User login
Suspecting Pituitary Disorders: “What's Next?”
Reinforcing mesh at ostomy site prevents parastomal hernia
For patients undergoing elective permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia, according to a report published in the April issue of Annals of Surgery.
The incidence of parastomal hernia is expected to rise because of the increasing number of cancer patients surviving with a colostomy, and the rising number of obese patients who have increased tension on the abdominal wall because of their elevated intra-abdominal pressure and larger abdominal radius. Researchers in the Netherlands performed a prospective randomized study, the PREVENT trial, to assess whether augmenting the abdominal wall at the ostomy site, using a lightweight mesh, would be safe, feasible, and effective at preventing parastomal hernia. They reported their findings after 1 year of follow-up; the study will continue until longer-term results are available at 5 years.
In the intervention group, a retromuscular space was created to accommodate the mesh by dissecting the muscle from the posterior fascia or peritoneum to the lateral border via a median laparotomy. An incision was made in the center of the mesh to allow passage of the colon, and the mesh was placed on the posterior rectus sheath and anchored laterally with two absorbable sutures. “On the medial side, the mesh was incorporated in the running suture closing the fascia, thus preventing contact between the mesh and the viscera,” the investigators said (Ann Surg. 2017;265:663-9).
The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group, a significant difference. There were no mesh-related complications such as infection, strictures, or adhesions. “The majority of the parastomal hernias that required surgical repair were in the control group, which supports the concept that if a hernia develops in a patient with mesh, it is smaller and less likely to cause complaints,” Dr. Brandsma and his associates said.
Significantly fewer patients in the mesh group (9%) than in the control group (21%) reported stoma-related complaints such as pain, leakage, and skin problems. Scores on measures of quality of life and pain severity were no different between the two study groups.
“Prophylactic augmentation of the abdominal wall with a retromuscular polypropylene mesh at the ostomy site is a safe and feasible procedure with no adverse events. It significantly reduces the incidence of parastomal hernia,” the investigators concluded.
This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
For patients undergoing elective permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia, according to a report published in the April issue of Annals of Surgery.
The incidence of parastomal hernia is expected to rise because of the increasing number of cancer patients surviving with a colostomy, and the rising number of obese patients who have increased tension on the abdominal wall because of their elevated intra-abdominal pressure and larger abdominal radius. Researchers in the Netherlands performed a prospective randomized study, the PREVENT trial, to assess whether augmenting the abdominal wall at the ostomy site, using a lightweight mesh, would be safe, feasible, and effective at preventing parastomal hernia. They reported their findings after 1 year of follow-up; the study will continue until longer-term results are available at 5 years.
In the intervention group, a retromuscular space was created to accommodate the mesh by dissecting the muscle from the posterior fascia or peritoneum to the lateral border via a median laparotomy. An incision was made in the center of the mesh to allow passage of the colon, and the mesh was placed on the posterior rectus sheath and anchored laterally with two absorbable sutures. “On the medial side, the mesh was incorporated in the running suture closing the fascia, thus preventing contact between the mesh and the viscera,” the investigators said (Ann Surg. 2017;265:663-9).
The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group, a significant difference. There were no mesh-related complications such as infection, strictures, or adhesions. “The majority of the parastomal hernias that required surgical repair were in the control group, which supports the concept that if a hernia develops in a patient with mesh, it is smaller and less likely to cause complaints,” Dr. Brandsma and his associates said.
Significantly fewer patients in the mesh group (9%) than in the control group (21%) reported stoma-related complaints such as pain, leakage, and skin problems. Scores on measures of quality of life and pain severity were no different between the two study groups.
“Prophylactic augmentation of the abdominal wall with a retromuscular polypropylene mesh at the ostomy site is a safe and feasible procedure with no adverse events. It significantly reduces the incidence of parastomal hernia,” the investigators concluded.
This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
For patients undergoing elective permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia, according to a report published in the April issue of Annals of Surgery.
The incidence of parastomal hernia is expected to rise because of the increasing number of cancer patients surviving with a colostomy, and the rising number of obese patients who have increased tension on the abdominal wall because of their elevated intra-abdominal pressure and larger abdominal radius. Researchers in the Netherlands performed a prospective randomized study, the PREVENT trial, to assess whether augmenting the abdominal wall at the ostomy site, using a lightweight mesh, would be safe, feasible, and effective at preventing parastomal hernia. They reported their findings after 1 year of follow-up; the study will continue until longer-term results are available at 5 years.
In the intervention group, a retromuscular space was created to accommodate the mesh by dissecting the muscle from the posterior fascia or peritoneum to the lateral border via a median laparotomy. An incision was made in the center of the mesh to allow passage of the colon, and the mesh was placed on the posterior rectus sheath and anchored laterally with two absorbable sutures. “On the medial side, the mesh was incorporated in the running suture closing the fascia, thus preventing contact between the mesh and the viscera,” the investigators said (Ann Surg. 2017;265:663-9).
The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group, a significant difference. There were no mesh-related complications such as infection, strictures, or adhesions. “The majority of the parastomal hernias that required surgical repair were in the control group, which supports the concept that if a hernia develops in a patient with mesh, it is smaller and less likely to cause complaints,” Dr. Brandsma and his associates said.
Significantly fewer patients in the mesh group (9%) than in the control group (21%) reported stoma-related complaints such as pain, leakage, and skin problems. Scores on measures of quality of life and pain severity were no different between the two study groups.
“Prophylactic augmentation of the abdominal wall with a retromuscular polypropylene mesh at the ostomy site is a safe and feasible procedure with no adverse events. It significantly reduces the incidence of parastomal hernia,” the investigators concluded.
This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
FROM THE ANNALS OF SURGERY
Key clinical point: For patients undergoing permanent colostomy, prophylactic augmentation of the abdominal wall using mesh at the ostomy site prevents the development of parastomal hernia.
Major finding: The primary end point – the incidence of parastomal hernia at 1 year – occurred in 3 patients (4.5%) in the intervention group and 16 (24.2%) in the control group.
Data source: A prospective, multicenter, randomized cohort study comparing prophylactic mesh against standard care in 133 adults undergoing elective end-colostomy during a 3-year period.
Disclosures: This study was supported by Canisius Wilhelmina Hospital’s surgery research fund, the Netherlands Organization for Health Research and Development, and Covidien/Medtronic. Dr. Brandsma and his associates reported having no relevant financial disclosures.
Anti-TNF agents show clinical benefit in refractory sarcoidosis
FROM SEMINARS IN ARTHRITIS & RHEUMATISM
Around two-thirds of patients with severe or refractory sarcoidosis show a significant clinical response to tumor necrosis factor (TNF) antagonists, according to findings from a retrospective, multicenter cohort study.
Biologic agents targeting TNF, such as etanercept, infliximab, and adalimumab, have been introduced as a third-line option for patients with disease that is refractory to other treatments. However, Yvan Jamilloux, MD, of the Hospices Civils de Lyon (France) and his coauthors reported that there are still insufficient data available on efficacy and safety of these drugs in the context of sarcoidosis.
Dr. Jamilloux and his colleagues analyzed data from 132 sarcoidosis patients who received TNF antagonists, 122 (92%) of whom had severe sarcoidosis (Semin Arthritis Rheum. 2017 Mar 8. doi: 10.1016/j.semarthrit.2017.03.005).
Overall, 64% of patients showed clinical improvements in response to TNF antagonists; 18% had a complete response, and 46% had a partial response. However, 33 (25%) patients showed no change, and 14 (11%) had continued disease progression despite treatment with TNF antagonists. In another 16 patients who received a second TNF antagonist, 10 (63%) had a complete or partial clinical response. The investigators could find no differences in response between anti-TNF agents or between monotherapy and a combination with an immunosuppressant.
Pulmonary involvement was associated with a significantly lower clinical response, but none of the other factors examined in a multivariate analysis (sex, age, ethnicity, organ involvement, disease duration, steroid dosage, or prior immunosuppressant use) distinguished responders and nonresponders.
The authors noted that these response rates were lower than those seen in the literature and suggested this may be attributable to the multicenter design, more patients with longer-lasting and more refractory disease, and longer times under biologic therapy (median 12 months).
