User login
Doc accused of impairment wins $3.7M for unproven complaint
A jury on May 2 awarded John M. Farmer, MD, $3.7 million for emotional distress and contract damages against Baptist Health Madisonville and Baptist Health Medical Group for a series of actions they took against Dr. Farmer after the impairment complaint.
“It’s been the worst thing that I’ve ever gone through in my entire life,” Dr. Farmer said in an interview. “My career was disrupted, because I couldn’t finish residency on time, and I had difficulty finding full-time employment comparable to what I expected to obtain immediately following residency. It continues to significantly impact my life and my job, because I remain subject to random drug testing at any time and must check in every day to see whether I have to get drug tested.”
Dr. Farmer was in his third year of residency at the hospital when the mother of two young patients accused the doctor of being “on something” during a visit with her children, said Kathleen DeLaney, an Indianapolis-based attorney who represented Dr. Farmer in the case.
According to the lawsuit, the hospital violated its fitness for duty and drug testing policy by not immediately notifying Dr. Farmer of the complaint nor immediately testing him to prove whether or not there was a factual basis for the allegation. Repercussions from the unproven complaint damaged Dr. Farmer’s personal and professional reputation. It severely limited his job prospects and earning potential, the suit alleged.
Baptist Health spokeswoman Rebecca Towles Brown said Baptist Health is exploring its legal options after the jury’s decision. “We strongly disagree with the allegations made against Baptist Health in this case and are disappointed in the jury’s verdict. Baptist Health followed its medical staff policies and appropriately responded to concerns raised about Dr. Farmer’s well-being and behavior on the date in question. We are evaluating our postverdict options, as we believe the facts as they occurred do not support the verdict. Our primary focus remains providing high-quality care to our patients and families.”
What sparked the complaint?
On Nov. 4, 2019, Dr. Farmer worked a full day in the clinic at Baptist Health, visiting and treating patients and interacting with colleagues, according to court documents. In the late afternoon, he conducted a routine appointment with two children while their mother, her boyfriend, and a medical student were present.
Following the afternoon appointment, the mother issued a complaint to an office manager that Dr. Farmer was impaired, noting that he was “touching his nose a lot,” according to the lawsuit.
The next morning, hospital administrators met with Dr. Farmer and asked whether he was impaired the day before, to which he replied, “Absolutely not,” court documents state. Dr. Farmer asked to be given a urine drug screen immediately, but administrators allegedly said he needed to be tested at the Kentucky Physicians Health Foundation in Louisville.
Dr. Farmer immediately made the 3-hour drive to the facility, and Baptist Health placed him on leave, pending the evaluation. The health foundation sent Dr. Farmer to a third-party vendor to complete a urine drug screen, which returned a result of “dilute.” (A “dilute” result occurs when the urine concentration is weak because of too much water in the urine and testers are unable to detect whether alcohol or drugs are present.)
He was then instructed to go to a separate alcohol treatment facility for a 96-hour evaluation, where he was ultimately diagnosed with mild alcohol use disorder, according to Ms. DeLaney. The facility did not recommend that he receive any inpatient care.
Hospital administrators later sent a letter to the Kentucky Board of Medical Licensure alerting them of the patient’s complaint. The board opened an investigation, and Farmer was required to sign an interim order in which he agreed not to practice medicine until approved by the board, according to court documents. The order was reported to the National Practitioner’s Data Bank.
To maintain his employment and complete his residency, Dr. Farmer was ultimately required to sign a 2-year agreement with Kentucky Physicians Health Foundation, which included regular testing, monitoring, and therapy. The board later extended the agreement to 5 years and made Dr. Farmer’s compliance a condition of retaining his medical license, according to legal records.
Dr. Farmer sued Baptist Health Medical Group and Baptist Heath Madisonville in 2021, alleging breach of contract and tortious interference with prospective business advantage.
At trial, coworkers, including Farmer’s attending physicians, testified that Dr. Farmer was not impaired on the date in question, Ms. DeLaney said. A key fact highlighted at trial is that Dr. Farmer has ADHD.
“My client has ADHD, so he’s normally a twitchy person,” she said. “There was lots of testimony about how he moves a lot and that he’s fidgety and doesn’t stand still. The two attending doctors that were supervising him at clinic that afternoon both said 100% he was not impaired; he was his usual self. They told the residency director that right after the incident. They both testified at trial they thought that would be the end of the matter.”
Baptist Health would not comment about whether it followed its fitness for duty and drug testing policy or whether leaders spoke with other medical professionals who worked with Dr. Farmer on the day of the complaint.
Dr. Farmer said he feels vindicated by the verdict and grateful to the jury.
“I intend to continue practicing as a family medicine doctor and hope to continue to grow and advance in my career,” he said.
Have you been falsely accused? Here’s what to do
Dr. Farmer is not alone in fighting back against allegations by hospitals regarding conduct associated with impairment.
In 2020, an ob.gyn. who had been accused of being under the influence while working won $4.75 million in fraud and defamation damages against St. Vincent Carmel (Ind.) Hospital and St. Vincent Carmel Medical Group for its treatment following an impairment complaint by a nurse.
It’s unclear how prevalent such scenarios are because frequently, physicians are embarrassed and keep quiet about the situation and how they were treated, said Louise B. Andrew, MD, JD, an emergency physician/internist and attorney who consults on physician health and wellness, litigation stress, and disability discrimination.
“Physicians are unlikely to reveal that it’s happened to them unless they happen to have had a good outcome” she said. “All we know is that we’re hearing more and more about it, and that might be because people are becoming more open and outraged when it happens. It’s quite easy for anyone in a hospital environment or in an office environment, for a competitor, a coworker, or even a disgruntled patient to allege a physician has ‘glazed eyes’ or ‘alcohol on the breath,’ and that’s all it takes to start the ball rolling.”
If you are falsely accused of being impaired at work or are suddenly confronted with a complaint, the first step is to remain calm, said Kernan Manion, MD, executive director for the Center for Physician Rights, a nonprofit organization that assists physicians who have been subject to unfair medical board, health program, or peer review processes.
“The first thing is to keep your wits about you,” he said, “because often, docs get frightened or angry, and they overact. You have to gain your composure and ask for documentation about the nature of the allegation.”
Obtain in writing any and all information that supports the allegation. Physicians who are asked to report to a physician health program should ask the reason they are being referred and whether it is for a medical evaluation or another type of evaluation, he said. If it’s a medical reason, the process needs to follow medical parameters in terms of confidentiality.
“The bottom line is that a doctor should not take everything at face value and follow the organization’s orders unquestioning,” Dr. Manion said. “They have a right to get their concerns addressed.”
Physicians who are accused of using substances on duty or being under the influence while working have to right to undergo testing immediately, Dr. Andrew said.
“If you’re told on the spot: ‘You need to submit to testing,’ then you should do it, but make sure it’s done properly,” she said. “Ensure that forensically, you give two samples and that they are sealed and the chain of evidence is maintained. The reason for that is if one of them is a false positive, the second one can be reviewed separately.”
If administrators do not allow for prompt testing, get yourself tested immediately on your own, she said.
As far as leaves of absence are concerned, ensure you know what type of leave is being executed, Dr. Manion said. Ask the nature of the leave and whether the leave counts as a suspension that will go against your medical license and be reportable to the NPDB. In such cases, the only reason to suspend a doctor’s privileges is because they are considered a danger to others, or, in other words, there’s been an allegation of unsafe care.
“If there is an allegation of unsafe care, the physician should ask for documentation of the patient safety issues in question and why they are being deemed unsafe to practice,” he said.
Ms. DeLaney recommended physicians not report to or communicate with any state medical association, physician health foundation, or licensing authority without first getting legal advice.
In addition, doctors will likely be tested for acute and long-term drug and alcohol use, so it’s a good idea to avoid any activity or substances that could result in a dilute sample or a positive result on a drug or alcohol test, she said.
As for broader solutions, it’s important that more physicians come out of the shadows and tell their stories when these injustices take place, said Dr. Andrew.
“Doctors need to be more open about this when it happens, which is not easy,” she said. “More need to be suing, which is certainly not cheap. Also, when they do come to settlements, they should not sign nondisclosure agreements so that they can talk about what happened and it can be publicized. This way, more doctors are aware of the types of tactics used against physicians and what other doctors have done that can help.”
A version of this article first appeared on Medscape.com.
A jury on May 2 awarded John M. Farmer, MD, $3.7 million for emotional distress and contract damages against Baptist Health Madisonville and Baptist Health Medical Group for a series of actions they took against Dr. Farmer after the impairment complaint.
“It’s been the worst thing that I’ve ever gone through in my entire life,” Dr. Farmer said in an interview. “My career was disrupted, because I couldn’t finish residency on time, and I had difficulty finding full-time employment comparable to what I expected to obtain immediately following residency. It continues to significantly impact my life and my job, because I remain subject to random drug testing at any time and must check in every day to see whether I have to get drug tested.”
Dr. Farmer was in his third year of residency at the hospital when the mother of two young patients accused the doctor of being “on something” during a visit with her children, said Kathleen DeLaney, an Indianapolis-based attorney who represented Dr. Farmer in the case.
According to the lawsuit, the hospital violated its fitness for duty and drug testing policy by not immediately notifying Dr. Farmer of the complaint nor immediately testing him to prove whether or not there was a factual basis for the allegation. Repercussions from the unproven complaint damaged Dr. Farmer’s personal and professional reputation. It severely limited his job prospects and earning potential, the suit alleged.
Baptist Health spokeswoman Rebecca Towles Brown said Baptist Health is exploring its legal options after the jury’s decision. “We strongly disagree with the allegations made against Baptist Health in this case and are disappointed in the jury’s verdict. Baptist Health followed its medical staff policies and appropriately responded to concerns raised about Dr. Farmer’s well-being and behavior on the date in question. We are evaluating our postverdict options, as we believe the facts as they occurred do not support the verdict. Our primary focus remains providing high-quality care to our patients and families.”
What sparked the complaint?
On Nov. 4, 2019, Dr. Farmer worked a full day in the clinic at Baptist Health, visiting and treating patients and interacting with colleagues, according to court documents. In the late afternoon, he conducted a routine appointment with two children while their mother, her boyfriend, and a medical student were present.
Following the afternoon appointment, the mother issued a complaint to an office manager that Dr. Farmer was impaired, noting that he was “touching his nose a lot,” according to the lawsuit.
The next morning, hospital administrators met with Dr. Farmer and asked whether he was impaired the day before, to which he replied, “Absolutely not,” court documents state. Dr. Farmer asked to be given a urine drug screen immediately, but administrators allegedly said he needed to be tested at the Kentucky Physicians Health Foundation in Louisville.
Dr. Farmer immediately made the 3-hour drive to the facility, and Baptist Health placed him on leave, pending the evaluation. The health foundation sent Dr. Farmer to a third-party vendor to complete a urine drug screen, which returned a result of “dilute.” (A “dilute” result occurs when the urine concentration is weak because of too much water in the urine and testers are unable to detect whether alcohol or drugs are present.)
He was then instructed to go to a separate alcohol treatment facility for a 96-hour evaluation, where he was ultimately diagnosed with mild alcohol use disorder, according to Ms. DeLaney. The facility did not recommend that he receive any inpatient care.
