User login
Long-term Intracranial Monitoring Reveals Circadian Pattern of Epileptic Discharges
Using the NeuroPace RNS system to monitor long-term epileptic-like activity, researchers have confirmed that there is a uniform circadian pattern to this brain activity. Studying 134 subjects, Spencer et al found the epileptiform activity peaked during normal sleeping hours. They also discovered a monophasic, nocturnally dominant rhythm in the neocortical areas of the brain and a more complex pattern, with a diurnal peak, in limbic sections of the brain. Some volunteers were also found to have a dual oscillator pattern to the brain activity, displaying a circadian and ultradian pattern.
Spencer D, Sun F, Brown S, Jobst, B, Wong V, Mirro E et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495-1502.
Using the NeuroPace RNS system to monitor long-term epileptic-like activity, researchers have confirmed that there is a uniform circadian pattern to this brain activity. Studying 134 subjects, Spencer et al found the epileptiform activity peaked during normal sleeping hours. They also discovered a monophasic, nocturnally dominant rhythm in the neocortical areas of the brain and a more complex pattern, with a diurnal peak, in limbic sections of the brain. Some volunteers were also found to have a dual oscillator pattern to the brain activity, displaying a circadian and ultradian pattern.
Spencer D, Sun F, Brown S, Jobst, B, Wong V, Mirro E et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495-1502.
Using the NeuroPace RNS system to monitor long-term epileptic-like activity, researchers have confirmed that there is a uniform circadian pattern to this brain activity. Studying 134 subjects, Spencer et al found the epileptiform activity peaked during normal sleeping hours. They also discovered a monophasic, nocturnally dominant rhythm in the neocortical areas of the brain and a more complex pattern, with a diurnal peak, in limbic sections of the brain. Some volunteers were also found to have a dual oscillator pattern to the brain activity, displaying a circadian and ultradian pattern.
Spencer D, Sun F, Brown S, Jobst, B, Wong V, Mirro E et al. Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia. 2016;57(9):1495-1502.
Finding the Links Between Tuberous Sclerosis Complex and Epilepsy
Patients with tuberous sclerosis complex (TSC) are at higher than average risk of developing epilepsy if they exhibit several systemic disease manifestations, according to a recent analysis of the TSC Natural History Database. After factoring out confounding variables like age, gender, and TSC mutation, Anna Jeong and Michael Wong of Washington University School of Medicine found that cardiac rhabdomyomas, retinal hamartomas, renal cysts, renal angiomyolipomas, shagreen patches, and facial angiofibromas increased the likelihood of TSC patients developing epilepsy.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(9):1443-1449.
Patients with tuberous sclerosis complex (TSC) are at higher than average risk of developing epilepsy if they exhibit several systemic disease manifestations, according to a recent analysis of the TSC Natural History Database. After factoring out confounding variables like age, gender, and TSC mutation, Anna Jeong and Michael Wong of Washington University School of Medicine found that cardiac rhabdomyomas, retinal hamartomas, renal cysts, renal angiomyolipomas, shagreen patches, and facial angiofibromas increased the likelihood of TSC patients developing epilepsy.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(9):1443-1449.
Patients with tuberous sclerosis complex (TSC) are at higher than average risk of developing epilepsy if they exhibit several systemic disease manifestations, according to a recent analysis of the TSC Natural History Database. After factoring out confounding variables like age, gender, and TSC mutation, Anna Jeong and Michael Wong of Washington University School of Medicine found that cardiac rhabdomyomas, retinal hamartomas, renal cysts, renal angiomyolipomas, shagreen patches, and facial angiofibromas increased the likelihood of TSC patients developing epilepsy.
Jeong A, Wong M. Systemic disease manifestations associated with epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(9):1443-1449.
Functional MRI Can Separate Types of Temporal Lobe Epilepsy
Performing resting state functioning MRIs can help distinguish temporal lobe epilepsy that’s accompanied by mesial temporal sclerosis (TLE-MTS) from temporal lobe epilepsy without the sclerosis. That conclusion was dreached by researchers who compared 34 TLE patients to 34 controls who were matched for age and gender and in whom the presence of mesial temporal sclerosis was definitively established by means of histologic examination of surgical tissue. More specifically, the investigators found that the fractional amplitude of low-frequency fluctuations (fALFF) in the blood oxygen level-dependent resting state fMRI was reduced in the ipsilateral amygdala and hippocampus among TLE patients with mesial temporal sclerosis. By contrast, among TLE patients without sclerosis, there was only marginally reduced fALFF in the ipsilateral amygdala but none in the hippocampus.
Reyes A, Thesen D, Wang X, Hahn D, Yoo D, Kuzniecky R et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-1484.
Performing resting state functioning MRIs can help distinguish temporal lobe epilepsy that’s accompanied by mesial temporal sclerosis (TLE-MTS) from temporal lobe epilepsy without the sclerosis. That conclusion was dreached by researchers who compared 34 TLE patients to 34 controls who were matched for age and gender and in whom the presence of mesial temporal sclerosis was definitively established by means of histologic examination of surgical tissue. More specifically, the investigators found that the fractional amplitude of low-frequency fluctuations (fALFF) in the blood oxygen level-dependent resting state fMRI was reduced in the ipsilateral amygdala and hippocampus among TLE patients with mesial temporal sclerosis. By contrast, among TLE patients without sclerosis, there was only marginally reduced fALFF in the ipsilateral amygdala but none in the hippocampus.
Reyes A, Thesen D, Wang X, Hahn D, Yoo D, Kuzniecky R et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-1484.
Performing resting state functioning MRIs can help distinguish temporal lobe epilepsy that’s accompanied by mesial temporal sclerosis (TLE-MTS) from temporal lobe epilepsy without the sclerosis. That conclusion was dreached by researchers who compared 34 TLE patients to 34 controls who were matched for age and gender and in whom the presence of mesial temporal sclerosis was definitively established by means of histologic examination of surgical tissue. More specifically, the investigators found that the fractional amplitude of low-frequency fluctuations (fALFF) in the blood oxygen level-dependent resting state fMRI was reduced in the ipsilateral amygdala and hippocampus among TLE patients with mesial temporal sclerosis. By contrast, among TLE patients without sclerosis, there was only marginally reduced fALFF in the ipsilateral amygdala but none in the hippocampus.
Reyes A, Thesen D, Wang X, Hahn D, Yoo D, Kuzniecky R et al. Resting-state functional MRI distinguishes temporal lobe epilepsy subtypes. Epilepsia. 2016;57(9):1475-1484.
Open Payments: Few dermatologists have significant conflicts of interest
A total of 8,333 dermatologists – 73% of those practicing in the United States – received $34.8 million from industry in 2014, mostly from pharmaceutical companies, according to an Oct. 5 report in JAMA Dermatology.
The bulk of the money went to a fraction of the dermatologists. Just 10% – 833 – collected $31.2 million. Eighty-three dermatologists – 1% of the total – pulled in about $15 million, each receiving at least $93,622. The 10 highest-paid dermatologists were mostly in private practice, not academia, and three were women (JAMA Dermatol. 2016 Oct 5. doi: 10.1001/jamadermatol.2016.3037).
Speaker fees accounted for 32% of the total payment amount, consulting fees for 22%, research payments for 17%, and food and beverage payments for 13%. Lesser amounts went towards travel and honoraria, among other things.
Almost $29 million came from pharmaceutical companies. AbbVie and Allergan led the way with payments of nearly $6 million each. Pharmaceutical company largesse is “not surprising” since companies have “financial incentives to promote their medications,” and having “thought leaders being advocates and spokespersons may help shift clinical practices,” said lead investigator Hao Feng, MD, a dermatology resident at New York University, and his colleagues. Recent studies “show that receipt of industry payments and industry-sponsored meals was associated with an increased rate of prescribing several class[es] of brand-name medications.”
The data come from the CMS Open Payments database, which records industry payments to physicians and is searchable by name. The investigators analyzed data from 2014 because it was the first year with a full 12 months of data.
For most dermatologists, industry payments didn’t amount to much: the overall median payment was $298, and 63% received less than $500. Almost 80% of the 208,613 payments in 2014 were for $50 or less.
The investigators were careful to note that industry payments are common in other specialties as well, with most of the money going to a select few. Dermatologists received 0.5% of the $6.5 billion that companies paid to U.S. physicians in 2014. Companies paid U.S. physicians $7.52 billion in 2015, according to CMS.
The Open Payments database was launched as part of the Affordable Care Act in the belief that transparency would combat the untoward effects of commercial interests and conflicts of interest on patient care, but it doesn’t catch everything. CMS only requires companies that make government-reimbursed products to report payments. For dermatologists, that means, for example, laser and other cosmetic device companies are exempt. Physicians also can direct payments to third parties. Such issues led Dr. Feng and his associates to conclude that CMS captures “only a fraction of … physician-industry financial relationships.”
Another problem, they said, is that CMS does not judge between industry payments that advance science and help patients and those that are “harmful,” so the database does little to counter public concerns about “dishonesty and selfishness” when doctors deal with industry. “Ultimately, the impact of financial disclosure from industry to dermatologists, and physicians in general, remains to be seen,” they wrote.
The investigators did not report any conflicts of interest.
Transparency for physician interactions with industry is important, especially given the findings showing individual consulting payments to dermatologists are as high as $249,643. However, the roll out of the Open Payments program has frustrated physicians and failed to provide the public with information of sufficient accuracy and meaning to use to make fair conclusions.
Some straightforward changes would substantially improve the situation. Physicians should have the opportunity to preview data before manufacturers transmit it to the CMS. The administrative burden inherent in the current CMS data review portal and the dispute process should be reduced. A common reporting method, including very clear definitions of meaningful categories of payments, should be standardized across companies. The CMS should issue clear guidance that reduces fear among manufacturers and decreases overreporting.
Jack Resneck Jr., MD, is professor and vice-chair of dermatology at the University of California, San Francisco. He serves on the American Medical Association Board of Trustees and made his comments in an editorial (JAMA Dermatol. 5 Oct. 2016).
Transparency for physician interactions with industry is important, especially given the findings showing individual consulting payments to dermatologists are as high as $249,643. However, the roll out of the Open Payments program has frustrated physicians and failed to provide the public with information of sufficient accuracy and meaning to use to make fair conclusions.
Some straightforward changes would substantially improve the situation. Physicians should have the opportunity to preview data before manufacturers transmit it to the CMS. The administrative burden inherent in the current CMS data review portal and the dispute process should be reduced. A common reporting method, including very clear definitions of meaningful categories of payments, should be standardized across companies. The CMS should issue clear guidance that reduces fear among manufacturers and decreases overreporting.
Jack Resneck Jr., MD, is professor and vice-chair of dermatology at the University of California, San Francisco. He serves on the American Medical Association Board of Trustees and made his comments in an editorial (JAMA Dermatol. 5 Oct. 2016).
Transparency for physician interactions with industry is important, especially given the findings showing individual consulting payments to dermatologists are as high as $249,643. However, the roll out of the Open Payments program has frustrated physicians and failed to provide the public with information of sufficient accuracy and meaning to use to make fair conclusions.
Some straightforward changes would substantially improve the situation. Physicians should have the opportunity to preview data before manufacturers transmit it to the CMS. The administrative burden inherent in the current CMS data review portal and the dispute process should be reduced. A common reporting method, including very clear definitions of meaningful categories of payments, should be standardized across companies. The CMS should issue clear guidance that reduces fear among manufacturers and decreases overreporting.
Jack Resneck Jr., MD, is professor and vice-chair of dermatology at the University of California, San Francisco. He serves on the American Medical Association Board of Trustees and made his comments in an editorial (JAMA Dermatol. 5 Oct. 2016).
A total of 8,333 dermatologists – 73% of those practicing in the United States – received $34.8 million from industry in 2014, mostly from pharmaceutical companies, according to an Oct. 5 report in JAMA Dermatology.
The bulk of the money went to a fraction of the dermatologists. Just 10% – 833 – collected $31.2 million. Eighty-three dermatologists – 1% of the total – pulled in about $15 million, each receiving at least $93,622. The 10 highest-paid dermatologists were mostly in private practice, not academia, and three were women (JAMA Dermatol. 2016 Oct 5. doi: 10.1001/jamadermatol.2016.3037).
Speaker fees accounted for 32% of the total payment amount, consulting fees for 22%, research payments for 17%, and food and beverage payments for 13%. Lesser amounts went towards travel and honoraria, among other things.
Almost $29 million came from pharmaceutical companies. AbbVie and Allergan led the way with payments of nearly $6 million each. Pharmaceutical company largesse is “not surprising” since companies have “financial incentives to promote their medications,” and having “thought leaders being advocates and spokespersons may help shift clinical practices,” said lead investigator Hao Feng, MD, a dermatology resident at New York University, and his colleagues. Recent studies “show that receipt of industry payments and industry-sponsored meals was associated with an increased rate of prescribing several class[es] of brand-name medications.”
The data come from the CMS Open Payments database, which records industry payments to physicians and is searchable by name. The investigators analyzed data from 2014 because it was the first year with a full 12 months of data.
For most dermatologists, industry payments didn’t amount to much: the overall median payment was $298, and 63% received less than $500. Almost 80% of the 208,613 payments in 2014 were for $50 or less.
The investigators were careful to note that industry payments are common in other specialties as well, with most of the money going to a select few. Dermatologists received 0.5% of the $6.5 billion that companies paid to U.S. physicians in 2014. Companies paid U.S. physicians $7.52 billion in 2015, according to CMS.
The Open Payments database was launched as part of the Affordable Care Act in the belief that transparency would combat the untoward effects of commercial interests and conflicts of interest on patient care, but it doesn’t catch everything. CMS only requires companies that make government-reimbursed products to report payments. For dermatologists, that means, for example, laser and other cosmetic device companies are exempt. Physicians also can direct payments to third parties. Such issues led Dr. Feng and his associates to conclude that CMS captures “only a fraction of … physician-industry financial relationships.”
Another problem, they said, is that CMS does not judge between industry payments that advance science and help patients and those that are “harmful,” so the database does little to counter public concerns about “dishonesty and selfishness” when doctors deal with industry. “Ultimately, the impact of financial disclosure from industry to dermatologists, and physicians in general, remains to be seen,” they wrote.
The investigators did not report any conflicts of interest.
A total of 8,333 dermatologists – 73% of those practicing in the United States – received $34.8 million from industry in 2014, mostly from pharmaceutical companies, according to an Oct. 5 report in JAMA Dermatology.
The bulk of the money went to a fraction of the dermatologists. Just 10% – 833 – collected $31.2 million. Eighty-three dermatologists – 1% of the total – pulled in about $15 million, each receiving at least $93,622. The 10 highest-paid dermatologists were mostly in private practice, not academia, and three were women (JAMA Dermatol. 2016 Oct 5. doi: 10.1001/jamadermatol.2016.3037).
Speaker fees accounted for 32% of the total payment amount, consulting fees for 22%, research payments for 17%, and food and beverage payments for 13%. Lesser amounts went towards travel and honoraria, among other things.
Almost $29 million came from pharmaceutical companies. AbbVie and Allergan led the way with payments of nearly $6 million each. Pharmaceutical company largesse is “not surprising” since companies have “financial incentives to promote their medications,” and having “thought leaders being advocates and spokespersons may help shift clinical practices,” said lead investigator Hao Feng, MD, a dermatology resident at New York University, and his colleagues. Recent studies “show that receipt of industry payments and industry-sponsored meals was associated with an increased rate of prescribing several class[es] of brand-name medications.”
The data come from the CMS Open Payments database, which records industry payments to physicians and is searchable by name. The investigators analyzed data from 2014 because it was the first year with a full 12 months of data.
For most dermatologists, industry payments didn’t amount to much: the overall median payment was $298, and 63% received less than $500. Almost 80% of the 208,613 payments in 2014 were for $50 or less.
The investigators were careful to note that industry payments are common in other specialties as well, with most of the money going to a select few. Dermatologists received 0.5% of the $6.5 billion that companies paid to U.S. physicians in 2014. Companies paid U.S. physicians $7.52 billion in 2015, according to CMS.
The Open Payments database was launched as part of the Affordable Care Act in the belief that transparency would combat the untoward effects of commercial interests and conflicts of interest on patient care, but it doesn’t catch everything. CMS only requires companies that make government-reimbursed products to report payments. For dermatologists, that means, for example, laser and other cosmetic device companies are exempt. Physicians also can direct payments to third parties. Such issues led Dr. Feng and his associates to conclude that CMS captures “only a fraction of … physician-industry financial relationships.”
Another problem, they said, is that CMS does not judge between industry payments that advance science and help patients and those that are “harmful,” so the database does little to counter public concerns about “dishonesty and selfishness” when doctors deal with industry. “Ultimately, the impact of financial disclosure from industry to dermatologists, and physicians in general, remains to be seen,” they wrote.
The investigators did not report any conflicts of interest.
Key clinical point:
Major finding: The bulk of the money went to 833 dermatologists who collected $31.2 million in 2014, mostly from pharmaceutical companies.
Data source: Review of the CMS Open Payments database
Disclosures: The investigators had no disclosures.
Tips for Sleep Hygiene
Click here to download the PDF.
Click here to download the PDF.
Click here to download the PDF.
VIDEO: The ‘artificial’ divide between biology and psychology
NEW YORK – “All psychology works through biology,” said the new head of the National Institute of Mental Health, Joshua A. Gordon, MD, PhD. “The divide is artificial at the level of neurocircuits.” All treatments for mental illness, from antidepressants to psychotherapy to emerging therapies, can be viewed through that lens.
In this video interview, conducted just days before he stepped into his new role, Dr. Gordon discusses how this biological view of the mind and brain will inform his approach to use of the Research Domain Criteria (RDoC) when reviewing grant applications, a process he said he is aware some researchers still resist.
“One thing that is important is that we really try to quantify and objectively evaluate behavior in the way that RDoC tries to do. RDoC essentially is a way to try to categorize behavior into its component building blocks, and then try to understand, yes, the biology underneath it,” Dr. Gordon said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW YORK – “All psychology works through biology,” said the new head of the National Institute of Mental Health, Joshua A. Gordon, MD, PhD. “The divide is artificial at the level of neurocircuits.” All treatments for mental illness, from antidepressants to psychotherapy to emerging therapies, can be viewed through that lens.
In this video interview, conducted just days before he stepped into his new role, Dr. Gordon discusses how this biological view of the mind and brain will inform his approach to use of the Research Domain Criteria (RDoC) when reviewing grant applications, a process he said he is aware some researchers still resist.