The researchers reported significant improvements in central nervous system, peripheral nervous system, heart, skin, and upper respiratory tract involvements based on declines in Extrapulmonary Physician Organ Severity Tool (ePOST) scores. There were also improvements in the eye, muscle, and lung, but these were not statistically significant.
TNF-antagonist therapy was associated with a high rate of adverse events. Around half of all patients (52%) experienced adverse events, such as pneumonia, urinary tract infections, bacterial sepsis, and herpes zoster. In 31 patients (23%), these led to treatment cessation.
Nine patients also had severe allergic reactions, four had paradoxical granulomatous reactions, three developed neutralizing antibodies against anti-TNF agents, two patients had demyelinating lesions, and one had a serum sickness-like reaction. All of these events led to discontinuation.
Overall, 128 (97%) of the patients in the study had received corticosteroids as first-line therapy, and 125 (95%) had received at least one second-line immunosuppressive drug over a median duration of 16 months. Most were treated with infliximab (91%) as the first-line TNF antagonist, followed by adalimumab (6%), etanercept (2%), and certolizumab pegol (1%).
Treatment with TNF antagonists was associated with significant reductions in corticosteroid use; the mean daily prednisone dose decreased from 23 mg/day to 11 mg/day over the median 20.5-month follow-up. This was seen even in the 33 patients who showed no change in their disease course after TNF-antagonist therapy.
No conflicts of interest were declared.
This uncontrolled, unblinded retrospective observational study reports the outcomes of anti-TNF therapy in a heterogenous group of refractory sarcoid patients, with only 12% of the severe sarcoidosis population studied having the indication for treatment based on lung involvement. Further, it is notable that the patients with primarily pulmonary involvement had a poorer response to anti-TNF therapy. Over half of the patients had an adverse event related to the treatment, with nearly a quarter having to discontinue therapy. Given the limitations of this type of study, the low numbers of pulmonary sarcoid patients included, the lack of an efficacy signal in pulmonary sarcoid, and the high rate of serious adverse events – the role of anti-TNF agents for pulmonary sarcoid remains unclear and limited. However
This uncontrolled, unblinded retrospective observational study reports the outcomes of anti-TNF therapy in a heterogenous group of refractory sarcoid patients, with only 12% of the severe sarcoidosis population studied having the indication for treatment based on lung involvement. Further, it is notable that the patients with primarily pulmonary involvement had a poorer response to anti-TNF therapy. Over half of the patients had an adverse event related to the treatment, with nearly a quarter having to discontinue therapy. Given the limitations of this type of study, the low numbers of pulmonary sarcoid patients included, the lack of an efficacy signal in pulmonary sarcoid, and the high rate of serious adverse events – the role of anti-TNF agents for pulmonary sarcoid remains unclear and limited. However
This uncontrolled, unblinded retrospective observational study reports the outcomes of anti-TNF therapy in a heterogenous group of refractory sarcoid patients, with only 12% of the severe sarcoidosis population studied having the indication for treatment based on lung involvement. Further, it is notable that the patients with primarily pulmonary involvement had a poorer response to anti-TNF therapy. Over half of the patients had an adverse event related to the treatment, with nearly a quarter having to discontinue therapy. Given the limitations of this type of study, the low numbers of pulmonary sarcoid patients included, the lack of an efficacy signal in pulmonary sarcoid, and the high rate of serious adverse events – the role of anti-TNF agents for pulmonary sarcoid remains unclear and limited. However
FROM SEMINARS IN ARTHRITIS & RHEUMATISM
Around two-thirds of patients with severe or refractory sarcoidosis show a significant clinical response to tumor necrosis factor (TNF) antagonists, according to findings from a retrospective, multicenter cohort study.
Biologic agents targeting TNF, such as etanercept, infliximab, and adalimumab, have been introduced as a third-line option for patients with disease that is refractory to other treatments. However, Yvan Jamilloux, MD, of the Hospices Civils de Lyon (France) and his coauthors reported that there are still insufficient data available on efficacy and safety of these drugs in the context of sarcoidosis.
Dr. Jamilloux and his colleagues analyzed data from 132 sarcoidosis patients who received TNF antagonists, 122 (92%) of whom had severe sarcoidosis (Semin Arthritis Rheum. 2017 Mar 8. doi: 10.1016/j.semarthrit.2017.03.005).
Overall, 64% of patients showed clinical improvements in response to TNF antagonists; 18% had a complete response, and 46% had a partial response. However, 33 (25%) patients showed no change, and 14 (11%) had continued disease progression despite treatment with TNF antagonists. In another 16 patients who received a second TNF antagonist, 10 (63%) had a complete or partial clinical response. The investigators could find no differences in response between anti-TNF agents or between monotherapy and a combination with an immunosuppressant.
Pulmonary involvement was associated with a significantly lower clinical response, but none of the other factors examined in a multivariate analysis (sex, age, ethnicity, organ involvement, disease duration, steroid dosage, or prior immunosuppressant use) distinguished responders and nonresponders.
The authors noted that these response rates were lower than those seen in the literature and suggested this may be attributable to the multicenter design, more patients with longer-lasting and more refractory disease, and longer times under biologic therapy (median 12 months).
The researchers reported significant improvements in central nervous system, peripheral nervous system, heart, skin, and upper respiratory tract involvements based on declines in Extrapulmonary Physician Organ Severity Tool (ePOST) scores. There were also improvements in the eye, muscle, and lung, but these were not statistically significant.
TNF-antagonist therapy was associated with a high rate of adverse events. Around half of all patients (52%) experienced adverse events, such as pneumonia, urinary tract infections, bacterial sepsis, and herpes zoster. In 31 patients (23%), these led to treatment cessation.
Nine patients also had severe allergic reactions, four had paradoxical granulomatous reactions, three developed neutralizing antibodies against anti-TNF agents, two patients had demyelinating lesions, and one had a serum sickness-like reaction. All of these events led to discontinuation.
Overall, 128 (97%) of the patients in the study had received corticosteroids as first-line therapy, and 125 (95%) had received at least one second-line immunosuppressive drug over a median duration of 16 months. Most were treated with infliximab (91%) as the first-line TNF antagonist, followed by adalimumab (6%), etanercept (2%), and certolizumab pegol (1%).
Treatment with TNF antagonists was associated with significant reductions in corticosteroid use; the mean daily prednisone dose decreased from 23 mg/day to 11 mg/day over the median 20.5-month follow-up. This was seen even in the 33 patients who showed no change in their disease course after TNF-antagonist therapy.
No conflicts of interest were declared.
FROM SEMINARS IN ARTHRITIS & RHEUMATISM
Around two-thirds of patients with severe or refractory sarcoidosis show a significant clinical response to tumor necrosis factor (TNF) antagonists, according to findings from a retrospective, multicenter cohort study.
Biologic agents targeting TNF, such as etanercept, infliximab, and adalimumab, have been introduced as a third-line option for patients with disease that is refractory to other treatments. However, Yvan Jamilloux, MD, of the Hospices Civils de Lyon (France) and his coauthors reported that there are still insufficient data available on efficacy and safety of these drugs in the context of sarcoidosis.
Dr. Jamilloux and his colleagues analyzed data from 132 sarcoidosis patients who received TNF antagonists, 122 (92%) of whom had severe sarcoidosis (Semin Arthritis Rheum. 2017 Mar 8. doi: 10.1016/j.semarthrit.2017.03.005).
Overall, 64% of patients showed clinical improvements in response to TNF antagonists; 18% had a complete response, and 46% had a partial response. However, 33 (25%) patients showed no change, and 14 (11%) had continued disease progression despite treatment with TNF antagonists. In another 16 patients who received a second TNF antagonist, 10 (63%) had a complete or partial clinical response. The investigators could find no differences in response between anti-TNF agents or between monotherapy and a combination with an immunosuppressant.
Pulmonary involvement was associated with a significantly lower clinical response, but none of the other factors examined in a multivariate analysis (sex, age, ethnicity, organ involvement, disease duration, steroid dosage, or prior immunosuppressant use) distinguished responders and nonresponders.
