User login
Survey finds chronic rhinosinusitis common, burdensome
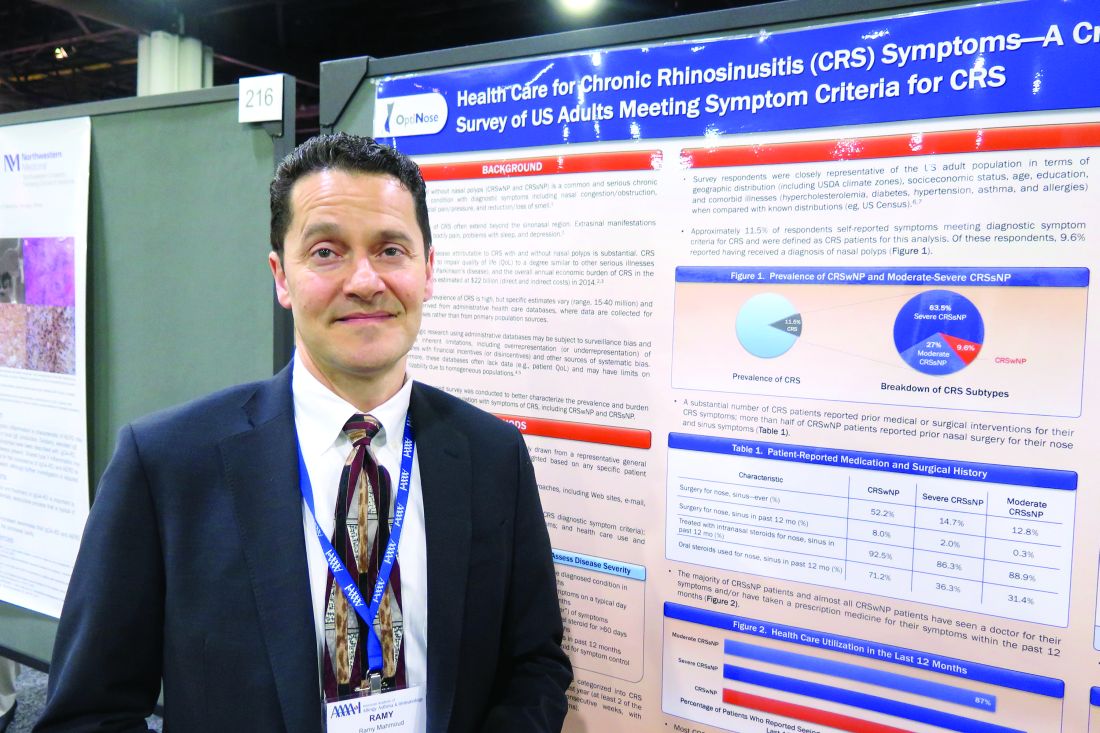
ATLANTA – Results from a large cross-sectional survey of adults who meet diagnostic symptom criteria for chronic rhinosinusitis (CRS) reveal that most report suffering significant symptoms, high rates of physician visits, and dissatisfaction with current intranasal steroid sprays.
“Chronic rhinosinusitis is common, it’s severe, and people are seeking a lot of medical care,” study author Ramy A. Mahmoud, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “It’s causing a lot of disruption in their lives, and they’re not satisfied with currently available intranasal steroids.”
In an effort to better characterize the prevalence and burden of illness in adults with symptoms of CRS, Dr. Mahmoud and his associates conducted a population-based survey of 10,336 U.S. adults drawn from a representative general panel of 4.3 million. They recruited participants via websites, email, phone, online communities, and social networks and collected information about nasal symptoms, frequency, duration, and severity/bother of nasal symptoms and health care use and treatments.
The researchers found that almost 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS. Of these, 63.5% were classified as having severe CRS without known nasal polyps (CRSsNP), 27% had moderate CRSsNP, and 9.6% had CRS with known nasal polyps (CRSwNP). In addition, 87%-99% of patients reported seeing a physician for their symptoms and/or taking a prescription medicine for their CRS symptoms in the past year. In fact, 60% of CRSwNP patients, 58% of severe CRSsNP patients, and 33% of moderate CRSsNP patients said they made more than five physician visits in the past year for nose and sinus symptoms.
The frequency of key CRS symptoms was similar in patients with and without nasal polyps, except that those with severe CRSsNP reported higher rates of facial pain/pressure (in the range of 60%-65%) and loss of smell/taste (46%-56%). The most frequently reported core symptoms were nasal congestion (94%-97%) and drainage (89%-92%). Despite currently available treatment options, up to 46% of CRS patients reported that they remain “extremely” affected by symptoms, and 79% of all CRS patients expressed concern about flare-ups. In addition, a large proportion of respondents expressed frustration with the inadequate symptom relief produced by their current intranasal corticosteroid (87% of CRSwNP patients, 86% of severe CRSsNP patients, and 63% of moderate CRSsNP patients).
Dr. Mahmoud said that the findings indicate a role for more and/or improved treatment options to meet the needs of this patient population. “People who have CRS have it pretty bad,” he said. “Very few people are not seeking a lot of medical care for their symptoms.”
The study was funded by OptiNose.
ATLANTA – Results from a large cross-sectional survey of adults who meet diagnostic symptom criteria for chronic rhinosinusitis (CRS) reveal that most report suffering significant symptoms, high rates of physician visits, and dissatisfaction with current intranasal steroid sprays.
“Chronic rhinosinusitis is common, it’s severe, and people are seeking a lot of medical care,” study author Ramy A. Mahmoud, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “It’s causing a lot of disruption in their lives, and they’re not satisfied with currently available intranasal steroids.”
In an effort to better characterize the prevalence and burden of illness in adults with symptoms of CRS, Dr. Mahmoud and his associates conducted a population-based survey of 10,336 U.S. adults drawn from a representative general panel of 4.3 million. They recruited participants via websites, email, phone, online communities, and social networks and collected information about nasal symptoms, frequency, duration, and severity/bother of nasal symptoms and health care use and treatments.
The researchers found that almost 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS. Of these, 63.5% were classified as having severe CRS without known nasal polyps (CRSsNP), 27% had moderate CRSsNP, and 9.6% had CRS with known nasal polyps (CRSwNP). In addition, 87%-99% of patients reported seeing a physician for their symptoms and/or taking a prescription medicine for their CRS symptoms in the past year. In fact, 60% of CRSwNP patients, 58% of severe CRSsNP patients, and 33% of moderate CRSsNP patients said they made more than five physician visits in the past year for nose and sinus symptoms.
The frequency of key CRS symptoms was similar in patients with and without nasal polyps, except that those with severe CRSsNP reported higher rates of facial pain/pressure (in the range of 60%-65%) and loss of smell/taste (46%-56%). The most frequently reported core symptoms were nasal congestion (94%-97%) and drainage (89%-92%). Despite currently available treatment options, up to 46% of CRS patients reported that they remain “extremely” affected by symptoms, and 79% of all CRS patients expressed concern about flare-ups. In addition, a large proportion of respondents expressed frustration with the inadequate symptom relief produced by their current intranasal corticosteroid (87% of CRSwNP patients, 86% of severe CRSsNP patients, and 63% of moderate CRSsNP patients).
Dr. Mahmoud said that the findings indicate a role for more and/or improved treatment options to meet the needs of this patient population. “People who have CRS have it pretty bad,” he said. “Very few people are not seeking a lot of medical care for their symptoms.”
The study was funded by OptiNose.
ATLANTA – Results from a large cross-sectional survey of adults who meet diagnostic symptom criteria for chronic rhinosinusitis (CRS) reveal that most report suffering significant symptoms, high rates of physician visits, and dissatisfaction with current intranasal steroid sprays.
“Chronic rhinosinusitis is common, it’s severe, and people are seeking a lot of medical care,” study author Ramy A. Mahmoud, MD, said in an interview at the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “It’s causing a lot of disruption in their lives, and they’re not satisfied with currently available intranasal steroids.”
In an effort to better characterize the prevalence and burden of illness in adults with symptoms of CRS, Dr. Mahmoud and his associates conducted a population-based survey of 10,336 U.S. adults drawn from a representative general panel of 4.3 million. They recruited participants via websites, email, phone, online communities, and social networks and collected information about nasal symptoms, frequency, duration, and severity/bother of nasal symptoms and health care use and treatments.
The researchers found that almost 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS. Of these, 63.5% were classified as having severe CRS without known nasal polyps (CRSsNP), 27% had moderate CRSsNP, and 9.6% had CRS with known nasal polyps (CRSwNP). In addition, 87%-99% of patients reported seeing a physician for their symptoms and/or taking a prescription medicine for their CRS symptoms in the past year. In fact, 60% of CRSwNP patients, 58% of severe CRSsNP patients, and 33% of moderate CRSsNP patients said they made more than five physician visits in the past year for nose and sinus symptoms.
The frequency of key CRS symptoms was similar in patients with and without nasal polyps, except that those with severe CRSsNP reported higher rates of facial pain/pressure (in the range of 60%-65%) and loss of smell/taste (46%-56%). The most frequently reported core symptoms were nasal congestion (94%-97%) and drainage (89%-92%). Despite currently available treatment options, up to 46% of CRS patients reported that they remain “extremely” affected by symptoms, and 79% of all CRS patients expressed concern about flare-ups. In addition, a large proportion of respondents expressed frustration with the inadequate symptom relief produced by their current intranasal corticosteroid (87% of CRSwNP patients, 86% of severe CRSsNP patients, and 63% of moderate CRSsNP patients).
Dr. Mahmoud said that the findings indicate a role for more and/or improved treatment options to meet the needs of this patient population. “People who have CRS have it pretty bad,” he said. “Very few people are not seeking a lot of medical care for their symptoms.”
The study was funded by OptiNose.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Nearly 12% of survey respondents self-reported symptoms that met diagnostic criteria for CRS.
Data source: A population-based survey of 10,336 US adults drawn from a representative general panel of 4.3 million.
Disclosures: The study was funded by OptiNose.
