User login
Low risk of bariatric surgery complications in IBD
Bariatric surgery is safe and feasible in patients with inflammatory bowel disease (IBD), with a low risk of postoperative complications vs. controls, according to results of a recent cohort study.
Besides a significantly higher risk of perioperative small-bowel obstruction and a 1-day increase in hospital stay, outcomes were comparable between patients with IBD and controls (Obes Surg. 2017 Oct 10. doi: 10.1007/s11695-017-2955-4).
Limitations of the retrospective study, according to the authors, included a potential underestimation of short-term postoperative complications, since the data set used in the study was limited to in-hospital stays and would not include events occurring after discharge.
Nevertheless, “our data show that it is reasonable to carefully proceed with bariatric interventions in obese IBD patients, especially those who are at higher risk of cardiovascular mortality and drastic need for weight reduction, to accrue benefits of weight loss,” wrote Fateh Bazerbachi, MD, of the Mayo Clinic, Rochester, Minn. and his coauthors.
Bariatric surgery is the “most effective solution” for obesity, and “appropriate candidates should not be deprived of this important, potentially life-saving procedure, if the intervention is deemed acceptably safe,” Dr. Bazerbachi and his colleagues noted.
Their cohort study included data for 314,864 adult patients in the Nationwide Inpatient Sample who underwent bariatric surgery between 2011 and 2013. Of that group, 790 patients had underlying IBD (459 Crohn’s disease, 331 ulcerative colitis). Remaining patients made up the comparator group.
The primary outcomes evaluated in the study included risks of systemic and technical complications. Risk of perioperative small-bowel obstruction was significantly higher in the IBD group (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4). However, the rates of other complications were similar between the two groups, data show.
Secondary outcomes in the study included length of hospital stay and mortality. Mean length of hospital stay was 3.4 days for IBD patients, vs. 2.5 days for the comparison group (P = .01), according to the report. Mortality was 0.25% for controls, while no deaths were reported in the IBD group.
In the future, bariatric surgeons may face increasing demand to treat IBD patients, given the increasing prevalence of obesity in the IBD patient population, Dr. Bazerbachi and his colleagues said.
Some surgeons may believe that bariatric intervention is more challenging in IBD patients, in part because of the underlying inflammatory state that might interfere with healing of wounds and recovery of bowel motility, they said. Bariatric surgery, however, can reduce body mass index, which in turn might make future IBD surgeries less challenging.
Another potential advantage is reduction in cardiovascular risk, which is elevated in IBD patients both due to obesity as well as the IBD condition, they added.
“Further studies are certainly needed to examine long-term outcomes of bariatric surgery on IBD and to determine whether cardiovascular mortality is reduced from these interventions in this susceptible cohort of obese IBD patients,” Dr. Bazerbachi and his colleagues wrote.
The authors declared that they had no conflicts of interest.
Bariatric surgery is safe and feasible in patients with inflammatory bowel disease (IBD), with a low risk of postoperative complications vs. controls, according to results of a recent cohort study.
Besides a significantly higher risk of perioperative small-bowel obstruction and a 1-day increase in hospital stay, outcomes were comparable between patients with IBD and controls (Obes Surg. 2017 Oct 10. doi: 10.1007/s11695-017-2955-4).
Limitations of the retrospective study, according to the authors, included a potential underestimation of short-term postoperative complications, since the data set used in the study was limited to in-hospital stays and would not include events occurring after discharge.
Nevertheless, “our data show that it is reasonable to carefully proceed with bariatric interventions in obese IBD patients, especially those who are at higher risk of cardiovascular mortality and drastic need for weight reduction, to accrue benefits of weight loss,” wrote Fateh Bazerbachi, MD, of the Mayo Clinic, Rochester, Minn. and his coauthors.
Bariatric surgery is the “most effective solution” for obesity, and “appropriate candidates should not be deprived of this important, potentially life-saving procedure, if the intervention is deemed acceptably safe,” Dr. Bazerbachi and his colleagues noted.
Their cohort study included data for 314,864 adult patients in the Nationwide Inpatient Sample who underwent bariatric surgery between 2011 and 2013. Of that group, 790 patients had underlying IBD (459 Crohn’s disease, 331 ulcerative colitis). Remaining patients made up the comparator group.
The primary outcomes evaluated in the study included risks of systemic and technical complications. Risk of perioperative small-bowel obstruction was significantly higher in the IBD group (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4). However, the rates of other complications were similar between the two groups, data show.
Secondary outcomes in the study included length of hospital stay and mortality. Mean length of hospital stay was 3.4 days for IBD patients, vs. 2.5 days for the comparison group (P = .01), according to the report. Mortality was 0.25% for controls, while no deaths were reported in the IBD group.
In the future, bariatric surgeons may face increasing demand to treat IBD patients, given the increasing prevalence of obesity in the IBD patient population, Dr. Bazerbachi and his colleagues said.
Some surgeons may believe that bariatric intervention is more challenging in IBD patients, in part because of the underlying inflammatory state that might interfere with healing of wounds and recovery of bowel motility, they said. Bariatric surgery, however, can reduce body mass index, which in turn might make future IBD surgeries less challenging.
Another potential advantage is reduction in cardiovascular risk, which is elevated in IBD patients both due to obesity as well as the IBD condition, they added.
“Further studies are certainly needed to examine long-term outcomes of bariatric surgery on IBD and to determine whether cardiovascular mortality is reduced from these interventions in this susceptible cohort of obese IBD patients,” Dr. Bazerbachi and his colleagues wrote.
The authors declared that they had no conflicts of interest.
Bariatric surgery is safe and feasible in patients with inflammatory bowel disease (IBD), with a low risk of postoperative complications vs. controls, according to results of a recent cohort study.
Besides a significantly higher risk of perioperative small-bowel obstruction and a 1-day increase in hospital stay, outcomes were comparable between patients with IBD and controls (Obes Surg. 2017 Oct 10. doi: 10.1007/s11695-017-2955-4).
Limitations of the retrospective study, according to the authors, included a potential underestimation of short-term postoperative complications, since the data set used in the study was limited to in-hospital stays and would not include events occurring after discharge.
Nevertheless, “our data show that it is reasonable to carefully proceed with bariatric interventions in obese IBD patients, especially those who are at higher risk of cardiovascular mortality and drastic need for weight reduction, to accrue benefits of weight loss,” wrote Fateh Bazerbachi, MD, of the Mayo Clinic, Rochester, Minn. and his coauthors.
Bariatric surgery is the “most effective solution” for obesity, and “appropriate candidates should not be deprived of this important, potentially life-saving procedure, if the intervention is deemed acceptably safe,” Dr. Bazerbachi and his colleagues noted.
Their cohort study included data for 314,864 adult patients in the Nationwide Inpatient Sample who underwent bariatric surgery between 2011 and 2013. Of that group, 790 patients had underlying IBD (459 Crohn’s disease, 331 ulcerative colitis). Remaining patients made up the comparator group.
The primary outcomes evaluated in the study included risks of systemic and technical complications. Risk of perioperative small-bowel obstruction was significantly higher in the IBD group (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4). However, the rates of other complications were similar between the two groups, data show.
Secondary outcomes in the study included length of hospital stay and mortality. Mean length of hospital stay was 3.4 days for IBD patients, vs. 2.5 days for the comparison group (P = .01), according to the report. Mortality was 0.25% for controls, while no deaths were reported in the IBD group.
In the future, bariatric surgeons may face increasing demand to treat IBD patients, given the increasing prevalence of obesity in the IBD patient population, Dr. Bazerbachi and his colleagues said.
Some surgeons may believe that bariatric intervention is more challenging in IBD patients, in part because of the underlying inflammatory state that might interfere with healing of wounds and recovery of bowel motility, they said. Bariatric surgery, however, can reduce body mass index, which in turn might make future IBD surgeries less challenging.
Another potential advantage is reduction in cardiovascular risk, which is elevated in IBD patients both due to obesity as well as the IBD condition, they added.
“Further studies are certainly needed to examine long-term outcomes of bariatric surgery on IBD and to determine whether cardiovascular mortality is reduced from these interventions in this susceptible cohort of obese IBD patients,” Dr. Bazerbachi and his colleagues wrote.
The authors declared that they had no conflicts of interest.
FROM OBESITY SURGERY
Key clinical point: Watch for perioperative small-bowel obstruction in IBD patients undergoing bariatric surgery.
Major finding: IBD patients had a higher risk of perioperative small bowel obstruction (adjusted odds ratio, 4.0; 95% confidence interval, 2.2-7.4) and a 1-day increase in hospital stay (P = .01), compared with controls.
Data source: Retrospective cohort study of Nationwide Inpatient Sample data including 790 patients with underlying IBD.
Disclosures: The authors declared that they had no conflicts of interest.