Hospital administrators later sent a letter to the Kentucky Board of Medical Licensure alerting them of the patient’s complaint. The board opened an investigation, and Farmer was required to sign an interim order in which he agreed not to practice medicine until approved by the board, according to court documents. The order was reported to the National Practitioner’s Data Bank.
To maintain his employment and complete his residency, Dr. Farmer was ultimately required to sign a 2-year agreement with Kentucky Physicians Health Foundation, which included regular testing, monitoring, and therapy. The board later extended the agreement to 5 years and made Dr. Farmer’s compliance a condition of retaining his medical license, according to legal records.
Dr. Farmer sued Baptist Health Medical Group and Baptist Heath Madisonville in 2021, alleging breach of contract and tortious interference with prospective business advantage.
At trial, coworkers, including Farmer’s attending physicians, testified that Dr. Farmer was not impaired on the date in question, Ms. DeLaney said. A key fact highlighted at trial is that Dr. Farmer has ADHD.
“My client has ADHD, so he’s normally a twitchy person,” she said. “There was lots of testimony about how he moves a lot and that he’s fidgety and doesn’t stand still. The two attending doctors that were supervising him at clinic that afternoon both said 100% he was not impaired; he was his usual self. They told the residency director that right after the incident. They both testified at trial they thought that would be the end of the matter.”
Baptist Health would not comment about whether it followed its fitness for duty and drug testing policy or whether leaders spoke with other medical professionals who worked with Dr. Farmer on the day of the complaint.
Dr. Farmer said he feels vindicated by the verdict and grateful to the jury.
“I intend to continue practicing as a family medicine doctor and hope to continue to grow and advance in my career,” he said.
Have you been falsely accused? Here’s what to do
Dr. Farmer is not alone in fighting back against allegations by hospitals regarding conduct associated with impairment.
In 2020, an ob.gyn. who had been accused of being under the influence while working won $4.75 million in fraud and defamation damages against St. Vincent Carmel (Ind.) Hospital and St. Vincent Carmel Medical Group for its treatment following an impairment complaint by a nurse.
It’s unclear how prevalent such scenarios are because frequently, physicians are embarrassed and keep quiet about the situation and how they were treated, said Louise B. Andrew, MD, JD, an emergency physician/internist and attorney who consults on physician health and wellness, litigation stress, and disability discrimination.
“Physicians are unlikely to reveal that it’s happened to them unless they happen to have had a good outcome” she said. “All we know is that we’re hearing more and more about it, and that might be because people are becoming more open and outraged when it happens. It’s quite easy for anyone in a hospital environment or in an office environment, for a competitor, a coworker, or even a disgruntled patient to allege a physician has ‘glazed eyes’ or ‘alcohol on the breath,’ and that’s all it takes to start the ball rolling.”
If you are falsely accused of being impaired at work or are suddenly confronted with a complaint, the first step is to remain calm, said Kernan Manion, MD, executive director for the Center for Physician Rights, a nonprofit organization that assists physicians who have been subject to unfair medical board, health program, or peer review processes.
“The first thing is to keep your wits about you,” he said, “because often, docs get frightened or angry, and they overact. You have to gain your composure and ask for documentation about the nature of the allegation.”
Obtain in writing any and all information that supports the allegation. Physicians who are asked to report to a physician health program should ask the reason they are being referred and whether it is for a medical evaluation or another type of evaluation, he said. If it’s a medical reason, the process needs to follow medical parameters in terms of confidentiality.
“The bottom line is that a doctor should not take everything at face value and follow the organization’s orders unquestioning,” Dr. Manion said. “They have a right to get their concerns addressed.”
Physicians who are accused of using substances on duty or being under the influence while working have to right to undergo testing immediately, Dr. Andrew said.
“If you’re told on the spot: ‘You need to submit to testing,’ then you should do it, but make sure it’s done properly,” she said. “Ensure that forensically, you give two samples and that they are sealed and the chain of evidence is maintained. The reason for that is if one of them is a false positive, the second one can be reviewed separately.”
If administrators do not allow for prompt testing, get yourself tested immediately on your own, she said.
As far as leaves of absence are concerned, ensure you know what type of leave is being executed, Dr. Manion said. Ask the nature of the leave and whether the leave counts as a suspension that will go against your medical license and be reportable to the NPDB. In such cases, the only reason to suspend a doctor’s privileges is because they are considered a danger to others, or, in other words, there’s been an allegation of unsafe care.
“If there is an allegation of unsafe care, the physician should ask for documentation of the patient safety issues in question and why they are being deemed unsafe to practice,” he said.
Ms. DeLaney recommended physicians not report to or communicate with any state medical association, physician health foundation, or licensing authority without first getting legal advice.
In addition, doctors will likely be tested for acute and long-term drug and alcohol use, so it’s a good idea to avoid any activity or substances that could result in a dilute sample or a positive result on a drug or alcohol test, she said.
As for broader solutions, it’s important that more physicians come out of the shadows and tell their stories when these injustices take place, said Dr. Andrew.
“Doctors need to be more open about this when it happens, which is not easy,” she said. “More need to be suing, which is certainly not cheap. Also, when they do come to settlements, they should not sign nondisclosure agreements so that they can talk about what happened and it can be publicized. This way, more doctors are aware of the types of tactics used against physicians and what other doctors have done that can help.”
A version of this article first appeared on Medscape.com.
A jury on May 2 awarded John M. Farmer, MD, $3.7 million for emotional distress and contract damages against Baptist Health Madisonville and Baptist Health Medical Group for a series of actions they took against Dr. Farmer after the impairment complaint.
“It’s been the worst thing that I’ve ever gone through in my entire life,” Dr. Farmer said in an interview. “My career was disrupted, because I couldn’t finish residency on time, and I had difficulty finding full-time employment comparable to what I expected to obtain immediately following residency. It continues to significantly impact my life and my job, because I remain subject to random drug testing at any time and must check in every day to see whether I have to get drug tested.”
Dr. Farmer was in his third year of residency at the hospital when the mother of two young patients accused the doctor of being “on something” during a visit with her children, said Kathleen DeLaney, an Indianapolis-based attorney who represented Dr. Farmer in the case.
According to the lawsuit, the hospital violated its fitness for duty and drug testing policy by not immediately notifying Dr. Farmer of the complaint nor immediately testing him to prove whether or not there was a factual basis for the allegation. Repercussions from the unproven complaint damaged Dr. Farmer’s personal and professional reputation. It severely limited his job prospects and earning potential, the suit alleged.
Baptist Health spokeswoman Rebecca Towles Brown said Baptist Health is exploring its legal options after the jury’s decision. “We strongly disagree with the allegations made against Baptist Health in this case and are disappointed in the jury’s verdict. Baptist Health followed its medical staff policies and appropriately responded to concerns raised about Dr. Farmer’s well-being and behavior on the date in question. We are evaluating our postverdict options, as we believe the facts as they occurred do not support the verdict. Our primary focus remains providing high-quality care to our patients and families.”
What sparked the complaint?
On Nov. 4, 2019, Dr. Farmer worked a full day in the clinic at Baptist Health, visiting and treating patients and interacting with colleagues, according to court documents. In the late afternoon, he conducted a routine appointment with two children while their mother, her boyfriend, and a medical student were present.
Following the afternoon appointment, the mother issued a complaint to an office manager that Dr. Farmer was impaired, noting that he was “touching his nose a lot,” according to the lawsuit.
The next morning, hospital administrators met with Dr. Farmer and asked whether he was impaired the day before, to which he replied, “Absolutely not,” court documents state. Dr. Farmer asked to be given a urine drug screen immediately, but administrators allegedly said he needed to be tested at the Kentucky Physicians Health Foundation in Louisville.
Dr. Farmer immediately made the 3-hour drive to the facility, and Baptist Health placed him on leave, pending the evaluation. The health foundation sent Dr. Farmer to a third-party vendor to complete a urine drug screen, which returned a result of “dilute.” (A “dilute” result occurs when the urine concentration is weak because of too much water in the urine and testers are unable to detect whether alcohol or drugs are present.)
He was then instructed to go to a separate alcohol treatment facility for a 96-hour evaluation, where he was ultimately diagnosed with mild alcohol use disorder, according to Ms. DeLaney. The facility did not recommend that he receive any inpatient care.
Hospital administrators later sent a letter to the Kentucky Board of Medical Licensure alerting them of the patient’s complaint. The board opened an investigation, and Farmer was required to sign an interim order in which he agreed not to practice medicine until approved by the board, according to court documents. The order was reported to the National Practitioner’s Data Bank.
To maintain his employment and complete his residency, Dr. Farmer was ultimately required to sign a 2-year agreement with Kentucky Physicians Health Foundation, which included regular testing, monitoring, and therapy. The board later extended the agreement to 5 years and made Dr. Farmer’s compliance a condition of retaining his medical license, according to legal records.
Dr. Farmer sued Baptist Health Medical Group and Baptist Heath Madisonville in 2021, alleging breach of contract and tortious interference with prospective business advantage.
At trial, coworkers, including Farmer’s attending physicians, testified that Dr. Farmer was not impaired on the date in question, Ms. DeLaney said. A key fact highlighted at trial is that Dr. Farmer has ADHD.
“My client has ADHD, so he’s normally a twitchy person,” she said. “There was lots of testimony about how he moves a lot and that he’s fidgety and doesn’t stand still. The two attending doctors that were supervising him at clinic that afternoon both said 100% he was not impaired; he was his usual self. They told the residency director that right after the incident. They both testified at trial they thought that would be the end of the matter.”
Baptist Health would not comment about whether it followed its fitness for duty and drug testing policy or whether leaders spoke with other medical professionals who worked with Dr. Farmer on the day of the complaint.
Dr. Farmer said he feels vindicated by the verdict and grateful to the jury.
“I intend to continue practicing as a family medicine doctor and hope to continue to grow and advance in my career,” he said.
Have you been falsely accused? Here’s what to do
Dr. Farmer is not alone in fighting back against allegations by hospitals regarding conduct associated with impairment.
In 2020, an ob.gyn. who had been accused of being under the influence while working won $4.75 million in fraud and defamation damages against St. Vincent Carmel (Ind.) Hospital and St. Vincent Carmel Medical Group for its treatment following an impairment complaint by a nurse.
It’s unclear how prevalent such scenarios are because frequently, physicians are embarrassed and keep quiet about the situation and how they were treated, said Louise B. Andrew, MD, JD, an emergency physician/internist and attorney who consults on physician health and wellness, litigation stress, and disability discrimination.
“Physicians are unlikely to reveal that it’s happened to them unless they happen to have had a good outcome” she said. “All we know is that we’re hearing more and more about it, and that might be because people are becoming more open and outraged when it happens. It’s quite easy for anyone in a hospital environment or in an office environment, for a competitor, a coworker, or even a disgruntled patient to allege a physician has ‘glazed eyes’ or ‘alcohol on the breath,’ and that’s all it takes to start the ball rolling.”
If you are falsely accused of being impaired at work or are suddenly confronted with a complaint, the first step is to remain calm, said Kernan Manion, MD, executive director for the Center for Physician Rights, a nonprofit organization that assists physicians who have been subject to unfair medical board, health program, or peer review processes.
“The first thing is to keep your wits about you,” he said, “because often, docs get frightened or angry, and they overact. You have to gain your composure and ask for documentation about the nature of the allegation.”
