User login
Management of Stable Chronic Obstructive Pulmonary Disease
From the Division of Pulmonary Critical Care Medicine, University of Florida, Gainesville, FL.
Abstract
- Objective:To review the management of stable chronic obstructive pulmonary disease (COPD).
- Methods: Review of the peer-reviewed literature.
- Results: Effective management of stable COPD requires the physician to apply a stepwise intensification of therapy depending on patient symptoms and functional reserve. Bronchodilators are the cornerstone of management. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
- Conclusion: Successful management of stable COPD requires a multidisciplinary approach that utilizes various medical therapies as well as nonpharmacologic interventions.
Key words: chronic obstructive pulmonary disease; exacerbation; bronchodilator; lung volume reduction; cough.
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease characterized by irreversible obstructive ventilatory defects [1–4]. It is a major cause of morbidity and mortality affecting 5% of the population in the United States and was the third leading cause of death in 2008 [5,6]. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. In this review, we will discuss the management of stable COPD in the context of 3 common clinical scenarios.
Case 1
A 65-year-old male with COPD underwent pulmonary function testing (PFT), which demonstrated an obstructive ventilatory defect (forced expiratory volume in 1 second/forced vital capacity ratio [FEV1/FVC], 0.45; FEV1, 2 L [65% of predicted]; and diffusing capacity of the lung for carbon monoxide [DLCO], 15 [65% of predicted]). He has dyspnea with strenuous exercise but is comfortable at rest and with minimal exercise. He has had 1 exacerbation in the last year that was treated on an outpatient basis with steroids and antibiotics. His medication regimen includes inhaled tiotropium once daily and inhaled albuterol as needed that he uses roughly twice a week.
What determines the appropriate therapy for a given COPD patient?
What is the approach to building a pharmacologic regimen for the patient with COPD?
The backbone of the pharmacologic regimen for COPD includes short- and long-acting bronchodilators. They are usually given in an inhaled form to maximize local effects on the lungs and minimize systemic side effects. There are 2 main classes of bronchodilators, beta agonists and muscarinic antagonists, and each targets specific receptors on the surface of airway smooth muscle cells. Beta agonists work by stimulating beta-2 receptors, resulting in bronchodilation, while muscarinic antagonists work by blocking the bronchoconstrictor action of M3 muscarinic receptors. Inhaled corticosteroids can be added to long-acting bronchodilator therapy but cannot be used as stand-alone therapy. Theophylline is an oral bronchodilator that is used infrequently due to its narrow therapeutic index, toxicity, and multiple drug interactions.
Who should be on short-acting bronchodilators? What is the best agent? Should it be scheduled or used as needed?
All patients with COPD should be an on inhaled short-acting bronchodilator as needed for relief of symptoms [7]. Both short-acting beta agonists (albuterol and levalbuterol) and short-acting muscarinic antagonists (ipratropium) have been shown in clinical trials and meta-analyses to improve symptoms and lung function in patients with stable COPD [9,10] and seem to have comparative efficacy when compared head-to-head in trials [11]. However, the airway bronchodilator effect achieved by both classes seems to be additive when used in combination and is also associated with less exacerbations compared to albuterol alone [12]. On the other hand, adding albuterol to ipratropium increased the bronchodilator response but did not reduce the exacerbation rate [11–13]. Inhaled short-acting beta agonists when used as needed rather than scheduled are associated with less medication use without any significant difference in symptoms or lung function [14].
The side effects related to using recommended doses of a short-acting bronchodilator are minimal. In retrospective studies, short-acting beta agonists increased the risk of severe cardiac arrhythmias [15]. Levalbuterol, the active enantiomer of albuterol (R-albuterol) developed for the theoretical benefits of reduced tachycardia, increased tolerability, and better or equal efficacy compared to racemic albuterol, failed to show a clinically significant difference in inducing tachycardia [16]. Beta agonist overuse is associated with tremor and in severe cases hypokalemia, which happens mainly when patients try to achieve maximal bronchodilation; the clinically used doses of beta agonists are associated with fewer side affects but achieve less than maximal bronchodilation [17]. Ipratropium can produce systemic anticholinergic side effects, urinary retention being the most clinically significant especially when combined with long-acting anticholinergic agents [18].
In light of the above discussion, a combination of short-acting beta agonist and muscarinic antagonist is recommended in all patients with COPD unless the patient is on a long-acting muscarinic antagonist [7,18]. In the latter case, a short-acting beta agonist used as a rescue inhaler is the best option. In our patient, albuterol was the choice for his short-acting bronchodilator as he was using the long-acting muscarinic antagonist tiotropium.
Are short-acting bronchodilators enough? What do we use for maintenance therapy?
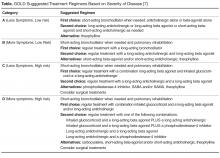
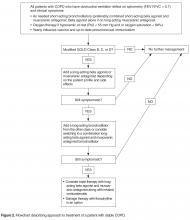
All patients with COPD who are category B or higher according to the modified GOLD staging system should be on a long-acting bronchodilator [7,19]: either a long-acting beta agonist (LABA) or long-acting muscarinic antagonist (LAMA). Long-acting bronchodilators work on the same receptors as their short-acting counterparts but have structural differences. Salmeterol is the prototype for long-acting selective beta-2 agonist. It is structurally similar to albuterol but has an elongated side chain that allows it to bind firmly to the area of beta receptors and stimulate them repetitively, resulting in an extendedduration of action [20]. Tiotropium on the other hand is a quaternary ammonium of ipratropium that is a nonselective muscarinic antagonist [21]. Compared to ipratropium, tiotropium dissociates more quickly from M2 receptors, which is responsible for the undesired anticholinergic effects, while at the same time it binds M1 and M3 receptors for a prolonged time, resulting in extended duration of action [21].
The currently available long-acting beta agonists include salmeterol, formoterol, aformoterol, olodatetol, and indacaterol. The last two have the advantage of once-daily dosing rather than twice [22,23]. LABAs have been shown to improve lung function, exacerbation rate, and quality of life in multiple clinical trials [22–24]. Vilanterol is another LABA that has a long duration of action and can be used once daily [25], but is only available in a combination with umeclidinium, a LAMA. Several LAMAs are approved for use in COPD, including the prototype tiotropium in addition to aclidinium, umeclidinium, and glycopyrronium. These have been shown in clinical trials to improve lung function, symptoms, and exacerbation rate [26–29].
Patients can be started on either a LAMA or LABA depending on patient needs and side effects [7]. Both have comparable side effects and efficacy as detailed below. Concerning side effects, there is conflicting data concerning an association of cardiovascular events with both classes of long-acting bronchodilators. While clinical trials failed to show an increased risk [24,30,31], several retrospective studies showed an increased risk of emergency room visits and hospitalizations due to tachyarrhythmias, heart failure, myocardial infarction, and stroke upon initiation of long-acting bronchodilators [32,33]. There was no difference in risk for adverse cardiovascular events between LABA and LAMA in one Canadian study, and slightly more with LABA in a study using an American database [32,33]. Urinary retention is another possible complication of LAMA supported by evidence from meta-analyses and retrospective studies but not clinical trials and should be discussed with patients upon initiation [34,35]. There have been concerns about increased mortality with the soft mist formulation of tiotropium that were put to rest by the tiotropium safety and performance in Respimat (TIOSPIR) trial, which showed no increased mortality compared to Handihaler [36].
As far as efficacy and benefits, tiotropium and salmeterol were compared head-to-head in a clinical trial, and tiotropium increased the time before developing first exacerbation and decreased the overall rate of exacerbations [37]. No difference in hospitalization rate or mortality was noted in one meta-analysis, although tiotropium was more effective in reducing exacerbations [38]. The choice of agent should be made based on patient comorbidities and side effects. For example, an elderly patient with severe benign prostatic hyperplasia and urinary retention should try a LABA while for a patient with severe tachycardia induced by albuterol, LAMA would be a better first agent.
What is the role of inhaled corticosteroids in COPD?
Inhaled corticosteroids (ICS) are believed to work in COPD by reducing airway inflammation [39]. ICS should not be used alone for COPD management and are always combined with LABA [7]. Several inhaled corticosteroid formulations are approved for use in COPD, including budesonide and fluticasone. ICS has been shown to decrease symptoms and exacerbations with modest effect on lung function and no change in mortality [40]. Side effects include oral candidiasis, dysphonia, and skin bruising [41]. There is also an increased risk of pneumonia [42]. ICS are best reserved for patients with a component of asthma or asthma–COPD overlap syndrome (ACOS) [43]. ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD [44].
What if the patient is still symptomatic on a LABA or LAMA?
For patients whose symptoms are not controlled on one class of long-acting bronchodilator, recommendations are to add a bronchodilator from the other class [7]. There are also multiple combined LAMA-LABA inhalers that are approved in the US and can possible improve adherence to therapy. These include tiotropium-oladeterol, umeclidinium-vilanterol, glycopyronnium-indacaterol, and glycopyrrolate-formoterol. In a large systematic review and meta-analysis comparing LABA-LAMA combination to either agent alone, there was a modest improvement in post bronchodilator FEV1 and quality of life with no change in hospital admissions, mortality, or side effects [45]. Interestingly, adding tiotropium to LABA reduced exacerbations although adding LABA to tiotropium did not [45].
Current guidelines recommend that patients in GOLD categories C and D that are not well controlled should receive a combination of LABA-ICS [7]. However, a new randomized trial showed better reduction of exacerbations and decreased occurrence of pneumonia in patients receiving LAMA-LABA compared to LABA-ICS [46]. In light of this new evidence, it is prudent to use a LAMA-LABA combination before adding ICS.
Triple therapy with LAMA, LABA, and ICS is a common approach for patients with severe uncontrolled disease and has been shown to decrease exacerbations and improve quality of life [7,47]. Adding tiotropium to LABA-ICS decreased exacerbations and improved quality of life and airflow in the landmark UPLIFT trial [26]. In another clinical trial, triple therapy with LAMA, LABA, and ICS compared to tiotropium alone decreased severe exacerbations, pre-bronchodilator FEV1, and morning symptoms [48].
Is there a role for theophylline? Other agents?
Theophylline
Theophylline is an oral adenosine diphosphate antagonist with indirect adrenergic activity, which is responsible for the bronchodilator therapeutic effect in patients with obstructive lung disease. It is also thought to work by an additional mechanism that decreases inflammation in the airways [49]. It has a serious side effect profile that includes ventricular arrhythmias, seizures, vomiting, and tremor [50]. It is metabolized in the liver and has multiple drug interactions and a narrow therapeutic index. It has been shown to improve lung function, gas exchange and symptoms in meta-analysis and clinical trials [51,52].
In light of the nature of the adverse effects and the wide array of safer and more effective pharmacologic agents available, theophylline should be avoided early on in patients with COPD. Its use can be justified as an add-on therapy in patients with refractory disease on triple therapy for symptomatic relief [50]. If used, the therapeutic range for COPD is 8–12 mcg/mL peak level measured 3 to 7 hours after morning dose and is usually achieved using a daily dose of 10 mg per kilogram of body weight for nonobese patients [53].
Systemic Steroids
Oral steroids are used in COPD exacerbations but should never be used chronically in COPD patients regardless of disease severity as they increase morbidity and mortality without improving symptoms or lung function [54,55]. The dose of systemic steroids should be tapered and finally discontinued.
Mucolytics
Classes of mucolytics include thiol derivatives, inhaled dornase alpha, hypertonic saline, and iodine preparations. Thiol derivatives such as N-acetylcysteine are the most widely studied [56].
There is no consistent evidence of beneficial role of mucolytics in COPD patient [7,56]. The PANTHEON trial showed decreased exacerbations with N-acetylcysteine (1.16 exacerbations per patient-year compared to 1.49 exacerbations per patient-year in the placebo group; risk ratio 0.78, 95% CI 0.67–0.90; P = 0.001) but had methodologic issues including high drop-out rate, exclusion of patients on oxygen, and a large of proportion of nonsmokers [57].
Chronic Antibiotics
There is no role for chronic antibiotics in the management of COPD [7]. Macrolides are an exception but are used for their anti-inflammatory effects rather than their antibiotic effects. They should be reserved for patient with frequent exacerbations on optimal therapy and will be discussed later in the review [58].
What nonpharmacologic treatments are recommended for COPD patients?
Smoking cessation, oxygen therapy for severe hypoxemia (resting O2 saturation ≤ 88 or PaO2 ≤ 55), vaccination for influenza and pneumococcus, and appropriate nutrition should be provided in all COPD patients. Pulmonary rehabilitation is indicated for patients in GOLD categories B, C, and D [7]. It improves symptoms, quality of life, exercise tolerance and health care utilization. Beneficial effects last for about 2 years [59,60].
What other diagnoses should be considered in patients who continue to be symptomatic on optimal therapy?
Other diseases that share the same risk factors as COPD and can contribute to dyspnea, including coronary heart disease, heart failure, thromboembolic disease, and pulmonary hypertension, should be considered. In addition, all patients with refractory disease should have a careful assessment of their inhaler technique, continued smoking, need for oxygen therapy, and associated deconditioning.
Case 2
A 70-year-old male with severe COPD on oxygen therapy and obstructive sleep apnea treated on nocturnal CPAP was seen in the pulmonary clinic for evaluation of his dyspnea. He was symptomatic with minimal activity and had chronic cough with some sputum production. He had been hospitalized 3 times over the past 12 months and had been to the emergency department (ED) the same number of times for dyspnea. Pertinent medications included as-needed albuterol inhaler, inhaled steroids, and tiotropium 18 mcg inhaled daily. He demonstrated good inhaler technique. On examination, his vital signs were pulse 99 bpm, SpO2 94% on 2L/min oxygen by nasal cannula, blood pressure 126/72 mm Hg, respiratory rate 15, and BMI 35 kg/m2. He appeared chronically ill but in no acute distress. No wheezing or rales were heard. He had no lower extremity edema. The remainder of the exam was within normal limits. His last pulmonary function test demonstrated moderate obstruction with significant bronchodilator response to 2 puffs of albuterol. The side effects of chronic steroid therapy were impressed upon the patient and 500 mg of roflumilast was started daily. Over the course of the next 3 months, he had no further exacerbations. Roflumilast was continued. He has not required any further hospitalizations, ED visits, or oral steroid use since the last clinic visit.
What is the significance of acute exacerbations of COPD?
Acute exacerbation of COPD (AECOPD) is a frequently observed complication for many patients with COPD [61,62]. AECOPD is associated with accelerated disease progression, augmented decline in health status and quality of life, and increased mortality [63]. Exacerbations account for most of the costs associated with COPD. Estimates suggest that the aggregate costs associated with the treatment of AECOPDs are between $3.2 and $3.8 billion, and that annual health care costs are 10-fold greater for patients with COPD associated with acute exacerbations than for patients with COPD but without exacerbations [64]. Hence, any intervention that could potentially minimize or prevent this complication will have far-reaching benefits to patients with COPD as well as provide significant cost saving.
How is acute exacerbation of COPD defined?
COPD exacerbation is defined as a baseline change of the patient’s dyspnea, cough, and/or sputum that is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD [65]. Exacerbation in clinical trials has been defined on the basis of whether an increase in the level of care beyond regular care is required primarily in the hospital or ED [66]. Frequent exacerbations are defined as 3 symptom-defined exacerbations per year or 2 per year if defined by the need for therapy with corticosteroids, antibiotics, or both [67].
What is the underlying pathophysiology?
AECOPD is associated with enhanced upper and lower airway and systemic inflammation. The bronchial mucosa of stable COPD patients have increased numbers of CD8+ lymphocytes and macrophages. In mild AECOPD, eosinophils are increased in the bronchial mucosa and modest elevation of neutrophils, T lymphocytes (CD3), and TNF alpha positive cells has also been reported [62]. With more severe AECOPD, airway neutrophils are increased. Oxidative stress is a key factor in the development of airway inflammation in COPD [61]. Patients with severe exacerbations have augmented large airway interleukin-8 (IL-8) levels and increased oxidative stress as demonstrated by markers such as hydrogen peroxide and 8-isoprostane [66].
How do acute exacerbations affect the course of the disease?
In general, as the severity of the underlying COPD increases, exacerbations become both more severe and more frequent. The quality of life of patients with frequent exacerbations is worse than patients with a history of less frequent exacerbations [68]. Frequent exacerbations have also been linked to a decline in lung function, with studies suggesting that there might be a decline of 7 mL in FEV1 per lower respiratory tract infection per year [59,69] and approxi-mately 8 mL per year in patients with frequent exacerbations as compared to those with sporadic exacerbations [70].
What are the triggers for COPD exacerbation?
Respiratory infections are estimated to trigger approximately two-thirds of exacerbations [62]. Viral and bacterial infections cause most exacerbations. The effect of the infective triggers is to increase inflammation, cause bronchoconstriction, edema, and mucus production, with a resultant increase in dynamic hyperinflation [71]. Thus, any intervention that reduces inflammation in COPD reduces the number and severity of exacerbations, whereas bronchodilators have an impact on exacerbation by their effects on reducing dynamic hyperinflation. The triggers for the one-third of exacerbations not triggered by infection are postulated to be related to other medical conditions, including pulmonary embolism, aspiration, heart failure, and myocardial ischemia [66].
What are the pharmacologic options available for prevention of AECOPD?
In recognition of the importance of preventing COPD exacerbations, the American College of Chest Physicians and Canadian Thoracic Society [65] have published an evidence-informed clinical guideline specifically examining the prevention of AECOPD, with the goal of assisting clinicians in providing optimal management for COPD patients. The following pharmacologic agents have been recognized as being effective at reducing the frequency of acute exacerbations without any impact on the severity of COPD itself.
Roflumilast
Phosphodiesterase 4 (PDE4) inhibition appears to have inflammatory modulating properties in the airways, although the exact mechanism of action is unclear. Some have proposed that it reduces inflammation by inhibiting the breakdown of intracellular cyclic adenosine monophosphate [72]. In 2 large clinical trials [73,74], daily use of a PDE4 inhibitor (roflumilast) showed a significant (15%–18%) reduction in yearly AECOPD incidence (approximate number needed to treat: 4). This benefit was seen in patients with GOLD stage 3–4 disease (FEV1 < 50% predicted) with the chronic bronchitic phenotype and who had experienced at least 1 exacerbation in the previous year.
Importantly, these clinical trials specifically prohibited the use of ICS and LAMAs. Thus, it remains unclear if PDE4 inhibition should be used as an add-on to ICS/LAMA therapy in patients who continue to have frequent AECOPD or whether PDE4 inhibition could be used instead of these standard therapies in patients with well-controlled daily symptoms without ICS or LAMA therapy but who experience frequent exacerbations.
Of note, earlier trials with roflumilast included patients with ICS and LAMA use [73,75]. These trials were focused on FEV1 improvement and found no benefit. It was only in post ad hoc analyses that a reduction in AECOPD in patients with frequent exacerbations was found among those taking roflumilast, regardless of ICS or LAMA use [76]. While roflumilast has documented benefit in improving lung function and reducing the rate of exacerbations, it has not been reported to decrease hospitalizations [64]. This indicates that although the drug reduces the total number of exacerbations, it may not be as useful in preventing episodes of severe exacerbations of COPD.
Although PDE4 inhibitors are easy to administer (a once-daily pill), they are associated with significant GI side effects (diarrhea, nausea, reduced appetite), weight loss, headache, and sleep disturbance [77]. Adverse effects tend to occur early during treatment, are reversible, and lessen over time with treatment [66]. Studies reported an average unexplained weight loss of 2 kg, and monitoring weight during treatment is advised. In addition, it is important to avoid roflumilast in underweight patients. Roflumilast should also be used with caution in depressed patients [65].
N-acetylcysteine
N-acetylcysteine (NAC) reduces the viscosity of respiratory secretions as a result of the cleavage of the disulfide bonds and has been studied as a mucolytic agent to aid in the elimination of respiratory secretions [78]. Oral NAC is quickly absorbed and is rapidly present in an active form in lung tissue and respiratory secretions after ingestion. NAC is well tolerated except for occasional patients with GI adverse effects. The role of NAC in preventing AECOPD has been studied for more than 3 decades [79–81], although the largest clinical trial to date was reported in 2014 [57]. Taken together, the combined data demonstrate a significant reduction in the rate of COPD exacerbations associated with the use of NAC when compared with placebo (OR, 0.61; CI, 0.37–0.99). Clinical guidelines suggest that in patients with moderate to severe COPD (FEV1/FVC < 0.7, and FEV1 < 80% predicted) receiving maintenance bronchodilator therapy combined with ICS and history of 2 more exacerbations in the previous 2 years, treatment with oral NAC can be administered to prevent AECOPD.
Macrolides
Continuous prophylactic use of antibiotics in older studies had no effect on the frequency of AECOPD [82,83]. But it is known that macrolide antibiotics have several antimicrobial, anti-inflammatory and immunomodulating effects and have been used for many years in the management of other chronic airway disease, including diffuse pan-bronchiolitis and cystic fibrosis [65]. One recent study showed that the use of once-daily, generic azithromycin 5 days/week appeared to have an impact on AECOPD incidence [84]. In this study, AECOPD was reduced from 1.83 to 1.48 per patient-year (RR, 0.83; 95% CI, 0.72–0.95: P = 0.01). Azithromycin also prevented severe AECOPD. Greater benefit was obtained with milder forms of the disease and in the elderly. Azithromycin did not appear to provide any benefit in those who continued to smoke (hazard ratio, 0.99) [85]. Other studies have shown that azithromycin was associated with an increased incidence of bacterial resistance and impaired hearing [86]. Overall data from the available clinical trials are robust and demonstrate that regular macrolide therapy definitely reduces the risk of AECOPD. But due to potential side effects macrolide therapy is an option rather than a strong recommendation [65]. The prescribing clinician also needs to consider the potential of prolongation of the QT interval [84].
Immunostimulants
Immunostimulants have also been reported to reduce frequency of AECOPD [87,88]. Bacterial lysates, reconstituted mixtures of bacterial antigens present in the lower airways of COPD patients, act as immuno-stimulants through the induction of cellular maturation, stimulating lymphocyte chemotaxis, and increasing opsonization when administered to individuals with COPD [66]. Studies have demonstrated a reduction in the severe complications of exacerbations and hospital admissions in COPD patients with OM-85, a detoxified oral immunoactive bacterial extract [87,88]. However, most of these trials were conducted prior to the routine use of long-acting bronchodilators and ICS in COPD. A recent study by Braido et al evaluated the efficacy of ismigen, a bacterial lysate, in reducing AECOPD [89] and found no difference in the exacerbation rate between ismigen and placebo or the time to first exacerbation. Additional studies are needed to examine the long-term effects of this therapy in patients receiving currently recommended COPD maintenance therapy [66].
β Blockers
Observational studies of beta-blocker use in preventing AECOPD have yielded encouraging results, with one study showing a reduction in AECOPD risk (incidence risk ratio, 0.73; CI 0.60–0.90) in patients receiving beta blockers versus those not on beta blockers [90]. Based on these findings, a clinical trial investigating the impact of metoprolol on risk of AECOPD is ongoing [91].
Proton Pump Inhibitors
Gastroesophageal reflux disease is an independent risk factor for exacerbations [92]. Two small, single-center studies [93,94] have shown that use of lansoprazole decreases the risk and frequency of AECOPD. However, data from the Predicting Outcome using Systemic Markers in Severe Exacerbations of COPD (PROMISE-COPD) study [66], which was a multicenter prospective observational study, suggested that patients with stable COPD receiving a proton pump inhibitor were at high risk of frequent and severe exacerbations [95]. Thus, at this stage, their definitive role needs to be defined, possibly with a randomized, placebo-controlled study.
Case 3
A 65-year-old male with severe COPD (FEV1/FVC 27, FEV1 25% of predicted, residual volume 170% of predicted for his age and height) was seen in the pulmonary clinic. His medications include a LABA/LAMA combination that he uses twice daily as advised. He uses his rescue albuterol inhaler roughly once a week. The patient complains of severe disabling shortness of breath with exertion and severe limitation of his quality of life because of his inability to lead a normal active life. He is on 2 L/min of oxygen at all times. He has received pulmonary rehabilitation in hopes of improving his quality of life but can only climb a flight of stairs before he must stop to rest. He asks about options but does not want to consider lung transplantation today. His most recent chest CT scan demonstrates upper lobe predominant emphysematous changes with no masses or nodules.
What are the patient's options at this time?
Lung volume reduction surgery (LVRS) attempts to reduce space-occupying severely diseased, hyperexpanded lung, thus allowing the relatively normal adjoining lung parenchyma to expand into the vacated space and function effectively [96].Hence, such therapies are suitable for patients with emphysematous lungs and not those with bronchitic-predominant COPD. LVRS offers a greater chance of improvement in exercise capacity, lung function, quality of life, and dyspnea in the correctly chosen patient population as compared with pharmacologic management alone [97]. However, the procedure is associated with risks, including higher short-term morbidity and mortality [97]. Patients with predominantly upper-lobe emphysema and a low maximal workload after rehabilitation were noted to have lower mortality, a greater probability of improvement in exercise capacity, and a greater probability of improvement in symptoms if they underwent surgery compared to medical therapy alone [97]. On the contrary, patients with predominantly non–upper-lobe emphysema and a high maximal workload after rehabilitation had higher mortality if they underwent surgery compared to receiving medical therapy alone [97]. Thus, a subgroup of patients with homogeneous emphysema symmetrically affecting the upper and lower lobes are considered to be unlikely to benefit from this surgery [97,98].
