User login
ASTRO guidelines lower age thresholds for APBI
The American Society for Radiation Oncology has issued new guidelines recommending accelerated partial breast irradiation brachytherapy (APBI) as an alternative to whole breast irradiation (WBI) after surgery in early-stage breast cancer patients, and lowering the age range of patients considered suitable for the procedure to people 50 and older, from 60.
With APBI, localized radiation is delivered to the region around the excised tissue, reducing treatment time and sparing healthy tissue. APBI may also be considered for patients 40 and older, according to ASTRO, if they meet all of the pathologic criteria for suitability listed in the guidelines for patients 50 and above.
The guidelines represent the first ASTRO update on APBI since 2009. In addition to expanding the age range for APBI treatment, the guidelines add low-risk ductal carcinoma in situ as an indication. The guidelines also address intraoperative radiation therapy, or IORT, in which patients receive low-energy photon or electron radiation during surgery (Pract Rad Oncol. 2016 Nov. 17 doi: 10.1016/j.prro.2016.09.007).
While IORT is suitable for patients with invasive cancer eligible for APBI, the guidelines say, patients considering this option should be counseled about the risk of recurrence compared with standard treatment, and, with photon IORT, about potential toxicity risk requiring follow-up. Though more than 40 studies were considered by the ASTRO committee, including large randomized trials comparing APBI with WBI, the new recommendations represent “more of a tweak than a revolution,” said Jay Harris, MD, of Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, one of the guideline authors.
Dr. Harris noted in an interview that two important randomized controlled trials comparing APBI and WBI are still underway, with full follow-up results expected in 2-3 years, after which more definitive recommendations can be made. For the intraoperative radiation advice contained in the guidelines, “we had evidence from two trials looking at different approaches,” Dr. Harris said. “One has long-term data using an electron beam in the operating room – this group showed that that approach seems reasonable in patients that we at ASTRO considered suitable in general for APBI. The other approach is low-dose photon radiation, for which we have only short-term follow-up, making us more hesitant to endorse it.” As for the new recommendation sanctioning APBI for ductal carcinoma, “There’s a lot of variation [in protocols] across the country, compared with invasive cancer,” Dr. Harris said. “We’re kind of all over the map with DCIS. This guideline presents another option.”
The guidelines were sponsored by ASTRO; two authors disclosed financial relationships with firms that make radiologic technology.
The American Society for Radiation Oncology has issued new guidelines recommending accelerated partial breast irradiation brachytherapy (APBI) as an alternative to whole breast irradiation (WBI) after surgery in early-stage breast cancer patients, and lowering the age range of patients considered suitable for the procedure to people 50 and older, from 60.
With APBI, localized radiation is delivered to the region around the excised tissue, reducing treatment time and sparing healthy tissue. APBI may also be considered for patients 40 and older, according to ASTRO, if they meet all of the pathologic criteria for suitability listed in the guidelines for patients 50 and above.
The guidelines represent the first ASTRO update on APBI since 2009. In addition to expanding the age range for APBI treatment, the guidelines add low-risk ductal carcinoma in situ as an indication. The guidelines also address intraoperative radiation therapy, or IORT, in which patients receive low-energy photon or electron radiation during surgery (Pract Rad Oncol. 2016 Nov. 17 doi: 10.1016/j.prro.2016.09.007).
While IORT is suitable for patients with invasive cancer eligible for APBI, the guidelines say, patients considering this option should be counseled about the risk of recurrence compared with standard treatment, and, with photon IORT, about potential toxicity risk requiring follow-up. Though more than 40 studies were considered by the ASTRO committee, including large randomized trials comparing APBI with WBI, the new recommendations represent “more of a tweak than a revolution,” said Jay Harris, MD, of Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, one of the guideline authors.
Dr. Harris noted in an interview that two important randomized controlled trials comparing APBI and WBI are still underway, with full follow-up results expected in 2-3 years, after which more definitive recommendations can be made. For the intraoperative radiation advice contained in the guidelines, “we had evidence from two trials looking at different approaches,” Dr. Harris said. “One has long-term data using an electron beam in the operating room – this group showed that that approach seems reasonable in patients that we at ASTRO considered suitable in general for APBI. The other approach is low-dose photon radiation, for which we have only short-term follow-up, making us more hesitant to endorse it.” As for the new recommendation sanctioning APBI for ductal carcinoma, “There’s a lot of variation [in protocols] across the country, compared with invasive cancer,” Dr. Harris said. “We’re kind of all over the map with DCIS. This guideline presents another option.”
The guidelines were sponsored by ASTRO; two authors disclosed financial relationships with firms that make radiologic technology.
The American Society for Radiation Oncology has issued new guidelines recommending accelerated partial breast irradiation brachytherapy (APBI) as an alternative to whole breast irradiation (WBI) after surgery in early-stage breast cancer patients, and lowering the age range of patients considered suitable for the procedure to people 50 and older, from 60.
With APBI, localized radiation is delivered to the region around the excised tissue, reducing treatment time and sparing healthy tissue. APBI may also be considered for patients 40 and older, according to ASTRO, if they meet all of the pathologic criteria for suitability listed in the guidelines for patients 50 and above.
The guidelines represent the first ASTRO update on APBI since 2009. In addition to expanding the age range for APBI treatment, the guidelines add low-risk ductal carcinoma in situ as an indication. The guidelines also address intraoperative radiation therapy, or IORT, in which patients receive low-energy photon or electron radiation during surgery (Pract Rad Oncol. 2016 Nov. 17 doi: 10.1016/j.prro.2016.09.007).
While IORT is suitable for patients with invasive cancer eligible for APBI, the guidelines say, patients considering this option should be counseled about the risk of recurrence compared with standard treatment, and, with photon IORT, about potential toxicity risk requiring follow-up. Though more than 40 studies were considered by the ASTRO committee, including large randomized trials comparing APBI with WBI, the new recommendations represent “more of a tweak than a revolution,” said Jay Harris, MD, of Brigham and Women’s Hospital and Dana-Farber Cancer Institute, Boston, one of the guideline authors.
Dr. Harris noted in an interview that two important randomized controlled trials comparing APBI and WBI are still underway, with full follow-up results expected in 2-3 years, after which more definitive recommendations can be made. For the intraoperative radiation advice contained in the guidelines, “we had evidence from two trials looking at different approaches,” Dr. Harris said. “One has long-term data using an electron beam in the operating room – this group showed that that approach seems reasonable in patients that we at ASTRO considered suitable in general for APBI. The other approach is low-dose photon radiation, for which we have only short-term follow-up, making us more hesitant to endorse it.” As for the new recommendation sanctioning APBI for ductal carcinoma, “There’s a lot of variation [in protocols] across the country, compared with invasive cancer,” Dr. Harris said. “We’re kind of all over the map with DCIS. This guideline presents another option.”
The guidelines were sponsored by ASTRO; two authors disclosed financial relationships with firms that make radiologic technology.
FROM PRACTICAL RADIATION ONCOLOGY
Newly available tissue containment system brings back power morcellation to advanced MIG surgeons
In the 11 studies that have examined the incidence of leiomyosarcoma (LMS) since the 2014 communications by the US Food and Drug Administration (FDA),1 Dr. Matthew Siedhoff and colleagues found that the cumulative LMS incidence in 318,006 women was 0.0017%, or approximately 1 in 600. This is according to data Dr. Seidhoff presented November 16, 2016, at the 45th annual AAGL Global Congress on MIGS.2 This reported risk is smaller than the 1 in 350 cited by the FDA in its 2014 notice.1 Dr. Seidhoff concluded that, particularly in women aged younger than 50 years, minimally invasive hysterectomy remains a safe option for the informed patient to consider.
Regardless of the incidence of unsuspected LMS found in the studies published since 2014, what have been the practice changes among gynecologic surgeons as a result of the FDA’s 2014 actions? Kerac N. Falk, MD, explored this question with his colleagues at the Icahn School of Medicine in New York, New York. He presented these findings to AAGL congress attendees on November 16 in Orlando, Florida.3 Notably, of 197 responders to a survey sent to members of the Society of Gynecologic Oncologists, 12.5% reported decreasing their use of power morcellation. A full 38.8% reported discontinuing altogether the use of a power morcellator.
One in 5 (20%) of gyn oncologists previously using power morcellation reported switching to laparotomy. Importantly, the drive for the switch was “driven by media, patient request, and the FDA rather than physician choice,” Falk said.
What if physicians, and patients, have another choice?
Here at the AAGL meeting, Olympus announced the FDA clearance, marketing, and initial training on its contained tissue extraction system—the PneumoLiner containment device and its accompanying laparoscopic PK Morcellator (FIGURE).
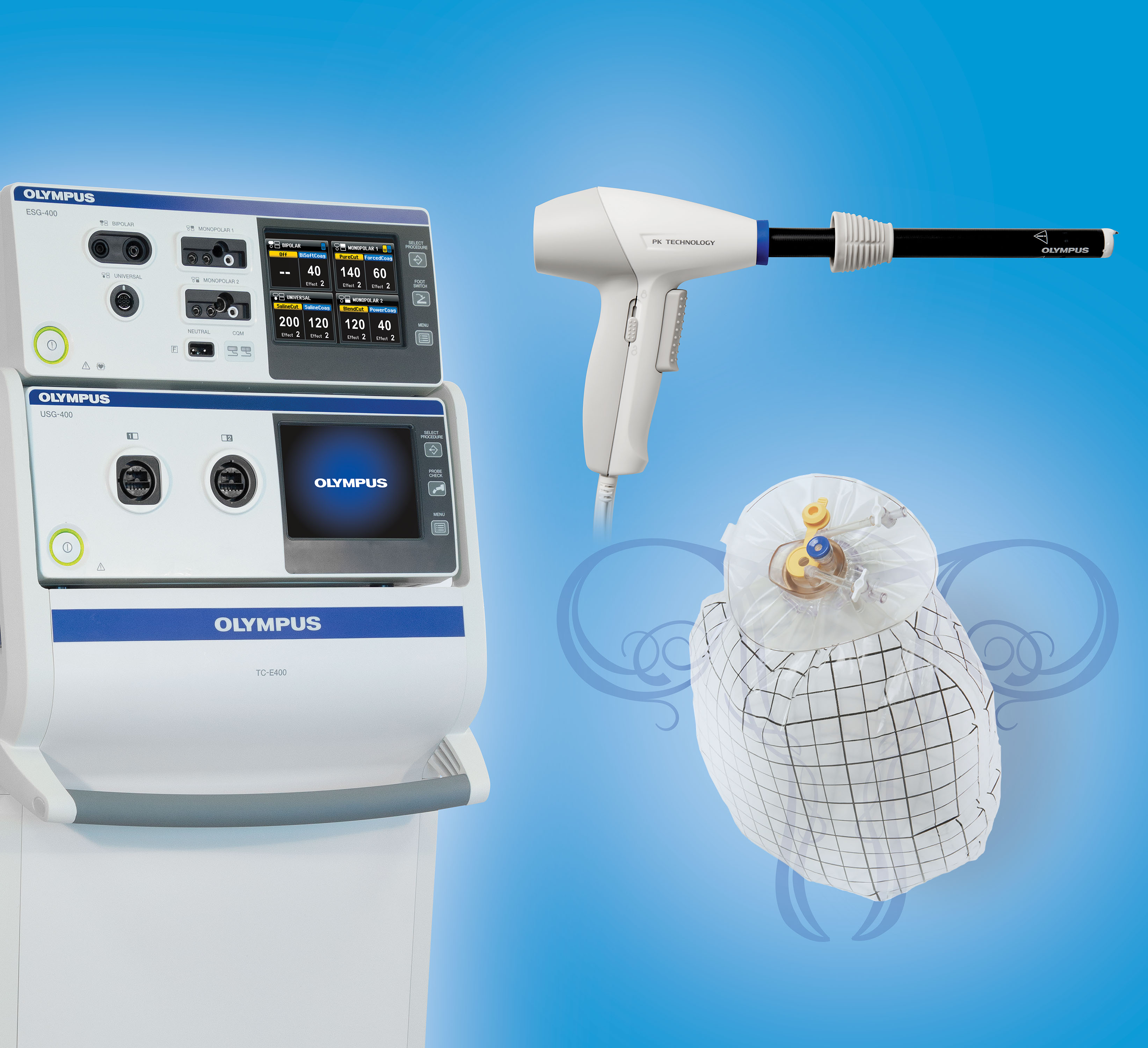
Both tools, FDA cleared in April and October 2016, respectively, provide low-risk, appropriate women with “an improved safety device,” said Jubilee Brown, MD, AAGL representative on power morcellation to the FDA and Associate Director of Gynecologic Oncology at the Levine Cancer Institute of the Carolinas HealthCare System in Charlotte, North Carolina.
“We have found at our institution that we have had to do more opens and minilaps on patients without the option for power morcellation, and this new device offers a way for us not to have to do that. Minimally invasive surgery stays truly minimally invasive surgery,” said Dr. Brown.
“Although I have a handful of patients who have chosen open surgery over minimally invasive surgery when MIS was appropriate, by and large most patients prefer the minimally invasive approach to surgery—especially when they learn that they have reduced risk for complications, blood loss, and pain; will be back to normal life faster; and will have less cosmetic incisions,” said Dr. Brown. “We all have tried workarounds, but for low-risk, appropriate patients under FDA guidelines, this new device by Olympus brings us back to minimally invasive options, and that is a good thing.”
How does the Olympus containment system work?
The PneumoLiner is the first containment device to receive FDA market clearance that is designed for use with certain laparoscopic morcellators to isolate uterine tissue that is not suspected to contain cancer.4
The containment bag and morcellator are not indicated for use in women with tissue that is known or suspected to contain malignancy; and should not be used for removal of uterine tissue containing suspected fibroids in patients who are peri- or post-menopausal, or candidates for en bloc tissue removal vaginally or via mini-laparotomy.4
When insufflated, the PneumoLiner bag allows for space and surgeon visibility throughout the power morcellation procedure while maintaining a barrier to the escape of fluids, cells, and tissue fragments. The bag creates a barrier between the targeted tissue and nontargeted abdominal contents, minimizing the risk of inadvertent damage to adjacent structures, says Olympus.4 See this video, which demonstrates the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Filtration, immersion, and stress testing were conducted to demonstrate the PneumoLiner as impermeable to human cells, that it maintains its integrity following morcellation, and that its mechanical strength withholds forces in excess of those demonstrated during actual use.5 To support FDA 510 clearance of the PK Morcellator, Olympus performed additional testing with both devices used together, demonstrating compatibility.4
Surgeon training on new device use is rigid, comprehensive, and not to be overlooked
“This is one of the rare times in my career when, right out of the gate, we have a very rigid and comprehensive way of educating and training physicians on the proper utilization of a new technology (avoiding the cart-before-the-horse phenomenon, in which a clinician is given the tools without the proper education),” said AAGL immediate past president Arnold Advincula, MD, “and it is important to underscore that.” Dr. Advincula is the Levine Family Professor, Vice-Chair of Women’s Health, and Chief of Gynecology at Sloane Women’s Hospital, Columbia University Medical Center/New York-Presbyterian Hospital, New York, New York.
Training plans
Instead of its sales force, Olympus is having surgeons train other surgeons. Clinical Education Specialists, a small, select group of individuals within the company, also will be able to train. The required training follows a rigid protocol that was validated and submitted to the FDA as part of the approval process, said Jerilyn Hitchings, Director of Procedure Marketing at Olympus. After training up to 40 surgeons in the past 3 weeks, Olympus expects to train another 100 at AAGL.