Obtain in writing any and all information that supports the allegation. Physicians who are asked to report to a physician health program should ask the reason they are being referred and whether it is for a medical evaluation or another type of evaluation, he said. If it’s a medical reason, the process needs to follow medical parameters in terms of confidentiality.
“The bottom line is that a doctor should not take everything at face value and follow the organization’s orders unquestioning,” Dr. Manion said. “They have a right to get their concerns addressed.”
Physicians who are accused of using substances on duty or being under the influence while working have to right to undergo testing immediately, Dr. Andrew said.
“If you’re told on the spot: ‘You need to submit to testing,’ then you should do it, but make sure it’s done properly,” she said. “Ensure that forensically, you give two samples and that they are sealed and the chain of evidence is maintained. The reason for that is if one of them is a false positive, the second one can be reviewed separately.”
If administrators do not allow for prompt testing, get yourself tested immediately on your own, she said.
As far as leaves of absence are concerned, ensure you know what type of leave is being executed, Dr. Manion said. Ask the nature of the leave and whether the leave counts as a suspension that will go against your medical license and be reportable to the NPDB. In such cases, the only reason to suspend a doctor’s privileges is because they are considered a danger to others, or, in other words, there’s been an allegation of unsafe care.
“If there is an allegation of unsafe care, the physician should ask for documentation of the patient safety issues in question and why they are being deemed unsafe to practice,” he said.
Ms. DeLaney recommended physicians not report to or communicate with any state medical association, physician health foundation, or licensing authority without first getting legal advice.
In addition, doctors will likely be tested for acute and long-term drug and alcohol use, so it’s a good idea to avoid any activity or substances that could result in a dilute sample or a positive result on a drug or alcohol test, she said.
As for broader solutions, it’s important that more physicians come out of the shadows and tell their stories when these injustices take place, said Dr. Andrew.
“Doctors need to be more open about this when it happens, which is not easy,” she said. “More need to be suing, which is certainly not cheap. Also, when they do come to settlements, they should not sign nondisclosure agreements so that they can talk about what happened and it can be publicized. This way, more doctors are aware of the types of tactics used against physicians and what other doctors have done that can help.”
A version of this article first appeared on Medscape.com.
Commentary: Enthesitis, synovitis, spondyloarthritis, and PsA, June 2023
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
Papular Acneform Eruption With Mucositis
The Diagnosis: Syphilis
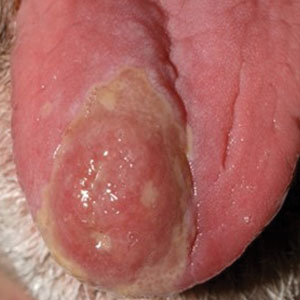
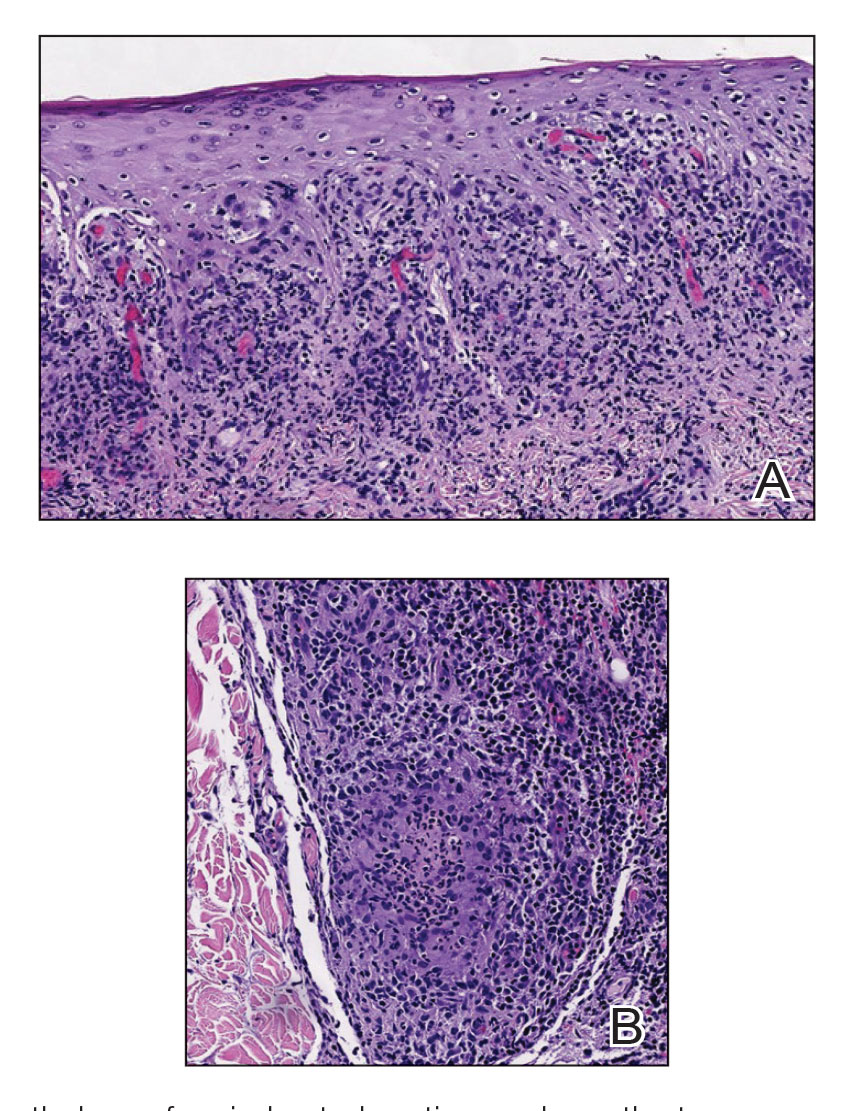
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.
Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
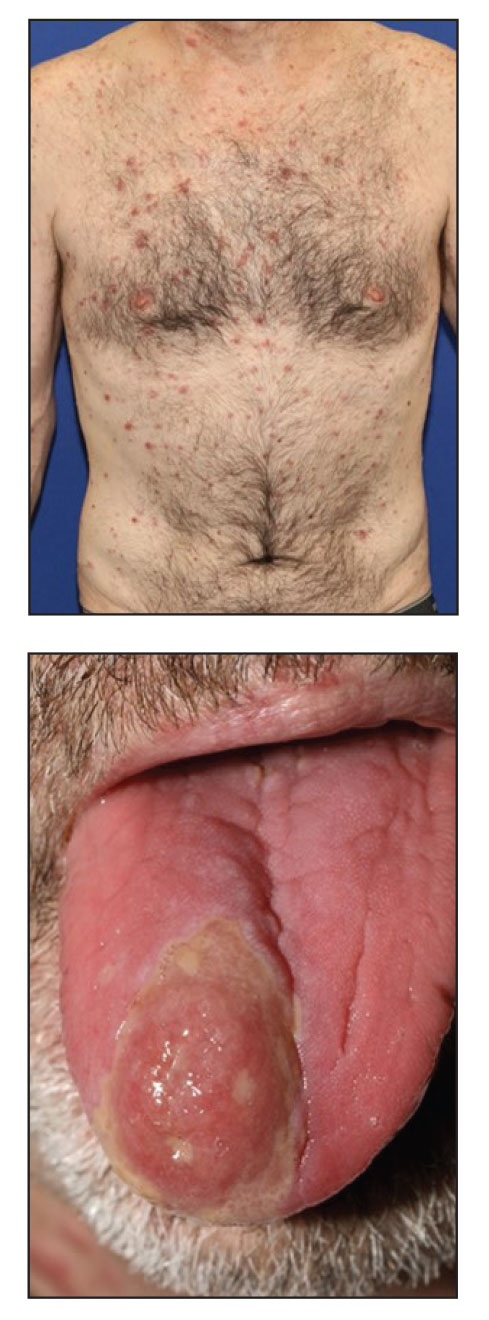
A 48-year-old man with a history of ulcerative colitis that was well-controlled with adalimumab presented with a generalized acneform eruption involving the face, chest (top) and back, as well as a well-defined ovoid ulcer on the anterior aspect of the tongue (bottom) of 2 months’ duration. Prior treatment with prednisone 60 mg daily for 14 days resulted in no improvement. He denied unintentional weight loss, cyclic fever, or arthritis. A complete blood cell count with differential showed mild anemia (hemoglobin, 11.6 g/dL [reference range, 13.2–16.6 g/dL]) with a differential cell count that was within reference range for each cell type. The erythrocyte sedimentation rate was elevated at 44 mm/h (reference range, 0–22 mm/h). A 4-mm punch biopsy specimen of an indurated cystic papule on the torso was obtained.
Commentary: Enthesitis, synovitis, spondyloarthritis, and PsA, June 2023
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
The relationship between enthesitis and synovitis is of considerable interest to both clinicians and researchers. This relationship is best evaluated using imaging, particularly ultrasonography, and could provide pathophysiologic insights. Balulu and colleagues recruited 158 patients with PsA who underwent sonographic assessment of 52 joints, 40 tendons, and 14 entheses as well as clinical evaluation. Overall, total sonographic enthesitis scores were significantly associated with total sonographic synovitis and sonographic tenosynovitis scores and also with older age, male sex, swollen joint count, C-reactive protein, physical occupation, and patient-reported outcomes. The association between enthesitis and synovitis was also demonstrated at the elbows, knees, and ankles. This study demonstrates that psoriatic enthesitis and synovitis are closely related and thus may share pathophysiologic mechanisms. Longitudinal studies in very early PsA using ultrasound might provide clues to confirm the hypothesis that psoriatic synovitis is secondary to enthesitis.
Another important domain that is increasingly studied is axial PsA. Currently, the evidence for treatment of axial PsA is extrapolated from that for axial spondyloarthritis (SpA), in the belief that the two diseases are pathophysiologically similar. However, there is increasing evidence for differences between axial PsA and axial SpA that might influence the choice of treatment. In a recent study, de Hooge and colleagues demonstrated that patients with axial PsA have lower severity of damage to the spine compared with those with axial SpA. Using data from 312 patients with PsA and 213 patients with SpA who underwent radiographic imaging assessment in the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS) and the Ghent and Belgian Inflammatory Arthritis and Spondylitis (Be-GIANT) study, respectively, they show that the proportion of patients with PsA vs SpA having spinal damage was comparable. Patients with SpA and spinal damage had higher modified Stoke Ankylosing Spondylitis Spine Scores, indicating more severe damage. These results are consistent with other published studies and indicate that patients with PsA have less severe spinal disease compared with other patients with axial SpA. Randomized controlled trials (RCTs) specifically investigating the treatment of axial PsA are currently underway. Nevertheless, post hoc analyses of data from PsA RCTs indicate that most drugs efficacious for PsA overall also provide benefit in axial disease.
In a recent report, Baraliakos and colleagues analyzed data from the SELECT-PsA 1 and SELECT-PsA 2 trials that evaluated the efficacy of upadacitinib in PsA. They show that, compared with placebo, 15 mg upadacitinib led to a greater improvement in axial symptoms. The improvement in overall Bath Ankylosing Spondylitis Disease Activity Index score at week 24 was significantly higher with 15 mg upadacitinib compared with placebo in both trials. However, these results are not definitive because there is yet no consensus on the definition of and outcome measures for axial PsA.
