolumab did so because of toxicity, he said.
“Around 20% of patients achieved an objective remission that included complete responses in all subgroups of patients; 15% of progressors and 23% of sorafenib-intolerant patients had an objective response,” Dr. Sangro said. In terms of overall response, about half of all patients in the sorafenib-experienced subgroups had a complete or partial response or stable disease: 51% in the dose-escalation subgroup and 54% in the dose-expansion subgroup.
Although tumor responses were associated with declines in alpha-fetoprotein levels, “it’s unlikely that these biomarkers will be useful either for monitoring or selecting patients for treatment,” he added. “Indeed, baseline alpha-fetoprotein levels were comparable between responders and nonresponders to nivolumab” Dr. Sangro said.
“We also showed there was some impact on HCV viral kinetics in infected individuals,” Dr. Sangro noted. “The overall safety profile for the HCC population is consistent with other tumor types in which nivolumab is approved; these include patients who are infected with hepatitis B or C viruses.”
The study showed that 36% (19/53) of HCV infected patients had a greater than 1 log decrease in viral load. No signs of additional antiviral activity were detected among HBV-infected patients already on effective antiviral treatment: only 5% (3/59) posted a up to 1 log decrease in HB surface antigen levels, and 11% (7/64) of patients had increases in viral load. “These increases occurred in the setting of low-level viremia.” Dr. Sangro said. “They were asymptomatic and [nivolumab] did not result in changes in hepatic parameters or other serious adverse events.”
With regard to adverse events (AEs), 77% of all patients had some treatment-related AEs, ranging from fatigue to rash to dry mouth to increased lab levels, but only 20% were grade 3 or 4, and 88% of those resolved in an average of 8 weeks, Dr. Sangro said.
More research into nivolumab for HCC is needed, Dr. Sangro said. “Ongoing and future studies in patients with advanced tumors will evaluate nivolumab in the first-line setting or in combination with other agents,” he said.
Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
WASHINGTON – A multinational clinical trial has found that the metastatic cancer agent nivolumab can improve long-term survival and durable tumor responses in patients with advanced hepatocellular carcinoma (HCC) whether or not they’ve had previous treatment with a chemotherapy agent already approved for advanced primary liver cancer, a principal investigator reported at the annual meeting of the American Association for the Study of Liver Diseases.
“Nivolumab has demonstrated clinically meaningful efficacy across etiologies in sorafenib-naive and -experienced patients with extended follow-up,” Bruno Sangro, MD, of the University of Navarra in Pamplona, Spain, said in reporting results of the CheckMate-040 trial. “The median overall survival is 15 and 15.6 months in patients who were sorafenib-experienced in both the dose-escalation and expansion cohorts.”
The dose-escalation cohort received 0.1 to 10 mg/kg of nivolumab (Opdivo) while the dose-expansion group received a steady dose of 3 mg/kg. In all, 262 patients participated in the trial, 80 of whom had never been on sorafenib (Nexavar) therapy. The survival outcome in these subgroups, Dr. Sangro said, “really speaks for the consistency and the robustness of the results.”
Trial participants had inoperable, usually metastatic HCC, with Child-Pugh scores up to and including 7 in the escalation group or up to and including 6 in the expansion group. Most of them were progressing to treatment with one or more prior systemic therapies, including sorafenib. Their aspartate aminotransferase and alanine aminotransferase scores were in the upper limits of normal, and bilirubin was less than or equal to 3 mg/dL. If they had hepatitis B (HBV), their viral load had to be less than 100 IU/mL and they had to be on effective antiviral therapy. Any history of hepatic encephalopathy or clinically significant ascites and an active HBV and hepatitis C (HCV) coinfection were grounds for exclusion.
“Most patients had to discontinue nivolumab because of disease progression,” Dr. Sangro noted, so that only 36 patients, or 14%, were continuing treatment at the time of this analysis. Thirteen patients in the total population that discontinued nivolumab did so because of toxicity, he said.
“Around 20% of patients achieved an objective remission that included complete responses in all subgroups of patients; 15% of progressors and 23% of sorafenib-intolerant patients had an objective response,” Dr. Sangro said. In terms of overall response, about half of all patients in the sorafenib-experienced subgroups had a complete or partial response or stable disease: 51% in the dose-escalation subgroup and 54% in the dose-expansion subgroup.
Although tumor responses were associated with declines in alpha-fetoprotein levels, “it’s unlikely that these biomarkers will be useful either for monitoring or selecting patients for treatment,” he added. “Indeed, baseline alpha-fetoprotein levels were comparable between responders and nonresponders to nivolumab” Dr. Sangro said.
“We also showed there was some impact on HCV viral kinetics in infected individuals,” Dr. Sangro noted. “The overall safety profile for the HCC population is consistent with other tumor types in which nivolumab is approved; these include patients who are infected with hepatitis B or C viruses.”
The study showed that 36% (19/53) of HCV infected patients had a greater than 1 log decrease in viral load. No signs of additional antiviral activity were detected among HBV-infected patients already on effective antiviral treatment: only 5% (3/59) posted a up to 1 log decrease in HB surface antigen levels, and 11% (7/64) of patients had increases in viral load. “These increases occurred in the setting of low-level viremia.” Dr. Sangro said. “They were asymptomatic and [nivolumab] did not result in changes in hepatic parameters or other serious adverse events.”
With regard to adverse events (AEs), 77% of all patients had some treatment-related AEs, ranging from fatigue to rash to dry mouth to increased lab levels, but only 20% were grade 3 or 4, and 88% of those resolved in an average of 8 weeks, Dr. Sangro said.
More research into nivolumab for HCC is needed, Dr. Sangro said. “Ongoing and future studies in patients with advanced tumors will evaluate nivolumab in the first-line setting or in combination with other agents,” he said.
Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
AT THE LIVER MEETING 2017
Key clinical point: Nivolumab demonstrated long-term survival, durable tumor responses, and manageable overall and hepatic safety profiles, regardless of prior sorafenib treatment, in patients with advanced hepatocellular carcinoma.
Major finding: The 18-month overall survival rate was 57% in sorafenib-naive patients and 46% (dose-escalation) and 44% (dose-expansion) in sorafenib-experienced patients.
Data source: CheckMate-040 phase 1/2 dose-escalation and -expansion trial of 262 patients.
Disclosures: Dr. Sangro disclosed relationships with Bayer Schering Pharma, Onxeo, Astra Zeneca, and Bristol-Myers Squibb. Bristol-Myers Squibb funded the trial, and Chrysalis Medical Communications assisted in reporting the study results.
‘Facebuddha’ analyzes psychology of social media through a Buddhist lens
The new book by Ravi Chandra, MD, is a concise introduction to Buddhism, and a forceful exposition on the power and danger of social networking – deftly interwoven with a moving account of the author’s personal life and professional growth as well as his arduous quest for identity.
