User login
Malpractice Counsel: Acute Pulmonary Embolism Masquerading as Acute Coronary Syndrome
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
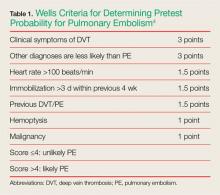
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
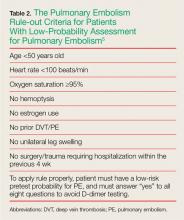
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
Case
A 37-year-old woman presented to the ED with a 90-minute history of chest tightness and shortness of breath. She admitted to feeling anxious but denied nausea, vomiting, or diaphoresis. The patient was in good health overall and had no history of similar symptoms. The only medication she took on a regular basis was a combination oral contraceptive (OC). Regarding the patient’s social history, she admitted to smoking one-half of a pack of cigarettes per day and occasional alcohol use.
On physical examination, the patient’s vital signs were: heart rate (HR), 102 beats/min; blood pressure, 118/64 mm Hg; respiratory rate, 20 breaths/min; and temperature, 98.6˚F. Oxygen saturation was 95% on room air. The head, eyes, ears, nose, and throat examination was normal. The cardiopulmonary examination revealed slight tachycardia with a regular rhythm but no murmurs, rubs, or gallops; the lungs were clear to auscultation bilaterally. The abdominal examination revealed a soft, nontender abdomen, without mass, and no guarding or rebound was present. An examination of the lower extremities was not documented.
The emergency physician (EP) ordered laboratory studies, which included a complete blood count (CBC), basic metabolic profile (BMP), and troponin I level. A chest X-ray and electrocardiogram (ECG) were also ordered. The chest X-ray was interpreted as normal, and the ECG revealed mild sinus tachycardia with nonspecific ST-T segment changes in V1 through V3. The CBC and BMP were all within normal limits, but the troponin I level was slightly elevated.
Given the patient’s clinical presentation and slightly elevated troponin I level, the EP was concerned for an acute coronary syndrome (ACS) and admitted the patient to the care of the on-call cardiologist. Prior to transfer, the patient was given 325 mg of aspirin by mouth, but no anticoagulation therapy was ordered. The cardiologist, who evaluated the patient after she was admitted to the inpatient floor, was concerned the patient had a pulmonary embolism (PE), and ordered a stat computed tomography angiography (CTA) scan of the chest. While the patient was undergoing the chest CTA scan, she went into cardiac arrest. Despite aggressive resuscitative measures, the patient could not be revived and was pronounced dead. An autopsy revealed a PE as the cause of death.
Plaintiff’s Claim
The patient’s estate sued the EP for failure to properly diagnose the PE, stating the hospital was vicariously liable for the EP’s actions. The emergency medicine (EM) expert for the plaintiff opined that the decedent’s symptoms should have prompted the EP to suspect she was suffering from a PE, and he should have immediately ordered anticoagulation, a D-dimer test, or a chest CTA scan. The expert cardiologist for the plaintiff stated the EP should have immediately started the patient on anticoagulation prior to the chest CTA scan.
The Defense
The defense EM expert stated the defendant’s diagnosis of ACS was appropriate given the patient’s overall clinical presentation, and the defense expert cardiologist stated the standard of care did not require the EP to administer anticoagulation prior to her diagnosis of PE, since the bleeding risks outweighed the benefits.
Verdict
At trial, the jury returned a defense verdict.
Discussion
This is not the first (nor probably the last) malpractice case in this column to involve a missed PE. While there have been improvements to the tools we currently possess to evaluate patients for suspected PE, it remains a difficult condition to reliably and timely identify in the ED. Although the two predominating symptoms—shortness of breath and chest pain—are common presentations in the ED, each is associated with large differential diagnoses.
Acute Coronary Syndrome Versus Pulmonary Embolism
From what we know of the published details of this case, the patient had only one risk factor for ACS (cigarette smoking) and two risk factors for PE (cigarette smoking and estrogen-containing contraceptive use). The only abnormal physical finding (tachycardia) was slightly more suggestive of PE than ACS. This patient’s primary complaint was chest fullness and shortness of breath. According to the Prospective Investigation of Pulmonary Embolism Diagnosis II study, shortness of breath is the most common complaint in PE (73%), followed by pleuritic chest pain (44%).1
In ACS, which is more common in men versus women and in patients of both sexes over age 55 years, the clinical presentation most commonly involves chest pain that patients describe as a pressure or fullness (as demonstrated in this patient). Unfortunately, in certain patient populations (eg, women, elderly patients, patients with diabetes mellitus) the presenting complaint can be shortness of breath, weakness, or nausea and vomiting. In a study evaluating how frequently an acute PE can mimic ACS, Kukla et al2 found that one-third of patients with an acute PE can present with all of the manifestations suggestive of ACS (ie, chest pain, ECG changes, and elevated troponin).
It is probably safe to assume the elevated troponin I level played a factor in influencing the EP to diagnose ACS, rather than pursuing an alternative diagnosis such as PE. Unfortunately, since both serum troponin T and I can be markers of right ventricle dysfunction, they are elevated in 30% to 50% of patients with moderate-to-large PE.3 However, neither serum troponin T nor troponin I is specific for myocardial infarction or unstable angina.
Pretest Probability: Wells Criteria
Determining pretest probability for any disease process is important when evaluating complaints in the ED; this is especially true for PE. One of the most frequently used tools for determining the likelihood of PE in ED patients is the Wells criteria (Table 1).4
Based on the published information available, the patient in this case would have scored a 1.5, placing her in the unlikely or low-risk category for PE. Patients whose Wells score places them in the low-risk group can benefit from serum D-dimer testing to help diagnose PE. However, serum D-dimer testing should not be ordered for patients in the likely or high-risk categories; these patients should instead be sent directly for imaging studies such as a chest CTA scan.
Pulmonary Embolism Rule-Out Criteria
For patients whose Wells criteria score places them in the “unlikely group,” the PE rule-out criteria (PERC) can be used to determine the need for ordering a D-dimer. If all eight criteria are met, no further testing is necessary to exclude PE from the differential diagnosis (Table 2).5
Summary
Evaluating chest pain and shortness of breath in the ED is a humbling experience for even the most seasoned EP. Thoroughly reviewing the patient’s history and physical examination, and determining the pretest probability of disease entities high on the differential diagnoses list, go a long way in helping make the correct diagnosis—and in turn initiating possible life-saving interventions and treatment.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
1. Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120(10):871-879.
2. Kukla P, Dlugopolski R, Krupa E, et al. How often pulmonary embolism mimics acute coronary syndrome? Kardiol Pol. 2011;69(3):235-240.
3. Meyer T, Binder L, Hruska N, Luthe H, Buchwald AB. Cardiac troponin I elevation in acute pulmonary embolism is associated with right ventricular dysfunction. J Am Coll Cardiol. 2000;36(5):1632-1636.
4. Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420.
5. Kline JA, Courtney DM, Kabrhel C, et al. Prospective multicenter evaluation of the pulmonary embolism rule-out criteria. J Thromb Haemost. 2008;6(5):772-780. doi: 10.1111/j.1538-7836.2008.02944.x.
First EDition: Zika—Not the Only Mosquito-Borne Virus to Worry About
BY DOUG BRUNK
FRONTLINE MEDICAL NEWS
As the spread of the Zika virus continues to garner attention in the national spotlight, two other mosquito-borne viral infections pose a potential threat to the United States: dengue fever and chikungunya.
At the annual meeting of the Pacific Dermatologic Association, Iris Z. Ahronowitz, MD, shared tips on how to spot and diagnose patients with these viral infections.
“You really need to use all the data at your disposal, including a thorough symptom history, a thorough exposure history, and of course, our most important tool in all of this: our eyes,” said Dr Ahronowitz, a dermatologist at the University of Southern California, Los Angeles. Reaching a diagnosis involves asking about epidemiologic exposure, symptoms, morphology, and performing confirmatory testing by polymerase chain reaction (PCR) and/or enzyme-linked immunosorbent assay (ELISA). “Unfortunately we are not getting these results very quickly,” she said. “Sometimes the turnaround time can be 3 weeks or longer.”
She discussed the case of a 32-year-old woman who had returned from travel to Central Mexico. Two days later, the patient developed fever, fatigue, and retro-orbital headache, as well as flushing macular erythema over the chest. Three days later, she developed a generalized morbilliform eruption. Her white blood cell count was 1.5 x 109/L, platelet count was 37 x 109/L, aspartate aminotransferase (AST) was 124 U/L, and alanine aminotransferase (ALT) was 87 U/L.
The differential diagnosis for morbilliform eruption plus fever in a returning traveler is extensive, Dr Ahronowitz said. It includes measles, chikungunya, West Nile virus, O’nyong-nyong virus, Mayaro virus, Sindbis virus, Ross river disease, Ebola/Marburg, dengue, and Zika. Bacterial/rickettsial possibilities include typhoid fever, typhus, and leptospirosis.
The patient was ultimately diagnosed with dengue virus, a mosquito-borne flavivirus. Five serotypes have been identified, the most recent in 2013. According to Dr Ahronowitz, dengue ranks as the most common febrile illness in travelers returning from the Caribbean, South America, and Southeast Asia. “There are up to 100 million cases every year, 40% of the world population is at risk, and an estimated 80% of people are asymptomatic carriers, which is facilitating the spread of this disease,” she said. The most common vector is Aedes aegypti, a daytime biting mosquito that is endemic to the tropics and subtropics. But a new vector is emerging, Aedes albopictus, which is common in temperate areas. “Both types of mosquitoes are in the United States, and they’re spreading rapidly,” she said. “This is probably due to a combination of climate change and international travel.”
Dengue classically presents with sudden onset of fever, headache, retro-orbital pain, and severe myalgia; 50% to 82% of cases develop a distinctive rash. “While most viruses have nonspecific lab abnormalities, one that can be very helpful to you with suspected dengue is thrombocytopenia,” she said. “The incubation period ranges from 3 to 14 days.”
Rashes associated with dengue are classically biphasic and sequential. The initial rash occurs within 24 to 48 hours of symptom onset and is often mistaken for sunburn, with a flushing erythema of the face, neck, and chest. Three to 5 days later, a subsequent rash develops that starts out as a generalized morbilliform eruption but becomes confluent with petechiae and islands of sparing. “It’s been described as ‘white islands in a sea of red,’” Dr Ahronowitz said.
A more severe form of the disease, dengue hemorrhagic fever, is characterized by extensive purpura and bleeding from mucosa, gastrointestinal tract, and injection sites. “The patients who get this have prior immunity to a different serotype,” she said. “This is thought to be due to a phenomenon called antibody-dependent enhancement, whereby the presence of preexisting antibodies facilitates entry of the virus and produces a more robust inflammatory response. Most of these patients, even the ones with severe dengue, recover fully. The most common long-term sequela we’re seeing is chronic fatigue.”
The diagnosis is made with viral PCR from serum less than 7 days from onset of symptoms, or immunoglobulin M (IgM)ELISA more than 4 days from onset of symptoms. The treatment is supportive care with fluid resuscitation and analgesia; there is no specific treatment. “Do not give nonsteroidal anti-inflammatory drugs (NSAIDs) which can potentiate hemorrhage; give acetaminophen for pain and fevers,” she advised. “A tetravalent vaccine is now available for dengue. Prevention is so important because there is no treatment.”
Next, Dr Ahronowitz discussed the case of a 38-year-old man who returned from travel to Bangladesh. Two days after returning he developed fever to 104˚F, headache, and cervical lymphadenopathy. Three days after returning, he developed severe pain in the wrist, knees, and ankles, and a rash. “This rash was not specific; it was a morbilliform eruption primarily on the chest,” she said.
The patient was ultimately diagnosed with chikungunya, a single-strand RNA mosquito-borne virus with the same vectors as dengue. “This has been wreaking havoc across the Caribbean in the past few years,” Dr Ahronowitz said. “Chikungunya was first identified in the Americas in 2013, and there have been hundreds of thousands of cases in the Caribbean.” The first case acquired in the United States occurred in Florida in the summer of 2014. As of January 2016 there were 679 imported cases of the infection in the United States. “Fortunately, this most recent epidemic is slowing down a bit, but it’s important to be aware of,” she said.
Clinical presentation of chikungunya includes an incubation period of 3 to 7 days, acute onset of high fevers, chills, and myalgia. Nonspecific exanthem around 3 days occurs in 40% to 75% of cases, and symmetric polyarthralgias are common in the fingers, wrists, and ankles. Labs may reveal lymphopenia, acute kidney injury, and elevated AST and ALT levels. Acute symptoms resolve within 7 to 10 days.
