User login
COVID led to rise in pregnancy-related deaths: New research
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
The rise in deaths was most pronounced among Black mothers.
In 2021, 1,205 women died from pregnancy-related causes, making the year one of the worst for maternal mortality in U.S. history, according to newly released data from the Centers for Disease Control and Prevention. Maternal mortality is defined as occurring during pregnancy, at delivery, or soon after delivery.
COVID was the driver of the increased death rate, according to a study published in the journal Obstetrics & Gynecology. The researchers noted that unvaccinated pregnant people are more likely to get severe COVID, and that prenatal and postnatal care were disrupted during the early part of the pandemic. From July 2021 to March 2023, the rate of women being vaccinated before pregnancy has risen from 22% to 70%, CDC data show.
Maternal mortality rates jumped the most among Black women, who in 2021 had a maternal mortality rate of nearly 70 deaths per 100,000 live births, which was 2.6 times the rate for White women.
Existing risks based on a mother’s age also increased from 2020 to 2021. The maternal mortality rates by age in 2021 per 100,000 live births were:
- 20.4 for women under age 25.
- 31.3 for women ages 25 to 39.
- 138.5 for women ages 40 and older.
Iffath Abbasi Hoskins, MD, FACOG, president of the American College of Obstetricians and Gynecologists, called the situation “stunning” and “preventable.”
The findings “send a resounding message that maternal health and evidence-based efforts to eliminate racial health inequities need to be, and remain, a top public health priority,” Dr. Hoskins said in a statement.
“The COVID-19 pandemic had a dramatic and tragic effect on maternal death rates, but we cannot let that fact obscure that there was – and still is – already a maternal mortality crisis to compound,” she said.
A version of this article first appeared on WebMD.com.
Retinopathy ‘emerging decades earlier’ in kids with type 2 diabetes than in adults
Nearly one in four children diagnosed with type 2 diabetes for 5 years or more develop diabetic retinopathy, according to a new report.
The global prevalence of diabetic retinopathy in pediatric patients with type 2 diabetes is about 7%, which appears to increase with age.
“In our clinical practice, we have seen an increase in children presenting with type 2 diabetes over the past few years. These patients present with multiple simultaneous comorbidities and complications like hypertension, fatty liver, and other conditions,” senior author M. Constantine Samaan, MD, told this news organization.
“The exact scale of diabetes-related eye disease was not clear, and we decided to quantify it,” said Dr. Samaan, associate professor of pediatrics at McMaster University and pediatric endocrinologist at McMaster Children’s Hospital in Hamilton, Ont.
“What we found was that in pediatric patients with type 2 diabetes, diabetic retinopathy is present in 1 in 14 youth. The risk of retinopathy increased significantly 5 years after diagnosis to almost one in four,” he noted.
“While we acknowledged that the number of diabetic retinopathy cases was relatively small and there was heterogeneity in studies, we were surprised that retinopathy rates rose so fast in the first few years after diabetes diagnosis,” Dr. Samaan indicated.
The findings signal that the increase in the prevalence of diabetic retinopathy is emerging decades earlier among children compared with adults with type 2 diabetes, the authors wrote in their article published online in JAMA Network Open.
“While the guidelines for eye care in children with type 2 diabetes recommend screening at diagnosis and annually afterward, these recommendations are not followed in almost half of these patients,” Dr. Samaan said. “There is a need to ensure that patients get screened to try and prevent or delay retinopathy onset and progression.”
Analyzing prevalence rates
Diabetic retinopathy is the leading cause of blindness in patients with type 2 diabetes. Between 21% and 39% of adults have diabetic retinopathy at diagnosis, with rates subsequently increasing, the authors wrote.
Dr. Samaan and colleagues conducted a systematic review and meta-analysis to estimate the global prevalence of diabetic retinopathy in pediatric patients with type 2 diabetes. They included studies that had a study population of at least 10 participants diagnosed at age 21 and younger, an observational study design, and prevalence data on diabetic retinopathy.
Among the 29 studies included, 6 were cross-sectional, 13 had a retrospective cohort design, and 10 had a prospective cohort design. Patients were diagnosed between age 6.5 and 21 years, and the diabetes duration ranged from 0 to 15 years after diagnosis.
The overall global prevalence of diabetic retinopathy in 5,924 pediatric patients was 7.0%. Prevalence varied by study design, ranging from 1.1% in cross-sectional studies to 6.5% in prospective cohort studies and 11.3% in retrospective cohort studies.
In the nine studies that reported diabetic retinopathy classification based on criteria, the prevalence of minimal-to-moderate nonproliferative diabetic retinopathy was 11.2%, the prevalence of severe nonproliferative diabetic retinopathy was 2.6%, the prevalence of proliferative diabetic retinopathy was 2.4%, and the prevalence of macular edema was 3.1%.
In the five studies that reported diabetic retinopathy diagnosis using fundoscopy, the prevalence was 0.5%. In the four studies that used 7-field stereoscopic fundus photography, the prevalence was 13.6%.
In the pooled analysis of 27 studies, the prevalence of diabetic retinopathy was 1.8% less than 2.5 years after diabetes diagnosis but more than doubled to 5.1% in years 2.5 to 5 and jumped to 28.8% more than 5 years after diagnosis.
Differences by sex, ethnicity
“We were also surprised that there was very limited evidence to understand the sex and race differences in retinopathy risk,” said Dr. Samaan. “Further research is warranted, considering that more girls develop type 2 diabetes than boys, and the risk of type 2 diabetes is higher in some racial groups.”
In addition, older age, longer diabetes duration, and higher hypertension prevalence were associated with diabetic retinopathy prevalence. There were no associations with obesity prevalence or mean age at diabetes diagnosis. However, patients who developed diabetic retinopathy had a higher mean A1c level of 1.4% compared to those without retinopathy.
Dr. Samaan and colleagues are continuing to research the comorbidities and complications that children with type 2 diabetes face as well as mechanisms that drive diabetes outcomes among children and adolescents.
For now, the findings highlight the importance of retinopathy screening and personalized diabetes treatment to protect vision, Dr. Samaan reiterated.
No funding source for the study was reported. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Nearly one in four children diagnosed with type 2 diabetes for 5 years or more develop diabetic retinopathy, according to a new report.
The global prevalence of diabetic retinopathy in pediatric patients with type 2 diabetes is about 7%, which appears to increase with age.
“In our clinical practice, we have seen an increase in children presenting with type 2 diabetes over the past few years. These patients present with multiple simultaneous comorbidities and complications like hypertension, fatty liver, and other conditions,” senior author M. Constantine Samaan, MD, told this news organization.
“The exact scale of diabetes-related eye disease was not clear, and we decided to quantify it,” said Dr. Samaan, associate professor of pediatrics at McMaster University and pediatric endocrinologist at McMaster Children’s Hospital in Hamilton, Ont.
“What we found was that in pediatric patients with type 2 diabetes, diabetic retinopathy is present in 1 in 14 youth. The risk of retinopathy increased significantly 5 years after diagnosis to almost one in four,” he noted.
“While we acknowledged that the number of diabetic retinopathy cases was relatively small and there was heterogeneity in studies, we were surprised that retinopathy rates rose so fast in the first few years after diabetes diagnosis,” Dr. Samaan indicated.
The findings signal that the increase in the prevalence of diabetic retinopathy is emerging decades earlier among children compared with adults with type 2 diabetes, the authors wrote in their article published online in JAMA Network Open.
“While the guidelines for eye care in children with type 2 diabetes recommend screening at diagnosis and annually afterward, these recommendations are not followed in almost half of these patients,” Dr. Samaan said. “There is a need to ensure that patients get screened to try and prevent or delay retinopathy onset and progression.”
Analyzing prevalence rates
Diabetic retinopathy is the leading cause of blindness in patients with type 2 diabetes. Between 21% and 39% of adults have diabetic retinopathy at diagnosis, with rates subsequently increasing, the authors wrote.
Dr. Samaan and colleagues conducted a systematic review and meta-analysis to estimate the global prevalence of diabetic retinopathy in pediatric patients with type 2 diabetes. They included studies that had a study population of at least 10 participants diagnosed at age 21 and younger, an observational study design, and prevalence data on diabetic retinopathy.
Among the 29 studies included, 6 were cross-sectional, 13 had a retrospective cohort design, and 10 had a prospective cohort design. Patients were diagnosed between age 6.5 and 21 years, and the diabetes duration ranged from 0 to 15 years after diagnosis.
The overall global prevalence of diabetic retinopathy in 5,924 pediatric patients was 7.0%. Prevalence varied by study design, ranging from 1.1% in cross-sectional studies to 6.5% in prospective cohort studies and 11.3% in retrospective cohort studies.
In the nine studies that reported diabetic retinopathy classification based on criteria, the prevalence of minimal-to-moderate nonproliferative diabetic retinopathy was 11.2%, the prevalence of severe nonproliferative diabetic retinopathy was 2.6%, the prevalence of proliferative diabetic retinopathy was 2.4%, and the prevalence of macular edema was 3.1%.
In the five studies that reported diabetic retinopathy diagnosis using fundoscopy, the prevalence was 0.5%. In the four studies that used 7-field stereoscopic fundus photography, the prevalence was 13.6%.
In the pooled analysis of 27 studies, the prevalence of diabetic retinopathy was 1.8% less than 2.5 years after diabetes diagnosis but more than doubled to 5.1% in years 2.5 to 5 and jumped to 28.8% more than 5 years after diagnosis.
Differences by sex, ethnicity
“We were also surprised that there was very limited evidence to understand the sex and race differences in retinopathy risk,” said Dr. Samaan. “Further research is warranted, considering that more girls develop type 2 diabetes than boys, and the risk of type 2 diabetes is higher in some racial groups.”
In addition, older age, longer diabetes duration, and higher hypertension prevalence were associated with diabetic retinopathy prevalence. There were no associations with obesity prevalence or mean age at diabetes diagnosis. However, patients who developed diabetic retinopathy had a higher mean A1c level of 1.4% compared to those without retinopathy.
Dr. Samaan and colleagues are continuing to research the comorbidities and complications that children with type 2 diabetes face as well as mechanisms that drive diabetes outcomes among children and adolescents.
For now, the findings highlight the importance of retinopathy screening and personalized diabetes treatment to protect vision, Dr. Samaan reiterated.
No funding source for the study was reported. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Nearly one in four children diagnosed with type 2 diabetes for 5 years or more develop diabetic retinopathy, according to a new report.
The global prevalence of diabetic retinopathy in pediatric patients with type 2 diabetes is about 7%, which appears to increase with age.
“In our clinical practice, we have seen an increase in children presenting with type 2 diabetes over the past few years. These patients present with multiple simultaneous comorbidities and complications like hypertension, fatty liver, and other conditions,” senior author M. Constantine Samaan, MD, told this news organization.
“The exact scale of diabetes-related eye disease was not clear, and we decided to quantify it,” said Dr. Samaan, associate professor of pediatrics at McMaster University and pediatric endocrinologist at McMaster Children’s Hospital in Hamilton, Ont.
“What we found was that in pediatric patients with type 2 diabetes, diabetic retinopathy is present in 1 in 14 youth. The risk of retinopathy increased significantly 5 years after diagnosis to almost one in four,” he noted.
“While we acknowledged that the number of diabetic retinopathy cases was relatively small and there was heterogeneity in studies, we were surprised that retinopathy rates rose so fast in the first few years after diabetes diagnosis,” Dr. Samaan indicated.
The findings signal that the increase in the prevalence of diabetic retinopathy is emerging decades earlier among children compared with adults with type 2 diabetes, the authors wrote in their article published online in JAMA Network Open.
