User login
E. coli, GBS account for majority of neonatal bacterial meningitis in Canada
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
FROM PEDIATRICS
Fighting Fatigue in MS
Q) Why do my patients with multiple sclerosis experience so much fatigue, and what can I do to help them?
Fatigue is an extremely common symptom of multiple sclerosis (MS) and one of the most disabling complications of the disease.1 More than 75% of patients with MS experience fatigue, which can worsen motor function, sleep quality, mood, and overall quality of life.1,2 Fatigue can also adversely affect employment; among patients with MS who reduce their work hours from full- to part-time, 90% do so because of fatigue.3
The
Patients with MS may have primary or secondary causes of fatigue. Primary fatigue is believed to result from the disease itself. Although it is not well understood, one hypothesis suggests that it is caused by an immune-related process involving inflammation and immune-mediated neurodegeneration.7 Another theory relates it to impaired nerve conduction.8
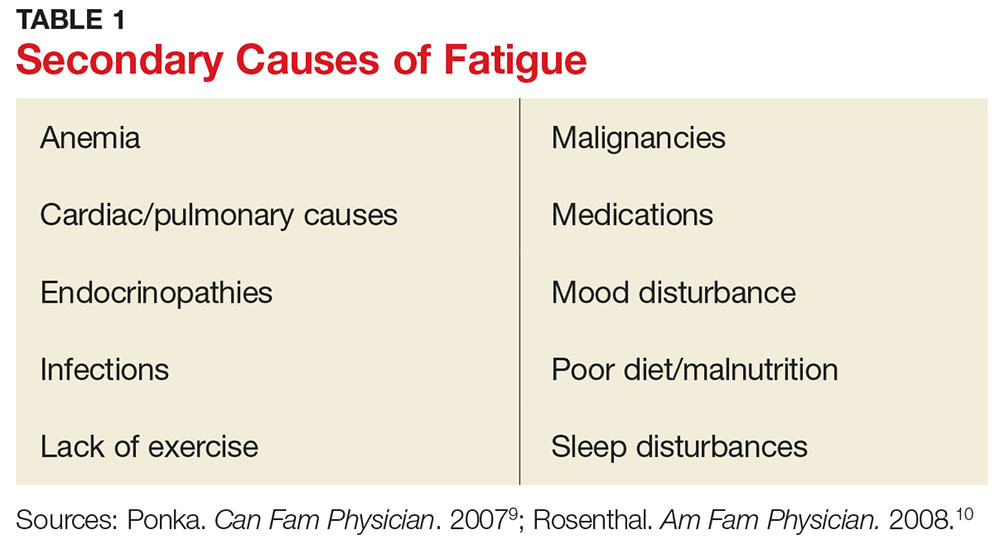
Secondary fatigue is unrelated to MS itself, and it is often treatable. Common causes include anemia, infection, or insomnia (see Table 1).9,10 These possibilities should be considered and ruled out in all patients with MS who complain of fatigue. A comprehensive history, exam, and evaluation performed by the clinician may help identify alternative reasons for fatigue.
Once any secondary causes have been addressed, primary fatigue should be evaluated and managed. One method for assessing the severity of fatigue and its impact on functional disability is to discuss it with the patient. The Fatigue Severity Scale can also be used as a measure; this self-assessment is quick, easy, and can be downloaded for free at www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf.11Identifying potential triggers of fatigue can help clinicians develop appropriate interventions. Heat intolerance is common and can precipitate or contribute to fatigue; cooling equipment can be a helpful solution (see Figure). Urinary tract infections frequently cause fatigue and can exacerbate many symptoms of MS. Bladder dysfunction and subsequent nocturnal wakening may contribute to the problem. Psychological stress is another common trigger; managing it can reduce fatigue.1,12 Screening for depression in patients with MS who complain of fatigue is imperative; if diagnosed, it must be addressed as the first line of treatment.1
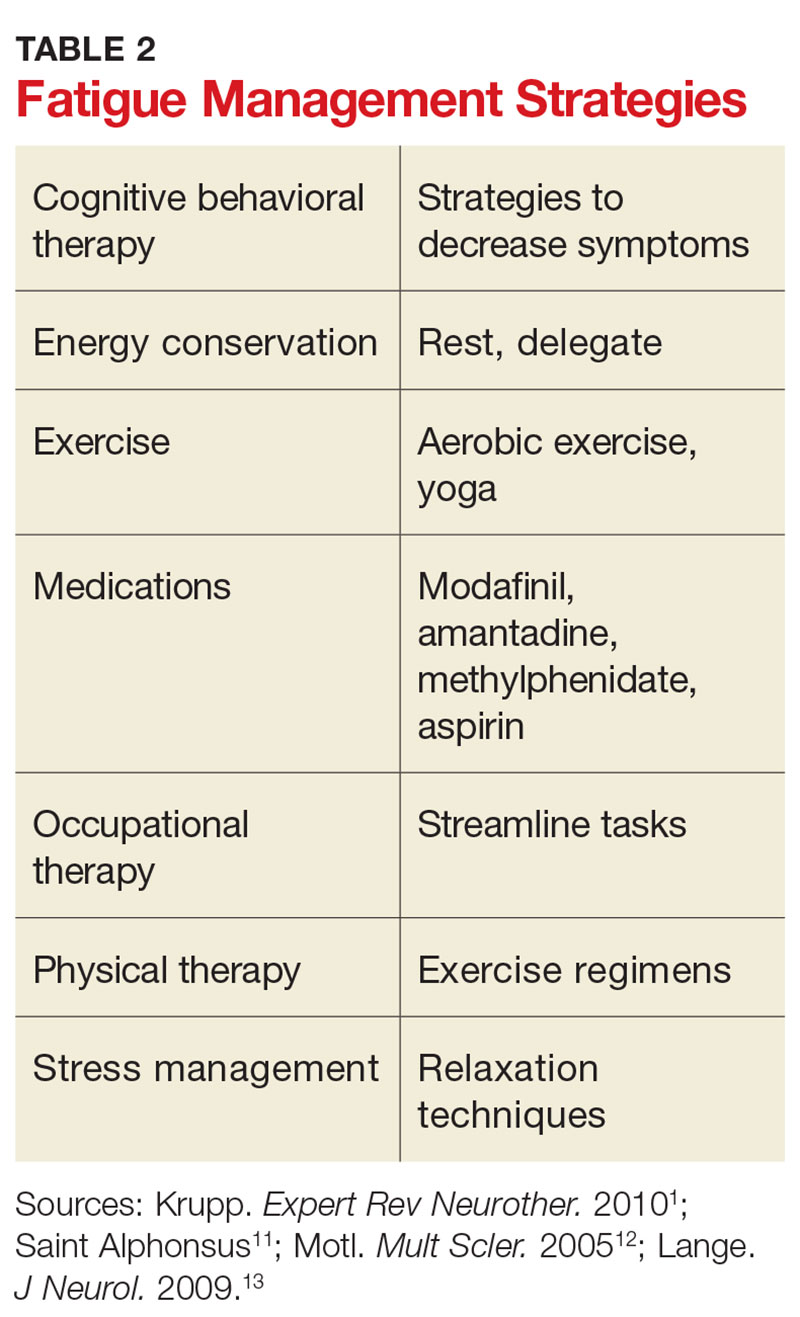
Other clinician-initiated intervention strategies include exercise, therapy, and medication. Modafinil is frequently prescribed for MS fatigue; small trials have demonstrated dramatic improvements with its use.13 Interestingly, aspirin has been shown to reduce fatigue in randomized controlled trials.14 This may be due to its indirect effects on neuroendocrine and autonomic responses, both of which are involved in the perception of fatigue.14 Additional interventions are listed in Table 2. As always, before prescribing any new medication, ensure that it is appropriate and that the patient’s other medical providers agree to the plan.
Counsel patients by emphasizing the importance of good sleep hygiene, a healthy diet, and avoidance of unhealthy habits. Taking an interdisciplinary approach can help patients with MS receive the best possible health care. While you may not be treating your patient’s disease, you will be managing much of his or her health care; treating the underlying causes of fatigue can significantly improve quality of life. —SA
Stephanie Agrella, MSN, RN, APRN, ANP-BC, MSCN
Director of Clinical Services, Multiple Scerlosis Clinic of Central Texas, Round Rock
1. Krupp B, Serafin D, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10(9):1437-1447.
2. Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367-368.
3. Dennett SL, Castelli-Haley J, Oleen-Burkey MK. The impact of multiple sclerosis on patient employment: a review of the medical literature. J Health Productivity. 2007;2(2):12-18.
4. Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
5. Kalb R. Multiple Sclerosis: The Questions You Have—The Answers You Need. New York, NY: Demos; 2012.
6. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435-437.
7. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15(3):210-220.
8. Davis S, Wilson T, White A, Frohman E. Thermoregulation in multiple sclerosis. J Appl Physiol. 2016;109(5):1531-1537.
9. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: fatigue. Can Fam Physician. 2007;53(5):892.
10. Rosenthal TC, Majeroni BA, Pretorius R, Malik K. Fatigue: an overview. Am Fam Physician. 2008;78(10):1173-1179.
11. Saint Alphonsus. Fatigue severity scale. www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf. Accessed May 16, 2017.
12. Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459-463.
13. Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009; 256(4):645-650.
14. Wingerchuk DM, Benarroch EE, O’Brien PC, et al. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005;64(7):1267-1269.
Q) Why do my patients with multiple sclerosis experience so much fatigue, and what can I do to help them?
Fatigue is an extremely common symptom of multiple sclerosis (MS) and one of the most disabling complications of the disease.1 More than 75% of patients with MS experience fatigue, which can worsen motor function, sleep quality, mood, and overall quality of life.1,2 Fatigue can also adversely affect employment; among patients with MS who reduce their work hours from full- to part-time, 90% do so because of fatigue.3
The
Patients with MS may have primary or secondary causes of fatigue. Primary fatigue is believed to result from the disease itself. Although it is not well understood, one hypothesis suggests that it is caused by an immune-related process involving inflammation and immune-mediated neurodegeneration.7 Another theory relates it to impaired nerve conduction.8
Secondary fatigue is unrelated to MS itself, and it is often treatable. Common causes include anemia, infection, or insomnia (see Table 1).9,10 These possibilities should be considered and ruled out in all patients with MS who complain of fatigue. A comprehensive history, exam, and evaluation performed by the clinician may help identify alternative reasons for fatigue.
Once any secondary causes have been addressed, primary fatigue should be evaluated and managed. One method for assessing the severity of fatigue and its impact on functional disability is to discuss it with the patient. The Fatigue Severity Scale can also be used as a measure; this self-assessment is quick, easy, and can be downloaded for free at www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf.11Identifying potential triggers of fatigue can help clinicians develop appropriate interventions. Heat intolerance is common and can precipitate or contribute to fatigue; cooling equipment can be a helpful solution (see Figure). Urinary tract infections frequently cause fatigue and can exacerbate many symptoms of MS. Bladder dysfunction and subsequent nocturnal wakening may contribute to the problem. Psychological stress is another common trigger; managing it can reduce fatigue.1,12 Screening for depression in patients with MS who complain of fatigue is imperative; if diagnosed, it must be addressed as the first line of treatment.1
Other clinician-initiated intervention strategies include exercise, therapy, and medication. Modafinil is frequently prescribed for MS fatigue; small trials have demonstrated dramatic improvements with its use.13 Interestingly, aspirin has been shown to reduce fatigue in randomized controlled trials.14 This may be due to its indirect effects on neuroendocrine and autonomic responses, both of which are involved in the perception of fatigue.14 Additional interventions are listed in Table 2. As always, before prescribing any new medication, ensure that it is appropriate and that the patient’s other medical providers agree to the plan.
