User login
Teriflunomide delays MS symptoms in radiologically isolated syndrome
BOSTON – , according to a double-blind, phase 3 trial presented in the Emerging Science session of the 2023 annual meeting of the American Academy of Neurology.
“These data add to the evidence that early immunomodulation offers clinical benefit even in the presymptomatic phase of MS,” reported Christine Lebrun-Frenay, MD, PhD, head of inflammatory neurological disorders research unit, University of Nice, France. This is the second study to show a benefit from a disease-modifying therapy in asymptomatic RIS patients. The ARISE study, which was presented at the 2022 European Committee for Treatment and Research in MS and has now been published, compared 240 mg of twice-daily dimethyl fumarate with placebo. Dimethyl fumarate was associated with an 82% (hazard ratio, 0.18; P = .007) reduction in the risk of a first demyelinating event after 96 weeks of follow-up.
TERIS trial data
In the new study, called TERIS, the design and outcomes were similar to the ARISE study. Eighty-nine patients meeting standard criteria for RIS were randomized to 14 mg of once-daily teriflunomide or placebo. The majority (71%) were female, and the mean age was 39.8 years. At the time of RIS diagnosis, the mean age was 38 years. At study entry, standardized MRI studies were performed of the brain and spinal cord.
During 2 years of follow-up, 8 of 28 demyelinating events were observed in the active treatment group. The remaining 20 occurred in the placebo group. This translated to a 63% reduction (HR, 0.37; P = .018) in favor of teriflunomide. When graphed, the curves separated at about 6 months and then widened progressively over time.
Distinct from clinically isolated syndrome (CIS), which describes individuals who have a symptomatic episode consistent with a demyelinating event, RIS is based primarily on an MRI that shows lesions highly suggestive of MS. Neither confirms the MS diagnosis, but both are associated with a high likelihood of eventually meeting MS diagnostic criteria. The ARISE and TERIS studies now support therapy to delay demyelinating events.
“With more and more people having brain scans for various reasons, such as headache or head trauma, more of these cases are being discovered,” Dr. Lebrun-Frenay said.
Caution warranted when interpreting the findings
The data support the theory that treatment should begin early in patients with a high likelihood of developing symptomatic MS on the basis of brain lesions. It is logical to assume that preventing damage to the myelin will reduce or delay permanent symptoms and permanent neurologic impairment, but Dr. Lebrun-Frenay suggested that the available data from ARISE and TERIS are not practice changing even though both were multicenter double-blind trials.
“More data from larger groups of patients are needed to confirm the findings,” she said. She expressed concern about not adhering to strict criteria to diagnosis RIS.
“It is important that medical professionals are cautious,” she said, citing the risk of misdiagnosis of pathology of MRI that leads to treatment of patients with a low risk of developing symptomatic MS.
Teriflunomide and dimethyl fumarate, which have long been available as first-line therapies in relapsing-remitting MS, are generally well tolerated. In the TERIS and ARISE studies, mild or moderate events occurred more commonly in the active treatment than the placebo arms, but there were no serious adverse events. However, both can produce more serious adverse events, which, in the case of teriflunomide, include liver toxicity leading to injury and liver failure.
Challenging the traditional definition of MS
The author of the ARISE study, Darin T. Okuda, MD, a professor of neurology at the UT Southwestern Medical Center, Dallas, indicated that his study, now reinforced by the TERIS study, challenges the definition of MS.
“Both ARISE and TERIS demonstrated a significant reduction in seminal clinical event rates related to inflammatory demyelination,” Dr. Okuda said in an interview. They provide evidence that patients are at high risk of the demyelinating events that characterize MS. Given the potential difficulty for accessing therapies of benefit, “how we define multiple sclerosis is highly important.”
“Individuals of younger age with abnormal spinal cord MRI studies along with other paraclinical features related to risk for a first event may be the most ideal group to treat,” he said. However, he agreed with Dr. Lebrun-Frenay that it is not yet clear which RIS patients are the most appropriate candidates.
“Gaining a more refined sense of who we should treat will require more work,” he said.
These data are likely to change the orientation toward RIS, according to Melina Hosseiny, MD, department of radiology, University of California, Los Angeles, Medical Center. She noted that the relationship between RIS and increased risk of MS has long been recognized, and the risk increases with specific features on imaging.
“Studies have shown that spinal cord lesions are associated with a greater than 50% chance of converting to MS,” said Dr. Hosseiny, who was the lead author of a review article on RIS. “Identifying such imaging findings can help identify patients who may benefit from disease-modifying medications.”
Dr. Lebrun-Frenay reports no potential conflicts of interest. Dr. Okuda has financial relationships with Alexion, Biogen, Celgene, EMD Serono, Genzyme, TG Therapeutics, and VielaBio. Dr. Hosseiny reports no potential conflicts of interest.
BOSTON – , according to a double-blind, phase 3 trial presented in the Emerging Science session of the 2023 annual meeting of the American Academy of Neurology.
“These data add to the evidence that early immunomodulation offers clinical benefit even in the presymptomatic phase of MS,” reported Christine Lebrun-Frenay, MD, PhD, head of inflammatory neurological disorders research unit, University of Nice, France. This is the second study to show a benefit from a disease-modifying therapy in asymptomatic RIS patients. The ARISE study, which was presented at the 2022 European Committee for Treatment and Research in MS and has now been published, compared 240 mg of twice-daily dimethyl fumarate with placebo. Dimethyl fumarate was associated with an 82% (hazard ratio, 0.18; P = .007) reduction in the risk of a first demyelinating event after 96 weeks of follow-up.
TERIS trial data
In the new study, called TERIS, the design and outcomes were similar to the ARISE study. Eighty-nine patients meeting standard criteria for RIS were randomized to 14 mg of once-daily teriflunomide or placebo. The majority (71%) were female, and the mean age was 39.8 years. At the time of RIS diagnosis, the mean age was 38 years. At study entry, standardized MRI studies were performed of the brain and spinal cord.
During 2 years of follow-up, 8 of 28 demyelinating events were observed in the active treatment group. The remaining 20 occurred in the placebo group. This translated to a 63% reduction (HR, 0.37; P = .018) in favor of teriflunomide. When graphed, the curves separated at about 6 months and then widened progressively over time.
Distinct from clinically isolated syndrome (CIS), which describes individuals who have a symptomatic episode consistent with a demyelinating event, RIS is based primarily on an MRI that shows lesions highly suggestive of MS. Neither confirms the MS diagnosis, but both are associated with a high likelihood of eventually meeting MS diagnostic criteria. The ARISE and TERIS studies now support therapy to delay demyelinating events.
“With more and more people having brain scans for various reasons, such as headache or head trauma, more of these cases are being discovered,” Dr. Lebrun-Frenay said.
Caution warranted when interpreting the findings
The data support the theory that treatment should begin early in patients with a high likelihood of developing symptomatic MS on the basis of brain lesions. It is logical to assume that preventing damage to the myelin will reduce or delay permanent symptoms and permanent neurologic impairment, but Dr. Lebrun-Frenay suggested that the available data from ARISE and TERIS are not practice changing even though both were multicenter double-blind trials.
“More data from larger groups of patients are needed to confirm the findings,” she said. She expressed concern about not adhering to strict criteria to diagnosis RIS.
“It is important that medical professionals are cautious,” she said, citing the risk of misdiagnosis of pathology of MRI that leads to treatment of patients with a low risk of developing symptomatic MS.
Teriflunomide and dimethyl fumarate, which have long been available as first-line therapies in relapsing-remitting MS, are generally well tolerated. In the TERIS and ARISE studies, mild or moderate events occurred more commonly in the active treatment than the placebo arms, but there were no serious adverse events. However, both can produce more serious adverse events, which, in the case of teriflunomide, include liver toxicity leading to injury and liver failure.
Challenging the traditional definition of MS
The author of the ARISE study, Darin T. Okuda, MD, a professor of neurology at the UT Southwestern Medical Center, Dallas, indicated that his study, now reinforced by the TERIS study, challenges the definition of MS.
“Both ARISE and TERIS demonstrated a significant reduction in seminal clinical event rates related to inflammatory demyelination,” Dr. Okuda said in an interview. They provide evidence that patients are at high risk of the demyelinating events that characterize MS. Given the potential difficulty for accessing therapies of benefit, “how we define multiple sclerosis is highly important.”
“Individuals of younger age with abnormal spinal cord MRI studies along with other paraclinical features related to risk for a first event may be the most ideal group to treat,” he said. However, he agreed with Dr. Lebrun-Frenay that it is not yet clear which RIS patients are the most appropriate candidates.
“Gaining a more refined sense of who we should treat will require more work,” he said.
These data are likely to change the orientation toward RIS, according to Melina Hosseiny, MD, department of radiology, University of California, Los Angeles, Medical Center. She noted that the relationship between RIS and increased risk of MS has long been recognized, and the risk increases with specific features on imaging.
“Studies have shown that spinal cord lesions are associated with a greater than 50% chance of converting to MS,” said Dr. Hosseiny, who was the lead author of a review article on RIS. “Identifying such imaging findings can help identify patients who may benefit from disease-modifying medications.”
Dr. Lebrun-Frenay reports no potential conflicts of interest. Dr. Okuda has financial relationships with Alexion, Biogen, Celgene, EMD Serono, Genzyme, TG Therapeutics, and VielaBio. Dr. Hosseiny reports no potential conflicts of interest.
BOSTON – , according to a double-blind, phase 3 trial presented in the Emerging Science session of the 2023 annual meeting of the American Academy of Neurology.
“These data add to the evidence that early immunomodulation offers clinical benefit even in the presymptomatic phase of MS,” reported Christine Lebrun-Frenay, MD, PhD, head of inflammatory neurological disorders research unit, University of Nice, France. This is the second study to show a benefit from a disease-modifying therapy in asymptomatic RIS patients. The ARISE study, which was presented at the 2022 European Committee for Treatment and Research in MS and has now been published, compared 240 mg of twice-daily dimethyl fumarate with placebo. Dimethyl fumarate was associated with an 82% (hazard ratio, 0.18; P = .007) reduction in the risk of a first demyelinating event after 96 weeks of follow-up.
TERIS trial data
In the new study, called TERIS, the design and outcomes were similar to the ARISE study. Eighty-nine patients meeting standard criteria for RIS were randomized to 14 mg of once-daily teriflunomide or placebo. The majority (71%) were female, and the mean age was 39.8 years. At the time of RIS diagnosis, the mean age was 38 years. At study entry, standardized MRI studies were performed of the brain and spinal cord.
During 2 years of follow-up, 8 of 28 demyelinating events were observed in the active treatment group. The remaining 20 occurred in the placebo group. This translated to a 63% reduction (HR, 0.37; P = .018) in favor of teriflunomide. When graphed, the curves separated at about 6 months and then widened progressively over time.
Distinct from clinically isolated syndrome (CIS), which describes individuals who have a symptomatic episode consistent with a demyelinating event, RIS is based primarily on an MRI that shows lesions highly suggestive of MS. Neither confirms the MS diagnosis, but both are associated with a high likelihood of eventually meeting MS diagnostic criteria. The ARISE and TERIS studies now support therapy to delay demyelinating events.
“With more and more people having brain scans for various reasons, such as headache or head trauma, more of these cases are being discovered,” Dr. Lebrun-Frenay said.
Caution warranted when interpreting the findings
The data support the theory that treatment should begin early in patients with a high likelihood of developing symptomatic MS on the basis of brain lesions. It is logical to assume that preventing damage to the myelin will reduce or delay permanent symptoms and permanent neurologic impairment, but Dr. Lebrun-Frenay suggested that the available data from ARISE and TERIS are not practice changing even though both were multicenter double-blind trials.
“More data from larger groups of patients are needed to confirm the findings,” she said. She expressed concern about not adhering to strict criteria to diagnosis RIS.
“It is important that medical professionals are cautious,” she said, citing the risk of misdiagnosis of pathology of MRI that leads to treatment of patients with a low risk of developing symptomatic MS.
Teriflunomide and dimethyl fumarate, which have long been available as first-line therapies in relapsing-remitting MS, are generally well tolerated. In the TERIS and ARISE studies, mild or moderate events occurred more commonly in the active treatment than the placebo arms, but there were no serious adverse events. However, both can produce more serious adverse events, which, in the case of teriflunomide, include liver toxicity leading to injury and liver failure.
Challenging the traditional definition of MS
The author of the ARISE study, Darin T. Okuda, MD, a professor of neurology at the UT Southwestern Medical Center, Dallas, indicated that his study, now reinforced by the TERIS study, challenges the definition of MS.
“Both ARISE and TERIS demonstrated a significant reduction in seminal clinical event rates related to inflammatory demyelination,” Dr. Okuda said in an interview. They provide evidence that patients are at high risk of the demyelinating events that characterize MS. Given the potential difficulty for accessing therapies of benefit, “how we define multiple sclerosis is highly important.”
“Individuals of younger age with abnormal spinal cord MRI studies along with other paraclinical features related to risk for a first event may be the most ideal group to treat,” he said. However, he agreed with Dr. Lebrun-Frenay that it is not yet clear which RIS patients are the most appropriate candidates.
