User login
Apomorphine pump dramatically decreases ‘off’ time in Parkinson’s
BOSTON – The most rigorous study of its kind suggests that apomorphine subcutaneous infusion (APO) via pump can substantially reduce ‘off’ time when conventional therapy fails to control dyskinesias in patients with Parkinson’s disease.
“When a patient´s off periods start to become refractory to adjustments to their oral medication, consider continuous dopaminergic drug delivery such as subcutaneous apomorphine infusion, which has proven efficacy and is generally well tolerated in this group of patients,” lead author Regina Katzenschlager, MD, head of the department of neurology and the Karl Landsteiner Institute for Neuroimmunological and Neurodegenerative Disorders at Danube Hospital in Vienna, said in an interview.
According to the study authors, previous open-label research has supported the use of apomorphine to reduce dyskinesias, off time, and the need for levodopa.
For the new study, researchers recruited 106 patients aged at least 30 years with a diagnosis of idiopathic Parkinson’s disease and motor fluctuations that were not adequately controlled by medication. They all were taking L-dopa and had an average off time of at least 3 hours a day.
The subjects, who came from 23 centers in seven countries, were randomly assigned to receive apomorphine infusion while they were awake (16 hours; 8 or less mg/hour) or placebo saline infusion.
“Apomorphine is administered via bespoke pumps, which are produced by several manufacturers,” Dr. Katzenschlager said. “The pump used in the trial is a mechanical delivery system and has been in clinical use in Europe for many years for this purpose. The pump is worn outside the body; no surgical procedure is required. It delivers the drug continuously into the subcutaneous fatty tissue, usually abdomen or thighs, via a tube and a thin metal or Teflon needle.”
The pump is about the size of a mobile phone, Dr. Katzenschlager said, and typically worn in a pouch attached to a belt.
The study abstract does not note the dose of apomorphine, and Dr. Katzenschlager declined to say how much of the drug is given to patients. The trial information on clinicaltrials.gov states only that a 5-mg/mL solution of APO-go was to be provided via prefilled syringe. The hourly flow rate of apomorphine and dosages of other Parkinson’s disease medications were adjusted during the first 4 weeks of the trial for efficacy and tolerability purposes.
Researchers report that daily off time declined over the 12-week trial (–0.58 hours for placebo vs. –2.47 hours for apomorphine), accounting for a difference of –1.89 hours (95% CI, –3.16 to –0.62; P = .0025).
“This was highly statistically significant. This degree of improvement has been proven to be clinically relevant to the patients,” Dr. Katzenschlager said. “This was reflected in the patients’ own assessment of the overall treatment effect in the study.”
The study examined Patient Global Impression of Change in apomorphine vs. placebo. The abstract released at the AAN meeting does not report the full results but says that the apomorphine patients had higher scores at the end of the study (P less than .001).
Why might this infusion approach work? “Apomorphine infusion achieves more than injections, which are intended as a rescue therapy for off periods in addition to the patients’ oral medication,” Dr. Katzenschlager said. “The continuous delivery of apomorphine resembles the natural state of dopamine levels in the brain in persons without Parkinson’s disease, where this is mostly stable. The administration via the pump system usually enables oral drugs to be reduced. This may contribute to the improvement in dyskinesias often observed and is a benefit to the patients in itself.”
The study authors noted that apomorphine “was generally well tolerated and no unexpected adverse events were observed.” Dr. Katzenschlager declined to discuss cost.
Britannia Pharmaceuticals, which manufactures APO-go, funded the study. Dr. Katzenschlager reports multiple disclosures, including research support and speaking/consulting fees from Brittania Pharmaceuticals.
BOSTON – The most rigorous study of its kind suggests that apomorphine subcutaneous infusion (APO) via pump can substantially reduce ‘off’ time when conventional therapy fails to control dyskinesias in patients with Parkinson’s disease.
“When a patient´s off periods start to become refractory to adjustments to their oral medication, consider continuous dopaminergic drug delivery such as subcutaneous apomorphine infusion, which has proven efficacy and is generally well tolerated in this group of patients,” lead author Regina Katzenschlager, MD, head of the department of neurology and the Karl Landsteiner Institute for Neuroimmunological and Neurodegenerative Disorders at Danube Hospital in Vienna, said in an interview.
According to the study authors, previous open-label research has supported the use of apomorphine to reduce dyskinesias, off time, and the need for levodopa.
For the new study, researchers recruited 106 patients aged at least 30 years with a diagnosis of idiopathic Parkinson’s disease and motor fluctuations that were not adequately controlled by medication. They all were taking L-dopa and had an average off time of at least 3 hours a day.
The subjects, who came from 23 centers in seven countries, were randomly assigned to receive apomorphine infusion while they were awake (16 hours; 8 or less mg/hour) or placebo saline infusion.
“Apomorphine is administered via bespoke pumps, which are produced by several manufacturers,” Dr. Katzenschlager said. “The pump used in the trial is a mechanical delivery system and has been in clinical use in Europe for many years for this purpose. The pump is worn outside the body; no surgical procedure is required. It delivers the drug continuously into the subcutaneous fatty tissue, usually abdomen or thighs, via a tube and a thin metal or Teflon needle.”
The pump is about the size of a mobile phone, Dr. Katzenschlager said, and typically worn in a pouch attached to a belt.
The study abstract does not note the dose of apomorphine, and Dr. Katzenschlager declined to say how much of the drug is given to patients. The trial information on clinicaltrials.gov states only that a 5-mg/mL solution of APO-go was to be provided via prefilled syringe. The hourly flow rate of apomorphine and dosages of other Parkinson’s disease medications were adjusted during the first 4 weeks of the trial for efficacy and tolerability purposes.
Researchers report that daily off time declined over the 12-week trial (–0.58 hours for placebo vs. –2.47 hours for apomorphine), accounting for a difference of –1.89 hours (95% CI, –3.16 to –0.62; P = .0025).
“This was highly statistically significant. This degree of improvement has been proven to be clinically relevant to the patients,” Dr. Katzenschlager said. “This was reflected in the patients’ own assessment of the overall treatment effect in the study.”
The study examined Patient Global Impression of Change in apomorphine vs. placebo. The abstract released at the AAN meeting does not report the full results but says that the apomorphine patients had higher scores at the end of the study (P less than .001).
Why might this infusion approach work? “Apomorphine infusion achieves more than injections, which are intended as a rescue therapy for off periods in addition to the patients’ oral medication,” Dr. Katzenschlager said. “The continuous delivery of apomorphine resembles the natural state of dopamine levels in the brain in persons without Parkinson’s disease, where this is mostly stable. The administration via the pump system usually enables oral drugs to be reduced. This may contribute to the improvement in dyskinesias often observed and is a benefit to the patients in itself.”
The study authors noted that apomorphine “was generally well tolerated and no unexpected adverse events were observed.” Dr. Katzenschlager declined to discuss cost.
Britannia Pharmaceuticals, which manufactures APO-go, funded the study. Dr. Katzenschlager reports multiple disclosures, including research support and speaking/consulting fees from Brittania Pharmaceuticals.
BOSTON – The most rigorous study of its kind suggests that apomorphine subcutaneous infusion (APO) via pump can substantially reduce ‘off’ time when conventional therapy fails to control dyskinesias in patients with Parkinson’s disease.
“When a patient´s off periods start to become refractory to adjustments to their oral medication, consider continuous dopaminergic drug delivery such as subcutaneous apomorphine infusion, which has proven efficacy and is generally well tolerated in this group of patients,” lead author Regina Katzenschlager, MD, head of the department of neurology and the Karl Landsteiner Institute for Neuroimmunological and Neurodegenerative Disorders at Danube Hospital in Vienna, said in an interview.
According to the study authors, previous open-label research has supported the use of apomorphine to reduce dyskinesias, off time, and the need for levodopa.
For the new study, researchers recruited 106 patients aged at least 30 years with a diagnosis of idiopathic Parkinson’s disease and motor fluctuations that were not adequately controlled by medication. They all were taking L-dopa and had an average off time of at least 3 hours a day.
The subjects, who came from 23 centers in seven countries, were randomly assigned to receive apomorphine infusion while they were awake (16 hours; 8 or less mg/hour) or placebo saline infusion.
“Apomorphine is administered via bespoke pumps, which are produced by several manufacturers,” Dr. Katzenschlager said. “The pump used in the trial is a mechanical delivery system and has been in clinical use in Europe for many years for this purpose. The pump is worn outside the body; no surgical procedure is required. It delivers the drug continuously into the subcutaneous fatty tissue, usually abdomen or thighs, via a tube and a thin metal or Teflon needle.”
The pump is about the size of a mobile phone, Dr. Katzenschlager said, and typically worn in a pouch attached to a belt.
The study abstract does not note the dose of apomorphine, and Dr. Katzenschlager declined to say how much of the drug is given to patients. The trial information on clinicaltrials.gov states only that a 5-mg/mL solution of APO-go was to be provided via prefilled syringe. The hourly flow rate of apomorphine and dosages of other Parkinson’s disease medications were adjusted during the first 4 weeks of the trial for efficacy and tolerability purposes.
