User login
There may be zebras running with the horses
Over the summer, I lectured in an internal medicine maintenance-of-certification course. Using a case-based discussion format, I presented real patients to review the breadth of rheumatic diseases that the course participants might encounter in their practices and on board examinations. At a break, one of the participants, obviously frustrated, approached me to say that he thought that I had mentioned too many uncommon situations, that he didn’t need to know secondary treatment options and disease mimics—I should just have reviewed the common aspects of the common diseases he would encounter in practice (and on the exam).
I was a bit taken aback. I try hard to avoid emphasizing the arcane details that only a subspecialist needs to know. I reconsidered my approach: should I only review the common and the expected? But without knowing something about the clinical permutations and the mimics of the common diseases we encounter, I don’t know how we can avoid making incorrect diagnoses due to the cognitive bias of familiarity. Common diseases are indeed common, but that doesn’t mean patients with less-common ones don’t find their way into our practices. As internists, we need to constantly humble ourselves with the knowledge that there is much in medicine, in every specialty, that we can’t remember, do not recognize, or just don’t know enough about. It is not just about “what will be on the test.” When a patient exhibits atypical features of the common disease that they have been diagnosed with, or doesn’t respond as expected to therapy, we need to remember that less-common mimics of that disease exist, even if we can’t remember the specific names or the pathophysiology.
Peripheral vascular disease is common, is rarely diagnosed early, and is usually related to atherosclerosis. Significant disease is generally manifested by symptoms of exertional limb ischemia or even pain at rest and stigmata of distal tissue hypoxia and necrosis from the decreased supply of oxygen and nutrients. But there are nonatherosclerotic causes of limb claudication, distal ischemia, and livedo reticularis. In my clinic, patients with those symptoms and findings are quite likely to have a form of large vessel arteritis, Raynaud phenomenon, or both. In a patient with Takayasu or giant cell arteritis, pain with repetitive arm use is more likely from subclavian occlusion than rotator cuff disease, whereas the latter is the far more common cause of arm pain with repetitive motion when patients see a physician for “arm pain” before the diagnosis of arteritis is considered. We need to entertain alternative diagnoses in order to perform the appropriate physical examination, obtain appropriate diagnostic studies, and initiate the correct therapy.
Eun et al, in this issue of the Journal, highlight and briefly discuss nine nonatherosclerotic entities that cause limb ischemia. The diagnostic assessment of these can be invasive and expensive, yet may be warranted to provide the appropriate therapy. The diagnosis not pursued will rarely be made in a timely manner, and the diagnosis not considered will not be pursued at all. And with these diseases, the patient’s history and examination usually provide the clues that the horse has stripes.
Eun et al highlight clinical features that may distinguish these entities from typical atherosclerotic peripheral vascular disease. Recognizing the features that are atypical for atherosclerosis implies that we know the more typical features of the disease. Knowing something about the uncommon as well as the common helps prepare us for the real test—the patient in front of us. And that patient doesn’t usually know that he or she is a zebra and not a horse.
Last month, I highlighted some staff changes in our editorial staff. I want to take this opportunity to introduce Craig Nielsen, MD, as our new deputy editor. Craig is a superb internist in both inpatient and outpatient arenas, a medical educator, and former director of our internal medicine residency program.
Over the summer, I lectured in an internal medicine maintenance-of-certification course. Using a case-based discussion format, I presented real patients to review the breadth of rheumatic diseases that the course participants might encounter in their practices and on board examinations. At a break, one of the participants, obviously frustrated, approached me to say that he thought that I had mentioned too many uncommon situations, that he didn’t need to know secondary treatment options and disease mimics—I should just have reviewed the common aspects of the common diseases he would encounter in practice (and on the exam).
I was a bit taken aback. I try hard to avoid emphasizing the arcane details that only a subspecialist needs to know. I reconsidered my approach: should I only review the common and the expected? But without knowing something about the clinical permutations and the mimics of the common diseases we encounter, I don’t know how we can avoid making incorrect diagnoses due to the cognitive bias of familiarity. Common diseases are indeed common, but that doesn’t mean patients with less-common ones don’t find their way into our practices. As internists, we need to constantly humble ourselves with the knowledge that there is much in medicine, in every specialty, that we can’t remember, do not recognize, or just don’t know enough about. It is not just about “what will be on the test.” When a patient exhibits atypical features of the common disease that they have been diagnosed with, or doesn’t respond as expected to therapy, we need to remember that less-common mimics of that disease exist, even if we can’t remember the specific names or the pathophysiology.
Peripheral vascular disease is common, is rarely diagnosed early, and is usually related to atherosclerosis. Significant disease is generally manifested by symptoms of exertional limb ischemia or even pain at rest and stigmata of distal tissue hypoxia and necrosis from the decreased supply of oxygen and nutrients. But there are nonatherosclerotic causes of limb claudication, distal ischemia, and livedo reticularis. In my clinic, patients with those symptoms and findings are quite likely to have a form of large vessel arteritis, Raynaud phenomenon, or both. In a patient with Takayasu or giant cell arteritis, pain with repetitive arm use is more likely from subclavian occlusion than rotator cuff disease, whereas the latter is the far more common cause of arm pain with repetitive motion when patients see a physician for “arm pain” before the diagnosis of arteritis is considered. We need to entertain alternative diagnoses in order to perform the appropriate physical examination, obtain appropriate diagnostic studies, and initiate the correct therapy.
Eun et al, in this issue of the Journal, highlight and briefly discuss nine nonatherosclerotic entities that cause limb ischemia. The diagnostic assessment of these can be invasive and expensive, yet may be warranted to provide the appropriate therapy. The diagnosis not pursued will rarely be made in a timely manner, and the diagnosis not considered will not be pursued at all. And with these diseases, the patient’s history and examination usually provide the clues that the horse has stripes.
Eun et al highlight clinical features that may distinguish these entities from typical atherosclerotic peripheral vascular disease. Recognizing the features that are atypical for atherosclerosis implies that we know the more typical features of the disease. Knowing something about the uncommon as well as the common helps prepare us for the real test—the patient in front of us. And that patient doesn’t usually know that he or she is a zebra and not a horse.
Last month, I highlighted some staff changes in our editorial staff. I want to take this opportunity to introduce Craig Nielsen, MD, as our new deputy editor. Craig is a superb internist in both inpatient and outpatient arenas, a medical educator, and former director of our internal medicine residency program.
Over the summer, I lectured in an internal medicine maintenance-of-certification course. Using a case-based discussion format, I presented real patients to review the breadth of rheumatic diseases that the course participants might encounter in their practices and on board examinations. At a break, one of the participants, obviously frustrated, approached me to say that he thought that I had mentioned too many uncommon situations, that he didn’t need to know secondary treatment options and disease mimics—I should just have reviewed the common aspects of the common diseases he would encounter in practice (and on the exam).
I was a bit taken aback. I try hard to avoid emphasizing the arcane details that only a subspecialist needs to know. I reconsidered my approach: should I only review the common and the expected? But without knowing something about the clinical permutations and the mimics of the common diseases we encounter, I don’t know how we can avoid making incorrect diagnoses due to the cognitive bias of familiarity. Common diseases are indeed common, but that doesn’t mean patients with less-common ones don’t find their way into our practices. As internists, we need to constantly humble ourselves with the knowledge that there is much in medicine, in every specialty, that we can’t remember, do not recognize, or just don’t know enough about. It is not just about “what will be on the test.” When a patient exhibits atypical features of the common disease that they have been diagnosed with, or doesn’t respond as expected to therapy, we need to remember that less-common mimics of that disease exist, even if we can’t remember the specific names or the pathophysiology.
Peripheral vascular disease is common, is rarely diagnosed early, and is usually related to atherosclerosis. Significant disease is generally manifested by symptoms of exertional limb ischemia or even pain at rest and stigmata of distal tissue hypoxia and necrosis from the decreased supply of oxygen and nutrients. But there are nonatherosclerotic causes of limb claudication, distal ischemia, and livedo reticularis. In my clinic, patients with those symptoms and findings are quite likely to have a form of large vessel arteritis, Raynaud phenomenon, or both. In a patient with Takayasu or giant cell arteritis, pain with repetitive arm use is more likely from subclavian occlusion than rotator cuff disease, whereas the latter is the far more common cause of arm pain with repetitive motion when patients see a physician for “arm pain” before the diagnosis of arteritis is considered. We need to entertain alternative diagnoses in order to perform the appropriate physical examination, obtain appropriate diagnostic studies, and initiate the correct therapy.
Eun et al, in this issue of the Journal, highlight and briefly discuss nine nonatherosclerotic entities that cause limb ischemia. The diagnostic assessment of these can be invasive and expensive, yet may be warranted to provide the appropriate therapy. The diagnosis not pursued will rarely be made in a timely manner, and the diagnosis not considered will not be pursued at all. And with these diseases, the patient’s history and examination usually provide the clues that the horse has stripes.
Eun et al highlight clinical features that may distinguish these entities from typical atherosclerotic peripheral vascular disease. Recognizing the features that are atypical for atherosclerosis implies that we know the more typical features of the disease. Knowing something about the uncommon as well as the common helps prepare us for the real test—the patient in front of us. And that patient doesn’t usually know that he or she is a zebra and not a horse.
Last month, I highlighted some staff changes in our editorial staff. I want to take this opportunity to introduce Craig Nielsen, MD, as our new deputy editor. Craig is a superb internist in both inpatient and outpatient arenas, a medical educator, and former director of our internal medicine residency program.
Neuronal protein could be multiple sclerosis blood biomarker
LONDON – Higher levels of a neuronal protein were found in the blood of patients with relapsing-remitting multiple sclerosis than in healthy subjects in a proof-of-concept study reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood levels of neurofilament light chain (NfL) were 28.1 vs. 12.5 pg/mL, respectively (P less than .0001), and were also found to be higher in relapsing-remitting multiple sclerosis (RRMS) patients with greater disease activity seen on MRI.
“These findings support a role for neurofilament light chain (NfL) as a peripheral biomarker for MS,” said the reporting investigator Jens Kuhle, MD, of University Hospital Basel (Switzerland).
“There is an urgent unmet need for reliable biomarkers of neurodegeneration, besides efforts that are being done in MRI, optical coherence tomography, and evoked potentials,” Dr. Kuhle said.
He added: “NfL is an exclusively neuronal protein. It is expressed in the cytosol of neurons, it is released upon cell injury into the CSF [cerebral spinal fluid], and obviously it also appears in the blood circulation.” NfL in the CSF thus reflects nerve damage and had been seen in patients with MS and several other neurologic diseases, including Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis.
Until recently, however, it was not possible to detect NfL in the blood, as levels are around 50 to 100 times lower than in the CSF, but Dr. Kuhle and his associates have shown that using an electrochemiluminescence immunoassay could detect increasing NfL levels with increasing disease activity (Mult Scler. 2016 Jan 11. doi: 10.1177/1352458515623365).
In the current study, a Single Molecule Array Immunoassay (Quanterix) was used. This is based on an enzyme-linked immunoassay with more than 280,000 wells and developed to be an ultrasensitive diagnostic platform to measure minute quantities of individual proteins. Dr. Kuhle noted that it had “significantly increased sensitivity to measure NfL in blood” when compared with conventional ELISA or electrochemiluminescence (Clin Chem Lab Med. 2016;54[10]:1655-61).
Two to three consecutive blood samples taken from 149 patients with RRMS participating in the phase III FREEDOMS trial were obtained and these were compared with samples from 29 similarly aged healthy individuals without MS obtained from a separate biobank.
Patients with two or more relapses in the past 24 months had significantly higher NfL levels than did those with one relapse or no relapses. Serum NfL also significantly increased with the Expanded Disability Status Scale score recorded at the time the blood samples were taken.
Furthermore, “blood NfL levels predicted subsequent brain atrophy rates,” Dr. Kuhle reported, with NfL levels at 6 months being highly predictive of brain volume changes at 24 months (P less than .0001).
And, in this preliminary dataset, patients who had been treated with fingolimod (Gilenya) versus placebo in the FREEDOMS trial had lower NfL levels at 6, 12, and 24 months.
Dr. Kuhle observed that the findings of this study were corroborated by other study data presented during the poster sessions at the ECTRIMS 2016 meeting.
The FREEDOMS trial was sponsored by Novartis. Dr. Kuhle disclosed receiving research support and consulting fees from Biogen, Novartis, and Protagen AG. He has also received speaker fees and travel expenses from Novartis and several other companies, and several of his coinvestigators were employees of Novartis.
LONDON – Higher levels of a neuronal protein were found in the blood of patients with relapsing-remitting multiple sclerosis than in healthy subjects in a proof-of-concept study reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood levels of neurofilament light chain (NfL) were 28.1 vs. 12.5 pg/mL, respectively (P less than .0001), and were also found to be higher in relapsing-remitting multiple sclerosis (RRMS) patients with greater disease activity seen on MRI.
“These findings support a role for neurofilament light chain (NfL) as a peripheral biomarker for MS,” said the reporting investigator Jens Kuhle, MD, of University Hospital Basel (Switzerland).
“There is an urgent unmet need for reliable biomarkers of neurodegeneration, besides efforts that are being done in MRI, optical coherence tomography, and evoked potentials,” Dr. Kuhle said.
He added: “NfL is an exclusively neuronal protein. It is expressed in the cytosol of neurons, it is released upon cell injury into the CSF [cerebral spinal fluid], and obviously it also appears in the blood circulation.” NfL in the CSF thus reflects nerve damage and had been seen in patients with MS and several other neurologic diseases, including Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis.
Until recently, however, it was not possible to detect NfL in the blood, as levels are around 50 to 100 times lower than in the CSF, but Dr. Kuhle and his associates have shown that using an electrochemiluminescence immunoassay could detect increasing NfL levels with increasing disease activity (Mult Scler. 2016 Jan 11. doi: 10.1177/1352458515623365).
In the current study, a Single Molecule Array Immunoassay (Quanterix) was used. This is based on an enzyme-linked immunoassay with more than 280,000 wells and developed to be an ultrasensitive diagnostic platform to measure minute quantities of individual proteins. Dr. Kuhle noted that it had “significantly increased sensitivity to measure NfL in blood” when compared with conventional ELISA or electrochemiluminescence (Clin Chem Lab Med. 2016;54[10]:1655-61).
Two to three consecutive blood samples taken from 149 patients with RRMS participating in the phase III FREEDOMS trial were obtained and these were compared with samples from 29 similarly aged healthy individuals without MS obtained from a separate biobank.
Patients with two or more relapses in the past 24 months had significantly higher NfL levels than did those with one relapse or no relapses. Serum NfL also significantly increased with the Expanded Disability Status Scale score recorded at the time the blood samples were taken.
Furthermore, “blood NfL levels predicted subsequent brain atrophy rates,” Dr. Kuhle reported, with NfL levels at 6 months being highly predictive of brain volume changes at 24 months (P less than .0001).
And, in this preliminary dataset, patients who had been treated with fingolimod (Gilenya) versus placebo in the FREEDOMS trial had lower NfL levels at 6, 12, and 24 months.
Dr. Kuhle observed that the findings of this study were corroborated by other study data presented during the poster sessions at the ECTRIMS 2016 meeting.