“We have strict criteria for training, including that they are advanced laparoscopists,” fulfilling a minimum number of surgeries within a 90-day period, said Hitchings.
The 1-hour training requires surgeons to perform a 4-step protocol and demonstrate unaided proficiency at training conclusion. Olympus will not sell its product to a facility until an advanced surgeon has been trained there, according to Hitchings. And many physicians are approaching their institutions inquiring about training now, she reports.
Applications for training will be submitted to the Olympus Professional Education Team for approval. “We want this to go well. We know that there are many physicians who are hoping that this becomes a good reality, and we want to make sure that it is done properly. The training process adheres to that agenda.”
Having the technology is step 1
OBG Management Board of Editors member Dr. Advincula struck an optimistic tone in his June 2016 Guest Editorial for the journal,6 indicating his belief that the “tissue morcellation pendulum,” which has swung toward non−minimally invasive approaches since 2014, can change direction. At the Olympus containment system official introduction event at AAGL, he expressed the same optimism:
“We now have technology that has the promise of being able to deliver to women a safe way to undergo tissue extraction, to undergo a minimally invasive surgical procedure. …When you combine innovation and education together, you ultimately are going to advance MIS worldwide. With all the things going on around the world today, I think it is important that women be able to have a choice, an autonomous choice with her physician. Having the technology that allows physicians to extract tissue safely, to give women the option of undergoing a minimally invasive surgery, is critically important. I look forward to seeing how we can continue to advance surgery. Partnership with clinicians and with industry, such as Olympus, is going to be key to the success of how we advance women’s health care in general.”
What do AAGL attendees have to say about it?
“It’s a good thing for the minimally invasive market,” said John B. Gebhart, MD, MS, vaginal hysterectomy representative at the star-studded Operating with the Stars event on Thursday, November 17, and Professor of Obstetrics and Gynecology at the Mayo Clinic in Rochester, Minnesota.
May Thomassee, MD, who practices in Lafayette, Louisiana, said that, for the past 3 years, she has been performing extracorporeal morcellation at her institution. “I think the new containment bag and morcellator devices that are attempting to be safer are a very good thing; however, my concern of introducing this new technology, such as bags, is that it may increase the cost within our health care system. We have had great success, and patients feel that they have options when we offer them—after informed consent and appropriate preoperative workup—an abdominal, vaginal, or extracorporeal morcellation approach.”
As a developer and proponent of the Extracorporeal Tissue Extraction (ExCITE) technique, Mireille Truong, MD, who demonstrated the ExCITE surgical approach with Dr. Advincula as part of the Research and Science Plenary here at AAGL, said, “I think the PneumoLiner is a great example of creativity and innovation. It’s good that we now have an FDA-approved device to offer and counsel patients as one of many options.” She added that, overall, the morcellation controversy has been “a positive experience. We have learned better as a community about how to approach new technology.”
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed November 17, 2016.
- Siedhoff MT, Doll KM, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation versus abdominal hysterectomy for presumed uterine leiomyomata: an updated decision analysis. J Minim Invasiv Gynecol. 2016;23(7):S4-S5.
- Mandelberger AH, Mathews S, Chuang L. Practice changes in power morcellation among gynecologic-oncologists since 2014. J Minim Invasiv Gynecol. 2016;23(7):S3.
Olympus introduces first-of-its-kind contained tissue extraction system, restoring healthcare option for gynecologists and women [press release]. November 16, 2016. Olympus website. http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=2079. Accessed November 16, 2016.
- FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. FDA website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed November 16, 2016.
- Advincula AP. Tissue extraction: can the pendulum change direction? OBG Manag. 2016;28(6):8, 10, 12.
In the 11 studies that have examined the incidence of leiomyosarcoma (LMS) since the 2014 communications by the US Food and Drug Administration (FDA),1 Dr. Matthew Siedhoff and colleagues found that the cumulative LMS incidence in 318,006 women was 0.0017%, or approximately 1 in 600. This is according to data Dr. Seidhoff presented November 16, 2016, at the 45th annual AAGL Global Congress on MIGS.2 This reported risk is smaller than the 1 in 350 cited by the FDA in its 2014 notice.1 Dr. Seidhoff concluded that, particularly in women aged younger than 50 years, minimally invasive hysterectomy remains a safe option for the informed patient to consider.
Regardless of the incidence of unsuspected LMS found in the studies published since 2014, what have been the practice changes among gynecologic surgeons as a result of the FDA’s 2014 actions? Kerac N. Falk, MD, explored this question with his colleagues at the Icahn School of Medicine in New York, New York. He presented these findings to AAGL congress attendees on November 16 in Orlando, Florida.3 Notably, of 197 responders to a survey sent to members of the Society of Gynecologic Oncologists, 12.5% reported decreasing their use of power morcellation. A full 38.8% reported discontinuing altogether the use of a power morcellator.
One in 5 (20%) of gyn oncologists previously using power morcellation reported switching to laparotomy. Importantly, the drive for the switch was “driven by media, patient request, and the FDA rather than physician choice,” Falk said.
What if physicians, and patients, have another choice?
Here at the AAGL meeting, Olympus announced the FDA clearance, marketing, and initial training on its contained tissue extraction system—the PneumoLiner containment device and its accompanying laparoscopic PK Morcellator (FIGURE).

Both tools, FDA cleared in April and October 2016, respectively, provide low-risk, appropriate women with “an improved safety device,” said Jubilee Brown, MD, AAGL representative on power morcellation to the FDA and Associate Director of Gynecologic Oncology at the Levine Cancer Institute of the Carolinas HealthCare System in Charlotte, North Carolina.
“We have found at our institution that we have had to do more opens and minilaps on patients without the option for power morcellation, and this new device offers a way for us not to have to do that. Minimally invasive surgery stays truly minimally invasive surgery,” said Dr. Brown.
“Although I have a handful of patients who have chosen open surgery over minimally invasive surgery when MIS was appropriate, by and large most patients prefer the minimally invasive approach to surgery—especially when they learn that they have reduced risk for complications, blood loss, and pain; will be back to normal life faster; and will have less cosmetic incisions,” said Dr. Brown. “We all have tried workarounds, but for low-risk, appropriate patients under FDA guidelines, this new device by Olympus brings us back to minimally invasive options, and that is a good thing.”
How does the Olympus containment system work?
The PneumoLiner is the first containment device to receive FDA market clearance that is designed for use with certain laparoscopic morcellators to isolate uterine tissue that is not suspected to contain cancer.4
The containment bag and morcellator are not indicated for use in women with tissue that is known or suspected to contain malignancy; and should not be used for removal of uterine tissue containing suspected fibroids in patients who are peri- or post-menopausal, or candidates for en bloc tissue removal vaginally or via mini-laparotomy.4
When insufflated, the PneumoLiner bag allows for space and surgeon visibility throughout the power morcellation procedure while maintaining a barrier to the escape of fluids, cells, and tissue fragments. The bag creates a barrier between the targeted tissue and nontargeted abdominal contents, minimizing the risk of inadvertent damage to adjacent structures, says Olympus.4 See this video, which demonstrates the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Filtration, immersion, and stress testing were conducted to demonstrate the PneumoLiner as impermeable to human cells, that it maintains its integrity following morcellation, and that its mechanical strength withholds forces in excess of those demonstrated during actual use.5 To support FDA 510 clearance of the PK Morcellator, Olympus performed additional testing with both devices used together, demonstrating compatibility.4
Surgeon training on new device use is rigid, comprehensive, and not to be overlooked
“This is one of the rare times in my career when, right out of the gate, we have a very rigid and comprehensive way of educating and training physicians on the proper utilization of a new technology (avoiding the cart-before-the-horse phenomenon, in which a clinician is given the tools without the proper education),” said AAGL immediate past president Arnold Advincula, MD, “and it is important to underscore that.” Dr. Advincula is the Levine Family Professor, Vice-Chair of Women’s Health, and Chief of Gynecology at Sloane Women’s Hospital, Columbia University Medical Center/New York-Presbyterian Hospital, New York, New York.
Training plans
Instead of its sales force, Olympus is having surgeons train other surgeons. Clinical Education Specialists, a small, select group of individuals within the company, also will be able to train. The required training follows a rigid protocol that was validated and submitted to the FDA as part of the approval process, said Jerilyn Hitchings, Director of Procedure Marketing at Olympus. After training up to 40 surgeons in the past 3 weeks, Olympus expects to train another 100 at AAGL.
“We have strict criteria for training, including that they are advanced laparoscopists,” fulfilling a minimum number of surgeries within a 90-day period, said Hitchings.
The 1-hour training requires surgeons to perform a 4-step protocol and demonstrate unaided proficiency at training conclusion. Olympus will not sell its product to a facility until an advanced surgeon has been trained there, according to Hitchings. And many physicians are approaching their institutions inquiring about training now, she reports.
Applications for training will be submitted to the Olympus Professional Education Team for approval. “We want this to go well. We know that there are many physicians who are hoping that this becomes a good reality, and we want to make sure that it is done properly. The training process adheres to that agenda.”
Having the technology is step 1
OBG Management Board of Editors member Dr. Advincula struck an optimistic tone in his June 2016 Guest Editorial for the journal,6 indicating his belief that the “tissue morcellation pendulum,” which has swung toward non−minimally invasive approaches since 2014, can change direction. At the Olympus containment system official introduction event at AAGL, he expressed the same optimism:
“We now have technology that has the promise of being able to deliver to women a safe way to undergo tissue extraction, to undergo a minimally invasive surgical procedure. …When you combine innovation and education together, you ultimately are going to advance MIS worldwide. With all the things going on around the world today, I think it is important that women be able to have a choice, an autonomous choice with her physician. Having the technology that allows physicians to extract tissue safely, to give women the option of undergoing a minimally invasive surgery, is critically important. I look forward to seeing how we can continue to advance surgery. Partnership with clinicians and with industry, such as Olympus, is going to be key to the success of how we advance women’s health care in general.”
What do AAGL attendees have to say about it?
“It’s a good thing for the minimally invasive market,” said John B. Gebhart, MD, MS, vaginal hysterectomy representative at the star-studded Operating with the Stars event on Thursday, November 17, and Professor of Obstetrics and Gynecology at the Mayo Clinic in Rochester, Minnesota.
May Thomassee, MD, who practices in Lafayette, Louisiana, said that, for the past 3 years, she has been performing extracorporeal morcellation at her institution. “I think the new containment bag and morcellator devices that are attempting to be safer are a very good thing; however, my concern of introducing this new technology, such as bags, is that it may increase the cost within our health care system. We have had great success, and patients feel that they have options when we offer them—after informed consent and appropriate preoperative workup—an abdominal, vaginal, or extracorporeal morcellation approach.”
As a developer and proponent of the Extracorporeal Tissue Extraction (ExCITE) technique, Mireille Truong, MD, who demonstrated the ExCITE surgical approach with Dr. Advincula as part of the Research and Science Plenary here at AAGL, said, “I think the PneumoLiner is a great example of creativity and innovation. It’s good that we now have an FDA-approved device to offer and counsel patients as one of many options.” She added that, overall, the morcellation controversy has been “a positive experience. We have learned better as a community about how to approach new technology.”
In the 11 studies that have examined the incidence of leiomyosarcoma (LMS) since the 2014 communications by the US Food and Drug Administration (FDA),1 Dr. Matthew Siedhoff and colleagues found that the cumulative LMS incidence in 318,006 women was 0.0017%, or approximately 1 in 600. This is according to data Dr. Seidhoff presented November 16, 2016, at the 45th annual AAGL Global Congress on MIGS.2 This reported risk is smaller than the 1 in 350 cited by the FDA in its 2014 notice.1 Dr. Seidhoff concluded that, particularly in women aged younger than 50 years, minimally invasive hysterectomy remains a safe option for the informed patient to consider.
Regardless of the incidence of unsuspected LMS found in the studies published since 2014, what have been the practice changes among gynecologic surgeons as a result of the FDA’s 2014 actions? Kerac N. Falk, MD, explored this question with his colleagues at the Icahn School of Medicine in New York, New York. He presented these findings to AAGL congress attendees on November 16 in Orlando, Florida.3 Notably, of 197 responders to a survey sent to members of the Society of Gynecologic Oncologists, 12.5% reported decreasing their use of power morcellation. A full 38.8% reported discontinuing altogether the use of a power morcellator.
One in 5 (20%) of gyn oncologists previously using power morcellation reported switching to laparotomy. Importantly, the drive for the switch was “driven by media, patient request, and the FDA rather than physician choice,” Falk said.
What if physicians, and patients, have another choice?
Here at the AAGL meeting, Olympus announced the FDA clearance, marketing, and initial training on its contained tissue extraction system—the PneumoLiner containment device and its accompanying laparoscopic PK Morcellator (FIGURE).

Both tools, FDA cleared in April and October 2016, respectively, provide low-risk, appropriate women with “an improved safety device,” said Jubilee Brown, MD, AAGL representative on power morcellation to the FDA and Associate Director of Gynecologic Oncology at the Levine Cancer Institute of the Carolinas HealthCare System in Charlotte, North Carolina.
“We have found at our institution that we have had to do more opens and minilaps on patients without the option for power morcellation, and this new device offers a way for us not to have to do that. Minimally invasive surgery stays truly minimally invasive surgery,” said Dr. Brown.
“Although I have a handful of patients who have chosen open surgery over minimally invasive surgery when MIS was appropriate, by and large most patients prefer the minimally invasive approach to surgery—especially when they learn that they have reduced risk for complications, blood loss, and pain; will be back to normal life faster; and will have less cosmetic incisions,” said Dr. Brown. “We all have tried workarounds, but for low-risk, appropriate patients under FDA guidelines, this new device by Olympus brings us back to minimally invasive options, and that is a good thing.”
How does the Olympus containment system work?
The PneumoLiner is the first containment device to receive FDA market clearance that is designed for use with certain laparoscopic morcellators to isolate uterine tissue that is not suspected to contain cancer.4
The containment bag and morcellator are not indicated for use in women with tissue that is known or suspected to contain malignancy; and should not be used for removal of uterine tissue containing suspected fibroids in patients who are peri- or post-menopausal, or candidates for en bloc tissue removal vaginally or via mini-laparotomy.4
When insufflated, the PneumoLiner bag allows for space and surgeon visibility throughout the power morcellation procedure while maintaining a barrier to the escape of fluids, cells, and tissue fragments. The bag creates a barrier between the targeted tissue and nontargeted abdominal contents, minimizing the risk of inadvertent damage to adjacent structures, says Olympus.4 See this video, which demonstrates the procedure.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Filtration, immersion, and stress testing were conducted to demonstrate the PneumoLiner as impermeable to human cells, that it maintains its integrity following morcellation, and that its mechanical strength withholds forces in excess of those demonstrated during actual use.5 To support FDA 510 clearance of the PK Morcellator, Olympus performed additional testing with both devices used together, demonstrating compatibility.4
Surgeon training on new device use is rigid, comprehensive, and not to be overlooked
“This is one of the rare times in my career when, right out of the gate, we have a very rigid and comprehensive way of educating and training physicians on the proper utilization of a new technology (avoiding the cart-before-the-horse phenomenon, in which a clinician is given the tools without the proper education),” said AAGL immediate past president Arnold Advincula, MD, “and it is important to underscore that.” Dr. Advincula is the Levine Family Professor, Vice-Chair of Women’s Health, and Chief of Gynecology at Sloane Women’s Hospital, Columbia University Medical Center/New York-Presbyterian Hospital, New York, New York.