Counsel patients by emphasizing the importance of good sleep hygiene, a healthy diet, and avoidance of unhealthy habits. Taking an interdisciplinary approach can help patients with MS receive the best possible health care. While you may not be treating your patient’s disease, you will be managing much of his or her health care; treating the underlying causes of fatigue can significantly improve quality of life. —SA
Stephanie Agrella, MSN, RN, APRN, ANP-BC, MSCN
Director of Clinical Services, Multiple Scerlosis Clinic of Central Texas, Round Rock
Q) Why do my patients with multiple sclerosis experience so much fatigue, and what can I do to help them?
Fatigue is an extremely common symptom of multiple sclerosis (MS) and one of the most disabling complications of the disease.1 More than 75% of patients with MS experience fatigue, which can worsen motor function, sleep quality, mood, and overall quality of life.1,2 Fatigue can also adversely affect employment; among patients with MS who reduce their work hours from full- to part-time, 90% do so because of fatigue.3
The
Patients with MS may have primary or secondary causes of fatigue. Primary fatigue is believed to result from the disease itself. Although it is not well understood, one hypothesis suggests that it is caused by an immune-related process involving inflammation and immune-mediated neurodegeneration.7 Another theory relates it to impaired nerve conduction.8
Secondary fatigue is unrelated to MS itself, and it is often treatable. Common causes include anemia, infection, or insomnia (see Table 1).9,10 These possibilities should be considered and ruled out in all patients with MS who complain of fatigue. A comprehensive history, exam, and evaluation performed by the clinician may help identify alternative reasons for fatigue.
Once any secondary causes have been addressed, primary fatigue should be evaluated and managed. One method for assessing the severity of fatigue and its impact on functional disability is to discuss it with the patient. The Fatigue Severity Scale can also be used as a measure; this self-assessment is quick, easy, and can be downloaded for free at www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf.11Identifying potential triggers of fatigue can help clinicians develop appropriate interventions. Heat intolerance is common and can precipitate or contribute to fatigue; cooling equipment can be a helpful solution (see Figure). Urinary tract infections frequently cause fatigue and can exacerbate many symptoms of MS. Bladder dysfunction and subsequent nocturnal wakening may contribute to the problem. Psychological stress is another common trigger; managing it can reduce fatigue.1,12 Screening for depression in patients with MS who complain of fatigue is imperative; if diagnosed, it must be addressed as the first line of treatment.1
Other clinician-initiated intervention strategies include exercise, therapy, and medication. Modafinil is frequently prescribed for MS fatigue; small trials have demonstrated dramatic improvements with its use.13 Interestingly, aspirin has been shown to reduce fatigue in randomized controlled trials.14 This may be due to its indirect effects on neuroendocrine and autonomic responses, both of which are involved in the perception of fatigue.14 Additional interventions are listed in Table 2. As always, before prescribing any new medication, ensure that it is appropriate and that the patient’s other medical providers agree to the plan.
Counsel patients by emphasizing the importance of good sleep hygiene, a healthy diet, and avoidance of unhealthy habits. Taking an interdisciplinary approach can help patients with MS receive the best possible health care. While you may not be treating your patient’s disease, you will be managing much of his or her health care; treating the underlying causes of fatigue can significantly improve quality of life. —SA
Stephanie Agrella, MSN, RN, APRN, ANP-BC, MSCN
Director of Clinical Services, Multiple Scerlosis Clinic of Central Texas, Round Rock
1. Krupp B, Serafin D, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10(9):1437-1447.
2. Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367-368.
3. Dennett SL, Castelli-Haley J, Oleen-Burkey MK. The impact of multiple sclerosis on patient employment: a review of the medical literature. J Health Productivity. 2007;2(2):12-18.
4. Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
5. Kalb R. Multiple Sclerosis: The Questions You Have—The Answers You Need. New York, NY: Demos; 2012.
6. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435-437.
7. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15(3):210-220.
8. Davis S, Wilson T, White A, Frohman E. Thermoregulation in multiple sclerosis. J Appl Physiol. 2016;109(5):1531-1537.
9. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: fatigue. Can Fam Physician. 2007;53(5):892.
10. Rosenthal TC, Majeroni BA, Pretorius R, Malik K. Fatigue: an overview. Am Fam Physician. 2008;78(10):1173-1179.
11. Saint Alphonsus. Fatigue severity scale. www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf. Accessed May 16, 2017.
12. Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459-463.
13. Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009; 256(4):645-650.
14. Wingerchuk DM, Benarroch EE, O’Brien PC, et al. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005;64(7):1267-1269.
1. Krupp B, Serafin D, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10(9):1437-1447.
2. Krupp L. Fatigue is intrinsic to multiple sclerosis (MS) and is the most commonly reported symptom of the disease. Mult Scler. 2006;12(4):367-368.
3. Dennett SL, Castelli-Haley J, Oleen-Burkey MK. The impact of multiple sclerosis on patient employment: a review of the medical literature. J Health Productivity. 2007;2(2):12-18.
4. Fatigue Guidelines Development Panel of the Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America; 1998.
5. Kalb R. Multiple Sclerosis: The Questions You Have—The Answers You Need. New York, NY: Demos; 2012.
6. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol. 1988;45(4):435-437.
7. Patejdl R, Penner IK, Noack TK, Zettl UK. Multiple sclerosis and fatigue: a review on the contribution of inflammation and immune-mediated neurodegeneration. Autoimmun Rev. 2016;15(3):210-220.
8. Davis S, Wilson T, White A, Frohman E. Thermoregulation in multiple sclerosis. J Appl Physiol. 2016;109(5):1531-1537.
9. Ponka D, Kirlew M. Top 10 differential diagnoses in family medicine: fatigue. Can Fam Physician. 2007;53(5):892.
10. Rosenthal TC, Majeroni BA, Pretorius R, Malik K. Fatigue: an overview. Am Fam Physician. 2008;78(10):1173-1179.
11. Saint Alphonsus. Fatigue severity scale. www.saintalphonsus.org/documents/boise/sleep-Fatigue-Severity-Scale.pdf. Accessed May 16, 2017.
12. Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459-463.
13. Lange R, Volkmer M, Heesen C, Liepert J. Modafinil effects in multiple sclerosis patients with fatigue. J Neurol. 2009; 256(4):645-650.
14. Wingerchuk DM, Benarroch EE, O’Brien PC, et al. A randomized controlled crossover trial of aspirin for fatigue in multiple sclerosis. Neurology. 2005;64(7):1267-1269.
OlympiAD’s positive results spell good news for olaparib in breast cancer
CHICAGO – The oral PARP inhibitor olaparib likely offers a new treatment option for patients with BRCA-related HER2-negative metastatic breast cancer, according to results of the randomized phase III OlympiAD trial reported at the annual meeting of the American Society of Clinical Oncology.
Inhibitors of PARP, or poly(ADP-ribose) polymerase, exploit defective DNA repair due to BRCA mutations, lead author Mark E. Robson, MD, clinic director of the clinical genetics service and medical oncologist at Memorial Sloan Kettering Cancer Center in New York, noted in a press briefing.
“PARP inhibitors have already been approved for the treatment of ovarian cancer in patients with mutations of BRCA1 or BRCA2, and recently in other circumstances. And there have been a couple of small studies that have suggested that breast cancer in BRCA mutation carriers could also be responsive to PARP inhibitors,” he said.
“This is the first phase III study that’s shown an advantage of a PARP inhibitor over standard-of-care chemotherapy in breast cancer patients with BRCA mutations,” Dr. Robson commented. “It was generally well tolerated, with less than 5% of patients discontinuing treatment for toxicity, and a lower rate of grade 3 or worse side effects.”
“It is our opinion that olaparib could be an effective treatment option for women with BRCA mutations who have metastatic HER2-negative breast cancer, including importantly women with BRCA mutations in triple-negative disease,” he concluded.
Findings going forward
Although the trial was positive, the absolute difference in progression-free survival was just 2.8 months, and the curves converged over time, raising questions about potentially more efficacious PARP inhibitors or strategies for getting greater benefit out of olaparib.
“There are three PARP inhibitors in the developmental stage in breast cancer, and there are noncomparative trials and no really good ways to make a decision about which one is better,” Dr. Robson commented.
Several strategies are being explored for enhancing the benefit of these drugs, he continued. “One is combining it with a conventional chemotherapy agent, which is hard to do because of overlapping bone marrow toxicity, so it’s tough to get full doses of chemotherapy and full doses of PARP inhibitor in. Another is to combine it with other targeted agents that interact with components of the DNA damage repair pathway … and there are certainly combination therapy trials that are underway. And then third is … a combination of olaparib with an immuno-oncology agent.”
Additionally, some studies are evaluating expansion of PARP inhibitors to populations such as patients with triple-negative breast cancer who do not have an identifiable BRCA mutation, on the assumption that they have similar, somatic DNA defects that might be susceptible to this class of agents, according to Dr. Robson.
“The studies have been small and at least the initial ones have not been particularly encouraging,” he said. “But as mentioned, combination approaches now are being evaluated as a way to potentially ‘soup up’ the effect in a broader group of patients.”
Expert perspective
The OlympiAD trial represents a “major step forward in breast cancer” in terms of both translational medicine and precision medicine, according to ASCO President Daniel F. Hayes, MD, FACP, FASCO.
Trials moving olaparib into earlier metastatic settings and possibly even the adjuvant setting will likely be conducted in the next year or 2, speculated Dr. Hayes, who is also clinical director of the breast oncology program and Stuart B. Padnos Professor in Breast Cancer Research at the University of Michigan Comprehensive Cancer Center in Ann Arbor.
Potential issues of long-term toxicity, such as secondary leukemias, will need to be kept in mind, especially if olaparib is moved to the curative treatment setting, he cautioned. And a better understanding of resistance (as suggested by the converging progression-free survival curves) and how to overcome it will be key. “That goes back to using it in different ways, clever ways, perhaps combining it with other sorts of therapies,” he said.
Study details
OlympiAD, which was funded by AstraZeneca, enrolled patients with HER2-negative metastatic breast cancer and a centrally confirmed germline (inherited) BRCA mutation who had received anthracyclines and taxanes, and up to two lines of chemotherapy for metastases.
They were randomized 2:1 to open-label treatment with either olaparib (300 mg., twice daily) or single-agent chemotherapy of the treating physician’s choice among three options (capecitabine, eribulin, or vinorelbine). A tablet formulation and dose of olaparib were used that differ from the currently approved capsule formulation and dose to reduce pill burden, Dr. Robson noted.
The patients were about equally split between BRCA1 and BRCA2 mutations, and between hormone receptor–positive disease and triple-negative disease, he reported. The majority (71%) had received chemotherapy for metastases, and a sizable minority (28%) had received prior platinum in the (neo)adjuvant or metastatic setting.
With a median follow-up of about 14.5 months, median progression-free survival assessed by central radiologic review was 7.0 months with olaparib and 4.2 months with chemotherapy (hazard ratio, 0.58; P = .0009).
The median time to investigator-reported second progression or death was also longer with olaparib (13.2 vs. 9.3 months; HR, 0.57; P = .0033).
Median overall survival was about 19-20 months in each group and not significantly different in an interim analysis.
The olaparib group had lower rates of grade 3 or worse adverse events (36.6% vs. 50.5%) and treatment discontinuation because of adverse events (4.9% vs. 7.7%).
The main adverse event of any grade with olaparib was nausea, which was usually mild; only about a quarter of affected patients required antiemetics, according to Dr. Robson. Patients also commonly experienced anemia, requiring transfusion in some cases, and fatigue.
Dr. Robson disclosed that he has a consulting or advisory role with McKesson and AstraZeneca; receives honoraria, travel, accommodations, and/or expenses from AstraZeneca; rand receives research funding (institutional) from AstraZeneca, Abbvie, Myriad Genetics, Biomarin, Medivation, and Tesaro.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. William J. Gradishar is the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. William J. Gradishar is the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. William J. Gradishar is the Betsy Bramsen Professor of Breast Oncology at Northwestern University, Chicago.
