User login
CMS makes economics of primary care ACOs more appealing
As you may have read, accountable care organizations have met uneven success over the last several years. But, when they are broken down into categories, physician-sponsored ACOs have done better, particularly those with a strong primary care core.
This is true for several reasons.
In this transition period from a fee-for-service payment system that rewards volume and expensive inpatient care to a pay-for-value system, some ACOs set up by health systems or specialists envisioned the savings coming through lower utilization of their services. They had an inherent impediment to fully committing to keeping people well and avoiding acute care. In contrast, primary care providers are free to be all in with population health value-based programs.
However, because the fee-for-service system has historically left primary care physicians at the bottom of the compensation food chain, we have a “can’t get there from here” dilemma. It is a cruel irony that the group best suited to stretch America’s health care dollar and benefit both professionally and financially usually does not have the capital to create and operate an ACO for the roughly 18 months before shared savings are distributed.
The Centers for Medicare & Medicaid Services has tried to mitigate this by offering financial support for small, non–health system ACOs, particularly those in rural areas. Some of those enrolled ACOs are primary care driven and have been among the most successful in the MSSP.
Nonetheless, the upfront costs, paired with the long delay for the sole economic return on the investment – shared savings – have combined to be deal killers for many promising would-be primary care ACOs.
New upfront payments are game changers
A successful ACO will be assigned one or more patient populations and be given a minimum of 50% of the savings for the overall costs for those populations, if the quality of their health is maintained or improved.
To excise avoidable waste, the ACO looks at gaps in care for those populations – frequent emergency department use for nonemergencies, avoidably high levels of diabetes and obesity, too-high readmission rates, unnecessarily high postacute care costs, etc. They then use evidence-based best team care practices – from patient self care and prevention, to multispecialty coordination and PCMH care management.
Why? Because these proved to give the highest impact on quality and reducing costs. To achieve significant shared savings, the costs are usually measured for a calendar year, then it takes about 6 months for the claims to be reported and paid. Thus, the shared savings check to the ACO will arrive about 18 months after all this is started.
The CMS has also figured out that primary care physician care coordination and management drive quality and savings. The agency knows that incentivizing this type of care, the very type calculated to create ACO success, will net significant savings to the Medicare program.
For example, the pilot project for preventing diabetes will be expanded, because Medicare hopes to save several thousand dollars a year per beneficiary in health care costs.
In a blog entry the day the expanded population health management codes were announced, the CMS acting administrator wrote that, “Over time, if the clinicians qualified to provide these services were to fully provide these services to all eligible beneficiaries, the increase would be as much as $4 billion or more in additional support for care coordination and patient-centered care.”
CMS revenue streams to support ACO success-driving activities include:
• Value-based screening and counseling codes to decrease downstream costs.
• Upward adjustment of evaluation and management reimbursement for assessment of care and care plan development for mobility-impaired patients.
• Annual wellness visits.
• Prolonged E&M services that accrue outside of a patient visit.
• Collaboration with mental health specialists.
• Comprehensive assessment and care planning for patients with cognitive impairment.
• Expansion of the diabetes prevention pilot program; diabetes prevention and diabetes education are two separate services.
• Transitional care management for high-risk patients post discharge.
• Structured obesity management.
The 2017 Medicare fee schedule smoothed some of the bumps in administering and being paid for chronic care management (CCM) services, and it added codes with increased reimbursement aligned with increased complexity of comorbidities/illness.
Perhaps the biggest new payment boost for primary care to engage in ACO high-value activities is actually the Merit-Based Incentive Payment System (MIPS) under MACRA, the Medicare Access and CHIP Reauthorization Act of 2015.
Under MACRA, all Medicare compensation for physicians will be determined by relative delivery of quality and efficient care. Experts are recommending that primary care physicians participate in non–risk-taking ACOs to optimize MIPS value scoring, while also reducing administrative burdens of compliance. Use an ACO’s analytics to support collaborative care and provide the reports required under MIPS.
Let’s be smart about it
According to Gordon Wilhoit, MD, a practicing physician and chief medical officer of an all–primary-care-physician ACO in South Carolina, “This is a no brainer. Start first with your MSSP ACO high-value game plan, then align the complementary care coordination codes, CCM, MIPS, and other revenue stream and reporting activities with it. Now, primary care physicians can finance their ACO and MIPS care coordination efforts with a stream of ongoing payments from these care management codes.
“One of my colleagues saw a 27% increase in revenues in 6 months just from providing and billing for this type of care,” Dr. Wilhoit explained. “And, not counting shared savings or MIPS incentive payments, our office’s reimbursement from these care management codes now exceeds our fee-for-service income, which has not decreased.”
Even with these payments, the CMS will reduce overall net expenditures. Your impact on health care will be more powerful as a manager of the team addressing patients’ overall health than reacting to patient sickness one at a time. The patients you impact the most may be ones you don’t actually see. Your empowerment to practice medicine the right way will continue to grow.
Now, finally, you may start getting compensation that takes away the last big hurdle to creating the infrastructure you need to succeed.
Mr. Bobbitt is a head of the health law group at the Smith Anderson law firm in Raleigh, N.C. He is president of, and Dr. Wilhoit is a consultant with, Value Health Partners, LLC, a health care strategic consulting company. He has years of experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at either bo@vhp.care or 919-906-4054.
As you may have read, accountable care organizations have met uneven success over the last several years. But, when they are broken down into categories, physician-sponsored ACOs have done better, particularly those with a strong primary care core.
This is true for several reasons.
In this transition period from a fee-for-service payment system that rewards volume and expensive inpatient care to a pay-for-value system, some ACOs set up by health systems or specialists envisioned the savings coming through lower utilization of their services. They had an inherent impediment to fully committing to keeping people well and avoiding acute care. In contrast, primary care providers are free to be all in with population health value-based programs.
However, because the fee-for-service system has historically left primary care physicians at the bottom of the compensation food chain, we have a “can’t get there from here” dilemma. It is a cruel irony that the group best suited to stretch America’s health care dollar and benefit both professionally and financially usually does not have the capital to create and operate an ACO for the roughly 18 months before shared savings are distributed.
The Centers for Medicare & Medicaid Services has tried to mitigate this by offering financial support for small, non–health system ACOs, particularly those in rural areas. Some of those enrolled ACOs are primary care driven and have been among the most successful in the MSSP.
Nonetheless, the upfront costs, paired with the long delay for the sole economic return on the investment – shared savings – have combined to be deal killers for many promising would-be primary care ACOs.
New upfront payments are game changers
A successful ACO will be assigned one or more patient populations and be given a minimum of 50% of the savings for the overall costs for those populations, if the quality of their health is maintained or improved.
To excise avoidable waste, the ACO looks at gaps in care for those populations – frequent emergency department use for nonemergencies, avoidably high levels of diabetes and obesity, too-high readmission rates, unnecessarily high postacute care costs, etc. They then use evidence-based best team care practices – from patient self care and prevention, to multispecialty coordination and PCMH care management.
Why? Because these proved to give the highest impact on quality and reducing costs. To achieve significant shared savings, the costs are usually measured for a calendar year, then it takes about 6 months for the claims to be reported and paid. Thus, the shared savings check to the ACO will arrive about 18 months after all this is started.
The CMS has also figured out that primary care physician care coordination and management drive quality and savings. The agency knows that incentivizing this type of care, the very type calculated to create ACO success, will net significant savings to the Medicare program.
For example, the pilot project for preventing diabetes will be expanded, because Medicare hopes to save several thousand dollars a year per beneficiary in health care costs.
In a blog entry the day the expanded population health management codes were announced, the CMS acting administrator wrote that, “Over time, if the clinicians qualified to provide these services were to fully provide these services to all eligible beneficiaries, the increase would be as much as $4 billion or more in additional support for care coordination and patient-centered care.”
CMS revenue streams to support ACO success-driving activities include:
• Value-based screening and counseling codes to decrease downstream costs.
• Upward adjustment of evaluation and management reimbursement for assessment of care and care plan development for mobility-impaired patients.
• Annual wellness visits.
• Prolonged E&M services that accrue outside of a patient visit.
• Collaboration with mental health specialists.
• Comprehensive assessment and care planning for patients with cognitive impairment.
• Expansion of the diabetes prevention pilot program; diabetes prevention and diabetes education are two separate services.
• Transitional care management for high-risk patients post discharge.
• Structured obesity management.
The 2017 Medicare fee schedule smoothed some of the bumps in administering and being paid for chronic care management (CCM) services, and it added codes with increased reimbursement aligned with increased complexity of comorbidities/illness.
Perhaps the biggest new payment boost for primary care to engage in ACO high-value activities is actually the Merit-Based Incentive Payment System (MIPS) under MACRA, the Medicare Access and CHIP Reauthorization Act of 2015.
Under MACRA, all Medicare compensation for physicians will be determined by relative delivery of quality and efficient care. Experts are recommending that primary care physicians participate in non–risk-taking ACOs to optimize MIPS value scoring, while also reducing administrative burdens of compliance. Use an ACO’s analytics to support collaborative care and provide the reports required under MIPS.
Let’s be smart about it
According to Gordon Wilhoit, MD, a practicing physician and chief medical officer of an all–primary-care-physician ACO in South Carolina, “This is a no brainer. Start first with your MSSP ACO high-value game plan, then align the complementary care coordination codes, CCM, MIPS, and other revenue stream and reporting activities with it. Now, primary care physicians can finance their ACO and MIPS care coordination efforts with a stream of ongoing payments from these care management codes.
“One of my colleagues saw a 27% increase in revenues in 6 months just from providing and billing for this type of care,” Dr. Wilhoit explained. “And, not counting shared savings or MIPS incentive payments, our office’s reimbursement from these care management codes now exceeds our fee-for-service income, which has not decreased.”
Even with these payments, the CMS will reduce overall net expenditures. Your impact on health care will be more powerful as a manager of the team addressing patients’ overall health than reacting to patient sickness one at a time. The patients you impact the most may be ones you don’t actually see. Your empowerment to practice medicine the right way will continue to grow.
Now, finally, you may start getting compensation that takes away the last big hurdle to creating the infrastructure you need to succeed.
Mr. Bobbitt is a head of the health law group at the Smith Anderson law firm in Raleigh, N.C. He is president of, and Dr. Wilhoit is a consultant with, Value Health Partners, LLC, a health care strategic consulting company. He has years of experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at either bo@vhp.care or 919-906-4054.
As you may have read, accountable care organizations have met uneven success over the last several years. But, when they are broken down into categories, physician-sponsored ACOs have done better, particularly those with a strong primary care core.
This is true for several reasons.
In this transition period from a fee-for-service payment system that rewards volume and expensive inpatient care to a pay-for-value system, some ACOs set up by health systems or specialists envisioned the savings coming through lower utilization of their services. They had an inherent impediment to fully committing to keeping people well and avoiding acute care. In contrast, primary care providers are free to be all in with population health value-based programs.
However, because the fee-for-service system has historically left primary care physicians at the bottom of the compensation food chain, we have a “can’t get there from here” dilemma. It is a cruel irony that the group best suited to stretch America’s health care dollar and benefit both professionally and financially usually does not have the capital to create and operate an ACO for the roughly 18 months before shared savings are distributed.
The Centers for Medicare & Medicaid Services has tried to mitigate this by offering financial support for small, non–health system ACOs, particularly those in rural areas. Some of those enrolled ACOs are primary care driven and have been among the most successful in the MSSP.
Nonetheless, the upfront costs, paired with the long delay for the sole economic return on the investment – shared savings – have combined to be deal killers for many promising would-be primary care ACOs.
New upfront payments are game changers
A successful ACO will be assigned one or more patient populations and be given a minimum of 50% of the savings for the overall costs for those populations, if the quality of their health is maintained or improved.
To excise avoidable waste, the ACO looks at gaps in care for those populations – frequent emergency department use for nonemergencies, avoidably high levels of diabetes and obesity, too-high readmission rates, unnecessarily high postacute care costs, etc. They then use evidence-based best team care practices – from patient self care and prevention, to multispecialty coordination and PCMH care management.
Why? Because these proved to give the highest impact on quality and reducing costs. To achieve significant shared savings, the costs are usually measured for a calendar year, then it takes about 6 months for the claims to be reported and paid. Thus, the shared savings check to the ACO will arrive about 18 months after all this is started.
The CMS has also figured out that primary care physician care coordination and management drive quality and savings. The agency knows that incentivizing this type of care, the very type calculated to create ACO success, will net significant savings to the Medicare program.
For example, the pilot project for preventing diabetes will be expanded, because Medicare hopes to save several thousand dollars a year per beneficiary in health care costs.
In a blog entry the day the expanded population health management codes were announced, the CMS acting administrator wrote that, “Over time, if the clinicians qualified to provide these services were to fully provide these services to all eligible beneficiaries, the increase would be as much as $4 billion or more in additional support for care coordination and patient-centered care.”
CMS revenue streams to support ACO success-driving activities include:
• Value-based screening and counseling codes to decrease downstream costs.
• Upward adjustment of evaluation and management reimbursement for assessment of care and care plan development for mobility-impaired patients.
• Annual wellness visits.
• Prolonged E&M services that accrue outside of a patient visit.
• Collaboration with mental health specialists.
• Comprehensive assessment and care planning for patients with cognitive impairment.
• Expansion of the diabetes prevention pilot program; diabetes prevention and diabetes education are two separate services.
• Transitional care management for high-risk patients post discharge.
• Structured obesity management.
The 2017 Medicare fee schedule smoothed some of the bumps in administering and being paid for chronic care management (CCM) services, and it added codes with increased reimbursement aligned with increased complexity of comorbidities/illness.
Perhaps the biggest new payment boost for primary care to engage in ACO high-value activities is actually the Merit-Based Incentive Payment System (MIPS) under MACRA, the Medicare Access and CHIP Reauthorization Act of 2015.
Under MACRA, all Medicare compensation for physicians will be determined by relative delivery of quality and efficient care. Experts are recommending that primary care physicians participate in non–risk-taking ACOs to optimize MIPS value scoring, while also reducing administrative burdens of compliance. Use an ACO’s analytics to support collaborative care and provide the reports required under MIPS.
Let’s be smart about it
According to Gordon Wilhoit, MD, a practicing physician and chief medical officer of an all–primary-care-physician ACO in South Carolina, “This is a no brainer. Start first with your MSSP ACO high-value game plan, then align the complementary care coordination codes, CCM, MIPS, and other revenue stream and reporting activities with it. Now, primary care physicians can finance their ACO and MIPS care coordination efforts with a stream of ongoing payments from these care management codes.
“One of my colleagues saw a 27% increase in revenues in 6 months just from providing and billing for this type of care,” Dr. Wilhoit explained. “And, not counting shared savings or MIPS incentive payments, our office’s reimbursement from these care management codes now exceeds our fee-for-service income, which has not decreased.”
Even with these payments, the CMS will reduce overall net expenditures. Your impact on health care will be more powerful as a manager of the team addressing patients’ overall health than reacting to patient sickness one at a time. The patients you impact the most may be ones you don’t actually see. Your empowerment to practice medicine the right way will continue to grow.
