User login
Smart bracelet may predict mood changes in bipolar disorder
BARCELONA – early research suggests.
In a small observational pilot study, researchers found the E4 wristband (Empatica Inc) was able to detect fluctuations in mood.
The results highlight the potential of EDA to serve as an objective BD biomarker, noted the investigators, led by Diego Hidalgo-Mazzei, MD, PhD, Bipolar and Depressive Disorders Unit, University of Barcelona.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress.
A need for objective markers
The evaluation of BD currently consists of clinical interviews, questionnaires, and scales, which largely rely on physician assessment, highlighting the need for objective biomarkers.
Previous studies show that EDA, which tracks changes in the skin due to sweat gland activity in response to psychological stimuli, is reduced in unipolar depression.
The researchers hypothesized that EDA could be a biomarker of mood changes in patients with BD. They recruited 38 patients experiencing manic (n = 12) or depressive (n = 9) episodes or who were euthymic (n = 17) and compared their responses with those of 19 healthy control persons.
Study participants were asked to wear the wristband continuously for approximately 48 hours to measure EDA, motion-based activity, blood volume pulse, and skin temperature.
The 48-hour monitoring session was determined by the battery life of the device, Dr. Hidalgo-Mazzei said in an interview.
The acute-phase patients in the study had three sessions at different time points – one during the acute state, another when the clinician determined there was a response to treatment, and again at remission. Euthymic patients and healthy control persons had a single monitoring session.
Dr. Hidalgo-Mazzei said the study’s protocol is unique because it involves unusually long sessions with the device. In this setup, each sensor collects a sample every second, resulting in highly detailed and granular data.
“At the end, it is a trade-off, as handling such an enormous amount of data for each session requires equally large preprocessing, computing power, and analysis,” he said.
Dr. Hidalgo-Mazzei characterized compliance with the device as “outstanding” for the majority of study participants.
Results showed that mean EDA was notably and significantly lower in BD patients during depressive episodes in comparison with those in other groups. Patients with depression also had significantly less frequent EDA peaks per minute (P = .001 for both).
There were also significant differences in EDA measures between baseline and after treatment in the acute BD groups.
Patients with depression had significant increases in mean EDA (P = .033), EDA peaks per minute (P = .002), and the mean amplitude of EDA peaks (P = .001) from baseline, while manic patients experienced a decrease in the mean amplitude of EDA peaks (P = .001).
It is important for the patient and doctor to know how and when mood fluctuations take place, said Dr. Hidalgo-Mazzei, because treatment for manic and depressive states differ.
“Until now, these mood swings have mostly been diagnosed subjectively, through interview with doctors or by questionnaires, and this had led to real difficulties.
“Arriving at the correct drug is difficult, with only around 30% to 40% of treated individuals having the expected response. We hope that the additional information these systems can provide will give us greater certainty in treating patients.”
However, Dr. Hidalgo-Mazzei said that is still a long way off, noting that this is an exploratory, observational study.
“We need to look at a larger sample and use machine learning to analyze all the biomarkers collected by the wearers to confirm the findings,” he said.
A true biomarker?
In a comment, Joseph F. Goldberg, MD, clinical professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York, said the study is an “interesting use of this technology to differentiate physiological correlates of mood states.”
However, he said the findings are limited and preliminary because the sample sizes were small and the measures weren’t repeated.
In addition, medications or other factors that may influence electrophysiologic activity, such as anxiety or panic, were not considered, and Dr. Goldberg noted the researchers did not compare the results with those in patients with other diagnoses.
“So, I don’t think one could call this a biomarker in the sense of having diagnostic specificity,” he said, making the comparison with body temperature, which “goes up in an infection; but fever alone doesn’t tell us much about the nature or cause of a presumed infection. More studies are needed before generalizable conclusion can be drawn.”
Also commenting on the research, Paolo Ossola, MD, PhD, assistant professor of psychiatry, department of medicine and surgery, University of Parma, Italy, described the study as exploratory but preliminary.
He said the researchers have “laid the foundation for a new approach to diagnosing and treating bipolar disorders.
“The shift from the subjective to the biological level could also promote understanding of the underlying mechanistic dynamics of mood swings.”
The study was funded by the Instituto de Salud Carlos III and a Baszucki Brain Research Fund grant from the Milken Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – early research suggests.
In a small observational pilot study, researchers found the E4 wristband (Empatica Inc) was able to detect fluctuations in mood.
The results highlight the potential of EDA to serve as an objective BD biomarker, noted the investigators, led by Diego Hidalgo-Mazzei, MD, PhD, Bipolar and Depressive Disorders Unit, University of Barcelona.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress.
A need for objective markers
The evaluation of BD currently consists of clinical interviews, questionnaires, and scales, which largely rely on physician assessment, highlighting the need for objective biomarkers.
Previous studies show that EDA, which tracks changes in the skin due to sweat gland activity in response to psychological stimuli, is reduced in unipolar depression.
The researchers hypothesized that EDA could be a biomarker of mood changes in patients with BD. They recruited 38 patients experiencing manic (n = 12) or depressive (n = 9) episodes or who were euthymic (n = 17) and compared their responses with those of 19 healthy control persons.
Study participants were asked to wear the wristband continuously for approximately 48 hours to measure EDA, motion-based activity, blood volume pulse, and skin temperature.
The 48-hour monitoring session was determined by the battery life of the device, Dr. Hidalgo-Mazzei said in an interview.
The acute-phase patients in the study had three sessions at different time points – one during the acute state, another when the clinician determined there was a response to treatment, and again at remission. Euthymic patients and healthy control persons had a single monitoring session.
Dr. Hidalgo-Mazzei said the study’s protocol is unique because it involves unusually long sessions with the device. In this setup, each sensor collects a sample every second, resulting in highly detailed and granular data.
“At the end, it is a trade-off, as handling such an enormous amount of data for each session requires equally large preprocessing, computing power, and analysis,” he said.
Dr. Hidalgo-Mazzei characterized compliance with the device as “outstanding” for the majority of study participants.
Results showed that mean EDA was notably and significantly lower in BD patients during depressive episodes in comparison with those in other groups. Patients with depression also had significantly less frequent EDA peaks per minute (P = .001 for both).
There were also significant differences in EDA measures between baseline and after treatment in the acute BD groups.
Patients with depression had significant increases in mean EDA (P = .033), EDA peaks per minute (P = .002), and the mean amplitude of EDA peaks (P = .001) from baseline, while manic patients experienced a decrease in the mean amplitude of EDA peaks (P = .001).
It is important for the patient and doctor to know how and when mood fluctuations take place, said Dr. Hidalgo-Mazzei, because treatment for manic and depressive states differ.
“Until now, these mood swings have mostly been diagnosed subjectively, through interview with doctors or by questionnaires, and this had led to real difficulties.
“Arriving at the correct drug is difficult, with only around 30% to 40% of treated individuals having the expected response. We hope that the additional information these systems can provide will give us greater certainty in treating patients.”
However, Dr. Hidalgo-Mazzei said that is still a long way off, noting that this is an exploratory, observational study.
“We need to look at a larger sample and use machine learning to analyze all the biomarkers collected by the wearers to confirm the findings,” he said.
A true biomarker?
In a comment, Joseph F. Goldberg, MD, clinical professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York, said the study is an “interesting use of this technology to differentiate physiological correlates of mood states.”
However, he said the findings are limited and preliminary because the sample sizes were small and the measures weren’t repeated.
In addition, medications or other factors that may influence electrophysiologic activity, such as anxiety or panic, were not considered, and Dr. Goldberg noted the researchers did not compare the results with those in patients with other diagnoses.
“So, I don’t think one could call this a biomarker in the sense of having diagnostic specificity,” he said, making the comparison with body temperature, which “goes up in an infection; but fever alone doesn’t tell us much about the nature or cause of a presumed infection. More studies are needed before generalizable conclusion can be drawn.”
Also commenting on the research, Paolo Ossola, MD, PhD, assistant professor of psychiatry, department of medicine and surgery, University of Parma, Italy, described the study as exploratory but preliminary.
He said the researchers have “laid the foundation for a new approach to diagnosing and treating bipolar disorders.
“The shift from the subjective to the biological level could also promote understanding of the underlying mechanistic dynamics of mood swings.”
The study was funded by the Instituto de Salud Carlos III and a Baszucki Brain Research Fund grant from the Milken Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – early research suggests.
In a small observational pilot study, researchers found the E4 wristband (Empatica Inc) was able to detect fluctuations in mood.
The results highlight the potential of EDA to serve as an objective BD biomarker, noted the investigators, led by Diego Hidalgo-Mazzei, MD, PhD, Bipolar and Depressive Disorders Unit, University of Barcelona.
The findings were presented at the 36th European College of Neuropsychopharmacology (ECNP) Congress.
A need for objective markers
The evaluation of BD currently consists of clinical interviews, questionnaires, and scales, which largely rely on physician assessment, highlighting the need for objective biomarkers.
Previous studies show that EDA, which tracks changes in the skin due to sweat gland activity in response to psychological stimuli, is reduced in unipolar depression.
The researchers hypothesized that EDA could be a biomarker of mood changes in patients with BD. They recruited 38 patients experiencing manic (n = 12) or depressive (n = 9) episodes or who were euthymic (n = 17) and compared their responses with those of 19 healthy control persons.
Study participants were asked to wear the wristband continuously for approximately 48 hours to measure EDA, motion-based activity, blood volume pulse, and skin temperature.
The 48-hour monitoring session was determined by the battery life of the device, Dr. Hidalgo-Mazzei said in an interview.
The acute-phase patients in the study had three sessions at different time points – one during the acute state, another when the clinician determined there was a response to treatment, and again at remission. Euthymic patients and healthy control persons had a single monitoring session.
Dr. Hidalgo-Mazzei said the study’s protocol is unique because it involves unusually long sessions with the device. In this setup, each sensor collects a sample every second, resulting in highly detailed and granular data.
“At the end, it is a trade-off, as handling such an enormous amount of data for each session requires equally large preprocessing, computing power, and analysis,” he said.
Dr. Hidalgo-Mazzei characterized compliance with the device as “outstanding” for the majority of study participants.
Results showed that mean EDA was notably and significantly lower in BD patients during depressive episodes in comparison with those in other groups. Patients with depression also had significantly less frequent EDA peaks per minute (P = .001 for both).
There were also significant differences in EDA measures between baseline and after treatment in the acute BD groups.
Patients with depression had significant increases in mean EDA (P = .033), EDA peaks per minute (P = .002), and the mean amplitude of EDA peaks (P = .001) from baseline, while manic patients experienced a decrease in the mean amplitude of EDA peaks (P = .001).
It is important for the patient and doctor to know how and when mood fluctuations take place, said Dr. Hidalgo-Mazzei, because treatment for manic and depressive states differ.
“Until now, these mood swings have mostly been diagnosed subjectively, through interview with doctors or by questionnaires, and this had led to real difficulties.
“Arriving at the correct drug is difficult, with only around 30% to 40% of treated individuals having the expected response. We hope that the additional information these systems can provide will give us greater certainty in treating patients.”
However, Dr. Hidalgo-Mazzei said that is still a long way off, noting that this is an exploratory, observational study.
“We need to look at a larger sample and use machine learning to analyze all the biomarkers collected by the wearers to confirm the findings,” he said.
A true biomarker?
In a comment, Joseph F. Goldberg, MD, clinical professor of psychiatry at Icahn School of Medicine at Mount Sinai, New York, said the study is an “interesting use of this technology to differentiate physiological correlates of mood states.”
However, he said the findings are limited and preliminary because the sample sizes were small and the measures weren’t repeated.
In addition, medications or other factors that may influence electrophysiologic activity, such as anxiety or panic, were not considered, and Dr. Goldberg noted the researchers did not compare the results with those in patients with other diagnoses.
“So, I don’t think one could call this a biomarker in the sense of having diagnostic specificity,” he said, making the comparison with body temperature, which “goes up in an infection; but fever alone doesn’t tell us much about the nature or cause of a presumed infection. More studies are needed before generalizable conclusion can be drawn.”
Also commenting on the research, Paolo Ossola, MD, PhD, assistant professor of psychiatry, department of medicine and surgery, University of Parma, Italy, described the study as exploratory but preliminary.
He said the researchers have “laid the foundation for a new approach to diagnosing and treating bipolar disorders.
“The shift from the subjective to the biological level could also promote understanding of the underlying mechanistic dynamics of mood swings.”
The study was funded by the Instituto de Salud Carlos III and a Baszucki Brain Research Fund grant from the Milken Foundation. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ECNP 2023
MS-tailored weight loss program achieves meaningful results
MILAN – results of a new trial show.
Seventy patients with MS and a high body mass index (BMI) were randomized to receive a multifaceted weight loss program or treatment as usual, resulting in almost two-thirds of the intervention group patients losing at least 5% of their body weight.
“Our weight loss intervention achieved clinically significant weight loss and improved mental quality of life,” said study presenter Sharon G. Lynch, MD, professor in the department of neurology at the University of Kansas Medical Center, Kansas City.
The results also showed weight loss “was associated with improved mobility, reduced fatigability, and improved physical quality of life.”
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Obesity linked to MS progression
Dr. Lynch noted that almost 40% of people with MS have comorbid obesity, and that obesity “is associated with developing MS in some studies, and also disease progression.” Moreover, MS and obesity share common chronic symptoms, particularly mobility problems, depression, fatigue, and reduced quality of life.
Despite this, no randomized controlled trials have been specifically designed to help people with MS lose weight, he noted.
The team therefore examined the efficacy of a behavioral weight loss program designed for patients with the disease, as well as the impact of weight loss on mobility and other chronic symptoms.
The participants were required to be English speaking, with access to a telephone and/or computer, and to have a confirmed diagnosis of MS. They also had to be aged 18-70 years, have a BMI of 29-50 kg/m2, and have a Patient-Determined Disease Steps (PDDS) score of less than 4.
The patients were randomly assigned to receive either a weight loss intervention or brief health education and treatment as usual. They were assessed at 6 months to measure their weight loss, mobility, and self-reported quality of life and perceived fatigability.
Dr. Lynch said the intervention was “fairly time intensive,” with an hour-long telehealth group weight loss session every week for 24 weeks, as well as monthly individual sessions for 6 months.
The participants were provided with a Fitbit activity tracker, a set of Bluetooth-enabled weighing scales, and access to the Lose It! weight loss app.
Caloric restriction was encouraged, with a focus on increasing intake of fruits and vegetables, alongside a target of 150 minutes per week of moderate to vigorous physical activity, in line with Centers for Disease Control and Prevention and American Heart Association recommendations.
Significant loss in body weight
Seventy individuals with MS took part in the trial, of whom 83% were female and 88% were White, 9% African American, and 3% Hispanic or Latino. The mean age was 46.7 years, and the mean number of years in education was 11.8.
The vast majority (96%) of the participants had relapsing remitting MS, at a mean disease duration of 10.9 years, and 82% had a score of 0 or 1 on the PDDS.
Dr. Lynch showed that participants in the intervention group lost, over the course of the study, 8.6% of their total body weight, compared with a loss of 0.7% among controls (P < .001).
Moreover, 65% of the intervention group lost at least 5% of their body weight, whereas 41% lost at least 10% of their body weight, which again was significantly higher than that seen in the control group (P < .001).
There was also a significant increase in moderate to vigorous physical activity in the intervention group as measured by accelerometry (P < .05), although Dr. Lynch pointed out this “did not necessarily correlate with their weight loss.”
Dr. Lynch showed there were significant differences across a range of anthropometric measures from baseline to follow-up between the intervention and control groups.
The adjusted difference in weight loss between the intervention and control participants was 7.8 kg, whereas the difference in reduction of BMI was 2.7 (P = .001 for both).
There was also a significant difference in the reduction in waist-to-hip ratio between the groups, at 0.033 in favor of the intervention, as well as a difference in the reduction in fat tissue, at 3.1% (P = .001 for both).
Further analyses showed weight loss was associated with significant improvements on the 6-minute walk test, at an r value of 0.48 (P = .015), and in the 25-foot walk test (r = 0.42; P = .015). Weight loss was also linked to reductions in perceived fatigability (r = 0.48; P = .005).
Dr. Lynch also reported that a 5% reduction in body weight was associated with a “clinically meaningful” improvement of 50 meters on the 6-minute walk test.
Finally, it was found the intervention was associated with a significant improvement in mental quality of life (P = .01), whereas weight loss specifically was linked to improved physical quality of life (P = .02).
“We believe that future studies should examine weight loss in people with MS who have more advanced disability,” Dr. Lynch said, and “we should examine the effects of weight loss on the underlying disease processes.”
She added they also “need to follow the patient for longer and see if they can maintain their weight loss.”
Emphasizing the social side of interventions
Session cochair Brian M. Sandroff, PhD, director of the Exercise Neurorehabilitation Research Laboratory at the Kessler Foundation, East Hanover, N.J., commented that the results are “really exciting.”
He said that the improvements across the range of measures assessed in the study were not surprising, “considering the intervention was multicomponent, and so had the potential to affect a number of different physical and cognitive domains.”
One factor in managing MS that came up again and again during the discussion at the end of the session was the social aspect of interventions, with Dr. Lynch saying the group sessions in particular were appreciated by participants in her trial.
Dr. Sandroff, who was not involved in the study, said that it has been questioned whether the social side should be controlled for when assessing interventions, or “maybe it’s something we should promote within our studies.”
He explained that being social “overcomes a lot of isolation-related issues among people with MS who have comorbidities,” which can result in “reduced quality of life and differences in symptomatic manifestations.”
“Providing that group setting might have lots of outcomes besides just a biological loss of mass” because of the intervention itself, Dr. Sandroff said.
The study was supported by grants from the National MS Society. Dr. Lynch declares relationships with Biogen, Genzyme, Teva, Sanofi, Novartis, Celgene, Roche, Immunic, National Institutes of Health, National Multiple Sclerosis Society, Patient-Centered Outcomes Research Institute, Anokion, TG Therapeutics, and Actelion. Other authors also declared relationships. Dr. Sandroff declared no relevant relationships.
A version of this article first appeared on Medscape.com.
MILAN – results of a new trial show.
Seventy patients with MS and a high body mass index (BMI) were randomized to receive a multifaceted weight loss program or treatment as usual, resulting in almost two-thirds of the intervention group patients losing at least 5% of their body weight.
“Our weight loss intervention achieved clinically significant weight loss and improved mental quality of life,” said study presenter Sharon G. Lynch, MD, professor in the department of neurology at the University of Kansas Medical Center, Kansas City.
The results also showed weight loss “was associated with improved mobility, reduced fatigability, and improved physical quality of life.”
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Obesity linked to MS progression
Dr. Lynch noted that almost 40% of people with MS have comorbid obesity, and that obesity “is associated with developing MS in some studies, and also disease progression.” Moreover, MS and obesity share common chronic symptoms, particularly mobility problems, depression, fatigue, and reduced quality of life.
Despite this, no randomized controlled trials have been specifically designed to help people with MS lose weight, he noted.