Psychological factors drive Crohn’s symptom reports
Psychological factors, rather than disease activity, were significantly associated with symptoms in children and teens with Crohn’s disease, based on data from 127 children aged 8-18 years.
Patients completed questionnaires on symptom severity and disability, as well as psychological measures assessing anxiety, depression, pain beliefs, and coping. Disease activity was measured by the Pediatric Crohn’s Disease Activity Index.
The researchers used a model to assess how psychology factors and disease activity impacted symptoms and disability.
The disability model showed significant associations with both psychological factors (P less than .001) and disease activity (P less than .05). However, the symptoms model showed a significant association only with psychological factors (P less than .001).
“One possible explanation for our findings is that many patients with Crohn’s disease report elevated levels of psychological distress,” wrote Miranda A. L. van Tilburg, PhD, of the University of North Carolina at Chapel Hill and colleagues.
Although the study was limited by the use of self-reports, “this finding is an important one because symptom presentation often plays an important role in treatment decisions, which could lead to unnecessary exposure to tests and treatments, with potential negative side effects,” the researchers wrote. “When confronted with a pediatric patient with Crohn’s disease who has high levels of psychological distress, independent of his or her inflammatory status, the clinician should consider incorporating behavioral techniques such as education, reassurance, and cognitive behavior therapy into the management plan,” they said.
The researchers had no financial conflicts to disclose.
Read the full study here: doi: 10.1016/j.jpeds.2017.01.058.
Psychological factors, rather than disease activity, were significantly associated with symptoms in children and teens with Crohn’s disease, based on data from 127 children aged 8-18 years.
Patients completed questionnaires on symptom severity and disability, as well as psychological measures assessing anxiety, depression, pain beliefs, and coping. Disease activity was measured by the Pediatric Crohn’s Disease Activity Index.
The researchers used a model to assess how psychology factors and disease activity impacted symptoms and disability.
The disability model showed significant associations with both psychological factors (P less than .001) and disease activity (P less than .05). However, the symptoms model showed a significant association only with psychological factors (P less than .001).
“One possible explanation for our findings is that many patients with Crohn’s disease report elevated levels of psychological distress,” wrote Miranda A. L. van Tilburg, PhD, of the University of North Carolina at Chapel Hill and colleagues.
Although the study was limited by the use of self-reports, “this finding is an important one because symptom presentation often plays an important role in treatment decisions, which could lead to unnecessary exposure to tests and treatments, with potential negative side effects,” the researchers wrote. “When confronted with a pediatric patient with Crohn’s disease who has high levels of psychological distress, independent of his or her inflammatory status, the clinician should consider incorporating behavioral techniques such as education, reassurance, and cognitive behavior therapy into the management plan,” they said.
The researchers had no financial conflicts to disclose.
Read the full study here: doi: 10.1016/j.jpeds.2017.01.058.
Psychological factors, rather than disease activity, were significantly associated with symptoms in children and teens with Crohn’s disease, based on data from 127 children aged 8-18 years.
Patients completed questionnaires on symptom severity and disability, as well as psychological measures assessing anxiety, depression, pain beliefs, and coping. Disease activity was measured by the Pediatric Crohn’s Disease Activity Index.
The researchers used a model to assess how psychology factors and disease activity impacted symptoms and disability.
The disability model showed significant associations with both psychological factors (P less than .001) and disease activity (P less than .05). However, the symptoms model showed a significant association only with psychological factors (P less than .001).
“One possible explanation for our findings is that many patients with Crohn’s disease report elevated levels of psychological distress,” wrote Miranda A. L. van Tilburg, PhD, of the University of North Carolina at Chapel Hill and colleagues.
Although the study was limited by the use of self-reports, “this finding is an important one because symptom presentation often plays an important role in treatment decisions, which could lead to unnecessary exposure to tests and treatments, with potential negative side effects,” the researchers wrote. “When confronted with a pediatric patient with Crohn’s disease who has high levels of psychological distress, independent of his or her inflammatory status, the clinician should consider incorporating behavioral techniques such as education, reassurance, and cognitive behavior therapy into the management plan,” they said.
The researchers had no financial conflicts to disclose.
Read the full study here: doi: 10.1016/j.jpeds.2017.01.058.
FROM JOURNAL OF PEDIATRICS
Influenza vaccine is underused in children with heart disease
The influenza vaccine is underused in children with heart disease; approximately one-third were vaccinated in a prospective study of 186 children in September and October 2012.
“Annual influenza vaccination is the most effective and safe means of preventing the disease,” and children with chronic diseases including heart conditions are at increased risk for complications that would require hospitalization, wrote Gilat Livni, MD, of Tel Aviv University and colleagues.
Overall, 59% of parents reported that their primary pediatrician recommended flu vaccination, and 53% of these parents complied. By contrast, only 13% of children whose pediatricians had not recommended vaccination received it.
“The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the researchers wrote. Parents’ misconceptions included the belief that the vaccine would cause flu (66%, of whom 30% had their child vaccinated); the belief that the vaccine would cause severe side effects (55%, of whom 26% had their child vaccinated), and the belief that the vaccine was unsafe (47%, 21% of whom had their child vaccinated).
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination,” the researchers said. “Recommending the vaccine should be made part of routine patient visits in fall and winter.”
The researchers had no financial conflicts to disclose. The findings were published online ahead of print in the Pediatric Infectious Disease Journal (Ped Infect Dis J. 2017. doi: 10.1097/INF.0000000000001579).
The influenza vaccine is underused in children with heart disease; approximately one-third were vaccinated in a prospective study of 186 children in September and October 2012.
“Annual influenza vaccination is the most effective and safe means of preventing the disease,” and children with chronic diseases including heart conditions are at increased risk for complications that would require hospitalization, wrote Gilat Livni, MD, of Tel Aviv University and colleagues.
Overall, 59% of parents reported that their primary pediatrician recommended flu vaccination, and 53% of these parents complied. By contrast, only 13% of children whose pediatricians had not recommended vaccination received it.
“The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the researchers wrote. Parents’ misconceptions included the belief that the vaccine would cause flu (66%, of whom 30% had their child vaccinated); the belief that the vaccine would cause severe side effects (55%, of whom 26% had their child vaccinated), and the belief that the vaccine was unsafe (47%, 21% of whom had their child vaccinated).
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination,” the researchers said. “Recommending the vaccine should be made part of routine patient visits in fall and winter.”
The researchers had no financial conflicts to disclose. The findings were published online ahead of print in the Pediatric Infectious Disease Journal (Ped Infect Dis J. 2017. doi: 10.1097/INF.0000000000001579).
The influenza vaccine is underused in children with heart disease; approximately one-third were vaccinated in a prospective study of 186 children in September and October 2012.
“Annual influenza vaccination is the most effective and safe means of preventing the disease,” and children with chronic diseases including heart conditions are at increased risk for complications that would require hospitalization, wrote Gilat Livni, MD, of Tel Aviv University and colleagues.
Overall, 59% of parents reported that their primary pediatrician recommended flu vaccination, and 53% of these parents complied. By contrast, only 13% of children whose pediatricians had not recommended vaccination received it.
“The failure of parents to receive information or advice from a physician regarding vaccination was strongly inversely related to vaccination of the child,” the researchers wrote. Parents’ misconceptions included the belief that the vaccine would cause flu (66%, of whom 30% had their child vaccinated); the belief that the vaccine would cause severe side effects (55%, of whom 26% had their child vaccinated), and the belief that the vaccine was unsafe (47%, 21% of whom had their child vaccinated).
“Our results emphasize the need to raise awareness among physicians and other medical health care personnel dealing with children with heart disease of the importance of properly counseling parents regarding influenza vaccination,” the researchers said. “Recommending the vaccine should be made part of routine patient visits in fall and winter.”
The researchers had no financial conflicts to disclose. The findings were published online ahead of print in the Pediatric Infectious Disease Journal (Ped Infect Dis J. 2017. doi: 10.1097/INF.0000000000001579).
FROM THE JOURNAL OF PEDIATRICS
VIDEO: Subclinical leaflet thrombosis frequent after TAVR, SAVR
WASHINGTON – Subclinical leaflet thrombosis, as evidenced by reduced leaflet motion and corresponding hypoattenuating lesions on high-resolution CT, occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically, according to a report presented at the annual meeting of the American College of Cardiology and simultaneously published March 19 in the Lancet.
In an observational cohort study involving 890 patients enrolled in two single-center registries, reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%) by four-dimensional, volume-rendered CT. The thrombosis was hemodynamically silent and undetected by transthoracic echocardiograms done in a large subgroup of the study participants. Mean aortic valve gradients were numerically higher in affected patients, but still fell within the normal range in most of them, said Raj R. Makkar, MD, of Cedars-Sinai Heart Institute, Los Angeles.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Makkar and his associates studied this issue by analyzing data in the RESOLVE (Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation) registry at Cedars-Sinai and the SAVORY (Subclinical Aortic Valve Biosprosthesis Thrombosis Assessed with Four-Dimensional CT) registry at the Rigshospitalet Heart Center in Copenhagen. Their study focused on enrolled patients who underwent either TAVR (626 patients) or SAVR (264 patients) and then had high-resolution CT scans done at various times after implantation. All the CT scans were analyzed in blinded fashion, and the study participants were followed for a mean of 540 days.
The prevalence of subclinical leaflet thrombosis was significantly higher in TAVR valves (13%) than in SAVR valves (4%). The severity of the thrombosis also was significantly higher in TAVR valves, when measured by the extent of leaflet motion restriction (71.0% vs. 56.9%).
One possible reason for this discrepancy is that traumatic injury to the pericardial leaflets, which theoretically could predispose to thrombus formation, may be more likely to occur during crimping and deployment of balloon-expandable and self-expanding stent valves in TAVR. Another possibility is that resection of the calcified native aortic valves during SAVR might improve flow dynamics after valve replacement, compared with leaving native aortic valve cusps in situ during TAVR. A third possibility is that inadvertent incomplete expansion or overexpansion of transcatheter valves might alter mechanical stress on the leaflets, predisposing them to thrombus formation, compared with the uniform expansion of valves placed surgically, the investigator said.
“Nevertheless, these findings should be interpreted in the context of findings from multiple randomized controlled trials showing similar mortality and stroke rates, better hemodynamics, and equivalent durability of transcatheter aortic valves at 5 years, compared with surgical valves,” he noted.
Patients taking novel oral anticoagulants (NOACs) or warfarin for anticoagulation before valve replacement were less likely to develop subclinical leaflet thrombosis than patients not taking those anticoagulants. And the thrombosis appeared to resolve in all patients treated with NOACs or warfarin for 30 days after it was found on CT, but it persisted or progressed in those not treated with anticoagulation. Thus, “anticoagulation with NOACs or warfarin was effective in prevention and treatment of reduced leaflet motion, but dual-antiplatelet therapy, which is the standard of care, was not,” the investigators said (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2.
“Our study challenges the American College of Cardiology and American Heart Association and European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines, which recommend dual-antiplatelet therapy after TAVR and do not recommend routine anticoagulation after TAVR,” he noted.
However, routine anticoagulation for all TAVR and SAVR patients, or even for all who develop leaflet thrombosis, cannot be recommended on the basis of results from a single observational study. The benefit of anticoagulation may not offset the risk of bleeding in this predominantly elderly population with multiple comorbidities, he said.
There were no significant differences between patients who developed subclinical leaflet thrombosis and those who did not regarding rates of death, MI, or stroke. However, “reduced leaflet wall motion was significantly associated with increased rates of all TIAs [transient ischemic attacks], nonprocedural TIAs, and post-CT TIAs.” Dr. Makkar said.
Based on his new findings, Dr. Makkar suggested that clinicians keep possible leaflet thrombosis on a replaced aortic valve in mind if they see clinical evidence for it, such as an increase in the valve’s pressure gradient or development of a TIA or stroke.* These events should trigger further investigation of the valve with CT angiography, and if thrombosis is then confirmed, the patient should receive treatment with an anticoagulant, although the appropriate duration of anticoagulant treatment remains unclear, he said in a video interview. Dr. Makkar also said that trials are needed to determine whether routine CT angiography assessment or routine prophylactic treatment with an anticoagulant is warranted because the antiplatelet therapy that patients currently receive after aortic valve replacement seems unable to prevent leaflet thrombosis.
Since this was an observational study based on data from nonrandomized registries, the findings cannot prove causality but only an association between leaflet thrombosis and TIA. They must be substantiated in further studies, including “the current Food and Drug Administration–mandated imaging substudies in randomized clinical trials.”
Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
Mitchel L. Zoler contributed to this report.
Correction, 3/20/17: An earlier version of this article misstated the clinical evidence for possible leaflet thrombosis on a replaced aortic valve.
The study by Dr. Makkar and his coinvestigators provides important new information to guide future research, but changing clinical practice or guidelines on the basis of this information would be premature.
In particular, whether to recommend anticoagulation therapy to TAVR and SAVR patients is unknown, given the risks of chronic anticoagulation. We do not yet know whether such a recommendation should be guided by CT or echocardiographic imaging, and, if so, at what intervals? We don’t yet know the optimal duration of anticoagulation treatment, or whether NOAC’s are as good as or better than warfarin in this setting. And we don’t yet know whether the risk of bleeding is offset by a reduction in stroke or any other meaningful clinical sequelae.
Jeroen J. Bax, MD, is in the department of cardiology at Leiden (the Netherlands) University Medical Center. Gregg W. Stone, MD, is at Columbia University Medical Center and the Cardiovascular Research Foundation, both in New York. Dr. Bax’s institution has received unrestricted research grants from Biotronik, Medtronic, Boston Scientific, and Edwards LifeScience; Dr. Stone reported ties to numerous industry sources. Dr. Bax and Dr. Stone made these remarks in an editorial accompanying the Lancet report (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2).
The study by Dr. Makkar and his coinvestigators provides important new information to guide future research, but changing clinical practice or guidelines on the basis of this information would be premature.
In particular, whether to recommend anticoagulation therapy to TAVR and SAVR patients is unknown, given the risks of chronic anticoagulation. We do not yet know whether such a recommendation should be guided by CT or echocardiographic imaging, and, if so, at what intervals? We don’t yet know the optimal duration of anticoagulation treatment, or whether NOAC’s are as good as or better than warfarin in this setting. And we don’t yet know whether the risk of bleeding is offset by a reduction in stroke or any other meaningful clinical sequelae.
Jeroen J. Bax, MD, is in the department of cardiology at Leiden (the Netherlands) University Medical Center. Gregg W. Stone, MD, is at Columbia University Medical Center and the Cardiovascular Research Foundation, both in New York. Dr. Bax’s institution has received unrestricted research grants from Biotronik, Medtronic, Boston Scientific, and Edwards LifeScience; Dr. Stone reported ties to numerous industry sources. Dr. Bax and Dr. Stone made these remarks in an editorial accompanying the Lancet report (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2).
The study by Dr. Makkar and his coinvestigators provides important new information to guide future research, but changing clinical practice or guidelines on the basis of this information would be premature.
In particular, whether to recommend anticoagulation therapy to TAVR and SAVR patients is unknown, given the risks of chronic anticoagulation. We do not yet know whether such a recommendation should be guided by CT or echocardiographic imaging, and, if so, at what intervals? We don’t yet know the optimal duration of anticoagulation treatment, or whether NOAC’s are as good as or better than warfarin in this setting. And we don’t yet know whether the risk of bleeding is offset by a reduction in stroke or any other meaningful clinical sequelae.
Jeroen J. Bax, MD, is in the department of cardiology at Leiden (the Netherlands) University Medical Center. Gregg W. Stone, MD, is at Columbia University Medical Center and the Cardiovascular Research Foundation, both in New York. Dr. Bax’s institution has received unrestricted research grants from Biotronik, Medtronic, Boston Scientific, and Edwards LifeScience; Dr. Stone reported ties to numerous industry sources. Dr. Bax and Dr. Stone made these remarks in an editorial accompanying the Lancet report (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2).
WASHINGTON – Subclinical leaflet thrombosis, as evidenced by reduced leaflet motion and corresponding hypoattenuating lesions on high-resolution CT, occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically, according to a report presented at the annual meeting of the American College of Cardiology and simultaneously published March 19 in the Lancet.
In an observational cohort study involving 890 patients enrolled in two single-center registries, reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%) by four-dimensional, volume-rendered CT. The thrombosis was hemodynamically silent and undetected by transthoracic echocardiograms done in a large subgroup of the study participants. Mean aortic valve gradients were numerically higher in affected patients, but still fell within the normal range in most of them, said Raj R. Makkar, MD, of Cedars-Sinai Heart Institute, Los Angeles.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Makkar and his associates studied this issue by analyzing data in the RESOLVE (Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation) registry at Cedars-Sinai and the SAVORY (Subclinical Aortic Valve Biosprosthesis Thrombosis Assessed with Four-Dimensional CT) registry at the Rigshospitalet Heart Center in Copenhagen. Their study focused on enrolled patients who underwent either TAVR (626 patients) or SAVR (264 patients) and then had high-resolution CT scans done at various times after implantation. All the CT scans were analyzed in blinded fashion, and the study participants were followed for a mean of 540 days.
The prevalence of subclinical leaflet thrombosis was significantly higher in TAVR valves (13%) than in SAVR valves (4%). The severity of the thrombosis also was significantly higher in TAVR valves, when measured by the extent of leaflet motion restriction (71.0% vs. 56.9%).
One possible reason for this discrepancy is that traumatic injury to the pericardial leaflets, which theoretically could predispose to thrombus formation, may be more likely to occur during crimping and deployment of balloon-expandable and self-expanding stent valves in TAVR. Another possibility is that resection of the calcified native aortic valves during SAVR might improve flow dynamics after valve replacement, compared with leaving native aortic valve cusps in situ during TAVR. A third possibility is that inadvertent incomplete expansion or overexpansion of transcatheter valves might alter mechanical stress on the leaflets, predisposing them to thrombus formation, compared with the uniform expansion of valves placed surgically, the investigator said.
“Nevertheless, these findings should be interpreted in the context of findings from multiple randomized controlled trials showing similar mortality and stroke rates, better hemodynamics, and equivalent durability of transcatheter aortic valves at 5 years, compared with surgical valves,” he noted.
Patients taking novel oral anticoagulants (NOACs) or warfarin for anticoagulation before valve replacement were less likely to develop subclinical leaflet thrombosis than patients not taking those anticoagulants. And the thrombosis appeared to resolve in all patients treated with NOACs or warfarin for 30 days after it was found on CT, but it persisted or progressed in those not treated with anticoagulation. Thus, “anticoagulation with NOACs or warfarin was effective in prevention and treatment of reduced leaflet motion, but dual-antiplatelet therapy, which is the standard of care, was not,” the investigators said (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2.
“Our study challenges the American College of Cardiology and American Heart Association and European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines, which recommend dual-antiplatelet therapy after TAVR and do not recommend routine anticoagulation after TAVR,” he noted.
However, routine anticoagulation for all TAVR and SAVR patients, or even for all who develop leaflet thrombosis, cannot be recommended on the basis of results from a single observational study. The benefit of anticoagulation may not offset the risk of bleeding in this predominantly elderly population with multiple comorbidities, he said.
There were no significant differences between patients who developed subclinical leaflet thrombosis and those who did not regarding rates of death, MI, or stroke. However, “reduced leaflet wall motion was significantly associated with increased rates of all TIAs [transient ischemic attacks], nonprocedural TIAs, and post-CT TIAs.” Dr. Makkar said.
Based on his new findings, Dr. Makkar suggested that clinicians keep possible leaflet thrombosis on a replaced aortic valve in mind if they see clinical evidence for it, such as an increase in the valve’s pressure gradient or development of a TIA or stroke.* These events should trigger further investigation of the valve with CT angiography, and if thrombosis is then confirmed, the patient should receive treatment with an anticoagulant, although the appropriate duration of anticoagulant treatment remains unclear, he said in a video interview. Dr. Makkar also said that trials are needed to determine whether routine CT angiography assessment or routine prophylactic treatment with an anticoagulant is warranted because the antiplatelet therapy that patients currently receive after aortic valve replacement seems unable to prevent leaflet thrombosis.
Since this was an observational study based on data from nonrandomized registries, the findings cannot prove causality but only an association between leaflet thrombosis and TIA. They must be substantiated in further studies, including “the current Food and Drug Administration–mandated imaging substudies in randomized clinical trials.”
Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
Mitchel L. Zoler contributed to this report.
Correction, 3/20/17: An earlier version of this article misstated the clinical evidence for possible leaflet thrombosis on a replaced aortic valve.
WASHINGTON – Subclinical leaflet thrombosis, as evidenced by reduced leaflet motion and corresponding hypoattenuating lesions on high-resolution CT, occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically, according to a report presented at the annual meeting of the American College of Cardiology and simultaneously published March 19 in the Lancet.
In an observational cohort study involving 890 patients enrolled in two single-center registries, reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%) by four-dimensional, volume-rendered CT. The thrombosis was hemodynamically silent and undetected by transthoracic echocardiograms done in a large subgroup of the study participants. Mean aortic valve gradients were numerically higher in affected patients, but still fell within the normal range in most of them, said Raj R. Makkar, MD, of Cedars-Sinai Heart Institute, Los Angeles.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Dr. Makkar and his associates studied this issue by analyzing data in the RESOLVE (Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and its Treatment with Anticoagulation) registry at Cedars-Sinai and the SAVORY (Subclinical Aortic Valve Biosprosthesis Thrombosis Assessed with Four-Dimensional CT) registry at the Rigshospitalet Heart Center in Copenhagen. Their study focused on enrolled patients who underwent either TAVR (626 patients) or SAVR (264 patients) and then had high-resolution CT scans done at various times after implantation. All the CT scans were analyzed in blinded fashion, and the study participants were followed for a mean of 540 days.
The prevalence of subclinical leaflet thrombosis was significantly higher in TAVR valves (13%) than in SAVR valves (4%). The severity of the thrombosis also was significantly higher in TAVR valves, when measured by the extent of leaflet motion restriction (71.0% vs. 56.9%).
One possible reason for this discrepancy is that traumatic injury to the pericardial leaflets, which theoretically could predispose to thrombus formation, may be more likely to occur during crimping and deployment of balloon-expandable and self-expanding stent valves in TAVR. Another possibility is that resection of the calcified native aortic valves during SAVR might improve flow dynamics after valve replacement, compared with leaving native aortic valve cusps in situ during TAVR. A third possibility is that inadvertent incomplete expansion or overexpansion of transcatheter valves might alter mechanical stress on the leaflets, predisposing them to thrombus formation, compared with the uniform expansion of valves placed surgically, the investigator said.
“Nevertheless, these findings should be interpreted in the context of findings from multiple randomized controlled trials showing similar mortality and stroke rates, better hemodynamics, and equivalent durability of transcatheter aortic valves at 5 years, compared with surgical valves,” he noted.
Patients taking novel oral anticoagulants (NOACs) or warfarin for anticoagulation before valve replacement were less likely to develop subclinical leaflet thrombosis than patients not taking those anticoagulants. And the thrombosis appeared to resolve in all patients treated with NOACs or warfarin for 30 days after it was found on CT, but it persisted or progressed in those not treated with anticoagulation. Thus, “anticoagulation with NOACs or warfarin was effective in prevention and treatment of reduced leaflet motion, but dual-antiplatelet therapy, which is the standard of care, was not,” the investigators said (Lancet 2017 Mar 19. doi: 10.1016/S0140-6736(17)30757-2.
“Our study challenges the American College of Cardiology and American Heart Association and European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines, which recommend dual-antiplatelet therapy after TAVR and do not recommend routine anticoagulation after TAVR,” he noted.
However, routine anticoagulation for all TAVR and SAVR patients, or even for all who develop leaflet thrombosis, cannot be recommended on the basis of results from a single observational study. The benefit of anticoagulation may not offset the risk of bleeding in this predominantly elderly population with multiple comorbidities, he said.
There were no significant differences between patients who developed subclinical leaflet thrombosis and those who did not regarding rates of death, MI, or stroke. However, “reduced leaflet wall motion was significantly associated with increased rates of all TIAs [transient ischemic attacks], nonprocedural TIAs, and post-CT TIAs.” Dr. Makkar said.
Based on his new findings, Dr. Makkar suggested that clinicians keep possible leaflet thrombosis on a replaced aortic valve in mind if they see clinical evidence for it, such as an increase in the valve’s pressure gradient or development of a TIA or stroke.* These events should trigger further investigation of the valve with CT angiography, and if thrombosis is then confirmed, the patient should receive treatment with an anticoagulant, although the appropriate duration of anticoagulant treatment remains unclear, he said in a video interview. Dr. Makkar also said that trials are needed to determine whether routine CT angiography assessment or routine prophylactic treatment with an anticoagulant is warranted because the antiplatelet therapy that patients currently receive after aortic valve replacement seems unable to prevent leaflet thrombosis.
Since this was an observational study based on data from nonrandomized registries, the findings cannot prove causality but only an association between leaflet thrombosis and TIA. They must be substantiated in further studies, including “the current Food and Drug Administration–mandated imaging substudies in randomized clinical trials.”
Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
Mitchel L. Zoler contributed to this report.
Correction, 3/20/17: An earlier version of this article misstated the clinical evidence for possible leaflet thrombosis on a replaced aortic valve.
AT ACC 17
Key clinical point: Subclinical leaflet thrombosis occurs frequently in bioprosthetic aortic valves implanted either by the transcatheter route or surgically.
Major finding: Reduced leaflet motion signaling the presence of thrombosis was detected in 106 patients (12%).
Data source: An observational cohort study involving 890 patients enrolled in two registries who underwent high-resolution CT to detect leaflet thrombosis after either TAVR or SAVR.
Disclosures: The RESOLVE study was funded by Cedars-Sinai Heart Institute and the SAVORY study was funded by Rigshospitalet. Dr. Makkar has received consultant fees and/or honoraria from Abbott Vascular, Medtronic, Cordis, and Entourage Medical, and research grants from Edwards Lifesciences and St. Jude Medical.
Evolocumab doesn’t make patients dumb
WASHINGTON – Concerns about possible adverse cognitive effects from the new lipid-lowering drug evolocumab and from aggressive lowering of LDL cholesterol were largely put to rest with results from a nearly 2,000-patient study showing that a median 20 months of treatment with evolocumab caused no cognitive or memory decline, either compared with baseline measures or compared with similar patients randomized to placebo treatment.
A subgroup analysis in the study also showed that, even among patients who achieved and sustained LDL cholesterol below 25 mg/dL, no hint arose of an adverse effect on memory or cognition, said Dr. Giugliano, a cardiologist at Brigham and Women’s Hospital in Boston.
“Every day in my office, patients tell me that statins make you dumb. Now I can comfortably tell my patients that we studied whether this drug [evolocumab] makes you dumb, and it doesn’t; nor did having an LDL cholesterol level below 25 mg/dL,” commented Sandra J. Lewis, MD, chief of cardiology at Legacy Good Samaritan Hospital in Portland, Ore.
The primary assessment tool Dr. Giugliano and his associates used in EBBINGHAUS was the Cambridge Neuropsychological Test Automated Battery (CANTAB) Assessments, a computer-based test of spatial working memory strategy index of executive function, and 1,204 patients underwent CANTAB testing both before they received their first dose of their assigned agent and also at the end of treatment. The scores of the patients on evolocumab virtually superimposed on those of patients in the placebo group, both at baseline and at the end of treatment, easily meeting the study’s prespecified definition of noninferiority, Dr. Giugliano reported at the annual meeting of the American College of Cardiology.
The CANTAB scores also showed no change from baseline over time in the individual subgroups of both evolocumab-treated and placebo-treated patients. The total absence of a signal of mental deterioration in the placebo patients who remained on statin treatment throughout the study provided new evidence that medium-term treatment with a statin was not linked with any change in memory or cognition.
EBBINGHAUS also assessed participants using several other memory and cognition tests, had patients complete an end-of-study survey of their perceptions of their mental function, and questioned participating physicians about patients’ mental performance. None of these measures showed any signals of impairment. A full description of the range of assessments used in EBBINGHAUS appeared recently in a published article (Clin Cardiol. 2017 Feb;40[2]:59-65).
Dr. Giugliano conceded that what remains unknown at this point is the possible impact of further prolonged treatment with a PCSK9 drug or from extended maintenance of LDL cholesterol levels at very low levels, effects that could continue for decades in the routine treatment of selected patients. He noted that patients who participated in FOURIER and in EBBINGHAUS are undergoing continued follow-up to gain better insight into the longer-term effects of their treatment.
EBBINGHAUS was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and received research support from Amgen and from several other drug companies. Dr. Bhatt has received research support from Amgen, from Sanofi Regeneron associated with research on another PCSK9 inhibitor, and from several other drug companies, Dr. Lewis had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Concerns about possible adverse cognitive effects from the new lipid-lowering drug evolocumab and from aggressive lowering of LDL cholesterol were largely put to rest with results from a nearly 2,000-patient study showing that a median 20 months of treatment with evolocumab caused no cognitive or memory decline, either compared with baseline measures or compared with similar patients randomized to placebo treatment.
A subgroup analysis in the study also showed that, even among patients who achieved and sustained LDL cholesterol below 25 mg/dL, no hint arose of an adverse effect on memory or cognition, said Dr. Giugliano, a cardiologist at Brigham and Women’s Hospital in Boston.
“Every day in my office, patients tell me that statins make you dumb. Now I can comfortably tell my patients that we studied whether this drug [evolocumab] makes you dumb, and it doesn’t; nor did having an LDL cholesterol level below 25 mg/dL,” commented Sandra J. Lewis, MD, chief of cardiology at Legacy Good Samaritan Hospital in Portland, Ore.
The primary assessment tool Dr. Giugliano and his associates used in EBBINGHAUS was the Cambridge Neuropsychological Test Automated Battery (CANTAB) Assessments, a computer-based test of spatial working memory strategy index of executive function, and 1,204 patients underwent CANTAB testing both before they received their first dose of their assigned agent and also at the end of treatment. The scores of the patients on evolocumab virtually superimposed on those of patients in the placebo group, both at baseline and at the end of treatment, easily meeting the study’s prespecified definition of noninferiority, Dr. Giugliano reported at the annual meeting of the American College of Cardiology.
The CANTAB scores also showed no change from baseline over time in the individual subgroups of both evolocumab-treated and placebo-treated patients. The total absence of a signal of mental deterioration in the placebo patients who remained on statin treatment throughout the study provided new evidence that medium-term treatment with a statin was not linked with any change in memory or cognition.
EBBINGHAUS also assessed participants using several other memory and cognition tests, had patients complete an end-of-study survey of their perceptions of their mental function, and questioned participating physicians about patients’ mental performance. None of these measures showed any signals of impairment. A full description of the range of assessments used in EBBINGHAUS appeared recently in a published article (Clin Cardiol. 2017 Feb;40[2]:59-65).
Dr. Giugliano conceded that what remains unknown at this point is the possible impact of further prolonged treatment with a PCSK9 drug or from extended maintenance of LDL cholesterol levels at very low levels, effects that could continue for decades in the routine treatment of selected patients. He noted that patients who participated in FOURIER and in EBBINGHAUS are undergoing continued follow-up to gain better insight into the longer-term effects of their treatment.
EBBINGHAUS was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and received research support from Amgen and from several other drug companies. Dr. Bhatt has received research support from Amgen, from Sanofi Regeneron associated with research on another PCSK9 inhibitor, and from several other drug companies, Dr. Lewis had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
WASHINGTON – Concerns about possible adverse cognitive effects from the new lipid-lowering drug evolocumab and from aggressive lowering of LDL cholesterol were largely put to rest with results from a nearly 2,000-patient study showing that a median 20 months of treatment with evolocumab caused no cognitive or memory decline, either compared with baseline measures or compared with similar patients randomized to placebo treatment.
A subgroup analysis in the study also showed that, even among patients who achieved and sustained LDL cholesterol below 25 mg/dL, no hint arose of an adverse effect on memory or cognition, said Dr. Giugliano, a cardiologist at Brigham and Women’s Hospital in Boston.
“Every day in my office, patients tell me that statins make you dumb. Now I can comfortably tell my patients that we studied whether this drug [evolocumab] makes you dumb, and it doesn’t; nor did having an LDL cholesterol level below 25 mg/dL,” commented Sandra J. Lewis, MD, chief of cardiology at Legacy Good Samaritan Hospital in Portland, Ore.
The primary assessment tool Dr. Giugliano and his associates used in EBBINGHAUS was the Cambridge Neuropsychological Test Automated Battery (CANTAB) Assessments, a computer-based test of spatial working memory strategy index of executive function, and 1,204 patients underwent CANTAB testing both before they received their first dose of their assigned agent and also at the end of treatment. The scores of the patients on evolocumab virtually superimposed on those of patients in the placebo group, both at baseline and at the end of treatment, easily meeting the study’s prespecified definition of noninferiority, Dr. Giugliano reported at the annual meeting of the American College of Cardiology.
The CANTAB scores also showed no change from baseline over time in the individual subgroups of both evolocumab-treated and placebo-treated patients. The total absence of a signal of mental deterioration in the placebo patients who remained on statin treatment throughout the study provided new evidence that medium-term treatment with a statin was not linked with any change in memory or cognition.
EBBINGHAUS also assessed participants using several other memory and cognition tests, had patients complete an end-of-study survey of their perceptions of their mental function, and questioned participating physicians about patients’ mental performance. None of these measures showed any signals of impairment. A full description of the range of assessments used in EBBINGHAUS appeared recently in a published article (Clin Cardiol. 2017 Feb;40[2]:59-65).
Dr. Giugliano conceded that what remains unknown at this point is the possible impact of further prolonged treatment with a PCSK9 drug or from extended maintenance of LDL cholesterol levels at very low levels, effects that could continue for decades in the routine treatment of selected patients. He noted that patients who participated in FOURIER and in EBBINGHAUS are undergoing continued follow-up to gain better insight into the longer-term effects of their treatment.
EBBINGHAUS was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and received research support from Amgen and from several other drug companies. Dr. Bhatt has received research support from Amgen, from Sanofi Regeneron associated with research on another PCSK9 inhibitor, and from several other drug companies, Dr. Lewis had no disclosures.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT ACC 17
Key clinical point:
Major finding: Patients on evolocumab for 20 months had noninferior memory and executive function test scores, compared with patients on placebo.
Data source: EBBINGHAUS, a multicenter randomized trial with 1,974 patients.
Disclosures: EBBINGHAUS was sponsored by Amgen, the company that markets evolocumab (Repatha). Dr. Giugliano has been a consultant to and received research support from Amgen and from several other drug companies.
VIDEO: iFR outperforms FFR in two major trials
WASHINGTON – Instantaneous wave-free ratio (iFR) is the new evidence-based standard of care for invasive physiologic assessment of stable coronary lesions of intermediate angiographic severity, supplanting the older fractional flow reserve (FFR) technology, Matthias Gotberg, MD, said at the annual meeting of the American College of Cardiology.
The virtually identical results of these two trials should encourage more interventional cardiologists to incorporate physiologic assessment of stable coronary lesions into their clinical practice instead of relying solely on anatomic assessment by angiography, which abundant evidence shows is insufficiently accurate in identifying hemodynamically significant lesions warranting revascularization.
FFR never really caught on because of its limitations. DEFINE-FLAIR and iFR-SWEDEHEART show that iFR overcomes those limitations, said Dr. Gotberg, director of the cardiac catheterization laboratory at Skane University Hospital in Lund, Sweden.
The two studies showed that iFR was noninferior to FFR in the 1-year composite endpoint of all-cause mortality, nonfatal MI, or unplanned revascularization. And iFR was associated with significantly shorter procedure times, less stent utilization, and markedly less patient discomfort because, unlike FFR, it doesn’t require administration of adenosine to induce hyperemia.
Indeed, in iFR-SWEDEHEART, which included more than 2,000 randomized patients in three Scandinavian countries, only 3% of the iFR group reported experiencing chest pain, dyspnea, or other forms of discomfort during their procedure, compared with 68% of the FFR group, Dr. Gotberg explains in this video interview.[[{"fid":"192539","view_mode":"medstat_image_full_text","attributes":{"height":"390","width":"100%","class":"media-element file-medstat-image-full-text","data-delta":"1"},"fields":{"format":"medstat_image_full_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_full_text"}}}]]
WASHINGTON – Instantaneous wave-free ratio (iFR) is the new evidence-based standard of care for invasive physiologic assessment of stable coronary lesions of intermediate angiographic severity, supplanting the older fractional flow reserve (FFR) technology, Matthias Gotberg, MD, said at the annual meeting of the American College of Cardiology.
The virtually identical results of these two trials should encourage more interventional cardiologists to incorporate physiologic assessment of stable coronary lesions into their clinical practice instead of relying solely on anatomic assessment by angiography, which abundant evidence shows is insufficiently accurate in identifying hemodynamically significant lesions warranting revascularization.
FFR never really caught on because of its limitations. DEFINE-FLAIR and iFR-SWEDEHEART show that iFR overcomes those limitations, said Dr. Gotberg, director of the cardiac catheterization laboratory at Skane University Hospital in Lund, Sweden.
The two studies showed that iFR was noninferior to FFR in the 1-year composite endpoint of all-cause mortality, nonfatal MI, or unplanned revascularization. And iFR was associated with significantly shorter procedure times, less stent utilization, and markedly less patient discomfort because, unlike FFR, it doesn’t require administration of adenosine to induce hyperemia.
Indeed, in iFR-SWEDEHEART, which included more than 2,000 randomized patients in three Scandinavian countries, only 3% of the iFR group reported experiencing chest pain, dyspnea, or other forms of discomfort during their procedure, compared with 68% of the FFR group, Dr. Gotberg explains in this video interview.[[{"fid":"192539","view_mode":"medstat_image_full_text","attributes":{"height":"390","width":"100%","class":"media-element file-medstat-image-full-text","data-delta":"1"},"fields":{"format":"medstat_image_full_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_full_text"}}}]]
WASHINGTON – Instantaneous wave-free ratio (iFR) is the new evidence-based standard of care for invasive physiologic assessment of stable coronary lesions of intermediate angiographic severity, supplanting the older fractional flow reserve (FFR) technology, Matthias Gotberg, MD, said at the annual meeting of the American College of Cardiology.
The virtually identical results of these two trials should encourage more interventional cardiologists to incorporate physiologic assessment of stable coronary lesions into their clinical practice instead of relying solely on anatomic assessment by angiography, which abundant evidence shows is insufficiently accurate in identifying hemodynamically significant lesions warranting revascularization.
FFR never really caught on because of its limitations. DEFINE-FLAIR and iFR-SWEDEHEART show that iFR overcomes those limitations, said Dr. Gotberg, director of the cardiac catheterization laboratory at Skane University Hospital in Lund, Sweden.
The two studies showed that iFR was noninferior to FFR in the 1-year composite endpoint of all-cause mortality, nonfatal MI, or unplanned revascularization. And iFR was associated with significantly shorter procedure times, less stent utilization, and markedly less patient discomfort because, unlike FFR, it doesn’t require administration of adenosine to induce hyperemia.
Indeed, in iFR-SWEDEHEART, which included more than 2,000 randomized patients in three Scandinavian countries, only 3% of the iFR group reported experiencing chest pain, dyspnea, or other forms of discomfort during their procedure, compared with 68% of the FFR group, Dr. Gotberg explains in this video interview.[[{"fid":"192539","view_mode":"medstat_image_full_text","attributes":{"height":"390","width":"100%","class":"media-element file-medstat-image-full-text","data-delta":"1"},"fields":{"format":"medstat_image_full_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_full_text"}}}]]
AT ACC 17
Cardiovascular disease most common cause of death in CRC survivors
SEATTLE – Improvements in diagnosis and treatment have lengthened the survival time of patients with colorectal cancer, but the majority of deaths from CRC occur within the first 5 years.
According to new findings presented at the annual Society of Surgical Oncology Cancer Symposium, CRC as a cause of death is surpassed by cardiovascular disease (CVD) and second primary cancers as time goes on.
Dr. Lewis explained that CRC as a cause of death begins to plateau over time and other causes become more important.
“As time goes on, colorectal cancer becomes less prominent, and by year 8, cardiovascular death surpasses it. By year 10, colorectal cancer is surpassed by second cancers and neurologic diseases.”
Information about long-term health problems in long-term colorectal cancer survivors is limited. To address this, Dr. Lewis and his colleagues sought to understand the trends and causes of death over time.
They analyzed causes of death in CRC patients who have survived 5 years and longer using the California Cancer Registry (2000-2011) that is linked to inpatient records. From this database, 139,743 patients with CRC were identified, with 97,604 (69.8%) having been treated for disease originating from the colon and 42,139 (30.2%) from the rectum.
The median age of the patients at the time of presentation was 68 years; at 5 years after diagnosis, 70 years; and at 10 years, 74 years. The 5-year overall survival was 59.1%, and it was during that 5 years that 95% of cancer-specific deaths occurred.
During the first 5 years, the major cause of death was CRC, accounting for nearly two-thirds of the mortality (n = 38,992, 65.4%). This was followed by cardiovascular disease (n = 7,140, 12.0%), second primary cancer (n = 3,775, 6.3%), neurologic disease (n = 2,329, 3.9%), and pulmonary disease (n = 2,307, 3.9%).
The most common second primary malignancies affecting CRC survivors were lung and hematologic cancers, followed by pancreatic and liver cancers.
Overall, in long-term survivors, cardiovascular disease was the major cause of death (n = 2,163, 24.0%) although nearly as many deaths were due to CRC (2,094, 23.2%). This was followed by neurologic disease (n = 1,174, 13.0%), secondary primary cancer (n = 1,146, 12.7%), and pulmonary disease (n = 765, 8.5%).
There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
SEATTLE – Improvements in diagnosis and treatment have lengthened the survival time of patients with colorectal cancer, but the majority of deaths from CRC occur within the first 5 years.
According to new findings presented at the annual Society of Surgical Oncology Cancer Symposium, CRC as a cause of death is surpassed by cardiovascular disease (CVD) and second primary cancers as time goes on.
Dr. Lewis explained that CRC as a cause of death begins to plateau over time and other causes become more important.
“As time goes on, colorectal cancer becomes less prominent, and by year 8, cardiovascular death surpasses it. By year 10, colorectal cancer is surpassed by second cancers and neurologic diseases.”
Information about long-term health problems in long-term colorectal cancer survivors is limited. To address this, Dr. Lewis and his colleagues sought to understand the trends and causes of death over time.
They analyzed causes of death in CRC patients who have survived 5 years and longer using the California Cancer Registry (2000-2011) that is linked to inpatient records. From this database, 139,743 patients with CRC were identified, with 97,604 (69.8%) having been treated for disease originating from the colon and 42,139 (30.2%) from the rectum.
The median age of the patients at the time of presentation was 68 years; at 5 years after diagnosis, 70 years; and at 10 years, 74 years. The 5-year overall survival was 59.1%, and it was during that 5 years that 95% of cancer-specific deaths occurred.
During the first 5 years, the major cause of death was CRC, accounting for nearly two-thirds of the mortality (n = 38,992, 65.4%). This was followed by cardiovascular disease (n = 7,140, 12.0%), second primary cancer (n = 3,775, 6.3%), neurologic disease (n = 2,329, 3.9%), and pulmonary disease (n = 2,307, 3.9%).
The most common second primary malignancies affecting CRC survivors were lung and hematologic cancers, followed by pancreatic and liver cancers.
Overall, in long-term survivors, cardiovascular disease was the major cause of death (n = 2,163, 24.0%) although nearly as many deaths were due to CRC (2,094, 23.2%). This was followed by neurologic disease (n = 1,174, 13.0%), secondary primary cancer (n = 1,146, 12.7%), and pulmonary disease (n = 765, 8.5%).
There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
SEATTLE – Improvements in diagnosis and treatment have lengthened the survival time of patients with colorectal cancer, but the majority of deaths from CRC occur within the first 5 years.
According to new findings presented at the annual Society of Surgical Oncology Cancer Symposium, CRC as a cause of death is surpassed by cardiovascular disease (CVD) and second primary cancers as time goes on.
Dr. Lewis explained that CRC as a cause of death begins to plateau over time and other causes become more important.
“As time goes on, colorectal cancer becomes less prominent, and by year 8, cardiovascular death surpasses it. By year 10, colorectal cancer is surpassed by second cancers and neurologic diseases.”
Information about long-term health problems in long-term colorectal cancer survivors is limited. To address this, Dr. Lewis and his colleagues sought to understand the trends and causes of death over time.
They analyzed causes of death in CRC patients who have survived 5 years and longer using the California Cancer Registry (2000-2011) that is linked to inpatient records. From this database, 139,743 patients with CRC were identified, with 97,604 (69.8%) having been treated for disease originating from the colon and 42,139 (30.2%) from the rectum.
The median age of the patients at the time of presentation was 68 years; at 5 years after diagnosis, 70 years; and at 10 years, 74 years. The 5-year overall survival was 59.1%, and it was during that 5 years that 95% of cancer-specific deaths occurred.
During the first 5 years, the major cause of death was CRC, accounting for nearly two-thirds of the mortality (n = 38,992, 65.4%). This was followed by cardiovascular disease (n = 7,140, 12.0%), second primary cancer (n = 3,775, 6.3%), neurologic disease (n = 2,329, 3.9%), and pulmonary disease (n = 2,307, 3.9%).
The most common second primary malignancies affecting CRC survivors were lung and hematologic cancers, followed by pancreatic and liver cancers.
Overall, in long-term survivors, cardiovascular disease was the major cause of death (n = 2,163, 24.0%) although nearly as many deaths were due to CRC (2,094, 23.2%). This was followed by neurologic disease (n = 1,174, 13.0%), secondary primary cancer (n = 1,146, 12.7%), and pulmonary disease (n = 765, 8.5%).
There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
AT SSO 2017
Key clinical point: Long-term colorectal cancer survivors generally will die from other causes.
Major finding: By year 8, cardiovascular disease surpasses colorectal cancer in survivors, as the leading cause of death.
Data source: Large cancer registry with almost 140,000 colorectal cancer patients.
Disclosures: There was no funding source disclosed in the abstract. Dr. Lewis had no disclosures.
Drugs demonstrate similar safety profile in ACS trial
WASHINGTON, DC—Results of a phase 2 trial suggest rivaroxaban and aspirin have similar safety profiles in patients with acute coronary syndrome (ACS) who are also taking a P2Y12 inhibitor.
Researchers found that, overall, the risk of bleeding was similar whether patients received aspirin or rivaroxaban.
There was no significant difference between the groups when the researchers used TIMI, GUSTO, or BARC bleeding definitions.
However, patients who received rivaroxaban did have a significantly increased risk of bleeding according to the ISTH major bleeding definition.
There was no significant difference between the treatment groups when it came to the study’s efficacy endpoints, although the researchers said the study was not adequately powered to assess efficacy.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published simultaneously in The Lancet.
The study, known as GEMINI-ACS-1, was funded by Janssen Research & Development and Bayer AG.
In this trial, researchers compared rivaroxaban (given at 2.5 mg twice daily) and aspirin (at 100 mg once daily) in patients with ACS who were also receiving clopidogrel or ticagrelor for the secondary prevention of cardiovascular events.
The trial included 3037 adults with unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction. Patients had positive cardiac biomarkers and either ischemic electrocardiographic changes or an atherosclerotic culprit lesion identified during angiography.
Within 10 days of hospital admission for ACS, the patients were randomized to receive aspirin (n=1518) or rivaroxaban (n=1519). Patients also received clopidogrel (n=1333) or ticagrelor (n=1704) based on investigator preference.
Patients received a minimum of 180 days of study treatment. The median treatment duration was 291 days.
Safety
The study’s primary endpoint was TIMI clinically significant bleeding not related to coronary artery bypass grafting (CABG) up to day 390.
This type of bleeding occurred in 5.3% of patients in the rivaroxaban group and 4.9% of patients in the aspirin group. The hazard ratio (HR) was 1.09 (P=0.5840).
The researchers also assessed other types of bleeding and used other bleeding definitions. The HRs for these endpoints (with aspirin as the reference) were as follows.
- TIMI major bleeding, HR=1.25 (P=0.6341)
- TIMI non-CABG major bleeding, HR=1.25 (P=0.6341)
- TIMI minor bleeding, HR=2.25 (P=0.1664)
- TIMI bleeding requiring medical attention, HR=1.01 (P=0.9581)
- TIMI insignificant bleeding, HR=0.84 (P=0.5504)
- GUSTO life-threatening or severe bleeding, HR=1.50 (P=0.6571)
- GUSTO life-threatening, severe, or moderate bleeding, HR=1.58 (P=0.3395)
- GUSTO life-threatening, severe, moderate, or mild bleeding, HR=1.04 (P=0.7869)
- BARC 3a and higher bleeding, HR=1.70 (P=0.1263)
- BARC 3b and higher bleeding, HR=1.38 (P=0.4882)
- ISTH major bleeding, HR=1.83 (P=0.0420)
Efficacy
Researchers also examined several exploratory efficacy endpoints. For the composite efficacy endpoint (which included cardiovascular death, myocardial infarction, stroke, and stent thrombosis), the 2 treatment groups had similar rates.