Symptoms fail to predict benefit of hormone therapy in older adults with subclinical hypothyroidism
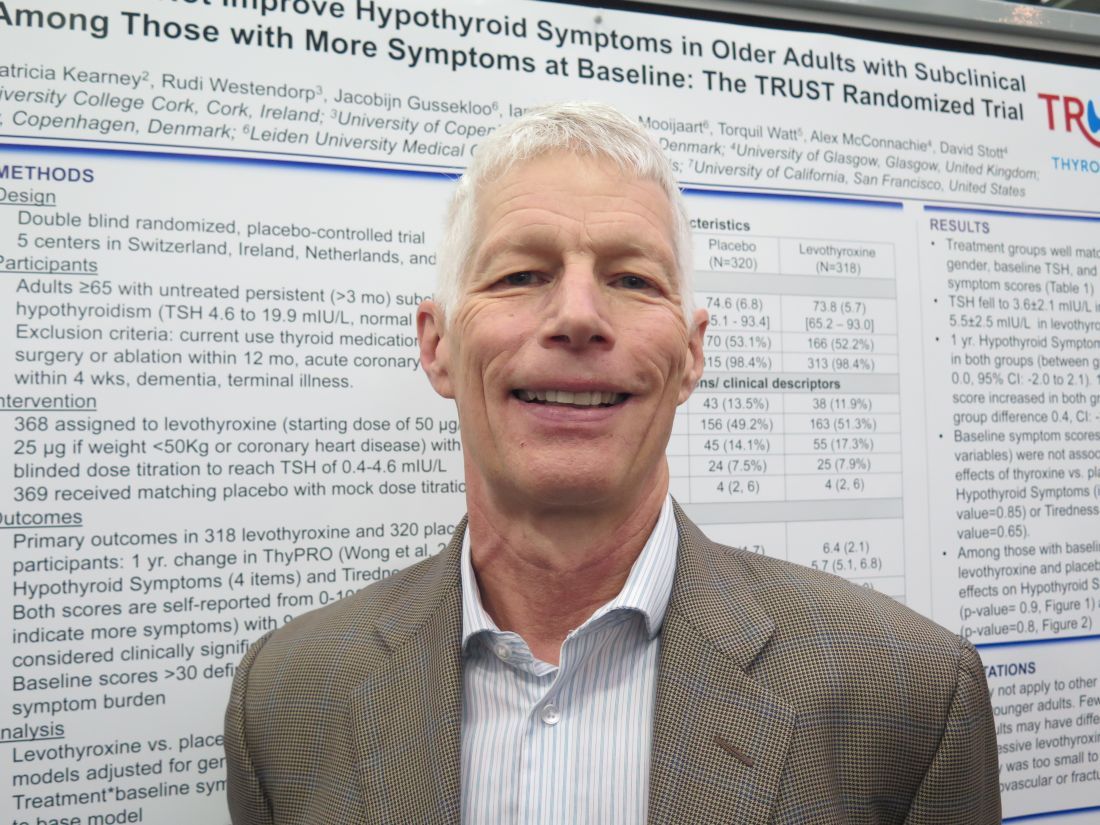
VICTORIA, B.C. – , according to findings from a study reported at the annual meeting of the American Thyroid Association.
“In the U.S., individuals are frequently treated either for just a number – just because their thyroid-stimulating hormone (TSH) is elevated – or for nonspecific hypothyroid-type symptoms, such as weight gain, cold intolerance, and such. It’s extremely common,” lead investigator Douglas Bauer, MD, professor and internist at the University of California, San Francisco, commented in an interview.
“On average, this study suggests that you shouldn’t be using hypothyroid-type symptomatology to treat subclinical hypothyroidism,” he said, while also acknowledging that not writing that prescription can be challenging. “It’s hard to do nothing, I know.”
Dr. Bauer and his coinvestigators performed a subgroup analysis of the randomized controlled TRUST trial (Thyroid Hormone Replacement for Subclinical Hypothyroidism), conducted in Switzerland, Ireland, the Netherlands, and the United Kingdom. In the trial, 737 adults aged 65 years or older with persistent subclinical hypothyroidism (TSH level, 4.60-19.99 mIU/L, with normal free thyroxine level) were given either levothyroxine or placebo on a double-blind basis.
Results for the entire trial population, previously reported, showed that at 1 year, patient-reported symptoms on a thyroid-specific quality-of-life questionnaire had improved by a similar extent in both groups, with no significant differences between them (N Engl J Med. 2017;376:2534-44).
The new analysis focused on two subgroups that might be especially expected to benefit: 132 patients with a hypothyroid symptoms score greater than 30 on a 100-point scale and 209 patients with a tiredness score greater than 30 on a 100-point scale.
Results reported in a poster session showed that at 1 year, scores had improved by about 10 points with levothyroxine and placebo alike, with no significant difference, in both the group with higher hypothyroid symptoms scores (P = .90) and the group with higher tiredness scores (P = .80).
“This provides additional evidence that it’s unlikely that the treatment of subclinical hypothyroidism, at least in this population, is going to lead to symptomatic improvement,” Dr. Bauer commented.
“I would speculate that we’ve overestimated the benefit [of thyroid hormone therapy] on symptoms based on the fact that previous studies haven’t been blinded,” he said, noting that the TRUST trial used a rigorous blinding protocol, even going so far as to change the appearance of placebo pills to convince patients in the placebo group that their dose was being titrated.
A potential criticism is that the treatment was not aggressive enough, with patients in the levothyroxine group achieving a mean TSH level of 3.6 mIU/L, according to Dr. Bauer. “I think many people treating with thyroxine would like to see this TSH fall into the 2 mIU/L to 3 mIU/L range,” he acknowledged.
Taken together, the trial’s overall and subgroup findings do not rule out potential benefit for certain patients, he cautioned. For example, patients with very high symptom burden and patients with baseline TSH levels greater than 10 mIU/L were too few for separate analysis, and younger adults were not included at all.
Additionally, treatment impact on other important clinical outcomes – cardiovascular events and fractures – could not be assessed in TRUST because of insufficient enrollment.
Dr. Bauer disclosed that he had no relevant conflicts of interest. The trial was funded by the European Union, Swiss National Science Foundation, Swiss Heart Foundation, and Velux Stiftung. Merck supplied study drug.
VICTORIA, B.C. – , according to findings from a study reported at the annual meeting of the American Thyroid Association.
“In the U.S., individuals are frequently treated either for just a number – just because their thyroid-stimulating hormone (TSH) is elevated – or for nonspecific hypothyroid-type symptoms, such as weight gain, cold intolerance, and such. It’s extremely common,” lead investigator Douglas Bauer, MD, professor and internist at the University of California, San Francisco, commented in an interview.
“On average, this study suggests that you shouldn’t be using hypothyroid-type symptomatology to treat subclinical hypothyroidism,” he said, while also acknowledging that not writing that prescription can be challenging. “It’s hard to do nothing, I know.”
Dr. Bauer and his coinvestigators performed a subgroup analysis of the randomized controlled TRUST trial (Thyroid Hormone Replacement for Subclinical Hypothyroidism), conducted in Switzerland, Ireland, the Netherlands, and the United Kingdom. In the trial, 737 adults aged 65 years or older with persistent subclinical hypothyroidism (TSH level, 4.60-19.99 mIU/L, with normal free thyroxine level) were given either levothyroxine or placebo on a double-blind basis.
Results for the entire trial population, previously reported, showed that at 1 year, patient-reported symptoms on a thyroid-specific quality-of-life questionnaire had improved by a similar extent in both groups, with no significant differences between them (N Engl J Med. 2017;376:2534-44).
The new analysis focused on two subgroups that might be especially expected to benefit: 132 patients with a hypothyroid symptoms score greater than 30 on a 100-point scale and 209 patients with a tiredness score greater than 30 on a 100-point scale.
Results reported in a poster session showed that at 1 year, scores had improved by about 10 points with levothyroxine and placebo alike, with no significant difference, in both the group with higher hypothyroid symptoms scores (P = .90) and the group with higher tiredness scores (P = .80).
“This provides additional evidence that it’s unlikely that the treatment of subclinical hypothyroidism, at least in this population, is going to lead to symptomatic improvement,” Dr. Bauer commented.
“I would speculate that we’ve overestimated the benefit [of thyroid hormone therapy] on symptoms based on the fact that previous studies haven’t been blinded,” he said, noting that the TRUST trial used a rigorous blinding protocol, even going so far as to change the appearance of placebo pills to convince patients in the placebo group that their dose was being titrated.
A potential criticism is that the treatment was not aggressive enough, with patients in the levothyroxine group achieving a mean TSH level of 3.6 mIU/L, according to Dr. Bauer. “I think many people treating with thyroxine would like to see this TSH fall into the 2 mIU/L to 3 mIU/L range,” he acknowledged.
Taken together, the trial’s overall and subgroup findings do not rule out potential benefit for certain patients, he cautioned. For example, patients with very high symptom burden and patients with baseline TSH levels greater than 10 mIU/L were too few for separate analysis, and younger adults were not included at all.
Additionally, treatment impact on other important clinical outcomes – cardiovascular events and fractures – could not be assessed in TRUST because of insufficient enrollment.
Dr. Bauer disclosed that he had no relevant conflicts of interest. The trial was funded by the European Union, Swiss National Science Foundation, Swiss Heart Foundation, and Velux Stiftung. Merck supplied study drug.
VICTORIA, B.C. – , according to findings from a study reported at the annual meeting of the American Thyroid Association.
“In the U.S., individuals are frequently treated either for just a number – just because their thyroid-stimulating hormone (TSH) is elevated – or for nonspecific hypothyroid-type symptoms, such as weight gain, cold intolerance, and such. It’s extremely common,” lead investigator Douglas Bauer, MD, professor and internist at the University of California, San Francisco, commented in an interview.
“On average, this study suggests that you shouldn’t be using hypothyroid-type symptomatology to treat subclinical hypothyroidism,” he said, while also acknowledging that not writing that prescription can be challenging. “It’s hard to do nothing, I know.”
Dr. Bauer and his coinvestigators performed a subgroup analysis of the randomized controlled TRUST trial (Thyroid Hormone Replacement for Subclinical Hypothyroidism), conducted in Switzerland, Ireland, the Netherlands, and the United Kingdom. In the trial, 737 adults aged 65 years or older with persistent subclinical hypothyroidism (TSH level, 4.60-19.99 mIU/L, with normal free thyroxine level) were given either levothyroxine or placebo on a double-blind basis.
Results for the entire trial population, previously reported, showed that at 1 year, patient-reported symptoms on a thyroid-specific quality-of-life questionnaire had improved by a similar extent in both groups, with no significant differences between them (N Engl J Med. 2017;376:2534-44).
The new analysis focused on two subgroups that might be especially expected to benefit: 132 patients with a hypothyroid symptoms score greater than 30 on a 100-point scale and 209 patients with a tiredness score greater than 30 on a 100-point scale.
Results reported in a poster session showed that at 1 year, scores had improved by about 10 points with levothyroxine and placebo alike, with no significant difference, in both the group with higher hypothyroid symptoms scores (P = .90) and the group with higher tiredness scores (P = .80).
“This provides additional evidence that it’s unlikely that the treatment of subclinical hypothyroidism, at least in this population, is going to lead to symptomatic improvement,” Dr. Bauer commented.
“I would speculate that we’ve overestimated the benefit [of thyroid hormone therapy] on symptoms based on the fact that previous studies haven’t been blinded,” he said, noting that the TRUST trial used a rigorous blinding protocol, even going so far as to change the appearance of placebo pills to convince patients in the placebo group that their dose was being titrated.
A potential criticism is that the treatment was not aggressive enough, with patients in the levothyroxine group achieving a mean TSH level of 3.6 mIU/L, according to Dr. Bauer. “I think many people treating with thyroxine would like to see this TSH fall into the 2 mIU/L to 3 mIU/L range,” he acknowledged.
Taken together, the trial’s overall and subgroup findings do not rule out potential benefit for certain patients, he cautioned. For example, patients with very high symptom burden and patients with baseline TSH levels greater than 10 mIU/L were too few for separate analysis, and younger adults were not included at all.
Additionally, treatment impact on other important clinical outcomes – cardiovascular events and fractures – could not be assessed in TRUST because of insufficient enrollment.
Dr. Bauer disclosed that he had no relevant conflicts of interest. The trial was funded by the European Union, Swiss National Science Foundation, Swiss Heart Foundation, and Velux Stiftung. Merck supplied study drug.
AT ATA 2017
Key clinical point: Hypothyroid-type symptoms do not predict treatment benefit in older adults with subclinical hypothyroidism.
Major finding: Patients had statistically indistinguishable improvements at 1 year in hypothyroid symptoms scores (P = .90) and Tiredness scores (P = .80) whether given levothyroxine or placebo.
Data source: A subgroup analysis of older adults with subclinical hypothyroidism having higher baseline levels of overall hypothyroid symptoms (n = 132) or tiredness (n = 209) (TRUST trial).
Disclosures: Dr. Bauer disclosed that he had no relevant conflicts of interest. The trial was funded by the European Union, Swiss National Science Foundation, Swiss Heart Foundation, and Velux Stiftung. Merck supplied study drug.
Abnormal potassium plus suspected ACS spell trouble
BARCELONA – A serum potassium level of at least 5.0 mmol/L or 3.5 mmol/L or less at admission for suspected acute coronary syndrome is a red flag for increased risk of in-hospital mortality and cardiac arrest, according to a Swedish study of nearly 33,000 consecutive patients.
That’s true even if, as so often ultimately proves to be the case, the patient turns out not to have ACS, Jonas Faxén, MD, of the Karolinska Institute, Stockholm, reported at the annual congress of the European Society of Cardiology.
“This study highlights that, if you have a patient in the emergency department with a possible ACS and potassium imbalance, you should really be cautious,” Dr. Faxén said.
He reported on 32,955 consecutive patients admitted to Stockholm County hospitals for suspected ACS during 2006-2011 and thereby enrolled in the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry.
Overall in-hospital mortality was 2.7%. In-hospital cardiac arrest occurred in 1.5% of patients. New-onset atrial fibrillation occurred in 2.4% of patients. These key outcomes were compared between the reference group – defined as patients with an admission serum potassium of 3.5 to less than 4.0 mmol/L – and patients with an admission serum potassium above or below those cutoffs.
In a multivariate logistic regression analysis adjusted for 24 potential confounders, including demographics, presentation characteristics, main diagnosis, comorbid conditions, medications on admission, and estimated glomerular filtration rate, patients with a serum potassium of 5.0 to less than 5.5 mmol/L were at 1.8-fold increased risk of in-hospital mortality. Those with a potassium of 5.5 mmol/L or greater were at 2.3-fold increased risk.
In contrast, a low rather than a high serum potassium was an independent risk factor cardiac arrest. An admission potassium of 3.0 to less than 3.5 mmol/L carried a 1.8-fold increased risk of in-hospital cardiac arrest, while a potassium of less than 3.0 was associated with a 2.7-fold increased risk.
A serum potassium below 3.0 mmol/L at admission also was associated with a 1.7-fold increased risk of new-onset atrial fibrillation.
These elevated risks of bad outcomes didn’t differ significantly between patients with ST-elevation MI, non-STEMI ACS, and those whose final diagnosis was not ACS, Dr. Faxén noted.
Session cochair David W. Walker, MD, medical director of the East Sussex (England) Healthcare NHS Trust, observed, “When I was a junior doctor I was always taught that when patients came onto coronary care we had to get their potassium to 4.5-5.0 mmol/L. I think you might want to change that advice now.”
“The implication would be that, if you intervene quickly in a patient with an abnormal potassium level, you might make a difference. Clearly, a potassium that’s too high is much worse than too low, since patients with in-hospital cardiac arrest can often be resuscitated,” Dr. Walker commented.
Dr. Faxén reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
BARCELONA – A serum potassium level of at least 5.0 mmol/L or 3.5 mmol/L or less at admission for suspected acute coronary syndrome is a red flag for increased risk of in-hospital mortality and cardiac arrest, according to a Swedish study of nearly 33,000 consecutive patients.
That’s true even if, as so often ultimately proves to be the case, the patient turns out not to have ACS, Jonas Faxén, MD, of the Karolinska Institute, Stockholm, reported at the annual congress of the European Society of Cardiology.
“This study highlights that, if you have a patient in the emergency department with a possible ACS and potassium imbalance, you should really be cautious,” Dr. Faxén said.
He reported on 32,955 consecutive patients admitted to Stockholm County hospitals for suspected ACS during 2006-2011 and thereby enrolled in the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry.
Overall in-hospital mortality was 2.7%. In-hospital cardiac arrest occurred in 1.5% of patients. New-onset atrial fibrillation occurred in 2.4% of patients. These key outcomes were compared between the reference group – defined as patients with an admission serum potassium of 3.5 to less than 4.0 mmol/L – and patients with an admission serum potassium above or below those cutoffs.
In a multivariate logistic regression analysis adjusted for 24 potential confounders, including demographics, presentation characteristics, main diagnosis, comorbid conditions, medications on admission, and estimated glomerular filtration rate, patients with a serum potassium of 5.0 to less than 5.5 mmol/L were at 1.8-fold increased risk of in-hospital mortality. Those with a potassium of 5.5 mmol/L or greater were at 2.3-fold increased risk.
In contrast, a low rather than a high serum potassium was an independent risk factor cardiac arrest. An admission potassium of 3.0 to less than 3.5 mmol/L carried a 1.8-fold increased risk of in-hospital cardiac arrest, while a potassium of less than 3.0 was associated with a 2.7-fold increased risk.
A serum potassium below 3.0 mmol/L at admission also was associated with a 1.7-fold increased risk of new-onset atrial fibrillation.
These elevated risks of bad outcomes didn’t differ significantly between patients with ST-elevation MI, non-STEMI ACS, and those whose final diagnosis was not ACS, Dr. Faxén noted.
Session cochair David W. Walker, MD, medical director of the East Sussex (England) Healthcare NHS Trust, observed, “When I was a junior doctor I was always taught that when patients came onto coronary care we had to get their potassium to 4.5-5.0 mmol/L. I think you might want to change that advice now.”
“The implication would be that, if you intervene quickly in a patient with an abnormal potassium level, you might make a difference. Clearly, a potassium that’s too high is much worse than too low, since patients with in-hospital cardiac arrest can often be resuscitated,” Dr. Walker commented.
Dr. Faxén reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
BARCELONA – A serum potassium level of at least 5.0 mmol/L or 3.5 mmol/L or less at admission for suspected acute coronary syndrome is a red flag for increased risk of in-hospital mortality and cardiac arrest, according to a Swedish study of nearly 33,000 consecutive patients.
That’s true even if, as so often ultimately proves to be the case, the patient turns out not to have ACS, Jonas Faxén, MD, of the Karolinska Institute, Stockholm, reported at the annual congress of the European Society of Cardiology.
“This study highlights that, if you have a patient in the emergency department with a possible ACS and potassium imbalance, you should really be cautious,” Dr. Faxén said.
He reported on 32,955 consecutive patients admitted to Stockholm County hospitals for suspected ACS during 2006-2011 and thereby enrolled in the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies) registry.
Overall in-hospital mortality was 2.7%. In-hospital cardiac arrest occurred in 1.5% of patients. New-onset atrial fibrillation occurred in 2.4% of patients. These key outcomes were compared between the reference group – defined as patients with an admission serum potassium of 3.5 to less than 4.0 mmol/L – and patients with an admission serum potassium above or below those cutoffs.
In a multivariate logistic regression analysis adjusted for 24 potential confounders, including demographics, presentation characteristics, main diagnosis, comorbid conditions, medications on admission, and estimated glomerular filtration rate, patients with a serum potassium of 5.0 to less than 5.5 mmol/L were at 1.8-fold increased risk of in-hospital mortality. Those with a potassium of 5.5 mmol/L or greater were at 2.3-fold increased risk.
In contrast, a low rather than a high serum potassium was an independent risk factor cardiac arrest. An admission potassium of 3.0 to less than 3.5 mmol/L carried a 1.8-fold increased risk of in-hospital cardiac arrest, while a potassium of less than 3.0 was associated with a 2.7-fold increased risk.
A serum potassium below 3.0 mmol/L at admission also was associated with a 1.7-fold increased risk of new-onset atrial fibrillation.
These elevated risks of bad outcomes didn’t differ significantly between patients with ST-elevation MI, non-STEMI ACS, and those whose final diagnosis was not ACS, Dr. Faxén noted.
Session cochair David W. Walker, MD, medical director of the East Sussex (England) Healthcare NHS Trust, observed, “When I was a junior doctor I was always taught that when patients came onto coronary care we had to get their potassium to 4.5-5.0 mmol/L. I think you might want to change that advice now.”
“The implication would be that, if you intervene quickly in a patient with an abnormal potassium level, you might make a difference. Clearly, a potassium that’s too high is much worse than too low, since patients with in-hospital cardiac arrest can often be resuscitated,” Dr. Walker commented.
Dr. Faxén reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
AT THE ESC CONGRESS 2017
Key clinical point:
Major finding: Hyperkalemia of 5.0 to less than 5.5 mmol/L at admission for suspected ACS was associated with close to a twofold increased risk of in-hospital mortality.
Data source: The SWEDEHEART study is an ongoing prospective registry of patients with cardiovascular disease admitted to Stockholm County hospitals.
Disclosures: The presenter reported having no financial conflicts regarding his study, which was funded by the Swedish Heart and Lung Foundation and the Stockholm County Council.
From cells to socioeconomics, meth worsens HIV outcomes
BERLIN – From cellular pathology to socioeconomics, methamphetamine and HIV are a devastating combination.
Either one is enough to ruin a life on its own. But together they can become a fatal ouroboros, Jordi Blanch, MD, said at the meeting of the World Psychiatric Congress. The drug sparks dangerous sexual behavior that ups HIV risk. It increases HIV-vulnerable receptors on immune cells, priming them for viral invasion. It interferes with the metabolism of antiretroviral drugs and grinds medication adherence into the dust.
And even when faced with the facts about these interactions with a serious disease, meth users find it almost impossible to leave the drug behind.
Methamphetamine was once almost exclusively a North American problem, said Dr. Blanch of the University of Barcelona. But in the last decade, the drug has jumped the pond, storming the beaches of Western Europe. Bolstered by imports from Asia, it’s now spreading eastward and down into Africa. Meth is challenging and surpassing alcohol as the drug of choice for HIV high-risk groups (particularly men who have sex with men). Like alcohol, it’s cheap and easy to find. Unlike alcohol, it delivers an incredibly potent, nearly instantaneous brain hit that amps up sexual desire and capacity while decreasing inhibition and executive function.
“When we look at the use of meth in the context of sexual relationships, it’s not hard to understand how it leads to all kinds of sexually transmitted infections, including HIV,” Dr. Blanch said.
A potent dopamine agonist, meth not only increases the neurotransmitter’s release, it blocks reuptake. It reduces the expression of dopamine transporters on the cell surface. At the same time, meth inhibits monoamine oxidase, normally a prime metabolizer. It even creates more dopamine: Methamphetamine increases the activity of tyrosine hydroxylase, the enzyme that catalyzes tyrosine into the dopamine precursor, l-dopa.
The neurologic response to smoking crystal meth – still the most popular way of ingesting the drug – is practically instantaneous. “It’s a very fast and intense euphoric high that, as we know, can have a lot of really bad side effects, like anxiety, restlessness, and even psychosis,” Dr. Blanch said. Its other side effects, though, are what make meth such a potent driver of risky sexual behavior.
“Men who have sex with men use it because it dramatically facilitates sexual functioning. It allows them to have sex for much longer. It decreases pain sensation, so this makes it easier to engage in anal sex, which is likely to be unprotected,” Dr. Blanch said. At the same time, the drug decreases higher-order thinking and increases impulsivity, driving even more behaviors that increase the risk of HIV, including group sex and the use of alcohol and injectable drugs together.
It is not just a cognitive-behavioral problem, though. Animal studies have found some intriguing pathophysiologic links between HIV viral activity and meth.
“Meth actually facilitates the infection,” Dr. Blanch said. “The risk of getting it is much higher, and the risk of it progressing with a high viral load is much higher.”
A 2015 review paper by Ryan Colby Passaro and his associates touches on some of these animal models (J Neuroimmune Pharmacol. 2015 Sep;10[3]:477-86). One of the most intriguing is a mouse study, which found that methamphetamine upregulated the HIV-1 coreceptors, CXCR4 and CCR5, not only on CD4+ T cells, but on monocytes, macrophages, dendritic cells, and, to some extent, astrocytes.
Cat and rhesus monkey data implicated this meth-related effect on CXCR4 and CCR5 as well. But the drug also was implicated in other cellular pathways – all of which serve to make immune cells more vulnerable to HIV attack. These findings support the observation that methamphetamine users with the disease frequently have higher viral loads than nonusers.
After a diagnosis, users may continue to use as a way of avoiding confronting their illness, or even to combat the accompanying physical fatigue, Dr. Blanch said. Like many illicit drug users, meth users often show poor compliance with medical follow-up and poor medication adherence. But even if they do take their antiretroviral medications, methamphetamine still has a way of exerting its power. Ritonavir and cobicistat both inhibit the metabolic pathway that breaks down methamphetamine; using meth with either of those drugs can increase meth concentrations by up to 10-fold, a combination that has killed many patients.
Unfortunately, Dr. Blanch said, it’s terribly difficult for users to give up meth, even in the face of contracting such a serious illness.
“In the beginning, after a diagnosis, they may stop using for a while. But then many start again,” he said. “We see this in study after study. But we have not so many studies on how to treat these patients.”
Trials of antidepressants and antipsychotics, and of replacement therapy with amphetamines or methylphenidate, have had mixed results.
“In my own clinic, we try to explain these problems of the interaction of meth and HIV. We have tried even to motivate our patients to use just on the weekend, for example, but they didn’t accept that,” he said. “Usually, we end up trying to make an agreement that the patient will use as little as possible and let them know how much it interferes with their treatment. But in my clinical experience, it’s not so easy. It’s hard to make any change. … very difficult.”
Dr. Blanch had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
BERLIN – From cellular pathology to socioeconomics, methamphetamine and HIV are a devastating combination.
Either one is enough to ruin a life on its own. But together they can become a fatal ouroboros, Jordi Blanch, MD, said at the meeting of the World Psychiatric Congress. The drug sparks dangerous sexual behavior that ups HIV risk. It increases HIV-vulnerable receptors on immune cells, priming them for viral invasion. It interferes with the metabolism of antiretroviral drugs and grinds medication adherence into the dust.
And even when faced with the facts about these interactions with a serious disease, meth users find it almost impossible to leave the drug behind.
Methamphetamine was once almost exclusively a North American problem, said Dr. Blanch of the University of Barcelona. But in the last decade, the drug has jumped the pond, storming the beaches of Western Europe. Bolstered by imports from Asia, it’s now spreading eastward and down into Africa. Meth is challenging and surpassing alcohol as the drug of choice for HIV high-risk groups (particularly men who have sex with men). Like alcohol, it’s cheap and easy to find. Unlike alcohol, it delivers an incredibly potent, nearly instantaneous brain hit that amps up sexual desire and capacity while decreasing inhibition and executive function.
“When we look at the use of meth in the context of sexual relationships, it’s not hard to understand how it leads to all kinds of sexually transmitted infections, including HIV,” Dr. Blanch said.
A potent dopamine agonist, meth not only increases the neurotransmitter’s release, it blocks reuptake. It reduces the expression of dopamine transporters on the cell surface. At the same time, meth inhibits monoamine oxidase, normally a prime metabolizer. It even creates more dopamine: Methamphetamine increases the activity of tyrosine hydroxylase, the enzyme that catalyzes tyrosine into the dopamine precursor, l-dopa.
The neurologic response to smoking crystal meth – still the most popular way of ingesting the drug – is practically instantaneous. “It’s a very fast and intense euphoric high that, as we know, can have a lot of really bad side effects, like anxiety, restlessness, and even psychosis,” Dr. Blanch said. Its other side effects, though, are what make meth such a potent driver of risky sexual behavior.
“Men who have sex with men use it because it dramatically facilitates sexual functioning. It allows them to have sex for much longer. It decreases pain sensation, so this makes it easier to engage in anal sex, which is likely to be unprotected,” Dr. Blanch said. At the same time, the drug decreases higher-order thinking and increases impulsivity, driving even more behaviors that increase the risk of HIV, including group sex and the use of alcohol and injectable drugs together.
It is not just a cognitive-behavioral problem, though. Animal studies have found some intriguing pathophysiologic links between HIV viral activity and meth.
“Meth actually facilitates the infection,” Dr. Blanch said. “The risk of getting it is much higher, and the risk of it progressing with a high viral load is much higher.”
A 2015 review paper by Ryan Colby Passaro and his associates touches on some of these animal models (J Neuroimmune Pharmacol. 2015 Sep;10[3]:477-86). One of the most intriguing is a mouse study, which found that methamphetamine upregulated the HIV-1 coreceptors, CXCR4 and CCR5, not only on CD4+ T cells, but on monocytes, macrophages, dendritic cells, and, to some extent, astrocytes.
Cat and rhesus monkey data implicated this meth-related effect on CXCR4 and CCR5 as well. But the drug also was implicated in other cellular pathways – all of which serve to make immune cells more vulnerable to HIV attack. These findings support the observation that methamphetamine users with the disease frequently have higher viral loads than nonusers.
After a diagnosis, users may continue to use as a way of avoiding confronting their illness, or even to combat the accompanying physical fatigue, Dr. Blanch said. Like many illicit drug users, meth users often show poor compliance with medical follow-up and poor medication adherence. But even if they do take their antiretroviral medications, methamphetamine still has a way of exerting its power. Ritonavir and cobicistat both inhibit the metabolic pathway that breaks down methamphetamine; using meth with either of those drugs can increase meth concentrations by up to 10-fold, a combination that has killed many patients.
Unfortunately, Dr. Blanch said, it’s terribly difficult for users to give up meth, even in the face of contracting such a serious illness.
“In the beginning, after a diagnosis, they may stop using for a while. But then many start again,” he said. “We see this in study after study. But we have not so many studies on how to treat these patients.”
Trials of antidepressants and antipsychotics, and of replacement therapy with amphetamines or methylphenidate, have had mixed results.
“In my own clinic, we try to explain these problems of the interaction of meth and HIV. We have tried even to motivate our patients to use just on the weekend, for example, but they didn’t accept that,” he said. “Usually, we end up trying to make an agreement that the patient will use as little as possible and let them know how much it interferes with their treatment. But in my clinical experience, it’s not so easy. It’s hard to make any change. … very difficult.”
Dr. Blanch had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
BERLIN – From cellular pathology to socioeconomics, methamphetamine and HIV are a devastating combination.
Either one is enough to ruin a life on its own. But together they can become a fatal ouroboros, Jordi Blanch, MD, said at the meeting of the World Psychiatric Congress. The drug sparks dangerous sexual behavior that ups HIV risk. It increases HIV-vulnerable receptors on immune cells, priming them for viral invasion. It interferes with the metabolism of antiretroviral drugs and grinds medication adherence into the dust.
And even when faced with the facts about these interactions with a serious disease, meth users find it almost impossible to leave the drug behind.
Methamphetamine was once almost exclusively a North American problem, said Dr. Blanch of the University of Barcelona. But in the last decade, the drug has jumped the pond, storming the beaches of Western Europe. Bolstered by imports from Asia, it’s now spreading eastward and down into Africa. Meth is challenging and surpassing alcohol as the drug of choice for HIV high-risk groups (particularly men who have sex with men). Like alcohol, it’s cheap and easy to find. Unlike alcohol, it delivers an incredibly potent, nearly instantaneous brain hit that amps up sexual desire and capacity while decreasing inhibition and executive function.
“When we look at the use of meth in the context of sexual relationships, it’s not hard to understand how it leads to all kinds of sexually transmitted infections, including HIV,” Dr. Blanch said.
A potent dopamine agonist, meth not only increases the neurotransmitter’s release, it blocks reuptake. It reduces the expression of dopamine transporters on the cell surface. At the same time, meth inhibits monoamine oxidase, normally a prime metabolizer. It even creates more dopamine: Methamphetamine increases the activity of tyrosine hydroxylase, the enzyme that catalyzes tyrosine into the dopamine precursor, l-dopa.
The neurologic response to smoking crystal meth – still the most popular way of ingesting the drug – is practically instantaneous. “It’s a very fast and intense euphoric high that, as we know, can have a lot of really bad side effects, like anxiety, restlessness, and even psychosis,” Dr. Blanch said. Its other side effects, though, are what make meth such a potent driver of risky sexual behavior.
“Men who have sex with men use it because it dramatically facilitates sexual functioning. It allows them to have sex for much longer. It decreases pain sensation, so this makes it easier to engage in anal sex, which is likely to be unprotected,” Dr. Blanch said. At the same time, the drug decreases higher-order thinking and increases impulsivity, driving even more behaviors that increase the risk of HIV, including group sex and the use of alcohol and injectable drugs together.
It is not just a cognitive-behavioral problem, though. Animal studies have found some intriguing pathophysiologic links between HIV viral activity and meth.
“Meth actually facilitates the infection,” Dr. Blanch said. “The risk of getting it is much higher, and the risk of it progressing with a high viral load is much higher.”
A 2015 review paper by Ryan Colby Passaro and his associates touches on some of these animal models (J Neuroimmune Pharmacol. 2015 Sep;10[3]:477-86). One of the most intriguing is a mouse study, which found that methamphetamine upregulated the HIV-1 coreceptors, CXCR4 and CCR5, not only on CD4+ T cells, but on monocytes, macrophages, dendritic cells, and, to some extent, astrocytes.
Cat and rhesus monkey data implicated this meth-related effect on CXCR4 and CCR5 as well. But the drug also was implicated in other cellular pathways – all of which serve to make immune cells more vulnerable to HIV attack. These findings support the observation that methamphetamine users with the disease frequently have higher viral loads than nonusers.
After a diagnosis, users may continue to use as a way of avoiding confronting their illness, or even to combat the accompanying physical fatigue, Dr. Blanch said. Like many illicit drug users, meth users often show poor compliance with medical follow-up and poor medication adherence. But even if they do take their antiretroviral medications, methamphetamine still has a way of exerting its power. Ritonavir and cobicistat both inhibit the metabolic pathway that breaks down methamphetamine; using meth with either of those drugs can increase meth concentrations by up to 10-fold, a combination that has killed many patients.
Unfortunately, Dr. Blanch said, it’s terribly difficult for users to give up meth, even in the face of contracting such a serious illness.
“In the beginning, after a diagnosis, they may stop using for a while. But then many start again,” he said. “We see this in study after study. But we have not so many studies on how to treat these patients.”
Trials of antidepressants and antipsychotics, and of replacement therapy with amphetamines or methylphenidate, have had mixed results.
“In my own clinic, we try to explain these problems of the interaction of meth and HIV. We have tried even to motivate our patients to use just on the weekend, for example, but they didn’t accept that,” he said. “Usually, we end up trying to make an agreement that the patient will use as little as possible and let them know how much it interferes with their treatment. But in my clinical experience, it’s not so easy. It’s hard to make any change. … very difficult.”
Dr. Blanch had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
EXPERT ANALYSIS FROM WPA 2017
Many women have unprotected sex in year after bariatric surgery
More than 40% of reproductive-age women reported having unprotected sex in the year after undergoing bariatric surgery, despite recommendations to avoid pregnancy for at least a year, a new study finds. Another 4% of women reported trying to conceive in the 12 months after surgery.
“We were surprised to find such a large percentage of women were not using contraception, and we were also surprised to find so many were actively trying to conceive,” the study’s lead author Marie N. Menke, MD, MPH, of the University of Pittsburgh, said in an interview. “Reproductive-age women who are considering bariatric surgery should be counseled that they should plan to use contraceptives after surgery for about 12-18 months.”
Pregnancy isn’t recommended over that period mainly because of the risks to the fetus, Dr. Menke said. “These risks are different from obesity in general. We don’t know exactly why surgery causes these risks, but ideally, patients would be weight-stable prior to conception.”
In 2013, the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic & Bariatric Surgery provided a Grade D recommendation for a 12-18 month delay in conception (Obesity [Silver Spring] 2013;21[suppl 1]:S1-27). A recent study provided more insight into the potential risks of pregnancy soon after weight-loss procedures. It reported that, compared with those who gave birth more than 4 years later, women who gave birth within 2 years of bariatric surgery had higher risks of premature birth, admission to neonatal intensive care units, and small-for-gestational-age infants (JAMA Surg. 2017 Feb 1;152[2]:1-8).
The current prospective cohort study included women who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals. The women, all aged 18-44 years, answered questions for as long as 7 years, until January 2015 (Obstet Gynecol. 2017 Oct 6. doi: 10.1097/AOG.0000000000002323).
The analysis included 710 women who provided conception data. The median body mass index of the women was 46.3 kg/m2. Most patients underwent Roux-en-Y gastric bypass (73%), followed by laparoscopic adjustable gastric banding (23%).
Researchers found that 4.3% of the women tried to conceive in the first year after surgery (95% confidence interval, 2.4-6.3), and 13.1% did so in the second year (95% CI, 9.3-17.0; P less than .001).
During the first year after surgery, 12.7% of women had no intercourse (95% CI, 9.4-16.0), 40.5% had only protected intercourse (95% CI, 35.6-45.4), and 41.5% (95% CI, 36.4-46.6) had unprotected intercourse while not trying to conceive.
Why are the unprotected sex numbers so high? “We wonder if some women simply feel that they cannot get pregnant,” Dr. Menke said. “Our group has previously reported that 42% of women who had attempted to conceive prior to bariatric surgery had a history of infertility. Some of these women went on to deliver a live birth, but many did not.”
The study reports that 183 of the 710 women did ultimately conceive and that the number may be as high as 237 because data were missing for 54 women who may have not wanted to report a nonlive birth. Of the women who reported conceiving, 68.9% had live births, 1.1% had stillbirths, 1.1% had ectopic pregnancies, 21.9% miscarried and 7.1% had abortions.
“We’d really like this research to be a reason to consider presurgical contraceptive counseling as one of the steps prior to bariatric surgery,” Dr. Menke said. “This may include a referral by the bariatric surgeon to a physician would who provide counseling, prescribe, or both.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
More than 40% of reproductive-age women reported having unprotected sex in the year after undergoing bariatric surgery, despite recommendations to avoid pregnancy for at least a year, a new study finds. Another 4% of women reported trying to conceive in the 12 months after surgery.
“We were surprised to find such a large percentage of women were not using contraception, and we were also surprised to find so many were actively trying to conceive,” the study’s lead author Marie N. Menke, MD, MPH, of the University of Pittsburgh, said in an interview. “Reproductive-age women who are considering bariatric surgery should be counseled that they should plan to use contraceptives after surgery for about 12-18 months.”
Pregnancy isn’t recommended over that period mainly because of the risks to the fetus, Dr. Menke said. “These risks are different from obesity in general. We don’t know exactly why surgery causes these risks, but ideally, patients would be weight-stable prior to conception.”
In 2013, the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic & Bariatric Surgery provided a Grade D recommendation for a 12-18 month delay in conception (Obesity [Silver Spring] 2013;21[suppl 1]:S1-27). A recent study provided more insight into the potential risks of pregnancy soon after weight-loss procedures. It reported that, compared with those who gave birth more than 4 years later, women who gave birth within 2 years of bariatric surgery had higher risks of premature birth, admission to neonatal intensive care units, and small-for-gestational-age infants (JAMA Surg. 2017 Feb 1;152[2]:1-8).
The current prospective cohort study included women who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals. The women, all aged 18-44 years, answered questions for as long as 7 years, until January 2015 (Obstet Gynecol. 2017 Oct 6. doi: 10.1097/AOG.0000000000002323).
The analysis included 710 women who provided conception data. The median body mass index of the women was 46.3 kg/m2. Most patients underwent Roux-en-Y gastric bypass (73%), followed by laparoscopic adjustable gastric banding (23%).
Researchers found that 4.3% of the women tried to conceive in the first year after surgery (95% confidence interval, 2.4-6.3), and 13.1% did so in the second year (95% CI, 9.3-17.0; P less than .001).
During the first year after surgery, 12.7% of women had no intercourse (95% CI, 9.4-16.0), 40.5% had only protected intercourse (95% CI, 35.6-45.4), and 41.5% (95% CI, 36.4-46.6) had unprotected intercourse while not trying to conceive.
Why are the unprotected sex numbers so high? “We wonder if some women simply feel that they cannot get pregnant,” Dr. Menke said. “Our group has previously reported that 42% of women who had attempted to conceive prior to bariatric surgery had a history of infertility. Some of these women went on to deliver a live birth, but many did not.”
The study reports that 183 of the 710 women did ultimately conceive and that the number may be as high as 237 because data were missing for 54 women who may have not wanted to report a nonlive birth. Of the women who reported conceiving, 68.9% had live births, 1.1% had stillbirths, 1.1% had ectopic pregnancies, 21.9% miscarried and 7.1% had abortions.
“We’d really like this research to be a reason to consider presurgical contraceptive counseling as one of the steps prior to bariatric surgery,” Dr. Menke said. “This may include a referral by the bariatric surgeon to a physician would who provide counseling, prescribe, or both.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
More than 40% of reproductive-age women reported having unprotected sex in the year after undergoing bariatric surgery, despite recommendations to avoid pregnancy for at least a year, a new study finds. Another 4% of women reported trying to conceive in the 12 months after surgery.
“We were surprised to find such a large percentage of women were not using contraception, and we were also surprised to find so many were actively trying to conceive,” the study’s lead author Marie N. Menke, MD, MPH, of the University of Pittsburgh, said in an interview. “Reproductive-age women who are considering bariatric surgery should be counseled that they should plan to use contraceptives after surgery for about 12-18 months.”
Pregnancy isn’t recommended over that period mainly because of the risks to the fetus, Dr. Menke said. “These risks are different from obesity in general. We don’t know exactly why surgery causes these risks, but ideally, patients would be weight-stable prior to conception.”
In 2013, the American Association of Clinical Endocrinologists, the Obesity Society, and the American Society for Metabolic & Bariatric Surgery provided a Grade D recommendation for a 12-18 month delay in conception (Obesity [Silver Spring] 2013;21[suppl 1]:S1-27). A recent study provided more insight into the potential risks of pregnancy soon after weight-loss procedures. It reported that, compared with those who gave birth more than 4 years later, women who gave birth within 2 years of bariatric surgery had higher risks of premature birth, admission to neonatal intensive care units, and small-for-gestational-age infants (JAMA Surg. 2017 Feb 1;152[2]:1-8).
The current prospective cohort study included women who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals. The women, all aged 18-44 years, answered questions for as long as 7 years, until January 2015 (Obstet Gynecol. 2017 Oct 6. doi: 10.1097/AOG.0000000000002323).
The analysis included 710 women who provided conception data. The median body mass index of the women was 46.3 kg/m2. Most patients underwent Roux-en-Y gastric bypass (73%), followed by laparoscopic adjustable gastric banding (23%).
Researchers found that 4.3% of the women tried to conceive in the first year after surgery (95% confidence interval, 2.4-6.3), and 13.1% did so in the second year (95% CI, 9.3-17.0; P less than .001).
During the first year after surgery, 12.7% of women had no intercourse (95% CI, 9.4-16.0), 40.5% had only protected intercourse (95% CI, 35.6-45.4), and 41.5% (95% CI, 36.4-46.6) had unprotected intercourse while not trying to conceive.
Why are the unprotected sex numbers so high? “We wonder if some women simply feel that they cannot get pregnant,” Dr. Menke said. “Our group has previously reported that 42% of women who had attempted to conceive prior to bariatric surgery had a history of infertility. Some of these women went on to deliver a live birth, but many did not.”