Social networking has exploded into a major global industry within the last decade, rapidly penetrating and dominating all aspects of our personal and social lives. Instead of promoting social interactions and connections, the lure of instant intimacy often proves illusory. For far too many, the virtual world deepens their sense of isolation and loneliness, fosters jealousy and narcissism, engenders a profound sense of insecurity, and leads to anxiety, depression, and much worse.
By juxtaposing the Buddha with social networking in the book title, Dr. Chandra expresses his hope and faith that Buddhism could serve as an effective tool for harnessing the force unleashed by these powerful new technologies, helping us to put the genie of our invention back into the bottle. Over the millenia, Buddhism has guided societies and individuals to overcome (“transcend”) crises and adversities, and could play a crucial role in negotiating these still largely uncharted territories.
Despite the popularity of terms such as Zen, meditation, transcendence, and mindfulness, Buddhism remains mysterious and exotic to most modern readers, and is laden with misconceptions and prejudices. This is regrettable, since Buddhism is the most clinically relevant of all major philosophical traditions, and its tenets are most compatible with modern neuroscience. The term philosophical is used here because, at its core, Buddhism represents an uncompromisingly rational approach to dealing with the “human condition.” Siddhartha Gautama (Buddha), its founder, admonished against speculating on questions that are “unanswerables,” such as eternity, existence after death, and the origin and ending of the universe. Instead, Siddhartha focused on identifying life’s vicissitudes (Dukkha, “bumpy rides in life,” commonly translated as “suffering”), clarifying forces responsible for these problems, delineating the ultimate goal, and specifying methods for achieving the goal (the “Four Noble Truths”). He then provided systematic paths for solving problems (the “Eightfold Noble Path”). His approaches are akin to what we clinicians strive to do on a daily basis, albeit on a grander scale: diagnosis, pathogenesis, treatment goals, and therapeutic approaches. The framework Siddhartha proposed is austere, rational, practical. It is exactly for this reason that Siddhartha has been called a great physician, a doctor, and a healer.
As a psychiatrist and a practicing Buddhist, Dr. Chandra is well positioned to critically examine these profoundly important issues (Buddhism and social networking), and he did an excellent job in “Facebuddha.” Impressively, Dr. Chandra’s discussions did not take place in a vacuum. They were not just dry intellectual exercises but were embedded in accounts of personal and clinical experiences, demonstrating the relevance of Buddhist thoughts and practices in real life.
Born in South India and raised by a physician mother in half a dozen American cities, Dr. Chandra experienced repeated uprooting and various types of racial/cultural discrimination. His childhood and adolescence were characterized by a long and arduous search for identity. That he has not only survived, but thrived, is a testament to his resilience and resourcefulness. His love of art and poetry played an important role, as did friendships and the support of Asian American communities. But, above all, it was the Buddha’s teachings and examples that have been the most significant sustaining forces in his life. His accounts are a personal testimony to the power of a 2,500-year-old tradition that is still alive and relevant in our postmodern world.
Dr. Chandra’s book is an endearing chronicle of a remarkable personal journey. Readers will appreciate the opportunity to witness glimpses of this journey and may reasonably expect that such explorations will continue, leading to new vistas that are not only fascinating to behold but also relevant to the practice of our profession.
Dr. Lin is professor emeritus of psychiatry, University of California, Los Angeles, and Distinguished Life Fellow, American Psychiatric Association. He was the founding director of the National Institute of Mental Health/Harbor-UCLA Research Center on the Psychobiology of Ethnicity, the Coastal Asian Pacific Mental Health Center, and the Long Beach Asian Pacific Mental Health Center. The honors Dr. Lin has received include the Kun-Po Soo Asian American Award, American Psychiatric Association; William Sargant Lecturer, Royal College of Psychiatrists, Great Britain; and honorary professor, Hunan (China) Medical University. Information about the book can be found at www.facebuddha.co.
The new book by Ravi Chandra, MD, is a concise introduction to Buddhism, and a forceful exposition on the power and danger of social networking – deftly interwoven with a moving account of the author’s personal life and professional growth as well as his arduous quest for identity.
Social networking has exploded into a major global industry within the last decade, rapidly penetrating and dominating all aspects of our personal and social lives. Instead of promoting social interactions and connections, the lure of instant intimacy often proves illusory. For far too many, the virtual world deepens their sense of isolation and loneliness, fosters jealousy and narcissism, engenders a profound sense of insecurity, and leads to anxiety, depression, and much worse.
By juxtaposing the Buddha with social networking in the book title, Dr. Chandra expresses his hope and faith that Buddhism could serve as an effective tool for harnessing the force unleashed by these powerful new technologies, helping us to put the genie of our invention back into the bottle. Over the millenia, Buddhism has guided societies and individuals to overcome (“transcend”) crises and adversities, and could play a crucial role in negotiating these still largely uncharted territories.
Despite the popularity of terms such as Zen, meditation, transcendence, and mindfulness, Buddhism remains mysterious and exotic to most modern readers, and is laden with misconceptions and prejudices. This is regrettable, since Buddhism is the most clinically relevant of all major philosophical traditions, and its tenets are most compatible with modern neuroscience. The term philosophical is used here because, at its core, Buddhism represents an uncompromisingly rational approach to dealing with the “human condition.” Siddhartha Gautama (Buddha), its founder, admonished against speculating on questions that are “unanswerables,” such as eternity, existence after death, and the origin and ending of the universe. Instead, Siddhartha focused on identifying life’s vicissitudes (Dukkha, “bumpy rides in life,” commonly translated as “suffering”), clarifying forces responsible for these problems, delineating the ultimate goal, and specifying methods for achieving the goal (the “Four Noble Truths”). He then provided systematic paths for solving problems (the “Eightfold Noble Path”). His approaches are akin to what we clinicians strive to do on a daily basis, albeit on a grander scale: diagnosis, pathogenesis, treatment goals, and therapeutic approaches. The framework Siddhartha proposed is austere, rational, practical. It is exactly for this reason that Siddhartha has been called a great physician, a doctor, and a healer.
As a psychiatrist and a practicing Buddhist, Dr. Chandra is well positioned to critically examine these profoundly important issues (Buddhism and social networking), and he did an excellent job in “Facebuddha.” Impressively, Dr. Chandra’s discussions did not take place in a vacuum. They were not just dry intellectual exercises but were embedded in accounts of personal and clinical experiences, demonstrating the relevance of Buddhist thoughts and practices in real life.
Born in South India and raised by a physician mother in half a dozen American cities, Dr. Chandra experienced repeated uprooting and various types of racial/cultural discrimination. His childhood and adolescence were characterized by a long and arduous search for identity. That he has not only survived, but thrived, is a testament to his resilience and resourcefulness. His love of art and poetry played an important role, as did friendships and the support of Asian American communities. But, above all, it was the Buddha’s teachings and examples that have been the most significant sustaining forces in his life. His accounts are a personal testimony to the power of a 2,500-year-old tradition that is still alive and relevant in our postmodern world.
