User login
Access AGA guidelines and clinical decision support tools on your mobile device
AGA’s gold-standard guidelines, clinical support tools, podcasts, and videos are now available for download via the AGA Clinical Guidelines App, part of AGA’s App Central (http://www.gastro.org/on-demand/aga-app-central). The AGA Clinical Guidelines App offers a quick snapshot of key recommendations, and allows you to input information in a step-by-step format to help you make the most informed decisions possible.
You can even take notes and bookmark information for future reference and quicker decision making.
Only the highest-quality scientific evidence is used to develop AGA’s guidelines. The app currently offers guidelines on:
• Hepatitis B Reactivation.
• Drug Therapy for Crohn’s.
• Constipation.
• IBS Drug Management.
• Colonoscopy after Polypectomy.
• Pancreatic Cysts.
Download the the AGA Clinical Guidelines App and AGA App Central on the Apple App Store or Google Play.
AGA’s gold-standard guidelines, clinical support tools, podcasts, and videos are now available for download via the AGA Clinical Guidelines App, part of AGA’s App Central (http://www.gastro.org/on-demand/aga-app-central). The AGA Clinical Guidelines App offers a quick snapshot of key recommendations, and allows you to input information in a step-by-step format to help you make the most informed decisions possible.
You can even take notes and bookmark information for future reference and quicker decision making.
Only the highest-quality scientific evidence is used to develop AGA’s guidelines. The app currently offers guidelines on:
• Hepatitis B Reactivation.
• Drug Therapy for Crohn’s.
• Constipation.
• IBS Drug Management.
• Colonoscopy after Polypectomy.
• Pancreatic Cysts.
Download the the AGA Clinical Guidelines App and AGA App Central on the Apple App Store or Google Play.
AGA’s gold-standard guidelines, clinical support tools, podcasts, and videos are now available for download via the AGA Clinical Guidelines App, part of AGA’s App Central (http://www.gastro.org/on-demand/aga-app-central). The AGA Clinical Guidelines App offers a quick snapshot of key recommendations, and allows you to input information in a step-by-step format to help you make the most informed decisions possible.
You can even take notes and bookmark information for future reference and quicker decision making.
Only the highest-quality scientific evidence is used to develop AGA’s guidelines. The app currently offers guidelines on:
• Hepatitis B Reactivation.
• Drug Therapy for Crohn’s.
• Constipation.
• IBS Drug Management.
• Colonoscopy after Polypectomy.
• Pancreatic Cysts.
Download the the AGA Clinical Guidelines App and AGA App Central on the Apple App Store or Google Play.
How to Tweet: a guide for physicians
Social media, and Twitter in particular, is reshaping the practice of medicine by bringing physicians, scientists, and patients together on a common platform. With the pressures for providers to remain current with new clinical developments within the framework of health reform and to navigate the shift from volume- to value-based, patient-centered care, immediate access to a dynamic information-exchange medium such as Twitter can have an impact on both the quality and efficiency of care.
Click on the PDF icon at the top of this introduction to read the full article.
Social media, and Twitter in particular, is reshaping the practice of medicine by bringing physicians, scientists, and patients together on a common platform. With the pressures for providers to remain current with new clinical developments within the framework of health reform and to navigate the shift from volume- to value-based, patient-centered care, immediate access to a dynamic information-exchange medium such as Twitter can have an impact on both the quality and efficiency of care.
Click on the PDF icon at the top of this introduction to read the full article.
Social media, and Twitter in particular, is reshaping the practice of medicine by bringing physicians, scientists, and patients together on a common platform. With the pressures for providers to remain current with new clinical developments within the framework of health reform and to navigate the shift from volume- to value-based, patient-centered care, immediate access to a dynamic information-exchange medium such as Twitter can have an impact on both the quality and efficiency of care.
Click on the PDF icon at the top of this introduction to read the full article.
Should surgeons change gloves during total laparoscopic hysterectomy?
ORLANDO – Many gynecologic surgeons change gloves, gowns, and even surgical drapes during total laparoscopic hysterectomy to prevent bacterial infections, but little data support the practice.
In a small study of women undergoing total laparoscopic hysterectomy, investigators found that the overall risk of infection from contaminated gowns, gloves, and instruments was very low, with bacterial growth below the infection threshold in 98.9% of samples and no surgical site infections reported during 6 weeks of follow-up after surgery.
“Tradition dictates that even after both fields have been prepped, we refer to the perineum and vagina as ‘dirty,’ and the abdomen as ‘clean,’ ” Dr. Shockley said, “And surgeons habitually change their gown and gloves when inadvertent contact with the perineum or vagina occurs.”
To elucidate the true pathogen picture, Dr. Shockley and her colleagues assessed 31 women undergoing total laparoscopic hysterectomy for a benign indication during 2016. They evaluated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus.
All patients received perioperative antibiotic prophylaxis and standard, separate perineovaginal and abdominal prep with chlorhexidine. Investigators swabbed the vaginal fornices and abdomen at six sites, as well as the surgeon’s gloves following placement of the uterine manipulator, tips of instruments used to close the vaginal cuff, uterine fundus after extraction, and surgeon’s gloves following removal of the uterus.
They detected no anaerobic bacterial growth from samples taken from the abdomen, in the vagina, or on the tips of instruments used for cuff closure. Similarly, there was no aerobic growth observed in the vagina of any patient. However, they did detect aerobic bacterial growth in the abdomen, which in all cases was consistent with skin flora.
Three patients demonstrated some growth with the surgeon’s gloves following manipulator placement. Nearly one-third – 32% – of surgeon’s gloves cultured bacteria after removal of the uterus. One sample yielded cumulative growth for a bacterial count considered high enough to potentially cause infection, defined as more than 5,000 colony-forming units (CFU) per milliliter. This was the highest growth sample out of the 180 samples collected.
Additionally, 39% of samples from the uterine fundus were positive, a higher percentage than at any other site, Dr. Shockley reported. “And the one sample with growth exceeding 5,000 CFU/mL – you guessed it – was from the same patient.”
Bacterial growth was scant on the instrument tips used to close the vaginal cuffs.
Overall, bacterial growth in 98.9% of samples was below the infection threshold. “We did not identify any post–surgical site infections during 6 weeks of follow-up,” Dr. Shockley said at the meeting sponsored by AAGL.
“This study does provide a good description and count of the bacteria encountered during total laparoscopic hysterectomy. They are unlikely to cause surgical site infections … but based on concentration and frequency of bacterial growth on the surgeon’s gloves after specimen extraction, we would recommend if you are going to change gloves, do it after this step, before turning your attention back to the abdomen for vaginal cuff closure,” she said.
But changing gloves after placing the Foley and uterine manipulator “seems to be a wasted exercise,” Dr. Shockley said. “There was no growth on the vaginal fornices of any patient.”
The bacteria on the gloves in those three cases developed very low colony counts. “Yes, there was growth after the removal of the specimen, but with the exception of one patient, the colony counts were all below 5,000,” she said. “I think we need more data to reassure ourselves [attire changes are] unnecessary at every step of the [total laparoscopic hysterectomy].”
The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
ORLANDO – Many gynecologic surgeons change gloves, gowns, and even surgical drapes during total laparoscopic hysterectomy to prevent bacterial infections, but little data support the practice.
In a small study of women undergoing total laparoscopic hysterectomy, investigators found that the overall risk of infection from contaminated gowns, gloves, and instruments was very low, with bacterial growth below the infection threshold in 98.9% of samples and no surgical site infections reported during 6 weeks of follow-up after surgery.
“Tradition dictates that even after both fields have been prepped, we refer to the perineum and vagina as ‘dirty,’ and the abdomen as ‘clean,’ ” Dr. Shockley said, “And surgeons habitually change their gown and gloves when inadvertent contact with the perineum or vagina occurs.”
To elucidate the true pathogen picture, Dr. Shockley and her colleagues assessed 31 women undergoing total laparoscopic hysterectomy for a benign indication during 2016. They evaluated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus.
All patients received perioperative antibiotic prophylaxis and standard, separate perineovaginal and abdominal prep with chlorhexidine. Investigators swabbed the vaginal fornices and abdomen at six sites, as well as the surgeon’s gloves following placement of the uterine manipulator, tips of instruments used to close the vaginal cuff, uterine fundus after extraction, and surgeon’s gloves following removal of the uterus.
