User login
Videos reduce need for anesthesia in kids undergoing radiotherapy
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
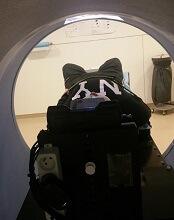
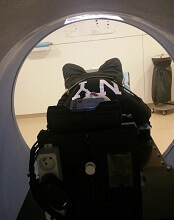
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious. ![]()
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious. ![]()
VIENNA, AUSTRIA—Children with cancer may not require general anesthesia prior to radiotherapy if they can watch videos during their treatment, according to research presented at the ESTRO 36 conference (abstract OC-0546).
Allowing children to watch videos during radiotherapy reduced but did not completely eliminate the use of anesthesia in this small study.
The use of videos proved less traumatic than anesthesia for children and their families, as well as making each treatment quicker and more cost-effective, according to study investigator Catia Aguas, of the Cliniques Universitaires Saint Luc in Brussels, Belgium.
“Being treated with radiotherapy means coming in for a treatment every weekday for 4 to 6 weeks,” Aguas noted. “The children need to remain motionless during treatment, and, on the whole, that means a general anesthesia. That, in turn, means they have to keep their stomach empty for 6 hours before the treatment.”
“We wanted to see if installing a projector and letting children watch a video of their choice would allow them to keep still enough that we would not need to give them anesthesia.”
The study included 12 children, ages 1.5 to 6 years, who were treated with radiotherapy using a Tomotherapy® treatment unit at the university hospital. Six children were treated before a video projector was installed in 2014, and 6 were treated after.
Before the video was available, general anesthesia was needed for 83.3% of children’s treatments. Once the projector was installed, anesthesia was needed in 33.3% of treatments.
“Radiotherapy can be very scary for children,” Aguas noted. “It’s a huge room full of machines and strange noises, and the worst part is that they’re in the room alone during their treatment. Before their radiotherapy treatment, they have already been through a series of tests and treatments, some of them painful, so when they arrive for radiotherapy, they don’t really feel very safe or confident.”
“Since we started using videos, children are a lot less anxious. Now they know that they’re going to watch a movie of their choice, they’re more relaxed, and, once the movie starts, it’s as though they travel to another world. Sponge Bob, Cars, and Barbie have been popular movie choices with our patients.”
The research also showed that treatments that used to take 1 hour or more now take around 15 to 20 minutes. This is partly because of the time saved by not having to prepare and administer anesthesia, but it is also because the children who know they are going to watch videos are more cooperative.
“Now, in our clinic, video has almost completely replaced anesthesia, resulting in reduced treatment times and reduction of stress for the young patients and their families,” Aguas said.
She also noted that the projector was inexpensive and simple to install.
“In radiotherapy, everything is usually very expensive, but, in this case, it was not,” Aguas said. “We bought a projector, and, with the help of college students, we created a support to fix the device to the patient couch. Using video is saving money and resources by reducing the need for anesthesia.”
Aguas and her colleagues continue to study children who have been treated since the projector was installed, and the team is extending the project to include adult patients who are claustrophobic or anxious. ![]()
Company stops developing hemophilia therapy
Dimension Therapeutics, Inc. has announced its decision to stop development of DTX101, a gene therapy product intended to treat moderate/severe to severe hemophilia B.
The decision was based on “disappointing” results from the phase 1/2 study of DTX101, according to Annalisa Jenkins, MBBS, chief executive officer of Dimension.
“[O]ur phase 1/2, open-label clinical study did not demonstrate an ability to achieve a minimum target product profile for continued development or future commercialization,” Dr Jenkins said.
Dimension previously reported results from this 6-patient study in February.
At that time, the data showed that DTX101 could increase factor IX (FIX) expression in adults with hemophilia B, allowing 3 of them to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients in the trial had alanine aminotransferase elevations, and 1 patient had a grade 4 adverse event as a result.
Dimension said it plans to present full results from this study, including results from ongoing immune and biomarker analyses, at a future scientific conference.
“[D]imension remains committed to the hemophilia community through continued investment in the company’s ongoing [investigational new drug]-enabling activities for DTX201 for hemophilia A, in collaboration with Bayer, and the follow-up of the 6 patients dosed with DTX101 in the phase 1/2 clinical trial through an extension study that will monitor all patients for a total of 5 years,” Dr Jenkins noted.
DTX101 was designed to deliver stable expression of FIX in patients with hemophilia B. It is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein. ![]()
Dimension Therapeutics, Inc. has announced its decision to stop development of DTX101, a gene therapy product intended to treat moderate/severe to severe hemophilia B.
The decision was based on “disappointing” results from the phase 1/2 study of DTX101, according to Annalisa Jenkins, MBBS, chief executive officer of Dimension.
“[O]ur phase 1/2, open-label clinical study did not demonstrate an ability to achieve a minimum target product profile for continued development or future commercialization,” Dr Jenkins said.
Dimension previously reported results from this 6-patient study in February.
At that time, the data showed that DTX101 could increase factor IX (FIX) expression in adults with hemophilia B, allowing 3 of them to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients in the trial had alanine aminotransferase elevations, and 1 patient had a grade 4 adverse event as a result.
Dimension said it plans to present full results from this study, including results from ongoing immune and biomarker analyses, at a future scientific conference.
“[D]imension remains committed to the hemophilia community through continued investment in the company’s ongoing [investigational new drug]-enabling activities for DTX201 for hemophilia A, in collaboration with Bayer, and the follow-up of the 6 patients dosed with DTX101 in the phase 1/2 clinical trial through an extension study that will monitor all patients for a total of 5 years,” Dr Jenkins noted.
DTX101 was designed to deliver stable expression of FIX in patients with hemophilia B. It is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein. ![]()
Dimension Therapeutics, Inc. has announced its decision to stop development of DTX101, a gene therapy product intended to treat moderate/severe to severe hemophilia B.
The decision was based on “disappointing” results from the phase 1/2 study of DTX101, according to Annalisa Jenkins, MBBS, chief executive officer of Dimension.
“[O]ur phase 1/2, open-label clinical study did not demonstrate an ability to achieve a minimum target product profile for continued development or future commercialization,” Dr Jenkins said.
Dimension previously reported results from this 6-patient study in February.
At that time, the data showed that DTX101 could increase factor IX (FIX) expression in adults with hemophilia B, allowing 3 of them to forgo prophylactic and on-demand treatment.
However, 5 of the 6 patients in the trial had alanine aminotransferase elevations, and 1 patient had a grade 4 adverse event as a result.
Dimension said it plans to present full results from this study, including results from ongoing immune and biomarker analyses, at a future scientific conference.
“[D]imension remains committed to the hemophilia community through continued investment in the company’s ongoing [investigational new drug]-enabling activities for DTX201 for hemophilia A, in collaboration with Bayer, and the follow-up of the 6 patients dosed with DTX101 in the phase 1/2 clinical trial through an extension study that will monitor all patients for a total of 5 years,” Dr Jenkins noted.
DTX101 was designed to deliver stable expression of FIX in patients with hemophilia B. It is a non-replicating, recombinant adeno-associated viral vector, AAVrh10, with a codon-optimized FIX gene expressing wild-type FIX protein. ![]()
When one patient decompensates, others on the ward may follow
CLINICAL QUESTION: How does the clinical decompensation of a ward patient affect the likelihood of another patient’s decompensation?
STUDY DESIGN: Observational cohort study.
SETTING: Thirteen geographically distinct, adult medical-surgical wards at an academic medical center.
SYNOPSIS: Of 83,723 admissions to medical-surgical wards, 4,286 patients experienced cardiac arrest (179) or were transferred to the ICU (4,107). When one or more of these events occurred, other patients on the same ward had an increased risk for either event over the subsequent 6 (OR 1.18; CI, 1.07-1.31) and 12 hours and the risk was higher if more than one event occurred. Importantly, for patients exposed to other patients on the same ward decompensating, there were no differences in the severity of illness for patients transferred to ICU or in overall mortality.