“While the guidelines for eye care in children with type 2 diabetes recommend screening at diagnosis and annually afterward, these recommendations are not followed in almost half of these patients,” Dr. Samaan said. “There is a need to ensure that patients get screened to try and prevent or delay retinopathy onset and progression.”
Analyzing prevalence rates
Diabetic retinopathy is the leading cause of blindness in patients with type 2 diabetes. Between 21% and 39% of adults have diabetic retinopathy at diagnosis, with rates subsequently increasing, the authors wrote.
Dr. Samaan and colleagues conducted a systematic review and meta-analysis to estimate the global prevalence of diabetic retinopathy in pediatric patients with type 2 diabetes. They included studies that had a study population of at least 10 participants diagnosed at age 21 and younger, an observational study design, and prevalence data on diabetic retinopathy.
Among the 29 studies included, 6 were cross-sectional, 13 had a retrospective cohort design, and 10 had a prospective cohort design. Patients were diagnosed between age 6.5 and 21 years, and the diabetes duration ranged from 0 to 15 years after diagnosis.
The overall global prevalence of diabetic retinopathy in 5,924 pediatric patients was 7.0%. Prevalence varied by study design, ranging from 1.1% in cross-sectional studies to 6.5% in prospective cohort studies and 11.3% in retrospective cohort studies.
In the nine studies that reported diabetic retinopathy classification based on criteria, the prevalence of minimal-to-moderate nonproliferative diabetic retinopathy was 11.2%, the prevalence of severe nonproliferative diabetic retinopathy was 2.6%, the prevalence of proliferative diabetic retinopathy was 2.4%, and the prevalence of macular edema was 3.1%.
In the five studies that reported diabetic retinopathy diagnosis using fundoscopy, the prevalence was 0.5%. In the four studies that used 7-field stereoscopic fundus photography, the prevalence was 13.6%.
In the pooled analysis of 27 studies, the prevalence of diabetic retinopathy was 1.8% less than 2.5 years after diabetes diagnosis but more than doubled to 5.1% in years 2.5 to 5 and jumped to 28.8% more than 5 years after diagnosis.
Differences by sex, ethnicity
“We were also surprised that there was very limited evidence to understand the sex and race differences in retinopathy risk,” said Dr. Samaan. “Further research is warranted, considering that more girls develop type 2 diabetes than boys, and the risk of type 2 diabetes is higher in some racial groups.”
In addition, older age, longer diabetes duration, and higher hypertension prevalence were associated with diabetic retinopathy prevalence. There were no associations with obesity prevalence or mean age at diabetes diagnosis. However, patients who developed diabetic retinopathy had a higher mean A1c level of 1.4% compared to those without retinopathy.
Dr. Samaan and colleagues are continuing to research the comorbidities and complications that children with type 2 diabetes face as well as mechanisms that drive diabetes outcomes among children and adolescents.
For now, the findings highlight the importance of retinopathy screening and personalized diabetes treatment to protect vision, Dr. Samaan reiterated.
No funding source for the study was reported. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New state bill could protect docs prescribing abortion pills to out-of-state patients
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
California lawmakers are considering legislation to protect California physicians and pharmacists who prescribe abortion pills to out-of-state patients. The proposed law would shield health care providers who are legally performing their jobs in California from facing prosecution in another state or being extradited.
State Sen. Nancy Skinner, who introduced the bill, said the legislation is necessary in a fractured, post-Roe legal landscape where doctors in some states can face felony charges or civil penalties for providing reproductive health care. It’s part of a package of 17 new bills aiming to “strengthen California’s standing as a safe haven for abortion, contraception, and pregnancy care,” according to a press release.
“I’m trying to protect our healthcare practitioners so they can do their jobs, without fear,” Ms. Skinner said in a statement on March 24.
Most abortions are banned in 14 states after the Supreme Court overturned Roe v. Wade. Lawmakers in those states have established a variety of penalties for doctors, pharmacists, and other clinicians to provide abortion care or assist patients in obtaining abortions, including jail time, fines, and loss of professional licenses.
As a result, doctors in restrictive states have anguished over having to delay treatment for patients experiencing miscarriages, ectopic pregnancies, and other conditions until their lives are enough at risk to satisfy exceptions to state abortion laws.
“As a physician, I believe everyone deserves the care they need, regardless of where they live,” said Daniel Grossman, MD, a University of California, San Francisco, ob.gyn. professor who directs the university’s Advancing New Standards in Reproductive Health program.
“Since the fall of Roe v. Wade, patients are being forced to travel long distances – often over 500 miles – to access abortion care in a clinic. People should be able to access this essential care closer to home, including by telemedicine, which has been shown to be safe and effective. I am hopeful that SB 345 will provide additional legal protections that would allow California clinicians to help patients in other states,” he stated.
Other states, including New York, Vermont, New Jersey, Massachusetts, and Connecticut, have passed or are considering similar legislation to protect doctors using telemedicine to prescribe abortion medication to out-of-state patients. These laws come amid a growing push by some states and anti-abortion groups to severely restrict access to abortion pills.
Wyoming is the first state to explicitly ban the pills, although a judge on March 22 blocked that ban. And, in a closely watched case, a conservative federal judge could soon rule to ban sales of mifepristone, one of the medications in a two-pill regimen approved for abortions early in pregnancy.
California’s legislation protects clinicians from losing their California professional licenses if an out-of-state medical board takes action against them. It also allows clinicians to sue anyone who tries to legally interfere with the care they are providing.
It also covers California physicians prescribing contraceptives or gender-affirming care to out-of-state patients. At least 21 states are considering restrictions on gender-affirming care for minors and another 9 states have passed them, according to the advocacy group Human Rights Campaign. Courts have blocked the restrictions in some states.
“It’s understandable that states like California want to reassure their doctors ... that, if one of their patients is caught in one of those states and can’t get help locally, they can step up to help and feel safe in doing so,” said Matthew Wynia, MD, MPH, FACP, director of the Center for Bioethics and Humanities at the University of Colorado at Denver, Aurora.
“This is also a crazy development in terms of the law. It’s just one part of the legal mayhem that was predicted when the Supreme Court overturned Roe,” Dr. Wynia said of the growing number of bills protecting in-state doctors. These bills “will almost certainly end up being litigated over issues of interstate commerce, cross-state licensure and practice compacts, FDA regulations and authorities, and maybe more. It’s a huge mess, in which both doctors and patients are being hurt.”
A version of this article first appeared on Medscape.com.
De Marco gift to CHEST makes more than one dream possible
As a member of CHEST leadership for years, Bob De Marco, MD, FCCP, ruminated over new, exciting ways to increase support of the philanthropic efforts of the American College of Chest Physicians.
Dr. De Marco knows all too well that the percentage of CHEST members who donate to support CHEST’s philanthropic initiatives is – in a word - underwhelming. For those who are involved, they do so greatly and with their whole selves, but Dr. De Marco believed more could be done.
In the months leading up to the CHEST Annual Meeting 2022 in Nashville, Dr. De Marco discussed fundraising with CHEST staff and was already thinking ahead to CHEST 2023 in Hawai’i.
“That’s when it hit me – we could leverage Hawai’i to get donations and to expose people to CHEST philanthropy,” said Dr. De Marco. “Hawai’i is a dream destination, and that might be the exact motivation it would take to get that first donation from someone.”
Having a good idea is one thing, but making sure it happens requires individual commitment. Dr. DeMarco personally pledged to cover the cost of first-class airfare for two to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu. For a minimum donation of $250 to CHEST between September and the end of 2022, each donor would be entered into a drawing for a chance to win this dream trip.
“I thought to myself, who wouldn’t want this prize?” said Dr. De Marco.
“You get to go to paradise for free – with a guest – and attend a top tier educational conference. Knowing your entry supported an organization as deserving as CHEST is the cherry on top,” he added.
In launching the Hawai’i trip fundraiser before and during CHEST 2022, attendees from around the world were introduced to CHEST’s philanthropic efforts and its mission to champion lung health. Over $180,000 was donated during this time period, in no small part because of the Hawai’i travel reward.
“I’m happy to say that the fundraiser did a lot better than I expected, and I was elated to see all of the new donors,” said Dr. De Marco.
“It’s my hope that those first-time donors continue their support for all that we do to provide grants – community, research, and diversity – and support CHEST initiatives that impact patient care and change lives.”
During CHEST 2022, Dr. De Marco and other donors reflected on the organization’s philanthropic accomplishments and impact over the past decades.
Former grant recipients were invited to celebrate with donors and speak to what they were able to accomplish because of the support they received.
The celebration also introduced new CHEST initiatives, the First 5 Minutes® program and Bridging Specialties™: Timely Diagnosis for ILD. The former improves patient care through strengthened patient/clinician relationships, and the latter aims to eliminate gaps in diagnosing complex lung diseases like pulmonary fibrosis.
To all who donated to CHEST in 2022, Dr. De Marco said, “A sincere thank you to each and every one of you for helping us fulfill our mission. To the first-time donors, hopefully this will inspire you and your friends to be an active part of the CHEST family.”
And, to the winner of the trip, Dr. De Marco said, “A sincere congratulations and I hope you enjoy beautiful Hawai’i and your time at the meeting.”
Those who are interested in getting involved and supporting the philanthropic work of CHEST can contact chestphilanthropy@chestnet.org.
Out of the 150+ donors who gave $250 or more to CHEST between September 2022 and the end of 2022, longtime friend of CHEST, Noah Dorsky, was the recipient of two first-class tickets to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu.
Noah donated specifically to the Mark J. Rosen, MD, Master FCCP Endowment in honor of his late friend, Dr. Mark J. Rosen, who served as CHEST President from 2006 to 2007 and died in 2019.
"Mark was a remarkable doctor and valued life-long friend,” Noah said. “My continued support for CHEST is my way of honoring his memory and how much he meant to me and others."
Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. Before serving as President, Dr. Rosen served on the CHEST Board of Regents for many years. He held positions as Chair or member on numerous CHEST committees, including Education, Nominations, Membership, Marketing, and Finance.
Following his passing, Dr. Rosen’s wife, Ilene, stayed engaged with CHEST by creating the endowment in his name and attending the CHEST Annual Meeting every year to award the Rosen Cup to the winners of the annual CHEST Challenge.
Congratulations, Noah, and thank you for your faithful giving to support the work of CHEST.
As a member of CHEST leadership for years, Bob De Marco, MD, FCCP, ruminated over new, exciting ways to increase support of the philanthropic efforts of the American College of Chest Physicians.
Dr. De Marco knows all too well that the percentage of CHEST members who donate to support CHEST’s philanthropic initiatives is – in a word - underwhelming. For those who are involved, they do so greatly and with their whole selves, but Dr. De Marco believed more could be done.
In the months leading up to the CHEST Annual Meeting 2022 in Nashville, Dr. De Marco discussed fundraising with CHEST staff and was already thinking ahead to CHEST 2023 in Hawai’i.
“That’s when it hit me – we could leverage Hawai’i to get donations and to expose people to CHEST philanthropy,” said Dr. De Marco. “Hawai’i is a dream destination, and that might be the exact motivation it would take to get that first donation from someone.”
Having a good idea is one thing, but making sure it happens requires individual commitment. Dr. DeMarco personally pledged to cover the cost of first-class airfare for two to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu. For a minimum donation of $250 to CHEST between September and the end of 2022, each donor would be entered into a drawing for a chance to win this dream trip.
“I thought to myself, who wouldn’t want this prize?” said Dr. De Marco.
“You get to go to paradise for free – with a guest – and attend a top tier educational conference. Knowing your entry supported an organization as deserving as CHEST is the cherry on top,” he added.