The FREEDOMS trial was sponsored by Novartis. Dr. Kuhle disclosed receiving research support and consulting fees from Biogen, Novartis, and Protagen AG. He has also received speaker fees and travel expenses from Novartis and several other companies, and several of his coinvestigators were employees of Novartis.
LONDON – Higher levels of a neuronal protein were found in the blood of patients with relapsing-remitting multiple sclerosis than in healthy subjects in a proof-of-concept study reported at the annual congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood levels of neurofilament light chain (NfL) were 28.1 vs. 12.5 pg/mL, respectively (P less than .0001), and were also found to be higher in relapsing-remitting multiple sclerosis (RRMS) patients with greater disease activity seen on MRI.
“These findings support a role for neurofilament light chain (NfL) as a peripheral biomarker for MS,” said the reporting investigator Jens Kuhle, MD, of University Hospital Basel (Switzerland).
“There is an urgent unmet need for reliable biomarkers of neurodegeneration, besides efforts that are being done in MRI, optical coherence tomography, and evoked potentials,” Dr. Kuhle said.
He added: “NfL is an exclusively neuronal protein. It is expressed in the cytosol of neurons, it is released upon cell injury into the CSF [cerebral spinal fluid], and obviously it also appears in the blood circulation.” NfL in the CSF thus reflects nerve damage and had been seen in patients with MS and several other neurologic diseases, including Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis.
Until recently, however, it was not possible to detect NfL in the blood, as levels are around 50 to 100 times lower than in the CSF, but Dr. Kuhle and his associates have shown that using an electrochemiluminescence immunoassay could detect increasing NfL levels with increasing disease activity (Mult Scler. 2016 Jan 11. doi: 10.1177/1352458515623365).
In the current study, a Single Molecule Array Immunoassay (Quanterix) was used. This is based on an enzyme-linked immunoassay with more than 280,000 wells and developed to be an ultrasensitive diagnostic platform to measure minute quantities of individual proteins. Dr. Kuhle noted that it had “significantly increased sensitivity to measure NfL in blood” when compared with conventional ELISA or electrochemiluminescence (Clin Chem Lab Med. 2016;54[10]:1655-61).
Two to three consecutive blood samples taken from 149 patients with RRMS participating in the phase III FREEDOMS trial were obtained and these were compared with samples from 29 similarly aged healthy individuals without MS obtained from a separate biobank.
Patients with two or more relapses in the past 24 months had significantly higher NfL levels than did those with one relapse or no relapses. Serum NfL also significantly increased with the Expanded Disability Status Scale score recorded at the time the blood samples were taken.
Furthermore, “blood NfL levels predicted subsequent brain atrophy rates,” Dr. Kuhle reported, with NfL levels at 6 months being highly predictive of brain volume changes at 24 months (P less than .0001).
And, in this preliminary dataset, patients who had been treated with fingolimod (Gilenya) versus placebo in the FREEDOMS trial had lower NfL levels at 6, 12, and 24 months.
Dr. Kuhle observed that the findings of this study were corroborated by other study data presented during the poster sessions at the ECTRIMS 2016 meeting.
The FREEDOMS trial was sponsored by Novartis. Dr. Kuhle disclosed receiving research support and consulting fees from Biogen, Novartis, and Protagen AG. He has also received speaker fees and travel expenses from Novartis and several other companies, and several of his coinvestigators were employees of Novartis.
Key clinical point:
Major finding: Blood NfL levels were significantly higher in MS patients than in healthy individuals (28.1 vs. 12.5 pg/mL; P less than .0001); NfL levels were also increased in patients with greater disease activity seen on MRI.
Data source: Proof-of-concept study involving blood samples taken from 149 patients with relapsing-remitting MS (RRMS) participating in the phase III FREEDOMS trial and 29 healthy individuals without MS from a separate biobank.
Disclosures: The FREEDOMS trial was sponsored by Novartis. Dr. Kuhle disclosed receiving research support and consulting fees from Biogen, Novartis, and Protagen AG. He has also received speaker fees and travel expenses from Novartis and several other companies, and several of his coinvestigators were employees of Novartis.
Pediatric Dermatology Consult - October 2016
BY CATALINA MATIZ, MD, AND ANDREA WALDMAN, MD
At the time of initial evaluation, the patient was prescribed a 2-week course of topical triamcinolone 0.1% ointment. Subsequently, the cutaneous lesions clinically improved, but did not completely resolve. Mother had no recollection of infant contact with metal, plastic, or additional topical emollients or medications. On further investigation, the patient’s mother revealed the recent conversion to a different brand of diaper. The diapers had a blue and green polka dot design corresponding with the distribution of our patient’s cutaneous lesions, highly suggestive of allergic contact dermatitis (ACD).
Diaper dermatitis represents a common complaint peaking at 9-12 months of age, estimated to occur in 25% of infants in the primary care office setting.1 Irritant contact dermatitis (ICD) predominates as the most common etiology of diaper dermatitis. ICD represents a nonimmunologic response, triggered by maceration and breakdown of the cutaneous barrier, directly resulting from chronic exposure to urine and feces.1 In contrast, ACD characterizes an immunologic reaction to specific offending allergen(s).
ACD can arise in the diaper area following contact with dyes, fragrances, or other constituents of disposable diapers, wet wipes, diaper creams, or medications. Alberta et al. initially described ACD in several infants, resulting from blue, green, and pink dyes present in various disposable diapers. The dermatitis promptly resolved upon switching to dye-free diapers.2 Furthermore, topical products applied to the diaper region such as barrier creams and wet wipes may trigger allergic sensitization. Sensitization to an allergen can occur after few exposures to years after introduction. Following the initial contact reaction, re-exposure to the offending allergen can elicit a quicker cutaneous response. Case reports have detailed cases of ACD presenting in neonates as early as 1 week of age.3
If ACD is suspected as an etiologic factor in diaper dermatitis, providers should consider constituents present in topical formulations, wet wipes, and components of the diaper itself. A specific example includes mercaptobenzothiazole, a rubber additive commonly found in elastic bands of disposable diapers.2 The most common potential allergens discovered in a recent review of 63 diaper wipes, 41 topical diaper preparations, and 3 top-selling diaper brands included botanical extracts (i.e. aloe vera), alpha-tocopherol, fragrances, propylene glycol, parabens, iodopropynyl butylcarbamate, and lanolin.4
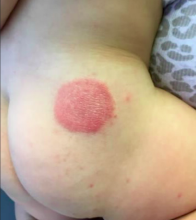
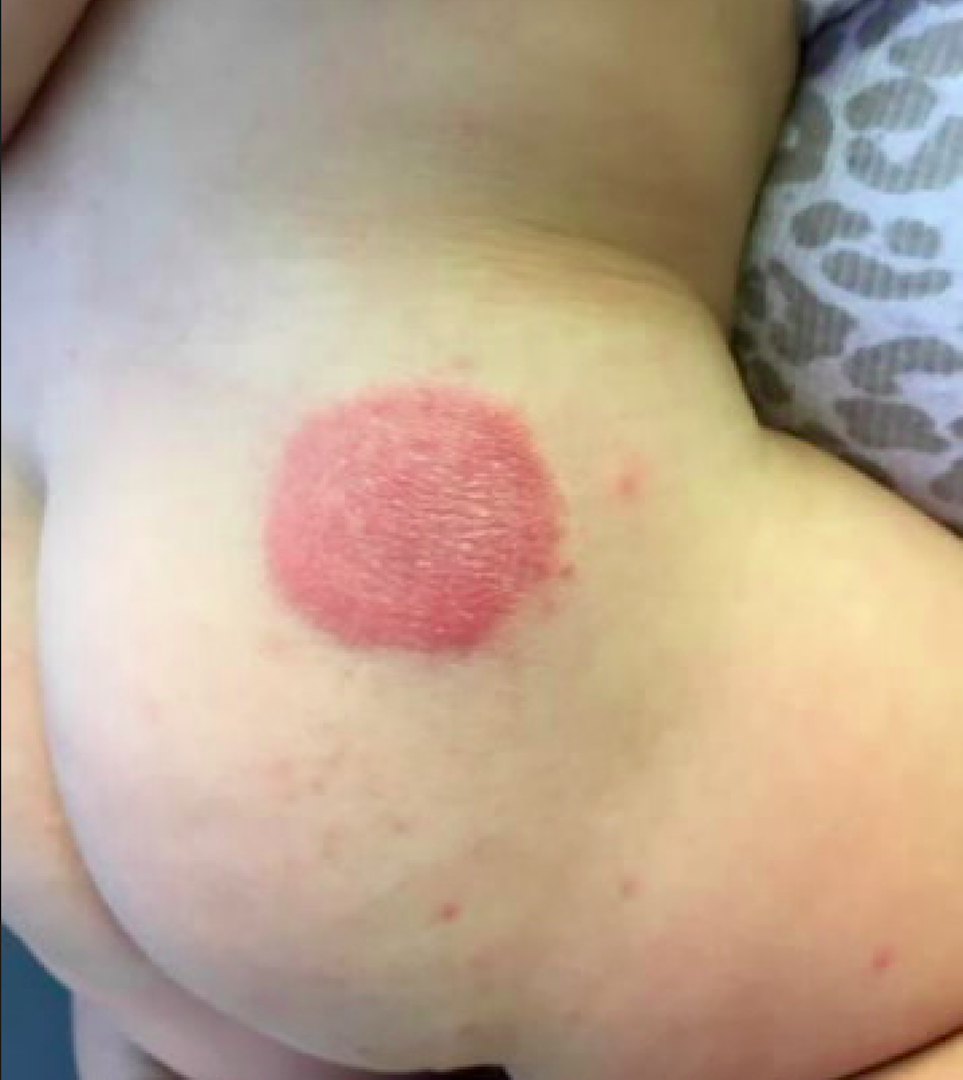
ACD may be difficult to diagnose. Obtaining a thorough clinical history is essential to diagnosis. ACD is often distinguished by its distinct cutaneous morphology, with initial lesions commonly characterized by sharply demarcated erythema associated with vesiculation and edema. The dermatitis progressively evolves to a mixed presentation of predominantly eczematous papules and plaques with crusting, scaling, and lichenification. Another clue suggestive of ACD is cutaneous involvement limited to areas of allergen contact, such as the geometric plaques corresponding to areas of skin exposure to blue and green dye in our patient.1
Differential diagnosis
In contrast to ACD, ICD commonly erupts in the perianal region in the presence of feces, especially during diarrheal episodes. Common clinical manifestations include skin maceration, erosions, erythema, papulation, and scaling. More severe presentations of irritant dermatitis include Jacquet’s diaper dermatitis, perianal pseudoverrucous papules and nodules and granuloma gluteale infantum.1 These cutaneous findings also may develop in older patients with irritated peristomal skin.5
Inflammatory dermatoses such as psoriasis and seborrheic dermatitis also may erupt in the diaper region. Psoriasis commonly involves the gluteal cleft and inguinal folds, appearing as well-demarcated, pink plaques with thin white scale.5 Persistence of plaques despite topical corticosteroid application and lack of pruritus further delineates this disorder. Family history of psoriasis may provide an additional clue to the diagnosis of psoriasis. Similarly, seborrheic dermatitis may present with diaper involvement characterized by well-demarcated erythematous plaques with minimal or absent greasy yellow scale. This dermatitis commonly erupts in infants and neonates less than 6 weeks old and resolves by 6-9 months of age.1
Langerhans cell histiocytosis (LCH) represents a serious condition important to consider in the differential diagnosis of persistent recalcitrant diaper dermatitis. Often confused with seborrheic dermatitis, the diaper dermatitis of LCH may be differentiated by petechiae, purpura, and/or ulceration and atrophy of the inguinal folds.5
Management
Avoidance of the allergen constitutes the gold standard of treatment for ACD. Initial treatment involves adequate-potency topical corticosteroids; for the diaper area, low- to mid-potency corticosteroids are recommended. Changing to dye-free diapers and/or avoiding contact with the suspected allergen also is advised. Epicutaneous patch testing is recommended if the dermatitis fails to improve despite removal of the suspected offending agent.5
References
1. Pediatr Dermatol. 2014 Nov;31 Suppl 1:19-24.
2. Pediatrics. 2005 Sep;116(3):e450-2.
3. Cutis. 1994 Nov;54(5):300-2.
4. Dermatitis. 2016 May-Jun;27(3):110-8.
5. “Neonatal and Infant Dermatology,” 3rd edition (Philadelphia: Elsevier, 2015, p. 251)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Dr. Waldman is a clinical research fellow at the hospital. Email them at pdnews@frontlinemedcom.com.
BY CATALINA MATIZ, MD, AND ANDREA WALDMAN, MD
At the time of initial evaluation, the patient was prescribed a 2-week course of topical triamcinolone 0.1% ointment. Subsequently, the cutaneous lesions clinically improved, but did not completely resolve. Mother had no recollection of infant contact with metal, plastic, or additional topical emollients or medications. On further investigation, the patient’s mother revealed the recent conversion to a different brand of diaper. The diapers had a blue and green polka dot design corresponding with the distribution of our patient’s cutaneous lesions, highly suggestive of allergic contact dermatitis (ACD).
Diaper dermatitis represents a common complaint peaking at 9-12 months of age, estimated to occur in 25% of infants in the primary care office setting.1 Irritant contact dermatitis (ICD) predominates as the most common etiology of diaper dermatitis. ICD represents a nonimmunologic response, triggered by maceration and breakdown of the cutaneous barrier, directly resulting from chronic exposure to urine and feces.1 In contrast, ACD characterizes an immunologic reaction to specific offending allergen(s).