They detected no anaerobic bacterial growth from samples taken from the abdomen, in the vagina, or on the tips of instruments used for cuff closure. Similarly, there was no aerobic growth observed in the vagina of any patient. However, they did detect aerobic bacterial growth in the abdomen, which in all cases was consistent with skin flora.
Three patients demonstrated some growth with the surgeon’s gloves following manipulator placement. Nearly one-third – 32% – of surgeon’s gloves cultured bacteria after removal of the uterus. One sample yielded cumulative growth for a bacterial count considered high enough to potentially cause infection, defined as more than 5,000 colony-forming units (CFU) per milliliter. This was the highest growth sample out of the 180 samples collected.
Additionally, 39% of samples from the uterine fundus were positive, a higher percentage than at any other site, Dr. Shockley reported. “And the one sample with growth exceeding 5,000 CFU/mL – you guessed it – was from the same patient.”
Bacterial growth was scant on the instrument tips used to close the vaginal cuffs.
Overall, bacterial growth in 98.9% of samples was below the infection threshold. “We did not identify any post–surgical site infections during 6 weeks of follow-up,” Dr. Shockley said at the meeting sponsored by AAGL.
“This study does provide a good description and count of the bacteria encountered during total laparoscopic hysterectomy. They are unlikely to cause surgical site infections … but based on concentration and frequency of bacterial growth on the surgeon’s gloves after specimen extraction, we would recommend if you are going to change gloves, do it after this step, before turning your attention back to the abdomen for vaginal cuff closure,” she said.
But changing gloves after placing the Foley and uterine manipulator “seems to be a wasted exercise,” Dr. Shockley said. “There was no growth on the vaginal fornices of any patient.”
The bacteria on the gloves in those three cases developed very low colony counts. “Yes, there was growth after the removal of the specimen, but with the exception of one patient, the colony counts were all below 5,000,” she said. “I think we need more data to reassure ourselves [attire changes are] unnecessary at every step of the [total laparoscopic hysterectomy].”
The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
ORLANDO – Many gynecologic surgeons change gloves, gowns, and even surgical drapes during total laparoscopic hysterectomy to prevent bacterial infections, but little data support the practice.
In a small study of women undergoing total laparoscopic hysterectomy, investigators found that the overall risk of infection from contaminated gowns, gloves, and instruments was very low, with bacterial growth below the infection threshold in 98.9% of samples and no surgical site infections reported during 6 weeks of follow-up after surgery.
“Tradition dictates that even after both fields have been prepped, we refer to the perineum and vagina as ‘dirty,’ and the abdomen as ‘clean,’ ” Dr. Shockley said, “And surgeons habitually change their gown and gloves when inadvertent contact with the perineum or vagina occurs.”
To elucidate the true pathogen picture, Dr. Shockley and her colleagues assessed 31 women undergoing total laparoscopic hysterectomy for a benign indication during 2016. They evaluated the type and quantity of bacteria found intraoperatively on the abdomen, vagina, surgical gloves, instrument tips, and uterus.
All patients received perioperative antibiotic prophylaxis and standard, separate perineovaginal and abdominal prep with chlorhexidine. Investigators swabbed the vaginal fornices and abdomen at six sites, as well as the surgeon’s gloves following placement of the uterine manipulator, tips of instruments used to close the vaginal cuff, uterine fundus after extraction, and surgeon’s gloves following removal of the uterus.
They detected no anaerobic bacterial growth from samples taken from the abdomen, in the vagina, or on the tips of instruments used for cuff closure. Similarly, there was no aerobic growth observed in the vagina of any patient. However, they did detect aerobic bacterial growth in the abdomen, which in all cases was consistent with skin flora.
Three patients demonstrated some growth with the surgeon’s gloves following manipulator placement. Nearly one-third – 32% – of surgeon’s gloves cultured bacteria after removal of the uterus. One sample yielded cumulative growth for a bacterial count considered high enough to potentially cause infection, defined as more than 5,000 colony-forming units (CFU) per milliliter. This was the highest growth sample out of the 180 samples collected.
Additionally, 39% of samples from the uterine fundus were positive, a higher percentage than at any other site, Dr. Shockley reported. “And the one sample with growth exceeding 5,000 CFU/mL – you guessed it – was from the same patient.”
Bacterial growth was scant on the instrument tips used to close the vaginal cuffs.
Overall, bacterial growth in 98.9% of samples was below the infection threshold. “We did not identify any post–surgical site infections during 6 weeks of follow-up,” Dr. Shockley said at the meeting sponsored by AAGL.
“This study does provide a good description and count of the bacteria encountered during total laparoscopic hysterectomy. They are unlikely to cause surgical site infections … but based on concentration and frequency of bacterial growth on the surgeon’s gloves after specimen extraction, we would recommend if you are going to change gloves, do it after this step, before turning your attention back to the abdomen for vaginal cuff closure,” she said.
But changing gloves after placing the Foley and uterine manipulator “seems to be a wasted exercise,” Dr. Shockley said. “There was no growth on the vaginal fornices of any patient.”
The bacteria on the gloves in those three cases developed very low colony counts. “Yes, there was growth after the removal of the specimen, but with the exception of one patient, the colony counts were all below 5,000,” she said. “I think we need more data to reassure ourselves [attire changes are] unnecessary at every step of the [total laparoscopic hysterectomy].”
The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Key clinical point:
Major finding: Bacterial concentrations did not exceed thresholds required to trigger potential infection in almost 99% of cultures.
Data source: A study of 31 women undergoing total laparoscopic hysterectomy for benign indications in 2016.
Disclosures: The study was supported by an educational grant from the Foundation of the AAGL Jerome J. Hoffman Endowment. Dr. Shockley reported having no relevant financial disclosures.
GLAGOV finds evolocumab plus statin drives unprecedented plaque regression
NEW ORLEANS – Adding the PCSK9 inhibitor evolocumab to maximum tolerated statin therapy in subjects with symptomatic coronary disease induced atheromatous plaque regression of a previously unheard-of scale in the phase III GLAGOV trial, Steven E. Nissen, MD, reported at the American Heart Association scientific sessions.
Over 18 months of follow-up, percent atheroma volume was unchanged in patients treated with maximum tolerated statin therapy. But in patients on maximum tolerated statin therapy plus evolocumab, mean atheroma volume decreased by 0.95%. A reduction in atheroma volume as small as 0.5% has been associated with a reduced rate of cardiovascular events in previous studies, according to GLAGOV principal investigator Stephen J. Nicholls, MBBS, PhD, of the University of Adelaide in Australia.
Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Among 423 patients on statin monotherapy, 47% had regression and 53% had progression of atheroma volume. In contrast, 64% of 423 patients on a statin plus evolocumab had atheroma regressions and 36% had atheroma progression.
What’s more, plaque regression was directly related to declines in LDL levels.
“We saw a linear relationship between lower LDL and greater regression with no tailing off of benefit at very low LDL levels. Down to 20 mg/dL, it’s continuous and linear,” the cardiologist said. “I thought there might be diminishing return at low LDL levels. We don’t see that.”
GLAGOV (Global Assessment of Plaque Regression with a PCSK9 antibody as Measured by Intravascular Ultrasound) was a double-blind, placebo-controlled, randomized trial including 846 evaluable patients with symptomatic CAD at 197 centers. All were on a stable maximum tolerated dose of a statin at baseline, at which point they underwent intravascular ultrasound (IVUS) and were assigned to subcutaneous evolocumab (Repatha) at 420 mg once monthly or placebo injections while continuing on their statin. At 18 months, participants had a follow-up IVUS of the originally imaged target vessel.
The mean LDL-cholesterol level was 93 mg/dL at the start of the study. With the addition of evolocumab, the mean LDL level dropped to 37 mg/dL; that’s a 60% further reduction below the level on statin alone.
The investigators conducted a post hoc exploratory subgroup analysis confined to 144 patients whose baseline LDL on statin monotherapy was less than 70 mg/dL. In this group, the addition of evolocumab caused the mean LDL level to plunge to 24 mg/dL. Mean atheroma volume in dual-treatment patients with a baseline LDL below 70 mg/dL decreased by 1.97% – double the reduction seen in the overall statin/evolocumab study arm. Further, 81% of patients on dual therapy who started out with an LDL below 70 mg/dL showed IVUS evidence of plaque regression, compared with 48% of those on statin monotherapy. For those whose baseline LDL level was below 70 mg/dL and who remained on statin monotherapy, there was no change in atheroma volume over time.