In launching the Hawai’i trip fundraiser before and during CHEST 2022, attendees from around the world were introduced to CHEST’s philanthropic efforts and its mission to champion lung health. Over $180,000 was donated during this time period, in no small part because of the Hawai’i travel reward.
“I’m happy to say that the fundraiser did a lot better than I expected, and I was elated to see all of the new donors,” said Dr. De Marco.
“It’s my hope that those first-time donors continue their support for all that we do to provide grants – community, research, and diversity – and support CHEST initiatives that impact patient care and change lives.”
During CHEST 2022, Dr. De Marco and other donors reflected on the organization’s philanthropic accomplishments and impact over the past decades.
Former grant recipients were invited to celebrate with donors and speak to what they were able to accomplish because of the support they received.
The celebration also introduced new CHEST initiatives, the First 5 Minutes® program and Bridging Specialties™: Timely Diagnosis for ILD. The former improves patient care through strengthened patient/clinician relationships, and the latter aims to eliminate gaps in diagnosing complex lung diseases like pulmonary fibrosis.
To all who donated to CHEST in 2022, Dr. De Marco said, “A sincere thank you to each and every one of you for helping us fulfill our mission. To the first-time donors, hopefully this will inspire you and your friends to be an active part of the CHEST family.”
And, to the winner of the trip, Dr. De Marco said, “A sincere congratulations and I hope you enjoy beautiful Hawai’i and your time at the meeting.”
Those who are interested in getting involved and supporting the philanthropic work of CHEST can contact chestphilanthropy@chestnet.org.
Out of the 150+ donors who gave $250 or more to CHEST between September 2022 and the end of 2022, longtime friend of CHEST, Noah Dorsky, was the recipient of two first-class tickets to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu.
Noah donated specifically to the Mark J. Rosen, MD, Master FCCP Endowment in honor of his late friend, Dr. Mark J. Rosen, who served as CHEST President from 2006 to 2007 and died in 2019.
"Mark was a remarkable doctor and valued life-long friend,” Noah said. “My continued support for CHEST is my way of honoring his memory and how much he meant to me and others."
Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. Before serving as President, Dr. Rosen served on the CHEST Board of Regents for many years. He held positions as Chair or member on numerous CHEST committees, including Education, Nominations, Membership, Marketing, and Finance.
Following his passing, Dr. Rosen’s wife, Ilene, stayed engaged with CHEST by creating the endowment in his name and attending the CHEST Annual Meeting every year to award the Rosen Cup to the winners of the annual CHEST Challenge.
Congratulations, Noah, and thank you for your faithful giving to support the work of CHEST.
As a member of CHEST leadership for years, Bob De Marco, MD, FCCP, ruminated over new, exciting ways to increase support of the philanthropic efforts of the American College of Chest Physicians.
Dr. De Marco knows all too well that the percentage of CHEST members who donate to support CHEST’s philanthropic initiatives is – in a word - underwhelming. For those who are involved, they do so greatly and with their whole selves, but Dr. De Marco believed more could be done.
In the months leading up to the CHEST Annual Meeting 2022 in Nashville, Dr. De Marco discussed fundraising with CHEST staff and was already thinking ahead to CHEST 2023 in Hawai’i.
“That’s when it hit me – we could leverage Hawai’i to get donations and to expose people to CHEST philanthropy,” said Dr. De Marco. “Hawai’i is a dream destination, and that might be the exact motivation it would take to get that first donation from someone.”
Having a good idea is one thing, but making sure it happens requires individual commitment. Dr. DeMarco personally pledged to cover the cost of first-class airfare for two to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu. For a minimum donation of $250 to CHEST between September and the end of 2022, each donor would be entered into a drawing for a chance to win this dream trip.
“I thought to myself, who wouldn’t want this prize?” said Dr. De Marco.
“You get to go to paradise for free – with a guest – and attend a top tier educational conference. Knowing your entry supported an organization as deserving as CHEST is the cherry on top,” he added.
In launching the Hawai’i trip fundraiser before and during CHEST 2022, attendees from around the world were introduced to CHEST’s philanthropic efforts and its mission to champion lung health. Over $180,000 was donated during this time period, in no small part because of the Hawai’i travel reward.
“I’m happy to say that the fundraiser did a lot better than I expected, and I was elated to see all of the new donors,” said Dr. De Marco.
“It’s my hope that those first-time donors continue their support for all that we do to provide grants – community, research, and diversity – and support CHEST initiatives that impact patient care and change lives.”
During CHEST 2022, Dr. De Marco and other donors reflected on the organization’s philanthropic accomplishments and impact over the past decades.
Former grant recipients were invited to celebrate with donors and speak to what they were able to accomplish because of the support they received.
The celebration also introduced new CHEST initiatives, the First 5 Minutes® program and Bridging Specialties™: Timely Diagnosis for ILD. The former improves patient care through strengthened patient/clinician relationships, and the latter aims to eliminate gaps in diagnosing complex lung diseases like pulmonary fibrosis.
To all who donated to CHEST in 2022, Dr. De Marco said, “A sincere thank you to each and every one of you for helping us fulfill our mission. To the first-time donors, hopefully this will inspire you and your friends to be an active part of the CHEST family.”
And, to the winner of the trip, Dr. De Marco said, “A sincere congratulations and I hope you enjoy beautiful Hawai’i and your time at the meeting.”
Those who are interested in getting involved and supporting the philanthropic work of CHEST can contact chestphilanthropy@chestnet.org.
Out of the 150+ donors who gave $250 or more to CHEST between September 2022 and the end of 2022, longtime friend of CHEST, Noah Dorsky, was the recipient of two first-class tickets to Hawai’i, hotel accommodations, and registration to CHEST 2023 in Honolulu.
Noah donated specifically to the Mark J. Rosen, MD, Master FCCP Endowment in honor of his late friend, Dr. Mark J. Rosen, who served as CHEST President from 2006 to 2007 and died in 2019.
"Mark was a remarkable doctor and valued life-long friend,” Noah said. “My continued support for CHEST is my way of honoring his memory and how much he meant to me and others."
Dr. Rosen’s distinguished career in pulmonary and critical care medicine spanned more than 4 decades, marked by his deep commitments to medical education and patient care. Before serving as President, Dr. Rosen served on the CHEST Board of Regents for many years. He held positions as Chair or member on numerous CHEST committees, including Education, Nominations, Membership, Marketing, and Finance.
Following his passing, Dr. Rosen’s wife, Ilene, stayed engaged with CHEST by creating the endowment in his name and attending the CHEST Annual Meeting every year to award the Rosen Cup to the winners of the annual CHEST Challenge.
Congratulations, Noah, and thank you for your faithful giving to support the work of CHEST.
Acetaminophen as Renoprotective Treatment in a Patient With Severe Malaria
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
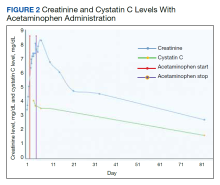
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
Renal impairment in severe falciparum malaria independently predicts a poor outcome in both adults and children.1 Prompt recognition of malaria-associated renal failure and immediate management with renal replacement therapy reduces mortality and can support the recovery of renal function.2-4 In addition, adjunctive treatment with acetaminophen has demonstrated improvement in the level of creatinine and reduced progression of kidney injury in a randomized, controlled trial of patients with severe falciparum malaria, particularly in patients with notable intravascular hemolysis.5 In this open-label, randomized controlled trial, 62 patients were randomly assigned to receive acetaminophen (n = 31) or no acetaminophen (n = 31).5 Antimalarial treatment was with IV artesunate, followed by artemether/lumefantrine. Median (IQR) reduction in creatinine after 72 hours was 23% (37, 18) in patients assigned to acetaminophen vs 14% (29, 0) in patients assigned to no acetaminophen (P = .04).5 Acetaminophen showed renoprotection without evidence of safety concerns in patients with severe falciparum malaria, especially those with prominent intravascular hemolysis.
Another study showed consistent findings in other malarial infections with prominent hemolysis, namely, Plasmodium knowlesi malaria. In the PACKNOW open-label, randomized controlled trial, 396 patients aged 12 to 96 years with knowlesi malaria of any severity were randomized to acetaminophen (500 mg or 1000 mg every 6 hours for 72 hours) vs no acetaminophen.6 All patients received artesunate and/or oral artemether-lumefantrine for malaria.6 No difference was seen overall in patients with acute kidney injury (AKI); however, in those with AKI and hemolysis, creatinine fell by a mean (SD) 34.5% (20.7) in the acetaminophen arm vs 25.9% (15.8) in the control arm (P = .04).6 Mixed-effects modeling demonstrated a benefit of acetaminophen at 72 hours (P = .04) and 1 week (P = .002) in patients with severe malaria and with AKI and hemolysis (P = .03 and P = .002, respectively).6
Earlier models suggest that the redox cycling of hemoproteins between ferric and ferryl states generates the radical species responsible for severe oxidative damage to the kidneys and subsequent renal impairment.7 Reduction of heme-ferryl radicals with therapeutic plasma concentrations of acetaminophen can inhibit this oxidative process.7 Rhabdomyolysis models treated with acetaminophen have shown reduced oxidative damage to the kidneys and improved renal functioning, supporting acetaminophen as a potential therapeutic option for disease processes involving hemoprotein-mediated oxidative injury.7 In this case report, we discuss the use of acetaminophen as a renoprotective treatment in a patient with renal impairment associated with severe falciparum malaria.
Case Presentation
A 50-year-old man with comorbidities, including hypertension, hyperlipidemia, and chronic kidney disease stage 2, with a baseline creatinine level of 1.4 mg/dL presented with severe falciparum malaria with renal impairment. About 7 months prior, the patient received treatment for his first known case of Plasmodium falciparum (P falciparum) infection. He again contracted P falciparum for a second time after traveling to a malaria-endemic country without taking prophylactic medication before travel.
The patient reported fevers, chills, night sweats, and progressive fatigue. His vital signs recorded a fever of 38.9 ºC with tachycardia and relative hypotension. A thin blood smear revealed P falciparum with approximately 8.5% parasitemia. Laboratory tests confirmed hemolytic anemia and thrombocytopenia reflected by consistently decreased hemoglobin, hematocrit, haptoglobin, and platelets with elevated lactate dehydrogenase and hyperbilirubinemia. Initial renal function testing included an elevated creatinine level of 3.4 mg/dL and an elevated blood urea nitrogen (BUN) level of 45 mg/dL.
The patient received multiple boluses of IV isotonic fluids and a single maximum dose of atovaquone and proguanil before procurement of IV artesunate to manage the malaria. Good response with IV artesunate lowered parasitemia from a high at admission of 10.5% to 0.1% before transitioning to oral artemether and lumefantrine. Concomitantly, the patient’s oliguric renal failure continued to progress early during the hospital stay, and he consented to anticipated dialysis.
To halt progression of his renal injury, salvage renal function, and avoid dialysis, the nephrology team considered acetaminophen 975 mg tablets every 6 hours for 72 hours per the Plewes and colleagues randomized trial.5 The patient met the criteria for severe falciparum malaria per the inclusion criteria in the Plewes and colleagues study and was deemed eligible for acetaminophen-based adjunctive treatment. The patient discussed and considered both dialysis and a trial of acetaminophen with the nephrology team, and he understood all the associated risks and benefits, including liver failure. The patient agreed to a trial of acetaminophen with close monitoring of his liver function.
Before starting acetaminophen, the patient’s aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels both measured 53 IU/L or 1.3 times the upper limit of normal (Figure 2).