CHICAGO – The oral PARP inhibitor olaparib likely offers a new treatment option for patients with BRCA-related HER2-negative metastatic breast cancer, according to results of the randomized phase III OlympiAD trial reported at the annual meeting of the American Society of Clinical Oncology.
Inhibitors of PARP, or poly(ADP-ribose) polymerase, exploit defective DNA repair due to BRCA mutations, lead author Mark E. Robson, MD, clinic director of the clinical genetics service and medical oncologist at Memorial Sloan Kettering Cancer Center in New York, noted in a press briefing.
“PARP inhibitors have already been approved for the treatment of ovarian cancer in patients with mutations of BRCA1 or BRCA2, and recently in other circumstances. And there have been a couple of small studies that have suggested that breast cancer in BRCA mutation carriers could also be responsive to PARP inhibitors,” he said.
“This is the first phase III study that’s shown an advantage of a PARP inhibitor over standard-of-care chemotherapy in breast cancer patients with BRCA mutations,” Dr. Robson commented. “It was generally well tolerated, with less than 5% of patients discontinuing treatment for toxicity, and a lower rate of grade 3 or worse side effects.”
“It is our opinion that olaparib could be an effective treatment option for women with BRCA mutations who have metastatic HER2-negative breast cancer, including importantly women with BRCA mutations in triple-negative disease,” he concluded.
Findings going forward
Although the trial was positive, the absolute difference in progression-free survival was just 2.8 months, and the curves converged over time, raising questions about potentially more efficacious PARP inhibitors or strategies for getting greater benefit out of olaparib.
“There are three PARP inhibitors in the developmental stage in breast cancer, and there are noncomparative trials and no really good ways to make a decision about which one is better,” Dr. Robson commented.
Several strategies are being explored for enhancing the benefit of these drugs, he continued. “One is combining it with a conventional chemotherapy agent, which is hard to do because of overlapping bone marrow toxicity, so it’s tough to get full doses of chemotherapy and full doses of PARP inhibitor in. Another is to combine it with other targeted agents that interact with components of the DNA damage repair pathway … and there are certainly combination therapy trials that are underway. And then third is … a combination of olaparib with an immuno-oncology agent.”
Additionally, some studies are evaluating expansion of PARP inhibitors to populations such as patients with triple-negative breast cancer who do not have an identifiable BRCA mutation, on the assumption that they have similar, somatic DNA defects that might be susceptible to this class of agents, according to Dr. Robson.
“The studies have been small and at least the initial ones have not been particularly encouraging,” he said. “But as mentioned, combination approaches now are being evaluated as a way to potentially ‘soup up’ the effect in a broader group of patients.”
Expert perspective
The OlympiAD trial represents a “major step forward in breast cancer” in terms of both translational medicine and precision medicine, according to ASCO President Daniel F. Hayes, MD, FACP, FASCO.
Trials moving olaparib into earlier metastatic settings and possibly even the adjuvant setting will likely be conducted in the next year or 2, speculated Dr. Hayes, who is also clinical director of the breast oncology program and Stuart B. Padnos Professor in Breast Cancer Research at the University of Michigan Comprehensive Cancer Center in Ann Arbor.
Potential issues of long-term toxicity, such as secondary leukemias, will need to be kept in mind, especially if olaparib is moved to the curative treatment setting, he cautioned. And a better understanding of resistance (as suggested by the converging progression-free survival curves) and how to overcome it will be key. “That goes back to using it in different ways, clever ways, perhaps combining it with other sorts of therapies,” he said.
Study details
OlympiAD, which was funded by AstraZeneca, enrolled patients with HER2-negative metastatic breast cancer and a centrally confirmed germline (inherited) BRCA mutation who had received anthracyclines and taxanes, and up to two lines of chemotherapy for metastases.
They were randomized 2:1 to open-label treatment with either olaparib (300 mg., twice daily) or single-agent chemotherapy of the treating physician’s choice among three options (capecitabine, eribulin, or vinorelbine). A tablet formulation and dose of olaparib were used that differ from the currently approved capsule formulation and dose to reduce pill burden, Dr. Robson noted.
The patients were about equally split between BRCA1 and BRCA2 mutations, and between hormone receptor–positive disease and triple-negative disease, he reported. The majority (71%) had received chemotherapy for metastases, and a sizable minority (28%) had received prior platinum in the (neo)adjuvant or metastatic setting.
With a median follow-up of about 14.5 months, median progression-free survival assessed by central radiologic review was 7.0 months with olaparib and 4.2 months with chemotherapy (hazard ratio, 0.58; P = .0009).
The median time to investigator-reported second progression or death was also longer with olaparib (13.2 vs. 9.3 months; HR, 0.57; P = .0033).
Median overall survival was about 19-20 months in each group and not significantly different in an interim analysis.
The olaparib group had lower rates of grade 3 or worse adverse events (36.6% vs. 50.5%) and treatment discontinuation because of adverse events (4.9% vs. 7.7%).
The main adverse event of any grade with olaparib was nausea, which was usually mild; only about a quarter of affected patients required antiemetics, according to Dr. Robson. Patients also commonly experienced anemia, requiring transfusion in some cases, and fatigue.
Dr. Robson disclosed that he has a consulting or advisory role with McKesson and AstraZeneca; receives honoraria, travel, accommodations, and/or expenses from AstraZeneca; rand receives research funding (institutional) from AstraZeneca, Abbvie, Myriad Genetics, Biomarin, Medivation, and Tesaro.
CHICAGO – The oral PARP inhibitor olaparib likely offers a new treatment option for patients with BRCA-related HER2-negative metastatic breast cancer, according to results of the randomized phase III OlympiAD trial reported at the annual meeting of the American Society of Clinical Oncology.
Inhibitors of PARP, or poly(ADP-ribose) polymerase, exploit defective DNA repair due to BRCA mutations, lead author Mark E. Robson, MD, clinic director of the clinical genetics service and medical oncologist at Memorial Sloan Kettering Cancer Center in New York, noted in a press briefing.
“PARP inhibitors have already been approved for the treatment of ovarian cancer in patients with mutations of BRCA1 or BRCA2, and recently in other circumstances. And there have been a couple of small studies that have suggested that breast cancer in BRCA mutation carriers could also be responsive to PARP inhibitors,” he said.
“This is the first phase III study that’s shown an advantage of a PARP inhibitor over standard-of-care chemotherapy in breast cancer patients with BRCA mutations,” Dr. Robson commented. “It was generally well tolerated, with less than 5% of patients discontinuing treatment for toxicity, and a lower rate of grade 3 or worse side effects.”
“It is our opinion that olaparib could be an effective treatment option for women with BRCA mutations who have metastatic HER2-negative breast cancer, including importantly women with BRCA mutations in triple-negative disease,” he concluded.
Findings going forward
Although the trial was positive, the absolute difference in progression-free survival was just 2.8 months, and the curves converged over time, raising questions about potentially more efficacious PARP inhibitors or strategies for getting greater benefit out of olaparib.
“There are three PARP inhibitors in the developmental stage in breast cancer, and there are noncomparative trials and no really good ways to make a decision about which one is better,” Dr. Robson commented.
Several strategies are being explored for enhancing the benefit of these drugs, he continued. “One is combining it with a conventional chemotherapy agent, which is hard to do because of overlapping bone marrow toxicity, so it’s tough to get full doses of chemotherapy and full doses of PARP inhibitor in. Another is to combine it with other targeted agents that interact with components of the DNA damage repair pathway … and there are certainly combination therapy trials that are underway. And then third is … a combination of olaparib with an immuno-oncology agent.”
Additionally, some studies are evaluating expansion of PARP inhibitors to populations such as patients with triple-negative breast cancer who do not have an identifiable BRCA mutation, on the assumption that they have similar, somatic DNA defects that might be susceptible to this class of agents, according to Dr. Robson.
“The studies have been small and at least the initial ones have not been particularly encouraging,” he said. “But as mentioned, combination approaches now are being evaluated as a way to potentially ‘soup up’ the effect in a broader group of patients.”
Expert perspective
The OlympiAD trial represents a “major step forward in breast cancer” in terms of both translational medicine and precision medicine, according to ASCO President Daniel F. Hayes, MD, FACP, FASCO.
Trials moving olaparib into earlier metastatic settings and possibly even the adjuvant setting will likely be conducted in the next year or 2, speculated Dr. Hayes, who is also clinical director of the breast oncology program and Stuart B. Padnos Professor in Breast Cancer Research at the University of Michigan Comprehensive Cancer Center in Ann Arbor.
Potential issues of long-term toxicity, such as secondary leukemias, will need to be kept in mind, especially if olaparib is moved to the curative treatment setting, he cautioned. And a better understanding of resistance (as suggested by the converging progression-free survival curves) and how to overcome it will be key. “That goes back to using it in different ways, clever ways, perhaps combining it with other sorts of therapies,” he said.
Study details
OlympiAD, which was funded by AstraZeneca, enrolled patients with HER2-negative metastatic breast cancer and a centrally confirmed germline (inherited) BRCA mutation who had received anthracyclines and taxanes, and up to two lines of chemotherapy for metastases.
They were randomized 2:1 to open-label treatment with either olaparib (300 mg., twice daily) or single-agent chemotherapy of the treating physician’s choice among three options (capecitabine, eribulin, or vinorelbine). A tablet formulation and dose of olaparib were used that differ from the currently approved capsule formulation and dose to reduce pill burden, Dr. Robson noted.
The patients were about equally split between BRCA1 and BRCA2 mutations, and between hormone receptor–positive disease and triple-negative disease, he reported. The majority (71%) had received chemotherapy for metastases, and a sizable minority (28%) had received prior platinum in the (neo)adjuvant or metastatic setting.
With a median follow-up of about 14.5 months, median progression-free survival assessed by central radiologic review was 7.0 months with olaparib and 4.2 months with chemotherapy (hazard ratio, 0.58; P = .0009).
The median time to investigator-reported second progression or death was also longer with olaparib (13.2 vs. 9.3 months; HR, 0.57; P = .0033).
Median overall survival was about 19-20 months in each group and not significantly different in an interim analysis.
The olaparib group had lower rates of grade 3 or worse adverse events (36.6% vs. 50.5%) and treatment discontinuation because of adverse events (4.9% vs. 7.7%).
The main adverse event of any grade with olaparib was nausea, which was usually mild; only about a quarter of affected patients required antiemetics, according to Dr. Robson. Patients also commonly experienced anemia, requiring transfusion in some cases, and fatigue.
Dr. Robson disclosed that he has a consulting or advisory role with McKesson and AstraZeneca; receives honoraria, travel, accommodations, and/or expenses from AstraZeneca; rand receives research funding (institutional) from AstraZeneca, Abbvie, Myriad Genetics, Biomarin, Medivation, and Tesaro.
AT ASCO 2017
Key clinical point:
Major finding: Progression-free survival was superior with olaparib as compared with standard single-agent chemotherapy (7.0 vs. 4.2 months; hazard ratio, 0.58; P = .0009).
Data source: An open-label randomized phase III trial among 302 patients with HER2-negative metastatic breast cancer and a germline BRCA mutation (OlympiAD trial).
Disclosures: Dr. Robson disclosed that he has a consulting or advisory role with McKesson and AstraZeneca; receives honoraria, travel, accommodations, and/or expenses from AstraZeneca; and receives research funding (institutional) from AstraZeneca, AbbVie, Myriad Genetics, Biomarin, Medivation, and Tesaro. The trial was funded by AstraZeneca.
EULAR program features novel treatments and targets in immune pathways and key overviews of the field
Novel treatments involving the interleukin-17, IL-23, and Janus kinase (JAK) pathways and the growing importance of early diagnosis and treatment will be some of the key themes covered in the scientific program at this year’s EULAR congress in Madrid, June 14-17.