Now, finally, you may start getting compensation that takes away the last big hurdle to creating the infrastructure you need to succeed.
Mr. Bobbitt is a head of the health law group at the Smith Anderson law firm in Raleigh, N.C. He is president of, and Dr. Wilhoit is a consultant with, Value Health Partners, LLC, a health care strategic consulting company. He has years of experience assisting physicians form integrated delivery systems. He has spoken and written nationally to primary care physicians on the strategies and practicalities of forming or joining ACOs. This article is meant to be educational and does not constitute legal advice. For additional information, readers may contact the author at either bo@vhp.care or 919-906-4054.
Psychiatric Comorbidities Are Common in Newly Diagnosed Pediatric Epilepsy
HOUSTON—Nearly one in three children diagnosed with new-onset epilepsy presents with psychiatric diagnoses at the onset, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
This finding “tells us that when kids are coming in, even if they are only having psychiatric symptoms at their onset of epilepsy, they should be referred for some treatment to help them possibly mitigate the development of these psychiatric diagnoses in the first year,” said Julia Doss, PsyD, Pediatric Psychologist at the Minnesota Epilepsy Group in Saint Paul.
About three years ago, the Minnesota Epilepsy Group, a private practice group that consults with the United Hospital and Children’s Hospitals and Clinics of Minnesota, launched a New Onset Pediatric Epilepsy (NOPE) clinic. At this clinic, referred patients undergo a psychologic evaluation, neuropsychologic testing, and medical evaluation in the same day. Psychologic assessment measures include the Clinical Interview with parent and patient, the Strengths and Difficulties Questionnaire (SDQ), and the Revised Children’s Anxiety and Depression Scale (RCADS).
Researchers evaluated 96 patients in the clinic who had presented for their first NOPE clinic visit within eight weeks of their epilepsy diagnosis. More than half were male, and patients ranged in age from 3 to 18. Dr. Doss and her colleagues separated the children into the following three groups: ages 3 to 6 (group 1), ages 7 to 11 (group 2), and ages 12 to 18 (group 3). Based on the Clinical Interview, none of the patients in group 1 screened positive for depression or anxiety, but 16% met criteria for some other behavioral disorder. However, among patients in group 2, the percentages who met criteria for depression, anxiety, and other behavioral disorders were 13%, 25%, and 13%, respectively. The corresponding percentages for patients in group 3 were 29%, 38%, and 10%.
Of the 96 patients evaluated, 64 parents completed all of the questions on the SDQ. The researchers observed significant correlations between parent response and diagnoses assigned on the Clinical Interview for behavior diagnoses and anxiety diagnoses) but not for depression diagnoses.
“Despite the correlations on both behavior and anxiety responses and clinical diagnoses assigned, parents still only reported significant concerns in about half of the children that were given diagnoses,” said Dr. Doss.
The comparison of RCADS scores between parent and child demonstrated moderate to strong correlation on the following scales: separation anxiety, generalized anxiety, obsessive/compulsive, and depression.
Small sample size was a key limitation in this study, said Dr. Doss. “Early evaluation or at least screening is necessary in all of our kids who present with an epilepsy diagnosis, because more than 30% develop psychiatric disorders within the first year of their diagnosis,” said Dr. Doss. “That’s one in three, so if we can start to better evaluate that early and get them funneled into treatment early, we might be able to prevent some of these problems from becoming lifelong issues.”
—Doug Brunk
Suggested Reading
Asato MR, Doss JL, Piloplys S. Clinic-friendly screening for cognitive and mental health problems in school-aged youth with epilepsy. Epilepsy Behav. 2015;48:97-102.
Piloplys S, Doss J, Siddarth P, et al. Risk factors for comorbid psychopathology in youth with psychogenic nonepileptic seizures. Seizure. 2016;38:32-37.
HOUSTON—Nearly one in three children diagnosed with new-onset epilepsy presents with psychiatric diagnoses at the onset, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
This finding “tells us that when kids are coming in, even if they are only having psychiatric symptoms at their onset of epilepsy, they should be referred for some treatment to help them possibly mitigate the development of these psychiatric diagnoses in the first year,” said Julia Doss, PsyD, Pediatric Psychologist at the Minnesota Epilepsy Group in Saint Paul.
About three years ago, the Minnesota Epilepsy Group, a private practice group that consults with the United Hospital and Children’s Hospitals and Clinics of Minnesota, launched a New Onset Pediatric Epilepsy (NOPE) clinic. At this clinic, referred patients undergo a psychologic evaluation, neuropsychologic testing, and medical evaluation in the same day. Psychologic assessment measures include the Clinical Interview with parent and patient, the Strengths and Difficulties Questionnaire (SDQ), and the Revised Children’s Anxiety and Depression Scale (RCADS).
Researchers evaluated 96 patients in the clinic who had presented for their first NOPE clinic visit within eight weeks of their epilepsy diagnosis. More than half were male, and patients ranged in age from 3 to 18. Dr. Doss and her colleagues separated the children into the following three groups: ages 3 to 6 (group 1), ages 7 to 11 (group 2), and ages 12 to 18 (group 3). Based on the Clinical Interview, none of the patients in group 1 screened positive for depression or anxiety, but 16% met criteria for some other behavioral disorder. However, among patients in group 2, the percentages who met criteria for depression, anxiety, and other behavioral disorders were 13%, 25%, and 13%, respectively. The corresponding percentages for patients in group 3 were 29%, 38%, and 10%.
Of the 96 patients evaluated, 64 parents completed all of the questions on the SDQ. The researchers observed significant correlations between parent response and diagnoses assigned on the Clinical Interview for behavior diagnoses and anxiety diagnoses) but not for depression diagnoses.
“Despite the correlations on both behavior and anxiety responses and clinical diagnoses assigned, parents still only reported significant concerns in about half of the children that were given diagnoses,” said Dr. Doss.
The comparison of RCADS scores between parent and child demonstrated moderate to strong correlation on the following scales: separation anxiety, generalized anxiety, obsessive/compulsive, and depression.
Small sample size was a key limitation in this study, said Dr. Doss. “Early evaluation or at least screening is necessary in all of our kids who present with an epilepsy diagnosis, because more than 30% develop psychiatric disorders within the first year of their diagnosis,” said Dr. Doss. “That’s one in three, so if we can start to better evaluate that early and get them funneled into treatment early, we might be able to prevent some of these problems from becoming lifelong issues.”
—Doug Brunk
Suggested Reading
Asato MR, Doss JL, Piloplys S. Clinic-friendly screening for cognitive and mental health problems in school-aged youth with epilepsy. Epilepsy Behav. 2015;48:97-102.
Piloplys S, Doss J, Siddarth P, et al. Risk factors for comorbid psychopathology in youth with psychogenic nonepileptic seizures. Seizure. 2016;38:32-37.
HOUSTON—Nearly one in three children diagnosed with new-onset epilepsy presents with psychiatric diagnoses at the onset, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
This finding “tells us that when kids are coming in, even if they are only having psychiatric symptoms at their onset of epilepsy, they should be referred for some treatment to help them possibly mitigate the development of these psychiatric diagnoses in the first year,” said Julia Doss, PsyD, Pediatric Psychologist at the Minnesota Epilepsy Group in Saint Paul.
About three years ago, the Minnesota Epilepsy Group, a private practice group that consults with the United Hospital and Children’s Hospitals and Clinics of Minnesota, launched a New Onset Pediatric Epilepsy (NOPE) clinic. At this clinic, referred patients undergo a psychologic evaluation, neuropsychologic testing, and medical evaluation in the same day. Psychologic assessment measures include the Clinical Interview with parent and patient, the Strengths and Difficulties Questionnaire (SDQ), and the Revised Children’s Anxiety and Depression Scale (RCADS).
Researchers evaluated 96 patients in the clinic who had presented for their first NOPE clinic visit within eight weeks of their epilepsy diagnosis. More than half were male, and patients ranged in age from 3 to 18. Dr. Doss and her colleagues separated the children into the following three groups: ages 3 to 6 (group 1), ages 7 to 11 (group 2), and ages 12 to 18 (group 3). Based on the Clinical Interview, none of the patients in group 1 screened positive for depression or anxiety, but 16% met criteria for some other behavioral disorder. However, among patients in group 2, the percentages who met criteria for depression, anxiety, and other behavioral disorders were 13%, 25%, and 13%, respectively. The corresponding percentages for patients in group 3 were 29%, 38%, and 10%.
Of the 96 patients evaluated, 64 parents completed all of the questions on the SDQ. The researchers observed significant correlations between parent response and diagnoses assigned on the Clinical Interview for behavior diagnoses and anxiety diagnoses) but not for depression diagnoses.
“Despite the correlations on both behavior and anxiety responses and clinical diagnoses assigned, parents still only reported significant concerns in about half of the children that were given diagnoses,” said Dr. Doss.
The comparison of RCADS scores between parent and child demonstrated moderate to strong correlation on the following scales: separation anxiety, generalized anxiety, obsessive/compulsive, and depression.
Small sample size was a key limitation in this study, said Dr. Doss. “Early evaluation or at least screening is necessary in all of our kids who present with an epilepsy diagnosis, because more than 30% develop psychiatric disorders within the first year of their diagnosis,” said Dr. Doss. “That’s one in three, so if we can start to better evaluate that early and get them funneled into treatment early, we might be able to prevent some of these problems from becoming lifelong issues.”
—Doug Brunk
Suggested Reading
Asato MR, Doss JL, Piloplys S. Clinic-friendly screening for cognitive and mental health problems in school-aged youth with epilepsy. Epilepsy Behav. 2015;48:97-102.
Piloplys S, Doss J, Siddarth P, et al. Risk factors for comorbid psychopathology in youth with psychogenic nonepileptic seizures. Seizure. 2016;38:32-37.
Dementia Prevalence Is Increased in Patients With Heart Failure
NEW ORLEANS—Elderly patients with heart failure have a significantly increased prevalence of dementia and mild cognitive impairment (MCI), compared with people of similar age without heart failure, according to an analysis of data collected from more than 6,000 US residents enrolled in a long-term observational study.
Patients diagnosed with either heart failure with reduced ejection fraction or heart failure with preserved ejection fraction had an 89% increased prevalence of dementia and a 41% increased prevalence of MCI, compared with people from the same cohort who did not develop heart failure. Investigators adjusted their analysis for several demographic and clinical variables, said Lucy S. Witt, MD, a researcher at the University of North Carolina in Chapel Hill, at the American Heart Association Scientific Sessions 2016. She speculated that the link between heart failure and dementia and MCI might result from impaired cerebral perfusion in patients with heart failure or from effects from heart failure medications.
“Our findings suggest that clinicians should have a higher suspicion for cognitive impairment in patients with heart failure, regardless of other, more classic risk factors,” she said. “This knowledge could prompt physicians to perform testing [for dementia and MCI], initiate conversations regarding the goals of care or advance care planning, and discuss appropriate living situations” for their patients with heart failure.
The analysis used data collected for the Atherosclerosis Risk in Communities (ARIC) study, which began in 1987 and enrolled a randomly selected representative cohort of approximately 16,000 women and men between ages 45 and 64 who resided in any of four US communities. Dr. Witt specifically focused on the data collected from 6,431 of the participants who returned for a fifth follow-up examination between 2011 and 2013, including 5,490 people without heart failure, whose average age was 76, and 941 participants with heart failure, whose average age was 78.
The adjusted rate of dementia prevalence at the fifth follow-up visit was 5.6% among those without heart failure and 7.0% in those with heart failure. The examinations also found MCI in an adjusted 21.5% of those without heart failure and in 26.2% of those with heart failure, said Dr. Witt. Adjustments were for factors such as age, sex, location, education, hypertension, diabetes, depression, alcohol and tobacco use, cerebrovascular disease, and marital status.
The relative risk for dementia among patients with heart failure was roughly similar, regardless of whether ARIC participants had a reduced or preserved left ventricular ejection fraction, she said.
ARIC is funded by the National Heart, Lung, and Blood Institute. Dr. Witt had no disclosures.
—Mitchel L. Zoler
Suggested Reading
Adelborg K, Horváth-Puhó E, Ording A, et al. Heart failure and risk of dementia: a Danish nationwide population-based cohort study. Eur J Heart Fail. 2016 Sep 9 [Epub ahead of print].
Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12-23.
Rusanen M, Kivipelto M, Levälahti E, et al. Heart diseases and long-term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimers Dis. 2014;42(1):183-191.
NEW ORLEANS—Elderly patients with heart failure have a significantly increased prevalence of dementia and mild cognitive impairment (MCI), compared with people of similar age without heart failure, according to an analysis of data collected from more than 6,000 US residents enrolled in a long-term observational study.
Patients diagnosed with either heart failure with reduced ejection fraction or heart failure with preserved ejection fraction had an 89% increased prevalence of dementia and a 41% increased prevalence of MCI, compared with people from the same cohort who did not develop heart failure. Investigators adjusted their analysis for several demographic and clinical variables, said Lucy S. Witt, MD, a researcher at the University of North Carolina in Chapel Hill, at the American Heart Association Scientific Sessions 2016. She speculated that the link between heart failure and dementia and MCI might result from impaired cerebral perfusion in patients with heart failure or from effects from heart failure medications.
“Our findings suggest that clinicians should have a higher suspicion for cognitive impairment in patients with heart failure, regardless of other, more classic risk factors,” she said. “This knowledge could prompt physicians to perform testing [for dementia and MCI], initiate conversations regarding the goals of care or advance care planning, and discuss appropriate living situations” for their patients with heart failure.
The analysis used data collected for the Atherosclerosis Risk in Communities (ARIC) study, which began in 1987 and enrolled a randomly selected representative cohort of approximately 16,000 women and men between ages 45 and 64 who resided in any of four US communities. Dr. Witt specifically focused on the data collected from 6,431 of the participants who returned for a fifth follow-up examination between 2011 and 2013, including 5,490 people without heart failure, whose average age was 76, and 941 participants with heart failure, whose average age was 78.
The adjusted rate of dementia prevalence at the fifth follow-up visit was 5.6% among those without heart failure and 7.0% in those with heart failure. The examinations also found MCI in an adjusted 21.5% of those without heart failure and in 26.2% of those with heart failure, said Dr. Witt. Adjustments were for factors such as age, sex, location, education, hypertension, diabetes, depression, alcohol and tobacco use, cerebrovascular disease, and marital status.
The relative risk for dementia among patients with heart failure was roughly similar, regardless of whether ARIC participants had a reduced or preserved left ventricular ejection fraction, she said.
ARIC is funded by the National Heart, Lung, and Blood Institute. Dr. Witt had no disclosures.
—Mitchel L. Zoler
Suggested Reading
Adelborg K, Horváth-Puhó E, Ording A, et al. Heart failure and risk of dementia: a Danish nationwide population-based cohort study. Eur J Heart Fail. 2016 Sep 9 [Epub ahead of print].
Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12-23.
Rusanen M, Kivipelto M, Levälahti E, et al. Heart diseases and long-term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimers Dis. 2014;42(1):183-191.
NEW ORLEANS—Elderly patients with heart failure have a significantly increased prevalence of dementia and mild cognitive impairment (MCI), compared with people of similar age without heart failure, according to an analysis of data collected from more than 6,000 US residents enrolled in a long-term observational study.