Discussion
AKI in malaria predominantly occurs with P falciparum infection and represents a significant independent factor in determining morbidity and mortality in adults with severe malaria.8 In severe malaria, any hemodynamic compromise likely contributes to the development of acute tubular necrosis (ATN) with insensible losses and poor intake decreasing renal perfusion.8 Direct tubular injury from hemoglobinuria or less commonly myoglobinuria from concomitant rhabdomyolysis may also drive malarial AKI.8 In addition, proposed mechanisms explaining the pathogenesis of malarial AKI include ATN secondary to disruptions in renal microvasculature, immune dysregulation with proinflammatory reactions within the kidneys, and metabolic disturbances.8 Oxidate tubular damage caused by the release of cell-free hemoglobin during red blood cell hemolysis represents 1 form of metabolic derangement possibly responsible for renal impairment.8 Acetaminophen administration may help mitigate this oxidative stress, especially in cases of significant hemolysis.5
In this case of severe falciparum malaria, the patient demonstrated renal impairment with measured falciparum parasitemia. His creatinine level and BUN appeared to stabilize and improve after 72 hours of acetaminophen administration. A recovery of urine output and improvement in cystatin C occurred during the 72 hours of acetaminophen usage. Despite the patient’s underlying chronic kidney disease, measured proteinuria, and significant changes in renal architecture revealed by ultrasound, he never showed signs of uremia, fluid overload, electrolyte derangements, or acidosis requiring urgent renal replacement therapy.
The patient’s treatment for severe falciparum malaria, including a combination of supportive management, acetaminophen, and IV antimalarials, resulted in the resolution of parasitemia and symptoms with some recovery of renal function without necessitating renal replacement therapy. Maximum daily doses of acetaminophen compared with the control in the Plewes and colleagues acetaminophen trial resulted in moderate increases in aminotransferases not rising to the criteria of hepatotoxicity described in Hy’s law.5 Following acetaminophen administration, in this case, AST and ALT levels peaked at 130 and 168 IU/L, 2.8 and 3.8 times the upper limits of normal, respectively. These mild, asymptomatic elevations in aminotransferases recovered to within normal limits, measuring 24 and 13 IU/L at the follow-up.
Conclusions
The demonstrated recovery in renal function, with only a transient, moderate increase in aminotransferases, supports the value of adjunctive acetaminophen as a renoprotective treatment in severe malaria. This simple, readily available treatment may significantly alter the morbidity and mortality associated with severe malaria.
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
1. von Seidlein L, Olaosebikan R, Hendriksen IC, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8): 1080-1090. doi:10.1093/cid/cis034
2. Trang TT, Phu NH, Vinh H, et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis. 1992;15(5):874-880. doi:10.1093/clind/15.5.874
3. Phu NH, Hien TT, Mai NT, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902. doi:10.1056/NEJMoa020074
4. Wiwanitkit V. Peritoneal dialysis in falciparum malaria-induced acute renal failure: an appraisal on Thai patients. Ren Fail. 2005;27(5):649. doi:10.1080/08860220500200924
5. Plewes K, Kingston HWF, Ghose A, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018;67(7):991-999. doi:10.1093/cid/ciy213
6. Cooper DJ, Grigg MJ, Plewes K, et al. The effect of regularly dosed acetaminophen vs no acetaminophen on renal function in plasmodium knowlesi malaria (PACKNOW): a randomized, controlled trial. Clin Infect Dis. 2022;75(8):1379-1388. doi:10.1093/cid/ciac152
7. Boutaud O, Moore KP, Reeder BJ, et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc Natl Acad Sci. 2010;107(6):2699-2704. doi:10.1073/pnas.0910174107
8. Chellappan A, Bhadauria DS. Acute kidney injury in malaria: an update. Clin Queries: Nephrol. 2016;5(1):26-32. doi:10.1016/j.cqn.2016.04.004
DMARDs taper-to-discontinuation trial deemed inconclusive
The small size of a new study of the feasibility of tapering conventional synthetic disease-modifying antirheumatic drug (csDMARD) doses to half for patients with rheumatoid arthritis in remission, and then to zero, makes suspect the validity of its finding of no statistical difference between continuing half doses and stopping altogether, according to one rheumatologist’s analysis.
In the open-label, randomized trial of 56 patients, which was published as a research letter in JAMA, more patients in the group that discontinued csDMARDs experienced flares within 1 year than did the half-dose group, but this difference was not statistically significant.
Most patients in the drug-free group did not experience disease flares, the authors note.
“The results show that in this population, a majority of patients remained flare-free for at least a year after csDMARD discontinuation. This highlights a potential for drug-free remission in a subgroup of RA patients, and the data provide a basis for shared decision-making in this patient group. We know that tapering is a common question from patients and thus think that the data are especially clinically relevant,” first author Siri Lillegraven, MD, MPH, PhD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at the Diakonhjemmet Hospital, Oslo, said in an interview.
While several studies have demonstrated that patients with RA can maintain remission on lower doses of medication, James O’Dell, MD, chief of the Division of Rheumatology at the University of Nebraska Medical Center, Omaha, urged caution in interpreting these results because the study was so small – just 56 patients. “Every analysis they did favored staying on treatment, but the confidence interval slightly crossed null, so they can’t say [that group] was superior,” Dr. O’Dell said in an interview. He was not involved in the research. “Had this study been double this size and they got the same results, they would have clearly shown that staying on medicines were superior,” he said.
Dr. Lillegraven acknowledged the impact of the trial’s small sample size. “This is a study with a limited study sample, and it is conceivable that a larger study might have shown a statistical difference between the groups,” she said.
In addition to the small number of patients in the study, Dr. O’Dell also noted that this study group was already a selected group of patients who had maintained remission on half-dose therapy for at least 1 year. Even then, “what they showed was that 39% of the patients who they discontinued [then] flared, compared with 17% when they didn’t taper [off medication],” he said. “That’s a pretty important clinical difference.”
While Dr. O’Dell thinks the study was too small to inform practice, he emphasized that tapering off full doses of medications can be beneficial for patients with RA that has been in remission for 6 months or longer. “It seems to take less medicines to keep somebody in remission than it did to get them there in the first place,” he said. “I come out strongly in favor of tapering medications in rheumatoid arthritis patients who are in remission, and that includes tapering and stopping biologics if patients are on conventional therapy,” he added, “but tapering patients off all of their conventional therapy is something that I think is a bridge too far.”
This trial was the second part of the ARCTIC REWIND study, which involved patients with RA that was in sustained remission, per their Disease Activity Score. In the first part of the trial, 160 participants from 10 hospitals in Norway were enrolled and were randomly assigned to either continue their standard csDMARD dosing or taper down to a half dose. Patients whose doses were tapered to a half dose and whose conditions were in remission for 1 year were eligible for the second half the study.
Of the 56 participants who were included, 26 discontinued csDMARD therapy, while 30 continued taking a half dose for 12 months of follow-up. Most patients in both groups had received methotrexate monotherapy (21 in the discontinuation group and 26 in the continued half-dosing group). Triple therapy (methotrexate, sulfasalazine, and hydroxychloroquine) was used by three patients in the discontinuation group and by two in the half-dose group. Two additional patients in the discontinuation group and two in the half-dose group took other mono/duo therapies. Clinic visits occurred every 4 months; visits were more frequent if there was an increase in disease activity. For patients who experienced a disease flare, full-dose csDMARD treatment was resumed.
Ten patients in the discontinuation group experienced flares during 1 year, compared with five patients in the half-dose group. The risk difference between the two groups was not statistically significant (RD, 21.5%; 95% CI, –3.4% to 49.7%). The median time to flare was 179 days in the discontinuation group and 133 days in the half-dose group.
Of those who experienced flares, 8 of 10 patients in the discontinuation group and 2 of 5 in the half-dose group regained remission when full-dose therapy was resumed.
The study was funded by the Research Council of Norway and the South-Eastern Norway Regional Health Authorities. Many of the authors disclosed financial ties to pharmaceutical companies. Dr. O’Dell disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The small size of a new study of the feasibility of tapering conventional synthetic disease-modifying antirheumatic drug (csDMARD) doses to half for patients with rheumatoid arthritis in remission, and then to zero, makes suspect the validity of its finding of no statistical difference between continuing half doses and stopping altogether, according to one rheumatologist’s analysis.
In the open-label, randomized trial of 56 patients, which was published as a research letter in JAMA, more patients in the group that discontinued csDMARDs experienced flares within 1 year than did the half-dose group, but this difference was not statistically significant.
Most patients in the drug-free group did not experience disease flares, the authors note.
“The results show that in this population, a majority of patients remained flare-free for at least a year after csDMARD discontinuation. This highlights a potential for drug-free remission in a subgroup of RA patients, and the data provide a basis for shared decision-making in this patient group. We know that tapering is a common question from patients and thus think that the data are especially clinically relevant,” first author Siri Lillegraven, MD, MPH, PhD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at the Diakonhjemmet Hospital, Oslo, said in an interview.
While several studies have demonstrated that patients with RA can maintain remission on lower doses of medication, James O’Dell, MD, chief of the Division of Rheumatology at the University of Nebraska Medical Center, Omaha, urged caution in interpreting these results because the study was so small – just 56 patients. “Every analysis they did favored staying on treatment, but the confidence interval slightly crossed null, so they can’t say [that group] was superior,” Dr. O’Dell said in an interview. He was not involved in the research. “Had this study been double this size and they got the same results, they would have clearly shown that staying on medicines were superior,” he said.
Dr. Lillegraven acknowledged the impact of the trial’s small sample size. “This is a study with a limited study sample, and it is conceivable that a larger study might have shown a statistical difference between the groups,” she said.
In addition to the small number of patients in the study, Dr. O’Dell also noted that this study group was already a selected group of patients who had maintained remission on half-dose therapy for at least 1 year. Even then, “what they showed was that 39% of the patients who they discontinued [then] flared, compared with 17% when they didn’t taper [off medication],” he said. “That’s a pretty important clinical difference.”
While Dr. O’Dell thinks the study was too small to inform practice, he emphasized that tapering off full doses of medications can be beneficial for patients with RA that has been in remission for 6 months or longer. “It seems to take less medicines to keep somebody in remission than it did to get them there in the first place,” he said. “I come out strongly in favor of tapering medications in rheumatoid arthritis patients who are in remission, and that includes tapering and stopping biologics if patients are on conventional therapy,” he added, “but tapering patients off all of their conventional therapy is something that I think is a bridge too far.”
This trial was the second part of the ARCTIC REWIND study, which involved patients with RA that was in sustained remission, per their Disease Activity Score. In the first part of the trial, 160 participants from 10 hospitals in Norway were enrolled and were randomly assigned to either continue their standard csDMARD dosing or taper down to a half dose. Patients whose doses were tapered to a half dose and whose conditions were in remission for 1 year were eligible for the second half the study.
Of the 56 participants who were included, 26 discontinued csDMARD therapy, while 30 continued taking a half dose for 12 months of follow-up. Most patients in both groups had received methotrexate monotherapy (21 in the discontinuation group and 26 in the continued half-dosing group). Triple therapy (methotrexate, sulfasalazine, and hydroxychloroquine) was used by three patients in the discontinuation group and by two in the half-dose group. Two additional patients in the discontinuation group and two in the half-dose group took other mono/duo therapies. Clinic visits occurred every 4 months; visits were more frequent if there was an increase in disease activity. For patients who experienced a disease flare, full-dose csDMARD treatment was resumed.
Ten patients in the discontinuation group experienced flares during 1 year, compared with five patients in the half-dose group. The risk difference between the two groups was not statistically significant (RD, 21.5%; 95% CI, –3.4% to 49.7%). The median time to flare was 179 days in the discontinuation group and 133 days in the half-dose group.
Of those who experienced flares, 8 of 10 patients in the discontinuation group and 2 of 5 in the half-dose group regained remission when full-dose therapy was resumed.