“One thing that is important is that we really try to quantify and objectively evaluate behavior in the way that RDoC tries to do. RDoC essentially is a way to try to categorize behavior into its component building blocks, and then try to understand, yes, the biology underneath it,” Dr. Gordon said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW YORK – “All psychology works through biology,” said the new head of the National Institute of Mental Health, Joshua A. Gordon, MD, PhD. “The divide is artificial at the level of neurocircuits.” All treatments for mental illness, from antidepressants to psychotherapy to emerging therapies, can be viewed through that lens.
In this video interview, conducted just days before he stepped into his new role, Dr. Gordon discusses how this biological view of the mind and brain will inform his approach to use of the Research Domain Criteria (RDoC) when reviewing grant applications, a process he said he is aware some researchers still resist.
“One thing that is important is that we really try to quantify and objectively evaluate behavior in the way that RDoC tries to do. RDoC essentially is a way to try to categorize behavior into its component building blocks, and then try to understand, yes, the biology underneath it,” Dr. Gordon said.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Emergency Imaging: Acute abdominal pain
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
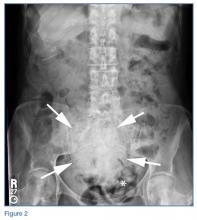
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
An 89-year-old woman with a history of coronary artery disease, diabetes mellitus, hypertension, chronic constipation, and glaucoma presented to the ED for evaluation of chest pain and headache. Upon arrival at the ED, the patient also began to experience unrelenting abdominal pain. Abdominal examination showed mild tenderness in the right lower quadrant upon palpation. An abdominal radiograph and a computed tomography (CT) scan were ordered; representative images are presented above (Figure 1a-1d).
What is the diagnosis? What is the preferred management for this patient?
Answer
The abdominal radiograph showed no evidence of bowel obstruction. There was, however, a round area of increased density in the pelvis, suggesting the presence of a soft-tissue mass (white arrows, Figure 2) directly adjacent to the sigmoid colon (white asterisk, Figure 2).
Giant Colonic Diverticula
Giant colonic diverticula (GCD) are diverticula larger than 4 cm. This is a rare manifestation of diverticular disease of the bowel and most commonly occurs within the sigmoid colon. The majority of patients who develop GCD are older than age 60 years.1
The clinical presentation of GCD is nonspecific but can include abdominal pain, vomiting, nausea, and fever in the acute setting.2 Chronic presentations of GCD include intermittent abdominal pain, bloating, and constipation. In two-thirds of patients, a palpable abdominal mass is found on physical examination.3
Diagnosis
Due to the nonspecific presentation of GCD, imaging studies are typically required for diagnosis. Although radiographs may show a dilated air-filled structure in the abdomen, differentiation from a normal air-filled bowel may be difficult. Computed tomography is the imaging modality of choice based on its ability to demonstrate the presence of a smooth-walled gas-containing structure that communicates with the bowel lumen. In addition, CT has the ability to visualize the fluid and stool that are often present within the diverticulum. In cases of acute inflammation, diverticular wall thickening also may be present on CT.
Though no longer routinely used, barium enema is another option for diagnosing GCD because it can also demonstrate communication between the giant diverticula and the bowel lumen. However, barium enema is not often used in the emergency setting due to an increased risk of perforation and peritonitis.1
Management
Complications caused by GCD occur in 15% to 35% of cases and most commonly include perforation with associated peritonitis and abscess formation.4 Due to associated morbidity, the preferred treatment is surgical management—even when GCD is found incidentally in asymptomatic patients. In uncomplicated cases, surgical resection of the diverticulum and adjacent colon is performed with primary colic anastomosis. In some cases, a diverting ileostomy is created. In the presence of perforation and/or abscess, percutaneous catheter drainage and two-stage colectomy with colostomy typically is performed.5
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
1. Zeina AR, Mahamid A, Nachtigal A, Ashkenazi I, Shapira-Rootman M. Giant colonic diverticulum: radiographic and MDCT characteristics. Insights Imaging. 2015;6(6):659-664. doi: 10.1007/s13244-015-0433-x.
2. Custer TJ, Blevins DV, Vara TM. Giant colonic diverticulum: a rare manifestation of a common disease. J Gastrointest Surg. 1999;3(5):543-548.
3. de Oliveira NC, Welch JP. Giant diverticula of the colon: a clinical assessment. Am J Gastroenterol. 1997;92(7):1092-1096.
4. Majeski J, Durst G Jr. Obstructing giant colonic diverticulum. South Med J. 2000;93(8):797-799.
5. Nigri G, Petrucciani N, Giannini G, et al. Giant colonic diverticulum: clinical presentation, diagnosis and treatment: systematic review of 166 cases. World J Gastroenterol. 2015;21(1):360-368. doi: 10.3748/wjg.v21.i1.360.
Supraglottitis
Case
A 39-year-old woman, previously in good health, presented to the ED with a chief complaint of severe sore throat, which she said had begun approximately 4 hours prior and was rapidly worsening. She thought her voice sounded muffled, and said she was now having difficulty swallowing her saliva. The patient denied fever but did admit to chills. She experienced onset of shortness of breath 30 minutes prior to arrival to the ED.
The patient stated that she was a house painter and had been working in the home of someone who had several dogs. While not previously allergic to animals, the patient was concerned exposure to the dogs might have contributed to her symptoms. Regarding her social history, the patient admitted to daily consumption of beer, but denied smoking cigarettes. She had no known drug allergies.
On physical examination, the patient was afebrile. Her vital signs were: heart rate, 125 beats/min; blood pressure, 137/74 mm Hg; and respiratory rate, 18 breaths/min. Oxygen saturation was 99% on room air. Overall, the patient appeared anxious and exhibited mild inspiratory stridor. Examination of the eyes and ears were normal. There was no obvious inflammation or swelling of the posterior pharynx; the tongue was normal; there was no swelling of the floor of the mouth; and the uvula was midline and without swelling.
The patient was noted to having difficulty handling her secretions. She exhibited full range of motion of her neck. Her trachea was tender upon palpation but without jugular venous distension or lymphadenopathy. The cardiac examination was significant for tachycardia with a regular rhythm and without murmurs, rubs, or gallops; the pulmonary examination was normal except for transmitted upper airway sounds. The patient’s abdominal, dermatological, and neurological examinations were all normal.
Based on the examination findings, the differential diagnosis included allergic reaction, angioedema, epiglottitis, and retropharyngeal abscess. An intravenous (IV) line was placed and blood was drawn for laboratory evaluation, which included a complete blood count, basic metabolic panel (BMP), and a quantitative pregnancy test. Given the patient’s history, the emergency
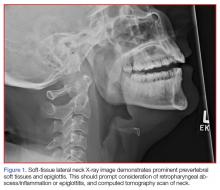
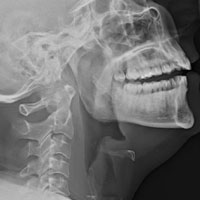
A portable soft-tissue lateral radiograph of the neck was obtained. Radiology services interpreted the film as showing “prominent prevertebral soft tissues and epiglottis.
At this point, the patient appeared relatively stable and without progression of symptoms. Since there was the possibility of an infectious etiology, she was given piperacillin/tazobactam, 4.5 g IV.
Laboratory evaluation results were significant for an elevated white blood cell count (WBC) of 14.8 ×109/L, but without a left shift; BMP results were within normal limits, and the pregnancy test was negative.
Based on these findings, otolaryngology services were consulted. The consulting otolaryngologist sprayed oxymetazoline and tetracaine into both of the patient’s nostrils and performed a flexible fiberoptic nasopharyngolaryngoscopy. During the procedure, a significant amount of diffuse supraglottic edema was noted, but no posterior pharyngeal wall edema.
Based on the presence of stridor, difficulty managing secretions, and significant amount of supraglottic edema, the patient was taken to the surgical suite for urgent airway control. She was given dexamethasone, 10 mg IV, and after some difficulty, the anesthesiologist orally intubated the patient with a 7.0-mm endotracheal tube. Examination during the procedure noted diffuse supraglottic edema but no other abnormalities.
The patient was transferred to the intensive care unit (ICU) and treated with IV piperacillin/tazobactam and dexamethasone. While in the ICU, the patient became extremely agitated and combative. After further inquiry into the patient’s social history, the patient’s husband reported that his wife drank 12 to 13 beers nightly. The patient required treatment for alcohol withdrawal with IV benzodiazepines, sedation, and physical restraints. By hospital day 9, she was extubated and tolerated fluids by mouth. On hospital day 10, her mental status had returned to baseline, her WBC was within normal limits, and she no longer complained of difficulty swallowing. The patient was discharged home on hospital day 11 with a final diagnosis of supraglottitis and alcohol withdrawal, and she was given a prescription for amoxicillin/clavulanate. Unfortunately, she did not return for her follow-up appointments.
Discussion
While the incidence of pediatric epiglottitis has decreased since the introduction of the Haemophilus influenzae type b (Hib) vaccine in 1985, adult epiglottitis continues to represent a potentially life-threatening condition whose incidence has remained constant over the past several decades.1,2 The incidence of supraglottitis in adults is now 2.5 times greater than the incidence in children.3,4
Several important differences exist in the presentation and management of adults who present with inflammation of the epiglottis as compared to children. Children commonly present with an acute onset of symptoms, and due to their smaller and more pliant airway anatomy, they often experience stridor and respiratory distress.3,5 The inflammation in children is typically confined to the epiglottis and aryepiglottic folds, while in adults the inflammation can affect not only the epiglottis, but also supraglottic structures such as the pharynx, uvula, and aryepiglottic folds. For this reason, in adults the condition is often referred to as “supraglottitis.”2,6 Adults with supraglottitis most likely present in their 30s, 40s, and 50s, while children present between the ages of 2 and 5 years old.1,3,7 In adults, men more commonly present with supraglottitis than women.1,2 Cigarette smokers and patients with hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease, or human immunodeficiency virus/AIDS are at increased risk for supraglottitis.3,4 The mortality rate for adults with supraglottitis ranges from 1.2% to 7.1%.3
Etiology
Prior to the use of the Hib vaccine, Hib was the most common cause of epiglottitis, and remains so for children.1 Currently, the most common cause of supraglottitis in adults is Group A beta-hemolytic Streptococci.2 Other etiologies include other bacteria (Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas species, Klebsiella pneumoniae, Pasteurella multocida, Neisseria species), viruses (herpes simplex, varicella, parainfluenza), trauma, and thermal injuries.1,4,5,8
Signs and Symptoms
Throat pain, dysphagia, odynophagia, and muffled voice are common complaints of adults presenting to the ED with supraglottitis.2,7 Fever is usually, but not always, present; the complaint of cough, however, is rare.2,3,4 Other less frequent complaints include hoarseness and drooling. Adults can also present with cervical lymphadenopathy, anterior neck tenderness, and cellulitis of the neck and chest.2,4 In general, the more severe cases will progress rapidly over a few hours. Due to the larger anatomy in adults, they are more likely than children to experience a gradual progression of symptoms, and supraglottitis will be missed on the initial presentation in up to 50% of adults.3,4 Stridor or respiratory compromise does occur in a minority of adult patients with supraglottitis. The need for artificial airway support (ie, endotracheal intubation, cricothyroidotomy) in adults ranges from 6.6% to 16%.9,10
Making the Diagnosis
The gold standard for diagnosing supraglottitis is direct laryngoscopy.3,4 This point is emphasized in our case report, since the CT scan was concerning for a retropharyngeal abscess, and not supraglottitis. The examination of the oropharynx is generally safer and better tolerated in adults compared to pediatric patients, since airway compromise is much less likely. On occasion, inflammation, erythema, and edema of the epiglottis, aryepiglottic folds, or arytenoid cartilages can be observed.5 More commonly, the supraglottic structures are not visualized, and the posterior oropharynx appears relatively normal. This should serve as a clue for possible supraglottitis.
In suspected cases of adult supraglottitis without emergent airway compromise, lateral soft-tissue radiographs can be obtained to look for the “thumb sign,” indicating a swollen epiglottis. In adult supraglottitis, the width of the epiglottis is usually greater than 8 mm.11 Other abnormal radiographic findings include arytenoid and aryepiglottic fold enlargement, thinning of the airway, and an increase in size of the prevertebral space. Plain film sensitivity rates range from 38% to 98%.
Complete blood count and throat cultures are not particularly helpful in adult cases. Blood cultures, while only about 30% sensitive in adults, should be considered as supraglottitis can result in secondary infection in the central nervous system, lungs, and surrounding structures.3,5
If available, otolaryngology services should be consulted to evaluate the airway, and IV antibiotics, such as a third-generation cephalosporin (eg, ceftriaxone, cefotaxime), should be initiated to include coverage of Hib.3 If methicillin-resistant S aureus is a concern, vancomycin should be added. Clindamycin or metronidazole should also be given if anaerobes are suspected.4,7 The location for performing the nasopharyngeal laryngoscopy varies, depending on the patient’s age (ie, pediatric vs adult), severity of symptoms, presence of airway compromise, and local practice and custom.
Advanced imaging studies (CT scan or magnetic resonance imaging) can help identify the presence of an abscess and delineate the extent of the infection, but are not indicated in the early diagnosis and management of suspected adult supraglottitis.4 As our case demonstrates, CT is neither highly sensitive nor specific for the diagnosis of epiglottitis. The role of ultrasound in the evaluation of suspected epiglottitis is still being developed. One recent study compared 15 healthy volunteers with 15 patients diagnosed with epiglottitis by an otolaryngologist using laryngoscopy.12 A statistically significant difference was observed in the anteroposterior diameter of the epiglottis at the midpoint and both lateral edges between the study subjects and healthy volunteers.12 While there was overlap in the ranges for the midpoint, there was no overlap in both lateral edges between the two groups.12
Treatment
The vast majority of adult cases of supraglottitis are managed medically without airway intervention. Patients presenting with a rapid onset of symptoms and in respiratory distress or with stridor, drooling, or cyanosis, should be managed with early airway intervention. The use of corticosteroids is controversial, and has not been proven beneficial in any prospective trials.1-4,6,7,13
Admission to a critical care unit is indicated initially, even in patients who are not intubated, as they can experience delayed airway compromise with progression of the infection and edema.13
Complications
Abscess formation is a serious complication of supraglottitis, is present in up to 30% of cases, and is more likely to be seen in adults than in children.13 Since the adult larynx and surrounding tissues are larger than in children, often the infection is present longer, which allows for an abscess to develop. The risk of abscess formation is increased in patients with DM or those in whom a foreign body is present.
Numerous organisms have been isolated from supraglottic abscesses in adults, and in addition to incision and drainage, antibiotics covering both gram-positive organisms and anaerobes should be initiated.5 The presence of a supraglottic abscess increases the need for emergent intubation.13 In addition, a supraglottic abscess increases the mortality rate to 30%.3 Other complications from supraglottitis include mediastinitis, cervical adenitis, meningitis, and pneumonia.4,5
Conclusion
While the incidence of epiglottitis in the pediatric patient population has fallen, the incidence in adults remains relatively stable. Clinicians should consider supraglottitis in the differential diagnosis of adults presenting with severe sore throat, dysphagia, or stridor. While airway compromise in adults is uncommon, it does occur. Soft-tissue lateral neck radiographs can help make the diagnosis, but the gold standard remains laryngoscopy. All patients should be started on IV antibiotics and admitted to the ICU initially for airway watch.
1. Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep. 2008;10(3):200-204.
2. Lichtor JL, Roche Rodriguez M, Aaronson NL, Spock T, Goodman TR, Baum ED. Epiglottitis: It hasn’t gone away. Anesthesiology. 2016;124(6):1404-1407. doi: 10.1097/ALN.0000000000001125.
3. Westerhuis B, Bietz MG, Lindemann J. Acute epiglottitis in adults: an under-recognized and life-threatening condition. S D Med. 2013;66(8):309-311, 313.
4. Al-Qudah M, Shetty S, Alomari M, Alqdah M. Acute adult supraglottitis: Current management and treatment. South Med J. 2010;103(8):800-804. doi: 10.1097/SMJ.0b013e3181e538d8.
5. Verbruggen K, Halewyck S, Deron P, Foulon I, Gordts F. Epiglottitis and related complications in adults. Case reports and review of the literature. B-ENT. 2012;8(2):143-148.
6. Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis. An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1647.
7. Bizaki AJ, Numminen J, Vasama JP, Laranne J, Rautiainen M. Acute supraglottitis in adults in Finland: review and analysis of 308 cases. Laryngoscope. 2011;121(10):2107-2113. doi: 10.1002/lary.22147.
8. Charles R, Fadden M, Brook J. Acute epiglottitis. BMJ. 2013;347:f5235. doi: 10.1136/bmj.f5235.
9. Ng HL, Sin LM, Li MF, Que TL, Anandaciva S. Acute epiglottitis in adults: a retrospective review of 106 patients in Hong Kong. Emerg Med J. 2008;25(5):253-255. doi: 10.1136/emj.2007.050153.
10. Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol. 1998;27(6):332-336.
11. Schumaker HM, Doris PE, Birnbaum G. Radiographic parameters in adult epiglottitis. Ann Emerg Med. 1984;13(8):588-590.
12. Ko DR, Chung YE, Park I, et al. Use of bedside sonography for diagnosing acute epiglottitis in the emergency department: a preliminary study. J Ultrasound Med. 2012;31(1):19-22.
13. Berger G, Landau T, Berger S, Finkelstein Y, Bernheim J, Ophir D. The rising incidence of adult acute epiglottitis and epiglottic abscess. Am J Otolaryngol. 2003;24(6):374-383.
Case
A 39-year-old woman, previously in good health, presented to the ED with a chief complaint of severe sore throat, which she said had begun approximately 4 hours prior and was rapidly worsening. She thought her voice sounded muffled, and said she was now having difficulty swallowing her saliva. The patient denied fever but did admit to chills. She experienced onset of shortness of breath 30 minutes prior to arrival to the ED.
The patient stated that she was a house painter and had been working in the home of someone who had several dogs. While not previously allergic to animals, the patient was concerned exposure to the dogs might have contributed to her symptoms. Regarding her social history, the patient admitted to daily consumption of beer, but denied smoking cigarettes. She had no known drug allergies.