No signals of any safety concerns arose in the group on a statin plus evolocumab. Of particular interest, there was no increase in new-onset diabetes, neurocognitive dysfunction, or myalgia in patients given dual therapy, compared with patients on a statin alone.
Dr. Santos, of the University of Sao Paolo in Brazil, noted that GLAGOV wasn’t powered to provide evidence of long-term safety or of hard clinical endpoints such as acute MI. For those outcomes, physicians must await the soon-to-come results of the nearly 28,000-patient FOURIER clinical outcomes trial.
The GLAGOV trial was sponsored by Amgen, the maker of evolocumab (Repatha). Dr. Santos reported serving as a consultant to and paid researcher for the company. Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies.
Simultaneously with Dr. Nissen’s presentation in New Orleans, the GLAGOV results were published online (JAMA. 2016 Nov 15. doi: 10.1001/jama.2016.16951).
NEW ORLEANS – Adding the PCSK9 inhibitor evolocumab to maximum tolerated statin therapy in subjects with symptomatic coronary disease induced atheromatous plaque regression of a previously unheard-of scale in the phase III GLAGOV trial, Steven E. Nissen, MD, reported at the American Heart Association scientific sessions.
Over 18 months of follow-up, percent atheroma volume was unchanged in patients treated with maximum tolerated statin therapy. But in patients on maximum tolerated statin therapy plus evolocumab, mean atheroma volume decreased by 0.95%. A reduction in atheroma volume as small as 0.5% has been associated with a reduced rate of cardiovascular events in previous studies, according to GLAGOV principal investigator Stephen J. Nicholls, MBBS, PhD, of the University of Adelaide in Australia.
Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Among 423 patients on statin monotherapy, 47% had regression and 53% had progression of atheroma volume. In contrast, 64% of 423 patients on a statin plus evolocumab had atheroma regressions and 36% had atheroma progression.
What’s more, plaque regression was directly related to declines in LDL levels.
“We saw a linear relationship between lower LDL and greater regression with no tailing off of benefit at very low LDL levels. Down to 20 mg/dL, it’s continuous and linear,” the cardiologist said. “I thought there might be diminishing return at low LDL levels. We don’t see that.”
GLAGOV (Global Assessment of Plaque Regression with a PCSK9 antibody as Measured by Intravascular Ultrasound) was a double-blind, placebo-controlled, randomized trial including 846 evaluable patients with symptomatic CAD at 197 centers. All were on a stable maximum tolerated dose of a statin at baseline, at which point they underwent intravascular ultrasound (IVUS) and were assigned to subcutaneous evolocumab (Repatha) at 420 mg once monthly or placebo injections while continuing on their statin. At 18 months, participants had a follow-up IVUS of the originally imaged target vessel.
The mean LDL-cholesterol level was 93 mg/dL at the start of the study. With the addition of evolocumab, the mean LDL level dropped to 37 mg/dL; that’s a 60% further reduction below the level on statin alone.
The investigators conducted a post hoc exploratory subgroup analysis confined to 144 patients whose baseline LDL on statin monotherapy was less than 70 mg/dL. In this group, the addition of evolocumab caused the mean LDL level to plunge to 24 mg/dL. Mean atheroma volume in dual-treatment patients with a baseline LDL below 70 mg/dL decreased by 1.97% – double the reduction seen in the overall statin/evolocumab study arm. Further, 81% of patients on dual therapy who started out with an LDL below 70 mg/dL showed IVUS evidence of plaque regression, compared with 48% of those on statin monotherapy. For those whose baseline LDL level was below 70 mg/dL and who remained on statin monotherapy, there was no change in atheroma volume over time.
No signals of any safety concerns arose in the group on a statin plus evolocumab. Of particular interest, there was no increase in new-onset diabetes, neurocognitive dysfunction, or myalgia in patients given dual therapy, compared with patients on a statin alone.
Dr. Santos, of the University of Sao Paolo in Brazil, noted that GLAGOV wasn’t powered to provide evidence of long-term safety or of hard clinical endpoints such as acute MI. For those outcomes, physicians must await the soon-to-come results of the nearly 28,000-patient FOURIER clinical outcomes trial.
The GLAGOV trial was sponsored by Amgen, the maker of evolocumab (Repatha). Dr. Santos reported serving as a consultant to and paid researcher for the company. Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies.
Simultaneously with Dr. Nissen’s presentation in New Orleans, the GLAGOV results were published online (JAMA. 2016 Nov 15. doi: 10.1001/jama.2016.16951).
NEW ORLEANS – Adding the PCSK9 inhibitor evolocumab to maximum tolerated statin therapy in subjects with symptomatic coronary disease induced atheromatous plaque regression of a previously unheard-of scale in the phase III GLAGOV trial, Steven E. Nissen, MD, reported at the American Heart Association scientific sessions.
Over 18 months of follow-up, percent atheroma volume was unchanged in patients treated with maximum tolerated statin therapy. But in patients on maximum tolerated statin therapy plus evolocumab, mean atheroma volume decreased by 0.95%. A reduction in atheroma volume as small as 0.5% has been associated with a reduced rate of cardiovascular events in previous studies, according to GLAGOV principal investigator Stephen J. Nicholls, MBBS, PhD, of the University of Adelaide in Australia.
Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Among 423 patients on statin monotherapy, 47% had regression and 53% had progression of atheroma volume. In contrast, 64% of 423 patients on a statin plus evolocumab had atheroma regressions and 36% had atheroma progression.
What’s more, plaque regression was directly related to declines in LDL levels.
“We saw a linear relationship between lower LDL and greater regression with no tailing off of benefit at very low LDL levels. Down to 20 mg/dL, it’s continuous and linear,” the cardiologist said. “I thought there might be diminishing return at low LDL levels. We don’t see that.”
GLAGOV (Global Assessment of Plaque Regression with a PCSK9 antibody as Measured by Intravascular Ultrasound) was a double-blind, placebo-controlled, randomized trial including 846 evaluable patients with symptomatic CAD at 197 centers. All were on a stable maximum tolerated dose of a statin at baseline, at which point they underwent intravascular ultrasound (IVUS) and were assigned to subcutaneous evolocumab (Repatha) at 420 mg once monthly or placebo injections while continuing on their statin. At 18 months, participants had a follow-up IVUS of the originally imaged target vessel.
The mean LDL-cholesterol level was 93 mg/dL at the start of the study. With the addition of evolocumab, the mean LDL level dropped to 37 mg/dL; that’s a 60% further reduction below the level on statin alone.
The investigators conducted a post hoc exploratory subgroup analysis confined to 144 patients whose baseline LDL on statin monotherapy was less than 70 mg/dL. In this group, the addition of evolocumab caused the mean LDL level to plunge to 24 mg/dL. Mean atheroma volume in dual-treatment patients with a baseline LDL below 70 mg/dL decreased by 1.97% – double the reduction seen in the overall statin/evolocumab study arm. Further, 81% of patients on dual therapy who started out with an LDL below 70 mg/dL showed IVUS evidence of plaque regression, compared with 48% of those on statin monotherapy. For those whose baseline LDL level was below 70 mg/dL and who remained on statin monotherapy, there was no change in atheroma volume over time.
No signals of any safety concerns arose in the group on a statin plus evolocumab. Of particular interest, there was no increase in new-onset diabetes, neurocognitive dysfunction, or myalgia in patients given dual therapy, compared with patients on a statin alone.
Dr. Santos, of the University of Sao Paolo in Brazil, noted that GLAGOV wasn’t powered to provide evidence of long-term safety or of hard clinical endpoints such as acute MI. For those outcomes, physicians must await the soon-to-come results of the nearly 28,000-patient FOURIER clinical outcomes trial.
The GLAGOV trial was sponsored by Amgen, the maker of evolocumab (Repatha). Dr. Santos reported serving as a consultant to and paid researcher for the company. Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies.
Simultaneously with Dr. Nissen’s presentation in New Orleans, the GLAGOV results were published online (JAMA. 2016 Nov 15. doi: 10.1001/jama.2016.16951).
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Data source: GLAGOV, a phase III, randomized, double-blind, 18-month intravascular ultrasound study of 846 evaluable patients with symptomatic CAD.
Disclosures: The GLAGOV trial was sponsored by Amgen, maker of evolocumab (Repatha). Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies. Dr. Santos reported serving as a consultant to and paid researcher for the company.
