User login
Rewriting the script on polypharmacy
Drugs are valuable when they effectively relieve symptoms or prevent illness, but we all know they are double-edged swords when it comes to cost, adverse effects, and drug interactions. This “downside” is not lost on older Americans—especially when you consider that more than a third of Americans, ages 62 to 85 years, take 5 or more prescription medications daily.1
Too often patients take prescription drugs that they either don’t need or that are harming them. That’s where deprescribing comes in. As this month’s feature article by McGrath and colleagues explains, deprescribing is the process of reducing or stopping unnecessary prescription medications.
The power of deprescribing. About a decade ago, a geriatrician/family physician friend of mine took over as medical director of a 160-bed nursing home. He lamented that the average number of prescription medications taken by the patients in the nursing home was 9.5. He and his team went to work deprescribing, and one year later, the average number of prescription medications per patient was 5.3. As far as he and the nursing staff could tell, the patients were doing just fine and were more alert and functional.
Another specialist, another Rx. In clinic, I saw a 54-year-old woman with the chief complaint of chronic, dry cough for which she had been on a specialist pilgrimage. A GI specialist prescribed omeprazole, an ENT physician prescribed fluticasone nasal spray and cetirizine, and a pulmonologist added an inhaled corticosteroid to the mix. (I’m not making this up!) I reviewed her medication list carefully and noted she had been placed on amitriptyline for insomnia shortly before the cough began. I was suspicious because the properties of anticholinergics can contribute to a cough. At my suggestion, she agreed to stop the amitriptyline (and endure some sleeplessness). Two weeks later, she returned with no cough. Over the next month, she stopped all 4 other medications, and the cough did not return.
Today in the office, a 64-year-old man complained of lightheadedness and fatigue and told me his blood pressure on home monitoring was consistently around 105/50 mm Hg. In addition to taking 3 antihypertensive medications, I discovered he had been prescribed doxazosin—an alpha blocker, which also lowers blood pressure—
I’m certain that you, too, have stories of successful deprescribing. Let’s remain alert to the problem of polypharmacy, keep meticulous medication lists, and deprescribe whenever it makes good sense. Doing so is essential to our roles as family physicians.
1. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
Drugs are valuable when they effectively relieve symptoms or prevent illness, but we all know they are double-edged swords when it comes to cost, adverse effects, and drug interactions. This “downside” is not lost on older Americans—especially when you consider that more than a third of Americans, ages 62 to 85 years, take 5 or more prescription medications daily.1
Too often patients take prescription drugs that they either don’t need or that are harming them. That’s where deprescribing comes in. As this month’s feature article by McGrath and colleagues explains, deprescribing is the process of reducing or stopping unnecessary prescription medications.
The power of deprescribing. About a decade ago, a geriatrician/family physician friend of mine took over as medical director of a 160-bed nursing home. He lamented that the average number of prescription medications taken by the patients in the nursing home was 9.5. He and his team went to work deprescribing, and one year later, the average number of prescription medications per patient was 5.3. As far as he and the nursing staff could tell, the patients were doing just fine and were more alert and functional.
Another specialist, another Rx. In clinic, I saw a 54-year-old woman with the chief complaint of chronic, dry cough for which she had been on a specialist pilgrimage. A GI specialist prescribed omeprazole, an ENT physician prescribed fluticasone nasal spray and cetirizine, and a pulmonologist added an inhaled corticosteroid to the mix. (I’m not making this up!) I reviewed her medication list carefully and noted she had been placed on amitriptyline for insomnia shortly before the cough began. I was suspicious because the properties of anticholinergics can contribute to a cough. At my suggestion, she agreed to stop the amitriptyline (and endure some sleeplessness). Two weeks later, she returned with no cough. Over the next month, she stopped all 4 other medications, and the cough did not return.
Today in the office, a 64-year-old man complained of lightheadedness and fatigue and told me his blood pressure on home monitoring was consistently around 105/50 mm Hg. In addition to taking 3 antihypertensive medications, I discovered he had been prescribed doxazosin—an alpha blocker, which also lowers blood pressure—
I’m certain that you, too, have stories of successful deprescribing. Let’s remain alert to the problem of polypharmacy, keep meticulous medication lists, and deprescribe whenever it makes good sense. Doing so is essential to our roles as family physicians.
Drugs are valuable when they effectively relieve symptoms or prevent illness, but we all know they are double-edged swords when it comes to cost, adverse effects, and drug interactions. This “downside” is not lost on older Americans—especially when you consider that more than a third of Americans, ages 62 to 85 years, take 5 or more prescription medications daily.1
Too often patients take prescription drugs that they either don’t need or that are harming them. That’s where deprescribing comes in. As this month’s feature article by McGrath and colleagues explains, deprescribing is the process of reducing or stopping unnecessary prescription medications.
The power of deprescribing. About a decade ago, a geriatrician/family physician friend of mine took over as medical director of a 160-bed nursing home. He lamented that the average number of prescription medications taken by the patients in the nursing home was 9.5. He and his team went to work deprescribing, and one year later, the average number of prescription medications per patient was 5.3. As far as he and the nursing staff could tell, the patients were doing just fine and were more alert and functional.
Another specialist, another Rx. In clinic, I saw a 54-year-old woman with the chief complaint of chronic, dry cough for which she had been on a specialist pilgrimage. A GI specialist prescribed omeprazole, an ENT physician prescribed fluticasone nasal spray and cetirizine, and a pulmonologist added an inhaled corticosteroid to the mix. (I’m not making this up!) I reviewed her medication list carefully and noted she had been placed on amitriptyline for insomnia shortly before the cough began. I was suspicious because the properties of anticholinergics can contribute to a cough. At my suggestion, she agreed to stop the amitriptyline (and endure some sleeplessness). Two weeks later, she returned with no cough. Over the next month, she stopped all 4 other medications, and the cough did not return.
Today in the office, a 64-year-old man complained of lightheadedness and fatigue and told me his blood pressure on home monitoring was consistently around 105/50 mm Hg. In addition to taking 3 antihypertensive medications, I discovered he had been prescribed doxazosin—an alpha blocker, which also lowers blood pressure—
I’m certain that you, too, have stories of successful deprescribing. Let’s remain alert to the problem of polypharmacy, keep meticulous medication lists, and deprescribe whenever it makes good sense. Doing so is essential to our roles as family physicians.
1. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
1. Qato DM, Wilder J, Schumm LP, et al. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176:473-482.
HCV on the Rise Among Women Giving Birth
Between 2009 and 2014, hepatitis C virus (HCV) infection among women giving birth rose 89%, from 1.8 to 3.4 per live births, according to a study published in Morbidity and Mortality Weekly Report. The researchers say, geographically, the increase in maternal HCV infection mirrors increases in HCV incidence among adults. The highest infection rate was in West Virginia, which had 22.6 per 1,000 live births. Next was Tennessee with 10.1. State infection rates varied widely: Hawaii had the lowest rate, of 0.7.
In Tennessee, the prevalence of maternal HCV infection increased 163%, from 3.8 per 1,000 in 2009 to 10 in 2014. But even among the 95 Tennessee counties, rates varied substantially. The highest rates were in the 52 Appalachian counties. Campbell County had 78 per 1,000 births. Compared with women in urban areas, pregnant women from rural areas had triple the odds of HCV infection. The rise in infection among pregnant women coincides with the rises in heroin and prescription opioid epidemics, which also disproportionately affect rural populations.
Analyzing the Tennessee births, researchers found that the odds of HCV infection were about 5 times higher among women who smoked cigarettes during pregnancy. Concurrent infections were another serious risk factor, with hepatitis B virus infection boosting the odds of HCV infection by nearly 17 times.
HCV infection is a growing—but modifiable—threat among pregnant women, the researchers say. The rise in infection is “particularly concerning,” in light of recent research that has found poor follow-up of HCV-exposed infants. The researchers cite a Philadelphia study that found only 16% of HCV-exposed infants were appropriately followed. That could mean that infected infants are going undetected, the researchers say. The rate of transmission from mothers to infants is estimated at 6%; it’s important for exposed infants to be followed for evidence of seroconversion. But anti-HCV antibody tests can’t be completed until 18 months because passively acquired maternal antibodies can persist. Testing for HCV ribonucleic acid can be performed earlier.
The CDC and the American College of Obstetricians and Gynecologists recommend selective screening of pregnant women at high risk for HCV infection, particularly those with a history of injection drug use or long-term hemodialysis.
Between 2009 and 2014, hepatitis C virus (HCV) infection among women giving birth rose 89%, from 1.8 to 3.4 per live births, according to a study published in Morbidity and Mortality Weekly Report. The researchers say, geographically, the increase in maternal HCV infection mirrors increases in HCV incidence among adults. The highest infection rate was in West Virginia, which had 22.6 per 1,000 live births. Next was Tennessee with 10.1. State infection rates varied widely: Hawaii had the lowest rate, of 0.7.
In Tennessee, the prevalence of maternal HCV infection increased 163%, from 3.8 per 1,000 in 2009 to 10 in 2014. But even among the 95 Tennessee counties, rates varied substantially. The highest rates were in the 52 Appalachian counties. Campbell County had 78 per 1,000 births. Compared with women in urban areas, pregnant women from rural areas had triple the odds of HCV infection. The rise in infection among pregnant women coincides with the rises in heroin and prescription opioid epidemics, which also disproportionately affect rural populations.
Analyzing the Tennessee births, researchers found that the odds of HCV infection were about 5 times higher among women who smoked cigarettes during pregnancy. Concurrent infections were another serious risk factor, with hepatitis B virus infection boosting the odds of HCV infection by nearly 17 times.
HCV infection is a growing—but modifiable—threat among pregnant women, the researchers say. The rise in infection is “particularly concerning,” in light of recent research that has found poor follow-up of HCV-exposed infants. The researchers cite a Philadelphia study that found only 16% of HCV-exposed infants were appropriately followed. That could mean that infected infants are going undetected, the researchers say. The rate of transmission from mothers to infants is estimated at 6%; it’s important for exposed infants to be followed for evidence of seroconversion. But anti-HCV antibody tests can’t be completed until 18 months because passively acquired maternal antibodies can persist. Testing for HCV ribonucleic acid can be performed earlier.
The CDC and the American College of Obstetricians and Gynecologists recommend selective screening of pregnant women at high risk for HCV infection, particularly those with a history of injection drug use or long-term hemodialysis.
Between 2009 and 2014, hepatitis C virus (HCV) infection among women giving birth rose 89%, from 1.8 to 3.4 per live births, according to a study published in Morbidity and Mortality Weekly Report. The researchers say, geographically, the increase in maternal HCV infection mirrors increases in HCV incidence among adults. The highest infection rate was in West Virginia, which had 22.6 per 1,000 live births. Next was Tennessee with 10.1. State infection rates varied widely: Hawaii had the lowest rate, of 0.7.
In Tennessee, the prevalence of maternal HCV infection increased 163%, from 3.8 per 1,000 in 2009 to 10 in 2014. But even among the 95 Tennessee counties, rates varied substantially. The highest rates were in the 52 Appalachian counties. Campbell County had 78 per 1,000 births. Compared with women in urban areas, pregnant women from rural areas had triple the odds of HCV infection. The rise in infection among pregnant women coincides with the rises in heroin and prescription opioid epidemics, which also disproportionately affect rural populations.
Analyzing the Tennessee births, researchers found that the odds of HCV infection were about 5 times higher among women who smoked cigarettes during pregnancy. Concurrent infections were another serious risk factor, with hepatitis B virus infection boosting the odds of HCV infection by nearly 17 times.
HCV infection is a growing—but modifiable—threat among pregnant women, the researchers say. The rise in infection is “particularly concerning,” in light of recent research that has found poor follow-up of HCV-exposed infants. The researchers cite a Philadelphia study that found only 16% of HCV-exposed infants were appropriately followed. That could mean that infected infants are going undetected, the researchers say. The rate of transmission from mothers to infants is estimated at 6%; it’s important for exposed infants to be followed for evidence of seroconversion. But anti-HCV antibody tests can’t be completed until 18 months because passively acquired maternal antibodies can persist. Testing for HCV ribonucleic acid can be performed earlier.
The CDC and the American College of Obstetricians and Gynecologists recommend selective screening of pregnant women at high risk for HCV infection, particularly those with a history of injection drug use or long-term hemodialysis.
EC approves therapy for relapsed/refractory BCP-ALL
The European Commission (EC) has approved inotuzumab ozogamicin (BESPONSA®) as monotherapy for adults with relapsed or refractory, CD22-positive B-cell precursor acute lymphoblastic leukemia (BCP-ALL).
Adults with Philadelphia chromosome-positive, relapsed/refractory, CD22-positive BCP-ALL should have failed treatment with at least one tyrosine kinase inhibitor before receiving inotuzumab ozogamicin.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The EC’s approval of inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory BCP-ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
The European Commission (EC) has approved inotuzumab ozogamicin (BESPONSA®) as monotherapy for adults with relapsed or refractory, CD22-positive B-cell precursor acute lymphoblastic leukemia (BCP-ALL).
Adults with Philadelphia chromosome-positive, relapsed/refractory, CD22-positive BCP-ALL should have failed treatment with at least one tyrosine kinase inhibitor before receiving inotuzumab ozogamicin.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The EC’s approval of inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory BCP-ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
The European Commission (EC) has approved inotuzumab ozogamicin (BESPONSA®) as monotherapy for adults with relapsed or refractory, CD22-positive B-cell precursor acute lymphoblastic leukemia (BCP-ALL).
Adults with Philadelphia chromosome-positive, relapsed/refractory, CD22-positive BCP-ALL should have failed treatment with at least one tyrosine kinase inhibitor before receiving inotuzumab ozogamicin.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The EC’s approval of inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory BCP-ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
A sheep in wolf’s clothing?