Commentary: Pregnancy, neoadjuvant treatment, and sexual function after BC diagnosis, June 2023
The advantages of neoadjuvant therapy (NAT), including the downstaging of the primary tumor/nodal burden and assessment of the tumor biology via response to chemotherapy, can have prognostic and therapeutic implications in the adjuvant setting. Additionally, trials in the neoadjuvant space allow rapid assessment of new agents that can help patients gain access to these therapies in an expedited fashion. Three-year outcomes from the neoadjuvant I-SPY2 trial have shown that achievement of pathologic complete response (pCR) after NAT is associated with an approximately 80% reduction in recurrence rate, regardless of molecular subtype or treatment regimen (including various novel therapy combinations).3 An analysis of individual data from 3710 patients with human epidermal growth factor receptor 2 (HER2)–positive early BC from 11 neoadjuvant trials evaluated additional prognostic factors to better characterize pCR (van Mackelenbergh et al). A total of 1497 patients (40%) had pCR, and these patients had improved event-free survival (hazard ratio 0.39; P < .001) and overall survival (hazard ratio 0.32 P < .001) compared to those with residual disease after NAT. Among patients who had pCR, tumor size at presentation (cT1-2 vs cT3-4) and nodal status (cN0 vs cN+) were independent prognostic factors for event-free survival (hazard ratio 0.67 [P = .007] and 0.72 [P = .039], respectively). These data support the role of pCR as an indicator of outcome post-NAT and, furthermore, identify additional features beyond pCR that can affect recurrence risk. It is valuable to take these other factors into account when considering patients for adjuvant therapies, even in the context of pCR.
Advances in detection modalities and treatments have led to improved survival after BC diagnosis, and as a result, more women in the survivorship setting are experiencing side effects that affect quality of life. The prevalence of sexual dysfunction is variable, perhaps owing to how this variable is defined and reported, and includes symptoms of low libido, dyspareunia, vaginal dryness, and anorgasmia.4Chang and colleagues performed a population-based study evaluating sexual dysfunction among a cohort of 19,709 BC survivors ≥ 18 years of age from the Utah Cancer Registry and 93,389 cancer-free women matched by age and birth state from the general population. BC survivors had a higher risk for sexual dysfunction (hazard ratio 1.60; 95% CI 1.51-1.70) compared with the general population, and this effect was more prominent within 1-5 years after diagnosis (hazard ratio 2.05; 95% CI 1.89-2.22) and in those < 50 years of age (hazard ratio 3.05; 95% CI 2.65-3.51). Furthermore, BC survivors who received chemotherapy and ET had an increased risk for sexual dysfunction (hazard ratio 1.16 and 1.46, respectively). These findings underscore the importance of recognition and communication regarding survivorship issues, such as sexual health, which can affect medication adherence, quality of life, and outcomes for patients.
Additional References
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.200535
- Anderson RA, Lambertini M, Hall PS, et al. Survival after breast cancer in women with a subsequent live birth: Influence of age at diagnosis and interval to subsequent pregnancy. Eur J Cancer. 2022;173:113-12 doi: 10.1016/j.ejca.20206.048
- I-SPY2 Trial Consortium. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6:1355-1362. doi: 10.1001/jamaoncol.2020.2535
- Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8:294-302. doi: 10.1111/j.1743-6109.2010.0203x
The advantages of neoadjuvant therapy (NAT), including the downstaging of the primary tumor/nodal burden and assessment of the tumor biology via response to chemotherapy, can have prognostic and therapeutic implications in the adjuvant setting. Additionally, trials in the neoadjuvant space allow rapid assessment of new agents that can help patients gain access to these therapies in an expedited fashion. Three-year outcomes from the neoadjuvant I-SPY2 trial have shown that achievement of pathologic complete response (pCR) after NAT is associated with an approximately 80% reduction in recurrence rate, regardless of molecular subtype or treatment regimen (including various novel therapy combinations).3 An analysis of individual data from 3710 patients with human epidermal growth factor receptor 2 (HER2)–positive early BC from 11 neoadjuvant trials evaluated additional prognostic factors to better characterize pCR (van Mackelenbergh et al). A total of 1497 patients (40%) had pCR, and these patients had improved event-free survival (hazard ratio 0.39; P < .001) and overall survival (hazard ratio 0.32 P < .001) compared to those with residual disease after NAT. Among patients who had pCR, tumor size at presentation (cT1-2 vs cT3-4) and nodal status (cN0 vs cN+) were independent prognostic factors for event-free survival (hazard ratio 0.67 [P = .007] and 0.72 [P = .039], respectively). These data support the role of pCR as an indicator of outcome post-NAT and, furthermore, identify additional features beyond pCR that can affect recurrence risk. It is valuable to take these other factors into account when considering patients for adjuvant therapies, even in the context of pCR.
Advances in detection modalities and treatments have led to improved survival after BC diagnosis, and as a result, more women in the survivorship setting are experiencing side effects that affect quality of life. The prevalence of sexual dysfunction is variable, perhaps owing to how this variable is defined and reported, and includes symptoms of low libido, dyspareunia, vaginal dryness, and anorgasmia.4Chang and colleagues performed a population-based study evaluating sexual dysfunction among a cohort of 19,709 BC survivors ≥ 18 years of age from the Utah Cancer Registry and 93,389 cancer-free women matched by age and birth state from the general population. BC survivors had a higher risk for sexual dysfunction (hazard ratio 1.60; 95% CI 1.51-1.70) compared with the general population, and this effect was more prominent within 1-5 years after diagnosis (hazard ratio 2.05; 95% CI 1.89-2.22) and in those < 50 years of age (hazard ratio 3.05; 95% CI 2.65-3.51). Furthermore, BC survivors who received chemotherapy and ET had an increased risk for sexual dysfunction (hazard ratio 1.16 and 1.46, respectively). These findings underscore the importance of recognition and communication regarding survivorship issues, such as sexual health, which can affect medication adherence, quality of life, and outcomes for patients.
Additional References
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.200535
- Anderson RA, Lambertini M, Hall PS, et al. Survival after breast cancer in women with a subsequent live birth: Influence of age at diagnosis and interval to subsequent pregnancy. Eur J Cancer. 2022;173:113-12 doi: 10.1016/j.ejca.20206.048
- I-SPY2 Trial Consortium. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6:1355-1362. doi: 10.1001/jamaoncol.2020.2535
- Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8:294-302. doi: 10.1111/j.1743-6109.2010.0203x
The advantages of neoadjuvant therapy (NAT), including the downstaging of the primary tumor/nodal burden and assessment of the tumor biology via response to chemotherapy, can have prognostic and therapeutic implications in the adjuvant setting. Additionally, trials in the neoadjuvant space allow rapid assessment of new agents that can help patients gain access to these therapies in an expedited fashion. Three-year outcomes from the neoadjuvant I-SPY2 trial have shown that achievement of pathologic complete response (pCR) after NAT is associated with an approximately 80% reduction in recurrence rate, regardless of molecular subtype or treatment regimen (including various novel therapy combinations).3 An analysis of individual data from 3710 patients with human epidermal growth factor receptor 2 (HER2)–positive early BC from 11 neoadjuvant trials evaluated additional prognostic factors to better characterize pCR (van Mackelenbergh et al). A total of 1497 patients (40%) had pCR, and these patients had improved event-free survival (hazard ratio 0.39; P < .001) and overall survival (hazard ratio 0.32 P < .001) compared to those with residual disease after NAT. Among patients who had pCR, tumor size at presentation (cT1-2 vs cT3-4) and nodal status (cN0 vs cN+) were independent prognostic factors for event-free survival (hazard ratio 0.67 [P = .007] and 0.72 [P = .039], respectively). These data support the role of pCR as an indicator of outcome post-NAT and, furthermore, identify additional features beyond pCR that can affect recurrence risk. It is valuable to take these other factors into account when considering patients for adjuvant therapies, even in the context of pCR.
Advances in detection modalities and treatments have led to improved survival after BC diagnosis, and as a result, more women in the survivorship setting are experiencing side effects that affect quality of life. The prevalence of sexual dysfunction is variable, perhaps owing to how this variable is defined and reported, and includes symptoms of low libido, dyspareunia, vaginal dryness, and anorgasmia.4Chang and colleagues performed a population-based study evaluating sexual dysfunction among a cohort of 19,709 BC survivors ≥ 18 years of age from the Utah Cancer Registry and 93,389 cancer-free women matched by age and birth state from the general population. BC survivors had a higher risk for sexual dysfunction (hazard ratio 1.60; 95% CI 1.51-1.70) compared with the general population, and this effect was more prominent within 1-5 years after diagnosis (hazard ratio 2.05; 95% CI 1.89-2.22) and in those < 50 years of age (hazard ratio 3.05; 95% CI 2.65-3.51). Furthermore, BC survivors who received chemotherapy and ET had an increased risk for sexual dysfunction (hazard ratio 1.16 and 1.46, respectively). These findings underscore the importance of recognition and communication regarding survivorship issues, such as sexual health, which can affect medication adherence, quality of life, and outcomes for patients.
Additional References
- Lambertini M, Blondeaux E, Bruzzone M, et al. Pregnancy after breast cancer: a systematic review and meta-analysis. J Clin Oncol. 2021;39:3293-3305. doi: 10.1200/JCO.200535
- Anderson RA, Lambertini M, Hall PS, et al. Survival after breast cancer in women with a subsequent live birth: Influence of age at diagnosis and interval to subsequent pregnancy. Eur J Cancer. 2022;173:113-12 doi: 10.1016/j.ejca.20206.048
- I-SPY2 Trial Consortium. Association of event-free and distant recurrence-free survival with individual-level pathologic complete response in neoadjuvant treatment of stages 2 and 3 breast cancer: three-year follow-up analysis for the I-SPY2 adaptively randomized clinical trial. JAMA Oncol. 2020;6:1355-1362. doi: 10.1001/jamaoncol.2020.2535
- Panjari M, Bell RJ, Davis SR. Sexual function after breast cancer. J Sex Med. 2011;8:294-302. doi: 10.1111/j.1743-6109.2010.0203x
CardioMEMS boosts QoL, curbs HF hospitalizations: MONITOR-HF
In the first randomized clinical trial of remote pulmonary artery pressure–guided monitoring and management of chronic heart failure (HF) in Europe, the intervention “substantially” improved quality of life (QoL) and reduced HF hospitalizations, new data show.
The CardioMEMS-HF system (Abbot Laboratories) used in the trial, called MONITOR-HF, remotely monitors changes in pulmonary artery pressure and provides an early warning of worsening HF.
Jasper Brugts, MD, PhD, of Erasmus MC University Medical Centre, Rotterdam, the Netherlands, said in an interview, “The concordance on outcomes of the three CardioMEMS trials across different eras, evolving GDMT [guideline-directed medical therapy], different conditions (pandemic), and different health care systems is reassuring and supportive of technologies such as CardioMEMS to improve patient monitoring to prevent HF hospitalizations and improve QoL.”
Dr. Brugts presented the study at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions.
(11 vs. 17), in comparison with standard of care, Dr. Brugts told meeting attendees.
Furthermore, CardioMEMS monitors hypervolemia as well as hypovolemia, enabling “fine-tuning of diuretics.”
The presentation drew such applause that one chairperson described it as “close to a standing ovation.” The study was published simultaneously in The Lancet.
Aggregate evidence
Early clinical evidence of the benefits of remote monitoring with the CardioMEMS-HF system was provided by the CHAMPION trial, which included patients with New York Heart Association (NYHA) class III heart failure.