“Gaining a more refined sense of who we should treat will require more work,” he said.
These data are likely to change the orientation toward RIS, according to Melina Hosseiny, MD, department of radiology, University of California, Los Angeles, Medical Center. She noted that the relationship between RIS and increased risk of MS has long been recognized, and the risk increases with specific features on imaging.
“Studies have shown that spinal cord lesions are associated with a greater than 50% chance of converting to MS,” said Dr. Hosseiny, who was the lead author of a review article on RIS. “Identifying such imaging findings can help identify patients who may benefit from disease-modifying medications.”
Dr. Lebrun-Frenay reports no potential conflicts of interest. Dr. Okuda has financial relationships with Alexion, Biogen, Celgene, EMD Serono, Genzyme, TG Therapeutics, and VielaBio. Dr. Hosseiny reports no potential conflicts of interest.
AT AAN 2023
Miliarial Gout in an Immunocompromised Patient
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.
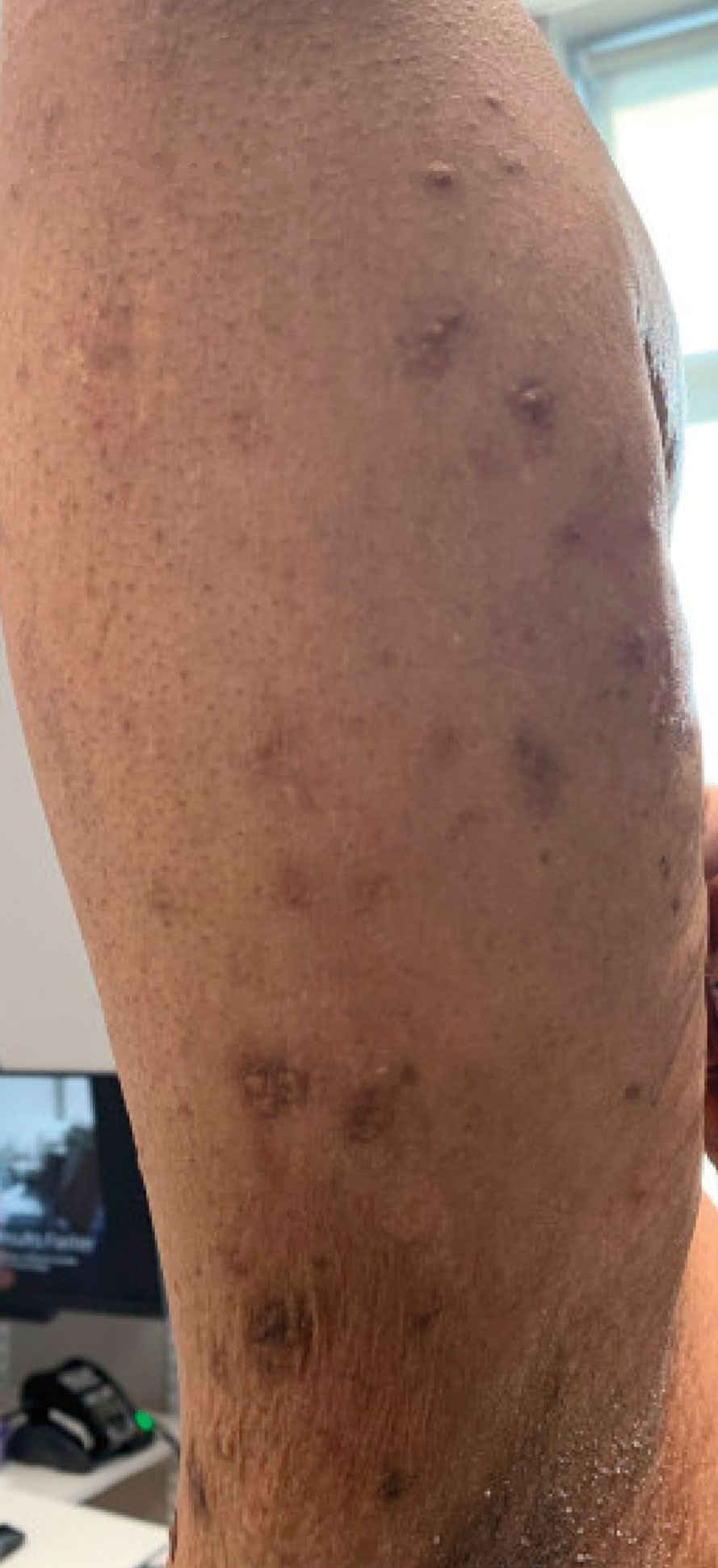
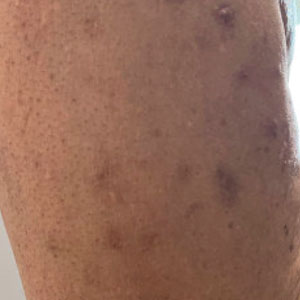
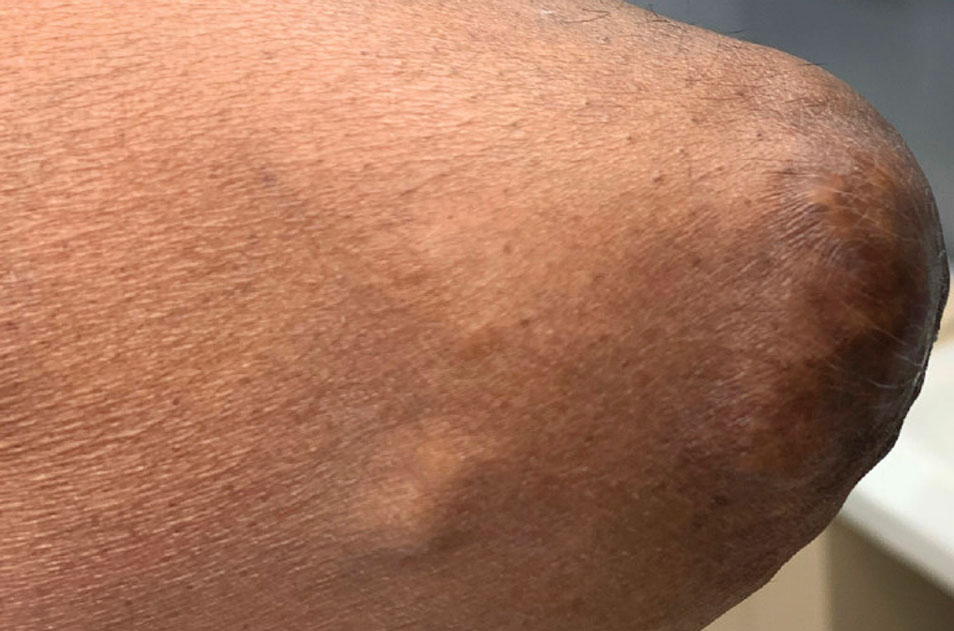
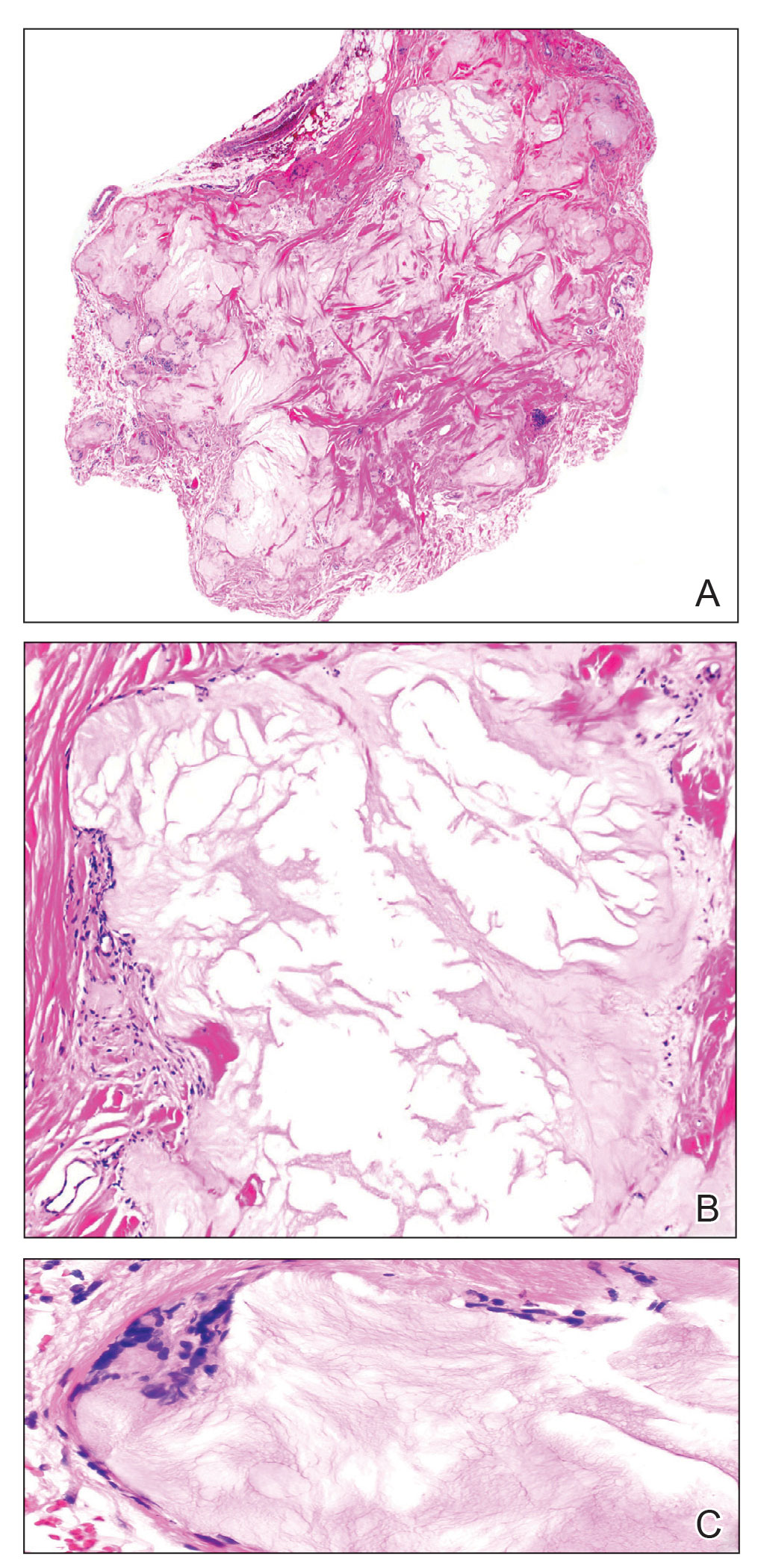
A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.
He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.
Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
To the Editor:
Miliarial gout is a rare intradermal manifestation of tophaceous gout. It was first described in 2007 when a patient presented with multiple small papules with a red base containing a white- to cream-colored substance,1 which has rarely been reported,1-6 according to a PubMed search of articles indexed for MEDLINE from 2007 to 2023 using the term miliarial gout. We describe a case of miliarial gout in a patient with a history of gout, uric acid levels within reference range, and immunocompromised status due to a prior orthotopic heart transplant.

A 59-year-old man presented with innumerable subcutaneous, firm, popcornlike clustered papules on the posterior surfaces of the upper arms and thighs of 5 years’ duration (Figure 1). The involved areas were sometimes painful on manipulation, but the patient was otherwise asymptomatic. His medical history was notable for tophaceous gout of more than 10 years’ duration, calcinosis cutis, adrenal insufficiency, essential hypertension, and an orthotopic heart transplant 2 years prior to the current presentation. At the current presentation he was taking tacrolimus, colchicine, febuxostat, and low-dose prednisone. The patient denied any other skin changes such as ulceration or bullae. In addition to the innumerable subcutaneous papules, he had much larger firm deep nodules bilaterally on the elbow (Figure 2). A complete blood cell count with differential and comprehensive metabolic panel results were within reference range. A 4-mm punch biopsy of the right posterior arm revealed dermal deposits consistent with gout on hematoxylin and eosin staining (Figure 3) but no calcium deposits on von Kossa staining, consistent with miliarial gout.

He was treated with 0.6 mg of colchicine daily, 80 mg of febuxostat twice daily, and 2.5 mg of prednisone daily. Unfortunately, the patient had difficulty affording his medications and therefore experienced frequent flares.

Gout is caused by inflammation that occurs from deposition of monosodium urate crystals in tissues, most commonly occurring in the skin and joints. Gout affects8.3 million individuals and is one of the most common rheumatic diseases of adulthood. The classic presentation of the acute form is monoarticular with associated swelling, erythema, and pain. The chronic form (also known as tophaceous gout) affects soft tissue and presents with smooth or multilobulated nodules.2 Miliarial gout is a rare variant of chronic tophaceous gout, and the diagnosis is based on atypical location, size, and distribution of tophi deposition.