Researchers report that daily off time declined over the 12-week trial (–0.58 hours for placebo vs. –2.47 hours for apomorphine), accounting for a difference of –1.89 hours (95% CI, –3.16 to –0.62; P = .0025).
“This was highly statistically significant. This degree of improvement has been proven to be clinically relevant to the patients,” Dr. Katzenschlager said. “This was reflected in the patients’ own assessment of the overall treatment effect in the study.”
The study examined Patient Global Impression of Change in apomorphine vs. placebo. The abstract released at the AAN meeting does not report the full results but says that the apomorphine patients had higher scores at the end of the study (P less than .001).
Why might this infusion approach work? “Apomorphine infusion achieves more than injections, which are intended as a rescue therapy for off periods in addition to the patients’ oral medication,” Dr. Katzenschlager said. “The continuous delivery of apomorphine resembles the natural state of dopamine levels in the brain in persons without Parkinson’s disease, where this is mostly stable. The administration via the pump system usually enables oral drugs to be reduced. This may contribute to the improvement in dyskinesias often observed and is a benefit to the patients in itself.”
The study authors noted that apomorphine “was generally well tolerated and no unexpected adverse events were observed.” Dr. Katzenschlager declined to discuss cost.
Britannia Pharmaceuticals, which manufactures APO-go, funded the study. Dr. Katzenschlager reports multiple disclosures, including research support and speaking/consulting fees from Brittania Pharmaceuticals.
AT AAN 2017
Key clinical point:
Major finding: Daily ‘off’ time declined over 12 weeks by –0.58 hours for placebo vs. –2.47 hours for apomorphine, accounting for a difference of –1.89 hours (95% CI, –3.16 to –0.62; P = .0025).
Data source: Double-blind, randomized, placebo-controlled, phase III study of 106 Parkinson’s disease patients.
Disclosures: Britannia Pharmaceuticals, which manufactures APO-go, funded the study.
Hitting BTK, PI3K pays off in B-cell malignancies
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
AT 14-ICML
Key clinical point:
Major finding: The objective response rate to the combination was 94% in 18 patients with chronic lymphocytic leukemia and 79% in 14 patients with mantle cell lymphoma.
Data source: A phase I/IB dose-escalation study.
Disclosures: The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
Rare Dual Lesion: Extraskeletal Osteosarcoma Developing Within a Simple Lipoma
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
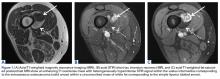
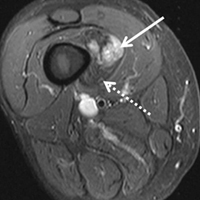
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
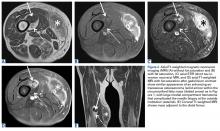
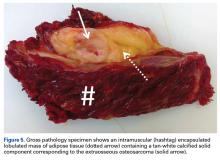
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
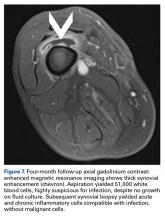
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
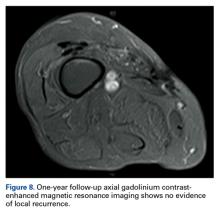
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
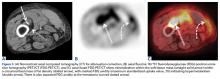
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
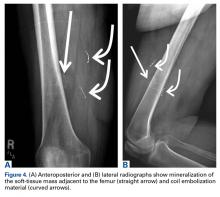
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Rare and histologically indistinguishable from osteosarcoma of bone.
- Most common presentation is an enlarging mass in the thigh or buttock.
- Secondary extraosseous osteosarcoma usually arises in the field of prior external beam radiation or brachytherapy.
- Radiographic pattern of mineralization is central amorphous or cloudlike.
- On cross sectional imaging, the soft-tissue mass is separate from the underlying bone and periosteum.
Aside from multiple myeloma, osteosarcoma is the most common primary malignancy of bone, but extraosseous osteosarcoma is rare and accounts for only 1% of soft-tissue sarcomas and only 4% of all osteosarcomas.1-3 Benign mesenchymal tumors, such as lipomas, are common, and they are estimated to outnumber their malignant counterparts by more than a factor of 100. However, the true ratio is unknown, as many clinically benign lipomas are not biopsied.4 Conventional lipoma is the most common lipoma and is biologically indolent. Conventional lipoma generally does not transform biologically into a more aggressive type of neoplasm—unlike atypical lipomatous tumors, which may demonstrate this type of evolution with multiple local recurrences.
This article is the first report of a case of radiation-associated extraosseous osteosarcoma that developed within a benign conventional lipoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
In March 2013, a 72-year-old woman presented to a general surgeon with a right thigh mass of several weeks’ duration. The patient, who had a remote history of thyroid carcinoma, underwent thyroidectomy in 1991, excision of melanoma of the chest in 1998, and resection and adjuvant external beam radiotherapy (30 fractions) for Merkel cell carcinoma of the right proximal lateral leg (malignancy images unavailable) at an outside institution in 2003. Regional lymph node dissection at the time was negative. The patient remained disease-free the next 10 years. On presentation, magnetic resonance imaging (MRI) showed a 2.2-cm mass encircled by a tumor of lipomatous tissue within the vastus intermedius muscle, adjacent to but separate from the right distal femur (Figures 1A-1C).
Physical examination revealed marked ecchymosis of the left groin at the access site for embolization as well as massive ecchymosis and swelling along the right distal thigh, medial knee, and medial lower leg. The neurovascular structures were intact with full motor function and sensation distally, as well as normal distal pulses. No inguinal adenopathy was identified. The proximal portion of the prior radiation tattoo was at the inferior extent of the lesion on MRI.
The patient was treated with doxorubicin and ifosfamide (2 cycles) while waiting for the hematoma to shrink. Contrast-enhanced MRI showed a 2.2-cm enhancing mass with isointense T1 signal and heterogeneously hyperintense STIR (short tau inversion recovery) signal surrounded by a circumscribed nonenhancing lipomatous tumor within the vastus intermedius muscle, adjacent to the distal femoral cortex. There was no invasion of the bone, and a fat plane between the enhancing mass and the femoral cortex was identified (Figures 2A-2E).
After 3 cycles of neoadjuvant chemotherapy with doxorubicin and ifosfamide, MRI showed a marked reduction in hematoma size, to 2.4 cm × 0.7 cm × 3.2 cm (estimated volume, ~3 mL), from 10 cm × 3.4 cm × 7.3 cm (estimated volume, ~130 mL), so the decision was made to proceed with surgery, excising the hematoma and sarcoma separately. First, wide resection of the hematoma yielded a 7-cm × 4-cm resection specimen with negative margins on frozen section. Subsequently, definitive radical resection of the tumor with wide margins yielded a 13-cm × 9-cm × 4-cm specimen. The resection specimen contained an intramuscular, mobile, encapsulated 2.0-cm × 1.5-cm × 1.0-cm mass with 2 components. The first was a tan-white solid mass containing thin deposits of calcified matrix, and the second, which surrounded the first, was composed of well-circumscribed soft yellow lobulated adipose tissue (Figure 5).
After surgery, the patient’s dermatologist performed a shave biopsy of a lentiginous lesion anterior to the knee. Subsequently, the patient began having increasing knee pain and developed, on the lower extremity, small areas of erythema that were attributed to mild cellulitis. Four months after surgery, emergent contrast-enhanced MRI showed enhancement of thickened synovium of the knee joint (Figure 7).
Since the lavage, the patient remained in good condition. There was no evidence of local recurrence on contrast-enhanced MRI (Figure 8), or metastases the first year, and she remained clinically free of disease the first 22 months of follow-up.
Discussion
Extraosseous osteosarcoma, typically a high-grade malignant neoplasm of the soft tissues that produces osteoid or cartilaginous matrix, is histologically indistinguishable from osteosarcoma of bone.