Valves and other methods of lung volume reduction such as coils, sealants, intrapulmonary vents, and thermal vapor in the bronchi or subsegmental airways have emerged as new techniques for nonsurgical lung volume reduction [99–104]. Endobronchial-valve therapy is associated with improvement in lung function and with clinical benefits that are greatest in the presence of heterogeneous lung involvement. This works by the same principle as with LVRS, by reduction of the most severely diseased lung units, expansion of the more viable, less emphysematous lung results in substantial improvements in lung mechanics [105,106]. The most important complications of this procedure include pneumonia, pneumothorax, hemoptysis and increased frequency of COPD exacerbation in the following thirty days. The fact that high-heterogeneity subgroup had greater improvements in both the FEV1 and distance on the 6-minute walk test than did patients with lower heterogeneity supports the use of quantitative high-resolution computed tomography (HRCT) in selecting patients for endobronchial-valve therapy [107].The HRCT scans also help in identifying those with complete fissures; a marker of lack of collateral ventilation (CV+) between different lobes. Presence of CV+ state predicts failure of endobronchial valve and all forms of endoscopic lung volume reduction strategies [108]. Bronchoscopic thermal vapor ablation (BTVA) therapy can potentially work on a subsegmental level and be successful for treatment of emphysema with lack of intact fissures on CT scans. Other methods that have the potential to be effective in those with collateral ventilation would be endoscopic coil therapy and polymeric lung volume reduction [106,109].Unfortunately, there are no randomized controlled trial data demonstrating clinically meaningful improvement following coil therapy or polymeric lung volume reduction in this CV+ patient population. Vapor therapy is perhaps the only technique that has been found to be effective in upper lobe predominant emphysema even with CV+ status [108].
Our patient has evidence of air trapping and emphysema based on a high residual volume. A CT scan of the chest can determine the nature of the emphysema (heterogeneous versus homogenous) and based on these findings, further determination of the best strategy for lung volume reduction can be made.
Is there a role for long-term oxygen therapy?
Long-term oxygen therapy (LTOT) used for > 15 hours a day is thought to reduce mortality among patients with chronic obstructive pulmonary disease (COPD) and severe resting hypoxemia [110–113].More recent studies have failed to show similar beneficial effects of LTOT. A recent study examined the effects of LTOT in randomized fashion and determined that supplemental oxygen for patients with stable COPD and resting or exercise-induced moderate desaturation did not affect the time to death or first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, the rate of all hospitalizations, the rate of all COPD exacerbations, or changes in measures of quality of life, depression, anxiety, or functional status [114].
Our patient is currently on long-term oxygen therapy and in spite of some uncertainty as to its benefit, it is prudent to order oxygen therapy until further clarification is available.
What is the role of pulmonary rehabilitation?
Pulmonary rehabilitation is an established treatment for patients with chronic lung disease [115]. Benefits include improvement in exercise tolerance, symptoms, and quality of life, with a reduction in the use of health care resources [116].A Spanish population-based cohort study that looked at the influence of regular physical activity on COPD showed that patients who reported low, moderate, or high physical activity had a lower risk of COPD admissions and all-cause mortality than patients with very low physical activity after adjusting for all confounders [117].
As previously mentioned, patients in GOLD categories B, C, and D should be offered pulmonary rehabilitation as part of their treatment [7]. The ideal patient is one who is not too sick to undergo rehabilitation and is motivated to his or her quality of life.
What is the current scope of lung transplantation in the management of severe COPD?
There is a indisputable role for lung transplantation in end-stage COPD. However, lung transplantation does not benefit all COPD patients. There is a subset of patients for whom the treatment provides a survival benefit. It has been reported that 79% of patients with an FEV1 < 16% predicted will survive at least 1 year additional after transplant, but only 11% of patients with an FEV1 > 25% will do so [118]. The pre-transplant BODE (body mass index, airflow obstruction/FEV1, dyspnea, and exercise capacity) index score is used to identify the patients who will benefit from lung transplantation [119,120]. International guidelines for the selection of lung transplant candidates identify the following patient characteristics [121]:
- The disease is progressive, despite maximal treatment including medication, pulmonary rehabilitation, and oxygen therapy
- The patient is not a candidate for endoscopic or surgical LVRS
- BODE index of 5 to 6
- The partial pressure of carbon dioxide is greater than 50 mm Hg or 6.6kPa and/or partial pressure of oxygen is less than 60 mm Hg or 8kPa
- FEV1 of 25% predicted
The perioperative mortality of lung transplantation surgery has been reduced to less than 10%. Risk of complications from surgery in the perioperative period, such as bronchial dehiscence, infectious complications, and acute rejection, have also been reduced but do occur. Chronic allograft dysfunction and the risk of lung cancer in cases of single lung transplant should be discussed with the patient before surgery [122].
How can we incorporate palliative care into the management plan for patients with COPD?
Among patients with end-stage COPD on home oxygen therapy who have required mechanical ventilation for an exacerbation, only 55% are alive at 1 year [123]. COPD patients at high risk of death within the next year of life as well as patients with refractory symptoms and unmet needs are candidates for early palliative care. Palliative care and palliative care specialists can aid in reducing symptom burden and improving quality of life among these patients and their family members and is recommended by multiple international societies for patients with advanced COPD [124,125]. In spite of these recommendations, the utilization of palliative care resources has been dismally low [126,127]. Improving physician-patient communication regarding palliative services and patients’ unmet care needs will help ensure that COPD patients receive adequate palliative care services at the appropriate time.
Conclusion
COPD is a leading cause of morbidity and mortality in the United States and represents a significant economic burden for both individuals and society. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. COPD management is guided by disease severity that is measured using the GOLD multimodal staging system and requires a multidisciplinary approach. Several classes of medication are available for treatment, and a step-wise approach should be applied in building an effective pharmacologic regimen. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
Corresponding author: Abhishek Biswas, MD, Division of Pulmonary and Critical Care Medicine, Rm. M452, University of Florida, 1600 SW Archer Rd, Gainesville, FL 32610, abiswas@ufl.edu.
Financial disclosures: None.
1. Segreti A, Stirpe E, Rogliani P, Cazzola M. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther 2014;18:381–8.
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182:598–604.
3. Aubier M, Marthan R, Berger P, et al. [COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms]. Rev Mal Respir 2010;27:1254–66.
4. Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J (Engl) 2010;123:3467–78.
5. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741–50.
6. Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep 2011;59:1–126.
7. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. Accessed at www.goldcopd.org.
8. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–54.
9. Wadbo M, Löfdahl CG, Larsson K, et al. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur Respir J 2002;20:1138–46.
10. Ram FS, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax 2003;58:580–4.
11. Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med 1996;100(1A):11S–8S.
12. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest 1994;105:1411–9.
13. Friedman M, Serby CW, Menjoge SS, et al. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest 1999;115:635–41.
14. Cook D, Guyatt G, Wong E, et al. Regular versus as-needed short-acting inhaled beta-agonist therapy for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:85–90.
15. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest 2012;142:305–11.
16. Scott VL, Frazee LA. Retrospective comparison of nebulized levalbuterol and albuterol for adverse events in patients with acute airflow obstruction. Am J Ther 2003;10:341–7.
17. Wong CS, Pavord ID, Williams J, et al. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet 1990;336:1396–9.
18. Cole JM, Sheehan AH, Jordan JK. Concomitant use of ipratropium and tiotropium in chronic obstructive pulmonary disease. Ann Pharmacother 2012;46:1717–21.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155 :179–91.
20. Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med 1992;327:1420–5.
21. Takahashi T, Belvisi MG, Patel H, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med 1994;150(6 Pt 1):1640–5.
22. Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010;182:155–62.
23. Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis 2014;9:697–714.
24. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–89.
25. Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest 2012;142:119–27.
26. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–54.
27. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013;1:524–33.
28. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012;40:830–6.
29. D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res 2011;12:156.
30. Antoniu SA. UPLIFT Study: the effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–54. Expert Opin Pharmacother 2009;10:719–22.
31. Nelson HS, Gross NJ, Levine B, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12-week, multicenter, randomized, double- blind, double-dummy, placebo- and active-controlled trial. Clin Ther 2007;29:2167–78.
32. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 2013;173:1175–85.
33. Aljaafareh A, Valle JR, Lin YL, et al. Risk of cardiovascular events after initiation of long-acting bronchodilators in patients with chronic obstructive lung disease: A population-based study. SAGE Open Med 2016;4:2050312116671337.
34. O’Connor AB. Tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2009;360:185–6.
35. Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest 2006;130:1695–703.
36. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013;369:1491–501.
37. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093–103.
38. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012(9):CD009157.
39. Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med 2005;5:3.
40. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012(7):CD002991.
41. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest 2004;126:213–9.
42. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:2407–16.
43. Lee SY, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016;11:2797–803.
44. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med 2015;373:1241–9.
45. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015:CD008989.
46. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016;374:2222–34.
47. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007;146:545–55.
48. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:741–50.
49. Gallelli L, Falcone D, Cannataro R, et al. Theophylline action on primary human bronchial epithelial cells under proinflammatory stimuli and steroidal drugs: a therapeutic rationale approach. Drug Des Devel Ther 2017;11:265–72.
50. Paloucek FP, Rodvold KA. Evaluation of theophylline overdoses and toxicities. Ann Emerg Med 1988;17:135–44.
51. Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002(4):CD003902.
52. Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med 1989;320:1521–5.
53. Devereux G, Cotton S, Barnes P, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials 2015;16:267.
54. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005(3):CD005374.
55. Horita N, Miyazawa N, Morita S, et al. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res 2014;15:37.
56. Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015(7):CD001287.
57. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014;2:187–94.
58. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008;178:1139–47.
59. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823– 32.
60. Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976–83.
61. Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med 2014;35:157–63.
62. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786–96.
63. Spencer S, Calverley PMA, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004;23:698–702.
64. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benef 2014;7:98.
65. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015;147:894–942.
66. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report. Respirology 2017;22:575–601.
67. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med 2013;11:181.
68. Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–22.
69. Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001;164:358–64.
70. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–52.
71. Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–21.
72. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 2011;163:53–67.
73. Calverley PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685–94.
74. Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374:695–703.
75. Lee S, Hui DSC, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology 2011;16:1249–57.
76. Calverley PM, Martinez FJ, Fabbri LM, et al. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis 2012;7:375–82.
77. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013(11):CD002309.
78. Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis 1964;90:721–9.
79. Boman G, Bäcker U, Larsson S, et al. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis 1983;64:405–15.
80. Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. European journal of clinical pharmacology. 1976;9:393–6.
81. Hansen NCG, Skriver A, Brorsen-Riis L, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med 1994;88:531–5.
82. Francis RS, Spicer CC. Chemotherapy in chronic bronchitis: Influence of daily penicillin and tetracycline on exacerbations and their cost: A report to the research committee of the British Tuberculosis Association by Their Chronic Bronchitis Subcommittee. BMJ 1960;1:297–303.
83. Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis. BMJ 1961;2:979.
84. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98.
85. Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014;189:1503–8.
86. Uzun S, Djamin RS, Kluytmans JAJW, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014;2:361–8.
87. Collet JP, Shapiro S, Ernst P, et al. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:1719–24.
88. Jing LI. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary. Age 2004;67:0–05.
89. Braido F, Tarantini F, Ghiglione V, et al. Bacterial lysate in the prevention of acute exacerbation of COPD and in respiratory recurrent infections. Int J Chron Obstruct Pulmon Dis 2007;2:335.
90. Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax 2016;71:8–14.
91. Bhatt SP, Connett JE, Voelker H, et al. β-Blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease (βLOCK COPD): a randomised controlled study protocol. BMJ Open 2016;6:e012292.
92. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128–8.
93. Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc 2009;57:1453–7.
94. Xiong W, Zhang Qs, Zhao W, et al. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exper Pathol 2016;97:107–13.
95. Baumeler L, Papakonstantinou E, Milenkovic B, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology 2016;21:883–90.
96 Sabanathan A, Sabanathan S, Shah R, Richardson J. Lung volume reduction surgery for emphysema: a review. J Cardiovasc Surg 1998;39:237.
97. Group NETTR. Patients at high risk of death after lung-volume–reduction surgery. N Engl J Med 2001;345:1075–83.
98. Group NETTR. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–73.
99. Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2014;148:2651–8.
100. Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA 2016;315:175–84.
101. Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015;20:319–26.
102. Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993–8.
103. Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771–8.
104. Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med 2001;163:1068–73.
105. Shah P, Slebos D, Cardoso P, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet 2011;378:997–1005.
106. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–44.
107. Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518–26.
108. Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: 12-month results from the STEP-UP randomized controlled study. Respiration 2016;92:397–403.
109. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651–62.
110. Group NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–8.
111. Council M. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981;1:681–6.
112. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179–91.
113. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65.
114. Group L-TOTTR. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016;375:1617–27.
115. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015(2):CD003793.
116. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362–8.
117. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61:772–8.
118. Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1156–63.
119. Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J 2010;36:74–80.
120. Cerón Navarro J, de Aguiar Quevedo K, Ansótegui Barrera E, et al. Functional outcomes after lung transplant in chronic obstructive pulmonary disease. Arch Bronconeumol 2015;51:109–14.
121. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1–15.
122. Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol 2008;3:1404–9.
123. Hajizadeh N, Goldfeld K, Crothers K. What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow- up study. Thorax 2015;70:294–6.
124. Siouta N, van Beek K, Preston N, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care 2016;15:18.
125. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–46.
126. Szekendi MK, Vaughn J, Lal A, et al. The prevalence of inpatients at thirty-three U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med 2016;19:360–72.
127. Rush B, Hertz P, Bond A, et al. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest 2017;151:41–6.
From the Division of Pulmonary Critical Care Medicine, University of Florida, Gainesville, FL.
Abstract
- Objective:To review the management of stable chronic obstructive pulmonary disease (COPD).
- Methods: Review of the peer-reviewed literature.
- Results: Effective management of stable COPD requires the physician to apply a stepwise intensification of therapy depending on patient symptoms and functional reserve. Bronchodilators are the cornerstone of management. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
- Conclusion: Successful management of stable COPD requires a multidisciplinary approach that utilizes various medical therapies as well as nonpharmacologic interventions.
Key words: chronic obstructive pulmonary disease; exacerbation; bronchodilator; lung volume reduction; cough.
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease characterized by irreversible obstructive ventilatory defects [1–4]. It is a major cause of morbidity and mortality affecting 5% of the population in the United States and was the third leading cause of death in 2008 [5,6]. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. In this review, we will discuss the management of stable COPD in the context of 3 common clinical scenarios.
Case 1
A 65-year-old male with COPD underwent pulmonary function testing (PFT), which demonstrated an obstructive ventilatory defect (forced expiratory volume in 1 second/forced vital capacity ratio [FEV1/FVC], 0.45; FEV1, 2 L [65% of predicted]; and diffusing capacity of the lung for carbon monoxide [DLCO], 15 [65% of predicted]). He has dyspnea with strenuous exercise but is comfortable at rest and with minimal exercise. He has had 1 exacerbation in the last year that was treated on an outpatient basis with steroids and antibiotics. His medication regimen includes inhaled tiotropium once daily and inhaled albuterol as needed that he uses roughly twice a week.
What determines the appropriate therapy for a given COPD patient?
What is the approach to building a pharmacologic regimen for the patient with COPD?
The backbone of the pharmacologic regimen for COPD includes short- and long-acting bronchodilators. They are usually given in an inhaled form to maximize local effects on the lungs and minimize systemic side effects. There are 2 main classes of bronchodilators, beta agonists and muscarinic antagonists, and each targets specific receptors on the surface of airway smooth muscle cells. Beta agonists work by stimulating beta-2 receptors, resulting in bronchodilation, while muscarinic antagonists work by blocking the bronchoconstrictor action of M3 muscarinic receptors. Inhaled corticosteroids can be added to long-acting bronchodilator therapy but cannot be used as stand-alone therapy. Theophylline is an oral bronchodilator that is used infrequently due to its narrow therapeutic index, toxicity, and multiple drug interactions.
Who should be on short-acting bronchodilators? What is the best agent? Should it be scheduled or used as needed?
All patients with COPD should be an on inhaled short-acting bronchodilator as needed for relief of symptoms [7]. Both short-acting beta agonists (albuterol and levalbuterol) and short-acting muscarinic antagonists (ipratropium) have been shown in clinical trials and meta-analyses to improve symptoms and lung function in patients with stable COPD [9,10] and seem to have comparative efficacy when compared head-to-head in trials [11]. However, the airway bronchodilator effect achieved by both classes seems to be additive when used in combination and is also associated with less exacerbations compared to albuterol alone [12]. On the other hand, adding albuterol to ipratropium increased the bronchodilator response but did not reduce the exacerbation rate [11–13]. Inhaled short-acting beta agonists when used as needed rather than scheduled are associated with less medication use without any significant difference in symptoms or lung function [14].
The side effects related to using recommended doses of a short-acting bronchodilator are minimal. In retrospective studies, short-acting beta agonists increased the risk of severe cardiac arrhythmias [15]. Levalbuterol, the active enantiomer of albuterol (R-albuterol) developed for the theoretical benefits of reduced tachycardia, increased tolerability, and better or equal efficacy compared to racemic albuterol, failed to show a clinically significant difference in inducing tachycardia [16]. Beta agonist overuse is associated with tremor and in severe cases hypokalemia, which happens mainly when patients try to achieve maximal bronchodilation; the clinically used doses of beta agonists are associated with fewer side affects but achieve less than maximal bronchodilation [17]. Ipratropium can produce systemic anticholinergic side effects, urinary retention being the most clinically significant especially when combined with long-acting anticholinergic agents [18].
In light of the above discussion, a combination of short-acting beta agonist and muscarinic antagonist is recommended in all patients with COPD unless the patient is on a long-acting muscarinic antagonist [7,18]. In the latter case, a short-acting beta agonist used as a rescue inhaler is the best option. In our patient, albuterol was the choice for his short-acting bronchodilator as he was using the long-acting muscarinic antagonist tiotropium.
Are short-acting bronchodilators enough? What do we use for maintenance therapy?
All patients with COPD who are category B or higher according to the modified GOLD staging system should be on a long-acting bronchodilator [7,19]: either a long-acting beta agonist (LABA) or long-acting muscarinic antagonist (LAMA). Long-acting bronchodilators work on the same receptors as their short-acting counterparts but have structural differences. Salmeterol is the prototype for long-acting selective beta-2 agonist. It is structurally similar to albuterol but has an elongated side chain that allows it to bind firmly to the area of beta receptors and stimulate them repetitively, resulting in an extendedduration of action [20]. Tiotropium on the other hand is a quaternary ammonium of ipratropium that is a nonselective muscarinic antagonist [21]. Compared to ipratropium, tiotropium dissociates more quickly from M2 receptors, which is responsible for the undesired anticholinergic effects, while at the same time it binds M1 and M3 receptors for a prolonged time, resulting in extended duration of action [21].
The currently available long-acting beta agonists include salmeterol, formoterol, aformoterol, olodatetol, and indacaterol. The last two have the advantage of once-daily dosing rather than twice [22,23]. LABAs have been shown to improve lung function, exacerbation rate, and quality of life in multiple clinical trials [22–24]. Vilanterol is another LABA that has a long duration of action and can be used once daily [25], but is only available in a combination with umeclidinium, a LAMA. Several LAMAs are approved for use in COPD, including the prototype tiotropium in addition to aclidinium, umeclidinium, and glycopyrronium. These have been shown in clinical trials to improve lung function, symptoms, and exacerbation rate [26–29].
Patients can be started on either a LAMA or LABA depending on patient needs and side effects [7]. Both have comparable side effects and efficacy as detailed below. Concerning side effects, there is conflicting data concerning an association of cardiovascular events with both classes of long-acting bronchodilators. While clinical trials failed to show an increased risk [24,30,31], several retrospective studies showed an increased risk of emergency room visits and hospitalizations due to tachyarrhythmias, heart failure, myocardial infarction, and stroke upon initiation of long-acting bronchodilators [32,33]. There was no difference in risk for adverse cardiovascular events between LABA and LAMA in one Canadian study, and slightly more with LABA in a study using an American database [32,33]. Urinary retention is another possible complication of LAMA supported by evidence from meta-analyses and retrospective studies but not clinical trials and should be discussed with patients upon initiation [34,35]. There have been concerns about increased mortality with the soft mist formulation of tiotropium that were put to rest by the tiotropium safety and performance in Respimat (TIOSPIR) trial, which showed no increased mortality compared to Handihaler [36].
As far as efficacy and benefits, tiotropium and salmeterol were compared head-to-head in a clinical trial, and tiotropium increased the time before developing first exacerbation and decreased the overall rate of exacerbations [37]. No difference in hospitalization rate or mortality was noted in one meta-analysis, although tiotropium was more effective in reducing exacerbations [38]. The choice of agent should be made based on patient comorbidities and side effects. For example, an elderly patient with severe benign prostatic hyperplasia and urinary retention should try a LABA while for a patient with severe tachycardia induced by albuterol, LAMA would be a better first agent.
What is the role of inhaled corticosteroids in COPD?
Inhaled corticosteroids (ICS) are believed to work in COPD by reducing airway inflammation [39]. ICS should not be used alone for COPD management and are always combined with LABA [7]. Several inhaled corticosteroid formulations are approved for use in COPD, including budesonide and fluticasone. ICS has been shown to decrease symptoms and exacerbations with modest effect on lung function and no change in mortality [40]. Side effects include oral candidiasis, dysphonia, and skin bruising [41]. There is also an increased risk of pneumonia [42]. ICS are best reserved for patients with a component of asthma or asthma–COPD overlap syndrome (ACOS) [43]. ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD [44].
What if the patient is still symptomatic on a LABA or LAMA?
For patients whose symptoms are not controlled on one class of long-acting bronchodilator, recommendations are to add a bronchodilator from the other class [7]. There are also multiple combined LAMA-LABA inhalers that are approved in the US and can possible improve adherence to therapy. These include tiotropium-oladeterol, umeclidinium-vilanterol, glycopyronnium-indacaterol, and glycopyrrolate-formoterol. In a large systematic review and meta-analysis comparing LABA-LAMA combination to either agent alone, there was a modest improvement in post bronchodilator FEV1 and quality of life with no change in hospital admissions, mortality, or side effects [45]. Interestingly, adding tiotropium to LABA reduced exacerbations although adding LABA to tiotropium did not [45].
Current guidelines recommend that patients in GOLD categories C and D that are not well controlled should receive a combination of LABA-ICS [7]. However, a new randomized trial showed better reduction of exacerbations and decreased occurrence of pneumonia in patients receiving LAMA-LABA compared to LABA-ICS [46]. In light of this new evidence, it is prudent to use a LAMA-LABA combination before adding ICS.
Triple therapy with LAMA, LABA, and ICS is a common approach for patients with severe uncontrolled disease and has been shown to decrease exacerbations and improve quality of life [7,47]. Adding tiotropium to LABA-ICS decreased exacerbations and improved quality of life and airflow in the landmark UPLIFT trial [26]. In another clinical trial, triple therapy with LAMA, LABA, and ICS compared to tiotropium alone decreased severe exacerbations, pre-bronchodilator FEV1, and morning symptoms [48].
Is there a role for theophylline? Other agents?
Theophylline
Theophylline is an oral adenosine diphosphate antagonist with indirect adrenergic activity, which is responsible for the bronchodilator therapeutic effect in patients with obstructive lung disease. It is also thought to work by an additional mechanism that decreases inflammation in the airways [49]. It has a serious side effect profile that includes ventricular arrhythmias, seizures, vomiting, and tremor [50]. It is metabolized in the liver and has multiple drug interactions and a narrow therapeutic index. It has been shown to improve lung function, gas exchange and symptoms in meta-analysis and clinical trials [51,52].
In light of the nature of the adverse effects and the wide array of safer and more effective pharmacologic agents available, theophylline should be avoided early on in patients with COPD. Its use can be justified as an add-on therapy in patients with refractory disease on triple therapy for symptomatic relief [50]. If used, the therapeutic range for COPD is 8–12 mcg/mL peak level measured 3 to 7 hours after morning dose and is usually achieved using a daily dose of 10 mg per kilogram of body weight for nonobese patients [53].
Systemic Steroids
Oral steroids are used in COPD exacerbations but should never be used chronically in COPD patients regardless of disease severity as they increase morbidity and mortality without improving symptoms or lung function [54,55]. The dose of systemic steroids should be tapered and finally discontinued.
Mucolytics
Classes of mucolytics include thiol derivatives, inhaled dornase alpha, hypertonic saline, and iodine preparations. Thiol derivatives such as N-acetylcysteine are the most widely studied [56].
There is no consistent evidence of beneficial role of mucolytics in COPD patient [7,56]. The PANTHEON trial showed decreased exacerbations with N-acetylcysteine (1.16 exacerbations per patient-year compared to 1.49 exacerbations per patient-year in the placebo group; risk ratio 0.78, 95% CI 0.67–0.90; P = 0.001) but had methodologic issues including high drop-out rate, exclusion of patients on oxygen, and a large of proportion of nonsmokers [57].
Chronic Antibiotics
There is no role for chronic antibiotics in the management of COPD [7]. Macrolides are an exception but are used for their anti-inflammatory effects rather than their antibiotic effects. They should be reserved for patient with frequent exacerbations on optimal therapy and will be discussed later in the review [58].
What nonpharmacologic treatments are recommended for COPD patients?
Smoking cessation, oxygen therapy for severe hypoxemia (resting O2 saturation ≤ 88 or PaO2 ≤ 55), vaccination for influenza and pneumococcus, and appropriate nutrition should be provided in all COPD patients. Pulmonary rehabilitation is indicated for patients in GOLD categories B, C, and D [7]. It improves symptoms, quality of life, exercise tolerance and health care utilization. Beneficial effects last for about 2 years [59,60].