A 25-year-old college student with no medical history sought care at our hospital for a nonproductive cough, subjective fevers, myalgia, and malaise that he’d developed 10 days earlier. The day before his visit, he’d also developed scratchy red eyes and a sore throat. He said he’d taken an over-the-counter cough suppressant to help with the cough, but his eyes and lips developed further redness and irritation.
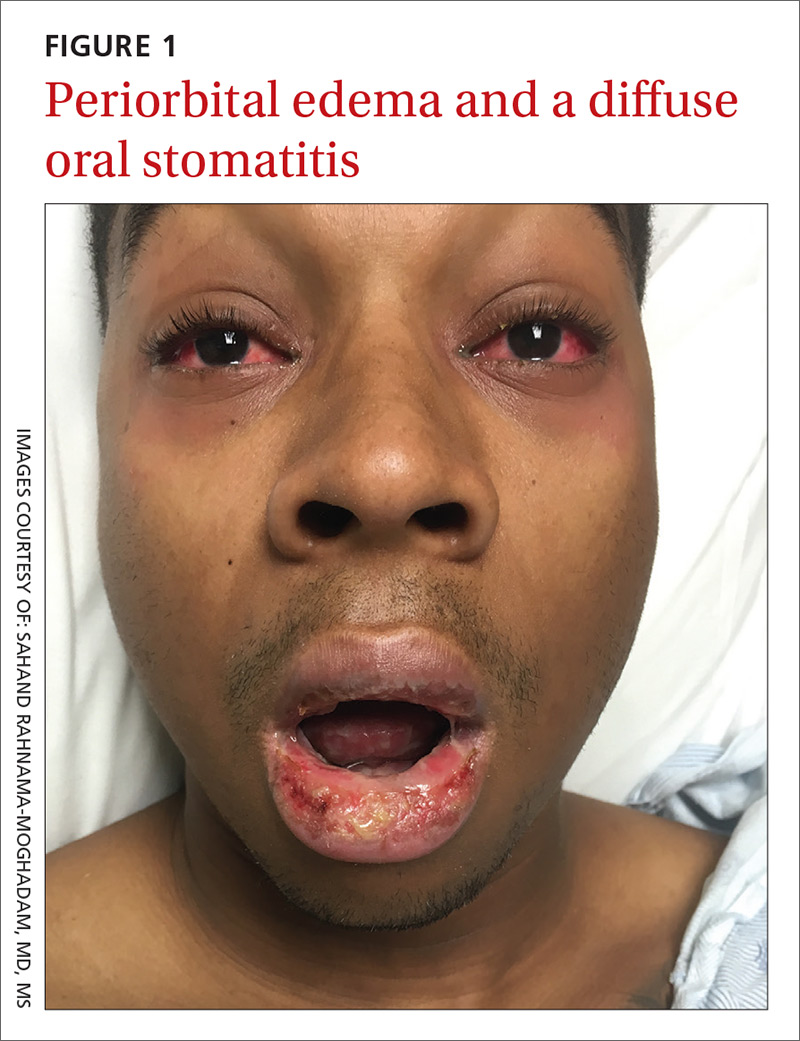
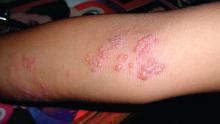
On examination, the patient demonstrated conjunctival suffusion, periorbital edema, diffuse oral stomatitis with pseudomembranous crusting, and nasal crusting (FIGURE 1). His vital signs were within normal limits, and he had no epithelial skin eruptions or erosions in any other mucosal regions.
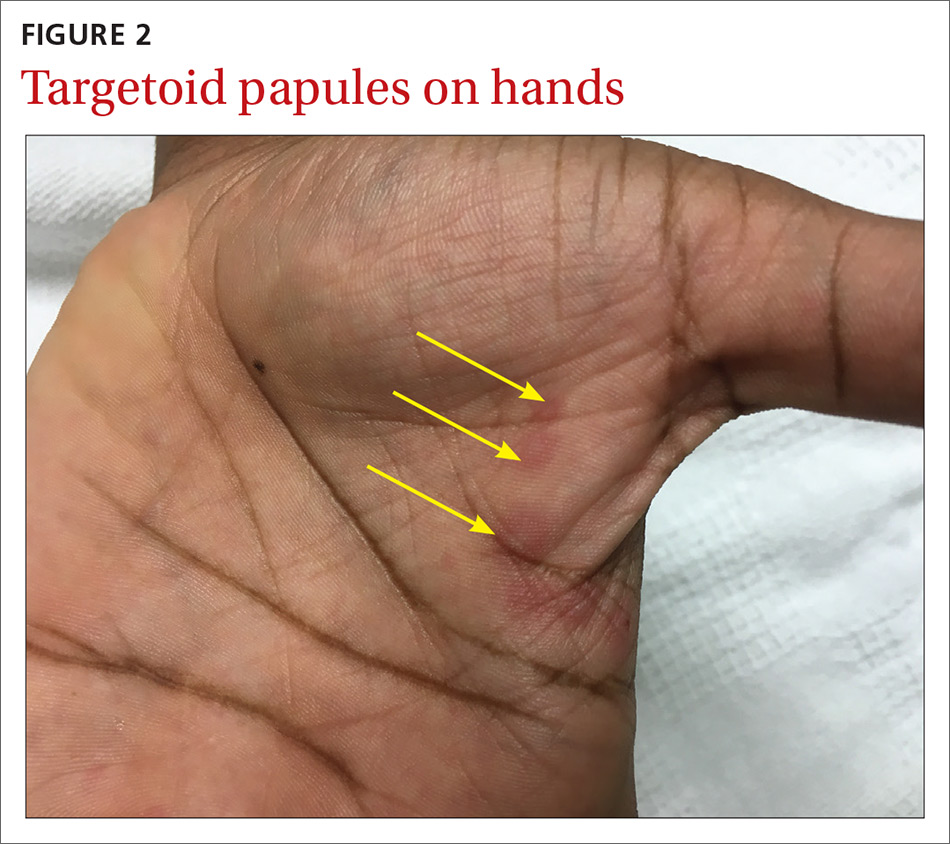
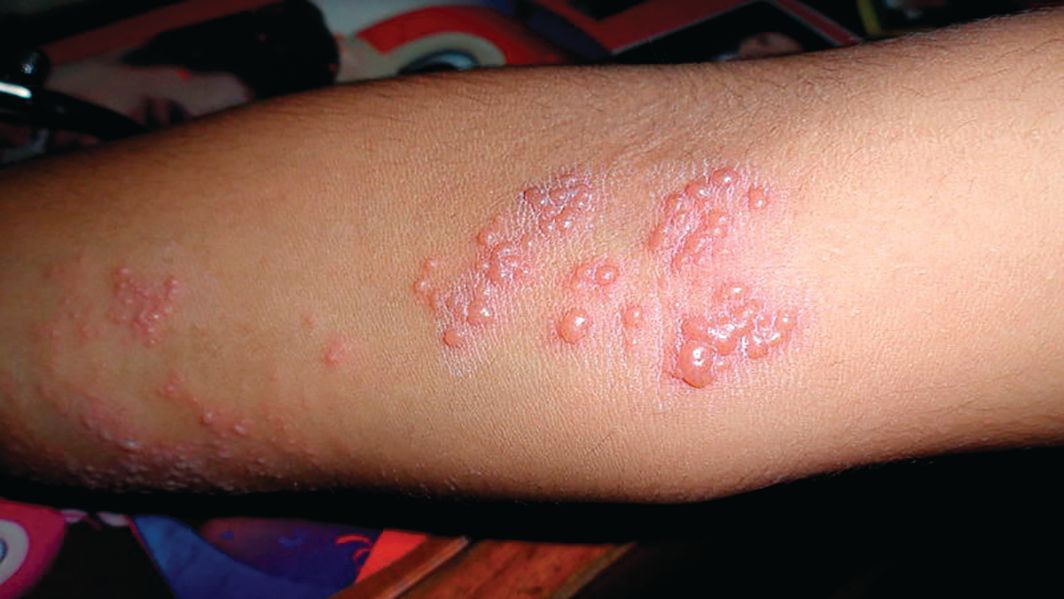
The patient was not currently sexually active and had one lifetime female sexual partner. He had no history of sexually transmitted infections or cold sores, and was not taking any medications, herbs, or supplements. During the initial 24 hours of admission, he developed 4 to 5 red targetoid papules on each hand (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: M pneumoniae-associated mucositis
The patient was admitted for observation to rule out Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). We felt that the degree of mucositis (extensive) compared to the number of targetoid papules on the hands (minimal) suggested a diagnosis of Mycoplasma pneumoniae-associated mucositis (MPAM), a subtype of erythema multiforme (EM) major. The patient’s prodrome of fever, cough, and malaise also supported a “walking pneumonia” diagnosis, such as MPAM.
Further testing. The patient had a normal chest x-ray and a negative respiratory virus polymerase chain reaction (PCR), but IgM serologies for Mycoplasma were elevated. Although the patient developed targetoid lesions on his hands during his first 24 hours in the hospital, he felt his constitutional symptoms had improved.
Exposure to Mycoplasma leads to an immune response
MPAM (also known as Fuchs’ syndrome and mycoplasma-associated mucositis with minimal skin manifestations) appears at some point during infection with M pneumoniae and causes severe ocular, oral, and sometimes genital symptoms with minimal skin manifestations.
MPAM is primarily seen in young males. In one systemic review of 202 cases, the average age of the patients was 11.9 years and 66% were male.1 Exposure to M pneumoniae is theorized to result in the production of autoantibodies to mycoplasma p1-adhesion molecules and to molecular mimicry of keratinocyte antigens located in the mucosa.1-3
Mycoplasma organisms have not been isolated from the cutaneous lesions of patients with MPAM; they have only been isolated from the respiratory tract, supporting the theory that MPAM is the body’s immune response to Mycoplasma, rather than a direct pathologic effect.4 This pathogenesis is distinct from that of SJS/TEN, which is thought to involve CD8+ T-cell-mediated keratinocyte apoptosis (programed cell death). In addition, SJS/TEN is almost always drug induced.
First up in the differential: Rule out SJS/TEN
When evaluating a patient like ours with a blistering eruption, the most important diagnosis to exclude is SJS/TEN. This condition is usually triggered by a medication, which was absent in this case. SJS/TEN begins with a host of constitutional symptoms and an erythematous blistering eruption, which may be preceded by atypical targetoid (2-zoned) flat papules along with erosions on 2 or more mucosal surfaces.
Patients with SJS/TEN are usually critically ill and may have a guarded prognosis. Patients with MPAM have a more favorable prognosis and are unlikely to be critically ill—as was the case with our patient.
EM major is often associated with Mycoplasma infections. Patients with EM major may have fever and arthralgias, as well as extensive mucous membrane involvement including that of the lips/mouth, eyes, and genitals.
Experts agree that EM is separate from the SJS/TEN continuum, and that patients with EM major, including those with MPAM, are not at risk of developing SJS/TEN.5 EM is characterized by the presence of the more characteristic ‘target’ or ‘iris’ 3-zoned lesion—a central dusky purpura, surrounded by an elevated edematous pale ring, rimmed by a red macular outer ring. EM major is defined as EM along with involvement of one or more mucosal regions.
In this case, the patient had acral target lesions and oral and ocular mucosal involvement characteristic of EM major, without widespread skin erosions or sloughing commonly seen with SJS/TEN.
Kawasaki’s disease occurs in young children and presents with conjunctivitis and oral changes. However, patients with Kawasaki’s disease generally have a fever for >5 days, a strawberry tongue (not a part of the morphology of EM major or MPAM), and palmoplantar erythema and desquamation that are not common with EM major or MPAM.1
Pemphigus vulgaris is uncommon in children and young adults. The disease does not present with diffuse mucositis nor diffuse blistering of the skin, but rather with discrete shallow erosions on the mucosa and the trunk along with flaccid bullae and erosions on the skin.
The morphologies of a fixed drug eruption (round purpuric patch) and toxic shock syndrome (diffuse macular erythema and widespread skin sloughing) are inconsistent with this patient’s diffuse mucositis, conjunctivitis, and targetoid lesions.
Confirm exposure to M pneumoniae
Testing with the purpose of ruling in MPAM is directed toward proving that the patient has been exposed to M pneumoniae. (Of note: M pneumoniae cannot be detected via routine commercial blood cultures.)
Serologic testing for elevated IgM antibodies to Mycoplasma is the most specific method. Various studies have found it to be positive in 100% of cases, but detection may be delayed for a couple of weeks while the body develops the requisite antibodies.4
Respiratory PCR for Mycoplasma is rapid and usually appropriately positive, but may be negative in cases where the patient has spontaneously cleared the infection or has been exposed to antibiotics before development of the eruption.4 An infiltrate on chest imaging is supportive of the diagnosis.
Skin biopsy will demonstrate either mucositis and necrosis of keratinocytes or EM-like necrosis, but does not suggest an etiology.
Strikingly different paths of care
Distinguishing between SJS/TEN and EM major (including MPAM) is crucial to guiding management. Patients with SJS/TEN need critical care, particularly of their eyes and genitourinary and respiratory systems. Specialist consultation is often required.
For EM major, patients require supportive care along with ongoing assurances that the eruption has a benign prognosis. Hospital admission is not mandatory as long as adequate supportive care and symptom control can be provided on an outpatient basis. Early consultation with Ophthalmology, Oral Medicine, and Urology may also be key.