Training plans
Instead of its sales force, Olympus is having surgeons train other surgeons. Clinical Education Specialists, a small, select group of individuals within the company, also will be able to train. The required training follows a rigid protocol that was validated and submitted to the FDA as part of the approval process, said Jerilyn Hitchings, Director of Procedure Marketing at Olympus. After training up to 40 surgeons in the past 3 weeks, Olympus expects to train another 100 at AAGL.
“We have strict criteria for training, including that they are advanced laparoscopists,” fulfilling a minimum number of surgeries within a 90-day period, said Hitchings.
The 1-hour training requires surgeons to perform a 4-step protocol and demonstrate unaided proficiency at training conclusion. Olympus will not sell its product to a facility until an advanced surgeon has been trained there, according to Hitchings. And many physicians are approaching their institutions inquiring about training now, she reports.
Applications for training will be submitted to the Olympus Professional Education Team for approval. “We want this to go well. We know that there are many physicians who are hoping that this becomes a good reality, and we want to make sure that it is done properly. The training process adheres to that agenda.”
Having the technology is step 1
OBG Management Board of Editors member Dr. Advincula struck an optimistic tone in his June 2016 Guest Editorial for the journal,6 indicating his belief that the “tissue morcellation pendulum,” which has swung toward non−minimally invasive approaches since 2014, can change direction. At the Olympus containment system official introduction event at AAGL, he expressed the same optimism:
“We now have technology that has the promise of being able to deliver to women a safe way to undergo tissue extraction, to undergo a minimally invasive surgical procedure. …When you combine innovation and education together, you ultimately are going to advance MIS worldwide. With all the things going on around the world today, I think it is important that women be able to have a choice, an autonomous choice with her physician. Having the technology that allows physicians to extract tissue safely, to give women the option of undergoing a minimally invasive surgery, is critically important. I look forward to seeing how we can continue to advance surgery. Partnership with clinicians and with industry, such as Olympus, is going to be key to the success of how we advance women’s health care in general.”
What do AAGL attendees have to say about it?
“It’s a good thing for the minimally invasive market,” said John B. Gebhart, MD, MS, vaginal hysterectomy representative at the star-studded Operating with the Stars event on Thursday, November 17, and Professor of Obstetrics and Gynecology at the Mayo Clinic in Rochester, Minnesota.
May Thomassee, MD, who practices in Lafayette, Louisiana, said that, for the past 3 years, she has been performing extracorporeal morcellation at her institution. “I think the new containment bag and morcellator devices that are attempting to be safer are a very good thing; however, my concern of introducing this new technology, such as bags, is that it may increase the cost within our health care system. We have had great success, and patients feel that they have options when we offer them—after informed consent and appropriate preoperative workup—an abdominal, vaginal, or extracorporeal morcellation approach.”
As a developer and proponent of the Extracorporeal Tissue Extraction (ExCITE) technique, Mireille Truong, MD, who demonstrated the ExCITE surgical approach with Dr. Advincula as part of the Research and Science Plenary here at AAGL, said, “I think the PneumoLiner is a great example of creativity and innovation. It’s good that we now have an FDA-approved device to offer and counsel patients as one of many options.” She added that, overall, the morcellation controversy has been “a positive experience. We have learned better as a community about how to approach new technology.”
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed November 17, 2016.
- Siedhoff MT, Doll KM, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation versus abdominal hysterectomy for presumed uterine leiomyomata: an updated decision analysis. J Minim Invasiv Gynecol. 2016;23(7):S4-S5.
- Mandelberger AH, Mathews S, Chuang L. Practice changes in power morcellation among gynecologic-oncologists since 2014. J Minim Invasiv Gynecol. 2016;23(7):S3.
Olympus introduces first-of-its-kind contained tissue extraction system, restoring healthcare option for gynecologists and women [press release]. November 16, 2016. Olympus website. http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=2079. Accessed November 16, 2016.
- FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. FDA website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed November 16, 2016.
- Advincula AP. Tissue extraction: can the pendulum change direction? OBG Manag. 2016;28(6):8, 10, 12.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Updated November 24, 2014. Accessed November 17, 2016.
- Siedhoff MT, Doll KM, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation versus abdominal hysterectomy for presumed uterine leiomyomata: an updated decision analysis. J Minim Invasiv Gynecol. 2016;23(7):S4-S5.
- Mandelberger AH, Mathews S, Chuang L. Practice changes in power morcellation among gynecologic-oncologists since 2014. J Minim Invasiv Gynecol. 2016;23(7):S3.
Olympus introduces first-of-its-kind contained tissue extraction system, restoring healthcare option for gynecologists and women [press release]. November 16, 2016. Olympus website. http://www.olympusamerica.com/corporate/corp_presscenter_headline.asp?pressNo=2079. Accessed November 16, 2016.
- FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. FDA website. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Updated April 7, 2016. Accessed November 16, 2016.
- Advincula AP. Tissue extraction: can the pendulum change direction? OBG Manag. 2016;28(6):8, 10, 12.
Direct-acting antiretrovirals in genotype 1 HCV patients with mental health conditions safe, well tolerated
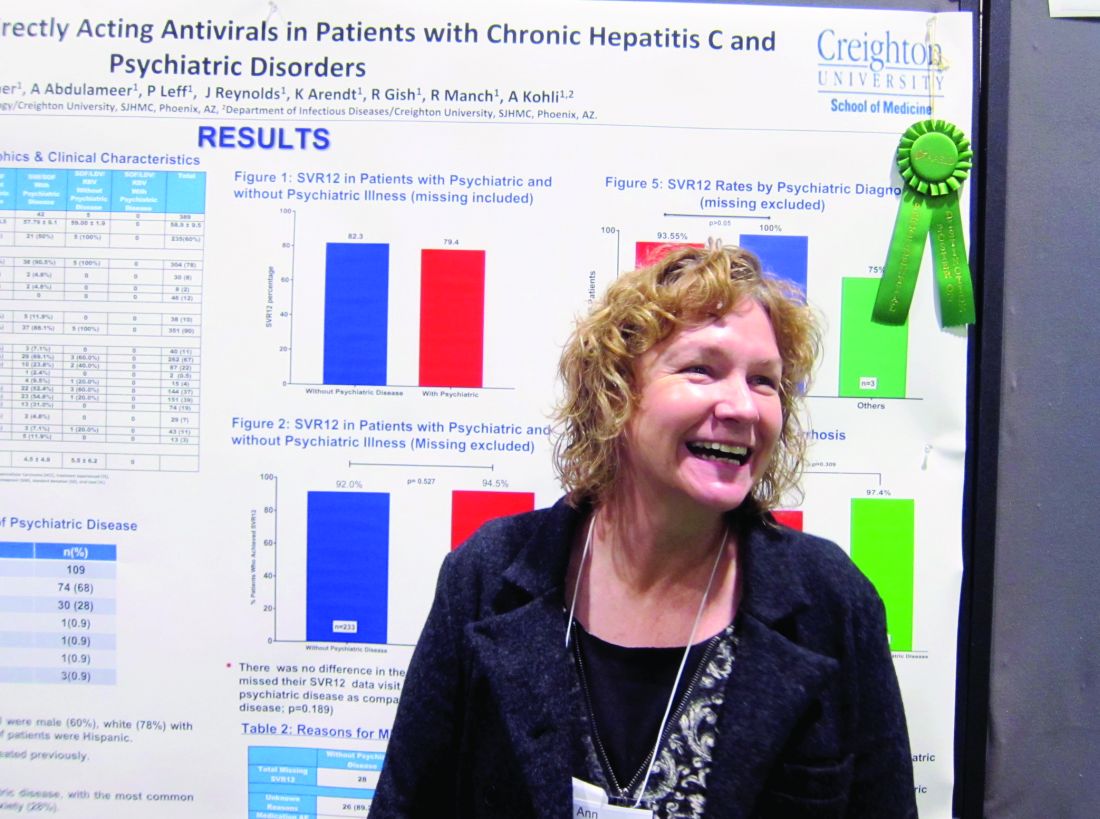
BOSTON – Direct-acting antiretroviral therapies are safe and well tolerated in hepatitis C virus patients with comorbid psychiatric conditions, a study showed.
The finding that sustained virologic response (SVR) rates at 1 year were similar in genotype 1 HCV patients with and without psychiatric comorbidities could help end the stigma against treating this patient subgroup, stemming from when treatment with interferon risked psychiatric decompensation. That’s according to nurse practitioner Anne Moore, winner of this year’s Poster of Distinction Award at the American Association for the Study of Liver Diseases annual meeting.
She and her colleagues reviewed patient records for 588 adults diagnosed with genotype 1 HCV in Arizona between 2013 and 2016. They found 389 patients who’d been treated with either a combination of sofosbuvir and ledipasvir (with or without ribavirin) or sofosbuvir and simeprevir. Patients coinfected with HIV were included in this group; those who’d had a liver transplant were not. Just over three-quarters of the patients were white, 60% were male, 10% were Hispanic, and the average age was 59 years. A third of the patients had been diagnosed with cirrhosis.
Because medical and mental health records typically are not integrated, Ms. Moore and her colleagues instead used medical records to evaluate which medications had been prescribed, in order to determine likely psychiatric diagnoses. They determined that 27% of the 389 patients had a comorbid psychiatric diagnosis. Of these, 68% had depression and 28% had anxiety. Only one person was considered to have bipolar disorder; Ms. Moore said it was possible this group was underrepresented in the study, but that some persons considered to have depression might have had bipolar disorder instead.
Rates of SVR at 1 year were similar across the study – 82.3% in those without a comorbid psychiatric diagnosis vs. 79.4% of those who did have one – with no additional risk of patients with psychiatric diagnoses being lost to follow-up. DAA therapy was well tolerated in both groups.
Ms. Moore said that winning the award was a “huge surprise” but interpreted it to mean that liver specialists are struggling to find the best treatment algorithms for HCV, partly because of the number of restrictions often placed by payers on access to DAAs, but also because it is often difficult to know who will adhere to treatment.
“Gastroenterologists and hepatologists are not used to dealing with patients who have psychiatric conditions. They don’t understand them well, and so there is a reluctance to ‘go there.’ If that’s what has been holding you back, it doesn’t have to. You can treat these patients successfully,” Ms. Moore said.
She had no relevant financial disclosures.
BOSTON – Direct-acting antiretroviral therapies are safe and well tolerated in hepatitis C virus patients with comorbid psychiatric conditions, a study showed.
The finding that sustained virologic response (SVR) rates at 1 year were similar in genotype 1 HCV patients with and without psychiatric comorbidities could help end the stigma against treating this patient subgroup, stemming from when treatment with interferon risked psychiatric decompensation. That’s according to nurse practitioner Anne Moore, winner of this year’s Poster of Distinction Award at the American Association for the Study of Liver Diseases annual meeting.
She and her colleagues reviewed patient records for 588 adults diagnosed with genotype 1 HCV in Arizona between 2013 and 2016. They found 389 patients who’d been treated with either a combination of sofosbuvir and ledipasvir (with or without ribavirin) or sofosbuvir and simeprevir. Patients coinfected with HIV were included in this group; those who’d had a liver transplant were not. Just over three-quarters of the patients were white, 60% were male, 10% were Hispanic, and the average age was 59 years. A third of the patients had been diagnosed with cirrhosis.
Because medical and mental health records typically are not integrated, Ms. Moore and her colleagues instead used medical records to evaluate which medications had been prescribed, in order to determine likely psychiatric diagnoses. They determined that 27% of the 389 patients had a comorbid psychiatric diagnosis. Of these, 68% had depression and 28% had anxiety. Only one person was considered to have bipolar disorder; Ms. Moore said it was possible this group was underrepresented in the study, but that some persons considered to have depression might have had bipolar disorder instead.
Rates of SVR at 1 year were similar across the study – 82.3% in those without a comorbid psychiatric diagnosis vs. 79.4% of those who did have one – with no additional risk of patients with psychiatric diagnoses being lost to follow-up. DAA therapy was well tolerated in both groups.
Ms. Moore said that winning the award was a “huge surprise” but interpreted it to mean that liver specialists are struggling to find the best treatment algorithms for HCV, partly because of the number of restrictions often placed by payers on access to DAAs, but also because it is often difficult to know who will adhere to treatment.
“Gastroenterologists and hepatologists are not used to dealing with patients who have psychiatric conditions. They don’t understand them well, and so there is a reluctance to ‘go there.’ If that’s what has been holding you back, it doesn’t have to. You can treat these patients successfully,” Ms. Moore said.
She had no relevant financial disclosures.
BOSTON – Direct-acting antiretroviral therapies are safe and well tolerated in hepatitis C virus patients with comorbid psychiatric conditions, a study showed.
The finding that sustained virologic response (SVR) rates at 1 year were similar in genotype 1 HCV patients with and without psychiatric comorbidities could help end the stigma against treating this patient subgroup, stemming from when treatment with interferon risked psychiatric decompensation. That’s according to nurse practitioner Anne Moore, winner of this year’s Poster of Distinction Award at the American Association for the Study of Liver Diseases annual meeting.
She and her colleagues reviewed patient records for 588 adults diagnosed with genotype 1 HCV in Arizona between 2013 and 2016. They found 389 patients who’d been treated with either a combination of sofosbuvir and ledipasvir (with or without ribavirin) or sofosbuvir and simeprevir. Patients coinfected with HIV were included in this group; those who’d had a liver transplant were not. Just over three-quarters of the patients were white, 60% were male, 10% were Hispanic, and the average age was 59 years. A third of the patients had been diagnosed with cirrhosis.
Because medical and mental health records typically are not integrated, Ms. Moore and her colleagues instead used medical records to evaluate which medications had been prescribed, in order to determine likely psychiatric diagnoses. They determined that 27% of the 389 patients had a comorbid psychiatric diagnosis. Of these, 68% had depression and 28% had anxiety. Only one person was considered to have bipolar disorder; Ms. Moore said it was possible this group was underrepresented in the study, but that some persons considered to have depression might have had bipolar disorder instead.
Rates of SVR at 1 year were similar across the study – 82.3% in those without a comorbid psychiatric diagnosis vs. 79.4% of those who did have one – with no additional risk of patients with psychiatric diagnoses being lost to follow-up. DAA therapy was well tolerated in both groups.
Ms. Moore said that winning the award was a “huge surprise” but interpreted it to mean that liver specialists are struggling to find the best treatment algorithms for HCV, partly because of the number of restrictions often placed by payers on access to DAAs, but also because it is often difficult to know who will adhere to treatment.
“Gastroenterologists and hepatologists are not used to dealing with patients who have psychiatric conditions. They don’t understand them well, and so there is a reluctance to ‘go there.’ If that’s what has been holding you back, it doesn’t have to. You can treat these patients successfully,” Ms. Moore said.
She had no relevant financial disclosures.
AT THE LIVER MEETING 2016
Key clinical point:
Major finding: Sustained virologic response rates at 1 year were similar between genotype 1 HCV patients with and without a comorbid psychiatric diagnosis.
Data source: A retrospective analysis of medical records from 2013 to 2016 for 588 adults with genotype 1 hepatitis C virus.
Disclosures: Ms. Moore had no relevant financial disclosures.