In the updated American College of Rheumatology criteria for gout published in 2020, tophi are defined as draining or chalklike subcutaneous nodules that typically are located in joints, ears, olecranon bursae, finger pads, and tendons.3 The term miliarial gout, which is not universally defined, is used to describe the morphology and distribution of tophi deposition in areas outside of the typical locations defined by the American College of Rheumatology criteria. Miliarial refers to the small, multilobulated, and disseminated presentation of tophi. The involvement of atypical locations distinguishes miliarial gout from chronic tophaceous gout.
The cause of tophi deposition in atypical locations is unknown. It is thought that patients with a history of sustained hyperuricemia have a much greater burden of urate crystal deposition, which can lead to involvement of atypical locations. Our patient had innumerable, discrete, 1- to 5-mm, multilobulated tophi located on the posterior upper arms and thighs even though his uric acid levels were within reference range over the last 5 years.
Miliarial gout is a rare entity.1 In 2007, Shukla et al1 coined the term miliarial gout when reporting the first known presentation of a patient with multiple tiny papules containing a white or creamlike substance scattered on an erythematous base. Other cases of miliarial gout have commonly involved the metacarpophalangeal joints of the hands, knees, abdomen, extensor forearms, and thighs.5 Similarly, our patient had disease involvement of the posterior upper arms and thighs. Furthermore, miliarial gout has been associated with carpal tunnel syndrome; monosodium urate crystal deposition in this space can lead to a clinical diagnosis of this condition.6
With a history of orthotopic heart transplant, it is possible that our patient’s immunocompromised status could have increased his susceptibility for the miliarial form of chronic tophaceous gout. Gout reportedly is the most common inflammatory arthritis in transplant recipients, with the highest prevalence following renal and heart transplantation.7 Pretransplant hyperuricemia is correlated with higher probabilities of posttransplant gout.8 In patients with a heart transplant, hyperuricemia may be due to diuretic use. Additionally, the presence of a gout diagnosis before transplant nearly triples the likelihood of posttransplant gout, which often is more severe than de novo gout, as seen in our patient. Calcineurin inhibitors, including tacrolimus, also can predispose patients to hyperuricemia and more severe forms of gout in the posttransplant phase by limiting fractional urate excretion within the first 3 months of therapy.7 Treatment with oral steroids, as in our patient, also has been identified as a potential inciting factor for the development of cutaneous tophaceous gout.9
Treatment with allopurinol and colchicine has been effective in patients with miliarial gout. Obesity and long-term treatment with furosemide (which our patient was not taking) are considered risk factors for the deposition of dermal and hypodermal urates.9 Our patient had a body mass index of 35 (≥30 indicates obesity); therefore, he also should be counseled on lifestyle modifications for optimal disease control.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
- Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity). J Cutan Med Surg. 2007;11:31-34.
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum. 2011;63:3136-3141.
- Neogi T, Jansen, TL, Dalbeth N, et al. 2015 gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
- Hung TL, Wang WM, Chiang CP. Miliarial gout: a rare presentation of extensive cutaneous tophi. QJM. 2016;109:811-812.
- Mireku KA, Burgy JR, Davis LS. Miliarial gout: a rare clinical presentation. J Am Acad Dermatol. 2014;71:E17-E18.
- Sadovici-Bobeica V, Mazur-Nicorici L, Nicorici A, et al. Chronic miliarial gout associated with carpal tunnel syndrome: a very rare clinical presentation. Eur J Case Rep Intern Med. 2018;5:000926.
- Schwab P, Lipton S, Kerr GS. Rheumatologic sequelae and challenges in organ transplantation. Best Pract Res Clin Rheumatol. 2010;24:329-340.
- Hernández-Molina G, Cachafeiro-Vilar A, Villa AR, et al. Gout in renal allograft recipients according to the pretransplant hyperuricemic status. Transplantation. 2008;86:1543-1547.
- Aguayo RS, Baradad M, Soria X, et al. Unilateral milia‐type intradermal tophi associated with underlying urate subcutaneous deposition: an uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38:622-625.
Practice Points
- Miliarial gout is a rare intradermal manifestation of tophaceous gout and often presents as multiple small papules containing a white- to cream-colored substance.
- Immunocompromised status may be a risk factor for miliarial gout, especially in patients with a history of gout or hyperuricemia.
- Effective treatments for miliarial gout include allopurinol and colchicine.
Adherence to cancer prevention guidance linked with reduced breast cancer recurrence, death risk
Following such recommendations surrounding smoking, physical activity (PA), eating fruits and vegetables and reducing or eliminating sugar-sweetened beverages seemed to be the most beneficial, wrote the authors of the paper published online in JAMA Network Open.
Rikki A. Cannioto, PhD, EdD, with the department of cancer prevention & control, Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., led the prospective cohort study of 1,340 patients.
The American Institute for Cancer Research and American Cancer Society regularly recommend and publish lifestyle modifications for cancer prevention. To conduct this study Dr. Cannioto and colleagues developed an aggregate lifestyle scoring index to investigate whether those recommendations have an effect on high-risk breast cancer survival.
Highest adherence vs. lowest cut death risk by more than half
The researchers found patients with highest vs. lowest lifestyle index scores saw a 37% reduction in cancer recurrence (hazard ratio, 0.63; 95% confidence interval, 0.48-0.82) and a 58% reduction in mortality (HR, 0.42; 95% CI, 0.30-0.59).
“As a person who has based her career on the belief that our modifiable lifestyle behaviors are associated with cancer survival, I was actually surprised about how strong these associations were, especially for breast cancer recurrence,” Dr. Cannioto said in an interview,
The author also expressed surprise about the associations that were seen “in patients diagnosed with triple-negative breast cancer and HER2-positive breast cancer, which are the two subtypes traditionally more aggressive and more difficult to treat.”
Most patients in the study were diagnosed with hormone receptor–positive breast cancer (873 [65.3%]); completed some education beyond high school (954 [71.2%]); were postmenopausal (696 [52.5%]); and self-identified as non-Hispanic White (1,118 [83.7%]).
Patients were drawn from the Diet, Exercise, Lifestyles, and Cancer Prognosis (DELCaP) study, a prospective, observational cohort study ancillary to a multicenter phase 3 trial led by the Southwest Oncology Group (SWOG). The DELCaP study was designed to examine lifestyles before diagnosis, during treatment, and at 1 and 2 years after treatment.
Never smoking, physical activity had strongest links
Never smoking and meeting or exceeding PA guidelines had the strongest and most consistent associations with outcomes; each factor was linked with a 44%-45% reduced risk of mortality and a 35% reduced risk of recurrence.
Strongest adherence to the alcohol and body mass index (BMI) recommendations were not significantly associated with improved outcomes.
Partial and full adherence to red and processed meat recommendations were associated with significant reductions in mortality, but not recurrence.
The authors note that, while medications are the foundation for breast cancer treatment, lifestyle interventions could be a safe and inexpensive additional strategy for delaying and preventing recurrence and death.
“Such developments could be especially impactful for patients diagnosed with more aggressive tumors that do not respond well to current therapies,” they write.
Dr. Cannioto says the guidelines around physical activity advise 150 minutes or more of moderate to vigorous intensity a week. But she noted that this research shows that any physical activity can lead to longer survival.
“The greatest benefits from physical activity occur from moving from a sedentary lifestyle to beginning to be active,” she said.
Dr. Cannioto acknowledged the homogeneity of the study population as a limitation and recommended the associations next be tested in a more racially and ethnically diverse population of breast cancer patients.
This work was supported by the National Cancer Institute, the Breast Cancer Research Foundation, and Amgen.
The authors report receiving grants from the Southwest Oncology Group and the National Cancer Institute during the conduct of the study and receiving personal fees, grants, or serving on the boards or independent monitoring committees of many pharmaceutical companies. A full list of disclosures is available with the paper.
Following such recommendations surrounding smoking, physical activity (PA), eating fruits and vegetables and reducing or eliminating sugar-sweetened beverages seemed to be the most beneficial, wrote the authors of the paper published online in JAMA Network Open.
Rikki A. Cannioto, PhD, EdD, with the department of cancer prevention & control, Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., led the prospective cohort study of 1,340 patients.
The American Institute for Cancer Research and American Cancer Society regularly recommend and publish lifestyle modifications for cancer prevention. To conduct this study Dr. Cannioto and colleagues developed an aggregate lifestyle scoring index to investigate whether those recommendations have an effect on high-risk breast cancer survival.
Highest adherence vs. lowest cut death risk by more than half
The researchers found patients with highest vs. lowest lifestyle index scores saw a 37% reduction in cancer recurrence (hazard ratio, 0.63; 95% confidence interval, 0.48-0.82) and a 58% reduction in mortality (HR, 0.42; 95% CI, 0.30-0.59).
“As a person who has based her career on the belief that our modifiable lifestyle behaviors are associated with cancer survival, I was actually surprised about how strong these associations were, especially for breast cancer recurrence,” Dr. Cannioto said in an interview,
The author also expressed surprise about the associations that were seen “in patients diagnosed with triple-negative breast cancer and HER2-positive breast cancer, which are the two subtypes traditionally more aggressive and more difficult to treat.”
Most patients in the study were diagnosed with hormone receptor–positive breast cancer (873 [65.3%]); completed some education beyond high school (954 [71.2%]); were postmenopausal (696 [52.5%]); and self-identified as non-Hispanic White (1,118 [83.7%]).
Patients were drawn from the Diet, Exercise, Lifestyles, and Cancer Prognosis (DELCaP) study, a prospective, observational cohort study ancillary to a multicenter phase 3 trial led by the Southwest Oncology Group (SWOG). The DELCaP study was designed to examine lifestyles before diagnosis, during treatment, and at 1 and 2 years after treatment.
Never smoking, physical activity had strongest links
Never smoking and meeting or exceeding PA guidelines had the strongest and most consistent associations with outcomes; each factor was linked with a 44%-45% reduced risk of mortality and a 35% reduced risk of recurrence.
Strongest adherence to the alcohol and body mass index (BMI) recommendations were not significantly associated with improved outcomes.
Partial and full adherence to red and processed meat recommendations were associated with significant reductions in mortality, but not recurrence.
The authors note that, while medications are the foundation for breast cancer treatment, lifestyle interventions could be a safe and inexpensive additional strategy for delaying and preventing recurrence and death.
“Such developments could be especially impactful for patients diagnosed with more aggressive tumors that do not respond well to current therapies,” they write.
Dr. Cannioto says the guidelines around physical activity advise 150 minutes or more of moderate to vigorous intensity a week. But she noted that this research shows that any physical activity can lead to longer survival.
“The greatest benefits from physical activity occur from moving from a sedentary lifestyle to beginning to be active,” she said.
Dr. Cannioto acknowledged the homogeneity of the study population as a limitation and recommended the associations next be tested in a more racially and ethnically diverse population of breast cancer patients.
This work was supported by the National Cancer Institute, the Breast Cancer Research Foundation, and Amgen.
The authors report receiving grants from the Southwest Oncology Group and the National Cancer Institute during the conduct of the study and receiving personal fees, grants, or serving on the boards or independent monitoring committees of many pharmaceutical companies. A full list of disclosures is available with the paper.
Following such recommendations surrounding smoking, physical activity (PA), eating fruits and vegetables and reducing or eliminating sugar-sweetened beverages seemed to be the most beneficial, wrote the authors of the paper published online in JAMA Network Open.
Rikki A. Cannioto, PhD, EdD, with the department of cancer prevention & control, Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., led the prospective cohort study of 1,340 patients.
The American Institute for Cancer Research and American Cancer Society regularly recommend and publish lifestyle modifications for cancer prevention. To conduct this study Dr. Cannioto and colleagues developed an aggregate lifestyle scoring index to investigate whether those recommendations have an effect on high-risk breast cancer survival.
Highest adherence vs. lowest cut death risk by more than half
The researchers found patients with highest vs. lowest lifestyle index scores saw a 37% reduction in cancer recurrence (hazard ratio, 0.63; 95% confidence interval, 0.48-0.82) and a 58% reduction in mortality (HR, 0.42; 95% CI, 0.30-0.59).
“As a person who has based her career on the belief that our modifiable lifestyle behaviors are associated with cancer survival, I was actually surprised about how strong these associations were, especially for breast cancer recurrence,” Dr. Cannioto said in an interview,
The author also expressed surprise about the associations that were seen “in patients diagnosed with triple-negative breast cancer and HER2-positive breast cancer, which are the two subtypes traditionally more aggressive and more difficult to treat.”
Most patients in the study were diagnosed with hormone receptor–positive breast cancer (873 [65.3%]); completed some education beyond high school (954 [71.2%]); were postmenopausal (696 [52.5%]); and self-identified as non-Hispanic White (1,118 [83.7%]).
Patients were drawn from the Diet, Exercise, Lifestyles, and Cancer Prognosis (DELCaP) study, a prospective, observational cohort study ancillary to a multicenter phase 3 trial led by the Southwest Oncology Group (SWOG). The DELCaP study was designed to examine lifestyles before diagnosis, during treatment, and at 1 and 2 years after treatment.
Never smoking, physical activity had strongest links
Never smoking and meeting or exceeding PA guidelines had the strongest and most consistent associations with outcomes; each factor was linked with a 44%-45% reduced risk of mortality and a 35% reduced risk of recurrence.