Keep in mind that patients may have severe stomatitis and pain that alter their ability to eat and perform normal activities. Thus, managing pain and ensuring adequate nutrition are crucial for successful support. While antibiotics treat active Mycoplasma infection, there is no clear evidence that antibiotics alter the course of the eruption, which is also consistent with the hypothesized pathogenesis.3,4
While there is no clear statistical evidence that systemic immune suppression alters the disease course, a large proportion (31%) of patients in a recent systematic review of MPAM were treated with corticosteroids, and a smaller, but noteworthy, percentage (9%) were treated with intravenous immunoglobulins (IVIG).4 There are reports of severe stomatitis that didn’t improve with supportive care, but that showed dramatic improvement with IVIG treatment.6,7
Our patient had difficulty controlling secretions and managing the painful mucositis of his mouth; he was initially unable to tolerate solid foods. Topical lidocaine solution for his mucositis caused burning and more discomfort, but acetaminophen-hydrocodone 300 mg-5 mg every 6 hours did relieve his pain. Wound care with a bland emollient and the application of non-stick dressings to his lips at night also helped to relieve some of the pain.
Because the patient’s oropharyngeal swelling made it hard for him to swallow, he received oral prednisone 0.5 mg/kg/d, which provided him with relief within 24 hours. The acute inflammation and eruption also subsided within 48 hours and the patient was discharged after 5 days of being hospitalized. He continued to recover as an outpatient, seeing his primary care physician within 2 weeks for final nutrition and wound care support. Two weeks after that, he had a dermatology appointment, and all of his lesions had re-epithelialized.
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, MS, University of Texas Health Science Center at San Antonio, 7323 Snowden Road, Apt. 1205, San Antonio, TX 78240; sahandazeez@gmail.com.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245.
2. Bressan S, Mion T, Andreola B, et al. Severe Mycoplasma pneumoniae-associated mucositis treated with immunoglobulins. Acta Paediatr. 2011;100:e238-e240.
3. Dinulos JG. What’s new with common, uncommon and rare rashes in childhood. Curr Opin Pediatr. 2015;27:261-266.
4. Meyer Sauteur PM, Goetschel P, Lautenschlager S. Mycoplasma pneumoniae and mucositis–part of the Stevens-Johnson syndrome spectrum. J Dtsh Dermatol Ges. 2012;10:740-746.
5. Figueira-Coelho J, Lourenço S, Pires AC, et al. Mycoplasma pneumoniae-associated mucositis with minimal skin manifestations. Am J Clin Dermatol. 2008;9:399-403.
6. Bressan S, Mion T, Andreola B, et al. Severe Mycoplasma pneumoniae-associated mucositis treated with immunoglobulins. Acta Paediatr. 2011;100:e238-e240.
7. Zipitis CS, Thalange N. Intravenous immunoglobulins for the management of Stevens-Johnson syndrome with minimal skin manifestations. Eur J Pediatr.2007;166:585-588.
A 25-year-old college student with no medical history sought care at our hospital for a nonproductive cough, subjective fevers, myalgia, and malaise that he’d developed 10 days earlier. The day before his visit, he’d also developed scratchy red eyes and a sore throat. He said he’d taken an over-the-counter cough suppressant to help with the cough, but his eyes and lips developed further redness and irritation.
On examination, the patient demonstrated conjunctival suffusion, periorbital edema, diffuse oral stomatitis with pseudomembranous crusting, and nasal crusting (FIGURE 1). His vital signs were within normal limits, and he had no epithelial skin eruptions or erosions in any other mucosal regions.
The patient was not currently sexually active and had one lifetime female sexual partner. He had no history of sexually transmitted infections or cold sores, and was not taking any medications, herbs, or supplements. During the initial 24 hours of admission, he developed 4 to 5 red targetoid papules on each hand (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: M pneumoniae-associated mucositis
The patient was admitted for observation to rule out Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). We felt that the degree of mucositis (extensive) compared to the number of targetoid papules on the hands (minimal) suggested a diagnosis of Mycoplasma pneumoniae-associated mucositis (MPAM), a subtype of erythema multiforme (EM) major. The patient’s prodrome of fever, cough, and malaise also supported a “walking pneumonia” diagnosis, such as MPAM.
Further testing. The patient had a normal chest x-ray and a negative respiratory virus polymerase chain reaction (PCR), but IgM serologies for Mycoplasma were elevated. Although the patient developed targetoid lesions on his hands during his first 24 hours in the hospital, he felt his constitutional symptoms had improved.
Exposure to Mycoplasma leads to an immune response
MPAM (also known as Fuchs’ syndrome and mycoplasma-associated mucositis with minimal skin manifestations) appears at some point during infection with M pneumoniae and causes severe ocular, oral, and sometimes genital symptoms with minimal skin manifestations.
MPAM is primarily seen in young males. In one systemic review of 202 cases, the average age of the patients was 11.9 years and 66% were male.1 Exposure to M pneumoniae is theorized to result in the production of autoantibodies to mycoplasma p1-adhesion molecules and to molecular mimicry of keratinocyte antigens located in the mucosa.1-3
Mycoplasma organisms have not been isolated from the cutaneous lesions of patients with MPAM; they have only been isolated from the respiratory tract, supporting the theory that MPAM is the body’s immune response to Mycoplasma, rather than a direct pathologic effect.4 This pathogenesis is distinct from that of SJS/TEN, which is thought to involve CD8+ T-cell-mediated keratinocyte apoptosis (programed cell death). In addition, SJS/TEN is almost always drug induced.
First up in the differential: Rule out SJS/TEN
When evaluating a patient like ours with a blistering eruption, the most important diagnosis to exclude is SJS/TEN. This condition is usually triggered by a medication, which was absent in this case. SJS/TEN begins with a host of constitutional symptoms and an erythematous blistering eruption, which may be preceded by atypical targetoid (2-zoned) flat papules along with erosions on 2 or more mucosal surfaces.
Patients with SJS/TEN are usually critically ill and may have a guarded prognosis. Patients with MPAM have a more favorable prognosis and are unlikely to be critically ill—as was the case with our patient.
EM major is often associated with Mycoplasma infections. Patients with EM major may have fever and arthralgias, as well as extensive mucous membrane involvement including that of the lips/mouth, eyes, and genitals.
Experts agree that EM is separate from the SJS/TEN continuum, and that patients with EM major, including those with MPAM, are not at risk of developing SJS/TEN.5 EM is characterized by the presence of the more characteristic ‘target’ or ‘iris’ 3-zoned lesion—a central dusky purpura, surrounded by an elevated edematous pale ring, rimmed by a red macular outer ring. EM major is defined as EM along with involvement of one or more mucosal regions.
In this case, the patient had acral target lesions and oral and ocular mucosal involvement characteristic of EM major, without widespread skin erosions or sloughing commonly seen with SJS/TEN.
Kawasaki’s disease occurs in young children and presents with conjunctivitis and oral changes. However, patients with Kawasaki’s disease generally have a fever for >5 days, a strawberry tongue (not a part of the morphology of EM major or MPAM), and palmoplantar erythema and desquamation that are not common with EM major or MPAM.1
Pemphigus vulgaris is uncommon in children and young adults. The disease does not present with diffuse mucositis nor diffuse blistering of the skin, but rather with discrete shallow erosions on the mucosa and the trunk along with flaccid bullae and erosions on the skin.
The morphologies of a fixed drug eruption (round purpuric patch) and toxic shock syndrome (diffuse macular erythema and widespread skin sloughing) are inconsistent with this patient’s diffuse mucositis, conjunctivitis, and targetoid lesions.
Confirm exposure to M pneumoniae
Testing with the purpose of ruling in MPAM is directed toward proving that the patient has been exposed to M pneumoniae. (Of note: M pneumoniae cannot be detected via routine commercial blood cultures.)
Serologic testing for elevated IgM antibodies to Mycoplasma is the most specific method. Various studies have found it to be positive in 100% of cases, but detection may be delayed for a couple of weeks while the body develops the requisite antibodies.4
Respiratory PCR for Mycoplasma is rapid and usually appropriately positive, but may be negative in cases where the patient has spontaneously cleared the infection or has been exposed to antibiotics before development of the eruption.4 An infiltrate on chest imaging is supportive of the diagnosis.
Skin biopsy will demonstrate either mucositis and necrosis of keratinocytes or EM-like necrosis, but does not suggest an etiology.
Strikingly different paths of care
Distinguishing between SJS/TEN and EM major (including MPAM) is crucial to guiding management. Patients with SJS/TEN need critical care, particularly of their eyes and genitourinary and respiratory systems. Specialist consultation is often required.
For EM major, patients require supportive care along with ongoing assurances that the eruption has a benign prognosis. Hospital admission is not mandatory as long as adequate supportive care and symptom control can be provided on an outpatient basis. Early consultation with Ophthalmology, Oral Medicine, and Urology may also be key.
Keep in mind that patients may have severe stomatitis and pain that alter their ability to eat and perform normal activities. Thus, managing pain and ensuring adequate nutrition are crucial for successful support. While antibiotics treat active Mycoplasma infection, there is no clear evidence that antibiotics alter the course of the eruption, which is also consistent with the hypothesized pathogenesis.3,4
While there is no clear statistical evidence that systemic immune suppression alters the disease course, a large proportion (31%) of patients in a recent systematic review of MPAM were treated with corticosteroids, and a smaller, but noteworthy, percentage (9%) were treated with intravenous immunoglobulins (IVIG).4 There are reports of severe stomatitis that didn’t improve with supportive care, but that showed dramatic improvement with IVIG treatment.6,7
Our patient had difficulty controlling secretions and managing the painful mucositis of his mouth; he was initially unable to tolerate solid foods. Topical lidocaine solution for his mucositis caused burning and more discomfort, but acetaminophen-hydrocodone 300 mg-5 mg every 6 hours did relieve his pain. Wound care with a bland emollient and the application of non-stick dressings to his lips at night also helped to relieve some of the pain.
Because the patient’s oropharyngeal swelling made it hard for him to swallow, he received oral prednisone 0.5 mg/kg/d, which provided him with relief within 24 hours. The acute inflammation and eruption also subsided within 48 hours and the patient was discharged after 5 days of being hospitalized. He continued to recover as an outpatient, seeing his primary care physician within 2 weeks for final nutrition and wound care support. Two weeks after that, he had a dermatology appointment, and all of his lesions had re-epithelialized.
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, MS, University of Texas Health Science Center at San Antonio, 7323 Snowden Road, Apt. 1205, San Antonio, TX 78240; sahandazeez@gmail.com.
A 25-year-old college student with no medical history sought care at our hospital for a nonproductive cough, subjective fevers, myalgia, and malaise that he’d developed 10 days earlier. The day before his visit, he’d also developed scratchy red eyes and a sore throat. He said he’d taken an over-the-counter cough suppressant to help with the cough, but his eyes and lips developed further redness and irritation.
On examination, the patient demonstrated conjunctival suffusion, periorbital edema, diffuse oral stomatitis with pseudomembranous crusting, and nasal crusting (FIGURE 1). His vital signs were within normal limits, and he had no epithelial skin eruptions or erosions in any other mucosal regions.
The patient was not currently sexually active and had one lifetime female sexual partner. He had no history of sexually transmitted infections or cold sores, and was not taking any medications, herbs, or supplements. During the initial 24 hours of admission, he developed 4 to 5 red targetoid papules on each hand (FIGURE 2).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: M pneumoniae-associated mucositis
The patient was admitted for observation to rule out Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN). We felt that the degree of mucositis (extensive) compared to the number of targetoid papules on the hands (minimal) suggested a diagnosis of Mycoplasma pneumoniae-associated mucositis (MPAM), a subtype of erythema multiforme (EM) major. The patient’s prodrome of fever, cough, and malaise also supported a “walking pneumonia” diagnosis, such as MPAM.
Further testing. The patient had a normal chest x-ray and a negative respiratory virus polymerase chain reaction (PCR), but IgM serologies for Mycoplasma were elevated. Although the patient developed targetoid lesions on his hands during his first 24 hours in the hospital, he felt his constitutional symptoms had improved.
Exposure to Mycoplasma leads to an immune response
MPAM (also known as Fuchs’ syndrome and mycoplasma-associated mucositis with minimal skin manifestations) appears at some point during infection with M pneumoniae and causes severe ocular, oral, and sometimes genital symptoms with minimal skin manifestations.
MPAM is primarily seen in young males. In one systemic review of 202 cases, the average age of the patients was 11.9 years and 66% were male.1 Exposure to M pneumoniae is theorized to result in the production of autoantibodies to mycoplasma p1-adhesion molecules and to molecular mimicry of keratinocyte antigens located in the mucosa.1-3
Mycoplasma organisms have not been isolated from the cutaneous lesions of patients with MPAM; they have only been isolated from the respiratory tract, supporting the theory that MPAM is the body’s immune response to Mycoplasma, rather than a direct pathologic effect.4 This pathogenesis is distinct from that of SJS/TEN, which is thought to involve CD8+ T-cell-mediated keratinocyte apoptosis (programed cell death). In addition, SJS/TEN is almost always drug induced.
First up in the differential: Rule out SJS/TEN
When evaluating a patient like ours with a blistering eruption, the most important diagnosis to exclude is SJS/TEN. This condition is usually triggered by a medication, which was absent in this case. SJS/TEN begins with a host of constitutional symptoms and an erythematous blistering eruption, which may be preceded by atypical targetoid (2-zoned) flat papules along with erosions on 2 or more mucosal surfaces.
Patients with SJS/TEN are usually critically ill and may have a guarded prognosis. Patients with MPAM have a more favorable prognosis and are unlikely to be critically ill—as was the case with our patient.
EM major is often associated with Mycoplasma infections. Patients with EM major may have fever and arthralgias, as well as extensive mucous membrane involvement including that of the lips/mouth, eyes, and genitals.