ACD can arise in the diaper area following contact with dyes, fragrances, or other constituents of disposable diapers, wet wipes, diaper creams, or medications. Alberta et al. initially described ACD in several infants, resulting from blue, green, and pink dyes present in various disposable diapers. The dermatitis promptly resolved upon switching to dye-free diapers.2 Furthermore, topical products applied to the diaper region such as barrier creams and wet wipes may trigger allergic sensitization. Sensitization to an allergen can occur after few exposures to years after introduction. Following the initial contact reaction, re-exposure to the offending allergen can elicit a quicker cutaneous response. Case reports have detailed cases of ACD presenting in neonates as early as 1 week of age.3
If ACD is suspected as an etiologic factor in diaper dermatitis, providers should consider constituents present in topical formulations, wet wipes, and components of the diaper itself. A specific example includes mercaptobenzothiazole, a rubber additive commonly found in elastic bands of disposable diapers.2 The most common potential allergens discovered in a recent review of 63 diaper wipes, 41 topical diaper preparations, and 3 top-selling diaper brands included botanical extracts (i.e. aloe vera), alpha-tocopherol, fragrances, propylene glycol, parabens, iodopropynyl butylcarbamate, and lanolin.4
ACD may be difficult to diagnose. Obtaining a thorough clinical history is essential to diagnosis. ACD is often distinguished by its distinct cutaneous morphology, with initial lesions commonly characterized by sharply demarcated erythema associated with vesiculation and edema. The dermatitis progressively evolves to a mixed presentation of predominantly eczematous papules and plaques with crusting, scaling, and lichenification. Another clue suggestive of ACD is cutaneous involvement limited to areas of allergen contact, such as the geometric plaques corresponding to areas of skin exposure to blue and green dye in our patient.1
Differential diagnosis
In contrast to ACD, ICD commonly erupts in the perianal region in the presence of feces, especially during diarrheal episodes. Common clinical manifestations include skin maceration, erosions, erythema, papulation, and scaling. More severe presentations of irritant dermatitis include Jacquet’s diaper dermatitis, perianal pseudoverrucous papules and nodules and granuloma gluteale infantum.1 These cutaneous findings also may develop in older patients with irritated peristomal skin.5
Inflammatory dermatoses such as psoriasis and seborrheic dermatitis also may erupt in the diaper region. Psoriasis commonly involves the gluteal cleft and inguinal folds, appearing as well-demarcated, pink plaques with thin white scale.5 Persistence of plaques despite topical corticosteroid application and lack of pruritus further delineates this disorder. Family history of psoriasis may provide an additional clue to the diagnosis of psoriasis. Similarly, seborrheic dermatitis may present with diaper involvement characterized by well-demarcated erythematous plaques with minimal or absent greasy yellow scale. This dermatitis commonly erupts in infants and neonates less than 6 weeks old and resolves by 6-9 months of age.1
Langerhans cell histiocytosis (LCH) represents a serious condition important to consider in the differential diagnosis of persistent recalcitrant diaper dermatitis. Often confused with seborrheic dermatitis, the diaper dermatitis of LCH may be differentiated by petechiae, purpura, and/or ulceration and atrophy of the inguinal folds.5
Management
Avoidance of the allergen constitutes the gold standard of treatment for ACD. Initial treatment involves adequate-potency topical corticosteroids; for the diaper area, low- to mid-potency corticosteroids are recommended. Changing to dye-free diapers and/or avoiding contact with the suspected allergen also is advised. Epicutaneous patch testing is recommended if the dermatitis fails to improve despite removal of the suspected offending agent.5
References
1. Pediatr Dermatol. 2014 Nov;31 Suppl 1:19-24.
2. Pediatrics. 2005 Sep;116(3):e450-2.
3. Cutis. 1994 Nov;54(5):300-2.
4. Dermatitis. 2016 May-Jun;27(3):110-8.
5. “Neonatal and Infant Dermatology,” 3rd edition (Philadelphia: Elsevier, 2015, p. 251)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Dr. Waldman is a clinical research fellow at the hospital. Email them at pdnews@frontlinemedcom.com.
BY CATALINA MATIZ, MD, AND ANDREA WALDMAN, MD
At the time of initial evaluation, the patient was prescribed a 2-week course of topical triamcinolone 0.1% ointment. Subsequently, the cutaneous lesions clinically improved, but did not completely resolve. Mother had no recollection of infant contact with metal, plastic, or additional topical emollients or medications. On further investigation, the patient’s mother revealed the recent conversion to a different brand of diaper. The diapers had a blue and green polka dot design corresponding with the distribution of our patient’s cutaneous lesions, highly suggestive of allergic contact dermatitis (ACD).
Diaper dermatitis represents a common complaint peaking at 9-12 months of age, estimated to occur in 25% of infants in the primary care office setting.1 Irritant contact dermatitis (ICD) predominates as the most common etiology of diaper dermatitis. ICD represents a nonimmunologic response, triggered by maceration and breakdown of the cutaneous barrier, directly resulting from chronic exposure to urine and feces.1 In contrast, ACD characterizes an immunologic reaction to specific offending allergen(s).
ACD can arise in the diaper area following contact with dyes, fragrances, or other constituents of disposable diapers, wet wipes, diaper creams, or medications. Alberta et al. initially described ACD in several infants, resulting from blue, green, and pink dyes present in various disposable diapers. The dermatitis promptly resolved upon switching to dye-free diapers.2 Furthermore, topical products applied to the diaper region such as barrier creams and wet wipes may trigger allergic sensitization. Sensitization to an allergen can occur after few exposures to years after introduction. Following the initial contact reaction, re-exposure to the offending allergen can elicit a quicker cutaneous response. Case reports have detailed cases of ACD presenting in neonates as early as 1 week of age.3
If ACD is suspected as an etiologic factor in diaper dermatitis, providers should consider constituents present in topical formulations, wet wipes, and components of the diaper itself. A specific example includes mercaptobenzothiazole, a rubber additive commonly found in elastic bands of disposable diapers.2 The most common potential allergens discovered in a recent review of 63 diaper wipes, 41 topical diaper preparations, and 3 top-selling diaper brands included botanical extracts (i.e. aloe vera), alpha-tocopherol, fragrances, propylene glycol, parabens, iodopropynyl butylcarbamate, and lanolin.4
ACD may be difficult to diagnose. Obtaining a thorough clinical history is essential to diagnosis. ACD is often distinguished by its distinct cutaneous morphology, with initial lesions commonly characterized by sharply demarcated erythema associated with vesiculation and edema. The dermatitis progressively evolves to a mixed presentation of predominantly eczematous papules and plaques with crusting, scaling, and lichenification. Another clue suggestive of ACD is cutaneous involvement limited to areas of allergen contact, such as the geometric plaques corresponding to areas of skin exposure to blue and green dye in our patient.1
Differential diagnosis
In contrast to ACD, ICD commonly erupts in the perianal region in the presence of feces, especially during diarrheal episodes. Common clinical manifestations include skin maceration, erosions, erythema, papulation, and scaling. More severe presentations of irritant dermatitis include Jacquet’s diaper dermatitis, perianal pseudoverrucous papules and nodules and granuloma gluteale infantum.1 These cutaneous findings also may develop in older patients with irritated peristomal skin.5
Inflammatory dermatoses such as psoriasis and seborrheic dermatitis also may erupt in the diaper region. Psoriasis commonly involves the gluteal cleft and inguinal folds, appearing as well-demarcated, pink plaques with thin white scale.5 Persistence of plaques despite topical corticosteroid application and lack of pruritus further delineates this disorder. Family history of psoriasis may provide an additional clue to the diagnosis of psoriasis. Similarly, seborrheic dermatitis may present with diaper involvement characterized by well-demarcated erythematous plaques with minimal or absent greasy yellow scale. This dermatitis commonly erupts in infants and neonates less than 6 weeks old and resolves by 6-9 months of age.1
Langerhans cell histiocytosis (LCH) represents a serious condition important to consider in the differential diagnosis of persistent recalcitrant diaper dermatitis. Often confused with seborrheic dermatitis, the diaper dermatitis of LCH may be differentiated by petechiae, purpura, and/or ulceration and atrophy of the inguinal folds.5
Management
Avoidance of the allergen constitutes the gold standard of treatment for ACD. Initial treatment involves adequate-potency topical corticosteroids; for the diaper area, low- to mid-potency corticosteroids are recommended. Changing to dye-free diapers and/or avoiding contact with the suspected allergen also is advised. Epicutaneous patch testing is recommended if the dermatitis fails to improve despite removal of the suspected offending agent.5
References
1. Pediatr Dermatol. 2014 Nov;31 Suppl 1:19-24.
2. Pediatrics. 2005 Sep;116(3):e450-2.
3. Cutis. 1994 Nov;54(5):300-2.
4. Dermatitis. 2016 May-Jun;27(3):110-8.
5. “Neonatal and Infant Dermatology,” 3rd edition (Philadelphia: Elsevier, 2015, p. 251)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Dr. Waldman is a clinical research fellow at the hospital. Email them at pdnews@frontlinemedcom.com.
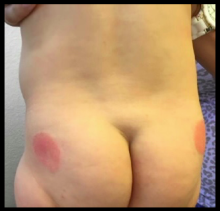
A 10-month-old previously healthy male presents to the dermatology clinic for evaluation of a persistent annular rash. The mother reports the development of two distinct symmetrical circular lesions on the upper lateral buttocks bilaterally 7 weeks earlier. Shortly after the lesions erupted, the pediatrician diagnosed him with tinea corporis. The patient’s rash markedly worsened over the following 3 weeks, despite the use of clotrimazole 1% cream and miconazole 2% cream. Patient’s mother denies any recent travel or pets residing in the home. Other than a history of mild atopic dermatitis in the mother, the family history is noncontributory.
The patient is a well-appearing infant who is attentive and in no acute distress. On skin examination there are two symmetric well-demarcated annular erythematous eczematous plaques on the upper lateral buttocks. The annular shape presents as geometric circles. There are no other lesions present on the cutaneous surface. Patient is afebrile and vital signs are within normal limits.
Continuous glucose monitoring recommended over finger sticks for type 1 diabetes
“Studies have found that people with type 1 diabetes who use CGMs [continuous glucose monitors] are able to maintain better control of their blood sugar without increasing episodes of hypoglycemia when blood sugar drops to dangerous levels, compared to those who self-monitor blood glucose with periodic finger sticks,” the chair of the guideline task force, Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said in a Sept. 26 statement.
The group recommended CGMs for well-controlled type 1 patients as well as those above hemoglobin A1c (HbA1c) targets, so long as they want and understand how to use the devices. It also suggested short-term, intermittent CGM use to help type 2 patients meet HbA1cgoals. Insulin pumps were recommended over multiple daily injections for type 1 patients above target HbA1clevels, as well as those who meet their target but continue to have severe hypoglycemia or high glucose variability. For patients with type 2 disease, pumps were suggested for cases of poor glycemic control despite intensive insulin therapy, oral agents, and other measures.
The Endocrine Society and others have been pushing Medicare to cover CGMs for a while; the new guideline seems to support the effort. Although the devices are used by type 1 patients and covered by some insurance plans, they are only indicated as finger-stick adjuncts, not replacements. For Medicare coverage, they would “need to serve a primary medical purpose and not be used adjunctively,” according to a recent review by Dexcom, a company seeking a primary monitoring indication for its CGM.
The Endocrine Society noted in its evidence-based guideline that standard capillary blood glucose measurements “offer only a limited perspective on the constant daily changes in blood glucose levels,” and, unlike continuous monitoring, “do not provide alarms that indicate when blood glucose levels are above or below various thresholds, and do not indicate trends in blood glucose levels.”
The society commissioned a pooled analysis of 11 randomized trials that showed a 0.3% reduction in HbA1c with real-time glucose monitoring, mostly in patients 15 years or older. Other studies cited by the group also showed better HbA1c control than with finger sticks, without an increased risk of hypoglycemia.
The guideline also suggested insulin pumps for type 1 patients who want greater flexibility and convenience and that insulin pump therapy should continue during hospitalizations. It also suggested encouraging patients to use the embedded bolus calculators in their pumps so long as they “have appropriate education regarding their use and limitations.”
The Endocrine Society funded the work, with cosponsorship from the American Association for Clinical Chemistry, the American Association of Diabetes Educators, and the European Society of Endocrinology. Several of the authors have industry ties to companies that make CGMs or insulin pumps. Dr. Peters is an advisor for Abbott, Becton Dickinson, AstraZeneca, Biodel, Medtronic, and other companies, as well as a speaker, investigator, and advisor for Janssen.
“Studies have found that people with type 1 diabetes who use CGMs [continuous glucose monitors] are able to maintain better control of their blood sugar without increasing episodes of hypoglycemia when blood sugar drops to dangerous levels, compared to those who self-monitor blood glucose with periodic finger sticks,” the chair of the guideline task force, Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said in a Sept. 26 statement.
The group recommended CGMs for well-controlled type 1 patients as well as those above hemoglobin A1c (HbA1c) targets, so long as they want and understand how to use the devices. It also suggested short-term, intermittent CGM use to help type 2 patients meet HbA1cgoals. Insulin pumps were recommended over multiple daily injections for type 1 patients above target HbA1clevels, as well as those who meet their target but continue to have severe hypoglycemia or high glucose variability. For patients with type 2 disease, pumps were suggested for cases of poor glycemic control despite intensive insulin therapy, oral agents, and other measures.
The Endocrine Society and others have been pushing Medicare to cover CGMs for a while; the new guideline seems to support the effort. Although the devices are used by type 1 patients and covered by some insurance plans, they are only indicated as finger-stick adjuncts, not replacements. For Medicare coverage, they would “need to serve a primary medical purpose and not be used adjunctively,” according to a recent review by Dexcom, a company seeking a primary monitoring indication for its CGM.
The Endocrine Society noted in its evidence-based guideline that standard capillary blood glucose measurements “offer only a limited perspective on the constant daily changes in blood glucose levels,” and, unlike continuous monitoring, “do not provide alarms that indicate when blood glucose levels are above or below various thresholds, and do not indicate trends in blood glucose levels.”
The society commissioned a pooled analysis of 11 randomized trials that showed a 0.3% reduction in HbA1c with real-time glucose monitoring, mostly in patients 15 years or older. Other studies cited by the group also showed better HbA1c control than with finger sticks, without an increased risk of hypoglycemia.
The guideline also suggested insulin pumps for type 1 patients who want greater flexibility and convenience and that insulin pump therapy should continue during hospitalizations. It also suggested encouraging patients to use the embedded bolus calculators in their pumps so long as they “have appropriate education regarding their use and limitations.”
The Endocrine Society funded the work, with cosponsorship from the American Association for Clinical Chemistry, the American Association of Diabetes Educators, and the European Society of Endocrinology. Several of the authors have industry ties to companies that make CGMs or insulin pumps. Dr. Peters is an advisor for Abbott, Becton Dickinson, AstraZeneca, Biodel, Medtronic, and other companies, as well as a speaker, investigator, and advisor for Janssen.
“Studies have found that people with type 1 diabetes who use CGMs [continuous glucose monitors] are able to maintain better control of their blood sugar without increasing episodes of hypoglycemia when blood sugar drops to dangerous levels, compared to those who self-monitor blood glucose with periodic finger sticks,” the chair of the guideline task force, Anne Peters, MD, a professor of medicine at the University of Southern California, Los Angeles, said in a Sept. 26 statement.
The group recommended CGMs for well-controlled type 1 patients as well as those above hemoglobin A1c (HbA1c) targets, so long as they want and understand how to use the devices. It also suggested short-term, intermittent CGM use to help type 2 patients meet HbA1cgoals. Insulin pumps were recommended over multiple daily injections for type 1 patients above target HbA1clevels, as well as those who meet their target but continue to have severe hypoglycemia or high glucose variability. For patients with type 2 disease, pumps were suggested for cases of poor glycemic control despite intensive insulin therapy, oral agents, and other measures.
The Endocrine Society and others have been pushing Medicare to cover CGMs for a while; the new guideline seems to support the effort. Although the devices are used by type 1 patients and covered by some insurance plans, they are only indicated as finger-stick adjuncts, not replacements. For Medicare coverage, they would “need to serve a primary medical purpose and not be used adjunctively,” according to a recent review by Dexcom, a company seeking a primary monitoring indication for its CGM.
The Endocrine Society noted in its evidence-based guideline that standard capillary blood glucose measurements “offer only a limited perspective on the constant daily changes in blood glucose levels,” and, unlike continuous monitoring, “do not provide alarms that indicate when blood glucose levels are above or below various thresholds, and do not indicate trends in blood glucose levels.”