What other diagnoses should be considered in patients who continue to be symptomatic on optimal therapy?
Other diseases that share the same risk factors as COPD and can contribute to dyspnea, including coronary heart disease, heart failure, thromboembolic disease, and pulmonary hypertension, should be considered. In addition, all patients with refractory disease should have a careful assessment of their inhaler technique, continued smoking, need for oxygen therapy, and associated deconditioning.
Case 2
A 70-year-old male with severe COPD on oxygen therapy and obstructive sleep apnea treated on nocturnal CPAP was seen in the pulmonary clinic for evaluation of his dyspnea. He was symptomatic with minimal activity and had chronic cough with some sputum production. He had been hospitalized 3 times over the past 12 months and had been to the emergency department (ED) the same number of times for dyspnea. Pertinent medications included as-needed albuterol inhaler, inhaled steroids, and tiotropium 18 mcg inhaled daily. He demonstrated good inhaler technique. On examination, his vital signs were pulse 99 bpm, SpO2 94% on 2L/min oxygen by nasal cannula, blood pressure 126/72 mm Hg, respiratory rate 15, and BMI 35 kg/m2. He appeared chronically ill but in no acute distress. No wheezing or rales were heard. He had no lower extremity edema. The remainder of the exam was within normal limits. His last pulmonary function test demonstrated moderate obstruction with significant bronchodilator response to 2 puffs of albuterol. The side effects of chronic steroid therapy were impressed upon the patient and 500 mg of roflumilast was started daily. Over the course of the next 3 months, he had no further exacerbations. Roflumilast was continued. He has not required any further hospitalizations, ED visits, or oral steroid use since the last clinic visit.
What is the significance of acute exacerbations of COPD?
Acute exacerbation of COPD (AECOPD) is a frequently observed complication for many patients with COPD [61,62]. AECOPD is associated with accelerated disease progression, augmented decline in health status and quality of life, and increased mortality [63]. Exacerbations account for most of the costs associated with COPD. Estimates suggest that the aggregate costs associated with the treatment of AECOPDs are between $3.2 and $3.8 billion, and that annual health care costs are 10-fold greater for patients with COPD associated with acute exacerbations than for patients with COPD but without exacerbations [64]. Hence, any intervention that could potentially minimize or prevent this complication will have far-reaching benefits to patients with COPD as well as provide significant cost saving.
How is acute exacerbation of COPD defined?
COPD exacerbation is defined as a baseline change of the patient’s dyspnea, cough, and/or sputum that is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD [65]. Exacerbation in clinical trials has been defined on the basis of whether an increase in the level of care beyond regular care is required primarily in the hospital or ED [66]. Frequent exacerbations are defined as 3 symptom-defined exacerbations per year or 2 per year if defined by the need for therapy with corticosteroids, antibiotics, or both [67].
What is the underlying pathophysiology?
AECOPD is associated with enhanced upper and lower airway and systemic inflammation. The bronchial mucosa of stable COPD patients have increased numbers of CD8+ lymphocytes and macrophages. In mild AECOPD, eosinophils are increased in the bronchial mucosa and modest elevation of neutrophils, T lymphocytes (CD3), and TNF alpha positive cells has also been reported [62]. With more severe AECOPD, airway neutrophils are increased. Oxidative stress is a key factor in the development of airway inflammation in COPD [61]. Patients with severe exacerbations have augmented large airway interleukin-8 (IL-8) levels and increased oxidative stress as demonstrated by markers such as hydrogen peroxide and 8-isoprostane [66].
How do acute exacerbations affect the course of the disease?
In general, as the severity of the underlying COPD increases, exacerbations become both more severe and more frequent. The quality of life of patients with frequent exacerbations is worse than patients with a history of less frequent exacerbations [68]. Frequent exacerbations have also been linked to a decline in lung function, with studies suggesting that there might be a decline of 7 mL in FEV1 per lower respiratory tract infection per year [59,69] and approxi-mately 8 mL per year in patients with frequent exacerbations as compared to those with sporadic exacerbations [70].
What are the triggers for COPD exacerbation?
Respiratory infections are estimated to trigger approximately two-thirds of exacerbations [62]. Viral and bacterial infections cause most exacerbations. The effect of the infective triggers is to increase inflammation, cause bronchoconstriction, edema, and mucus production, with a resultant increase in dynamic hyperinflation [71]. Thus, any intervention that reduces inflammation in COPD reduces the number and severity of exacerbations, whereas bronchodilators have an impact on exacerbation by their effects on reducing dynamic hyperinflation. The triggers for the one-third of exacerbations not triggered by infection are postulated to be related to other medical conditions, including pulmonary embolism, aspiration, heart failure, and myocardial ischemia [66].
What are the pharmacologic options available for prevention of AECOPD?
In recognition of the importance of preventing COPD exacerbations, the American College of Chest Physicians and Canadian Thoracic Society [65] have published an evidence-informed clinical guideline specifically examining the prevention of AECOPD, with the goal of assisting clinicians in providing optimal management for COPD patients. The following pharmacologic agents have been recognized as being effective at reducing the frequency of acute exacerbations without any impact on the severity of COPD itself.
Roflumilast
Phosphodiesterase 4 (PDE4) inhibition appears to have inflammatory modulating properties in the airways, although the exact mechanism of action is unclear. Some have proposed that it reduces inflammation by inhibiting the breakdown of intracellular cyclic adenosine monophosphate [72]. In 2 large clinical trials [73,74], daily use of a PDE4 inhibitor (roflumilast) showed a significant (15%–18%) reduction in yearly AECOPD incidence (approximate number needed to treat: 4). This benefit was seen in patients with GOLD stage 3–4 disease (FEV1 < 50% predicted) with the chronic bronchitic phenotype and who had experienced at least 1 exacerbation in the previous year.
Importantly, these clinical trials specifically prohibited the use of ICS and LAMAs. Thus, it remains unclear if PDE4 inhibition should be used as an add-on to ICS/LAMA therapy in patients who continue to have frequent AECOPD or whether PDE4 inhibition could be used instead of these standard therapies in patients with well-controlled daily symptoms without ICS or LAMA therapy but who experience frequent exacerbations.
Of note, earlier trials with roflumilast included patients with ICS and LAMA use [73,75]. These trials were focused on FEV1 improvement and found no benefit. It was only in post ad hoc analyses that a reduction in AECOPD in patients with frequent exacerbations was found among those taking roflumilast, regardless of ICS or LAMA use [76]. While roflumilast has documented benefit in improving lung function and reducing the rate of exacerbations, it has not been reported to decrease hospitalizations [64]. This indicates that although the drug reduces the total number of exacerbations, it may not be as useful in preventing episodes of severe exacerbations of COPD.
Although PDE4 inhibitors are easy to administer (a once-daily pill), they are associated with significant GI side effects (diarrhea, nausea, reduced appetite), weight loss, headache, and sleep disturbance [77]. Adverse effects tend to occur early during treatment, are reversible, and lessen over time with treatment [66]. Studies reported an average unexplained weight loss of 2 kg, and monitoring weight during treatment is advised. In addition, it is important to avoid roflumilast in underweight patients. Roflumilast should also be used with caution in depressed patients [65].
N-acetylcysteine
N-acetylcysteine (NAC) reduces the viscosity of respiratory secretions as a result of the cleavage of the disulfide bonds and has been studied as a mucolytic agent to aid in the elimination of respiratory secretions [78]. Oral NAC is quickly absorbed and is rapidly present in an active form in lung tissue and respiratory secretions after ingestion. NAC is well tolerated except for occasional patients with GI adverse effects. The role of NAC in preventing AECOPD has been studied for more than 3 decades [79–81], although the largest clinical trial to date was reported in 2014 [57]. Taken together, the combined data demonstrate a significant reduction in the rate of COPD exacerbations associated with the use of NAC when compared with placebo (OR, 0.61; CI, 0.37–0.99). Clinical guidelines suggest that in patients with moderate to severe COPD (FEV1/FVC < 0.7, and FEV1 < 80% predicted) receiving maintenance bronchodilator therapy combined with ICS and history of 2 more exacerbations in the previous 2 years, treatment with oral NAC can be administered to prevent AECOPD.
Macrolides
Continuous prophylactic use of antibiotics in older studies had no effect on the frequency of AECOPD [82,83]. But it is known that macrolide antibiotics have several antimicrobial, anti-inflammatory and immunomodulating effects and have been used for many years in the management of other chronic airway disease, including diffuse pan-bronchiolitis and cystic fibrosis [65]. One recent study showed that the use of once-daily, generic azithromycin 5 days/week appeared to have an impact on AECOPD incidence [84]. In this study, AECOPD was reduced from 1.83 to 1.48 per patient-year (RR, 0.83; 95% CI, 0.72–0.95: P = 0.01). Azithromycin also prevented severe AECOPD. Greater benefit was obtained with milder forms of the disease and in the elderly. Azithromycin did not appear to provide any benefit in those who continued to smoke (hazard ratio, 0.99) [85]. Other studies have shown that azithromycin was associated with an increased incidence of bacterial resistance and impaired hearing [86]. Overall data from the available clinical trials are robust and demonstrate that regular macrolide therapy definitely reduces the risk of AECOPD. But due to potential side effects macrolide therapy is an option rather than a strong recommendation [65]. The prescribing clinician also needs to consider the potential of prolongation of the QT interval [84].
Immunostimulants
Immunostimulants have also been reported to reduce frequency of AECOPD [87,88]. Bacterial lysates, reconstituted mixtures of bacterial antigens present in the lower airways of COPD patients, act as immuno-stimulants through the induction of cellular maturation, stimulating lymphocyte chemotaxis, and increasing opsonization when administered to individuals with COPD [66]. Studies have demonstrated a reduction in the severe complications of exacerbations and hospital admissions in COPD patients with OM-85, a detoxified oral immunoactive bacterial extract [87,88]. However, most of these trials were conducted prior to the routine use of long-acting bronchodilators and ICS in COPD. A recent study by Braido et al evaluated the efficacy of ismigen, a bacterial lysate, in reducing AECOPD [89] and found no difference in the exacerbation rate between ismigen and placebo or the time to first exacerbation. Additional studies are needed to examine the long-term effects of this therapy in patients receiving currently recommended COPD maintenance therapy [66].
β Blockers
Observational studies of beta-blocker use in preventing AECOPD have yielded encouraging results, with one study showing a reduction in AECOPD risk (incidence risk ratio, 0.73; CI 0.60–0.90) in patients receiving beta blockers versus those not on beta blockers [90]. Based on these findings, a clinical trial investigating the impact of metoprolol on risk of AECOPD is ongoing [91].
Proton Pump Inhibitors
Gastroesophageal reflux disease is an independent risk factor for exacerbations [92]. Two small, single-center studies [93,94] have shown that use of lansoprazole decreases the risk and frequency of AECOPD. However, data from the Predicting Outcome using Systemic Markers in Severe Exacerbations of COPD (PROMISE-COPD) study [66], which was a multicenter prospective observational study, suggested that patients with stable COPD receiving a proton pump inhibitor were at high risk of frequent and severe exacerbations [95]. Thus, at this stage, their definitive role needs to be defined, possibly with a randomized, placebo-controlled study.
Case 3
A 65-year-old male with severe COPD (FEV1/FVC 27, FEV1 25% of predicted, residual volume 170% of predicted for his age and height) was seen in the pulmonary clinic. His medications include a LABA/LAMA combination that he uses twice daily as advised. He uses his rescue albuterol inhaler roughly once a week. The patient complains of severe disabling shortness of breath with exertion and severe limitation of his quality of life because of his inability to lead a normal active life. He is on 2 L/min of oxygen at all times. He has received pulmonary rehabilitation in hopes of improving his quality of life but can only climb a flight of stairs before he must stop to rest. He asks about options but does not want to consider lung transplantation today. His most recent chest CT scan demonstrates upper lobe predominant emphysematous changes with no masses or nodules.
What are the patient's options at this time?
Lung volume reduction surgery (LVRS) attempts to reduce space-occupying severely diseased, hyperexpanded lung, thus allowing the relatively normal adjoining lung parenchyma to expand into the vacated space and function effectively [96].Hence, such therapies are suitable for patients with emphysematous lungs and not those with bronchitic-predominant COPD. LVRS offers a greater chance of improvement in exercise capacity, lung function, quality of life, and dyspnea in the correctly chosen patient population as compared with pharmacologic management alone [97]. However, the procedure is associated with risks, including higher short-term morbidity and mortality [97]. Patients with predominantly upper-lobe emphysema and a low maximal workload after rehabilitation were noted to have lower mortality, a greater probability of improvement in exercise capacity, and a greater probability of improvement in symptoms if they underwent surgery compared to medical therapy alone [97]. On the contrary, patients with predominantly non–upper-lobe emphysema and a high maximal workload after rehabilitation had higher mortality if they underwent surgery compared to receiving medical therapy alone [97]. Thus, a subgroup of patients with homogeneous emphysema symmetrically affecting the upper and lower lobes are considered to be unlikely to benefit from this surgery [97,98].
Valves and other methods of lung volume reduction such as coils, sealants, intrapulmonary vents, and thermal vapor in the bronchi or subsegmental airways have emerged as new techniques for nonsurgical lung volume reduction [99–104]. Endobronchial-valve therapy is associated with improvement in lung function and with clinical benefits that are greatest in the presence of heterogeneous lung involvement. This works by the same principle as with LVRS, by reduction of the most severely diseased lung units, expansion of the more viable, less emphysematous lung results in substantial improvements in lung mechanics [105,106]. The most important complications of this procedure include pneumonia, pneumothorax, hemoptysis and increased frequency of COPD exacerbation in the following thirty days. The fact that high-heterogeneity subgroup had greater improvements in both the FEV1 and distance on the 6-minute walk test than did patients with lower heterogeneity supports the use of quantitative high-resolution computed tomography (HRCT) in selecting patients for endobronchial-valve therapy [107].The HRCT scans also help in identifying those with complete fissures; a marker of lack of collateral ventilation (CV+) between different lobes. Presence of CV+ state predicts failure of endobronchial valve and all forms of endoscopic lung volume reduction strategies [108]. Bronchoscopic thermal vapor ablation (BTVA) therapy can potentially work on a subsegmental level and be successful for treatment of emphysema with lack of intact fissures on CT scans. Other methods that have the potential to be effective in those with collateral ventilation would be endoscopic coil therapy and polymeric lung volume reduction [106,109].Unfortunately, there are no randomized controlled trial data demonstrating clinically meaningful improvement following coil therapy or polymeric lung volume reduction in this CV+ patient population. Vapor therapy is perhaps the only technique that has been found to be effective in upper lobe predominant emphysema even with CV+ status [108].
Our patient has evidence of air trapping and emphysema based on a high residual volume. A CT scan of the chest can determine the nature of the emphysema (heterogeneous versus homogenous) and based on these findings, further determination of the best strategy for lung volume reduction can be made.
Is there a role for long-term oxygen therapy?
Long-term oxygen therapy (LTOT) used for > 15 hours a day is thought to reduce mortality among patients with chronic obstructive pulmonary disease (COPD) and severe resting hypoxemia [110–113].More recent studies have failed to show similar beneficial effects of LTOT. A recent study examined the effects of LTOT in randomized fashion and determined that supplemental oxygen for patients with stable COPD and resting or exercise-induced moderate desaturation did not affect the time to death or first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, the rate of all hospitalizations, the rate of all COPD exacerbations, or changes in measures of quality of life, depression, anxiety, or functional status [114].
Our patient is currently on long-term oxygen therapy and in spite of some uncertainty as to its benefit, it is prudent to order oxygen therapy until further clarification is available.
What is the role of pulmonary rehabilitation?
Pulmonary rehabilitation is an established treatment for patients with chronic lung disease [115]. Benefits include improvement in exercise tolerance, symptoms, and quality of life, with a reduction in the use of health care resources [116].A Spanish population-based cohort study that looked at the influence of regular physical activity on COPD showed that patients who reported low, moderate, or high physical activity had a lower risk of COPD admissions and all-cause mortality than patients with very low physical activity after adjusting for all confounders [117].
As previously mentioned, patients in GOLD categories B, C, and D should be offered pulmonary rehabilitation as part of their treatment [7]. The ideal patient is one who is not too sick to undergo rehabilitation and is motivated to his or her quality of life.
What is the current scope of lung transplantation in the management of severe COPD?
There is a indisputable role for lung transplantation in end-stage COPD. However, lung transplantation does not benefit all COPD patients. There is a subset of patients for whom the treatment provides a survival benefit. It has been reported that 79% of patients with an FEV1 < 16% predicted will survive at least 1 year additional after transplant, but only 11% of patients with an FEV1 > 25% will do so [118]. The pre-transplant BODE (body mass index, airflow obstruction/FEV1, dyspnea, and exercise capacity) index score is used to identify the patients who will benefit from lung transplantation [119,120]. International guidelines for the selection of lung transplant candidates identify the following patient characteristics [121]:
- The disease is progressive, despite maximal treatment including medication, pulmonary rehabilitation, and oxygen therapy
- The patient is not a candidate for endoscopic or surgical LVRS
- BODE index of 5 to 6
- The partial pressure of carbon dioxide is greater than 50 mm Hg or 6.6kPa and/or partial pressure of oxygen is less than 60 mm Hg or 8kPa
- FEV1 of 25% predicted
The perioperative mortality of lung transplantation surgery has been reduced to less than 10%. Risk of complications from surgery in the perioperative period, such as bronchial dehiscence, infectious complications, and acute rejection, have also been reduced but do occur. Chronic allograft dysfunction and the risk of lung cancer in cases of single lung transplant should be discussed with the patient before surgery [122].
How can we incorporate palliative care into the management plan for patients with COPD?
Among patients with end-stage COPD on home oxygen therapy who have required mechanical ventilation for an exacerbation, only 55% are alive at 1 year [123]. COPD patients at high risk of death within the next year of life as well as patients with refractory symptoms and unmet needs are candidates for early palliative care. Palliative care and palliative care specialists can aid in reducing symptom burden and improving quality of life among these patients and their family members and is recommended by multiple international societies for patients with advanced COPD [124,125]. In spite of these recommendations, the utilization of palliative care resources has been dismally low [126,127]. Improving physician-patient communication regarding palliative services and patients’ unmet care needs will help ensure that COPD patients receive adequate palliative care services at the appropriate time.
Conclusion
COPD is a leading cause of morbidity and mortality in the United States and represents a significant economic burden for both individuals and society. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. COPD management is guided by disease severity that is measured using the GOLD multimodal staging system and requires a multidisciplinary approach. Several classes of medication are available for treatment, and a step-wise approach should be applied in building an effective pharmacologic regimen. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
Corresponding author: Abhishek Biswas, MD, Division of Pulmonary and Critical Care Medicine, Rm. M452, University of Florida, 1600 SW Archer Rd, Gainesville, FL 32610, abiswas@ufl.edu.
Financial disclosures: None.
From the Division of Pulmonary Critical Care Medicine, University of Florida, Gainesville, FL.
Abstract
- Objective:To review the management of stable chronic obstructive pulmonary disease (COPD).
- Methods: Review of the peer-reviewed literature.
- Results: Effective management of stable COPD requires the physician to apply a stepwise intensification of therapy depending on patient symptoms and functional reserve. Bronchodilators are the cornerstone of management. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
- Conclusion: Successful management of stable COPD requires a multidisciplinary approach that utilizes various medical therapies as well as nonpharmacologic interventions.
Key words: chronic obstructive pulmonary disease; exacerbation; bronchodilator; lung volume reduction; cough.
Chronic obstructive pulmonary disease (COPD) is a systemic inflammatory disease characterized by irreversible obstructive ventilatory defects [1–4]. It is a major cause of morbidity and mortality affecting 5% of the population in the United States and was the third leading cause of death in 2008 [5,6]. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. In this review, we will discuss the management of stable COPD in the context of 3 common clinical scenarios.
Case 1
A 65-year-old male with COPD underwent pulmonary function testing (PFT), which demonstrated an obstructive ventilatory defect (forced expiratory volume in 1 second/forced vital capacity ratio [FEV1/FVC], 0.45; FEV1, 2 L [65% of predicted]; and diffusing capacity of the lung for carbon monoxide [DLCO], 15 [65% of predicted]). He has dyspnea with strenuous exercise but is comfortable at rest and with minimal exercise. He has had 1 exacerbation in the last year that was treated on an outpatient basis with steroids and antibiotics. His medication regimen includes inhaled tiotropium once daily and inhaled albuterol as needed that he uses roughly twice a week.
What determines the appropriate therapy for a given COPD patient?
What is the approach to building a pharmacologic regimen for the patient with COPD?
The backbone of the pharmacologic regimen for COPD includes short- and long-acting bronchodilators. They are usually given in an inhaled form to maximize local effects on the lungs and minimize systemic side effects. There are 2 main classes of bronchodilators, beta agonists and muscarinic antagonists, and each targets specific receptors on the surface of airway smooth muscle cells. Beta agonists work by stimulating beta-2 receptors, resulting in bronchodilation, while muscarinic antagonists work by blocking the bronchoconstrictor action of M3 muscarinic receptors. Inhaled corticosteroids can be added to long-acting bronchodilator therapy but cannot be used as stand-alone therapy. Theophylline is an oral bronchodilator that is used infrequently due to its narrow therapeutic index, toxicity, and multiple drug interactions.
Who should be on short-acting bronchodilators? What is the best agent? Should it be scheduled or used as needed?
All patients with COPD should be an on inhaled short-acting bronchodilator as needed for relief of symptoms [7]. Both short-acting beta agonists (albuterol and levalbuterol) and short-acting muscarinic antagonists (ipratropium) have been shown in clinical trials and meta-analyses to improve symptoms and lung function in patients with stable COPD [9,10] and seem to have comparative efficacy when compared head-to-head in trials [11]. However, the airway bronchodilator effect achieved by both classes seems to be additive when used in combination and is also associated with less exacerbations compared to albuterol alone [12]. On the other hand, adding albuterol to ipratropium increased the bronchodilator response but did not reduce the exacerbation rate [11–13]. Inhaled short-acting beta agonists when used as needed rather than scheduled are associated with less medication use without any significant difference in symptoms or lung function [14].
The side effects related to using recommended doses of a short-acting bronchodilator are minimal. In retrospective studies, short-acting beta agonists increased the risk of severe cardiac arrhythmias [15]. Levalbuterol, the active enantiomer of albuterol (R-albuterol) developed for the theoretical benefits of reduced tachycardia, increased tolerability, and better or equal efficacy compared to racemic albuterol, failed to show a clinically significant difference in inducing tachycardia [16]. Beta agonist overuse is associated with tremor and in severe cases hypokalemia, which happens mainly when patients try to achieve maximal bronchodilation; the clinically used doses of beta agonists are associated with fewer side affects but achieve less than maximal bronchodilation [17]. Ipratropium can produce systemic anticholinergic side effects, urinary retention being the most clinically significant especially when combined with long-acting anticholinergic agents [18].
In light of the above discussion, a combination of short-acting beta agonist and muscarinic antagonist is recommended in all patients with COPD unless the patient is on a long-acting muscarinic antagonist [7,18]. In the latter case, a short-acting beta agonist used as a rescue inhaler is the best option. In our patient, albuterol was the choice for his short-acting bronchodilator as he was using the long-acting muscarinic antagonist tiotropium.
Are short-acting bronchodilators enough? What do we use for maintenance therapy?
All patients with COPD who are category B or higher according to the modified GOLD staging system should be on a long-acting bronchodilator [7,19]: either a long-acting beta agonist (LABA) or long-acting muscarinic antagonist (LAMA). Long-acting bronchodilators work on the same receptors as their short-acting counterparts but have structural differences. Salmeterol is the prototype for long-acting selective beta-2 agonist. It is structurally similar to albuterol but has an elongated side chain that allows it to bind firmly to the area of beta receptors and stimulate them repetitively, resulting in an extendedduration of action [20]. Tiotropium on the other hand is a quaternary ammonium of ipratropium that is a nonselective muscarinic antagonist [21]. Compared to ipratropium, tiotropium dissociates more quickly from M2 receptors, which is responsible for the undesired anticholinergic effects, while at the same time it binds M1 and M3 receptors for a prolonged time, resulting in extended duration of action [21].
The currently available long-acting beta agonists include salmeterol, formoterol, aformoterol, olodatetol, and indacaterol. The last two have the advantage of once-daily dosing rather than twice [22,23]. LABAs have been shown to improve lung function, exacerbation rate, and quality of life in multiple clinical trials [22–24]. Vilanterol is another LABA that has a long duration of action and can be used once daily [25], but is only available in a combination with umeclidinium, a LAMA. Several LAMAs are approved for use in COPD, including the prototype tiotropium in addition to aclidinium, umeclidinium, and glycopyrronium. These have been shown in clinical trials to improve lung function, symptoms, and exacerbation rate [26–29].
Patients can be started on either a LAMA or LABA depending on patient needs and side effects [7]. Both have comparable side effects and efficacy as detailed below. Concerning side effects, there is conflicting data concerning an association of cardiovascular events with both classes of long-acting bronchodilators. While clinical trials failed to show an increased risk [24,30,31], several retrospective studies showed an increased risk of emergency room visits and hospitalizations due to tachyarrhythmias, heart failure, myocardial infarction, and stroke upon initiation of long-acting bronchodilators [32,33]. There was no difference in risk for adverse cardiovascular events between LABA and LAMA in one Canadian study, and slightly more with LABA in a study using an American database [32,33]. Urinary retention is another possible complication of LAMA supported by evidence from meta-analyses and retrospective studies but not clinical trials and should be discussed with patients upon initiation [34,35]. There have been concerns about increased mortality with the soft mist formulation of tiotropium that were put to rest by the tiotropium safety and performance in Respimat (TIOSPIR) trial, which showed no increased mortality compared to Handihaler [36].
As far as efficacy and benefits, tiotropium and salmeterol were compared head-to-head in a clinical trial, and tiotropium increased the time before developing first exacerbation and decreased the overall rate of exacerbations [37]. No difference in hospitalization rate or mortality was noted in one meta-analysis, although tiotropium was more effective in reducing exacerbations [38]. The choice of agent should be made based on patient comorbidities and side effects. For example, an elderly patient with severe benign prostatic hyperplasia and urinary retention should try a LABA while for a patient with severe tachycardia induced by albuterol, LAMA would be a better first agent.
What is the role of inhaled corticosteroids in COPD?
Inhaled corticosteroids (ICS) are believed to work in COPD by reducing airway inflammation [39]. ICS should not be used alone for COPD management and are always combined with LABA [7]. Several inhaled corticosteroid formulations are approved for use in COPD, including budesonide and fluticasone. ICS has been shown to decrease symptoms and exacerbations with modest effect on lung function and no change in mortality [40]. Side effects include oral candidiasis, dysphonia, and skin bruising [41]. There is also an increased risk of pneumonia [42]. ICS are best reserved for patients with a component of asthma or asthma–COPD overlap syndrome (ACOS) [43]. ACOS is characterized by persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD [44].
What if the patient is still symptomatic on a LABA or LAMA?
For patients whose symptoms are not controlled on one class of long-acting bronchodilator, recommendations are to add a bronchodilator from the other class [7]. There are also multiple combined LAMA-LABA inhalers that are approved in the US and can possible improve adherence to therapy. These include tiotropium-oladeterol, umeclidinium-vilanterol, glycopyronnium-indacaterol, and glycopyrrolate-formoterol. In a large systematic review and meta-analysis comparing LABA-LAMA combination to either agent alone, there was a modest improvement in post bronchodilator FEV1 and quality of life with no change in hospital admissions, mortality, or side effects [45]. Interestingly, adding tiotropium to LABA reduced exacerbations although adding LABA to tiotropium did not [45].
Current guidelines recommend that patients in GOLD categories C and D that are not well controlled should receive a combination of LABA-ICS [7]. However, a new randomized trial showed better reduction of exacerbations and decreased occurrence of pneumonia in patients receiving LAMA-LABA compared to LABA-ICS [46]. In light of this new evidence, it is prudent to use a LAMA-LABA combination before adding ICS.
Triple therapy with LAMA, LABA, and ICS is a common approach for patients with severe uncontrolled disease and has been shown to decrease exacerbations and improve quality of life [7,47]. Adding tiotropium to LABA-ICS decreased exacerbations and improved quality of life and airflow in the landmark UPLIFT trial [26]. In another clinical trial, triple therapy with LAMA, LABA, and ICS compared to tiotropium alone decreased severe exacerbations, pre-bronchodilator FEV1, and morning symptoms [48].
Is there a role for theophylline? Other agents?
Theophylline
Theophylline is an oral adenosine diphosphate antagonist with indirect adrenergic activity, which is responsible for the bronchodilator therapeutic effect in patients with obstructive lung disease. It is also thought to work by an additional mechanism that decreases inflammation in the airways [49]. It has a serious side effect profile that includes ventricular arrhythmias, seizures, vomiting, and tremor [50]. It is metabolized in the liver and has multiple drug interactions and a narrow therapeutic index. It has been shown to improve lung function, gas exchange and symptoms in meta-analysis and clinical trials [51,52].
In light of the nature of the adverse effects and the wide array of safer and more effective pharmacologic agents available, theophylline should be avoided early on in patients with COPD. Its use can be justified as an add-on therapy in patients with refractory disease on triple therapy for symptomatic relief [50]. If used, the therapeutic range for COPD is 8–12 mcg/mL peak level measured 3 to 7 hours after morning dose and is usually achieved using a daily dose of 10 mg per kilogram of body weight for nonobese patients [53].
Systemic Steroids
Oral steroids are used in COPD exacerbations but should never be used chronically in COPD patients regardless of disease severity as they increase morbidity and mortality without improving symptoms or lung function [54,55]. The dose of systemic steroids should be tapered and finally discontinued.
Mucolytics
Classes of mucolytics include thiol derivatives, inhaled dornase alpha, hypertonic saline, and iodine preparations. Thiol derivatives such as N-acetylcysteine are the most widely studied [56].
There is no consistent evidence of beneficial role of mucolytics in COPD patient [7,56]. The PANTHEON trial showed decreased exacerbations with N-acetylcysteine (1.16 exacerbations per patient-year compared to 1.49 exacerbations per patient-year in the placebo group; risk ratio 0.78, 95% CI 0.67–0.90; P = 0.001) but had methodologic issues including high drop-out rate, exclusion of patients on oxygen, and a large of proportion of nonsmokers [57].
Chronic Antibiotics
There is no role for chronic antibiotics in the management of COPD [7]. Macrolides are an exception but are used for their anti-inflammatory effects rather than their antibiotic effects. They should be reserved for patient with frequent exacerbations on optimal therapy and will be discussed later in the review [58].
What nonpharmacologic treatments are recommended for COPD patients?
Smoking cessation, oxygen therapy for severe hypoxemia (resting O2 saturation ≤ 88 or PaO2 ≤ 55), vaccination for influenza and pneumococcus, and appropriate nutrition should be provided in all COPD patients. Pulmonary rehabilitation is indicated for patients in GOLD categories B, C, and D [7]. It improves symptoms, quality of life, exercise tolerance and health care utilization. Beneficial effects last for about 2 years [59,60].
What other diagnoses should be considered in patients who continue to be symptomatic on optimal therapy?
Other diseases that share the same risk factors as COPD and can contribute to dyspnea, including coronary heart disease, heart failure, thromboembolic disease, and pulmonary hypertension, should be considered. In addition, all patients with refractory disease should have a careful assessment of their inhaler technique, continued smoking, need for oxygen therapy, and associated deconditioning.
Case 2
A 70-year-old male with severe COPD on oxygen therapy and obstructive sleep apnea treated on nocturnal CPAP was seen in the pulmonary clinic for evaluation of his dyspnea. He was symptomatic with minimal activity and had chronic cough with some sputum production. He had been hospitalized 3 times over the past 12 months and had been to the emergency department (ED) the same number of times for dyspnea. Pertinent medications included as-needed albuterol inhaler, inhaled steroids, and tiotropium 18 mcg inhaled daily. He demonstrated good inhaler technique. On examination, his vital signs were pulse 99 bpm, SpO2 94% on 2L/min oxygen by nasal cannula, blood pressure 126/72 mm Hg, respiratory rate 15, and BMI 35 kg/m2. He appeared chronically ill but in no acute distress. No wheezing or rales were heard. He had no lower extremity edema. The remainder of the exam was within normal limits. His last pulmonary function test demonstrated moderate obstruction with significant bronchodilator response to 2 puffs of albuterol. The side effects of chronic steroid therapy were impressed upon the patient and 500 mg of roflumilast was started daily. Over the course of the next 3 months, he had no further exacerbations. Roflumilast was continued. He has not required any further hospitalizations, ED visits, or oral steroid use since the last clinic visit.
What is the significance of acute exacerbations of COPD?
Acute exacerbation of COPD (AECOPD) is a frequently observed complication for many patients with COPD [61,62]. AECOPD is associated with accelerated disease progression, augmented decline in health status and quality of life, and increased mortality [63]. Exacerbations account for most of the costs associated with COPD. Estimates suggest that the aggregate costs associated with the treatment of AECOPDs are between $3.2 and $3.8 billion, and that annual health care costs are 10-fold greater for patients with COPD associated with acute exacerbations than for patients with COPD but without exacerbations [64]. Hence, any intervention that could potentially minimize or prevent this complication will have far-reaching benefits to patients with COPD as well as provide significant cost saving.
How is acute exacerbation of COPD defined?
COPD exacerbation is defined as a baseline change of the patient’s dyspnea, cough, and/or sputum that is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD [65]. Exacerbation in clinical trials has been defined on the basis of whether an increase in the level of care beyond regular care is required primarily in the hospital or ED [66]. Frequent exacerbations are defined as 3 symptom-defined exacerbations per year or 2 per year if defined by the need for therapy with corticosteroids, antibiotics, or both [67].
What is the underlying pathophysiology?
AECOPD is associated with enhanced upper and lower airway and systemic inflammation. The bronchial mucosa of stable COPD patients have increased numbers of CD8+ lymphocytes and macrophages. In mild AECOPD, eosinophils are increased in the bronchial mucosa and modest elevation of neutrophils, T lymphocytes (CD3), and TNF alpha positive cells has also been reported [62]. With more severe AECOPD, airway neutrophils are increased. Oxidative stress is a key factor in the development of airway inflammation in COPD [61]. Patients with severe exacerbations have augmented large airway interleukin-8 (IL-8) levels and increased oxidative stress as demonstrated by markers such as hydrogen peroxide and 8-isoprostane [66].
How do acute exacerbations affect the course of the disease?
In general, as the severity of the underlying COPD increases, exacerbations become both more severe and more frequent. The quality of life of patients with frequent exacerbations is worse than patients with a history of less frequent exacerbations [68]. Frequent exacerbations have also been linked to a decline in lung function, with studies suggesting that there might be a decline of 7 mL in FEV1 per lower respiratory tract infection per year [59,69] and approxi-mately 8 mL per year in patients with frequent exacerbations as compared to those with sporadic exacerbations [70].
What are the triggers for COPD exacerbation?
Respiratory infections are estimated to trigger approximately two-thirds of exacerbations [62]. Viral and bacterial infections cause most exacerbations. The effect of the infective triggers is to increase inflammation, cause bronchoconstriction, edema, and mucus production, with a resultant increase in dynamic hyperinflation [71]. Thus, any intervention that reduces inflammation in COPD reduces the number and severity of exacerbations, whereas bronchodilators have an impact on exacerbation by their effects on reducing dynamic hyperinflation. The triggers for the one-third of exacerbations not triggered by infection are postulated to be related to other medical conditions, including pulmonary embolism, aspiration, heart failure, and myocardial ischemia [66].
What are the pharmacologic options available for prevention of AECOPD?
In recognition of the importance of preventing COPD exacerbations, the American College of Chest Physicians and Canadian Thoracic Society [65] have published an evidence-informed clinical guideline specifically examining the prevention of AECOPD, with the goal of assisting clinicians in providing optimal management for COPD patients. The following pharmacologic agents have been recognized as being effective at reducing the frequency of acute exacerbations without any impact on the severity of COPD itself.
Roflumilast
Phosphodiesterase 4 (PDE4) inhibition appears to have inflammatory modulating properties in the airways, although the exact mechanism of action is unclear. Some have proposed that it reduces inflammation by inhibiting the breakdown of intracellular cyclic adenosine monophosphate [72]. In 2 large clinical trials [73,74], daily use of a PDE4 inhibitor (roflumilast) showed a significant (15%–18%) reduction in yearly AECOPD incidence (approximate number needed to treat: 4). This benefit was seen in patients with GOLD stage 3–4 disease (FEV1 < 50% predicted) with the chronic bronchitic phenotype and who had experienced at least 1 exacerbation in the previous year.
Importantly, these clinical trials specifically prohibited the use of ICS and LAMAs. Thus, it remains unclear if PDE4 inhibition should be used as an add-on to ICS/LAMA therapy in patients who continue to have frequent AECOPD or whether PDE4 inhibition could be used instead of these standard therapies in patients with well-controlled daily symptoms without ICS or LAMA therapy but who experience frequent exacerbations.
Of note, earlier trials with roflumilast included patients with ICS and LAMA use [73,75]. These trials were focused on FEV1 improvement and found no benefit. It was only in post ad hoc analyses that a reduction in AECOPD in patients with frequent exacerbations was found among those taking roflumilast, regardless of ICS or LAMA use [76]. While roflumilast has documented benefit in improving lung function and reducing the rate of exacerbations, it has not been reported to decrease hospitalizations [64]. This indicates that although the drug reduces the total number of exacerbations, it may not be as useful in preventing episodes of severe exacerbations of COPD.
Although PDE4 inhibitors are easy to administer (a once-daily pill), they are associated with significant GI side effects (diarrhea, nausea, reduced appetite), weight loss, headache, and sleep disturbance [77]. Adverse effects tend to occur early during treatment, are reversible, and lessen over time with treatment [66]. Studies reported an average unexplained weight loss of 2 kg, and monitoring weight during treatment is advised. In addition, it is important to avoid roflumilast in underweight patients. Roflumilast should also be used with caution in depressed patients [65].
N-acetylcysteine
N-acetylcysteine (NAC) reduces the viscosity of respiratory secretions as a result of the cleavage of the disulfide bonds and has been studied as a mucolytic agent to aid in the elimination of respiratory secretions [78]. Oral NAC is quickly absorbed and is rapidly present in an active form in lung tissue and respiratory secretions after ingestion. NAC is well tolerated except for occasional patients with GI adverse effects. The role of NAC in preventing AECOPD has been studied for more than 3 decades [79–81], although the largest clinical trial to date was reported in 2014 [57]. Taken together, the combined data demonstrate a significant reduction in the rate of COPD exacerbations associated with the use of NAC when compared with placebo (OR, 0.61; CI, 0.37–0.99). Clinical guidelines suggest that in patients with moderate to severe COPD (FEV1/FVC < 0.7, and FEV1 < 80% predicted) receiving maintenance bronchodilator therapy combined with ICS and history of 2 more exacerbations in the previous 2 years, treatment with oral NAC can be administered to prevent AECOPD.
Macrolides
Continuous prophylactic use of antibiotics in older studies had no effect on the frequency of AECOPD [82,83]. But it is known that macrolide antibiotics have several antimicrobial, anti-inflammatory and immunomodulating effects and have been used for many years in the management of other chronic airway disease, including diffuse pan-bronchiolitis and cystic fibrosis [65]. One recent study showed that the use of once-daily, generic azithromycin 5 days/week appeared to have an impact on AECOPD incidence [84]. In this study, AECOPD was reduced from 1.83 to 1.48 per patient-year (RR, 0.83; 95% CI, 0.72–0.95: P = 0.01). Azithromycin also prevented severe AECOPD. Greater benefit was obtained with milder forms of the disease and in the elderly. Azithromycin did not appear to provide any benefit in those who continued to smoke (hazard ratio, 0.99) [85]. Other studies have shown that azithromycin was associated with an increased incidence of bacterial resistance and impaired hearing [86]. Overall data from the available clinical trials are robust and demonstrate that regular macrolide therapy definitely reduces the risk of AECOPD. But due to potential side effects macrolide therapy is an option rather than a strong recommendation [65]. The prescribing clinician also needs to consider the potential of prolongation of the QT interval [84].
Immunostimulants
Immunostimulants have also been reported to reduce frequency of AECOPD [87,88]. Bacterial lysates, reconstituted mixtures of bacterial antigens present in the lower airways of COPD patients, act as immuno-stimulants through the induction of cellular maturation, stimulating lymphocyte chemotaxis, and increasing opsonization when administered to individuals with COPD [66]. Studies have demonstrated a reduction in the severe complications of exacerbations and hospital admissions in COPD patients with OM-85, a detoxified oral immunoactive bacterial extract [87,88]. However, most of these trials were conducted prior to the routine use of long-acting bronchodilators and ICS in COPD. A recent study by Braido et al evaluated the efficacy of ismigen, a bacterial lysate, in reducing AECOPD [89] and found no difference in the exacerbation rate between ismigen and placebo or the time to first exacerbation. Additional studies are needed to examine the long-term effects of this therapy in patients receiving currently recommended COPD maintenance therapy [66].
β Blockers
Observational studies of beta-blocker use in preventing AECOPD have yielded encouraging results, with one study showing a reduction in AECOPD risk (incidence risk ratio, 0.73; CI 0.60–0.90) in patients receiving beta blockers versus those not on beta blockers [90]. Based on these findings, a clinical trial investigating the impact of metoprolol on risk of AECOPD is ongoing [91].
Proton Pump Inhibitors
Gastroesophageal reflux disease is an independent risk factor for exacerbations [92]. Two small, single-center studies [93,94] have shown that use of lansoprazole decreases the risk and frequency of AECOPD. However, data from the Predicting Outcome using Systemic Markers in Severe Exacerbations of COPD (PROMISE-COPD) study [66], which was a multicenter prospective observational study, suggested that patients with stable COPD receiving a proton pump inhibitor were at high risk of frequent and severe exacerbations [95]. Thus, at this stage, their definitive role needs to be defined, possibly with a randomized, placebo-controlled study.
Case 3
A 65-year-old male with severe COPD (FEV1/FVC 27, FEV1 25% of predicted, residual volume 170% of predicted for his age and height) was seen in the pulmonary clinic. His medications include a LABA/LAMA combination that he uses twice daily as advised. He uses his rescue albuterol inhaler roughly once a week. The patient complains of severe disabling shortness of breath with exertion and severe limitation of his quality of life because of his inability to lead a normal active life. He is on 2 L/min of oxygen at all times. He has received pulmonary rehabilitation in hopes of improving his quality of life but can only climb a flight of stairs before he must stop to rest. He asks about options but does not want to consider lung transplantation today. His most recent chest CT scan demonstrates upper lobe predominant emphysematous changes with no masses or nodules.
What are the patient's options at this time?
Lung volume reduction surgery (LVRS) attempts to reduce space-occupying severely diseased, hyperexpanded lung, thus allowing the relatively normal adjoining lung parenchyma to expand into the vacated space and function effectively [96].Hence, such therapies are suitable for patients with emphysematous lungs and not those with bronchitic-predominant COPD. LVRS offers a greater chance of improvement in exercise capacity, lung function, quality of life, and dyspnea in the correctly chosen patient population as compared with pharmacologic management alone [97]. However, the procedure is associated with risks, including higher short-term morbidity and mortality [97]. Patients with predominantly upper-lobe emphysema and a low maximal workload after rehabilitation were noted to have lower mortality, a greater probability of improvement in exercise capacity, and a greater probability of improvement in symptoms if they underwent surgery compared to medical therapy alone [97]. On the contrary, patients with predominantly non–upper-lobe emphysema and a high maximal workload after rehabilitation had higher mortality if they underwent surgery compared to receiving medical therapy alone [97]. Thus, a subgroup of patients with homogeneous emphysema symmetrically affecting the upper and lower lobes are considered to be unlikely to benefit from this surgery [97,98].
Valves and other methods of lung volume reduction such as coils, sealants, intrapulmonary vents, and thermal vapor in the bronchi or subsegmental airways have emerged as new techniques for nonsurgical lung volume reduction [99–104]. Endobronchial-valve therapy is associated with improvement in lung function and with clinical benefits that are greatest in the presence of heterogeneous lung involvement. This works by the same principle as with LVRS, by reduction of the most severely diseased lung units, expansion of the more viable, less emphysematous lung results in substantial improvements in lung mechanics [105,106]. The most important complications of this procedure include pneumonia, pneumothorax, hemoptysis and increased frequency of COPD exacerbation in the following thirty days. The fact that high-heterogeneity subgroup had greater improvements in both the FEV1 and distance on the 6-minute walk test than did patients with lower heterogeneity supports the use of quantitative high-resolution computed tomography (HRCT) in selecting patients for endobronchial-valve therapy [107].The HRCT scans also help in identifying those with complete fissures; a marker of lack of collateral ventilation (CV+) between different lobes. Presence of CV+ state predicts failure of endobronchial valve and all forms of endoscopic lung volume reduction strategies [108]. Bronchoscopic thermal vapor ablation (BTVA) therapy can potentially work on a subsegmental level and be successful for treatment of emphysema with lack of intact fissures on CT scans. Other methods that have the potential to be effective in those with collateral ventilation would be endoscopic coil therapy and polymeric lung volume reduction [106,109].Unfortunately, there are no randomized controlled trial data demonstrating clinically meaningful improvement following coil therapy or polymeric lung volume reduction in this CV+ patient population. Vapor therapy is perhaps the only technique that has been found to be effective in upper lobe predominant emphysema even with CV+ status [108].
Our patient has evidence of air trapping and emphysema based on a high residual volume. A CT scan of the chest can determine the nature of the emphysema (heterogeneous versus homogenous) and based on these findings, further determination of the best strategy for lung volume reduction can be made.
Is there a role for long-term oxygen therapy?
Long-term oxygen therapy (LTOT) used for > 15 hours a day is thought to reduce mortality among patients with chronic obstructive pulmonary disease (COPD) and severe resting hypoxemia [110–113].More recent studies have failed to show similar beneficial effects of LTOT. A recent study examined the effects of LTOT in randomized fashion and determined that supplemental oxygen for patients with stable COPD and resting or exercise-induced moderate desaturation did not affect the time to death or first hospitalization, time to first COPD exacerbation, time to first hospitalization for a COPD exacerbation, the rate of all hospitalizations, the rate of all COPD exacerbations, or changes in measures of quality of life, depression, anxiety, or functional status [114].
Our patient is currently on long-term oxygen therapy and in spite of some uncertainty as to its benefit, it is prudent to order oxygen therapy until further clarification is available.
What is the role of pulmonary rehabilitation?
Pulmonary rehabilitation is an established treatment for patients with chronic lung disease [115]. Benefits include improvement in exercise tolerance, symptoms, and quality of life, with a reduction in the use of health care resources [116].A Spanish population-based cohort study that looked at the influence of regular physical activity on COPD showed that patients who reported low, moderate, or high physical activity had a lower risk of COPD admissions and all-cause mortality than patients with very low physical activity after adjusting for all confounders [117].
As previously mentioned, patients in GOLD categories B, C, and D should be offered pulmonary rehabilitation as part of their treatment [7]. The ideal patient is one who is not too sick to undergo rehabilitation and is motivated to his or her quality of life.
What is the current scope of lung transplantation in the management of severe COPD?
There is a indisputable role for lung transplantation in end-stage COPD. However, lung transplantation does not benefit all COPD patients. There is a subset of patients for whom the treatment provides a survival benefit. It has been reported that 79% of patients with an FEV1 < 16% predicted will survive at least 1 year additional after transplant, but only 11% of patients with an FEV1 > 25% will do so [118]. The pre-transplant BODE (body mass index, airflow obstruction/FEV1, dyspnea, and exercise capacity) index score is used to identify the patients who will benefit from lung transplantation [119,120]. International guidelines for the selection of lung transplant candidates identify the following patient characteristics [121]:
- The disease is progressive, despite maximal treatment including medication, pulmonary rehabilitation, and oxygen therapy
- The patient is not a candidate for endoscopic or surgical LVRS
- BODE index of 5 to 6
- The partial pressure of carbon dioxide is greater than 50 mm Hg or 6.6kPa and/or partial pressure of oxygen is less than 60 mm Hg or 8kPa
- FEV1 of 25% predicted
The perioperative mortality of lung transplantation surgery has been reduced to less than 10%. Risk of complications from surgery in the perioperative period, such as bronchial dehiscence, infectious complications, and acute rejection, have also been reduced but do occur. Chronic allograft dysfunction and the risk of lung cancer in cases of single lung transplant should be discussed with the patient before surgery [122].
How can we incorporate palliative care into the management plan for patients with COPD?
Among patients with end-stage COPD on home oxygen therapy who have required mechanical ventilation for an exacerbation, only 55% are alive at 1 year [123]. COPD patients at high risk of death within the next year of life as well as patients with refractory symptoms and unmet needs are candidates for early palliative care. Palliative care and palliative care specialists can aid in reducing symptom burden and improving quality of life among these patients and their family members and is recommended by multiple international societies for patients with advanced COPD [124,125]. In spite of these recommendations, the utilization of palliative care resources has been dismally low [126,127]. Improving physician-patient communication regarding palliative services and patients’ unmet care needs will help ensure that COPD patients receive adequate palliative care services at the appropriate time.
Conclusion
COPD is a leading cause of morbidity and mortality in the United States and represents a significant economic burden for both individuals and society. The goals in COPD management are to provide symptom relief, improve the quality of life, preserve lung function, and reduce the frequency of exacerbations and mortality. COPD management is guided by disease severity that is measured using the GOLD multimodal staging system and requires a multidisciplinary approach. Several classes of medication are available for treatment, and a step-wise approach should be applied in building an effective pharmacologic regimen. In addition to pharmacologic therapies, nonpharmacologic therapies, including smoking cessation, vaccinations, proper nutrition, and maintaining physical activity, are an important part of long-term management. Those who continue to be symptomatic despite appropriate maximal therapy may be candidates for lung volume reduction. Palliative care services for COPD patients, which can aid in reducing symptom burden and improving quality of life, should not be overlooked.
Corresponding author: Abhishek Biswas, MD, Division of Pulmonary and Critical Care Medicine, Rm. M452, University of Florida, 1600 SW Archer Rd, Gainesville, FL 32610, abiswas@ufl.edu.
Financial disclosures: None.
1. Segreti A, Stirpe E, Rogliani P, Cazzola M. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther 2014;18:381–8.
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182:598–604.
3. Aubier M, Marthan R, Berger P, et al. [COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms]. Rev Mal Respir 2010;27:1254–66.
4. Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J (Engl) 2010;123:3467–78.
5. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741–50.
6. Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep 2011;59:1–126.
7. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. Accessed at www.goldcopd.org.
8. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–54.
9. Wadbo M, Löfdahl CG, Larsson K, et al. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur Respir J 2002;20:1138–46.
10. Ram FS, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax 2003;58:580–4.
11. Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med 1996;100(1A):11S–8S.
12. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest 1994;105:1411–9.
13. Friedman M, Serby CW, Menjoge SS, et al. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest 1999;115:635–41.
14. Cook D, Guyatt G, Wong E, et al. Regular versus as-needed short-acting inhaled beta-agonist therapy for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:85–90.
15. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest 2012;142:305–11.
16. Scott VL, Frazee LA. Retrospective comparison of nebulized levalbuterol and albuterol for adverse events in patients with acute airflow obstruction. Am J Ther 2003;10:341–7.
17. Wong CS, Pavord ID, Williams J, et al. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet 1990;336:1396–9.
18. Cole JM, Sheehan AH, Jordan JK. Concomitant use of ipratropium and tiotropium in chronic obstructive pulmonary disease. Ann Pharmacother 2012;46:1717–21.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155 :179–91.
20. Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med 1992;327:1420–5.
21. Takahashi T, Belvisi MG, Patel H, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med 1994;150(6 Pt 1):1640–5.
22. Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010;182:155–62.
23. Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis 2014;9:697–714.
24. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–89.
25. Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest 2012;142:119–27.
26. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–54.
27. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013;1:524–33.
28. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012;40:830–6.
29. D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res 2011;12:156.
30. Antoniu SA. UPLIFT Study: the effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–54. Expert Opin Pharmacother 2009;10:719–22.
31. Nelson HS, Gross NJ, Levine B, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12-week, multicenter, randomized, double- blind, double-dummy, placebo- and active-controlled trial. Clin Ther 2007;29:2167–78.
32. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 2013;173:1175–85.
33. Aljaafareh A, Valle JR, Lin YL, et al. Risk of cardiovascular events after initiation of long-acting bronchodilators in patients with chronic obstructive lung disease: A population-based study. SAGE Open Med 2016;4:2050312116671337.
34. O’Connor AB. Tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2009;360:185–6.
35. Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest 2006;130:1695–703.
36. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013;369:1491–501.
37. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093–103.
38. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012(9):CD009157.
39. Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med 2005;5:3.
40. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012(7):CD002991.
41. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest 2004;126:213–9.
42. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:2407–16.
43. Lee SY, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016;11:2797–803.
44. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med 2015;373:1241–9.
45. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015:CD008989.
46. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016;374:2222–34.
47. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007;146:545–55.
48. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:741–50.
49. Gallelli L, Falcone D, Cannataro R, et al. Theophylline action on primary human bronchial epithelial cells under proinflammatory stimuli and steroidal drugs: a therapeutic rationale approach. Drug Des Devel Ther 2017;11:265–72.
50. Paloucek FP, Rodvold KA. Evaluation of theophylline overdoses and toxicities. Ann Emerg Med 1988;17:135–44.
51. Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002(4):CD003902.
52. Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med 1989;320:1521–5.
53. Devereux G, Cotton S, Barnes P, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials 2015;16:267.
54. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005(3):CD005374.
55. Horita N, Miyazawa N, Morita S, et al. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res 2014;15:37.
56. Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015(7):CD001287.
57. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014;2:187–94.
58. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008;178:1139–47.
59. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823– 32.
60. Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976–83.
61. Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med 2014;35:157–63.
62. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786–96.
63. Spencer S, Calverley PMA, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004;23:698–702.
64. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benef 2014;7:98.
65. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015;147:894–942.
66. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report. Respirology 2017;22:575–601.
67. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med 2013;11:181.
68. Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–22.
69. Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001;164:358–64.
70. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–52.
71. Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–21.
72. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 2011;163:53–67.
73. Calverley PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685–94.
74. Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374:695–703.
75. Lee S, Hui DSC, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology 2011;16:1249–57.
76. Calverley PM, Martinez FJ, Fabbri LM, et al. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis 2012;7:375–82.
77. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013(11):CD002309.
78. Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis 1964;90:721–9.
79. Boman G, Bäcker U, Larsson S, et al. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis 1983;64:405–15.
80. Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. European journal of clinical pharmacology. 1976;9:393–6.
81. Hansen NCG, Skriver A, Brorsen-Riis L, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med 1994;88:531–5.
82. Francis RS, Spicer CC. Chemotherapy in chronic bronchitis: Influence of daily penicillin and tetracycline on exacerbations and their cost: A report to the research committee of the British Tuberculosis Association by Their Chronic Bronchitis Subcommittee. BMJ 1960;1:297–303.
83. Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis. BMJ 1961;2:979.
84. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98.
85. Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014;189:1503–8.
86. Uzun S, Djamin RS, Kluytmans JAJW, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014;2:361–8.
87. Collet JP, Shapiro S, Ernst P, et al. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:1719–24.
88. Jing LI. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary. Age 2004;67:0–05.
89. Braido F, Tarantini F, Ghiglione V, et al. Bacterial lysate in the prevention of acute exacerbation of COPD and in respiratory recurrent infections. Int J Chron Obstruct Pulmon Dis 2007;2:335.
90. Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax 2016;71:8–14.
91. Bhatt SP, Connett JE, Voelker H, et al. β-Blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease (βLOCK COPD): a randomised controlled study protocol. BMJ Open 2016;6:e012292.
92. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128–8.
93. Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc 2009;57:1453–7.
94. Xiong W, Zhang Qs, Zhao W, et al. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exper Pathol 2016;97:107–13.
95. Baumeler L, Papakonstantinou E, Milenkovic B, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology 2016;21:883–90.
96 Sabanathan A, Sabanathan S, Shah R, Richardson J. Lung volume reduction surgery for emphysema: a review. J Cardiovasc Surg 1998;39:237.
97. Group NETTR. Patients at high risk of death after lung-volume–reduction surgery. N Engl J Med 2001;345:1075–83.
98. Group NETTR. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–73.
99. Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2014;148:2651–8.
100. Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA 2016;315:175–84.
101. Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015;20:319–26.
102. Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993–8.
103. Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771–8.
104. Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med 2001;163:1068–73.
105. Shah P, Slebos D, Cardoso P, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet 2011;378:997–1005.
106. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–44.
107. Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518–26.
108. Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: 12-month results from the STEP-UP randomized controlled study. Respiration 2016;92:397–403.
109. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651–62.
110. Group NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–8.
111. Council M. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981;1:681–6.
112. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179–91.
113. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65.
114. Group L-TOTTR. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016;375:1617–27.
115. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015(2):CD003793.
116. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362–8.
117. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61:772–8.
118. Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1156–63.
119. Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J 2010;36:74–80.
120. Cerón Navarro J, de Aguiar Quevedo K, Ansótegui Barrera E, et al. Functional outcomes after lung transplant in chronic obstructive pulmonary disease. Arch Bronconeumol 2015;51:109–14.
121. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1–15.
122. Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol 2008;3:1404–9.
123. Hajizadeh N, Goldfeld K, Crothers K. What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow- up study. Thorax 2015;70:294–6.
124. Siouta N, van Beek K, Preston N, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care 2016;15:18.
125. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–46.
126. Szekendi MK, Vaughn J, Lal A, et al. The prevalence of inpatients at thirty-three U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med 2016;19:360–72.
127. Rush B, Hertz P, Bond A, et al. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest 2017;151:41–6.
1. Segreti A, Stirpe E, Rogliani P, Cazzola M. Defining phenotypes in COPD: an aid to personalized healthcare. Mol Diagn Ther 2014;18:381–8.
2. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182:598–604.
3. Aubier M, Marthan R, Berger P, et al. [COPD and inflammation: statement from a French expert group: inflammation and remodelling mechanisms]. Rev Mal Respir 2010;27:1254–66.
4. Wang ZL. Evolving role of systemic inflammation in comorbidities of chronic obstructive pulmonary disease. Chin Med J (Engl) 2010;123:3467–78.
5. Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741–50.
6. Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep 2011;59:1–126.
7. Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global strategy for the diagnosis, management, and prevention of COPD 2017. Accessed at www.goldcopd.org.
8. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD Assessment Test. Eur Respir J 2009;34:648–54.
9. Wadbo M, Löfdahl CG, Larsson K, et al. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur Respir J 2002;20:1138–46.
10. Ram FS, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax 2003;58:580–4.
11. Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. Am J Med 1996;100(1A):11S–8S.
12. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. An 85-day multicenter trial. COMBIVENT Inhalation Aerosol Study Group. Chest 1994;105:1411–9.
13. Friedman M, Serby CW, Menjoge SS, et al. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone in COPD. Chest 1999;115:635–41.
14. Cook D, Guyatt G, Wong E, et al. Regular versus as-needed short-acting inhaled beta-agonist therapy for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:85–90.
15. Wilchesky M, Ernst P, Brophy JM, et al. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest 2012;142:305–11.
16. Scott VL, Frazee LA. Retrospective comparison of nebulized levalbuterol and albuterol for adverse events in patients with acute airflow obstruction. Am J Ther 2003;10:341–7.
17. Wong CS, Pavord ID, Williams J, et al. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet 1990;336:1396–9.
18. Cole JM, Sheehan AH, Jordan JK. Concomitant use of ipratropium and tiotropium in chronic obstructive pulmonary disease. Ann Pharmacother 2012;46:1717–21.
19. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155 :179–91.
20. Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salmeterol with albuterol in the treatment of mild-to-moderate asthma. N Engl J Med 1992;327:1420–5.
21. Takahashi T, Belvisi MG, Patel H, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med 1994;150(6 Pt 1):1640–5.
22. Donohue JF, Fogarty C, Lötvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010;182:155–62.
23. Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy and symptomatic benefit of olodaterol once daily delivered via Respimat versus placebo and formoterol twice daily in patients with GOLD 2-4 COPD: results from two replicate 48-week studies. Int J Chron Obstruct Pulmon Dis 2014;9:697–714.
24. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–89.
25. Hanania NA, Feldman G, Zachgo W, et al. The efficacy and safety of the novel long-acting β2 agonist vilanterol in patients with COPD: a randomized placebo-controlled trial. Chest 2012;142:119–27.
26. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–54.
27. Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med 2013;1:524–33.
28. Jones PW, Singh D, Bateman ED, et al. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J 2012;40:830–6.
29. D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res 2011;12:156.
30. Antoniu SA. UPLIFT Study: the effects of long-term therapy with inhaled tiotropium in chronic obstructive pulmonary disease. Evaluation of: Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2008;359:1543–54. Expert Opin Pharmacother 2009;10:719–22.
31. Nelson HS, Gross NJ, Levine B, et al. Cardiac safety profile of nebulized formoterol in adults with COPD: a 12-week, multicenter, randomized, double- blind, double-dummy, placebo- and active-controlled trial. Clin Ther 2007;29:2167–78.
32. Gershon A, Croxford R, Calzavara A, et al. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med 2013;173:1175–85.
33. Aljaafareh A, Valle JR, Lin YL, et al. Risk of cardiovascular events after initiation of long-acting bronchodilators in patients with chronic obstructive lung disease: A population-based study. SAGE Open Med 2016;4:2050312116671337.
34. O’Connor AB. Tiotropium in chronic obstructive pulmonary disease. N Engl J Med 2009;360:185–6.
35. Kesten S, Jara M, Wentworth C, Lanes S. Pooled clinical trial analysis of tiotropium safety. Chest 2006;130:1695–703.
36. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med 2013;369:1491–501.
37. Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093–103.
38. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012(9):CD009157.
39. Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med 2005;5:3.
40. Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012(7):CD002991.
41. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest 2004;126:213–9.
42. Drummond MB, Dasenbrook EC, Pitz MW, et al. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. JAMA 2008;300:2407–16.
43. Lee SY, Park HY, Kim EK, et al. Combination therapy of inhaled steroids and long-acting beta2-agonists in asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2016;11:2797–803.
44. Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med 2015;373:1241–9.
45. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015:CD008989.
46. Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016;374:2222–34.
47. Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2007;146:545–55.
48. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:741–50.
49. Gallelli L, Falcone D, Cannataro R, et al. Theophylline action on primary human bronchial epithelial cells under proinflammatory stimuli and steroidal drugs: a therapeutic rationale approach. Drug Des Devel Ther 2017;11:265–72.
50. Paloucek FP, Rodvold KA. Evaluation of theophylline overdoses and toxicities. Ann Emerg Med 1988;17:135–44.
51. Ram FS, Jones PW, Castro AA, et al. Oral theophylline for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002(4):CD003902.
52. Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med 1989;320:1521–5.
53. Devereux G, Cotton S, Barnes P, et al. Use of low-dose oral theophylline as an adjunct to inhaled corticosteroids in preventing exacerbations of chronic obstructive pulmonary disease: study protocol for a randomised controlled trial. Trials 2015;16:267.
54. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005(3):CD005374.
55. Horita N, Miyazawa N, Morita S, et al. Evidence suggesting that oral corticosteroids increase mortality in stable chronic obstructive pulmonary disease. Respir Res 2014;15:37.
56. Poole P, Chong J, Cates CJ. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015(7):CD001287.
57. Zheng JP, Wen FQ, Bai CX, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med 2014;2:187–94.
58. Seemungal TA, Wilkinson TM, Hurst JR, et al. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med 2008;178:1139–47.
59. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823– 32.
60. Güell R, Casan P, Belda J, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976–83.
61. Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med 2014;35:157–63.
62. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet 2007;370:786–96.
63. Spencer S, Calverley PMA, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004;23:698–702.
64. Blanchette CM, Gross NJ, Altman P. Rising costs of COPD and the potential for maintenance therapy to slow the trend. Am Health Drug Benef 2014;7:98.
65. Criner GJ, Bourbeau J, Diekemper RL, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015;147:894–942.
66. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report. Respirology 2017;22:575–601.
67. Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med 2013;11:181.
68. Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–22.
69. Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV1 decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001;164:358–64.
70. Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–52.
71. Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–21.
72. Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol 2011;163:53–67.
73. Calverley PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685–94.
74. Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374:695–703.
75. Lee S, Hui DSC, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology 2011;16:1249–57.
76. Calverley PM, Martinez FJ, Fabbri LM, et al. Does roflumilast decrease exacerbations in severe COPD patients not controlled by inhaled combination therapy? The REACT study protocol. Int J Chron Obstruct Pulmon Dis 2012;7:375–82.
77. Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013(11):CD002309.
78. Sheffner AL, Medler EM, Jacobs LW, Sarett HP. The in vitro reduction in viscosity of human tracheobronchial secretions by acetylcysteine. Am Rev Respir Dis 1964;90:721–9.
79. Boman G, Bäcker U, Larsson S, et al. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis 1983;64:405–15.
80. Grassi C, Morandini GC. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. European journal of clinical pharmacology. 1976;9:393–6.
81. Hansen NCG, Skriver A, Brorsen-Riis L, et al. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med 1994;88:531–5.
82. Francis RS, Spicer CC. Chemotherapy in chronic bronchitis: Influence of daily penicillin and tetracycline on exacerbations and their cost: A report to the research committee of the British Tuberculosis Association by Their Chronic Bronchitis Subcommittee. BMJ 1960;1:297–303.
83. Francis RS, May JR, Spicer CC. Chemotherapy of bronchitis. BMJ 1961;2:979.
84. Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98.
85. Han MK, Tayob N, Murray S, et al. Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy. Am J Respir Crit Care Med 2014;189:1503–8.
86. Uzun S, Djamin RS, Kluytmans JAJW, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2014;2:361–8.
87. Collet JP, Shapiro S, Ernst P, et al. Effects of an immunostimulating agent on acute exacerbations and hospitalizations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997;156:1719–24.
88. Jing LI. Protective effect of a bacterial extract against acute exacerbation in patients with chronic bronchitis accompanied by chronic obstructive pulmonary. Age 2004;67:0–05.
89. Braido F, Tarantini F, Ghiglione V, et al. Bacterial lysate in the prevention of acute exacerbation of COPD and in respiratory recurrent infections. Int J Chron Obstruct Pulmon Dis 2007;2:335.
90. Bhatt SP, Wells JM, Kinney GL, et al. β-Blockers are associated with a reduction in COPD exacerbations. Thorax 2016;71:8–14.
91. Bhatt SP, Connett JE, Voelker H, et al. β-Blockers for the prevention of acute exacerbations of chronic obstructive pulmonary disease (βLOCK COPD): a randomised controlled study protocol. BMJ Open 2016;6:e012292.
92. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010;363:1128–8.
93. Sasaki T, Nakayama K, Yasuda H, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients. J Am Geriatr Soc 2009;57:1453–7.
94. Xiong W, Zhang Qs, Zhao W, et al. A 12-month follow-up study on the preventive effect of oral lansoprazole on acute exacerbation of chronic obstructive pulmonary disease. Int J Exper Pathol 2016;97:107–13.
95. Baumeler L, Papakonstantinou E, Milenkovic B, et al. Therapy with proton-pump inhibitors for gastroesophageal reflux disease does not reduce the risk for severe exacerbations in COPD. Respirology 2016;21:883–90.
96 Sabanathan A, Sabanathan S, Shah R, Richardson J. Lung volume reduction surgery for emphysema: a review. J Cardiovasc Surg 1998;39:237.
97. Group NETTR. Patients at high risk of death after lung-volume–reduction surgery. N Engl J Med 2001;345:1075–83.
98. Group NETTR. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–73.
99. Decker MR, Leverson GE, Jaoude WA, Maloney JD. Lung volume reduction surgery since the National Emphysema Treatment Trial: study of Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2014;148:2651–8.
100. Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA 2016;315:175–84.
101. Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015;20:319–26.
102. Snell GI, Hopkins P, Westall G, et al. A feasibility and safety study of bronchoscopic thermal vapor ablation: a novel emphysema therapy. Ann Thorac Surg 2009;88:1993–8.
103. Ingenito EP, Berger RL, Henderson AC, et al. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771–8.
104. Ingenito EP, Loring SH, Moy ML, et al. Comparison of physiological and radiological screening for lung volume reduction surgery. Am J Respir Crit Care Med 2001;163:1068–73.
105. Shah P, Slebos D, Cardoso P, et al. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet 2011;378:997–1005.
106. Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233–44.
107. Wan IY, Toma TP, Geddes DM, et al. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518–26.
108. Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: 12-month results from the STEP-UP randomized controlled study. Respiration 2016;92:397–403.
109. Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651–62.
110. Group NOTT. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–8.
111. Council M. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981;1:681–6.
112. Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179–91.
113. Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65.
114. Group L-TOTTR. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016;375:1617–27.
115. McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015(2):CD003793.
116. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000;355:362–8.
117. Garcia-Aymerich J, Lange P, Benet M, et al. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006;61:772–8.
118. Thabut G, Ravaud P, Christie JD, et al. Determinants of the survival benefit of lung transplantation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1156–63.
119. Lahzami S, Bridevaux PO, Soccal PM, et al. Survival impact of lung transplantation for COPD. Eur Respir J 2010;36:74–80.
120. Cerón Navarro J, de Aguiar Quevedo K, Ansótegui Barrera E, et al. Functional outcomes after lung transplant in chronic obstructive pulmonary disease. Arch Bronconeumol 2015;51:109–14.
121. Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015;34:1–15.
122. Minai OA, Shah S, Mazzone P, et al. Bronchogenic carcinoma after lung transplantation: characteristics and outcomes. J Thorac Oncol 2008;3:1404–9.
123. Hajizadeh N, Goldfeld K, Crothers K. What happens to patients with COPD with long-term oxygen treatment who receive mechanical ventilation for COPD exacerbation? A 1-year retrospective follow- up study. Thorax 2015;70:294–6.
124. Siouta N, van Beek K, Preston N, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care 2016;15:18.
125. Celli BR, MacNee W; ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–46.
126. Szekendi MK, Vaughn J, Lal A, et al. The prevalence of inpatients at thirty-three U.S. hospitals appropriate for and receiving referral to palliative care. J Palliat Med 2016;19:360–72.
127. Rush B, Hertz P, Bond A, et al. Use of palliative care in patients with end-stage COPD and receiving home oxygen: national trends and barriers to care in the United States. Chest 2017;151:41–6.
Anhedonia emerges as a major transdiagnostic treatment target
SAN FRANCISCO – Anhedonia is the symptom dimension that cuts most strongly across the diagnostic boundaries of anxiety and depression and contributes most to the characteristically poor quality of life in both, Emily C. Livermore reported at the annual conference of the Anxiety and Depression Association of America.
“Our results suggest that anhedonia may have a disproportionate impact on disability from depression and anxiety and may be an important target for tailoring treatment and assessing treatment outcomes,” declared Ms. Livermore, a doctoral student in clinical psychology at Stanford (Calif.) University.
She presented a study of 121 adults with anxiety or depressive symptoms. The study was conducted under the auspices of the National Institute of Mental Health’s Research Domain Criteria (RDoC) initiative. The RDoC program is intended to promote a new way of studying mental disorders based on dimensional psychological constructs independent of traditional psychiatric diagnoses, which in some circles are now dismissed as “silos.” In keeping with the RDoC goals, Ms. Livermore and her coinvestigators examined diagnosis-independent dimensions of symptoms and how they affected quality of life.
The investigators obtained a comprehensive picture of the participants’ symptoms and quality of life by having them complete the Penn State Worry Questionnaire, the Mood and Anxiety Symptoms Questionnaire, the Depression Anxiety Stress Scale, and the World Health Organization Quality of Life – Brief Version.
The investigators then mapped the symptoms and their interconnections in order to identify what they called transdiagnostic symptom factor dimensions. They found four of them, which they termed anhedonia, worry, tension, and anxious arousal, a dimension encompassing physical symptoms including shortness of breath and heart palpitations.
Next, using regression analyses, they examined the relationship between levels of those four symptom factor dimensions and the four quality of life domains captured in the WHO instrument, namely, physical, psychologic, environmental, and social quality of life. Study participants averaged unhealthily low quality of life scores on two of these domains – the psychological and social – as defined by scores more than one standard deviation below normative.
Of the four symptom dimensions, anhedonia stood out as having moderate to-strong negative associations with all four quality of life domains. The other three symptom dimensions showed no or only weak associations with the four quality of life domains, with the exception of anxious arousal, which displayed a moderate relationship with physical quality of life.
Ms. Livermore reported having no financial conflicts regarding the study, which was funded by the National Institute of Mental Health.
SAN FRANCISCO – Anhedonia is the symptom dimension that cuts most strongly across the diagnostic boundaries of anxiety and depression and contributes most to the characteristically poor quality of life in both, Emily C. Livermore reported at the annual conference of the Anxiety and Depression Association of America.
“Our results suggest that anhedonia may have a disproportionate impact on disability from depression and anxiety and may be an important target for tailoring treatment and assessing treatment outcomes,” declared Ms. Livermore, a doctoral student in clinical psychology at Stanford (Calif.) University.
She presented a study of 121 adults with anxiety or depressive symptoms. The study was conducted under the auspices of the National Institute of Mental Health’s Research Domain Criteria (RDoC) initiative. The RDoC program is intended to promote a new way of studying mental disorders based on dimensional psychological constructs independent of traditional psychiatric diagnoses, which in some circles are now dismissed as “silos.” In keeping with the RDoC goals, Ms. Livermore and her coinvestigators examined diagnosis-independent dimensions of symptoms and how they affected quality of life.
The investigators obtained a comprehensive picture of the participants’ symptoms and quality of life by having them complete the Penn State Worry Questionnaire, the Mood and Anxiety Symptoms Questionnaire, the Depression Anxiety Stress Scale, and the World Health Organization Quality of Life – Brief Version.
The investigators then mapped the symptoms and their interconnections in order to identify what they called transdiagnostic symptom factor dimensions. They found four of them, which they termed anhedonia, worry, tension, and anxious arousal, a dimension encompassing physical symptoms including shortness of breath and heart palpitations.
Next, using regression analyses, they examined the relationship between levels of those four symptom factor dimensions and the four quality of life domains captured in the WHO instrument, namely, physical, psychologic, environmental, and social quality of life. Study participants averaged unhealthily low quality of life scores on two of these domains – the psychological and social – as defined by scores more than one standard deviation below normative.
Of the four symptom dimensions, anhedonia stood out as having moderate to-strong negative associations with all four quality of life domains. The other three symptom dimensions showed no or only weak associations with the four quality of life domains, with the exception of anxious arousal, which displayed a moderate relationship with physical quality of life.
Ms. Livermore reported having no financial conflicts regarding the study, which was funded by the National Institute of Mental Health.
SAN FRANCISCO – Anhedonia is the symptom dimension that cuts most strongly across the diagnostic boundaries of anxiety and depression and contributes most to the characteristically poor quality of life in both, Emily C. Livermore reported at the annual conference of the Anxiety and Depression Association of America.
“Our results suggest that anhedonia may have a disproportionate impact on disability from depression and anxiety and may be an important target for tailoring treatment and assessing treatment outcomes,” declared Ms. Livermore, a doctoral student in clinical psychology at Stanford (Calif.) University.
She presented a study of 121 adults with anxiety or depressive symptoms. The study was conducted under the auspices of the National Institute of Mental Health’s Research Domain Criteria (RDoC) initiative. The RDoC program is intended to promote a new way of studying mental disorders based on dimensional psychological constructs independent of traditional psychiatric diagnoses, which in some circles are now dismissed as “silos.” In keeping with the RDoC goals, Ms. Livermore and her coinvestigators examined diagnosis-independent dimensions of symptoms and how they affected quality of life.
The investigators obtained a comprehensive picture of the participants’ symptoms and quality of life by having them complete the Penn State Worry Questionnaire, the Mood and Anxiety Symptoms Questionnaire, the Depression Anxiety Stress Scale, and the World Health Organization Quality of Life – Brief Version.