On physical examination, the patient was afebrile. Her vital signs were: heart rate, 125 beats/min; blood pressure, 137/74 mm Hg; and respiratory rate, 18 breaths/min. Oxygen saturation was 99% on room air. Overall, the patient appeared anxious and exhibited mild inspiratory stridor. Examination of the eyes and ears were normal. There was no obvious inflammation or swelling of the posterior pharynx; the tongue was normal; there was no swelling of the floor of the mouth; and the uvula was midline and without swelling.
The patient was noted to having difficulty handling her secretions. She exhibited full range of motion of her neck. Her trachea was tender upon palpation but without jugular venous distension or lymphadenopathy. The cardiac examination was significant for tachycardia with a regular rhythm and without murmurs, rubs, or gallops; the pulmonary examination was normal except for transmitted upper airway sounds. The patient’s abdominal, dermatological, and neurological examinations were all normal.
Based on the examination findings, the differential diagnosis included allergic reaction, angioedema, epiglottitis, and retropharyngeal abscess. An intravenous (IV) line was placed and blood was drawn for laboratory evaluation, which included a complete blood count, basic metabolic panel (BMP), and a quantitative pregnancy test. Given the patient’s history, the emergency
A portable soft-tissue lateral radiograph of the neck was obtained. Radiology services interpreted the film as showing “prominent prevertebral soft tissues and epiglottis.
At this point, the patient appeared relatively stable and without progression of symptoms. Since there was the possibility of an infectious etiology, she was given piperacillin/tazobactam, 4.5 g IV.
Laboratory evaluation results were significant for an elevated white blood cell count (WBC) of 14.8 ×109/L, but without a left shift; BMP results were within normal limits, and the pregnancy test was negative.
Based on these findings, otolaryngology services were consulted. The consulting otolaryngologist sprayed oxymetazoline and tetracaine into both of the patient’s nostrils and performed a flexible fiberoptic nasopharyngolaryngoscopy. During the procedure, a significant amount of diffuse supraglottic edema was noted, but no posterior pharyngeal wall edema.
Based on the presence of stridor, difficulty managing secretions, and significant amount of supraglottic edema, the patient was taken to the surgical suite for urgent airway control. She was given dexamethasone, 10 mg IV, and after some difficulty, the anesthesiologist orally intubated the patient with a 7.0-mm endotracheal tube. Examination during the procedure noted diffuse supraglottic edema but no other abnormalities.
The patient was transferred to the intensive care unit (ICU) and treated with IV piperacillin/tazobactam and dexamethasone. While in the ICU, the patient became extremely agitated and combative. After further inquiry into the patient’s social history, the patient’s husband reported that his wife drank 12 to 13 beers nightly. The patient required treatment for alcohol withdrawal with IV benzodiazepines, sedation, and physical restraints. By hospital day 9, she was extubated and tolerated fluids by mouth. On hospital day 10, her mental status had returned to baseline, her WBC was within normal limits, and she no longer complained of difficulty swallowing. The patient was discharged home on hospital day 11 with a final diagnosis of supraglottitis and alcohol withdrawal, and she was given a prescription for amoxicillin/clavulanate. Unfortunately, she did not return for her follow-up appointments.
Discussion
While the incidence of pediatric epiglottitis has decreased since the introduction of the Haemophilus influenzae type b (Hib) vaccine in 1985, adult epiglottitis continues to represent a potentially life-threatening condition whose incidence has remained constant over the past several decades.1,2 The incidence of supraglottitis in adults is now 2.5 times greater than the incidence in children.3,4
Several important differences exist in the presentation and management of adults who present with inflammation of the epiglottis as compared to children. Children commonly present with an acute onset of symptoms, and due to their smaller and more pliant airway anatomy, they often experience stridor and respiratory distress.3,5 The inflammation in children is typically confined to the epiglottis and aryepiglottic folds, while in adults the inflammation can affect not only the epiglottis, but also supraglottic structures such as the pharynx, uvula, and aryepiglottic folds. For this reason, in adults the condition is often referred to as “supraglottitis.”2,6 Adults with supraglottitis most likely present in their 30s, 40s, and 50s, while children present between the ages of 2 and 5 years old.1,3,7 In adults, men more commonly present with supraglottitis than women.1,2 Cigarette smokers and patients with hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease, or human immunodeficiency virus/AIDS are at increased risk for supraglottitis.3,4 The mortality rate for adults with supraglottitis ranges from 1.2% to 7.1%.3
Etiology
Prior to the use of the Hib vaccine, Hib was the most common cause of epiglottitis, and remains so for children.1 Currently, the most common cause of supraglottitis in adults is Group A beta-hemolytic Streptococci.2 Other etiologies include other bacteria (Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas species, Klebsiella pneumoniae, Pasteurella multocida, Neisseria species), viruses (herpes simplex, varicella, parainfluenza), trauma, and thermal injuries.1,4,5,8
Signs and Symptoms
Throat pain, dysphagia, odynophagia, and muffled voice are common complaints of adults presenting to the ED with supraglottitis.2,7 Fever is usually, but not always, present; the complaint of cough, however, is rare.2,3,4 Other less frequent complaints include hoarseness and drooling. Adults can also present with cervical lymphadenopathy, anterior neck tenderness, and cellulitis of the neck and chest.2,4 In general, the more severe cases will progress rapidly over a few hours. Due to the larger anatomy in adults, they are more likely than children to experience a gradual progression of symptoms, and supraglottitis will be missed on the initial presentation in up to 50% of adults.3,4 Stridor or respiratory compromise does occur in a minority of adult patients with supraglottitis. The need for artificial airway support (ie, endotracheal intubation, cricothyroidotomy) in adults ranges from 6.6% to 16%.9,10
Making the Diagnosis
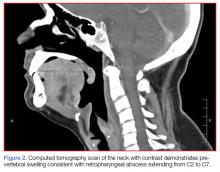
The gold standard for diagnosing supraglottitis is direct laryngoscopy.3,4 This point is emphasized in our case report, since the CT scan was concerning for a retropharyngeal abscess, and not supraglottitis. The examination of the oropharynx is generally safer and better tolerated in adults compared to pediatric patients, since airway compromise is much less likely. On occasion, inflammation, erythema, and edema of the epiglottis, aryepiglottic folds, or arytenoid cartilages can be observed.5 More commonly, the supraglottic structures are not visualized, and the posterior oropharynx appears relatively normal. This should serve as a clue for possible supraglottitis.
In suspected cases of adult supraglottitis without emergent airway compromise, lateral soft-tissue radiographs can be obtained to look for the “thumb sign,” indicating a swollen epiglottis. In adult supraglottitis, the width of the epiglottis is usually greater than 8 mm.11 Other abnormal radiographic findings include arytenoid and aryepiglottic fold enlargement, thinning of the airway, and an increase in size of the prevertebral space. Plain film sensitivity rates range from 38% to 98%.
Complete blood count and throat cultures are not particularly helpful in adult cases. Blood cultures, while only about 30% sensitive in adults, should be considered as supraglottitis can result in secondary infection in the central nervous system, lungs, and surrounding structures.3,5
If available, otolaryngology services should be consulted to evaluate the airway, and IV antibiotics, such as a third-generation cephalosporin (eg, ceftriaxone, cefotaxime), should be initiated to include coverage of Hib.3 If methicillin-resistant S aureus is a concern, vancomycin should be added. Clindamycin or metronidazole should also be given if anaerobes are suspected.4,7 The location for performing the nasopharyngeal laryngoscopy varies, depending on the patient’s age (ie, pediatric vs adult), severity of symptoms, presence of airway compromise, and local practice and custom.
Advanced imaging studies (CT scan or magnetic resonance imaging) can help identify the presence of an abscess and delineate the extent of the infection, but are not indicated in the early diagnosis and management of suspected adult supraglottitis.4 As our case demonstrates, CT is neither highly sensitive nor specific for the diagnosis of epiglottitis. The role of ultrasound in the evaluation of suspected epiglottitis is still being developed. One recent study compared 15 healthy volunteers with 15 patients diagnosed with epiglottitis by an otolaryngologist using laryngoscopy.12 A statistically significant difference was observed in the anteroposterior diameter of the epiglottis at the midpoint and both lateral edges between the study subjects and healthy volunteers.12 While there was overlap in the ranges for the midpoint, there was no overlap in both lateral edges between the two groups.12
Treatment
The vast majority of adult cases of supraglottitis are managed medically without airway intervention. Patients presenting with a rapid onset of symptoms and in respiratory distress or with stridor, drooling, or cyanosis, should be managed with early airway intervention. The use of corticosteroids is controversial, and has not been proven beneficial in any prospective trials.1-4,6,7,13
Admission to a critical care unit is indicated initially, even in patients who are not intubated, as they can experience delayed airway compromise with progression of the infection and edema.13
Complications
Abscess formation is a serious complication of supraglottitis, is present in up to 30% of cases, and is more likely to be seen in adults than in children.13 Since the adult larynx and surrounding tissues are larger than in children, often the infection is present longer, which allows for an abscess to develop. The risk of abscess formation is increased in patients with DM or those in whom a foreign body is present.
Numerous organisms have been isolated from supraglottic abscesses in adults, and in addition to incision and drainage, antibiotics covering both gram-positive organisms and anaerobes should be initiated.5 The presence of a supraglottic abscess increases the need for emergent intubation.13 In addition, a supraglottic abscess increases the mortality rate to 30%.3 Other complications from supraglottitis include mediastinitis, cervical adenitis, meningitis, and pneumonia.4,5
Conclusion
While the incidence of epiglottitis in the pediatric patient population has fallen, the incidence in adults remains relatively stable. Clinicians should consider supraglottitis in the differential diagnosis of adults presenting with severe sore throat, dysphagia, or stridor. While airway compromise in adults is uncommon, it does occur. Soft-tissue lateral neck radiographs can help make the diagnosis, but the gold standard remains laryngoscopy. All patients should be started on IV antibiotics and admitted to the ICU initially for airway watch.
Case
A 39-year-old woman, previously in good health, presented to the ED with a chief complaint of severe sore throat, which she said had begun approximately 4 hours prior and was rapidly worsening. She thought her voice sounded muffled, and said she was now having difficulty swallowing her saliva. The patient denied fever but did admit to chills. She experienced onset of shortness of breath 30 minutes prior to arrival to the ED.
The patient stated that she was a house painter and had been working in the home of someone who had several dogs. While not previously allergic to animals, the patient was concerned exposure to the dogs might have contributed to her symptoms. Regarding her social history, the patient admitted to daily consumption of beer, but denied smoking cigarettes. She had no known drug allergies.
On physical examination, the patient was afebrile. Her vital signs were: heart rate, 125 beats/min; blood pressure, 137/74 mm Hg; and respiratory rate, 18 breaths/min. Oxygen saturation was 99% on room air. Overall, the patient appeared anxious and exhibited mild inspiratory stridor. Examination of the eyes and ears were normal. There was no obvious inflammation or swelling of the posterior pharynx; the tongue was normal; there was no swelling of the floor of the mouth; and the uvula was midline and without swelling.
The patient was noted to having difficulty handling her secretions. She exhibited full range of motion of her neck. Her trachea was tender upon palpation but without jugular venous distension or lymphadenopathy. The cardiac examination was significant for tachycardia with a regular rhythm and without murmurs, rubs, or gallops; the pulmonary examination was normal except for transmitted upper airway sounds. The patient’s abdominal, dermatological, and neurological examinations were all normal.
Based on the examination findings, the differential diagnosis included allergic reaction, angioedema, epiglottitis, and retropharyngeal abscess. An intravenous (IV) line was placed and blood was drawn for laboratory evaluation, which included a complete blood count, basic metabolic panel (BMP), and a quantitative pregnancy test. Given the patient’s history, the emergency
A portable soft-tissue lateral radiograph of the neck was obtained. Radiology services interpreted the film as showing “prominent prevertebral soft tissues and epiglottis.
At this point, the patient appeared relatively stable and without progression of symptoms. Since there was the possibility of an infectious etiology, she was given piperacillin/tazobactam, 4.5 g IV.
Laboratory evaluation results were significant for an elevated white blood cell count (WBC) of 14.8 ×109/L, but without a left shift; BMP results were within normal limits, and the pregnancy test was negative.
Based on these findings, otolaryngology services were consulted. The consulting otolaryngologist sprayed oxymetazoline and tetracaine into both of the patient’s nostrils and performed a flexible fiberoptic nasopharyngolaryngoscopy. During the procedure, a significant amount of diffuse supraglottic edema was noted, but no posterior pharyngeal wall edema.
Based on the presence of stridor, difficulty managing secretions, and significant amount of supraglottic edema, the patient was taken to the surgical suite for urgent airway control. She was given dexamethasone, 10 mg IV, and after some difficulty, the anesthesiologist orally intubated the patient with a 7.0-mm endotracheal tube. Examination during the procedure noted diffuse supraglottic edema but no other abnormalities.
The patient was transferred to the intensive care unit (ICU) and treated with IV piperacillin/tazobactam and dexamethasone. While in the ICU, the patient became extremely agitated and combative. After further inquiry into the patient’s social history, the patient’s husband reported that his wife drank 12 to 13 beers nightly. The patient required treatment for alcohol withdrawal with IV benzodiazepines, sedation, and physical restraints. By hospital day 9, she was extubated and tolerated fluids by mouth. On hospital day 10, her mental status had returned to baseline, her WBC was within normal limits, and she no longer complained of difficulty swallowing. The patient was discharged home on hospital day 11 with a final diagnosis of supraglottitis and alcohol withdrawal, and she was given a prescription for amoxicillin/clavulanate. Unfortunately, she did not return for her follow-up appointments.
Discussion
While the incidence of pediatric epiglottitis has decreased since the introduction of the Haemophilus influenzae type b (Hib) vaccine in 1985, adult epiglottitis continues to represent a potentially life-threatening condition whose incidence has remained constant over the past several decades.1,2 The incidence of supraglottitis in adults is now 2.5 times greater than the incidence in children.3,4
Several important differences exist in the presentation and management of adults who present with inflammation of the epiglottis as compared to children. Children commonly present with an acute onset of symptoms, and due to their smaller and more pliant airway anatomy, they often experience stridor and respiratory distress.3,5 The inflammation in children is typically confined to the epiglottis and aryepiglottic folds, while in adults the inflammation can affect not only the epiglottis, but also supraglottic structures such as the pharynx, uvula, and aryepiglottic folds. For this reason, in adults the condition is often referred to as “supraglottitis.”2,6 Adults with supraglottitis most likely present in their 30s, 40s, and 50s, while children present between the ages of 2 and 5 years old.1,3,7 In adults, men more commonly present with supraglottitis than women.1,2 Cigarette smokers and patients with hypertension, diabetes mellitus (DM), chronic obstructive pulmonary disease, or human immunodeficiency virus/AIDS are at increased risk for supraglottitis.3,4 The mortality rate for adults with supraglottitis ranges from 1.2% to 7.1%.3
Etiology
Prior to the use of the Hib vaccine, Hib was the most common cause of epiglottitis, and remains so for children.1 Currently, the most common cause of supraglottitis in adults is Group A beta-hemolytic Streptococci.2 Other etiologies include other bacteria (Haemophilus influenzae, Streptococcus pneumoniae, Staphylococcus aureus, Pseudomonas species, Klebsiella pneumoniae, Pasteurella multocida, Neisseria species), viruses (herpes simplex, varicella, parainfluenza), trauma, and thermal injuries.1,4,5,8
Signs and Symptoms
Throat pain, dysphagia, odynophagia, and muffled voice are common complaints of adults presenting to the ED with supraglottitis.2,7 Fever is usually, but not always, present; the complaint of cough, however, is rare.2,3,4 Other less frequent complaints include hoarseness and drooling. Adults can also present with cervical lymphadenopathy, anterior neck tenderness, and cellulitis of the neck and chest.2,4 In general, the more severe cases will progress rapidly over a few hours. Due to the larger anatomy in adults, they are more likely than children to experience a gradual progression of symptoms, and supraglottitis will be missed on the initial presentation in up to 50% of adults.3,4 Stridor or respiratory compromise does occur in a minority of adult patients with supraglottitis. The need for artificial airway support (ie, endotracheal intubation, cricothyroidotomy) in adults ranges from 6.6% to 16%.9,10
Making the Diagnosis
The gold standard for diagnosing supraglottitis is direct laryngoscopy.3,4 This point is emphasized in our case report, since the CT scan was concerning for a retropharyngeal abscess, and not supraglottitis. The examination of the oropharynx is generally safer and better tolerated in adults compared to pediatric patients, since airway compromise is much less likely. On occasion, inflammation, erythema, and edema of the epiglottis, aryepiglottic folds, or arytenoid cartilages can be observed.5 More commonly, the supraglottic structures are not visualized, and the posterior oropharynx appears relatively normal. This should serve as a clue for possible supraglottitis.
In suspected cases of adult supraglottitis without emergent airway compromise, lateral soft-tissue radiographs can be obtained to look for the “thumb sign,” indicating a swollen epiglottis. In adult supraglottitis, the width of the epiglottis is usually greater than 8 mm.11 Other abnormal radiographic findings include arytenoid and aryepiglottic fold enlargement, thinning of the airway, and an increase in size of the prevertebral space. Plain film sensitivity rates range from 38% to 98%.
Complete blood count and throat cultures are not particularly helpful in adult cases. Blood cultures, while only about 30% sensitive in adults, should be considered as supraglottitis can result in secondary infection in the central nervous system, lungs, and surrounding structures.3,5
If available, otolaryngology services should be consulted to evaluate the airway, and IV antibiotics, such as a third-generation cephalosporin (eg, ceftriaxone, cefotaxime), should be initiated to include coverage of Hib.3 If methicillin-resistant S aureus is a concern, vancomycin should be added. Clindamycin or metronidazole should also be given if anaerobes are suspected.4,7 The location for performing the nasopharyngeal laryngoscopy varies, depending on the patient’s age (ie, pediatric vs adult), severity of symptoms, presence of airway compromise, and local practice and custom.