Conventional lipoma, the most common subtype of lipoma, is a benign mesenchymal tumor. Other subtypes are hibernoma, fibrolipoma, angiolipoma, myelolipoma, spindle-cell lipoma, pleomorphic lipoma, and atypical lipomatous tumor.7 Atypical lipomatous tumor and well-differentiated liposarcoma are distinguished from each other by location: The World Health Organization recommends the term atypical lipomatous tumor for tumors that arise in the extremities and trunk lesions and well-differentiated liposarcoma for neoplasms that develop in the retroperitoneum, peritoneum, mediastinum, spermatic cord, and thoracic cavity.8 On PET, hypermetabolic activity is nonspecific and can be seen in malignant tumors and some benign reactive processes, such as evolving heterotopic ossification. However, simple lipomas, including those with mature ossification or dystrophic calcification, do not manifest increased FDG avidity.9
We are not aware of any published cases of extraosseous osteosarcoma arising within a conventional lipoma. A limited number of cases of coexisting conventional lipoma and spindle-cell lipoma or liposarcoma have been reported.10-13 Retroperitoneal liposarcoma with areas of dedifferentiation into osteosarcoma has also been described.14 Development of malignant fibrous histiocytoma and liposarcoma have also been reported within intraosseous lipomas.15 One theory is based on premalignancy as a biological concept as opposed to a morphologic one. In other words, lesions that may be considered morphologically benign may already have the biological phenotype for malignancy that is not yet reflected morphologically.16 However, it has been suggested that such findings may instead result from initial sampling error or histologic misdiagnosis.17,18There is a spectrum of findings on imaging studies of extraosseous osteosarcoma. Plain radiographs show a soft-tissue density with variable degrees of central calcification that reflects mineralization of deposited neoplastic bone. The pattern of calcification is characteristically amorphous or cloudlike, as opposed to the ring-and-arc observed in cartilage matrix. On CT, the soft-tissue mass of extraosseous osteosarcoma is separate from the underlying bone and periosteum—a defining characteristic that distinguishes it from conventional intramedullary and juxtacortical osteosarcoma.6 The central pattern of amorphous calcification helps to differentiate extraosseous osteosarcoma from heterotopic ossification, which characteristically demonstrates zonation, with trabecular architecture and mature cortical bone peripherally.1 Enhancement of extraskeletal osteosarcoma tends to be heterogeneous and depends on the quantity of necrosis. Extraskeletal osteosarcoma tends to be isointense on T1-weighted MRI and mildly hyperintense on T2-weighted MRI.1,6 Areas of very low signal intensity on both T1- and T2-weighted MRI may reflect mineralization.19 If intratumoral hemorrhage has occurred, there may be signal intensity of blood products of various ages.1,3 Tumors with abundant hemorrhage can be mistaken for hematoma. FDG-PET radiotracer accumulation tends to be intense peripherally with variable central activity depending on quantity of necrosis and hemorrhage.1The radiologic differential diagnosis includes myositis ossificans, chondrosseous lipoma, parosteal lipoma (ossifying variant), liposarcoma with metaplastic bone, dedifferentiated liposarcoma with osteosarcoma or chondrosarcoma component, and malignant mesenchymoma. Other common soft-tissue sarcomas, such as fibrosarcoma, leiomyosarcoma, and pleomorphic undifferentiated sarcoma, are excluded by the presence of fat within the tumor. The radiographic pattern of osteoid matrix produced by the tumor in our patient may be seen in heterotopic ossification, but the absence of mature ossification with zonation was evidence against heterotopic ossification, and microscopically it was neoplastic rather than reactive osteoid. In addition, it is possible that, because of the small size of the soft-tissue component, it was difficult to appreciate the less mature osteoid matrix peripherally. The lack of characteristic rings and arcs helps exclude benign and malignant cartilage containing neoplasms. Malignant mesenchymoma is a diagnosis of exclusion, and such tumors are usually better classified as sarcomas that have undergone heterologous differentiation.
The histologic diagnosis of extraosseous osteosarcoma requires identification of malignant mesenchymal cells that secrete neoplastic osteoid that may or may not mineralize. It is important to exclude the possibility that the malignant bone-forming tumor is part of a different type of sarcoma, the most common being dedifferentiated liposarcoma. Immunohistochemistry can be helpful in this situation, as dedifferentiated liposarcomas demonstrate nuclear expression of MDM2, CDK4, and p16, a constellation of findings rare in conventional and extraosseous osteosarcoma.20-23 Osteosarcoma has not previously been reported as arising in a lipoma; in our patient’s case, we excluded the possibility that the fatty component represented an underlying atypical lipomatous tumor/well-differentiated or dedifferentiated liposarcoma on the basis of morphology and lack of expression of MDM2, CDK4, and p16.
Although histologically identical to osteosarcoma of bone, extraosseous osteosarcoma is treated differently because of its relatively decreased chemosensitivity and radiosensitivity. Treatment tends to be focused on limb-sparing wide local excision, and local recurrence complicates about 50% of cases.1 Neoadjuvant or adjuvant treatment with radiation or chemotherapy is often provided.6 Platinum and doxorubicin chemotherapeutic agents, which are first-line treatments for osteosarcoma of bone, tend to be less effective in extraosseous osteosarcoma, and ifosfamide is more often used instead.5
Primary extraosseous osteosarcoma classically has a poor prognosis, with 2- to 3-year mortality of 50%, and prognosis tends to be worse for secondary radiation-induced sarcomas than for primary sarcomas.2,6 However, with there being improved treatment protocols involving surgery and chemoradiation, more recent 5-year survival rates without metastatic disease are between 60% and 80%, though there is no definite consensus regarding the optimal systemic therapy regimen.1,24 In a 2014 review of 53 patients who presented with localized disease, Choi and colleagues25 identified a 3-year cumulative 39% incidence of death caused by disease, and in 2016 Sio and colleagues26 reported that 55% of patients, most of whom had stage 3 disease, were alive at median follow-up of 45 months. Similar to osteosarcoma of bone, metastases may develop up to 10 years after primary treatment and are most commonly to the lung (80%-88%). Because extraosseous osteosarcoma is rare, no definite prognostic factors have been determined, but metastases at presentation and large tumor size (>5 cm) likely portend a worse prognosis.2,3,27 Fibroblastic and chondroblastic subtypes may have a slightly better prognosis.6,28
Conclusion
Extraosseous osteosarcoma is a rare malignancy that should be considered in the appropriate clinical and imaging scenario. This article is the first report of a case of a radiation-associated extraosseous osteosarcoma that developed within a lipoma with preoperative and postoperative multimodality imaging.
Am J Orthop. 2017;46(3):E200-E206. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
1. Mc Auley G, Jagannathan J, O’Regan K, et al. Extraskeletal osteosarcoma: spectrum of imaging findings. AJR Am J Roentgenol. 2012;198(1):W31-W37.
2. Vikram S, Salih S, Krishnan A, et al. Radiation-induced extra-osseous osteosarcoma—a case report and review of literature. Indian J Surg Oncol. 2013;4(4):374-377.
3. Rosenberg AE. Extraskeletal osteosarcoma. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed. Lyon, France: IARC; 2013:161-162.
4. Ramnani BG, Kumar A, Chandak S, Ranjan A, Patel MK. Clinicopathological profile of benign soft tissue tumours: a study in a tertiary care hospital in Western India. J Clin Diagn Res. 2014;8(10):FC01-FC04.
5. Ahmad SA, Patel SR, Ballo MT, et al. Extraosseous osteosarcoma: response to treatment and long-term outcome. J Clin Oncol. 2002;20(2):521-527.
6. Mavrogenis AF, Papadogeorgou E, Papagelopoulos PJ. Extraskeletal osteosarcoma: a case report. Acta Orthop Traumatol Turc. 2012;46(3):215-219.
7. Morell N, Quinn RH. Lipoma. orthoinfo.aaos.org/topic.cfm?topic=a00631. Published 2012. Accessed December 28, 2014.
8. Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, Foster WC, Temple HT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99-104.
9. Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661-670.
10. Laliotis A, De Bree E, Vasilaki S, Papadakis M, Melissas J. Co-existence of intramuscular spindle cell lipoma with an intramuscular ordinary lipoma: report of a case. Pol J Pathol. 2013;64(3):224-227.
11. Wright C. Liposarcoma arising in a simple lipoma. J Pathol Bacteriol. 1948;60:483-487.
12. Sampson CC, Saunders EH, Green WE, Laurey JR. Liposarcoma developing in a lipoma. Arch Pathol. 1960;69:506-510.
13. Sternberg SS. Liposarcoma arising within a subcutaneous lipoma. Cancer. 1952;5(5):975-978.
14. Ho L, Wassef H, Chang D, Boswell W, Henderson R, Seto J. Liposarcoma of the retroperitoneum with dedifferentiation to osteosarcoma: a case report. Clin Nucl Med. 2011;36(5):400-402.
15. Milgram JW. Malignant transformation in bone lipomas. Skeletal Radiol. 1990;19(5):347-352.
16. Mentzel T. Biological continuum of benign, atypical, and malignant mesenchymal neoplasms—does it exist? J Pathol. 2000;190(5):523-525.
17. Murphey MD, Carroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of the AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
18. Zornig C, Schröder S. Does malignant transformation of benign soft-tissue tumours occur? A clinicomorphological study of ten initially misdiagnosed soft-tissue sarcomas. J Cancer Res Clin Oncol. 1992;118(2):166-169.
19. Dönmez FY, Tüzün U, Başaran C, Tunaci M, Bilgiç B, Acunaş G. MRI findings in parosteal osteosarcoma: correlation with histopathology. Diagn Interv Radiol. 2008;14(3):142-152.
20. Mariño-Enriquez A, Hornick JL, Dal Cin P, Cibas ES, Qian X. Dedifferentiated liposarcoma and pleomorphic liposarcoma: a comparative study of cytomorphology and MDM2/CDK4 expression on fine-needle aspiration. Cancer Cytopathol. 2014;122(2):128-137.
21. Yoshida A, Ushiku T, Motoi T, et al. MDM2 and CDK4 immunohistochemical coexpression in high-grade osteosarcoma: correlation with a dedifferentiated subtype. Am J Surg Pathol. 2012;36(3):423-431.
22. Thway K, Flora R, Shah C, Olmos D, Fisher C. Diagnostic utility of p16, CDK4, and MDM2 as an immunohistochemical panel in distinguishing well-differentiated and dedifferentiated liposarcomas from other adipocytic tumors. Am J Surg Pathol. 2012;36(3):462-469.
23. Lokka S, Scheel AH, Dango S, et al. Challenging dedifferentiated liposarcoma identified by MDM2-amplification, a report of two cases. BMC Clin Pathol. 2014;14:36.
24. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015.
25. Choi LE, Healey JH, Kuk D, Brennan MF. Analysis of outcomes in extraskeletal osteosarcoma: a review of fifty-three cases. J Bone Joint Surg Am. 2014;96(1):e2.
26. Sio TT, Vu CC, Sohawon S, et al. Extraskeletal osteosarcoma: an international Rare Cancer Network study. Am J Clin Oncol. 2016;39(1):32-36.
27. Bane BL, Evans HL, Ro JY, et al. Extraskeletal osteosarcoma. A clinicopathologic review of 26 cases. Cancer. 1990;65(12):2762-2770.
28. Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76(11):2253-2259.
Exercise tops NSAIDs for knee osteoarthritis
LAS VEGAS – After participating in an 8-week neuromuscular exercise therapy program, patients with mild to moderate knee osteoarthritis showed significantly greater symptomatic improvement at 12 months of follow-up than if they had been instructed to treat with analgesics and anti-inflammatory agents in the randomized EXERPHARMA trial.
“Neuromuscular exercise could be the superior choice for long-term relief of symptoms such as swelling, stiffness, and catching, while avoiding the potential side effects of analgesics and anti-inflammatory drugs,” Anders Holsgaard-Larsen, MD, said in his presentation of the 1-year study results at the World Congress on Osteoarthritis.
Ninety-three patients with mild to moderate medial knee osteoarthritis – “a group we commonly see in primary care,” he noted – were randomized to the structured 8-week neuromuscular exercise therapy program or to 8 weeks of instruction in the appropriate use of NSAIDs and acetaminophen. The exercise program entailed two hour-long, physical therapist-supervised sessions per week, which included functional, proprioceptive, strength, and endurance exercises of three or four progressive degrees of difficulty.
The initial results obtained at the conclusion of the 8-week interventions – change in knee joint load while walking – have been published (Osteoarthritis Cartilage. 2017 Apr;25[4]:470-80). There was no significant difference between the two study groups. But outcomes at the prespecified 12-month follow-up designed to capture any late improvement were a different story, Dr. Holsgaard-Larsen said at the congress sponsored by the Osteoarthritis Research Society International.
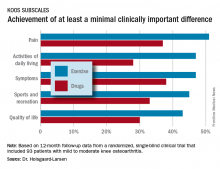
The neuromuscular exercise therapy group showed a significantly greater improvement on the Knee Injury and Osteoarthritis Scores (KOOS) symptom subscale at 12 months: a mean 10.9-point improvement from baseline, compared with a 3.3-point improvement with drug therapy.
That being said, the prespecified primary endpoint at 12 months was change on the activities of daily living subscale – and this between-group difference didn’t reach statistical significance. So technically EXERPHARMA was a negative study, according to the investigator.
That comment caused audience member Theodore P. Pincus, MD, to rise in protest.
“I might argue that perhaps we’ve gotten too focused on P-values rather than looking at the whole picture. In my opinion, your hypothesis is more validated than you seem to think,” said Dr. Pincus, professor of rheumatology at Rush University in Chicago.
The EXERPHARMA trial was funded by the Danish Rheumatism Association and other noncommercial research support. Dr. Holsgaard-Larsen reported having no financial conflicts of interest.
LAS VEGAS – After participating in an 8-week neuromuscular exercise therapy program, patients with mild to moderate knee osteoarthritis showed significantly greater symptomatic improvement at 12 months of follow-up than if they had been instructed to treat with analgesics and anti-inflammatory agents in the randomized EXERPHARMA trial.
“Neuromuscular exercise could be the superior choice for long-term relief of symptoms such as swelling, stiffness, and catching, while avoiding the potential side effects of analgesics and anti-inflammatory drugs,” Anders Holsgaard-Larsen, MD, said in his presentation of the 1-year study results at the World Congress on Osteoarthritis.
Ninety-three patients with mild to moderate medial knee osteoarthritis – “a group we commonly see in primary care,” he noted – were randomized to the structured 8-week neuromuscular exercise therapy program or to 8 weeks of instruction in the appropriate use of NSAIDs and acetaminophen. The exercise program entailed two hour-long, physical therapist-supervised sessions per week, which included functional, proprioceptive, strength, and endurance exercises of three or four progressive degrees of difficulty.
The initial results obtained at the conclusion of the 8-week interventions – change in knee joint load while walking – have been published (Osteoarthritis Cartilage. 2017 Apr;25[4]:470-80). There was no significant difference between the two study groups. But outcomes at the prespecified 12-month follow-up designed to capture any late improvement were a different story, Dr. Holsgaard-Larsen said at the congress sponsored by the Osteoarthritis Research Society International.
The neuromuscular exercise therapy group showed a significantly greater improvement on the Knee Injury and Osteoarthritis Scores (KOOS) symptom subscale at 12 months: a mean 10.9-point improvement from baseline, compared with a 3.3-point improvement with drug therapy.
That being said, the prespecified primary endpoint at 12 months was change on the activities of daily living subscale – and this between-group difference didn’t reach statistical significance. So technically EXERPHARMA was a negative study, according to the investigator.
That comment caused audience member Theodore P. Pincus, MD, to rise in protest.
“I might argue that perhaps we’ve gotten too focused on P-values rather than looking at the whole picture. In my opinion, your hypothesis is more validated than you seem to think,” said Dr. Pincus, professor of rheumatology at Rush University in Chicago.
The EXERPHARMA trial was funded by the Danish Rheumatism Association and other noncommercial research support. Dr. Holsgaard-Larsen reported having no financial conflicts of interest.
LAS VEGAS – After participating in an 8-week neuromuscular exercise therapy program, patients with mild to moderate knee osteoarthritis showed significantly greater symptomatic improvement at 12 months of follow-up than if they had been instructed to treat with analgesics and anti-inflammatory agents in the randomized EXERPHARMA trial.
“Neuromuscular exercise could be the superior choice for long-term relief of symptoms such as swelling, stiffness, and catching, while avoiding the potential side effects of analgesics and anti-inflammatory drugs,” Anders Holsgaard-Larsen, MD, said in his presentation of the 1-year study results at the World Congress on Osteoarthritis.
Ninety-three patients with mild to moderate medial knee osteoarthritis – “a group we commonly see in primary care,” he noted – were randomized to the structured 8-week neuromuscular exercise therapy program or to 8 weeks of instruction in the appropriate use of NSAIDs and acetaminophen. The exercise program entailed two hour-long, physical therapist-supervised sessions per week, which included functional, proprioceptive, strength, and endurance exercises of three or four progressive degrees of difficulty.
The initial results obtained at the conclusion of the 8-week interventions – change in knee joint load while walking – have been published (Osteoarthritis Cartilage. 2017 Apr;25[4]:470-80). There was no significant difference between the two study groups. But outcomes at the prespecified 12-month follow-up designed to capture any late improvement were a different story, Dr. Holsgaard-Larsen said at the congress sponsored by the Osteoarthritis Research Society International.
The neuromuscular exercise therapy group showed a significantly greater improvement on the Knee Injury and Osteoarthritis Scores (KOOS) symptom subscale at 12 months: a mean 10.9-point improvement from baseline, compared with a 3.3-point improvement with drug therapy.
That being said, the prespecified primary endpoint at 12 months was change on the activities of daily living subscale – and this between-group difference didn’t reach statistical significance. So technically EXERPHARMA was a negative study, according to the investigator.
That comment caused audience member Theodore P. Pincus, MD, to rise in protest.
“I might argue that perhaps we’ve gotten too focused on P-values rather than looking at the whole picture. In my opinion, your hypothesis is more validated than you seem to think,” said Dr. Pincus, professor of rheumatology at Rush University in Chicago.
The EXERPHARMA trial was funded by the Danish Rheumatism Association and other noncommercial research support. Dr. Holsgaard-Larsen reported having no financial conflicts of interest.
AT OARSI 2017
Key clinical point:
Major finding: At 12 months of follow-up, patients with knee osteoarthritis who had been assigned to an 8-week structured neuromuscular exercise therapy program had a mean 10.9-point improvement on the KOOS symptom subscale, significantly better than the 3.3-point improvement in patients assigned to primary therapy with NSAIDs and acetaminophen.
Data source: This randomized, single-blind clinical trial included 93 patients with mild to moderate knee osteoarthritis.
Disclosures: The EXERPHARMA trial was funded by the Danish Rheumatism Association and other noncommercial research support. The presenter reported having no financial conflicts of interest.
Poll: How have you changed opioid prescribing in your practice?
[polldaddy:9772633]
[polldaddy:9772633]
[polldaddy:9772633]
GOP senators shutting out physician input on reform – ASCO CEO
WASHINGTON – Physician associations are having an easy time finding people on Capitol Hill willing to listen when it comes to health care reform, but it appears that the conversations are falling on deaf ears, according to Clifford Hudis, MD, CEO of the American Society of Clinical Oncology.
“I am dismayed that our opinions are not being valued,” Dr. Hudis said at a policy summit hosted by the National Comprehensive Cancer Network.
He was critical of the current skyrocketing prices of oncology treatments but noted that, the way the current market is set up, there is no incentive anywhere in the system to put any pressure on manufacturers.