The study reports that 183 of the 710 women did ultimately conceive and that the number may be as high as 237 because data were missing for 54 women who may have not wanted to report a nonlive birth. Of the women who reported conceiving, 68.9% had live births, 1.1% had stillbirths, 1.1% had ectopic pregnancies, 21.9% miscarried and 7.1% had abortions.
“We’d really like this research to be a reason to consider presurgical contraceptive counseling as one of the steps prior to bariatric surgery,” Dr. Menke said. “This may include a referral by the bariatric surgeon to a physician would who provide counseling, prescribe, or both.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: In the first year after surgery, 4.3% of women surveyed tried to conceive (95% CI, 2.4-6.3), and another 41.5% had unprotected intercourse (95% CI, 36.4-46.6).
Data source: Prospective cohort study of 710 women, aged 18-44 years, who underwent bariatric surgery for the first time during 2005-2009 at 10 U.S. hospitals.
Disclosures: The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Menke reported having no relevant disclosures. Her coauthors reported financial relationships with pharmaceutical and medical device companies with products to treat metabolic diseases.
System automates classification of RBCs
Scientists say they have developed an automated system that can identify shapes of red blood cells (RBCs).
The team found their system could classify sickled RBCs “with high accuracy,” which suggests it could be used to help monitor patients with sickle cell disease.
“We have developed the first deep learning tool that can automatically identify and classify red blood cell alteration, hence providing direct quantitative evidence of the severity of the disease,” said George Karniadakis, PhD, of Brown University in Providence, Rhode Island.
Dr Karniadakis and his colleagues described their tool in PLOS Computational Biology.
The researchers wanted to automate the process of identifying RBC shape. So they developed a computational framework that employs a machine-learning tool known as a deep convolutional neural network (CNN).
The framework uses 3 steps to classify the shapes of RBCs in microscopic images of blood.
First, it distinguishes RBCs from the background of each image and from each other. Then, for each cell detected, it zooms in or out until all cell images are a uniform size. Finally, it uses deep CNNs to categorize RBCs by shape.
The researchers validated their new tool using 7000 microscopy images from 8 patients with sickle cell disease. The method successfully classified RBC shape for both oxygenated and deoxygenated cells.
The researchers plan to further improve their deep CNN tool and test it in other diseases that alter the shape and size of RBCs, such as diabetes and HIV. They also plan to explore its usefulness in characterizing cancer cells. ![]()
Scientists say they have developed an automated system that can identify shapes of red blood cells (RBCs).
The team found their system could classify sickled RBCs “with high accuracy,” which suggests it could be used to help monitor patients with sickle cell disease.
“We have developed the first deep learning tool that can automatically identify and classify red blood cell alteration, hence providing direct quantitative evidence of the severity of the disease,” said George Karniadakis, PhD, of Brown University in Providence, Rhode Island.
Dr Karniadakis and his colleagues described their tool in PLOS Computational Biology.
The researchers wanted to automate the process of identifying RBC shape. So they developed a computational framework that employs a machine-learning tool known as a deep convolutional neural network (CNN).
The framework uses 3 steps to classify the shapes of RBCs in microscopic images of blood.
First, it distinguishes RBCs from the background of each image and from each other. Then, for each cell detected, it zooms in or out until all cell images are a uniform size. Finally, it uses deep CNNs to categorize RBCs by shape.
The researchers validated their new tool using 7000 microscopy images from 8 patients with sickle cell disease. The method successfully classified RBC shape for both oxygenated and deoxygenated cells.
The researchers plan to further improve their deep CNN tool and test it in other diseases that alter the shape and size of RBCs, such as diabetes and HIV. They also plan to explore its usefulness in characterizing cancer cells. ![]()
Scientists say they have developed an automated system that can identify shapes of red blood cells (RBCs).
The team found their system could classify sickled RBCs “with high accuracy,” which suggests it could be used to help monitor patients with sickle cell disease.
“We have developed the first deep learning tool that can automatically identify and classify red blood cell alteration, hence providing direct quantitative evidence of the severity of the disease,” said George Karniadakis, PhD, of Brown University in Providence, Rhode Island.
Dr Karniadakis and his colleagues described their tool in PLOS Computational Biology.
The researchers wanted to automate the process of identifying RBC shape. So they developed a computational framework that employs a machine-learning tool known as a deep convolutional neural network (CNN).
The framework uses 3 steps to classify the shapes of RBCs in microscopic images of blood.
First, it distinguishes RBCs from the background of each image and from each other. Then, for each cell detected, it zooms in or out until all cell images are a uniform size. Finally, it uses deep CNNs to categorize RBCs by shape.
The researchers validated their new tool using 7000 microscopy images from 8 patients with sickle cell disease. The method successfully classified RBC shape for both oxygenated and deoxygenated cells.
The researchers plan to further improve their deep CNN tool and test it in other diseases that alter the shape and size of RBCs, such as diabetes and HIV. They also plan to explore its usefulness in characterizing cancer cells. ![]()
Aptiom Receives FDA approval for Partial-Onset Seizures in Children
FDA has approved Aptiom (eslicarbazepine) for an expanded indication to treat partial-onset seizures in children.
Indications: Aptiom was previously approved form the treatment of partial onset seizures in adults. The new approval is for children ages 4 years to 17 years with the same disorder. It is a once daily immediate release anti-epilepsy drug that can be taken whole or crushed, with or without food. The approval is based in part on extrapolation of previous data to support its use in the pediatric population.
Dosage and administration. The recommended dosage of APTIOM is based on
body weight and is administered orally once daily. The package insert says to increase the dose in weekly intervals based on clinical response and tolerability to the recommended maintenance dosage. The initial pediatric dose is 200 mg/day for a patient 11 to 21 kg, with 200 mg/day as a maximum titration increment. Maximum dose for a child in this weight range in 400 to 600 mg/day.
Adverse effects. The most common reactions in adults include dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor. Adverse reactions are similar in pediatric patients.
Sunovion’s Aptiom (eslicarbazepine acetate) Receives FDA Approval for Expanded Indication to Treat Partial-Onset Seizures in Children and Adolescents 4 Years of Age and Older
http://www.sunovion.us/featured-news/press-releases/20170914a.pdf. Accessed October 16, 2017
FDA has approved Aptiom (eslicarbazepine) for an expanded indication to treat partial-onset seizures in children.
Indications: Aptiom was previously approved form the treatment of partial onset seizures in adults. The new approval is for children ages 4 years to 17 years with the same disorder. It is a once daily immediate release anti-epilepsy drug that can be taken whole or crushed, with or without food. The approval is based in part on extrapolation of previous data to support its use in the pediatric population.
Dosage and administration. The recommended dosage of APTIOM is based on
body weight and is administered orally once daily. The package insert says to increase the dose in weekly intervals based on clinical response and tolerability to the recommended maintenance dosage. The initial pediatric dose is 200 mg/day for a patient 11 to 21 kg, with 200 mg/day as a maximum titration increment. Maximum dose for a child in this weight range in 400 to 600 mg/day.
Adverse effects. The most common reactions in adults include dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor. Adverse reactions are similar in pediatric patients.
Sunovion’s Aptiom (eslicarbazepine acetate) Receives FDA Approval for Expanded Indication to Treat Partial-Onset Seizures in Children and Adolescents 4 Years of Age and Older
http://www.sunovion.us/featured-news/press-releases/20170914a.pdf. Accessed October 16, 2017
FDA has approved Aptiom (eslicarbazepine) for an expanded indication to treat partial-onset seizures in children.
Indications: Aptiom was previously approved form the treatment of partial onset seizures in adults. The new approval is for children ages 4 years to 17 years with the same disorder. It is a once daily immediate release anti-epilepsy drug that can be taken whole or crushed, with or without food. The approval is based in part on extrapolation of previous data to support its use in the pediatric population.
Dosage and administration. The recommended dosage of APTIOM is based on
body weight and is administered orally once daily. The package insert says to increase the dose in weekly intervals based on clinical response and tolerability to the recommended maintenance dosage. The initial pediatric dose is 200 mg/day for a patient 11 to 21 kg, with 200 mg/day as a maximum titration increment. Maximum dose for a child in this weight range in 400 to 600 mg/day.
Adverse effects. The most common reactions in adults include dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor. Adverse reactions are similar in pediatric patients.
Sunovion’s Aptiom (eslicarbazepine acetate) Receives FDA Approval for Expanded Indication to Treat Partial-Onset Seizures in Children and Adolescents 4 Years of Age and Older
http://www.sunovion.us/featured-news/press-releases/20170914a.pdf. Accessed October 16, 2017
Why is Psychogenic Nonepileptic Seizure Diagnosis Missed?
The diagnosis of psychogenic nonepileptic seizure (PNES) is often delayed—and a history of head trauma can contribute to that delay.
- 49 adult patients who were admitted to the epilepsy unit at the Jefferson Comprehensive Epilepsy Center for PNES were studied to determine how long it took for a correct diagnosis to be reached.
- Of the 49 patients, 39 were women, 10 were men; the study period was 2012 to 2016.
- On average, it took 3 years (median) to arrive at the correct diagnosis.
- A comparison of patients who received an early diagnosis to those whose diagnosis was delayed found that a history of head trauma was the only significant difference between the 2 groups.
- 2 of 19 patients had an early diagnosis (7%) and 11 of 28 patients experienced a delayed diagnosis, which was associated with head trauma (P=0.02).
Asadi-Pooya AA, Tinker J. Delay in diagnosis of psychogenic nonepileptic seizures in adults: A post hoc study [published online ahead of print Sept 15, 2017]. Epilepsy Behav. doi: 10.1016/j.yebeh.2017.08.005.
The diagnosis of psychogenic nonepileptic seizure (PNES) is often delayed—and a history of head trauma can contribute to that delay.
- 49 adult patients who were admitted to the epilepsy unit at the Jefferson Comprehensive Epilepsy Center for PNES were studied to determine how long it took for a correct diagnosis to be reached.
- Of the 49 patients, 39 were women, 10 were men; the study period was 2012 to 2016.
- On average, it took 3 years (median) to arrive at the correct diagnosis.
- A comparison of patients who received an early diagnosis to those whose diagnosis was delayed found that a history of head trauma was the only significant difference between the 2 groups.
- 2 of 19 patients had an early diagnosis (7%) and 11 of 28 patients experienced a delayed diagnosis, which was associated with head trauma (P=0.02).
Asadi-Pooya AA, Tinker J. Delay in diagnosis of psychogenic nonepileptic seizures in adults: A post hoc study [published online ahead of print Sept 15, 2017]. Epilepsy Behav. doi: 10.1016/j.yebeh.2017.08.005.
The diagnosis of psychogenic nonepileptic seizure (PNES) is often delayed—and a history of head trauma can contribute to that delay.
- 49 adult patients who were admitted to the epilepsy unit at the Jefferson Comprehensive Epilepsy Center for PNES were studied to determine how long it took for a correct diagnosis to be reached.
- Of the 49 patients, 39 were women, 10 were men; the study period was 2012 to 2016.
- On average, it took 3 years (median) to arrive at the correct diagnosis.
- A comparison of patients who received an early diagnosis to those whose diagnosis was delayed found that a history of head trauma was the only significant difference between the 2 groups.
- 2 of 19 patients had an early diagnosis (7%) and 11 of 28 patients experienced a delayed diagnosis, which was associated with head trauma (P=0.02).
Asadi-Pooya AA, Tinker J. Delay in diagnosis of psychogenic nonepileptic seizures in adults: A post hoc study [published online ahead of print Sept 15, 2017]. Epilepsy Behav. doi: 10.1016/j.yebeh.2017.08.005.
Federal Report Finds Increased Prevalence of Epilepsy
Three million adults and 470,000 children have epilepsy according to the latest statistics from the Centers for Disease Control and Prevention (CDC). These figures, based on the 2015 National Health Interview Survey, translate into 1.2% of the US population, an all-time high. Previous estimates, based on 2010 data, indicated that 2.3 million adults and 450,000 children had the disorder.
- The CDC report provides the first ever estimates of the prevalence of epilepsy state by state, with the agency reporting higher volumes in more densely populated states, as expected.
- The lowest prevalence of the neurological disease was noted in Wyoming, with 5900 active cases.
- California was estimated to have 427,700 cases of the disease.
- CDC believes increases from 2010 to 2015 are likely due to population growth.
- Among the limitations of the analysis is the fact that the estimates are based on self-reports, which are subject to bias.
- Underreporting by the public may reflect their reluctance to admit to a disorder because of its stigma and because they fear it could lead to driver’s license restrictions.
Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy — United States, 2015. MMWR; 2017;66(31):821-825.
Three million adults and 470,000 children have epilepsy according to the latest statistics from the Centers for Disease Control and Prevention (CDC). These figures, based on the 2015 National Health Interview Survey, translate into 1.2% of the US population, an all-time high. Previous estimates, based on 2010 data, indicated that 2.3 million adults and 450,000 children had the disorder.
- The CDC report provides the first ever estimates of the prevalence of epilepsy state by state, with the agency reporting higher volumes in more densely populated states, as expected.
- The lowest prevalence of the neurological disease was noted in Wyoming, with 5900 active cases.
- California was estimated to have 427,700 cases of the disease.
- CDC believes increases from 2010 to 2015 are likely due to population growth.
- Among the limitations of the analysis is the fact that the estimates are based on self-reports, which are subject to bias.
- Underreporting by the public may reflect their reluctance to admit to a disorder because of its stigma and because they fear it could lead to driver’s license restrictions.
Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy — United States, 2015. MMWR; 2017;66(31):821-825.
Three million adults and 470,000 children have epilepsy according to the latest statistics from the Centers for Disease Control and Prevention (CDC). These figures, based on the 2015 National Health Interview Survey, translate into 1.2% of the US population, an all-time high. Previous estimates, based on 2010 data, indicated that 2.3 million adults and 450,000 children had the disorder.
- The CDC report provides the first ever estimates of the prevalence of epilepsy state by state, with the agency reporting higher volumes in more densely populated states, as expected.
- The lowest prevalence of the neurological disease was noted in Wyoming, with 5900 active cases.
- California was estimated to have 427,700 cases of the disease.
- CDC believes increases from 2010 to 2015 are likely due to population growth.
- Among the limitations of the analysis is the fact that the estimates are based on self-reports, which are subject to bias.
- Underreporting by the public may reflect their reluctance to admit to a disorder because of its stigma and because they fear it could lead to driver’s license restrictions.
Zack MM, Kobau R. National and state estimates of the numbers of adults and children with active epilepsy — United States, 2015. MMWR; 2017;66(31):821-825.
Study: EHR malpractice claims rising
Malpractice claims involving the use of electronic health records (EHRs) are on the rise, according to data from The Doctors Company.
Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016, according an analysis published Oct. 16 by The Doctors Company, a national medical malpractice insurer.
The majority of EHR-related claims during 2014-2016 stemmed from incidents in a doctor’s office or a hospital clinic (35%), while the second most common location was a patient’s room. Malpractice claims involving EHRs were most commonly alleged against ob.gyns, followed by family physicians, and orthopedists. Diagnosis errors and improper medication management were the top most frequent allegations associated with EHR claims.
The analysis shows that while digitization of medicine has improved patient safety, it also has a dark side – as evidenced by the emergence of new kinds of errors, said Robert M. Wachter, MD, a professor at the University of California, San Francisco, and a member of the board of governors for The Doctors Company.
“This study makes an important contribution by chronicling actual errors, such as wrong medications selected from an autopick list, and helps point the way to changes ranging from physician education to EHR software design,” Dr. Wachter said in a statement.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Malpractice claims involving the use of electronic health records (EHRs) are on the rise, according to data from The Doctors Company.
Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016, according an analysis published Oct. 16 by The Doctors Company, a national medical malpractice insurer.
The majority of EHR-related claims during 2014-2016 stemmed from incidents in a doctor’s office or a hospital clinic (35%), while the second most common location was a patient’s room. Malpractice claims involving EHRs were most commonly alleged against ob.gyns, followed by family physicians, and orthopedists. Diagnosis errors and improper medication management were the top most frequent allegations associated with EHR claims.
The analysis shows that while digitization of medicine has improved patient safety, it also has a dark side – as evidenced by the emergence of new kinds of errors, said Robert M. Wachter, MD, a professor at the University of California, San Francisco, and a member of the board of governors for The Doctors Company.
“This study makes an important contribution by chronicling actual errors, such as wrong medications selected from an autopick list, and helps point the way to changes ranging from physician education to EHR software design,” Dr. Wachter said in a statement.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Malpractice claims involving the use of electronic health records (EHRs) are on the rise, according to data from The Doctors Company.
Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016, according an analysis published Oct. 16 by The Doctors Company, a national medical malpractice insurer.
The majority of EHR-related claims during 2014-2016 stemmed from incidents in a doctor’s office or a hospital clinic (35%), while the second most common location was a patient’s room. Malpractice claims involving EHRs were most commonly alleged against ob.gyns, followed by family physicians, and orthopedists. Diagnosis errors and improper medication management were the top most frequent allegations associated with EHR claims.
The analysis shows that while digitization of medicine has improved patient safety, it also has a dark side – as evidenced by the emergence of new kinds of errors, said Robert M. Wachter, MD, a professor at the University of California, San Francisco, and a member of the board of governors for The Doctors Company.
“This study makes an important contribution by chronicling actual errors, such as wrong medications selected from an autopick list, and helps point the way to changes ranging from physician education to EHR software design,” Dr. Wachter said in a statement.
agallegos@frontlinemedcom.com
On Twitter @legal_med
Key clinical point:
Major finding: Cases in which EHRs were a factor grew from 2 claims during 2007-2010 to 161 claims from 2011 to December 2016
Data source: Review of 163 claims from 2007 to 2016 in The Doctors Company database.
Disclosures: The study was funded by The Doctors Company.