Patients diagnosed with either heart failure with reduced ejection fraction or heart failure with preserved ejection fraction had an 89% increased prevalence of dementia and a 41% increased prevalence of MCI, compared with people from the same cohort who did not develop heart failure. Investigators adjusted their analysis for several demographic and clinical variables, said Lucy S. Witt, MD, a researcher at the University of North Carolina in Chapel Hill, at the American Heart Association Scientific Sessions 2016. She speculated that the link between heart failure and dementia and MCI might result from impaired cerebral perfusion in patients with heart failure or from effects from heart failure medications.
“Our findings suggest that clinicians should have a higher suspicion for cognitive impairment in patients with heart failure, regardless of other, more classic risk factors,” she said. “This knowledge could prompt physicians to perform testing [for dementia and MCI], initiate conversations regarding the goals of care or advance care planning, and discuss appropriate living situations” for their patients with heart failure.
The analysis used data collected for the Atherosclerosis Risk in Communities (ARIC) study, which began in 1987 and enrolled a randomly selected representative cohort of approximately 16,000 women and men between ages 45 and 64 who resided in any of four US communities. Dr. Witt specifically focused on the data collected from 6,431 of the participants who returned for a fifth follow-up examination between 2011 and 2013, including 5,490 people without heart failure, whose average age was 76, and 941 participants with heart failure, whose average age was 78.
The adjusted rate of dementia prevalence at the fifth follow-up visit was 5.6% among those without heart failure and 7.0% in those with heart failure. The examinations also found MCI in an adjusted 21.5% of those without heart failure and in 26.2% of those with heart failure, said Dr. Witt. Adjustments were for factors such as age, sex, location, education, hypertension, diabetes, depression, alcohol and tobacco use, cerebrovascular disease, and marital status.
The relative risk for dementia among patients with heart failure was roughly similar, regardless of whether ARIC participants had a reduced or preserved left ventricular ejection fraction, she said.
ARIC is funded by the National Heart, Lung, and Blood Institute. Dr. Witt had no disclosures.
—Mitchel L. Zoler
Suggested Reading
Adelborg K, Horváth-Puhó E, Ording A, et al. Heart failure and risk of dementia: a Danish nationwide population-based cohort study. Eur J Heart Fail. 2016 Sep 9 [Epub ahead of print].
Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12-23.
Rusanen M, Kivipelto M, Levälahti E, et al. Heart diseases and long-term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimers Dis. 2014;42(1):183-191.
Study Identifies Predictors of Poor Outcome in Status Epilepticus
HOUSTON—Predictors of poor outcomes in patients with status epilepticus admitted to the neurointensive care unit include complex partial status epilepticus (CPSE), refractory status epilepticus, or the development of nonconvulsive status epilepticus (NCSE), according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Not a lot of data exist as to what predicts the poor outcomes and what’s known about the outcome in patients with status epilepticus,” said Advait Mahulikar, MD, a neurology resident at Wayne State University in Detroit.
Dr. Mahulikar and colleagues retrospectively reviewed data from 100 patients with status epilepticus who were admitted to the neurointensive care unit at Detroit Medical Center from November 2013 to January 2016. Variables of interest included patient demographics, initial presentation, refractoriness to treatment, presence or absence of underlying etiology, past history of epilepsy, and use of benzodiazepines on admission. NCSE was another variable of interest, either from initial presentation or developed during the course of convulsive status epilepticus. A good outcome was defined as a Glasgow Outcome Scale (GOS) score of 4 or 5
The mean age of the 100 patients was 58; 53% were male, 84% were African American, and 70% had a history of epilepsy. The median hospital length of stay was seven days and the median neurointensive care unit length of stay was three days. Good outcomes occurred in 69 patients.
Neither age nor gender predicted poor outcome, and there was no difference in outcome between structural and nonstructural causes of status epilepticus. However, prior history of epilepsy was a strong negative predictor of poor outcome. Fourteen out of 70 patients (20%) with a prior history of epilepsy had a poor outcome. “The theory is that [these patients] were already on treatment for epilepsy in the past and that affected their outcome in a positive way,” said Dr. Mahulikar.
When outcome was analyzed based on status semiology on initial presentation, poor outcome was observed in 16 of the 37 patients (43%) with CPSE, nine of 48 patients (19%) with generalized convulsive status epilepticus, all patients with myoclonic status epilepticus (n = 2), and three of nine (33%) who had NCSE. The type of status epilepticus was unknown for four patients, one of whom had an unknown outcome. NCSE at any time during the hospital course was seen in 31 patients; 14 (45%) had a poor outcome.
The mean number of ventilator days was higher in patients with NCSE than in those without NCSE (9.2 vs 1.6 days) and also higher in those with new-onset seizures than in those without (7.8 vs. 2.9 days). Analysis of methods of treatment revealed that only seven of 31 (22.5%) patients who received adequate benzodiazepine dosing had poor outcomes.
“The take-home message is to diagnose NCSE as early as possible,” said Dr. Mahulikar. Neurologists may attribute some cases incorrectly to metabolic or autoimmune causes on their initial presentation. “Treat aggressively at the beginning,” Dr. Mahulikar advised.
—Doug Brunk
Suggested Reading
Power KN, Gramstad A, Gilhus NE, Englesen BA. Prognostic factors of status epilepticus in adults. Epileptic Disord. 2016;18(3):297-304.
Sutter R, De Marchis GM, Semmlack S, et al. Anesthetics and outcome in status epilepticus: A matched two-center cohort study. CNS Drugs. 2017;31(1):655-674.
HOUSTON—Predictors of poor outcomes in patients with status epilepticus admitted to the neurointensive care unit include complex partial status epilepticus (CPSE), refractory status epilepticus, or the development of nonconvulsive status epilepticus (NCSE), according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Not a lot of data exist as to what predicts the poor outcomes and what’s known about the outcome in patients with status epilepticus,” said Advait Mahulikar, MD, a neurology resident at Wayne State University in Detroit.
Dr. Mahulikar and colleagues retrospectively reviewed data from 100 patients with status epilepticus who were admitted to the neurointensive care unit at Detroit Medical Center from November 2013 to January 2016. Variables of interest included patient demographics, initial presentation, refractoriness to treatment, presence or absence of underlying etiology, past history of epilepsy, and use of benzodiazepines on admission. NCSE was another variable of interest, either from initial presentation or developed during the course of convulsive status epilepticus. A good outcome was defined as a Glasgow Outcome Scale (GOS) score of 4 or 5
The mean age of the 100 patients was 58; 53% were male, 84% were African American, and 70% had a history of epilepsy. The median hospital length of stay was seven days and the median neurointensive care unit length of stay was three days. Good outcomes occurred in 69 patients.
Neither age nor gender predicted poor outcome, and there was no difference in outcome between structural and nonstructural causes of status epilepticus. However, prior history of epilepsy was a strong negative predictor of poor outcome. Fourteen out of 70 patients (20%) with a prior history of epilepsy had a poor outcome. “The theory is that [these patients] were already on treatment for epilepsy in the past and that affected their outcome in a positive way,” said Dr. Mahulikar.
When outcome was analyzed based on status semiology on initial presentation, poor outcome was observed in 16 of the 37 patients (43%) with CPSE, nine of 48 patients (19%) with generalized convulsive status epilepticus, all patients with myoclonic status epilepticus (n = 2), and three of nine (33%) who had NCSE. The type of status epilepticus was unknown for four patients, one of whom had an unknown outcome. NCSE at any time during the hospital course was seen in 31 patients; 14 (45%) had a poor outcome.
The mean number of ventilator days was higher in patients with NCSE than in those without NCSE (9.2 vs 1.6 days) and also higher in those with new-onset seizures than in those without (7.8 vs. 2.9 days). Analysis of methods of treatment revealed that only seven of 31 (22.5%) patients who received adequate benzodiazepine dosing had poor outcomes.
“The take-home message is to diagnose NCSE as early as possible,” said Dr. Mahulikar. Neurologists may attribute some cases incorrectly to metabolic or autoimmune causes on their initial presentation. “Treat aggressively at the beginning,” Dr. Mahulikar advised.
—Doug Brunk
Suggested Reading
Power KN, Gramstad A, Gilhus NE, Englesen BA. Prognostic factors of status epilepticus in adults. Epileptic Disord. 2016;18(3):297-304.
Sutter R, De Marchis GM, Semmlack S, et al. Anesthetics and outcome in status epilepticus: A matched two-center cohort study. CNS Drugs. 2017;31(1):655-674.
HOUSTON—Predictors of poor outcomes in patients with status epilepticus admitted to the neurointensive care unit include complex partial status epilepticus (CPSE), refractory status epilepticus, or the development of nonconvulsive status epilepticus (NCSE), according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Not a lot of data exist as to what predicts the poor outcomes and what’s known about the outcome in patients with status epilepticus,” said Advait Mahulikar, MD, a neurology resident at Wayne State University in Detroit.
Dr. Mahulikar and colleagues retrospectively reviewed data from 100 patients with status epilepticus who were admitted to the neurointensive care unit at Detroit Medical Center from November 2013 to January 2016. Variables of interest included patient demographics, initial presentation, refractoriness to treatment, presence or absence of underlying etiology, past history of epilepsy, and use of benzodiazepines on admission. NCSE was another variable of interest, either from initial presentation or developed during the course of convulsive status epilepticus. A good outcome was defined as a Glasgow Outcome Scale (GOS) score of 4 or 5
The mean age of the 100 patients was 58; 53% were male, 84% were African American, and 70% had a history of epilepsy. The median hospital length of stay was seven days and the median neurointensive care unit length of stay was three days. Good outcomes occurred in 69 patients.
Neither age nor gender predicted poor outcome, and there was no difference in outcome between structural and nonstructural causes of status epilepticus. However, prior history of epilepsy was a strong negative predictor of poor outcome. Fourteen out of 70 patients (20%) with a prior history of epilepsy had a poor outcome. “The theory is that [these patients] were already on treatment for epilepsy in the past and that affected their outcome in a positive way,” said Dr. Mahulikar.
When outcome was analyzed based on status semiology on initial presentation, poor outcome was observed in 16 of the 37 patients (43%) with CPSE, nine of 48 patients (19%) with generalized convulsive status epilepticus, all patients with myoclonic status epilepticus (n = 2), and three of nine (33%) who had NCSE. The type of status epilepticus was unknown for four patients, one of whom had an unknown outcome. NCSE at any time during the hospital course was seen in 31 patients; 14 (45%) had a poor outcome.
The mean number of ventilator days was higher in patients with NCSE than in those without NCSE (9.2 vs 1.6 days) and also higher in those with new-onset seizures than in those without (7.8 vs. 2.9 days). Analysis of methods of treatment revealed that only seven of 31 (22.5%) patients who received adequate benzodiazepine dosing had poor outcomes.
“The take-home message is to diagnose NCSE as early as possible,” said Dr. Mahulikar. Neurologists may attribute some cases incorrectly to metabolic or autoimmune causes on their initial presentation. “Treat aggressively at the beginning,” Dr. Mahulikar advised.
—Doug Brunk
Suggested Reading
Power KN, Gramstad A, Gilhus NE, Englesen BA. Prognostic factors of status epilepticus in adults. Epileptic Disord. 2016;18(3):297-304.
Sutter R, De Marchis GM, Semmlack S, et al. Anesthetics and outcome in status epilepticus: A matched two-center cohort study. CNS Drugs. 2017;31(1):655-674.
Trump administration reinstates global gag rule on abortion
President Trump has reinstated and expanded the Mexico City policy prohibiting foreign nongovernmental organizations from offering counseling or referrals for abortion services if they receive funding from the U.S. government.
Mr. Trump’s executive order on Jan. 23 was not unexpected given his conservative platform on reproductive health care; however, he has taken the policy – also known as the global gag rule – further than his predecessors.
Originally instituted under President Reagan, the Mexico City Policy required overseas organizations to certify that they would not “perform or actively promote abortion as a method of family planning” using non–U.S. funds in order to receive family planning aid from the U.S. government. The policy has been rescinded and enforced periodically since 1984, based on the ideologic slant of successive U.S. administrations.
The Trump iteration of the Mexico City Policy “extends the requirements of the reinstated memorandum to global health assistance furnished by all departments and agencies,” according to the White House.
Planned Parenthood Global condemned the expanded policy, which now affects international organizations working on any U.S.–funded global health initiative, including HIV/AIDS prevention and treatment, maternal and child health, and Zika virus programs. These groups will be stripped of all U.S. aid if they also provide abortion counseling, referrals, or services for abortion, even with their own funds.
“This is an unprecedented move, and the most extreme executive action we’ve seen of its kind,” Latanya Mapp Frett, executive director, said in a statement. “The global gag rule, as it existed under previous anti–women’s health presidents, was deeply harmful. But this action will be catastrophic for all communities, especially those relying on U.S. funding to address HIV/AIDS and maternal health care, and the fight against Zika.”
The expansion of the Mexico City Policy “certainly does presage negative things for abortion and contraception” under the new administration, said Sarah W. Prager, MD, of the department of obstetrics and gynecology at the University of Washington, Seattle. “Another example is the [Jan. 24] passage in the House of Representatives of H.R. 7, which would make permanent the Hyde Amendment and deny our most disadvantaged women access to abortion.”
The American Association of Pro-Life Obstetricians and Gynecologists took a different stance. “The Mexico City Policy was instituted to prevent the U.S. from forcing taxpayers to fund abortion overseas,” said Donna J. Harrison, MD, executive director. “This policy has been intermittently in place since 1984 and represents no departure from previous policies implemented by pro-life presidents.”
AAPLOG supports reinstating this policy because elective abortion “is not a part of essential women’s health care, and, in fact, is an elective procedure,” Dr. Harrison said.
President Trump has reinstated and expanded the Mexico City policy prohibiting foreign nongovernmental organizations from offering counseling or referrals for abortion services if they receive funding from the U.S. government.
Mr. Trump’s executive order on Jan. 23 was not unexpected given his conservative platform on reproductive health care; however, he has taken the policy – also known as the global gag rule – further than his predecessors.
Originally instituted under President Reagan, the Mexico City Policy required overseas organizations to certify that they would not “perform or actively promote abortion as a method of family planning” using non–U.S. funds in order to receive family planning aid from the U.S. government. The policy has been rescinded and enforced periodically since 1984, based on the ideologic slant of successive U.S. administrations.
The Trump iteration of the Mexico City Policy “extends the requirements of the reinstated memorandum to global health assistance furnished by all departments and agencies,” according to the White House.
Planned Parenthood Global condemned the expanded policy, which now affects international organizations working on any U.S.–funded global health initiative, including HIV/AIDS prevention and treatment, maternal and child health, and Zika virus programs. These groups will be stripped of all U.S. aid if they also provide abortion counseling, referrals, or services for abortion, even with their own funds.
“This is an unprecedented move, and the most extreme executive action we’ve seen of its kind,” Latanya Mapp Frett, executive director, said in a statement. “The global gag rule, as it existed under previous anti–women’s health presidents, was deeply harmful. But this action will be catastrophic for all communities, especially those relying on U.S. funding to address HIV/AIDS and maternal health care, and the fight against Zika.”