The annual EULAR congress’ traditional spirit of giving congress attendees a thorough scientific update of the evidence published in peer-reviewed journals across the broad spectrum of rheumatic diseases is reflected in the wide range of state-of-the-art lectures, clinical and basic science symposia, practical workshops, and special interest sessions running throughout the packed 4-day congress, said João Eurico Cabral da Fonseca, MD, PhD, chair of the Scientific Programme Committee.
“Our program is driven by novelty and not by a particular area we need to cover,” said Dr. Fonseca of the rheumatology and metabolic bone disease department at the Santa Maria Hospital in Lisbon.
“There has been a lot of research in the past year on the IL-17 and IL-23 pathway, on the use of IL-6 inhibitors in vasculitis, and exploring the several diseases in rheumatology where the inhibition of the JAK pathway and other intracellular pathways will be relevant,” he said.
Some of these advances and innovation in rheumatology will be highlighted in the many “What is New” (WIN) and “How to Treat” (HOT) sessions scattered throughout the scientific program. WIN sessions are a review of the evidence that has been published during the year on a specific area of rheumatology, whereas the purpose of the HOT sessions is to update attendees on the new research in that space while also allowing experts to impart some of their hands-on experience in the area.
“For the HOT and WIN sessions, we invite people to present who are not only scientifically active but are clinically active in order to give some input, particularly for the HOT sessions. They are also usually well skilled in speaking to and engaging with large audiences.”
In WIN and HOT sessions to be held on the afternoon of Saturday, June 17, Josef Smolen, MD, of the Medical University of Vienna will update attendees on the latest developments in the treatment of rheumatoid arthritis.
Dr. Smolen’s talk will be followed by a presentation from pediatric rheumatologist Nico Wulffraat, MD, PhD, of the Wilhelmina Children’s Hospital, Utrecht, the Netherlands on the latest developments in juvenile idiopathic arthritis.
Another WIN session that has been popular with attendees in previous years is EULAR’s collaborative session with The Lancet. The purpose of the collaborative session with The Lancet is twofold: to give attendees an excellent state-of-the-art session on the latest developments in rheumatoid arthritis and also to showcase to the wider global medical community the latest developments in the field of rheumatology, Dr. Fonseca said.
“The long-term goal is to distribute the information we’re gathering in rheumatology journals and at the congress to a broader audience,” he said, noting the relevance of bringing the innovations in rheumatology to audiences outside the field.
The Lancet session this year is on Saturday morning and will focus on the pathogenesis and treatment of rheumatoid arthritis. High-profile speakers at this session include Iain McInnes, PhD, professor of experimental medicine and rheumatology at the University of Glasgow, who will be presenting a WIN session entitled “Dissecting the pathogenesis of rheumatoid arthritis – what have therapeutics taught us?” and EULAR President Gerd Burmester, MD, director of the department of rheumatology and clinical immunology and professor of medicine at Charité University Hospital and Free University and Humboldt University of Berlin, who will present the WIN session “Don’t delay – new treatment concepts in rheumatoid arthritis.”
The importance of diagnosing and treating patients early is a message that is close to EULAR’s heart, Dr. Fonseca said.
The organization, which celebrates its 70th birthday this year, will launch its first awareness campaign‚ “Don’t delay, connect today!” at the congress. The message of the campaign is that “early diagnosis and access to treatment are the key to preventing further damage and burden on individuals and society.”
He said that while the sessions cover all the major rheumatology disciplines, there are some particularly interesting sessions on psoriatic arthritis and spondyloarthritis.
“There’s a lot more interest in these areas than compared to 5 years ago,” he said in an interview. On the morning of Thursday, June 15, there will be an abstract session titled “PsA: A fascinating disease,” followed by a session the next morning called “PsA: The options grow!”
Attendees can also join a poster tour on Thursday morning to discover exactly what progress has been made in the management of spondyloarthritis.
There are new developments in systemic diseases such as lupus and scleroderma that will be highlighted at this year’s congress. However, osteoarthritis is still waiting for its time in the sun, Dr. Landewé said.
“I would say keep an eye on OA over the next few years. ... There are not many sessions this year, but I am very certain there are many new developments on the horizon, perhaps not at this congress, but in the next couple of years,” he said.
Perhaps the pièce de résistance of the scientific program is the conference highlights session on the last day of the congress. Attendees will need to arrive early to get a seat as this session represents a huge effort by two experts who are selected by the Scientific Programme Committee to summarize the most important research published since EULAR 2016 from a clinical, translational, and basic science perspective.
This year, Loreto Carmona, MD, PhD, an epidemiologist and rheumatologist from the Musculoskeletal Health Institute in Madrid, will take the podium to present the clinical highlights. She will be followed by Thomas Dörner, MD, of the Charité University Hospital, Berlin, who will present the translational and basic science highlights.
“This session is a very useful one for delegates as it simplifies the major bits of the congress,” Dr. Fonseca said.
Novel treatments involving the interleukin-17, IL-23, and Janus kinase (JAK) pathways and the growing importance of early diagnosis and treatment will be some of the key themes covered in the scientific program at this year’s EULAR congress in Madrid, June 14-17.
The annual EULAR congress’ traditional spirit of giving congress attendees a thorough scientific update of the evidence published in peer-reviewed journals across the broad spectrum of rheumatic diseases is reflected in the wide range of state-of-the-art lectures, clinical and basic science symposia, practical workshops, and special interest sessions running throughout the packed 4-day congress, said João Eurico Cabral da Fonseca, MD, PhD, chair of the Scientific Programme Committee.
“Our program is driven by novelty and not by a particular area we need to cover,” said Dr. Fonseca of the rheumatology and metabolic bone disease department at the Santa Maria Hospital in Lisbon.
“There has been a lot of research in the past year on the IL-17 and IL-23 pathway, on the use of IL-6 inhibitors in vasculitis, and exploring the several diseases in rheumatology where the inhibition of the JAK pathway and other intracellular pathways will be relevant,” he said.
Some of these advances and innovation in rheumatology will be highlighted in the many “What is New” (WIN) and “How to Treat” (HOT) sessions scattered throughout the scientific program. WIN sessions are a review of the evidence that has been published during the year on a specific area of rheumatology, whereas the purpose of the HOT sessions is to update attendees on the new research in that space while also allowing experts to impart some of their hands-on experience in the area.
“For the HOT and WIN sessions, we invite people to present who are not only scientifically active but are clinically active in order to give some input, particularly for the HOT sessions. They are also usually well skilled in speaking to and engaging with large audiences.”
In WIN and HOT sessions to be held on the afternoon of Saturday, June 17, Josef Smolen, MD, of the Medical University of Vienna will update attendees on the latest developments in the treatment of rheumatoid arthritis.
Dr. Smolen’s talk will be followed by a presentation from pediatric rheumatologist Nico Wulffraat, MD, PhD, of the Wilhelmina Children’s Hospital, Utrecht, the Netherlands on the latest developments in juvenile idiopathic arthritis.
Another WIN session that has been popular with attendees in previous years is EULAR’s collaborative session with The Lancet. The purpose of the collaborative session with The Lancet is twofold: to give attendees an excellent state-of-the-art session on the latest developments in rheumatoid arthritis and also to showcase to the wider global medical community the latest developments in the field of rheumatology, Dr. Fonseca said.
“The long-term goal is to distribute the information we’re gathering in rheumatology journals and at the congress to a broader audience,” he said, noting the relevance of bringing the innovations in rheumatology to audiences outside the field.
The Lancet session this year is on Saturday morning and will focus on the pathogenesis and treatment of rheumatoid arthritis. High-profile speakers at this session include Iain McInnes, PhD, professor of experimental medicine and rheumatology at the University of Glasgow, who will be presenting a WIN session entitled “Dissecting the pathogenesis of rheumatoid arthritis – what have therapeutics taught us?” and EULAR President Gerd Burmester, MD, director of the department of rheumatology and clinical immunology and professor of medicine at Charité University Hospital and Free University and Humboldt University of Berlin, who will present the WIN session “Don’t delay – new treatment concepts in rheumatoid arthritis.”
The importance of diagnosing and treating patients early is a message that is close to EULAR’s heart, Dr. Fonseca said.
The organization, which celebrates its 70th birthday this year, will launch its first awareness campaign‚ “Don’t delay, connect today!” at the congress. The message of the campaign is that “early diagnosis and access to treatment are the key to preventing further damage and burden on individuals and society.”
He said that while the sessions cover all the major rheumatology disciplines, there are some particularly interesting sessions on psoriatic arthritis and spondyloarthritis.
“There’s a lot more interest in these areas than compared to 5 years ago,” he said in an interview. On the morning of Thursday, June 15, there will be an abstract session titled “PsA: A fascinating disease,” followed by a session the next morning called “PsA: The options grow!”
Attendees can also join a poster tour on Thursday morning to discover exactly what progress has been made in the management of spondyloarthritis.
There are new developments in systemic diseases such as lupus and scleroderma that will be highlighted at this year’s congress. However, osteoarthritis is still waiting for its time in the sun, Dr. Landewé said.
“I would say keep an eye on OA over the next few years. ... There are not many sessions this year, but I am very certain there are many new developments on the horizon, perhaps not at this congress, but in the next couple of years,” he said.
Perhaps the pièce de résistance of the scientific program is the conference highlights session on the last day of the congress. Attendees will need to arrive early to get a seat as this session represents a huge effort by two experts who are selected by the Scientific Programme Committee to summarize the most important research published since EULAR 2016 from a clinical, translational, and basic science perspective.
This year, Loreto Carmona, MD, PhD, an epidemiologist and rheumatologist from the Musculoskeletal Health Institute in Madrid, will take the podium to present the clinical highlights. She will be followed by Thomas Dörner, MD, of the Charité University Hospital, Berlin, who will present the translational and basic science highlights.
“This session is a very useful one for delegates as it simplifies the major bits of the congress,” Dr. Fonseca said.
Novel treatments involving the interleukin-17, IL-23, and Janus kinase (JAK) pathways and the growing importance of early diagnosis and treatment will be some of the key themes covered in the scientific program at this year’s EULAR congress in Madrid, June 14-17.
The annual EULAR congress’ traditional spirit of giving congress attendees a thorough scientific update of the evidence published in peer-reviewed journals across the broad spectrum of rheumatic diseases is reflected in the wide range of state-of-the-art lectures, clinical and basic science symposia, practical workshops, and special interest sessions running throughout the packed 4-day congress, said João Eurico Cabral da Fonseca, MD, PhD, chair of the Scientific Programme Committee.
“Our program is driven by novelty and not by a particular area we need to cover,” said Dr. Fonseca of the rheumatology and metabolic bone disease department at the Santa Maria Hospital in Lisbon.
“There has been a lot of research in the past year on the IL-17 and IL-23 pathway, on the use of IL-6 inhibitors in vasculitis, and exploring the several diseases in rheumatology where the inhibition of the JAK pathway and other intracellular pathways will be relevant,” he said.
Some of these advances and innovation in rheumatology will be highlighted in the many “What is New” (WIN) and “How to Treat” (HOT) sessions scattered throughout the scientific program. WIN sessions are a review of the evidence that has been published during the year on a specific area of rheumatology, whereas the purpose of the HOT sessions is to update attendees on the new research in that space while also allowing experts to impart some of their hands-on experience in the area.
“For the HOT and WIN sessions, we invite people to present who are not only scientifically active but are clinically active in order to give some input, particularly for the HOT sessions. They are also usually well skilled in speaking to and engaging with large audiences.”
In WIN and HOT sessions to be held on the afternoon of Saturday, June 17, Josef Smolen, MD, of the Medical University of Vienna will update attendees on the latest developments in the treatment of rheumatoid arthritis.