Intuitively, when one patient becomes critically ill, other patients receive less attention. Though the effect was small, this study highlights that diversion of resources may increase other patients to greater risk. Surprisingly, the increased risk was not significant during night-time hours, when resources are more limited, but data collection may have been also affected.
BOTTOM LINE: When a patient becomes critically ill, another patient on the same ward is more likely to decompensate within the next 6-12 hours, but the effect is small.
CITATIONS: Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316(24):2674-5.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: How does the clinical decompensation of a ward patient affect the likelihood of another patient’s decompensation?
STUDY DESIGN: Observational cohort study.
SETTING: Thirteen geographically distinct, adult medical-surgical wards at an academic medical center.
SYNOPSIS: Of 83,723 admissions to medical-surgical wards, 4,286 patients experienced cardiac arrest (179) or were transferred to the ICU (4,107). When one or more of these events occurred, other patients on the same ward had an increased risk for either event over the subsequent 6 (OR 1.18; CI, 1.07-1.31) and 12 hours and the risk was higher if more than one event occurred. Importantly, for patients exposed to other patients on the same ward decompensating, there were no differences in the severity of illness for patients transferred to ICU or in overall mortality.
Intuitively, when one patient becomes critically ill, other patients receive less attention. Though the effect was small, this study highlights that diversion of resources may increase other patients to greater risk. Surprisingly, the increased risk was not significant during night-time hours, when resources are more limited, but data collection may have been also affected.
BOTTOM LINE: When a patient becomes critically ill, another patient on the same ward is more likely to decompensate within the next 6-12 hours, but the effect is small.
CITATIONS: Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316(24):2674-5.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: How does the clinical decompensation of a ward patient affect the likelihood of another patient’s decompensation?
STUDY DESIGN: Observational cohort study.
SETTING: Thirteen geographically distinct, adult medical-surgical wards at an academic medical center.
SYNOPSIS: Of 83,723 admissions to medical-surgical wards, 4,286 patients experienced cardiac arrest (179) or were transferred to the ICU (4,107). When one or more of these events occurred, other patients on the same ward had an increased risk for either event over the subsequent 6 (OR 1.18; CI, 1.07-1.31) and 12 hours and the risk was higher if more than one event occurred. Importantly, for patients exposed to other patients on the same ward decompensating, there were no differences in the severity of illness for patients transferred to ICU or in overall mortality.
Intuitively, when one patient becomes critically ill, other patients receive less attention. Though the effect was small, this study highlights that diversion of resources may increase other patients to greater risk. Surprisingly, the increased risk was not significant during night-time hours, when resources are more limited, but data collection may have been also affected.
BOTTOM LINE: When a patient becomes critically ill, another patient on the same ward is more likely to decompensate within the next 6-12 hours, but the effect is small.
CITATIONS: Volchenboum SL, Mayampurath A, Göksu-Gürsoy G, et al. Association between in-hospital critical illness events and outcomes in patients on the same ward. JAMA. 2016;316(24):2674-5.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Principles learned from a successful improvement program can increase compliance and reduce hospital acquired VTEs (HA-VTEs) across multiple institutions
CLINICAL QUESTION: Can a single institution’s VTE prophylaxis program be scaled to increase prophylaxis and reduce HA-VTEs across multiple institutions?
STUDY DESIGN: prospective, unblinded, open-intervention study
SETTING: Inpatient medical and surgical services at five independent, cooperating academic hospitals
SYNOPSIS: Each site used common principles to develop their own multi-pronged VTE prophylaxis program including structured order-sets, simplified risk-assessment, feedback to providers, and education programs.
306,906 inpatient discharges were evaluated with average VTE prophylaxis bundle compliance reaching 89% across all institutions. HA-VTE rates declined from 0.90% to 0.69% (RR, 0.76; CI, 0.68-0.85) – equivalent to averting 81 pulmonary emboli and 89 deep venous thrombi. Of note, HA-VTE rates only declined at three of the five institutions with the greatest improvement at those with the highest baseline rates. Further, while HA-VTE rates improved across all patient populations, the incidence reduction was statistically significant in Oncologic and Surgical populations.
BOTTOM LINE: Hospital systems can reduce HA-VTE and increase VTE prophylaxis by implementing a bundle of interventions and these efforts are highest yield for Oncologic and Surgical populations.
CITATIONS: Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11:S22-8.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: Can a single institution’s VTE prophylaxis program be scaled to increase prophylaxis and reduce HA-VTEs across multiple institutions?
STUDY DESIGN: prospective, unblinded, open-intervention study
SETTING: Inpatient medical and surgical services at five independent, cooperating academic hospitals
SYNOPSIS: Each site used common principles to develop their own multi-pronged VTE prophylaxis program including structured order-sets, simplified risk-assessment, feedback to providers, and education programs.
306,906 inpatient discharges were evaluated with average VTE prophylaxis bundle compliance reaching 89% across all institutions. HA-VTE rates declined from 0.90% to 0.69% (RR, 0.76; CI, 0.68-0.85) – equivalent to averting 81 pulmonary emboli and 89 deep venous thrombi. Of note, HA-VTE rates only declined at three of the five institutions with the greatest improvement at those with the highest baseline rates. Further, while HA-VTE rates improved across all patient populations, the incidence reduction was statistically significant in Oncologic and Surgical populations.
BOTTOM LINE: Hospital systems can reduce HA-VTE and increase VTE prophylaxis by implementing a bundle of interventions and these efforts are highest yield for Oncologic and Surgical populations.
CITATIONS: Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11:S22-8.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
CLINICAL QUESTION: Can a single institution’s VTE prophylaxis program be scaled to increase prophylaxis and reduce HA-VTEs across multiple institutions?
STUDY DESIGN: prospective, unblinded, open-intervention study
SETTING: Inpatient medical and surgical services at five independent, cooperating academic hospitals
SYNOPSIS: Each site used common principles to develop their own multi-pronged VTE prophylaxis program including structured order-sets, simplified risk-assessment, feedback to providers, and education programs.
306,906 inpatient discharges were evaluated with average VTE prophylaxis bundle compliance reaching 89% across all institutions. HA-VTE rates declined from 0.90% to 0.69% (RR, 0.76; CI, 0.68-0.85) – equivalent to averting 81 pulmonary emboli and 89 deep venous thrombi. Of note, HA-VTE rates only declined at three of the five institutions with the greatest improvement at those with the highest baseline rates. Further, while HA-VTE rates improved across all patient populations, the incidence reduction was statistically significant in Oncologic and Surgical populations.
BOTTOM LINE: Hospital systems can reduce HA-VTE and increase VTE prophylaxis by implementing a bundle of interventions and these efforts are highest yield for Oncologic and Surgical populations.
CITATIONS: Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11:S22-8.
Dr. Anstett is Hospital Medicine Fellow in Quality and Systems Leadership, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Changing ethics of pediatric health care: The last 50 years
The ethics of pediatric health care have changed radically in the past 50 years. “History,” they say, “is written by the victors.” So, if you are not careful, you will only get part of the story. Clinical ethicists learn to seek out, involve, and empower the voices of all stakeholders. To fully appreciate how much things have changed, you must learn more than one side of the story. Indeed, piecing together the history of medical ethics reminds me of the Indian story of five blind men describing an elephant, in which each can only describe a part of an ultimately much bigger animal.
If you ask philosophers about the history of medical ethics, they will point to events 50 years ago as the beginning of the modern era. In the 1960s, physicians tended to be paternalistic authoritarians. Some considered it best not to even tell a patient that he had cancer. There was minimal patient education provided. Medications were prescribed as orders for the patient to follow. Medical research had harmed volunteers, and new protections were needed.
In 1995, the American Academy of Pediatrics Section on Bioethics emphasized the importance of obtaining the child’s assent in addition to the parent’s consent.2 Most states have passed laws permitting minors to give consent for treatment for pregnancy, sexually transmitted diseases, drug addiction, and mental health care.
Technology alters ethics
At the same time, technology has been changing medicine. New life sustaining technologies in the 1960s – such as dialysis and ventilators – created new issues of extreme financial cost, allocation of scarce resources, and even the existential question of when life ends. In 1968, an ad hoc committee at Harvard created criteria for what is colloquially called “brain death.”3 Many landmark legal cases further developed the ethics of end-of-life care.