The expansion of the Mexico City Policy “certainly does presage negative things for abortion and contraception” under the new administration, said Sarah W. Prager, MD, of the department of obstetrics and gynecology at the University of Washington, Seattle. “Another example is the [Jan. 24] passage in the House of Representatives of H.R. 7, which would make permanent the Hyde Amendment and deny our most disadvantaged women access to abortion.”
The American Association of Pro-Life Obstetricians and Gynecologists took a different stance. “The Mexico City Policy was instituted to prevent the U.S. from forcing taxpayers to fund abortion overseas,” said Donna J. Harrison, MD, executive director. “This policy has been intermittently in place since 1984 and represents no departure from previous policies implemented by pro-life presidents.”
AAPLOG supports reinstating this policy because elective abortion “is not a part of essential women’s health care, and, in fact, is an elective procedure,” Dr. Harrison said.
President Trump has reinstated and expanded the Mexico City policy prohibiting foreign nongovernmental organizations from offering counseling or referrals for abortion services if they receive funding from the U.S. government.
Mr. Trump’s executive order on Jan. 23 was not unexpected given his conservative platform on reproductive health care; however, he has taken the policy – also known as the global gag rule – further than his predecessors.
Originally instituted under President Reagan, the Mexico City Policy required overseas organizations to certify that they would not “perform or actively promote abortion as a method of family planning” using non–U.S. funds in order to receive family planning aid from the U.S. government. The policy has been rescinded and enforced periodically since 1984, based on the ideologic slant of successive U.S. administrations.
The Trump iteration of the Mexico City Policy “extends the requirements of the reinstated memorandum to global health assistance furnished by all departments and agencies,” according to the White House.
Planned Parenthood Global condemned the expanded policy, which now affects international organizations working on any U.S.–funded global health initiative, including HIV/AIDS prevention and treatment, maternal and child health, and Zika virus programs. These groups will be stripped of all U.S. aid if they also provide abortion counseling, referrals, or services for abortion, even with their own funds.
“This is an unprecedented move, and the most extreme executive action we’ve seen of its kind,” Latanya Mapp Frett, executive director, said in a statement. “The global gag rule, as it existed under previous anti–women’s health presidents, was deeply harmful. But this action will be catastrophic for all communities, especially those relying on U.S. funding to address HIV/AIDS and maternal health care, and the fight against Zika.”
The expansion of the Mexico City Policy “certainly does presage negative things for abortion and contraception” under the new administration, said Sarah W. Prager, MD, of the department of obstetrics and gynecology at the University of Washington, Seattle. “Another example is the [Jan. 24] passage in the House of Representatives of H.R. 7, which would make permanent the Hyde Amendment and deny our most disadvantaged women access to abortion.”
The American Association of Pro-Life Obstetricians and Gynecologists took a different stance. “The Mexico City Policy was instituted to prevent the U.S. from forcing taxpayers to fund abortion overseas,” said Donna J. Harrison, MD, executive director. “This policy has been intermittently in place since 1984 and represents no departure from previous policies implemented by pro-life presidents.”
AAPLOG supports reinstating this policy because elective abortion “is not a part of essential women’s health care, and, in fact, is an elective procedure,” Dr. Harrison said.
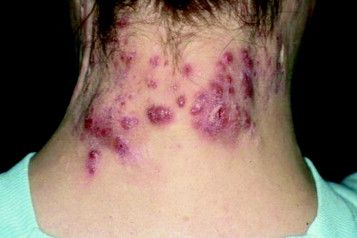
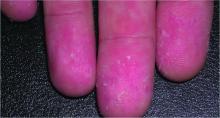
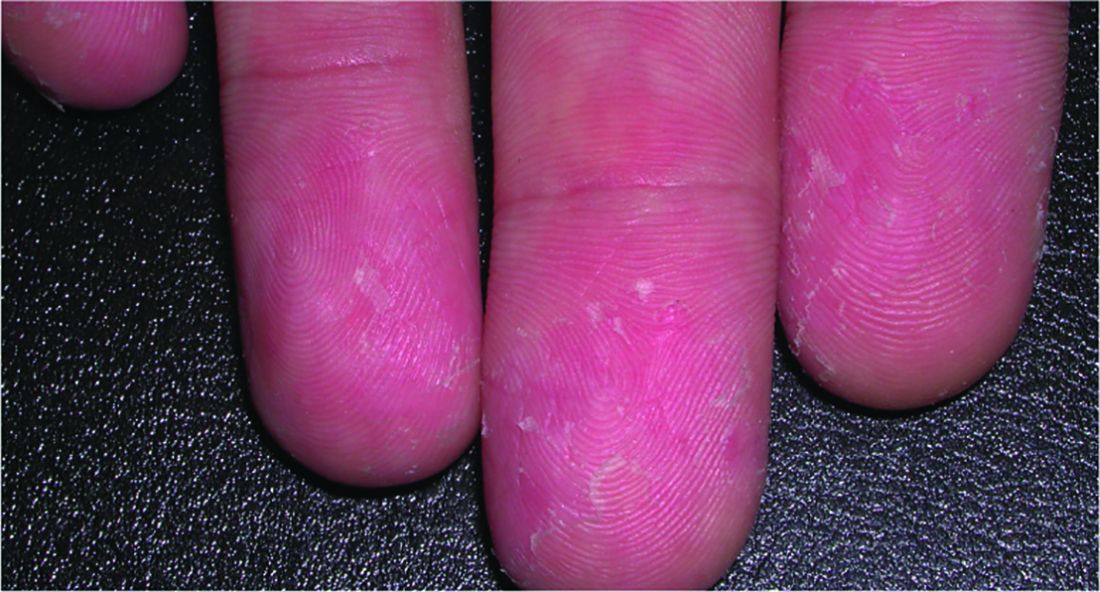
Incidence of IBD is elevated in hidradenitis suppurativa patients
Individuals with hidradenitis suppurativa (HS) may be at significantly greater risk of inflammatory bowel conditions such as Crohn’s disease and ulcerative colitis, according to a Danish populationwide cohort study.
“In HS patients presenting with gastrointestinal complaints, timely referral for gastroenterological evaluation of IBD [inflammatory bowel disease] may be appropriate,” Alexander Egeberg, MD, of the University of Copenhagen and his coauthors wrote.
Similarly, the incidence of ulcerative colitis was about 1.8-fold higher among individuals with HS, compared with controls (1.3% vs. 0.7%; OR, 1.75; P less than .002), and the incidence of “unspecified” inflammatory bowel disease was 3.4-fold higher (0.7% vs. 0.1%; OR, 3.40; P less than .007), according to a paper published online in the Journal of Investigative Dermatology.
Dr. Egeberg and his coauthors reported that HS and inflammatory bowel disease similarities suggest a shared pathogenesis, including the worsening effect of smoking on both conditions; the appearance of scarring and sinus tract formation; the apparent involvement of T-helper 17 cells, interleukin-23, and tumor necrosis factor; and the coinvolvement of genes such as SULT1B1 and SULT1E1 (J Invest Dermatol. 2017 Jan 13. doi: 10.1016/j.jid.2016.11.040).
“Finally, an increased prevalence of spondylarthropathy has been reported in patients with IBD as well as in those with HS, raising the hypothesis that genetic, epigenetic, and/or environmental factors cooperate to lead to dysregulated inflammatory pathways across these immune-mediated diseases,” the authors wrote.
While previous study evidence linking HS and IBD has been inconsistent, the authors said their findings suggested an increased incidence of IBD in individuals with HS – ranging from 0.13 to 0.97 per 1,000 HS patients per year.
They acknowledged that the study population was predominantly of Northern European descent, and the results might not be generalizable to patients of other ethnicities. They also noted that patients with HS identified by hospital diagnoses may therefore have had greater comorbidity than patients sampled from a population setting.
The study was funded by Eli Lilly, and one author is an employee of Eli Lilly. Five other authors declared research funding, grants, consultancies, honoraria, consultancy, and board positions for various pharmaceutical companies, including Eli Lilly.
Individuals with hidradenitis suppurativa (HS) may be at significantly greater risk of inflammatory bowel conditions such as Crohn’s disease and ulcerative colitis, according to a Danish populationwide cohort study.
“In HS patients presenting with gastrointestinal complaints, timely referral for gastroenterological evaluation of IBD [inflammatory bowel disease] may be appropriate,” Alexander Egeberg, MD, of the University of Copenhagen and his coauthors wrote.
Similarly, the incidence of ulcerative colitis was about 1.8-fold higher among individuals with HS, compared with controls (1.3% vs. 0.7%; OR, 1.75; P less than .002), and the incidence of “unspecified” inflammatory bowel disease was 3.4-fold higher (0.7% vs. 0.1%; OR, 3.40; P less than .007), according to a paper published online in the Journal of Investigative Dermatology.
Dr. Egeberg and his coauthors reported that HS and inflammatory bowel disease similarities suggest a shared pathogenesis, including the worsening effect of smoking on both conditions; the appearance of scarring and sinus tract formation; the apparent involvement of T-helper 17 cells, interleukin-23, and tumor necrosis factor; and the coinvolvement of genes such as SULT1B1 and SULT1E1 (J Invest Dermatol. 2017 Jan 13. doi: 10.1016/j.jid.2016.11.040).
“Finally, an increased prevalence of spondylarthropathy has been reported in patients with IBD as well as in those with HS, raising the hypothesis that genetic, epigenetic, and/or environmental factors cooperate to lead to dysregulated inflammatory pathways across these immune-mediated diseases,” the authors wrote.
While previous study evidence linking HS and IBD has been inconsistent, the authors said their findings suggested an increased incidence of IBD in individuals with HS – ranging from 0.13 to 0.97 per 1,000 HS patients per year.
They acknowledged that the study population was predominantly of Northern European descent, and the results might not be generalizable to patients of other ethnicities. They also noted that patients with HS identified by hospital diagnoses may therefore have had greater comorbidity than patients sampled from a population setting.
The study was funded by Eli Lilly, and one author is an employee of Eli Lilly. Five other authors declared research funding, grants, consultancies, honoraria, consultancy, and board positions for various pharmaceutical companies, including Eli Lilly.
Individuals with hidradenitis suppurativa (HS) may be at significantly greater risk of inflammatory bowel conditions such as Crohn’s disease and ulcerative colitis, according to a Danish populationwide cohort study.
“In HS patients presenting with gastrointestinal complaints, timely referral for gastroenterological evaluation of IBD [inflammatory bowel disease] may be appropriate,” Alexander Egeberg, MD, of the University of Copenhagen and his coauthors wrote.
Similarly, the incidence of ulcerative colitis was about 1.8-fold higher among individuals with HS, compared with controls (1.3% vs. 0.7%; OR, 1.75; P less than .002), and the incidence of “unspecified” inflammatory bowel disease was 3.4-fold higher (0.7% vs. 0.1%; OR, 3.40; P less than .007), according to a paper published online in the Journal of Investigative Dermatology.
Dr. Egeberg and his coauthors reported that HS and inflammatory bowel disease similarities suggest a shared pathogenesis, including the worsening effect of smoking on both conditions; the appearance of scarring and sinus tract formation; the apparent involvement of T-helper 17 cells, interleukin-23, and tumor necrosis factor; and the coinvolvement of genes such as SULT1B1 and SULT1E1 (J Invest Dermatol. 2017 Jan 13. doi: 10.1016/j.jid.2016.11.040).
“Finally, an increased prevalence of spondylarthropathy has been reported in patients with IBD as well as in those with HS, raising the hypothesis that genetic, epigenetic, and/or environmental factors cooperate to lead to dysregulated inflammatory pathways across these immune-mediated diseases,” the authors wrote.
While previous study evidence linking HS and IBD has been inconsistent, the authors said their findings suggested an increased incidence of IBD in individuals with HS – ranging from 0.13 to 0.97 per 1,000 HS patients per year.
They acknowledged that the study population was predominantly of Northern European descent, and the results might not be generalizable to patients of other ethnicities. They also noted that patients with HS identified by hospital diagnoses may therefore have had greater comorbidity than patients sampled from a population setting.
The study was funded by Eli Lilly, and one author is an employee of Eli Lilly. Five other authors declared research funding, grants, consultancies, honoraria, consultancy, and board positions for various pharmaceutical companies, including Eli Lilly.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Key clinical point: Individuals with hidradenitis suppurativa may be at significantly greater risk of inflammatory bowel conditions such as Crohn’s disease and ulcerative colitis.
Major finding: The baseline prevalence of Crohn’s disease is twofold higher, and ulcerative colitis is about 1.8-fold higher, in individuals with hidradenitis suppurativa, compared with the general population.
Data source: A population-based cohort study in 7,732 patients with a hospital diagnosis of hidradenitis suppurativa, and 4,354,137 controls.
Disclosures: The study was funded by Eli Lilly, and one author is an employee of Eli Lilly. Five other authors declared research funding, grants, consultancies, honoraria, consultancy, and board positions for various pharmaceutical companies, including Eli Lilly.
Evidence supports efficacy of topical skin products for photoaging reversal
MIAMI – Topical skin care products are evolving along with evidence in the literature supporting their efficacy; these products include serums, lotions, and cleansers with DNA repair enzymes or epidermal growth factor as active ingredients, according to Ron Moy, MD.
Importantly, some of these formulations not only show efficacy to reverse the signs of photodamage to skin and to promote rejuvenation, but may have a role in skin cancer prevention as well, said Dr. Moy, a dermatologist and facial plastic surgeon in private practice in Beverly Hills, Calif.
More investigators are assessing the mechanisms and potential advantages of DNA repair, Dr. Moy said at the Orlando Dermatology Aesthetic and Clinical Conference. In 2015, the Nobel Prize in Chemistry was awarded to scientists who discovered key mechanisms underlying DNA repair. “Since then, DNA repair has gotten a lot more attention,” he said.
Evidence suggests a person’s DNA repair capability can modulate colon cancer risk. Also, smokers with a low level of DNA repair enzymes carry a higher risk for lung cancer, and DNA repair genes can predict ovarian cancer and lung cancer survival, Dr. Moy said.
“It is still not entirely understood how everything ties together,” he said. “But the work on DNA repair is convincing, more convincing than work on antioxidants or retinoids.” Although antioxidants look favorable in experimental and animal studies, for example, “generally the published work on antioxidants does not give you good clinical results,” he said.
DNA repair enzymes have a beneficial effect on the proto-oncogene hyperexpression in human skin and ultraviolet light telomere shortening, according to an experimental pilot study in the Journal of Drugs in Dermatology (2013 Sep;12[9]:1017-21).
Also, a DNA repair enzyme derived from bacteria, T4 endonuclease V, showed promise in an older study of 30 patients with xeroderma pigmentosum (Lancet. 2001 Mar 24;357[9260]:926-9). Those affected carry a higher risk overall for any skin cancer, compared with the general population. The DNA repair enzyme group had fewer new actinic keratoses and fewer new basal cell carcinoma, squamous cell carcinomas, and melanoma lesions at 1 year, compared with a vehicle-only group at 1 year.
Another class of DNA repair molecules, photolyases, is newer than T4 endonuclease V and “might work even better,” Dr. Moy said.