Besides the rash, other cutaneous signs of the disease include aphthous-like ulcers and anogenital ulcers, particularly around the scrotum. Other patients may present with facial hyperpigmentation, also known as “brownie nose,” that appears with the rash. In babies, bullous lesions can occur. More than 20% of patients who acquire chikungunya still have severe joint pain 1 year after initial presentation. “This can be really debilitating,” she said. “A subset of patients will develop an inflammatory seronegative rheumatoid-like arthritis. It’s generally not a fatal condition except in the extremes of age and in people with a lot of comorbidities. Most people recover fully.”
As in dengue, clinicians can diagnose chikungunya by viral culture in the first 3 days of illness, and by reverse transcription PCR in the first 8 days of illness. On serology, IgM is positive by 5 days of symptom onset.
“If testing is not available locally, contact the Centers for Disease Control and Prevention,” Dr Ahronowitz said. “Treatment is supportive. Evaluate for and treat potential coinfections, including dengue, malaria, and bacterial infections. If dengue is in the differential diagnosis, avoid NSAIDs.” A new vaccine for chikungunya is currently in phase II trials.
BY DOUG BRUNK
FRONTLINE MEDICAL NEWS
As the spread of the Zika virus continues to garner attention in the national spotlight, two other mosquito-borne viral infections pose a potential threat to the United States: dengue fever and chikungunya.
At the annual meeting of the Pacific Dermatologic Association, Iris Z. Ahronowitz, MD, shared tips on how to spot and diagnose patients with these viral infections.
“You really need to use all the data at your disposal, including a thorough symptom history, a thorough exposure history, and of course, our most important tool in all of this: our eyes,” said Dr Ahronowitz, a dermatologist at the University of Southern California, Los Angeles. Reaching a diagnosis involves asking about epidemiologic exposure, symptoms, morphology, and performing confirmatory testing by polymerase chain reaction (PCR) and/or enzyme-linked immunosorbent assay (ELISA). “Unfortunately we are not getting these results very quickly,” she said. “Sometimes the turnaround time can be 3 weeks or longer.”
She discussed the case of a 32-year-old woman who had returned from travel to Central Mexico. Two days later, the patient developed fever, fatigue, and retro-orbital headache, as well as flushing macular erythema over the chest. Three days later, she developed a generalized morbilliform eruption. Her white blood cell count was 1.5 x 109/L, platelet count was 37 x 109/L, aspartate aminotransferase (AST) was 124 U/L, and alanine aminotransferase (ALT) was 87 U/L.
The differential diagnosis for morbilliform eruption plus fever in a returning traveler is extensive, Dr Ahronowitz said. It includes measles, chikungunya, West Nile virus, O’nyong-nyong virus, Mayaro virus, Sindbis virus, Ross river disease, Ebola/Marburg, dengue, and Zika. Bacterial/rickettsial possibilities include typhoid fever, typhus, and leptospirosis.
The patient was ultimately diagnosed with dengue virus, a mosquito-borne flavivirus. Five serotypes have been identified, the most recent in 2013. According to Dr Ahronowitz, dengue ranks as the most common febrile illness in travelers returning from the Caribbean, South America, and Southeast Asia. “There are up to 100 million cases every year, 40% of the world population is at risk, and an estimated 80% of people are asymptomatic carriers, which is facilitating the spread of this disease,” she said. The most common vector is Aedes aegypti, a daytime biting mosquito that is endemic to the tropics and subtropics. But a new vector is emerging, Aedes albopictus, which is common in temperate areas. “Both types of mosquitoes are in the United States, and they’re spreading rapidly,” she said. “This is probably due to a combination of climate change and international travel.”
Dengue classically presents with sudden onset of fever, headache, retro-orbital pain, and severe myalgia; 50% to 82% of cases develop a distinctive rash. “While most viruses have nonspecific lab abnormalities, one that can be very helpful to you with suspected dengue is thrombocytopenia,” she said. “The incubation period ranges from 3 to 14 days.”
Rashes associated with dengue are classically biphasic and sequential. The initial rash occurs within 24 to 48 hours of symptom onset and is often mistaken for sunburn, with a flushing erythema of the face, neck, and chest. Three to 5 days later, a subsequent rash develops that starts out as a generalized morbilliform eruption but becomes confluent with petechiae and islands of sparing. “It’s been described as ‘white islands in a sea of red,’” Dr Ahronowitz said.
A more severe form of the disease, dengue hemorrhagic fever, is characterized by extensive purpura and bleeding from mucosa, gastrointestinal tract, and injection sites. “The patients who get this have prior immunity to a different serotype,” she said. “This is thought to be due to a phenomenon called antibody-dependent enhancement, whereby the presence of preexisting antibodies facilitates entry of the virus and produces a more robust inflammatory response. Most of these patients, even the ones with severe dengue, recover fully. The most common long-term sequela we’re seeing is chronic fatigue.”
The diagnosis is made with viral PCR from serum less than 7 days from onset of symptoms, or immunoglobulin M (IgM)ELISA more than 4 days from onset of symptoms. The treatment is supportive care with fluid resuscitation and analgesia; there is no specific treatment. “Do not give nonsteroidal anti-inflammatory drugs (NSAIDs) which can potentiate hemorrhage; give acetaminophen for pain and fevers,” she advised. “A tetravalent vaccine is now available for dengue. Prevention is so important because there is no treatment.”
Next, Dr Ahronowitz discussed the case of a 38-year-old man who returned from travel to Bangladesh. Two days after returning he developed fever to 104˚F, headache, and cervical lymphadenopathy. Three days after returning, he developed severe pain in the wrist, knees, and ankles, and a rash. “This rash was not specific; it was a morbilliform eruption primarily on the chest,” she said.
The patient was ultimately diagnosed with chikungunya, a single-strand RNA mosquito-borne virus with the same vectors as dengue. “This has been wreaking havoc across the Caribbean in the past few years,” Dr Ahronowitz said. “Chikungunya was first identified in the Americas in 2013, and there have been hundreds of thousands of cases in the Caribbean.” The first case acquired in the United States occurred in Florida in the summer of 2014. As of January 2016 there were 679 imported cases of the infection in the United States. “Fortunately, this most recent epidemic is slowing down a bit, but it’s important to be aware of,” she said.
Clinical presentation of chikungunya includes an incubation period of 3 to 7 days, acute onset of high fevers, chills, and myalgia. Nonspecific exanthem around 3 days occurs in 40% to 75% of cases, and symmetric polyarthralgias are common in the fingers, wrists, and ankles. Labs may reveal lymphopenia, acute kidney injury, and elevated AST and ALT levels. Acute symptoms resolve within 7 to 10 days.
Besides the rash, other cutaneous signs of the disease include aphthous-like ulcers and anogenital ulcers, particularly around the scrotum. Other patients may present with facial hyperpigmentation, also known as “brownie nose,” that appears with the rash. In babies, bullous lesions can occur. More than 20% of patients who acquire chikungunya still have severe joint pain 1 year after initial presentation. “This can be really debilitating,” she said. “A subset of patients will develop an inflammatory seronegative rheumatoid-like arthritis. It’s generally not a fatal condition except in the extremes of age and in people with a lot of comorbidities. Most people recover fully.”
As in dengue, clinicians can diagnose chikungunya by viral culture in the first 3 days of illness, and by reverse transcription PCR in the first 8 days of illness. On serology, IgM is positive by 5 days of symptom onset.
“If testing is not available locally, contact the Centers for Disease Control and Prevention,” Dr Ahronowitz said. “Treatment is supportive. Evaluate for and treat potential coinfections, including dengue, malaria, and bacterial infections. If dengue is in the differential diagnosis, avoid NSAIDs.” A new vaccine for chikungunya is currently in phase II trials.
BY DOUG BRUNK
FRONTLINE MEDICAL NEWS
As the spread of the Zika virus continues to garner attention in the national spotlight, two other mosquito-borne viral infections pose a potential threat to the United States: dengue fever and chikungunya.
At the annual meeting of the Pacific Dermatologic Association, Iris Z. Ahronowitz, MD, shared tips on how to spot and diagnose patients with these viral infections.
“You really need to use all the data at your disposal, including a thorough symptom history, a thorough exposure history, and of course, our most important tool in all of this: our eyes,” said Dr Ahronowitz, a dermatologist at the University of Southern California, Los Angeles. Reaching a diagnosis involves asking about epidemiologic exposure, symptoms, morphology, and performing confirmatory testing by polymerase chain reaction (PCR) and/or enzyme-linked immunosorbent assay (ELISA). “Unfortunately we are not getting these results very quickly,” she said. “Sometimes the turnaround time can be 3 weeks or longer.”
She discussed the case of a 32-year-old woman who had returned from travel to Central Mexico. Two days later, the patient developed fever, fatigue, and retro-orbital headache, as well as flushing macular erythema over the chest. Three days later, she developed a generalized morbilliform eruption. Her white blood cell count was 1.5 x 109/L, platelet count was 37 x 109/L, aspartate aminotransferase (AST) was 124 U/L, and alanine aminotransferase (ALT) was 87 U/L.
The differential diagnosis for morbilliform eruption plus fever in a returning traveler is extensive, Dr Ahronowitz said. It includes measles, chikungunya, West Nile virus, O’nyong-nyong virus, Mayaro virus, Sindbis virus, Ross river disease, Ebola/Marburg, dengue, and Zika. Bacterial/rickettsial possibilities include typhoid fever, typhus, and leptospirosis.
The patient was ultimately diagnosed with dengue virus, a mosquito-borne flavivirus. Five serotypes have been identified, the most recent in 2013. According to Dr Ahronowitz, dengue ranks as the most common febrile illness in travelers returning from the Caribbean, South America, and Southeast Asia. “There are up to 100 million cases every year, 40% of the world population is at risk, and an estimated 80% of people are asymptomatic carriers, which is facilitating the spread of this disease,” she said. The most common vector is Aedes aegypti, a daytime biting mosquito that is endemic to the tropics and subtropics. But a new vector is emerging, Aedes albopictus, which is common in temperate areas. “Both types of mosquitoes are in the United States, and they’re spreading rapidly,” she said. “This is probably due to a combination of climate change and international travel.”
Dengue classically presents with sudden onset of fever, headache, retro-orbital pain, and severe myalgia; 50% to 82% of cases develop a distinctive rash. “While most viruses have nonspecific lab abnormalities, one that can be very helpful to you with suspected dengue is thrombocytopenia,” she said. “The incubation period ranges from 3 to 14 days.”
Rashes associated with dengue are classically biphasic and sequential. The initial rash occurs within 24 to 48 hours of symptom onset and is often mistaken for sunburn, with a flushing erythema of the face, neck, and chest. Three to 5 days later, a subsequent rash develops that starts out as a generalized morbilliform eruption but becomes confluent with petechiae and islands of sparing. “It’s been described as ‘white islands in a sea of red,’” Dr Ahronowitz said.
A more severe form of the disease, dengue hemorrhagic fever, is characterized by extensive purpura and bleeding from mucosa, gastrointestinal tract, and injection sites. “The patients who get this have prior immunity to a different serotype,” she said. “This is thought to be due to a phenomenon called antibody-dependent enhancement, whereby the presence of preexisting antibodies facilitates entry of the virus and produces a more robust inflammatory response. Most of these patients, even the ones with severe dengue, recover fully. The most common long-term sequela we’re seeing is chronic fatigue.”
The diagnosis is made with viral PCR from serum less than 7 days from onset of symptoms, or immunoglobulin M (IgM)ELISA more than 4 days from onset of symptoms. The treatment is supportive care with fluid resuscitation and analgesia; there is no specific treatment. “Do not give nonsteroidal anti-inflammatory drugs (NSAIDs) which can potentiate hemorrhage; give acetaminophen for pain and fevers,” she advised. “A tetravalent vaccine is now available for dengue. Prevention is so important because there is no treatment.”
Next, Dr Ahronowitz discussed the case of a 38-year-old man who returned from travel to Bangladesh. Two days after returning he developed fever to 104˚F, headache, and cervical lymphadenopathy. Three days after returning, he developed severe pain in the wrist, knees, and ankles, and a rash. “This rash was not specific; it was a morbilliform eruption primarily on the chest,” she said.
The patient was ultimately diagnosed with chikungunya, a single-strand RNA mosquito-borne virus with the same vectors as dengue. “This has been wreaking havoc across the Caribbean in the past few years,” Dr Ahronowitz said. “Chikungunya was first identified in the Americas in 2013, and there have been hundreds of thousands of cases in the Caribbean.” The first case acquired in the United States occurred in Florida in the summer of 2014. As of January 2016 there were 679 imported cases of the infection in the United States. “Fortunately, this most recent epidemic is slowing down a bit, but it’s important to be aware of,” she said.
Clinical presentation of chikungunya includes an incubation period of 3 to 7 days, acute onset of high fevers, chills, and myalgia. Nonspecific exanthem around 3 days occurs in 40% to 75% of cases, and symmetric polyarthralgias are common in the fingers, wrists, and ankles. Labs may reveal lymphopenia, acute kidney injury, and elevated AST and ALT levels. Acute symptoms resolve within 7 to 10 days.
Besides the rash, other cutaneous signs of the disease include aphthous-like ulcers and anogenital ulcers, particularly around the scrotum. Other patients may present with facial hyperpigmentation, also known as “brownie nose,” that appears with the rash. In babies, bullous lesions can occur. More than 20% of patients who acquire chikungunya still have severe joint pain 1 year after initial presentation. “This can be really debilitating,” she said. “A subset of patients will develop an inflammatory seronegative rheumatoid-like arthritis. It’s generally not a fatal condition except in the extremes of age and in people with a lot of comorbidities. Most people recover fully.”
As in dengue, clinicians can diagnose chikungunya by viral culture in the first 3 days of illness, and by reverse transcription PCR in the first 8 days of illness. On serology, IgM is positive by 5 days of symptom onset.
“If testing is not available locally, contact the Centers for Disease Control and Prevention,” Dr Ahronowitz said. “Treatment is supportive. Evaluate for and treat potential coinfections, including dengue, malaria, and bacterial infections. If dengue is in the differential diagnosis, avoid NSAIDs.” A new vaccine for chikungunya is currently in phase II trials.
The Long Hot Summer of 2016
Months of extremely high temperatures throughout the United States made the summer of 2016 one of the hottest summers on record. The summer may also be remembered for the excessive amounts of hot air generated in the run-up to the 2016 presidential election. But most oppressive of all has been the failure of Congress to appropriate funds for Zika virus research, prevention, and treatment before it recessed for vacation.
Emergency physicians (EPs) in the United States are already dealing with frightened, symptomatic patients who may have been exposed to the Zika, dengue, or chikungunya viruses, transmitted by the bite of the Aedes aegypti mosquito. In the First EDition section of this issue, dermatologist Iris Z. Ahronowitz, MD, describes some of the similarities in the acute clinical presentations of those infections (see page 438). But among this group of related viruses, only Zika has been positively linked to microcephaly and severely underdeveloped, damaged brains in babies born to women who are infected during pregnancy. An increasing number of newborn babies severely affected by Zika virus in utero began appearing in South America in late 2015. By summer’s end (September 21, 2016), the Centers for Disease Control and Prevention reports of Zika virus disease in the United States included over 3,300 travel-related cases, 43 locally acquired mosquito-borne cases, 28 sexually transmitted cases, and eight cases of Guillain-Barré syndrome (http://www.cdc.gov/zika/geo/united-states.html). Most importantly, as of September 15, 2016, there have been 20 live-born infants with birth defects and five pregnancy losses with birth defects—numbers that do not reflect the outcomes of ongoing pregnancies.
The life expectancy of babies severely affected by Zika virus and the nature and extent of disability in less physically affected babies are presently unknown. But according to The Washington Post (http://wapo.st/29Y5CnR), estimates of the cost of caring for a severely affected Zika baby through adulthood run as high as $10 million or more, and as high a total price as we will pay for the congressional intransigence this summer, such cost estimates do not even consider the terrible human suffering these babies will experience or the anguish their parents may have for the rest of their lives.
Emergency physicians are all too familiar with the emotional and behavioral problems that complicate our efforts to manage acute medical problems of children and adults born with autism or Down syndrome when they present to the ED. Most such congenital illnesses are not preventable, but when one potentially is, delaying needed resources because of partisan politics is unconscionable.
By summer’s end, as the last of leftover Ebola dollars were being spent on Zika-related programs, Democrats and Republicans finally appeared to be reaching a consensus to provide $1.1 billion of the $1.9 billion originally requested by the President long before the long hot summer began. This sudden agreement may be driven by the importance both parties place on winning the Florida vote in the upcoming election. But whatever the reason, Zika funding now will help prevent untold hardships and suffering in the years to come. In the meantime, EPs will continue to evaluate, diagnose, counsel, and, hopefully someday soon, be able to treat all who come to our EDs with Zika infection.
Months of extremely high temperatures throughout the United States made the summer of 2016 one of the hottest summers on record. The summer may also be remembered for the excessive amounts of hot air generated in the run-up to the 2016 presidential election. But most oppressive of all has been the failure of Congress to appropriate funds for Zika virus research, prevention, and treatment before it recessed for vacation.
Emergency physicians (EPs) in the United States are already dealing with frightened, symptomatic patients who may have been exposed to the Zika, dengue, or chikungunya viruses, transmitted by the bite of the Aedes aegypti mosquito. In the First EDition section of this issue, dermatologist Iris Z. Ahronowitz, MD, describes some of the similarities in the acute clinical presentations of those infections (see page 438). But among this group of related viruses, only Zika has been positively linked to microcephaly and severely underdeveloped, damaged brains in babies born to women who are infected during pregnancy. An increasing number of newborn babies severely affected by Zika virus in utero began appearing in South America in late 2015. By summer’s end (September 21, 2016), the Centers for Disease Control and Prevention reports of Zika virus disease in the United States included over 3,300 travel-related cases, 43 locally acquired mosquito-borne cases, 28 sexually transmitted cases, and eight cases of Guillain-Barré syndrome (http://www.cdc.gov/zika/geo/united-states.html). Most importantly, as of September 15, 2016, there have been 20 live-born infants with birth defects and five pregnancy losses with birth defects—numbers that do not reflect the outcomes of ongoing pregnancies.
The life expectancy of babies severely affected by Zika virus and the nature and extent of disability in less physically affected babies are presently unknown. But according to The Washington Post (http://wapo.st/29Y5CnR), estimates of the cost of caring for a severely affected Zika baby through adulthood run as high as $10 million or more, and as high a total price as we will pay for the congressional intransigence this summer, such cost estimates do not even consider the terrible human suffering these babies will experience or the anguish their parents may have for the rest of their lives.
Emergency physicians are all too familiar with the emotional and behavioral problems that complicate our efforts to manage acute medical problems of children and adults born with autism or Down syndrome when they present to the ED. Most such congenital illnesses are not preventable, but when one potentially is, delaying needed resources because of partisan politics is unconscionable.
By summer’s end, as the last of leftover Ebola dollars were being spent on Zika-related programs, Democrats and Republicans finally appeared to be reaching a consensus to provide $1.1 billion of the $1.9 billion originally requested by the President long before the long hot summer began. This sudden agreement may be driven by the importance both parties place on winning the Florida vote in the upcoming election. But whatever the reason, Zika funding now will help prevent untold hardships and suffering in the years to come. In the meantime, EPs will continue to evaluate, diagnose, counsel, and, hopefully someday soon, be able to treat all who come to our EDs with Zika infection.
Months of extremely high temperatures throughout the United States made the summer of 2016 one of the hottest summers on record. The summer may also be remembered for the excessive amounts of hot air generated in the run-up to the 2016 presidential election. But most oppressive of all has been the failure of Congress to appropriate funds for Zika virus research, prevention, and treatment before it recessed for vacation.
Emergency physicians (EPs) in the United States are already dealing with frightened, symptomatic patients who may have been exposed to the Zika, dengue, or chikungunya viruses, transmitted by the bite of the Aedes aegypti mosquito. In the First EDition section of this issue, dermatologist Iris Z. Ahronowitz, MD, describes some of the similarities in the acute clinical presentations of those infections (see page 438). But among this group of related viruses, only Zika has been positively linked to microcephaly and severely underdeveloped, damaged brains in babies born to women who are infected during pregnancy. An increasing number of newborn babies severely affected by Zika virus in utero began appearing in South America in late 2015. By summer’s end (September 21, 2016), the Centers for Disease Control and Prevention reports of Zika virus disease in the United States included over 3,300 travel-related cases, 43 locally acquired mosquito-borne cases, 28 sexually transmitted cases, and eight cases of Guillain-Barré syndrome (http://www.cdc.gov/zika/geo/united-states.html). Most importantly, as of September 15, 2016, there have been 20 live-born infants with birth defects and five pregnancy losses with birth defects—numbers that do not reflect the outcomes of ongoing pregnancies.
The life expectancy of babies severely affected by Zika virus and the nature and extent of disability in less physically affected babies are presently unknown. But according to The Washington Post (http://wapo.st/29Y5CnR), estimates of the cost of caring for a severely affected Zika baby through adulthood run as high as $10 million or more, and as high a total price as we will pay for the congressional intransigence this summer, such cost estimates do not even consider the terrible human suffering these babies will experience or the anguish their parents may have for the rest of their lives.
Emergency physicians are all too familiar with the emotional and behavioral problems that complicate our efforts to manage acute medical problems of children and adults born with autism or Down syndrome when they present to the ED. Most such congenital illnesses are not preventable, but when one potentially is, delaying needed resources because of partisan politics is unconscionable.
By summer’s end, as the last of leftover Ebola dollars were being spent on Zika-related programs, Democrats and Republicans finally appeared to be reaching a consensus to provide $1.1 billion of the $1.9 billion originally requested by the President long before the long hot summer began. This sudden agreement may be driven by the importance both parties place on winning the Florida vote in the upcoming election. But whatever the reason, Zika funding now will help prevent untold hardships and suffering in the years to come. In the meantime, EPs will continue to evaluate, diagnose, counsel, and, hopefully someday soon, be able to treat all who come to our EDs with Zika infection.
Direct Anterior Versus Posterior Simultaneous Bilateral Total Hip Arthroplasties: No Major Differences at 90 Days
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
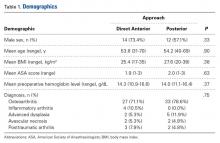
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
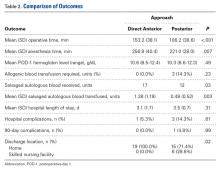
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
End-stage osteoarthritis of the hip is a debilitating disease that is reliably treated with total hip arthroplasty (THA).1 Up to 35% of patients who undergo THA eventually require contralateral THA.2,3 In patients who present with advanced bilateral disease and undergo unilateral THA, the risk of ultimately requiring a contralateral procedure is as high as 97%.3-6 In patients with bilateral hip disease, function is not fully optimized until both hips have been replaced, particularly in the setting of fixed flexion contractures.7-9 Naturally, there has been some interest in simultaneous bilateral THAs for select patients.
The potential benefits of bilateral THAs over staged procedures include faster overall rehabilitation, exposure to a single anesthetic, reduced hospital length of stay (LOS), and cost savings.10-12 However, opinion on recommending bilateral THAs is mixed. Although bilateral procedures historically have been fraught with perioperative complications,13,14 advances in surgical and anesthetic techniques have led to improved outcomes.15 Whether surgical approach is a factor in these outcomes is unclear.
The popularity of the direct anterior (DA) approach for THA has increased in recent years.16 Although the relative advantages of various approaches remain in debate, one potential benefit of the DA approach is supine positioning, which allows simultaneous bilateral THAs to be performed without the need for repositioning before proceeding with the contralateral side. However, simultaneous bilateral THAs performed through the DA approach and those performed through other surgical approaches are lacking in comparative outcomes data.17In this study, we evaluated operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who had simultaneous bilateral THAs through either the DA approach or the posterior approach.
Methods
Study Design
This single-center study was conducted at the Mayo Clinic in Rochester, Minnesota. After obtaining approval from our Institutional Review Board, we performed a retrospective cohort analysis. We used our institution’s total joint registry to identify all patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach. The first bilateral THAs to use the DA approach at our institution were performed in 2012. To ensure that the DA and posterior groups’ perioperative management would be similar, we included only cases performed between 2012 and 2014.
There were 19 patients in the DA group and 21 in the posterior group. The groups were similar in mean age (54 vs 54 years; P = .90), sex (73% vs 57% males; P = .33), body mass index (BMI; 25 vs 28 kg/m2; P = .38), preoperative hemoglobin level (14.3 vs 14.0 g/dL; P = .37), preoperative diagnosis (71.1% vs 78.6% degenerative joint disease; P = .75), and American Society of Anesthesiologists (ASA) score (1.9 vs 2.0; P = .63) (Table 1).
Patient Care
All cases were performed by 1 of 3 dedicated arthroplasty surgeons (Dr. Taunton, Dr. Sierra, Dr. Trousdale). Dr. Taunton exclusively uses the DA approach, and Dr. Sierra and Dr. Trousdale exclusively use the posterior approach. Patients in both groups received preoperative medical clearance and attended the same preoperative education class.
Patients in the DA group were positioned supine on an orthopedic table that allows hyperextension and adduction of the operative leg. Both hips were prepared and draped simultaneously. The most symptomatic hip was operated on first, with a sterile drape covering the contralateral hip. Between hips, fluoroscopy was moved to the other side of the operative suite, but no changes in positioning or preparation were necessary. A deep drain was placed on each side, and then was removed the morning of postoperative day 1. The same set of instruments was used on both sides.
Patients in the posterior group were positioned lateral on a regular operative table with hip rests. The most symptomatic hip was operated on first. After wound closure and dressing application, the patient was flipped to allow access to the contralateral hip and was prepared and draped again. The same instruments were used on each side. Drains were not used.
All patients received the same comprehensive multimodal pain management, which combined general and epidural anesthesia (remaining in place until postoperative day 2) and included an oral pain regimen of scheduled acetaminophen and as-needed tramadol and oxycodone. In all cases, intraoperative blood salvage and intravenous tranexamic acid (1 g at time of incision on first hip, 1 g at wound closure on second hip) were used. Preoperative autologous blood donation was not used. For deep vein thrombosis prophylaxis, patients were treated with bilateral sequential compression devices while hospitalized, but chemoprophylaxis was different between groups. Patients in the DA group received prophylactic low-molecular-weight heparin for 10 days, followed by twice-daily aspirin (325 mg) for 4 weeks. Patients in the posterior group received warfarin (goal international normalized ratio, 1.7-2.2) for 3 weeks, followed by twice-daily aspirin (325 mg) for 3 weeks. The decision to transfuse allogenic red blood cells was made by the treating surgeon, based on standardized hospital protocols, wherein patients are transfused for hemoglobin levels under 7.0 g/dL, or for hemoglobin levels less than 8.0 g/dL in the presence of persistent symptoms. All patients received care on an orthopedic specialty floor and were assisted by the same physical therapists. Discharge disposition was coordinated with the same group of social workers.
Two to 3 months after surgery, patients returned for routine examination and radiographs. All patients were followed up for at least 90 days.
Statistical Analysis
All outcomes were analyzed with appropriate summary statistics. Chi-square tests or logistic regression analyses (for categorical outcomes) were used to compare baseline covariates with perioperative outcomes, and 2-sample tests or Wilcoxon rank-sum tests were used to compare outcomes measured on a continuous scale. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated as appropriate. Operative time was calculated by adding time from incision to wound closure for both hips (room turnover time between hips was not included). Anesthesia time was defined as total time patients were in the operating room. All statistical tests were 2-sided, and the threshold for statistical significance was set at α = 0.05.
Results
Compared with patients who underwent simultaneous bilateral THAs through the posterior approach, patients who underwent simultaneous bilateral THAs through the DA approach had longer mean operative times (153 vs 106 min; P < .001) and anesthesia times (257 vs 221 min; P = .007). The 2 groups’ hospital stays were similar in length (3.1 vs 3.5 days; P = .31), but patients in the DA group were more likely to be discharged home (100.0% vs 71.4%; P = .02) (Table 2).
Patients in the DA group were more likely to have sufficient intraoperative blood salvage for autologous transfusion (89.5% vs 57.1%; OR, 6.4; 95% CI, 1.16-34.94; P = .03) (Table 3) and received more mean units of salvaged autologous blood (1.4 vs 0.5; P = .003) (Table 2). Allogenic blood was not given to any patients in the DA group, but 3 patients in the posterior group (14.3%) required allogenic blood transfusion (P = .23) (Table 2). Salvaged autologous and allogenic blood transfusion was not associated with sex, age 60 years or older, or hospital LOS of 4 days or more (Table 3). The groups’ mean hemoglobin levels, measured the morning of postoperative day 1, were similar: 10.6 g/dL (range, 8.5-12.4 g/dL) for the DA group and 10.3 g/dL (range 8.6-12.3 g/dL) for the posterior group (Table 2).
In-hospital complications were uncommon in both groups (5% vs 14%; P = .61) (Table 2). One patient in the posterior group sustained a unilateral dislocation the day of surgery, and closed reduction was required; other complications (1 ileus, 2 tachyarrhythmias) did not require intervention. Ninety-day complications were also rare; 1 patient in the posterior group developed a hematoma with wound drainage, and this was successfully managed conservatively. There were no reoperations or readmissions in either group (Table 2).
Discussion
Although bilateral procedures account for less than 1% of THAs in the United States,11 debate about their role in patients with severe bilateral hip disease continues. The potential benefits of a single episode of care must be weighed against the slightly increased risk for systemic complications.7,10-15 Recent innovations in perioperative management have been shown to minimize complications,15 but it is unclear whether surgical approach affects perioperative outcomes. Our goals in this study were to evaluate operative times, transfusion requirements, hospital discharge data, and 90-day complication rates in patients who underwent simultaneous bilateral THAs through either the DA approach or the posterior approach.
Patients in our DA group had longer operative and anesthesia times. Other studies have found longer operative times for the DA approach relative to the posterior approach in unilateral THAs.18 One potential benefit of the DA approach in the setting of simultaneous bilateral THAs is the ability to prepare and drape both sides before surgery and thereby keep the interruption between hips to a minimum. In the present study, however, time saved during turnover between hips was overshadowed by the time added for each THA.
Although it was uncommon for complications to occur within 90 days after surgery in this study, many patients are needed to fully investigate these rare occurrences. Because of inherent selection bias, these risks are difficult to directly compare in patients who undergo unilateral procedures. Although small studies have failed to clarify the issue,7,19,20 a recent review of the almost 20,000 bilateral THA cases in the US Nationwide Inpatient Sample database found that bilateral (vs unilateral) THAs were associated with increased risk of local and systemic complications.11 Therefore, bilateral THAs should be reserved for select cases, with attention given to excluding patients with preexisting cardiopulmonary disease and providing appropriate preoperative counseling.
Most studies have reported a higher transfusion rate in bilateral THAs relative to staged procedures.7,21-23 Allogenic blood transfusion leads to immune suppression, coagulopathy, and other systemic effects in general, and has been specifically associated with infection in patients who undergo total joint arthroplasty.24-29 Parvizi and colleagues17 reported reduced blood loss and fewer blood transfusions in patients who had simultaneous bilateral THAs through the DA approach, compared with the direct lateral approach. Patients in our DA group received more salvaged autologous blood, which we suppose was a function of longer operative times. However, postoperative hemoglobin levels and allogenic blood transfusion rates were statistically similar between the 2 groups. It is important to consider the increased risk of required allogenic blood transfusion associated with simultaneous bilateral THAs, but it is not fully clear if this risk is lower in THAs performed through the DA approach relative to other approaches. In our experience, the required transfusion risk is limited in DA and posterior approaches with use of contemporary perioperative blood management strategies.
Although hospital LOS is longer with simultaneous bilateral THAs than with unilateral THAs, historically it is shorter than the combined LOS of staged bilateral THAs.20 Patients in our study had a relatively short LOS after bilateral THAs, and there was no difference in LOS between groups. However, patients were more likely to be discharged home after bilateral THAs through the DA approach vs the posterior approach. Although discharge location was not affected by age, sex, ASA score, or LOS, unrecognized social factors unrelated to surgical approach likely influenced this finding.
This study should be interpreted in light of important limitations. Foremost, although data were prospectively collected, we examined them retrospectively. Thus, it is possible there may be unaccounted for differences between our DA and posterior THA groups. For example, the DA and posterior approaches were used by different surgeons with differing experience, technique, and preferences, all of which could have affected outcomes. Furthermore, our sample was relatively small (simultaneous bilateral THAs are performed relatively infrequently). Last, although anesthesia, pain management, blood conservation, and physical therapy were similar for the 2 groups, there was no standardized protocol for determining eligibility for simultaneous bilateral THAs.
In conclusion, we found that simultaneous bilateral THAs can be safely performed through either the DA approach or the posterior approach. Although the transition between hips is shorter with the DA approach, this time savings is overshadowed by the increased duration of each procedure. Transfusion rates are low in both groups, and in-hospital and 90-day complications are quite rare. Furthermore, patients can routinely be discharged home without elevating readmission rates. We will continue to perform simultaneous bilateral THAs through the DA approach or the posterior approach, according to surgeon preference.
Am J Orthop. 2016;45(6):E373-E378. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508-1519.
2. Sayeed SA, Johnson AJ, Jaffe DE, Mont MA. Incidence of contralateral THA after index THA for osteoarthritis. Clin Orthop Relat Res. 2012;470(2):535-540.
3. Sayeed SA, Trousdale RT, Barnes SA, Kaufman KR, Pagnano MW. Joint arthroplasty within 10 years after primary Charnley total hip arthroplasty. Am J Orthop. 2009;38(8):E141-E143.
4. Goker B, Doughan AM, Schnitzer TJ, Block JA. Quantification of progressive joint space narrowing in osteoarthritis of the hip: longitudinal analysis of the contralateral hip after total hip arthroplasty. Arthritis Rheum. 2000;43(5):988-994.
5. Husted H, Overgaard S, Laursen JO, et al. Need for bilateral arthroplasty for coxarthrosis. 1,477 replacements in 1,199 patients followed for 0-14 years. Acta Orthop Scand. 1996;67(5):421-423.
6. Ritter MA, Carr K, Herbst SA, et al. Outcome of the contralateral hip following total hip arthroplasty for osteoarthritis. J Arthroplasty. 1996;11(3):242-246.
7. Alfaro- Adrián J, Bayona F, Rech JA, Murray DW. One- or two-stage bilateral total hip replacement. J Arthroplasty. 1999;14(4):439-445.
8. Wykman A, Olsson E. Walking ability after total hip replacement. A comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74(1):53-56.
9. Yoshii T, Jinno T, Morita S, et al. Postoperative hip motion and functional recovery after simultaneous bilateral total hip arthroplasty for bilateral osteoarthritis. J Orthop Sci. 2009;14(2):161-166.
10. Lorenze M, Huo MH, Zatorski LE, Keggi KJ. A comparison of the cost effectiveness of one-stage versus two-stage bilateral total hip replacement. Orthopedics. 1998;21(12):1249-1252.
11. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148.
12. Reuben JD, Meyers SJ, Cox DD, Elliott M, Watson M, Shim SD. Cost comparison between bilateral simultaneous, staged, and unilateral total joint arthroplasty. J Arthroplasty. 1998;13(2):172-179.
13. Bracy D, Wroblewski BM. Bilateral Charnley arthroplasty as a single procedure. A report on 400 patients. J Bone Joint Surg Br. 1981;63(3):354-356.
14. Ritter MA, Randolph JC. Bilateral total hip arthroplasty: a simultaneous procedure. Acta Orthop Scand. 1976;47(2):203-208.
15. Ritter MA, Stringer EA. Bilateral total hip arthroplasty: a single procedure. Clin Orthop Relat Res. 1980;(149):185-190.
16. Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;(441):115-124.
17. Parvizi J, Rasouli MR, Jaberi M, et al. Does the surgical approach in one stage bilateral total hip arthroplasty affect blood loss? Int Orthop. 2013;37(12):2357-2362.
18. Poehling-Monaghan KL, Kamath AF, Taunton MJ, Pagnano MW. Direct anterior versus miniposterior THA with the same advanced perioperative protocols: surprising early clinical results. Clin Orthop Relat Res. 2015;473(2):623-631.
19. Macaulay W, Salvati EA, Sculco TP, Pellicci PM. Single-stage bilateral total hip arthroplasty. J Am Acad Orthop Surg. 2002;10(3):217-221.
20. Romagnoli S, Zacchetti S, Perazzo P, Verde F, Banfi G, Viganò M. Simultaneous bilateral total hip arthroplasties do not lead to higher complication or allogeneic transfusion rates compared to unilateral procedures. Int Orthop. 2013;37(11):2125-2130.
21. Salvati EA, Hughes P, Lachiewicz P. Bilateral total hip-replacement arthroplasty in one stage. J Bone Joint Surg Am. 1978;60(5):640-644.
22. Parvizi J, Chaudhry S, Rasouli MR, et al. Who needs autologous blood donation in joint replacement? J Knee Surg. 2011;24(1):25-31.
23. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
24. Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. Br J Surg. 1986;73(10):783-785.
25. Blumberg N, Heal JM. Immunomodulation by blood transfusion: an evolving scientific and clinical challenge. Am J Med. 1996;101(3):299-308.
26. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409-417.
27. Iturbe T, Cornudella R, de Miguel R, et al. Hypercoagulability state in hip and knee surgery: influence of ABO antigenic system and allogenic transfusion. Transfus Sci. 1999;20(1):17-20.
28. Murphy P, Heal JM, Blumberg N. Infection or suspected infection after hip replacement surgery with autologous or homologous blood transfusions. Transfusion. 1991;31(3):212-217.
29. Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. J Bone Joint Surg Br. 2012;94(11 suppl A):8-10.
Biopsy scalp area for alopecia diagnosis
BOSTON – Getting a proper scalp biopsy and providing the dermatopathologist with a good supporting history are important elements in diagnosing a patient with hair loss, according to Eleanor Knopp, MD, a dermatologist and dermatopathologist with Group Health Permanente, Seattle.
The keys to a good scalp biopsy in a patient with alopecia are to take an adequate sample of scalp in both size and degree of involvement. With regard to where to biopsy, “it’s important ... to select an area of advanced thinning if you’re doing a biopsy of a nonscarring alopecia,” Dr. Knopp said at the American Academy of Dermatology summer meeting. She advised being “generous” with an anesthetic, preferably one containing epinephrine, to help keep the wound dry and to help with visualization during the procedure.
Evaluating for the presence or absence of follicular ostia with a dermatoscope helps distinguish scarring from nonscarring alopecia. Scarring alopecias typically show loss of follicular ostia, she noted.
While this method is effective at identifying nonscarring areas in white patients, it can be difficult to appreciate the disappearance of follicular ostia with a dermatoscope in patients of African descent or patients with darkly pigmented skin. In these patients, eccrine ostia appear as white pinpoint dots under a dermatoscope and mimic the appearance of follicular ostia, despite the presence of scarring alopecia, she noted.
In this situation, Dr. Knopp said the threshold for biopsying patients with darkly pigmented skin should be lower to rule out an early cicatricial alopecia.
For any specimen sent to the dermatopathologist, it is important to note patient characteristics, including age and race, duration of the condition, and clinical pattern. Not only is race helpful for interpreting what is seen in the specimen, but certain racial groups have higher predilections for certain diseases. There are also differences in normal hair densities depending on race although there can be a wide range even within a race, she added. Providing a photo of the involved area of the scalp is also a good idea, she added.
When biopsying a scarring alopecia, Dr. Knopp said that her preference is to find an area of relatively early thinning with visible erythema, and scale if it is present, “so that you know you have active inflammatory disease, but it’s not so advanced that you’re just seeing end-stage changes of scarring.”
It is worth having a discussion with the dermatopathologist about sectioning specimens, Dr. Knopp said. The consensus among most dermatopathologists is that horizontal sections are “absolutely the way to go for nonscarring alopecias,” but some dermatopathologists strongly prefer vertical sections, especially in cicatricial alopecias. Clinicians can always choose among the many reference laboratories to obtain the type of sections they prefer.
In cases of cicatricial alopecia, Dr. Knopp cautioned that clinicians may see “juicy pustules or fluctuant nodules” and consider these findings indicative of a highly active area of disease, but these changes may obscure early findings that are helpful to a pathologist. A better choice for a biopsy site is an area of early involvement that is not too inflamed and not so advanced that it is just scar, she noted.
Another potential pitfall is the temptation to biopsy a tuft of hairs. If there is polytrichia or compounding of follicles, it may be tempting to fit the punch tool over what are also sometimes called “doll’s hairs.” But those structures are nonspecific, end-stage features of many different cicatricial alopecias, including lichen planopilaris, central centrifugal cicatricial alopecia, and even lupus. Instead, Dr. Knopp recommended taking a specimen at the periphery where compounding is not present but where there is thinning and active inflammation.
Dr. Knopp, also with the University of Washington, Seattle, reported no financial relationships.
BOSTON – Getting a proper scalp biopsy and providing the dermatopathologist with a good supporting history are important elements in diagnosing a patient with hair loss, according to Eleanor Knopp, MD, a dermatologist and dermatopathologist with Group Health Permanente, Seattle.
The keys to a good scalp biopsy in a patient with alopecia are to take an adequate sample of scalp in both size and degree of involvement. With regard to where to biopsy, “it’s important ... to select an area of advanced thinning if you’re doing a biopsy of a nonscarring alopecia,” Dr. Knopp said at the American Academy of Dermatology summer meeting. She advised being “generous” with an anesthetic, preferably one containing epinephrine, to help keep the wound dry and to help with visualization during the procedure.
Evaluating for the presence or absence of follicular ostia with a dermatoscope helps distinguish scarring from nonscarring alopecia. Scarring alopecias typically show loss of follicular ostia, she noted.
While this method is effective at identifying nonscarring areas in white patients, it can be difficult to appreciate the disappearance of follicular ostia with a dermatoscope in patients of African descent or patients with darkly pigmented skin. In these patients, eccrine ostia appear as white pinpoint dots under a dermatoscope and mimic the appearance of follicular ostia, despite the presence of scarring alopecia, she noted.
In this situation, Dr. Knopp said the threshold for biopsying patients with darkly pigmented skin should be lower to rule out an early cicatricial alopecia.
For any specimen sent to the dermatopathologist, it is important to note patient characteristics, including age and race, duration of the condition, and clinical pattern. Not only is race helpful for interpreting what is seen in the specimen, but certain racial groups have higher predilections for certain diseases. There are also differences in normal hair densities depending on race although there can be a wide range even within a race, she added. Providing a photo of the involved area of the scalp is also a good idea, she added.
When biopsying a scarring alopecia, Dr. Knopp said that her preference is to find an area of relatively early thinning with visible erythema, and scale if it is present, “so that you know you have active inflammatory disease, but it’s not so advanced that you’re just seeing end-stage changes of scarring.”
It is worth having a discussion with the dermatopathologist about sectioning specimens, Dr. Knopp said. The consensus among most dermatopathologists is that horizontal sections are “absolutely the way to go for nonscarring alopecias,” but some dermatopathologists strongly prefer vertical sections, especially in cicatricial alopecias. Clinicians can always choose among the many reference laboratories to obtain the type of sections they prefer.
In cases of cicatricial alopecia, Dr. Knopp cautioned that clinicians may see “juicy pustules or fluctuant nodules” and consider these findings indicative of a highly active area of disease, but these changes may obscure early findings that are helpful to a pathologist. A better choice for a biopsy site is an area of early involvement that is not too inflamed and not so advanced that it is just scar, she noted.
Another potential pitfall is the temptation to biopsy a tuft of hairs. If there is polytrichia or compounding of follicles, it may be tempting to fit the punch tool over what are also sometimes called “doll’s hairs.” But those structures are nonspecific, end-stage features of many different cicatricial alopecias, including lichen planopilaris, central centrifugal cicatricial alopecia, and even lupus. Instead, Dr. Knopp recommended taking a specimen at the periphery where compounding is not present but where there is thinning and active inflammation.
Dr. Knopp, also with the University of Washington, Seattle, reported no financial relationships.
BOSTON – Getting a proper scalp biopsy and providing the dermatopathologist with a good supporting history are important elements in diagnosing a patient with hair loss, according to Eleanor Knopp, MD, a dermatologist and dermatopathologist with Group Health Permanente, Seattle.
The keys to a good scalp biopsy in a patient with alopecia are to take an adequate sample of scalp in both size and degree of involvement. With regard to where to biopsy, “it’s important ... to select an area of advanced thinning if you’re doing a biopsy of a nonscarring alopecia,” Dr. Knopp said at the American Academy of Dermatology summer meeting. She advised being “generous” with an anesthetic, preferably one containing epinephrine, to help keep the wound dry and to help with visualization during the procedure.
Evaluating for the presence or absence of follicular ostia with a dermatoscope helps distinguish scarring from nonscarring alopecia. Scarring alopecias typically show loss of follicular ostia, she noted.
While this method is effective at identifying nonscarring areas in white patients, it can be difficult to appreciate the disappearance of follicular ostia with a dermatoscope in patients of African descent or patients with darkly pigmented skin. In these patients, eccrine ostia appear as white pinpoint dots under a dermatoscope and mimic the appearance of follicular ostia, despite the presence of scarring alopecia, she noted.
In this situation, Dr. Knopp said the threshold for biopsying patients with darkly pigmented skin should be lower to rule out an early cicatricial alopecia.
For any specimen sent to the dermatopathologist, it is important to note patient characteristics, including age and race, duration of the condition, and clinical pattern. Not only is race helpful for interpreting what is seen in the specimen, but certain racial groups have higher predilections for certain diseases. There are also differences in normal hair densities depending on race although there can be a wide range even within a race, she added. Providing a photo of the involved area of the scalp is also a good idea, she added.
When biopsying a scarring alopecia, Dr. Knopp said that her preference is to find an area of relatively early thinning with visible erythema, and scale if it is present, “so that you know you have active inflammatory disease, but it’s not so advanced that you’re just seeing end-stage changes of scarring.”
It is worth having a discussion with the dermatopathologist about sectioning specimens, Dr. Knopp said. The consensus among most dermatopathologists is that horizontal sections are “absolutely the way to go for nonscarring alopecias,” but some dermatopathologists strongly prefer vertical sections, especially in cicatricial alopecias. Clinicians can always choose among the many reference laboratories to obtain the type of sections they prefer.
In cases of cicatricial alopecia, Dr. Knopp cautioned that clinicians may see “juicy pustules or fluctuant nodules” and consider these findings indicative of a highly active area of disease, but these changes may obscure early findings that are helpful to a pathologist. A better choice for a biopsy site is an area of early involvement that is not too inflamed and not so advanced that it is just scar, she noted.
Another potential pitfall is the temptation to biopsy a tuft of hairs. If there is polytrichia or compounding of follicles, it may be tempting to fit the punch tool over what are also sometimes called “doll’s hairs.” But those structures are nonspecific, end-stage features of many different cicatricial alopecias, including lichen planopilaris, central centrifugal cicatricial alopecia, and even lupus. Instead, Dr. Knopp recommended taking a specimen at the periphery where compounding is not present but where there is thinning and active inflammation.
Dr. Knopp, also with the University of Washington, Seattle, reported no financial relationships.
Further evidence links Zika, Guillain-Barré syndrome
Evidence of Zika virus found in Colombian patients with Guillain-Barré syndrome supports the theory that Zika virus infection and Guillain-Barré syndrome are related and could occur parainfectiously, according to a study published in the New England Journal of Medicine.
“Our study provides virologic evidence of [Zika virus] infection in patients with Guillain-Barré syndrome,” wrote Beatriz Parra, PhD, of the Hospital Universitario del Valle in Valle del Cauca, Colombia, and her coauthors (N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMoa1605564).
Results indicated that 66 (97%) of subjects had symptoms consistent with a Zika virus infection prior to the onset of Guillain-Barré syndrome. The median number of days between onset of Zika-like symptoms and the onset of Guillain-Barré syndrome was found to be 7 days (interquartile range, 3-10 days). Seventeen (40%) of the 42 patients who underwent laboratory testing tested positive for Zika virus RNA in their sample, with 16 of those 17 positive tests coming from urine samples. Additionally, 18 of the 42 laboratory-tested subjects had “clinical and immunologic findings [that] supported” a Zika virus infection.
“The onset of the Guillain-Barré syndrome can parallel the onset of systemic manifestations of [Zika virus] infection, indicating a so-called parainfectious onset, which suggests that factors different from the known postinfectious mechanisms may be present in [Zika virus]–related Guillain-Barré syndrome,” the authors explained, adding that 20 (48%) of the 42 laboratory-tested subjects had a parainfectious onset.
“RT-PCR testing of urine is a valuable diagnostic tool for the identification of [Zika virus] infection in patients with Guillain-Barré syndrome,” Dr. Parra and her coauthors concluded.
The study was funded by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.
Dr. Parra and her colleagues report in the Journal the results of a prospective study of 68 Colombian patients who had a syndrome consistent with the Guillain-Barré syndrome, 66 of whom had previously had symptoms of Zika virus (ZIKV) infection. Major strengths of this study include the documentation of a temporal relationship between the Guillain-Barré syndrome and ZIKV infection (marked by a substantial increase in the incidence of the Guillain-Barré syndrome after the introduction of ZIKV, from 20 to 90 cases per month throughout Colombia), the criteria applied for the diagnosis of the Guillain-Barré syndrome, and the molecular and serologic flavivirus data from analyses of serum, cerebrospinal fluid, and urine.
The difficulties in diagnosing ZIKV infection are borne out in this study, as only 17 patients had definitive laboratory evidence of recent ZIKV infection. Of these 17 patients, only 14 had electrophysiologic data consistent with the Guillain-Barré syndrome and therefore could have met Brighton level 1 diagnostic criteria for the syndrome. Among the 25 ZIKV PCR–negative patients, dengue virus (DENV) IgG antibodies were present in the cerebrospinal fluid of 12 patients and in the serum of 10 patients, and serum DENV IgM test results were positive in 1. These data raise the possibility of primary DENV infection and false-positive ZIKV serologic test results from cross reactivity.
Overall, the study by Dr. Parra and her colleagues supports the association between ZIKV and the Guillain-Barré syndrome, although confirmation in another cohort would strengthen this assertion. Although high rates of seropositivity may prove protective against further waves of ZIKV-related Guillain-Barré syndrome in Central and South America, the ZIKV pandemic is just beginning in North America and Africa, and an increase in the incidence of the Guillain-Barré syndrome may follow.
Jennifer A. Frontera, MD, is with the Cerebrovascular Center at the Cleveland Clinic in Cleveland. Ivan R.F. da Silva. MD, is with the Federal Fluminense University in Niterói, Brazil. These comments were adapted from their editorial accompanying the study ( N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMe1611840 ).
Dr. Parra and her colleagues report in the Journal the results of a prospective study of 68 Colombian patients who had a syndrome consistent with the Guillain-Barré syndrome, 66 of whom had previously had symptoms of Zika virus (ZIKV) infection. Major strengths of this study include the documentation of a temporal relationship between the Guillain-Barré syndrome and ZIKV infection (marked by a substantial increase in the incidence of the Guillain-Barré syndrome after the introduction of ZIKV, from 20 to 90 cases per month throughout Colombia), the criteria applied for the diagnosis of the Guillain-Barré syndrome, and the molecular and serologic flavivirus data from analyses of serum, cerebrospinal fluid, and urine.
The difficulties in diagnosing ZIKV infection are borne out in this study, as only 17 patients had definitive laboratory evidence of recent ZIKV infection. Of these 17 patients, only 14 had electrophysiologic data consistent with the Guillain-Barré syndrome and therefore could have met Brighton level 1 diagnostic criteria for the syndrome. Among the 25 ZIKV PCR–negative patients, dengue virus (DENV) IgG antibodies were present in the cerebrospinal fluid of 12 patients and in the serum of 10 patients, and serum DENV IgM test results were positive in 1. These data raise the possibility of primary DENV infection and false-positive ZIKV serologic test results from cross reactivity.
Overall, the study by Dr. Parra and her colleagues supports the association between ZIKV and the Guillain-Barré syndrome, although confirmation in another cohort would strengthen this assertion. Although high rates of seropositivity may prove protective against further waves of ZIKV-related Guillain-Barré syndrome in Central and South America, the ZIKV pandemic is just beginning in North America and Africa, and an increase in the incidence of the Guillain-Barré syndrome may follow.
Jennifer A. Frontera, MD, is with the Cerebrovascular Center at the Cleveland Clinic in Cleveland. Ivan R.F. da Silva. MD, is with the Federal Fluminense University in Niterói, Brazil. These comments were adapted from their editorial accompanying the study ( N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMe1611840 ).
Dr. Parra and her colleagues report in the Journal the results of a prospective study of 68 Colombian patients who had a syndrome consistent with the Guillain-Barré syndrome, 66 of whom had previously had symptoms of Zika virus (ZIKV) infection. Major strengths of this study include the documentation of a temporal relationship between the Guillain-Barré syndrome and ZIKV infection (marked by a substantial increase in the incidence of the Guillain-Barré syndrome after the introduction of ZIKV, from 20 to 90 cases per month throughout Colombia), the criteria applied for the diagnosis of the Guillain-Barré syndrome, and the molecular and serologic flavivirus data from analyses of serum, cerebrospinal fluid, and urine.
The difficulties in diagnosing ZIKV infection are borne out in this study, as only 17 patients had definitive laboratory evidence of recent ZIKV infection. Of these 17 patients, only 14 had electrophysiologic data consistent with the Guillain-Barré syndrome and therefore could have met Brighton level 1 diagnostic criteria for the syndrome. Among the 25 ZIKV PCR–negative patients, dengue virus (DENV) IgG antibodies were present in the cerebrospinal fluid of 12 patients and in the serum of 10 patients, and serum DENV IgM test results were positive in 1. These data raise the possibility of primary DENV infection and false-positive ZIKV serologic test results from cross reactivity.
Overall, the study by Dr. Parra and her colleagues supports the association between ZIKV and the Guillain-Barré syndrome, although confirmation in another cohort would strengthen this assertion. Although high rates of seropositivity may prove protective against further waves of ZIKV-related Guillain-Barré syndrome in Central and South America, the ZIKV pandemic is just beginning in North America and Africa, and an increase in the incidence of the Guillain-Barré syndrome may follow.
Jennifer A. Frontera, MD, is with the Cerebrovascular Center at the Cleveland Clinic in Cleveland. Ivan R.F. da Silva. MD, is with the Federal Fluminense University in Niterói, Brazil. These comments were adapted from their editorial accompanying the study ( N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMe1611840 ).
Evidence of Zika virus found in Colombian patients with Guillain-Barré syndrome supports the theory that Zika virus infection and Guillain-Barré syndrome are related and could occur parainfectiously, according to a study published in the New England Journal of Medicine.
“Our study provides virologic evidence of [Zika virus] infection in patients with Guillain-Barré syndrome,” wrote Beatriz Parra, PhD, of the Hospital Universitario del Valle in Valle del Cauca, Colombia, and her coauthors (N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMoa1605564).
Results indicated that 66 (97%) of subjects had symptoms consistent with a Zika virus infection prior to the onset of Guillain-Barré syndrome. The median number of days between onset of Zika-like symptoms and the onset of Guillain-Barré syndrome was found to be 7 days (interquartile range, 3-10 days). Seventeen (40%) of the 42 patients who underwent laboratory testing tested positive for Zika virus RNA in their sample, with 16 of those 17 positive tests coming from urine samples. Additionally, 18 of the 42 laboratory-tested subjects had “clinical and immunologic findings [that] supported” a Zika virus infection.
“The onset of the Guillain-Barré syndrome can parallel the onset of systemic manifestations of [Zika virus] infection, indicating a so-called parainfectious onset, which suggests that factors different from the known postinfectious mechanisms may be present in [Zika virus]–related Guillain-Barré syndrome,” the authors explained, adding that 20 (48%) of the 42 laboratory-tested subjects had a parainfectious onset.
“RT-PCR testing of urine is a valuable diagnostic tool for the identification of [Zika virus] infection in patients with Guillain-Barré syndrome,” Dr. Parra and her coauthors concluded.
The study was funded by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.
Evidence of Zika virus found in Colombian patients with Guillain-Barré syndrome supports the theory that Zika virus infection and Guillain-Barré syndrome are related and could occur parainfectiously, according to a study published in the New England Journal of Medicine.
“Our study provides virologic evidence of [Zika virus] infection in patients with Guillain-Barré syndrome,” wrote Beatriz Parra, PhD, of the Hospital Universitario del Valle in Valle del Cauca, Colombia, and her coauthors (N Engl J Med. 2016 Oct 5. doi: 10.1056/NEJMoa1605564).
Results indicated that 66 (97%) of subjects had symptoms consistent with a Zika virus infection prior to the onset of Guillain-Barré syndrome. The median number of days between onset of Zika-like symptoms and the onset of Guillain-Barré syndrome was found to be 7 days (interquartile range, 3-10 days). Seventeen (40%) of the 42 patients who underwent laboratory testing tested positive for Zika virus RNA in their sample, with 16 of those 17 positive tests coming from urine samples. Additionally, 18 of the 42 laboratory-tested subjects had “clinical and immunologic findings [that] supported” a Zika virus infection.
“The onset of the Guillain-Barré syndrome can parallel the onset of systemic manifestations of [Zika virus] infection, indicating a so-called parainfectious onset, which suggests that factors different from the known postinfectious mechanisms may be present in [Zika virus]–related Guillain-Barré syndrome,” the authors explained, adding that 20 (48%) of the 42 laboratory-tested subjects had a parainfectious onset.
“RT-PCR testing of urine is a valuable diagnostic tool for the identification of [Zika virus] infection in patients with Guillain-Barré syndrome,” Dr. Parra and her coauthors concluded.
The study was funded by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.
Key clinical point:
Major finding: 97% of subjects had Zika-like symptoms prior to onset of GBS, and 40% of GBS patients who underwent RT-PCR testing were positive for Zika virus.
Data source: Study of 68 GBS patients from six hospitals in Colombia, with 42 patients undergoing RT-PCR analysis.
Disclosures: Funding provided by the Bart McLean Fund for Neuroimmunology Research, Johns Hopkins Project Restore, and the Universidad del Valle. Dr. Parra reported no relevant financial disclosures.
‘Practice-changing’ treatments emerging in AML

NEW YORK—We are “finally” making progress in the treatment of acute myeloid leukemia (AML), according to a speaker at the NCCN 11th Annual Congress: Hematologic Malignancies.
Jessica K. Altman, MD, said a number of developments have resulted in improved AML treatment, including a better understanding of biology and prognostic assessment, continued advances in transplant, and updating standard treatments and incorporating novel agents in both relapsed/refractory and newly diagnosed patients.
“There are a couple of practice-changing treatments in acute myeloid leukemia, 2 of which happened over the last decade: daunorubicin intensification and the use of FLT3 inhibitors,” said Dr Altman, an associate professor at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
Dr Altman went on to explain that novel therapies for AML can be divided into 2 basic categories. There are agents that don’t depend on mutation status (like daunorubicin) and those that are mutation-specific (like FLT3 inhibitors).
Therapies not dependent on mutational complexity
The therapies that are not dependent on mutational complexity include anti-CD33 antibodies, BCL‐2 inhibitors, a dose-intensified anthracycline regimen, and different formulations of 7+3, including CPX‐351.
Escalated daunorubicin
Randomized trials of escalated daunorubicin (90 vs 45 mg/m2) have demonstrated benefit in complete responses (CRs) and overall survival (OS) in intermediate-risk patients and patients with core-binding factor mutation. They have demonstrated benefit in OS in FLT3 ITD+ patients.
In patients up to 65 years of age, 60–90 mg/m2 of daunorubicin is now standard.
“It’s still not clear to me—and I don’t know if it ever will be—if 90 is equivalent to 60,” Dr Altman said.
CPX-351
CPX-351 is a liposomal formulation of cytarabine and daunorubicin. In a randomized, phase 3 study of older adults with secondary AML, the median OS was 9.56 months for patients treated with CPX-351 and 5.95 months for patients on the 7+3 regimen (P=0.005).
The median event-free survival was significantly better with CPX-351 (P=0.021), as was the rate of CR + CR with incomplete blood count recovery (CRi). The rate of CR + CRi was 47.7% with CPX-351 and 33.3% for 7+3 (P=0.016).
A similar number of patients went on to transplant in each arm. Grade 3-5 adverse events were similar in frequency and severity in both arms—92% with CPX-351 vs 91% with 7+3.
SGN-CD33A
CD33 is not a new target in myeloid leukemia, Dr Altman pointed out. Gemtuzumab ozogamicin has been studied, approved by the US Food and Drug Administration, and then withdrawn.
However, an increasing number of studies with gemtuzumab are underway, she said, and the agent may once again have a place in the AML armamentarium.
The newest CD33 construct is SGN-CD33A, a stable dipeptide linker that enables uniform drug loading of a pyrrolobenzodiazepine dimer that crosslinks DNA and leads to cell death.
“Single-agent data was quite promising,” Dr Altman noted, with a CR + CRi rate of 41% in previously treated patients and 58% in 12 treatment-naïve patients.
These results prompted a combination study of SGN-CD33A with hypomethylating agents.
“Results were higher than expected with a hypomethylating agent,” Dr Altman pointed out.
The CR + CRi + CR with incomplete platelet recovery was 58%. And the median relapse-free survival was 7.7 months.
A phase 3 randomized trial of SGN-CD33A is planned.
Venetoclax
BCL-2 inhibitors are the fourth type of agent not dependent on mutation complexity. Venetoclax (ABT‐199) is a small‐molecule BCL-2 inhibitor that leads to the initiation of apoptosis.
In a phase 1b trial of venetoclax in combination with a hypomethylating agent, the overall CR rate was 35%, and the CRi rate was 35%.
“Again, higher than what would be expected with a hypomethylating agent alone,” Dr Altman said.
In a phase 1b/2 trial of venetoclax in combination with low‐dose cytarabine, the CR + CRi rate was 54%. Patients responded even if they had prior exposure to hypomethylating agents.
Mutation-specific novel agents
The FLT3 inhibitor midostaurin and the IDH inhibitors AG-120 and AG-221 are among the most exciting mutation-specific agents and the ones most progressed, according to Dr Altman.
FLT3-ITD is mutated in about 30% of AML patients and carries an unfavorable prognosis, and the IDH mutation occurs in about 10% and confers a favorable prognosis.
Midostaurin
A phase 3, randomized, double-blind study of daunorubicin/cytarabine induction and high-dose cytarabine consolidation with midostaurin (PKC412) or placebo had a 59% CR rate by day 60 in the midostaurin arm, compared with 53% in the placebo arm.
“The CR rate was slightly higher in the midostaurin arm,” Dr Altman said, “but what’s the most remarkable about this study is the difference in overall survival.”
The median OS in the midostaurin arm was 74.7 months, compared with 25.6 months in the placebo arm (P=0.0074).
“The major take-home message from this clinical trial,” Dr Altman said, “is that midostaurin improved the overall survival when added to standard therapy and represents a new standard of care.”
AG-120 and AG-221
Two IDH inhibitors that have substantial data available are the IDH1 inhibitor AG-120 and the IDH2 inhibitor AG-221.
As of October 2015, 78 patients had been treated with AG-120 in a phase 1 trial, yielding an overall response rate of 35% and a CR rate of 15%.
As of September 2015, 209 patients had been treated with AG-221 in a phase 1/2 trial, and 66 are still on study. The overall response rate was 37% in 159 adults with relapsed/refractory AML, with a median duration of response of 6.9 months. The CR rate was 18%.
Investigators have initiated a phase 3 study of AG-221 compared to conventional care regimens. ![]()

NEW YORK—We are “finally” making progress in the treatment of acute myeloid leukemia (AML), according to a speaker at the NCCN 11th Annual Congress: Hematologic Malignancies.
Jessica K. Altman, MD, said a number of developments have resulted in improved AML treatment, including a better understanding of biology and prognostic assessment, continued advances in transplant, and updating standard treatments and incorporating novel agents in both relapsed/refractory and newly diagnosed patients.
“There are a couple of practice-changing treatments in acute myeloid leukemia, 2 of which happened over the last decade: daunorubicin intensification and the use of FLT3 inhibitors,” said Dr Altman, an associate professor at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
Dr Altman went on to explain that novel therapies for AML can be divided into 2 basic categories. There are agents that don’t depend on mutation status (like daunorubicin) and those that are mutation-specific (like FLT3 inhibitors).
Therapies not dependent on mutational complexity
The therapies that are not dependent on mutational complexity include anti-CD33 antibodies, BCL‐2 inhibitors, a dose-intensified anthracycline regimen, and different formulations of 7+3, including CPX‐351.
Escalated daunorubicin
Randomized trials of escalated daunorubicin (90 vs 45 mg/m2) have demonstrated benefit in complete responses (CRs) and overall survival (OS) in intermediate-risk patients and patients with core-binding factor mutation. They have demonstrated benefit in OS in FLT3 ITD+ patients.
In patients up to 65 years of age, 60–90 mg/m2 of daunorubicin is now standard.
“It’s still not clear to me—and I don’t know if it ever will be—if 90 is equivalent to 60,” Dr Altman said.
CPX-351
CPX-351 is a liposomal formulation of cytarabine and daunorubicin. In a randomized, phase 3 study of older adults with secondary AML, the median OS was 9.56 months for patients treated with CPX-351 and 5.95 months for patients on the 7+3 regimen (P=0.005).
The median event-free survival was significantly better with CPX-351 (P=0.021), as was the rate of CR + CR with incomplete blood count recovery (CRi). The rate of CR + CRi was 47.7% with CPX-351 and 33.3% for 7+3 (P=0.016).
A similar number of patients went on to transplant in each arm. Grade 3-5 adverse events were similar in frequency and severity in both arms—92% with CPX-351 vs 91% with 7+3.
SGN-CD33A
CD33 is not a new target in myeloid leukemia, Dr Altman pointed out. Gemtuzumab ozogamicin has been studied, approved by the US Food and Drug Administration, and then withdrawn.
However, an increasing number of studies with gemtuzumab are underway, she said, and the agent may once again have a place in the AML armamentarium.
The newest CD33 construct is SGN-CD33A, a stable dipeptide linker that enables uniform drug loading of a pyrrolobenzodiazepine dimer that crosslinks DNA and leads to cell death.
“Single-agent data was quite promising,” Dr Altman noted, with a CR + CRi rate of 41% in previously treated patients and 58% in 12 treatment-naïve patients.
These results prompted a combination study of SGN-CD33A with hypomethylating agents.
“Results were higher than expected with a hypomethylating agent,” Dr Altman pointed out.
The CR + CRi + CR with incomplete platelet recovery was 58%. And the median relapse-free survival was 7.7 months.
A phase 3 randomized trial of SGN-CD33A is planned.
Venetoclax
BCL-2 inhibitors are the fourth type of agent not dependent on mutation complexity. Venetoclax (ABT‐199) is a small‐molecule BCL-2 inhibitor that leads to the initiation of apoptosis.
In a phase 1b trial of venetoclax in combination with a hypomethylating agent, the overall CR rate was 35%, and the CRi rate was 35%.
“Again, higher than what would be expected with a hypomethylating agent alone,” Dr Altman said.
In a phase 1b/2 trial of venetoclax in combination with low‐dose cytarabine, the CR + CRi rate was 54%. Patients responded even if they had prior exposure to hypomethylating agents.
Mutation-specific novel agents
The FLT3 inhibitor midostaurin and the IDH inhibitors AG-120 and AG-221 are among the most exciting mutation-specific agents and the ones most progressed, according to Dr Altman.
FLT3-ITD is mutated in about 30% of AML patients and carries an unfavorable prognosis, and the IDH mutation occurs in about 10% and confers a favorable prognosis.
Midostaurin
A phase 3, randomized, double-blind study of daunorubicin/cytarabine induction and high-dose cytarabine consolidation with midostaurin (PKC412) or placebo had a 59% CR rate by day 60 in the midostaurin arm, compared with 53% in the placebo arm.
“The CR rate was slightly higher in the midostaurin arm,” Dr Altman said, “but what’s the most remarkable about this study is the difference in overall survival.”
The median OS in the midostaurin arm was 74.7 months, compared with 25.6 months in the placebo arm (P=0.0074).
“The major take-home message from this clinical trial,” Dr Altman said, “is that midostaurin improved the overall survival when added to standard therapy and represents a new standard of care.”
AG-120 and AG-221
Two IDH inhibitors that have substantial data available are the IDH1 inhibitor AG-120 and the IDH2 inhibitor AG-221.
As of October 2015, 78 patients had been treated with AG-120 in a phase 1 trial, yielding an overall response rate of 35% and a CR rate of 15%.
As of September 2015, 209 patients had been treated with AG-221 in a phase 1/2 trial, and 66 are still on study. The overall response rate was 37% in 159 adults with relapsed/refractory AML, with a median duration of response of 6.9 months. The CR rate was 18%.
Investigators have initiated a phase 3 study of AG-221 compared to conventional care regimens. ![]()

NEW YORK—We are “finally” making progress in the treatment of acute myeloid leukemia (AML), according to a speaker at the NCCN 11th Annual Congress: Hematologic Malignancies.
Jessica K. Altman, MD, said a number of developments have resulted in improved AML treatment, including a better understanding of biology and prognostic assessment, continued advances in transplant, and updating standard treatments and incorporating novel agents in both relapsed/refractory and newly diagnosed patients.
“There are a couple of practice-changing treatments in acute myeloid leukemia, 2 of which happened over the last decade: daunorubicin intensification and the use of FLT3 inhibitors,” said Dr Altman, an associate professor at Northwestern University Feinberg School of Medicine in Chicago, Illinois.
Dr Altman went on to explain that novel therapies for AML can be divided into 2 basic categories. There are agents that don’t depend on mutation status (like daunorubicin) and those that are mutation-specific (like FLT3 inhibitors).
Therapies not dependent on mutational complexity
The therapies that are not dependent on mutational complexity include anti-CD33 antibodies, BCL‐2 inhibitors, a dose-intensified anthracycline regimen, and different formulations of 7+3, including CPX‐351.
Escalated daunorubicin
Randomized trials of escalated daunorubicin (90 vs 45 mg/m2) have demonstrated benefit in complete responses (CRs) and overall survival (OS) in intermediate-risk patients and patients with core-binding factor mutation. They have demonstrated benefit in OS in FLT3 ITD+ patients.
In patients up to 65 years of age, 60–90 mg/m2 of daunorubicin is now standard.
“It’s still not clear to me—and I don’t know if it ever will be—if 90 is equivalent to 60,” Dr Altman said.
CPX-351
CPX-351 is a liposomal formulation of cytarabine and daunorubicin. In a randomized, phase 3 study of older adults with secondary AML, the median OS was 9.56 months for patients treated with CPX-351 and 5.95 months for patients on the 7+3 regimen (P=0.005).
The median event-free survival was significantly better with CPX-351 (P=0.021), as was the rate of CR + CR with incomplete blood count recovery (CRi). The rate of CR + CRi was 47.7% with CPX-351 and 33.3% for 7+3 (P=0.016).
A similar number of patients went on to transplant in each arm. Grade 3-5 adverse events were similar in frequency and severity in both arms—92% with CPX-351 vs 91% with 7+3.
SGN-CD33A
CD33 is not a new target in myeloid leukemia, Dr Altman pointed out. Gemtuzumab ozogamicin has been studied, approved by the US Food and Drug Administration, and then withdrawn.
However, an increasing number of studies with gemtuzumab are underway, she said, and the agent may once again have a place in the AML armamentarium.
The newest CD33 construct is SGN-CD33A, a stable dipeptide linker that enables uniform drug loading of a pyrrolobenzodiazepine dimer that crosslinks DNA and leads to cell death.
“Single-agent data was quite promising,” Dr Altman noted, with a CR + CRi rate of 41% in previously treated patients and 58% in 12 treatment-naïve patients.
These results prompted a combination study of SGN-CD33A with hypomethylating agents.
“Results were higher than expected with a hypomethylating agent,” Dr Altman pointed out.
The CR + CRi + CR with incomplete platelet recovery was 58%. And the median relapse-free survival was 7.7 months.
A phase 3 randomized trial of SGN-CD33A is planned.
Venetoclax
BCL-2 inhibitors are the fourth type of agent not dependent on mutation complexity. Venetoclax (ABT‐199) is a small‐molecule BCL-2 inhibitor that leads to the initiation of apoptosis.
In a phase 1b trial of venetoclax in combination with a hypomethylating agent, the overall CR rate was 35%, and the CRi rate was 35%.
“Again, higher than what would be expected with a hypomethylating agent alone,” Dr Altman said.
In a phase 1b/2 trial of venetoclax in combination with low‐dose cytarabine, the CR + CRi rate was 54%. Patients responded even if they had prior exposure to hypomethylating agents.
Mutation-specific novel agents
The FLT3 inhibitor midostaurin and the IDH inhibitors AG-120 and AG-221 are among the most exciting mutation-specific agents and the ones most progressed, according to Dr Altman.
FLT3-ITD is mutated in about 30% of AML patients and carries an unfavorable prognosis, and the IDH mutation occurs in about 10% and confers a favorable prognosis.
Midostaurin
A phase 3, randomized, double-blind study of daunorubicin/cytarabine induction and high-dose cytarabine consolidation with midostaurin (PKC412) or placebo had a 59% CR rate by day 60 in the midostaurin arm, compared with 53% in the placebo arm.
“The CR rate was slightly higher in the midostaurin arm,” Dr Altman said, “but what’s the most remarkable about this study is the difference in overall survival.”
The median OS in the midostaurin arm was 74.7 months, compared with 25.6 months in the placebo arm (P=0.0074).
“The major take-home message from this clinical trial,” Dr Altman said, “is that midostaurin improved the overall survival when added to standard therapy and represents a new standard of care.”
AG-120 and AG-221
Two IDH inhibitors that have substantial data available are the IDH1 inhibitor AG-120 and the IDH2 inhibitor AG-221.
As of October 2015, 78 patients had been treated with AG-120 in a phase 1 trial, yielding an overall response rate of 35% and a CR rate of 15%.
As of September 2015, 209 patients had been treated with AG-221 in a phase 1/2 trial, and 66 are still on study. The overall response rate was 37% in 159 adults with relapsed/refractory AML, with a median duration of response of 6.9 months. The CR rate was 18%.
Investigators have initiated a phase 3 study of AG-221 compared to conventional care regimens. ![]()
Drug granted conditional approval to treat CLL in Canada

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()

of venetoclax (Venclexta)
Photo courtesy of AbbVie
Health Canada has issued a Notice of Compliance with Conditions (NOC/c) for the BCL-2 inhibitor venetoclax (Venclexta™).
This means venetoclax is conditionally approved for use in patients with previously treated chronic lymphocytic leukemia (CLL) who have 17p deletion or no other available treatment options.
An NOC/c is authorization to market a drug with the condition that the sponsor perform additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Venetoclax (previously ABT‐199) is being developed by AbbVie and Genentech, a member of the Roche Group. The drug is jointly commercialized by the companies in the US and by AbbVie outside of the US.