The boundaries were even less clear at the beginning of life. Technological advances in ventilators, improvements in nursing care by neonatal intensive care unit nurses, and the whole new subspecialty of neonatology progressively lowered the gestational age for survival. The distinction between clinical care and experimental care was again blurry as neonatologists sought to overcome previously unknown complications, like retinopathy of prematurity resulting from too much oxygen and bronchopulmonary dysplasia from the ventilator. Many babies survived with profound physical and neurological compromise. The ethical dilemmas were continuously present.
Change in the status of children
There is more to the story than philosophy, law, and technology. Pediatric ethics has been profoundly impacted by a change in the status of the children. One change from 50 years ago has been the social response to child abuse.4 Norms changed. Before, fathers pretty much could raise their children any way they saw fit, including corporal punishment. Neighbors didn’t intervene. The proverb was “spare the rod and spoil the child,” but abuse was not motivated by discipline. It was cruel, authoritarian, and demeaning. The landmark article describing the Battered Child Syndrome was published in 1962.5 By 1967, the local Society for the Prevention of Cruelty to Children had become nearly obsolete, but understaffed local government agencies were just beginning to respond. In 1974, federal action produced the Child Abuse Prevention and Treatment Act.6 Medical personnel became mandatory reporters, developed expertise, and, in 2009, child abuse became a boarded subspecialty in pediatrics.
Then, in 1971, a documentary “Who Should Survive?” explored the ethical decision making for babies with birth defects.7 The harms of institutionalization became recognized. The benefits of early intervention and special education have been established. Support for an Individual Education Plan has progressed through successive laws beginning in 1975 until the Individuals with Disabilities Education Improvement Act of 2004.
This is just another example of how ethics develops from a philosophical ideal to a change in social status, followed by consciousness raising, civil rights legislation, enabling legislation, funding, and program development and implementation.
It takes a village of philosophers, activists, lawyers, legislators, physicians, and other experts to implement ethics. There are also countervailing forces. The mainstreaming of children with disabilities is one factor in the movement of children into private schools and the pressure for a voucher system, as advocated by the new Secretary of Education, Betty DeVos.
There also has been a change in the status of children as future providers. Historically, children were relied upon to provide for the parents in their old age. With decreases in infant mortality, the availability of birth control, and legalized abortions, smaller families became the social norm. Worldwide, there has been a marked drop in fertility rates in developed nations. Governmental programs such as Social Security, particularly with the introduction of Medicare in 1965, meant that the elderly were less dependent on their descendants. China found that acceptance of the One Child policy was heavily dependent on convincing parents that the State would provide for them in their old age. The modern political state has assumed duties previously performed by the family.
More recent changes
Pediatric health care is strongly impacted by public health measures. Infant mortality has been reduced by improved nutrition and public health, not medication and surgery. Mass immunization programs were viewed as an appropriate function of civic government.
The introduction of polio vaccine in the 1950s made a large impact. Families lined up at any opportunity to get the vaccine. Polio went from hundreds of thousands of cases of paralysis each summer down to zero cases of wild polio transmitted within the western hemisphere. Measles cases went from 450,000 cases a year in the early 1960s down to zero, until a fraudulent link to autism led to a significant number of parents not immunizing their children. Vaccine refusal, previously a rare ethical issue related to religious liberty, became corrupted by efforts at boutique medicine and alternative facts. In modern America, the ethics of individualism and personal rights have eclipsed civic responsibility. With herd immunity compromised, a blip up to 100 cases of measles per year was histrionically described as a huge epidemic. That spin shows ignorance of the historical record, but the risk was enough for the liberal state of California in 2015 to ban philosophical exemptions to vaccination with one of the strictest state laws in the nation.
Ethics is about values. So, as I look at the changes over 50 years, the areas that have failed to make progress are illuminating. Mental health care for children has not made the same progress achieved with vaccines and cancer therapy. My most recent clinical ethics case involved a teenager who had made a suicidal gesture by taking a handful of pills. The nurses were caught between caring for their patient and meeting the demands of an upset, authoritarian parent in a world where customer satisfaction is critical. I spent much of the night exploring hospital policy and state law. I solicited and listened to widely disparate interpretations of law, medical ethics, and hospital policy from the floor nurse, the nursing supervisor, the nursing staff on the adult inpatient psychiatric unit, three ED docs, a social worker, a government agency, and a judge’s representative. The physician of 1967 was captain of the ship and would not recognize the chaotic teamwork of modern medicine. The exercise showed me how little progress we have made in mental health care for adolescents during my 25 years of practice.
It also reminded me that I have the luxury to debate ethical minutia like vaccine hesitancy and adolescent consent in a world with Syrian refugee camps and starvation in South Sudan. Mahatma Gandhi said, “There are people in the world so hungry that God cannot appear to them except in the form of bread.” That, unfortunately, has not changed in 50 years.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@frontlinemedcom.com.
References
1. www.hhs.gov/ohrp/regulations-and-policy/belmont-report/
2. Pediatrics 1995;95:314-7.
3. JAMA. 1968;205(6):337-40.
4. Family Law Quarterly. 2008 Fall;42(3):449-63.
5. JAMA. 1962;181(1):17-24.
6. National Child Abuse and Neglect Training and Publications Project (2014). The Child Abuse Prevention and Treatment Act: 40 years of safeguarding America’s children. Washington: U.S. Department of Health and Human Services, Children’s Bureau.
7. Kennedy Inst Ethics J. 2006 Sep;16(3):205-24.
The ethics of pediatric health care have changed radically in the past 50 years. “History,” they say, “is written by the victors.” So, if you are not careful, you will only get part of the story. Clinical ethicists learn to seek out, involve, and empower the voices of all stakeholders. To fully appreciate how much things have changed, you must learn more than one side of the story. Indeed, piecing together the history of medical ethics reminds me of the Indian story of five blind men describing an elephant, in which each can only describe a part of an ultimately much bigger animal.
If you ask philosophers about the history of medical ethics, they will point to events 50 years ago as the beginning of the modern era. In the 1960s, physicians tended to be paternalistic authoritarians. Some considered it best not to even tell a patient that he had cancer. There was minimal patient education provided. Medications were prescribed as orders for the patient to follow. Medical research had harmed volunteers, and new protections were needed.
In 1995, the American Academy of Pediatrics Section on Bioethics emphasized the importance of obtaining the child’s assent in addition to the parent’s consent.2 Most states have passed laws permitting minors to give consent for treatment for pregnancy, sexually transmitted diseases, drug addiction, and mental health care.
Technology alters ethics
At the same time, technology has been changing medicine. New life sustaining technologies in the 1960s – such as dialysis and ventilators – created new issues of extreme financial cost, allocation of scarce resources, and even the existential question of when life ends. In 1968, an ad hoc committee at Harvard created criteria for what is colloquially called “brain death.”3 Many landmark legal cases further developed the ethics of end-of-life care.
The boundaries were even less clear at the beginning of life. Technological advances in ventilators, improvements in nursing care by neonatal intensive care unit nurses, and the whole new subspecialty of neonatology progressively lowered the gestational age for survival. The distinction between clinical care and experimental care was again blurry as neonatologists sought to overcome previously unknown complications, like retinopathy of prematurity resulting from too much oxygen and bronchopulmonary dysplasia from the ventilator. Many babies survived with profound physical and neurological compromise. The ethical dilemmas were continuously present.
Change in the status of children
There is more to the story than philosophy, law, and technology. Pediatric ethics has been profoundly impacted by a change in the status of the children. One change from 50 years ago has been the social response to child abuse.4 Norms changed. Before, fathers pretty much could raise their children any way they saw fit, including corporal punishment. Neighbors didn’t intervene. The proverb was “spare the rod and spoil the child,” but abuse was not motivated by discipline. It was cruel, authoritarian, and demeaning. The landmark article describing the Battered Child Syndrome was published in 1962.5 By 1967, the local Society for the Prevention of Cruelty to Children had become nearly obsolete, but understaffed local government agencies were just beginning to respond. In 1974, federal action produced the Child Abuse Prevention and Treatment Act.6 Medical personnel became mandatory reporters, developed expertise, and, in 2009, child abuse became a boarded subspecialty in pediatrics.