Five percent of patients in the rivaroxaban group and 4.7% of patients in the aspirin group experienced one of these cardiovascular events. The HR was 1.06 (P=0.7316).
There was no significant difference between the treatment arms for the individual components of the efficacy endpoint or for all-cause death.
The HRs were 1.12 (P=0.7401) for cardiovascular death, 1.15 (P=0.4872) for myocardial infarction, 0.58 (P=0.2506) for stroke, 1.37 (P=0.4917) for definite stent thrombosis, and 0.95 (P=0.8771) for all-cause death. ![]()
WASHINGTON, DC—Results of a phase 2 trial suggest rivaroxaban and aspirin have similar safety profiles in patients with acute coronary syndrome (ACS) who are also taking a P2Y12 inhibitor.
Researchers found that, overall, the risk of bleeding was similar whether patients received aspirin or rivaroxaban.
There was no significant difference between the groups when the researchers used TIMI, GUSTO, or BARC bleeding definitions.
However, patients who received rivaroxaban did have a significantly increased risk of bleeding according to the ISTH major bleeding definition.
There was no significant difference between the treatment groups when it came to the study’s efficacy endpoints, although the researchers said the study was not adequately powered to assess efficacy.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published simultaneously in The Lancet.
The study, known as GEMINI-ACS-1, was funded by Janssen Research & Development and Bayer AG.
In this trial, researchers compared rivaroxaban (given at 2.5 mg twice daily) and aspirin (at 100 mg once daily) in patients with ACS who were also receiving clopidogrel or ticagrelor for the secondary prevention of cardiovascular events.
The trial included 3037 adults with unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction. Patients had positive cardiac biomarkers and either ischemic electrocardiographic changes or an atherosclerotic culprit lesion identified during angiography.
Within 10 days of hospital admission for ACS, the patients were randomized to receive aspirin (n=1518) or rivaroxaban (n=1519). Patients also received clopidogrel (n=1333) or ticagrelor (n=1704) based on investigator preference.
Patients received a minimum of 180 days of study treatment. The median treatment duration was 291 days.
Safety
The study’s primary endpoint was TIMI clinically significant bleeding not related to coronary artery bypass grafting (CABG) up to day 390.
This type of bleeding occurred in 5.3% of patients in the rivaroxaban group and 4.9% of patients in the aspirin group. The hazard ratio (HR) was 1.09 (P=0.5840).
The researchers also assessed other types of bleeding and used other bleeding definitions. The HRs for these endpoints (with aspirin as the reference) were as follows.
- TIMI major bleeding, HR=1.25 (P=0.6341)
- TIMI non-CABG major bleeding, HR=1.25 (P=0.6341)
- TIMI minor bleeding, HR=2.25 (P=0.1664)
- TIMI bleeding requiring medical attention, HR=1.01 (P=0.9581)
- TIMI insignificant bleeding, HR=0.84 (P=0.5504)
- GUSTO life-threatening or severe bleeding, HR=1.50 (P=0.6571)
- GUSTO life-threatening, severe, or moderate bleeding, HR=1.58 (P=0.3395)
- GUSTO life-threatening, severe, moderate, or mild bleeding, HR=1.04 (P=0.7869)
- BARC 3a and higher bleeding, HR=1.70 (P=0.1263)
- BARC 3b and higher bleeding, HR=1.38 (P=0.4882)
- ISTH major bleeding, HR=1.83 (P=0.0420)
Efficacy
Researchers also examined several exploratory efficacy endpoints. For the composite efficacy endpoint (which included cardiovascular death, myocardial infarction, stroke, and stent thrombosis), the 2 treatment groups had similar rates.
Five percent of patients in the rivaroxaban group and 4.7% of patients in the aspirin group experienced one of these cardiovascular events. The HR was 1.06 (P=0.7316).
There was no significant difference between the treatment arms for the individual components of the efficacy endpoint or for all-cause death.
The HRs were 1.12 (P=0.7401) for cardiovascular death, 1.15 (P=0.4872) for myocardial infarction, 0.58 (P=0.2506) for stroke, 1.37 (P=0.4917) for definite stent thrombosis, and 0.95 (P=0.8771) for all-cause death. ![]()
WASHINGTON, DC—Results of a phase 2 trial suggest rivaroxaban and aspirin have similar safety profiles in patients with acute coronary syndrome (ACS) who are also taking a P2Y12 inhibitor.
Researchers found that, overall, the risk of bleeding was similar whether patients received aspirin or rivaroxaban.
There was no significant difference between the groups when the researchers used TIMI, GUSTO, or BARC bleeding definitions.
However, patients who received rivaroxaban did have a significantly increased risk of bleeding according to the ISTH major bleeding definition.
There was no significant difference between the treatment groups when it came to the study’s efficacy endpoints, although the researchers said the study was not adequately powered to assess efficacy.
These results were presented at the American College of Cardiology’s 66th Annual Scientific Session and published simultaneously in The Lancet.
The study, known as GEMINI-ACS-1, was funded by Janssen Research & Development and Bayer AG.
In this trial, researchers compared rivaroxaban (given at 2.5 mg twice daily) and aspirin (at 100 mg once daily) in patients with ACS who were also receiving clopidogrel or ticagrelor for the secondary prevention of cardiovascular events.
The trial included 3037 adults with unstable angina, non-ST segment elevation myocardial infarction, or ST segment elevation myocardial infarction. Patients had positive cardiac biomarkers and either ischemic electrocardiographic changes or an atherosclerotic culprit lesion identified during angiography.
Within 10 days of hospital admission for ACS, the patients were randomized to receive aspirin (n=1518) or rivaroxaban (n=1519). Patients also received clopidogrel (n=1333) or ticagrelor (n=1704) based on investigator preference.
Patients received a minimum of 180 days of study treatment. The median treatment duration was 291 days.
Safety
The study’s primary endpoint was TIMI clinically significant bleeding not related to coronary artery bypass grafting (CABG) up to day 390.
This type of bleeding occurred in 5.3% of patients in the rivaroxaban group and 4.9% of patients in the aspirin group. The hazard ratio (HR) was 1.09 (P=0.5840).
The researchers also assessed other types of bleeding and used other bleeding definitions. The HRs for these endpoints (with aspirin as the reference) were as follows.
- TIMI major bleeding, HR=1.25 (P=0.6341)
- TIMI non-CABG major bleeding, HR=1.25 (P=0.6341)
- TIMI minor bleeding, HR=2.25 (P=0.1664)
- TIMI bleeding requiring medical attention, HR=1.01 (P=0.9581)
- TIMI insignificant bleeding, HR=0.84 (P=0.5504)
- GUSTO life-threatening or severe bleeding, HR=1.50 (P=0.6571)
- GUSTO life-threatening, severe, or moderate bleeding, HR=1.58 (P=0.3395)
- GUSTO life-threatening, severe, moderate, or mild bleeding, HR=1.04 (P=0.7869)
- BARC 3a and higher bleeding, HR=1.70 (P=0.1263)
- BARC 3b and higher bleeding, HR=1.38 (P=0.4882)
- ISTH major bleeding, HR=1.83 (P=0.0420)
Efficacy
Researchers also examined several exploratory efficacy endpoints. For the composite efficacy endpoint (which included cardiovascular death, myocardial infarction, stroke, and stent thrombosis), the 2 treatment groups had similar rates.
Five percent of patients in the rivaroxaban group and 4.7% of patients in the aspirin group experienced one of these cardiovascular events. The HR was 1.06 (P=0.7316).
There was no significant difference between the treatment arms for the individual components of the efficacy endpoint or for all-cause death.
The HRs were 1.12 (P=0.7401) for cardiovascular death, 1.15 (P=0.4872) for myocardial infarction, 0.58 (P=0.2506) for stroke, 1.37 (P=0.4917) for definite stent thrombosis, and 0.95 (P=0.8771) for all-cause death. ![]()
PE patients rarely receive CDT or ST, study shows
WASHINGTON, DC—New research suggests US patients with pulmonary embolism (PE) rarely receive catheter-directed thrombolysis (CDT) or systemic thrombolysis (ST).
Investigators analyzed data from more than 100,000 patients who were hospitalized for PE and found that roughly 2% received CDT or ST.
These findings were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 904-12).
“For years, ST and CDT have been available for use in patients with PE,” said study investigator Srinath Adusumalli, MD, of the University of Pennsylvania in Philadelphia.
“However, there has been little research done to understand how these therapies are being utilized in the real world. Our initial data suggest that, in fact, both ST and CDT are used infrequently to treat PE, including in young, critically ill patients who may experience the highest clinical benefit from those therapies.”
Dr Adusumalli and his colleagues performed a retrospective study in which they collected data from the OptumInsight national commercial insurance claims database.
The team identified 100,744 patients who had been hospitalized with PE during a 10-year period (2004-2014). About 2% of these patients (2.2%, n=2175) received either CDT (n=761) or ST (n=1414).
During the period studied, the number of PE hospitalizations increased by 306% (P<0.001), while the number of patients treated with CDT increased by 197% (P=0.001) and the number treated with ST increased by 514% (P<0.001).
The investigators said these findings are clinically useful and could impact decisions about patient care, but more research is needed.
“This study is the first in a 2-step research plan,” noted Bram Geller, MD, of the University of Pennsylvania.
“[O]ur next phase will be to actually evaluate the safety and clinical effectiveness of CDT versus ST by exploring patient outcomes in the OptumInsight commercial insurance claims database.” ![]()
WASHINGTON, DC—New research suggests US patients with pulmonary embolism (PE) rarely receive catheter-directed thrombolysis (CDT) or systemic thrombolysis (ST).