The society commissioned a pooled analysis of 11 randomized trials that showed a 0.3% reduction in HbA1c with real-time glucose monitoring, mostly in patients 15 years or older. Other studies cited by the group also showed better HbA1c control than with finger sticks, without an increased risk of hypoglycemia.
The guideline also suggested insulin pumps for type 1 patients who want greater flexibility and convenience and that insulin pump therapy should continue during hospitalizations. It also suggested encouraging patients to use the embedded bolus calculators in their pumps so long as they “have appropriate education regarding their use and limitations.”
The Endocrine Society funded the work, with cosponsorship from the American Association for Clinical Chemistry, the American Association of Diabetes Educators, and the European Society of Endocrinology. Several of the authors have industry ties to companies that make CGMs or insulin pumps. Dr. Peters is an advisor for Abbott, Becton Dickinson, AstraZeneca, Biodel, Medtronic, and other companies, as well as a speaker, investigator, and advisor for Janssen.
Update in perioperative cardiac medicine
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
Perioperative medicine is an evolving field with a rapidly growing body of literature. Because physicians and patients are often concerned about cardiac risk, we focus this review on perioperative cardiology.
The information we present here is derived from presentations at the Perioperative Medicine Summit and the annual meetings of the Society of Hospital Medicine and Society of General Internal Medicine in 2016. We surveyed perioperative literature from January 2015 through March 2016 and chose the final articles by consensus, based on relevance to clinicians who provide preoperative evaluations and postoperative care to surgical patients.
We have divided this review into four sections:
- Preoperative cardiac risk assessment
- Medical therapy to reduce postoperative cardiac complications (beta-blockers, statins, and angiotensin II receptor blockers [ARBs])
- Perioperative management of patients with a coronary stent on antiplatelet therapy
- Perioperative bridging anticoagulation.
PREOPERATIVE ASSESSMENT OF CARDIAC RISK
Functionally independent patients do better
Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
Functional capacity is an independent predictor of perioperative death and is included in the algorithm of the current joint American College of Cardiology/American Heart Association (ACC/AHA) guidelines,1 but it is not in the Revised Cardiac Risk Index2 or the American Society of Anesthesiologists (ASA) classification.3
The study. Visnjevac et al4 performed a retrospective, observational cohort study of 12,324 patients who underwent noncardiac surgery, stratifying rates of all-cause mortality and 30-day postoperative complications based on ASA class and functional capacity.
The ASA physical status classification is defined as:
- 1—Normal healthy patient
- 2—Patient with mild systemic disease
- 3—Patient with severe systemic disease
- 4—Patient with severe systemic disease that is a constant threat to life
- 5—Moribund patient not expected to survive without surgery.
Functional capacity was defined as the ability to perform all activities of daily living. It was prospectively assessed during the patient interview by pre-anesthesia personnel and entered into the database of the Veterans Affairs Surgical Quality Improvement Program.
Results. Within each ASA class, the mortality rate was significantly lower for functionally independent patients than for partially or fully dependent patients:
- In class 2—odds ratio (OR) 0.14 for functionally independent patients
- In class 3—OR 0.29 for functionally independent patients
- In class 4—OR 0.5 for functionally independent patients.
The mortality rate was higher for dependent patients than for independent patients who were one ASA class higher, despite the higher class having greater rates of comorbidity.
Adding functional capacity to the ASA classification improved the area under the receiver operating curve from 0.811 to 0.848 (a perfect test would have a value of 1.0), suggesting that physicians should incorporate functional capacity into their preoperative evaluation, perhaps by increasing a patient’s ASA class to the next higher class if he or she is functionally dependent.
Angina portends poor outcomes
Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
Coronary artery disease is a risk factor for adverse perioperative outcomes, but the risk varies depending on whether the patient has had a myocardial infarction (and how long ago) and whether he or she has anginal symptoms (and how severe they are).
The study. Pandey et al5 used data from the American College of Surgeons National Surgical Quality Improvement Program to evaluate the impact of stable angina in 1,568 patients who underwent noncardiac surgery after a myocardial infarction.
Results. Postoperative myocardial infarction or cardiac arrest occurred in 5.5% of patients. The incidence was significantly greater in those who had anginal symptoms before surgery than in those without symptoms (8.4% vs 5%, P = .035); reintervention rates and length of stay were also higher in this group. In multivariate analysis, preoperative angina remained a significant predictor of postoperative myocardial infarction (OR 2.49, 95% confidence interval [CI] 1.20–5.81) and reintervention (OR 2.4, 95% CI 1.44–3.82.
The authors cautioned against relying on predictive tools such as the Revised Cardiac Risk Index that do not consider stable angina and previous myocardial infarction as separate independent risk factors.
Implications for clinical practice. While functional capacity is an integral part of the ACC/AHA guideline algorithm,1 the findings of these two studies suggest that other current tools to calculate perioperative risk (ASA class and Revised Cardiac Risk Index) could be improved by including functional capacity and stable angina.
PERIOPERATIVE MEDICAL THERAPY
Beta-blockers help only those at high risk and may harm others
Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
Beta-blockers have been used perioperatively for nearly 2 decades to try to reduce rates of postoperative major adverse cardiovascular events. However, in view of recent trials, fewer patients are likely to benefit from this intervention than has been thought.
The study. Friedell et al6 retrospectively analyzed data from 343,645 patients in Veterans Affairs hospitals to determine the effect of beta-blockers on major adverse cardiac event rates after major noncardiac surgery. Beta-blockers were considered to have been used perioperatively if given any time between 8 hours before and 24 hours after surgery. The outcome studied was the mortality rate at 30 days.
The authors derived a novel risk score and used multivariate analysis to attempt to adjust for confounding factors. The risk score was based on four risk factors identified a priori:
- Serum creatinine level > 2.0 mg/dL
- Coronary artery disease
- Diabetes
- Surgery in a major body cavity (abdomen or chest).
Results. In this cohort, 43.2% of patients had received a beta-blocker. The unadjusted mortality rates by risk category for patients receiving or not receiving a beta-blocker were:
- No risk factors: 1.0% with a beta-blocker vs 0.6% without
- One or two risk factors: 1.7% vs 1.5%
- Three or four risk factors: 2.3% vs 4.5%.
After adjustment for confounding factors, the 30-day mortality rate was higher in low-risk patients and lower in high-risk patients who received beta-blockers. Odds ratios for death in beta-blocker users (entire cohort) by risk category were:
- No risk factors: 1.19
- One or two risk factors 0.97
- Three or four risk factors 0.76.
In the 3.8% of the total cohort who underwent cardiac surgery, beta-blockers had no significant effect—beneficial or harmful—in any risk group.
Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
The study. Jørgensen et al7 investigated the association between chronic beta-blocker use for the treatment of hypertension and 30-day rates of mortality and major adverse cardiac events. Eligible patients (N = 55,320) were at least 20 years old and were undergoing any type of noncardiac surgery. The authors established that hypertension was present through use of an algorithm based on the International Classification of Diseases (10th edition). Patients with existing cardiovascular disease and renal disease were excluded. The authors used multivariate analysis to adjust for confounding factors.
Results. Twenty-six percent of the patients were on chronic beta-blocker therapy for hypertension. The mortality rate at 30 days was 1.93% in patients treated with a beta-blocker alone or in combination with other antihypertensive drugs; the rate was 1.32% for patients receiving any combination of renin-angiotensin system inhibitor, calcium antagonist, or thiazide, but no beta-blocker. Similarly, the 30-day major adverse cardiac event rates were 1.32% with beta-blockers and 0.84% without beta-blockers.
In subgroup analysis, each medication combination that included a beta-blocker was associated with higher rates of death and major adverse cardiac events than the same combination without a beta-blocker. Odds ratios for major adverse cardiac events with beta-blocker combinations ranged from 1.22 to 2.16 compared with regimens with no beta-blocker.
Implications for clinical practice. These two studies added to a growing chorus of concerns about the value and safety of beta-blockers in surgical patients. Friedell et al6 made an observation that was remarkably similar to one reported by Lindenauer et al8 in 2005: when patients were stratified by baseline risk of death, only those with the highest baseline risk benefited from beta-blocker therapy. Those in the lowest risk group actually were harmed by beta-blocker use, ie, the mortality rate was higher.
More interesting is the novel observation by Jørgensen et al7 that even in patients with no known cardiovascular disease who are on chronic beta-blocker therapy—presumably on stable doses and not solely for perioperative risk reduction—rates of mortality and major adverse cardiac events were higher than for patients not on chronic beta-blocker therapy.
The current studies support a cautious, selective approach to the perioperative use of beta-blockers—they should be used only in high-risk patients undergoing high-risk surgery, as has been proposed by the ACC/AHA.1
Statins protect
Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
The study9 was a comprehensive meta-analysis of randomized controlled trials and observational studies of the effects of HMG-CoA reductase inhibitors (statins) on perioperative outcomes in patients undergoing vascular surgery (but not for intracranial or coronary artery disease). Twenty-four studies were included, 4 randomized controlled trials and 20 observational studies (including 16 cohort and 4 case-controlled studies), with a total of 22,536 patients, 8,052 receiving statins and 15,484 not receiving statins.
Results. Although there was no significant difference in cardiovascular mortality rates, patients receiving statins had significantly lower rates of all-cause mortality, myocardial infarction, stroke, and a composite of myocardial infarction, stroke, and death at 30 days postoperatively than patients not receiving statins. Additionally, there was no difference in the incidence of kidney injury between groups. The possibility of publication bias was thought to be low for all of these outcomes.
Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
The study. The Vascular Events in Non-cardiac Surgery Patients Cohort Evaluation (VISION) study10,11 was an international prospective cohort study of more than 40,000 patients age 45 and older undergoing major noncardiac surgery with either general or regional anesthesia. Postoperative troponin measurements were obtained in all patients 6 to 12 hours after surgery and for the first 3 postoperative days. The authors evaluated the effect of preoperative statin use on cardiovascular outcomes at 30 days after surgery using a multivariate logistic model and propensity score analysis to correct for confounding factors. Statin use was defined as exposure within 7 days before surgery or 3 days after.
Results. In the 15,478 patients included in the analysis, statin use conferred a significant reduction in the primary outcome (composite of all-cause mortality, myocardial injury after noncardiac surgery, or stroke); the absolute risk reduction was 2.0%. Statin users also had a significantly lower risk of all-cause mortality, cardiovascular mortality, and myocardial injury after noncardiac surgery, but not of postoperative myocardial infarction or stroke. This analysis did not address the type of statin, dosing, or safety markers such as liver and muscle function.
Implications for clinical practice. With largely observational data and a few small randomized trials, these meta-analyses provide important information with respect to perioperative cardiovascular protection by statins. Starting a statin before surgery and continuing it perioperatively seems appropriate in patients at high risk (as recommended by the ACC/AHA guidelines1). Based on other data, the benefit may be evident in as little as 5 days, as this is when statins appear to reach their plateau with regard to their vascular pleiotropic effects.12 The incidence of adverse effects of statins, including muscle and liver injury, appears to be low in the perioperative setting.13
Given the inconsistent data regarding perioperative beta-blocker therapy, statins may very well be the most important perioperative medication with respect to cardiovascular risk reduction. However, a large randomized trial would help to confirm this belief.
Restart angiotensin II receptor blockers soon after surgery
Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
A concern about perioperative use of ARBs is that they impair the renin-angiotensin-aldosterone system, which maintains blood pressure under general anesthesia. ARB-induced intraoperative hypotension is particularly difficult to control, as it is often refractory to treatment with conventional adrenergic vasopressors.
The study. Lee et al14 conducted a retrospective cohort trial to evaluate the effects of continuing to withhold ARBs postoperatively. Of the 30,173 patients admitted for surgery in the Veterans Affairs system from 1999 through 2011 who were taking an ARB before surgery and who met the inclusion criteria, 10,205 (33.8%) were not restarted on their medication by postoperative day 2.
Results. The mortality rate at 30 days was higher in those whose ARBs were withheld than in those in whom it was resumed, with a multivariable-adjusted hazard ratio of 1.74 (95% CI 1.47–2.06; P < .001). The risk of withholding ARBs was more pronounced in younger patients (hazard ratio 2.52; 95% CI 1.69–3.76 in those under age 60) than in older patients (hazard ratio 1.42, 95% CI 1.09–1.85 in those over age 75).
Implications for clinical practice. While not addressing whether to continue or withhold ARBs preoperatively, this retrospective study presented evidence that delay in resuming chronic ARB therapy after surgery was common and appeared to be associated with a higher 30-day mortality rate. The ACC/AHA guidelines1 state:
- Continuing angiotensin-converting enzyme (ACE) inhibitors or ARBs perioperatively is reasonable (class IIa recommendation, level of evidence B) (Table 1).
- If an ACE inhibitor or ARB is withheld before surgery, it is reasonable to restart it postoperatively as soon as clinically feasible (class IIa recommendation, level of evidence C).
Close attention to medication reconciliation in the postoperative period is necessary to facilitate early resumption of ARBs.
CORONARY STENTS AND ANTIPLATELET THERAPY IN NONCARDIAC SURGERY PATIENTS
Considerations in the management of noncardiac surgery patients with stents include risks of stent thrombosis, bleeding, and potentially delaying procedures to continue uninterrupted dual antiplatelet therapy. Evidence is evolving regarding the risks of perioperative complications in patients with bare-metal stents and drug-eluting stents, as well as the optimal timing before noncardiac surgery.
Bare-metal vs drug-eluting stents
Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
The study. Bangalore et al15 compared the safety of drug-eluting vs bare-metal stents in noncardiac surgery patients and investigated adverse events stratified by time since stent placement. This was a retrospective observational study of 8,415 patients in the Massachusetts claims database who underwent noncardiac surgery 1 year or less after percutaneous coronary intervention.
Results. There was no significant difference in the incidence of the primary outcome (composite of death, myocardial infarction, and bleeding) between the two groups.
With drug-eluting stents, patients had lower 30-day postoperative mortality rates, and their rate of the primary outcome decreased with time from percutaneous coronary intervention to surgery, being lowest beyond 90 days:
- 8.6% in days 1–30
- 7.5% in days 31–90
- 5.2% in days 91–180
- 5.8% in days 181–365 (P = .02).
With bare-metal stents, the event rate remained high over time:
- 8.2% in days 1–30
- 6.6% in days 31–90
- 8.1% in days 91–180
- 8.8% in days 181–365 (P = .60).
This study did not report information about perioperative antiplatelet management and was limited to first-generation drug-eluting stents.
Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
The study. Saia et al16 retrospectively examined predictors of periprocedural ischemic and bleeding events among cardiac and noncardiac surgical patients who had previously undergone percutaneous coronary intervention. They also assessed the risks associated with stent type and time from percutaneous coronary intervention to surgery.
Of 39,362 patients, 13,128 underwent procedures during the 5-year study period. The cumulative incidence of surgery was 3.6% at 30 days, 14% at 1 year, and 40% at 5 years after percutaneous coronary intervention. Almost 30% of the procedures were done urgently.