Then, in 1971, a documentary “Who Should Survive?” explored the ethical decision making for babies with birth defects.7 The harms of institutionalization became recognized. The benefits of early intervention and special education have been established. Support for an Individual Education Plan has progressed through successive laws beginning in 1975 until the Individuals with Disabilities Education Improvement Act of 2004.
This is just another example of how ethics develops from a philosophical ideal to a change in social status, followed by consciousness raising, civil rights legislation, enabling legislation, funding, and program development and implementation.
It takes a village of philosophers, activists, lawyers, legislators, physicians, and other experts to implement ethics. There are also countervailing forces. The mainstreaming of children with disabilities is one factor in the movement of children into private schools and the pressure for a voucher system, as advocated by the new Secretary of Education, Betty DeVos.
There also has been a change in the status of children as future providers. Historically, children were relied upon to provide for the parents in their old age. With decreases in infant mortality, the availability of birth control, and legalized abortions, smaller families became the social norm. Worldwide, there has been a marked drop in fertility rates in developed nations. Governmental programs such as Social Security, particularly with the introduction of Medicare in 1965, meant that the elderly were less dependent on their descendants. China found that acceptance of the One Child policy was heavily dependent on convincing parents that the State would provide for them in their old age. The modern political state has assumed duties previously performed by the family.
More recent changes
Pediatric health care is strongly impacted by public health measures. Infant mortality has been reduced by improved nutrition and public health, not medication and surgery. Mass immunization programs were viewed as an appropriate function of civic government.
The introduction of polio vaccine in the 1950s made a large impact. Families lined up at any opportunity to get the vaccine. Polio went from hundreds of thousands of cases of paralysis each summer down to zero cases of wild polio transmitted within the western hemisphere. Measles cases went from 450,000 cases a year in the early 1960s down to zero, until a fraudulent link to autism led to a significant number of parents not immunizing their children. Vaccine refusal, previously a rare ethical issue related to religious liberty, became corrupted by efforts at boutique medicine and alternative facts. In modern America, the ethics of individualism and personal rights have eclipsed civic responsibility. With herd immunity compromised, a blip up to 100 cases of measles per year was histrionically described as a huge epidemic. That spin shows ignorance of the historical record, but the risk was enough for the liberal state of California in 2015 to ban philosophical exemptions to vaccination with one of the strictest state laws in the nation.
Ethics is about values. So, as I look at the changes over 50 years, the areas that have failed to make progress are illuminating. Mental health care for children has not made the same progress achieved with vaccines and cancer therapy. My most recent clinical ethics case involved a teenager who had made a suicidal gesture by taking a handful of pills. The nurses were caught between caring for their patient and meeting the demands of an upset, authoritarian parent in a world where customer satisfaction is critical. I spent much of the night exploring hospital policy and state law. I solicited and listened to widely disparate interpretations of law, medical ethics, and hospital policy from the floor nurse, the nursing supervisor, the nursing staff on the adult inpatient psychiatric unit, three ED docs, a social worker, a government agency, and a judge’s representative. The physician of 1967 was captain of the ship and would not recognize the chaotic teamwork of modern medicine. The exercise showed me how little progress we have made in mental health care for adolescents during my 25 years of practice.
It also reminded me that I have the luxury to debate ethical minutia like vaccine hesitancy and adolescent consent in a world with Syrian refugee camps and starvation in South Sudan. Mahatma Gandhi said, “There are people in the world so hungry that God cannot appear to them except in the form of bread.” That, unfortunately, has not changed in 50 years.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@frontlinemedcom.com.
References
1. www.hhs.gov/ohrp/regulations-and-policy/belmont-report/
2. Pediatrics 1995;95:314-7.
3. JAMA. 1968;205(6):337-40.
4. Family Law Quarterly. 2008 Fall;42(3):449-63.
5. JAMA. 1962;181(1):17-24.
6. National Child Abuse and Neglect Training and Publications Project (2014). The Child Abuse Prevention and Treatment Act: 40 years of safeguarding America’s children. Washington: U.S. Department of Health and Human Services, Children’s Bureau.
7. Kennedy Inst Ethics J. 2006 Sep;16(3):205-24.
The ethics of pediatric health care have changed radically in the past 50 years. “History,” they say, “is written by the victors.” So, if you are not careful, you will only get part of the story. Clinical ethicists learn to seek out, involve, and empower the voices of all stakeholders. To fully appreciate how much things have changed, you must learn more than one side of the story. Indeed, piecing together the history of medical ethics reminds me of the Indian story of five blind men describing an elephant, in which each can only describe a part of an ultimately much bigger animal.
If you ask philosophers about the history of medical ethics, they will point to events 50 years ago as the beginning of the modern era. In the 1960s, physicians tended to be paternalistic authoritarians. Some considered it best not to even tell a patient that he had cancer. There was minimal patient education provided. Medications were prescribed as orders for the patient to follow. Medical research had harmed volunteers, and new protections were needed.
In 1995, the American Academy of Pediatrics Section on Bioethics emphasized the importance of obtaining the child’s assent in addition to the parent’s consent.2 Most states have passed laws permitting minors to give consent for treatment for pregnancy, sexually transmitted diseases, drug addiction, and mental health care.
Technology alters ethics
At the same time, technology has been changing medicine. New life sustaining technologies in the 1960s – such as dialysis and ventilators – created new issues of extreme financial cost, allocation of scarce resources, and even the existential question of when life ends. In 1968, an ad hoc committee at Harvard created criteria for what is colloquially called “brain death.”3 Many landmark legal cases further developed the ethics of end-of-life care.
The boundaries were even less clear at the beginning of life. Technological advances in ventilators, improvements in nursing care by neonatal intensive care unit nurses, and the whole new subspecialty of neonatology progressively lowered the gestational age for survival. The distinction between clinical care and experimental care was again blurry as neonatologists sought to overcome previously unknown complications, like retinopathy of prematurity resulting from too much oxygen and bronchopulmonary dysplasia from the ventilator. Many babies survived with profound physical and neurological compromise. The ethical dilemmas were continuously present.
Change in the status of children
There is more to the story than philosophy, law, and technology. Pediatric ethics has been profoundly impacted by a change in the status of the children. One change from 50 years ago has been the social response to child abuse.4 Norms changed. Before, fathers pretty much could raise their children any way they saw fit, including corporal punishment. Neighbors didn’t intervene. The proverb was “spare the rod and spoil the child,” but abuse was not motivated by discipline. It was cruel, authoritarian, and demeaning. The landmark article describing the Battered Child Syndrome was published in 1962.5 By 1967, the local Society for the Prevention of Cruelty to Children had become nearly obsolete, but understaffed local government agencies were just beginning to respond. In 1974, federal action produced the Child Abuse Prevention and Treatment Act.6 Medical personnel became mandatory reporters, developed expertise, and, in 2009, child abuse became a boarded subspecialty in pediatrics.
Then, in 1971, a documentary “Who Should Survive?” explored the ethical decision making for babies with birth defects.7 The harms of institutionalization became recognized. The benefits of early intervention and special education have been established. Support for an Individual Education Plan has progressed through successive laws beginning in 1975 until the Individuals with Disabilities Education Improvement Act of 2004.
This is just another example of how ethics develops from a philosophical ideal to a change in social status, followed by consciousness raising, civil rights legislation, enabling legislation, funding, and program development and implementation.
It takes a village of philosophers, activists, lawyers, legislators, physicians, and other experts to implement ethics. There are also countervailing forces. The mainstreaming of children with disabilities is one factor in the movement of children into private schools and the pressure for a voucher system, as advocated by the new Secretary of Education, Betty DeVos.