MIGS for infertility: Surgery can address QoL and pathology concerns that ART can’t, remind expert surgeons at AAGL 2016
Although patients have more assisted reproductive techniques (ART) available in recent years, management of infertility through minimally invasive surgical avenues can confront quality of life (QoL) and pathology concerns with birth rates equal to those with ART. This was a main takeaway in a packed ballroom in Orlando, Florida, at the 45th Global Congress of the AAGL. In this session, G. David Adamson, MD, brought together 3 top minimally invasive gynecologic surgeons to discuss clinical decisions in the overall and specific surgical management of: endometriomas and endometriosis, including deeply infiltrating disease; pelvic adhesions; distal tubal injury/occlusion; and proximal tubal occlusion by hysteroscopy.
Tommaso Falcone, MD, maintained that many patients (up to 85%) have pain with endometriomas, and addressing QoL for these women, with surgery versus managing their infertility only with in vitro fertilization (IVF), is an important consideration. Dr. Adamson noted that, “although there are no RCT data to guide management of endometriomas, we do have reasonable data to counsel patients on surgery versus IVF, with clinical considerations including patient age, presence of pain, and size of the endometrioma.”
Antonio Gargiulo, MD, advised attendees that when counseling patients on the role of laparoscopy in adhesiolysis to consider (1) that adhesions interfere with gamete and embryo transplant, (2) retrospective data from a small study show a positive effect of adhesiolysis in infertility, and (3) that the effect is dependent on the ASRM Adhesion Score. Regarding laparoscopy for distal tubal inclusion, he noted that case selection is important, as surgery can restore anatomic integrity but not functional integrity. In addition, he pointed out that neosalpingectomy before IVF should be considered in young women with mild hydrosalpinges when male factor infertility is present.
For proximal tubal occlusion, Dr. Gargiulo noted that hysteroscopy catheterization has diagnostic and therapeutic value, with contraindications including infection, inflammation, male factor infertility, and prior tubal surgery.
“Surgeons must offer and understand ART alternatives so that they can offer patient-centered choices,” said Dr. Gargiulo.
Finally, when does Dr. Leila Adamyan of the Federal State Institution Research Center for Obstetrics, Gynecology, and Perinatology of the V.I. Kulakov Russian Federation perform myomectomy before IVF? In the presence of:
- submucosal myoma
- myoma greater than 4 cm in size
- multiple myoma.
When sarcoma is suspected, she advises the use of endobags.
Although patients have more assisted reproductive techniques (ART) available in recent years, management of infertility through minimally invasive surgical avenues can confront quality of life (QoL) and pathology concerns with birth rates equal to those with ART. This was a main takeaway in a packed ballroom in Orlando, Florida, at the 45th Global Congress of the AAGL. In this session, G. David Adamson, MD, brought together 3 top minimally invasive gynecologic surgeons to discuss clinical decisions in the overall and specific surgical management of: endometriomas and endometriosis, including deeply infiltrating disease; pelvic adhesions; distal tubal injury/occlusion; and proximal tubal occlusion by hysteroscopy.
Tommaso Falcone, MD, maintained that many patients (up to 85%) have pain with endometriomas, and addressing QoL for these women, with surgery versus managing their infertility only with in vitro fertilization (IVF), is an important consideration. Dr. Adamson noted that, “although there are no RCT data to guide management of endometriomas, we do have reasonable data to counsel patients on surgery versus IVF, with clinical considerations including patient age, presence of pain, and size of the endometrioma.”
Antonio Gargiulo, MD, advised attendees that when counseling patients on the role of laparoscopy in adhesiolysis to consider (1) that adhesions interfere with gamete and embryo transplant, (2) retrospective data from a small study show a positive effect of adhesiolysis in infertility, and (3) that the effect is dependent on the ASRM Adhesion Score. Regarding laparoscopy for distal tubal inclusion, he noted that case selection is important, as surgery can restore anatomic integrity but not functional integrity. In addition, he pointed out that neosalpingectomy before IVF should be considered in young women with mild hydrosalpinges when male factor infertility is present.
For proximal tubal occlusion, Dr. Gargiulo noted that hysteroscopy catheterization has diagnostic and therapeutic value, with contraindications including infection, inflammation, male factor infertility, and prior tubal surgery.
“Surgeons must offer and understand ART alternatives so that they can offer patient-centered choices,” said Dr. Gargiulo.
Finally, when does Dr. Leila Adamyan of the Federal State Institution Research Center for Obstetrics, Gynecology, and Perinatology of the V.I. Kulakov Russian Federation perform myomectomy before IVF? In the presence of:
- submucosal myoma
- myoma greater than 4 cm in size
- multiple myoma.
When sarcoma is suspected, she advises the use of endobags.
Although patients have more assisted reproductive techniques (ART) available in recent years, management of infertility through minimally invasive surgical avenues can confront quality of life (QoL) and pathology concerns with birth rates equal to those with ART. This was a main takeaway in a packed ballroom in Orlando, Florida, at the 45th Global Congress of the AAGL. In this session, G. David Adamson, MD, brought together 3 top minimally invasive gynecologic surgeons to discuss clinical decisions in the overall and specific surgical management of: endometriomas and endometriosis, including deeply infiltrating disease; pelvic adhesions; distal tubal injury/occlusion; and proximal tubal occlusion by hysteroscopy.
Tommaso Falcone, MD, maintained that many patients (up to 85%) have pain with endometriomas, and addressing QoL for these women, with surgery versus managing their infertility only with in vitro fertilization (IVF), is an important consideration. Dr. Adamson noted that, “although there are no RCT data to guide management of endometriomas, we do have reasonable data to counsel patients on surgery versus IVF, with clinical considerations including patient age, presence of pain, and size of the endometrioma.”
Antonio Gargiulo, MD, advised attendees that when counseling patients on the role of laparoscopy in adhesiolysis to consider (1) that adhesions interfere with gamete and embryo transplant, (2) retrospective data from a small study show a positive effect of adhesiolysis in infertility, and (3) that the effect is dependent on the ASRM Adhesion Score. Regarding laparoscopy for distal tubal inclusion, he noted that case selection is important, as surgery can restore anatomic integrity but not functional integrity. In addition, he pointed out that neosalpingectomy before IVF should be considered in young women with mild hydrosalpinges when male factor infertility is present.
For proximal tubal occlusion, Dr. Gargiulo noted that hysteroscopy catheterization has diagnostic and therapeutic value, with contraindications including infection, inflammation, male factor infertility, and prior tubal surgery.
“Surgeons must offer and understand ART alternatives so that they can offer patient-centered choices,” said Dr. Gargiulo.
Finally, when does Dr. Leila Adamyan of the Federal State Institution Research Center for Obstetrics, Gynecology, and Perinatology of the V.I. Kulakov Russian Federation perform myomectomy before IVF? In the presence of:
- submucosal myoma
- myoma greater than 4 cm in size
- multiple myoma.
When sarcoma is suspected, she advises the use of endobags.
Distinguishing early puberty from pathology
SAN FRANCISCO – You have a female patient come in with apparent breast development but no dark pubic hair – and she’s 7 years old. Is it a case of early puberty, a warning sign to test for possible conditions, or an unremarkable departure from typical development that does not require any intervention?
The answer to situations such as these varies, explained Dennis Styne, MD, professor of pediatrics, and Yocha Dehe Endowed Chair in Pediatric Endocrinology, at the University of California, Davis.
“We don’t know why puberty begins when it does even though we know many of the controlling factors,” Dr. Styne said at the annual meeting of the American Academy of Pediatrics, but it’s important to understand when “early” is so early that you should order lab evaluations, as opposed to simply letting an outlier’s body development continue as it would.
Normal puberty
Dr. Styne reviewed the Tanner stages of puberty for girls’ breast and pubic hair development and boys’ genital and pubic hair development, noting that the classic lower ages of pubertal onset are age 8 years in girls and 9 years in boys. Yet the normal curve may actually start earlier than those ages for U.S. girls, he noted. He shared the results of a 1997 Pediatrics study of 17,077 girls, in which by age 7 years, more than a quarter of black girls (27%) and 7% of white girls had reached at least Tanner stage 2. At age 8, nearly half of black girls (48%) and 15% of white girls had reached at least stage 2 (Pediatrics. 1997 Apr;99[4]:505-12).
Further, breast development, menarche, and early pubic hair development (pubarche) all occur earlier with increased body mass index, which has been increasing among children overall. Another study identified earlier breast development without increased body mass index: Stage 2 development occurred an average 10 months earlier in girls in 2006 than in 1991, regardless of BMI, even though no difference in LH or FSH levels occurred at these ages and estradiol level was even lower. The authors of that Danish study concluded some other factors besides pubertal hormones had to account for the increasingly earlier breast development in girls. Endocrine-disrupting chemicals are a possible cause.
Similarly, puberty in boys is occurring a bit earlier, but less dramatically so: A 2012 study of 4,131 boys found that 5.75% of black boys, 0.54% of white boys, and 1.16% of Hispanic boys had stage 2 pubic hair development by age 6. Meanwhile, 10.9% of black boys, 2% of white boys, and 2.5% of Hispanic boys began puberty with stage 2 pubic hair development at age 8 (Pediatrics 2012;130:e1058-68).
But the boys differ in one key way from the girls: Boys with obesity tend to begin puberty later than those with normal or overweight BMIs, even though overweight boys begin puberty earlier than those with normal weights.
This leaves age 8 years as a normal age to begin puberty in boys but leaves the ages for girls’ start less certain – perhaps 7 years for white girls and 6 years for black girls – but still controversial.
When to be concerned
Various neurotransmitters in the central nervous system control puberty by suppression during childhood, until a trigger for onset occurs that remains mysterious. But gene mutations, such as MKRN3 in girls, as well as brain tumors or trauma, can remove that disinhibition, prompting further investigation. Brain tumors causing precocious puberty are more common in boys than girls.
Rapid growth and bone age advancement, elevated serum levels of sex steroids, and breast development in girls could all indicate precocious puberty. If these signs are accompanied by a rise in gonadotropin values to pubertal levels, early central precocious puberty, which follows the normal course of puberty except that it is earlier, is likely.
With both sex steroids and gonadotropins in the pubertal range, a GnRH agonist could be used to control gonadal steroid production and stop bone age advancement, allowing children to reach a greater adult height if started before age 6 years. If the early puberty is slowly progressing and more subtle, no treatment at all may be necessary if there are no pathologic findings.
Without proper testing, however, a physician might as well be guessing at the cause of the early development.
“You need a highly sensitive assay, and you need pediatric standards, so you’ll probably have to send blood samples out to a national laboratory,” Dr. Styne said.
If sex hormones are being secreted at a higher rate with suppression of gonadotropins, the source is most likely autonomous secretion by the gonads or the adrenal glands.
“If you see a boy with precious puberty, with testes that are not as big as they should be for the pubertal testosterone levels, it could be that the source is the adrenal glands,” Dr. Styne said.
Meanwhile, about 75% of boys will have gynecomastia to some degree during puberty, likely because of a subtle early pubertal imbalance between estrogen and testosterone, Dr. Styne said. The condition usually regresses, but “if it doesn’t regress, there’s a chance scar tissue will develop and remain, leading to the need for surgical correction. Klinefelter syndrome must be ruled out in cases of gynecomastia or, alternatively, rarer cases of disorders of sexual development.
Dr. Styne reported that he had no relevant financial disclosures.
SAN FRANCISCO – You have a female patient come in with apparent breast development but no dark pubic hair – and she’s 7 years old. Is it a case of early puberty, a warning sign to test for possible conditions, or an unremarkable departure from typical development that does not require any intervention?
The answer to situations such as these varies, explained Dennis Styne, MD, professor of pediatrics, and Yocha Dehe Endowed Chair in Pediatric Endocrinology, at the University of California, Davis.
“We don’t know why puberty begins when it does even though we know many of the controlling factors,” Dr. Styne said at the annual meeting of the American Academy of Pediatrics, but it’s important to understand when “early” is so early that you should order lab evaluations, as opposed to simply letting an outlier’s body development continue as it would.
Normal puberty
Dr. Styne reviewed the Tanner stages of puberty for girls’ breast and pubic hair development and boys’ genital and pubic hair development, noting that the classic lower ages of pubertal onset are age 8 years in girls and 9 years in boys. Yet the normal curve may actually start earlier than those ages for U.S. girls, he noted. He shared the results of a 1997 Pediatrics study of 17,077 girls, in which by age 7 years, more than a quarter of black girls (27%) and 7% of white girls had reached at least Tanner stage 2. At age 8, nearly half of black girls (48%) and 15% of white girls had reached at least stage 2 (Pediatrics. 1997 Apr;99[4]:505-12).
Further, breast development, menarche, and early pubic hair development (pubarche) all occur earlier with increased body mass index, which has been increasing among children overall. Another study identified earlier breast development without increased body mass index: Stage 2 development occurred an average 10 months earlier in girls in 2006 than in 1991, regardless of BMI, even though no difference in LH or FSH levels occurred at these ages and estradiol level was even lower. The authors of that Danish study concluded some other factors besides pubertal hormones had to account for the increasingly earlier breast development in girls. Endocrine-disrupting chemicals are a possible cause.
Similarly, puberty in boys is occurring a bit earlier, but less dramatically so: A 2012 study of 4,131 boys found that 5.75% of black boys, 0.54% of white boys, and 1.16% of Hispanic boys had stage 2 pubic hair development by age 6. Meanwhile, 10.9% of black boys, 2% of white boys, and 2.5% of Hispanic boys began puberty with stage 2 pubic hair development at age 8 (Pediatrics 2012;130:e1058-68).
But the boys differ in one key way from the girls: Boys with obesity tend to begin puberty later than those with normal or overweight BMIs, even though overweight boys begin puberty earlier than those with normal weights.
This leaves age 8 years as a normal age to begin puberty in boys but leaves the ages for girls’ start less certain – perhaps 7 years for white girls and 6 years for black girls – but still controversial.
When to be concerned
Various neurotransmitters in the central nervous system control puberty by suppression during childhood, until a trigger for onset occurs that remains mysterious. But gene mutations, such as MKRN3 in girls, as well as brain tumors or trauma, can remove that disinhibition, prompting further investigation. Brain tumors causing precocious puberty are more common in boys than girls.
Rapid growth and bone age advancement, elevated serum levels of sex steroids, and breast development in girls could all indicate precocious puberty. If these signs are accompanied by a rise in gonadotropin values to pubertal levels, early central precocious puberty, which follows the normal course of puberty except that it is earlier, is likely.
With both sex steroids and gonadotropins in the pubertal range, a GnRH agonist could be used to control gonadal steroid production and stop bone age advancement, allowing children to reach a greater adult height if started before age 6 years. If the early puberty is slowly progressing and more subtle, no treatment at all may be necessary if there are no pathologic findings.
Without proper testing, however, a physician might as well be guessing at the cause of the early development.
“You need a highly sensitive assay, and you need pediatric standards, so you’ll probably have to send blood samples out to a national laboratory,” Dr. Styne said.
If sex hormones are being secreted at a higher rate with suppression of gonadotropins, the source is most likely autonomous secretion by the gonads or the adrenal glands.
“If you see a boy with precious puberty, with testes that are not as big as they should be for the pubertal testosterone levels, it could be that the source is the adrenal glands,” Dr. Styne said.