Dr. Smolen’s talk will be followed by a presentation from pediatric rheumatologist Nico Wulffraat, MD, PhD, of the Wilhelmina Children’s Hospital, Utrecht, the Netherlands on the latest developments in juvenile idiopathic arthritis.
Another WIN session that has been popular with attendees in previous years is EULAR’s collaborative session with The Lancet. The purpose of the collaborative session with The Lancet is twofold: to give attendees an excellent state-of-the-art session on the latest developments in rheumatoid arthritis and also to showcase to the wider global medical community the latest developments in the field of rheumatology, Dr. Fonseca said.
“The long-term goal is to distribute the information we’re gathering in rheumatology journals and at the congress to a broader audience,” he said, noting the relevance of bringing the innovations in rheumatology to audiences outside the field.
The Lancet session this year is on Saturday morning and will focus on the pathogenesis and treatment of rheumatoid arthritis. High-profile speakers at this session include Iain McInnes, PhD, professor of experimental medicine and rheumatology at the University of Glasgow, who will be presenting a WIN session entitled “Dissecting the pathogenesis of rheumatoid arthritis – what have therapeutics taught us?” and EULAR President Gerd Burmester, MD, director of the department of rheumatology and clinical immunology and professor of medicine at Charité University Hospital and Free University and Humboldt University of Berlin, who will present the WIN session “Don’t delay – new treatment concepts in rheumatoid arthritis.”
The importance of diagnosing and treating patients early is a message that is close to EULAR’s heart, Dr. Fonseca said.
The organization, which celebrates its 70th birthday this year, will launch its first awareness campaign‚ “Don’t delay, connect today!” at the congress. The message of the campaign is that “early diagnosis and access to treatment are the key to preventing further damage and burden on individuals and society.”
He said that while the sessions cover all the major rheumatology disciplines, there are some particularly interesting sessions on psoriatic arthritis and spondyloarthritis.
“There’s a lot more interest in these areas than compared to 5 years ago,” he said in an interview. On the morning of Thursday, June 15, there will be an abstract session titled “PsA: A fascinating disease,” followed by a session the next morning called “PsA: The options grow!”
Attendees can also join a poster tour on Thursday morning to discover exactly what progress has been made in the management of spondyloarthritis.
There are new developments in systemic diseases such as lupus and scleroderma that will be highlighted at this year’s congress. However, osteoarthritis is still waiting for its time in the sun, Dr. Landewé said.
“I would say keep an eye on OA over the next few years. ... There are not many sessions this year, but I am very certain there are many new developments on the horizon, perhaps not at this congress, but in the next couple of years,” he said.
Perhaps the pièce de résistance of the scientific program is the conference highlights session on the last day of the congress. Attendees will need to arrive early to get a seat as this session represents a huge effort by two experts who are selected by the Scientific Programme Committee to summarize the most important research published since EULAR 2016 from a clinical, translational, and basic science perspective.
This year, Loreto Carmona, MD, PhD, an epidemiologist and rheumatologist from the Musculoskeletal Health Institute in Madrid, will take the podium to present the clinical highlights. She will be followed by Thomas Dörner, MD, of the Charité University Hospital, Berlin, who will present the translational and basic science highlights.
“This session is a very useful one for delegates as it simplifies the major bits of the congress,” Dr. Fonseca said.
CDC: First-trimester Zika infection had highest rate of birth defects
One in 12 infant or fetus born to mothers from the U.S. territories with laboratory-confirmed Zika infection during the first trimester had a birth defect possibly-associated with the infection, officials from the Centers for Disease Control and Prevention reported.
Overall, there were 3,930 pregnant women with laboratory evidence of possible Zika infection reported in the U.S. territories during Jan. 1, 2016-May 24, 2017. Of the 2,549 completed pregnancies, 122 resulted in a fetus or infant with possible Zika-related birth defects. The greatest number of birth defects was for maternal infections in the first trimester at 8%, followed by 5% in the second trimester, and 4% in the third trimester (MMWR. 2017, June 8. doi: 10.15585/mmwr.mm6623e1).
“These data indicate that Zika virus is associated with risks to pregnant women and their babies, even when the infection is identified later during pregnancy,” Dr. Schuchat said. “Although we are still learning about the full range of birth defects that can occur within a woman infected with Zika during pregnancy, we know that it causes brain abnormalities, vision problems, and other consequences of brain damage that might require long-term specialized care.”
In depth analysis of Zika side effects among the studied population found that 108 (89%) of the 122 fetuses or infants with infection confirmed by nucleic acid testing were diagnosed with brain abnormalities and/or microcephaly.
Researchers also found potential gaps in the evaluation of infants at birth with possible congenital Zika virus infections in the U.S. territories, according to Peggy Honein, PhD, a coleader of the CDC Pregnancy and Birth Defects Task Force.
“There are still opportunities to ensure every health care provider is aware of how to screen for exposure to Zika, the need for comprehensive evaluation of infants, and how to monitor and provide follow-up care,” Dr. Honein said. “Identification and follow-ups with laboratory evidence of Zika infection during pregnancy can facilitate timely and appropriate clinical intervention services and help assess their future needs.”
This research was limited by the size of the population analyzed, which was small and, therefore, may not be the full scope of the Zika population. The clinical guidance for infants was also changed in August of 2016, which may have affected reporting, officials said.
To help collect more accurate data, U.S. territories will begin using the same standard case definition as used by the U.S. states and Washington, DC starting June 22, 2017.
One of the investigators reported personal fees from Population Services International, Dexis Consulting Group, and Public Health Institute outside the submitted work. The other investigators reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
One in 12 infant or fetus born to mothers from the U.S. territories with laboratory-confirmed Zika infection during the first trimester had a birth defect possibly-associated with the infection, officials from the Centers for Disease Control and Prevention reported.
Overall, there were 3,930 pregnant women with laboratory evidence of possible Zika infection reported in the U.S. territories during Jan. 1, 2016-May 24, 2017. Of the 2,549 completed pregnancies, 122 resulted in a fetus or infant with possible Zika-related birth defects. The greatest number of birth defects was for maternal infections in the first trimester at 8%, followed by 5% in the second trimester, and 4% in the third trimester (MMWR. 2017, June 8. doi: 10.15585/mmwr.mm6623e1).
“These data indicate that Zika virus is associated with risks to pregnant women and their babies, even when the infection is identified later during pregnancy,” Dr. Schuchat said. “Although we are still learning about the full range of birth defects that can occur within a woman infected with Zika during pregnancy, we know that it causes brain abnormalities, vision problems, and other consequences of brain damage that might require long-term specialized care.”
In depth analysis of Zika side effects among the studied population found that 108 (89%) of the 122 fetuses or infants with infection confirmed by nucleic acid testing were diagnosed with brain abnormalities and/or microcephaly.
Researchers also found potential gaps in the evaluation of infants at birth with possible congenital Zika virus infections in the U.S. territories, according to Peggy Honein, PhD, a coleader of the CDC Pregnancy and Birth Defects Task Force.
“There are still opportunities to ensure every health care provider is aware of how to screen for exposure to Zika, the need for comprehensive evaluation of infants, and how to monitor and provide follow-up care,” Dr. Honein said. “Identification and follow-ups with laboratory evidence of Zika infection during pregnancy can facilitate timely and appropriate clinical intervention services and help assess their future needs.”
This research was limited by the size of the population analyzed, which was small and, therefore, may not be the full scope of the Zika population. The clinical guidance for infants was also changed in August of 2016, which may have affected reporting, officials said.
To help collect more accurate data, U.S. territories will begin using the same standard case definition as used by the U.S. states and Washington, DC starting June 22, 2017.
One of the investigators reported personal fees from Population Services International, Dexis Consulting Group, and Public Health Institute outside the submitted work. The other investigators reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
One in 12 infant or fetus born to mothers from the U.S. territories with laboratory-confirmed Zika infection during the first trimester had a birth defect possibly-associated with the infection, officials from the Centers for Disease Control and Prevention reported.
Overall, there were 3,930 pregnant women with laboratory evidence of possible Zika infection reported in the U.S. territories during Jan. 1, 2016-May 24, 2017. Of the 2,549 completed pregnancies, 122 resulted in a fetus or infant with possible Zika-related birth defects. The greatest number of birth defects was for maternal infections in the first trimester at 8%, followed by 5% in the second trimester, and 4% in the third trimester (MMWR. 2017, June 8. doi: 10.15585/mmwr.mm6623e1).
“These data indicate that Zika virus is associated with risks to pregnant women and their babies, even when the infection is identified later during pregnancy,” Dr. Schuchat said. “Although we are still learning about the full range of birth defects that can occur within a woman infected with Zika during pregnancy, we know that it causes brain abnormalities, vision problems, and other consequences of brain damage that might require long-term specialized care.”
In depth analysis of Zika side effects among the studied population found that 108 (89%) of the 122 fetuses or infants with infection confirmed by nucleic acid testing were diagnosed with brain abnormalities and/or microcephaly.
Researchers also found potential gaps in the evaluation of infants at birth with possible congenital Zika virus infections in the U.S. territories, according to Peggy Honein, PhD, a coleader of the CDC Pregnancy and Birth Defects Task Force.
“There are still opportunities to ensure every health care provider is aware of how to screen for exposure to Zika, the need for comprehensive evaluation of infants, and how to monitor and provide follow-up care,” Dr. Honein said. “Identification and follow-ups with laboratory evidence of Zika infection during pregnancy can facilitate timely and appropriate clinical intervention services and help assess their future needs.”
This research was limited by the size of the population analyzed, which was small and, therefore, may not be the full scope of the Zika population. The clinical guidance for infants was also changed in August of 2016, which may have affected reporting, officials said.
To help collect more accurate data, U.S. territories will begin using the same standard case definition as used by the U.S. states and Washington, DC starting June 22, 2017.
One of the investigators reported personal fees from Population Services International, Dexis Consulting Group, and Public Health Institute outside the submitted work. The other investigators reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
FROM MMWR
The changing face of JIA sets the tone for pediatric sessions at EULAR
The heterogeneous nature of juvenile idiopathic arthritis (JIA), the use of biologics in childhood rheumatic diseases, and a look at the long-term outcomes for children with JIA are just some of the highlights from the pediatric rheumatology sessions at this year’s EULAR Congress in Madrid, June 14-17.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Tadej Avcin, MD, PhD, said that a bench-to-bedside session on the heterogeneity of JIA on the afternoon of Thursday, June 15, would explore the biologic basis of the disease, the role of cytokine profiling, and the clinical variability in the disease.
“By highlighting the heterogeneity of JIA, we hope this session will contribute to the further understanding of differences between JIA subtypes, as well as contribute some scientific background for the further classification of children with JIA,” he said.
Another “not to miss” session from the pediatric program that will be held on the afternoon of Friday, June 16, is the open issues session on the use of biologic agents in JIA, according to Dr. Avcin, professor of pediatrics and head of the department of allergology, rheumatology, and clinical immunology at University Children’s Hospital, University Medical Center, Ljubljana, Slovenia.
Speaking on the long-term side effects of biologics, Joost Swart, MD, from Utrecht in the Netherlands will present some novel data from the large ongoing pharmacovigilance project Pharmachild that follows children aged 3-10 years who have been treated with methotrexate or a biologic.
At the same session, Pierre Quartier, MD, from Paris will take delegates through data on autoimmune phenomena that can occur in children who are on biologic treatment.
“We know that, in treating children with biologics, they can sometimes develop antidrug antibodies and various induced autoimmune phenomena. We would like to highlight this aspect so that physicians can have more of an overview of possible immune-mediated adverse effects in their patients,” Dr. Avcin said.
The session will also address what Dr. Avcin describes as an emerging and important clinical question: When and how do you discontinue treatment in children with sustained remission?
It’s a question he hopes Gerd Horneff, MD, from Germany will be able to shed some light on when he shares data on how frequently children experience disease flares after discontinuing treatment.