There also has been a change in the status of children as future providers. Historically, children were relied upon to provide for the parents in their old age. With decreases in infant mortality, the availability of birth control, and legalized abortions, smaller families became the social norm. Worldwide, there has been a marked drop in fertility rates in developed nations. Governmental programs such as Social Security, particularly with the introduction of Medicare in 1965, meant that the elderly were less dependent on their descendants. China found that acceptance of the One Child policy was heavily dependent on convincing parents that the State would provide for them in their old age. The modern political state has assumed duties previously performed by the family.
More recent changes
Pediatric health care is strongly impacted by public health measures. Infant mortality has been reduced by improved nutrition and public health, not medication and surgery. Mass immunization programs were viewed as an appropriate function of civic government.
The introduction of polio vaccine in the 1950s made a large impact. Families lined up at any opportunity to get the vaccine. Polio went from hundreds of thousands of cases of paralysis each summer down to zero cases of wild polio transmitted within the western hemisphere. Measles cases went from 450,000 cases a year in the early 1960s down to zero, until a fraudulent link to autism led to a significant number of parents not immunizing their children. Vaccine refusal, previously a rare ethical issue related to religious liberty, became corrupted by efforts at boutique medicine and alternative facts. In modern America, the ethics of individualism and personal rights have eclipsed civic responsibility. With herd immunity compromised, a blip up to 100 cases of measles per year was histrionically described as a huge epidemic. That spin shows ignorance of the historical record, but the risk was enough for the liberal state of California in 2015 to ban philosophical exemptions to vaccination with one of the strictest state laws in the nation.
Ethics is about values. So, as I look at the changes over 50 years, the areas that have failed to make progress are illuminating. Mental health care for children has not made the same progress achieved with vaccines and cancer therapy. My most recent clinical ethics case involved a teenager who had made a suicidal gesture by taking a handful of pills. The nurses were caught between caring for their patient and meeting the demands of an upset, authoritarian parent in a world where customer satisfaction is critical. I spent much of the night exploring hospital policy and state law. I solicited and listened to widely disparate interpretations of law, medical ethics, and hospital policy from the floor nurse, the nursing supervisor, the nursing staff on the adult inpatient psychiatric unit, three ED docs, a social worker, a government agency, and a judge’s representative. The physician of 1967 was captain of the ship and would not recognize the chaotic teamwork of modern medicine. The exercise showed me how little progress we have made in mental health care for adolescents during my 25 years of practice.
It also reminded me that I have the luxury to debate ethical minutia like vaccine hesitancy and adolescent consent in a world with Syrian refugee camps and starvation in South Sudan. Mahatma Gandhi said, “There are people in the world so hungry that God cannot appear to them except in the form of bread.” That, unfortunately, has not changed in 50 years.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at pdnews@frontlinemedcom.com.
References
1. www.hhs.gov/ohrp/regulations-and-policy/belmont-report/
2. Pediatrics 1995;95:314-7.
3. JAMA. 1968;205(6):337-40.
4. Family Law Quarterly. 2008 Fall;42(3):449-63.
5. JAMA. 1962;181(1):17-24.
6. National Child Abuse and Neglect Training and Publications Project (2014). The Child Abuse Prevention and Treatment Act: 40 years of safeguarding America’s children. Washington: U.S. Department of Health and Human Services, Children’s Bureau.
7. Kennedy Inst Ethics J. 2006 Sep;16(3):205-24.
Second generation ALK inhibitors show good response in second-line NSCLC
GENEVA – Second-generation inhibitors of the anaplastic lymphoma kinase (ALK) are associated with high response rates in patients with non–small cell lung cancer (NSCLC) who have disease progression after treatment with the first-generation ALK inhibitor crizotinib (Xalkori), investigators reported.
Of 117 patients with ALK-positive NSCLC that progressed while on treatment with the ALK-targeting tyrosine-kinase inhibitor (TKI) crizotinib, both ceritinib (Zykadia) and alectinib (Alecensa) were associated with high combined partial and complete response rates, reported Christian Britschgi, MD, PhD, of University Hospital Zürich, and his colleagues.
“We can confirm the high rate of response of ceritinib and alectinib as second TKIs,” Dr. Britschgi said in an interview.
“With ceritinib or alectinib in the first line, however, it’s hard to draw any conclusions because there are very few patients treated with these drugs in the first line who were included in the trials,” he added.
ALK rearrangements occur in 3%-5% of lung adenocarcinomas, and these mutations are potential targets for TKIs directed against ALK.
The investigators analyzed the clinical characteristics of response to, and disease progression on, available ALK-TKIs. They also performed next-generation sequencing on a small group of tumor samples to investigate mechanisms of resistance and to look for new mutations that could be targetable.
They collected data on patients with ALK-positive NSCLC who were receiving palliative care and looked at clinical data and biopsy samples collected before treatment, and where available, post–ALK-TKI therapy.
They collected clinical data on 139 patients, 117 of whom had undergone first-line treatment with chemotherapy (74%), ALK-TKIs (25%), or other therapies (1%). Of this group, 92 received second-line therapy – chemotherapy (17%), ALK-TKIs (74%), or immunotherapies/other (9%).
Third-line treatments were given to 55 patients, 20% of whom received chemotherapy, 75% of whom received ALK-TKIs, and 5% of whom received other therapies.
Twenty-four patients survived long enough to receive fourth-line treatments, which included chemotherapy in 8 patients (33%), ALK-TKIs in 15 (63%), or other therapies in 1 patient.
In all, 102 patients received crizotinib, all but one in the first line and the remaining patient in the second line. The overall response rate was 58.8% (59.4% for the 101 first-line patients). Median progression-free survival (PFS) in these patients was 11.7 months.
Ceritinib was given to 2 patients in the first line, 7 in the second line, and 28 in the third line. The overall response rate was 48.6% (50% in the two patients who received it in the first line, and 50% in patients who received it after crizotinib). The median PFS was 6.5 months.
For the 26 patients who received alectinib – 3 in the first line and 23 after crizotinib – the overall response rate was 100% when the drug was used in the first line, and 60.9% when used in the second and subsequent lines. Median PFS was 12.4 months.
Only a few patients to date have had next-generation sequencing of tumors performed, Dr. Britschgi said. One patient who developed resistance while on alectinib was found to have a point mutation (ALK p.lle117Ser) that was not found in a pretreatment biopsy sample, indicating that it had emerged during therapy. This patient was then started on and had a response to lorlatinib.
A second patient who had received several lines of therapy including chemotherapy and TKIs and was enrolled in the expanded access program for lorlatinib developed hepatic progression while on that drug. A biopsy showed that the tumor carried epidermal growth-factor receptor variant 3, which is known to be highly mutagenic and is associated with glioblastoma. The patient was then put on nivolumab (Opdivo) and had a good response, but had further progression, and was then put on alectinib and again had a good response that is currently ongoing, Dr. Britschgi said.
The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer and Roche.
GENEVA – Second-generation inhibitors of the anaplastic lymphoma kinase (ALK) are associated with high response rates in patients with non–small cell lung cancer (NSCLC) who have disease progression after treatment with the first-generation ALK inhibitor crizotinib (Xalkori), investigators reported.
Of 117 patients with ALK-positive NSCLC that progressed while on treatment with the ALK-targeting tyrosine-kinase inhibitor (TKI) crizotinib, both ceritinib (Zykadia) and alectinib (Alecensa) were associated with high combined partial and complete response rates, reported Christian Britschgi, MD, PhD, of University Hospital Zürich, and his colleagues.
“We can confirm the high rate of response of ceritinib and alectinib as second TKIs,” Dr. Britschgi said in an interview.
“With ceritinib or alectinib in the first line, however, it’s hard to draw any conclusions because there are very few patients treated with these drugs in the first line who were included in the trials,” he added.
ALK rearrangements occur in 3%-5% of lung adenocarcinomas, and these mutations are potential targets for TKIs directed against ALK.
The investigators analyzed the clinical characteristics of response to, and disease progression on, available ALK-TKIs. They also performed next-generation sequencing on a small group of tumor samples to investigate mechanisms of resistance and to look for new mutations that could be targetable.