Meanwhile, about 75% of boys will have gynecomastia to some degree during puberty, likely because of a subtle early pubertal imbalance between estrogen and testosterone, Dr. Styne said. The condition usually regresses, but “if it doesn’t regress, there’s a chance scar tissue will develop and remain, leading to the need for surgical correction. Klinefelter syndrome must be ruled out in cases of gynecomastia or, alternatively, rarer cases of disorders of sexual development.
Dr. Styne reported that he had no relevant financial disclosures.
SAN FRANCISCO – You have a female patient come in with apparent breast development but no dark pubic hair – and she’s 7 years old. Is it a case of early puberty, a warning sign to test for possible conditions, or an unremarkable departure from typical development that does not require any intervention?
The answer to situations such as these varies, explained Dennis Styne, MD, professor of pediatrics, and Yocha Dehe Endowed Chair in Pediatric Endocrinology, at the University of California, Davis.
“We don’t know why puberty begins when it does even though we know many of the controlling factors,” Dr. Styne said at the annual meeting of the American Academy of Pediatrics, but it’s important to understand when “early” is so early that you should order lab evaluations, as opposed to simply letting an outlier’s body development continue as it would.
Normal puberty
Dr. Styne reviewed the Tanner stages of puberty for girls’ breast and pubic hair development and boys’ genital and pubic hair development, noting that the classic lower ages of pubertal onset are age 8 years in girls and 9 years in boys. Yet the normal curve may actually start earlier than those ages for U.S. girls, he noted. He shared the results of a 1997 Pediatrics study of 17,077 girls, in which by age 7 years, more than a quarter of black girls (27%) and 7% of white girls had reached at least Tanner stage 2. At age 8, nearly half of black girls (48%) and 15% of white girls had reached at least stage 2 (Pediatrics. 1997 Apr;99[4]:505-12).
Further, breast development, menarche, and early pubic hair development (pubarche) all occur earlier with increased body mass index, which has been increasing among children overall. Another study identified earlier breast development without increased body mass index: Stage 2 development occurred an average 10 months earlier in girls in 2006 than in 1991, regardless of BMI, even though no difference in LH or FSH levels occurred at these ages and estradiol level was even lower. The authors of that Danish study concluded some other factors besides pubertal hormones had to account for the increasingly earlier breast development in girls. Endocrine-disrupting chemicals are a possible cause.
Similarly, puberty in boys is occurring a bit earlier, but less dramatically so: A 2012 study of 4,131 boys found that 5.75% of black boys, 0.54% of white boys, and 1.16% of Hispanic boys had stage 2 pubic hair development by age 6. Meanwhile, 10.9% of black boys, 2% of white boys, and 2.5% of Hispanic boys began puberty with stage 2 pubic hair development at age 8 (Pediatrics 2012;130:e1058-68).
But the boys differ in one key way from the girls: Boys with obesity tend to begin puberty later than those with normal or overweight BMIs, even though overweight boys begin puberty earlier than those with normal weights.
This leaves age 8 years as a normal age to begin puberty in boys but leaves the ages for girls’ start less certain – perhaps 7 years for white girls and 6 years for black girls – but still controversial.
When to be concerned
Various neurotransmitters in the central nervous system control puberty by suppression during childhood, until a trigger for onset occurs that remains mysterious. But gene mutations, such as MKRN3 in girls, as well as brain tumors or trauma, can remove that disinhibition, prompting further investigation. Brain tumors causing precocious puberty are more common in boys than girls.
Rapid growth and bone age advancement, elevated serum levels of sex steroids, and breast development in girls could all indicate precocious puberty. If these signs are accompanied by a rise in gonadotropin values to pubertal levels, early central precocious puberty, which follows the normal course of puberty except that it is earlier, is likely.
With both sex steroids and gonadotropins in the pubertal range, a GnRH agonist could be used to control gonadal steroid production and stop bone age advancement, allowing children to reach a greater adult height if started before age 6 years. If the early puberty is slowly progressing and more subtle, no treatment at all may be necessary if there are no pathologic findings.
Without proper testing, however, a physician might as well be guessing at the cause of the early development.
“You need a highly sensitive assay, and you need pediatric standards, so you’ll probably have to send blood samples out to a national laboratory,” Dr. Styne said.
If sex hormones are being secreted at a higher rate with suppression of gonadotropins, the source is most likely autonomous secretion by the gonads or the adrenal glands.
“If you see a boy with precious puberty, with testes that are not as big as they should be for the pubertal testosterone levels, it could be that the source is the adrenal glands,” Dr. Styne said.
Meanwhile, about 75% of boys will have gynecomastia to some degree during puberty, likely because of a subtle early pubertal imbalance between estrogen and testosterone, Dr. Styne said. The condition usually regresses, but “if it doesn’t regress, there’s a chance scar tissue will develop and remain, leading to the need for surgical correction. Klinefelter syndrome must be ruled out in cases of gynecomastia or, alternatively, rarer cases of disorders of sexual development.
Dr. Styne reported that he had no relevant financial disclosures.
AT AAP 16
Guselkumab appears safe, effective for psoriatic arthritis
WASHINGTON – The investigational anti-interleukin-23 monoclonal antibody guselkumab was safe and well tolerated, and demonstrated significant improvement on a variety of measures in patients with active psoriatic arthritis in a randomized, placebo-controlled phase IIa study.
The primary endpoint of ACR20 at 24 weeks was achieved in 58% of 100 patients randomized to receive treatment with guselkumab versus 18.4% of 49 patients who received placebo, Atul A. Deodhar, MD, reported in a late-breaking abstract presentation at the annual meeting of the American College of Rheumatology.
For example, PASI75 response at 24 weeks was 78.6% for guselkumab and 12.5% for placebo, and the ACR50 response at 24 weeks was 34% vs. 10.2%, respectively. The HAQ-DI score mean change from baseline was –0.42 vs. –0.06, respectively.
Further, ACR20 response was seen as early as week 4 (21% vs. 0% for placebo), and the effect increased over time, reaching the maximum effect by week 16 (60% vs. 16.3%), Dr. Deodhar said.
Subjects in the double-blind, multicenter study had active psoriatic arthritis and at least 3% body surface area of plaque psoriasis despite treatment with standard-of-care therapies. Patients with prior exposure to anti–tumor necrosis factor–alpha agents were included. They received either 100 mg guselkumab delivered subcutaneously or placebo at week 0 and 4, then every 8 weeks thereafter through week 44.
Patients with less than 5% improvement in swollen and tender joint count at week 16 were allowed early escape to open-label ustekinumab (Stelara), and at week 24 all remaining patients crossed over to receive guselkumab, which was repeated at week 28 and every 8 weeks thereafter through week 44.
Treatment was well tolerated; the proportion of patients with one or more adverse events was similar in the treatment and placebo groups (36% and 32.7%, respectively), with infections being the most common adverse event, occurring in 17% and 20.4% of patients in the groups, respectively.
“And even though 10% and 14% of the patients received systemic treatment, no patient received [intravenous] antibiotics,” he said.
Severe adverse events occurred in two patients – one in each group – and included a knee injury and myocardial infarction. Specific information about which group these occurred in is not available as the study is ongoing and investigators remain blinded, Dr. Deodhar noted.
No serious infections, malignancies, or deaths occurred through week 24, he said.
“Guselkumab is the first anti-IL23 biologic to demonstrate efficacy in psoriatic arthritis. In patients with active psoriatic arthritis ... treatment with guselkumab shows significant improvements in joint symptoms, physical function, psoriasis, enthesitis, dactylitis, and quality of life,” he concluded, adding that there were no unexpected safety findings in this population.
On Nov. 17, Janssen Biotech, Inc. announced the submission of a Biologics License Application to the Food and Drug Administration seeking approval of guselkumab for the treatment of adults with moderate to severe plaque psoriasis based on findings from prior studies for that indication.
The current study was sponsored by Janssen Research & Development. Dr. Deodhar reported financial relationships with AbbVie, Amgen, Boehringer Ingelheim, Janssen, Novartis, Pfizer, and UCB. Two other authors also reported financial ties to industry, including Janssen. Four of the seven authors of the abstract were employees of Janssen.
WASHINGTON – The investigational anti-interleukin-23 monoclonal antibody guselkumab was safe and well tolerated, and demonstrated significant improvement on a variety of measures in patients with active psoriatic arthritis in a randomized, placebo-controlled phase IIa study.
The primary endpoint of ACR20 at 24 weeks was achieved in 58% of 100 patients randomized to receive treatment with guselkumab versus 18.4% of 49 patients who received placebo, Atul A. Deodhar, MD, reported in a late-breaking abstract presentation at the annual meeting of the American College of Rheumatology.
For example, PASI75 response at 24 weeks was 78.6% for guselkumab and 12.5% for placebo, and the ACR50 response at 24 weeks was 34% vs. 10.2%, respectively. The HAQ-DI score mean change from baseline was –0.42 vs. –0.06, respectively.
Further, ACR20 response was seen as early as week 4 (21% vs. 0% for placebo), and the effect increased over time, reaching the maximum effect by week 16 (60% vs. 16.3%), Dr. Deodhar said.
Subjects in the double-blind, multicenter study had active psoriatic arthritis and at least 3% body surface area of plaque psoriasis despite treatment with standard-of-care therapies. Patients with prior exposure to anti–tumor necrosis factor–alpha agents were included. They received either 100 mg guselkumab delivered subcutaneously or placebo at week 0 and 4, then every 8 weeks thereafter through week 44.
Patients with less than 5% improvement in swollen and tender joint count at week 16 were allowed early escape to open-label ustekinumab (Stelara), and at week 24 all remaining patients crossed over to receive guselkumab, which was repeated at week 28 and every 8 weeks thereafter through week 44.
Treatment was well tolerated; the proportion of patients with one or more adverse events was similar in the treatment and placebo groups (36% and 32.7%, respectively), with infections being the most common adverse event, occurring in 17% and 20.4% of patients in the groups, respectively.
“And even though 10% and 14% of the patients received systemic treatment, no patient received [intravenous] antibiotics,” he said.
Severe adverse events occurred in two patients – one in each group – and included a knee injury and myocardial infarction. Specific information about which group these occurred in is not available as the study is ongoing and investigators remain blinded, Dr. Deodhar noted.
No serious infections, malignancies, or deaths occurred through week 24, he said.
“Guselkumab is the first anti-IL23 biologic to demonstrate efficacy in psoriatic arthritis. In patients with active psoriatic arthritis ... treatment with guselkumab shows significant improvements in joint symptoms, physical function, psoriasis, enthesitis, dactylitis, and quality of life,” he concluded, adding that there were no unexpected safety findings in this population.
On Nov. 17, Janssen Biotech, Inc. announced the submission of a Biologics License Application to the Food and Drug Administration seeking approval of guselkumab for the treatment of adults with moderate to severe plaque psoriasis based on findings from prior studies for that indication.
The current study was sponsored by Janssen Research & Development. Dr. Deodhar reported financial relationships with AbbVie, Amgen, Boehringer Ingelheim, Janssen, Novartis, Pfizer, and UCB. Two other authors also reported financial ties to industry, including Janssen. Four of the seven authors of the abstract were employees of Janssen.
WASHINGTON – The investigational anti-interleukin-23 monoclonal antibody guselkumab was safe and well tolerated, and demonstrated significant improvement on a variety of measures in patients with active psoriatic arthritis in a randomized, placebo-controlled phase IIa study.
The primary endpoint of ACR20 at 24 weeks was achieved in 58% of 100 patients randomized to receive treatment with guselkumab versus 18.4% of 49 patients who received placebo, Atul A. Deodhar, MD, reported in a late-breaking abstract presentation at the annual meeting of the American College of Rheumatology.
For example, PASI75 response at 24 weeks was 78.6% for guselkumab and 12.5% for placebo, and the ACR50 response at 24 weeks was 34% vs. 10.2%, respectively. The HAQ-DI score mean change from baseline was –0.42 vs. –0.06, respectively.
Further, ACR20 response was seen as early as week 4 (21% vs. 0% for placebo), and the effect increased over time, reaching the maximum effect by week 16 (60% vs. 16.3%), Dr. Deodhar said.
Subjects in the double-blind, multicenter study had active psoriatic arthritis and at least 3% body surface area of plaque psoriasis despite treatment with standard-of-care therapies. Patients with prior exposure to anti–tumor necrosis factor–alpha agents were included. They received either 100 mg guselkumab delivered subcutaneously or placebo at week 0 and 4, then every 8 weeks thereafter through week 44.
Patients with less than 5% improvement in swollen and tender joint count at week 16 were allowed early escape to open-label ustekinumab (Stelara), and at week 24 all remaining patients crossed over to receive guselkumab, which was repeated at week 28 and every 8 weeks thereafter through week 44.
Treatment was well tolerated; the proportion of patients with one or more adverse events was similar in the treatment and placebo groups (36% and 32.7%, respectively), with infections being the most common adverse event, occurring in 17% and 20.4% of patients in the groups, respectively.
“And even though 10% and 14% of the patients received systemic treatment, no patient received [intravenous] antibiotics,” he said.
Severe adverse events occurred in two patients – one in each group – and included a knee injury and myocardial infarction. Specific information about which group these occurred in is not available as the study is ongoing and investigators remain blinded, Dr. Deodhar noted.
No serious infections, malignancies, or deaths occurred through week 24, he said.
“Guselkumab is the first anti-IL23 biologic to demonstrate efficacy in psoriatic arthritis. In patients with active psoriatic arthritis ... treatment with guselkumab shows significant improvements in joint symptoms, physical function, psoriasis, enthesitis, dactylitis, and quality of life,” he concluded, adding that there were no unexpected safety findings in this population.
On Nov. 17, Janssen Biotech, Inc. announced the submission of a Biologics License Application to the Food and Drug Administration seeking approval of guselkumab for the treatment of adults with moderate to severe plaque psoriasis based on findings from prior studies for that indication.
The current study was sponsored by Janssen Research & Development. Dr. Deodhar reported financial relationships with AbbVie, Amgen, Boehringer Ingelheim, Janssen, Novartis, Pfizer, and UCB. Two other authors also reported financial ties to industry, including Janssen. Four of the seven authors of the abstract were employees of Janssen.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: ACR20 at 24 weeks was achieved in 58% of 100 patients randomized to receive treatment with guselkumab vs. 18.4% of 49 patients who received placebo.
Data source: A randomized, placebo-controlled phase IIa study of 149 patients.
Disclosures: The current study was sponsored by Janssen Research & Development. Dr. Deodhar reported financial relationships with AbbVie, Amgen, Boehringer Ingelheim, Janssen, Novartis, Pfizer, and UCB. Two other authors also reported financial ties to industry, including Janssen. Four of the seven authors of the abstract were employees of Janssen.
FDA approves vaginal insert to treat dyspareunia in menopause
Prasterone (Intrarosa), a vaginal insert containing dehydroepiandrosterone (DHEA) to treat dyspareunia in menopause caused by vulvar and vaginal atrophy, has won Food and Drug Administration approval.
It’s the first FDA-approved product containing the active ingredient prasterone, known also as DHEA. It will be marketed by Endoceutics Inc., a Quebec-based pharmaceutical company focused on women’s health.
The approval is based on the results of two 12-week placebo-controlled trials of 406 healthy, postmenopausal women, ranging in age from 40 to 80 years, who identified dyspareunia as their most bothersome symptom of VVA. During the trials, prasterone reduced the severity of pain experienced during sexual intercourse, when compared with placebo. Safety of the treatment was established in four 12-week placebo-controlled trials and one 52-week open-label trial. The most common adverse events were vaginal discharge and abnormal Pap smear, according to the FDA.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Prasterone (Intrarosa), a vaginal insert containing dehydroepiandrosterone (DHEA) to treat dyspareunia in menopause caused by vulvar and vaginal atrophy, has won Food and Drug Administration approval.
It’s the first FDA-approved product containing the active ingredient prasterone, known also as DHEA. It will be marketed by Endoceutics Inc., a Quebec-based pharmaceutical company focused on women’s health.
The approval is based on the results of two 12-week placebo-controlled trials of 406 healthy, postmenopausal women, ranging in age from 40 to 80 years, who identified dyspareunia as their most bothersome symptom of VVA. During the trials, prasterone reduced the severity of pain experienced during sexual intercourse, when compared with placebo. Safety of the treatment was established in four 12-week placebo-controlled trials and one 52-week open-label trial. The most common adverse events were vaginal discharge and abnormal Pap smear, according to the FDA.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Prasterone (Intrarosa), a vaginal insert containing dehydroepiandrosterone (DHEA) to treat dyspareunia in menopause caused by vulvar and vaginal atrophy, has won Food and Drug Administration approval.
It’s the first FDA-approved product containing the active ingredient prasterone, known also as DHEA. It will be marketed by Endoceutics Inc., a Quebec-based pharmaceutical company focused on women’s health.
The approval is based on the results of two 12-week placebo-controlled trials of 406 healthy, postmenopausal women, ranging in age from 40 to 80 years, who identified dyspareunia as their most bothersome symptom of VVA. During the trials, prasterone reduced the severity of pain experienced during sexual intercourse, when compared with placebo. Safety of the treatment was established in four 12-week placebo-controlled trials and one 52-week open-label trial. The most common adverse events were vaginal discharge and abnormal Pap smear, according to the FDA.
mschneider@frontlinemedcom.com
On Twitter @maryellenny
Fewer Zika-infected pregnancies reported for second week in a row
The number of new Zika cases reported among pregnant women in the United States dropped for the second week in a row, according to data from the Centers for Disease Control and Prevention.
For the week ending Nov. 10, there were 124 new cases of pregnant women with laboratory evidence of Zika virus infection reported to the CDC: 30 in the states and the District of Columbia and 94 in the U.S. territories. The previous week (Nov. 3), the number of new cases was 146, which came on the heels of a new weekly high of 288 for the week of Oct. 27.
The total number of pregnant women with Zika now stands at 3,538 for the year: 1,087 for the states and 2,451 for the territories, the CDC said.
Among all Americans, there have been 36,323 cases of Zika reported to the CDC: 4,255 have occurred in the states/D.C. and 32,068 in the territories. About 98% of territorial cases have occurred in Puerto Rico, the CDC said.
No new cases of infants with Zika-related birth defects were reported for the week ending Nov. 10, so the totals hold at 26 infants born with Zika-related birth defects and five Zika-related pregnancy losses, according to the CDC.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of new Zika cases reported among pregnant women in the United States dropped for the second week in a row, according to data from the Centers for Disease Control and Prevention.
For the week ending Nov. 10, there were 124 new cases of pregnant women with laboratory evidence of Zika virus infection reported to the CDC: 30 in the states and the District of Columbia and 94 in the U.S. territories. The previous week (Nov. 3), the number of new cases was 146, which came on the heels of a new weekly high of 288 for the week of Oct. 27.
The total number of pregnant women with Zika now stands at 3,538 for the year: 1,087 for the states and 2,451 for the territories, the CDC said.
Among all Americans, there have been 36,323 cases of Zika reported to the CDC: 4,255 have occurred in the states/D.C. and 32,068 in the territories. About 98% of territorial cases have occurred in Puerto Rico, the CDC said.
No new cases of infants with Zika-related birth defects were reported for the week ending Nov. 10, so the totals hold at 26 infants born with Zika-related birth defects and five Zika-related pregnancy losses, according to the CDC.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
The number of new Zika cases reported among pregnant women in the United States dropped for the second week in a row, according to data from the Centers for Disease Control and Prevention.
For the week ending Nov. 10, there were 124 new cases of pregnant women with laboratory evidence of Zika virus infection reported to the CDC: 30 in the states and the District of Columbia and 94 in the U.S. territories. The previous week (Nov. 3), the number of new cases was 146, which came on the heels of a new weekly high of 288 for the week of Oct. 27.
The total number of pregnant women with Zika now stands at 3,538 for the year: 1,087 for the states and 2,451 for the territories, the CDC said.
Among all Americans, there have been 36,323 cases of Zika reported to the CDC: 4,255 have occurred in the states/D.C. and 32,068 in the territories. About 98% of territorial cases have occurred in Puerto Rico, the CDC said.
No new cases of infants with Zika-related birth defects were reported for the week ending Nov. 10, so the totals hold at 26 infants born with Zika-related birth defects and five Zika-related pregnancy losses, according to the CDC.
The CDC is no longer reporting adverse pregnancy outcomes for the territories because Puerto Rico is not using the same “inclusion criteria to monitor brain abnormalities and other adverse pregnancy outcomes.” As of Sept. 29 – the date of the last territorial report – there had been one liveborn infant and one pregnancy loss related to Zika.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
ACR 2015 Workforce Study: Fewer rheumatologists, more patients, and the struggle to bridge the gap
WASHINGTON – The mass retirement of Baby Boomer workhorses, the changing face of a Millennial workforce, and the graying of America will deliver a triple-whammy to rheumatology over the next 15 years: By 2030, the United States will be short 4,700 full-time rheumatologists – just about as many as are currently in practice.
“This is drastic,” Marcy Bolster, MD, said at the annual meeting of the American College of Rheumatology. “We need to take action now or we will not be able to meet the expected increases in patient demand.”
Dr. Bolster, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston, was one of 10 clinicians who discussed the ACR’s massive new project, “The 2015 Workforce Study of Rheumatology Specialists in the United States,” a comprehensive assessment of the current supply of rheumatologists in this country, and a sobering prediction of how many will be needed in the future.
Released at the ACR meeting in Washington, the 2015 survey is the first that ACR has conducted since 2005. The project drew on a number of sources: questionnaires sent out to the entire ACR membership; published research, position papers, and government reports; the Institute of Medicine; and state licensure and National Resident Matching Program data. Four online questionnaires surveyed not only rheumatology clinicians and fellows, but other health professionals, adult and young patients with rheumatic diseases, and the parents of pediatric patients.
The survey response rate of 31% among practicing rheumatologists was not as high as the Committee on Rheumatology Training and Workforce Issues would have liked, said Daniel Battafarano, DO, a study cochair. But fellows had a 95% response rate, offering the study a very solid look at the near future of the specialty.
The 2015 results were a striking contrast to the 2005 report, which examined supply and demand up to 2025. It came to a brighter conclusion: that a relative balance would be maintained. Not so now.
The reasons for this projected imbalance are not overly complicated, and are actually recapitulated in other areas of medicine, said Dr. Battafarano, chief of rheumatology at San Antonio (Tex.) Military Medical Center. Baby Boomer senior physicians are tired of working 70 hours a week, and moving toward retirement in unprecedented numbers. In fact, according to the report, 50% of today’s full-time rheumatologists will retire within the next 15 years, and 80% of those plan to cut back their workload by about 25%.
It’s not that new blood isn’t coming into the profession. In fact, Dr. Bolster said during her presentation, the percentage of internal medicine residents moving into rheumatology will stay stable, at about 4%. But the number isn’t projected to increase as patient demand does, and these new doctors will look and practice very differently than the new doctors of 30 or 40 years ago.
“The majority of medical school graduates now – about 60% – are women,” Dr. Battafarano said. “And they are entering the workforce at a very challenging time of life, simultaneously building careers and families.”
Studies have also demonstrated that women have different practice patterns than men. They tend to spend more time with patients, so they sometimes see fewer in a day. In fact, in its supply assessment, the ACR study characterized women rheumatologists as 0.7 of a full-time equivalent position – an important factor in the projected supply gap.
But the projected workforce shortage does not hinge on the practice patterns of women rheumatologists, Dr. Battafarano cautioned. Part of it is based on his own generation of work-a-holism. In general, young physicians now place more importance on a healthy work-life balance than did his generation, and this he sees as a healthy way to approach a new career.
“I’ll admit it: Baby Boomers are dysfunctionally working too hard, and they miss the importance of a health work/life balance. That’s changing, and that’s a good thing.”
His protégé, Katrina Lawrence-Wolff, DO, agrees. A second-year rheumatology fellow, Dr. Lawrence-Wolff is also expecting her first child. She presented the breakout results focusing on the adult rheumatologist supply/demand picture.
“None of us think working 80 hours a week is a good idea for us personally, or a good way to give good medical care to patients,” she said in an interview. “We would rather have more time with family, more time for volunteering in our community, or to do advocacy work. We want to be flexible, and we do understand that this attitude may change the level of our reimbursement. But we are willing to forgo some salary to become a happier, better-balanced person.”
International medical graduates also affect this snapshot of the future, Dr. Bolster said during her lecture. More than half of new medical graduates are international students, and 17% of those intend to leave the United States after their education is complete.
Shortages will affect all regions, but some more than others
All of these issues are now converging at a time when patient demand is expected to soar. In the United States, about 22.5 million adults and 300,000 children have a rheumatic disease. According to the study, that number will increase by 61% by 2030.
Dr. Lawrence-Wolff used population statistics to really put the numbers needed to care for these patients into perspective. Studies in the United States, Canada, and Europe generally agree that the ideal rheumatologist:patient ratio is somewhere around 2 per 100,000 adults. “In 2005, when we were felt to be balanced in this way, our ratio was 1.67/100,000 patients.”
This ratio will look very different by 2025, she said, and the regional imbalances already seen will be magnified. These regional differences aren’t a surprise, Dr. Lawrence-Wolff noted. They directly reflect the density of academic rheumatology training centers. “Most practicing rheumatologists tend to stay in the region where they received their training,” she said, “so we have more clinicians in areas with more academic centers.”
For example, the Northeast U.S. hosts a highly enriched rheumatologist population, with a rheumatologist:patient ratio of 3.7/100,000. By 2025, this will be reduced to 1.6/100,000 – still acceptable, but more than a 50% decrease.
That same decrease will be much more drastically felt in regions that already have a paucity of rheumatologists. In the Southwest, the current ratio is 1.2/100,000; in 10 years, this will be 0.64/100,000. Even areas that are moderately well supplied now will suffer. Both the Northwest and North Central regions have a ratio of about 1.6/100,000. Both those regions will sink into the range of 0.6-0.5/100,000.
“All regions are going to decline below the 1.67 threshold by 2030,” she said. “Every single one.”
Troubles for pediatric rheumatology workforce
If things are concerning in the world of adult rheumatology, they’re downright alarming in the world of pediatric rheumatology, said Dr. Battafarano, who broke out the pediatric subspecialty results.
These clinicians are already scarce, with only 287 currently practicing full-time in 2015. By 2030, 461 will be needed, but the supply is expected to drop to 231.
The pediatric supply problem starts much earlier in the academic process, he said. For years, about 50% of the slots of pediatric rheumatology fellows have gone unfilled. In this world, recruitment woes will drive supply problems more than retirement. “As a whole, pediatric rheumatologists are younger,” Dr. Battafarano said, with only 32% planning to retire by 2030.
“Recruitment into adult rheumatology isn’t a problem, but on the pediatric side, it’s been very hard to recruit. This isn’t unique to rheumatology; it’s being seen in other pediatric subspecialties as well.”
Reimbursement and work overload are at the root of it, he believes.
“It translates pretty well to income. Reimbursement for chronic diseases, like what we see, is predominately Medicaid. The reimbursement rate and income for subspecialty pediatrics is definitely lower than it is in other academic subspecialties. And I have to think that time spent observing an exhausted, overworked physician isn’t helpful either.”
The mandatory 3-year pediatric rheumatology fellowship may also be a deal killer for some potential recruits, Dr. Battafarano said. The prevailing thought has always been to include a year of academic research in the pediatric track, which extends it beyond the 2-year fellowship that adult rheumatologists experience.
“So you marry the 3-year fellowship with workload, quality of life, student debt, and income and you get a combination that’s just less appealing than some other areas.”
Adjusting that fellowship track is one way to potentially improve the pediatric supply picture, he said. “One of the things I recommended is adding a 2-year clinical track. There are ways to do research that can be folded into a clinical setting that wouldn’t require an entire year. The majority of adult fellowships are 2 years with concurrent research, so there is already a precedent.”
In fact, Dr. Lawrence-Wolff is doing just that, he said. “She will do clinical research that’s integrated into her practice, but her primary role is to learn to be a clinical rheumatologist.”
Ideas for stretching rheumatologic care
Dr. Battafarano had some other practical suggestions for improving recruitment into the field. “We have to offer some incentives. I’d like to see us exploring the potential of loan repayment and visa programs.”
Overall, expanding the musculoskeletal expertise of primary care providers should also be on the table, Dr. Lawrence-Wolff said. “It’s possible that we can help our primary care colleagues extend rheumatology care by treating things like osteoarthritis and gout.”
Rheumatologists also can’t afford to ignore the expanding-role of mid-level providers, she said. “We would like to recruit more nurse practitioners and physician assistants into the specialty. The numbers we hope will go up, even if the percentage remains the same as it is, about 2%-5%.”
Skillfully leveraging these clinicians’ strengths will be the key to successfully employing them.
“We see different ways to utilize them to stretch our care. One suggestion is having them in the clinic to see more patients per day, but also to use them to see lower-level patients so the rheumatologist can take care of the more complex cases.”
They could also serve patients who have multiple regularly scheduled checkups for chronic illness. “If you have a patient who needs to be seen four times a year, the NP or PA can see that person, check the labs and determine if the patent is stable, and doesn’t really need to see the rheumatologist.”
Technology will invariably come into play as well, Dr. Battafarano said.
“We envision this as a multipronged approach that includes telehealth and ‘E-consults,’ although we don’t precisely know what that will look like. But other specialties – and primary care as well – are going through very similar trends here. We are all talking about working with other providers to reach more patients, and telemedicine is a key area of investigation. We really are all in the same boat.”
Finally, Dr. Battafarano urges his fellow senior clinicians to consider severing professional ties gradually. “It’s not just a dearth of bodies we’re facing, but a sudden depletion of valuable experience and clinical wisdom. In my practice, for example, the three of us each have more than 30 years’ experience. That’s close to a century of experience, and two of them want to retire. It’s such a brain-drain on a terrific practice to lose our colleagues overnight.”
For some reason, he said, the locum tenens model has never really caught on in rheumatology, and he’d like to see that idea explored and embraced. It’s a perfect way to keep experienced hands in the mix, both seeing patients and mentoring young rheumatologists, he added.
“Even if we’re in our 60s and 70s, we’re not brain-dead yet. A lot of us want to keep contributing, just not full-time.”
None of the clinicians quoted in this article had any relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The mass retirement of Baby Boomer workhorses, the changing face of a Millennial workforce, and the graying of America will deliver a triple-whammy to rheumatology over the next 15 years: By 2030, the United States will be short 4,700 full-time rheumatologists – just about as many as are currently in practice.
“This is drastic,” Marcy Bolster, MD, said at the annual meeting of the American College of Rheumatology. “We need to take action now or we will not be able to meet the expected increases in patient demand.”
Dr. Bolster, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston, was one of 10 clinicians who discussed the ACR’s massive new project, “The 2015 Workforce Study of Rheumatology Specialists in the United States,” a comprehensive assessment of the current supply of rheumatologists in this country, and a sobering prediction of how many will be needed in the future.
Released at the ACR meeting in Washington, the 2015 survey is the first that ACR has conducted since 2005. The project drew on a number of sources: questionnaires sent out to the entire ACR membership; published research, position papers, and government reports; the Institute of Medicine; and state licensure and National Resident Matching Program data. Four online questionnaires surveyed not only rheumatology clinicians and fellows, but other health professionals, adult and young patients with rheumatic diseases, and the parents of pediatric patients.
The survey response rate of 31% among practicing rheumatologists was not as high as the Committee on Rheumatology Training and Workforce Issues would have liked, said Daniel Battafarano, DO, a study cochair. But fellows had a 95% response rate, offering the study a very solid look at the near future of the specialty.
The 2015 results were a striking contrast to the 2005 report, which examined supply and demand up to 2025. It came to a brighter conclusion: that a relative balance would be maintained. Not so now.
The reasons for this projected imbalance are not overly complicated, and are actually recapitulated in other areas of medicine, said Dr. Battafarano, chief of rheumatology at San Antonio (Tex.) Military Medical Center. Baby Boomer senior physicians are tired of working 70 hours a week, and moving toward retirement in unprecedented numbers. In fact, according to the report, 50% of today’s full-time rheumatologists will retire within the next 15 years, and 80% of those plan to cut back their workload by about 25%.
It’s not that new blood isn’t coming into the profession. In fact, Dr. Bolster said during her presentation, the percentage of internal medicine residents moving into rheumatology will stay stable, at about 4%. But the number isn’t projected to increase as patient demand does, and these new doctors will look and practice very differently than the new doctors of 30 or 40 years ago.
“The majority of medical school graduates now – about 60% – are women,” Dr. Battafarano said. “And they are entering the workforce at a very challenging time of life, simultaneously building careers and families.”
Studies have also demonstrated that women have different practice patterns than men. They tend to spend more time with patients, so they sometimes see fewer in a day. In fact, in its supply assessment, the ACR study characterized women rheumatologists as 0.7 of a full-time equivalent position – an important factor in the projected supply gap.
But the projected workforce shortage does not hinge on the practice patterns of women rheumatologists, Dr. Battafarano cautioned. Part of it is based on his own generation of work-a-holism. In general, young physicians now place more importance on a healthy work-life balance than did his generation, and this he sees as a healthy way to approach a new career.
“I’ll admit it: Baby Boomers are dysfunctionally working too hard, and they miss the importance of a health work/life balance. That’s changing, and that’s a good thing.”
His protégé, Katrina Lawrence-Wolff, DO, agrees. A second-year rheumatology fellow, Dr. Lawrence-Wolff is also expecting her first child. She presented the breakout results focusing on the adult rheumatologist supply/demand picture.
“None of us think working 80 hours a week is a good idea for us personally, or a good way to give good medical care to patients,” she said in an interview. “We would rather have more time with family, more time for volunteering in our community, or to do advocacy work. We want to be flexible, and we do understand that this attitude may change the level of our reimbursement. But we are willing to forgo some salary to become a happier, better-balanced person.”
International medical graduates also affect this snapshot of the future, Dr. Bolster said during her lecture. More than half of new medical graduates are international students, and 17% of those intend to leave the United States after their education is complete.
Shortages will affect all regions, but some more than others
All of these issues are now converging at a time when patient demand is expected to soar. In the United States, about 22.5 million adults and 300,000 children have a rheumatic disease. According to the study, that number will increase by 61% by 2030.
Dr. Lawrence-Wolff used population statistics to really put the numbers needed to care for these patients into perspective. Studies in the United States, Canada, and Europe generally agree that the ideal rheumatologist:patient ratio is somewhere around 2 per 100,000 adults. “In 2005, when we were felt to be balanced in this way, our ratio was 1.67/100,000 patients.”
This ratio will look very different by 2025, she said, and the regional imbalances already seen will be magnified. These regional differences aren’t a surprise, Dr. Lawrence-Wolff noted. They directly reflect the density of academic rheumatology training centers. “Most practicing rheumatologists tend to stay in the region where they received their training,” she said, “so we have more clinicians in areas with more academic centers.”
For example, the Northeast U.S. hosts a highly enriched rheumatologist population, with a rheumatologist:patient ratio of 3.7/100,000. By 2025, this will be reduced to 1.6/100,000 – still acceptable, but more than a 50% decrease.
That same decrease will be much more drastically felt in regions that already have a paucity of rheumatologists. In the Southwest, the current ratio is 1.2/100,000; in 10 years, this will be 0.64/100,000. Even areas that are moderately well supplied now will suffer. Both the Northwest and North Central regions have a ratio of about 1.6/100,000. Both those regions will sink into the range of 0.6-0.5/100,000.
“All regions are going to decline below the 1.67 threshold by 2030,” she said. “Every single one.”
Troubles for pediatric rheumatology workforce
If things are concerning in the world of adult rheumatology, they’re downright alarming in the world of pediatric rheumatology, said Dr. Battafarano, who broke out the pediatric subspecialty results.
These clinicians are already scarce, with only 287 currently practicing full-time in 2015. By 2030, 461 will be needed, but the supply is expected to drop to 231.
The pediatric supply problem starts much earlier in the academic process, he said. For years, about 50% of the slots of pediatric rheumatology fellows have gone unfilled. In this world, recruitment woes will drive supply problems more than retirement. “As a whole, pediatric rheumatologists are younger,” Dr. Battafarano said, with only 32% planning to retire by 2030.
“Recruitment into adult rheumatology isn’t a problem, but on the pediatric side, it’s been very hard to recruit. This isn’t unique to rheumatology; it’s being seen in other pediatric subspecialties as well.”
Reimbursement and work overload are at the root of it, he believes.
“It translates pretty well to income. Reimbursement for chronic diseases, like what we see, is predominately Medicaid. The reimbursement rate and income for subspecialty pediatrics is definitely lower than it is in other academic subspecialties. And I have to think that time spent observing an exhausted, overworked physician isn’t helpful either.”
The mandatory 3-year pediatric rheumatology fellowship may also be a deal killer for some potential recruits, Dr. Battafarano said. The prevailing thought has always been to include a year of academic research in the pediatric track, which extends it beyond the 2-year fellowship that adult rheumatologists experience.
“So you marry the 3-year fellowship with workload, quality of life, student debt, and income and you get a combination that’s just less appealing than some other areas.”
Adjusting that fellowship track is one way to potentially improve the pediatric supply picture, he said. “One of the things I recommended is adding a 2-year clinical track. There are ways to do research that can be folded into a clinical setting that wouldn’t require an entire year. The majority of adult fellowships are 2 years with concurrent research, so there is already a precedent.”
In fact, Dr. Lawrence-Wolff is doing just that, he said. “She will do clinical research that’s integrated into her practice, but her primary role is to learn to be a clinical rheumatologist.”
Ideas for stretching rheumatologic care
Dr. Battafarano had some other practical suggestions for improving recruitment into the field. “We have to offer some incentives. I’d like to see us exploring the potential of loan repayment and visa programs.”
Overall, expanding the musculoskeletal expertise of primary care providers should also be on the table, Dr. Lawrence-Wolff said. “It’s possible that we can help our primary care colleagues extend rheumatology care by treating things like osteoarthritis and gout.”
Rheumatologists also can’t afford to ignore the expanding-role of mid-level providers, she said. “We would like to recruit more nurse practitioners and physician assistants into the specialty. The numbers we hope will go up, even if the percentage remains the same as it is, about 2%-5%.”
Skillfully leveraging these clinicians’ strengths will be the key to successfully employing them.
“We see different ways to utilize them to stretch our care. One suggestion is having them in the clinic to see more patients per day, but also to use them to see lower-level patients so the rheumatologist can take care of the more complex cases.”
They could also serve patients who have multiple regularly scheduled checkups for chronic illness. “If you have a patient who needs to be seen four times a year, the NP or PA can see that person, check the labs and determine if the patent is stable, and doesn’t really need to see the rheumatologist.”
Technology will invariably come into play as well, Dr. Battafarano said.
“We envision this as a multipronged approach that includes telehealth and ‘E-consults,’ although we don’t precisely know what that will look like. But other specialties – and primary care as well – are going through very similar trends here. We are all talking about working with other providers to reach more patients, and telemedicine is a key area of investigation. We really are all in the same boat.”
Finally, Dr. Battafarano urges his fellow senior clinicians to consider severing professional ties gradually. “It’s not just a dearth of bodies we’re facing, but a sudden depletion of valuable experience and clinical wisdom. In my practice, for example, the three of us each have more than 30 years’ experience. That’s close to a century of experience, and two of them want to retire. It’s such a brain-drain on a terrific practice to lose our colleagues overnight.”
For some reason, he said, the locum tenens model has never really caught on in rheumatology, and he’d like to see that idea explored and embraced. It’s a perfect way to keep experienced hands in the mix, both seeing patients and mentoring young rheumatologists, he added.
“Even if we’re in our 60s and 70s, we’re not brain-dead yet. A lot of us want to keep contributing, just not full-time.”
None of the clinicians quoted in this article had any relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – The mass retirement of Baby Boomer workhorses, the changing face of a Millennial workforce, and the graying of America will deliver a triple-whammy to rheumatology over the next 15 years: By 2030, the United States will be short 4,700 full-time rheumatologists – just about as many as are currently in practice.
“This is drastic,” Marcy Bolster, MD, said at the annual meeting of the American College of Rheumatology. “We need to take action now or we will not be able to meet the expected increases in patient demand.”
Dr. Bolster, director of the rheumatology fellowship training program at Massachusetts General Hospital, Boston, was one of 10 clinicians who discussed the ACR’s massive new project, “The 2015 Workforce Study of Rheumatology Specialists in the United States,” a comprehensive assessment of the current supply of rheumatologists in this country, and a sobering prediction of how many will be needed in the future.
Released at the ACR meeting in Washington, the 2015 survey is the first that ACR has conducted since 2005. The project drew on a number of sources: questionnaires sent out to the entire ACR membership; published research, position papers, and government reports; the Institute of Medicine; and state licensure and National Resident Matching Program data. Four online questionnaires surveyed not only rheumatology clinicians and fellows, but other health professionals, adult and young patients with rheumatic diseases, and the parents of pediatric patients.
The survey response rate of 31% among practicing rheumatologists was not as high as the Committee on Rheumatology Training and Workforce Issues would have liked, said Daniel Battafarano, DO, a study cochair. But fellows had a 95% response rate, offering the study a very solid look at the near future of the specialty.
The 2015 results were a striking contrast to the 2005 report, which examined supply and demand up to 2025. It came to a brighter conclusion: that a relative balance would be maintained. Not so now.
The reasons for this projected imbalance are not overly complicated, and are actually recapitulated in other areas of medicine, said Dr. Battafarano, chief of rheumatology at San Antonio (Tex.) Military Medical Center. Baby Boomer senior physicians are tired of working 70 hours a week, and moving toward retirement in unprecedented numbers. In fact, according to the report, 50% of today’s full-time rheumatologists will retire within the next 15 years, and 80% of those plan to cut back their workload by about 25%.
It’s not that new blood isn’t coming into the profession. In fact, Dr. Bolster said during her presentation, the percentage of internal medicine residents moving into rheumatology will stay stable, at about 4%. But the number isn’t projected to increase as patient demand does, and these new doctors will look and practice very differently than the new doctors of 30 or 40 years ago.
“The majority of medical school graduates now – about 60% – are women,” Dr. Battafarano said. “And they are entering the workforce at a very challenging time of life, simultaneously building careers and families.”
Studies have also demonstrated that women have different practice patterns than men. They tend to spend more time with patients, so they sometimes see fewer in a day. In fact, in its supply assessment, the ACR study characterized women rheumatologists as 0.7 of a full-time equivalent position – an important factor in the projected supply gap.
But the projected workforce shortage does not hinge on the practice patterns of women rheumatologists, Dr. Battafarano cautioned. Part of it is based on his own generation of work-a-holism. In general, young physicians now place more importance on a healthy work-life balance than did his generation, and this he sees as a healthy way to approach a new career.
“I’ll admit it: Baby Boomers are dysfunctionally working too hard, and they miss the importance of a health work/life balance. That’s changing, and that’s a good thing.”
His protégé, Katrina Lawrence-Wolff, DO, agrees. A second-year rheumatology fellow, Dr. Lawrence-Wolff is also expecting her first child. She presented the breakout results focusing on the adult rheumatologist supply/demand picture.
“None of us think working 80 hours a week is a good idea for us personally, or a good way to give good medical care to patients,” she said in an interview. “We would rather have more time with family, more time for volunteering in our community, or to do advocacy work. We want to be flexible, and we do understand that this attitude may change the level of our reimbursement. But we are willing to forgo some salary to become a happier, better-balanced person.”
International medical graduates also affect this snapshot of the future, Dr. Bolster said during her lecture. More than half of new medical graduates are international students, and 17% of those intend to leave the United States after their education is complete.
Shortages will affect all regions, but some more than others
All of these issues are now converging at a time when patient demand is expected to soar. In the United States, about 22.5 million adults and 300,000 children have a rheumatic disease. According to the study, that number will increase by 61% by 2030.
Dr. Lawrence-Wolff used population statistics to really put the numbers needed to care for these patients into perspective. Studies in the United States, Canada, and Europe generally agree that the ideal rheumatologist:patient ratio is somewhere around 2 per 100,000 adults. “In 2005, when we were felt to be balanced in this way, our ratio was 1.67/100,000 patients.”
This ratio will look very different by 2025, she said, and the regional imbalances already seen will be magnified. These regional differences aren’t a surprise, Dr. Lawrence-Wolff noted. They directly reflect the density of academic rheumatology training centers. “Most practicing rheumatologists tend to stay in the region where they received their training,” she said, “so we have more clinicians in areas with more academic centers.”
For example, the Northeast U.S. hosts a highly enriched rheumatologist population, with a rheumatologist:patient ratio of 3.7/100,000. By 2025, this will be reduced to 1.6/100,000 – still acceptable, but more than a 50% decrease.
That same decrease will be much more drastically felt in regions that already have a paucity of rheumatologists. In the Southwest, the current ratio is 1.2/100,000; in 10 years, this will be 0.64/100,000. Even areas that are moderately well supplied now will suffer. Both the Northwest and North Central regions have a ratio of about 1.6/100,000. Both those regions will sink into the range of 0.6-0.5/100,000.
“All regions are going to decline below the 1.67 threshold by 2030,” she said. “Every single one.”
Troubles for pediatric rheumatology workforce
If things are concerning in the world of adult rheumatology, they’re downright alarming in the world of pediatric rheumatology, said Dr. Battafarano, who broke out the pediatric subspecialty results.
These clinicians are already scarce, with only 287 currently practicing full-time in 2015. By 2030, 461 will be needed, but the supply is expected to drop to 231.
The pediatric supply problem starts much earlier in the academic process, he said. For years, about 50% of the slots of pediatric rheumatology fellows have gone unfilled. In this world, recruitment woes will drive supply problems more than retirement. “As a whole, pediatric rheumatologists are younger,” Dr. Battafarano said, with only 32% planning to retire by 2030.
“Recruitment into adult rheumatology isn’t a problem, but on the pediatric side, it’s been very hard to recruit. This isn’t unique to rheumatology; it’s being seen in other pediatric subspecialties as well.”