Results of the subsequent GUIDE-HF trial, which aimed to test a broader population of patients with NYHA class II–IV heart failure and either increased N-terminal-pro-B-type natriuretic peptide (NT-proBNP) concentrations or hospitalization, were inconclusive.
However, a pre–COVID-19 impact analysis of GUIDE-HF indicated a possible benefit, which was primarily driven by a lower HF hospitalization rate, compared with the control group. That finding was the basis for an expanded indication for the system from the U.S. Food and Drug Administration.
The 2022 FDA indication permits the use of CardioMEMS for patients with NYHA class II HF and for those with worsening HF, as assessed by elevated natriuretic peptide levels.
From United States to Europe
Aware that most CardioMEMS data came from U.S. trials, the investigators embarked on the current trial, MONITOR-HF, an open-label, randomized trial in 25 centers in the Netherlands. Eligible patients had chronic NYHA class III HF, irrespective of ejection fraction, and had previously undergone hospitalization for HF.
A total of 348 patients were randomly assigned to either CardioMEMS-HF or standard of care (SoC) between 2019 and 2022.The median age of the patients was 69 years, and the median ejection fraction was 30%.
All patients were scheduled to be seen by their clinician at 3 months, 6 months, and every 6 months thereafter for up to 48 months.
The primary endpoint was the mean difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score at 12 months
That difference between groups was 7.13 (+7.05 in the CardioMEMS group and –0.08 in the SoC group).
In the responder analysis, the odds ratio of an improvement of at least 5 points in the KCCQ overall summary score was 1.69 in the CardioMEMS group vs. the SoC group; the OR of a deterioration of at least 5 points was 0.45.
Subgroup analyses showed no relevant heterogeneity in the treatment effect on total HF hospitalizations and, notably, no significant interaction in patients with an EF below 40% and an EF above 40%.
There was a significant reduction in the median NT-proBNP change from baseline only in the remote monitoring group (800 pg/mL) and a smaller, nonsignificant difference with SoC.
Both groups received highly appropriate background guideline–directed medical therapy throughout the study. There were no significant between-group differences at 12 months.
Freedom from device-related or system-related complications and sensor failure were 97.7% and 98.8%, respectively.
Two sensor failures occurred during a mean follow-up 1.8 years. The percentage of failures was comparable to CHAMPION and GUIDE-HF trials.
The trial was not powered to assess a mortality benefit.
Pick the right patients
“As in the U.S. trials, there will be side effects, so select the right patients, because [remote monitoring] is not without risk,” Dr. Brugts told meeting attendees.
That point also was made by Christiane E. Angermann of University and University Hospital Würzburg, Germany, in a related editorial in The Lancet.
“To reproduce these results on a large scale in real-life health care, diligent patient selection should identify those at high risk of heart failure–related hospitalization who agree with the concept of daily data collection and are able and motivated to comply with treatment recommendations even if asymptomatic,” Dr. Angermann writes.
“Without direct interaction between health care providers and patients, and timely treatment modification triggered by abnormal monitoring results, the care cycle might break and the potential benefits from early detection of decompensation would be lost.”
Val Rakita, MD, assistant professor of medicine at Temple University, Philadelphia, a specialist in advanced heart failure and main implanter of the CardioMEMS device at Temple University Hospital, commented on the study for this article.
“This study confirms the previous data that the device is very safe and effective in preventing HF hospitalizations and improving patients’ quality of life, even in a different population with more modern background guideline-directed medical therapy.”
Nevertheless, he noted, “Studies have yet to confirm a mortality benefit, despite logic telling us that preventing heart failure hospitalizations should also improve patient survival. More studies are needed to see if a survival benefit can be proven over a longer follow-up period.”
Overall, he said, “Remote monitoring allows more precise management of medications, prevention of hospitalizations, and improvement in quality of life, and I am an advocate for it in my practice.”
Not everyone is an advocate, however. In a commentary published last year, John M. Mandrola, MD, a cardiac electrophysiologist at Baptist Medical Associates in Louisville, Ky., said the expanded FDA indication for the device is the result of “dubious trial analysis, spin, lax regulation, and the growth of low-value care.”
Others also have questioned the device’s value in the clinic.
But at least for now, as Dr. Angermann writes, “Scientific evidence supports the use of the CardioMEMS-HF system to enhance remote patient management in heart failure care. For more widespread application, technological advancements are desirable to provide more comfort for patients and reusable external device components, thereby improving care experience and saving resources.”
The MONITOR-HF trial is funded by the Dutch Ministry of Health and Health Care institute. Dr. Brugts has an independent research grant from Abbott (investigator-sponsored study) and has had speaker engagements or has participated in advisory boards for Abbott and other pharmaceutical companies. Dr. Angermann has received personal fees from Abbott for serving as chair of the steering committee for the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) and consulting fees, honoraria, and travel costs from Abbott. Dr. Rakita has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the first randomized clinical trial of remote pulmonary artery pressure–guided monitoring and management of chronic heart failure (HF) in Europe, the intervention “substantially” improved quality of life (QoL) and reduced HF hospitalizations, new data show.
The CardioMEMS-HF system (Abbot Laboratories) used in the trial, called MONITOR-HF, remotely monitors changes in pulmonary artery pressure and provides an early warning of worsening HF.
Jasper Brugts, MD, PhD, of Erasmus MC University Medical Centre, Rotterdam, the Netherlands, said in an interview, “The concordance on outcomes of the three CardioMEMS trials across different eras, evolving GDMT [guideline-directed medical therapy], different conditions (pandemic), and different health care systems is reassuring and supportive of technologies such as CardioMEMS to improve patient monitoring to prevent HF hospitalizations and improve QoL.”
Dr. Brugts presented the study at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions.
(11 vs. 17), in comparison with standard of care, Dr. Brugts told meeting attendees.
Furthermore, CardioMEMS monitors hypervolemia as well as hypovolemia, enabling “fine-tuning of diuretics.”
The presentation drew such applause that one chairperson described it as “close to a standing ovation.” The study was published simultaneously in The Lancet.
Aggregate evidence
Early clinical evidence of the benefits of remote monitoring with the CardioMEMS-HF system was provided by the CHAMPION trial, which included patients with New York Heart Association (NYHA) class III heart failure.
Results of the subsequent GUIDE-HF trial, which aimed to test a broader population of patients with NYHA class II–IV heart failure and either increased N-terminal-pro-B-type natriuretic peptide (NT-proBNP) concentrations or hospitalization, were inconclusive.
However, a pre–COVID-19 impact analysis of GUIDE-HF indicated a possible benefit, which was primarily driven by a lower HF hospitalization rate, compared with the control group. That finding was the basis for an expanded indication for the system from the U.S. Food and Drug Administration.
The 2022 FDA indication permits the use of CardioMEMS for patients with NYHA class II HF and for those with worsening HF, as assessed by elevated natriuretic peptide levels.
From United States to Europe
Aware that most CardioMEMS data came from U.S. trials, the investigators embarked on the current trial, MONITOR-HF, an open-label, randomized trial in 25 centers in the Netherlands. Eligible patients had chronic NYHA class III HF, irrespective of ejection fraction, and had previously undergone hospitalization for HF.
A total of 348 patients were randomly assigned to either CardioMEMS-HF or standard of care (SoC) between 2019 and 2022.The median age of the patients was 69 years, and the median ejection fraction was 30%.
All patients were scheduled to be seen by their clinician at 3 months, 6 months, and every 6 months thereafter for up to 48 months.
The primary endpoint was the mean difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score at 12 months
That difference between groups was 7.13 (+7.05 in the CardioMEMS group and –0.08 in the SoC group).
In the responder analysis, the odds ratio of an improvement of at least 5 points in the KCCQ overall summary score was 1.69 in the CardioMEMS group vs. the SoC group; the OR of a deterioration of at least 5 points was 0.45.
Subgroup analyses showed no relevant heterogeneity in the treatment effect on total HF hospitalizations and, notably, no significant interaction in patients with an EF below 40% and an EF above 40%.
There was a significant reduction in the median NT-proBNP change from baseline only in the remote monitoring group (800 pg/mL) and a smaller, nonsignificant difference with SoC.
Both groups received highly appropriate background guideline–directed medical therapy throughout the study. There were no significant between-group differences at 12 months.
Freedom from device-related or system-related complications and sensor failure were 97.7% and 98.8%, respectively.
Two sensor failures occurred during a mean follow-up 1.8 years. The percentage of failures was comparable to CHAMPION and GUIDE-HF trials.
The trial was not powered to assess a mortality benefit.
Pick the right patients
“As in the U.S. trials, there will be side effects, so select the right patients, because [remote monitoring] is not without risk,” Dr. Brugts told meeting attendees.
That point also was made by Christiane E. Angermann of University and University Hospital Würzburg, Germany, in a related editorial in The Lancet.
“To reproduce these results on a large scale in real-life health care, diligent patient selection should identify those at high risk of heart failure–related hospitalization who agree with the concept of daily data collection and are able and motivated to comply with treatment recommendations even if asymptomatic,” Dr. Angermann writes.
“Without direct interaction between health care providers and patients, and timely treatment modification triggered by abnormal monitoring results, the care cycle might break and the potential benefits from early detection of decompensation would be lost.”
Val Rakita, MD, assistant professor of medicine at Temple University, Philadelphia, a specialist in advanced heart failure and main implanter of the CardioMEMS device at Temple University Hospital, commented on the study for this article.
“This study confirms the previous data that the device is very safe and effective in preventing HF hospitalizations and improving patients’ quality of life, even in a different population with more modern background guideline-directed medical therapy.”
Nevertheless, he noted, “Studies have yet to confirm a mortality benefit, despite logic telling us that preventing heart failure hospitalizations should also improve patient survival. More studies are needed to see if a survival benefit can be proven over a longer follow-up period.”
Overall, he said, “Remote monitoring allows more precise management of medications, prevention of hospitalizations, and improvement in quality of life, and I am an advocate for it in my practice.”
Not everyone is an advocate, however. In a commentary published last year, John M. Mandrola, MD, a cardiac electrophysiologist at Baptist Medical Associates in Louisville, Ky., said the expanded FDA indication for the device is the result of “dubious trial analysis, spin, lax regulation, and the growth of low-value care.”
Others also have questioned the device’s value in the clinic.
But at least for now, as Dr. Angermann writes, “Scientific evidence supports the use of the CardioMEMS-HF system to enhance remote patient management in heart failure care. For more widespread application, technological advancements are desirable to provide more comfort for patients and reusable external device components, thereby improving care experience and saving resources.”
The MONITOR-HF trial is funded by the Dutch Ministry of Health and Health Care institute. Dr. Brugts has an independent research grant from Abbott (investigator-sponsored study) and has had speaker engagements or has participated in advisory boards for Abbott and other pharmaceutical companies. Dr. Angermann has received personal fees from Abbott for serving as chair of the steering committee for the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) and consulting fees, honoraria, and travel costs from Abbott. Dr. Rakita has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the first randomized clinical trial of remote pulmonary artery pressure–guided monitoring and management of chronic heart failure (HF) in Europe, the intervention “substantially” improved quality of life (QoL) and reduced HF hospitalizations, new data show.
The CardioMEMS-HF system (Abbot Laboratories) used in the trial, called MONITOR-HF, remotely monitors changes in pulmonary artery pressure and provides an early warning of worsening HF.