Venetoclax is currently under evaluation in phase 3 trials for the treatment of relapsed, refractory, and previously untreated CLL.
Phase 2 trial
Results from a phase 2 trial of venetoclax in CLL (M13-982, NCT01889186) were published in The Lancet Oncology in June. The trial enrolled 107 patients with relapsed or refractory CLL and 17p deletion.
Patients received venetoclax at 400 mg once daily following a weekly ramp-up schedule for the first 5 weeks. The primary endpoint was overall response rate, as determined by an independent review committee.
At a median follow-up of 12.1 months, 85 patients had responded to treatment, for an overall response rate of 79%.
Eight patients (8%) achieved a complete response or complete response with incomplete count recovery, 3 (3%) had a near-partial response, and 74 (69%) had a partial response. Twenty-two patients (21%) did not respond.
At the time of analysis, the median duration of response had not been reached. The same was true for progression-free survival and overall survival. The progression-free survival estimate for 12 months was 72%, and the overall survival estimate was 87%.
The incidence of treatment-emergent adverse was 96%. The most frequent grade 3/4 adverse events were neutropenia (40%), infection (20%), anemia (18%), and thrombocytopenia (15%).
The incidence of serious adverse events was 55%. The most common of these events were pyrexia (7%), autoimmune hemolytic anemia (7%), pneumonia (6%), and febrile neutropenia (5%).
Grade 3 laboratory tumor lysis syndrome (TLS) was reported in 5 patients during the ramp-up period only. Three of these patients continued on venetoclax, but 2 patients required a dose interruption of 1 day each.
In the past, TLS has caused deaths in patients receiving venetoclax. In response, AbbVie stopped dose-escalation in patients receiving the drug and suspended enrollment in phase 1 trials.
However, researchers subsequently found that a modified dosing schedule, prophylaxis, and patient monitoring can reduce the risk of TLS. ![]()
What Hospitalists Can Really Learn from Aviation
The aviation safety model is often discussed in a healthcare context but in a way that may miss the most important points, a new article in BMJ Quality & Safety suggests.
The article, “Learning from Near Misses in Aviation: So Much More to It Than You Thought” by Robert Wears, MD, PhD, MS, of University of Florida’s Department of Emergency Medicine, suggests healthcare still has important lessons to learn from aviation. The article focuses on a book called Close Calls: Managing Risk and Resilience in Airline Flight Safety by Carl Macrae.
“Although the book itself is about airlines, it has important lessons for improving safety in healthcare, especially with respect to management of incidents or ‘near misses,’” Dr. Wears writes. “Its rich descriptions and detailed explanation of the practical, everyday work of flight safety investigators should be required reading for anyone interested in patient safety. It will destroy many of the myths and misconceptions about reporting systems and learning from incidents that have caused us to expend so much effort for such meager results; it will also overturn the normative model of safety prevalent in healthcare.”
Dr. Wears says he wanted to write the article for two reasons.
“First, the patient safety orthodoxy has been obsessed with systems for reporting incidents, accidents, hazards, general ‘hiccups’ in clinical work for years, but almost nothing of value has come from this effort despite frequent badgering of physicians to report more,” he says. “Second, mainstream patient safety has also been enamored of the aviation safety model, but its ideas about how aviation safety is actually accomplished are naive and simplistic.”
He emphasizes that patient safety efforts to date have focused on the wrong things: too much on acquiring and storing reports and too little on analyzing them to develop an understanding of the systems in which hazards to patients arise.
“Making sense of incidents is far more important than classifying, counting, or trending them,” Dr. Wears says.
Hospitalists are on the front line of these issues, of course.
“Hospitalists regularly encounter hazards to patients in their daily work and, for the most part, successfully manage to mitigate or work around them, but the hazards remain in the system, only to pop up again sometime later. … A rich description of how a successful and effective safety reporting and analysis effort really works—not how we imagine it to work—could help us exchange our current wasteful and ineffective approach for something better,” he says.
Reference
- Wears R. Learning from near misses in aviation: so much more to it than you thought [published online ahead of print September 1, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2016-005990.
The aviation safety model is often discussed in a healthcare context but in a way that may miss the most important points, a new article in BMJ Quality & Safety suggests.
The article, “Learning from Near Misses in Aviation: So Much More to It Than You Thought” by Robert Wears, MD, PhD, MS, of University of Florida’s Department of Emergency Medicine, suggests healthcare still has important lessons to learn from aviation. The article focuses on a book called Close Calls: Managing Risk and Resilience in Airline Flight Safety by Carl Macrae.
“Although the book itself is about airlines, it has important lessons for improving safety in healthcare, especially with respect to management of incidents or ‘near misses,’” Dr. Wears writes. “Its rich descriptions and detailed explanation of the practical, everyday work of flight safety investigators should be required reading for anyone interested in patient safety. It will destroy many of the myths and misconceptions about reporting systems and learning from incidents that have caused us to expend so much effort for such meager results; it will also overturn the normative model of safety prevalent in healthcare.”
Dr. Wears says he wanted to write the article for two reasons.
“First, the patient safety orthodoxy has been obsessed with systems for reporting incidents, accidents, hazards, general ‘hiccups’ in clinical work for years, but almost nothing of value has come from this effort despite frequent badgering of physicians to report more,” he says. “Second, mainstream patient safety has also been enamored of the aviation safety model, but its ideas about how aviation safety is actually accomplished are naive and simplistic.”
He emphasizes that patient safety efforts to date have focused on the wrong things: too much on acquiring and storing reports and too little on analyzing them to develop an understanding of the systems in which hazards to patients arise.
“Making sense of incidents is far more important than classifying, counting, or trending them,” Dr. Wears says.
Hospitalists are on the front line of these issues, of course.
“Hospitalists regularly encounter hazards to patients in their daily work and, for the most part, successfully manage to mitigate or work around them, but the hazards remain in the system, only to pop up again sometime later. … A rich description of how a successful and effective safety reporting and analysis effort really works—not how we imagine it to work—could help us exchange our current wasteful and ineffective approach for something better,” he says.
Reference
- Wears R. Learning from near misses in aviation: so much more to it than you thought [published online ahead of print September 1, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2016-005990.
The aviation safety model is often discussed in a healthcare context but in a way that may miss the most important points, a new article in BMJ Quality & Safety suggests.
The article, “Learning from Near Misses in Aviation: So Much More to It Than You Thought” by Robert Wears, MD, PhD, MS, of University of Florida’s Department of Emergency Medicine, suggests healthcare still has important lessons to learn from aviation. The article focuses on a book called Close Calls: Managing Risk and Resilience in Airline Flight Safety by Carl Macrae.
“Although the book itself is about airlines, it has important lessons for improving safety in healthcare, especially with respect to management of incidents or ‘near misses,’” Dr. Wears writes. “Its rich descriptions and detailed explanation of the practical, everyday work of flight safety investigators should be required reading for anyone interested in patient safety. It will destroy many of the myths and misconceptions about reporting systems and learning from incidents that have caused us to expend so much effort for such meager results; it will also overturn the normative model of safety prevalent in healthcare.”
Dr. Wears says he wanted to write the article for two reasons.
“First, the patient safety orthodoxy has been obsessed with systems for reporting incidents, accidents, hazards, general ‘hiccups’ in clinical work for years, but almost nothing of value has come from this effort despite frequent badgering of physicians to report more,” he says. “Second, mainstream patient safety has also been enamored of the aviation safety model, but its ideas about how aviation safety is actually accomplished are naive and simplistic.”
He emphasizes that patient safety efforts to date have focused on the wrong things: too much on acquiring and storing reports and too little on analyzing them to develop an understanding of the systems in which hazards to patients arise.
“Making sense of incidents is far more important than classifying, counting, or trending them,” Dr. Wears says.
Hospitalists are on the front line of these issues, of course.
“Hospitalists regularly encounter hazards to patients in their daily work and, for the most part, successfully manage to mitigate or work around them, but the hazards remain in the system, only to pop up again sometime later. … A rich description of how a successful and effective safety reporting and analysis effort really works—not how we imagine it to work—could help us exchange our current wasteful and ineffective approach for something better,” he says.
Reference
- Wears R. Learning from near misses in aviation: so much more to it than you thought [published online ahead of print September 1, 2016]. BMJ Qual Saf. doi:10.1136/bmjqs-2016-005990.
Educating Patients about Sleep Tools
One of the biggest complaints of hospital patients today is poor sleep, which is not conducive to healing or good health in general.
“The reason I’m interested, as a cardiologist, is that sleep disorders are associated with an increased risk of cardiovascular mortality,” says Peter M. Farrehi, MD, assistant professor of internal medicine at the University of Michigan and lead author of a recent sleep study published in The American Journal of Medicine.
Most information about sleeping in the hospital comes from ICU studies, he says.
Dr. Farrehi wanted to actually test an intervention rather than simply survey patients. All patients received an eye mask, ear plugs, and a white-noise machine, then were randomized to receive an education-based script on the importance of using these sleep-enhancing tools or a discussion about the general benefits of sleep.
“To avoid bias in the study both from the research staff and also hospital staff, I didn't want only the intervention to have the tools,” he says. “This was a double-blind, randomized control trial in the hospital, which is really unusual.”
Patients in the group that was taught about the sleep-enhancing tools had a statistically significant difference in their perceptions of fatigue and a trend toward improving their sleep and wake disturbances.
Dr. Farrehi suggests hospitalists talk to their patients complaining of poor sleep about these sleep tools. If they are not available in their hospital, hospitalists might refer their medical director to this paper to see if there is any interest in purchasing these sleep tools.
Reference
- 1. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of sleep tool education during hospitalization: a randomized controlled trial [published online ahead of print August 23, 2016]. Am J Med. doi:10.1016/j.amjmed.2016.08.001.
One of the biggest complaints of hospital patients today is poor sleep, which is not conducive to healing or good health in general.
“The reason I’m interested, as a cardiologist, is that sleep disorders are associated with an increased risk of cardiovascular mortality,” says Peter M. Farrehi, MD, assistant professor of internal medicine at the University of Michigan and lead author of a recent sleep study published in The American Journal of Medicine.
Most information about sleeping in the hospital comes from ICU studies, he says.
Dr. Farrehi wanted to actually test an intervention rather than simply survey patients. All patients received an eye mask, ear plugs, and a white-noise machine, then were randomized to receive an education-based script on the importance of using these sleep-enhancing tools or a discussion about the general benefits of sleep.
“To avoid bias in the study both from the research staff and also hospital staff, I didn't want only the intervention to have the tools,” he says. “This was a double-blind, randomized control trial in the hospital, which is really unusual.”
Patients in the group that was taught about the sleep-enhancing tools had a statistically significant difference in their perceptions of fatigue and a trend toward improving their sleep and wake disturbances.
Dr. Farrehi suggests hospitalists talk to their patients complaining of poor sleep about these sleep tools. If they are not available in their hospital, hospitalists might refer their medical director to this paper to see if there is any interest in purchasing these sleep tools.
Reference
- 1. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of sleep tool education during hospitalization: a randomized controlled trial [published online ahead of print August 23, 2016]. Am J Med. doi:10.1016/j.amjmed.2016.08.001.
One of the biggest complaints of hospital patients today is poor sleep, which is not conducive to healing or good health in general.
“The reason I’m interested, as a cardiologist, is that sleep disorders are associated with an increased risk of cardiovascular mortality,” says Peter M. Farrehi, MD, assistant professor of internal medicine at the University of Michigan and lead author of a recent sleep study published in The American Journal of Medicine.
Most information about sleeping in the hospital comes from ICU studies, he says.
Dr. Farrehi wanted to actually test an intervention rather than simply survey patients. All patients received an eye mask, ear plugs, and a white-noise machine, then were randomized to receive an education-based script on the importance of using these sleep-enhancing tools or a discussion about the general benefits of sleep.
“To avoid bias in the study both from the research staff and also hospital staff, I didn't want only the intervention to have the tools,” he says. “This was a double-blind, randomized control trial in the hospital, which is really unusual.”
Patients in the group that was taught about the sleep-enhancing tools had a statistically significant difference in their perceptions of fatigue and a trend toward improving their sleep and wake disturbances.
Dr. Farrehi suggests hospitalists talk to their patients complaining of poor sleep about these sleep tools. If they are not available in their hospital, hospitalists might refer their medical director to this paper to see if there is any interest in purchasing these sleep tools.
Reference
- 1. Farrehi PM, Clore KR, Scott JR, Vanini G, Clauw DJ. Efficacy of sleep tool education during hospitalization: a randomized controlled trial [published online ahead of print August 23, 2016]. Am J Med. doi:10.1016/j.amjmed.2016.08.001.