Strongest adherence to the alcohol and body mass index (BMI) recommendations were not significantly associated with improved outcomes.
Partial and full adherence to red and processed meat recommendations were associated with significant reductions in mortality, but not recurrence.
The authors note that, while medications are the foundation for breast cancer treatment, lifestyle interventions could be a safe and inexpensive additional strategy for delaying and preventing recurrence and death.
“Such developments could be especially impactful for patients diagnosed with more aggressive tumors that do not respond well to current therapies,” they write.
Dr. Cannioto says the guidelines around physical activity advise 150 minutes or more of moderate to vigorous intensity a week. But she noted that this research shows that any physical activity can lead to longer survival.
“The greatest benefits from physical activity occur from moving from a sedentary lifestyle to beginning to be active,” she said.
Dr. Cannioto acknowledged the homogeneity of the study population as a limitation and recommended the associations next be tested in a more racially and ethnically diverse population of breast cancer patients.
This work was supported by the National Cancer Institute, the Breast Cancer Research Foundation, and Amgen.
The authors report receiving grants from the Southwest Oncology Group and the National Cancer Institute during the conduct of the study and receiving personal fees, grants, or serving on the boards or independent monitoring committees of many pharmaceutical companies. A full list of disclosures is available with the paper.
FROM JAMA NETWORK OPEN
COVID-19 and psoriasis: Is there a link?
.
Psoriasis has several well-established triggers, including stress, skin injury, cold or warm air, and allergies. Illnesses like strep throat can also cause a psoriasis flare in some people – and it appears COVID may also do so. “Psoriasis flares have long been associated with bacterial and viral infections, particularly a form of psoriasis called guttate, which is characterized by tons of tiny red scaly bumps all over the body,” said Joel M. Gelfand, MD, a professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. “Infection with COVID-19 has been associated with flares of guttate and pustular psoriasis, and even psoriasis that affects 100% of the skin ... in many published case reports.”
Israeli researchers recently found that psoriasis patients have a slightly higher risk of getting COVID, although they are not at higher risk of hospitalization or death, which could be related to treatment with immune-modulating therapy, which can increase their risk of infections.
How could COVID cause psoriasis to flare?
Psoriasis is an autoimmune condition, and inflammation can cause symptoms.
Investigators for a study from Albany (N.Y.) Medical College and Weirton (Pa.) Medical Center found that people in the study who were already diagnosed with the skin condition had an unexpected flare within a week to a month after testing positive for COVID. New psoriasis after a COVID infection was also found. The researchers think this could be because COVID causes inflammation in the body, which negatively affects previously well-controlled psoriasis. They also think it’s possible that COVID-related inflammation could trigger a genetic tendency to have psoriasis, which may explain why it can appear for the first time after a positive test.
“A viral infection like COVID-19 can signal the release of proinflammatory factors that can appear as rashes, such as with psoriasis.” said Robert O. Carpenter, MD, director of wellness at Texas A&M University in Bryan.
What are the symptoms of COVID-related psoriasis?
The signs are the same as those of any form of psoriasis.
For a patient with psoriasis, will COVID automatically make it worse?
Not necessarily.
“Psoriasis is a common condition, so people should be aware that new psoriasis that develops may not be related to COVID-19,” said Esther Freeman MD, PhD, director of global health dermatology at Massachusetts General Hospital in Boston.
As with every aspect of COVID, doctors and scientists are still learning about how serious and widespread a problem psoriasis after COVID-19 may be. “We have seen case reports that psoriasis can flare after COVID-19,” said Dr. Freeman, who is also an associate professor of dermatology at Harvard Medical School. “I will say, this has not been a tidal wave – more like sporadic cases here and there. So I do not think psoriasis flares are a major post-COVID finding, nor do they necessarily mean you have long COVID. That being said, we know that many different infections can cause psoriasis flares, and so, in that respect, it’s not that surprising that SARS-CoV-2, like other infections, could trigger a psoriasis flare.”
Could getting COVID more than once cause psoriasis to flare? It’s possible.
“Your body can change after having COVID-19,” said Dr. Carpenter. “We don’t know the long-term implications, but having COVID-19 repeatedly can increase the risk of long COVID, which can cause many systemic changes in your body.”
Another important point: For patients who take biologics for treating psoriasis, getting vaccinated and boosted for COVID is an important step to take to help protect themselves.
Is psoriasis itself a potential symptom of COVID?
“Yes, but we don’t know the frequency at which this may occur, and a causal relationship is difficult to establish from just case reports,” said Dr. Gelfand, who’s also medical director of the clinical studies unit in the department of dermatology at his university. “Typically, if a patient presents with a flare of psoriasis, particularly guttate, pustular, or erythrodermic forms, an infectious trigger should be considered, and testing for strep and possibly COVID-19 may be appropriate.”
A version of this article first appeared on Medscape.com.
.
Psoriasis has several well-established triggers, including stress, skin injury, cold or warm air, and allergies. Illnesses like strep throat can also cause a psoriasis flare in some people – and it appears COVID may also do so. “Psoriasis flares have long been associated with bacterial and viral infections, particularly a form of psoriasis called guttate, which is characterized by tons of tiny red scaly bumps all over the body,” said Joel M. Gelfand, MD, a professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. “Infection with COVID-19 has been associated with flares of guttate and pustular psoriasis, and even psoriasis that affects 100% of the skin ... in many published case reports.”
Israeli researchers recently found that psoriasis patients have a slightly higher risk of getting COVID, although they are not at higher risk of hospitalization or death, which could be related to treatment with immune-modulating therapy, which can increase their risk of infections.
How could COVID cause psoriasis to flare?
Psoriasis is an autoimmune condition, and inflammation can cause symptoms.
Investigators for a study from Albany (N.Y.) Medical College and Weirton (Pa.) Medical Center found that people in the study who were already diagnosed with the skin condition had an unexpected flare within a week to a month after testing positive for COVID. New psoriasis after a COVID infection was also found. The researchers think this could be because COVID causes inflammation in the body, which negatively affects previously well-controlled psoriasis. They also think it’s possible that COVID-related inflammation could trigger a genetic tendency to have psoriasis, which may explain why it can appear for the first time after a positive test.
“A viral infection like COVID-19 can signal the release of proinflammatory factors that can appear as rashes, such as with psoriasis.” said Robert O. Carpenter, MD, director of wellness at Texas A&M University in Bryan.
What are the symptoms of COVID-related psoriasis?
The signs are the same as those of any form of psoriasis.
For a patient with psoriasis, will COVID automatically make it worse?
Not necessarily.
“Psoriasis is a common condition, so people should be aware that new psoriasis that develops may not be related to COVID-19,” said Esther Freeman MD, PhD, director of global health dermatology at Massachusetts General Hospital in Boston.
As with every aspect of COVID, doctors and scientists are still learning about how serious and widespread a problem psoriasis after COVID-19 may be. “We have seen case reports that psoriasis can flare after COVID-19,” said Dr. Freeman, who is also an associate professor of dermatology at Harvard Medical School. “I will say, this has not been a tidal wave – more like sporadic cases here and there. So I do not think psoriasis flares are a major post-COVID finding, nor do they necessarily mean you have long COVID. That being said, we know that many different infections can cause psoriasis flares, and so, in that respect, it’s not that surprising that SARS-CoV-2, like other infections, could trigger a psoriasis flare.”
Could getting COVID more than once cause psoriasis to flare? It’s possible.
“Your body can change after having COVID-19,” said Dr. Carpenter. “We don’t know the long-term implications, but having COVID-19 repeatedly can increase the risk of long COVID, which can cause many systemic changes in your body.”
Another important point: For patients who take biologics for treating psoriasis, getting vaccinated and boosted for COVID is an important step to take to help protect themselves.
Is psoriasis itself a potential symptom of COVID?
“Yes, but we don’t know the frequency at which this may occur, and a causal relationship is difficult to establish from just case reports,” said Dr. Gelfand, who’s also medical director of the clinical studies unit in the department of dermatology at his university. “Typically, if a patient presents with a flare of psoriasis, particularly guttate, pustular, or erythrodermic forms, an infectious trigger should be considered, and testing for strep and possibly COVID-19 may be appropriate.”
A version of this article first appeared on Medscape.com.
.
Psoriasis has several well-established triggers, including stress, skin injury, cold or warm air, and allergies. Illnesses like strep throat can also cause a psoriasis flare in some people – and it appears COVID may also do so. “Psoriasis flares have long been associated with bacterial and viral infections, particularly a form of psoriasis called guttate, which is characterized by tons of tiny red scaly bumps all over the body,” said Joel M. Gelfand, MD, a professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia. “Infection with COVID-19 has been associated with flares of guttate and pustular psoriasis, and even psoriasis that affects 100% of the skin ... in many published case reports.”
Israeli researchers recently found that psoriasis patients have a slightly higher risk of getting COVID, although they are not at higher risk of hospitalization or death, which could be related to treatment with immune-modulating therapy, which can increase their risk of infections.
How could COVID cause psoriasis to flare?
Psoriasis is an autoimmune condition, and inflammation can cause symptoms.
Investigators for a study from Albany (N.Y.) Medical College and Weirton (Pa.) Medical Center found that people in the study who were already diagnosed with the skin condition had an unexpected flare within a week to a month after testing positive for COVID. New psoriasis after a COVID infection was also found. The researchers think this could be because COVID causes inflammation in the body, which negatively affects previously well-controlled psoriasis. They also think it’s possible that COVID-related inflammation could trigger a genetic tendency to have psoriasis, which may explain why it can appear for the first time after a positive test.
“A viral infection like COVID-19 can signal the release of proinflammatory factors that can appear as rashes, such as with psoriasis.” said Robert O. Carpenter, MD, director of wellness at Texas A&M University in Bryan.
What are the symptoms of COVID-related psoriasis?
The signs are the same as those of any form of psoriasis.
For a patient with psoriasis, will COVID automatically make it worse?
Not necessarily.
“Psoriasis is a common condition, so people should be aware that new psoriasis that develops may not be related to COVID-19,” said Esther Freeman MD, PhD, director of global health dermatology at Massachusetts General Hospital in Boston.
As with every aspect of COVID, doctors and scientists are still learning about how serious and widespread a problem psoriasis after COVID-19 may be. “We have seen case reports that psoriasis can flare after COVID-19,” said Dr. Freeman, who is also an associate professor of dermatology at Harvard Medical School. “I will say, this has not been a tidal wave – more like sporadic cases here and there. So I do not think psoriasis flares are a major post-COVID finding, nor do they necessarily mean you have long COVID. That being said, we know that many different infections can cause psoriasis flares, and so, in that respect, it’s not that surprising that SARS-CoV-2, like other infections, could trigger a psoriasis flare.”
Could getting COVID more than once cause psoriasis to flare? It’s possible.
“Your body can change after having COVID-19,” said Dr. Carpenter. “We don’t know the long-term implications, but having COVID-19 repeatedly can increase the risk of long COVID, which can cause many systemic changes in your body.”
Another important point: For patients who take biologics for treating psoriasis, getting vaccinated and boosted for COVID is an important step to take to help protect themselves.
Is psoriasis itself a potential symptom of COVID?
“Yes, but we don’t know the frequency at which this may occur, and a causal relationship is difficult to establish from just case reports,” said Dr. Gelfand, who’s also medical director of the clinical studies unit in the department of dermatology at his university. “Typically, if a patient presents with a flare of psoriasis, particularly guttate, pustular, or erythrodermic forms, an infectious trigger should be considered, and testing for strep and possibly COVID-19 may be appropriate.”
A version of this article first appeared on Medscape.com.
Plasma monitoring supports earlier osimertinib treatment in lung cancer patients
Previous studies have suggested that molecular progression of disease in patients with EGFR-mutant NSCLC, as measured by sequential plasma EGFR T790M, may precede radiological progression, as measured by Response Evaluation Criteria in Solid Tumors (RECIST).
However, the impact of these measures on timing of treatment changes and patient outcomes has not been examined, wrote Jordi Remon, MD, of Paris (France)–Saclay University and colleagues, in Annals of Oncology.
The European Organization for Research Treatment and Cancer Lung Cancer Group designed a phase 2 clinical trial known as APPLE to evaluate the use of sequential plasma EGFR T790M and determine the optimal sequencing for gefitinib and osimertinib in patients with EGFR-mutant NSCLC.
The researchers reported results from two randomized arms of the APPLE trial. In arm B, 52 patients received gefitinib until emergence of circulating tumor DNA (ctDNA) EGFR T790M mutation, based on the cobas EGFR test v2 (a real-time PCR test), or progression of disease based on Response Evaluation Criteria in Solid Tumors (RECIST). In arm C, 51 patients received gefitinib until disease progression based on RECIST. Both arms then switched to osimertinib. Patients randomized to a third arm (arm A) received osimertinib upfront until progression of disease based on RECIST, and they were not included in the current study.
The primary endpoint was progression-free survival (PFS) while receiving osimertinib at 18 months in patients who were originally randomized to gefitinib, then switched to osimertinib at the emergence of circulating tumor DNA. Secondary endpoints included PFS, overall response rate, overall survival, and brain PFS.