The investigators then mapped the symptoms and their interconnections in order to identify what they called transdiagnostic symptom factor dimensions. They found four of them, which they termed anhedonia, worry, tension, and anxious arousal, a dimension encompassing physical symptoms including shortness of breath and heart palpitations.
Next, using regression analyses, they examined the relationship between levels of those four symptom factor dimensions and the four quality of life domains captured in the WHO instrument, namely, physical, psychologic, environmental, and social quality of life. Study participants averaged unhealthily low quality of life scores on two of these domains – the psychological and social – as defined by scores more than one standard deviation below normative.
Of the four symptom dimensions, anhedonia stood out as having moderate to-strong negative associations with all four quality of life domains. The other three symptom dimensions showed no or only weak associations with the four quality of life domains, with the exception of anxious arousal, which displayed a moderate relationship with physical quality of life.
Ms. Livermore reported having no financial conflicts regarding the study, which was funded by the National Institute of Mental Health.
AT THE ANXIETY AND DEPRESSION CONFERENCE 2017
Key clinical point:
Major finding: Anhedonia has a disproportionate negative impact on all of the major quality of life domains in both anxiety and depression.
Data source: A cross-sectional study of 121 adults with clinically significant anxiety or depression symptoms.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was funded by the National Institute of Mental Health.
Cutting CAUTIs in Critical Care
From the Tucson Medical Center, Tucson, AZ.
Abstract
- Objective: To describe a quality improvement project to reduce catheter-associated urinary tract infections (CAUTIs) in an intensive care unit (ICU).
- Methods: Descriptive report.
- Results: CAUTIs are a common health care–associated infection that results in increased length of stay, patient discomfort, excess health care costs, and sometime mortality. However, many cases of CAUTIs are preventable. To address this problem at our institution, we enrolled in the Hospital Engagement Network (HEN) collaborative for the reduction of CAUTIs, utilizing the Comprehensive Unit-based Safety Program (CUSP) as the platform for our project. This article describes our project implementation, challenges encountered, and the lasting improvement we have achieved at our facility.
- Conclusion: By challenging the ICU culture, providing nursing with alternatives to urinary catheters, and promoting physician engagement, we were able to reduce catheter utilization and CAUTI rates in the ICU.
Hospital-acquired infections (HAIs) are important causes of morbidity and mortality in the United States [1]. Among HAIs, urinary tract infections are the 4th most common, with almost all cases caused by urethral instrumentation [2]. Catheter-associated urinary tract infections (CAUTIs) are associated with an increased hospital length of stay of 2 to 4 days and a cost of $400 million to $500 million annually [3]. As of 2015, the Centers for Medicare and Medicaid Services no longer reimburses hospitals for treating CAUTIs.
CAUTIs are a particular challenge in the intensive care unit (ICU) due to the high urinary catheter utilization rates. In our mixed medical/surgical ICU, the catheter utilization rate was 84% in 2012 and was the setting for the majority of CAUTIs in our hospital. The risk of CAUTI can be reduced by ensuring that catheters are used only when needed and removed as soon as possible; that catheters are placed using proper aseptic technique; and that the closed sterile drainage system is maintained. In 2013 we launched a project to improve our CAUTI rates and enrolled in the Hospital Engagement Network (HEN) collaborative for the reduction of CAUTIs, utilizing the Comprehensive Unit-based Safety Program (CUSP) [4] as the platform for our project. This article describes our project implementation, the challenges we encountered, and the lasting improvement we have achieved.
Setting
Tucson Medical Center is a 600-bed tertiary care hospital, the largest in southern Arizona, with over 1000 independent medical providers. The medical center is a locally governed, nonprofit teaching hospital that has been providing care to the city of Tucson, southern Arizona, southwest New Mexico, and northern Mexico for the past 70 years. There are 2 adult critical care units: a cardiovascular ICU and a mixed medical/surgical ICU. We focused our efforts and interventions on the mixed ICU, a 16-bed unit that includes medical, surgical (neuro, general and vascular), and neurological patient populations that had 19 CAUTIs in 2012, versus 2 CAUTIs in the cardiovascular ICU.
Project
Initial Phase
The first steps in our project were to develop our unit-based team, identify project goals, and review our current nursing practice and processes. First, using the template from the CUSP platform, we assembled a team that consisted of the chief nursing officer (executive sponsor), ICU medical director, nurse manager, infection control manager, infection control nurse, 4 nurse champions (2 two night shift 2 day shift), and a patient care technician.
The second step was to identify a realistic and achievable goal. A goal of a 20% reduction from our current utilization rate was selected. As our catheter utilization rates were consistently above 90%, we aimed to for a rate of less than 70%. In addition, we sought to reduce our CAUTI standardized infection ratio (number of health care–associated infections observed divided by the national predicted number) from 3.875 to less than 1.0.
In reviewing our current nursing practice and processes, we utilized the CUSP data collection tool and adapted it to meet our institutional needs. Figure 1 shows the original CUSP data collection tool, which is organized around 5 key questions about the catheter (eg, Is catheter present? Where it was placed? Why does the patient has a catheter today?) as well as lists appropriate and inappropriate indications.
To implement the guidelines, we provided education to the nursing staff via emails, placed posters on the unit, and discussed appropriate and inappropriate indications during bedside conversations using the audit tool. As the project continued, these guidelines were reinforced daily when the question “why does your patient have a catheter today?” was posed to the nurses during the audit. Our chief nursing officer supported our implementation efforts by including a CAUTI prevention lecture with her monthly house-wide nursing education series called “lunch and learn.”
We added additional questions to the tool as we learned more about the practices and processes that were currently in use. For example, “accurate measurement of urinary output in the critically ill patient” was the most common reason given by nurses for keeping a catheter in. Upon further questioning, however, the common response was that “the doctor ordered it.” By adding “MD order” to the audit tool, we were able to track actual orders versus nurses falling back on old patterns. This data collection item also provided us the names and groups of physicians to approach and educate on our project goals. Two other helpful items added to the tool related to the catheter seal and stat lock (catheter securement device) placement. The data provided by these questions helped us recognize areas for improvement in nursing practice, supply issues, and the impact of other departments. For example, auditing showed that most of our catheters were placed in the emergency department (ED) and surgery. This gave us an opportunity to reach out to these units to discuss CAUTI reduction strategies. For example, after review of the ED catheter supplies, we discovered that they did not have a closed catheter insertion system with a urometer drainage bag. Therefore, when a patient was transferred to the ICU, the integrity of the urinary collection system had to be broken to place a urometer. Evidence has shown that breaking the integrity of the system increases a patient’s risk for a CAUTI [1]. Once this problem was identified, the ED inventory was changed to include the urometer as part of the closed system urinary insertion kit.
Active Phase
After the implementation phase, the next 15 months were dedicated to daily rounding and bedside auditing, the foundation of our project. Rounding was done by the unit manager or nurse champion and involved talking with the bedside nurse and completing the audit tool. These bedside conversations were an opportunity to review the HICPAC guidelines, identify education needs, and reinforce best practices. During these discussions, the nurses often would identify reasons to remove catheters.
The CAUTI team met monthly to review the previous month’s data, other observed opportunities for improvement, and any patient CAUTI information provided by our infection control nurse liaison. We conducted root cause analysis when CAUTIs developed, in which we reviewed the patient’s chart and sought to identify possible interventions that could have reduced the number of catheter days. Our findings were shared in staff meetings, newsletters, and through quality bulletin boards. We also recognized improved performance. Tokens that could be cashed in at the cafeteria for snacks or drinks were awarded to nurses who removed a urinary catheter. We also organized a celebration on the unit the first time we had 3 months without a CAUTI.
Challenges Encountered
Culture change is challenging. The entrenched mindset was that “If a patient is sick enough to be in an ICU, then they are sick enough to need a urinary catheter.” Standard nursing practice typically included placement of a urinary catheter immediately on arrival to the ICU if not already present. Over the years, placing a urinary catheter had become the norm in the ICU, with nurses noting concern about obtaining accurate measurement of urine output and prevention of skin breakdown from incontinence. We had to continually address these concerns to make progress on the project. By providing alternatives to urinary catheters, such as incontinence pads, external male collection devices in varying sizes, moisture barrier products, and scales to measure urine output, nurses were more willing to comply with catheter removal.
We worked with our wound and ostomy nurses to ensure we were providing the proper moisture barrier products and presented research to support that incontinence did not need to lead to pressure ulcers. The wound care team helped with guiding the use of products for incontinent patients to prevent incontinence-associated dermatitis and potential skin breakdown. Our administration financially supported our program, allowing us to bring in and trial supplies. As we identified products for use, we were able to place them into floor stock and make them easily available to nursing. Items such as wicking pads, skin protective creams, and alternatives to catheters were a vital part of our bedside toolkit to maintain our patient’s skin integrity.
Both nurses and physicians were concerned about accurate measurement of output, specifically in surgical patients. The use of scales to weigh and measure output from an incontinent patient’s pads was helpful but sometimes inconvenient. From our surgeons' perspective, not having immediate hourly measurements of urine output to monitor risk for hypovolemia from third spacing of fluid or from abdominal compartment syndrome was not acceptable. Because of this concern, we did not see a decrease in early catheter removal among surgical patients. Daily conversations with nurses and surgeons at the bedside continue to be key to removing catheters as soon as the surgeon is comfortable that the patient is out of risk for hypovolemia.
Outcomes
Within the first month we saw an immediate drop in catheter utilization and had zero CAUTIs, but during the next 2 months there was a return to our previous rates (Figure 2 and Figure 3 [figures show combined mixed and cardiovascular ICU rates due to reporting requirements]).
Although nursing is at the heart of this engagement, it is the combined efforts of all disciplines that promote the reduction of CAUTIs and improve patient outcomes. When our CAUTI counts plateaued at 10 annually in 2014–2015, we reached out to physicians and found that we had not adequately educated our medical and surgical staff of our project and goals. With the backing of a supportive and vocal ICU director, physician engagement has increased and there is more attention paid to catheter removal by our ICU intensivists. This collaborative approach has helped lower our rates even further in 2016 (n = 3). We achieved our CAUTI SIR goal of less than 1.0 , and changed our current goal to less than 0.5 (Figure 3).
In addition to greater intensivist engagement, the ED reduced their urinary catheter insertion rate from 12% to 4% for all patients transferring to an inpatient status. As previously mentioned, they are now placing catheters from kits that include urometers, so we do not have to break the integrity of the closed system after the patient it transferred to the ICU. We are also collaborating with surgical services to reduce catheter use. This is still a work in progress that requires collaboration with surgeons and hospitalists in changing departmental norms.
Conclusion
Through a combined effort involving a number of departments across the hospital, we were able to reduce catheter utilization and CAUTI rates in the ICU. We have seen a culture shift, with more ICU nursing staff questioning the use of catheters and requesting to have them removed during daily bedside rounds or simply removing them based on our nursing-driven protocol. Currently, both critical care units have been actively working on reducing CAUTI rates and have gone 310 days without a CAUTI.
Reluctance among ICU nurses to remove urinary catheters has declined; however, it is easy to fall back on the convenience of catheters. We have found that each rise in utilization rates and CAUTIs pointed to the need to refocus our effort on the daily bedside conversations. Unless we can eliminate the need for urinary catheters, there will always be a risk of a CAUTI. However, with advances in catheter technology, alternatives to catheters, and nursing education, the reduction in this hospital-acquired infection can be realized.
Acknowledgments: The author thanks our devoted infection control manager (now director), Nina Espinoza Mazzola, BSM, CIC. Our attaining success at the bedside is a reflection of her commitment as a resource and in providing support for nursing practice.
Corresponding author: Jennifer C. Tuttle, RN, MSNEd, CNRN, Tucson Medical Center, 5301 E. Grant Rd, Tucson, AZ 85712.
Financial disclosures: None.
1. Nicolle LE. Catheter-associated urinary tract infections. Antimicrob Resist Infect Control 2014;3:23.
2. Centers for Disease Control and Prevention (CDC). Urinary tract infection (catheter-associated urinary tract infection [cauti] and non-catheter-associated urinary tract infection [uti]) and other urinary system infection [usi]) events. 2017. Accessed at www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf.
3. Centers for Disease Control and Prevention. Catheter-associated urinary tract infection (cauti) toolkit. Accessed 5 Mar 2017 at www.cdc.gov/HAI/pdfs/toolkits/CAUTItoolkit_3_10.pdf.
4. On the CUSP implementation guide. Accessed at http://web.mhanet.com/cauti-implementation_guide_508.pdf.
5. Healthcare Infection Control Practice Advisory Committee (HICPAC). Guidelines for the prevention of catheter associated urinary tract infections 2009. Accessed 25 Feb 2017 at www.cdc.gov/infectioncontrol/pdf/guidelines/cauti-guidelines.pdf.
From the Tucson Medical Center, Tucson, AZ.
Abstract
- Objective: To describe a quality improvement project to reduce catheter-associated urinary tract infections (CAUTIs) in an intensive care unit (ICU).
- Methods: Descriptive report.
- Results: CAUTIs are a common health care–associated infection that results in increased length of stay, patient discomfort, excess health care costs, and sometime mortality. However, many cases of CAUTIs are preventable. To address this problem at our institution, we enrolled in the Hospital Engagement Network (HEN) collaborative for the reduction of CAUTIs, utilizing the Comprehensive Unit-based Safety Program (CUSP) as the platform for our project. This article describes our project implementation, challenges encountered, and the lasting improvement we have achieved at our facility.
- Conclusion: By challenging the ICU culture, providing nursing with alternatives to urinary catheters, and promoting physician engagement, we were able to reduce catheter utilization and CAUTI rates in the ICU.
Hospital-acquired infections (HAIs) are important causes of morbidity and mortality in the United States [1]. Among HAIs, urinary tract infections are the 4th most common, with almost all cases caused by urethral instrumentation [2]. Catheter-associated urinary tract infections (CAUTIs) are associated with an increased hospital length of stay of 2 to 4 days and a cost of $400 million to $500 million annually [3]. As of 2015, the Centers for Medicare and Medicaid Services no longer reimburses hospitals for treating CAUTIs.
CAUTIs are a particular challenge in the intensive care unit (ICU) due to the high urinary catheter utilization rates. In our mixed medical/surgical ICU, the catheter utilization rate was 84% in 2012 and was the setting for the majority of CAUTIs in our hospital. The risk of CAUTI can be reduced by ensuring that catheters are used only when needed and removed as soon as possible; that catheters are placed using proper aseptic technique; and that the closed sterile drainage system is maintained. In 2013 we launched a project to improve our CAUTI rates and enrolled in the Hospital Engagement Network (HEN) collaborative for the reduction of CAUTIs, utilizing the Comprehensive Unit-based Safety Program (CUSP) [4] as the platform for our project. This article describes our project implementation, the challenges we encountered, and the lasting improvement we have achieved.
Setting
Tucson Medical Center is a 600-bed tertiary care hospital, the largest in southern Arizona, with over 1000 independent medical providers. The medical center is a locally governed, nonprofit teaching hospital that has been providing care to the city of Tucson, southern Arizona, southwest New Mexico, and northern Mexico for the past 70 years. There are 2 adult critical care units: a cardiovascular ICU and a mixed medical/surgical ICU. We focused our efforts and interventions on the mixed ICU, a 16-bed unit that includes medical, surgical (neuro, general and vascular), and neurological patient populations that had 19 CAUTIs in 2012, versus 2 CAUTIs in the cardiovascular ICU.
Project
Initial Phase
The first steps in our project were to develop our unit-based team, identify project goals, and review our current nursing practice and processes. First, using the template from the CUSP platform, we assembled a team that consisted of the chief nursing officer (executive sponsor), ICU medical director, nurse manager, infection control manager, infection control nurse, 4 nurse champions (2 two night shift 2 day shift), and a patient care technician.
The second step was to identify a realistic and achievable goal. A goal of a 20% reduction from our current utilization rate was selected. As our catheter utilization rates were consistently above 90%, we aimed to for a rate of less than 70%. In addition, we sought to reduce our CAUTI standardized infection ratio (number of health care–associated infections observed divided by the national predicted number) from 3.875 to less than 1.0.
In reviewing our current nursing practice and processes, we utilized the CUSP data collection tool and adapted it to meet our institutional needs. Figure 1 shows the original CUSP data collection tool, which is organized around 5 key questions about the catheter (eg, Is catheter present? Where it was placed? Why does the patient has a catheter today?) as well as lists appropriate and inappropriate indications.
To implement the guidelines, we provided education to the nursing staff via emails, placed posters on the unit, and discussed appropriate and inappropriate indications during bedside conversations using the audit tool. As the project continued, these guidelines were reinforced daily when the question “why does your patient have a catheter today?” was posed to the nurses during the audit. Our chief nursing officer supported our implementation efforts by including a CAUTI prevention lecture with her monthly house-wide nursing education series called “lunch and learn.”
We added additional questions to the tool as we learned more about the practices and processes that were currently in use. For example, “accurate measurement of urinary output in the critically ill patient” was the most common reason given by nurses for keeping a catheter in. Upon further questioning, however, the common response was that “the doctor ordered it.” By adding “MD order” to the audit tool, we were able to track actual orders versus nurses falling back on old patterns. This data collection item also provided us the names and groups of physicians to approach and educate on our project goals. Two other helpful items added to the tool related to the catheter seal and stat lock (catheter securement device) placement. The data provided by these questions helped us recognize areas for improvement in nursing practice, supply issues, and the impact of other departments. For example, auditing showed that most of our catheters were placed in the emergency department (ED) and surgery. This gave us an opportunity to reach out to these units to discuss CAUTI reduction strategies. For example, after review of the ED catheter supplies, we discovered that they did not have a closed catheter insertion system with a urometer drainage bag. Therefore, when a patient was transferred to the ICU, the integrity of the urinary collection system had to be broken to place a urometer. Evidence has shown that breaking the integrity of the system increases a patient’s risk for a CAUTI [1]. Once this problem was identified, the ED inventory was changed to include the urometer as part of the closed system urinary insertion kit.
Active Phase
After the implementation phase, the next 15 months were dedicated to daily rounding and bedside auditing, the foundation of our project. Rounding was done by the unit manager or nurse champion and involved talking with the bedside nurse and completing the audit tool. These bedside conversations were an opportunity to review the HICPAC guidelines, identify education needs, and reinforce best practices. During these discussions, the nurses often would identify reasons to remove catheters.
The CAUTI team met monthly to review the previous month’s data, other observed opportunities for improvement, and any patient CAUTI information provided by our infection control nurse liaison. We conducted root cause analysis when CAUTIs developed, in which we reviewed the patient’s chart and sought to identify possible interventions that could have reduced the number of catheter days. Our findings were shared in staff meetings, newsletters, and through quality bulletin boards. We also recognized improved performance. Tokens that could be cashed in at the cafeteria for snacks or drinks were awarded to nurses who removed a urinary catheter. We also organized a celebration on the unit the first time we had 3 months without a CAUTI.
Challenges Encountered
Culture change is challenging. The entrenched mindset was that “If a patient is sick enough to be in an ICU, then they are sick enough to need a urinary catheter.” Standard nursing practice typically included placement of a urinary catheter immediately on arrival to the ICU if not already present. Over the years, placing a urinary catheter had become the norm in the ICU, with nurses noting concern about obtaining accurate measurement of urine output and prevention of skin breakdown from incontinence. We had to continually address these concerns to make progress on the project. By providing alternatives to urinary catheters, such as incontinence pads, external male collection devices in varying sizes, moisture barrier products, and scales to measure urine output, nurses were more willing to comply with catheter removal.
We worked with our wound and ostomy nurses to ensure we were providing the proper moisture barrier products and presented research to support that incontinence did not need to lead to pressure ulcers. The wound care team helped with guiding the use of products for incontinent patients to prevent incontinence-associated dermatitis and potential skin breakdown. Our administration financially supported our program, allowing us to bring in and trial supplies. As we identified products for use, we were able to place them into floor stock and make them easily available to nursing. Items such as wicking pads, skin protective creams, and alternatives to catheters were a vital part of our bedside toolkit to maintain our patient’s skin integrity.
Both nurses and physicians were concerned about accurate measurement of output, specifically in surgical patients. The use of scales to weigh and measure output from an incontinent patient’s pads was helpful but sometimes inconvenient. From our surgeons' perspective, not having immediate hourly measurements of urine output to monitor risk for hypovolemia from third spacing of fluid or from abdominal compartment syndrome was not acceptable. Because of this concern, we did not see a decrease in early catheter removal among surgical patients. Daily conversations with nurses and surgeons at the bedside continue to be key to removing catheters as soon as the surgeon is comfortable that the patient is out of risk for hypovolemia.
Outcomes
Within the first month we saw an immediate drop in catheter utilization and had zero CAUTIs, but during the next 2 months there was a return to our previous rates (Figure 2 and Figure 3 [figures show combined mixed and cardiovascular ICU rates due to reporting requirements]).
Although nursing is at the heart of this engagement, it is the combined efforts of all disciplines that promote the reduction of CAUTIs and improve patient outcomes. When our CAUTI counts plateaued at 10 annually in 2014–2015, we reached out to physicians and found that we had not adequately educated our medical and surgical staff of our project and goals. With the backing of a supportive and vocal ICU director, physician engagement has increased and there is more attention paid to catheter removal by our ICU intensivists. This collaborative approach has helped lower our rates even further in 2016 (n = 3). We achieved our CAUTI SIR goal of less than 1.0 , and changed our current goal to less than 0.5 (Figure 3).
In addition to greater intensivist engagement, the ED reduced their urinary catheter insertion rate from 12% to 4% for all patients transferring to an inpatient status. As previously mentioned, they are now placing catheters from kits that include urometers, so we do not have to break the integrity of the closed system after the patient it transferred to the ICU. We are also collaborating with surgical services to reduce catheter use. This is still a work in progress that requires collaboration with surgeons and hospitalists in changing departmental norms.
Conclusion
Through a combined effort involving a number of departments across the hospital, we were able to reduce catheter utilization and CAUTI rates in the ICU. We have seen a culture shift, with more ICU nursing staff questioning the use of catheters and requesting to have them removed during daily bedside rounds or simply removing them based on our nursing-driven protocol. Currently, both critical care units have been actively working on reducing CAUTI rates and have gone 310 days without a CAUTI.
Reluctance among ICU nurses to remove urinary catheters has declined; however, it is easy to fall back on the convenience of catheters. We have found that each rise in utilization rates and CAUTIs pointed to the need to refocus our effort on the daily bedside conversations. Unless we can eliminate the need for urinary catheters, there will always be a risk of a CAUTI. However, with advances in catheter technology, alternatives to catheters, and nursing education, the reduction in this hospital-acquired infection can be realized.
Acknowledgments: The author thanks our devoted infection control manager (now director), Nina Espinoza Mazzola, BSM, CIC. Our attaining success at the bedside is a reflection of her commitment as a resource and in providing support for nursing practice.
Corresponding author: Jennifer C. Tuttle, RN, MSNEd, CNRN, Tucson Medical Center, 5301 E. Grant Rd, Tucson, AZ 85712.
Financial disclosures: None.
From the Tucson Medical Center, Tucson, AZ.
Abstract
- Objective: To describe a quality improvement project to reduce catheter-associated urinary tract infections (CAUTIs) in an intensive care unit (ICU).
- Methods: Descriptive report.
- Results: CAUTIs are a common health care–associated infection that results in increased length of stay, patient discomfort, excess health care costs, and sometime mortality. However, many cases of CAUTIs are preventable. To address this problem at our institution, we enrolled in the Hospital Engagement Network (HEN) collaborative for the reduction of CAUTIs, utilizing the Comprehensive Unit-based Safety Program (CUSP) as the platform for our project. This article describes our project implementation, challenges encountered, and the lasting improvement we have achieved at our facility.
- Conclusion: By challenging the ICU culture, providing nursing with alternatives to urinary catheters, and promoting physician engagement, we were able to reduce catheter utilization and CAUTI rates in the ICU.
Hospital-acquired infections (HAIs) are important causes of morbidity and mortality in the United States [1]. Among HAIs, urinary tract infections are the 4th most common, with almost all cases caused by urethral instrumentation [2]. Catheter-associated urinary tract infections (CAUTIs) are associated with an increased hospital length of stay of 2 to 4 days and a cost of $400 million to $500 million annually [3]. As of 2015, the Centers for Medicare and Medicaid Services no longer reimburses hospitals for treating CAUTIs.
CAUTIs are a particular challenge in the intensive care unit (ICU) due to the high urinary catheter utilization rates. In our mixed medical/surgical ICU, the catheter utilization rate was 84% in 2012 and was the setting for the majority of CAUTIs in our hospital. The risk of CAUTI can be reduced by ensuring that catheters are used only when needed and removed as soon as possible; that catheters are placed using proper aseptic technique; and that the closed sterile drainage system is maintained. In 2013 we launched a project to improve our CAUTI rates and enrolled in the Hospital Engagement Network (HEN) collaborative for the reduction of CAUTIs, utilizing the Comprehensive Unit-based Safety Program (CUSP) [4] as the platform for our project. This article describes our project implementation, the challenges we encountered, and the lasting improvement we have achieved.