The authors noted that these response rates were lower than those seen in the literature and suggested this may be attributable to the multicenter design, more patients with longer-lasting and more refractory disease, and longer times under biologic therapy (median 12 months).
The researchers reported significant improvements in central nervous system, peripheral nervous system, heart, skin, and upper respiratory tract involvements based on declines in Extrapulmonary Physician Organ Severity Tool (ePOST) scores. There were also improvements in the eye, muscle, and lung, but these were not statistically significant.
TNF-antagonist therapy was associated with a high rate of adverse events. Around half of all patients (52%) experienced adverse events, such as pneumonia, urinary tract infections, bacterial sepsis, and herpes zoster. In 31 patients (23%), these led to treatment cessation.
Nine patients also had severe allergic reactions, four had paradoxical granulomatous reactions, three developed neutralizing antibodies against anti-TNF agents, two patients had demyelinating lesions, and one had a serum sickness-like reaction. All of these events led to discontinuation.
Overall, 128 (97%) of the patients in the study had received corticosteroids as first-line therapy, and 125 (95%) had received at least one second-line immunosuppressive drug over a median duration of 16 months. Most were treated with infliximab (91%) as the first-line TNF antagonist, followed by adalimumab (6%), etanercept (2%), and certolizumab pegol (1%).
Treatment with TNF antagonists was associated with significant reductions in corticosteroid use; the mean daily prednisone dose decreased from 23 mg/day to 11 mg/day over the median 20.5-month follow-up. This was seen even in the 33 patients who showed no change in their disease course after TNF-antagonist therapy.
No conflicts of interest were declared.
Key clinical point:
Major finding: A total of 18% had a complete response, and 46% had a partial response, to TNF antagonists.
Data source: A retrospective, multicenter study in 132 sarcoidosis patients who received TNF antagonists.
Disclosures: No conflicts of interest were declared.
HIV vaccine could prevent 30 million cases by 2035
Global cases of HIV from 2015 to 2035 would be reduced by over 50% if the Joint United Nations Program on HIV/AIDS 95/95/95 target is met and a moderately effective HIV vaccine is introduced by 2020, according to new research published in Proceedings of the National Academy of Sciences.
A custom model based on current rates of diagnosis and treatment in 127 countries predicts that a total of 49 million new cases of HIV would occur globally from 2015 to 2035, investigators said. Achieving the UNAIDS goal of 95% disease diagnosis, 95% antiretroviral coverage, and 95% viral suppression by 2030 would avert 25 million cases by 2035. Achieving the more modest 90/90/90 target would avert 22 million cases within the same time period.
“Recent results from the HVTN 100 vaccine trial have bolstered optimism for the development and deployment of an HIV vaccine in the near term,” the investigators said. “HIV vaccination would enable a strategic shift from reactive to proactive control, as suggested by our finding that an HIV vaccine with even moderate efficacy rolled out in 2020 could avert 17 million new infections by 2035 relative to expectations under status quo interventions.”
Find the full study in PNAS (doi: 10.1073/pnas.1620788114)
lfranki@frontlinemedcom.com
On Twitter @IDPractitioner
Global cases of HIV from 2015 to 2035 would be reduced by over 50% if the Joint United Nations Program on HIV/AIDS 95/95/95 target is met and a moderately effective HIV vaccine is introduced by 2020, according to new research published in Proceedings of the National Academy of Sciences.
A custom model based on current rates of diagnosis and treatment in 127 countries predicts that a total of 49 million new cases of HIV would occur globally from 2015 to 2035, investigators said. Achieving the UNAIDS goal of 95% disease diagnosis, 95% antiretroviral coverage, and 95% viral suppression by 2030 would avert 25 million cases by 2035. Achieving the more modest 90/90/90 target would avert 22 million cases within the same time period.
“Recent results from the HVTN 100 vaccine trial have bolstered optimism for the development and deployment of an HIV vaccine in the near term,” the investigators said. “HIV vaccination would enable a strategic shift from reactive to proactive control, as suggested by our finding that an HIV vaccine with even moderate efficacy rolled out in 2020 could avert 17 million new infections by 2035 relative to expectations under status quo interventions.”
Find the full study in PNAS (doi: 10.1073/pnas.1620788114)
lfranki@frontlinemedcom.com
On Twitter @IDPractitioner
Global cases of HIV from 2015 to 2035 would be reduced by over 50% if the Joint United Nations Program on HIV/AIDS 95/95/95 target is met and a moderately effective HIV vaccine is introduced by 2020, according to new research published in Proceedings of the National Academy of Sciences.
A custom model based on current rates of diagnosis and treatment in 127 countries predicts that a total of 49 million new cases of HIV would occur globally from 2015 to 2035, investigators said. Achieving the UNAIDS goal of 95% disease diagnosis, 95% antiretroviral coverage, and 95% viral suppression by 2030 would avert 25 million cases by 2035. Achieving the more modest 90/90/90 target would avert 22 million cases within the same time period.
“Recent results from the HVTN 100 vaccine trial have bolstered optimism for the development and deployment of an HIV vaccine in the near term,” the investigators said. “HIV vaccination would enable a strategic shift from reactive to proactive control, as suggested by our finding that an HIV vaccine with even moderate efficacy rolled out in 2020 could avert 17 million new infections by 2035 relative to expectations under status quo interventions.”
Find the full study in PNAS (doi: 10.1073/pnas.1620788114)
lfranki@frontlinemedcom.com
On Twitter @IDPractitioner
Reversible Cutaneous Side Effects of Vismodegib Treatment
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
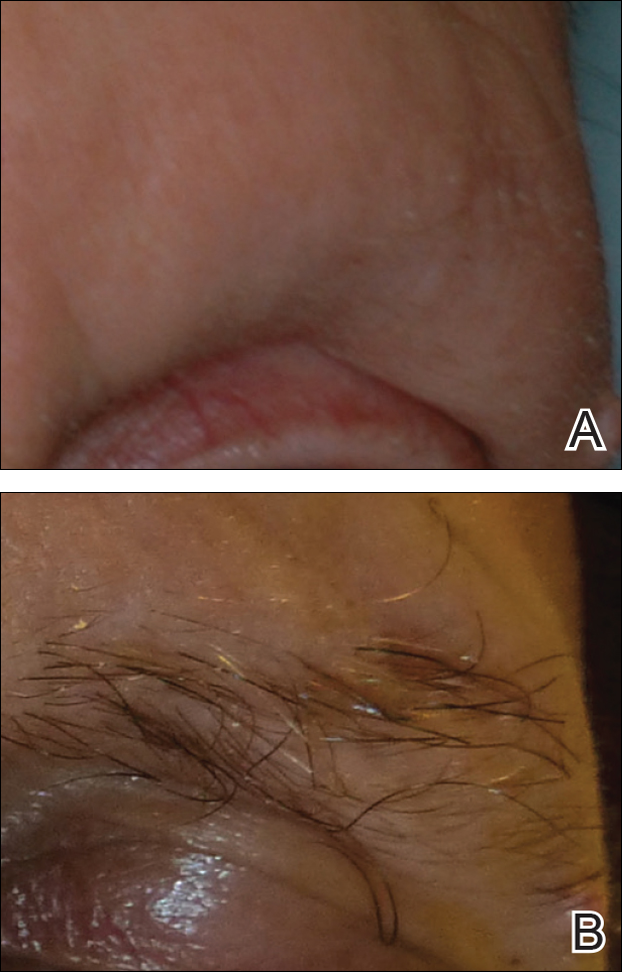
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).
A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).

A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).

A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
Practice Points
- Hair loss is a common late side effect of vismodegib usage and is reversible, but regrowth takes many months.
- Mild folliculitis that resolves spontaneously has been observed in patients using vismodegib.
- Dermal hypersensitivity has been observed in patients on vismodegib, though the exact frequency of this type of dermatitis is not known.
Real-world EGFR and ALK testing of NSCLC falls short
ORLANDO – A large proportion of patients with advanced non–small cell lung cancer (NSCLC) are not being tested for tumor associated–epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations according to national guidelines. This situation may be leading to suboptimal treatment, a large retrospective cohort study suggests.
Guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend testing before first-line therapy for all treatment-eligible patients with nonsquamous histology and for those patients with squamous histology who are nonsmokers or who have mixed cell types or small tumor samples. Additionally, the guidelines recommend that results be made available within 2 weeks of the lab’s receipt of the sample so that they can be used to inform treatment decisions.
Overall, 22% of patients with nonsquamous tumors had no evidence of EGFR and ALK testing in their records. The large majority of patients with squamous tumors did not have any evidence of testing either, and it was unclear how well testing corresponded with the criteria.
In roughly a third of cases in which testing was done, the time between diagnosis of advanced disease and availability of test results exceeded 4 weeks. Among patients with positive test results, those whose results came back after the start of first-line therapy, were about half as likely to appropriately receive a therapy that targeted their tumor’s molecular aberration.
“We observed variation in adherence to [the American Society of Clinical Oncology] and [the National Comprehensive Cancer Network] guidelines around biomarker testing in advanced NSCLC, and we saw significant variation in testing in the squamous population and the nonsquamous population across practices,” presenting author Jay Rughani, manager of Life Sciences at Flatiron Health, New York, commented in an interview. Observed delays in availability of test results were mainly driven by delays between diagnosis and submission of samples to the lab for testing.
“There may be an opportunity to educate the oncology community around testing, certainly for all nonsquamous patients, because this is a case where they all should have been tested,” he said. “And there is also an opportunity to ensure testing of the appropriate squamous cell patients, while discouraging the testing of the majority who aren’t candidates, so there may be an opportunity for education around smoking status.”
Slow uptake of the national guidelines is unlikely to explain the observed variations in testing, according to Mr. Rughani. “Since we looked at patients diagnosed after Jan. 1, 2014, our impression was that the guidelines were sort of disseminated enough and widely known enough by that point, particularly around EGFR and ALK, that we wouldn’t expect any lag there. If we had done this for PD-L1 [programmed death ligand 1] testing, perhaps we might have thought about some lag in adoption.”
The impact of variations in testing and receipt of inappropriate initial therapy on clinical outcomes is yet to be determined. “As a follow-on, some of the work we have been doing is trying to understand, for these separate cohorts of patients, depending on what they received in the front line, what their overall survival was and what their surrogate endpoints were,” Mr. Rughani concluded.
Study details
For the study, the investigators identified 16,316 patients with advanced NSCLC from 206 community clinics across the United States participating in the Flatiron Network. All patients were treated between 2014 and 2016.
Cross-checking of the total Flatiron population against the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results databases suggested that it is a good national representation, according to Mr. Rughani.
A record review showed that the rate of EGFR and ALK testing among study patients ranged widely across clinics, from 0% to 100% for both the nonsquamous cases and the squamous cases, according to results reported in a poster session. The median was 79% for the former and 16% for the latter.
Overall, 22% of the nonsquamous cohort and 79% of the squamous cohort did not have any evidence of testing in their records. For the latter, a sampling of records was unable to verify whether testing was appropriately matched to eligibility criteria.
When testing was performed, 35% of EGFR test results and 37% of ALK test results were not available to the treating clinician until more than 4 weeks after the date of the advanced cancer diagnosis.
“The delays were mostly attributed to nonlab factors. When we isolated the time that the lab took to turn it around, it was under 2 weeks for the vast majority of patients,” Mr. Rughani reported. Possible nonlab culprit factors include clinic work flows, insurance-related issues, and families’ and patients’ hesitancy to be tested, he said.
Delays in receipt of positive test results appeared to influence choice of first-line therapy. Among patients in whom these results were available before first-line therapy, 80% of those found to have an EGFR-mutated tumor received an EGFR–tyrosine kinase inhibitor, and 77% of those found to have ALK-rearranged tumors received an ALK inhibitor.
In sharp contrast, among patients in whom positive test results did not become available until after the start of first-line therapy, respective values were just 43% and 42%.
“Anecdotally, we saw that some patients would go on to Avastin [bevacizumab] in the front line when the results were delayed, and then, ultimately, they would have the opportunity to receive an EGFR[–tyrosine kinase inhibitor] or something like that in later lines,” commented Mr. Rughani. “So, that impacted treatment decisions there.”
Mr. Rughani disclosed stock and other ownership interests in Flatiron Health.
ORLANDO – A large proportion of patients with advanced non–small cell lung cancer (NSCLC) are not being tested for tumor associated–epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations according to national guidelines. This situation may be leading to suboptimal treatment, a large retrospective cohort study suggests.
Guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend testing before first-line therapy for all treatment-eligible patients with nonsquamous histology and for those patients with squamous histology who are nonsmokers or who have mixed cell types or small tumor samples. Additionally, the guidelines recommend that results be made available within 2 weeks of the lab’s receipt of the sample so that they can be used to inform treatment decisions.
Overall, 22% of patients with nonsquamous tumors had no evidence of EGFR and ALK testing in their records. The large majority of patients with squamous tumors did not have any evidence of testing either, and it was unclear how well testing corresponded with the criteria.
In roughly a third of cases in which testing was done, the time between diagnosis of advanced disease and availability of test results exceeded 4 weeks. Among patients with positive test results, those whose results came back after the start of first-line therapy, were about half as likely to appropriately receive a therapy that targeted their tumor’s molecular aberration.
“We observed variation in adherence to [the American Society of Clinical Oncology] and [the National Comprehensive Cancer Network] guidelines around biomarker testing in advanced NSCLC, and we saw significant variation in testing in the squamous population and the nonsquamous population across practices,” presenting author Jay Rughani, manager of Life Sciences at Flatiron Health, New York, commented in an interview. Observed delays in availability of test results were mainly driven by delays between diagnosis and submission of samples to the lab for testing.
“There may be an opportunity to educate the oncology community around testing, certainly for all nonsquamous patients, because this is a case where they all should have been tested,” he said. “And there is also an opportunity to ensure testing of the appropriate squamous cell patients, while discouraging the testing of the majority who aren’t candidates, so there may be an opportunity for education around smoking status.”
Slow uptake of the national guidelines is unlikely to explain the observed variations in testing, according to Mr. Rughani. “Since we looked at patients diagnosed after Jan. 1, 2014, our impression was that the guidelines were sort of disseminated enough and widely known enough by that point, particularly around EGFR and ALK, that we wouldn’t expect any lag there. If we had done this for PD-L1 [programmed death ligand 1] testing, perhaps we might have thought about some lag in adoption.”
The impact of variations in testing and receipt of inappropriate initial therapy on clinical outcomes is yet to be determined. “As a follow-on, some of the work we have been doing is trying to understand, for these separate cohorts of patients, depending on what they received in the front line, what their overall survival was and what their surrogate endpoints were,” Mr. Rughani concluded.
Study details
For the study, the investigators identified 16,316 patients with advanced NSCLC from 206 community clinics across the United States participating in the Flatiron Network. All patients were treated between 2014 and 2016.
Cross-checking of the total Flatiron population against the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results databases suggested that it is a good national representation, according to Mr. Rughani.
A record review showed that the rate of EGFR and ALK testing among study patients ranged widely across clinics, from 0% to 100% for both the nonsquamous cases and the squamous cases, according to results reported in a poster session. The median was 79% for the former and 16% for the latter.
Overall, 22% of the nonsquamous cohort and 79% of the squamous cohort did not have any evidence of testing in their records. For the latter, a sampling of records was unable to verify whether testing was appropriately matched to eligibility criteria.
When testing was performed, 35% of EGFR test results and 37% of ALK test results were not available to the treating clinician until more than 4 weeks after the date of the advanced cancer diagnosis.
“The delays were mostly attributed to nonlab factors. When we isolated the time that the lab took to turn it around, it was under 2 weeks for the vast majority of patients,” Mr. Rughani reported. Possible nonlab culprit factors include clinic work flows, insurance-related issues, and families’ and patients’ hesitancy to be tested, he said.
Delays in receipt of positive test results appeared to influence choice of first-line therapy. Among patients in whom these results were available before first-line therapy, 80% of those found to have an EGFR-mutated tumor received an EGFR–tyrosine kinase inhibitor, and 77% of those found to have ALK-rearranged tumors received an ALK inhibitor.
In sharp contrast, among patients in whom positive test results did not become available until after the start of first-line therapy, respective values were just 43% and 42%.
“Anecdotally, we saw that some patients would go on to Avastin [bevacizumab] in the front line when the results were delayed, and then, ultimately, they would have the opportunity to receive an EGFR[–tyrosine kinase inhibitor] or something like that in later lines,” commented Mr. Rughani. “So, that impacted treatment decisions there.”
Mr. Rughani disclosed stock and other ownership interests in Flatiron Health.
ORLANDO – A large proportion of patients with advanced non–small cell lung cancer (NSCLC) are not being tested for tumor associated–epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) alterations according to national guidelines. This situation may be leading to suboptimal treatment, a large retrospective cohort study suggests.
Guidelines from the American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend testing before first-line therapy for all treatment-eligible patients with nonsquamous histology and for those patients with squamous histology who are nonsmokers or who have mixed cell types or small tumor samples. Additionally, the guidelines recommend that results be made available within 2 weeks of the lab’s receipt of the sample so that they can be used to inform treatment decisions.
Overall, 22% of patients with nonsquamous tumors had no evidence of EGFR and ALK testing in their records. The large majority of patients with squamous tumors did not have any evidence of testing either, and it was unclear how well testing corresponded with the criteria.
In roughly a third of cases in which testing was done, the time between diagnosis of advanced disease and availability of test results exceeded 4 weeks. Among patients with positive test results, those whose results came back after the start of first-line therapy, were about half as likely to appropriately receive a therapy that targeted their tumor’s molecular aberration.
“We observed variation in adherence to [the American Society of Clinical Oncology] and [the National Comprehensive Cancer Network] guidelines around biomarker testing in advanced NSCLC, and we saw significant variation in testing in the squamous population and the nonsquamous population across practices,” presenting author Jay Rughani, manager of Life Sciences at Flatiron Health, New York, commented in an interview. Observed delays in availability of test results were mainly driven by delays between diagnosis and submission of samples to the lab for testing.
“There may be an opportunity to educate the oncology community around testing, certainly for all nonsquamous patients, because this is a case where they all should have been tested,” he said. “And there is also an opportunity to ensure testing of the appropriate squamous cell patients, while discouraging the testing of the majority who aren’t candidates, so there may be an opportunity for education around smoking status.”
Slow uptake of the national guidelines is unlikely to explain the observed variations in testing, according to Mr. Rughani. “Since we looked at patients diagnosed after Jan. 1, 2014, our impression was that the guidelines were sort of disseminated enough and widely known enough by that point, particularly around EGFR and ALK, that we wouldn’t expect any lag there. If we had done this for PD-L1 [programmed death ligand 1] testing, perhaps we might have thought about some lag in adoption.”
The impact of variations in testing and receipt of inappropriate initial therapy on clinical outcomes is yet to be determined. “As a follow-on, some of the work we have been doing is trying to understand, for these separate cohorts of patients, depending on what they received in the front line, what their overall survival was and what their surrogate endpoints were,” Mr. Rughani concluded.
Study details
For the study, the investigators identified 16,316 patients with advanced NSCLC from 206 community clinics across the United States participating in the Flatiron Network. All patients were treated between 2014 and 2016.
Cross-checking of the total Flatiron population against the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results databases suggested that it is a good national representation, according to Mr. Rughani.
A record review showed that the rate of EGFR and ALK testing among study patients ranged widely across clinics, from 0% to 100% for both the nonsquamous cases and the squamous cases, according to results reported in a poster session. The median was 79% for the former and 16% for the latter.
Overall, 22% of the nonsquamous cohort and 79% of the squamous cohort did not have any evidence of testing in their records. For the latter, a sampling of records was unable to verify whether testing was appropriately matched to eligibility criteria.
When testing was performed, 35% of EGFR test results and 37% of ALK test results were not available to the treating clinician until more than 4 weeks after the date of the advanced cancer diagnosis.
“The delays were mostly attributed to nonlab factors. When we isolated the time that the lab took to turn it around, it was under 2 weeks for the vast majority of patients,” Mr. Rughani reported. Possible nonlab culprit factors include clinic work flows, insurance-related issues, and families’ and patients’ hesitancy to be tested, he said.
Delays in receipt of positive test results appeared to influence choice of first-line therapy. Among patients in whom these results were available before first-line therapy, 80% of those found to have an EGFR-mutated tumor received an EGFR–tyrosine kinase inhibitor, and 77% of those found to have ALK-rearranged tumors received an ALK inhibitor.
In sharp contrast, among patients in whom positive test results did not become available until after the start of first-line therapy, respective values were just 43% and 42%.
“Anecdotally, we saw that some patients would go on to Avastin [bevacizumab] in the front line when the results were delayed, and then, ultimately, they would have the opportunity to receive an EGFR[–tyrosine kinase inhibitor] or something like that in later lines,” commented Mr. Rughani. “So, that impacted treatment decisions there.”
Mr. Rughani disclosed stock and other ownership interests in Flatiron Health.
Key clinical point:
Major finding: Overall, 22% of patients with nonsquamous advanced NSCLC had no evidence of EGFR and ALK tumor testing in their records.
Data source: A retrospective cohort study of 16,316 community oncology patients with advanced NSCLC.
Disclosures: Mr. Rughani disclosed that he is an employee of and has stock or other ownership interests in Flatiron Health.
U.S. influenza activity remains steady
The decline in U.S. influenza activity that started in February paused during the week ending March 11, according to the U.S. Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) stayed at 3.7% for a second consecutive week after declining for 3 weeks in a row. The peak for the season, 5.2%, came during the week ending Feb. 11, CDC data show. The national baseline is 2.2%.
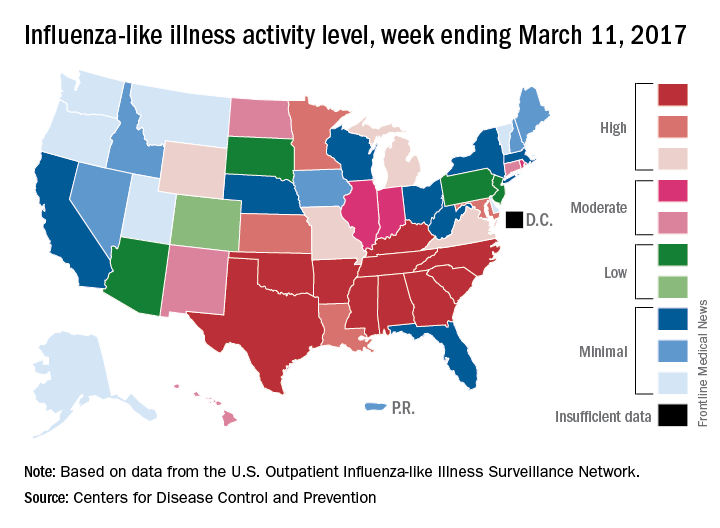
Five ILI-related pediatric deaths were reported to the CDC for the week – all of which occurred during previous weeks – bringing the total to 53 for the 2016-2017 season, the CDC said.
The decline in U.S. influenza activity that started in February paused during the week ending March 11, according to the U.S. Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) stayed at 3.7% for a second consecutive week after declining for 3 weeks in a row. The peak for the season, 5.2%, came during the week ending Feb. 11, CDC data show. The national baseline is 2.2%.

Five ILI-related pediatric deaths were reported to the CDC for the week – all of which occurred during previous weeks – bringing the total to 53 for the 2016-2017 season, the CDC said.
The decline in U.S. influenza activity that started in February paused during the week ending March 11, according to the U.S. Centers for Disease Control and Prevention.
The proportion of outpatient visits for influenza-like illness (ILI) stayed at 3.7% for a second consecutive week after declining for 3 weeks in a row. The peak for the season, 5.2%, came during the week ending Feb. 11, CDC data show. The national baseline is 2.2%.

Five ILI-related pediatric deaths were reported to the CDC for the week – all of which occurred during previous weeks – bringing the total to 53 for the 2016-2017 season, the CDC said.