“There is evidence that DNA enzymes are very effective and helpful in preventing skin cancer,” he pointed out. One mechanism is the ability of exogenous DNA repair enzymes to bolster intrinsic DNA in the fight against carcinogenesis, according to a review article Dr. Moy and his colleagues published in the Journal of Drugs in Dermatology (2015 Mar;14[3]:297-303).
The role of human epidermal growth factor for improving skin appearance and tightening is another area of active research and promise.
“It basically thickens and tightens skin,” Dr. Moy explained. “It works better on thinner skin, including the eyelids and neck.” Ultrasound objectively demonstrates gains in skin thickness. Studies also show improvement in acne scars following application of epidermal growth factor.
In addition, epidermal growth factor can enhance the appearance of solar purpura. In fact, this is the dermatologic condition with the most convincing evidence supporting its use so far, Dr. Moy said. Clinical studies have shown that epidermal growth factor along with other active ingredients also can improve acne scars and eye bags.
During the question and answer session at the ODAC conference, moderator Susan Weinkle, MD, a dermatologist and Mohs surgeon in private practice in Bradenton, Fla., asked Dr. Moy which epidermal growth factor product he recommends.
“There are a lot of different growth factors. Epidermal growth factors are not all the same,” said Dr. Moy, who is the founder of DNA EGF Renewal, a company that manufactures skin care products containing epidermal growth factor and DNA repair enzymes.
When pressed for the name of his growth factor product by Dr. Weinkle and an uproar from the audience, Dr. Moy chose to stay noncommercial, pointing attendees to his website instead: www.dnaegfrenewal.com.
Dr. Moy is founder of DNA EGF Renewal in Beverly Hills, Calif.
MIAMI – Topical skin care products are evolving along with evidence in the literature supporting their efficacy; these products include serums, lotions, and cleansers with DNA repair enzymes or epidermal growth factor as active ingredients, according to Ron Moy, MD.
Importantly, some of these formulations not only show efficacy to reverse the signs of photodamage to skin and to promote rejuvenation, but may have a role in skin cancer prevention as well, said Dr. Moy, a dermatologist and facial plastic surgeon in private practice in Beverly Hills, Calif.
More investigators are assessing the mechanisms and potential advantages of DNA repair, Dr. Moy said at the Orlando Dermatology Aesthetic and Clinical Conference. In 2015, the Nobel Prize in Chemistry was awarded to scientists who discovered key mechanisms underlying DNA repair. “Since then, DNA repair has gotten a lot more attention,” he said.
Evidence suggests a person’s DNA repair capability can modulate colon cancer risk. Also, smokers with a low level of DNA repair enzymes carry a higher risk for lung cancer, and DNA repair genes can predict ovarian cancer and lung cancer survival, Dr. Moy said.
“It is still not entirely understood how everything ties together,” he said. “But the work on DNA repair is convincing, more convincing than work on antioxidants or retinoids.” Although antioxidants look favorable in experimental and animal studies, for example, “generally the published work on antioxidants does not give you good clinical results,” he said.
DNA repair enzymes have a beneficial effect on the proto-oncogene hyperexpression in human skin and ultraviolet light telomere shortening, according to an experimental pilot study in the Journal of Drugs in Dermatology (2013 Sep;12[9]:1017-21).
Also, a DNA repair enzyme derived from bacteria, T4 endonuclease V, showed promise in an older study of 30 patients with xeroderma pigmentosum (Lancet. 2001 Mar 24;357[9260]:926-9). Those affected carry a higher risk overall for any skin cancer, compared with the general population. The DNA repair enzyme group had fewer new actinic keratoses and fewer new basal cell carcinoma, squamous cell carcinomas, and melanoma lesions at 1 year, compared with a vehicle-only group at 1 year.
Another class of DNA repair molecules, photolyases, is newer than T4 endonuclease V and “might work even better,” Dr. Moy said.
“There is evidence that DNA enzymes are very effective and helpful in preventing skin cancer,” he pointed out. One mechanism is the ability of exogenous DNA repair enzymes to bolster intrinsic DNA in the fight against carcinogenesis, according to a review article Dr. Moy and his colleagues published in the Journal of Drugs in Dermatology (2015 Mar;14[3]:297-303).
The role of human epidermal growth factor for improving skin appearance and tightening is another area of active research and promise.
“It basically thickens and tightens skin,” Dr. Moy explained. “It works better on thinner skin, including the eyelids and neck.” Ultrasound objectively demonstrates gains in skin thickness. Studies also show improvement in acne scars following application of epidermal growth factor.
In addition, epidermal growth factor can enhance the appearance of solar purpura. In fact, this is the dermatologic condition with the most convincing evidence supporting its use so far, Dr. Moy said. Clinical studies have shown that epidermal growth factor along with other active ingredients also can improve acne scars and eye bags.
During the question and answer session at the ODAC conference, moderator Susan Weinkle, MD, a dermatologist and Mohs surgeon in private practice in Bradenton, Fla., asked Dr. Moy which epidermal growth factor product he recommends.
“There are a lot of different growth factors. Epidermal growth factors are not all the same,” said Dr. Moy, who is the founder of DNA EGF Renewal, a company that manufactures skin care products containing epidermal growth factor and DNA repair enzymes.
When pressed for the name of his growth factor product by Dr. Weinkle and an uproar from the audience, Dr. Moy chose to stay noncommercial, pointing attendees to his website instead: www.dnaegfrenewal.com.
Dr. Moy is founder of DNA EGF Renewal in Beverly Hills, Calif.
MIAMI – Topical skin care products are evolving along with evidence in the literature supporting their efficacy; these products include serums, lotions, and cleansers with DNA repair enzymes or epidermal growth factor as active ingredients, according to Ron Moy, MD.
Importantly, some of these formulations not only show efficacy to reverse the signs of photodamage to skin and to promote rejuvenation, but may have a role in skin cancer prevention as well, said Dr. Moy, a dermatologist and facial plastic surgeon in private practice in Beverly Hills, Calif.
More investigators are assessing the mechanisms and potential advantages of DNA repair, Dr. Moy said at the Orlando Dermatology Aesthetic and Clinical Conference. In 2015, the Nobel Prize in Chemistry was awarded to scientists who discovered key mechanisms underlying DNA repair. “Since then, DNA repair has gotten a lot more attention,” he said.
Evidence suggests a person’s DNA repair capability can modulate colon cancer risk. Also, smokers with a low level of DNA repair enzymes carry a higher risk for lung cancer, and DNA repair genes can predict ovarian cancer and lung cancer survival, Dr. Moy said.
“It is still not entirely understood how everything ties together,” he said. “But the work on DNA repair is convincing, more convincing than work on antioxidants or retinoids.” Although antioxidants look favorable in experimental and animal studies, for example, “generally the published work on antioxidants does not give you good clinical results,” he said.
DNA repair enzymes have a beneficial effect on the proto-oncogene hyperexpression in human skin and ultraviolet light telomere shortening, according to an experimental pilot study in the Journal of Drugs in Dermatology (2013 Sep;12[9]:1017-21).
Also, a DNA repair enzyme derived from bacteria, T4 endonuclease V, showed promise in an older study of 30 patients with xeroderma pigmentosum (Lancet. 2001 Mar 24;357[9260]:926-9). Those affected carry a higher risk overall for any skin cancer, compared with the general population. The DNA repair enzyme group had fewer new actinic keratoses and fewer new basal cell carcinoma, squamous cell carcinomas, and melanoma lesions at 1 year, compared with a vehicle-only group at 1 year.
Another class of DNA repair molecules, photolyases, is newer than T4 endonuclease V and “might work even better,” Dr. Moy said.
“There is evidence that DNA enzymes are very effective and helpful in preventing skin cancer,” he pointed out. One mechanism is the ability of exogenous DNA repair enzymes to bolster intrinsic DNA in the fight against carcinogenesis, according to a review article Dr. Moy and his colleagues published in the Journal of Drugs in Dermatology (2015 Mar;14[3]:297-303).
The role of human epidermal growth factor for improving skin appearance and tightening is another area of active research and promise.
“It basically thickens and tightens skin,” Dr. Moy explained. “It works better on thinner skin, including the eyelids and neck.” Ultrasound objectively demonstrates gains in skin thickness. Studies also show improvement in acne scars following application of epidermal growth factor.
In addition, epidermal growth factor can enhance the appearance of solar purpura. In fact, this is the dermatologic condition with the most convincing evidence supporting its use so far, Dr. Moy said. Clinical studies have shown that epidermal growth factor along with other active ingredients also can improve acne scars and eye bags.
During the question and answer session at the ODAC conference, moderator Susan Weinkle, MD, a dermatologist and Mohs surgeon in private practice in Bradenton, Fla., asked Dr. Moy which epidermal growth factor product he recommends.
“There are a lot of different growth factors. Epidermal growth factors are not all the same,” said Dr. Moy, who is the founder of DNA EGF Renewal, a company that manufactures skin care products containing epidermal growth factor and DNA repair enzymes.
When pressed for the name of his growth factor product by Dr. Weinkle and an uproar from the audience, Dr. Moy chose to stay noncommercial, pointing attendees to his website instead: www.dnaegfrenewal.com.
Dr. Moy is founder of DNA EGF Renewal in Beverly Hills, Calif.
EXPERT ANALYSIS FROM ODAC 2017
Is SUDEP Preventable in Children?
HOUSTON—Sudden unexpected death in epilepsy (SUDEP) in children is rare, but can it be avoided? The majority of pediatric SUDEP cases may occur in children with global developmental delay, early-onset epilepsy, or with seizures requiring polytherapy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Every death of a person with epilepsy is devastating, the death of a child even more so. If we can understand who is at higher risk of SUDEP, then our clinical and basic science researchers can work to find preventative techniques, and hopefully we can apply those techniques to patients who are most at risk,” said Elizabeth Donner, MD, Director of the Comprehensive Epilepsy Program at the Hospital for Sick Children and Associate Professor of Pediatrics at the University of Toronto.
The incidence of SUDEP in children is estimated to be 0.43 per 1,000 patient years of epilepsy, more than 10 times the rate of sudden death in children overall. Since the numbers are relatively low, it has been debatable whether doctors should discuss SUDEP with their patients. Recent studies, however, suggest that SUDEP may be more common and potentially avoidable. To determine potential risk factors for pediatric SUDEP, Dr. Donner and her colleagues developed a national, multicenter prospective population registry for SUDEP.
Researchers collected data from the Canadian Pediatric Epilepsy Network, the Canadian Pediatric Surveillance Program, and the Ontario Forensic Pathology Service. They reviewed demographics, clinical features, circumstances surrounding death, and autopsy findings.
Researchers sought to include children with epilepsy with an unexpected death between January 1, 2014, and December 31, 2015. Inclusion criteria were age 18 or younger at death; a history of two or more seizures; and death that was sudden, unexpected, and occurred during normal circumstances. Autopsies, when available, determined that there was no anatomical or toxicological cause of death. Investigators excluded deaths due to trauma and drowning and status epilepticus.
The majority of deaths occurred in children between the ages of 5 and 10, and 52% were boys. In addition, all children had seizure onset before age 5, and median age of seizure onset was about 6 months. Seven of the children had genetic abnormalities. One child was seizure-free for 12 months and was not being treated with antiepileptic medications.
The investigators identified 21 cases of definite, probable, or possible pediatric SUDEP: 10 cases of definite SUDEP, two cases of definite SUDEP plus, six cases of probable SUDEP, and three cases of possible SUDEP (ie, an autopsy was not performed). Additionally, 10 of 12 children were having tonic clonic seizures six months prior to death. Researchers also found that in 10 of 17 cases, the parents reported that their child had a recent infection. Nearly all of the deaths occurred during sleep and were unwitnessed.
“It may be worth looking at whether infection in children with epilepsy changes their risk of sudden death,” said Dr. Donner. “We really have a lot more work to do with this limited number of cases, and we are still identifying more cases and working to better understand the data.”
—Erica Tricarico
Suggested Reading
Donner EJ, Waddell B, Osland K, et al. After sudden unexpected death in epilepsy: Lessons learned and the road forward. Epilepsia. 2016;57(Suppl 1):46-53.
HOUSTON—Sudden unexpected death in epilepsy (SUDEP) in children is rare, but can it be avoided? The majority of pediatric SUDEP cases may occur in children with global developmental delay, early-onset epilepsy, or with seizures requiring polytherapy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Every death of a person with epilepsy is devastating, the death of a child even more so. If we can understand who is at higher risk of SUDEP, then our clinical and basic science researchers can work to find preventative techniques, and hopefully we can apply those techniques to patients who are most at risk,” said Elizabeth Donner, MD, Director of the Comprehensive Epilepsy Program at the Hospital for Sick Children and Associate Professor of Pediatrics at the University of Toronto.
The incidence of SUDEP in children is estimated to be 0.43 per 1,000 patient years of epilepsy, more than 10 times the rate of sudden death in children overall. Since the numbers are relatively low, it has been debatable whether doctors should discuss SUDEP with their patients. Recent studies, however, suggest that SUDEP may be more common and potentially avoidable. To determine potential risk factors for pediatric SUDEP, Dr. Donner and her colleagues developed a national, multicenter prospective population registry for SUDEP.
Researchers collected data from the Canadian Pediatric Epilepsy Network, the Canadian Pediatric Surveillance Program, and the Ontario Forensic Pathology Service. They reviewed demographics, clinical features, circumstances surrounding death, and autopsy findings.
Researchers sought to include children with epilepsy with an unexpected death between January 1, 2014, and December 31, 2015. Inclusion criteria were age 18 or younger at death; a history of two or more seizures; and death that was sudden, unexpected, and occurred during normal circumstances. Autopsies, when available, determined that there was no anatomical or toxicological cause of death. Investigators excluded deaths due to trauma and drowning and status epilepticus.
The majority of deaths occurred in children between the ages of 5 and 10, and 52% were boys. In addition, all children had seizure onset before age 5, and median age of seizure onset was about 6 months. Seven of the children had genetic abnormalities. One child was seizure-free for 12 months and was not being treated with antiepileptic medications.
The investigators identified 21 cases of definite, probable, or possible pediatric SUDEP: 10 cases of definite SUDEP, two cases of definite SUDEP plus, six cases of probable SUDEP, and three cases of possible SUDEP (ie, an autopsy was not performed). Additionally, 10 of 12 children were having tonic clonic seizures six months prior to death. Researchers also found that in 10 of 17 cases, the parents reported that their child had a recent infection. Nearly all of the deaths occurred during sleep and were unwitnessed.
“It may be worth looking at whether infection in children with epilepsy changes their risk of sudden death,” said Dr. Donner. “We really have a lot more work to do with this limited number of cases, and we are still identifying more cases and working to better understand the data.”
—Erica Tricarico
Suggested Reading
Donner EJ, Waddell B, Osland K, et al. After sudden unexpected death in epilepsy: Lessons learned and the road forward. Epilepsia. 2016;57(Suppl 1):46-53.