Results. The 30-day rate of postoperative cardiac death was 2.5%, nonfatal myocardial infarction 1.5%, and serious bleeding events 6.5%. Older drug-eluting stents were associated with higher risks of adverse events than newer drug-eluting stents at any time point (odds ratio 2.1 at 0–180 days, 1.9 at 6–12 months, and 1.45 after 12 months). Surgery performed 6 to 12 months after percutaneous coronary intervention had lower rates of adverse outcomes than surgery performed within 6 months. Beyond 6 months from percutaneous coronary intervention, bare-metal stents and newer drug-eluting stents did not have significantly different adverse event rates; however, newer drug-eluting stents appeared safer than bare-metal stents from 0 to 180 days.
Limitations of this study included lack of information regarding periprocedural antiplatelet management and a relatively small subset of newer drug-eluting stent patients.
Implications for clinical practice. These studies added to earlier work that demonstrated that the risk of perioperative adverse events differs by both the stent type and the time from percutaneous coronary intervention to noncardiac surgery. In patients with a drug-eluting stent, the risk levels off 90 days after percutaneous coronary intervention, suggesting that the previously recommended 12 months of uninterrupted dual antiplatelet therapy (per the 2014 ACC/AHA guidelines1) may not be needed, particularly with newer-generation drug-eluting stents. Based on new evidence, the ACC/AHA guidelines regarding perioperative management of dual antiplatelet therapy in noncardiac surgery patients were updated,17 as noted below.
An update to the ACC/AHA guidelines on dual antiplatelet therapy
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29. DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]
The 2016 update17 provides the following recommendations for patients with coronary stents who undergo noncardiac surgery:
- Delay elective surgery for 30 days after placement of a bare-metal stent (class I recommendation, level of evidence B).
- It is optimal to delay elective surgery 6 months after drug-eluting stent placement (class I recommendation, level of evidence B).
- If dual antiplatelet therapy must be discontinued, then continue aspirin if possible and restart the P2Y12 inhibitor as soon as possible postoperatively (class I recommendation, level of evidence C ).
- A consensus decision among treating clinicians is useful regarding the risks of surgery and discontinuation or continuation of antiplatelet therapy (class IIa recommendation, level of evidence C).
- If dual antiplatelet therapy must be discontinued, then elective surgery should not be performed less than 30 days after bare-metal stent placement, or less than 3 months after drug-eluting stent placement (class III recommendation, level of evidence B).
- Elective surgery after drug-eluting stent placement when the P2Y12 inhibitor must be discontinued may be considered 3 months after drug-eluting stent placement if the risk of surgical delay is greater than the risk of stent thrombosis (class IIb recommendation, level of evidence C).
The basic differences are the new recommendations for a minimum of 6 months of dual antiplatelet therapy as opposed to 12 months after drug-eluting stent placement before elective noncardiac surgery, and to allow surgery after 3 months (as opposed to 6 months) if the risk of delaying surgery outweighs the risk of stent thrombosis or myocardial infarction.
PERIOPERATIVE ANTICOAGULATION
The optimal perioperative management of patients with atrial fibrillation who are on warfarin is uncertain. The American College of Chest Physicians guidelines18 categorized patients with atrial fibrillation into low, moderate, and high thromboembolic risk. Based primarily on observational data, these guidelines recommended perioperative bridging anticoagulation for those at high risk but not for those at low risk. For intermediate-risk patients, there were insufficient data to make any recommendation.
Bridging may not benefit those at intermediate risk
Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
The study. The Bridging Anticoagulation in Patients Who Require Temporary Interruption of Warfarin Therapy for an Elective Invasive Procedure or Surgery (BRIDGE) trial19 was the first randomized controlled trial to examine the effects of perioperative bridging anticoagulation in patients with atrial fibrillation without mechanical heart valves.
Results. In 1,884 patients undergoing elective surgery, the incidence of arterial thromboembolism was 0.4% in the no-bridging group and 0.3% in the bridging group (95% CI −0.6 to 0.8; P = .01 for noninferiority). Major bleeding occurred in 1.3% of patients in the no-bridging group and 3.2% in the bridging group (95% CI 0.20–0.78; P = .005 for superiority).
These results suggest that the risks of bridging therapy are greater than the benefits. Of note, the mean CHADS2 score (1 point each for congestive heart failure, hypertension, age ≥ 75 years, and diabetes mellitus; 2 points for previous stroke or transient ischemic attack; a total score > 2 indicates significant risk of stroke) for patients enrolled in this trial was 2.3, and it may be difficult to extrapolate these results to the limited number of patients at highest risk, ie, who have a CHADS2 score of 5 or 6. Also, this study did not address patients with arterial or venous thromboembolism.
Implications for clinical practice. Despite the limitations noted above, this study does provide guidance for management of the intermediate-risk group with atrial fibrillation as defined by the American College of Chest Physicians: a no-bridging strategy is the best option.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64:e77–e137.
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999; 100:1043–1049.
- Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA 1961; 178:261–266.
- Visnjevac O, Davari-Farid S, Lee J, et al. The effect of adding functional classification to ASA status for predicting 30-day mortality. Anesth Analg 2015; 121:110–116.
- Pandey A, Sood A, Sammon JD, et al. Effect of preoperative angina pectoris on cardiac outcomes in patients with previous myocardial infarction undergoing major noncardiac surgery (data from ACS-NSQIP). Am J Cardiol 2015; 115:1080–1084.
- Friedell ML, Van Way CW 3rd, Freyberg RW, Almenoff PL. ß-blockade and operative mortality in noncardiac surgery: harmful or helpful? JAMA Surg 2015; 150:658–663.
- Jørgensen ME, Hlatky MA, Køber L, et al. ß-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med 2015; 175:1923–1931.
- Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005; 353:349–361.
- Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg 2015; 61:519–532.
- Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I; Devereaux PJ, Chan MT, Alonso-Coello P, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2012; 307:2295–2304.
- Berwanger O, Le Manach Y, Suzumura EA, et al. Association between pre-operative statin use and major cardiovascular complications among patients undergoing non-cardiac surgery: the VISION study. Eur Heart J 2016; 37:177–185.
- Laufs U, Wassmann S, Hilgers S, Ribaudo N, Bohm M, Nickenig G. Rapid effects on vascular function after initiation and withdrawal of atorvastatin in healthy, normocholesterolemic men. Am J Cardiol 2001; 88:1306–1307.
- Schouten O, Kertai MD, Bax JJ, et al. Safety of perioperative statin use in high-risk patients undergoing major vascular surgery. Am J Cardiol 2005; 95:658–660.
- Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology 2015; 123:288–306.
- Bangalore S, Silbaugh TS, Normand SL, Lovett AF, Welt FG, Resnic FS. Drug-eluting stents versus bare metal stents prior to noncardiac surgery. Catheter Cardiovasc Interv 2015; 85:533–541.
- Saia F, Belotti LM, Guastaroba P, et al. Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stents: how important is the interplay between stent type and time from stenting to surgery? Circ Cardiovasc Qual Outcomes 2015; 9:39–47.
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. Circulation 2016 Mar 29 DOI: 10.1161/CIR.0000000000000404. [Epub ahead of print]. Accessed August 16, 2016.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S. Erratum in Chest 2012; 141:1129.
- Douketis JD, Spyropoulos AC, Kaatz S, et al; BRIDGE Investigators. Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 2015; 373:823–833.
KEY POINTS
- Outcomes are worse in patients with poor functional capacity or stable angina, and these factors should be considered in preoperative risk assessment.
- Perioperative use of beta-blockers may benefit only patients at highest risk and may harm other patients.
- Statins seem to provide perioperative protection.
- If an ARB is withheld for surgery, it should be restarted soon after.
- For patients with a coronary stent, the type of stent and duration of dual antiplatelet therapy need to be considered before noncardiac surgery.
- Bridging anticoagulant therapy should not be used in patients at intermediate or low risk of thromboembolism.
The ABCs of managing systolic heart failure: Past, present, and future
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
Managing heart failure is a challenge. To aid clinicians in this task, the American College of Cardiology Foundation (ACC) and the American Heart Association (AHA) publish evidence-based guidelines, most recently in 2013.1 Since then, new drugs and devices have been shown to improve survival and reduce hospitalizations.
This paper reviews the ABCs of outpatient management of systolic heart failure (or heart failure with reduced ejection fraction), including the results of major trials and recommendations.

A common and serious condition
Heart failure is a debilitating syndrome that takes a significant physical and mental toll on those affected.
And it is common. An American age 40 or older faces a 20% lifetime risk of heart failure.1 An estimated 5.1 million Americans have clinical signs and symptoms of heart failure, and 900,000 new cases are diagnosed each year.2 By 2030 the prevalence of heart failure is projected to increase by 46%, and 9 million Americans will have been diagnosed with it.2
The severity of heart failure can be described using either the functional classification devised by the New York Heart Association (NYHA; Table 1) or the stages defined by the ACC and AHA.1,3 Though survival rates have improved, there is a direct correlation between worsening symptoms and death.4
Heart failure is the leading cause of hospitalizations annually. It accounts for $30 billion in healthcare costs, with direct medical costs accounting for 68% and another $1.8 billion associated with clinic visits, most often with primary care providers. By 2030, the cost is projected to increase by 127% to $69.7 billion—$244 per person in the United States.2
ACE inhibitors
The renin-angiotensin-aldosterone system has been studied for over 100 years.5
In heart failure with reduced ejection fraction, this system is upregulated as an adaptive mechanism to maintain hemodynamic homeostasis.6–8 However, prolonged activation of the renin-angiotensin-aldosterone system can lead to deleterious cardiovascular effects such as myocyte hypertrophy, myocardial fibrosis, sodium conservation, and fluid overload.8,9 Angiotensin II is a potent vasoconstrictor and plays a role in cardiovascular remodeling, leading to worsening progression of heart failure.6
CONSENSUS (the Cooperative North Scandinavian Enalapril Survival Study) examined the effect of the angiotensin-converting enzyme (ACE) inhibitor enalapril on survival in 253 patients with NYHA class IV heart failure. Participants were randomized to receive either enalapril or placebo. At 6 months, the mortality rate was 26% in the enalapril group vs 44% in the placebo group, an 18% absolute risk reduction and a 41% relative risk reduction (P = .002). At 12 months, the relative risk reduction in mortality was 30% (P = .001).10
SOLVD (the Study of Left Ventricular Dysfunction) extended the use of ACE inhibitors to all patients with heart failure, not just those in NYHA class IV. It randomized 1,284 patients with heart failure of any NYHA class and an ejection fraction less than 35% to receive either enalapril or placebo, and demonstrated a 16% relative risk reduction in mortality in the enalapril group, with mortality rates of 36% vs 39.7% (P = .0036).11
Recommendations. The benefits of ACE inhibition have been demonstrated in patients with mild, moderate, and severe heart failure. Thus, the guidelines recommend ACE inhibitors (Table 2) for all patients with heart failure with reduced ejection fraction.1
Angiotensin II receptor blockers
Angiotensin II receptor blockers (ARBs) (Table 3) have been proven to be suitable alternatives for patients with heart failure with reduced ejection fraction who cannot tolerate ACE inhibitors.
Val-HefT (the Valsartan HF Trial)12 randomized 5,010 patients in a double-blind fashion to receive either valsartan or placebo, with background therapy that included beta-blockers, digoxin, diuretics, and ACE inhibitors. There was a 13% reduction of the combined primary end point of mortality and morbidity and a 24% reduction in heart failure hospitalizations in the valsartan group.12
Subgroup analysis compared patients on the basis of use of ACE inhibitors and beta-blockers at study entry. Valsartan had a favorable effect in the subgroups using beta-blockers alone, ACE inhibitors alone, and neither drug. However, when patients received all three (a beta-blocker, an ACE inhibitor, and valsartan), the mortality rate was significantly increased (P = .009).12 This finding conflicted with those of other studies, which found a small benefit of combining an ACE inhibitor and an ARB.
CHARM-Added (the Candesartan in HF Assessment of Reduction in Mortality and Morbidity trial)13 investigated whether adding the ARB candesartan to an ACE inhibitor would improve clinical outcomes. In the study, 2,548 patients in NYHA class II, III, or IV with a left ventricular ejection fraction of less than 40% who were receiving ACE inhibitors were randomized to either candesartan or placebo. The addition of candesartan resulted in a significant reduction in cardiovascular mortality and heart failure hospitalizations, but with the downside of higher rates of hyperkalemia and serum creatinine elevation.13
Recommendations. The 2013 guidelines recommend that ARBs be used in patients who cannot tolerate an ACE inhibitor due to cough. However, routine combined use of ARBs, ACE inhibitors, and aldosterone antagonists is not recommended and may cause harm.1
Aldosterone receptor antagonists
Elevated levels of aldosterone lead to fluid retention, loss of magnesium and potassium, and myocardial fibrosis.
RALES (the Randomized Aldactone Evaluation Study)14 tested the hypothesis that the aldosterone receptor antagonist spironolactone (25 mg daily) would reduce deaths from all causes in patients with severe heart failure receiving standard medications including an ACE inhibitor. RALES included 1,663 patients in NYHA class III or IV with a left ventricular ejection fraction of 35% or less, randomized to receive 25 mg of spironolactone or matching placebo. This study found a 30% relative risk reduction and an 11% absolute risk reduction in all-cause mortality, a 31% relative risk reduction and a 10% absolute risk reduction in cardiac mortality, and 30% fewer cardiac-related hospitalizations in the spironolactone group.14
Eplerenone, an aldosterone receptor antagonist that lacks the antiandrogenic side effects of spironolactone, has also been shown to be beneficial. Its efficacy in patients with left ventricular systolic dysfunction was first established in postmyocardial infarction patients.15
EMPHASIS-HF (the Eplerenone in Mild Patients Hospitalized and Survival Study in Heart Failure)16 broadened the application of eplerenone (and aldosterone antagonists in general), investigating the effects of eplerenone in 2,737 NYHA class II patients, regardless of ischemic etiology. The composite end point of cardiovascular death or heart failure hospitalization occurred in 18.3% of the eplerenone group vs 25.9% of the placebo group (P < .001). A total of 12.5% of patients in the eplerenone group died, compared with 15.5% in the placebo group (P = .008). Hospitalizations were also fewer in the eplerenone group.
Recommendations. The 2013 guidelines recommend aldosterone receptor antagonists (Table 4) for patients with NYHA class II, III, or IV heart failure who have an ejection fraction of 35% or less, to reduce morbidity and mortality (class IA recommendation).1 The guidelines also recommend that these agents not be used in patients with renal insufficiency (serum creatinine > 2.5 mg/dL in men or > 2.0 mg/dL in women; an estimated glomerular filtration rate < 30 mL/min/1.73 m2); or a serum potassium level above 5 mmol/L.1
Angiotensin-neprilysin inhibitor (the future)
Research has identified neprilysin as another potential target in the treatment of heart failure and has sought to combine inhibition of angiotensin and neprilysin.
Neprilysin, a neutral endopeptidase, is associated with degradation of several natural vasoactive peptides such as natriuretic peptide, bradykinin, and adrenomedullin. Neprilysin inhibition increases these substances and counters the neurohormonal overactivation that leads to vasoconstriction, sodium retention, and cardiac remodeling.17
The ARB valsartan has been combined with the neprilysin inhibitor sacubitril to create the first angiotensin-neprilysin inhibitor (ARNI) (Table 5). The combination was selected to minimize the potential for angioedema.