Dr. Chandra’s book is an endearing chronicle of a remarkable personal journey. Readers will appreciate the opportunity to witness glimpses of this journey and may reasonably expect that such explorations will continue, leading to new vistas that are not only fascinating to behold but also relevant to the practice of our profession.
Dr. Lin is professor emeritus of psychiatry, University of California, Los Angeles, and Distinguished Life Fellow, American Psychiatric Association. He was the founding director of the National Institute of Mental Health/Harbor-UCLA Research Center on the Psychobiology of Ethnicity, the Coastal Asian Pacific Mental Health Center, and the Long Beach Asian Pacific Mental Health Center. The honors Dr. Lin has received include the Kun-Po Soo Asian American Award, American Psychiatric Association; William Sargant Lecturer, Royal College of Psychiatrists, Great Britain; and honorary professor, Hunan (China) Medical University. Information about the book can be found at www.facebuddha.co.
The new book by Ravi Chandra, MD, is a concise introduction to Buddhism, and a forceful exposition on the power and danger of social networking – deftly interwoven with a moving account of the author’s personal life and professional growth as well as his arduous quest for identity.
Social networking has exploded into a major global industry within the last decade, rapidly penetrating and dominating all aspects of our personal and social lives. Instead of promoting social interactions and connections, the lure of instant intimacy often proves illusory. For far too many, the virtual world deepens their sense of isolation and loneliness, fosters jealousy and narcissism, engenders a profound sense of insecurity, and leads to anxiety, depression, and much worse.
By juxtaposing the Buddha with social networking in the book title, Dr. Chandra expresses his hope and faith that Buddhism could serve as an effective tool for harnessing the force unleashed by these powerful new technologies, helping us to put the genie of our invention back into the bottle. Over the millenia, Buddhism has guided societies and individuals to overcome (“transcend”) crises and adversities, and could play a crucial role in negotiating these still largely uncharted territories.
Despite the popularity of terms such as Zen, meditation, transcendence, and mindfulness, Buddhism remains mysterious and exotic to most modern readers, and is laden with misconceptions and prejudices. This is regrettable, since Buddhism is the most clinically relevant of all major philosophical traditions, and its tenets are most compatible with modern neuroscience. The term philosophical is used here because, at its core, Buddhism represents an uncompromisingly rational approach to dealing with the “human condition.” Siddhartha Gautama (Buddha), its founder, admonished against speculating on questions that are “unanswerables,” such as eternity, existence after death, and the origin and ending of the universe. Instead, Siddhartha focused on identifying life’s vicissitudes (Dukkha, “bumpy rides in life,” commonly translated as “suffering”), clarifying forces responsible for these problems, delineating the ultimate goal, and specifying methods for achieving the goal (the “Four Noble Truths”). He then provided systematic paths for solving problems (the “Eightfold Noble Path”). His approaches are akin to what we clinicians strive to do on a daily basis, albeit on a grander scale: diagnosis, pathogenesis, treatment goals, and therapeutic approaches. The framework Siddhartha proposed is austere, rational, practical. It is exactly for this reason that Siddhartha has been called a great physician, a doctor, and a healer.
As a psychiatrist and a practicing Buddhist, Dr. Chandra is well positioned to critically examine these profoundly important issues (Buddhism and social networking), and he did an excellent job in “Facebuddha.” Impressively, Dr. Chandra’s discussions did not take place in a vacuum. They were not just dry intellectual exercises but were embedded in accounts of personal and clinical experiences, demonstrating the relevance of Buddhist thoughts and practices in real life.
Born in South India and raised by a physician mother in half a dozen American cities, Dr. Chandra experienced repeated uprooting and various types of racial/cultural discrimination. His childhood and adolescence were characterized by a long and arduous search for identity. That he has not only survived, but thrived, is a testament to his resilience and resourcefulness. His love of art and poetry played an important role, as did friendships and the support of Asian American communities. But, above all, it was the Buddha’s teachings and examples that have been the most significant sustaining forces in his life. His accounts are a personal testimony to the power of a 2,500-year-old tradition that is still alive and relevant in our postmodern world.
Dr. Chandra’s book is an endearing chronicle of a remarkable personal journey. Readers will appreciate the opportunity to witness glimpses of this journey and may reasonably expect that such explorations will continue, leading to new vistas that are not only fascinating to behold but also relevant to the practice of our profession.
Dr. Lin is professor emeritus of psychiatry, University of California, Los Angeles, and Distinguished Life Fellow, American Psychiatric Association. He was the founding director of the National Institute of Mental Health/Harbor-UCLA Research Center on the Psychobiology of Ethnicity, the Coastal Asian Pacific Mental Health Center, and the Long Beach Asian Pacific Mental Health Center. The honors Dr. Lin has received include the Kun-Po Soo Asian American Award, American Psychiatric Association; William Sargant Lecturer, Royal College of Psychiatrists, Great Britain; and honorary professor, Hunan (China) Medical University. Information about the book can be found at www.facebuddha.co.
Nemolizumab continues to crush itch in 64-week atopic dermatitis study
GENEVA – Nemolizumab, a humanized monoclonal antibody that inhibits interleukin-31 signaling, maintained its early dramatic antipruritic effect in patients with moderate to severe atopic dermatitis (AD) throughout a year-long unblinded extension of a large, high-profile, 12-week, phase 2 randomized trial, Thomas Ruzicka, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In contrast, the 52-week extension study also showed the improvement in the dermatitis aspect of AD as measured by Eczema Area and Severity Index (EASI) scores was more gradual and less robust.
The study, 64 weeks in total, also demonstrated that what really matters to patients with moderate or severe AD is relief from the itch. Scores on the Dermatology Life Quality Index showed marked improvement even if their improvement in EASI scores was suboptimal.
“The dermatitis scores improved. But the most concerning symptom to patients is the pruritus; that’s what worsens their quality of life. And this improves dramatically very early in the course of the study,” Dr. Ruzicka explained. “If they drop their itch they are very happy. They don’t care about the redness of the skin so much as the pruritus. This is what really bothers them, as has been shown in epidemiologic studies.”
The original 12-week, phase 2, randomized, double-blind, dose-ranging trial drew extensive attention because it demonstrated convincingly for the first time that the inflammatory cytokine interleukin-31 plays a key role in the pathobiology of AD by promoting skin barrier dysfunction, pruritus, and the inflammatory response in AD – and that a biologic agent, nemolizumab, could inhibit those disease mechanisms (N Engl J Med. 2017 Mar 2;376[9]:826-35).
At a dose of 0.5 mg/kg administered by subcutaneous injection every 4 weeks, nemolizumab, a humanized monoclonal antibody directed against interleukin-31 receptor A, reduced scores on the pruritus visual-analogue scale by 60% as early as 4 weeks and maintained that effect through 12 weeks. At that point, the double-blind study ended and the 52-week extension began. Patients on nemolizumab in the double-blind phase stayed on the same dosage for the extension, while those who’d been on placebo were switched to nemolizumab at 0.1, 0.5, or 2.0 mg/kg every 4 weeks.
A total of 211 patients with moderate to severe AD inadequately controlled by topical therapies enrolled in the extension study. The combined 64-week experience convinced investigators and the sponsor, Chugai Pharmaceutical, that 0.5 mg/kg every 4 weeks is the optimal dose to take forward into planned advanced-stage clinical trials.
“At this dosage the IL-31 receptor is saturated, so higher dosages aren’t needed,” according to Dr. Ruzicka.
By week 64, patients in the 0.5-mg/kg arm of the study showed further gradual improvement in their pruritus visual-analogue score, from a 60% reduction from baseline at 12 weeks to close to an 80% reduction at week 64.
Roughly half of nemolizumab-treated patients achieved an EASI-50 response within the first 4 weeks of the double-blind phase of the study and stayed in that response zone throughout the extension study.
The EASI-75 and -90 responses followed a pattern different from EASI-50. Patients didn’t leap to those more robust levels of response early and then plateau. Instead, the EASI-75 and -90 responses were achieved via a gradual climb in efficacy throughout the study, such that by week 64 roughly 35%-40% of patients on the various nemolizumab dosages had reached EASI-75.
In the extension study, patients were permitted to use a mild topical steroid or topical calcineurin inhibitor as needed, and a potent or very potent topical steroid as rescue medication. Roughly half of participants resorted to any topical steroid at least once during the 64 months. An important new observation gleaned in the long-term study was that the patients who did use topical steroids were twice as likely to reach an EASI 75 response.
Roughly 20% of patients on the three highest dosages of nemolizumab achieved a static Investigator’s Global Assessment score of 0 or 1, meaning clear or almost clear, by week 64.
Scores on the Dermatology Life Quality Index fell steadily over the course of 64 weeks, from a baseline of about 16 to 5 at study’s close.
The side effect profile of nemolizumab was essentially the same as seen in the placebo arm during the double-blind phase. About one-quarter of patients experienced nasopharyngitis over the course of 64 weeks of treatment. A similar fraction reported exacerbations of their atopic dermatitis; however, these events were front-loaded in the first weeks of the initial double-blind study phase and appeared to have been triggered by the drug washout period prior to the patients’ receiving the first dose of nemolizumab, according to Dr. Ruzicka. No new safety signals emerged during the additional 52 weeks of treatment.
The study was funded by Chugai. Dr. Ruzicka reported serving as a paid company adviser.
GENEVA – Nemolizumab, a humanized monoclonal antibody that inhibits interleukin-31 signaling, maintained its early dramatic antipruritic effect in patients with moderate to severe atopic dermatitis (AD) throughout a year-long unblinded extension of a large, high-profile, 12-week, phase 2 randomized trial, Thomas Ruzicka, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In contrast, the 52-week extension study also showed the improvement in the dermatitis aspect of AD as measured by Eczema Area and Severity Index (EASI) scores was more gradual and less robust.
The study, 64 weeks in total, also demonstrated that what really matters to patients with moderate or severe AD is relief from the itch. Scores on the Dermatology Life Quality Index showed marked improvement even if their improvement in EASI scores was suboptimal.
“The dermatitis scores improved. But the most concerning symptom to patients is the pruritus; that’s what worsens their quality of life. And this improves dramatically very early in the course of the study,” Dr. Ruzicka explained. “If they drop their itch they are very happy. They don’t care about the redness of the skin so much as the pruritus. This is what really bothers them, as has been shown in epidemiologic studies.”
The original 12-week, phase 2, randomized, double-blind, dose-ranging trial drew extensive attention because it demonstrated convincingly for the first time that the inflammatory cytokine interleukin-31 plays a key role in the pathobiology of AD by promoting skin barrier dysfunction, pruritus, and the inflammatory response in AD – and that a biologic agent, nemolizumab, could inhibit those disease mechanisms (N Engl J Med. 2017 Mar 2;376[9]:826-35).
At a dose of 0.5 mg/kg administered by subcutaneous injection every 4 weeks, nemolizumab, a humanized monoclonal antibody directed against interleukin-31 receptor A, reduced scores on the pruritus visual-analogue scale by 60% as early as 4 weeks and maintained that effect through 12 weeks. At that point, the double-blind study ended and the 52-week extension began. Patients on nemolizumab in the double-blind phase stayed on the same dosage for the extension, while those who’d been on placebo were switched to nemolizumab at 0.1, 0.5, or 2.0 mg/kg every 4 weeks.
A total of 211 patients with moderate to severe AD inadequately controlled by topical therapies enrolled in the extension study. The combined 64-week experience convinced investigators and the sponsor, Chugai Pharmaceutical, that 0.5 mg/kg every 4 weeks is the optimal dose to take forward into planned advanced-stage clinical trials.
“At this dosage the IL-31 receptor is saturated, so higher dosages aren’t needed,” according to Dr. Ruzicka.
By week 64, patients in the 0.5-mg/kg arm of the study showed further gradual improvement in their pruritus visual-analogue score, from a 60% reduction from baseline at 12 weeks to close to an 80% reduction at week 64.
Roughly half of nemolizumab-treated patients achieved an EASI-50 response within the first 4 weeks of the double-blind phase of the study and stayed in that response zone throughout the extension study.
The EASI-75 and -90 responses followed a pattern different from EASI-50. Patients didn’t leap to those more robust levels of response early and then plateau. Instead, the EASI-75 and -90 responses were achieved via a gradual climb in efficacy throughout the study, such that by week 64 roughly 35%-40% of patients on the various nemolizumab dosages had reached EASI-75.
In the extension study, patients were permitted to use a mild topical steroid or topical calcineurin inhibitor as needed, and a potent or very potent topical steroid as rescue medication. Roughly half of participants resorted to any topical steroid at least once during the 64 months. An important new observation gleaned in the long-term study was that the patients who did use topical steroids were twice as likely to reach an EASI 75 response.
Roughly 20% of patients on the three highest dosages of nemolizumab achieved a static Investigator’s Global Assessment score of 0 or 1, meaning clear or almost clear, by week 64.
Scores on the Dermatology Life Quality Index fell steadily over the course of 64 weeks, from a baseline of about 16 to 5 at study’s close.
The side effect profile of nemolizumab was essentially the same as seen in the placebo arm during the double-blind phase. About one-quarter of patients experienced nasopharyngitis over the course of 64 weeks of treatment. A similar fraction reported exacerbations of their atopic dermatitis; however, these events were front-loaded in the first weeks of the initial double-blind study phase and appeared to have been triggered by the drug washout period prior to the patients’ receiving the first dose of nemolizumab, according to Dr. Ruzicka. No new safety signals emerged during the additional 52 weeks of treatment.
The study was funded by Chugai. Dr. Ruzicka reported serving as a paid company adviser.