Advanced imaging studies (CT scan or magnetic resonance imaging) can help identify the presence of an abscess and delineate the extent of the infection, but are not indicated in the early diagnosis and management of suspected adult supraglottitis.4 As our case demonstrates, CT is neither highly sensitive nor specific for the diagnosis of epiglottitis. The role of ultrasound in the evaluation of suspected epiglottitis is still being developed. One recent study compared 15 healthy volunteers with 15 patients diagnosed with epiglottitis by an otolaryngologist using laryngoscopy.12 A statistically significant difference was observed in the anteroposterior diameter of the epiglottis at the midpoint and both lateral edges between the study subjects and healthy volunteers.12 While there was overlap in the ranges for the midpoint, there was no overlap in both lateral edges between the two groups.12
Treatment
The vast majority of adult cases of supraglottitis are managed medically without airway intervention. Patients presenting with a rapid onset of symptoms and in respiratory distress or with stridor, drooling, or cyanosis, should be managed with early airway intervention. The use of corticosteroids is controversial, and has not been proven beneficial in any prospective trials.1-4,6,7,13
Admission to a critical care unit is indicated initially, even in patients who are not intubated, as they can experience delayed airway compromise with progression of the infection and edema.13
Complications
Abscess formation is a serious complication of supraglottitis, is present in up to 30% of cases, and is more likely to be seen in adults than in children.13 Since the adult larynx and surrounding tissues are larger than in children, often the infection is present longer, which allows for an abscess to develop. The risk of abscess formation is increased in patients with DM or those in whom a foreign body is present.
Numerous organisms have been isolated from supraglottic abscesses in adults, and in addition to incision and drainage, antibiotics covering both gram-positive organisms and anaerobes should be initiated.5 The presence of a supraglottic abscess increases the need for emergent intubation.13 In addition, a supraglottic abscess increases the mortality rate to 30%.3 Other complications from supraglottitis include mediastinitis, cervical adenitis, meningitis, and pneumonia.4,5
Conclusion
While the incidence of epiglottitis in the pediatric patient population has fallen, the incidence in adults remains relatively stable. Clinicians should consider supraglottitis in the differential diagnosis of adults presenting with severe sore throat, dysphagia, or stridor. While airway compromise in adults is uncommon, it does occur. Soft-tissue lateral neck radiographs can help make the diagnosis, but the gold standard remains laryngoscopy. All patients should be started on IV antibiotics and admitted to the ICU initially for airway watch.
1. Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep. 2008;10(3):200-204.
2. Lichtor JL, Roche Rodriguez M, Aaronson NL, Spock T, Goodman TR, Baum ED. Epiglottitis: It hasn’t gone away. Anesthesiology. 2016;124(6):1404-1407. doi: 10.1097/ALN.0000000000001125.
3. Westerhuis B, Bietz MG, Lindemann J. Acute epiglottitis in adults: an under-recognized and life-threatening condition. S D Med. 2013;66(8):309-311, 313.
4. Al-Qudah M, Shetty S, Alomari M, Alqdah M. Acute adult supraglottitis: Current management and treatment. South Med J. 2010;103(8):800-804. doi: 10.1097/SMJ.0b013e3181e538d8.
5. Verbruggen K, Halewyck S, Deron P, Foulon I, Gordts F. Epiglottitis and related complications in adults. Case reports and review of the literature. B-ENT. 2012;8(2):143-148.
6. Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis. An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1647.
7. Bizaki AJ, Numminen J, Vasama JP, Laranne J, Rautiainen M. Acute supraglottitis in adults in Finland: review and analysis of 308 cases. Laryngoscope. 2011;121(10):2107-2113. doi: 10.1002/lary.22147.
8. Charles R, Fadden M, Brook J. Acute epiglottitis. BMJ. 2013;347:f5235. doi: 10.1136/bmj.f5235.
9. Ng HL, Sin LM, Li MF, Que TL, Anandaciva S. Acute epiglottitis in adults: a retrospective review of 106 patients in Hong Kong. Emerg Med J. 2008;25(5):253-255. doi: 10.1136/emj.2007.050153.
10. Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol. 1998;27(6):332-336.
11. Schumaker HM, Doris PE, Birnbaum G. Radiographic parameters in adult epiglottitis. Ann Emerg Med. 1984;13(8):588-590.
12. Ko DR, Chung YE, Park I, et al. Use of bedside sonography for diagnosing acute epiglottitis in the emergency department: a preliminary study. J Ultrasound Med. 2012;31(1):19-22.
13. Berger G, Landau T, Berger S, Finkelstein Y, Bernheim J, Ophir D. The rising incidence of adult acute epiglottitis and epiglottic abscess. Am J Otolaryngol. 2003;24(6):374-383.
1. Glynn F, Fenton JE. Diagnosis and management of supraglottitis (epiglottitis). Curr Infect Dis Rep. 2008;10(3):200-204.
2. Lichtor JL, Roche Rodriguez M, Aaronson NL, Spock T, Goodman TR, Baum ED. Epiglottitis: It hasn’t gone away. Anesthesiology. 2016;124(6):1404-1407. doi: 10.1097/ALN.0000000000001125.
3. Westerhuis B, Bietz MG, Lindemann J. Acute epiglottitis in adults: an under-recognized and life-threatening condition. S D Med. 2013;66(8):309-311, 313.
4. Al-Qudah M, Shetty S, Alomari M, Alqdah M. Acute adult supraglottitis: Current management and treatment. South Med J. 2010;103(8):800-804. doi: 10.1097/SMJ.0b013e3181e538d8.
5. Verbruggen K, Halewyck S, Deron P, Foulon I, Gordts F. Epiglottitis and related complications in adults. Case reports and review of the literature. B-ENT. 2012;8(2):143-148.
6. Mayo-Smith MF, Spinale JW, Donskey CJ, Yukawa M, Li RH, Schiffman FJ. Acute epiglottitis. An 18-year experience in Rhode Island. Chest. 1995;108(6):1640-1647.
7. Bizaki AJ, Numminen J, Vasama JP, Laranne J, Rautiainen M. Acute supraglottitis in adults in Finland: review and analysis of 308 cases. Laryngoscope. 2011;121(10):2107-2113. doi: 10.1002/lary.22147.
8. Charles R, Fadden M, Brook J. Acute epiglottitis. BMJ. 2013;347:f5235. doi: 10.1136/bmj.f5235.
9. Ng HL, Sin LM, Li MF, Que TL, Anandaciva S. Acute epiglottitis in adults: a retrospective review of 106 patients in Hong Kong. Emerg Med J. 2008;25(5):253-255. doi: 10.1136/emj.2007.050153.
10. Solomon P, Weisbrod M, Irish JC, Gullane PJ. Adult epiglottitis: the Toronto Hospital experience. J Otolaryngol. 1998;27(6):332-336.
11. Schumaker HM, Doris PE, Birnbaum G. Radiographic parameters in adult epiglottitis. Ann Emerg Med. 1984;13(8):588-590.
12. Ko DR, Chung YE, Park I, et al. Use of bedside sonography for diagnosing acute epiglottitis in the emergency department: a preliminary study. J Ultrasound Med. 2012;31(1):19-22.
13. Berger G, Landau T, Berger S, Finkelstein Y, Bernheim J, Ophir D. The rising incidence of adult acute epiglottitis and epiglottic abscess. Am J Otolaryngol. 2003;24(6):374-383.
Nontraumatic Splenic Rupture
Case
A 25-year-old college student presented to the ED following a near-syncopal episode. The patient stated he had felt lightheaded and had fallen to his knees immediately after taking a shower earlier that morning, but did not experience any loss of consciousness or injury. He denied a history of syncope or any recent trauma or fatigue. A review of the patient’s systems was negative. His medical history was remarkable for irritable bowel syndrome; he had no surgical history. Regarding his social history, he admitted to occasional alcohol use but denied any tobacco or illicit drug use. He was not on any current prescription or over-the-counter medications and denied any allergies.
The patient’s initial vital signs at presentation were: blood pressure, 112/58 mm Hg; heart rate, 86 beats/min; temperature, 97.9°F; and respiratory rate, 18 breaths/min. Oxygen saturation was 100% on room air. The patient reported pain in his left shoulder, epigastric region, and right flank. He rated his pain as a “4” on a 0-to-10 pain scale.
On physical examination, the patient was alert and oriented; he was thin and had mild pallor. His head, eyes, ears, nose, and throat; cardiac; pulmonary; and neurological examinations were normal. The abdominal examination revealed a soft, minimally tender epigastrium but with normal bowel sounds. Initial laboratory studies were remarkable for low hemoglobin (Hgb; 12.0 g/dL) and elevated aspartate transaminase (105 U/L), alanine aminotransferase (168 U/L), total bilirubin (1.6 mg/dL), and glucose (179 mg/dL) levels. The patient’s troponin I and lipase levels were within normal range. An electrocardiogram was unremarkable.
Given the patient’s elevated hepatic enzymes, right upper quadrant ultrasound was obtained, which demonstrated a normal gallbladder, a moderate amount of complicated free fluid (with hyper-echoic densities suggestive of coagulated blood) in all four quadrants, and splenomegaly measuring 13.7 cm (Figure 1a and 1b).
The patient’s status, including his vital signs, remained stable throughout his entire ED course. However, repeat laboratory studies taken 4 hours aft
Positive:
- Epstein-Barr virus (EBV)
- Viral capsid antigen (VCA) immunoglobulin G
- VCA immunoglobulin M
Negative:
- Mononuclear spot test
- Human immunodeficiency virus
- Hepatitis B and C
- Antinuclear antibodies
- Venereal disease research laboratory test
The rest of the patient’s recovery was uneventful, and he was discharged home in stable condition on hospital day 3.
Discussion
Although the spleen is the most common intra-abdominal organ that can rupture with blunt abdominal trauma, splenic rupture in the absence of trauma is very rare. Nontraumatic splenic rupture (NSR) has been associated with pathological and nonpathological spleens.1,2 A systemic review of NSRs showed that 7% of the 845 patients in the review had completely normal spleens; the remaining 93% had some form of splenic pathology.1
Etiology
The top three causes of splenic enlargement associated with NSR include hematologic malignancies, viral infections, and inflammation.1,2 Although viruses, such as EBV and cytomegalovirus, represent almost 15% of the pathological causes of NSR, it is not uncommon for a patient to have multiple pathological processes present.1 Our patient’s enlarged spleen was due to acute infectious mononucleosis.
Signs and Symptoms
Diagnosing NSR can be challenging and it is often missed or discovered incidentally during evaluation (as was initially the case with our patient).3 Several signs and symptoms present in our patient were red herrings that warranted closer analysis. The patient’s complaint of left shoulder pain suggested left hemidiaphragm irritation from the NSR. Furthermore, our patient’s near-syncopal episode was possibly due to acute vagal simulation from the initial contact of blood with the peritoneal cavity.4 The maximal vagal stimulus was likely transient, as our patient returned to baseline after a brief near-syncopal episode.
As illustrated in our case, though tachycardia is common in splenic rupture, not all patients present with this sign. The absence of tachycardia in our patient can be explained by the elevation of his baseline enteric vagal tone due to the continued presence of blood in the peritoneum.5 There are also other factors associated with the absence of tachycardia. For example, a well-conditioned athlete presenting with states of shock due to splenic rupture may not show signs of tachycardia.6
San Francisco Syncope Rule
The San Francisco Syncope Rule (SFSR) is a clinical decision-making risk-stratification tool used to determine outcomes and disposition of ED patients presenting with syncope.7 It is important to note that if we had used a straightforward application of the SFSR upon our patient’s initial presentation, the results would have been negative, suggesting he was not at risk for short-term serious outcomes.7
Imaging Studies
As demonstrated in our patient, a quick point-of-care (POC) bedside ultrasound scan can reveal the presence of free fluid in the abdomen to help with the diagnosis. On ultrasound, the presence of free fluid in the right upper quadrant is more commonly found in the hepatorenal recess, whereas in the left upper quadrant free fluid is seen sub-diaphragmatic/suprasplenic first before fluid is seen in the splenorenal recess. Bedside ultrasound can accurately detect as little as 100 mL of free fluid in the abdominal cavity, with a 90% sensitivity and 99% specificity.8
An ultrasound is highly sensitive as a preliminary screening tool to identify the presence of free intraperitoneal fluid and has some limited utility in identifying any disruption in the splenic echotexture that may suggest a laceration or hematoma. Ultrasound, however, has poor specificity in identifying solid organ injuries.9
Computed tomography scanning is the imaging modality of choice for assessing splenic injuries, and should be obtained to confirm the presence of a solid organ injury, as well as to grade the degree of injury and thereby determine the need for surgical intervention.10 It is worth noting that in a hemodynamically unstable patient, exploratory laparotomy may be embarked upon without a CT scan and positive free fluid on ultrasound.
Splenic Injury Scale
Splenic injury is classified on a scale of 1 (mild injury) to 5 (severe injury) (Table).11
Conclusion
This case illustrates an uncommon presentation of NSR and underscores the importance of considering NSR in the differential diagnoses of patients presenting with abdominal pain—a sign with such a broad differential that NSR could easily be missed during evaluation. Based on its high sensitivity and specificity in detecting the presence of free fluid in the abdominal cavity, POC ultrasound imaging should be used to evaluate patients presenting with abdominal pain and syncopal or near-syncopal symptoms. This case further demonstrates that the absence of tachycardia or signs of shock should not rule out NSR.
1. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;96(10):1114-1121. doi: 10.1002/bjs.6737.
2. Aubrey-Bassler FK, Sowers N. 613 cases of splenic rupture without risk factors or previously diagnosed disease: a systematic review. BMC Emerg Med. 2012;12:11. doi: 10.1186/1471-227X-12-11.
3. Schattner A, Meital A, Mavor E. Red-flag syncope: spontaneous splenic rupture. Am J Med. 2014;127(6):501-502. doi: 10.1016/j.amjmed.2014.02.024.
4. Moya A, Sutton R, Ammirati F, et al; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671. doi: 10.1093/eurheartj/ehp298.
5. Rana MS, Khalid U, Law S. Paradoxical bradycardia in a patient with haemorrhagic shock secondary to blunt abdominal trauma. BMJ Case Rep. 2010;2010. doi: 10.1136/bcr.04.2010.2872.
6. Kiss O, Sydó N, Vargha P, et al. Prevalence of physiological and pathological electrocardiographic findings in Hungarian athletes. Acta Physiol Hung. 2015;102(2):228-237. doi: 10.1556/036.102.2015.2.13.
7. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232.
8. Ma OJ, Mateer JR, Ogata M, Kefer MP, Wittmann D, Aprahamian C. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38(6):879-885.
9. Kendall JL, Faragher J, Hewitt GJ, Burcham G, Haukoos JS. Emergency Department Ultrasound Is not a Sensitive Detector of Solid Organ Injury. West J Emerg Med. 2009;10(1):1-5.
10. Hassan R, Abd Aziz A, Md Ralib AR, Saat A. Computed tomography of blunt spleen injury: a pictorial review. Malays J Med Sci. 2011;18(1):60-67.
11. Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38(3):323-324.
12. Cirocchi R, Boselli C, Corsi A, et al. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care. 2013;17(5):R185. doi: 10.1186/cc12868.
Case
A 25-year-old college student presented to the ED following a near-syncopal episode. The patient stated he had felt lightheaded and had fallen to his knees immediately after taking a shower earlier that morning, but did not experience any loss of consciousness or injury. He denied a history of syncope or any recent trauma or fatigue. A review of the patient’s systems was negative. His medical history was remarkable for irritable bowel syndrome; he had no surgical history. Regarding his social history, he admitted to occasional alcohol use but denied any tobacco or illicit drug use. He was not on any current prescription or over-the-counter medications and denied any allergies.
The patient’s initial vital signs at presentation were: blood pressure, 112/58 mm Hg; heart rate, 86 beats/min; temperature, 97.9°F; and respiratory rate, 18 breaths/min. Oxygen saturation was 100% on room air. The patient reported pain in his left shoulder, epigastric region, and right flank. He rated his pain as a “4” on a 0-to-10 pain scale.
On physical examination, the patient was alert and oriented; he was thin and had mild pallor. His head, eyes, ears, nose, and throat; cardiac; pulmonary; and neurological examinations were normal. The abdominal examination revealed a soft, minimally tender epigastrium but with normal bowel sounds. Initial laboratory studies were remarkable for low hemoglobin (Hgb; 12.0 g/dL) and elevated aspartate transaminase (105 U/L), alanine aminotransferase (168 U/L), total bilirubin (1.6 mg/dL), and glucose (179 mg/dL) levels. The patient’s troponin I and lipase levels were within normal range. An electrocardiogram was unremarkable.
Given the patient’s elevated hepatic enzymes, right upper quadrant ultrasound was obtained, which demonstrated a normal gallbladder, a moderate amount of complicated free fluid (with hyper-echoic densities suggestive of coagulated blood) in all four quadrants, and splenomegaly measuring 13.7 cm (Figure 1a and 1b).
The patient’s status, including his vital signs, remained stable throughout his entire ED course. However, repeat laboratory studies taken 4 hours aft
Positive:
- Epstein-Barr virus (EBV)
- Viral capsid antigen (VCA) immunoglobulin G
- VCA immunoglobulin M
Negative:
- Mononuclear spot test
- Human immunodeficiency virus
- Hepatitis B and C
- Antinuclear antibodies
- Venereal disease research laboratory test
The rest of the patient’s recovery was uneventful, and he was discharged home in stable condition on hospital day 3.
Discussion
Although the spleen is the most common intra-abdominal organ that can rupture with blunt abdominal trauma, splenic rupture in the absence of trauma is very rare. Nontraumatic splenic rupture (NSR) has been associated with pathological and nonpathological spleens.1,2 A systemic review of NSRs showed that 7% of the 845 patients in the review had completely normal spleens; the remaining 93% had some form of splenic pathology.1
Etiology
The top three causes of splenic enlargement associated with NSR include hematologic malignancies, viral infections, and inflammation.1,2 Although viruses, such as EBV and cytomegalovirus, represent almost 15% of the pathological causes of NSR, it is not uncommon for a patient to have multiple pathological processes present.1 Our patient’s enlarged spleen was due to acute infectious mononucleosis.
Signs and Symptoms
Diagnosing NSR can be challenging and it is often missed or discovered incidentally during evaluation (as was initially the case with our patient).3 Several signs and symptoms present in our patient were red herrings that warranted closer analysis. The patient’s complaint of left shoulder pain suggested left hemidiaphragm irritation from the NSR. Furthermore, our patient’s near-syncopal episode was possibly due to acute vagal simulation from the initial contact of blood with the peritoneal cavity.4 The maximal vagal stimulus was likely transient, as our patient returned to baseline after a brief near-syncopal episode.