Jasper Brugts, MD, PhD, of Erasmus MC University Medical Centre, Rotterdam, the Netherlands, said in an interview, “The concordance on outcomes of the three CardioMEMS trials across different eras, evolving GDMT [guideline-directed medical therapy], different conditions (pandemic), and different health care systems is reassuring and supportive of technologies such as CardioMEMS to improve patient monitoring to prevent HF hospitalizations and improve QoL.”
Dr. Brugts presented the study at the Heart Failure Association of the European Society of Cardiology (HFA-ESC) 2023 sessions.
(11 vs. 17), in comparison with standard of care, Dr. Brugts told meeting attendees.
Furthermore, CardioMEMS monitors hypervolemia as well as hypovolemia, enabling “fine-tuning of diuretics.”
The presentation drew such applause that one chairperson described it as “close to a standing ovation.” The study was published simultaneously in The Lancet.
Aggregate evidence
Early clinical evidence of the benefits of remote monitoring with the CardioMEMS-HF system was provided by the CHAMPION trial, which included patients with New York Heart Association (NYHA) class III heart failure.
Results of the subsequent GUIDE-HF trial, which aimed to test a broader population of patients with NYHA class II–IV heart failure and either increased N-terminal-pro-B-type natriuretic peptide (NT-proBNP) concentrations or hospitalization, were inconclusive.
However, a pre–COVID-19 impact analysis of GUIDE-HF indicated a possible benefit, which was primarily driven by a lower HF hospitalization rate, compared with the control group. That finding was the basis for an expanded indication for the system from the U.S. Food and Drug Administration.
The 2022 FDA indication permits the use of CardioMEMS for patients with NYHA class II HF and for those with worsening HF, as assessed by elevated natriuretic peptide levels.
From United States to Europe
Aware that most CardioMEMS data came from U.S. trials, the investigators embarked on the current trial, MONITOR-HF, an open-label, randomized trial in 25 centers in the Netherlands. Eligible patients had chronic NYHA class III HF, irrespective of ejection fraction, and had previously undergone hospitalization for HF.
A total of 348 patients were randomly assigned to either CardioMEMS-HF or standard of care (SoC) between 2019 and 2022.The median age of the patients was 69 years, and the median ejection fraction was 30%.
All patients were scheduled to be seen by their clinician at 3 months, 6 months, and every 6 months thereafter for up to 48 months.
The primary endpoint was the mean difference in the Kansas City Cardiomyopathy Questionnaire (KCCQ) summary score at 12 months
That difference between groups was 7.13 (+7.05 in the CardioMEMS group and –0.08 in the SoC group).
In the responder analysis, the odds ratio of an improvement of at least 5 points in the KCCQ overall summary score was 1.69 in the CardioMEMS group vs. the SoC group; the OR of a deterioration of at least 5 points was 0.45.
Subgroup analyses showed no relevant heterogeneity in the treatment effect on total HF hospitalizations and, notably, no significant interaction in patients with an EF below 40% and an EF above 40%.
There was a significant reduction in the median NT-proBNP change from baseline only in the remote monitoring group (800 pg/mL) and a smaller, nonsignificant difference with SoC.
Both groups received highly appropriate background guideline–directed medical therapy throughout the study. There were no significant between-group differences at 12 months.
Freedom from device-related or system-related complications and sensor failure were 97.7% and 98.8%, respectively.
Two sensor failures occurred during a mean follow-up 1.8 years. The percentage of failures was comparable to CHAMPION and GUIDE-HF trials.
The trial was not powered to assess a mortality benefit.
Pick the right patients
“As in the U.S. trials, there will be side effects, so select the right patients, because [remote monitoring] is not without risk,” Dr. Brugts told meeting attendees.
That point also was made by Christiane E. Angermann of University and University Hospital Würzburg, Germany, in a related editorial in The Lancet.
“To reproduce these results on a large scale in real-life health care, diligent patient selection should identify those at high risk of heart failure–related hospitalization who agree with the concept of daily data collection and are able and motivated to comply with treatment recommendations even if asymptomatic,” Dr. Angermann writes.
“Without direct interaction between health care providers and patients, and timely treatment modification triggered by abnormal monitoring results, the care cycle might break and the potential benefits from early detection of decompensation would be lost.”
Val Rakita, MD, assistant professor of medicine at Temple University, Philadelphia, a specialist in advanced heart failure and main implanter of the CardioMEMS device at Temple University Hospital, commented on the study for this article.
“This study confirms the previous data that the device is very safe and effective in preventing HF hospitalizations and improving patients’ quality of life, even in a different population with more modern background guideline-directed medical therapy.”
Nevertheless, he noted, “Studies have yet to confirm a mortality benefit, despite logic telling us that preventing heart failure hospitalizations should also improve patient survival. More studies are needed to see if a survival benefit can be proven over a longer follow-up period.”
Overall, he said, “Remote monitoring allows more precise management of medications, prevention of hospitalizations, and improvement in quality of life, and I am an advocate for it in my practice.”
Not everyone is an advocate, however. In a commentary published last year, John M. Mandrola, MD, a cardiac electrophysiologist at Baptist Medical Associates in Louisville, Ky., said the expanded FDA indication for the device is the result of “dubious trial analysis, spin, lax regulation, and the growth of low-value care.”
Others also have questioned the device’s value in the clinic.
But at least for now, as Dr. Angermann writes, “Scientific evidence supports the use of the CardioMEMS-HF system to enhance remote patient management in heart failure care. For more widespread application, technological advancements are desirable to provide more comfort for patients and reusable external device components, thereby improving care experience and saving resources.”
The MONITOR-HF trial is funded by the Dutch Ministry of Health and Health Care institute. Dr. Brugts has an independent research grant from Abbott (investigator-sponsored study) and has had speaker engagements or has participated in advisory boards for Abbott and other pharmaceutical companies. Dr. Angermann has received personal fees from Abbott for serving as chair of the steering committee for the CardioMEMS European Monitoring Study for Heart Failure (MEMS-HF) and consulting fees, honoraria, and travel costs from Abbott. Dr. Rakita has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ESC HEART FAILURE 2023
Losing weight may bolster AFib ablation’s chances for success: LEAF interim results
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.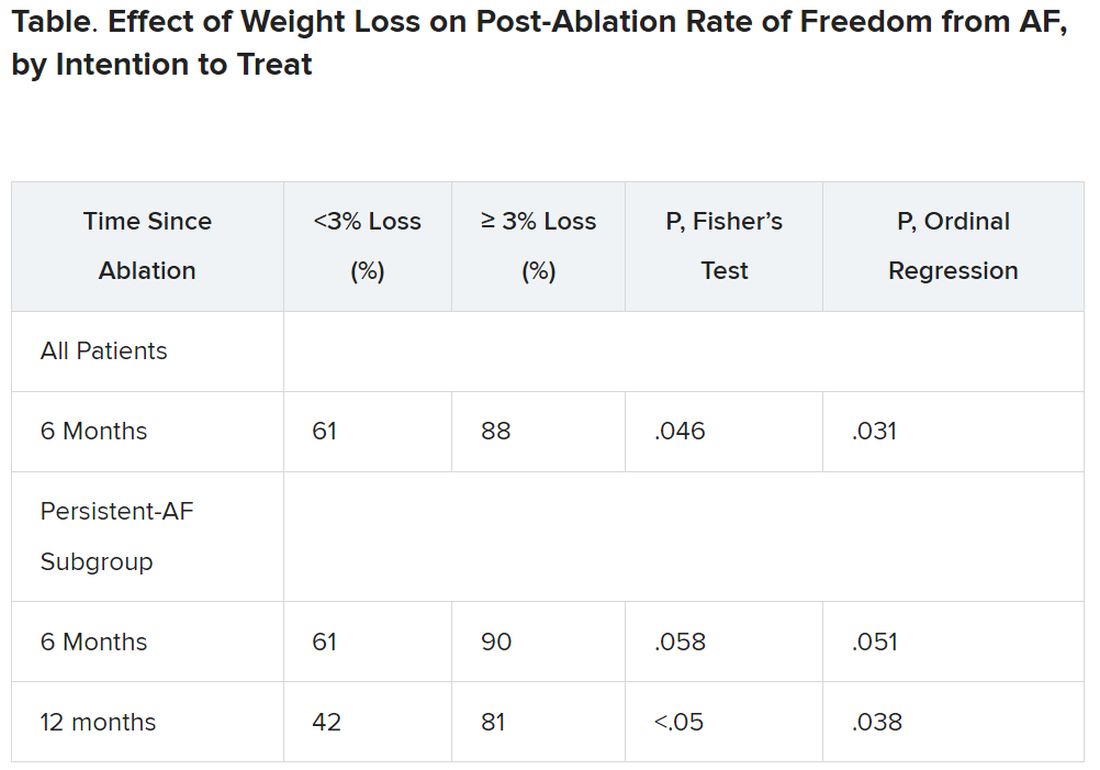
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis suggests.
The finding comes from a small study that entered such patients with paroxysmal and especially persistent AFib who were candidates for ablation. Those shedding at least 3% of body weight in the months before the procedure while engaged in a structured risk-factor modification (RFM) program were “dramatically” more likely to be AFib-free 6 months later.
The improved ablation efficacy, compared with results in similar patients who didn’t lose as much weight, was most pronounced among those whose AFib had been the persistent form, reported investigators at the annual scientific sessions of the Heart Rhythm Society, held in New Orleans.
Of note, ablations in the study were consistently limited, as much as possible, to standard pulmonary-vein isolation (PVI).
Associations between AFib and obesity and other behavioral and lifestyle-related risk factors are well recognized, but the limited studies of their effect on AFib ablation success have been inconsistent. The current analysis, the group says, points specifically to preablation weight loss as means to improving AFib-ablation outcomes.
“Adjunctive therapy focused on weight loss should be incorporated in the treatment plan for obese patients undergoing ablation for atrial fibrillation,” Jeffrey J. Goldberger, MD, MBA, of the University of Miami, said when presenting the new results at the HRS sessions.
Such a plan is entirely consistent with recent guidelines and especially a 2020 American Heart Association (AHA) consensus statement, but is inconsistently and perhaps even seldom realized in clinical practice.
Dramatic increase in success
Even modest weight loss before ablation may help, proposed Dr. Goldberger, who directs his institution’s Center for Atrial Fibrillation. Decreases for the greater-weight-loss group actually averaged less than 6% of baseline body weight.
Yet it was apparently enough to improve ablation outcomes significantly: Eighty-eight percent were free of AFib 6 months after the procedure, compared with 61% for patients who lost less than 3% of their preablation weight.
For improving ablation success, he said, “We’re talking about a moderate amount of weight loss. These patients are not going from being obese to being thin. They’re still quite overweight.”
In an analysis limited to the four-fifths of patients with persistent AFib, “we saw the same pattern,” Dr. Goldberger said at a media presentation prior to his formal report at the HRS sessions.
Moreover, that subgroup’s benefit persisted out to 12 months, at which time 42% and 81% of patients with less and greater weight loss, respectively, were free of AFib. That represents, he said, “a really tremendous – dramatic, actually – increase in success of pulmonary vein isolation in those who lost weight.”
“We’ve known for a long time that weight loss is important for preventing atrial fibrillation or increasing the success rates of the different treatments we use,” Cynthia M. Tracy, MD, said in an interview. “Probably in some studies, weight loss has been as effective as antiarrhythmics.”
A loss of 3% body weight “is not a lot,” she said. In the current analysis, “It’s notable that it made that much difference with even a fairly modest amount of weight loss.”
Now when asked, “ ‘How much do I have to lose before you’ll consider doing my ablation?’ we have a bit more concrete data to give patients and doctors as to what amount might be beneficial,” said Dr. Tracy of George Washington University Hospital, Washington, who is not associated with the study.
Evolving view of AFib
The findings are emblematic of the profession’s evolving view of AFib and its management, Dr. Goldberger observed at the press conference. Should clinicians think of AFib as similar to “a disease like Wolff-Parkinson-White syndrome,” in which the patient usually has a successful ablation, and then “we expect that to last in perpetuity with no further interventions?”
Or, he said, “is atrial fibrillation more a disease like coronary artery disease, where even if they have an intervention, the disease process is still ongoing and requires long-term disease management? I think it’s pretty clear that we’re dealing with the latter case.”
Dr. Goldberger’s report was an interim analysis of an ongoing randomized trial called LEAF (Liraglutide Effect on Atrial Fibrillation), which is comparing patients with AFib assigned to “take” vs. “not take” the GLP-1 receptor agonist liraglutide, an antidiabetic (Victoza) and weight-loss (Saxenda) drug. The trial aims to assess the drug’s apparent ability to shrink atrial epicardial adipose tissue which, Dr. Goldberger said, is thought to contribute to AFib development and influence AFib-ablation outcomes.
It’s unknown and a limitation of the current analysis, he said, whether the observed link between improved preablation–weight ablation success “is specifically related to weight loss, liraglutide treatment, or both.”
As the invited discussant for Dr. Goldberger’s presentation, David Frankel, MD, observed that studies have been inconsistent on whether substantial weight loss may improve the results of AFib rhythm-control therapy.
Those finding such an association, including LEAF and the influential LEGACY study, differed from others showing a null effect by including “a comprehensive risk factor management” program, observed Dr. Frankel, of the Hospital of the University of Pennsylvania and Penn Heart and Vascular Center, Philadelphia.
Rather than focusing solely on weight loss or sleep apnea as AFib risk factors, he said, the studies linking weight loss to AFib rhythm control also included “hypertension, diabetes, hyperlipidemia, smoking cessation, and alcohol reduction,” Dr. Frankel said. “So it seems clear that to significantly impact AF recurrence, we need to focus on all these contributors to metabolic syndrome.”
Comprehensive risk-factor management
LEAF entered patients with AFib, 79% of whom had persistent AF and the rest paroxysmal AF, who followed the RFM program and were randomly assigned also to take liraglutide or placebo. The “nurse-practitioner-led” RFM program, conducted both in-clinic and online, featured “established goals for each patient” using AHA diet and lifestyle recommendations, an exercise prescription, dietary counseling, evaluation and treatment of sleep apnea, and measures to control any diabetes, hyperlipidemia, or hypertension, Dr. Goldberger said. And patients “were counseled on alcohol reduction and smoking cessation as necessary.”
After 3 months, 29 and 30 patients – regardless of randomization assignment – had lost < 3% and at least 3% of baseline body weight, respectively.
Catheter ablation achieved PVI in all patients. A 3-month blanking period followed, after which they went off antiarrhythmic meds.
It’s very difficult for patients to lose 10% or more of body weight, “and it would not happen overnight,” Dr. Tracy observed. “These are symptomatic patients, for the most part, if they get referred to an electrophysiologist. So you don’t want to defer them indefinitely.”
The current findings, she said, point to “a more realistic target,” suggesting that weight loss of at least 3% should improve AFib ablation’s chances for success.
Dr. Goldberger disclosed ties to Medtronic. Dr. Frankel disclosed ties to Medtronic, Stryker, Biosense Webster, and Boston Scientific. Dr. Tracy reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023
AI & U
Since the November 2022 release of a much-discussed artificial intelligence (AI)-based chatbot, I have been curious what all the buzz is about. I decided to engage my well-connected software-savvy son-in-law to hear where he thought things were going.
He started by suggesting that I pose a question to the chatbot about something of which I had some current knowledge. I had recently researched the concept of primal beliefs and so we asked the chatbot to write a short essay about when an individual develops his/her primal beliefs.
In a matter of seconds the “machine” spit out a very readable document that included all the information that had taken me several hours to unearth and digest. And ... it included the references that I had determined to be valid and appropriate. It was an impressive performance to say the least.
Obviously, a technological development with this capability is sending tremors through the educational establishment. One can easily think of several human skills that an AI like this might eventually make superfluous. It will also make it increasingly difficult for educators to determine a students’ true abilities – research, synthesis, and writing to name just a few. But, of course, one could question whether we will need to teach and then test for these skills that the chatbot can perform more quickly. I’m going to leave it to the educators to struggle with that question.
In the long term you and I may find that AI is a serious threat to our existence as health care providers. In the meantime
My first thought is that if I were having trouble arriving at a diagnosis, I might appreciate having a chatbot to ask for help. Of course this would require that I had already taken a history, done a good exam, and ordered some obvious lab and imaging studies. It would also mean that I had decent knowledge and understanding of basic pathophysiology and was capable of thinking broadly enough to ask a question that would give me the greatest chance of getting the correct answer.
Knowing how to ask the right question is a skill that can be taught. For example, my wife is a successful and experienced online shopper but she acknowledges that when we have medical questions, I can often find the answer more quickly than she can. My relative success usually hinges on my choice of the key word(s) to begin the search, clearly the result of my medical training.
Once I have received a list of possible diagnoses from the chatbot, I must then be able to evaluate the validity and applicability of the references it has supplied. That too is a skill that can be taught. And, for the moment the critical importance of having these two skills suggests that graduating from medical school will continue to give us some job security in the face of expanding AI.
The same process I could used to coax the chatbot to arrive at a diagnosis could be applied when faced with a therapeutic question. Is surgery better than a pharmacological approach? If I need help with a dosage regimen, I could find this information online now. But, wouldn’t it be quicker and maybe better if I asked the chatbot to do the research for me and print a short essay on the pros and cons of different management approaches?
Once I’ve made the diagnosis, crafted a management plan, and now want to hand the patient a document in his/her primary language and at his/her reading skill level describing the diagnosis and giving detailed instructions to follow, this would seem to be a piece of cake for a chatbot given the appropriate commands. Hopefully I would remember to include the disclaimer that “This document was created with the help of a chatbot.”
Clearly, there is nothing to prevent our patients from asking the chatbot the same questions I have posed. And, no doubt, this will happen. It is already happening in a more cumbersome fashion when patients research their own symptoms. However, in the short term I believe we will retain the upper hand.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Since the November 2022 release of a much-discussed artificial intelligence (AI)-based chatbot, I have been curious what all the buzz is about. I decided to engage my well-connected software-savvy son-in-law to hear where he thought things were going.
He started by suggesting that I pose a question to the chatbot about something of which I had some current knowledge. I had recently researched the concept of primal beliefs and so we asked the chatbot to write a short essay about when an individual develops his/her primal beliefs.
In a matter of seconds the “machine” spit out a very readable document that included all the information that had taken me several hours to unearth and digest. And ... it included the references that I had determined to be valid and appropriate. It was an impressive performance to say the least.
Obviously, a technological development with this capability is sending tremors through the educational establishment. One can easily think of several human skills that an AI like this might eventually make superfluous. It will also make it increasingly difficult for educators to determine a students’ true abilities – research, synthesis, and writing to name just a few. But, of course, one could question whether we will need to teach and then test for these skills that the chatbot can perform more quickly. I’m going to leave it to the educators to struggle with that question.
In the long term you and I may find that AI is a serious threat to our existence as health care providers. In the meantime
My first thought is that if I were having trouble arriving at a diagnosis, I might appreciate having a chatbot to ask for help. Of course this would require that I had already taken a history, done a good exam, and ordered some obvious lab and imaging studies. It would also mean that I had decent knowledge and understanding of basic pathophysiology and was capable of thinking broadly enough to ask a question that would give me the greatest chance of getting the correct answer.
Knowing how to ask the right question is a skill that can be taught. For example, my wife is a successful and experienced online shopper but she acknowledges that when we have medical questions, I can often find the answer more quickly than she can. My relative success usually hinges on my choice of the key word(s) to begin the search, clearly the result of my medical training.
Once I have received a list of possible diagnoses from the chatbot, I must then be able to evaluate the validity and applicability of the references it has supplied. That too is a skill that can be taught. And, for the moment the critical importance of having these two skills suggests that graduating from medical school will continue to give us some job security in the face of expanding AI.
The same process I could used to coax the chatbot to arrive at a diagnosis could be applied when faced with a therapeutic question. Is surgery better than a pharmacological approach? If I need help with a dosage regimen, I could find this information online now. But, wouldn’t it be quicker and maybe better if I asked the chatbot to do the research for me and print a short essay on the pros and cons of different management approaches?
Once I’ve made the diagnosis, crafted a management plan, and now want to hand the patient a document in his/her primary language and at his/her reading skill level describing the diagnosis and giving detailed instructions to follow, this would seem to be a piece of cake for a chatbot given the appropriate commands. Hopefully I would remember to include the disclaimer that “This document was created with the help of a chatbot.”
Clearly, there is nothing to prevent our patients from asking the chatbot the same questions I have posed. And, no doubt, this will happen. It is already happening in a more cumbersome fashion when patients research their own symptoms. However, in the short term I believe we will retain the upper hand.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Since the November 2022 release of a much-discussed artificial intelligence (AI)-based chatbot, I have been curious what all the buzz is about. I decided to engage my well-connected software-savvy son-in-law to hear where he thought things were going.
He started by suggesting that I pose a question to the chatbot about something of which I had some current knowledge. I had recently researched the concept of primal beliefs and so we asked the chatbot to write a short essay about when an individual develops his/her primal beliefs.
In a matter of seconds the “machine” spit out a very readable document that included all the information that had taken me several hours to unearth and digest. And ... it included the references that I had determined to be valid and appropriate. It was an impressive performance to say the least.
Obviously, a technological development with this capability is sending tremors through the educational establishment. One can easily think of several human skills that an AI like this might eventually make superfluous. It will also make it increasingly difficult for educators to determine a students’ true abilities – research, synthesis, and writing to name just a few. But, of course, one could question whether we will need to teach and then test for these skills that the chatbot can perform more quickly. I’m going to leave it to the educators to struggle with that question.
In the long term you and I may find that AI is a serious threat to our existence as health care providers. In the meantime
My first thought is that if I were having trouble arriving at a diagnosis, I might appreciate having a chatbot to ask for help. Of course this would require that I had already taken a history, done a good exam, and ordered some obvious lab and imaging studies. It would also mean that I had decent knowledge and understanding of basic pathophysiology and was capable of thinking broadly enough to ask a question that would give me the greatest chance of getting the correct answer.
Knowing how to ask the right question is a skill that can be taught. For example, my wife is a successful and experienced online shopper but she acknowledges that when we have medical questions, I can often find the answer more quickly than she can. My relative success usually hinges on my choice of the key word(s) to begin the search, clearly the result of my medical training.