Reimbursement and work overload are at the root of it, he believes.
“It translates pretty well to income. Reimbursement for chronic diseases, like what we see, is predominately Medicaid. The reimbursement rate and income for subspecialty pediatrics is definitely lower than it is in other academic subspecialties. And I have to think that time spent observing an exhausted, overworked physician isn’t helpful either.”
The mandatory 3-year pediatric rheumatology fellowship may also be a deal killer for some potential recruits, Dr. Battafarano said. The prevailing thought has always been to include a year of academic research in the pediatric track, which extends it beyond the 2-year fellowship that adult rheumatologists experience.
“So you marry the 3-year fellowship with workload, quality of life, student debt, and income and you get a combination that’s just less appealing than some other areas.”
Adjusting that fellowship track is one way to potentially improve the pediatric supply picture, he said. “One of the things I recommended is adding a 2-year clinical track. There are ways to do research that can be folded into a clinical setting that wouldn’t require an entire year. The majority of adult fellowships are 2 years with concurrent research, so there is already a precedent.”
In fact, Dr. Lawrence-Wolff is doing just that, he said. “She will do clinical research that’s integrated into her practice, but her primary role is to learn to be a clinical rheumatologist.”
Ideas for stretching rheumatologic care
Dr. Battafarano had some other practical suggestions for improving recruitment into the field. “We have to offer some incentives. I’d like to see us exploring the potential of loan repayment and visa programs.”
Overall, expanding the musculoskeletal expertise of primary care providers should also be on the table, Dr. Lawrence-Wolff said. “It’s possible that we can help our primary care colleagues extend rheumatology care by treating things like osteoarthritis and gout.”
Rheumatologists also can’t afford to ignore the expanding-role of mid-level providers, she said. “We would like to recruit more nurse practitioners and physician assistants into the specialty. The numbers we hope will go up, even if the percentage remains the same as it is, about 2%-5%.”
Skillfully leveraging these clinicians’ strengths will be the key to successfully employing them.
“We see different ways to utilize them to stretch our care. One suggestion is having them in the clinic to see more patients per day, but also to use them to see lower-level patients so the rheumatologist can take care of the more complex cases.”
They could also serve patients who have multiple regularly scheduled checkups for chronic illness. “If you have a patient who needs to be seen four times a year, the NP or PA can see that person, check the labs and determine if the patent is stable, and doesn’t really need to see the rheumatologist.”
Technology will invariably come into play as well, Dr. Battafarano said.
“We envision this as a multipronged approach that includes telehealth and ‘E-consults,’ although we don’t precisely know what that will look like. But other specialties – and primary care as well – are going through very similar trends here. We are all talking about working with other providers to reach more patients, and telemedicine is a key area of investigation. We really are all in the same boat.”
Finally, Dr. Battafarano urges his fellow senior clinicians to consider severing professional ties gradually. “It’s not just a dearth of bodies we’re facing, but a sudden depletion of valuable experience and clinical wisdom. In my practice, for example, the three of us each have more than 30 years’ experience. That’s close to a century of experience, and two of them want to retire. It’s such a brain-drain on a terrific practice to lose our colleagues overnight.”
For some reason, he said, the locum tenens model has never really caught on in rheumatology, and he’d like to see that idea explored and embraced. It’s a perfect way to keep experienced hands in the mix, both seeing patients and mentoring young rheumatologists, he added.
“Even if we’re in our 60s and 70s, we’re not brain-dead yet. A lot of us want to keep contributing, just not full-time.”
None of the clinicians quoted in this article had any relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
ACGME seeks to return trainees’ maximum shifts to 24 hours
First-year residents may be permitted to work up to 24 consecutive hours – 8 hours longer than they can now – under a proposal from a task force of the Accreditation Council for Graduate Medical Education (ACGME).
The ACGME Duty Hour Task Force proposes to raise first-year trainees’ work hour limit from 16 hours, reverting to the 24-hour maximum that remained in effect until 2011 – and the existing limit in place for all other residents.
“There is better team continuity of care provided with less micromanagement of resident duty hours,” said Thomas J. Nasca, MD, ACGME chief executive and vice chairman of the task force.
The ACGME instituted the 16-hour cap for first-year residents in the wake of a December 2008 report released by the Institute of Medicine (IOM), “Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.” The ACGME also prohibited 30-hour shifts, which some trainees had been clocking.
“Every 5 years, the ACGME reviews its program requirements,” Dr. Nasca said. “That’s a promise we made to the community and to the public” in July 2003. The most recent changes occurred in July 2011.
Since then, some faculty have contended that shorter work hour limits for the first-year residents are accelerating the frequency of patient transitions and disrupting continuity of care. Many educators also noted that residents would gain more knowledge by monitoring a hospitalized patient during the initial 24 hours.
The proposed changes are “encouraging and courageous,” according to R. James Valentine, MD, FACS, president of the Western Surgical Association.
“This is a real world scenario,” said Dr. Valentine, professor of vascular surgery at Vanderbilt University, Nashville, Tenn. “Medicine is such a complex system. It is not easily constrained by time limitations.”
Requiring a physician to transition a patient’s care at a specific time may help promote the trainee’s well-being; however, it also “robs the resident of the opportunity to see the disease progress and to see the response to the treatment that is being offered. The new rules help strike a balance between bedside education and rest,” he added.
The proposed revisions to training requirements also include phasing out the term “duty” hours in favor of “clinical experience and education,” highlighting that residents’ responsibility to patients takes precedence over any adherence to a schedule or clock. Working a certain number of hospital hours is only one aspect of delivering safe and quality care, the ACGME Duty Hour Task Force noted in its proposal.
The proposal does not change the following, which all are averaged over a period of 4 weeks: a maximum of 80 hours per week, 1 day free from clinical experience or education in 7, and in-house call no more often than every third night.
“It is important to note that the absence of a common 16-hour limit does not imply that programs may no longer configure their clinical schedules in 16-hour increments if that is the preferred option for a given setting or clinical context,” Dr. Nasca wrote in a Nov. 4 letter to the graduate medical education community.
In its December 2008 report, the IOM noted that revamping residents’ work hours alone offered no guarantee of patient safety. It called for increased supervision by seasoned physicians, restrictions on patient caseloads based on residents’ levels of experience and specialty, overlap in schedules around shift changes to decrease the possibility of error in transitioning patients from one provider to another, and broad-based research to examine the outcomes from these changes.
“Surgical residents, when they’re operating, come under very direct supervision by attending physicians,” said Jay Bhattacharya, MD, professor of medicine at Stanford (Calif.) University, who served on the 2008 IOM committee. “That supervision is generally pretty good. Even for a tired resident, the supervision makes it so that the fatigue the trainee faces doesn’t endanger the patient.”
Acknowledging the emerging evidence that physicians are at heightened risk for burnout and perhaps depression, the proposal underscores the need for residency programs and institutions to prioritize the well-being of residents as well as nurses and other hospital personnel.
“Burnout and depression impair a physician’s ability to provide excellent care. Self-care is an important aspect of professionalism, and a skill that must be learned and nurtured under the guidance and role modeling of faculty members,” Dr. Nasca wrote in his Nov. 4 letter.
If the proposal is approved by the ACGME board of directors in February, the changes will be rolled out in July.
The proposal is open to public comment through Dec. 19; comments will be reviewed and considered before a final set of proposed requirements are sent to the ACGME board for approval.
“We’re here to serve the public, and so the focus is on the comments that reflect the most recent and comprehensive evidence and educational outcomes rather than the individual opinions we receive,” Dr. Nasca said.
First-year residents may be permitted to work up to 24 consecutive hours – 8 hours longer than they can now – under a proposal from a task force of the Accreditation Council for Graduate Medical Education (ACGME).
The ACGME Duty Hour Task Force proposes to raise first-year trainees’ work hour limit from 16 hours, reverting to the 24-hour maximum that remained in effect until 2011 – and the existing limit in place for all other residents.
“There is better team continuity of care provided with less micromanagement of resident duty hours,” said Thomas J. Nasca, MD, ACGME chief executive and vice chairman of the task force.
The ACGME instituted the 16-hour cap for first-year residents in the wake of a December 2008 report released by the Institute of Medicine (IOM), “Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.” The ACGME also prohibited 30-hour shifts, which some trainees had been clocking.
“Every 5 years, the ACGME reviews its program requirements,” Dr. Nasca said. “That’s a promise we made to the community and to the public” in July 2003. The most recent changes occurred in July 2011.
Since then, some faculty have contended that shorter work hour limits for the first-year residents are accelerating the frequency of patient transitions and disrupting continuity of care. Many educators also noted that residents would gain more knowledge by monitoring a hospitalized patient during the initial 24 hours.
The proposed changes are “encouraging and courageous,” according to R. James Valentine, MD, FACS, president of the Western Surgical Association.
“This is a real world scenario,” said Dr. Valentine, professor of vascular surgery at Vanderbilt University, Nashville, Tenn. “Medicine is such a complex system. It is not easily constrained by time limitations.”
Requiring a physician to transition a patient’s care at a specific time may help promote the trainee’s well-being; however, it also “robs the resident of the opportunity to see the disease progress and to see the response to the treatment that is being offered. The new rules help strike a balance between bedside education and rest,” he added.
The proposed revisions to training requirements also include phasing out the term “duty” hours in favor of “clinical experience and education,” highlighting that residents’ responsibility to patients takes precedence over any adherence to a schedule or clock. Working a certain number of hospital hours is only one aspect of delivering safe and quality care, the ACGME Duty Hour Task Force noted in its proposal.
The proposal does not change the following, which all are averaged over a period of 4 weeks: a maximum of 80 hours per week, 1 day free from clinical experience or education in 7, and in-house call no more often than every third night.
“It is important to note that the absence of a common 16-hour limit does not imply that programs may no longer configure their clinical schedules in 16-hour increments if that is the preferred option for a given setting or clinical context,” Dr. Nasca wrote in a Nov. 4 letter to the graduate medical education community.
In its December 2008 report, the IOM noted that revamping residents’ work hours alone offered no guarantee of patient safety. It called for increased supervision by seasoned physicians, restrictions on patient caseloads based on residents’ levels of experience and specialty, overlap in schedules around shift changes to decrease the possibility of error in transitioning patients from one provider to another, and broad-based research to examine the outcomes from these changes.
“Surgical residents, when they’re operating, come under very direct supervision by attending physicians,” said Jay Bhattacharya, MD, professor of medicine at Stanford (Calif.) University, who served on the 2008 IOM committee. “That supervision is generally pretty good. Even for a tired resident, the supervision makes it so that the fatigue the trainee faces doesn’t endanger the patient.”
Acknowledging the emerging evidence that physicians are at heightened risk for burnout and perhaps depression, the proposal underscores the need for residency programs and institutions to prioritize the well-being of residents as well as nurses and other hospital personnel.
“Burnout and depression impair a physician’s ability to provide excellent care. Self-care is an important aspect of professionalism, and a skill that must be learned and nurtured under the guidance and role modeling of faculty members,” Dr. Nasca wrote in his Nov. 4 letter.
If the proposal is approved by the ACGME board of directors in February, the changes will be rolled out in July.
The proposal is open to public comment through Dec. 19; comments will be reviewed and considered before a final set of proposed requirements are sent to the ACGME board for approval.
“We’re here to serve the public, and so the focus is on the comments that reflect the most recent and comprehensive evidence and educational outcomes rather than the individual opinions we receive,” Dr. Nasca said.
First-year residents may be permitted to work up to 24 consecutive hours – 8 hours longer than they can now – under a proposal from a task force of the Accreditation Council for Graduate Medical Education (ACGME).
The ACGME Duty Hour Task Force proposes to raise first-year trainees’ work hour limit from 16 hours, reverting to the 24-hour maximum that remained in effect until 2011 – and the existing limit in place for all other residents.
“There is better team continuity of care provided with less micromanagement of resident duty hours,” said Thomas J. Nasca, MD, ACGME chief executive and vice chairman of the task force.
The ACGME instituted the 16-hour cap for first-year residents in the wake of a December 2008 report released by the Institute of Medicine (IOM), “Resident Duty Hours: Enhancing Sleep, Supervision, and Safety.” The ACGME also prohibited 30-hour shifts, which some trainees had been clocking.
“Every 5 years, the ACGME reviews its program requirements,” Dr. Nasca said. “That’s a promise we made to the community and to the public” in July 2003. The most recent changes occurred in July 2011.
Since then, some faculty have contended that shorter work hour limits for the first-year residents are accelerating the frequency of patient transitions and disrupting continuity of care. Many educators also noted that residents would gain more knowledge by monitoring a hospitalized patient during the initial 24 hours.
The proposed changes are “encouraging and courageous,” according to R. James Valentine, MD, FACS, president of the Western Surgical Association.
“This is a real world scenario,” said Dr. Valentine, professor of vascular surgery at Vanderbilt University, Nashville, Tenn. “Medicine is such a complex system. It is not easily constrained by time limitations.”
Requiring a physician to transition a patient’s care at a specific time may help promote the trainee’s well-being; however, it also “robs the resident of the opportunity to see the disease progress and to see the response to the treatment that is being offered. The new rules help strike a balance between bedside education and rest,” he added.
The proposed revisions to training requirements also include phasing out the term “duty” hours in favor of “clinical experience and education,” highlighting that residents’ responsibility to patients takes precedence over any adherence to a schedule or clock. Working a certain number of hospital hours is only one aspect of delivering safe and quality care, the ACGME Duty Hour Task Force noted in its proposal.
The proposal does not change the following, which all are averaged over a period of 4 weeks: a maximum of 80 hours per week, 1 day free from clinical experience or education in 7, and in-house call no more often than every third night.
“It is important to note that the absence of a common 16-hour limit does not imply that programs may no longer configure their clinical schedules in 16-hour increments if that is the preferred option for a given setting or clinical context,” Dr. Nasca wrote in a Nov. 4 letter to the graduate medical education community.
In its December 2008 report, the IOM noted that revamping residents’ work hours alone offered no guarantee of patient safety. It called for increased supervision by seasoned physicians, restrictions on patient caseloads based on residents’ levels of experience and specialty, overlap in schedules around shift changes to decrease the possibility of error in transitioning patients from one provider to another, and broad-based research to examine the outcomes from these changes.
“Surgical residents, when they’re operating, come under very direct supervision by attending physicians,” said Jay Bhattacharya, MD, professor of medicine at Stanford (Calif.) University, who served on the 2008 IOM committee. “That supervision is generally pretty good. Even for a tired resident, the supervision makes it so that the fatigue the trainee faces doesn’t endanger the patient.”
Acknowledging the emerging evidence that physicians are at heightened risk for burnout and perhaps depression, the proposal underscores the need for residency programs and institutions to prioritize the well-being of residents as well as nurses and other hospital personnel.
“Burnout and depression impair a physician’s ability to provide excellent care. Self-care is an important aspect of professionalism, and a skill that must be learned and nurtured under the guidance and role modeling of faculty members,” Dr. Nasca wrote in his Nov. 4 letter.
If the proposal is approved by the ACGME board of directors in February, the changes will be rolled out in July.
The proposal is open to public comment through Dec. 19; comments will be reviewed and considered before a final set of proposed requirements are sent to the ACGME board for approval.
“We’re here to serve the public, and so the focus is on the comments that reflect the most recent and comprehensive evidence and educational outcomes rather than the individual opinions we receive,” Dr. Nasca said.