As illustrated in our case, though tachycardia is common in splenic rupture, not all patients present with this sign. The absence of tachycardia in our patient can be explained by the elevation of his baseline enteric vagal tone due to the continued presence of blood in the peritoneum.5 There are also other factors associated with the absence of tachycardia. For example, a well-conditioned athlete presenting with states of shock due to splenic rupture may not show signs of tachycardia.6
San Francisco Syncope Rule
The San Francisco Syncope Rule (SFSR) is a clinical decision-making risk-stratification tool used to determine outcomes and disposition of ED patients presenting with syncope.7 It is important to note that if we had used a straightforward application of the SFSR upon our patient’s initial presentation, the results would have been negative, suggesting he was not at risk for short-term serious outcomes.7
Imaging Studies
As demonstrated in our patient, a quick point-of-care (POC) bedside ultrasound scan can reveal the presence of free fluid in the abdomen to help with the diagnosis. On ultrasound, the presence of free fluid in the right upper quadrant is more commonly found in the hepatorenal recess, whereas in the left upper quadrant free fluid is seen sub-diaphragmatic/suprasplenic first before fluid is seen in the splenorenal recess. Bedside ultrasound can accurately detect as little as 100 mL of free fluid in the abdominal cavity, with a 90% sensitivity and 99% specificity.8
An ultrasound is highly sensitive as a preliminary screening tool to identify the presence of free intraperitoneal fluid and has some limited utility in identifying any disruption in the splenic echotexture that may suggest a laceration or hematoma. Ultrasound, however, has poor specificity in identifying solid organ injuries.9
Computed tomography scanning is the imaging modality of choice for assessing splenic injuries, and should be obtained to confirm the presence of a solid organ injury, as well as to grade the degree of injury and thereby determine the need for surgical intervention.10 It is worth noting that in a hemodynamically unstable patient, exploratory laparotomy may be embarked upon without a CT scan and positive free fluid on ultrasound.
Splenic Injury Scale
Splenic injury is classified on a scale of 1 (mild injury) to 5 (severe injury) (Table).11
Conclusion
This case illustrates an uncommon presentation of NSR and underscores the importance of considering NSR in the differential diagnoses of patients presenting with abdominal pain—a sign with such a broad differential that NSR could easily be missed during evaluation. Based on its high sensitivity and specificity in detecting the presence of free fluid in the abdominal cavity, POC ultrasound imaging should be used to evaluate patients presenting with abdominal pain and syncopal or near-syncopal symptoms. This case further demonstrates that the absence of tachycardia or signs of shock should not rule out NSR.
Case
A 25-year-old college student presented to the ED following a near-syncopal episode. The patient stated he had felt lightheaded and had fallen to his knees immediately after taking a shower earlier that morning, but did not experience any loss of consciousness or injury. He denied a history of syncope or any recent trauma or fatigue. A review of the patient’s systems was negative. His medical history was remarkable for irritable bowel syndrome; he had no surgical history. Regarding his social history, he admitted to occasional alcohol use but denied any tobacco or illicit drug use. He was not on any current prescription or over-the-counter medications and denied any allergies.
The patient’s initial vital signs at presentation were: blood pressure, 112/58 mm Hg; heart rate, 86 beats/min; temperature, 97.9°F; and respiratory rate, 18 breaths/min. Oxygen saturation was 100% on room air. The patient reported pain in his left shoulder, epigastric region, and right flank. He rated his pain as a “4” on a 0-to-10 pain scale.
On physical examination, the patient was alert and oriented; he was thin and had mild pallor. His head, eyes, ears, nose, and throat; cardiac; pulmonary; and neurological examinations were normal. The abdominal examination revealed a soft, minimally tender epigastrium but with normal bowel sounds. Initial laboratory studies were remarkable for low hemoglobin (Hgb; 12.0 g/dL) and elevated aspartate transaminase (105 U/L), alanine aminotransferase (168 U/L), total bilirubin (1.6 mg/dL), and glucose (179 mg/dL) levels. The patient’s troponin I and lipase levels were within normal range. An electrocardiogram was unremarkable.
Given the patient’s elevated hepatic enzymes, right upper quadrant ultrasound was obtained, which demonstrated a normal gallbladder, a moderate amount of complicated free fluid (with hyper-echoic densities suggestive of coagulated blood) in all four quadrants, and splenomegaly measuring 13.7 cm (Figure 1a and 1b).
The patient’s status, including his vital signs, remained stable throughout his entire ED course. However, repeat laboratory studies taken 4 hours aft
Positive:
- Epstein-Barr virus (EBV)
- Viral capsid antigen (VCA) immunoglobulin G
- VCA immunoglobulin M
Negative:
- Mononuclear spot test
- Human immunodeficiency virus
- Hepatitis B and C
- Antinuclear antibodies
- Venereal disease research laboratory test
The rest of the patient’s recovery was uneventful, and he was discharged home in stable condition on hospital day 3.
Discussion
Although the spleen is the most common intra-abdominal organ that can rupture with blunt abdominal trauma, splenic rupture in the absence of trauma is very rare. Nontraumatic splenic rupture (NSR) has been associated with pathological and nonpathological spleens.1,2 A systemic review of NSRs showed that 7% of the 845 patients in the review had completely normal spleens; the remaining 93% had some form of splenic pathology.1
Etiology
The top three causes of splenic enlargement associated with NSR include hematologic malignancies, viral infections, and inflammation.1,2 Although viruses, such as EBV and cytomegalovirus, represent almost 15% of the pathological causes of NSR, it is not uncommon for a patient to have multiple pathological processes present.1 Our patient’s enlarged spleen was due to acute infectious mononucleosis.
Signs and Symptoms
Diagnosing NSR can be challenging and it is often missed or discovered incidentally during evaluation (as was initially the case with our patient).3 Several signs and symptoms present in our patient were red herrings that warranted closer analysis. The patient’s complaint of left shoulder pain suggested left hemidiaphragm irritation from the NSR. Furthermore, our patient’s near-syncopal episode was possibly due to acute vagal simulation from the initial contact of blood with the peritoneal cavity.4 The maximal vagal stimulus was likely transient, as our patient returned to baseline after a brief near-syncopal episode.
As illustrated in our case, though tachycardia is common in splenic rupture, not all patients present with this sign. The absence of tachycardia in our patient can be explained by the elevation of his baseline enteric vagal tone due to the continued presence of blood in the peritoneum.5 There are also other factors associated with the absence of tachycardia. For example, a well-conditioned athlete presenting with states of shock due to splenic rupture may not show signs of tachycardia.6
San Francisco Syncope Rule
The San Francisco Syncope Rule (SFSR) is a clinical decision-making risk-stratification tool used to determine outcomes and disposition of ED patients presenting with syncope.7 It is important to note that if we had used a straightforward application of the SFSR upon our patient’s initial presentation, the results would have been negative, suggesting he was not at risk for short-term serious outcomes.7
Imaging Studies
As demonstrated in our patient, a quick point-of-care (POC) bedside ultrasound scan can reveal the presence of free fluid in the abdomen to help with the diagnosis. On ultrasound, the presence of free fluid in the right upper quadrant is more commonly found in the hepatorenal recess, whereas in the left upper quadrant free fluid is seen sub-diaphragmatic/suprasplenic first before fluid is seen in the splenorenal recess. Bedside ultrasound can accurately detect as little as 100 mL of free fluid in the abdominal cavity, with a 90% sensitivity and 99% specificity.8
An ultrasound is highly sensitive as a preliminary screening tool to identify the presence of free intraperitoneal fluid and has some limited utility in identifying any disruption in the splenic echotexture that may suggest a laceration or hematoma. Ultrasound, however, has poor specificity in identifying solid organ injuries.9
Computed tomography scanning is the imaging modality of choice for assessing splenic injuries, and should be obtained to confirm the presence of a solid organ injury, as well as to grade the degree of injury and thereby determine the need for surgical intervention.10 It is worth noting that in a hemodynamically unstable patient, exploratory laparotomy may be embarked upon without a CT scan and positive free fluid on ultrasound.
Splenic Injury Scale
Splenic injury is classified on a scale of 1 (mild injury) to 5 (severe injury) (Table).11
Conclusion
This case illustrates an uncommon presentation of NSR and underscores the importance of considering NSR in the differential diagnoses of patients presenting with abdominal pain—a sign with such a broad differential that NSR could easily be missed during evaluation. Based on its high sensitivity and specificity in detecting the presence of free fluid in the abdominal cavity, POC ultrasound imaging should be used to evaluate patients presenting with abdominal pain and syncopal or near-syncopal symptoms. This case further demonstrates that the absence of tachycardia or signs of shock should not rule out NSR.
1. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;96(10):1114-1121. doi: 10.1002/bjs.6737.
2. Aubrey-Bassler FK, Sowers N. 613 cases of splenic rupture without risk factors or previously diagnosed disease: a systematic review. BMC Emerg Med. 2012;12:11. doi: 10.1186/1471-227X-12-11.
3. Schattner A, Meital A, Mavor E. Red-flag syncope: spontaneous splenic rupture. Am J Med. 2014;127(6):501-502. doi: 10.1016/j.amjmed.2014.02.024.
4. Moya A, Sutton R, Ammirati F, et al; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671. doi: 10.1093/eurheartj/ehp298.
5. Rana MS, Khalid U, Law S. Paradoxical bradycardia in a patient with haemorrhagic shock secondary to blunt abdominal trauma. BMJ Case Rep. 2010;2010. doi: 10.1136/bcr.04.2010.2872.
6. Kiss O, Sydó N, Vargha P, et al. Prevalence of physiological and pathological electrocardiographic findings in Hungarian athletes. Acta Physiol Hung. 2015;102(2):228-237. doi: 10.1556/036.102.2015.2.13.
7. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232.
8. Ma OJ, Mateer JR, Ogata M, Kefer MP, Wittmann D, Aprahamian C. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38(6):879-885.
9. Kendall JL, Faragher J, Hewitt GJ, Burcham G, Haukoos JS. Emergency Department Ultrasound Is not a Sensitive Detector of Solid Organ Injury. West J Emerg Med. 2009;10(1):1-5.
10. Hassan R, Abd Aziz A, Md Ralib AR, Saat A. Computed tomography of blunt spleen injury: a pictorial review. Malays J Med Sci. 2011;18(1):60-67.
11. Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38(3):323-324.
12. Cirocchi R, Boselli C, Corsi A, et al. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care. 2013;17(5):R185. doi: 10.1186/cc12868.
1. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Systematic review of atraumatic splenic rupture. Br J Surg. 2009;96(10):1114-1121. doi: 10.1002/bjs.6737.
2. Aubrey-Bassler FK, Sowers N. 613 cases of splenic rupture without risk factors or previously diagnosed disease: a systematic review. BMC Emerg Med. 2012;12:11. doi: 10.1186/1471-227X-12-11.
3. Schattner A, Meital A, Mavor E. Red-flag syncope: spontaneous splenic rupture. Am J Med. 2014;127(6):501-502. doi: 10.1016/j.amjmed.2014.02.024.
4. Moya A, Sutton R, Ammirati F, et al; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631-2671. doi: 10.1093/eurheartj/ehp298.
5. Rana MS, Khalid U, Law S. Paradoxical bradycardia in a patient with haemorrhagic shock secondary to blunt abdominal trauma. BMJ Case Rep. 2010;2010. doi: 10.1136/bcr.04.2010.2872.
6. Kiss O, Sydó N, Vargha P, et al. Prevalence of physiological and pathological electrocardiographic findings in Hungarian athletes. Acta Physiol Hung. 2015;102(2):228-237. doi: 10.1556/036.102.2015.2.13.
7. Quinn JV, Stiell IG, McDermott DA, Sellers KL, Kohn MA, Wells GA. Derivation of the San Francisco Syncope Rule to predict patients with short-term serious outcomes. Ann Emerg Med. 2004;43(2):224-232.
8. Ma OJ, Mateer JR, Ogata M, Kefer MP, Wittmann D, Aprahamian C. Prospective analysis of a rapid trauma ultrasound examination performed by emergency physicians. J Trauma. 1995;38(6):879-885.
9. Kendall JL, Faragher J, Hewitt GJ, Burcham G, Haukoos JS. Emergency Department Ultrasound Is not a Sensitive Detector of Solid Organ Injury. West J Emerg Med. 2009;10(1):1-5.
10. Hassan R, Abd Aziz A, Md Ralib AR, Saat A. Computed tomography of blunt spleen injury: a pictorial review. Malays J Med Sci. 2011;18(1):60-67.
11. Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38(3):323-324.
12. Cirocchi R, Boselli C, Corsi A, et al. Is non-operative management safe and effective for all splenic blunt trauma? A systematic review. Crit Care. 2013;17(5):R185. doi: 10.1186/cc12868.
Hypertension in the ED
Case Scenarios
Case 1
You had just started your shift, and your first patient presented with symptoms of headache and dizziness, and a blood pressure (BP) of 240/130 mm Hg, without any vomiting or visual symptoms. Physical examination revealed an alert, pleasant 65-year-old black man whose ocular, neurological, and cardiovascular (CV) examinations were normal. The patient reported a history of borderline hypertension, but had never taken any medications for it.
After placing some initial orders, including an electrocardiogram (ECG), basic metabolic panel (BMP), and head computed tomography (CT) scan, and ordering 10 mg intravenous (IV) prochlorperazine and 25 mg IV diphenhydramine to treat the patient’s headache, you are left asking yourself what steps you should take next.
Case 2
Your next patient was a 90-year-old white woman who had been referred to the ED by her primary care physician (PCP) for “hypertensive urgency.” She had no complaints to report. Similar to the first patient, this patient’s physical examination was also normal, with the exception of a persistently elevated BP of 220/140 mm Hg. Her history was significant for congestive heart failure (CHF), but she exhibited no current CV signs or symptoms. The patient had been taking furosemide but was not on any other antihypertensive medications.
Case 3
In the room next to your 90-year-old patient is a 32-year-old uninsured hypertensive white woman. During the history taking, the patient stated that she was trying to become pregnant and was not currently using any form of contraception. Similar to the second patient, she had no complaints to report. Regarding her reason for presentation, the patient stated that when she had her BP checked at a pharmacy earlier that day, the reading was “too high,” and the pharmacist had advised her to go to the ED. She seemed anxious but otherwise well. Her initial BP at presentation was 240/100 mm Hg, but her physical examination was otherwise normal.
Hypertensive Emergencies
As emergency physicians (EPs), we see hypertensive patients every day. According to the US Centers for Disease Control and Prevention, 33% of American adults have hypertension, which is defined by a BP of ≥140/90 mm Hg (Table 1).
Almost 25% of total annual US adult ED visits are directly or indirectly related to hypertension, and about 1% of all ED visits are due solely to elevated BP.3 In an ambulatory care survey for 2007, moderate or severe hypertensive BP readings were found to be more common in patients presenting to the ED (43.5%) than to primary care clinics (27%).4 Patients presenting to the ED with hypertensive BP readings disproportionately represented patients who were older, male, non-Hispanic black, Medicare beneficiaries, or uninsured. Certainly, some patients presenting to the ED have hypertensive BP readings due to pain or anxiety, but multiple studies have suggested that 50% to 70% of ED patients who have hypertensive BP readings will be diagnosed with hypertension on office follow-up.5,6 While a minority of these patients present to the ED with hypertensive emergencies, the majority present either without symptoms of hypertension or with only mild headache. Given the disease burden of hypertension combined with the benefits of treatment, it is worthwhile for the practicing EP to review the most up-to-date guidelines on outpatient management of hypertension.
When a patient presents to the ED with a hypertensive BP reading, the initial priority of the EP is to exclude hypertensive emergency. Hypertensive emergencies are defined by the presence of hypertension (generally grade 3/severe hypertension with BP ≥180/110 mm Hg; see Table 1) in conjunction with evidence of target organ damage.
Target Organ Manifestations
The acuity and/or presence of target organ damage are not always clear on initial ED evaluation. For instance, when a patient who has no history of primary care presents to the ED with severe hypertension, laboratory evaluation may demonstrate protein and blood in his or her urine and an elevated serum creatinine level. In the absence of values from past laboratory studies, it is difficult to determine whether these test results represent normal laboratory parameters for this patient due to longstanding hypertensive kidney disease (ie, hypertensive nephrosclerosis) or if they represent a true hypertensive emergency, (ie, hypertensive emergency-related nephropathy).7 In patients with severe hypertension and possibly new acute kidney injury, it is probably safest to either assume hypertensive emergency-related nephropathy and to treat accordingly or consult with nephrology services. The picture of hypertensive emergency-related nephropathy often only becomes clear after renal biopsy results and improvement in renal parameters with BP control.
The ocular manifestations of hypertensive emergency require detailed fundoscopy, which at times can be challenging in the ED. In assessing for cardiac target organ damage, at our institution, we typically ask patients if they have experienced symptoms of dyspnea and chest pain or pressure. Generally, we also evaluate cardiac enzymes, B-type natriuretic peptide, and order ECG and chest X-ray studies when suggested by history or physical examination. Alarmingly, a study of 161 ED hypertensive (average BP of 183/109 mm Hg), asymptomatic, predominantly black patients found that 146 (90.7%) had subclinical hypertensive heart disease on point-of-care echocardiogram.8
Neurological/Hypertensive Encephalopathy
Hypertensive encephalopathy is a diagnosis of exclusion as alternate causes of confusion and headache, such as intracranial hemorrhage, are excluded and mental status improves with titrated BP control. Nonetheless, it is difficult to confidently state from the literature that patients who present with headache but have a normal mental status in the presence of severe hypertension are not on an early spectrum of hypertensive encephalopathy. Therefore, it is likely that the degree of symptoms should define whether target organ damage exists, though there is certainly a spectrum of hypertensive emergency—the strict definition of which is not always clear.
When a hypertensive emergency is diagnosed, management typically involves the use of antihypertensive IV medication in the intensive care unit. While such management is outside the scope of this paper, Adebayo and Rogers9 have published an excellent review of the care of hypertensive emergencies.