Once I have received a list of possible diagnoses from the chatbot, I must then be able to evaluate the validity and applicability of the references it has supplied. That too is a skill that can be taught. And, for the moment the critical importance of having these two skills suggests that graduating from medical school will continue to give us some job security in the face of expanding AI.
The same process I could used to coax the chatbot to arrive at a diagnosis could be applied when faced with a therapeutic question. Is surgery better than a pharmacological approach? If I need help with a dosage regimen, I could find this information online now. But, wouldn’t it be quicker and maybe better if I asked the chatbot to do the research for me and print a short essay on the pros and cons of different management approaches?
Once I’ve made the diagnosis, crafted a management plan, and now want to hand the patient a document in his/her primary language and at his/her reading skill level describing the diagnosis and giving detailed instructions to follow, this would seem to be a piece of cake for a chatbot given the appropriate commands. Hopefully I would remember to include the disclaimer that “This document was created with the help of a chatbot.”
Clearly, there is nothing to prevent our patients from asking the chatbot the same questions I have posed. And, no doubt, this will happen. It is already happening in a more cumbersome fashion when patients research their own symptoms. However, in the short term I believe we will retain the upper hand.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
Safety remains top parent concern for HPV vaccine
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
FROM PEDIATRICS
AxSpA effects may be more severe for Black patients
CLEVELAND – Documenting the prevalence of axial spondyloarthritis (axSpA) among Black Americans has been difficult because of little published data, but new research suggests that when Black Americans do have the disease, it seems to be more severe.
Iman Abutineh, MD, of the University of Tennessee, Memphis, discussed her team’s work at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
A total of 244 patients with axSpA were identified, including 168 (69%) males, 78 (32%) Black patients, and 143 (59%) White patients.
Average age of onset for patients overall was 27.7 years, and age at diagnosis was 36.1 years with a 7-year delay in diagnosis. Sixty-six (27%) patients had nonradiographic axSpA, 83% were on tumor necrosis factor inhibitors, and 38% were prescribed glucocorticoids.
The researchers found several differences by race.
White patients were more likely to be HLA-B27 positive (77% vs. 59%; P = .010). White patients also had higher prevalence of psoriasis, coronary artery disease, and family history of SpA. White females had a higher prevalence of inflammatory bowel disease, fibromyalgia, depression, and lower grades of sacroiliitis.
Black patients had more hip involvement
A higher percentage of Black patients had elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hip involvement. In comparing hip involvement, the researchers found that 39 (39%) White males had hip involvement as did 9 (21%) White females. In comparison, 22 (45%) Black men in the study and 14 (54%) Black women showed hip involvement (P = .035).
After adjustment for age, sex, HLA-B27, and insurance status, Black patients had higher grades of sacroiliitis with an odds ratio of 2.32 (95% confidence interval, 1.23-4.44). Black patients had a numerically longer delay in diagnosis, compared with Whites (7.93 vs. 6.64 years), but this did not achieve statistical significance (P = .454), the researchers wrote.
Study addresses racial disparities
“Traditionally we think of axial spondyloarthritis largely in Caucasian males who are HLA-B27 positive,” Dr. Abutineh said, “and we found that there is still a significant portion of patients who don’t meet the criteria that do have disease that is very significant.”
Although actual prevalence was not clear from this study, Dr. Abutineh said their data suggest a 3-to-1 ratio of White-to-Black patients with spondyloarthritis, “but of the Black patients who are diagnosed, their disease is almost always more severe. That points to why it’s important to have a high index of suspicion for this disease in that patient population because if you miss it, it could be detrimental to the patients.”
Swetha Alexander, MD, a rheumatology fellow at the University of Utah, Salt Lake City, who was not part of the study, said in an interview, “It is an excellent and timely study addressing the racial disparities and inequities surrounding axSpA diagnosis. It highlights the delay in diagnosis and increased burden of disease among Black Americans.”
She said the study may prompt a further look into barriers to care for Black Americans and their beliefs regarding seeking health care for their pain.
Higher rates of nonradiographic axSpA among Black patients
The rate of nonradiographic axSpA among Black Americans was more than twice that of their White counterparts, which, Dr. Alexander noted, could make it more difficult to diagnose axSpA in that population.
The odds ratio for Black patients having nonradiographic axSpA, compared with Whites, was 2.265 (95% CI, 1.082-4.999; P = .035), after adjustment for age, sex, and HLA-B27 status.
Adult patients with axSpA were identified from rheumatology clinics at four major hospital systems and one private clinic in Shelby County, Tenn., between 2011 and 2021. Patients met modified New York (mNY) or Assessment of Spondyloarthritis International Society (ASAS) criteria as assessed by reviewers.
The authors and Dr. Alexander reported no relevant financial relationships.
CLEVELAND – Documenting the prevalence of axial spondyloarthritis (axSpA) among Black Americans has been difficult because of little published data, but new research suggests that when Black Americans do have the disease, it seems to be more severe.
Iman Abutineh, MD, of the University of Tennessee, Memphis, discussed her team’s work at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
A total of 244 patients with axSpA were identified, including 168 (69%) males, 78 (32%) Black patients, and 143 (59%) White patients.
Average age of onset for patients overall was 27.7 years, and age at diagnosis was 36.1 years with a 7-year delay in diagnosis. Sixty-six (27%) patients had nonradiographic axSpA, 83% were on tumor necrosis factor inhibitors, and 38% were prescribed glucocorticoids.
The researchers found several differences by race.
White patients were more likely to be HLA-B27 positive (77% vs. 59%; P = .010). White patients also had higher prevalence of psoriasis, coronary artery disease, and family history of SpA. White females had a higher prevalence of inflammatory bowel disease, fibromyalgia, depression, and lower grades of sacroiliitis.
Black patients had more hip involvement
A higher percentage of Black patients had elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hip involvement. In comparing hip involvement, the researchers found that 39 (39%) White males had hip involvement as did 9 (21%) White females. In comparison, 22 (45%) Black men in the study and 14 (54%) Black women showed hip involvement (P = .035).
After adjustment for age, sex, HLA-B27, and insurance status, Black patients had higher grades of sacroiliitis with an odds ratio of 2.32 (95% confidence interval, 1.23-4.44). Black patients had a numerically longer delay in diagnosis, compared with Whites (7.93 vs. 6.64 years), but this did not achieve statistical significance (P = .454), the researchers wrote.
Study addresses racial disparities
“Traditionally we think of axial spondyloarthritis largely in Caucasian males who are HLA-B27 positive,” Dr. Abutineh said, “and we found that there is still a significant portion of patients who don’t meet the criteria that do have disease that is very significant.”
Although actual prevalence was not clear from this study, Dr. Abutineh said their data suggest a 3-to-1 ratio of White-to-Black patients with spondyloarthritis, “but of the Black patients who are diagnosed, their disease is almost always more severe. That points to why it’s important to have a high index of suspicion for this disease in that patient population because if you miss it, it could be detrimental to the patients.”
Swetha Alexander, MD, a rheumatology fellow at the University of Utah, Salt Lake City, who was not part of the study, said in an interview, “It is an excellent and timely study addressing the racial disparities and inequities surrounding axSpA diagnosis. It highlights the delay in diagnosis and increased burden of disease among Black Americans.”
She said the study may prompt a further look into barriers to care for Black Americans and their beliefs regarding seeking health care for their pain.
Higher rates of nonradiographic axSpA among Black patients
The rate of nonradiographic axSpA among Black Americans was more than twice that of their White counterparts, which, Dr. Alexander noted, could make it more difficult to diagnose axSpA in that population.
The odds ratio for Black patients having nonradiographic axSpA, compared with Whites, was 2.265 (95% CI, 1.082-4.999; P = .035), after adjustment for age, sex, and HLA-B27 status.
Adult patients with axSpA were identified from rheumatology clinics at four major hospital systems and one private clinic in Shelby County, Tenn., between 2011 and 2021. Patients met modified New York (mNY) or Assessment of Spondyloarthritis International Society (ASAS) criteria as assessed by reviewers.
The authors and Dr. Alexander reported no relevant financial relationships.
CLEVELAND – Documenting the prevalence of axial spondyloarthritis (axSpA) among Black Americans has been difficult because of little published data, but new research suggests that when Black Americans do have the disease, it seems to be more severe.
Iman Abutineh, MD, of the University of Tennessee, Memphis, discussed her team’s work at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
A total of 244 patients with axSpA were identified, including 168 (69%) males, 78 (32%) Black patients, and 143 (59%) White patients.
Average age of onset for patients overall was 27.7 years, and age at diagnosis was 36.1 years with a 7-year delay in diagnosis. Sixty-six (27%) patients had nonradiographic axSpA, 83% were on tumor necrosis factor inhibitors, and 38% were prescribed glucocorticoids.
The researchers found several differences by race.
White patients were more likely to be HLA-B27 positive (77% vs. 59%; P = .010). White patients also had higher prevalence of psoriasis, coronary artery disease, and family history of SpA. White females had a higher prevalence of inflammatory bowel disease, fibromyalgia, depression, and lower grades of sacroiliitis.
Black patients had more hip involvement
A higher percentage of Black patients had elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hip involvement. In comparing hip involvement, the researchers found that 39 (39%) White males had hip involvement as did 9 (21%) White females. In comparison, 22 (45%) Black men in the study and 14 (54%) Black women showed hip involvement (P = .035).
After adjustment for age, sex, HLA-B27, and insurance status, Black patients had higher grades of sacroiliitis with an odds ratio of 2.32 (95% confidence interval, 1.23-4.44). Black patients had a numerically longer delay in diagnosis, compared with Whites (7.93 vs. 6.64 years), but this did not achieve statistical significance (P = .454), the researchers wrote.
Study addresses racial disparities
“Traditionally we think of axial spondyloarthritis largely in Caucasian males who are HLA-B27 positive,” Dr. Abutineh said, “and we found that there is still a significant portion of patients who don’t meet the criteria that do have disease that is very significant.”
Although actual prevalence was not clear from this study, Dr. Abutineh said their data suggest a 3-to-1 ratio of White-to-Black patients with spondyloarthritis, “but of the Black patients who are diagnosed, their disease is almost always more severe. That points to why it’s important to have a high index of suspicion for this disease in that patient population because if you miss it, it could be detrimental to the patients.”
Swetha Alexander, MD, a rheumatology fellow at the University of Utah, Salt Lake City, who was not part of the study, said in an interview, “It is an excellent and timely study addressing the racial disparities and inequities surrounding axSpA diagnosis. It highlights the delay in diagnosis and increased burden of disease among Black Americans.”
She said the study may prompt a further look into barriers to care for Black Americans and their beliefs regarding seeking health care for their pain.
Higher rates of nonradiographic axSpA among Black patients
The rate of nonradiographic axSpA among Black Americans was more than twice that of their White counterparts, which, Dr. Alexander noted, could make it more difficult to diagnose axSpA in that population.
The odds ratio for Black patients having nonradiographic axSpA, compared with Whites, was 2.265 (95% CI, 1.082-4.999; P = .035), after adjustment for age, sex, and HLA-B27 status.
Adult patients with axSpA were identified from rheumatology clinics at four major hospital systems and one private clinic in Shelby County, Tenn., between 2011 and 2021. Patients met modified New York (mNY) or Assessment of Spondyloarthritis International Society (ASAS) criteria as assessed by reviewers.
The authors and Dr. Alexander reported no relevant financial relationships.
AT SPARTAN 2023