Another pediatric highlight is a morning session on Saturday, June 17, that will address the long-term outcomes of children with JIA.
A presentation by Marion van Rossum, MD, PhD, from the Netherlands will explore whether there are certain clinical or laboratory markers that can help identify children who are more likely to respond well to treatment, compared with other children.
Dirk Foell, MD, from Germany will follow with a session on immunological markers of remission in JIA.
As Dr. Avcin explained, immunological markers such as S100 proteins have shown promise as a biomarker of subclinical active disease.
“Even if a child appears to have clinically inactive disease, elevated levels of these markers may help predict which children will remain in remission after discontinuing treatment and which children may be at an increased risk of a disease flare,” he said.
Rounding off the session, Berit Flatø, MD, PhD, from Norway will present delegates with data from an epidemiological study of long-term outcomes of children with JIA as they move into adulthood.
“Dr. Flatø will present the long-term outcome data from children followed for up to 20 years,” Dr. Avcin said. “Biologics have been in pediatric rheumatology for around 17 years so we will be able to see what is the outcome of children with JIA moving into adulthood with our current treatment protocols.”
In the afternoon, on Friday, pediatric experts will team up with their adult rheumatology colleagues in a “challenges in clinical practice” session to update delegates on life-threatening presentations of rheumatic diseases.
“We will highlight life-threatening presentations that are of particular interest in children, like macrophage activation syndrome and complications of systemic connective tissue diseases and systemic vasculitides like Kawasaki disease and Takayasu’s arteritis,” Dr. Avcin said.
The heterogeneous nature of juvenile idiopathic arthritis (JIA), the use of biologics in childhood rheumatic diseases, and a look at the long-term outcomes for children with JIA are just some of the highlights from the pediatric rheumatology sessions at this year’s EULAR Congress in Madrid, June 14-17.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Tadej Avcin, MD, PhD, said that a bench-to-bedside session on the heterogeneity of JIA on the afternoon of Thursday, June 15, would explore the biologic basis of the disease, the role of cytokine profiling, and the clinical variability in the disease.
“By highlighting the heterogeneity of JIA, we hope this session will contribute to the further understanding of differences between JIA subtypes, as well as contribute some scientific background for the further classification of children with JIA,” he said.
Another “not to miss” session from the pediatric program that will be held on the afternoon of Friday, June 16, is the open issues session on the use of biologic agents in JIA, according to Dr. Avcin, professor of pediatrics and head of the department of allergology, rheumatology, and clinical immunology at University Children’s Hospital, University Medical Center, Ljubljana, Slovenia.
Speaking on the long-term side effects of biologics, Joost Swart, MD, from Utrecht in the Netherlands will present some novel data from the large ongoing pharmacovigilance project Pharmachild that follows children aged 3-10 years who have been treated with methotrexate or a biologic.
At the same session, Pierre Quartier, MD, from Paris will take delegates through data on autoimmune phenomena that can occur in children who are on biologic treatment.
“We know that, in treating children with biologics, they can sometimes develop antidrug antibodies and various induced autoimmune phenomena. We would like to highlight this aspect so that physicians can have more of an overview of possible immune-mediated adverse effects in their patients,” Dr. Avcin said.
The session will also address what Dr. Avcin describes as an emerging and important clinical question: When and how do you discontinue treatment in children with sustained remission?
It’s a question he hopes Gerd Horneff, MD, from Germany will be able to shed some light on when he shares data on how frequently children experience disease flares after discontinuing treatment.
Another pediatric highlight is a morning session on Saturday, June 17, that will address the long-term outcomes of children with JIA.
A presentation by Marion van Rossum, MD, PhD, from the Netherlands will explore whether there are certain clinical or laboratory markers that can help identify children who are more likely to respond well to treatment, compared with other children.
Dirk Foell, MD, from Germany will follow with a session on immunological markers of remission in JIA.
As Dr. Avcin explained, immunological markers such as S100 proteins have shown promise as a biomarker of subclinical active disease.
“Even if a child appears to have clinically inactive disease, elevated levels of these markers may help predict which children will remain in remission after discontinuing treatment and which children may be at an increased risk of a disease flare,” he said.
Rounding off the session, Berit Flatø, MD, PhD, from Norway will present delegates with data from an epidemiological study of long-term outcomes of children with JIA as they move into adulthood.
“Dr. Flatø will present the long-term outcome data from children followed for up to 20 years,” Dr. Avcin said. “Biologics have been in pediatric rheumatology for around 17 years so we will be able to see what is the outcome of children with JIA moving into adulthood with our current treatment protocols.”
In the afternoon, on Friday, pediatric experts will team up with their adult rheumatology colleagues in a “challenges in clinical practice” session to update delegates on life-threatening presentations of rheumatic diseases.
“We will highlight life-threatening presentations that are of particular interest in children, like macrophage activation syndrome and complications of systemic connective tissue diseases and systemic vasculitides like Kawasaki disease and Takayasu’s arteritis,” Dr. Avcin said.
The heterogeneous nature of juvenile idiopathic arthritis (JIA), the use of biologics in childhood rheumatic diseases, and a look at the long-term outcomes for children with JIA are just some of the highlights from the pediatric rheumatology sessions at this year’s EULAR Congress in Madrid, June 14-17.
EULAR Standing Committee Chairperson for Paediatric Rheumatology Tadej Avcin, MD, PhD, said that a bench-to-bedside session on the heterogeneity of JIA on the afternoon of Thursday, June 15, would explore the biologic basis of the disease, the role of cytokine profiling, and the clinical variability in the disease.
“By highlighting the heterogeneity of JIA, we hope this session will contribute to the further understanding of differences between JIA subtypes, as well as contribute some scientific background for the further classification of children with JIA,” he said.
Another “not to miss” session from the pediatric program that will be held on the afternoon of Friday, June 16, is the open issues session on the use of biologic agents in JIA, according to Dr. Avcin, professor of pediatrics and head of the department of allergology, rheumatology, and clinical immunology at University Children’s Hospital, University Medical Center, Ljubljana, Slovenia.
Speaking on the long-term side effects of biologics, Joost Swart, MD, from Utrecht in the Netherlands will present some novel data from the large ongoing pharmacovigilance project Pharmachild that follows children aged 3-10 years who have been treated with methotrexate or a biologic.
At the same session, Pierre Quartier, MD, from Paris will take delegates through data on autoimmune phenomena that can occur in children who are on biologic treatment.
“We know that, in treating children with biologics, they can sometimes develop antidrug antibodies and various induced autoimmune phenomena. We would like to highlight this aspect so that physicians can have more of an overview of possible immune-mediated adverse effects in their patients,” Dr. Avcin said.
The session will also address what Dr. Avcin describes as an emerging and important clinical question: When and how do you discontinue treatment in children with sustained remission?
It’s a question he hopes Gerd Horneff, MD, from Germany will be able to shed some light on when he shares data on how frequently children experience disease flares after discontinuing treatment.
Another pediatric highlight is a morning session on Saturday, June 17, that will address the long-term outcomes of children with JIA.
A presentation by Marion van Rossum, MD, PhD, from the Netherlands will explore whether there are certain clinical or laboratory markers that can help identify children who are more likely to respond well to treatment, compared with other children.
Dirk Foell, MD, from Germany will follow with a session on immunological markers of remission in JIA.
As Dr. Avcin explained, immunological markers such as S100 proteins have shown promise as a biomarker of subclinical active disease.
“Even if a child appears to have clinically inactive disease, elevated levels of these markers may help predict which children will remain in remission after discontinuing treatment and which children may be at an increased risk of a disease flare,” he said.
Rounding off the session, Berit Flatø, MD, PhD, from Norway will present delegates with data from an epidemiological study of long-term outcomes of children with JIA as they move into adulthood.
“Dr. Flatø will present the long-term outcome data from children followed for up to 20 years,” Dr. Avcin said. “Biologics have been in pediatric rheumatology for around 17 years so we will be able to see what is the outcome of children with JIA moving into adulthood with our current treatment protocols.”
In the afternoon, on Friday, pediatric experts will team up with their adult rheumatology colleagues in a “challenges in clinical practice” session to update delegates on life-threatening presentations of rheumatic diseases.
“We will highlight life-threatening presentations that are of particular interest in children, like macrophage activation syndrome and complications of systemic connective tissue diseases and systemic vasculitides like Kawasaki disease and Takayasu’s arteritis,” Dr. Avcin said.
EMEUNET tailors EULAR experience for young rheumatologists
Young rheumatologists and researchers will find plenty of relevant content at this year’s EULAR Congress in Madrid, June 14-17, thanks to a dedicated presentation track. Other tailored opportunities include networking events, mentorship for first-time attendees to help them make the most of their EULAR experience, and a unique opportunity for small group discussion and networking with key opinion leaders in rheumatology in the so-called mentor-mentee meetings.
The Young Rheumatologists track provides three sessions with a special focus on researchers and clinicians who are early in their careers, Sofia Ramiro, MD, PhD, explained in an interview. Dr. Ramiro chairs the steering committee of the Emerging Eular Network (EMEUNET), a network of young clinicians and researchers in the field of rheumatology in Europe.
Another session will zero in on osteoarthritis, vasculitis, spondyloarthritis, and rheumatoid arthritis, with a shorter lecture format and more time left for a question-and-answer session and discussion. With a group of younger rheumatologists in attendance, “the sessions are somewhat more informal,” promoting a comfortable and interactive environment for discussion and learning, Dr. Ramiro said.
The third session in the Young Rheumatologists track will consist of case discussions focused on how to counsel and take care of women who have rheumatoid arthritis and would like to become pregnant. Two patient cases will be presented and discussed by leaders in the field. “Again, the idea is to make these presentations as real-world as possible,” Dr. Ramiro said.
The EMEUNET booth will be in the EULAR Village, and, for the first time, the booth will be incorporated in the EULAR booth, as a “pillar” under the bigger EULAR umbrella. Dr. Ramiro said to be sure to stay tuned for a surprise associated with EULAR’s 70th anniversary. On the evening of Thursday, June 15, EMEUNET will host a networking event.
On the morning of Friday, June 16, mentor-mentee meetings organized by EMEUNET link five to six young attendees with mentors, according to area of interest. Sign up is available online, allowing small group discussion with leaders in academic rheumatology. This year, meetings will be led by Iain McInnes, PhD (Glasgow, Scotland), Josef Smolen, MD (Vienna), and William Dixon, MBBS, PhD (Manchester, England). Mentorship topics can include the incorporation of research into a clinical career, general career advice, and insight into international collaboration, Dr. Ramiro said.
“These are usually very well-attended meetings and very popular,” she said. “People who have participated in them always give us very good feedback and are very enthusiastic about how easily accessible these very famous key opinion leaders are and what good advice they give to them.”
Finally, the Ambassador program helps first-time attendees get the most out of EULAR. “I think that we all know that the first time we attend such a huge conference the experience can be daunting,” Dr. Ramiro said. Now in its third year, the ambassador program pairs an EMEUNET member with up to six first-timers. The ambassador helps the newcomers decide which sessions to attend and remains available through mobile phone, social media, and the meeting app throughout the meeting.
All of EMEUNET’s activities during EULAR support the organization’s aim of “widening collaboration and fostering collaboration among young researchers and clinicians,” Dr. Ramiro said. “The ultimate aim is to improve and promote education in the area of our diseases and to foster research collaborations,” she said of the 1,500-member strong organization.
Young rheumatologists and researchers will find plenty of relevant content at this year’s EULAR Congress in Madrid, June 14-17, thanks to a dedicated presentation track. Other tailored opportunities include networking events, mentorship for first-time attendees to help them make the most of their EULAR experience, and a unique opportunity for small group discussion and networking with key opinion leaders in rheumatology in the so-called mentor-mentee meetings.