GENEVA – Nemolizumab, a humanized monoclonal antibody that inhibits interleukin-31 signaling, maintained its early dramatic antipruritic effect in patients with moderate to severe atopic dermatitis (AD) throughout a year-long unblinded extension of a large, high-profile, 12-week, phase 2 randomized trial, Thomas Ruzicka, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
In contrast, the 52-week extension study also showed the improvement in the dermatitis aspect of AD as measured by Eczema Area and Severity Index (EASI) scores was more gradual and less robust.
The study, 64 weeks in total, also demonstrated that what really matters to patients with moderate or severe AD is relief from the itch. Scores on the Dermatology Life Quality Index showed marked improvement even if their improvement in EASI scores was suboptimal.
“The dermatitis scores improved. But the most concerning symptom to patients is the pruritus; that’s what worsens their quality of life. And this improves dramatically very early in the course of the study,” Dr. Ruzicka explained. “If they drop their itch they are very happy. They don’t care about the redness of the skin so much as the pruritus. This is what really bothers them, as has been shown in epidemiologic studies.”
The original 12-week, phase 2, randomized, double-blind, dose-ranging trial drew extensive attention because it demonstrated convincingly for the first time that the inflammatory cytokine interleukin-31 plays a key role in the pathobiology of AD by promoting skin barrier dysfunction, pruritus, and the inflammatory response in AD – and that a biologic agent, nemolizumab, could inhibit those disease mechanisms (N Engl J Med. 2017 Mar 2;376[9]:826-35).
At a dose of 0.5 mg/kg administered by subcutaneous injection every 4 weeks, nemolizumab, a humanized monoclonal antibody directed against interleukin-31 receptor A, reduced scores on the pruritus visual-analogue scale by 60% as early as 4 weeks and maintained that effect through 12 weeks. At that point, the double-blind study ended and the 52-week extension began. Patients on nemolizumab in the double-blind phase stayed on the same dosage for the extension, while those who’d been on placebo were switched to nemolizumab at 0.1, 0.5, or 2.0 mg/kg every 4 weeks.
A total of 211 patients with moderate to severe AD inadequately controlled by topical therapies enrolled in the extension study. The combined 64-week experience convinced investigators and the sponsor, Chugai Pharmaceutical, that 0.5 mg/kg every 4 weeks is the optimal dose to take forward into planned advanced-stage clinical trials.
“At this dosage the IL-31 receptor is saturated, so higher dosages aren’t needed,” according to Dr. Ruzicka.
By week 64, patients in the 0.5-mg/kg arm of the study showed further gradual improvement in their pruritus visual-analogue score, from a 60% reduction from baseline at 12 weeks to close to an 80% reduction at week 64.
Roughly half of nemolizumab-treated patients achieved an EASI-50 response within the first 4 weeks of the double-blind phase of the study and stayed in that response zone throughout the extension study.
The EASI-75 and -90 responses followed a pattern different from EASI-50. Patients didn’t leap to those more robust levels of response early and then plateau. Instead, the EASI-75 and -90 responses were achieved via a gradual climb in efficacy throughout the study, such that by week 64 roughly 35%-40% of patients on the various nemolizumab dosages had reached EASI-75.
In the extension study, patients were permitted to use a mild topical steroid or topical calcineurin inhibitor as needed, and a potent or very potent topical steroid as rescue medication. Roughly half of participants resorted to any topical steroid at least once during the 64 months. An important new observation gleaned in the long-term study was that the patients who did use topical steroids were twice as likely to reach an EASI 75 response.
Roughly 20% of patients on the three highest dosages of nemolizumab achieved a static Investigator’s Global Assessment score of 0 or 1, meaning clear or almost clear, by week 64.
Scores on the Dermatology Life Quality Index fell steadily over the course of 64 weeks, from a baseline of about 16 to 5 at study’s close.
The side effect profile of nemolizumab was essentially the same as seen in the placebo arm during the double-blind phase. About one-quarter of patients experienced nasopharyngitis over the course of 64 weeks of treatment. A similar fraction reported exacerbations of their atopic dermatitis; however, these events were front-loaded in the first weeks of the initial double-blind study phase and appeared to have been triggered by the drug washout period prior to the patients’ receiving the first dose of nemolizumab, according to Dr. Ruzicka. No new safety signals emerged during the additional 52 weeks of treatment.
The study was funded by Chugai. Dr. Ruzicka reported serving as a paid company adviser.
AT THE EADV CONGRESS
Key clinical point:
Major finding: The early dramatic antipruritic effect demonstrated by nemolizumab for atopic dermatitis in a 12-week randomized trial was maintained throughout an additional 52 weeks in an extension study.
Data source: This analysis focused on 211 patients with moderate to severe atopic dermatitis who participated in a 52-week open-label extension study after completing a previously reported 12-week, double-blind, placebo-controlled phase.
Disclosures: The presenter is a paid medical adviser to Chugai Pharmaceutical, which funded the study.
Top research to be presented at AAGL
The 46th AAGL Global Congress on Minimally Invasive Gynecologic Surgery starts Nov. 12, 2017, in National Harbor, Md., and attendees will have a chance to hear presentations on more than 300 studies, plus numerous virtual posters.
Dr. Charles E. Miller, a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL, offered his top picks for not-to-be-missed research at this year’s meeting.
Cesarean-induced isthmoceles
On Wednesday, Nov. 15, at 12:17 p.m., researchers from West Virginia University in Morgantown and Universidad Autónoma de Nuevo León in Mexico will present data from a prospective study on the anatomy of cesarean-induced isthmoceles. The paper won the Golden Hysteroscope Award for best paper on hysteroscopy. It is being presented during the Open Communications 13 session on reproductive medicine.
“Isthmocele has become such a hot topic,” Dr. Miller said. “Besides the implications in terms of pelvic pain and abnormal bleeding, it can be a cause of infertility as fluid goes into the endometrial cavity and impacts implantation.”
Dr. Miller will be performing a telesurgery featuring robotic-assisted excision and repair of a cesarean section isthmocele on Thursday, Nov. 16, as part of General Session V from 8:30 a.m. to 12:30 p.m.
Rectosigmoid endometriosis
On Wednesday, Nov. 15, at 12:50 p.m., researchers from the University of Pittsburgh will show a surgical video on anterior discoid resection for rectosigmoid endometriosis. They use various laparoscopic instruments and techniques to assess and resect the nodule, including a “squeeze” technique, barbed suture, and a V-shaped closure. The video, which won the Golden Laparoscope Award for best surgical video, is being presented during the Plenary 6 session on endometriosis and adenomyosis.
“There is great debate in just how aggressively patients should be treated when a woman has deep infiltrative endometriosis involving the rectosigmoid area,” Dr. Miller said.
Most of the research in this area is from single-institution studies that do not always completely describe the procedure, leaving surgeons “unsure of which way to go,” Dr. Miller said. In addition, because many patients with endometriosis are young, surgeons need to consider how the procedure will impact them in 20 or even 50 years. While more aggressive than shaving, discoid resection is less aggressive than standard bowel resection.