Asymptomatic Hypertension
The American College of Emergency Physicians (ACEP) has developed two clinical policies on the evaluation and management of asymptomatic hypertension in the ED. The original, published in 2006, advised that initially high BP readings of ED patients should be repeated: two separate high readings are adequate for screening, and those patients with hypertension should be referred for follow-up. Furthermore, ACEP policies note that initiating treatment in the ED is not necessary when patients are referred for follow-up. If treatment for hypertension is initiated in the ED, ACEP recommends that such management should attempt only to gradually lower BP, and not to normalize it during the initial ED visit.10
The 2013 update to ACEP’s clinical policy on managing asymptomatic hypertension expanded on the original policy. The updated policy advised against routine testing for target organ damage in patients who have asymptomatic severe hypertension. However, ACEP policy notes that evaluating serum creatinine in these patients with poor follow-up may influence patient disposition.11
The 2013 policy further stated that medical intervention is not required in ED patients who have asymptomatic severe hypertension, but may be considered in patients with poor follow-up. The policies emphasize that all asymptomatic hypertensive patients should be referred for follow-up. The literature cited for the recommendation that ED patients with asymptomatic severe hypertension do not require routine investigation stems from two observational studies. These studies found that screening asymptomatic ED patients who presented with severe hypertension revealed serum creatinine abnormalities in approximately 6%, which impacted patient disposition, though it was not clear from the study results whether admission correlated to meaningful patient outcomes.12,13
Patient Disposition
Since ACEP’s 2013 clinical policy, a study from the Cleveland Clinic has been published. This retrospective cohort study reviewed 6 years of data looking at all patients in its system with a BP of ≥180/110 mm Hg, and compared those office patients discharged to home to those referred to the ED or directly admitted to the inpatient hospital solely on the basis of severe hypertension.14 The study found that 0.5% of 387 patients referred to the ED by primary care clinics for asymptomatic severe hypertension had confirmed acute kidney injury on BMP.14 The Cleveland Clinic study also found that 2.1% of patients had evidence of target organ damage and 5.5% had any abnormal results.14 In addition, referral to the ED from the clinic for hypertension was associated with a slightly higher rate of major adverse CV events at 7 days (2 of 426 [0.5%] versus 61 of 58,109 [0.1%]; P = .02).14
The results of the Cleveland Clinic study confirm that in the absence of target organ damage, hypertension is probably best managed in the outpatient setting. The European Task Force hypertensive guidelines state “hospitalization for hypertension is regarded as inappropriate in most European countries.”15 However, from 2006 to 2012, 26% of US ED patients with primary diagnoses of hypertension were admitted to the hospital.3 In Canada’s most populous province of Ontario, from 2002 to 2011, approximately 8% of hypertensive patients were admitted.16 Part of this discrepancy may be due to the sometimes ambiguous nature of the presentation of patients with hypertension, making it unclear whether a true hypertensive emergency exists. Many patients perceive visual symptoms, headache, dizziness, and even chest pressure as the result of their elevated BP—without clear findings on fundoscopy, ECG, or cardiac marker testing. Perhaps more of these patients would be discharged if EPs felt comfortable initiating appropriate initial antihypertensive treatment.
Management
Initiating Antihypertensive Treatment
Some EPs may feel that an accurate diagnosis of hypertension requires repeat BP testing in the primary care office setting, and for this reason are reluctant to initiate antihypertensive treatment in the ED. The most recent guidelines by the Joint National Committee (JNC 8) do not address how many BP readings are necessary to diagnose hypertension, but JNC 7 suggested that diagnosis of hypertension requires two separate office visits.17 Evidence cited in ACEP’s first clinical policy states that two separate BP measurements in the ED are adequate for screening—but not necessarily for initiating treatment.10 However, European and British outpatient clinical recommendations advocate initiation of antihypertensive medication for a single visit in patients who have an elevated BP categorized as grade 3/severe hypertension (BP of ≥180/110 mm Hg).15,18 Furthermore, for patients with severe hypertension seen in the ED, as many as 97% are likely to have true hypertension at office follow-up.6 Those ED patients presenting with severe hypertension are very likely to have a true diagnosis of hypertension.
A recent retrospective analysis of a group of hypertensive ED patients by Brody et al19 found that patients prescribed BP medications by an EP were more likely to have improved BP control at follow-up 2 weeks later. In their study, the decision to prescribe antihypertensive medications were at the discretion of the EP. Seventy-six patients were given one or more prescriptions for antihypertensive therapy, compared to a control group of 141 patients who were not given a prescription. On follow-up at 2 weeks, there was an 11 mm Hg greater reduction of BP in the group who received prescriptions compared to the control group. None of the patients in either group on follow-up had experienced any new neurological deficits, ischemic events, life-threatening anaphylactic reactions, or clinically significant hypotension.
The Cleveland Clinic study14 also reported on those patents given who received new prescriptions from the ED. Similar to the study by Brody et al,19 none of the 82 patients discharged to home from the ED with a new antihypertensive prescription had any major adverse event at 30-day follow-up.14
Pharmacological Treatment Recommendations
When choosing to treat patients with new prescriptions for antihypertensives, it is important to follow the most current outpatient treatment recommendations. In 2014, JNC 8 released new guidelines for the outpatient management of adults with hypertension.20 The panel issued recommendations based on its systematic review of randomized controlled trials on antihypertensive treatments. The key recommendations are as follows:
- In patients aged 60 years or older, initiate pharmacological treatment at a BP of ≥150/90 mm Hg.
- In patients aged 18 to 59 years, initiate pharmacological treatment at a BP of ≥140/90 mm Hg.
- In the general nonblack population, initial antihypertensive treatment should include a thiazide-type diuretic, a calcium channel blocker (CCB), an angiotensin-converting enzyme inhibitor (ACE-I), or an angiotensin receptor blocker (ARB).
- In the general black population, initial treatment should include a thiazide-type diuretic or a CCB.
- In patients with chronic kidney disease (CKD) (including black patients), initial (or add-on) antihypertensive treatment should include an ACE-I or ARB to improve kidney outcomes—but not both.
- If goal BP is not reached within 1 month of initial treatment, increase the dose of the initial drug or add a second agent (eg, thiazide-type diuretic, CCB, ACE-I, or ARB). If goal is not reached with two drugs, use the third drug from that list if no contraindications exist, but do not use both an ACE-I and an ARB together in the same patient.
Of note, JNC 8, in departure from JNC 7, no longer recommends beta-blockers as first-line therapy for isolated hypertension (there may be compelling alternate indications, such as atrial fibrillation or postmyocardial infarction (MI), such that a beta-blocker would still be the first medication considered). The reason for this stems from a single randomized controlled trial of 9,193 patients that found that despite equivalent BP reduction, use of a beta-blocker in comparison to an ARB resulted in a higher rate of a composite outcome of death, MI, or stroke.21 The main difference was a 25% relative risk reduction for stroke with use of an ARB (losartan) in comparison to a beta-blocker (atenolol). The most recent European guidelines still include beta-blockers among its first-line recommended BP medications, but do acknowledge that they are not as effective in reducing stroke incidence as other alternative medications.15 The European guidelines otherwise include the same list of first-line agents. The British guidelines mirror JNC 8 in terms of first-line antihypertensive medication choices.18
Since the release of JNC 8, the Systolic Blood Pressure Intervention Trial (SPRINT) has been published, and will likely impact future national recommendations on BP management. The SPRINT study was a randomized controlled trial enrolling over 9,000 hypertensive nondiabetic patients older than age 50 years that treated individuals to a standard BP goal (systolic BP of 140 mm Hg) versus an intensive BP goal (systolic BP of 120 mm Hg) over a 3.5-year period. The trial was stopped early for safety as a 25% mortality reduction was observed in the intensive treatment group (1.65 vs 2.19 deaths/y).22 This was in contrast to previous trials that had mostly failed to show this sort of benefit, though previous trials were smaller in number or included only diabetic patients.23 While it is likely that this trial may influence lowering treatment thresholds from the office, it is not likely to impact care from the ED.
The recommendations of JNC 8 do not necessarily coincide with current US EP practice. In the study by Brody et al,19 of patients provided ED antihypertensive prescriptions, 54% received thiazide-type diuretics, 26% ACE-I, 10% CCBs, and 6% beta-blockers. This is noteworthy because 96% of those in the study were black patients who would benefit most from either a thiazide or a CCB. Another recent study of ED patients showed that of patients who were both treated in the ED and discharged with antihypertensive medications, 34% received a diuretic prescription, 32% clonidine, 15% a beta-blocker, 19% an ARB or ACE-I, 12% a CCB, and 2% hydrazine.24 These results are important because according to many published guidelines, including JNC 8, clonidine is only considered one of several fourth-line options for severe resistant hypertension.15,18,20 Since clonidine use can be complicated by rebound hypertension, it is not an ideal agent to be prescribed de novo to patients in the ED. This is particularly true if these patients are not already on maximum doses of the three most recommended agents previously noted, or if there are concerns over patient compliance.
Of the drug classes recommended by JNC 8, Table 3 lists the absolute and relative contraindications.
In clinical trials, amlodipine is among the most effective BP medications and is considered first-line therapy for all groups of patients with hypertension.15,18,20 A simplistic approach for most patients presenting with severe asymptomatic hypertension (BP of ≥180/110 mm Hg) not currently on treatment would be to recheck the BP and assure it remains elevated over the period of the ED visit.
Conclusion
Hypertension is among the most common medical conditions for which emergency patients seek care. The ACEP clinical policies provide guidance on the appropriate work-up and treatment of these patients. Given the occasional lack of clarity on whether a patient’s presentation is on the spectrum of more acute/serious, EPs may feel more comfortable in discharging patients with poor follow-up if they are able to safely prescribe antihypertensive treatment. Prior to prescribing treatment, EPs should refer to the JNC 8 guidelines to appropriately start antihypertensive treatment in select patient groups in the ED. The guidelines of JNC 8 are therefore worth referring to in order to appropriately start treatment in select patient groups from the ED.
Case Scenarios Continued
Case 1
[The 65-year-old black man who presented with headache and dizziness, and had an initial BP of 240/130 mm Hg.]
After treating the patient with prochlorperazine and diphenhydramine, his headache resolved. His BP improved but remained elevated at 190/120 mm Hg. On further questioning, the patient reported a history of similar headaches and wondered whether it was related to his BP. The head CT scan was negative for any acute hemorrhage, infarct, or mass; the ECG only showed evidence of left ventricular hypertrophy; and the BMP showed normal renal function.
After a long discussion with the patient, you agreed to start him on amlodipine 5 mg/d and referred him for follow-up with a local PCP.
Case 2
[The 90-year-old white woman with a history of CHF and an initial BP of 220/140 mm Hg at presentation.]
The BMP evaluation showed a baseline creatinine level of 1.3 mg/dL. Given this patient’s history of CHF, amlodipine would not be the ideal next agent to prescribe. After discussion with her PCP, you elected to start her on losartan at 25 mg/d, and instructed her to follow-up with her PCP within 1 week.
Case 3
[The 32-year-old white woman who presented at the advice of a pharmacist and had an initial BP of 240/100 mm Hg.]While reviewing the patient’s work-up and history, you noted her plans to become pregnant, and recalled a recent review on BP management, noting the contraindications associated with ARB or ACE-I in pregnancy. Based on the patient’s uninsured status and poor follow-up, you considered prescribing amlodipine. Prior to issuing the prescription, you performed a repeat BP check and noted that the patient’s BP had decreased to 130/85 mm Hg. Given the marked improvement in the patient’s BP during her ED course, you were not convinced that she truly had hypertension.
Instead of prescribing an antihypertensive agent, which may not ultimately benefit this patient, you advised her to seek follow-up care at an outpatient clinic to have her BP rechecked. The patient agreed, and you referred her to a local free clinic.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
2. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2013;362(9395):1527-1535.
3. McNaughton CD, Self WH, Zhu Y, Janke AT, Storrow AB, Levy P. Incidence of hypertension-related emergency department visits in the United States, 2006 to 2012. Am J Cardiol. 2015;116(11):1717-1723. doi: 10.1016/j.amjcard.2015.09.007.
4. Niska RW. Blood pressure measurements at emergency department visits by adults: United States, 2007-2008. NCHS Data Brief. 2011;(72):1-8.
5. Chernow SM, Iserson KV, Criss E. Use of the emergency department for hypertension screening: a prospective study. Ann Emerg Med. 1987;16(2):180-182.
6. Backer HD, Decker L, Ackerson L. Reproducibility of increased blood pressure during an emergency department or urgent care visit. Ann Emerg Med. 2003.41(4):507-512.
7. Nonaka K, Ubara Y, Sumida K, et al. Clinical and pathological evaluation of hypertensive emergency-related nephropathy. Intern Med. 2013;52(1):45-53.
8. Levy P, Ye H, Compton S, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60(4):467-474.e1. doi: 10.1016/j.annemergmed.2012.03.030.
9. Adebayo O, Rogers RL. Hypertensive emergencies in the emergency department. Emerg Med Clin North Am. 2015;33(3):539-551. doi: 10.1016/j.emc.2015.04.005.
10. Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-234. doi: 10.1016/j.annemergmed.2005.10.003
11. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68. doi: 10.1016/j.annemergmed.2013.05.012.
12. Karras DJ, Kruus LK, Cienki JJ, et al. Evaluation and treatment of patients with severely elevated blood pressure in academic emergency departments: a multicenter study. Ann Emerg Med. 2006;47(3):230-236.
13. Nishijima DK, Paladino L, Sinert R. Routine testing in patients with asymptomatic elevated blood pressure in the ED. Am J Emerg Med. 2010;28(2):235-242. doi: 10.1016/j.ajem.2008.11.015.
14. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509.
15. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151.
16. Masood S, Austin PC, Atzema CL. A population-based analysis of outcomes in patients with a primary diagnosis of hypertension in the emergency department. Ann Emerg Med. 2016;68(3):258-267.e5. doi: 10.1016/j.annemergmed.2016.04.060.
17. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. Erratum in: JAMA. 2003;290(2):197.
18. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891.
19. Brody A, Rahman T, Reed B, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22(5):632-635. doi: 10.1111/acem.12660.
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014;311(17):1809.
21. Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
22. PRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939.
23. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286.
24. Levy PD, Mahn JJ, Miller J, et al. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med. 2015;33(9):1219-1224. doi: 10.1016/j.ajem.2015.05.036.
25. Leung AA, Wright A, Pazo V, Karson A, Bates DW. Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med. 2011;124(11):1064-1072. doi: 10.1016/j.amjmed.2011.06.031.
26. Mann JFE, Hilgers KF. Renin-angiotensin system inhibition in the treatment of hypertension. http://www.uptodate.com/contents/renin-angiotensin-system-inhibition-in-the-treatment-of-hypertension. Accessed September 21, 2016.
Case Scenarios
Case 1
You had just started your shift, and your first patient presented with symptoms of headache and dizziness, and a blood pressure (BP) of 240/130 mm Hg, without any vomiting or visual symptoms. Physical examination revealed an alert, pleasant 65-year-old black man whose ocular, neurological, and cardiovascular (CV) examinations were normal. The patient reported a history of borderline hypertension, but had never taken any medications for it.
After placing some initial orders, including an electrocardiogram (ECG), basic metabolic panel (BMP), and head computed tomography (CT) scan, and ordering 10 mg intravenous (IV) prochlorperazine and 25 mg IV diphenhydramine to treat the patient’s headache, you are left asking yourself what steps you should take next.
Case 2
Your next patient was a 90-year-old white woman who had been referred to the ED by her primary care physician (PCP) for “hypertensive urgency.” She had no complaints to report. Similar to the first patient, this patient’s physical examination was also normal, with the exception of a persistently elevated BP of 220/140 mm Hg. Her history was significant for congestive heart failure (CHF), but she exhibited no current CV signs or symptoms. The patient had been taking furosemide but was not on any other antihypertensive medications.
Case 3
In the room next to your 90-year-old patient is a 32-year-old uninsured hypertensive white woman. During the history taking, the patient stated that she was trying to become pregnant and was not currently using any form of contraception. Similar to the second patient, she had no complaints to report. Regarding her reason for presentation, the patient stated that when she had her BP checked at a pharmacy earlier that day, the reading was “too high,” and the pharmacist had advised her to go to the ED. She seemed anxious but otherwise well. Her initial BP at presentation was 240/100 mm Hg, but her physical examination was otherwise normal.
Hypertensive Emergencies
As emergency physicians (EPs), we see hypertensive patients every day. According to the US Centers for Disease Control and Prevention, 33% of American adults have hypertension, which is defined by a BP of ≥140/90 mm Hg (Table 1).
Almost 25% of total annual US adult ED visits are directly or indirectly related to hypertension, and about 1% of all ED visits are due solely to elevated BP.3 In an ambulatory care survey for 2007, moderate or severe hypertensive BP readings were found to be more common in patients presenting to the ED (43.5%) than to primary care clinics (27%).4 Patients presenting to the ED with hypertensive BP readings disproportionately represented patients who were older, male, non-Hispanic black, Medicare beneficiaries, or uninsured. Certainly, some patients presenting to the ED have hypertensive BP readings due to pain or anxiety, but multiple studies have suggested that 50% to 70% of ED patients who have hypertensive BP readings will be diagnosed with hypertension on office follow-up.5,6 While a minority of these patients present to the ED with hypertensive emergencies, the majority present either without symptoms of hypertension or with only mild headache. Given the disease burden of hypertension combined with the benefits of treatment, it is worthwhile for the practicing EP to review the most up-to-date guidelines on outpatient management of hypertension.
When a patient presents to the ED with a hypertensive BP reading, the initial priority of the EP is to exclude hypertensive emergency. Hypertensive emergencies are defined by the presence of hypertension (generally grade 3/severe hypertension with BP ≥180/110 mm Hg; see Table 1) in conjunction with evidence of target organ damage.
Target Organ Manifestations
The acuity and/or presence of target organ damage are not always clear on initial ED evaluation. For instance, when a patient who has no history of primary care presents to the ED with severe hypertension, laboratory evaluation may demonstrate protein and blood in his or her urine and an elevated serum creatinine level. In the absence of values from past laboratory studies, it is difficult to determine whether these test results represent normal laboratory parameters for this patient due to longstanding hypertensive kidney disease (ie, hypertensive nephrosclerosis) or if they represent a true hypertensive emergency, (ie, hypertensive emergency-related nephropathy).7 In patients with severe hypertension and possibly new acute kidney injury, it is probably safest to either assume hypertensive emergency-related nephropathy and to treat accordingly or consult with nephrology services. The picture of hypertensive emergency-related nephropathy often only becomes clear after renal biopsy results and improvement in renal parameters with BP control.
The ocular manifestations of hypertensive emergency require detailed fundoscopy, which at times can be challenging in the ED. In assessing for cardiac target organ damage, at our institution, we typically ask patients if they have experienced symptoms of dyspnea and chest pain or pressure. Generally, we also evaluate cardiac enzymes, B-type natriuretic peptide, and order ECG and chest X-ray studies when suggested by history or physical examination. Alarmingly, a study of 161 ED hypertensive (average BP of 183/109 mm Hg), asymptomatic, predominantly black patients found that 146 (90.7%) had subclinical hypertensive heart disease on point-of-care echocardiogram.8
Neurological/Hypertensive Encephalopathy
Hypertensive encephalopathy is a diagnosis of exclusion as alternate causes of confusion and headache, such as intracranial hemorrhage, are excluded and mental status improves with titrated BP control. Nonetheless, it is difficult to confidently state from the literature that patients who present with headache but have a normal mental status in the presence of severe hypertension are not on an early spectrum of hypertensive encephalopathy. Therefore, it is likely that the degree of symptoms should define whether target organ damage exists, though there is certainly a spectrum of hypertensive emergency—the strict definition of which is not always clear.
When a hypertensive emergency is diagnosed, management typically involves the use of antihypertensive IV medication in the intensive care unit. While such management is outside the scope of this paper, Adebayo and Rogers9 have published an excellent review of the care of hypertensive emergencies.
Asymptomatic Hypertension
The American College of Emergency Physicians (ACEP) has developed two clinical policies on the evaluation and management of asymptomatic hypertension in the ED. The original, published in 2006, advised that initially high BP readings of ED patients should be repeated: two separate high readings are adequate for screening, and those patients with hypertension should be referred for follow-up. Furthermore, ACEP policies note that initiating treatment in the ED is not necessary when patients are referred for follow-up. If treatment for hypertension is initiated in the ED, ACEP recommends that such management should attempt only to gradually lower BP, and not to normalize it during the initial ED visit.10
The 2013 update to ACEP’s clinical policy on managing asymptomatic hypertension expanded on the original policy. The updated policy advised against routine testing for target organ damage in patients who have asymptomatic severe hypertension. However, ACEP policy notes that evaluating serum creatinine in these patients with poor follow-up may influence patient disposition.11
The 2013 policy further stated that medical intervention is not required in ED patients who have asymptomatic severe hypertension, but may be considered in patients with poor follow-up. The policies emphasize that all asymptomatic hypertensive patients should be referred for follow-up. The literature cited for the recommendation that ED patients with asymptomatic severe hypertension do not require routine investigation stems from two observational studies. These studies found that screening asymptomatic ED patients who presented with severe hypertension revealed serum creatinine abnormalities in approximately 6%, which impacted patient disposition, though it was not clear from the study results whether admission correlated to meaningful patient outcomes.12,13
Patient Disposition
Since ACEP’s 2013 clinical policy, a study from the Cleveland Clinic has been published. This retrospective cohort study reviewed 6 years of data looking at all patients in its system with a BP of ≥180/110 mm Hg, and compared those office patients discharged to home to those referred to the ED or directly admitted to the inpatient hospital solely on the basis of severe hypertension.14 The study found that 0.5% of 387 patients referred to the ED by primary care clinics for asymptomatic severe hypertension had confirmed acute kidney injury on BMP.14 The Cleveland Clinic study also found that 2.1% of patients had evidence of target organ damage and 5.5% had any abnormal results.14 In addition, referral to the ED from the clinic for hypertension was associated with a slightly higher rate of major adverse CV events at 7 days (2 of 426 [0.5%] versus 61 of 58,109 [0.1%]; P = .02).14
The results of the Cleveland Clinic study confirm that in the absence of target organ damage, hypertension is probably best managed in the outpatient setting. The European Task Force hypertensive guidelines state “hospitalization for hypertension is regarded as inappropriate in most European countries.”15 However, from 2006 to 2012, 26% of US ED patients with primary diagnoses of hypertension were admitted to the hospital.3 In Canada’s most populous province of Ontario, from 2002 to 2011, approximately 8% of hypertensive patients were admitted.16 Part of this discrepancy may be due to the sometimes ambiguous nature of the presentation of patients with hypertension, making it unclear whether a true hypertensive emergency exists. Many patients perceive visual symptoms, headache, dizziness, and even chest pressure as the result of their elevated BP—without clear findings on fundoscopy, ECG, or cardiac marker testing. Perhaps more of these patients would be discharged if EPs felt comfortable initiating appropriate initial antihypertensive treatment.
Management
Initiating Antihypertensive Treatment
Some EPs may feel that an accurate diagnosis of hypertension requires repeat BP testing in the primary care office setting, and for this reason are reluctant to initiate antihypertensive treatment in the ED. The most recent guidelines by the Joint National Committee (JNC 8) do not address how many BP readings are necessary to diagnose hypertension, but JNC 7 suggested that diagnosis of hypertension requires two separate office visits.17 Evidence cited in ACEP’s first clinical policy states that two separate BP measurements in the ED are adequate for screening—but not necessarily for initiating treatment.10 However, European and British outpatient clinical recommendations advocate initiation of antihypertensive medication for a single visit in patients who have an elevated BP categorized as grade 3/severe hypertension (BP of ≥180/110 mm Hg).15,18 Furthermore, for patients with severe hypertension seen in the ED, as many as 97% are likely to have true hypertension at office follow-up.6 Those ED patients presenting with severe hypertension are very likely to have a true diagnosis of hypertension.
A recent retrospective analysis of a group of hypertensive ED patients by Brody et al19 found that patients prescribed BP medications by an EP were more likely to have improved BP control at follow-up 2 weeks later. In their study, the decision to prescribe antihypertensive medications were at the discretion of the EP. Seventy-six patients were given one or more prescriptions for antihypertensive therapy, compared to a control group of 141 patients who were not given a prescription. On follow-up at 2 weeks, there was an 11 mm Hg greater reduction of BP in the group who received prescriptions compared to the control group. None of the patients in either group on follow-up had experienced any new neurological deficits, ischemic events, life-threatening anaphylactic reactions, or clinically significant hypotension.
The Cleveland Clinic study14 also reported on those patents given who received new prescriptions from the ED. Similar to the study by Brody et al,19 none of the 82 patients discharged to home from the ED with a new antihypertensive prescription had any major adverse event at 30-day follow-up.14
Pharmacological Treatment Recommendations
When choosing to treat patients with new prescriptions for antihypertensives, it is important to follow the most current outpatient treatment recommendations. In 2014, JNC 8 released new guidelines for the outpatient management of adults with hypertension.20 The panel issued recommendations based on its systematic review of randomized controlled trials on antihypertensive treatments. The key recommendations are as follows:
- In patients aged 60 years or older, initiate pharmacological treatment at a BP of ≥150/90 mm Hg.
- In patients aged 18 to 59 years, initiate pharmacological treatment at a BP of ≥140/90 mm Hg.
- In the general nonblack population, initial antihypertensive treatment should include a thiazide-type diuretic, a calcium channel blocker (CCB), an angiotensin-converting enzyme inhibitor (ACE-I), or an angiotensin receptor blocker (ARB).
- In the general black population, initial treatment should include a thiazide-type diuretic or a CCB.
- In patients with chronic kidney disease (CKD) (including black patients), initial (or add-on) antihypertensive treatment should include an ACE-I or ARB to improve kidney outcomes—but not both.
- If goal BP is not reached within 1 month of initial treatment, increase the dose of the initial drug or add a second agent (eg, thiazide-type diuretic, CCB, ACE-I, or ARB). If goal is not reached with two drugs, use the third drug from that list if no contraindications exist, but do not use both an ACE-I and an ARB together in the same patient.
Of note, JNC 8, in departure from JNC 7, no longer recommends beta-blockers as first-line therapy for isolated hypertension (there may be compelling alternate indications, such as atrial fibrillation or postmyocardial infarction (MI), such that a beta-blocker would still be the first medication considered). The reason for this stems from a single randomized controlled trial of 9,193 patients that found that despite equivalent BP reduction, use of a beta-blocker in comparison to an ARB resulted in a higher rate of a composite outcome of death, MI, or stroke.21 The main difference was a 25% relative risk reduction for stroke with use of an ARB (losartan) in comparison to a beta-blocker (atenolol). The most recent European guidelines still include beta-blockers among its first-line recommended BP medications, but do acknowledge that they are not as effective in reducing stroke incidence as other alternative medications.15 The European guidelines otherwise include the same list of first-line agents. The British guidelines mirror JNC 8 in terms of first-line antihypertensive medication choices.18
Since the release of JNC 8, the Systolic Blood Pressure Intervention Trial (SPRINT) has been published, and will likely impact future national recommendations on BP management. The SPRINT study was a randomized controlled trial enrolling over 9,000 hypertensive nondiabetic patients older than age 50 years that treated individuals to a standard BP goal (systolic BP of 140 mm Hg) versus an intensive BP goal (systolic BP of 120 mm Hg) over a 3.5-year period. The trial was stopped early for safety as a 25% mortality reduction was observed in the intensive treatment group (1.65 vs 2.19 deaths/y).22 This was in contrast to previous trials that had mostly failed to show this sort of benefit, though previous trials were smaller in number or included only diabetic patients.23 While it is likely that this trial may influence lowering treatment thresholds from the office, it is not likely to impact care from the ED.
The recommendations of JNC 8 do not necessarily coincide with current US EP practice. In the study by Brody et al,19 of patients provided ED antihypertensive prescriptions, 54% received thiazide-type diuretics, 26% ACE-I, 10% CCBs, and 6% beta-blockers. This is noteworthy because 96% of those in the study were black patients who would benefit most from either a thiazide or a CCB. Another recent study of ED patients showed that of patients who were both treated in the ED and discharged with antihypertensive medications, 34% received a diuretic prescription, 32% clonidine, 15% a beta-blocker, 19% an ARB or ACE-I, 12% a CCB, and 2% hydrazine.24 These results are important because according to many published guidelines, including JNC 8, clonidine is only considered one of several fourth-line options for severe resistant hypertension.15,18,20 Since clonidine use can be complicated by rebound hypertension, it is not an ideal agent to be prescribed de novo to patients in the ED. This is particularly true if these patients are not already on maximum doses of the three most recommended agents previously noted, or if there are concerns over patient compliance.
Of the drug classes recommended by JNC 8, Table 3 lists the absolute and relative contraindications.
In clinical trials, amlodipine is among the most effective BP medications and is considered first-line therapy for all groups of patients with hypertension.15,18,20 A simplistic approach for most patients presenting with severe asymptomatic hypertension (BP of ≥180/110 mm Hg) not currently on treatment would be to recheck the BP and assure it remains elevated over the period of the ED visit.
Conclusion
Hypertension is among the most common medical conditions for which emergency patients seek care. The ACEP clinical policies provide guidance on the appropriate work-up and treatment of these patients. Given the occasional lack of clarity on whether a patient’s presentation is on the spectrum of more acute/serious, EPs may feel more comfortable in discharging patients with poor follow-up if they are able to safely prescribe antihypertensive treatment. Prior to prescribing treatment, EPs should refer to the JNC 8 guidelines to appropriately start antihypertensive treatment in select patient groups in the ED. The guidelines of JNC 8 are therefore worth referring to in order to appropriately start treatment in select patient groups from the ED.
Case Scenarios Continued
Case 1
[The 65-year-old black man who presented with headache and dizziness, and had an initial BP of 240/130 mm Hg.]
After treating the patient with prochlorperazine and diphenhydramine, his headache resolved. His BP improved but remained elevated at 190/120 mm Hg. On further questioning, the patient reported a history of similar headaches and wondered whether it was related to his BP. The head CT scan was negative for any acute hemorrhage, infarct, or mass; the ECG only showed evidence of left ventricular hypertrophy; and the BMP showed normal renal function.
After a long discussion with the patient, you agreed to start him on amlodipine 5 mg/d and referred him for follow-up with a local PCP.
Case 2
[The 90-year-old white woman with a history of CHF and an initial BP of 220/140 mm Hg at presentation.]
The BMP evaluation showed a baseline creatinine level of 1.3 mg/dL. Given this patient’s history of CHF, amlodipine would not be the ideal next agent to prescribe. After discussion with her PCP, you elected to start her on losartan at 25 mg/d, and instructed her to follow-up with her PCP within 1 week.
Case 3
[The 32-year-old white woman who presented at the advice of a pharmacist and had an initial BP of 240/100 mm Hg.]While reviewing the patient’s work-up and history, you noted her plans to become pregnant, and recalled a recent review on BP management, noting the contraindications associated with ARB or ACE-I in pregnancy. Based on the patient’s uninsured status and poor follow-up, you considered prescribing amlodipine. Prior to issuing the prescription, you performed a repeat BP check and noted that the patient’s BP had decreased to 130/85 mm Hg. Given the marked improvement in the patient’s BP during her ED course, you were not convinced that she truly had hypertension.
Instead of prescribing an antihypertensive agent, which may not ultimately benefit this patient, you advised her to seek follow-up care at an outpatient clinic to have her BP rechecked. The patient agreed, and you referred her to a local free clinic.
Case Scenarios
Case 1
You had just started your shift, and your first patient presented with symptoms of headache and dizziness, and a blood pressure (BP) of 240/130 mm Hg, without any vomiting or visual symptoms. Physical examination revealed an alert, pleasant 65-year-old black man whose ocular, neurological, and cardiovascular (CV) examinations were normal. The patient reported a history of borderline hypertension, but had never taken any medications for it.
After placing some initial orders, including an electrocardiogram (ECG), basic metabolic panel (BMP), and head computed tomography (CT) scan, and ordering 10 mg intravenous (IV) prochlorperazine and 25 mg IV diphenhydramine to treat the patient’s headache, you are left asking yourself what steps you should take next.
Case 2
Your next patient was a 90-year-old white woman who had been referred to the ED by her primary care physician (PCP) for “hypertensive urgency.” She had no complaints to report. Similar to the first patient, this patient’s physical examination was also normal, with the exception of a persistently elevated BP of 220/140 mm Hg. Her history was significant for congestive heart failure (CHF), but she exhibited no current CV signs or symptoms. The patient had been taking furosemide but was not on any other antihypertensive medications.
Case 3
In the room next to your 90-year-old patient is a 32-year-old uninsured hypertensive white woman. During the history taking, the patient stated that she was trying to become pregnant and was not currently using any form of contraception. Similar to the second patient, she had no complaints to report. Regarding her reason for presentation, the patient stated that when she had her BP checked at a pharmacy earlier that day, the reading was “too high,” and the pharmacist had advised her to go to the ED. She seemed anxious but otherwise well. Her initial BP at presentation was 240/100 mm Hg, but her physical examination was otherwise normal.
Hypertensive Emergencies
As emergency physicians (EPs), we see hypertensive patients every day. According to the US Centers for Disease Control and Prevention, 33% of American adults have hypertension, which is defined by a BP of ≥140/90 mm Hg (Table 1).
Almost 25% of total annual US adult ED visits are directly or indirectly related to hypertension, and about 1% of all ED visits are due solely to elevated BP.3 In an ambulatory care survey for 2007, moderate or severe hypertensive BP readings were found to be more common in patients presenting to the ED (43.5%) than to primary care clinics (27%).4 Patients presenting to the ED with hypertensive BP readings disproportionately represented patients who were older, male, non-Hispanic black, Medicare beneficiaries, or uninsured. Certainly, some patients presenting to the ED have hypertensive BP readings due to pain or anxiety, but multiple studies have suggested that 50% to 70% of ED patients who have hypertensive BP readings will be diagnosed with hypertension on office follow-up.5,6 While a minority of these patients present to the ED with hypertensive emergencies, the majority present either without symptoms of hypertension or with only mild headache. Given the disease burden of hypertension combined with the benefits of treatment, it is worthwhile for the practicing EP to review the most up-to-date guidelines on outpatient management of hypertension.
When a patient presents to the ED with a hypertensive BP reading, the initial priority of the EP is to exclude hypertensive emergency. Hypertensive emergencies are defined by the presence of hypertension (generally grade 3/severe hypertension with BP ≥180/110 mm Hg; see Table 1) in conjunction with evidence of target organ damage.
Target Organ Manifestations
The acuity and/or presence of target organ damage are not always clear on initial ED evaluation. For instance, when a patient who has no history of primary care presents to the ED with severe hypertension, laboratory evaluation may demonstrate protein and blood in his or her urine and an elevated serum creatinine level. In the absence of values from past laboratory studies, it is difficult to determine whether these test results represent normal laboratory parameters for this patient due to longstanding hypertensive kidney disease (ie, hypertensive nephrosclerosis) or if they represent a true hypertensive emergency, (ie, hypertensive emergency-related nephropathy).7 In patients with severe hypertension and possibly new acute kidney injury, it is probably safest to either assume hypertensive emergency-related nephropathy and to treat accordingly or consult with nephrology services. The picture of hypertensive emergency-related nephropathy often only becomes clear after renal biopsy results and improvement in renal parameters with BP control.
The ocular manifestations of hypertensive emergency require detailed fundoscopy, which at times can be challenging in the ED. In assessing for cardiac target organ damage, at our institution, we typically ask patients if they have experienced symptoms of dyspnea and chest pain or pressure. Generally, we also evaluate cardiac enzymes, B-type natriuretic peptide, and order ECG and chest X-ray studies when suggested by history or physical examination. Alarmingly, a study of 161 ED hypertensive (average BP of 183/109 mm Hg), asymptomatic, predominantly black patients found that 146 (90.7%) had subclinical hypertensive heart disease on point-of-care echocardiogram.8
Neurological/Hypertensive Encephalopathy
Hypertensive encephalopathy is a diagnosis of exclusion as alternate causes of confusion and headache, such as intracranial hemorrhage, are excluded and mental status improves with titrated BP control. Nonetheless, it is difficult to confidently state from the literature that patients who present with headache but have a normal mental status in the presence of severe hypertension are not on an early spectrum of hypertensive encephalopathy. Therefore, it is likely that the degree of symptoms should define whether target organ damage exists, though there is certainly a spectrum of hypertensive emergency—the strict definition of which is not always clear.
When a hypertensive emergency is diagnosed, management typically involves the use of antihypertensive IV medication in the intensive care unit. While such management is outside the scope of this paper, Adebayo and Rogers9 have published an excellent review of the care of hypertensive emergencies.