They collected data on patients with ALK-positive NSCLC who were receiving palliative care and looked at clinical data and biopsy samples collected before treatment, and where available, post–ALK-TKI therapy.
They collected clinical data on 139 patients, 117 of whom had undergone first-line treatment with chemotherapy (74%), ALK-TKIs (25%), or other therapies (1%). Of this group, 92 received second-line therapy – chemotherapy (17%), ALK-TKIs (74%), or immunotherapies/other (9%).
Third-line treatments were given to 55 patients, 20% of whom received chemotherapy, 75% of whom received ALK-TKIs, and 5% of whom received other therapies.
Twenty-four patients survived long enough to receive fourth-line treatments, which included chemotherapy in 8 patients (33%), ALK-TKIs in 15 (63%), or other therapies in 1 patient.
In all, 102 patients received crizotinib, all but one in the first line and the remaining patient in the second line. The overall response rate was 58.8% (59.4% for the 101 first-line patients). Median progression-free survival (PFS) in these patients was 11.7 months.
Ceritinib was given to 2 patients in the first line, 7 in the second line, and 28 in the third line. The overall response rate was 48.6% (50% in the two patients who received it in the first line, and 50% in patients who received it after crizotinib). The median PFS was 6.5 months.
For the 26 patients who received alectinib – 3 in the first line and 23 after crizotinib – the overall response rate was 100% when the drug was used in the first line, and 60.9% when used in the second and subsequent lines. Median PFS was 12.4 months.
Only a few patients to date have had next-generation sequencing of tumors performed, Dr. Britschgi said. One patient who developed resistance while on alectinib was found to have a point mutation (ALK p.lle117Ser) that was not found in a pretreatment biopsy sample, indicating that it had emerged during therapy. This patient was then started on and had a response to lorlatinib.
A second patient who had received several lines of therapy including chemotherapy and TKIs and was enrolled in the expanded access program for lorlatinib developed hepatic progression while on that drug. A biopsy showed that the tumor carried epidermal growth-factor receptor variant 3, which is known to be highly mutagenic and is associated with glioblastoma. The patient was then put on nivolumab (Opdivo) and had a good response, but had further progression, and was then put on alectinib and again had a good response that is currently ongoing, Dr. Britschgi said.
The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer and Roche.
GENEVA – Second-generation inhibitors of the anaplastic lymphoma kinase (ALK) are associated with high response rates in patients with non–small cell lung cancer (NSCLC) who have disease progression after treatment with the first-generation ALK inhibitor crizotinib (Xalkori), investigators reported.
Of 117 patients with ALK-positive NSCLC that progressed while on treatment with the ALK-targeting tyrosine-kinase inhibitor (TKI) crizotinib, both ceritinib (Zykadia) and alectinib (Alecensa) were associated with high combined partial and complete response rates, reported Christian Britschgi, MD, PhD, of University Hospital Zürich, and his colleagues.
“We can confirm the high rate of response of ceritinib and alectinib as second TKIs,” Dr. Britschgi said in an interview.
“With ceritinib or alectinib in the first line, however, it’s hard to draw any conclusions because there are very few patients treated with these drugs in the first line who were included in the trials,” he added.
ALK rearrangements occur in 3%-5% of lung adenocarcinomas, and these mutations are potential targets for TKIs directed against ALK.
The investigators analyzed the clinical characteristics of response to, and disease progression on, available ALK-TKIs. They also performed next-generation sequencing on a small group of tumor samples to investigate mechanisms of resistance and to look for new mutations that could be targetable.
They collected data on patients with ALK-positive NSCLC who were receiving palliative care and looked at clinical data and biopsy samples collected before treatment, and where available, post–ALK-TKI therapy.
They collected clinical data on 139 patients, 117 of whom had undergone first-line treatment with chemotherapy (74%), ALK-TKIs (25%), or other therapies (1%). Of this group, 92 received second-line therapy – chemotherapy (17%), ALK-TKIs (74%), or immunotherapies/other (9%).
Third-line treatments were given to 55 patients, 20% of whom received chemotherapy, 75% of whom received ALK-TKIs, and 5% of whom received other therapies.
Twenty-four patients survived long enough to receive fourth-line treatments, which included chemotherapy in 8 patients (33%), ALK-TKIs in 15 (63%), or other therapies in 1 patient.
In all, 102 patients received crizotinib, all but one in the first line and the remaining patient in the second line. The overall response rate was 58.8% (59.4% for the 101 first-line patients). Median progression-free survival (PFS) in these patients was 11.7 months.
Ceritinib was given to 2 patients in the first line, 7 in the second line, and 28 in the third line. The overall response rate was 48.6% (50% in the two patients who received it in the first line, and 50% in patients who received it after crizotinib). The median PFS was 6.5 months.
For the 26 patients who received alectinib – 3 in the first line and 23 after crizotinib – the overall response rate was 100% when the drug was used in the first line, and 60.9% when used in the second and subsequent lines. Median PFS was 12.4 months.
Only a few patients to date have had next-generation sequencing of tumors performed, Dr. Britschgi said. One patient who developed resistance while on alectinib was found to have a point mutation (ALK p.lle117Ser) that was not found in a pretreatment biopsy sample, indicating that it had emerged during therapy. This patient was then started on and had a response to lorlatinib.
A second patient who had received several lines of therapy including chemotherapy and TKIs and was enrolled in the expanded access program for lorlatinib developed hepatic progression while on that drug. A biopsy showed that the tumor carried epidermal growth-factor receptor variant 3, which is known to be highly mutagenic and is associated with glioblastoma. The patient was then put on nivolumab (Opdivo) and had a good response, but had further progression, and was then put on alectinib and again had a good response that is currently ongoing, Dr. Britschgi said.
The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer and Roche.
FROM ELCC
Key clinical point:
Major finding: Overall response rates after crizotinib were 50% for ceritinib and 60.9% for alectinib.
Data source: A retrospective review of data on 117 patients with ALK-positive NSCLC in Swiss and Italian hospitals.
Disclosures: The study was supported by University Hospital Zurich and Novartis. Dr. Britschgi reported no relevant disclosures. Coauthor Sacha Rothschild, MD, PhD, disclosed institutional compensation for advisory boards from Novartis, Pfizer, and Roche.
FDA: Fluoroquinolone use not linked to retina detachment, aortic problems
The Food and Drug Administration has found no evidence of a link between fluoroquinolone usage and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling side effects of oral and injectable fluoroquinolone antibiotics.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
However, after reviewing patient cases and study findings, the FDA said the evidence didn’t support an association between fluoroquinolone usage and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
Serious side effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a “pins and needles” feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
The Food and Drug Administration has found no evidence of a link between fluoroquinolone usage and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling side effects of oral and injectable fluoroquinolone antibiotics.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
However, after reviewing patient cases and study findings, the FDA said the evidence didn’t support an association between fluoroquinolone usage and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
Serious side effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a “pins and needles” feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
The Food and Drug Administration has found no evidence of a link between fluoroquinolone usage and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling side effects of oral and injectable fluoroquinolone antibiotics.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
However, after reviewing patient cases and study findings, the FDA said the evidence didn’t support an association between fluoroquinolone usage and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
Serious side effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a “pins and needles” feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
SLE linked to subsequent risk of malignant melanoma
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
AT SID 2017
Key clinical point: Compared with controls, patients with systemic lupus erythematosus (SLE) were at significantly increased risk of later being diagnosed with malignant melanoma.
Major finding: Ten patients with SLE (0.4%) were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), a statistically significant difference (P = .03).
Data source: Electronic medical record reviews of 2,351 patients with SLE and 1,676 patients with systemic sclerosis (controls) between 2000 and 2016.
Disclosures: The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
Contact dermatitis in children: The top 10 allergens
Contact dermatitis should be suspected in patients with eczematous dermatitis atypical in location, geometric or symmetric in distribution, or unresponsive to or worsened by common therapies, Dr. Catalina Matiz emphasized.