Renal cell carcinoma approval adds another notch to cabozantinib’s belt
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
In April this year, the US Food and Drug Administration awarded regulatory approval to cabozantinib for the treatment of advanced renal cell carcinoma patients previously treated with anti-angiogenic therapy.1 The small-molecule inhibitor, which targets multiple kinases, including the vascular endothelial growth factor receptors (VEGFRs) and the hepatocyte growth factor receptor (MET), had previously been approved for the treatment of medullary thyroid carcinoma in 2012.
Click on the PDF icon below for the full article.
AAGL 2016: Conference social highlights
The 2016 AAGL Global Congress kicked off in Orlando, Florida, with a jam-packed day of postgraduate courses on Monday, November 14. On Tuesday, Scientific Program Chair Kevin J. E. Stepp, MD, introduced the keynote speaker, and the 45th annual meeting of the AAGL was off and running. Many of the meeting's major events and individual sessions were captured through social media, and a few of those posts are captured here. We look forward to seeing you at next year’s meeting, where we hope you’ll be social with us once again!
View the story & AAGL: Conference Social Highlights on Storify
The 2016 AAGL Global Congress kicked off in Orlando, Florida, with a jam-packed day of postgraduate courses on Monday, November 14. On Tuesday, Scientific Program Chair Kevin J. E. Stepp, MD, introduced the keynote speaker, and the 45th annual meeting of the AAGL was off and running. Many of the meeting's major events and individual sessions were captured through social media, and a few of those posts are captured here. We look forward to seeing you at next year’s meeting, where we hope you’ll be social with us once again!
View the story & AAGL: Conference Social Highlights on Storify
The 2016 AAGL Global Congress kicked off in Orlando, Florida, with a jam-packed day of postgraduate courses on Monday, November 14. On Tuesday, Scientific Program Chair Kevin J. E. Stepp, MD, introduced the keynote speaker, and the 45th annual meeting of the AAGL was off and running. Many of the meeting's major events and individual sessions were captured through social media, and a few of those posts are captured here. We look forward to seeing you at next year’s meeting, where we hope you’ll be social with us once again!
View the story & AAGL: Conference Social Highlights on Storify
Two doses of HPV vaccine may be noninferior to three
A two-dose schedule of the 9-valent human papillomavirus (HPV) vaccine in children aged 9-14 years is noninferior to a three-dose schedule in adolescent girls and women (aged 16-26 years), based on immunogenicity measurements.
Many countries have poor HPV vaccination rates, in part because the current regimen requires three doses over a 6-month span, and it can be challenging in some areas for children to make three health care visits in the required time span. “Using an effective two-dose regimen entailing fewer visits could improve adherence to HPV vaccination programs. Coadministration of the 9-valent HPV vaccine with diphtheria, tetanus, pertussis, polio, and meningococcal vaccines could also be completed at the same visit,” reported Ole-Erik Iversen, MD, PhD, of the University of Bergen (Norway) and his colleagues (JAMA. 2016 Nov 21. doi: 10.1001/jama.2016.17615).
The researchers measured serum anti-HPV antibodies 1 month after the final dose. At least 98% of the participants in each group seroconverted to a response against all 9 HPV subtypes, and analysis of the antibody geometric mean titers revealed that the groups who received two doses had noninferior responses to the control group of adolescent girls and young women who received three doses.
Antibody geometric mean titers against all 9 HPV types were higher in subgroups of boys and girls (aged 9-10 years, aged 11-12 years, and aged 13-14 years) who received two doses, compared with girls and young women who received three doses. “These observations suggest that the overall results of the primary immunogenicity analyses may be applicable across the entire studied age range of girls and boys,” Dr. Iversen and his associates wrote.
The study cannot prove that the two-dose regimen has equal efficacy to the three-dose regimen in preventing HPV infection, only that the immunogenicity is noninferior, they said.
The study was sponsored by Merck, which manufactures the vaccine. Study authors have financial ties to Merck and a number of other pharmaceutical companies.
Evidence now supports a two-dose schedule in adolescents (aged 9-14 years) for all three licensed HPV vaccines. When the vaccination series is initiated before the age of 15 years, two doses administered at a 0- and 6-month interval or at a 0- and 12-month interval were found to be just as immunogenic as (or even better than) three doses.
The coverage of HPV vaccination in the United States is lower than that for other vaccines recommended for adolescents, such as quadrivalent meningococcal conjugate vaccine and tetanus, diphtheria, and acellular pertussis vaccine. In 2015, three-dose HPV vaccination coverage among 13- to 17-year-olds was only 41.9% for girls and 28.1% for boys; at least one-dose coverage was 62.8% for girls and 49.8% for boys.
Going forward, a two-dose schedule should make it easier to complete the recommended vaccination series. A two-dose schedule (at 0 and 6-12 months) will decrease health care appointments needed for HPV vaccination and facilitate clinicians’ ability to deliver vaccine at preventive health visits. Nevertheless, efforts will be needed to increase vaccine initiation and ensure delivery of the second dose.
Lauri E. Markowitz, MD, is at the division of viral diseases, National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, Atlanta. Elizabeth R. Unger, MD, MPH, is at the division of high-consequence pathogens and pathology, National Center for Emerging and Zoonotic Infectious Diseases at the CDC. Elissa Meites, PhD, MD, is at the division of viral diseases, National Center for Immunization and Respiratory Diseases at the CDC. Their comments were excerpted from an editorial accompanying the article by Iversen et al. (JAMA. 2016 Nov 21. doi: 10.1001/jama.2016.16393). The authors declared no financial conflicts of interest.
Evidence now supports a two-dose schedule in adolescents (aged 9-14 years) for all three licensed HPV vaccines. When the vaccination series is initiated before the age of 15 years, two doses administered at a 0- and 6-month interval or at a 0- and 12-month interval were found to be just as immunogenic as (or even better than) three doses.
The coverage of HPV vaccination in the United States is lower than that for other vaccines recommended for adolescents, such as quadrivalent meningococcal conjugate vaccine and tetanus, diphtheria, and acellular pertussis vaccine. In 2015, three-dose HPV vaccination coverage among 13- to 17-year-olds was only 41.9% for girls and 28.1% for boys; at least one-dose coverage was 62.8% for girls and 49.8% for boys.
Going forward, a two-dose schedule should make it easier to complete the recommended vaccination series. A two-dose schedule (at 0 and 6-12 months) will decrease health care appointments needed for HPV vaccination and facilitate clinicians’ ability to deliver vaccine at preventive health visits. Nevertheless, efforts will be needed to increase vaccine initiation and ensure delivery of the second dose.
Lauri E. Markowitz, MD, is at the division of viral diseases, National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, Atlanta. Elizabeth R. Unger, MD, MPH, is at the division of high-consequence pathogens and pathology, National Center for Emerging and Zoonotic Infectious Diseases at the CDC. Elissa Meites, PhD, MD, is at the division of viral diseases, National Center for Immunization and Respiratory Diseases at the CDC. Their comments were excerpted from an editorial accompanying the article by Iversen et al. (JAMA. 2016 Nov 21. doi: 10.1001/jama.2016.16393). The authors declared no financial conflicts of interest.
Evidence now supports a two-dose schedule in adolescents (aged 9-14 years) for all three licensed HPV vaccines. When the vaccination series is initiated before the age of 15 years, two doses administered at a 0- and 6-month interval or at a 0- and 12-month interval were found to be just as immunogenic as (or even better than) three doses.
The coverage of HPV vaccination in the United States is lower than that for other vaccines recommended for adolescents, such as quadrivalent meningococcal conjugate vaccine and tetanus, diphtheria, and acellular pertussis vaccine. In 2015, three-dose HPV vaccination coverage among 13- to 17-year-olds was only 41.9% for girls and 28.1% for boys; at least one-dose coverage was 62.8% for girls and 49.8% for boys.
Going forward, a two-dose schedule should make it easier to complete the recommended vaccination series. A two-dose schedule (at 0 and 6-12 months) will decrease health care appointments needed for HPV vaccination and facilitate clinicians’ ability to deliver vaccine at preventive health visits. Nevertheless, efforts will be needed to increase vaccine initiation and ensure delivery of the second dose.