Patients entered the study between November 2017 and February 2020. A total of 75% and 65% of those in arms B and C, respectively, were female, approximately 65% had the mutation EGFR Del19, and approximately one-third had baseline brain metastases. In arm B, 17% of patients switched to osimertinib based on the emergence of ctDNA T790M mutation before progressive disease based on RECIST. The median time to molecular disease progression was 266 days.
More patients in arm B met the primary endpoint of PFS while receiving osimertinib at 18 months (67.2%) than in arm C (53.5%), after a median follow-up of 30 months.
As for secondary endpoints, the median PFS in the two arms was 22.0 months and 20.2 months, respectively. Median overall survival was 42.8 months in arm C and was not reached in arm B. The median brain PFS was 24.4 months for arm B and 21.4 months for arm C.
The benefits seen in the osimertinib patients may be due in part to the timing of the switch to correspond with molecular or radiological disease progression, the researchers wrote in their discussion.
In the future, more research is needed to determine whether molecular monitoring may impact patients’ outcomes, compared with monitoring based on radiological progression, they said.
The findings were limited by several factors, mainly the rapid evolution in the treatment landscape of EGFR-mutant NSCLC, the researchers noted.
Osimertinib is currently considered the preferred first-line treatment by most physicians, they said. “The APPLE trial is the first prospective study supporting the role of dynamic adaptive strategies based on ctDNA monitoring in patients with EGFR-mutant advanced NSCLC.”
The study was supported by AstraZeneca. Lead author Dr. Remon had no financial conflicts to disclose. Corresponding author Dr. Dziadziuszko disclosed honoraria for consultancy or lectures from AstraZeneca, Roche, Novartis, MSD, Takeda, Pfizer, Amgen, and Bristol-Myers Squibb.
Previous studies have suggested that molecular progression of disease in patients with EGFR-mutant NSCLC, as measured by sequential plasma EGFR T790M, may precede radiological progression, as measured by Response Evaluation Criteria in Solid Tumors (RECIST).
However, the impact of these measures on timing of treatment changes and patient outcomes has not been examined, wrote Jordi Remon, MD, of Paris (France)–Saclay University and colleagues, in Annals of Oncology.
The European Organization for Research Treatment and Cancer Lung Cancer Group designed a phase 2 clinical trial known as APPLE to evaluate the use of sequential plasma EGFR T790M and determine the optimal sequencing for gefitinib and osimertinib in patients with EGFR-mutant NSCLC.
The researchers reported results from two randomized arms of the APPLE trial. In arm B, 52 patients received gefitinib until emergence of circulating tumor DNA (ctDNA) EGFR T790M mutation, based on the cobas EGFR test v2 (a real-time PCR test), or progression of disease based on Response Evaluation Criteria in Solid Tumors (RECIST). In arm C, 51 patients received gefitinib until disease progression based on RECIST. Both arms then switched to osimertinib. Patients randomized to a third arm (arm A) received osimertinib upfront until progression of disease based on RECIST, and they were not included in the current study.
The primary endpoint was progression-free survival (PFS) while receiving osimertinib at 18 months in patients who were originally randomized to gefitinib, then switched to osimertinib at the emergence of circulating tumor DNA. Secondary endpoints included PFS, overall response rate, overall survival, and brain PFS.
Patients entered the study between November 2017 and February 2020. A total of 75% and 65% of those in arms B and C, respectively, were female, approximately 65% had the mutation EGFR Del19, and approximately one-third had baseline brain metastases. In arm B, 17% of patients switched to osimertinib based on the emergence of ctDNA T790M mutation before progressive disease based on RECIST. The median time to molecular disease progression was 266 days.
More patients in arm B met the primary endpoint of PFS while receiving osimertinib at 18 months (67.2%) than in arm C (53.5%), after a median follow-up of 30 months.
As for secondary endpoints, the median PFS in the two arms was 22.0 months and 20.2 months, respectively. Median overall survival was 42.8 months in arm C and was not reached in arm B. The median brain PFS was 24.4 months for arm B and 21.4 months for arm C.
The benefits seen in the osimertinib patients may be due in part to the timing of the switch to correspond with molecular or radiological disease progression, the researchers wrote in their discussion.
In the future, more research is needed to determine whether molecular monitoring may impact patients’ outcomes, compared with monitoring based on radiological progression, they said.
The findings were limited by several factors, mainly the rapid evolution in the treatment landscape of EGFR-mutant NSCLC, the researchers noted.
Osimertinib is currently considered the preferred first-line treatment by most physicians, they said. “The APPLE trial is the first prospective study supporting the role of dynamic adaptive strategies based on ctDNA monitoring in patients with EGFR-mutant advanced NSCLC.”
The study was supported by AstraZeneca. Lead author Dr. Remon had no financial conflicts to disclose. Corresponding author Dr. Dziadziuszko disclosed honoraria for consultancy or lectures from AstraZeneca, Roche, Novartis, MSD, Takeda, Pfizer, Amgen, and Bristol-Myers Squibb.
Previous studies have suggested that molecular progression of disease in patients with EGFR-mutant NSCLC, as measured by sequential plasma EGFR T790M, may precede radiological progression, as measured by Response Evaluation Criteria in Solid Tumors (RECIST).
However, the impact of these measures on timing of treatment changes and patient outcomes has not been examined, wrote Jordi Remon, MD, of Paris (France)–Saclay University and colleagues, in Annals of Oncology.
The European Organization for Research Treatment and Cancer Lung Cancer Group designed a phase 2 clinical trial known as APPLE to evaluate the use of sequential plasma EGFR T790M and determine the optimal sequencing for gefitinib and osimertinib in patients with EGFR-mutant NSCLC.
The researchers reported results from two randomized arms of the APPLE trial. In arm B, 52 patients received gefitinib until emergence of circulating tumor DNA (ctDNA) EGFR T790M mutation, based on the cobas EGFR test v2 (a real-time PCR test), or progression of disease based on Response Evaluation Criteria in Solid Tumors (RECIST). In arm C, 51 patients received gefitinib until disease progression based on RECIST. Both arms then switched to osimertinib. Patients randomized to a third arm (arm A) received osimertinib upfront until progression of disease based on RECIST, and they were not included in the current study.
The primary endpoint was progression-free survival (PFS) while receiving osimertinib at 18 months in patients who were originally randomized to gefitinib, then switched to osimertinib at the emergence of circulating tumor DNA. Secondary endpoints included PFS, overall response rate, overall survival, and brain PFS.
Patients entered the study between November 2017 and February 2020. A total of 75% and 65% of those in arms B and C, respectively, were female, approximately 65% had the mutation EGFR Del19, and approximately one-third had baseline brain metastases. In arm B, 17% of patients switched to osimertinib based on the emergence of ctDNA T790M mutation before progressive disease based on RECIST. The median time to molecular disease progression was 266 days.
More patients in arm B met the primary endpoint of PFS while receiving osimertinib at 18 months (67.2%) than in arm C (53.5%), after a median follow-up of 30 months.
As for secondary endpoints, the median PFS in the two arms was 22.0 months and 20.2 months, respectively. Median overall survival was 42.8 months in arm C and was not reached in arm B. The median brain PFS was 24.4 months for arm B and 21.4 months for arm C.
The benefits seen in the osimertinib patients may be due in part to the timing of the switch to correspond with molecular or radiological disease progression, the researchers wrote in their discussion.
In the future, more research is needed to determine whether molecular monitoring may impact patients’ outcomes, compared with monitoring based on radiological progression, they said.
The findings were limited by several factors, mainly the rapid evolution in the treatment landscape of EGFR-mutant NSCLC, the researchers noted.
Osimertinib is currently considered the preferred first-line treatment by most physicians, they said. “The APPLE trial is the first prospective study supporting the role of dynamic adaptive strategies based on ctDNA monitoring in patients with EGFR-mutant advanced NSCLC.”
The study was supported by AstraZeneca. Lead author Dr. Remon had no financial conflicts to disclose. Corresponding author Dr. Dziadziuszko disclosed honoraria for consultancy or lectures from AstraZeneca, Roche, Novartis, MSD, Takeda, Pfizer, Amgen, and Bristol-Myers Squibb.
FROM ANNALS OF ONCOLOGY
New drugs in primary care: Lessons learned from COVID-19
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
AT INTERNAL MEDICINE 2023
“Terrific progress”: Adding blinatumomab for infant leukemia
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
.
Two-year disease-free and overall survival measures, as well as the percentage of children who had complete minimal residual disease (MRD) responses, were substantially higher among the 30 infants in the study than in historical controls treated with the same chemotherapy backbone in an earlier trial, Interfant-06.
“These outcome data are very promising, given the poor survival and lack of improvements in outcomes among infants with KMT2A-rearranged ALL in recent decades,” said the investigators, led by Inge M. van der Sluis, MD, PhD, a hematologist-oncologist at Princess Maxima Center for Pediatric Oncology in Utrecht, the Netherlands.
“The low incidence of relapse after treatment with blinatumomab is remarkable, given that in historical controls relapses occur frequently and early during therapy,” the investigators stated. Although the “follow-up time was relatively short” in the study, “it included the period historically defined” as being at high risk of relapse, they said.
The team suggested that future research should assess whether infants benefit from multiple courses of blinatumomab, rather than the one course used in the study, and whether blinatumomab plus chemotherapy can replace stem cell transplants for high-risk infants.
Pediatric community responds
There was excitement on Twitter about the results; a number of pediatric blood cancer specialists were impressed and posted the study on that platform. Comments included, “Wow! After years of stagnation, a huge step forward for infant leukemia” and “great news for infant lymphoblastic leukemia.”
Akshay Sharma, MBBS, a pediatric bone marrow transplant and cellular therapy specialist at St. Jude Children’s Research Hospital, Memphis, also posted. He said in an interview that the findings are “very exciting.”
The “outcomes of children diagnosed with leukemia in their infancy, particularly if they have a KMT2A rearrangement, have been dismal. This is terrific progress and a testament to the role that immunotherapy and novel agents will be playing in treatment of several malignant diseases in the decade to come,” he said.
Another poster, Pratik “Tik” Patel, MD, a pediatric hematology/oncology fellow at Emory University in Atlanta, told this news organization that the study “is welcome news to pediatric oncologists” and highlights “the success in incorporating newer immune-based therapeutics upfront in treatment rather than in relapsed/refractory settings.”
The National Cancer Institute–funded Children’s Oncology Group is thinking the same way. The group is launching a large, randomized trial to test if adding blinatumomab to chemotherapy upfront for B-cell acute lymphoblastic leukemia and lymphoblastic lymphoma improves outcomes in children and young adults aged 1-31 years. Results are due after 2029.
Study details
Blinatumomab is an expensive “T-cell engager” that helps cytotoxic CD3+T cells link to and destroy leukemic CD19+ B cells. Past studies have shown that it’s safe and works in older children and adults with B-lineage ALL after intensive chemotherapy, but until now the approach hadn’t been tested in infants, the investigators said.
The 30 subjects in the study were under a year old and newly diagnosed with KMT2A-rearranged ALL. They were treated with the Interfant-06 chemotherapy regimen – cytosine arabinoside and other agents – plus one postinduction course of blinatumomab at 15 micrograms/m2 per day as a 4-week continuous infusion. Eight of nine high-risk patients had allogeneic hematopoietic stem cell transplants.
Overall survival was 93.3% over a median follow up of 26.3 months, substantially higher than the 65.8% in the Interfant-06 trial. Two-year disease-free survival was 81.6% versus 49.4% in Interfant-06.
Sixteen patients (53%) were MRD negative after blinatumomab infusion and 12 (40%) had low levels of MRD. All of the children who continued chemotherapy went on to become MRD negative.
There were no permanent blinatumomab discontinuations and no treatment related deaths. Serious toxic effects were consistent with those in older patients and included four fevers, four infections, and one case each of hypertension and vomiting.
There were no cases of severe cytokine release syndrome (CRS) because of the low tumor burden of the subjects. Likewise, there were no obvious neurologic adverse events – like CRS, a particular concern with blinatumomab – but “we cannot rule out underreporting of mild neurologic symptoms that may have been unrecognized in infants,” the investigators said.
Patients who relapsed in the study had CNS involvement at relapse. “This underscores the need for adequate intrathecal chemotherapy during the blinatumomab infusion, because the efficacy of blinatumomab for the treatment of CNS leukemia may be limited,” they said.
The work was supported by Amgen, the maker of blinatumomab, as well as the Princess Maxima Center Foundation, the Danish Childhood Cancer Foundation, and others. Dr. Sluis is a consultant and researcher for Amgen. Five other authors were also consultants/advisers/researchers for the company. Dr. Sharma and Dr. Patel didn’t have any relevant disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
FDA puts partial hold on investigational alopecia areata drug deuruxolitinib
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
The in a press release on May 2.
The announcement came after a pulmonary embolism occurred with the 12-mg twice-daily dose in one of the long-term open-label extension (OLE) studies, the company, Sun Pharmaceutical Industries, said.