Experts agree that EM is separate from the SJS/TEN continuum, and that patients with EM major, including those with MPAM, are not at risk of developing SJS/TEN.5 EM is characterized by the presence of the more characteristic ‘target’ or ‘iris’ 3-zoned lesion—a central dusky purpura, surrounded by an elevated edematous pale ring, rimmed by a red macular outer ring. EM major is defined as EM along with involvement of one or more mucosal regions.
In this case, the patient had acral target lesions and oral and ocular mucosal involvement characteristic of EM major, without widespread skin erosions or sloughing commonly seen with SJS/TEN.
Kawasaki’s disease occurs in young children and presents with conjunctivitis and oral changes. However, patients with Kawasaki’s disease generally have a fever for >5 days, a strawberry tongue (not a part of the morphology of EM major or MPAM), and palmoplantar erythema and desquamation that are not common with EM major or MPAM.1
Pemphigus vulgaris is uncommon in children and young adults. The disease does not present with diffuse mucositis nor diffuse blistering of the skin, but rather with discrete shallow erosions on the mucosa and the trunk along with flaccid bullae and erosions on the skin.
The morphologies of a fixed drug eruption (round purpuric patch) and toxic shock syndrome (diffuse macular erythema and widespread skin sloughing) are inconsistent with this patient’s diffuse mucositis, conjunctivitis, and targetoid lesions.
Confirm exposure to M pneumoniae
Testing with the purpose of ruling in MPAM is directed toward proving that the patient has been exposed to M pneumoniae. (Of note: M pneumoniae cannot be detected via routine commercial blood cultures.)
Serologic testing for elevated IgM antibodies to Mycoplasma is the most specific method. Various studies have found it to be positive in 100% of cases, but detection may be delayed for a couple of weeks while the body develops the requisite antibodies.4
Respiratory PCR for Mycoplasma is rapid and usually appropriately positive, but may be negative in cases where the patient has spontaneously cleared the infection or has been exposed to antibiotics before development of the eruption.4 An infiltrate on chest imaging is supportive of the diagnosis.
Skin biopsy will demonstrate either mucositis and necrosis of keratinocytes or EM-like necrosis, but does not suggest an etiology.
Strikingly different paths of care
Distinguishing between SJS/TEN and EM major (including MPAM) is crucial to guiding management. Patients with SJS/TEN need critical care, particularly of their eyes and genitourinary and respiratory systems. Specialist consultation is often required.
For EM major, patients require supportive care along with ongoing assurances that the eruption has a benign prognosis. Hospital admission is not mandatory as long as adequate supportive care and symptom control can be provided on an outpatient basis. Early consultation with Ophthalmology, Oral Medicine, and Urology may also be key.
Keep in mind that patients may have severe stomatitis and pain that alter their ability to eat and perform normal activities. Thus, managing pain and ensuring adequate nutrition are crucial for successful support. While antibiotics treat active Mycoplasma infection, there is no clear evidence that antibiotics alter the course of the eruption, which is also consistent with the hypothesized pathogenesis.3,4
While there is no clear statistical evidence that systemic immune suppression alters the disease course, a large proportion (31%) of patients in a recent systematic review of MPAM were treated with corticosteroids, and a smaller, but noteworthy, percentage (9%) were treated with intravenous immunoglobulins (IVIG).4 There are reports of severe stomatitis that didn’t improve with supportive care, but that showed dramatic improvement with IVIG treatment.6,7
Our patient had difficulty controlling secretions and managing the painful mucositis of his mouth; he was initially unable to tolerate solid foods. Topical lidocaine solution for his mucositis caused burning and more discomfort, but acetaminophen-hydrocodone 300 mg-5 mg every 6 hours did relieve his pain. Wound care with a bland emollient and the application of non-stick dressings to his lips at night also helped to relieve some of the pain.
Because the patient’s oropharyngeal swelling made it hard for him to swallow, he received oral prednisone 0.5 mg/kg/d, which provided him with relief within 24 hours. The acute inflammation and eruption also subsided within 48 hours and the patient was discharged after 5 days of being hospitalized. He continued to recover as an outpatient, seeing his primary care physician within 2 weeks for final nutrition and wound care support. Two weeks after that, he had a dermatology appointment, and all of his lesions had re-epithelialized.
CORRESPONDENCE
Sahand Rahnama-Moghadam, MD, MS, University of Texas Health Science Center at San Antonio, 7323 Snowden Road, Apt. 1205, San Antonio, TX 78240; sahandazeez@gmail.com.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245.
2. Bressan S, Mion T, Andreola B, et al. Severe Mycoplasma pneumoniae-associated mucositis treated with immunoglobulins. Acta Paediatr. 2011;100:e238-e240.
3. Dinulos JG. What’s new with common, uncommon and rare rashes in childhood. Curr Opin Pediatr. 2015;27:261-266.
4. Meyer Sauteur PM, Goetschel P, Lautenschlager S. Mycoplasma pneumoniae and mucositis–part of the Stevens-Johnson syndrome spectrum. J Dtsh Dermatol Ges. 2012;10:740-746.
5. Figueira-Coelho J, Lourenço S, Pires AC, et al. Mycoplasma pneumoniae-associated mucositis with minimal skin manifestations. Am J Clin Dermatol. 2008;9:399-403.
6. Bressan S, Mion T, Andreola B, et al. Severe Mycoplasma pneumoniae-associated mucositis treated with immunoglobulins. Acta Paediatr. 2011;100:e238-e240.
7. Zipitis CS, Thalange N. Intravenous immunoglobulins for the management of Stevens-Johnson syndrome with minimal skin manifestations. Eur J Pediatr.2007;166:585-588.
1. Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae-induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245.
2. Bressan S, Mion T, Andreola B, et al. Severe Mycoplasma pneumoniae-associated mucositis treated with immunoglobulins. Acta Paediatr. 2011;100:e238-e240.
3. Dinulos JG. What’s new with common, uncommon and rare rashes in childhood. Curr Opin Pediatr. 2015;27:261-266.
4. Meyer Sauteur PM, Goetschel P, Lautenschlager S. Mycoplasma pneumoniae and mucositis–part of the Stevens-Johnson syndrome spectrum. J Dtsh Dermatol Ges. 2012;10:740-746.
5. Figueira-Coelho J, Lourenço S, Pires AC, et al. Mycoplasma pneumoniae-associated mucositis with minimal skin manifestations. Am J Clin Dermatol. 2008;9:399-403.
6. Bressan S, Mion T, Andreola B, et al. Severe Mycoplasma pneumoniae-associated mucositis treated with immunoglobulins. Acta Paediatr. 2011;100:e238-e240.
7. Zipitis CS, Thalange N. Intravenous immunoglobulins for the management of Stevens-Johnson syndrome with minimal skin manifestations. Eur J Pediatr.2007;166:585-588.
Recalcitrant genital papules
A 21-year-old man presented to the dermatology clinic with a 2-month history of painless genital and perianal lesions. The patient reported having unprotected sex in recent months, but had no prior history of oral, penile, or anal mucosal lesions or ulcers. He was not on any medications or immunosuppressive agents and noted that the lesions did not represent a recurrence. He also reported a nonspecific, asymptomatic rash on his trunk and extremities that had been present for an unknown period of time.
The patient indicated that his primary care physician had looked at the genital/perianal lesions and told him they were genital warts. Previous treatments included an over-the-counter wart medication, cryotherapy, and a course of imiquimod, but none had helped.
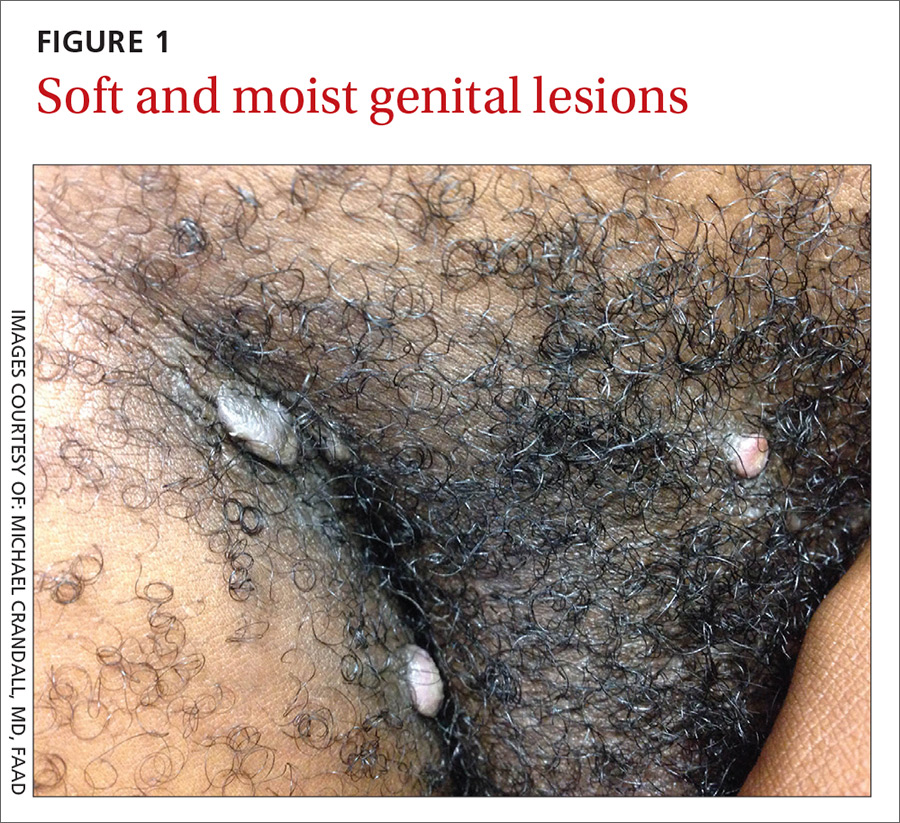
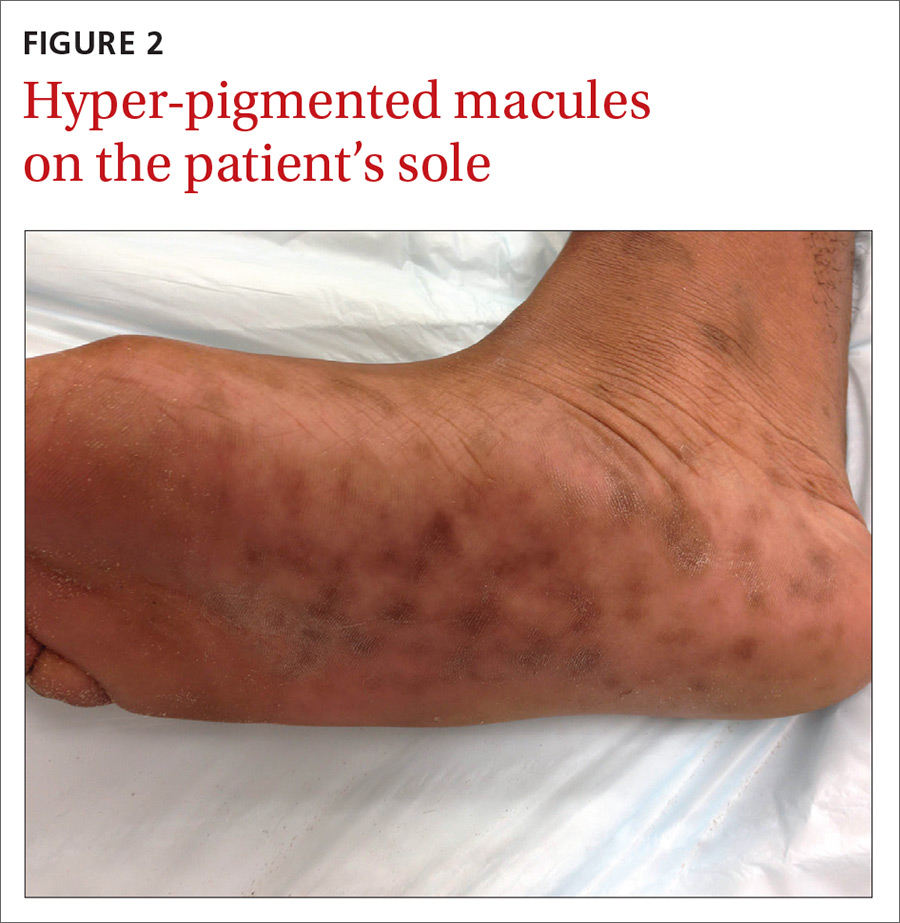
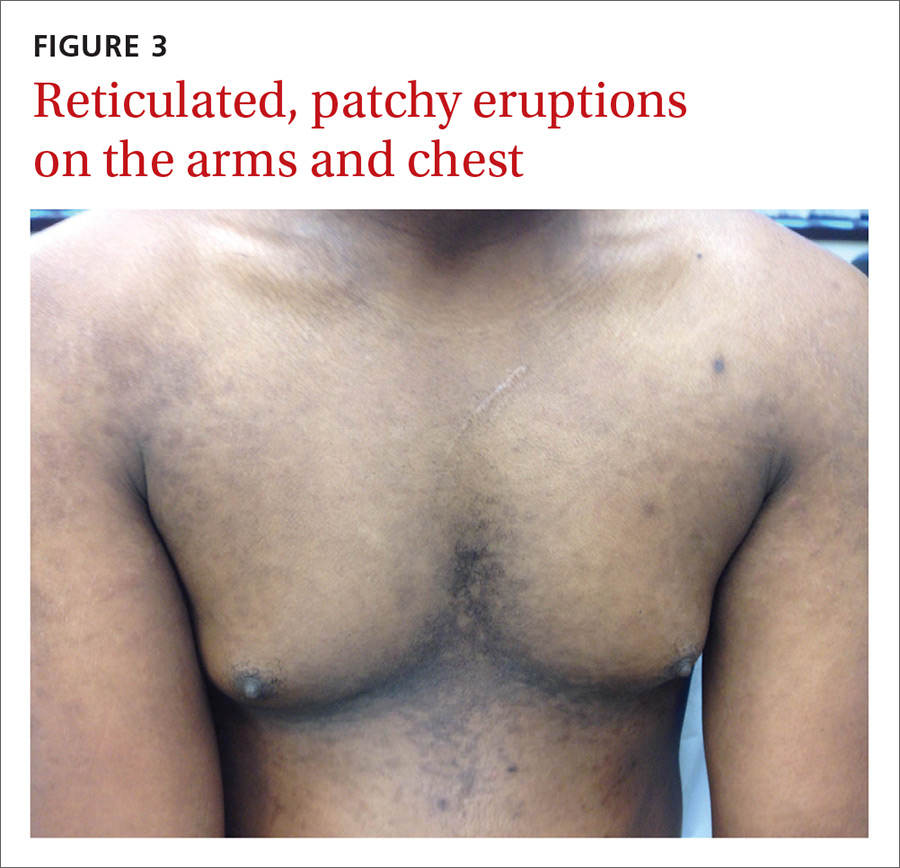
The physical examination revealed multiple soft, moist, beefy papules and plaques around the genital area (FIGURE 1) and perianal region. In addition, there were multiple hyper-pigmented macules on the patient’s palms and soles (FIGURE 2), and reticulated, patchy eruptions on his arms, chest (FIGURE 3), and back.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Secondary syphilis
The appearance of the genital and perianal lesions was consistent with condylomata lata—a cutaneous sign of secondary syphilis—rather than genital warts. The presence of a rash on the patient’s trunk and extremities further supported this diagnosis. We did a rapid plasma reagin (RPR) test and a Treponema pallidum particle agglutination test; we also tested for human immunodeficiency virus (HIV). The patient’s RPR titer was 1:128, and the T pallidum antibody test came back positive. HIV-1 and HIV-2 serology were negative.