Allergic contact dermatitis is a complex disorder characterized by a type IV delayed-type hypersensitivity reaction that occurs when a patient’s skin is exposed to a substance easily penetrates the skin barrier.
Increasingly recognized in the United States, many pediatric patients are becoming sensitized to contact allergens found in personal care products, including skin care products, topical medications, and clothing.
A study published by Hill et al. in 2016 reviewed pediatric patch test studies to determine the top contact allergens in children (Expert Rev Clin Immunol. 2016;12[5]:551-61).
Dr. Matiz presented the top 10 allergens discovered by this group, and offered practical advice for allergen avoidance.
Topping the list in descending order are the following:
- Tixocortol pivalate (a corticosteroid).
- Propylene glycol.
- Methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI).
- Formaldehyde.
- Cocamidopropyl betaine.
- Lanolin.
- Benzalkonium chloride.
- Fragrance and balsam of peru.
- Neomycin.
- Nickel.
Dr. Matiz educated meeting attendees on allergen-containing products and clinical correlations suggestive of allergic sensitization to common allergens. Tixocortol pivilate is a corticosteroid present in Class A corticosteroids including hydrocortisone acetate. She highlighted the cross reactivity between class A and class D2 corticosteroids including hydrocortisone butyrate and valerate.
“Usually topical corticosteroid–contact sensitivity manifests as a failure to improve or worsening of existing dermatitis,” emphasized Dr. Matiz of departments of dermatology and pediatrics at the university.
Propylene glycol, benzalkonium chloride, and neomycin also represent top contact allergens frequently found in topical medications. Similarly, lanolin contact sensitivity often presents as refractory or worsening atopic dermatitis. “Lanolin, also known as wool alcohol, is commonly found in emollients, medications, and personal care products used by atopic dermatitis patients,” Dr. Matiz stressed.
Methylchloroisothiazolinone/methylisothiazolinone represent preservatives commonly found in wet wipes, hypoallergenic, and sensitive skin products. This was the allergen of the year in 2013 and was subsequently removed from many wet wipe formulations and products in response.
In addition to acute management of allergic contact dermatitis with corticosteroids, Dr. Matiz further emphasized the importance of preemptive avoidance of the top ten allergens.
She recommends patch testing if no clinical improvement is evident after 8 weeks of common allergen avoidance, for definitive allergen identification.
Dr. Matiz said she had no relevant financial disclosures.
Contact dermatitis should be suspected in patients with eczematous dermatitis atypical in location, geometric or symmetric in distribution, or unresponsive to or worsened by common therapies, Dr. Catalina Matiz emphasized.
Allergic contact dermatitis is a complex disorder characterized by a type IV delayed-type hypersensitivity reaction that occurs when a patient’s skin is exposed to a substance easily penetrates the skin barrier.
Increasingly recognized in the United States, many pediatric patients are becoming sensitized to contact allergens found in personal care products, including skin care products, topical medications, and clothing.
A study published by Hill et al. in 2016 reviewed pediatric patch test studies to determine the top contact allergens in children (Expert Rev Clin Immunol. 2016;12[5]:551-61).
Dr. Matiz presented the top 10 allergens discovered by this group, and offered practical advice for allergen avoidance.
Topping the list in descending order are the following:
- Tixocortol pivalate (a corticosteroid).
- Propylene glycol.
- Methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI).
- Formaldehyde.
- Cocamidopropyl betaine.
- Lanolin.
- Benzalkonium chloride.
- Fragrance and balsam of peru.
- Neomycin.
- Nickel.
Dr. Matiz educated meeting attendees on allergen-containing products and clinical correlations suggestive of allergic sensitization to common allergens. Tixocortol pivilate is a corticosteroid present in Class A corticosteroids including hydrocortisone acetate. She highlighted the cross reactivity between class A and class D2 corticosteroids including hydrocortisone butyrate and valerate.
“Usually topical corticosteroid–contact sensitivity manifests as a failure to improve or worsening of existing dermatitis,” emphasized Dr. Matiz of departments of dermatology and pediatrics at the university.
Propylene glycol, benzalkonium chloride, and neomycin also represent top contact allergens frequently found in topical medications. Similarly, lanolin contact sensitivity often presents as refractory or worsening atopic dermatitis. “Lanolin, also known as wool alcohol, is commonly found in emollients, medications, and personal care products used by atopic dermatitis patients,” Dr. Matiz stressed.
Methylchloroisothiazolinone/methylisothiazolinone represent preservatives commonly found in wet wipes, hypoallergenic, and sensitive skin products. This was the allergen of the year in 2013 and was subsequently removed from many wet wipe formulations and products in response.
In addition to acute management of allergic contact dermatitis with corticosteroids, Dr. Matiz further emphasized the importance of preemptive avoidance of the top ten allergens.
She recommends patch testing if no clinical improvement is evident after 8 weeks of common allergen avoidance, for definitive allergen identification.
Dr. Matiz said she had no relevant financial disclosures.
Contact dermatitis should be suspected in patients with eczematous dermatitis atypical in location, geometric or symmetric in distribution, or unresponsive to or worsened by common therapies, Dr. Catalina Matiz emphasized.
Allergic contact dermatitis is a complex disorder characterized by a type IV delayed-type hypersensitivity reaction that occurs when a patient’s skin is exposed to a substance easily penetrates the skin barrier.
Increasingly recognized in the United States, many pediatric patients are becoming sensitized to contact allergens found in personal care products, including skin care products, topical medications, and clothing.
A study published by Hill et al. in 2016 reviewed pediatric patch test studies to determine the top contact allergens in children (Expert Rev Clin Immunol. 2016;12[5]:551-61).
Dr. Matiz presented the top 10 allergens discovered by this group, and offered practical advice for allergen avoidance.
Topping the list in descending order are the following:
- Tixocortol pivalate (a corticosteroid).
- Propylene glycol.
- Methylchloroisothiazolinone (MCI)/methylisothiazolinone (MI).
- Formaldehyde.
- Cocamidopropyl betaine.
- Lanolin.
- Benzalkonium chloride.
- Fragrance and balsam of peru.
- Neomycin.
- Nickel.
Dr. Matiz educated meeting attendees on allergen-containing products and clinical correlations suggestive of allergic sensitization to common allergens. Tixocortol pivilate is a corticosteroid present in Class A corticosteroids including hydrocortisone acetate. She highlighted the cross reactivity between class A and class D2 corticosteroids including hydrocortisone butyrate and valerate.
“Usually topical corticosteroid–contact sensitivity manifests as a failure to improve or worsening of existing dermatitis,” emphasized Dr. Matiz of departments of dermatology and pediatrics at the university.
Propylene glycol, benzalkonium chloride, and neomycin also represent top contact allergens frequently found in topical medications. Similarly, lanolin contact sensitivity often presents as refractory or worsening atopic dermatitis. “Lanolin, also known as wool alcohol, is commonly found in emollients, medications, and personal care products used by atopic dermatitis patients,” Dr. Matiz stressed.
Methylchloroisothiazolinone/methylisothiazolinone represent preservatives commonly found in wet wipes, hypoallergenic, and sensitive skin products. This was the allergen of the year in 2013 and was subsequently removed from many wet wipe formulations and products in response.
In addition to acute management of allergic contact dermatitis with corticosteroids, Dr. Matiz further emphasized the importance of preemptive avoidance of the top ten allergens.
She recommends patch testing if no clinical improvement is evident after 8 weeks of common allergen avoidance, for definitive allergen identification.
Dr. Matiz said she had no relevant financial disclosures.
High-dose oral vitamin D3 significantly reduced effects of sunburn
PORTLAND, ORE. – When given within an hour, a single dose of at least 100,000 IU vitamin D3 rapidly attenuates sunburn, according to the results of a randomized, double-blind, placebo-controlled pilot study of 25 healthy adults.
This is the first in vivo study to evaluate whether vitamin D3 can modulate acute inflammation in target tissues, Jeffrey F. Scott, MD, wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings “have broad implications for the role of vitamin D in skin homeostasis, and suggest that oral vitamin D may be clinically therapeutic for its immunomodulatory properties,” he and his coauthors concluded.