PARADIGM-HF (the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial)17 examined whether combined angiotensin-neprilysin inhibition was superior to ACE inhibition alone with enalapril in patients with chronic heart failure.
In PARADIGM-HF, 10,521 patients with NYHA class II, III, or IV heart failure were randomized to receive either sacubitril-valsartan or enalapril. The group receiving sacubitril-valsartan had significantly fewer deaths from cardiovascular causes and heart failure hospitalizations.17 An improvement in quality of life and NYHA functional class was also observed in the sacubitril-valsartan group.17
Sacubitril-valsartan underwent priority review by the US Food and Drug Administration and has been approved. Currently, it is indicated for the treatment of heart failure with reduced ejection fraction and NYHA class II, III, or IV symptoms. It should be avoided in patients who have previously experienced angioedema with an ACE inhibitor or ARB, in patients receiving aliskiren for diabetes, and in patients with hypersensitivity reactions to either of its components. Simultaneous use of sacubitril-valsartan and an ACE inhibitor should be avoided, and a washout period is recommended when transitioning from an ACE inhibitor to this combined agent.

Beta-blockers
In heart failure, there is increased sympathetic activation and associated elevations in norepinephrine levels, which may lead to deleterious long-term effects on cardiac function and structure. Beta-adrenergic receptor blockade is now known to be cardioprotective, but it was not always so; beta-blockers used to be contraindicated in patients with heart failure.
An early experience using beta-blockers in heart failure was described in 1975.18,19 The first study to report a survival benefit of treating systolic heart failure with a beta-blocker was published in 1979.20 Later, small controlled trials demonstrated a reduction in heart failure symptoms and improvement in left ventricular function and in NYHA functional class.21 Larger clinical trials have demonstrated a tremendous survival benefit with beta-blockers in heart failure, specifically carvedilol, extended-release metoprolol, and bisoprolol.
The US Carvedilol Heart Failure Study Group trial22 evaluated whether beta-blocker use in heart failure patients would reduce the rates of morbidity and mortality.22 The trial included 1,094 patients with symptomatic heart failure for at least 3 months and a left ventricular ejection fraction of 35% or less on background therapy including vasodilators, ACE inhibitors, and digoxin. Patients were randomized to receive either carvedilol or placebo. Carvedilol use was associated with a dramatic 65% risk reduction in mortality (7.8% with placebo vs 3.2% with carvedilol, P < .001) and a 27% risk reduction in hospitalizations (19.6% vs 14.1%, P = .036), leading to early trial termination.
CIBIS-II (the Cardiac Insufficiency Bisoprolol Study II)23 investigated the effects of beta-blockers on survival and morbidity. CIBIS-II included 2,647 NYHA class III or IV patients with a left ventricular ejection fraction less than 35% on background medical therapy that included diuretics and ACE inhibitors. This trial was also terminated early, after demonstrating a significant survival benefit with bisoprolol.
MERIT-HF (the Metoprolol Extended Release Randomized Intervention Trial in Congestive Heart Failure)24 evaluated if once-daily metoprolol would lower mortality rates in patients with symptomatic heart failure. The study enrolled 3,991 NYHA class II–IV patients with chronic heart failure and a left ventricular ejection fraction of 40% or less. Like the previous two beta-blocker trials, MERIT-HF was terminated early, as it demonstrated a 34% reduction in all-cause mortality (7.2% risk of death per patient-year vs 11.0%, P = .00009).
The beta-blocker trials have shown that when added to background therapy, beta-blockers improve survival and reduce hospitalizations. However, when prescribing a beta-blocker, it is important to understand that not all beta-blockers are equal in the treatment of heart failure.
COMET (the Carvedilol or Metoprolol European Trial)25 was the only head-to-head randomized control trial evaluating clinical outcomes in patients receiving carvedilol or metoprolol tartrate (not metoprolol succinate). In COMET, 1,511 patients with NYHA class II, III, or IV heart failure with a left ventricular ejection fraction of 35% or less were randomized to carvedilol or metoprolol tartrate. The primary end point of all-cause mortality occurred in 34% of the carvedilol group and 40% of the metoprolol tartrate group (P = .0017). There was no significant difference with regard to the composite end point of mortality and all-cause admissions.
Recommendations. The 2013 guidelines give a class IA recommendation for starting a beta-blocker (carvedilol, bisoprolol, or metoprolol succinate, Table 6) in patients with current or prior symptoms of heart failure.1 Beta-blockers should be initiated with caution or avoided in patients with acutely decompensated heart failure with evidence of fluid overload.
Brain-type natriuretic peptide
Brain-type natriuretic peptide (BNP) or its amino-terminal cleavage product (NT-proBNP) originates in cardiomyocytes and is released by several triggers, most commonly cardiomyocyte stretch in the setting of volume or pressure overload.26 The biologic significance of BNP includes natriuresis and vasodilation, renin-angiotensin system inhibition, and sympathetic nervous system modulation.26
TIME-CHF (the Trial of Intensified vs. Standard Medical Therapy in Elderly Patients With Congestive HF)27 investigated whether 18-month outcomes would be better if treatment were guided by N-terminal BNP levels rather than by symptoms. The BNP-guided strategy was not associated with a reduction in hospitalization or a survival benefit.
BATTLESCARRED (the NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death trial)28 in 2009 showed that a BNP-guided management strategy significantly reduced mortality rates in patients under age 75 compared with standard medical therapy.
PROTECT (the Use of NT-proBNP Testing to Guide HF Therapy in the Outpatient Setting study)29 also showed that a BNP-guided strategy was superior to usual care and was associated with reduced cardiovascular events and improved quality of life.29
GUIDE IT-HF (the Guiding Evidence Based Therapy Using Biomarker Intensified Treatment in Heart Failure study), currently ongoing, is designed to assess the safety, efficacy and cost-effectiveness of a biomarker-guided strategy in 1,100 high-risk patients with heart failure with reduced ejection fraction.
Recommendations. The 2013 ACC/AHA guidelines give a class IA recommendation for the use of BNP to support clinical decision-making, particularly in cases of clinical uncertainty.1 BNP can also be used to establish prognosis or disease severity in chronic heart failure and to achieve optimal dosage of goal-directed medical therapy for euvolemic patients followed in a structured heart failure program.1

Heart failure clinics
Continuity of care upon discharge from the hospital is currently in a state of evolution. Those diagnosed with heart failure can now experience more comprehensive posthospital care by virtue of disease management clinics. The name may vary by institution, but whether it is called a “diuresis clinic,” “bridge clinic,” or “heart failure clinic,” the goal is to improve guideline-driven care, educate the patient, and reduce heart failure hospitalizations. Heart failure clinics are designed to provide a smooth transition from inpatient to outpatient care and to encourage patient self-accountability in health maintenance thereafter.
Studies have shown that heart failure clinics are associated with better medication dosing, fewer hospitalizations, and lower healthcare costs.30–32
Chronotropy: If inhibition
An elevated resting heart rate has been shown to be associated with increased cardiovascular morbidity and mortality.33 Studies have shown that slowing the heart rate improves myocardial contraction and energy supply and reduces energy expenditure.34 Ivabradine, a selective If (the f is for “funny”) channel inhibitor, slows the heart rate without other known cardiovascular effects.
SHIFT (the Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial)35 investigated whether isolated heart rate reduction with ivabradine would reduce adverse clinical outcomes in patients with symptomatic heart failure. SHIFT randomized 6,505 patients with a left ventricular ejection fraction of 35% or less, in sinus rhythm, with a heart rate of at least 70 beats per minute, on optimal medical therapy, and hospitalized within 12 months of enrollment to receive ivabradine or placebo. The primary end point was a composite of cardiovascular mortality and hospital admission for worsening heart failure. Outcomes varied by heart rates achieved, with the best outcomes in those with the lowest heart rates at trial conclusion.
Ivabradine (Table 7) is indicated for patients with symptomatic heart failure with a left ventricular ejection fraction less than 35%, in sinus rhythm, with a resting heart rate of at least 70 beats per minute, and either on a maximally tolerated beta-blocker or with a contraindication to beta-blockers.
Ivabradine should be avoided in patients who are in acute decompensated heart failure or are hypotensive (blood pressure < 90/50 mm Hg), as well as in patients with a significant conduction abnormality (sick sinus syndrome, sinoatrial block, third-degree atrioventricular block), hepatic impairment, or bradycardia (resting heart rate < 60 beats per minute).

Digoxin
Digoxin has been used in treating systolic heart failure for more than 70 years.36,37
DIG (Digoxin Investigative Group trial)38 evaluated the long-term effect of digoxin on rates of mortality and hospitalization for heart failure over a 3-year period. In patients with a left ventricular ejection fraction less than 45%, digoxin had no effect on overall mortality when combined with diuretics and ACE inhibitors. However, the risk of hospitalization for worsening heart failure was significantly reduced with digoxin treatment.38
Recommendations. Digoxin should be considered when patients are on guideline-recommended therapy but heart failure symptoms persist. It is commonly initiated at a dose of 0.125 to 0.25 mg. The target therapeutic range for digoxin is 0.5 to 0.9 ng/mL.1 Digoxin toxicity can occur in patients with renal impairment, hypokalemia, hypomagnesemia, and hypothyroidism.
The 2013 ACC/AHA guidelines give a class IIA recommendation (treatment is “reasonable”) for digoxin in patients with heart failure with reduced ejection fraction unless contraindicated, to decrease hospitalizations for heart failure.1
Diuretics
Clinical manifestations of volume overload in patients with heart failure are from excess salt and water retention leading to inappropriate volume expansion in both the vascular and extravascular space. Diuretics (Table 8) are the foundation of heart failure treatment. Most patients are first initiated on a combination of a loop diuretic and a low-sodium diet to improve symptoms.
The 2013 ACC/AHA guidelines give a class I recommendation for diuretics in patients with heart failure with reduced ejection fraction who have evidence of fluid retention, unless contraindicated, to improve symptoms.1
Devices: ICDs
Patients with heart failure are at increased risk of sudden death and ventricular arrhythmias.39 Previously, antiarrhythmic drugs were considered the standard of care for nonsustained ventricular tachycardia after myocardial infarction.
MADIT (the Multicenter Automatic Defibrillator Implantation Trial) investigated whether prophylactic implantation of an internal cardiac defibrillator would improve 5-year survival rates in patients with heart failure. Eligible patients had had a Q-wave or enzyme-positive myocardial infarction within 3 weeks of study entry. They also had had an episode of asymptomatic nonsustained ventricular tachycardia unrelated to an acute myocardial infarction. Additionally, the patients had a left ventricular ejection fraction less than 35%, and inducible, sustained, nonsuppressible ventricular tachyarrhythmia on electrophysiologic testing.40
During the study, 15 patients in the defibrillator group died vs 39 in the conventional therapy group (P = .009).40
MADIT II evaluated the potential survival benefit of a prophylactically implanted defibrillator in the absence of electrophysiologic testing to induce arrhythmias.41 MADIT II included 1,232 patients with prior myocardial infarctions and a left ventricular ejection fracton of 30% or less. Patients were randomized to receive an implanted cardioverter-defibrillator or conventional medical therapy. The primary end point was death from any cause.41
The mortality rate was 19.8% in the conventional therapy group vs 14.2% in the defibrillator group (hazard ratio 0.69, P = .016).41 Thus, MADIT-II confirmed the benefits of prophylactic implantable cardioverter-defibrillator therapy seen in the original MADIT, and additionally eliminated the need for an electrophysiology test prior to device implantation.
SCD-HeFT (the Sudden Cardiac Death in Heart Failure Trial) evaluated whether amiodarone or a conservatively programmed shock-only, single-lead implanted cardioverter-defibrillator would decrease the risk of death (all-cause) in a population with mild to moderate heart failure with ischemic and nonischemic causes.42 In this trial, 2,521 patients with an ejection fraction of 35% or less, in NYHA class II or III, and with stable heart failure were randomized to receive a single-chamber implantable cardioverter-defibrillator, amiodarone, or placebo.
There were 244 deaths in the placebo group, 240 deaths in the amiodarone group (P = .53 compared with placebo), and 182 deaths in the defibrillator group (P = .007 compared with placebo).42
Recommendations. The 2013 ACC/AHA guideline1 gives implantable defibrillator therapy a class IA recommendation for the primary prevention of sudden cardiac death in selected patients with nonischemic cardiomyopathy or ischemic cardiomyopathy at least 40 days after a myocardial infarction and 90 days after percutaneous coronary intervention or coronary artery bypass grafting; with a left ventricular ejection fraction of 35% or less; and NYHA class II or III symptoms on chronic goal-directed medical management.
This therapy receives a class IB recommendation for primary prevention of sudden cardiac death to reduce total mortality in selected patients at least 40 days after myocardial infarction with a left ventricular ejection fraction of 30% or less and NYHA class I symptoms while receiving goal-directed medical therapy.
Implantable cardioverter-defibrillators are not recommended in patients who otherwise have a life expectancy of less than 1 year.
Devices: Cardiac resynchronization therapy
From 25% to 30% of heart failure patients have an intraventricular conduction abnormality,43,44 which can result in abnormalities of systolic and diastolic function. Biventricular pacing, in which a pacing lead is placed in the coronary sinus in addition to the right atrium and right ventricle, optimizes synchronization of ventricular contraction.43,44
MUSTIC (the Multisite Stimulation in Cardiomyopathies study) was a randomized trial designed to assess the efficacy of biventricular pacing (also known as cardiac resynchronization therapy) in heart failure patients.44 Entry criteria included NYHA class III heart failure for at least 1 month, left ventricular ejection fraction less than 35%, left ventricular end-diastolic diameter greater than 60 mm, and QRS duration longer than 150 ms. Patients were followed up at 9 and 12 months with 6-minute walking distance, peak oxygen consumption, changes in NYHA class, and left ventricular systolic function by echocardiography or radionuclide testing. Quality of life was assessed by the Minnesota Living With Heart Failure Questionnaire.
At 12 months, patients could walk significantly farther in 6 minutes, and their peak oxygen consumption had increased. They also reported significant improvement in quality of life, and NYHA class improved by 25%. MUSTIC was the first study to show a benefit in exercise tolerance, quality of life, improvement in cardiac performance, and reduction in heart failure symptoms with the use of biventricular pacing at 1 year.
MIRACLE (the Multicenter InSync Randomized Clinical Evaluation) validated the findings seen in MUSTIC by using a larger population size and a double-blinded method.45 Compared with a control group, patients who underwent cardiac resynchronization therapy could walk farther in 6 minutes and scored better in NYHA class, quality of life, and left ventricular ejection fraction.45
Recommendations. The 2013 ACC/AHA guidelines1 give cardiac resynchronization therapy a class IA/B indication for NYHA class II, III, or IV patients on goal-directed medical therapy in sinus rhythm with left ventricular ejection fraction 35% or less, left bundle branch block, and QRS duration of 150 ms or more.1
Devices: Implantable sensors
The future of ambulatory heart failure management may include implantable pulmonary artery pressure sensors.