Asymptomatic Hypertension
The American College of Emergency Physicians (ACEP) has developed two clinical policies on the evaluation and management of asymptomatic hypertension in the ED. The original, published in 2006, advised that initially high BP readings of ED patients should be repeated: two separate high readings are adequate for screening, and those patients with hypertension should be referred for follow-up. Furthermore, ACEP policies note that initiating treatment in the ED is not necessary when patients are referred for follow-up. If treatment for hypertension is initiated in the ED, ACEP recommends that such management should attempt only to gradually lower BP, and not to normalize it during the initial ED visit.10
The 2013 update to ACEP’s clinical policy on managing asymptomatic hypertension expanded on the original policy. The updated policy advised against routine testing for target organ damage in patients who have asymptomatic severe hypertension. However, ACEP policy notes that evaluating serum creatinine in these patients with poor follow-up may influence patient disposition.11
The 2013 policy further stated that medical intervention is not required in ED patients who have asymptomatic severe hypertension, but may be considered in patients with poor follow-up. The policies emphasize that all asymptomatic hypertensive patients should be referred for follow-up. The literature cited for the recommendation that ED patients with asymptomatic severe hypertension do not require routine investigation stems from two observational studies. These studies found that screening asymptomatic ED patients who presented with severe hypertension revealed serum creatinine abnormalities in approximately 6%, which impacted patient disposition, though it was not clear from the study results whether admission correlated to meaningful patient outcomes.12,13
Patient Disposition
Since ACEP’s 2013 clinical policy, a study from the Cleveland Clinic has been published. This retrospective cohort study reviewed 6 years of data looking at all patients in its system with a BP of ≥180/110 mm Hg, and compared those office patients discharged to home to those referred to the ED or directly admitted to the inpatient hospital solely on the basis of severe hypertension.14 The study found that 0.5% of 387 patients referred to the ED by primary care clinics for asymptomatic severe hypertension had confirmed acute kidney injury on BMP.14 The Cleveland Clinic study also found that 2.1% of patients had evidence of target organ damage and 5.5% had any abnormal results.14 In addition, referral to the ED from the clinic for hypertension was associated with a slightly higher rate of major adverse CV events at 7 days (2 of 426 [0.5%] versus 61 of 58,109 [0.1%]; P = .02).14
The results of the Cleveland Clinic study confirm that in the absence of target organ damage, hypertension is probably best managed in the outpatient setting. The European Task Force hypertensive guidelines state “hospitalization for hypertension is regarded as inappropriate in most European countries.”15 However, from 2006 to 2012, 26% of US ED patients with primary diagnoses of hypertension were admitted to the hospital.3 In Canada’s most populous province of Ontario, from 2002 to 2011, approximately 8% of hypertensive patients were admitted.16 Part of this discrepancy may be due to the sometimes ambiguous nature of the presentation of patients with hypertension, making it unclear whether a true hypertensive emergency exists. Many patients perceive visual symptoms, headache, dizziness, and even chest pressure as the result of their elevated BP—without clear findings on fundoscopy, ECG, or cardiac marker testing. Perhaps more of these patients would be discharged if EPs felt comfortable initiating appropriate initial antihypertensive treatment.
Management
Initiating Antihypertensive Treatment
Some EPs may feel that an accurate diagnosis of hypertension requires repeat BP testing in the primary care office setting, and for this reason are reluctant to initiate antihypertensive treatment in the ED. The most recent guidelines by the Joint National Committee (JNC 8) do not address how many BP readings are necessary to diagnose hypertension, but JNC 7 suggested that diagnosis of hypertension requires two separate office visits.17 Evidence cited in ACEP’s first clinical policy states that two separate BP measurements in the ED are adequate for screening—but not necessarily for initiating treatment.10 However, European and British outpatient clinical recommendations advocate initiation of antihypertensive medication for a single visit in patients who have an elevated BP categorized as grade 3/severe hypertension (BP of ≥180/110 mm Hg).15,18 Furthermore, for patients with severe hypertension seen in the ED, as many as 97% are likely to have true hypertension at office follow-up.6 Those ED patients presenting with severe hypertension are very likely to have a true diagnosis of hypertension.
A recent retrospective analysis of a group of hypertensive ED patients by Brody et al19 found that patients prescribed BP medications by an EP were more likely to have improved BP control at follow-up 2 weeks later. In their study, the decision to prescribe antihypertensive medications were at the discretion of the EP. Seventy-six patients were given one or more prescriptions for antihypertensive therapy, compared to a control group of 141 patients who were not given a prescription. On follow-up at 2 weeks, there was an 11 mm Hg greater reduction of BP in the group who received prescriptions compared to the control group. None of the patients in either group on follow-up had experienced any new neurological deficits, ischemic events, life-threatening anaphylactic reactions, or clinically significant hypotension.
The Cleveland Clinic study14 also reported on those patents given who received new prescriptions from the ED. Similar to the study by Brody et al,19 none of the 82 patients discharged to home from the ED with a new antihypertensive prescription had any major adverse event at 30-day follow-up.14
Pharmacological Treatment Recommendations
When choosing to treat patients with new prescriptions for antihypertensives, it is important to follow the most current outpatient treatment recommendations. In 2014, JNC 8 released new guidelines for the outpatient management of adults with hypertension.20 The panel issued recommendations based on its systematic review of randomized controlled trials on antihypertensive treatments. The key recommendations are as follows:
- In patients aged 60 years or older, initiate pharmacological treatment at a BP of ≥150/90 mm Hg.
- In patients aged 18 to 59 years, initiate pharmacological treatment at a BP of ≥140/90 mm Hg.
- In the general nonblack population, initial antihypertensive treatment should include a thiazide-type diuretic, a calcium channel blocker (CCB), an angiotensin-converting enzyme inhibitor (ACE-I), or an angiotensin receptor blocker (ARB).
- In the general black population, initial treatment should include a thiazide-type diuretic or a CCB.
- In patients with chronic kidney disease (CKD) (including black patients), initial (or add-on) antihypertensive treatment should include an ACE-I or ARB to improve kidney outcomes—but not both.
- If goal BP is not reached within 1 month of initial treatment, increase the dose of the initial drug or add a second agent (eg, thiazide-type diuretic, CCB, ACE-I, or ARB). If goal is not reached with two drugs, use the third drug from that list if no contraindications exist, but do not use both an ACE-I and an ARB together in the same patient.
Of note, JNC 8, in departure from JNC 7, no longer recommends beta-blockers as first-line therapy for isolated hypertension (there may be compelling alternate indications, such as atrial fibrillation or postmyocardial infarction (MI), such that a beta-blocker would still be the first medication considered). The reason for this stems from a single randomized controlled trial of 9,193 patients that found that despite equivalent BP reduction, use of a beta-blocker in comparison to an ARB resulted in a higher rate of a composite outcome of death, MI, or stroke.21 The main difference was a 25% relative risk reduction for stroke with use of an ARB (losartan) in comparison to a beta-blocker (atenolol). The most recent European guidelines still include beta-blockers among its first-line recommended BP medications, but do acknowledge that they are not as effective in reducing stroke incidence as other alternative medications.15 The European guidelines otherwise include the same list of first-line agents. The British guidelines mirror JNC 8 in terms of first-line antihypertensive medication choices.18
Since the release of JNC 8, the Systolic Blood Pressure Intervention Trial (SPRINT) has been published, and will likely impact future national recommendations on BP management. The SPRINT study was a randomized controlled trial enrolling over 9,000 hypertensive nondiabetic patients older than age 50 years that treated individuals to a standard BP goal (systolic BP of 140 mm Hg) versus an intensive BP goal (systolic BP of 120 mm Hg) over a 3.5-year period. The trial was stopped early for safety as a 25% mortality reduction was observed in the intensive treatment group (1.65 vs 2.19 deaths/y).22 This was in contrast to previous trials that had mostly failed to show this sort of benefit, though previous trials were smaller in number or included only diabetic patients.23 While it is likely that this trial may influence lowering treatment thresholds from the office, it is not likely to impact care from the ED.
The recommendations of JNC 8 do not necessarily coincide with current US EP practice. In the study by Brody et al,19 of patients provided ED antihypertensive prescriptions, 54% received thiazide-type diuretics, 26% ACE-I, 10% CCBs, and 6% beta-blockers. This is noteworthy because 96% of those in the study were black patients who would benefit most from either a thiazide or a CCB. Another recent study of ED patients showed that of patients who were both treated in the ED and discharged with antihypertensive medications, 34% received a diuretic prescription, 32% clonidine, 15% a beta-blocker, 19% an ARB or ACE-I, 12% a CCB, and 2% hydrazine.24 These results are important because according to many published guidelines, including JNC 8, clonidine is only considered one of several fourth-line options for severe resistant hypertension.15,18,20 Since clonidine use can be complicated by rebound hypertension, it is not an ideal agent to be prescribed de novo to patients in the ED. This is particularly true if these patients are not already on maximum doses of the three most recommended agents previously noted, or if there are concerns over patient compliance.
Of the drug classes recommended by JNC 8, Table 3 lists the absolute and relative contraindications.
In clinical trials, amlodipine is among the most effective BP medications and is considered first-line therapy for all groups of patients with hypertension.15,18,20 A simplistic approach for most patients presenting with severe asymptomatic hypertension (BP of ≥180/110 mm Hg) not currently on treatment would be to recheck the BP and assure it remains elevated over the period of the ED visit.
Conclusion
Hypertension is among the most common medical conditions for which emergency patients seek care. The ACEP clinical policies provide guidance on the appropriate work-up and treatment of these patients. Given the occasional lack of clarity on whether a patient’s presentation is on the spectrum of more acute/serious, EPs may feel more comfortable in discharging patients with poor follow-up if they are able to safely prescribe antihypertensive treatment. Prior to prescribing treatment, EPs should refer to the JNC 8 guidelines to appropriately start antihypertensive treatment in select patient groups in the ED. The guidelines of JNC 8 are therefore worth referring to in order to appropriately start treatment in select patient groups from the ED.
Case Scenarios Continued
Case 1
[The 65-year-old black man who presented with headache and dizziness, and had an initial BP of 240/130 mm Hg.]
After treating the patient with prochlorperazine and diphenhydramine, his headache resolved. His BP improved but remained elevated at 190/120 mm Hg. On further questioning, the patient reported a history of similar headaches and wondered whether it was related to his BP. The head CT scan was negative for any acute hemorrhage, infarct, or mass; the ECG only showed evidence of left ventricular hypertrophy; and the BMP showed normal renal function.
After a long discussion with the patient, you agreed to start him on amlodipine 5 mg/d and referred him for follow-up with a local PCP.
Case 2
[The 90-year-old white woman with a history of CHF and an initial BP of 220/140 mm Hg at presentation.]
The BMP evaluation showed a baseline creatinine level of 1.3 mg/dL. Given this patient’s history of CHF, amlodipine would not be the ideal next agent to prescribe. After discussion with her PCP, you elected to start her on losartan at 25 mg/d, and instructed her to follow-up with her PCP within 1 week.
Case 3
[The 32-year-old white woman who presented at the advice of a pharmacist and had an initial BP of 240/100 mm Hg.]While reviewing the patient’s work-up and history, you noted her plans to become pregnant, and recalled a recent review on BP management, noting the contraindications associated with ARB or ACE-I in pregnancy. Based on the patient’s uninsured status and poor follow-up, you considered prescribing amlodipine. Prior to issuing the prescription, you performed a repeat BP check and noted that the patient’s BP had decreased to 130/85 mm Hg. Given the marked improvement in the patient’s BP during her ED course, you were not convinced that she truly had hypertension.
Instead of prescribing an antihypertensive agent, which may not ultimately benefit this patient, you advised her to seek follow-up care at an outpatient clinic to have her BP rechecked. The patient agreed, and you referred her to a local free clinic.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
2. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2013;362(9395):1527-1535.
3. McNaughton CD, Self WH, Zhu Y, Janke AT, Storrow AB, Levy P. Incidence of hypertension-related emergency department visits in the United States, 2006 to 2012. Am J Cardiol. 2015;116(11):1717-1723. doi: 10.1016/j.amjcard.2015.09.007.
4. Niska RW. Blood pressure measurements at emergency department visits by adults: United States, 2007-2008. NCHS Data Brief. 2011;(72):1-8.
5. Chernow SM, Iserson KV, Criss E. Use of the emergency department for hypertension screening: a prospective study. Ann Emerg Med. 1987;16(2):180-182.
6. Backer HD, Decker L, Ackerson L. Reproducibility of increased blood pressure during an emergency department or urgent care visit. Ann Emerg Med. 2003.41(4):507-512.
7. Nonaka K, Ubara Y, Sumida K, et al. Clinical and pathological evaluation of hypertensive emergency-related nephropathy. Intern Med. 2013;52(1):45-53.
8. Levy P, Ye H, Compton S, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60(4):467-474.e1. doi: 10.1016/j.annemergmed.2012.03.030.
9. Adebayo O, Rogers RL. Hypertensive emergencies in the emergency department. Emerg Med Clin North Am. 2015;33(3):539-551. doi: 10.1016/j.emc.2015.04.005.
10. Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-234. doi: 10.1016/j.annemergmed.2005.10.003
11. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68. doi: 10.1016/j.annemergmed.2013.05.012.
12. Karras DJ, Kruus LK, Cienki JJ, et al. Evaluation and treatment of patients with severely elevated blood pressure in academic emergency departments: a multicenter study. Ann Emerg Med. 2006;47(3):230-236.
13. Nishijima DK, Paladino L, Sinert R. Routine testing in patients with asymptomatic elevated blood pressure in the ED. Am J Emerg Med. 2010;28(2):235-242. doi: 10.1016/j.ajem.2008.11.015.
14. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509.
15. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151.
16. Masood S, Austin PC, Atzema CL. A population-based analysis of outcomes in patients with a primary diagnosis of hypertension in the emergency department. Ann Emerg Med. 2016;68(3):258-267.e5. doi: 10.1016/j.annemergmed.2016.04.060.
17. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. Erratum in: JAMA. 2003;290(2):197.
18. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891.
19. Brody A, Rahman T, Reed B, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22(5):632-635. doi: 10.1111/acem.12660.
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014;311(17):1809.
21. Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
22. PRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939.
23. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286.
24. Levy PD, Mahn JJ, Miller J, et al. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med. 2015;33(9):1219-1224. doi: 10.1016/j.ajem.2015.05.036.
25. Leung AA, Wright A, Pazo V, Karson A, Bates DW. Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med. 2011;124(11):1064-1072. doi: 10.1016/j.amjmed.2011.06.031.
26. Mann JFE, Hilgers KF. Renin-angiotensin system inhibition in the treatment of hypertension. http://www.uptodate.com/contents/renin-angiotensin-system-inhibition-in-the-treatment-of-hypertension. Accessed September 21, 2016.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):1-8.
2. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2013;362(9395):1527-1535.
3. McNaughton CD, Self WH, Zhu Y, Janke AT, Storrow AB, Levy P. Incidence of hypertension-related emergency department visits in the United States, 2006 to 2012. Am J Cardiol. 2015;116(11):1717-1723. doi: 10.1016/j.amjcard.2015.09.007.
4. Niska RW. Blood pressure measurements at emergency department visits by adults: United States, 2007-2008. NCHS Data Brief. 2011;(72):1-8.
5. Chernow SM, Iserson KV, Criss E. Use of the emergency department for hypertension screening: a prospective study. Ann Emerg Med. 1987;16(2):180-182.
6. Backer HD, Decker L, Ackerson L. Reproducibility of increased blood pressure during an emergency department or urgent care visit. Ann Emerg Med. 2003.41(4):507-512.
7. Nonaka K, Ubara Y, Sumida K, et al. Clinical and pathological evaluation of hypertensive emergency-related nephropathy. Intern Med. 2013;52(1):45-53.
8. Levy P, Ye H, Compton S, et al. Subclinical hypertensive heart disease in black patients with elevated blood pressure in an inner-city emergency department. Ann Emerg Med. 2012;60(4):467-474.e1. doi: 10.1016/j.annemergmed.2012.03.030.
9. Adebayo O, Rogers RL. Hypertensive emergencies in the emergency department. Emerg Med Clin North Am. 2015;33(3):539-551. doi: 10.1016/j.emc.2015.04.005.
10. Decker WW, Godwin SA, Hess EP, Lenamond CC, Jagoda AS; American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Asymptomatic Hypertension in the ED. Clinical policy: critical issues in the evaluation and management of adult patients with asymptomatic hypertension in the emergency department. Ann Emerg Med. 2006;47(3):237-234. doi: 10.1016/j.annemergmed.2005.10.003
11. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68. doi: 10.1016/j.annemergmed.2013.05.012.
12. Karras DJ, Kruus LK, Cienki JJ, et al. Evaluation and treatment of patients with severely elevated blood pressure in academic emergency departments: a multicenter study. Ann Emerg Med. 2006;47(3):230-236.
13. Nishijima DK, Paladino L, Sinert R. Routine testing in patients with asymptomatic elevated blood pressure in the ED. Am J Emerg Med. 2010;28(2):235-242. doi: 10.1016/j.ajem.2008.11.015.
14. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509.
15. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. doi: 10.1093/eurheartj/eht151.
16. Masood S, Austin PC, Atzema CL. A population-based analysis of outcomes in patients with a primary diagnosis of hypertension in the emergency department. Ann Emerg Med. 2016;68(3):258-267.e5. doi: 10.1016/j.annemergmed.2016.04.060.
17. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. Erratum in: JAMA. 2003;290(2):197.
18. Krause T, Lovibond K, Caulfield M, McCormack T, Williams B; Guideline Development Group. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. doi: 10.1136/bmj.d4891.
19. Brody A, Rahman T, Reed B, et al. Safety and efficacy of antihypertensive prescription at emergency department discharge. Acad Emerg Med. 2015;22(5):632-635. doi: 10.1111/acem.12660.
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014;311(17):1809.
21. Dahlöf B, Devereux RB, Kjeldsen SE, et al; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995-1003.
22. PRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. doi: 10.1056/NEJMoa1511939.
23. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575-1585. doi: 10.1056/NEJMoa1001286.
24. Levy PD, Mahn JJ, Miller J, et al. Blood pressure treatment and outcomes in hypertensive patients without acute target organ damage: a retrospective cohort. Am J Emerg Med. 2015;33(9):1219-1224. doi: 10.1016/j.ajem.2015.05.036.
25. Leung AA, Wright A, Pazo V, Karson A, Bates DW. Risk of thiazide-induced hyponatremia in patients with hypertension. Am J Med. 2011;124(11):1064-1072. doi: 10.1016/j.amjmed.2011.06.031.
26. Mann JFE, Hilgers KF. Renin-angiotensin system inhibition in the treatment of hypertension. http://www.uptodate.com/contents/renin-angiotensin-system-inhibition-in-the-treatment-of-hypertension. Accessed September 21, 2016.