HOUSTON—Sudden unexpected death in epilepsy (SUDEP) in children is rare, but can it be avoided? The majority of pediatric SUDEP cases may occur in children with global developmental delay, early-onset epilepsy, or with seizures requiring polytherapy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Every death of a person with epilepsy is devastating, the death of a child even more so. If we can understand who is at higher risk of SUDEP, then our clinical and basic science researchers can work to find preventative techniques, and hopefully we can apply those techniques to patients who are most at risk,” said Elizabeth Donner, MD, Director of the Comprehensive Epilepsy Program at the Hospital for Sick Children and Associate Professor of Pediatrics at the University of Toronto.
The incidence of SUDEP in children is estimated to be 0.43 per 1,000 patient years of epilepsy, more than 10 times the rate of sudden death in children overall. Since the numbers are relatively low, it has been debatable whether doctors should discuss SUDEP with their patients. Recent studies, however, suggest that SUDEP may be more common and potentially avoidable. To determine potential risk factors for pediatric SUDEP, Dr. Donner and her colleagues developed a national, multicenter prospective population registry for SUDEP.
Researchers collected data from the Canadian Pediatric Epilepsy Network, the Canadian Pediatric Surveillance Program, and the Ontario Forensic Pathology Service. They reviewed demographics, clinical features, circumstances surrounding death, and autopsy findings.
Researchers sought to include children with epilepsy with an unexpected death between January 1, 2014, and December 31, 2015. Inclusion criteria were age 18 or younger at death; a history of two or more seizures; and death that was sudden, unexpected, and occurred during normal circumstances. Autopsies, when available, determined that there was no anatomical or toxicological cause of death. Investigators excluded deaths due to trauma and drowning and status epilepticus.
The majority of deaths occurred in children between the ages of 5 and 10, and 52% were boys. In addition, all children had seizure onset before age 5, and median age of seizure onset was about 6 months. Seven of the children had genetic abnormalities. One child was seizure-free for 12 months and was not being treated with antiepileptic medications.
The investigators identified 21 cases of definite, probable, or possible pediatric SUDEP: 10 cases of definite SUDEP, two cases of definite SUDEP plus, six cases of probable SUDEP, and three cases of possible SUDEP (ie, an autopsy was not performed). Additionally, 10 of 12 children were having tonic clonic seizures six months prior to death. Researchers also found that in 10 of 17 cases, the parents reported that their child had a recent infection. Nearly all of the deaths occurred during sleep and were unwitnessed.
“It may be worth looking at whether infection in children with epilepsy changes their risk of sudden death,” said Dr. Donner. “We really have a lot more work to do with this limited number of cases, and we are still identifying more cases and working to better understand the data.”
—Erica Tricarico
Suggested Reading
Donner EJ, Waddell B, Osland K, et al. After sudden unexpected death in epilepsy: Lessons learned and the road forward. Epilepsia. 2016;57(Suppl 1):46-53.
Watch for cutaneous manifestations of tropical infectious diseases
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: A retrospective cohort analysis
Empiric antimicrobial therapy often consists of the combination of gram-positive coverage with vancomycin (VAN) and gram-negative coverage, specifically an antipseudomonal beta-lactam such as piperacillin-tazobactam (PTZ). Literature from a variety of patient populations reports nephrotoxicity associated with VAN, targeting troughs greater than 15 µg/mL, that occur in 5% to 43% of patients.1 In a study of critically ill patients, acute kidney injury (AKI) was found in 21% of patients receiving VAN, with increasing duration of VAN treatment, greater VAN levels, concomitant vasoactive medication administration, and intermittent infusion methods being associated with higher odds of AKI.2 A recent report from adult internal medicine patients estimated the incidence of VAN-associated nephrotoxicity at 13.6% and implicated concomitant PTZ therapy as a key factor in these patients.3
Further studies have explored the interaction between empiric beta-lactam and VAN therapy, showing mixed results. Reports of AKI associated with the combination of VAN and PTZ range from 16.3% to 34.8%,4-8 while the cefepime-VAN combination is reported to range from 12.5% to 13.3%.5,6 While VAN monotherapy groups were well represented, only 1 study7 compared the PTZ-VAN combination to a control group of PTZ monotherapy.
The primary objective of this study was to evaluate the differences in AKI incidence between patients treated with VAN and with PTZ, alone and in combination.
METHODS
This is a retrospective cohort study of adult patients conducted at the University of Kentucky Chandler Medical Center (UKMC) from September 1, 2010 through August 31, 2014. Patients were included if they were at least 18 years of age on admission; remained hospitalized for at least 48 hours; received VAN combined with PTZ (VAN/PTZ), VAN alone, or PTZ alone; and had at least 48 hours of therapy (and 48 hours of overlapping therapy in the VAN/PTZ group). Patients were excluded if they had underlying diagnosis of chronic kidney disease according to the International Classification of Diseases 9 (ICD-9) code, were receiving renal replacement therapy before admission, had a diagnosis of cystic fibrosis, or were pregnant. Additionally, patients were excluded if they presented with AKI, defined as an initial creatinine clearance less than 30 mL/min, or if baseline creatinine clearance was greater than 4 times the standard deviation from the mean; serum creatinine values were not obtained during admission; and if AKI occurred prior to therapy initiation, within 48 hours of initiation, or more than 7 days after treatment was discontinued. Patients were followed throughout their stay until time of discharge.
Data Source
Patient data were collected from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). The EDT contains clinical data from the inpatient population of UKMC from 2006 to present. Data stored and updated nightly by the EDT includes: demographics, financial classification (Medicare, Medicaid, private insurance), provider-level detail (service line), medical diagnosis (ICD-9 codes), medical procedures (Current Procedural Terminology [CPT] codes), lab tests and results, medication administration details, visit details (age, length of stay, etc), and vital signs. This study was approved by the UKMC Institutional Review Board.
Data collected for each patient included: demographic data, visit details (length of stay, admitting and primary diagnosis codes, etc.), severity of underlying illness as defined by the Charlson Comorbidity Index (CCI), all serum creatinine levels drawn per visit, medication administration information (dose, date, and time administered), all VAN trough levels, receipt of other nephrotoxic agents, blood pressures, and receipt of vasopressors.
Outcome Ascertainment
The definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss, End-stage) criteria,9 with risk defined as a 25% to 50% decrease in estimated glomerular filtration rate (GFR), injury as a 50% to 75% decrease in estimated GFR, and failure defined as a greater than 75% decrease in estimated GFR. Loss and end-stage classifications were not assessed because of this study’s follow-up period. The adjusted Cockcroft and Gault equation10 was used to estimate GFR due to the inconsistency of weight availability in the dataset and concordance with the institution’s practice. Baseline creatinine clearance was calculated with the first serum creatinine obtained, and the minimum creatinine clearance was calculated using the maximum serum creatinine during each patient’s visit. The percent decrease in creatinine clearance was calculated from these 2 values. AKI status was defined as meeting any of the RIFLE criteria. Mortality was assessed for all patients and defined as the composite of inhospital mortality and discharge or transfer to hospice care.
Exposure Ascertainment
Hypotension exposure was defined as experiencing 1 of the following: mean arterial blood pressure less than 60 mm Hg, a diagnosis of hypotension by a physician, or receipt of vasopressors or inotropic agents. Days of therapy for each drug were obtained and combination days of therapy were calculated by including only those days in which the patient received both medications. Total days of therapy were calculated by the sum of all days receiving at least 1 study agent. Exposure to other nephrotoxic agents (eg, acyclovir, angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor antagonists, aminoglycosides, amphotericin B, cyclosporine, foscarnet, loop diuretics, nonsteroidal anti-inflammatory drugs, sulfonamides, tacrolimus, and tenofovir) were defined as receipt of at least 1 dose of the agent during hospitalization.
Statistical Analysis
Characteristics between groups were described with basic descriptive statistics. Continuous variables were compared with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test. Categorical variables were compared with chi-square or Fisher exact test. Yearly AKI trends were assessed with Pearson correlation coefficient. To control for differences in underlying severity of illness between groups, a subanalysis was performed in which the cohort was split into 4 groups (0, 1, 2 to 4, and ≥5 points) based on CCI. Univariate models for all covariates were created with probability of AKI as the outcome. Covariates significant after univariate were incorporated into the multivariate model, which was subsequently adjusted to achieve the highest predictive accuracy by minimizing the Akaike information criterion (AIC). Nephrotoxic agent exposures were included in the final multivariate model regardless of statistical significance in univariate analysis. Model fit was assessed with a standardized Hosmer-Lemeshow goodness-of-fit test.11 All statistical analyses were completed with RStudio v 0.98 running R v 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).12 All tests were 2-tailed and significance was defined at an alpha of 0.05.
RESULTS
Of 17,879 patients initially screened, 11,650 patients were evaluated, of which 5,497 received VAN and PTZ (VAN/PTZ), 3,055 received VAN alone, and 3,098 received PTZ alone. Table 1 contains basic demographic information. The mean age of patients was 52.5 years ± 16.8 years with 6,242 (53.6%) males. Patients receiving VAN/PTZ had higher CCIs than either monotherapy group and had significantly increased length of hospitalization. While patients in the combination therapy group were more likely to experience hypotension, concomitant nephrotoxic agent exposure was more common in the VAN monotherapy group.
RIFLE-defined AKI occurred in 1,647 (14.1%) across the entire cohort. AKI occurred in 21% of VAN/PTZ patients, 8.3% of VAN patients, and 7.8% of PTZ patients (P < 0.0001). RIFLE-defined risk, injury, and failure occurred more frequently in the VAN/PTZ cohort compared to the VAN and PTZ monotherapy groups (Figure). There were no differences in AKI rates between years studied (r2 = 0.4732, P = 0.2). Patients in the VAN/PTZ group experienced AKI on average of 8.0 days after treatment initiation, compared to 8.7 days and 5.2 days for VAN and PTZ monotherapy groups, respectively. The composite of inhospital mortality and transfer-to-hospice care was more common in VAN/PTZ patients (9.6%) compared to monotherapy groups (VAN, 3.9%; PTZ, 3.4%), most likely due to the increased severity of illness.
In the subgroup analysis of patients with similar CCI, AKI incidence increased with severity of illness. When CCI was 0, 7.5% of patients experienced AKI compared to 11.2%, 16.4%, and 18.9% of patients when CCI was 1, 2 to 4, and ≥5, respectively (P < 0.0001). VAN/PTZ (range = 12.1% to 26.5%) was associated with greater AKI incidence than either VAN (range = 4.8% to 11.5%) or PTZ (range = 3.8% to 10.4%) alone in each subgroup (P < 0.0001 for all subgroups).
Factors associated with AKI in univariate analyses included treatment with VAN/PTZ, days of therapy, baseline creatinine clearance, transfer from outside hospitals, CCI, admission type, length of hospitalization, dehydration exposure, and hypotension exposure. Exposure to aminoglycosides, amphotericin B, ACE inhibitors, nonsteroidal anti-inflammatory drugs, tacrolimus, foscarnet, loop diuretics, sulfonamides, and tenofovir were all associated with increased odds of AKI in simple univariate logistic regression. Gender, age, year of treatment, angiotensin II receptor antagonist exposure, and cyclosporine exposure were not significantly associated with AKI incidence.
After multivariate logistic regression, monotherapy with VAN or PTZ was associated with decreased odds of AKI compared to VAN/PTZ therapy (aORVAN,0.48; 95% CIVAN,0.41-0.57; aORPTZ, 0.43; 95% CIPTZ, 0.37-0.50). No difference in AKI incidence was observed between VAN and PTZ groups (aORPTZ:VAN, 0.88; 95% CI, 0.73-1.08). Table 2 describes the relationship between AKI and other covariates included in the model. Increased odds of AKI were seen with concomitant administration of ACE inhibitors, amphotericin B, tacrolimus, loop diuretics, and tenofovir. Radio-contrast dye administration was associated with lower odds of AKI. Patients admitted urgently and emergently were at higher risk of AKI, while those admitted via the trauma center were less likely to experience AKI compared to patients who were electively admitted. Increased length of stay and duration of therapy were both associated with increased likelihood of AKI, independent of treatment group; however, durations of therapy beyond 12 days was not associated with increased AKI. Hypotension, as defined, and diagnosed dehydration both independently increased AKI odds. Aside from those older than 80 years of age, increasing age was not associated with increased AKI risk. Male gender was associated with a slight decrease in AKI rate. No evidence of overfitting was observed with the standardized Hosmer-Lemeshow P-value of 0.683, and the model provides good predictive accuracy with a C-statistic of 0.788.
CONCLUSIONS
Acute kidney injury secondary to VAN therapy is a well-characterized adverse effect, while AKI incidence secondary to PTZ is less understood. Additionally, there appears to be an additive effect when these agents are used in combination. This is the largest review of AKI in patients receiving VAN,PTZ, or the combination of both agents.
There is increasing evidence suggesting greater nephrotoxicity in patients treated with the combination of VAN and antipseudomonal beta-lactams. The mechanism for the apparent increase in nephrotoxicity with this drug combination is not well understood and needs further study in both animal models and humans.
Acute kidney injury rates related to VAN vary widely, with recent studies in critically ill and internal medicine patients estimated at 21% and 13.6%, respectively.2,3 In our VAN monotherapy cohort, the AKI rate was 8.3%, with 2.3% of patients experiencing a greater than 50% decrease in creatinine clearance. Piperacillin-tazobactam-related AKI rates are not well characterized; however, a small retrospective analysis estimated that 11.1% of PTZ patients experienced acute renal failure (defined as either increase in serum creatinine greater than 0.5 mg/dL or 50% increase from baseline).13 In the present study, we found the PTZ-related AKI rate to be 7.8%, which may be due to a more stringent definition of AKI. Additionally, Hellwig et al13 found that PTZ monotherapy was associated with higher AKI rates compared to VAN monotherapy (11.1% vs 4.9%; P = 0.014). This was not replicated in our study, with VAN and PTZ monotherapy having similar AKI rates (8.3% and 7.8%, respectively) and an adjusted aOR of 0.88 (95% CI 0.0.73-1.08) for AKI in PTZ- compared to VAN-treated patients. The estimated AKI incidence of 21% in the combination therapy group at our institution is consistent with literature that ranges from 16.3% to 34.8%.4-8,13
To control for differences in baseline severity of illness, we performed a subgroup analysis of patients with similar CCI scores. The finding of increased AKI in patients receiving combination VAN and PTZ was consistent in each subgroup, suggesting that the increase in AKI is independent of illness severity.
This study is not without limitations. As with all retrospective studies, it is difficult to determine a causal link between VAN and PTZ combination therapy and increased AKI incidence due to confounding. We employed a rigorous study design that controlled for major confounders of AKI, such as concomitant nephrotoxic exposure, hypotension, and renal disease. Severity of illness was measured with CCI, which may not accurately capture the severity of illness at treatment initiation. Alternatives, such as acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) scores, may more accurately reflect critical illness on presentation; however, this study was not focused specifically on critically ill patients. In addition to baseline comorbidity, we controlled for hypotension and dehydration as a surrogate marker for critical illness. In the subgroup analysis of patients with similar CCI, the effect of VAN/PTZ on AKI compared to VAN or PTZ monotherapy was consistent in each group. Nephrotoxic potential of agents was assumed to be equal, which is not necessarily true. Additionally, the binary representation of nephrotoxic exposure does not describe the amount of the agent received; as such, our estimations of AKI odds may be artificially elevated. Approximately one-quarter of the patients in this study were transferred from an outside hospital, for which no data regarding initial treatment are available. This may lead to exposure misclassification. We attempted to control for this factor in the regression model and found that, after controlling for other covariates, hospital transfer was associated with increasing odds of AKI. Finally, data were collected retrospectively from the electronic medical record and are subject to inaccuracies documented in the chart; however, any bias introduced should be nondifferential.