Lauri E. Markowitz, MD, is at the division of viral diseases, National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, Atlanta. Elizabeth R. Unger, MD, MPH, is at the division of high-consequence pathogens and pathology, National Center for Emerging and Zoonotic Infectious Diseases at the CDC. Elissa Meites, PhD, MD, is at the division of viral diseases, National Center for Immunization and Respiratory Diseases at the CDC. Their comments were excerpted from an editorial accompanying the article by Iversen et al. (JAMA. 2016 Nov 21. doi: 10.1001/jama.2016.16393). The authors declared no financial conflicts of interest.
A two-dose schedule of the 9-valent human papillomavirus (HPV) vaccine in children aged 9-14 years is noninferior to a three-dose schedule in adolescent girls and women (aged 16-26 years), based on immunogenicity measurements.
Many countries have poor HPV vaccination rates, in part because the current regimen requires three doses over a 6-month span, and it can be challenging in some areas for children to make three health care visits in the required time span. “Using an effective two-dose regimen entailing fewer visits could improve adherence to HPV vaccination programs. Coadministration of the 9-valent HPV vaccine with diphtheria, tetanus, pertussis, polio, and meningococcal vaccines could also be completed at the same visit,” reported Ole-Erik Iversen, MD, PhD, of the University of Bergen (Norway) and his colleagues (JAMA. 2016 Nov 21. doi: 10.1001/jama.2016.17615).
The researchers measured serum anti-HPV antibodies 1 month after the final dose. At least 98% of the participants in each group seroconverted to a response against all 9 HPV subtypes, and analysis of the antibody geometric mean titers revealed that the groups who received two doses had noninferior responses to the control group of adolescent girls and young women who received three doses.
Antibody geometric mean titers against all 9 HPV types were higher in subgroups of boys and girls (aged 9-10 years, aged 11-12 years, and aged 13-14 years) who received two doses, compared with girls and young women who received three doses. “These observations suggest that the overall results of the primary immunogenicity analyses may be applicable across the entire studied age range of girls and boys,” Dr. Iversen and his associates wrote.
The study cannot prove that the two-dose regimen has equal efficacy to the three-dose regimen in preventing HPV infection, only that the immunogenicity is noninferior, they said.
The study was sponsored by Merck, which manufactures the vaccine. Study authors have financial ties to Merck and a number of other pharmaceutical companies.
A two-dose schedule of the 9-valent human papillomavirus (HPV) vaccine in children aged 9-14 years is noninferior to a three-dose schedule in adolescent girls and women (aged 16-26 years), based on immunogenicity measurements.
Many countries have poor HPV vaccination rates, in part because the current regimen requires three doses over a 6-month span, and it can be challenging in some areas for children to make three health care visits in the required time span. “Using an effective two-dose regimen entailing fewer visits could improve adherence to HPV vaccination programs. Coadministration of the 9-valent HPV vaccine with diphtheria, tetanus, pertussis, polio, and meningococcal vaccines could also be completed at the same visit,” reported Ole-Erik Iversen, MD, PhD, of the University of Bergen (Norway) and his colleagues (JAMA. 2016 Nov 21. doi: 10.1001/jama.2016.17615).
The researchers measured serum anti-HPV antibodies 1 month after the final dose. At least 98% of the participants in each group seroconverted to a response against all 9 HPV subtypes, and analysis of the antibody geometric mean titers revealed that the groups who received two doses had noninferior responses to the control group of adolescent girls and young women who received three doses.
Antibody geometric mean titers against all 9 HPV types were higher in subgroups of boys and girls (aged 9-10 years, aged 11-12 years, and aged 13-14 years) who received two doses, compared with girls and young women who received three doses. “These observations suggest that the overall results of the primary immunogenicity analyses may be applicable across the entire studied age range of girls and boys,” Dr. Iversen and his associates wrote.
The study cannot prove that the two-dose regimen has equal efficacy to the three-dose regimen in preventing HPV infection, only that the immunogenicity is noninferior, they said.
The study was sponsored by Merck, which manufactures the vaccine. Study authors have financial ties to Merck and a number of other pharmaceutical companies.
FROM JAMA
Key clinical point:
Major finding: Antibody geometric mean titers against all 9 HPV types were higher in subgroups of boys and girls (aged 9-10 years, aged 11-12 years, and aged 13-14 years) who received two doses, compared with girls and young women who received three doses.
Data source: Prospective, randomized trial of 1,377 children and young adults.
Disclosures: The study was sponsored by Merck, which manufactures the vaccine. Study authors have financial ties to Merck and a number of other pharmaceutical companies.
AURA-LV study: Rapid remission with voclosporin for lupus nephritis
WASHINGTON – The investigational calcineurin inhibitor voclosporin, given in addition to mycophenolate mofetil and low-dose steroids, was associated with rapid and complete remissions in lupus nephritis patients in the randomized, controlled AURA-LV study.
Aurinia Urinary Protein Reduction Active – Lupus With Voclosporin (AURA-LV) included 265 subjects in over 20 countries with active lupus nephritis. Trial participants received low-dose voclosporin (23.7 mg b.i.d.) or high-dose voclosporin (39.5 mg b.i.d.) in addition to mycophenolate mofetil (2 g/day) and low-dose steroids. Patients began on 20-25 mg of a steroid with a taper to 5 mg at week 8 and 2.5 mg at week 16-24.
Complete remission occurred at 24 weeks in 32.6% of 89 subjects who received 23.7 mg of voclosporin twice daily and standard of care therapy and in 19.3% of 88 control subjects who received placebo and standard of care therapy (odds ratio, 2.03), Mary Anne Dooley, MD, reported at the annual meeting of the American College of Rheumatology.
The complete remission rate was 27.3% in the 88 subjects who received the higher dose (39.5 mg b.i.d.) of voclosporin. The difference between the high-dose voclosporin group and the control group was not statistically significant.
The “very exciting findings” of this study – the first lupus nephritis study to meet its primary endpoint of complete remission – are important, because “partial remission is insufficient for our patients,” she said.
“Clinical trials over the past 10 years have really shown that we’re not reaching a large group of patients. ... more than 40% of patients are complete nonresponders at 6 months,” she said. While attainment of partial remission has improved, half of those who achieve partial remission have been shown to have a 50% increase in the risk of end-stage renal disease in 10 years.
Complete remission was defined as urine protein/creatinine ratio of no more than 0.5 mg/mg using first morning void with an estimated glomerular filtration rate of at least 60 mL/min without a decrease of 20% or more, sustained low-dose steroids (at or below 10 mg/day) and no use of rescue medications.
Partial remission was a composite of reduction in protein/creatinine ratio of at least 50%, no use of rescue medication, and stability of renal function. Both the low- and high-dose voclosporin groups had outcomes that were superior to standard-of-care therapy, with 69.7% partial remission with low-dose voclosporin, 65.9% partial remission with high-dose voclosporin, and 49.4% partial remission with placebo, said Dr. Dooley, a rheumatologist in Chapel Hill, N.C.
“Patients began responding literally within weeks [to voclosporin] ... and we saw significant responses by 7-8 weeks. This was during the time period when the steroids rapidly decreased,” she said, noting that the steroid dosing at baseline was a median of 25 mg vs. 2.5 mg at 16 weeks.
Study subjects met ACR criteria for lupus and had biopsy-proven lupus nephritis, including proliferative nephritis class III/IV or class V alone or in combination with proliferative disease. All were treated with 2 g/day of mycophenolate mofetil, and the steroid taper “was such that by 10 weeks, patients were down to 5 mg, and that by 24 weeks the median dose was 2.5 mg,” she said.
Adverse events, most commonly infection and gastrointestinal disorders, occurred in 90% of study subjects. Infections occurred in 56.2% of those in the low-dose group, 63.6% of those in the high-dose group, and 50% of controls. GI disorders occurred in 41.6%, 52.3%, and 36.4% of patients in the groups, respectively.
Serious adverse events were more common in the voclosporin groups, occurring in 25.8% and 25% of patients in the low- and high-dose groups, respectively, compared with 15.8% of patients in the control group.
Ten of the 13 deaths occurred in the low-dose voclosporin group (3 due to infection, 3 due to thromboembolism, and 4 due to “other” causes); 2 occurred in the high-dose voclosporin group (1 each due to infection and thromboembolism); and 1 death due to thromboembolism occurred in the control group. As most of the deaths were clustered in the low-dose arm, and 11 of the 13 deaths occurred in areas with “compromised access to standard of care,” the deaths were not considered to be directly related to voclosporin therapy.
Patients who died had “a statistically different clinical baseline picture with higher levels of proteinuria or difficulty with comorbid conditions and some signs of poor nutrition,” Dr. Dooley said.
The findings of the study will be used as the basis for planned subsequent studies of the use of voclosporin in lupus nephritis, she said.
Voclosporin is an analogue of cyclosporin A that may allow flat dosing and a potentially improved safety profile compared with other calcineurin inhibitors.
Aurinia Pharmaceuticals, the maker of voclosporin, announced in early November 2016 that the twice-daily 23.7 mg voclosporin dose will advance to a global 52-week double-blind, placebo-controlled phase III study in the second quarter of 2017. Voclosporin has already received fast track designation from the Food and Drug Administration.
Dr. Dooley reported a financial relationship with Aurinia, which sponsored the study.
WASHINGTON – The investigational calcineurin inhibitor voclosporin, given in addition to mycophenolate mofetil and low-dose steroids, was associated with rapid and complete remissions in lupus nephritis patients in the randomized, controlled AURA-LV study.
Aurinia Urinary Protein Reduction Active – Lupus With Voclosporin (AURA-LV) included 265 subjects in over 20 countries with active lupus nephritis. Trial participants received low-dose voclosporin (23.7 mg b.i.d.) or high-dose voclosporin (39.5 mg b.i.d.) in addition to mycophenolate mofetil (2 g/day) and low-dose steroids. Patients began on 20-25 mg of a steroid with a taper to 5 mg at week 8 and 2.5 mg at week 16-24.
Complete remission occurred at 24 weeks in 32.6% of 89 subjects who received 23.7 mg of voclosporin twice daily and standard of care therapy and in 19.3% of 88 control subjects who received placebo and standard of care therapy (odds ratio, 2.03), Mary Anne Dooley, MD, reported at the annual meeting of the American College of Rheumatology.
The complete remission rate was 27.3% in the 88 subjects who received the higher dose (39.5 mg b.i.d.) of voclosporin. The difference between the high-dose voclosporin group and the control group was not statistically significant.
The “very exciting findings” of this study – the first lupus nephritis study to meet its primary endpoint of complete remission – are important, because “partial remission is insufficient for our patients,” she said.
“Clinical trials over the past 10 years have really shown that we’re not reaching a large group of patients. ... more than 40% of patients are complete nonresponders at 6 months,” she said. While attainment of partial remission has improved, half of those who achieve partial remission have been shown to have a 50% increase in the risk of end-stage renal disease in 10 years.
Complete remission was defined as urine protein/creatinine ratio of no more than 0.5 mg/mg using first morning void with an estimated glomerular filtration rate of at least 60 mL/min without a decrease of 20% or more, sustained low-dose steroids (at or below 10 mg/day) and no use of rescue medications.
Partial remission was a composite of reduction in protein/creatinine ratio of at least 50%, no use of rescue medication, and stability of renal function. Both the low- and high-dose voclosporin groups had outcomes that were superior to standard-of-care therapy, with 69.7% partial remission with low-dose voclosporin, 65.9% partial remission with high-dose voclosporin, and 49.4% partial remission with placebo, said Dr. Dooley, a rheumatologist in Chapel Hill, N.C.
“Patients began responding literally within weeks [to voclosporin] ... and we saw significant responses by 7-8 weeks. This was during the time period when the steroids rapidly decreased,” she said, noting that the steroid dosing at baseline was a median of 25 mg vs. 2.5 mg at 16 weeks.
Study subjects met ACR criteria for lupus and had biopsy-proven lupus nephritis, including proliferative nephritis class III/IV or class V alone or in combination with proliferative disease. All were treated with 2 g/day of mycophenolate mofetil, and the steroid taper “was such that by 10 weeks, patients were down to 5 mg, and that by 24 weeks the median dose was 2.5 mg,” she said.
Adverse events, most commonly infection and gastrointestinal disorders, occurred in 90% of study subjects. Infections occurred in 56.2% of those in the low-dose group, 63.6% of those in the high-dose group, and 50% of controls. GI disorders occurred in 41.6%, 52.3%, and 36.4% of patients in the groups, respectively.
Serious adverse events were more common in the voclosporin groups, occurring in 25.8% and 25% of patients in the low- and high-dose groups, respectively, compared with 15.8% of patients in the control group.
Ten of the 13 deaths occurred in the low-dose voclosporin group (3 due to infection, 3 due to thromboembolism, and 4 due to “other” causes); 2 occurred in the high-dose voclosporin group (1 each due to infection and thromboembolism); and 1 death due to thromboembolism occurred in the control group. As most of the deaths were clustered in the low-dose arm, and 11 of the 13 deaths occurred in areas with “compromised access to standard of care,” the deaths were not considered to be directly related to voclosporin therapy.
Patients who died had “a statistically different clinical baseline picture with higher levels of proteinuria or difficulty with comorbid conditions and some signs of poor nutrition,” Dr. Dooley said.
The findings of the study will be used as the basis for planned subsequent studies of the use of voclosporin in lupus nephritis, she said.
Voclosporin is an analogue of cyclosporin A that may allow flat dosing and a potentially improved safety profile compared with other calcineurin inhibitors.
Aurinia Pharmaceuticals, the maker of voclosporin, announced in early November 2016 that the twice-daily 23.7 mg voclosporin dose will advance to a global 52-week double-blind, placebo-controlled phase III study in the second quarter of 2017. Voclosporin has already received fast track designation from the Food and Drug Administration.
Dr. Dooley reported a financial relationship with Aurinia, which sponsored the study.
WASHINGTON – The investigational calcineurin inhibitor voclosporin, given in addition to mycophenolate mofetil and low-dose steroids, was associated with rapid and complete remissions in lupus nephritis patients in the randomized, controlled AURA-LV study.
Aurinia Urinary Protein Reduction Active – Lupus With Voclosporin (AURA-LV) included 265 subjects in over 20 countries with active lupus nephritis. Trial participants received low-dose voclosporin (23.7 mg b.i.d.) or high-dose voclosporin (39.5 mg b.i.d.) in addition to mycophenolate mofetil (2 g/day) and low-dose steroids. Patients began on 20-25 mg of a steroid with a taper to 5 mg at week 8 and 2.5 mg at week 16-24.
Complete remission occurred at 24 weeks in 32.6% of 89 subjects who received 23.7 mg of voclosporin twice daily and standard of care therapy and in 19.3% of 88 control subjects who received placebo and standard of care therapy (odds ratio, 2.03), Mary Anne Dooley, MD, reported at the annual meeting of the American College of Rheumatology.
The complete remission rate was 27.3% in the 88 subjects who received the higher dose (39.5 mg b.i.d.) of voclosporin. The difference between the high-dose voclosporin group and the control group was not statistically significant.
The “very exciting findings” of this study – the first lupus nephritis study to meet its primary endpoint of complete remission – are important, because “partial remission is insufficient for our patients,” she said.
“Clinical trials over the past 10 years have really shown that we’re not reaching a large group of patients. ... more than 40% of patients are complete nonresponders at 6 months,” she said. While attainment of partial remission has improved, half of those who achieve partial remission have been shown to have a 50% increase in the risk of end-stage renal disease in 10 years.
Complete remission was defined as urine protein/creatinine ratio of no more than 0.5 mg/mg using first morning void with an estimated glomerular filtration rate of at least 60 mL/min without a decrease of 20% or more, sustained low-dose steroids (at or below 10 mg/day) and no use of rescue medications.
Partial remission was a composite of reduction in protein/creatinine ratio of at least 50%, no use of rescue medication, and stability of renal function. Both the low- and high-dose voclosporin groups had outcomes that were superior to standard-of-care therapy, with 69.7% partial remission with low-dose voclosporin, 65.9% partial remission with high-dose voclosporin, and 49.4% partial remission with placebo, said Dr. Dooley, a rheumatologist in Chapel Hill, N.C.
“Patients began responding literally within weeks [to voclosporin] ... and we saw significant responses by 7-8 weeks. This was during the time period when the steroids rapidly decreased,” she said, noting that the steroid dosing at baseline was a median of 25 mg vs. 2.5 mg at 16 weeks.
Study subjects met ACR criteria for lupus and had biopsy-proven lupus nephritis, including proliferative nephritis class III/IV or class V alone or in combination with proliferative disease. All were treated with 2 g/day of mycophenolate mofetil, and the steroid taper “was such that by 10 weeks, patients were down to 5 mg, and that by 24 weeks the median dose was 2.5 mg,” she said.