The company stated that the FDA has placed the Investigational New Drug testing for deuruxolitinib on partial clinical hold, and the agency is requiring that study participants who are currently on the 12-mg twice-daily dose in the OLE studies stop taking that dose. The hold covers only the 12-mg dose.
No hold on 8-mg dose
“There have been no thrombotic events reported to date for the 8-mg b.i.d. dose and U.S. FDA has not placed the 8-mg b.i.d. dose on hold,” the company said in the statement.
The statement added, “We are taking immediate steps to transition the patients in the OLE studies to the 8-mg b.i.d. dose arm in the ongoing studies.”
The company said that no thromboembolic events were observed in the phase 2 or phase 3 trials and said that it will work closely with the FDA to address its concerns. A formal letter detailing the FDA’s concerns is expected within 30 days.
Deuruxolitinib is an investigational oral selective inhibitor of Janus kinase 1 (JAK1) and JAK2 enzymes.
The FDA has granted deuruxolitinib breakthrough therapy designation for the treatment of adult patients with moderate to severe alopecia areata as well as fast-track designation for the treatment of alopecia areata.
In March, this news organization reported from the annual meeting of the American Academy of Dermatology that, based on phase 3 studies that demonstrate robust hair growth in about one-third of patients, deuruxolitinib has the potential to become the second JAK inhibitor available for the treatment of alopecia areata. If approved, it will join baricitinib (Olumiant), which received FDA approval almost 1 year ago.
Also at the AAD annual meeting, this news organization reported that principal investigator Brett A. King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Conn., in his presentation on the results of THRIVE-AA2, one of the two phase 3 trials of deuruxolitinib, displayed several before-and-after photos and said, “The photos tell the whole story. This is why there is so much excitement about these drugs.” Dr King also was a principal investigator in studies of baricitinib.
With one exception, labeling for baricitinib and other JAK inhibitors with dermatologic indications includes a boxed warning listing serious adverse events including the risk for major adverse cardiac events and thrombosis, including pulmonary embolism, based on the risks in a rheumatoid arthritis study.
A version of this article first appeared on Medscape.com.
Should CGM be used for those without diabetes?
Dallas Waldon doesn’t have diabetes, but she says she benefits from continuous glucose monitoring (CGM). “I’m a huge fan of CGMs and used them before, during, and after my pregnancy, [up to] 6 months postpartum, I’m down 11 pounds from my prepregnancy weight,” said Ms. Waldon, a manager for a land-buying company who lives in El Dorado, Calif.
“CGMs bring a certain level of accountability to what you’re eating. You can’t pretend you didn’t eat that cookie while making the kids’ lunch, or that the latte you had was ‘just coffee,’ ” she said. “You have the hard numbers to answer to, and that makes you think twice before putting anything in your mouth.”
Ms. Waldon is not alone. Although CGMs are typically used by people with type 1 diabetes, and increasingly those with type 2 diabetes, some endocrinologists say they are seeing an increased demand for CGM use from individuals who don’t have diabetes.
This allows users to monitor the glucose level and see how eating and exercise affects it. The companies claim that CGM use will help motivate individuals to eat better and maximize their exercise, and therefore consequently lose weight.
These lifestyle programs typically offer users the FreeStyle Libre (Abbott Laboratories). It uses a coin-sized sensor, generally worn on the upper arm, which lasts 14 days and measures glucose in the interstitial fluid. Users can read their glucose levels via an app on their smartphones as many times a day as they want. The FreeStyle Libre is worn by many people with diabetes and is a simple CGM to use, said Anne Peters, MD, professor of clinical medicine, University of Southern California, Los Angeles.
This growing demand for CGM use among healthy people is driven by an increasing “fascination” for monitoring every bodily function, as can be seen by the popularity of smart devices such as Fitbits and Apple watches, Dr. Peters added. These devices allow users to see their heart rates, review their sleep patterns, and monitor their pulses in real time; a CGM is an extension of that by providing up-to-the-minute glucose monitoring.
‘Everyone wants a CGM’
“Everyone wants a CGM,” Dr. Peters said, noting that even family members of her patients with diabetes are asking for them. She admits that their use can be effective for those who are prediabetic so they can see their glycemic responses to food. For instance, someone who typically eats oatmeal for breakfast may see their blood glucose increase, meaning they might want to lower their carbs.
David Klonoff, MD, medical director of the Diabetes Research Institute, Mills-Peninsula Medical Center, San Mateo, Calif., agrees that there has been an increase in use by people who don’t have diabetes as CGMs offer information they wouldn’t otherwise have access to “without having to prick themselves many times.”
People are using CGMs to monitor how high their blood glucose rises after eating certain foods, the length of time it takes to reach peak levels, and how quickly levels drop, he added. Elite athletes are using CGMs to ensure that they are consuming enough calories to avoid hypoglycemia, Dr. Klonoff said.
David T. Ahn, MD, program director at the Mary & Dick Allen Diabetes Center at Hoag Hospital in Newport Beach, Calif., also believes that the devices can provide useful information. “I find that CGM helps people learn a lot about nutrition and how lifestyle choices like food, activity, and stress impact their own physiology,” he stated.
“For example, comparing glucose spikes after different [types of] meals can deepen people’s understanding of carbohydrates vs. protein, or high glycemic index foods vs. low glycemic index foods,” he continued. “In addition, if a patient chooses to follow a very low-carbohydrate diet and/or an intermittent fasting diet, a CGM can be a powerful tool to measure consistency with that lifestyle choice.”
And for a person without diabetes, wearing a CGM provides a way to have personalized information on other physiologic measures, part of the quantified self movement where users log and track their blood pressure, urine output, and oxygen saturation, among other things, Dr. Klonoff said.
But does knowing all this result in behavioral changes?
Dr. Ahn isn’t sure. “For many people, being able to see glucose excursions throughout the day and after meals can be extremely educational and motivating. But much like the Fitbit or Apple Watch, simply wearing [a CGM] ... does not translate to behavior change. The CGM data patterns in someone without diabetes can start to become predictable over time, leading to a drop-off in utility/adherence after the initial education period,” he said.
Dr. Peters said she too isn’t convinced about the long-term worth of CGM in promoting or sustaining behavioral change, as the “novelty” of tracking may wear off after a few months.
And there’s no scientific proof that CGM use in those without diabetes has any impact.
“While there are many programs that offer coaching with CGM data, we need more studies to determine if this leads to improved outcomes like weight loss and prevention, or delay in the development of diabetes,” said Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, FADCES, FCCP, director of education and training in diabetes technology at Cleveland Clinic Diabetes Center.
A 2019 study published in the Journal of Clinical Endocrinology and Metabolism found that the blood glucose of individuals without diabetes using a CGM was in the “ideal” range between 70 mg/dL and 140 mg/dL 96% of the time. “Their glucose was beautifully controlled,” said Dr. Peters, who was one of the study authors.
Currently there aren’t any studies evaluating patterns among healthy individuals wearing CGMs, Dr. Klonoff noted, but he predicts that those studies will be done in the future to examine metabolic patterns that might contribute to someone developing prediabetes or diabetes.
“More data are needed from studies that focus on individuals at risk for diabetes to better understand the role of CGM in these cases, and how to best interpret and utilize the results,” said Fida Bacha, MD, a diabetes and endocrinology specialist and associate professor of pediatrics at Baylor College of Medicine in Houston.
“If clear metrics are identified to predict the progression to diabetes, then this would be worthwhile for early detection and prevention of the disease,” Dr. Bacha said.
Are CGMs too expensive, and can the information overwhelm some?
The biggest obstacle to many people using CGM is cost. “The main downside of using a CGM without diabetes is cost, since insurance won’t usually cover a CGM if the patient does not have diabetes,” said Marilyn Tan, MD, FACE, chief of the Endocrine Clinic, Stanford Health Clinic, Palo Alto, Calif. “Even for patients with diabetes but not on insulin, CGM coverage can be challenging, as out-of-pocket costs for CGM are variable.”
The lifestyle companies mentioned above charge $139-$399 per month, which covers two CGM sensors, each one good for 14 days. Users need to subscribe to a plan for service and delivery. Additional services such as nutrition counseling may be available at an additional cost. Because CGMs in the United States require a prescription, these companies offer web screening and access to a web-based provider.
If healthy patients feel that the informational value of CGMs is worth the money, then they shouldn’t be discouraged, the experts believe.
“There’s little risk of harm with wearing a CGM,” Dr. Tan said, although she acknowledges that “[t]oo much information can also be overwhelming for some individuals.”
Users need to consult with their clinicians to ensure they understand the readings, Dr. Peters said. “You have to tell them not to overreact if the device reads low [glucose] or not to freak out if they get an alarm.” A high glucose reading, indicating hyperglycemia, can be caused by a steroid injection, or older people may experience a postprandial high after eating, she added. “They need to talk to their healthcare provider to interpret the data especially if they are out of [the ideal glucose] range.”
Dr. Klonoff agreed that there is a risk of people trying to “medicalize” too much information. “If you have a fever, you don’t have to go to a doctor to know you have an infection,” he said.
And the point, he added, is not to obsess over the individual numbers but to look for patterns particularly as predictors of metabolic syndrome. If a patient’s glucose is primarily in range, he or she wouldn’t necessarily worry about diabetes. But if it’s out of range more than 10% of the time, it might mean that patient is at risk for diabetes. “It might be time to counsel the patient to eat healthier and exercise more,” he said. “It’s never wrong to steer people to a healthier lifestyle.”
But another issue is whether the numbers from CGMs are entirely accurate in people without diabetes. A 2020 study published in the American Journal of Clinical Nutrition had 16 adults without diabetes wear both the Dexcom G4 Platinum CGM and Abbott FreeStyle Libre Pro for 28 days.
Researchers found that mean postprandial glucose was higher with the Dexcom than with the Abbott system, suggesting that “postprandial glycemic excursions were somewhat inconsistent between the CGMs.” The authors concluded that it may be too early to personalize meal recommendations via CGM.
Dr. Isaacs said perhaps the happy medium is for those without diabetes to just use CGMs occasionally. “It’s ... unclear [if] the right [use] of CGM needs to be continuous or if periodic use, such as once every 3 months, is enough for benefits,” she concluded.
A version of this article first appeared on Medscape.com.
Dallas Waldon doesn’t have diabetes, but she says she benefits from continuous glucose monitoring (CGM). “I’m a huge fan of CGMs and used them before, during, and after my pregnancy, [up to] 6 months postpartum, I’m down 11 pounds from my prepregnancy weight,” said Ms. Waldon, a manager for a land-buying company who lives in El Dorado, Calif.
“CGMs bring a certain level of accountability to what you’re eating. You can’t pretend you didn’t eat that cookie while making the kids’ lunch, or that the latte you had was ‘just coffee,’ ” she said. “You have the hard numbers to answer to, and that makes you think twice before putting anything in your mouth.”
Ms. Waldon is not alone. Although CGMs are typically used by people with type 1 diabetes, and increasingly those with type 2 diabetes, some endocrinologists say they are seeing an increased demand for CGM use from individuals who don’t have diabetes.
This allows users to monitor the glucose level and see how eating and exercise affects it. The companies claim that CGM use will help motivate individuals to eat better and maximize their exercise, and therefore consequently lose weight.
These lifestyle programs typically offer users the FreeStyle Libre (Abbott Laboratories). It uses a coin-sized sensor, generally worn on the upper arm, which lasts 14 days and measures glucose in the interstitial fluid. Users can read their glucose levels via an app on their smartphones as many times a day as they want. The FreeStyle Libre is worn by many people with diabetes and is a simple CGM to use, said Anne Peters, MD, professor of clinical medicine, University of Southern California, Los Angeles.
This growing demand for CGM use among healthy people is driven by an increasing “fascination” for monitoring every bodily function, as can be seen by the popularity of smart devices such as Fitbits and Apple watches, Dr. Peters added. These devices allow users to see their heart rates, review their sleep patterns, and monitor their pulses in real time; a CGM is an extension of that by providing up-to-the-minute glucose monitoring.
‘Everyone wants a CGM’
“Everyone wants a CGM,” Dr. Peters said, noting that even family members of her patients with diabetes are asking for them. She admits that their use can be effective for those who are prediabetic so they can see their glycemic responses to food. For instance, someone who typically eats oatmeal for breakfast may see their blood glucose increase, meaning they might want to lower their carbs.
David Klonoff, MD, medical director of the Diabetes Research Institute, Mills-Peninsula Medical Center, San Mateo, Calif., agrees that there has been an increase in use by people who don’t have diabetes as CGMs offer information they wouldn’t otherwise have access to “without having to prick themselves many times.”
People are using CGMs to monitor how high their blood glucose rises after eating certain foods, the length of time it takes to reach peak levels, and how quickly levels drop, he added. Elite athletes are using CGMs to ensure that they are consuming enough calories to avoid hypoglycemia, Dr. Klonoff said.
David T. Ahn, MD, program director at the Mary & Dick Allen Diabetes Center at Hoag Hospital in Newport Beach, Calif., also believes that the devices can provide useful information. “I find that CGM helps people learn a lot about nutrition and how lifestyle choices like food, activity, and stress impact their own physiology,” he stated.