Postsurgical pain control
On Tuesday, Nov. 14, at 3:46 p.m., researchers from the University of Pittsburgh, Oregon Health & Science University, Southern California Permanente Medical Group, and the University of Wisconsin, Madison, will present results from a prospective, double-blind, randomized study comparing intravenous acetaminophen with placebo for postsurgical pain control and patient satisfaction after laparoscopic hysterectomy. Their findings indicate no difference in either pain or satisfaction, casting doubt on routine use during hysterectomy. The study, which won the Jay M. Cooper Award for best paper on minimally invasive gynecology by a fellow, will be presented during the Open Communications 9 session on laparoscopy.
On Tuesday, Nov. 14, at 1:21 p.m., researchers from the University of Maryland, Baltimore; Mercy Medical Center, Baltimore; and Yoyodyne General Services, New York, will present a single-center, double-blind, randomized, placebo-controlled trial to assess the use of a single belladonna and opium suppository placed after laparoscopic or robotic hysterectomy to control postoperative pain. As with acetaminophen, they also found that the suppositories did not significantly lower pain or narcotic use. However, the belladonna/opium suppository reduced time to discharge from the postanesthesia care unit in phase I. The research, which won the Jerome J. Hoffman Award for best abstract by a resident or fellow, will be presented during Session 2 of the Virtual Posters.
“Here again are two treatments that really have minimal basis,” Dr. Miller said. “In the days of cost containment, is there really any reason for either?”
Cervical ripening
Dr. Miller also recommended that attendees take note of a randomized controlled trial evaluating whether misoprostol oral is as effective as vaginal tablets for cervical ripening. Researchers at Cairo University in Egypt considered this question among more than 350 women who were undergoing operative hysterectomy for various indications. They found no statistically significant difference in efficacy and similar adverse effects.
“There has been some concern raised about, is there a better way?” Dr. Miller said. “This is especially important as we move hysteroscopy to the office.”
The cervical priming study, which won the Robert B. Hunt Award for best paper published in the Journal of Minimally Invasive Gynecology between September 2016 and August 2017, will be presented on Tuesday, Nov. 14, at 7:10 a.m. during the journal’s editorial/advisory board breakfast. You can read the full article online (J Minim Invasive Gynecol. 2016 Nov - Dec;23[7]:1107-12).
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The 46th AAGL Global Congress on Minimally Invasive Gynecologic Surgery starts Nov. 12, 2017, in National Harbor, Md., and attendees will have a chance to hear presentations on more than 300 studies, plus numerous virtual posters.
Dr. Charles E. Miller, a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL, offered his top picks for not-to-be-missed research at this year’s meeting.
Cesarean-induced isthmoceles
On Wednesday, Nov. 15, at 12:17 p.m., researchers from West Virginia University in Morgantown and Universidad Autónoma de Nuevo León in Mexico will present data from a prospective study on the anatomy of cesarean-induced isthmoceles. The paper won the Golden Hysteroscope Award for best paper on hysteroscopy. It is being presented during the Open Communications 13 session on reproductive medicine.
“Isthmocele has become such a hot topic,” Dr. Miller said. “Besides the implications in terms of pelvic pain and abnormal bleeding, it can be a cause of infertility as fluid goes into the endometrial cavity and impacts implantation.”
Dr. Miller will be performing a telesurgery featuring robotic-assisted excision and repair of a cesarean section isthmocele on Thursday, Nov. 16, as part of General Session V from 8:30 a.m. to 12:30 p.m.
Rectosigmoid endometriosis
On Wednesday, Nov. 15, at 12:50 p.m., researchers from the University of Pittsburgh will show a surgical video on anterior discoid resection for rectosigmoid endometriosis. They use various laparoscopic instruments and techniques to assess and resect the nodule, including a “squeeze” technique, barbed suture, and a V-shaped closure. The video, which won the Golden Laparoscope Award for best surgical video, is being presented during the Plenary 6 session on endometriosis and adenomyosis.
“There is great debate in just how aggressively patients should be treated when a woman has deep infiltrative endometriosis involving the rectosigmoid area,” Dr. Miller said.
Most of the research in this area is from single-institution studies that do not always completely describe the procedure, leaving surgeons “unsure of which way to go,” Dr. Miller said. In addition, because many patients with endometriosis are young, surgeons need to consider how the procedure will impact them in 20 or even 50 years. While more aggressive than shaving, discoid resection is less aggressive than standard bowel resection.
Postsurgical pain control
On Tuesday, Nov. 14, at 3:46 p.m., researchers from the University of Pittsburgh, Oregon Health & Science University, Southern California Permanente Medical Group, and the University of Wisconsin, Madison, will present results from a prospective, double-blind, randomized study comparing intravenous acetaminophen with placebo for postsurgical pain control and patient satisfaction after laparoscopic hysterectomy. Their findings indicate no difference in either pain or satisfaction, casting doubt on routine use during hysterectomy. The study, which won the Jay M. Cooper Award for best paper on minimally invasive gynecology by a fellow, will be presented during the Open Communications 9 session on laparoscopy.
On Tuesday, Nov. 14, at 1:21 p.m., researchers from the University of Maryland, Baltimore; Mercy Medical Center, Baltimore; and Yoyodyne General Services, New York, will present a single-center, double-blind, randomized, placebo-controlled trial to assess the use of a single belladonna and opium suppository placed after laparoscopic or robotic hysterectomy to control postoperative pain. As with acetaminophen, they also found that the suppositories did not significantly lower pain or narcotic use. However, the belladonna/opium suppository reduced time to discharge from the postanesthesia care unit in phase I. The research, which won the Jerome J. Hoffman Award for best abstract by a resident or fellow, will be presented during Session 2 of the Virtual Posters.
“Here again are two treatments that really have minimal basis,” Dr. Miller said. “In the days of cost containment, is there really any reason for either?”
Cervical ripening
Dr. Miller also recommended that attendees take note of a randomized controlled trial evaluating whether misoprostol oral is as effective as vaginal tablets for cervical ripening. Researchers at Cairo University in Egypt considered this question among more than 350 women who were undergoing operative hysterectomy for various indications. They found no statistically significant difference in efficacy and similar adverse effects.
“There has been some concern raised about, is there a better way?” Dr. Miller said. “This is especially important as we move hysteroscopy to the office.”
The cervical priming study, which won the Robert B. Hunt Award for best paper published in the Journal of Minimally Invasive Gynecology between September 2016 and August 2017, will be presented on Tuesday, Nov. 14, at 7:10 a.m. during the journal’s editorial/advisory board breakfast. You can read the full article online (J Minim Invasive Gynecol. 2016 Nov - Dec;23[7]:1107-12).
mschneider@frontlinemedcom.com
On Twitter @maryellenny
The 46th AAGL Global Congress on Minimally Invasive Gynecologic Surgery starts Nov. 12, 2017, in National Harbor, Md., and attendees will have a chance to hear presentations on more than 300 studies, plus numerous virtual posters.