Appearance of the lesions was a giveaway. Condylomata lata are flat-topped, broad papules that are usually located on folds of moist skin (particularly the genitals and anus), and have a smooth, gray, moist surface. Although they can be lobulated, they do not have the classic digitate projections that are characteristic of genital warts. A nonpruritic, symmetric, “raw ham”-colored papular eruption on a patient’s trunk, palms, and soles is also characteristic of secondary syphilis.1 In this case, the reticular pattern on the patient’s chest represented the commonly seen lenticular rash of secondary syphilis.
Cutaneous lesions of secondary syphilis contain numerous spirochetes (T pallidum) and are highly infectious. Systemic symptoms of secondary syphilis may include fatigue, generalized lymphadenopathy, arthralgia, myalgia, pharyngitis, and headache.
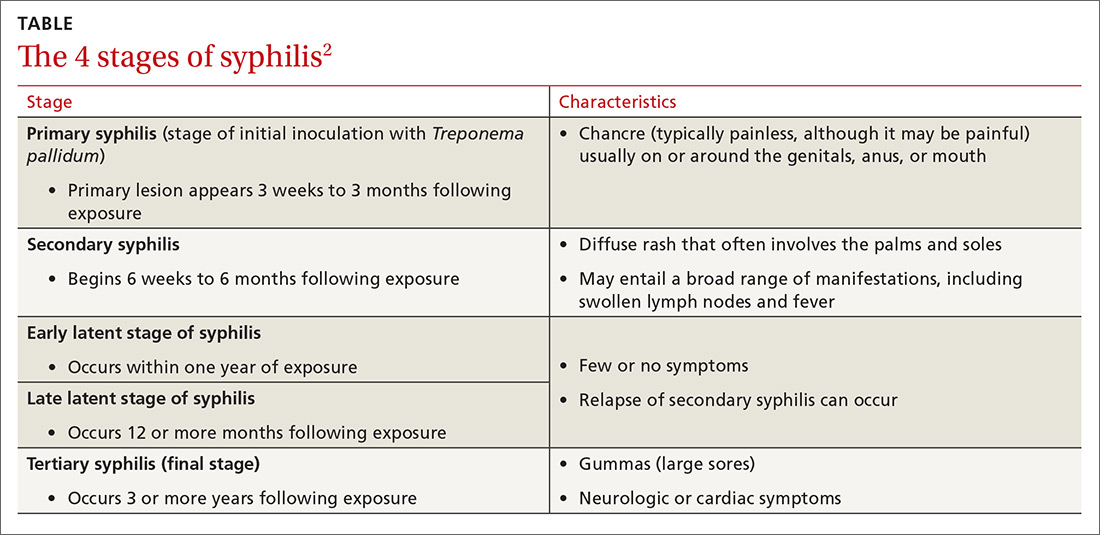
Some patients may report having a recent chancre—a painless, self-limiting ulcer in the genital area—which is characteristic of primary syphilis (see “Single nontender ulcer on the glans,” J Fam Pract. 2017;66:253-255). For more on the stages of syphilis, see the TABLE2. Our patient did not remember ever having a chancre.
Increase in cases. Rates of primary and secondary syphilis have increased in the past decade. In 2014, approximately 20,000 syphilis cases were reported—a record high since 1994.3 Men who have sex with men are particularly affected; however, increases in infection rates have also been noted in women and across people of all ages and ethnicities.3
Rule out other causes of genital lesions
Condyloma acuminata, commonly called genital warts, are localized human papilloma virus (HPV) infections that appear as discrete, gray to pale pink, lobulated papules that may coalesce to form a large, cauliflower-like mass. They are sexually transmitted and commonly involve the genital and anal areas. While physicians may confuse condylomata lata with genital warts, diffuse skin rashes and constitutional symptoms are not usually seen with genital warts.4
Fordyce spots are small, whitish, raised papules on the glans or the shaft of the penis or the vulva of the vagina. They may also appear on the lips and oral mucosa. They are a result of prominent sebaceous glands and are harmless. They are not infectious or sexually transmitted.5,6
Lymphogranuloma venereum is an uncommon sexually transmitted disease caused by Chlamydia trachomatis. It is characterized by genital papules or ulcers, followed by bilateral, suppurative, inguinal adenitis known as buboes. The buboes may breakdown, form multiple fistulous openings, and discharge purulent material.6
Acute HIV may present with flu-like symptoms and well-circumscribed maculopapular rashes on the face, neck, and upper trunk. The palms and soles may also be affected. Patients with HIV may also develop genital plaque-like lesions from herpes simplex virus-2, genital warts from HPV, molluscum contagiosum, and, not uncommonly, anogenital malignancies.7,8
Confluent and reticulated papillomatosis (CARP) is a disorder that occurs predominantly in young adults and teenagers, with cosmetically displeasing brown scaling macules that may coalesce to form patches or plaques affecting the neck, chest, back, and axillae. It is often mistaken for tinea versicolor.9 In this case, the eruption on the chest closely resembled CARP, but a diagnosis of CARP would not have explained the genital lesions.
Confirm diagnosis with treponemal tests
Syphilis is often a clinical diagnosis with pathologic confirmation. Patients suspected of having syphilis should be screened with nontreponemal tests, such as the Venereal Disease Research Laboratory (VDRL) test or the RPR test, which become positive within 3 weeks of developing primary syphilis.
Diagnosis is confirmed with specific treponemal testing, such as with a fluorescent treponemal antibody absorption assay or the T pallidum particle agglutination test. HIV testing is recommended for all patients with syphilis.
Penicillin G is the mainstay of treatment
Proper selection of penicillin is paramount in the treatment of syphilis. Primary, secondary, and early latent syphilis are treated with an intramuscular injection of 2.4 million units of long-acting benzathine penicillin G. Patients with late latent or latent syphilis of unknown duration are treated with 3 doses of the same injection at weekly intervals, totaling 7.2 million units of benzathine penicillin G.10 Certain penicillin preparations (eg, combinations of benzathine penicillin and procaine penicillin) are not appropriate treatments because they do not provide adequate amounts of the antibiotic.
Watch for this reaction. Approximately 30% of patients following penicillin treatment for spirochete infection develop a Jarisch-Herxheimer reaction (JHR).11 JHR is characterized by an abrupt onset of fever, chills, myalgia, tachycardia, vasodilatation with flushing, exacerbated maculopapular skin rash, or mild hypotension. Care for JHR is generally supportive.
Our patient received an intramuscular injection of 2.4 million units of long-acting benzathine penicillin G. His skin eruption and condylomata lata lesions were completely resolved at follow-up 6 months later.
As recommended by the Centers for Disease Control and Prevention,10 our patient’s RPR titers were repeated at 6 months and again at 12 months to verify a four-fold decline, indicating successful treatment.
CORRESPONDENCE
Anne Bartels, MD, General Medicine, Naval Hospital Camp Lejeune, 100 Brewster Blvd., Camp Lejeune, NC 28547; Anne.k.bartels.mil@mail.mil.
1. James WD, Berger TG, Elston DM. Secondary syphilis. In: James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, Pa: Elsevier; 2011:348-350.
2. Centers for Disease Control and Prevention. Syphilis—CDC Fact Sheet. Available at: https://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Accessed May 31, 2017.
3. Centers for Disease Control and Prevention. Syphilis. November 17, 2015. Available at: http://www.cdc.gov/std/stats14/syphilis.htm. Accessed March 30, 2017.
4. Karnes JB, Usatine RP. Management of external genital warts. Am Fam Physician. 2014;90:312-318.
5. DuVivier A. Disorders of the sebaceous, sweat and apocrine glands. In: DuVivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, Pa: Elsevier; 2013:326-330.
6. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002;78:90-92.
7. Altman K, Vanness E, Westergaard RP. Cutaneous manifestations of human immunodeficiency virus: a clinical update. Curr Infect Dis Rep. 2015;17:464.
8. Maurer TA. Dermatologic manifestations of HIV infection. Top HIV Med. 2005;13:149-154.
9. Hudacek KD, Haque MS, Hochberg AL, et al. An unusual variant of confluent and reticulated papillomatosis masquerading as tinea versicolor. Arch Dermatol. 2012;148:505-508.
10. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. Available at: https://www.cdc.gov/std/treatment/2010/STD-Treatment-2010-RR5912.pdf#. Accessed June 8, 2017.
11. Yang CJ, Lee NY, Lin YH, et al. Jarisch-Herxheimer reaction after penicillin therapy among patients with syphilis in the era of the hiv infection epidemic: incidence and risk factors. Clin Infect Dis. 2010;51:976-979.
A 21-year-old man presented to the dermatology clinic with a 2-month history of painless genital and perianal lesions. The patient reported having unprotected sex in recent months, but had no prior history of oral, penile, or anal mucosal lesions or ulcers. He was not on any medications or immunosuppressive agents and noted that the lesions did not represent a recurrence. He also reported a nonspecific, asymptomatic rash on his trunk and extremities that had been present for an unknown period of time.
The patient indicated that his primary care physician had looked at the genital/perianal lesions and told him they were genital warts. Previous treatments included an over-the-counter wart medication, cryotherapy, and a course of imiquimod, but none had helped.
The physical examination revealed multiple soft, moist, beefy papules and plaques around the genital area (FIGURE 1) and perianal region. In addition, there were multiple hyper-pigmented macules on the patient’s palms and soles (FIGURE 2), and reticulated, patchy eruptions on his arms, chest (FIGURE 3), and back.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Secondary syphilis
The appearance of the genital and perianal lesions was consistent with condylomata lata—a cutaneous sign of secondary syphilis—rather than genital warts. The presence of a rash on the patient’s trunk and extremities further supported this diagnosis. We did a rapid plasma reagin (RPR) test and a Treponema pallidum particle agglutination test; we also tested for human immunodeficiency virus (HIV). The patient’s RPR titer was 1:128, and the T pallidum antibody test came back positive. HIV-1 and HIV-2 serology were negative.
Appearance of the lesions was a giveaway. Condylomata lata are flat-topped, broad papules that are usually located on folds of moist skin (particularly the genitals and anus), and have a smooth, gray, moist surface. Although they can be lobulated, they do not have the classic digitate projections that are characteristic of genital warts. A nonpruritic, symmetric, “raw ham”-colored papular eruption on a patient’s trunk, palms, and soles is also characteristic of secondary syphilis.1 In this case, the reticular pattern on the patient’s chest represented the commonly seen lenticular rash of secondary syphilis.
Cutaneous lesions of secondary syphilis contain numerous spirochetes (T pallidum) and are highly infectious. Systemic symptoms of secondary syphilis may include fatigue, generalized lymphadenopathy, arthralgia, myalgia, pharyngitis, and headache.
Some patients may report having a recent chancre—a painless, self-limiting ulcer in the genital area—which is characteristic of primary syphilis (see “Single nontender ulcer on the glans,” J Fam Pract. 2017;66:253-255). For more on the stages of syphilis, see the TABLE2. Our patient did not remember ever having a chancre.