Investigators analyzed data from more than 100,000 patients who were hospitalized for PE and found that roughly 2% received CDT or ST.
These findings were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 904-12).
“For years, ST and CDT have been available for use in patients with PE,” said study investigator Srinath Adusumalli, MD, of the University of Pennsylvania in Philadelphia.
“However, there has been little research done to understand how these therapies are being utilized in the real world. Our initial data suggest that, in fact, both ST and CDT are used infrequently to treat PE, including in young, critically ill patients who may experience the highest clinical benefit from those therapies.”
Dr Adusumalli and his colleagues performed a retrospective study in which they collected data from the OptumInsight national commercial insurance claims database.
The team identified 100,744 patients who had been hospitalized with PE during a 10-year period (2004-2014). About 2% of these patients (2.2%, n=2175) received either CDT (n=761) or ST (n=1414).
During the period studied, the number of PE hospitalizations increased by 306% (P<0.001), while the number of patients treated with CDT increased by 197% (P=0.001) and the number treated with ST increased by 514% (P<0.001).
The investigators said these findings are clinically useful and could impact decisions about patient care, but more research is needed.
“This study is the first in a 2-step research plan,” noted Bram Geller, MD, of the University of Pennsylvania.
“[O]ur next phase will be to actually evaluate the safety and clinical effectiveness of CDT versus ST by exploring patient outcomes in the OptumInsight commercial insurance claims database.” ![]()
WASHINGTON, DC—New research suggests US patients with pulmonary embolism (PE) rarely receive catheter-directed thrombolysis (CDT) or systemic thrombolysis (ST).
Investigators analyzed data from more than 100,000 patients who were hospitalized for PE and found that roughly 2% received CDT or ST.
These findings were presented at the American College of Cardiology’s 66th Annual Scientific Session (abstract 904-12).
“For years, ST and CDT have been available for use in patients with PE,” said study investigator Srinath Adusumalli, MD, of the University of Pennsylvania in Philadelphia.
“However, there has been little research done to understand how these therapies are being utilized in the real world. Our initial data suggest that, in fact, both ST and CDT are used infrequently to treat PE, including in young, critically ill patients who may experience the highest clinical benefit from those therapies.”
Dr Adusumalli and his colleagues performed a retrospective study in which they collected data from the OptumInsight national commercial insurance claims database.
The team identified 100,744 patients who had been hospitalized with PE during a 10-year period (2004-2014). About 2% of these patients (2.2%, n=2175) received either CDT (n=761) or ST (n=1414).
During the period studied, the number of PE hospitalizations increased by 306% (P<0.001), while the number of patients treated with CDT increased by 197% (P=0.001) and the number treated with ST increased by 514% (P<0.001).
The investigators said these findings are clinically useful and could impact decisions about patient care, but more research is needed.
“This study is the first in a 2-step research plan,” noted Bram Geller, MD, of the University of Pennsylvania.
“[O]ur next phase will be to actually evaluate the safety and clinical effectiveness of CDT versus ST by exploring patient outcomes in the OptumInsight commercial insurance claims database.” ![]()
BNP flags high atrial fibrillation prevalence in ESUS stroke
HOUSTON – Following an embolic stroke of undetermined source, a patient’s serum level of brain natriuretic peptide may identify patients at high risk of having covert atrial fibrillation that warrants assessment by prolonged arrhythmia monitoring, based on a post hoc analysis of data collected from 373 patients.
The analysis showed that in older patients with a recent embolic stroke of undetermined source (ESUS) who had a serum brain natriuretic peptide (BNP) level of 100 pg/mL or greater, the prevalence of atrial fibrillation (AF) detected by up to 30 days of Holter monitoring was 33%. In contrast the AF prevalence was 4% among similar ESUS patients with a lower BNP level, Rolf Wachter, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“This evidence is pretty compelling,” commented Hooman Kamel, MD, a neurologist at Weill Cornell Medicine in New York. “We already use other lab tests that have less evidence supporting their use than this, and checking BNP is a simple lab test.”
The results showed that monitoring identified AF in 5% of the control patients and in 14% of those who underwent extended monitoring, a statistically significant difference (Lancet Neurol. 2017 Apr;16[4]:282-90).
Dr. Wachter and his colleagues had baseline BNP measurements for 373 of the patients and analyzed the prevalence of AF based on these levels. The patients averaged 72 years old. Among those with BNP values of at least 100 pg/mL, prolonged arrhythmia monitoring found AF in 33%, while briefer, conventional monitoring found AF in about 4%. This meant that for every three patients receiving prolonged arrhythmia scrutiny in this subgroup, the assessment found one patient with AF. But among patients with a serum BNP of less than 100 pg/mL, prolonged arrhythmia assessment yielded a 10% rate of AF detection, compared with about 4% in the controls, meaning that for every 18 patients screened prolonged Holter monitoring detected one additional case of AF, he reported.
This relationship presumably exists because higher BNP levels identify patients with structural heart abnormalities, a subgroup at increased risk for AF, Dr. Wachter explained. He cautioned that, because this was a post hoc analysis, he would like to see the findings confirmed in prospective studies.
Dr. Wachter has been a speaker on behalf of Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo and has received research support from Boehringer Ingelheim. The FIND-AFRANDOMISED trial received funding from Boehringer Ingelheim.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
HOUSTON – Following an embolic stroke of undetermined source, a patient’s serum level of brain natriuretic peptide may identify patients at high risk of having covert atrial fibrillation that warrants assessment by prolonged arrhythmia monitoring, based on a post hoc analysis of data collected from 373 patients.
The analysis showed that in older patients with a recent embolic stroke of undetermined source (ESUS) who had a serum brain natriuretic peptide (BNP) level of 100 pg/mL or greater, the prevalence of atrial fibrillation (AF) detected by up to 30 days of Holter monitoring was 33%. In contrast the AF prevalence was 4% among similar ESUS patients with a lower BNP level, Rolf Wachter, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“This evidence is pretty compelling,” commented Hooman Kamel, MD, a neurologist at Weill Cornell Medicine in New York. “We already use other lab tests that have less evidence supporting their use than this, and checking BNP is a simple lab test.”
The results showed that monitoring identified AF in 5% of the control patients and in 14% of those who underwent extended monitoring, a statistically significant difference (Lancet Neurol. 2017 Apr;16[4]:282-90).
Dr. Wachter and his colleagues had baseline BNP measurements for 373 of the patients and analyzed the prevalence of AF based on these levels. The patients averaged 72 years old. Among those with BNP values of at least 100 pg/mL, prolonged arrhythmia monitoring found AF in 33%, while briefer, conventional monitoring found AF in about 4%. This meant that for every three patients receiving prolonged arrhythmia scrutiny in this subgroup, the assessment found one patient with AF. But among patients with a serum BNP of less than 100 pg/mL, prolonged arrhythmia assessment yielded a 10% rate of AF detection, compared with about 4% in the controls, meaning that for every 18 patients screened prolonged Holter monitoring detected one additional case of AF, he reported.
This relationship presumably exists because higher BNP levels identify patients with structural heart abnormalities, a subgroup at increased risk for AF, Dr. Wachter explained. He cautioned that, because this was a post hoc analysis, he would like to see the findings confirmed in prospective studies.
Dr. Wachter has been a speaker on behalf of Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo and has received research support from Boehringer Ingelheim. The FIND-AFRANDOMISED trial received funding from Boehringer Ingelheim.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
HOUSTON – Following an embolic stroke of undetermined source, a patient’s serum level of brain natriuretic peptide may identify patients at high risk of having covert atrial fibrillation that warrants assessment by prolonged arrhythmia monitoring, based on a post hoc analysis of data collected from 373 patients.
The analysis showed that in older patients with a recent embolic stroke of undetermined source (ESUS) who had a serum brain natriuretic peptide (BNP) level of 100 pg/mL or greater, the prevalence of atrial fibrillation (AF) detected by up to 30 days of Holter monitoring was 33%. In contrast the AF prevalence was 4% among similar ESUS patients with a lower BNP level, Rolf Wachter, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“This evidence is pretty compelling,” commented Hooman Kamel, MD, a neurologist at Weill Cornell Medicine in New York. “We already use other lab tests that have less evidence supporting their use than this, and checking BNP is a simple lab test.”
The results showed that monitoring identified AF in 5% of the control patients and in 14% of those who underwent extended monitoring, a statistically significant difference (Lancet Neurol. 2017 Apr;16[4]:282-90).
Dr. Wachter and his colleagues had baseline BNP measurements for 373 of the patients and analyzed the prevalence of AF based on these levels. The patients averaged 72 years old. Among those with BNP values of at least 100 pg/mL, prolonged arrhythmia monitoring found AF in 33%, while briefer, conventional monitoring found AF in about 4%. This meant that for every three patients receiving prolonged arrhythmia scrutiny in this subgroup, the assessment found one patient with AF. But among patients with a serum BNP of less than 100 pg/mL, prolonged arrhythmia assessment yielded a 10% rate of AF detection, compared with about 4% in the controls, meaning that for every 18 patients screened prolonged Holter monitoring detected one additional case of AF, he reported.
This relationship presumably exists because higher BNP levels identify patients with structural heart abnormalities, a subgroup at increased risk for AF, Dr. Wachter explained. He cautioned that, because this was a post hoc analysis, he would like to see the findings confirmed in prospective studies.
Dr. Wachter has been a speaker on behalf of Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo and has received research support from Boehringer Ingelheim. The FIND-AFRANDOMISED trial received funding from Boehringer Ingelheim.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE INTERNATIONAL STROKE CONFERENCE
Key clinical point:
Major finding: Following ESUS, patients with a BNP of 100 pg/mL or greater had a 33% prevalence of AF.
Data source: A post hoc analysis of 373 German patients enrolled in the FIND-AFRANDOMISED trial.
Disclosures: Dr. Wachter has been a speaker on behalf of Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Daiichi Sankyo and has received research support from Boehringer Ingelheim. The FIND-AFRANDOMISED trial received funding from Boehringer Ingelheim.