“Right now, the market forces, such as they are, not only allow this but encourage it and probably will continue to do so for a little longer,” he said, adding that the environment is such that “a majority of Americans want the government to do something about price. Think about that for a minute. That is a call for regulation, indirectly or directly. That’s a call for oversight. It’s a call for something different from the traditional open workings of a free market.”
He noted that, at least conceptually, the Trump administration is following in the footsteps of the Obama administration with an emphasis on value over volume, shared risk, and looking for improved outcomes.
In that vein, Dr. Hudis said there are rumblings that the much-criticized Part B demonstration that would put more emphasis on value-based payments to physicians for Part B drugs could return in some form, though he had no further details.
“Our position is that physicians should be accountable for utilization, for quality of care, and not for the price of the drug at market entry,” he said, noting ASCO’s opposition to the Part B demonstration as it was originally proposed.
That demonstration also highlighted another issue of value, particularly when there are no traditional market forces in play to help exert downward pressure on prices.
Defining value is problematic, and Dr. Hudis criticized the many value frameworks – including ASCO’s – as a signal that there is not enough being done to let the market truly make its determination on what is value.
“When we superimpose something like the value framework on this, we are essentially admitting a kind of defeat,” he said. “We are admitting that there won’t be traditional bid-counterbid price setting. There will instead be a declaration of value ultimately leading to pricing.”
“They are a declaration that somebody, through opinion or formula, will start to tell you collectively what a therapy is worth,” he added. “That’s like somebody telling you what an automobile is worth. It doesn’t really work that way in the rest of your world. You make a judgment for yourself about what something is worth. … One thing I know for sure is that for most patients, most new drugs are not worth their current price.”
WASHINGTON – Physician associations are having an easy time finding people on Capitol Hill willing to listen when it comes to health care reform, but it appears that the conversations are falling on deaf ears, according to Clifford Hudis, MD, CEO of the American Society of Clinical Oncology.
“I am dismayed that our opinions are not being valued,” Dr. Hudis said at a policy summit hosted by the National Comprehensive Cancer Network.
He was critical of the current skyrocketing prices of oncology treatments but noted that, the way the current market is set up, there is no incentive anywhere in the system to put any pressure on manufacturers.
“Right now, the market forces, such as they are, not only allow this but encourage it and probably will continue to do so for a little longer,” he said, adding that the environment is such that “a majority of Americans want the government to do something about price. Think about that for a minute. That is a call for regulation, indirectly or directly. That’s a call for oversight. It’s a call for something different from the traditional open workings of a free market.”
He noted that, at least conceptually, the Trump administration is following in the footsteps of the Obama administration with an emphasis on value over volume, shared risk, and looking for improved outcomes.
In that vein, Dr. Hudis said there are rumblings that the much-criticized Part B demonstration that would put more emphasis on value-based payments to physicians for Part B drugs could return in some form, though he had no further details.
“Our position is that physicians should be accountable for utilization, for quality of care, and not for the price of the drug at market entry,” he said, noting ASCO’s opposition to the Part B demonstration as it was originally proposed.
That demonstration also highlighted another issue of value, particularly when there are no traditional market forces in play to help exert downward pressure on prices.
Defining value is problematic, and Dr. Hudis criticized the many value frameworks – including ASCO’s – as a signal that there is not enough being done to let the market truly make its determination on what is value.
“When we superimpose something like the value framework on this, we are essentially admitting a kind of defeat,” he said. “We are admitting that there won’t be traditional bid-counterbid price setting. There will instead be a declaration of value ultimately leading to pricing.”
“They are a declaration that somebody, through opinion or formula, will start to tell you collectively what a therapy is worth,” he added. “That’s like somebody telling you what an automobile is worth. It doesn’t really work that way in the rest of your world. You make a judgment for yourself about what something is worth. … One thing I know for sure is that for most patients, most new drugs are not worth their current price.”
WASHINGTON – Physician associations are having an easy time finding people on Capitol Hill willing to listen when it comes to health care reform, but it appears that the conversations are falling on deaf ears, according to Clifford Hudis, MD, CEO of the American Society of Clinical Oncology.
“I am dismayed that our opinions are not being valued,” Dr. Hudis said at a policy summit hosted by the National Comprehensive Cancer Network.
He was critical of the current skyrocketing prices of oncology treatments but noted that, the way the current market is set up, there is no incentive anywhere in the system to put any pressure on manufacturers.
“Right now, the market forces, such as they are, not only allow this but encourage it and probably will continue to do so for a little longer,” he said, adding that the environment is such that “a majority of Americans want the government to do something about price. Think about that for a minute. That is a call for regulation, indirectly or directly. That’s a call for oversight. It’s a call for something different from the traditional open workings of a free market.”
He noted that, at least conceptually, the Trump administration is following in the footsteps of the Obama administration with an emphasis on value over volume, shared risk, and looking for improved outcomes.
In that vein, Dr. Hudis said there are rumblings that the much-criticized Part B demonstration that would put more emphasis on value-based payments to physicians for Part B drugs could return in some form, though he had no further details.
“Our position is that physicians should be accountable for utilization, for quality of care, and not for the price of the drug at market entry,” he said, noting ASCO’s opposition to the Part B demonstration as it was originally proposed.
That demonstration also highlighted another issue of value, particularly when there are no traditional market forces in play to help exert downward pressure on prices.
Defining value is problematic, and Dr. Hudis criticized the many value frameworks – including ASCO’s – as a signal that there is not enough being done to let the market truly make its determination on what is value.
“When we superimpose something like the value framework on this, we are essentially admitting a kind of defeat,” he said. “We are admitting that there won’t be traditional bid-counterbid price setting. There will instead be a declaration of value ultimately leading to pricing.”
“They are a declaration that somebody, through opinion or formula, will start to tell you collectively what a therapy is worth,” he added. “That’s like somebody telling you what an automobile is worth. It doesn’t really work that way in the rest of your world. You make a judgment for yourself about what something is worth. … One thing I know for sure is that for most patients, most new drugs are not worth their current price.”
AT NCCN POLICY SUMMIT
HCV/HIV-coinfected teens rapidly progress to advanced liver disease
MADRID – Roughly one in four children with vertically transmitted hepatitis C virus (HCV)/HIV coinfection will progress to advanced hepatic fibrosis by age 20 years despite treatment with pegylated interferon plus ribavirin, Carolina Fernández McPhee, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This rate was nearly five times higher than in matched children with vertically acquired HCV monoinfection in a multicenter retrospective study, according to Dr. McPhee of Hospital General Universitario Gregorio Marañón in Madrid.
She presented a multicenter retrospective study of liver disease progression in 71 HCV/HIV coinfected children and 71 age- and sex-matched HCV-monoinfected children. The coinfected children are being followed in CORISPES (the Spanish Cohort of HIV-infected Children), where they receive state-of-the-art care.
All was quiet through age 9 years, with no progression to liver fibrosis in either patient group. Among patients followed to age 20 years, however, 9 (24%) of 38 HCV/HIV-coinfected patients showed progression to advanced fibrosis, compared with just 3 (6%) of 54 patients with HCV only.
Of HCV/HIV coinfected patients, 73% were infected with the hard to treat viral genotypes 1 or 4, compared with 93% of patients with HCV-only.
In the study group, 22 patients with HCV/HIV and 52 patients with HCV-only underwent treatment with pegylated interferon and ribavirin. At the time of treatment, three coinfected patients already had cirrhosis, another eight had moderate to advanced fibrosis, and half had no or mild fibrosis. In contrast, only one patient with HCV monoinfection had cirrhosis, four had moderate to advanced fibrosis, and the rest – nearly 90% of the total group – had no or mild fibrosis.
At treatment initiation, 96% of the HCV/HIV group were on antiretroviral therapy, 86% showed suppression of HIV RNA, 44% had AIDS, and 32% had a CD4 count below 500 cells/mm3.
The sustained viral response rate was similar in the two groups of patients – 41% in the HCV/HIV group and 42% in HCV-only patients – despite the fact that the HCV monoinfected patients had a higher prevalence of the tough to treat genotypes.
Roughly 12 million people worldwide are coinfected with HCV and HIV.
Dr. McPhee reported having no relevant financial disclosures.
MADRID – Roughly one in four children with vertically transmitted hepatitis C virus (HCV)/HIV coinfection will progress to advanced hepatic fibrosis by age 20 years despite treatment with pegylated interferon plus ribavirin, Carolina Fernández McPhee, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This rate was nearly five times higher than in matched children with vertically acquired HCV monoinfection in a multicenter retrospective study, according to Dr. McPhee of Hospital General Universitario Gregorio Marañón in Madrid.
She presented a multicenter retrospective study of liver disease progression in 71 HCV/HIV coinfected children and 71 age- and sex-matched HCV-monoinfected children. The coinfected children are being followed in CORISPES (the Spanish Cohort of HIV-infected Children), where they receive state-of-the-art care.
All was quiet through age 9 years, with no progression to liver fibrosis in either patient group. Among patients followed to age 20 years, however, 9 (24%) of 38 HCV/HIV-coinfected patients showed progression to advanced fibrosis, compared with just 3 (6%) of 54 patients with HCV only.
Of HCV/HIV coinfected patients, 73% were infected with the hard to treat viral genotypes 1 or 4, compared with 93% of patients with HCV-only.
In the study group, 22 patients with HCV/HIV and 52 patients with HCV-only underwent treatment with pegylated interferon and ribavirin. At the time of treatment, three coinfected patients already had cirrhosis, another eight had moderate to advanced fibrosis, and half had no or mild fibrosis. In contrast, only one patient with HCV monoinfection had cirrhosis, four had moderate to advanced fibrosis, and the rest – nearly 90% of the total group – had no or mild fibrosis.
At treatment initiation, 96% of the HCV/HIV group were on antiretroviral therapy, 86% showed suppression of HIV RNA, 44% had AIDS, and 32% had a CD4 count below 500 cells/mm3.
The sustained viral response rate was similar in the two groups of patients – 41% in the HCV/HIV group and 42% in HCV-only patients – despite the fact that the HCV monoinfected patients had a higher prevalence of the tough to treat genotypes.
Roughly 12 million people worldwide are coinfected with HCV and HIV.
Dr. McPhee reported having no relevant financial disclosures.
MADRID – Roughly one in four children with vertically transmitted hepatitis C virus (HCV)/HIV coinfection will progress to advanced hepatic fibrosis by age 20 years despite treatment with pegylated interferon plus ribavirin, Carolina Fernández McPhee, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This rate was nearly five times higher than in matched children with vertically acquired HCV monoinfection in a multicenter retrospective study, according to Dr. McPhee of Hospital General Universitario Gregorio Marañón in Madrid.
She presented a multicenter retrospective study of liver disease progression in 71 HCV/HIV coinfected children and 71 age- and sex-matched HCV-monoinfected children. The coinfected children are being followed in CORISPES (the Spanish Cohort of HIV-infected Children), where they receive state-of-the-art care.
All was quiet through age 9 years, with no progression to liver fibrosis in either patient group. Among patients followed to age 20 years, however, 9 (24%) of 38 HCV/HIV-coinfected patients showed progression to advanced fibrosis, compared with just 3 (6%) of 54 patients with HCV only.
Of HCV/HIV coinfected patients, 73% were infected with the hard to treat viral genotypes 1 or 4, compared with 93% of patients with HCV-only.
In the study group, 22 patients with HCV/HIV and 52 patients with HCV-only underwent treatment with pegylated interferon and ribavirin. At the time of treatment, three coinfected patients already had cirrhosis, another eight had moderate to advanced fibrosis, and half had no or mild fibrosis. In contrast, only one patient with HCV monoinfection had cirrhosis, four had moderate to advanced fibrosis, and the rest – nearly 90% of the total group – had no or mild fibrosis.
At treatment initiation, 96% of the HCV/HIV group were on antiretroviral therapy, 86% showed suppression of HIV RNA, 44% had AIDS, and 32% had a CD4 count below 500 cells/mm3.
The sustained viral response rate was similar in the two groups of patients – 41% in the HCV/HIV group and 42% in HCV-only patients – despite the fact that the HCV monoinfected patients had a higher prevalence of the tough to treat genotypes.
Roughly 12 million people worldwide are coinfected with HCV and HIV.
Dr. McPhee reported having no relevant financial disclosures.
AT ESPID 2017
Key clinical point:
Major finding: By age 20 years, 24% of a group of patients with vertically transmitted HCV/HIV coinfection had progressed to advanced hepatic fibrosis, compared with 6% of patients with vertically transmitted HCV monoinfection.
Data source: This retrospective, multicenter, observational Spanish study included 71 children with vertically transmitted HCV/HIV coinfection and 71 with vertically transmitted HCV monoinfection.
Disclosures: Dr. McPhee reported having no relevant financial disclosures.
ODYSSEY: Alirocumab improves lipids but not glycemic targets in 2DM
SAN DIEGO – The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab helped correct dyslipidemia but did not improve glucose control in patients with type 2 diabetes mellitus, investigators reported at the annual scientific sessions of the American Diabetes Association.
In the international, double-blind ODYSSEY DM-Insulin trial, 24 weeks of alirocumab (Praluent, Sanofi and Regeneron) therapy cut low-density lipoprotein (LDL) cholesterol levels by an average of 49% more than placebo (P less than .0001), said Lawrence A. Leiter, MD, professor of medicine and nutritional sciences at the University of Toronto. Alirocumab also significantly reduced non–high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a) levels. However, hemoglobin A1c, fasting plasma glucose, total insulin dose, and number of antihyperglycemic drugs remained nearly identical between the trial arms throughout follow-up.
About 94% of patients in each arm completed the trial. Most were in their mid-60s, white, obese, and already on a moderate or high-intensity statin, with baseline fasting plasma glucose levels of about 150 mg per dL and HbA1c levels of 7.5%. The most common treatment-associated adverse events were myalgia (4%) and arthralgia (3%). Rates of local and systemic allergic drug reactions, neurologic or neurocognitive events, and elevated transaminases were low and similar between groups, according to Dr. Leiter, who is also director of the lipid clinic at the Li Ka Shing Knowledge Institute at St. Michael’s Hospital.
Robert R. Henry, MD, who is professor of medicine at the University of California, San Diego, discussed the ODYSSEY DM-Dyslipidemia trial which compared alirocumab with usual care in patients with type 2 diabetes whose mixed dyslipidemia was inadequately controlled with maximum tolerable statin therapy. In all, 413 patients received open-label alirocumab (75 mg–150 mg) or placebo plus optional ezetimibe, fenofibrate, omega-3 fatty acids, or nicotinic acid every 2 weeks for 24 weeks. At the end of treatment, non-HDL cholesterol dropped about 33% more with alirocumab than usual care (P less than .0001). Alirocumab also produced significant declines in LDL cholesterol, apolipoprotein B, total cholesterol, and lipoprotein(a), and a 6% increase in HDL cholesterol as compared with usual care. Once again, alirocumab induced no changes in HbA1c or fasting plasma glucose levels. The most common treatment-related adverse events were urinary tract infections, diarrhea, and nasopharyngitis.
Sanofi US and Regeneron Pharmaceuticals make alirocumab and funded the trials. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Henry disclosed consulting and advisory relationships with Sanofi and many other pharmaceutical companies.
SAN DIEGO – The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab helped correct dyslipidemia but did not improve glucose control in patients with type 2 diabetes mellitus, investigators reported at the annual scientific sessions of the American Diabetes Association.
In the international, double-blind ODYSSEY DM-Insulin trial, 24 weeks of alirocumab (Praluent, Sanofi and Regeneron) therapy cut low-density lipoprotein (LDL) cholesterol levels by an average of 49% more than placebo (P less than .0001), said Lawrence A. Leiter, MD, professor of medicine and nutritional sciences at the University of Toronto. Alirocumab also significantly reduced non–high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a) levels. However, hemoglobin A1c, fasting plasma glucose, total insulin dose, and number of antihyperglycemic drugs remained nearly identical between the trial arms throughout follow-up.
About 94% of patients in each arm completed the trial. Most were in their mid-60s, white, obese, and already on a moderate or high-intensity statin, with baseline fasting plasma glucose levels of about 150 mg per dL and HbA1c levels of 7.5%. The most common treatment-associated adverse events were myalgia (4%) and arthralgia (3%). Rates of local and systemic allergic drug reactions, neurologic or neurocognitive events, and elevated transaminases were low and similar between groups, according to Dr. Leiter, who is also director of the lipid clinic at the Li Ka Shing Knowledge Institute at St. Michael’s Hospital.
Robert R. Henry, MD, who is professor of medicine at the University of California, San Diego, discussed the ODYSSEY DM-Dyslipidemia trial which compared alirocumab with usual care in patients with type 2 diabetes whose mixed dyslipidemia was inadequately controlled with maximum tolerable statin therapy. In all, 413 patients received open-label alirocumab (75 mg–150 mg) or placebo plus optional ezetimibe, fenofibrate, omega-3 fatty acids, or nicotinic acid every 2 weeks for 24 weeks. At the end of treatment, non-HDL cholesterol dropped about 33% more with alirocumab than usual care (P less than .0001). Alirocumab also produced significant declines in LDL cholesterol, apolipoprotein B, total cholesterol, and lipoprotein(a), and a 6% increase in HDL cholesterol as compared with usual care. Once again, alirocumab induced no changes in HbA1c or fasting plasma glucose levels. The most common treatment-related adverse events were urinary tract infections, diarrhea, and nasopharyngitis.
Sanofi US and Regeneron Pharmaceuticals make alirocumab and funded the trials. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Henry disclosed consulting and advisory relationships with Sanofi and many other pharmaceutical companies.
SAN DIEGO – The proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor alirocumab helped correct dyslipidemia but did not improve glucose control in patients with type 2 diabetes mellitus, investigators reported at the annual scientific sessions of the American Diabetes Association.
In the international, double-blind ODYSSEY DM-Insulin trial, 24 weeks of alirocumab (Praluent, Sanofi and Regeneron) therapy cut low-density lipoprotein (LDL) cholesterol levels by an average of 49% more than placebo (P less than .0001), said Lawrence A. Leiter, MD, professor of medicine and nutritional sciences at the University of Toronto. Alirocumab also significantly reduced non–high-density lipoprotein cholesterol, apolipoprotein B, and lipoprotein(a) levels. However, hemoglobin A1c, fasting plasma glucose, total insulin dose, and number of antihyperglycemic drugs remained nearly identical between the trial arms throughout follow-up.
About 94% of patients in each arm completed the trial. Most were in their mid-60s, white, obese, and already on a moderate or high-intensity statin, with baseline fasting plasma glucose levels of about 150 mg per dL and HbA1c levels of 7.5%. The most common treatment-associated adverse events were myalgia (4%) and arthralgia (3%). Rates of local and systemic allergic drug reactions, neurologic or neurocognitive events, and elevated transaminases were low and similar between groups, according to Dr. Leiter, who is also director of the lipid clinic at the Li Ka Shing Knowledge Institute at St. Michael’s Hospital.
Robert R. Henry, MD, who is professor of medicine at the University of California, San Diego, discussed the ODYSSEY DM-Dyslipidemia trial which compared alirocumab with usual care in patients with type 2 diabetes whose mixed dyslipidemia was inadequately controlled with maximum tolerable statin therapy. In all, 413 patients received open-label alirocumab (75 mg–150 mg) or placebo plus optional ezetimibe, fenofibrate, omega-3 fatty acids, or nicotinic acid every 2 weeks for 24 weeks. At the end of treatment, non-HDL cholesterol dropped about 33% more with alirocumab than usual care (P less than .0001). Alirocumab also produced significant declines in LDL cholesterol, apolipoprotein B, total cholesterol, and lipoprotein(a), and a 6% increase in HDL cholesterol as compared with usual care. Once again, alirocumab induced no changes in HbA1c or fasting plasma glucose levels. The most common treatment-related adverse events were urinary tract infections, diarrhea, and nasopharyngitis.
Sanofi US and Regeneron Pharmaceuticals make alirocumab and funded the trials. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly and Company, and several other pharmaceutical companies. Dr. Henry disclosed consulting and advisory relationships with Sanofi and many other pharmaceutical companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point:
Major finding: After 24 weeks of treatment, average low-density lipoprotein levels fell by about 43%-49% (P less than .0001, compared with placebo, in each trial).
Data source: ODYSSEY DM-Insulin included 441 patients on insulin for type 2 diabetes who had elevated LDL levels and other cardiovascular risk factors. ODYSSEY DM-Dyslipidemia included 413 patients with type 2 diabetes and mixed dyslipidemia despite maximally tolerated statins.
Disclosures: Sanofi and Regeneron Pharmaceuticals funded the trial. Dr. Leiter disclosed research grants and consulting relationships with Regeneron, Sanofi, Eli Lilly, and several other pharmaceutical companies.
Aspirin triples major bleeding risk after age 75 years
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
In patients with stroke with a cardiac source of embolism who qualify for oral anticoagulation, we obsess about the association between benefit and bleeding risk. Specific risk scores were developed to assess the bleeding risk for patients with atrial fibrillation who qualified for anticoagulation. However, similar risk scores are not applied for patients who undergo long-term prevention with antiplatelet therapy.
On the basis of this study’s results, the benefit-risk association in long-term antiplatelet therapy should be evaluated every 3-5 years in patients older than 75 years, and PPIs should be used in patients on antiplatelet therapy who are at least 75 years old or in patients with a history of gastrointestinal bleeds.
Hans-Christoph Diener, MD, of the department of neurology at the University Duisburg-Essen in Essen, Germany, made these remarks in an accompanying editorial (Lancet. 2017 Jun 13. doi: 10.1016/S0140-6736[17]31507-6). He disclosed relationships with multiple companies, including AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Novartis.
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
Older adults who take aspirin daily are at greater risk for serious bleeding than previously thought, based on data from roughly 3,000 patients.
“The risk of upper gastrointestinal bleeding on antiplatelet treatment increases with age, but it is uncertain whether older age alone is a sufficient indicator of high risk to justify routine coprescription of PPIs [proton pump inhibitors],” wrote Linxin Li, DPhil, of the University of Oxford (England) and her colleagues.
To assess the rate of bleeding among older adults on long-term aspirin therapy, Dr. Li and her colleagues reviewed data from the Oxford Vascular Study, a prospective population-based study of 3,166 patients. Of those, 1,584 were younger than 75 years, with an average age of 61 years, and 1,582 were at least 75 years old, with average age of 83 years. Patients were followed at 30 days, 6 months, and 1, 5, and 10 years to determine bleeding, recurrent ischemic events, and disability (Lancet. 2017. doi: 10.1016/S0140-6736[17]30770-5).
In addition, more than twice the major upper GI bleeds were disabling or fatal in adults aged 75 years and older than in the younger patients (62% vs. 25%).
Only a third of the patients in the study were taking proton pump inhibitors (PPIs), partly because current clinical guidelines don’t specifically recommend their use and partly in the absence of an accepted definition of which patients are at high risk for upper GI bleeding, the researchers said. They estimated that the number needed to treat with PPIs to prevent a major GI bleed after 5 years decreased with age: “80 for patients younger than 65 years, 75 for patients aged 65-74 years, 23 for patients aged 75-84 years, and 21 for patients aged 85 years or older.” In addition, the number needed to treat with PPIs to prevent a disabling or fatal upper GI bleed after 5 years was 338 for patients younger than 65 years but dropped to 25 for patients aged 85 years and older.
The findings were limited by the observational nature of the study and inability to show that increased risk of bleeding was caused by aspirin alone, the researchers said. However, based on the data, “age 75 years would be an appropriate threshold to start a PPI both in patients newly initiated on antiplatelet drugs and in patients on established treatment,” they wrote.
The study data were taken from the Oxford Vascular Study, which was funded by the National Institute of Health Research and several other research institutions. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
FROM THE LANCET
Key clinical point:
Major finding: The annual rate of life-threatening or fatal bleeding episodes was less than 0.5% for patients younger than 65 years but rose to 1.5% in those aged 75-84 years and 2.5% in those aged 85 years and older.
Data source: A prospective, population-based cohort study of 3,166 adults who had one transient ischemic attack, ischemic stroke, or MI and who were treated with antiplatelet drugs.
Disclosures: The study data were taken from the Oxford Vascular Study, which was funded by the Wellcome Trust, Wolfson Foundation, British Heart Foundation, Dunhill Medical Trust, the National Institute of Health Research (NIHR), and the NIHR Oxford Biomedical Research Centre. Corresponding author Peter Rothwell, MD, disclosed financial relationships with Bayer.
USPSTF recommends screening children, adolescents for obesity
Children and adolescents aged 6 years and older should be screened for obesity and referred to comprehensive, intensive behavioral interventions with at least 26 hours of intervention contact, according to a U.S. Preventive Services Task Force Recommendation Statement that was published online June 20 in JAMA.
This updated recommendation is largely consistent with the previous 2010 recommendation “but includes the word ‘adolescents’ to further clarify the population to which this recommendation applies,” according to a press release accompanying the Recommendation Statement and the Evidence Report on which it is based.
The behavioral interventions that proved most beneficial included at least 26 hours of contact over a period of 2-12 months. Those that included 52 or more hours of contact achieved even greater weight loss, as well as some improvements in cardiovascular and metabolic risk factors (JAMA. 2017 Jun 20. doi: 10.1001/jama.2017.6803).
In general, children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
The components of these comprehensive interventions varied, but the most successful ones included sessions involving both the child and the parent (separately, together, or both); offered both family and group sessions; provided education regarding healthy eating, exercising, and reading food labels; encouraged stimulus-control measures such as limiting access to unhealthy foods and limiting screen time (that is, physical inactivity); and included supervised physical activity. Additional beneficial components are assisting patients to identify and accomplish goals, self-monitor, and problem-solve, as well as teaching them coping skills and addressing their body image.
In contrast to behavioral interventions, pharmacotherapy was not endorsed by the USPSTF. The current evidence was deemed inadequate to determine whether the slight weight loss achieved with pharmacotherapy is clinically significant and whether it outweighs the harms of the medications.
The two agents currently used in this regard are metformin, which is not Food and Drug Administration–approved for this purpose, and orlistat, which is approved for patients aged 12 years and older. Orlistat in particular frequently causes adverse events including fatty or oily stools, abdominal pain or cramping, flatus with stool discharge, and fecal incontinence, Dr. Grossman and his associates said.
The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures.
Letter to the Editor
It is not surprising that there are so many overweight and obese children, despite the fact that in order to prevent bad feelings, children have to be much more overweight than adults to be classified as obese or overweight.
If one is waiting until age 6 years to screen for obesity, that horse will be long out of the barn. The problem begins when the rapid weight gain of infants does not slow down in the 2nd and 3rd years of life. This is also when bad food choices and eating habits often begin.
The other problem is that body mass index is a terrible tool and so is any indicator that tries to define obesity using only height and weight. None of these distinguish between the muscular child and the slender child with a large belly. Waist to height or body volume measurements are far better indicators.
Lastly, parents have come to see the mildly overweight child as the norm because so many children are. Until parents see pictures of children from a few decades ago and are educated as to what normal looks like, we will have great difficulty making a dent in this problem.