Adverse events, most commonly infection and gastrointestinal disorders, occurred in 90% of study subjects. Infections occurred in 56.2% of those in the low-dose group, 63.6% of those in the high-dose group, and 50% of controls. GI disorders occurred in 41.6%, 52.3%, and 36.4% of patients in the groups, respectively.
Serious adverse events were more common in the voclosporin groups, occurring in 25.8% and 25% of patients in the low- and high-dose groups, respectively, compared with 15.8% of patients in the control group.
Ten of the 13 deaths occurred in the low-dose voclosporin group (3 due to infection, 3 due to thromboembolism, and 4 due to “other” causes); 2 occurred in the high-dose voclosporin group (1 each due to infection and thromboembolism); and 1 death due to thromboembolism occurred in the control group. As most of the deaths were clustered in the low-dose arm, and 11 of the 13 deaths occurred in areas with “compromised access to standard of care,” the deaths were not considered to be directly related to voclosporin therapy.
Patients who died had “a statistically different clinical baseline picture with higher levels of proteinuria or difficulty with comorbid conditions and some signs of poor nutrition,” Dr. Dooley said.
The findings of the study will be used as the basis for planned subsequent studies of the use of voclosporin in lupus nephritis, she said.
Voclosporin is an analogue of cyclosporin A that may allow flat dosing and a potentially improved safety profile compared with other calcineurin inhibitors.
Aurinia Pharmaceuticals, the maker of voclosporin, announced in early November 2016 that the twice-daily 23.7 mg voclosporin dose will advance to a global 52-week double-blind, placebo-controlled phase III study in the second quarter of 2017. Voclosporin has already received fast track designation from the Food and Drug Administration.
Dr. Dooley reported a financial relationship with Aurinia, which sponsored the study.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The complete remission rate at 24 weeks was 32.6% vs. 19.3% in patients receiving low-dose voclosporin vs. controls (odds ratio, 2.03).
Data source: The randomized, controlled AURA-LV study of 265 lupus nephritis patients.
Disclosures: Dr. Dooley reported a financial relationship with Aurinia Pharmaceuticals, which sponsored the study.
Toxicity analysis of docetaxel, cisplatin, and 5- fluorouracil neoadjuvant chemotherapy in Indian patients with head and neck cancers
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Background There is a lack of data that systematically address toxicity with docetaxel, cisplatin, and 5-fluorouracil (TPF) regimen in routine care.
Objective To detect, profile, and quantify the toxicity in Indian patients with head and neck cancers who received neoadjuvant TPF chemotherapy in a routine clinical practice (non-trial setting).
Methods 58 patients with locally advanced head and neck cancer who received TPF chemotherapy were selected for this analysis. They received 2 cycles of TPF chemotherapy every 21 days. The patients were monitored for the occurrence of adverse drug reactions in accordance with Common Terminology Criteria for Adverse Events (version 4.03) during the hospitalization (median length of stay in cycle 1, 10 days), daily (at least until day 8 after chemotherapy initiation), then at days 15 and 20. Descriptive statistics was done and factors predicting for toxicity were identified using logistic regression analysis.
Results The cumulative rate of grade ¦3 anemia, neutropenia, and thrombocytopenia were 12.1%, 56.9%, and 5.2%, respectively. The cumulative incidence of febrile neutropenia was 20.7% (12 of 58 patients). The cumulative incidences of mucositis and diarrhea were 67.2% and 74.1%, respectively. There was no mortality associated with induction chemotherapy, and all of the patients completed the planned 2 cycles of TPF. None of the tested factors predicted for any of the adverse events considered in the study.
Limitations Small, single-center study
Conclusion The incidence of TPF-related toxicity in Indian patients in routine practice is high, and the toxicities differ substantially from the toxicities seen in trial settings.
Click on the PDF icon at the top of this introduction to read the full article.
Risk-reducing salpingectomy at benign hysterectomy: Have surgeons embraced this practice?
According to its January 2015 Committee Opinion, the American College of Obstetricians and Gynecologists supported the following recommendations and conclusions regarding salpingectomy for ovarian cancer prevention1:
- The surgeon and patient should discuss the potential benefits of the removal of the fallopian tubes during a hysterectomy in women at population risk of ovarian cancer who are not having an oophorectomy.
- When counseling women about laparoscopic sterilization methods, clinicians can communicate that bilateral salpingectomy can be considered a method that provides effective contraception.
- Prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients.
- Randomized controlled trials are needed to support the validity of this approach to reduce the incidence of ovarian cancer.
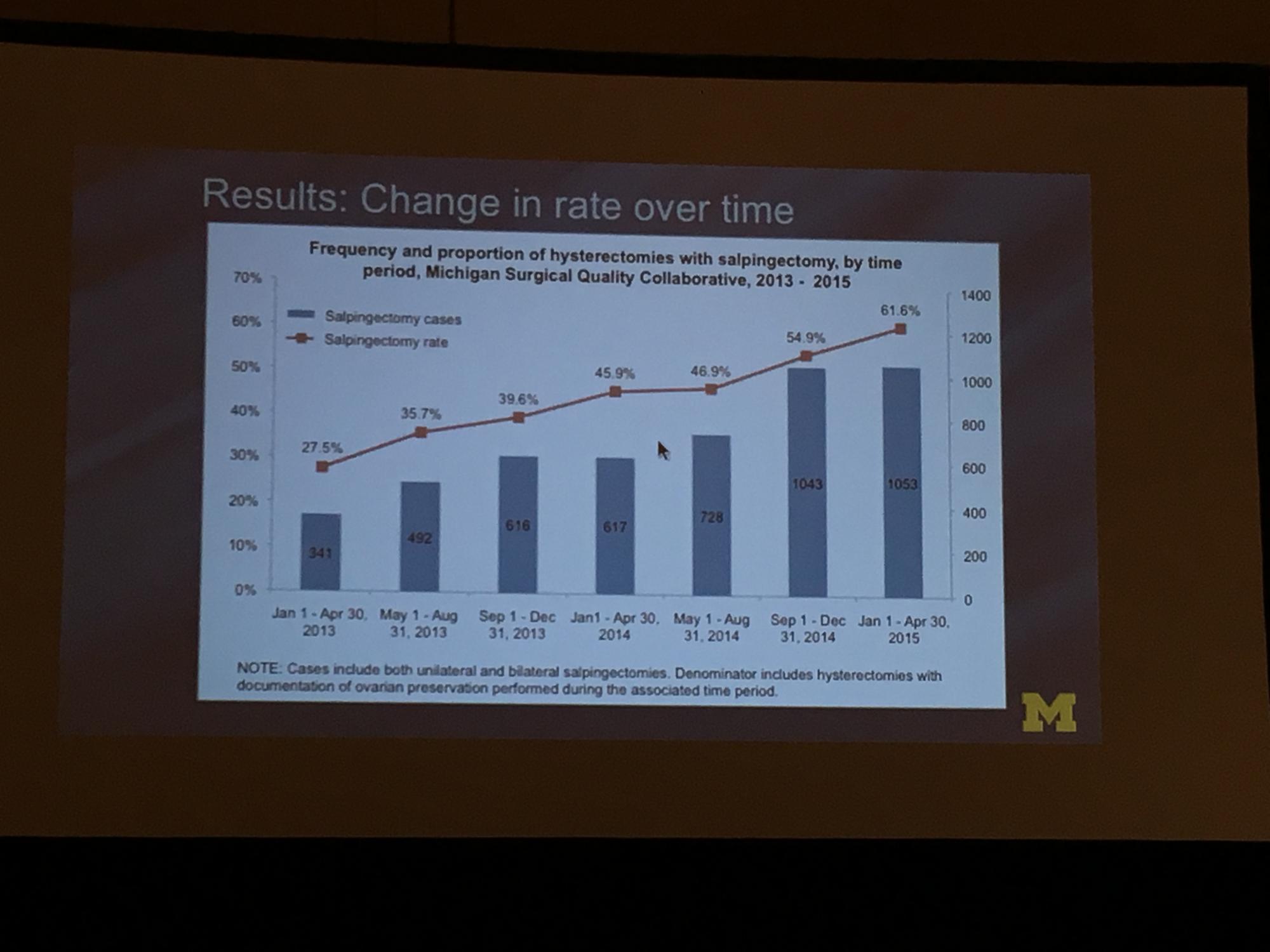
To determine the change in rate of salpingectomy performed at benign hysterectomy at Michigan hospitals, Sara Till, MD, MPH, and colleagues from the University of Michigan Health System performed a retrospective cross-sectioned study of data from the Michigan Surgical Quality Collaborative. They examined hysterectomies performed for all surgical routes between January 2013 and April 2015. Exclusion criteria included malignancy and obstetric indication. The primary objective was to measure salpingectomy at the time of hysterectomy with ovarian preservation. Measures studied included demographics; comorbidities; perioperative and postoperative results; and hospital/surgeon-related data; including surgeon volume, hospital type (ie, teaching), and hospital size.2
During the study period (January 1, 2013, to April 30, 2015), 18,642 hysterectomies were performed for benign indications, of which 55.7% (n = 10,382) were ovarian conserving. Among patients who underwent ovarian conserving hysterectomy, 44.9% (n = 4,668) had salpingectomy, with rates increasing steadily from 26.4% to 61.1% across the study period (P<.001). Salpingectomy was more likely with a laparoscopic approach (odds ratio [OR], 2.93; 95% confidence interval [CI], 2.69–3.20) and among women aged <60 years (OR, 2.60; 95% CI, 1.42–1.98), but did not vary with surgeon volume. After adjustments for age, body mass index, and surgical approach using a mixed model, the researchers found substantial variation in rates of salpingectomy across hospital sites, ranging from 3.7% to 88.3%. Variation in adjusted salpingectomy rates was not associated with academic affiliation or hospital size.2
Dr. Till and colleagues concluded that there was a substantial rise in risk-reducing salpingectomy from January 1, 2013, to April 30, 2015, and that there is substantial variation in the practice of salpingectomy, which is not accounted for by patient, surgeon, or hospital characteristics.2
- American College of Obstetricians and Gynecologists, Committee on Gynecologic Practice. Salpingectomy for ovarian cancer prevention. Committee Opinion No. 620 [published correction appears in: Obstet Gynecol. 2016;127(2):405]. Obstet Gynecol. 2015;125(1):279–281.