Setting
Tucson Medical Center is a 600-bed tertiary care hospital, the largest in southern Arizona, with over 1000 independent medical providers. The medical center is a locally governed, nonprofit teaching hospital that has been providing care to the city of Tucson, southern Arizona, southwest New Mexico, and northern Mexico for the past 70 years. There are 2 adult critical care units: a cardiovascular ICU and a mixed medical/surgical ICU. We focused our efforts and interventions on the mixed ICU, a 16-bed unit that includes medical, surgical (neuro, general and vascular), and neurological patient populations that had 19 CAUTIs in 2012, versus 2 CAUTIs in the cardiovascular ICU.
Project
Initial Phase
The first steps in our project were to develop our unit-based team, identify project goals, and review our current nursing practice and processes. First, using the template from the CUSP platform, we assembled a team that consisted of the chief nursing officer (executive sponsor), ICU medical director, nurse manager, infection control manager, infection control nurse, 4 nurse champions (2 two night shift 2 day shift), and a patient care technician.
The second step was to identify a realistic and achievable goal. A goal of a 20% reduction from our current utilization rate was selected. As our catheter utilization rates were consistently above 90%, we aimed to for a rate of less than 70%. In addition, we sought to reduce our CAUTI standardized infection ratio (number of health care–associated infections observed divided by the national predicted number) from 3.875 to less than 1.0.
In reviewing our current nursing practice and processes, we utilized the CUSP data collection tool and adapted it to meet our institutional needs. Figure 1 shows the original CUSP data collection tool, which is organized around 5 key questions about the catheter (eg, Is catheter present? Where it was placed? Why does the patient has a catheter today?) as well as lists appropriate and inappropriate indications.
To implement the guidelines, we provided education to the nursing staff via emails, placed posters on the unit, and discussed appropriate and inappropriate indications during bedside conversations using the audit tool. As the project continued, these guidelines were reinforced daily when the question “why does your patient have a catheter today?” was posed to the nurses during the audit. Our chief nursing officer supported our implementation efforts by including a CAUTI prevention lecture with her monthly house-wide nursing education series called “lunch and learn.”
We added additional questions to the tool as we learned more about the practices and processes that were currently in use. For example, “accurate measurement of urinary output in the critically ill patient” was the most common reason given by nurses for keeping a catheter in. Upon further questioning, however, the common response was that “the doctor ordered it.” By adding “MD order” to the audit tool, we were able to track actual orders versus nurses falling back on old patterns. This data collection item also provided us the names and groups of physicians to approach and educate on our project goals. Two other helpful items added to the tool related to the catheter seal and stat lock (catheter securement device) placement. The data provided by these questions helped us recognize areas for improvement in nursing practice, supply issues, and the impact of other departments. For example, auditing showed that most of our catheters were placed in the emergency department (ED) and surgery. This gave us an opportunity to reach out to these units to discuss CAUTI reduction strategies. For example, after review of the ED catheter supplies, we discovered that they did not have a closed catheter insertion system with a urometer drainage bag. Therefore, when a patient was transferred to the ICU, the integrity of the urinary collection system had to be broken to place a urometer. Evidence has shown that breaking the integrity of the system increases a patient’s risk for a CAUTI [1]. Once this problem was identified, the ED inventory was changed to include the urometer as part of the closed system urinary insertion kit.
Active Phase
After the implementation phase, the next 15 months were dedicated to daily rounding and bedside auditing, the foundation of our project. Rounding was done by the unit manager or nurse champion and involved talking with the bedside nurse and completing the audit tool. These bedside conversations were an opportunity to review the HICPAC guidelines, identify education needs, and reinforce best practices. During these discussions, the nurses often would identify reasons to remove catheters.
The CAUTI team met monthly to review the previous month’s data, other observed opportunities for improvement, and any patient CAUTI information provided by our infection control nurse liaison. We conducted root cause analysis when CAUTIs developed, in which we reviewed the patient’s chart and sought to identify possible interventions that could have reduced the number of catheter days. Our findings were shared in staff meetings, newsletters, and through quality bulletin boards. We also recognized improved performance. Tokens that could be cashed in at the cafeteria for snacks or drinks were awarded to nurses who removed a urinary catheter. We also organized a celebration on the unit the first time we had 3 months without a CAUTI.
Challenges Encountered
Culture change is challenging. The entrenched mindset was that “If a patient is sick enough to be in an ICU, then they are sick enough to need a urinary catheter.” Standard nursing practice typically included placement of a urinary catheter immediately on arrival to the ICU if not already present. Over the years, placing a urinary catheter had become the norm in the ICU, with nurses noting concern about obtaining accurate measurement of urine output and prevention of skin breakdown from incontinence. We had to continually address these concerns to make progress on the project. By providing alternatives to urinary catheters, such as incontinence pads, external male collection devices in varying sizes, moisture barrier products, and scales to measure urine output, nurses were more willing to comply with catheter removal.
We worked with our wound and ostomy nurses to ensure we were providing the proper moisture barrier products and presented research to support that incontinence did not need to lead to pressure ulcers. The wound care team helped with guiding the use of products for incontinent patients to prevent incontinence-associated dermatitis and potential skin breakdown. Our administration financially supported our program, allowing us to bring in and trial supplies. As we identified products for use, we were able to place them into floor stock and make them easily available to nursing. Items such as wicking pads, skin protective creams, and alternatives to catheters were a vital part of our bedside toolkit to maintain our patient’s skin integrity.
Both nurses and physicians were concerned about accurate measurement of output, specifically in surgical patients. The use of scales to weigh and measure output from an incontinent patient’s pads was helpful but sometimes inconvenient. From our surgeons' perspective, not having immediate hourly measurements of urine output to monitor risk for hypovolemia from third spacing of fluid or from abdominal compartment syndrome was not acceptable. Because of this concern, we did not see a decrease in early catheter removal among surgical patients. Daily conversations with nurses and surgeons at the bedside continue to be key to removing catheters as soon as the surgeon is comfortable that the patient is out of risk for hypovolemia.
Outcomes
Within the first month we saw an immediate drop in catheter utilization and had zero CAUTIs, but during the next 2 months there was a return to our previous rates (Figure 2 and Figure 3 [figures show combined mixed and cardiovascular ICU rates due to reporting requirements]).
Although nursing is at the heart of this engagement, it is the combined efforts of all disciplines that promote the reduction of CAUTIs and improve patient outcomes. When our CAUTI counts plateaued at 10 annually in 2014–2015, we reached out to physicians and found that we had not adequately educated our medical and surgical staff of our project and goals. With the backing of a supportive and vocal ICU director, physician engagement has increased and there is more attention paid to catheter removal by our ICU intensivists. This collaborative approach has helped lower our rates even further in 2016 (n = 3). We achieved our CAUTI SIR goal of less than 1.0 , and changed our current goal to less than 0.5 (Figure 3).
In addition to greater intensivist engagement, the ED reduced their urinary catheter insertion rate from 12% to 4% for all patients transferring to an inpatient status. As previously mentioned, they are now placing catheters from kits that include urometers, so we do not have to break the integrity of the closed system after the patient it transferred to the ICU. We are also collaborating with surgical services to reduce catheter use. This is still a work in progress that requires collaboration with surgeons and hospitalists in changing departmental norms.
Conclusion
Through a combined effort involving a number of departments across the hospital, we were able to reduce catheter utilization and CAUTI rates in the ICU. We have seen a culture shift, with more ICU nursing staff questioning the use of catheters and requesting to have them removed during daily bedside rounds or simply removing them based on our nursing-driven protocol. Currently, both critical care units have been actively working on reducing CAUTI rates and have gone 310 days without a CAUTI.
Reluctance among ICU nurses to remove urinary catheters has declined; however, it is easy to fall back on the convenience of catheters. We have found that each rise in utilization rates and CAUTIs pointed to the need to refocus our effort on the daily bedside conversations. Unless we can eliminate the need for urinary catheters, there will always be a risk of a CAUTI. However, with advances in catheter technology, alternatives to catheters, and nursing education, the reduction in this hospital-acquired infection can be realized.
Acknowledgments: The author thanks our devoted infection control manager (now director), Nina Espinoza Mazzola, BSM, CIC. Our attaining success at the bedside is a reflection of her commitment as a resource and in providing support for nursing practice.
Corresponding author: Jennifer C. Tuttle, RN, MSNEd, CNRN, Tucson Medical Center, 5301 E. Grant Rd, Tucson, AZ 85712.
Financial disclosures: None.
1. Nicolle LE. Catheter-associated urinary tract infections. Antimicrob Resist Infect Control 2014;3:23.
2. Centers for Disease Control and Prevention (CDC). Urinary tract infection (catheter-associated urinary tract infection [cauti] and non-catheter-associated urinary tract infection [uti]) and other urinary system infection [usi]) events. 2017. Accessed at www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf.
3. Centers for Disease Control and Prevention. Catheter-associated urinary tract infection (cauti) toolkit. Accessed 5 Mar 2017 at www.cdc.gov/HAI/pdfs/toolkits/CAUTItoolkit_3_10.pdf.
4. On the CUSP implementation guide. Accessed at http://web.mhanet.com/cauti-implementation_guide_508.pdf.
5. Healthcare Infection Control Practice Advisory Committee (HICPAC). Guidelines for the prevention of catheter associated urinary tract infections 2009. Accessed 25 Feb 2017 at www.cdc.gov/infectioncontrol/pdf/guidelines/cauti-guidelines.pdf.
1. Nicolle LE. Catheter-associated urinary tract infections. Antimicrob Resist Infect Control 2014;3:23.
2. Centers for Disease Control and Prevention (CDC). Urinary tract infection (catheter-associated urinary tract infection [cauti] and non-catheter-associated urinary tract infection [uti]) and other urinary system infection [usi]) events. 2017. Accessed at www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf.
3. Centers for Disease Control and Prevention. Catheter-associated urinary tract infection (cauti) toolkit. Accessed 5 Mar 2017 at www.cdc.gov/HAI/pdfs/toolkits/CAUTItoolkit_3_10.pdf.
4. On the CUSP implementation guide. Accessed at http://web.mhanet.com/cauti-implementation_guide_508.pdf.
5. Healthcare Infection Control Practice Advisory Committee (HICPAC). Guidelines for the prevention of catheter associated urinary tract infections 2009. Accessed 25 Feb 2017 at www.cdc.gov/infectioncontrol/pdf/guidelines/cauti-guidelines.pdf.
Skin cancer procedures up by 35% since 2012
The number of skin cancer procedures in 2016 was up by 10.5% since 2015 and by 35% since 2012, according to the American Society for Dermatologic Surgery.
Of the estimated 3.5 million skin cancer treatments provided by dermatologic surgeons in 2016, just over 227,000, or 6.5%, were for melanoma – a 4% increase over those diagnosed in 2015. Since 2012, the annual number of melanoma procedures has risen by 55%. The 3.29 million nonmelanoma procedures performed in 2016 represent a 10% increase over 2015, the ASDS said in a report on its 2016 Survey on Dermatologic Procedures.
“The public is increasingly aware of the need to have any new or suspicious lesions checked,” ASDS President Thomas Rohrer, MD, said in a written statement.
In addition to the skin cancer treatments, ASDS members also performed over 7 million cosmetic procedures in 2016, including 2.8 million involving laser, light, and energy-based devices. Additionally, 1.7 million involving neuromodulators, and 1.35 million involved soft-tissue fillers, the ASDS said.
The procedures survey was conducted Jan. 4 to Feb. 8, 2017, and included 627 physicians’ responses, which were then generalized to represent all of the almost 6,100 ASDS members.
The number of skin cancer procedures in 2016 was up by 10.5% since 2015 and by 35% since 2012, according to the American Society for Dermatologic Surgery.
Of the estimated 3.5 million skin cancer treatments provided by dermatologic surgeons in 2016, just over 227,000, or 6.5%, were for melanoma – a 4% increase over those diagnosed in 2015. Since 2012, the annual number of melanoma procedures has risen by 55%. The 3.29 million nonmelanoma procedures performed in 2016 represent a 10% increase over 2015, the ASDS said in a report on its 2016 Survey on Dermatologic Procedures.
“The public is increasingly aware of the need to have any new or suspicious lesions checked,” ASDS President Thomas Rohrer, MD, said in a written statement.
In addition to the skin cancer treatments, ASDS members also performed over 7 million cosmetic procedures in 2016, including 2.8 million involving laser, light, and energy-based devices. Additionally, 1.7 million involving neuromodulators, and 1.35 million involved soft-tissue fillers, the ASDS said.
The procedures survey was conducted Jan. 4 to Feb. 8, 2017, and included 627 physicians’ responses, which were then generalized to represent all of the almost 6,100 ASDS members.
The number of skin cancer procedures in 2016 was up by 10.5% since 2015 and by 35% since 2012, according to the American Society for Dermatologic Surgery.
Of the estimated 3.5 million skin cancer treatments provided by dermatologic surgeons in 2016, just over 227,000, or 6.5%, were for melanoma – a 4% increase over those diagnosed in 2015. Since 2012, the annual number of melanoma procedures has risen by 55%. The 3.29 million nonmelanoma procedures performed in 2016 represent a 10% increase over 2015, the ASDS said in a report on its 2016 Survey on Dermatologic Procedures.
“The public is increasingly aware of the need to have any new or suspicious lesions checked,” ASDS President Thomas Rohrer, MD, said in a written statement.
In addition to the skin cancer treatments, ASDS members also performed over 7 million cosmetic procedures in 2016, including 2.8 million involving laser, light, and energy-based devices. Additionally, 1.7 million involving neuromodulators, and 1.35 million involved soft-tissue fillers, the ASDS said.
The procedures survey was conducted Jan. 4 to Feb. 8, 2017, and included 627 physicians’ responses, which were then generalized to represent all of the almost 6,100 ASDS members.
Factors tied to parents’ intent to vaccinate teens for HPV
in a large study, said Kahee A. Mohammed, MD, of Saint Louis (Missouri) University Center for Outcomes Research, and associates.
Messages to boost HPV vaccination rates may need to be targeted based on maternal education, non-Hispanic white ethnicity, and provider recommendations, the researchers said.
Among unvaccinated girls, independent variables predicting parents’ intent to vaccinate teens for HPV were Hispanic race/ethnicity (AOR, 1.57), compared with non-Hispanic whites; mothers with less than a high school diploma (AOR, 1.86), compared with mothers with a college education; and a provider recommendation for HPV vaccine (AOR, 1.38).
Also, mothers with some college education were more likely to intend to vaccinate their sons (AOR, 1.21), but less likely to intend to vaccinate their daughters (AOR, .69) than mothers with a college education.
About 7% of the survey respondents said “not sure/don’t know” regarding their intent to vaccinate their teens. The largest percentage had boys (66%), were non-Hispanic whites (47%), lived in the South (38%), lived above the poverty line (62%), the mother was a college graduate (31%), and had never received a recommendation for HPV vaccination from a health care provider (75%).
“Health care providers should actively engage in discussions with parents about HPV and strongly recommend the vaccine to all eligible patients concurrently with other routinely administered vaccinations to dispel any potential negative assumptions or opinions regarding HPV vaccination, especially among girls,” Dr. Mohammed and his associates said.
Read more at (Prev Chronic Dis. 2017. doi: 10.5888/pcd14.160314).
in a large study, said Kahee A. Mohammed, MD, of Saint Louis (Missouri) University Center for Outcomes Research, and associates.
Messages to boost HPV vaccination rates may need to be targeted based on maternal education, non-Hispanic white ethnicity, and provider recommendations, the researchers said.
Among unvaccinated girls, independent variables predicting parents’ intent to vaccinate teens for HPV were Hispanic race/ethnicity (AOR, 1.57), compared with non-Hispanic whites; mothers with less than a high school diploma (AOR, 1.86), compared with mothers with a college education; and a provider recommendation for HPV vaccine (AOR, 1.38).
Also, mothers with some college education were more likely to intend to vaccinate their sons (AOR, 1.21), but less likely to intend to vaccinate their daughters (AOR, .69) than mothers with a college education.
About 7% of the survey respondents said “not sure/don’t know” regarding their intent to vaccinate their teens. The largest percentage had boys (66%), were non-Hispanic whites (47%), lived in the South (38%), lived above the poverty line (62%), the mother was a college graduate (31%), and had never received a recommendation for HPV vaccination from a health care provider (75%).
“Health care providers should actively engage in discussions with parents about HPV and strongly recommend the vaccine to all eligible patients concurrently with other routinely administered vaccinations to dispel any potential negative assumptions or opinions regarding HPV vaccination, especially among girls,” Dr. Mohammed and his associates said.
Read more at (Prev Chronic Dis. 2017. doi: 10.5888/pcd14.160314).
in a large study, said Kahee A. Mohammed, MD, of Saint Louis (Missouri) University Center for Outcomes Research, and associates.
Messages to boost HPV vaccination rates may need to be targeted based on maternal education, non-Hispanic white ethnicity, and provider recommendations, the researchers said.
Among unvaccinated girls, independent variables predicting parents’ intent to vaccinate teens for HPV were Hispanic race/ethnicity (AOR, 1.57), compared with non-Hispanic whites; mothers with less than a high school diploma (AOR, 1.86), compared with mothers with a college education; and a provider recommendation for HPV vaccine (AOR, 1.38).
Also, mothers with some college education were more likely to intend to vaccinate their sons (AOR, 1.21), but less likely to intend to vaccinate their daughters (AOR, .69) than mothers with a college education.
About 7% of the survey respondents said “not sure/don’t know” regarding their intent to vaccinate their teens. The largest percentage had boys (66%), were non-Hispanic whites (47%), lived in the South (38%), lived above the poverty line (62%), the mother was a college graduate (31%), and had never received a recommendation for HPV vaccination from a health care provider (75%).
“Health care providers should actively engage in discussions with parents about HPV and strongly recommend the vaccine to all eligible patients concurrently with other routinely administered vaccinations to dispel any potential negative assumptions or opinions regarding HPV vaccination, especially among girls,” Dr. Mohammed and his associates said.
Read more at (Prev Chronic Dis. 2017. doi: 10.5888/pcd14.160314).
FROM PREVENTING CHRONIC DISEASE
FDA approves Sapien 3 transcatheter valve for bioprosthetic valve failure
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
The Food and Drug Administration announced June 5 the approval of an expanded indication for the Sapien 3 Transcatheter Heart Valve (THV) for patients with symptomatic heart disease caused by failure of a previously placed bioprosthetic aortic or mitral valve who have a risk of death or severe complications from repeat surgery.
This is the first FDA approval for the expanded use of the Sapien 3 THV as a valve-in-valve treatment. Such procedures provide an alternative to repeat surgery.
“For the first time, a regulatory agency is approving a transcatheter heart valve as a valve-in-valve treatment when bioprosthetic mitral or aortic valves fail in patients who are at high or greater risk of complications from repeat surgery,” Bram Zuckerman, MD, director of the division of cardiovascular devices at the FDA’s Center for Devices and Radiological Health, said in a press release. “This new approval offers U.S. patients with failing surgical bioprosthetic aortic or mitral valves a less-invasive treatment option.”
Originally, the FDA approved the Sapien 3 THV for transcatheter aortic valve replacement (TAVR) as an alternative to surgical aortic valve replacement for patients with native aortic stenosis whose risk for death or severe complications from surgery is high or greater. Then in 2016, the FDA expanded the TAVR indication for Sapien 3 THV to include patients who are at intermediate surgical risk for death or complications.
Read the full press release on the FDA’s website.
Impairment persists despite treatment in adult ADHD
SAN DIEGO – Despite treatment with short- or long-acting medications, adults with attention-deficit/hyperactivity disorder report more impairment than do non-ADHD adults across several domains of daily life, and at certain times of day.
The findings, from a study presented at the annual meeting of the American Psychiatric Association, suggest that adults with ADHD have burdens that may persist despite medication.
The studies compared a cohort of 616 adults with a self-reported ADHD diagnosis and at least 6 months on medication, including short-acting stimulants, long-acting agents, or a combination of these. The researchers also recruited a comparison cohort of 200 non-ADHD adults.
“Interestingly, there was not only a difference between ADHD and non-ADHD groups, but there was also significant impairment reported among patients who are currently being treated for ADHD,” Alexandra Khachatryan, MPH, of Shire Pharmaceuticals, the study’s senior author, said in an interview. Ms. Khachatryan and her colleagues presented the findings at the APA.
For example, 44% of the ADHD respondents reported that the afternoon was the most challenging time of day, compared with 29% of non-ADHD participants (P less than .001). Mid-morning also was significantly more challenging for the ADHD group, with 26% reporting difficulties, compared with 17% of the non-ADHD cohort (P less than .01).
Other statistically significant between-group differences were seen related to managing affect and emotions, sustaining effort, working memory and recall, and interpersonal relationships.
“In addition to the burden patients report across the day, they also expressed significant challenges with psychosocial functioning and managing the demands of work, social, and family life despite treatment,” said Norman Atkins, PhD, of Shire, a coauthor of the study.
A separate poster by the same research group, using the same study data from the cohort of 616 currently treated adult ADHD patients (mean age 39, 70% female) looked at self-reported impairment across daily life domains by patients under different medication regimens.
Patients in the cohort were treated with short-acting stimulants (n = 166), long-acting stimulants (n = 201), or augmentation strategies (n = 249). The researchers found that afternoons and evenings were most difficult for patients regardless of treatment approach.
Ms. Khachatryan said the study was intended to help clinicians “understand what we’re offering patients and if we’re adequately meeting the needs of patients across the day. And we found that adults experience burden across the day despite being treated, and what they report as the most challenging times of day are the afternoon and evening hours,” when work, family, and household obligations are likely to be present.
Dr. Atkins added: “From an ADHD management perspective, the key takeaway is that these impairments occur across multiple settings and are most problematic at certain times of the day. It’s important for providers to have a meaningful conversation with their patients about their day-to-day challenges to fully appreciate how ADHD impacts their functioning so they can best optimize care.”
The researchers acknowledged as limitations of their study its high number of women participants, potentially reducing the generalizability of its findings; the reliance on self-reported outcomes; and between-group differences for the ADHD and non-ADHD groups that included differences in mean age (39 vs. 43, respectively) and full-time employment status (57% vs. 42%).
The study was sponsored by Shire Pharmaceuticals, with three of its five coauthors employed by the company.
SAN DIEGO – Despite treatment with short- or long-acting medications, adults with attention-deficit/hyperactivity disorder report more impairment than do non-ADHD adults across several domains of daily life, and at certain times of day.
The findings, from a study presented at the annual meeting of the American Psychiatric Association, suggest that adults with ADHD have burdens that may persist despite medication.
The studies compared a cohort of 616 adults with a self-reported ADHD diagnosis and at least 6 months on medication, including short-acting stimulants, long-acting agents, or a combination of these. The researchers also recruited a comparison cohort of 200 non-ADHD adults.
“Interestingly, there was not only a difference between ADHD and non-ADHD groups, but there was also significant impairment reported among patients who are currently being treated for ADHD,” Alexandra Khachatryan, MPH, of Shire Pharmaceuticals, the study’s senior author, said in an interview. Ms. Khachatryan and her colleagues presented the findings at the APA.
For example, 44% of the ADHD respondents reported that the afternoon was the most challenging time of day, compared with 29% of non-ADHD participants (P less than .001). Mid-morning also was significantly more challenging for the ADHD group, with 26% reporting difficulties, compared with 17% of the non-ADHD cohort (P less than .01).
Other statistically significant between-group differences were seen related to managing affect and emotions, sustaining effort, working memory and recall, and interpersonal relationships.
“In addition to the burden patients report across the day, they also expressed significant challenges with psychosocial functioning and managing the demands of work, social, and family life despite treatment,” said Norman Atkins, PhD, of Shire, a coauthor of the study.
A separate poster by the same research group, using the same study data from the cohort of 616 currently treated adult ADHD patients (mean age 39, 70% female) looked at self-reported impairment across daily life domains by patients under different medication regimens.
Patients in the cohort were treated with short-acting stimulants (n = 166), long-acting stimulants (n = 201), or augmentation strategies (n = 249). The researchers found that afternoons and evenings were most difficult for patients regardless of treatment approach.
Ms. Khachatryan said the study was intended to help clinicians “understand what we’re offering patients and if we’re adequately meeting the needs of patients across the day. And we found that adults experience burden across the day despite being treated, and what they report as the most challenging times of day are the afternoon and evening hours,” when work, family, and household obligations are likely to be present.
Dr. Atkins added: “From an ADHD management perspective, the key takeaway is that these impairments occur across multiple settings and are most problematic at certain times of the day. It’s important for providers to have a meaningful conversation with their patients about their day-to-day challenges to fully appreciate how ADHD impacts their functioning so they can best optimize care.”
The researchers acknowledged as limitations of their study its high number of women participants, potentially reducing the generalizability of its findings; the reliance on self-reported outcomes; and between-group differences for the ADHD and non-ADHD groups that included differences in mean age (39 vs. 43, respectively) and full-time employment status (57% vs. 42%).
The study was sponsored by Shire Pharmaceuticals, with three of its five coauthors employed by the company.
SAN DIEGO – Despite treatment with short- or long-acting medications, adults with attention-deficit/hyperactivity disorder report more impairment than do non-ADHD adults across several domains of daily life, and at certain times of day.
The findings, from a study presented at the annual meeting of the American Psychiatric Association, suggest that adults with ADHD have burdens that may persist despite medication.
The studies compared a cohort of 616 adults with a self-reported ADHD diagnosis and at least 6 months on medication, including short-acting stimulants, long-acting agents, or a combination of these. The researchers also recruited a comparison cohort of 200 non-ADHD adults.
“Interestingly, there was not only a difference between ADHD and non-ADHD groups, but there was also significant impairment reported among patients who are currently being treated for ADHD,” Alexandra Khachatryan, MPH, of Shire Pharmaceuticals, the study’s senior author, said in an interview. Ms. Khachatryan and her colleagues presented the findings at the APA.