“For example, comparing glucose spikes after different [types of] meals can deepen people’s understanding of carbohydrates vs. protein, or high glycemic index foods vs. low glycemic index foods,” he continued. “In addition, if a patient chooses to follow a very low-carbohydrate diet and/or an intermittent fasting diet, a CGM can be a powerful tool to measure consistency with that lifestyle choice.”
And for a person without diabetes, wearing a CGM provides a way to have personalized information on other physiologic measures, part of the quantified self movement where users log and track their blood pressure, urine output, and oxygen saturation, among other things, Dr. Klonoff said.
But does knowing all this result in behavioral changes?
Dr. Ahn isn’t sure. “For many people, being able to see glucose excursions throughout the day and after meals can be extremely educational and motivating. But much like the Fitbit or Apple Watch, simply wearing [a CGM] ... does not translate to behavior change. The CGM data patterns in someone without diabetes can start to become predictable over time, leading to a drop-off in utility/adherence after the initial education period,” he said.
Dr. Peters said she too isn’t convinced about the long-term worth of CGM in promoting or sustaining behavioral change, as the “novelty” of tracking may wear off after a few months.
And there’s no scientific proof that CGM use in those without diabetes has any impact.
“While there are many programs that offer coaching with CGM data, we need more studies to determine if this leads to improved outcomes like weight loss and prevention, or delay in the development of diabetes,” said Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, FADCES, FCCP, director of education and training in diabetes technology at Cleveland Clinic Diabetes Center.
A 2019 study published in the Journal of Clinical Endocrinology and Metabolism found that the blood glucose of individuals without diabetes using a CGM was in the “ideal” range between 70 mg/dL and 140 mg/dL 96% of the time. “Their glucose was beautifully controlled,” said Dr. Peters, who was one of the study authors.
Currently there aren’t any studies evaluating patterns among healthy individuals wearing CGMs, Dr. Klonoff noted, but he predicts that those studies will be done in the future to examine metabolic patterns that might contribute to someone developing prediabetes or diabetes.
“More data are needed from studies that focus on individuals at risk for diabetes to better understand the role of CGM in these cases, and how to best interpret and utilize the results,” said Fida Bacha, MD, a diabetes and endocrinology specialist and associate professor of pediatrics at Baylor College of Medicine in Houston.
“If clear metrics are identified to predict the progression to diabetes, then this would be worthwhile for early detection and prevention of the disease,” Dr. Bacha said.
Are CGMs too expensive, and can the information overwhelm some?
The biggest obstacle to many people using CGM is cost. “The main downside of using a CGM without diabetes is cost, since insurance won’t usually cover a CGM if the patient does not have diabetes,” said Marilyn Tan, MD, FACE, chief of the Endocrine Clinic, Stanford Health Clinic, Palo Alto, Calif. “Even for patients with diabetes but not on insulin, CGM coverage can be challenging, as out-of-pocket costs for CGM are variable.”
The lifestyle companies mentioned above charge $139-$399 per month, which covers two CGM sensors, each one good for 14 days. Users need to subscribe to a plan for service and delivery. Additional services such as nutrition counseling may be available at an additional cost. Because CGMs in the United States require a prescription, these companies offer web screening and access to a web-based provider.
If healthy patients feel that the informational value of CGMs is worth the money, then they shouldn’t be discouraged, the experts believe.
“There’s little risk of harm with wearing a CGM,” Dr. Tan said, although she acknowledges that “[t]oo much information can also be overwhelming for some individuals.”
Users need to consult with their clinicians to ensure they understand the readings, Dr. Peters said. “You have to tell them not to overreact if the device reads low [glucose] or not to freak out if they get an alarm.” A high glucose reading, indicating hyperglycemia, can be caused by a steroid injection, or older people may experience a postprandial high after eating, she added. “They need to talk to their healthcare provider to interpret the data especially if they are out of [the ideal glucose] range.”
Dr. Klonoff agreed that there is a risk of people trying to “medicalize” too much information. “If you have a fever, you don’t have to go to a doctor to know you have an infection,” he said.
And the point, he added, is not to obsess over the individual numbers but to look for patterns particularly as predictors of metabolic syndrome. If a patient’s glucose is primarily in range, he or she wouldn’t necessarily worry about diabetes. But if it’s out of range more than 10% of the time, it might mean that patient is at risk for diabetes. “It might be time to counsel the patient to eat healthier and exercise more,” he said. “It’s never wrong to steer people to a healthier lifestyle.”
But another issue is whether the numbers from CGMs are entirely accurate in people without diabetes. A 2020 study published in the American Journal of Clinical Nutrition had 16 adults without diabetes wear both the Dexcom G4 Platinum CGM and Abbott FreeStyle Libre Pro for 28 days.
Researchers found that mean postprandial glucose was higher with the Dexcom than with the Abbott system, suggesting that “postprandial glycemic excursions were somewhat inconsistent between the CGMs.” The authors concluded that it may be too early to personalize meal recommendations via CGM.
Dr. Isaacs said perhaps the happy medium is for those without diabetes to just use CGMs occasionally. “It’s ... unclear [if] the right [use] of CGM needs to be continuous or if periodic use, such as once every 3 months, is enough for benefits,” she concluded.
A version of this article first appeared on Medscape.com.
Dallas Waldon doesn’t have diabetes, but she says she benefits from continuous glucose monitoring (CGM). “I’m a huge fan of CGMs and used them before, during, and after my pregnancy, [up to] 6 months postpartum, I’m down 11 pounds from my prepregnancy weight,” said Ms. Waldon, a manager for a land-buying company who lives in El Dorado, Calif.
“CGMs bring a certain level of accountability to what you’re eating. You can’t pretend you didn’t eat that cookie while making the kids’ lunch, or that the latte you had was ‘just coffee,’ ” she said. “You have the hard numbers to answer to, and that makes you think twice before putting anything in your mouth.”
Ms. Waldon is not alone. Although CGMs are typically used by people with type 1 diabetes, and increasingly those with type 2 diabetes, some endocrinologists say they are seeing an increased demand for CGM use from individuals who don’t have diabetes.
This allows users to monitor the glucose level and see how eating and exercise affects it. The companies claim that CGM use will help motivate individuals to eat better and maximize their exercise, and therefore consequently lose weight.
These lifestyle programs typically offer users the FreeStyle Libre (Abbott Laboratories). It uses a coin-sized sensor, generally worn on the upper arm, which lasts 14 days and measures glucose in the interstitial fluid. Users can read their glucose levels via an app on their smartphones as many times a day as they want. The FreeStyle Libre is worn by many people with diabetes and is a simple CGM to use, said Anne Peters, MD, professor of clinical medicine, University of Southern California, Los Angeles.
This growing demand for CGM use among healthy people is driven by an increasing “fascination” for monitoring every bodily function, as can be seen by the popularity of smart devices such as Fitbits and Apple watches, Dr. Peters added. These devices allow users to see their heart rates, review their sleep patterns, and monitor their pulses in real time; a CGM is an extension of that by providing up-to-the-minute glucose monitoring.
‘Everyone wants a CGM’
“Everyone wants a CGM,” Dr. Peters said, noting that even family members of her patients with diabetes are asking for them. She admits that their use can be effective for those who are prediabetic so they can see their glycemic responses to food. For instance, someone who typically eats oatmeal for breakfast may see their blood glucose increase, meaning they might want to lower their carbs.
David Klonoff, MD, medical director of the Diabetes Research Institute, Mills-Peninsula Medical Center, San Mateo, Calif., agrees that there has been an increase in use by people who don’t have diabetes as CGMs offer information they wouldn’t otherwise have access to “without having to prick themselves many times.”
People are using CGMs to monitor how high their blood glucose rises after eating certain foods, the length of time it takes to reach peak levels, and how quickly levels drop, he added. Elite athletes are using CGMs to ensure that they are consuming enough calories to avoid hypoglycemia, Dr. Klonoff said.
David T. Ahn, MD, program director at the Mary & Dick Allen Diabetes Center at Hoag Hospital in Newport Beach, Calif., also believes that the devices can provide useful information. “I find that CGM helps people learn a lot about nutrition and how lifestyle choices like food, activity, and stress impact their own physiology,” he stated.
“For example, comparing glucose spikes after different [types of] meals can deepen people’s understanding of carbohydrates vs. protein, or high glycemic index foods vs. low glycemic index foods,” he continued. “In addition, if a patient chooses to follow a very low-carbohydrate diet and/or an intermittent fasting diet, a CGM can be a powerful tool to measure consistency with that lifestyle choice.”
And for a person without diabetes, wearing a CGM provides a way to have personalized information on other physiologic measures, part of the quantified self movement where users log and track their blood pressure, urine output, and oxygen saturation, among other things, Dr. Klonoff said.
But does knowing all this result in behavioral changes?
Dr. Ahn isn’t sure. “For many people, being able to see glucose excursions throughout the day and after meals can be extremely educational and motivating. But much like the Fitbit or Apple Watch, simply wearing [a CGM] ... does not translate to behavior change. The CGM data patterns in someone without diabetes can start to become predictable over time, leading to a drop-off in utility/adherence after the initial education period,” he said.
Dr. Peters said she too isn’t convinced about the long-term worth of CGM in promoting or sustaining behavioral change, as the “novelty” of tracking may wear off after a few months.
And there’s no scientific proof that CGM use in those without diabetes has any impact.
“While there are many programs that offer coaching with CGM data, we need more studies to determine if this leads to improved outcomes like weight loss and prevention, or delay in the development of diabetes,” said Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, FADCES, FCCP, director of education and training in diabetes technology at Cleveland Clinic Diabetes Center.
A 2019 study published in the Journal of Clinical Endocrinology and Metabolism found that the blood glucose of individuals without diabetes using a CGM was in the “ideal” range between 70 mg/dL and 140 mg/dL 96% of the time. “Their glucose was beautifully controlled,” said Dr. Peters, who was one of the study authors.
Currently there aren’t any studies evaluating patterns among healthy individuals wearing CGMs, Dr. Klonoff noted, but he predicts that those studies will be done in the future to examine metabolic patterns that might contribute to someone developing prediabetes or diabetes.
“More data are needed from studies that focus on individuals at risk for diabetes to better understand the role of CGM in these cases, and how to best interpret and utilize the results,” said Fida Bacha, MD, a diabetes and endocrinology specialist and associate professor of pediatrics at Baylor College of Medicine in Houston.
“If clear metrics are identified to predict the progression to diabetes, then this would be worthwhile for early detection and prevention of the disease,” Dr. Bacha said.
Are CGMs too expensive, and can the information overwhelm some?
The biggest obstacle to many people using CGM is cost. “The main downside of using a CGM without diabetes is cost, since insurance won’t usually cover a CGM if the patient does not have diabetes,” said Marilyn Tan, MD, FACE, chief of the Endocrine Clinic, Stanford Health Clinic, Palo Alto, Calif. “Even for patients with diabetes but not on insulin, CGM coverage can be challenging, as out-of-pocket costs for CGM are variable.”
The lifestyle companies mentioned above charge $139-$399 per month, which covers two CGM sensors, each one good for 14 days. Users need to subscribe to a plan for service and delivery. Additional services such as nutrition counseling may be available at an additional cost. Because CGMs in the United States require a prescription, these companies offer web screening and access to a web-based provider.
If healthy patients feel that the informational value of CGMs is worth the money, then they shouldn’t be discouraged, the experts believe.
“There’s little risk of harm with wearing a CGM,” Dr. Tan said, although she acknowledges that “[t]oo much information can also be overwhelming for some individuals.”
Users need to consult with their clinicians to ensure they understand the readings, Dr. Peters said. “You have to tell them not to overreact if the device reads low [glucose] or not to freak out if they get an alarm.” A high glucose reading, indicating hyperglycemia, can be caused by a steroid injection, or older people may experience a postprandial high after eating, she added. “They need to talk to their healthcare provider to interpret the data especially if they are out of [the ideal glucose] range.”
Dr. Klonoff agreed that there is a risk of people trying to “medicalize” too much information. “If you have a fever, you don’t have to go to a doctor to know you have an infection,” he said.
And the point, he added, is not to obsess over the individual numbers but to look for patterns particularly as predictors of metabolic syndrome. If a patient’s glucose is primarily in range, he or she wouldn’t necessarily worry about diabetes. But if it’s out of range more than 10% of the time, it might mean that patient is at risk for diabetes. “It might be time to counsel the patient to eat healthier and exercise more,” he said. “It’s never wrong to steer people to a healthier lifestyle.”
But another issue is whether the numbers from CGMs are entirely accurate in people without diabetes. A 2020 study published in the American Journal of Clinical Nutrition had 16 adults without diabetes wear both the Dexcom G4 Platinum CGM and Abbott FreeStyle Libre Pro for 28 days.
Researchers found that mean postprandial glucose was higher with the Dexcom than with the Abbott system, suggesting that “postprandial glycemic excursions were somewhat inconsistent between the CGMs.” The authors concluded that it may be too early to personalize meal recommendations via CGM.