The team therefore examined the efficacy of a behavioral weight loss program designed for patients with the disease, as well as the impact of weight loss on mobility and other chronic symptoms.
The participants were required to be English speaking, with access to a telephone and/or computer, and to have a confirmed diagnosis of MS. They also had to be aged 18-70 years, have a BMI of 29-50 kg/m2, and have a Patient-Determined Disease Steps (PDDS) score of less than 4.
The patients were randomly assigned to receive either a weight loss intervention or brief health education and treatment as usual. They were assessed at 6 months to measure their weight loss, mobility, and self-reported quality of life and perceived fatigability.
Dr. Lynch said the intervention was “fairly time intensive,” with an hour-long telehealth group weight loss session every week for 24 weeks, as well as monthly individual sessions for 6 months.
The participants were provided with a Fitbit activity tracker, a set of Bluetooth-enabled weighing scales, and access to the Lose It! weight loss app.
Caloric restriction was encouraged, with a focus on increasing intake of fruits and vegetables, alongside a target of 150 minutes per week of moderate to vigorous physical activity, in line with Centers for Disease Control and Prevention and American Heart Association recommendations.
Significant loss in body weight
Seventy individuals with MS took part in the trial, of whom 83% were female and 88% were White, 9% African American, and 3% Hispanic or Latino. The mean age was 46.7 years, and the mean number of years in education was 11.8.
The vast majority (96%) of the participants had relapsing remitting MS, at a mean disease duration of 10.9 years, and 82% had a score of 0 or 1 on the PDDS.
Dr. Lynch showed that participants in the intervention group lost, over the course of the study, 8.6% of their total body weight, compared with a loss of 0.7% among controls (P < .001).
Moreover, 65% of the intervention group lost at least 5% of their body weight, whereas 41% lost at least 10% of their body weight, which again was significantly higher than that seen in the control group (P < .001).
There was also a significant increase in moderate to vigorous physical activity in the intervention group as measured by accelerometry (P < .05), although Dr. Lynch pointed out this “did not necessarily correlate with their weight loss.”
Dr. Lynch showed there were significant differences across a range of anthropometric measures from baseline to follow-up between the intervention and control groups.
The adjusted difference in weight loss between the intervention and control participants was 7.8 kg, whereas the difference in reduction of BMI was 2.7 (P = .001 for both).
There was also a significant difference in the reduction in waist-to-hip ratio between the groups, at 0.033 in favor of the intervention, as well as a difference in the reduction in fat tissue, at 3.1% (P = .001 for both).
Further analyses showed weight loss was associated with significant improvements on the 6-minute walk test, at an r value of 0.48 (P = .015), and in the 25-foot walk test (r = 0.42; P = .015). Weight loss was also linked to reductions in perceived fatigability (r = 0.48; P = .005).
Dr. Lynch also reported that a 5% reduction in body weight was associated with a “clinically meaningful” improvement of 50 meters on the 6-minute walk test.
Finally, it was found the intervention was associated with a significant improvement in mental quality of life (P = .01), whereas weight loss specifically was linked to improved physical quality of life (P = .02).
“We believe that future studies should examine weight loss in people with MS who have more advanced disability,” Dr. Lynch said, and “we should examine the effects of weight loss on the underlying disease processes.”
She added they also “need to follow the patient for longer and see if they can maintain their weight loss.”
Emphasizing the social side of interventions
Session cochair Brian M. Sandroff, PhD, director of the Exercise Neurorehabilitation Research Laboratory at the Kessler Foundation, East Hanover, N.J., commented that the results are “really exciting.”
He said that the improvements across the range of measures assessed in the study were not surprising, “considering the intervention was multicomponent, and so had the potential to affect a number of different physical and cognitive domains.”
One factor in managing MS that came up again and again during the discussion at the end of the session was the social aspect of interventions, with Dr. Lynch saying the group sessions in particular were appreciated by participants in her trial.
Dr. Sandroff, who was not involved in the study, said that it has been questioned whether the social side should be controlled for when assessing interventions, or “maybe it’s something we should promote within our studies.”
He explained that being social “overcomes a lot of isolation-related issues among people with MS who have comorbidities,” which can result in “reduced quality of life and differences in symptomatic manifestations.”
“Providing that group setting might have lots of outcomes besides just a biological loss of mass” because of the intervention itself, Dr. Sandroff said.
The study was supported by grants from the National MS Society. Dr. Lynch declares relationships with Biogen, Genzyme, Teva, Sanofi, Novartis, Celgene, Roche, Immunic, National Institutes of Health, National Multiple Sclerosis Society, Patient-Centered Outcomes Research Institute, Anokion, TG Therapeutics, and Actelion. Other authors also declared relationships. Dr. Sandroff declared no relevant relationships.
A version of this article first appeared on Medscape.com.
MILAN – results of a new trial show.
Seventy patients with MS and a high body mass index (BMI) were randomized to receive a multifaceted weight loss program or treatment as usual, resulting in almost two-thirds of the intervention group patients losing at least 5% of their body weight.
“Our weight loss intervention achieved clinically significant weight loss and improved mental quality of life,” said study presenter Sharon G. Lynch, MD, professor in the department of neurology at the University of Kansas Medical Center, Kansas City.
The results also showed weight loss “was associated with improved mobility, reduced fatigability, and improved physical quality of life.”
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS meeting.
Obesity linked to MS progression
Dr. Lynch noted that almost 40% of people with MS have comorbid obesity, and that obesity “is associated with developing MS in some studies, and also disease progression.” Moreover, MS and obesity share common chronic symptoms, particularly mobility problems, depression, fatigue, and reduced quality of life.
Despite this, no randomized controlled trials have been specifically designed to help people with MS lose weight, he noted.
The team therefore examined the efficacy of a behavioral weight loss program designed for patients with the disease, as well as the impact of weight loss on mobility and other chronic symptoms.
The participants were required to be English speaking, with access to a telephone and/or computer, and to have a confirmed diagnosis of MS. They also had to be aged 18-70 years, have a BMI of 29-50 kg/m2, and have a Patient-Determined Disease Steps (PDDS) score of less than 4.
The patients were randomly assigned to receive either a weight loss intervention or brief health education and treatment as usual. They were assessed at 6 months to measure their weight loss, mobility, and self-reported quality of life and perceived fatigability.
Dr. Lynch said the intervention was “fairly time intensive,” with an hour-long telehealth group weight loss session every week for 24 weeks, as well as monthly individual sessions for 6 months.
The participants were provided with a Fitbit activity tracker, a set of Bluetooth-enabled weighing scales, and access to the Lose It! weight loss app.
Caloric restriction was encouraged, with a focus on increasing intake of fruits and vegetables, alongside a target of 150 minutes per week of moderate to vigorous physical activity, in line with Centers for Disease Control and Prevention and American Heart Association recommendations.
Significant loss in body weight
Seventy individuals with MS took part in the trial, of whom 83% were female and 88% were White, 9% African American, and 3% Hispanic or Latino. The mean age was 46.7 years, and the mean number of years in education was 11.8.
The vast majority (96%) of the participants had relapsing remitting MS, at a mean disease duration of 10.9 years, and 82% had a score of 0 or 1 on the PDDS.
Dr. Lynch showed that participants in the intervention group lost, over the course of the study, 8.6% of their total body weight, compared with a loss of 0.7% among controls (P < .001).
Moreover, 65% of the intervention group lost at least 5% of their body weight, whereas 41% lost at least 10% of their body weight, which again was significantly higher than that seen in the control group (P < .001).
There was also a significant increase in moderate to vigorous physical activity in the intervention group as measured by accelerometry (P < .05), although Dr. Lynch pointed out this “did not necessarily correlate with their weight loss.”
Dr. Lynch showed there were significant differences across a range of anthropometric measures from baseline to follow-up between the intervention and control groups.
The adjusted difference in weight loss between the intervention and control participants was 7.8 kg, whereas the difference in reduction of BMI was 2.7 (P = .001 for both).
There was also a significant difference in the reduction in waist-to-hip ratio between the groups, at 0.033 in favor of the intervention, as well as a difference in the reduction in fat tissue, at 3.1% (P = .001 for both).
Further analyses showed weight loss was associated with significant improvements on the 6-minute walk test, at an r value of 0.48 (P = .015), and in the 25-foot walk test (r = 0.42; P = .015). Weight loss was also linked to reductions in perceived fatigability (r = 0.48; P = .005).
Dr. Lynch also reported that a 5% reduction in body weight was associated with a “clinically meaningful” improvement of 50 meters on the 6-minute walk test.
Finally, it was found the intervention was associated with a significant improvement in mental quality of life (P = .01), whereas weight loss specifically was linked to improved physical quality of life (P = .02).
“We believe that future studies should examine weight loss in people with MS who have more advanced disability,” Dr. Lynch said, and “we should examine the effects of weight loss on the underlying disease processes.”
She added they also “need to follow the patient for longer and see if they can maintain their weight loss.”
Emphasizing the social side of interventions
Session cochair Brian M. Sandroff, PhD, director of the Exercise Neurorehabilitation Research Laboratory at the Kessler Foundation, East Hanover, N.J., commented that the results are “really exciting.”
He said that the improvements across the range of measures assessed in the study were not surprising, “considering the intervention was multicomponent, and so had the potential to affect a number of different physical and cognitive domains.”
One factor in managing MS that came up again and again during the discussion at the end of the session was the social aspect of interventions, with Dr. Lynch saying the group sessions in particular were appreciated by participants in her trial.
Dr. Sandroff, who was not involved in the study, said that it has been questioned whether the social side should be controlled for when assessing interventions, or “maybe it’s something we should promote within our studies.”
He explained that being social “overcomes a lot of isolation-related issues among people with MS who have comorbidities,” which can result in “reduced quality of life and differences in symptomatic manifestations.”
“Providing that group setting might have lots of outcomes besides just a biological loss of mass” because of the intervention itself, Dr. Sandroff said.
The study was supported by grants from the National MS Society. Dr. Lynch declares relationships with Biogen, Genzyme, Teva, Sanofi, Novartis, Celgene, Roche, Immunic, National Institutes of Health, National Multiple Sclerosis Society, Patient-Centered Outcomes Research Institute, Anokion, TG Therapeutics, and Actelion. Other authors also declared relationships. Dr. Sandroff declared no relevant relationships.
A version of this article first appeared on Medscape.com.
AT ECTRIMS 2023
Debate: Is lasting remission of type 2 diabetes feasible in the real-world setting?
The prospect of remission of type 2 diabetes (T2D) has captured the hearts and minds of many patients with T2D and health care professionals, including myself.
I have changed my narrative when supporting my patients with T2D. I used to say that T2D is a progressive condition, but considering seminal recent evidence like the DiRECT trial, I now say that T2D can be a progressive condition. Through significant weight loss, patients can reverse it and achieve remission of T2D. This has given my patients hope that their T2D is no longer an inexorable condition. And hope, of course, is a powerful enabler of change.
However,
I therefore relished the opportunity to attend a debate on this topic at the annual meeting of the European Association for the Study of Diabetes in Hamburg, Germany, between Roy Taylor, MD, principal investigator for the DiRECT study and professor of medicine and metabolism at the University of Newcastle, England, and Kamlesh Khunti, MD, PhD, professor of primary care diabetes at the University of Leicester, England.
Remarkable weight loss
Dr. Taylor powerfully recapitulated the initial results of the DiRECT study. T2D remission was achieved in 46% of participants who underwent a low-energy formula diet (around 850 calories daily) for 3-5 months. After 2 years’ follow-up, an impressive 36% of participants were still in remission. Dr. Taylor then discussed unpublished 5-year extension follow-up data of the DiRECT study. Average weight loss in the remaining intervention group was 6.1 kg. I echo Taylor’s sentiment that this finding is remarkable in the context of a dietary study.
Overall, 13% of participants were still in remission, and this cohort maintained an average weight loss of 8.9 kg. Dr. Taylor concluded that lasting remission of T2D is indeed feasible in a primary care setting.
Yet he acknowledged that although remission appears feasible in the longer term, it was not necessarily easy, or indeed possible, for everyone. He used a wonderful analogy about climbing Mount Everest: It is feasible, but not everyone can or wants to climb it. And even if you try, you might not reach the top.
This analogy perfectly encapsulates the challenges I have observed when my patients have striven for T2D remission. In my opinion, intensive weight management with a low-energy formula diet is not a panacea for T2D but another tool in our toolbox to offer patients.
He also described some “jaw-dropping” results regarding incidence of cancer: There were no cases of cancer in the intervention group during the 5-year period, but there were eight cases of cancer in the control group. The latter figure is consistent with published data for cancer incidence in patients with T2D and the body mass index (BMI) inclusion criteria for the DiRECT study (a BMI of 27-45 kg/m2). Obesity is an established risk factor for 13 types of cancer, and excess body fat entails an approximately 17% increased risk for cancer-specific mortality. This indeed is a powerful motivator to facilitate meaningful lifestyle change.
In primary care, we also need to be aware that most weight regain usually occurs secondary to a life event (for example, financial, family, or illness). We should reiterate to our patients that weight regain is not a failure; it is just part of life. Once the life event has passed, rapid weight loss can be attempted again. In the “rescue plans” that were integral to the DiRECT study, participants were offered further periods of total diet replacement, depending on quantity of weight gain. In fact, 50% of participants in DiRECT required rescue therapy, and their outcomes, reassuringly, were the same as the other 50%.
Dr. Taylor also quoted data from the ReTUNE study suggesting that weight regain was less of an issue for those with initial BMI of 21-27, and there is “more bang for your buck” in approaching remission of T2D in patients with lower BMI. The fact that people with normal or near-normal BMI can also reverse their T2D was also a game changer for my clinical practice; the concept of an individual or personal fat threshold that results in T2D offers a pragmatic explanation to patients with T2D who are frustrated by the lack of improvements in cardiometabolic parameters despite significant weight loss.
Finally, Dr. Taylor acknowledged the breadth of the definition of T2D remission: A1c < 48 mmol/mol at least 2 months off all antidiabetic medication. This definition includes A1c values within the “prediabetes” range: 42-47 mmol/mol.
He cited 10-year cardiovascular risk data driven by hypertension and dyslipidemia before significant weight loss and compared it with 10-year cardiovascular risk data after significant weight loss. Cardiovascular risk profile was more favorable after weight loss, compared with controls with prediabetes without weight loss, even though some of the intervention group who lost significant weight still had an A1c of 42-47 mmol/mol. Dr. Taylor suggested that we not label these individuals who have lost significant weight as having prediabetes. Instead “postdiabetes” should be preferred, because these patients had more favorable cardiovascular profiles.
This is a very important take-home message for primary care: prediabetes is more than just dysglycemia.
New terminology proposed
Dr. Khunti outlined a recent large, systematic review that concluded that the definition of T2D remission encompassed substantial heterogeneity. This heterogeneity complicates the interpretation of previous research on T2D remission and complicates the implementation of remission pathways into routine clinical practice. Furthermore, Dr. Khunti highlighted a recent consensus report on the definition and interpretation of remission in T2D that explicitly stated that the underlying pathophysiology of T2D is rarely normalized completely by interventions, thus reducing the possibility of lasting remission.
Dr. Khunti also challenged the cardiovascular benefits seen after T2D remission. Recent Danish registry data were presented, demonstrating a twofold increased risk for major adverse cardiovascular events over 5 years in individuals who achieved remission of T2D, but not on glucose-lowering drug therapy.
Adherence to strict dietary interventions in the longer term was also addressed. Diet-induced weight loss causes changes in circulating hormones such as ghrelin, glucose-dependent insulinotropic polypeptide (GIP), and leptin, which mediate appetite and drive hunger and an increased preference for energy-dense foods (that is, high-fat or sugary foods), all of which encourage weight regain. Dr. Khunti suggested that other interventions, such as glucagon-like peptide 1 (GLP-1) receptor agonists or bariatric surgery, specifically target some of these hormonal responses.
The challenges in recruitment and retention for lifestyle studies were also discussed; they reflect the challenges of behavioral programs in primary care. The DiRECT study had 20% participation of screened candidates and an attrition rate approaching 30%. The seminal Diabetes Prevention Program study and Finnish Diabetes Prevention Study had similar results. At a population level, individuals do not appear to want to participate in behavioral programs.
Dr. Khunti also warned that the review of annual care processes for diabetes is declining for patients who had achieved remission, possibly because of a false sense of reassurance among health care professionals. It is essential that all those in remission remain under at least annual follow-up, because there is still a risk for future microvascular and macrovascular complications, especially in the event of weight regain.
Dr. Khunti concluded by proposing new terminology for remission: remission of hyperglycemia or euglycemia, aiming for A1c < 48 mmol/mol with or without glucose-lowering therapy. I do agree with this; it reflects the zeitgeist of cardiorenal protective diabetes therapies and is analogous to rheumatoid arthritis, where remission is defined as no disease activity while on therapy. But one size does not fit all.
Sir William Osler’s words provide a fitting conclusion: “If it were not for the great variability among individuals, medicine might as well be a science and not an art.”
Dr. Fernando has disclosed that he has received speakers’ fees from Eli Lilly and Novo Nordisk.
Dr. Fernando is a general practitioner near Edinburgh, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education.
A version of this article first appeared on Medscape.com.
The prospect of remission of type 2 diabetes (T2D) has captured the hearts and minds of many patients with T2D and health care professionals, including myself.
I have changed my narrative when supporting my patients with T2D. I used to say that T2D is a progressive condition, but considering seminal recent evidence like the DiRECT trial, I now say that T2D can be a progressive condition. Through significant weight loss, patients can reverse it and achieve remission of T2D. This has given my patients hope that their T2D is no longer an inexorable condition. And hope, of course, is a powerful enabler of change.
However,
I therefore relished the opportunity to attend a debate on this topic at the annual meeting of the European Association for the Study of Diabetes in Hamburg, Germany, between Roy Taylor, MD, principal investigator for the DiRECT study and professor of medicine and metabolism at the University of Newcastle, England, and Kamlesh Khunti, MD, PhD, professor of primary care diabetes at the University of Leicester, England.
Remarkable weight loss
Dr. Taylor powerfully recapitulated the initial results of the DiRECT study. T2D remission was achieved in 46% of participants who underwent a low-energy formula diet (around 850 calories daily) for 3-5 months. After 2 years’ follow-up, an impressive 36% of participants were still in remission. Dr. Taylor then discussed unpublished 5-year extension follow-up data of the DiRECT study. Average weight loss in the remaining intervention group was 6.1 kg. I echo Taylor’s sentiment that this finding is remarkable in the context of a dietary study.
Overall, 13% of participants were still in remission, and this cohort maintained an average weight loss of 8.9 kg. Dr. Taylor concluded that lasting remission of T2D is indeed feasible in a primary care setting.
Yet he acknowledged that although remission appears feasible in the longer term, it was not necessarily easy, or indeed possible, for everyone. He used a wonderful analogy about climbing Mount Everest: It is feasible, but not everyone can or wants to climb it. And even if you try, you might not reach the top.
This analogy perfectly encapsulates the challenges I have observed when my patients have striven for T2D remission. In my opinion, intensive weight management with a low-energy formula diet is not a panacea for T2D but another tool in our toolbox to offer patients.