The study was funded by the Research Council of Norway and the South-Eastern Norway Regional Health Authorities. Many of the authors disclosed financial ties to pharmaceutical companies. Dr. O’Dell disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The small size of a new study of the feasibility of tapering conventional synthetic disease-modifying antirheumatic drug (csDMARD) doses to half for patients with rheumatoid arthritis in remission, and then to zero, makes suspect the validity of its finding of no statistical difference between continuing half doses and stopping altogether, according to one rheumatologist’s analysis.
In the open-label, randomized trial of 56 patients, which was published as a research letter in JAMA, more patients in the group that discontinued csDMARDs experienced flares within 1 year than did the half-dose group, but this difference was not statistically significant.
Most patients in the drug-free group did not experience disease flares, the authors note.
“The results show that in this population, a majority of patients remained flare-free for at least a year after csDMARD discontinuation. This highlights a potential for drug-free remission in a subgroup of RA patients, and the data provide a basis for shared decision-making in this patient group. We know that tapering is a common question from patients and thus think that the data are especially clinically relevant,” first author Siri Lillegraven, MD, MPH, PhD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases at the Diakonhjemmet Hospital, Oslo, said in an interview.
While several studies have demonstrated that patients with RA can maintain remission on lower doses of medication, James O’Dell, MD, chief of the Division of Rheumatology at the University of Nebraska Medical Center, Omaha, urged caution in interpreting these results because the study was so small – just 56 patients. “Every analysis they did favored staying on treatment, but the confidence interval slightly crossed null, so they can’t say [that group] was superior,” Dr. O’Dell said in an interview. He was not involved in the research. “Had this study been double this size and they got the same results, they would have clearly shown that staying on medicines were superior,” he said.
Dr. Lillegraven acknowledged the impact of the trial’s small sample size. “This is a study with a limited study sample, and it is conceivable that a larger study might have shown a statistical difference between the groups,” she said.
In addition to the small number of patients in the study, Dr. O’Dell also noted that this study group was already a selected group of patients who had maintained remission on half-dose therapy for at least 1 year. Even then, “what they showed was that 39% of the patients who they discontinued [then] flared, compared with 17% when they didn’t taper [off medication],” he said. “That’s a pretty important clinical difference.”
While Dr. O’Dell thinks the study was too small to inform practice, he emphasized that tapering off full doses of medications can be beneficial for patients with RA that has been in remission for 6 months or longer. “It seems to take less medicines to keep somebody in remission than it did to get them there in the first place,” he said. “I come out strongly in favor of tapering medications in rheumatoid arthritis patients who are in remission, and that includes tapering and stopping biologics if patients are on conventional therapy,” he added, “but tapering patients off all of their conventional therapy is something that I think is a bridge too far.”
This trial was the second part of the ARCTIC REWIND study, which involved patients with RA that was in sustained remission, per their Disease Activity Score. In the first part of the trial, 160 participants from 10 hospitals in Norway were enrolled and were randomly assigned to either continue their standard csDMARD dosing or taper down to a half dose. Patients whose doses were tapered to a half dose and whose conditions were in remission for 1 year were eligible for the second half the study.
Of the 56 participants who were included, 26 discontinued csDMARD therapy, while 30 continued taking a half dose for 12 months of follow-up. Most patients in both groups had received methotrexate monotherapy (21 in the discontinuation group and 26 in the continued half-dosing group). Triple therapy (methotrexate, sulfasalazine, and hydroxychloroquine) was used by three patients in the discontinuation group and by two in the half-dose group. Two additional patients in the discontinuation group and two in the half-dose group took other mono/duo therapies. Clinic visits occurred every 4 months; visits were more frequent if there was an increase in disease activity. For patients who experienced a disease flare, full-dose csDMARD treatment was resumed.
Ten patients in the discontinuation group experienced flares during 1 year, compared with five patients in the half-dose group. The risk difference between the two groups was not statistically significant (RD, 21.5%; 95% CI, –3.4% to 49.7%). The median time to flare was 179 days in the discontinuation group and 133 days in the half-dose group.
Of those who experienced flares, 8 of 10 patients in the discontinuation group and 2 of 5 in the half-dose group regained remission when full-dose therapy was resumed.
The study was funded by the Research Council of Norway and the South-Eastern Norway Regional Health Authorities. Many of the authors disclosed financial ties to pharmaceutical companies. Dr. O’Dell disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA
Safety, efficacy of analgesics for low back pain ‘uncertain’
Higher-quality randomized controlled trials of head-to-head comparisons are needed, study investigator Michael A. Wewege, PhD candidate, research fellow, University of New South Wales and Neuroscience Research Australia, Sydney, said in an interview.
“Until then, doctors should use caution when prescribing analgesic medicines for adults with nonspecific acute low back pain. They should use this new evidence in line with their own expertise and the patient sitting in front of them when making any decision about a medication,” he added.
The findings were published online in the BMJ.
Poor quality evidence
Analgesics such as ibuprofen, acetaminophen, and codeine are widely used to treat nonspecific low-back pain, which is defined as pain lasting less than 6 weeks, but evidence for the comparative efficacy of these agents is limited.
To fill this knowledge gap, the researchers conducted a systematic review and analysis of controlled trials comparing analgesics with another analgesic, placebo, or no treatment in patients with acute, nonspecific low back pain.
The review involved 98 randomized controlled trials that included 15,134 adults (49% women) aged 30-60 years with pain duration ranging from 24 hours to 21 days. The median baseline pain intensity was 65 on a pain scale of 0-100.
Of the included trials, 39% were placebo controlled, 67% masked both participants and clinicians, and 41% reported industry sponsorship.
The studies compared an analgesic medicine with another analgesic, placebo, or no treatment comprised of usual care or being placed on a wait list.
Study medications, which had to be approved in the United States, Europe, or Australia, included nonsteroidal anti-inflammatory drugs, paracetamol, opioids, anticonvulsants, antidepressants, muscle relaxants, and corticosteroids.
These drugs were administered systemically as a single drug or in combination formulations, at any dose.
Researchers used a network meta-analysis, which combines direct and indirect information across a network of randomized clinical trials to estimate the comparative effectiveness of multiple treatments.
The primary outcomes were reductions in low back pain intensity (measured with a visual analogue scale), numerical rating scale or another ordinal scale, and safety as indicated by the number of participants who had any adverse event.
Investigators found several medications were associated with large reductions in pain intensity, compared with placebo, though with low or very low confidence.
Low or very low confidence was found for reduced pain intensity after treatment with tolperisone (mean difference, −26.1; 95% confidence interval, −34.0 to −18.2), aceclofenac plus tizanidine (mean difference, −26.1; 95% CI, −38.5 to −13.6), pregabalin (mean difference, −24.7; 95% CI, −34.6 to −14.7), and 14 other medicines, compared with placebo, the researchers report.
In addition, they found low or very low confidence for no difference between the effects of several of these medications.
Increased adverse events had moderate to very low confidence with tramadol (risk ratio, 2.6; 95% CI, 1.5-4.5), paracetamol plus sustained release tramadol (RR, 2.4; 95% CI, 1.5-3.8), baclofen (RR, 2.3; 95% CI, 1.5-3.4), and paracetamol plus tramadol (RR, 2.1; 95% CI, 1.3-3.4), compared with placebo, the investigators add.
“These medicines could increase the risk of adverse events, compared with other medicines with moderate to low confidence. Moderate to low confidence was also noted for secondary outcomes and secondary analysis of medicine classes,” the researchers note.
The review suggested 14 additional comparisons favored the treatment over placebo, all with very low confidence except for one with low confidence.
In the 68 trials that included the number of participants reporting an adverse event, there was moderate confidence for increased adverse events with the opioid tramadol (RR, 2.6; 95% CI, 1.5-4.5), paracetamol plus sustained release tramadol (RR, 2.4; 95% CI, 1.5-3.8), paracetamol plus tramadol (RR, 2.1; 95% CI, 1.3-3.4), and low confidence for baclofen (RR, 2.3; 1.5-3.4), compared with placebo.
The review also uncovered moderate to low confidence for secondary outcomes, which included low back-specific function, serious adverse events, and acceptability (number of participants who dropped out).
Unexpected findings
The new results were somewhat unexpected, said Mr. Wewege.
“When we set out to do this review, we envisioned the evidence would be a lot more comprehensive. We didn’t think it would be so disconnected and there would be so few trials looking at the different comparisons that would lead us to have low confidence in most of the findings.”
Various factors contributed to this low confidence, he said. One was the risk of bias – about 90% of trials had some concerns or high risk of bias. Another factor was the heterogeneity in effect estimates.
Most of the evidence is based on studies comparing different analgesics to placebo, Mr. Wewege noted. The lack of head-to-head drug comparisons is because “the easiest way to get a drug approved is just to demonstrate it’s better than placebo,” he said.
In addition to these new findings, clinicians should consider a medication’s availability, their own expertise, and patient preferences when selecting an analgesic, said Mr. Wewege. He noted most patients with acute low back pain get better within a few weeks without any intervention.
“Patients should be reassured that things will heal naturally and that they are not going to be in pain forever,” he said.
Determining optimal treatment is key
Chris Gilligan, MD, associate chief medical officer, Brigham and Women’s Hospital, and associate professor of anesthesia, Harvard Medical School, both in Boston, said determining which medications are optimal is “key,” as acute low back pain is very common and analgesics are used frequently.
The new review does provide information on which medications have the strongest evidence for pain reduction, said Dr. Gilligan. “On the one hand, it directionally points you towards certain medications, and even certain classes of medication, for comparative effectiveness.”
However, he said, the confidence for this effectiveness is low or very low, “so I wouldn’t overweight it.”
The data on adverse effects, where the confidence is mostly moderate to low, might have more of an influence on prescribing, he said.
“For example, there’s some indication tramadol may be more closely associated with adverse events in patients with acute low back pain and that would add to our caution about using tramadol; it’s not that we would never use it, but [we]would take that into account.”
Dr. Gilligan agrees clinicians should be cautious about prescribing analgesics for low back pain. One reason for being conservative in terms of treatments, he noted, is that “acute low back pain has a very favorable natural history.”
While clinical practice guidelines recommend nonpharmacologic therapies as first- and second-line treatment for acute, nonspecific low back pain, Dr. Gilligan noted that as with drugs, evidence for nondrug therapies also has low or very low confidence.
The study received funding from a 2020 Exercise Physiology Research (Consumables) Grant from the University of New South Wales, which was used to obtain translations of studies published in languages other than English.
Mr. Wewege was supported by a Postgraduate Scholarship from the National Health and Medical Research Council of Australia, a School of Medical Sciences Top-Up Scholarship from the University of New South Wales, and a PhD Supplementary Scholarship from Neuroscience Research Australia. Dr. Gilligan reports that he conducts clinical trials with companies and groups, including the National Institutes of Health related to medications, devices, and procedures for pain.
A version of this article first appeared on Medscape.com.
Higher-quality randomized controlled trials of head-to-head comparisons are needed, study investigator Michael A. Wewege, PhD candidate, research fellow, University of New South Wales and Neuroscience Research Australia, Sydney, said in an interview.
“Until then, doctors should use caution when prescribing analgesic medicines for adults with nonspecific acute low back pain. They should use this new evidence in line with their own expertise and the patient sitting in front of them when making any decision about a medication,” he added.
The findings were published online in the BMJ.
Poor quality evidence
Analgesics such as ibuprofen, acetaminophen, and codeine are widely used to treat nonspecific low-back pain, which is defined as pain lasting less than 6 weeks, but evidence for the comparative efficacy of these agents is limited.
To fill this knowledge gap, the researchers conducted a systematic review and analysis of controlled trials comparing analgesics with another analgesic, placebo, or no treatment in patients with acute, nonspecific low back pain.
The review involved 98 randomized controlled trials that included 15,134 adults (49% women) aged 30-60 years with pain duration ranging from 24 hours to 21 days. The median baseline pain intensity was 65 on a pain scale of 0-100.
Of the included trials, 39% were placebo controlled, 67% masked both participants and clinicians, and 41% reported industry sponsorship.
The studies compared an analgesic medicine with another analgesic, placebo, or no treatment comprised of usual care or being placed on a wait list.
Study medications, which had to be approved in the United States, Europe, or Australia, included nonsteroidal anti-inflammatory drugs, paracetamol, opioids, anticonvulsants, antidepressants, muscle relaxants, and corticosteroids.
These drugs were administered systemically as a single drug or in combination formulations, at any dose.
Researchers used a network meta-analysis, which combines direct and indirect information across a network of randomized clinical trials to estimate the comparative effectiveness of multiple treatments.
The primary outcomes were reductions in low back pain intensity (measured with a visual analogue scale), numerical rating scale or another ordinal scale, and safety as indicated by the number of participants who had any adverse event.
Investigators found several medications were associated with large reductions in pain intensity, compared with placebo, though with low or very low confidence.
Low or very low confidence was found for reduced pain intensity after treatment with tolperisone (mean difference, −26.1; 95% confidence interval, −34.0 to −18.2), aceclofenac plus tizanidine (mean difference, −26.1; 95% CI, −38.5 to −13.6), pregabalin (mean difference, −24.7; 95% CI, −34.6 to −14.7), and 14 other medicines, compared with placebo, the researchers report.
In addition, they found low or very low confidence for no difference between the effects of several of these medications.
Increased adverse events had moderate to very low confidence with tramadol (risk ratio, 2.6; 95% CI, 1.5-4.5), paracetamol plus sustained release tramadol (RR, 2.4; 95% CI, 1.5-3.8), baclofen (RR, 2.3; 95% CI, 1.5-3.4), and paracetamol plus tramadol (RR, 2.1; 95% CI, 1.3-3.4), compared with placebo, the investigators add.
“These medicines could increase the risk of adverse events, compared with other medicines with moderate to low confidence. Moderate to low confidence was also noted for secondary outcomes and secondary analysis of medicine classes,” the researchers note.
The review suggested 14 additional comparisons favored the treatment over placebo, all with very low confidence except for one with low confidence.
In the 68 trials that included the number of participants reporting an adverse event, there was moderate confidence for increased adverse events with the opioid tramadol (RR, 2.6; 95% CI, 1.5-4.5), paracetamol plus sustained release tramadol (RR, 2.4; 95% CI, 1.5-3.8), paracetamol plus tramadol (RR, 2.1; 95% CI, 1.3-3.4), and low confidence for baclofen (RR, 2.3; 1.5-3.4), compared with placebo.
The review also uncovered moderate to low confidence for secondary outcomes, which included low back-specific function, serious adverse events, and acceptability (number of participants who dropped out).
Unexpected findings
The new results were somewhat unexpected, said Mr. Wewege.
“When we set out to do this review, we envisioned the evidence would be a lot more comprehensive. We didn’t think it would be so disconnected and there would be so few trials looking at the different comparisons that would lead us to have low confidence in most of the findings.”
Various factors contributed to this low confidence, he said. One was the risk of bias – about 90% of trials had some concerns or high risk of bias. Another factor was the heterogeneity in effect estimates.
Most of the evidence is based on studies comparing different analgesics to placebo, Mr. Wewege noted. The lack of head-to-head drug comparisons is because “the easiest way to get a drug approved is just to demonstrate it’s better than placebo,” he said.
In addition to these new findings, clinicians should consider a medication’s availability, their own expertise, and patient preferences when selecting an analgesic, said Mr. Wewege. He noted most patients with acute low back pain get better within a few weeks without any intervention.
“Patients should be reassured that things will heal naturally and that they are not going to be in pain forever,” he said.
Determining optimal treatment is key
Chris Gilligan, MD, associate chief medical officer, Brigham and Women’s Hospital, and associate professor of anesthesia, Harvard Medical School, both in Boston, said determining which medications are optimal is “key,” as acute low back pain is very common and analgesics are used frequently.
The new review does provide information on which medications have the strongest evidence for pain reduction, said Dr. Gilligan. “On the one hand, it directionally points you towards certain medications, and even certain classes of medication, for comparative effectiveness.”
However, he said, the confidence for this effectiveness is low or very low, “so I wouldn’t overweight it.”
The data on adverse effects, where the confidence is mostly moderate to low, might have more of an influence on prescribing, he said.
“For example, there’s some indication tramadol may be more closely associated with adverse events in patients with acute low back pain and that would add to our caution about using tramadol; it’s not that we would never use it, but [we]would take that into account.”
Dr. Gilligan agrees clinicians should be cautious about prescribing analgesics for low back pain. One reason for being conservative in terms of treatments, he noted, is that “acute low back pain has a very favorable natural history.”
While clinical practice guidelines recommend nonpharmacologic therapies as first- and second-line treatment for acute, nonspecific low back pain, Dr. Gilligan noted that as with drugs, evidence for nondrug therapies also has low or very low confidence.
The study received funding from a 2020 Exercise Physiology Research (Consumables) Grant from the University of New South Wales, which was used to obtain translations of studies published in languages other than English.
Mr. Wewege was supported by a Postgraduate Scholarship from the National Health and Medical Research Council of Australia, a School of Medical Sciences Top-Up Scholarship from the University of New South Wales, and a PhD Supplementary Scholarship from Neuroscience Research Australia. Dr. Gilligan reports that he conducts clinical trials with companies and groups, including the National Institutes of Health related to medications, devices, and procedures for pain.
A version of this article first appeared on Medscape.com.
Higher-quality randomized controlled trials of head-to-head comparisons are needed, study investigator Michael A. Wewege, PhD candidate, research fellow, University of New South Wales and Neuroscience Research Australia, Sydney, said in an interview.
“Until then, doctors should use caution when prescribing analgesic medicines for adults with nonspecific acute low back pain. They should use this new evidence in line with their own expertise and the patient sitting in front of them when making any decision about a medication,” he added.
The findings were published online in the BMJ.
Poor quality evidence
Analgesics such as ibuprofen, acetaminophen, and codeine are widely used to treat nonspecific low-back pain, which is defined as pain lasting less than 6 weeks, but evidence for the comparative efficacy of these agents is limited.
To fill this knowledge gap, the researchers conducted a systematic review and analysis of controlled trials comparing analgesics with another analgesic, placebo, or no treatment in patients with acute, nonspecific low back pain.
The review involved 98 randomized controlled trials that included 15,134 adults (49% women) aged 30-60 years with pain duration ranging from 24 hours to 21 days. The median baseline pain intensity was 65 on a pain scale of 0-100.
Of the included trials, 39% were placebo controlled, 67% masked both participants and clinicians, and 41% reported industry sponsorship.
The studies compared an analgesic medicine with another analgesic, placebo, or no treatment comprised of usual care or being placed on a wait list.
Study medications, which had to be approved in the United States, Europe, or Australia, included nonsteroidal anti-inflammatory drugs, paracetamol, opioids, anticonvulsants, antidepressants, muscle relaxants, and corticosteroids.
These drugs were administered systemically as a single drug or in combination formulations, at any dose.
Researchers used a network meta-analysis, which combines direct and indirect information across a network of randomized clinical trials to estimate the comparative effectiveness of multiple treatments.
The primary outcomes were reductions in low back pain intensity (measured with a visual analogue scale), numerical rating scale or another ordinal scale, and safety as indicated by the number of participants who had any adverse event.
Investigators found several medications were associated with large reductions in pain intensity, compared with placebo, though with low or very low confidence.
Low or very low confidence was found for reduced pain intensity after treatment with tolperisone (mean difference, −26.1; 95% confidence interval, −34.0 to −18.2), aceclofenac plus tizanidine (mean difference, −26.1; 95% CI, −38.5 to −13.6), pregabalin (mean difference, −24.7; 95% CI, −34.6 to −14.7), and 14 other medicines, compared with placebo, the researchers report.
In addition, they found low or very low confidence for no difference between the effects of several of these medications.
Increased adverse events had moderate to very low confidence with tramadol (risk ratio, 2.6; 95% CI, 1.5-4.5), paracetamol plus sustained release tramadol (RR, 2.4; 95% CI, 1.5-3.8), baclofen (RR, 2.3; 95% CI, 1.5-3.4), and paracetamol plus tramadol (RR, 2.1; 95% CI, 1.3-3.4), compared with placebo, the investigators add.
“These medicines could increase the risk of adverse events, compared with other medicines with moderate to low confidence. Moderate to low confidence was also noted for secondary outcomes and secondary analysis of medicine classes,” the researchers note.
The review suggested 14 additional comparisons favored the treatment over placebo, all with very low confidence except for one with low confidence.
In the 68 trials that included the number of participants reporting an adverse event, there was moderate confidence for increased adverse events with the opioid tramadol (RR, 2.6; 95% CI, 1.5-4.5), paracetamol plus sustained release tramadol (RR, 2.4; 95% CI, 1.5-3.8), paracetamol plus tramadol (RR, 2.1; 95% CI, 1.3-3.4), and low confidence for baclofen (RR, 2.3; 1.5-3.4), compared with placebo.
The review also uncovered moderate to low confidence for secondary outcomes, which included low back-specific function, serious adverse events, and acceptability (number of participants who dropped out).
Unexpected findings
The new results were somewhat unexpected, said Mr. Wewege.
“When we set out to do this review, we envisioned the evidence would be a lot more comprehensive. We didn’t think it would be so disconnected and there would be so few trials looking at the different comparisons that would lead us to have low confidence in most of the findings.”
Various factors contributed to this low confidence, he said. One was the risk of bias – about 90% of trials had some concerns or high risk of bias. Another factor was the heterogeneity in effect estimates.
Most of the evidence is based on studies comparing different analgesics to placebo, Mr. Wewege noted. The lack of head-to-head drug comparisons is because “the easiest way to get a drug approved is just to demonstrate it’s better than placebo,” he said.
In addition to these new findings, clinicians should consider a medication’s availability, their own expertise, and patient preferences when selecting an analgesic, said Mr. Wewege. He noted most patients with acute low back pain get better within a few weeks without any intervention.
“Patients should be reassured that things will heal naturally and that they are not going to be in pain forever,” he said.
Determining optimal treatment is key
Chris Gilligan, MD, associate chief medical officer, Brigham and Women’s Hospital, and associate professor of anesthesia, Harvard Medical School, both in Boston, said determining which medications are optimal is “key,” as acute low back pain is very common and analgesics are used frequently.
The new review does provide information on which medications have the strongest evidence for pain reduction, said Dr. Gilligan. “On the one hand, it directionally points you towards certain medications, and even certain classes of medication, for comparative effectiveness.”
However, he said, the confidence for this effectiveness is low or very low, “so I wouldn’t overweight it.”
The data on adverse effects, where the confidence is mostly moderate to low, might have more of an influence on prescribing, he said.
“For example, there’s some indication tramadol may be more closely associated with adverse events in patients with acute low back pain and that would add to our caution about using tramadol; it’s not that we would never use it, but [we]would take that into account.”
Dr. Gilligan agrees clinicians should be cautious about prescribing analgesics for low back pain. One reason for being conservative in terms of treatments, he noted, is that “acute low back pain has a very favorable natural history.”
While clinical practice guidelines recommend nonpharmacologic therapies as first- and second-line treatment for acute, nonspecific low back pain, Dr. Gilligan noted that as with drugs, evidence for nondrug therapies also has low or very low confidence.
The study received funding from a 2020 Exercise Physiology Research (Consumables) Grant from the University of New South Wales, which was used to obtain translations of studies published in languages other than English.
Mr. Wewege was supported by a Postgraduate Scholarship from the National Health and Medical Research Council of Australia, a School of Medical Sciences Top-Up Scholarship from the University of New South Wales, and a PhD Supplementary Scholarship from Neuroscience Research Australia. Dr. Gilligan reports that he conducts clinical trials with companies and groups, including the National Institutes of Health related to medications, devices, and procedures for pain.
A version of this article first appeared on Medscape.com.
FROM BMJ
Dupilumab moves forward as possible COPD treatment
of more than 900 adults with uncontrolled chronic obstructive pulmonary disease.
In the study, known as the BOREAS trial, dupilumab met its primary and secondary endpoints, with a significant reduction compared with placebo in exacerbations for adults with chronic obstructive pulmonary disease (COPD) that was uncontrolled despite use of the maximal standard-of-care inhaled therapy (triple therapy), according to a press release from manufacturers Regeneron and Sanofi.
Dupilumab, which inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, is currently approved in multiple countries for certain patients with conditions including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, or prurigo nodularis in different age groups. The drug is not an immunosuppressant, and would be the first biologic approved for COPD, according to the manufacturers.
In the BOREAS trial, 468 adults with COPD who were current or former smokers aged 40-80 years were randomized to dupilumab and 471 to placebo; both groups continued to receive maximal standard of care.
Over 52 weeks, patients in the dupilumab group experienced a 30% reduction in moderate to severe COPD exacerbations compared with placebo (P = .0005).
In addition, patients treated with dupilumab met the key secondary endpoints of significant improvement in lung function from baseline to 12 weeks compared with placebo (160 mL vs. 77 mL, P < .0001); this difference persisted at 52 weeks (P = .0003).
Dupilumab also met endpoints for improvement in patient-reported health-related quality of life based on the St. George’s Respiratory Questionnaire (SGRQ) and reduction in the severity of respiratory symptoms of COPD based on the Evaluation Respiratory Symptoms: COPD (E-RS: COPD) Scale, according to the companies’ statement.
The results represent a previously unreported magnitude of improvement for COPD patients treated with a biologic, principal investigator George D. Yancopoulos, MD, said in the statement. “These results also validate the role type 2 inflammation plays in driving COPD in these patients, advancing the scientific community’s understanding of the underlying biology of this disease,” he added.
The safety results in the BOREAS trial were generally consistent with the known safety profile of Dupixent in its approved indications. Overall adverse event rates were similar for dupilumab and placebo patients (77% and 76%, respectively) and the overall safety profiles were consistent with the currently approved dupilumab indications, according to the manufacturers.
The adverse events that were more common in dupilumab patients compared with placebo patients were headache (8.1% vs. 6.8%), diarrhea (5.3% vs. 3.6%), and back pain (5.1% vs. 3.4%).
Adverse events leading to deaths were similar between the groups (1.7% in placebo patients and 1.5% in dupilumab patients).
Complete safety and efficacy results from the BOREAS trial are scheduled to be presented in a future scientific forum, and a second phase 3 trial of dupilumab for COPD, known as NOTUS, is ongoing, with data expected in 2024, according to the manufacturers.
The Boreas trial was sponsored by Sanofi and Regeneron Pharmaceuticals.
of more than 900 adults with uncontrolled chronic obstructive pulmonary disease.
In the study, known as the BOREAS trial, dupilumab met its primary and secondary endpoints, with a significant reduction compared with placebo in exacerbations for adults with chronic obstructive pulmonary disease (COPD) that was uncontrolled despite use of the maximal standard-of-care inhaled therapy (triple therapy), according to a press release from manufacturers Regeneron and Sanofi.
Dupilumab, which inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, is currently approved in multiple countries for certain patients with conditions including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, or prurigo nodularis in different age groups. The drug is not an immunosuppressant, and would be the first biologic approved for COPD, according to the manufacturers.
In the BOREAS trial, 468 adults with COPD who were current or former smokers aged 40-80 years were randomized to dupilumab and 471 to placebo; both groups continued to receive maximal standard of care.
Over 52 weeks, patients in the dupilumab group experienced a 30% reduction in moderate to severe COPD exacerbations compared with placebo (P = .0005).
In addition, patients treated with dupilumab met the key secondary endpoints of significant improvement in lung function from baseline to 12 weeks compared with placebo (160 mL vs. 77 mL, P < .0001); this difference persisted at 52 weeks (P = .0003).
Dupilumab also met endpoints for improvement in patient-reported health-related quality of life based on the St. George’s Respiratory Questionnaire (SGRQ) and reduction in the severity of respiratory symptoms of COPD based on the Evaluation Respiratory Symptoms: COPD (E-RS: COPD) Scale, according to the companies’ statement.
The results represent a previously unreported magnitude of improvement for COPD patients treated with a biologic, principal investigator George D. Yancopoulos, MD, said in the statement. “These results also validate the role type 2 inflammation plays in driving COPD in these patients, advancing the scientific community’s understanding of the underlying biology of this disease,” he added.
The safety results in the BOREAS trial were generally consistent with the known safety profile of Dupixent in its approved indications. Overall adverse event rates were similar for dupilumab and placebo patients (77% and 76%, respectively) and the overall safety profiles were consistent with the currently approved dupilumab indications, according to the manufacturers.
The adverse events that were more common in dupilumab patients compared with placebo patients were headache (8.1% vs. 6.8%), diarrhea (5.3% vs. 3.6%), and back pain (5.1% vs. 3.4%).
Adverse events leading to deaths were similar between the groups (1.7% in placebo patients and 1.5% in dupilumab patients).
Complete safety and efficacy results from the BOREAS trial are scheduled to be presented in a future scientific forum, and a second phase 3 trial of dupilumab for COPD, known as NOTUS, is ongoing, with data expected in 2024, according to the manufacturers.
The Boreas trial was sponsored by Sanofi and Regeneron Pharmaceuticals.
of more than 900 adults with uncontrolled chronic obstructive pulmonary disease.
In the study, known as the BOREAS trial, dupilumab met its primary and secondary endpoints, with a significant reduction compared with placebo in exacerbations for adults with chronic obstructive pulmonary disease (COPD) that was uncontrolled despite use of the maximal standard-of-care inhaled therapy (triple therapy), according to a press release from manufacturers Regeneron and Sanofi.
Dupilumab, which inhibits the signaling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) pathways, is currently approved in multiple countries for certain patients with conditions including atopic dermatitis, asthma, chronic rhinosinusitis with nasal polyps, eosinophilic esophagitis, or prurigo nodularis in different age groups. The drug is not an immunosuppressant, and would be the first biologic approved for COPD, according to the manufacturers.
In the BOREAS trial, 468 adults with COPD who were current or former smokers aged 40-80 years were randomized to dupilumab and 471 to placebo; both groups continued to receive maximal standard of care.
Over 52 weeks, patients in the dupilumab group experienced a 30% reduction in moderate to severe COPD exacerbations compared with placebo (P = .0005).
In addition, patients treated with dupilumab met the key secondary endpoints of significant improvement in lung function from baseline to 12 weeks compared with placebo (160 mL vs. 77 mL, P < .0001); this difference persisted at 52 weeks (P = .0003).
Dupilumab also met endpoints for improvement in patient-reported health-related quality of life based on the St. George’s Respiratory Questionnaire (SGRQ) and reduction in the severity of respiratory symptoms of COPD based on the Evaluation Respiratory Symptoms: COPD (E-RS: COPD) Scale, according to the companies’ statement.
The results represent a previously unreported magnitude of improvement for COPD patients treated with a biologic, principal investigator George D. Yancopoulos, MD, said in the statement. “These results also validate the role type 2 inflammation plays in driving COPD in these patients, advancing the scientific community’s understanding of the underlying biology of this disease,” he added.
The safety results in the BOREAS trial were generally consistent with the known safety profile of Dupixent in its approved indications. Overall adverse event rates were similar for dupilumab and placebo patients (77% and 76%, respectively) and the overall safety profiles were consistent with the currently approved dupilumab indications, according to the manufacturers.
The adverse events that were more common in dupilumab patients compared with placebo patients were headache (8.1% vs. 6.8%), diarrhea (5.3% vs. 3.6%), and back pain (5.1% vs. 3.4%).
Adverse events leading to deaths were similar between the groups (1.7% in placebo patients and 1.5% in dupilumab patients).
Complete safety and efficacy results from the BOREAS trial are scheduled to be presented in a future scientific forum, and a second phase 3 trial of dupilumab for COPD, known as NOTUS, is ongoing, with data expected in 2024, according to the manufacturers.
The Boreas trial was sponsored by Sanofi and Regeneron Pharmaceuticals.
Life’s Essential 8: Higher scores extend health span
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report on
This study leveraged the UK Biobank and included more than 135,000 U.K. adults with a mean age of 55. The AHA metric was defined as including the following lifestyle behavioral factors:
- Not smoking.
- Regular physical activity.
- Healthy weight.
- Healthy diet.
- Healthy sleep (defined as an average of 7-9 hours nightly).
- Blood pressure in a healthy range.
- Blood glucose in a healthy range.
- Non-HDL cholesterol in a healthy range.
This study was just published in JAMA Internal Medicine. I’d like to acknowledge that I’m a coauthor of this study, along with my colleagues at Tulane.
We divided the study population into three groups: those with low, moderate, and high scores on the Life’s Essential 8 metric – low, moderate, and high cardiovascular health. Overall, the average life expectancy free of chronic disease was estimated to be age 50, with 25 additional years in men and 30 additional years in women.
We saw large differences across the Life’s Essential 8 metric group. Men with high cardiovascular health scores tended to have an additional 7 years of life expectancy free of chronic disease, compared with those who had poorer scores. In women, the difference was about 9.5 years between high scores and lower scores. Also, the number of years lived with chronic disease was compressed in those with high cardiovascular health scores. They tended to have fewer years living with those chronic diseases but more years living free of chronic diseases.
We were interested in how these results might differ by socioeconomic status, educational level, and income level, as well as the Townsend deprivation index. We were intrigued by the finding that the gain in life expectancy free of chronic disease was very similar across all socioeconomic strata – those with lower education and lower income gained as much in terms of chronic disease–free life expectancy as those who were in the higher socioeconomic strata.
Overall, the findings make a compelling case for the importance of lifestyle factors in extending health span and years free of chronic disease. It can be motivating to tell our patients that a healthy lifestyle not only extends life expectancy but also extends years of health free of chronic disease.
Nonetheless, we do have many disparities in life expectancy and health span. So it will be very important to population health to narrow those health disparities through education about the importance of lifestyle factors, more research on implementation of lifestyle factors and behaviors, and public policy to make a healthy lifestyle both affordable and accessible to all people across all of these socioeconomic groups.
Thank you so much for your attention.
JoAnn E. Manson, MD, DrPH, is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report on
This study leveraged the UK Biobank and included more than 135,000 U.K. adults with a mean age of 55. The AHA metric was defined as including the following lifestyle behavioral factors:
- Not smoking.
- Regular physical activity.
- Healthy weight.
- Healthy diet.
- Healthy sleep (defined as an average of 7-9 hours nightly).
- Blood pressure in a healthy range.
- Blood glucose in a healthy range.
- Non-HDL cholesterol in a healthy range.
This study was just published in JAMA Internal Medicine. I’d like to acknowledge that I’m a coauthor of this study, along with my colleagues at Tulane.
We divided the study population into three groups: those with low, moderate, and high scores on the Life’s Essential 8 metric – low, moderate, and high cardiovascular health. Overall, the average life expectancy free of chronic disease was estimated to be age 50, with 25 additional years in men and 30 additional years in women.
We saw large differences across the Life’s Essential 8 metric group. Men with high cardiovascular health scores tended to have an additional 7 years of life expectancy free of chronic disease, compared with those who had poorer scores. In women, the difference was about 9.5 years between high scores and lower scores. Also, the number of years lived with chronic disease was compressed in those with high cardiovascular health scores. They tended to have fewer years living with those chronic diseases but more years living free of chronic diseases.
We were interested in how these results might differ by socioeconomic status, educational level, and income level, as well as the Townsend deprivation index. We were intrigued by the finding that the gain in life expectancy free of chronic disease was very similar across all socioeconomic strata – those with lower education and lower income gained as much in terms of chronic disease–free life expectancy as those who were in the higher socioeconomic strata.
Overall, the findings make a compelling case for the importance of lifestyle factors in extending health span and years free of chronic disease. It can be motivating to tell our patients that a healthy lifestyle not only extends life expectancy but also extends years of health free of chronic disease.
Nonetheless, we do have many disparities in life expectancy and health span. So it will be very important to population health to narrow those health disparities through education about the importance of lifestyle factors, more research on implementation of lifestyle factors and behaviors, and public policy to make a healthy lifestyle both affordable and accessible to all people across all of these socioeconomic groups.
Thank you so much for your attention.
JoAnn E. Manson, MD, DrPH, is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
This is Dr. JoAnn Manson, professor of medicine at Harvard Medical School and Brigham and Women’s Hospital. I’d like to talk with you about a recent report on
This study leveraged the UK Biobank and included more than 135,000 U.K. adults with a mean age of 55. The AHA metric was defined as including the following lifestyle behavioral factors:
- Not smoking.
- Regular physical activity.
- Healthy weight.
- Healthy diet.
- Healthy sleep (defined as an average of 7-9 hours nightly).
- Blood pressure in a healthy range.
- Blood glucose in a healthy range.
- Non-HDL cholesterol in a healthy range.
This study was just published in JAMA Internal Medicine. I’d like to acknowledge that I’m a coauthor of this study, along with my colleagues at Tulane.
We divided the study population into three groups: those with low, moderate, and high scores on the Life’s Essential 8 metric – low, moderate, and high cardiovascular health. Overall, the average life expectancy free of chronic disease was estimated to be age 50, with 25 additional years in men and 30 additional years in women.
We saw large differences across the Life’s Essential 8 metric group. Men with high cardiovascular health scores tended to have an additional 7 years of life expectancy free of chronic disease, compared with those who had poorer scores. In women, the difference was about 9.5 years between high scores and lower scores. Also, the number of years lived with chronic disease was compressed in those with high cardiovascular health scores. They tended to have fewer years living with those chronic diseases but more years living free of chronic diseases.
We were interested in how these results might differ by socioeconomic status, educational level, and income level, as well as the Townsend deprivation index. We were intrigued by the finding that the gain in life expectancy free of chronic disease was very similar across all socioeconomic strata – those with lower education and lower income gained as much in terms of chronic disease–free life expectancy as those who were in the higher socioeconomic strata.
Overall, the findings make a compelling case for the importance of lifestyle factors in extending health span and years free of chronic disease. It can be motivating to tell our patients that a healthy lifestyle not only extends life expectancy but also extends years of health free of chronic disease.
Nonetheless, we do have many disparities in life expectancy and health span. So it will be very important to population health to narrow those health disparities through education about the importance of lifestyle factors, more research on implementation of lifestyle factors and behaviors, and public policy to make a healthy lifestyle both affordable and accessible to all people across all of these socioeconomic groups.
Thank you so much for your attention.
JoAnn E. Manson, MD, DrPH, is professor of medicine and the Michael and Lee Bell Professor of Women’s Health, Harvard Medical School, Boston.
A version of this article first appeared on Medscape.com.
Autism rates trending upwards, CDC reports
Childhood autism rates have ticked up once again, according to the latest data from Centers for Disease Control and Prevention.
According to the CDC, 1 in 36 (2.8%) 8-year-old children have been identified with autism spectrum disorder (ASD) – up from the previous 2018 estimate of 1 in 44 (2.3%).
The updated data come from 11 communities in the Autism and Developmental Disabilities Monitoring (ADDM) network and were published online in Morbidity and Mortality Weekly Report.
A separate report in the MMWR on 4-year-old children in the same 11 communities highlights the impact of COVID-19, showing disruptions in progress in early autism detection.
In the early months of the pandemic, 4-year-old children were less likely to have an evaluation or be identified with ASD than 8-year-old children when they were the same age. This coincides with interruptions in childcare and health care services during the COVID-19 pandemic.
“Disruptions due to the pandemic in the timely evaluation of children and delays in connecting children to the services and support they need could have long-lasting effects,” Karen Remley, MD, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a statement.
“The data in this report can help communities better understand how the pandemic impacted early identification of autism in young children and anticipate future needs as these children get older,” Dr. Remley noted.
Shifting demographics
The latest data also show that ASD prevalence among Asian, Black, and Hispanic children was at least 30% higher in 2020 than in 2018, and ASD prevalence among White children was 14.6% higher than in 2018.
For the first time, according to the CDC, the percentage of 8-year-old Asian/Pacific Islander (3.3%), Hispanic (3.2%) and Black (2.9%) children identified with autism was higher than the percentage of 8-year-old White children (2.4%).
This is the opposite of racial and ethnic differences seen in previous ADDM reports for 8-year-olds. These shifts may reflect improved screening, awareness, and access to services among historically underserved groups, the CDC said.
Disparities for co-occurring intellectual disability have also persisted, with a higher percentage of Black children with autism identified with intellectual disability compared with White, Hispanic, or Asian/Pacific Islander children with autism. These differences could relate in part to access to services that diagnose and support children with autism, the CDC noted.
Overall, autism prevalence within the 11 ADDM communities was nearly four times higher for boys than girls. However, it’s the first time that the prevalence of autism among 8-year-old girls has topped 1%.
Community differences
Autism prevalence in the 11 ADDM communities ranged from 1 in 43 (2.3%) children in Maryland to 1 in 22 (4.5%) in California – variations that could be due to how communities identify children with autism.
This variability affords an opportunity to compare local policies and models for delivering diagnostic and interventional services that could enhance autism identification and provide more comprehensive support to people with autism, the CDC said.
A version of this article first appeared on Medscape.com.
Childhood autism rates have ticked up once again, according to the latest data from Centers for Disease Control and Prevention.
According to the CDC, 1 in 36 (2.8%) 8-year-old children have been identified with autism spectrum disorder (ASD) – up from the previous 2018 estimate of 1 in 44 (2.3%).
The updated data come from 11 communities in the Autism and Developmental Disabilities Monitoring (ADDM) network and were published online in Morbidity and Mortality Weekly Report.
A separate report in the MMWR on 4-year-old children in the same 11 communities highlights the impact of COVID-19, showing disruptions in progress in early autism detection.
In the early months of the pandemic, 4-year-old children were less likely to have an evaluation or be identified with ASD than 8-year-old children when they were the same age. This coincides with interruptions in childcare and health care services during the COVID-19 pandemic.
“Disruptions due to the pandemic in the timely evaluation of children and delays in connecting children to the services and support they need could have long-lasting effects,” Karen Remley, MD, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a statement.
“The data in this report can help communities better understand how the pandemic impacted early identification of autism in young children and anticipate future needs as these children get older,” Dr. Remley noted.
Shifting demographics
The latest data also show that ASD prevalence among Asian, Black, and Hispanic children was at least 30% higher in 2020 than in 2018, and ASD prevalence among White children was 14.6% higher than in 2018.
For the first time, according to the CDC, the percentage of 8-year-old Asian/Pacific Islander (3.3%), Hispanic (3.2%) and Black (2.9%) children identified with autism was higher than the percentage of 8-year-old White children (2.4%).
This is the opposite of racial and ethnic differences seen in previous ADDM reports for 8-year-olds. These shifts may reflect improved screening, awareness, and access to services among historically underserved groups, the CDC said.
Disparities for co-occurring intellectual disability have also persisted, with a higher percentage of Black children with autism identified with intellectual disability compared with White, Hispanic, or Asian/Pacific Islander children with autism. These differences could relate in part to access to services that diagnose and support children with autism, the CDC noted.
Overall, autism prevalence within the 11 ADDM communities was nearly four times higher for boys than girls. However, it’s the first time that the prevalence of autism among 8-year-old girls has topped 1%.
Community differences
Autism prevalence in the 11 ADDM communities ranged from 1 in 43 (2.3%) children in Maryland to 1 in 22 (4.5%) in California – variations that could be due to how communities identify children with autism.
This variability affords an opportunity to compare local policies and models for delivering diagnostic and interventional services that could enhance autism identification and provide more comprehensive support to people with autism, the CDC said.
A version of this article first appeared on Medscape.com.
Childhood autism rates have ticked up once again, according to the latest data from Centers for Disease Control and Prevention.
According to the CDC, 1 in 36 (2.8%) 8-year-old children have been identified with autism spectrum disorder (ASD) – up from the previous 2018 estimate of 1 in 44 (2.3%).
The updated data come from 11 communities in the Autism and Developmental Disabilities Monitoring (ADDM) network and were published online in Morbidity and Mortality Weekly Report.
A separate report in the MMWR on 4-year-old children in the same 11 communities highlights the impact of COVID-19, showing disruptions in progress in early autism detection.
In the early months of the pandemic, 4-year-old children were less likely to have an evaluation or be identified with ASD than 8-year-old children when they were the same age. This coincides with interruptions in childcare and health care services during the COVID-19 pandemic.
“Disruptions due to the pandemic in the timely evaluation of children and delays in connecting children to the services and support they need could have long-lasting effects,” Karen Remley, MD, director of CDC’s National Center on Birth Defects and Developmental Disabilities, said in a statement.
“The data in this report can help communities better understand how the pandemic impacted early identification of autism in young children and anticipate future needs as these children get older,” Dr. Remley noted.
Shifting demographics
The latest data also show that ASD prevalence among Asian, Black, and Hispanic children was at least 30% higher in 2020 than in 2018, and ASD prevalence among White children was 14.6% higher than in 2018.
For the first time, according to the CDC, the percentage of 8-year-old Asian/Pacific Islander (3.3%), Hispanic (3.2%) and Black (2.9%) children identified with autism was higher than the percentage of 8-year-old White children (2.4%).
This is the opposite of racial and ethnic differences seen in previous ADDM reports for 8-year-olds. These shifts may reflect improved screening, awareness, and access to services among historically underserved groups, the CDC said.
Disparities for co-occurring intellectual disability have also persisted, with a higher percentage of Black children with autism identified with intellectual disability compared with White, Hispanic, or Asian/Pacific Islander children with autism. These differences could relate in part to access to services that diagnose and support children with autism, the CDC noted.
Overall, autism prevalence within the 11 ADDM communities was nearly four times higher for boys than girls. However, it’s the first time that the prevalence of autism among 8-year-old girls has topped 1%.
Community differences
Autism prevalence in the 11 ADDM communities ranged from 1 in 43 (2.3%) children in Maryland to 1 in 22 (4.5%) in California – variations that could be due to how communities identify children with autism.
This variability affords an opportunity to compare local policies and models for delivering diagnostic and interventional services that could enhance autism identification and provide more comprehensive support to people with autism, the CDC said.
A version of this article first appeared on Medscape.com.