Dr. Isaacs said perhaps the happy medium is for those without diabetes to just use CGMs occasionally. “It’s ... unclear [if] the right [use] of CGM needs to be continuous or if periodic use, such as once every 3 months, is enough for benefits,” she concluded.
A version of this article first appeared on Medscape.com.
ASCO updates treatment guidelines for anxiety and depression
Since the last guidelines, published in 2014, screening and assessment for depression and anxiety have improved, and a large new evidence base has emerged. To ensure the most up-to-date recommendations, a group of experts spanning psychology, psychiatry, medical and surgical oncology, internal medicine, and nursing convened to review the current literature on managing depression and anxiety. The review included 61 studies – 16 meta-analyses, 44 randomized controlled trials, and one systematic review – published between 2013 and 2021.
“The purpose of this guideline update is to gather and examine the evidence published since the 2014 guideline ... [with a] focus on management and treatment only.” The overall goal is to provide “the most effective and least resource-intensive intervention based on symptom severity” for patients with cancer, the experts write.
The new clinical practice guideline addresses the following question: What are the recommended treatment approaches in the management of anxiety and/or depression in survivors of adult cancer?
After an extensive literature search and analysis, the study was published online in the Journal of Clinical Oncology.
The expert panel’s recommendations fell into three broad categories – general management principles, treatment and care options for depressive symptoms, and treatment and care options for anxiety symptoms – with the guidelines for managing depression and anxiety largely mirroring each other.
The authors caution, however, that the guidelines “were developed in the context of mental health care being available and may not be applicable within other resource settings.”
General management principals
All patients with cancer, along with their caregivers, family members, or trusted confidants, should be offered information and resources on depression and anxiety. The panel gave this a “strong” recommendation but provided the caveat that the “information should be culturally informed and linguistically appropriate and can include a conversation between clinician and patient.”
Clinicians should select the most effective and least intensive intervention based on symptom severity when selecting treatment – what the panelists referred to as a stepped-care model. History of psychiatric diagnoses or substance use as well as prior responses to mental health treatment are some of the factors that may inform treatment choice.
For patients experiencing both depression and anxiety symptoms, treatment of depressive symptoms should be prioritized.
When referring a patient for further evaluation or care, clinicians “should make every effort to reduce barriers and facilitate patient follow-through,” the authors write. And health care professionals should regularly assess the treatment responses for patients receiving psychological or pharmacological interventions.
Overall, the treatments should be “supervised by a psychiatrist, and primary care or oncology providers work collaboratively with a nurse care manager to provide psychological interventions and monitor treatment compliance and outcomes,” the panelists write. “This type of collaborative care is found to be superior to usual care and is more cost-effective than face-to-face and pharmacologic treatment for depression.”
Treatment and care options for depressive and anxiety symptoms
For patients with moderate to severe depression symptoms, the panelists again stressed that clinicians should provide “culturally informed and linguistically appropriate information.” This information may include the frequency and symptoms of depression as well as signs these symptoms may be getting worse, with contact information for the medical team provided.
Among patients with moderate symptoms, clinicians can offer patients a range of individual or group therapy options, including cognitive-behavioral therapy (CBT), behavioral activation, mindfulness-based stress reduction, or structured physical activity and exercise. For patients with severe symptoms of depression, clinicians should offer individual therapy with one of these four treatment options: CBT, behavioral activation, mindfulness-based stress reduction, or interpersonal therapy.
The panelists offered almost identical recommendations for patients with anxiety, except mindfulness-based stress reduction was an option for patients with severe symptoms.
Clinicians can also provide pharmacologic options to treat depression or anxiety in certain patients, though the panelists provided the caveat that evidence for pharmacologic management is weak.
“These guidelines make no recommendations about any specific pharmacologic regimen being better than another,” the experts wrote. And “patients should be warned of potential harm or adverse effects.”
Overall, the panelists noted that, as highlighted in the 2014 ASCO guideline, the updated version continues to stress the importance of providing education on coping with stress, anxiety, and depression.
And “for individuals with elevated symptoms, validation and normalizing patients’ experiences is crucial,” the panelists write.
Although the timing of screening is not the focus of this updated review, the experts recognized that “how and when patients with cancer and survivors are screened are important determinants of timely management of anxiety and depression.”
And unlike the prior guideline, “pharmacotherapy is not recommended as a first-line treatment, neither alone nor in combination,” the authors say.
Overall, the panelists emphasize how widespread the mental health care crisis is and that problems accessing mental health care remain. “The choice of intervention to offer patients facing such obstacles should be based on shared decision-making, taking into account availability, accessibility, patient preference, likelihood of adverse events, adherence, and cost,” the experts conclude.
A version of this article first appeared on Medscape.com.
Since the last guidelines, published in 2014, screening and assessment for depression and anxiety have improved, and a large new evidence base has emerged. To ensure the most up-to-date recommendations, a group of experts spanning psychology, psychiatry, medical and surgical oncology, internal medicine, and nursing convened to review the current literature on managing depression and anxiety. The review included 61 studies – 16 meta-analyses, 44 randomized controlled trials, and one systematic review – published between 2013 and 2021.
“The purpose of this guideline update is to gather and examine the evidence published since the 2014 guideline ... [with a] focus on management and treatment only.” The overall goal is to provide “the most effective and least resource-intensive intervention based on symptom severity” for patients with cancer, the experts write.
The new clinical practice guideline addresses the following question: What are the recommended treatment approaches in the management of anxiety and/or depression in survivors of adult cancer?
After an extensive literature search and analysis, the study was published online in the Journal of Clinical Oncology.
The expert panel’s recommendations fell into three broad categories – general management principles, treatment and care options for depressive symptoms, and treatment and care options for anxiety symptoms – with the guidelines for managing depression and anxiety largely mirroring each other.
The authors caution, however, that the guidelines “were developed in the context of mental health care being available and may not be applicable within other resource settings.”
General management principals
All patients with cancer, along with their caregivers, family members, or trusted confidants, should be offered information and resources on depression and anxiety. The panel gave this a “strong” recommendation but provided the caveat that the “information should be culturally informed and linguistically appropriate and can include a conversation between clinician and patient.”
Clinicians should select the most effective and least intensive intervention based on symptom severity when selecting treatment – what the panelists referred to as a stepped-care model. History of psychiatric diagnoses or substance use as well as prior responses to mental health treatment are some of the factors that may inform treatment choice.
For patients experiencing both depression and anxiety symptoms, treatment of depressive symptoms should be prioritized.
When referring a patient for further evaluation or care, clinicians “should make every effort to reduce barriers and facilitate patient follow-through,” the authors write. And health care professionals should regularly assess the treatment responses for patients receiving psychological or pharmacological interventions.
Overall, the treatments should be “supervised by a psychiatrist, and primary care or oncology providers work collaboratively with a nurse care manager to provide psychological interventions and monitor treatment compliance and outcomes,” the panelists write. “This type of collaborative care is found to be superior to usual care and is more cost-effective than face-to-face and pharmacologic treatment for depression.”
Treatment and care options for depressive and anxiety symptoms
For patients with moderate to severe depression symptoms, the panelists again stressed that clinicians should provide “culturally informed and linguistically appropriate information.” This information may include the frequency and symptoms of depression as well as signs these symptoms may be getting worse, with contact information for the medical team provided.
Among patients with moderate symptoms, clinicians can offer patients a range of individual or group therapy options, including cognitive-behavioral therapy (CBT), behavioral activation, mindfulness-based stress reduction, or structured physical activity and exercise. For patients with severe symptoms of depression, clinicians should offer individual therapy with one of these four treatment options: CBT, behavioral activation, mindfulness-based stress reduction, or interpersonal therapy.
The panelists offered almost identical recommendations for patients with anxiety, except mindfulness-based stress reduction was an option for patients with severe symptoms.
Clinicians can also provide pharmacologic options to treat depression or anxiety in certain patients, though the panelists provided the caveat that evidence for pharmacologic management is weak.
“These guidelines make no recommendations about any specific pharmacologic regimen being better than another,” the experts wrote. And “patients should be warned of potential harm or adverse effects.”
Overall, the panelists noted that, as highlighted in the 2014 ASCO guideline, the updated version continues to stress the importance of providing education on coping with stress, anxiety, and depression.
And “for individuals with elevated symptoms, validation and normalizing patients’ experiences is crucial,” the panelists write.
Although the timing of screening is not the focus of this updated review, the experts recognized that “how and when patients with cancer and survivors are screened are important determinants of timely management of anxiety and depression.”
And unlike the prior guideline, “pharmacotherapy is not recommended as a first-line treatment, neither alone nor in combination,” the authors say.
Overall, the panelists emphasize how widespread the mental health care crisis is and that problems accessing mental health care remain. “The choice of intervention to offer patients facing such obstacles should be based on shared decision-making, taking into account availability, accessibility, patient preference, likelihood of adverse events, adherence, and cost,” the experts conclude.
A version of this article first appeared on Medscape.com.
Since the last guidelines, published in 2014, screening and assessment for depression and anxiety have improved, and a large new evidence base has emerged. To ensure the most up-to-date recommendations, a group of experts spanning psychology, psychiatry, medical and surgical oncology, internal medicine, and nursing convened to review the current literature on managing depression and anxiety. The review included 61 studies – 16 meta-analyses, 44 randomized controlled trials, and one systematic review – published between 2013 and 2021.
“The purpose of this guideline update is to gather and examine the evidence published since the 2014 guideline ... [with a] focus on management and treatment only.” The overall goal is to provide “the most effective and least resource-intensive intervention based on symptom severity” for patients with cancer, the experts write.
The new clinical practice guideline addresses the following question: What are the recommended treatment approaches in the management of anxiety and/or depression in survivors of adult cancer?
After an extensive literature search and analysis, the study was published online in the Journal of Clinical Oncology.
The expert panel’s recommendations fell into three broad categories – general management principles, treatment and care options for depressive symptoms, and treatment and care options for anxiety symptoms – with the guidelines for managing depression and anxiety largely mirroring each other.
The authors caution, however, that the guidelines “were developed in the context of mental health care being available and may not be applicable within other resource settings.”
General management principals
All patients with cancer, along with their caregivers, family members, or trusted confidants, should be offered information and resources on depression and anxiety. The panel gave this a “strong” recommendation but provided the caveat that the “information should be culturally informed and linguistically appropriate and can include a conversation between clinician and patient.”
Clinicians should select the most effective and least intensive intervention based on symptom severity when selecting treatment – what the panelists referred to as a stepped-care model. History of psychiatric diagnoses or substance use as well as prior responses to mental health treatment are some of the factors that may inform treatment choice.
For patients experiencing both depression and anxiety symptoms, treatment of depressive symptoms should be prioritized.
When referring a patient for further evaluation or care, clinicians “should make every effort to reduce barriers and facilitate patient follow-through,” the authors write. And health care professionals should regularly assess the treatment responses for patients receiving psychological or pharmacological interventions.
Overall, the treatments should be “supervised by a psychiatrist, and primary care or oncology providers work collaboratively with a nurse care manager to provide psychological interventions and monitor treatment compliance and outcomes,” the panelists write. “This type of collaborative care is found to be superior to usual care and is more cost-effective than face-to-face and pharmacologic treatment for depression.”
Treatment and care options for depressive and anxiety symptoms
For patients with moderate to severe depression symptoms, the panelists again stressed that clinicians should provide “culturally informed and linguistically appropriate information.” This information may include the frequency and symptoms of depression as well as signs these symptoms may be getting worse, with contact information for the medical team provided.
Among patients with moderate symptoms, clinicians can offer patients a range of individual or group therapy options, including cognitive-behavioral therapy (CBT), behavioral activation, mindfulness-based stress reduction, or structured physical activity and exercise. For patients with severe symptoms of depression, clinicians should offer individual therapy with one of these four treatment options: CBT, behavioral activation, mindfulness-based stress reduction, or interpersonal therapy.
The panelists offered almost identical recommendations for patients with anxiety, except mindfulness-based stress reduction was an option for patients with severe symptoms.
Clinicians can also provide pharmacologic options to treat depression or anxiety in certain patients, though the panelists provided the caveat that evidence for pharmacologic management is weak.
“These guidelines make no recommendations about any specific pharmacologic regimen being better than another,” the experts wrote. And “patients should be warned of potential harm or adverse effects.”
Overall, the panelists noted that, as highlighted in the 2014 ASCO guideline, the updated version continues to stress the importance of providing education on coping with stress, anxiety, and depression.
And “for individuals with elevated symptoms, validation and normalizing patients’ experiences is crucial,” the panelists write.
Although the timing of screening is not the focus of this updated review, the experts recognized that “how and when patients with cancer and survivors are screened are important determinants of timely management of anxiety and depression.”
And unlike the prior guideline, “pharmacotherapy is not recommended as a first-line treatment, neither alone nor in combination,” the authors say.
Overall, the panelists emphasize how widespread the mental health care crisis is and that problems accessing mental health care remain. “The choice of intervention to offer patients facing such obstacles should be based on shared decision-making, taking into account availability, accessibility, patient preference, likelihood of adverse events, adherence, and cost,” the experts conclude.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY