User login
Strategy could reduce myelosuppression in AML
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197. ![]()
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197. ![]()
Researchers believe they may have found a way to prevent chemotherapy-induced myelosuppression in acute myeloid leukemia (AML).
The team found that priming mice with the FLT3 inhibitor quizartinib protected multipotent progenitor cells (MPPs) from subsequent treatment with fluorouracil (5-FU) or gemcitabine.
And treatment with quizartinib followed by 5-FU proved more effective against AML than standard induction with cytarabine and doxorubicin.
Samuel Taylor, of the University of Western Australia in Crawley, Australia, and his colleagues reported these results in Science Translational Medicine.
The researchers first found that quizartinib induced “rapid and transient” quiescence of MPPs in C57BL/6 mice.
Quizartinib also provided MPPs with “marked protection” from 5-FU. In these experiments, a 10 mg/kg dose of quizartinib was given to mice at the same time as a 150 mg/kg dose of 5-FU. This treatment provided MPPs with 4- to 5-fold greater protection than vehicle control.
Subsequent experiments revealed the optimal dose and schedule for quizartinib. A priming dose of 30 mg/kg given 6 hours before 5-FU provided “slightly greater” protection to hematopoietic stem and progenitor cells than a 10 mg/kg dose, with significantly greater protection observed for short-term hematopoietic stem cells.
The researchers then showed that priming with quizartinib allowed for “rapid recovery of bone marrow cellularity” after treatment with 5-FU. Bone marrow cells were fully restored by day 8 after treatment in quizartinib-primed mice but not in vehicle-primed mice.
Quizartinib priming also protected mice from multiple rounds of treatment with 5-FU (15 cycles in some mice) and from myelosuppression induced by gemcitabine.
Finally, the researchers tested quizartinib followed by 5-FU in mouse models of AML. They found the treatment was more effective than treatment with cytarabine and doxorubicin in both FLT3-ITD(F692L)/NPM1c AML and NPM1c/NrasG12D AML.
FLT3-ITD(F692L)/NPM1c AML
The researchers transplanted 15 non-irradiated B6.CD45.1 mice with 3 × 105 spleen cells each from a FLT3-ITD(F691L)/NPM1c mouse that succumbed to AML at 6 weeks of age. Sixteen days after transplant, the mice were given one of the following:
- No treatment
- 10-day cycles of quizartinib (30 mg/kg) followed 6 hours later by 5-FU (150 mg/kg)
- Cytarabine plus doxorubicin (5+3).
All 5 of the untreated mice died within 30 days of transplantation, exhibiting high white blood cell (WBC) counts and splenomegaly.
The 5+3 mice received 2 cycles of treatment (days 16 to 21 and 36 to 41). All 5 had died by day 56 after transplantation, with high WBC counts and splenomegaly.
One the other hand, 4 of the 5 mice in the quizartinib/5-FU arm were still healthy at 176 days after transplantation and 80 days after stopping treatment. There were no detectable CD45.2+ AML cells when the mice were last bled on day 160, and they had normal WBC counts. There were no AML cells detectable in the animals’ bone marrow after they were killed at day 176.
The quizartinib/5-FU mouse that died before day 176 is believed to have developed resistance to 5-FU. This animal died 121 days after transplantation.
NPM1c/ NrasG12D AML
For another AML model, the researchers crossed NPM1c-mutant mice with NrasG12D-mutant mice. The team transplanted spleen cells from NPM1c/NrasG12D leukemic mice into 15 non-irradiated B6.CD45.1 recipient mice.
Fifteen days after transplantation, the NPM1c/ NrasG12D mice received one of the following:
- No treatment
- Quizartinib and 5-FU as above
- Cytarabine plus doxorubicin (5+3).
All 5 untreated mice died by day 32 after transplantation, and all 5 mice that received 5+3 died by day 35. Both groups of mice had high WBC counts and splenomegaly.
Mice in the quizartinib/5-FU arm initially received 4 cycles of treatment, starting on days 15, 25, 35, and 45 after transplantation. On day 53, they had minimal or undetectable numbers of CD45.2+ AML cells, and WBC counts were normal or slightly below normal.
At day 81—a month after stopping treatment—4 of the mice had detectable CD45.2+ AML cells in their blood. So they restarted treatment the next day. After 4 additional cycles, AML cells were undetectable in all 5 mice. At day 146—a month after stopping the second round of treatment—AML cells again became detectable in the blood.
The mice did not receive any additional treatment. One died at day 196, and 1 was killed at day 197 due to weight loss related to feeding difficulties (but this mouse did not show signs of AML).
The other 3 mice were “active and healthy” until they were killed at day 214. However, they had “high proportions” of CD45.2+ myeloid cells in their blood since day 183. And 2 of the mice had increased WBC counts from day 197. ![]()
Study suggests malaria is undertreated
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.” ![]()
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.” ![]()
A large study suggests the use of rapid diagnostic tests (RDTs) for malaria can reduce overuse of artemisinin combination therapies (ACTs), but malaria may also go untreated.
Researchers analyzed data from more than 500,000 patient visits across malaria-endemic regions of Africa and Afghanistan.
They found that, in most settings, the introduction of RDTs improved antimalarial targeting.
However, a substantial number of patients who tested positive for malaria appeared to go untreated, and negative test results prompted a shift to antibiotic prescriptions.
Katia Bruxvoort, PhD, of London School of Hygiene & Tropical Medicine in the UK, and her colleagues reported these results in the American Journal of Tropical Medicine and Hygiene.
The researchers analyzed drug prescriptions written from 2007 to 2013 in 562,368 patient encounters documented in 10 related studies. Eight studies were conducted in sub-Saharan Africa (Cameroon, Ghana, Nigeria, Tanzania, and Uganda), and 2 were conducted in Afghanistan.
Overall, RDTs appeared to limit—though not eliminate—routine prescription of ACTs to patients presenting with fever but not malaria.
In most cases, fewer than 30% of patients who tested negative for malaria still received ACTs. However, in Cameroon and Ghana, 39% to 49% of patients who tested negative for malaria received ACTs.
“[I]n many places, a reduction in the use of ACTs was accompanied by an increase in the use of antibiotics, which may drive up the risk of antibiotic-resistant infections,” Dr Bruxvoort noted.
Overall, 75% of patients studied left the clinic with either an antibiotic or an ACT.
In most areas studied, antibiotics were given to 40% to 80% of patients who had tested negative for malaria.
The researchers believe the shift to antibiotic use after ruling out malaria may indicate that many patients and providers are not comfortable treating a fever using only supportive care (taking a fever-reducing drug and drinking plenty of fluids).
“A key challenge is that we don’t currently have a reliable way to determine which fevers are evidence of a bacterial infection that requires a specific antibiotic treatment and which fevers will resolve with supportive care only,” Dr Bruxvoort said.
In addition, Dr Bruxvoort and her colleagues were surprised to find that, in 5 of the 8 African studies, more than 20% of patients who tested positive for malaria were not prescribed ACTs.
“Drug supply issues did not seem to be a problem in most of the areas where these patients sought treatment,” Dr Bruxvoort said. “There might be other reasons either patients or providers are not using ACTs in these contexts, but the issue of undertreating malaria, even when there is clear evidence of the disease, is troubling and deserves further study.” ![]()
Drug receives orphan designation for ocular GVHD
The US Food and Drug Administration (FDA) has granted orphan drug designation to OCU300 (brimonidine tartrate) for the treatment of ocular graft-versus-host disease (oGVHD) occurring after allogeneic hematopoietic stem cell transplant.
Brimonidine tartrate is an alpha adrenergic agonist that is already FDA-approved to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Ocugen Inc. is repurposing the drug as OCU300 for the treatment of oGVHD under the FDA’s 505(b)(2) regulatory pathway.
“We are very excited to receive the first-ever orphan drug designation by the FDA for oGVHD, emphasizing the unmet medical need for patients with this disease,” said Shankar Musunuri, PhD, chairman, chief executive officer, and co-founder of Ocugen Inc.
“This is a significant milestone that will allow us to further advance the clinical development of OCU300, with a proprietary nanoemulsion, into a phase 3 clinical trial in the near future.”
According to Ocugen, OCU300 produced a beneficial effect in 90% of patients with oGVHD in an observational study, and the drug did not produce significant side effects.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to OCU300 (brimonidine tartrate) for the treatment of ocular graft-versus-host disease (oGVHD) occurring after allogeneic hematopoietic stem cell transplant.
Brimonidine tartrate is an alpha adrenergic agonist that is already FDA-approved to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Ocugen Inc. is repurposing the drug as OCU300 for the treatment of oGVHD under the FDA’s 505(b)(2) regulatory pathway.
“We are very excited to receive the first-ever orphan drug designation by the FDA for oGVHD, emphasizing the unmet medical need for patients with this disease,” said Shankar Musunuri, PhD, chairman, chief executive officer, and co-founder of Ocugen Inc.
“This is a significant milestone that will allow us to further advance the clinical development of OCU300, with a proprietary nanoemulsion, into a phase 3 clinical trial in the near future.”
According to Ocugen, OCU300 produced a beneficial effect in 90% of patients with oGVHD in an observational study, and the drug did not produce significant side effects.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted orphan drug designation to OCU300 (brimonidine tartrate) for the treatment of ocular graft-versus-host disease (oGVHD) occurring after allogeneic hematopoietic stem cell transplant.
Brimonidine tartrate is an alpha adrenergic agonist that is already FDA-approved to lower intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
Ocugen Inc. is repurposing the drug as OCU300 for the treatment of oGVHD under the FDA’s 505(b)(2) regulatory pathway.
“We are very excited to receive the first-ever orphan drug designation by the FDA for oGVHD, emphasizing the unmet medical need for patients with this disease,” said Shankar Musunuri, PhD, chairman, chief executive officer, and co-founder of Ocugen Inc.
“This is a significant milestone that will allow us to further advance the clinical development of OCU300, with a proprietary nanoemulsion, into a phase 3 clinical trial in the near future.”
According to Ocugen, OCU300 produced a beneficial effect in 90% of patients with oGVHD in an observational study, and the drug did not produce significant side effects.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
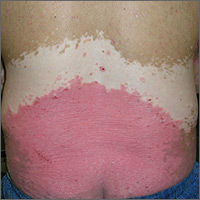
Rash and skin discoloration
The FP diagnosed plaque psoriasis as the cause of the erythema and scale. The FP suspected vitiligo or postinflammatory hypopigmentation as the cause of the discoloration. (It’s not unusual for patients with psoriasis to have vitiligo, as well.) The FP referred the patient to a dermatologist for a diagnosis, but never received feedback.
When diagnosing psoriasis, it’s crucial to determine if it’s accompanied by psoriatic arthritis. That’s because the presence of arthritis requires systemic therapy to prevent further joint damage. In this case, the patient denied having joint pain and morning stiffness.
Smoking tobacco and drinking excessive amounts of alcohol are both risk factors for psoriasis. The FP started treatment by discussing lifestyle changes and emphasizing the importance of abstinence from tobacco. While the patient wouldn’t set a quit date, he agreed to cut his intake down to half a pack of cigarettes a day. He also agreed to limit his consumption of alcohol to 2 beers a day. In addition, the FP prescribed a 454-g tub of 0.1% triamcinolone ointment to be applied twice daily. The ointment is more effective, but some patients may prefer the less greasy feel of a cream.
At a follow-up appointment a year later, the patient’s plaque psoriasis was under better control. His hands, however, showed extensive depigmentation. This confirmed the diagnosis of vitiligo, but the patient was not bothered by the appearance of it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed plaque psoriasis as the cause of the erythema and scale. The FP suspected vitiligo or postinflammatory hypopigmentation as the cause of the discoloration. (It’s not unusual for patients with psoriasis to have vitiligo, as well.) The FP referred the patient to a dermatologist for a diagnosis, but never received feedback.
When diagnosing psoriasis, it’s crucial to determine if it’s accompanied by psoriatic arthritis. That’s because the presence of arthritis requires systemic therapy to prevent further joint damage. In this case, the patient denied having joint pain and morning stiffness.
Smoking tobacco and drinking excessive amounts of alcohol are both risk factors for psoriasis. The FP started treatment by discussing lifestyle changes and emphasizing the importance of abstinence from tobacco. While the patient wouldn’t set a quit date, he agreed to cut his intake down to half a pack of cigarettes a day. He also agreed to limit his consumption of alcohol to 2 beers a day. In addition, the FP prescribed a 454-g tub of 0.1% triamcinolone ointment to be applied twice daily. The ointment is more effective, but some patients may prefer the less greasy feel of a cream.
At a follow-up appointment a year later, the patient’s plaque psoriasis was under better control. His hands, however, showed extensive depigmentation. This confirmed the diagnosis of vitiligo, but the patient was not bothered by the appearance of it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed plaque psoriasis as the cause of the erythema and scale. The FP suspected vitiligo or postinflammatory hypopigmentation as the cause of the discoloration. (It’s not unusual for patients with psoriasis to have vitiligo, as well.) The FP referred the patient to a dermatologist for a diagnosis, but never received feedback.
When diagnosing psoriasis, it’s crucial to determine if it’s accompanied by psoriatic arthritis. That’s because the presence of arthritis requires systemic therapy to prevent further joint damage. In this case, the patient denied having joint pain and morning stiffness.
Smoking tobacco and drinking excessive amounts of alcohol are both risk factors for psoriasis. The FP started treatment by discussing lifestyle changes and emphasizing the importance of abstinence from tobacco. While the patient wouldn’t set a quit date, he agreed to cut his intake down to half a pack of cigarettes a day. He also agreed to limit his consumption of alcohol to 2 beers a day. In addition, the FP prescribed a 454-g tub of 0.1% triamcinolone ointment to be applied twice daily. The ointment is more effective, but some patients may prefer the less greasy feel of a cream.
At a follow-up appointment a year later, the patient’s plaque psoriasis was under better control. His hands, however, showed extensive depigmentation. This confirmed the diagnosis of vitiligo, but the patient was not bothered by the appearance of it.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Psoriasis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 878-895.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
U.S. opioid, heroin overdose deaths may be one-fifth higher than reported
Christopher J. Ruhm, PhD, of the University of Virginia, Charlottesville, conducted an analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014. The analysis suggests that, in 2014, national opioid mortality rates were actually 24% greater than reported and that heroin overdose fatality rates were 22% higher than reported (Am J Prev Med. 2017 Aug 7. doi: 10.1016/j.amepre.2017.06.009).
To correct for this, Dr. Ruhm extrapolated the drug category in these unspecified cases by using information from death certificate reports where at least one specific drug category was identified as the cause of death. The variables considered included sex, ethnicity, marital status, education, day of the week indicators, location of death (such as hospital inpatient or ED) or arriving at the hospital already deceased, and interactions among these variables.
“The corrected rates provide the best currently available information on geographic variation in opioid and heroin involved fatality rates,” Dr. Ruhm noted.
The differences between corrected and reported rates of fatal opioid and heroin overdose varied significantly between states; in some cases, the correct rates were more than double the reported rates.
In Pennsylvania, for example, the corrected rates of opioid and heroin overdose fatalities were 108% and 107% greater than the reported rates, respectively; in Mississippi, the corrected rates were 107% and 139% higher than reported. Alabama, Indiana, and Louisiana also showed large disparities between corrected and reported rates.
Dr. Ruhm also noted significant variability between the states in whether a specific drug was mentioned on the death certificate or not. In Rhode Island, Connecticut, and New Hampshire, a drug category was mentioned for more than 99% of drug poisoning deaths; but in Pennsylvania, Indiana, Mississippi, Louisiana, and Alabama, this was reported in only about half of these incidents.
There was a general trend toward underreporting in the South, while rates of nonreporting were lower in parts of the Northeast and the West.
Dr. Ruhm suggested that additional training and standardization could be helpful in states with low specification rates, “particularly because this is a bigger problem when death certificates are completed by coroners rather than medical examiners and in states without centralized oversight.”
Overall, the growth in opioid-involved drug deaths from 2008 to 2014 was similar between the reported and corrected rates. However, in the case of heroin-related mortality, the increase was underestimated by the reported data by about 18%. Again, this difference varied between states, with substantial underestimates seen in Pennsylvania, Indiana, New Jersey, Louisiana, and Alabama.
“Understanding the inaccuracies resulting from the lack of specificity of drug involvement on death certificates is also important because federal policies often target states believed to have especially severe opioid or heroin problems,” Dr. Ruhm explained. “More fundamentally, geographic disparities in drug poisoning deaths are substantial, and a correct assessment of them is almost certainly a prerequisite for designing policies to address the fatal drug epidemic.”
Dr. Ruhm declared no conflicts of interest.
Christopher J. Ruhm, PhD, of the University of Virginia, Charlottesville, conducted an analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014. The analysis suggests that, in 2014, national opioid mortality rates were actually 24% greater than reported and that heroin overdose fatality rates were 22% higher than reported (Am J Prev Med. 2017 Aug 7. doi: 10.1016/j.amepre.2017.06.009).
To correct for this, Dr. Ruhm extrapolated the drug category in these unspecified cases by using information from death certificate reports where at least one specific drug category was identified as the cause of death. The variables considered included sex, ethnicity, marital status, education, day of the week indicators, location of death (such as hospital inpatient or ED) or arriving at the hospital already deceased, and interactions among these variables.
“The corrected rates provide the best currently available information on geographic variation in opioid and heroin involved fatality rates,” Dr. Ruhm noted.
The differences between corrected and reported rates of fatal opioid and heroin overdose varied significantly between states; in some cases, the correct rates were more than double the reported rates.
In Pennsylvania, for example, the corrected rates of opioid and heroin overdose fatalities were 108% and 107% greater than the reported rates, respectively; in Mississippi, the corrected rates were 107% and 139% higher than reported. Alabama, Indiana, and Louisiana also showed large disparities between corrected and reported rates.
Dr. Ruhm also noted significant variability between the states in whether a specific drug was mentioned on the death certificate or not. In Rhode Island, Connecticut, and New Hampshire, a drug category was mentioned for more than 99% of drug poisoning deaths; but in Pennsylvania, Indiana, Mississippi, Louisiana, and Alabama, this was reported in only about half of these incidents.
There was a general trend toward underreporting in the South, while rates of nonreporting were lower in parts of the Northeast and the West.
Dr. Ruhm suggested that additional training and standardization could be helpful in states with low specification rates, “particularly because this is a bigger problem when death certificates are completed by coroners rather than medical examiners and in states without centralized oversight.”
Overall, the growth in opioid-involved drug deaths from 2008 to 2014 was similar between the reported and corrected rates. However, in the case of heroin-related mortality, the increase was underestimated by the reported data by about 18%. Again, this difference varied between states, with substantial underestimates seen in Pennsylvania, Indiana, New Jersey, Louisiana, and Alabama.
“Understanding the inaccuracies resulting from the lack of specificity of drug involvement on death certificates is also important because federal policies often target states believed to have especially severe opioid or heroin problems,” Dr. Ruhm explained. “More fundamentally, geographic disparities in drug poisoning deaths are substantial, and a correct assessment of them is almost certainly a prerequisite for designing policies to address the fatal drug epidemic.”
Dr. Ruhm declared no conflicts of interest.
Christopher J. Ruhm, PhD, of the University of Virginia, Charlottesville, conducted an analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014. The analysis suggests that, in 2014, national opioid mortality rates were actually 24% greater than reported and that heroin overdose fatality rates were 22% higher than reported (Am J Prev Med. 2017 Aug 7. doi: 10.1016/j.amepre.2017.06.009).
To correct for this, Dr. Ruhm extrapolated the drug category in these unspecified cases by using information from death certificate reports where at least one specific drug category was identified as the cause of death. The variables considered included sex, ethnicity, marital status, education, day of the week indicators, location of death (such as hospital inpatient or ED) or arriving at the hospital already deceased, and interactions among these variables.
“The corrected rates provide the best currently available information on geographic variation in opioid and heroin involved fatality rates,” Dr. Ruhm noted.
The differences between corrected and reported rates of fatal opioid and heroin overdose varied significantly between states; in some cases, the correct rates were more than double the reported rates.
In Pennsylvania, for example, the corrected rates of opioid and heroin overdose fatalities were 108% and 107% greater than the reported rates, respectively; in Mississippi, the corrected rates were 107% and 139% higher than reported. Alabama, Indiana, and Louisiana also showed large disparities between corrected and reported rates.
Dr. Ruhm also noted significant variability between the states in whether a specific drug was mentioned on the death certificate or not. In Rhode Island, Connecticut, and New Hampshire, a drug category was mentioned for more than 99% of drug poisoning deaths; but in Pennsylvania, Indiana, Mississippi, Louisiana, and Alabama, this was reported in only about half of these incidents.
There was a general trend toward underreporting in the South, while rates of nonreporting were lower in parts of the Northeast and the West.
Dr. Ruhm suggested that additional training and standardization could be helpful in states with low specification rates, “particularly because this is a bigger problem when death certificates are completed by coroners rather than medical examiners and in states without centralized oversight.”
Overall, the growth in opioid-involved drug deaths from 2008 to 2014 was similar between the reported and corrected rates. However, in the case of heroin-related mortality, the increase was underestimated by the reported data by about 18%. Again, this difference varied between states, with substantial underestimates seen in Pennsylvania, Indiana, New Jersey, Louisiana, and Alabama.
“Understanding the inaccuracies resulting from the lack of specificity of drug involvement on death certificates is also important because federal policies often target states believed to have especially severe opioid or heroin problems,” Dr. Ruhm explained. “More fundamentally, geographic disparities in drug poisoning deaths are substantial, and a correct assessment of them is almost certainly a prerequisite for designing policies to address the fatal drug epidemic.”
Dr. Ruhm declared no conflicts of interest.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Death certificates for drug overdose that fail to specify the drug ivolved may be responsible for a significant underestimation of opioid and heroin fatality rates in the United States.
Major finding: In 2014, national opioid mortality rates were actually 24% greater than reported, and heroin overdose fatality rates were 22% higher than reported.
Data source: Analysis using improved estimates of state-level opioid and heroin overdose fatalities during 2014 and of changes over time during 2008-2014.
Disclosures: No conflicts of interest were declared.
C-section raises hysterectomy complication risk later
Women who have at least one cesarean delivery have a more than 30% risk of a complication requiring reoperation after benign hysterectomy later in life, compared with women who have had vaginal deliveries only, according to a study of more than 7,600 women in a Danish patient registry.
Cesarean delivery is the most common major surgery performed in the world, and the rate is rapidly increasing, with the global average cesarean rate estimated at 18.6%, and rates as high as 52% in some European countries. However, the impact cesarean deliveries have on surgical complications later in life has not been thoroughly studied. The study authors said this might be the first population study of the association of cesarean delivery with hysterectomy complications.
Of the 388 women (5%) who had a hysterectomy and then a reoperation within 30 days, the risk increased with the number of previous cesarean deliveries. Those who had vaginal-only deliveries had reoperation rates of 4.4%, compared with 6.2% for those who had one cesarean delivery and 6.8% for those who had two or more. That represents increased risks of 31% and 35% for women who had one cesarean delivery and two or more cesarean deliveries, respectively, compared with women who had only vaginal deliveries.
Likewise, surgical complications were 16% more frequent in women who had one previous cesarean delivery and 30% more likely in women with two or more cesarean deliveries. Women who had two or more cesarean deliveries were almost twice as likely (odds ratio, 1.93) to receive a blood transfusion.
“Our results imply that information on long-term associations should be made more readily available to women, clinicians, and policymakers and suggest that decisions on cesarean delivery should take into account not only immediate maternal and neonatal influences, but also women’s health in the long term, including an increased risk of reoperation and complications associated with surgery later in life,” the researchers wrote. “The results support policies and clinical efforts to prevent cesarean deliveries that are not medically indicated.”
The study noted some limitations, including the observational design, which did not allow for elimination of all potential confounding factors.
The researchers reported having no relevant financial disclosures.
Women who have at least one cesarean delivery have a more than 30% risk of a complication requiring reoperation after benign hysterectomy later in life, compared with women who have had vaginal deliveries only, according to a study of more than 7,600 women in a Danish patient registry.
Cesarean delivery is the most common major surgery performed in the world, and the rate is rapidly increasing, with the global average cesarean rate estimated at 18.6%, and rates as high as 52% in some European countries. However, the impact cesarean deliveries have on surgical complications later in life has not been thoroughly studied. The study authors said this might be the first population study of the association of cesarean delivery with hysterectomy complications.
Of the 388 women (5%) who had a hysterectomy and then a reoperation within 30 days, the risk increased with the number of previous cesarean deliveries. Those who had vaginal-only deliveries had reoperation rates of 4.4%, compared with 6.2% for those who had one cesarean delivery and 6.8% for those who had two or more. That represents increased risks of 31% and 35% for women who had one cesarean delivery and two or more cesarean deliveries, respectively, compared with women who had only vaginal deliveries.
Likewise, surgical complications were 16% more frequent in women who had one previous cesarean delivery and 30% more likely in women with two or more cesarean deliveries. Women who had two or more cesarean deliveries were almost twice as likely (odds ratio, 1.93) to receive a blood transfusion.
“Our results imply that information on long-term associations should be made more readily available to women, clinicians, and policymakers and suggest that decisions on cesarean delivery should take into account not only immediate maternal and neonatal influences, but also women’s health in the long term, including an increased risk of reoperation and complications associated with surgery later in life,” the researchers wrote. “The results support policies and clinical efforts to prevent cesarean deliveries that are not medically indicated.”
The study noted some limitations, including the observational design, which did not allow for elimination of all potential confounding factors.
The researchers reported having no relevant financial disclosures.
Women who have at least one cesarean delivery have a more than 30% risk of a complication requiring reoperation after benign hysterectomy later in life, compared with women who have had vaginal deliveries only, according to a study of more than 7,600 women in a Danish patient registry.
Cesarean delivery is the most common major surgery performed in the world, and the rate is rapidly increasing, with the global average cesarean rate estimated at 18.6%, and rates as high as 52% in some European countries. However, the impact cesarean deliveries have on surgical complications later in life has not been thoroughly studied. The study authors said this might be the first population study of the association of cesarean delivery with hysterectomy complications.
Of the 388 women (5%) who had a hysterectomy and then a reoperation within 30 days, the risk increased with the number of previous cesarean deliveries. Those who had vaginal-only deliveries had reoperation rates of 4.4%, compared with 6.2% for those who had one cesarean delivery and 6.8% for those who had two or more. That represents increased risks of 31% and 35% for women who had one cesarean delivery and two or more cesarean deliveries, respectively, compared with women who had only vaginal deliveries.
Likewise, surgical complications were 16% more frequent in women who had one previous cesarean delivery and 30% more likely in women with two or more cesarean deliveries. Women who had two or more cesarean deliveries were almost twice as likely (odds ratio, 1.93) to receive a blood transfusion.
“Our results imply that information on long-term associations should be made more readily available to women, clinicians, and policymakers and suggest that decisions on cesarean delivery should take into account not only immediate maternal and neonatal influences, but also women’s health in the long term, including an increased risk of reoperation and complications associated with surgery later in life,” the researchers wrote. “The results support policies and clinical efforts to prevent cesarean deliveries that are not medically indicated.”
The study noted some limitations, including the observational design, which did not allow for elimination of all potential confounding factors.
The researchers reported having no relevant financial disclosures.
FROM JAMA SURGERY
Key clinical point:
Major finding: The rate of complications after hysterectomy was 4.4% for women who had vaginal birth only, 6.2% for those who had one cesarean delivery, and 6.8% for those who had two or more cesarean deliveries.
Data source: Danish National Patient Registry–based cohort study of 7,685 women who gave birth from 1993 to 2012.
Disclosures: The researchers reported having no financial disclosures.
FDA approves first spironolactone oral suspension
The Food and Drug Administration has approved CaroSpir, the first oral suspension form of spironolactone, the aldosterone antagonist that was first approved in 1960, according to an announcement from CMP Pharma.
CaroSpir is intended for the treatment of New York Heart Association class III-IV heart failure and reduced ejection fraction, usually in combination with other treatments. CaroSpir is also indicated as an add-on medication for the treatment of hypertension, and for the treatment of edema in cirrhotic patients who have not adequately responded to fluid and sodium restriction.
“CaroSpir provides a stable, ready to use, and consistent liquid treatment option for adult patients. Up until now, these patients have been prescribed a pharmacy-compounded liquid form of spironolactone. The dosing inconsistencies of compounded liquids have long been a persistent challenge for physicians,” Gerald Sakowski, CEO at CMP Pharma, said in the press release.
Find the full press release on the CMP Pharma website.
The Food and Drug Administration has approved CaroSpir, the first oral suspension form of spironolactone, the aldosterone antagonist that was first approved in 1960, according to an announcement from CMP Pharma.
CaroSpir is intended for the treatment of New York Heart Association class III-IV heart failure and reduced ejection fraction, usually in combination with other treatments. CaroSpir is also indicated as an add-on medication for the treatment of hypertension, and for the treatment of edema in cirrhotic patients who have not adequately responded to fluid and sodium restriction.
“CaroSpir provides a stable, ready to use, and consistent liquid treatment option for adult patients. Up until now, these patients have been prescribed a pharmacy-compounded liquid form of spironolactone. The dosing inconsistencies of compounded liquids have long been a persistent challenge for physicians,” Gerald Sakowski, CEO at CMP Pharma, said in the press release.
Find the full press release on the CMP Pharma website.
The Food and Drug Administration has approved CaroSpir, the first oral suspension form of spironolactone, the aldosterone antagonist that was first approved in 1960, according to an announcement from CMP Pharma.
CaroSpir is intended for the treatment of New York Heart Association class III-IV heart failure and reduced ejection fraction, usually in combination with other treatments. CaroSpir is also indicated as an add-on medication for the treatment of hypertension, and for the treatment of edema in cirrhotic patients who have not adequately responded to fluid and sodium restriction.
“CaroSpir provides a stable, ready to use, and consistent liquid treatment option for adult patients. Up until now, these patients have been prescribed a pharmacy-compounded liquid form of spironolactone. The dosing inconsistencies of compounded liquids have long been a persistent challenge for physicians,” Gerald Sakowski, CEO at CMP Pharma, said in the press release.
Find the full press release on the CMP Pharma website.
Inactivated flu vaccine more effective than live version
The inactivated influenza vaccine is more effective than the quadrivalent live attenuated vaccine, according to a study conducted by the Influenza Vaccine Effectiveness Network.
After poor live vaccine performance in young children during the 2013-2014 flu season, the A(H1N1)pdm09 strain was changed. In response to earlier reports that the A(H1N1)pdm09 vaccine strain had poor thermostability, it was updated to A/Bolivia/559/2013 (an A/California/7/2009-like virus) for the 2015-2016 influenza season. Unfortunately, the changes did not increase the vaccine’s immunogenicity. At study sites in Michigan, Pennsylvania, Texas, Washington, and Wisconsin, 6,879 patients aged 6 months or older who presented for acute respiratory illness with a cough of 7 or fewer days were tested for influenza. Of those, 1,309 (19%) tested positive. Vaccination histories were taken from parent interviews and electronic health records.
“Although the quadrivalent live attenuated vaccine remains licensed in the United States, the [Advisory Committee on Immunization Practices] did not recommend this vaccine for the 2016-2017 influenza season,” the investigators wrote.
Partial funding for the research came from the Centers for Disease Control and Prevention and the National Institutes of Health. Dr. Jackson reported receiving a grant from Medimmune.
Read more in the New England Journal of Medicine (2017;377:534-43).
The inactivated influenza vaccine is more effective than the quadrivalent live attenuated vaccine, according to a study conducted by the Influenza Vaccine Effectiveness Network.
After poor live vaccine performance in young children during the 2013-2014 flu season, the A(H1N1)pdm09 strain was changed. In response to earlier reports that the A(H1N1)pdm09 vaccine strain had poor thermostability, it was updated to A/Bolivia/559/2013 (an A/California/7/2009-like virus) for the 2015-2016 influenza season. Unfortunately, the changes did not increase the vaccine’s immunogenicity. At study sites in Michigan, Pennsylvania, Texas, Washington, and Wisconsin, 6,879 patients aged 6 months or older who presented for acute respiratory illness with a cough of 7 or fewer days were tested for influenza. Of those, 1,309 (19%) tested positive. Vaccination histories were taken from parent interviews and electronic health records.
“Although the quadrivalent live attenuated vaccine remains licensed in the United States, the [Advisory Committee on Immunization Practices] did not recommend this vaccine for the 2016-2017 influenza season,” the investigators wrote.
Partial funding for the research came from the Centers for Disease Control and Prevention and the National Institutes of Health. Dr. Jackson reported receiving a grant from Medimmune.
Read more in the New England Journal of Medicine (2017;377:534-43).
The inactivated influenza vaccine is more effective than the quadrivalent live attenuated vaccine, according to a study conducted by the Influenza Vaccine Effectiveness Network.
After poor live vaccine performance in young children during the 2013-2014 flu season, the A(H1N1)pdm09 strain was changed. In response to earlier reports that the A(H1N1)pdm09 vaccine strain had poor thermostability, it was updated to A/Bolivia/559/2013 (an A/California/7/2009-like virus) for the 2015-2016 influenza season. Unfortunately, the changes did not increase the vaccine’s immunogenicity. At study sites in Michigan, Pennsylvania, Texas, Washington, and Wisconsin, 6,879 patients aged 6 months or older who presented for acute respiratory illness with a cough of 7 or fewer days were tested for influenza. Of those, 1,309 (19%) tested positive. Vaccination histories were taken from parent interviews and electronic health records.
“Although the quadrivalent live attenuated vaccine remains licensed in the United States, the [Advisory Committee on Immunization Practices] did not recommend this vaccine for the 2016-2017 influenza season,” the investigators wrote.
Partial funding for the research came from the Centers for Disease Control and Prevention and the National Institutes of Health. Dr. Jackson reported receiving a grant from Medimmune.
Read more in the New England Journal of Medicine (2017;377:534-43).
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Researchers identify ‘congenital NAD deficiency disorders’
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Shi et al. report that a deficiency of nicotinamide adenine dinucleotide (NAD) causes congenital malformations, suggesting that interventions to raise NAD levels during fetal and early postnatal development might further reduce the incidence of congenital anomalies.
Regardless of how NAD depletion leads to congenital malformations (whether by compromising the detection of DNA damage by PARP proteins, reducing the supply of nucleotides, or both), dietary supplementation with NAD precursors merits further study. At high doses, niacin can cause flushing and gastrointestinal symptoms, but it has few side effects at lower doses.
Nicotinamide mononucleotide, nicotinamide riboside, and nicotinamide itself are better tolerated than niacin and are generally considered to be safe as dietary supplements, but the doses of NAD precursors required to reduce the risk of congenital malformations in humans are not known. Also unknown is the extent to which raising dietary levels of NAD would limit cognitive impairment in infants with congenital malformations.
Matthew G. Vander Heiden, MD, PhD, is with the Massachusetts Institute of Technology, Cambridge, Mass., and the Dana Farber Cancer Center, Boston. He reported receiving personal fees from Agios Pharmaceuticals and Aeglea Biotherapeutics outside the submitted work. These comments are adapted from an editorial (N Engl J Med. 2007;377:509-11).
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Mutations that disrupt de novo synthesis of nicotinamide adenine dinucleotide (NAD) were associated with multiple congenital malformations in humans and mice, and supplementing niacin during gestation prevented these malformations in mice, new research suggests.
The malformations include vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities (VACTERL), “a nonrandom combination of congenital defects without a known cause,” wrote Hongjun Shi, PhD, of Victor Chang Cardiac Research Institute, New South Wales, Australia, and colleagues (N Engl J Med. 2017;377:544-52).
Numerous genetic and environmental factors can potentially cause NAD deficiency during gestation and the investigators suggested collectively referring to the resulting malformations as “congenital NAD deficiency disorders.”
Congenital defects can occur together in newborns more often than would be expected by chance, but “in many such cases, it has proved difficult to identify a genetic cause,” the investigators noted. Using genomic sequencing, they looked for possible pathogenic gene variants within four unrelated families in which a person was born with multiple congenital malformations. Next, they evaluated the function of the variants by testing in vitro enzyme activity and measuring relevant plasma metabolites. Finally, they used the CRISPR (clustered regularly interspaced short palindromic repeats)–Cas9 system to create mouse models with similar variants.
This approach identified variants in two genes encoding enzymes of the kynurenine pathway: 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU). Three patients had homozygous variants associated with loss-of-function changes in these proteins. A fourth patient had heterozygous variants in the gene encoding KYNU.
“The mutant enzymes had greatly reduced activity in vitro,” the researchers wrote. Patients had decreased circulating levels of NAD, which tryptophan synthesizes through the kynurenine pathway. Notably, mouse embryos lacking the mouse equivalents of HAAO or KYNU also had congenital defects associated with NAD deficiency. Preventing NAD deficiency during gestation averted these defects in mice.
“The NAD de novo synthesis pathway catabolizes tryptophan,” the researchers added. “Although metabolite levels upstream of the block are elevated, and the metabolites have postnatal functions, we found that it is the deficiency in embryonic NAD, downstream of the block, that is disrupting embryogenesis.”
The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Major congenital defects affecting unrelated families were associated with variants in genes encoding 3-hydroxyanthranilic acid 3,4-dioxygenase (HAAO) and kynureninase (KYNU).
Data source: Genomic sequencing of four unrelated families in which a person was born with multiple congenital malformations, plus in vitro measurements of enzyme activity and plasma metabolites and studies of mouse models created with the CRISPR–Cas9 system.
Disclosures: The study was supported by the Australian and New South Wales governments and foundations. The investigators reported having no other financial disclosures.
Cosmetic Corner: Dermatologists Weigh in on Men’s Products
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s products. Consideration must be given to:
- bareMinerals SPF 30 Natural Sunscreen
Bare Escentuals Beauty, Inc
“I recommend this product to my male patients when they are not wearing a hat. It protects the scalp from UV damage without a heavy greasy finish.”—Shari Lipner, MD, PhD, New York, New York
- Ducray Alopexy 5% For Men
Pierre Fabre Laboratories
“This dermatologist-dispensed product for men addresses chronic hair loss as well as thinning hair. It contains an optimal level of minoxidil 5% in an elegant unscented formulation and is designed to spray on smoothly and evenly.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Scrub
Kiehl’s
“This product is great for oily skin and enlarged pores. The particles in the product allow one to get a deep-clean feeling.”—Gary Goldenberg, MD, New York, New York
- Physical Matte UV Defense SPF 50
SkinCeuticals
“For men I like to keep things simple. I recommend what I use with the single most important thing being daily sun protection. SkinCeuticals Physical Matte UV Defense SPF 50 is my favorite and I use it after I shave. It goes on smoothly and has a natural tint along with a high SPF.”—Jerome Potozkin, MD, Danville, California
- Ultimate Brushless Shave Cream
Kiehl’s
“I recommend this product for men with frequent irritation from shaving. This cream-based product helps to provide a close shave without as much irritation from other gel-based products. A small amount goes a long way!”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s products. Consideration must be given to:
- bareMinerals SPF 30 Natural Sunscreen
Bare Escentuals Beauty, Inc
“I recommend this product to my male patients when they are not wearing a hat. It protects the scalp from UV damage without a heavy greasy finish.”—Shari Lipner, MD, PhD, New York, New York
- Ducray Alopexy 5% For Men
Pierre Fabre Laboratories
“This dermatologist-dispensed product for men addresses chronic hair loss as well as thinning hair. It contains an optimal level of minoxidil 5% in an elegant unscented formulation and is designed to spray on smoothly and evenly.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Scrub
Kiehl’s
“This product is great for oily skin and enlarged pores. The particles in the product allow one to get a deep-clean feeling.”—Gary Goldenberg, MD, New York, New York
- Physical Matte UV Defense SPF 50
SkinCeuticals
“For men I like to keep things simple. I recommend what I use with the single most important thing being daily sun protection. SkinCeuticals Physical Matte UV Defense SPF 50 is my favorite and I use it after I shave. It goes on smoothly and has a natural tint along with a high SPF.”—Jerome Potozkin, MD, Danville, California
- Ultimate Brushless Shave Cream
Kiehl’s
“I recommend this product for men with frequent irritation from shaving. This cream-based product helps to provide a close shave without as much irritation from other gel-based products. A small amount goes a long way!”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on men’s products. Consideration must be given to:
- bareMinerals SPF 30 Natural Sunscreen
Bare Escentuals Beauty, Inc
“I recommend this product to my male patients when they are not wearing a hat. It protects the scalp from UV damage without a heavy greasy finish.”—Shari Lipner, MD, PhD, New York, New York
- Ducray Alopexy 5% For Men
Pierre Fabre Laboratories
“This dermatologist-dispensed product for men addresses chronic hair loss as well as thinning hair. It contains an optimal level of minoxidil 5% in an elegant unscented formulation and is designed to spray on smoothly and evenly.”—Jeannette Graf, MD, Great Neck, New York
- Facial Fuel Energizing Scrub
Kiehl’s
“This product is great for oily skin and enlarged pores. The particles in the product allow one to get a deep-clean feeling.”—Gary Goldenberg, MD, New York, New York
- Physical Matte UV Defense SPF 50
SkinCeuticals
“For men I like to keep things simple. I recommend what I use with the single most important thing being daily sun protection. SkinCeuticals Physical Matte UV Defense SPF 50 is my favorite and I use it after I shave. It goes on smoothly and has a natural tint along with a high SPF.”—Jerome Potozkin, MD, Danville, California
- Ultimate Brushless Shave Cream
Kiehl’s
“I recommend this product for men with frequent irritation from shaving. This cream-based product helps to provide a close shave without as much irritation from other gel-based products. A small amount goes a long way!”—Anthony M. Rossi, MD, New York, New York
Cutis invites readers to send us their recommendations. Athlete’s foot treatments and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please email your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.