He also described some “jaw-dropping” results regarding incidence of cancer: There were no cases of cancer in the intervention group during the 5-year period, but there were eight cases of cancer in the control group. The latter figure is consistent with published data for cancer incidence in patients with T2D and the body mass index (BMI) inclusion criteria for the DiRECT study (a BMI of 27-45 kg/m2). Obesity is an established risk factor for 13 types of cancer, and excess body fat entails an approximately 17% increased risk for cancer-specific mortality. This indeed is a powerful motivator to facilitate meaningful lifestyle change.
In primary care, we also need to be aware that most weight regain usually occurs secondary to a life event (for example, financial, family, or illness). We should reiterate to our patients that weight regain is not a failure; it is just part of life. Once the life event has passed, rapid weight loss can be attempted again. In the “rescue plans” that were integral to the DiRECT study, participants were offered further periods of total diet replacement, depending on quantity of weight gain. In fact, 50% of participants in DiRECT required rescue therapy, and their outcomes, reassuringly, were the same as the other 50%.
Dr. Taylor also quoted data from the ReTUNE study suggesting that weight regain was less of an issue for those with initial BMI of 21-27, and there is “more bang for your buck” in approaching remission of T2D in patients with lower BMI. The fact that people with normal or near-normal BMI can also reverse their T2D was also a game changer for my clinical practice; the concept of an individual or personal fat threshold that results in T2D offers a pragmatic explanation to patients with T2D who are frustrated by the lack of improvements in cardiometabolic parameters despite significant weight loss.
Finally, Dr. Taylor acknowledged the breadth of the definition of T2D remission: A1c < 48 mmol/mol at least 2 months off all antidiabetic medication. This definition includes A1c values within the “prediabetes” range: 42-47 mmol/mol.
He cited 10-year cardiovascular risk data driven by hypertension and dyslipidemia before significant weight loss and compared it with 10-year cardiovascular risk data after significant weight loss. Cardiovascular risk profile was more favorable after weight loss, compared with controls with prediabetes without weight loss, even though some of the intervention group who lost significant weight still had an A1c of 42-47 mmol/mol. Dr. Taylor suggested that we not label these individuals who have lost significant weight as having prediabetes. Instead “postdiabetes” should be preferred, because these patients had more favorable cardiovascular profiles.
This is a very important take-home message for primary care: prediabetes is more than just dysglycemia.
New terminology proposed
Dr. Khunti outlined a recent large, systematic review that concluded that the definition of T2D remission encompassed substantial heterogeneity. This heterogeneity complicates the interpretation of previous research on T2D remission and complicates the implementation of remission pathways into routine clinical practice. Furthermore, Dr. Khunti highlighted a recent consensus report on the definition and interpretation of remission in T2D that explicitly stated that the underlying pathophysiology of T2D is rarely normalized completely by interventions, thus reducing the possibility of lasting remission.
Dr. Khunti also challenged the cardiovascular benefits seen after T2D remission. Recent Danish registry data were presented, demonstrating a twofold increased risk for major adverse cardiovascular events over 5 years in individuals who achieved remission of T2D, but not on glucose-lowering drug therapy.
Adherence to strict dietary interventions in the longer term was also addressed. Diet-induced weight loss causes changes in circulating hormones such as ghrelin, glucose-dependent insulinotropic polypeptide (GIP), and leptin, which mediate appetite and drive hunger and an increased preference for energy-dense foods (that is, high-fat or sugary foods), all of which encourage weight regain. Dr. Khunti suggested that other interventions, such as glucagon-like peptide 1 (GLP-1) receptor agonists or bariatric surgery, specifically target some of these hormonal responses.
The challenges in recruitment and retention for lifestyle studies were also discussed; they reflect the challenges of behavioral programs in primary care. The DiRECT study had 20% participation of screened candidates and an attrition rate approaching 30%. The seminal Diabetes Prevention Program study and Finnish Diabetes Prevention Study had similar results. At a population level, individuals do not appear to want to participate in behavioral programs.
Dr. Khunti also warned that the review of annual care processes for diabetes is declining for patients who had achieved remission, possibly because of a false sense of reassurance among health care professionals. It is essential that all those in remission remain under at least annual follow-up, because there is still a risk for future microvascular and macrovascular complications, especially in the event of weight regain.
Dr. Khunti concluded by proposing new terminology for remission: remission of hyperglycemia or euglycemia, aiming for A1c < 48 mmol/mol with or without glucose-lowering therapy. I do agree with this; it reflects the zeitgeist of cardiorenal protective diabetes therapies and is analogous to rheumatoid arthritis, where remission is defined as no disease activity while on therapy. But one size does not fit all.
Sir William Osler’s words provide a fitting conclusion: “If it were not for the great variability among individuals, medicine might as well be a science and not an art.”
Dr. Fernando has disclosed that he has received speakers’ fees from Eli Lilly and Novo Nordisk.
Dr. Fernando is a general practitioner near Edinburgh, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education.
A version of this article first appeared on Medscape.com.
The prospect of remission of type 2 diabetes (T2D) has captured the hearts and minds of many patients with T2D and health care professionals, including myself.
I have changed my narrative when supporting my patients with T2D. I used to say that T2D is a progressive condition, but considering seminal recent evidence like the DiRECT trial, I now say that T2D can be a progressive condition. Through significant weight loss, patients can reverse it and achieve remission of T2D. This has given my patients hope that their T2D is no longer an inexorable condition. And hope, of course, is a powerful enabler of change.
However,
I therefore relished the opportunity to attend a debate on this topic at the annual meeting of the European Association for the Study of Diabetes in Hamburg, Germany, between Roy Taylor, MD, principal investigator for the DiRECT study and professor of medicine and metabolism at the University of Newcastle, England, and Kamlesh Khunti, MD, PhD, professor of primary care diabetes at the University of Leicester, England.
Remarkable weight loss
Dr. Taylor powerfully recapitulated the initial results of the DiRECT study. T2D remission was achieved in 46% of participants who underwent a low-energy formula diet (around 850 calories daily) for 3-5 months. After 2 years’ follow-up, an impressive 36% of participants were still in remission. Dr. Taylor then discussed unpublished 5-year extension follow-up data of the DiRECT study. Average weight loss in the remaining intervention group was 6.1 kg. I echo Taylor’s sentiment that this finding is remarkable in the context of a dietary study.
Overall, 13% of participants were still in remission, and this cohort maintained an average weight loss of 8.9 kg. Dr. Taylor concluded that lasting remission of T2D is indeed feasible in a primary care setting.
Yet he acknowledged that although remission appears feasible in the longer term, it was not necessarily easy, or indeed possible, for everyone. He used a wonderful analogy about climbing Mount Everest: It is feasible, but not everyone can or wants to climb it. And even if you try, you might not reach the top.
This analogy perfectly encapsulates the challenges I have observed when my patients have striven for T2D remission. In my opinion, intensive weight management with a low-energy formula diet is not a panacea for T2D but another tool in our toolbox to offer patients.
He also described some “jaw-dropping” results regarding incidence of cancer: There were no cases of cancer in the intervention group during the 5-year period, but there were eight cases of cancer in the control group. The latter figure is consistent with published data for cancer incidence in patients with T2D and the body mass index (BMI) inclusion criteria for the DiRECT study (a BMI of 27-45 kg/m2). Obesity is an established risk factor for 13 types of cancer, and excess body fat entails an approximately 17% increased risk for cancer-specific mortality. This indeed is a powerful motivator to facilitate meaningful lifestyle change.
In primary care, we also need to be aware that most weight regain usually occurs secondary to a life event (for example, financial, family, or illness). We should reiterate to our patients that weight regain is not a failure; it is just part of life. Once the life event has passed, rapid weight loss can be attempted again. In the “rescue plans” that were integral to the DiRECT study, participants were offered further periods of total diet replacement, depending on quantity of weight gain. In fact, 50% of participants in DiRECT required rescue therapy, and their outcomes, reassuringly, were the same as the other 50%.
Dr. Taylor also quoted data from the ReTUNE study suggesting that weight regain was less of an issue for those with initial BMI of 21-27, and there is “more bang for your buck” in approaching remission of T2D in patients with lower BMI. The fact that people with normal or near-normal BMI can also reverse their T2D was also a game changer for my clinical practice; the concept of an individual or personal fat threshold that results in T2D offers a pragmatic explanation to patients with T2D who are frustrated by the lack of improvements in cardiometabolic parameters despite significant weight loss.
Finally, Dr. Taylor acknowledged the breadth of the definition of T2D remission: A1c < 48 mmol/mol at least 2 months off all antidiabetic medication. This definition includes A1c values within the “prediabetes” range: 42-47 mmol/mol.
He cited 10-year cardiovascular risk data driven by hypertension and dyslipidemia before significant weight loss and compared it with 10-year cardiovascular risk data after significant weight loss. Cardiovascular risk profile was more favorable after weight loss, compared with controls with prediabetes without weight loss, even though some of the intervention group who lost significant weight still had an A1c of 42-47 mmol/mol. Dr. Taylor suggested that we not label these individuals who have lost significant weight as having prediabetes. Instead “postdiabetes” should be preferred, because these patients had more favorable cardiovascular profiles.
This is a very important take-home message for primary care: prediabetes is more than just dysglycemia.
New terminology proposed
Dr. Khunti outlined a recent large, systematic review that concluded that the definition of T2D remission encompassed substantial heterogeneity. This heterogeneity complicates the interpretation of previous research on T2D remission and complicates the implementation of remission pathways into routine clinical practice. Furthermore, Dr. Khunti highlighted a recent consensus report on the definition and interpretation of remission in T2D that explicitly stated that the underlying pathophysiology of T2D is rarely normalized completely by interventions, thus reducing the possibility of lasting remission.
Dr. Khunti also challenged the cardiovascular benefits seen after T2D remission. Recent Danish registry data were presented, demonstrating a twofold increased risk for major adverse cardiovascular events over 5 years in individuals who achieved remission of T2D, but not on glucose-lowering drug therapy.
Adherence to strict dietary interventions in the longer term was also addressed. Diet-induced weight loss causes changes in circulating hormones such as ghrelin, glucose-dependent insulinotropic polypeptide (GIP), and leptin, which mediate appetite and drive hunger and an increased preference for energy-dense foods (that is, high-fat or sugary foods), all of which encourage weight regain. Dr. Khunti suggested that other interventions, such as glucagon-like peptide 1 (GLP-1) receptor agonists or bariatric surgery, specifically target some of these hormonal responses.
The challenges in recruitment and retention for lifestyle studies were also discussed; they reflect the challenges of behavioral programs in primary care. The DiRECT study had 20% participation of screened candidates and an attrition rate approaching 30%. The seminal Diabetes Prevention Program study and Finnish Diabetes Prevention Study had similar results. At a population level, individuals do not appear to want to participate in behavioral programs.
Dr. Khunti also warned that the review of annual care processes for diabetes is declining for patients who had achieved remission, possibly because of a false sense of reassurance among health care professionals. It is essential that all those in remission remain under at least annual follow-up, because there is still a risk for future microvascular and macrovascular complications, especially in the event of weight regain.
Dr. Khunti concluded by proposing new terminology for remission: remission of hyperglycemia or euglycemia, aiming for A1c < 48 mmol/mol with or without glucose-lowering therapy. I do agree with this; it reflects the zeitgeist of cardiorenal protective diabetes therapies and is analogous to rheumatoid arthritis, where remission is defined as no disease activity while on therapy. But one size does not fit all.
Sir William Osler’s words provide a fitting conclusion: “If it were not for the great variability among individuals, medicine might as well be a science and not an art.”
Dr. Fernando has disclosed that he has received speakers’ fees from Eli Lilly and Novo Nordisk.
Dr. Fernando is a general practitioner near Edinburgh, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education.
A version of this article first appeared on Medscape.com.
Abatacept, certolizumab: Best biologics in early RA
TOPLINE:
In combination with methotrexate, both abatacept (Orencia) and certolizumab pegol (Cimzia), but not tocilizumab (Actemra), showed superiority over different combinations of active conventional disease-modifying antirheumatic drugs (DMARDs) for promoting remission in patients with early, untreated rheumatoid arthritis.
METHODOLOGY:
The study population included 812 adults from sites in six European countries who had treatment-naive early RA (less than 24 months’ duration) and moderate to severe disease.
Participants were randomly assigned to open-label treatment with methotrexate plus one of four treatments:
- Active conventional therapy (oral , tapered quickly and discontinued after 9 months, or , hydroxychloroquine, and intra-articular glucocorticoid injections in swollen joints).
- Certolizumab pegol.
- Abatacept.
- Tocilizumab.
In all the biologic-treated groups, intra-articular glucocorticoid injections were allowed on demand up to week 12; thereafter, up to 40 mg were allowed every 12 weeks. In all groups, intra-articular glucocorticoids were prohibited in weeks 20-24 and weeks 44-48 to minimize their influence on week 24 and week 48 outcomes.
TAKEAWAY:
- Clinical remission rates at week 48 based on Clinical Disease Activity Index scores of 2.8 or less were 59.3% with abatacept and 52.3% with certolizumab, which were significantly greater than the rate of 39.2% seen with active conventional therapy. The 51.9% rate seen with tocilizumab was not superior to active conventional therapy.
- The co–primary outcome of change in van der Heijde-modified Sharp Score from baseline to week 48 was relatively low across all groups: 0.45 for active conventional therapy, and 0.62, 0.47, and 0.50 for abatacept, certolizumab pegol, and tocilizumab, respectively.
- No new safety signals appeared, nor did any significantly increased risk associated with glucocorticoid use; at least one adverse event was reported in 88.3%, 89.6%, 85.8%, and 96.7% of patients taking conventional therapy, certolizumab pegol, abatacept, and tocilizumab, respectively.
IN PRACTICE:
The results suggest that both abatacept and certolizumab pegol yield higher remission rates than optimized conventional therapy, and “should be considered when the management of patients with newly diagnosed RA is decided, both in clinical practice and in treatment recommendations,” the authors write.
SOURCE:
The lead author on the study was Mikkel Østergaard, MD, PhD, of the Center for Rheumatology and Spine Diseases, Rigshospitalet, Copenhagen. The study was published online in Annals of the Rheumatic Disease.
LIMITATIONS:
The open-label study design could influence some subjective outcomes, and conventional therapy included two slightly different strategies based on national recommendations for the individual countries.
DISCLOSURES:
The study was funded by multiple public sources to centers from countries participating in the study, as well as the Icelandic Society for Rheumatology, the Swedish Rheumatism Association, and the Research Fund of University Hospital, Reykjavik, Iceland. UCB and Bristol-Myers Squibb provided certolizumab pegol and abatacept, respectively, at no cost, but were not otherwise involved in the study. Many authors, including Dr. Østergaard, report financial relationships with multiple pharmaceutical companies. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
In combination with methotrexate, both abatacept (Orencia) and certolizumab pegol (Cimzia), but not tocilizumab (Actemra), showed superiority over different combinations of active conventional disease-modifying antirheumatic drugs (DMARDs) for promoting remission in patients with early, untreated rheumatoid arthritis.
METHODOLOGY:
The study population included 812 adults from sites in six European countries who had treatment-naive early RA (less than 24 months’ duration) and moderate to severe disease.
Participants were randomly assigned to open-label treatment with methotrexate plus one of four treatments:
- Active conventional therapy (oral , tapered quickly and discontinued after 9 months, or , hydroxychloroquine, and intra-articular glucocorticoid injections in swollen joints).
- Certolizumab pegol.
- Abatacept.
- Tocilizumab.
In all the biologic-treated groups, intra-articular glucocorticoid injections were allowed on demand up to week 12; thereafter, up to 40 mg were allowed every 12 weeks. In all groups, intra-articular glucocorticoids were prohibited in weeks 20-24 and weeks 44-48 to minimize their influence on week 24 and week 48 outcomes.
TAKEAWAY:
- Clinical remission rates at week 48 based on Clinical Disease Activity Index scores of 2.8 or less were 59.3% with abatacept and 52.3% with certolizumab, which were significantly greater than the rate of 39.2% seen with active conventional therapy. The 51.9% rate seen with tocilizumab was not superior to active conventional therapy.
- The co–primary outcome of change in van der Heijde-modified Sharp Score from baseline to week 48 was relatively low across all groups: 0.45 for active conventional therapy, and 0.62, 0.47, and 0.50 for abatacept, certolizumab pegol, and tocilizumab, respectively.
- No new safety signals appeared, nor did any significantly increased risk associated with glucocorticoid use; at least one adverse event was reported in 88.3%, 89.6%, 85.8%, and 96.7% of patients taking conventional therapy, certolizumab pegol, abatacept, and tocilizumab, respectively.
IN PRACTICE:
The results suggest that both abatacept and certolizumab pegol yield higher remission rates than optimized conventional therapy, and “should be considered when the management of patients with newly diagnosed RA is decided, both in clinical practice and in treatment recommendations,” the authors write.
SOURCE:
The lead author on the study was Mikkel Østergaard, MD, PhD, of the Center for Rheumatology and Spine Diseases, Rigshospitalet, Copenhagen. The study was published online in Annals of the Rheumatic Disease.
LIMITATIONS:
The open-label study design could influence some subjective outcomes, and conventional therapy included two slightly different strategies based on national recommendations for the individual countries.
DISCLOSURES:
The study was funded by multiple public sources to centers from countries participating in the study, as well as the Icelandic Society for Rheumatology, the Swedish Rheumatism Association, and the Research Fund of University Hospital, Reykjavik, Iceland. UCB and Bristol-Myers Squibb provided certolizumab pegol and abatacept, respectively, at no cost, but were not otherwise involved in the study. Many authors, including Dr. Østergaard, report financial relationships with multiple pharmaceutical companies. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
In combination with methotrexate, both abatacept (Orencia) and certolizumab pegol (Cimzia), but not tocilizumab (Actemra), showed superiority over different combinations of active conventional disease-modifying antirheumatic drugs (DMARDs) for promoting remission in patients with early, untreated rheumatoid arthritis.
METHODOLOGY:
The study population included 812 adults from sites in six European countries who had treatment-naive early RA (less than 24 months’ duration) and moderate to severe disease.
Participants were randomly assigned to open-label treatment with methotrexate plus one of four treatments:
- Active conventional therapy (oral , tapered quickly and discontinued after 9 months, or , hydroxychloroquine, and intra-articular glucocorticoid injections in swollen joints).
- Certolizumab pegol.
- Abatacept.
- Tocilizumab.
In all the biologic-treated groups, intra-articular glucocorticoid injections were allowed on demand up to week 12; thereafter, up to 40 mg were allowed every 12 weeks. In all groups, intra-articular glucocorticoids were prohibited in weeks 20-24 and weeks 44-48 to minimize their influence on week 24 and week 48 outcomes.
TAKEAWAY:
- Clinical remission rates at week 48 based on Clinical Disease Activity Index scores of 2.8 or less were 59.3% with abatacept and 52.3% with certolizumab, which were significantly greater than the rate of 39.2% seen with active conventional therapy. The 51.9% rate seen with tocilizumab was not superior to active conventional therapy.