In our large retrospective study of combination empiric therapy with VAN and PTZ, we found that combination therapy was associated with more than double the odds of AKI occurring compared to either monotherapy with VAN or PTZ. Increasing duration of therapy was also associated with increases in AKI. These findings demonstrate the need for judicious use of combination therapy and strengthen the need for antimicrobial de-escalation when appropriate to avoid deleterious effects.
Acknowledgments
The authors thank Chantal Le Rutter, MPA, for copyediting services.
Disclosures
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
1. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734-744. PubMed
2. Hanrahan TP, Harlow G, Hutchinson J, et al. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med. 2014;42:2527-2536. PubMed
3. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653-661. PubMed
4. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34:670-676. PubMed
5. Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014;20:O384-O389. PubMed
6. Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34:662-669. PubMed
7. Kim T, Kandiah S, Patel M, et al. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8:579. PubMed
8. Davies SW, Efird JT, Guidry CA, et al. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt). 2016;17:38-47. PubMed
9. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. PubMed
10. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011;31:658-664. PubMed
11. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67-80. PubMed
12. R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
13. Hellwig T, Hammerquist R, Loecker B, Shields J. Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Abstracts of the Society of Critical Care Medicine 41st Critical Care Congress. February 4-8, 2012. Houston, Texas. Crit Care Med. 2011;39:1-264.
Empiric antimicrobial therapy often consists of the combination of gram-positive coverage with vancomycin (VAN) and gram-negative coverage, specifically an antipseudomonal beta-lactam such as piperacillin-tazobactam (PTZ). Literature from a variety of patient populations reports nephrotoxicity associated with VAN, targeting troughs greater than 15 µg/mL, that occur in 5% to 43% of patients.1 In a study of critically ill patients, acute kidney injury (AKI) was found in 21% of patients receiving VAN, with increasing duration of VAN treatment, greater VAN levels, concomitant vasoactive medication administration, and intermittent infusion methods being associated with higher odds of AKI.2 A recent report from adult internal medicine patients estimated the incidence of VAN-associated nephrotoxicity at 13.6% and implicated concomitant PTZ therapy as a key factor in these patients.3
Further studies have explored the interaction between empiric beta-lactam and VAN therapy, showing mixed results. Reports of AKI associated with the combination of VAN and PTZ range from 16.3% to 34.8%,4-8 while the cefepime-VAN combination is reported to range from 12.5% to 13.3%.5,6 While VAN monotherapy groups were well represented, only 1 study7 compared the PTZ-VAN combination to a control group of PTZ monotherapy.
The primary objective of this study was to evaluate the differences in AKI incidence between patients treated with VAN and with PTZ, alone and in combination.
METHODS
This is a retrospective cohort study of adult patients conducted at the University of Kentucky Chandler Medical Center (UKMC) from September 1, 2010 through August 31, 2014. Patients were included if they were at least 18 years of age on admission; remained hospitalized for at least 48 hours; received VAN combined with PTZ (VAN/PTZ), VAN alone, or PTZ alone; and had at least 48 hours of therapy (and 48 hours of overlapping therapy in the VAN/PTZ group). Patients were excluded if they had underlying diagnosis of chronic kidney disease according to the International Classification of Diseases 9 (ICD-9) code, were receiving renal replacement therapy before admission, had a diagnosis of cystic fibrosis, or were pregnant. Additionally, patients were excluded if they presented with AKI, defined as an initial creatinine clearance less than 30 mL/min, or if baseline creatinine clearance was greater than 4 times the standard deviation from the mean; serum creatinine values were not obtained during admission; and if AKI occurred prior to therapy initiation, within 48 hours of initiation, or more than 7 days after treatment was discontinued. Patients were followed throughout their stay until time of discharge.
Data Source
Patient data were collected from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). The EDT contains clinical data from the inpatient population of UKMC from 2006 to present. Data stored and updated nightly by the EDT includes: demographics, financial classification (Medicare, Medicaid, private insurance), provider-level detail (service line), medical diagnosis (ICD-9 codes), medical procedures (Current Procedural Terminology [CPT] codes), lab tests and results, medication administration details, visit details (age, length of stay, etc), and vital signs. This study was approved by the UKMC Institutional Review Board.
Data collected for each patient included: demographic data, visit details (length of stay, admitting and primary diagnosis codes, etc.), severity of underlying illness as defined by the Charlson Comorbidity Index (CCI), all serum creatinine levels drawn per visit, medication administration information (dose, date, and time administered), all VAN trough levels, receipt of other nephrotoxic agents, blood pressures, and receipt of vasopressors.
Outcome Ascertainment
The definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss, End-stage) criteria,9 with risk defined as a 25% to 50% decrease in estimated glomerular filtration rate (GFR), injury as a 50% to 75% decrease in estimated GFR, and failure defined as a greater than 75% decrease in estimated GFR. Loss and end-stage classifications were not assessed because of this study’s follow-up period. The adjusted Cockcroft and Gault equation10 was used to estimate GFR due to the inconsistency of weight availability in the dataset and concordance with the institution’s practice. Baseline creatinine clearance was calculated with the first serum creatinine obtained, and the minimum creatinine clearance was calculated using the maximum serum creatinine during each patient’s visit. The percent decrease in creatinine clearance was calculated from these 2 values. AKI status was defined as meeting any of the RIFLE criteria. Mortality was assessed for all patients and defined as the composite of inhospital mortality and discharge or transfer to hospice care.
Exposure Ascertainment
Hypotension exposure was defined as experiencing 1 of the following: mean arterial blood pressure less than 60 mm Hg, a diagnosis of hypotension by a physician, or receipt of vasopressors or inotropic agents. Days of therapy for each drug were obtained and combination days of therapy were calculated by including only those days in which the patient received both medications. Total days of therapy were calculated by the sum of all days receiving at least 1 study agent. Exposure to other nephrotoxic agents (eg, acyclovir, angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor antagonists, aminoglycosides, amphotericin B, cyclosporine, foscarnet, loop diuretics, nonsteroidal anti-inflammatory drugs, sulfonamides, tacrolimus, and tenofovir) were defined as receipt of at least 1 dose of the agent during hospitalization.
Statistical Analysis
Characteristics between groups were described with basic descriptive statistics. Continuous variables were compared with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test. Categorical variables were compared with chi-square or Fisher exact test. Yearly AKI trends were assessed with Pearson correlation coefficient. To control for differences in underlying severity of illness between groups, a subanalysis was performed in which the cohort was split into 4 groups (0, 1, 2 to 4, and ≥5 points) based on CCI. Univariate models for all covariates were created with probability of AKI as the outcome. Covariates significant after univariate were incorporated into the multivariate model, which was subsequently adjusted to achieve the highest predictive accuracy by minimizing the Akaike information criterion (AIC). Nephrotoxic agent exposures were included in the final multivariate model regardless of statistical significance in univariate analysis. Model fit was assessed with a standardized Hosmer-Lemeshow goodness-of-fit test.11 All statistical analyses were completed with RStudio v 0.98 running R v 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).12 All tests were 2-tailed and significance was defined at an alpha of 0.05.
RESULTS
Of 17,879 patients initially screened, 11,650 patients were evaluated, of which 5,497 received VAN and PTZ (VAN/PTZ), 3,055 received VAN alone, and 3,098 received PTZ alone. Table 1 contains basic demographic information. The mean age of patients was 52.5 years ± 16.8 years with 6,242 (53.6%) males. Patients receiving VAN/PTZ had higher CCIs than either monotherapy group and had significantly increased length of hospitalization. While patients in the combination therapy group were more likely to experience hypotension, concomitant nephrotoxic agent exposure was more common in the VAN monotherapy group.
RIFLE-defined AKI occurred in 1,647 (14.1%) across the entire cohort. AKI occurred in 21% of VAN/PTZ patients, 8.3% of VAN patients, and 7.8% of PTZ patients (P < 0.0001). RIFLE-defined risk, injury, and failure occurred more frequently in the VAN/PTZ cohort compared to the VAN and PTZ monotherapy groups (Figure). There were no differences in AKI rates between years studied (r2 = 0.4732, P = 0.2). Patients in the VAN/PTZ group experienced AKI on average of 8.0 days after treatment initiation, compared to 8.7 days and 5.2 days for VAN and PTZ monotherapy groups, respectively. The composite of inhospital mortality and transfer-to-hospice care was more common in VAN/PTZ patients (9.6%) compared to monotherapy groups (VAN, 3.9%; PTZ, 3.4%), most likely due to the increased severity of illness.
In the subgroup analysis of patients with similar CCI, AKI incidence increased with severity of illness. When CCI was 0, 7.5% of patients experienced AKI compared to 11.2%, 16.4%, and 18.9% of patients when CCI was 1, 2 to 4, and ≥5, respectively (P < 0.0001). VAN/PTZ (range = 12.1% to 26.5%) was associated with greater AKI incidence than either VAN (range = 4.8% to 11.5%) or PTZ (range = 3.8% to 10.4%) alone in each subgroup (P < 0.0001 for all subgroups).
Factors associated with AKI in univariate analyses included treatment with VAN/PTZ, days of therapy, baseline creatinine clearance, transfer from outside hospitals, CCI, admission type, length of hospitalization, dehydration exposure, and hypotension exposure. Exposure to aminoglycosides, amphotericin B, ACE inhibitors, nonsteroidal anti-inflammatory drugs, tacrolimus, foscarnet, loop diuretics, sulfonamides, and tenofovir were all associated with increased odds of AKI in simple univariate logistic regression. Gender, age, year of treatment, angiotensin II receptor antagonist exposure, and cyclosporine exposure were not significantly associated with AKI incidence.
After multivariate logistic regression, monotherapy with VAN or PTZ was associated with decreased odds of AKI compared to VAN/PTZ therapy (aORVAN,0.48; 95% CIVAN,0.41-0.57; aORPTZ, 0.43; 95% CIPTZ, 0.37-0.50). No difference in AKI incidence was observed between VAN and PTZ groups (aORPTZ:VAN, 0.88; 95% CI, 0.73-1.08). Table 2 describes the relationship between AKI and other covariates included in the model. Increased odds of AKI were seen with concomitant administration of ACE inhibitors, amphotericin B, tacrolimus, loop diuretics, and tenofovir. Radio-contrast dye administration was associated with lower odds of AKI. Patients admitted urgently and emergently were at higher risk of AKI, while those admitted via the trauma center were less likely to experience AKI compared to patients who were electively admitted. Increased length of stay and duration of therapy were both associated with increased likelihood of AKI, independent of treatment group; however, durations of therapy beyond 12 days was not associated with increased AKI. Hypotension, as defined, and diagnosed dehydration both independently increased AKI odds. Aside from those older than 80 years of age, increasing age was not associated with increased AKI risk. Male gender was associated with a slight decrease in AKI rate. No evidence of overfitting was observed with the standardized Hosmer-Lemeshow P-value of 0.683, and the model provides good predictive accuracy with a C-statistic of 0.788.
CONCLUSIONS
Acute kidney injury secondary to VAN therapy is a well-characterized adverse effect, while AKI incidence secondary to PTZ is less understood. Additionally, there appears to be an additive effect when these agents are used in combination. This is the largest review of AKI in patients receiving VAN,PTZ, or the combination of both agents.
There is increasing evidence suggesting greater nephrotoxicity in patients treated with the combination of VAN and antipseudomonal beta-lactams. The mechanism for the apparent increase in nephrotoxicity with this drug combination is not well understood and needs further study in both animal models and humans.
Acute kidney injury rates related to VAN vary widely, with recent studies in critically ill and internal medicine patients estimated at 21% and 13.6%, respectively.2,3 In our VAN monotherapy cohort, the AKI rate was 8.3%, with 2.3% of patients experiencing a greater than 50% decrease in creatinine clearance. Piperacillin-tazobactam-related AKI rates are not well characterized; however, a small retrospective analysis estimated that 11.1% of PTZ patients experienced acute renal failure (defined as either increase in serum creatinine greater than 0.5 mg/dL or 50% increase from baseline).13 In the present study, we found the PTZ-related AKI rate to be 7.8%, which may be due to a more stringent definition of AKI. Additionally, Hellwig et al13 found that PTZ monotherapy was associated with higher AKI rates compared to VAN monotherapy (11.1% vs 4.9%; P = 0.014). This was not replicated in our study, with VAN and PTZ monotherapy having similar AKI rates (8.3% and 7.8%, respectively) and an adjusted aOR of 0.88 (95% CI 0.0.73-1.08) for AKI in PTZ- compared to VAN-treated patients. The estimated AKI incidence of 21% in the combination therapy group at our institution is consistent with literature that ranges from 16.3% to 34.8%.4-8,13
To control for differences in baseline severity of illness, we performed a subgroup analysis of patients with similar CCI scores. The finding of increased AKI in patients receiving combination VAN and PTZ was consistent in each subgroup, suggesting that the increase in AKI is independent of illness severity.
This study is not without limitations. As with all retrospective studies, it is difficult to determine a causal link between VAN and PTZ combination therapy and increased AKI incidence due to confounding. We employed a rigorous study design that controlled for major confounders of AKI, such as concomitant nephrotoxic exposure, hypotension, and renal disease. Severity of illness was measured with CCI, which may not accurately capture the severity of illness at treatment initiation. Alternatives, such as acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) scores, may more accurately reflect critical illness on presentation; however, this study was not focused specifically on critically ill patients. In addition to baseline comorbidity, we controlled for hypotension and dehydration as a surrogate marker for critical illness. In the subgroup analysis of patients with similar CCI, the effect of VAN/PTZ on AKI compared to VAN or PTZ monotherapy was consistent in each group. Nephrotoxic potential of agents was assumed to be equal, which is not necessarily true. Additionally, the binary representation of nephrotoxic exposure does not describe the amount of the agent received; as such, our estimations of AKI odds may be artificially elevated. Approximately one-quarter of the patients in this study were transferred from an outside hospital, for which no data regarding initial treatment are available. This may lead to exposure misclassification. We attempted to control for this factor in the regression model and found that, after controlling for other covariates, hospital transfer was associated with increasing odds of AKI. Finally, data were collected retrospectively from the electronic medical record and are subject to inaccuracies documented in the chart; however, any bias introduced should be nondifferential.
In our large retrospective study of combination empiric therapy with VAN and PTZ, we found that combination therapy was associated with more than double the odds of AKI occurring compared to either monotherapy with VAN or PTZ. Increasing duration of therapy was also associated with increases in AKI. These findings demonstrate the need for judicious use of combination therapy and strengthen the need for antimicrobial de-escalation when appropriate to avoid deleterious effects.