- Till SR, Edwards MG, Kobernik EK, Kamdar NS, As-Sanie S, Morgan DM. Implementation rate of risk-reducing salpingectomy at time of benign hysterectomy. Poster presented at: AAGL Global Congress of Minimally Invasive Gynecology; November 16, 2016; Orlando, Florida. J Minim Invasiv Gynecol. 2016;23(7 suppl):S1.
According to its January 2015 Committee Opinion, the American College of Obstetricians and Gynecologists supported the following recommendations and conclusions regarding salpingectomy for ovarian cancer prevention1:
- The surgeon and patient should discuss the potential benefits of the removal of the fallopian tubes during a hysterectomy in women at population risk of ovarian cancer who are not having an oophorectomy.
- When counseling women about laparoscopic sterilization methods, clinicians can communicate that bilateral salpingectomy can be considered a method that provides effective contraception.
- Prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients.
- Randomized controlled trials are needed to support the validity of this approach to reduce the incidence of ovarian cancer.
To determine the change in rate of salpingectomy performed at benign hysterectomy at Michigan hospitals, Sara Till, MD, MPH, and colleagues from the University of Michigan Health System performed a retrospective cross-sectioned study of data from the Michigan Surgical Quality Collaborative. They examined hysterectomies performed for all surgical routes between January 2013 and April 2015. Exclusion criteria included malignancy and obstetric indication. The primary objective was to measure salpingectomy at the time of hysterectomy with ovarian preservation. Measures studied included demographics; comorbidities; perioperative and postoperative results; and hospital/surgeon-related data; including surgeon volume, hospital type (ie, teaching), and hospital size.2
During the study period (January 1, 2013, to April 30, 2015), 18,642 hysterectomies were performed for benign indications, of which 55.7% (n = 10,382) were ovarian conserving. Among patients who underwent ovarian conserving hysterectomy, 44.9% (n = 4,668) had salpingectomy, with rates increasing steadily from 26.4% to 61.1% across the study period (P<.001). Salpingectomy was more likely with a laparoscopic approach (odds ratio [OR], 2.93; 95% confidence interval [CI], 2.69–3.20) and among women aged <60 years (OR, 2.60; 95% CI, 1.42–1.98), but did not vary with surgeon volume. After adjustments for age, body mass index, and surgical approach using a mixed model, the researchers found substantial variation in rates of salpingectomy across hospital sites, ranging from 3.7% to 88.3%. Variation in adjusted salpingectomy rates was not associated with academic affiliation or hospital size.2
Dr. Till and colleagues concluded that there was a substantial rise in risk-reducing salpingectomy from January 1, 2013, to April 30, 2015, and that there is substantial variation in the practice of salpingectomy, which is not accounted for by patient, surgeon, or hospital characteristics.2
According to its January 2015 Committee Opinion, the American College of Obstetricians and Gynecologists supported the following recommendations and conclusions regarding salpingectomy for ovarian cancer prevention1:
- The surgeon and patient should discuss the potential benefits of the removal of the fallopian tubes during a hysterectomy in women at population risk of ovarian cancer who are not having an oophorectomy.
- When counseling women about laparoscopic sterilization methods, clinicians can communicate that bilateral salpingectomy can be considered a method that provides effective contraception.
- Prophylactic salpingectomy may offer clinicians the opportunity to prevent ovarian cancer in their patients.
- Randomized controlled trials are needed to support the validity of this approach to reduce the incidence of ovarian cancer.
To determine the change in rate of salpingectomy performed at benign hysterectomy at Michigan hospitals, Sara Till, MD, MPH, and colleagues from the University of Michigan Health System performed a retrospective cross-sectioned study of data from the Michigan Surgical Quality Collaborative. They examined hysterectomies performed for all surgical routes between January 2013 and April 2015. Exclusion criteria included malignancy and obstetric indication. The primary objective was to measure salpingectomy at the time of hysterectomy with ovarian preservation. Measures studied included demographics; comorbidities; perioperative and postoperative results; and hospital/surgeon-related data; including surgeon volume, hospital type (ie, teaching), and hospital size.2
During the study period (January 1, 2013, to April 30, 2015), 18,642 hysterectomies were performed for benign indications, of which 55.7% (n = 10,382) were ovarian conserving. Among patients who underwent ovarian conserving hysterectomy, 44.9% (n = 4,668) had salpingectomy, with rates increasing steadily from 26.4% to 61.1% across the study period (P<.001). Salpingectomy was more likely with a laparoscopic approach (odds ratio [OR], 2.93; 95% confidence interval [CI], 2.69–3.20) and among women aged <60 years (OR, 2.60; 95% CI, 1.42–1.98), but did not vary with surgeon volume. After adjustments for age, body mass index, and surgical approach using a mixed model, the researchers found substantial variation in rates of salpingectomy across hospital sites, ranging from 3.7% to 88.3%. Variation in adjusted salpingectomy rates was not associated with academic affiliation or hospital size.2
Dr. Till and colleagues concluded that there was a substantial rise in risk-reducing salpingectomy from January 1, 2013, to April 30, 2015, and that there is substantial variation in the practice of salpingectomy, which is not accounted for by patient, surgeon, or hospital characteristics.2
- American College of Obstetricians and Gynecologists, Committee on Gynecologic Practice. Salpingectomy for ovarian cancer prevention. Committee Opinion No. 620 [published correction appears in: Obstet Gynecol. 2016;127(2):405]. Obstet Gynecol. 2015;125(1):279–281.
- Till SR, Edwards MG, Kobernik EK, Kamdar NS, As-Sanie S, Morgan DM. Implementation rate of risk-reducing salpingectomy at time of benign hysterectomy. Poster presented at: AAGL Global Congress of Minimally Invasive Gynecology; November 16, 2016; Orlando, Florida. J Minim Invasiv Gynecol. 2016;23(7 suppl):S1.
- American College of Obstetricians and Gynecologists, Committee on Gynecologic Practice. Salpingectomy for ovarian cancer prevention. Committee Opinion No. 620 [published correction appears in: Obstet Gynecol. 2016;127(2):405]. Obstet Gynecol. 2015;125(1):279–281.
- Till SR, Edwards MG, Kobernik EK, Kamdar NS, As-Sanie S, Morgan DM. Implementation rate of risk-reducing salpingectomy at time of benign hysterectomy. Poster presented at: AAGL Global Congress of Minimally Invasive Gynecology; November 16, 2016; Orlando, Florida. J Minim Invasiv Gynecol. 2016;23(7 suppl):S1.