- The co–primary outcome of change in van der Heijde-modified Sharp Score from baseline to week 48 was relatively low across all groups: 0.45 for active conventional therapy, and 0.62, 0.47, and 0.50 for abatacept, certolizumab pegol, and tocilizumab, respectively.
- No new safety signals appeared, nor did any significantly increased risk associated with glucocorticoid use; at least one adverse event was reported in 88.3%, 89.6%, 85.8%, and 96.7% of patients taking conventional therapy, certolizumab pegol, abatacept, and tocilizumab, respectively.
IN PRACTICE:
The results suggest that both abatacept and certolizumab pegol yield higher remission rates than optimized conventional therapy, and “should be considered when the management of patients with newly diagnosed RA is decided, both in clinical practice and in treatment recommendations,” the authors write.
SOURCE:
The lead author on the study was Mikkel Østergaard, MD, PhD, of the Center for Rheumatology and Spine Diseases, Rigshospitalet, Copenhagen. The study was published online in Annals of the Rheumatic Disease.
LIMITATIONS:
The open-label study design could influence some subjective outcomes, and conventional therapy included two slightly different strategies based on national recommendations for the individual countries.
DISCLOSURES:
The study was funded by multiple public sources to centers from countries participating in the study, as well as the Icelandic Society for Rheumatology, the Swedish Rheumatism Association, and the Research Fund of University Hospital, Reykjavik, Iceland. UCB and Bristol-Myers Squibb provided certolizumab pegol and abatacept, respectively, at no cost, but were not otherwise involved in the study. Many authors, including Dr. Østergaard, report financial relationships with multiple pharmaceutical companies. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Do screening mammograms in women aged 70 and older improve stage at diagnosis or breast cancer–specific mortality?
Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443

Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.

Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
EXPERT COMMENTARY
A screening test is performed to detect potential health disorders or diseases in people who do not have any symptoms of disease. The goal of screening is to detect the condition early enough to treat it most effectively, and ultimately to decrease morbidity and mortality related to the disease. Overdiagnosis refers to the finding of a cancer that would not have caused clinical problems during a person’s lifetime.
Current guidelines for the early detection of breast cancer vary considerably, including recommendations for what age to initiate screening, the cadence of screening (annual or biannual), the use of ancillary screening for people with dense breasts, and importantly the upper age limit for which screening is advised. The US Preventive Services Task Force recommends continuing screening to age 74. The American Cancer Society suggests ongoing screening if life expectancy is estimated at more than 10 years, and the American College of Physicians recommends stopping screening at age 75, or younger if life expectancy is less than 10 years. The American College of Obstetricians and Gynecologists states that women at average risk of breast cancer should continue screening mammography until at least age 75.
Overdiagnosis is a difficult concept for clinicians to understand let alone explain to our patients. Recently, Richman and colleagues published the results of their study aimed at estimating overdiagnosis associated with breast cancer screening among older women.1 As Dr. Otis Brawley, former Chief Medical and Scientific Officer of the American Cancer Society and current Distinguished Professor of Oncology and Epidemiology at Johns Hopkins University, states in the editorial that accompanies the study by Richman and colleagues, “Some tumors are not destined to grow, spread, and kill due to their genomics or their microenvironment. A second type of overdiagnosis involves small tumors that do have the potential to grow but will not grow fast enough to bother the patient within their natural lifetime.”2
Although screening mammography in older women results in frequent false positives that require additional imaging as well as biopsies, we have become more aware of the potential of overdiagnosis as an important downside of screening mammography in an elderly population.
Continue to: Details of the study...
Details of the study
Using the SEER registry to identify breast cancers linked to a 5% sample of Medicare beneficiaries, Richman and colleagues (funded by the National Cancer Institute and based at Yale University) conducted a retrospectivecohort study to estimate the likelihood of overdiagnosis associated with screening mammography among older women over 15 years of follow-up. Specifically, they assessed the difference in cumulative incidence of in situ and invasive breast cancer among women aged 70 years and older without a history of breast cancer when screened in 2002. During the subsequent 3 years, participants either continued screening (screened group) or did not (unscreened group). Women were followed through 2017.
Among almost 55,000 women followed, 88% were White, 6% were Black, and 3% were Hispanic. Mean follow-up was 13.7 years among women aged 70 to 74 years at baseline. For those aged 75 to 84 at baseline, mean follow-up was 10 years, and for those aged 85 years and older, mean follow-up was 5.7 years.
Estimated rates of overdiagnosis. Overall, among women aged 70 to 74 at baseline who were eventually diagnosed with breast cancer, the investigators estimated that 31% of these cancers were overdiagnosed. The corresponding percentage of breast cancers estimated to represent overdiagnosis climbed to 47% for those aged 75 to 84 years at baseline and to 54% for those aged 85 years and older at baseline.
The investigators assessed the impact of greater screening among women with a first-degree relative with a diagnosis of breast cancer and determined that this did not explain their results. With respect to cancer stage, the investigators noted that overdiagnosis was more prevalent among in situ and localized invasive cancers compared with those with regional or distant spread. Of note, the incidence of cancer with regional or distant spread was neither higher nor lower among those who were screened. Finally, the investigators did not observe significant differences in breast cancer–specific mortality by screening status.
The proportion of cancers that were overdiagnosed was particularly high among women with in situ as well as those with localized invasive disease. The investigators pointed out that as many as 90% of women aged 80 and older diagnosed with localized cancer undergo surgery, and almost two-thirds of those older than 70 years have radiation therapy for early-stage disease. In addition to the burdens associated with these treatments for overdiagnosed cancers in older women, simply being diagnosed with breast cancer profoundly affects the health and well-being of women, resulting in anxiety and substantial reductions in quality of life.
The authors also noted that some studies suggest that, among breast cancers diagnosed with screening, chemotherapy is less likely to be employed among older women, a screening benefit that must be weighed against the high likelihood of overdiagnosis. However, this benefit is unlikely to be meaningful for the majority of patients in this study who presented with in situ or early invasive lesions since chemotherapy often is not recommended for such women.
Study strengths and limitations
If screening mammography is effective, the incidence of advanced-stage tumors and breast cancer–specific mortality should be reduced in screened populations. Accordingly, in this large, long-term study using reliable sources of data, the findings that the incidence of advanced-stage disease as well as breast cancer–specific mortality were similar in the screened and unscreened cohorts provides powerful evidence that screening mammography is not effective in older women.3
As the authors pointed out, their findings regarding a high prevalence of overdiagnosis associated with screening mammography in older women are consistent with findings of other studies, some of which used different methodology.
The authors acknowledged that some women in their Medicare cohort who initially continued screening likely stopped screening subsequently, while some who initially did not continue screening might have been screened subsequently. They went on to indicate that if patients were completely adherent with subsequent screening (or not getting screened) the likelihood that cancers among screened women were overdiagnosed would be even higher.
Lead-time bias occurs when screening finds a cancer earlier than that cancer would have been diagnosed because of symptoms. This study followed the cohorts over a long timeframe to reduce the possibility that lead time was inappropriately identified as overdiagnosis. They also observed that, among women aged 85 and older, most cohort members had died by the end of study follow-up; accordingly, lead time is not likely to have explained their findings.
Limitations. The authors acknowledged that miscoding the mammogram type (screening vs diagnostic) could result in higher estimates of overdiagnosis. In their most conservative sensitivity analysis, the overdiagnosis rates could be as low as 15% for women aged 70 to 74, 36% for those aged 75 to 84, and 44% for people aged 85 and older.
Because this was an observational cohort study, unmeasured differences in breast cancer risk and underlying health factors may have been confounders. Specifically, people with severe life-threatening conditions that limited their expected life span may have chosen not to undergo regular screening. Although the authors did attempt to adjust for these factors, there may have been unrecognized confounders. This study was designed to estimate overdiagnosis, and therefore the specific benefits and harms of screening could not be addressed based on the data collected. ●
The high prevalence of overdiagnosis and lack of a breast cancer–specific mortality benefit among older women who undergo screening mammography is sobering. Clinician recommendations and shared decision making with our patients regarding screening mammography should take into consideration overdiagnosis and the considerable harms associated with overtreatment. Although we may recognize that overdiagnosed cancers are often indolent tumors with a long presymptomatic phase, in older women, even finding a biologically aggressive cancer may represent overdiagnosis if life expectancy is limited.
BARBARA LEVY, MD, MSCP; ANDREW M. KAUNITZ, MD, MSCP.
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443
- Richman IB, Long JB, Soulos PR, et al. Estimating breast cancer overdiagnosis after screening mammography among older women in the United States. Ann Intern Med. 2023;176:1172-1180. doi:10.7326/M23-0133
- Brawley OW, Ramalingam R. Understanding the varying biological behaviors of breast and other types of cancer to avoid overdiagnosis. Ann Intern Med. 2023;176:1273-1274. doi:10.7326/M23-18953
- Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer—lessons in cancer dynamics. N Engl J Med. 2015;373:1685-1687. doi:10.1056/NEJM p1510443
Making time to care for patients with diabetes
Can busy primary care offices continue to care for patients with diabetes? No one would argue that it is involved and takes effort, and health care providers are bankrupt when it comes to sparing additional time for this chronic disease. With roughly 37 million people living with diabetes and 96 million with prediabetes or early type 2 diabetes, and just over 8,000 practicing endocrinologists in the United States, we all need to make time especially in primary care to provide insight and holistic care. With limited time and budget, how do we do this?
First, decide to be involved in caring for patients with diabetes. Diabetes is best managed by interprofessional care teams, so you’re not going it alone. These teams may include physicians; pharmacists; physician assistants; advanced practice nurses; registered nurses; certified diabetes care and education specialists (CDCES); dietitians; and other professionals such as social workers, behavioral health professionals, medical assistants, and community health workers. Know which professionals are available to serve on your team, either within your clinic or as a consultant, and reach out to them to share the care and ease the burden. Remember to refer to these professionals to reinforce the diabetes intervention message to the patient.
Second, incorporate “diabetes only” appointments into your schedule, allowing time to focus on current comprehensive diabetes treatment goals, barriers/inertia for care. Remember to have short-interval follow-up as needed to keep that patient engaged to achieve their targets. Instruct your office staff to create diabetes appointment templates and reminders to patients to bring diabetes-related technologies, medication lists, and diabetes questions to the appointment. When I implemented this change, my patients welcomed the focus on their diabetes health, and they knew we were prioritizing this disease that they have for a lifetime. These appointments did not take away from their other conditions; rather, they often reminded me to stay focused on their diabetes and associated coconditions.
Taking the time to establish efficient workflows before implementing diabetes care saves countless hours later and immediately maximizes health care provider–patient interactions. Assign specific staff duties and expectations related to diabetes appointments, such as downloading diabetes technology, medication reconciliation, laboratory data, point-of-care hemoglobin A1c, basic foot exam, and patient goals for diabetes care. This allows the prescriber to focus on the glycemic, cardiologic, renal, and metabolic goals and overcome the therapeutic inertia that plagues us all.
Incorporating diabetes-related technology into clinical practice can be a significant time-saver but requires initial onboarding. Set aside a few hours to create a technology clinic flow, and designate at least one team member to be responsible for obtaining patient data before, during, or after encounters. If possible, obtaining data ahead of the visit will enhance efficiency, allowing for meaningful discussion of blood glucose and lifestyle patterns. Diabetes technology reveals the gaps in care and enhances our ability to identify the areas where glycemic intervention is needed. In addition, it reveals the impact of food choices, activity level, stress, and medication adherence to the person living with diabetes.
Finally, be proactive about therapeutic inertia. This is defined as a prescribers’ failure to intensify or deintensify a patient’s treatment when appropriate to do so. Causes of therapeutic inertia can be placed at the primary care physician level, including time constraints or inexperience in treating diabetes; the patient level, such as concerns about side effects or new treatment regimens; or a systemic level, such as availability of medications or their costs. Be real with yourself: We all have inertia and can identify areas to overcome. Never let inertia be traced back to you.
Not all inertia lives with the health care provider. Patients bring apprehension and concerns, have questions, and just want to share the frustrations associated with living their best life with the disease. Don’t assume that you know what your patients’ treatment barriers are; ask them. If you don’t have an answer, then note it and come up with one by the next follow-up. Remember that this is a chronic disease – a marathon, not a sprint. You don’t have to solve everything at one appointment; rather, keep the momentum going.
Let’s put this into clinical practice. For the next patient with diabetes who comes into your office, discuss with them your intention to prioritize their diabetes by having an appointment set aside to specifically focus on their individual goals and targets for their disease. Have the patient list any barriers and treatment goals they would like to review; flag your schedule to indicate it is a diabetes-only visit; and orient your staff to reconcile diabetes medications and record the patient’s last eye exam, urine albumin-to-creatinine ratio, A1c result, and blood glucose data. During this encounter, identify the patient’s personal targets for control, examine their feet, and review or order necessary laboratory metrics. Explore the patient-reported barriers and make inroads to remove or alleviate these. Advance treatment intervention, and schedule follow-up: every 4-6 weeks if the A1c is > 9%, every 2 months if it’s 7% to < 9%, and every 3-6 months if it’s < 7%. Utilize team diabetes care, such as CDCES referrals, dietitians, online resources, and community members, to help reinforce care and enhance engagement.
We need to take steps in our clinical practice to make the necessary space to accommodate this pervasive disease affecting nearly one-third of our population. Take a moment to look up and determine what needs to be in place so that you can take care of the people in your practice with diabetes. Laying the groundwork for implementing diabetes-only appointments can be time-consuming, but establishing consistent procedures, developing efficient workflows, and clearly defining roles and responsibilities is well worth the effort. This solid foundation equips the office, health care providers, and staff to care for persons with diabetes and will be invaluable to ensure that time for this care is available in the day-to-day clinical practice.
A version of this article first appeared on Medscape.com.
Can busy primary care offices continue to care for patients with diabetes? No one would argue that it is involved and takes effort, and health care providers are bankrupt when it comes to sparing additional time for this chronic disease. With roughly 37 million people living with diabetes and 96 million with prediabetes or early type 2 diabetes, and just over 8,000 practicing endocrinologists in the United States, we all need to make time especially in primary care to provide insight and holistic care. With limited time and budget, how do we do this?
First, decide to be involved in caring for patients with diabetes. Diabetes is best managed by interprofessional care teams, so you’re not going it alone. These teams may include physicians; pharmacists; physician assistants; advanced practice nurses; registered nurses; certified diabetes care and education specialists (CDCES); dietitians; and other professionals such as social workers, behavioral health professionals, medical assistants, and community health workers. Know which professionals are available to serve on your team, either within your clinic or as a consultant, and reach out to them to share the care and ease the burden. Remember to refer to these professionals to reinforce the diabetes intervention message to the patient.
Second, incorporate “diabetes only” appointments into your schedule, allowing time to focus on current comprehensive diabetes treatment goals, barriers/inertia for care. Remember to have short-interval follow-up as needed to keep that patient engaged to achieve their targets. Instruct your office staff to create diabetes appointment templates and reminders to patients to bring diabetes-related technologies, medication lists, and diabetes questions to the appointment. When I implemented this change, my patients welcomed the focus on their diabetes health, and they knew we were prioritizing this disease that they have for a lifetime. These appointments did not take away from their other conditions; rather, they often reminded me to stay focused on their diabetes and associated coconditions.
Taking the time to establish efficient workflows before implementing diabetes care saves countless hours later and immediately maximizes health care provider–patient interactions. Assign specific staff duties and expectations related to diabetes appointments, such as downloading diabetes technology, medication reconciliation, laboratory data, point-of-care hemoglobin A1c, basic foot exam, and patient goals for diabetes care. This allows the prescriber to focus on the glycemic, cardiologic, renal, and metabolic goals and overcome the therapeutic inertia that plagues us all.
Incorporating diabetes-related technology into clinical practice can be a significant time-saver but requires initial onboarding. Set aside a few hours to create a technology clinic flow, and designate at least one team member to be responsible for obtaining patient data before, during, or after encounters. If possible, obtaining data ahead of the visit will enhance efficiency, allowing for meaningful discussion of blood glucose and lifestyle patterns. Diabetes technology reveals the gaps in care and enhances our ability to identify the areas where glycemic intervention is needed. In addition, it reveals the impact of food choices, activity level, stress, and medication adherence to the person living with diabetes.
Finally, be proactive about therapeutic inertia. This is defined as a prescribers’ failure to intensify or deintensify a patient’s treatment when appropriate to do so. Causes of therapeutic inertia can be placed at the primary care physician level, including time constraints or inexperience in treating diabetes; the patient level, such as concerns about side effects or new treatment regimens; or a systemic level, such as availability of medications or their costs. Be real with yourself: We all have inertia and can identify areas to overcome. Never let inertia be traced back to you.
Not all inertia lives with the health care provider. Patients bring apprehension and concerns, have questions, and just want to share the frustrations associated with living their best life with the disease. Don’t assume that you know what your patients’ treatment barriers are; ask them. If you don’t have an answer, then note it and come up with one by the next follow-up. Remember that this is a chronic disease – a marathon, not a sprint. You don’t have to solve everything at one appointment; rather, keep the momentum going.
Let’s put this into clinical practice. For the next patient with diabetes who comes into your office, discuss with them your intention to prioritize their diabetes by having an appointment set aside to specifically focus on their individual goals and targets for their disease. Have the patient list any barriers and treatment goals they would like to review; flag your schedule to indicate it is a diabetes-only visit; and orient your staff to reconcile diabetes medications and record the patient’s last eye exam, urine albumin-to-creatinine ratio, A1c result, and blood glucose data. During this encounter, identify the patient’s personal targets for control, examine their feet, and review or order necessary laboratory metrics. Explore the patient-reported barriers and make inroads to remove or alleviate these. Advance treatment intervention, and schedule follow-up: every 4-6 weeks if the A1c is > 9%, every 2 months if it’s 7% to < 9%, and every 3-6 months if it’s < 7%. Utilize team diabetes care, such as CDCES referrals, dietitians, online resources, and community members, to help reinforce care and enhance engagement.
We need to take steps in our clinical practice to make the necessary space to accommodate this pervasive disease affecting nearly one-third of our population. Take a moment to look up and determine what needs to be in place so that you can take care of the people in your practice with diabetes. Laying the groundwork for implementing diabetes-only appointments can be time-consuming, but establishing consistent procedures, developing efficient workflows, and clearly defining roles and responsibilities is well worth the effort. This solid foundation equips the office, health care providers, and staff to care for persons with diabetes and will be invaluable to ensure that time for this care is available in the day-to-day clinical practice.
A version of this article first appeared on Medscape.com.
Can busy primary care offices continue to care for patients with diabetes? No one would argue that it is involved and takes effort, and health care providers are bankrupt when it comes to sparing additional time for this chronic disease. With roughly 37 million people living with diabetes and 96 million with prediabetes or early type 2 diabetes, and just over 8,000 practicing endocrinologists in the United States, we all need to make time especially in primary care to provide insight and holistic care. With limited time and budget, how do we do this?