The Young Rheumatologists track provides three sessions with a special focus on researchers and clinicians who are early in their careers, Sofia Ramiro, MD, PhD, explained in an interview. Dr. Ramiro chairs the steering committee of the Emerging Eular Network (EMEUNET), a network of young clinicians and researchers in the field of rheumatology in Europe.
Another session will zero in on osteoarthritis, vasculitis, spondyloarthritis, and rheumatoid arthritis, with a shorter lecture format and more time left for a question-and-answer session and discussion. With a group of younger rheumatologists in attendance, “the sessions are somewhat more informal,” promoting a comfortable and interactive environment for discussion and learning, Dr. Ramiro said.
The third session in the Young Rheumatologists track will consist of case discussions focused on how to counsel and take care of women who have rheumatoid arthritis and would like to become pregnant. Two patient cases will be presented and discussed by leaders in the field. “Again, the idea is to make these presentations as real-world as possible,” Dr. Ramiro said.
The EMEUNET booth will be in the EULAR Village, and, for the first time, the booth will be incorporated in the EULAR booth, as a “pillar” under the bigger EULAR umbrella. Dr. Ramiro said to be sure to stay tuned for a surprise associated with EULAR’s 70th anniversary. On the evening of Thursday, June 15, EMEUNET will host a networking event.
On the morning of Friday, June 16, mentor-mentee meetings organized by EMEUNET link five to six young attendees with mentors, according to area of interest. Sign up is available online, allowing small group discussion with leaders in academic rheumatology. This year, meetings will be led by Iain McInnes, PhD (Glasgow, Scotland), Josef Smolen, MD (Vienna), and William Dixon, MBBS, PhD (Manchester, England). Mentorship topics can include the incorporation of research into a clinical career, general career advice, and insight into international collaboration, Dr. Ramiro said.
“These are usually very well-attended meetings and very popular,” she said. “People who have participated in them always give us very good feedback and are very enthusiastic about how easily accessible these very famous key opinion leaders are and what good advice they give to them.”
Finally, the Ambassador program helps first-time attendees get the most out of EULAR. “I think that we all know that the first time we attend such a huge conference the experience can be daunting,” Dr. Ramiro said. Now in its third year, the ambassador program pairs an EMEUNET member with up to six first-timers. The ambassador helps the newcomers decide which sessions to attend and remains available through mobile phone, social media, and the meeting app throughout the meeting.
All of EMEUNET’s activities during EULAR support the organization’s aim of “widening collaboration and fostering collaboration among young researchers and clinicians,” Dr. Ramiro said. “The ultimate aim is to improve and promote education in the area of our diseases and to foster research collaborations,” she said of the 1,500-member strong organization.
Young rheumatologists and researchers will find plenty of relevant content at this year’s EULAR Congress in Madrid, June 14-17, thanks to a dedicated presentation track. Other tailored opportunities include networking events, mentorship for first-time attendees to help them make the most of their EULAR experience, and a unique opportunity for small group discussion and networking with key opinion leaders in rheumatology in the so-called mentor-mentee meetings.
The Young Rheumatologists track provides three sessions with a special focus on researchers and clinicians who are early in their careers, Sofia Ramiro, MD, PhD, explained in an interview. Dr. Ramiro chairs the steering committee of the Emerging Eular Network (EMEUNET), a network of young clinicians and researchers in the field of rheumatology in Europe.
Another session will zero in on osteoarthritis, vasculitis, spondyloarthritis, and rheumatoid arthritis, with a shorter lecture format and more time left for a question-and-answer session and discussion. With a group of younger rheumatologists in attendance, “the sessions are somewhat more informal,” promoting a comfortable and interactive environment for discussion and learning, Dr. Ramiro said.
The third session in the Young Rheumatologists track will consist of case discussions focused on how to counsel and take care of women who have rheumatoid arthritis and would like to become pregnant. Two patient cases will be presented and discussed by leaders in the field. “Again, the idea is to make these presentations as real-world as possible,” Dr. Ramiro said.
The EMEUNET booth will be in the EULAR Village, and, for the first time, the booth will be incorporated in the EULAR booth, as a “pillar” under the bigger EULAR umbrella. Dr. Ramiro said to be sure to stay tuned for a surprise associated with EULAR’s 70th anniversary. On the evening of Thursday, June 15, EMEUNET will host a networking event.
On the morning of Friday, June 16, mentor-mentee meetings organized by EMEUNET link five to six young attendees with mentors, according to area of interest. Sign up is available online, allowing small group discussion with leaders in academic rheumatology. This year, meetings will be led by Iain McInnes, PhD (Glasgow, Scotland), Josef Smolen, MD (Vienna), and William Dixon, MBBS, PhD (Manchester, England). Mentorship topics can include the incorporation of research into a clinical career, general career advice, and insight into international collaboration, Dr. Ramiro said.
“These are usually very well-attended meetings and very popular,” she said. “People who have participated in them always give us very good feedback and are very enthusiastic about how easily accessible these very famous key opinion leaders are and what good advice they give to them.”
Finally, the Ambassador program helps first-time attendees get the most out of EULAR. “I think that we all know that the first time we attend such a huge conference the experience can be daunting,” Dr. Ramiro said. Now in its third year, the ambassador program pairs an EMEUNET member with up to six first-timers. The ambassador helps the newcomers decide which sessions to attend and remains available through mobile phone, social media, and the meeting app throughout the meeting.
All of EMEUNET’s activities during EULAR support the organization’s aim of “widening collaboration and fostering collaboration among young researchers and clinicians,” Dr. Ramiro said. “The ultimate aim is to improve and promote education in the area of our diseases and to foster research collaborations,” she said of the 1,500-member strong organization.
Supreme Court: Faith-based hospitals are exempt from federal pension requirements
The U.S. Supreme Court has ruled that faith-based hospitals are exempt from federal pension requirements, holding that employees of religious-affiliated entities are not protected under the Employee Retirement Income Security Act (ERISA). The unanimous decision overturns rulings in the three circuit courts of appeals.
The opinion preserves the status quo, so it should not have an immediate effect on the affected employers and employees, said Ronald J. Mann, a law professor at Columbia University in New York who has written about one case, Advocate Health Care Network v. Stapleton, for Scotusblog.com.
The high court’s opinion centers on logic and the plain language of the ERISA statute, which is sensible, said Paul Secunda, a law professor and director of the labor and employment law program at Marquette University in Milwaukee. However, the decision does not address the larger question that matters to physicians and hospitals, he said.
“Some of these church-affiliated hospitals look an awful lot like their secular competitors,” he said. “They have thousands of employees. They make billions of dollars in revenue.”
Associate Justice Sonia Sotomayor raised similar concerns in a concurring opinion to the decision. While she agreed with her fellow justices on the ruling, she said the case outcome was still troubling.
“Despite their relationship to churches, organizations such as petitioners operate for-profit subsidiaries ... and compete in the secular market with companies that must bear the cost of complying with ERISA,” Justice Sotomayor wrote.
The Supreme Court ruling comes after current and former employees of three hospital chains – Dignity Health, Advocate Health Care, and Saint Peter’s Healthcare System – sued their employers in an effort to make them comply with ERISA. The employees argued that pension plans established by the large health care providers should not fall under ERISA’s “church plan” exception because the plans were not established by churches. ERISA requires that all private employers offering pension plans adhere to federal requirements designed to ensure plan solvency and protect plan participants.
The hospitals argued that pension plans do not have to be established by a church for the ERISA exemption to apply because the plans are maintained by qualifying church-affiliated organizations. The defendants based their position on a 1980 ERISA amendment that included the “maintained” exception. Since the amendment, the federal agencies charged with interpreting ERISA – the IRS, the Department of Labor, and the Pension Benefit Guaranty Corporation – have issued opinion after opinion reaffirming that view, the defendants noted.
Courts of appeals for the Third, Seventh, and Ninth circuits agreed with the employees, concluding that ERISA’s “plain text” requires that a pension plan be established by a church to qualify for the church-plan exemption.
The Supreme Court disagreed, however, ruling that a defined-benefit pension plan maintained by a principal-purpose organization – one controlled by or associate
However, the hospital sector has changed dramatically since the 1980 ERISA amendment, Mr. Secunda pointed out. “If you think back to when this reform was passed ... almost 40 years ago, we have a whole different animal [today] with regard to these large church-oriented hospital organizations.”
Justice Sotomayor raised similar concerns. “These organizations thus bear little resemblance to those Congress considered when enacting the 1980 amendment to the church plan definition. This current reality might prompt Congress to take a different path,” she wrote.
The legal battle over whether faith-based hospitals should comply with ERISA is not likely over, according to Mr. Secunda.
“This is not the end of the story,” he said. “This is just the beginning, and now the next big issue to be decided will be, okay, principal purpose organizations come under the church plan exemption, but do these large billion-dollar hospitals qualify? Are they principal purpose organizations? I think that’s a very open question.”
agallegos@frontlinemedcom.com
On Twitter @legal_med
The U.S. Supreme Court has ruled that faith-based hospitals are exempt from federal pension requirements, holding that employees of religious-affiliated entities are not protected under the Employee Retirement Income Security Act (ERISA). The unanimous decision overturns rulings in the three circuit courts of appeals.
The opinion preserves the status quo, so it should not have an immediate effect on the affected employers and employees, said Ronald J. Mann, a law professor at Columbia University in New York who has written about one case, Advocate Health Care Network v. Stapleton, for Scotusblog.com.
The high court’s opinion centers on logic and the plain language of the ERISA statute, which is sensible, said Paul Secunda, a law professor and director of the labor and employment law program at Marquette University in Milwaukee. However, the decision does not address the larger question that matters to physicians and hospitals, he said.
“Some of these church-affiliated hospitals look an awful lot like their secular competitors,” he said. “They have thousands of employees. They make billions of dollars in revenue.”
Associate Justice Sonia Sotomayor raised similar concerns in a concurring opinion to the decision. While she agreed with her fellow justices on the ruling, she said the case outcome was still troubling.
“Despite their relationship to churches, organizations such as petitioners operate for-profit subsidiaries ... and compete in the secular market with companies that must bear the cost of complying with ERISA,” Justice Sotomayor wrote.
The Supreme Court ruling comes after current and former employees of three hospital chains – Dignity Health, Advocate Health Care, and Saint Peter’s Healthcare System – sued their employers in an effort to make them comply with ERISA. The employees argued that pension plans established by the large health care providers should not fall under ERISA’s “church plan” exception because the plans were not established by churches. ERISA requires that all private employers offering pension plans adhere to federal requirements designed to ensure plan solvency and protect plan participants.
The hospitals argued that pension plans do not have to be established by a church for the ERISA exemption to apply because the plans are maintained by qualifying church-affiliated organizations. The defendants based their position on a 1980 ERISA amendment that included the “maintained” exception. Since the amendment, the federal agencies charged with interpreting ERISA – the IRS, the Department of Labor, and the Pension Benefit Guaranty Corporation – have issued opinion after opinion reaffirming that view, the defendants noted.
Courts of appeals for the Third, Seventh, and Ninth circuits agreed with the employees, concluding that ERISA’s “plain text” requires that a pension plan be established by a church to qualify for the church-plan exemption.
The Supreme Court disagreed, however, ruling that a defined-benefit pension plan maintained by a principal-purpose organization – one controlled by or associate
However, the hospital sector has changed dramatically since the 1980 ERISA amendment, Mr. Secunda pointed out. “If you think back to when this reform was passed ... almost 40 years ago, we have a whole different animal [today] with regard to these large church-oriented hospital organizations.”
Justice Sotomayor raised similar concerns. “These organizations thus bear little resemblance to those Congress considered when enacting the 1980 amendment to the church plan definition. This current reality might prompt Congress to take a different path,” she wrote.
The legal battle over whether faith-based hospitals should comply with ERISA is not likely over, according to Mr. Secunda.