Increase in cases. Rates of primary and secondary syphilis have increased in the past decade. In 2014, approximately 20,000 syphilis cases were reported—a record high since 1994.3 Men who have sex with men are particularly affected; however, increases in infection rates have also been noted in women and across people of all ages and ethnicities.3
Rule out other causes of genital lesions
Condyloma acuminata, commonly called genital warts, are localized human papilloma virus (HPV) infections that appear as discrete, gray to pale pink, lobulated papules that may coalesce to form a large, cauliflower-like mass. They are sexually transmitted and commonly involve the genital and anal areas. While physicians may confuse condylomata lata with genital warts, diffuse skin rashes and constitutional symptoms are not usually seen with genital warts.4
Fordyce spots are small, whitish, raised papules on the glans or the shaft of the penis or the vulva of the vagina. They may also appear on the lips and oral mucosa. They are a result of prominent sebaceous glands and are harmless. They are not infectious or sexually transmitted.5,6
Lymphogranuloma venereum is an uncommon sexually transmitted disease caused by Chlamydia trachomatis. It is characterized by genital papules or ulcers, followed by bilateral, suppurative, inguinal adenitis known as buboes. The buboes may breakdown, form multiple fistulous openings, and discharge purulent material.6
Acute HIV may present with flu-like symptoms and well-circumscribed maculopapular rashes on the face, neck, and upper trunk. The palms and soles may also be affected. Patients with HIV may also develop genital plaque-like lesions from herpes simplex virus-2, genital warts from HPV, molluscum contagiosum, and, not uncommonly, anogenital malignancies.7,8
Confluent and reticulated papillomatosis (CARP) is a disorder that occurs predominantly in young adults and teenagers, with cosmetically displeasing brown scaling macules that may coalesce to form patches or plaques affecting the neck, chest, back, and axillae. It is often mistaken for tinea versicolor.9 In this case, the eruption on the chest closely resembled CARP, but a diagnosis of CARP would not have explained the genital lesions.
Confirm diagnosis with treponemal tests
Syphilis is often a clinical diagnosis with pathologic confirmation. Patients suspected of having syphilis should be screened with nontreponemal tests, such as the Venereal Disease Research Laboratory (VDRL) test or the RPR test, which become positive within 3 weeks of developing primary syphilis.
Diagnosis is confirmed with specific treponemal testing, such as with a fluorescent treponemal antibody absorption assay or the T pallidum particle agglutination test. HIV testing is recommended for all patients with syphilis.
Penicillin G is the mainstay of treatment
Proper selection of penicillin is paramount in the treatment of syphilis. Primary, secondary, and early latent syphilis are treated with an intramuscular injection of 2.4 million units of long-acting benzathine penicillin G. Patients with late latent or latent syphilis of unknown duration are treated with 3 doses of the same injection at weekly intervals, totaling 7.2 million units of benzathine penicillin G.10 Certain penicillin preparations (eg, combinations of benzathine penicillin and procaine penicillin) are not appropriate treatments because they do not provide adequate amounts of the antibiotic.
Watch for this reaction. Approximately 30% of patients following penicillin treatment for spirochete infection develop a Jarisch-Herxheimer reaction (JHR).11 JHR is characterized by an abrupt onset of fever, chills, myalgia, tachycardia, vasodilatation with flushing, exacerbated maculopapular skin rash, or mild hypotension. Care for JHR is generally supportive.
Our patient received an intramuscular injection of 2.4 million units of long-acting benzathine penicillin G. His skin eruption and condylomata lata lesions were completely resolved at follow-up 6 months later.
As recommended by the Centers for Disease Control and Prevention,10 our patient’s RPR titers were repeated at 6 months and again at 12 months to verify a four-fold decline, indicating successful treatment.
CORRESPONDENCE
Anne Bartels, MD, General Medicine, Naval Hospital Camp Lejeune, 100 Brewster Blvd., Camp Lejeune, NC 28547; Anne.k.bartels.mil@mail.mil.
A 21-year-old man presented to the dermatology clinic with a 2-month history of painless genital and perianal lesions. The patient reported having unprotected sex in recent months, but had no prior history of oral, penile, or anal mucosal lesions or ulcers. He was not on any medications or immunosuppressive agents and noted that the lesions did not represent a recurrence. He also reported a nonspecific, asymptomatic rash on his trunk and extremities that had been present for an unknown period of time.
The patient indicated that his primary care physician had looked at the genital/perianal lesions and told him they were genital warts. Previous treatments included an over-the-counter wart medication, cryotherapy, and a course of imiquimod, but none had helped.
The physical examination revealed multiple soft, moist, beefy papules and plaques around the genital area (FIGURE 1) and perianal region. In addition, there were multiple hyper-pigmented macules on the patient’s palms and soles (FIGURE 2), and reticulated, patchy eruptions on his arms, chest (FIGURE 3), and back.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Secondary syphilis
The appearance of the genital and perianal lesions was consistent with condylomata lata—a cutaneous sign of secondary syphilis—rather than genital warts. The presence of a rash on the patient’s trunk and extremities further supported this diagnosis. We did a rapid plasma reagin (RPR) test and a Treponema pallidum particle agglutination test; we also tested for human immunodeficiency virus (HIV). The patient’s RPR titer was 1:128, and the T pallidum antibody test came back positive. HIV-1 and HIV-2 serology were negative.
Appearance of the lesions was a giveaway. Condylomata lata are flat-topped, broad papules that are usually located on folds of moist skin (particularly the genitals and anus), and have a smooth, gray, moist surface. Although they can be lobulated, they do not have the classic digitate projections that are characteristic of genital warts. A nonpruritic, symmetric, “raw ham”-colored papular eruption on a patient’s trunk, palms, and soles is also characteristic of secondary syphilis.1 In this case, the reticular pattern on the patient’s chest represented the commonly seen lenticular rash of secondary syphilis.
Cutaneous lesions of secondary syphilis contain numerous spirochetes (T pallidum) and are highly infectious. Systemic symptoms of secondary syphilis may include fatigue, generalized lymphadenopathy, arthralgia, myalgia, pharyngitis, and headache.
Some patients may report having a recent chancre—a painless, self-limiting ulcer in the genital area—which is characteristic of primary syphilis (see “Single nontender ulcer on the glans,” J Fam Pract. 2017;66:253-255). For more on the stages of syphilis, see the TABLE2. Our patient did not remember ever having a chancre.
Increase in cases. Rates of primary and secondary syphilis have increased in the past decade. In 2014, approximately 20,000 syphilis cases were reported—a record high since 1994.3 Men who have sex with men are particularly affected; however, increases in infection rates have also been noted in women and across people of all ages and ethnicities.3
Rule out other causes of genital lesions
Condyloma acuminata, commonly called genital warts, are localized human papilloma virus (HPV) infections that appear as discrete, gray to pale pink, lobulated papules that may coalesce to form a large, cauliflower-like mass. They are sexually transmitted and commonly involve the genital and anal areas. While physicians may confuse condylomata lata with genital warts, diffuse skin rashes and constitutional symptoms are not usually seen with genital warts.4
Fordyce spots are small, whitish, raised papules on the glans or the shaft of the penis or the vulva of the vagina. They may also appear on the lips and oral mucosa. They are a result of prominent sebaceous glands and are harmless. They are not infectious or sexually transmitted.5,6
Lymphogranuloma venereum is an uncommon sexually transmitted disease caused by Chlamydia trachomatis. It is characterized by genital papules or ulcers, followed by bilateral, suppurative, inguinal adenitis known as buboes. The buboes may breakdown, form multiple fistulous openings, and discharge purulent material.6
Acute HIV may present with flu-like symptoms and well-circumscribed maculopapular rashes on the face, neck, and upper trunk. The palms and soles may also be affected. Patients with HIV may also develop genital plaque-like lesions from herpes simplex virus-2, genital warts from HPV, molluscum contagiosum, and, not uncommonly, anogenital malignancies.7,8
Confluent and reticulated papillomatosis (CARP) is a disorder that occurs predominantly in young adults and teenagers, with cosmetically displeasing brown scaling macules that may coalesce to form patches or plaques affecting the neck, chest, back, and axillae. It is often mistaken for tinea versicolor.9 In this case, the eruption on the chest closely resembled CARP, but a diagnosis of CARP would not have explained the genital lesions.
Confirm diagnosis with treponemal tests
Syphilis is often a clinical diagnosis with pathologic confirmation. Patients suspected of having syphilis should be screened with nontreponemal tests, such as the Venereal Disease Research Laboratory (VDRL) test or the RPR test, which become positive within 3 weeks of developing primary syphilis.
Diagnosis is confirmed with specific treponemal testing, such as with a fluorescent treponemal antibody absorption assay or the T pallidum particle agglutination test. HIV testing is recommended for all patients with syphilis.
Penicillin G is the mainstay of treatment
Proper selection of penicillin is paramount in the treatment of syphilis. Primary, secondary, and early latent syphilis are treated with an intramuscular injection of 2.4 million units of long-acting benzathine penicillin G. Patients with late latent or latent syphilis of unknown duration are treated with 3 doses of the same injection at weekly intervals, totaling 7.2 million units of benzathine penicillin G.10 Certain penicillin preparations (eg, combinations of benzathine penicillin and procaine penicillin) are not appropriate treatments because they do not provide adequate amounts of the antibiotic.
Watch for this reaction. Approximately 30% of patients following penicillin treatment for spirochete infection develop a Jarisch-Herxheimer reaction (JHR).11 JHR is characterized by an abrupt onset of fever, chills, myalgia, tachycardia, vasodilatation with flushing, exacerbated maculopapular skin rash, or mild hypotension. Care for JHR is generally supportive.
Our patient received an intramuscular injection of 2.4 million units of long-acting benzathine penicillin G. His skin eruption and condylomata lata lesions were completely resolved at follow-up 6 months later.
As recommended by the Centers for Disease Control and Prevention,10 our patient’s RPR titers were repeated at 6 months and again at 12 months to verify a four-fold decline, indicating successful treatment.
CORRESPONDENCE
Anne Bartels, MD, General Medicine, Naval Hospital Camp Lejeune, 100 Brewster Blvd., Camp Lejeune, NC 28547; Anne.k.bartels.mil@mail.mil.
1. James WD, Berger TG, Elston DM. Secondary syphilis. In: James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, Pa: Elsevier; 2011:348-350.
2. Centers for Disease Control and Prevention. Syphilis—CDC Fact Sheet. Available at: https://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Accessed May 31, 2017.
3. Centers for Disease Control and Prevention. Syphilis. November 17, 2015. Available at: http://www.cdc.gov/std/stats14/syphilis.htm. Accessed March 30, 2017.
4. Karnes JB, Usatine RP. Management of external genital warts. Am Fam Physician. 2014;90:312-318.
5. DuVivier A. Disorders of the sebaceous, sweat and apocrine glands. In: DuVivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, Pa: Elsevier; 2013:326-330.
6. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002;78:90-92.
7. Altman K, Vanness E, Westergaard RP. Cutaneous manifestations of human immunodeficiency virus: a clinical update. Curr Infect Dis Rep. 2015;17:464.
8. Maurer TA. Dermatologic manifestations of HIV infection. Top HIV Med. 2005;13:149-154.
9. Hudacek KD, Haque MS, Hochberg AL, et al. An unusual variant of confluent and reticulated papillomatosis masquerading as tinea versicolor. Arch Dermatol. 2012;148:505-508.
10. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. Available at: https://www.cdc.gov/std/treatment/2010/STD-Treatment-2010-RR5912.pdf#. Accessed June 8, 2017.
11. Yang CJ, Lee NY, Lin YH, et al. Jarisch-Herxheimer reaction after penicillin therapy among patients with syphilis in the era of the hiv infection epidemic: incidence and risk factors. Clin Infect Dis. 2010;51:976-979.
1. James WD, Berger TG, Elston DM. Secondary syphilis. In: James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Philadelphia, Pa: Elsevier; 2011:348-350.
2. Centers for Disease Control and Prevention. Syphilis—CDC Fact Sheet. Available at: https://www.cdc.gov/std/syphilis/stdfact-syphilis.htm. Accessed May 31, 2017.
3. Centers for Disease Control and Prevention. Syphilis. November 17, 2015. Available at: http://www.cdc.gov/std/stats14/syphilis.htm. Accessed March 30, 2017.
4. Karnes JB, Usatine RP. Management of external genital warts. Am Fam Physician. 2014;90:312-318.
5. DuVivier A. Disorders of the sebaceous, sweat and apocrine glands. In: DuVivier A. Atlas of Clinical Dermatology. 4th ed. Philadelphia, Pa: Elsevier; 2013:326-330.
6. Mabey D, Peeling RW. Lymphogranuloma venereum. Sex Transm Infect. 2002;78:90-92.
7. Altman K, Vanness E, Westergaard RP. Cutaneous manifestations of human immunodeficiency virus: a clinical update. Curr Infect Dis Rep. 2015;17:464.
8. Maurer TA. Dermatologic manifestations of HIV infection. Top HIV Med. 2005;13:149-154.
9. Hudacek KD, Haque MS, Hochberg AL, et al. An unusual variant of confluent and reticulated papillomatosis masquerading as tinea versicolor. Arch Dermatol. 2012;148:505-508.
10. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines, 2010. Available at: https://www.cdc.gov/std/treatment/2010/STD-Treatment-2010-RR5912.pdf#. Accessed June 8, 2017.
11. Yang CJ, Lee NY, Lin YH, et al. Jarisch-Herxheimer reaction after penicillin therapy among patients with syphilis in the era of the hiv infection epidemic: incidence and risk factors. Clin Infect Dis. 2010;51:976-979.
Herpes zoster raises risk of stroke, MI
Herpes zoster infection raised the risk of stroke by 35% and of myocardial infarction by 59%, in a South Korean nationwide study.
In addition, the risks of stroke and MI were highest in the first year following herpes zoster infection and decreased over time, said Min-Chul Kim, MD, the University of Ulsan, Seoul, South Korea, and associates.