First, decide to be involved in caring for patients with diabetes. Diabetes is best managed by interprofessional care teams, so you’re not going it alone. These teams may include physicians; pharmacists; physician assistants; advanced practice nurses; registered nurses; certified diabetes care and education specialists (CDCES); dietitians; and other professionals such as social workers, behavioral health professionals, medical assistants, and community health workers. Know which professionals are available to serve on your team, either within your clinic or as a consultant, and reach out to them to share the care and ease the burden. Remember to refer to these professionals to reinforce the diabetes intervention message to the patient.
Second, incorporate “diabetes only” appointments into your schedule, allowing time to focus on current comprehensive diabetes treatment goals, barriers/inertia for care. Remember to have short-interval follow-up as needed to keep that patient engaged to achieve their targets. Instruct your office staff to create diabetes appointment templates and reminders to patients to bring diabetes-related technologies, medication lists, and diabetes questions to the appointment. When I implemented this change, my patients welcomed the focus on their diabetes health, and they knew we were prioritizing this disease that they have for a lifetime. These appointments did not take away from their other conditions; rather, they often reminded me to stay focused on their diabetes and associated coconditions.
Taking the time to establish efficient workflows before implementing diabetes care saves countless hours later and immediately maximizes health care provider–patient interactions. Assign specific staff duties and expectations related to diabetes appointments, such as downloading diabetes technology, medication reconciliation, laboratory data, point-of-care hemoglobin A1c, basic foot exam, and patient goals for diabetes care. This allows the prescriber to focus on the glycemic, cardiologic, renal, and metabolic goals and overcome the therapeutic inertia that plagues us all.
Incorporating diabetes-related technology into clinical practice can be a significant time-saver but requires initial onboarding. Set aside a few hours to create a technology clinic flow, and designate at least one team member to be responsible for obtaining patient data before, during, or after encounters. If possible, obtaining data ahead of the visit will enhance efficiency, allowing for meaningful discussion of blood glucose and lifestyle patterns. Diabetes technology reveals the gaps in care and enhances our ability to identify the areas where glycemic intervention is needed. In addition, it reveals the impact of food choices, activity level, stress, and medication adherence to the person living with diabetes.
Finally, be proactive about therapeutic inertia. This is defined as a prescribers’ failure to intensify or deintensify a patient’s treatment when appropriate to do so. Causes of therapeutic inertia can be placed at the primary care physician level, including time constraints or inexperience in treating diabetes; the patient level, such as concerns about side effects or new treatment regimens; or a systemic level, such as availability of medications or their costs. Be real with yourself: We all have inertia and can identify areas to overcome. Never let inertia be traced back to you.
Not all inertia lives with the health care provider. Patients bring apprehension and concerns, have questions, and just want to share the frustrations associated with living their best life with the disease. Don’t assume that you know what your patients’ treatment barriers are; ask them. If you don’t have an answer, then note it and come up with one by the next follow-up. Remember that this is a chronic disease – a marathon, not a sprint. You don’t have to solve everything at one appointment; rather, keep the momentum going.
Let’s put this into clinical practice. For the next patient with diabetes who comes into your office, discuss with them your intention to prioritize their diabetes by having an appointment set aside to specifically focus on their individual goals and targets for their disease. Have the patient list any barriers and treatment goals they would like to review; flag your schedule to indicate it is a diabetes-only visit; and orient your staff to reconcile diabetes medications and record the patient’s last eye exam, urine albumin-to-creatinine ratio, A1c result, and blood glucose data. During this encounter, identify the patient’s personal targets for control, examine their feet, and review or order necessary laboratory metrics. Explore the patient-reported barriers and make inroads to remove or alleviate these. Advance treatment intervention, and schedule follow-up: every 4-6 weeks if the A1c is > 9%, every 2 months if it’s 7% to < 9%, and every 3-6 months if it’s < 7%. Utilize team diabetes care, such as CDCES referrals, dietitians, online resources, and community members, to help reinforce care and enhance engagement.
We need to take steps in our clinical practice to make the necessary space to accommodate this pervasive disease affecting nearly one-third of our population. Take a moment to look up and determine what needs to be in place so that you can take care of the people in your practice with diabetes. Laying the groundwork for implementing diabetes-only appointments can be time-consuming, but establishing consistent procedures, developing efficient workflows, and clearly defining roles and responsibilities is well worth the effort. This solid foundation equips the office, health care providers, and staff to care for persons with diabetes and will be invaluable to ensure that time for this care is available in the day-to-day clinical practice.
A version of this article first appeared on Medscape.com.
Are migraine preventives underused in young adults?
, according to recent research published in the journal Headache.
“Approximately two-fifths of young adults with migraine were prescribed preventive medications, and this did not differ between pediatric and adult neurologists,” Hannah F. J. Shapiro MD, of the department of neurology at the University of California, San Francisco, and the UCSF Benioff Children’s Hospitals, and colleagues wrote in their study. “This finding suggests that pediatric neurologists are providing comparable care to adult neurologists for young adults with migraine; however, this may represent the underuse of preventive medications in this patient population.”
Dr. Shapiro and colleagues conducted a retrospective study of 767 patients (mean age 20.3 years) at Mass General Brigham Hospital in Boston between 2017 and 2021 who received care from a pediatric or adult neurologist for episodic migraine. The majority of patients in the study were white (72.2%), non-Hispanic (82.1%) women (80.3%) with episodic migraine (72.8%), some of whom experienced a psychiatric comorbidity (12.7%), and had a 3.88 mean clinic visits for migraine. Researchers assessed prescription of migraine preventive medication as a primary outcome, with a secondary outcome of comparing the rate of migraine preventive prescriptions written by pediatric and adult neurologists.
Overall, 290 patients (37.8%) received care from a pediatric neurologist, and 131 of those 290 patients (45.2%) received preventive medications (95% confidence interval, 39.5%-51.0%). The remaining 477 patients received care from an adult neurologist; of these, 206 patients (43.2%) received preventive medications (95% CI, 39.0%-47.7%; P = .591). The most common preventive medication prescribed was topiramate, which was prescribed in 19.1% of cases by adult neurologists and 15.2% of cases by pediatric neurologists. Other preventive medications included tricyclic antidepressants such as amitriptyline and nortriptyline; pediatric neurologists prescribed amitriptyline more often than adult neurologists (14.5% vs. 5.5%; P < .001), and adult neurologists prescribed nortriptyline more often than pediatric neurologists (12.8% vs. 2.4%; P < .001).
Dr. Shapiro and colleagues performed a mixed effects logistic regression analysis of potential confounders, and found no significant association between clinician specialty and use of preventive medication (adjusted odds ratio, 1.20; 95% CI, 0.62-2.31), while factors such as female sex (aOR, 1.69; 95% CI, 1.07-2.66) and number of visits (aOR, 1.64; 95% CI, 1.49-1.80) carried associations with preventive medication use.
The finding that pediatric and adult neurologists use similar preventive medications is a positive one because “patients who continue care into adulthood with a pediatric neurologist should receive comparable care to the care they would receive with an adult neurologist,” Dr. Shapiro and colleagues said. “It is even more pertinent now for pediatric neurologists to have comfort prescribing preventive medication to young adults, as the newer calcitonin gene-related peptide (CGRP) pathway antagonists are currently only FDA approved for use in patients aged 18 years or older.”
Roadblocks may prevent adoption
M. Cristina Victorio, MD, a pediatric neurologist and director of the headache program at Akron (Ohio) Children’s, said in an interview that the study is well-designed, but the results cannot be generalized as the study is retrospective, was conducted at a single institution, and data about nutraceuticals and drug-free neuromodulation devices were excluded from the analysis.
Another aspect of the study to consider is that episodic migraine, defined as between 0 and 14 migraine days per month, comprised most of the diagnoses in this study, while preventive medication is usually considered in patients with migraines occurring at least 6 days per month. “[I]f migraine is only once every other month or once a month, preventive treatment may not be recommended,” she said.
There is also the element of patient preference, which is “difficult to obtain” in a retrospective study, she noted.
Citing the authors’ comments about pediatric neurologists’ comfortability prescribing preventive medications, including CGRP antagonists, Dr. Victorio said she offers CGRP antagonists to “young adult patients who have failed at least two of the guideline-recommended preventive medications.”
However, pediatric neurologists may encounter roadblocks to prescribing these medications. “A big challenge is access, as it requires prior authorization as well as writing a letter of appeal or medical necessity, which can be a nuisance for clinicians who are already inundated with clinical responsibilities,” she said.
More education is needed
“As a pediatric headache specialist and knowing the results of this study, my colleagues and I have a role in educating all clinicians as well as trainees on headache management to improve and provide optimal care for young adult patients with migraine,” Dr. Victorio said.
In her experience, more clinic visits usually mean a need for preventative medication, and psychiatric morbidities are common. “I differ in the sense that as a headache specialist I am comfortable offering various preventive treatment options when indicated, so I do not believe I am underutilizing,” she said.
Dr. Victorio said she prescribes topiramate, amitriptyline, and propranolol as migraine preventatives for adolescents and young adults, but recommends cyproheptadine for younger children “due to lesser side effects, tolerability, and convenience of formulation (both liquid and tablet forms are available), which can be challenging for younger children who are unable to swallow pills.”
“Cognizant that there are patients who are reluctant to take daily prescription medication and that consideration for preventive treatment includes patient’s preference, I include the use of nutraceuticals and drug-free neuromodulation devices when discussing preventive treatment options,” she added, noting that children and adolescents “[m]ore often than not” prefer nutraceuticals like magnesium and vitamin B2.
“I think the bottom line is that all clinicians managing young adults with migraine should know when to consider starting preventive migraine medication,” Dr. Victorio said. “Not offering preventive treatment to young adults specifically for those who have frequent migraine attacks, or those who have severe migraine despite adequate acute treatment, or those with significant adverse reactions to acute medications will only put these patients at risk to progression to chronic migraine (meaning having migraine more often than not – at least 15 days per month), and increases headache-related disability and reduces quality of life.”
The authors report no relevant financial disclosures. This study was supported by Harvard University and an award from the National Institutes of Health. Dr. Victorio reports being on the advisory board for Theranica Bio-electronics, has received honorarium serving as an author of the Merck Manual, and is involved in industry-sponsored clinical trials through Akron Children’s Hospital.
, according to recent research published in the journal Headache.
“Approximately two-fifths of young adults with migraine were prescribed preventive medications, and this did not differ between pediatric and adult neurologists,” Hannah F. J. Shapiro MD, of the department of neurology at the University of California, San Francisco, and the UCSF Benioff Children’s Hospitals, and colleagues wrote in their study. “This finding suggests that pediatric neurologists are providing comparable care to adult neurologists for young adults with migraine; however, this may represent the underuse of preventive medications in this patient population.”
Dr. Shapiro and colleagues conducted a retrospective study of 767 patients (mean age 20.3 years) at Mass General Brigham Hospital in Boston between 2017 and 2021 who received care from a pediatric or adult neurologist for episodic migraine. The majority of patients in the study were white (72.2%), non-Hispanic (82.1%) women (80.3%) with episodic migraine (72.8%), some of whom experienced a psychiatric comorbidity (12.7%), and had a 3.88 mean clinic visits for migraine. Researchers assessed prescription of migraine preventive medication as a primary outcome, with a secondary outcome of comparing the rate of migraine preventive prescriptions written by pediatric and adult neurologists.
Overall, 290 patients (37.8%) received care from a pediatric neurologist, and 131 of those 290 patients (45.2%) received preventive medications (95% confidence interval, 39.5%-51.0%). The remaining 477 patients received care from an adult neurologist; of these, 206 patients (43.2%) received preventive medications (95% CI, 39.0%-47.7%; P = .591). The most common preventive medication prescribed was topiramate, which was prescribed in 19.1% of cases by adult neurologists and 15.2% of cases by pediatric neurologists. Other preventive medications included tricyclic antidepressants such as amitriptyline and nortriptyline; pediatric neurologists prescribed amitriptyline more often than adult neurologists (14.5% vs. 5.5%; P < .001), and adult neurologists prescribed nortriptyline more often than pediatric neurologists (12.8% vs. 2.4%; P < .001).
Dr. Shapiro and colleagues performed a mixed effects logistic regression analysis of potential confounders, and found no significant association between clinician specialty and use of preventive medication (adjusted odds ratio, 1.20; 95% CI, 0.62-2.31), while factors such as female sex (aOR, 1.69; 95% CI, 1.07-2.66) and number of visits (aOR, 1.64; 95% CI, 1.49-1.80) carried associations with preventive medication use.
The finding that pediatric and adult neurologists use similar preventive medications is a positive one because “patients who continue care into adulthood with a pediatric neurologist should receive comparable care to the care they would receive with an adult neurologist,” Dr. Shapiro and colleagues said. “It is even more pertinent now for pediatric neurologists to have comfort prescribing preventive medication to young adults, as the newer calcitonin gene-related peptide (CGRP) pathway antagonists are currently only FDA approved for use in patients aged 18 years or older.”
Roadblocks may prevent adoption
M. Cristina Victorio, MD, a pediatric neurologist and director of the headache program at Akron (Ohio) Children’s, said in an interview that the study is well-designed, but the results cannot be generalized as the study is retrospective, was conducted at a single institution, and data about nutraceuticals and drug-free neuromodulation devices were excluded from the analysis.
Another aspect of the study to consider is that episodic migraine, defined as between 0 and 14 migraine days per month, comprised most of the diagnoses in this study, while preventive medication is usually considered in patients with migraines occurring at least 6 days per month. “[I]f migraine is only once every other month or once a month, preventive treatment may not be recommended,” she said.
There is also the element of patient preference, which is “difficult to obtain” in a retrospective study, she noted.
Citing the authors’ comments about pediatric neurologists’ comfortability prescribing preventive medications, including CGRP antagonists, Dr. Victorio said she offers CGRP antagonists to “young adult patients who have failed at least two of the guideline-recommended preventive medications.”
However, pediatric neurologists may encounter roadblocks to prescribing these medications. “A big challenge is access, as it requires prior authorization as well as writing a letter of appeal or medical necessity, which can be a nuisance for clinicians who are already inundated with clinical responsibilities,” she said.
More education is needed
“As a pediatric headache specialist and knowing the results of this study, my colleagues and I have a role in educating all clinicians as well as trainees on headache management to improve and provide optimal care for young adult patients with migraine,” Dr. Victorio said.
In her experience, more clinic visits usually mean a need for preventative medication, and psychiatric morbidities are common. “I differ in the sense that as a headache specialist I am comfortable offering various preventive treatment options when indicated, so I do not believe I am underutilizing,” she said.
Dr. Victorio said she prescribes topiramate, amitriptyline, and propranolol as migraine preventatives for adolescents and young adults, but recommends cyproheptadine for younger children “due to lesser side effects, tolerability, and convenience of formulation (both liquid and tablet forms are available), which can be challenging for younger children who are unable to swallow pills.”
“Cognizant that there are patients who are reluctant to take daily prescription medication and that consideration for preventive treatment includes patient’s preference, I include the use of nutraceuticals and drug-free neuromodulation devices when discussing preventive treatment options,” she added, noting that children and adolescents “[m]ore often than not” prefer nutraceuticals like magnesium and vitamin B2.
“I think the bottom line is that all clinicians managing young adults with migraine should know when to consider starting preventive migraine medication,” Dr. Victorio said. “Not offering preventive treatment to young adults specifically for those who have frequent migraine attacks, or those who have severe migraine despite adequate acute treatment, or those with significant adverse reactions to acute medications will only put these patients at risk to progression to chronic migraine (meaning having migraine more often than not – at least 15 days per month), and increases headache-related disability and reduces quality of life.”
The authors report no relevant financial disclosures. This study was supported by Harvard University and an award from the National Institutes of Health. Dr. Victorio reports being on the advisory board for Theranica Bio-electronics, has received honorarium serving as an author of the Merck Manual, and is involved in industry-sponsored clinical trials through Akron Children’s Hospital.
, according to recent research published in the journal Headache.
“Approximately two-fifths of young adults with migraine were prescribed preventive medications, and this did not differ between pediatric and adult neurologists,” Hannah F. J. Shapiro MD, of the department of neurology at the University of California, San Francisco, and the UCSF Benioff Children’s Hospitals, and colleagues wrote in their study. “This finding suggests that pediatric neurologists are providing comparable care to adult neurologists for young adults with migraine; however, this may represent the underuse of preventive medications in this patient population.”
Dr. Shapiro and colleagues conducted a retrospective study of 767 patients (mean age 20.3 years) at Mass General Brigham Hospital in Boston between 2017 and 2021 who received care from a pediatric or adult neurologist for episodic migraine. The majority of patients in the study were white (72.2%), non-Hispanic (82.1%) women (80.3%) with episodic migraine (72.8%), some of whom experienced a psychiatric comorbidity (12.7%), and had a 3.88 mean clinic visits for migraine. Researchers assessed prescription of migraine preventive medication as a primary outcome, with a secondary outcome of comparing the rate of migraine preventive prescriptions written by pediatric and adult neurologists.
Overall, 290 patients (37.8%) received care from a pediatric neurologist, and 131 of those 290 patients (45.2%) received preventive medications (95% confidence interval, 39.5%-51.0%). The remaining 477 patients received care from an adult neurologist; of these, 206 patients (43.2%) received preventive medications (95% CI, 39.0%-47.7%; P = .591). The most common preventive medication prescribed was topiramate, which was prescribed in 19.1% of cases by adult neurologists and 15.2% of cases by pediatric neurologists. Other preventive medications included tricyclic antidepressants such as amitriptyline and nortriptyline; pediatric neurologists prescribed amitriptyline more often than adult neurologists (14.5% vs. 5.5%; P < .001), and adult neurologists prescribed nortriptyline more often than pediatric neurologists (12.8% vs. 2.4%; P < .001).
Dr. Shapiro and colleagues performed a mixed effects logistic regression analysis of potential confounders, and found no significant association between clinician specialty and use of preventive medication (adjusted odds ratio, 1.20; 95% CI, 0.62-2.31), while factors such as female sex (aOR, 1.69; 95% CI, 1.07-2.66) and number of visits (aOR, 1.64; 95% CI, 1.49-1.80) carried associations with preventive medication use.
The finding that pediatric and adult neurologists use similar preventive medications is a positive one because “patients who continue care into adulthood with a pediatric neurologist should receive comparable care to the care they would receive with an adult neurologist,” Dr. Shapiro and colleagues said. “It is even more pertinent now for pediatric neurologists to have comfort prescribing preventive medication to young adults, as the newer calcitonin gene-related peptide (CGRP) pathway antagonists are currently only FDA approved for use in patients aged 18 years or older.”
Roadblocks may prevent adoption
M. Cristina Victorio, MD, a pediatric neurologist and director of the headache program at Akron (Ohio) Children’s, said in an interview that the study is well-designed, but the results cannot be generalized as the study is retrospective, was conducted at a single institution, and data about nutraceuticals and drug-free neuromodulation devices were excluded from the analysis.