The CardioMEMS is a permanently implantable pressure measurement system designed to provide daily pulmonary artery pressure measurements in an ambulatory setting with a goal of reducing heart failure-related hospitalizations. Through a transvenous delivery system, an implantable, battery-free sensor is positioned in the distal pulmonary artery.46,47
CHAMPION (the CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients trial) was one of the first major trials to assess the safety and efficacy of implantable pulmonary artery pressure monitoring systems.46 The study device was associated with a significant reduction in mean pulmonary artery pressures, fewer heart failure hospitalizations, and better quality of life. The length of stay for heart failure-related hospitalizations was also significantly shorter in the CardioMEMs group.46

Exercise
Patients with heart failure routinely experience a decline in functional capacity. This decline manifests as reduced exercise tolerance and poor quality of life, usually resulting in a physician recommendation to rest and paradoxical deconditioning and possible progression of symptoms.
Several studies have shown that cardiac rehabilitation has improved outcomes in heart failure patients.48 Cardiac rehabilitation is a supervised program that helps patients with exercise training, healthy living, education, and psychosocial counseling.
HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) is the largest randomized trial performed to determine whether aerobic exercise training reduces all-cause mortality or all-cause hospitalization and improves quality of life in patients with stable heart failure.49 Although the reduction in end points was initially not statistically significant, after adjusting for highly prognostic predictors of poor outcomes (cardiopulmonary exercise time, left ventricular ejection fraction, atrial fibrillation, and depression), exercise training was found to reduce the incidence of all-cause mortality or all-cause hospitalization by 11% (P = .03).49
Recommendations. Based on the results of HF-ACTION and several smaller studies, the ACC/AHA guidelines give exercise training a class IA recommendation as a safe and effective activity for patients with heart failure who are able to participate, to improve functional status.1 A class IIA recommendation is given to cardiac rehabilitation for the improvement of functional capacity, exercise duration, quality of life, and mortality rates.1
End-stage heart failure: Recognition
Despite adequate titration of goal-directed medical therapy, a portion of patients with heart failure with reduced ejection fraction ultimately progress to stage D, also termed “advanced” heart failure. The 5-year survival rate for patients with heart failure overall is 50%, but the 1-year mortality rate for those with advanced heart failure exceeds 50%.50
Because the high rates of morbidity and mortality can potentially be lowered, recognition of heart failure disease progression is imperative so that patients can be promptly referred for therapies such as inotropic infusion, mechanical circulatory support, and cardiac transplant, as well as end-of-life care such as hospice.1
The ACC/AHA1 have published clinical events and findings useful in identifying patients with advanced heart failure:
- Two or more hospitalizations or emergency department visits for heart failure in the past year
- Progressive deterioration in renal function (eg, elevation in creatinine or blood urea nitrogen)
- Weight loss without other cause
- Intolerance to ACE inhibitors due to hypotension or worsening renal function
- Inability to tolerate beta-blockers due to worsening heart failure or hypotension
- Systolic blood pressure often below 90 mm Hg
- Persistent dyspnea with dressing or bathing requiring rest
- Inability to walk one block on level ground due to dyspnea or fatigue
- Recent need to escalate diuretics to maintain volume status, often reaching daily dose equivalent to furosemide more than 160 mg/day or use of supplemental metolazone
- Progressive decline in serum sodium, usually to below 133 mmol/L
- Frequent shocks from implanted cardiac defibrillator.
End-stage heart failure: Left ventricular assist devices
For patients with refractory heart failure despite optimal medical management, advanced therapies such as heart transplant or ventricular assist devices have been proven to be durable options. These mechanical circulatory support devices “unload” the diseased ventricle and maintain cardiac output to vital organs.51 They were initially designed as temporary support to allow ventricular recovery or as a bridge to cardiac transplant. However, they have also evolved into permanent (“destination”) therapy.52
REMATCH (the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive HF trial) was the landmark study that showed that left ventricular assist device implantation resulted in a survival benefit and an improved quality of life in patients with advanced heart failure ineligible for cardiac transplant, compared with medical management.50 Implantation of a left ventricular assist device was associated with a 27% absolute reduction in the 1-year mortality rate.50
Since the National Institutes of Health’s artificial heart program was launched in 1964, there has been tremendous progress in the development of mechanical circulatory devices.50 The results of REMATCH were promising, but the 2-year survival rate was still only 23%, leaving a lot to be desired.
The HeartMate II (Thoratec) trial compared an axial continuous-flow device vs the previously established pulsatile left ventricular assist device, and noted a 2-year survival of 58% with the continuous flow device vs 24% with the pulsatile device (P = .008).53
ADVANCE (Evaluation of the HeartWare Left Ventricular Assist Device for the Treatment of Advanced Heart Failure) showed similar efficacy of the HVAD (Heartware), a centrifugal continuous-flow LVAD currently in use.54
The next generation of continuous-flow left ventricular assist devices are currently in clinical trials in the United States and include the axial flow MVAD (Heartware) and centrifugal flow Heartmate III (Thoratec).
We emphasize the importance of early identification of patients with advanced disease who may qualify for and benefit from such therapies.

The management of heart failure is evolving. In the 1960s, the standard heart failure medical regimen included digoxin, diuretics, and the recommendation of rest. This contrasts with the current era, in which medical regimens include neurohormonal blockade, diuretics, and the promotion of physical activity.55 Since the publication of the 2013 heart failure guidelines, new medical and device options have emerged that have been proven to either improve survival or reduce hospitalizations. The development of clinical guidelines promotes evidence-based practice and overcomes the inertia of practice patterns based on anecdotal evidence.
Several approaches to the management of heart failure have been recommended. A major effort should be made to identify those at risk for heart failure (stage A) and to implement risk factor modification. Treatment of hypertension, diabetes mellitus, and dyslipidemia decreases the risk of heart failure.1
For patients with evidence of structural heart disease with and without symptoms, Figure 1 summarizes a guideline approach to the management of heart failure. It should be stressed that guidelines are meant to guide management, but do not serve as a substitute for sound clinical judgment.
Heart failure is the common final pathway of all cardiac pathology, and understanding the neurohormonal response and maladaptive physiology has led to the development of novel therapeutics and devices. At present, the field of cardiology may not be able to remove the “failure” from heart failure, but we can make every effort to prevent failure of treatment delivery and reduce resource utilization and morbidity associated with this syndrome.
Acknowledgments: We would like to thank Chankya Dahagam and Cynthia Obenwa for their valuable contribution in the preparation of this manuscript.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation 2015; 133:e38–e360.
- Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006; 113:2851–2860.
- Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation 2007; 115:1563–1570.
- Tigerstedt R, Bergman PQ. Niere und Kreislauf. Skand Arch Physiol 1898; 8:223–271.
- Unger T, Li J. The role of the renin-angiotensin-aldosterone system in heart failure. J Renin Angiotensin Aldosterone Syst 2004; 5(suppl 1):S7–S10.
- Cohn JN, Levine TB, Francis GS, Goldsmith S. Neurohumoral control mechanisms in congestive heart failure. Am Heart J 1981; 102:509–514.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation 1991; 83:1849–1865.
- The CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345:1667–1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003; 362:767–771.
- Pitt B, Zannad F, Remme WJ, et al.The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2010; 364:11–21.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J 1975; 37:1022–1036.
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003; 107:1570–1575.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet 1979; 1:1374–1376.
- Klapholz M. Beta-blocker use for the stages of heart failure. Mayo Clin Proc 2009; 84:718–729.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med 1996; 334:1349–1355.
- The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999; 353:9–13.
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Poole-Wilson PA, Swedberg K, Cleland JG, et al; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003; 362:7–13.
- Kim H-N, Januzzi JL Jr. Natriuretic peptide testing in heart failure. Circulation 2011; 123:2015–2019.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF) randomized trial. JAMA 2009; 301:383–392.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro–B-type natriuretic peptide-guided treatment for chronic heart failure: results From the BATTLESCARRED (NT-proBNP–Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol 2009; 55:53–60.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro–B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol 2011; 58:1881-1889.
- Whellan DJ, Gaulden L, Gattis WA, et al. The benefit of implementing a heart failure disease management program. Arch Intern Med 2001; 161:2223–2228.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- Grady KL, Dracup K, Kennedy G, et al. Team management of patients with heart filure: a statement for healthcare professionals from the Cardiovascular Nursing Council of the American Heart Association. Circulation 2000; 102:2443–2456.
- Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 1987; 113:1489-1494.
- Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exper Ther 2004; 308:236–240.
- Böhm M, Swedberg K, Komajda M, et al; SHIFT Investigators. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010; 376:886–894.
- Batterman RC, DeGraff AC. Comparative study on the use of the purified digitalis glycosides, digoxin, digitoxin, and lanatoside C, for the management of ambulatory patients with congestive heart failure. Am Heart J 1947; 34:663–673.
- Ouyang AJ, Lv YN, Zhong HL, et al. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am J Cardiol 2015; 115:901–906.
- Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997; 336:525–533.
- Aleong RG, Mulvahill MJ, Halder I, et al. Left ventricular dilatation increases the risk of ventricular arrhythmias in patients with reduced systolic function. J Am Heart Assoc 2015; 4:e001566.
- Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996; 335:1933–1940.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Greenberg B, Mehra MR. All patients with heart failure and intraventricular conduction defect or dyssynchrony should not receive cardiac resynchronization therapy. Circulation 2006; 114:2685–2691.
- Linde C, Leclercq C, Rex S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002; 40:111–118.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- Abraham WT, Adamson PB, Bourge RC, et al; CHAMPION Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377:658–666.
- Loh JP, Barbash IM, Waksman R. Overview of the 2011 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee Meeting on the CardioMEMS Champion Heart Failure Monitoring System. J Am Coll Cardiol 2013; 61:1571–1576.
- Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013; 1:540–547.
- O’Connor CM, Whellan DJ, Lee KL, et al; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009; 301:1439–1450.
- Rose EA, Gelijns AC, Moskowitz AJ, et al; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001; 345:1435–1443.
- Givertz MM. Ventricular assist devices: important information for patients and families. Circulation 2011; 124:e305–e311.
- Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg 2010; 89:1205–1210.
- Slaughter MS, Rogers JG, Milano CA, et al; HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009; 361:2241–2251.
- Aaronson KD, Slaughter MS, Miller LW, et al; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125:3191–3200.
- Katz AM. The “modern” view of heart failure: how did we get here? Circ Heart Fail 2008; 1:63–71.
KEY POINTS
- Most patients with systolic heart failure (also called heart failure with reduced ejection fraction) should receive either an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker. Most should also receive a beta-blocker (carvedilol, metoprolol succinate, or bisoprolol).
- If symptoms persist or progress despite these treatments, an aldosterone receptor antagonist (spironolactone or eplerenone) is recommended.
- Since the publication of the ACC/AHA guidelines in 2013, the combination of sacubitril and valsartan has been approved, as has ivabradine.
- Patients with advanced heart failure should be identified early for consideration of resynchronization therapy, an implantable cardiac defibrillator, digoxin, a left ventricular assist device, or heart transplant.
- B-type natriuretic peptide levels can be used to guide therapy.
Obstructive sleep apnea
To the Editor: Thanks for the concise review of obstructive sleep apnea (OSA) in the January 2016 issue.1 I offer the following comments and questions:
1. Risk factors for OSA include large neck circumference, which in Table 1 is defined as larger than 40 cm (15.75 inches), which would include shirt collar sizes 16 and above. In the second paragraph of the text, large neck circumference is defined as greater than 17 inches in men, which would include collar sizes above 17. The definition of a large neck as larger than 40 cm must obviously be more sensitive for predicting OSA, and the definition of greater than 17 inches more specific. Which do the authors use in clinical practice?
2. The American Academy of Sleep Medicine is quoted as recommending home OSA screening “if direct monitoring of the response to non-[continuous positive airway pressure] treatments for sleep apnea is needed.”2 However, the need for direct monitoring would seem to be a contraindication to home testing rather than an indication. If this statement is correct as written, would the authors explain why and how specific non-CPAP treatments for OSA are more amenable to monitoring at home than in the sleep lab?
3. Patients with Parkinson disease are at risk for both OSA and hypotension, making them generally an exception to the association of OSA with hypertension.3
4. The home overnight OSA test often consists of a pulse oximeter worn for 8 hours at night, taped to a finger.4 This simple, inexpensive test for OSA detects episodes of apnea or hypopnea that result in arterial desaturation. Is it beneficial to also document episodes of apnea or hypopnea that do not result in arterial desaturation? These episodes are included in the 17% false-negative rate for home OSA testing mentioned in the text. Are these episodes important clinically, other than for prognosis in patients who may go on to develop apneic episodes severe enough to cause desaturation?
5. Lastly, the authors may wish to comment on the importance of diagnosing and treating OSA in patients who plan to have elective surgery under general anesthesia, which can lead to profound sleep apnea in the recovery room, with associated morbidity and death.5
- Manne MB, Rutecki G. Obstructive sleep apnea: who should be tested, and how? Cleve Clin J Med 2016; 83:25–27.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Sheu JJ, Lee HC, Lin HC, Kao LT, Chung SD. A 5-year follow-up study on the relationship between obstructive sleep apnea and Parkinson disease. J Clin Sleep Med 2015; 11:1403–1408.
- Lux L, Boehlecke B, Lohr KN. Effectiveness of portable monitoring devices for diagnosing obstructive sleep apnea: update of a systematic review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep 01. www.ncbi.nlm.nih.gov/books/NBK299250. Accessed August 31, 2016.
- Xará D, Mendonça J, Pereira H, Santos A, Abelha FJ. Adverse respiratory events after general anesthesia in patients at high risk of obstructive sleep apnea syndrome. Braz J Anesthesiol 2015; 65:359–366.
To the Editor: Thanks for the concise review of obstructive sleep apnea (OSA) in the January 2016 issue.1 I offer the following comments and questions:
1. Risk factors for OSA include large neck circumference, which in Table 1 is defined as larger than 40 cm (15.75 inches), which would include shirt collar sizes 16 and above. In the second paragraph of the text, large neck circumference is defined as greater than 17 inches in men, which would include collar sizes above 17. The definition of a large neck as larger than 40 cm must obviously be more sensitive for predicting OSA, and the definition of greater than 17 inches more specific. Which do the authors use in clinical practice?
2. The American Academy of Sleep Medicine is quoted as recommending home OSA screening “if direct monitoring of the response to non-[continuous positive airway pressure] treatments for sleep apnea is needed.”2 However, the need for direct monitoring would seem to be a contraindication to home testing rather than an indication. If this statement is correct as written, would the authors explain why and how specific non-CPAP treatments for OSA are more amenable to monitoring at home than in the sleep lab?
3. Patients with Parkinson disease are at risk for both OSA and hypotension, making them generally an exception to the association of OSA with hypertension.3
4. The home overnight OSA test often consists of a pulse oximeter worn for 8 hours at night, taped to a finger.4 This simple, inexpensive test for OSA detects episodes of apnea or hypopnea that result in arterial desaturation. Is it beneficial to also document episodes of apnea or hypopnea that do not result in arterial desaturation? These episodes are included in the 17% false-negative rate for home OSA testing mentioned in the text. Are these episodes important clinically, other than for prognosis in patients who may go on to develop apneic episodes severe enough to cause desaturation?