Higher doses of vitamin D3 also produced significant decreases in skin levels of tumor necrosis factor–alpha (TNF-alpha) (P = .02) and inducible nitric oxide synthase (iNOS) (P = .04) compared with placebo, reported Dr. Scott, a resident in dermatology at University Hospitals Cleveland Medical Center.
Notably, 48 hours after sunburn, hematoxylin and eosin histology of punch biopsies showed that participants who received 200,000 IU vitamin D3 had the least structural damage to the skin, while placebo recipients had the most damage. Expression profiling also linked vitamin D3 treatment with upregulation of genes associated with skin barrier repair.
Studies continue to document diverse biologic effects of vitamin D, including “modulation of immune response, inflammatory disease, cardiovascular health, and carcinogenesis,” the researchers wrote. Vitamin D3 also has been shown to suppress inflammatory mediators and induce autophagy, they added. Previously, their group showed that oral D3 induced similar protective effects in a mouse model of chemical skin injury. Treatment inhibited proinflammatory cytokines and chemokines within the skin, including iNOS and TNF-alpha.
The current study also included a control phase in which participants underwent experimental sunburn on the right arm without any treatment. Forty-eight hours later, punch biopsies revealed high levels of iNOS and TNF-alpha, with increased expression of proinflammatory genes, the researchers wrote.
During the subsequent experimental phase, seven participants were assigned to receive placebo, and six were assigned to receive 50,000 IU, 100,000 IU, or 200,000 IU of vitamin D3. After treatment, participants with the highest serum D3 levels had significantly decreased skin redness compared with participants with lower serum D3 levels (P less than .05). Higher vitamin D3 serum levels were also associated with significant (P less than .05) upregulation of skin barrier repair genes and of arginase-1, a cytosolic enzyme that helps mediate anti-inflammatory activity.
“Arginase-1 may be a clinically useful tissue biomarker for monitoring the immunomodulatory effects of vitamin D3 in humans,” the researchers concluded.
The National Institute of Arthritis Musculoskeletal and Skin Diseases and the National Institutes of Health supported the work. Dr. Scott had no relevant financial disclosures.
PORTLAND, ORE. – When given within an hour, a single dose of at least 100,000 IU vitamin D3 rapidly attenuates sunburn, according to the results of a randomized, double-blind, placebo-controlled pilot study of 25 healthy adults.
This is the first in vivo study to evaluate whether vitamin D3 can modulate acute inflammation in target tissues, Jeffrey F. Scott, MD, wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings “have broad implications for the role of vitamin D in skin homeostasis, and suggest that oral vitamin D may be clinically therapeutic for its immunomodulatory properties,” he and his coauthors concluded.
Higher doses of vitamin D3 also produced significant decreases in skin levels of tumor necrosis factor–alpha (TNF-alpha) (P = .02) and inducible nitric oxide synthase (iNOS) (P = .04) compared with placebo, reported Dr. Scott, a resident in dermatology at University Hospitals Cleveland Medical Center.
Notably, 48 hours after sunburn, hematoxylin and eosin histology of punch biopsies showed that participants who received 200,000 IU vitamin D3 had the least structural damage to the skin, while placebo recipients had the most damage. Expression profiling also linked vitamin D3 treatment with upregulation of genes associated with skin barrier repair.
Studies continue to document diverse biologic effects of vitamin D, including “modulation of immune response, inflammatory disease, cardiovascular health, and carcinogenesis,” the researchers wrote. Vitamin D3 also has been shown to suppress inflammatory mediators and induce autophagy, they added. Previously, their group showed that oral D3 induced similar protective effects in a mouse model of chemical skin injury. Treatment inhibited proinflammatory cytokines and chemokines within the skin, including iNOS and TNF-alpha.
The current study also included a control phase in which participants underwent experimental sunburn on the right arm without any treatment. Forty-eight hours later, punch biopsies revealed high levels of iNOS and TNF-alpha, with increased expression of proinflammatory genes, the researchers wrote.
During the subsequent experimental phase, seven participants were assigned to receive placebo, and six were assigned to receive 50,000 IU, 100,000 IU, or 200,000 IU of vitamin D3. After treatment, participants with the highest serum D3 levels had significantly decreased skin redness compared with participants with lower serum D3 levels (P less than .05). Higher vitamin D3 serum levels were also associated with significant (P less than .05) upregulation of skin barrier repair genes and of arginase-1, a cytosolic enzyme that helps mediate anti-inflammatory activity.
“Arginase-1 may be a clinically useful tissue biomarker for monitoring the immunomodulatory effects of vitamin D3 in humans,” the researchers concluded.
The National Institute of Arthritis Musculoskeletal and Skin Diseases and the National Institutes of Health supported the work. Dr. Scott had no relevant financial disclosures.
PORTLAND, ORE. – When given within an hour, a single dose of at least 100,000 IU vitamin D3 rapidly attenuates sunburn, according to the results of a randomized, double-blind, placebo-controlled pilot study of 25 healthy adults.
This is the first in vivo study to evaluate whether vitamin D3 can modulate acute inflammation in target tissues, Jeffrey F. Scott, MD, wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings “have broad implications for the role of vitamin D in skin homeostasis, and suggest that oral vitamin D may be clinically therapeutic for its immunomodulatory properties,” he and his coauthors concluded.
Higher doses of vitamin D3 also produced significant decreases in skin levels of tumor necrosis factor–alpha (TNF-alpha) (P = .02) and inducible nitric oxide synthase (iNOS) (P = .04) compared with placebo, reported Dr. Scott, a resident in dermatology at University Hospitals Cleveland Medical Center.
Notably, 48 hours after sunburn, hematoxylin and eosin histology of punch biopsies showed that participants who received 200,000 IU vitamin D3 had the least structural damage to the skin, while placebo recipients had the most damage. Expression profiling also linked vitamin D3 treatment with upregulation of genes associated with skin barrier repair.
Studies continue to document diverse biologic effects of vitamin D, including “modulation of immune response, inflammatory disease, cardiovascular health, and carcinogenesis,” the researchers wrote. Vitamin D3 also has been shown to suppress inflammatory mediators and induce autophagy, they added. Previously, their group showed that oral D3 induced similar protective effects in a mouse model of chemical skin injury. Treatment inhibited proinflammatory cytokines and chemokines within the skin, including iNOS and TNF-alpha.
The current study also included a control phase in which participants underwent experimental sunburn on the right arm without any treatment. Forty-eight hours later, punch biopsies revealed high levels of iNOS and TNF-alpha, with increased expression of proinflammatory genes, the researchers wrote.
During the subsequent experimental phase, seven participants were assigned to receive placebo, and six were assigned to receive 50,000 IU, 100,000 IU, or 200,000 IU of vitamin D3. After treatment, participants with the highest serum D3 levels had significantly decreased skin redness compared with participants with lower serum D3 levels (P less than .05). Higher vitamin D3 serum levels were also associated with significant (P less than .05) upregulation of skin barrier repair genes and of arginase-1, a cytosolic enzyme that helps mediate anti-inflammatory activity.
“Arginase-1 may be a clinically useful tissue biomarker for monitoring the immunomodulatory effects of vitamin D3 in humans,” the researchers concluded.
The National Institute of Arthritis Musculoskeletal and Skin Diseases and the National Institutes of Health supported the work. Dr. Scott had no relevant financial disclosures.
Key clinical point: A single dose of at least 100,000 IU vitamin D3 rapidly attenuated experimental sunburn.
Major finding: Twenty-four hours after sunburn, recipients of 100,000 IU or 200,000 IU D3 had a marked, sustained reduction in skin redness compared with the placebo and 50,000 IU groups. Higher doses of D3 produced significant decreases in skin levels of tumor necrosis factor–alpha and inducible nitric oxide synthase, compared with placebo.
Data source: A double-blind, randomized, placebo-controlled pilot study of 25 healthy adults.
Disclosures: The National Institute of Arthritis Musculoskeletal and Skin Diseases and the National Institutes of Health supported the work. Dr. Scott had no relevant financial conflicts.