Acknowledgments
The authors thank Chantal Le Rutter, MPA, for copyediting services.
Disclosures
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
Empiric antimicrobial therapy often consists of the combination of gram-positive coverage with vancomycin (VAN) and gram-negative coverage, specifically an antipseudomonal beta-lactam such as piperacillin-tazobactam (PTZ). Literature from a variety of patient populations reports nephrotoxicity associated with VAN, targeting troughs greater than 15 µg/mL, that occur in 5% to 43% of patients.1 In a study of critically ill patients, acute kidney injury (AKI) was found in 21% of patients receiving VAN, with increasing duration of VAN treatment, greater VAN levels, concomitant vasoactive medication administration, and intermittent infusion methods being associated with higher odds of AKI.2 A recent report from adult internal medicine patients estimated the incidence of VAN-associated nephrotoxicity at 13.6% and implicated concomitant PTZ therapy as a key factor in these patients.3
Further studies have explored the interaction between empiric beta-lactam and VAN therapy, showing mixed results. Reports of AKI associated with the combination of VAN and PTZ range from 16.3% to 34.8%,4-8 while the cefepime-VAN combination is reported to range from 12.5% to 13.3%.5,6 While VAN monotherapy groups were well represented, only 1 study7 compared the PTZ-VAN combination to a control group of PTZ monotherapy.
The primary objective of this study was to evaluate the differences in AKI incidence between patients treated with VAN and with PTZ, alone and in combination.
METHODS
This is a retrospective cohort study of adult patients conducted at the University of Kentucky Chandler Medical Center (UKMC) from September 1, 2010 through August 31, 2014. Patients were included if they were at least 18 years of age on admission; remained hospitalized for at least 48 hours; received VAN combined with PTZ (VAN/PTZ), VAN alone, or PTZ alone; and had at least 48 hours of therapy (and 48 hours of overlapping therapy in the VAN/PTZ group). Patients were excluded if they had underlying diagnosis of chronic kidney disease according to the International Classification of Diseases 9 (ICD-9) code, were receiving renal replacement therapy before admission, had a diagnosis of cystic fibrosis, or were pregnant. Additionally, patients were excluded if they presented with AKI, defined as an initial creatinine clearance less than 30 mL/min, or if baseline creatinine clearance was greater than 4 times the standard deviation from the mean; serum creatinine values were not obtained during admission; and if AKI occurred prior to therapy initiation, within 48 hours of initiation, or more than 7 days after treatment was discontinued. Patients were followed throughout their stay until time of discharge.
Data Source
Patient data were collected from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). The EDT contains clinical data from the inpatient population of UKMC from 2006 to present. Data stored and updated nightly by the EDT includes: demographics, financial classification (Medicare, Medicaid, private insurance), provider-level detail (service line), medical diagnosis (ICD-9 codes), medical procedures (Current Procedural Terminology [CPT] codes), lab tests and results, medication administration details, visit details (age, length of stay, etc), and vital signs. This study was approved by the UKMC Institutional Review Board.
Data collected for each patient included: demographic data, visit details (length of stay, admitting and primary diagnosis codes, etc.), severity of underlying illness as defined by the Charlson Comorbidity Index (CCI), all serum creatinine levels drawn per visit, medication administration information (dose, date, and time administered), all VAN trough levels, receipt of other nephrotoxic agents, blood pressures, and receipt of vasopressors.
Outcome Ascertainment
The definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss, End-stage) criteria,9 with risk defined as a 25% to 50% decrease in estimated glomerular filtration rate (GFR), injury as a 50% to 75% decrease in estimated GFR, and failure defined as a greater than 75% decrease in estimated GFR. Loss and end-stage classifications were not assessed because of this study’s follow-up period. The adjusted Cockcroft and Gault equation10 was used to estimate GFR due to the inconsistency of weight availability in the dataset and concordance with the institution’s practice. Baseline creatinine clearance was calculated with the first serum creatinine obtained, and the minimum creatinine clearance was calculated using the maximum serum creatinine during each patient’s visit. The percent decrease in creatinine clearance was calculated from these 2 values. AKI status was defined as meeting any of the RIFLE criteria. Mortality was assessed for all patients and defined as the composite of inhospital mortality and discharge or transfer to hospice care.
Exposure Ascertainment
Hypotension exposure was defined as experiencing 1 of the following: mean arterial blood pressure less than 60 mm Hg, a diagnosis of hypotension by a physician, or receipt of vasopressors or inotropic agents. Days of therapy for each drug were obtained and combination days of therapy were calculated by including only those days in which the patient received both medications. Total days of therapy were calculated by the sum of all days receiving at least 1 study agent. Exposure to other nephrotoxic agents (eg, acyclovir, angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor antagonists, aminoglycosides, amphotericin B, cyclosporine, foscarnet, loop diuretics, nonsteroidal anti-inflammatory drugs, sulfonamides, tacrolimus, and tenofovir) were defined as receipt of at least 1 dose of the agent during hospitalization.
Statistical Analysis
Characteristics between groups were described with basic descriptive statistics. Continuous variables were compared with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test. Categorical variables were compared with chi-square or Fisher exact test. Yearly AKI trends were assessed with Pearson correlation coefficient. To control for differences in underlying severity of illness between groups, a subanalysis was performed in which the cohort was split into 4 groups (0, 1, 2 to 4, and ≥5 points) based on CCI. Univariate models for all covariates were created with probability of AKI as the outcome. Covariates significant after univariate were incorporated into the multivariate model, which was subsequently adjusted to achieve the highest predictive accuracy by minimizing the Akaike information criterion (AIC). Nephrotoxic agent exposures were included in the final multivariate model regardless of statistical significance in univariate analysis. Model fit was assessed with a standardized Hosmer-Lemeshow goodness-of-fit test.11 All statistical analyses were completed with RStudio v 0.98 running R v 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).12 All tests were 2-tailed and significance was defined at an alpha of 0.05.
RESULTS
Of 17,879 patients initially screened, 11,650 patients were evaluated, of which 5,497 received VAN and PTZ (VAN/PTZ), 3,055 received VAN alone, and 3,098 received PTZ alone. Table 1 contains basic demographic information. The mean age of patients was 52.5 years ± 16.8 years with 6,242 (53.6%) males. Patients receiving VAN/PTZ had higher CCIs than either monotherapy group and had significantly increased length of hospitalization. While patients in the combination therapy group were more likely to experience hypotension, concomitant nephrotoxic agent exposure was more common in the VAN monotherapy group.
RIFLE-defined AKI occurred in 1,647 (14.1%) across the entire cohort. AKI occurred in 21% of VAN/PTZ patients, 8.3% of VAN patients, and 7.8% of PTZ patients (P < 0.0001). RIFLE-defined risk, injury, and failure occurred more frequently in the VAN/PTZ cohort compared to the VAN and PTZ monotherapy groups (Figure). There were no differences in AKI rates between years studied (r2 = 0.4732, P = 0.2). Patients in the VAN/PTZ group experienced AKI on average of 8.0 days after treatment initiation, compared to 8.7 days and 5.2 days for VAN and PTZ monotherapy groups, respectively. The composite of inhospital mortality and transfer-to-hospice care was more common in VAN/PTZ patients (9.6%) compared to monotherapy groups (VAN, 3.9%; PTZ, 3.4%), most likely due to the increased severity of illness.
In the subgroup analysis of patients with similar CCI, AKI incidence increased with severity of illness. When CCI was 0, 7.5% of patients experienced AKI compared to 11.2%, 16.4%, and 18.9% of patients when CCI was 1, 2 to 4, and ≥5, respectively (P < 0.0001). VAN/PTZ (range = 12.1% to 26.5%) was associated with greater AKI incidence than either VAN (range = 4.8% to 11.5%) or PTZ (range = 3.8% to 10.4%) alone in each subgroup (P < 0.0001 for all subgroups).
Factors associated with AKI in univariate analyses included treatment with VAN/PTZ, days of therapy, baseline creatinine clearance, transfer from outside hospitals, CCI, admission type, length of hospitalization, dehydration exposure, and hypotension exposure. Exposure to aminoglycosides, amphotericin B, ACE inhibitors, nonsteroidal anti-inflammatory drugs, tacrolimus, foscarnet, loop diuretics, sulfonamides, and tenofovir were all associated with increased odds of AKI in simple univariate logistic regression. Gender, age, year of treatment, angiotensin II receptor antagonist exposure, and cyclosporine exposure were not significantly associated with AKI incidence.
After multivariate logistic regression, monotherapy with VAN or PTZ was associated with decreased odds of AKI compared to VAN/PTZ therapy (aORVAN,0.48; 95% CIVAN,0.41-0.57; aORPTZ, 0.43; 95% CIPTZ, 0.37-0.50). No difference in AKI incidence was observed between VAN and PTZ groups (aORPTZ:VAN, 0.88; 95% CI, 0.73-1.08). Table 2 describes the relationship between AKI and other covariates included in the model. Increased odds of AKI were seen with concomitant administration of ACE inhibitors, amphotericin B, tacrolimus, loop diuretics, and tenofovir. Radio-contrast dye administration was associated with lower odds of AKI. Patients admitted urgently and emergently were at higher risk of AKI, while those admitted via the trauma center were less likely to experience AKI compared to patients who were electively admitted. Increased length of stay and duration of therapy were both associated with increased likelihood of AKI, independent of treatment group; however, durations of therapy beyond 12 days was not associated with increased AKI. Hypotension, as defined, and diagnosed dehydration both independently increased AKI odds. Aside from those older than 80 years of age, increasing age was not associated with increased AKI risk. Male gender was associated with a slight decrease in AKI rate. No evidence of overfitting was observed with the standardized Hosmer-Lemeshow P-value of 0.683, and the model provides good predictive accuracy with a C-statistic of 0.788.
CONCLUSIONS
Acute kidney injury secondary to VAN therapy is a well-characterized adverse effect, while AKI incidence secondary to PTZ is less understood. Additionally, there appears to be an additive effect when these agents are used in combination. This is the largest review of AKI in patients receiving VAN,PTZ, or the combination of both agents.
There is increasing evidence suggesting greater nephrotoxicity in patients treated with the combination of VAN and antipseudomonal beta-lactams. The mechanism for the apparent increase in nephrotoxicity with this drug combination is not well understood and needs further study in both animal models and humans.
Acute kidney injury rates related to VAN vary widely, with recent studies in critically ill and internal medicine patients estimated at 21% and 13.6%, respectively.2,3 In our VAN monotherapy cohort, the AKI rate was 8.3%, with 2.3% of patients experiencing a greater than 50% decrease in creatinine clearance. Piperacillin-tazobactam-related AKI rates are not well characterized; however, a small retrospective analysis estimated that 11.1% of PTZ patients experienced acute renal failure (defined as either increase in serum creatinine greater than 0.5 mg/dL or 50% increase from baseline).13 In the present study, we found the PTZ-related AKI rate to be 7.8%, which may be due to a more stringent definition of AKI. Additionally, Hellwig et al13 found that PTZ monotherapy was associated with higher AKI rates compared to VAN monotherapy (11.1% vs 4.9%; P = 0.014). This was not replicated in our study, with VAN and PTZ monotherapy having similar AKI rates (8.3% and 7.8%, respectively) and an adjusted aOR of 0.88 (95% CI 0.0.73-1.08) for AKI in PTZ- compared to VAN-treated patients. The estimated AKI incidence of 21% in the combination therapy group at our institution is consistent with literature that ranges from 16.3% to 34.8%.4-8,13
To control for differences in baseline severity of illness, we performed a subgroup analysis of patients with similar CCI scores. The finding of increased AKI in patients receiving combination VAN and PTZ was consistent in each subgroup, suggesting that the increase in AKI is independent of illness severity.
This study is not without limitations. As with all retrospective studies, it is difficult to determine a causal link between VAN and PTZ combination therapy and increased AKI incidence due to confounding. We employed a rigorous study design that controlled for major confounders of AKI, such as concomitant nephrotoxic exposure, hypotension, and renal disease. Severity of illness was measured with CCI, which may not accurately capture the severity of illness at treatment initiation. Alternatives, such as acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) scores, may more accurately reflect critical illness on presentation; however, this study was not focused specifically on critically ill patients. In addition to baseline comorbidity, we controlled for hypotension and dehydration as a surrogate marker for critical illness. In the subgroup analysis of patients with similar CCI, the effect of VAN/PTZ on AKI compared to VAN or PTZ monotherapy was consistent in each group. Nephrotoxic potential of agents was assumed to be equal, which is not necessarily true. Additionally, the binary representation of nephrotoxic exposure does not describe the amount of the agent received; as such, our estimations of AKI odds may be artificially elevated. Approximately one-quarter of the patients in this study were transferred from an outside hospital, for which no data regarding initial treatment are available. This may lead to exposure misclassification. We attempted to control for this factor in the regression model and found that, after controlling for other covariates, hospital transfer was associated with increasing odds of AKI. Finally, data were collected retrospectively from the electronic medical record and are subject to inaccuracies documented in the chart; however, any bias introduced should be nondifferential.
In our large retrospective study of combination empiric therapy with VAN and PTZ, we found that combination therapy was associated with more than double the odds of AKI occurring compared to either monotherapy with VAN or PTZ. Increasing duration of therapy was also associated with increases in AKI. These findings demonstrate the need for judicious use of combination therapy and strengthen the need for antimicrobial de-escalation when appropriate to avoid deleterious effects.
Acknowledgments
The authors thank Chantal Le Rutter, MPA, for copyediting services.
Disclosures
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
1. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734-744. PubMed
2. Hanrahan TP, Harlow G, Hutchinson J, et al. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med. 2014;42:2527-2536. PubMed
3. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653-661. PubMed
4. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34:670-676. PubMed
5. Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014;20:O384-O389. PubMed
6. Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34:662-669. PubMed
7. Kim T, Kandiah S, Patel M, et al. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8:579. PubMed
8. Davies SW, Efird JT, Guidry CA, et al. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt). 2016;17:38-47. PubMed
9. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. PubMed
10. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011;31:658-664. PubMed
11. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67-80. PubMed
12. R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
13. Hellwig T, Hammerquist R, Loecker B, Shields J. Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Abstracts of the Society of Critical Care Medicine 41st Critical Care Congress. February 4-8, 2012. Houston, Texas. Crit Care Med. 2011;39:1-264.
1. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734-744. PubMed
2. Hanrahan TP, Harlow G, Hutchinson J, et al. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med. 2014;42:2527-2536. PubMed
3. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653-661. PubMed
4. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34:670-676. PubMed
5. Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014;20:O384-O389. PubMed
6. Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34:662-669. PubMed
7. Kim T, Kandiah S, Patel M, et al. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8:579. PubMed
8. Davies SW, Efird JT, Guidry CA, et al. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt). 2016;17:38-47. PubMed
9. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. PubMed
10. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011;31:658-664. PubMed
11. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67-80. PubMed
12. R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
13. Hellwig T, Hammerquist R, Loecker B, Shields J. Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Abstracts of the Society of Critical Care Medicine 41st Critical Care Congress. February 4-8, 2012. Houston, Texas. Crit Care Med. 2011;39:1-264.
© 2017 Society of Hospital Medicine