LVADs achieve cardiac palliation in muscular dystrophies
At one time, respiratory failure was the primary cause of death in young men and boys with muscular dystrophies, but since improvements in ventilator support have addressed this problem, cardiac complications such as cardiomyopathy have become the main cause of death in this group, with the highest risk of death in people with Duchenne muscular dystrophy (DMD). Researchers from Rome have reported that the novel use of ventricular assist devices in this population can prolong life.
Gianluigi Perri, MD, PhD, of University Hospital and Bambino Gesù Children Hospital in Rome, and his coauthors, shared their experience treating seven patients with dystrophinopathies and dilated cardiomyopathy (DCM) with left ventricular assist devices (LVADs) from February 2011 to February 2016 (J Thorac Cardiovasc Surg. 2017 March;153:669-74). “Our experience indicates that the use of an LVAD as destination therapy in patients with dystrophinopathies with end-stage DCM is feasible, suggesting that it may be suitable as a palliative therapy for the treatment of these patients with no other therapeutic options,” Dr. Perri and his coauthors said.
Heart transplantation is considered the procedure of choice for children with severe advanced heart failure, but transplantation is contraindicated for children with dystrophinopathies because of the risk of respiratory failure and progression of skeletal myopathy leads to limited functional capacity. Hence, Dr. Perri and his coauthors developed their alternative treatment for end-stage heart failure in these children. They used the Jarvik 2000 LVAD (Jarvik Heart Inc., New York) as destination therapy.
Six of the seven patients they operated on had DMD and one had beta-2 sarcoglycan deficit. Their ages ranged from 14.2 to 23.4 years. Two patients had early complications: retropharyngeal bleeding and cholecystectomy; and abdominal bleeding and splenectomy. Two different patients had late complications: gastrostomy; and osteolysis and infection at the pedestal site. Three patients died after the operation: one of stroke at 15 months; one of severe bleeding about 28 months later; and one of lung infection 45 months afterward. Follow-up for the surviving patients ranged from about 2 months to 40 months. Median hospital stay was 77 days.
Dr. Perri and his coauthors noted that the DMD Care Considerations Working Group expanded acceptable therapies for DMD cardiomyopathy to include novel treatments such as mechanical circulatory support and implantable cardioverter-defibrillators.
“Although the best approach remains unclear, it does seem clear that treatment should be more aggressive,” the researchers said. The limited life expectancy of these patients makes transplantation a complicated choice when a shortage of donors is a concern. “Therefore, the alternative therapeutic option is the use of LVAD,” Dr. Perri and his coauthors said.
These patients need care at centers “with a high level of experience of patients with DMD,” the researchers stated. Common comorbidities such as severe kyphoscoliosis and respiratory muscle weakness in this population increase surgical risks.
Dr. Perri and his coauthors used a surgical technique that involved avoiding the left thoracotomy approach common in adults who undergo VAD implantation, because of respiratory insufficiency in these younger patients. They also used cardiopulmonary bypass in all but one patient who had a minimally invasive off-pump procedure through a left anterior minithoracotomy.
The researchers “strongly suggest” noninvasive ventilation after surgery to assist in pulmonary function often compromised by scoliosis and muscle weakness. “Our experience shows that postoperative care can be extremely challenging and is often burdened by unexpected complications,” they noted.
Kyphoscoliosis poses challenges when placing drains, and complications of these patients should be treated only in a specialized center. “Indeed, one of our patients died in a peripheral hospital because they underwent bronchoscopic examination with an endoscope that caused severe and intractable retropharyngeal bleeding,” they said.
The researchers no relevant financial relationships to disclose.
Almost all young men living with Duchenne muscular dystrophy will develop heart failure, but for many of these patients, continuous-flow left ventricular assist devices can provide “reliable support” for up to a decade, David L. S. Morales, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:675-6)
“The current series demonstrates, as has been shown at our institute as well as others, that one can provide an effective therapy for certain patients with DMD and heart failure,” Dr. Morales said of the work of Dr. Perri and coauthors. Dr. Morales added that maximizing outcomes in this population hinges on finding the appropriate time point for intervention in the disease process.
While “there is still much to be learned,” Dr. Morales said, Dr. Perri and his coauthors have shown that LVAD therapy is an option in patients with DMD and heart failure who have failed other treatments. “These young men may, therefore, have the option to extend their lives and possibly have the opportunity to benefit from the impressive medical advances being made,” he said. “Perhaps they and their families have been provided hope.”
Dr. Morales disclosed relationships with Berlin Heart, HeartWare and Oregon Total Artificial Heart.
Almost all young men living with Duchenne muscular dystrophy will develop heart failure, but for many of these patients, continuous-flow left ventricular assist devices can provide “reliable support” for up to a decade, David L. S. Morales, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:675-6)
“The current series demonstrates, as has been shown at our institute as well as others, that one can provide an effective therapy for certain patients with DMD and heart failure,” Dr. Morales said of the work of Dr. Perri and coauthors. Dr. Morales added that maximizing outcomes in this population hinges on finding the appropriate time point for intervention in the disease process.
While “there is still much to be learned,” Dr. Morales said, Dr. Perri and his coauthors have shown that LVAD therapy is an option in patients with DMD and heart failure who have failed other treatments. “These young men may, therefore, have the option to extend their lives and possibly have the opportunity to benefit from the impressive medical advances being made,” he said. “Perhaps they and their families have been provided hope.”
Dr. Morales disclosed relationships with Berlin Heart, HeartWare and Oregon Total Artificial Heart.
Almost all young men living with Duchenne muscular dystrophy will develop heart failure, but for many of these patients, continuous-flow left ventricular assist devices can provide “reliable support” for up to a decade, David L. S. Morales, MD, of the Heart Institute at Cincinnati Children’s Hospital Medical Center, said in his invited commentary (J Thorac Cardiovasc Surg. 2017;153:675-6)
“The current series demonstrates, as has been shown at our institute as well as others, that one can provide an effective therapy for certain patients with DMD and heart failure,” Dr. Morales said of the work of Dr. Perri and coauthors. Dr. Morales added that maximizing outcomes in this population hinges on finding the appropriate time point for intervention in the disease process.
While “there is still much to be learned,” Dr. Morales said, Dr. Perri and his coauthors have shown that LVAD therapy is an option in patients with DMD and heart failure who have failed other treatments. “These young men may, therefore, have the option to extend their lives and possibly have the opportunity to benefit from the impressive medical advances being made,” he said. “Perhaps they and their families have been provided hope.”
Dr. Morales disclosed relationships with Berlin Heart, HeartWare and Oregon Total Artificial Heart.
At one time, respiratory failure was the primary cause of death in young men and boys with muscular dystrophies, but since improvements in ventilator support have addressed this problem, cardiac complications such as cardiomyopathy have become the main cause of death in this group, with the highest risk of death in people with Duchenne muscular dystrophy (DMD). Researchers from Rome have reported that the novel use of ventricular assist devices in this population can prolong life.
Gianluigi Perri, MD, PhD, of University Hospital and Bambino Gesù Children Hospital in Rome, and his coauthors, shared their experience treating seven patients with dystrophinopathies and dilated cardiomyopathy (DCM) with left ventricular assist devices (LVADs) from February 2011 to February 2016 (J Thorac Cardiovasc Surg. 2017 March;153:669-74). “Our experience indicates that the use of an LVAD as destination therapy in patients with dystrophinopathies with end-stage DCM is feasible, suggesting that it may be suitable as a palliative therapy for the treatment of these patients with no other therapeutic options,” Dr. Perri and his coauthors said.
Heart transplantation is considered the procedure of choice for children with severe advanced heart failure, but transplantation is contraindicated for children with dystrophinopathies because of the risk of respiratory failure and progression of skeletal myopathy leads to limited functional capacity. Hence, Dr. Perri and his coauthors developed their alternative treatment for end-stage heart failure in these children. They used the Jarvik 2000 LVAD (Jarvik Heart Inc., New York) as destination therapy.