“This is not the end of the story,” he said. “This is just the beginning, and now the next big issue to be decided will be, okay, principal purpose organizations come under the church plan exemption, but do these large billion-dollar hospitals qualify? Are they principal purpose organizations? I think that’s a very open question.”
agallegos@frontlinemedcom.com
On Twitter @legal_med
The U.S. Supreme Court has ruled that faith-based hospitals are exempt from federal pension requirements, holding that employees of religious-affiliated entities are not protected under the Employee Retirement Income Security Act (ERISA). The unanimous decision overturns rulings in the three circuit courts of appeals.
The opinion preserves the status quo, so it should not have an immediate effect on the affected employers and employees, said Ronald J. Mann, a law professor at Columbia University in New York who has written about one case, Advocate Health Care Network v. Stapleton, for Scotusblog.com.
The high court’s opinion centers on logic and the plain language of the ERISA statute, which is sensible, said Paul Secunda, a law professor and director of the labor and employment law program at Marquette University in Milwaukee. However, the decision does not address the larger question that matters to physicians and hospitals, he said.
“Some of these church-affiliated hospitals look an awful lot like their secular competitors,” he said. “They have thousands of employees. They make billions of dollars in revenue.”
Associate Justice Sonia Sotomayor raised similar concerns in a concurring opinion to the decision. While she agreed with her fellow justices on the ruling, she said the case outcome was still troubling.
“Despite their relationship to churches, organizations such as petitioners operate for-profit subsidiaries ... and compete in the secular market with companies that must bear the cost of complying with ERISA,” Justice Sotomayor wrote.
The Supreme Court ruling comes after current and former employees of three hospital chains – Dignity Health, Advocate Health Care, and Saint Peter’s Healthcare System – sued their employers in an effort to make them comply with ERISA. The employees argued that pension plans established by the large health care providers should not fall under ERISA’s “church plan” exception because the plans were not established by churches. ERISA requires that all private employers offering pension plans adhere to federal requirements designed to ensure plan solvency and protect plan participants.
The hospitals argued that pension plans do not have to be established by a church for the ERISA exemption to apply because the plans are maintained by qualifying church-affiliated organizations. The defendants based their position on a 1980 ERISA amendment that included the “maintained” exception. Since the amendment, the federal agencies charged with interpreting ERISA – the IRS, the Department of Labor, and the Pension Benefit Guaranty Corporation – have issued opinion after opinion reaffirming that view, the defendants noted.
Courts of appeals for the Third, Seventh, and Ninth circuits agreed with the employees, concluding that ERISA’s “plain text” requires that a pension plan be established by a church to qualify for the church-plan exemption.
The Supreme Court disagreed, however, ruling that a defined-benefit pension plan maintained by a principal-purpose organization – one controlled by or associate
However, the hospital sector has changed dramatically since the 1980 ERISA amendment, Mr. Secunda pointed out. “If you think back to when this reform was passed ... almost 40 years ago, we have a whole different animal [today] with regard to these large church-oriented hospital organizations.”
Justice Sotomayor raised similar concerns. “These organizations thus bear little resemblance to those Congress considered when enacting the 1980 amendment to the church plan definition. This current reality might prompt Congress to take a different path,” she wrote.
The legal battle over whether faith-based hospitals should comply with ERISA is not likely over, according to Mr. Secunda.
“This is not the end of the story,” he said. “This is just the beginning, and now the next big issue to be decided will be, okay, principal purpose organizations come under the church plan exemption, but do these large billion-dollar hospitals qualify? Are they principal purpose organizations? I think that’s a very open question.”
agallegos@frontlinemedcom.com
On Twitter @legal_med
Sjögren’s syndrome most common extrahepatic PBC manifestation
Extrahepatic manifestations of primary biliary cholangitis (PBC) occur in 73% of patients, with Sjögren’s syndrome, thyroid dysfunction, and systemic sclerosis being the most common, according to a literature review from Sara Chalifoux, MD, and her associates.
Sjögren’s syndrome occurs in 3.5%-73% of PBC patients, usually presenting with dry eyes and oral complications. Sjögren’s treatment in PBC patients involves symptom management associated with exocrine gland infiltration.
Thyroid diseases are present in 5.6%-23.6% of PBC patients. Hashimoto’s thyroiditis is the most common hypothyroidism in PBC patients, presenting with symptoms such as constipation, bradycardia, oligomenorrhea, and inability to concentrate. Grave’s disease is the most common hyperthyroidism, presenting with symptoms such as palpitations, tremulousness, heat intolerance, and weight loss.
Systemic sclerosis occurs in 1.4%-12.3% of PBC patients. Multiple studies found that limited cutaneous systemic sclerosis was more common in PBC patients than was the diffuse form of the disease.
Other diseases that may have a connection to PBC but lack solid, compelling evidence to make a firm association include rheumatoid arthritis, systemic lupus erythematosus, and celiac disease. While many PBC patients have irritable bowel disorder, there is no significant association between the two conditions.
“The patient care team should include practitioners in rheumatology, endocrinology, pulmonology, and cardiology when indicated. Patients should follow up regularly with their primary care physicians. As some of these extrahepatic manifestations can lead to diseases with a poor prognosis, vigilant screening and close follow-up will lead to prompt identification and treatment,” the investigators noted.
The investigators reported no financial conflicts of interest.
Find the full study in Gut and Liver (doi: 10.5009/gnl16365).
Extrahepatic manifestations of primary biliary cholangitis (PBC) occur in 73% of patients, with Sjögren’s syndrome, thyroid dysfunction, and systemic sclerosis being the most common, according to a literature review from Sara Chalifoux, MD, and her associates.
Sjögren’s syndrome occurs in 3.5%-73% of PBC patients, usually presenting with dry eyes and oral complications. Sjögren’s treatment in PBC patients involves symptom management associated with exocrine gland infiltration.
Thyroid diseases are present in 5.6%-23.6% of PBC patients. Hashimoto’s thyroiditis is the most common hypothyroidism in PBC patients, presenting with symptoms such as constipation, bradycardia, oligomenorrhea, and inability to concentrate. Grave’s disease is the most common hyperthyroidism, presenting with symptoms such as palpitations, tremulousness, heat intolerance, and weight loss.
Systemic sclerosis occurs in 1.4%-12.3% of PBC patients. Multiple studies found that limited cutaneous systemic sclerosis was more common in PBC patients than was the diffuse form of the disease.
Other diseases that may have a connection to PBC but lack solid, compelling evidence to make a firm association include rheumatoid arthritis, systemic lupus erythematosus, and celiac disease. While many PBC patients have irritable bowel disorder, there is no significant association between the two conditions.
“The patient care team should include practitioners in rheumatology, endocrinology, pulmonology, and cardiology when indicated. Patients should follow up regularly with their primary care physicians. As some of these extrahepatic manifestations can lead to diseases with a poor prognosis, vigilant screening and close follow-up will lead to prompt identification and treatment,” the investigators noted.
The investigators reported no financial conflicts of interest.
Find the full study in Gut and Liver (doi: 10.5009/gnl16365).
Extrahepatic manifestations of primary biliary cholangitis (PBC) occur in 73% of patients, with Sjögren’s syndrome, thyroid dysfunction, and systemic sclerosis being the most common, according to a literature review from Sara Chalifoux, MD, and her associates.
Sjögren’s syndrome occurs in 3.5%-73% of PBC patients, usually presenting with dry eyes and oral complications. Sjögren’s treatment in PBC patients involves symptom management associated with exocrine gland infiltration.
Thyroid diseases are present in 5.6%-23.6% of PBC patients. Hashimoto’s thyroiditis is the most common hypothyroidism in PBC patients, presenting with symptoms such as constipation, bradycardia, oligomenorrhea, and inability to concentrate. Grave’s disease is the most common hyperthyroidism, presenting with symptoms such as palpitations, tremulousness, heat intolerance, and weight loss.
Systemic sclerosis occurs in 1.4%-12.3% of PBC patients. Multiple studies found that limited cutaneous systemic sclerosis was more common in PBC patients than was the diffuse form of the disease.
Other diseases that may have a connection to PBC but lack solid, compelling evidence to make a firm association include rheumatoid arthritis, systemic lupus erythematosus, and celiac disease. While many PBC patients have irritable bowel disorder, there is no significant association between the two conditions.
“The patient care team should include practitioners in rheumatology, endocrinology, pulmonology, and cardiology when indicated. Patients should follow up regularly with their primary care physicians. As some of these extrahepatic manifestations can lead to diseases with a poor prognosis, vigilant screening and close follow-up will lead to prompt identification and treatment,” the investigators noted.
The investigators reported no financial conflicts of interest.
Find the full study in Gut and Liver (doi: 10.5009/gnl16365).
FROM GUT AND LIVER
Low-dose aspirin bests dual-antiplatelet therapy in TAVR
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.
All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.
All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
PARIS – Single-antiplatelet therapy with low-dose aspirin following transcatheter aortic valve replacement (TAVR) reduced the occurrence of major adverse events, compared with guideline-recommended dual-antiplatelet therapy (DAPT), in the randomized ARTE trial.
The TAVR guideline recommendation for DAPT with low-dose aspirin plus clopidogrel is not based on evidence. It relies on expert opinion. ARTE (Aspirin Versus Aspirin + Clopidogrel Following TAVR) is the first sizable randomized trial to address the safety and efficacy of aspirin alone versus DAPT in the setting of TAVR, Josep Rodés-Cabau, MD, noted in presenting the ARTE results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
ARTE was a multicenter, prospective, international open-label study of 222 TAVR patients who were randomized to 3 months of single-antiplatelet therapy (SAPT) with aspirin at 80-100 mg/day or to DAPT with aspirin at 80-100 mg/day plus clopidogrel at 75 mg/day after a single 300-mg loading dose. Participants had a mean Society of Thoracic Surgery Predicted Risk of Mortality score of 6.3%. The vast majority of participants received the balloon-expandable Edwards Lifesciences Sapien XT valve. The remainder got the Sapien 3 valve.
The primary outcome was the 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack. It occurred in 15.3% of the DAPT group and 7.2% on SAPT, a difference that didn’t reach statistical significance (P = .065) because of small patient numbers.
All subjects were on a proton pump inhibitor. The type, timing, and severity of bleeding events differed between the two study arms. All 4 bleeding events in the SAPT group were vascular in nature, while 5 of the 12 in the DAPT group were gastrointestinal. All the bleeding events in the SAPT group occurred within 72 hours after TAVR, whereas 5 of 12 in the DAPT recipients occurred later. Only one patient on SAPT experienced life-threatening bleeding, compared with seven DAPT patients who did.
“There were two prior smaller studies before ours,” according to Dr. Rodés-Cabau of Laval University in Quebec City. “One showed no differences, and an Italian one showed a tendency toward more bleeding with DAPT. So, I think there has been no sign to date that adding clopidogrel protects this group of patients from anything.”
Discussant Luis Nombela-Franco, MD, an interventional cardiologist at San Carlos Hospital in Madrid, pronounced the ARTE trial guideline-changing despite its limitations.
ARTE was supported by grants from Edwards Lifesciences and the Quebec Heart and Lung Institute.
Simultaneous with Dr. Rodés-Cabau’s presentation in Paris, the ARTE trial was published online (JACC Cardiovasc Interv. 2017 May 11. pii: S1936-8798[17]30812-9).
AT EUROPCR
Key clinical point:
Major finding: The 3-month composite of death, MI, major or life-threatening bleeding, or stroke or transient ischemic attack occurred in 15.3% of TAVR patients randomized to DAPT with low-dose aspirin plus clopidogrel, compared with 7.2% on aspirin only.
Data source: A randomized, multicenter, international, prospective open-label trial in 222 TAVR patients.
Disclosures: The presenter reported receiving research grants from Edwards Lifesciences and the Quebec Heart and Lung Institute, which supported the ARTE trial.