The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it. Similarly, the incidence of MI was 0.80 per 1,000 person-years higher among patients with the infection than among those without it. The risks of both stroke and MI were highest during the first year after herpes onset and decreased over time. In contrast, the risks of stroke and MI were evenly distributed over time in the control group, the investigators said in a letter to the editor (J Am Coll Cardiol. 2017;70[2]:293-300). Several possible reasons have been proposed to explain a causal association between this infection and cardiovascular disease. Herpes zoster is “the only virus for which there is clear evidence of viral DNA and antigen in areas of ischemia or infarction in cerebral arteries,” Dr. Kim and associates noted.
Viral replication adjacent to a cerebral or cardiac artery could cause inflammation of the vessel, followed by subsequent thrombosis and rupture. Or, repeated subclinical reactivation of the virus could weaken the arteries. It is also possible that herpes zoster reactivation alters patients’ immunologic status, making them vulnerable to cerebrovascular or cardiovascular events, the investigators said.
This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
Herpes zoster infection raised the risk of stroke by 35% and of myocardial infarction by 59%, in a South Korean nationwide study.
In addition, the risks of stroke and MI were highest in the first year following herpes zoster infection and decreased over time, said Min-Chul Kim, MD, the University of Ulsan, Seoul, South Korea, and associates.
The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it. Similarly, the incidence of MI was 0.80 per 1,000 person-years higher among patients with the infection than among those without it. The risks of both stroke and MI were highest during the first year after herpes onset and decreased over time. In contrast, the risks of stroke and MI were evenly distributed over time in the control group, the investigators said in a letter to the editor (J Am Coll Cardiol. 2017;70[2]:293-300). Several possible reasons have been proposed to explain a causal association between this infection and cardiovascular disease. Herpes zoster is “the only virus for which there is clear evidence of viral DNA and antigen in areas of ischemia or infarction in cerebral arteries,” Dr. Kim and associates noted.
Viral replication adjacent to a cerebral or cardiac artery could cause inflammation of the vessel, followed by subsequent thrombosis and rupture. Or, repeated subclinical reactivation of the virus could weaken the arteries. It is also possible that herpes zoster reactivation alters patients’ immunologic status, making them vulnerable to cerebrovascular or cardiovascular events, the investigators said.
This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
Herpes zoster infection raised the risk of stroke by 35% and of myocardial infarction by 59%, in a South Korean nationwide study.
In addition, the risks of stroke and MI were highest in the first year following herpes zoster infection and decreased over time, said Min-Chul Kim, MD, the University of Ulsan, Seoul, South Korea, and associates.
The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it. Similarly, the incidence of MI was 0.80 per 1,000 person-years higher among patients with the infection than among those without it. The risks of both stroke and MI were highest during the first year after herpes onset and decreased over time. In contrast, the risks of stroke and MI were evenly distributed over time in the control group, the investigators said in a letter to the editor (J Am Coll Cardiol. 2017;70[2]:293-300). Several possible reasons have been proposed to explain a causal association between this infection and cardiovascular disease. Herpes zoster is “the only virus for which there is clear evidence of viral DNA and antigen in areas of ischemia or infarction in cerebral arteries,” Dr. Kim and associates noted.
Viral replication adjacent to a cerebral or cardiac artery could cause inflammation of the vessel, followed by subsequent thrombosis and rupture. Or, repeated subclinical reactivation of the virus could weaken the arteries. It is also possible that herpes zoster reactivation alters patients’ immunologic status, making them vulnerable to cerebrovascular or cardiovascular events, the investigators said.
This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Herpes zoster infection raised the risk of stroke by 35% and of MI by 59% in a nationwide study in South Korea.
Major finding: The incidence of stroke was 1.34 per 1,000 person-years higher among patients with the infection than among those without it, and the incidence of MI was 0.80 per 1,000 person-years higher.
Data source: A cohort study of CV risk in 519,880 adults in South Korea.
Disclosures: This study was supported by the Korea Health Technology Research and Development Project, the Korea Health Industry Development Institute, and the Republic of Korea Ministry of Health & Welfare. Dr. Kim and associates reported having no relevant financial disclosures.
Which combined OC to prescribe with CV safety in mind?
ILLUSTRATIVE CASE
A 28-year-old woman presents to your office for a routine health maintenance examination. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a patient, physicians often have “go-to” favorites—tried and true agents that are easy to prescribe on a busy clinic day. However, some of these may be placing patients at increased risk for venous thromboembolic events.
In general, when compared with nonusers, women who use COCs have a 2- to 4-fold increase in risk of venous thromboembolism (VTE) and an increased risk of myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk of VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk of VTE than other preparations, despite similar estrogen content.7 The US Food and Drug Administration (FDA) produced a similar statement that same year, recommending that physicians carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks of ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One combined oral contraceptive comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks of pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included just under 5 million women 15 to 49 years of age, living in France, with at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks of first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 mcg) or high-dose estrogen (30-40 mcg) combined with one of 5 progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 (95% confidence interval [CI], 0.67-0.85) for PE, 0.82 (95% CI, 0.7-0.96) for ischemic stroke, and 0.56 (95% CI, 0.39-0.79) for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs of PE but not arterial events (2.16; 95% CI, 1.93-2.41 for desogestrel and 1.63; 95% CI, 1.34-1.97 for gestodene). The ARR with levonorgestrel use as opposed to desogestrel for PE was 19/100,000 person-years of use (NNH=5263); the ARR with levonorgestrel use as opposed to gestodene was 12/100,000 person-years of use (NNH=8333). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk of PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks of PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen and levonorgestrel confer lowest risk of 3 CV conditions
Prior studies have shown that COCs increase the risk of PE and may also increase the risks of ischemic stroke and MI.
CAVEATS
A cohort study, no contraceptive start date, and incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk of VTE is highest in the first 3 months to one year of COC use.
CHALLENGES TO IMPLEMENTATION
Low-dose estrogen is associated with increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 mcg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. US Food and Drug Administration. Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints. Available at: https://www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed February 23, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP Monthly report on safety concerns, guidelines and general matters. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed February 23, 2017.
8. US Food and Drug Administration. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed February 23, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
ILLUSTRATIVE CASE
A 28-year-old woman presents to your office for a routine health maintenance examination. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a patient, physicians often have “go-to” favorites—tried and true agents that are easy to prescribe on a busy clinic day. However, some of these may be placing patients at increased risk for venous thromboembolic events.
In general, when compared with nonusers, women who use COCs have a 2- to 4-fold increase in risk of venous thromboembolism (VTE) and an increased risk of myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk of VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk of VTE than other preparations, despite similar estrogen content.7 The US Food and Drug Administration (FDA) produced a similar statement that same year, recommending that physicians carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks of ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One combined oral contraceptive comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks of pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included just under 5 million women 15 to 49 years of age, living in France, with at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks of first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 mcg) or high-dose estrogen (30-40 mcg) combined with one of 5 progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 (95% confidence interval [CI], 0.67-0.85) for PE, 0.82 (95% CI, 0.7-0.96) for ischemic stroke, and 0.56 (95% CI, 0.39-0.79) for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs of PE but not arterial events (2.16; 95% CI, 1.93-2.41 for desogestrel and 1.63; 95% CI, 1.34-1.97 for gestodene). The ARR with levonorgestrel use as opposed to desogestrel for PE was 19/100,000 person-years of use (NNH=5263); the ARR with levonorgestrel use as opposed to gestodene was 12/100,000 person-years of use (NNH=8333). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk of PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks of PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen and levonorgestrel confer lowest risk of 3 CV conditions
Prior studies have shown that COCs increase the risk of PE and may also increase the risks of ischemic stroke and MI.
CAVEATS
A cohort study, no contraceptive start date, and incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk of VTE is highest in the first 3 months to one year of COC use.
CHALLENGES TO IMPLEMENTATION
Low-dose estrogen is associated with increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 mcg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 28-year-old woman presents to your office for a routine health maintenance examination. She is currently using an oral contraceptive containing desogestrel and ethinyl estradiol for contraception and is inquiring about a refill for the coming year. What would you recommend?
When choosing a combined oral contraceptive (COC) for a patient, physicians often have “go-to” favorites—tried and true agents that are easy to prescribe on a busy clinic day. However, some of these may be placing patients at increased risk for venous thromboembolic events.
In general, when compared with nonusers, women who use COCs have a 2- to 4-fold increase in risk of venous thromboembolism (VTE) and an increased risk of myocardial infarction (MI) and stroke.2,3 More specifically, higher doses of estrogen combined with the progesterones gestodene, desogestrel, and levonorgestrel, are associated with a higher risk of VTE.2-6
In 2012, the European Medicines Agency warned that COCs containing drospirenone were associated with a higher risk of VTE than other preparations, despite similar estrogen content.7 The US Food and Drug Administration (FDA) produced a similar statement that same year, recommending that physicians carefully consider the risks and benefits before prescribing contraceptives containing drospirenone.8
The risks of ischemic stroke and MI have not been clearly established for varying doses of estrogen and different progesterones. This large observational study fills that informational gap by providing risk estimates for the various COC options.
STUDY SUMMARY
One combined oral contraceptive comes out ahead
The authors used an observational cohort model to determine the effects of different doses of estrogen combined with different progesterones in COCs on the risks of pulmonary embolism (PE), ischemic stroke, and MI.1 Data were collected from the French national health insurance database and the French national hospital discharge database.9,10 The study included just under 5 million women 15 to 49 years of age, living in France, with at least one prescription filled for COCs between July 2010 and September 2012.
The investigators calculated the absolute and relative risks of first PE, ischemic stroke, and MI in women using COC formulations containing either low-dose estrogen (20 mcg) or high-dose estrogen (30-40 mcg) combined with one of 5 progesterones (norethisterone, norgestrel, levonorgestrel, desogestrel, gestodene). The relative risk (RR) was adjusted for confounding factors, including age, complimentary universal health insurance, socioeconomic status, hypertension, diabetes, and consultation with a gynecologist in the previous year.
The absolute risk per 100,000 woman-years for all COC use was 33 for PE, 19 for ischemic stroke, and 7 for MI with a composite risk of 60. The RRs for low-dose estrogen vs high-dose estrogen were 0.75 (95% confidence interval [CI], 0.67-0.85) for PE, 0.82 (95% CI, 0.7-0.96) for ischemic stroke, and 0.56 (95% CI, 0.39-0.79) for MI. The absolute risk reduction (ARR) with low-dose estrogen vs high-dose estrogen was 14/100,000 person-years of use; the number needed to harm (NNH) was 7143.
Compared with levonorgestrel, desogestrel and gestodene were associated with higher RRs of PE but not arterial events (2.16; 95% CI, 1.93-2.41 for desogestrel and 1.63; 95% CI, 1.34-1.97 for gestodene). The ARR with levonorgestrel use as opposed to desogestrel for PE was 19/100,000 person-years of use (NNH=5263); the ARR with levonorgestrel use as opposed to gestodene was 12/100,000 person-years of use (NNH=8333). The authors concluded that for the same progesterone, using a lower dose of estrogen decreases risk of PE, ischemic stroke, and MI, and that oral contraceptives containing levonorgestrel and low-dose estrogen resulted in the lowest overall risks of PE and arterial thromboembolism.
WHAT’S NEW?
Low-dose estrogen and levonorgestrel confer lowest risk of 3 CV conditions
Prior studies have shown that COCs increase the risk of PE and may also increase the risks of ischemic stroke and MI.
CAVEATS
A cohort study, no contraceptive start date, and incomplete tobacco use data
This is an observational cohort study, so it is subject to confounding factors and biases. It does, however, include a very large population, which improves validity. The study did not account for COC start date, which may be confounding because the risk of VTE is highest in the first 3 months to one year of COC use.
CHALLENGES TO IMPLEMENTATION
Low-dose estrogen is associated with increased vaginal spotting
One potential challenge to implementing this practice changer may be the increased rate of vaginal spotting associated with low-dose estrogen. COCs containing 20 mcg of estrogen are associated with spotting in approximately two-thirds of menstrual cycles over the course of a year.14 That said, women may prefer to endure the spotting in light of the improved safety profile of a lower-dose estrogen pill.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. US Food and Drug Administration. Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints. Available at: https://www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed February 23, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP Monthly report on safety concerns, guidelines and general matters. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed February 23, 2017.
8. US Food and Drug Administration. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed February 23, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
1. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.
2. Lidegaard Ø, Løkkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: national follow-up study. BMJ. 2009;339:b2890.
3. Lidegaard Ø, Løkkegaard E, Jensen A, et al. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257-2266.
4. Stegeman BH, de Bastos M, Rosendaal FR, et al. Different combined oral contraceptives and the risk of venous thrombosis: systematic review and network meta-analysis. BMJ. 2013;347:f5298.
5. US Food and Drug Administration. Combined Hormonal Contraceptives (CHCs) and the Risk of Cardiovascular Disease Endpoints. Available at: https://www.fda.gov/downloads/drugs/drugsafety/ucm277384. Accessed February 23, 2017.
6. Seeger JD, Loughlin J, Eng PM, et al. Risk of thromboembolism in women taking ethinyl estradiol/drospirenone and other oral contraceptives. Obstet Gynecol. 2007;110:587-593.
7. European Medicines Agency. PhVWP Monthly report on safety concerns, guidelines and general matters. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/01/WC500121387.pdf. Accessed February 23, 2017.
8. US Food and Drug Administration. FDA Drug Safety Communication: Updated information about the risk of blood clots in women taking birth control pills containing drospirenone. 2012. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm299305.htm. Accessed February 23, 2017.
9. Tuppin P, de Roquefeuil L, Weill A, et al. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286-290.
10. Moulis G, Lapeyre-Mestre M, Palmaro A, et al. French health insurance databases: what interest for medical research? Rev Med Interne. 2015;36:411-417.
11. Farmer RD, Lawrenson RA, Thompson CR, et al. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet. 1997;349:83-88.
12. Lidegaard Ø, Nielsen LH, Skovlund CW, et al. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423.
13. Zhang G, Xu X, Su W, et al. Smoking and risk of venous thromboembolism: a systematic review. Southeast Asian J Trop Med Public Health. 2014;45:736-745.
14. Akerlund M, Røde A, Westergaard J. Comparative profiles of reliability, cycle control and side effects of two oral contraceptive formulations containing 150 micrograms desogestrel and either 30 micrograms or 20 micrograms ethinyl oestradiol. Br J Obstet Gynaecol. 1993;100:832-838.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
When prescribing combined oral contraceptives, choose one containing levonorgestrel and low-dose estrogen (20 mcg) to minimize the risks of pulmonary embolism, ischemic stroke, and myocardial infarction.
STRENGTH OF RECOMMENDATION
B: Based on a good quality, patient-oriented cohort study.
Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002.1
Measles: Why it’s still a threat
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
In April of this year, 3 counties in Minnesota reported a measles outbreak, illustrating the danger of vaccine hesitancy that exists in some communities, resulting in low rates of childhood immunization. Fifty people—mostly children under the age of 5 and almost all unimmunized—have been diagnosed with measles since this outbreak began. As of early May, 11 had been hospitalized. Most of those infected have been American-born children of Somali immigrants.1,2
At the time of the outbreak, only 42% of the Somali children had been immunized against measles, compared with 88.5% of non-Somalis in Minnesota.2 Because of concern about the number of Somali children being diagnosed with autism, a condition apparently not recognized in Somalia, Somali parents living in Minnesota began questioning why this was occurring.
High profile anti-vaccine advocates reportedly visited the community and advised these parents that the measles-mumps-rubella (MMR) vaccine was the cause of this rise in autism incidence and encouraged them to avoid the vaccine.2 This series of events led to low vaccination rates in what was once a well-vaccinated community. The outbreak appears to have started with a Somali child who visited Africa and then returned to his community while incubating measles.
The clinical course of measles. Measles is an acute viral respiratory illness, which, after an incubation period of
Measles is not a benign childhood illness. Before the licensure of live measles vaccine in 1963, an average of 549,000 measles cases were reported in the United States each year.3 That number is likely an underestimate due to inconsistent reporting, with a more plausible number of infections annually being 3 to 4 million.3 These regular epidemics led each year to about 48,000 people being hospitalized from complications, 1000 developing chronic disability from acute measles encephalitis, and about 500 dying from measles-related complications. Today, worldwide, an estimated 134,200 individuals die from measles each year.3
Where the risk is greatest. In the year 2000, measles was declared eliminated from the United States, meaning that endemic transmission was no longer occurring. Since then, the annual number of cases has ranged from a low of 37 in 2004 to a high of 667 in 2014.3 Most measles cases have occurred in unvaccinated individuals and primarily through importation by people infected in other countries who then transmit the infection upon entry or reentry to this country. In the United States, measles is more likely to spread and cause outbreaks in communities where large groups of people are unvaccinated.
Laboratory confirmation of measles is important to establish a correct clinical diagnosis, as well as to verify the infection for public health purposes. Confirmation is achieved by detecting in a patient’s blood sample the measles-specific IgM antibody or measles RNA by real-time polymerase chain reaction (RT-PCR). Obtain both a serum sample and a throat swab (or nasopharyngeal swab) from patients you suspect may have measles. Urine samples may also contain virus, and can be useful. The local health department can offer advice on how to collect and process these laboratory specimens.
Measles is a preventable infection
The Centers for Disease Control and Prevention (CDC) recommends routine childhood immunization with MMR vaccine, with the first dose given at age 12 through 15 months, and the second dose at 4 through 6 years of age (or at least 28 days following the first dose).3,5 Others for whom the vaccine is recommended are included in the TABLE.3
Because the MMR vaccine is a modified live-virus vaccine, it is contraindicated for pregnant women and those with severe immune deficiencies. It is also contraindicated for individuals who have ever had a life-threatening allergic reaction to the antibiotic neomycin or to any other MMR vaccine component.4 That these high-risk groups cannot receive protection from the vaccine underscores the importance of maintaining community herd immunity at a high level to prevent the spread of infection.
In response to this latest outbreak, the Minnesota Department of Health (MDH) has augmented its routine recommendations regarding measles vaccine,1 including advising that:
- All children 12 months and older who have not received the MMR vaccine and all adults born in 1957 (or later) who have not received the vaccine or ever had the measles should get the first dose as soon as possible.
- Children who live in counties where measles cases have occurred and who have received their first dose of the MMR vaccine at least 28 days ago should get their second dose as soon as possible.
- All Somali Minnesotan children statewide who received their first dose of the vaccine at least 28 days ago should get their second as soon as possible.
- Health care providers statewide may recommend an early (before age 4 years) second dose of the vaccine during routine appointments for children.
Preventing measles outbreaks and minimizing community impact
Measures family physicians can take to protect their staff, patients, and community from measles (and other infectious diseases) include ensuring that all staff are fully immunized as recommended by the CDC,6 vaccinating all patients according to the recommended immunization schedules, implementing and enforcing good infection control practices in the clinical setting, and taking appropriate measures to diagnose and manage individuals with suspected measles. These measures are described on the CDC Web site.7
Measles virus, commonly believed to be the most infectious agent known, is often transmitted in medical facilities. An individual can become infected simply by entering a closed space that had been occupied by someone with measles several hours earlier. In your facility, physically separate those with fever and rash from other patients as soon as possible and, if measles is suspected, care for them in an isolation room or one that can be kept unused afterwards.
Any time you suspect that a patient has measles, immediately inform the local public health department. The health department should conduct an investigation to find susceptible individuals, provide immunizations for case contacts (and immune globulin for unvaccinated pregnant women and those who are severely immunosuppressed), and implement isolation and quarantine measures as indicated by the situation.
There is no antiviral medication for measles. Aim treatment at controlling symptoms and addressing any complicating bacterial infections. Children who have severe illness should receive vitamin A at recommended doses.3
Outbreaks such as the one in Minnesota demonstrate the importance of family physicians working in collaboration with public health officials to minimize the effect of infectious illnesses on the community.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
1. Minnesota Department of Health. MDH expands advice for measles vaccination to make sure more children in state are protected. Available at: http://www.health.state.mn.us/news/pressrel/2017/measles050417.html. Accessed May 24, 2017.
2. Offit PA. Did Anti-Vaxxers Spark a Measles Outbreak in an Immigrant Community? Daily Beast. Available at: http://www.thedailybeast.com/articles/2017/05/13/did-anti-vaxxers-spark-a-measles-outbreak-in-an-immigrant-community. Accessed May 24, 2017.
3. CDC. Measles. For healthcare professionals. Available at: https://www.cdc.gov/measles/hcp/index.html. Accessed May 24, 2017.
4. CDC. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2013;62:1-34. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6204a1.htm. Accessed May 24, 2017.
5. CDC. Immunization schedules. Available at: https://www.cdc.gov/vaccines/schedules/hcp/child-adolescent.html. Accessed May 24, 2017.
6. CDC. Recommended vaccines for health care workers. Available at: https://www.cdc.gov/vaccines/adults/rec-vac/hcw.html. Accessed May 24, 2017.
7. CDC. Infection control in health care facilities. Available at: https://www.cdc.gov/flu/professionals/infectioncontrol/index.htm. Accessed May 24, 2017.
Insulin degludec decreases rate, severity of hypoglycemic episodes
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Given the risks associated with hypoglycemia and the concerns about this adverse effect among patients and their families, any basal insulin that reduces the rate of hypoglycemia represents an advance in the treatment of diabetes.
Both studies were limited in that they had relatively high dropout rates of approximately 20% each. However, it appeared that the patients who completed the study were not substantially different from those who dropped out.
These remarks are from an editorial by Elizabeth R. Seaquist, MD, and Lisa S. Chow, MD, that was published along with the research reports (JAMA 2017;318[1]:31-2).
Dr Seaquist reported a variety of sources of funding from Eli Lilly, Locemia, Medtronic, Sanofi, and Zucera; serving as a member of the International Hypoglycemia Study Group; and serving on the examination committee for the American Board of Internal Medicine. Dr. Chow reported research funding from Eli Lilly.
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Insulin degludec decreased both the rate and the severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine, in two head-to-head trials sponsored by the maker of insulin degludec and reported online July 3 in JAMA.
The two trials had identical randomized double-blind crossover designs. The first involved 501 adults with type 1 diabetes treated at 84 sites in the United States and 6 sites in Poland, and the second involved 721 adults with type 2 diabetes treated at 152 sites in the United States. All the study participants were at risk for hypoglycemia by virtue of experiencing one or more severe hypoglycemic episodes within the preceding year, having moderate chronic renal failure, having a 15-year or longer duration of diabetes, being unaware of their hypoglycemic symptoms, or experiencing severe hypoglycemia within the 3 months preceding baseline.
In both trials, patients were randomized to one type of once-daily insulin for 32 weeks (a 16-week titration period followed by a 16-week maintenance period) and then crossed over to the other type of insulin for 32 weeks. The primary end point in both studies was the rate of overall severe hypoglycemia during the maintenance period. This was defined as either an episode requiring the assistance of another person to administer aid or an episode in which blood glucose measured less than 56 mg/dL.
In the first trial, rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE), demonstrating not just the noninferiority but also the superiority of insulin degludec. In addition, a significantly lower proportion of patients had hypoglycemia with insulin degludec (32.8%) than with insulin glargine (43.1%), said Wendy Lane, MD, of Mountain Diabetes and Endocrine Center, Asheville NC, and her associates.
Regarding the severity of hypoglycemia, the rate of severe episodes was significantly lower with insulin degludec (69 episodes per 100 PYE) than with insulin glargine (92 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was significantly lower with insulin degludec (10.3%) than with insulin glargine (17.1%).
Both types of insulin reduced hemoglobin A1c levels to 7% or below. Rates of adverse events and serious adverse events were similar between the two study groups, and there were no differences between them in weight change, blood pressure, pulse rate, or laboratory findings, the investigators said (JAMA.2017;318[1]:33-44).
In the second trial, rates of hypoglycemia again were significantly lower with insulin degludec than with insulin glargine (186 vs. 265 episodes per 100 PYE). In addition, the proportion of patients who experienced a severe hypoglycemic episode was 1.6% vs. 2.4%, respectively, said Carol Wysham, MD, of Rockwood Clinic University of Washington, Spokane, and her associates.
As in the first trial, both types of insulin reduced HbA1c levels to the same degree, and rates of adverse events and of serious adverse events were comparable, Dr. Wysham and her associates said (JAMA. 2017;318[1]:45-56).
The dropout rates were similar and higher than expected in both trials, at approximately 20%. Dr. Lane and her associates noted that this may have resulted from “the demanding nature” of the studies, including their 64-week duration; demand for close monitoring of blood glucose; and the requirement of using vials and syringes to maintain treatment blinding, instead of more convenient injector devices.
Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Insulin degludec decreases the rate and severity of hypoglycemic episodes in adults with type 1 and type 2 diabetes, compared with insulin glargine.
Major finding: Rates of hypoglycemia were significantly lower with insulin degludec (2,201 episodes per 100 person-years of exposure) than with insulin glargine (2,463 episodes per 100 PYE) in type 1 diabetes and in type 2 diabetes (185.6 vs. 265.4 episodes per 100 PYE).
Data source: Two separate multicenter, randomized, double-blind crossover trials involving 501 adults with type 1 and 721 with type 2 diabetes.
Disclosures: Both trials were funded by Novo Nordisk, maker of insulin degludec. Dr. Lane reported ties to Novo Nordisk, Insulet Corporation, and Eli Lilly, and her associates reported ties to numerous industry sources. Dr. Wysham reported ties to Novo Nordisk, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, and Sanofi, and her associates reported ties to numerous industry sources.
Best Practices: Understanding the Role of Delayed Cord Clamping During Cord Blood Collection
Click Here to Read Supplement.
Topics Include:
- The Role of Cord Blood Stem Cells
- Impact of DCC in Cord Blood Collection
- Combining DCC and Cord Blood Collection
- When to Clamp the Umbilical Cord
Faculty/Faculty Disclosure:
Fung Lam, MD
Chair-GYN Quality, Director of Medical Education (OB-GYN), California-Pacific Medical Center
Senior Partner, Golden Gate Obstetrics & Gynecology
Clinical Professor-Department of Obstetrics, Gynecology and Reproductive Sciences
University of California, San Francisco
San Francisco, California
Click Here to Read Supplement.
Topics Include:
- The Role of Cord Blood Stem Cells
- Impact of DCC in Cord Blood Collection
- Combining DCC and Cord Blood Collection
- When to Clamp the Umbilical Cord
Faculty/Faculty Disclosure:
Fung Lam, MD
Chair-GYN Quality, Director of Medical Education (OB-GYN), California-Pacific Medical Center
Senior Partner, Golden Gate Obstetrics & Gynecology
Clinical Professor-Department of Obstetrics, Gynecology and Reproductive Sciences
University of California, San Francisco
San Francisco, California
Click Here to Read Supplement.
Topics Include:
- The Role of Cord Blood Stem Cells
- Impact of DCC in Cord Blood Collection
- Combining DCC and Cord Blood Collection
- When to Clamp the Umbilical Cord
Faculty/Faculty Disclosure:
Fung Lam, MD
Chair-GYN Quality, Director of Medical Education (OB-GYN), California-Pacific Medical Center
Senior Partner, Golden Gate Obstetrics & Gynecology
Clinical Professor-Department of Obstetrics, Gynecology and Reproductive Sciences
University of California, San Francisco
San Francisco, California