Richard H. Feuille Jr., MD
This USPSTF recommendation simply confirms what pediatric clinicians always do in the everyday care for children and adolescents: monitor growth, counsel on healthy lifestyles, and refer for specialized care when appropriate.
Intensive behavioral interventions are impractical for many families and frequently aren’t covered by insurance. At best, implementing this recommendation will have only a modest effect on obesity in the United States. At worst, it could divert attention and resources away from population-health approaches to prevention and toward weight management programs that are not well equipped to meet the demand and very often don’t exist within local communities.
Improving neighborhood walkability, increasing the availability of healthy foods, and providing safe physical spaces would be more effective at reducing childhood obesity, as would improving school nutrition and curtailing the marketing of sugar-sweetened drinks and other unhealthy foods to children.
Rachel L. J. Thornton, MD, PhD, Raquel G. Hernandez, MD, MPH; Tina L. Cheng, MD, MPH, are in the department of pediatrics at Johns Hopkins University, Baltimore. They reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the USPSTF report (JAMA. 2017;317:2378-80).
This USPSTF recommendation simply confirms what pediatric clinicians always do in the everyday care for children and adolescents: monitor growth, counsel on healthy lifestyles, and refer for specialized care when appropriate.
Intensive behavioral interventions are impractical for many families and frequently aren’t covered by insurance. At best, implementing this recommendation will have only a modest effect on obesity in the United States. At worst, it could divert attention and resources away from population-health approaches to prevention and toward weight management programs that are not well equipped to meet the demand and very often don’t exist within local communities.
Improving neighborhood walkability, increasing the availability of healthy foods, and providing safe physical spaces would be more effective at reducing childhood obesity, as would improving school nutrition and curtailing the marketing of sugar-sweetened drinks and other unhealthy foods to children.
Rachel L. J. Thornton, MD, PhD, Raquel G. Hernandez, MD, MPH; Tina L. Cheng, MD, MPH, are in the department of pediatrics at Johns Hopkins University, Baltimore. They reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the USPSTF report (JAMA. 2017;317:2378-80).
This USPSTF recommendation simply confirms what pediatric clinicians always do in the everyday care for children and adolescents: monitor growth, counsel on healthy lifestyles, and refer for specialized care when appropriate.
Intensive behavioral interventions are impractical for many families and frequently aren’t covered by insurance. At best, implementing this recommendation will have only a modest effect on obesity in the United States. At worst, it could divert attention and resources away from population-health approaches to prevention and toward weight management programs that are not well equipped to meet the demand and very often don’t exist within local communities.
Improving neighborhood walkability, increasing the availability of healthy foods, and providing safe physical spaces would be more effective at reducing childhood obesity, as would improving school nutrition and curtailing the marketing of sugar-sweetened drinks and other unhealthy foods to children.
Rachel L. J. Thornton, MD, PhD, Raquel G. Hernandez, MD, MPH; Tina L. Cheng, MD, MPH, are in the department of pediatrics at Johns Hopkins University, Baltimore. They reported having no relevant financial disclosures. They made these remarks in an editorial accompanying the USPSTF report (JAMA. 2017;317:2378-80).
Children and adolescents aged 6 years and older should be screened for obesity and referred to comprehensive, intensive behavioral interventions with at least 26 hours of intervention contact, according to a U.S. Preventive Services Task Force Recommendation Statement that was published online June 20 in JAMA.
This updated recommendation is largely consistent with the previous 2010 recommendation “but includes the word ‘adolescents’ to further clarify the population to which this recommendation applies,” according to a press release accompanying the Recommendation Statement and the Evidence Report on which it is based.
The behavioral interventions that proved most beneficial included at least 26 hours of contact over a period of 2-12 months. Those that included 52 or more hours of contact achieved even greater weight loss, as well as some improvements in cardiovascular and metabolic risk factors (JAMA. 2017 Jun 20. doi: 10.1001/jama.2017.6803).
In general, children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
The components of these comprehensive interventions varied, but the most successful ones included sessions involving both the child and the parent (separately, together, or both); offered both family and group sessions; provided education regarding healthy eating, exercising, and reading food labels; encouraged stimulus-control measures such as limiting access to unhealthy foods and limiting screen time (that is, physical inactivity); and included supervised physical activity. Additional beneficial components are assisting patients to identify and accomplish goals, self-monitor, and problem-solve, as well as teaching them coping skills and addressing their body image.
In contrast to behavioral interventions, pharmacotherapy was not endorsed by the USPSTF. The current evidence was deemed inadequate to determine whether the slight weight loss achieved with pharmacotherapy is clinically significant and whether it outweighs the harms of the medications.
The two agents currently used in this regard are metformin, which is not Food and Drug Administration–approved for this purpose, and orlistat, which is approved for patients aged 12 years and older. Orlistat in particular frequently causes adverse events including fatty or oily stools, abdominal pain or cramping, flatus with stool discharge, and fecal incontinence, Dr. Grossman and his associates said.
The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures.
Letter to the Editor
It is not surprising that there are so many overweight and obese children, despite the fact that in order to prevent bad feelings, children have to be much more overweight than adults to be classified as obese or overweight.
If one is waiting until age 6 years to screen for obesity, that horse will be long out of the barn. The problem begins when the rapid weight gain of infants does not slow down in the 2nd and 3rd years of life. This is also when bad food choices and eating habits often begin.
The other problem is that body mass index is a terrible tool and so is any indicator that tries to define obesity using only height and weight. None of these distinguish between the muscular child and the slender child with a large belly. Waist to height or body volume measurements are far better indicators.
Lastly, parents have come to see the mildly overweight child as the norm because so many children are. Until parents see pictures of children from a few decades ago and are educated as to what normal looks like, we will have great difficulty making a dent in this problem.
Richard H. Feuille Jr., MD
Children and adolescents aged 6 years and older should be screened for obesity and referred to comprehensive, intensive behavioral interventions with at least 26 hours of intervention contact, according to a U.S. Preventive Services Task Force Recommendation Statement that was published online June 20 in JAMA.
This updated recommendation is largely consistent with the previous 2010 recommendation “but includes the word ‘adolescents’ to further clarify the population to which this recommendation applies,” according to a press release accompanying the Recommendation Statement and the Evidence Report on which it is based.
The behavioral interventions that proved most beneficial included at least 26 hours of contact over a period of 2-12 months. Those that included 52 or more hours of contact achieved even greater weight loss, as well as some improvements in cardiovascular and metabolic risk factors (JAMA. 2017 Jun 20. doi: 10.1001/jama.2017.6803).
In general, children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
The components of these comprehensive interventions varied, but the most successful ones included sessions involving both the child and the parent (separately, together, or both); offered both family and group sessions; provided education regarding healthy eating, exercising, and reading food labels; encouraged stimulus-control measures such as limiting access to unhealthy foods and limiting screen time (that is, physical inactivity); and included supervised physical activity. Additional beneficial components are assisting patients to identify and accomplish goals, self-monitor, and problem-solve, as well as teaching them coping skills and addressing their body image.
In contrast to behavioral interventions, pharmacotherapy was not endorsed by the USPSTF. The current evidence was deemed inadequate to determine whether the slight weight loss achieved with pharmacotherapy is clinically significant and whether it outweighs the harms of the medications.
The two agents currently used in this regard are metformin, which is not Food and Drug Administration–approved for this purpose, and orlistat, which is approved for patients aged 12 years and older. Orlistat in particular frequently causes adverse events including fatty or oily stools, abdominal pain or cramping, flatus with stool discharge, and fecal incontinence, Dr. Grossman and his associates said.
The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at https://www.uspreventiveservicestaskforce.org/Page/Name/conflict-of-interest-disclosures.
Letter to the Editor
It is not surprising that there are so many overweight and obese children, despite the fact that in order to prevent bad feelings, children have to be much more overweight than adults to be classified as obese or overweight.
If one is waiting until age 6 years to screen for obesity, that horse will be long out of the barn. The problem begins when the rapid weight gain of infants does not slow down in the 2nd and 3rd years of life. This is also when bad food choices and eating habits often begin.
The other problem is that body mass index is a terrible tool and so is any indicator that tries to define obesity using only height and weight. None of these distinguish between the muscular child and the slender child with a large belly. Waist to height or body volume measurements are far better indicators.
Lastly, parents have come to see the mildly overweight child as the norm because so many children are. Until parents see pictures of children from a few decades ago and are educated as to what normal looks like, we will have great difficulty making a dent in this problem.
Richard H. Feuille Jr., MD
FROM JAMA
Key clinical point: with at least 26 hours of contact.
Major finding: Children and adolescents who received intensive behavioral intervention showed absolute reductions in BMI z scores of 0.20 and maintained their baseline weight within approximately 5 pounds, while control subjects showed small or no reductions in BMI z scores and typically gained a mean of 5-17 pounds.
Data source: A review of the literature since the previous USPSTF recommendation statement in 2010, including 45 studies of lifestyle-based interventions involving 7,099 overweight and obese children.
Disclosures: The USPSTF is an independent voluntary group supported by the U.S. Agency for Healthcare Research and Quality as mandated by Congress. The authors’ conflicts of interest are available at www.uspreventiveservicestaskforce.org.