5. Lastly, the authors may wish to comment on the importance of diagnosing and treating OSA in patients who plan to have elective surgery under general anesthesia, which can lead to profound sleep apnea in the recovery room, with associated morbidity and death.5
To the Editor: Thanks for the concise review of obstructive sleep apnea (OSA) in the January 2016 issue.1 I offer the following comments and questions:
1. Risk factors for OSA include large neck circumference, which in Table 1 is defined as larger than 40 cm (15.75 inches), which would include shirt collar sizes 16 and above. In the second paragraph of the text, large neck circumference is defined as greater than 17 inches in men, which would include collar sizes above 17. The definition of a large neck as larger than 40 cm must obviously be more sensitive for predicting OSA, and the definition of greater than 17 inches more specific. Which do the authors use in clinical practice?
2. The American Academy of Sleep Medicine is quoted as recommending home OSA screening “if direct monitoring of the response to non-[continuous positive airway pressure] treatments for sleep apnea is needed.”2 However, the need for direct monitoring would seem to be a contraindication to home testing rather than an indication. If this statement is correct as written, would the authors explain why and how specific non-CPAP treatments for OSA are more amenable to monitoring at home than in the sleep lab?
3. Patients with Parkinson disease are at risk for both OSA and hypotension, making them generally an exception to the association of OSA with hypertension.3
4. The home overnight OSA test often consists of a pulse oximeter worn for 8 hours at night, taped to a finger.4 This simple, inexpensive test for OSA detects episodes of apnea or hypopnea that result in arterial desaturation. Is it beneficial to also document episodes of apnea or hypopnea that do not result in arterial desaturation? These episodes are included in the 17% false-negative rate for home OSA testing mentioned in the text. Are these episodes important clinically, other than for prognosis in patients who may go on to develop apneic episodes severe enough to cause desaturation?
5. Lastly, the authors may wish to comment on the importance of diagnosing and treating OSA in patients who plan to have elective surgery under general anesthesia, which can lead to profound sleep apnea in the recovery room, with associated morbidity and death.5
- Manne MB, Rutecki G. Obstructive sleep apnea: who should be tested, and how? Cleve Clin J Med 2016; 83:25–27.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Sheu JJ, Lee HC, Lin HC, Kao LT, Chung SD. A 5-year follow-up study on the relationship between obstructive sleep apnea and Parkinson disease. J Clin Sleep Med 2015; 11:1403–1408.
- Lux L, Boehlecke B, Lohr KN. Effectiveness of portable monitoring devices for diagnosing obstructive sleep apnea: update of a systematic review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep 01. www.ncbi.nlm.nih.gov/books/NBK299250. Accessed August 31, 2016.
- Xará D, Mendonça J, Pereira H, Santos A, Abelha FJ. Adverse respiratory events after general anesthesia in patients at high risk of obstructive sleep apnea syndrome. Braz J Anesthesiol 2015; 65:359–366.
- Manne MB, Rutecki G. Obstructive sleep apnea: who should be tested, and how? Cleve Clin J Med 2016; 83:25–27.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007; 3:737–747.
- Sheu JJ, Lee HC, Lin HC, Kao LT, Chung SD. A 5-year follow-up study on the relationship between obstructive sleep apnea and Parkinson disease. J Clin Sleep Med 2015; 11:1403–1408.
- Lux L, Boehlecke B, Lohr KN. Effectiveness of portable monitoring devices for diagnosing obstructive sleep apnea: update of a systematic review. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004 Sep 01. www.ncbi.nlm.nih.gov/books/NBK299250. Accessed August 31, 2016.
- Xará D, Mendonça J, Pereira H, Santos A, Abelha FJ. Adverse respiratory events after general anesthesia in patients at high risk of obstructive sleep apnea syndrome. Braz J Anesthesiol 2015; 65:359–366.
AT Children’s Project Advisors Elected to National Academy of Sciences
Longtime scientific advisors to the AT (Ataxia Telangiectasia) Children’s Project Nathaniel Heintz, PhD, and Michael Kastan, MD, PhD, have been elected to the National Academy of Sciences.
Longtime scientific advisors to the AT (Ataxia Telangiectasia) Children’s Project Nathaniel Heintz, PhD, and Michael Kastan, MD, PhD, have been elected to the National Academy of Sciences.
Longtime scientific advisors to the AT (Ataxia Telangiectasia) Children’s Project Nathaniel Heintz, PhD, and Michael Kastan, MD, PhD, have been elected to the National Academy of Sciences.
Penn State Graduate Student Hopes to Help Families Find NORD at Time of Rare Disease Diagnosis
Kerri Nelson, a graduate student in the Department of Nursing at Pennsylvania State University and mother of a child with a rare disorder, is conducting a survey with NORD to learn how patients and families find rare disease information that is helpful to them.
“When I began my Doctorate of Nursing Practice, I found the National Organization for Rare Disorders (NORD) and felt that this organization would have been beneficial two years prior when my son was diagnosed with a rare disorder,” she wrote on the NORD blog. As a result, Ms. Nelson got the idea to help those with newly diagnosed children find the NORD website. She has designed a toolkit to be distributed to patients and families at Penn State’s Milton S. Hershey Medical Center. She also developed a brief survey that is posted on the NORD website.
Kerri Nelson, a graduate student in the Department of Nursing at Pennsylvania State University and mother of a child with a rare disorder, is conducting a survey with NORD to learn how patients and families find rare disease information that is helpful to them.
“When I began my Doctorate of Nursing Practice, I found the National Organization for Rare Disorders (NORD) and felt that this organization would have been beneficial two years prior when my son was diagnosed with a rare disorder,” she wrote on the NORD blog. As a result, Ms. Nelson got the idea to help those with newly diagnosed children find the NORD website. She has designed a toolkit to be distributed to patients and families at Penn State’s Milton S. Hershey Medical Center. She also developed a brief survey that is posted on the NORD website.
Kerri Nelson, a graduate student in the Department of Nursing at Pennsylvania State University and mother of a child with a rare disorder, is conducting a survey with NORD to learn how patients and families find rare disease information that is helpful to them.
“When I began my Doctorate of Nursing Practice, I found the National Organization for Rare Disorders (NORD) and felt that this organization would have been beneficial two years prior when my son was diagnosed with a rare disorder,” she wrote on the NORD blog. As a result, Ms. Nelson got the idea to help those with newly diagnosed children find the NORD website. She has designed a toolkit to be distributed to patients and families at Penn State’s Milton S. Hershey Medical Center. She also developed a brief survey that is posted on the NORD website.
Make the Diagnosis - October 2016
Erythema multiforme
Erythema multiforme (EM) is a hypersensitivity reaction. Cutaneous lesions generally occur symmetrically on the extensor surfaces of the acral extremities and spread centripetally. Lesions are polymorphous and may be macular, papular, or bullous, with or without mucosal involvement. Classic targetoid lesions have a dusky central area, a paler edematous ring, and an erythematous outermost ring. EM can be triggered by multiple causes. 90% of cases are infectious, with herpes simplex virus infection most common and mycoplasma pneumoniae second. Medications (sulfonamides, anticonvulsants, etc), malignancy, autoimmune disease, immunizations, radiation, and sarcoidosis may also cause EM.
The differential diagnosis for EM includes urticaria, Stevens-Johnson syndrome, fixed drug eruption, bullous pemphigoid, paraneoplastic pemphigus, acute febrile neutophilic dermatosis (Sweet's syndrome), polymorphous light eruption (PMLE), and cutaneous small vessel vasculitis. The lesions of EM are fixed and all arise within 72 hours of onset, which can help distinguish between urticaria. The distribution of the lesions can help rule out Stevens-Johnson syndrome, which classically begins centrally and spreads distally and often will involve mucosal surfaces. Fixed drug eruption generally presents with fewer lesions and a preceding medication history. Bullous pemphigoid and paraneoplastic pemphigus are both chronic in their course and have distinguishing histopathologic characteristics that will not be present in erythema multiforme. Sweet's syndrome is also associated with infection; however, histology reveals a dense neutrophilic infiltrate with dermal edema on biopsy. History can be useful in distinguishing EM from PMLE: preceding infection strongly suggests EM, while ultraviolet radiation exposure is more likely to result in PMLE. Lastly, EM differs from cutaneous small vessel vasculitis through both histopathologic examination and direct immunofluorescence.
EM is usually a self-limited condition of about two weeks without significant sequelae, however, it may vary in both the length and severity of its course. A small subset of patients may experience episodic eruptions over a period of years, in what is known as recurrent EM. An even smaller subset of patients may continuously suffer from the lesions for months or even years, which is termed persistent EM. Treatment is aimed at the underlying condition or cause. Offending medications should be discontinued. Patients with mild cases of EM generally fare well with symptomatic treatment alone; however, more severe cases may require hospitalization for hydration, analgesia, and antiviral therapy. The use of systemic corticosteroids for EM is controversial and is generally reserved for severe cases with mucosal involvement. Prophylactic antiviral therapy is indicated for those with recurrent erythema multiforme of viral origin who suffer from six or more episodes per year. Very rarely, erythema multiforme may include ocular involvement leading to serious long-term sequelae, so patients with ocular symptoms should be immediately referred to an ophthalmologist for evaluation.
The patient's biopsy revealed necrotic keratinocytes and exocytosis of lymphocytes and was consistent with EM. She improved with oral antiviral therapy and topical steroid cream.
Erythema multiforme
Erythema multiforme (EM) is a hypersensitivity reaction. Cutaneous lesions generally occur symmetrically on the extensor surfaces of the acral extremities and spread centripetally. Lesions are polymorphous and may be macular, papular, or bullous, with or without mucosal involvement. Classic targetoid lesions have a dusky central area, a paler edematous ring, and an erythematous outermost ring. EM can be triggered by multiple causes. 90% of cases are infectious, with herpes simplex virus infection most common and mycoplasma pneumoniae second. Medications (sulfonamides, anticonvulsants, etc), malignancy, autoimmune disease, immunizations, radiation, and sarcoidosis may also cause EM.
The differential diagnosis for EM includes urticaria, Stevens-Johnson syndrome, fixed drug eruption, bullous pemphigoid, paraneoplastic pemphigus, acute febrile neutophilic dermatosis (Sweet's syndrome), polymorphous light eruption (PMLE), and cutaneous small vessel vasculitis. The lesions of EM are fixed and all arise within 72 hours of onset, which can help distinguish between urticaria. The distribution of the lesions can help rule out Stevens-Johnson syndrome, which classically begins centrally and spreads distally and often will involve mucosal surfaces. Fixed drug eruption generally presents with fewer lesions and a preceding medication history. Bullous pemphigoid and paraneoplastic pemphigus are both chronic in their course and have distinguishing histopathologic characteristics that will not be present in erythema multiforme. Sweet's syndrome is also associated with infection; however, histology reveals a dense neutrophilic infiltrate with dermal edema on biopsy. History can be useful in distinguishing EM from PMLE: preceding infection strongly suggests EM, while ultraviolet radiation exposure is more likely to result in PMLE. Lastly, EM differs from cutaneous small vessel vasculitis through both histopathologic examination and direct immunofluorescence.
EM is usually a self-limited condition of about two weeks without significant sequelae, however, it may vary in both the length and severity of its course. A small subset of patients may experience episodic eruptions over a period of years, in what is known as recurrent EM. An even smaller subset of patients may continuously suffer from the lesions for months or even years, which is termed persistent EM. Treatment is aimed at the underlying condition or cause. Offending medications should be discontinued. Patients with mild cases of EM generally fare well with symptomatic treatment alone; however, more severe cases may require hospitalization for hydration, analgesia, and antiviral therapy. The use of systemic corticosteroids for EM is controversial and is generally reserved for severe cases with mucosal involvement. Prophylactic antiviral therapy is indicated for those with recurrent erythema multiforme of viral origin who suffer from six or more episodes per year. Very rarely, erythema multiforme may include ocular involvement leading to serious long-term sequelae, so patients with ocular symptoms should be immediately referred to an ophthalmologist for evaluation.
The patient's biopsy revealed necrotic keratinocytes and exocytosis of lymphocytes and was consistent with EM. She improved with oral antiviral therapy and topical steroid cream.
Erythema multiforme
Erythema multiforme (EM) is a hypersensitivity reaction. Cutaneous lesions generally occur symmetrically on the extensor surfaces of the acral extremities and spread centripetally. Lesions are polymorphous and may be macular, papular, or bullous, with or without mucosal involvement. Classic targetoid lesions have a dusky central area, a paler edematous ring, and an erythematous outermost ring. EM can be triggered by multiple causes. 90% of cases are infectious, with herpes simplex virus infection most common and mycoplasma pneumoniae second. Medications (sulfonamides, anticonvulsants, etc), malignancy, autoimmune disease, immunizations, radiation, and sarcoidosis may also cause EM.
The differential diagnosis for EM includes urticaria, Stevens-Johnson syndrome, fixed drug eruption, bullous pemphigoid, paraneoplastic pemphigus, acute febrile neutophilic dermatosis (Sweet's syndrome), polymorphous light eruption (PMLE), and cutaneous small vessel vasculitis. The lesions of EM are fixed and all arise within 72 hours of onset, which can help distinguish between urticaria. The distribution of the lesions can help rule out Stevens-Johnson syndrome, which classically begins centrally and spreads distally and often will involve mucosal surfaces. Fixed drug eruption generally presents with fewer lesions and a preceding medication history. Bullous pemphigoid and paraneoplastic pemphigus are both chronic in their course and have distinguishing histopathologic characteristics that will not be present in erythema multiforme. Sweet's syndrome is also associated with infection; however, histology reveals a dense neutrophilic infiltrate with dermal edema on biopsy. History can be useful in distinguishing EM from PMLE: preceding infection strongly suggests EM, while ultraviolet radiation exposure is more likely to result in PMLE. Lastly, EM differs from cutaneous small vessel vasculitis through both histopathologic examination and direct immunofluorescence.
EM is usually a self-limited condition of about two weeks without significant sequelae, however, it may vary in both the length and severity of its course. A small subset of patients may experience episodic eruptions over a period of years, in what is known as recurrent EM. An even smaller subset of patients may continuously suffer from the lesions for months or even years, which is termed persistent EM. Treatment is aimed at the underlying condition or cause. Offending medications should be discontinued. Patients with mild cases of EM generally fare well with symptomatic treatment alone; however, more severe cases may require hospitalization for hydration, analgesia, and antiviral therapy. The use of systemic corticosteroids for EM is controversial and is generally reserved for severe cases with mucosal involvement. Prophylactic antiviral therapy is indicated for those with recurrent erythema multiforme of viral origin who suffer from six or more episodes per year. Very rarely, erythema multiforme may include ocular involvement leading to serious long-term sequelae, so patients with ocular symptoms should be immediately referred to an ophthalmologist for evaluation.
The patient's biopsy revealed necrotic keratinocytes and exocytosis of lymphocytes and was consistent with EM. She improved with oral antiviral therapy and topical steroid cream.
A healthy 30 year old female with a history of herpes simplex virus on the lips presented with three days of pruritic targetoid lesions on the arms and legs. She reports similar episodes in the past.