Six of the seven patients they operated on had DMD and one had beta-2 sarcoglycan deficit. Their ages ranged from 14.2 to 23.4 years. Two patients had early complications: retropharyngeal bleeding and cholecystectomy; and abdominal bleeding and splenectomy. Two different patients had late complications: gastrostomy; and osteolysis and infection at the pedestal site. Three patients died after the operation: one of stroke at 15 months; one of severe bleeding about 28 months later; and one of lung infection 45 months afterward. Follow-up for the surviving patients ranged from about 2 months to 40 months. Median hospital stay was 77 days.
Dr. Perri and his coauthors noted that the DMD Care Considerations Working Group expanded acceptable therapies for DMD cardiomyopathy to include novel treatments such as mechanical circulatory support and implantable cardioverter-defibrillators.
“Although the best approach remains unclear, it does seem clear that treatment should be more aggressive,” the researchers said. The limited life expectancy of these patients makes transplantation a complicated choice when a shortage of donors is a concern. “Therefore, the alternative therapeutic option is the use of LVAD,” Dr. Perri and his coauthors said.
These patients need care at centers “with a high level of experience of patients with DMD,” the researchers stated. Common comorbidities such as severe kyphoscoliosis and respiratory muscle weakness in this population increase surgical risks.
Dr. Perri and his coauthors used a surgical technique that involved avoiding the left thoracotomy approach common in adults who undergo VAD implantation, because of respiratory insufficiency in these younger patients. They also used cardiopulmonary bypass in all but one patient who had a minimally invasive off-pump procedure through a left anterior minithoracotomy.
The researchers “strongly suggest” noninvasive ventilation after surgery to assist in pulmonary function often compromised by scoliosis and muscle weakness. “Our experience shows that postoperative care can be extremely challenging and is often burdened by unexpected complications,” they noted.
Kyphoscoliosis poses challenges when placing drains, and complications of these patients should be treated only in a specialized center. “Indeed, one of our patients died in a peripheral hospital because they underwent bronchoscopic examination with an endoscope that caused severe and intractable retropharyngeal bleeding,” they said.
The researchers no relevant financial relationships to disclose.
At one time, respiratory failure was the primary cause of death in young men and boys with muscular dystrophies, but since improvements in ventilator support have addressed this problem, cardiac complications such as cardiomyopathy have become the main cause of death in this group, with the highest risk of death in people with Duchenne muscular dystrophy (DMD). Researchers from Rome have reported that the novel use of ventricular assist devices in this population can prolong life.
Gianluigi Perri, MD, PhD, of University Hospital and Bambino Gesù Children Hospital in Rome, and his coauthors, shared their experience treating seven patients with dystrophinopathies and dilated cardiomyopathy (DCM) with left ventricular assist devices (LVADs) from February 2011 to February 2016 (J Thorac Cardiovasc Surg. 2017 March;153:669-74). “Our experience indicates that the use of an LVAD as destination therapy in patients with dystrophinopathies with end-stage DCM is feasible, suggesting that it may be suitable as a palliative therapy for the treatment of these patients with no other therapeutic options,” Dr. Perri and his coauthors said.
Heart transplantation is considered the procedure of choice for children with severe advanced heart failure, but transplantation is contraindicated for children with dystrophinopathies because of the risk of respiratory failure and progression of skeletal myopathy leads to limited functional capacity. Hence, Dr. Perri and his coauthors developed their alternative treatment for end-stage heart failure in these children. They used the Jarvik 2000 LVAD (Jarvik Heart Inc., New York) as destination therapy.
Six of the seven patients they operated on had DMD and one had beta-2 sarcoglycan deficit. Their ages ranged from 14.2 to 23.4 years. Two patients had early complications: retropharyngeal bleeding and cholecystectomy; and abdominal bleeding and splenectomy. Two different patients had late complications: gastrostomy; and osteolysis and infection at the pedestal site. Three patients died after the operation: one of stroke at 15 months; one of severe bleeding about 28 months later; and one of lung infection 45 months afterward. Follow-up for the surviving patients ranged from about 2 months to 40 months. Median hospital stay was 77 days.
Dr. Perri and his coauthors noted that the DMD Care Considerations Working Group expanded acceptable therapies for DMD cardiomyopathy to include novel treatments such as mechanical circulatory support and implantable cardioverter-defibrillators.
“Although the best approach remains unclear, it does seem clear that treatment should be more aggressive,” the researchers said. The limited life expectancy of these patients makes transplantation a complicated choice when a shortage of donors is a concern. “Therefore, the alternative therapeutic option is the use of LVAD,” Dr. Perri and his coauthors said.
These patients need care at centers “with a high level of experience of patients with DMD,” the researchers stated. Common comorbidities such as severe kyphoscoliosis and respiratory muscle weakness in this population increase surgical risks.
Dr. Perri and his coauthors used a surgical technique that involved avoiding the left thoracotomy approach common in adults who undergo VAD implantation, because of respiratory insufficiency in these younger patients. They also used cardiopulmonary bypass in all but one patient who had a minimally invasive off-pump procedure through a left anterior minithoracotomy.
The researchers “strongly suggest” noninvasive ventilation after surgery to assist in pulmonary function often compromised by scoliosis and muscle weakness. “Our experience shows that postoperative care can be extremely challenging and is often burdened by unexpected complications,” they noted.
Kyphoscoliosis poses challenges when placing drains, and complications of these patients should be treated only in a specialized center. “Indeed, one of our patients died in a peripheral hospital because they underwent bronchoscopic examination with an endoscope that caused severe and intractable retropharyngeal bleeding,” they said.
The researchers no relevant financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: A left ventricular assist device can be used as destination therapy in patients with Duchenne muscular dystrophy dystrophinopathies and end-stage dilated cardiomyopathy.
Major finding: Four of seven patients who had LVAD survived long term, and survival for the three who died ranged from 15 to 44 months.
Data source: Single-center, retrospective review of seven patients with DMD who had LVAD for DCM from February 2011 to February 2016.
Disclosure: Dr. Perri and his coauthors reported having no relevant financial disclosures.
For Latinos, misperceptions and lack of medical care make preventing melanoma risky business
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – Ignorance and exposure are teaming up to put Latinos in the bull’s-eye of skin cancer.
Many believe that they are not at risk for either melanoma or nonmelanoma skin cancers – and too often, their physicians believe the same, Maritza Perez, MD, said at the annual meeting of the American Academy of Dermatology. Because of such incorrect perceptions, Latino patients get little counseling about risky behaviors, and so their exposure to those dangers continues unabated.
“The behavior of many Hispanic patients is very risky,” said Dr. Perez, a clinical professor of dermatology, at Mount Sinai Medical Center, New York. “They don’t wear sunscreen. They don’t do skin self-exams. They use tanning beds. And because of these beliefs, they don’t educate their children about sun safety.”
A research letter published in the Journal of the American Academy of Dermatology in 2011 broke down levels of skin cancer awareness by race and ethnicity among 165 whites, Hispanics, blacks, and Asians surveyed in New York City (64[1]:198-200). Compared with whites, Hispanics were significantly less likely to have ever had a doctor perform a full body skin exam (21% vs. 61%) or to have performed a self-exam (37% vs. 54%). Significantly fewer believed that skin cancer could occur in darker skin (78% vs. 91%). Only 8% had heard of the ABCDs of early melanoma detection, compared with 27% of whites. And about half as many Hispanics said they wore sunscreen (55% vs. 96%).
Unfortunately, Dr. Perez said, doctors aren’t correcting these misperceptions. Many physicians display a similar lack of understanding. They may correctly believe that the risk for skin cancer is less among Hispanics than it is among whites overall, but fail to communicate individual risk.
What these physicians may not understand, Dr. Perez said, is that the Hispanic population comprises an incredible variety of ethnic backgrounds. The population’s centuries-long genetic mixing bowl means there is no “typical” Hispanic skin. Instead, it includes every Fitzpatrick skin type, from fair-skinned redheads to the darkest brown and black skins.
Inadequate healthcare access exerts yet another damaging force. Like other ethnic minorities, many Hispanic patients lack insurance or adequate access to medical care. Instead of seeking regular primary care that would include skin cancer screenings, they tend to rely on urgent care or emergency departments to address emergent health issues, Dr. Perez said. When primary and preventive care falls by the wayside, melanomas that could be diagnosed at a curable stage invariably progress.