Another aspect of the study to consider is that episodic migraine, defined as between 0 and 14 migraine days per month, comprised most of the diagnoses in this study, while preventive medication is usually considered in patients with migraines occurring at least 6 days per month. “[I]f migraine is only once every other month or once a month, preventive treatment may not be recommended,” she said.
There is also the element of patient preference, which is “difficult to obtain” in a retrospective study, she noted.
Citing the authors’ comments about pediatric neurologists’ comfortability prescribing preventive medications, including CGRP antagonists, Dr. Victorio said she offers CGRP antagonists to “young adult patients who have failed at least two of the guideline-recommended preventive medications.”
However, pediatric neurologists may encounter roadblocks to prescribing these medications. “A big challenge is access, as it requires prior authorization as well as writing a letter of appeal or medical necessity, which can be a nuisance for clinicians who are already inundated with clinical responsibilities,” she said.
More education is needed
“As a pediatric headache specialist and knowing the results of this study, my colleagues and I have a role in educating all clinicians as well as trainees on headache management to improve and provide optimal care for young adult patients with migraine,” Dr. Victorio said.
In her experience, more clinic visits usually mean a need for preventative medication, and psychiatric morbidities are common. “I differ in the sense that as a headache specialist I am comfortable offering various preventive treatment options when indicated, so I do not believe I am underutilizing,” she said.
Dr. Victorio said she prescribes topiramate, amitriptyline, and propranolol as migraine preventatives for adolescents and young adults, but recommends cyproheptadine for younger children “due to lesser side effects, tolerability, and convenience of formulation (both liquid and tablet forms are available), which can be challenging for younger children who are unable to swallow pills.”
“Cognizant that there are patients who are reluctant to take daily prescription medication and that consideration for preventive treatment includes patient’s preference, I include the use of nutraceuticals and drug-free neuromodulation devices when discussing preventive treatment options,” she added, noting that children and adolescents “[m]ore often than not” prefer nutraceuticals like magnesium and vitamin B2.
“I think the bottom line is that all clinicians managing young adults with migraine should know when to consider starting preventive migraine medication,” Dr. Victorio said. “Not offering preventive treatment to young adults specifically for those who have frequent migraine attacks, or those who have severe migraine despite adequate acute treatment, or those with significant adverse reactions to acute medications will only put these patients at risk to progression to chronic migraine (meaning having migraine more often than not – at least 15 days per month), and increases headache-related disability and reduces quality of life.”
The authors report no relevant financial disclosures. This study was supported by Harvard University and an award from the National Institutes of Health. Dr. Victorio reports being on the advisory board for Theranica Bio-electronics, has received honorarium serving as an author of the Merck Manual, and is involved in industry-sponsored clinical trials through Akron Children’s Hospital.
FROM HEADACHE
From failure to hope: Tracking the changing landscape of Alzheimer’s therapies
In 2014 neurologist Jeffrey L. Cummings, MD, startled the Alzheimer’s disease research world with a paper that laid bare the alarmingly high failure rate of Alzheimer’s disease therapies in development.
Publishing in the journal Alzheimer’s Research & Therapy, Dr. Cummings and his colleagues determined that 99.6% of all therapies tested between 2002 and 2012 had failed. Since downloaded some 75,000 times, Dr. Cumming’s “99% paper,” as it came to be nicknamed, led him to look more deeply and thoroughly at Alzheimer’s disease drugs in the pipeline, and describe them in a readable, user-friendly way.
His “Alzheimer’s Drug Development Pipeline” report, in the journal Alzheimer’s & Dementia, classifies therapies by their targets, their mechanisms of action, and where they stand in the development process.
Heavy on color-coded visuals, this snapshot of Alzheimer’s disease therapies is widely consulted by industry, researchers, and clinicians. Over time this report – which first documented a crisis – has come to show something more optimistic: an increasingly crowded pipeline reflecting a broad array of treatment approaches. Dr. Cummings wants more people to know that Alzheimer’s disease drug research, which now includes the first two Food and Drug Administration–approved monoclonal antibodies against amyloid-beta, is not the bleak landscape that it was in recent memory.
Lately, with the help of a grant from the National Institute on Aging, Dr. Cummings and his group have been working to expand on their reports to build an even more user-friendly database that can be searched by people in all corners of the neurodegenerative disease world. Dr. Cummings says he plans for this public-facing database to be up and running by year end.
Neurology Reviews spoke with Dr. Cummings, who is a member of the publication’s Editorial Advisory Board, about the genesis of his influential drug-tracking effort, how it has evolved, and what has been learned from it over the years.
How did all this begin?
Already in 2014 there was a dialogue going on was about the high failure rate for Alzheimer’s drugs. And I thought: “there’s probably a number that can be assigned to that.” And when it turned out to be 99.6%, that generated a huge amount of interest. That’s when I realized what interests me also interests the world. And that I was uniquely positioned after that point to do something annually.
How do you create your annual report, and how do you classify the drugs in it when some might act on little-understood pathways or mechanisms?
We capture information available on clinicaltrials.gov. We are notified immediately of any new Alzheimer-related trials, and we automate everything that is possible to automate. But there is still some human curation required. Most of that is around mechanisms. If it’s a monoclonal antibody directed at amyloid-beta, that’s not difficult to categorize. But with the small molecules especially, it can be more complicated.
We often look to see how the sponsor describes the drug and what their perception of the primary target is. A resource of great importance to us is CADRO, Common Alzheimer’s and Related Dementias Disease Research Ontology, which describes about 20 mechanisms that a group of scientists sponsored by the National Institutes of Health and the Alzheimer’s Association have agreed on. Inflammation, epigenetics, and oxidation are just a few that most people know. CADRO is organized in a very specific way that allows us to go to the mechanism and relate it to the target. But we do try to be humble and acknowledge we probably make some errors in this.
Are you able to capture every Alzheimer’s drug in development globally?
If they’re on clinicaltrials.gov, they’re in our database. But we think there’s about 15% of drugs in the world that aren’t for some reason on clinicaltrials.gov – so we know we are comprehensive, but not quite exhaustive. I’m in kind of quandary about whether to search for that other 15%. But we do always acknowledge that we’re not 100% exhaustive.
Who are the report’s main readers?
Drug developers use it for investor discussions, and also to understand the competition and the landscape. The competition might be a drug with the same mechanism, and the landscape might be drugs coming into the Alzheimer’s disease world. So if someone is developing a PDE-5 inhibitor for mild dementia, for example, they can see that other people are working on a PDE-5 inhibitor for moderate dementia, and there’s no overlap. Investors use the report to make decisions about which horse in the race to bet on. And of course it’s used by academics and clinicians to learn which are the new drugs in the pipeline, which drugs have fallen out of the pipeline, how are biomarkers changing trials, what are the new outcomes.
It’s really become a community project. Investigators will email me and say “Jeff, we’re in phase 1, make sure it’s on your map.” Or, “you forgot our agent! We’re disappointed.” When that occurs it’s because they were not in a trial on the index date – the 1 day in our publication when everything we say in the paper is true. A trial initiated 1 day later won’t make the report for that year.
What about patients and families? Are they able to use the report as well?
One of the things we want to expand with the new database is its usefulness for patients. Among the new data display approaches that we have is a world map where you can go click on a dot near your home and find active trials. That’s something patients and families want to know, right? There’s 140 drugs in clinical trials, there must be one for me, how would I get to it? Soon we will have quite a good public portal so if you want to go in and see what new monoclonal antibodies are in phase 2, you can do that with drop-down menus. It’s a very easy to use site that anyone can explore.
Looking back at your last decade tracking drugs, what are some lessons learned and what are some of the more exciting drug categories to emerge?
My answer to this question is: Biologics rule. The main successes have been in biologics, in the monoclonal antibodies against amyloid, like the two FDA-approved agents lecanemab and aducanumab. But I think that the monoclonals, while I’m really happy to have them, are a first step. If you look back at tacrine, the first drug approved in 1993 for Alzheimer’s disease, it was a very difficult drug with lots of side effects. But then within 3 years we had donepezil, which was a very benign drug. I feel that a similar evolution is likely with regard to these antibodies. The first ones, we know, have big challenges, and you learn from those challenges and you just keep improving them. But you have to start somewhere, and you have to validate that target. Now I think that amyloid is validated.
What other approaches are interesting to you?
We have seen dramatic imaging results with marked reductions in neurofibrillary tangles from an antisense oligonucleotide aimed at tau protein. And there are two very active areas in the pipeline: inflammation and synaptic plasticity. Each has roughly 20 drugs apiece in development across all phases. And as you know, both synaptic plasticity and inflammation are represented across neurodegenerative conditions.
Your annual report has always focused on drugs to treat Alzheimer’s disease. Will the new database cover other types of dementia and neurodegenerative diseases?
That’s an obvious next step. I’m hoping that late this year we will have funding to expand the database into frontotemporal lobar degenerations, which will include all the tauopathies. And there’s also an overlap with TDP-43 diseases, so we’ll bring all of that in too. We have a new initiative on Parkinson’s disease and dementia with Lewy bodies that I hope will materialize by next year. My goal is that this will eventually become a neurodegenerative disease therapies database. The really interesting drugs right now are being tested in more than one neurodegenerative disease, and we should look at those more carefully. It will be more feasible to do that if they’re on the same data set.
What about other therapy classes?
We aim to be more serious about devices.
What will you call the database?
The Clinical Trial Observatory. We may start by calling it the Alzheimer’s Disease Clinical Trial Observatory. But the intention, obviously, is to go way beyond Alzheimer’s disease. The database is managed by a terrific team of data scientists at Cleveland Clinic, led by Feixiong Cheng, PhD.
The annual pipeline report is very much associated with you. Is the database going to be different?
Right now, I’m like the grandfather of this project. I won’t be around forever. This will have to pass on, and we’re already talking about succession. We’re thinking about how to make sure this community resource continues to be a community resource. Also, over all these years the annual report reflected my perspective. But with a database, many more people will be able to share their perspectives. I happen to think that “biologics rule,” but others might look at the data, see different scientific currents, and draw different conclusions. That will create a rich dialogue.
Do you think your reports have changed people’s perspectives on Alzheimer’s disease therapies? There’s a widely held idea that the field is exclusively focused on amyloid, or even dead-ended, but the papers seem to show something different.
We think this effort has helped, and will continue to help and foster investment and growth in treatments for our patients. It really does show how diverse the clinical trials landscape is now. People are surprised to learn of the number and diversity of approaches. Just last week I was presenting at the Center for Brain Health in Dallas and there was a doctor in the audience who was a caregiver to his wife with Alzheimer’s disease. He came up afterwards and said, “I had no idea there were so many drugs in clinical trials,” because there’s no way to find out if you don’t know about this resource.
Dr. Cummings discloses consulting for a range of companies working in Alzheimer’s therapies and diagnostics, including Acadia, Alkahest, AlphaCognition, AriBio, Avanir, Axsome, Behren, Biogen, Biohaven, Cassava, Cerecin, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, United Neuroscience, and Unlearn AI. He has received several grants from the National Institute on Aging.
In 2014 neurologist Jeffrey L. Cummings, MD, startled the Alzheimer’s disease research world with a paper that laid bare the alarmingly high failure rate of Alzheimer’s disease therapies in development.
Publishing in the journal Alzheimer’s Research & Therapy, Dr. Cummings and his colleagues determined that 99.6% of all therapies tested between 2002 and 2012 had failed. Since downloaded some 75,000 times, Dr. Cumming’s “99% paper,” as it came to be nicknamed, led him to look more deeply and thoroughly at Alzheimer’s disease drugs in the pipeline, and describe them in a readable, user-friendly way.
His “Alzheimer’s Drug Development Pipeline” report, in the journal Alzheimer’s & Dementia, classifies therapies by their targets, their mechanisms of action, and where they stand in the development process.
Heavy on color-coded visuals, this snapshot of Alzheimer’s disease therapies is widely consulted by industry, researchers, and clinicians. Over time this report – which first documented a crisis – has come to show something more optimistic: an increasingly crowded pipeline reflecting a broad array of treatment approaches. Dr. Cummings wants more people to know that Alzheimer’s disease drug research, which now includes the first two Food and Drug Administration–approved monoclonal antibodies against amyloid-beta, is not the bleak landscape that it was in recent memory.
Lately, with the help of a grant from the National Institute on Aging, Dr. Cummings and his group have been working to expand on their reports to build an even more user-friendly database that can be searched by people in all corners of the neurodegenerative disease world. Dr. Cummings says he plans for this public-facing database to be up and running by year end.
Neurology Reviews spoke with Dr. Cummings, who is a member of the publication’s Editorial Advisory Board, about the genesis of his influential drug-tracking effort, how it has evolved, and what has been learned from it over the years.
How did all this begin?
Already in 2014 there was a dialogue going on was about the high failure rate for Alzheimer’s drugs. And I thought: “there’s probably a number that can be assigned to that.” And when it turned out to be 99.6%, that generated a huge amount of interest. That’s when I realized what interests me also interests the world. And that I was uniquely positioned after that point to do something annually.
How do you create your annual report, and how do you classify the drugs in it when some might act on little-understood pathways or mechanisms?
We capture information available on clinicaltrials.gov. We are notified immediately of any new Alzheimer-related trials, and we automate everything that is possible to automate. But there is still some human curation required. Most of that is around mechanisms. If it’s a monoclonal antibody directed at amyloid-beta, that’s not difficult to categorize. But with the small molecules especially, it can be more complicated.
We often look to see how the sponsor describes the drug and what their perception of the primary target is. A resource of great importance to us is CADRO, Common Alzheimer’s and Related Dementias Disease Research Ontology, which describes about 20 mechanisms that a group of scientists sponsored by the National Institutes of Health and the Alzheimer’s Association have agreed on. Inflammation, epigenetics, and oxidation are just a few that most people know. CADRO is organized in a very specific way that allows us to go to the mechanism and relate it to the target. But we do try to be humble and acknowledge we probably make some errors in this.
Are you able to capture every Alzheimer’s drug in development globally?
If they’re on clinicaltrials.gov, they’re in our database. But we think there’s about 15% of drugs in the world that aren’t for some reason on clinicaltrials.gov – so we know we are comprehensive, but not quite exhaustive. I’m in kind of quandary about whether to search for that other 15%. But we do always acknowledge that we’re not 100% exhaustive.
Who are the report’s main readers?
Drug developers use it for investor discussions, and also to understand the competition and the landscape. The competition might be a drug with the same mechanism, and the landscape might be drugs coming into the Alzheimer’s disease world. So if someone is developing a PDE-5 inhibitor for mild dementia, for example, they can see that other people are working on a PDE-5 inhibitor for moderate dementia, and there’s no overlap. Investors use the report to make decisions about which horse in the race to bet on. And of course it’s used by academics and clinicians to learn which are the new drugs in the pipeline, which drugs have fallen out of the pipeline, how are biomarkers changing trials, what are the new outcomes.
It’s really become a community project. Investigators will email me and say “Jeff, we’re in phase 1, make sure it’s on your map.” Or, “you forgot our agent! We’re disappointed.” When that occurs it’s because they were not in a trial on the index date – the 1 day in our publication when everything we say in the paper is true. A trial initiated 1 day later won’t make the report for that year.
What about patients and families? Are they able to use the report as well?
One of the things we want to expand with the new database is its usefulness for patients. Among the new data display approaches that we have is a world map where you can go click on a dot near your home and find active trials. That’s something patients and families want to know, right? There’s 140 drugs in clinical trials, there must be one for me, how would I get to it? Soon we will have quite a good public portal so if you want to go in and see what new monoclonal antibodies are in phase 2, you can do that with drop-down menus. It’s a very easy to use site that anyone can explore.
Looking back at your last decade tracking drugs, what are some lessons learned and what are some of the more exciting drug categories to emerge?
My answer to this question is: Biologics rule. The main successes have been in biologics, in the monoclonal antibodies against amyloid, like the two FDA-approved agents lecanemab and aducanumab. But I think that the monoclonals, while I’m really happy to have them, are a first step. If you look back at tacrine, the first drug approved in 1993 for Alzheimer’s disease, it was a very difficult drug with lots of side effects. But then within 3 years we had donepezil, which was a very benign drug. I feel that a similar evolution is likely with regard to these antibodies. The first ones, we know, have big challenges, and you learn from those challenges and you just keep improving them. But you have to start somewhere, and you have to validate that target. Now I think that amyloid is validated.
What other approaches are interesting to you?
We have seen dramatic imaging results with marked reductions in neurofibrillary tangles from an antisense oligonucleotide aimed at tau protein. And there are two very active areas in the pipeline: inflammation and synaptic plasticity. Each has roughly 20 drugs apiece in development across all phases. And as you know, both synaptic plasticity and inflammation are represented across neurodegenerative conditions.
Your annual report has always focused on drugs to treat Alzheimer’s disease. Will the new database cover other types of dementia and neurodegenerative diseases?
That’s an obvious next step. I’m hoping that late this year we will have funding to expand the database into frontotemporal lobar degenerations, which will include all the tauopathies. And there’s also an overlap with TDP-43 diseases, so we’ll bring all of that in too. We have a new initiative on Parkinson’s disease and dementia with Lewy bodies that I hope will materialize by next year. My goal is that this will eventually become a neurodegenerative disease therapies database. The really interesting drugs right now are being tested in more than one neurodegenerative disease, and we should look at those more carefully. It will be more feasible to do that if they’re on the same data set.
What about other therapy classes?
We aim to be more serious about devices.
What will you call the database?
The Clinical Trial Observatory. We may start by calling it the Alzheimer’s Disease Clinical Trial Observatory. But the intention, obviously, is to go way beyond Alzheimer’s disease. The database is managed by a terrific team of data scientists at Cleveland Clinic, led by Feixiong Cheng, PhD.
The annual pipeline report is very much associated with you. Is the database going to be different?
Right now, I’m like the grandfather of this project. I won’t be around forever. This will have to pass on, and we’re already talking about succession. We’re thinking about how to make sure this community resource continues to be a community resource. Also, over all these years the annual report reflected my perspective. But with a database, many more people will be able to share their perspectives. I happen to think that “biologics rule,” but others might look at the data, see different scientific currents, and draw different conclusions. That will create a rich dialogue.
Do you think your reports have changed people’s perspectives on Alzheimer’s disease therapies? There’s a widely held idea that the field is exclusively focused on amyloid, or even dead-ended, but the papers seem to show something different.
We think this effort has helped, and will continue to help and foster investment and growth in treatments for our patients. It really does show how diverse the clinical trials landscape is now. People are surprised to learn of the number and diversity of approaches. Just last week I was presenting at the Center for Brain Health in Dallas and there was a doctor in the audience who was a caregiver to his wife with Alzheimer’s disease. He came up afterwards and said, “I had no idea there were so many drugs in clinical trials,” because there’s no way to find out if you don’t know about this resource.
Dr. Cummings discloses consulting for a range of companies working in Alzheimer’s therapies and diagnostics, including Acadia, Alkahest, AlphaCognition, AriBio, Avanir, Axsome, Behren, Biogen, Biohaven, Cassava, Cerecin, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, United Neuroscience, and Unlearn AI. He has received several grants from the National Institute on Aging.
In 2014 neurologist Jeffrey L. Cummings, MD, startled the Alzheimer’s disease research world with a paper that laid bare the alarmingly high failure rate of Alzheimer’s disease therapies in development.
Publishing in the journal Alzheimer’s Research & Therapy, Dr. Cummings and his colleagues determined that 99.6% of all therapies tested between 2002 and 2012 had failed. Since downloaded some 75,000 times, Dr. Cumming’s “99% paper,” as it came to be nicknamed, led him to look more deeply and thoroughly at Alzheimer’s disease drugs in the pipeline, and describe them in a readable, user-friendly way.
His “Alzheimer’s Drug Development Pipeline” report, in the journal Alzheimer’s & Dementia, classifies therapies by their targets, their mechanisms of action, and where they stand in the development process.
Heavy on color-coded visuals, this snapshot of Alzheimer’s disease therapies is widely consulted by industry, researchers, and clinicians. Over time this report – which first documented a crisis – has come to show something more optimistic: an increasingly crowded pipeline reflecting a broad array of treatment approaches. Dr. Cummings wants more people to know that Alzheimer’s disease drug research, which now includes the first two Food and Drug Administration–approved monoclonal antibodies against amyloid-beta, is not the bleak landscape that it was in recent memory.
Lately, with the help of a grant from the National Institute on Aging, Dr. Cummings and his group have been working to expand on their reports to build an even more user-friendly database that can be searched by people in all corners of the neurodegenerative disease world. Dr. Cummings says he plans for this public-facing database to be up and running by year end.
Neurology Reviews spoke with Dr. Cummings, who is a member of the publication’s Editorial Advisory Board, about the genesis of his influential drug-tracking effort, how it has evolved, and what has been learned from it over the years.
How did all this begin?
Already in 2014 there was a dialogue going on was about the high failure rate for Alzheimer’s drugs. And I thought: “there’s probably a number that can be assigned to that.” And when it turned out to be 99.6%, that generated a huge amount of interest. That’s when I realized what interests me also interests the world. And that I was uniquely positioned after that point to do something annually.
How do you create your annual report, and how do you classify the drugs in it when some might act on little-understood pathways or mechanisms?
We capture information available on clinicaltrials.gov. We are notified immediately of any new Alzheimer-related trials, and we automate everything that is possible to automate. But there is still some human curation required. Most of that is around mechanisms. If it’s a monoclonal antibody directed at amyloid-beta, that’s not difficult to categorize. But with the small molecules especially, it can be more complicated.
We often look to see how the sponsor describes the drug and what their perception of the primary target is. A resource of great importance to us is CADRO, Common Alzheimer’s and Related Dementias Disease Research Ontology, which describes about 20 mechanisms that a group of scientists sponsored by the National Institutes of Health and the Alzheimer’s Association have agreed on. Inflammation, epigenetics, and oxidation are just a few that most people know. CADRO is organized in a very specific way that allows us to go to the mechanism and relate it to the target. But we do try to be humble and acknowledge we probably make some errors in this.
Are you able to capture every Alzheimer’s drug in development globally?
If they’re on clinicaltrials.gov, they’re in our database. But we think there’s about 15% of drugs in the world that aren’t for some reason on clinicaltrials.gov – so we know we are comprehensive, but not quite exhaustive. I’m in kind of quandary about whether to search for that other 15%. But we do always acknowledge that we’re not 100% exhaustive.
Who are the report’s main readers?
Drug developers use it for investor discussions, and also to understand the competition and the landscape. The competition might be a drug with the same mechanism, and the landscape might be drugs coming into the Alzheimer’s disease world. So if someone is developing a PDE-5 inhibitor for mild dementia, for example, they can see that other people are working on a PDE-5 inhibitor for moderate dementia, and there’s no overlap. Investors use the report to make decisions about which horse in the race to bet on. And of course it’s used by academics and clinicians to learn which are the new drugs in the pipeline, which drugs have fallen out of the pipeline, how are biomarkers changing trials, what are the new outcomes.
It’s really become a community project. Investigators will email me and say “Jeff, we’re in phase 1, make sure it’s on your map.” Or, “you forgot our agent! We’re disappointed.” When that occurs it’s because they were not in a trial on the index date – the 1 day in our publication when everything we say in the paper is true. A trial initiated 1 day later won’t make the report for that year.
What about patients and families? Are they able to use the report as well?
One of the things we want to expand with the new database is its usefulness for patients. Among the new data display approaches that we have is a world map where you can go click on a dot near your home and find active trials. That’s something patients and families want to know, right? There’s 140 drugs in clinical trials, there must be one for me, how would I get to it? Soon we will have quite a good public portal so if you want to go in and see what new monoclonal antibodies are in phase 2, you can do that with drop-down menus. It’s a very easy to use site that anyone can explore.
Looking back at your last decade tracking drugs, what are some lessons learned and what are some of the more exciting drug categories to emerge?
My answer to this question is: Biologics rule. The main successes have been in biologics, in the monoclonal antibodies against amyloid, like the two FDA-approved agents lecanemab and aducanumab. But I think that the monoclonals, while I’m really happy to have them, are a first step. If you look back at tacrine, the first drug approved in 1993 for Alzheimer’s disease, it was a very difficult drug with lots of side effects. But then within 3 years we had donepezil, which was a very benign drug. I feel that a similar evolution is likely with regard to these antibodies. The first ones, we know, have big challenges, and you learn from those challenges and you just keep improving them. But you have to start somewhere, and you have to validate that target. Now I think that amyloid is validated.
What other approaches are interesting to you?
We have seen dramatic imaging results with marked reductions in neurofibrillary tangles from an antisense oligonucleotide aimed at tau protein. And there are two very active areas in the pipeline: inflammation and synaptic plasticity. Each has roughly 20 drugs apiece in development across all phases. And as you know, both synaptic plasticity and inflammation are represented across neurodegenerative conditions.
Your annual report has always focused on drugs to treat Alzheimer’s disease. Will the new database cover other types of dementia and neurodegenerative diseases?
That’s an obvious next step. I’m hoping that late this year we will have funding to expand the database into frontotemporal lobar degenerations, which will include all the tauopathies. And there’s also an overlap with TDP-43 diseases, so we’ll bring all of that in too. We have a new initiative on Parkinson’s disease and dementia with Lewy bodies that I hope will materialize by next year. My goal is that this will eventually become a neurodegenerative disease therapies database. The really interesting drugs right now are being tested in more than one neurodegenerative disease, and we should look at those more carefully. It will be more feasible to do that if they’re on the same data set.
What about other therapy classes?
We aim to be more serious about devices.
What will you call the database?
The Clinical Trial Observatory. We may start by calling it the Alzheimer’s Disease Clinical Trial Observatory. But the intention, obviously, is to go way beyond Alzheimer’s disease. The database is managed by a terrific team of data scientists at Cleveland Clinic, led by Feixiong Cheng, PhD.
The annual pipeline report is very much associated with you. Is the database going to be different?
Right now, I’m like the grandfather of this project. I won’t be around forever. This will have to pass on, and we’re already talking about succession. We’re thinking about how to make sure this community resource continues to be a community resource. Also, over all these years the annual report reflected my perspective. But with a database, many more people will be able to share their perspectives. I happen to think that “biologics rule,” but others might look at the data, see different scientific currents, and draw different conclusions. That will create a rich dialogue.
Do you think your reports have changed people’s perspectives on Alzheimer’s disease therapies? There’s a widely held idea that the field is exclusively focused on amyloid, or even dead-ended, but the papers seem to show something different.
We think this effort has helped, and will continue to help and foster investment and growth in treatments for our patients. It really does show how diverse the clinical trials landscape is now. People are surprised to learn of the number and diversity of approaches. Just last week I was presenting at the Center for Brain Health in Dallas and there was a doctor in the audience who was a caregiver to his wife with Alzheimer’s disease. He came up afterwards and said, “I had no idea there were so many drugs in clinical trials,” because there’s no way to find out if you don’t know about this resource.
Dr. Cummings discloses consulting for a range of companies working in Alzheimer’s therapies and diagnostics, including Acadia, Alkahest, AlphaCognition, AriBio, Avanir, Axsome, Behren, Biogen, Biohaven, Cassava, Cerecin, Cortexyme, Diadem, EIP Pharma, Eisai, GemVax, Genentech, Green Valley, Grifols, Janssen, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Otsuka, PRODEO, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, United Neuroscience, and Unlearn AI. He has received several grants from the National Institute on Aging.
What’s Eating You? Phlebotomine Sandflies and Leishmania Parasites
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.
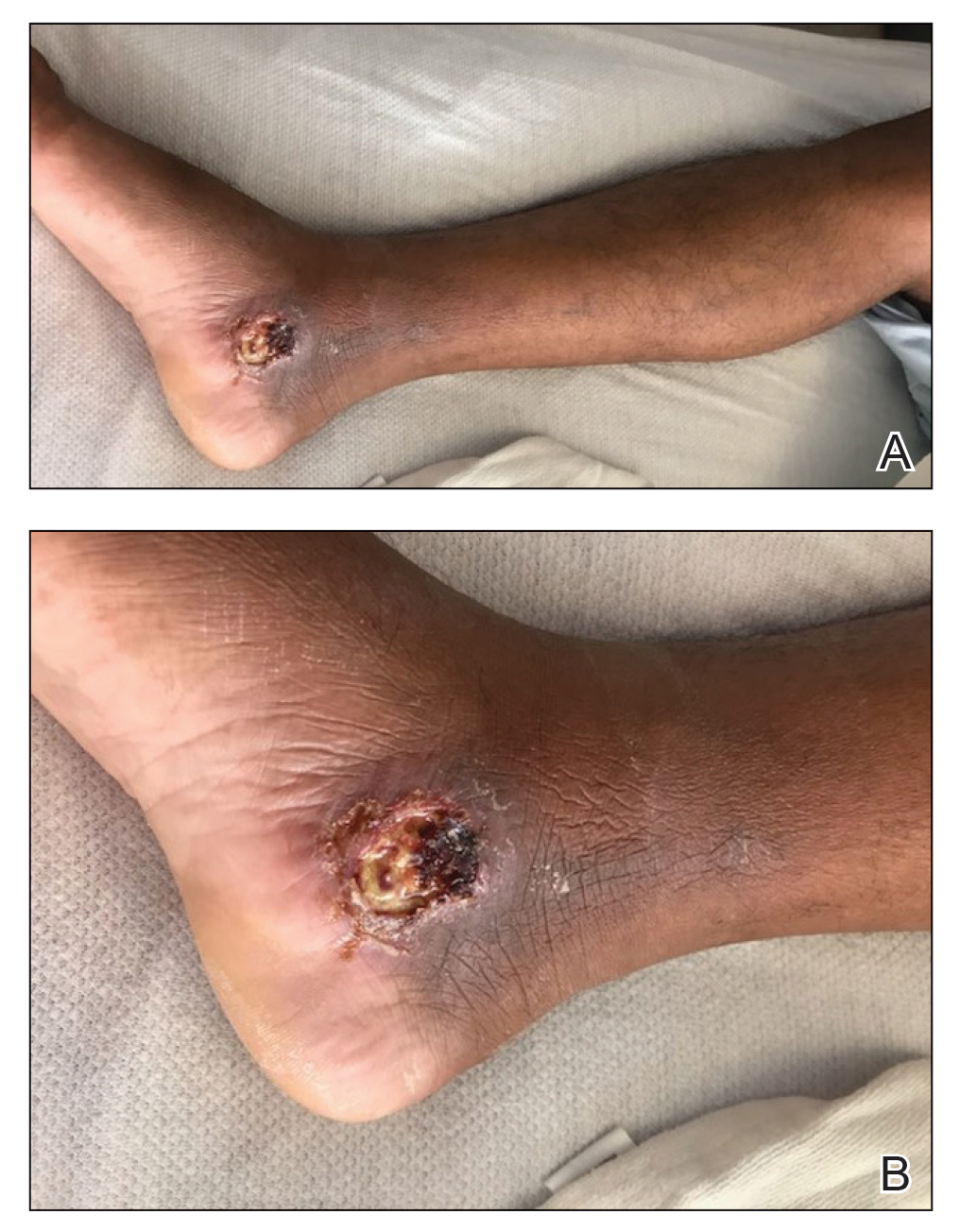
Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.
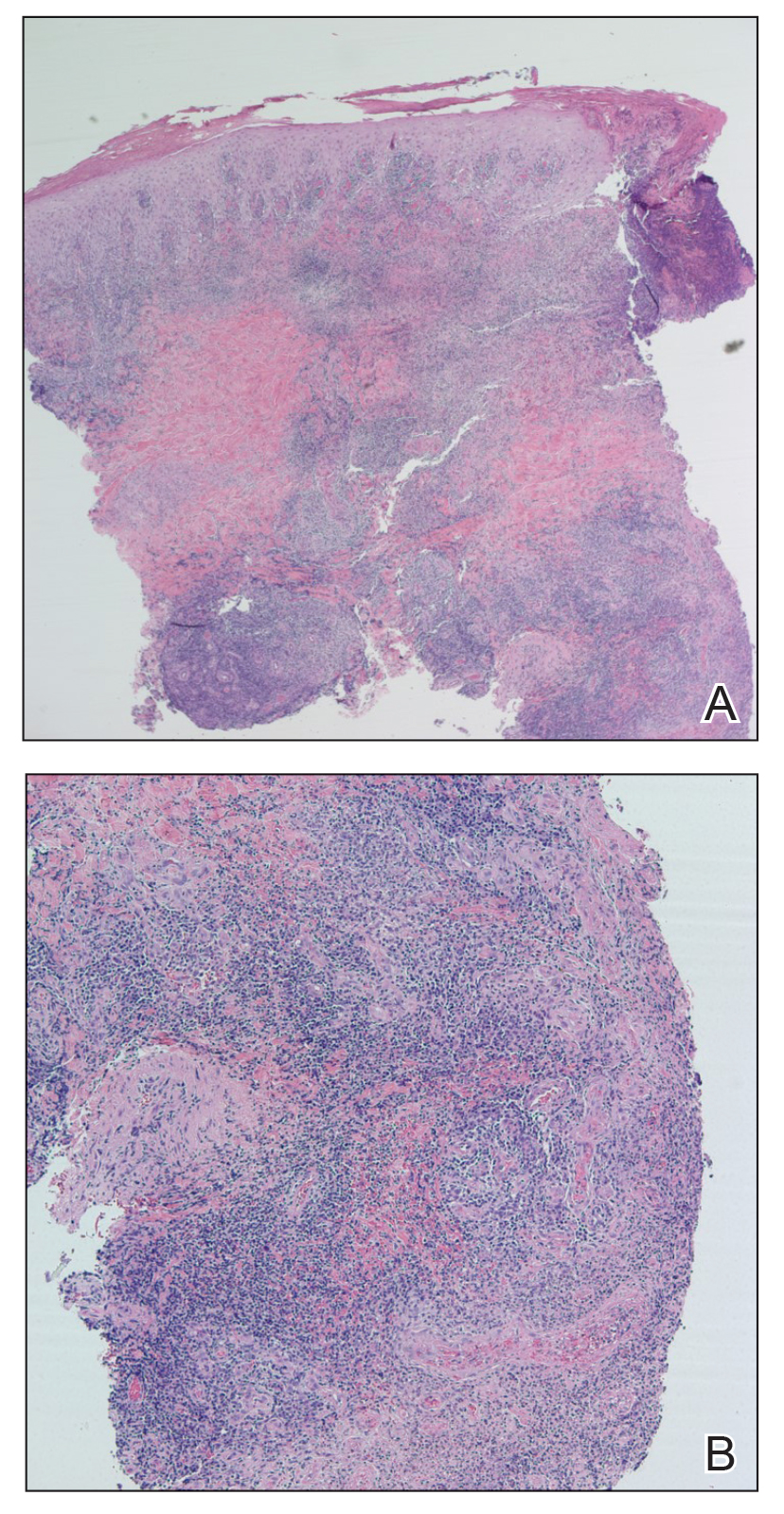
Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
The genus Leishmania comprises protozoan parasites that cause approximately 2 million new cases of leishmaniasis each year across 98 countries.1 These protozoa are obligate intracellular parasites of phlebotomine sandfly species that transmit leishmaniasis and result in a considerable parasitic cause of fatalities globally, second only to malaria.2,3
Phlebotomine sandflies primarily live in tropical and subtropical regions and function as vectors for many pathogens in addition to Leishmania species, such as Bartonella species and arboviruses.3 In 2004, it was noted that the majority of leishmaniasis cases affected developing countries: 90% of visceral leishmaniasis cases occurred in Bangladesh, India, Nepal, Sudan, and Brazil, and 90% of cutaneous leishmaniasis cases occurred in Afghanistan, Algeria, Brazil, Iran, Peru, Saudi Arabia, and Syria.4 Of note, with recent environmental changes, phlebotomine sandflies have gradually migrated to more northerly latitudes, extending into Europe.5
Twenty Leishmania species and 30 sandfly species have been identified as causes of leishmaniasis.4Leishmania infection occurs when an infected sandfly bites a mammalian host and transmits the parasite’s flagellated form, known as a promastigote. Host inflammatory cells, such as monocytes and dendritic cells, phagocytize parasites that enter the skin. The interaction between parasites and dendritic cells become an important factor in the outcome of Leishmania infection in the host because dendritic cells promote development of CD4 and CD8 T lymphocytes with specificity to target Leishmania parasites and protect the host.1
The number of cases of leishmaniasis has increased worldwide, most likely due to changes in the environment and human behaviors such as urbanization, the creation of new settlements, and migration from rural to urban areas.3,5 Important risk factors in individual patients include malnutrition; low-quality housing and sanitation; a history of migration or travel; and immunosuppression, such as that caused by HIV co-infection.2,5
Case Report
An otherwise healthy 25-year-old Bangladeshi man presented to our community hospital for evaluation of a painful leg ulcer of 1 month’s duration. The patient had migrated from Bangladesh to Panama, then to Costa Rica, followed by Guatemala, Honduras, Mexico, and, last, Texas. In Texas, he was identified by the US Immigration and Customs Enforcement, transported to a detention facility, and transferred to this hospital shortly afterward.
The patient reported that, during his extensive migration, he had lived in the jungle and reported what he described as mosquito bites on the legs. He subsequently developed a 3-cm ulcerated and crusted plaque with rolled borders on the right medial ankle (Figure 1). In addition, he had a palpable nodular cord on the medial leg from the ankle lesion to the mid thigh that was consistent with lymphocutaneous spread. Ultrasonography was negative for deep-vein thrombosis.

Because the patient’s recent migration from Central America was highly concerning for microbial infection, vancomycin and piperacillin-tazobactam were started empirically on admission. A punch biopsy from the right medial ankle was nondiagnostic, showing acute and chronic necrotizing inflammation along with numerous epithelioid histiocytes with a vaguely granulomatous appearance (Figure 2). A specimen from the right medial ankle that had already been taken by an astute border patrol medical provider was sent to the Centers for Disease Control and Prevention (CDC) for polymerase chain reaction analysis following admission and was found to be positive for Leishmania panamensis.