For example, 44% of the ADHD respondents reported that the afternoon was the most challenging time of day, compared with 29% of non-ADHD participants (P less than .001). Mid-morning also was significantly more challenging for the ADHD group, with 26% reporting difficulties, compared with 17% of the non-ADHD cohort (P less than .01).
Other statistically significant between-group differences were seen related to managing affect and emotions, sustaining effort, working memory and recall, and interpersonal relationships.
“In addition to the burden patients report across the day, they also expressed significant challenges with psychosocial functioning and managing the demands of work, social, and family life despite treatment,” said Norman Atkins, PhD, of Shire, a coauthor of the study.
A separate poster by the same research group, using the same study data from the cohort of 616 currently treated adult ADHD patients (mean age 39, 70% female) looked at self-reported impairment across daily life domains by patients under different medication regimens.
Patients in the cohort were treated with short-acting stimulants (n = 166), long-acting stimulants (n = 201), or augmentation strategies (n = 249). The researchers found that afternoons and evenings were most difficult for patients regardless of treatment approach.
Ms. Khachatryan said the study was intended to help clinicians “understand what we’re offering patients and if we’re adequately meeting the needs of patients across the day. And we found that adults experience burden across the day despite being treated, and what they report as the most challenging times of day are the afternoon and evening hours,” when work, family, and household obligations are likely to be present.
Dr. Atkins added: “From an ADHD management perspective, the key takeaway is that these impairments occur across multiple settings and are most problematic at certain times of the day. It’s important for providers to have a meaningful conversation with their patients about their day-to-day challenges to fully appreciate how ADHD impacts their functioning so they can best optimize care.”
The researchers acknowledged as limitations of their study its high number of women participants, potentially reducing the generalizability of its findings; the reliance on self-reported outcomes; and between-group differences for the ADHD and non-ADHD groups that included differences in mean age (39 vs. 43, respectively) and full-time employment status (57% vs. 42%).
The study was sponsored by Shire Pharmaceuticals, with three of its five coauthors employed by the company.
AT APA
Isotretinoin not associated with increased depression risk, in meta-analysis
A meta-analysis of 31 studies examining the relationship between isotretinoin treatment for acne and depression found no significant association. Rather, the treatment of acne was associated with improved symptoms of depression.
Researchers have been evaluating isotretinoin’s possible association with depression since 1983. Many studies have failed to find an association or have been inconclusive. One study found a statistically significant association between isotretinoin treatment and depression, but there are also depression risks associated with not treating a patient with severe acne.
Gathering the existing literature into a meta-analysis allowed the researchers to assess possible confounding factors in the individual studies, such as sex, length of treatment, and cumulative isotretinoin dose.
“In our meta-analysis, we pooled the results of 1,411 patients who received depression evaluations at baseline and after treatment, which revealed a significant improvement in the depression scores,” wrote Yu-Chen Huang, MD, of the department of dermatology, Taipei Medical University, Taiwan, and Ying-Chih Cheng, MD, of National Taiwan University. The 31 studies included 3 population-based studies, 8 controlled studies, and 20 prospective, open-label studies, and were published through September 2016.
Prevalence of depression also significantly dropped after treatment (relative risk, .588), they wrote, but they pointed out that “some studies described newly developed depression during treatment.” They referred to one controlled study in which “new onset of depression was noted in both the isotretinoin and antibiotic groups, implying that depression is associated with acne, independently of isotretinoin” (World J Psychiatr. 2016 Mar 22;6[1]:136-42). “Thus, physicians should consider the possibility of depression among all acne patients regardless of the treatment method,” they added.
They also concluded that some patients “might be more prone to depression regardless of acne or other conditions. Thus, closely monitoring acne patients for depression is essential to identify patients at a high risk.”
The full study can be found at: J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.
A meta-analysis of 31 studies examining the relationship between isotretinoin treatment for acne and depression found no significant association. Rather, the treatment of acne was associated with improved symptoms of depression.
Researchers have been evaluating isotretinoin’s possible association with depression since 1983. Many studies have failed to find an association or have been inconclusive. One study found a statistically significant association between isotretinoin treatment and depression, but there are also depression risks associated with not treating a patient with severe acne.
Gathering the existing literature into a meta-analysis allowed the researchers to assess possible confounding factors in the individual studies, such as sex, length of treatment, and cumulative isotretinoin dose.
“In our meta-analysis, we pooled the results of 1,411 patients who received depression evaluations at baseline and after treatment, which revealed a significant improvement in the depression scores,” wrote Yu-Chen Huang, MD, of the department of dermatology, Taipei Medical University, Taiwan, and Ying-Chih Cheng, MD, of National Taiwan University. The 31 studies included 3 population-based studies, 8 controlled studies, and 20 prospective, open-label studies, and were published through September 2016.
Prevalence of depression also significantly dropped after treatment (relative risk, .588), they wrote, but they pointed out that “some studies described newly developed depression during treatment.” They referred to one controlled study in which “new onset of depression was noted in both the isotretinoin and antibiotic groups, implying that depression is associated with acne, independently of isotretinoin” (World J Psychiatr. 2016 Mar 22;6[1]:136-42). “Thus, physicians should consider the possibility of depression among all acne patients regardless of the treatment method,” they added.
They also concluded that some patients “might be more prone to depression regardless of acne or other conditions. Thus, closely monitoring acne patients for depression is essential to identify patients at a high risk.”
The full study can be found at: J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.
A meta-analysis of 31 studies examining the relationship between isotretinoin treatment for acne and depression found no significant association. Rather, the treatment of acne was associated with improved symptoms of depression.
Researchers have been evaluating isotretinoin’s possible association with depression since 1983. Many studies have failed to find an association or have been inconclusive. One study found a statistically significant association between isotretinoin treatment and depression, but there are also depression risks associated with not treating a patient with severe acne.
Gathering the existing literature into a meta-analysis allowed the researchers to assess possible confounding factors in the individual studies, such as sex, length of treatment, and cumulative isotretinoin dose.
“In our meta-analysis, we pooled the results of 1,411 patients who received depression evaluations at baseline and after treatment, which revealed a significant improvement in the depression scores,” wrote Yu-Chen Huang, MD, of the department of dermatology, Taipei Medical University, Taiwan, and Ying-Chih Cheng, MD, of National Taiwan University. The 31 studies included 3 population-based studies, 8 controlled studies, and 20 prospective, open-label studies, and were published through September 2016.
Prevalence of depression also significantly dropped after treatment (relative risk, .588), they wrote, but they pointed out that “some studies described newly developed depression during treatment.” They referred to one controlled study in which “new onset of depression was noted in both the isotretinoin and antibiotic groups, implying that depression is associated with acne, independently of isotretinoin” (World J Psychiatr. 2016 Mar 22;6[1]:136-42). “Thus, physicians should consider the possibility of depression among all acne patients regardless of the treatment method,” they added.
They also concluded that some patients “might be more prone to depression regardless of acne or other conditions. Thus, closely monitoring acne patients for depression is essential to identify patients at a high risk.”
The full study can be found at: J Am Acad Dermatol. 2017 Jun;76[6]:1068-76.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
It matters how you phrase a child’s flu vaccine recommendation
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
Vaccine hesitant parents were more likely to change their minds about flu vaccine for their children when pediatricians or pediatric nurse practitioners used a presumptive recommendation that their children get the vaccine, pursued the recommendation if the parent was resistant, and combined their recommendation for the flu vaccine with other childhood vaccines, said Annika M. Hofstetter, MD, PhD, of the University of Washington, Seattle, and her associates.
The researchers recruited 17 pediatricians and pediatric nurse practitioners from eight primary care pediatric practices in the Seattle area to take part in 50 videotaped visits with parents during the 2011-2012 and 2013-2014 flu seasons.
(83% vs. 33%; P less than .01), Dr. Hofstetter and her colleagues said. The various communication patterns did not appear to negatively affect the way parents rated their visit experiences.
Read more at Vaccine. 2017;35:2709-15.
FROM VACCINE
Former Pharma reps’ new mission: To school docs on high drug costs
As a drug salesman, Mike Courtney worked hard to make health care expensive. He wined and dined doctors, golfed with them and bought lunch for their entire staffs – all to promote pills often costing thousands of dollars a year.
Now he’s on a different mission. When Mr. Courtney calls on doctors these days, he champions generic drugs that frequently cost pennies and work just as well as the kinds of pricey brands he used to push.
Instead of Big Pharma, he works for Capital District Physicians’ Health Plan (CDPHP), an Albany, N.Y., insurer. Instead of maximizing pill profits, his job is to save millions of dollars by educating doctors about expensive prescriptions and the stratagems used to sell them.
“Having come from Big Pharma, I do really feel my soul has been cleansed,” laughs Mr. Courtney, who formerly worked for Pfizer and Johnson & Johnson. “I do feel like I’m more in touch with the physicians” and plan members, he added.
Costs for prescription drugs have been rising faster than those for any other health segment, marked by high-profile cases such as the reported 400% increase for Mylan’s EpiPen and 5,000% spike for Turing Pharmaceuticals’ Daraprim.
Health plans and others paying those costs are fighting back. Many have tried to give doctors academic research on pill effectiveness or simply removed high-cost drugs from coverage lists.
Consumer groups and medical societies have tried to spread the word about expensive drugs. Startup GoodRx lets patients compare retail prices online.
CDPHP is one of the few insurers to have taken the battle against pricey pills a step further. It is recruiting across enemy lines, hiring former pharma representatives and staffing what may be a new job category: a sales force for cost-effective medicine.
“Insurers are taking matters into their own hands,” said Lea Prevel Katsanis, a marketing professor at Canada’s Concordia University who specializes in the pharmaceutical industry. “They’re saying, ‘We can’t really rely on drug companies to talk to doctors about what’s cost-efficient.’ ”
If insurance companies can curb drug costs, premiums paid by employers, taxpayers, and consumers need not rise as fast.
Two years ago, when one company increased the cost of a common diabetes medicine to 20 times what it had been a few years earlier, Mr. Courtney and five other former pharma and medical-device reps working for CDPHP knew what to do.
Valeant Pharmaceuticals had cranked up the price of one common dosage of its Glumetza medicine for lowering blood sugar to an astonishing $81,270 a year, according to Truven Health Analytics, a data firm. Meanwhile a similar, generic version can be bought for as little as a penny a pill.
Because Glumetza was on CDPHP’s list of approved drugs, the insurer and its members had to pay for it when doctors prescribed it, resulting in millions in extra costs and stinging copayments for patients.
Eric Schnakenberg, MD, an upstate New York family medicine doctor, was shocked when patients began complaining about what he assumed was an inexpensive prescription. Doctors are famously unaware about the cost of the care they order, a situation exploited by drug sellers and other vendors.
While physicians’ electronic prescribing programs and even pharmaceutical guides like the Physicians’ Desk Reference contain prescribing information – some are even peppered with ads – they contain no specific information about prices. Drug sales reps who visit their offices don’t highlight high prices as they drop off free samples, and drug makers can quietly, but substantially, hike the price of a drug from one year to the next.
“As physicians, we’re blindsided by that,” Dr. Schnakenberg said. “We get patient complaints saying, ‘Hey, I can’t afford this,’ and we say: ‘It’s cheap!’ ”
After Mr. Courtney and his colleagues alerted doctors to what Valeant was up to, all but a handful of the 60 plan members who were taking Glumetza switched to metformin, the generic alternative. That saved about $5 million in a year.
Following an outcry over its practices, Valeant agreed last year to raise annual prices by no more than single-digit percentages, the company said through a spokesman. But such hikes could still outpace the inflation rate.
Using ‘Those Powers For Good’
Cardiologist John Bennett got the idea to hire pharma reps a few years ago, after he became CDPHP’s chief executive. He knew reps are smart, genial, and motivated. Overhiring by pharma had put many back on the job market.
His sales pitch to them, he says half-jokingly, was: “You know everything they taught you in Big Pharma? How would you like to use those powers for good?”
Pharma companies spend billions on TV ads, doctor blandishments, and expensive salespeople to keep prescriptions flowing.
Pfizer, Johnson & Johnson, and other sellers responded to critics a few years ago by restricting gifts of entertainment, coffee mugs, and some meals. But the industry’s ethics code still allows lavish consulting contracts for doctors and sponsorship of physician conferences as well as meals for doctors and their staffs who listen to an “informational presentation” from sales reps touting expensive pills.
“When those products go generic, nobody’s promoting them anymore,” Mr. Courtney said. Generics makers lack big marketing budgets. CDPHP’s remedy: The insurer promotes generics with its own reps.
“It’s a great idea,” said Alan Sorensen, an economist at the University of Wisconsin who has studied drug prices. “Even a small moving of the needle on their [doctors’] prescribing behavior can have a pretty big impact on costs.”
At first the team concentrated on educating doctors about cheaper alternatives to Lipitor, a widely prescribed cholesterol-lowering medicine, and Nexium, for stomach problems. That saved around $10 million the first year, much in the form of copayments that would have been owed by plan members.
Recently the plan has focused on Seroquel, a branded antipsychotic that costs far more than a similar generic. Switching to the generic saves $600 to $1,000 a month, estimates Eileen Wood, the insurer’s vice president of pharmacy and health quality.
CDPHP’s repurposed reps have helped keep the insurer’s annual drug-cost increases to single-digit percentages, whereas without them and other measures “we would certainly be well into double-digit” increases, she said.
Educating doctors about drug costs is part of a larger push for “transparency” in an industry where Princeton economist Uwe Reinhardt says consumers face the same experience as somebody shopping in Macy’s blindfolded.
Current research by the University of Wisconsin’s Mr. Sorensen finds physicians with access to data about drug prices and insurance coverage are more likely to prescribe generics.
That gives Mr. Courtney and his colleagues a fighting chance, even if, he said, “we don’t have the freewheeling, unlimited green Amex card like I did back in the day.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
As a drug salesman, Mike Courtney worked hard to make health care expensive. He wined and dined doctors, golfed with them and bought lunch for their entire staffs – all to promote pills often costing thousands of dollars a year.
Now he’s on a different mission. When Mr. Courtney calls on doctors these days, he champions generic drugs that frequently cost pennies and work just as well as the kinds of pricey brands he used to push.
Instead of Big Pharma, he works for Capital District Physicians’ Health Plan (CDPHP), an Albany, N.Y., insurer. Instead of maximizing pill profits, his job is to save millions of dollars by educating doctors about expensive prescriptions and the stratagems used to sell them.
“Having come from Big Pharma, I do really feel my soul has been cleansed,” laughs Mr. Courtney, who formerly worked for Pfizer and Johnson & Johnson. “I do feel like I’m more in touch with the physicians” and plan members, he added.
Costs for prescription drugs have been rising faster than those for any other health segment, marked by high-profile cases such as the reported 400% increase for Mylan’s EpiPen and 5,000% spike for Turing Pharmaceuticals’ Daraprim.
Health plans and others paying those costs are fighting back. Many have tried to give doctors academic research on pill effectiveness or simply removed high-cost drugs from coverage lists.
Consumer groups and medical societies have tried to spread the word about expensive drugs. Startup GoodRx lets patients compare retail prices online.
CDPHP is one of the few insurers to have taken the battle against pricey pills a step further. It is recruiting across enemy lines, hiring former pharma representatives and staffing what may be a new job category: a sales force for cost-effective medicine.
“Insurers are taking matters into their own hands,” said Lea Prevel Katsanis, a marketing professor at Canada’s Concordia University who specializes in the pharmaceutical industry. “They’re saying, ‘We can’t really rely on drug companies to talk to doctors about what’s cost-efficient.’ ”
If insurance companies can curb drug costs, premiums paid by employers, taxpayers, and consumers need not rise as fast.
Two years ago, when one company increased the cost of a common diabetes medicine to 20 times what it had been a few years earlier, Mr. Courtney and five other former pharma and medical-device reps working for CDPHP knew what to do.
Valeant Pharmaceuticals had cranked up the price of one common dosage of its Glumetza medicine for lowering blood sugar to an astonishing $81,270 a year, according to Truven Health Analytics, a data firm. Meanwhile a similar, generic version can be bought for as little as a penny a pill.
Because Glumetza was on CDPHP’s list of approved drugs, the insurer and its members had to pay for it when doctors prescribed it, resulting in millions in extra costs and stinging copayments for patients.
Eric Schnakenberg, MD, an upstate New York family medicine doctor, was shocked when patients began complaining about what he assumed was an inexpensive prescription. Doctors are famously unaware about the cost of the care they order, a situation exploited by drug sellers and other vendors.
While physicians’ electronic prescribing programs and even pharmaceutical guides like the Physicians’ Desk Reference contain prescribing information – some are even peppered with ads – they contain no specific information about prices. Drug sales reps who visit their offices don’t highlight high prices as they drop off free samples, and drug makers can quietly, but substantially, hike the price of a drug from one year to the next.
“As physicians, we’re blindsided by that,” Dr. Schnakenberg said. “We get patient complaints saying, ‘Hey, I can’t afford this,’ and we say: ‘It’s cheap!’ ”
After Mr. Courtney and his colleagues alerted doctors to what Valeant was up to, all but a handful of the 60 plan members who were taking Glumetza switched to metformin, the generic alternative. That saved about $5 million in a year.
Following an outcry over its practices, Valeant agreed last year to raise annual prices by no more than single-digit percentages, the company said through a spokesman. But such hikes could still outpace the inflation rate.
Using ‘Those Powers For Good’
Cardiologist John Bennett got the idea to hire pharma reps a few years ago, after he became CDPHP’s chief executive. He knew reps are smart, genial, and motivated. Overhiring by pharma had put many back on the job market.
His sales pitch to them, he says half-jokingly, was: “You know everything they taught you in Big Pharma? How would you like to use those powers for good?”
Pharma companies spend billions on TV ads, doctor blandishments, and expensive salespeople to keep prescriptions flowing.
Pfizer, Johnson & Johnson, and other sellers responded to critics a few years ago by restricting gifts of entertainment, coffee mugs, and some meals. But the industry’s ethics code still allows lavish consulting contracts for doctors and sponsorship of physician conferences as well as meals for doctors and their staffs who listen to an “informational presentation” from sales reps touting expensive pills.
“When those products go generic, nobody’s promoting them anymore,” Mr. Courtney said. Generics makers lack big marketing budgets. CDPHP’s remedy: The insurer promotes generics with its own reps.
“It’s a great idea,” said Alan Sorensen, an economist at the University of Wisconsin who has studied drug prices. “Even a small moving of the needle on their [doctors’] prescribing behavior can have a pretty big impact on costs.”
At first the team concentrated on educating doctors about cheaper alternatives to Lipitor, a widely prescribed cholesterol-lowering medicine, and Nexium, for stomach problems. That saved around $10 million the first year, much in the form of copayments that would have been owed by plan members.
Recently the plan has focused on Seroquel, a branded antipsychotic that costs far more than a similar generic. Switching to the generic saves $600 to $1,000 a month, estimates Eileen Wood, the insurer’s vice president of pharmacy and health quality.
CDPHP’s repurposed reps have helped keep the insurer’s annual drug-cost increases to single-digit percentages, whereas without them and other measures “we would certainly be well into double-digit” increases, she said.
Educating doctors about drug costs is part of a larger push for “transparency” in an industry where Princeton economist Uwe Reinhardt says consumers face the same experience as somebody shopping in Macy’s blindfolded.
Current research by the University of Wisconsin’s Mr. Sorensen finds physicians with access to data about drug prices and insurance coverage are more likely to prescribe generics.
That gives Mr. Courtney and his colleagues a fighting chance, even if, he said, “we don’t have the freewheeling, unlimited green Amex card like I did back in the day.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.
As a drug salesman, Mike Courtney worked hard to make health care expensive. He wined and dined doctors, golfed with them and bought lunch for their entire staffs – all to promote pills often costing thousands of dollars a year.
Now he’s on a different mission. When Mr. Courtney calls on doctors these days, he champions generic drugs that frequently cost pennies and work just as well as the kinds of pricey brands he used to push.
Instead of Big Pharma, he works for Capital District Physicians’ Health Plan (CDPHP), an Albany, N.Y., insurer. Instead of maximizing pill profits, his job is to save millions of dollars by educating doctors about expensive prescriptions and the stratagems used to sell them.
“Having come from Big Pharma, I do really feel my soul has been cleansed,” laughs Mr. Courtney, who formerly worked for Pfizer and Johnson & Johnson. “I do feel like I’m more in touch with the physicians” and plan members, he added.
Costs for prescription drugs have been rising faster than those for any other health segment, marked by high-profile cases such as the reported 400% increase for Mylan’s EpiPen and 5,000% spike for Turing Pharmaceuticals’ Daraprim.
Health plans and others paying those costs are fighting back. Many have tried to give doctors academic research on pill effectiveness or simply removed high-cost drugs from coverage lists.
Consumer groups and medical societies have tried to spread the word about expensive drugs. Startup GoodRx lets patients compare retail prices online.
CDPHP is one of the few insurers to have taken the battle against pricey pills a step further. It is recruiting across enemy lines, hiring former pharma representatives and staffing what may be a new job category: a sales force for cost-effective medicine.
“Insurers are taking matters into their own hands,” said Lea Prevel Katsanis, a marketing professor at Canada’s Concordia University who specializes in the pharmaceutical industry. “They’re saying, ‘We can’t really rely on drug companies to talk to doctors about what’s cost-efficient.’ ”
If insurance companies can curb drug costs, premiums paid by employers, taxpayers, and consumers need not rise as fast.
Two years ago, when one company increased the cost of a common diabetes medicine to 20 times what it had been a few years earlier, Mr. Courtney and five other former pharma and medical-device reps working for CDPHP knew what to do.
Valeant Pharmaceuticals had cranked up the price of one common dosage of its Glumetza medicine for lowering blood sugar to an astonishing $81,270 a year, according to Truven Health Analytics, a data firm. Meanwhile a similar, generic version can be bought for as little as a penny a pill.
Because Glumetza was on CDPHP’s list of approved drugs, the insurer and its members had to pay for it when doctors prescribed it, resulting in millions in extra costs and stinging copayments for patients.
Eric Schnakenberg, MD, an upstate New York family medicine doctor, was shocked when patients began complaining about what he assumed was an inexpensive prescription. Doctors are famously unaware about the cost of the care they order, a situation exploited by drug sellers and other vendors.
While physicians’ electronic prescribing programs and even pharmaceutical guides like the Physicians’ Desk Reference contain prescribing information – some are even peppered with ads – they contain no specific information about prices. Drug sales reps who visit their offices don’t highlight high prices as they drop off free samples, and drug makers can quietly, but substantially, hike the price of a drug from one year to the next.
“As physicians, we’re blindsided by that,” Dr. Schnakenberg said. “We get patient complaints saying, ‘Hey, I can’t afford this,’ and we say: ‘It’s cheap!’ ”
After Mr. Courtney and his colleagues alerted doctors to what Valeant was up to, all but a handful of the 60 plan members who were taking Glumetza switched to metformin, the generic alternative. That saved about $5 million in a year.
Following an outcry over its practices, Valeant agreed last year to raise annual prices by no more than single-digit percentages, the company said through a spokesman. But such hikes could still outpace the inflation rate.
Using ‘Those Powers For Good’
Cardiologist John Bennett got the idea to hire pharma reps a few years ago, after he became CDPHP’s chief executive. He knew reps are smart, genial, and motivated. Overhiring by pharma had put many back on the job market.
His sales pitch to them, he says half-jokingly, was: “You know everything they taught you in Big Pharma? How would you like to use those powers for good?”
Pharma companies spend billions on TV ads, doctor blandishments, and expensive salespeople to keep prescriptions flowing.
Pfizer, Johnson & Johnson, and other sellers responded to critics a few years ago by restricting gifts of entertainment, coffee mugs, and some meals. But the industry’s ethics code still allows lavish consulting contracts for doctors and sponsorship of physician conferences as well as meals for doctors and their staffs who listen to an “informational presentation” from sales reps touting expensive pills.
“When those products go generic, nobody’s promoting them anymore,” Mr. Courtney said. Generics makers lack big marketing budgets. CDPHP’s remedy: The insurer promotes generics with its own reps.
“It’s a great idea,” said Alan Sorensen, an economist at the University of Wisconsin who has studied drug prices. “Even a small moving of the needle on their [doctors’] prescribing behavior can have a pretty big impact on costs.”
At first the team concentrated on educating doctors about cheaper alternatives to Lipitor, a widely prescribed cholesterol-lowering medicine, and Nexium, for stomach problems. That saved around $10 million the first year, much in the form of copayments that would have been owed by plan members.
Recently the plan has focused on Seroquel, a branded antipsychotic that costs far more than a similar generic. Switching to the generic saves $600 to $1,000 a month, estimates Eileen Wood, the insurer’s vice president of pharmacy and health quality.
CDPHP’s repurposed reps have helped keep the insurer’s annual drug-cost increases to single-digit percentages, whereas without them and other measures “we would certainly be well into double-digit” increases, she said.
Educating doctors about drug costs is part of a larger push for “transparency” in an industry where Princeton economist Uwe Reinhardt says consumers face the same experience as somebody shopping in Macy’s blindfolded.
Current research by the University of Wisconsin’s Mr. Sorensen finds physicians with access to data about drug prices and insurance coverage are more likely to prescribe generics.
That gives Mr. Courtney and his colleagues a fighting chance, even if, he said, “we don’t have the freewheeling, unlimited green Amex card like I did back in the day.”
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a national health policy news service that is part of the nonpartisan Henry J. Kaiser Family Foundation.