“We know that the only way of curing melanoma is with a scalpel. And the only way to remove it is by treating early disease. We’re not doing that. Our melanoma patients are diagnosed at younger ages with more advanced disease with more lymph node involvement than Caucasians, so there is also more mortality. We achieve early-stage diagnosis in 91% of Caucasians, but only 74% of Hispanics.”
A 2011 paper on racial and ethnic variations in the incidence and survival of melanoma, based on national cancer registry data covering almost 70% of the U.S. population, from 1999-2006, provided more information on the differences between the white and Hispanic populations (J Am Acad Dermatol. 2011 Nov;65[5 Suppl 1]:S26-37). Compared with non-Hispanic whites, Hispanics presented with thicker tumors (more than 1 mm, 35% vs. 25%), more regional involvement (12% vs. 8%), and more distant metastasis (7% vs. 4%).
Because adult Hispanic patients lack knowledge about their melanoma risk, they aren’t improving the outlook for their children, Dr. Perez said. The Hispanic demographic in the United States is already a young one. According on 2014 data cited by the Pew Research Center, 58% of Hispanics in the United States are aged 33 years or younger; 32% are younger than 18 years.
These young people are already endangering their health with unsafe sun behavior, Dr. Perez said. A 2007 study surveyed 369 white Hispanic and white non-Hispanic high school students in Miami about sun protection behaviors and skin cancer risk. The Hispanic teens were 2.5 times more likely to have used a tanning bed in the previous year; they were also less likely to wear sunscreen and protective clothing. The Hispanic students generally believed they were less likely to get skin cancer than the Caucasian students. They were 60% less likely to have heard of a skin self-exam and 70% less likely to have been told how to do one (Arch Dermatol. 2007;143[8]:983-8).
The oil to calm these troubled waters is education, Dr. Perez said. She takes this commitment very seriously, and said a simple conversation is the first step.
“I tell all my patients, no matter what ethnicity you are or what skin type you have, you can get skin cancer and you need regular, complete skin exams. And I teach them to do this for themselves.”
A senior vice-president for the Skin Cancer Foundation, Dr. Perez is coauthor of “Understanding Melanoma: What You Need to Know,” which is now in its fifth edition.
The book, originally published in 1996, is aimed at melanoma patients and their families. It covers the four types of melanoma and their causes and risk factors. Information on melanoma diagnosis, staging, treatment options, prognosis, and hereditary and genetic factors is also included, as well as guidelines for prevention.
The updated edition contains information on the latest immunotherapy and genetically targeted treatments, including ipilimumab (Yervoy), pembrolizumab (Keytruda), nivolumab (Opdivo), vemurafenib (Zelboraf), dabrafenib (Tafinlar) and trametinib (Mekinist). The book is available for download for a nominal fee.
She has also committed to educating physicians about the issue.
“If we want to decrease the incidence of melanoma in Latinos, decrease the tumor depth at diagnosis and bring down the higher mortality, we have to first educate the doctors who are taking care of these patients and correct the message delivered to Latinos by telling them that they are as prone to skin cancer as Caucasians. We simply have to get the message across that, just like everyone else, they need protection from the sun by applying sunblocks, using sunglasses, and covering their bodies with sun-protective clothing and large-rim hats. And we have to make medical care more accessible so that these people can be diagnosed and saved. This is what we need to do now. But I don’t know how many decades it will take to turn the tables.”
Dr. Perez had no disclosures relevant to her lecture.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM AAD 17
Clinicians still seek the best uses for apremilast
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Oral apremilast is a drug in search of a compelling indication, Craig L. Leonardi, MD, declared at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
“Apremilast is the least effective systemic agent for the treatment of psoriasis. That’s a statement of fact. I think the drug is mismatched for the treatment of moderate to severe plaque psoriasis. In fact, many of us gave the company that advice early on, but it was a program that was set in stone at that point,” according to Dr. Leonardi, a dermatologist at Saint Louis University and a prominent psoriasis clinical trialist.
“You have a drug that’s modest with regard to clearing psoriasis and it’s modest in controlling psoriatic arthritis. So you have to ask yourself what’s the patient population for this drug. I would just say that in my hands – and this is off-label – I use it only in patients with mild to moderate psoriasis,” Dr. Leonardi said.
“We all have psoriasis patients who come in with 3% or 4% of their skin involved, they’re actually using the class I topical steroids that I prescribe for them, yet they’re still not clear or almost clear and they want more. At that point, that’s when I might take them down this pathway and put them on apremilast along with a topical steroid. And I think that’s the appropriate place for this drug. I don’t use it in patients with 10% or more body surface area involvement,” he said.
He added that he would like to be able to prescribe apremilast in conjunction with a biologic agent in patients with more severe psoriasis to obtain synergistic efficacy, but payers balk at that because, at close to $3,000 per month, apremilast costs far more than methotrexate and other generic conventional disease-modifying antirheumatic drugs.
“The insurance industry is all over this drug. They require preauthorization, and they tell me, ‘No way, have a nice day,’ ” according to the dermatologist.
Dr. Leonardi described a couple of other practical caveats regarding apremilast. In patients with an estimated glomerular filtration rate below 30 mL/min per 1.73 m2, a dose reduction to 30 mg once daily in the morning is necessary. And apremilast is not recommended for use in patients who are on a strong inducer of the CYP 450 enzyme, such as rifampin or phenytoin.
An intriguing side effect of apremilast is that it causes weight loss: A 5%-10% weight loss was seen in the major psoriasis clinical trials. But it’s been established that there is no correlation at all between the weight loss and clinical efficacy for skin clearance.
What efficacy can be anticipated?
In the pivotal phase III ESTEEM I trial conducted in 844 patients with moderate to severe psoriasis (J Am Acad Dermatol. 2015 Jul;73[1]:37-49), the overall PASI-75 response rate at week 16 in patients assigned to apremilast at 30 mg twice daily was 33%, significantly better than the 5% placebo response rate, but substantially less than what’s achieved with the tumor necrosis factor inhibitors and other injectable biologic agents.
Moreover, in the pivotal phase III PALACE 1 study of apremilast for the treatment of psoriatic arthritis, the primary outcome of at least a 20% improvement in the modified American College of Rheumatology response criteria at week 16 was achieved in 40% of patients randomized to apremilast at 30 mg twice daily, compared with 19% on placebo (Ann Rheum Dis. 2014 Jun;73[6]:1020-6). Again, that doesn’t approach the efficacy of a TNF inhibitor, Dr. Leonardi noted.
On the plus side, an oral agent such as apremilast is an attractive option for treatment-adherent patients. Plus, the drug has a favorable safety profile and is well tolerated, with mild to moderate side effects that appear early and are self-limited. In ESTEEM I, for example, more than 96% of patients had either no or only mild to moderate adverse events. The incidence of the two most common adverse events, diarrhea and nausea, at 19% and 16%, respectively, was more than double that in placebo-treated controls, but rates of other adverse events were similar in the two treatment arms.
Symposium codirector Linda Stein Gold, MD, director of dermatology research at the Henry Ford Health System in Detroit, said a clinical trial of apremilast conducted specifically in patients with moderate psoriasis has recently been completed. When those results become available they should shore up the drug’s use in that population.
Finding potential in off-label use
“This drug may have been brought to market for psoriasis, but I think its utility is so much more in other diseases where inflammation is an important mechanism,” said Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “Most of my use of apremilast is off-label for atopic dermatitis, for lichen planus, and I’ve tried it in a lot of patients where it has worked for discoid lupus. There’s so much potential for apremilast,” said Dr. Bhatia.
Dr. Leonardi remained unpersuaded.
“Your glass of water is half full, mine is half empty in this case,” he replied. “This drug has been approved now for at least 3 years, and we are still looking at the occasional favorable case report that flies up. I’ve got to say this drug is having a hard time finding a place outside of psoriasis, but we’ll all see.”
Dr. Leonardi reported having financial relationships with more than a dozen pharmaceutical companies, including Celgene, which markets apremilast. Dr. Stein Gold, too, has received research grants from and serves as a consultant to numerous drug companies, including Celgene. Dr. Bhatia declared having financial relationships with more than two dozen.
SDEF and this news organization are owned by the same parent company.