Dr. Charles E. Miller, a minimally invasive gynecologic surgeon in Naperville, Ill., and a past president of the AAGL, offered his top picks for not-to-be-missed research at this year’s meeting.
Cesarean-induced isthmoceles
On Wednesday, Nov. 15, at 12:17 p.m., researchers from West Virginia University in Morgantown and Universidad Autónoma de Nuevo León in Mexico will present data from a prospective study on the anatomy of cesarean-induced isthmoceles. The paper won the Golden Hysteroscope Award for best paper on hysteroscopy. It is being presented during the Open Communications 13 session on reproductive medicine.
“Isthmocele has become such a hot topic,” Dr. Miller said. “Besides the implications in terms of pelvic pain and abnormal bleeding, it can be a cause of infertility as fluid goes into the endometrial cavity and impacts implantation.”
Dr. Miller will be performing a telesurgery featuring robotic-assisted excision and repair of a cesarean section isthmocele on Thursday, Nov. 16, as part of General Session V from 8:30 a.m. to 12:30 p.m.
Rectosigmoid endometriosis
On Wednesday, Nov. 15, at 12:50 p.m., researchers from the University of Pittsburgh will show a surgical video on anterior discoid resection for rectosigmoid endometriosis. They use various laparoscopic instruments and techniques to assess and resect the nodule, including a “squeeze” technique, barbed suture, and a V-shaped closure. The video, which won the Golden Laparoscope Award for best surgical video, is being presented during the Plenary 6 session on endometriosis and adenomyosis.
“There is great debate in just how aggressively patients should be treated when a woman has deep infiltrative endometriosis involving the rectosigmoid area,” Dr. Miller said.
Most of the research in this area is from single-institution studies that do not always completely describe the procedure, leaving surgeons “unsure of which way to go,” Dr. Miller said. In addition, because many patients with endometriosis are young, surgeons need to consider how the procedure will impact them in 20 or even 50 years. While more aggressive than shaving, discoid resection is less aggressive than standard bowel resection.
Postsurgical pain control
On Tuesday, Nov. 14, at 3:46 p.m., researchers from the University of Pittsburgh, Oregon Health & Science University, Southern California Permanente Medical Group, and the University of Wisconsin, Madison, will present results from a prospective, double-blind, randomized study comparing intravenous acetaminophen with placebo for postsurgical pain control and patient satisfaction after laparoscopic hysterectomy. Their findings indicate no difference in either pain or satisfaction, casting doubt on routine use during hysterectomy. The study, which won the Jay M. Cooper Award for best paper on minimally invasive gynecology by a fellow, will be presented during the Open Communications 9 session on laparoscopy.
On Tuesday, Nov. 14, at 1:21 p.m., researchers from the University of Maryland, Baltimore; Mercy Medical Center, Baltimore; and Yoyodyne General Services, New York, will present a single-center, double-blind, randomized, placebo-controlled trial to assess the use of a single belladonna and opium suppository placed after laparoscopic or robotic hysterectomy to control postoperative pain. As with acetaminophen, they also found that the suppositories did not significantly lower pain or narcotic use. However, the belladonna/opium suppository reduced time to discharge from the postanesthesia care unit in phase I. The research, which won the Jerome J. Hoffman Award for best abstract by a resident or fellow, will be presented during Session 2 of the Virtual Posters.
“Here again are two treatments that really have minimal basis,” Dr. Miller said. “In the days of cost containment, is there really any reason for either?”
Cervical ripening
Dr. Miller also recommended that attendees take note of a randomized controlled trial evaluating whether misoprostol oral is as effective as vaginal tablets for cervical ripening. Researchers at Cairo University in Egypt considered this question among more than 350 women who were undergoing operative hysterectomy for various indications. They found no statistically significant difference in efficacy and similar adverse effects.
“There has been some concern raised about, is there a better way?” Dr. Miller said. “This is especially important as we move hysteroscopy to the office.”
The cervical priming study, which won the Robert B. Hunt Award for best paper published in the Journal of Minimally Invasive Gynecology between September 2016 and August 2017, will be presented on Tuesday, Nov. 14, at 7:10 a.m. during the journal’s editorial/advisory board breakfast. You can read the full article online (J Minim Invasive Gynecol. 2016 Nov - Dec;23[7]:1107-12).
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Case study suggests maternal inheritance of PBC susceptibility
A case report involving a family with four sisters with primary biliary cholangitis (PBC) suggests that there may be a maternal inheritance of susceptibility, according to Saeam Shin, MD, and associates at Hallym University in Seoul, South Korea.
In the first case, a 56-year-old woman was diagnosed with PBC, and afterwards, her three sisters, her brother, and her half-sister born to a different mother were evaluated for PBC as well. The second and fourth sisters showed no symptoms, but they were antimitochondrial-antibody (AMA) positive and were diagnosed with PBC. The third sister had been admitted to a different hospital for acute hepatitis of unknown origin – after receiving a positive AMA test, she also was diagnosed with PBC.
The brother and half-sister evaluated for PBC showed no symptoms and had negative AMA tests. The four sisters diagnosed showed good response to ursodeoxycholic acid in liver biochemistry tests and have continued on that medication without complication.
“If one patient is diagnosed with PBC, screening with AMA and liver function tests should be recommended to other family members for the early detection and management of this condition, especially for female relatives,” the investigators wrote.
Find the full case report in the World Journal of Gastroenterology (doi: 10.3748/wjg.v23.i39.7191).
A case report involving a family with four sisters with primary biliary cholangitis (PBC) suggests that there may be a maternal inheritance of susceptibility, according to Saeam Shin, MD, and associates at Hallym University in Seoul, South Korea.
In the first case, a 56-year-old woman was diagnosed with PBC, and afterwards, her three sisters, her brother, and her half-sister born to a different mother were evaluated for PBC as well. The second and fourth sisters showed no symptoms, but they were antimitochondrial-antibody (AMA) positive and were diagnosed with PBC. The third sister had been admitted to a different hospital for acute hepatitis of unknown origin – after receiving a positive AMA test, she also was diagnosed with PBC.
The brother and half-sister evaluated for PBC showed no symptoms and had negative AMA tests. The four sisters diagnosed showed good response to ursodeoxycholic acid in liver biochemistry tests and have continued on that medication without complication.
“If one patient is diagnosed with PBC, screening with AMA and liver function tests should be recommended to other family members for the early detection and management of this condition, especially for female relatives,” the investigators wrote.
Find the full case report in the World Journal of Gastroenterology (doi: 10.3748/wjg.v23.i39.7191).
A case report involving a family with four sisters with primary biliary cholangitis (PBC) suggests that there may be a maternal inheritance of susceptibility, according to Saeam Shin, MD, and associates at Hallym University in Seoul, South Korea.