Given the concern for mucocutaneous leishmaniasis with this particular species, otolaryngology was consulted; however, the patient did not demonstrate mucocutaneous disease. Because of the elevated risk for persistent disease with L panamensis, systemic therapy was indicated and administered: IV amphotericin B 200 mg on days 1 through 5 and again on day 10. Improvement in the ulcer was seen after the 10-day regimen was completed.
Comment
Leishmaniasis can be broadly classified by geographic region or clinical presentation. Under the geographic region system, leishmaniasis can be categorized as Old World or New World. Old World leishmaniasis primarily is transmitted by Phlebotomus sandflies and carries the parasites Leishmania major and Leishmania tropica, among others. New World leishmaniasis is caused by Lutzomyia sandflies, which carry Leishmania mexicana, Leishmania braziliensis, Leishmania amazonensis, and others.6
Our patient presented with cutaneous leishmaniasis, one of 4 primary clinical disease forms of leishmaniasis; the other 3 forms under this classification system are diffuse cutaneous, mucocutaneous, and visceral leishmaniasis, also known as kala-azar.3,6 Cutaneous leishmaniasis is limited to the skin, particularly the face and extremities. This form is more common with Old World vectors, with most cases occurring in Peru, Brazil, and the Middle East. In Old World cutaneous leishmaniasis, the disease begins with a solitary nodule at the site of the bite that ulcerates and can continue to spread in a sporotrichoid pattern. This cutaneous form tends to heal slowly over months to years with residual scarring. New World cutaneous leishmaniasis can present with a variety of clinical manifestations, including ulcerative, sarcoidlike, miliary, and nodular lesions.6,7
The diffuse form of cutaneous leishmaniasis begins in a similar manner to the Old World cutaneous form: a single nodule spreads widely over the body, especially the nose, and covers the patient’s skin with keloidal or verrucous lesions that do not ulcerate. These nodules contain large groupings of Leishmania-filled foamy macrophages. Often, patients with diffuse cutaneous leishmaniasis are immunosuppressed and are unable to develop an immune response to leishmanin and other skin antigens.6,7
Mucocutaneous leishmaniasis predominantly is caused by the New World species L braziliensis but also has been attributed to L amazonensis, L panamensis, and L guyanensis. This form manifests as mucosal lesions that can develop simultaneously with cutaneous lesions but more commonly appear months to years after resolution of the skin infection. Patients often present with ulceration of the lip, nose, and oropharynx, and destruction of the nasopharynx can result in severe consequences such as obstruction of the airway and perforation of the nasal septum (also known as espundia).6,7
The most severe presentation of leishmaniasis is the visceral form (kala-azar), which presents with parasitic infection of the liver, spleen, and bone marrow. Most commonly caused by Leishmania donovani, Leishmania infantum, and Leishmania chagasi, this form has a long incubation period spanning months to years before presenting with diarrhea, hepatomegaly, splenomegaly, darkening of the skin (in Hindi, kala-azar means “black fever”), pancytopenia, lymphadenopathy, nephritis, and intestinal hemorrhage, among other severe manifestations. Visceral leishmaniasis has a poor prognosis: patients succumb to disease within 2 years if not treated.6,7
Diagnosis—Diagnosing leishmaniasis starts with a complete personal and medical history, paying close attention to travel and exposures. Diagnosis is most successfully performed by polymerase chain reaction analysis, which is both highly sensitive and specific but also can be determined by culture using Novy-McNeal-Nicolle medium or by light microscopy. Histologic findings include the marquee sign, which describes an array of amastigotes (promastigotes that have developed into the intracellular tissue-stage form) with kinetoplasts surrounding the periphery of parasitized histiocytes. Giemsa staining can be helpful in identifying organisms.2,6,7
The diagnosis in our case was challenging, as none of the above findings were seen in our patient. The specimen taken by the border patrol medical provider was negative on Gram, Giemsa, and Grocott-Gömöri methenamine silver staining; no amastigotes were identified. Another diagnostic modality (not performed in our patient) is the Montenegro delayed skin-reaction test, which often is positive in patients with cutaneous leishmaniasis but also yields a positive result in patients who have been cured of Leishmania infection.6
An important consideration in the diagnostic workup of leishmaniasis is that collaboration with the CDC can be helpful, such as in our case, as they provide clear guidance for specimen collection and processing.2
Treatment—Treating leishmaniasis is challenging and complex. Even the initial decision to treat depends on several factors, including the form of infection. Most visceral and mucocutaneous infections should be treated due to both the lack of self-resolution of these forms and the higher risk for a potentially life-threatening disease course; in contrast, cutaneous forms require further consideration before initiating treatment. Some indicators for treating cutaneous leishmaniasis include widespread infection, intention to decrease scarring, and lesions with the potential to cause further complications (eg, on the face or ears or close to joints).6-8
The treatment of choice for cutaneous and mucocutaneous leishmaniasis is pentavalent antimony; however, this drug can only be obtained in the United States for investigational use, requiring approval by the CDC. A 20-day intravenous or intramuscular course of 20 mg/kg per day typically is used for cutaneous cases; a 28-day course typically is used for mucosal forms.
Amphotericin B is not only the treatment of choice for visceral leishmaniasis but also is an important alternative therapy for patients with mucosal leishmaniasis or who are co-infected with HIV. Patients with visceral infection also should receive supportive care for any concomitant afflictions, such as malnutrition or other infections. Although different regimens have been described, the US Food and Drug Administration has created outlines of specific intravenous infusion schedules for liposomal amphotericin B in immunocompetent and immunosuppressed patients.8 Liposomal amphotericin B also has a more favorable toxicity profile than conventional amphotericin B deoxycholate, which is otherwise effective in combating visceral leishmaniasis.6-8
Other treatments that have been attempted include pentamidine, miltefosine, thermotherapy, oral itraconazole and fluconazole, rifampicin, metronidazole and cotrimoxazole, dapsone, photodynamic therapy, thermotherapy, topical paromomycin formulations, intralesional pentavalent antimony, and laser cryotherapy. Notable among these other agents is miltefosine, a US Food and Drug Administration–approved oral medication for adults and adolescents (used off-label for patients younger than 12 years) with cutaneous leishmaniasis caused by L braziliensis, L panamensis, or L guyanensis. Other oral options mentioned include the so-called azole antifungal medications, which historically have produced variable results. From the CDC’s reports, ketoconazole was moderately effective in Guatemala and Panama,8 whereas itraconazole did not demonstrate efficacy in Colombia, and the efficacy of fluconazole was inconsistent in different countries.8 When considering one of the local (as opposed to oral and parenteral) therapies mentioned, the extent of cutaneous findings as well as the risk of mucosal spread should be factored in.6-8
Understandably, a number of considerations can come into play in determining the appropriate treatment modality, including body region affected, clinical form, severity, and Leishmania species.6-8 Our case is of particular interest because it demonstrates the complexities behind the diagnosis and treatment of cutaneous leishmaniasis, with careful consideration geared toward the species; for example, because our patient was infected with L panamensis, which is known to cause mucocutaneous disease, the infectious disease service decided to pursue systemic therapy with amphotericin B rather than topical treatment.
Prevention—Vector control is the primary means of preventing leishmaniasis under 2 umbrellas: environmental management and synthetic insecticides. The goal of environmental management is to eliminate the phlebotomine sandfly habitat; this was the primary method of vector control until 1940. Until that time, tree stumps were removed, indoor cracks and crevices were filled to prevent sandfly emergence, and areas around animal shelters were cleaned. These methods were highly dependent on community awareness and involvement; today, they can be combined with synthetic insecticides to offer maximum protection.
Synthetic insecticides include indoor sprays, treated nets, repellents, and impregnated dog collars, all of which control sandflies. However, the use of these insecticides in endemic areas, such as India, has driven development of insecticide resistance in many sandfly vector species.3
As of 2020, 5 vaccines against Leishmania have been created. Two are approved–one in Brazil and one in Uzbekistan–for human use as immunotherapy, while the other 3 have been developed to immunize dogs in Brazil. However, the effectiveness of these vaccines is under debate. First, one of the vaccines used as immunotherapy for cutaneous leishmaniasis must be used in combination with conventional chemotherapy; second, long-term effects of the canine vaccine are unknown.1 A preventive vaccine for humans is under development.1,3
Final Thoughts
Leishmaniasis remains a notable parasitic disease that is increasing in prevalence worldwide. Clinicians should be aware of this disease because early detection and treatment are essential to control infection.3 Health care providers in the United States should be especially aware of this condition among patients who have a history of travel or migration; those in Texas should recognize the current endemic status of leishmaniasis there.4,6
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
- Coutinho De Oliveira B, Duthie MS, Alves Pereira VR. Vaccines for leishmaniasis and the implications of their development for American tegumentary leishmaniasis. Hum Vaccin Immunother. 2020;16:919-930. doi:10.1080/21645515.2019.1678998
- Chan CX, Simmons BJ, Call JE, et al. Cutaneous leishmaniasis successfully treated with miltefosine. Cutis. 2020;106:206-209. doi:10.12788/cutis.0086
- Balaska S, Fotakis EA, Chaskopoulou A, et al. Chemical control and insecticide resistance status of sand fly vectors worldwide. PLoS Negl Trop Dis. 2021;15:E0009586. doi:10.1371/journal.pntd.0009586
- Desjeux P. Leishmaniasis. Nat Rev Microbiol. 2004;2:692. doi:10.1038/nrmicro981
- Michelutti A, Toniolo F, Bertola M, et al. Occurrence of Phlebotomine sand flies (Diptera: Psychodidae) in the northeastern plain of Italy. Parasit Vectors. 2021;14:164. doi:10.1186/s13071-021-04652-2
- Alkihan A, Hocker TLH. Infectious diseases: parasites and other creatures: protozoa. In: Alikhan A, Hocker TLH, eds. Review of Dermatology. Elsevier; 2024:329-331.
- Dinulos JGH. Infestations and bites. In: Habif TP, ed. Clinical Dermatology. Elsevier; 2016:630-634.
- Centers for Disease Control and Prevention. Leishmaniasis: resources for health professionals. US Department of Health and Human Services. March 20, 2023. Accessed October 5, 2023. https://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#:~:text=Liposomal%20amphotericin%20B%20is%20FDA,treatment%20of%20choice%20for%20U.S
Practice Points
- The Phlebotomus and Lutzomyia genera of sandflies are vectors of Leishmania parasites, which can result in an array of clinical findings associated with leishmaniasis.
- Treatment options for leishmaniasis differ based on whether the infection is considered uncomplicated or complicated, which depends on the species of Leishmania; the number, size, and location of the lesion(s); and host immune status.
- All US practitioners should be aware of this pathogen, especially with regard to patients who have a history of travel to other countries. Health care professionals in states such as Texas and Oklahoma should be especially cognizant because these constitute one of the few areas in the United States where locally acquired cases of leishmaniasis have been reported.
Atopic Dermatitis Triggered by Omalizumab and Treated With Dupilumab
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.
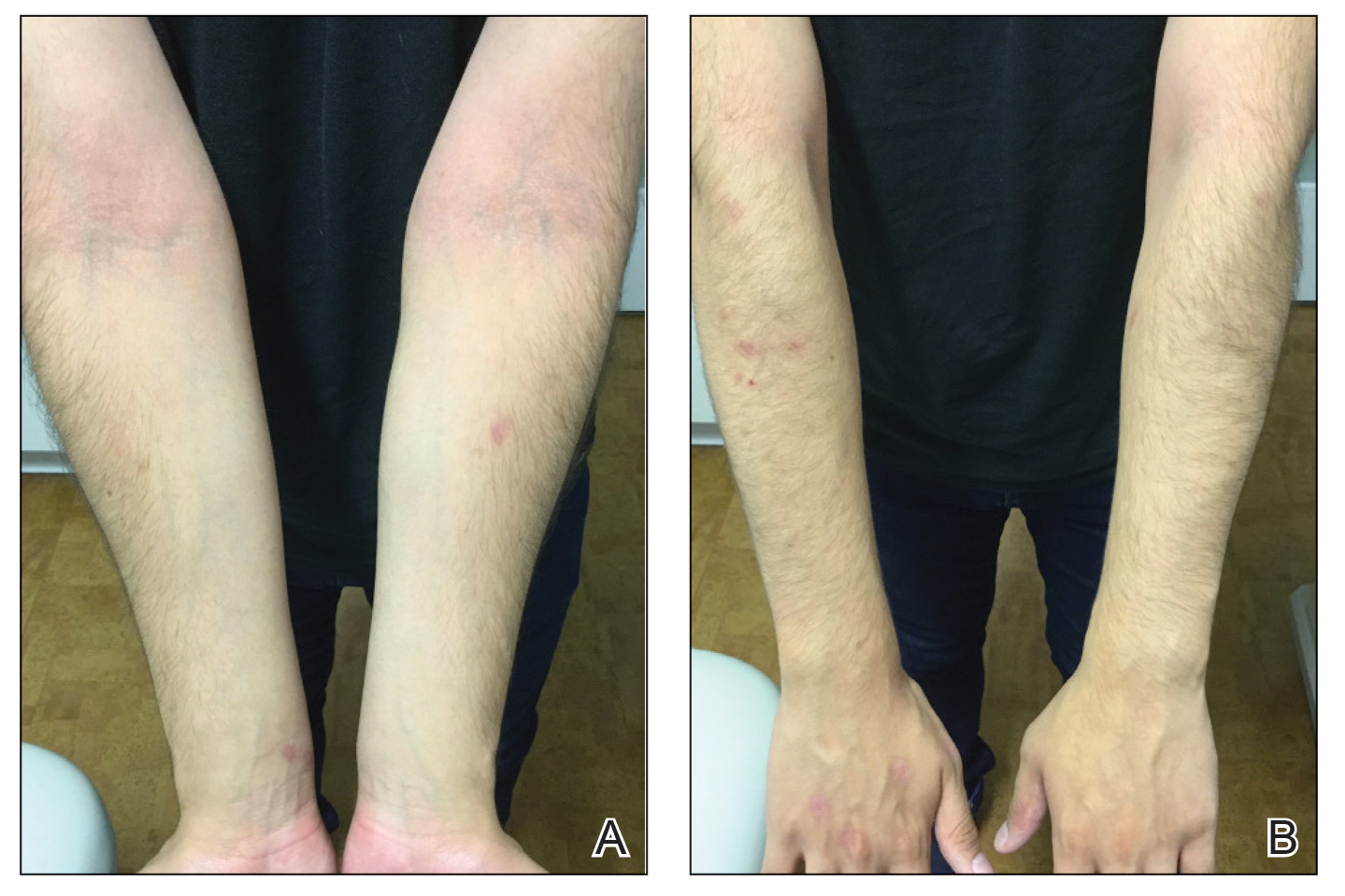
Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
To the Editor:
A 16-year-old adolescent boy presented to our pediatric dermatology clinic for evaluation of long-standing mild atopic dermatitis (AD) that had become severe over the last year after omalizumab was initiated for severe asthma. The patient had a history of multiple hospitalizations for severe asthma. Despite excellent control of asthma with omalizumab given every 2 weeks, he developed widespread eczematous plaques on the neck, trunk, and extremities over the course of a year. The AD often was complicated by superimposed folliculitis due to scratching from severe pruritus. Treatment with topical corticosteroids including triamcinolone ointment 0.1% to AD on the body, plus clobetasol ointment 0.05% for prurigolike lesions on the legs resulted in modest improvement; however, the AD consistently recurred within a few days after the biweekly omalizumab injection (Figure 1). When the omalizumab injections were delayed, the flares temporarily improved, and when injections were decreased to once monthly, the exacerbations subsided partially but not fully.

Because omalizumab resulted in dramatic improvement in the patient’s asthma, there was hesitation to discontinue it initially; however, the patient and his parents in conjunction with the dermatology and pulmonary teams decided to transition to dupilumab. The patient reported vast improvement of AD 1 month after initiation of dupilumab (Figure 2), which remained well controlled more than 1 year later. Mid-potency topical corticosteroids for the treatment of occasional mild eczematous flares on the extremities were used. The patient’s asthma has remained well controlled on dupilumab without any exacerbations.

Omalizumab is a recombinant DNA-derived humanized monoclonal antibody that binds both circulating and membrane-bound IgE. It has been proposed as a possible treatment for severe and/or recalcitrant AD, with mixed treatment results.1 A case series and review of 174 patients demonstrated a moderate to complete AD response to treatment with omalizumab in 74.1% of patients.2 The Atopic Dermatitis Anti-IgE Pediatric Trial (ADAPT) showed a statistically significant reduction in the Scoring Atopic Dermatitis (SCORAD) index (P=.01), along with improved quality of life in children treated with omalizumab vs those treated with placebo.3 However, a prior randomized, placebo-controlled, double-blind study did not show a significant difference in clinical disease parameters in patients treated with omalizumab.4
The humanized monoclonal antibody dupilumab, an anti–IL-4/IL-13 agent, has demonstrated more consistent efficacy for the treatment of AD in children and adults.1 Dupilumab is effective for both intrinsic and extrinsic AD1 because its clinical efficacy is unrelated to circulating levels of IgE in the bloodstream. Although IgE may have a role in childhood AD, our case demonstrated a different pathophysiologic mechanism independent of IgE. Our patient’s AD flares occurred within a few days of omalizumab injection, which may have resulted in a paradoxical increase in basophil sensitivity to other cytokines such as IL-335 and led to an increase in IL-4/IL-13 production within the skin. In our patient, this increase was successfully blocked by dupilumab. Furthermore, omalizumab has been shown to modulate helper T cell (TH2) cytokine response such as thymic stromal lymphopoietin.6 A cytokine imbalance could have exacerbated AD in our case.
Although additional work to clarify the pathogenesis of AD is needed, it is important to recognize the potential for the occurrence of paradoxical AD flares in patients treated with omalizumab, which is analogous to the well-documented entity of tumor necrosis factor α inhibitor–induced psoriasis. It is equally important to recognize the potential benefit for patients treated with dupilumab.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
- Nygaard U, Vestergaard C, Deleuran M. Emerging treatment options in atopic dermatitis: systemic therapies. Dermatology. 2017;233:344-357.
- Holm JG, Agner T, Sand C, et al. Omalizumab for atopic dermatitis: case series and a systematic review of the literature. Int J Dermatol. 2017;56:18-26.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. 2020;174:29-37.
- Heil PM, Maurer D, Klein B, et al. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8:990-998.
- Imai Y. Interleukin-33 in atopic dermatitis. J Dermatol Sci. 2019;96:2-7.
- Iyengar SR, Hoyte EG, Loza A, et al. Immunologic effects of omalizumab in children with severe refractory atopic dermatitis: a randomized, placebo-controlled clinical trial. Int Arch Allergy Immunol. 2013;162:89-93.
Practice Points
- Monoclonal antibodies are promising therapies for atopic conditions, although its efficacy for atopic dermatitis (AD) is debated and the side-effect profile is not entirely known.
- Omalizumab may cause a paradoxical exacerbation of AD in select patients analogous to tumor necrosis factor α inhibitor–induced psoriasis.