In the first case, a 56-year-old woman was diagnosed with PBC, and afterwards, her three sisters, her brother, and her half-sister born to a different mother were evaluated for PBC as well. The second and fourth sisters showed no symptoms, but they were antimitochondrial-antibody (AMA) positive and were diagnosed with PBC. The third sister had been admitted to a different hospital for acute hepatitis of unknown origin – after receiving a positive AMA test, she also was diagnosed with PBC.
The brother and half-sister evaluated for PBC showed no symptoms and had negative AMA tests. The four sisters diagnosed showed good response to ursodeoxycholic acid in liver biochemistry tests and have continued on that medication without complication.
“If one patient is diagnosed with PBC, screening with AMA and liver function tests should be recommended to other family members for the early detection and management of this condition, especially for female relatives,” the investigators wrote.
Find the full case report in the World Journal of Gastroenterology (doi: 10.3748/wjg.v23.i39.7191).
FROM THE WORLD JOURNAL OF GASTROENTEROLOGY
Does Anyone Really Understand Nutrition Labels?
In 1990, nutrition labeling—that handy chart that gives us the information we need to make healthy choices—was added to nearly all packaged foods. But according to researchers from the FDA, Tufts University, and the National Cancer Institute, many people lack the health literacy to understand the information and use it as intended.
The researchers analyzed data on 3,185 U.S. adults from the Health Information National Trends Survey, conducted in 2013. Participants were asked to view an ice-cream nutrition label and answer 4 questions that tested their ability to apply basic arithmetic and understanding of percentages to interpret the label. They also reported their intake of sugar-sweetened soft drinks, fruits, and vegetables.
About one-quarter of the participants could not determine the calorie content of the full ice-cream container; 42% could not estimate the effect on daily calorie intake of foregoing 1 serving; 41% could not calculate the percentage daily value of calories in a single serving; and 21% could not estimate the number of servings equal to 60 g of carbohydrates.
Higher scores of label understanding were associated with consuming more vegetables and fewer sugar-sweetened drinks. After adjusting for demographic factors, only the link with soft drinks remained significant.
Across all educational levels, people had the most trouble with the questions about health recommendations and daily value. As in other studies, low educational attainment was associated with poor understanding of nutrition labels. More than one-third of participants with less than a high school diploma could not correctly answer any of the questions. Less than 9% could answer all 4 correctly. However, only 54% of participants with a 4-year college degree could answer all the questions correctly.
One obvious way to improve things, the researchers suggest, is to make the nutrition label easier to use. They note that the FDA tried to do this in 2016, in addition to reflecting current nutrition science and public health research. For instance, certain label elements, like calories and serving size, are now larger and in a bold font. Serving sizes have been updated to more accurately reflect the amount of food and drink people usually consume. To help consumers better understand serving size, 2 columns are used for foods that can be eaten in 1 or multiple sittings, such as a bag of potato chips, so people will better grasp how many calories they consume in 1 sitting.
Still, understanding nutrition labels is not the same as using the nutrition information for selecting food, the researchers point out. Participants who answered all 4 questions correctly might not necessarily use the labels when buying food.
In 1990, nutrition labeling—that handy chart that gives us the information we need to make healthy choices—was added to nearly all packaged foods. But according to researchers from the FDA, Tufts University, and the National Cancer Institute, many people lack the health literacy to understand the information and use it as intended.
The researchers analyzed data on 3,185 U.S. adults from the Health Information National Trends Survey, conducted in 2013. Participants were asked to view an ice-cream nutrition label and answer 4 questions that tested their ability to apply basic arithmetic and understanding of percentages to interpret the label. They also reported their intake of sugar-sweetened soft drinks, fruits, and vegetables.
About one-quarter of the participants could not determine the calorie content of the full ice-cream container; 42% could not estimate the effect on daily calorie intake of foregoing 1 serving; 41% could not calculate the percentage daily value of calories in a single serving; and 21% could not estimate the number of servings equal to 60 g of carbohydrates.
Higher scores of label understanding were associated with consuming more vegetables and fewer sugar-sweetened drinks. After adjusting for demographic factors, only the link with soft drinks remained significant.
Across all educational levels, people had the most trouble with the questions about health recommendations and daily value. As in other studies, low educational attainment was associated with poor understanding of nutrition labels. More than one-third of participants with less than a high school diploma could not correctly answer any of the questions. Less than 9% could answer all 4 correctly. However, only 54% of participants with a 4-year college degree could answer all the questions correctly.
One obvious way to improve things, the researchers suggest, is to make the nutrition label easier to use. They note that the FDA tried to do this in 2016, in addition to reflecting current nutrition science and public health research. For instance, certain label elements, like calories and serving size, are now larger and in a bold font. Serving sizes have been updated to more accurately reflect the amount of food and drink people usually consume. To help consumers better understand serving size, 2 columns are used for foods that can be eaten in 1 or multiple sittings, such as a bag of potato chips, so people will better grasp how many calories they consume in 1 sitting.
Still, understanding nutrition labels is not the same as using the nutrition information for selecting food, the researchers point out. Participants who answered all 4 questions correctly might not necessarily use the labels when buying food.
In 1990, nutrition labeling—that handy chart that gives us the information we need to make healthy choices—was added to nearly all packaged foods. But according to researchers from the FDA, Tufts University, and the National Cancer Institute, many people lack the health literacy to understand the information and use it as intended.
The researchers analyzed data on 3,185 U.S. adults from the Health Information National Trends Survey, conducted in 2013. Participants were asked to view an ice-cream nutrition label and answer 4 questions that tested their ability to apply basic arithmetic and understanding of percentages to interpret the label. They also reported their intake of sugar-sweetened soft drinks, fruits, and vegetables.
About one-quarter of the participants could not determine the calorie content of the full ice-cream container; 42% could not estimate the effect on daily calorie intake of foregoing 1 serving; 41% could not calculate the percentage daily value of calories in a single serving; and 21% could not estimate the number of servings equal to 60 g of carbohydrates.
Higher scores of label understanding were associated with consuming more vegetables and fewer sugar-sweetened drinks. After adjusting for demographic factors, only the link with soft drinks remained significant.
Across all educational levels, people had the most trouble with the questions about health recommendations and daily value. As in other studies, low educational attainment was associated with poor understanding of nutrition labels. More than one-third of participants with less than a high school diploma could not correctly answer any of the questions. Less than 9% could answer all 4 correctly. However, only 54% of participants with a 4-year college degree could answer all the questions correctly.
One obvious way to improve things, the researchers suggest, is to make the nutrition label easier to use. They note that the FDA tried to do this in 2016, in addition to reflecting current nutrition science and public health research. For instance, certain label elements, like calories and serving size, are now larger and in a bold font. Serving sizes have been updated to more accurately reflect the amount of food and drink people usually consume. To help consumers better understand serving size, 2 columns are used for foods that can be eaten in 1 or multiple sittings, such as a bag of potato chips, so people will better grasp how many calories they consume in 1 sitting.
Still, understanding nutrition labels is not the same as using the nutrition information for selecting food, the researchers point out. Participants who answered all 4 questions correctly might not necessarily use the labels when buying food.